1199 SEIU Benefit Funds Laboratory Management Program PRIOR AUTHORIZATION CODE LIST FOR OUTPATIENT MOLECULAR AND GENOMIC LABORATORY TESTS
|
|
|
- Jodie Campbell
- 8 years ago
- Views:
Transcription
1 1199 SEIU Benefit Funds Laboratory Management Program PRIOR AUTHORIZATION CODE LIST FOR OUTPATIENT MOLECULAR AND GENOMIC LABORATORY TESTS Effective January 1, 2016 Administered by evicore healthcare Refer to website at or call toll free at (844) ˆ Non-Infectious Molecular Pathology Procedures: Tier 1 Codes* ; full sequence and full duplication/deletion APC (adenomatous polyposis coli) (eg, familial adenomatosis polyposis [FAP], attenuated FAP) gene ; full gene sequence APC (adenomatous polyposis coli) (eg, familial adenomatosis polyposis [FAP], attenuated FAP) gene ; duplication/deletion Long QT syndrome gene analyses (eg, KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, KCNJ2, CACNA1C, CAV3, SCN4B, AKAP, SNTA1, and ANK2); full sequence Long QT syndrome gene analyses (eg, KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, KCNJ2, CACNA1C, CAV3, SCN4B, AKAP, SNTA1, and ANK2); duplication/deletion MGMT (O-6-methylguanine-DNA methyltransferase) (eg, glioblastoma multiforme), methylation ; full sequence and common duplication/deletion in BRCA1 (ie, exon 13 del 3.835kb, exon 13 dup 6kb, exon del 26kb, exon 22 del 510bp, exon 8-9 del 7.1kb) MTHFR (5,10-methylenetetrahydrofolate reductase) (eg, hereditary hypercoagulability) gene, common (eg, 677T, 1298C) ; 185delAG, 5385insC, 6174delT ; uncommon duplication/deletion MLH1 (mutl homolog 1, colon cancer, nonpolyposis type 2) (eg, hereditary non-polyposis colorectal cancer, Lynch syndrome) gene ; full sequence MLH1 (mutl homolog 1, colon cancer, nonpolyposis type 2) (eg, hereditary non-polyposis colorectal cancer, Lynch syndrome) gene ; duplication/deletion BRCA1 (breast cancer 1) (eg, hereditary breast and ovarian cancer) gene ; full sequence and common duplication/deletion (ie, exon 13 del 3.835kb, exon 13 dup 6kb, exon del 26kb, exon 22 del 510bp, exon 8-9 del 7.1kb) MSH2 (muts homolog 2, colon cancer, nonpolyposis type 1) (eg, hereditary non-polyposis colorectal cancer, Lynch syndrome) gene ; full sequence BRCA1 (breast cancer 1) (eg, hereditary breast and ovarian cancer) gene ; known familial variant BRCA2 (breast cancer 2) (eg, hereditary breast and ovarian cancer) gene ; full sequence BRCA2 (breast cancer 2) (eg, hereditary breast and ovarian cancer) gene ; known familial variant CFTR (cystic fibrosis transmembrane conductance regulator) (eg, cystic fibrosis) gene ; duplication/deletion CFTR (cystic fibrosis transmembrane conductance regulator) (eg, cystic fibrosis) gene ; full gene sequence CYP2C19 (cytochrome P450, family 2, subfamily C, polypeptide 19) (eg, drug metabolism), gene, common (eg, *2, *3, *4, *8, *17) MSH2 (muts homolog 2, colon cancer, nonpolyposis type 1) (eg, hereditary non-polyposis colorectal cancer, Lynch syndrome) gene ; duplication/deletion MSH6 (muts homolog 6 [E. coli]) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) gene ; full sequence MSH6 (muts homolog 6 [E. coli]) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) gene ; duplication/deletion PCA3/KLK3 (prostate cancer antigen 3 [non-protein coding]/kallikrein-related peptidase 3 [prostate specific antigen]) ratio (eg, prostate cancer) PMS2 (postmeiotic segregation increased 2 [S. cerevisiae]) (eg, hereditary non-polyposis colorectal cancer, Lynch syndrome) gene ; full sequence PMS2 (postmeiotic segregation increased 2 [S. cerevisiae]) (eg, hereditary non-polyposis colorectal cancer, Lynch syndrome) gene ; duplication/deletion Page 1
2 CYP2D6 (cytochrome P450, family 2, subfamily D, polypeptide 6) (eg, drug metabolism), gene, common (eg, *2, *3, *4, *5, *6, *9, *10, *17, *19, *29, *35, *41, *1XN, *2XN, *4XN) CYP2C9 (cytochrome P450, family 2, subfamily C, polypeptide 9) (eg, drug metabolism), gene, common (eg, *2, *3, *5, *6) Cytogenomic constitutional (genome-wide) microarray ; interrogation of genomic regions for copy number (eg, bacterial artificial chromosome [BAC] or oligo-based comparative genomic hybridization [CGH] microarray ) Cytogenomic constitutional (genome-wide) microarray ; interrogation of genomic regions for copy number and single nucleotide polymorphism (SNP) for chromosomal abnormalities PTEN (phosphatase and tensin homolog) (eg, Cowden syndrome, PTEN hamartoma tumor syndrome) gene ; full sequence PTEN (phosphatase and tensin homolog) (eg, Cowden syndrome, PTEN hamartoma tumor syndrome) gene ; duplication/deletion variant PMP22 (peripheral myelin protein 22) (eg, Charcot-Marie- Tooth, hereditary neuropathy with liability to pressure palsies) gene ; full sequence VKORC1 (vitamin K epoxide reductase complex, subunit 1) (eg, warfarin metabolism), gene, common variant(s) (eg, -1639G>A, c c>t) Molecular Pathology Procedures: Tier 2 and Unlisted Codes* Molecular pathology procedure, Level Molecular pathology procedure, Level Molecular pathology procedure, Level Molecular pathology procedure, Level Molecular pathology procedure, Level Molecular pathology procedure, Level Molecular pathology procedure, Level Unlisted molecular pathology procedure Molecular pathology procedure, Level Unlisted chemistry procedure Molecular pathology procedure, Level Aortic dysfunction or dilation (eg, Marfan syndrome, Loeys Dietz syndrome, Ehler Danlos syndrome type IV, arterial tortuosity syndrome); genomic sequence panel, must include sequencing of at least 9 genes, including FBN1, TGFBR1, TGFBR2, COL3A1, MYH11, ACTA2, SLC2A10, SMAD3, and MYLK Genomic Sequencing Procedures* 81434ˆ Hereditary retinal disorders (eg, retinitis pigmentosa, Leber congenital amaurosis, cone-rod dystrophy), genomic sequence panel, must include sequencing of at least 15 genes, including ABCA4, CNGA1, CRB1, EYS, PDE6A, PDE6B, PRPF31, PRPH2, RDH12, RHO, RP1, RP2, RPE65, RPGR, and USH2A Aortic dysfunction or dilation (eg, Marfan syndrome, Loeys Dietz syndrome, Ehler Danlos syndrome type IV, arterial tortuosity syndrome); duplication/deletion panel, must include analyses for TGFBR1, TGFBR2, MYH11, and COL3A Hereditary colon cancer disorders (eg, Lynch syndrome, PTEN hamartoma syndrome, Cowden syndrome, familial adenomatosis polyposis); genomic sequence panel, must include sequencing of at least 10 genes, including APC, BMPR1A, CDH1, MLH1, MSH2, MSH6, MUTYH, PTEN, SMAD4, and STK Ashkenazi Jewish associated disorders (eg, Bloom syndrome, Canavan disease, cystic fibrosis, familial dysautonomia, Fanconi anemia group C, Gaucher disease, Tay-Sachs disease), genomic sequence panel, must include sequencing of at least 9 genes, including ASPA, BLM, CFTR, FANCC, GBA, HEXA, IKBKAP, MCOLN1, and SMPD Hereditary colon cancer disorders (eg, Lynch syndrome, PTEN hamartoma syndrome, Cowden syndrome, familial adenomatosis polyposis); duplication/deletion panel, must include of at least 5 genes, including MLH1, MSH2, EPCAM, SMAD4, and STK ˆ Hereditary neuroendocrine tumor disorders (eg, medullary thyroid carcinoma, parathyroid carcinoma, malignant pheochromocytoma or paraganglioma); genomic sequence panel, must include sequencing of at least 6 genes, including MAX, SDHB, SDHC, SDHD, TMEM127, and VHL Page 2
3 , each comparator exome (eg, parents, siblings) (List separately in addition to code for primary procedure) heritable disorder or syndrome); re-evaluation of previously obtained exome sequence (eg, updated knowledge or unrelated condition/syndrome) 81438ˆ Hereditary neuroendocrine tumor disorders (eg, medullary thyroid carcinoma, parathyroid carcinoma, malignant pheochromocytoma or paraganglioma); duplication/deletion panel, must include analyses for SDHB, SDHC, SDHD, and VHL Nuclear encoded mitochondrial genes (eg, neurologic or myopathic phenotypes), genomic sequence panel, must include of at least 100 genes, including BCS1L, C10orf2, COQ2, COX10, DGUOK, MPV17, OPA1, PDSS2, POLG, POLG2, RRM2B, SCO1, SCO2, SLC25A4, SUCLA2, SUCLG1, TAZ, TK2, and TYMP Fetal chromosomal aneuploidy (eg, trisomy 21, monosomy X) genomic sequence panel, circulating cell-free fetal DNA in maternal blood, must include of chromosomes 13, 18, and 21, each comparator genome (eg, parents, siblings) (List separately in addition to code for primary procedure) heritable disorder or syndrome); re-evaluation of previously obtained genome sequence (eg, updated knowledge or unrelated condition/syndrome) Hearing loss (eg, nonsyndromic hearing loss, Usher syndrome, Pendred syndrome); genomic sequence panel, must include sequencing of at least 60 genes, including CDH23, CLRN1, GJB2, GPR98, MTRNR1, MYO7A, MYO15A, PCDH15, OTOF, SLC26A4, TMC1, TMPRSS3, USH1C, USH1G, USH2A, and WFS1 Hearing loss (eg, nonsyndromic hearing loss, Usher syndrome, Pendred syndrome); duplication/deletion panel, must include copy number analyses for STRC and DFNB1 deletions in GJB2 and GJB6 genes Hereditary breast cancer-related disorders (eg, hereditary breast cancer, hereditary ovarian cancer, hereditary endometrial cancer); genomic sequence panel, must include sequencing of at least 14 genes, including ATM, BRCA1, BRCA2, BRIP1, CDH1, MLH1, MSH2, MSH6, NBN, PALB2, PTEN, RAD51C, STK11, and TP53 Hereditary breast cancer-related disorders (eg, hereditary breast cancer, hereditary ovarian cancer, hereditary endometrial cancer); duplication/deletion panel, must include analyses for BRCA1, BRCA2, MLH1, MSH2, and STK ˆ Noonan spectrum disorders (eg, Noonan syndrome, cardio-facio-cutaneous syndrome, Costello syndrome, LEOPARD syndrome, Noonan-like syndrome), genomic sequence panel, must include sequencing of at least 12 genes, including BRAF, CBL, HRAS, KRAS, MAP2K1, MAP2K2, NRAS, PTPN11, RAF1, RIT1, SHOC2, and SOS1 Targeted genomic sequence panel, solid organ neoplasm, DNA, and RNA when performed, 5-50 genes (eg, ALK, BRAF, CDKN2A, EGFR, ERBB2, KIT, KRAS, NRAS, MET, PDGFRA, PDGFRB, PGR, PIK3CA, PTEN, RET), interrogation for sequence and copy number or rearrangements, if performed Targeted genomic sequence panel, hematolymphoid neoplasm or disorder, DNA, and RNA when performed, 5-50 genes (eg, BRAF, CEBPA, DNMT3A, EZH2, FLT3, IDH1, IDH2, JAK2, KRAS, KIT, MLL, NRAS, NPM1, NOTCH1), interrogation for sequence, and copy number or rearrangements, or isoform expression or mrna expression levels, if performed Targeted genomic sequence panel, solid organ or hematolymphoid neoplasm, DNA, and RNA when performed, 51 or greater genes (eg, ALK, BRAF, CDKN2A, CEBPA, DNMT3A, EGFR, ERBB2, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MLL, NPM1, NRAS, MET, NOTCH1, PDGFRA, PDGFRB, PGR, PIK3CA, PTEN, RET), interrogation for sequence and copy number or rearrangements, if performed Whole mitochondrial genome (eg, Leigh syndrome, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes [MELAS], myoclonic epilepsy with ragged-red fibers [MERFF], neuropathy, ataxia, and retinitis pigmentosa [NARP], Leber hereditary optic neuropathy [LHON]), genomic sequence, must include sequence of entire mitochondrial genome with heteroplasmy detection Whole mitochondrial genome large deletion panel (eg, Kearns-Sayre syndrome, chronic progressive external ophthalmoplegia), including heteroplasmy detection, if performed X-linked intellectual disability (XLID) (eg, syndromic and non-syndromic XLID); genomic sequence panel, must include sequencing of at least 60 genes, including ARX, ATRX, CDKL5, FGD1, FMR1, HUWE1, IL1RAPL, KDM5C, L1CAM, MECP2, MED12, MID1, OCRL, RPS6KA3, and SLC16A2 X-linked intellectual disability (XLID) (eg, syndromic and non-syndromic XLID); duplication/deletion gene, must include of at least 60 genes, including ARX, ATRX, CDKL5, FGD1, FMR1, HUWE1, IL1RAPL, KDM5C, L1CAM, MECP2, MED12, MID1, OCRL, RPS6KA3, and SLC16A2 Page 3
4 81490ˆ Multianalyte Assay with Alogrithmic Analyses (MAAA)* Autoimmune (rheumatoid arthritis), of 12 biomarkers using immunoassays, utilizing serum, prognostic algorithm reported as a disease activity score 81540ˆ Oncology (tumor of unknown origin), mrna, gene expression profiling by real-time RT-PCR of 92 genes (87 content and 5 housekeeping) to classify tumor into main cancer type and subtype, utilizing formalin-fixed paraffin-embedded tissue, algorithm reported as a probability of a predicted main cancer type and subtype 81493ˆ ˆ 81528ˆ 81535ˆ 81536ˆ Coronary artery disease, mrna, gene expression profiling by real-time RT-PCR of 23 genes, utilizing whole peripheral blood, algorithm reported as a risk score Oncology (tissue of origin), microarray gene expression profiling of > 2000 genes, utilizing formalin-fixed paraffin-embedded tissue, algorithm reported as tissue similarity scores Fetal aneuploidy (trisomy 21, 18, and 13) DNA sequence of selected regions using maternal plasma, algorithm reported as a risk score for each trisomy Oncology (breast), mrna, gene expression profiling by real-time RT-PCR of 21 genes, utilizing formalin-fixed paraffin embedded tissue, algorithm reported as recurrence score Oncology (colon), mrna, gene expression profiling by real-time RT-PCR of 12 genes (7 content and 5 housekeeping), utilizing formalinfixed paraffin-embedded tissue, algorithm reported as a recurrence score Oncology (colorectal) screening, quantitative realtime target and signal amplification of 10 DNA markers (KRAS mutations, promoter methylation of NDRG4 and BMP3) and fecal hemoglobin, utilizing stool, algorithm reported as a positive or negative result Oncology (gynecologic), live tumor cell culture and chemotherapeutic response by DAPI stain and morphology, predictive algorithm reported as a drug response score; first single drug or drug combination Oncology (gynecologic), live tumor cell culture and chemotherapeutic response by DAPI stain and morphology, predictive algorithm reported as a drug response score; each additional single drug or drug combination (List separately in addition to code for primary procedure) 81545ˆ 81595ˆ Oncology (thyroid), gene expression of 142 genes, utilizing fine needle aspirate, algorithm reported as a categorical result (eg, benign or suspicious) Cardiology (heart transplant), mrna, gene expression profiling by real-time quantitative PCR of 20 genes (11 content and 9 housekeeping), utilizing subfraction of peripheral blood, algorithm reported as a rejection risk score Unlisted multianalyte assay with algorithmic 0004M 0006M 0007M 0008M 0009Mˆ Scoliosis, DNA of 53 single nucleotide polymorphisms (SNPs), using saliva, prognostic algorithm reported as a risk score Oncology (hepatic), mrna expression levels of 161 genes, utilizing fresh hepatocellular carcinoma tumor tissue, with alpha-fetoprotein level, algorithm reported as a risk classifier Oncology (gastrointestinal neuroendocrine tumors), realtime PCR expression of 51 genes, utilizing whole peripheral blood, algorithm reported as a nomogram of tumor disease index Oncology (breast), mrna of 58 genes using hybrid capture, on formalin-fixed paraffin-embedded (FFPE) tissue, prognostic algorithm reported as a risk score Fetal aneuploidy (trisomy 21, and 18) DNA sequence of selected regions using maternal plasma, algorithm reported as a risk score for each trisomy 81538ˆ G0464 G9143 Oncology (lung), mass spectrometric 8-protein signature, including amyloid A, utilizing serum, prognostic and predictive algorithm reported as good versus poor overall survival Colorectal cancer screening; stool-based dna and fecal occult hemoglobin (e.g., kras, ndrg4 and bmp3) Warfarin responsiveness testing by genetic technique using any method, any number of specimen(s) 0010Mˆ S3846 S3852 S3721 Prostate cancer antigen 3 (pca3) testing S3854 S3800 S3840 Molecular Laboratory Procedures with HCPCS Codes* Genetic testing for amyotrophic lateral sclerosis (als) Dna for germline mutations of the ret proto-oncogene for susceptibility to multiple endocrine neoplasia type 2 S3861 S3865 Oncology (High-Grade Prostate Cancer), biochemical assay of four proteins (Total PSA, Free PSA, Intact PSA and human kallikrein 2 [hk2]) plus patient age, digital rectal examination status, and no history of positive prostate biopsy, utilizing plasma, prognostic algorithm reported as a probability score Genetic testing for hemoglobin e beta-thalassemia Dna for apoe epsilon 4 allele for susceptibility to alzheimer's disease Gene expression profiling panel for use in the management of breast cancer treatment Genetic testing, sodium channel, voltage-gated, type v, alpha subunit (scn5a) and for suspected brugada syndrome Comprehensive gene sequence for hypertrophic cardiomyopathy Page 4
5 S3841 Genetic testing for retinoblastoma S3866 Genetic for a specific gene mutation for hypertrophic cardiomyopathy (hcm) in an individual with a known hcm mutation in the family S3842 Genetic testing for von hippel-lindau disease S3870 Comparative genomic hybridization (cgh) microarray testing for developmental delay, autism spectrum disorder and/or intellectual disability S3845 Genetic testing for alpha-thalassemia S3890 Dna, fecal, for colorectal cancer screening *CPT is a registered trademark of the American Medical Association. Narrative is short description of code. ˆ Inclusive New 2016 CPT Codes Any CPT/HCPCS code within the range of and S3844-S3853 included with a required prior authorization code will be reviewed and those results are binding Please review list regularly as codes are subject to change Page 5
July 14, 2014. Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244
 ASCP s Statements before the Centers for Medicare and Medicaid Services Regarding Recommendations for Crosswalking/Gapfilling New CPT s for the CLFS for CY 2015 and the Revaluation of the Clinical Laboratory
ASCP s Statements before the Centers for Medicare and Medicaid Services Regarding Recommendations for Crosswalking/Gapfilling New CPT s for the CLFS for CY 2015 and the Revaluation of the Clinical Laboratory
JOHNS HOPKINS HEALTHCARE
 Page 1 of 15 ACTION: New Policy: Effective Date: 08/26/2003 Revising : Review Dates: 03/15/2004, 10/22/2004, Superseding : 10/21/2005, 10/19/2006, 01/07/2008, Archiving : 01/05/2009, 01/07/2011, 06/07/2013,
Page 1 of 15 ACTION: New Policy: Effective Date: 08/26/2003 Revising : Review Dates: 03/15/2004, 10/22/2004, Superseding : 10/21/2005, 10/19/2006, 01/07/2008, Archiving : 01/05/2009, 01/07/2011, 06/07/2013,
MEDICAL POLICY No. 91540-R10 GENETICS: COUNSELING, TESTING, SCREENING
 GENETICS: COUNSELING, TESTING, SCREENING Effective Date: February 26, 2015 Review Dates: 8/07, 10/07, 8/08, 8/09, 10/09, 2/10, 8/10, 8/11, 10/11, 10/12, 10/13, 11/14 Date Of Origin: August 8, 2007 Status:
GENETICS: COUNSELING, TESTING, SCREENING Effective Date: February 26, 2015 Review Dates: 8/07, 10/07, 8/08, 8/09, 10/09, 2/10, 8/10, 8/11, 10/11, 10/12, 10/13, 11/14 Date Of Origin: August 8, 2007 Status:
Molecular Pathology/Molecular Diagnostics/Genetic Testing
 Policy Number GEN01252012RP Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 01/28/2015 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable
Policy Number GEN01252012RP Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 01/28/2015 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable
TEST AND PRICE LIST FOR NEXT GENERATION SEQUENCING
 AIP ALK APC Mutation Detection Techniques Ai Saple Type TAT ATM BAP1 BLM BMPR1A BRCA1 BRCA2 BRIP1 BUB1B CDC73 CDH1 CDK4 CDKN1C CDKN2A CEBPA CEP57 CHEK2 CYLD DDB2 DICER1 DIS3L2 EGFR EPCAM ERCC2 ERCC3 ERCC4
AIP ALK APC Mutation Detection Techniques Ai Saple Type TAT ATM BAP1 BLM BMPR1A BRCA1 BRCA2 BRIP1 BUB1B CDC73 CDH1 CDK4 CDKN1C CDKN2A CEBPA CEP57 CHEK2 CYLD DDB2 DICER1 DIS3L2 EGFR EPCAM ERCC2 ERCC3 ERCC4
CPT Code Changes for 2015 PATHOLOGY/LABORATORY
 Changes for 2015 PATHOLOGY/LABORATORY Rick Oliver, CPCO, CPC, MT(ASCP) Compliance Director- Laboratory and Pathology McKesson Business Performance Services This commentary is a summary prepared by McKesson
Changes for 2015 PATHOLOGY/LABORATORY Rick Oliver, CPCO, CPC, MT(ASCP) Compliance Director- Laboratory and Pathology McKesson Business Performance Services This commentary is a summary prepared by McKesson
RMHP Prior Authorization List Effective October 1, 2015 rev 7/18/2016
 Effective October 1, 2015 rev 7/18/2016 *This list applies to all services for which RMHP is the primary payer. * Services that are not a benefit of the Member's Evidence of Coverage will not be authorized.
Effective October 1, 2015 rev 7/18/2016 *This list applies to all services for which RMHP is the primary payer. * Services that are not a benefit of the Member's Evidence of Coverage will not be authorized.
Health First Health Plans Authorization List
 Health First Health Plans Authorization List Effective April 15, 2016 IMPORTANT CONTACTS FOR AUTHORIZATIONS SUBMITTED TO THE HEALTH PLAN Submit online requests via your secure account at MyHFHP.org/login
Health First Health Plans Authorization List Effective April 15, 2016 IMPORTANT CONTACTS FOR AUTHORIZATIONS SUBMITTED TO THE HEALTH PLAN Submit online requests via your secure account at MyHFHP.org/login
Section: Genetic Testing Last Reviewed Date: October 2015. Policy No: 73 Effective Date: November 1, 2015
 Medical Policy Manual Topic: Genetic Cancer Susceptibility Panels Using Next Generation Sequencing Date of Origin: July 2014 Section: Genetic Testing Last Reviewed Date: October 2015 Policy No: 73 Effective
Medical Policy Manual Topic: Genetic Cancer Susceptibility Panels Using Next Generation Sequencing Date of Origin: July 2014 Section: Genetic Testing Last Reviewed Date: October 2015 Policy No: 73 Effective
Launching a Cancer Genetic Laboratory to Enhance Diagnosis and Treatment
 Launching a Cancer Genetic Laboratory to Enhance Diagnosis and Treatment Arthur L. Beaudet, M.D. Department of Molecular and Human Genetics Baylor College of Medicine ORIGIN AND PRECEDENT Decades of experience
Launching a Cancer Genetic Laboratory to Enhance Diagnosis and Treatment Arthur L. Beaudet, M.D. Department of Molecular and Human Genetics Baylor College of Medicine ORIGIN AND PRECEDENT Decades of experience
GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110
 GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110 COVERAGE: Pre- and post-genetic test counseling may be eligible for coverage in addition to the genetic
GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110 COVERAGE: Pre- and post-genetic test counseling may be eligible for coverage in addition to the genetic
Test Information Sheet
 Test Information Sheet GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 888-729-1206 Fax: 301-710-6594 E-mail: wecare@genedx.com www.genedx.com/oncology OncoGene Dx: High/Moderate Risk Panel Sequence
Test Information Sheet GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 888-729-1206 Fax: 301-710-6594 E-mail: wecare@genedx.com www.genedx.com/oncology OncoGene Dx: High/Moderate Risk Panel Sequence
2014 CPT Code Updates
 2014 CPT Code Updates This publication is a summary of The American Medical Association Current Procedural Terminology (CPT) codes published in ARUP's Laboratory Test Directory effective January 1, 2014.
2014 CPT Code Updates This publication is a summary of The American Medical Association Current Procedural Terminology (CPT) codes published in ARUP's Laboratory Test Directory effective January 1, 2014.
NEW YORK STATE MEDICAID PROGRAM LABORATORY PROCEDURE CODES
 NEW YORK STATE MEDICAID PROGRAM LABORATORY PROCEDURE S Table of Contents GENERAL INFORMATION AND RULES...3 ORGAN OR DISEASE ORIENTED PANELS... 14 THERAPEUTIC DRUG ASSAYS... 15 EVOCATIVE/SUPPRESSION TESTING...
NEW YORK STATE MEDICAID PROGRAM LABORATORY PROCEDURE S Table of Contents GENERAL INFORMATION AND RULES...3 ORGAN OR DISEASE ORIENTED PANELS... 14 THERAPEUTIC DRUG ASSAYS... 15 EVOCATIVE/SUPPRESSION TESTING...
La diagnostica molecolare nelle neoplasie colo-rettali. A do Scarpa. U iversita di Vero a
 La diagnostica molecolare nelle neoplasie colo-rettali C-NET A do Scarpa e Dipartime to Pato ogia e U iversita di Vero a La stessa malattia puo avere diverse patogenesi cucchiaio moneta forchetta Gastrointestinal
La diagnostica molecolare nelle neoplasie colo-rettali C-NET A do Scarpa e Dipartime to Pato ogia e U iversita di Vero a La stessa malattia puo avere diverse patogenesi cucchiaio moneta forchetta Gastrointestinal
THE LEADING EDGE FALL 2015 ISSUE. Welcome... 1. ICD-10 is Here for Pathology... 2. 2016 CLFS for Drugs of Abuse Testing... 5
 FALL 2015 ISSUE Welcome... 1 ICD-10 is Here for Pathology... 2 2016 CLFS for Drugs of Abuse Testing... 5 Common Causes of Enrollment Delays... 10 ICD-10 is Here!... 12 High Deductible Plans Grow Despite
FALL 2015 ISSUE Welcome... 1 ICD-10 is Here for Pathology... 2 2016 CLFS for Drugs of Abuse Testing... 5 Common Causes of Enrollment Delays... 10 ICD-10 is Here!... 12 High Deductible Plans Grow Despite
G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics
 G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics One of every 500 newborns has bilateral permanent sensorineural hearing loss 40 db which makes it the most common
G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics One of every 500 newborns has bilateral permanent sensorineural hearing loss 40 db which makes it the most common
Genetic Testing for Lynch Syndrome/Colorectal Cancer and Polyposis Syndromes
 Genetic Testing for Lynch Syndrome/Colorectal Cancer and Polyposis Syndromes Policy Number: Original Effective Date: MM.02.007 09/01/2011 Line(s) of Business: Current Effective Date: HMO; PPO 09/01/2011
Genetic Testing for Lynch Syndrome/Colorectal Cancer and Polyposis Syndromes Policy Number: Original Effective Date: MM.02.007 09/01/2011 Line(s) of Business: Current Effective Date: HMO; PPO 09/01/2011
Common Cancers & Hereditary Syndromes
 Common Cancers & Hereditary Syndromes Elizabeth Hoodfar, MS, LCGC Regional Cancer Genetics Coordinator Kaiser Permanente Northern California Detect clinical characteristics of hereditary cancer syndromes.
Common Cancers & Hereditary Syndromes Elizabeth Hoodfar, MS, LCGC Regional Cancer Genetics Coordinator Kaiser Permanente Northern California Detect clinical characteristics of hereditary cancer syndromes.
CLINICAL GUIDELINES. Lab Management Program. Effective January 15, 2016
 CLINICAL GUIDELINES Lab Management Program Effective January 15, 2016 CareCore National, LLC d/b/a evicore healthcare (evicore) Clinical guidelines for medical necessity review of lab management services.
CLINICAL GUIDELINES Lab Management Program Effective January 15, 2016 CareCore National, LLC d/b/a evicore healthcare (evicore) Clinical guidelines for medical necessity review of lab management services.
Hereditary Breast Cancer Panels. High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel
 P A T I E N T G U I D E Hereditary Breast Cancer Panels High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel B a y l o r M i r a c a G e n e t i c s L a b o r a t
P A T I E N T G U I D E Hereditary Breast Cancer Panels High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel B a y l o r M i r a c a G e n e t i c s L a b o r a t
Name of Policy: Genetic Testing for Inherited Cancer Predisposition and/or Pharmacogenetics related to Cancer Treatment
 Name of Policy: Genetic Testing for Inherited Cancer Predisposition and/or Pharmacogenetics related to Cancer Treatment Policy #: 133 Latest Review Date: April 2015 Category: Laboratory Policy Grade: D
Name of Policy: Genetic Testing for Inherited Cancer Predisposition and/or Pharmacogenetics related to Cancer Treatment Policy #: 133 Latest Review Date: April 2015 Category: Laboratory Policy Grade: D
Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss
 Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_hereditary_hearing_loss 10/2013 8/2015 8/2016
Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_hereditary_hearing_loss 10/2013 8/2015 8/2016
Test Information Sheet
 Test Information Sheet GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 888-729-1206 Fax: 301-710-6594 E-mail: wecare@genedx.com www.genedx.com/oncology OncoGene Dx: Breast/Ovarian Cancer Panel Sequence
Test Information Sheet GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 888-729-1206 Fax: 301-710-6594 E-mail: wecare@genedx.com www.genedx.com/oncology OncoGene Dx: Breast/Ovarian Cancer Panel Sequence
CancerTREATMENT NGS+ NSCLC Summary Report Page 1 of 7 PATIENT SPECIMEN PHYSICIAN
 Page 1 of 7 POSITIVE TEST RESULTS Biomarker Result DETECTED NEGATIVE TEST RESULTS FDA Approved Therapies Targeting Molecular Pathway TEST DESCRIPTION: CancerTREATMENT NGS+ uses Next Generation Sequencing
Page 1 of 7 POSITIVE TEST RESULTS Biomarker Result DETECTED NEGATIVE TEST RESULTS FDA Approved Therapies Targeting Molecular Pathway TEST DESCRIPTION: CancerTREATMENT NGS+ uses Next Generation Sequencing
Corporate Medical Policy Carrier Testing for Genetic Disease
 Corporate Medical Policy Carrier Testing for Genetic Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: carrier_testing_for_genetic_disease 12/2013 8/2015 8/2016 8/2015 Description
Corporate Medical Policy Carrier Testing for Genetic Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: carrier_testing_for_genetic_disease 12/2013 8/2015 8/2016 8/2015 Description
6/10/2015. Hereditary Predisposition for Breast Cancer: Looking at BRCA1/BRCA2 Testing & Beyond. Hereditary Cancers. BRCA1 and BRCA2 Review
 Hereditary Predisposition for Breast Cancer: Looking at BRCA1/BRCA2 Testing & Beyond Arturo Anguiano MD, FACMG International Medical Director, Medical Affairs Vice Chairman, Genetics; Medical Director,
Hereditary Predisposition for Breast Cancer: Looking at BRCA1/BRCA2 Testing & Beyond Arturo Anguiano MD, FACMG International Medical Director, Medical Affairs Vice Chairman, Genetics; Medical Director,
MEDICAL POLICY Genetic Testing
 POLICY.........PG0041 EFFECTIVE......01/01/12 LAST REVIEW... 04/22/16 MEDICAL POLICY Genetic Testing GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by
POLICY.........PG0041 EFFECTIVE......01/01/12 LAST REVIEW... 04/22/16 MEDICAL POLICY Genetic Testing GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by
Nuovi Scenari in Oncologia. G. Zoppoli X-Files in Nutrizione Clinica e Artificiale, 08/06/2012
 Nuovi Scenari in Oncologia G. Zoppoli X-Files in Nutrizione Clinica e Artificiale, 08/06/2012 WHAT is cancer? «Cancer is a genetic disease of the somatic cell» [B. Vogelstein] Ten years ago Now [Cell.
Nuovi Scenari in Oncologia G. Zoppoli X-Files in Nutrizione Clinica e Artificiale, 08/06/2012 WHAT is cancer? «Cancer is a genetic disease of the somatic cell» [B. Vogelstein] Ten years ago Now [Cell.
Genetic Tests and Disease States Included in Humana s Genetic Guidance Program
 81161 81200 81201 Dystrophin (DMD) (e.g., Duchenne/Becker muscular dystrophy) deletion analysis and duplication analysis, if performed Aspartoacylase (ASPA) (e.g., Canavan disease) gene analysis, common
81161 81200 81201 Dystrophin (DMD) (e.g., Duchenne/Becker muscular dystrophy) deletion analysis and duplication analysis, if performed Aspartoacylase (ASPA) (e.g., Canavan disease) gene analysis, common
CPT Generic Test Test Description
 CPT Generic Test Test Description Code Name 1 81200 ASPA Gene Test for leukodystrophies (Canavan disease), an autosomal recessive disease (most common in Ashkenazi Jewish population) with life expectancy
CPT Generic Test Test Description Code Name 1 81200 ASPA Gene Test for leukodystrophies (Canavan disease), an autosomal recessive disease (most common in Ashkenazi Jewish population) with life expectancy
Genetic Testing for Familial Adenomatous Polyposis and MYH-Associated Polyposis (Lynch Syndrome)
 Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
LAB MANAGEMENT CRITERIA. 1199SEIU Funds. Effective July 16, 2015
 LAB MANAGEMENT CRITERIA 1199SEIU Funds Effective July 16, 2015 Clinical criteria for medical necessity review of lab management services. Please note the following: CPT Copyright 2015 American Medical
LAB MANAGEMENT CRITERIA 1199SEIU Funds Effective July 16, 2015 Clinical criteria for medical necessity review of lab management services. Please note the following: CPT Copyright 2015 American Medical
PROVIDER POLICIES & PROCEDURES
 PROVIDER POLICIES & PROCEDURES BRCA GENETIC TESTING The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance Program (CMAP) on the requirements for
PROVIDER POLICIES & PROCEDURES BRCA GENETIC TESTING The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance Program (CMAP) on the requirements for
Hereditary Breast Cancer Testing. Diagnostic
 Hereditary Cancer Testing Diagnostic New solutions for hereditary breast cancer. Identifying and understanding the genetic contribution to breast cancer allows for individualized disease management and
Hereditary Cancer Testing Diagnostic New solutions for hereditary breast cancer. Identifying and understanding the genetic contribution to breast cancer allows for individualized disease management and
Genetic Testing for Hereditary Hearing Loss
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Genetic Testing for Hereditary Hearing Loss Number 12.04.87 Effective
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Genetic Testing for Hereditary Hearing Loss Number 12.04.87 Effective
Invasive Prenatal (Fetal) Diagnostic Testing
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Invasive Prenatal (Fetal) Diagnostic Testing Number 12.04.116
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Invasive Prenatal (Fetal) Diagnostic Testing Number 12.04.116
Médecine de précision médecine personnalisée en Oncologie. Fabien Calvo, Directeur Recherche et Innovation, INCa, Directeur ITMO Cancer, AVIESAN
 Médecine de précision médecine personnalisée en Oncologie Fabien Calvo, Directeur Recherche et Innovation, INCa, Directeur ITMO Cancer, AVIESAN Successful targeted drug development Rapid identification
Médecine de précision médecine personnalisée en Oncologie Fabien Calvo, Directeur Recherche et Innovation, INCa, Directeur ITMO Cancer, AVIESAN Successful targeted drug development Rapid identification
Clinical Policy Title: Genomic tests in sensorineural hearing loss
 Clinical Policy Title: Genomic tests in sensorineural hearing loss Clinical Policy Number: 02.01.18 Effective Date: January 1, 2016 Initial Review Date: September 16, 2015 Most Recent Review Date: September
Clinical Policy Title: Genomic tests in sensorineural hearing loss Clinical Policy Number: 02.01.18 Effective Date: January 1, 2016 Initial Review Date: September 16, 2015 Most Recent Review Date: September
Medical Policy Manual. Topic: Genetic Testing for Hereditary Breast and/or Ovarian Cancer. Date of Origin: January 27, 2011
 Medical Policy Manual Topic: Genetic Testing for Hereditary Breast and/or Ovarian Cancer Date of Origin: January 27, 2011 Section: Genetic Testing Last Reviewed Date: May 2015 Policy No: 02 Effective Date:
Medical Policy Manual Topic: Genetic Testing for Hereditary Breast and/or Ovarian Cancer Date of Origin: January 27, 2011 Section: Genetic Testing Last Reviewed Date: May 2015 Policy No: 02 Effective Date:
Contents. molecular biology techniques. - Mutations in Factor II. - Mutations in MTHFR gene. - Breast cencer genes. - p53 and breast cancer
 Contents Introduction: biology and medicine, two separated compartments What we need to know: - boring basics in DNA/RNA structure and overview of particular aspects of molecular biology techniques - How
Contents Introduction: biology and medicine, two separated compartments What we need to know: - boring basics in DNA/RNA structure and overview of particular aspects of molecular biology techniques - How
A Decision Support Tool to Facilitate Cancer Risk Assessment and Referral for Genetics Services. Kristen Vogel Postula, MS, CGC & Leigh Baumgart, PhD
 A Decision Support Tool to Facilitate Cancer Risk Assessment and Referral for Genetics Services Kristen Vogel Postula, MS, CGC & Leigh Baumgart, PhD Importance of Family History Increasing awareness of
A Decision Support Tool to Facilitate Cancer Risk Assessment and Referral for Genetics Services Kristen Vogel Postula, MS, CGC & Leigh Baumgart, PhD Importance of Family History Increasing awareness of
Reconsideration Code 86386. Reconsideration Code Description Nuclear Matrix Protein 22 (NMP22), qualitative
 Calendar Year 2013 Centers for Medicare and Medicaid Services (CMS) New and Reconsidered Clinical Laboratory Fee Schedule (CLFS) Test Codes And Final Payment Determinations Reconsideration Code 86386 Reconsideration
Calendar Year 2013 Centers for Medicare and Medicaid Services (CMS) New and Reconsidered Clinical Laboratory Fee Schedule (CLFS) Test Codes And Final Payment Determinations Reconsideration Code 86386 Reconsideration
Microsatellite Instability (MSI) A New Paradigm in Cancer Treatment. Lynch Syndrome OUTLINE. GI Molecular Pathology
 OUTLINE GI Molecular Pathology Microsatellite Instability and Lynch Syndrome GI Cancer Genotyping KRAS Mutations in Colorectal Cancer A. John Iafrate MD-PhD Department of Pathology Massachusetts General
OUTLINE GI Molecular Pathology Microsatellite Instability and Lynch Syndrome GI Cancer Genotyping KRAS Mutations in Colorectal Cancer A. John Iafrate MD-PhD Department of Pathology Massachusetts General
Prevalenza delle mutazioni TMEM127 nel feocromocitoma sporadico
 Prevalenza delle mutazioni TMEM127 nel feocromocitoma sporadico Giuseppe Opocher Veneto Institute of Oncology and Department of Medical and Surgical Sciences, University of Padova, Italy Sympathetic paraganglia
Prevalenza delle mutazioni TMEM127 nel feocromocitoma sporadico Giuseppe Opocher Veneto Institute of Oncology and Department of Medical and Surgical Sciences, University of Padova, Italy Sympathetic paraganglia
Name of Policy: Genetic Testing for Inherited Cancer Predisposition and/or Pharmacogenetics related to Cancer Treatment
 Name of Policy: Genetic Testing for Inherited Cancer Predisposition and/or Pharmacogenetics related to Cancer Treatment Policy #: 133 Latest Review Date: April 2015 Category: Laboratory Policy Grade: D
Name of Policy: Genetic Testing for Inherited Cancer Predisposition and/or Pharmacogenetics related to Cancer Treatment Policy #: 133 Latest Review Date: April 2015 Category: Laboratory Policy Grade: D
Genomic Medicine The Future of Cancer Care. Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America
 Genomic Medicine The Future of Cancer Care Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America Personalized Medicine Personalized health care is a broad term for interventions
Genomic Medicine The Future of Cancer Care Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America Personalized Medicine Personalized health care is a broad term for interventions
National Medical Policy
 National Medical Policy Subject: Policy Number: Genetic Testing for BRCA1 and BRCA2 NMP136 Effective Date*: April 2004 Updated: September 2015 This National Medical Policy is subject to the terms in the
National Medical Policy Subject: Policy Number: Genetic Testing for BRCA1 and BRCA2 NMP136 Effective Date*: April 2004 Updated: September 2015 This National Medical Policy is subject to the terms in the
Gene mutation and molecular medicine Chapter 15
 Gene mutation and molecular medicine Chapter 15 Lecture Objectives What Are Mutations? How Are DNA Molecules and Mutations Analyzed? How Do Defective Proteins Lead to Diseases? What DNA Changes Lead to
Gene mutation and molecular medicine Chapter 15 Lecture Objectives What Are Mutations? How Are DNA Molecules and Mutations Analyzed? How Do Defective Proteins Lead to Diseases? What DNA Changes Lead to
***Next Generation sequencing testing options available effective April 18 th, 2016***
 ***Next Generation sequencing testing options available effective April 18 th, 2016*** Genetic Test Next Generation Sequencing NF1-RASopathy Panel Testing NF1-only NGS testing and copy number analysis
***Next Generation sequencing testing options available effective April 18 th, 2016*** Genetic Test Next Generation Sequencing NF1-RASopathy Panel Testing NF1-only NGS testing and copy number analysis
Ovarian Cancer Genetic Testing: Why, When, How?
 Ovarian Cancer Genetic Testing: Why, When, How? Jeffrey Dungan, MD Associate Professor Division of Clinical Genetics Department of Obstetrics & Gynecology Northwestern University Feinberg School of Medicine
Ovarian Cancer Genetic Testing: Why, When, How? Jeffrey Dungan, MD Associate Professor Division of Clinical Genetics Department of Obstetrics & Gynecology Northwestern University Feinberg School of Medicine
Objectives Role of Medical Genetics in Hearing Loss Evaluation. 5 y.o. boy with severe SNHL
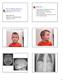 Objectives Role of Medical Genetics in Hearing Loss Evaluation Millan Patel, MD UBC Dept. of Medical Genetics October 22, 2010 Case presentation to illustrate importance of defining syndromic hearing loss
Objectives Role of Medical Genetics in Hearing Loss Evaluation Millan Patel, MD UBC Dept. of Medical Genetics October 22, 2010 Case presentation to illustrate importance of defining syndromic hearing loss
Molecular Genetic Testing in Public Health and Clinical Settings
 Molecular Genetic Testing in Public Health and Clinical Settings Ira M. Lubin, PhD, FACMG Division of Laboratory Systems NCPDCID, CCID Centers for Disease Control and Prevention Atlanta, Georgia Disclaimers
Molecular Genetic Testing in Public Health and Clinical Settings Ira M. Lubin, PhD, FACMG Division of Laboratory Systems NCPDCID, CCID Centers for Disease Control and Prevention Atlanta, Georgia Disclaimers
Breast cancer and the role of low penetrance alleles: a focus on ATM gene
 Modena 18-19 novembre 2010 Breast cancer and the role of low penetrance alleles: a focus on ATM gene Dr. Laura La Paglia Breast Cancer genetic Other BC susceptibility genes TP53 PTEN STK11 CHEK2 BRCA1
Modena 18-19 novembre 2010 Breast cancer and the role of low penetrance alleles: a focus on ATM gene Dr. Laura La Paglia Breast Cancer genetic Other BC susceptibility genes TP53 PTEN STK11 CHEK2 BRCA1
Name of Policy: Genetic and Protein Biomarkers for the Diagnosis and Cancer Risk Assessment of Prostate Cancer
 Name of Policy: Genetic and Protein Biomarkers for the Diagnosis and Cancer Risk Assessment of Prostate Cancer Policy #: 534 Latest Review Date: June 2015 Category: Laboratory Policy Grade: B Background/Definitions:
Name of Policy: Genetic and Protein Biomarkers for the Diagnosis and Cancer Risk Assessment of Prostate Cancer Policy #: 534 Latest Review Date: June 2015 Category: Laboratory Policy Grade: B Background/Definitions:
Progress and Prospects in Ovarian Cancer Screening and Prevention
 Progress and Prospects in Ovarian Cancer Screening and Prevention Rebecca Stone, MD MS Assistant Professor Kelly Gynecologic Oncology Service The Johns Hopkins Hospital 1 No Disclosures 4/12/2016 2 Ovarian
Progress and Prospects in Ovarian Cancer Screening and Prevention Rebecca Stone, MD MS Assistant Professor Kelly Gynecologic Oncology Service The Johns Hopkins Hospital 1 No Disclosures 4/12/2016 2 Ovarian
micrornas Non protein coding, endogenous RNAs of 21-22nt length Evolutionarily conserved
 microrna 2 micrornas Non protein coding, endogenous RNAs of 21-22nt length Evolutionarily conserved Regulate gene expression by binding complementary regions at 3 regions of target mrnas Act as negative
microrna 2 micrornas Non protein coding, endogenous RNAs of 21-22nt length Evolutionarily conserved Regulate gene expression by binding complementary regions at 3 regions of target mrnas Act as negative
Molecular Diagnostics in Thyroid Cancer
 Disclosure Nothing to disclose Jonathan George, MD, MPH Assistant Professor Head and Neck Oncologic & Endocrine Surgery Molecular Diagnostics in Thyroid Cancer Current Practices & Future Trends UCSF Medical
Disclosure Nothing to disclose Jonathan George, MD, MPH Assistant Professor Head and Neck Oncologic & Endocrine Surgery Molecular Diagnostics in Thyroid Cancer Current Practices & Future Trends UCSF Medical
Prior Authorization Updates
 Prior Authorization Updates Managed Health Services (MHS) requires prior authorization as a condition of payment for many services. This Notice contains information regarding such prior authorization requirements
Prior Authorization Updates Managed Health Services (MHS) requires prior authorization as a condition of payment for many services. This Notice contains information regarding such prior authorization requirements
Risk stratification for colorectal cancer especially: the difference between sporadic disease and polyposis syndromes. Dr. med. Henrik Csaba Horváth
 Risk stratification for colorectal cancer especially: the difference between sporadic disease and polyposis syndromes Dr. med. Henrik Csaba Horváth Why is risk stratification for colorectal cancer (CRC)
Risk stratification for colorectal cancer especially: the difference between sporadic disease and polyposis syndromes Dr. med. Henrik Csaba Horváth Why is risk stratification for colorectal cancer (CRC)
The following chapter is called "Preimplantation Genetic Diagnosis (PGD)".
 Slide 1 Welcome to chapter 9. The following chapter is called "Preimplantation Genetic Diagnosis (PGD)". The author is Dr. Maria Lalioti. Slide 2 The learning objectives of this chapter are: To learn the
Slide 1 Welcome to chapter 9. The following chapter is called "Preimplantation Genetic Diagnosis (PGD)". The author is Dr. Maria Lalioti. Slide 2 The learning objectives of this chapter are: To learn the
What is Cancer? Cancer is a genetic disease: Cancer typically involves a change in gene expression/function:
 Cancer is a genetic disease: Inherited cancer Sporadic cancer What is Cancer? Cancer typically involves a change in gene expression/function: Qualitative change Quantitative change Any cancer causing genetic
Cancer is a genetic disease: Inherited cancer Sporadic cancer What is Cancer? Cancer typically involves a change in gene expression/function: Qualitative change Quantitative change Any cancer causing genetic
Corporate Medical Policy Molecular Markers in Fine Needle Aspirates of the Thyroid
 Corporate Medical Policy Molecular Markers in Fine Needle Aspirates of the Thyroid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_markers_in_fine_needle_aspirates_of_the_thyroid
Corporate Medical Policy Molecular Markers in Fine Needle Aspirates of the Thyroid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_markers_in_fine_needle_aspirates_of_the_thyroid
Genes and Cancer. What are genes? Dominant vs. recessive genes
 Genes and Cancer Advances in science have improved our knowledge of the inner workings of cells, the basic building blocks of the body. All living things are made of cells. Complex animals such as humans
Genes and Cancer Advances in science have improved our knowledge of the inner workings of cells, the basic building blocks of the body. All living things are made of cells. Complex animals such as humans
Description of Procedure or Service. gene_based_tests_for_screening_detection_or_management_of_prostate_cancer 4/2009 8/2015 8/2016 8/2015
 Corporate Medical Policy Gene-Based Tests for Screening, Detection, and/or Management File Name: Origination: Last CAP Review: Next CAP Review: Last Review: gene_based_tests_for_screening_detection_or_management_of_prostate_cancer
Corporate Medical Policy Gene-Based Tests for Screening, Detection, and/or Management File Name: Origination: Last CAP Review: Next CAP Review: Last Review: gene_based_tests_for_screening_detection_or_management_of_prostate_cancer
Epi procolon The Blood Test for Colorectal Cancer Screening
 Epi procolon The Blood Test for Colorectal Cancer Screening Epi procolon is an approved blood test for colorectal cancer screening. The US Preventive Services Task Force, the American Cancer Society and
Epi procolon The Blood Test for Colorectal Cancer Screening Epi procolon is an approved blood test for colorectal cancer screening. The US Preventive Services Task Force, the American Cancer Society and
The Genetics of Early- Onset Breast Cancer. Cecelia Bellcross, Ph.D., M.S.,C.G.C. Department of Human Genetics Emory University School of Medicine
 The Genetics of Early- Onset Breast Cancer Cecelia Bellcross, Ph.D., M.S.,C.G.C. Department of Human Genetics Emory University School of Medicine All cancers are genetic BUT Not all cancers are hereditary
The Genetics of Early- Onset Breast Cancer Cecelia Bellcross, Ph.D., M.S.,C.G.C. Department of Human Genetics Emory University School of Medicine All cancers are genetic BUT Not all cancers are hereditary
White paper Evaluation of BRAF (V600E) Mutation by Immunohistochemical Staining with anti-braf V600E (VE1) Antibody: A Comparison with Sanger
 White paper Evaluation of BRAF (V600E) Mutation by Immunohistochemical Staining with anti-braf V600E (VE1) Antibody: A Comparison with Sanger Sequencing 2 Evaluation of BRAF (V600E) Mutation by Immunohistochemical
White paper Evaluation of BRAF (V600E) Mutation by Immunohistochemical Staining with anti-braf V600E (VE1) Antibody: A Comparison with Sanger Sequencing 2 Evaluation of BRAF (V600E) Mutation by Immunohistochemical
190.25 - Alpha-fetoprotein
 Other Names/Abbreviations AFP 190.25 - Alpha-fetoprotein Alpha-fetoprotein (AFP) is a polysaccharide found in some carcinomas. It is effective as a biochemical marker for monitoring the response of certain
Other Names/Abbreviations AFP 190.25 - Alpha-fetoprotein Alpha-fetoprotein (AFP) is a polysaccharide found in some carcinomas. It is effective as a biochemical marker for monitoring the response of certain
Coding and Payment Guide for Laboratory Services. An essential coding, billing, and payment resource for laboratory and pathology services
 Coding and Payment Guide for Laboratory Services An essential coding, billing, and payment resource for laboratory and pathology services Contents Introduction............................... 1 Coding Systems...............................
Coding and Payment Guide for Laboratory Services An essential coding, billing, and payment resource for laboratory and pathology services Contents Introduction............................... 1 Coding Systems...............................
Validation parameters: An introduction to measures of
 Validation parameters: An introduction to measures of test accuracy Types of tests All tests are fundamentally quantitative Sometimes we use the quantitative result directly However, it is often necessary
Validation parameters: An introduction to measures of test accuracy Types of tests All tests are fundamentally quantitative Sometimes we use the quantitative result directly However, it is often necessary
Next-generation sequencing can replace Sanger sequencing in clinical diagnostics.
 Next-generation sequencing can replace Sanger sequencing in clinical diagnostics. Birgit Sikkema-Raddatz Department of Genetics, University Medical Center Groningen The Netherlands Groningen GRONINGEN
Next-generation sequencing can replace Sanger sequencing in clinical diagnostics. Birgit Sikkema-Raddatz Department of Genetics, University Medical Center Groningen The Netherlands Groningen GRONINGEN
Tumour Markers. What are Tumour Markers? How Are Tumour Markers Used?
 Dr. Anthony C.H. YING What are? Tumour markers are substances that can be found in the body when cancer is present. They are usually found in the blood or urine. They can be products of cancer cells or
Dr. Anthony C.H. YING What are? Tumour markers are substances that can be found in the body when cancer is present. They are usually found in the blood or urine. They can be products of cancer cells or
Targeted. sequencing solutions. Accurate, scalable, fast TARGETED
 Targeted TARGETED Sequencing sequencing solutions Accurate, scalable, fast Sequencing for every lab, every budget, every application Ion Torrent semiconductor sequencing Ion Torrent technology has pioneered
Targeted TARGETED Sequencing sequencing solutions Accurate, scalable, fast Sequencing for every lab, every budget, every application Ion Torrent semiconductor sequencing Ion Torrent technology has pioneered
Nuevas tecnologías basadas en biomarcadores para oncología
 Nuevas tecnologías basadas en biomarcadores para oncología Simposio ASEBIO 14 de marzo 2013, PCB Jose Jimeno, MD, PhD Co-Founder / Vice Chairman Pangaea Biotech SL Barcelona, Spain PANGAEA BIOTECH BUSINESS
Nuevas tecnologías basadas en biomarcadores para oncología Simposio ASEBIO 14 de marzo 2013, PCB Jose Jimeno, MD, PhD Co-Founder / Vice Chairman Pangaea Biotech SL Barcelona, Spain PANGAEA BIOTECH BUSINESS
http://www.springer.com/3-540-22006-2
 http://www.springer.com/3-540-22006-2 1 Molecular Pathology Laboratory of the Future Christopher A. Moskaluk 1.1 The Past The integration of laboratory analysis with human medicine has traditionally been
http://www.springer.com/3-540-22006-2 1 Molecular Pathology Laboratory of the Future Christopher A. Moskaluk 1.1 The Past The integration of laboratory analysis with human medicine has traditionally been
Downstream Outcomes of New Molecular Diagnostics CPT Coding System Codes
 Downstream Outcomes of New Molecular Diagnostics CPT Coding System Codes Michael Watson, MS, PhD, FACMG Advisory Committee on Heritable Disorders of Newborns and Children April 9, 2014 Disclosures As Executive
Downstream Outcomes of New Molecular Diagnostics CPT Coding System Codes Michael Watson, MS, PhD, FACMG Advisory Committee on Heritable Disorders of Newborns and Children April 9, 2014 Disclosures As Executive
Genomic Analysis of Mature B-cell Malignancies
 Genomic Analysis of Mature B-cell Malignancies Update and Lessons Learned Omar Abdel-Wahab, MD Memorial Sloan Kettering Cancer Center Human Oncology and Pathogenesis Program and Leukemia Service Disclaimer:
Genomic Analysis of Mature B-cell Malignancies Update and Lessons Learned Omar Abdel-Wahab, MD Memorial Sloan Kettering Cancer Center Human Oncology and Pathogenesis Program and Leukemia Service Disclaimer:
How To Get A Cell Print
 QUICK CELL CAPTURE AND CHARACTERIZATION GUIDE FOR CELLSEARCH CUSTOMERS CellSave EDTA Blood sample Rare cell capture Enumeration Single protein marker Cell capture for molecular characterization CELLSEARCH
QUICK CELL CAPTURE AND CHARACTERIZATION GUIDE FOR CELLSEARCH CUSTOMERS CellSave EDTA Blood sample Rare cell capture Enumeration Single protein marker Cell capture for molecular characterization CELLSEARCH
GENETIC TESTING AND MARFAN SYNDROME
 GENETIC TESTING AND MARFAN SYNDROME Genetic testing for mutations in fibrillin-1 (FBN1) and other genes has become an important and reliable option to aid in the diagnosis of Marfan syndrome and related
GENETIC TESTING AND MARFAN SYNDROME Genetic testing for mutations in fibrillin-1 (FBN1) and other genes has become an important and reliable option to aid in the diagnosis of Marfan syndrome and related
Becker Muscular Dystrophy
 Muscular Dystrophy A Case Study of Positional Cloning Described by Benjamin Duchenne (1868) X-linked recessive disease causing severe muscular degeneration. 100 % penetrance X d Y affected male Frequency
Muscular Dystrophy A Case Study of Positional Cloning Described by Benjamin Duchenne (1868) X-linked recessive disease causing severe muscular degeneration. 100 % penetrance X d Y affected male Frequency
Cancer Genomics & Precision Medicine in the 21 st Century. Lee J. Helman, MD Scientific Director for Clinical Research CCR, NCI
 Cancer Genomics & Precision Medicine in the 21 st Century Lee J. Helman, MD Scientific Director for Clinical Research CCR, NCI Outline Define terms Describe vision for how genetic characterization of tumors
Cancer Genomics & Precision Medicine in the 21 st Century Lee J. Helman, MD Scientific Director for Clinical Research CCR, NCI Outline Define terms Describe vision for how genetic characterization of tumors
Fredrik.Enlund@gu.se Sahlgrenska universitetssjukhuset
 Fredrik.Enlund@gu.se Sahlgrenska universitetssjukhuset 1 Techniques for sarcoma diagnostics Molecular Pathology of Solid Tumors Uppsala 120924 Klinisk Molekylär Patologi Klinisk Patologi och Cytologi Gene
Fredrik.Enlund@gu.se Sahlgrenska universitetssjukhuset 1 Techniques for sarcoma diagnostics Molecular Pathology of Solid Tumors Uppsala 120924 Klinisk Molekylär Patologi Klinisk Patologi och Cytologi Gene
Breast and Lung Cancer Biomarker Research at ASCO: Changing Treatment Patterns
 July 2013 Edition Vol. 7, Issue 7 Breast and Lung Cancer Biomarker Research at ASCO: Changing Treatment Patterns By Julie Katz, MPH, MPhil Biomarkers played a prominent role in the research presented in
July 2013 Edition Vol. 7, Issue 7 Breast and Lung Cancer Biomarker Research at ASCO: Changing Treatment Patterns By Julie Katz, MPH, MPhil Biomarkers played a prominent role in the research presented in
Genetic Testing for Susceptibility to Breast and Ovarian Cancer (BRCA1 and BRCA 2)
 Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. M issouri Care, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois,
Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. M issouri Care, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois,
Human Genome Organization: An Update. Genome Organization: An Update
 Human Genome Organization: An Update Genome Organization: An Update Highlights of Human Genome Project Timetable Proposed in 1990 as 3 billion dollar joint venture between DOE and NIH with 15 year completion
Human Genome Organization: An Update Genome Organization: An Update Highlights of Human Genome Project Timetable Proposed in 1990 as 3 billion dollar joint venture between DOE and NIH with 15 year completion
Gebruik van predictieve markers voor targeted therapy in de algemene praktijk
 Gebruik van predictieve markers voor targeted therapy in de algemene praktijk Gerrit A. Meijer, MD, PhD Professor of Pathology Chair of the Department of Pathology VU Universtiy Medical Center Amsterdam
Gebruik van predictieve markers voor targeted therapy in de algemene praktijk Gerrit A. Meijer, MD, PhD Professor of Pathology Chair of the Department of Pathology VU Universtiy Medical Center Amsterdam
ACMG Practice Guideline
 June 2007 Vol. 9 No. 6 ACMG Practice Guideline Indications for genetic referral: a guide for healthcare providers Beth A. Pletcher, MD 1, Helga V. Toriello, PhD 2, Sarah J. Noblin, MS, CGC 3, Laurie H.
June 2007 Vol. 9 No. 6 ACMG Practice Guideline Indications for genetic referral: a guide for healthcare providers Beth A. Pletcher, MD 1, Helga V. Toriello, PhD 2, Sarah J. Noblin, MS, CGC 3, Laurie H.
MAJOR PARADIGM SHIFT IN EARLY 1990S IN UNDERSTANDING RENAL CANCER
 Renal tumours WHO 4 MAJOR PARADIGM SHIFT IN EARLY 1990S IN UNDERSTANDING RENAL CANCER Molecular differential pathology of renal cell tumours G. KOVACS A CLASSIFICATION BASED ON UNDERSTANDING THE GENETIC
Renal tumours WHO 4 MAJOR PARADIGM SHIFT IN EARLY 1990S IN UNDERSTANDING RENAL CANCER Molecular differential pathology of renal cell tumours G. KOVACS A CLASSIFICATION BASED ON UNDERSTANDING THE GENETIC
***Next Generation sequencing testing options available effective April 18 th, 2016***
 ***Next Generation sequencing testing options available effective April 18 th, 2016*** Genetic Test Next Generation Sequencing NF1-RASopathy Panel Testing NF1-only NGS testing and copy number analysis
***Next Generation sequencing testing options available effective April 18 th, 2016*** Genetic Test Next Generation Sequencing NF1-RASopathy Panel Testing NF1-only NGS testing and copy number analysis
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA
 CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives
 What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives What does the career involve? Explore family histories to identify risks Reducing risks
What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives What does the career involve? Explore family histories to identify risks Reducing risks
NEIGE. diagnosis In oncogenetics. Nicolas Sévenet 02 juillet 2012. n.sevenet@bordeaux.unicancer.fr
 NEIGE g for molecular NExt g generation sequencing diagnosis In oncogenetics Nicolas Sévenet 02 juillet 2012 n.sevenet@bordeaux.unicancer.fr t@b d i f Reports 15 years Next generation sequencing 06/2011
NEIGE g for molecular NExt g generation sequencing diagnosis In oncogenetics Nicolas Sévenet 02 juillet 2012 n.sevenet@bordeaux.unicancer.fr t@b d i f Reports 15 years Next generation sequencing 06/2011
Opportunities and Challenges in Translating Novel Discoveries into Useful Clinical Tests
 Opportunities and Challenges in Translating Novel Discoveries into Useful Clinical Tests James H. Doroshow, M.D. NCI Deputy Director for Clinical and Translational Research NCI Workshop: Evidence Needed
Opportunities and Challenges in Translating Novel Discoveries into Useful Clinical Tests James H. Doroshow, M.D. NCI Deputy Director for Clinical and Translational Research NCI Workshop: Evidence Needed
Advances in Diagnostic and Molecular Testing in Prostate Cancer
 Advances in Diagnostic and Molecular Testing in Prostate Cancer Ashley E. Ross MD PhD Assistant Professor Urology, Oncology, Pathology Johns Hopkins School of Medicine September 24, 2015 1 Disclosures
Advances in Diagnostic and Molecular Testing in Prostate Cancer Ashley E. Ross MD PhD Assistant Professor Urology, Oncology, Pathology Johns Hopkins School of Medicine September 24, 2015 1 Disclosures
GENETIC CONSIDERATIONS IN CANCER TREATMENT AND SURVIVORSHIP
 GENETIC CONSIDERATIONS IN CANCER TREATMENT AND SURVIVORSHIP WHO IS AT HIGH RISK OF HEREDITARY CANCER? Hereditary Cancer accounts for a small proportion of all cancer or approximately 5-10% THE DEVELOPMENT
GENETIC CONSIDERATIONS IN CANCER TREATMENT AND SURVIVORSHIP WHO IS AT HIGH RISK OF HEREDITARY CANCER? Hereditary Cancer accounts for a small proportion of all cancer or approximately 5-10% THE DEVELOPMENT
RENAL CANCER PATHOLOGY WHAT REALLY MATTERS? STEWART FLEMING UNIVERSITY OF DUNDEE
 RENAL CANCER PATHOLOGY WHAT REALLY MATTERS? STEWART FLEMING UNIVERSITY OF DUNDEE MAJOR PARADIGM SHIFT IN EARLY 1990S IN UNDERSTANDING RENAL CANCER Molecular differential pathology of renal cell tumours
RENAL CANCER PATHOLOGY WHAT REALLY MATTERS? STEWART FLEMING UNIVERSITY OF DUNDEE MAJOR PARADIGM SHIFT IN EARLY 1990S IN UNDERSTANDING RENAL CANCER Molecular differential pathology of renal cell tumours
The RNA strategy. RNA as a tool and target in human disease diagnosis and therapy.
 The RNA strategy RNA as a tool and target in human disease diagnosis and therapy. The Laboratory of RNA Biology and Biotechnology at the Centre for Integrative Biology (CIBIO) of the University of Trento,
The RNA strategy RNA as a tool and target in human disease diagnosis and therapy. The Laboratory of RNA Biology and Biotechnology at the Centre for Integrative Biology (CIBIO) of the University of Trento,
Sequencing and microarrays for genome analysis: complementary rather than competing?
 Sequencing and microarrays for genome analysis: complementary rather than competing? Simon Hughes, Richard Capper, Sandra Lam and Nicole Sparkes Introduction The human genome is comprised of more than
Sequencing and microarrays for genome analysis: complementary rather than competing? Simon Hughes, Richard Capper, Sandra Lam and Nicole Sparkes Introduction The human genome is comprised of more than
