ATCC PRIMARY CELL Culture Guide. tips and techniques for culturing primary cells
|
|
|
- Annabelle Fitzgerald
- 9 years ago
- Views:
Transcription
1 ATCC PRIMARY CELL Culture Guide tips and techniques for culturing primary cells The Essentials of Life Science Research Globally Delivered
2 Table of Contents ATCC Primary Cell Solutions...1 ATCC Primary Human Endothelial Cell Solutions...5 ATCC Primary Human Smooth Muscle Cell Solutions...9 ATCC Primary Human Epithelial Cell Solutions...12 ATCC Primary Human Fibroblast Solutions...15 ATCC Primary Human Keratinocyte Solutions...19 ATCC Primary Human Melanocyte Solutions...22 ATCC Human Mesenchymal Stem Cell and Differentiation Solutions...25 References
3 ATCC PRIMARY CELL SOLUTIONS Guide to Culturing Human Primary Cells Primary cells and cell types Primary cell cultures more closely mimic the physiological state of cells in vivo and generate more relevant data representing living systems. Primary cultures consist of cells that have been freshly derived from a living organism and are maintained for growth in vitro. Primary cells can be categorized according to the genus from which they are isolated, as well as by species or tissue type. Each mammalian tissue type is derived from the embryonic germ layer consisting of ectoderm, endoderm and mesoderm, which differentiate into the many cell types that organize into tertiary structures such as skin, muscle, internal organs, bone and cartilage, the nervous system, blood and blood vessels 1. The cell types most frequently found in primary cell culture are epithelial cells, fibroblasts, keratinocytes, melanocytes, endothelial cells, muscle cells, hematopoietic and mesenchymal stem cells. Basic properties of primary cells Once adapted to in vitro culture conditions, primary cells undergo a limited, predetermined number of cell divisions before entering senescence. The number of times a primary cell culture can be passaged is minimal due to the Hayflick Limit, nutrient requirements and culture conditions, and the expertise by which they are manipulated and subcultured. In contrast, cell lines that have been immortalized by viral, htert or tumorigenic transformation typically undergo unlimited cell division and have an infinite lifespan. And, unlike tumor cell lines cultured in medium containing 10% to 20% serum, primary cell cultures are fastidious, requiring optimized growth conditions, including the addition of tissue specific cytokines and growth factors for propagation in serum-free or low-serum growth media. Benefits of primary cells Primary cell cultures are commonly used as in vitro tools for pre-clinical and investigative biological research, such as studies of inter- and intracellular communication, developmental biology, and elucidation of disease mechanisms, such as cancer, Parkinson s disease, and diabetes. Historically, investigators have employed immortalized cell lines in research related to tissue function; however, the use of cell lines containing gross mutations and chromosomal abnormalities provides poor indicators of normal cell phenotype and progression of early-stage disease. The use of primary cells, maintained for only short periods of time in vitro, now serves as the best representative of the main functional component of the tissue (in vivo) from which they are derived. Isolation of primary cells The isolation and purification of peripheral blood cells can be easily achieved by differential centrifugation or by positive sorting using magnetic beads. On the other hand, the isolation of a pure population of cells from primary tissue is often difficult to perform, and requires knowledge of how the cell strata should be teased apart into a suspension containing only one predominant cell type. Diagram 1 is an illustration of some of the basic steps used to establish a primary cell culture 14. Primary cell culture Growth requirements Primary cells, except for those derived from peripheral blood, are anchorage-dependent, adherent cells, meaning they require a surface in order to grow properly in vitro. In most cases, primary cells are cultured in a flat un-coated plastic vessel, but sometimes a microcarrier, which can greatly increase the surface area, can be used. A complete cell culture media, composed of a basal medium supplemented page 1 [email protected] Phone
4 Diagram 1. Basic steps used to isolate cells from primary tissue Tissue Acquisition Dissection Disaggregation Incubation & Growth with appropriate growth factors and cytokines, is required. During establishment of primary cultures, it may be useful to include an antibiotic in the growth medium to inhibit contamination introduced from the host tissue. These may include a mixture of gentamicin, penicillin, streptomycin and amphotericin B. However, long-term use of antibiotics is not advised, since some reagents, such as amphotericin B, may be toxic to cells over time. Maintenance The maintenance phase begins when cells have attached to the surface of the culture dish. Attachment usually occurs about 24 hours after initiation of the culture. When initiating a culture of cryopreserved primary cells, it is important to remove the spent media once the cells have attached because DMSO is harmful to primary cells and may cause a drop in post-thaw viability. When cells have reached a desired percent of cellular confluence and are actively proliferating, it is time to subculture. It is best to subculture primary cell cultures before reaching 100% confluence, since post-confluent cells may undergo differentiation and exhibit slower proliferation after passaging. Cellular confluence Cellular confluence refers to the percentage of the culture vessel inhabited by attached cells. For example, 100% cellular confluence means the surface area is completely covered by cells, while 50% confluence means roughly half of the surface is covered. It is an important parameter to track and assess in primary cell culture because different cell types require different confluence end points, at which point they need to be subcultured. Levels of cellular confluence Process primary tissue, removing fatty and necrotic cells Mechanical or enzymatic disaggregation. Enzymes used: Trypsin Collagenase II Elastase Hyaluronidase DNase Incubate dispersed cells Change medium 24 hours after initiation to remove loose debris & unattached cells Separation & Purification Further purification of primary cells achieved by: Selective media Remove cells at different levels of attachment Immunomagnetic beads Human smooth muscle cells between 20% and 30% confluence Human melanocyte cells between 50% and 60% confluence Human keratinocytes at nearly 100% confluence (note the formation of vacuoles and differentiated cells) Subculture Anchorage-dependent cells grow in monolayers and need to be subcultured at regular intervals to maintain exponential growth. Subculturing procedures, including recommended split-ratios and medium replenishment (feeding) schedules for each ATCC primary cell culture, are provided on the page 2
5 product information sheet provided with each cell (available online at Subcultivation of monolayers involves the breakage of both inter- and intracellular cell-to-surface bonds. Most adherent primary cells require the digestion of their protein attachment bonds with a low concentration of a proteolytic enzyme such as trypsin/edta. After the cells have been dissociated and dispersed into a single-cell suspension, they are counted and diluted to the appropriate concentration and transferred to fresh culture vessels where they will reattach and divide. Cell Counting Hemocytometers are commonly used to estimate cell number and determine cell viability with the aid of an exclusion dye such as Trypan Blue or Erythrosin B. A hemocytometer is a fairly thick glass slide with two counting chambers, one on each side. Each counting chamber has a mirrored surface with a 3 3 mm grid consisting of 9 counting squares. The chambers have raised sides that will hold a coverslip exactly 0.1 mm above the chamber floor. Each of the 9 counting squares holds a volume of ml. Alternatively, counting cells can also be achieved by using an automated cell counter, such as Vi-CELL. For a detailed description of cell counting using a hemocytometer, refer to the ATCC Animal Cell Culture Guide: Tips and Techniques for Continuous Cell Lines available online at Cryopreservation and Recovery Special attention is needed to cryopreserve and thaw primary cells in order to minimize cell damage and death during each process. Cryopreservation of human cells is best achieved with the use of a cryoprotectant, such as DMSO or glycerol. Most primary cell cultures can be cryopreserved in a mixture of 80% complete growth medium supplemented with 10% FBS and 10% DMSO. The freezing process should be slow, at a rate of -1 C per minute, to minimize the formation of ice crystals within the cells. Once frozen, cultures are stored in the vapor phase of liquid nitrogen, or below -130 C. Additional information about freezing cells can be found in ATCC Technical Bulletin No. 3: Cryogenic Preservation of Animal Cells, available online at Thawing cryopreserved cells is a rapid process and is accomplished by immersing frozen cells in a 37 C water bath for about 1 to 2 minutes. Care should be taken not to centrifuge primary cells upon thaw, since they are extremely sensitive to damage during recovery from cryopreservation. It is best to plate cells directly upon thaw, and allow cultures to attach for the first 24 hours before changing the medium to remove residual DMSO. Challenges of primary cell isolation and culture There are several challenges associated with the use of primary cells. One of the greatest hurdles primary cell culturists face is limited cell accessibility due to issues with donor tissue supply, difficulty with cell isolation/purification, quality assurance and consistency, and contamination risks. Data comparability is also a serious issue with primary cell use, and arises out of variability among reagents used and the procedures implemented by individual scientists and laboratories to isolate and culture primary cells. A comparison between primary cells and continuous cell lines is described in Table 1. page 3 [email protected] Phone
6 Table 1. Comparison between primary cells and continuous cell lines Properties Primary cells Continuous cell lines Life span & cell proliferation Consistency Genetic Integrity Biological relevance Ease of use (freeze-thaw & use) Finite; limited to a small number of cell doublings Variability exists between donors and preparations Retains in vivo tissue genetic makeup through cell doublings More closely mimics the physiology of cells in vivo Requires optimized culture conditions and careful handling Infinite when handled properly Minimal variability Subject to genetic drift as cells divide Relevance can drift over time as cells divide Well established conditions and robust protocols exist Time & expense to use More time and less abundance of cells Less time and more abundance of cells ATCC Primary Cell Solutions ATCC has provided a solution to help investigators overcome the high cost and inconsistency found in routine primary cell culture with the development of ATCC Primary Cell Solutions, a standardized cell culture system that includes high quality cells, media, supplements, reagents and protocols. ATCC Primary Cell Solutions focuses on providing researchers with superior quality from a trusted source each lot of ATCC Primary Cell Solutions primary cells is: Cryopreserved at early passage ATCC Primary Cell Solutions primary cells are frozen at passages 1 through 3 Early passage material ensures higher viability and optimal plating efficiency Performance tested ATCC Primary Cell Solutions cells, media, kit supplements, and reagents are tested for optimal reliability Growth and morphology are assessed to ensure all components work synergistically Quality controlled to ensure safety, purity, and functionality Sterility testing - all cells are tested for bacteria, yeast, fungi, and Mycoplasma Viral testing - HIV-1, HIV-2, HBV, and HCV tissue screening is performed at isolation Viability and Growth - viability and growth of each lot of cells is checked before freezing and after-thawing Staining - staining for cell-specific marker expression is determined and performed for some cell types ATCC Primary Cell Solutions basal media and cell-specific growth kits are designed to support recovery and proliferation of ATCC Primary Cell Solutions primary cells in vitro. Used as a system, ATCC Primary Cell Solutions primary cells, basal media and cell-specific growth kits provide all the components needed to be successful in primary cell research. (Detailed formulations for each growth kit can be found at page 4
7 Endothelial Cells ATCC Primary Human Endothelial Cell Solutions Introduction Endothelial cells form the endothelium - the thin layer of cells that line the interior surface of blood vessels forming a smooth anticoagulant surface that functions as a selective filter to regulate the passage of gases, fluid, immune cells and various molecules 14. Human endothelial cells can be isolated from human umbilical vein, the aorta, the pulmonary and coronary arteries, and the skin, and serve as useful tools in the study of angiogenesis, cancer therapy, wound healing, burn therapy, highthroughput and high-content screening projects, cell signaling studies, gene expression profiling, toxicology screening, tissue engineering and regeneration. ATCC Primary Human Endothelial Cells can be cultured in complete growth medium containing either bovine brain extract (BBE) or vascular endothelial growth factor (VEGF). Use of the Endothelial Cell Growth Kit-VEGF (ATCC PCS ) will support a faster rate of proliferation, while the Endothelial Cell Growth Kit-BBE (ATCC PCS ) is recommended if a less defined cell culture medium is desired. Cell Culture Protocols Materials Needed Primary cells and complete growth medium Endothelial Cells Growth Kit Options* Basal Medium Umbilical Vein Endothelial Cells, Normal, Human (ATCC PCS ) Umbilical Vein Endothelial Cells, Normal, Human, Pooled (ATCC PCS ) Aortic Endothelial Cells; Normal, Human (ATCC PCS ) Coronary Artery Endothelial Cells, Normal, Human (ATCC PCS ), Pulmonary Artery Endothelial Cells, Normal, Human (ATCC PCS ), Dermal Microvascular Endothelial Cells, Normal, Human, Neonatal (ATCC PCS ) Choose ONE: Endothelial Cell Growth Kit- BBE (ATCC PCS ) Endothelial Cell Growth Kit- VEGF (ATCC PCS ) For Microvascular Endothelial Cells, Choose ONE: Microvascular Endothelial Cell Growth Kit-BBE (ATCC PCS ) Microvascular Endothelial Cell Growth Kit-VEGF (ATCC PCS ) Reagents for Subculture D-PBS (ATCC ) Trypsin-EDTA for Primary Cells (ATCC PCS ) Trypsin Neutralizing Solution (ATCC PCS ) *Phenol red and antibiotics may be added if desired, and are listed in the appendix. page 5 [email protected] Phone Primary Dermal Micovascular Endothelial Cells, Normal, Human, Neonatal (ATCC PCS ) Vascular Cell Basal Medium (ATCC PCS )
8 Preparation of Complete Growth Media Endothelial Cells 1. Obtain one growth kit from the freezer; make sure that the caps of all components are tight. 2. Thaw the components of the growth kit just prior to adding them to the basal medium. It is necessary to warm the L-glutamine component in a 37 C water bath and shake to dissolve any precipitates prior to adding to the basal medium. 3. Obtain one bottle of Vascular Cell Basal Medium from cold storage. 4. Decontaminate the external surfaces of all growth kit component vials and the basal medium bottle by spraying them with 70% ethanol. 5. Using aseptic technique, and working in a laminar flow hood or biosafety cabinet, transfer the volume of each growth kit component (volumes for each growth kit are provided on the product information sheet; the following table represents the Endothelial Cell Growth Kit-VEGF) to the bottle of basal medium using a separate sterile pipette for each transfer. 6. Tightly cap the bottle of complete growth medium and swirl the contents gently to assure a homogeneous solution. Do not shake forcefully to avoid foaming. Label and date the bottle. 7. Complete growth media should be stored in the dark at 2 C to 8 C (do not freeze). When stored under these conditions, complete growth media is stable for 30 days. Endothelial Cell Growth Kit-VEGF (ATCC PCS ) Component Volume Final Concentration rh VEGF 0.5 ml 5 ng/ml rh EGF 0.5 ml 5 ng/ml rh FGF basic 0.5 ml 5 ng/ml rh IGF ml 15 ng/ml L-glutamine 25.0 ml 10 mm Heparin sulfate 0.5 ml 0.75 Units/mL Hydrocortisone hemisuccinate 0.5 ml 1 µg/ml Fetal Bovine Serum 10.0 ml 2% Ascorbic acid 0.5 ml 50 µg/ml Handling Procedure for Frozen Cells and Initiation of Cultures 1. Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC Primary Human Endothelial Cells. 2. Using the total number of viable cells, determine how much surface area can be inoculated to achieve an initial seeding density between 2,500 and 5,000 cells per cm 2 for most Primary Human Endothelial Cells offered by ATCC; the exception being Primary Dermal Microvascular Endothelial Cells, which should be seeded at a density of 5,000 cells per cm Prepare the desired combination of flasks. Add 5 ml of complete growth media per 25 cm 2 of surface area. Place Primary Dermal Microvascular Endothelial Cells dual stained for von Willebrand factor (green) as a marker for endothelial cells and smooth muscle α-actin (red) the flasks in a 37 C, 5% CO 2, humidified incubator and allow the media to pre-equilibrate to temperature and ph for 30 minutes prior to adding cells. page 6
9 Endothelial Cells 4. While the culture flasks equilibrate, remove one vial of ATCC Primary Human Endothelial Cells from storage and thaw the cells by gentle agitation in a 37 C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 1 to 2 minutes). 5. Remove the vial from the water bath as soon as the contents are thawed, and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point onward should be carried out under strict aseptic conditions. 6. Add the appropriate volume of complete growth media [volume = (1 ml x number of flasks to be seeded) 1 ml] into a sterile conical tube. Using a sterile pipette, transfer the cells from the cryovial to the conical tube. Gently pipette the cells to homogenize the suspension. Do not centrifuge. 7. Transfer 1.0 ml of the cell suspension to each of the pre-equilibrated culture flasks prepared in steps 1 to 3 of Handling Procedure for Frozen Cells and Initiation of Culture. Pipette several times, then cap and gently rock each flask to evenly distribute the cells. 8. Place the seeded culture flasks in the incubator at 37 C with a 5% CO 2 atmosphere. Incubate for at least 24 hours before processing the cells further. Maintenance 1. Before beginning, pre-warm complete growth media in a 37 C water bath. This will take between 10 and 30 minutes, depending on the volume. If using a small volume of medium (50 ml or less), warm only the volume needed in a sterile conical tube. Avoid warming complete growth media multiple times hours after seeding, remove the cells from the incubator and view each flask under the microscope to determine percent cellular confluence. 3. Carefully remove the spent media without disturbing the monolayer. 4. Add 5 ml of fresh, pre-warmed complete growth media per 25 cm 2 of surface area and return the flasks to the incubator. 5. After 24 to 48 hours, view each flask under the microscope to determine percent cellular confluence. If not ready to passage, repeat steps 3 and 4 as described above. When cultures have reached approximately 80% confluence, and are actively proliferating (many mitotic figures are visible), it is time to subculture. Subculture endothelial cells before reaching confluence; postconfluent primary endothelial cells may exhibit slower proliferation after passaging. Subculture 1. Passage Primary Human Endothelial Cells when cultures have reached approximately 80% confluence. 2. Warm both the Trypsin-EDTA for Primary Cells (ATCC PCS ) and the Trypsin Neutralizing Solution (ATCC PCS ) to room temperature prior to dissociation. Warm complete growth medium to 37 C prior to use with the cells. 3. For each flask, carefully aspirate the spent media without disturbing the monolayer. 4. Rinse the cell layer two times with 3 to 5 ml D-PBS (ATCC ) to remove residual traces of serum. 5. Add pre-warmed trypsin-edta solution (1 to 2 ml for every 25 cm 2 ) to each flask. 6. Gently rock each flask to ensure complete coverage of the trypsin-edta solution over the cells, and then aspirate the excess fluid off of the monolayer. 7. Observe the cells under the microscope. When the cells pull away from each other and round up page 7 [email protected] Phone
10 Endothelial Cells (typically within 3 to 5 minutes), remove the flask from the microscope and gently tap it from several sides to promote detachment of the cells from the flask surface. 8. When the majority of cells appear to have detached, quickly add an equal volume of Trypsin Neutralizing Solution (ATCC PCS ) to each flask. Gently pipette or swirl the culture to ensure all of the trypsin-edta solution has been neutralized. 9. Transfer the dissociated cells to a sterile centrifuge tube and set aside while processing any remaining cells in the flask. 10. Add 3 to 5 ml D-PBS (ATCC ) to the flask to collect any additional cells that might have been left behind. 11. Transfer the cell/d-pbs suspension to the centrifuge tube containing the trypsin-edtadissociated cells. 12. Repeat steps 10 and 11 as needed until all cells have been collected from the flask. 13. Centrifuge the cells at 150 x g for 3 to 5 minutes. 14. Aspirate the neutralized dissociation solution from the cell pellet and resuspend the cells in 2 to 8 ml fresh, pre-warmed, complete growth medium. 15. Count the cells and seed new flasks at a density of 2,500 to 5,000 cells per cm 2 ; however, be sure to seed Primary Dermal Microvascular Endothelial Cells at 5,000 cells per cm Place newly seeded flasks in a 37 C, 5% CO 2, incubator for at least 24 to 48 hours before processing the cells further. Refer to Maintenance for guidelines on feeding. page 8
11 Smooth Muscle Cells ATCC Primary Human Smooth Muscle Cell Solutions Introduction Vascular tissue is made up of a diverse population of cell types, including endothelial cells, smooth muscle cells, pericytes, fibroblasts, and other connective tissue cell types. Together these cell types form tight junctions or connections, which allow for permeability for both passive and active transport across the vessel wall. Vascular smooth muscle cells make up the smooth muscle layer of blood vessels and can be co-cultured with vascular endothelium 14. Smooth muscle cells isolated from human ascending (thoracic) and descending (abdominal) aorta, coronary artery, and pulmonary artery are useful for studying vascular diseases such as thrombosis and atherosclerosis. Cell Culture Protocols Materials Needed Primary cells and complete growth medium Smooth Muscle Cells Growth Kit Options* Basal Medium Aortic Smooth Muscle Cells, Normal, Human (ATCC PCS ) Coronary Artery Smooth Muscle Cells, Normal, Human (ATCC PCS ) Pulmonary Artery Smooth Muscle Cells, Normal, Human (ATCC PCS ) Reagents for Subculture D-PBS (ATCC ) Trypsin-EDTA for Primary Cells (ATCC PCS ) Trypsin Neutralizing Solution (ATCC PCS ) Vascular Smooth Muscle Cell Growth Kit (ATCC PCS ) *Phenol red and antibiotics may be added if desired, and are listed in the appendix. Preparation of Complete Growth Media 1. Obtain one growth kit from the freezer; make sure that the caps of all components are tight. 2. Thaw the components of the growth kit just prior to adding them to the basal medium. It is necessary to warm the L-glutamine component in a 37 C water bath and shake to dissolve any precipitates prior to adding to the basal medium. 3. Obtain one bottle of Vascular Cell Basal Medium from cold storage. 4. Decontaminate the external surfaces of all growth kit component vials and the basal medium bottle by spraying them with 70% ethanol. 5. Using aseptic technique, and working in a laminar flow hood or biosafety cabinet, transfer the volume of each growth kit Primary Aortic Smooth Muscle Cells, Normal, Human (ATCC PCS ) Vascular Cell Basal Medium (ATCC PCS ) ATCC Primary Cell Solutions aortic smooth muscle cells (ATCC PCS ) dual stained for von Willebrand factor (green) as a marker for endothelial cells and smooth muscle α-actin (red) after differentiation. page 9 [email protected] Phone
12 Smooth Muscle Cells component, as indicated in the following table to the bottle of basal medium using a separate sterile pipette for each transfer. Vascular Smooth Muscle Cell Growth Kit (ATCC PCS ) Component Volume Final Concentration rh FGF-basic 0.5 ml 5 ng/ml rh Insulin 0.5 ml 5 μg/ml Ascorbic acid 0.5 ml 50 µg/ml L-glutamine 25.0 ml 10 mm rh EGF 0.5 ml 5 ng/ml Fetal Bovine Serum 25.0 ml 5% 6. Tightly cap the bottle of complete growth medium and swirl the contents gently to assure a homogeneous solution. Do not shake forcefully to avoid foaming. Label and date the bottle. 7. Complete growth media should be stored in the dark at 2 C to 8 C (do not freeze). When stored under these conditions, complete growth media is stable for 30 days. Handling Procedure for Frozen Cells and Initiation of Cultures 1. Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC Primary Human Smooth Muscle Cells. 2. Using the total number of viable cells, determine how much surface area can be inoculated to achieve an initial seeding density between 2,500 and 5,000 cells per cm Prepare the desired combination of flasks. Add 5 ml of complete growth media per 25 cm 2 of surface area. Place the flasks in a 37 C, 5% CO 2, humidified incubator and allow the media to preequilibrate to temperature and ph for 30 minutes prior to adding cells. 4. While the culture flasks equilibrate, remove one vial of ATCC Primary Human Smooth Muscle Cells from storage and thaw the cells by gentle agitation in a 37 C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 1 to 2 minutes). 5. Remove the vial from the water bath as soon as the contents are thawed, and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point onward should be carried out under strict aseptic conditions. 6. Add the appropriate volume of complete growth media [volume = (1 ml x number of flasks to be seeded) 1 ml] into a sterile conical tube. Using a sterile pipette, transfer the cells from the cryovial to the conical tube. Gently pipette the cells to homogenize the suspension. Do not centrifuge. 7. Transfer 1.0 ml of the cell suspension to each of the pre-equilibrated culture flasks prepared in steps 1 to 3 of Handling Procedure for Frozen Cells and Initiation of Culture. Pipette several times, then cap and gently rock each flask to evenly distribute the cells. 8. Place the seeded culture flasks in the incubator at 37 C with a 5% CO 2 atmosphere. Incubate for at least 24 hours before processing the cells further. Maintenance 1. Before beginning, pre-warm complete growth media in a 37 C water bath. This will take between 10 and 30 minutes, depending on the volume. If using a small volume of medium (50 ml or less), warm only the volume needed in a sterile conical tube. Avoid warming complete growth media page 10
13 Smooth Muscle Cells multiple times to 36 hours after seeding, remove the cells from the incubator and view each flask under the microscope to determine percent cellular confluence. 3. Carefully remove the spent media without disturbing the monolayer. 4. Add 5 ml of fresh, pre-warmed complete growth media per 25 cm 2 of surface area and return the flasks to the incubator. 5. After 24 to 48 hours, view each flask under the microscope to determine percent cellular confluence. If not ready to passage, repeat steps 3 and 4 as described above. When cultures have reached approximately 80% to 90% confluence, and are actively proliferating (many mitotic figures are visible), it is time to subculture. Vascular smooth muscle cells are contact inhibited; post-confluent vascular smooth muscle cells may not proliferate after passaging. Subculture Note: Cells are typically ready to passage after 7 to 9 days in culture when inoculated with 2,500 cells/cm2. 1. Passage normal vascular smooth muscle cells when the culture has reached approximately 80% confluence. 2. Warm both the Trypsin-EDTA for Primary Cells (ATCC PCS ) and the Trypsin Neutralizing Solution (ATCC PCS ) to room temperature prior to dissociation. Warm complete growth medium to 37 C prior to use with the cells. 3. For each flask, carefully aspirate the spent media without disturbing the monolayer. 4. Rinse the cell layer two times with 3 to 5 ml D-PBS (ATCC ) to remove residual traces of serum. 5. Add pre-warmed trypsin-edta solution (1 to 2 ml for every 25 cm 2 ) to each flask. 6. Gently rock each flask to ensure complete coverage of the trypsin-edta solution over the cells, and then aspirate the excess fluid off of the monolayer. 7. Observe the cells under the microscope. When the cells pull away from each other and round up (typically within 1 to 3 minutes), remove the flask from the microscope and gently tap it from several sides to promote detachment of the cells from the flask surface. 8. When the majority of cells appear to have detached, quickly add an equal volume of Trypsin Neutralizing Solution (ATCC PCS ) to each flask. Gently pipette or swirl the culture to ensure all of the trypsin-edta solution has been neutralized. 9. Transfer the dissociated cells to a sterile centrifuge tube and set aside while processing any remaining cells in the flask. 10. Add 3 to 5 ml D-PBS (ATCC ) to the flask to collect any additional cells that might have been left behind. 11. Transfer the cell/d-pbs suspension to the centrifuge tube containing the trypsin-edtadissociated cells. 12. Repeat steps 10 and 11 as needed until all cells have been collected from the flask. 13. Centrifuge the cells at 150 x g for 3 to 5 minutes. 14. Aspirate the neutralized dissociation solution from the cell pellet and resuspend the cells in 2 to 8 ml fresh, pre-warmed, complete growth medium. 15. Count the cells and seed new flasks at a density of 2,500 to 5,000 cells per cm Place newly seeded flasks in a 37 C, 5% CO 2, incubator for at least 24 to 48 hours before processing the cells further. Refer to Maintenance for guidelines on feeding. page 11 [email protected] Phone
14 ATCC Primary Human Epithelial Cell Solutions Introduction Epithelia are tissues found throughout the body. They line the cavities of glands and organs in the body and also cover flat surfaces, such as skin. Epithelial cells are polarized cells that discriminate between an apical and basolateral compartment. They perform a variety of functions depending on their location, including boundary and protection, sensory, secretion, transportation, absorption and diffusion. One challenging aspect of culturing primary epithelial cells is overgrowth of stromal cells. Stromal Epithelial Cells fibroblasts can be inhibited by culturing explants in low serum or serum-free culture media, limiting the calcium concentration in the growth medium, selectively inhibiting fibroblasts with agents, or by targeting with antimesodermal antibodies 14. ATCC Primary Cell Solutions offers epithelial cells isolated from human bronchi, trachea and small airways, as well as the cornea, prostate and kidneys. The usefulness of such cultures has been found in studies related to inflammation, microbial infection and pathogenesis including influenza, cancer, toxicology, gene regulation and tissue development, cell-matrix interactions, as well as application in toxicology testing and drug screening/development. Cell Culture Protocols Materials Needed Primary cells and complete growth medium Epithelial Cells Growth Kit Options* Basal Medium Small Airway Epithelial Cells; Normal, Human (ATCC PCS ) Bronchial/Tracheal Epithelial Cells; Normal, Human (ATCC PCS ) Renal Proximal Tubule Epithelial Cells; Normal, Human (ATCC PCS ) Renal Cortical Epithelial Cells; Normal, Human (ATCC PCS ) Renal Mixed Epithelial Cells; Normal, Human (ATCC PCS ) Prostate Epithelial Cells; Normal, Human (ATCC PCS ) Corneal Epithelial Cells; Normal, Human (ATCC PCS ) Reagents for Subculture D-PBS (ATCC ) Trypsin-EDTA for Primary Cells (ATCC PCS ) Trypsin Neutralizing Solution (ATCC PCS ) Small Airway Epithelial Cell Growth Kit (ATCC PCS ) Airway Epithelial Cell Basal Bronchial Epithelial Cell Growth Kit (ATCC PCS ) Renal Epithelial Cell Growth Kit (ATCC PCS ) Prostate Epithelial Cell Growth Kit (ATCC PCS ) Corneal Epithelial Cell Growth Kit (ATCC PCS ) *Phenol red and antibiotics may be added if desired, and are listed in the appendix. Primary Prostate Epithelial Cells; Normal, Human (ATCC PCS ) Medium (ATCC PCS ) Renal Epithelial Cell Basal Medium (ATCC PCS ) Prostate Epithelial Cell Basal Medium (ATCC PCS ) Corneal Epithelial Cell Basal Medium (ATCC PCS ) page 12
15 Epithelial Cells Preparation of Complete Growth Media 1. Obtain one Epithelial Cell Growth Kit (corresponding to the epithelial cell type being used) from the freezer; make sure that the caps of all components are tight. 2. Thaw the components of the growth kit just prior to adding them to the basal medium. 3. Obtain one bottle of Epithelial Cell Basal Medium (corresponding to the epithelial cell being used) from cold storage. 4. Decontaminate the external surfaces of all growth kit component vials and the basal medium bottle by spraying them with 70% ethanol. 5. Using aseptic technique and working in a laminar flow hood or biosafety cabinet, transfer the indicated volume of each growth kit component as indicated in the culture s corresponding product information sheet, to the bottle of basal medium using a separate sterile pipette for each transfer. 6. Tightly cap the bottle of complete growth medium and swirl the contents gently to assure a homogeneous solution. Do not shake forcefully to avoid foaming. Label and date the bottle. 7. Complete growth media should be stored in the dark at 2 C to 8 C (do not freeze). When stored under these conditions, complete growth media is stable for 30 days. Handling Procedure for Frozen Cells and Initiation of Cultures 1. Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC Primary Human Epithelial Cells. 2. Using the total number of viable cells, determine how much surface area can be inoculated to achieve an initial seeding density of 5,000 cells per cm Prepare the desired combination of flasks. Add 5 ml of complete growth medium per 25 cm 2 of surface area. Place the flasks in a 37 C, 5% CO 2, humidified incubator and allow the media to preequilibrate to temperature and ph for 30 minutes prior to adding cells. 4. While the culture flasks equilibrate, remove one vial of ATCC Primary Human Epithelial Cells from storage and thaw the cells by gentle agitation in a 37 C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 1 to 2 minutes). 5. Remove the vial from the water bath as soon as the contents are thawed, and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point onward should be carried out under strict aseptic conditions. 6. Add the appropriate volume of complete growth medium [volume = (1 ml x number of flasks to be seeded) 1 ml] into a sterile conical tube. Using a sterile pipette, transfer the cells from the cryovial to the conical tube. Gently pipette the cells to homogenize the suspension. Do not centrifuge. 7. Transfer 1.0 ml of the cell suspension to each of the pre-equilibrated culture flasks prepared in steps 1 to 3 of Handling Procedure for Frozen Cells and Initiation of Culture. Pipette several times, then cap and gently rock each flask to evenly distribute the cells. 8. Place the seeded culture flasks in the incubator at 37 C, 5% CO 2 atmosphere. Incubate for at least 24 hours before processing the cells further. Maintenance 1. Before beginning, pre-warm complete growth media in a 37 C water bath. This will take between 10 and 30 minutes, depending on the volume. If using a small volume of medium (50 ml or less), page 13 [email protected] Phone
16 Epithelial Cells warm only the volume needed in a sterile conical tube. Avoid warming complete growth media multiple times hours after seeding, remove the cells from the incubator and view each flask under the microscope to determine percent cellular confluence. 3. Carefully remove the spent media without disturbing the monolayer. 4. Add 5 ml of fresh, pre-warmed complete growth medium per 25 cm 2 of surface area and return the flasks to the incubator. 5. After 24 to 48 hours, view each flask under the microscope to determine percent cellular confluence. If not ready to passage, repeat steps 3 and 4 as described above. When cultures have reached about 80% confluence, and are actively proliferating (many mitotic figures are visible), it is time to subculture. Subculture 1. Passage normal human epithelial cells when the culture has reached about 80% confluence. 2. Warm both the Trypsin-EDTA for Primary Cells (ATCC PCS ) and the Trypsin Neutralizing Solution (ATCC PCS ) to room temperature prior to dissociation. Warm the complete growth medium to 37 C prior to use with the cells. 3. For each flask, carefully aspirate the spent media without disturbing the monolayer. 4. Rinse the cell layer one time with 3 to 5 ml D-PBS (ATCC ) to remove residual medium. 5. Add pre-warmed trypsin-edta solution (1 to 2 ml for every 25 cm 2 ) to each flask. 6. Gently rock each flask to ensure complete coverage of the trypsin-edta solution over the cells, and then aspirate the excess fluid off of the monolayer. 7. Observe the cells under the microscope. When the cells pull away from each other and round up (typically within 1 to 3 minutes), remove the flask from the microscope and gently tap it from several sides to promote detachment of the cells from the flask surface. 8. When the majority of cells appear to have detached, quickly add an equal volume of the Trypsin Neutralizing Solution (ATCC PCS ) to each flask. Gently pipette or swirl the culture to ensure all of the trypsin-edta solution has been neutralized. 9. Transfer the dissociated cells to a sterile centrifuge tube and set aside while processing any remaining cells in the culture flask. 10. Add 3 to 5 ml D-PBS (ATCC ) to the tissue culture flask to collect any additional cells that might have been left behind. 11. Transfer the cell/d-pbs suspension to the centrifuge tube containing the trypsin-edtadissociated cells. 12. Repeat steps 10 and 11 as needed until all cells have been collected from the flask. 13. Centrifuge the cells at 150 x g for 3 to 5 minutes. 14. Aspirate neutralized dissociation solution from the cell pellet and resuspend the cells in 2 to 8 ml fresh, pre-warmed, complete growth medium. 15. Count the cells and seed new culture flasks at a density of 5,000 viable cells per cm Place newly seeded flasks in a 37 C, 5% CO 2 incubator for at least 24 to 48 hours before processing the cells further. Refer to Maintenance for guidelines on feeding. page 14
17 Fibroblasts ATCC Primary Human Fibroblast Solutions Introduction Fibroblasts play an important role in maintaining the structural integrity of connective tissue, and synthesize extracellular matrix proteins such as collagens, glycosaminoglycans, and glycoproteins 15,16. Fibroblasts are morphologically heterogeneous, and their appearance is dependent on in vivo location and activity. Injury of tissue displays a proliferative stimulus for fibroblasts and induces them to produce wound healing proteins. Fibroblasts used in primary cell culture are commonly isolated from the dermis layer of human neonatal foreskin or adult skin. They are frequently used in studies related to wound healing, tissue engineering and regeneration applications, and the induction of pluripotent stem (ips) cells. Fibroblasts, treated with mitomycin C to inhibit proliferation, have been extensively used as feeder layers to enhance the cultivation of human stem cells and keratinocytes in vitro. ATCC Primary Human Fibroblasts can be cultured in complete growth medium with or without serum. The use of Fibroblast Growth Kit Serum-Free creates a completely defined medium for the serum-free culture of human fibroblasts. The rate of proliferation is equal to or greater than media supplemented using FBS (at concentrations ranging from 2% to 10%) through 10 population doublings. Cell Culture Protocols Materials Needed Primary cells and complete growth medium Fibroblasts Growth Kit Options* Basal Medium Dermal Fiboblasts; Normal, Human Neonatal (ATCC PCS ) Dermal Fiboblasts; Normal, Human, Adult (ATCC PCS ) Dermal Fiboblasts; Normal, Human Neonatal, Mitomycin C Treated (ATCC PCS )** Reagents for Subculture D-PBS (ATCC ) Trypsin-EDTA for Primary Cells (ATCC PCS ) Trypsin Neutralizing Solution (ATCC PCS ) Choose ONE: Fibroblast Growth Kit Serum Free (ATCC PCS ) Fibroblast Growth Kit Low Serum (ATCC PCS ) Primary adult human dermal fibroblasts (ATCC PCS ) cultured in serumfree medium. Fibroblast Basal Medium (ATCC PCS ) *Phenol red and antibiotics may be added if desired, and are listed in the appendix. **0.1% Gelatin Solution (ATCC PCS ) is also needed for culturing Mitomycin C Treated Dermal Fibroblasts, Normal, Human Neonatal (ATCC PCS ). Preparation of Complete Growth Media 1. Obtain one growth kit from the freezer; make sure that the caps of all containers are tight. 2. Thaw the components of the growth kit just prior to adding them to the basal medium. It is necessary to warm the L-glutamine component in a 37 C water bath, and shake to dissolve any precipitates prior to adding to the basal medium. 3. Obtain one bottle of Fibroblast Basal Medium from cold storage. page 15 [email protected] Phone
18 Fibroblasts 4. Decontaminate the external surfaces of all growth kit component vials and the basal medium bottle by spraying them with 70% ethanol. 5. Using aseptic technique and working in a laminar flow hood or biosafety cabinet, transfer the volume of each growth kit component, as indicated in the following tables, to the bottle of basal medium using a separate sterile pipette for each transfer. Fibroblast Growth Kit Serum-Free (ATCC PCS ) Component Volume Final Concentration L-glutamine ml 7.5 mm Hydrocortisone Hemisuccinate 0.5 ml 1 μg/ml HLL Supplement 1.25 ml rh FGF β 0.5 ml 5 ng/ml rh EGF / TGF β-1 Supplement 0.5 ml HSA 500 μg/ml Linoleic Acid 0.6 μm Lecithin 0.6 μg/ml 5 ng/ml 30 pg/ml rh Insulin 0.5 ml 5 μg/ml Ascorbic acid 0.5 ml 50 μg/ml Fibroblast Growth Kit Low Serum (ATCC PCS ) Component Volume Final Concentration rh FGF β 0.5 ml 5 ng/ml L-glutamine ml 7.5 mm Ascorbic acid 0.5 ml 50 μg/ml Hydrocortisone Hemisuccinate 0.5 ml 1 μg/ml rh Insulin 0.5 ml 5 μg/ml Fetal Bovine Serum 10.0 ml 2% 6. Tightly cap the bottle of complete growth medium and swirl the contents gently to assure a homogeneous solution. Do not shake forcefully to avoid foaming. Label and date the bottle. 7. Complete growth media should be stored in the dark at 2 C to 8 C (do not freeze). When stored under these conditions, complete growth media is stable for 30 days. Note: Gelatin-Coated Tissue Culture Flasks are needed to culture Mitomycin C Treated Dermal Fibroblasts (ATCC PCS ) in order to achieve optimal cell attachment. Culture flasks should be prepared before thawing cells. Non-coated tissue culture flasks can also be used, if needed. Handling Procedure for Frozen Cells and Initiation of Cultures 1. Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC Primary Human Fibroblasts. 2. Using the total number of viable cells reported, determine how much surface area can be inoculated to achieve an initial seeding density of 2,500 to 5,000 cells per cm 2 for untreated fibroblasts. Note: Mitomycin C treated fibroblasts should be seeded at a density of 20,000 to 40,000 cells per cm 2 for use with stem cells. page 16
19 Fibroblasts 3. Prepare the desired combination of flasks. Add 5mL of complete growth medium per 25 cm 2 of surface area. Place the flasks in a 37 C, 5% CO 2, humidified incubator and allow the media to preequilibrate to temperature and ph for 30 minutes prior to adding cells. 4. While the culture flasks equilibrate, remove one vial of corresponding ATCC Primary Human Fibroblasts from storage and thaw the cells by gentle agitation in a 37 C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 1 to 2 minutes). 5. Remove the vial from the water bath as soon as the contents are thawed, and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point onward should be carried out under strict aseptic conditions. 6. Add the appropriate volume of complete growth media [volume = (1 ml x number of flasks to be seeded) 1 ml] into a sterile conical tube. Using a sterile pipette, transfer the cells from the cryovial to the conical tube. Gently pipette the cells to homogenize the suspension. Do not centrifuge. 7. Transfer 1 ml of the cell suspension to each of the pre-equilibrated culture flasks prepared in steps 1 to 3 of Handling Procedure for Frozen Cells and Initiation of Cultures. Pipette several times, then cap and gently rock each flask to evenly distribute the cells. 8. Place the seeded culture flasks in the incubator at 37 C, 5% CO 2 atmosphere. Incubate at least 24 hours before processing the cells further. Maintenance 1. Before beginning, pre-warm complete growth media in a 37 C water bath. This will take between 10 and 30 minutes, depending on the volume. If using a small volume of medium (50 ml or less), warm only the volume needed in a sterile conical tube. Avoid warming complete growth media multiple times hours after seeding, remove the cells from the incubator and view each flask under the microscope to determine percent cellular confluence. 3. Carefully remove the spent media without disturbing the monolayer. 4. Add 5 ml of fresh, pre-warmed complete growth media per 25 cm 2 of surface area and return the flasks to the incubator. 5. After 24 to 48 hours, view each flask under the microscope to determine percent cellular confluence. If not ready to passage, repeat steps 3 and 4 as described above. When cultures have reached 80% to 100% confluence, and are actively proliferating (many mitotic figures are visible), it is time to subculture. Fibroblasts are not a contact inhibited cell type. Subculture 1. Passage normal human fibroblasts when the cells have reached approximately 80% to 100% confluence and are actively proliferating. Note: Mitomycin C treated fibroblasts are mitotically arrested and cannot be subcultured. 2. Warm both the Trypsin-EDTA for Primary Cells (ATCC PCS ) and the Trypsin Neutralizing Solution (ATCC PCS ) to room temperature prior to dissociation. Warm the complete growth medium to 37 C prior to use with the cells. 3. For each flask, carefully aspirate the spent media without disturbing the monolayer. 4. Rinse the cell layer two times with 3 to 5 ml of D-PBS per 25 cm 2 of surface area (ATCC ) to remove any residual traces of serum. Rinse the cell layer one time with 3 to 5 ml of D-PBS if page 17 [email protected] Phone
20 serum-free culture conditions are used. 5. Add pre-warmed trypsin-edta solution (1 to 2 ml for every 25 cm 2 ) to each flask. Fibroblasts 6. Gently rock each flask to ensure complete coverage of the trypsin-edta solution over the cells, and then aspirate the excess fluid off of the monolayer. 7. Observe the cells under the microscope. When the cells pull away from each other and round up (typically within about 3 to 5 minutes), remove the flask from the microscope and gently tap it from several sides to promote detachment of the cells from the flask surface. 8. When the majority of cells appear to have detached, quickly add to each flask, a volume of the Trypsin Neutralizing Solution (ATCC PCS ) equal to the volume of trypsin-edta solution used previously. Gently pipette or swirl the culture to ensure all of the trypsin-edta solution has been neutralized. 9. Transfer the dissociated cells to a sterile centrifuge tube and set aside while processing any remaining cells in the culture flask. 10. Add 3 to 5 ml D-PBS (ATCC ) to the tissue culture flask to collect any additional cells that might have been left behind. 11. Transfer the cell/d-pbs suspension to the centrifuge tube containing the trypsin-edtadissociated cells. 12. Repeat steps 10 and 11 as needed until all cells have been collected from the flask. 13. Centrifuge the cells at 150 x g for 3 to 5 minutes. 14. Aspirate the neutralized dissociation solution from the cell pellet and resuspend the cells in 2 to 8 ml fresh, pre-warmed, complete growth medium. 15. Count the cells and seed new culture flasks at a density of 2,500 to 5,000 cells per cm Place newly seeded flasks in a 37 C, 5% CO 2 incubator for at least 24 to 48 hours before processing the cells further. Refer to Maintenance for guidelines on feeding. Inoculation of Mitomycin-C-treated Fibroblast Feeder Layers with Co-culture Cells of Interest 1. Aspirate the complete fibroblast growth medium from the feeder cell layer. 2. Add pre-warmed medium appropriate for the cell type of interest (e.g., embryonic stem cell). 3. Return the culture flask to the incubator at 37 C, 5% CO 2. Allow to equilibrate for at least 30 minutes before seeding the cells of interest. For more information regarding the use of feeder cells with stem cell lines, refer to ATCC Human ES/iPS Cell Culture Guide: Protocols for Feeder-free and Feeder-dependent Systems available online at page 18
21 Keratinocytes ATCC Primary Human Keratinocyte Solutions Introduction Keratinocytes are the most common type of skin cells making up the majority of the epidermis. Dividing keratinocytes produce keratin (a protein that provides strength to skin, hair and nails) and migrate to the surface of the skin, the stratum corneum, where they serve as the most abundant cells in the skin s outermost layer 17. Once present in the stratum corneum, keratinocytes differentiate, stop dividing as cornified cells 18 and eventually undergo apoptosis. Keratinocytes can be isolated from different locations of the body. However, the most utilized keratinocytes in primary cell culture have been isolated from the epidermis of human juvenile foreskin or human adult skin, the latter of which is significant in the study of adult diseases such as psoriasis and skin cancer 14. Keratinocytes cultured in serum-free, low calcium growth medium will maintain the cells in an un-differentiated, proliferative state while inhibiting fibroblast outgrowth. Keratinocytes are useful for a number of scientific applications including the study of growth factor behavior, wound healing, toxicity/irritancy studies, and use as target cells for derivation of induced pluripotent stem cells. Cell Culture Protocols Materials Needed Primary cells and complete growth medium Keratinocytes Growth Kit Options* Basal Medium Epidermal Keratinocyte; Normal, Human, Neonatal Foreskin (ATCC PCS ) Epidermal Keratinocyte; Normal, Human, Adult (ATCC PCS ) Reagents for Subculture D-PBS (ATCC ) Trypsin-EDTA for Primary Cells (ATCC PCS ) Trypsin Neutralizing Solution (ATCC PCS ) page 19 Keratinocyte Growth Kit (ATCC PCS ) *Phenol red and antibiotics may be added if desired, and are listed in the appendix. [email protected] Phone Primary Epidermal Keratinocytes; Normal, Human, Adult (ATCC PCS ) Dermal Cell Basal Medium (ATCC PCS ) Preparation of Complete Growth Media 1. Obtain one Keratinocyte Growth Kit from the freezer; make sure that the caps of all components are tight. 2. Thaw the components of the growth kit just prior to adding them to the basal medium. It is necessary to warm the L-glutamine component in a 37 C water bath and shake to dissolve any precipitates prior to adding to the basal medium. 3. Obtain one bottle of Dermal Cell Basal Medium from cold storage. 4. Decontaminate the external surfaces of all growth kit component vials and the basal medium bottle by spraying them with 70% ethanol. 5. Using aseptic technique and working in a laminar flow hood or biosafety cabinet, transfer the indicated volume of each growth kit component, as indicated in the following table, to the bottle of basal medium using a separate sterile pipette for each transfer.
22 Keratinocyte Growth Kit (ATCC PCS ) Component Volume Final Concentration Bovine Pituitary Extract (BPE) 2.0 ml 0.4% rh TGF-α 0.5 ml 0.5 ng/ml L-Glutamine 15.0 ml 6 mm Hydrocortisone Hemisuccinate 0.5 ml 100 ng/ml rh Insulin 0.5 ml 5 μg/ml Epinephrine 0.5 ml 1.0 μm Apo-Transferrin 0.5 ml 5 μg/ml Keratinocytes 6. Tightly cap the bottle of complete growth medium and swirl the contents gently to assure a homogeneous solution. Do not shake forcefully to avoid foaming. Label and date the bottle. 7. Complete growth media should be stored in the dark at 2 C to 8 C (do not freeze). When stored under these conditions, complete growth media is stable for 30 days. Handling Procedure for Frozen Cells and Initiation of Cultures 1. Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC Primary Human Keratinocytes. 2. Using the total number of viable cells, determine how much surface area can be inoculated to achieve an initial seeding density between 2,500 and 5,000 cells per cm Prepare the desired combination of flasks. Add 5 ml of complete growth medium per 25 cm 2 of surface area. Place the flasks in a 37 C, 5% CO 2, humidified incubator and allow the media to preequilibrate to temperature and ph for 30 minutes prior to adding cells. 4. While the culture flasks equilibrate, remove one vial of ATCC Primary Human Keratinocytes from storage and thaw the cells by gentle agitation in a 37 C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 1 to 2 minutes). 5. Remove the vial from the water bath as soon as the contents are thawed, and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point onward should be carried out under strict aseptic conditions. 6. Add the appropriate volume of complete growth medium [volume = (1 ml x number of flasks to be seeded) 1 ml] into a sterile conical tube. Using a sterile pipette, transfer the cells from the cryovial to the conical tube. Gently pipette the cells to homogenize the suspension. Do not centrifuge. 7. Transfer 1.0 ml of the cell suspension to each of the pre-equilibrated culture flasks prepared in steps 1 to 3 of Handling Procedure for Frozen Cells and Initiation of Culture. Pipette several times, then cap and gently rock each flask to evenly distribute the cells. 8. Place the seeded culture flasks in the incubator at 37 C, 5% CO 2 atmosphere. Incubate for at least 24 hours before processing the cells further. Maintenance 1. Before beginning, pre-warm complete growth media in a 37 C water bath. This will take between 10 and 30 minutes, depending on the volume. If using a small volume of medium (50 ml or less), warm only the volume needed in a sterile conical tube. Avoid warming complete growth media page 20
23 Keratinocytes multiple times hours after seeding, remove the cells from the incubator and view each flask under the microscope to determine percent cellular confluence. 3. Carefully remove the spent media without disturbing the monolayer. 4. Add 5 ml of fresh, pre-warmed complete growth medium per 25 cm 2 of surface area and return the flasks to the incubator. 5. After 24 to 48 hours, view each flask under the microscope to determine percent cellular confluence. If not ready to passage, repeat steps 3 and 4 as described above. When cultures have reached approximately 80% confluence, and are actively proliferating (many mitotic figures are visible), it is time to subculture. Keratinocytes will begin to terminally differentiate once they become 100% confluent. Subculture 1. Passage normal keratinocytes when the culture has reached approximately 70% to 80% confluence. 2. Warm both the Trypsin-EDTA for Primary Cells (ATCC PCS ) and the Trypsin Neutralizing Solution (ATCC PCS ) to room temperature prior to dissociation. Warm the complete growth medium to 37 C prior to use with the cells. 3. For each flask, carefully aspirate the spent media without disturbing the monolayer. 4. Rinse the cell layer one time with 3 to 5 ml D-PBS (ATCC ) to remove residual medium. 5. Add pre-warmed trypsin-edta solution (1 to 2 ml for every 25 cm 2 ) to each flask. 6. Gently rock each flask to ensure complete coverage of the trypsin-edta solution over the cells, and then aspirate the excess fluid off of the monolayer. 7. Observe the cells under the microscope. When the cells pull away from each other and round up (typically within 3 to 6 minutes), remove the flask from the microscope and gently tap it from several sides to promote detachment of the cells from the flask surface. Note: If cells are difficult to detach, incubate each flask containing cells and the trypsin-edta solution at 37 C to facilitate dispersal. 8. When the majority of cells appear to have detached, quickly add an equal volume of the Trypsin Neutralizing Solution (ATCC PCS ) to each flask. Gently pipette or swirl the culture to ensure all of the trypsin-edta solution has been neutralized. 9. Transfer the dissociated cells to a sterile centrifuge tube and set aside while processing any remaining cells in the culture flask. 10. Add 3 to 5 ml D-PBS (ATCC ) to the tissue culture flask to collect any additional cells that might have been left behind. 11. Transfer the cell/d-pbs suspension to the centrifuge tube containing the trypsin-edtadissociated cells. 12. Repeat steps 10 and 11 as needed until all cells have been collected from the flask. 13. Centrifuge the cells at 150 x g for 3 to 5 minutes. 14. Aspirate neutralized dissociation solution from the cell pellet and resuspend the cells in 2 to 8 ml fresh, pre-warmed, complete growth medium. 15. Count the cells and seed new culture flasks at a density of 2,500 to 5,000 cells per cm Place newly seeded flasks in a 37 C, 5% CO 2 incubator for at least 24 to 48 hours before processing the cells further. Refer to Maintenance for guidelines on feeding. page 21 [email protected] Phone
24 ATCC Primary Human Melanocyte Solutions Introduction Melanocytes are found mainly in the epidermis, but may occur elsewhere in the body such as in the matrix of the hair. They are specialized skin cells that produce the pigment melanin, which gives skin its color and protects it from the hazardous effects of UV radiation. The most useful melanocytes for research are isolated from the epidermis of human juvenile foreskin or human adult skin. Special care must be taken to prevent contamination of melanocyte cultures with keratinocytes or fibroblasts found in the epidermis. Lowering the concentration of calcium in the medium to 60 μm and selecting for adherent melanocytes in hormone-supplemented Melanocytes medium aids in the isolation of melanocytes from epidermal tissue 14. Melanocytes are frequently used in the in vitro study of wound healing, and as testing models for toxicity/irritancy studies, melanoma, dermal response to UV radiation, psoriasis and other skin diseases, and cosmetic research (e.g., skin lightening compounds, skin protecting compounds). Cell Culture Protocols Materials Needed Primary cells and complete growth medium Melanocytes Growth Kit Options Basal Medium Epidermal Melanocytes; Normal, Human, Neonatal (ATCC PCS ) Epidermal Melanocytes; Normal, Human, Adult (ATCC PCS ) Reagents for Subculture D-PBS (ATCC ) Trypsin-EDTA for Primary Cells (ATCC PCS ) Trypsin Neutralizing Solution (ATCC PCS ) Melanocyte Growth Kit (ATCC PCS ) Adult Melanocyte Growth Kit (ATCC PCS ) *Phenol red and antibiotics may be added if desired, and are listed in the appendix. Adult human melanocytes (ATCC PCS ). Dermal Cell Basal Medium (ATCC PCS ) Preparation of Complete Growth Media 1. Obtain one Melanocyte Growth Kit (corresponding to the melanocyte being used) from the freezer; make sure that the caps of all components are tight. 2. Thaw the components of the growth kit just prior to adding them to the basal medium. It is necessary to warm the L-glutamine component in a 37 C water bath and shake to dissolve any precipitates prior to adding to the basal medium. 3. Obtain one bottle of Dermal Cell Basal Medium from cold storage. 4. Decontaminate the external surfaces of all growth kit component vials and the basal medium bottle by spraying them with 70% ethanol. 5. Using aseptic technique and working in a laminar flow hood or biosafety cabinet, transfer the indicated volume of each growth kit component, as indicated in the following table, to the bottle of basal medium using a separate sterile pipette for each transfer. page 22
25 Melanocytes Melanocyte Growth Kit (ATCC PCS ) Component Volume Final Concentration rh Insulin 0.5 ml 5 μg/ml Ascorbic Acid 0.5 ml 50 μg/ml L-Glutamine 15.0 ml 6 mm Epinephrine 0.5 ml 1.0 μm Calcium Chloride 80 μl 0.2 mm M8 Supplement 5 ml Proprietary formulation 6. Tightly cap the bottle of complete growth medium and swirl the contents gently to assure a homogeneous solution. Do not shake forcefully to avoid foaming. Label and date the bottle. 7. Complete growth media should be stored in the dark at 2 C to 8 C (do not freeze). When stored under these conditions, complete growth media is stable for 30 days. Handling Procedure for Frozen Cells and Initiation of Cultures 1. Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC Primary Human Melanocytes. 2. Using the total number of viable cells, determine how much surface area can be inoculated to achieve an initial seeding density of 5,000 cells per cm Prepare the desired combination of flasks. Add 5 ml of complete growth medium per 25 cm 2 of surface area. Place the flasks in a 37 C, 5% CO 2, humidified incubator and allow the media to preequilibrate to temperature and ph for 30 minutes prior to adding cells. 4. While the culture flasks equilibrate, remove one vial of ATCC Primary Human Melanocytes from storage and thaw the cells by gentle agitation in a 37 C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 1 to 2 minutes). 5. Remove the vial from the water bath as soon as the contents are thawed, and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point onward should be carried out under strict aseptic conditions. 6. Add the appropriate volume of complete growth medium [volume = (1 ml x number of flasks to be seeded) 1 ml] into a sterile conical tube. Using a sterile pipette, transfer the cells from the cryovial to the conical tube. Gently pipette the cells to homogenize the suspension. Do not centrifuge. 7. Transfer 1.0 ml of the cell suspension to each of the pre-equilibrated culture flasks prepared in steps 1 to 3 of Handling Procedure for Frozen Cells and Initiation of Culture. Pipette several times, then cap and gently rock each flask to evenly distribute the cells. 8. Place the seeded culture flasks in the incubator at 37 C, 5% CO 2 atmosphere. Incubate for at least 24 hours before processing the cells further. Maintenance 1. Before beginning, pre-warm complete growth media in a 37 C water bath. This will take between 10 and 30 minutes, depending on the volume. If using a small volume of medium (50 ml or less), warm only the volume needed in a sterile conical tube. Avoid warming complete growth media multiple times. page 23 [email protected] Phone
26 Melanocytes to 36 hours after seeding, remove the cells from the incubator and view each flask under the microscope to determine percent cellular confluence. 3. Carefully remove the spent media without disturbing the monolayer. 4. Add 5 ml of fresh, pre-warmed complete growth medium per 25 cm 2 of surface area and return the flasks to the incubator. 5. After 24 to 48 hours, view each flask under the microscope to determine percent cellular confluence. If not ready to passage, repeat steps 3 and 4 as described above. When cultures have reached 80% to 90% confluence, and are actively proliferating (many mitotic figures are visible), it is time to subculture. Melanocytes are not contact inhibited; however, they will proliferate best if the cells are passaged prior to 100% confluence. Melanocytes from lightly pigmented tissue should reach 80% confluence in 7 to 9 days. Subculture 1. Passage normal melanocytes when the culture has reached approximately 80% to 90% confluence. 2. Warm both the Trypsin-EDTA for Primary Cells (ATCC PCS ) and the Trypsin Neutralizing Solution (ATCC PCS ) to room temperature prior to dissociation. Warm the complete growth medium to 37 C prior to use with the cells. 3. For each flask, carefully aspirate the spent media without disturbing the monolayer. 4. Rinse the cell layer two times with 3 to 5 ml D-PBS (ATCC ) to remove residual medium. 5. Add pre-warmed trypsin-edta solution (1 to 2 ml for every 25 cm 2 ) to each flask. 6. Gently rock each flask to ensure complete coverage of the trypsin-edta solution over the cells, and then aspirate the excess fluid off of the monolayer. 7. Observe the cells under the microscope. When the cells pull away from each other and round up (typically within 1 to 3 minutes), remove the flask from the microscope and gently tap it from several sides to promote detachment of the cells from the flask surface. Note: Melanocytes are sensitive to overtrypsinization. 8. When the majority of cells appear to have detached, quickly add an equal volume of the Trypsin Neutralizing Solution (ATCC PCS ) to each flask. Gently pipette or swirl the culture to ensure all of the trypsin-edta solution has been neutralized. 9. Transfer the dissociated cells to a sterile centrifuge tube and set aside while processing any remaining cells in the culture flask. 10. Add 3 to 5 ml D-PBS (ATCC ) to the tissue culture flask to collect any additional cells that might have been left behind. 11. Transfer the cell/d-pbs suspension to the centrifuge tube containing the trypsin-edtadissociated cells. 12. Repeat steps 10 and 11 as needed until all cells have been collected from the flask. 13. Centrifuge the cells at 150 x g for 3 to 5 minutes. 14. Aspirate neutralized dissociation solution from the cell pellet and resuspend the cells in 2 to 8 ml fresh, pre-warmed, complete growth medium. 15. Count the cells and seed new culture flasks at a density of 2,500 to 5,000 cells per cm Place newly seeded flasks in a 37 C, 5% CO 2 incubator for at least 24 to 48 hours before processing the cells further. Refer to Maintenance for guidelines on feeding. page 24
27 Human Mesenchymal Stem Cells ATCC Human Mesenchymal Stem Cell and Differentiation Solutions Introduction ATCC Human Mesenchymal Stem Cells (MSCs) are self-renewing multipotent adult stem cells. MSCs are traditionally found in the bone marrow, but can also be isolated from other tissues including adipose, human umbilical cord or cord blood, and peripheral blood. Adipose-derived mesenchymal stem cells are isolated from human adipose (fat) tissues by lipoaspiration or biopsy. Umbilical cord derived mesenchymal stem cells are isolated from Wharton s Jelly found in human umbilical cord. Although MSCs from primary adipose tissue and Wharton s Jelly are considered to be relatively easy to obtain, isolation of consistent stem cell populations from Adipose-Derived Mesenchymal Stem Cells, Normal, Human (ATCC PCS ). such material is costly and time-consuming. Lipoaspirates and umbilical cord tissue represent a heterogeneous mixture of cell types, including adipocytes, endothelial cells, smooth muscle, pericytes and progenitor cells 2. The inherent heterogeneity of this source material, then, yields a high potential for cellular contamination to predominate cultures. To ensure the purity of ATCC Primary Cell Solutions Normal Human Mesenchymal Stem Cells, each batch is isolated from single-donor tissue, cryopreserved in the second passage, and tested for: 1. Authentication of growth and morphology, including adherence to plastic when cultured in optimized growth medium consisting of Mesenchymal Stem Cell Basal Medium (ATCC PCS ) supplemented with Mesenchymal Stem Cell Growth Kit-Low Serum (ATCC PCS ) 2. Verification of surface antigen expression 13, including a total of 10 markers: Positive ( 95%) for CD29, CD44, CD73, CD90, CD105, and CD166; and, negative ( 2%) for CD14, CD31, CD34, and CD45 3. Confirmation of multi-lineage differentiation into osteoblasts, adipocytes, and chondrocytes using optimized differentiation kits and protocols MSCs are useful tools for non-controversial stem cell differentiation research and for the creation of induced pluripotent stem (ips) cell lines 8. MSCs have also found applications in tissue engineering, 3,14,5,11, cell therapy 12, and regenerative medicine 6,7,9, % Percent Cells Positive 100% 80% 60% 40% 20% 0% CD29 CD44 CD73 CD90 CD105 CD166 CD14 CD31 CD34 CD45 Surface Antigen ATCC Primary Cell Solutions adipose-derived mesenchymal stem cells were taken from liquid nitrogen and cultures initiated. A sample for analysis by flow cytometry was taken when the culture was initiated and then after 48-hours of growth. The cells must test positive for CD29, CD44, CD73, CD90, CD105, and CD166 (greater than 95% of the cell population expresses these markers by flow cytometry). The cells must test negative for CD14, CD31, CD34, and CD45 (less than 2% of cell population expresses these markers by flow cytometry). Fresh from Cryo 48 Hours Growth page 25 [email protected] Phone
28 Human Mesenchymal Stem Cells Cell Culture Protocols Materials Needed Primary cells and complete growth medium Mesenchymal Stem Cells Growth Kit Options Basal Medium Adipose-Derived Mesenchymal Stem Cells, Normal, Human (ATCC PCS ) Umbilical Cord-Derived Mesenchymal Stem Cells; Normal, Human (ATCC PCS ) Reagents for Subculture D-PBS (ATCC ) Trypsin-EDTA for Primary Cells (ATCC PCS ) Trypsin Neutralizing Solution (ATCC PCS ) Mesenchymal Stem Cell Growth Kit-Low Serum(ATCC PCS ) Mesenchymal Stem Cell Basal Medium (ATCC PCS ) Preparation of Complete Growth Media 1. Obtain one Mesenchymal Stem Cell Growth Kit Low Serum from the freezer; make sure that the caps of all components are tight. 2. Thaw the components of the growth kit just prior to adding them to the basal medium. 3. Obtain one bottle of Mesenchymal Stem Cell Basal Medium from cold storage. 4. Decontaminate the external surfaces of all growth kit component vials and the basal medium bottle by spraying them with 70% ethanol. 5. Using aseptic technique and working in a laminar flow hood or biosafety cabinet, transfer the indicated volume of each growth kit component, as indicated in the following table, to the bottle of basal medium using a separate sterile pipette for each transfer. Mesenchymal Stem Cell Growth Kit Low Serum (ATCC PCS ) Component Volume Final Concentration MSC Supplement 10 ml 2% FBS 5 ng/ml rh FGF basic 5 ng/ml rh FGF acidic 5 ng/ml rh EGF L-Alanyl-L-Glutamine 6 ml 2.4 mm 6. Tightly cap the bottle of complete growth medium and swirl the contents gently to assure a homogeneous solution. Do not shake forcefully to avoid foaming. Label and date the bottle. 7. Complete growth media should be stored in the dark at 2 C to 8 C (do not freeze). When stored under these conditions, complete growth media is stable for two weeks. Handling Procedure for Frozen Cells and Initiation of Cultures 1. Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC Human Mesenchymal Stem Cells. 2. Using the total number of viable cells, determine how much surface area can be inoculated to achieve an initial seeding density of 5,000 cells per cm Prepare the desired combination of flasks. Add 5 ml of complete growth medium per 25 cm 2 of page 26
29 Human Mesenchymal Stem Cells surface area. Place the flasks in a 37 C, 5% CO 2, humidified incubator and allow the media to preequilibrate to temperature and ph for 30 minutes prior to adding cells. 4. While the culture flasks equilibrate, remove one vial of ATCC Human Mesenchymal Stem Cells from storage and thaw the cells by gentle agitation in a 37 C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 1 to 2 minutes). 5. Remove the vial from the water bath as soon as the contents are thawed, and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point onward should be carried out under strict aseptic conditions. 6. Add the appropriate volume of complete growth medium [volume = (1 ml x number of flasks to be seeded) 1 ml] into a sterile conical tube. Using a sterile pipette, transfer the cells from the cryovial to the conical tube. Gently pipette the cells to homogenize the suspension. Do not centrifuge. 7. Transfer 1.0 ml of the cell suspension to each of the pre-equilibrated culture flasks prepared in steps 1 to 3 of Handling Procedure for Frozen Cells and Initiation of Culture. Pipette several times, then cap and gently rock each flask to evenly distribute the cells. 8. Place the seeded culture flasks in the incubator at 37 C, 5% CO 2 atmosphere. Incubate for at least 24 hours before processing the cells further. Maintenance 1. Before beginning, pre-warm complete growth media in a 37 C water bath. This will take between 10 and 30 minutes, depending on the volume. If using a small volume of medium (50 ml or less), warm only the volume needed in a sterile conical tube. Avoid warming complete growth media multiple times hours after seeding, remove the cells from the incubator and view each flask under the microscope to determine percent cellular confluence. 3. Carefully remove the spent media without disturbing the monolayer. 4. Add 5 ml of fresh, pre-warmed complete growth medium per 25 cm 2 of surface area and return the flasks to the incubator. 5. After 24 to 48 hours, view each flask under the microscope to determine percent cellular confluence. If not ready to passage, repeat steps 3 and 4 as described above. When cultures have reached approximately 70% to 80% confluence, and are actively proliferating (many mitotic figures are visible), it is time to subculture. Subculture 1. Passage normal mesenchymal stem cells when the culture has reached approximately 70% to 80% confluence. 2. Warm both the Trypsin-EDTA for Primary Cells (ATCC PCS ) and the Trypsin Neutralizing Solution (ATCC PCS ) to room temperature prior to dissociation. Warm the complete growth medium to 37 C prior to use with the cells. 3. For each flask, carefully aspirate the spent media without disturbing the monolayer. 4. Rinse the cell layer one time with 3 to 5 ml D-PBS (ATCC ) to remove residual medium. 5. Add pre-warmed trypsin-edta solution (1 to 2 ml for every 25 cm 2 ) to each flask. 6. Gently rock each flask to ensure complete coverage of the trypsin-edta solution over the cells, page 27 [email protected] Phone Note: Adipose- and umbilical cordderived stem cells are contact inhibited. It is essential that the cells be subcultured BEFORE reaching confluence as postconfluent cells exhibit changes in morphology, slower proliferation, and reduced differentiation capacity after passaging.
30 and then aspirate the excess fluid off of the monolayer. Human Mesenchymal Stem Cell Differentiation 7. Observe the cells under the microscope. When the cells pull away from each other and round up (typically within 1 to 3 minutes), remove the flask from the microscope and gently tap it from several sides to promote detachment of the cells from the flask surface. 8. When the majority of cells appear to have detached, quickly add an equal volume of the Trypsin Neutralizing Solution (ATCC PCS ) to each flask. Gently pipette or swirl the culture to ensure all of the trypsin-edta solution has been neutralized. 9. Transfer the dissociated cells to a sterile centrifuge tube and set aside while processing any remaining cells in the culture flask. 10. Add 3 to 5 ml D-PBS (ATCC ) to the tissue culture flask to collect any additional cells that might have been left behind. 11. Transfer the cell/d-pbs suspension to the centrifuge tube containing the trypsin-edtadissociated cells. 12. Repeat steps 10 and 11 as needed until all cells have been collected from the flask. 13. Centrifuge the cells at 150 x g for 3 to 5 minutes. 14. Aspirate neutralized dissociation solution from the cell pellet and resuspend the cells in 2 to 8 ml fresh, pre-warmed, complete growth medium. 15. Count the cells and seed new culture flasks at a density of 5,000 viable cells per cm Place newly seeded flasks in a 37 C, 5% CO 2 incubator for at least 24 to 48 hours before processing the cells further. Refer to Maintenance for guidelines on feeding. Adipose-derived Mesenchymal Stem Cell Differentiation Protocols Adipocyte Differentiation Materials Needed The Adipocyte Differentiation Toolkit (ATCC PCS ) contains medium and reagents designed to induce adipogenesis in actively proliferating Adipose-Derived Mesenchymal Stem Cells (ATCC PCS ) with high efficiency, and support maturation of derived adipocytes during lipid accumulation. Preparing Cells for Adipocyte Differentiation 1. Follow the instructions for the growth of Adipose-Derived Mesenchymal Stem Cells (ATCC PCS ). It is recommended that the cells not be passaged more than four (4) times before initiating adipocyte differentiation. 2. When cells are 70-80% confluent, passage them into a tissue culture plate at a density of 18,000 cells/cm 2. Adjust the number of cells and volume of media according to the tissue culture plate used. Example: For a 6-well tissue culture plate with a surface area of 9.5 cm 2 /well, add a total of 171,000 viable cells to each well containing 2 ml of Mesenchymal Stem Cell Basal Medium (ATCC PCS ) supplemented with Mesenchymal Stem Cell Growth Kit Low Serum (ATCC PCS ) components. 3. Gently rock the plate back and forth and side to side to evenly distribute cells before incubation. Do not swirl. 4. Incubate the cells at 37 C with 5% CO 2 for 48 hours before initiating adipocyte differentiation. Adipocyte Differentiation Media Preparation The adipocyte differentiation process requires two separate media preparations: one for initiation and page 28
31 Human Mesenchymal Stem Cell Differentiation one for maintenance. Stock solutions of these media can be prepared in tandem in advance as follows: 1. Thaw all three components of the differentiation kit and warm to 37 C in a water bath. 2. Decontaminate the external surfaces of all three kit components by spraying them with 70% ethanol. 3. Using aseptic technique and working in a laminar flow hood or biosafety cabinet: a. Transfer 15 ml of Adipocyte Basal Medium and 1 ml of AD Supplement to a sterile 50 ml conical tube, using a separate sterile pipette for each transfer. This is your working stock of Adipocyte Differentiation Initiation Medium used during the first 96 hours of differentiation. b. Add 5 ml of ADM Supplement to the remaining 85 ml of Adipocyte Basal Medium. This is your working stock of Adipocyte Differentiation Maintenance Medium. 4. Tightly cap the each container of media and swirl the contents gently to assure a homogeneous solution. Do not shake forcefully to avoid foaming. Label and date the bottle. 5. Each container of differentiation medium should be stored in the dark at 2 C to 8 C (do not freeze). When stored under these conditions, the differentiation media is stable for up to three weeks. Adipocyte Differentiation Procedure A. Initiation Phase 1. After incubating the prepared Adipose-Derived Mesenchymal Stem Cells for (as described above), carefully aspirate the media from the wells. 2. Immediately rinse the cells once by adding 2 ml of room-temperature D-PBS (ATCC ) to each well, then carefully aspirate the PBS from the wells. 3. Add 2 ml of pre-warmed (37 C) Adipocyte Differentiation Initiation Medium to each well to begin the adipocyte differentiation process. 4. Incubate the cells at 37 C with 5% CO 2 for 48 hours. 5. Feed the cells by carefully removing half the volume of media (1 ml) from each well and adding another 2 ml of pre-warmed (37 C) Adipocyte Differentiation Initiation Medium to each well. Important: DO NOT TILT plate during aspiration. It is important that the cell monolayer is not exposed to air during this and subsequent steps to ensure that developing lipid vesicles do not burst. B. Maintenance Phase 6. Incubate the cells at 37 C with 5% CO 2 for 48 hours. 7. Carefully remove 2 ml of media from each well (leaving 1 ml) and replace with 2 ml of pre-warmed (37 C) Adipocyte Differentiation Maintenance Medium in each well. Important: DO NOT TILT plate during aspiration. It is important that the cell monolayer is not exposed to air during this and subsequent steps to ensure that page 29 [email protected] Phone Note: It may be necessary to shake the AD Supplement and the ADM Supplement upon warming to help re-dissolve any components that may have precipitated out of solution upon freezing. Note: It is recommended that you transfer the required volume of media to a sterile tube for prewarming prior to each feeding rather than repeatedly re-warming the entire working stock. Adipocytes differentiated from Adiposederived Mesenchymal Stem Cells (PCS ) and stained with Oil Red O to detect the formation of lipid droplets.
32 Human Mesenchymal Stem Cell Differentiation developing lipid vesicles do not burst. 8. Repeat Steps 6 and 7 every 3-4 days for another 11 days until adipocytes reach full maturity. (Full maturity will be reached 15 days after the beginning of initiation phase, or 17 days from initial plating of cells.) 9. Cells can be used at any phase of adipocyte differentiation as predicated upon experimental design. To confirm lipid accumulation, cells can be fixed and stained with Oil Red O. Additional differentiation protocols, including Chondrocyte and Osteocyte differentiation of Adipose-derived Mesenchymal Stem Cells, are available online at ATCC Adipose-Derived Mesenchymal Stem Cells were expanded in Complete Growth Medium and then induced to differentiate to chondrocytes using the Chondrocyte Differentiation Tool. Passage 3 cells, day 21 following differentiation (100X magnification), stained with Alcian Blue. page 30
33 References 1. Stein GS, et. al. Human Stem Cell Technology and Biology: A Research Guide and Laboratory Manual. Wiley & Sons, Hoboken, New Jersey, Zuk PA, et al. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 7(2): ), Zuk PA, et al. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 13: , Ogawa R. The Importance of Adipose-Derived Stem Cell and Vascularized Tissue Regeneration in the Field of Tissue Transplantation. Cur. Stem Cell Res. 1:13-20, Shaeffler A & Buechler C. Concise review: adipose tissue-derived stromal cells basic and clinical applications for novel cell-based therapies. Stem Cells 25: , Kang SK, et al. Neurogenesis of Rhesus adipose stromal cells. J. Cell Sci. 117: , Safford KM, et al. Stem cell therepy for neurologic disorders: Therapeutic potential of adiposederived stem cells. Current Drug Targets 6:57-62, Safford KM, et al. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp. Neurol. 187: , Kingham PJ, et al. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 207: , Xu, et al. Myelin-forming ability of Schwann cell-like cells induced from rat adipose-derived stem cells in vitro. Brain Res. 1239:49-55, Seo MJ, et al. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem. Biophys. Res. Commun. 328: , Timper K, et al. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem. Biophys. Res. Commun. 341: , Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy 8(4): , Freshney, RI. Culture of Animal Cells: A Manual of Basic Technique, 6th Ed. John Wiley & Sons, Hoboken, New Jersey, Histology Text Atlas Book. Chapter 3: Connective Tissue. Downloaded from Visual Histology.com at on March 26, Elias JA, et. al. Regulation of Human Lung Fibroblast Glycosaminoglycan Production by Recombinant Interferons, Tumor Necrosis Factor, and Lymphotoxin. J. Clin. Invest. 81: , Rieger MM. Keratinocyte function and skin health. Cosmetics and Toiletries, July 01, Downloaded from the WWW on March 26, 2012 from Access My Library at accessmylibrary.com/article/print/1g Bensouilah J, et. al. Aromadermatology: Aromatherapy in the Treatment and Care of Common Skin Conditions. Blackwell Publishers, Chapter 1 downloaded from the WWW on March 26, 2012 at Have Questions? Search ATCC Frequently Asked Questions online at for answers related to primary cells, or contact ATCC Technical Services at [email protected] for additional information. page 31 [email protected] Phone
34 Cell Type Endothelial Cells Smooth Muscle Cells Epithelial Cells Fibroblasts Product Name ATCC No. Species Umbilical Vein Endothelial Cells; Normal, Human Umbilical Vein Endothelial Cells; Normal, Human, Pooled Number of viable cells-post thaw Passage at freezing Cells tested upon thaw to achieve PCS Human 5 x PDL PCS Human 5 x PDL Aortic Endothelial Cells; Normal, Human PCS Human 5 x PDL Coronary Artery Endothelial Cells; Normal, Human Pulmonary Artery Endothelial Cells; Normal, Human Dermal Microvascular Endothelial Cells; Normal, Human, Neonatal Aortic Smooth Muscle Cells, Normal, Human Coronary Artery Smooth Muscle Cells, Normal, Human Pulmonary Artery Smooth Muscle Cells, Normal, Human Small Airway Epithelial Cells; Normal, Human Bronchial/Tracheal Epithelial Cells; Normal, Human Renal Proximal Tubule Epithelial Cells; Normal, Human Renal Cortical Epithelial Cells; Normal, Human Renal Mixed Epithelial Cells; Normal, Human PCS Human 5 x PDL PCS Human 5 x PDL PCS Human 5 x PDL PCS Human 5 x PDL PCS Human 5 x PDL PCS Human 5 x PDL Basal media Growth kit Reagents and supplements Additional Reagents Applications Vascular Cell Basal Medium (ATCC PCS ) Vascular Cell Basal Medium (ATCC PCS ) Vascular Cell Basal Medium (ATCC PCS ) PCS Human 5 x PDL Airway Epithelial Cell Basal Medium (ATCC PCS Human 5 x PDL PCS ) PCS Human 5 x PDL PCS Human 5 x PDL PCS Human 5 x PDL Prostate Epithelial Cells; Normal, Human PCS Human 5 x PDL Corneal Epithelial Cells; Normal, Human PCS Human 5 x passages Mammary Epithelial Cells; Normal, Human PCS Human 5 x 10⁵ 2 15 PDL Dermal Fibroblasts, Normal, Human Neonatal Dermal Fibroblasts, Normal, Human Neonatal, Mitomicin C treated PCS Human 5 x PCS Human 3 x Dermal Fibroblasts, Normal, Human Adult PCS Human 5 x Renal Epithelial Cell Basal Medium (ATCC PCS ) Prostate Epithelial Cell Basal Medium (ATCC PCS ) Corneal Epithelial Cell Basal Medium (ATCC PCS ) Mammary Epithelial Cell Medium (ATCC No. PCS ) 10 PDL in serum-free medium No growth or divison beyond 4 weeks 10 PDL in serum-free medium Fibroblast Basal Medium (ATCC PCS ) Endothelial Cell Growth Kit-BBE (ATCC PCS ) or Endothelial Cell Growth Kit-VEGF (ATCC PCS ) Phenol Red (ATCC PCS ); D-PBS (ATCC ); Trypsin-EDTA for Primary Cells (ATCC PCS ); Trypsin Neutralizing Solution (ATCC PCS ) Microvascular Endothelial Cell Growth Kit-BBE (ATCC PCS ) or Microvascular Endothelial Cell Growth Kit-VEGF (ATCC PCS ) Vascular Smooth Muscle Cell Growth Kit (ATCC PCS ) Small Airway Epithelial Cell Growth Kit (ATCC PCS ) or Bronchial Epithelial Cell Growth kit (ATCC PCS ) Renal Epithelial Cell Growth Kit (ATCC PCS ) Prostate Epithelial Cell Growth Kit (ATCC PCS ) Corneal Epithelial Cell Growth Kit (ATCC PCS ) Mammary Epithelial Cell Growth Kit (ATCC No. PCS ) Fibroblast Growth Kit Serum-Free (ATCC PCS ) or Fibroblast Growth Kit Low Serum (ATCC PCS ) Phenol Red (ATCC PCS ); D-PBS (ATCC ); Trypsin-EDTA for Primary Cells (ATCC PCS ); Trypsin Neutralizing Solution (ATCC PCS ) Phenol Red (ATCC PCS ); D-PBS (ATCC ); Trypsin-EDTA for Primary Cells (ATCC PCS ); Trypsin Neutralizing Solution (ATCC PCS ) Phenol Red (ATCC PCS ); D-PBS (ATCC ); Trypsin-EDTA for Primary Cells (ATCC PCS ); Trypsin Neutralizing Solution (ATCC PCS ) NA NA NA 0.1% Gelatin Solution (ATCC PCS ) only for use with Mitomicin C treated Dermal Fibroblasts Physiological and pharmacological investigations, such as macromolecule transport, blood coagulation, angiogenesis, and fibrinolysis. Studies of vascular diseases such as thrombosis, atherosclerosis, metabolism, and hypertension; stent-graft compatibility testing; membrane conductance models. Studies of microvascular functions and cutaneous inflammation Studies of vascular diseases such as thrombosis, and atherosclerosis Asthma, airway inflammation, and would healing, pulmonary fibrosis, COPD, Cancer, Toxicology, intracellular ph regulations, IL-1b effect to stimulate airway epithelial cell growth, ICAM-1 expression. In vitro studies of osmoregualtion and excretion, renal fibrosis, inflammation, as well as applications in drug screening/ development, e.g. hypertension, diabetes, oncology, autoimmune disease, and toxicology screening. Hormonal regulation of the prostate, the secretory function of prostate cells, and prostate cancer Cell de-differentiation, toxicology testing and drug development. A normal in vitro control, useful for studying stages of breast cancer development, three dimensional culture and carcinogen screening Wound healing studies, tissue engineering and regeneration applications, as well as induction of pluripotent stem (ipscs). Feeder cells for use with human stem cells and keratinocytes Wound healing studies, tissue engineering and regeneration applications, as well as induction of pluripotent stem (ipscs). Keratinocytes Epidermal Keratinocytes, Normal, Human, Neonatal Foreskin Epidermal Keratinocytes, Normal, Human, Adult PCS Human 5 x PDL PCS Human 5 x PDL Dermal Cell Basal Medium (ATCC PCS ) Keratinocyte Growth Kit (ATCC PCS ) Phenol Red (ATCC PCS ); D-PBS (ATCC ); Trypsin-EDTA for Primary Cells (ATCC PCS ); Trypsin Neutralizing Solution (ATCC PCS ) NA Studies of growth factor activity, wound healing, toxicity/irritancy studies, and use as target cells for derivation of induced pluripotent stem cells Melanocytes Epidermal Melanocytes, Normal, Human, Neonatal Foreskin Epidermal Melanocytes, Normal, Human, Adult PCS Human 5 x PDL PCS Human 5 x PDL Dermal Cell Basal Medium (ATCC PCS ) Melanocyte Growth Kit (ATCC PCS ) Phenol Red (ATCC PCS ); D-PBS (ATCC ); Trypsin-EDTA for Primary Cells (ATCC PCS ); Trypsin Neutralizing Solution (ATCC PCS ) NA Wound healing, testing models for toxicity/irritancy studies, melanoma, dermal response to UV radiation, psoriasis and other skin diseases, and cosmetic research Mesenchymal Stem Cells Umbilical Cord-Derived Mesenchymal Stem Cells, Normal, Human Adipose-Derived Mesenchymal Stem Cells, Normal, Human Bone Marrow-Derived Mesenchymal Stem Cells; Normal, Human Primary Subcutaneous Preadipocyte; Normal, Human PCS Human 5 x PDL Adipocyte Differentiation Tool (ATCC PCS ) Mesenchymal Stem Cell Growth Kit- Chondrocyte Differentiation Tool Mesenchymal Stem Cell Low Serum (ATCC PCS ) Phenol Red (ATCC PCS ); D-PBS (ATCC (ATCC PCS ) ); Trypsin-EDTA for Primary Cells (ATCC PCS Human 1 x PDL Basal Medium (ATCC PCS ); Trypsin Neutralizing Solution Osteocyte Differentiation Tool PCS ) (ATCC PCS ) (ATCC PCS ) PCS Coming soon Human 1 x 10⁶ 2 10 PDL PCS Human 1 x 10⁶ 2 15 PDL Fibroblast Basal Medium (ATCC No. PCS ) Mesenchymal Stem Cell Growth Kit for Bone Marrow MSCs (ATCC No. PCS ) Coming soon Fibroblast Growth Kit Low Serum (ATCC No. PCS ) D-PBS (ATCC No ); Trypsin-EDTA for Primary Cells (ATCC No. PCS ); Trypsin Neutralizing Solution (ATCC No. PCS ) Adipocyte Differentiation Toolkit for BM-MSCs (ATCC No. PCS ) Coming soon Adipocyte Differentiation Tool (ATCC No. PCS ) Adult stem cell differentiation research, induced pluripotent stem cell lines, tissue engineering, cell therapy, and regenerative medicine Useful as an in vitro model for the study of multipotent stem cell biology, differentiation, and regenerative medicine and tissue engineering Differentiation research, tissue engineering, cell therapy, and regenerative medicine
35 10801 University Blvd. Manassas, VA Tel Fax Web Or contact your local distributor PC American Type Culture Collection. The ATCC trademark and trade name, any and all ATCC catalog numbers and any other trademarks listed in this publication are trademarks of American Type Culture Collection unless indicated otherwise. Vi-Cell is a registered trademark of Beckman Coulter. The products in this publication are intended for research use only.
Follicle Dermal Papilla Cell
 Follicle Dermal Papilla Cell Instruction Manual Product Size Catalog Number Human Follicle Dermal Papilla Cells (HFDPC) 500,000 cryopreserved cells 500,000 proliferating cells C-12071 C-12072 Product Description
Follicle Dermal Papilla Cell Instruction Manual Product Size Catalog Number Human Follicle Dermal Papilla Cells (HFDPC) 500,000 cryopreserved cells 500,000 proliferating cells C-12071 C-12072 Product Description
Human Adult Mesothelial Cell Manual
 Human Adult Mesothelial Cell Manual INSTRUCTION MANUAL ZBM0025.01 SHIPPING CONDITIONS Human Adult Mesothelial Cells Orders are delivered via Federal Express courier. All US and Canada orders are shipped
Human Adult Mesothelial Cell Manual INSTRUCTION MANUAL ZBM0025.01 SHIPPING CONDITIONS Human Adult Mesothelial Cells Orders are delivered via Federal Express courier. All US and Canada orders are shipped
Human Umbilical Cord-derived Multipotent Mesenchymal Stromal Cells (huc-msc) Handling Instructions
 Human Umbilical Cord-derived Multipotent Mesenchymal Stromal Cells (huc-msc) Order No.: 19401-005, 19401-010 Handling Instructions For preclinical ex vivo use. Not intended for therapeutic use. Table of
Human Umbilical Cord-derived Multipotent Mesenchymal Stromal Cells (huc-msc) Order No.: 19401-005, 19401-010 Handling Instructions For preclinical ex vivo use. Not intended for therapeutic use. Table of
Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual
 Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.03 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+ Progenitor Cells, cryopreserved Cryopreserved human umbilical
Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.03 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+ Progenitor Cells, cryopreserved Cryopreserved human umbilical
Poietics human mesenchymal stem cells Instructions for use
 Poietics human mesenchymal stem cells Instructions for use www.lonza.com U.S. Scientific Support: 800-521-0390 [email protected] EU/ROW Scientific Support: +49-221-99199-400 [email protected]
Poietics human mesenchymal stem cells Instructions for use www.lonza.com U.S. Scientific Support: 800-521-0390 [email protected] EU/ROW Scientific Support: +49-221-99199-400 [email protected]
Mesenchymal Stem Cells
 Mesenchymal Stem Cells Instruction Manual Product Size Catalog Number from Bone Marrow (hmsc-bm) from Umbilical Cord Matrix (hmsc-uc) from Adipose Tissue (hmsc-at) 500,000 cryopreserved cells 500,000 proliferating
Mesenchymal Stem Cells Instruction Manual Product Size Catalog Number from Bone Marrow (hmsc-bm) from Umbilical Cord Matrix (hmsc-uc) from Adipose Tissue (hmsc-at) 500,000 cryopreserved cells 500,000 proliferating
Human Hepatic Sinusoidal Endothelial Cell Manual
 Human Hepatic Sinusoidal Endothelial Cell Manual INSTRUCTION MANUAL SHIPPING CONDITIONS ZBM0093.00 Human Hepatic Sinusoidal Cells. Orders are delivered via Federal Express courier. All US and Canada orders
Human Hepatic Sinusoidal Endothelial Cell Manual INSTRUCTION MANUAL SHIPPING CONDITIONS ZBM0093.00 Human Hepatic Sinusoidal Cells. Orders are delivered via Federal Express courier. All US and Canada orders
Xpert TM Animal Tissue Culture Teaching Kit
 Xpert TM Animal Tissue Culture Teaching Kit Product Code: CCK005 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques
Xpert TM Animal Tissue Culture Teaching Kit Product Code: CCK005 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques
MTT Cell Proliferation Assay
 ATCC 30-1010K Store at 4 C This product is intended for laboratory research purposes only. It is not intended for use in humans, animals or for diagnostics. Introduction Measurement of cell viability and
ATCC 30-1010K Store at 4 C This product is intended for laboratory research purposes only. It is not intended for use in humans, animals or for diagnostics. Introduction Measurement of cell viability and
Human CD4+T Cell Care Manual
 Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.02 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.02 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Human Peripheral Blood Mononuclear Cell (PBMC) Manual
 Human Peripheral Blood Mononuclear Cell (PBMC) Manual INSTRUCTION MANUAL ZBM0063.04 SHIPPING CONDITIONS Human Peripheral Blood Mononuclear Cells, cryopreserved Cryopreserved human peripheral blood mononuclear
Human Peripheral Blood Mononuclear Cell (PBMC) Manual INSTRUCTION MANUAL ZBM0063.04 SHIPPING CONDITIONS Human Peripheral Blood Mononuclear Cells, cryopreserved Cryopreserved human peripheral blood mononuclear
HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs)
 HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs) OBJECTIVE: can be cryopreserved in a liquid nitrogen (LN 2 ) freezer for long-term storage. This Standard Operating Procedure (SOP)
HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs) OBJECTIVE: can be cryopreserved in a liquid nitrogen (LN 2 ) freezer for long-term storage. This Standard Operating Procedure (SOP)
GASTRIC ORGANOID CULTURE PROTOCOL
 GASTRIC ORGANOID CULTURE PROTOCOL THIS PROTOCOL PROVIDES THE PROCEDURE FOR SUBCULTURING NORMAL HUMAN GASTRIC ORGANOIDS WHICH WAS DERIVED FROM THE SUBMERGED METHOD AS DESCRIBED IN BARKER N, ET AL. LGR5+VE
GASTRIC ORGANOID CULTURE PROTOCOL THIS PROTOCOL PROVIDES THE PROCEDURE FOR SUBCULTURING NORMAL HUMAN GASTRIC ORGANOIDS WHICH WAS DERIVED FROM THE SUBMERGED METHOD AS DESCRIBED IN BARKER N, ET AL. LGR5+VE
Instructions. Torpedo sirna. Material. Important Guidelines. Specifications. Quality Control
 is a is a state of the art transfection reagent, specifically designed for the transfer of sirna and mirna into a variety of eukaryotic cell types. is a state of the art transfection reagent, specifically
is a is a state of the art transfection reagent, specifically designed for the transfer of sirna and mirna into a variety of eukaryotic cell types. is a state of the art transfection reagent, specifically
Generation and Culture of Neural Progenitor Cells using the STEMdiff Neural System
 Generation and Culture of Neural Progenitor Cells using the STEMdiff Neural System i Table of Contents 1.0 Introduction... 1 2.0 Materials, Reagents and Equipment... 2 2.1 Materials Required for Neural
Generation and Culture of Neural Progenitor Cells using the STEMdiff Neural System i Table of Contents 1.0 Introduction... 1 2.0 Materials, Reagents and Equipment... 2 2.1 Materials Required for Neural
Required Materials. Stemgent Reprogramming-Qualified NuFF Cells GSC-3002G 4 x 10 6 cells/vial Liquid Nitrogen
 Page 1 Overview This protocol for microrna-enhanced mrna reprogramming describes the combined use of the Stemgent mrna Reprogramming Kit, the Stemgent microrna Booster Kit, and the Stemgent Stemfect RNA
Page 1 Overview This protocol for microrna-enhanced mrna reprogramming describes the combined use of the Stemgent mrna Reprogramming Kit, the Stemgent microrna Booster Kit, and the Stemgent Stemfect RNA
TransIT -2020 Transfection Reagent
 Quick Reference Protocol, MSDS and Certificate of Analysis available at mirusbio.com/5400 INTRODUCTION TransIT -2020 Transfection Reagent is a high-performance, animal-origin free, broad spectrum reagent
Quick Reference Protocol, MSDS and Certificate of Analysis available at mirusbio.com/5400 INTRODUCTION TransIT -2020 Transfection Reagent is a high-performance, animal-origin free, broad spectrum reagent
Chondrogenical Differentiation of Umbilical Cord Lining. Stem Cells
 Chondrogenical Differentiation of Umbilical Cord Lining Stem Cells He Xi, Masilamani Jeyakumar, Phan Toan Thang Bioengineering, National University of Bioengineering Abstract Cartilage defects, such as
Chondrogenical Differentiation of Umbilical Cord Lining Stem Cells He Xi, Masilamani Jeyakumar, Phan Toan Thang Bioengineering, National University of Bioengineering Abstract Cartilage defects, such as
MEF Starter Nucleofector Kit
 page 1 of 7 MEF Starter Nucleofector Kit for Mouse Embryonic Fibroblasts (MEF) MEF display significant phenotypic variations which depend on the strain, the genetic background of the mice they are isolated
page 1 of 7 MEF Starter Nucleofector Kit for Mouse Embryonic Fibroblasts (MEF) MEF display significant phenotypic variations which depend on the strain, the genetic background of the mice they are isolated
STANDARD OPERATING PROCEDURE
 STANDARD OPERATING PROCEDURE Title: Antibody Production at Strategic Diagnostics Inc. SOP#: M-119 Version #: 1 Date Approved: August 6, 2009 Author: Strategic Diagnostic Inc. Date Modified: 1. PURPOSE
STANDARD OPERATING PROCEDURE Title: Antibody Production at Strategic Diagnostics Inc. SOP#: M-119 Version #: 1 Date Approved: August 6, 2009 Author: Strategic Diagnostic Inc. Date Modified: 1. PURPOSE
Cell Culture Experiments
 Cell Culture Experiments Cell cultures are another way to observe and analyze biological systems. These in vitro (in glass; outside the living body and in an artificial environment) models allow experimental
Cell Culture Experiments Cell cultures are another way to observe and analyze biological systems. These in vitro (in glass; outside the living body and in an artificial environment) models allow experimental
Xpert TM Karyotyping Teaching Kit
 Xpert TM Karyotyping Teaching Kit Product Code: CCK012 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques and good
Xpert TM Karyotyping Teaching Kit Product Code: CCK012 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques and good
BIOTECHNOLOGY I MAMMALIAN CELL CULTURE
 LAB 5 Mammalian Cell Culture Tim Warmke and Catherine Sweeny, former SLCC-FV Biotechnology students, helped in the writing and initial teaching of this lab. STUDENT GUIDE GOAL The purpose of this lab is
LAB 5 Mammalian Cell Culture Tim Warmke and Catherine Sweeny, former SLCC-FV Biotechnology students, helped in the writing and initial teaching of this lab. STUDENT GUIDE GOAL The purpose of this lab is
Transfection reagent for visualizing lipofection with DNA. For ordering information, MSDS, publications and application notes see www.biontex.
 METAFECTENE FluoR Transfection reagent for visualizing lipofection with DNA For ordering information, MSDS, publications and application notes see www.biontex.com Description Cat. No. Size METAFECTENE
METAFECTENE FluoR Transfection reagent for visualizing lipofection with DNA For ordering information, MSDS, publications and application notes see www.biontex.com Description Cat. No. Size METAFECTENE
Basic Science in Medicine
 Medical Journal of th e Islamic Republic of Iran Volume 18 Number 3 Fall 1383 November 2004 Basic Science in Medicine ] EXPANSION OF HUMAN CORD BLOOD PRIMITIVE PROGENITORS IN SERUM-FREE MEDIA USING HUMAN
Medical Journal of th e Islamic Republic of Iran Volume 18 Number 3 Fall 1383 November 2004 Basic Science in Medicine ] EXPANSION OF HUMAN CORD BLOOD PRIMITIVE PROGENITORS IN SERUM-FREE MEDIA USING HUMAN
ab139418 Propidium Iodide Flow Cytometry Kit for Cell Cycle Analysis
 ab139418 Propidium Iodide Flow Cytometry Kit for Cell Cycle Analysis Instructions for Use To determine cell cycle status in tissue culture cell lines by measuring DNA content using a flow cytometer. This
ab139418 Propidium Iodide Flow Cytometry Kit for Cell Cycle Analysis Instructions for Use To determine cell cycle status in tissue culture cell lines by measuring DNA content using a flow cytometer. This
Fast, easy and effective transfection reagent for mammalian cells
 METAFECTENE EASY + Fast, easy and effective transfection reagent for mammalian cells For ordering information, MSDS, publications and application notes see www.biontex.com Description Cat. No. Size METAFECTENE
METAFECTENE EASY + Fast, easy and effective transfection reagent for mammalian cells For ordering information, MSDS, publications and application notes see www.biontex.com Description Cat. No. Size METAFECTENE
Animal Cell Culture. Third Edition. A Practical Approach OXJORD VNIVVRSITY 1'RVSS
 Animal Cell Culture Third Edition A Practical Approach Edited by John R. W. Masters 3rd Floor Research Laboratories, University College London OXJORD VNIVVRSITY 1'RVSS Contents List of protocols page xiii
Animal Cell Culture Third Edition A Practical Approach Edited by John R. W. Masters 3rd Floor Research Laboratories, University College London OXJORD VNIVVRSITY 1'RVSS Contents List of protocols page xiii
Growth and Maintenance of Insect Cell Lines
 Insect Cell Lines Version K July 12, 2002 25-0127 Growth and Maintenance of Insect Cell Lines www.invitrogen.com [email protected] ii Table of Contents Table of Contents... iii Important Information...iv
Insect Cell Lines Version K July 12, 2002 25-0127 Growth and Maintenance of Insect Cell Lines www.invitrogen.com [email protected] ii Table of Contents Table of Contents... iii Important Information...iv
MEF Nucleofector Kit 1 and 2
 page 1 of 7 MEF Nucleofector Kit 1 and 2 for Mouse Embryonic Fibroblasts (MEF) MEF display significant phenotypic variations which depend on the strain, the genetic background of the they are isolated
page 1 of 7 MEF Nucleofector Kit 1 and 2 for Mouse Embryonic Fibroblasts (MEF) MEF display significant phenotypic variations which depend on the strain, the genetic background of the they are isolated
Cell Migration, Chemotaxis and Invasion Assay Protocol
 Cell Migration, Chemotaxis and Invasion Assay Protocol Hilary Sherman, Pilar Pardo and Todd Upton Corning Life Sciences 900 Chelmsford Street Lowell, MA 01851 Introduction Cell migration, the movement
Cell Migration, Chemotaxis and Invasion Assay Protocol Hilary Sherman, Pilar Pardo and Todd Upton Corning Life Sciences 900 Chelmsford Street Lowell, MA 01851 Introduction Cell migration, the movement
STEMCELL Quality Control Kits
 STEMCELL Quality Control Kits i Table of Contents 1.0 Introduction...1 2.0 Thawing Cells, Plating and Colony Enumeration...2 2.1 Supplies and reagents included in the QC kit...2 2.2 Additional reagents
STEMCELL Quality Control Kits i Table of Contents 1.0 Introduction...1 2.0 Thawing Cells, Plating and Colony Enumeration...2 2.1 Supplies and reagents included in the QC kit...2 2.2 Additional reagents
BioArchive Freezing Curve for Cord Blood
 BioArchive Freezing Curve for Cord Blood Determination of the Specific Freezing in the BioArchive System to Provide the Highest Viability of Cord Blood MNC as Measured by -C Assays Introduction Freezing
BioArchive Freezing Curve for Cord Blood Determination of the Specific Freezing in the BioArchive System to Provide the Highest Viability of Cord Blood MNC as Measured by -C Assays Introduction Freezing
RiboZol RNA Extraction Reagents
 RiboZol RNA Extraction Reagents Code Description Size N580-30ML-SAMPLE Ribozol TM RNA Extraction Reagent 30 ml N580-30ML Ribozol TM RNA Extraction Reagent 30 ml N580-100ML Ribozol TM RNA Extraction Reagent
RiboZol RNA Extraction Reagents Code Description Size N580-30ML-SAMPLE Ribozol TM RNA Extraction Reagent 30 ml N580-30ML Ribozol TM RNA Extraction Reagent 30 ml N580-100ML Ribozol TM RNA Extraction Reagent
Related topics: Application Note 27 Data Analysis of Tube Formation Assays.
 Tube Formation Assays in µ-slide Angiogenesis Related topics: Application Note 27 Data Analysis of Tube Formation Assays. Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview...
Tube Formation Assays in µ-slide Angiogenesis Related topics: Application Note 27 Data Analysis of Tube Formation Assays. Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview...
Introduction to animal cell culture
 Introduction to animal cell culture Make proteins: commercial scale Stem and cancer cells Antibody production: monoclonals CELL CULTURE Why do it? Primary Human and animal Cell culture Gene Therapy Embryo
Introduction to animal cell culture Make proteins: commercial scale Stem and cancer cells Antibody production: monoclonals CELL CULTURE Why do it? Primary Human and animal Cell culture Gene Therapy Embryo
Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum
 SUPPLEMENTAL PROTOCOL WHITE PAPER Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum Bee Na Lee, Ph.D., Beckman Coulter Life Sciences Process
SUPPLEMENTAL PROTOCOL WHITE PAPER Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum Bee Na Lee, Ph.D., Beckman Coulter Life Sciences Process
Osteoblast Differentiation and Mineralization
 Osteoblast Differentiation and Mineralization Application Note Background Osteoblasts are specialized fibroblasts that secrete and mineralize the bone matrix. They develop from mesenchymal precursors.
Osteoblast Differentiation and Mineralization Application Note Background Osteoblasts are specialized fibroblasts that secrete and mineralize the bone matrix. They develop from mesenchymal precursors.
PROTOCOL. Immunostaining for Flow Cytometry. Background. Materials and equipment required.
 PROTOCOL Immunostaining for Flow Cytometry 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 Background The combination of single cell analysis using flow cytometry and the specificity of antibody-based
PROTOCOL Immunostaining for Flow Cytometry 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 Background The combination of single cell analysis using flow cytometry and the specificity of antibody-based
Cellartis Protocol Culturing of hes Cells
 Cellartis Protocol Culturing of hes Cells This material was cultured and frozen using Cellartis protocols. WiCell recommends that stem cells should be thawed and established in the conditions in which
Cellartis Protocol Culturing of hes Cells This material was cultured and frozen using Cellartis protocols. WiCell recommends that stem cells should be thawed and established in the conditions in which
EXTRACTION OF DNA FROM CALF THYMUS CELLS Revised 2/1/96 Introduction
 Revised 2/1/96 Introduction Cells may be classified into two primary types depending on whether they have a discrete nucleus (eukaryotic) or do not (prokaryotic). Prokaryotes include bacteria, such as
Revised 2/1/96 Introduction Cells may be classified into two primary types depending on whether they have a discrete nucleus (eukaryotic) or do not (prokaryotic). Prokaryotes include bacteria, such as
Workshop 14-16 February 2006
 Theoretical and practical approaches of Hepatocyte primary culture Workshop 14-16 February 2006 Lecture (2) Disaggregation & purification of target cells Coarse organizer Dr. Abo bakr Mohamed Eltayeb General
Theoretical and practical approaches of Hepatocyte primary culture Workshop 14-16 February 2006 Lecture (2) Disaggregation & purification of target cells Coarse organizer Dr. Abo bakr Mohamed Eltayeb General
3D Cell Culture mimsys G
 3D Cell Culture mimsys G xeno-free & nutrient permeable hydrogel for 3D cell culture mimsys G is a xeno-free and non-immunogenic, easy to handle hydrogel for cell encapsulation in 3D experiments in vitro
3D Cell Culture mimsys G xeno-free & nutrient permeable hydrogel for 3D cell culture mimsys G is a xeno-free and non-immunogenic, easy to handle hydrogel for cell encapsulation in 3D experiments in vitro
Stem cells possess 2 main characteristics: Sources of pluripotent stem cells: -Long-term self renewal. -The inner cell mass of the blastocyst.
 Stem cells possess 2 main characteristics: -Long-term self renewal. - They give rise to all types of differentiate cells. Sources of pluripotent stem cells: -The inner cell mass of the blastocyst. - Fetal
Stem cells possess 2 main characteristics: -Long-term self renewal. - They give rise to all types of differentiate cells. Sources of pluripotent stem cells: -The inner cell mass of the blastocyst. - Fetal
Effective Methods For Culturing Breast Cancer Cell Lines
 Abstract Effective Methods For Culturing Breast Cancer Cell Lines William H. Marshall II Life Science Team Cell culturing is one of the most useful and prolific techniques practiced in biological science
Abstract Effective Methods For Culturing Breast Cancer Cell Lines William H. Marshall II Life Science Team Cell culturing is one of the most useful and prolific techniques practiced in biological science
Mouse ES Cell Nucleofector Kit
 page 1 of 7 Mouse ES Cell Nucleofector Kit for Mouse Embryonic Stem Cells Cell type Origin Cells derived from mouse blastocysts. Morphology Round cells growing in clumps. Important remarks! 1. This protocol
page 1 of 7 Mouse ES Cell Nucleofector Kit for Mouse Embryonic Stem Cells Cell type Origin Cells derived from mouse blastocysts. Morphology Round cells growing in clumps. Important remarks! 1. This protocol
Standard Operating Procedures (SOPs) for the production and testing of embryonic stem cell lines in Taiwan Stem Cell Bank
 Standard Operating Procedures (SOPs) for the production and testing of embryonic stem cell lines in Taiwan Stem Cell Bank Bioresource Collection and Research Centre (BCRC) at Food Industry Research and
Standard Operating Procedures (SOPs) for the production and testing of embryonic stem cell lines in Taiwan Stem Cell Bank Bioresource Collection and Research Centre (BCRC) at Food Industry Research and
Unit 1 Higher Human Biology Summary Notes
 Unit 1 Higher Human Biology Summary Notes a. Cells tissues organs body systems Division of labour occurs in multicellular organisms (rather than each cell carrying out every function) Most cells become
Unit 1 Higher Human Biology Summary Notes a. Cells tissues organs body systems Division of labour occurs in multicellular organisms (rather than each cell carrying out every function) Most cells become
Modulating Glucose Uptake in Skeletal Myotubes:
 icell Skeletal Myoblasts Application Protocol Introduction Modulating Glucose Uptake in Skeletal Myotubes: Insulin Induction with Bioluminescent Glucose Uptake Analysis The skeletal muscle is one of the
icell Skeletal Myoblasts Application Protocol Introduction Modulating Glucose Uptake in Skeletal Myotubes: Insulin Induction with Bioluminescent Glucose Uptake Analysis The skeletal muscle is one of the
STEM CELL FELLOWSHIP
 Module I: The Basic Principles of Stem Cells 1. Basics of Stem Cells a. Understanding the development of embryonic stem cells i. Embryonic stem cells ii. Embryonic germ cells iii. Differentiated stem cell
Module I: The Basic Principles of Stem Cells 1. Basics of Stem Cells a. Understanding the development of embryonic stem cells i. Embryonic stem cells ii. Embryonic germ cells iii. Differentiated stem cell
Aseptic Technique. A GMP/GTP Training Module
 Aseptic Technique A GMP/GTP Training Module Aseptic Technique The GMP Facility manufactures products for clinical use These products must meet a number of requirements, one of which is that they are sterile
Aseptic Technique A GMP/GTP Training Module Aseptic Technique The GMP Facility manufactures products for clinical use These products must meet a number of requirements, one of which is that they are sterile
Clonetics Endothelial Cell System Technical Information & Instructions
 Lonza Walkersville, Inc. Walkersville, MD 21793-0127 USA U.S. Scientific Support: 800 521 0390 [email protected] EU/ROW Scientific Support: +32 87 321 611 [email protected] Document
Lonza Walkersville, Inc. Walkersville, MD 21793-0127 USA U.S. Scientific Support: 800 521 0390 [email protected] EU/ROW Scientific Support: +32 87 321 611 [email protected] Document
QuickZyme Soluble Collagen Assay
 QuickZyme Soluble Collagen Assay Version June 2012 FOR RESEARCH USE ONLY NOT FOR USE IN DIAGNOSTIC PROCEDURES This package insert must be read in its entirety before using this product. Introduction Collagen
QuickZyme Soluble Collagen Assay Version June 2012 FOR RESEARCH USE ONLY NOT FOR USE IN DIAGNOSTIC PROCEDURES This package insert must be read in its entirety before using this product. Introduction Collagen
Amaxa 4D-Nucleofector Protocol for Mouse Embryonic Stem [ES] Cells For 4D-Nucleofector X Unit Transfection in suspension
![Amaxa 4D-Nucleofector Protocol for Mouse Embryonic Stem [ES] Cells For 4D-Nucleofector X Unit Transfection in suspension Amaxa 4D-Nucleofector Protocol for Mouse Embryonic Stem [ES] Cells For 4D-Nucleofector X Unit Transfection in suspension](/thumbs/30/14702458.jpg) For 4D-Nucleofector X Unit Transfection in suspension Cells derived from mouse blastocysts; round cells, growing in clumps s 1. This protocol is meant to provide an outline for the handling and the Nucleofection
For 4D-Nucleofector X Unit Transfection in suspension Cells derived from mouse blastocysts; round cells, growing in clumps s 1. This protocol is meant to provide an outline for the handling and the Nucleofection
Basics of Cell Culture A student laboratory manual
 Basics of Cell Culture A student laboratory manual By Golnar Afshar PhD Table of Contents: Introduction 3 Laboratory Exercises Laboratory Safety 3 Aseptic Technique Exercise30 30 Laboratory Notebooks5
Basics of Cell Culture A student laboratory manual By Golnar Afshar PhD Table of Contents: Introduction 3 Laboratory Exercises Laboratory Safety 3 Aseptic Technique Exercise30 30 Laboratory Notebooks5
INTERFERin in vitro sirna/mirna transfection reagent PROTOCOL. 1 Standard sirna transfection of adherent cells... 2
 INTERFERin in vitro sirna/mirna transfection reagent PROTOCOL DESCRIPTION INTERFERin is a powerful sirna/mirna transfection reagent that ensures efficient gene silencing and reproducible transfection in
INTERFERin in vitro sirna/mirna transfection reagent PROTOCOL DESCRIPTION INTERFERin is a powerful sirna/mirna transfection reagent that ensures efficient gene silencing and reproducible transfection in
Intestinal Epithelial Organoid Culture with IntestiCult Organoid Growth Medium (Mouse)
 TECHNICL ULLETIN Intestinal Epithelial Organoid Culture with IntestiCult Organoid Growth Medium (Mouse) Introduction The use of three-dimensional (3D) culture systems continues to increase, due in large
TECHNICL ULLETIN Intestinal Epithelial Organoid Culture with IntestiCult Organoid Growth Medium (Mouse) Introduction The use of three-dimensional (3D) culture systems continues to increase, due in large
Stem Cell Research: Adult or Somatic Stem Cells
 Chiang 1 Stem Cell Research: Adult or Somatic Stem Cells Abstract Kelly Chiang Cluster 7 Dr. LeFebvre 07/26/10 Over the past few decades, stem cells have been a controversial topic in the scientific field.
Chiang 1 Stem Cell Research: Adult or Somatic Stem Cells Abstract Kelly Chiang Cluster 7 Dr. LeFebvre 07/26/10 Over the past few decades, stem cells have been a controversial topic in the scientific field.
DNA Isolation Kit for Cells and Tissues
 DNA Isolation Kit for Cells and Tissues for 10 isolations of 00 mg each for tissue or 5 x 10 7 cultured cells Cat. No. 11 81 770 001 Principle Starting material Application Time required Results Benefits
DNA Isolation Kit for Cells and Tissues for 10 isolations of 00 mg each for tissue or 5 x 10 7 cultured cells Cat. No. 11 81 770 001 Principle Starting material Application Time required Results Benefits
ATCC ANIMAL CELL CULTURE GUIDE
 ATCC ANIMAL CELL CULTURE GUIDE tips and techniques for continuous cell lines The Essentials of Life Science Research Globally Delivered Table of Contents This guide contains general technical information
ATCC ANIMAL CELL CULTURE GUIDE tips and techniques for continuous cell lines The Essentials of Life Science Research Globally Delivered Table of Contents This guide contains general technical information
6 Characterization of Casein and Bovine Serum Albumin
 6 Characterization of Casein and Bovine Serum Albumin (BSA) Objectives: A) To separate a mixture of casein and bovine serum albumin B) to characterize these proteins based on their solubilities as a function
6 Characterization of Casein and Bovine Serum Albumin (BSA) Objectives: A) To separate a mixture of casein and bovine serum albumin B) to characterize these proteins based on their solubilities as a function
NCL Method ITA-6. Leukocyte Proliferation Assay
 NCL Method ITA-6 Leukocyte Proliferation Assay Nanotechnology Characterization Laboratory National Cancer Institute-Frederick SAIC-Frederick Frederick, MD 21702 (301) 846-6939 [email protected] December
NCL Method ITA-6 Leukocyte Proliferation Assay Nanotechnology Characterization Laboratory National Cancer Institute-Frederick SAIC-Frederick Frederick, MD 21702 (301) 846-6939 [email protected] December
BD PuraMatrix Peptide Hydrogel
 BD PuraMatrix Peptide Hydrogel Catalog No. 354250 Guidelines for Use FOR RESEARCH USE ONLY NOT FOR CLINICAL, DIAGNOSTIC OR THERAPEUTIC USE SPC-354250-G rev 2.0 1 TABLE OF CONTENTS Intended Use... 2 Materials
BD PuraMatrix Peptide Hydrogel Catalog No. 354250 Guidelines for Use FOR RESEARCH USE ONLY NOT FOR CLINICAL, DIAGNOSTIC OR THERAPEUTIC USE SPC-354250-G rev 2.0 1 TABLE OF CONTENTS Intended Use... 2 Materials
Cell Culture Protocol for Biogelx Peptide Hydrogel 2D and 3D Cell Culture PRO/BGX/001
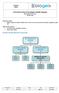 Protocol (PRO) Cell Culture Protocol for Hydrogel 2D and 3D Cell Culture PRO/BGX/001 Materials Provided Gel solution (stiffness of Gel will vary as per type of product requested), packaged in a glass vial.
Protocol (PRO) Cell Culture Protocol for Hydrogel 2D and 3D Cell Culture PRO/BGX/001 Materials Provided Gel solution (stiffness of Gel will vary as per type of product requested), packaged in a glass vial.
Bacterial Transformation with Green Fluorescent Protein. Table of Contents Fall 2012
 Bacterial Transformation with Green Fluorescent Protein pglo Version Table of Contents Bacterial Transformation Introduction..1 Laboratory Exercise...3 Important Laboratory Practices 3 Protocol...... 4
Bacterial Transformation with Green Fluorescent Protein pglo Version Table of Contents Bacterial Transformation Introduction..1 Laboratory Exercise...3 Important Laboratory Practices 3 Protocol...... 4
CHEF Genomic DNA Plug Kits Instruction Manual
 CHEF Genomic DNA Plug Kits Instruction Manual Catalog Numbers 170-3591 170-3592 170-3593 For Technical Service Call Your Local Bio-Rad Office or in the U.S. Call 1-800-4BIORAD (1-800-424-6723) Table of
CHEF Genomic DNA Plug Kits Instruction Manual Catalog Numbers 170-3591 170-3592 170-3593 For Technical Service Call Your Local Bio-Rad Office or in the U.S. Call 1-800-4BIORAD (1-800-424-6723) Table of
Day -1 RETRIEVAL OF OVARIES AND OOCYTE COLLECTION AND MATURATION
 Day -1 RETRIEVAL OF OVARIES AND OOCYTE COLLECTION AND MATURATION OVARY COLLECTION Materials and Equipment Needed Thermos with 2 containers with 0.5 L of transport saline in each. Appropriate attire as
Day -1 RETRIEVAL OF OVARIES AND OOCYTE COLLECTION AND MATURATION OVARY COLLECTION Materials and Equipment Needed Thermos with 2 containers with 0.5 L of transport saline in each. Appropriate attire as
Glycolysis Cell-Based Assay Kit
 Glycolysis Cell-Based Assay Kit Item No. 600450 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Glycolysis Cell-Based Assay Kit Item No. 600450 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
RealLine HCV PCR Qualitative - Uni-Format
 Instructions for use PCR KIT FOR EXTRACTION OF RNA AND REAL TIME PCR DETECTION KIT FOR HEPATITIS C VIRUS RNA Research Use Only Qualitative Uni Format VBD0798 48 tests valid from: December 2013 Rev11122013
Instructions for use PCR KIT FOR EXTRACTION OF RNA AND REAL TIME PCR DETECTION KIT FOR HEPATITIS C VIRUS RNA Research Use Only Qualitative Uni Format VBD0798 48 tests valid from: December 2013 Rev11122013
An Overview of Cells and Cell Research
 An Overview of Cells and Cell Research 1 An Overview of Cells and Cell Research Chapter Outline Model Species and Cell types Cell components Tools of Cell Biology Model Species E. Coli: simplest organism
An Overview of Cells and Cell Research 1 An Overview of Cells and Cell Research Chapter Outline Model Species and Cell types Cell components Tools of Cell Biology Model Species E. Coli: simplest organism
Animal Tissues. I. Epithelial Tissue
 Animal Tissues There are four types of tissues found in animals: epithelial tissue, connective tissue, muscle tissue, and nervous tissue. In this lab you will learn the major characteristics of each tissue
Animal Tissues There are four types of tissues found in animals: epithelial tissue, connective tissue, muscle tissue, and nervous tissue. In this lab you will learn the major characteristics of each tissue
Growth and Maintenance of T-REx Cell Lines
 USER GUIDE Growth and Maintenance of T-REx Cell Lines Catalog Numbers R710-07, R714-07, R718-07, R722-07 Document Part Number 25-0272 Publication Number MAN0000106 Revision 3.0 For Research Use Only. Not
USER GUIDE Growth and Maintenance of T-REx Cell Lines Catalog Numbers R710-07, R714-07, R718-07, R722-07 Document Part Number 25-0272 Publication Number MAN0000106 Revision 3.0 For Research Use Only. Not
Section B: Epithelial Tissue 1. Where are epithelial tissues found within the body? 2. What are the functions of the epithelial tissues?
 Tissue worksheet Name Section A: Intro to Histology Cells are the smallest units of life. In complex organisms, cells group together with one another based on similar structure and function to form tissues.
Tissue worksheet Name Section A: Intro to Histology Cells are the smallest units of life. In complex organisms, cells group together with one another based on similar structure and function to form tissues.
GM23226*A. Certificate of Analysis
 GM23226*A Certificate of Analysis Product Description Human fibroblast line reprogrammed with four factors (Oct 4, Sox 2, c-myc and Klf-4) using retroviral vector Publication(s) describing ipsc establishment
GM23226*A Certificate of Analysis Product Description Human fibroblast line reprogrammed with four factors (Oct 4, Sox 2, c-myc and Klf-4) using retroviral vector Publication(s) describing ipsc establishment
Gentra Puregene Handbook
 Third Edition June 2011 Gentra Puregene Handbook For purification of archive-quality DNA from human whole blood bone marrow buffy coat buccal cells body fluids cultured cells tissue mouse tail yeast bacteria
Third Edition June 2011 Gentra Puregene Handbook For purification of archive-quality DNA from human whole blood bone marrow buffy coat buccal cells body fluids cultured cells tissue mouse tail yeast bacteria
Transformation of the bacterium E. coli. using a gene for Green Fluorescent Protein
 Transformation of the bacterium E. coli using a gene for Green Fluorescent Protein Background In molecular biology, transformation refers to a form of genetic exchange in which the genetic material carried
Transformation of the bacterium E. coli using a gene for Green Fluorescent Protein Background In molecular biology, transformation refers to a form of genetic exchange in which the genetic material carried
Cell Phone Radiation Effects on Cancer. Tyler Barkich Central Catholic High School Grade 11
 Cell Phone Radiation Effects on Cancer Tyler Barkich Central Catholic High School Grade 11 Cancer Overview Cancer cells are cells that grow and divide at an irregular, unregulated pace. Apoptosis does
Cell Phone Radiation Effects on Cancer Tyler Barkich Central Catholic High School Grade 11 Cancer Overview Cancer cells are cells that grow and divide at an irregular, unregulated pace. Apoptosis does
MLX BCG Buccal Cell Genomic DNA Extraction Kit. Performance Characteristics
 MLX BCG Buccal Cell Genomic DNA Extraction Kit Performance Characteristics Monolythix, Inc. 4720 Calle Carga Camarillo, CA 93012 Tel: (805) 484-8478 monolythix.com Page 2 of 9 MLX BCG Buccal Cell Genomic
MLX BCG Buccal Cell Genomic DNA Extraction Kit Performance Characteristics Monolythix, Inc. 4720 Calle Carga Camarillo, CA 93012 Tel: (805) 484-8478 monolythix.com Page 2 of 9 MLX BCG Buccal Cell Genomic
Mouse IFN-gamma ELISpot Kit
 Page 1 of 8 Mouse IFN-gamma ELISpot Kit Without Plates With Plates With Sterile Plates Quantity Catalog Nos. 862.031.001 862.031.001P 862.031.001S 1 x 96 tests 862.031.005 862.031.005P 862.031.005S 5 x
Page 1 of 8 Mouse IFN-gamma ELISpot Kit Without Plates With Plates With Sterile Plates Quantity Catalog Nos. 862.031.001 862.031.001P 862.031.001S 1 x 96 tests 862.031.005 862.031.005P 862.031.005S 5 x
PRODUCT INFORMATION...
 VIROMER RED In vitro plasmid DNA and mrna Standard Transfection PRODUCT INFORMATION... 2 GENERAL... 2 RED OR YELLOW?... 3 PROTOCOL GUIDELINES... 4 GENERAL REMARKS... 4 CELL CULTURE AND PLATING... 4 FORWARD/REVERSE
VIROMER RED In vitro plasmid DNA and mrna Standard Transfection PRODUCT INFORMATION... 2 GENERAL... 2 RED OR YELLOW?... 3 PROTOCOL GUIDELINES... 4 GENERAL REMARKS... 4 CELL CULTURE AND PLATING... 4 FORWARD/REVERSE
Islet Viability Assessment by Single Cell Flow Cytometry
 Islet Viability Assessment by Single Cell Flow Cytometry Page 1 of 8 Purpose: To comprehensively assess the viability of the islet cell preparation prior to transplantation. Tissue Samples: A sample containing
Islet Viability Assessment by Single Cell Flow Cytometry Page 1 of 8 Purpose: To comprehensively assess the viability of the islet cell preparation prior to transplantation. Tissue Samples: A sample containing
Green Fluorescent Protein (GFP): Genetic Transformation, Synthesis and Purification of the Recombinant Protein
 Green Fluorescent Protein (GFP): Genetic Transformation, Synthesis and Purification of the Recombinant Protein INTRODUCTION Green Fluorescent Protein (GFP) is a novel protein produced by the bioluminescent
Green Fluorescent Protein (GFP): Genetic Transformation, Synthesis and Purification of the Recombinant Protein INTRODUCTION Green Fluorescent Protein (GFP) is a novel protein produced by the bioluminescent
UltraClean Soil DNA Isolation Kit
 PAGE 1 UltraClean Soil DNA Isolation Kit Catalog # 12800-50 50 preps New improved PCR inhibitor removal solution (IRS) included Instruction Manual (New Alternative Protocol maximizes yields) Introduction
PAGE 1 UltraClean Soil DNA Isolation Kit Catalog # 12800-50 50 preps New improved PCR inhibitor removal solution (IRS) included Instruction Manual (New Alternative Protocol maximizes yields) Introduction
BioResearch. RAFT 3D Cell Culture Kit Protocol
 BioResearch RAFT 3D Cell Culture Kit Protocol RAFT 3D Cell Culture Kit This protocol describes in detail how to produce RAFT 3D Cell Cultures in 96-well plates, 24-well plates and 24-well inserts. It is
BioResearch RAFT 3D Cell Culture Kit Protocol RAFT 3D Cell Culture Kit This protocol describes in detail how to produce RAFT 3D Cell Cultures in 96-well plates, 24-well plates and 24-well inserts. It is
Optimal Conditions for F(ab ) 2 Antibody Fragment Production from Mouse IgG2a
 Optimal Conditions for F(ab ) 2 Antibody Fragment Production from Mouse IgG2a Ryan S. Stowers, 1 Jacqueline A. Callihan, 2 James D. Bryers 2 1 Department of Bioengineering, Clemson University, Clemson,
Optimal Conditions for F(ab ) 2 Antibody Fragment Production from Mouse IgG2a Ryan S. Stowers, 1 Jacqueline A. Callihan, 2 James D. Bryers 2 1 Department of Bioengineering, Clemson University, Clemson,
Biotechnology. Srivatsan Kidambi, Ph.D.
 Stem Stem Cell Cell Engineering-What, Biology and it Application Why, How?? to Biotechnology Srivatsan Kidambi, Ph.D. Assistant Professor Department of Chemical & Biomolecular Engineering University of
Stem Stem Cell Cell Engineering-What, Biology and it Application Why, How?? to Biotechnology Srivatsan Kidambi, Ph.D. Assistant Professor Department of Chemical & Biomolecular Engineering University of
AAGPs TM Anti-Aging Glyco Peptides. Enhancing Cell, Tissue and Organ Integrity Molecular and biological attributes of lead AAGP molecule
 AAGPs TM Anti-Aging Glyco Peptides Enhancing Cell, Tissue and Organ Integrity Molecular and biological attributes of lead AAGP molecule 1 Acknowledgements This presentation was prepared by Dr. Samer Hussein
AAGPs TM Anti-Aging Glyco Peptides Enhancing Cell, Tissue and Organ Integrity Molecular and biological attributes of lead AAGP molecule 1 Acknowledgements This presentation was prepared by Dr. Samer Hussein
IgM ELISA. For the quantitative determination of IgM in human serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures.
 IgM ELISA For the quantitative determination of IgM in human serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calibrator
IgM ELISA For the quantitative determination of IgM in human serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calibrator
Especially developed for the transfection of mammalian cells with sirna or mirna
 METAFECTENE SI Especially developed for the transfection of mammalian cells with sirna or mirna For ordering information, MSDS, publications and application notes see www.biontex.com Description Cat. No.
METAFECTENE SI Especially developed for the transfection of mammalian cells with sirna or mirna For ordering information, MSDS, publications and application notes see www.biontex.com Description Cat. No.
Amaxa Mouse/Rat Hepatocyte Nucleofector Kit
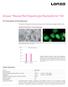 Amaxa Mouse/Rat Hepatocyte Nucleofector Kit For Primary Mouse and Rat Hepatocytes This protocol is designed for freshly isolated mouse and rat hepatocytes; polygonal, adherent cells Example for Nucleofection
Amaxa Mouse/Rat Hepatocyte Nucleofector Kit For Primary Mouse and Rat Hepatocytes This protocol is designed for freshly isolated mouse and rat hepatocytes; polygonal, adherent cells Example for Nucleofection
Annexin V-EGFP Apoptosis Detection Kit
 ab14153 Annexin V-EGFP Apoptosis Detection Kit Instructions for Use For the rapid, sensitive and accurate measurement of apoptosis in various samples This product is for research use only and is not intended
ab14153 Annexin V-EGFP Apoptosis Detection Kit Instructions for Use For the rapid, sensitive and accurate measurement of apoptosis in various samples This product is for research use only and is not intended
Isolation of Caffeine from Tea
 Isolation of Caffeine from Tea Introduction A number of interesting, biologically active compounds have been isolated from plants. Isolating some of these natural products, as they are called, can require
Isolation of Caffeine from Tea Introduction A number of interesting, biologically active compounds have been isolated from plants. Isolating some of these natural products, as they are called, can require
Basic pluripotent stem cell culture protocols
 Basic pluripotent stem cell culture protocols Maria Borowski, Maria Giovino-Doherty, Lan Ji, Meng-Jiao Shi, Kelly P. Smith and Joseph Laning, Massachusetts Stem Cell Bank, University of Massachusetts Medical
Basic pluripotent stem cell culture protocols Maria Borowski, Maria Giovino-Doherty, Lan Ji, Meng-Jiao Shi, Kelly P. Smith and Joseph Laning, Massachusetts Stem Cell Bank, University of Massachusetts Medical
Classic Immunoprecipitation
 292PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 [email protected] A Geno Technology, Inc. (USA) brand name Classic Immunoprecipitation Utilizes Protein A/G Agarose for Antibody Binding (Cat.
292PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 [email protected] A Geno Technology, Inc. (USA) brand name Classic Immunoprecipitation Utilizes Protein A/G Agarose for Antibody Binding (Cat.
Corning Matrigel Matrix Frequently Asked Questions.
 Corning Matrigel Matrix Frequently Asked Questions. What is matrix? matrix is a reconstituted basement membrane preparation that is extracted from the Engelbreth-Holm-Swarm (EHS) mouse sarcoma, a tumor
Corning Matrigel Matrix Frequently Asked Questions. What is matrix? matrix is a reconstituted basement membrane preparation that is extracted from the Engelbreth-Holm-Swarm (EHS) mouse sarcoma, a tumor
Steatosis Colorimetric Assay Kit
 Steatosis Colorimetric Assay Kit Item No. 10012643 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
Steatosis Colorimetric Assay Kit Item No. 10012643 www.caymanchem.com Customer Service 800.364.9897 Technical Support 888.526.5351 1180 E. Ellsworth Rd Ann Arbor, MI USA TABLE OF CONTENTS GENERAL INFORMATION
SOLIDscript Solid Phase cdna Synthesis Kit Instruction Manual
 Toll Free: 866-252-7771 752A Lincoln Blvd. Phone: 732-469-7771 Fax: 732-469-7782 Middlesex, NJ 08846 Web: www.purebiotechllc.com SOLIDscript Solid Phase cdna Synthesis Kit Instruction Manual Product: SOLIDscript
Toll Free: 866-252-7771 752A Lincoln Blvd. Phone: 732-469-7771 Fax: 732-469-7782 Middlesex, NJ 08846 Web: www.purebiotechllc.com SOLIDscript Solid Phase cdna Synthesis Kit Instruction Manual Product: SOLIDscript
Basics of Cell Culture An instructor manual
 Basics of Cell Culture An instructor manual By Golnar Afshar PhD Table of Contents: Basics of Cell Culture 3 Laboratory Exercises 17 Laboratory Safety 3 Aseptic Technique Exercise 18 Cell Culture Laboratory
Basics of Cell Culture An instructor manual By Golnar Afshar PhD Table of Contents: Basics of Cell Culture 3 Laboratory Exercises 17 Laboratory Safety 3 Aseptic Technique Exercise 18 Cell Culture Laboratory
