Genetic and Genomic Testing (Disease Specific) MPM 7.1
|
|
|
- Deborah Kory Atkins
- 9 years ago
- Views:
Transcription
1 Disclaimer Page 1 of 24 Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans or the plan may have broader or more limited benefits than those listed in these criteria. Description Genetic testing is the use of specific assays to determine the genetic status of individuals already suspected to be at high risk for a particular inherited condition. High risk means that the individual has a known family history or classic symptoms of the disorder. Genetic testing includes a variety of techniques that test for genetic diseases and analyzes genetic risk factors that may contribute to disease. Techniques involve the examination of a blood sample, or other body fluid, or tissue to indicate the presence, absence, or alteration (mutation) of genes linked to specific diseases or conditions. The main difference between genetic and genomic tests is that genetic tests look at sequence variants in single genes while genomic tests look at the expression of multiple genes in a single assay. Genetic testing typically refers to inherited disorders. Genomic testing usually refers to tests that look at expression profiles of multiple genes in a particular tissue affected by an acquired disease (e.g., a tumor), and in many cases, are cancer tests. 1 Recognizing the differences as described above, for the purposes of this, the term genetic testing is considered interchangeable with genomic testing and is used throughout. Coverage Determination Prior Authorization is required for the following tests: Allomap, BRCA 1, BRCA 2, BART, Colaris, Colaris AP, and Oncotype DX. Guidelines for these tests are located on pages 7, 8 and 9. For Benefit Certification and billing purposes, S codes should be utilized (see pages 12 and 13). Log on to Pres Online to submit a request: Prior Authorization is not required for all other testing listed under Covered Diagnoses. However, all claims are subject to retrospective review. Genetic testing may not be a benefit on all plans. Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage.
2 Page 2 of 25 The following basic guidelines apply: General population screening using genetic testing is not covered unless specified in this Policy or the member s contract. If there is a conflict between this Policy and the member s contract, the contract will govern. Metabolic disease/genetic inborn errors of metabolism testing is covered for newborn screening for genetic disorders as mandated by state guidelines. 2 Presbyterian considers multigene cancer panels experimental and investigational because there is insufficient evidence of their clinical validity and utility. They therefore are not covered except those that are required by Medicare. Otherwise only individual tests that are medically necessary and prior authorized are covered. Genetic testing is covered for certain diagnoses when all of the following criteria have been met. (See pages 4-9 for a list of diagnoses and the type of genetic testing covered.) Genetic testing should be ordered by specialized physicians and/or certified genetic counselor qualified to interpret the testing results. Appropriate documentation of patient consent should be obtained. After physical examination and routine testing, the diagnosis remains uncertain. The member is at risk for a genetic disease, either with a direct risk factor for the development of an inheritable disease (known family history), or demonstrating signs/symptoms of a genetic disease. The genetic test result has a potential to affect the course of treatment for the member. Consultations with qualified genetic counselors and physicians should be part of the treatment plan in order for the patient to receive the appropriate interpretation of the genetic testing. Unless otherwise stated, a printed three generation pedigree should be part of the genetic consultation and should be available for review. 51 The genetic test is considered a proven method to 1. identify or rule out an inheritable disease, or 2. to detect an inherited or acquired disease-related genotype, mutation, phenotype or karyotype for clinical purposes. Genetic testing for a specific disease is only covered once in a person s lifetime. Coverage will be extended if additional tests are developed that expand the ability to find mutations in patients who have been
3 Page 3 of 25 previously tested, and if the test is considered a proven method. Carrier and predictive testing is covered for certain genetic diseases when there is an affected family member of first or second-degree relation who has an identified mutation or genetic disease, and the information will help with medical or reproductive decision-making. In some circumstances, testing may also be covered when the patient is the reproductive partner of a person with a positive genetic test and the couple intends to have a baby. Prenatal or preimplantation genetic testing is covered for certain genetic diseases if there is an increased risk (known family history) that an offspring will have a genetic or chromosomal disorder. (Please note: Preimplantation genetic testing, as part of assisted reproductive techniques such as in-vitro fertilization, may not be a covered benefit. Refer to the member s specific benefit plan to determine coverage.) Exclusions Genetic testing of PHP members is not covered when the test is performed primarily for the medical management of other family members. Additional expenses for banking of genetic material is not covered. The following testing is not covered by PHP: APOE testing for use as adjunct test in clinical evaluation of patients with dementia of unknown etiology or for risk assessment of Alzheimer 87, 96 Disease in asymptomatic individuals. Chromosomal Microarray Analysis (CMA) for the Genetic Evaluation of Patients with Developmental Delay/Intellectual Disability or Autism Spectrum Disorders. Evidence on the clinical benefit of CMA testing is largely anecdotal. Systematic studies of the impact of CMA analysis on patient outcomes are lacking. Therefore, chromosomal microarray analysis is considered investigational for all indications, including but not limited to evaluation of children with cognitive delay/intellectual disability or autism spectrum disorders and prenatal genetic testing. CDKN2A Testing (Melaris ) (test for familial melanoma) 12 CYP2D6 Genotyping (for dose management of Tamoxifen during treatment for breast cancer) 65 decode Prostate Cancer (for assessment of prostate cancer risk or prostate cancer aggressiveness) 85 Decision DX-UM for Uveal Melanoma(Used to determine risk for metastasis in patients with diagnosis of Uveal Melanoma). Reviewed by the TAC Committee and determined to be experimental/investigational. Epidermal Growth Factor Receptor (EGFR) Gene Amplification Analysis by FISH (for predicting response to non-small cell lung cancer drug therapy). EGFR Sequence Variant Analysis is a covered benefit for this
4 Page 4 of 25 diagnosis (see page 6) 66 exagen BC (breast cancer prognostic test) exagen/bd (inflammatory bowel disease expression profile) exagen/bs (irritable bowel syndrome expression profile) Familion (for Brugada syndrome, short QT syndrome, or catecholaminergic polymorphic ventricular tachycardia. Familion is covered for long QT syndrome; see page 6) 68 H/I (HOXB13:1L17BR) Gene Expression Ratio (used for breast cancer recurrence risk) 32 JAK2 testing (for chronic myeloproliferative disorders for children. JAK2 testing is covered for adults.) 18 Mammoprint assay used for the prognosis of breast cancer recurrence. Mammostrat (used for breast cancer recurrence risk) 32 Methylenetetrahydrofolate reductase (MTHFR) C677T (used for risk assessment for venous thromboembolism or obstetric complications) 20 Methylenetetrahydrofolate reductase (MTHFR) (to predict response to anti-folate chemotherapy (includes Methotrexate) 56 Mitochondrial DNA (mtdna) Whole Genome Scanning/Sequencing (See Mitochondrial Disorders under Covered Diagnoses for diagnoses covered) 97,103 PathFinder TG (RedPath Integrated Pathology) (wide scope of 80, 98 application in cancer diagnosis) PGxPredict : Rituximab (for prediction of response to treatment of follicular non-hodgkin s lymphoma) 73 PreGen-Plus ProOncTumorSourceDX (to identify tissue or origin for metastatic tumor) 89 TargetNow by Caris DX (to identify biological markers in an individual s cancer that are associated with targets for certain drugs) TheraGuide 5-FU (used to determine risk of adverse reaction to 5-FU related chemotherapy) 29 Covered Diagnoses and types of genetic testing covered for each diagnosis Genetic testing may be covered when any of the following diagnoses are suspected, either because of symptomatology and/or family history. The basic guidelines on page 2 must be met. The results of the genetic testing must have potential to impact medical and reproductive decision making. Please see Background section on pages 10 and 11 for a list of the different types of genetic tests. In some instances, more specific criteria are required for coverage of a genetic test. See pages 7 through 10 for those. Newborn Screening for genetic disorders, as mandated by state guidelines. Guidelines can be accessed at the following web site 2 : Adrenoleukodystrophy, X-linked (includes adrenomyeloneuropathy)
5 Page 5 of 25 (diagnostic, carrier, prenatal or preimplantation) 83 AlloMap (used to determine cardiac transplant rejection) 57. See page 7 for specific guidelines. To be used in lieu of biopsy. Prior Authorization is required. Angelman syndrome (diagnostic testing for children) 58 Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy (ARVD/C). Genetic testing for sequence variants (DSG2, DSP and PKP2) are covered for diagnostic and predictive purposes as stated below: o Members with confirmed diagnosis (using International Task Force diagnostic criteria) to facilitate genetic screening for ARVD/C in atrisk relatives; or o Members who are at-risk relatives (meaning first-degree or seconddegree relative) or of a proband with a confirmed diagnosis of ARVD/C (as defined above). 102 Azoospermia or severe oligozoospermia (Y chromosome microdeletions) (diagnostic). 79 Breast and ovarian cancer BRCA1, BRCA2, and BART: see pages 7 and 8 for specific guidelines. 13,54,60,101 Prior Authorization is required. Breast cancer recurrence: see page 8 for specific guidelines for Oncotype DX. 32 Prior Authorization is required. Breast cancer, invasive: Invitrogen SPOT_Light HER2 CISH covered to predict response to trastuzumab treatment. 86 Canavan disease (diagnostic, carrier, prenatal or preimplantation testing) 33,59 Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) (diagnostic, predictive, prenatal and preimplantation) 61,84 Charcot-Marie-Tooth, Type 1A (diagnostic, prenatal or preimplantation testing) 11,34 Chromosomal imbalances for patients, see page 8 for specific guidelines for Comparative Genomic Hybridization (CGH) Microarray Testing. 64 Chromosome 22q11.2 deletion syndrome (diagnostic, prenatal and preimplantation) 62 Chronic myelogenous leukemia (CML), BCR-ABL Testing (Diagnostic and monitoring response to therapy) 10 Chronic Myeloproliferative Disorders (polycythemia vera, essential thrombocytopenia and primary Myelofibrosis), JAK2 testing for adults (diagnostic testing) 18 Colorectal Cancer Drug Therapy (KRAS Sequence Variant Analysis) (predictive) 71 Cystic Fibrosis (diagnostic, carrier, prenatal or preimplantation testing) 6,59 Deafness (Congenital) (diagnostic, carrier or prenatal testing) 35 Ehlers-Danlos syndrome (diagnostic, predictive, prenatal or 30, 49 preimplantation testing)
6 Page 6 of 25 Familial adenomatous polyposis (diagnostic, predictive, prenatal or 7, 47 preimplantation testing): see page 9 for guidelines. Familial dysautonomia (diagnostic, carrier, prenatal or preimplantation) 59 Fanconi s anemia (diagnostic testing) 47 Fragile X syndrome (diagnostic, carrier, prenatal or preimplantation 15, 26 testing) Gaucher disease (carrier, prenatal or preimplantation testing) 36 HLA associated diseases (diagnostic) 69 Hemachromatosis, hereditary (diagnostic or carrier testing) 16,37 Hemoglobinopathies (thalassemias and sickle cell anemia) (diagnostic or carrier testing) 43,44 Hemophilias (diagnostic, carrier, prenatal or preimplantation testing) 38,39 Hereditary non-polyposis colorectal cancer (HNPCC) Lynch syndrome: See pages 8 and 9 for colorectal cancer screening guidelines (diagnostic, predictive, prenatal or preimplantation testing) 8, 47,81,100 Prior Authorization is required. Huntington Chorea/Disease (diagnostic, predictive, prenatal or preimplantation testing) 17 Long QT syndrome (Familion ) (diagnostic or predictive testing) 3,68 Lung cancer, non-small cell. Epidermal Growth Factor Receptor (EGFR) Sequence Variant Analysis and KRAS Sequence Variant Analysis (for predicting response to non-small cell lung cancer drug therapy) (predictive) 67,70,82 MYH associated polyposis (diagnostic, predictive, prenatal or preimplantation testing) 47,88 See page 9 for guidelines. Prior Authorization is required. Mitochondrial disorders: Leber hereditary optic neuropathy (LHON), Leigh syndrome, neurogenic muscle weakness with ataxia and retinitis pigmentosa (NARP), mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS), myoclonic epilepsy with ragged-red fibers (MERRF) (diagnostic, prenatal or preimplantation 19, 97, 103 testing) Myotonic dystrophy (diagnostic, predictive, prenatal or preimplantation testing) 27,72 Niemann-Pick disease (diagnostic, carrier, prenatal or preimplantation testing) 40 47, 52 Neurofibromatosis (diagnostic, prenatal and preimplantation testing) Osteogenesis Imperfecta Types 1 to IV (diagnostic, prenatal, preimplantation testing) 63 PTEN hamartoma tumor syndrome (diagnostic, predictive, prenatal or preimplantation testing) for patients at high risk of breast, thyroid, endometrial and renal cancers. 99 Prader-Willi syndrome (diagnostic, prenatal and preimplantation testing) 21 Primary dystonia Type 1 (diagnostic, prenatal or preimplantation
7 Page 7 of 25 testing) 22,28 Retinoblastoma (diagnostic, predictive and prenatal testing) 41 RET Proto-Oncogene Point Mutations (diagnostic or predictive testing) 42 Rett syndrome (diagnostic, prenatal or preimplantation) 53 Spinal Muscular Atrophy (diagnostic, carrier, prenatal or preimplantation) 74 75,76,77, Spinocerebellar Ataxia (diagnosis, predictive) Tay-Sachs disease (diagnostic, carrier, prenatal or preimplantation testing) 9,59 Thrombosis panel for risk assessment for venous thromboembolism (VTE) or obstetric complications: see page 6 for specific guidelines. 14,20,23,24 von Hippel-Lindau disease (diagnostic, predictive, prenatal or preimplantation testing) 45 Uniparental disomy (UPD) for Chromosomes 7,11,14 or 15 (diagnostic, prenatal or preimplantation). See page 8 for specific guidelines. 78 Warfarin, Pharmacogenetic Testing: This test has been reviewed by the Technology Assessment Committee and the Committee. The practical value of this testing is limited or yet to be determined; therefore, the TAC and MPC do not endorse this testing for widespread use. A small volume of clinically appropriate testing is already occurring and will be continue to be covered. 104 Additional criteria for specific genetic testing: AlloMap is is a noninvasive option to monitor cardiac transplant rejection, and is used in lieu of biopsy. 57 AlloMap testing protocols will vary with facilities. PHP covers AlloMap when ALL of the following criteria are met. Prior Authorization is required. o Member is age 15 years or older o Member is >55 days post-cardiac transplant with stable allograft function and low probability of moderate/severe acute cellular rejection at the time of testing. BRCA1, BRCA2, and BART for breast or ovarian cancer: Prior Authorization is required. Prior Authorization for BART will not be given unless BRCA1 and BRCA2 have been done and are negative. Basic guidelines for BRCA1 and BRCA2 testing are listed below. Refer to NCCN Practice Guidelines in Oncology for Hereditary Breast and/or Ovarian Cancer for greater detail. Genetic testing criteria for BRCA1 and BRCA2 is located on page 6 at the following web address: 13,54,60,101 g.pdf BRCA1 and BRCA2 are covered per NCCN guidelines in the following
8 Page 8 of 25 circumstances: Member from a family with a known BRCA1/BRCA2 mutation Personal history of breast cancer and one or more of the following: Diagnosed at 45 years of age Diagnosed at 50 years of age with at least one close blood relative with breast cancer 50 years of age and/or 1 close blood relative with epithelial ovarian/fallopian tube/primary peritoneal cancer at any age Two breast primaries when first breast cancer diagnosis occurred before age 50 Diagnosed age <60 with a triple negative breast cancer Diagnosed age <50 with a limited family history (Individuals with limited family history, such as fewer than 2 first or second degree female relatives surviving beyond 45 years in either lineage may have underestimated probablility of a familial mutation) Diagnosed at any age, when 2 close blood relatives with breast and/or epithelial ovarian, cancer at any age Diagnosed at any age with 2 close blood relatives with pancreatic cancer at any age. Close male blood relative with breast cancer For an individual of ethnicity associated with higher mutation frequency (e.g., Ashkenazi Jewish, Icelandic, Swedish, Hungarian or other). Personal history of epithelial ovarian, fallopian tube, and primary peritoneal cancer Personal history of male breast cancer Personal history of pancreatic cancer at any age with 2 close blood relatives with breast and/or ovarian and/or pancreatic cancer at any age Family history only: First or second degree blood relative meeting any of the above criteria Third degree blood relative with breast cancer and/or ovarian, fallopian tube, and primary peritoneal cancer with 2 close blood relatives with breast cancer ( at least one with breast cancer 50 y) ovarian cancer Predicting breast cancer recurrence: Oncotype DX Recurrence Score Assay is a genomic test. Prior Authorization is required. Oncotype DX is covered in the following circumstances: o Woman newly diagnosed with Stage I or II breast cancer o Node negative, estrogen receptor positive 32 o Pedigree and genetic counseling not necessary for this test Colorectal cancer (CRC) screening is covered for patients meeting the requirements below. Prior Authorization is required. o PHP covers genetic testing for Lynch syndrome. Prior Authorization is required. Lynch syndrome (HNPCC) is the most
9 Page 9 of 25 common of the familial colon cancer syndromes and the identification of individuals with Lynch syndrome and their relatives leads to reduced cancer mortality. The Amsterdam Criteria II and the revised Bethesda guidelines were developed to improve identification of individuals with Lynch syndrome who will benefit from genetic testing. However, these criteria are stringent and difficult to use in a clinical practice setting. The following requirements must be met: 1. Familial adenomatous polyposis has been excluded as a diagnosis; and 2. Before gene sequencing is ordered for a member who has been identified as potentially having Lynch Syndrome, the following requirements should be met and available for review: A three generation pedigree The person being initially tested in a family with suspected Lynch syndrome should have the diagnosis of colorectal cancer or endometrial cancer. If a person with endometrial cancer is the first family member with an HNPCC-related cancer, there must also be a first-degree relative with documented colon cancer. HNPCC or Lynch-related tumors refer to colorectal, endometrial, gastric, ovarian, pancreas, ureter, renal pelvis, biliary tract, small bowel CA, as well as brain and sebaceous skin tumors. The person to be tested should have had immunohistochemistry (IHC) testing of the four MMR proteins (MSH1, MSH6, MLH1, PMS2) and one of the MMR proteins should be absent by IHC. There are exceptions that may need to be reviewed with peer consultants. IHC stains for MMR are available at TriCore Reference Laboratories, and are typically obtained post-operatively on colon tumor pathologies. If MLH1 is absent by IHC prior to gene sequencing, testing for b-raf is recommended. If testing for b-raf is negative, then promoter methylation assay is recommended. 3. Genetic counseling and testing is also available to PHP members who have a first or second-degree relative in which a deleterious Lynch syndrome mutation has been identified. 4. All other requests for testing should be referred to the Medical Directors who will utilize the Amsterdam II and revised Bethesda guidelines in determination of coverage for genetic testing. A three-generation pedigree demonstrating a family history of colon cancer or other HNPCC/Lynch-related cancers should be submitted with the request. Note: Gene sequencing for the 4 MMR proteins are carried out at a number of reference laboratories. It is best to order the specific gene which shows an abnormal IHC. It is not necessary to sequence all genes at the same time. The majority of mutations occur in either MLH1
10 Page 10 of 25 or MSH o Gene sequencing, including Colaris AP, is covered for the detection of mutations in the APC and MYH genes which cause adenomatous polyposis syndromes, including familial adenomatous polyposis (FAP), attenuated FAP (AFAP) and MYH-associated polyposis (MAP). Prior Authorization is required. The following criteria must be met: o Member has a personal history of suspected FAP, AFAP or MAP; or o Member has a first- or second-degree relative with disease 7,47,55, 88 causing mutation for FAP, AFAP or MAP. Comparative Genomic Hybridization (CGH) Microarray Testing for Chromosomal Imbalances is covered as an adjunct to a conventional karyotype analysis for members suspected of having a genetic syndrome (i.e., have congenital anomalies, dysmorphic features, mental retardation, developmental delays or disabilities), when the test results will impact medical or reproductive decision making. 64 Thrombosis panel for risk assessment for venous thromboembolism (VTE) or obstetric complications -- Factor V Leiden and Prothrombin G20210A are covered in the following circumstances: o Patients with obstetric complications of abnormal placenta vasculature o Patients with VTE with a personal or family history of recurrent VTE. 14,20,23,24 Uniparental disomy (UPD) for Chromosomes 7, 11, 14, 15 o For neonates, infants, children or adults symptomatic for Beckwith- Wiedermann syndrome for UPD for chromosome 11 o For prenatal testing among fetuses with inherited Robertsonian translocations, supernumerary marker chromosomes, or level III mosaicism involving chromosomes 7, 14 or 15 for UPD for chromosomes 7, 14 or Background Genetics refers to the study of genes and their role in inheritance the way certain traits or conditions are passed down from one generation to another. Genetics involves scientific studies of single genes and their effects. Genes (units of heredity) carry the instructions for making proteins, which direct the activities of cells and functions of the body. Genes influence traits such as hair and eye color as well as health and disease development. Genetics determines much (but not all) of a person s health status; environmental differences also play a part. 48 A genome is defined as all the genetic material in the chromosomes of a particular organism. Genomics is a relatively new term describing the study
11 Page 11 of 25 of multiple genes from the same person, including interactions of those genes with each other and the person s environment. Genomics involves the scientific study of complex diseases such as heart disease, asthma, diabetes and cancer because they are caused more by a combination of genetic and environmental factors. Genomics is offering new possibilities for therapies and treatment of some diseases, as well as new diagnostic methods. The major tools and methods related to genomics studies are bioinformatics, genetic analysis, measurement of gene expression, and determination of gene function. 48 Types of genetic tests: Newborn screening: Used just after birth to identify genetic disorders that can be treated early in life. Diagnostic testing: Used to identify or rule out a specific genetic or chromosomal condition. In many cases, genetic testing is used to confirm a diagnosis when a particular condition is suspected based on physical signs and symptoms. Diagnostic testing can be performed before birth or at any time during a person s life, but is not available for all genes or all genetic conditions. The results of a diagnostic test can influence a person s choices about health care and the management of the disorder. Carrier testing: Used to identify people who carry one copy of a gene mutation that, when present in two copies, causes a genetic disorder. This type of testing is offered to individuals who have a family history of a genetic disorder and to people in certain ethnic groups with an increased risk of specific genetic conditions. If both parents are tested, the test can provide information about a couple s risk of having a child with a genetic condition. Prenatal testing: Used to detect changes in a fetus genes or chromosomes before birth. This type of testing is offered during pregnancy if there is an increased risk that the baby will have a genetic or chromosomal disorder. In some cases, prenatal testing can lessen a couple s uncertainty or help them make decisions about a pregnancy. It cannot identify all possible inherited disorders and birth defects. Preimplantation testing: A specialized technique that can reduce the risk of having a child with a particular genetic or chromosomal disorder. It is used to detect genetic changes in embryos created using assisted reproductive techniques such as in-vitro fertilization. Only embryos without certain genetic changes are implanted in the uterus to initiate a pregnancy.
12 Page 12 of 25 Predictive and presymptomatic testing: Used to detect gene mutations associated with disorders that appear after birth, often later in life. These tests can be helpful to people who have a family member with a genetic disorder, but who have no features of the disorder themselves at the time of testing. The results of the testing can provide information about a person s risk of developing a specific disorder and help with making decisions about medical care. 46 Medical Terms Assay: A laboratory test to find and measure the amount of a specific substance. First-degree relative: Parents, children, siblings (blood relatives). Gene expression: The process by which proteins are made from the instructions encoded in DNA. Second-degree relative: Grandparents, aunts and uncles, nieces and nephews, grandchildren, half-sibling (blood relatives) Third-degree relative: Great-grandparents, great-aunts, great-uncles, and first cousins (blood relatives) Three-Generation Pedigree: A pictorial representation of diseases within a family to assess hereditary influences on disease or to help identify relatively rare conditions that may not be considered in a differential diagnosis. 51 Coding HCPCS Codes S0265 S3800 S3818 S3819 S3820 S3822 S3823 The coding listed in this is for reference only. Covered and noncovered codes are included in this list. Description Genetic counseling, under physician supervision, each 15 minutes Genetic testing for amyotrophic lateral sclerosis (ALS) Complete gene sequence analysis; BRCA1 gene Complete gene sequence analysis; BRCA2 gene Complete BRCA1 and BRCA2 gene sequence analysis for susceptibility to breast and ovarian cancer Single mutation analysis (in individual with a known BRCA1 or BRCA2 mutation in the family) for susceptibility to breast and ovarian cancer Three-mutation BRCA1 and BRCA2 analysis for susceptibility to breast and ovarian cancer in Ashkenazi individuals
13 Page 13 of 25 S3828 S3829 S3830 S3831 S3833 S3834 S3835 S3837 S3840 S3841 S3842 S3843 S3844 S3845 S3846 S3847 S3848 S3849 S3850 S3851 S3852 S3853 S3854 S3855 S3860 S3861 S3862 Complete gene sequence analysis; MLH1 gene (Colaris ) Complete gene sequence analysis; MSH2 gene (Colaris ) Complete MLH1 and MLH2 gene sequence analysis for hereditary nonpolyposis colorectal cancer (HNPCC) genetic testing (Colaris ) Single-mutation analysis (in an individual with known MLH1 and MLH2 mutation in the family) for hereditary nonpolyposis colorectal cancer (HNPCC) genetic testing (Colaris ) Complete APC gene sequence analysis for susceptibility to familial adenomatous polyposis (FAP) and attenuated FAP (Colaris AP ) Single-mutation analysis (in individuals with a known APC mutation in the family) for susceptibility to familial adenomatous polyposis (FAP) and attenuated FAP (Colaris AP ) Complete gene sequence analysis for cystic fibrosis Complete gene sequence analysis for hemochromatosis genetic testing DNA analysis for germline mutations of the RET proto-oncogene for susceptibility to multiple endocrine neoplasia type 2 Genetic testing for retinoblastoma Genetic testing for von Hippel-Lindau disease DNA analysis of the F5 gene for susceptibility to Factor V Leiden thrombophilia DNA analysis of the connexin26 gene (GJB2) for susceptibility to congenital, profound deafness Genetic testing for alpha-thalassemia Genetic testing for hemoglobin E beta-thalassemia Genetic testing for Tay-Sachs disease Genetic testing for Gaucher disease Genetic testing for Niemann-Pick disease Genetic testing for sickle cell anemia Genetic testing for Canavan disease DNA analysis for APOE epilson 4 allelle for susceptibility to Alzheimer s disease Genetic testing for myotonic muscular dystrophy Genetic expression profiling panel for use in the management of breast cancer treatment (Oncotype DX) Genetic testing for detection of mutations in the presenilin, 1 gene Genetic testing, comprehensive cardiac ion channel analysis, for variants in 5 major cardiac ion channel genes for individuals with high index of suspicion for familiar long QT syndrome (LQTS) or related syndromes Genetic testing, sodium channel, voltage-gated, type V, alpha subunit (SCN5A) and variants for suspected Brugada syndrome Genetic testing, family-specific ion channel analysis, for blood-relatives of individuals (index case) who have previously tested positive for a genetic variant of a cardiac ion channel syndrome using either one of the above test configurations or confirmed results from another laboratory
14 Page 14 of 25 S3890 DNA analysis, fecal, for colorectal cancer screening CPT Codes Description Molecular diagnostics; isolation or extraction of highly purified nucleic acid Molecular diagnostics; separation by gel electrophoresis (e.g. agarose, polyacrylamide) Molecular diagnostics; amplification of patient nucleic acid, each nucleic acid sequence Molecular diagnostics; mutation identification by sequencing, single segment, each segment Molecular diagnostics; signal amplification of patient nucleic acid, each nucleic acid sequence Molecular diagnostics; separation and identification by high resolution technique (eg, capillary electrophoresis) Molecular diagnostics; interpretation and report Molecular diagnostics; RNA stabilization Mutation identification by enzymatic ligation or primer extension, single segment, each segment (eg, oligonucleotide ligation assay (OLA), single base chain extension (SBCE), or allele-specific primer extension (ASPE)) Chromosome analysis for breakage syndromes Chromosome analysis Molecular cytogenetics Chromosome analysis Array-based evaluation of multiple molecular probes ICD-9 Diagnosis Codes Description Malignant neoplasm of stomach Malignant neoplasm of small intestine, including duodenum Malignant neoplasm of colon Malignant neoplasm of liver and intrahepatic bile ducts Malignant neoplasm of trachea, bronchus and lung Malignant neoplasm of connective and other soft tissue site unspecified
15 Page 15 of 25 ICD-9 Diagnosis Codes Description Malignant neoplasm of female breast Malignant neoplasm of uterus, part unspecified Malignant neoplasm of body of uterus Malignant neoplasm of other specified sites of body of uterus Malignant neoplasm of ovary and other uterine adnexa [ Malignant neoplasm of Fallopian tube Malignant neoplasm of bladder Malignant neoplasm of renal pelvis Malignant neoplasm of ureter Malignant Neoplasm of Eye: eyeball except conjunctiva, cornea, retina, and choroid Malignant neoplasm of retina 193 Malignant neoplasm of thyroid gland 202.0, Lymphoma (malignant), follicular Multiple myeloma and immunoproliferative neoplasms (without remission) Multiple myeloma and immunoproliferative neoplasms (in remission) Acute lymphoid leukemia, without mention of having achieved remission Chronic lymphoid leukemia, without mention of having achieved remission Acute myeloid leukemia, without mention of having achieved remission Chronic myeloid leukemia, without mention of having achieved remission Benign neoplasm of colon Benign neoplasm of adrenal gland Carcinoma in situ of breast Neurofibromatosis, type I Neurofibromatosis, type II Polycythemia vera Essential thrombocythemia Myelodysplastic syndrome, unspecified Myelofibrosis with myeloid metaplasia Other lymphatic and hematopoietic tissues Other anterior pituitary disorders Multiple endocrine neoplasia [MEN] type Dwarfism, not elsewhere classified Other disturbances of aromatic amino-acid metabolism Lipidoses Disorders of iron metabolism Cystic Fibrosis Other amyloidosis
16 Page 16 of 25 ICD-9 Diagnosis Codes Description Mucopolysaccaridosis Disorders of fatty acid oxidation Disorders of mitochondrial metabolism Other hemolytic anemias due to enzyme deficiency Thalassemias Sickle-cell trait Other hemoglobinopathies Constitutional aplastic anemia Congenital factor VIII disorder Congenital factor IX disorder Congenital deficiency of other clotting factors Congenital neutropenia Cyclic neutropenia Primary hypercoagulable state Infantism autism, current or active state or residual state Mental retardation Leukodystrophy Cerebral lipidoses Other specified cerebral degenerations in childhood Huntington s chorea Genetic torsion dystonia Friedreich s ataxia Hereditary spastic paraplegia Other cerebellar ataxia Other spinocerebellar diseases Spinal muscular atrophy Generalized convulsive epilepsy Peroneal muscular atrophy Hereditary sensory neuropathy Hereditary progressive muscular dystrophy Myotonic disorders Pigmentary retinal dystrophy Other optic neuritis Hearing loss Long QT syndrome Cardiac dysrhythmia, unspecified (use for ARVD/C)
17 Page 17 of 25 ICD-9 Diagnosis Codes Description Budd-Chiari syndrome Regional enteritis Ulcerative colitis Acute pancreatitis Chronic pancreatitis Nephrotic syndrome Azoospermia Oligospermia Muscle weakness Reduction deformity of brain Other specified anomalies of nervous system (familial dysautonomia) Polycystic kidney, autosomal recessive Acrocephalosyndactyly Anomalies of skull and face bones Chondrodystrophy [achondroplasia] Osteogenesis imperfecta Ehlers-Danlos syndrome Other specified anomalies of muscle, tendon, fascia, and connective tissue Ichthyosis congenita Velo-cardio-facial syndrome Other hamartoses, not elsewhere classified Prader-Willi syndrome Marfan syndrome Fragile X syndrome Other specified anomalies Lack of coordination Short stature Other nonspecific abnormal serum enzyme levels [hyper-amylasemia] Complications of transplanted organ, heart V10.05 Personal history of malignant neoplasm of large intestine V10.06 Personal history of malignant neoplasm of rectum, rectosigmoid junction, and anus V10.3 Personal history of malignant neoplasm of breast V10.42 Personal history of malignant neoplasm of other parts of uterus V10.43 Personal history of malignant neoplasm of ovary V12.72 Personal history of colonic polyps V13.69 Personal history of other congenital malformations V16.0 Family history of malignant neoplasm of gastrointestinal tract
18 Page 18 of 25 ICD-9 Diagnosis Codes Description V17.2 Family history of neurological diseases V17.41 Family history of sudden cardiac death, other cardiovascular diseases V17.49 V18.4 Family history of mental retardation V18.51 Family history, colonic polyps V18.59 Family history of other digestive disorders V18.9 Family history of genetic disease carrier V19.5 Family history of congenital anomalies V26.31 Testing of female for genetic disease carrier status V26.32 Other genetic testing of female V26.33 Genetic counseling V26.34 Testing of male for genetic disease carrier status V26.39 Other genetic testing of male V28.0 Screening for chromosomal anomalies by amniocentesis V29.3 Observation for suspected genetic or metabolic condition V42.1 Organ or tissue replaced by transplant, heart V77.0 Special screening for thyroid disorders V77.6 Special screening for cystic fibrosis V77.7 Special screening for other inborn errors of metabolism V77.91 Screening for lipoid disorders V77.99 Special screening for other and unspecified endocrine, nutritional, metabolic, and immunity disorders V78.1 Special screening for other and unspecified deficiency anemia V78.2 Special screening for sickle-cell disease or trait V78.3 Special screening for other hemoglobinopathies V78.8 Special screening for other disorders of blood and blood-forming organs V80.0 Special screening for neurological conditions V80.3 Special screening for ear diseases V82.4 Maternal postnatal screening for chromosomal anomalies V83.01 Asymptomatic hemophilia A carrier V83.02 Symptomatic hemophilia A carrier V83.81 Cystic fibrosis gene carrier V83.89 Other genetic carrier status V84.09 Genetic susceptibility to other malignant neoplasm V84.81 Genetic susceptibility to other disease V84.89 References: 1. Hayes Health Information, copyright Winifred S. Hayes, Inc Gene Testing. 2. Section NMSA 1978, Chapter 359, Section 6. Tests Required for
19 Page 19 of 25 Newborn Infants. Accessed on at: 3. Hayes Brief. Copyright Winifred S. Hayes, Inc Genetic Testing Using the Familion Test (PGxHealth ) for Diagnosis of Inherited Channelopathies. October 24, Archived January 20, Hayes Directory. Copyright Winifred S. Hayes, Inc Genetic Testing for Susceptibility to Alzheimer s Disease. May 13, Archived November 12, Hayes Directory. Copyright Winifred S. Hayes, Inc Genetic Testing for Susceptibility to Hereditary Nonpolyposis Colorectal Cancer. December 15, Archived August 31, Hayes Directory. Copyright Winifred S. Hayes, Inc Genetic Carrier Testing for Cystic Fibrosis. June 7, Update Search: August 1, Archived July 7, Hayes Directory. Copyright Winifred S. Hayes, Inc Genetic Testing for Susceptibility to Familial Adenomatous Polyposis. January 21, Archived November 12, Hayes Directory. Copyright Winifred S. Hayes, Inc Genetic Testing for Susceptibility to Hereditary Nonpolyposis Colorectal Cancer. December 15, Update Search January 29, Archived August 31, Hayes Directory. Copyright Winifred S. Hayes, Inc Genetic Testing for Tay-Sachs Disease. August 25, Archived November 12, Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc BCR-ABL Testing in Chronic Myelogenous Leukemia (CML). September 30, Update Search: September 3, Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc Charcot-Marie-Tooth Type 1A (PMP22). August 5, Update Search: July 30, Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc CDKN2A Testing for Malignant Melanoma. July 29, Update Search: July 21, Hayes Genetic Test Evaluation Report Copyright Winifred S. Hayes, Inc Comprehensive Screening for Large Rearrangements in BRCA1/2 for Assessment of Breast Cancer Risk. March 31, Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc Factor V Leiden (FVL) for Risk Assessment for Venous Thromboembolism (VTE) or Obstetric Complications. October 1, Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc Fragile X Syndrome (FMR1). August 7, Update Search: July 29, Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc HFE-Associated Hereditary Hemochromatosis (HFE-HHC). June 4, Update Search: June 19, Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc Huntington Chorea/Disease (HD) for Diagnostic, Predictive, and Prenatal or Preimplantation Genetic Diagnosis Purposes. April 29, 2008.
20 Page 20 of Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc Janus kinase 2 (JAK2) Sequence Variants (including V617F) for Chronic Myeloproliferative Disorders. May 27, Update Search: June 1, Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc LHON (Leber Hereditary Optic Neuropathy) for Diagnosis and Risk of Transmission. March 30, Update Search: March 19, Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc Methylenetetrahydrofolate Reductase (MTHFR) C677T for Risk Assessment for Venous Thromboembolism (VTE) or Obstetric Complications. October 3, Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc Prader-Willi Syndrome (PWS). October 15, Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc Primary Dystonia Type 1 (DYT1/TOR1A).July 7, Update Search: July 21, Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc Prothrombin G20210A for Risk Assessment for Venous Thromboembolism (VTE) or Obstetric Complications. October 2, Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc Thrombosis Panel for Risk Assessment for Venous Thromboembolism (VTE) or Obstetric Complications. October 1, Hayes Genetic Test Evaluation Synopsis. Copyright Winifred S. Hayes, Inc Familial Thrombosis. April 30, Hayes Genetic Test Evaluation Synopsis. Copyright Winifred S. Hayes, Inc Fragile X Syndrome (FMR1) April 8, Hayes Genetic Test Evaluation Synopsis. Copyright Winifred S. Hayes, Inc Myotonic Dystrophy Types 1 and 2. December 7, Hayes Genetic Test Evaluation Synopsis. Copyright Winifred S. Hayes, Inc Primary Dystonia Type 1 (TOR1A). April 8, Hayes Genetic Test Evaluation Report. Copyright Winifred S. Hayes, Inc TheraGuide 5-FU (Myriad Genetics) for Predicting Toxicity to 5- Fluorouracil (5-FU)/Capecitabine-based Chemotherapy. May 5, Hayes Search and Summary. Copyright Winifred S. Hayes, Inc Genetic Testing for Vascular (Type IV) Ehlers-Danlos Syndrome. October 19, Hayes Search and Summary. Copyright Winifred S. Hayes, Inc SHOX (Short Stature HOmeoboX-Containing) Gene Deletion/Mutation Testing for the Prenatal Diagnosis of Leri-Weill Syndrome. February 22, Hayes Special Report. Copyright Winifred S. Hayes, Inc Gene Expression Profiling for Prognosis of Recurrence of Primary Breast Cancer. January Update Search December 6, Matalon R. GeneReviews. Canavan Disease. Posted Last Revision: Pagon RA, Bird TC, et al. GeneReviews. Charcot-Marie-Tooth Neuropathy Type 1. Posted Last Revision Smith R, Van Camp G. GeneReviews. Deafness and Hereditary Hearing Loss Overview. Posted Last Revision
21 Page 21 of Pastores GM, Hughes DA. GeneReviews. Gaucher Disease. Posted Last Update Kowdley KV, et al. GeneReviews. HFE-Associated Hereditary Hemochromatosis. Posted Last Update Brower C, Thompson AR. GeneReviews. Hemophilia A. Posted Last Update Brower C, Thompson AR. GeneReviews. Hemophilia B. Posted Last Update Patterson M. GeneReviews. Niemann-Pick Disease Type C. Posted Last Revision Lohmann DR, Gallie BL. GeneReviews. Retinoblastoma. Posted Last Update Wiesner GL, Snow-Bailey K. GeneReviews. Multiple Endocrine Neoplasia Type 2. Posted Last Update Bender MA, Hobbs W, Vichinsky E, Schlis K. GeneReviews. Sickle Cell Disease. Posted Last Update Galanello R, Cao A. GeneReviews. Alpha-Thalassemia. Posted Last Update Schimke RN, Collins DL, Stolle CA. GeneReviews. Von Hippel-Lindau Syndrome. Posted Last Update Genetics Home Reference, US National Library of Medicine. What are the types of genetic tests? No date posted. 47. Lindor NM, McMaster ML, Lindor CJ, Greene MH. Concise Handbook of Familial Cancer Susceptibility Syndromes, Second Edition. Journal of the National Cancer Institute Monographs, No. 38, National Human Genome Research Institute, National Institutes of Health. Frequently Asked Questions about Genetic and Genomic Science. Last Reviewed: March 27, Wenstrup R, De Paepe A. GeneReviews. Ehlers-Danlos Syndrome, Classic Type. Posted Last Update Pepin MG, Byers PH. GeneReviews. Ehlers-Danlos Syndrome, Vascular Type. Posted Last Update: Wattendorf DJ, Hadley DW. Family History: The Three Generation Pedigree. American Family Physician, Vol. 72, No. 3, August 1, Friedman, JM. GeneReviews. Neurofibromatosis1. Posted Last Update: Christodoulou, J. GeneReviews. MECP2-Related Disorders. Posted Last Revision: NCCN Practice Guidelines in Oncology. Hereditary Breast and/or Ovarian Cancer, v /04/09 National Comprehensive Cancer Network, Inc. Accessed : American Gastroenterological Association. Colorectal Cancer Screening and Surveillance: Clinical Guidelines and Rationale Update Based on New Evidence
22 Page 22 of 25 Additional references used for revision of Agency for Healthcare Research and Quality (AHRQ) Technology Assessment Program. Reviews of Selected Pharmacogenetic Tests for Non-Cancer and Cancer Conditions. Final Report. November 12, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. AlloMap Molecular Expression (XDx Inc.) for Detection of Heart Transplant Rejection. November 18, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Angelman Syndrome (AS). November 7, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Ashkenazi Jewish Genetic Carrier Screening Panel. February 18, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Breast Cancer Susceptibility 1 and 2 (BRCA 1/2) Sequence Variant Testing for Susceptibility to Hereditary Breast Cancer. January 5, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL). April 16, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Chromosome 22q11.2 Deletion Syndrome. November 20, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. COL1A1 and COL1A2 Gene Testing for Osteogenesis Imperfecta Types I to IV. February 20, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Comparative Genomic Hybridization (CGH) Microarray Testing for Chromosomal Imbalances. October 28, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. CYP2D6 Genotyping for Dose Management of Tamoxifen. September 5, Update Search: August 11, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Epidermal Growth Factor Receptor (EGFR) Gene Amplification Analysis by Fluorescence in situ Hybridization (FISH) for Predicting Response to Non-Small Cell Lung Cancer (NSCLC) Drug Therapy. September 17, Update Search: September 30, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Epidermal Growth Factor Receptor (EGFR) Sequence Variant Analysis for Predicting Response to Non-Small Cell Lung Cancer (NSCLC) Drug Therapy. July 11, Update Search: July 21, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Familion Test (PGxHealth) for Diagnosis of Cardiac Channelopathies. October 24, Update Search December 18, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. HLA-B*5701 Screening for Abacavir (Ziagen ) Hypersensitivity in HIV Patients. October 10, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. KRAS Sequence Variant Analysis for Non-Small Cell Lung Cancer (NSCLC).
23 Page 23 of 25 October 7, Update Search November 24, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. KRAS Sequence Variant Analysis for Predicting Response to Colorectal Cancer Drug Therapy. November 10, Update Search: December 10, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Myotonic Dystrophy Types 1 and 2. March 9, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. PGxPredict : Rituximab Test (PGxHealth) for FCGR3A Variants in Follicular CD20-Positive Non-Hodgkin s Lymphoma. November 4, Update Search: December 10, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Spinal Muscular Atrophy. January 23, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Spinocerebellar Ataxia Type 1. February 9, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Spinocerebellar Ataxia Type 2. February 23, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Spinocerebellar Ataxia Type 3/Machado-Joseph Disease. February 26, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Uniparental Disomy (UPD) for Chromosomes 6, 7, 11, 14, or 15. October 31, Update Search: December 10, Hayes Genetic Test Evaluation Report Winifred S. Hayes, Inc. Y Chromosome Microdeletions in Male Infertility. November 14, Update Search: December 10, Hayes Genetic Test Evaluation Synopsis Winifred S. Hayes, Inc. PathFinderTG Test (RedPath Integrated Pathology Inc.) for the Diagnosis of Breast Cancer. May 30, NCCN Practice Guidelines in Oncology v Colorectal Cancer Screening National Comprehensive Cancer Network, Inc. October 23, NCCN Practice Guidelines in Oncology v Non-Small Cell Lung Cancer. December 15, Moser HW, Moser AB, Steinberg, SJ, Raymond GV. GeneReviews. X-Linked Adrenoleukodystrophy. Initial Posting: March 26, Last Update: July 2, Oberstein SAJ, Boon EMJ, Dichgans M. GeneReviews. CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy). Initial posting: March 15, Last Update: July 23, 2009 Additional References used for revision of : 85. Hayes Genetic Test Evaluation Report, Copyright 2009 Winifred S. Hayes, Inc. decode Prostate Cancer for Prostate Cancer Risk. October 2, Hayes Genetic Test Evaluation Report. Copyright 2009 Winifred S. Hayes, Inc. Invitrogen SPOT-Light HER2 CISH Kit. April 21, 2009.
24 Page 24 of Hayes Genetic Test Evaluation Report. Copyright 2009 Winifred S. Hayes, Inc. Late-Onset Familial Alzheimer Disease (AD2). October 2, Hayes Genetic Test Evaluation Report. Copyright 2009 Winifred S. Hayes, Inc. MYH-Associated Polyposis (MAP). October 9, Hayes Genetic Test Evaluation Report. Copyright 2009 Winifred S. Hayes, Inc. ProOnc TumorSourceDX - mirview mets (Prometheus Laboratories; Rosetta Genomics) microrna Analysis for Carcinoma of Unknown Primary Origin (CUP). August 5, Hayes Genetic Test Evaluation Report. Copyright 2009 Winifred S. Hayes, Inc. Spinocerebellar Ataxia Type 6. March 11, Hayes Genetic Test Evaluation Report. Copyright 2009 Winifred S. Hayes, Inc. Spinocerebellar Ataxia Type 7. April 29, Hayes Genetic Test Evaluation Report. Copyright 2009 Winifred S. Hayes, Inc. Spinocerebellar Ataxia Type 8. May 14, Hayes Genetic Test Evaluation Report. Copyright 2009 Winifred S. Hayes, Inc. Spinocerebellar Ataxia Type 10. June 2, Hayes Genetic Test Evaluation Report. Copyright 2009 Winifred S. Hayes, Inc. Spinocerebellar Ataxia Type 12. June 11, Hayes Genetic Test Evaluation Report. Copyright 2009 Winifred S. Hayes, Inc. Spinocerebellar Ataxia Type 17. June 25, Hayes Genetic Test Evaluation Synopsis. Copyright 2009 Winifred S. Hayes, Inc. Early-Onset Familial Alzheimer Disease. April 24, Hayes Genetic Test Evaluation Synopsis. Copyright 2009 Winifred S. Hayes, Inc. Mitochondrial DNA (mtdna) Whole Genome Scanning/Sequencing. July 2, Hayes Genetic Test Evaluation Report. Copyright 2009 Winifred S. Hayes, Inc. PathFinderTG Test for Pancreatic Cancer. December 7, Hayes Genetic Test Evaluation Synopsis. Copyright 2009 Winifred S. Hayes, Inc. PTEN Hamartoma Tumor Syndrome. May 1, Hayes Special Report. Copyright 2009 Winifred S. Hayes, Inc. Genetic Testing for Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer [HNPCC]). August Hayes Genetic Test Evaluation Report. Copyright 2009 Winifred S. Hayes, Inc. BRCA1 and BRCA2 Sequence Variant Analysis for Susceptibility to Hereditary Ovarian Cancer. December 16, Hayes Genetic Test Evaluation Report. Copyright 2009 Winifred S. Hayes, Inc. Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy (ARVD/C). November 6, Chinnery PF. GeneReviews. Mitochondrial Disorders Overview. Initial Posting: June 8, Last Update: February 21, Centers for Medicare and Medicaid Services. Decision Memo for Pharmacogenomic Testing for Warfarin Response (CAG-00400N). August 3, References used for revision NCCN Guidelines Version Hereditary Breast and/or Ovarian Cancer
25 Page 25 of 25 Syndrome Accessed on the Internet on NCCN Guidelines Version Breast and/or Ovarian Cancer Syndrome. Accessed on the Internet References used for revision Hayes Decision DX-UM Gene Expression Assay for Risk Stratification of Patients with Uveal Melanoma. Published April 15, American Academy of Ophthalmology, Uveal Melanoma, Copyright Regence August 2010, Chromosomal Microarray Analysis for the Genetic Evaluation of Patients with Developmental Delay/Intellectual Disability or Autism Spectrum Disorders. Reviewed by: 1. Michael Crossey, MD. Clinical Pathologist, TriCore Reference Labs. Albuquerque, NM. August Paul Duncan, MD. Hematology Oncology Associates. Albuquerque, NM. August, September, December 2008, November 2009 Approval Signatures: Clinical Quality Committee: Norman White MD Medical Director: Pedro Cardona MD Approval Date: March 24, 2016 Publication History : Original Effective Date : Review and Revision : Review and Revision : Annual Review & Revision : Revision Updated BRCA1/BRCA2 guidelines : Revised BRCA1/BRCA2, BART guidelines : Revised added Decision Dx-UM for Uveal Melanoma,Mammoprint and Chromosomal Microarray Analysis are not covered : Language added re: Non coverage of multigene panels. This is intended to represent clinical guidelines describing medical appropriateness and is developed to assist Presbyterian Health Plan and Presbyterian Insurance Company, Inc. (Presbyterian) Health Services staff and Presbyterian medical directors in determination of coverage. The is not a treatment guide and should not be used as such. For those instances where a member does not meet the criteria described in these guidelines, additional information supporting medical necessity is welcome and may be utilized by the medical director in reviewing the case. Please note that all Presbyterian Medical Policies are available on the Internet at:
Genetic Testing for Familial Adenomatous Polyposis and MYH-Associated Polyposis (Lynch Syndrome)
 Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Common Cancers & Hereditary Syndromes
 Common Cancers & Hereditary Syndromes Elizabeth Hoodfar, MS, LCGC Regional Cancer Genetics Coordinator Kaiser Permanente Northern California Detect clinical characteristics of hereditary cancer syndromes.
Common Cancers & Hereditary Syndromes Elizabeth Hoodfar, MS, LCGC Regional Cancer Genetics Coordinator Kaiser Permanente Northern California Detect clinical characteristics of hereditary cancer syndromes.
Genetic Testing for Lynch Syndrome/Colorectal Cancer and Polyposis Syndromes
 Genetic Testing for Lynch Syndrome/Colorectal Cancer and Polyposis Syndromes Policy Number: Original Effective Date: MM.02.007 09/01/2011 Line(s) of Business: Current Effective Date: HMO; PPO 09/01/2011
Genetic Testing for Lynch Syndrome/Colorectal Cancer and Polyposis Syndromes Policy Number: Original Effective Date: MM.02.007 09/01/2011 Line(s) of Business: Current Effective Date: HMO; PPO 09/01/2011
GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110
 GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110 COVERAGE: Pre- and post-genetic test counseling may be eligible for coverage in addition to the genetic
GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110 COVERAGE: Pre- and post-genetic test counseling may be eligible for coverage in addition to the genetic
Non-covered ICD-10-CM Codes for All Lab NCDs
 Non-covered ICD-10-CM s for All Lab NCDs This section lists codes that are never covered by Medicare for a diagnostic lab testing service. If a code from this section is given as the reason for the test,
Non-covered ICD-10-CM s for All Lab NCDs This section lists codes that are never covered by Medicare for a diagnostic lab testing service. If a code from this section is given as the reason for the test,
GENETIC CONSIDERATIONS IN CANCER TREATMENT AND SURVIVORSHIP
 GENETIC CONSIDERATIONS IN CANCER TREATMENT AND SURVIVORSHIP WHO IS AT HIGH RISK OF HEREDITARY CANCER? Hereditary Cancer accounts for a small proportion of all cancer or approximately 5-10% THE DEVELOPMENT
GENETIC CONSIDERATIONS IN CANCER TREATMENT AND SURVIVORSHIP WHO IS AT HIGH RISK OF HEREDITARY CANCER? Hereditary Cancer accounts for a small proportion of all cancer or approximately 5-10% THE DEVELOPMENT
CLINICAL GUIDELINES. Lab Management Program. Effective January 15, 2016
 CLINICAL GUIDELINES Lab Management Program Effective January 15, 2016 CareCore National, LLC d/b/a evicore healthcare (evicore) Clinical guidelines for medical necessity review of lab management services.
CLINICAL GUIDELINES Lab Management Program Effective January 15, 2016 CareCore National, LLC d/b/a evicore healthcare (evicore) Clinical guidelines for medical necessity review of lab management services.
Genetic Mutations. Indicator 4.8: Compare the consequences of mutations in body cells with those in gametes.
 Genetic Mutations Indicator 4.8: Compare the consequences of mutations in body cells with those in gametes. Agenda Warm UP: What is a mutation? Body cell? Gamete? Notes on Mutations Karyotype Web Activity
Genetic Mutations Indicator 4.8: Compare the consequences of mutations in body cells with those in gametes. Agenda Warm UP: What is a mutation? Body cell? Gamete? Notes on Mutations Karyotype Web Activity
Genetic Testing in Research & Healthcare
 We Innovate Healthcare Genetic Testing in Research & Healthcare We Innovate Healthcare Genetic Testing in Research and Healthcare Human genetic testing is a growing science. It is used to study genes
We Innovate Healthcare Genetic Testing in Research & Healthcare We Innovate Healthcare Genetic Testing in Research and Healthcare Human genetic testing is a growing science. It is used to study genes
Mendelian inheritance and the
 Mendelian inheritance and the most common genetic diseases Cornelia Schubert, MD, University of Goettingen, Dept. Human Genetics EUPRIM-Net course Genetics, Immunology and Breeding Mangement German Primate
Mendelian inheritance and the most common genetic diseases Cornelia Schubert, MD, University of Goettingen, Dept. Human Genetics EUPRIM-Net course Genetics, Immunology and Breeding Mangement German Primate
Advice about familial aspects of breast cancer and epithelial ovarian cancer a guide for health professionals DECEMBER 2010
 Advice about familial aspects of breast cancer and epithelial ovarian cancer a guide for health professionals DECEMBER 2010 This guide has three parts: 1. Information for health professionals 2. Tables
Advice about familial aspects of breast cancer and epithelial ovarian cancer a guide for health professionals DECEMBER 2010 This guide has three parts: 1. Information for health professionals 2. Tables
PROVIDER POLICIES & PROCEDURES
 PROVIDER POLICIES & PROCEDURES BRCA GENETIC TESTING The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance Program (CMAP) on the requirements for
PROVIDER POLICIES & PROCEDURES BRCA GENETIC TESTING The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance Program (CMAP) on the requirements for
Corporate Medical Policy Genetic Testing for Fanconi Anemia
 Corporate Medical Policy Genetic Testing for Fanconi Anemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_fanconi_anemia 03/2015 3/2016 3/2017 3/2016 Description
Corporate Medical Policy Genetic Testing for Fanconi Anemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_fanconi_anemia 03/2015 3/2016 3/2017 3/2016 Description
CHAPTER 2. Neoplasms (C00-D49) March 2014. 2014 MVP Health Care, Inc.
 Neoplasms (C00-D49) March 2014 2014 MVP Health Care, Inc. CHAPTER SPECIFIC CATEGORY CODE BLOCKS C00-C14 Malignant neoplasms of lip, oral cavity and pharynx C15-C26 Malignant neoplasms of digestive organs
Neoplasms (C00-D49) March 2014 2014 MVP Health Care, Inc. CHAPTER SPECIFIC CATEGORY CODE BLOCKS C00-C14 Malignant neoplasms of lip, oral cavity and pharynx C15-C26 Malignant neoplasms of digestive organs
patient education Fact Sheet PFS007: BRCA1 and BRCA2 Mutations MARCH 2015
 patient education Fact Sheet PFS007: BRCA1 and BRCA2 Mutations MARCH 2015 BRCA1 and BRCA2 Mutations Cancer is a complex disease thought to be caused by several different factors. A few types of cancer
patient education Fact Sheet PFS007: BRCA1 and BRCA2 Mutations MARCH 2015 BRCA1 and BRCA2 Mutations Cancer is a complex disease thought to be caused by several different factors. A few types of cancer
BRCA in Men. Mary B. Daly,M.D.,Ph.D. June 25, 2010
 BRCA in Men Mary B. Daly,M.D.,Ph.D. June 25, 2010 BRCA in Men Inheritance patterns of BRCA1/2 Cancer Risks for men with BRCA1/2 mutations Risk management recommendations for men with BRCA1/2 mutations
BRCA in Men Mary B. Daly,M.D.,Ph.D. June 25, 2010 BRCA in Men Inheritance patterns of BRCA1/2 Cancer Risks for men with BRCA1/2 mutations Risk management recommendations for men with BRCA1/2 mutations
Patient Information. for Childhood
 Patient Information Genetic Testing for Childhood Hearing Loss Introduction This document describes the most common genetic cause of childhood hearing loss and explains the role of genetic testing. Childhood
Patient Information Genetic Testing for Childhood Hearing Loss Introduction This document describes the most common genetic cause of childhood hearing loss and explains the role of genetic testing. Childhood
Overview of testing for Lynch syndrome/hnpcc
 Overview of testing for Lynch syndrome/hnpcc This overview provides detailed information about interpreting MSI/IHC testing and genetic testing for Lynch syndrome/hnpcc. It is intended to be a reference
Overview of testing for Lynch syndrome/hnpcc This overview provides detailed information about interpreting MSI/IHC testing and genetic testing for Lynch syndrome/hnpcc. It is intended to be a reference
Ovarian Cancer Genetic Testing: Why, When, How?
 Ovarian Cancer Genetic Testing: Why, When, How? Jeffrey Dungan, MD Associate Professor Division of Clinical Genetics Department of Obstetrics & Gynecology Northwestern University Feinberg School of Medicine
Ovarian Cancer Genetic Testing: Why, When, How? Jeffrey Dungan, MD Associate Professor Division of Clinical Genetics Department of Obstetrics & Gynecology Northwestern University Feinberg School of Medicine
Optional Tests Offered Before and During Pregnancy
 Plano Women s Healthcare Optional Tests Offered Before and During Pregnancy Alpha-Fetoprotein Test (AFP) and Quad Screen These are screening tests that can assess your baby s risk of having such birth
Plano Women s Healthcare Optional Tests Offered Before and During Pregnancy Alpha-Fetoprotein Test (AFP) and Quad Screen These are screening tests that can assess your baby s risk of having such birth
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA
 CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
Screening guidelines tool
 Screening guidelines tool Disclaimer: This material is intended as a general summary of screening and management recommendations; it is not intended to be comprehensive. Colorectal cancer (CRC) screening
Screening guidelines tool Disclaimer: This material is intended as a general summary of screening and management recommendations; it is not intended to be comprehensive. Colorectal cancer (CRC) screening
Estimated New Cases of Leukemia, Lymphoma, Myeloma 2014
 ABOUT BLOOD CANCERS Leukemia, Hodgkin lymphoma (HL), non-hodgkin lymphoma (NHL), myeloma, myelodysplastic syndromes (MDS) and myeloproliferative neoplasms (MPNs) are types of cancer that can affect the
ABOUT BLOOD CANCERS Leukemia, Hodgkin lymphoma (HL), non-hodgkin lymphoma (NHL), myeloma, myelodysplastic syndromes (MDS) and myeloproliferative neoplasms (MPNs) are types of cancer that can affect the
Overview of Genetic Testing and Screening
 Integrating Genetics into Your Practice Webinar Series Overview of Genetic Testing and Screening Genetic testing is an important tool in the screening and diagnosis of many conditions. New technology is
Integrating Genetics into Your Practice Webinar Series Overview of Genetic Testing and Screening Genetic testing is an important tool in the screening and diagnosis of many conditions. New technology is
Test Information Sheet
 Test Information Sheet GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 888-729-1206 Fax: 301-710-6594 E-mail: [email protected] www.genedx.com/oncology OncoGene Dx: High/Moderate Risk Panel Sequence
Test Information Sheet GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 888-729-1206 Fax: 301-710-6594 E-mail: [email protected] www.genedx.com/oncology OncoGene Dx: High/Moderate Risk Panel Sequence
Objectives Role of Medical Genetics in Hearing Loss Evaluation. 5 y.o. boy with severe SNHL
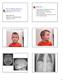 Objectives Role of Medical Genetics in Hearing Loss Evaluation Millan Patel, MD UBC Dept. of Medical Genetics October 22, 2010 Case presentation to illustrate importance of defining syndromic hearing loss
Objectives Role of Medical Genetics in Hearing Loss Evaluation Millan Patel, MD UBC Dept. of Medical Genetics October 22, 2010 Case presentation to illustrate importance of defining syndromic hearing loss
Diagnostic Scoring System for LQTS
 Medical Coverage Policy Genetic Testing: Congenital Long QT Syndrome Device/Equipment Drug Medical Surgery Test Other Effective Date: 2/15/2011 Policy Last Updated: 2/21/2012 Prospective review is recommended/required.
Medical Coverage Policy Genetic Testing: Congenital Long QT Syndrome Device/Equipment Drug Medical Surgery Test Other Effective Date: 2/15/2011 Policy Last Updated: 2/21/2012 Prospective review is recommended/required.
The following chapter is called "Preimplantation Genetic Diagnosis (PGD)".
 Slide 1 Welcome to chapter 9. The following chapter is called "Preimplantation Genetic Diagnosis (PGD)". The author is Dr. Maria Lalioti. Slide 2 The learning objectives of this chapter are: To learn the
Slide 1 Welcome to chapter 9. The following chapter is called "Preimplantation Genetic Diagnosis (PGD)". The author is Dr. Maria Lalioti. Slide 2 The learning objectives of this chapter are: To learn the
Tumour Markers. What are Tumour Markers? How Are Tumour Markers Used?
 Dr. Anthony C.H. YING What are? Tumour markers are substances that can be found in the body when cancer is present. They are usually found in the blood or urine. They can be products of cancer cells or
Dr. Anthony C.H. YING What are? Tumour markers are substances that can be found in the body when cancer is present. They are usually found in the blood or urine. They can be products of cancer cells or
What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives
 What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives What does the career involve? Explore family histories to identify risks Reducing risks
What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives What does the career involve? Explore family histories to identify risks Reducing risks
LAB MANAGEMENT CRITERIA. 1199SEIU Funds. Effective July 16, 2015
 LAB MANAGEMENT CRITERIA 1199SEIU Funds Effective July 16, 2015 Clinical criteria for medical necessity review of lab management services. Please note the following: CPT Copyright 2015 American Medical
LAB MANAGEMENT CRITERIA 1199SEIU Funds Effective July 16, 2015 Clinical criteria for medical necessity review of lab management services. Please note the following: CPT Copyright 2015 American Medical
Frequently Asked Questions About Ovarian Cancer
 Media Contact: Gerri Gomez Howard Cell: 303-748-3933 [email protected] Frequently Asked Questions About Ovarian Cancer What is ovarian cancer? Ovarian cancer is a cancer that forms in tissues
Media Contact: Gerri Gomez Howard Cell: 303-748-3933 [email protected] Frequently Asked Questions About Ovarian Cancer What is ovarian cancer? Ovarian cancer is a cancer that forms in tissues
Georgia Department of Human Resources BACKGROUND INFORMATION FOR NON-STATE AGENCY CHILD
 Georgia Department of Human Resources BACKGROUND INFORMATION FOR NON-STATE AGENCY CHILD Responsible Party Telephone Number Date Name of Child Date of Birth Time of Birth Sex Resident County Placement County
Georgia Department of Human Resources BACKGROUND INFORMATION FOR NON-STATE AGENCY CHILD Responsible Party Telephone Number Date Name of Child Date of Birth Time of Birth Sex Resident County Placement County
Carrier Screening For Genetic Diseases Preconception Consent (Female and/or Male Partner)
 Carrier Screening For Genetic Diseases Preconception Consent (Female and/or Male Partner) The goal of our practice at ARMS is to make sure that you receive optimal care to improve your chances of having
Carrier Screening For Genetic Diseases Preconception Consent (Female and/or Male Partner) The goal of our practice at ARMS is to make sure that you receive optimal care to improve your chances of having
Hereditary Breast Cancer Panels. High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel
 P A T I E N T G U I D E Hereditary Breast Cancer Panels High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel B a y l o r M i r a c a G e n e t i c s L a b o r a t
P A T I E N T G U I D E Hereditary Breast Cancer Panels High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel B a y l o r M i r a c a G e n e t i c s L a b o r a t
UNIT 13 (OPTION) Genetic Abnormalities
 Unit 13 Genetic Abnormailities 1 UNIT 13 (OPTION) Genetic Abnormalities Originally developed by: Hildur Helgedottir RN, MN Revised (2000) by: Marlene Reimer RN, PhD, CCN (C) Associate Professor Faculty
Unit 13 Genetic Abnormailities 1 UNIT 13 (OPTION) Genetic Abnormalities Originally developed by: Hildur Helgedottir RN, MN Revised (2000) by: Marlene Reimer RN, PhD, CCN (C) Associate Professor Faculty
190.25 - Alpha-fetoprotein
 Other Names/Abbreviations AFP 190.25 - Alpha-fetoprotein Alpha-fetoprotein (AFP) is a polysaccharide found in some carcinomas. It is effective as a biochemical marker for monitoring the response of certain
Other Names/Abbreviations AFP 190.25 - Alpha-fetoprotein Alpha-fetoprotein (AFP) is a polysaccharide found in some carcinomas. It is effective as a biochemical marker for monitoring the response of certain
Co-pay assistance organizations offering assistance
 Acromegaly Acute Exacerbations of Multiple Sclerosis Acute Porphyrias Advanced Idiopathic Parkinson' s Disease Age-Related Macular Degeneration www.theassistancefund.org Alcohol Dependence Alpha-1 Antitrypsin
Acromegaly Acute Exacerbations of Multiple Sclerosis Acute Porphyrias Advanced Idiopathic Parkinson' s Disease Age-Related Macular Degeneration www.theassistancefund.org Alcohol Dependence Alpha-1 Antitrypsin
A Guide to Prenatal Genetic Testing
 Patient Education Page 29 A Guide to Prenatal Genetic Testing This section describes prenatal tests that give information about your baby s health. It is your choice whether or not to have these tests
Patient Education Page 29 A Guide to Prenatal Genetic Testing This section describes prenatal tests that give information about your baby s health. It is your choice whether or not to have these tests
Corporate Medical Policy Carrier Testing for Genetic Disease
 Corporate Medical Policy Carrier Testing for Genetic Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: carrier_testing_for_genetic_disease 12/2013 8/2015 8/2016 8/2015 Description
Corporate Medical Policy Carrier Testing for Genetic Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: carrier_testing_for_genetic_disease 12/2013 8/2015 8/2016 8/2015 Description
COLORECTAL CANCER SCREENING
 COLORECTAL CANCER SCREENING By Douglas K. Rex, M.D., FACG & Suthat Liangpunsakul, M.D. Division of Gastroenterology and Hepatology, Department of Medicine Indiana University School of Medicine Indianapolis,
COLORECTAL CANCER SCREENING By Douglas K. Rex, M.D., FACG & Suthat Liangpunsakul, M.D. Division of Gastroenterology and Hepatology, Department of Medicine Indiana University School of Medicine Indianapolis,
CPT Generic Test Test Description
 CPT Generic Test Test Description Code Name 1 81200 ASPA Gene Test for leukodystrophies (Canavan disease), an autosomal recessive disease (most common in Ashkenazi Jewish population) with life expectancy
CPT Generic Test Test Description Code Name 1 81200 ASPA Gene Test for leukodystrophies (Canavan disease), an autosomal recessive disease (most common in Ashkenazi Jewish population) with life expectancy
NORD Guides for Physicians #1. Physician s Guide to. Tyrosinemia. Type 1
 NORD Guides for Physicians #1 The National Organization for Rare Disorders Physician s Guide to Tyrosinemia Type 1 The original version of this booklet was made possible by donations in honor of Danielle
NORD Guides for Physicians #1 The National Organization for Rare Disorders Physician s Guide to Tyrosinemia Type 1 The original version of this booklet was made possible by donations in honor of Danielle
Known Donor Questionnaire
 Known Donor Questionnaire Your donor s answers to these questions will provide you with a wealth of information about his health. You ll probably need assistance from a health care provider to interpret
Known Donor Questionnaire Your donor s answers to these questions will provide you with a wealth of information about his health. You ll probably need assistance from a health care provider to interpret
Preimplantation Genetic Diagnosis. Evaluation for single gene disorders
 Preimplantation Genetic Diagnosis Evaluation for single gene disorders What is Preimplantation Genetic Diagnosis? Preimplantation genetic diagnosis or PGD is a technology that allows genetic testing of
Preimplantation Genetic Diagnosis Evaluation for single gene disorders What is Preimplantation Genetic Diagnosis? Preimplantation genetic diagnosis or PGD is a technology that allows genetic testing of
MEDICAL GENETICS GENERAL OBJECTIVE SPECIFIC OBJECTIVES
 SUBJECT MEDICAL GENETICS CREDITS Total: 4.5 Theory 2.5 Practical 2 GENERAL OBJECTIVE To provide students with terminology and knowledge from the field of human genetics that will enable them to understand
SUBJECT MEDICAL GENETICS CREDITS Total: 4.5 Theory 2.5 Practical 2 GENERAL OBJECTIVE To provide students with terminology and knowledge from the field of human genetics that will enable them to understand
What is Cancer? Cancer is a genetic disease: Cancer typically involves a change in gene expression/function:
 Cancer is a genetic disease: Inherited cancer Sporadic cancer What is Cancer? Cancer typically involves a change in gene expression/function: Qualitative change Quantitative change Any cancer causing genetic
Cancer is a genetic disease: Inherited cancer Sporadic cancer What is Cancer? Cancer typically involves a change in gene expression/function: Qualitative change Quantitative change Any cancer causing genetic
Pathology ICD-10-CM Coding Tip Sheet Overview of Key Chapter Updates for Pathology and Top 25 codes
 Pathology ICD-10-CM Coding Tip Sheet Overview of Key Chapter Updates for Pathology and Top 25 codes Chapter 2 Neoplasms (C00-D49) Classification improvements Code expansions Significant expansions or revisions
Pathology ICD-10-CM Coding Tip Sheet Overview of Key Chapter Updates for Pathology and Top 25 codes Chapter 2 Neoplasms (C00-D49) Classification improvements Code expansions Significant expansions or revisions
Report series: General cancer information
 Fighting cancer with information Report series: General cancer information Eastern Cancer Registration and Information Centre ECRIC report series: General cancer information Cancer is a general term for
Fighting cancer with information Report series: General cancer information Eastern Cancer Registration and Information Centre ECRIC report series: General cancer information Cancer is a general term for
Risk stratification for colorectal cancer especially: the difference between sporadic disease and polyposis syndromes. Dr. med. Henrik Csaba Horváth
 Risk stratification for colorectal cancer especially: the difference between sporadic disease and polyposis syndromes Dr. med. Henrik Csaba Horváth Why is risk stratification for colorectal cancer (CRC)
Risk stratification for colorectal cancer especially: the difference between sporadic disease and polyposis syndromes Dr. med. Henrik Csaba Horváth Why is risk stratification for colorectal cancer (CRC)
Genetic Aspects of Mental Retardation and Developmental Disabilities
 Prepared by: Chahira Kozma, MD Associate Professor of Pediatrics Medical Director/DCHRP [email protected] [email protected] Genetic Aspects of Mental Retardation and Developmental Disabilities
Prepared by: Chahira Kozma, MD Associate Professor of Pediatrics Medical Director/DCHRP [email protected] [email protected] Genetic Aspects of Mental Retardation and Developmental Disabilities
Genetic testing. The difference diagnostics can make. The British In Vitro Diagnostics Association
 6 Genetic testing The difference diagnostics can make The British In Vitro Diagnostics Association Genetic INTRODUCTION testing The Department of Health published Our Inheritance, Our Future - Realising
6 Genetic testing The difference diagnostics can make The British In Vitro Diagnostics Association Genetic INTRODUCTION testing The Department of Health published Our Inheritance, Our Future - Realising
The effect of the introduction of ICD-10 on cancer mortality trends in England and Wales
 The effect of the introduction of ICD-10 on cancer mortality trends in Anita Brock, Clare Griffiths and Cleo Rooney, Offi ce for INTRODUCTION From January 2001 deaths in have been coded to the Tenth Revision
The effect of the introduction of ICD-10 on cancer mortality trends in Anita Brock, Clare Griffiths and Cleo Rooney, Offi ce for INTRODUCTION From January 2001 deaths in have been coded to the Tenth Revision
ORGAN SYSTEMS OF THE BODY
 ORGAN SYSTEMS OF THE BODY DEFINITIONS AND CONCEPTS A. Organ a structure made up of two or more kinds of tissues organized in such a way that they can together perform a more complex function that can any
ORGAN SYSTEMS OF THE BODY DEFINITIONS AND CONCEPTS A. Organ a structure made up of two or more kinds of tissues organized in such a way that they can together perform a more complex function that can any
Gene mutation and molecular medicine Chapter 15
 Gene mutation and molecular medicine Chapter 15 Lecture Objectives What Are Mutations? How Are DNA Molecules and Mutations Analyzed? How Do Defective Proteins Lead to Diseases? What DNA Changes Lead to
Gene mutation and molecular medicine Chapter 15 Lecture Objectives What Are Mutations? How Are DNA Molecules and Mutations Analyzed? How Do Defective Proteins Lead to Diseases? What DNA Changes Lead to
Biochemistry of Cancer Cell
 Biochemistry of Cancer Cell Prof. Taha Kumosani Prof. Taha Kumosani http://biochemistry4all.com/taha/5.htm Cancer: an Overview Paleopathologists Dinosaur bones Egyptians Papyrus Autopsis Hippocrates Carcinoma
Biochemistry of Cancer Cell Prof. Taha Kumosani Prof. Taha Kumosani http://biochemistry4all.com/taha/5.htm Cancer: an Overview Paleopathologists Dinosaur bones Egyptians Papyrus Autopsis Hippocrates Carcinoma
REI Pearls: Pitfalls of Genetic Testing in Miscarriage
 The Skinny: Genetic testing of miscarriage tissue is controversial and some people question if testing is helpful or not. This summary will: 1) outline the arguments for and against genetic testing; 2)
The Skinny: Genetic testing of miscarriage tissue is controversial and some people question if testing is helpful or not. This summary will: 1) outline the arguments for and against genetic testing; 2)
Description Code Recommendation Description Code. All natural death 001-799 IPH All natural death A00-R99
 Natural death Description Code Recommendation Description Code All natural death 001-799 IPH All natural death A00-R99 Infectious and parasitic diseases 001-139 CDC, EUROSTAT, CBS & VG Infectious and parasitic
Natural death Description Code Recommendation Description Code All natural death 001-799 IPH All natural death A00-R99 Infectious and parasitic diseases 001-139 CDC, EUROSTAT, CBS & VG Infectious and parasitic
Test Information Sheet
 Test Information Sheet GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 888-729-1206 Fax: 301-710-6594 E-mail: [email protected] www.genedx.com/oncology OncoGene Dx: Breast/Ovarian Cancer Panel Sequence
Test Information Sheet GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 888-729-1206 Fax: 301-710-6594 E-mail: [email protected] www.genedx.com/oncology OncoGene Dx: Breast/Ovarian Cancer Panel Sequence
MEDICAL POLICY SUBJECT: GENETIC TESTING FOR HEREDITARY BRCA MUTATIONS. POLICY NUMBER: 2.02.06 CATEGORY: Laboratory Test
 MEDICAL POLICY SUBJECT: GENETIC TESTING FOR, 10/15/15 PAGE: 1 OF: 12 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
MEDICAL POLICY SUBJECT: GENETIC TESTING FOR, 10/15/15 PAGE: 1 OF: 12 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
C CS. California Children Services Alameda County
 C CS California Children Services Alameda County The California Children Services (CCS) Program strives to assure access to medical services essential to the health and well-being of children with catastrophic
C CS California Children Services Alameda County The California Children Services (CCS) Program strives to assure access to medical services essential to the health and well-being of children with catastrophic
Update in Hematology Oncology Targeted Therapies. Mark Holguin
 Update in Hematology Oncology Targeted Therapies Mark Holguin 25 years ago Why I chose oncology People How to help people with possibly the most difficult thing they may have to deal with Science Turning
Update in Hematology Oncology Targeted Therapies Mark Holguin 25 years ago Why I chose oncology People How to help people with possibly the most difficult thing they may have to deal with Science Turning
ACMG Practice Guideline
 June 2007 Vol. 9 No. 6 ACMG Practice Guideline Indications for genetic referral: a guide for healthcare providers Beth A. Pletcher, MD 1, Helga V. Toriello, PhD 2, Sarah J. Noblin, MS, CGC 3, Laurie H.
June 2007 Vol. 9 No. 6 ACMG Practice Guideline Indications for genetic referral: a guide for healthcare providers Beth A. Pletcher, MD 1, Helga V. Toriello, PhD 2, Sarah J. Noblin, MS, CGC 3, Laurie H.
Neonatal Hypotonia. Clinical Approach to Floppy Baby
 Neonatal Hypotonia Clinical Approach to Floppy Baby Hypotonia in the newborn is a common presenting feature of systemic illness or neurologic dysfunction at any level of the central or peripheral nervous
Neonatal Hypotonia Clinical Approach to Floppy Baby Hypotonia in the newborn is a common presenting feature of systemic illness or neurologic dysfunction at any level of the central or peripheral nervous
Preimplantation genetic diagnosis new method of screening of 24 chromosomes with the Array CGH method...2
 August 2012 content 8 Preimplantation genetic diagnosis new method of screening of 24 chromosomes with the Array CGH method...2 Maintaining fertility new opportunities in GENNET...3 Hysteroscopy without
August 2012 content 8 Preimplantation genetic diagnosis new method of screening of 24 chromosomes with the Array CGH method...2 Maintaining fertility new opportunities in GENNET...3 Hysteroscopy without
Lecture 6: Single nucleotide polymorphisms (SNPs) and Restriction Fragment Length Polymorphisms (RFLPs)
 Lecture 6: Single nucleotide polymorphisms (SNPs) and Restriction Fragment Length Polymorphisms (RFLPs) Single nucleotide polymorphisms or SNPs (pronounced "snips") are DNA sequence variations that occur
Lecture 6: Single nucleotide polymorphisms (SNPs) and Restriction Fragment Length Polymorphisms (RFLPs) Single nucleotide polymorphisms or SNPs (pronounced "snips") are DNA sequence variations that occur
NEOPLASMS C00 D49. Presented by Jan Halloran CCS
 NEOPLASMS C00 D49 Presented by Jan Halloran CCS 1 INTRODUCTION A neoplasm is a new or abnormal growth. In the ICD-10-CM classification system, neoplastic disease is classified in categories C00 through
NEOPLASMS C00 D49 Presented by Jan Halloran CCS 1 INTRODUCTION A neoplasm is a new or abnormal growth. In the ICD-10-CM classification system, neoplastic disease is classified in categories C00 through
Epi procolon The Blood Test for Colorectal Cancer Screening
 Epi procolon The Blood Test for Colorectal Cancer Screening Epi procolon is an approved blood test for colorectal cancer screening. The US Preventive Services Task Force, the American Cancer Society and
Epi procolon The Blood Test for Colorectal Cancer Screening Epi procolon is an approved blood test for colorectal cancer screening. The US Preventive Services Task Force, the American Cancer Society and
Genetics Review for USMLE (Part 2)
 Single Gene Disorders Genetics Review for USMLE (Part 2) Some Definitions Alleles variants of a given DNA sequence at a particular location (locus) in the genome. Often used more narrowly to describe alternative
Single Gene Disorders Genetics Review for USMLE (Part 2) Some Definitions Alleles variants of a given DNA sequence at a particular location (locus) in the genome. Often used more narrowly to describe alternative
It s not something you want to think about, but it s something you want to prepare for.
 It s not something you want to think about, but it s something you want to prepare for. StemCyte cord blood banking offers your family a new lifesaving treatment alternative Why Bank Take the once-in-alifetime
It s not something you want to think about, but it s something you want to prepare for. StemCyte cord blood banking offers your family a new lifesaving treatment alternative Why Bank Take the once-in-alifetime
Becker Muscular Dystrophy
 Muscular Dystrophy A Case Study of Positional Cloning Described by Benjamin Duchenne (1868) X-linked recessive disease causing severe muscular degeneration. 100 % penetrance X d Y affected male Frequency
Muscular Dystrophy A Case Study of Positional Cloning Described by Benjamin Duchenne (1868) X-linked recessive disease causing severe muscular degeneration. 100 % penetrance X d Y affected male Frequency
GENETIC TESTING AND MARFAN SYNDROME
 GENETIC TESTING AND MARFAN SYNDROME Genetic testing for mutations in fibrillin-1 (FBN1) and other genes has become an important and reliable option to aid in the diagnosis of Marfan syndrome and related
GENETIC TESTING AND MARFAN SYNDROME Genetic testing for mutations in fibrillin-1 (FBN1) and other genes has become an important and reliable option to aid in the diagnosis of Marfan syndrome and related
Influences on Birth Defects
 Influences on Birth Defects FACTS About 150,000 babies are born each year with birth defects. The parents of one out of every 28 babies receive the frightening news that their baby has a birth defect There
Influences on Birth Defects FACTS About 150,000 babies are born each year with birth defects. The parents of one out of every 28 babies receive the frightening news that their baby has a birth defect There
Hereditary Breast Cancer. Nicole Kounalakis, MD Assistant Professor of Surgery University of Colorado Medical Center
 Hereditary Breast Cancer Nicole Kounalakis, MD Assistant Professor of Surgery University of Colorado Medical Center Outline Background Assessing risk of patient Syndromes BRCA 1,2 Li Fraumeni Cowden Hereditary
Hereditary Breast Cancer Nicole Kounalakis, MD Assistant Professor of Surgery University of Colorado Medical Center Outline Background Assessing risk of patient Syndromes BRCA 1,2 Li Fraumeni Cowden Hereditary
Cancer is the leading cause of death for Canadians aged 35 to 64 and is also the leading cause of critical illness claims in Canada.
 Underwriting cancer In this issue of the Decision, we provide an overview of Canadian cancer statistics and the information we use to make an underwriting decision. The next few issues will deal with specific
Underwriting cancer In this issue of the Decision, we provide an overview of Canadian cancer statistics and the information we use to make an underwriting decision. The next few issues will deal with specific
INTRODUCTION TO THE UK CURRICULUM IN CLINICAL GENETICS
 INTRODUCTION TO THE UK CURRICULUM IN CLINICAL GENETICS Clinical Geneticists work in multidisciplinary regional genetic centres in the UK, in close collaboration with laboratory scientists, clinical co-workers
INTRODUCTION TO THE UK CURRICULUM IN CLINICAL GENETICS Clinical Geneticists work in multidisciplinary regional genetic centres in the UK, in close collaboration with laboratory scientists, clinical co-workers
Understanding Your Risk of Ovarian Cancer
 Understanding Your Risk of Ovarian Cancer A WOMAN S GUIDE This brochure is made possible through partnership support from Project Hope for Ovarian Cancer Research and Education. Project HOPE FOR OVARIAN
Understanding Your Risk of Ovarian Cancer A WOMAN S GUIDE This brochure is made possible through partnership support from Project Hope for Ovarian Cancer Research and Education. Project HOPE FOR OVARIAN
Cancer Screening and Early Detection Guidelines
 Cancer Screening and Early Detection Guidelines Guillermo Tortolero Luna, MD, PhD Director Cancer Control and Population Sciences Program University of Puerto Rico Comprehensive Cancer Center ASPPR Clinical
Cancer Screening and Early Detection Guidelines Guillermo Tortolero Luna, MD, PhD Director Cancer Control and Population Sciences Program University of Puerto Rico Comprehensive Cancer Center ASPPR Clinical
Obstetrical Ultrasound and Prenatal Diagnostic Center
 Obstetrical Ultrasound and Prenatal Diagnostic Center Prenatal Diagnosis: Options and Opportunities Learn about various screening options including Early Risk Assessment (ERA), now available to women of
Obstetrical Ultrasound and Prenatal Diagnostic Center Prenatal Diagnosis: Options and Opportunities Learn about various screening options including Early Risk Assessment (ERA), now available to women of
The Developing Person Through the Life Span 8e by Kathleen Stassen Berger
 The Developing Person Through the Life Span 8e by Kathleen Stassen Berger Chapter 3 Heredity and Environment PowerPoint Slides developed by Martin Wolfger and Michael James Ivy Tech Community College-Bloomington
The Developing Person Through the Life Span 8e by Kathleen Stassen Berger Chapter 3 Heredity and Environment PowerPoint Slides developed by Martin Wolfger and Michael James Ivy Tech Community College-Bloomington
Medical Surgical Nursing (Elsevier)
 1 of 6 I. The Musculoskeletal System Medical Surgical Nursing (Elsevier) 1. Med/Surg: Musculoskeletal System: The Comprehensive Health History 2. Med/Surg: Musculoskeletal System: A Nursing Approach to
1 of 6 I. The Musculoskeletal System Medical Surgical Nursing (Elsevier) 1. Med/Surg: Musculoskeletal System: The Comprehensive Health History 2. Med/Surg: Musculoskeletal System: A Nursing Approach to
National Coverage Determination (NCD) for Tumor Antigen by Immunoassay - CA 125 (190.28)
 National Coverage Determination (NCD) for Tumor Antigen by Immunoassay - CA 125 (190.28) Tracking Information Publication Number Manual Section Number 100-3 190.28 Manual Section Title Tumor Antigen by
National Coverage Determination (NCD) for Tumor Antigen by Immunoassay - CA 125 (190.28) Tracking Information Publication Number Manual Section Number 100-3 190.28 Manual Section Title Tumor Antigen by
Minimum standards for ICSI use, screening, patient information and follow-up in WA fertility clinics. January 2006
 Minimum standards for ICSI use, screening, patient information and follow-up in WA fertility clinics January 2006 1. BACKGROUND ICSI has been shown to be effective for male factor infertility and it also
Minimum standards for ICSI use, screening, patient information and follow-up in WA fertility clinics January 2006 1. BACKGROUND ICSI has been shown to be effective for male factor infertility and it also
About The Causes of Hearing Loss
 About 1 in 500 infants is born with or develops hearing loss during early childhood. Hearing loss has many causes: some are genetic (that is, caused by a baby s genes) or non-genetic (such as certain infections
About 1 in 500 infants is born with or develops hearing loss during early childhood. Hearing loss has many causes: some are genetic (that is, caused by a baby s genes) or non-genetic (such as certain infections
Critical Illness with Term Assurance
 AIG Life Critical Illness with Term Assurance Our comprehensive Critical Illness with Term Assurance delivers more value and quality to the customer and their family than ever before. It is designed to
AIG Life Critical Illness with Term Assurance Our comprehensive Critical Illness with Term Assurance delivers more value and quality to the customer and their family than ever before. It is designed to
Ovarian Cancer. in Georgia, 1999-2003. Georgia Department of Human Resources Division of Public Health
 Ovarian Cancer in Georgia, 1999-23 Georgia Department of Human Resources Division of Public Health Acknowledgments Georgia Department of Human Resources......B. J. Walker, Commissioner Division of Public
Ovarian Cancer in Georgia, 1999-23 Georgia Department of Human Resources Division of Public Health Acknowledgments Georgia Department of Human Resources......B. J. Walker, Commissioner Division of Public
A Source of Hard- to- Find Pa3ents and Caregivers For Researchers. Peter Ziedins [email protected] 514-426- 9295 www.rarepa;entvoice.
 A Source of Hard- to- Find Pa3ents and Caregivers For Researchers Peter Ziedins [email protected] 514-426- 9295 www.rarepa;entvoice.com About RPV Rare Pa3ent Voice, LLC was formed to provide
A Source of Hard- to- Find Pa3ents and Caregivers For Researchers Peter Ziedins [email protected] 514-426- 9295 www.rarepa;entvoice.com About RPV Rare Pa3ent Voice, LLC was formed to provide
Information leaflet. Centrum voor Medische Genetica. Version 1/20150504 Design by Ben Caljon, UZ Brussel. Universitair Ziekenhuis Brussel
 Information on genome-wide genetic testing Array Comparative Genomic Hybridization (array CGH) Single Nucleotide Polymorphism array (SNP array) Massive Parallel Sequencing (MPS) Version 120150504 Design
Information on genome-wide genetic testing Array Comparative Genomic Hybridization (array CGH) Single Nucleotide Polymorphism array (SNP array) Massive Parallel Sequencing (MPS) Version 120150504 Design
Medicare Coverage of Genomic Testing
 Medicare Coverage of Genomic Testing Louis B. Jacques, MD Director, DID/CAG/OCSQ With acknowledgements to Jeff Roche, MD Social Security Act 1862(a)(1)(A) Notwithstanding any other provision of this title,
Medicare Coverage of Genomic Testing Louis B. Jacques, MD Director, DID/CAG/OCSQ With acknowledgements to Jeff Roche, MD Social Security Act 1862(a)(1)(A) Notwithstanding any other provision of this title,
