July 14, Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244
|
|
|
- Annice Hardy
- 8 years ago
- Views:
Transcription
1 ASCP s Statements before the Centers for Medicare and Medicaid Services Regarding Recommendations for Crosswalking/Gapfilling New CPT s for the CLFS for CY 2015 and the Revaluation of the Clinical Laboratory Fee Schedule July 14, 2014 Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244
2 ASCP s Statement before the Centers for Medicare and Medicaid Services Regarding the Revaluation of the Clinical Laboratory Fee Schedule July 14, 2014 Thank you for the opportunity to comment on the revaluation of the Clinical Laboratory Fee Schedule (CLFS), mandated by Sec. 216, Improving Medicare Policies for Clinical Diagnostic Laboratory Tests, of P.L , the Protecting Access to Medicare Act (PAMA) of My name is Lee Hilborne, MD, MPH, FASCP, DLM(ASCP) CM and I am a Professor of Pathology and Laboratory Medicine at the David Geffen School of Medicine at the University of California Los Angeles, Medical Director for Quest Diagnostics, Southern California, Deputy Director for Global Health at the RAND Corporation in Santa Monica, CA, and a former president of the American Society for Clinical Pathology (ASCP). It is in that latter capacity that I am here today on behalf of the ASCP to provide the Centers for Medicare and Medicaid Services (CMS) with our initial recommendations on the re-valuation of the Clinical Laboratory Fee Schedule (CLFS). The ASCP is a 501(c)(3) nonprofit medical specialty society representing more than 100,000 members. Our members are board certified pathologists, other physicians, clinical scientists, certified medical technologists and technicians, and educators. ASCP is one of our nation s largest medical specialty societies and is the world s largest organization representing the field of laboratory medicine and pathology. As the leading provider of continuing education for pathologists and medical laboratory personnel, ASCP enhances the quality of the profession through comprehensive educational programs, publications, and self-assessment materials. Section 216 of PAMA will result in the most significant changes in the way laboratory tests are reimbursed by Medicare since the creation of the Clinical Laboratory Fee Schedule (CLFS) in The reform of the CLFS will incorporate pricing and volume data from private payors and will ultimately identify a new price for each of the approximately 1250 CPT codes on the CLFS. These prices will be based on the median of the data CMS obtains from applicable laboratories. As ASCP represents the entire laboratory testing workforce, we have a significant interest in the reshaping of the CLFS. As you work toward developing the proposed rules, ASCP encourages the agency to implement Sec. 216 of PAMA in a manner that minimizes disruption of laboratory services and operations. This is necessary to maintain the stability of the laboratory market and to ensure patient access to quality testing. Key Points We offer the following comments as an overview of some of the key points that we would urge CMS to keep in mind as it moves forward with its development of the proposed regulations.
3 ASCP Statement before CMS Public Meeting on CLFS Revaluation July 14, 2014 (1) Full Representation of the Entire Market for Laboratory Services One of the outstanding issues created by the new statute relates to who must report data and how that data should be analyzed. ASCP believes that it is imperative to create payment rates that properly reflect the market for laboratory services. For this to occur, the major clinical laboratory sectors must be adequately represented in the data collected. This includes reference or independent laboratories, hospital laboratories, and physician office laboratories. Due to the nature of financial relationships between clinical laboratories and payors, we are especially concerned about the possibility that hospital laboratories will not report sufficient data to aid in a proper revaluation of the CLFS. Robust participation in the data reporting of hospital laboratories is imperative to a proper revaluation of the CLFS. Therefore, ASCP urges CMS to require hospital and physician office laboratories to report private payor data that is not part of a bundled payment. Moreover, given the nature of payment rearrangements, ASCP is further concerned that even if the Agency were to require hospital laboratories to report data, the data collected from these entities will likely underrepresent their presence in the market for laboratory services. This could distort the ability of CMS to set payment rates that properly captures the true median price of laboratory services on then CLFS. Moreover, to ensure patient access to quality testing services at all laboratory sites, ASCP believes that it is imperative for prices to accurately represent rates everywhere in accordance with their respective market share. Therefore, ASCP respectfully encourages CMS to weight the data received in accordance with that laboratory type s respective market share. If hospital laboratories represent 50 percent of the market for testing services, then the data for these facilities should be weighted to 50 percent. This will help adjust the data to ensure that CMS s calculated median pricing accurately reflects the true market median price. CMS could further weight the data by location to ensure that calculated rates are reflective of the true urban-rural dynamics existing in the market for laboratory services. (2) Burden and Penalties ASCP is concerned that the current software capabilities for many clinical laboratories, most likely hospital laboratories, small independent laboratories and physician-owned laboratories may not support the reporting of required data. The cost and time necessary to upgrade or reconfigure laboratory software systems to support the reporting of payment rate and volume data may prove costly and difficult, if not impossible, for some laboratories to provide. Still, we believe the inclusions of data from these providers are critical to calculating an accurate median payment for each laboratory service on the CLFS. While this data are critically important, we further believe that weighting the data by market share may provide the agency with greater flexibility to provide regulatory relief to those laboratories that are most likely to struggle with the reporting requirements. We strongly urge CMS to consider weighting the data to market share. Further, we believe that the potential penalties are unrealistically punitive, i.e., $10,000 per day per infraction. Therefore, we would strongly urge CMS to address in its regulations ways in which it can minimize the imposition of excessive penalties as well as create exemptions or lesser penalties for those applicable laboratories making good-faith efforts to comply with the reporting requirements or who make accidental or inadvertent mistakes in reporting data. Page 2
4 ASCP Statement before CMS Public Meeting on CLFS Revaluation July 14, 2014 (3) Timeframes and Transparency Given the complexity of the data reporting system, ASCP encourages CMS to provide at least six months for laboratories to report data, once the initial collection period begins. This will provide laboratories with the time necessary to verify the adequacy and accuracy of the data collected. ASCP would also encourage CMS to establish an electronic reporting mechanism to collect this data and to establish a pilot phase to test the system s ability to collect the data. Further, to foster transparency, we urge CMS to publish the pilot median rates to allow stakeholders to review and comment on test-specific calculations. (4) Allow for Inclusion of Cost Data Another concern for ASCP is the cost of providing services. We note that payment rates of private payors may not adequately cover costs. Under a revalued CLFS, the newly calculated median prices may not accurately reflect the cost of providing the service. This could result in access problems in cases where price is significantly out-of-line with cost. ASCP urges CMS to establish a process that allows CMS to factor in cost as well as price. Moreover, we urge CMS to create an appeals mechanism to allow stakeholders to seek reconsideration of payment rates that may not adequately cover cost. (5) Adherence to Local Coverage Determination Process ASCP supports provisions in PAMA requiring adherence to Section 1869(f)(2)(B) of the Social Security Act as well as regulations found at 42 CFR 426. ASCP believes it to be critical that Medicare Administrative Contractors (MACs) adhere to the Local Coverage Determination Process (LCDs) requirements, not just for new decisions but also for LCDs up for review. This is needed to address some of the problems that have arisen with transparency and the ability of stakeholders to provide comment. Moreover, to enhance the ability of stakeholders to provide comment and to increase transparency, ASCP urges CMS to utilize the four MACs allowed by statute for the purposes of establishing coverage policies. (6) Utilizing HCPCS s ASCP urges CMS to utilize HCPCS Level 1 CPT codes to the extent possible when implementing new codes. Moreover, ASCP does not support the process utilized by the MolDx program to distinguish between FDA-cleared or approved tests and Laboratory Developed Tests. (7) GAO Study Lastly, we recognize that the following comment pertains mostly to the Government Accountability Office, but we would encourage the agency to work with the GAO to also address in its analysis an assessment of the impact of the new payment rates on hospital laboratories, including an examination of any related outsourcing or discontinuation of laboratory services that may occur in reaction to the changes in the fee schedule. That you for the opportunity today to address some of ASCP s initial concerns regard to the upcoming revaluation of the CLFS. ASCP looks forward to working collaboratively with the agency. Thank you. Page 3
5 ASCP Presumptive Drug Class Screening Drug screen, any number of drug classes from Drug Class List A; any number of non-tlc devices or procedures, (eg, immunoassay) capable of being read by optical observation including instrumented-assisted when performed (eg, dipsticks, cups, cards, cartridges), per date of service G0434 $19.84 This code is intended for presumptive drug screening procedures with one unit of service per date of service for all drugs tested. Methodology is direct optical observation, including instrumentassisted read. Drug screen, any number of drug classes from Drug Class List A; single drug class method, by instrumented test systems (eg, discrete multichannel chemistry analyzers utilizing immunoassay or enzyme assay), per date of service G0431 minus 39% $99.20 less $38.69 $60.51 The crosswalk recommendation is intended to reflect the services performed for the typical patient. Here, the average number of drugs included in this panel is 11. The recommendation reflects the current required resources. Drug screen, presumptive, single drug class from Drug Class List B, by immunoassay (eg, ELISA) or non-tlc chromatography without mass spectrometry (eg, GC, HPLC), each procedure Drug screen, any number of drug classes, presumptive, single or multiple drug class method; thin layer chromatography procedure(s) (TLC) (eg, acid, neutral, alkaloid plate), per date of service $ $25.23 Procedures for the drugs on List B require additional resources than those on List A. Units of service are comparable. The methodology for this presumptive drug screen utilizes a thin layer chromatography, as does our recommended crosswalk of CPT Drug screen, any number of drug classes, presumptive, single or multiple drug class method; not otherwise specified presumptive procedure (eg, TOF, MALDI, LDTD, DESI, DART), each procedure $24.63 This new CPT code utilizes a new methdology not previously listed in the presumptive drug section. Therefore, it should be crosswalked to a similar methodology CPT code within the Path/Lab section of the CPT Manual: CPT DEFINITIVE DRUG ASSAYS Alcohols $14.74 Crosswalk to the specific alcohol code, CPT Alcohol biomarkers; 1 to $14.74 A crosswalk to the specific alcohol code, CPT 82055, should be utilized. Alcohol biomarkers; 3 or more % $ $1.47 $16.21 This new definitive drug assay CPT code should be crosswalked to the specific alcohol code, CPT However, as additional resources are required to complete this procedure an increase about the base code is appropriate. 1
6 ASCP Alkaloids, not otherwise specified $24.63 Amphetamines; 1 to $21.20 Amphetamines; 3 to 4 Amphetamines; 5 or more Anabolic steroids; % % $ $2.12 $23.32 $ $4.24 $25.44 $35.22 ASCP recommends crosswalking this code to the methodology code, CPT 82542, generally used for alkaloids. A crosswalk to the specific Amphetamines code, CPT 82145, should be used. ASCP recommends a crosswalk to the specific Amphetamines code, CPT 82145, should be used plus an additional 10% to capture the additional resources utlized to identify additional drugs and/or metabolites. ASCP recommends a crosswalk to the specific Amphetamines code, CPT 82145, should be used plus an additional 20% to capture the additional resources utlized to identify additional drugs and/or metabolites. A crosswalk to the specific Dihydrotestosterone code, CPT 82651, should be used. Anabolic steroids; 3 or more $ $3.52 $38.74 A crosswalk to the specific Dihydrotestosterone code, CPT 82651, should be used plus 10% to capture the additional resources utlized to identify additional drugs and/or metabolites. Analgesics, non-opioid; 1 to 2 drugs $27.61 A crosswalk to the specific code for Acetaminophen, CPT 82003, should be used. Analgesics, non-opioid; 3 to 5 drugs $ $2.76 $30.37 A crosswalk to the specific code for Acetaminophen, CPT 82003, should be used plus 10% to cover the additional resources utlized to identify additional drugs and/or metabolites. Analgesics, non-opioid; 6 or more % $ $5.52 $33.13 A crosswalk to the specific code for Acetaminophen, CPT 82003, should be used plus 20% to cover the additional resources utlized to identify additional drugs and/or metabolites. Antidepressants, serotonergic class; 1 to 2 drugs $24.63 We recommend crosswalking this presumptive drug screen to a method code, CPT Antidepressants, serotonergic class; 3 to $ $2.46 $27.09 A crosswalk to method code, CPT 82492, plus 10% is recommended to capture the additional resources utilized to identify additional drugs and/or metabolites beyond the base code. 2
7 ASCP Antidepressants, serotonergic class; 6 or more % $ $4.92 $29.55 A crosswalk to method code, CPT 82492, plus 20% is recommended to capture the additional resources utilized to identify additional drugs and/or metabolites beyond the base code. Antidepressants, tricyclic and other cyclicals; 1 to 2 drugs $24.42 This code should be crosswalked to existing code for Amitriptyline, CPT Antidepressants, tricyclic and other cyclicals; 3 to $ $2.44 $26.86 A crosswalk to the Amitriptyline code, CPT 80152, plus an additional 10% to capture the additional resources utilized to test for additional drugs and/or metabolites beyond the base code. Antidepressants, tricyclic and other cyclicals; 6 or more % $ $4.88 $29.30 A crosswalk to the Amitriptyline code, CPT 80152, plus an additional 20% to capture the additional resources utilized to test for additional drugs and/or metabolites beyond the base code. Antidepressants, not otherwise specified $24.63 Antiepileptics, not otherwise specified; 1 to 3 drugs $19.87 ASCP recommends crosswalking this new code to method code, CPT A crosswalk to the code for antiepileptic Carbamazapine, CPT 80156, should be utitlized. Antiepileptics, not otherwise specified; 4 to $ $1.98 $21.85 A crosswalk to the code for antiepileptic Carbamazapine, CPT 80156, should be utilized plus an additional 10% to cover the additional resources involved in testing for additional drugs and/or metabolites. Antiepileptics, not otherwise specified; 7 or more % $ $3.97 $23.84 A crosswalk to the code for antiepileptic Carbamazapine, CPT 80156, should be utilized plus an additional 20% to cover the additional resources involved in testing for additional drugs and/or metabolites. Antipsychotics, not otherwise specified; 1 to 3 drugs $25.23 A crosswalk to the code for Clozapine, CPT 80159, is recommended. Antipsychotics, not otherwise specified; 4 to $ $2.52 $27.75 A crosswalk to the code for Clozapine, CPT 80159, is recommended plus an additional 10% to cover the additional resources expended to test for additional drugs and/or metabolites. 3
8 ASCP Antipsychotics, not otherwise specified; 7 or more % $ $5.04 $30.27 A crosswalk to the code for Clozapine, CPT 80159, is recommended plus an additional 20% to cover the additional resources expended to test for additional drugs and/or metabolites. Barbiturates $15.62 A crosswalk to the code for Barbiturates, CPT 82205, is recommended. Benzodiazepines; $25.23 A crosswalk to the code for Benzodiazepines, CPT 80154, is recommended. Benzodiazepines; 13 or more $ $2.52 $27.75 A crosswalk to the code for Benzodiazepines, CPT 80154, is recommended plus an additional 10% to cover the additional resources expended to test for additional drugs and/or metabolites. Buprenorphine $26.54 A crosswalk to the code for Opiates, CPT 83925, is recommended. Cannabinoids, natural $24.63 We recommend crosswalking to the method code CPT Cannabinoids, synthetic; 1 to $24.63 We recommend crosswalking to the method code CPT Cannabinoids, synthetic; 4 to 6 Cannabinoids, synthetic; 7 or more % $ $2.46 $27.09 $ $4.92 $29.55 A crosswalk to method code CPT plus an additional 10% to cover the additional resources utilized to test for additional drugs and/or metabolites. A crosswalk to method code CPT plus an additional 20% to cover the additional resources utilized to test for additional drugs and/or metabolites. Cocaine $20.68 ASCP recommends a crosswalk to the code for Cocaine, CPT Fentanyl $26.54 ASCP recommends a crosswalk to the code for Opiates, CPT Gabapentin, non-blood $18.09 A crosswalk to the code specific to Gabapentin, CPT 80171, is recommended. Heroin metabolite $26.54 ASCP recommends a crosswalk to the code for Opiates, CPT Ketamine and norketamine $24.63 Crosswalk to the method code
9 ASCP Methadone $22.28 A crosswalk to the code for Methadone, CPT 83840, is recommended. Methylenedioxyamphetamines (MDA, MDEA, MDMA) $21.20 A crosswalk to the code for Amphetamines, CPT 82145, is recommended. Methylphenidate $24.63 ASCP recommends crosswalking to the method code CPT Opiates, one or more $26.54 ASCP recommends a crosswalk to the code for Opiates, CPT Opioids and opiate analogs; 1 to $26.54 ASCP recommends a crosswalk to the code for Opiates, CPT Opioids and opiate analogs; 3 to 4 Opioids and opiate analogs; 5 or more % $ $2.65 $29.19 $ $5.30 $31.84 ASCP recommends a crosswalk to the Opiates code plus an additional 10% to cover the additional resources used to test for additional drugs and/or metabolites. ASCP recommends a crosswalk to the Opiates code plus an additional 20% to cover the additional resources used to test for additional drugs and/or metabolites. Oxycodone $26.54 ASCP recommends a crosswalk to the code for Opiates, CPT Phencyclidine $20.05 Crosswalk to the specific code for Phencyclidine Pregabalin $24.63 ASCP recommends crosswalking to the method code CPT Propoxyphene $26.54 ASCP recommends a crosswalk to the code for Opiates, CPT Sedative hypnotics (non-benzodiazepines) $24.63 ASCP recommends crosswalking to the method code CPT Skeletal muscle relaxants; $24.63 ASCP recommends crosswalking to the method code CPT Skeletal muscle relaxants; 3 or more $ $2.46 $27.09 ASCP recommends crosswalking to the method code CPT plus and additional 10% to cover the additional resources utitlized to test for more drugs and/or metabolites. Stimulants, synthetic $24.63 ASCP recommends crosswalking to the method code CPT Tapentadol $26.54 ASCP recommends a crosswalk to the code for Opiates, CPT
10 ASCP Tramadol $26.54 ASCP recommends a crosswalk to the code for Opiates, CPT Stereoisomer (enantiomer) analysis, single drug class $24.63 ASCP recommends crosswalking to the method code CPT Drug(s) or substance(s), definitive, qualitative or quantitative, not otherwise specified; 1 to $24.63 ASCP recommends crosswalking to the method code CPT Drug(s) or substance(s), definitive, qualitative or quantitative, not otherwise specified; 4 to $ $2.46 $27.09 ASCP recommends crosswalking to the method code CPT plus an additional 10% to cover the additional resources utitlized to test for more drugs and/or metabolites. 801XY47 Drug(s) or substance(s), definitive, qualitative or quantitative, not otherwise specified; 7 or more % $ $4.92 $29.55 ASCP recommends crosswalking to the method code CPT plus an additional 20% to cover the additional resources utitlized to test for more drugs and/or metabolites. THERAPEUTIC DRUG ASSAYS - 801XX 801XX Digoxin; free $18.12 A crosswalk to the code for Digoxin, CPT 80162, is recommended. 801XX Valproic acid (dipropylacetic acid); free $18.49 A crosswalk to the code for Dipropylacetic acid (valproic acid) is recommended. Moleculars Tier 1 812XX FLT3 (FMS-related tyrosine kinase 3) (eg, acute myeloid leukemia), gene analysis tyrosine kinase domain (TKD) variants (eg, D835, I836) $ FLT3 TKD uses similar methodology to and also is similar in resources in that covers the same number of variants and is in the same gene code family. 812XX MLH1 (mutl homolog 1, colon cancer, nonpolyposis type 2) (eg, hereditary non-polyposis colorectal cancer, Lynch syndrome) gene analysis; promoter methylation analysis $ MLH1 promoter methylation analysis is similar in resources to MLH1 in that it covers the same number of variants and is in the same genecode family. 8131X3 PCA3/KLK3 (prostate cancer antigen 3 [non-protein coding]/kallikrein-related peptidase 3 [prostate specific antigen]) ratio (eg, prostate cancer) $ ASCP supports crosswalking PCA3 to 81293, MLH1 known variants. This crosswalk reflects similar resources as identified by a Palmetto cost analysis of $256. Based on its analysis, Palmetto set a rate of $260, which is similar to the $ of
11 ASCP Genomic Sequencing Procedures - CMS Aortic dysfunction or dilation (eg, Marfan syndrome, Loeys Dietz syndrome, Ehler Danlos syndrome type IV, arterial tortuosity syndrome); genomic sequence analysis panel, must include sequencing of at least 9 genes, including FBN1, TGFBR1, TGFBR2, COL3A1, MYH11, ACTA2, SLC2A10, SMAD3, and MYLK Aortic dysfunction or dilation (eg, Marfan syndrome, Loeys Dietz syndrome, Ehler Danlos syndrome type IV, arterial tortuosity syndrome); duplication/deletion analysis, panel must include analyses for TGFBR1, TGFBR2, MYH11, and COL3A1 Exome (eg, unexplained constitutional or heritable disorder or syndrome); sequence analysis Exome (eg, unexplained constitutional or heritable disorder or syndrome); sequence analysis, each comparator exome (eg, parents, siblings) (List separately in addition to code for primary procedure) Exome (eg, unexplained constitutional or heritable disorder or syndrome); re-evaluation of previously obtained exome sequence (eg, updated knowledge or unrelated condition/syndrome) Fetal chromosomal aneuploidy (eg, trisomy 21, monosomy X) genomic sequence analysis panel, circulating cell-free fetal DNA in maternal blood, must include analysis of chromosomes 13, 18, and 21 Genome (eg, unexplained constitutional or heritable disorder or syndrome); sequence analysis 7
12 ASCP Genome (eg, unexplained constitutional or heritable disorder or syndrome); sequence analysis, each comparator genome (eg, parents, siblings) (List separately in addition to code for primary procedure) Genome (eg, unexplained constitutional or heritable disorder or syndrome); re-evaluation of previously obtained genome sequence (eg, updated knowledge or unrelated condition/syndrome) Hearing loss (eg, nonsyndromic hearing loss, Usher syndrome, Pendred syndrome); genomic sequence analysis panel, must include sequencing of at least 60 genes, including CDH23, CLRN1, GJB2, GPR98, MTRNR1, MYO7A, MYO15A, PCDH15, OTOF, SLC26A4, TMC1, TMPRSS3, USH1C, USH1G, USH2A, and WFS1 Hearing loss (eg, nonsyndromic hearing loss, Usher syndrome, Pendred syndrome); duplication/ deletion analysis panel, must include copy number analyses for STRC and DFNB1 deletions in GJB2 and GJB6 genes Hereditary colon cancer syndromes, (eg, Lynch syndrome, familial adenomatosis polyposis); genomic sequence analysis panel, must include analysis of at least 7 genes, including APC, CHEK2, MLH1, MSH2, MSH6, MUTYH, and PMS2 Hereditary colon cancer syndromes, (eg, Lynch syndrome, familial adenomatosis polyposis); duplication/deletion gene analysis panel, must include analysis of at least 8 genes, including APC, MLH1, MSH2, MSH6, PMS2, EPCAM, CHEK2, and MUTYH 8
13 ASCP Nuclear encoded mitochondrial genes (eg, neurologic or myopathic phenotypes), genomic sequence panel, must include analysis of at least 100 genes. Including, BCSIL, C10orf2, COQ2, COX 10, DGUOK, MPV17, OPA1, PDSS2 POLG, POLG2, RRM2B, SCO1, SCO2, SLC25A4, SUCLA2, SUCLG1, TAZ, TK2, and TYMP Targeted genomic sequence analysis panel, solid organ neoplasm, DNA analysis, 5-50 genes (eg, ALK, BRAF, CDKN2A, EGFR, ERBB2, KIT, KRAS, NRAS, MET, PDGFRA, PDGFRB, PGR, PIK3CA, PTEN, RET), interrogation for sequence variants and copy number variants or rearrangements, if performed Targeted genomic sequence analysis panel, hematolymphoid neoplasm or disorder, DNA and RNA analysis when performed, 5-50 genes (eg, BRAF, CEBPA, DNMAT3A, EZH2, FLT3, IDH1, IDH2, JAK2, KRAS, KIT, MLL, NRAS, NPM1, NOTCH1), interrogation for sequence variants, and copy number variants or rearrangements, or isoform expression or mrna expression levels, if performed Targeted genomic sequence analysis panel, solid organ or hematolymphoid neoplasm, DNA and RNA analysis when performed, 51 or greater genes (eg, ALK, BRAF, CDKN2A, CEBPA, DNMT3A, EGFR, ERBB2, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MLL, NPM1, NRAS, MET, NOTCH1, PDGFRA, PDGFRB, PGR, PIK3CA, PTEN, RET), interrogation for sequence variants, and copy number variants or rearrangements, if performed 9
14 ASCP Whole mitochondrial genome (eg, Leigh syndrome, mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes [MELAS], myoclonic epilepsy with ragged-red fibers [MERFF], neuropathy, ataxis and retinitis pigmentosa [NARP], Leber hereditary optic neuropathy [LHON], genomic sequence, must include sequence analysis of entire mitochondrial genome with heteroplasmy detection Whole mitochondrial genome large deletion analysis panel (eg, Kearns-Sayre syndrome, chronic progressive external ophthalmoplegia), including heteroplasmy detection, if performed X-linked intellectual disability (XLID) (eg, syndromic and nonsyndromic XLID); genomic sequence analysis panel, must include sequencing of at least 60 genes, including ARX, ATRX, CDKL5, FGD1, FMR1, HUWE1, IL1RAPL, KCM5C, L1CAM, MECP2, MED12, MID1, OCRL, RPS6KA3, and SLC16A2 X-linked intellectual disability (XLID) (eg, syndromic and nonsyndromic XLID); duplication/deletion gene analysis, must include analysis of at least 60 genes, including ARX, ATRX, CDKL5, FGD1, FMR1, HUWE1, IL1RAPL, KCM5C, L1CAM, MECP2, MED12, MID1, OCRL, RPS6KA3, and SLC16A2 Multianalyte Assays with Algorithmic Analyses (MAAA, Category I) Administrative s not listed 815XX Oncology (breast), mrna, gene expression profiling by realtime RT-PCR of 21 genes, utilizing formalin-fixed paraffin embedded tissue, algorithm reported as recurrence score Oncotype DX Breast Cancer Assay (Genomic Health, Inc.) 10
15 ASCP CHEMISTRY - CMS 830XX 830XX Growth stimulation expressed gene 2 (ST2, interleukin I receptor like-1) $30.01 The ST2 assay is a quantitative 2-site manual enzyme-linked immunosorbent assay (ELISA) and is an FDA 510K-cleared in vitro diagnostic procedure used for cardiac patient management. The test should be crosswalked to Galectin-3, CPT 82777, as it is similar in methodology and clinical utility. MICROBIOLOGY 875XX 875XX 875XX Infectious agent detection by nucleic acid (DNA or RNA); gastrointestinal pathogen (eg, Clostridium difficile, E. coli, Salmonella, Shigella, norovirus, Giardia), includes multiplex reverse transcription, when performed, and multiplex amplified probe technique, multiple types or subtypes, 3-5 targets Infectious agent detection by nucleic acid (DNA or RNA); gastrointestinal pathogen (eg, Clostridium difficile, E. coli, Salmonella, Shigella, norovirus, Giardia), includes multiplex reverse transcription, when performed, and multiplex amplified probe technique, multiple types or subtypes, 6-11 targets Infectious agent detection by nucleic acid (DNA or RNA); gastrointestinal pathogen (eg, Clostridium difficile, E. coli, Salmonella, Shigella, norovirus, Giardia), includes multiplex reverse transcription, when performed, and multiplex amplified probe technique, multiple types or subtypes, targets $ $ $ This gastrointestinal microorganism multiplex nucleic acid-based assay should be crosswalked to CPT Both assays involve multiplex reverse transcription and amplified probe technique for 3-5 targets. This gastrointestinal microorganism multiplex nucleic acid-based assay should be crosswalked to CPT Both assays involve multiplex reverse transcription and amplified probe technique for 6-11 targets. This gastrointestinal microorganism multiplex nucleic acid-based assay should be crosswalked to CPT Both assays involve multiplex reverse transcription and amplified probe technique for targets. 875XX Infectious agent detection by nucleic acid (DNA or RNA); Human Papillomavirus (HPV), low-risk types (eg 6,11,42,43,44) $47.87 The three new HPV CPT codes were added to provide specificity to the type of procedures and to antigens tested. The technology is the same as that of CPT Therefore, this new code should be crossed walking to
16 ASCP 875XX Infectious agent detection by nucleic acid (DNA or RNA); Human Papillomavirus (HPV), high-risk types (eg 16,18,31,33,35,39,45,51,52,56,58,59,68) $47.87 The three new HPV CPT codes were added to provide specificity to the type of procedures and to antigens tested. The technology is the same as that of CPT Therefore, this new code should be crosswalked to XX Infectious agent detection by nucleic acid (DNA or RNA); Human Papillomavirus (HPV), types 16 and 18 only, includes type 45 if performed $47.87 The three new HPV CPT codes were added to provide specificity to the type of procedures and to antigens tested. The technology is the same as that of CPT Therefore, this new code should be crossedwalked to XX Infectious agent antigen detection by immunoassay with direct optical observation; HIV-1 antigen(s), with HIV-1 and HIV-2 antibodies $32.86 The new code should be crosswalked to similar immunoassay, CPT 87389, HIV antigen and HIV antibodies in a single result. The new code is somewhat different than the proposed crosswalk in that differentiates between the antigen and antibodies providing two results. Other Procedures 89XXX Cryopreservation; mature oocytes GXXXX G s Colorectal cancer screening; stool-based DNA and fecal occult hemoglobin (e.g., KRAS, NDRG4 and BMP3) gap-fill no listed in the 2014 CLFS The procedure is similar to Cryopreservation; embryo(s). 12
June 2016 Updated Crosswalk:
 June 2016 Updated Crosswalk: 2016 HCPCS G0279 G6001 G6002 G6003 G6004 deleted f Radiology Coding BCBSNC currently considers this service investigational and will not cover either the HCPCS CPT s. Diagnostic
June 2016 Updated Crosswalk: 2016 HCPCS G0279 G6001 G6002 G6003 G6004 deleted f Radiology Coding BCBSNC currently considers this service investigational and will not cover either the HCPCS CPT s. Diagnostic
CPT Code Changes for 2015 PATHOLOGY/LABORATORY
 Changes for 2015 PATHOLOGY/LABORATORY Rick Oliver, CPCO, CPC, MT(ASCP) Compliance Director- Laboratory and Pathology McKesson Business Performance Services This commentary is a summary prepared by McKesson
Changes for 2015 PATHOLOGY/LABORATORY Rick Oliver, CPCO, CPC, MT(ASCP) Compliance Director- Laboratory and Pathology McKesson Business Performance Services This commentary is a summary prepared by McKesson
1199 SEIU Benefit Funds Laboratory Management Program PRIOR AUTHORIZATION CODE LIST FOR OUTPATIENT MOLECULAR AND GENOMIC LABORATORY TESTS
 1199 SEIU Benefit Funds Laboratory Management Program PRIOR AUTHORIZATION CODE LIST FOR OUTPATIENT MOLECULAR AND GENOMIC LABORATORY TESTS Effective January 1, 2016 Administered by evicore healthcare Refer
1199 SEIU Benefit Funds Laboratory Management Program PRIOR AUTHORIZATION CODE LIST FOR OUTPATIENT MOLECULAR AND GENOMIC LABORATORY TESTS Effective January 1, 2016 Administered by evicore healthcare Refer
PAYMENT POLICY STATEMENT
 PAYMENT POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 01/01/2014 04/05/2017 04/05/2016 Policy Name Policy Number Drug Screening Tests PY-0020 Policy Type
PAYMENT POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 01/01/2014 04/05/2017 04/05/2016 Policy Name Policy Number Drug Screening Tests PY-0020 Policy Type
Clinical Drug Screening and/or Drug Testing
 Manual: Policy Title: Reimbursement Policy Clinical Drug Screening and/or Drug Testing Section: Laboratory & Pathology Subsection: None Date of Origin: 10/26/2011 Policy Number: RPM016 Last Updated: 1/8/2016
Manual: Policy Title: Reimbursement Policy Clinical Drug Screening and/or Drug Testing Section: Laboratory & Pathology Subsection: None Date of Origin: 10/26/2011 Policy Number: RPM016 Last Updated: 1/8/2016
Reconsideration Code 86386. Reconsideration Code Description Nuclear Matrix Protein 22 (NMP22), qualitative
 Calendar Year 2013 Centers for Medicare and Medicaid Services (CMS) New and Reconsidered Clinical Laboratory Fee Schedule (CLFS) Test Codes And Final Payment Determinations Reconsideration Code 86386 Reconsideration
Calendar Year 2013 Centers for Medicare and Medicaid Services (CMS) New and Reconsidered Clinical Laboratory Fee Schedule (CLFS) Test Codes And Final Payment Determinations Reconsideration Code 86386 Reconsideration
Toxicology CPT Code Changes for 2016
 Beginning January 1, 2016, CMS deleted all 2015 drug testing G codes and will continue to not recognize the AMA CPT codes for drug testing. CMS created three G codes for presumptive testing and four G
Beginning January 1, 2016, CMS deleted all 2015 drug testing G codes and will continue to not recognize the AMA CPT codes for drug testing. CMS created three G codes for presumptive testing and four G
July 1, 2015 VIA EMAIL
 July 1, 2015 VIA EMAIL Marc Hartstein Director, Hospital and Ambulatory Policy Group Centers for Medicare and Medicaid Services 7500 Security Boulevard Mail Stop C4-01-26 Baltimore, MD 21244 Dear Marc:
July 1, 2015 VIA EMAIL Marc Hartstein Director, Hospital and Ambulatory Policy Group Centers for Medicare and Medicaid Services 7500 Security Boulevard Mail Stop C4-01-26 Baltimore, MD 21244 Dear Marc:
Reimbursement for Molecular Diagnostics
 Reimbursement for Molecular Diagnostics Michael Pollock SLA Annual Conference June 11, 2013 San Diego Overview of Changes to Molecular Diagnostic Test Reimbursement Molecular pathology (MoPath) codes provide
Reimbursement for Molecular Diagnostics Michael Pollock SLA Annual Conference June 11, 2013 San Diego Overview of Changes to Molecular Diagnostic Test Reimbursement Molecular pathology (MoPath) codes provide
Confirmatory Drug Testing for Substance Abuse and Chronic Pain Treatment
 Clinical Position Statement Confirmatory Drug Testing for Substance Abuse and Chronic Pain Treatment CLINICAL POSITION STATEMENT In most cases, immunoassay (qualitative) drug testing is sufficient for
Clinical Position Statement Confirmatory Drug Testing for Substance Abuse and Chronic Pain Treatment CLINICAL POSITION STATEMENT In most cases, immunoassay (qualitative) drug testing is sufficient for
JOHNS HOPKINS HEALTHCARE
 Page 1 of 15 ACTION: New Policy: Effective Date: 08/26/2003 Revising : Review Dates: 03/15/2004, 10/22/2004, Superseding : 10/21/2005, 10/19/2006, 01/07/2008, Archiving : 01/05/2009, 01/07/2011, 06/07/2013,
Page 1 of 15 ACTION: New Policy: Effective Date: 08/26/2003 Revising : Review Dates: 03/15/2004, 10/22/2004, Superseding : 10/21/2005, 10/19/2006, 01/07/2008, Archiving : 01/05/2009, 01/07/2011, 06/07/2013,
The CPT code for Human papillomavirus (HPV) has changed from 87621 to 87624. This code (87624) covers testing for HPV High Risk genotypes.
 January 2015 2015 CPT Code Updates Dear Client: The American Medical Association (AMA) publishes the Current Procedural Terminology (CPT) Coding Manual on an annual basis. For 2015, the AMA made several
January 2015 2015 CPT Code Updates Dear Client: The American Medical Association (AMA) publishes the Current Procedural Terminology (CPT) Coding Manual on an annual basis. For 2015, the AMA made several
Launching a Cancer Genetic Laboratory to Enhance Diagnosis and Treatment
 Launching a Cancer Genetic Laboratory to Enhance Diagnosis and Treatment Arthur L. Beaudet, M.D. Department of Molecular and Human Genetics Baylor College of Medicine ORIGIN AND PRECEDENT Decades of experience
Launching a Cancer Genetic Laboratory to Enhance Diagnosis and Treatment Arthur L. Beaudet, M.D. Department of Molecular and Human Genetics Baylor College of Medicine ORIGIN AND PRECEDENT Decades of experience
G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics
 G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics One of every 500 newborns has bilateral permanent sensorineural hearing loss 40 db which makes it the most common
G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics One of every 500 newborns has bilateral permanent sensorineural hearing loss 40 db which makes it the most common
2014 CPT Code Updates
 2014 CPT Code Updates This publication is a summary of The American Medical Association Current Procedural Terminology (CPT) codes published in ARUP's Laboratory Test Directory effective January 1, 2014.
2014 CPT Code Updates This publication is a summary of The American Medical Association Current Procedural Terminology (CPT) codes published in ARUP's Laboratory Test Directory effective January 1, 2014.
Downstream Outcomes of New Molecular Diagnostics CPT Coding System Codes
 Downstream Outcomes of New Molecular Diagnostics CPT Coding System Codes Michael Watson, MS, PhD, FACMG Advisory Committee on Heritable Disorders of Newborns and Children April 9, 2014 Disclosures As Executive
Downstream Outcomes of New Molecular Diagnostics CPT Coding System Codes Michael Watson, MS, PhD, FACMG Advisory Committee on Heritable Disorders of Newborns and Children April 9, 2014 Disclosures As Executive
July 18, 2016: Advisory Panel on Clinical Laboratory Diagnostic Tests Meeting on ADLT Designation and Coding.
 Healthcare Alert Summary: PAMA Final Rule Written by Thomas Barker, Brian P. Carey June 29, 2016 Market Based Payment for Clinical Diagnostic Laboratory Tests Summary On June 17, 2016 the Centers of Medicare
Healthcare Alert Summary: PAMA Final Rule Written by Thomas Barker, Brian P. Carey June 29, 2016 Market Based Payment for Clinical Diagnostic Laboratory Tests Summary On June 17, 2016 the Centers of Medicare
Agenda. Regulatory Success. Regulatory Success. Regulatory Success. Antiquated Reimbursement Methodology. Coding Transparency Initiatives
 VALUE-BASED REIMBURSEMENT FOR PERSONALIZED MEDICINE: WHERE DO WE STAND? overview US health care value-based purchasing reforms, explore implications for diagnostics and personalized medicines Michael Longacre,
VALUE-BASED REIMBURSEMENT FOR PERSONALIZED MEDICINE: WHERE DO WE STAND? overview US health care value-based purchasing reforms, explore implications for diagnostics and personalized medicines Michael Longacre,
RE: CMS 1621 P, Medicare Clinical Diagnostic Laboratory Tests Payment System Proposed Rule; (Vol. 80, No.190), October 1, 2015.
 Andrew M. Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-1621-P P.O. Box 8016 Baltimore, MD 21244-8016 RE: CMS 1621 P, Medicare
Andrew M. Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-1621-P P.O. Box 8016 Baltimore, MD 21244-8016 RE: CMS 1621 P, Medicare
Genomic Medicine Education Initiatives of the College of American Pathologists
 Genomic Medicine Education Initiatives of the College of American Pathologists Debra G.B. Leonard, MD, PhD, FCAP Chair, Personalized Healthcare Committee, CAP Professor of Pathology, Weill Cornell Medical
Genomic Medicine Education Initiatives of the College of American Pathologists Debra G.B. Leonard, MD, PhD, FCAP Chair, Personalized Healthcare Committee, CAP Professor of Pathology, Weill Cornell Medical
Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss
 Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_hereditary_hearing_loss 10/2013 8/2015 8/2016
Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_hereditary_hearing_loss 10/2013 8/2015 8/2016
Payers those financially responsible for the cost of
 Reimbursement Challenges with In Vitro Diagnostic Tests: Fitting a Square Peg into a Round Hole by Paul Radensky Payers those financially responsible for the cost of healthcare are critical market regulators
Reimbursement Challenges with In Vitro Diagnostic Tests: Fitting a Square Peg into a Round Hole by Paul Radensky Payers those financially responsible for the cost of healthcare are critical market regulators
Genomic Medicine The Future of Cancer Care. Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America
 Genomic Medicine The Future of Cancer Care Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America Personalized Medicine Personalized health care is a broad term for interventions
Genomic Medicine The Future of Cancer Care Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America Personalized Medicine Personalized health care is a broad term for interventions
9/19/2014. Challenges Facing Pathology Today A CAP Pathologist s View Presenter: Sang Wu, MD, FCAP MGMA Annual Conference, Las Vegas, NV.
 sub-brand here Challenges Facing Pathology Today A CAP Pathologist s View Presenter: Sang Wu, MD, FCAP MGMA Annual Conference, Las Vegas, NV October 2014 cap.org v. # Introduction 2 Introduction 3 1 Course
sub-brand here Challenges Facing Pathology Today A CAP Pathologist s View Presenter: Sang Wu, MD, FCAP MGMA Annual Conference, Las Vegas, NV October 2014 cap.org v. # Introduction 2 Introduction 3 1 Course
Health First Health Plans Authorization List
 Health First Health Plans Authorization List Effective April 15, 2016 IMPORTANT CONTACTS FOR AUTHORIZATIONS SUBMITTED TO THE HEALTH PLAN Submit online requests via your secure account at MyHFHP.org/login
Health First Health Plans Authorization List Effective April 15, 2016 IMPORTANT CONTACTS FOR AUTHORIZATIONS SUBMITTED TO THE HEALTH PLAN Submit online requests via your secure account at MyHFHP.org/login
May 8, 2013. The Honorable Fred Upton Chairman House Committee on Energy and Commerce United States House of Representatives Washington, DC 20151
 May 8, 2013 The Honorable Dave Camp Chairman House Committee on Ways and Means United States House of Representatives Washington D.C. 20515 The Honorable Fred Upton Chairman House Committee on Energy and
May 8, 2013 The Honorable Dave Camp Chairman House Committee on Ways and Means United States House of Representatives Washington D.C. 20515 The Honorable Fred Upton Chairman House Committee on Energy and
2015 CPT Code Changes ACL Test
 Changes ACL Test Test Description UCOMP DRUG SCREEN COMPLETE 80100 80303 G0431 UDINV DRUG INVESTIGATION 80100 80303 G0431 UTLC THIN LAYER CHROMATOGRAPHY DRUG SCREEN 80100 80303 G0431 UNIC NICOTINE, URINE
Changes ACL Test Test Description UCOMP DRUG SCREEN COMPLETE 80100 80303 G0431 UDINV DRUG INVESTIGATION 80100 80303 G0431 UTLC THIN LAYER CHROMATOGRAPHY DRUG SCREEN 80100 80303 G0431 UNIC NICOTINE, URINE
Molecular Pathology/Molecular Diagnostics/Genetic Testing
 Policy Number GEN01252012RP Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 01/28/2015 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable
Policy Number GEN01252012RP Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 01/28/2015 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable
MEDICAL POLICY No. 91540-R10 GENETICS: COUNSELING, TESTING, SCREENING
 GENETICS: COUNSELING, TESTING, SCREENING Effective Date: February 26, 2015 Review Dates: 8/07, 10/07, 8/08, 8/09, 10/09, 2/10, 8/10, 8/11, 10/11, 10/12, 10/13, 11/14 Date Of Origin: August 8, 2007 Status:
GENETICS: COUNSELING, TESTING, SCREENING Effective Date: February 26, 2015 Review Dates: 8/07, 10/07, 8/08, 8/09, 10/09, 2/10, 8/10, 8/11, 10/11, 10/12, 10/13, 11/14 Date Of Origin: August 8, 2007 Status:
TEST AND PRICE LIST FOR NEXT GENERATION SEQUENCING
 AIP ALK APC Mutation Detection Techniques Ai Saple Type TAT ATM BAP1 BLM BMPR1A BRCA1 BRCA2 BRIP1 BUB1B CDC73 CDH1 CDK4 CDKN1C CDKN2A CEBPA CEP57 CHEK2 CYLD DDB2 DICER1 DIS3L2 EGFR EPCAM ERCC2 ERCC3 ERCC4
AIP ALK APC Mutation Detection Techniques Ai Saple Type TAT ATM BAP1 BLM BMPR1A BRCA1 BRCA2 BRIP1 BUB1B CDC73 CDH1 CDK4 CDKN1C CDKN2A CEBPA CEP57 CHEK2 CYLD DDB2 DICER1 DIS3L2 EGFR EPCAM ERCC2 ERCC3 ERCC4
Coding and Billing for HIV Services in Healthcare Facilities
 P a g e 1 Coding and Billing for HIV Services in Healthcare Facilities The Hawai i State Department of Health STD/AIDS Prevention Branch is pleased to provide you information on billing and reimbursement
P a g e 1 Coding and Billing for HIV Services in Healthcare Facilities The Hawai i State Department of Health STD/AIDS Prevention Branch is pleased to provide you information on billing and reimbursement
Genetic Testing for Lynch Syndrome/Colorectal Cancer and Polyposis Syndromes
 Genetic Testing for Lynch Syndrome/Colorectal Cancer and Polyposis Syndromes Policy Number: Original Effective Date: MM.02.007 09/01/2011 Line(s) of Business: Current Effective Date: HMO; PPO 09/01/2011
Genetic Testing for Lynch Syndrome/Colorectal Cancer and Polyposis Syndromes Policy Number: Original Effective Date: MM.02.007 09/01/2011 Line(s) of Business: Current Effective Date: HMO; PPO 09/01/2011
Drug Testing to Support Pain Management
 NATIONAL REFERENCE LABORATORY Drug Testing to Support Pain Management 500 Chipeta Way, Salt Lake City, UT 84108 (800) 522-2787 (801) 583-2787 www.aruplab.com www.arupconsult.com ARUP is an enterprise of
NATIONAL REFERENCE LABORATORY Drug Testing to Support Pain Management 500 Chipeta Way, Salt Lake City, UT 84108 (800) 522-2787 (801) 583-2787 www.aruplab.com www.arupconsult.com ARUP is an enterprise of
Genetic Testing for Familial Adenomatous Polyposis and MYH-Associated Polyposis (Lynch Syndrome)
 Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Coding and Payment Guide for Laboratory Services. An essential coding, billing, and payment resource for laboratory and pathology services
 Coding and Payment Guide for Laboratory Services An essential coding, billing, and payment resource for laboratory and pathology services Contents Introduction............................... 1 Coding Systems...............................
Coding and Payment Guide for Laboratory Services An essential coding, billing, and payment resource for laboratory and pathology services Contents Introduction............................... 1 Coding Systems...............................
ANNUAL NOTICE TO PHYSICIANS
 NOVEMBER 2014 ANNUAL NOTICE TO PHYSICIANS Dear Physician Colleague: Genoptix Medical Laboratory is committed to complying with all applicable laws and regulations governing the health care industry. We
NOVEMBER 2014 ANNUAL NOTICE TO PHYSICIANS Dear Physician Colleague: Genoptix Medical Laboratory is committed to complying with all applicable laws and regulations governing the health care industry. We
RMHP Prior Authorization List Effective October 1, 2015 rev 7/18/2016
 Effective October 1, 2015 rev 7/18/2016 *This list applies to all services for which RMHP is the primary payer. * Services that are not a benefit of the Member's Evidence of Coverage will not be authorized.
Effective October 1, 2015 rev 7/18/2016 *This list applies to all services for which RMHP is the primary payer. * Services that are not a benefit of the Member's Evidence of Coverage will not be authorized.
This is the written version of our Hot Topic video presentation available at: MayoMedicalLaboratories.com/hot-topics
 This is the written version of our Hot Topic video presentation available at: MayoMedicalLaboratories.com/hot-topics Welcome to Mayo Medical Laboratories Hot Topics. These presentations provide short discussion
This is the written version of our Hot Topic video presentation available at: MayoMedicalLaboratories.com/hot-topics Welcome to Mayo Medical Laboratories Hot Topics. These presentations provide short discussion
Opportunities and Challenges in Translating Novel Discoveries into Useful Clinical Tests
 Opportunities and Challenges in Translating Novel Discoveries into Useful Clinical Tests James H. Doroshow, M.D. NCI Deputy Director for Clinical and Translational Research NCI Workshop: Evidence Needed
Opportunities and Challenges in Translating Novel Discoveries into Useful Clinical Tests James H. Doroshow, M.D. NCI Deputy Director for Clinical and Translational Research NCI Workshop: Evidence Needed
Genetic Testing for Hereditary Hearing Loss
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Genetic Testing for Hereditary Hearing Loss Number 12.04.87 Effective
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Genetic Testing for Hereditary Hearing Loss Number 12.04.87 Effective
Toxicology/DAU Testing by Mass Spectrometry
 Toxicology/DAU Testing by Mass Spectrometry AACC Conference: Mass Spectrometry in the Clinical Lab September 17, 2013 Kara Lynch, PhD, DABCC University of California San Francisco Disclosures Research
Toxicology/DAU Testing by Mass Spectrometry AACC Conference: Mass Spectrometry in the Clinical Lab September 17, 2013 Kara Lynch, PhD, DABCC University of California San Francisco Disclosures Research
NEW YORK STATE MEDICAID PROGRAM LABORATORY PROCEDURE CODES
 NEW YORK STATE MEDICAID PROGRAM LABORATORY PROCEDURE S Table of Contents GENERAL INFORMATION AND RULES...3 ORGAN OR DISEASE ORIENTED PANELS... 14 THERAPEUTIC DRUG ASSAYS... 15 EVOCATIVE/SUPPRESSION TESTING...
NEW YORK STATE MEDICAID PROGRAM LABORATORY PROCEDURE S Table of Contents GENERAL INFORMATION AND RULES...3 ORGAN OR DISEASE ORIENTED PANELS... 14 THERAPEUTIC DRUG ASSAYS... 15 EVOCATIVE/SUPPRESSION TESTING...
CERT: Documentation of Clinical Diagnostic Tests
 CERT: Documentation of Clinical Diagnostic Tests May 29, 2014 Cahaba Government Benefit Administrators, LLC Provider Outreach and Education Disclaimer This resource is not a legal document. The presentation
CERT: Documentation of Clinical Diagnostic Tests May 29, 2014 Cahaba Government Benefit Administrators, LLC Provider Outreach and Education Disclaimer This resource is not a legal document. The presentation
Molecular diagnostics is now used for a wide range of applications, including:
 Molecular Diagnostics: A Dynamic and Rapidly Broadening Market Molecular diagnostics is now used for a wide range of applications, including: Human clinical molecular diagnostic testing Veterinary molecular
Molecular Diagnostics: A Dynamic and Rapidly Broadening Market Molecular diagnostics is now used for a wide range of applications, including: Human clinical molecular diagnostic testing Veterinary molecular
URINE DRUG TESTING. Effective December 1 st, 2012
 URINE DRUG TESTING Effective December 1 st, 2012 Policy Neighborhood Health Plan (NHP) reimburses medically appropriate urine drug testing (UDT) to detect the parent drug and/or its metabolite(s) to demonstrate
URINE DRUG TESTING Effective December 1 st, 2012 Policy Neighborhood Health Plan (NHP) reimburses medically appropriate urine drug testing (UDT) to detect the parent drug and/or its metabolite(s) to demonstrate
Médecine de précision médecine personnalisée en Oncologie. Fabien Calvo, Directeur Recherche et Innovation, INCa, Directeur ITMO Cancer, AVIESAN
 Médecine de précision médecine personnalisée en Oncologie Fabien Calvo, Directeur Recherche et Innovation, INCa, Directeur ITMO Cancer, AVIESAN Successful targeted drug development Rapid identification
Médecine de précision médecine personnalisée en Oncologie Fabien Calvo, Directeur Recherche et Innovation, INCa, Directeur ITMO Cancer, AVIESAN Successful targeted drug development Rapid identification
Guideline Development The American Society of Clinical Oncology
 Assessing Genomic Sequencing Information for Health Care Decision Making Decision Making Once Evidence is Assessed/Graded Evaluated Guideline Development The American Society of Clinical Oncology Gary
Assessing Genomic Sequencing Information for Health Care Decision Making Decision Making Once Evidence is Assessed/Graded Evaluated Guideline Development The American Society of Clinical Oncology Gary
La diagnostica molecolare nelle neoplasie colo-rettali. A do Scarpa. U iversita di Vero a
 La diagnostica molecolare nelle neoplasie colo-rettali C-NET A do Scarpa e Dipartime to Pato ogia e U iversita di Vero a La stessa malattia puo avere diverse patogenesi cucchiaio moneta forchetta Gastrointestinal
La diagnostica molecolare nelle neoplasie colo-rettali C-NET A do Scarpa e Dipartime to Pato ogia e U iversita di Vero a La stessa malattia puo avere diverse patogenesi cucchiaio moneta forchetta Gastrointestinal
Nuovi Scenari in Oncologia. G. Zoppoli X-Files in Nutrizione Clinica e Artificiale, 08/06/2012
 Nuovi Scenari in Oncologia G. Zoppoli X-Files in Nutrizione Clinica e Artificiale, 08/06/2012 WHAT is cancer? «Cancer is a genetic disease of the somatic cell» [B. Vogelstein] Ten years ago Now [Cell.
Nuovi Scenari in Oncologia G. Zoppoli X-Files in Nutrizione Clinica e Artificiale, 08/06/2012 WHAT is cancer? «Cancer is a genetic disease of the somatic cell» [B. Vogelstein] Ten years ago Now [Cell.
Department of Health and Human Services. No. 190 October 1, 2015. Part III
 Vol. 80 Thursday, No. 190 October 1, 2015 Part III Department of Health and Human Services Centers for Medicare & Medicaid Services 42 CFR Part 414 Medicare Program; Medicare Clinical Diagnostic Laboratory
Vol. 80 Thursday, No. 190 October 1, 2015 Part III Department of Health and Human Services Centers for Medicare & Medicaid Services 42 CFR Part 414 Medicare Program; Medicare Clinical Diagnostic Laboratory
Common Cancers & Hereditary Syndromes
 Common Cancers & Hereditary Syndromes Elizabeth Hoodfar, MS, LCGC Regional Cancer Genetics Coordinator Kaiser Permanente Northern California Detect clinical characteristics of hereditary cancer syndromes.
Common Cancers & Hereditary Syndromes Elizabeth Hoodfar, MS, LCGC Regional Cancer Genetics Coordinator Kaiser Permanente Northern California Detect clinical characteristics of hereditary cancer syndromes.
Urine Drug Testing Methadone 101 Methadone for hospitalists
 Urine Drug Testing Methadone 101 Methadone for hospitalists Dr. Patricia Mark MB, BCh LEARNING OBJECTIVES Clarify the purpose of urine drug testing Distinguish between UDT for detection of illicit drug
Urine Drug Testing Methadone 101 Methadone for hospitalists Dr. Patricia Mark MB, BCh LEARNING OBJECTIVES Clarify the purpose of urine drug testing Distinguish between UDT for detection of illicit drug
Subject: Clinical Laboratory Personnel Standards: Draft Proposed Regulations:
 Ronald Chapman, MD, MPH Director, California Department of Public Health and State Health Officer California Department of Public Health Post Office Box 997377, MS-0507 Sacramento, CA 97377 Subject: Clinical
Ronald Chapman, MD, MPH Director, California Department of Public Health and State Health Officer California Department of Public Health Post Office Box 997377, MS-0507 Sacramento, CA 97377 Subject: Clinical
How To Treat The Sando Syndrome
 Consultation on Drugs for Rare Diseases Rare Disease Day Symposium Hannover Medical School (MHH), Solidarity Rare but Strong Together Roland Seifert, MHH @ Dear Sir, I am writing hoping that there might
Consultation on Drugs for Rare Diseases Rare Disease Day Symposium Hannover Medical School (MHH), Solidarity Rare but Strong Together Roland Seifert, MHH @ Dear Sir, I am writing hoping that there might
Coding guide for routine HIV testing in health care settings
 Coding guide f routine HIV testing in health care settings Background In September of 2006, CDC issued recommendations f Human immunodeficiency virus (HIV) testing in health care settings. The Revised
Coding guide f routine HIV testing in health care settings Background In September of 2006, CDC issued recommendations f Human immunodeficiency virus (HIV) testing in health care settings. The Revised
CancerTREATMENT NGS+ NSCLC Summary Report Page 1 of 7 PATIENT SPECIMEN PHYSICIAN
 Page 1 of 7 POSITIVE TEST RESULTS Biomarker Result DETECTED NEGATIVE TEST RESULTS FDA Approved Therapies Targeting Molecular Pathway TEST DESCRIPTION: CancerTREATMENT NGS+ uses Next Generation Sequencing
Page 1 of 7 POSITIVE TEST RESULTS Biomarker Result DETECTED NEGATIVE TEST RESULTS FDA Approved Therapies Targeting Molecular Pathway TEST DESCRIPTION: CancerTREATMENT NGS+ uses Next Generation Sequencing
Section: Laboratory Last Reviewed Date: December 2015. Policy No: 68 Effective Date: April 1, 2016
 Medical Policy Manual Topic: Urine Drug Testing for Substance Abuse and Chronic Pain Date of Origin: December 2015 Section: Laboratory Last Reviewed Date: December 2015 Policy No: 68 Effective Date: April
Medical Policy Manual Topic: Urine Drug Testing for Substance Abuse and Chronic Pain Date of Origin: December 2015 Section: Laboratory Last Reviewed Date: December 2015 Policy No: 68 Effective Date: April
Local Coverage Determination (LCD) for Qualitative Drug Screening (L30574)
 Local Coverage Determination (LCD) for Qualitative Drug Screening (L30574) Contractor Information Contractor Name First Coast Service Options, Inc. Back to Top Contractor Number 09102 Contractor Type MAC
Local Coverage Determination (LCD) for Qualitative Drug Screening (L30574) Contractor Information Contractor Name First Coast Service Options, Inc. Back to Top Contractor Number 09102 Contractor Type MAC
The following chapter is called "Preimplantation Genetic Diagnosis (PGD)".
 Slide 1 Welcome to chapter 9. The following chapter is called "Preimplantation Genetic Diagnosis (PGD)". The author is Dr. Maria Lalioti. Slide 2 The learning objectives of this chapter are: To learn the
Slide 1 Welcome to chapter 9. The following chapter is called "Preimplantation Genetic Diagnosis (PGD)". The author is Dr. Maria Lalioti. Slide 2 The learning objectives of this chapter are: To learn the
March 19, 2014. Dear Dr. Duvall, Dr. Hambrick, and Ms. Smith,
 Dr. Daniel Duvall, Medical Officer Center for Medicare, Hospital and Ambulatory Policy Group Centers for Medicare and Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244 Dr. Edith Hambrick,
Dr. Daniel Duvall, Medical Officer Center for Medicare, Hospital and Ambulatory Policy Group Centers for Medicare and Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244 Dr. Edith Hambrick,
2013 Coding and Reimbursement Update
 2013 Coding and Reimbursement Update HCCA Compliance Institute 2013 Washington, DC April 21, 2013 2 Presented by Diana W. Voorhees, M.A., CLS, MT, SH, CLCP Principal/CEO DV & Associates, Inc. 5200 Highland
2013 Coding and Reimbursement Update HCCA Compliance Institute 2013 Washington, DC April 21, 2013 2 Presented by Diana W. Voorhees, M.A., CLS, MT, SH, CLCP Principal/CEO DV & Associates, Inc. 5200 Highland
GENETIC TESTING AND MARFAN SYNDROME
 GENETIC TESTING AND MARFAN SYNDROME Genetic testing for mutations in fibrillin-1 (FBN1) and other genes has become an important and reliable option to aid in the diagnosis of Marfan syndrome and related
GENETIC TESTING AND MARFAN SYNDROME Genetic testing for mutations in fibrillin-1 (FBN1) and other genes has become an important and reliable option to aid in the diagnosis of Marfan syndrome and related
! ~<?- OFFICE OF INSPECTOR GENERAL
 $'''''''"'~ DEPARTMENT OF HEALTH AND HUMAN SERVICES! ~
$'''''''"'~ DEPARTMENT OF HEALTH AND HUMAN SERVICES! ~
Test Information Sheet
 Test Information Sheet GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 888-729-1206 Fax: 301-710-6594 E-mail: wecare@genedx.com www.genedx.com/oncology OncoGene Dx: High/Moderate Risk Panel Sequence
Test Information Sheet GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 888-729-1206 Fax: 301-710-6594 E-mail: wecare@genedx.com www.genedx.com/oncology OncoGene Dx: High/Moderate Risk Panel Sequence
TABLE OF CONTENTS. Introduction...1. Chapter1 AdvancesinTreatment...2. Chapter2 MedicinesinDevelopment...11. Chapter3 ValueandSpending...
 CANCER TABLE OF CONTENTS Introduction...1 Chapter1 AdvancesinTreatment...2 Chapter2 MedicinesinDevelopment......11 Chapter3 ValueandSpending......15 Chapter4 Conclusion...22 INTRODUCTION Researchers and
CANCER TABLE OF CONTENTS Introduction...1 Chapter1 AdvancesinTreatment...2 Chapter2 MedicinesinDevelopment......11 Chapter3 ValueandSpending......15 Chapter4 Conclusion...22 INTRODUCTION Researchers and
U.S. Clinical Laboratory and Pathology Testing 2013-2015:
 U.S. Clinical Laboratory and Pathology Testing 2013-2015: Market Analysis, Trends, and Forecasts Now more than ever, it s critical to understand the industry at a macro level. Prepare your organization
U.S. Clinical Laboratory and Pathology Testing 2013-2015: Market Analysis, Trends, and Forecasts Now more than ever, it s critical to understand the industry at a macro level. Prepare your organization
August 29, 2014. Dear Administrator Tavenner:
 Ms. Marilyn Tavenner Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1612-P 7500 Security Boulevard Baltimore, MD 21244-1850 Submitted electronically:
Ms. Marilyn Tavenner Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1612-P 7500 Security Boulevard Baltimore, MD 21244-1850 Submitted electronically:
Appropriate Use of UDT to Improve Patient Care
 Published on OpioidRisk (http://www.opioidrisk.com) Home > Urine Drug Testing Urine Drug Testing This guide provides: Download Entire Guide [1] Appropriate use of Urine Drug Testing (UDT) to improve patient
Published on OpioidRisk (http://www.opioidrisk.com) Home > Urine Drug Testing Urine Drug Testing This guide provides: Download Entire Guide [1] Appropriate use of Urine Drug Testing (UDT) to improve patient
DUI in Southern Ohio MATT 2006
 DUI in Southern Ohio MATT 2006 Laureen J. Marinetti, M.S., Ph.D. Chief Toxicologist Montgomery County Coroner s s Office & Miami Valley Regional Crime Lab (MVRCL) Dayton, Ohio Region The MVRCL is a regional
DUI in Southern Ohio MATT 2006 Laureen J. Marinetti, M.S., Ph.D. Chief Toxicologist Montgomery County Coroner s s Office & Miami Valley Regional Crime Lab (MVRCL) Dayton, Ohio Region The MVRCL is a regional
CAP Accreditation Checklists 2015 Edition
 CAP Accreditation Checklists 2015 Edition The College of American Pathologists (CAP) accreditation checklists contain the CAP accreditation program requirements, developed on more than 50 years of insight
CAP Accreditation Checklists 2015 Edition The College of American Pathologists (CAP) accreditation checklists contain the CAP accreditation program requirements, developed on more than 50 years of insight
DRUG SCREENING POLICY AND PROCEDURES Effective August 31, 2015
 DRUG SCREENING POLICY AND PROCEDURES Effective August 31, 2015 The signatory parties recognize that drug abuse is an illness that creates serious problems for workers, their families, the workplace and
DRUG SCREENING POLICY AND PROCEDURES Effective August 31, 2015 The signatory parties recognize that drug abuse is an illness that creates serious problems for workers, their families, the workplace and
Hillsborough Community College - Ybor City Campus 1025C Laboratory Exercise 9: Testing Urine for the Presence of Drugs Introduction
 Hillsborough Community College - Ybor City Campus 1025C Laboratory Exercise 9: Testing Urine for the Presence of Drugs Introduction Drug testing beyond the health care and criminal justice systems has
Hillsborough Community College - Ybor City Campus 1025C Laboratory Exercise 9: Testing Urine for the Presence of Drugs Introduction Drug testing beyond the health care and criminal justice systems has
Generation Drug Free Workplace Policy for Contractor Personnel
 Generation Drug Free Workplace Policy for Contractor Personnel Revision: 04 Date: 05-06-15 Submitted: Electronic approval pages are archived in CSR Portfolio. Contact K. Kemp for a copy. Reviewed: Reviewed:
Generation Drug Free Workplace Policy for Contractor Personnel Revision: 04 Date: 05-06-15 Submitted: Electronic approval pages are archived in CSR Portfolio. Contact K. Kemp for a copy. Reviewed: Reviewed:
Drug Utilization Is On The Rise
 Objectives Identify the clinical issues related to opioid prescribing for chronic pain indications Define the clinical needs and expectations of urine drug testing in pain management Address preanalytical
Objectives Identify the clinical issues related to opioid prescribing for chronic pain indications Define the clinical needs and expectations of urine drug testing in pain management Address preanalytical
2015 Medicare Physician Fee Schedule Putting the Pieces Together for GI Colleen M. Schmitt, MD, MHA, FASGE ASGE President
 2015 Medicare Physician Fee Schedule Putting the Pieces Together for GI Colleen M. Schmitt, MD, MHA, FASGE ASGE President Glenn D. Littenberg, MD, MACP Chair, ASGE Practice Management Committee and CPT
2015 Medicare Physician Fee Schedule Putting the Pieces Together for GI Colleen M. Schmitt, MD, MHA, FASGE ASGE President Glenn D. Littenberg, MD, MACP Chair, ASGE Practice Management Committee and CPT
Objectives Role of Medical Genetics in Hearing Loss Evaluation. 5 y.o. boy with severe SNHL
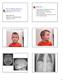 Objectives Role of Medical Genetics in Hearing Loss Evaluation Millan Patel, MD UBC Dept. of Medical Genetics October 22, 2010 Case presentation to illustrate importance of defining syndromic hearing loss
Objectives Role of Medical Genetics in Hearing Loss Evaluation Millan Patel, MD UBC Dept. of Medical Genetics October 22, 2010 Case presentation to illustrate importance of defining syndromic hearing loss
Number 2.04.513 Effective Date April 1, 2016 Revision Date(s) 05/04/16; 03/09/16; 04/24/15; 8/11/14 Replaces 2.04.98 (not adopted)
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES CODING DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES APPENDIX HISTORY Drug Testing in Pain Management and Substance Abuse Treatment
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES CODING DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES APPENDIX HISTORY Drug Testing in Pain Management and Substance Abuse Treatment
MAD-MR: 12-13 SPECIALTY SERVICES EFF: 9-1-12 MEDICATION ASSISTED TREATMENT FOR OPIOID ADDICTION INDEX
 INDEX 8.325.11 8.325.11.1 ISSUING AGENCY...1 8.325.11.2 SCOPE...1 8.325.11.3 STATUTORY AUTHORITY...1 8.325.11.4 DURATION...1 8.325.11.5 EFFECTIVE DATE...1 8.325.11.6 OBJECTIVE...1 8.325.11.7 DEFINITIONS...1
INDEX 8.325.11 8.325.11.1 ISSUING AGENCY...1 8.325.11.2 SCOPE...1 8.325.11.3 STATUTORY AUTHORITY...1 8.325.11.4 DURATION...1 8.325.11.5 EFFECTIVE DATE...1 8.325.11.6 OBJECTIVE...1 8.325.11.7 DEFINITIONS...1
SMF Awareness Seminar 2014
 SMF Awareness Seminar 2014 Clinical Evaluation for In Vitro Diagnostic Medical Devices Dr Jiang Naxin Health Sciences Authority Medical Device Branch 1 In vitro diagnostic product means Definition of IVD
SMF Awareness Seminar 2014 Clinical Evaluation for In Vitro Diagnostic Medical Devices Dr Jiang Naxin Health Sciences Authority Medical Device Branch 1 In vitro diagnostic product means Definition of IVD
PROVIDER POLICIES & PROCEDURES
 PROVIDER POLICIES & PROCEDURES BRCA GENETIC TESTING The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance Program (CMAP) on the requirements for
PROVIDER POLICIES & PROCEDURES BRCA GENETIC TESTING The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance Program (CMAP) on the requirements for
Assessment and Diagnosis of DSM-5 Substance-Related Disorders
 Assessment and Diagnosis of DSM-5 Substance-Related Disorders Jason H. King, PhD (listed on p. 914 of DSM-5 as a Collaborative Investigator) j.king@lecutah.com or 801-404-8733 www.lecutah.com D I S C L
Assessment and Diagnosis of DSM-5 Substance-Related Disorders Jason H. King, PhD (listed on p. 914 of DSM-5 as a Collaborative Investigator) j.king@lecutah.com or 801-404-8733 www.lecutah.com D I S C L
targeted therapy a guide for the patient
 targeted therapy FOR LUNG CANCER a guide for the patient TABLE OF CONTENTS lung cancer basics... 2-3 Gene changes... 4-5 Testing... 7-8 Targeted therapy... 9-11 Drugs Targeting EGFR... 12 Drugs Targeting
targeted therapy FOR LUNG CANCER a guide for the patient TABLE OF CONTENTS lung cancer basics... 2-3 Gene changes... 4-5 Testing... 7-8 Targeted therapy... 9-11 Drugs Targeting EGFR... 12 Drugs Targeting
ST. VINCENT'S. MEDICAL CENTER St. Vincent's Healthcare
 ST. VINCENT'S MEDICAL CENTER St. Vincent's Healthcare Medical Technology St. Vincent s Schools of Medical Science Throughout Northeast Florida and Southern Georgia, St. Vincent s HealthCare is well known
ST. VINCENT'S MEDICAL CENTER St. Vincent's Healthcare Medical Technology St. Vincent s Schools of Medical Science Throughout Northeast Florida and Southern Georgia, St. Vincent s HealthCare is well known
PtProtect Pain Medication Management Program Monitors Patient Compliance
 PHYSICIAN UPDATE 2013 Edition PtProtect Pain Medication Management Program Monitors Patient Compliance The PtProtect (Patient Protect) program offers pain medication management panels designed to improve
PHYSICIAN UPDATE 2013 Edition PtProtect Pain Medication Management Program Monitors Patient Compliance The PtProtect (Patient Protect) program offers pain medication management panels designed to improve
LAKE COUNTY SCHOOLS RECEIPT OF DRUG-FREE WORKPLACE POLICY
 LAKE COUNTY SCHOOLS RECEIPT OF DRUG-FREE WORKPLACE POLICY I hereby acknowledge receipt of the Lake County School Board s Drug-Free Workplace Policy. I Understand that the name, address and telephone number
LAKE COUNTY SCHOOLS RECEIPT OF DRUG-FREE WORKPLACE POLICY I hereby acknowledge receipt of the Lake County School Board s Drug-Free Workplace Policy. I Understand that the name, address and telephone number
Name of Policy: Urine Drug Testing in Pain Management
 Name of Policy: Urine Drug Testing in Pain Management Policy #: 566 Latest Review Date: February 2015 Category: Laboratory Policy Grade: B Background/Definitions: As a general rule, benefits are payable
Name of Policy: Urine Drug Testing in Pain Management Policy #: 566 Latest Review Date: February 2015 Category: Laboratory Policy Grade: B Background/Definitions: As a general rule, benefits are payable
OUTPATIENT SUBSTANCE USE DISORDER SERVICES FEE-FOR-SERVICE
 OUTPATIENT SUBSTANCE USE DISORDER SERVICES FEE-FOR-SERVICE Brief Coverage Statement Outpatient Substance Use Disorder (SUD) Fee-For-Service (FFS) Treatment Services are available for the treatment of substance
OUTPATIENT SUBSTANCE USE DISORDER SERVICES FEE-FOR-SERVICE Brief Coverage Statement Outpatient Substance Use Disorder (SUD) Fee-For-Service (FFS) Treatment Services are available for the treatment of substance
Epi procolon The Blood Test for Colorectal Cancer Screening
 Epi procolon The Blood Test for Colorectal Cancer Screening Epi procolon is an approved blood test for colorectal cancer screening. The US Preventive Services Task Force, the American Cancer Society and
Epi procolon The Blood Test for Colorectal Cancer Screening Epi procolon is an approved blood test for colorectal cancer screening. The US Preventive Services Task Force, the American Cancer Society and
THE FUTURE OF COVERAGE AND PAYMENT FOR PERSONALIZED MEDICINE DIAGNOSTICS
 THE FUTURE OF COVERAGE AND PAYMENT FOR PERSONALIZED MEDICINE DIAGNOSTICS EXECUTIVE SUMMARY THE FUTURE OF COVERAGE AND PAYMENT FOR PERSONALIZED MEDICINE DIAGNOSTICS EXECUTIVE SUMMARY The past decade has
THE FUTURE OF COVERAGE AND PAYMENT FOR PERSONALIZED MEDICINE DIAGNOSTICS EXECUTIVE SUMMARY THE FUTURE OF COVERAGE AND PAYMENT FOR PERSONALIZED MEDICINE DIAGNOSTICS EXECUTIVE SUMMARY The past decade has
What is New in Oncology. Michael J Messino, MD Cancer Care of WNC An affiliate of Mission hospitals
 What is New in Oncology Michael J Messino, MD Cancer Care of WNC An affiliate of Mission hospitals Personalized Medicine Personalized Genomics Genomic Medicine Precision Medicine Definition Application
What is New in Oncology Michael J Messino, MD Cancer Care of WNC An affiliate of Mission hospitals Personalized Medicine Personalized Genomics Genomic Medicine Precision Medicine Definition Application
A Decision Support Tool to Facilitate Cancer Risk Assessment and Referral for Genetics Services. Kristen Vogel Postula, MS, CGC & Leigh Baumgart, PhD
 A Decision Support Tool to Facilitate Cancer Risk Assessment and Referral for Genetics Services Kristen Vogel Postula, MS, CGC & Leigh Baumgart, PhD Importance of Family History Increasing awareness of
A Decision Support Tool to Facilitate Cancer Risk Assessment and Referral for Genetics Services Kristen Vogel Postula, MS, CGC & Leigh Baumgart, PhD Importance of Family History Increasing awareness of
Course Curriculum for Master Degree in Medical Laboratory Sciences/Clinical Biochemistry
 Course Curriculum for Master Degree in Medical Laboratory Sciences/Clinical Biochemistry The Master Degree in Medical Laboratory Sciences /Clinical Biochemistry, is awarded by the Faculty of Graduate Studies
Course Curriculum for Master Degree in Medical Laboratory Sciences/Clinical Biochemistry The Master Degree in Medical Laboratory Sciences /Clinical Biochemistry, is awarded by the Faculty of Graduate Studies
Genetic Testing: Scientific Background for Policymakers
 Genetic Testing: Scientific Background for Policymakers Amanda K. Sarata Specialist in Health Policy December 19, 2011 CRS Report for Congress Prepared for Members and Committees of Congress Congressional
Genetic Testing: Scientific Background for Policymakers Amanda K. Sarata Specialist in Health Policy December 19, 2011 CRS Report for Congress Prepared for Members and Committees of Congress Congressional
About Our Products. Blood Products. Purified Infectious/Inactivated Agents. Native & Recombinant Viral Proteins. DNA Controls and Primers for PCR
 About Our Products Purified Infectious/Inactivated Agents ABI produces a variety of specialized reagents, allowing researchers to choose the best preparations for their studies. Available reagents include
About Our Products Purified Infectious/Inactivated Agents ABI produces a variety of specialized reagents, allowing researchers to choose the best preparations for their studies. Available reagents include
OUTPATIENT SUBSTANCE USE DISORDER SERVICES FEE-FOR-SERVICE
 OUTPATIENT SUBSTANCE USE DISORDER SERVICES FEE-FOR-SERVICE BRIEF COVERAGE STATEMENT This benefit coverage standard describes outpatient Substance Use Disorder services (known as SUD Fee-For-Service (FFS)
OUTPATIENT SUBSTANCE USE DISORDER SERVICES FEE-FOR-SERVICE BRIEF COVERAGE STATEMENT This benefit coverage standard describes outpatient Substance Use Disorder services (known as SUD Fee-For-Service (FFS)
Affordable Care Act and Adolescents and Young Adults
 Affordable Care Act and Adolescents and Young Adults Overview of Summit Welcome and Introductions Affordable Care Act 101 Affordable Care Act and Impact on Adolescents and Young Adults Federal Update on
Affordable Care Act and Adolescents and Young Adults Overview of Summit Welcome and Introductions Affordable Care Act 101 Affordable Care Act and Impact on Adolescents and Young Adults Federal Update on
Section: Genetic Testing Last Reviewed Date: October 2015. Policy No: 73 Effective Date: November 1, 2015
 Medical Policy Manual Topic: Genetic Cancer Susceptibility Panels Using Next Generation Sequencing Date of Origin: July 2014 Section: Genetic Testing Last Reviewed Date: October 2015 Policy No: 73 Effective
Medical Policy Manual Topic: Genetic Cancer Susceptibility Panels Using Next Generation Sequencing Date of Origin: July 2014 Section: Genetic Testing Last Reviewed Date: October 2015 Policy No: 73 Effective
Implementation of Point-of-Care Rapid Urine Testing for Drugs of Abuse in the Emergency Department of an Academic Medical Center
 Clinical Chemistry / POC Testing f o r Dr u g s o f Ab u s e in t h e ED Implementation of Point-of-Care Rapid Urine Testing for Drugs of Abuse in the Emergency Department of an Academic Medical Center
Clinical Chemistry / POC Testing f o r Dr u g s o f Ab u s e in t h e ED Implementation of Point-of-Care Rapid Urine Testing for Drugs of Abuse in the Emergency Department of an Academic Medical Center
How To Get A Cell Print
 QUICK CELL CAPTURE AND CHARACTERIZATION GUIDE FOR CELLSEARCH CUSTOMERS CellSave EDTA Blood sample Rare cell capture Enumeration Single protein marker Cell capture for molecular characterization CELLSEARCH
QUICK CELL CAPTURE AND CHARACTERIZATION GUIDE FOR CELLSEARCH CUSTOMERS CellSave EDTA Blood sample Rare cell capture Enumeration Single protein marker Cell capture for molecular characterization CELLSEARCH
Medicare Coverage of Genomic Testing
 Medicare Coverage of Genomic Testing Louis B. Jacques, MD Director, DID/CAG/OCSQ With acknowledgements to Jeff Roche, MD Social Security Act 1862(a)(1)(A) Notwithstanding any other provision of this title,
Medicare Coverage of Genomic Testing Louis B. Jacques, MD Director, DID/CAG/OCSQ With acknowledgements to Jeff Roche, MD Social Security Act 1862(a)(1)(A) Notwithstanding any other provision of this title,
