American College of Radiology Mammography Accreditation Program
|
|
|
- Raymond Mathews
- 9 years ago
- Views:
Transcription
1 American College of Radiology Mammography Accreditation Program
2 Objectives Overview of the ACR Mammography Accreditation Program Interrelationship between mammography accreditation, FDA certification and annual inspections How to successfully apply for accreditation What to do if you do not succeed Analysis of accreditation deficiencies
3 ACR Mammography Accreditation Program Began in 1987 QC Manual first published in 1990 Judy M. Destouet, M.D. chairs Committee on Mammography Accreditation Currently accredit over 12,000 units at 9000 facilities
4 Facility Requirements Under MQSA All mammography facilities must be Accredited Certified Inspected Complementary, not duplicative
5 Accrediting Bodies Responsibilities Under MQSA Facility standards review Clinical image review Physics survey review Random clinical image review On-site visits Mechanism to deal with complaints Reporting and record keeping Maintain reasonable fees
6 ACR has Updated all Application Materials and Final Reports Forms Revised to be consistent with Final Rules (FDA regulates accrediting bodies, too) New personnel sections no longer ask for CV s, training certificates Streamlined: redundant and unnecessary questions eliminated Reports Take advantage of capabilities of ACR s new accreditation software Clear instructions for proceeding after deficiencies
7 What Must a Facility Do to Pass Accreditation?
8 Know When Your MQSA and Accreditation Certificates Expire Against the law to perform mammo without a current MQSA certificate Medicare will not reimburse under expired certificate Look for ACR expiration dates on certificate and unit label
9 Meet All Application Deadlines Renewal notices sent out 8 months prior to accreditation expiration ACR must receive the complete entry application within 6 months prior to expiration You have 45 calendar days to return completed testing to ACR This guarantees completion of the review process before accreditation expiration
10 Follow Instructions Submitting Clinical Images Images should be negative BI-RADS assessment category 1 nothing to comment on breasts are symmetrical no masses, architectural disturbances or suspicious calcifications BI-RADS assessment category 2 ( benign ) will be accepted with prior approval from ACR Examples of your facility s best work Within 30 days of the phantom image and within the time period on the QC chart Supervising radiologist should review & approve the images
11 Submit Appropriate Density Images Fatty BI-RADS Composition Cat 1 Composed Almost Entirely of Fat BI-RADS Composition Cat 2 Scattered Fibroglandular Densities Dense BI-RADS Composition Cat 3 Heterogeneously Dense BI-RADS Composition Cat 4 Extremely Dense
12 Clinical Image Quality Evaluated in Eight Categories Positioning* Compression* Exposure level Contrast Sharpness Noise Artifacts Exam identification Review evaluation criteria in Clinical Image Evaluation section of 1999 QC Manual before submitting images (*Primary reasons for accreditation failure)
13 Follow Instructions Submitting Phantom Images & Dosimeter Make a test exposure without the dosimeter Dosimeter or disk should not cover fibers, specks or masses Criteria used by ACR Phantom Image Reviewers is in 1999 ACR QC Manual
14 Phantom Results Have Improved % of films not meeting ACR criteria Approx 11% did not meet criteria between 1993 and approx 16% 1994 approx 16% 1995 approx 16% 1996 approx 7% 1997 approx 6% 1998 approx 6% 1999 approx 7%
15 26-50 Fail Rates 50% 45% 40% 35% 30% 25% 20% 15% 10% 5% 0% 43% over 300 Total Overall Phantom Fail Rates % 16% 11% 8% 6% 6% 8% 8% 16% 17% 18% 11% Average Dose
16 Other Things to Keep in Mind
17 Notify ACR When Installing a New Mammography Unit Before use Physicist must conduct Equipment Evaluation Call ACR for instructions to accredit the unit If more than 1 year left on accreditation Full testing (clinical-phantom-dose-processor) Reduced fee If approved, same exp dates as other unit(s) If less than 1 year left on accreditation Early renewal of entire facility (all units) Usual fee If approved, exp date for all units is old +3 yrs
18 Equipment Evaluation Must be done by qualified medical physicist This evaluation is an acceptance test; evaluates different features in addition to those tested as part of the medical physicist s annual QC survey Must be done (& all problems fixed) before equipment used on patients Must be submitted to ACR during initial application of new (or used) mammo unit
19 FDA Final Rules Equipment Standards: section (b) X-ray units (e.g., flat and parallel compression) Film and intensifying screens Film processing Film viewing Quality Assurance - Equipment: section (e) Facilities must conduct these tests at proscribed intervals and equipment must meet performance standards Different from equipment standards
20 ACR Has a Form to Help You Supplement Your Annual QC Survey Report for the Equipment Evaluation Section VI (MQSA Requirements for Mammography Equipment) in physicist s chapter of QC Manual Also downloadable from the ACR website Simple Yes/No/NA checklist format
21
22 When Must a Facility Have an Equipment Evaluation Done? After installation of new (or used) x-ray unit or processor After x-ray unit or processor disassembled and reassembled at the same or new location After x-ray tube replacement After collimator replacement After filter replacement After AEC unit or sensor replacement
23 FDA Policy Guidance Help System Revised May 2001
24 You Have 2 Attempts at Accreditation Before Failing A first deficiency is NOT a Failure (ACR does not notify FDA) Do NOT have to discontinue mammo Take corrective action on your own Repeat deficient test (if >2 months on MQSA certificate) Reinstate (if < 2 months on MQSA certificate) Appeal Withdraw
25 A 2 nd Deficiency is a Fail ACR notifies FDA after a 2 nd deficiency FDA sends cease mammo letter Facility may Reinstate by reapplying and retesting in all areas after Submitting corrective action plan to ACR ACR reviews and approves completion ACR sends facility testing materials ACR notifies FDA of Reinstatement FDA sends interim notice and 6-mo provisional certificate (if necessary)
26 A 3 rd Deficiency is a 2 nd Failure Closer Oversight Necessary ACR notifies FDA after a 3 rd deficiency FDA sends cease mammo letter Facility must have Scheduled On-Site Survey (SOSS) in order to Reinstate Submit corrective action plan to ACR ACR reviews and approves completion SOSS radiologist, medical physicist, ACR staff technologist After SOSS, if no other corrective action needed, facility may reinstate
27 Accreditation Approval Rates 2000 After Initial or Renewal application 69% approved 31% had a deficiency After Reinstating 90% approved 10% had a deficiency resulting in failure Majority of deficiencies are due to poor clinical image quality
28 Alex, I ll take RADIOLOGY, please, for $200. OK, John. The American College of Radiology wants increased Medicare reimbursement for this breast imaging procedure.
29 What is mammography?
30
Update on MQSA and ACR Mammography Accreditation
 MQSA - Who s Who Update on MQSA and ACR Mammography Accreditation The Law: Mammography Quality Standards Act (MQSA) The Regulator: US Food and Drug Administration (FDA) Pamela L. Platt, BSRT(R)(M)(CV)
MQSA - Who s Who Update on MQSA and ACR Mammography Accreditation The Law: Mammography Quality Standards Act (MQSA) The Regulator: US Food and Drug Administration (FDA) Pamela L. Platt, BSRT(R)(M)(CV)
MQSA, FDA Regulations, and Inspections
 The American College of Radiology Mammography Accreditation Program: Frequently Asked Questions (Updated: December 15, 2015) Table of Contents MQSA, FDA Regulations, and Inspections... 2 ACR Mammography
The American College of Radiology Mammography Accreditation Program: Frequently Asked Questions (Updated: December 15, 2015) Table of Contents MQSA, FDA Regulations, and Inspections... 2 ACR Mammography
Annual MQSA Inspection Questions NMQAAC April 19, 2004
 1 1 1 1 1 1 1 1 0 1 0 1 0 1 0 1 Annual MQSA Inspection Questions NMQAAC April 1, 00 1.0 Inspection Information 1.1 Name and Address 1. Equipment Registration.0 Facility Inspection Download.0 Facility Inspections.0
1 1 1 1 1 1 1 1 0 1 0 1 0 1 0 1 Annual MQSA Inspection Questions NMQAAC April 1, 00 1.0 Inspection Information 1.1 Name and Address 1. Equipment Registration.0 Facility Inspection Download.0 Facility Inspections.0
IAC Ch 41, p.1. Procedure means a stereotactically guided breast biopsy performed on a patient for diagnostic purposes.
 IAC Ch 41, p.1 641 41.7 (136C) X-ray machines used for stereotactically guided breast biopsy. 41.7(1) Definitions. In addition to the definitions provided in rules 641 38.2(136C), 641 40.2(136C), and 641
IAC Ch 41, p.1 641 41.7 (136C) X-ray machines used for stereotactically guided breast biopsy. 41.7(1) Definitions. In addition to the definitions provided in rules 641 38.2(136C), 641 40.2(136C), and 641
Role of the Medical Physicist in Clinical Implementation of Breast Tomosynthesis
 Role of the Medical Physicist in Clinical Implementation of Breast Tomosynthesis Bob Liu, Ph.D. Department of Radiology Massachusetts General Hospital And Harvard Medical School Digital Breast Tomosynthesis
Role of the Medical Physicist in Clinical Implementation of Breast Tomosynthesis Bob Liu, Ph.D. Department of Radiology Massachusetts General Hospital And Harvard Medical School Digital Breast Tomosynthesis
The American College of Radiology Stereotactic Breast Biopsy Accreditation Program: Frequently Asked Questions (Updated: August 24, 2015)
 The American College of Radiology Stereotactic Breast Biopsy Accreditation Program: Frequently Asked Questions (Updated: August 24, 2015) Table of Contents Stereotactic Breast Biopsy Accreditation Program
The American College of Radiology Stereotactic Breast Biopsy Accreditation Program: Frequently Asked Questions (Updated: August 24, 2015) Table of Contents Stereotactic Breast Biopsy Accreditation Program
Quality Control of Full Field Digital Mammography Units
 Quality Control of Full Field Digital Mammography Units Melissa C. Martin, M.S., FACMP, FACR, FAAPM [email protected] 310-612-8127 ACMP Annual Meeting Virginia Beach, VA May 2, 2009 History of
Quality Control of Full Field Digital Mammography Units Melissa C. Martin, M.S., FACMP, FACR, FAAPM [email protected] 310-612-8127 ACMP Annual Meeting Virginia Beach, VA May 2, 2009 History of
Surveying and QC of Stereotactic Breast Biopsy Units for ACR Accreditation
 Surveying and QC of Stereotactic Breast Biopsy Units for ACR Accreditation LORAD Stereotactic Breast Biopsy System AAPM Spring Clinical Meeting Phoenix, AZ March 17, 2013 Melissa C. Martin, M.S., FACR,
Surveying and QC of Stereotactic Breast Biopsy Units for ACR Accreditation LORAD Stereotactic Breast Biopsy System AAPM Spring Clinical Meeting Phoenix, AZ March 17, 2013 Melissa C. Martin, M.S., FACR,
Update on ACR Digital Mammography QC Manual
 Update on ACR Digital Mammography QC Manual Priscilla F. Butler, M.S. Medical Physicist and Senior Director, ACR, Reston, VA (with thanks to Eric Berns, Ph.D.) Overview Phantom Specifications QC Manual
Update on ACR Digital Mammography QC Manual Priscilla F. Butler, M.S. Medical Physicist and Senior Director, ACR, Reston, VA (with thanks to Eric Berns, Ph.D.) Overview Phantom Specifications QC Manual
SUBCHAPTER 22 QUALITY ASSURANCE PROGRAMS FOR MEDICAL DIAGNOSTIC X-RAY INSTALLATIONS
 Note: This is a courtesy copy and is not the official version of this rule. The official, legally effective version of this rule is available through www.lexisnexic.com/bookstore (Phone: (800) 223-1940).
Note: This is a courtesy copy and is not the official version of this rule. The official, legally effective version of this rule is available through www.lexisnexic.com/bookstore (Phone: (800) 223-1940).
Dose Measurement in Mammography; What are we measuring? David E. Hintenlang, Ph.D. DABR University of Florida
 Dose Measurement in Mammography; What are we measuring? David E. Hintenlang, Ph.D. DABR University of Florida Average Glandular Dose Required measurement performed by medical physicist as part of Mammography
Dose Measurement in Mammography; What are we measuring? David E. Hintenlang, Ph.D. DABR University of Florida Average Glandular Dose Required measurement performed by medical physicist as part of Mammography
Q.A. Collectible. Sponsored by CRCPD s Committee on Quality Assurance in Diagnostic X-Ray (H-7)
 Q.A. Collectible Sponsored by CRCPD s Committee on Quality Assurance in Diagnostic X-Ray (H-7) Mammography Phantom Image Quality Evaluation (from the American College of Radiology 1999 Mammography Quality
Q.A. Collectible Sponsored by CRCPD s Committee on Quality Assurance in Diagnostic X-Ray (H-7) Mammography Phantom Image Quality Evaluation (from the American College of Radiology 1999 Mammography Quality
Sustaining a High-Quality Breast MRI Practice
 Sustaining a High-Quality Breast MRI Practice Christoph Lee, MD, MSHS Associate Professor of Radiology Adjunct Associate Professor, Health Services University of Washington September 11, 2015 Overview
Sustaining a High-Quality Breast MRI Practice Christoph Lee, MD, MSHS Associate Professor of Radiology Adjunct Associate Professor, Health Services University of Washington September 11, 2015 Overview
The Mammography Quality Standards Act Final Regulations Document #1
 Compliance Guidance The Mammography Quality Standards Act Final Regulations Document #1 Document issued on: March 19, 1999 This document supersedes document Draft Compliance Guidance August 27, 1998 U.S.
Compliance Guidance The Mammography Quality Standards Act Final Regulations Document #1 Document issued on: March 19, 1999 This document supersedes document Draft Compliance Guidance August 27, 1998 U.S.
Breast MRI Quality Control
 Donna M. Reeve, MS, DABR, DABMP Department of Imaging Physics Educational Objectives Discuss the importance of breast MRI quality control (QC). Provide an overview of the new ACR Breast MRI Accreditation
Donna M. Reeve, MS, DABR, DABMP Department of Imaging Physics Educational Objectives Discuss the importance of breast MRI quality control (QC). Provide an overview of the new ACR Breast MRI Accreditation
REGULATION: QUALITY ASSURANCE PROGRAMS FOR MEDICAL DIAGNOSTIC X-RAY INSTALLATIONS N.J.A.C. 7:28-22
 REGULATION: QUALITY ASSURANCE PROGRAMS FOR MEDICAL DIAGNOSTIC X-RAY INSTALLATIONS N.J.A.C. 7:28-22 New Jersey Department of Environmental Protection Bureau of Radiological Health PO Box 415 Trenton NJ
REGULATION: QUALITY ASSURANCE PROGRAMS FOR MEDICAL DIAGNOSTIC X-RAY INSTALLATIONS N.J.A.C. 7:28-22 New Jersey Department of Environmental Protection Bureau of Radiological Health PO Box 415 Trenton NJ
MQSA and ACR Digital Breast Tomosynthesis Mammography Accreditation
 US FFDM Mammography Facilities and Units MQSA and ACR Digital Breast Tomosynthesis Mammography Accreditation Pamela L. Platt, BSRT(R)(M)(CV) FDA Liaison, ACR Breast Imaging Accreditation Program In 2000
US FFDM Mammography Facilities and Units MQSA and ACR Digital Breast Tomosynthesis Mammography Accreditation Pamela L. Platt, BSRT(R)(M)(CV) FDA Liaison, ACR Breast Imaging Accreditation Program In 2000
2/28/2011. MIPPA overview and CMS requirements. CT accreditation. Today s agenda. About MIPPA. Computed Tomography
 Today s agenda Computed Tomography Presented by: Dina Hernandez, BSRT, RT (R), CT, QM Krista Bush, RT, MBA Leonard Lucey, JD ACR Quality & Safety MIPPA overview and CMS requirements CT accreditation How
Today s agenda Computed Tomography Presented by: Dina Hernandez, BSRT, RT (R), CT, QM Krista Bush, RT, MBA Leonard Lucey, JD ACR Quality & Safety MIPPA overview and CMS requirements CT accreditation How
The American College of Radiology Breast Ultrasound Accreditation Program: Frequently Asked Questions
 The American College of Radiology Breast Ultrasound Accreditation Program: Frequently Asked Questions Table of Contents APPLICATION - GENERAL... 1 MOVED FACILITIES AND UNITS... 3 EQUIPMENT... 4 PERSONNEL...
The American College of Radiology Breast Ultrasound Accreditation Program: Frequently Asked Questions Table of Contents APPLICATION - GENERAL... 1 MOVED FACILITIES AND UNITS... 3 EQUIPMENT... 4 PERSONNEL...
Introduction. Digital Mammography Detector QC FFDM. What is FFDM QC and why is it. important? What to know before you start. Eric A. Berns, Ph.D.
 Slide 1 Digital Mammography Detector QC Slide 2 Introduction What is FFDM QC and why is it important? Eric A. Berns, Ph.D. What to know before you start [email protected] Overview and compare QC tests
Slide 1 Digital Mammography Detector QC Slide 2 Introduction What is FFDM QC and why is it important? Eric A. Berns, Ph.D. What to know before you start [email protected] Overview and compare QC tests
Mammography. For 35+ years, screenfilm has been the gold standard for breast cancer detection
 Digital Mammography Upright Stereo Mammography For 35+ years, screenfilm has been the gold standard for breast cancer detection IN THE BEGINNING Mammography technology has come a long way since the first
Digital Mammography Upright Stereo Mammography For 35+ years, screenfilm has been the gold standard for breast cancer detection IN THE BEGINNING Mammography technology has come a long way since the first
Personalized Breast Screening Service
 Frequently Asked Questions WHAT IS BREAST DENSITY? Breasts are made up of a mixture of fibrous, glandular and fatty tissue. Your breasts are considered if you have predominantly fibrous or glandular tissue
Frequently Asked Questions WHAT IS BREAST DENSITY? Breasts are made up of a mixture of fibrous, glandular and fatty tissue. Your breasts are considered if you have predominantly fibrous or glandular tissue
ADMINISTRATIVE RULES OF THE VERMONT BOARD OF RADIOLOGIC TECHNOLOGY
 ADMINISTRATIVE RULES OF THE VERMONT BOARD OF RADIOLOGIC TECHNOLOGY Part 1. GENERAL INFORMATION 1.1 The Board's Purpose The State Board of Radiologic Technology (the Board) has been created and given powers
ADMINISTRATIVE RULES OF THE VERMONT BOARD OF RADIOLOGIC TECHNOLOGY Part 1. GENERAL INFORMATION 1.1 The Board's Purpose The State Board of Radiologic Technology (the Board) has been created and given powers
Digital Breast Tomosynthesis QC Requirements
 Digital Breast Tomosynthesis QC Requirements AAPM Spring Clinical Meeting March 8, 2015 Michael S Glaser, MS, DABR Alliance Medical Physics, LLC Learning Objectives 1. GE SenoClaire - Physicist & Technologist
Digital Breast Tomosynthesis QC Requirements AAPM Spring Clinical Meeting March 8, 2015 Michael S Glaser, MS, DABR Alliance Medical Physics, LLC Learning Objectives 1. GE SenoClaire - Physicist & Technologist
Medicare Part B. Mammograms - Updated Billing Guide for Screening and Diagnostic Tests
 Mammograms - Updated Billing Guide for Screening and Diagnostic Tests This article from Medicare B News Issue 223 dated October 21, 2005 is being updated and reprinted to ensure that the Noridian Administrative
Mammograms - Updated Billing Guide for Screening and Diagnostic Tests This article from Medicare B News Issue 223 dated October 21, 2005 is being updated and reprinted to ensure that the Noridian Administrative
Hologic Selenia Dimensions C-View Software Module. October 24, 2012
 Hologic Selenia Dimensions C-View Software Module October 24, 2012 Introduction and Agenda Peter Soltani, Ph.D. Senior VP & GM, Breast Health Hologic, Inc. Agenda Technology Overview Clinical Overview
Hologic Selenia Dimensions C-View Software Module October 24, 2012 Introduction and Agenda Peter Soltani, Ph.D. Senior VP & GM, Breast Health Hologic, Inc. Agenda Technology Overview Clinical Overview
D. FREQUENTLY ASKED QUESTIONS
 ACR BI-RADS ATLAS D. FREQUENTLY ASKED QUESTIONS 1. Under MQSA, is it necessary to include a numeric assessment code (i.e., 0, 1, 2, 3, 4, 5, or 6) in addition to the assessment category in all mammography
ACR BI-RADS ATLAS D. FREQUENTLY ASKED QUESTIONS 1. Under MQSA, is it necessary to include a numeric assessment code (i.e., 0, 1, 2, 3, 4, 5, or 6) in addition to the assessment category in all mammography
How To Compare Mammography To A 3D System
 Radiology Advisory Panel Meeting Hologic Selenia Dimensions 3D System with C-View Software Module FDA Review Robert Ochs, PhD Branch Chief Mammography, Ultrasound, and Imaging Software Branch Division
Radiology Advisory Panel Meeting Hologic Selenia Dimensions 3D System with C-View Software Module FDA Review Robert Ochs, PhD Branch Chief Mammography, Ultrasound, and Imaging Software Branch Division
Digital Mammography Update: Design and Characteristics of Current Systems
 : Design and Characteristics of Current Systems Kalpana M. Kanal, Ph.D., DABR Assistant Professor Department of Radiology University of Washington Seattle, Washington AAPM Annual Meeting 2009 Anaheim,
: Design and Characteristics of Current Systems Kalpana M. Kanal, Ph.D., DABR Assistant Professor Department of Radiology University of Washington Seattle, Washington AAPM Annual Meeting 2009 Anaheim,
MAMMOGRAPHY GOALS AND OBJECTIVES
 MAMMOGRAPHY GOALS AND OBJECTIVES GOALS: After completion of the mammography rotations, the resident will be able to: 1. Demonstrate learning of the knowledge-based objectives-(practice Base Learning) 2.
MAMMOGRAPHY GOALS AND OBJECTIVES GOALS: After completion of the mammography rotations, the resident will be able to: 1. Demonstrate learning of the knowledge-based objectives-(practice Base Learning) 2.
Quality Control and Maintenance Programs
 Quality Control and Maintenance Programs Cari Borrás, D.Sc., FACR, FAAPM Visiting Professor DOIN-DEN / UFPE Recife, Pernambuco, Brazil Co-Chair, IUPESM Health Technology Task Group 1 Medical Imaging Equipment
Quality Control and Maintenance Programs Cari Borrás, D.Sc., FACR, FAAPM Visiting Professor DOIN-DEN / UFPE Recife, Pernambuco, Brazil Co-Chair, IUPESM Health Technology Task Group 1 Medical Imaging Equipment
Aspire FCRm/CRm Fujifilm CR for Mammography
 : May 2013 Fujifilm Medical Systems U.S.A., Inc. 2 CONTENTS FOREWORD... 5 Introduction to Measurements... 6 S value... 7 Overview of Testing... 8 Section A Getting Started... 9 0 Set-up & Baseline Images...10
: May 2013 Fujifilm Medical Systems U.S.A., Inc. 2 CONTENTS FOREWORD... 5 Introduction to Measurements... 6 S value... 7 Overview of Testing... 8 Section A Getting Started... 9 0 Set-up & Baseline Images...10
Mammography. What is Mammography?
 Scan for mobile link. Mammography Mammography is a specific type of breast imaging that uses low-dose x-rays to detect cancer early before women experience symptoms when it is most treatable. Tell your
Scan for mobile link. Mammography Mammography is a specific type of breast imaging that uses low-dose x-rays to detect cancer early before women experience symptoms when it is most treatable. Tell your
BREAST IMAGING. Developed by the Ad Hoc Committee on Resident and Fellow Education of the Society of Breast Imaging
 BREAST IMAGING Developed by the Ad Hoc Committee on Resident and Fellow Education of the Society of Breast Imaging Stephen A. Feig, M.D., Chair Ferris Hall, M.D. Debra Ikeda, M.D. Ellen Mendelson, M.D.
BREAST IMAGING Developed by the Ad Hoc Committee on Resident and Fellow Education of the Society of Breast Imaging Stephen A. Feig, M.D., Chair Ferris Hall, M.D. Debra Ikeda, M.D. Ellen Mendelson, M.D.
Radiation Safety Issues for Radiologic Technologists
 Radiation Safety Issues for Radiologic Technologists Greg Sackett, M.S., CHP Medical Physicist Radiation Worker Risks? 1 Patient Risks? Acute Effects? Delayed Effects? Patient Questions? Radiation Dose
Radiation Safety Issues for Radiologic Technologists Greg Sackett, M.S., CHP Medical Physicist Radiation Worker Risks? 1 Patient Risks? Acute Effects? Delayed Effects? Patient Questions? Radiation Dose
A Guide to Breast Imaging: The Latest Technology for Screening and Detecting Breast Cancer
 A Guide to Breast Imaging: The Latest Technology for Screening and Detecting Breast Cancer Sally Herschorn, MD Associate Professor of Radiology University of Vermont College of Medicine Medical Director
A Guide to Breast Imaging: The Latest Technology for Screening and Detecting Breast Cancer Sally Herschorn, MD Associate Professor of Radiology University of Vermont College of Medicine Medical Director
Compliance Guidance for QUALITY ASSURANCE MANUAL (2 nd Edition)
 Compliance Guidance for QUALITY ASSURANCE MANUAL (2 nd Edition) New Jersey Department of Environmental Protection Bureau of Radiological Health PO Box 415 Trenton NJ 08625 FAX 609-984-5811 Website: http://www.state.nj.us/dep/rpp
Compliance Guidance for QUALITY ASSURANCE MANUAL (2 nd Edition) New Jersey Department of Environmental Protection Bureau of Radiological Health PO Box 415 Trenton NJ 08625 FAX 609-984-5811 Website: http://www.state.nj.us/dep/rpp
AOAC Research Institute PERFORMANCE TESTED METHODS SM PROGRAM. POLICIES and PROCEDURES
 AOAC Research Institute PERFORMANCE TESTED METHODS SM PROGRAM POLICIES and PROCEDURES Notice: AOAC Research Institute reserves the right to modify the program at any time. Participants are required to
AOAC Research Institute PERFORMANCE TESTED METHODS SM PROGRAM POLICIES and PROCEDURES Notice: AOAC Research Institute reserves the right to modify the program at any time. Participants are required to
Mammography: Dosimetry ACMP Annual Meeting Virginia Beach Saturday, May 2, 2009 Lawrence N. Rothenberg, Ph.D. Department of Medical Physics Memorial
 Mammography: Dosimetry ACMP Annual Meeting Virginia Beach Saturday, May 2, 2009 Lawrence N. Rothenberg, Ph.D. Department of Medical Physics Memorial Sloan-Kettering Cancer Center New York, NY [email protected]
Mammography: Dosimetry ACMP Annual Meeting Virginia Beach Saturday, May 2, 2009 Lawrence N. Rothenberg, Ph.D. Department of Medical Physics Memorial Sloan-Kettering Cancer Center New York, NY [email protected]
Department of Veterans Affairs VHA HANDBOOK 1105.03. Washington DC 20420 April 28, 2011 MAMMOGRAPHY PROGRAM PROCEDURES AND STANDARDS
 Department of Veterans Affairs VHA HANDBOOK 1105.03 Veterans Health Administration Transmittal Sheet Washington DC 20420 April 28, 2011 MAMMOGRAPHY PROGRAM PROCEDURES AND STANDARDS 1. REASON FOR ISSUE.
Department of Veterans Affairs VHA HANDBOOK 1105.03 Veterans Health Administration Transmittal Sheet Washington DC 20420 April 28, 2011 MAMMOGRAPHY PROGRAM PROCEDURES AND STANDARDS 1. REASON FOR ISSUE.
Moving Forward What does this mean for the Medical Physicist and the Imaging Community?
 Moving Forward What does this mean for the Medical Physicist and the Imaging Community? John M. Boone, Ph.D., FAAPM, FACR Professor and Vice Chairman of Radiology University of California Davis Medical
Moving Forward What does this mean for the Medical Physicist and the Imaging Community? John M. Boone, Ph.D., FAAPM, FACR Professor and Vice Chairman of Radiology University of California Davis Medical
SECTION 1: REQUIREMENTS FOR CERTIFICATES OF COMPLIANCE FOR CLASSES OF RADIATION APPARATUS
 Department of Health and Human services Population Health Radiation Protection Act 2005 Section 17 CERTIFICATE OF COMPLIANCE: STANDARD FOR RADIATION APPARATUS - X-RAY MEDICAL DIAGNOSTIC (MAMMOGRAPHY) SECTION
Department of Health and Human services Population Health Radiation Protection Act 2005 Section 17 CERTIFICATE OF COMPLIANCE: STANDARD FOR RADIATION APPARATUS - X-RAY MEDICAL DIAGNOSTIC (MAMMOGRAPHY) SECTION
SOP #: Revision #: Current Version Implementation Date: Page #: Page 1 of 10 Last Reviewed/Update Date: Expiration
 Implementation Page #: Page 1 of 10 Last Reviewed/Update 1. Purpose and Scope The purpose of this document is to describe the Medical Physics and Radiation Safety program at Boston University (BU) and
Implementation Page #: Page 1 of 10 Last Reviewed/Update 1. Purpose and Scope The purpose of this document is to describe the Medical Physics and Radiation Safety program at Boston University (BU) and
ACR AAPM SIIM PRACTICE PARAMETER FOR DETERMINANTS OF IMAGE QUALITY IN DIGITAL MAMMOGRAPHY
 The American College of Radiology, with more than 30,000 members, is the principal organization of radiologists, radiation oncologists, and clinical medical physicists in the United States. The College
The American College of Radiology, with more than 30,000 members, is the principal organization of radiologists, radiation oncologists, and clinical medical physicists in the United States. The College
BREAST CHARACTERISTICS AND DOSIMETRIC DATA IN X RAY MAMMOGRAPHY - A LARGE SAMPLE WORLDWIDE SURVEY
 BREAST CHARACTERISTICS AND DOSIMETRIC DATA IN X RAY MAMMOGRAPHY - A LARGE SAMPLE WORLDWIDE SURVEY N. GEERAERT a,b,c, R. KLAUSZ a, S. MULLER a, I. BLOCH c, H. BOSMANS b a GE Healthcare, Buc, France b Department
BREAST CHARACTERISTICS AND DOSIMETRIC DATA IN X RAY MAMMOGRAPHY - A LARGE SAMPLE WORLDWIDE SURVEY N. GEERAERT a,b,c, R. KLAUSZ a, S. MULLER a, I. BLOCH c, H. BOSMANS b a GE Healthcare, Buc, France b Department
Digital Mammogram National Database
 Digital Mammogram National Database Professor Michael Brady FRS FREng Medical Vision Laboratory Oxford University Chairman: Mirada Solutions Ltd PharmaGrid 2/7/03 ediamond aims construct a federated database
Digital Mammogram National Database Professor Michael Brady FRS FREng Medical Vision Laboratory Oxford University Chairman: Mirada Solutions Ltd PharmaGrid 2/7/03 ediamond aims construct a federated database
Coronis 5MP Mammo. The standard of care for digital mammography
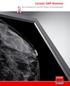 Coronis 5MP Mammo The standard of care for digital mammography The standard of care For thousands of women every day, details make all the difference. This understanding, along with many years of commitment
Coronis 5MP Mammo The standard of care for digital mammography The standard of care For thousands of women every day, details make all the difference. This understanding, along with many years of commitment
Name: Date: Team: Lab Experiment # 3. Focused Grid Positioning Errors. Computed Radiography and Direct Digital Radiography
 Name: Date: Team: Lab Experiment # 3 Focused Grid Positioning Errors Computed Radiography and Direct Digital Radiography Purpose This experiment is designed to demonstrate the effect of off-level error,
Name: Date: Team: Lab Experiment # 3 Focused Grid Positioning Errors Computed Radiography and Direct Digital Radiography Purpose This experiment is designed to demonstrate the effect of off-level error,
NEW JERSEY RADIOLOGIC TECHNOLOGY BOARD OF EXAMINERS (BOARD) ACCREDITATION STANDARDS FOR SCHOOLS OF RADIATION THERAPY TECHNOLOGY
 NEW JERSEY RADIOLOGIC TECHNOLOGY BOARD OF EXAMINERS (BOARD) ACCREDITATION STANDARDS FOR SCHOOLS OF RADIATION THERAPY TECHNOLOGY This document contains the Board s accreditation standards for schools of
NEW JERSEY RADIOLOGIC TECHNOLOGY BOARD OF EXAMINERS (BOARD) ACCREDITATION STANDARDS FOR SCHOOLS OF RADIATION THERAPY TECHNOLOGY This document contains the Board s accreditation standards for schools of
CT: Size Specific Dose Estimate (SSDE): Why We Need Another CT Dose Index. Acknowledgements
 CT: Size Specific Dose Estimate (SSDE): Why We Need Another CT Dose Index Keith J. Strauss, MSc, FAAPM, FACR Clinical Imaging Physicist Cincinnati Children s Hospital University of Cincinnati College of
CT: Size Specific Dose Estimate (SSDE): Why We Need Another CT Dose Index Keith J. Strauss, MSc, FAAPM, FACR Clinical Imaging Physicist Cincinnati Children s Hospital University of Cincinnati College of
RADIATION PROTECTION PROGRAM (RPP) GUIDANCE
 RADIATION PROTECTION PROGRAM (RPP) GUIDANCE The Colorado Rules and Regulations Pertaining to Radiation Control, (6 CCR 1007-1) are the regulations developed by the Colorado Department of Public Health
RADIATION PROTECTION PROGRAM (RPP) GUIDANCE The Colorado Rules and Regulations Pertaining to Radiation Control, (6 CCR 1007-1) are the regulations developed by the Colorado Department of Public Health
Radiation Rule Guide. Veterinary Facilities. Hawaii Administrative Rules, Chapter 11-45, Radiation Control. Hawaii State Department of Health
 Radiation Rule Guide Veterinary Facilities Hawaii Administrative Rules, Chapter 11-45, Radiation Control Hawaii State Department of Health MAY 2009 Radiation Rule Guide for Veterinary Facilities Hawaii
Radiation Rule Guide Veterinary Facilities Hawaii Administrative Rules, Chapter 11-45, Radiation Control Hawaii State Department of Health MAY 2009 Radiation Rule Guide for Veterinary Facilities Hawaii
ACR BI-RADS ATLAS MAMMOGRAPHY MAMMOGRAPHY II. REPORTING SYSTEM. American College of Radiology 121
 ACR BI-RADS ATLAS II. REPORTING SYSTEM American College of Radiology 121 2013 122 American College of Radiology ACR BI-RADS ATLAS A. REPORT ORGANIZATION (Guidance chapter, see page 147) The reporting system
ACR BI-RADS ATLAS II. REPORTING SYSTEM American College of Radiology 121 2013 122 American College of Radiology ACR BI-RADS ATLAS A. REPORT ORGANIZATION (Guidance chapter, see page 147) The reporting system
PRACTICAL TIPS IN ENSURING RADIATION SAFETY IN THE USE OF MEDICAL DIAGNOSTIC X-RAY EQUIPMENT
 PRACTICAL TIPS IN ENSURING RADIATION SAFETY IN THE USE OF MEDICAL DIAGNOSTIC X-RAY EQUIPMENT Although the medical uses of X-rays to examine a patient without surgery became an amazing medical breakthrough,
PRACTICAL TIPS IN ENSURING RADIATION SAFETY IN THE USE OF MEDICAL DIAGNOSTIC X-RAY EQUIPMENT Although the medical uses of X-rays to examine a patient without surgery became an amazing medical breakthrough,
PET Accreditation Program Requirements
 PET Accreditation Program Requirements OVERVIEW... 2 MEDICARE IMPROVEMENT F PATIENTS AND PROVIDERS ACT OF 2008 (MIPPA)... 2 MANDATY ACCREDITATION TIME REQUIREMENTS... 3 WITHDRAWN, ADDED, REPLACEMENT UNITS...
PET Accreditation Program Requirements OVERVIEW... 2 MEDICARE IMPROVEMENT F PATIENTS AND PROVIDERS ACT OF 2008 (MIPPA)... 2 MANDATY ACCREDITATION TIME REQUIREMENTS... 3 WITHDRAWN, ADDED, REPLACEMENT UNITS...
Quality Assurance. The selection of the equipment. Equipment Specifications. Medical Exposure Directive 97/43 Euratom. Quality Assurance Programme
 Medical Exposure Directive 97/43 Euratom Quality Assurance Ministry of Health, Radiation Protection Department, Luxembourg Alexandra Schreiner Medical Physicist Quality Assurance (QA): All those planned
Medical Exposure Directive 97/43 Euratom Quality Assurance Ministry of Health, Radiation Protection Department, Luxembourg Alexandra Schreiner Medical Physicist Quality Assurance (QA): All those planned
Applicable Models ME511L, ME551i2, MS31i2, MS33i2, MS51i2, MS53i2
 Version 3.1 Applicable Models ME511L, ME551i2, MS31i2, MS33i2, MS51i2, MS53i2 MQSA Quality Control Manual for Monochrome Monitors for Mammography Foreword Purpose of this Document This document is for
Version 3.1 Applicable Models ME511L, ME551i2, MS31i2, MS33i2, MS51i2, MS53i2 MQSA Quality Control Manual for Monochrome Monitors for Mammography Foreword Purpose of this Document This document is for
portable x-ray survey report
 department of HealtH and Human services centers for medicare & medicaid services form approved omb no. 0938-0027 portable x-ray survey report provider number H1 date surveyed (1) initial (2) survey W H2
department of HealtH and Human services centers for medicare & medicaid services form approved omb no. 0938-0027 portable x-ray survey report provider number H1 date surveyed (1) initial (2) survey W H2
Nuclear Medicine Accreditation Program Requirements
 Nuclear Medicine Accreditation Program Requirements OVERVIEW... 2 MEDICARE IMPROVEMENT F PATIENTS AND PROVIDERS ACT OF 2008 (MIPPA)... 2 MANDATY ACCREDITATION TIME REQUIREMENTS... 3 WITHDRAWN, ADDED, REPLACEMENT
Nuclear Medicine Accreditation Program Requirements OVERVIEW... 2 MEDICARE IMPROVEMENT F PATIENTS AND PROVIDERS ACT OF 2008 (MIPPA)... 2 MANDATY ACCREDITATION TIME REQUIREMENTS... 3 WITHDRAWN, ADDED, REPLACEMENT
2012 LACC Clinical Obligations & Grading System. Attendance Requirement
 2012 LACC Clinical Obligations & Grading System Attendance Requirement The Radiology program has a Monday through Friday schedule during the fall, winter, spring and summer semesters. Students are required
2012 LACC Clinical Obligations & Grading System Attendance Requirement The Radiology program has a Monday through Friday schedule during the fall, winter, spring and summer semesters. Students are required
Agency for Health Care Administration
 Page 1 of 20 FED - H0000 - INITIAL COMMENTS Title INITIAL COMMENTS CFR Type Memo Tag FED - H0005 - COMPLIANCE WITH FED/STATE/LOCAL LAWS Title COMPLIANCE WITH FED/STATE/LOCAL LAWS CFR 486.100 Type Condition
Page 1 of 20 FED - H0000 - INITIAL COMMENTS Title INITIAL COMMENTS CFR Type Memo Tag FED - H0005 - COMPLIANCE WITH FED/STATE/LOCAL LAWS Title COMPLIANCE WITH FED/STATE/LOCAL LAWS CFR 486.100 Type Condition
Transnational Association of Christian Colleges and Schools
 Transnational Association of Christian Colleges and Schools Steps Toward Accreditation Transnational Association of Christian Colleges and Schools (TRACS) is recognized by the United States Department
Transnational Association of Christian Colleges and Schools Steps Toward Accreditation Transnational Association of Christian Colleges and Schools (TRACS) is recognized by the United States Department
Board of Addiction and Prevention Professionals (BAPP)
 Board of Addiction and Prevention Professionals (BAPP) Overview of Proposed Additions and Changes to the Administrative Rules Chemical dependency will change to addiction throughout the entire document
Board of Addiction and Prevention Professionals (BAPP) Overview of Proposed Additions and Changes to the Administrative Rules Chemical dependency will change to addiction throughout the entire document
Radiation safety in dental radiography
 Radiation safety in dental radiography Dental Radiography Series The goal of dental radiography is to obtain diagnostic information while keeping the exposure to the patient and dental staff at minimum
Radiation safety in dental radiography Dental Radiography Series The goal of dental radiography is to obtain diagnostic information while keeping the exposure to the patient and dental staff at minimum
VI. FREQUENTLY ASKED QUESTIONS CONCERNING BREAST IMAGING AUDITS
 ACR BI-RADS ATLAS VI. FREQUENTLY ASKED QUESTIONS CONCERNING BREAST IMAGING AUDITS American College of Radiology 55 ACR BI-RADS ATLAS A. All Breast Imaging Modalities 1. According to the BI-RADS Atlas,
ACR BI-RADS ATLAS VI. FREQUENTLY ASKED QUESTIONS CONCERNING BREAST IMAGING AUDITS American College of Radiology 55 ACR BI-RADS ATLAS A. All Breast Imaging Modalities 1. According to the BI-RADS Atlas,
ADMISSIONS AND EDUCATIONAL STANDARDS. Adopted July 2007
 TITLE 4. ADMISSIONS AND EDUCATIONAL STANDARDS Adopted July 2007 DIVISION 2. ACCREDITED LAW SCHOOL RULES Chapter 1. General Provisions Rule 4.100 Authority The Committee of Bar Examiners ( the Committee
TITLE 4. ADMISSIONS AND EDUCATIONAL STANDARDS Adopted July 2007 DIVISION 2. ACCREDITED LAW SCHOOL RULES Chapter 1. General Provisions Rule 4.100 Authority The Committee of Bar Examiners ( the Committee
Radiology Physician Performance Measurement Set
 American College of Radiology/ Physician Consortium for Performance Improvement / National Committee for Quality Assurance Radiology Physician Performance Measurement Set October 2007 Radiology Work Group
American College of Radiology/ Physician Consortium for Performance Improvement / National Committee for Quality Assurance Radiology Physician Performance Measurement Set October 2007 Radiology Work Group
Compliance Guidance for FLUOROSCOPIC QUALITY CONTROL
 Compliance Guidance for FLUOROSCOPIC QUALITY CONTROL New Jersey Department of Environmental Protection Bureau of Radiological Health PO Box 415 Trenton NJ 08625 FAX 609-984-5811 Website: http://www.state.nj.us/dep/rpp
Compliance Guidance for FLUOROSCOPIC QUALITY CONTROL New Jersey Department of Environmental Protection Bureau of Radiological Health PO Box 415 Trenton NJ 08625 FAX 609-984-5811 Website: http://www.state.nj.us/dep/rpp
STATE OF OKLAHOMA RADIOLOGIST ASSISTANT LICENSURE ACT TITLE 59, SECTIONS 541 541.9
 STATE OF OKLAHOMA RADIOLOGIST ASSISTANT LICENSURE ACT TITLE 59, SECTIONS 541 541.9 SECTION 541. This act shall be known and may be cited as the Radiologist Assistant Licensure Act. SECTION 541.1. A. A
STATE OF OKLAHOMA RADIOLOGIST ASSISTANT LICENSURE ACT TITLE 59, SECTIONS 541 541.9 SECTION 541. This act shall be known and may be cited as the Radiologist Assistant Licensure Act. SECTION 541.1. A. A
Highmark Provider Privileging Requirements
 Highmark Provider Privileging Requirements Copyright 2008 by Highmark Inc. All rights reserved. No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or
Highmark Provider Privileging Requirements Copyright 2008 by Highmark Inc. All rights reserved. No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or
CHECKLIST ISO/IEC 17021:2011 Conformity Assessment Requirements for Bodies Providing Audit and Certification of Management Systems
 Date(s) of Evaluation: CHECKLIST ISO/IEC 17021:2011 Conformity Assessment Requirements for Bodies Providing Audit and Certification of Management Systems Assessor(s) & Observer(s): Organization: Area/Field
Date(s) of Evaluation: CHECKLIST ISO/IEC 17021:2011 Conformity Assessment Requirements for Bodies Providing Audit and Certification of Management Systems Assessor(s) & Observer(s): Organization: Area/Field
Part 89 PRACTICE OF RADIOLOGIC TECHNOLOGY (Statutory authority: Public Health Law, Sections 3504, 3510(1)(g), 3502(4), 3507(2) and (7))
 Part 89 PRACTICE OF RADIOLOGIC TECHNOLOGY (Statutory authority: Public Health Law, Sections 3504, 3510(1)(g), 3502(4), 3507(2) and (7)) Section PRACTICE OF RADIOLOGIC TECHNOLOGY 89.0 Purpose and scope
Part 89 PRACTICE OF RADIOLOGIC TECHNOLOGY (Statutory authority: Public Health Law, Sections 3504, 3510(1)(g), 3502(4), 3507(2) and (7)) Section PRACTICE OF RADIOLOGIC TECHNOLOGY 89.0 Purpose and scope
U.S. ELECTION ASSISTANCE COMMISSION
 U.S. ELECTION ASSISTANCE COMMISSION Voting System Testing and Certification Program Manual Flowcharts Registration I. Initial Registration submits Registration Application Form (EAC Form 001C) EAC sends
U.S. ELECTION ASSISTANCE COMMISSION Voting System Testing and Certification Program Manual Flowcharts Registration I. Initial Registration submits Registration Application Form (EAC Form 001C) EAC sends
Breast Density Legislation: Implications for primary care providers
 Breast Density Legislation: Implications for primary care providers Deborah J. Rhodes MD Associate Professor of Medicine 2012 MFMER slide-1 Disclosure Relevant financial relationship(s) None Off-label
Breast Density Legislation: Implications for primary care providers Deborah J. Rhodes MD Associate Professor of Medicine 2012 MFMER slide-1 Disclosure Relevant financial relationship(s) None Off-label
QUESTIONS AND ANSWERS LIMITED-SCOPE X-RAY OPERATORS Rules and Regulations Pertaining to Radiation Control (6CCR 1007-1)
 QUESTIONS AND ANSWERS LIMITED-SCOPE X-RAY OPERATORS Rules and Regulations Pertaining to Radiation Control (6CCR 1007-1) QUESTION1: Does the CDPHE require any paperwork for individuals who are already limited-scope
QUESTIONS AND ANSWERS LIMITED-SCOPE X-RAY OPERATORS Rules and Regulations Pertaining to Radiation Control (6CCR 1007-1) QUESTION1: Does the CDPHE require any paperwork for individuals who are already limited-scope
SUBCHAPTER B: ENVIRONMENTAL TESTING LABORATORY ACCREDITATION
 Texas Commission on Environmental Quality Page 1 SUBCHAPTER B: ENVIRONMENTAL TESTING LABORATORY ACCREDITATION ''25.9, 25.10, 25.12, 25.14, 25.16, 25.18, 25.20, 25.22, 25.24, 25.26, 25.30, 25.32, 25.34,
Texas Commission on Environmental Quality Page 1 SUBCHAPTER B: ENVIRONMENTAL TESTING LABORATORY ACCREDITATION ''25.9, 25.10, 25.12, 25.14, 25.16, 25.18, 25.20, 25.22, 25.24, 25.26, 25.30, 25.32, 25.34,
MQSA Quality Control Manual for Monochrome Displays for Mammography
 MQSA Quality Control Manual for Monochrome Displays for Mammography Ver1.0 Applicable Models LMD-DM50, LMD-DM30 Sony Corporation - 1 - MQSA Quality Control Manual for Monochrome Displays for Mammography
MQSA Quality Control Manual for Monochrome Displays for Mammography Ver1.0 Applicable Models LMD-DM50, LMD-DM30 Sony Corporation - 1 - MQSA Quality Control Manual for Monochrome Displays for Mammography
X-RAY REGULATORY GUIDE
 Minnesota Department of Health Radiation Control, X-ray Unit Protecting, maintaining and improving the health of all Minnesotans by promoting radiation safety through guidance and collaboration with the
Minnesota Department of Health Radiation Control, X-ray Unit Protecting, maintaining and improving the health of all Minnesotans by promoting radiation safety through guidance and collaboration with the
Quality control tests of diagnostic radiology equipment in Hungary, and its radiation protection aspects
 Quality control tests of diagnostic radiology equipment in Hungary, and its radiation protection aspects Tamás Porubszky *, Csaba Váradi, László Ballay, Olivér Turák, Géza Gáspárdy, István Turai Frédéric
Quality control tests of diagnostic radiology equipment in Hungary, and its radiation protection aspects Tamás Porubszky *, Csaba Váradi, László Ballay, Olivér Turák, Géza Gáspárdy, István Turai Frédéric
Radiation safety in dental radiography
 Radiation safety in dental radiography Kodak s dental radiograph series The goal of dental radiography is to obtain diagnostic information while keeping the exposure to the patient and dental staff at
Radiation safety in dental radiography Kodak s dental radiograph series The goal of dental radiography is to obtain diagnostic information while keeping the exposure to the patient and dental staff at
IAEA-TECDOC-1447 Optimization of the radiological protection of patients: Image quality and dose in mammography (coordinated research in Europe)
 IAEA-TECDOC-1447 Optimization of the radiological protection of patients: Image quality and dose in mammography (coordinated research in Europe) Results of the Coordinated Research Project on Optimization
IAEA-TECDOC-1447 Optimization of the radiological protection of patients: Image quality and dose in mammography (coordinated research in Europe) Results of the Coordinated Research Project on Optimization
Analysis of Causes for Reject X-Ray Films as a Quality Assurance Element in Diagnostic Radiology in Jos University Teaching Hospital(JUTH)
 Analysis of Causes for Reject X-Ray Films as a Quality Assurance Element in Diagnostic Radiology in Jos University Teaching Hospital(JUTH) By S.O ANIKOH Dept. of Physics, University of Jos, Jos. HABILA
Analysis of Causes for Reject X-Ray Films as a Quality Assurance Element in Diagnostic Radiology in Jos University Teaching Hospital(JUTH) By S.O ANIKOH Dept. of Physics, University of Jos, Jos. HABILA
X-ray (Radiography) - Bone
 Scan for mobile link. X-ray (Radiography) - Bone Bone x-ray uses a very small dose of ionizing radiation to produce pictures of any bone in the body. It is commonly used to diagnose fractured bones or
Scan for mobile link. X-ray (Radiography) - Bone Bone x-ray uses a very small dose of ionizing radiation to produce pictures of any bone in the body. It is commonly used to diagnose fractured bones or
The Regulations on Certification of Civil Aircraft Maintenance Personnel Training Organizations
 The Civil Aviation Administration of China CAAC Decree No. 154 The Regulations on Certification of Civil Aircraft Maintenance Personnel Training Organizations (Formulated on August 26, 2005) CCAR-147 The
The Civil Aviation Administration of China CAAC Decree No. 154 The Regulations on Certification of Civil Aircraft Maintenance Personnel Training Organizations (Formulated on August 26, 2005) CCAR-147 The
33-10-06-03. General requirements. 1. Administrative Controls.
 33-10-06-03. General requirements. 1. Administrative Controls. a. Registrant. The registrant shall be responsible for directing the operation of the x-ray systems which have been registered with the department.
33-10-06-03. General requirements. 1. Administrative Controls. a. Registrant. The registrant shall be responsible for directing the operation of the x-ray systems which have been registered with the department.
Frequently Asked Questions: The following regulations are provided to answer some of the many questions that are asked of this office.
 Steven L. Beshear Governor CABINET FOR HEALTH AND FAMILY SERVICES DEPARTMENT FOR PUBLIC HEALTH Division of Public Health Protection and Safety Radiation Health Branch 275 East Main Street, HS 1C-A Frankfort,
Steven L. Beshear Governor CABINET FOR HEALTH AND FAMILY SERVICES DEPARTMENT FOR PUBLIC HEALTH Division of Public Health Protection and Safety Radiation Health Branch 275 East Main Street, HS 1C-A Frankfort,
X-ray (Radiography) - Abdomen
 Scan for mobile link. X-ray (Radiography) - Abdomen Abdominal x-ray uses a very small dose of ionizing radiation to produce pictures of the inside of the abdominal cavity. It is used to evaluate the stomach,
Scan for mobile link. X-ray (Radiography) - Abdomen Abdominal x-ray uses a very small dose of ionizing radiation to produce pictures of the inside of the abdominal cavity. It is used to evaluate the stomach,
