ONCOLOGY LETTERS 8: , 2014
|
|
|
- Alyson Baldwin
- 10 years ago
- Views:
Transcription
1 2012 Salvage therapy with mitoxantrone, etoposide, bleomycin and dexamethasone for refractory or relapsed aggressive non-hodgkin's lymphoma patients with a poor performance status or comorbidity XUEDE LIN *, XI SHI *, WUCHA ZENG, MIN ZHENG and LIMING HUANG Department of Chemotherapy, The First Affiliated Hospital, Fujian Medical University, Fuzhou, Fujian , P.R. China Received January 3, 2014; Accepted August 7, 2014 DOI: /ol Abstract. The treatment of refractory or relapsed aggressive non- Hodgkin's lymphoma (NHL) in patients in a state of poor health is difficult due to their ineligibility to receive intensive salvage chemotherapy. In the present study, 16 refractory or relapsed aggressive NHL patients with a poor performance status or comorbidities were treated with mitoxantrone, etoposide, bleomycin and dexamethasone (MEBD) therapy. The treatment consisted of 10 mg/m 2 intravenous (IV) mitoxantrone on day 1, 75 mg/m 2 IV etoposide on days 1-3, 20 mg IV dexamethasone on days 1-4 and 15 mg intramuscular bleomycin on days 1, 4, 8 and 12, every 21 days. The efficacy and toxicity of the regimen were evaluated. The overall response rate was 68.8%, with a complete response rate of 18.8% and a partial response rate of 50.0%. The efficacy of the treatment for B cell lymphoma was greater than that for T cell lymphoma. The median progression-free survival time for the patients was 16.7 months and the median overall survival time was 22.4 months. The one year overall survival rate was 62.5% and the two year overall survival rate was 43.8%. The most common toxicity symptom was myelosuppression. In conclusion, refractory or relapsed aggressive NHL patients with a poor performance status or comorbidity are eligible for chemotherapy. MEBD therapy is an effective and feasible salvage regimen for NHL patients in a state of poor health. Introduction The majority of aggressive non Hodgkin's lymphoma (NHL) cases originate from B cells, with ~10% arising from T cells (1). Correspondence to: Dr Xi Shi, Department of Chemotherapy, The First Affiliated Hospital, Fujian Medical University, 20 Chazhong Road, Fuzhou, Fujian , P.R. China [email protected] * Contributed equally Key words: mitoxantrone, salvage therapy, non-hodgkin's lymphoma, poor performance status, comorbidity The standard first-line chemotherapy for the majority of aggressive NHL cases is cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) or R-CHOP, a combination of CHOP and rituximab, a monoclonal antibody to cluster of differentiation 20 (2,3). Although the majority of patients with aggressive NHL are responsive to the initial chemotherapy, 40 to 60% either fail to achieve a complete response (CR) following firstline chemotherapy or relapse subsequent to obtaining CR (4). The current standard treatment strategy for refractory or relapsed NHL is high-dose therapy and autologous stem cell transplantation (HD-ASCT) with curative intent in patients without comorbidities (5,6). However, HD-ASCT is only suitable for fit, young patients who are chemosensitive to salvage chemotherapy. In the absence of hematopoietic stem cell transplantation, the majority of the current treatment strategies for refractory or relapsed NHL are palliative (7-9). The majority of patients are not eligible for ASCT due to refractory disease, age, a poor performance status, comorbidities and other individual reasons (5,10,11). Therefore, alternative salvage approaches have to be employed in these patients. The standard salvage chemotherapy for these NHL patients has not been determined. Prior to the advent of novel chemotherapeutic or targeted agents, the ideal approach for these patients remains as a chemotherapeutic regimen with a high response rate and less toxicity, and containing chemotherapeutic agents that are not cross-resistant to previous therapy. For refractory or relapsed aggressive NHL patients with a poor performance status or comorbidities, treatment efficacy and quality of life require careful simultaneous consideration. In the present study, the mitoxantrone, etoposide, bleomycin and dexamethasone (MEBD) regimen, which is composed of myelosuppressive (mitoxantrone and etoposide) and non myelosuppressive (bleomycin and dexamethasone) drugs, was used to treat a group of such patients, and the response rates and toxicities were investigated. Patients and methods Patients. A retrospective analysis of 16 patients treated in the First Affiliated Hospital, Fujian Medical University (Fuzhou, China) between 2009 and 2012 was conducted. All patients had pathologically confirmed aggressive NHL and
2 LIN et al: MEBD AS SALVAGE THERAPY FOR NHL 2013 had been previously treated with at least one anthracyclinebased chemotherapeutic agent. All patients had either an Eastern Cooperative Oncology Group (ECOG) performance status (12) of or comorbidities. Among the patients with comorbidities, one presented with bronchiectasis, one with deep venous thrombosis, two with diabetes and five with chronic hepatitis B infection. Patients with primary central nervous system lymphoma or testicular involvement were not included in the present study. Prior to MEBD chemotherapy, all patients were staged according to the Ann Arbor classification (13), with physical examination, bone marrow biopsy and computed tomography (CT) scans of the neck, chest, abdomen and pelvis. Serum lactate dehydrogenase (LDH) and β2-microglobulin levels were also analyzed. In addition, baseline electrocardiogram (ECG) and ultrasonic cardiogram examinations were performed. The patients were required to have adequate bone marrow, hepatic and renal function, defined as a white blood cell count of 3,500/mm 3, an absolute neutrophil count of 1,500/mm 3, a platelet count of 100,000/ mm 3, alanine aminotransferase or aspartate aminotransferase levels <2.0 times the upper normal limit, a bilirubin level of 1.5 times the upper normal limit and a serum creatinine level of 1.5 times the upper normal limit. This study was approved by the ethics committee of the First Affiliated Hospital, Fujian Medical University. Treatment schedule. Once written informed consent had been obtained, all patients received systemic chemotherapy with the MEBD regimen, consisting of 10 mg/m 2 intravenous (IV) mitoxantrone on day 1, 75 mg/m 2 IV etoposide on days 1-3, 20 mg IV dexamethasone on days 1-4 and intramuscular 15 mg bleomycin on days 1, 4, 8 and 12, and the cycles were repeated every 21 days. If toxicity occurred, the dose was adjusted according to the physician. If hematological toxicity occurred, prophylactic granulocyte colony-stimulating factor (G-CSF) was used in subsequent cycles. The treatment was continued until either a maximum of six cycles, disease progression, the occurrence of unacceptable toxicity or the decision of the patient to withdraw. During the chemotherapy period, low molecular weight heparin calcium injections (0.3 ml; 3075 AXaIU, twice daily) were provided for the patient with deep venous thrombosis, insulin was administered to the diabetes patients, with monitored blood glucose levels, and entecavir tablets (0.5 mg, daily) were used in the patients with hepatitis B infection for 6 months following chemotherapy, with monitored blood hepatitis B virus DNA concentrations. Response and toxicity evaluation. For the response evaluation, CT scans were performed every two cycles of chemotherapy until the end of the treatment and every two months during follow up. Bone marrow biopsies were performed every two cycles of chemotherapy until the end of the treatment and according to the physician during follow-up. Blood, ECG and ultrasonic cardiogram results were monitored for signs of hematological or cardiac toxicity. Tumor responses, including complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) were assessed by the Response Evaluation Criteria In Solid Tumors (14). The overall response rate (ORR) was calculated as the CR plus PR. The progression-free survival (PFS) time was calculated as the time period between the date of MEBD chemotherapy and the date of disease progression. The overall survival (OS) time was calculated as the time period between the initial MEBD treatment and the time point at which the patient succumbed to the disease. Toxicity was graded by the National Cancer Institute Common Terminology Criteria, version 3.0 (15). Results Patient characteristics. A total of 16 refractory or relapsed aggressive NHL patients with a poor performance status or comorbidity were treated. The baseline characteristics of the patients and the previous treatments received are summarized in Table I. The median age of the patients was 55 years (range, years), and 11 patients were male and five were female. Seven patients had ECOG performance status scores of Nine patients presented with comorbidities. Histologically, 11 patients (68.8%) presented with diffuse large B-cell lymphoma (DLBCL) and five patients (31.2%) with peripheral T-cell lymphoma (PTCL). Two patients (12.5%) were at stage I or II and 14 patients (87.5%) were at stage III or IV. A total of 13 patients (81.3%) had elevated serum LDH levels and all 16 patients (100%) had elevated serum β2-microglobulin levels. With regard to previous chemotherapy, 13 patients (81.3%) had been administered CHOP chemotherapy, two patients (12.5%) had received R-CHOP chemotherapy and one patient (6.25%) had experienced CHOP + etoposide chemotherapy. Among these patients, six (37.5%) had been treated by at least one further regimen in addition to anthracycline-based chemotherapy. Six patients (37.5%) were refractory to previous chemotherapy and 10 patients (62.5%) had relapsed subsequent to previous chemotherapy. Treatment response and survival time. Out of the 16 patients, three (18.8%) achieved a CR, eight (50.0%) obtained PR, one (6.2%) exhibited SD and four (25.0%) developed PD. The ORR (CR + PR) was 68.8%. Among the 11 DLBCL patients, a CR was achieved in 18.2% (2/11) and PR in 63.6% (7/11); thus ORR was reached in 81.8% patients (9/11). Among the five PTCL patients, the CR rate was 20.0% (1/5), the PR rate was 20.0% (1/5) and the ORR was therefore 40.0%. The patients with refractory and relapsed aggressive NHL were all responsive to the MEBD chemotherapy. The treatment results are summarized in Table II. The median PFS time was 16.7 months and the median OS time was 22.4 months. The one year overall survival rate was 62.5% and the two year overall survival rate was 43.8%. At present, 10 patients remain alive and three of these patients remain with a CR. One patient has remained in CR for 47 months thus far. Toxicity. The side effects of chemotherapy are presented in Table III. The hematological toxicity was severe, with grade 3/4 neutropenia observed in 11 patients (68.8%) and two febrile neutropenia cases (12.5%). Grade 3/4 thrombocytopenia occurred in 18.8% of cases, but only grade 1/2 anemia was observed. The majority of non-hematological toxicity consisted of hepatic dysfunction and gastrointestinal reactions, which were mild and transient. Grade 1/2 interstitial pneumonia occurred in four patients (25.0%). Grade 1 arrhythmia
3 2014 Table I. Patient characteristics. Table II. Patient responses to MEBD. Characteristic was identified in two patients (12.5%). No renal damage or treatment-related mortality were detected. Discussion Value Median age (range), years 55 (35-79) Gender, n Male 11 Female 5 ECOG performance status, n Stage, n I/II 2 III/IV 14 B-symptoms, n 9 LDH level Elevated 13 Normal 3 β2-microglobulin level, n Elevated 16 Normal 0 Bulky mass, n 3 Bone marrow involvement, n 1 Disease status, n Relapsed 10 Refractory 6 Previous chemotherapy, n CHOP 13 R-CHOP 2 CHOP-E 1 2 regimens 6 Previous high-dose chemotherapy, n 2 Previous radiotherapy, n 2 NHL subtype, n Diffuse large B-cell lymphoma 11 Peripheral T-cell lymphoma 5 ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisolone; CHOP-E, CHOP + etoposide; NHL, non Hodgkin's lymphoma. As the majority of patients have an incomplete or only temporary response to salvage therapy, the treatment of refractory or relapsed aggressive NHL remains a problem. Salvage Response, n (%) Patient characteristic CR PR SD PD All patients 3 (18.8) 8 (50.0) 1 (6.3) 4 (25.0) Histological type B-Cell 2 (18.2) 7 (63.6) 0 (0.0) 2 (18.2) T-Cell 1 (20.0) 1 (20.0) 1 (20.0) 2 (40.0) Pre-treatment status Relapsed 3 (30.0) 5 (50.0) 0 (0.0) 2 (20.0) Refractory 0 (0.0) 3 (50.0) 1 (16.7) 2 (33.3) MEBD, mitoxantrone, etoposide, bleomycin and dexamethasone; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease. Table III. Toxicity. Number of patients (%) Adverse effect Grade 1 Grade 2 Grade 3 Grade 4 Leukopenia 3 (18.8) 2 (12.5) 4 (25.0) 7 (43.8) Neutropenia 2 (12.5) 3 (18.8) 5 (31.3) 6 (37.5) Anemia 6 (37.5) 3 (18.8) 0 (0.0) 0 (0.0) Thrombocytopenia 8 (50.0) 5 (31.3) 2 (12.5) 1 (6.3) Febrile neutropenia 0 (0.0) 0 (0.0) 2 (12.5) 0 (0.0) AST/ALT elevation 5 (31.3) 1 (6.3) 0 (0.0) 0 (0.0) Bilirubin elevation 2 (12.5) 0 (0.0) 0 (0.0) 0 (0.0) Mucositis 0 (0.0) 1 (6.3) 0 (0.0) 0 (0.0) Nausea 6 (37.5) 3 (13.8) 0 (0.0) 0 (0.0) Vomiting 4 (25.0) 0 (0.0) 0 (0.0) 0 (0.0) Constipation 7 (43.8) 2 (12.5) 0 (0.0) 0 (0.0) Arrhythmia 2 (12.5) 0 (0.0) 0 (0.0) 0 (0.0) Interstitial pneumonia 1 (6.3) 3 (18.8) 0 (0.0) 0 (0.0) AST, aspartate aminotransferase; ALT, alanine aminotransferase. chemotherapy regimens normally contain different drugs from those used previously, usually employing non anthracycline containing regimens to prevent drug resistance and cumulative toxicity. A number of salvage chemotherapy regimens have been developed to treat refractory or relapsed aggressive NHL. Currently, the majority of the effective salvage approaches use ifosfamide, cytarabine/platinum or gemcitabine. The most commonly used salvage regimens are dexamethasone, cytarabine and cisplatin (DHAP) (16-18), etoposide, methylprednisone, cytarabine and cisplatin (ESHAP) (16,19), carmustine, etoposide, cytarabine and melphalan (mini BEAM) (20,21), ifosfamide, carboplatin and etoposide (ICE)(18,22,23) and gemcitabine, dexamethasone and cisplatin (GDP) (16,24 26). These regimens have
4 LIN et al: MEBD AS SALVAGE THERAPY FOR NHL 2015 comparable efficacy, resulting in ORRs of 37-68% and complete remission rates of 12 to 37% (19, 21 27). However, the high incidence of hematological toxicity and nephrotoxicity limits the application of these drug regimens in elderly, heavily treated or unfit patients (27). Mitoxantrone, an anthracenedione antibiotic, exhibits similar clinical activity to the anthracyclines. Mitoxantrone intercalates into DNA through hydrogen bonding, resulting in crosslinks and strand breaks. In addition, mitoxantrone interferes with RNA and is a potent inhibitor of topoisomerase II, an enzyme responsible for uncoiling and repairing of damaged DNA (28,29). In preclinical lymphoma models, the potent activity of mitoxantrone has been demonstrated, with the drug appearing to be clinically active against follicular and aggressive lymphomas (39-32). However, controversy remains with regard to the superiority of mitoxantrone or anthracyclines in the treatment of elderly NHL patients (33,34). It has been hypothesized to that mitoxantrone retains the antineoplastic effects of the anthracyclines, but with less potential for cardiotoxicity, as mitoxantrone does not have the amino sugar of doxorubicin or the characteristic ring structure of the classical anthracyclines (28,35). Mitoxantrone has only partial cross resistance with anthracyclines, such as Adriamycin (36,37), and the efficacy of mitoxantrone appears to be less affected by multidrug resistance than Adriamycin or etoposide (36,38,39). In theory, anthracycline resistant tumors are sensitive to mitoxantrone, and mitoxantrone may exert a synergistic effect with etoposide. The first-line use of etoposide, mitoxantrone, cyclophosphamide, vincristine, prednisolone and bleomycin (VNCOP-B) has produced an 83% ORR and a 58% CR rate in elderly patients with aggressive NHL (40). However, in this study, grade 4 neutropenia was shown to occur in 29% patients on this regimen. The combined use of etoposide, mitoxantrone and prednisone has achieved a 38% ORR among refractory or relapsed NHL patients, with relatively low toxicity (41). Therefore, in the present study, these regimens were modified and a novel combination chemotherapy regimen, MEBD, was developed, which comprises myelosuppressive (mitoxantrone and etoposide) and non myelosuppressive (bleomycin and dexamethasone) drugs in order to increase efficacy and reduce toxicity. In the present study, MEBD treatment, used as a salvage chemotherapeutic regimen in patients with aggressive NHL, achieved a 68.8% ORR and an 18.8% CR rate, with a median PFS of 16.7 months and a median OS of 22.4 months. Certain patients achieved long term survival. The preliminary results appear comparable with those from patients treated with the aforementioned intensive salvage regimens, such as DHAP, ESHAP, mini BEAM, ICE and GDP. Furthermore, the results of the present study were obtained from refractory or relapsed patients with poor health conditions, with 87.5% patients at stage III or IV. The results demonstrate that MEBD is an efficacious salvage regimen for patients with aggressive NHL who are in a state of poor health. MEBD therapy appeared to have greater efficacy in B cell lymphoma than in T cell lymphoma. For the DLBCL patients, the CR rate was 18.2% and the PR rate was 63.6%, thus the ORR was 81.8%. Among the PTCL patients, the CR was 20.0% and the PR was 20.0%, therefore the ORR was 40.0%. The refractory and relapsed NHL patients who had experienced anthracycline based chemotherapy were observed to respond to MEBD treatment. This demonstrates that mitoxantrone containing regimens have little cross resistance to anthracyclines. As the present study was limited by the low number of patients, further large group studies are required in order to draw definite conclusions. Due to the poor health conditions of the patients in the present study, hematological toxicity remained severe even if a moderate dosage of mitoxantrone and etoposide was used and the non myelosuppressive agents bleomycin and dexamethasone were applied. Grade 3/4 neutropenia was identified in 68.8% of patients and grade 3/4 thrombocytopenia was observed in 18.8% of cases. Drug doses had to be adjusted for these patients, with prophylactic G-CSF used in the following cycles of chemotherapy. Cardiotoxicity was mild even if all patients had previously received anthracycline-based chemotherapy. Only two patients (12.5%) presented with grade 1 arrhythmia. Hepatic dysfunction was also mild; five patients (31.3%) exhibited grade 1 and one patient (6.3%) exhibited grade 2 transient toxicity. The gastrointestinal reactions that were detected were not severe and were controllable. Interstitial pneumonia occurred in 25.0% of patients due to the use of bleomycin. Although the interstitial pneumonia identified was classified as grade 1/2 and curable, the toxicity impeded the continuation of MEBD chemotherapy. The bleomycin dose modification required to balance the treatment efficacy and the lung toxicity requires further investigation. In VNCOP-B treatment, 10 mg/m 2 IV bleomycin was used only once every four weeks and no interstitial pneumonia was detected (40). In conclusion, refractory or relapsed aggressive NHL patients with a poor performance status or comorbidity remain eligible for chemotherapy. MEBD is an effective and feasible salvage regimen with long-term survival efficacy for patients in a state of poor health. The most severe toxicity symptom is myelosuppression and prophylactic measures are recommended to prevent hematological toxicity. References 1. Armitage JO and Weisenburger DD: New approach to classifying non-hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol 16: , Maxwell SA and Mousavi-Fard S: Non-Hodgkin's B-cell lymphoma: advances in molecular strategies targeting drug resistance. Exp Biol Med (Maywood) 238: , Foss FM: Treatment strategies for peripheral T-cell lymphomas. Best Pract Res Clin Haematol 26: 43-56, Fisher RI, Gaynor ER, Dahlberg S, et al: Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-hodgkin's lymphoma. N Engl J Med 328: , Gangatharan S and Kuruvilla J: Relapsed and refractory aggressive NHL: time for a change. Transfus Apher Sci 49: 72-79, Lunning MA, Moskowitz AJ and Horwitz S: Strategies for relapsed peripheral T-cell lymphoma: the tail that wags the curve. J Clin Oncol 31: , Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al; European Bone Marrow Transplantation (EBMT) Lymphoma Registry: An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant 31: , 2003.
5 Sinha R, Nastoupil L and Flowers CR: Treatment Strategies for Patients with Diffuse Large B-Cell Lymphoma: Past, Present, and Future. Blood Lymphat Cancer 2012: 87-98, Mak V, Hamm J, Chhanabhai M, et al: Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol 31: , Friedberg JW: Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program 2011: , Raut LS and Chakrabarti PP: Management of relapsed-refractory diffuse large B cell lymphoma. South Asian J Cancer 3: 66-70, Oken MM, Creech RH, Tormey DC, et al: Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5: , Armitage JO: Staging Non-Hodgkin Lymphoma. CA Cancer J Clin 55: , Therasse P, Arbuck SG, Eisenhauer EA, et al: New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: , Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS. August 9, Prichard M, Harris T, Williams ME, et al: Treatment strategies for relapsed and refractory aggressive non-hodgkin's lymphoma. Expert Opin Pharmacother 10: , Velasquez WS, Cabanillas F, Salvador P, et al: Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP). Blood 71: , Gisselbrecht C, Glass B, Mounier N, et al: Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 28: , Velasquez WS, McLaughlin P, Tucker S, et al: ESHAP - an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol 12: , Caballero MD, Amigo ML, Hernández JM, et al: Alternating mini-beam/eshap as salvage therapy for refractory non Hodgkin's lymphomas. Ann Hematol 74: 79-82, Girouard C, Dufresne J, Imrie K, et al: Salvage chemotherapy with mini-beam for relapsed or refractory non-hodgkin's lymphoma prior to autologous bone marrow transplantation. Ann Oncol 8: , Moskowitz CH, Bertino JR, Glassman JR, et al: Ifosfamide, carboplatin, and etoposide: a highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant-eligible patients with non-hodgkin's lymphoma. J Clin Oncol 17: , Aurer I, Mitrović Z, Nemet D, et al: Treatment of relapsed or refractory aggressive non-hodgkin lymphoma with two ifosfamidebased regimens, IMVP and ICE. J Chemother 20: , Crump M, Baetz T, Couban S, et al: Gemcitabine, dexamethasone, and cisplatin in patients with recurrent or refractory aggressive histology B-cell non-hodgkin lymphoma: a Phase II study by the National Cancer Institute of Canada Clinical Trials Group (NCIC- CTG). Cancer 101: , Crump M, Shepherd L and Lin B: A randomized phase III study of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin as salvage chemotherapy followed by posttransplantation rituximab maintenance therapy versus observation for treatment of aggressive B-Cell and T-Cell non-hodgkin's lymphoma. Clin Lymphoma 6: 56-60, Kuruvilla J, Nagy T, Pintilie M, et al: Similar response rates and superior early progression-free survival with gemcitabine, dexamethasone, and cisplatin salvage therapy compared with carmustine, etoposide, cytarabine, and melphalan salvage therapy prior to autologous stem cell transplantation for recurrent or refractory Hodgkin lymphoma. Cancer 106: , Hernandez-Ilizaliturri FJ and Czuczman MS: Therapeutic options in relapsed or refractory diffuse large B-cell lymphoma. Part 1. current treatment approaches. Oncology (Williston Park) 23: , Wallace RE, Murdock KC, Angier RB and Durr FE: Activity of a novel anthracenedione, 1,4-dihydroxy- 5,8-bis ((2-[(2-hydroxyethyl)amino]ethyl) amino)-9,10-anthracenedione dihydrochloride, against experimental tumors in mice. Cancer Res 39: , Armitage JO: The role of mitoxantrone in non-hodgkin's lymphoma. Oncology (Williston Park) 16: , , discussion , 514, Coltman CA Jr, Coltman TM, Balcerzak SP, et al: Mitoxantrone in refractory nonhodgkin's lymphoma. A Southwest Oncology Group study. Semin Oncol 11 (3 Suppl 1): 50-53, Gams RA, Bryan S, Dukart G, et al: Mitoxantrone in malignant lymphoma. Invest New Drugs 3: , Silver RT, Case DC Jr, Wheeler RH, et al: Multicenter clinical trial of mitoxantrone in non-hodgkin's lymphoma and Hodgkin's disease. J Clin Oncol 9: , Chamorey E, Gressin R, Peyrade F, et al: Prospective randomized study comparing MEMID with a chop-like regimen in elderly patients with aggressive non-hodgkin's lymphoma. Oncology 69: 19-26, Mainwaring PN, Cunningham D, Gregory W, et al: Mitoxantrone is superior to doxorubicin in a multiagent weekly regimen for patients older than 60 with high-grade lymphoma: results of a BNLI randomized trial of PAdriaCEBO versus PMitCEBO. Blood 97: , Bennett JM, Muss HB, Doroshow JH, et al: A randomized multicenter trial comparing mitoxantrone, cyclophosphamide, and fluorouracil with doxorubicin, cyclophosphamide, and fluorouracil in the therapy of metastatic breast carcinoma. J Clin Oncol 6: , Larsson R and Nygren P: Cytotoxic activity of topoisomerase II inhibitors in primary cultures of tumor cells from patients with human hematologic and solid tumors. Cancer 74: , Klumper E, Pieters R, den Boer ML, et al: In vitro anthracycline cross-resistance pattern in childhood acute lymphoblastic leukaemia. Br J Cancer 71: , Cole SP, Sparks KE, Fraser K, et al: Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res 54: , Testi R, Mattii L, Di Simone D, et al: Evaluation of resistance index of several anticancer agents on parental and resistant P-388 cell lines. Leuk Res 19: , Zinzani PL, Storti S, Zaccaria A, et al: Elderly Aggressive Histology Non-Hodgkin's Lymphoma: First-Line VNCOP-B Regimen Experience on 350 Patients. Blood 94: 33-38, Doorduijn JK, Spruit P, van Der Holt B, et al: Etoposide, mitoxantrone and prednisone: a salvage regimen with low toxicity for refractory or relapsed non-hodgkin's lymphoma. Haematologica 85: , 2000.
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003)
 Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
Hodgkin Lymphoma Disease Specific Biology and Treatment Options. John Kuruvilla
 Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Salvage Chemotherapy with Dexamethasone, Etoposide, Ifosfamide and Cisplatin (DVIP) for Relapsing and Refractory Non-Hodgkin s Lymphoma
 IMAJ VOL 11 January 9 Salvage Chemotherapy with Dexamethasone, Etoposide, Ifosfamide and Cisplatin (DVIP) for Relapsing and Refractory Non-Hodgkin s Lymphoma Ariela Dortort Lazar MD 1,, Ofer Shpilberg
IMAJ VOL 11 January 9 Salvage Chemotherapy with Dexamethasone, Etoposide, Ifosfamide and Cisplatin (DVIP) for Relapsing and Refractory Non-Hodgkin s Lymphoma Ariela Dortort Lazar MD 1,, Ofer Shpilberg
What is non-hodgkin lymphoma, how is it treated, and what is the unmet need?
 What is non-hodgkin lymphoma, how is it treated, and what is the unmet need? Tim Illidge BSc PhD MRCP FRCR FRCPath Institute of Cancer Sciences, University of Manchester Manchester Cancer Research Centre,
What is non-hodgkin lymphoma, how is it treated, and what is the unmet need? Tim Illidge BSc PhD MRCP FRCR FRCPath Institute of Cancer Sciences, University of Manchester Manchester Cancer Research Centre,
Bendamustine for the fourth-line treatment of multiple myeloma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine for the fourth-line treatment of multiple myeloma Contents Summary 1 Background 2 Epidemiology 3 Cost 6 References 7 Summary There is no standard
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine for the fourth-line treatment of multiple myeloma Contents Summary 1 Background 2 Epidemiology 3 Cost 6 References 7 Summary There is no standard
Frequency of NHL Subtypes in Adults
 Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Cycle frequency: Every four weeks Total number of cycles: 6-8
 Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY
 CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY 26.1 Introduction rituximab Subsequent to the completion of drafts for the guidelines earlier in 2004,
CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY 26.1 Introduction rituximab Subsequent to the completion of drafts for the guidelines earlier in 2004,
亞 東 紀 念 醫 院 Follicular Lymphoma 臨 床 指 引
 前 言 : 惡 性 淋 巴 瘤 ( 或 簡 稱 淋 巴 癌 ) 乃 由 體 內 淋 巴 系 統 包 括 淋 巴 細 胞 淋 巴 管 淋 巴 腺 及 一 些 淋 巴 器 官 或 組 織 如 脾 臟 胸 腺 及 扁 桃 腺 等 所 長 出 的 惡 性 腫 瘤 依 腫 瘤 病 理 組 織 型 態 的 不 同 可 分 為 何 杰 金 氏 淋 巴 瘤 (Hodgkin s disease) 與 非 何 杰 金
前 言 : 惡 性 淋 巴 瘤 ( 或 簡 稱 淋 巴 癌 ) 乃 由 體 內 淋 巴 系 統 包 括 淋 巴 細 胞 淋 巴 管 淋 巴 腺 及 一 些 淋 巴 器 官 或 組 織 如 脾 臟 胸 腺 及 扁 桃 腺 等 所 長 出 的 惡 性 腫 瘤 依 腫 瘤 病 理 組 織 型 態 的 不 同 可 分 為 何 杰 金 氏 淋 巴 瘤 (Hodgkin s disease) 與 非 何 杰 金
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
Supplementary Online Content
 Supplementary Online Content Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving
Supplementary Online Content Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving
PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA
 2012 1 31,, PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA Version 1.0 2012 DIVISION OF HAEMATOLOGY / ONCOLOGY DEPARTMENT OF MEDICINE KAOHSING VETERAN GENERAL HOSPTIAL General Guide Diagnosis 1.Adequate
2012 1 31,, PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA Version 1.0 2012 DIVISION OF HAEMATOLOGY / ONCOLOGY DEPARTMENT OF MEDICINE KAOHSING VETERAN GENERAL HOSPTIAL General Guide Diagnosis 1.Adequate
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Guidelines for the Management of Follicular Lymphoma
 Guidelines for the Management of Follicular Lymphoma Scope The following guidance for first- and second-line therapy applies to follicular lymphoma histological grades 1, 2 and 3a according to the World
Guidelines for the Management of Follicular Lymphoma Scope The following guidance for first- and second-line therapy applies to follicular lymphoma histological grades 1, 2 and 3a according to the World
IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN
 + IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
+ IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
Aggressive lymphomas. Michael Crump Princess Margaret Hospital
 Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
DE Tsai 1, HCF Moore 1, CL Hardy 2, DL Porter 1, EY Loh 1, DJ Vaughn 1, S Luger 1, SJ Schuster 1 and EA Stadtmauer 1
 Bone Marrow Transplantation, (1999) 24, 521 526 1999 Stockton Press All rights reserved 0268 3369/99 $15.00 http://www.stockton-press.co.uk/bmt Rituximab (anti-cd20 monoclonal antibody) therapy for progressive
Bone Marrow Transplantation, (1999) 24, 521 526 1999 Stockton Press All rights reserved 0268 3369/99 $15.00 http://www.stockton-press.co.uk/bmt Rituximab (anti-cd20 monoclonal antibody) therapy for progressive
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics
 Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics Disclosure(s) I do not intend to discuss an off-label use
Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics Disclosure(s) I do not intend to discuss an off-label use
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
MALIGNANT LYMPHOMAS. Dr. Olga Vujovic (Updated August 2010)
 MALIGNANT LYMPHOMAS Dr. Olga Vujovic (Updated August 2010) Malignant lymphomas consist of Hodgkin and non-hodgkin lymphomas. The current management of these diseases involves a multi-disciplinary approach.
MALIGNANT LYMPHOMAS Dr. Olga Vujovic (Updated August 2010) Malignant lymphomas consist of Hodgkin and non-hodgkin lymphomas. The current management of these diseases involves a multi-disciplinary approach.
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS
 MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
Summary & Conclusion
 The prognostic value of angiogenesis markers in patients with non-hodgkin lymphoma. Summary & Conclusion The current study aims to asses the prognostic value of some angiogenesis markers in patients with
The prognostic value of angiogenesis markers in patients with non-hodgkin lymphoma. Summary & Conclusion The current study aims to asses the prognostic value of some angiogenesis markers in patients with
Michael Crump MD. Lymphoma Site Leader Princess Margaret Hospital University of Toronto
 Evolution of Lymphoma Therapy: What can we expect for the rest of the millenium decade? Michael Crump MD Lymphoma Site Leader Princess Margaret Hospital University of Toronto disclaimers Served on advisory
Evolution of Lymphoma Therapy: What can we expect for the rest of the millenium decade? Michael Crump MD Lymphoma Site Leader Princess Margaret Hospital University of Toronto disclaimers Served on advisory
FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma
 Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer
 Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
cancer cancer Hessamfar-Bonarek M et al. Int. J. Epidemiol. 2010;39:135-146
 Hematopoietic Stem Cell Transplant in HIV- related lymphoma Song Zhao, MD PhD Hematology-Oncology Program University of Washington/FHCRC Underlying Causes of Death in HIV-infected Adults 2000 2005 cancer
Hematopoietic Stem Cell Transplant in HIV- related lymphoma Song Zhao, MD PhD Hematology-Oncology Program University of Washington/FHCRC Underlying Causes of Death in HIV-infected Adults 2000 2005 cancer
Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma. Claire Vines, 2016 Pharm.D. Candidate
 + Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma Claire Vines, 2016 Pharm.D. Candidate + Disclosure I have no conflicts of interest to disclose. + Objectives Summarize NCCN
+ Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma Claire Vines, 2016 Pharm.D. Candidate + Disclosure I have no conflicts of interest to disclose. + Objectives Summarize NCCN
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
6/3/2013. Follicular and Other Slow Growing Lymphomas. Stephen Ansell, MD, PhD Mayo Clinic
 Follicular and Other Slow Growing Lymphomas Stephen Ansell, MD, PhD Mayo Clinic 1 Learning Objectives Start with an overview of Follicular and other slow growing lymphomas Discuss current and emerging
Follicular and Other Slow Growing Lymphomas Stephen Ansell, MD, PhD Mayo Clinic 1 Learning Objectives Start with an overview of Follicular and other slow growing lymphomas Discuss current and emerging
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer
 Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
Lymphoma: The Roleof Nurses in the Treatment Process
 Lymphoma: The Roleof Nurses in the Treatment Process Sarah Liptrott MSc,BN(Hons), RN Istituto Europeo di Oncologia, Milan (IT) EBMT Swiss Study Day 2014, Zurich, Switzerland LymphomaManagement Watch &
Lymphoma: The Roleof Nurses in the Treatment Process Sarah Liptrott MSc,BN(Hons), RN Istituto Europeo di Oncologia, Milan (IT) EBMT Swiss Study Day 2014, Zurich, Switzerland LymphomaManagement Watch &
DIFFUSE LARGE B-CELL LYMPHOMA
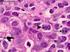 DIFFUSE LARGE B-CELL LYMPHOMA Executive Summary Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-hodgkin lymphoma (NHL), constituting up to 40% of all cases globally.[1] This subtype
DIFFUSE LARGE B-CELL LYMPHOMA Executive Summary Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-hodgkin lymphoma (NHL), constituting up to 40% of all cases globally.[1] This subtype
Treatment of low-grade non-hodgkin lymphoma
 Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Outline of thesis and future perspectives.
 Outline of thesis and future perspectives. This thesis is divided into two different sections. The B- section involves reviews and studies on B- cell non- Hodgkin lymphoma [NHL] and radioimmunotherapy
Outline of thesis and future perspectives. This thesis is divided into two different sections. The B- section involves reviews and studies on B- cell non- Hodgkin lymphoma [NHL] and radioimmunotherapy
A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on
 A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on examination. She also has fever, weight loss, and sweats. What
A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on examination. She also has fever, weight loss, and sweats. What
New Treatment Options for Breast Cancer
 New Treatment Options for Breast Cancer Brandon Vakiner, PharmD., BCOP Clinical Pharmacy Specialist - Oncology The University of Iowa Hospitals and Clinics Assistant Professor (Clinical) University of
New Treatment Options for Breast Cancer Brandon Vakiner, PharmD., BCOP Clinical Pharmacy Specialist - Oncology The University of Iowa Hospitals and Clinics Assistant Professor (Clinical) University of
Sonneveld, P; de Ridder, M; van der Lelie, H; et al. J Clin Oncology, 13 (10) : 2530-2539 Oct 1995
 Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
DECISION AND SUMMARY OF RATIONALE
 DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Clofarabine in the treatment of relapsed acute myeloid leukaemia (AML) The application was for clofarabine to remain in
DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Clofarabine in the treatment of relapsed acute myeloid leukaemia (AML) The application was for clofarabine to remain in
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma
 a report by Martin Dreyling Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma Head, Lymphoma Section, Department of Medicine III, University Hospital Großhadern, Ludwig Maximilians-University
a report by Martin Dreyling Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma Head, Lymphoma Section, Department of Medicine III, University Hospital Großhadern, Ludwig Maximilians-University
Mantle Cell Lymphoma Understanding Your Treatment Options
 New Developments in Mantle Cell Lymphoma John P. Leonard, M.D. Richard T. Silver Distinguished Professor of Hematology and Medical Oncology Associate Dean for Clinical Research Vice Chairman, Department
New Developments in Mantle Cell Lymphoma John P. Leonard, M.D. Richard T. Silver Distinguished Professor of Hematology and Medical Oncology Associate Dean for Clinical Research Vice Chairman, Department
Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with relatively good performance status
 Available online at www.sciencedirect.com Journal of the Chinese Medical Association 74 (2011) 209e214 Original Article Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with
Available online at www.sciencedirect.com Journal of the Chinese Medical Association 74 (2011) 209e214 Original Article Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with
Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy
 Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy Yoichi Kitamura, MD Kazuhiko Hayashi, MD Kazumi Uchida,
Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy Yoichi Kitamura, MD Kazuhiko Hayashi, MD Kazumi Uchida,
Treating myeloma. Dr Rachel Hall Royal Bournemouth Hospital
 Treating myeloma Dr Rachel Hall Royal Bournemouth Hospital Treatment overview When to treat? Aim of treatment Which treatment? Monitoring response to treatment Prevention of complications What happens
Treating myeloma Dr Rachel Hall Royal Bournemouth Hospital Treatment overview When to treat? Aim of treatment Which treatment? Monitoring response to treatment Prevention of complications What happens
Avastin in Metastatic Breast Cancer
 Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
IMMUNOMEDICS, INC. February 2016. Advanced Antibody-Based Therapeutics. Oncology Autoimmune Diseases
 IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases February 2016 Forward-Looking Statements This presentation, in addition to historical information, contains certain
IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases February 2016 Forward-Looking Statements This presentation, in addition to historical information, contains certain
Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study
 Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited
 rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited 06 June 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited 06 June 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Background. t 1/2 of 3.7 4.7 days allows once-daily dosing (1.5 mg) with consistent serum concentration 2,3 No interaction with CYP3A4 inhibitors 4
 Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Current Multiple Myeloma Treatment Adapted From the NCCN Guidelines
 Current Multiple Myeloma Treatment Adapted From the NCCN Guidelines Diagnosis Survival 3-5 yrs Survival
Current Multiple Myeloma Treatment Adapted From the NCCN Guidelines Diagnosis Survival 3-5 yrs Survival
Cure versus control: Which is the best strategy?
 Cure versus control: Which is the best strategy? Barcelona 8-9-2012 Mario Boccadoro DIVISIONE UNIVERSITARIA DI EMATOLOGIA AZIENDA OSPEDALIERA SAN GIOVANNI TORINO, ITALY MULTIPLE MYELOMA Cure versus control
Cure versus control: Which is the best strategy? Barcelona 8-9-2012 Mario Boccadoro DIVISIONE UNIVERSITARIA DI EMATOLOGIA AZIENDA OSPEDALIERA SAN GIOVANNI TORINO, ITALY MULTIPLE MYELOMA Cure versus control
The Blood Cancer Twice As Likely To Affect African Americans: Multiple Myeloma
 The Blood Cancer Twice As Likely To Affect African Americans: Multiple Myeloma 11 th Annual National Leadership Summit on Health Disparities Innovation Towards Reducing Disparities Congressional Black
The Blood Cancer Twice As Likely To Affect African Americans: Multiple Myeloma 11 th Annual National Leadership Summit on Health Disparities Innovation Towards Reducing Disparities Congressional Black
CHILDHOOD CANCER SURVIVOR STUDY Analysis Concept Proposal
 CHILDHOOD CANCER SURVIVOR STUDY Analysis Concept Proposal 1. STUDY TITLE: Longitudinal Assessment of Chronic Health Conditions: The Aging of Childhood Cancer Survivors 2. WORKING GROUP AND INVESTIGATORS:
CHILDHOOD CANCER SURVIVOR STUDY Analysis Concept Proposal 1. STUDY TITLE: Longitudinal Assessment of Chronic Health Conditions: The Aging of Childhood Cancer Survivors 2. WORKING GROUP AND INVESTIGATORS:
New Targets and Treatments for Follicular Lymphoma. Disclosures
 Winship Cancer Institute of Emory University New Targets and Treatments for Follicular Lymphoma Jonathon B. Cohen, MD, MS Assistant Professor Div of BMT, Emory University Disclosures Consulting fees from:
Winship Cancer Institute of Emory University New Targets and Treatments for Follicular Lymphoma Jonathon B. Cohen, MD, MS Assistant Professor Div of BMT, Emory University Disclosures Consulting fees from:
Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment.
 1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
NATIONAL CANCER INSTITUTE. Lenalidomide or Observation in Treating Patients With Asymptomatic High-Risk Smoldering Multiple Myeloma
 NATIONAL CANCER INSTITUTE Lenalidomide or Observation in Treating Patients With Asymptomatic High-Risk Smoldering Multiple Myeloma Basic Trial Information Phase Type Status Age Sponsor Protocol IDs Phase
NATIONAL CANCER INSTITUTE Lenalidomide or Observation in Treating Patients With Asymptomatic High-Risk Smoldering Multiple Myeloma Basic Trial Information Phase Type Status Age Sponsor Protocol IDs Phase
West of Scotland Cancer Network Chemotherapy Protocol. Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021)
 West of Scotland Cancer Network Chemotherapy Protocol Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021) Indication Palliative chemotherapy for malignant mesothelioma of the pleura Eligibility
West of Scotland Cancer Network Chemotherapy Protocol Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021) Indication Palliative chemotherapy for malignant mesothelioma of the pleura Eligibility
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
BCCA Protocol Summary for Palliative Therapy for Metastatic Breast Cancer using Trastuzumab Emtansine (KADCYLA)
 BCCA Protocol Summary for Palliative Therapy for Metastatic Breast Cancer using Trastuzumab Emtansine (KADCYLA) Protocol Code Tumour Group Contact Physician UBRAVKAD Breast Dr Stephen Chia ELIGIBILITY:
BCCA Protocol Summary for Palliative Therapy for Metastatic Breast Cancer using Trastuzumab Emtansine (KADCYLA) Protocol Code Tumour Group Contact Physician UBRAVKAD Breast Dr Stephen Chia ELIGIBILITY:
Lymphomas after organ transplantation
 Produced 21.03.2011 Revision due 21.03.2011 Lymphomas after organ transplantation People who have undergone an organ transplant are more at risk of developing lymphoma known as post-transplant lymphoproliferative
Produced 21.03.2011 Revision due 21.03.2011 Lymphomas after organ transplantation People who have undergone an organ transplant are more at risk of developing lymphoma known as post-transplant lymphoproliferative
Stem Cell Transplantation
 Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Long Term Low Dose Maintenance Chemotherapy in the Treatment of Acute Myeloid Leukemia
 Long Term Low Dose Chemotherapy in the Treatment of Acute Myeloid Leukemia Murat TOMBULO LU*, Seçkin ÇA IRGAN* * Department of Hematology, Faculty of Medicine, Ege University, zmir, TURKEY ABSTRACT In
Long Term Low Dose Chemotherapy in the Treatment of Acute Myeloid Leukemia Murat TOMBULO LU*, Seçkin ÇA IRGAN* * Department of Hematology, Faculty of Medicine, Ege University, zmir, TURKEY ABSTRACT In
Audience Response Question?
 Presenter Disclosure Information Session 4: 3:30 PM - 4:15 PM Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman, MD The following relationships
Presenter Disclosure Information Session 4: 3:30 PM - 4:15 PM Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman, MD The following relationships
Non-Hodgkin Lymphoma Richard Orlowski, MD
 Non-Hodgkin Lymphoma Richard Orlowski, MD The American Cancer Society (ACS) estimates that 69,740 Americans will be diagnosed with non-hodgkin lymphoma (NHL) in 2013. Excluding non-melanoma skin cancers,
Non-Hodgkin Lymphoma Richard Orlowski, MD The American Cancer Society (ACS) estimates that 69,740 Americans will be diagnosed with non-hodgkin lymphoma (NHL) in 2013. Excluding non-melanoma skin cancers,
Interesting Case Series. Periorbital Richter Syndrome
 Interesting Case Series Periorbital Richter Syndrome MarkGorman,MRCS,MSc, a Julia Ruston, MRCS, b and Sarath Vennam, BMBS a a Division of Plastic Surgery, Royal Devon and Exeter Hospital, Exeter, Devon,
Interesting Case Series Periorbital Richter Syndrome MarkGorman,MRCS,MSc, a Julia Ruston, MRCS, b and Sarath Vennam, BMBS a a Division of Plastic Surgery, Royal Devon and Exeter Hospital, Exeter, Devon,
STEM CELL TRANSPLANTATION IN MULTIPLE MYELOMA
 STEM CELL TRANSPLANTATION IN MULTIPLE MYELOMA Sundar Jagannath MD Professor of Medicine St. Vincent s Comprehensive Cancer Center New York, NY Where is transplant today in the management of Myeloma? Autologous
STEM CELL TRANSPLANTATION IN MULTIPLE MYELOMA Sundar Jagannath MD Professor of Medicine St. Vincent s Comprehensive Cancer Center New York, NY Where is transplant today in the management of Myeloma? Autologous
Lauren Berger: Why is it so important for patients to get an accurate diagnosis of their blood cancer subtype?
 Hello, I m Lauren Berger and I m the Senior Director of Patient Services Programs at The Leukemia & Lymphoma Society. I m pleased to welcome Dr. Rebecca Elstrom. Dr. Elstrom is an Assistant Professor in
Hello, I m Lauren Berger and I m the Senior Director of Patient Services Programs at The Leukemia & Lymphoma Society. I m pleased to welcome Dr. Rebecca Elstrom. Dr. Elstrom is an Assistant Professor in
Social inequalities impacts of care management and survival in patients with non-hodgkin lymphomas (ISO-LYMPH)
 Session 3 : Epidemiology and public health Social inequalities impacts of care management and survival in patients with non-hodgkin lymphomas (ISO-LYMPH) Le Guyader-Peyrou Sandra Bergonie Institut Context:
Session 3 : Epidemiology and public health Social inequalities impacts of care management and survival in patients with non-hodgkin lymphomas (ISO-LYMPH) Le Guyader-Peyrou Sandra Bergonie Institut Context:
BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP)
 BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP) Protocol Code Tumour Group Contact Physician UGUTIP Genitourinary Dr.
BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP) Protocol Code Tumour Group Contact Physician UGUTIP Genitourinary Dr.
Audience Response Question? Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management. Presenter Disclosure Information
 Welcome to Master Class for Oncologists Session 4: 10:00 AM - 10:45 AM Miami, FL December 18, 2009 Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman,
Welcome to Master Class for Oncologists Session 4: 10:00 AM - 10:45 AM Miami, FL December 18, 2009 Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman,
PREMEDICATIONS: Agent(s) Dose Route Schedule
 BCCA Protocol Summary for the Treatment of Relapsed or Refractory Advanced Stage Aggressive B-Cell Non-Hodgkin s Lymphoma with Ifosfamide, CARBOplatin, Etoposide and rituximab Protocol Code Tumour Group
BCCA Protocol Summary for the Treatment of Relapsed or Refractory Advanced Stage Aggressive B-Cell Non-Hodgkin s Lymphoma with Ifosfamide, CARBOplatin, Etoposide and rituximab Protocol Code Tumour Group
HOVON Staging and Response Criteria for Non-Hodgkin s Lymphomas Page 1
 HOVON Staging and Response Criteria for Non-Hodgkin s Lymphomas Page 1 This document describes the minimally required staging and evaluation procedures and response criteria that will be applied in all
HOVON Staging and Response Criteria for Non-Hodgkin s Lymphomas Page 1 This document describes the minimally required staging and evaluation procedures and response criteria that will be applied in all
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); [email protected] Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); [email protected] Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
David Loew, LCL MabThera
 MabThera The star continues to rise David Loew, LCL MabThera MabThera the star continues to raise Group sales (CHF bn) 4,5 4,0 3,5 3,0 2,5 2,0 1,5 1,0 0,5 0,0 2001 2002 2003 2004 2005 Outstanding clinical
MabThera The star continues to rise David Loew, LCL MabThera MabThera the star continues to raise Group sales (CHF bn) 4,5 4,0 3,5 3,0 2,5 2,0 1,5 1,0 0,5 0,0 2001 2002 2003 2004 2005 Outstanding clinical
Non-Hodgkin s lymphomas (NHLs) are a
 Oncology 33 Non-Hodgkin s lymphoma in the elderly The incidence of non-hodgkin s lymphoma (NHL) is increasing, and this increase is even more rapid in the older population. Although treatment of NHL in
Oncology 33 Non-Hodgkin s lymphoma in the elderly The incidence of non-hodgkin s lymphoma (NHL) is increasing, and this increase is even more rapid in the older population. Although treatment of NHL in
New Evaluation Criteria for Response and Toxicity in Lung Cancer Treatment
 Lung Cancer New Evaluation Criteria for Response and Toxicity in Lung Cancer Treatment JMAJ 46(12): 554 558, 2003 Masahiko SHIBUYA Chief, Division of Respiratory Medicine, Tokyo Metropolitan Komagome Hospital
Lung Cancer New Evaluation Criteria for Response and Toxicity in Lung Cancer Treatment JMAJ 46(12): 554 558, 2003 Masahiko SHIBUYA Chief, Division of Respiratory Medicine, Tokyo Metropolitan Komagome Hospital
Malignant Lymphomas and Plasma Cell Myeloma
 Malignant Lymphomas and Plasma Cell Myeloma Dr. Bruce F. Burns Dept. of Pathology and Lab Medicine Overview definitions - lymphoma lymphoproliferative disorder plasma cell myeloma pathogenesis - translocations
Malignant Lymphomas and Plasma Cell Myeloma Dr. Bruce F. Burns Dept. of Pathology and Lab Medicine Overview definitions - lymphoma lymphoproliferative disorder plasma cell myeloma pathogenesis - translocations
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof.
 Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Breast Pathway Group FEC 60 (Fluorouracil / Epirubicin / Cyclophosphamide) in Early Breast Cancer in Elderly / Frail
 Breast Pathway Group FEC 60 (Fluorouracil / Epirubicin / Cyclophosphamide) in Early Breast Cancer in Elderly / Frail Indication: Neoadjuvant or adjuvant therapy for elderly and frail patients with breast
Breast Pathway Group FEC 60 (Fluorouracil / Epirubicin / Cyclophosphamide) in Early Breast Cancer in Elderly / Frail Indication: Neoadjuvant or adjuvant therapy for elderly and frail patients with breast
Treatment results with Bortezomib in multiple myeloma
 Treatment results with Bortezomib in multiple myeloma Prof. Dr. Orhan Sezer Hamburg University Medical Center Circulating proteasome levels are an independent prognostic factor in MM 1.0 Probability of
Treatment results with Bortezomib in multiple myeloma Prof. Dr. Orhan Sezer Hamburg University Medical Center Circulating proteasome levels are an independent prognostic factor in MM 1.0 Probability of
Schedule: Drug Dose iv/infusion/oral q Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8
 Carboplatin/Gemcitabine Lung Cancer (non-small cell) - Advanced Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8 Cycle frequency: Every three weeks Total
Carboplatin/Gemcitabine Lung Cancer (non-small cell) - Advanced Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8 Cycle frequency: Every three weeks Total
Follicular Lymphoma. Aruna K. Reddy, MD. Hematology& Oncology Peace Health Southwest Medical Center
 Follicular Lymphoma Aruna K. Reddy, MD Hematology& Oncology Peace Health Southwest Medical Center Follicular Lymphoma Malignant neoplasm resulting from clonal proliferation of malignant B-cells Second
Follicular Lymphoma Aruna K. Reddy, MD Hematology& Oncology Peace Health Southwest Medical Center Follicular Lymphoma Malignant neoplasm resulting from clonal proliferation of malignant B-cells Second
Rituximab in the Management of Follicular Lymphoma
 Hong Kong J Radiol. 2011;14(Suppl):S63-7 REVIEW ARTICLE Rituximab in the Management of Follicular Lymphoma YL Kwong Department of Medicine, Professorial Block, Queen Mary Hospital, Pokfulam Road, Hong
Hong Kong J Radiol. 2011;14(Suppl):S63-7 REVIEW ARTICLE Rituximab in the Management of Follicular Lymphoma YL Kwong Department of Medicine, Professorial Block, Queen Mary Hospital, Pokfulam Road, Hong
Schedule: Drug Dose iv/infusion/oral q Doxorubicin 25mg/m 2 iv bolus Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/2hrs Days 1 & 2
 Doxorubicin/Cisplatin Osteosarcoma - neoadjuvant Doxorubicin 25mg/m 2 iv bolus Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/2hrs Days 1 & 2 (3 before surgery) Ensure adequate renal function Pre & post-hydration,
Doxorubicin/Cisplatin Osteosarcoma - neoadjuvant Doxorubicin 25mg/m 2 iv bolus Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/2hrs Days 1 & 2 (3 before surgery) Ensure adequate renal function Pre & post-hydration,
Advances In Chemotherapy For Hormone Refractory Prostate Cancer. TAX 327 study results & SWOG 99-16 study results presented at ASCO 2004
 Ronald de Wit Rotterdam Cancer Institute The Netherlands Advances In Chemotherapy For Hormone Refractory Prostate Cancer TAX 327 study results & SWOG 99-16 study results presented at Slide 1 Prostate Cancer
Ronald de Wit Rotterdam Cancer Institute The Netherlands Advances In Chemotherapy For Hormone Refractory Prostate Cancer TAX 327 study results & SWOG 99-16 study results presented at Slide 1 Prostate Cancer
Leukaemia and lymphoma what s the difference?
 Freephone helpline 0808 808 5555 [email protected] www.lymphomas.org.uk Leukaemia and lymphoma what s the difference? This is a difficult question to answer simply but it is one that is often
Freephone helpline 0808 808 5555 [email protected] www.lymphomas.org.uk Leukaemia and lymphoma what s the difference? This is a difficult question to answer simply but it is one that is often
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer
 Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
Introduction. About 10,500 new cases of acute myelogenous leukemia are diagnosed each
 Introduction 1.1 Introduction: About 10,500 new cases of acute myelogenous leukemia are diagnosed each year in the United States (Hope et al., 2003). Acute myelogenous leukemia has several names, including
Introduction 1.1 Introduction: About 10,500 new cases of acute myelogenous leukemia are diagnosed each year in the United States (Hope et al., 2003). Acute myelogenous leukemia has several names, including
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
