Mycobacteria: Part 2
|
|
|
- Ashlynn Gilmore
- 7 years ago
- Views:
Transcription
1 This is the written version of our Hot Topic video presentation available at: MayoMedicalLaboratories.com/hot-topics Welcome to Mayo Medical Laboratories Hot Topics. These presentations provide short discussion of current topics and may be helpful to you in your practice. 1
2 Our speaker for this program is Dr. Nancy Wengenack, Director of the Mycology and Mycobacteriology Laboratories and Associate Professor of Laboratory Medicine and Pathology in the Division of Clinical Microbiology at Mayo Clinic in Rochester, Minnesota. This is the second of a 3-part series on tuberculosis. In Part 2, Dr. Wengenack compares the strengths and limitations of the various assays available for identification of Mycobacterium tuberculosis complex. Thank you, Cara. In this Hot Topic presentation, I will be discussing methods for the detection and identification of Mycobacterium tuberculosis complex. 2
3 For this program, I wish to acknowledge the receipt of research support from Trek Diagnostics 3
4 The objectives of this Hot Topic are to describe methods available for the identification of Mycobacterium tuberculosis after growth on a culture plate or in broth medium. The specific methods to be discussed are nucleic acid hybridization probes, line probes, DNA sequencing, and MALDI-TOF mass spectrometry. In addition, I will discuss methods available for the direct detection of Mycobacterium tuberculosis complex from specimens without the need to wait for growth in culture. These methods include the Mycobacterium tuberculosis Direct test, line probe assays, laboratorydeveloped PCR assays, and the Cepheid GeneXpert MTB/RIF PCR assay. 4
5 First, I will begin by discussing the identification of Mycobacterium tuberculosis complex after growth of colonies on a culture plate or in broth medium. 5
6 Traditionally mycobacteria including Mycobacterium tuberculosis have been identified after growth in culture using clues such as colonial morphology (in other words, is it rough and buff as discussed in the previous Hot Topic), or growth characteristics (is it a rapidly growing mycobacterium or a slowly growing mycobacterium), or perhaps through the use of selected biochemical tests such as niacin accumulation, nitrate reduction, or pyrazinamidase activity. All of these methods have the potential to be extremely slow and in the past, identification of Mycobacterium tuberculosis complex from culture could require 4 to 8 weeks after the organism grew in culture. In addition, high performance-liquid chromatography (HPLC) or gas-liquid chromatograph (GLC) have been used generally by the CDC or in state public health labs. The chromatographic methods targeted the unique mycolic acids which make up the mycobacterial cell wall and by comparing the mycolic acid pattern of the unknown isolate to those in reference libraries, the mycobacteria could be identified. HPLC and GLC have largely given way to other methods of identification for mycobacteria because of the large numbers of mycobacterial species that have been identified in the past 20 years. Mycolic acid patterns are no longer able to discriminate between the large number of closely related species of mycobacteria. Molecular methods are now the gold standard for the identification of mycobacteria including Mycobacterium tuberculosis complex from culture because they allow for rapid identification and they are able to discriminate between the majority of the 150 species of mycobacteria currently recognized today. 6
7 Nucleic acid hybridization probes are available for the identification of Mycobacterium tuberculosis complex, Mycobacterium avium complex, Mycobacterium gordonae, and Mycobacterium kansasii. There is no PCR or other amplification step that occurs when utilizing these probes so a large amount of target nucleic acid is required. That is why the probes are only useful for mycobacteria growing in culture and they cannot be used for the direct detection of mycobacteria from patient specimens. The probes are FDAapproved products and they are rapid with results available within approximately 2 hours after the growth of the organism in culture, they are highly sensitive and specific, and they are technically simple to utilize. The main limitation associated with the probes is that they are only available for the 4 mycobacterial species or complexes listed on this slide so other tools are needed for the identification of the majority of mycobacteria seen in the clinical laboratory today. 7
8 This slide provides an overview of how the nucleic acid hybridization probes function. The laboratorian obtains an RNA template from the organism to be identified by lysing a small amount of the organism after it is picked from a culture plate using an inoculating loop. A DNA-hybridization probe labeled with a chemiluminescent moiety is added to the solution containing the RNA template. If the hybridization probe sequence is complementary to the RNA target, it will bind tightly and specifically. Then, any unbound probe remaining in the solution is hydrolyzed to remove the potential for nonspecific signal and the DNA-RNA hybrid is detected by measuring a chemiluminescence signal. 8
9 The mycobacterial nucleic acid hybridization probes have excellent sensitivity and specificity as shown in the table on this slide. Sensitivity values range from 93% for Mycobacterium kansasii, up to 100% for Mycobacterium intracellulare. Specificity is also extremely good attaining 99% to 100% for all targeted species. There have been some minor cross-reactions reported with uncommon species of nontuberculous mycobacteria, but this can largely be eliminated by strictly controlling the hybridization time and temperature. 9
10 Another type of probe technology that provided identification of mycobacteria from culture isolates are the line probe assays available from Hain Lifesciences or Innogenetics. These tests have genus- and species-specific probes bound to nitrocellulose membranes in a strip format. DNA from the lysed culture extract is added to the strip and allowed to hybridize with the probe. Nonhybridized nucleic acids are washed off and the strip is read to determine which genus- or speciesspecific probe the unknown isolate was able to hybridize with. Several different strip formats are available. For example, the Hain group offers GenoType Mycobacterium CM and AS strips, which allow for the identification of Mycobacterium tuberculosis complex and 29 species of nontuberculous mycobacteria on 2 strips. The GenoType MTBC strip allows for speciation of the members of the Mycobacterium tuberculosis complex, and the GenoType MTBDR plus strip allows for identification of Mycobacterium tuberculosis complex plus selected drug resistance markers including rpob, katg, and inha. A major limitation of the line probe assays is that they are not cleared by the US FDA and, therefore, the manufacturers will not sell these strips for clinical diagnostic use in the United States. 10
11 So what can a laboratory do if it has an acid fast organism growing in culture, but it does not react with the available nucleic acid hybridization probes? Well, the current gold standard for the identification of mycobacteria is DNA sequencing. The most popular method used is traditional Sanger dideoxy sequencing and there are various targets which have proven to be useful for mycobacteria. These include the 16S ribosomal DNA gene, the beta subunit of the bacterial RNA polymerase or rpob gene, and the heat shock protein hsp65 gene. Other gene targets have also been described in the literature. Using the 16S rdna gene target as an example, the technique utilized broad range primers that amplify the 16S gene or a portion of the 16S gene, usually the first 500-base pairs. Sequence evaluation of a hypervariable region of the 16S target found between the primers allows for the species-specific identification of a wide variety of mycobacterial species when the unknown sequence is interrogated against a known mycobacterial sequence library. The typical workflow used for sequencing of mycobacteria in our laboratory includes selection of the organism to be sequenced from the culture plate. This is done working inside of a biological safety cabinet in a biosafety level 3 laboratory as a precaution in case the organism growing on the culture plate is Mycobacterium tuberculosis. After selection, the organism is lysed and heated to render it nonviable. Then the mycobacterial DNA is amplified using a PCR reaction followed by a template cleaning step. Then a second PCR reaction is done to incorporate the dideoxynucleotides 11
12 required for traditional Sanger sequencing, followed by a second clean-up step to remove unincorporated nucleotides. Cycle sequencing is completed using a capillary electrophoresis instrument and the mycobacterial DNA sequence is available for analysis by the laboratory staff. 11
13 Mycobacterial sequence libraries commonly utilized in our laboratory are the MicroSeq library from Applied Biosystems (now Life Technologies), a custom laboratory-specific mycobacterial library that has been developed over a couple of decades using type strains and well-characterized clinical isolates, and the NCBI GenBank database, which can be queried using a BLAST search. In addition, there are several vended, curated, web-based database tools available for library searching and comparison. These include products from SmartGene and isentio. The turnaround time for sequencing can be as fast as 8 hours after growth of the organism on a culture plate. In our laboratory, we batch isolates into groups of 96 for efficiency's sake. This means that we select colonies to be sequenced from culture plates in the morning, perform PCR amplification in the afternoon, perform the electrophoresis of the cleaned PCR products on the sequencing instrument overnight with analysis and reporting of the sequence results occurring early the next morning so the information is available for the physicians as they do their morning rounds. 12
14 The advantages of DNA sequencing for the identification of mycobacteria are that it allows for the objective identification of a wide variety of mycobacteria species including Mycobacterium tuberculosis. Also, a fairly rapid turnaround time is possible with same day or next day identification to the species level after the organism grows in culture. Limitations of sequencing include the fact that it s fairly labor-intensive and requires highly skilled, trained technologists for performance. In addition, the equipment and reagents costs are not insignificant. Finally, I cannot stress strongly enough how dependent the use of a high-quality library database. Sequencing is generally used for the identification of nontuberculous mycobacteria, but Mycobacterium tuberculosis complex can also be identified by sequence analysis if the specific nucleic acid hybridization probe is not available. 13
15 Another technology which is becoming popular in clinical microbiology laboratories is matrix-assisted laser-desorption time of flight mass spectrometry or MALDI-TOF MS. This technology is changing the way that we identify microbes in the laboratory and it has already been established as an important method in our laboratories for the identification of aerobic bacteria and yeast. We will soon be implementing it for the identification of mycobacteria as well. 14
16 This slide shows the 2 MALDI-TOF MS systems that I am aware of that are commercially available for clinical laboratories to use for the identification of microorganisms. The Bruker BioTyper system is shown on the left, and the biomerieux Vitek MS system is pictured on the right. Both systems are FDA-cleared for the identification of a limited menu of bacteria. Neither system has clearance for the identification of mycobacteria at this time, although clinical trial studies in this area are being planned. Laboratories utilizing this technology must perform a thorough validation and verification study to ensure that mycobacteria are correctly identified. 15
17 This slide depicts the workflow utilized in our laboratory for the identification of mycobacteria using MALDI-TOF mass spectrometry. Working a biosafety level 3 laboratory, the organisms to be identified are selected using a 10-microliter disposable loop. The organism is then added to a small tube containing zirconium beads and 70% ethanol. Exposure to the ethanol for a period of time followed by bead beading renders the organism nonviable and makes it safe to complete the remainder of the processing steps in the biosafety level 2 laboratory. The solution is centrifuged to pellet the proteins and the supernatant is decanted followed by a speed vac step to dry the pellet. Finally, formic acid and acetonitrile are added and the solution is spotted onto the MALDI-TOF plate followed by analysis in the mass spectrometer. 16
18 There are a number of advantages to using MALDI-TOF mass spectrometry for the identification of mycobacteria, including the fact that the work flow for the laboratory is similar regardless of the type of organism you are looking for. Bacteria, yeast, mycobacteria, and molds can all be analyzed on the instrument with only minor changes to the preprocessing steps required to spotting on the plate. In addition, the technology is cost effective and green with very few consumable used. The turnaround time for a result is pretty rapid with only an hour or so required to analyze 24 specimens and the throughput available in a single day per instrument is fairly high. Only a single colony of organism is needed and the instrument itself has small footprint. The chemicals used in the process are extremely low volume and the exposure risk to laboratory personnel is small because organisms are inactivated before they are applied to the plate. Finally, the system is adaptable by the user with the ability to create and expand databases to supplement the manufacturer s database. 17
19 Limitations of MALDI-TOF MS for the identification of mycobacteria include the need to grow the organism in culture first, which is often a rate limiting step. MALDI-TOF requires a pure isolate so mixtures of organisms often give a no identification result. In addition, the phase of growth and the media used can affect the spectra produced. For best performance, the spectral library needs to be composed of spectra produced under comparable conditions to your everyday working practices in the laboratory. The manufacturer s databases need expansion for less common organisms and instrument downtime, whether for scheduled maintenance or due to a breakdown, can be a problem if you are using a single instrument and if it has been the main method of organism identification in your laboratory. Regulatory issues can be a concern for groups of organisms which have not yet received FDA-clearance and, specifically for mycobacteria, the technique may, at times, be a little slower than sequencing because of the need to have more growth for MALDI-TOF MS than for sequencing. 18
20 In this slide, I will talk in very general terms about the costs associated with identifying microorganisms including mycobacteria with MALDI-TOF MS. The instrument itself costs approximately $200,000 dollars, while the steel plates are about $500 each or a total of $5,000 for a set of 10. A significant cost is associated with the service contract and annual maintenance cost, which will be in the neighborhood of $25,000 but which are very important to purchase in our experience. Remember, the good news is that mass spectrometry can be used for the identification of bacteria, mycobacteria, and molds on the same platform; next generation MALDI- TOF instruments will likely be linked with susceptibility platforms as well, which will further increase the utility for the laboratory. 19
21 In the remaining slides, I will discuss the direct identification of Mycobacterium tuberculosis complex without waiting for growth of the organism in culture. 20
22 The Centers for Disease Control and Prevention recommend that nucleic acid amplification testing be performed on at least 1 respiratory specimen, preferably the first specimen, from each patient with suspected pulmonary tuberculosis. The testing is recommended if it would alter case management or if it would alter TB control activities. Nucleic acid amplification testing does not replace the need for performing a mycobacterial culture because culture usually has better sensitivity. 21
23 One nucleic acid amplification test which is FDA-approved for the direct detection of Mycobacterium tuberculosis complex is the Mycobacterium tuberculosis Direct Test or the MTD test from Hologic GenProbe. This test utilizes transcription-mediated amplification of Mycobacterium tuberculosis complex ribosomal RNA directly from respiratory specimens. You may frequently hear this test referred to as the TB Probe assay because it is from the same company the produces the nucleic acid hybridization probes. This is a misnomer, however, because this is actually a nucleic acid amplification test. The MTD test has excellent clinical specificity at 99% to 100% and the clinical sensitivity is also good at 91% to 95% for smear-positive respiratory specimens and 83 to 100% for smear-negative respiratory specimens. 22
24 The limitations of the MTD test include the observation that this is a technically difficult test for laboratory technologists to perform. It is an open PCR system, which requires opening of tubes and transfer of amplified products so the risk for a falsepositive result due to sample cross-contamination or amplicon contamination is high. In addition, potential inhibitors from specimen components is a concern. A negative MTD test does not rule-out Mycobacterium tuberculosis infection and a mycobacterial culture still needs to be performed. In addition, the MTD test detects the presence of nucleic acid, but it doesn t indicate if the organism is still viable. Cross reactions can occur with some rare mycobacteria and the test can be costly. 23
25 Two of the line probe assays described previously from Hain Lifesciences or Innogenetics can also detect Mycobacterium tuberculosis complex directly from respiratory specimens, but as discussed earlier they are not cleared by the US FDA and, therefore, the manufacturers will not sell these strips for clinical diagnostic use in the United States. 24
26 Several laboratories including ours offers laboratory-developed PCR tests for the direct detection of Mycobacterium tuberculosis. These are generally closed PCR systems, which significantly reduce the opportunity for false-positives due to cross contamination. In addition, the tests are reported to have good sensitivity and specificity, although this can be difficult to judge since each test is developed and verified independently. Often these laboratory-developed real-time PCR assays are less expensive that the commercially available test and they can sometimes be utilized on a wider variety of specimen types including smear-negative respiratory specimens and nonrespiratory specimens including formalin-fixed, paraffin-embedded tissue blocks from Pathology. 25
27 The workflow for all of our real-time PCR assays is the same and is depicted in this slide. We begin with the patient specimen or a culture isolate, which undergo a processing step to lyse and inactivate the organism. Next the specimen is extracted to isolate the nucleic acids, which are in turn amplified using real-time PCR. Sequencespecific detection is achieved using fluorescence resonance energy transfer (or FRET) probes. The entire process requires about 4 hours from start to finish and, therefore, the result can be available the same day that the specimen is submitted. 26
28 A direct head-to-head comparison of our laboratory-developed real-time PCR assay with the Hologic GenProbe MTD test demonstrated that the 2 assays show a high degree of concordance with a 99.3% agreement rate for 542 specimens including 52 that were previously found to be positive by the MTD test. The kappa coefficient is 0.96, which indicates almost perfect agreement between the 2 tests. 27
29 Limitations of a laboratory-developed PCR assay for Mycobacterium tuberculosis complex include some of those limitations found for the commercially available tests, namely a lower sensitivity for smear-negative specimens, the fact that a negative result does not rule-out Mycobacterium tuberculosis infection, and a mycobacterial culture is still needed, and the fact that nucleic acid amplification tests detect the presence of amplifiable nucleic acid, but they don t indicate if the organism is still viable. In addition, the cost of an LDT test can also be high. 28
30 The most recent FDA-cleared tests available for the direct detection of Mycobacterium tuberculosis complex directly from respiratory specimens is the Cepheid Xpert MTB/RIF test, which is endorsed by the World Health Organization and which runs on the Cepheid GeneXpert system. This test provides direct detection of Mycobacterium tuberculosis complex and provides information about rifampin resistance. The schematic shown on this slide depicts how simple the Xpert test is to perform. The patient s sputum specimen is inactivated and liquefied by the addition of the sample prep reagent. Once inactivated, 2 ml of the sample is transferred into an MTB/RIF test cartridge and the cartridge is inserted into the GeneXpert instrument. This is all the technologist has to do and the sample is extracted and amplified inside the instrument with no hands-on work required. At the completion of the test, which requires about 2 hours from start to finish, a report is generated which indicates whether Mycobacterium tuberculosis complex is present and whether mutations in the rpob gene have been detected, which correlate with rifampin resistance. 29
31 The have been a large number of studies published on the Xpert MTB/RIF assay performance including a meta-analysis of 18 studies with over 10,000 patients presented in the Journal of Infection in 2012 by Chang et al. Their analysis indicated that the sensitivity of the Xpert assay for smear-positive, pulmonary tuberculosis is approximately 91%, while the sensitivity for smear-negative disease is expectedly lower at approximately 74%. The specificity of the test is excellent at 98%. For extrapulmonary tuberculosis, the sensitivity and specificity of the Xpert assay are lower at 80% and 86%, respectively. Finally, the time to diagnosis for the Xpert assay compared favorably with nonspecific smear microscopy and the Xpert assay, both resulted on the day of specimen submission, while broth culture for tuberculosis required an average of 16 days and solid media cultures required an average of 20 days to complete. 30
32 As mentioned earlier, the Xpert MTB/RIF assay provides information about potential rifampin resistance. The target is the rpob gene, which encodes the beta subunit of the bacterial RNA polymerase. Mutations in an 81-base pair region of the rpob gene are responsible for approximately 96% of rifampin resistance in Mycobacterium tuberculosis. Rifampin resistance also is a predictor of multidrug-resistant tuberculosis since the majority of rifampin-resistant isolates will also be isoniazid-resistant. Some false-rifampin resistance has been reported in the literature by users of the Xpert MTB/RIF assay, so the CDC recommends reporting Xpert-rifampin resistant results as a preliminary result pending confirmation with sequencing. In addition, the positive-predictive value of the assay is lower in low-prevalence settings. Growthbased drug susceptibility testing is also still required. 31
33 Strengths of the Xpert MTB/RIF assay include good sensitivity and specificity for respiratory specimens, a rapid 2-hour turnaround time, the ability to detect Mycobacterium tuberculosis complex and rifampin resistance directly, and the use of a closed PCR system with a low risk of cross-contamination. The GeneXpert platform is multifunctional and can be used for other tests such as Clostridium difficile or HIV viral load testing. The test is extremely simple for operators to perform and no advanced biosafety equipment is needed, making this test extremely attractive in developing countries with a high burden of TB. 32
34 Limitations of the Xpert MTB/RIF assay include the need to still perform a culture, the possibility of false-positive rifampin resistance requiring confirmation by sequencing, and the lower sensitivity and specificity for extrapulmonary specimens. Cost considerations include the need to purchase the instrument and the cartridges, which can be as high as $65 each in the US. The cartridges often have a discounted price of around $10 in countries with high TB burden and in developing countries. The need for continuous electrical power and air conditioning can also make its use a challenge in developing countries. Sample storage is limited to 3 days at room temperature or 7 days under refrigerated conditions. Finally as with the other PCR assays discussed, the test cannot differentiate between live and nonviable Mycobacterium Tuberculosis, so it cannot be used to monitor response to treatment. 33
35 So in summary, I will conclude by reiterating that molecular diagnostics are enabling clinical microbiology laboratories to detect and identify Mycobacterium tuberculosis complex both after growth in culture and directly from specimens with good sensitivity and specificity. In addition, molecular methods allow for the rapid speciation between members of the Mycobacterium tuberculosis complex and for the detection of mutations associated with drug resistance. 34
36 I thank you very much for taking the time to listen to this Hot Topic presentation and I hope you will join me for the last installment in this series when I discuss drug susceptibility testing for Mycobacterium tuberculosis complex. 35
37 36
Laboratory Techniques for the Diagnosis of TB what s available? Dr. Annette Jepson
 Laboratory Techniques for the Diagnosis of TB what s available? Dr. Annette Jepson Outline of Topics to be Covered Laboratory processing of sputum samples Culture techniques Molecular diagnostics New developments
Laboratory Techniques for the Diagnosis of TB what s available? Dr. Annette Jepson Outline of Topics to be Covered Laboratory processing of sputum samples Culture techniques Molecular diagnostics New developments
Performance Evaluation and Clinical Application of MTB NAAT in Orange County, CA. Minoo Ghajar Orange County Public Health Laboratory
 Performance Evaluation and Clinical Application of MTB NAAT in Orange County, CA Minoo Ghajar Orange County Public Health Laboratory TB Case Count and Rate: Orange County, CA and the United States, 2012
Performance Evaluation and Clinical Application of MTB NAAT in Orange County, CA Minoo Ghajar Orange County Public Health Laboratory TB Case Count and Rate: Orange County, CA and the United States, 2012
Applications for MALDI-TOF MS in Clinical Microbiology. MALDI-TOF is not a Cocktail. The Ideal Identification System 9/10/2012
 Applications for MALDI-TOF MS in Clinical Microbiology Carey-Ann Burnham and David Winkler SWACM 2012 St. Louis, MO Identification of Microorganisms Historical Perspective Microbial identification based
Applications for MALDI-TOF MS in Clinical Microbiology Carey-Ann Burnham and David Winkler SWACM 2012 St. Louis, MO Identification of Microorganisms Historical Perspective Microbial identification based
19th Swiss TB Symposium Münchenwiler, 25.03.2010
 19 th SWISS TUBERCULOSIS SYMPOSIUM Update on culture and identification Münchenwiler, 25.03.2010 Dr. Thomas Bodmer, M.D. Institute for Infectious Diseases Culture of mycobacteria Cultivation of MTB in
19 th SWISS TUBERCULOSIS SYMPOSIUM Update on culture and identification Münchenwiler, 25.03.2010 Dr. Thomas Bodmer, M.D. Institute for Infectious Diseases Culture of mycobacteria Cultivation of MTB in
Essentials of Real Time PCR. About Sequence Detection Chemistries
 Essentials of Real Time PCR About Real-Time PCR Assays Real-time Polymerase Chain Reaction (PCR) is the ability to monitor the progress of the PCR as it occurs (i.e., in real time). Data is therefore collected
Essentials of Real Time PCR About Real-Time PCR Assays Real-time Polymerase Chain Reaction (PCR) is the ability to monitor the progress of the PCR as it occurs (i.e., in real time). Data is therefore collected
Mobile Lab-Diagnostik
 Mobile Lab-Diagnostik Jenaer Technologietag 2013 Alere Technologies GmbH, Jena; 11.11.2013, Torsten Schulz ALERE TECHNOLOGIES Alere Technologies GmbH former CLONDIAG GmbH 1998/1999 Foundation; 2001 first
Mobile Lab-Diagnostik Jenaer Technologietag 2013 Alere Technologies GmbH, Jena; 11.11.2013, Torsten Schulz ALERE TECHNOLOGIES Alere Technologies GmbH former CLONDIAG GmbH 1998/1999 Foundation; 2001 first
NON-COMMERCIAL CULTURE AND DRUG-SUSCEPTIBILITY TESTING METHODS FOR SCREENING OF PATIENTS AT RISK OF MULTI-DRUG RESISTANT TUBERCULOSIS
 NON-COMMERCIAL CULTURE AND DRUG-SUSCEPTIBILITY TESTING METHODS FOR SCREENING OF PATIENTS AT RISK OF MULTI-DRUG RESISTANT TUBERCULOSIS - POLICY STATEMENT - July 2010 TABLE OF CONTENTS 1. Background... 1
NON-COMMERCIAL CULTURE AND DRUG-SUSCEPTIBILITY TESTING METHODS FOR SCREENING OF PATIENTS AT RISK OF MULTI-DRUG RESISTANT TUBERCULOSIS - POLICY STATEMENT - July 2010 TABLE OF CONTENTS 1. Background... 1
Protocol. Introduction to TaqMan and SYBR Green Chemistries for Real-Time PCR
 Protocol Introduction to TaqMan and SYBR Green Chemistries for Real-Time PCR Copyright 2008, 2010 Applied Biosystems. All rights reserved. Ambion and Applied Biosystems products are for Research Use Only.
Protocol Introduction to TaqMan and SYBR Green Chemistries for Real-Time PCR Copyright 2008, 2010 Applied Biosystems. All rights reserved. Ambion and Applied Biosystems products are for Research Use Only.
Bacterial Transformation with Green Fluorescent Protein. Table of Contents Fall 2012
 Bacterial Transformation with Green Fluorescent Protein pglo Version Table of Contents Bacterial Transformation Introduction..1 Laboratory Exercise...3 Important Laboratory Practices 3 Protocol...... 4
Bacterial Transformation with Green Fluorescent Protein pglo Version Table of Contents Bacterial Transformation Introduction..1 Laboratory Exercise...3 Important Laboratory Practices 3 Protocol...... 4
TEST METHOD VERIFICATION AND VALIDATION
 1 TEST METHOD VERIFICATION AND VALIDATION SWACM 2014 MICHAEL LOEFFELHOLZ, PH.D., ABMM DEPT. PATHOLOGY UNIV TEXAS MEDICAL BRANCH GALVESTON, TX 2 OBJECTIVES Define validation and verification Describe components
1 TEST METHOD VERIFICATION AND VALIDATION SWACM 2014 MICHAEL LOEFFELHOLZ, PH.D., ABMM DEPT. PATHOLOGY UNIV TEXAS MEDICAL BRANCH GALVESTON, TX 2 OBJECTIVES Define validation and verification Describe components
Mycoplasma Testing Products & Services. M-175 CELLshipper Mycoplasma Detection Kit (In-house sample preparation and slide fixation)
 Cell Culture Testing M-1500 Real-Time PCR with Broth Enrichment A specific and sensitive method for the detection of mycoplasma using Real-Time PCR coupled with a pre-enrichment procedure to enhance method
Cell Culture Testing M-1500 Real-Time PCR with Broth Enrichment A specific and sensitive method for the detection of mycoplasma using Real-Time PCR coupled with a pre-enrichment procedure to enhance method
Application Guide... 2
 Protocol for GenomePlex Whole Genome Amplification from Formalin-Fixed Parrafin-Embedded (FFPE) tissue Application Guide... 2 I. Description... 2 II. Product Components... 2 III. Materials to be Supplied
Protocol for GenomePlex Whole Genome Amplification from Formalin-Fixed Parrafin-Embedded (FFPE) tissue Application Guide... 2 I. Description... 2 II. Product Components... 2 III. Materials to be Supplied
GeneXpert Technology. Indira Soundiram 2012
 GeneXpert Technology Indira Soundiram 2012 A Better Way to Platform Design GeneXpert Infinity-48 GeneXpert Module GX-I GX-II GX-IV GX-XVI 2 Defining Molecular Diagnostics Any Test Any Time Any Sample Any
GeneXpert Technology Indira Soundiram 2012 A Better Way to Platform Design GeneXpert Infinity-48 GeneXpert Module GX-I GX-II GX-IV GX-XVI 2 Defining Molecular Diagnostics Any Test Any Time Any Sample Any
50 g 650 L. *Average yields will vary depending upon a number of factors including type of phage, growth conditions used and developmental stage.
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Phage DNA Isolation Kit Product # 46800, 46850 Product Insert
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Phage DNA Isolation Kit Product # 46800, 46850 Product Insert
VLLM0421c Medical Microbiology I, practical sessions. Protocol to topic J10
 Topic J10+11: Molecular-biological methods + Clinical virology I (hepatitis A, B & C, HIV) To study: PCR, ELISA, your own notes from serology reactions Task J10/1: DNA isolation of the etiological agent
Topic J10+11: Molecular-biological methods + Clinical virology I (hepatitis A, B & C, HIV) To study: PCR, ELISA, your own notes from serology reactions Task J10/1: DNA isolation of the etiological agent
Molecular diagnostics is now used for a wide range of applications, including:
 Molecular Diagnostics: A Dynamic and Rapidly Broadening Market Molecular diagnostics is now used for a wide range of applications, including: Human clinical molecular diagnostic testing Veterinary molecular
Molecular Diagnostics: A Dynamic and Rapidly Broadening Market Molecular diagnostics is now used for a wide range of applications, including: Human clinical molecular diagnostic testing Veterinary molecular
Introduction To Real Time Quantitative PCR (qpcr)
 Introduction To Real Time Quantitative PCR (qpcr) SABiosciences, A QIAGEN Company www.sabiosciences.com The Seminar Topics The advantages of qpcr versus conventional PCR Work flow & applications Factors
Introduction To Real Time Quantitative PCR (qpcr) SABiosciences, A QIAGEN Company www.sabiosciences.com The Seminar Topics The advantages of qpcr versus conventional PCR Work flow & applications Factors
Single Nucleotide Polymorphisms (SNPs)
 Single Nucleotide Polymorphisms (SNPs) Additional Markers 13 core STR loci Obtain further information from additional markers: Y STRs Separating male samples Mitochondrial DNA Working with extremely degraded
Single Nucleotide Polymorphisms (SNPs) Additional Markers 13 core STR loci Obtain further information from additional markers: Y STRs Separating male samples Mitochondrial DNA Working with extremely degraded
Lab 10: Bacterial Transformation, part 2, DNA plasmid preps, Determining DNA Concentration and Purity
 Lab 10: Bacterial Transformation, part 2, DNA plasmid preps, Determining DNA Concentration and Purity Today you analyze the results of your bacterial transformation from last week and determine the efficiency
Lab 10: Bacterial Transformation, part 2, DNA plasmid preps, Determining DNA Concentration and Purity Today you analyze the results of your bacterial transformation from last week and determine the efficiency
UltraClean Soil DNA Isolation Kit
 PAGE 1 UltraClean Soil DNA Isolation Kit Catalog # 12800-50 50 preps New improved PCR inhibitor removal solution (IRS) included Instruction Manual (New Alternative Protocol maximizes yields) Introduction
PAGE 1 UltraClean Soil DNA Isolation Kit Catalog # 12800-50 50 preps New improved PCR inhibitor removal solution (IRS) included Instruction Manual (New Alternative Protocol maximizes yields) Introduction
510K Summary. This summary of 510(k) safety and effectiveness information is being submitted in accordance with the requirements of 21 CFR 807.92.
 510K Summary This summary of 510(k) safety and effectiveness information is being submitted in accordance with the requirements of 21 CFR 807.92. Submitter: Contact: One Lambda, Incorporated 21001 Kittridge
510K Summary This summary of 510(k) safety and effectiveness information is being submitted in accordance with the requirements of 21 CFR 807.92. Submitter: Contact: One Lambda, Incorporated 21001 Kittridge
PCR and Sequencing Reaction Clean-Up Kit (Magnetic Bead System) 50 preps Product #60200
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com PCR and Sequencing Reaction Clean-Up Kit (Magnetic Bead System)
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com PCR and Sequencing Reaction Clean-Up Kit (Magnetic Bead System)
Genomic DNA Extraction Kit INSTRUCTION MANUAL
 Genomic DNA Extraction Kit INSTRUCTION MANUAL Table of Contents Introduction 3 Kit Components 3 Storage Conditions 4 Recommended Equipment and Reagents 4 Introduction to the Protocol 4 General Overview
Genomic DNA Extraction Kit INSTRUCTION MANUAL Table of Contents Introduction 3 Kit Components 3 Storage Conditions 4 Recommended Equipment and Reagents 4 Introduction to the Protocol 4 General Overview
BacReady TM Multiplex PCR System
 BacReady TM Multiplex PCR System Technical Manual No. 0191 Version 10112010 I Description.. 1 II Applications 2 III Key Features.. 2 IV Shipping and Storage. 2 V Simplified Procedures. 2 VI Detailed Experimental
BacReady TM Multiplex PCR System Technical Manual No. 0191 Version 10112010 I Description.. 1 II Applications 2 III Key Features.. 2 IV Shipping and Storage. 2 V Simplified Procedures. 2 VI Detailed Experimental
How many of you have checked out the web site on protein-dna interactions?
 How many of you have checked out the web site on protein-dna interactions? Example of an approximately 40,000 probe spotted oligo microarray with enlarged inset to show detail. Find and be ready to discuss
How many of you have checked out the web site on protein-dna interactions? Example of an approximately 40,000 probe spotted oligo microarray with enlarged inset to show detail. Find and be ready to discuss
Real-Time PCR Vs. Traditional PCR
 Real-Time PCR Vs. Traditional PCR Description This tutorial will discuss the evolution of traditional PCR methods towards the use of Real-Time chemistry and instrumentation for accurate quantitation. Objectives
Real-Time PCR Vs. Traditional PCR Description This tutorial will discuss the evolution of traditional PCR methods towards the use of Real-Time chemistry and instrumentation for accurate quantitation. Objectives
AxyPrep TM Mag PCR Clean-up Protocol
 AxyPrep TM Mag PCR Clean-up Protocol Intro The AxyPrep Mag PCR Clean-up kit utilizes a unique paramagnetic bead technology for rapid, high-throughput purification of PCR amplicons. Using this kit, PCR
AxyPrep TM Mag PCR Clean-up Protocol Intro The AxyPrep Mag PCR Clean-up kit utilizes a unique paramagnetic bead technology for rapid, high-throughput purification of PCR amplicons. Using this kit, PCR
World Health Organization 2011
 2011 1 Automated Real-time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System Policy Statement WHO Library Cataloguing-in-Publication
2011 1 Automated Real-time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System Policy Statement WHO Library Cataloguing-in-Publication
ab185916 Hi-Fi cdna Synthesis Kit
 ab185916 Hi-Fi cdna Synthesis Kit Instructions for Use For cdna synthesis from various RNA samples This product is for research use only and is not intended for diagnostic use. Version 1 Last Updated 1
ab185916 Hi-Fi cdna Synthesis Kit Instructions for Use For cdna synthesis from various RNA samples This product is for research use only and is not intended for diagnostic use. Version 1 Last Updated 1
Guidance for Industry and FDA Staff Commercially Distributed Analyte Specific Reagents (ASRs): Frequently Asked Questions
 Guidance for Industry and FDA Staff Commercially Distributed Analyte Specific Reagents (ASRs): Frequently Asked Questions Document issued on: September 14, 2007 The draft of this guidance document was
Guidance for Industry and FDA Staff Commercially Distributed Analyte Specific Reagents (ASRs): Frequently Asked Questions Document issued on: September 14, 2007 The draft of this guidance document was
Next Generation Sequencing in Public Health Laboratories. 2014 Survey Results
 Next Generation Sequencing in Public Health Laboratories 2014 Survey Results MAY 2015 This project was 100% funded with federal funds from a federal program of $215,972. This publication was supported
Next Generation Sequencing in Public Health Laboratories 2014 Survey Results MAY 2015 This project was 100% funded with federal funds from a federal program of $215,972. This publication was supported
Biotechnology and Recombinant DNA (Chapter 9) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College
 Biotechnology and Recombinant DNA (Chapter 9) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Primary Source for figures and content: Eastern Campus Tortora, G.J. Microbiology
Biotechnology and Recombinant DNA (Chapter 9) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Primary Source for figures and content: Eastern Campus Tortora, G.J. Microbiology
INTERPRETATION INFORMATION SHEET
 Creative Testing Solutions 2424 West Erie Dr. 2205 Highway 121 10100 Martin Luther King Jr. St. No. Tempe, AZ 85282 Bedford, TX 76021 St. Petersburg, FL 33716 INTERPRETATION INFORMATION SHEET Human Immunodeficiency
Creative Testing Solutions 2424 West Erie Dr. 2205 Highway 121 10100 Martin Luther King Jr. St. No. Tempe, AZ 85282 Bedford, TX 76021 St. Petersburg, FL 33716 INTERPRETATION INFORMATION SHEET Human Immunodeficiency
Green Fluorescent Protein (GFP): Genetic Transformation, Synthesis and Purification of the Recombinant Protein
 Green Fluorescent Protein (GFP): Genetic Transformation, Synthesis and Purification of the Recombinant Protein INTRODUCTION Green Fluorescent Protein (GFP) is a novel protein produced by the bioluminescent
Green Fluorescent Protein (GFP): Genetic Transformation, Synthesis and Purification of the Recombinant Protein INTRODUCTION Green Fluorescent Protein (GFP) is a novel protein produced by the bioluminescent
Medical Microbiology Culture Media :
 Lecture 3 Dr. Ismail I. Daood Medical Microbiology Culture Media : Culture media are used for recognition and identification (diagnosis) of microorganisms. The media are contained in plates (Petri dishes),
Lecture 3 Dr. Ismail I. Daood Medical Microbiology Culture Media : Culture media are used for recognition and identification (diagnosis) of microorganisms. The media are contained in plates (Petri dishes),
PNA BRAF Mutation Detection Kit
 - PNA BRAF Mutation Detection Kit Catalog Number KA2102 50 tests/kit Version: 01 Intended for research use only www.abnova.com Introduction and Background Intended use The PNA BRAF Mutation Detection Kit
- PNA BRAF Mutation Detection Kit Catalog Number KA2102 50 tests/kit Version: 01 Intended for research use only www.abnova.com Introduction and Background Intended use The PNA BRAF Mutation Detection Kit
Transformation of the bacterium E. coli. using a gene for Green Fluorescent Protein
 Transformation of the bacterium E. coli using a gene for Green Fluorescent Protein Background In molecular biology, transformation refers to a form of genetic exchange in which the genetic material carried
Transformation of the bacterium E. coli using a gene for Green Fluorescent Protein Background In molecular biology, transformation refers to a form of genetic exchange in which the genetic material carried
REAL TIME PCR USING SYBR GREEN
 REAL TIME PCR USING SYBR GREEN 1 THE PROBLEM NEED TO QUANTITATE DIFFERENCES IN mrna EXPRESSION SMALL AMOUNTS OF mrna LASER CAPTURE SMALL AMOUNTS OF TISSUE PRIMARY CELLS PRECIOUS REAGENTS 2 THE PROBLEM
REAL TIME PCR USING SYBR GREEN 1 THE PROBLEM NEED TO QUANTITATE DIFFERENCES IN mrna EXPRESSION SMALL AMOUNTS OF mrna LASER CAPTURE SMALL AMOUNTS OF TISSUE PRIMARY CELLS PRECIOUS REAGENTS 2 THE PROBLEM
ZR DNA Sequencing Clean-up Kit
 INSTRUCTION MANUAL ZR DNA Sequencing Clean-up Kit Catalog Nos. D40 & D4051 Highlights Simple 2 Minute Bind, Wash, Elute Procedure Flexible 6-20 µl Elution Volumes Allow for Direct Loading of Samples with
INSTRUCTION MANUAL ZR DNA Sequencing Clean-up Kit Catalog Nos. D40 & D4051 Highlights Simple 2 Minute Bind, Wash, Elute Procedure Flexible 6-20 µl Elution Volumes Allow for Direct Loading of Samples with
Technical Note. Roche Applied Science. No. LC 18/2004. Assay Formats for Use in Real-Time PCR
 Roche Applied Science Technical Note No. LC 18/2004 Purpose of this Note Assay Formats for Use in Real-Time PCR The LightCycler Instrument uses several detection channels to monitor the amplification of
Roche Applied Science Technical Note No. LC 18/2004 Purpose of this Note Assay Formats for Use in Real-Time PCR The LightCycler Instrument uses several detection channels to monitor the amplification of
JIANGSU CARTMAY INDUSTRIAL CO.,LTD www.labfurniture.asia mail: info@labfurniture.asia
 The basic layout, the main functions and instrumentation concept of micro Inspection Division laboratory, 1, Virology Laboratory 1. Functions: for the city to monitor the prevalence of HIV disease, dealing
The basic layout, the main functions and instrumentation concept of micro Inspection Division laboratory, 1, Virology Laboratory 1. Functions: for the city to monitor the prevalence of HIV disease, dealing
THE MICROBIAL ID BREAKTHROUGH: How DNA Sequencing Services Help Prevent Catastrophic Cleanroom Shutdowns
 A WHITE PAPER THE MICROBIAL ID BREAKTHROUGH: How DNA Sequencing Services Help Prevent Catastrophic Cleanroom Shutdowns By Dennis Champagne Director, Laboratory Services, Microtest, Inc. A WHITE PAPER THE
A WHITE PAPER THE MICROBIAL ID BREAKTHROUGH: How DNA Sequencing Services Help Prevent Catastrophic Cleanroom Shutdowns By Dennis Champagne Director, Laboratory Services, Microtest, Inc. A WHITE PAPER THE
TransformAid Bacterial Transformation Kit
 Home Contacts Order Catalog Support Search Alphabetical Index Numerical Index Restriction Endonucleases Modifying Enzymes PCR Kits Markers Nucleic Acids Nucleotides & Oligonucleotides Media Transfection
Home Contacts Order Catalog Support Search Alphabetical Index Numerical Index Restriction Endonucleases Modifying Enzymes PCR Kits Markers Nucleic Acids Nucleotides & Oligonucleotides Media Transfection
Monitoring Genetic and Metabolic Potential for In Situ Bioremediation: Mass Spectrometry. Progress Report
 Monitoring Genetic and Metabolic Potential for In Situ Bioremediation: Mass Spectrometry September 1997 Progress Report Principal Investigator Michelle V. Buchanan (423) 574-4868 (Phone) (423) 576-8559
Monitoring Genetic and Metabolic Potential for In Situ Bioremediation: Mass Spectrometry September 1997 Progress Report Principal Investigator Michelle V. Buchanan (423) 574-4868 (Phone) (423) 576-8559
Quantification of Mycobacterium Tuberculosis. 150 tests
 Quantification of Mycobacterium Tuberculosis rep 13E12 For general laboratory and research use only 150 tests Introduction to Mycobacterium Tuberculosis Mycobacterium tuberculosis is the bacterium that
Quantification of Mycobacterium Tuberculosis rep 13E12 For general laboratory and research use only 150 tests Introduction to Mycobacterium Tuberculosis Mycobacterium tuberculosis is the bacterium that
IIID 14. Biotechnology in Fish Disease Diagnostics: Application of the Polymerase Chain Reaction (PCR)
 IIID 14. Biotechnology in Fish Disease Diagnostics: Application of the Polymerase Chain Reaction (PCR) Background Infectious diseases caused by pathogenic organisms such as bacteria, viruses, protozoa,
IIID 14. Biotechnology in Fish Disease Diagnostics: Application of the Polymerase Chain Reaction (PCR) Background Infectious diseases caused by pathogenic organisms such as bacteria, viruses, protozoa,
HiPer RT-PCR Teaching Kit
 HiPer RT-PCR Teaching Kit Product Code: HTBM024 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 4 hours Agarose Gel Electrophoresis: 45 minutes Storage Instructions: The
HiPer RT-PCR Teaching Kit Product Code: HTBM024 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 4 hours Agarose Gel Electrophoresis: 45 minutes Storage Instructions: The
Annex to the Accreditation Certificate D-PL-13372-01-00 according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH German Accreditation Body Annex to the Accreditation Certificate D-PL-13372-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 26.03.2012 to 25.03.2017
Deutsche Akkreditierungsstelle GmbH German Accreditation Body Annex to the Accreditation Certificate D-PL-13372-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 26.03.2012 to 25.03.2017
Human Peripheral Blood Mononuclear Cell (PBMC) Manual
 Human Peripheral Blood Mononuclear Cell (PBMC) Manual INSTRUCTION MANUAL ZBM0063.04 SHIPPING CONDITIONS Human Peripheral Blood Mononuclear Cells, cryopreserved Cryopreserved human peripheral blood mononuclear
Human Peripheral Blood Mononuclear Cell (PBMC) Manual INSTRUCTION MANUAL ZBM0063.04 SHIPPING CONDITIONS Human Peripheral Blood Mononuclear Cells, cryopreserved Cryopreserved human peripheral blood mononuclear
Genomic DNA Clean & Concentrator Catalog Nos. D4010 & D4011
 Page 0 INSTRUCTION MANUAL Catalog Nos. D4010 & D4011 Highlights Quick (5 minute) spin column recovery of large-sized DNA (e.g., genomic, mitochondrial, plasmid (BAC/PAC), viral, phage, (wga)dna, etc.)
Page 0 INSTRUCTION MANUAL Catalog Nos. D4010 & D4011 Highlights Quick (5 minute) spin column recovery of large-sized DNA (e.g., genomic, mitochondrial, plasmid (BAC/PAC), viral, phage, (wga)dna, etc.)
Co Extra (GM and non GM supply chains: Their CO EXistence and TRAceability) Outcomes of Co Extra
 GM and non GM supply chains: Their CO EXistence and TRAceability Outcomes of Co Extra Comparison of different real time PCR chemistries and their suitability for detection and quantification of genetically
GM and non GM supply chains: Their CO EXistence and TRAceability Outcomes of Co Extra Comparison of different real time PCR chemistries and their suitability for detection and quantification of genetically
SYBR Green Realtime PCR Master Mix -Plus-
 Instruction manual SYBR Green Realtime PCR Master Mix -Plus- 0810 F0925K SYBR Green Realtime PCR Master Mix -Plus- Contents QPK-212T 1mLx1 QPK-212 1mLx5 Store at -20 C, protected from light [1] Introduction
Instruction manual SYBR Green Realtime PCR Master Mix -Plus- 0810 F0925K SYBR Green Realtime PCR Master Mix -Plus- Contents QPK-212T 1mLx1 QPK-212 1mLx5 Store at -20 C, protected from light [1] Introduction
Drive Process Productivity
 Solutions for the Biopharmaceutical Industry Drive Process Productivity Innovative solutions from a proven technology leader Your partner in productivity Whether you are developing a process for a novel
Solutions for the Biopharmaceutical Industry Drive Process Productivity Innovative solutions from a proven technology leader Your partner in productivity Whether you are developing a process for a novel
Diagnosis of HIV-1 Infection. Estelle Piwowar-Manning HPTN Central Laboratory The Johns Hopkins University
 Diagnosis of HIV-1 Infection Estelle Piwowar-Manning HPTN Central Laboratory The Johns Hopkins University Tests Used to Diagnose HIV-1 Infection HIV antibody (today s topic) HIV p24 antigen HIV DNA HIV
Diagnosis of HIV-1 Infection Estelle Piwowar-Manning HPTN Central Laboratory The Johns Hopkins University Tests Used to Diagnose HIV-1 Infection HIV antibody (today s topic) HIV p24 antigen HIV DNA HIV
ABSTRACT. Promega Corporation, Updated September 2008. http://www.promega.com/pubhub. 1 Campbell-Staton, S.
 A Modified Wizard SV Genomic DNA Purification System Protocol to Purify Genomic DNA... A Modified Wizard SV Genomic DNA Purification System Protocol to Purify Genomic DNA from Shed Reptile Skin ABSTRACT
A Modified Wizard SV Genomic DNA Purification System Protocol to Purify Genomic DNA... A Modified Wizard SV Genomic DNA Purification System Protocol to Purify Genomic DNA from Shed Reptile Skin ABSTRACT
Microarray Technology
 Microarrays And Functional Genomics CPSC265 Matt Hudson Microarray Technology Relatively young technology Usually used like a Northern blot can determine the amount of mrna for a particular gene Except
Microarrays And Functional Genomics CPSC265 Matt Hudson Microarray Technology Relatively young technology Usually used like a Northern blot can determine the amount of mrna for a particular gene Except
AURAMINE O STAIN. Preanalytical Considerations
 AURAMINE O STAIN Preanalytical Considerations I. PRINCIPLE Acid-fast mycobacteria resist decolorization by acid-alcohol after primary staining owing to the high lipid (mycolic acid) content in their cell
AURAMINE O STAIN Preanalytical Considerations I. PRINCIPLE Acid-fast mycobacteria resist decolorization by acid-alcohol after primary staining owing to the high lipid (mycolic acid) content in their cell
The Power of Next-Generation Sequencing in Your Hands On the Path towards Diagnostics
 The Power of Next-Generation Sequencing in Your Hands On the Path towards Diagnostics The GS Junior System The Power of Next-Generation Sequencing on Your Benchtop Proven technology: Uses the same long
The Power of Next-Generation Sequencing in Your Hands On the Path towards Diagnostics The GS Junior System The Power of Next-Generation Sequencing on Your Benchtop Proven technology: Uses the same long
Genolution Pharmaceuticals, Inc. Life Science and Molecular Diagnostic Products
 Genolution Pharmaceuticals, Inc. Revolution through genes, And Solution through genes. Life Science and Molecular Diagnostic Products www.genolution1.com TEL; 02-3010-8670, 8672 Geno-Serum Hepatitis B
Genolution Pharmaceuticals, Inc. Revolution through genes, And Solution through genes. Life Science and Molecular Diagnostic Products www.genolution1.com TEL; 02-3010-8670, 8672 Geno-Serum Hepatitis B
The Financial Benefits of the MicroSEQ Microbial Identification System
 White paper Financial Benefits of the MicroSEQ Microbial Identification System The Financial Benefits of the MicroSEQ Microbial Identification System Up to 160% ROI over 3 years, Break-even as soon as
White paper Financial Benefits of the MicroSEQ Microbial Identification System The Financial Benefits of the MicroSEQ Microbial Identification System Up to 160% ROI over 3 years, Break-even as soon as
HiPer Ion Exchange Chromatography Teaching Kit
 HiPer Ion Exchange Chromatography Teaching Kit Product Code: HTC001 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 5-6 hours Storage Instructions: The kit is stable for
HiPer Ion Exchange Chromatography Teaching Kit Product Code: HTC001 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 5-6 hours Storage Instructions: The kit is stable for
CHECKLIST OF KEY ACTIONS FOR THE USE OF LIQUID MEDIA FOR CULTURE AND DRUG SUSCEPTIBILITY TESTING (DST)
 CHECKLIST OF FOR THE USE OF LIQUID MEDIA FOR CULTURE AND DRUG SUSCEPTIBILITY TESTING (DST) Rationale for liquid culture systems Laboratory diagnosis of tuberculosis (TB) relies on the direct microscopic
CHECKLIST OF FOR THE USE OF LIQUID MEDIA FOR CULTURE AND DRUG SUSCEPTIBILITY TESTING (DST) Rationale for liquid culture systems Laboratory diagnosis of tuberculosis (TB) relies on the direct microscopic
Guidelines for TB Blood Testing. Minnesota Department of Health TB Prevention and Control Program June 2011
 Guidelines for TB Blood Testing Minnesota Department of Health TB Prevention and Control Program June 2011 Outline Interferon-Gamma Release Assays aka TB blood tests 1. What are they? 2. What are the current
Guidelines for TB Blood Testing Minnesota Department of Health TB Prevention and Control Program June 2011 Outline Interferon-Gamma Release Assays aka TB blood tests 1. What are they? 2. What are the current
BD Affirm VPIII. Microbial Identification System
 BD Affirm VPIII Microbial Identification System The Only Diagnostic Test that Differentiates and Identifies 3 Vaginitis Pathogens from a Single Sample, with DNA Certainty. Translating the power and the
BD Affirm VPIII Microbial Identification System The Only Diagnostic Test that Differentiates and Identifies 3 Vaginitis Pathogens from a Single Sample, with DNA Certainty. Translating the power and the
Lyme (IgG and IgM) Antibody Confirmation
 Pathology & Laboratory Medicine Lyme (IgG and IgM) Antibody Confirmation TEST UPDATE: New Test Notification Date: 1/9/2013 Effective Date: 1/7/2013 CONTACT INFO Call 802-847-5121 800-991-2799 email labmarketing@vtmednet.org
Pathology & Laboratory Medicine Lyme (IgG and IgM) Antibody Confirmation TEST UPDATE: New Test Notification Date: 1/9/2013 Effective Date: 1/7/2013 CONTACT INFO Call 802-847-5121 800-991-2799 email labmarketing@vtmednet.org
Core Functions and Capabilities. Laboratory Services
 Core Functions and Capabilities British Columbia Centre for Disease Control Laboratory Services Understanding the role and value of British Columbia s public health laboratory in protecting our community
Core Functions and Capabilities British Columbia Centre for Disease Control Laboratory Services Understanding the role and value of British Columbia s public health laboratory in protecting our community
Quantifying Bacterial Concentration using a Calibrated Growth Curve
 BTEC 4200 Lab 2. Quantifying Bacterial Concentration using a Calibrated Growth Curve Background and References Bacterial concentration can be measured by several methods, all of which you have studied
BTEC 4200 Lab 2. Quantifying Bacterial Concentration using a Calibrated Growth Curve Background and References Bacterial concentration can be measured by several methods, all of which you have studied
RealLine HCV PCR Qualitative - Uni-Format
 Instructions for use PCR KIT FOR EXTRACTION OF RNA AND REAL TIME PCR DETECTION KIT FOR HEPATITIS C VIRUS RNA Research Use Only Qualitative Uni Format VBD0798 48 tests valid from: December 2013 Rev11122013
Instructions for use PCR KIT FOR EXTRACTION OF RNA AND REAL TIME PCR DETECTION KIT FOR HEPATITIS C VIRUS RNA Research Use Only Qualitative Uni Format VBD0798 48 tests valid from: December 2013 Rev11122013
Department of Microbiology Vidyasagar University Midnapore - 721 102 West Bengal
 S'yllabus for the I 'year FG Diploma Course in QUALITY CONTROL AND ASSURANCE IN MICROBIAL TECHNOLOGY (Semester Based: 400 marks in two semesters) Department of Microbiology Vidyasagar University Midnapore
S'yllabus for the I 'year FG Diploma Course in QUALITY CONTROL AND ASSURANCE IN MICROBIAL TECHNOLOGY (Semester Based: 400 marks in two semesters) Department of Microbiology Vidyasagar University Midnapore
Specimen and Data Repository for TB Diagnostic Research in Children. Sharon Nachman SUNY Stony Brook
 Specimen and Data Repository for TB Diagnostic Research in Children Sharon Nachman SUNY Stony Brook Why have repositories? We need to develop a prospective system that will allow for collection of data
Specimen and Data Repository for TB Diagnostic Research in Children Sharon Nachman SUNY Stony Brook Why have repositories? We need to develop a prospective system that will allow for collection of data
How is genome sequencing done?
 How is genome sequencing done? Using 454 Sequencing on the Genome Sequencer FLX System, DNA from a genome is converted into sequence data through four primary steps: Step One DNA sample preparation; Step
How is genome sequencing done? Using 454 Sequencing on the Genome Sequencer FLX System, DNA from a genome is converted into sequence data through four primary steps: Step One DNA sample preparation; Step
Biological Sciences Initiative
 Biological Sciences Initiative HHMI Student Activities Measuring Antibiotic Resistance Introduction: You might be aware that antibiotics were once thought of as a magic bullet; a nearly perfect drug for
Biological Sciences Initiative HHMI Student Activities Measuring Antibiotic Resistance Introduction: You might be aware that antibiotics were once thought of as a magic bullet; a nearly perfect drug for
Genetic Analysis. Phenotype analysis: biological-biochemical analysis. Genotype analysis: molecular and physical analysis
 Genetic Analysis Phenotype analysis: biological-biochemical analysis Behaviour under specific environmental conditions Behaviour of specific genetic configurations Behaviour of progeny in crosses - Genotype
Genetic Analysis Phenotype analysis: biological-biochemical analysis Behaviour under specific environmental conditions Behaviour of specific genetic configurations Behaviour of progeny in crosses - Genotype
ZR-96 DNA Sequencing Clean-up Kit Catalog Nos. D4052 & D4053
 INSTRUCTION MANUAL ZR-96 DNA Sequencing Clean-up Kit Catalog Nos. D4052 & D4053 Highlights Simple 10 Minute Bind, Wash, Elute Procedure Flexible 15-20 µl Elution Volumes Allow for Direct Loading of Samples
INSTRUCTION MANUAL ZR-96 DNA Sequencing Clean-up Kit Catalog Nos. D4052 & D4053 Highlights Simple 10 Minute Bind, Wash, Elute Procedure Flexible 15-20 µl Elution Volumes Allow for Direct Loading of Samples
March 19, 2014. Dear Dr. Duvall, Dr. Hambrick, and Ms. Smith,
 Dr. Daniel Duvall, Medical Officer Center for Medicare, Hospital and Ambulatory Policy Group Centers for Medicare and Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244 Dr. Edith Hambrick,
Dr. Daniel Duvall, Medical Officer Center for Medicare, Hospital and Ambulatory Policy Group Centers for Medicare and Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244 Dr. Edith Hambrick,
Fluorescent dyes for use with the
 Detection of Multiple Reporter Dyes in Real-time, On-line PCR Analysis with the LightCycler System Gregor Sagner, Cornelia Goldstein, and Rob van Miltenburg Roche Molecular Biochemicals, Penzberg, Germany
Detection of Multiple Reporter Dyes in Real-time, On-line PCR Analysis with the LightCycler System Gregor Sagner, Cornelia Goldstein, and Rob van Miltenburg Roche Molecular Biochemicals, Penzberg, Germany
Just the Facts: A Basic Introduction to the Science Underlying NCBI Resources
 1 of 8 11/7/2004 11:00 AM National Center for Biotechnology Information About NCBI NCBI at a Glance A Science Primer Human Genome Resources Model Organisms Guide Outreach and Education Databases and Tools
1 of 8 11/7/2004 11:00 AM National Center for Biotechnology Information About NCBI NCBI at a Glance A Science Primer Human Genome Resources Model Organisms Guide Outreach and Education Databases and Tools
Path-ID Multiplex One-Step RT-PCR Kit
 USER GUIDE Path-ID Multiplex One-Step RT-PCR Kit TaqMan probe-based multiplex one-step real-time RT-PCR detection of RNA targets Catalog Numbers 4428206, 4428207, 4440022 Publication Part Number 4440907
USER GUIDE Path-ID Multiplex One-Step RT-PCR Kit TaqMan probe-based multiplex one-step real-time RT-PCR detection of RNA targets Catalog Numbers 4428206, 4428207, 4440022 Publication Part Number 4440907
Separation of Amino Acids by Paper Chromatography
 Separation of Amino Acids by Paper Chromatography Chromatography is a common technique for separating chemical substances. The prefix chroma, which suggests color, comes from the fact that some of the
Separation of Amino Acids by Paper Chromatography Chromatography is a common technique for separating chemical substances. The prefix chroma, which suggests color, comes from the fact that some of the
ELISA BIO 110 Lab 1. Immunity and Disease
 ELISA BIO 110 Lab 1 Immunity and Disease Introduction The principal role of the mammalian immune response is to contain infectious disease agents. This response is mediated by several cellular and molecular
ELISA BIO 110 Lab 1 Immunity and Disease Introduction The principal role of the mammalian immune response is to contain infectious disease agents. This response is mediated by several cellular and molecular
DNA Sequence Analysis
 DNA Sequence Analysis Two general kinds of analysis Screen for one of a set of known sequences Determine the sequence even if it is novel Screening for a known sequence usually involves an oligonucleotide
DNA Sequence Analysis Two general kinds of analysis Screen for one of a set of known sequences Determine the sequence even if it is novel Screening for a known sequence usually involves an oligonucleotide
IMBB 2013. Genomic DNA purifica8on
 IMBB 2013 Genomic DNA purifica8on Why purify DNA? The purpose of DNA purifica8on from the cell/8ssue is to ensure it performs well in subsequent downstream applica8ons, e.g. Polymerase Chain Reac8on (PCR),
IMBB 2013 Genomic DNA purifica8on Why purify DNA? The purpose of DNA purifica8on from the cell/8ssue is to ensure it performs well in subsequent downstream applica8ons, e.g. Polymerase Chain Reac8on (PCR),
Raw Milk Quality Tests Do They Predict Fluid Milk Shelf-life or Is it time for new tests?
 Raw Milk Quality Tests Do They Predict Fluid Milk Shelf-life or Is it time for new tests? Martin Wiedmann Milk Quality Improvement Program November 3, 2011 Fluid milk shelf life What defines shelf life
Raw Milk Quality Tests Do They Predict Fluid Milk Shelf-life or Is it time for new tests? Martin Wiedmann Milk Quality Improvement Program November 3, 2011 Fluid milk shelf life What defines shelf life
RealStar HBV PCR Kit 1.0 11/2012
 RealStar HBV PCR Kit 1.0 11/2012 RealStar HBV PCR Kit 1.0 For research use only! (RUO) Product No.: 201003 96 rxns INS-201000-GB-02 Store at -25 C... -15 C November 2012 altona Diagnostics GmbH Mörkenstraße
RealStar HBV PCR Kit 1.0 11/2012 RealStar HBV PCR Kit 1.0 For research use only! (RUO) Product No.: 201003 96 rxns INS-201000-GB-02 Store at -25 C... -15 C November 2012 altona Diagnostics GmbH Mörkenstraße
Fungal DNA sequencing from laser capture microdissection samples
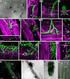 APPLICATION NOTE Laser capture microdissection and Sanger sequencing Fungal DNA sequencing from laser capture microdissection samples Introduction The direct identification of human fungal pathogens by
APPLICATION NOTE Laser capture microdissection and Sanger sequencing Fungal DNA sequencing from laser capture microdissection samples Introduction The direct identification of human fungal pathogens by
Forensic DNA Testing Terminology
 Forensic DNA Testing Terminology ABI 310 Genetic Analyzer a capillary electrophoresis instrument used by forensic DNA laboratories to separate short tandem repeat (STR) loci on the basis of their size.
Forensic DNA Testing Terminology ABI 310 Genetic Analyzer a capillary electrophoresis instrument used by forensic DNA laboratories to separate short tandem repeat (STR) loci on the basis of their size.
Recommended Procedures for the Extraction of RNA. Jan Pedersen USDA, APHIS, VS, National Veterinary Services Laboratories, Ames, IA 50010
 Recommended Procedures for the Extraction of RNA Jan Pedersen USDA, APHIS, VS, National Veterinary Services Laboratories, Ames, IA 50010 RNA Extraction Isolates RNA from other cellular components in the
Recommended Procedures for the Extraction of RNA Jan Pedersen USDA, APHIS, VS, National Veterinary Services Laboratories, Ames, IA 50010 RNA Extraction Isolates RNA from other cellular components in the
How Does a Doctor Test for AIDS?
 Edvo-Kit #S-70 How Does a Doctor Test for AIDS? S-70 Experiment Objective: The Human Immunodefi ciency Virus (HIV) is an infectious agent that causes Acquired Immunodefi ciency Syndrome (AIDS) in humans.
Edvo-Kit #S-70 How Does a Doctor Test for AIDS? S-70 Experiment Objective: The Human Immunodefi ciency Virus (HIV) is an infectious agent that causes Acquired Immunodefi ciency Syndrome (AIDS) in humans.
Genetic Engineering and Biotechnology
 1 So, what is biotechnology?? The use of living organisms to carry out defined chemical processes for industrial or commercial application. The office of Technology Assessment of the U.S. Congress defines
1 So, what is biotechnology?? The use of living organisms to carry out defined chemical processes for industrial or commercial application. The office of Technology Assessment of the U.S. Congress defines
Lab Exercise 3: Media, incubation, and aseptic technique
 Lab Exercise 3: Media, incubation, and aseptic technique Objectives 1. Compare the different types of media. 2. Describe the different formats of media, plate, tube etc. 3. Explain how to sterilize it,
Lab Exercise 3: Media, incubation, and aseptic technique Objectives 1. Compare the different types of media. 2. Describe the different formats of media, plate, tube etc. 3. Explain how to sterilize it,
IMA Knowledge June, 2015
 Tuberculosis is a major public health problem in India. Early diagnosis and complete treatment of TB is the corner-stone of TB prevention and control strategy. India's Revised National TB Control program(rntcp)
Tuberculosis is a major public health problem in India. Early diagnosis and complete treatment of TB is the corner-stone of TB prevention and control strategy. India's Revised National TB Control program(rntcp)
HBV Quantitative Real Time PCR Kit
 Revision No.: ZJ0002 Issue Date: Aug 7 th, 2008 HBV Quantitative Real Time PCR Kit Cat. No.: HD-0002-01 For Use with LightCycler 1.0/LightCycler2.0/LightCycler480 (Roche) Real Time PCR Systems (Pls ignore
Revision No.: ZJ0002 Issue Date: Aug 7 th, 2008 HBV Quantitative Real Time PCR Kit Cat. No.: HD-0002-01 For Use with LightCycler 1.0/LightCycler2.0/LightCycler480 (Roche) Real Time PCR Systems (Pls ignore
Mitochondrial DNA Analysis
 Mitochondrial DNA Analysis Lineage Markers Lineage markers are passed down from generation to generation without changing Except for rare mutation events They can help determine the lineage (family tree)
Mitochondrial DNA Analysis Lineage Markers Lineage markers are passed down from generation to generation without changing Except for rare mutation events They can help determine the lineage (family tree)
Welcome to Implementing Inquirybased Microbial Project. Veronica Ardi, PhD
 Welcome to Implementing Inquirybased Microbial Project Veronica Ardi, PhD Microbiology Laboratory Courses CourseSmart: ebook resources http://instructors.coursesmart.com/ Microbiology Laboratory Courses
Welcome to Implementing Inquirybased Microbial Project Veronica Ardi, PhD Microbiology Laboratory Courses CourseSmart: ebook resources http://instructors.coursesmart.com/ Microbiology Laboratory Courses
Human Herpes Virus 1 (Herpes simplex type 1) genesig Standard Kit. DNA testing. Everything... Everyone... Everywhere...
 TM Primerdesign Ltd TM Primerdesign Ltd Human Herpes Virus 1 (Herpes simplex type 1) Capsid assembly and DNA maturation gene genesig Standard Kit 150 tests DNA testing Everything... Everyone... Everywhere...
TM Primerdesign Ltd TM Primerdesign Ltd Human Herpes Virus 1 (Herpes simplex type 1) Capsid assembly and DNA maturation gene genesig Standard Kit 150 tests DNA testing Everything... Everyone... Everywhere...
User Manual. CelluLyser Lysis and cdna Synthesis Kit. Version 1.4 Oct 2012 From cells to cdna in one tube
 User Manual CelluLyser Lysis and cdna Synthesis Kit Version 1.4 Oct 2012 From cells to cdna in one tube CelluLyser Lysis and cdna Synthesis Kit Table of contents Introduction 4 Contents 5 Storage 5 Additionally
User Manual CelluLyser Lysis and cdna Synthesis Kit Version 1.4 Oct 2012 From cells to cdna in one tube CelluLyser Lysis and cdna Synthesis Kit Table of contents Introduction 4 Contents 5 Storage 5 Additionally
DNA SPOOLING 1 ISOLATION OF DNA FROM ONION
 DNA SPOOLING 1 ISOLATION OF DNA FROM ONION INTRODUCTION This laboratory protocol will demonstrate several basic steps required for isolation of chromosomal DNA from cells. To extract the chromosomal DNA,
DNA SPOOLING 1 ISOLATION OF DNA FROM ONION INTRODUCTION This laboratory protocol will demonstrate several basic steps required for isolation of chromosomal DNA from cells. To extract the chromosomal DNA,
Clinical description 2 Laboratory test for diagnosis 3. Incubation period 4 Mode of transmission 4 Period of communicability 4
 Tuberculosis Contents Epidemiology in New Zealand 2 Case definition 2 Clinical description 2 Laboratory test for diagnosis 3 Case classification 3 Spread of infection 4 Incubation period 4 Mode of transmission
Tuberculosis Contents Epidemiology in New Zealand 2 Case definition 2 Clinical description 2 Laboratory test for diagnosis 3 Case classification 3 Spread of infection 4 Incubation period 4 Mode of transmission
ncounter Gene Expression Assay Manual Total RNA and Cell Lysate Protocols
 ncounter Gene Expression Assay Manual Total RNA and Cell Lysate Protocols v.20090807 For research use only. Not for use in diagnostic procedures. Limited License Subject to the terms and conditions of
ncounter Gene Expression Assay Manual Total RNA and Cell Lysate Protocols v.20090807 For research use only. Not for use in diagnostic procedures. Limited License Subject to the terms and conditions of
