* Author to whom correspondence should be addressed; Tel.: ; Fax:
|
|
|
- Calvin Tate
- 3 years ago
- Views:
From this document you will learn the answers to the following questions:
What matrix is used to store the data from newborn testing?
What does NSQAP help NBS labs do?
What organization is responsible for the NSQAP?
Transcription
1 Int. J. Neonatal Screen. 2015, 1, 13-26; doi: /ijns Review International Journal of Neonatal Screening ISSN X The Newborn Screening Quality Assurance Program at the Centers for Disease Control and Prevention: Thirty-Five Year Experience Assuring Newborn Screening Laboratory Quality Víctor R. De Jesús *, Joanne V. Mei, Suzanne K. Cordovado and Carla D. Cuthbert Newborn Screening and Molecular Biology Branch, Division of Laboratory Sciences, National Center for Environmental Health, US Centers for Disease Control and Prevention, 4770 Buford Highway, NE, Mail Stop F-19, Atlanta, GA 30341, USA; s: (J.V.M.); (S.K.C.); (C.D.C.) These authors contributed equally to this work. OPEN ACCESS * Author to whom correspondence should be addressed; vdejesus@cdc.gov; Tel.: ; Fax: Academic Editor: Ralph Fingerhut Received: 25 February 2015/ Accepted: 27 March 2015 / Published: 17 April 2015 Abstract: Newborn screening is the largest genetic testing effort in the United States and is considered one of the ten great public health achievements during the first 10 years of the 21st century. For over 35 years, the Newborn Screening Quality Assurance Program (NSQAP) at the US Centers for Disease Control and Prevention has helped NBS laboratories ensure that their testing does not delay diagnosis, minimizes false-positive reports, and sustains high-quality testing performance. It is a multi-component program that provides comprehensive quality assurance services for dried blood spot testing. The NSQAP, the Biochemical Mass Spectrometry Laboratory (BMSL), the Molecular Quality Improvement Program (MQIP) and the Newborn Screening Translation Research Initiative (NSTRI), aid screening laboratories achieve technical proficiency and maintain confidence in their performance while processing large volumes of specimens daily. The accuracy of screening tests could be the difference between life and death for many babies; in other instances, identifying newborns with a disorder means that they can be treated and thus avoid life-long disability or severe cognitive impairment. Thousands of newborns and their families have benefited from reliable and accurate testing that has been accomplished by a network of screening laboratories and the NSQAP, BMSL, MQIP and NSTRI.
2 Int. J. Neonatal Screen. 2015, 1 14 Keywords: newborn screening; quality assurance; dried blood spots; Newborn Screening Quality Assurance Program 1. Introduction Newborn screening (NBS) is the largest genetic testing effort in the United States and is considered one of the ten great public health achievements during the first 10 years of the 21st century [1]. Screening tests are designed to detect asymptomatic newborns at risk for a disease from those who are not at risk. Effective screening of newborns, combined with follow-up diagnostic confirmatory testing and treatment, helps prevent morbidity and mortality. In the United States, public health laboratories or their associated laboratories routinely screen dried blood spot (DBS) specimens for inborn errors of metabolism and other disorders that require medical intervention [2]. The Centers for Disease Control and Prevention s Newborn Screening Quality Assurance Program (NSQAP) helps NBS laboratories ensure that testing accurately detects these disorders, does not delay diagnosis, minimizes false-positive reports, and sustains high-quality testing performance [3]. For over 35 years, NSQAP has performed this essential public health service, ensuring the quality and accuracy of screening tests for more than 4 million babies born each year in the United States. All NBS laboratories in the United States must meet strict quality assurance (QA) criteria in order to perform testing on human specimens through the Clinical Laboratory Improvement Amendments (CLIA) [4]. They must participate in proficiency testing (PT) programs designed to evaluate the quality of laboratory performance on a periodic basis using specimens in the dried blood matrix. Proficiency testing examines the analytical performance of individual laboratories for specific assays, and is used to monitor laboratories continuing performance at one point in time. All PT challenges are designed to mimic actual newborn specimens, and thus should be assayed following the individual laboratories standard operating procedures. The NSQAP provides comprehensive QA services that include PT and quality control (QC) materials for DBS testing. Thus, NSQAP enables laboratories to meet the QA requirement for verifying test accuracy and provides technical guidance to participating laboratories to help assure that no screen-positive cases are misclassified during routine screening activities. Participation in NSQAP allows laboratories to gain testing confidence through an external QA program for comparative analysis of peer performance within and among methods [2]. All PT and QC materials simulate, as closely as possible, specimens that are appropriate for a variety of assay systems. These DBS materials are certified for homogeneity, accuracy, stability, and suitability for assays from different commercial sources. Moreover, NSQAP provides training in the preparation of DBS materials and some test methods. The program staff compiles and distributes summary data reports each year for the comparative assessment of methods used by participants. This review presents the NSQAP s expansion timeline (Section 1.1) and current program statistics (Section 1.2). In Sections 2 and 3 we present a detailed description of NSQAP s PT and QC programs, highlighting our practices in support of NBS laboratory quality. Section 4 details our program s expansion since 2009, where two new laboratories were created to enhance programmatic support to
3 Int. J. Neonatal Screen. 2015, 1 15 newborn screening laboratories. In Section 5 we present a preview of our program s future activities in response to current NBS laboratory needs Newborn Screening Quality Assurance Program Timeline The NSQAP began providing services to newborn screening laboratories in 1978, when the first thyroxine and thyroid-stimulating hormone QC materials in DBS were distributed (Figure 1). The first PT surveys for phenylketonuria (PKU) and Congenital Hypothyroidism were conducted in That same year, the NSQAP Filter Paper Evaluation Project was established after the development of a DBS-based quantitative radio-isotopic test to measure and monitor the performance characteristics of filter paper used to collect newborn screening specimens under a standardized protocol. This work resulted in the development of the laboratory standard, Clinical and Laboratory Standards Institute NBS01-A6 Approved Standard, that addresses issues associated with specimen collection, the filter paper collection device, and the transfer of blood onto filter paper [5]. The first distribution of QC materials for phenylalanine occurred in In 1988, NSQAP began distributing HIV-1 QC and PT materials for HIV antibodies in DBS as part of the seroprevalence survey among childbearing women [6]. Galactose QC materials were initially offered in 1988, followed by the first galactosemia PT survey in Figure 1. Newborn Screening Quality Assurance Program Timeline. The first congenital adrenal hyperplasia (CAH) PT survey using 17-α-hydroxyprogesterone was distributed to NSQAP participants in 1990, followed by distribution of CAH QC materials in Additionally, in 1991, proficiency testing for sickle cell disease and other hemoglobinopathies were added to the program. In 1994, NSQAP established the QA program for DNA confirmatory methods using DBS specimens for hemoglobins A, S, C, E and D. After a pilot PT survey, methionine was added in 1995 to both the growing amino acids PT panel and to the amino acid DBS QC materials. Molecular
4 Int. J. Neonatal Screen. 2015, 1 16 methods for detecting the cystic fibrosis (CF) mutation, F508del (p.phe508del) in DBS were established in the NSQAP laboratory in 1997, along with the first biotinidase deficiency PT survey. The introduction of tandem mass spectrometry (MS/MS)-based methods for the detection of phenylalanine in DBS [7] revolutionized the practice of newborn screening for amino acid, fatty acid oxidation, and organic acid metabolic disorders. In 2001, NSQAP launched a pilot PT survey for laboratories testing DBS by MS/MS for the detection of amino acid, fatty acid oxidation, and organic acid disorders. The program began distributing the galactose-1-phosphate uridyltransferase (GALT) PT survey, as well as panels of DBS specimens for immunoreactive trypsinogen (IRT) in In 2004, NSQAP added a PT program for DBS that mimicked CF newborn specimens with elevated IRT levels and the F508del (p.phe508del) mutation. The program s expansion continued in 2005, when a pilot PT survey for Toxoplasma gondii antibodies was launched for laboratories performing Toxoplasmosis screening [8]. In 2006, NSQAP launched the CAH PT pilot survey to support laboratories evaluating a second tier MS/MS-based CAH assay [9], which involved the detection of other steroid markers and the establishment of a clinical algorithm to identify CAH-affected newborns. In 2007, NSQAP expanded its CF PT surveys to include separate DBS panels to test for CF mutations using molecular methods. IRT became an independent PT program and was not included in the CF Mutation Detection surveys. The expanded CF Mutation Detection survey enhanced the NSQAP s expertise in molecular biology-based technology while establishing a solid foundation to assist participating laboratories with their DNA-based screening assays. In 2008, a PT survey for succinylacetone (a specific marker for Tyrosinemia type 1) was launched. In addition, in 2008, QC materials for lysosomal storage disorders (LSD) screening using DBS were offered to NBS laboratories, in collaboration with the Newborn Screening Translation Research Initiative (NSTRI) at CDC [10]. In 2011, NSQAP, in collaboration with NSTRI, began a PT program for T-cell receptor excision circle (TREC) analysis in DBS to detect Severe Combined Immunodeficiency (SCID). NSTRI initially worked with the Wisconsin and Massachusetts newborn screening programs to develop methods and DBS reference materials for the detection of TREC. Materials were prepared from human blood, including cord blood from unaffected individuals and modified adult blood depleted of mononuclear cells or leukocytes. Through the Model Performance Evaluation Survey (MPES) operated by NSTRI, the DBS reference materials were offered to all US and international laboratories that conducted routine NBS for SCID. Over one dozen US and international NBS laboratories evaluated the reference materials in seven collaborative surveys. Using the cumulative experience of the laboratories that tested the DBS TREC reference materials through MPES, NSQAP initiated the TREC PT survey for US laboratories-only with seven participants. To further enhance NSQAP s molecular expertise and capacity, the Molecular Quality Improvement Program (MQIP) was created in 2011, allowing expanded molecular characterization including CFTR gene sequencing and large deletion analysis of the CF Mutation Detection PT materials (Section 4.1). In addition, in 2011, MQIP, in collaboration with the Association of Public Health Laboratories (APHL), launched a laboratory site visit program to assess the molecular components of the newborn screening laboratory. In addition, the Molecular Resources website, a joint effort of MQIP and APHL, was created in 2012, to provide screening specific molecular resources to newborn screening laboratories. The Biochemical Mass Spectrometry Laboratory (BMSL) was created in 2011 (Section 4.2). Its mission is to work with public health laboratories, academic institutions and clinical laboratories to
5 Int. J. Neonatal Screen. 2015, 1 17 develop new MS-based assays to detect and monitor metabolic disorders, and enhance newborn screening laboratory performance through innovative approaches for biochemical marker detection. In 2013 a pilot PT survey for two LSDs, Krabbe and Pompe disease, was started by NSTRI and NSQAP for US domestic laboratories. NSTRI prepared DBS specimens from human blood, including cord blood from unaffected individuals and leuko-depleted adult blood restored with lymphoblast cells derived from patients with either Krabbe or Pompe. The LSD PT program currently has seven participants. The BMSL and NSQAP collaborated with the Genetic Disease Laboratory Branch of the California Department of Public Health to develop DBS QC materials for monitoring the performance of GALT screening tests [11]. The collaboration led to the launch of a GALT QC program in In addition, in 2014 the TREC PT program was expanded to all newborn screening laboratories worldwide and enrollment grew to thirty-eight laboratories NSQAP Biomarker Coverage, Participant Enrollments and Program Statistics The NSQAP offers DBS QA materials for 26 of 31 primary (core) disorders (two disorders are screened by point-of-care technologies), and 24 of 26 secondary disorders in the US Department of Health and Human Services Recommended Uniform Screening Panel (RUSP) [12] (Tables 1 and 2). Additionally, NSQAP offers DBS QA materials for X-linked adrenoleukodystrophy (X-ALD), HIV and Toxoplasma gondii antibodies, and six lysosomal storage disorders. Moreover, DBS QA materials are offered for selected second-tier tests. These include CAH by MS/MS, CF mutation testing by DNA-based technology including 71 CFTR mutations covering all of the 23 CFTR mutations recommended for screening by the American College of Medical Genetics (ACMG), branched-chain amino acids for MSUD by MS/MS, and second-tier propionic acidemia/methylmalonic acidemia. NSQAP serves 612 laboratories in 71 countries (as of December 2014). Annually, NSQAP produces approximately one million DBS to meet the QA and PT needs of its participating newborn screening laboratories worldwide. Enrollment in the NSQAP program is open to laboratories actively screening newborn populations using the DBS matrix. A Request for Participation Form may be downloaded from NSQAP s website ( Table 1. US Recommended Uniform Screening Panel Core Conditions. Core Condition ACMG Code NSQAP QA Materials (Y/N) Propionic acidemia PROP Y Methylmalonic acidemia (methylmalonyl-coa mutase) MUT Y Methylmalonic acidemia (cobalamin disorders) Cbl A,B Y Isovaleric acidemia IVA Y 3-Methylcrotonyl-CoA carboxylase deficiency 3-MCC Y 3-Hydroxy-3-methyglutaric aciduria HMG Y Holocarboxylase synthase deficiency MCD Y β-ketothiolase deficiency βkt Y Glutaric acidemia type I GA1 Y Carnitine uptake defect/carnitine transport defect CUD Y Medium-chain acyl-coa dehydrogenase deficiency MCAD Y Very long-chain acyl-coa dehydrogenase deficiency VLCAD Y
6 Int. J. Neonatal Screen. 2015, 1 18 Table 1. Cont. Core Condition ACMG Code NSQAP QA Materials (Y/N) Long-chain L-3 hydroxyacyl-coa dehydrogenase deficiency LCHAD Y Trifunctional protein deficiency TFP Y Argininosuccinic aciduria ASA Y Citrullinemia type I CIT Y Maple syrup urine disease MSUD Y Homocystinuria HCY Y Classic phenylketonuria PKU Y Tyrosinemia type I TYR I Y Primary congenital hypothyroidism CH Y Congenital adrenal hyperplasia CAH Y Biotinidase deficiency BIOT Y Cystic fibrosis CF Y Classic galactosemia GALT Y Severe combined immunodeficiencies SCID Y S,S Disease (Sickle cell anemia) Hb SS N S, beta-thalassemia Hb S/β Th N S,C Disease Hb S/C N Critical congenital heart disease CCHD N/A Hearing loss HEAR N/A Table 2. US Recommended Uniform Screening Panel Secondary Conditions. Secondary Condition ACMG Code NSQAP QA Materials (Y/N) Methylmalonic acidemia with homocystinuria Cbl C,D Y Malonic acidemia MAL Y Isobutyrylglycinuria IBG Y 2-Methylbutyrylglycinuria 2MBG Y 3-Methylglutaconic aciduria 3MGA Y 2-Methyl-3-hydroxybutyric aciduria 2M3HBA Y Short-chain acyl-coa dehydrogenase deficiency SCAD Y Medium/short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency M/SCHAD Y Glutaric acidemia type II GA2 Y Medium-chain ketoacyl-coa thiolase deficiency MCAT Y 2,4 Dienoyl-CoA reductase deficiency DE RED Y Carnitine palmitoyltransferase type I deficiency CPT IA Y Carnitine palmitoyltransferase type II deficiency CPT II Y Carnitine acylcarnitine translocase deficiency CACT Y Argininemia ARG Y Citrullinemia type II CIT II Y Hypermethioninemia MET Y Benign hyperphenylalaninemia H-PHE Y
7 Int. J. Neonatal Screen. 2015, 1 19 Table 2. Cont. Secondary Condition ACMG Code NSQAP QA Materials (Y/N) Biopterin defect in cofactor biosynthesis BIOPT (BS) Y Biopterin defect in cofactor regeneration BIOPT (REG) Y Tyrosinemia type II TYR II Y Tyrosinemia type III TYR III Y Various other hemoglobinopathies Var Hb Y Galactoepimerase deficiency GALE N Galactokinase deficiency GALK N T-cell related lymphocyte deficiencies - Y 2. NSQAP Proficiency Testing Program The NSQAP provides NBS laboratories with quarterly panels of five blind-coded DBS specimens in all PT programs (Table 3). Data is returned to the program through either a web-based, secured reporting system, or by . Reports of individual laboratory performance and summary data by analyte and method are available within one week of the closure of the internet data-reporting system. For PT challenges, laboratories report the cutoff value for each analyte tested. While there is variability in cutoff values among laboratories, participants are evaluated by their choice of whether a specimen s results are in-range or out-of-range based on their laboratory cutoff. The reported analytical values are also an important component of the overall grading algorithm because specimen assessments are based on the expected analytical values [3]. Table 3. NSQAP Proficiency Testing (PT) Programs as of December PT Program (Number of Participants) Amino Acids (444) Acylcarnitines (315) Hormones (376) Immunoreactive Trypsinogen (211) CF DNA (64) Hemoglobins * (73) UDOT * (65) Galactose-1-phosphate Uridyltransferase (125) Biotinidase Deficiency (199) Second Tier CAH (17) Lysosomal Storage Disorders * (7) SCID (40) Toxoplasmosis gondii (10) HIV (28) * Limited enrollment. Analytes Included in Each Program Arginine, Leucine, Phenylalanine, Tyrosine, Valine, Citrulline, Succinylacetone, Methionine C0(L), C3, C3DC, C4, C4OH, C5, C5:1, C5DC, C5OH, C6, C8, C10, C10:1, C10:2, C14, C14:1, C16, C16OH, C18, C18:1, C18OH Thyroid-Stimulating Hormone, Thyroxine, Total Galactose, 17- α -Hydroxyprogesterone Immunoreactive Trypsinogen 71 Mutations Varied Varied GALT Biotinidase 17-Hydroxyprogesterone, 4-Androstenedione, Cortisol, 11-Deoxycortisol, 21-Deoxycortisol Pompe, Krabbe T-cell Excision Circles (TREC) Anti-Toxoplasma gondii Immunoglobulins M and G Anti-HIV-1 Antibodies
8 Int. J. Neonatal Screen. 2015, 1 20 NSQAP summarizes annual false-positive and false-negative rates for PT challenges for laboratories based on qualitative results (clinical assessments) for analytes or disorders. When false-negative misclassifications are detected, laboratories receive immediate notification from NSQAP supervisory staff so that the source of the error can be investigated and steps can be taken to eliminate the risk of the error occurring again. False-positive results are identified as a tool for use by the laboratory in examining their performance metrics. 3. NSQAP Quality Control Program The NSQAP QC materials allow participants to monitor the long-term stability of their commercial assay kits or in-house methods. They are intended to supplement the participants method- or kit-control materials. The NSQAP provides NBS laboratories with QC DBS specimens two times per year (Table 4). Sets of DBS QC materials consist of three or four levels of single or multiple analytes. The materials are prepared from pooled, adult donor blood that has been hematocrit-adjusted. Each pool is divided into equal portions so that the sets of QC materials are prepared from the same blood matrix. The whole blood is stored at 20 C for two weeks to lyse the red blood cells. Before dispensing, the blood is thoroughly mixed to assure a homogenous preparation. A customized robotic liquid handling system applies 100-µL of whole blood aliquots onto blood collection devices (filter paper). The DBS are dried at ambient temperature overnight and packed the next day by layering each filter paper card between sheets of glassine paper. The QC materials are placed in low-gas permeable bags with several desiccant packets and stored at 20 C until distribution to participating laboratories. The concentration of analyte(s) in each set of QC materials is certified by NSQAP and BMSL and certification pages accompany each shipment of DBS QC materials. Table 4. NSQAP Quality Control (QC) Programs as of December QC Program (Number of Participants) Amino Acids (398) Acylcarnitines (284) Thyroid-Stimulating Hormone (323) Thyroxine (77) 17-Hydroxyprogesterone (211) Immunoreactive Trypsinogen (185) Galactose-1-phosphate Uridyltransferase (88) Lysosomal Storage Disorders (42) HIV-1 (28) X-ALD (4) Analytes Included in Each Program Arginine, Leucine, Phenylalanine, Tyrosine, Valine, Citrulline, Succinylacetone, Methionine, Total Galactose C0, C2, C3, C3DC, C4, C4OH, C5, C5DC, C5OH, C6, C8, C10, C12, C14, C16, C16OH, C18, C18OH Thyroid-Stimulating Hormone Thyroxine 17-Hydroxyprogesterone Immunoreactive Trypsinogen GALT Pompe, Krabbe, Niemann Pick A/B, Fabry, Gaucher, Mucopolysaccharidosis I Anti-HIV-1 Antibodies 24:0 Lysophosphatidylcholine, 26:0 Lysophosphatidylcholine
9 Int. J. Neonatal Screen. 2015, Program Expansions: In 2011, the Biochemical Mass Spectrometry Laboratory (BMSL) and the Molecular Quality Improvement Program (MQIP) were established at CDC to support the increasing needs of participants in the fields of mass spectrometry and molecular biology. The BMSL and MQIP collaborate and support the mission of NSQAP to provide quality assurance to newborn screening laboratories Molecular Quality Improvement Program (MQIP) NBS laboratory methods have traditionally involved biochemical assays that detect the presence or absence of analytes or enzyme activities that indicate a particular disorder or subset of disorders. With the introduction of CF mutation screening and more recently SCID screening, the first primary NBS molecular assay, molecular technology has become part of routine NBS. The MQIP collaborates with NSQAP to provide molecular expertise in support of DNA-based PT programs and QA, and works closely with NBS laboratories to assist with the incorporation of primary and second-tier molecular assays into their routine workflow by providing technical assistance and molecular specific resources. The MQIP laboratories use cutting-edge technology to comprehensively characterize disease causing mutations from DBS materials. In addition, MQIP offers a variety of resources for QA to support laboratories using molecular methods for NBS. The Molecular Assessment Program is an invited personalized site visit to assess the molecular components of the newborn screening laboratory covering all phases of testing including pre-analytical, analytical and post-analytical. The assessment also offers customized approaches for implementing new molecular methods. The MAP team is comprised of scientists from the MQIP, US newborn screening public health programs and a representative from APHL. The NBS Molecular Resources Website is another resource the MQIP has developed in collaboration with APHL [13]. The website contains molecular information tailored to the newborn screening laboratory s needs and includes the MAP site visit portal and checklists, detailed information on molecular assays currently being utilized in newborn screening laboratories in the U.S., a section listing automation resources for molecular screening, and archived materials from past molecular training workshops and webinars. The MQIP also hosts an annual Newborn Screening Molecular Training Course and Laboratory Workshop which consists of both lecture and laboratory segments that directly relate to the detection of newborn disorders using molecular methods Biochemical Mass Spectrometry Laboratory (BMSL) MS/MS has become the standard for the screening of fatty acid, amino acid and organic acid metabolism disorders in newborns worldwide. Largely, laboratories follow the analytical methods described by Chace [7], who introduced the use of MS/MS for the detection of phenylalanine (biomarker for phenylketonuria) in DBS. These methods are used to detect more than fifty diagnostic metabolites, covering over forty metabolic disorders in newborns. Many of these disorders are included in the US Recommended Uniform Screening Panel (RUSP). MS/MS also enables the introduction of second-tier tests to improve disease detection specificity.
10 Int. J. Neonatal Screen. 2015, 1 22 The BMSL, together with the NSQAP and the APHL, provides MS/MS QA services to ensure the quality of MS/MS-based screening activities worldwide. BMSL provides QC and PT DBS materials for galactosemia, amino acids disorders, fatty acid oxidation disorders, organic acid disorders, galactose-1-phosphate uridyltransferase, biotinidase, and second-tier assays for CAH and MSUD. The BMSL consults with public health laboratories to ascertain which markers should be included in NSQAP QA panels. It also houses the filter paper evaluation program, which includes the routine evaluations offered to filter paper manufacturers. The BMSL and the NSQAP expanded their coverage of MS/MS-detected analytes in the offered QC and PT panels to now cover all primary biomarkers for all MS/MS-detectable core and secondary target disorders listed in the US RUSP. Furthermore, MS/MS-based second-tier testing materials have been developed successfully to improve newborn screening assay specificity for selected biomarkers. These activities, in addition to ensuring the quality of filter paper blood collection devices, support newborn screening laboratory efforts to identify newborns with inborn errors of metabolism, and provide a high level of confidence in the laboratory analytical phase Training Courses One of the most important activities conducted by the CDC is hands-on laboratory trainings, and they are an integral part of the program s mission. To date, our programs have developed three courses that offer an in-depth, hands-on training for NBS laboratorians in order to meet this critical need. The BMSL offers an in-depth laboratory workshop on newborn screening using MS/MS technology. The 5-day intensive workshop, titled Newborn Screening by Tandem Mass spectrometry (MS/MS): A Hands-On Course in Understanding Laboratory Issues and Interpreting Test Results., is co-sponsored by APHL, and is conducted at the BMSL s laboratories. The course features four MS/MS hands-on exercises designed to enhance understanding of the didactic portions of the workshop. The exercises cover routine MS/MS newborn screening tests, methods and MS source optimization strategies, as well as second-tier testing using MS/MS methods. To date, over 25 laboratorians have benefited from the MS/MS training course. The MQIP offers a comprehensive hands-on training workshop using molecular technology for newborn screening. This 5-day workshop, titled The Newborn Screening Molecular Training Workshop is co-sponsored by APHL and incorporates lecture, group discussion and laboratory activities. The course offers lectures on newborn disorders that include a molecular component such as galactosemia, hemoglobinopathies, CF, MSUD, SCID and MCAD, as well as how to design a molecular laboratory such as setting up unidirectional workflow, comparing genotyping instrument platforms, and molecular data reporting and clinical interpretation. The laboratory activities include DNA extraction, gel electrophoresis, DNA quantitation, PCR-based mutation detection and genotyping, and liquid handling robotics. Since its inception, 52 participants have attended the Molecular Training Workshop. The NSTRI conducts specialized SCID training for newborn screening programs teaching laboratorians how to produce and analyze DBS reference materials for the TREC assay used to detect SCID. These hands-on workshops are vital for the transfer of technology to states so that they can produce their own reference materials. As of December 2014, over 40 laboratorians have participated in the SCID training workshop.
11 Int. J. Neonatal Screen. 2015, Future Directions The NSQAP is in constant consultation with its participants and other stakeholders to stay abreast of near and future quality assurance needs. Every candidate analyte is evaluated for stability in the DBS matrix prior to its formal introduction into the NSQAP routine offerings. Depending on the analyte, NSQAP develops analytical methods, or uses commercially available kits to characterize new QA DBS materials. NSQAP sends pilot QA DBS materials to domestic and international partner laboratories that test them with their methods to assure that the materials are suitable for use by newborn screening methods worldwide. In 2015, BMSL and NSQAP will launch a QA program for X-linked adrenoleukodystrophy (X-ALD), the most common human peroxisomal disorder. Individuals affected with X-ALD exhibit an accumulation of very long-chain fatty acids (VLCFA) due to their reduced oxidation within peroxisomes. The accumulation of C26:0 VLCFA has been shown to result in elevations of C26:0-lysophosphatidylcholine (C26:0-LPC) in X-ALD patients [14]. Currently, New York State is screening its population for X-ALD using the DBS matrix, and other jurisdictions are expected to initiate X-ALD newborn screening in the next two years. The BMSL and NSQAP will also introduce a new PT program for glucose-6-phosphate dehydrogenase (G6PD) deficiency, the most common human enzyme defect [15]. It affects over 400 million people worldwide, most commonly males due to X-linked heredity. Individuals affected with G6PD deficiency have an increased risk of significant neonatal hyperbilirubinemia due to hemolytic crisis or baseline increase in red blood cell breakdown [16]. It is expected that the G6PD PT program will launch in The BMSL is investigating the addition of the amino acids ornithine and glycine for introduction into the NSQAP amino acids QC program. Ornithine is used by one newborn screening program to screen for argininemia through the use of an arginine/ornithine ratio [17]. Glycine levels can be measured by MS/MS in the DBS sample to screen for non-ketotic hyperglycinemia (NKHG). While NKHG is not one of the disorders in the US RUSP, early diagnosis of NKHG might be particularly significant for atypical forms of the disease where early intervention may prove beneficial. Glycine is also measured by some newborn screening laboratories to assist in the diagnosis of NKHG, even though elevated glycine levels may not detectable by MS/MS newborn screening assays [18]. Both amino acids are currently under evaluation for stability and suitability for routine use in the NSQAP amino acids QC materials. The MQIP is working towards developing a sustainable resource of QA materials to support NSQAP s participants engaged in second-tier mutation detection for specific disorders such as CF and galactosemia. In order to offer molecular QA materials, donor samples that naturally contain the human genome with disease-specific mutations must be collected to test the accuracy of newborn disorder genotyping methods. Thus, QA materials cannot be created without collecting blood from affected donor participants; however, donors are often difficult to find due to the rarity of the mutations. In addition, blood collections only result in a finite number of DBS for QA. Using immortalized cell lines from these rare donors would allow for an infinite supply of a rare resource that can then be used to create DBS that will meet this need. The MQIP in support of the NSQAP is working towards creating DBS made from immortalized cell lines that will work robustly with different DNA extraction methods and a wide variety of genotyping methods and platforms. Once validated, it will be feasible for NSQAP to provide PT and PT and QC materials for screening methods that involve genotyping a panel of mutations.
12 Int. J. Neonatal Screen. 2015, Discussion NSQAP is a proactive, multi-component QA program for a specialized area of public health testing [2]. NSQAP, BMSL, MQIP and NSTRI provide PT testing, QC materials, filter paper evaluations, special consultations, and technical assistance to public health and private laboratories engaged in NBS. The PT data-reporting is handled by an internet web site that permits participants to report their results online for each analyte, receive timely performance evaluation reports, and allows storage and retrieval access for historical data-report summaries. The NSQAP serves as a focal point for NBS laboratories to address a variety of problems that may be encountered with the performance of laboratory-developed tests, and commercial kits/products with their manufacturers. Prompt consultation with the laboratories participating in the NSQAP may reduce the risk of catastrophic consequences of a delayed diagnosis to the newborn. The NSQAP, BMSL, MQIP and NSTRI are designed to help screening laboratories achieve excellent technical proficiency and maintain confidence in their performance while processing large volumes of specimens daily. The programs continually strive to produce certified DBS materials for reference and QC analysis, to improve the quality and scope of services, and to provide immediate consultative and technical assistance. Over 14 million DBS QA materials have been distributed globally during 36 years of operation. Through our interactive efforts with the program s participants, it works to meet their growing and changing NBS needs, always with an eye on future technological advances. The accuracy of screening tests marks the difference between life and death for many babies; in other instances, identifying newborns with a disorder means that they can be treated and thus avoid life-long disability or severe cognitive impairment. In the United States, over 12,000 babies each year are saved from deleterious health outcomes through newborn screening and early identification. Thousands of newborns and their immediate families have benefited from reliable and accurate testing that has been accomplished by a network of screening laboratories and the NSQAP, BMSL, MQIP and NSTRI. Acknowledgments The authors would like to thank the staff of the Newborn Screening and Molecular Biology Branch at the US Centers for Disease Control and Prevention. The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention, or the authors affiliated institutions. Use of trade names is for identification only and does not imply endorsement. Author Contributions V.D.J. designed the overall concept, and contributed the majority of the writing of the manuscript. J.V.M, S.K.C. and C.D.C. contributed writing and editing of the manuscript. Conflicts of Interest The authors declare no conflict of interest.
13 Int. J. Neonatal Screen. 2015, 1 25 References 1. US Centers for Disease Control and Prevention. Ten great public health achievements--united States, MMWR 2011, 60, De Jesus, V.R.; Mei, J.V.; Bell, C.J.; Hannon, W.H. Improving and assuring newborn screening laboratory quality worldwide: 30-year experience at the Centers for Disease Control and Prevention. Semin. Perinatol. 2010, 34, Zobel, S. Newborn Screening Quality Assurance Program 2012 Annual Summary Report. Zobel, S., Ed. US Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013; Volume Clinical Laboratory Improvement Amendments (CLIA). Available online: (accessed on 13 April 2015). 5. Clinical and laboratory Standards Institute (CLSI). NBS01-A6 Blood Collection on Filter Paper for Newborn Screening Programs; Approved Standard - Sixth Edition; CLSI: Wayne, PA, USA, Gwinn, M.; Pappaioanou, M.; George, J.R.; Hannon, W.H.; Wasser, S.C.; Redus, M.A.; Hoff, R.; Grady, G.F.; Willoughby, A.; Novello, A.C.; et al. Prevalence of HIV infection in childbearing women in the United States. Surveillance using newborn blood samples. JAMA 1991, 265, Chace, D.H.; Millington, D.S.; Terada, N.; Kahler, S.G.; Roe, C.R.; Hofman, L.F. Rapid diagnosis of phenylketonuria by quantitative analysis for phenylalanine and tyrosine in neonatal blood spots by tandem mass spectrometry. Clin. Chem. 1993, 39, Mei, J.V.; Li, L.; Rasmussen, S.A.; Collier, S.; Frias, J.L.; Honein, M.A.; Shawe, G.M.; Lorey, F.; Meyerg, R.; Chaing, S.; et al. Effect of specimen storage conditions on newborn dried blood spots used to assess Toxoplasma gondii immunoglobulin M (IgM). Clin. Chim. Acta 2011, 412, Lacey, J.M.; Minutti, C.Z.; Magera, M.J.; Tauscher, A.L.; Casetta, B.; McCann, M.; Lymp, J.; Hahn, S.H.; Rinaldo, P.; Matern, D. Improved specificity of newborn screening for congenital adrenal hyperplasia by second-tier steroid profiling using tandem mass spectrometry. Clin. Chem. 2004, 50, De Jesus, V.R.; Zhang, X.K.; Keutzer, J.; Bodamer, O.A.; Muhl, A.; Orsini, J.J.; Caggana, M.; Vogt, R.F.; Hannon, W.H. Development and evaluation of quality control dried blood spot materials in newborn screening for lysosomal storage disorders. Clin. Chem. 2009, 55, Adam, B.W.; Flores, S.R.; Hou, Y.; Allen, T.W.; de Jesus, V.R. Galactose-1-Phosphate uridyltransferase dried blood spot quality control materials for newborn screening tests. Clin. Biochem. 2015, doi: /j.clinbiochem US Department of Health and Human Services Secretary s Advisory Committee on Heritable Disorders in Newborns and Children. Recommended Uniform Screening Panel. Available online: dex.html (accessed on 22 December 2014). 13. Association of Public Health Laboratories. Molecular Resources. Available online: (accessed on 21 January 2015).
14 Int. J. Neonatal Screen. 2015, Hubbard, W.C.; Moser, A.B.; Liu, A.C.; Jones, R.O.; Steinberg, S.J.; Lorey, F.; Panny, S.R.; Vogt, R.F.; Macaya, D.; Turgeon, C.T.; et al. Newborn screening for X-linked adrenoleukodystrophy (X-ALD): validation of a combined liquid chromatography-tandem mass spectrometric (LC-MS/MS) method. Mol. Gen. Metabol. 2009, 97, Bernardo, J.; Nock, M. Pediatric Provider Insight into Newborn Screening for Glucose-6-Phosphate Dehydrogenase Deficiency. Clin. Peds. 2014, doi: / Beutler, E. Glucose-6-Phosphate dehydrogenase deficiency: A historical perspective. Blood 2008, 111, Jay, A.; Seeterlin, M.; Stanley, E.; Grier, R. Case Report of Argininemia: The Utility of the Arginine/Ornithine Ratio for Newborn Screening (NBS). JIMD Rep. 2013, 9, Tan, E.S.; Wiley, V.; Carpenter, K.; Wilcken, B. Non-Ketotic hyperglycinemia is usually not detectable by tandem mass spectrometry newborn screening. Mol. Gen. Metabol. 2007, 90, by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (
NEWBORN. Quality Assurance Program. Annual Summary Report volume 32 SCREENING 2014
 NEWBORN Annual Summary Report volume 32 SCREENING 2014 Quality Assurance Program National Center for Environmental Health Division of Laboratory Sciences Centers for Disease Control and Prevention National
NEWBORN Annual Summary Report volume 32 SCREENING 2014 Quality Assurance Program National Center for Environmental Health Division of Laboratory Sciences Centers for Disease Control and Prevention National
Newborn Screening in Saskatchewan Information for Health Care Providers
 Newborn Screening in Saskatchewan Information for Health Care Providers 2 Newborn screening: a healthy start leads to a healthier life Since the mid-1960s, health care providers have offered newborn screening
Newborn Screening in Saskatchewan Information for Health Care Providers 2 Newborn screening: a healthy start leads to a healthier life Since the mid-1960s, health care providers have offered newborn screening
Newborn Screening in Manitoba. Information for Health Care Providers
 Newborn Screening in Manitoba Information for Health Care Providers Newborn screening: a healthy start leads to a healthier life Health care professionals have provided newborn screening for phenylketonuria
Newborn Screening in Manitoba Information for Health Care Providers Newborn screening: a healthy start leads to a healthier life Health care professionals have provided newborn screening for phenylketonuria
MICHIGAN NEWBORN SCREENING PROGRAM
 Michigan Department of Community Health MICHIGAN NEWBORN SCREENING PROGRAM Annual Report 2011 Acknowledgements State of Michigan Governor Rick Snyder Michigan Department of Community Health Director James
Michigan Department of Community Health MICHIGAN NEWBORN SCREENING PROGRAM Annual Report 2011 Acknowledgements State of Michigan Governor Rick Snyder Michigan Department of Community Health Director James
COLLECTION OF NEWBORN SCREENING SPECIMENS
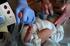 COLLECTION OF NEWBORN SCREENING SPECIMENS Newborn Screening is Mandatory Newborn screening has the potential to save or greatly improve the life of a newborn; therefore, Newborn Screening is mandatory
COLLECTION OF NEWBORN SCREENING SPECIMENS Newborn Screening is Mandatory Newborn screening has the potential to save or greatly improve the life of a newborn; therefore, Newborn Screening is mandatory
Alabama Newborn Screening Program
 Alabama Newborn Screening Program Blood Spot Screening Hearing Screening Pulse Oximetry Screening Delivering You the Facts Alabama Department of Public Health Newborn Screening Program www.adph.org/newbornscreening
Alabama Newborn Screening Program Blood Spot Screening Hearing Screening Pulse Oximetry Screening Delivering You the Facts Alabama Department of Public Health Newborn Screening Program www.adph.org/newbornscreening
State of Board of Health Agenda March 23, 2012 9:00 a.m. Perimeter Center 9960 Mayland Drive Richmond, Virginia 23233
 State of Board of Health Agenda March 23, 2012 9:00 a.m. Perimeter Center 9960 Mayland Drive Richmond, Virginia 23233 Welcome and Introductions Review of Agenda Approval of December 2011 Minutes Commissioner
State of Board of Health Agenda March 23, 2012 9:00 a.m. Perimeter Center 9960 Mayland Drive Richmond, Virginia 23233 Welcome and Introductions Review of Agenda Approval of December 2011 Minutes Commissioner
This program is cosponsored by the Centers for Disease Control and Prevention (CDC) and the Association of Public Health Laboratories (APHL).
 PROFICIENCY TESTING TREC Quarterly Report Volume 4, No. 4 November 2014 INTRODUCTION This report is the Quarterly summary of all data reported within the specified data-reporting period for the Quarter
PROFICIENCY TESTING TREC Quarterly Report Volume 4, No. 4 November 2014 INTRODUCTION This report is the Quarterly summary of all data reported within the specified data-reporting period for the Quarter
Genetics in Family Medicine: The Australian Handbook for General Practitioners. Newborn screening
 Genetics in Family Medicine: The Australian Handbook for General Practitioners GP s role 3 The newborn screening process 3 Storage of newborn screening cards 3 results 5 Where no further testing is required
Genetics in Family Medicine: The Australian Handbook for General Practitioners GP s role 3 The newborn screening process 3 Storage of newborn screening cards 3 results 5 Where no further testing is required
MEDICAL NUTRITION FOR INHERITED METABOLIC DISEASE Corporate Medical Policy
 MEDICAL NUTRITION FOR INHERITED METABOLIC DISEASE Corporate Medical Policy File name: Medical Nutrition for Inherited Metabolic Disease File code: UM.NUT.02 Origination: 10/1998 Last Review: 11/2013 Next
MEDICAL NUTRITION FOR INHERITED METABOLIC DISEASE Corporate Medical Policy File name: Medical Nutrition for Inherited Metabolic Disease File code: UM.NUT.02 Origination: 10/1998 Last Review: 11/2013 Next
Newborn screening policy and guidelines
 Newborn screening policy and guidelines 2011 Newborn screening policy and guidelines 2011 Newborn screening policy and guidelines 2011 Contacts Detailed newborn screening program information including
Newborn screening policy and guidelines 2011 Newborn screening policy and guidelines 2011 Newborn screening policy and guidelines 2011 Contacts Detailed newborn screening program information including
Texas Newborn Screening Performance Measures Project
 Texas Newborn Screening Performance Measures Project Susan Tanksley, PhD MSGRCC Annual Meeting July 14, 2011 The Texas Newborn Screening Performance Measure Project (TNSPMP) is funded through a cooperative
Texas Newborn Screening Performance Measures Project Susan Tanksley, PhD MSGRCC Annual Meeting July 14, 2011 The Texas Newborn Screening Performance Measure Project (TNSPMP) is funded through a cooperative
Inborn Errors of Metabolism
 351 Inborn Errors of Metabolism Definition/ cut-off value Inherited metabolic disorders caused by a defect in the enzymes or their cofactors that metabolize protein, carbohydrate, or fat. Inborn errors
351 Inborn Errors of Metabolism Definition/ cut-off value Inherited metabolic disorders caused by a defect in the enzymes or their cofactors that metabolize protein, carbohydrate, or fat. Inborn errors
Fatty Acid Oxidation Disorders Galactosemia Biotinidase Deficiency
 Fatty Acid Oxidation Disorders Galactosemia Biotinidase Deficiency Dr. Kathy Grange, MD Division of Genetics and Genomic Medicine Department of Pediatrics Washington University School of Medicine What
Fatty Acid Oxidation Disorders Galactosemia Biotinidase Deficiency Dr. Kathy Grange, MD Division of Genetics and Genomic Medicine Department of Pediatrics Washington University School of Medicine What
Newborn Screening for Pompe Disease and other Lysosomal Storage Disorders:
 Newborn Screening for Pompe Disease and other Lysosomal Storage Disorders: CDC Reference Methods and Materials Hui Zhou, MD, PhD Newborn Screening Translational Research Initiative (NSTRI) Newborn Screening
Newborn Screening for Pompe Disease and other Lysosomal Storage Disorders: CDC Reference Methods and Materials Hui Zhou, MD, PhD Newborn Screening Translational Research Initiative (NSTRI) Newborn Screening
Your newborn baby s blood test
 Newborn Screening Free health checks for your baby Your newborn baby s blood test The Newborn Metabolic Screening Programme All babies are checked at birth to see that all is well. Some of your baby s
Newborn Screening Free health checks for your baby Your newborn baby s blood test The Newborn Metabolic Screening Programme All babies are checked at birth to see that all is well. Some of your baby s
Newborn Screening Test
 Important Information for Parents about the Newborn Screening Test Newborn Screening Branch Genetic Disease Screening Program http://cdph.ca.gov/nbs California Department of Public Health Publication Date:
Important Information for Parents about the Newborn Screening Test Newborn Screening Branch Genetic Disease Screening Program http://cdph.ca.gov/nbs California Department of Public Health Publication Date:
Newborn Screening In America:
 Newborn Screening In America: Problems and Policies Vani Kilakkathi 2012 Council for Responsible Genetics 5 Upland Road, Suite 3 Cambridge, MA 02140 Email: crg@gene-watch.org www.councilforresponsiblegenetics.org
Newborn Screening In America: Problems and Policies Vani Kilakkathi 2012 Council for Responsible Genetics 5 Upland Road, Suite 3 Cambridge, MA 02140 Email: crg@gene-watch.org www.councilforresponsiblegenetics.org
Practitioner s Manual
 Oregon Practitioner s Manual The Oregon Practitioner s Manual Oregon Health & Science University Cary Harding, MD Stephen L. LaFranchi, MD Gregory Thomas, MD Michael Wall, MD Oregon Health Authority Public
Oregon Practitioner s Manual The Oregon Practitioner s Manual Oregon Health & Science University Cary Harding, MD Stephen L. LaFranchi, MD Gregory Thomas, MD Michael Wall, MD Oregon Health Authority Public
Expanded newborn screening and confirmatory follow-up testing for inborn errors of metabolism detected by tandem mass spectrometry
 DOI 10.1515/cclm-2012-0472 Clin Chem Lab Med 2013; 51(1): 157 176 Review Tomris Ozben * Expanded newborn screening and confirmatory follow-up testing for inborn errors of metabolism detected by tandem
DOI 10.1515/cclm-2012-0472 Clin Chem Lab Med 2013; 51(1): 157 176 Review Tomris Ozben * Expanded newborn screening and confirmatory follow-up testing for inborn errors of metabolism detected by tandem
Organisation of Biochemical Genetic Testing in Estonia
 Organisation of Biochemical Genetic Testing in Estonia Katrin Õunap Department of Pediatrics, University of Tartu Department of Genetics, Tartu University Hospital Basel, 09 th May 2008 ESTONIA 1,35 million
Organisation of Biochemical Genetic Testing in Estonia Katrin Õunap Department of Pediatrics, University of Tartu Department of Genetics, Tartu University Hospital Basel, 09 th May 2008 ESTONIA 1,35 million
STEP 2 IMPLEMENTATION: HIE TOOLS USER GUIDE HIE TOOLS USER GUIDE
 STEP 2 IMPLEMENTATION: HIE TOOLS USER GUIDE HIE TOOLS USER GUIDE LIST OF REVISIONS REVISION NO. DATE REVISED BY: PAGE/S DESCRIPTION 1 TBD GDSP 13 Initial Version II STEP 2 IMPLEMENTATION: HIE TOOLS USER
STEP 2 IMPLEMENTATION: HIE TOOLS USER GUIDE HIE TOOLS USER GUIDE LIST OF REVISIONS REVISION NO. DATE REVISED BY: PAGE/S DESCRIPTION 1 TBD GDSP 13 Initial Version II STEP 2 IMPLEMENTATION: HIE TOOLS USER
Four Years of Expanded Newborn Screening in. Portugal with MS/MS. National Institute of Health Portugal www.insa.pt 1
 Four Years of Expanded Newborn Screening in Portugal with MS/MS National Institute of Health Portugal www.insa.pt 1 Laura Vilarinho PhD, Hugo Rocha MSc, Carmen Sousa MSc, Ana Marcão PhD, Helena Fonseca
Four Years of Expanded Newborn Screening in Portugal with MS/MS National Institute of Health Portugal www.insa.pt 1 Laura Vilarinho PhD, Hugo Rocha MSc, Carmen Sousa MSc, Ana Marcão PhD, Helena Fonseca
Newborn Screening Update for Health Care Practitioners
 Newborn Screening Update for Health Care Practitioners Changes in regulations affecting your practice for newborns transferred to neonatal intensive care units. Changes for newborn screening of premature,
Newborn Screening Update for Health Care Practitioners Changes in regulations affecting your practice for newborns transferred to neonatal intensive care units. Changes for newborn screening of premature,
Molecular Genetic Testing in Public Health and Clinical Settings
 Molecular Genetic Testing in Public Health and Clinical Settings Ira M. Lubin, PhD, FACMG Division of Laboratory Systems NCPDCID, CCID Centers for Disease Control and Prevention Atlanta, Georgia Disclaimers
Molecular Genetic Testing in Public Health and Clinical Settings Ira M. Lubin, PhD, FACMG Division of Laboratory Systems NCPDCID, CCID Centers for Disease Control and Prevention Atlanta, Georgia Disclaimers
Inborn Errors of Metabolism
 Intensive Care Nursery House Staff Manual Inborn Errors of Metabolism INTRODUCTION and PATHOPHYSIOLOGY: Inborn Errors of Metabolism (IEM) comprise a group of disorders in which a single gene defect causes
Intensive Care Nursery House Staff Manual Inborn Errors of Metabolism INTRODUCTION and PATHOPHYSIOLOGY: Inborn Errors of Metabolism (IEM) comprise a group of disorders in which a single gene defect causes
On Time / Every Time. A Partnership of Safety and Reliability for Newborn Screening
 On Time / Every Time A Partnership of Safety and Reliability for Newborn Screening Welcome As a result of this program, the participant will be able to: Discuss the importance of obtaining a quality specimen
On Time / Every Time A Partnership of Safety and Reliability for Newborn Screening Welcome As a result of this program, the participant will be able to: Discuss the importance of obtaining a quality specimen
Newborn screening: the genomic challenge
 Newborn screening: the genomic challenge The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published Version Accessed
Newborn screening: the genomic challenge The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published Version Accessed
Senate Bill No. 1555 CHAPTER 484
 Senate Bill No. 1555 CHAPTER 484 An act to amend Sections 124977 and 125055 of, to add Sections 1604.6 and 125002 to, and to add Article 4 (commencing with Section 123370) to Chapter 1 of Part 2 of Division
Senate Bill No. 1555 CHAPTER 484 An act to amend Sections 124977 and 125055 of, to add Sections 1604.6 and 125002 to, and to add Article 4 (commencing with Section 123370) to Chapter 1 of Part 2 of Division
A fine inheritance, but it s not all in the bag
 A fine inheritance, but it s not all in the bag Dr Guy Besley, formerly Willink Biochemical Genetics Unit, Manchester Children s Hospital, Manchester M27 4HA, UK. The Willink Unit, Manchester - a fine
A fine inheritance, but it s not all in the bag Dr Guy Besley, formerly Willink Biochemical Genetics Unit, Manchester Children s Hospital, Manchester M27 4HA, UK. The Willink Unit, Manchester - a fine
Gene mutation and molecular medicine Chapter 15
 Gene mutation and molecular medicine Chapter 15 Lecture Objectives What Are Mutations? How Are DNA Molecules and Mutations Analyzed? How Do Defective Proteins Lead to Diseases? What DNA Changes Lead to
Gene mutation and molecular medicine Chapter 15 Lecture Objectives What Are Mutations? How Are DNA Molecules and Mutations Analyzed? How Do Defective Proteins Lead to Diseases? What DNA Changes Lead to
National Survey of Pre- and Post- Analytical Performance Measures Used in Newborn Screening Programs
 National Survey of Pre- and Post- Analytical Performance Measures Used in Newborn Screening Programs Simran Tiwana, MBA* Susan Tanksley, PhD* Brad Therrell, PhD Donna Williams, BS* Mirsa Douglass, MBA*
National Survey of Pre- and Post- Analytical Performance Measures Used in Newborn Screening Programs Simran Tiwana, MBA* Susan Tanksley, PhD* Brad Therrell, PhD Donna Williams, BS* Mirsa Douglass, MBA*
Genetic Testing in Research & Healthcare
 We Innovate Healthcare Genetic Testing in Research & Healthcare We Innovate Healthcare Genetic Testing in Research and Healthcare Human genetic testing is a growing science. It is used to study genes
We Innovate Healthcare Genetic Testing in Research & Healthcare We Innovate Healthcare Genetic Testing in Research and Healthcare Human genetic testing is a growing science. It is used to study genes
Suggested Reporting Language for the HIV Laboratory Diagnostic Testing Algorithm
 Suggested Reporting Language for the HIV Laboratory Diagnostic Testing Algorithm November 2013 Introduction In March 2010, the Centers for Disease Control and Prevention (CDC) and the Association of Public
Suggested Reporting Language for the HIV Laboratory Diagnostic Testing Algorithm November 2013 Introduction In March 2010, the Centers for Disease Control and Prevention (CDC) and the Association of Public
Newborn Screening Issues
 Newborn Screening Issues - The Public Health Aspect - Dr. Helmut Brand Msc, MFPHM Institute of Public Health NRW, Bielefeld Public Health Genetics is like Mr Tur Tur, the phantom giant: the further you
Newborn Screening Issues - The Public Health Aspect - Dr. Helmut Brand Msc, MFPHM Institute of Public Health NRW, Bielefeld Public Health Genetics is like Mr Tur Tur, the phantom giant: the further you
Newborn Screening Program Study On Comprehensive and Sustainable Long-Term Storage and Use
 Public Health Laboratory, Newborn Screening Program 601 Robert Street North St. Paul, MN 55155-2531 651-201-5466 www.health.state.mn.us/newbornscreening Newborn Screening Program Study On Comprehensive
Public Health Laboratory, Newborn Screening Program 601 Robert Street North St. Paul, MN 55155-2531 651-201-5466 www.health.state.mn.us/newbornscreening Newborn Screening Program Study On Comprehensive
A LEAN SIX SIGMA APPROACH TO CONTINUOUS QUALITY IMPROVEMENT IN THE TEXAS NEWBORN SCREENING PROGRAM
 A LEAN SIX SIGMA APPROACH TO CONTINUOUS QUALITY IMPROVEMENT IN THE TEXAS NEWBORN SCREENING PROGRAM PATRICIA HUNT V. Telles, T. Odoms, P. Trevino Gonzales, A. Vinyard, L. Cao, S. Tupy, B. Reilly, P. Hunt,
A LEAN SIX SIGMA APPROACH TO CONTINUOUS QUALITY IMPROVEMENT IN THE TEXAS NEWBORN SCREENING PROGRAM PATRICIA HUNT V. Telles, T. Odoms, P. Trevino Gonzales, A. Vinyard, L. Cao, S. Tupy, B. Reilly, P. Hunt,
A newsletter of the Newborn Screening Program and the Newborn Screening Laboratory
 NEWBORN SCREENING August 2001 Be Kind To Tiny Feet A newsletter of the Newborn Screening Program and the Newborn Screening Laboratory HEMOGLOBINOPATHIES Second Edition Follow-up procedures for hemoglobinopathy
NEWBORN SCREENING August 2001 Be Kind To Tiny Feet A newsletter of the Newborn Screening Program and the Newborn Screening Laboratory HEMOGLOBINOPATHIES Second Edition Follow-up procedures for hemoglobinopathy
Newborn Screening Steering Committee. Newborn Screening Expert Group
 NEWBORN SCREENING: TOWARD A UNIFORM SCREENING PANEL AND SYSTEM 1 Jose Cordero, MD, MPH Centers for Disease Control and Prevention Health Resources and Services Administration Newborn Screening Steering
NEWBORN SCREENING: TOWARD A UNIFORM SCREENING PANEL AND SYSTEM 1 Jose Cordero, MD, MPH Centers for Disease Control and Prevention Health Resources and Services Administration Newborn Screening Steering
Newborn Blood Spot Standards for newborn blood spot screening
 Newborn Blood Spot Standards for newborn blood spot screening Version 1.0 / August 2013 About the NHS Newborn Blood Spot Screening Programme The NHS Newborn Blood Spot Screening Programme has responsibility
Newborn Blood Spot Standards for newborn blood spot screening Version 1.0 / August 2013 About the NHS Newborn Blood Spot Screening Programme The NHS Newborn Blood Spot Screening Programme has responsibility
UPLC/MSMS in the analysis of physiological steroids. May 2012. Anders Feldthus. Anders_Feldthus@Waters.com. 2012 Waters Corporation 1
 UPLC/MSMS in the analysis of physiological steroids Anders Feldthus Anders_Feldthus@Waters.com May 2012 2012 Waters Corporation 1 Clinical Chemistry Mass Spectrometry Citations (1955-2006) 70 Number of
UPLC/MSMS in the analysis of physiological steroids Anders Feldthus Anders_Feldthus@Waters.com May 2012 2012 Waters Corporation 1 Clinical Chemistry Mass Spectrometry Citations (1955-2006) 70 Number of
STATE STATUTES AND REGULATIONS ON DIETARY TREATMENT OF DISORDERS IDENTIFIED THROUGH NEWBORN SCREENING JURISDICTION AND CITATION
 Alabama AC 22 20 3 AAC 40 20 10.01 et seq. PKU, CH, GALT, CAH, hearing loss, hemoglobinopathy, BD, CF, AAD, FAOD, OAD, and other heritable diseases Statutes authorize board of health to create regulations
Alabama AC 22 20 3 AAC 40 20 10.01 et seq. PKU, CH, GALT, CAH, hearing loss, hemoglobinopathy, BD, CF, AAD, FAOD, OAD, and other heritable diseases Statutes authorize board of health to create regulations
Hb A distribution in cord blood
 2 ND European Hemoglobinopathy Forum: Insights on the Diagnosis of Hemoglobin disorders November 29th, 2011 Madrid Hb A distribution in cord blood (normal vs β + or β o thalassemia carriers) Giovanni Ivaldi
2 ND European Hemoglobinopathy Forum: Insights on the Diagnosis of Hemoglobin disorders November 29th, 2011 Madrid Hb A distribution in cord blood (normal vs β + or β o thalassemia carriers) Giovanni Ivaldi
Tennessee Newborn Screening Program
 Tennessee Newborn Screening Program Guide for Practitioners State of Tennessee Department of Health Family Health and Wellness 710 James Robertson Parkway Nashville, TN 37243 Phone 615-532-8462 1-855-202-1357
Tennessee Newborn Screening Program Guide for Practitioners State of Tennessee Department of Health Family Health and Wellness 710 James Robertson Parkway Nashville, TN 37243 Phone 615-532-8462 1-855-202-1357
One Hundred Tenth Congress of the United States of America
 S. 1858 One Hundred Tenth Congress of the United States of America AT THE SECOND SESSION Begun and held at the City of Washington on Thursday, the third day of January, two thousand and eight An Act To
S. 1858 One Hundred Tenth Congress of the United States of America AT THE SECOND SESSION Begun and held at the City of Washington on Thursday, the third day of January, two thousand and eight An Act To
Georgia Newborn Screening Manual
 Georgia Newborn Screening Manual for Metabolic Diseases & Hemoglobinopathies A Practitioner s Guide Georgia Department of Human Resources Division of Public Health Family Health Branch GEORGIA NEWBORN
Georgia Newborn Screening Manual for Metabolic Diseases & Hemoglobinopathies A Practitioner s Guide Georgia Department of Human Resources Division of Public Health Family Health Branch GEORGIA NEWBORN
Newborn Screening and Health Information Technology
 Newborn Screening and Health Information Technology Alan E Zuckerman MD FAAP Georgetown University Medical Center SACHDNC HIT Workgroup Co-Chair AAP Council on Clinical Information Technology (COCIT) Executive
Newborn Screening and Health Information Technology Alan E Zuckerman MD FAAP Georgetown University Medical Center SACHDNC HIT Workgroup Co-Chair AAP Council on Clinical Information Technology (COCIT) Executive
Organic Acid Disorders
 Genetic Fact Sheets for Parents Organic Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Genetic Fact Sheets for Parents Organic Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Sickle cell anemia: Altered beta chain Single AA change (#6 Glu to Val) Consequence: Protein polymerizes Change in RBC shape ---> phenotypes
 Protein Structure Polypeptide: Protein: Therefore: Example: Single chain of amino acids 1 or more polypeptide chains All polypeptides are proteins Some proteins contain >1 polypeptide Hemoglobin (O 2 binding
Protein Structure Polypeptide: Protein: Therefore: Example: Single chain of amino acids 1 or more polypeptide chains All polypeptides are proteins Some proteins contain >1 polypeptide Hemoglobin (O 2 binding
New Estimates of the Economic Benefits of Newborn Screening for Congenital Hypothyroidism in the US
 The findings and conclusions in this presentation have not been formally disseminated by the Centers for Disease Control and Prevention and should not be construed to represent any agency determination
The findings and conclusions in this presentation have not been formally disseminated by the Centers for Disease Control and Prevention and should not be construed to represent any agency determination
Best Practices for Maintaining Quality in Molecular Diagnostics Gyorgy Abel, MD, PhD
 Best Practices for Maintaining Quality in Molecular Diagnostics Gyorgy Abel, MD, PhD Director, Clinical Chemistry Molecular Diagnostics / Immunology Department of Laboratory Medicine Lahey Clinic Medical
Best Practices for Maintaining Quality in Molecular Diagnostics Gyorgy Abel, MD, PhD Director, Clinical Chemistry Molecular Diagnostics / Immunology Department of Laboratory Medicine Lahey Clinic Medical
Coding and Billing for HIV Services in Healthcare Facilities
 P a g e 1 Coding and Billing for HIV Services in Healthcare Facilities The Hawai i State Department of Health STD/AIDS Prevention Branch is pleased to provide you information on billing and reimbursement
P a g e 1 Coding and Billing for HIV Services in Healthcare Facilities The Hawai i State Department of Health STD/AIDS Prevention Branch is pleased to provide you information on billing and reimbursement
HEALTH RESEARCH, INC. New York State Department of Health Wadsworth Center/Division of Genetics
 HEALTH RESEARCH, INC. New York State Department of Health Wadsworth Center/Division of Genetics New York-Mid-Atlantic Consortium for Genetic and Newborn Screening Services Program Evaluation Consultant
HEALTH RESEARCH, INC. New York State Department of Health Wadsworth Center/Division of Genetics New York-Mid-Atlantic Consortium for Genetic and Newborn Screening Services Program Evaluation Consultant
SICKLE CELL ANEMIA & THE HEMOGLOBIN GENE TEACHER S GUIDE
 AP Biology Date SICKLE CELL ANEMIA & THE HEMOGLOBIN GENE TEACHER S GUIDE LEARNING OBJECTIVES Students will gain an appreciation of the physical effects of sickle cell anemia, its prevalence in the population,
AP Biology Date SICKLE CELL ANEMIA & THE HEMOGLOBIN GENE TEACHER S GUIDE LEARNING OBJECTIVES Students will gain an appreciation of the physical effects of sickle cell anemia, its prevalence in the population,
Viral Hepatitis. 2009 APHL survey report
 Issues in Brief: viral hepatitis testing Association of Public Health Laboratories May Viral Hepatitis Testing 9 APHL survey report In order to characterize the role that the nation s public health laboratories
Issues in Brief: viral hepatitis testing Association of Public Health Laboratories May Viral Hepatitis Testing 9 APHL survey report In order to characterize the role that the nation s public health laboratories
Exhibit A Scope of Work. Vendor agrees to provide to the California Department of Public Health (CDPH) the services described herein.
 1. Service Overview Vendor agrees to provide to the (CDPH) the services described herein. The Vendor shall provide diagnosis, treatment, and outcome data for metabolic patients identified through the Newborn
1. Service Overview Vendor agrees to provide to the (CDPH) the services described herein. The Vendor shall provide diagnosis, treatment, and outcome data for metabolic patients identified through the Newborn
Newborn Screening in New York State. A Guide for Health Professionals
 New York State Department of Health Newborn Screening in New York State A Guide for Health Professionals Newborn Screening Program Wadsworth Center New York State Department of Health Empire State Plaza
New York State Department of Health Newborn Screening in New York State A Guide for Health Professionals Newborn Screening Program Wadsworth Center New York State Department of Health Empire State Plaza
Newborn Screening for Severe Combined Immunodeficiency Disorder Secretary s Advisory Committee on Heritable Disorders in Newborns and Children
 Newborn Screening for Severe Combined Immunodeficiency Disorder Secretary s Advisory Committee on Heritable Disorders in Newborns and Children REPORT Executive Summary In January 2010, the Secretary s
Newborn Screening for Severe Combined Immunodeficiency Disorder Secretary s Advisory Committee on Heritable Disorders in Newborns and Children REPORT Executive Summary In January 2010, the Secretary s
Medical Record - Infant Screening
 A Second-Tier Screening Approach for CAH: Oklahoma s Experience Kathy Kirk, RN, MSN Theresa Steckel, BSN, RN Pam King, MPA, RN Debbie Kline, BS Edd Rhoades, MD, MPH Kenneth Copeland, MD U.S. Newborn Screening
A Second-Tier Screening Approach for CAH: Oklahoma s Experience Kathy Kirk, RN, MSN Theresa Steckel, BSN, RN Pam King, MPA, RN Debbie Kline, BS Edd Rhoades, MD, MPH Kenneth Copeland, MD U.S. Newborn Screening
NORD Guides for Physicians #1. Physician s Guide to. Tyrosinemia. Type 1
 NORD Guides for Physicians #1 The National Organization for Rare Disorders Physician s Guide to Tyrosinemia Type 1 The original version of this booklet was made possible by donations in honor of Danielle
NORD Guides for Physicians #1 The National Organization for Rare Disorders Physician s Guide to Tyrosinemia Type 1 The original version of this booklet was made possible by donations in honor of Danielle
Blood Testing Protocols. Disclaimer
 Blood Testing Protocols / Page 2 Blood Testing Protocols Here are the specific test protocols recommend by Dr. J.E. Williams. You may request these from your doctor or visit www.readyourbloodtest.com to
Blood Testing Protocols / Page 2 Blood Testing Protocols Here are the specific test protocols recommend by Dr. J.E. Williams. You may request these from your doctor or visit www.readyourbloodtest.com to
in hiv diagnostics the role of phls
 Issues in Brief: HIV Diagnostics UPDATE Association of Public Health Laboratories August 2011 Conference calls Focus on New trends in hiv diagnostics the role of phls In February 2011, the Association
Issues in Brief: HIV Diagnostics UPDATE Association of Public Health Laboratories August 2011 Conference calls Focus on New trends in hiv diagnostics the role of phls In February 2011, the Association
Algorithm for detecting Zika virus (ZIKV) 1
 Algorithm for detecting Zika virus (ZIKV) 1 This algorithm is addressed to laboratories with established capacity (molecular, antigenic and/or serological) to detect dengue (DENV), Zika (ZIKV) 2, and chikungunya
Algorithm for detecting Zika virus (ZIKV) 1 This algorithm is addressed to laboratories with established capacity (molecular, antigenic and/or serological) to detect dengue (DENV), Zika (ZIKV) 2, and chikungunya
Volume 22 Number 22. C43-A ISBN 1-56238-475-9 ISSN 0273-3099 Gas Chromatography/Mass Spectrometry (GC/MS) Confirmation of Drugs; Approved Guideline
 ISBN 1-56238-475-9 ISSN 0273-3099 Gas Chromatography/Mass Spectrometry (GC/MS) Confirmation of Drugs; Approved Guideline Volume 22 Number 22 Larry D. Bowers, Ph.D., DABCC, Chairholder David A. Armbruster,
ISBN 1-56238-475-9 ISSN 0273-3099 Gas Chromatography/Mass Spectrometry (GC/MS) Confirmation of Drugs; Approved Guideline Volume 22 Number 22 Larry D. Bowers, Ph.D., DABCC, Chairholder David A. Armbruster,
The Contribution of large Healthcare Systems to Improving Treatment for Patients with Rare Diseases
 Uniting Rare Diseases Advancing Rare Disease Research: The Intersection of Patient Registries, Biospecimen Repositories and Clinical Data Keynote address: The Contribution of large Healthcare Systems to
Uniting Rare Diseases Advancing Rare Disease Research: The Intersection of Patient Registries, Biospecimen Repositories and Clinical Data Keynote address: The Contribution of large Healthcare Systems to
Michigan Department of Health and Human Services
 Michigan Department of Health and Human Services Newborn Screening Guide for Hospitals January 2016 Michigan Department of Health and Human Services Newborn Screening 201 Townsend Street PO Box 30195 Lansing,
Michigan Department of Health and Human Services Newborn Screening Guide for Hospitals January 2016 Michigan Department of Health and Human Services Newborn Screening 201 Townsend Street PO Box 30195 Lansing,
State Policy and Finance Framework for Newborn Screening Programs:
 State Policy and Finance Framework for Newborn Screening Programs: Case Studies of Select States Presentation by: Kay Johnson, MPH, EdM President, Johnson Group Consulting Research Assistant Professor,
State Policy and Finance Framework for Newborn Screening Programs: Case Studies of Select States Presentation by: Kay Johnson, MPH, EdM President, Johnson Group Consulting Research Assistant Professor,
Genetic testing. The difference diagnostics can make. The British In Vitro Diagnostics Association
 6 Genetic testing The difference diagnostics can make The British In Vitro Diagnostics Association Genetic INTRODUCTION testing The Department of Health published Our Inheritance, Our Future - Realising
6 Genetic testing The difference diagnostics can make The British In Vitro Diagnostics Association Genetic INTRODUCTION testing The Department of Health published Our Inheritance, Our Future - Realising
CAP Accreditation Checklists 2015 Edition
 CAP Accreditation Checklists 2015 Edition The College of American Pathologists (CAP) accreditation checklists contain the CAP accreditation program requirements, developed on more than 50 years of insight
CAP Accreditation Checklists 2015 Edition The College of American Pathologists (CAP) accreditation checklists contain the CAP accreditation program requirements, developed on more than 50 years of insight
APPLICATION OF GEOGRAPHIC INFORMATION SYSTEM IN TSH NEONATAL SCREENING FOR MONITORING OF IODINE DEFICIENCY AREAS IN THAILAND
 SOUTHEAST ASIAN J TROP MED PUBLIC HEALTH APPLICATION OF GEOGRAPHIC INFORMATION SYSTEM IN TSH NEONATAL SCREENING FOR MONITORING OF IODINE DEFICIENCY AREAS IN THAILAND Wiyada Charoensiriwatana 1, Pongsant
SOUTHEAST ASIAN J TROP MED PUBLIC HEALTH APPLICATION OF GEOGRAPHIC INFORMATION SYSTEM IN TSH NEONATAL SCREENING FOR MONITORING OF IODINE DEFICIENCY AREAS IN THAILAND Wiyada Charoensiriwatana 1, Pongsant
Newborn screening sample collection guidelines
 Newborn screening sample collection guidelines Detailed information about the newborn screening program, including correct sample collection techniques, can be found in the e-learning tool available at:
Newborn screening sample collection guidelines Detailed information about the newborn screening program, including correct sample collection techniques, can be found in the e-learning tool available at:
The National Antimicrobial Resistance Monitoring System (NARMS)
 The National Antimicrobial Resistance Monitoring System (NARMS) Strategic Plan 2012-2016 Table of Contents Background... 2 Mission... 3 Overview of Accomplishments, 1996-2011... 4 Strategic Goals and Objectives...
The National Antimicrobial Resistance Monitoring System (NARMS) Strategic Plan 2012-2016 Table of Contents Background... 2 Mission... 3 Overview of Accomplishments, 1996-2011... 4 Strategic Goals and Objectives...
Guidelines for the Retention, Storage, and Use of Residual Dried Blood Spot Samples after Newborn Screening Analysis:
 BIOCHEMICAL AND MOLECULAR MEDICINE 57, 116 124 (1996) ARTICLE NO. 0017 Guidelines for the Retention, Storage, and Use of Residual Dried Blood Spot Samples after Newborn Screening Analysis: Statement of
BIOCHEMICAL AND MOLECULAR MEDICINE 57, 116 124 (1996) ARTICLE NO. 0017 Guidelines for the Retention, Storage, and Use of Residual Dried Blood Spot Samples after Newborn Screening Analysis: Statement of
Differential Diagnosis of NAFLD- A Short Summary:
 Differential Diagnosis of NAFLD- A Short Summary: Almost a fifth of our general pediatric population is now classified as overweight in the United States. When such children present with elevated liver
Differential Diagnosis of NAFLD- A Short Summary: Almost a fifth of our general pediatric population is now classified as overweight in the United States. When such children present with elevated liver
Birth defects. Report by the Secretariat
 EXECUTIVE BOARD EB126/10 126th Session 3 December 2009 Provisional agenda item 4.7 Birth defects Report by the Secretariat 1. In May 2009 the Executive Board at its 125th session considered an agenda item
EXECUTIVE BOARD EB126/10 126th Session 3 December 2009 Provisional agenda item 4.7 Birth defects Report by the Secretariat 1. In May 2009 the Executive Board at its 125th session considered an agenda item
The Search for Causes and Cures
 ISSUE REPORT BIRTH DEFECTS AND DEVELOPMENTAL DISABILITIES: The Search for Causes and Cures JULY 2005 PREVENTING EPIDEMICS. PROTECTING PEOPLE. REPORT AUTHORS Kim Elliott, MA Deputy Director Trust for America
ISSUE REPORT BIRTH DEFECTS AND DEVELOPMENTAL DISABILITIES: The Search for Causes and Cures JULY 2005 PREVENTING EPIDEMICS. PROTECTING PEOPLE. REPORT AUTHORS Kim Elliott, MA Deputy Director Trust for America
An introduction to metabolic disorders
 12 An introduction to metabolic disorders Individual inborn or congenital errors of metabolism are very rare. However, more than 1400 inherited metabolic diseases have been described. Many of these disorders
12 An introduction to metabolic disorders Individual inborn or congenital errors of metabolism are very rare. However, more than 1400 inherited metabolic diseases have been described. Many of these disorders
Intended Use: The kit is designed to detect the 5 different mutations found in Asian population using seven different primers.
 Unzipping Genes MBPCR014 Beta-Thalassemia Detection Kit P r o d u c t I n f o r m a t i o n Description: Thalassemia is a group of genetic disorders characterized by quantitative defects in globin chain
Unzipping Genes MBPCR014 Beta-Thalassemia Detection Kit P r o d u c t I n f o r m a t i o n Description: Thalassemia is a group of genetic disorders characterized by quantitative defects in globin chain
A New Model for development: USAId MANAgeMeNt & process reform JUNe 2014
 A New Model for Development: USAID Management & Process Reform June 2014 Four years ago, President Obama elevated development alongside diplomacy and defense as a core pillar of America s national security
A New Model for Development: USAID Management & Process Reform June 2014 Four years ago, President Obama elevated development alongside diplomacy and defense as a core pillar of America s national security
Colorado s Integrated Data System: one state s approach to a comprehensive health information exchange for short-term and long-term follow-up
 Colorado s Integrated Data System: one state s approach to a comprehensive health information exchange for short-term and long-term follow-up Erica L. Wright, MS, CGC Certified Genetic Counselor, Instructor
Colorado s Integrated Data System: one state s approach to a comprehensive health information exchange for short-term and long-term follow-up Erica L. Wright, MS, CGC Certified Genetic Counselor, Instructor
PREVENTIVE HEALTHCARE GUIDELINES INTRODUCTION
 PREVENTIVE HEALTHCARE GUIDELINES INTRODUCTION Health Plan of Nevada and Sierra Health and Life suggest that health plan members get certain screening tests, exams and shots to stay healthy. This document
PREVENTIVE HEALTHCARE GUIDELINES INTRODUCTION Health Plan of Nevada and Sierra Health and Life suggest that health plan members get certain screening tests, exams and shots to stay healthy. This document
Size Exclusion Chromatography
 Size Exclusion Chromatography Size Exclusion Chromatography Instructors Stan Hitomi Coordinator Math & Science San Ramon Valley Unified School District Danville, CA Kirk Brown Lead Instructor, Edward Teller
Size Exclusion Chromatography Size Exclusion Chromatography Instructors Stan Hitomi Coordinator Math & Science San Ramon Valley Unified School District Danville, CA Kirk Brown Lead Instructor, Edward Teller
USPSTF Grade A B Recommendations
 USPSTF Grade Recommendations bdominal aortic aneurysm screening: men The USPSTF recommends one-time screening for abdominal aortic aneurysm by ultrasonography in men aged 65 to 75 who have ever smoked.
USPSTF Grade Recommendations bdominal aortic aneurysm screening: men The USPSTF recommends one-time screening for abdominal aortic aneurysm by ultrasonography in men aged 65 to 75 who have ever smoked.
Structures of Proteins. Primary structure - amino acid sequence
 Structures of Proteins Primary structure - amino acid sequence Secondary structure chain of covalently linked amino acids folds into regularly repeating structures. Secondary structure is the result of
Structures of Proteins Primary structure - amino acid sequence Secondary structure chain of covalently linked amino acids folds into regularly repeating structures. Secondary structure is the result of
Course Curriculum for Master Degree in Medical Laboratory Sciences/Clinical Biochemistry
 Course Curriculum for Master Degree in Medical Laboratory Sciences/Clinical Biochemistry The Master Degree in Medical Laboratory Sciences /Clinical Biochemistry, is awarded by the Faculty of Graduate Studies
Course Curriculum for Master Degree in Medical Laboratory Sciences/Clinical Biochemistry The Master Degree in Medical Laboratory Sciences /Clinical Biochemistry, is awarded by the Faculty of Graduate Studies
ENTERAL FORMULAE AND PARENTERAL NUTRITIONAL SOLUTIONS, DME
 ENTERAL FORMULAE AND PARENTERAL NUTRITIONAL SOLUTIONS, DME Policy NHP only reimburses participating DME vendors for the provision of medically necessary enteral and parenteral formulae and nutritional
ENTERAL FORMULAE AND PARENTERAL NUTRITIONAL SOLUTIONS, DME Policy NHP only reimburses participating DME vendors for the provision of medically necessary enteral and parenteral formulae and nutritional
Hepatitis and Retrovirus. LIAISON XL Accurate detection of HIV infection. HIV Ab/Ag FOR OUTSIDE THE US AND CANADA ONLY
 Hepatitis and Retrovirus LIAISON XL Accurate detection of HIV infection HIV Ab/Ag FOR OUTSIDE THE US AND CANADA ONLY LIAISON XL HIV Ab/Ag is Your solution LIAISON XL murex HIV Ab/Ag main features The LIAISON
Hepatitis and Retrovirus LIAISON XL Accurate detection of HIV infection HIV Ab/Ag FOR OUTSIDE THE US AND CANADA ONLY LIAISON XL HIV Ab/Ag is Your solution LIAISON XL murex HIV Ab/Ag main features The LIAISON
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA
 CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
INTERPRETATION INFORMATION SHEET
 Creative Testing Solutions 2424 West Erie Dr. 2205 Highway 121 10100 Martin Luther King Jr. St. No. Tempe, AZ 85282 Bedford, TX 76021 St. Petersburg, FL 33716 INTERPRETATION INFORMATION SHEET Human Immunodeficiency
Creative Testing Solutions 2424 West Erie Dr. 2205 Highway 121 10100 Martin Luther King Jr. St. No. Tempe, AZ 85282 Bedford, TX 76021 St. Petersburg, FL 33716 INTERPRETATION INFORMATION SHEET Human Immunodeficiency
Sutter Health Support Services Shared Laboratory Position Description. Incumbent: Laboratory Date: October 23, 2006 Written By: Michele Leonard
 Sutter Health Support Services Shared Laboratory Position Description Position Title: Clinical Laboratory Scientist, Microbiologist Incumbent: Entity: SHSS Shared Laboratory Reports To: Director of Operations,
Sutter Health Support Services Shared Laboratory Position Description Position Title: Clinical Laboratory Scientist, Microbiologist Incumbent: Entity: SHSS Shared Laboratory Reports To: Director of Operations,
CFTR Related Metabolic Syndrome (CRMS) Definition: Challenges in Application
 CFTR Related Metabolic Syndrome (CRMS) Definition: Challenges in Application Richard B Parad, MD, MPH 1 and Marci Sontag, PhD 2 1 Department of Newborn Medicine, Brigham and Women's Hospital, Harvard Medical
CFTR Related Metabolic Syndrome (CRMS) Definition: Challenges in Application Richard B Parad, MD, MPH 1 and Marci Sontag, PhD 2 1 Department of Newborn Medicine, Brigham and Women's Hospital, Harvard Medical
NEWBORN GENETIC SCREENING: EVOLVING TECHNOLOGIES AND PUBLIC HEALTH POLICY
 NEWBORN GENETIC SCREENING: EVOLVING TECHNOLOGIES AND PUBLIC HEALTH POLICY Patricia C. Kuszler, MD, JD * University of Washington School of Law William H. Gates Hall, Box 353020 Seattle, Washington U.S.A
NEWBORN GENETIC SCREENING: EVOLVING TECHNOLOGIES AND PUBLIC HEALTH POLICY Patricia C. Kuszler, MD, JD * University of Washington School of Law William H. Gates Hall, Box 353020 Seattle, Washington U.S.A
PUBLIC HEALTH IMPROVEMENT PARTNERSHIP
 PUBLIC HEALTH IMPROVEMENT PARTNERSHIP PUBLIC HEALTH ACTIVITIES & SERVICES INVENTORY TECHNICAL NOTES HEALTHY FAMILY DEVELOPMENT Nurse-Family Partnership Nurse-Family Partnership is a voluntary program of
PUBLIC HEALTH IMPROVEMENT PARTNERSHIP PUBLIC HEALTH ACTIVITIES & SERVICES INVENTORY TECHNICAL NOTES HEALTHY FAMILY DEVELOPMENT Nurse-Family Partnership Nurse-Family Partnership is a voluntary program of
Molecular Assessment of Dried Blood Spot Quality during Development of a Novel Automated. Screening
 Molecular Assessment of Dried Blood Spot Quality during Development of a Novel Automated in situ TREC qpcr Assay for SCID Screening J Bai, T Henry, J Benfer, S Berberich, T Kreman, and L DesJardin State
Molecular Assessment of Dried Blood Spot Quality during Development of a Novel Automated in situ TREC qpcr Assay for SCID Screening J Bai, T Henry, J Benfer, S Berberich, T Kreman, and L DesJardin State
General Information. Our Background Automated Phone Services Holiday Coverage Licenses & Accreditations
 General Information Our Background Automated Phone Services Holiday Coverage Licenses & Accreditations Our Background Foundation Laboratory is an advanced, accredited and certified clinical diagnostic
General Information Our Background Automated Phone Services Holiday Coverage Licenses & Accreditations Our Background Foundation Laboratory is an advanced, accredited and certified clinical diagnostic
Health Information Technology Survey for Newborn Screening Programs SUMMARY DATA REPORT
 Health Information Technology Survey for Newborn Screening Programs SUMMARY DATA REPORT NOVEMBER 2014 The development of this report was 100% supported with federal funds from a $975,000 Cooperative Agreement
Health Information Technology Survey for Newborn Screening Programs SUMMARY DATA REPORT NOVEMBER 2014 The development of this report was 100% supported with federal funds from a $975,000 Cooperative Agreement
Dried Blood Spot Specimens for Serological Testing of Rodents
 IDEXX BioResearch l Laboratory Animal Diagnostics Dried Blood Spot Specimens for Serological Testing of Rodents Mathew H. Myles, Earl K. Steffen, Cynthia L. Besch-Williford, Beth A. Bauer, Robert S. Livingston,
IDEXX BioResearch l Laboratory Animal Diagnostics Dried Blood Spot Specimens for Serological Testing of Rodents Mathew H. Myles, Earl K. Steffen, Cynthia L. Besch-Williford, Beth A. Bauer, Robert S. Livingston,
SCID Pilot Study but first
 SCID Pilot Study but first SCID Screening by Immunoassay using the Guthrie Specimen D Janik and K Pass Biggs Lab Intent Develop an immunoassay for SCID that can be used as a first tier screening instrument
SCID Pilot Study but first SCID Screening by Immunoassay using the Guthrie Specimen D Janik and K Pass Biggs Lab Intent Develop an immunoassay for SCID that can be used as a first tier screening instrument
Quality Assurance Guidelines for Testing Using Rapid HIV Antibody Tests Waived Under the Clinical Laboratory Improvement Amendments of 1988
 Quality Assurance Guidelines for Testing Using Rapid HIV Antibody Tests Waived Under the Clinical Laboratory Improvement Amendments of 1988 U.S. Department of Health and Human Services Centers for Disease
Quality Assurance Guidelines for Testing Using Rapid HIV Antibody Tests Waived Under the Clinical Laboratory Improvement Amendments of 1988 U.S. Department of Health and Human Services Centers for Disease
