Name of Policy: Diagnosis and Treatment of Chronic Cerebrospinal Venous Insufficiency in Multiple Sclerosis
|
|
|
- Brandon Homer Davidson
- 8 years ago
- Views:
Transcription
1 Name of Policy: Diagnosis and Treatment of Chronic Cerebrospinal Venous Insufficiency in Multiple Sclerosis Policy #: 477 Latest Review Date: June 2015 Category: Medical Policy Grade: C Background/Definitions: As a general rule, benefits are payable under Blue Cross and Blue Shield of Alabama health plans only in cases of medical necessity and only if services or supplies are not investigational, provided the customer group contracts have such coverage. The following Association Technology Evaluation Criteria must be met for a service/supply to be considered for coverage: 1. The technology must have final approval from the appropriate government regulatory bodies; 2. The scientific evidence must permit conclusions concerning the effect of the technology on health outcomes; 3. The technology must improve the net health outcome; 4. The technology must be as beneficial as any established alternatives; 5. The improvement must be attainable outside the investigational setting. Medical Necessity means that health care services (e.g., procedures, treatments, supplies, devices, equipment, facilities or drugs) that a physician, exercising prudent clinical judgment, would provide to a patient for the purpose of preventing, evaluating, diagnosing or treating an illness, injury or disease or its symptoms, and that are: 1. In accordance with generally accepted standards of medical practice; and 2. Clinically appropriate in terms of type, frequency, extent, site and duration and considered effective for the patient s illness, injury or disease; and 3. Not primarily for the convenience of the patient, physician or other health care provider; and 4. Not more costly than an alternative service or sequence of services at least as likely to produce equivalent therapeutic or diagnostic results as to the diagnosis or treatment of that patient s illness, injury or disease. Page 1 of 12
2 Description of Procedure or Service: Chronic cerebrospinal venous insufficiency (CCSVI) may be associated with multiple sclerosis (MS), although this is controversial and an active area of research. Correction of CCSVI has been attempted via percutaneous venoplasty. The intent of this procedure is to relieve MS symptoms by improving venous drainage of the central nervous system. Correction of CCSVI by this method may be referred to as the Liberation Procedure. Multiple sclerosis (MS) is generally considered a chronic inflammatory demyelinating disease of the central nervous system (brain, spinal cord, optic nerve) felt to be triggered by an autoimmune response to myelin. However, in part due to the periventricular predilection of the lesions of multiple sclerosis, vascular etiologies (chronic cerebrospinal venous insufficiency [CCSVI]) have also been considered. The core foundation of this vascular theory is that there is abnormal venous drainage from the brain due to outflow obstruction in the draining jugular vein and/or azygos veins. This abnormal venous drainage, which is characterized by special ultrasound criteria, is said to cause intracerebral flow disturbance or outflow problems that lead to periventricular deposits. In the CCSVI theory, these deposits have a similarity to the iron deposits seen around the veins in the legs in patients with chronic deep vein thrombosis. Balloon dilatation, with or without stenting, has been proposed as a means to treat the outflow problems, thereby alleviating CCSVI and MS complaints. The following five criteria were defined by Zamboni et al as features of CCSVI. In order to make the diagnosis of CCSVI, at least two of the five criteria need to be present: 1. Reflux constantly present (for a duration >0.8 s) in the supine and upright positions at the level of an internal jugular or vertebral vein. This parameter was evaluated during a short breath-hold following normal breathing and not under Valsalva maneuver. 2. Reflux at the level of veins of the deep cerebral system (for a duration >0.5 s). This was evaluated with the patient in the sitting and supine positions, and venous flow was enhanced by inviting the patient to breath in. 3. Stenosis (<0.3 cm), valve abnormalities and septa on B-mode imaging. 4. Absence of flow at the level of the internal jugular or vertebral vein despite numerous deep inspirations. 5. No increase in the diameter of the internal jugular vein when changing from an upright to a supine position (lack of Δ-). Policy: The identification and subsequent treatment of chronic cerebrospinal venous insufficiency (CCSVI) in patients with multiple sclerosis does not meet Blue Cross and Blue Shield of Alabama s medical criteria for coverage and is considered investigational. Blue Cross and Blue Shield of Alabama does not approve or deny procedures, services, testing, or equipment for our members. Our decisions concern coverage only. The decision of whether or not to have a certain test, treatment or procedure is one made between the physician and his/her patient. Blue Cross and Blue Shield of Alabama administers benefits based on the member s contract and corporate medical policies. Physicians should always exercise their best Page 2 of 12
3 medical judgment in providing the care they feel is most appropriate for their patients. Needed care should not be delayed or refused because of a coverage determination. Key Points: This policy is based on a literature search through April Diagnosis of CCSVI and Association with MS Recent interest in the role of chronic cerebrospinal venous insufficiency (CCSVI) in multiple sclerosis (MS) followed reports from a European vascular surgeon, Zamboni (et al). He used ultrasound and catheter-based venography to describe venous insufficiency in the internal jugular veins, vertebral veins, and deep cerebral veins of MS patients and described the finding as CCSVI. Using five ultrasound criteria, Zamboni defined the condition of CCSVI. In initial research reports, Zamboni and colleagues reported both sensitivity and specificity of 100% in separating MS patients from controls when applying these criteria. Since the development of the Zamboni criteria for CCSVI, there have been numerous research studies that have attempted to compare the rate of CCSVI in MS, normal patients, and patients with other neurologic diagnoses. The following review includes some of the largest of these studies, as well as any relevant systematic reviews and meta-analysis. In 2014, a meta-analysis on the association between CCSVI and MS was published by Tsivgoulis et al. Nineteen studies with a total of 1250 MS patients and 899 healthy controls were included in the meta-analysis. CCSVI was associated with MS in the pooled analysis with an odds ratio (OR) of 8.35 (95% confidence interval [CI], 3.44 to 20.31; p<0.001). Heterogeneity across studies was considerable with a reported I 2 of 80.1%. In additional sensitivity analyses, the OR associating CCSVI and MS decreased. In the most conservative sensitivity analysis, which excluded eight studies (studies by Zamboni or associated groups and studies conducted in Italy or by advocates of endovascular procedures for CCSVI), MS was not associated with CCSVI with an OR of 1.35 (95% CI, 0.62 to 2.93; p=0.453). Additionally, heterogeneity was not found with a reported I 2 of 0%. Zwischenberger et al in 2013 reported on a meta-analysis of the association between MS and CCSVI diagnosed by ultrasound. Included in the meta-analysis were 13 studies with a total of 1141 MS patients and 738 healthy controls. Initial analysis demonstrated CCSVI was associated with MS with an OR of 2.57 (p<0.001), but heterogeneity was significant with a reported I 2 of 82.7% (p<0.001). In a subsequent analysis of nine studies with four outliers (studies with disproportionately high ORs) removed, the OR decreased, but still associated CCSVI with MS with an OR of (p<0.001) and heterogeneity decreased with an I 2 of 18 (p=0.279). A systematic review of the association between CCSVI and MS was published in 2011 by Laupacis et al. This review included eight studies that used ultrasound to diagnose CCSVI by the Zamboni criteria and compared the rate of CCSVI in patients with MS to those without MS. These studies were mostly small, with the median number of patients with MS of 50. There was a large degree of heterogeneity across studies in the rate of CCSVI among MS patients. Two smaller studies reported a rate of 0% for CCSVI in a total of 20 and 56 patients with MS. In Page 3 of 12
4 contrast, the original study by Zamboni et al reported a 100% rate of CCSVI in 109 patients with MS. A small study of 25 patients also reported a very high rate of CCSVI at 84% (21/25). There was no obvious reason identified for this large discrepancy in CCSVI rates; the authors hypothesized that the most likely reason was variability in ultrasound technique and interpretation. There was a significant association of CCSVI with MS in combined analysis, with an odds ratio of 13.5 (95% confidence interval [CI]: 2.6 to 71.4). There was a large amount of heterogeneity in this measure as well, with a reported I2 0f 89%. Several sensitivity analyses were performed, with marked variability of the odds ratio from a low of 3.7 to more than 58,000, depending on the analysis. However, in all cases the association of CCSVI with MS remained significant. A second systematic review published in 2011 that included a smaller number of studies (n=4) came to similar conclusions. The rate of CCSVI in MS patients ranged from 7 to 100%, and the rate in non-ms patients ranged from 2 to 36%. There was a significant association between MS and CCSVI but with a high degree of heterogeneity (I 2 =96%) and an odds ratio for association that varied widely, from approximately two to more than 26,000. Clinical Studies The largest study performed to date is a U.S. study by Zivadinov and colleagues that used ultrasound to evaluate CCSVI in 499 subjects. A subject was considered CCSVI-positive if two or more venous hemodynamic (VH) criteria were fulfilled. The authors studies of transcranial and extracranial echo-colored Doppler (ECD) were carried out in 499 enrolled subjects: 289 with MS, 163 healthy controls (HC), 26 other neurologic diseases (OND), 21 clinically isolated syndromes (CIS). Prevalence rates for CCSVI were calculated in three ways: first, using only the subjects for whom diagnosis was certain (i.e., borderline subjects were excluded); second, including the borderline subjects in the no CCSVI group; and finally, taking into account subjects who presented any of the VH criteria. CCSVI prevalence with borderline cases included in the no CCSVI group was 56.1% in MS, 42.3% in OND, 38.1% in CIS, and 22.7% in HC (p<0.001). The CCSVI prevalence figures were 62.5% for MS, 45.8% for OND, 42.1% for CIS, and 25.5% for HC when borderline cases were excluded (p<0.001). The prevalence of one or more positive VH criteria was the highest in MS (81.3%), followed by CIS (76.2%), OND (65.4%), and HC (55.2%) (p<0.001). CCSVI prevalence was higher in patients with progressive than in nonprogressive MS (p=0.004). The authors concluded that their findings were consistent with an increased prevalence of CCSVI in MS but with modest sensitivity and specificity. They also noted that their findings point against CCSVI having a primary causative role in the development of MS. Zivadinov et al also reported on a substudy from the original study to explore any relationship between CCSVI and intracranial MS pathology as determined by magnetic resonance imaging (MRI). This substudy included 228 MS patients (162 relapsing-remitting and 66 secondaryprogressive MS subtypes) and 73 HCs who had MRI imaging within 30 days of ultrasound imaging for CCSVI. In the MS group, 131 (57.5%) patients were considered CCSVI-positive and 21 (9.2%) were considered having borderline CCSVI. In the HC group, 19 (26%) were CCSVIpositive and six (8.2%) had borderline CCSVI. CCSVI was not significantly correlated with MRI imaging results on lesion burden and brain atrophy in MS patients and HCs. There was also no association between CCSVI and MRI imaging markers of inflammatory and neurodegenerative processes. Page 4 of 12
5 In an additional report from the Zivadinov study, Weinstock et al analyzed data from the MS subjects to examine the association between CCSVI and disability status, as measured by the Kurtzke Expanded Disability Status Scale (EDSS) and MS severity scale (MSSS). CCSVI was not associated with disability status. However, there was an association between CCSVI and secondary or progressive MS versus nonprogressive MS, which included relapsing-remitting MS (p=0.004, OR: 2.34, CI: 1.3 to 4.2). Barreto et al conducted a single-center, prospective, case control study of 206 MS and 70 non- MS patients to examine rates of CCSVI using color and spectral Doppler, B-mode imaging, with neurosonologists blinded to patients clinical characteristics. Rates of CCSVI and extracranial or intracranial venous flow rates were not significantly different between MS and non-ms patients. In MS patients, CCSVI was found in 3.88% versus 7.14% of non-ms patients. Floris et al used the Zamboni criteria to assess 74 patients with a diagnosis of MS and 34 healthy controls. All patients underwent Doppler ultrasound of the neck and transcranial Doppler ultrasound. A total of 34 patients were identified with CCSVI. The rate of CCSVI in MS patients was numerically higher than in normal controls (55% vs. 35%), but this difference did not reach statistical significance (p=0.09). There were 12 of 74 patients 16% in the MS group who had normal ultrasound exams and an additional 28% (21 patients) who had some abnormalities on ultrasound but did not meet the criteria for CCSVI. Centonze et al evaluated CCSVI by the Zamboni criteria in 84 patients with MS and 56 healthy controls. The rate of CCSVI was 50% in the MS patients versus 36% in controls (p=0.12). These authors also reported that there were no differences between MS patients that did and did not meet the criteria for CCSVI on demographic and clinical characteristics. There were also no differences between MS patients that did and did not meet CCSVI criteria in terms of disease severity, functional status, or quality of life. Section Summary The relationship between CCSVI and MS is unclear. The initial reports of excellent discrimination of MS patients from non-ms patients using CCSVI ultrasound criteria has not been replicated in subsequent studies. There is an extremely large variability in the literature in the rate of CCSVI among MS patients, ranging from 0 to 100%. Many of these studies report higher rates of CCSVI in MS patients, but others do not. Systematic reviews and meta-analyses have reported that the combined odds ratio for an association is significantly increased; however, there is a very large degree of heterogeneity in these studies that has not been explained. If there is an association, it is unclear whether this is a causative factor for MS or whether the ultrasound findings are a result of MS and/or related processes. Treatment of CCSVI with Percutaneous Venoplasty The latest literature review for this Policy identified one randomized controlled trial (RCT) that compared outcomes in patients who underwent venous angioplasty versus a sham-operated concurrent control group. Page 5 of 12
6 In 2014, Siddiqui et al published results from a prospective, double-blind, sham-controlled RCT of venous angioplasty in MS patients with CCSVI. This trial enrolled nine patients in intervention group and 10 in the sham-controlled group. All patients met the criteria for diagnosis of CCSVI. The primary end points of the trial included safety at 24 hours and 30 days postangioplasty; greater than 75% restoration of venous outflow at 30 days; the presence of new MS lesions; and relapse rate over six months. Secondary end points included changes in disability scores, brain volume, cognitive test scores, and QOL measures. All patients tolerated the procedures well; no operative or postoperative complications were identified. One patient in the angioplasty group experienced an episode of symptomatic bradycardia. No significant differences were observed in venous outflow characteristics between the treated and control groups, nor were any significant improvements observed in clinical disease scores among treated patients compared with controls. The results of this RCT are limited by the small number of patients. However, the failure to show a beneficial effect of venous angioplasty on MS activity supports a lack of efficacy for this treatment. Some experts have suggested that the results show clinical equipoise no longer exists with regard to venous angioplasty and CCSVI and that further clinical study of the former would be unethical. The authors of a 2015 comprehensive literature review of venous angioplasty as treatment of CCSVI in MS concur with the editorial. They indicate that this procedure has no proven efficacy, may exacerbate underlying disease activity, and state that the treatment should no longer be offered, even in clinical trials. In a 2012 Cochrane review, Van Zuuren et al found no randomized controlled trials (RCTs) on the treatment of CCSVI in MS patients. While there are ongoing clinical trials, the reviewers concluded the efficacy or safety of percutaneous transluminal angioplasty (PTA) for CCSVI treatment in MS patients could not be determined. Hubbard et al prospectively followed 259 MS patients treated with venous angioplasty for CCSVI. Patients completed the Multiple Sclerosis Impact Scale (MSIS-29) one month before, and one and six months after angioplasty. MSIS scores significantly improved at each evaluation after angioplasty on both the physical and psychologic scales (p<0.01). Symptoms recurred in 15 patients (6.3%). In a case series of 65 patients with MS and CCSVI, Zamboni et al reported clinical improvement following catheter-based venoplasty. Patients were subdivided by MS clinical course into relapsing remitting (n=35), secondary progressive (n=20), and primary progressive (n=10) MS, and all patients underwent percutaneous transluminal angioplasty (PTA). Mean follow-up was 18 months. In this study, outpatient endovascular treatment of CCSVI was noted to be feasible, with a minor complication rate. Postoperative venous pressure was significantly lower. The endovascular treatment was noted to improve MS clinical outcome measures, especially in the relapsing remitting group: the rate of relapse-free patients changed from 27% to 50% postoperatively (p<0.001). The Multiple Sclerosis Functional Composite at one year improved significantly in relapsing remitting patients (p<0.008) but not in primary progressive or secondary progressive. Physical quality of life (QOL) improved significantly in relapsing remitting (p<0.01) and in primary progressive patients (p<0.03), with a positive trend in secondary progressive (p<0.08). The authors concluded that PTA of venous strictures in patients Page 6 of 12
7 with CCSVI is safe, and especially in patients with relapsing remitting disease, the clinical course was positively influenced by treatment. The authors also indicated these results were from a pilot study and that a subsequent randomized controlled study is warranted. Zamboni et al also reported a smaller series of eight patients with ultrasound criteria for CCSVI undergoing immediate venoplasty compared to seven patients undergoing delayed venoplasty. There were improvements on the EDSS (expanded disability status scale) for both groups following treatment, but no difference between groups in the first six months comparing immediate versus delayed treatment subjects. The relapse rate during the initial six months was 0.12% in the treatment group versus 0.66% in the control group, but this difference did not meet statistical significance. There were also trends toward improvement for the immediate treatment group on magnetic resonance imaging (MRI) scans, such as the number of T2 lesions, but these differences also did not reach statistical significance. No short-term adverse events were reported following the procedure, but the rate of restenosis at one year was 27% in treated patients. Adverse events The initial small case series of venoplasty reported few adverse events. However, a number of larger case series have now been published that report on complications following endovascular interventions for CCSVI. Burton et al described five patients who had undergone venoplasty and presented with complications of the procedure. The complications were internal jugular vein stent thrombosis, cerebral sinovenous thrombosis, stent migration, cranial nerve injury, and injury associated with venous catheterization. There was not a denominator in these studies to determine the rate of these events. Petrov et al reported on the safety profile of 495 venoplasty procedures performed in 461 patients with MS, including 98 stent implantations. There were no deaths, major bleeding events, or acute exacerbations of MS. The most common procedure-related complication was vein dissection, which occurred in 3.0% of cases. Other complications included cardiac arrhythmias (1.2%), groin hematoma (1.0%), vein rupture (0.4%), and acute stent thrombosis (1.6%). Mandato et al reported adverse events within 30 days of endovascular intervention for 240 patients with MS over an eight-month period. Neck pain occurred in 15.6% of patients, most commonly following stent implantation. Headache occurred in 8.2% of patients and was persistent past 30 days in one patient (0.4%). Intraprocedural arrhythmias occurred in 1.3%, and one patient was diagnosed with a stress-induced cardiomyopathy following the procedure. An U.S. Food and Drug Administration (FDA) alert was issued in May 2012 concerning the potential for adverse events following endovascular interventions for MS. Reports of adverse events obtained by the FDA included death, stroke, detachment and/or migration of stents, vein damage, thrombosis, cranial nerve damage, and abdominal bleeding. This alert included the caveat that clinical trials of this procedure require FDA approval and an investigational device exemption due to potential for harms. Page 7 of 12
8 Section Summary A prospective, double-blind, sham-controlled RCT of venous angioplasty in MS patients (N=20) with CCSVI published in 2014 showed no significant differences in venous outflow characteristics between the treated and control groups, nor any significant improvements in clinical disease scores among treated patients compared with controls. The results of this RCT are limited by the small number of patients. The efficacy of venoplasty for CCSVI has been evaluated only in small case series and one very small trial of immediate versus delayed treatment. These studies report improvements in symptoms and disease-specific quality-of-life measures. However, this evidence is insufficient to determine the efficacy of venoplasty because of the small amount of literature and the lack of controlled studies. Randomized controlled trials (RCTs) are needed to adequately assess efficacy, especially when subjective patient-reported outcomes are used as the primary endpoint. A few case series of several hundred patients have reported on adverse events. These studies establish that adverse events are uncommon following venoplasty, but serious adverse events do occur. The FDA issued an alert in May 2012, noting the existence of serious complications, including death, and the need for ongoing monitoring. It is not currently possible to estimate the rate of serious adverse events such as death or major bleeding with confidence. Summary The association of CCSVI with MS is uncertain. The rate of CCSVI in MS patients varies widely in the literature for unclear reasons, from 0 to 100%. Some studies report higher rates of CCSVI in patients with MS compared to non-ms patients, but others do not. If there is an association between MS and CCSVI, it is not known whether this is a causative factor for MS or a secondary result of the disease. It also appears that CCSVI can occur in other disorders, and is not specific for MS. Treatment of CCSVI with endovascular interventions has been attempted. Some currently available studies report improvement in patient-reported symptoms following treatment, but this evidence is not sufficient to establish efficacy. A prospective, double-blind, sham-controlled randomized controlled trial (RCT) of venous angioplasty in MS patients (N=20) with CCSVI published in 2014 showed no significant differences in venous outflow characteristics between the treated and control groups, nor any significant improvements in clinical disease scores among treated patients compared with controls. The results of this RCT are limited by the small number of patients. However, the failure to show a beneficial effect of venous angioplasty on blood flow or symptoms supports a lack of efficacy for this treatment. Adverse events occur at a low overall rate, but serious adverse events can occur, and the FDA issued an alert in 2012 concerning the potential for serious adverse events. Practice Guidelines and Position Statements The Cardiovascular and Interventional Radiological Society of Europe (CIRSE) commentary on the treatment of chronic cerebrospinal venous insufficiency notes that: Page 8 of 12
9 Thus far, no trial data are available, and there is currently no randomized controlled trial (RCT) in progress. Therefore, the basis for this new treatment rests on anecdotal evidence and successful testimonies by patients on the Internet. CIRSE believes that this is not a sound basis on which to offer a new treatment, which could have possible procedure-related complications, to an often desperate patient population. The Society for Interventional Radiology (SIR) published a position statement on the association of CCSVI with MS and the efficacy of endovascular treatments. Their recommendations included the following statements: At present, SIR considers the published literature to be inconclusive on whether CCSVI is a clinically important factor in the development and/or progression of MS, and on whether balloon angioplasty and/or stent placement are clinically effective in patients with MS. SIR strongly supports the urgent performance of high-quality clinical research to determine the safety and efficacy of interventional MS therapies, and is actively working to promote and expedite the completion. The U.K. National Institute for Health and Clinical Excellence (NICE) published a guidance document on the use of percutaneous venoplasty to treat CCSVI in patients with MS. This document contained the following statements on the diagnosis and treatment of CCSVI: Current evidence on the efficacy of percutaneous venoplasty for chronic cerebrospinal venous insufficiency (CCSVI) for multiple sclerosis (MS) is inadequate in quality and quantity. Therefore, this procedure should only be used in the context of research. NICE encourages further research on percutaneous venoplasty for CCSVI for MS, in the form of robust controlled clinical trials. Studies should clearly define selection criteria and patient characteristics. They should also clearly define technical success which may include measurement of pressure gradients across treated vein segments before and after venoplasty. Outcomes should include clinical and quality of life measures. The European Society of Neurosonology and Cerebral Hemodynamics (ESNCH) issued a statement on CCSVI and MS in The ESNCH statement indicates the proposed criteria for the diagnosis of CCSVI is questionable due to methodological and technological errors, and lack of validation. The statement strongly discourages any interventional treatment for CCSVI in MS, such as transluminal angioplasty and/or stenting, due to lack of evidence and risk of serious complications. U.S. Preventive Services Task Force Recommendations Not applicable. Key Words: Chronic cerebrospinal venous insufficiency, CCSVI, Liberation Procedure, CCSVI interventions, balloon venoplasty, catheter-based venoplasty, PTA of venous strictures in patients with CCSVI Page 9 of 12
10 Approved by Governing Bodies: Endovascular correction of CCSVI is a surgical procedure and as such is not subject to FDA approval. However, in 2012, FDA issued an alert concerning the potential for serious adverse events with the treatment for CCSVI. Benefit Application: Coverage is subject to member s specific benefits. Group specific policy will supersede this policy when applicable. ITS: Home Policy provisions apply. FEP: Special benefit consideration may apply. Refer to member s benefit plan. FEP does not consider investigational if FDA approved and will be reviewed for medical necessity. Current Coding: CPT Codes: Transluminal balloon angioplasty, open, venous Transluminal balloon angioplasty, percutaneous; venous Transluminal balloon angioplasty, venous (e.g., subclavian stenosis), radiological supervision and interpretation References: 1. Baracchini C, Valdueza JM, Del Sette M et al. CCSVI and MS: a statement from the European Society of neurosonology and cerebral hemodynamics. J Neurol 2012; 259(12): Barreto AD, Brod SA, Bui TT et al. Chronic cerebrospinal venous insufficiency: Casecontrol neurosonography results. Ann Neurol. Dec Bourdette DN, Cohen JA. Venous angioplasty for "CCSVI" in multiple sclerosis: ending a therapeutic misadventure. Neurology. Jul ;83(5): Burton JM, Alikhani K, Goyal M et al. Complications in MS patients after CCSVI procedures abroad (Calgary, AB). Can. J. Neurol. Sci. 2011; 38(5): Centonze D, Floris R, Stefanini M et al. Proposed chronic cerebrospinal venous insufficiency criteria do not predict multiple sclerosis risk or severity. Ann. Neurol. 2011; 70(1): Doepp F, Paul F, Valdueza JM et al. No cerebrospinal venous congestion in patients with multiple sclerosis. Ann Neurol 2010;68(2): Floris R, Centonze D, Fabiano S et al. Prevalence study of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis: preliminary data. Radiol Med Fox RJ, Rae-Grant A. Chronic cerebrospinal venous insufficiency: have we found the cause and cure of MS? Neurology 2011 Apr 13. [Epub ahead of print] 9. Hubbard D, Ponec D, Gooding J et al. Clinical improvement after extracranial venoplasty in multiple sclerosis. J Vasc Interv Radiol 2012; 23(10): Laupacis A, Lillie E, Dueck A et al. Association between chronic cerebrospinal venous insufficiency and multiple sclerosis: a meta-analysis. CMAJ 2011; 183(16):E Page 10 of 12
11 11. Mandato KD, Hegener PF, Siskin GP et al. Safety of endovascular treatment of chronic cerebrospinal venous insufficiency: a report of 240 patients with multiple sclerosis. J. Vasc. Interv. Radiol. 2012; 23(1): Mayer CA, Pfeilschifter W, Lorenz MW et al. The perfect crime? CCSVI not leaving a trace in MS. J Neurol Neurosurg Psychiatry 2011; 82(4): National Institute for Health and Clinical Excellence (NICE). Percutaneous venoplasty for chronic cerebrospinal venous insufficiency for multiple sclerosis. March Available online at: Petrov I, Grozdinski L, Kaninski G et al. Safety profile of endovascular treatment for chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Endovasc Ther 2011; 18(3): Reekers JA, Lee MJ, Belli AM et al. Cardiovascular and Interventional Radiological Society of Europe commentary on the treatment of chronic cerebrospinal venous insufficiency. Cardiovasc Intervent Radiol 2011;34(1): Siddiqui AH, Zivadinov R, Benedict RH, et al. Prospective randomized trial of venous angioplasty in MS (PREMiSe). Neurology. Jul ;83(5): Thapar A, Lane T, Nicholas R et al. Systematic review of sonographic chronic cerebrospinal venous insufficiency findings in multiple sclerosis. Phlebology 2011; 26(8): Tsivgoulis G, Faissner S, Voumvourakis K, et al. "Liberation treatment" for chronic cerebrospinal venous insufficiency in multiple sclerosis: the truth will set you free. Brain Behav. Jan 2015;5(1): Tsivgoulis G, Sergentanis TN, Chan A et al. Chronic cerebrospinal venous insufficiency and multiple sclerosis: a comprehensive meta-analysis of case-control studies. Ther Adv Neurol Disord 2014; 7(2): U.S. Food and Drug Administration (FDA). FDA News Release: FDA issues alert on potential dangers of unproven treatment for multiple sclerosis. Available online at: elivery Last accessed April 18, van Zuuren EJ, Fedorowicz Z, Pucci E et al. Percutaneous transluminal angioplasty for treatment of chronic cerebrospinal venous insufficiency (CCSVI) in multiple sclerosis patients. Cochrane Database Syst Rev 2012; 12:CD Vedantham S, Benenati JF, Kundu S et al. Interventional endovascular management of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis: a position statement by the Society of Interventional Radiology, endorsed by the Canadian Interventional Radiology Association. J. Vasc. Interv. Radiol. 2010; 21(9): Weinstock-Guttman B, Ramanathan M, Marr K et al. Clinical correlates of chronic cerebrospinal venous insufficiency in multiple sclerosis. BMC Neurol 2012; 12: Zamboni P, Galeotti R, Menegatti E et al. A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency. J Vasc Surg 2009; 50(6): Zamboni P, Galeotti R, Weinstock-Guttman B et al. Venous angioplasty in patients with multiple sclerosis: results of a pilot study. Eur. J. Vasc. Endovasc. Surg. 2012; 43(1): Zamboni R, Galeotti R, Menegatti E et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2009;80(4): Zivadinov R, Cutter G, Marr K et al. No association between conventional brain MR imaging and chronic cerebrospinal venous insufficiency in multiple sclerosis. AJNR Am J Neuroradiol 2012; 33(10): Page 11 of 12
12 28. Zivadinov R, Mar K, Cutter G et al. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurology 2011 Apr 13. [Epub ahead of print] 29. Zwischenberger BA, Beasley MM, Davenport DL et al. Meta-analysis of the correlation between chronic cerebrospinal venous insufficiency and multiple sclerosis. Vasc Endovascular Surg 2013; 47(8): Policy History: Medical Policy Panel, June 2011 Medical Policy Group, June 2011 (2) Medical Policy Administration Committee, July 2011 Available for comment July 6 through August 22, 2011 Medical Policy Panel, June 2012 Medical Policy Group, July 2012 (2): Updated Description, Key Points, References Medical Policy Group, June 2013 (2): Update to Key Points and References Medical Policy Group, October 2013 (2): Removed ICD-9 Diagnosis codes; no change to policy statement. Medical Policy Panel, June 2014 Medical Policy Group, June 2014 (4): Updated Key Points and References. No change to the policy statement. Medical Policy Panel, May 2015 Medical Policy Group, June 2015 (2): 2015 Updates to Key Points, Approved by Governing Bodies, and Reference; no change to policy statement. This medical policy is not an authorization, certification, explanation of benefits, or a contract. Eligibility and benefits are determined on a caseby-case basis according to the terms of the member s plan in effect as of the date services are rendered. All medical policies are based on (i) research of current medical literature and (ii) review of common medical practices in the treatment and diagnosis of disease as of the date hereof. Physicians and other providers are solely responsible for all aspects of medical care and treatment, including the type, quality, and levels of care and treatment. This policy is intended to be used for adjudication of claims (including pre-admission certification, pre-determinations, and pre-procedure review) in Blue Cross and Blue Shield s administration of plan contracts. Page 12 of 12
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Diagnosis and Treatment of CCSVI in MS Page 1 of 12 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Diagnosis and Treatment of Chronic Cerebrospinal Venous Insufficiency
Diagnosis and Treatment of CCSVI in MS Page 1 of 12 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Diagnosis and Treatment of Chronic Cerebrospinal Venous Insufficiency
OHTAC Recommendation
 OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
Summary Diagnosis and Treatment of Chronic Cerebrospinal Venous Insufficiency (CCSVI) in People with Multiple Sclerosis (MS)
 ETMIS 2012; Vol. 8: N o 7 Summary Diagnosis and Treatment of Chronic Cerebrospinal Venous Insufficiency (CCSVI) in People with Multiple Sclerosis (MS) March 2012 A production of the Institut national d
ETMIS 2012; Vol. 8: N o 7 Summary Diagnosis and Treatment of Chronic Cerebrospinal Venous Insufficiency (CCSVI) in People with Multiple Sclerosis (MS) March 2012 A production of the Institut national d
PRO BALLOON VENOPLASTY IS A PROMISING TECHNOLOGY FOR TREATMENT OF MS
 PRO BALLOON VENOPLASTY IS A PROMISING TECHNOLOGY FOR TREATMENT OF MS Manish Mehta MD MPH The Vascular Group The Institute for Vascular Health & Disease Albany Medical Center SVS 2012 DISCLOSURES Research,
PRO BALLOON VENOPLASTY IS A PROMISING TECHNOLOGY FOR TREATMENT OF MS Manish Mehta MD MPH The Vascular Group The Institute for Vascular Health & Disease Albany Medical Center SVS 2012 DISCLOSURES Research,
Name of Policy: Reconstructive versus Cosmetic Surgery
 Name of Policy: Reconstructive versus Cosmetic Surgery Policy #: 106 Latest Review Date: February 2010 Category: Administrative Policy Grade: Background/Definitions: As a general rule, benefits are payable
Name of Policy: Reconstructive versus Cosmetic Surgery Policy #: 106 Latest Review Date: February 2010 Category: Administrative Policy Grade: Background/Definitions: As a general rule, benefits are payable
HealthPACT Health Policy Advisory Committee on Technology Australia and New Zealand Technology Brief
 HealthPACT Health Policy Advisory Committee on Technology Australia and New Zealand Technology Brief Percutaneous venoplasty for the treatment of chronic cerebrospinal venous insufficiency (CCSVI) in multiple
HealthPACT Health Policy Advisory Committee on Technology Australia and New Zealand Technology Brief Percutaneous venoplasty for the treatment of chronic cerebrospinal venous insufficiency (CCSVI) in multiple
Study could hold key to MS treatment
 Page 1 of 5 "We're very encouraged, but can't say it's a 100 percent thing yet," says Dr. Robert Zivadinov, principal investigator of the new MS theory. Harry Scull Jr./Buffalo News Study could hold key
Page 1 of 5 "We're very encouraged, but can't say it's a 100 percent thing yet," says Dr. Robert Zivadinov, principal investigator of the new MS theory. Harry Scull Jr./Buffalo News Study could hold key
Refer to https://www.bcbsal.org/providers/hcreform/hcrpreventivecod ing.pdf for the Quick Reference Guide for HCR Preventive Care Services
 Refer to https://www.bcbsal.org/providers/hcreform/hcrpreventivecod ing.pdf for the Quick Reference Guide for HCR Preventive Care Services Name of Policy: Preventive Care Services under Health Care Reform
Refer to https://www.bcbsal.org/providers/hcreform/hcrpreventivecod ing.pdf for the Quick Reference Guide for HCR Preventive Care Services Name of Policy: Preventive Care Services under Health Care Reform
Guidelines for the Management of Patients Following Endoluminal Vein Dilation Procedures for the Treatment of Multiple Sclerosis
 Guidelines for the Management of Patients Following Endoluminal Vein Dilation Procedures for the Treatment of Multiple Sclerosis Report Submitted to the Minister of Health and Long-Term Care By the Ontario
Guidelines for the Management of Patients Following Endoluminal Vein Dilation Procedures for the Treatment of Multiple Sclerosis Report Submitted to the Minister of Health and Long-Term Care By the Ontario
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Name of Policy: Medical Criteria for Physical/Occupational Therapy and Osteopathic/Chiropractic Manipulative Treatment
 Name of Policy: Medical Criteria for Physical/Occupational Therapy and Osteopathic/Chiropractic Manipulative Treatment Policy #: 132 Latest Review Date: January 2015 Category: Administrative Policy Grade:
Name of Policy: Medical Criteria for Physical/Occupational Therapy and Osteopathic/Chiropractic Manipulative Treatment Policy #: 132 Latest Review Date: January 2015 Category: Administrative Policy Grade:
Publicly-funded clinical trials on endovascular treatment of multiple sclerosis? The views of a citizens council
 Publicly-funded clinical trials on endovascular treatment of multiple sclerosis? The views of a citizens council EXECUTIVE SUMMARY Background: There is controversy in Canada about treating multiple sclerosis
Publicly-funded clinical trials on endovascular treatment of multiple sclerosis? The views of a citizens council EXECUTIVE SUMMARY Background: There is controversy in Canada about treating multiple sclerosis
John E. O Toole, Marjorie C. Wang, and Michael G. Kaiser
 Hypothermia and Human Spinal Cord Injury: Updated Position Statement and Evidence Based Recommendations from the AANS/CNS Joint Sections on Disorders of the Spine & Peripheral Nerves and Neurotrauma &
Hypothermia and Human Spinal Cord Injury: Updated Position Statement and Evidence Based Recommendations from the AANS/CNS Joint Sections on Disorders of the Spine & Peripheral Nerves and Neurotrauma &
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
Version History. Previous Versions. Policy Title. Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Multiple Sclerosis Society of Canada. CADA Presentation May 4, 2011
 Multiple Sclerosis Society of Canada CADA Presentation May 4, 2011 1 MS Society s Mission To be a leader in finding a cure for multiple sclerosis and enabling people affected by MS to enhance their quality
Multiple Sclerosis Society of Canada CADA Presentation May 4, 2011 1 MS Society s Mission To be a leader in finding a cure for multiple sclerosis and enabling people affected by MS to enhance their quality
SUMMARY REPORT. CIHR and MS Society of Canada Joint Invitational Meeting on Multiple Sclerosis Research August 26, 2010, Ottawa, Ontario
 SUMMARY REPORT CIHR and MS Society of Joint Invitational Meeting on Multiple Sclerosis Research August 26, 2010, Ottawa, Ontario Overview The Canadian Institutes of Health Research (CIHR), in collaboration
SUMMARY REPORT CIHR and MS Society of Joint Invitational Meeting on Multiple Sclerosis Research August 26, 2010, Ottawa, Ontario Overview The Canadian Institutes of Health Research (CIHR), in collaboration
Non-surgical treatment of severe varicose veins
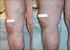 Non-surgical treatment of severe varicose veins Yasu Harasaki UCHSC Department of Surgery General Surgery Grand Rounds March 19, 2007 Definition Dilated, palpable, subcutaneous veins generally >3mm in
Non-surgical treatment of severe varicose veins Yasu Harasaki UCHSC Department of Surgery General Surgery Grand Rounds March 19, 2007 Definition Dilated, palpable, subcutaneous veins generally >3mm in
Summary chapter 2 chapter 2
 Summary Multiple Sclerosis (MS) is a chronic disease of the brain and the spinal cord. The cause of MS is unknown. MS usually starts in young adulthood. In the course of the disease progression of neurological
Summary Multiple Sclerosis (MS) is a chronic disease of the brain and the spinal cord. The cause of MS is unknown. MS usually starts in young adulthood. In the course of the disease progression of neurological
Background report on whether treating MS/CCSVI by cerebrospinal vein dilatation complies with established medical science and medical practice
 Report Background report on whether treating MS/CCSVI by cerebrospinal vein dilatation complies with established medical science and medical practice ICD-10 code: G35 Multiple sclerosis Multiple sclerosis
Report Background report on whether treating MS/CCSVI by cerebrospinal vein dilatation complies with established medical science and medical practice ICD-10 code: G35 Multiple sclerosis Multiple sclerosis
ß-interferon and. ABN Guidelines for 2007 Treatment of Multiple Sclerosis with. Glatiramer Acetate
 ABN Guidelines for 2007 Treatment of Multiple Sclerosis with ß-interferon and Glatiramer Acetate Published by the Association of British Neurologists Ormond House, 27 Boswell Street, London WC1N 3JZ Contents
ABN Guidelines for 2007 Treatment of Multiple Sclerosis with ß-interferon and Glatiramer Acetate Published by the Association of British Neurologists Ormond House, 27 Boswell Street, London WC1N 3JZ Contents
Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty
 Round Table: Antithrombotic therapy beyond ACS Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty M. Matsagkas, MD, PhD, EBSQ-Vasc Associate Professor
Round Table: Antithrombotic therapy beyond ACS Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty M. Matsagkas, MD, PhD, EBSQ-Vasc Associate Professor
Clinical Medical Policy Cognitive Rehabilitation
 Benefit Coverage Outpatient cognitive rehabilitation is considered to be the most appropriate setting for members who have sustained a traumatic brain injury or an acute brain insult. Covered Benefit for
Benefit Coverage Outpatient cognitive rehabilitation is considered to be the most appropriate setting for members who have sustained a traumatic brain injury or an acute brain insult. Covered Benefit for
How To Cover Occupational Therapy
 Guidelines for Medical Necessity Determination for Occupational Therapy These Guidelines for Medical Necessity Determination (Guidelines) identify the clinical information MassHealth needs to determine
Guidelines for Medical Necessity Determination for Occupational Therapy These Guidelines for Medical Necessity Determination (Guidelines) identify the clinical information MassHealth needs to determine
Committee Approval Date: September 12, 2014 Next Review Date: September 2015
 Medication Policy Manual Policy No: dru361 Topic: Pradaxa, dabigatran Date of Origin: September 12, 2014 Committee Approval Date: September 12, 2014 Next Review Date: September 2015 Effective Date: November
Medication Policy Manual Policy No: dru361 Topic: Pradaxa, dabigatran Date of Origin: September 12, 2014 Committee Approval Date: September 12, 2014 Next Review Date: September 2015 Effective Date: November
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: spinal_cord_stimulation 3/1980 10/2015 10/2016 10/2015 Description of Procedure or Service Spinal cord stimulation
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: spinal_cord_stimulation 3/1980 10/2015 10/2016 10/2015 Description of Procedure or Service Spinal cord stimulation
Summary HTA. Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C. HTA-Report Summary
 Summary HTA HTA-Report Summary Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C Health policy background Multiple sclerosis (MS) is a chronic inflammatory disease
Summary HTA HTA-Report Summary Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C Health policy background Multiple sclerosis (MS) is a chronic inflammatory disease
AUBMC Multiple Sclerosis Center
 AUBMC Multiple Sclerosis Center 1 AUBMC Multiple Sclerosis Center The vision of the American University of Beirut Medical Center (AUBMC) is to be the leading academic medical center in Lebanon and the
AUBMC Multiple Sclerosis Center 1 AUBMC Multiple Sclerosis Center The vision of the American University of Beirut Medical Center (AUBMC) is to be the leading academic medical center in Lebanon and the
MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION. POLICY NUMBER: 8.01.19 CATEGORY: Therapy/Rehabilitation
 MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
Policy #: 111 Latest Review Date: January 2010
 Name of Policy: Co-surgeons and Team Surgeons Policy #: 111 Latest Review Date: January 2010 Category: Administrative Policy Grade: N/A Background: As a general rule, benefits are payable under Blue Cross
Name of Policy: Co-surgeons and Team Surgeons Policy #: 111 Latest Review Date: January 2010 Category: Administrative Policy Grade: N/A Background: As a general rule, benefits are payable under Blue Cross
on behalf of the AUGMENT-HF Investigators
 One Year Follow-Up Results from AUGMENT-HF: A Multicenter Randomized Controlled Clinical Trial of the Efficacy of Left Ventricular Augmentation with Algisyl-LVR in the Treatment of Heart Failure* Douglas
One Year Follow-Up Results from AUGMENT-HF: A Multicenter Randomized Controlled Clinical Trial of the Efficacy of Left Ventricular Augmentation with Algisyl-LVR in the Treatment of Heart Failure* Douglas
Name of Policy: Antigen Leukocyte Cellular Antibody Test (ALCAT)
 Name of Policy: Antigen Leukocyte Cellular Antibody Test (ALCAT) Policy #: 165 Latest Review Date: February 2015 Category: Laboratory Policy Grade: C Background/Definitions: As a general rule, benefits
Name of Policy: Antigen Leukocyte Cellular Antibody Test (ALCAT) Policy #: 165 Latest Review Date: February 2015 Category: Laboratory Policy Grade: C Background/Definitions: As a general rule, benefits
Endovascular Treatment for Chronic Cerebrospinal Venous Insufficiency in Patients with Multiple Sclerosis
 Vascular Disease Management Published on Vascular Disease Management (http://vasculardiseasemanagement.com) Endovascular Treatment for Chronic Cerebrospinal Venous Insufficiency in Patients with Multiple
Vascular Disease Management Published on Vascular Disease Management (http://vasculardiseasemanagement.com) Endovascular Treatment for Chronic Cerebrospinal Venous Insufficiency in Patients with Multiple
Chapter 10. Summary & Future perspectives
 Summary & Future perspectives 123 Multiple sclerosis is a chronic disorder of the central nervous system, characterized by inflammation and axonal degeneration. All current therapies modulate the peripheral
Summary & Future perspectives 123 Multiple sclerosis is a chronic disorder of the central nervous system, characterized by inflammation and axonal degeneration. All current therapies modulate the peripheral
STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY
 STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY Per Medical Board decision March 18, 2008: These credentialing standards do NOT apply to peripheral angiography performed in the context
STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY Per Medical Board decision March 18, 2008: These credentialing standards do NOT apply to peripheral angiography performed in the context
Vascular Technology (VT) Content Outline Anatomy & physiology 20% Cerebrovascular Cerebrovascular normal anatomy Evaluate the cerebrovascular vessels
 Vascular Technology (VT) Content Outline Anatomy & physiology 20% normal anatomy Evaluate the cerebrovascular vessels hemodynamics Evaluate the cerebrovascular vessels for normal perfusion normal anatomy
Vascular Technology (VT) Content Outline Anatomy & physiology 20% normal anatomy Evaluate the cerebrovascular vessels hemodynamics Evaluate the cerebrovascular vessels for normal perfusion normal anatomy
ESC Guidelines on the diagnosis and treatment of peripheral artery diseases Lower extremity artery disease. Erich Minar Medical University Vienna
 ESC Guidelines on the diagnosis and treatment of peripheral artery diseases Lower extremity artery disease Erich Minar Medical University Vienna for the Task Force on the Diagnosis and Treatment of Peripheral
ESC Guidelines on the diagnosis and treatment of peripheral artery diseases Lower extremity artery disease Erich Minar Medical University Vienna for the Task Force on the Diagnosis and Treatment of Peripheral
Duration of Dual Antiplatelet Therapy After Coronary Stenting
 Duration of Dual Antiplatelet Therapy After Coronary Stenting C. DEAN KATSAMAKIS, DO, FACC, FSCAI INTERVENTIONAL CARDIOLOGIST ADVOCATE LUTHERAN GENERAL HOSPITAL INTRODUCTION Coronary artery stents are
Duration of Dual Antiplatelet Therapy After Coronary Stenting C. DEAN KATSAMAKIS, DO, FACC, FSCAI INTERVENTIONAL CARDIOLOGIST ADVOCATE LUTHERAN GENERAL HOSPITAL INTRODUCTION Coronary artery stents are
Clinical guidance for MRI referral
 MRI for cervical radiculopathy Referral by a medical practitioner (excluding a specialist or consultant physician) for a scan of spine for a patient 16 years or older for suspected: cervical radiculopathy
MRI for cervical radiculopathy Referral by a medical practitioner (excluding a specialist or consultant physician) for a scan of spine for a patient 16 years or older for suspected: cervical radiculopathy
PROVIDER POLICIES & PROCEDURES
 PROVIDER POLICIES & PROCEDURES SCLEROTHERAPY TREATMENT OF SUPERFICIAL VARICOSE VEINS OF THE LEGS The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance
PROVIDER POLICIES & PROCEDURES SCLEROTHERAPY TREATMENT OF SUPERFICIAL VARICOSE VEINS OF THE LEGS The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance
CLINICAL STUDY ABSTRACT
 CLINICAL STUDY Changes of Cine Cerebrospinal Fluid Dynamics in Patients with Multiple Sclerosis Treated with Percutaneous Transluminal Angioplasty: A Case-control Study Robert Zivadinov, MD, PhD, Christopher
CLINICAL STUDY Changes of Cine Cerebrospinal Fluid Dynamics in Patients with Multiple Sclerosis Treated with Percutaneous Transluminal Angioplasty: A Case-control Study Robert Zivadinov, MD, PhD, Christopher
Clinical Medical Policy Cognitive Rehabilitation
 Benefit Coverage Outpatient cognitive rehabilitation is considered to be the most appropriate setting for members who have sustained a traumatic brain injury or an acute brain insult. Cognitive rehabilitation
Benefit Coverage Outpatient cognitive rehabilitation is considered to be the most appropriate setting for members who have sustained a traumatic brain injury or an acute brain insult. Cognitive rehabilitation
A blood sample will be collected annually for up to 2 years for JCV antibody testing.
 Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Trauma Insurance Claims Seminar Invitation
 Trauma Insurance Claims Seminar Invitation Introduction Since the development of Trauma Insurance in Australia in the 1980s, the product has evolved at a great pace. Some of the challenges faced by claims
Trauma Insurance Claims Seminar Invitation Introduction Since the development of Trauma Insurance in Australia in the 1980s, the product has evolved at a great pace. Some of the challenges faced by claims
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual. Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014
 Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Clinically isolated syndrome (CIS)
 Clinically isolated syndrome (CIS) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 We hope you find the information in this factsheet helpful. If you would
Clinically isolated syndrome (CIS) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 We hope you find the information in this factsheet helpful. If you would
Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple Sclerosis
 Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Spinal Cord Stimulation (SCS) Therapy: Fact Sheet
 Spinal Cord Stimulation (SCS) Therapy: Fact Sheet What is SCS Therapy? Spinal cord stimulation (SCS) may be a life-changing 1 surgical option for patients to control their chronic neuropathic pain and
Spinal Cord Stimulation (SCS) Therapy: Fact Sheet What is SCS Therapy? Spinal cord stimulation (SCS) may be a life-changing 1 surgical option for patients to control their chronic neuropathic pain and
Relapsing-remitting multiple sclerosis Ambulatory with or without aid
 AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
A Definition of Multiple Sclerosis
 English 182 READING PRACTICE by Alyx Meltzer, Spring 2009 Vocabulary Preview (see bolded, underlined words) gait: (n) a particular way of walking transient: (adj) temporary; synonym = transitory remission:
English 182 READING PRACTICE by Alyx Meltzer, Spring 2009 Vocabulary Preview (see bolded, underlined words) gait: (n) a particular way of walking transient: (adj) temporary; synonym = transitory remission:
2.1 Who first described NMO?
 History & Discovery 54 2 History & Discovery 2.1 Who first described NMO? 2.2 What is the difference between NMO and Multiple Sclerosis? 2.3 How common is NMO? 2.4 Who is affected by NMO? 2.1 Who first
History & Discovery 54 2 History & Discovery 2.1 Who first described NMO? 2.2 What is the difference between NMO and Multiple Sclerosis? 2.3 How common is NMO? 2.4 Who is affected by NMO? 2.1 Who first
What You Should Know About Cerebral Aneurysms
 What You Should Know About Cerebral Aneurysms From the Cerebrovascular Imaging and Interventions Committee of the American Heart Association Cardiovascular Radiology Council Randall T. Higashida, M.D.,
What You Should Know About Cerebral Aneurysms From the Cerebrovascular Imaging and Interventions Committee of the American Heart Association Cardiovascular Radiology Council Randall T. Higashida, M.D.,
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook
 National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
MEDICAL POLICY No. 91608-R1 MENTAL HEALTH RESIDENTIAL TREATMENT: ADULT
 MENTAL HEALTH RESIDENTIAL TREATMENT: ADULT Effective Date: June 4, 2015 Review Dates: 5/14, 5/15 Date Of Origin: May 12, 2014 Status: Current Summary of Changes Clarifications: Pg 4, Description, updated
MENTAL HEALTH RESIDENTIAL TREATMENT: ADULT Effective Date: June 4, 2015 Review Dates: 5/14, 5/15 Date Of Origin: May 12, 2014 Status: Current Summary of Changes Clarifications: Pg 4, Description, updated
NOVOSTE BETA-CATH SYSTEM
 HOSPITAL INPATIENT AND OUTPATIENT BILLING GUIDE FOR THE NOVOSTE BETA-CATH SYSTEM INTRAVASCULAR BRACHYTHERAPY DEVICE This guide is intended solely for use as a tool to help hospital billing staff resolve
HOSPITAL INPATIENT AND OUTPATIENT BILLING GUIDE FOR THE NOVOSTE BETA-CATH SYSTEM INTRAVASCULAR BRACHYTHERAPY DEVICE This guide is intended solely for use as a tool to help hospital billing staff resolve
AUBAGIO (teriflunomide) oral tablet
 AUBAGIO (teriflunomide) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
AUBAGIO (teriflunomide) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
Clinical Medical Policy Outpatient Rehab Therapies (PT & OT) for Members With Special Needs
 Benefit Coverage Rehabilitative services, (PT, OT,) are covered for members with neurodevelopmental disorders when recommended by a medical provider to address a specific condition, deficit, or dysfunction,
Benefit Coverage Rehabilitative services, (PT, OT,) are covered for members with neurodevelopmental disorders when recommended by a medical provider to address a specific condition, deficit, or dysfunction,
Extracranial Jugular Venous Insufficiency in Multiple Sclerosis Patients Treated with Interferon Beta
 ORIGINAL ARTICLE Extracranial Jugular Venous Insufficiency in Multiple Sclerosis Patients Treated with Interferon Beta Bălaşa Rodica, Bajkó Z, Stan Camelia First Neurological Department, University of
ORIGINAL ARTICLE Extracranial Jugular Venous Insufficiency in Multiple Sclerosis Patients Treated with Interferon Beta Bălaşa Rodica, Bajkó Z, Stan Camelia First Neurological Department, University of
Key Words: Multiple Sclerosis, CCSVI, X-Ray C1-C2, Venous Compressive syndrome
 C1-C2 X-Ray assessment of misalignment parameters in patients with Chronic Cerebra-spinal Venous Insufficiency and Multiple Sclerosis versus patients with other pathologies. Mandolesi S (1), Marceca G
C1-C2 X-Ray assessment of misalignment parameters in patients with Chronic Cerebra-spinal Venous Insufficiency and Multiple Sclerosis versus patients with other pathologies. Mandolesi S (1), Marceca G
Subclavian Steal Syndrome By Marta Thorup
 Subclavian Steal Syndrome By Marta Thorup Definition Subclavian steal syndrome (SSS), is a constellation of signs and symptoms that arise from retrograde flow of blood in the vertebral artery, due to proximal
Subclavian Steal Syndrome By Marta Thorup Definition Subclavian steal syndrome (SSS), is a constellation of signs and symptoms that arise from retrograde flow of blood in the vertebral artery, due to proximal
Issued and entered this _6th_ day of October 2010 by Ken Ross Commissioner ORDER I PROCEDURAL BACKGROUND
 STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX Petitioner
STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX Petitioner
Intrathecal Baclofen for CNS Spasticity
 Intrathecal Baclofen for CNS Spasticity Last Review Date: November 13, 2015 Number: MG.MM.ME.31bC5 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Intrathecal Baclofen for CNS Spasticity Last Review Date: November 13, 2015 Number: MG.MM.ME.31bC5 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
PCORI Workshop on Treatment for Multiple Sclerosis. Breakout Group Topics and Questions Draft 3-27-15
 PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft 3-27-15 Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man
PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft 3-27-15 Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man
Imaging of Thoracic Endovascular Stent-Grafts
 Imaging of Thoracic Endovascular Stent-Grafts Tariq Hameed, M.D. Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, Indiana Disclosures: No relevant financial
Imaging of Thoracic Endovascular Stent-Grafts Tariq Hameed, M.D. Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, Indiana Disclosures: No relevant financial
MEDICAL ASSOCIATES HEALTH PLANS HEALTH CARE SERVI CES POLICY AND PROCEDURE MANUAL POLICY NUMBER: PP 60
 POLICY TITLE: CRITERIA UTILIZED IN DETERMINATION OF MEDICAL NECESSITY. POLICY STATEMENT: MAHP utilizes evidence based medicine in its decision making process. This policy is to establish and maintain medical
POLICY TITLE: CRITERIA UTILIZED IN DETERMINATION OF MEDICAL NECESSITY. POLICY STATEMENT: MAHP utilizes evidence based medicine in its decision making process. This policy is to establish and maintain medical
THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT
 THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT Stroke Prevention in Atrial Fibrillation Gregory Albers, M.D. Director Stanford Stroke Center Professor of Neurology and Neurological
THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT Stroke Prevention in Atrial Fibrillation Gregory Albers, M.D. Director Stanford Stroke Center Professor of Neurology and Neurological
How To Use A Drug In Multiple Sclerosis
 Revised (2009) guidelines for prescribing in multiple sclerosis INTRODUCTION In January 2001, the (ABN) first published guidelines for the use of licensed disease modifying treatments (ß-interferon and
Revised (2009) guidelines for prescribing in multiple sclerosis INTRODUCTION In January 2001, the (ABN) first published guidelines for the use of licensed disease modifying treatments (ß-interferon and
Version History. Previous Versions. for secondary progressive MS (SPMS) Policy Title. Drugs for MS.Drug facts box Interferon beta 1b
 Version History Policy Title Drugs for MS.Drug facts box Interferon beta 1b for secondary progressive MS (SPMS) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review
Version History Policy Title Drugs for MS.Drug facts box Interferon beta 1b for secondary progressive MS (SPMS) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review
A list of FDA-approved testosterone products can be found by searching for testosterone at http://www.accessdata.fda.gov/scripts/cder/drugsatfda/.
 FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke
FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON
 Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis
 May 25, 2015 Contact: Shikha Virdi 905-919-0200 ext. 5504 Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis Rebif now reimbursed under Ontario Drug Benefit Program
May 25, 2015 Contact: Shikha Virdi 905-919-0200 ext. 5504 Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis Rebif now reimbursed under Ontario Drug Benefit Program
Interferons (Avonex, Betaferon, Rebif ) for relapsing remitting multiple sclerosis (RRMS)
 Interferons (Avonex, Betaferon, Rebif ) for relapsing remitting multiple sclerosis (RRMS) Review Question: What happens when people with RRMS take interferons? The short answer: This review found that
Interferons (Avonex, Betaferon, Rebif ) for relapsing remitting multiple sclerosis (RRMS) Review Question: What happens when people with RRMS take interferons? The short answer: This review found that
Alberta s chiropractors: Spine care experts Patient satisfaction and research synopsis
 www.albertachiro.com 11203 70 Street NW Edmonton, AB T5B 1T1 Telephone: 780.420.0932 Fax: 780.425.6583 Alberta s chiropractors: Spine care experts Patient satisfaction and research synopsis Chiropractic
www.albertachiro.com 11203 70 Street NW Edmonton, AB T5B 1T1 Telephone: 780.420.0932 Fax: 780.425.6583 Alberta s chiropractors: Spine care experts Patient satisfaction and research synopsis Chiropractic
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
NeuroStar TMS Therapy Patient Guide for Treating Depression
 NeuroStar TMS Therapy Patient Guide for Treating Depression This NeuroStar TMS Therapy Patient Guide for Treating Depression provides important safety and use information for you to consider about treating
NeuroStar TMS Therapy Patient Guide for Treating Depression This NeuroStar TMS Therapy Patient Guide for Treating Depression provides important safety and use information for you to consider about treating
ABOUT XARELTO CLINICAL STUDIES
 ABOUT XARELTO CLINICAL STUDIES FAST FACTS Xarelto (rivaroxaban) is a novel, oral direct Factor Xa inhibitor. On September 30, 2008, the European Commission granted marketing approval for Xarelto for the
ABOUT XARELTO CLINICAL STUDIES FAST FACTS Xarelto (rivaroxaban) is a novel, oral direct Factor Xa inhibitor. On September 30, 2008, the European Commission granted marketing approval for Xarelto for the
Reversibility of Acute Demyelinating Lesions in relapsingremitting
 Reversibility of Acute Demyelinating Lesions in relapsingremitting Multiple Sclerosis Omar A. Khan ( Division of Neuroimmunology, Department of Neurology, Neurology and Research Services. Veterans Affairs
Reversibility of Acute Demyelinating Lesions in relapsingremitting Multiple Sclerosis Omar A. Khan ( Division of Neuroimmunology, Department of Neurology, Neurology and Research Services. Veterans Affairs
33 % of whiplash patients develop. headaches originating from the upper. cervical spine
 33 % of whiplash patients develop headaches originating from the upper cervical spine - Dr Nikolai Bogduk Spine, 1995 1 Physical Treatments for Headache: A Structured Review Headache: The Journal of Head
33 % of whiplash patients develop headaches originating from the upper cervical spine - Dr Nikolai Bogduk Spine, 1995 1 Physical Treatments for Headache: A Structured Review Headache: The Journal of Head
Medicare C/D Medical Coverage Policy
 Varicose Vein Treatment Medicare C/D Medical Coverage Policy Origination Date: June 1, 1993 Review Date: September 16, 2015 Next Review: September, 2017 DESCRIPTION OF PROCEDURE OR SERVICE Varicose veins
Varicose Vein Treatment Medicare C/D Medical Coverage Policy Origination Date: June 1, 1993 Review Date: September 16, 2015 Next Review: September, 2017 DESCRIPTION OF PROCEDURE OR SERVICE Varicose veins
Section 6. Medical Management Program
 Section 6. Medical Management Program Introduction Molina Healthcare maintains a medical management program to ensure patient safety as well as detect and prevent fraud, waste and abuse in its programs.
Section 6. Medical Management Program Introduction Molina Healthcare maintains a medical management program to ensure patient safety as well as detect and prevent fraud, waste and abuse in its programs.
THE MEDICAL TREATMENT GUIDELINES
 THE MEDICAL TREATMENT GUIDELINES I. INTRODUCTION A. About the Medical Treatment Guidelines. On December 1, 2010, the NYS Workers' Compensation Board is implementing new regulations and Medical Treatment
THE MEDICAL TREATMENT GUIDELINES I. INTRODUCTION A. About the Medical Treatment Guidelines. On December 1, 2010, the NYS Workers' Compensation Board is implementing new regulations and Medical Treatment
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
PATIENT INFORMATION BOOKLET
 PATIENT INFORMATION BOOKLET Wingspan Stent System with Gateway PTA Balloon Catheter TABLE OF CONTENTS Definitions... 2 What is the Purpose of This Booklet?... 3 What is an Intracranial Lesion?... 3 Who
PATIENT INFORMATION BOOKLET Wingspan Stent System with Gateway PTA Balloon Catheter TABLE OF CONTENTS Definitions... 2 What is the Purpose of This Booklet?... 3 What is an Intracranial Lesion?... 3 Who
What is Multiple Sclerosis? Gener al information
 What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
The Nuts and Bolts of Multiple Sclerosis. Rebecca Milholland, M.D., Ph.D. Center for Neurosciences
 The Nuts and Bolts of Multiple Sclerosis Rebecca Milholland, M.D., Ph.D. Center for Neurosciences Objectives Discuss which patients are at risk for Multiple Sclerosis Discuss the diagnostic criteria for
The Nuts and Bolts of Multiple Sclerosis Rebecca Milholland, M.D., Ph.D. Center for Neurosciences Objectives Discuss which patients are at risk for Multiple Sclerosis Discuss the diagnostic criteria for
OVERVIEW This policy is to document the criteria for coverage of services at the acute inpatient rehabilitation level of care.
 Medical Coverage Policy Acute Inpatient Rehabilitation Level of Care EFFECTIVE DATE: 07 06 2010 POLICY LAST UPDATED: 06 04 2013 sad OVERVIEW This policy is to document the criteria for coverage of services
Medical Coverage Policy Acute Inpatient Rehabilitation Level of Care EFFECTIVE DATE: 07 06 2010 POLICY LAST UPDATED: 06 04 2013 sad OVERVIEW This policy is to document the criteria for coverage of services
Renovascular Disease. Renal Artery and Arteriosclerosis
 Other names: Renal Artery Stenosis (RAS) Renal Vascular Hypertension (RVH) Renal Artery Aneurysm (RAA) How does the normal kidney work? The blood passes through the kidneys to remove the body s waste.
Other names: Renal Artery Stenosis (RAS) Renal Vascular Hypertension (RVH) Renal Artery Aneurysm (RAA) How does the normal kidney work? The blood passes through the kidneys to remove the body s waste.
Behavioral and Physical Treatments for Tension-type and Cervicogenic Headache
 Evidence Report: Behavioral and Physical Treatments for Tension-type and Cervicogenic Headache Douglas C. McCrory, MD, MHSc Donald B. Penzien, PhD Vic Hasselblad, PhD Rebecca N. Gray, DPhil Duke University
Evidence Report: Behavioral and Physical Treatments for Tension-type and Cervicogenic Headache Douglas C. McCrory, MD, MHSc Donald B. Penzien, PhD Vic Hasselblad, PhD Rebecca N. Gray, DPhil Duke University
CDEC FINAL RECOMMENDATION
 CDEC FINAL RECOMMENDATION RIVAROXABAN (Xarelto Bayer Inc.) New Indication: Pulmonary Embolism Note: The Canadian Drug Expert Committee (CDEC) previously reviewed rivaroxaban for the treatment of deep vein
CDEC FINAL RECOMMENDATION RIVAROXABAN (Xarelto Bayer Inc.) New Indication: Pulmonary Embolism Note: The Canadian Drug Expert Committee (CDEC) previously reviewed rivaroxaban for the treatment of deep vein
Understanding. Multiple Sclerosis. Tim, diagnosed in 2004.
 Understanding Multiple Sclerosis Tim, diagnosed in 2004. What Is Multiple Sclerosis? Multiple sclerosis (MS) is a neurologic disorder that affects the central nervous system (CNS). The CNS includes the
Understanding Multiple Sclerosis Tim, diagnosed in 2004. What Is Multiple Sclerosis? Multiple sclerosis (MS) is a neurologic disorder that affects the central nervous system (CNS). The CNS includes the
Version History. Previous Versions. Drugs for MS.Drug facts box fampridine Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fampridine Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fampridine Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
How do you decide on rate versus rhythm control?
 How do you decide on rate versus rhythm control? Dr. Mark O Neill Consultant Cardiologist & Electrophysiologist Assumptions Camm et al. EHJ 2010;Sept 25 epub Choice of strategy: Criteria for consideration
How do you decide on rate versus rhythm control? Dr. Mark O Neill Consultant Cardiologist & Electrophysiologist Assumptions Camm et al. EHJ 2010;Sept 25 epub Choice of strategy: Criteria for consideration
Your Guide to Express Critical Illness Insurance Definitions
 Your Guide to Express Critical Illness Insurance Definitions Your Guide to EXPRESS Critical Illness Insurance Definitions This guide to critical illness definitions will help you understand the illnesses
Your Guide to Express Critical Illness Insurance Definitions Your Guide to EXPRESS Critical Illness Insurance Definitions This guide to critical illness definitions will help you understand the illnesses
Provided by the American Venous Forum: veinforum.org
 CHAPTER 17 SURGICAL THERAPY FOR DEEP VALVE INCOMPETENCE Original author: Seshadri Raju Abstracted by Gary W. Lemmon Introduction Deep vein valvular incompetence happens when the valves in the veins (tubes
CHAPTER 17 SURGICAL THERAPY FOR DEEP VALVE INCOMPETENCE Original author: Seshadri Raju Abstracted by Gary W. Lemmon Introduction Deep vein valvular incompetence happens when the valves in the veins (tubes
Sensitive and reproducible clinical rating
 CLINICAL AND MRI MARKERS OF MS DISEASE PROGRESSION * Richard A. Rudick, MD ABSTRACT Sensitive and reproducible measures of multiple sclerosis (MS) severity and progression are important in the treatment
CLINICAL AND MRI MARKERS OF MS DISEASE PROGRESSION * Richard A. Rudick, MD ABSTRACT Sensitive and reproducible measures of multiple sclerosis (MS) severity and progression are important in the treatment
CARE MANAGEMENT FOR LATE LIFE DEPRESSION IN URBAN CHINESE PRIMARY CARE CLINICS
 CARE MANAGEMENT FOR LATE LIFE DEPRESSION IN URBAN CHINESE PRIMARY CARE CLINICS Dept of Public Health Sciences February 6, 2015 Yeates Conwell, MD Dept of Psychiatry, University of Rochester Shulin Chen,
CARE MANAGEMENT FOR LATE LIFE DEPRESSION IN URBAN CHINESE PRIMARY CARE CLINICS Dept of Public Health Sciences February 6, 2015 Yeates Conwell, MD Dept of Psychiatry, University of Rochester Shulin Chen,
Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy. Spine Volume 21(16) August 15, 1996, pp 1877-1883
 Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy 1 Spine Volume 21(16) August 15, 1996, pp 1877-1883 Saal, Joel S. MD; Saal, Jeffrey A. MD; Yurth, Elizabeth F. MD FROM
Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy 1 Spine Volume 21(16) August 15, 1996, pp 1877-1883 Saal, Joel S. MD; Saal, Jeffrey A. MD; Yurth, Elizabeth F. MD FROM
Clinical Commissioning Policy Statement: Percutaneous mitral valve leaflet repair for mitral regurgitation April 2013. Reference: NHSCB/A09/PS/b
 Clinical Commissioning Policy Statement: Percutaneous mitral valve leaflet repair for mitral regurgitation April 2013 Reference: NHS Commissioning Board Clinical Commissioning Policy Statement: Percutaneous
Clinical Commissioning Policy Statement: Percutaneous mitral valve leaflet repair for mitral regurgitation April 2013 Reference: NHS Commissioning Board Clinical Commissioning Policy Statement: Percutaneous
