Note. 2.0 Background. 1.0 Introduction RECOMMENDATION
|
|
|
- Juliana Long
- 9 years ago
- Views:
Transcription
1 Interim recommendations for the reporting of extensively drug resistant and pan-drug resistant isolates of Enterobacteriaceae, Pseudomonas aeruginosa, Acinetobacter spp. and Stenotrophomonas maltophilia German GJ 1, Jamieson FB 2, Gilmour M 3, Almohri H 4, Bullard J 5, Domingo MC 6, Fuller J 7, Girouard G 8, Haldane D 9, Hoang L 10, Levett PN 11, Longtin J 6, Melano R 2, Needle R 12, Patel SN 2, Rebbapragada A 13, Reyes RC 14, and Mulvey MR 3* Note The recommendations in this publication should be considered preliminary for one year from the publication date. Comments regarding the document should be sent to Dr. Michael Mulvey. All comments received will be reviewed by the Canadian Public Health Laboratory Network Antimicrobial Resistance Subcommittee before the final recommendations are drafted and released. Suggested citation: German GJ, Jamieson FB, Gilmour M, Almohri H, Bullard J, Domingo MC, Fuller J, Girouard G, Haldane D, Hoang L, Levett PN, Longtin J, Melano R, Needle R, Patel SN, Rebbapragada A, Reyes RC, and Mulvey MR. Interim Recommendations for the Reporting of Extensively Drug Resistant and Pan Drug Resistant Isolates of Enterobacteriaceae, Pseudomonas aeruginosa, Acinetobacter spp. and Stenotrophomonas maltophilia. Can Comm Dis Rep 2016;42:91-7. Affiliations 1 Health PEI, Charlottetown, PEI 2 Public Health Ontario Laboratories, Toronto, ON 3 National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, MB 4 LifeLabs, Toronto, ON 5 Cadham Provincial Laboratory, Winnipeg, MB 6 Laboratoire de santé publique du Québec, INSPQ, Ste-Anne-de-Bellevue, QC 7 Alberta Provincial Laboratory for Public Health, Edmonton, AB 8 Centre hospitalier universitaire Dr-Georges-L.-Dumont, Moncton, NB 9 Queen Elizabeth II Health Science Centre, Halifax, NS 10 BC Centre for Disease Control Public Health Laboratory, Vancouver, BC 11 Saskatchewan Disease Control Laboratory, Regina, SK 12 Newfoundland Public Health Laboratory, St. John s, NL 13 Dynacare, Brampton, ON 1.0 Introduction 2.0 Background 14 LifeLabs, Burnaby, BC *Correspondence: Michael. [email protected] These recommendations are produced under the auspices and authority of the Canadian Public Health Laboratory Network, Antimicrobial Resistance Working Group. They represent a consensus of peer reviewed information and expert opinion on the most appropriate ways to test for and report a multi-drug resistant phenotype in common Gram-negative pathogens. These recommendations were developed for use by all Canadian non-veterinary clinical microbiology laboratories to provide standardization for provincial and national surveillance programs. Antimicrobial resistance is a growing concern for human health as bacterial pathogens continue to accumulate genes and genomic alterations that confer resistance to antimicrobials. Most concerning is the occurrence of multiple resistance traits within individual key pathogens, which greatly limits, if not entirely eliminates the arsenal of effective treatment options for those infections, thereby leading to poor clinical outcomes. In Canada, we have observed these highly resistant strains in Enterobacteriaceae, Acinetobacter spp., Stenotrophomonas CCDR April 7, 2016 Volume 42-4 Page 91
2 maltophilia, and Pseudomonas aeruginosa (1-3). There is a need for laboratories to classify organisms that are resistant to multiple antimicrobials in order to consistently and accurately share the information locally, nationally, and internationally with the medical community, public health authorities and policy makers. More specifically, classification as multi-drug resistant is commonly an actionable finding within hospital Infection Prevention & Control programs. Recently, there has been a proposal to internationally standardize these definitions in selected Gram-positive and Gram-negative organisms (4), yet this proposal for interim definitions has not yet led to a revised definition or national recommendations. The goal of this document is to provide Canadian laboratories with a framework for consistent reporting and monitoring of multi-drug resistant organisms (MDRO), extensively drug resistant organisms (XDRO), and pan-drug resistant organisms (PDRO). The recommendations were based on an interim international proposal published in 2012 for Gram-negative organisms (4). This document modifies the following for the Canadian setting: 1) Resistance was used instead of non-susceptibility (Intermediate and Resistant) to better match which antimicrobials will be clinically used for treating resistant infections; antimicrobials that are more easily tested in the laboratory; and those that would limit unnecessary reference testing. 2) MDRO rules are separated for commonly used antimicrobials in the community setting for urine infections and non-urine infections. 3) Rather than all classes of antimicrobials being considered in the definitions, only relevant classes that are commonly tested in Canadian clinical laboratories were considered. Also within a class of antimicrobials, resistance to the most commonly used antimicrobial for treating severe infections (i.e. meropenem or imipenem) was considered rather than an inferior drug for infections (i.e. ertapenem for the carbapenems). 4) Since XDRO definitions will fluctuate from country to country based on 2 nd and 3 rd line available antimicrobials, adjustments were made for antimicrobials available/approved for use in Canada rather than all drug categories listed in the Clinical Laboratory Standards Institute (CLSI) (5). The justification for these modifications can be found in Appendix 1. Over time as new antimicrobials become available, previously available antimicrobials lose effectiveness, or no longer available, the definitions will necessitate periodic review. The recommendations stated herein are considered interim and are open for stakeholder consultation such that future recommendations evolve to accommodate public health, community care, and acute care partners. 3.0 Recommendations for Antimicrobial Susceptibility Testing 3.1 A resistant interpretation of an isolate can be determined using disk diffusion, broth microdilution, or agar dilution following CLSI guidelines for the testing of Enterobacteriaceae, Pseudomonas aeruginosa, Acinetobacter spp. and Stenotrophomonas maltophilia (5). A Health Canada or Federal Drug Administration (FDA) approved automated method or gradient diffusion strips can also be used for the generation of the antimicrobial susceptibility data. XDRO, and PDRO. It is understood that some laboratories use automated methods with Food and Drug Administration (FDA; breakpoints that may differ from the CLSI recommendations. A laboratory using FDA breakpoints should include the breakpoint difference in any report for MDRO, XDRO, and PDRO. 3.3 Certain species of Enterobacteriaceae should not be tested for particular antimicrobial agents because of intrinsic resistance to the agent (Table 1, Exceptions). 4.0 Definitions of Screening/Testing for MDRO, XDRO and PDRO These interim recommendations are to be applied only to clinical/diagnostic specimens. However, acute care and long term care facilities, and by extension health authorities, may choose to still apply the definitions of MDRO/XDRO/PDRO for screening purposes as determined by their own fiscal situation and local health resources. If isolates are part of a specialized surveillance program (e.g. in-patient screening), it should be clearly indicated in the laboratory report that the MDRO/XDRO/ PDRO is pertinent for colonization or carriage status only. 4.1 Enterobacteriaceae Multi-Drug Resistance Definition It is recognized that laboratories may not test Gram-negative isolates for all classes of antimicrobial agents and therefore would not be able to determine MDRO, XDRO, and PDRO. Therefore, we have included a category of multi-drug resistant organisms (MDRO) that should be considered for screening isolates for XDRO or PDRO There are four rules for MDRO status in Enterobacteriaceae which takes into consideration the specific specimen type (Table 1). 4.2 Acinetobacter spp. or Pseudomonas aeruginosa Multi-drug Resistance Definition An isolate should be considered MDRO if resistant to THREE of the FIVE antimicrobial agents listed below (Table 2): Piperacillin-tazobactam OR piperacillin (specifically for P. aeruginosa) 3. Ceftazidime OR cefepime 4. Imipenem OR meropenem 5. Tobramycin 4.3 Stenotrophomonas maltophilia Multi-Drug Resistance Definition S. maltophilia is intrinsically resistant to all carbapenems and most cephalosporins. A clinical isolate should be considered an MDRO if it is resistant to trimethoprim-sulfamethoxazole and subsequent susceptibility testing indicates it is also resistant to an oral anti-microbial (minocycline or levofloxacin) [Table 2]. 3.2 Current CLSI breakpoints (M100) for resistance should be used when determining the designations of MDRO, Page 92 CCDR April 7, 2016 Volume 42-4
3 Table 1: Rules for the determination of Multi-Drug-, Extensively Drug-, Pan-Drug Resistant Organisms in Enterobacteriaceae from clinical isolates a Rule Speciman Antimicrobial Groups Interpretation 1 Urine Cefixime OR Amoxicillin-clavulanate Trimethoprim-Sulfamethoxazole Nitrofurantoin 2 Non-Urine (Cefixime OR Amoxicillin-clavulanate) 3 All Meropenem b ( OR 4 All Tobramycin ) Gentamicin Piperacillin-Tazobactam ( OR ) 5.0 Confirmation of XDRO 5.1 Enterobacteriaceae XDRO Definition THREE of the FOUR groups = MDRO THREE of the THREE groups = MDRO a very broad spectrum antimicrobial and resistance to one of two commonly used and unrelated drug classes = MDRO two commonly susceptible drug classes and resistance to one of two commonly used and unrelated drug classes = MDRO 5 All Tobramycin Gentamicin Piperacillin-Tazobactam Imipenem OR Meropenem Cefepime OR (cefotaxime/ ceftriaxone) ceftazidime FOUR of the SIX antimicrobial groups = XDRO Trimethoprim-Sulfamethoxazole 6 All Same groups listed in rule #5 Resistance to SIX of SIX antimicrobial groups = PDRO Abbreviations: MDRO, multi-drug resistant organisms; XDRO, extensively drug resistant organisms; PDRO, pan-drug resistant organisms a Expert rules modified from Leclercq et al., 2013 (7) b Imipenem can be substituted for meropenem with the exception of Proteus spp An isolate that has been determined to be an MDRO should be considered an XDRO by testing/assessing resistance to other antimicrobial agents listed in this section Unlike the definition of MDRO for Enterobacteriaceae, the type of specimen does not need to be considered for the definition of XDRO An isolate of Enterobacteriaceae should be considered an XDRO when the isolate is resistant to FOUR of the SIX antimicrobial agents listed below (Table 1): 1. Tobramycin gentamicin 2. Piperacillin-tazobactam 3. Imipenem OR meropenem 4. Cefepime OR (cefotaxime/ceftriaxone) ceftazidime Pseudomonas aeruginosa XDRO Definition A P. aeruginosa should be considered an XDRO when the isolate is resistant to FOUR of the SIX antimicrobial agents listed below (Table 2): 1. Tobramycin 2. Piperacillin OR piperacillin-tazobactam 3. Imipenem OR meropenem OR doripenem 4. Cefepime OR ceftazidime Colistin A P. aeruginosa should be considered a PDRO when the isolate is resistant to ALL of the antimicrobial agents listed in Acinetobacter spp. XDRO Definition An Acinetobacter spp. should be considered an XDRO when the isolate is resistant to SIX of the EIGHT antimicrobial agents listed below (Table 2): 1. Gentamicin OR Tobramycin 2. Piperacillin-tazobactam 3. Imipenem OR meropenem OR doripenem 4. Cefepime OR ceftazidime Colistin 7. Doxycycline OR minocycline 8. (note: intrinsically resistant to trimethoprim) 5.4 Stenotrophomonas maltophilia XDRO Definition A S. maltophilia should be considered an XDRO if resistant to three oral antimicrobials (trimethoprim-sulfamethoxazole, minocycline, and levofloxacin). The isolate should be referred for complete antimicrobial susceptibility testing to exclude a PDRO (see Table 2). CCDR April 7, 2016 Volume 42-4 Page 93
4 6.0 Confirmation of PDRO An Enterobacteriaceae, P. aeruginosa, Acinetobacter spp. should be considered a PDRO when the isolate is resistant to ALL antimicrobial agents listed in Table 1 (rule 6), section 5.2.1, or 5.3.1, respectively. S. maltophilia should be considered a PDRO if it is resistant to all of the following: trimethoprim-sulfamethoxazole, levofloxacin, ceftazidime, and chloramphenicol. 7.0 Reporting to Reference Laboratories 7.1 Any laboratory identifying a MDRO that cannot confirm an XDRO or PDRO using additional antimicrobial susceptibility tests should send the isolate to a reference (provincial) laboratory (See Appendix 2). 7.2 The reference (provincial) laboratory should be notified of any XDR or PDR organisms identified and the isolate should be forwarded to the reference laboratory, and should include the following information: 1. Age of patient 2. Gender of patient 3. Type of clinical specimen (blood, respiratory, skin/soft tissue, or urine) 4. Date of collection 5. Antimicrobial susceptibility testing results from submitting laboratory 6. Out of Canada travel history in the last 3 months. Travel history is dated from the time of the first isolation of the organism. This is highly recommended for inpatients and desirable for outpatients. All countries traveled should be listed. 7.3 If multiple clinical isolates of the same species and susceptibility pattern are recovered from the same patient, send the isolate from the most invasive site where possible. Additional isolates of the same species and susceptibility pattern should be reported/sent to a reference laboratory no more frequently than every 7 days after the first isolate. Annotating as an MDRO/ XDRO/PDRO on the clinical report should continue for each isolate regardless number of isolates or time interval between specimens. Table 2: Definitions for the determination of Multi-Drug-, Extensively Drug-, Pan-Drug Resistant Organisms in select organisms MDRO XDRO / PDRO Definition Antimicrobial Groups Definitions Antimicrobial Groups THREE of the FIVE antimicrobial groups THREE of the FIVE antimicrobial groups BOTH antimicrobial groups Piperacillin-tazobactam OR piperacillin Ceftazidime OR cefepime Imipenem OR meropenem Tobramycin Piperacillin-tazobactam Ceftazidime OR cefepime Imipenem OR meropenem Tobramycin Minocycline OR levofloxacin Organism: Pseudomonas aeruginosa FOUR of the SIX antimicrobial groups = XDRO SIX of the SIX antimicrobial groups = PDRO Organism: Acinetobacter spp. SIX of the EIGHT antimicrobial groups = XDRO all groups = PDRO Organism: Stenotrophomonas maltophilia the FIRST THREE antimicrobial groups = XDRO all antimicrobial groups = PDRO Abbreviations: MRDO, multi-drug resistant organsims; XDRO, extensively drug resistant organisms; PDRO, pan-drug resistant organisms Tobramycin Piperacillin-tazobactam OR piperacillin Imipenem OR meropenem OR doripenem Cefepime OR ceftazidime Colistin Gentamicin OR tobramycin Piperacillin-tazobactam Imipenem OR meropenem OR doripenem Cefepime OR ceftazidime Colistin Doxycycline OR minocycline Minocycline Levofloxacin Ceftazidime Chloramphenicol Page 94 CCDR April 7, 2016 Volume 42-4
5 7.4 It is suggested that reports of clinical specimens found to contain XDRO or PDRO isolates incorporate the term Extensively Drug Resistant Organism or Pan-Drug Resistant Organism within the body of the clinical report. 7.5 Any XDRO or PDRO isolate identified should be reported to public health according to local, regional, and provincial regulations with the additional information outlined in The originating laboratory should retain the XDRO or PDRO isolates for at least six months, or as required by provincial or local regulations. 7.7 The reference (provincial) laboratory should report all of the data to the National Microbiology Laboratory as defined in 7.2. Acknowledgements The subcommittee would like to acknowledge the work of Dr. John Conly (University of Alberta), Dr. Charles Frenette (McGill University), and all the other members of the Canadian Infectious Disease Steering Committee Antimicrobial Resistance Surveillance Task Group. We also appreciate the support of Dr. George Zhanel (University of Manitoba) of the Canadian Antimicrobial Resistance Alliance for feedback on earlier versions of the document. We thank members of the Canadian Public Health Laboratory Network Laboratory Director s Council for review and final approval of the document. We would also like to thank Ms. Sandra Radons-Arneson from our secretariat for her support. 2. Mataseje LF, Bryce E, Roscoe D, Boyd DA, Embree J, Gravel D, Katz K, Kibsey P, Kuhn M, Mounchili A, Simor A, Taylor G, Thomas E, Turgeon N, Mulvey MR, Canadian Nosocomial Infection Surveillance Program Carbapenem-resistant Gram-negative bacilli in Canada : results from the Canadian Nosocomial Infection Surveillance Program (CNISP). J Antimicrob Chemother 67: Tien HC, Battad A, Bryce EA, Fuller J, Mulvey M, Bernard K, Brisebois R, Doucet JJ, Rizoli SB, Fowler R, Simor A Multi-drug resistant Acinetobacter infections in critically injured Canadian forces soldiers. BMC Infect Dis 7: Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson- Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL Multidrugresistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18: Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty- Third Informational Supplement M100-S25. CLSI, Wayne, PA, USA, Mosby s Medical Dictionary 9th ed 2012 St. Louis, MO: Mosby Elsevier. 7. Leclercq R, Canton R, Brown DFJ, Giske CG, Heisig P, MacGowan AP, Mouton JW, Nordmann P, Rodloff AC, Rossolini GM, Soussy CJ, Steinbakk M, Winstanley TG, Kahlmeter G EUCAST expert rules in antimicrobial susceptibility testing. Clin Microbiol Infect 19: Conflict of interest None. Funding This work was supported in kind by all laboratories of the authors and the Canadian Public Health Laboratory Network Antimicrobial Resistance Subcommittee. References 1. Laupland KB, Parkins MD, Church DL, Gregson DB, Louie TJ, Conly JM, Elsayed S, Pitout JDD Population-based epidemiological study of infections caused by carbapenemresistant Pseudomonas aeruginosa in the Calgary Health Region: importance of metallo-beta-lactamase (MBL)- producing strains. J Infect Dis 192: CCDR April 7, 2016 Volume 42-4 Page 95
6 Appendix 1 Methodology for Developing the Recommendations The article published by Magiorakos and colleagues (2012) was used as the main reference for the development of these Canadian recommendations. Drs. German and Mulvey developed the initial framework for the document, which was reviewed by the Canadian Public Health Laboratory Network (CPHLN) AMR Working Group members and invited collaborators. Two main considerations were discussed by the working group members: (i) formulation of a recommendation that focused on antimicrobial drugs commonly used in Canada; and (ii) creation of a document that is easy to use by frontline laboratories, which predominantly utilize automated methods for generating antimicrobial susceptibility data. Three rounds of discussion and document revision took place with the working group. This included discussion and suggestions from the Communicable and Infectious Disease Steering Committee (CIDSC) AMR Task Group from the Pan-Canadian Public Health Network. The final draft recommendations were reviewed by the CPHLN Executive. Major variation with recommendations in this document as compared to Magiorakos et. al. (2012) was as follows: 1. The working group decided to focus on Gram-negative isolates to keep the recommendations straightforward and achievable. It was decided that recommendations for Gram-positive organisms would be addressed in a future document; 2. Stenotrophomonas maltophilia was added as an additional Gram-negative organism to be considered for the reporting of MDRO, XDRO and PDRO in the Canadian document; 3. Although the definition of MDRO in Gram-negative organisms is an important consideration, given the treatment complications that can be associated with these infections, it was decided at a provincial and national level to voluntarily report only XDRO and PDRO isolates and use the identification of an MDRO as a screening test to direct further testing and reporting of resistant isolates. This was done to ensure frontline laboratories could easily report their findings to reference laboratories, or request additional tests of antimicrobial drugs not covered under the frontline antimicrobial drug panel needed to confirm XDRO/PDRO. 4. A great deal of discussion focused on the value of using the definition of resistance, as defined by CLSI (2015), rather than that of non-susceptibility proposed by Magiorakos et. al. (2012). It was decided to use the CLSI definition of resistance based on the main arguments put forward, which were: (i) front-line laboratories may have difficulty analyzing intermediate resistance data in the context of MDRO/ XDRO/PDRO; (ii) there were concerns about the reporting of these organisms in relation to public health. A stringent definition of resistance was determined to be the most feasible solution. 5. It was noted that laboratories may have to use FDA breakpoints, which may differ from the CLSI definitions. It was requested in the recommendations that these differences be noted in the report to the reference laboratory. 6. The exhaustive antimicrobial agents listed in the Tables of the Magiorakos et. al. (2012) publication was simplified to reflect the antimicrobial agents commonly used and available in Canada. 7. Ertapenem was removed as a marker for carbapenem resistance in Enterobacteriaceae. The specificity of ertapennem is lower than that of meropenem and imipenem and is not commonly used in a clinical laboratory setting. 8. With the exception of Acinetobacter spp. and S. maltophilia, the tetracyclines were removed from the list of antimicrobials to be considered as they are not frequently tested in frontline laboratories nor are they commonly used to treat serious infections. 9. The Canadian recommendations requested additional clinical information that were not included in the Magiorakos et. al. (2012) publication. Page 96 CCDR April 7, 2016 Volume 42-4
7 Appendix 2 Reference Laboratory Contact Information Dr. Linda Hoang BC Public Health Microbiology & Reference Laboratory, 655 West 12th Avenue, Vancouver, BC, V5Z 4R4 [email protected] Dr. Jeff Fuller Alberta Provincial Laboratory for Public Health, Alberta Health Services, 2B3.13 WMC, Street, Edmonton, AB, T6G 2J2 [email protected] Dr. Paul Levett Saskatchewan Disease Control Laboratory, 5 Research Drive, Regina, SK, S4S 0A4 [email protected] Dr. Jared Bullard Cadham Provincial Laboratory, 750 William Avenue, Winnipeg, MB, R3E 3J7 [email protected] Dr. Samir Patel Public Health Ontario Laboratories, 661 University Avenue, Ste Toronto, ON, N5G 1M1 [email protected] Dr. Gabriel Girouard Centre Hospitalier Universitaire Dr-Georges-L-Dumont, 330 Avenue Universite, Moncton, NB, E1C 2Z3 [email protected] Dr. David Haldane Queen Elizabeth II Health Science Centre, 5788 University Avenue, Halifax, NS, B3H 1V8 [email protected] Dr. Greg German Queen Elizabeth Hospital 60 Riverside Drive Charlottetown, PE C1A 8T5 [email protected] Robert Needle Newfoundland Public Health Laboratory, Dr. L.A. Miller Centre, 100 Forest Road, St. John s, NL, A1A 3Z9 [email protected] Dr. Michael Mulvey National Microbiology Laboratory 1015 Arlington Street Winnipeg, MB, R3E 3R2 [email protected] Dr. Brigitte Lefebvre Laboratoire de santé publique du Québec, Institut national de santé publique du Québec, chemin Sainte-Marie, Ste-Anne-de-Bellevue (Québec) H9X 3R5 [email protected] CCDR April 7, 2016 Volume 42-4 Page 97
The Antimicrobial Susceptibility Testing Device Manufacturer: Development Process, Timelines, and Challenges
 The Antimicrobial Susceptibility Testing Device Manufacturer: Development Process, Timelines, and Challenges Sheila Farnham, MT(ASCP) President Susceptibility Testing Manufacturers Association January
The Antimicrobial Susceptibility Testing Device Manufacturer: Development Process, Timelines, and Challenges Sheila Farnham, MT(ASCP) President Susceptibility Testing Manufacturers Association January
2013 Indiana Healthcare Provider and Hospital Administrator Multi-Drug Resistant Organism Survey
 2013 Indiana Healthcare Provider and Hospital Administrator Multi-Drug Resistant Organism Survey Antibiotic resistance is a global issue that has significant impact in the field of infectious diseases.
2013 Indiana Healthcare Provider and Hospital Administrator Multi-Drug Resistant Organism Survey Antibiotic resistance is a global issue that has significant impact in the field of infectious diseases.
Prevalence of Metallo-β-lactamases Encoding Genes Among Pseudomonas
 Avicenna J Clin Microb Infec. 2014 May; 1(1): e19216. Published online 2014 April 28. DOI: 10.17795/ajcmi-19216 Research Article Prevalence of Metallo-β-lactamases Encoding Genes Among Pseudomonas aeruginosa
Avicenna J Clin Microb Infec. 2014 May; 1(1): e19216. Published online 2014 April 28. DOI: 10.17795/ajcmi-19216 Research Article Prevalence of Metallo-β-lactamases Encoding Genes Among Pseudomonas aeruginosa
Date: November 30, 2010
 Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Date: November 30, 2010 To: Through: From: Subject: Drug Name(s): Application
Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Date: November 30, 2010 To: Through: From: Subject: Drug Name(s): Application
HUSRES Annual Report 2010 Martti Vaara www.huslab.fi www.intra.hus.fi
 HUSRES Annual Report 2010 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara, 2/2011 1 The basis of this HUSRES 2010 report is the HUSLAB/Whonet database 2010, which contains susceptibility data
HUSRES Annual Report 2010 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara, 2/2011 1 The basis of this HUSRES 2010 report is the HUSLAB/Whonet database 2010, which contains susceptibility data
Drug Use Review. Edward Cox, M.D. Director Office of Antimicrobial Products
 Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Drug Use Review Date: April 5, 2012 To: Through: Edward Cox, M.D. Director
Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Drug Use Review Date: April 5, 2012 To: Through: Edward Cox, M.D. Director
HUSRES Annual Report 2008 Martti Vaara. www.huslab.fi www.intra.hus.fi
 HUSRES Annual Report 2008 Martti Vaara www.huslab.fi www.intra.hus.fi The basis of this HUSRES 2008 report is the HUSLAB/Whonet database 2008, which contains susceptibility data on about 180.000 bacteria
HUSRES Annual Report 2008 Martti Vaara www.huslab.fi www.intra.hus.fi The basis of this HUSRES 2008 report is the HUSLAB/Whonet database 2008, which contains susceptibility data on about 180.000 bacteria
How To Treat Mrsa From A Dead Body
 HUSRES Annual Report 2012 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2013 1 The basis of this HUSRES 2012 report is the HUSLAB/Whonet database 2012, which contains susceptibility data on
HUSRES Annual Report 2012 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2013 1 The basis of this HUSRES 2012 report is the HUSLAB/Whonet database 2012, which contains susceptibility data on
ANTIBIOTICS IN SEPSIS
 ANTIBIOTICS IN SEPSIS Jennifer Curello, PharmD, BCPS Clinical Pharmacist, Infectious Diseases Antimicrobial Stewardship Program Ronald Reagan UCLA Medical Center October 27, 2014 The power of antibiotics
ANTIBIOTICS IN SEPSIS Jennifer Curello, PharmD, BCPS Clinical Pharmacist, Infectious Diseases Antimicrobial Stewardship Program Ronald Reagan UCLA Medical Center October 27, 2014 The power of antibiotics
Attachment A. Electricity Rate Comparison Annual Report. May 1, 2011 Rates
 Electricity Rate Comparison Annual Report May 1, 2011 Rates List of Tables Table 1 Residential Monthly Bills... 4 Table 2 Small Power Monthly Bills... 5 Table 3 Medium Power Monthly Bills... 6 Table 4
Electricity Rate Comparison Annual Report May 1, 2011 Rates List of Tables Table 1 Residential Monthly Bills... 4 Table 2 Small Power Monthly Bills... 5 Table 3 Medium Power Monthly Bills... 6 Table 4
Global Spread of Carbapenemase- Producing Klebsiella pneumoniae
 Global Spread of Carbapenemase- Producing Klebsiella pneumoniae These pathogens arose in the mid-1990s and continue to spread, leaving few options for treating infected patients John Quale John Quale is
Global Spread of Carbapenemase- Producing Klebsiella pneumoniae These pathogens arose in the mid-1990s and continue to spread, leaving few options for treating infected patients John Quale John Quale is
British Society for Antimicrobial Chemotherapy
 British Society for Antimicrobial Chemotherapy BSAC to actively support the EUCAST Disc Diffusion Method for Antimicrobial Susceptibility Testing in preference to the current BSAC Disc Diffusion Method
British Society for Antimicrobial Chemotherapy BSAC to actively support the EUCAST Disc Diffusion Method for Antimicrobial Susceptibility Testing in preference to the current BSAC Disc Diffusion Method
Resolving Customer Complaints
 Resolving Customer Complaints When a Problem Occurs - We Can Help As an MCAP Client you have come to expect a high level of customer service. If you have a complaint regarding our service or policy, we
Resolving Customer Complaints When a Problem Occurs - We Can Help As an MCAP Client you have come to expect a high level of customer service. If you have a complaint regarding our service or policy, we
Optimizing Antimicrobial Susceptibility Test Reporting
 JOURNAL OF CLINICAL MICROBIOLOGY, Sept. 2011, p. S15 S19 0095-1137/11/$12.00 doi:10.1128/jcm.00712-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. VOL. 49, NO. 9 SUPPL. Optimizing
JOURNAL OF CLINICAL MICROBIOLOGY, Sept. 2011, p. S15 S19 0095-1137/11/$12.00 doi:10.1128/jcm.00712-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. VOL. 49, NO. 9 SUPPL. Optimizing
2016 CARE. Chartered Accountancy Reciprocity Examination
 2016 CARE Chartered Accountancy Reciprocity Examination Information for Applicants Seeking to Qualify as Chartered Professional Accountants, Chartered Accountants and as Public Accountants in Ontario Preface
2016 CARE Chartered Accountancy Reciprocity Examination Information for Applicants Seeking to Qualify as Chartered Professional Accountants, Chartered Accountants and as Public Accountants in Ontario Preface
Antibiotic resistance does it matter in paediatric clinical practice? Jette Bangsborg Department of Clinical Microbiology Herlev Hospital
 Antibiotic resistance does it matter in paediatric clinical practice? Jette Bangsborg Department of Clinical Microbiology Herlev Hospital Background The Department of Clinical Microbiology at Herlev Hospital
Antibiotic resistance does it matter in paediatric clinical practice? Jette Bangsborg Department of Clinical Microbiology Herlev Hospital Background The Department of Clinical Microbiology at Herlev Hospital
Updated ECDC Public Health Microbiology Strategy and Work Plan 2012-2016
 Updated ECDC Public Health Microbiology Strategy and Work Plan 2012-2016 Marc Struelens Microbiology Coordination Section, Resource Management and Coordination Unit Ole Heuer Surveillance Section, Surveillance
Updated ECDC Public Health Microbiology Strategy and Work Plan 2012-2016 Marc Struelens Microbiology Coordination Section, Resource Management and Coordination Unit Ole Heuer Surveillance Section, Surveillance
COMPARISON OF ELECTRICITY PRICES IN MAJOR NORTH AMERICAN CITIES. Rates in effect April 1, 2011
 COMPARISON OF ELECTRICITY PRICES IN MAJOR NORTH AMERICAN CITIES Rates in effect April 1, 2011 Amended November 2011 TABLE OF CONTENTS INTRODUCTION 3 METHOD 7 HIGHLIGHTS 9 Residential Customers 9 Small-Power
COMPARISON OF ELECTRICITY PRICES IN MAJOR NORTH AMERICAN CITIES Rates in effect April 1, 2011 Amended November 2011 TABLE OF CONTENTS INTRODUCTION 3 METHOD 7 HIGHLIGHTS 9 Residential Customers 9 Small-Power
Received 22 March 2006/Returned for modification 2 May 2006/Accepted 14 September 2006
 JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2006, p. 4085 4094 Vol. 44, No. 11 0095-1137/06/$08.00 0 doi:10.1128/jcm.00614-06 Copyright 2006, American Society for Microbiology. All Rights Reserved. Two-Center
JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2006, p. 4085 4094 Vol. 44, No. 11 0095-1137/06/$08.00 0 doi:10.1128/jcm.00614-06 Copyright 2006, American Society for Microbiology. All Rights Reserved. Two-Center
Cluster of urinary tract infections due to XDR Pseudomonas aeruginosa. Dr Marie Hallin Service de Microbiologie Hôpital Erasme
 Dr Marie Hallin Service de Microbiologie Hôpital Erasme ULB-Hôpital Erasme: 800 beds Nephrology/Renal transplantation 3300 urines/month 15/05/10-2 Urines 13/05/10: BG100504717 (M) 1946 ER WBC: 123/µL RBC:
Dr Marie Hallin Service de Microbiologie Hôpital Erasme ULB-Hôpital Erasme: 800 beds Nephrology/Renal transplantation 3300 urines/month 15/05/10-2 Urines 13/05/10: BG100504717 (M) 1946 ER WBC: 123/µL RBC:
Liofilchem - Antibiotic Disk Interpretative Criteria and Quality Control - F14013 - Rev.7 / 20.02.2013
 Liofilchem Antibiotic Disk Interpretative Criteria and Quality Control F0 Rev.7 /.02. Amikacin AK Amoxicillin + Clavulanic acid AUG (+) ATCC 352 Coagulasenegative staphylococci Amoxicillin + Clavulanic
Liofilchem Antibiotic Disk Interpretative Criteria and Quality Control F0 Rev.7 /.02. Amikacin AK Amoxicillin + Clavulanic acid AUG (+) ATCC 352 Coagulasenegative staphylococci Amoxicillin + Clavulanic
How To Treat Mrsa In Finnish
 HUSRES Annual Report 2013 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2014 1 The basis of this HUSRES 2013 report is the HUSLAB/Whonet database 2013, which contains susceptibility data on
HUSRES Annual Report 2013 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2014 1 The basis of this HUSRES 2013 report is the HUSLAB/Whonet database 2013, which contains susceptibility data on
COMPARISON OF ELECTRICITY PRICES IN MAJOR NORTH AMERICAN CITIES. Rates in effect April 1, 2015 0,0272
 COMPARISON OF ELECTRICITY PRICES IN MAJOR NORTH AMERICAN CITIES Rates in effect April 1, 2015 0,0272 TABLE OF CONTENTS INTRODUCTION 3 METHOD 7 HIGHLIGHTS 9 Residential Customers 9 Small-Power Customers
COMPARISON OF ELECTRICITY PRICES IN MAJOR NORTH AMERICAN CITIES Rates in effect April 1, 2015 0,0272 TABLE OF CONTENTS INTRODUCTION 3 METHOD 7 HIGHLIGHTS 9 Residential Customers 9 Small-Power Customers
SEA-HLM-415 Distribution: General. Establishment of national laboratory-based surveillance of antimicrobial resistance
 SEA-HLM-415 Distribution: General Establishment of national laboratory-based surveillance of antimicrobial resistance World Health Organization 2011 All rights reserved. Requests for publications, or for
SEA-HLM-415 Distribution: General Establishment of national laboratory-based surveillance of antimicrobial resistance World Health Organization 2011 All rights reserved. Requests for publications, or for
Tigecycline and intravenous fosfomycin zone breakpoints equivalent to the EUCAST MIC criteria for Enterobacteriaceae
 Brief Original Article Tigecycline and intravenous fosfomycin zone breakpoints equivalent to the EUCAST MIC criteria for Enterobacteriaceae Fernando Pasteran, Celeste Lucero, Melina Rapoport, Leonor Guerriero,
Brief Original Article Tigecycline and intravenous fosfomycin zone breakpoints equivalent to the EUCAST MIC criteria for Enterobacteriaceae Fernando Pasteran, Celeste Lucero, Melina Rapoport, Leonor Guerriero,
Management of Extended Spectrum Beta- Lactamase (ESBL) Producing Enterobacteriaceae in health care settings
 Management of Extended Spectrum Beta- Lactamase (ESBL) Producing Enterobacteriaceae in health care settings Dr. Mary Vearncombe PIDAC-IPC February 2012 Objectives: To provide an overview of the RP/AP Annex
Management of Extended Spectrum Beta- Lactamase (ESBL) Producing Enterobacteriaceae in health care settings Dr. Mary Vearncombe PIDAC-IPC February 2012 Objectives: To provide an overview of the RP/AP Annex
Review of Healthcare-Associated Infection (HAI) and Multidrug-Resistant Organism (MDRO) Reporting Requirements in the United States PRESENTED BY:
 Review of Healthcare-Associated Infection (HAI) and Multidrug-Resistant Organism (MDRO) Reporting Requirements in the United States PRESENTED BY: BYRAN DAI MASTERS OF HEALTH SCIENCE CANDIDATE 14 JHSPH
Review of Healthcare-Associated Infection (HAI) and Multidrug-Resistant Organism (MDRO) Reporting Requirements in the United States PRESENTED BY: BYRAN DAI MASTERS OF HEALTH SCIENCE CANDIDATE 14 JHSPH
The 14th EURL-AR Proficiency Test - enterococci, staphylococci and E. coli 2013. Lina Cavaco Susanne Karlsmose Rene S. Hendriksen Frank M.
 The 14th EURL-AR Proficiency Test - enterococci, staphylococci and E. coli 2013 Lina Cavaco Susanne Karlsmose Rene S. Hendriksen Frank M. Aarestrup The 14TH EURL-AR Proficiency Test Enterococci, Staphylococci
The 14th EURL-AR Proficiency Test - enterococci, staphylococci and E. coli 2013 Lina Cavaco Susanne Karlsmose Rene S. Hendriksen Frank M. Aarestrup The 14TH EURL-AR Proficiency Test Enterococci, Staphylococci
Nosocomial Antibiotic Resistance in Multiple Gram-Negative Species: Experience at One Hospital with Squeezing the Resistance Balloon at Multiple Sites
 INVITED ARTICLE HEALTHCARE EPIDEMIOLOGY Robert A. Weinstein, Section Editor Nosocomial Antibiotic Resistance in Multiple Gram-Negative Species: Experience at One Hospital with Squeezing the Resistance
INVITED ARTICLE HEALTHCARE EPIDEMIOLOGY Robert A. Weinstein, Section Editor Nosocomial Antibiotic Resistance in Multiple Gram-Negative Species: Experience at One Hospital with Squeezing the Resistance
Canadian Public Health Laboratory Network. Core Functions of Canadian Public Health Laboratories
 Canadian Public Health Laboratory Network Core Functions of Canadian Public Health Laboratories Canadian Public Health Laboratory Network The CPHLN Core Functions of Canadian Public Health Laboratories
Canadian Public Health Laboratory Network Core Functions of Canadian Public Health Laboratories Canadian Public Health Laboratory Network The CPHLN Core Functions of Canadian Public Health Laboratories
Standards Benefit the New Brunswick Lab Repository. Canada Health Infoway 2014
 Standards Benefit the New Brunswick Lab Repository Canada Health Infoway 2014 Lorie Carey & Marion Long November 27, 2014 Do We Need a Central Lab Repository? Improving patient care 1 Between 60% and 70%
Standards Benefit the New Brunswick Lab Repository Canada Health Infoway 2014 Lorie Carey & Marion Long November 27, 2014 Do We Need a Central Lab Repository? Improving patient care 1 Between 60% and 70%
Urinary tract infections caused by extended spectrum β-lactamase (ESBL) producing Escherichia coli and Klebsiella pneumoniae
 African Journal of Biotechnology Vol. 10(73), pp. 16661-16666, 21 November, 2011 Available online at http://www.academicjournals.org/ajb DOI: 10.5897/AJB11.2449 ISSN 1684 5315 2011 Academic Journals Full
African Journal of Biotechnology Vol. 10(73), pp. 16661-16666, 21 November, 2011 Available online at http://www.academicjournals.org/ajb DOI: 10.5897/AJB11.2449 ISSN 1684 5315 2011 Academic Journals Full
Appendix B: Provincial Case Definitions for Reportable Diseases
 Infectious Diseases Protocol Appendix B: Provincial Case Definitions for Reportable Diseases Disease: Verotoxin-producing E. coli infection indicator conditions, including Hemolytic Uremic Syndrome (HUS)
Infectious Diseases Protocol Appendix B: Provincial Case Definitions for Reportable Diseases Disease: Verotoxin-producing E. coli infection indicator conditions, including Hemolytic Uremic Syndrome (HUS)
Streptococcus pneumoniae IgG AB (13 Serotypes), MAID... 7
 Volume 12 December 2011 Table of Contents Summary of Test Changes... 3 Test Changes... 6 Human Anti-Mouse AB (HAMA), ELISA... 6 Hepatitis E Antibody (IgG, IgM)... 6 Hepatitis E Antibody (IgG)... 6 Hepatitis
Volume 12 December 2011 Table of Contents Summary of Test Changes... 3 Test Changes... 6 Human Anti-Mouse AB (HAMA), ELISA... 6 Hepatitis E Antibody (IgG, IgM)... 6 Hepatitis E Antibody (IgG)... 6 Hepatitis
HUSRES Annual Report 2015
 HUSRES Annual Report 2015 1 This report is based on the HUSLAB/Whonet database 2015, which contains data on more than 190.000 microbes. EUCAST 2015 clinical breakpoints have mainly been used. The doses
HUSRES Annual Report 2015 1 This report is based on the HUSLAB/Whonet database 2015, which contains data on more than 190.000 microbes. EUCAST 2015 clinical breakpoints have mainly been used. The doses
Practice Guidelines. Updated Guideline on Diagnosis and Treatment of Intra-abdominal Infections
 Updated Guideline on Diagnosis and Treatment of Intra-abdominal Infections CARRIE ARMSTRONG Guideline source: Surgical Infection Society, Infectious Diseases Society of America Literature search described?
Updated Guideline on Diagnosis and Treatment of Intra-abdominal Infections CARRIE ARMSTRONG Guideline source: Surgical Infection Society, Infectious Diseases Society of America Literature search described?
Urinary Tract Infections
 Urinary Tract Infections Overview A urine culture must ALWAYS be interpreted in the context of the urinalysis and patient symptoms. If a patient has no signs of infection on urinalysis, no symptoms of
Urinary Tract Infections Overview A urine culture must ALWAYS be interpreted in the context of the urinalysis and patient symptoms. If a patient has no signs of infection on urinalysis, no symptoms of
Version 12 May 2013. BSAC Methods for Antimicrobial Susceptibility Testing. All enquiries to: Mandy Wootton. Email: [email protected].
 BSAC Methods for Antimicrobial Susceptibility Testing Version 12 May 2013 All enquiries to: Mandy Wootton Email: [email protected] Telephone: +44 (0) 2920 746581 Contents Page Working Party members
BSAC Methods for Antimicrobial Susceptibility Testing Version 12 May 2013 All enquiries to: Mandy Wootton Email: [email protected] Telephone: +44 (0) 2920 746581 Contents Page Working Party members
Investment Dealers Association of Canada
 2 Investment Dealers Association of Canada Dual Registration of Life Insurance Agents and Securities Salespersons The respective securities and insurance legislation and governing bodies of each of the
2 Investment Dealers Association of Canada Dual Registration of Life Insurance Agents and Securities Salespersons The respective securities and insurance legislation and governing bodies of each of the
Workers Compensation How to Make a Claim
 Workers Compensation How to Make a Claim HOW TO MAKE A WORKERS COMPENSATION CLAIM dśğĩžůůžǁ ŝŷőŝŷĩžƌŵăɵžŷǁ ŝůůśğůɖljžƶƚžŵăŭğăǁ ŽƌŬĞƌƐΖĐŽŵƉĞŶƐĂƟŽŶĐůĂŝŵĨŽƌLJŽƵƌƐĞůĨŽƌ to help your fellow workers establish
Workers Compensation How to Make a Claim HOW TO MAKE A WORKERS COMPENSATION CLAIM dśğĩžůůžǁ ŝŷőŝŷĩžƌŵăɵžŷǁ ŝůůśğůɖljžƶƚžŵăŭğăǁ ŽƌŬĞƌƐΖĐŽŵƉĞŶƐĂƟŽŶĐůĂŝŵĨŽƌLJŽƵƌƐĞůĨŽƌ to help your fellow workers establish
Professional Standards For Dietitians In Canada
 Professional Standards For Dietitians In Canada Developed by: Dietitians of Canada College of Dietitians of Ontario in collaboration and with financial support from: British Columbia Dietitians' and Nutritionists'
Professional Standards For Dietitians In Canada Developed by: Dietitians of Canada College of Dietitians of Ontario in collaboration and with financial support from: British Columbia Dietitians' and Nutritionists'
ANTIBIOTIC RESISTANCE THREATS. in the United States, 2013
 ANTIBIOTIC RESISTANCE THREATS in the United States, 2013 TABLE OF CONTENTS Foreword... 5 Executive Summary.... 6 Section 1: The Threat of Antibiotic Resistance... 11 Introduction.... 11 National Summary
ANTIBIOTIC RESISTANCE THREATS in the United States, 2013 TABLE OF CONTENTS Foreword... 5 Executive Summary.... 6 Section 1: The Threat of Antibiotic Resistance... 11 Introduction.... 11 National Summary
Revenue Requirement Application 2004/05 and 2005/06. Volume 2. Appendix D. Electric Rates Comparison
 Revenue Requirement Application 00/0 and 00/0 Volume Appendix D. Electric Rates Comparison Table of Contents 1 Consumer Price Index... 1 Rates Comparison... List of Figures None. List of Tables Table 1A.
Revenue Requirement Application 00/0 and 00/0 Volume Appendix D. Electric Rates Comparison Table of Contents 1 Consumer Price Index... 1 Rates Comparison... List of Figures None. List of Tables Table 1A.
CANADIAN ASSOCIATION OF INSURANCE WOMEN ASSOCIATION CANADIENNE DES FEMMES D ASSURANCE 2014-2015 CONTACT LIST
 CANADIAN OF WOMEN CANADIENNE DES FEMMES D ASSURANCE 2014-2015 CONTACT LIST CAIW EXECUTIVE BOARD COMPANY INFORMATION PHONE, FAX & EMAIL INFORMATION PRESIDENT Tracy Fata, BSc, FCIP, CRM Portage Mutual Ins.
CANADIAN OF WOMEN CANADIENNE DES FEMMES D ASSURANCE 2014-2015 CONTACT LIST CAIW EXECUTIVE BOARD COMPANY INFORMATION PHONE, FAX & EMAIL INFORMATION PRESIDENT Tracy Fata, BSc, FCIP, CRM Portage Mutual Ins.
ANTIMICROBIAL AGENT CLASSES AND SUBCLASSES
 ANTIMICROBIAL AGENT CLASSES AND SUBCLASSES FOR USE WITH CLSI DOCUMENTS M2 AND M7 Beta-lactams: penicillins e penicillin a penicillin beta-lactam/beta-lactamase inhibitor combinations aminopenicillin a
ANTIMICROBIAL AGENT CLASSES AND SUBCLASSES FOR USE WITH CLSI DOCUMENTS M2 AND M7 Beta-lactams: penicillins e penicillin a penicillin beta-lactam/beta-lactamase inhibitor combinations aminopenicillin a
Recommendations for Metrics for Multidrug-Resistant Organisms in Healthcare Settings: SHEA/HICPAC Position Paper
 infection control and hospital epidemiology october 2008, vol. 29, no. 10 shea/hicpac position paper Recommendations for Metrics for Multidrug-Resistant Organisms in Healthcare Settings: SHEA/HICPAC Position
infection control and hospital epidemiology october 2008, vol. 29, no. 10 shea/hicpac position paper Recommendations for Metrics for Multidrug-Resistant Organisms in Healthcare Settings: SHEA/HICPAC Position
Antimicrobial Activity and Spectrum of PPI-0903M (T-91825), a Novel Cephalosporin, Tested against a Worldwide Collection of Clinical Strains
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2005, p. 3501 3512 Vol. 49, No. 8 0066-4804/05/$08.00 0 doi:10.1128/aac.49.8.3501 3512.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2005, p. 3501 3512 Vol. 49, No. 8 0066-4804/05/$08.00 0 doi:10.1128/aac.49.8.3501 3512.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
TITLE 410 INDIANA STATE DEPARTMENT OF HEALTH. LSA Document #15-39 SUMMARY/RESPONSE TO COMMENTS
 TITLE 410 INDIANA STATE DEPARTMENT OF HEALTH LSA Document #15-39 SUMMARY/RESPONSE TO COMMENTS The Indiana State Department of Health s (ISDH) Executive Board preliminarily adopted the Disease Reporting
TITLE 410 INDIANA STATE DEPARTMENT OF HEALTH LSA Document #15-39 SUMMARY/RESPONSE TO COMMENTS The Indiana State Department of Health s (ISDH) Executive Board preliminarily adopted the Disease Reporting
Corporate Membership Package 2016
 Corporate Membership Package 2016 February 2016 Dear Industry Partner: RE: CORPORATE MEMBERSHIP INVITATION CANADIAN ASSOCIATION OF MEDICAL DEVICE REPROCESSING (CAMDR) The (CAMDR) invites you to be part
Corporate Membership Package 2016 February 2016 Dear Industry Partner: RE: CORPORATE MEMBERSHIP INVITATION CANADIAN ASSOCIATION OF MEDICAL DEVICE REPROCESSING (CAMDR) The (CAMDR) invites you to be part
Study of bacteria isolated from urinary tract infections and determination of their susceptibility to antibiotics
 Jundishapur Journal of Microbiology (2009); 2(3): 118-123 118 Original article Study of bacteria isolated from urinary tract infections and determination of their susceptibility to antibiotics Mansour
Jundishapur Journal of Microbiology (2009); 2(3): 118-123 118 Original article Study of bacteria isolated from urinary tract infections and determination of their susceptibility to antibiotics Mansour
Imipenem and meropenem resistance amongst ESBL producing Escherichia coli and Klebsiella pneumoniae clinical isolates
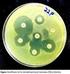 International Research Journal of Microbiology (IRJM) (ISSN: 2141-5463) Vol. 3(10) pp. 339-344, October 2012 Available online http://www.interesjournals.org/irjm Copyright 2012 International Research Journals
International Research Journal of Microbiology (IRJM) (ISSN: 2141-5463) Vol. 3(10) pp. 339-344, October 2012 Available online http://www.interesjournals.org/irjm Copyright 2012 International Research Journals
BD Phoenix Automated Microbiology System
 BD Phoenix Automated Microbiology System Resistance Detection Workflow Efficiency Analysis and Communication BD Diagnostics 7 Loveton Circle Sparks, MD 115-0999 800.638.8663 www.bd.com/ds CHROMagar is
BD Phoenix Automated Microbiology System Resistance Detection Workflow Efficiency Analysis and Communication BD Diagnostics 7 Loveton Circle Sparks, MD 115-0999 800.638.8663 www.bd.com/ds CHROMagar is
NewYork-Presbyterian Hospital Sites: Columbia University Medical Center Guideline: Medication Use Manual Page 1 of 12
 Page 1 of 12 TITLE: ANTIBIOTICS IN ADULT PATIENTS EMPIRIC USE GUIDELINES, COLUMBIA UNIVERSITY MEDICAL CENTER MEDICATION GUIDELINE PURPOSE: These are the 2011 guidelines for the empiric use of antibiotics
Page 1 of 12 TITLE: ANTIBIOTICS IN ADULT PATIENTS EMPIRIC USE GUIDELINES, COLUMBIA UNIVERSITY MEDICAL CENTER MEDICATION GUIDELINE PURPOSE: These are the 2011 guidelines for the empiric use of antibiotics
Guidelines for Antimicrobial Stewardship in Hospitals in Ireland. A Strategy for the Control of Antimicrobial Resistance in Ireland
 A Strategy for the Control of Antimicrobial Resistance in Ireland Guidelines for Antimicrobial Stewardship in Hospitals in Ireland Hospital Antimicrobial Stewardship Working Group Guidelines for Antimicrobial
A Strategy for the Control of Antimicrobial Resistance in Ireland Guidelines for Antimicrobial Stewardship in Hospitals in Ireland Hospital Antimicrobial Stewardship Working Group Guidelines for Antimicrobial
Thermo Scientific Sensititre. Susceptibility and Identification System. Customizable testing solutions. maximum performance
 Thermo Scientific Sensititre Susceptibility and Identification System Customizable testing solutions maximum performance True MIC results the key to battling resistance The Thermo Scientific Sensititre
Thermo Scientific Sensititre Susceptibility and Identification System Customizable testing solutions maximum performance True MIC results the key to battling resistance The Thermo Scientific Sensititre
Beta-Lactamase Encoding Gene Among Extended Spectrum B-Lactamase Positive Wound Isolates of Pseudomonas
 Arch Pediatr Infect Dis. 2015 October; 3(4): e26362. Published online 2015 October 12. doi: 10.5812/pedinfect.26362 Research Article Molecular Detection of bla VEB-1 Beta-Lactamase Encoding Gene Among
Arch Pediatr Infect Dis. 2015 October; 3(4): e26362. Published online 2015 October 12. doi: 10.5812/pedinfect.26362 Research Article Molecular Detection of bla VEB-1 Beta-Lactamase Encoding Gene Among
Establishing an Evidence Base for Future Directions in Settlement
 Establishing an Evidence Base for Future Directions in Settlement By: Lori Wilkinson, Jill Bucklaschuk & Janine Bramadat Victoria Esses, Leah Hamilton and Li Zong Presented at the Vision National Settlement
Establishing an Evidence Base for Future Directions in Settlement By: Lori Wilkinson, Jill Bucklaschuk & Janine Bramadat Victoria Esses, Leah Hamilton and Li Zong Presented at the Vision National Settlement
Ms. Cecilie Lord (Chair) Assistant Deputy Minister Health Strategies Division Alberta Health 10025 Jasper Avenue, 24th Floor Edmonton, AB T5J 2N3
 Ms. Cecilie Lord (Chair) Health Strategies Division 10025 Jasper Avenue, 24th Floor Mr. Ian Potter (Vice-Chair) Health Promotion and Programs Branch Room A1614, 16th Floor Tunney s Pasture (AL1916A) Ms.
Ms. Cecilie Lord (Chair) Health Strategies Division 10025 Jasper Avenue, 24th Floor Mr. Ian Potter (Vice-Chair) Health Promotion and Programs Branch Room A1614, 16th Floor Tunney s Pasture (AL1916A) Ms.
Frequently Asked Questions
 Guidelines for Testing and Treatment of Gonorrhea in Ontario, 2013 Frequently Asked Questions Table of Contents Background... 1 Treatment Recommendations... 2 Treatment of Contacts... 4 Administration
Guidelines for Testing and Treatment of Gonorrhea in Ontario, 2013 Frequently Asked Questions Table of Contents Background... 1 Treatment Recommendations... 2 Treatment of Contacts... 4 Administration
ACCEPTED CLINICAL AND ECONOMIC IMPACT OF COMMON MULTIDRUG-RESISTANT GRAM-NEGATIVE BACILLI. On behalf of ReAct, Action on Antibiotic Resistance
 AAC Accepts, published online ahead of print on 10 December 2007 Antimicrob. Agents Chemother. doi:10.1128/aac.01169-07 Copyright 2007, American Society for Microbiology and/or the Listed Authors/Institutions.
AAC Accepts, published online ahead of print on 10 December 2007 Antimicrob. Agents Chemother. doi:10.1128/aac.01169-07 Copyright 2007, American Society for Microbiology and/or the Listed Authors/Institutions.
Application deadline: march 31 Apply for a $2,000 bursary!
 VIVRE À FOND LA FRANCOPHONIE CANADIENNE THREE-WEEK PROGRAM for Grades 8 and 9 Application deadline: march 31 Apply for a $2,000 bursary! Follow us! #destinationclic www.fb.com/destination.clic @OLP-PLO
VIVRE À FOND LA FRANCOPHONIE CANADIENNE THREE-WEEK PROGRAM for Grades 8 and 9 Application deadline: march 31 Apply for a $2,000 bursary! Follow us! #destinationclic www.fb.com/destination.clic @OLP-PLO
GUIDELINES EXECUTIVE SUMMARY
 GUIDELINES Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship Timothy
GUIDELINES Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship Timothy
CLOSING THE COVERAGE GAP. Pan-Canadian Pharmacare
 CLOSING THE COVERAGE GAP Pan-Canadian Pharmacare Prescription drug coverage for all Canadians While the vast majority of Canadians have access to prescription drugs, some Canadians can t afford their medications.
CLOSING THE COVERAGE GAP Pan-Canadian Pharmacare Prescription drug coverage for all Canadians While the vast majority of Canadians have access to prescription drugs, some Canadians can t afford their medications.
TREATMENT OF PERITONEAL DIALYSIS (PD) RELATED PERITONITIS. General Principles
 WA HOME DIALYSIS PROGRAM (WAHDIP) GUIDELINES General Principles 1. PD related peritonitis is an EMERGENCY early empiric treatment followed by close review is essential 2. When culture results and sensitivities
WA HOME DIALYSIS PROGRAM (WAHDIP) GUIDELINES General Principles 1. PD related peritonitis is an EMERGENCY early empiric treatment followed by close review is essential 2. When culture results and sensitivities
APPROVAL PROCESS FOR NURSE PRACTITIONER EDUCATION PROGRAMS
 APPROVAL PROCESS FOR NURSE PRACTITIONER EDUCATION PROGRAMS 2015 This Regulatory Document was approved by ARNNL Council in 2015. Approval Process for Nurse Practitioner Education Programs Table of Contents
APPROVAL PROCESS FOR NURSE PRACTITIONER EDUCATION PROGRAMS 2015 This Regulatory Document was approved by ARNNL Council in 2015. Approval Process for Nurse Practitioner Education Programs Table of Contents
Advances in Medical Sciences Vol. 58(1) 2013 pp 164-171 DOI: 10.2478/v10039-012-0064-0 Medical University of Bialystok, Poland
 Advances in Medical Sciences Vol. 58(1) 2013 pp 164-171 DOI: 10.2478/v10039-012-0064-0 Medical University of Bialystok, Poland Rep-PCR genotyping of infectious Acinetobacter spp. strains from patients
Advances in Medical Sciences Vol. 58(1) 2013 pp 164-171 DOI: 10.2478/v10039-012-0064-0 Medical University of Bialystok, Poland Rep-PCR genotyping of infectious Acinetobacter spp. strains from patients
Open Access Article pissn 0976 3325 eissn 2229 6816
 Original Article A STUDY OF EXTENDED SPECTRUM β-lactamase (ESBL) AND AmpC β-lactamase PRODUCING KLEBSIELLA PNEUMONIAE IN NEONATAL INTENSIVE CARE UNIT AT TERTIARY CARE HOSPITAL, AHMEDABAD Modi Dhara J 1,
Original Article A STUDY OF EXTENDED SPECTRUM β-lactamase (ESBL) AND AmpC β-lactamase PRODUCING KLEBSIELLA PNEUMONIAE IN NEONATAL INTENSIVE CARE UNIT AT TERTIARY CARE HOSPITAL, AHMEDABAD Modi Dhara J 1,
Integrated Surveillance of Antimicrobial Resistance
 ii iii WORLD HEALTH ORGANIZATION Integrated Surveillance of Antimicrobial Resistance Guidance from a WHO Advisory Group World Health Organization, Geneva iv WHO Library Cataloguing-in-Publication Data
ii iii WORLD HEALTH ORGANIZATION Integrated Surveillance of Antimicrobial Resistance Guidance from a WHO Advisory Group World Health Organization, Geneva iv WHO Library Cataloguing-in-Publication Data
PRIORITY RESEARCH TOPICS
 PRIORITY RESEARCH TOPICS Understanding all the issues associated with antimicrobial resistance is probably impossible, but it is clear that there are a number of key issues about which we need more information.
PRIORITY RESEARCH TOPICS Understanding all the issues associated with antimicrobial resistance is probably impossible, but it is clear that there are a number of key issues about which we need more information.
Version 11.1 May 2012
 BSAC Methods for Antimicrobial Susceptibility Testing Version 11.1 May 2012 All enquiries to: Jenny Andrews at: + 44 (0) 121 507 5693 Email: [email protected] Contents Page Working Party members 5
BSAC Methods for Antimicrobial Susceptibility Testing Version 11.1 May 2012 All enquiries to: Jenny Andrews at: + 44 (0) 121 507 5693 Email: [email protected] Contents Page Working Party members 5
Diagnosis, Treatment and Prevention of Typhoid Fever in the Children of Bangladesh: A Microbiologist s View.
 Diagnosis, Treatment and Prevention of Typhoid Fever in the Children of Bangladesh: A Microbiologist s View. Samir K Saha, Ph.D. Department of Microbiology Dhaka Shishu Hospital Bangladesh Typhoid Fever:Salmonella
Diagnosis, Treatment and Prevention of Typhoid Fever in the Children of Bangladesh: A Microbiologist s View. Samir K Saha, Ph.D. Department of Microbiology Dhaka Shishu Hospital Bangladesh Typhoid Fever:Salmonella
Overview How BC teacher salaries rank among the provinces and territories in 2011
 13 BARGAINING PROPOSAL BRITISH COLUMBIA TEACHERS FEDERATION Document Number: U102 Date: Time: Overview How BC teacher salaries rank among the provinces and territories in 2011 Minimum The minimum salary
13 BARGAINING PROPOSAL BRITISH COLUMBIA TEACHERS FEDERATION Document Number: U102 Date: Time: Overview How BC teacher salaries rank among the provinces and territories in 2011 Minimum The minimum salary
Disc Diffusion Susceptibility Methods
 Disc Diffusion Susceptibility Methods Introduction When a filter paper disc impregnated with a chemical is placed on agar the chemical will diffuse from the disc into the agar. This diffusion will place
Disc Diffusion Susceptibility Methods Introduction When a filter paper disc impregnated with a chemical is placed on agar the chemical will diffuse from the disc into the agar. This diffusion will place
DEGREE PROGRAM DEVELOPMENT STATUS CHART
 DEGREE PROGRAM DEVELOPMENT STATUS CHART For As of September 18th, 2003: This information is accurate (to the best of our knowledge) as provided to CAMRT by Provincial Member Associations and Education
DEGREE PROGRAM DEVELOPMENT STATUS CHART For As of September 18th, 2003: This information is accurate (to the best of our knowledge) as provided to CAMRT by Provincial Member Associations and Education
The National Antimicrobial Resistance Monitoring System (NARMS)
 The National Antimicrobial Resistance Monitoring System (NARMS) Strategic Plan 2012-2016 Table of Contents Background... 2 Mission... 3 Overview of Accomplishments, 1996-2011... 4 Strategic Goals and Objectives...
The National Antimicrobial Resistance Monitoring System (NARMS) Strategic Plan 2012-2016 Table of Contents Background... 2 Mission... 3 Overview of Accomplishments, 1996-2011... 4 Strategic Goals and Objectives...
If you have an accident
 LABOUR PROGRAM If you have an accident What to do and how to do it LT-058-03-05 This publication is available in multiple formats (large print, audio cassette, braille and diskette) in English and French.
LABOUR PROGRAM If you have an accident What to do and how to do it LT-058-03-05 This publication is available in multiple formats (large print, audio cassette, braille and diskette) in English and French.
CHAPTER 4. Eye Care in the Private Sector: Innovation at the Service of Patients
 CHAPTER 4 Eye Care in the Private Sector: Innovation at the Service of Patients In Canada, it is professionals working essentially in private practices who provide patients with the eye and vision care
CHAPTER 4 Eye Care in the Private Sector: Innovation at the Service of Patients In Canada, it is professionals working essentially in private practices who provide patients with the eye and vision care
Short Report: Failure of Burkholderia pseudomallei to Grow in an Automated Blood Culture System
 Accepted for Publication, Published online October 13, 2014; doi:10.4269/ajtmh.14-0018. The latest version is at http://ajtmh.org/cgi/doi/10.4269/ajtmh.14-0018 In order to provide our readers with timely
Accepted for Publication, Published online October 13, 2014; doi:10.4269/ajtmh.14-0018. The latest version is at http://ajtmh.org/cgi/doi/10.4269/ajtmh.14-0018 In order to provide our readers with timely
REGISTERED NURSING ASSOCATIONS CONTACT INFO
 REGISTERED NURSING ASSOCATIONS CONTACT INFO CANADIAN COUNCIL OF REGISTERED NURSE REGULATORS Greenbank North Post Office PO Box 30005 Ottawa, ON K2H 1A3 [email protected] Tel: 613.447.5253 COLLEGE OF REGISTERED
REGISTERED NURSING ASSOCATIONS CONTACT INFO CANADIAN COUNCIL OF REGISTERED NURSE REGULATORS Greenbank North Post Office PO Box 30005 Ottawa, ON K2H 1A3 [email protected] Tel: 613.447.5253 COLLEGE OF REGISTERED
GLOBAL OVERVIEW OF ANTIMICROBIAL RESISTANCE STD AGENTS
 GLOBAL OVERVIEW OF ANTIMICROBIAL RESISTANCE STD AGENTS IN Dr.P.Bhalla Director Professor and Head Dept. of Microbiology Maulana Azad Medical College New Delhi, INDIA STDs & RTIs l HIV l Gonococcal infections/
GLOBAL OVERVIEW OF ANTIMICROBIAL RESISTANCE STD AGENTS IN Dr.P.Bhalla Director Professor and Head Dept. of Microbiology Maulana Azad Medical College New Delhi, INDIA STDs & RTIs l HIV l Gonococcal infections/
Victims Compensation Claim Status of All Pending Claims and Claims Decided Within the Last Three Years
 Claim#:021914-174 Initials: J.T. Last4SSN: 6996 DOB: 5/3/1970 Crime Date: 4/30/2013 Status: Claim is currently under review. Decision expected within 7 days Claim#:041715-334 Initials: M.S. Last4SSN: 2957
Claim#:021914-174 Initials: J.T. Last4SSN: 6996 DOB: 5/3/1970 Crime Date: 4/30/2013 Status: Claim is currently under review. Decision expected within 7 days Claim#:041715-334 Initials: M.S. Last4SSN: 2957
Antimicrobial Stewardship for Hospital Acquired Infection Prevention: Focus on C. difficile infection
 Antimicrobial Stewardship for Hospital Acquired Infection Prevention: Focus on C. difficile infection Emi Minejima, PharmD Assistant Professor of Clinical Pharmacy USC School of Pharmacy [email protected]
Antimicrobial Stewardship for Hospital Acquired Infection Prevention: Focus on C. difficile infection Emi Minejima, PharmD Assistant Professor of Clinical Pharmacy USC School of Pharmacy [email protected]
Memorial University of Newfoundland 2,550 2,550 8,800 8,800. University of Prince Edward Island 5,360 5,360 11,600 11,600
 TUITION FEES BY CANADIAN UNIVERSITY University tuition fees for full-time Canadian and international students in an arts and humanities program (unless otherwise indicated) at the undergraduate level at
TUITION FEES BY CANADIAN UNIVERSITY University tuition fees for full-time Canadian and international students in an arts and humanities program (unless otherwise indicated) at the undergraduate level at
Original ABSTRACT RESUMEN
 Original Rafael Cantón 1,2, Elena Loza 1, Javier Aznar 3, Jorge Calvo 4, Emilia Cercenado 5, Ramón Cisterna 6, Fernando González Romo 7, José Luis López Hontangas 8, Carmen Rubio Calvo 9, Ana Isabel Suárez
Original Rafael Cantón 1,2, Elena Loza 1, Javier Aznar 3, Jorge Calvo 4, Emilia Cercenado 5, Ramón Cisterna 6, Fernando González Romo 7, José Luis López Hontangas 8, Carmen Rubio Calvo 9, Ana Isabel Suárez
Ceftriaxone Therapy Vs. Ciprofloxacin In Treatment Of Typhoid Fever In Adult Patients.
 Dec QMJ VOL.5 No.8 Ceftriaxone Therapy Vs. Ciprofloxacin In Treatment Of Typhoid Fever In Adult Patients. Radhi F.Alshaibani* الخلاصھ لا زالت حمى التایفوید سببا مھما من اسباب الامراض وفقدان ساعات العمل
Dec QMJ VOL.5 No.8 Ceftriaxone Therapy Vs. Ciprofloxacin In Treatment Of Typhoid Fever In Adult Patients. Radhi F.Alshaibani* الخلاصھ لا زالت حمى التایفوید سببا مھما من اسباب الامراض وفقدان ساعات العمل
Recommendations for the control of Multi-drug resistant Gram-negatives: carbapenem resistant Enterobacteriaceae. November 2013
 Recommendations for the control of Multi-drug resistant Gram-negatives: carbapenem resistant Enterobacteriaceae November 2013 ISBN: Print: 978-1-921983-55-9 Electronic: 978-1-921983-56-6 Suggested citation:
Recommendations for the control of Multi-drug resistant Gram-negatives: carbapenem resistant Enterobacteriaceae November 2013 ISBN: Print: 978-1-921983-55-9 Electronic: 978-1-921983-56-6 Suggested citation:
Objective 1A: Increase the adoption and effective use of health IT products, systems, and services
 1275 K Street, NW, Suite 1000 Washington, DC 20005-4006 Phone: 202/789-1890 Fax: 202/789-1899 [email protected] www.apic.org February 4, 2015 Karen DeSalvo, MD, MPH, MSc National Coordinator for Health
1275 K Street, NW, Suite 1000 Washington, DC 20005-4006 Phone: 202/789-1890 Fax: 202/789-1899 [email protected] www.apic.org February 4, 2015 Karen DeSalvo, MD, MPH, MSc National Coordinator for Health
