Medical Policy Manual. Topic: Interspinous and Interlaminar Stabilization/Distraction Devices (Spacers) Date of Origin: October 2006.
|
|
|
- Earl Alexander
- 10 years ago
- Views:
Transcription
1 Medical Policy Manual Topic: Interspinous and Interlaminar Stabilization/Distraction Devices (Spacers) Section: Surgery Date of Origin: October 2006 Last Reviewed Date: December 2015 Policy No: 155 Effective Date: January 1, 2016 IMPORTANT REMINDER Medical Policies are developed to provide guidance for members and providers regarding coverage in accordance with contract terms. Benefit determinations are based in all cases on the applicable contract language. To the extent there may be any conflict between the Medical Policy and contract language, the contract language takes precedence. PLEASE NOTE: Contracts exclude from coverage, among other things, services or procedures that are considered investigational or cosmetic. Providers may bill members for services or procedures that are considered investigational or cosmetic. Providers are encouraged to inform members before rendering such services that the members are likely to be financially responsible for the cost of these services. DESCRIPTION Surgical decompression with or without fusion is the standard surgical treatment for patients with moderate to severe lumbar spinal stenosis. Lumbar interspinous process decompression (IPD), also known as interspinous distraction or posterior spinal distraction, and interlaminar stabilization have been proposed as minimally invasive alternatives to laminectomy and fusion. One type of interspinous process spacers are inserted between the spinous processes through a small (4 8 cm) incision. The supraspinous ligament is maintained and assists in holding the implant in place. No laminotomy, laminectomy or foraminotomy is performed. Other interspinous spacers require removal of the interspinous ligament and are secured around the upper and lower spinous processes. Interlaminar spacers are implanted midline between adjacent lamina and spinous processes following surgical decompression at the affected level(s). These implants have two sets of wings that are placed around the inferior and superior spinous processes. These devices are intended to restrict painful motion while enabling otherwise normal motion. The devices theoretically enlarge the neural foramen, decompresses the cauda equina, and act as spacers between the spinous processes to maintain the flexion of the spinal interspace. 1 SUR155
2 Proponents of these spacers list the advantages compared with standard surgical decompression techniques to be the option of local anesthesia, shorter hospital stay and rehabilitation period, preservation of local bone and soft tissue, reduced risk of epidural scarring and cerebrospinal fluid leakage, and reversibility that does not limit future treatment options. The potential complications of spacers are implant dislodgement, incorrect positioning of implant, fracture of the spinous process, foreign body reaction (e.g., allergic reaction to titanium alloy), and mechanical failure of the implant. Regulatory Status There are a number of interspinous process and interlaminar spacers that are under investigation: Device name Manufacturer FDA Approved? Aperius -PercLID System Kyphon/Medtronic No Coflex Interlaminar Stabilization Paradigm Spine As a stand-alone spacer No Device* (formerly Interspinous U) As adjunct to fusion - No DIAM Spinal Stabilization System Medtronic Sofamor Danek No IDE only Falena Interspinous Decompression Mikai Spine No Device FLEXUS Globus Medical No IDE only Helifix Interspinous Spacer System Alphatec Spine No In-Space Synthes No IDE only NL-Prow Interspinous Spacer Non-Linear Technologies No Stenofix Synthes No Superion Interspinous Spacer System VertiFlex, Inc. No IDE only Wallis System Zimmer Spine (formerly Abbott Spine) No IDE only X-STOP Interspinous Process Kyphon/Medtronic Spine Yes Decompression (IPD ) System X-STOP PEEK (polyetheretherketone) Medtronic Yes Note: This policy addresses only IPD devices. Dynamic stabilization devices across pedicle screws are considered in separate medical policies (see Cross References below). *The Coflex-F device is a fusion device and is not address in this policy. MEDICAL POLICY CRITERIA Interspinous process and interlaminar distraction/stabilization devices are considered investigational for all indications. 2 SUR155
3 SCIENTIFIC EVIDENCE The primary beneficial outcomes of interest for treatment of low back pain are relief of pain and improved function. Both of these outcomes are subjective and can be influenced by nonspecific effects, placebo response, and the variable natural history of the disease. Therefore, data from large, blinded, randomized controlled trials (RCTs) with sufficient long-term follow-up are required to control for the placebo effect, determine its magnitude, and determine whether any treatment effect from interspinous process and interlaminar distraction/stabilization spacers provides a significant advantage over conventional surgical decompression or nonsurgical treatment. In addition, adverse effects related to complications, such as spinous process fracture and implant dislodgement or breakage, must be considered in evaluating the net health impact of spacers compared with conventional surgical decompression with or without fusion. Literature Appraisal The focus of this literature appraisal is on systematic reviews, randomized trials, and nonrandomized comparative studies. There are currently no long-term clinical trials for either interspinous process distraction or interlaminar stabilization spacers. The published clinical trial data are relatively sparse and consist largely of small, non-randomized, uncontrolled studies with short-term follow-up. Of the few studies with a control group, most compare spacers with conservative medical management. Systematic Reviews Two systematic reviews of studies that compared spacers to traditional decompression surgery for lumbar spinal stenosis were published in [1,2] Both noted that outcomes seem promising, but that the level of evidence is low. The authors call for well-designed, large randomized studies with long-term follow-up and consistent outcome measures. In 2014, Wu et al [3] conducted a meta-analysis of 2 RCTs [4,5] and 3 non-randomized prospective comparative studies [6-8]. There were 204 patients in the interspinous spacer group and 217 patients in the decompressive surgery group. The interspinous spacers that were studied were the X-STOP, Aperius, Coflex, DIAM, and distraxion. Pooled analysis showed no significant difference at 12 and 24 months between the spacer and decompression groups for low back pain, leg pain, Oswestry Disability Index (ODI), Roland Disability Questionnaire (RDQ) or complications. However, the traditional decompressive surgery group had a significantly lower incidence of reoperation, with 11 of 160 cases requiring reoperation compared to 31 of 161 cases in the interspinous spacer group (relative risk [RR] 3.34; 95% CI: 1.77, 6.31). Several limitations to this meta-analysis were listed, with the primary concern being the small number of studies in the published literature comparing spacers and traditional decompression surgery. Although risk of bias was analyzed, no narrative critical appraisal of the included articles was provided. The authors noted the high reoperation rate associated with spacer use and stated that the indications, risks, and benefits of these devices required careful consideration before surgery. A 2015 meta-analysis by Hong et al. included 20 studies with 3,155 patients in the interspinous spacers group and 50,983 patients treated with open decompression. [9] Devices studied were the X- STOP, DiAM, Aperius, Coflex, Wallis, and SPIRE. Results of this meta-analysis were similar to those obtained in the more selective analysis by Wu et al. There was no significant difference between the 2 procedures for improvement rate, ODI, or visual analog scale (VAS) for back or leg 3 SUR155
4 pain. Although secondary outcomes such as operative and hospitalization time, perioperative blood loss, and postoperative complication rate were superior in the spacer group, reoperation rate was higher in that group (16.5% vs 8.7%). Because of the higher reoperation rate the authors concluded that, while the use of spacers may be a viable technique, they could not conclude that it had replaced open decompression surgery as the gold standard for treatment of lumbar spinal stenosis. Randomized Controlled Trials (RCTs) Spacers Compared with Nonoperative Treatment The U.S. Food and Drug Administration (FDA) approval of the X STOP Interspinous Process Decompression System was based on laboratory, mechanical and cadaver studies, and a multicenter, prospective randomized controlled clinical study. [10-12] In this clinical study, patients were randomized to either the XSTOP at one (n=64) or two (n=36) levels or to a control group (n=91) which received continued non-operative therapy which included bed rest, a lumbar corset and a varied number of epidural injections. The Symptom Severity and Physical Function scores were measured at six weeks, six months, one year and two years. The scores for the X STOP patients were significantly higher than the scores for the control group at each follow-up point. At two years, the mean Symptom Severity score for the X-STOP and the control groups was 45.4% above baseline scores and 7.4 (p<0.001), respectively. The mean Physical Function score changes were 44.3% and -0.4% (p<0.001), respectively. While these short-term results are promising, the study precludes scientific conclusions related to long-term health outcomes. The following are additional reports on various subsets of the participants in this RCT: o o o A subsequent article was been published by the same authors using the 2-year quality of life date (SF-36) data from this trial. [13] As with other reports, the X STOP group showed improvements (by single-factor ANOVA or t-test) in both physical and mental component scores compared to both baseline and control subjects. However, in this report the authors considered the patients from both treatment and control groups who went on to have laminectomy within the 2-year follow-up period as lost to follow-up rather than as treatment failures; thus, the beneficial outcomes reported are misleadingly inflated. The article also notes a conflict of interest for the two primary authors of these articles. Anderson and colleagues reported two year outcomes of a subset of patients in the original randomized trial reported above. [14] This subset consisted of patients in the randomized trial whose symptoms were due to degenerative spondylolisthesis at one or two levels. The overall success was defined as a case in which all outcome measures (i.e., Zurich Claudication Questionnaire (ZCQ), Patient Satisfaction Survey, Short Form-36 (SF-36) scores, and additional surgery) were met. In the X-STOP group (n=42) 63.4% of patients met success criteria while 12.9% of the control group (n=33) met success criteria. The difference was statistically significant. Five patients (12%) in the X-STOP group and four patients (12%) in the control group underwent laminotomy during the follow-up period. Again, short-term results were encouraging but long-term outcomes are needed. In 2006, Kondrashov and Zucherman published the four year outcomes of another subset of patients in the randomized trial noted above. [15] Eighteen patients from one center were selected from the original nine-center sample based on the availability of preoperative Oswestry Disability Index (ODI) scores and willingness to complete the ODI at four years 4 SUR155
5 following surgery. Using a 15 point improvement from baseline ODI score as a success criterion, 14 out of 18 patients (78%) had successful outcomes at the 4-year follow-up. The outcomes of the original control group were not included in this article. This intermediateterm study suffered from the same design flaws noted previously, specifically, the small size, lack of a control group for comparison, and lack of long-term health outcomes. In 2014 Puzzilli et al. reported a multicenter controlled trial of X-STOP versus non-surgical management. [16] A total of 542 patients with lumbar spinal stenosis (LSS) and intermittent claudication relieved on flexion were enrolled. All patients had failed a 6-month trial of conservative therapy (medical and/or physical). Initially patients were randomized, but randomization to conservative management was terminated after the first 120 patients due to poor outcomes. These patients were followed for a minimum of 3 years. By 3 years, the overall failure rate was 12.3% of X-STOP patients, with 24 of 422 requiring device removal, compared to 50% of patients with continued non-surgical management with 38 of 120 patients having decompression and/or spinal fixation surgery. Spacers Compared with Decompression Surgery In 2015, Lonne et al. reported a trial of X-STOP versus minimally invasive decompression in 96 patients with symptoms of neurogenic intermittent claudication relieved on flexion. [17] Intention-totreat analysis showed no significant differences between the groups in primary and secondary outcome measures at up to 2-year follow-up. However, the number of patients having secondary surgery due to persistent or recurrent symptoms was significantly higher in the X-STOP group (25% vs 5%, odds ratio = 6.5). In addition, 2 patients had fracture of the spinous process and 1 had dislocation of the implant. [18] Three patients in the decompression group had secondary surgery during the first hospital stay due to hematoma. Mean days of rehabilitation were 66 for X-STOP and 48 for surgical decompression. The study was terminated after planned mid-term analysis due to the higher reoperation rate with X-STOP. A 2-year outcomes of double-blind RCT (the FELIX trial) comparing the use of the coflex spacer without bony decompression to surgical decompression were reported in [19] Functional outcomes were measured by ZCQ and Modified Roland-Morris Disability Questionnaire (RMDS), and pain was measured with VAS and McGill Pain Questionnaire. All 159 participants had intermittent neurogenic claudication due to lumbar spinal stenosis. Surgery time was shorter, but reoperation rates due to absence of recovery were higher in the coflex group compared with the bony decompression group (29% vs 8%, p<0.001). For patients with 2-level surgery, the reoperation rate was 38% for coflex versus 6% for bony decompression (p<0.05). At 2 years, reoperations due to absence of recovery had been performed in 33% of the coflex group compared with 8% of the bony decompression group. VAS back pain at final follow-up was also higher in the coflex group (36 mm vs 28 mm/100). A number of methodological limitations were reported that limit interpretation and generalizability of the study findings. Differences may not have been found due to the lack of power, though the authors were not certain that a larger sample size would lead to a different study result. To the contrary, the higher reoperation rate and the higher intensity of [low back pain] in the [spacer] group do suggest inferiority compared to classical decompression. In 2014 Marsh et al reported a randomized controlled trial that compared decompression alone (n=30) versus decompression with a Wallis implant (n=30). [20] Follow-up at an average of 40 months showed no significant differences between the groups in VAS for back or leg pain or in the ODI. Improvement in back pain was 3.5 out of 10 with the Wallis implant compared with 2.7 without 5 SUR155
6 (p=0.1926). Improvement in ODI was 19.3 with the Wallis implant compared with 10.6 without (p=0.0787). Additional study in a larger population is needed. The 2-year outcomes of the pivotal investigational device exemption (IDE) trial for the coflex Interlaminar Technology were published in This was a non-blinded randomized multi-center non-inferiority trial that compared implantation of the coflex spacer with decompression and posterolateral fusion with pedicle screw fixation. [21,22] The condition treated was back pain due to spinal stenosis or low-grade degeneration spondylolisthesis. A total of 322 patients were randomized to undergo either laminectomy and coflex insertion (n=215) or laminectomy and fusion (n=107). At a minimum of 2 years follow-up, non-inferiority was reported, with 66.2% success with coflex and 57.7% success with fusion (p=0.999). There was no statistically significant between-group differences in pain and function scores. The percentage of device-related adverse events was the same (5.6%) for both groups, and the rate of spinous process fractures was not significantly different between the groups (14% for coflex and 12% for fusion). The vast majority of spinous process fractures were asymptomatic. A separate article reported similar outcomes for the spondylolisthesis subgroup in the study. [23] The overall reoperation rate was 10.7% in the coflex group and 7.5% in the fusion control (p=0.426). One limitation of this study was the lack of participant blinding to the treatment allocation; however, since the postoperative protocols are different for these procedures, blinding can be difficult to maintain. In addition, the 2-year follow-up does not permit conclusion about long-term outcomes. In 2013, Stromqvist et al. reported the 2-year outcomes of a noninferiority randomized trial of 100 patients with symptomatic one- or two-level lumbar spinal stenosis with neurogenic claudication relieved on flexion. [4] Patients were randomized in a 1:1 ratio to undergo either X-STOP implantation or conventional surgical decompression. At 6, 12, and 24 months follow-up, there was no significant difference in scores for symptoms and function, or for complication rates. Reoperation rates were significantly higher (p<0.04) in the X-STOP group (n=13; 26%) than in the decompression group (n=3; 6%). (The X-STOP patients who later underwent decompression were not considered to be treatment failures.) For the reasons noted above, longer-term data is needed to determine the durability of treatment effects and to compare the long-term reoperation rates. Richter et al. also published 2-year follow-up for 60 patients who underwent decompressive surgery with or without implantation of the Coflex device. [6,24] Though comparative, this study was not a randomized trial; treatment was allocated at the discretion of the surgeon. The authors reported no significant between-group differences in any outcome measures, and concluded that additional placement of a Coflex interspinous device does not improve the already good clinical outcomes after decompression surgery for LSS in this 24-month follow up interval. Comparisons Between Spacers Preliminary [25] and 2-year follow-up [26] results have been published from an FDA-regulated multicenter randomized IDE non-inferiority trial comparing the Superion interspinous spacer to the X-STOP. [25] At baseline, all patients (N=391) had intermittent neurogenic claudication despite 6 months nonsurgical management. The FDA-mandated primary endpoint of this trial was non-inferiority to X-STOP at 2 years, with additional postmarket surveillance for 10 years. The reported outcome was a composite of clinically significant improvement in at least 2 of 3 ZCQ domain scores compared with baseline, freedom from reoperation, revision, removal, or supplemental fixation at the index level, freedom from epidural steroid injection or nerve block within 12 weeks of the 2-year visit, freedom from rhizotomy or 6 SUR155
7 spinal cord stimulator at any level, and freedom from major implant or procedure-related complications. The primary non-inferiority endpoint was met, with a Bayesian posterior probability of However, 111 patients (28%, 54 Superion and 57 XSTOP) were withdrawn from the study during follow-up due to a protocol-defined secondary intervention. Modified intent-to-treat analysis showed clinical success (improvement 20 mm/100) for leg pain in 76% to 77% of patients and for back pain in 67% to 68% of patients, with no significant differences between groups. At 2-years, ODI success was achieved in 63% of Superion patients and 67% of XSTOP patients (p=0.061). Rates of complications and reoperations (44 [23.2%] Superion and 38 [18.9%] XSTOP) were similar between groups. Spinous process fractures, reportedly asymptomatic, occurred in 16.4% of Superion patients and 8.5% of XSTOP patients. Interpretation of this study is limited by the lack of blinding and lack of control groups treated by surgical decompression or medical management. Clinical Practice Guidelines North American Spine Society The 2014 revised clinical guidelines from the North American Spine Society (NASS) on lumbar spinal stenosis concluded that there is insufficient evidence at this time to make a recommendation for or against the placement of an interspinous process spacing device in patients with lumbar spinal stenosis (Grade of Recommendation I - Insufficient Evidence) [27] The 2014 revised NASS clinical guidelines on degenerative lumbar spondylolisthesis concluded that there is insufficient and conflicting evidence to make a recommendation for or against the efficacy of interspinous spacers versus medical/interventional treatment in the management of degenerative lumbar spondylolisthesis patients. (Grade of Recommendation I - Insufficient Evidence) [28] Summary Current evidence is insufficient to permit conclusions about whether any beneficial effect from interspinous process distraction or interlaminar stabilization spacers provides a significant advantage over surgical decompression, which is the current standard of care for surgical treatment of lumbar spinal stenosis. In addition, the complication rates and reoperation rates for these spacers compared with those of decompression surgery is unknown. Therefore, use of interspinous process and interlaminar stabilization/distraction spacers is considered investigational. [29] REFERENCES 1. Kabir, SM, Gupta, SR, Casey, AT. Lumbar interspinous spacers: a systematic review of clinical and biomechanical evidence. Spine (Phila Pa 1976) Dec 1;35(25):E PMID: Yi, X, McPherson, B. Application of X STOP device in the treatment of lumbar spinal stenosis. Pain Physician Sep-Oct;13(5):E PMID: Wu, AM, Zhou, Y, Li, QL, et al. Interspinous spacer versus traditional decompressive surgery for lumbar spinal stenosis: a systematic review and meta-analysis. United States, p. e SUR155
8 4. Stromqvist, BH, Berg, S, Gerdhem, P, et al. X-stop versus decompressive surgery for lumbar neurogenic intermittent claudication: randomized controlled trial with 2-year follow-up. Spine (Phila Pa 1976) Aug 1;38(17): PMID: Moojen, WA, Arts, MP, Jacobs, WC, et al. Interspinous process device versus standard conventional surgical decompression for lumbar spinal stenosis: randomized controlled trial. BMJ. 2013;347:f6415. PMID: Richter, A, Halm, HF, Hauck, M, Quante, M. 2-year Follow-up After Decompressive Surgery With and Without Implantation of an Interspinous Device for Lumbar Spinal Stenosis: A Prospective Controlled Study. J Spinal Disord Tech May 24. PMID: Kim, KA, McDonald, M, Pik, JH, Khoueir, P, Wang, MY. Dynamic intraspinous spacer technology for posterior stabilization: case-control study on the safety, sagittal angulation, and pain outcome at 1-year follow-up evaluation. United States, p. E7. 8. Beyer, F, Yagdiran, A, Neu, P, Kaulhausen, T, Eysel, P, Sobottke, R. Percutaneous interspinous spacer versus open decompression: a 2-year follow-up of clinical outcome and quality of life. Eur Spine J Sep;22(9): PMID: Hong, P, Liu, Y, Li, H. Comparison of the efficacy and safety between interspinous process distraction device and open decompression surgery in treating lumbar spinal stenosis: a meta analysis. Journal of investigative surgery : the official journal of the Academy of Surgical Research Feb;28(1):40-9. PMID: U.S. Food and Drug Administration. Summary of safety and effectiveness data: X STOP Interspinous Process Decompression System. [cited 03/31/2015]; Available from: Zucherman, JF, Hsu, KY, Hartjen, CA, et al. A prospective randomized multi-center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant: 1-year results. Eur Spine J Feb;13(1): PMID: Zucherman, JF, Hsu, KY, Hartjen, CA, et al. A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine (Phila Pa 1976) Jun 15;30(12): PMID: Hsu, KY, Zucherman, JF, Hartjen, CA, et al. Quality of life of lumbar stenosis-treated patients in whom the X STOP interspinous device was implanted. J Neurosurg Spine Dec;5(6): PMID: Anderson, PA, Tribus, CB, Kitchel, SH. Treatment of neurogenic claudication by interspinous decompression: application of the X STOP device in patients with lumbar degenerative spondylolisthesis. J Neurosurg Spine Jun;4(6): PMID: Kondrashov, DG, Hannibal, M, Hsu, KY, Zucherman, JF. Interspinous process decompression with the X-STOP device for lumbar spinal stenosis: a 4-year follow-up study. J Spinal Disord Tech Jul;19(5): PMID: Puzzilli, F, Gazzeri, R, Galarza, M, et al. Interspinous spacer decompression (X-STOP) for lumbar spinal stenosis and degenerative disk disease: a multicenter study with a minimum 3-year follow-up. Clinical neurology and neurosurgery Sep;124: PMID: Lonne, G, Johnsen, LG, Rossvoll, I, et al. Minimally invasive decompression versus x-stop in lumbar spinal stenosis: a randomized controlled multicenter study. United States, p Lonne, G, Johnsen, LG, Aas, E, et al. Comparing cost-effectiveness of X-stop to minimally Invasive Decompression in Lumbar Spinal Stenosis: A Randomized Controlled Trial. Spine (Phila Pa 1976) Jan 20. PMID: SUR155
9 19. Moojen, WA, Arts, MP, Jacobs, WC, et al. IPD without bony decompression versus conventional surgical decompression for lumbar spinal stenosis: 2-year results of a double-blind randomized controlled trial. Eur Spine J Jan 14. PMID: Marsh, GD, Mahir, S, Leyte, A. A prospective randomised controlled trial to assess the efficacy of dynamic stabilisation of the lumbar spine with the Wallis ligament. Eur Spine J Oct;23(10): PMID: U.S. Food and Drug Administration. Summary of safety and effectiveness data: coflex Interlaminar Technology [cited 03/31/2015]; Available from: Davis, RJ, Errico, TJ, Bae, H, Auerbach, JD. Decompression and Coflex interlaminar stabilization compared with decompression and instrumented spinal fusion for spinal stenosis and low-grade degenerative spondylolisthesis: two-year results from the prospective, randomized, multicenter, Food and Drug Administration Investigational Device Exemption trial. Spine (Phila Pa 1976) Aug 15;38(18): PMID: Davis, R, Auerbach, JD, Bae, H, Errico, TJ. Can low-grade spondylolisthesis be effectively treated by either coflex interlaminar stabilization or laminectomy and posterior spinal fusion? Two-year clinical and radiographic results from the randomized, prospective, multicenter US investigational device exemption trial: clinical article. J Neurosurg Spine Aug;19(2): PMID: Richter, A, Schutz, C, Hauck, M, Halm, H. Does an interspinous device (Coflex) improve the outcome of decompressive surgery in lumbar spinal stenosis? One-year follow up of a prospective case control study of 60 patients. Eur Spine J Feb;19(2): PMID: Miller, LE, Block, JE. Interspinous spacer implant in patients with lumbar spinal stenosis: preliminary results of a multicenter, randomized, controlled trial. Pain research and treatment. 2012;2012: PMID: Patel, VV, Whang, PG, Haley, TR, et al. Superion Interspinous Process Spacer for Intermittent Neurogenic Claudication Secondary to Moderate Lumbar Spinal Stenosis: Two-Year Results From a Randomized Controlled FDA-IDE Pivotal Trial. Spine (Phila Pa 1976) Dec 9. PMID: North American Spine Society (NASS). Evidence-Based Clinical Guidelines for Multidisciplinary Spine Care: Diagnosis and Treatment of Degenerative Lumbar Spinal Stenosis. [cited 03/31/2015]; Available from: North American Spine Society (NASS). Evidence-Based Clinical Guidelines for Multidisciplinary Spine Care: Diagnosis and Treatment of Degenerative Lumbar Spondylolisthesis. [cited 04/14/2015]; Available from: BlueCross BlueShield Association Medical Policy Reference Manual "Interspinous Distraction Devices (Spacers)." Policy No CROSS REFERENCES Dynamic Stabilization of the Spine, Surgery, Policy No. 143 Total Facet Arthroplasty, Surgery, Policy No. 171 Interspinous Fixation (Fusion) Devices, Surgery, Policy No SUR155
10 Image-Guided Minimally Invasive Spinal Decompression (IG-MSD) for Spinal Stenosis, Surgery, Policy No. 176 Lumbar Spinal Fusion, Surgery, Policy No. 187 CODES NUMBER DESCRIPTION CPT 0171T Insertion of posterior spinous process distraction device (including necessary removal of bone or ligament for insertion and imaging guidance), lumbar; single level 0172T Insertion of posterior spinous process distraction device (including necessary removal of bone or ligament for insertion and imaging guidance), lumbar; each additional level (list separately in addition to code for primary procedure) Unlisted procedure, spine HCPCS C1821 Interspinous process distraction device (implantable) 10 SUR155
X Stop Spinal Stenosis Decompression
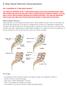 X Stop Spinal Stenosis Decompression Am I a candidate for X Stop spinal surgery? You may be a candidate for the X Stop spinal surgery if you have primarily leg pain rather than mostly back pain and your
X Stop Spinal Stenosis Decompression Am I a candidate for X Stop spinal surgery? You may be a candidate for the X Stop spinal surgery if you have primarily leg pain rather than mostly back pain and your
Issued and entered this _6th_ day of October 2010 by Ken Ross Commissioner ORDER I PROCEDURAL BACKGROUND
 STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX Petitioner
STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX Petitioner
Title: Interspinous Process Decompression with the X-Stop Device for Lumbar Spinal Stenosis: A Retrospective Review. Authors: Jennifer R.
 Title: Interspinous Process Decompression with the X-Stop Device for Lumbar Spinal Stenosis: A Retrospective Review. Authors: Jennifer R. Madonia-Barr, MS, PA-C and David L. Kramer, MD Institution: Connecticut
Title: Interspinous Process Decompression with the X-Stop Device for Lumbar Spinal Stenosis: A Retrospective Review. Authors: Jennifer R. Madonia-Barr, MS, PA-C and David L. Kramer, MD Institution: Connecticut
Subject: Implanted Devices for Spinal Stenosis Policy #: SURG.00092 Current Effective Date: 07/13/2011 Status: Reviewed Last Review Date: 05/19/2011
 1 of 5 6/18/2012 11:08 AM Medical Policy Subject: Implanted Devices for Spinal Stenosis Policy #: SURG.00092 Current Effective Date: 07/13/2011 Status: Reviewed Last Review Date: 05/19/2011 Description/Scope
1 of 5 6/18/2012 11:08 AM Medical Policy Subject: Implanted Devices for Spinal Stenosis Policy #: SURG.00092 Current Effective Date: 07/13/2011 Status: Reviewed Last Review Date: 05/19/2011 Description/Scope
LUMBAR SPINAL STENOSIS OBSERVATIONS, EVIDENCE, AND TRENDS FULILLING THE UNMET CLINICAL NEED WRITTEN BY: HALLETT MATHEWS, MD, MBA
 LUMBAR SPINAL STENOSIS OBSERVATIONS, EVIDENCE, AND TRENDS FULILLING THE UNMET CLINICAL NEED WRITTEN BY: HALLETT MATHEWS, MD, MBA Overview of Lumbar Spinal Stenosis Spine stabilization, which has equated
LUMBAR SPINAL STENOSIS OBSERVATIONS, EVIDENCE, AND TRENDS FULILLING THE UNMET CLINICAL NEED WRITTEN BY: HALLETT MATHEWS, MD, MBA Overview of Lumbar Spinal Stenosis Spine stabilization, which has equated
Motion Preservation. Hansen Yuan, MD President, Spine Arthroplasty Society
 Motion Preservation Procedure Codes Hansen Yuan, MD President, Spine Arthroplasty Society Who are we? The Spine Arthroplasty Society (SAS) is a group of medical and associated specialists devoted to the
Motion Preservation Procedure Codes Hansen Yuan, MD President, Spine Arthroplasty Society Who are we? The Spine Arthroplasty Society (SAS) is a group of medical and associated specialists devoted to the
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Interspinous Fixation (Fusion) Devices Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Interspinous Fixation (Fusion) Devices Lumbar Spine
Interspinous Fixation (Fusion) Devices Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Interspinous Fixation (Fusion) Devices Lumbar Spine
Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances?
 Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances? Do you experience weakness, tingling, numbness, stiffness, or cramping in your legs, buttocks or
Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances? Do you experience weakness, tingling, numbness, stiffness, or cramping in your legs, buttocks or
visualized. The correct level is then identified again. With the use of a microscope and
 SURGERY FOR SPINAL STENOSIS Laminectomy A one inch (or longer for extensive stenosis) incision is made in the middle of the back over the effected region of the spine. The muscles over the bone are moved
SURGERY FOR SPINAL STENOSIS Laminectomy A one inch (or longer for extensive stenosis) incision is made in the middle of the back over the effected region of the spine. The muscles over the bone are moved
PARADIGM SPINE. Brochure. coflex Interlaminar Stabilization
 PARADIGM SPINE Brochure Interlaminar Stabilization SPINAL STENOSIS WITH BACK PAIN THE RATIONALE FOR STABILIZATION For the treatment of spinal stenosis, surgeons have various treatment options. The continuum
PARADIGM SPINE Brochure Interlaminar Stabilization SPINAL STENOSIS WITH BACK PAIN THE RATIONALE FOR STABILIZATION For the treatment of spinal stenosis, surgeons have various treatment options. The continuum
ISPI Newsletter Archive Lumbar Spine Surgery
 ISPI Newsletter Archive Lumbar Spine Surgery January 2005 Effects of Charite Artificial Disc on the Implanted and Adjacent Spinal Segments Mechanics Using a Hybrid Testing Protocol Spine. 30(24):2755-2764,
ISPI Newsletter Archive Lumbar Spine Surgery January 2005 Effects of Charite Artificial Disc on the Implanted and Adjacent Spinal Segments Mechanics Using a Hybrid Testing Protocol Spine. 30(24):2755-2764,
10801 Sixth St, Suite 120 Rancho Cucamonga, CA 91730 Tel (909) 890-2000 Fax (909) 890-2003 Visit our web site at: www.iehp.org.
 Percutaneous Image-Guided Lumbar Decompression for Lumbar Spinal Stenosis (PILD), Including the Commercial Procedure Known as Minimally Invasive Lumbar Decompression (mild -Vertos Medical) Policy: Based
Percutaneous Image-Guided Lumbar Decompression for Lumbar Spinal Stenosis (PILD), Including the Commercial Procedure Known as Minimally Invasive Lumbar Decompression (mild -Vertos Medical) Policy: Based
Dynamic stabilization of the lumbar spine Robert W. Molinari
 Dynamic stabilization of the lumbar spine Robert W. Molinari Purpose of review This is a review of the recent literature involving dynamic posterior stabilization in the lumbar spine. Recent findings As
Dynamic stabilization of the lumbar spine Robert W. Molinari Purpose of review This is a review of the recent literature involving dynamic posterior stabilization in the lumbar spine. Recent findings As
INTERSPINOUS SPACERS IN THE LUMBAR SPINE
 INTERSPINOUS SPACERS IN THE LUMBAR SPINE Policy Number: 2015M0017A Effective Date: July 1, 2015 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION COVERAGE RATIONALE/CLINICAL CONSIDERATIONS
INTERSPINOUS SPACERS IN THE LUMBAR SPINE Policy Number: 2015M0017A Effective Date: July 1, 2015 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION COVERAGE RATIONALE/CLINICAL CONSIDERATIONS
Surgical Treatment for Lumbar Spinal Stenosis Dynamic Interspinous Distraction Interlaminar Stabilization Implant - Coflex
 International 31st Course For Percutaneous Endoscopic Spinal Surgery And Complementary Minimal Invasive Techniques Zurich, Switzerland January 24-25, 2013 Surgical Treatment for Lumbar Spinal Stenosis
International 31st Course For Percutaneous Endoscopic Spinal Surgery And Complementary Minimal Invasive Techniques Zurich, Switzerland January 24-25, 2013 Surgical Treatment for Lumbar Spinal Stenosis
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Imaged-Guided Minimally Invasive Lumbar Page 1 of 9 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Image-Guided Minimally Invasive Lumbar Professional Institutional
Imaged-Guided Minimally Invasive Lumbar Page 1 of 9 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Image-Guided Minimally Invasive Lumbar Professional Institutional
Minimally Invasive Spine Surgery For Your Patients
 Minimally Invasive Spine Surgery For Your Patients Lukas P. Zebala, M.D. Assistant Professor Orthopaedic and Neurological Spine Surgery Department of Orthopaedic Surgery Washington University School of
Minimally Invasive Spine Surgery For Your Patients Lukas P. Zebala, M.D. Assistant Professor Orthopaedic and Neurological Spine Surgery Department of Orthopaedic Surgery Washington University School of
Image-Guided Minimally Invasive Lumbar Decompression for Spinal Stenosis
 Image-Guided Minimally Invasive Lumbar Decompression for Spinal Stenosis Policy Number: 7.01.126 Last Review: 6/2015 Origination: 5/2010 Next Review: 6/2016 Policy Blue Cross and Blue Shield of Kansas
Image-Guided Minimally Invasive Lumbar Decompression for Spinal Stenosis Policy Number: 7.01.126 Last Review: 6/2015 Origination: 5/2010 Next Review: 6/2016 Policy Blue Cross and Blue Shield of Kansas
Effective Date: 01/01/2012 Revision Date: 07/24/2013 Comments: Policy Accepted during 2013 Annual Review with no changes.
 Health Plan Coverage Policy ARBenefits Approval: 01/01/2012 Effective Date: 01/01/2012 Revision Date: 07/24/2013 Comments: Policy Accepted during 2013 Annual Review with no changes. Title: Minimally Invasive,
Health Plan Coverage Policy ARBenefits Approval: 01/01/2012 Effective Date: 01/01/2012 Revision Date: 07/24/2013 Comments: Policy Accepted during 2013 Annual Review with no changes. Title: Minimally Invasive,
Update to the Treatment of Degenerative Cervical Disc Disease
 Update to the Treatment of Degenerative Cervical Disc Disease Michael Lynn, MD Neurosurgeon, Southeastern Neurosurgical & Spine Institute Adjunct Assistant Clinical Professor of Bioengineering, Clemson
Update to the Treatment of Degenerative Cervical Disc Disease Michael Lynn, MD Neurosurgeon, Southeastern Neurosurgical & Spine Institute Adjunct Assistant Clinical Professor of Bioengineering, Clemson
IP'Lumbar Spinal Stenosis
 A Patient's G uide to IP'Lumbar Spinal Stenosis X*STOP Interspinous Process Decompression This patient information guide is made possible through cooperation between your physician and St. Francis Medical
A Patient's G uide to IP'Lumbar Spinal Stenosis X*STOP Interspinous Process Decompression This patient information guide is made possible through cooperation between your physician and St. Francis Medical
Treatment pathways in lumbar spinal stenosis. By Prof. Dr. H. Michael Mayer
 Treatment pathways in lumbar spinal stenosis By Prof. Dr. H. Michael Mayer Content The pathology of spinal stenosis 4 The treatment of spinal stenosis 6 Spinal stenosis treatment options and outcomes 8
Treatment pathways in lumbar spinal stenosis By Prof. Dr. H. Michael Mayer Content The pathology of spinal stenosis 4 The treatment of spinal stenosis 6 Spinal stenosis treatment options and outcomes 8
Information for the Patient About Surgical
 Information for the Patient About Surgical Decompression and Stabilization of the Spine Aging and the Spine Daily wear and tear, along with disc degeneration due to aging and injury, are common causes
Information for the Patient About Surgical Decompression and Stabilization of the Spine Aging and the Spine Daily wear and tear, along with disc degeneration due to aging and injury, are common causes
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: lumbar_spine_fusion_surgery 9/2010 5/2015 5/2016 5/2015 Description of Procedure or Service Low back pain
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: lumbar_spine_fusion_surgery 9/2010 5/2015 5/2016 5/2015 Description of Procedure or Service Low back pain
SPINAL STENOSIS Information for Patients WHAT IS SPINAL STENOSIS?
 SPINAL STENOSIS Information for Patients WHAT IS SPINAL STENOSIS? The spinal canal is best imagined as a bony tube through which nerve fibres pass. The tube is interrupted between each pair of adjacent
SPINAL STENOSIS Information for Patients WHAT IS SPINAL STENOSIS? The spinal canal is best imagined as a bony tube through which nerve fibres pass. The tube is interrupted between each pair of adjacent
Low Back Pain (LBP) Prevalence. Low Back Pain (LBP) Prevalence. Lumbar Fusion: Where is the Evidence?
 15 th Annual Cleveland Clinic Pain Management Symposium Sarasota, Florida Lumbar Fusion: Where is the Evidence? Gordon R. Bell, M.D. Director, Cleveland Clinic Low Back Pain (LBP) Prevalence Lifetime prevalence:
15 th Annual Cleveland Clinic Pain Management Symposium Sarasota, Florida Lumbar Fusion: Where is the Evidence? Gordon R. Bell, M.D. Director, Cleveland Clinic Low Back Pain (LBP) Prevalence Lifetime prevalence:
The recent Spine Patient Outcomes Research Trial
 SPINE Volume 38, Number 18, pp 1529-1539 2013, Lippincott Williams & Wilkins RANDOMIZED TRIAL Decompression and Coflex Interlaminar Stabilization Compared With Decompression and Instrumented Spinal Fusion
SPINE Volume 38, Number 18, pp 1529-1539 2013, Lippincott Williams & Wilkins RANDOMIZED TRIAL Decompression and Coflex Interlaminar Stabilization Compared With Decompression and Instrumented Spinal Fusion
James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE:
 James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE: 1. I have been strongly advised to carefully read and consider this operative permit. I realize that it is important that I understand
James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE: 1. I have been strongly advised to carefully read and consider this operative permit. I realize that it is important that I understand
Lumbar Laminectomy and Interspinous Process Fusion
 Lumbar Laminectomy and Interspinous Process Fusion Introduction Low back and leg pain caused by pinched nerves in the back is a common condition that limits your ability to move, walk, and work. This condition
Lumbar Laminectomy and Interspinous Process Fusion Introduction Low back and leg pain caused by pinched nerves in the back is a common condition that limits your ability to move, walk, and work. This condition
John E. O Toole, Marjorie C. Wang, and Michael G. Kaiser
 Hypothermia and Human Spinal Cord Injury: Updated Position Statement and Evidence Based Recommendations from the AANS/CNS Joint Sections on Disorders of the Spine & Peripheral Nerves and Neurotrauma &
Hypothermia and Human Spinal Cord Injury: Updated Position Statement and Evidence Based Recommendations from the AANS/CNS Joint Sections on Disorders of the Spine & Peripheral Nerves and Neurotrauma &
SURGICAL TREATMENT FOR SPINE PAIN
 CLINICAL POLICY SURGICAL TREATMENT FOR SPINE PAIN Policy Number: SURGERY 075.11 T2 Effective Date: April 1, 2015 Table of Contents CONDITIONS OF COVERAGE... COVERAGE RATIONALE... BACKGROUND... CLINICAL
CLINICAL POLICY SURGICAL TREATMENT FOR SPINE PAIN Policy Number: SURGERY 075.11 T2 Effective Date: April 1, 2015 Table of Contents CONDITIONS OF COVERAGE... COVERAGE RATIONALE... BACKGROUND... CLINICAL
Patient Guide to Lower Back Surgery
 The following is a sampling of products offered by Zimmer Spine for use in Open Lumbar Fusion procedures. Patient Guide to Lower Back Surgery Open Lumbar Fusion Dynesys The Dynesys Dynamic Stabilization
The following is a sampling of products offered by Zimmer Spine for use in Open Lumbar Fusion procedures. Patient Guide to Lower Back Surgery Open Lumbar Fusion Dynesys The Dynesys Dynamic Stabilization
Spinal Surgery Functional Status and Quality of Life Outcome Specifications 2015 (01/01/2013 to 12/31/2013 Dates of Procedure) September 2014
 Description Methodology For patients ages 18 years and older who undergo a lumbar discectomy/laminotomy or lumbar spinal fusion procedure during the measurement year, the following measures will be calculated:
Description Methodology For patients ages 18 years and older who undergo a lumbar discectomy/laminotomy or lumbar spinal fusion procedure during the measurement year, the following measures will be calculated:
Lumbar Spinal Stenosis Materclass: Surgical management of lumbar spinal stenosis:
 Lumbar Spinal Stenosis Materclass: Surgical management of lumbar spinal stenosis: Presented By: Michelle Emsley Senior Spinal In-Patient Physiotherapist Learning objectives Indications Evidence Post operative
Lumbar Spinal Stenosis Materclass: Surgical management of lumbar spinal stenosis: Presented By: Michelle Emsley Senior Spinal In-Patient Physiotherapist Learning objectives Indications Evidence Post operative
Minimally Invasive Lumbar Fusion
 Minimally Invasive Lumbar Fusion Biomechanical Evaluation (1) coflex-f screw Biomechanical Evaluation (1) coflex-f intact Primary Stability intact Primary Stability Extension Neutral Position Flexion Coflex
Minimally Invasive Lumbar Fusion Biomechanical Evaluation (1) coflex-f screw Biomechanical Evaluation (1) coflex-f intact Primary Stability intact Primary Stability Extension Neutral Position Flexion Coflex
Advances In Spine Care. James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery
 Advances In Spine Care James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery Introduction The Spine - A common source of problems Back pain is the #2 presenting
Advances In Spine Care James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery Introduction The Spine - A common source of problems Back pain is the #2 presenting
Surgical Guideline for Lumbar Fusion (Arthrodesis)
 I. Introduction Surgical Guideline for Lumbar Fusion (Arthrodesis) The purpose of this guideline is: A. To provide utilization review staff with the information necessary to make recommendations about
I. Introduction Surgical Guideline for Lumbar Fusion (Arthrodesis) The purpose of this guideline is: A. To provide utilization review staff with the information necessary to make recommendations about
Artificial Intervertebral Disc: Lumbar Spine
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Artificial Intervertebral Disc: Lumbar Spine Number 7.01.87 Effective
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Artificial Intervertebral Disc: Lumbar Spine Number 7.01.87 Effective
Reimbursement Overview Supporting Adjunctive Use of the coflex-f Implant During Lumbar Fusion Procedures
 Interlaminar Technology Reimbursement Overview Supporting Adjunctive Use of the coflex-f Implant During Lumbar Fusion Procedures Coding Recommendations Overview Implant Description & Device Type Differentiation
Interlaminar Technology Reimbursement Overview Supporting Adjunctive Use of the coflex-f Implant During Lumbar Fusion Procedures Coding Recommendations Overview Implant Description & Device Type Differentiation
Discectomy. Cervical discectomy is considered not medically necessary for the treatment of cervical herniated disc
 Discectomy Policy Number: 7.01.146 Last Review: 12/2014 Origination: 12/2014 Next Review: 12/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Discectomy when it
Discectomy Policy Number: 7.01.146 Last Review: 12/2014 Origination: 12/2014 Next Review: 12/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Discectomy when it
SURGICAL TREATMENT FOR SPINE PAIN
 MEDICAL POLICY SURGICAL TREATMENT FOR SPINE PAIN Policy Number: 2012T0547C Effective Date: April 1, 2012 Table of Contents COVERAGE RATIONALE... BACKGROUND... CLINICAL EVIDENCE... U.S. FOOD AND DRUG ADMINISTRATION...
MEDICAL POLICY SURGICAL TREATMENT FOR SPINE PAIN Policy Number: 2012T0547C Effective Date: April 1, 2012 Table of Contents COVERAGE RATIONALE... BACKGROUND... CLINICAL EVIDENCE... U.S. FOOD AND DRUG ADMINISTRATION...
Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery
 Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery 221 Madison Ave Morristown, New Jersey 07960 (973) 538 4444 Fax (973) 538 0420 Patient
Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery 221 Madison Ave Morristown, New Jersey 07960 (973) 538 4444 Fax (973) 538 0420 Patient
SURGICAL TREATMENT FOR SPINE PAIN
 Lots of MEDICAL POLICY SURGICAL TREATMENT FOR SPINE PAIN Policy Number: 2015T0547J Effective Date: April 1, 2015 Table of Contents BENEFIT CONSIDERATIONS COVERAGE RATIONALE APPLICABLE CODES.. DESCRIPTION
Lots of MEDICAL POLICY SURGICAL TREATMENT FOR SPINE PAIN Policy Number: 2015T0547J Effective Date: April 1, 2015 Table of Contents BENEFIT CONSIDERATIONS COVERAGE RATIONALE APPLICABLE CODES.. DESCRIPTION
Spinal Cord Stimulators (SCS) for Injured Workers with Chronic Back and Leg
 Spinal Cord Stimulators (SCS) for Injured Workers with Chronic Back and Leg Pain after Lumbar Surgery A Prospective Study to Describe Costs, Complications, and Patient Outcomes: Final Report September
Spinal Cord Stimulators (SCS) for Injured Workers with Chronic Back and Leg Pain after Lumbar Surgery A Prospective Study to Describe Costs, Complications, and Patient Outcomes: Final Report September
SPINE SERVICE ROTATION ROTATION SPECIFIC OBJECTIVES (RSO) DEPT. OF ORTHOPEDICS AND PHYSICAL REHABILITATION UNIVERSITY OF MASSACHUSETTS
 SPINE SERVICE ROTATION ROTATION SPECIFIC OBJECTIVES (RSO) DEPT. OF ORTHOPEDICS AND PHYSICAL REHABILITATION UNIVERSITY OF MASSACHUSETTS The purpose of this RSO is to outline and clarify the objectives of
SPINE SERVICE ROTATION ROTATION SPECIFIC OBJECTIVES (RSO) DEPT. OF ORTHOPEDICS AND PHYSICAL REHABILITATION UNIVERSITY OF MASSACHUSETTS The purpose of this RSO is to outline and clarify the objectives of
Lumbar Spinal Stenosis
 Copyright 2009 American Academy of Orthopaedic Surgeons Lumbar Spinal Stenosis Almost everyone will experience low back pain at some point in their lives. A common cause of low back pain is lumbar spinal
Copyright 2009 American Academy of Orthopaedic Surgeons Lumbar Spinal Stenosis Almost everyone will experience low back pain at some point in their lives. A common cause of low back pain is lumbar spinal
Utilization and Cost of Surgery for Lumbar Spinal Stenosis in a Commercially Insured Population
 Utilization and Cost of Surgery for Lumbar Spinal Stenosis in a Commercially Insured Population Prepared by Milliman, Inc. Kathryn Fitch, RN, MEd Principal and Healthcare Management Consultant Helen Blumen,
Utilization and Cost of Surgery for Lumbar Spinal Stenosis in a Commercially Insured Population Prepared by Milliman, Inc. Kathryn Fitch, RN, MEd Principal and Healthcare Management Consultant Helen Blumen,
OUTLINE. Anatomy Approach to LBP Discogenic LBP. Treatment. Herniated Nucleus Pulposus Annular Tear. Non-Surgical Surgical
 DISCOGENIC PAIN OUTLINE Anatomy Approach to LBP Discogenic LBP Herniated Nucleus Pulposus Annular Tear Treatment Non-Surgical Surgical Facet Joints: bear 20% of weight Discs bear 80% of weight Neural Foramen
DISCOGENIC PAIN OUTLINE Anatomy Approach to LBP Discogenic LBP Herniated Nucleus Pulposus Annular Tear Treatment Non-Surgical Surgical Facet Joints: bear 20% of weight Discs bear 80% of weight Neural Foramen
mild Lumbar Decompression for the Treatment of Lumbar Spinal Stenosis
 The Neuroradiology Journal 24: 620-626, 2011 www.centauro.it mild Lumbar Decompression for the Treatment of Lumbar Spinal Stenosis D.F. SCHOMER 1, D. SOLSBERg 1, W. WONg 2, B.W. CHOPkO 3 1 Radiology Imaging
The Neuroradiology Journal 24: 620-626, 2011 www.centauro.it mild Lumbar Decompression for the Treatment of Lumbar Spinal Stenosis D.F. SCHOMER 1, D. SOLSBERg 1, W. WONg 2, B.W. CHOPkO 3 1 Radiology Imaging
White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants
 White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants For Health Plans, Medical Management Organizations and TPAs Executive Summary Back pain is one of the most
White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants For Health Plans, Medical Management Organizations and TPAs Executive Summary Back pain is one of the most
Balloon Kyphoplasty. Balloon Kyphoplasty is a minimally invasive procedure to treat vertebral body compression fractures.
 Balloon Kyphoplasty Overview Balloon Kyphoplasty is a minimally invasive procedure to treat vertebral body compression fractures. The technique is designed to: Reduce and stabilise the fracture in a controlled
Balloon Kyphoplasty Overview Balloon Kyphoplasty is a minimally invasive procedure to treat vertebral body compression fractures. The technique is designed to: Reduce and stabilise the fracture in a controlled
Spinal Cord Stimulation (SCS) Therapy: Fact Sheet
 Spinal Cord Stimulation (SCS) Therapy: Fact Sheet What is SCS Therapy? Spinal cord stimulation (SCS) may be a life-changing 1 surgical option for patients to control their chronic neuropathic pain and
Spinal Cord Stimulation (SCS) Therapy: Fact Sheet What is SCS Therapy? Spinal cord stimulation (SCS) may be a life-changing 1 surgical option for patients to control their chronic neuropathic pain and
Minimally Invasive Spine Surgery What is it and how will it benefit patients?
 Minimally Invasive Spine Surgery What is it and how will it benefit patients? Dr Raoul Pope MBChB, FRACS, Neurosurgeon and Minimally Invasive Spine Surgeon Concord Hospital and Mater Private Hospital Sydney
Minimally Invasive Spine Surgery What is it and how will it benefit patients? Dr Raoul Pope MBChB, FRACS, Neurosurgeon and Minimally Invasive Spine Surgeon Concord Hospital and Mater Private Hospital Sydney
Subject: BlueCross BlueShield of North Carolina Lumbar Spine Fusion Surgery Notification
 , 2010 Don W. Bradley, M.D. Senior Vice President, Healthcare & Chief Medical Officer Blue Cross and Blue Shield of North Carolina 5901 Chapel Hill Road Durham, NC 27707 Subject: BlueCross BlueShield of
, 2010 Don W. Bradley, M.D. Senior Vice President, Healthcare & Chief Medical Officer Blue Cross and Blue Shield of North Carolina 5901 Chapel Hill Road Durham, NC 27707 Subject: BlueCross BlueShield of
ARTIFICIAL DISC REPLACEMENT FOR DEGENERATIVE DISC DISEASE OF THE LUMBAR SPINE. A Technology Assessment
 ARTIFICIAL DISC REPLACEMENT FOR DEGENERATIVE DISC DISEASE OF THE LUMBAR SPINE A Technology Assessment INTRODUCTION The California Technology Assessment Forum has been asked to update its review of the
ARTIFICIAL DISC REPLACEMENT FOR DEGENERATIVE DISC DISEASE OF THE LUMBAR SPINE A Technology Assessment INTRODUCTION The California Technology Assessment Forum has been asked to update its review of the
Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization
 APPROVED IRN10389-1.1-04 Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization Because it involves accessing the spine through the patient s side, the Direct Lateral approach
APPROVED IRN10389-1.1-04 Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization Because it involves accessing the spine through the patient s side, the Direct Lateral approach
Complications in Adult Deformity Surgery
 Complications in Adult Deformity Surgery Proximal Junctional Kyphosis: Thoracolumbar and Cervicothoracic Sigurd Berven, MD Professor in Residence UC San Francisco Disclosures Research/Institutional Support:
Complications in Adult Deformity Surgery Proximal Junctional Kyphosis: Thoracolumbar and Cervicothoracic Sigurd Berven, MD Professor in Residence UC San Francisco Disclosures Research/Institutional Support:
Degenerative Spine Solutions
 Degenerative Spine Solutions The Backbone for Your Surgical Needs Aesculap Spine Backbone for Your Degenerative Spine Needs Comprehensive operative solutions, unique product technology and world-class
Degenerative Spine Solutions The Backbone for Your Surgical Needs Aesculap Spine Backbone for Your Degenerative Spine Needs Comprehensive operative solutions, unique product technology and world-class
The outcome of Microscopic Selective Decompression of Degenerative Lumbar Spinal Stenosis
 Bahrain Medical Bulletin, Vol.28, No.4, December 2006 The outcome of Microscopic Selective Decompression of Degenerative Lumbar Spinal Stenosis A.Aziz Mohammed, CABS, FRCS (Ortho, Tr)* Tariq El Kalifa,
Bahrain Medical Bulletin, Vol.28, No.4, December 2006 The outcome of Microscopic Selective Decompression of Degenerative Lumbar Spinal Stenosis A.Aziz Mohammed, CABS, FRCS (Ortho, Tr)* Tariq El Kalifa,
Overview Anatomy of the spinal canal What is spinal stenosis? > 1
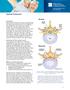 Spinal Stenosis Overview Spinal stenosis is the narrowing of your spinal canal and nerve root canal along with the enlargement of your facet joints. Most commonly it is caused by osteoarthritis and your
Spinal Stenosis Overview Spinal stenosis is the narrowing of your spinal canal and nerve root canal along with the enlargement of your facet joints. Most commonly it is caused by osteoarthritis and your
EXPERIMENTAL AND THERAPEUTIC MEDICINE 5: 567-571, 2013
 EXPERIMENTAL AND THERAPEUTIC MEDICINE 5: 567-571, 2013 Treatment of multilevel degenerative lumbar spinal stenosis with spondylolisthesis using a combination of microendoscopic discectomy and minimally
EXPERIMENTAL AND THERAPEUTIC MEDICINE 5: 567-571, 2013 Treatment of multilevel degenerative lumbar spinal stenosis with spondylolisthesis using a combination of microendoscopic discectomy and minimally
.org. Fractures of the Thoracic and Lumbar Spine. Cause. Description
 Fractures of the Thoracic and Lumbar Spine Page ( 1 ) Spinal fractures can vary widely in severity. While some fractures are very serious injuries that require emergency treatment, other fractures can
Fractures of the Thoracic and Lumbar Spine Page ( 1 ) Spinal fractures can vary widely in severity. While some fractures are very serious injuries that require emergency treatment, other fractures can
Treating Bulging Discs & Sciatica. Alexander Ching, MD
 Treating Bulging Discs & Sciatica Alexander Ching, MD Disclosures Depuy Spine Teaching and courses K2 Spine Complex Spine Study Group Disclosures Take 2 I am a spine surgeon I like spine surgery I believe
Treating Bulging Discs & Sciatica Alexander Ching, MD Disclosures Depuy Spine Teaching and courses K2 Spine Complex Spine Study Group Disclosures Take 2 I am a spine surgeon I like spine surgery I believe
Back & Neck Pain Survival Guide
 Back & Neck Pain Survival Guide www.kleinpeterpt.com Zachary - 225-658-7751 Baton Rouge - 225-768-7676 Kleinpeter Physical Therapy - Spine Care Program Finally! A Proven Assessment & Treatment Program
Back & Neck Pain Survival Guide www.kleinpeterpt.com Zachary - 225-658-7751 Baton Rouge - 225-768-7676 Kleinpeter Physical Therapy - Spine Care Program Finally! A Proven Assessment & Treatment Program
How To Understand The Anatomy Of A Lumbar Spine
 Sciatica: Low back and Leg Pain Diagnosis and Treatment Options Presented by Devesh Ramnath, MD Orthopaedic Associates Of Dallas Baylor Spine Center Sciatica Compression of the spinal nerves in the back
Sciatica: Low back and Leg Pain Diagnosis and Treatment Options Presented by Devesh Ramnath, MD Orthopaedic Associates Of Dallas Baylor Spine Center Sciatica Compression of the spinal nerves in the back
Measure Title X RAY PRIOR TO MRI OR CAT SCAN IN THE EVAULATION OF LOWER BACK PAIN Disease State Back pain Indicator Classification Utilization
 Client HMSA: PQSR 2009 Measure Title X RAY PRIOR TO MRI OR CAT SCAN IN THE EVAULATION OF LOWER BACK PAIN Disease State Back pain Indicator Classification Utilization Strength of Recommendation Organizations
Client HMSA: PQSR 2009 Measure Title X RAY PRIOR TO MRI OR CAT SCAN IN THE EVAULATION OF LOWER BACK PAIN Disease State Back pain Indicator Classification Utilization Strength of Recommendation Organizations
Moderator and Panelists
 Panelists Moderator and Panelists Moderator Dr. Hallett Mathews Executive Vice President Chief Medical Officer Paradigm Spine, LLC Panelist Dr. John Peloza Orthopedic Surgeon The Center for Spine Care
Panelists Moderator and Panelists Moderator Dr. Hallett Mathews Executive Vice President Chief Medical Officer Paradigm Spine, LLC Panelist Dr. John Peloza Orthopedic Surgeon The Center for Spine Care
Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy. Spine Volume 21(16) August 15, 1996, pp 1877-1883
 Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy 1 Spine Volume 21(16) August 15, 1996, pp 1877-1883 Saal, Joel S. MD; Saal, Jeffrey A. MD; Yurth, Elizabeth F. MD FROM
Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy 1 Spine Volume 21(16) August 15, 1996, pp 1877-1883 Saal, Joel S. MD; Saal, Jeffrey A. MD; Yurth, Elizabeth F. MD FROM
Position Statement: The Use of Total Ankle Replacement for the Treatment of Arthritic Conditions of the Ankle
 Position Statement: The Use of Total Ankle Replacement for the Treatment of Arthritic Conditions of the Ankle Position Statement The (AOFAS) endorses the use of total ankle replacement surgery for treatment
Position Statement: The Use of Total Ankle Replacement for the Treatment of Arthritic Conditions of the Ankle Position Statement The (AOFAS) endorses the use of total ankle replacement surgery for treatment
.org. Herniated Disk in the Lower Back. Anatomy. Description
 Herniated Disk in the Lower Back Page ( 1 ) Sometimes called a slipped or ruptured disk, a herniated disk most often occurs in your lower back. It is one of the most common causes of low back pain, as
Herniated Disk in the Lower Back Page ( 1 ) Sometimes called a slipped or ruptured disk, a herniated disk most often occurs in your lower back. It is one of the most common causes of low back pain, as
DUKE ORTHOPAEDIC SURGERY GOALS AND OBJECTIVES SPINE SERVICE
 GOALS AND OBJECTIVES PATIENT CARE Able to perform a complete musculoskeletal and neurologic examination on the patient including cervical spine, thoracic spine, and lumbar spine. The neurologic examination
GOALS AND OBJECTIVES PATIENT CARE Able to perform a complete musculoskeletal and neurologic examination on the patient including cervical spine, thoracic spine, and lumbar spine. The neurologic examination
https://www.laserspineinstitute.com/back_problems/foraminal_stenosis/e...
 Questions? Call toll free 1-866-249-1627 Contact us today. We're here for you seven days a week. MRI Review Consultation Live help Call 1-866-249-1627 Chat Live Home Laser Spine Institute Laser Spine Institute's
Questions? Call toll free 1-866-249-1627 Contact us today. We're here for you seven days a week. MRI Review Consultation Live help Call 1-866-249-1627 Chat Live Home Laser Spine Institute Laser Spine Institute's
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF).
 Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
Consent for Lumbar Spine Surgery and Fusion at
 STEPHEN MARANO, M.D. JAMES COOK PA-C Consent for Lumbar Spine Surgery and Fusion at Patient Name: Patient Diagnosis: Lumbar Spinal Stenosis (Narrowing of the canal where the spinal cord and nerves are
STEPHEN MARANO, M.D. JAMES COOK PA-C Consent for Lumbar Spine Surgery and Fusion at Patient Name: Patient Diagnosis: Lumbar Spinal Stenosis (Narrowing of the canal where the spinal cord and nerves are
Patients with pain in the neck, arm, low back, or leg (sciatica) may benefit from ESI. Specifically, those with the following conditions:
 Overview An epidural steroid injection (ESI) is a minimally invasive procedure that can help relieve neck, arm, back, and leg pain caused by inflamed spinal nerves. ESI may be performed to relieve pain
Overview An epidural steroid injection (ESI) is a minimally invasive procedure that can help relieve neck, arm, back, and leg pain caused by inflamed spinal nerves. ESI may be performed to relieve pain
Open Discectomy. North American Spine Society Public Education Series
 Open Discectomy North American Spine Society Public Education Series What Is Open Discectomy? Open discectomy is the most common surgical treatment for ruptured or herniated discs of the lumbar spine.
Open Discectomy North American Spine Society Public Education Series What Is Open Discectomy? Open discectomy is the most common surgical treatment for ruptured or herniated discs of the lumbar spine.
Diagnosis and Treatment of Degenerative Lumbar Spondylolisthesis 2 nd Edition
 Diagnosis and Treatment of Degenerative Lumbar Spondylolisthesis NASS Clinical Guidelines Evidence-Based Clinical Guidelines for Multidisciplinary Spine Care Diagnosis and Treatment of Degenerative Lumbar
Diagnosis and Treatment of Degenerative Lumbar Spondylolisthesis NASS Clinical Guidelines Evidence-Based Clinical Guidelines for Multidisciplinary Spine Care Diagnosis and Treatment of Degenerative Lumbar
Spine Trauma: When to Transfer. Alexander Ching, MD Director, Orthopaedic Spine Trauma OHSU
 Spine Trauma: When to Transfer Alexander Ching, MD Director, Orthopaedic Spine Trauma OHSU Disclosures Depuy Spine Consultant (teaching and courses) Department education and research funds Atlas Spine
Spine Trauma: When to Transfer Alexander Ching, MD Director, Orthopaedic Spine Trauma OHSU Disclosures Depuy Spine Consultant (teaching and courses) Department education and research funds Atlas Spine
Instructions for Use: coflex Interlaminar Technology Caution: Federal law restricts this device to sale by or on the order of a physician
 Instructions for Use: Interlaminar Technology Caution: Federal law restricts this device to sale by or on the order of a physician How Supplied Implant Components Sterile Surgical instruments Non-Sterile
Instructions for Use: Interlaminar Technology Caution: Federal law restricts this device to sale by or on the order of a physician How Supplied Implant Components Sterile Surgical instruments Non-Sterile
SPINE AND NECK SURGERY: MAKING A DECISION THAT S RIGHT FOR YOU
 1. GET THE FACTS: Back and neck pain affects 8 out of 10 people at some point in their life. Acute back and neck pain comes on suddenly and usually lasts from a few days to a few weeks. Chronic back and
1. GET THE FACTS: Back and neck pain affects 8 out of 10 people at some point in their life. Acute back and neck pain comes on suddenly and usually lasts from a few days to a few weeks. Chronic back and
Lumbar Spinal Stenosis
 Lumbar Spinal Stenosis Introduction Lumbar spinal stenosis is defined as reduction in the diameter of the spinal canal, lateral nerve canals or neural foramina. The stenosis may involve multiple level
Lumbar Spinal Stenosis Introduction Lumbar spinal stenosis is defined as reduction in the diameter of the spinal canal, lateral nerve canals or neural foramina. The stenosis may involve multiple level
Hybrid Technique for Posterior Lumbar Interbody Fusion: A Combination of Open Decompression and Percutaneous Pedicle Screw Fixation
 135 2013 Chinese Orthopaedic Association and Wiley Publishing Asia Pty Ltd SURGICAL TECHNIQUE Hybrid Technique for Posterior Lumbar Interbody Fusion: A Combination of Open Decompression and Percutaneous
135 2013 Chinese Orthopaedic Association and Wiley Publishing Asia Pty Ltd SURGICAL TECHNIQUE Hybrid Technique for Posterior Lumbar Interbody Fusion: A Combination of Open Decompression and Percutaneous
1 REVISOR 5223.0070. (4) Pain associated with rigidity (loss of motion or postural abnormality) or
 1 REVISOR 5223.0070 5223.0070 MUSCULOSKELETAL SCHEDULE; BACK. Subpart 1. Lumbar spine. The spine rating is inclusive of leg symptoms except for gross motor weakness, bladder or bowel dysfunction, or sexual
1 REVISOR 5223.0070 5223.0070 MUSCULOSKELETAL SCHEDULE; BACK. Subpart 1. Lumbar spine. The spine rating is inclusive of leg symptoms except for gross motor weakness, bladder or bowel dysfunction, or sexual
Sample Treatment Protocol
 Sample Treatment Protocol 1 Adults with acute episode of LBP Definition: Acute episode Back pain lasting
Sample Treatment Protocol 1 Adults with acute episode of LBP Definition: Acute episode Back pain lasting
Spinal Surgery for Chronic Low Back Pain: Review of Clinical Evidence and Guidelines
 ASERNIP S Australian Safety and Efficacy Register of New Interventional Procedures - Surgical Rapid Review Spinal Surgery for Chronic Low Back Pain: Review of Clinical Evidence and Guidelines June 2014
ASERNIP S Australian Safety and Efficacy Register of New Interventional Procedures - Surgical Rapid Review Spinal Surgery for Chronic Low Back Pain: Review of Clinical Evidence and Guidelines June 2014
Patient Guide to Neck Surgery
 The following is a sampling of products offered by Zimmer Spine for use in Anterior Cervical Fusion procedures. Patient Guide to Neck Surgery Anterior Cervical Fusion Trinica Select With the Trinica and
The following is a sampling of products offered by Zimmer Spine for use in Anterior Cervical Fusion procedures. Patient Guide to Neck Surgery Anterior Cervical Fusion Trinica Select With the Trinica and
Polymethylmethacrylate (PMMA) Augmentation Of A Cannulated And Fenestrated Pedicle
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861. Volume 13, Issue 5 Ver. III. (May. 2014), PP 77-81 Polymethylmethacrylate (PMMA) Augmentation Of A Cannulated
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861. Volume 13, Issue 5 Ver. III. (May. 2014), PP 77-81 Polymethylmethacrylate (PMMA) Augmentation Of A Cannulated
