SF 424 R&R Form. Section 5 Applicant Information. DUNS Number. Legal Name Virginia Commonwealth University. Department and Division Leave Blank
|
|
|
- Juliet Lyons
- 8 years ago
- Views:
Transcription
1 Section 5 Applicant Information DUNS Number Legal Name Virginia Commonwealth University Department and Division Leave Blank Street1 800 East Leigh Street, Suite Street2 PO Box City Richmond County Richmond City State SELECT VA from dropdown Country SELECT USA from dropdown Zip Person to be Contacted First Name Susan Last Name Robb SF 424 R&R Form
2 Phone Fax Section 6 Employer Identification Section 7 Type of Applicant Select Letter H from dropdown Section 8 Select type of Application New = New Resubmission = Formerly called Revision Renewal = Competing Continuation Continuation = Non-competing Continuation (Progress Report) Revision = Competing Supplemental Section 9 Auto-filled Section10 Auto-filled if available Section 11 Title(limitedto82characters) Section 12 Areas Affected by Project Enter areas Section 13 Enter Start and End Dates - MM/DD/YYYY Section 14 Congressional Districts 3 (for Richmond, VA). You will need to look up any others Section 15 - Project/PI Information First - PI First Name Last - PI Last Name Position/Title - PI Title Organization Name: Auto-filled Department - PI Department Division - PI Division (if applicable)
3 Street1 - PI Address Street2 PI Address City Richmond County Richmond City State Select VA from dropdown Country Select UNITED STATES from dropdown ZIP PI Zip Code Phone Number PI Phone Number Fax Number PI Fax Number PI Section 16 A Total Estimated Project Funding for the entire period (2 years) B Total Federal & Non-Federal Funds C Estimated Program Income Section 17 Select: b. NO Program is not covered by E.O Section 18 Read and then check I Agree Section 19 First Name Susan Last Name Robb Department Office of Sponsored Programs Street1 800 East Leigh Street, Suite Street2 PO Box City Richmond
4 County Richmond City State Select VA from dropdown Country Select UNITED STATES from dropdown Zip/Postal Code Phone Number Fax Number
5 RESEARCH & RELATED Other Project Information Form 1. Human Subjects? Check Yes or No. If Yes, Answer all of 1a. Human Subjects Assurance Number FWA Vertebrate Animals? Check Yes or No. If Yes, Answer all of 2a. Animal Welfare Assurance Number A Questions 3 through 5 must be answered by the PI. Question 6 Upload Project Summary file in pdf format Question 7 Upload Project Narrative file in pdf format (This is the relevance statement) Question 8 Upload Bibliography and References Cited file in pdf format Question 9 Upload Facilities & Other Resources file in pdf format Question 10 Upload Equipment file in pdf format Question 11 Upload any Other Attachments/Appendices in pdf format
6 RESEARCH & RELATED Project/Performance Site Location(s) Form Organization Virginia Commonwealth University Street 800 East Leigh Street, Suite City Richmond County Richmond City State Select VA from dropdown Country Select UNITED STATES from dropdown Zip/Postal Code Continue filling out other Performance Sites.
7 RESEARCH & RELATED Senior/Key Person Profile Form First Name - PI First Name Last Name - PI Last Name Position/Title - PI Title Department - PI Department Organization Name Virginia Commonwealth University Division - PI Division (if applicable) Street1 - PI Address Street2 PI Address City Richmond County Richmond City State Select VA from dropdown Country Select UNITED STATES from dropdown Zip/Postal Code PI Zip Phone Number PI Phone Number Fax Number PI Fax Number PI Credential PI COMMONS USER NAME IN ALL CAPS for NIH applications, leave blank for other agencies Project Role: PD/PI should be preselected for PI Biographical Sketch - Attach Biographical Sketch in pdf file
8 PHS 398 Cover Page Supplement Form Section 1 Auto-filled except for New Investigator question. Answer Yes or No. Section 2 Human Subjects Questions Answer Yes or No Section 3 Applicant Organization Contact -Top section should be auto-filled, but if not First Name Susan Last Name Robb Phone Number Fax Number dirospa@vcu.edu Title Director, Assoc. V.P. Office for Research of Sponsored Administration Programs and Compliance Street1 800 East Leigh Street, Suite Street2 PO Box City Richmond County Richmond City State Select VA from dropdown Country Select UNITED STATES from dropdown Zip/Postal Code Section 4 Answer Yes or No. If Yes, list Cell Lines in Boxes.
9 Section 1 Application Type Select 1 PHS 398 Research Plan Form Section 2 Research Plan Attachments Upload Specific Aims file in pdf format Upload Background and Significance in pdf format Upload Preliminary Studies in pdf format Upload Research Design and Methods in pdf format Upload Inclusion Enrollment Report in pdf format** Upload Progress Report Publication List in pdf format** Upload Protection of Human Subjects in pdf format** Upload Inclusion of Women and Minorities in pdf format** Upload Targeted/Planned Enrollment in pdf format** Upload Inclusion of Children in pdf format** Upload Vertebrate Animals in pdf format** Upload Select Agent Research in pdf format** Upload Multiple PI Leadership Plan in pdf format** Upload Consortium/Contractual Arrangements in pdf format** Upload Letters of Support in pdf format** Upload Resource Sharing Plan in pdf format Appendix Upload appendices as needed** **As needed
10 PHS 398 Checklist Form Section 1 - Application Type Should be auto-filled New = New Resubmission = Formerly called Revision Renewal = Competing Continuation Continuation = Non-competing Continuation (Progress Report) Revision = Competing Supplemental Federal Identifier = Proposal or Award Number or Grants.gov Tracking Number for Changed/Corrected Applications Section 2 Change of Investigator/Change of Institution Answer as appropriate Section 3 Inventions and Patents (for renewal applications only) Answer as appropriate Section 4 Program Income Answer and complete as appropriate Section 5- Assurances/Certifications No action to be taken on the part of the PI
11 Upload Cover Letter in pdf format PHS 398 Cover Letter Form
12 PHS 398 Modular Budget Form Budget Period 1 - Enter Start and End Dates for Budget Period 1 Section A. Select $25,000 increment amount Enter Consortium F&A amount Total Direct Costs will be calculated Section B Enter one of the following, as applicable: Select appropriate F&A rate from listing On-Campus Research MTDC Base 49 found on Off-Campus Research MTDC Base 26 On-Campus Instruction MTDC Base 34 Off-Campus Instruction MTDC Base 18.7 On-Campus Other MTDC Base 30 Off-Campus Other MTDC Base 18 Training 8 Base and Dollar Amounts should be entered as calculated. Cognizant Agency Name, POC Name and Phone Number Department of Health and Human Services, Jay Andrew Mervis, Lee, (301) Indirect Cost Rate Agreement Date 01/22/ /08/2012 Repeat for each year. Totals will be automatically calculated in Cumulative Budget section Cumulative Budget Information: Personnel Justification Upload Personnel Justification in pdf format Consortium Justification Upload Consortium Justification in pdf format Additional Narrative Justification Upload Budget Justification in pdf format
13 RESEARCH & RELATED BUDGET Section A and B Organizational DUNS: For VCU Budget Data: For Subawardee Budget Data: Enter Subawardee s DUNS number Budget Type: Select either Project (complete budget) or Subaward/Consortium (if the data to be entered is for a subaward/consortium) Enter name of Organization: Virginia Commonwealth University Or Subawardee Name Start Date: Enter Start Date End Date: Enter End Date Enter budget data in the form for Budget Period 1 through Section H: Section H: Enter one of the following, as applicable: Select appropriate F&A rate from listing On-Campus Research MTDC Base 49% found on On-Campus Research MTDC Base 49.5% Off-Campus Research MTDC Base 26% On-Campus Instruction MTDC Base 34% Off-Campus Instruction MTDC Base 26% On-Campus Other MTDC Base 30% Off-Campus Other MTDC Base 26% Cognizant Federal Agency: Department of Health and Human Services, Jay Andrew Mervis, Lee, (301) Budget Justification: Upload Budget Justification in pdf format
Grant Application Package
 Opportunity Title: Offering Agency: CFDA Number: CFDA Description: Opportunity Number: Competition ID: Opportunity Open Date: Opportunity Close Date: Agency Contact: Grant Application Package Reducing
Opportunity Title: Offering Agency: CFDA Number: CFDA Description: Opportunity Number: Competition ID: Opportunity Open Date: Opportunity Close Date: Agency Contact: Grant Application Package Reducing
Small Business Electronic Applications: Annotated SF424 (R&R) Form Set
 Small Business Electronic Applications: Annotated SF424 (R&R) Form Set FORMS Included in SBIR and STTR applications: Federal-wide Forms SF424 (R&R) Cover Component [Page 2] Project/Performance Site Location(s)
Small Business Electronic Applications: Annotated SF424 (R&R) Form Set FORMS Included in SBIR and STTR applications: Federal-wide Forms SF424 (R&R) Cover Component [Page 2] Project/Performance Site Location(s)
How to Use FOA As aBiroprint Application Form
 A Walk Through The SF424 (R&R) Presented by Scott Cooper & Emily Linde Office of Policy for Extramural Research Administration OPERA SF424 (R&R) Complete application includes combination of (R&R) components
A Walk Through The SF424 (R&R) Presented by Scott Cooper & Emily Linde Office of Policy for Extramural Research Administration OPERA SF424 (R&R) Complete application includes combination of (R&R) components
Johns Hopkins University School of Medicine Office of Research Administration
 Checklist for T Applications (Institutional Training Grants) Please read RFA and Individual NIH Institute Guidelines This document is designed only to serve as a project management tool. It does NOT replace
Checklist for T Applications (Institutional Training Grants) Please read RFA and Individual NIH Institute Guidelines This document is designed only to serve as a project management tool. It does NOT replace
NATIONAL SCIENCE FOUNDATION Grants.Gov Application Guide A Guide for Preparation and Submission of NSF Application via Grants.
 This document has been archived and replaced by grantsgovguide309. NATIONAL SCIENCE FOUNDATION A Guide for Preparation and Submission of NSF Application via Grants.gov June 15, 2006 Summary of Significant
This document has been archived and replaced by grantsgovguide309. NATIONAL SCIENCE FOUNDATION A Guide for Preparation and Submission of NSF Application via Grants.gov June 15, 2006 Summary of Significant
Crystal Miller, MPA Assistant Director Sponsored Programs Administration. March 26 & 27, 2009 April 3, 2009
 Crystal Miller, MPA Assistant Director Sponsored Programs Administration March 26 & 27, 2009 April 3, 2009 NIH Funding Current Announcements Timelines and Process Challenge Grants Other NIH Stimulus Opportunities
Crystal Miller, MPA Assistant Director Sponsored Programs Administration March 26 & 27, 2009 April 3, 2009 NIH Funding Current Announcements Timelines and Process Challenge Grants Other NIH Stimulus Opportunities
1 - Issue tracker... 1. 2 - Proposal submission Cayuse... 4 WISPER...30 Final Steps...44 3 JIT...46. 4 - Project set- up...47
 Grants Management Desk Manual 1 - Issue tracker... 1 2 - Proposal submission Cayuse... 4 WISPER...30 Final Steps...44 3 JIT...46 4 - Project set- up...47 5 Subcontracts Issuing a Subcontract (needs some
Grants Management Desk Manual 1 - Issue tracker... 1 2 - Proposal submission Cayuse... 4 WISPER...30 Final Steps...44 3 JIT...46 4 - Project set- up...47 5 Subcontracts Issuing a Subcontract (needs some
esnap Progress Reports: Submission via the era Commons UC Irvine Office of Research Sponsored Projects Administration
 esnap Progress Reports: Submission via the era Commons UC Irvine Office of Research Sponsored Projects Administration Overview esnap: Electronic Streamlined Non-competing Award Process Progress Reports
esnap Progress Reports: Submission via the era Commons UC Irvine Office of Research Sponsored Projects Administration Overview esnap: Electronic Streamlined Non-competing Award Process Progress Reports
Office of Sponsored Projects NIH INSTITUTIONAL TRAINING GRANT GUIDE RUTH L. KIRSCHSTEIN NATIONAL SERVICE AWARDS (NRSA) IN COEUS
 Office of Sponsored Projects NIH INSTITUTIONAL TRAINING GRANT GUIDE RUTH L. KIRSCHSTEIN NATIONAL SERVICE AWARDS (NRSA) IN COEUS Coeus Premium 4.5.1.X Document Version 4 Updated June 2013 Contents I. ABOUT
Office of Sponsored Projects NIH INSTITUTIONAL TRAINING GRANT GUIDE RUTH L. KIRSCHSTEIN NATIONAL SERVICE AWARDS (NRSA) IN COEUS Coeus Premium 4.5.1.X Document Version 4 Updated June 2013 Contents I. ABOUT
NIH Electronic Grant Submission. Maqsood Wani
 NIH Electronic Grant Submission Maqsood Wani Electronic Submission Process New Process New Forms but It s Still About the Science! Our philosophy and policy has not changed! 2 The Plan OMB set the following
NIH Electronic Grant Submission Maqsood Wani Electronic Submission Process New Process New Forms but It s Still About the Science! Our philosophy and policy has not changed! 2 The Plan OMB set the following
INFOED PROPOSAL DEVELOPMENT TIPS. Version 12
 INFOED PROPOSAL DEVELOPMENT TIPS General: We recommend using Mozilla Firefox as your browser. These tips are geared toward NIH proposals, although other sponsors are available. We recommend uploading PDFs
INFOED PROPOSAL DEVELOPMENT TIPS General: We recommend using Mozilla Firefox as your browser. These tips are geared toward NIH proposals, although other sponsors are available. We recommend uploading PDFs
Scleroderma Foundation Research Grant Application Instructions
 1. Application Scleroderma Foundation Research Grant Application Instructions Grant applications that do not meet these guidelines will not be reviewed. Please download our text format version. Grant Application
1. Application Scleroderma Foundation Research Grant Application Instructions Grant applications that do not meet these guidelines will not be reviewed. Please download our text format version. Grant Application
Charles Eason SBIR/STTR Specialist Tech Futures Group (707) 863-7846 charles.eason@solano.edu
 Charles Eason SBIR/STTR Specialist Tech Futures Group (707) 863-7846 charles.eason@solano.edu ShapeMaker Technology Rapid Prototyping Process called Thick Layer Object Manufacturing Can produce objects
Charles Eason SBIR/STTR Specialist Tech Futures Group (707) 863-7846 charles.eason@solano.edu ShapeMaker Technology Rapid Prototyping Process called Thick Layer Object Manufacturing Can produce objects
2016 EARLY INVESTIGATOR GRANT APPLICATION TITLE PAGE
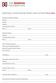 TITLE PAGE Principal Investigator, Degree: Division: Address: City/State/Zip/Country: Phone: Email: Institution Address: City/State/Zip/Country: Phone: Email: Title of Proposed Project: Amount of Funding
TITLE PAGE Principal Investigator, Degree: Division: Address: City/State/Zip/Country: Phone: Email: Institution Address: City/State/Zip/Country: Phone: Email: Title of Proposed Project: Amount of Funding
Creating the Detailed Budget in Coeus: Rates/Boundaries Budget Persons The Running Man
 Creating the Detailed Budget in Coeus: Select Edit>Budget Select New If any investigator has multiple appointments, a window will pop up. Select the primary appointment for this proposal. OH Rate Type
Creating the Detailed Budget in Coeus: Select Edit>Budget Select New If any investigator has multiple appointments, a window will pop up. Select the primary appointment for this proposal. OH Rate Type
 OMB Number: 4040-0004 Expiration Date: 01/31/2009 Application for Federal Assistance SF-424 Version 02 * 1. Type of Submission: Preapplication Application Changed/Corrected Application * 2. Type of Application:
OMB Number: 4040-0004 Expiration Date: 01/31/2009 Application for Federal Assistance SF-424 Version 02 * 1. Type of Submission: Preapplication Application Changed/Corrected Application * 2. Type of Application:
http://grants.nih.gov/grants/electronicreceipt/faq_full.htm
 Page 1 of 20 Glossary & Acronyms Frequently Asked Questions Applying Electronically Public FAQs NIH Staff FAQs Last Revised: September 27, 2012 Related NIH Staff FAQs Back to List On This Page: I. Preparing
Page 1 of 20 Glossary & Acronyms Frequently Asked Questions Applying Electronically Public FAQs NIH Staff FAQs Last Revised: September 27, 2012 Related NIH Staff FAQs Back to List On This Page: I. Preparing
Tips and Guidelines for an NIH Proposal
 Tips and Guidelines for an NIH Proposal FORMAT SPECIFICATIONS All file attachments must be in PDF format, and should have descriptive filenames of 50 characters or less, using only standard characters.
Tips and Guidelines for an NIH Proposal FORMAT SPECIFICATIONS All file attachments must be in PDF format, and should have descriptive filenames of 50 characters or less, using only standard characters.
CDMRP ereceipt System User Guide
 CDMRP ereceipt System User Guide Congressionally Directed Medical Research Programs (CDMRP) Last Updated: January 2011 Table of Contents Overview... 3 Purpose... 3 Help Desk Contact Information... 3 System
CDMRP ereceipt System User Guide Congressionally Directed Medical Research Programs (CDMRP) Last Updated: January 2011 Table of Contents Overview... 3 Purpose... 3 Help Desk Contact Information... 3 System
Process of a Grant Or Agency Application
 1.0 Purpose: To present the procedures for the processing of a Grants.gov application. 2.0 Procedure: Grants.gov application 2.1 Responsibility: Principal Investigator (PI) 2.1.1 Searches for Funding Opportunity.
1.0 Purpose: To present the procedures for the processing of a Grants.gov application. 2.0 Procedure: Grants.gov application 2.1 Responsibility: Principal Investigator (PI) 2.1.1 Searches for Funding Opportunity.
Grant Writing Workshop for Young Investigators
 Grant Writing Workshop for Young Investigators Elizabeth R. Albro, Ph.D. Associate Commissioner Teaching and Learning Division National Center for Education Research Getting Started Recognize that competing
Grant Writing Workshop for Young Investigators Elizabeth R. Albro, Ph.D. Associate Commissioner Teaching and Learning Division National Center for Education Research Getting Started Recognize that competing
AUTOMATED PROPOSAL COMPLIANCE CHECKS PERFORMED BY SYSTEM AS OF JULY 24TH, 2015.*
 BLANK N/A = The system runs a compliance check and an Error or Warning message will be displayed (as noted in the Error/ Warning column) if the proposal fails the compliance check. = The system does not
BLANK N/A = The system runs a compliance check and an Error or Warning message will be displayed (as noted in the Error/ Warning column) if the proposal fails the compliance check. = The system does not
Moving to the Top: Establishing Your Research Program at BWH MODULE 5: Money: How to Spend it January 28, 2010
 Moving to the Top: Establishing Your Research Program at BWH MODULE 5: Money: How to Spend it January 28, 2010 Recommended Reading: Chapter 4, Academic Scientists at Work TOPICS: 1) Get to know your research
Moving to the Top: Establishing Your Research Program at BWH MODULE 5: Money: How to Spend it January 28, 2010 Recommended Reading: Chapter 4, Academic Scientists at Work TOPICS: 1) Get to know your research
APPLICATION GUIDELINES MIZUTANI FOUNDATION FOR GLYCOSCIENCE THE 23rd RESEARCH GRANT (for 2016)
 APPLICATION GUIDELINES MIZUTANI FOUNDATION FOR GLYCOSCIENCE THE 23rd RESEARCH GRANT (for 2016) ================================== General Information: Mizutani Foundation for Glycoscience is a non-profit
APPLICATION GUIDELINES MIZUTANI FOUNDATION FOR GLYCOSCIENCE THE 23rd RESEARCH GRANT (for 2016) ================================== General Information: Mizutani Foundation for Glycoscience is a non-profit
2015 Grant Application Guidelines for A Awards
 2015 Grant Application Guidelines for A Awards In this packet you will find the following: Award Overview and Eligibility - page 2, 3 Application Information and Outline - pages 4, 5 Important Dates and
2015 Grant Application Guidelines for A Awards In this packet you will find the following: Award Overview and Eligibility - page 2, 3 Application Information and Outline - pages 4, 5 Important Dates and
Instructions for Submission: Research Grant Applications National Multiple Sclerosis Society 2016
 Instructions for Submission: Research Grant Applications National Multiple Sclerosis Society 2016 INTRODUCTION Please read these instructions and follow them carefully. Applications that are incomplete
Instructions for Submission: Research Grant Applications National Multiple Sclerosis Society 2016 INTRODUCTION Please read these instructions and follow them carefully. Applications that are incomplete
ENHANCING PEER REVIEW Changes to Application Forms and Instructions October 6, 2009
 ENHANCING PEER REVIEW Changes to Application Forms and Instructions October 6, 2009 NIH Peer Review System Cornerstone of the NIH Extramural Mission Standard of Excellence Worldwide Collaboration between
ENHANCING PEER REVIEW Changes to Application Forms and Instructions October 6, 2009 NIH Peer Review System Cornerstone of the NIH Extramural Mission Standard of Excellence Worldwide Collaboration between
SCHOOL OF MEDICINE OFFICE OF RESEARCH ADMINISTRATION
 SCHOOL OF MEDICINE OFFICE OF RESEARCH ADMINISTRATION Step By Step Guide for: Coeus Proposal Development (Coeus PD) (in place of eis) for Paper (Hard) Copy and Electronic (non Grants.gov) submission Effective
SCHOOL OF MEDICINE OFFICE OF RESEARCH ADMINISTRATION Step By Step Guide for: Coeus Proposal Development (Coeus PD) (in place of eis) for Paper (Hard) Copy and Electronic (non Grants.gov) submission Effective
Grants and Cooperative Agreements with Subawards you must enter the detailed subaward budget.
 `Grants and Contracts Office Grants and Contracts Office One Gustave L. Levy Place Box 1075 New York, NY 10029-6574 Phone: 212. 824-8300 Facsimile: 212.241-3294 Email: grants@mssm.edu Memorandum To: All
`Grants and Contracts Office Grants and Contracts Office One Gustave L. Levy Place Box 1075 New York, NY 10029-6574 Phone: 212. 824-8300 Facsimile: 212.241-3294 Email: grants@mssm.edu Memorandum To: All
FINANCIAL ASSISTANCE FUNDING OPPORTUNITY ANNOUNCEMENT. Small Business Innovation Research (SBIR) Small Business Technology Transfer (STTR)
 FINANCIAL ASSISTANCE FUNDING OPPORTUNITY ANNOUNCEMENT Small Business Innovation Research (SBIR) Small Business Technology Transfer (STTR) FY 2012 Phase II Funding Opportunity Number: DE-FOA-0000676 Announcement
FINANCIAL ASSISTANCE FUNDING OPPORTUNITY ANNOUNCEMENT Small Business Innovation Research (SBIR) Small Business Technology Transfer (STTR) FY 2012 Phase II Funding Opportunity Number: DE-FOA-0000676 Announcement
The PCORI Application Process
 The PCORI Application Process James Hulbert Pre- Award Manager February 19, 2015 Welcome! James Hulbert Pre-Award Manager 2 Session Objectives List and define each sec>on of the PCORI applica>on State the
The PCORI Application Process James Hulbert Pre- Award Manager February 19, 2015 Welcome! James Hulbert Pre-Award Manager 2 Session Objectives List and define each sec>on of the PCORI applica>on State the
Q: What is the best way to structure my multi project application from a budget perspective?
 BUDGET Q and A related to ASSIST NIH era Items of Interest September 16, 2013 Sheri Cummins The following questions are contained in this article: What is the best way to structure my multi project application
BUDGET Q and A related to ASSIST NIH era Items of Interest September 16, 2013 Sheri Cummins The following questions are contained in this article: What is the best way to structure my multi project application
T 32 Training Grants and the Department Administrator
 T 32 Training Grants and the Department Administrator 1 Glenda Bullock, Manager of Business Operations, Division of Hematology, Washington University Brenda Kavanaugh, Assistant Director, Office of Research
T 32 Training Grants and the Department Administrator 1 Glenda Bullock, Manager of Business Operations, Division of Hematology, Washington University Brenda Kavanaugh, Assistant Director, Office of Research
General Application Instructions. Fiscal Year 2015. Defense Health Program Department of Defense Congressionally Directed Medical Research Programs
 General Application Instructions Fiscal Year 2015 Defense Health Program Department of Defense Congressionally Directed Medical Research Programs Table of Contents I. HELPFUL INFORMATION... 2 A. Tips for
General Application Instructions Fiscal Year 2015 Defense Health Program Department of Defense Congressionally Directed Medical Research Programs Table of Contents I. HELPFUL INFORMATION... 2 A. Tips for
Division of Nutritional Sciences Grant Proposal Preparation and Submission Guidelines
 Division of Nutritional Sciences Grant Proposal Preparation and Submission Guidelines A. DNS Grant and Contract Administrator and DNS Office of Administrative Support (OAS) and what they will or can do
Division of Nutritional Sciences Grant Proposal Preparation and Submission Guidelines A. DNS Grant and Contract Administrator and DNS Office of Administrative Support (OAS) and what they will or can do
Instructions for Completing a DOE SBIR/STTR Phase I Grant Application
 U.S. Department of Energy (DOE) Instructions for Completing a DOE SBIR/STTR Phase I Grant Application (Please refer to the respective Funding Opportunity Announcement for Fast-Track and Phase II guidance)
U.S. Department of Energy (DOE) Instructions for Completing a DOE SBIR/STTR Phase I Grant Application (Please refer to the respective Funding Opportunity Announcement for Fast-Track and Phase II guidance)
Research Performance Progress Report (RPPR) Overview. Office of Grants and Contracts Administration
 Research Performance Progress Report (RPPR) Overview Office of Grants and Contracts Administration Overview on RPPR NIH Implementation of RPPR OMB mandated that federal agencies implement a federal-wide
Research Performance Progress Report (RPPR) Overview Office of Grants and Contracts Administration Overview on RPPR NIH Implementation of RPPR OMB mandated that federal agencies implement a federal-wide
March 7, 2016, NIH Webinar. THE FOLLOWING IS AN UNEDITED ROUGH DRAFT TRANSLATION FROM THE CART PROVIDER'S OUTPUT
 March 7, 2016, NIH Webinar. ****************DISCLAIMER!!!**************** THE FOLLOWING IS AN UNEDITED ROUGH DRAFT TRANSLATION FROM THE CART PROVIDER'S OUTPUT FILE. THIS TRANSCRIPT IS NOT VERBATIM AND
March 7, 2016, NIH Webinar. ****************DISCLAIMER!!!**************** THE FOLLOWING IS AN UNEDITED ROUGH DRAFT TRANSLATION FROM THE CART PROVIDER'S OUTPUT FILE. THIS TRANSCRIPT IS NOT VERBATIM AND
Transferring Grants. between Institutions. November 2014. You will learn: Grant Transfers
 Transferring Grants between Institutions November 2014 You will learn: Grant Transfers General principles Who is involved Specifics on transferring in and out How an administrator can help Resources available
Transferring Grants between Institutions November 2014 You will learn: Grant Transfers General principles Who is involved Specifics on transferring in and out How an administrator can help Resources available
Research Resources (R 2 ) Office
 Grant Prep Boot Camp Robyn Gershon, MHS, DrPH Associate Dean for Research Resources Columbia University Mailman School of Public Health Research Resources (R 2 ) Office 1 Grant Prep Battleplan Initiate
Grant Prep Boot Camp Robyn Gershon, MHS, DrPH Associate Dean for Research Resources Columbia University Mailman School of Public Health Research Resources (R 2 ) Office 1 Grant Prep Battleplan Initiate
Program Announcement. Neurofibromatosis Research Program
 Program Announcement Department of Defense Congressionally Directed Medical Research Programs Neurofibromatosis Research Program New Investigator Award Funding Opportunity Number: W81XWH-13-NFRP-NIA Catalog
Program Announcement Department of Defense Congressionally Directed Medical Research Programs Neurofibromatosis Research Program New Investigator Award Funding Opportunity Number: W81XWH-13-NFRP-NIA Catalog
Proposal Development Guide
 Proposal Development Guide Contents The Proposal Tab... 3 Document Overview Panel... 3 Required Fields for Saving Document Panel... 3 Institutional Fields (Conditionally Required)... 9 Sponsor & Program
Proposal Development Guide Contents The Proposal Tab... 3 Document Overview Panel... 3 Required Fields for Saving Document Panel... 3 Institutional Fields (Conditionally Required)... 9 Sponsor & Program
NIH Research Performance Progress Reports (RPPR)
 NIH Research Performance Progress Reports (RPPR) Helpful Hints for the UW Research Community This document is meant as a tool for research administrators to collect information from PIs for the new RPPR
NIH Research Performance Progress Reports (RPPR) Helpful Hints for the UW Research Community This document is meant as a tool for research administrators to collect information from PIs for the new RPPR
SF424 (R&R) Application Guide for NIH and Other PHS Agencies
 U.S. Department of Health and Human Services Public Health Service SF424 (R&R) Application Guide for NIH and Other PHS Agencies A guide developed and maintained by NIH for preparing and submitting applications
U.S. Department of Health and Human Services Public Health Service SF424 (R&R) Application Guide for NIH and Other PHS Agencies A guide developed and maintained by NIH for preparing and submitting applications
Program Announcement. Ovarian Cancer Research Program
 Program Announcement for the Department of Defense Defense Health Program Congressionally Directed Medical Research Programs Ovarian Cancer Research Program Ovarian Cancer Academy Early-Career Investigator
Program Announcement for the Department of Defense Defense Health Program Congressionally Directed Medical Research Programs Ovarian Cancer Research Program Ovarian Cancer Academy Early-Career Investigator
System-to-System FAQs User Role(s): Central, Department, Principal Investigator Last Updated: 06/16/2015
 GMAS System-to-System FAQs Please note that similar issues below could be resulting from other issues, and resolutions may not work. In those scenarios contact the helpdesk (contactgmas@harvard.edu) with
GMAS System-to-System FAQs Please note that similar issues below could be resulting from other issues, and resolutions may not work. In those scenarios contact the helpdesk (contactgmas@harvard.edu) with
DEFENSE EXPERIMENTAL PROGRAM TO STIMULATE COMPETITIVE RESEARCH (DEPSCoR)
 PROGRAM ANNOUNCEMENT THE DEPARTMENT OF DEFENSE (DoD) FISCAL YEAR 2009 DEFENSE EXPERIMENTAL PROGRAM TO STIMULATE COMPETITIVE RESEARCH (DEPSCoR) Broad Agency Announcement No.: W911NF-09-R-0003 FULL PROPOSAL
PROGRAM ANNOUNCEMENT THE DEPARTMENT OF DEFENSE (DoD) FISCAL YEAR 2009 DEFENSE EXPERIMENTAL PROGRAM TO STIMULATE COMPETITIVE RESEARCH (DEPSCoR) Broad Agency Announcement No.: W911NF-09-R-0003 FULL PROPOSAL
Biostatistical support from CTSI RDC/BRC is already budgeted for Scholars and does not need to be included
 Budget Instructions for Tufts CTSI KL2 Application The proposed budget must be submitted using the NIH 398 forms and include a budget justification as described below. Please download the Form Page 4:
Budget Instructions for Tufts CTSI KL2 Application The proposed budget must be submitted using the NIH 398 forms and include a budget justification as described below. Please download the Form Page 4:
FY 2016 Funding Opportunity Announcement (FOA): PART II
 Department of Health and Human Services Substance Abuse and Mental Health Services Administration FY 2016 Funding Opportunity Announcement (FOA): PART II General Policies and Procedures Applicable to all
Department of Health and Human Services Substance Abuse and Mental Health Services Administration FY 2016 Funding Opportunity Announcement (FOA): PART II General Policies and Procedures Applicable to all
Instructions for Application Submission Postdoctoral Fellowship
 Instructions for Application Submission Postdoctoral Fellowship 2016 INTRODUCTION Please read these instructions and follow them carefully. Applications that are incomplete exceed the page limitations,
Instructions for Application Submission Postdoctoral Fellowship 2016 INTRODUCTION Please read these instructions and follow them carefully. Applications that are incomplete exceed the page limitations,
Great Grant Writing: Learning the Fundamentals of Effective Proposal Development
 Great Grant Writing: Learning the Fundamentals of Effective Proposal Development Jill Morris-IPR Grants Program Manager Jeff Agnoli-Education, Funding and Research Development-Office of the Vice President
Great Grant Writing: Learning the Fundamentals of Effective Proposal Development Jill Morris-IPR Grants Program Manager Jeff Agnoli-Education, Funding and Research Development-Office of the Vice President
FINAL FORMAT RESEARCH PERFORMANCE PROGRESS REPORT
 FINAL FORMAT RESEARCH PERFORMANCE PROGRESS REPORT Background Effective with publication of this Notice in the Federal Register (Volume 75, Number 8, Pages 1816-1819), agencies will be able to utilize a
FINAL FORMAT RESEARCH PERFORMANCE PROGRESS REPORT Background Effective with publication of this Notice in the Federal Register (Volume 75, Number 8, Pages 1816-1819), agencies will be able to utilize a
Questions and Answers from the Informational Conference Calls for RFA CE12-001 Grant for Injury Control Research Centers
 Questions and Answers from the Informational Conference Calls for RFA CE12-001 Grant for Injury Control Research Centers Two informational conference calls for potential applicants for funding opportunity
Questions and Answers from the Informational Conference Calls for RFA CE12-001 Grant for Injury Control Research Centers Two informational conference calls for potential applicants for funding opportunity
Program Announcement. Clinical and Rehabilitative Medicine Research Program
 Program Announcement for the Department of Defense Congressionally Directed Medical Research Programs Clinical and Rehabilitative Medicine Research Program Vision Prosthesis Pilot Study Award Funding Opportunity
Program Announcement for the Department of Defense Congressionally Directed Medical Research Programs Clinical and Rehabilitative Medicine Research Program Vision Prosthesis Pilot Study Award Funding Opportunity
NIH Research Performance Progress Reports (RPPR) Helpful Hints for completing the RPPR
 This document is a tool for research administrators to collect information from PIs for the new RPPR format, and to give some tips/explanations for areas that look/feel different than esnap. NIH has an
This document is a tool for research administrators to collect information from PIs for the new RPPR format, and to give some tips/explanations for areas that look/feel different than esnap. NIH has an
FastLane Proposal Preparation. 1. PI receives password from Office of Sponsored Research. 2. PI accesses FastLane homepage <www.fastlane.nsf.gov>.
 1. PI receives password from Office of Sponsored Research. 2. PI accesses FastLane homepage . 3. PI clicks on Proposal Preparation. 4. PI types in last name, SSN, and password, then
1. PI receives password from Office of Sponsored Research. 2. PI accesses FastLane homepage . 3. PI clicks on Proposal Preparation. 4. PI types in last name, SSN, and password, then
PennERA Proposals v12 Upgrade
 PennERA Proposals v12 Upgrade Agenda General Information Breaking News and Important Reminders New Features and Improvements Demo: New Portal Navigation Demo: Proposal Creation Known Issues Resources Contact
PennERA Proposals v12 Upgrade Agenda General Information Breaking News and Important Reminders New Features and Improvements Demo: New Portal Navigation Demo: Proposal Creation Known Issues Resources Contact
NIH and Other PHS Agency Research Performance Progress Report (RPPR) Instruction Guide
 NIH and Other PHS Agency Research Performance Progress Report (RPPR) Instruction Guide Document Version 9.5.0 July 17, 2015 Forms Approved Through 08/31/2015 OMB No. 0925-0002 CONTACT US Document Comments:
NIH and Other PHS Agency Research Performance Progress Report (RPPR) Instruction Guide Document Version 9.5.0 July 17, 2015 Forms Approved Through 08/31/2015 OMB No. 0925-0002 CONTACT US Document Comments:
Program Announcement. Autism Research Program
 Program Announcement for the Defense Health Program Department of Defense Congressionally Directed Medical Research Programs Autism Research Program Pilot Award Funding Opportunity Number: W81XWH-13-ARP-PA
Program Announcement for the Defense Health Program Department of Defense Congressionally Directed Medical Research Programs Autism Research Program Pilot Award Funding Opportunity Number: W81XWH-13-ARP-PA
STTR PROGRAMMATIC INVESTMENT GRANT APPLICATION
 2016 STTR PROGRAMMATIC INVESTMENT GRANT APPLICATION Application Deadline: November 16, 2015 November 30, 2015 BLADDER BRAIN BREAST COLORECTAL HEAD & NECK LUNG OVARY PANCREAS PROSTATE SARCOMA APPLICATION
2016 STTR PROGRAMMATIC INVESTMENT GRANT APPLICATION Application Deadline: November 16, 2015 November 30, 2015 BLADDER BRAIN BREAST COLORECTAL HEAD & NECK LUNG OVARY PANCREAS PROSTATE SARCOMA APPLICATION
Instructions for Accessing the Inclusion Management System (IMS) via Commons Status. Document Version 2.1.0
 Instructions for Accessing the Inclusion Management System (IMS) via Commons Status Document Version 2.1.0 July 17, 2015 CONTACT US Document Comments: We value your feedback on this document. Please email
Instructions for Accessing the Inclusion Management System (IMS) via Commons Status Document Version 2.1.0 July 17, 2015 CONTACT US Document Comments: We value your feedback on this document. Please email
READING THE FUNDING OPPORTUNITY ANNOUNCEMENT (FOA)
 READING THE FUNDING OPPORTUNITY ANNOUNCEMENT (FOA) Overview of Federal, Non-Profit and Private Foundation Funding Opportunity Announcements Office of Sponsored Projects WHAT IS A FUNDING OPPORTUNITY ANNOUNCEMENT?
READING THE FUNDING OPPORTUNITY ANNOUNCEMENT (FOA) Overview of Federal, Non-Profit and Private Foundation Funding Opportunity Announcements Office of Sponsored Projects WHAT IS A FUNDING OPPORTUNITY ANNOUNCEMENT?
 OMB Number: 4040-0004 Expiration Date: 01/31/2009 Application for Federal Assistance SF-424 Version 02 * 1. Type of Submission: * 2. Type of Application: Preapplication New * If Revision, select appropriate
OMB Number: 4040-0004 Expiration Date: 01/31/2009 Application for Federal Assistance SF-424 Version 02 * 1. Type of Submission: * 2. Type of Application: Preapplication New * If Revision, select appropriate
UFIRST Training Manual
 UFIRST Training Manual Division of Sponsored Programs UF Training & Organizational Development Page 1 TABLE OF CONTENTS UFIRST 5 INTRODUCTION 5 ADDITIONAL RESOURCES 5 HOW TO USE THIS MANUAL 5 ACKNOWLEDGEMENTS
UFIRST Training Manual Division of Sponsored Programs UF Training & Organizational Development Page 1 TABLE OF CONTENTS UFIRST 5 INTRODUCTION 5 ADDITIONAL RESOURCES 5 HOW TO USE THIS MANUAL 5 ACKNOWLEDGEMENTS
KRIEGER SCHOOL OF ARTS & SCIENCES GRANT PROPOSAL GUIDE
 KRIEGER SCHOOL OF ARTS & SCIENCES GRANT PROPOSAL GUIDE Presented by the Grant Proposal Guide Workgroup Fall, 2011 GRANT PROPOSAL GUIDE TABLE OF CONTENTS INTRODUCTION... 4 NEW TO DOING PROPOSALS... 5 NEW
KRIEGER SCHOOL OF ARTS & SCIENCES GRANT PROPOSAL GUIDE Presented by the Grant Proposal Guide Workgroup Fall, 2011 GRANT PROPOSAL GUIDE TABLE OF CONTENTS INTRODUCTION... 4 NEW TO DOING PROPOSALS... 5 NEW
HIGHER Ground. Women s Leadership Development Program. A program of The Grace E. Harris Leadership Institute at Virginia Commonwealth University
 HIGHER Ground Women s Leadership Development Program A program of The Grace E. Harris Leadership Institute at Virginia Commonwealth University V i r g i n i a C o m m o n w e a l t h U n i v e r s i t
HIGHER Ground Women s Leadership Development Program A program of The Grace E. Harris Leadership Institute at Virginia Commonwealth University V i r g i n i a C o m m o n w e a l t h U n i v e r s i t
Instructions for Application Submission National MS Society-American Academy of Neurology (AAN) Clinician Scientist Development Award 2016
 Instructions for Application Submission National MS Society-American Academy of Neurology (AAN) Clinician Scientist Development Award 2016 INTRODUCTION Please read these instructions and follow them carefully.
Instructions for Application Submission National MS Society-American Academy of Neurology (AAN) Clinician Scientist Development Award 2016 INTRODUCTION Please read these instructions and follow them carefully.
National Institutes of Health/Office of Extramural Research. esnap User Guide
 National Institutes of Health/Office of Extramural Research esnap User Guide Version 2.2.3.0 August 01, 2003 Table of Contents Introduction...4 Logging In to esnap...4 Logging In...4 Logging Out...5 Username
National Institutes of Health/Office of Extramural Research esnap User Guide Version 2.2.3.0 August 01, 2003 Table of Contents Introduction...4 Logging In to esnap...4 Logging In...4 Logging Out...5 Username
Department of Health and Human Services Part 1. Overview Information
 Department of Health and Human Services Part 1. Overview Information Participating Organization(s) U.S. Food and Drug Administration (FDA) The FDA does not follow the NIH Page Limitation Guidelines or
Department of Health and Human Services Part 1. Overview Information Participating Organization(s) U.S. Food and Drug Administration (FDA) The FDA does not follow the NIH Page Limitation Guidelines or
Clinical Trials in Thalassemia Cell and Gene Therapy Grant Award
 Clinical Trials in Thalassemia Cell and Gene Therapy Grant Award The Cooley s Anemia Foundation invites national and international applicants to apply for grants to facilitate clinical trials in Cell and
Clinical Trials in Thalassemia Cell and Gene Therapy Grant Award The Cooley s Anemia Foundation invites national and international applicants to apply for grants to facilitate clinical trials in Cell and
NIH era Items of Interest - September 16, 2013 Budgets Make My Brain Hurt
 NIH era Items of Interest - September 16, 2013 Budgets Make My Brain Hurt I have budget phobia. There I said it. It is a bit embarrassing to admit I work with grants after all, but it s true. I'm actually
NIH era Items of Interest - September 16, 2013 Budgets Make My Brain Hurt I have budget phobia. There I said it. It is a bit embarrassing to admit I work with grants after all, but it s true. I'm actually
Cystic Fibrosis Foundation STUDENT TRAINEESHIP GRANT
 Cystic Fibrosis Foundation STUDENT TRAINEESHIP GRANT Policies and Guidelines CFF Grants and Contracts Office 6931 Arlington Road Bethesda, MD 20814 (301) 841-2637 grants@cff.org STUDENT TRAINEESHIP GRANT
Cystic Fibrosis Foundation STUDENT TRAINEESHIP GRANT Policies and Guidelines CFF Grants and Contracts Office 6931 Arlington Road Bethesda, MD 20814 (301) 841-2637 grants@cff.org STUDENT TRAINEESHIP GRANT
CROHN S & COLITIS FOUNDATION OF AMERICA. Senior Research Award INSTRUCTIONS. Effective September 2013
 CROHN S & COLITIS FOUNDATION OF AMERICA Senior Research Award INSTRUCTIONS Effective September 2013 Crohn s & Colitis Foundation of America National Office Research & Scientific Programs Department 733
CROHN S & COLITIS FOUNDATION OF AMERICA Senior Research Award INSTRUCTIONS Effective September 2013 Crohn s & Colitis Foundation of America National Office Research & Scientific Programs Department 733
Title of project: Please observe the size limitation of the box and limit the title to a maximum of two lines.
 Medical Grant Application Information Thank you for your interest in The Children's Heart Foundation (CHF). The CHF supports clinical and basic science research in congenital heart disease, including,
Medical Grant Application Information Thank you for your interest in The Children's Heart Foundation (CHF). The CHF supports clinical and basic science research in congenital heart disease, including,
Title Title should be short and descriptive of the proposed research. Maximum space limit is 81 characters, including spaces.
 DETAILED GUIDELINES FOR THE NIH PROPOSAL (NOTE: While these guidelines are specific to the NIH proposal, the principles stated here are also applicable for proposals to other major research funders) This
DETAILED GUIDELINES FOR THE NIH PROPOSAL (NOTE: While these guidelines are specific to the NIH proposal, the principles stated here are also applicable for proposals to other major research funders) This
SAMPLE. PROGRAM ELIGIBILITY INFORMATION: (Responses to selected fields displayed below. For some grant programs this section may be blank.
 Grant Application Date Submitted: Proposal Type: If renewal, current grant: Resubmission? 1st or 2nd: TITLE OF PROJECT (s exceeding 81 characters, including spaces and punctuation, will be truncated.)
Grant Application Date Submitted: Proposal Type: If renewal, current grant: Resubmission? 1st or 2nd: TITLE OF PROJECT (s exceeding 81 characters, including spaces and punctuation, will be truncated.)
American Venous Forum
 American Venous Forum Principal Investigator (Last, first, middle): LEAVE BLANK FOR AVF RESEARCH REVIEW COMMITTEE USE ONLY. RESEARCH REVIEW COMMITTEE Grant Deadline Date Received BSN-JOBST INC. Grant Application
American Venous Forum Principal Investigator (Last, first, middle): LEAVE BLANK FOR AVF RESEARCH REVIEW COMMITTEE USE ONLY. RESEARCH REVIEW COMMITTEE Grant Deadline Date Received BSN-JOBST INC. Grant Application
Program Announcement. Bone Marrow Failure Research Program
 Program Announcement for the Department of Defense Defense Health Program Congressionally Directed Medical Research Programs Bone Marrow Failure Research Program Idea Development Award Funding Opportunity
Program Announcement for the Department of Defense Defense Health Program Congressionally Directed Medical Research Programs Bone Marrow Failure Research Program Idea Development Award Funding Opportunity
DEPARTMENT OF VETERANS AFFAIRS Veterans Health Administration Office of Academic Affiliations Washington, DC
 July 11, 2013 DEPARTMENT OF VETERANS AFFAIRS Veterans Health Administration Office of Academic Affiliations Washington, DC PROGRAM ANNOUNCEMENT Post-doctoral Nurse Fellowship Program 1. PURPOSE AND GOAL
July 11, 2013 DEPARTMENT OF VETERANS AFFAIRS Veterans Health Administration Office of Academic Affiliations Washington, DC PROGRAM ANNOUNCEMENT Post-doctoral Nurse Fellowship Program 1. PURPOSE AND GOAL
Noncompeting Continuation (NCC) Progress Report - Research & Related (R&R) Grants
 HRSA EHB USER GUIDE Noncompeting Continuation (NCC) Progress Report - Research & Related (R&R) Grants User Guide for Grantees Last updated on: 03/09/2010 Table of Contents 1. INTRODUCTION... 6 1.1. DOCUMENT
HRSA EHB USER GUIDE Noncompeting Continuation (NCC) Progress Report - Research & Related (R&R) Grants User Guide for Grantees Last updated on: 03/09/2010 Table of Contents 1. INTRODUCTION... 6 1.1. DOCUMENT
Preparing a Budget for a Research Grant Proposal. Office of Sponsored Projects Faculty Education Working Group http://www.dartmouth.
 Preparing a Budget for a Research Grant Proposal Office of Sponsored Projects Faculty Education Working Group http://www.dartmouth.edu/~osp Table of Contents 1. SOLICITATIONS 2. THE PURPOSE & IMPORTANCE
Preparing a Budget for a Research Grant Proposal Office of Sponsored Projects Faculty Education Working Group http://www.dartmouth.edu/~osp Table of Contents 1. SOLICITATIONS 2. THE PURPOSE & IMPORTANCE
Program Announcement. Multiple Sclerosis Research Program
 Program Announcement for the Defense Health Program Defense Medical Research and Development Program Department of Defense Congressionally Directed Medical Research Programs Multiple Sclerosis Research
Program Announcement for the Defense Health Program Defense Medical Research and Development Program Department of Defense Congressionally Directed Medical Research Programs Multiple Sclerosis Research
Advanced Education Nursing Traineeship (AENT) Program - FY 2014 Funding Opportunity Announcement. Frequently Asked Questions
 Advanced Education Nursing Traineeship (AENT) Program - FY 2014 Funding Opportunity Announcement Frequently Asked Questions 1. How do I register for the Division of Nursing Listserve? Interested parties
Advanced Education Nursing Traineeship (AENT) Program - FY 2014 Funding Opportunity Announcement Frequently Asked Questions 1. How do I register for the Division of Nursing Listserve? Interested parties
Office of the Vice President for Research (OVPR) FY17 Pilot/Seed Grants to Attract External Funding. Request for Proposals
 Office of the Vice President for Research (OVPR) FY17 Pilot/Seed Grants to Attract External Funding Request for Proposals The Office of the Vice President for Research (OVPR) invites grant proposals for
Office of the Vice President for Research (OVPR) FY17 Pilot/Seed Grants to Attract External Funding Request for Proposals The Office of the Vice President for Research (OVPR) invites grant proposals for
Farm Business Management and Benchmarking (FBMB) Competitive Grants Program
 Farm Business Management and Benchmarking (FBMB) Competitive Grants Program Fiscal Year (FY) 2016 Request for Applications (RFA) APPLICATION DEADLINE: April 11, 2016 ELIGIBILITY: See Part III, A of RFA
Farm Business Management and Benchmarking (FBMB) Competitive Grants Program Fiscal Year (FY) 2016 Request for Applications (RFA) APPLICATION DEADLINE: April 11, 2016 ELIGIBILITY: See Part III, A of RFA
Research Performance Progress Report (RPPR) Dave Bowen Denise Clarke Office of Sponsored Programs
 Research Performance Progress Report (RPPR) Dave Bowen Denise Clarke Office of Sponsored Programs RPPR Structure 2 RPPR- Component instructions The basic RPPR Component Instructions apply to the following
Research Performance Progress Report (RPPR) Dave Bowen Denise Clarke Office of Sponsored Programs RPPR Structure 2 RPPR- Component instructions The basic RPPR Component Instructions apply to the following
Step-by-Step Guide to Submitting a SBIR/STTR Phase I Proposal in FastLane
 Step-by-Step Guide to Submitting a SBIR/STTR Phase I Proposal in FastLane ADA & 508 Compliance Assistance - Please Call the FastLane Help Desk at 1-800-673-6188. STOP: Do you have the required registrations?
Step-by-Step Guide to Submitting a SBIR/STTR Phase I Proposal in FastLane ADA & 508 Compliance Assistance - Please Call the FastLane Help Desk at 1-800-673-6188. STOP: Do you have the required registrations?
Program Announcement. Epilepsy Research Program
 Program Announcement for the Department of Defense Defense Health Program Congressionally Directed Medical Research Programs Joint Program Committee 6/ Combat Casualty Care Research Program Epilepsy Research
Program Announcement for the Department of Defense Defense Health Program Congressionally Directed Medical Research Programs Joint Program Committee 6/ Combat Casualty Care Research Program Epilepsy Research
Application Submission System & Interface for Submission Tracking (ASSIST) User Guide
 Application Submission System & Interface for Submission Tracking (ASSIST) User Guide System Version 2.14.1 Document Version 6.2.0 August 5, 2015 CONTACT US Document Comments: We value your feedback on
Application Submission System & Interface for Submission Tracking (ASSIST) User Guide System Version 2.14.1 Document Version 6.2.0 August 5, 2015 CONTACT US Document Comments: We value your feedback on
Program Announcement To DOE National Laboratories LAB 01-09 Scientific Discovery through Advanced Computing: Climate Change Prediction Program
 Program Announcement To DOE National Laboratories LAB 01-09 Scientific Discovery through Advanced Computing: Climate Change Prediction Program The Office of Biological and Environmental Research (OBER)
Program Announcement To DOE National Laboratories LAB 01-09 Scientific Discovery through Advanced Computing: Climate Change Prediction Program The Office of Biological and Environmental Research (OBER)
FINANCIAL ASSISTANCE FUNDING OPPORTUNITY ANNOUNCEMENT. U. S. Department of Energy Office of Science Office of Biological & Environmental Research
 FINANCIAL ASSISTANCE FUNDING OPPORTUNITY ANNOUNCEMENT U. S. Department of Energy Office of Science Office of Biological & Environmental Research Systems Biology Enabled Research on the Roles of Microbial
FINANCIAL ASSISTANCE FUNDING OPPORTUNITY ANNOUNCEMENT U. S. Department of Energy Office of Science Office of Biological & Environmental Research Systems Biology Enabled Research on the Roles of Microbial
RESEARCH ADMINISTRATION & FINANCE. Lunch & Learn
 RESEARCH ADMINISTRATION & FINANCE Lunch & Learn October 21, 2015 era Enhancements: Updates in esubmission Updates in ASSIST Updates in era Commons Discussion Topics DHHS NIH & AHRQ: Plannned changes to
RESEARCH ADMINISTRATION & FINANCE Lunch & Learn October 21, 2015 era Enhancements: Updates in esubmission Updates in ASSIST Updates in era Commons Discussion Topics DHHS NIH & AHRQ: Plannned changes to
Sponsored Programs New Developments and Important Reminders
 Sponsored Programs New Developments and Important Reminders Bonnie Pendergraft Rebecca Trahan Office of Sponsored Programs March 22, 2011 1 Training Session Overview NSF Updates NIH Updates Senior Personnel
Sponsored Programs New Developments and Important Reminders Bonnie Pendergraft Rebecca Trahan Office of Sponsored Programs March 22, 2011 1 Training Session Overview NSF Updates NIH Updates Senior Personnel
PS15-1505: Enhancing HIV Prevention Communication and Mobilization Efforts through Strategic Partnerships. Procurement and Grants Management Overview
 PS15-1505: Enhancing HIV Prevention Communication and Mobilization Efforts through Strategic Partnerships Procurement and Grants Management Overview Constance Jarvis Grants Management Specialist Procurement
PS15-1505: Enhancing HIV Prevention Communication and Mobilization Efforts through Strategic Partnerships Procurement and Grants Management Overview Constance Jarvis Grants Management Specialist Procurement
The What, When, and How of Grants.gov Finding Your Way with the Electronic Grant Application Process
 The What, When, and How of Grants.gov Finding Your Way with the Electronic Grant Application Process Presented by Charles Moore Office of Sponsored Projects Administration cmoore@email.uky.edu What is
The What, When, and How of Grants.gov Finding Your Way with the Electronic Grant Application Process Presented by Charles Moore Office of Sponsored Projects Administration cmoore@email.uky.edu What is
Program Announcement. Prostate Cancer Research Program
 Program Announcement for the Department of Defense Defense Health Program Congressionally Directed Medical Research Programs Prostate Cancer Research Program Collaborative Undergraduate Historically Black
Program Announcement for the Department of Defense Defense Health Program Congressionally Directed Medical Research Programs Prostate Cancer Research Program Collaborative Undergraduate Historically Black
OGRD Guideline 6 NIH Electronic Progress Report Guide
 OGRD Guideline 6 NIH Electronic Progress Report Guide WASHINGTON STATE UNIVERSITY The Office of Research Office of Grant and Research Development Revised March 2014 As per NIH NOT OD 13 061: Grantee institutions
OGRD Guideline 6 NIH Electronic Progress Report Guide WASHINGTON STATE UNIVERSITY The Office of Research Office of Grant and Research Development Revised March 2014 As per NIH NOT OD 13 061: Grantee institutions
How to Calculate Your NIH Cap Salary Level
 Brown University Office of Sponsored Projects NIH Cap Procedure Overview Entering NIH Salary Cap Information at Proposal Stage This document outlines the procedure for correctly completing the NIH Cap
Brown University Office of Sponsored Projects NIH Cap Procedure Overview Entering NIH Salary Cap Information at Proposal Stage This document outlines the procedure for correctly completing the NIH Cap
Plant Feedstock Genomics for Bioenergy: A Joint Research Funding Opportunity Announcement USDA, DOE
 FINANCIAL ASSISTANCE FUNDING OPPORTUNITY ANNOUNCEMENT U. S. Department of Energy Office of Science Office of Biological and Environmental Research U. S. Department of Agriculture National Institute of
FINANCIAL ASSISTANCE FUNDING OPPORTUNITY ANNOUNCEMENT U. S. Department of Energy Office of Science Office of Biological and Environmental Research U. S. Department of Agriculture National Institute of
SUBRECIPIENT CERTIFICATION FORM
 Our records indicate that your organization is currently being considered for receipt of a subaward under sponsored project funds awarded to the University of Washington. Please check all applicable Certifications
Our records indicate that your organization is currently being considered for receipt of a subaward under sponsored project funds awarded to the University of Washington. Please check all applicable Certifications
