Monoclonal Antibody Therapy for Lymphoma: Targeting CD20
|
|
|
- Abel Chapman
- 8 years ago
- Views:
Transcription
1 Monoclonal Antibody Therapy for Lymphoma: Targeting CD20 Session Chair: Jonathan W. Freidberg, MD Speakers: Michele Ghielmini, MD; Jonathan W. Friedberg, MD; and John P. Leonard, MD Multimodality Therapies and Optimal Schedule of Antibodies: Rituximab in Lymphoma as an Example Michele Ghielmini Rituximab was the first humanized antibody widely used on patients, so research on its optimal use was a clinical challenge. Many studies have been performed to optimize its dose and schedule, and more are ongoing. The dose of 375 mg/m 2 has become standard, mainly because it shows activity and has little associated toxicity. The combination of rituximab with chemotherapy has been shown to prolong remission in all types of lymphomas, and in patients with diffuse large B-cell lymphoma it can improve survival. As a single agent, particularly when the treatment is prolonged over several months, results are similar to chemotherapy but with fewer side effects. Finally, used as maintenance therapy it can prolong the duration of chemotherapy-obtained remissions. Based on available data, the administration of 375 mg/m 2 before each chemotherapy cycle can be recommended for first line treatment of patients with curable B-cell lymphomas and for patients with high-risk indolent lymphoma who are rituximab-naïve. Singleagent treatment at a prolonged schedule is recommended for cases of indolent disease not in need of urgent response and for patients who are unlikely to tolerate chemotherapy. Since its first use in humans more than 10 years ago, rituximab has become an essential component of the treatment of all types of B-cell lymphoma. Its modulating effects on the normal immune system have also prompted its increasing use in non-neoplastic hematologic diseases (such as idiopathic thrombocytopenic purpura or cryoglobulinemia) and in non-hematologic diseases (such as rheumatoid arthritis or other autoimmune diseases). Despite its widespread use, the optimal schedule for drug administration, either alone or in combination with other modalities, is still not completely defined. This review focuses on the data available to define the most appropriate way of using rituximab in patients with lymphoma. Michele Ghielmini, MD, Oncology Inst. of Southern Switzerland, Ospedale San Giovanni, Bellinzona 6500, Switzerland; Phone +41 (91) , Fax +41 (91) , mghielmini@ticino.com Rituximab as Single Agent In 1997 rituximab at the schedule of 375 mg/m 2 weekly for 4 consecutive weeks was approved by the US Food and Drug Administration (FDA) for the treatment of indolent lymphoma. This original schedule was developed based on two phase I studies demonstrating that this dose was safe and active. 1 The rationale for this schedule was based mostly on empiric and logistic considerations. At the time of FDA approval, no other treatment regimen had been tested, but because of its efficacy and safety, 2 it remained the standard drug schedule for several years. Nevertheless, several considerations led to the initiation of studies to improve the effect of rituximab. All aspects of the schedule could in fact be challenged: the unitary dose, the number of infusions, the duration of the treatment, the interval between administrations, and the speed of the infusion (Figures 1 3). Hematology
2 Figure 1. Schedules of rituximab used as single agent to induce remission. a) 375 mg/m 2 weekly, wk 1 4 (1) b) 500 mg/m 2 weekly, wk 1 4 (5) c) 375 mg/m 2 3x/week, wk 1 4 (35) d) 2250 mg/m 2 weekly, wk 1 4 (6) e) 375 mg/m 2 weekly, wk 1 6 (9) f) 375 mg/m 2 weekly, wk 1 8 (10) The height of the bars is proportional to the dose administered. Figure 3. Schedules of rituximab used as single agent to consolidate or prolong remission. a) 375 mg/m 2 weekly, wk 1-4, 2 months after end of induction (29) b) 375 mg/m 2 weekly, wk 1-4, every 6 months for 3 times (10) c) 375 mg/m 2 at month 2, 4, 6, 8 after end of induction (13) d) 375 mg/m 2 every 3 months for 2 years (24) e) 375 mg/m 2 every time the rituximab blood levels falls under 25 mg/ml (16) f) 375 mg/m 2 every 2 months for 2 years (ongoing PRIMA study) Figure 2. Schedules of rituximab in combination with chemotherapy. a) 375 mg/m 2 on d1 of each chemotherapy cycle (20) b) 375 mg/m 2 on d1 of chemotherapy, starting from cycle 2 (37) c) 375 mg/m 2 on d-7 and -3 of first cycle, and -3 of cycle 2, 4, 6, and 2x on week 4 after the end of treatment (36) d) 375 mg/m 2 on d-7 and -3 of first cycle, then on d1 of each cycle (38) e) 375mg/m 2 twice between high-dose cycles, and twice after the last myeloablative chemotherapy (30) Preclinical data on dose and schedule Rituximab has many proposed mechanisms of action. Some of these actions depend on the activation of complement and of the host immune system, such that in vitro studies might not be representative of the drug s in vivo efficacy. The majority of in vitro studies suggest that there is a doseresponse relationship up to the mg/ml threshold, after which the curve levels off, possibly due to a saturation of the receptors or of other effector mechanisms. 3 This does not mean that mg/ml should be the target concentration to be reached in humans: persistent blood levels of rituximab suggest saturation of all available CD20, so that any measurable rituximab level means that sufficient drug is present in the patient. Nevertheless, because pharmacokinetic (PK) data from the pivotal trial identified 25 mg/ml as the median 3-month level associated with response, 4 a schedule maintaining this level in the blood could be considered as an empiric target. Unitary dose In the original phase I trials, no dose-limiting toxicity was seen and the dose of 375 mg/m 2 was chosen based on the limited availability of the drug for the first phase II trial. Only a limited number of studies have since investigated the effect of higher or lower dosages. Coiffier et al, in a small randomized study comparing 375 mg/m 2 with 500 mg/m 2 in aggressive lymphomas, could establish no difference in the response rate and response duration of the two schedules. 5 In patients with chronic lymphocytic leukemia (CLL), O Brien et al escalated the dose of rituximab from 322 American Society of Hematology
3 375 mg/m 2 to 2250 mg/m 2 weekly for 4 consecutive weeks. 6 Even though the study was not randomized, a comparison among the small cohorts of the study suggest a dose-response relationship in this disease without any increase in toxicity. Despite these encouraging data, no similar trial was carried out in other B-lymphoproliferative diseases, possibly because of what has been called the economical dose-limiting toxicity. The data generated in CLL patients cannot be extrapolated to solid lymphoprolipherative diseases, both because CLL cells express low levels of CD20 on their surface and because the disease is associated with a uniquely high level of circulating CD20 antigen. 7 Duration of the weekly treatment Although the response rate with 4 weeks of treatment was satisfactory and comparable to other single-agent chemotherapies, it soon became evident that the duration of the responses and the proportion of complete responses (CR) were somehow lower than those observed with chemotherapy. In an effort to improve upon these results, investigators tried to extend the treatment to 6 or 8 weeks duration. 8,9 Unfortunately this was done only in small pilot phase II trials, which suggested some benefit in CR rate (even leading to the registration of the 8-week extended schedule), but which were not further confirmed. Overall, we do not have sufficient evidence today to recommend an extension of the weekly treatment beyond the standard 4 weeks. Optimal duration of rituximab treatment The PK analysis in the first pivotal study of rituximab indicated that patients maintaining a higher and more prolonged blood level of rituximab had an increased chance of responding. 4 This suggested a relationship between the duration of exposure of the tumor cells to the drug and response and led to investigation of extended treatment beyond the standard 4 weeks. Hainsworth et al piloted a strategy of scheduled re-treatment, consisting of a regular 4- week rituximab treatment every 6 months for a total of 4 courses. In two phase II studies (one in follicular and small lymphocytic lymphoma [FL], the other in CLL), they showed that the duration of response was much higher than expected using this strategy when compared to the standard 1-month treatment. 10,11 In a subsequent randomized study in relapsed FL patients, in which patients could receive further rituximab courses at scheduled 6-month intervals or only when needed for relapse, they showed that, even though the duration of remission was significantly longer with scheduled re-treatment, the overall rituximab benefit was not different in the two arms. 12 In deciding on the optimal approach, one must balance the financial advantage of less therapy (20% less rituximab used in the group treated when needed) against the advantage to the patient of fewer relapses and longer disease-free intervals. The ECOG is now running a trial to verify on a larger patient set the equivalence in the duration of rituximab benefit of a scheduled maintenance compared to re-treatment at relapse: the RESORT study randomizes newly diagnosed FL patients responding to rituximab to either one of these two schedules. A different approach was chosen by the Swiss Group for Clinical Cancer Research (SAKK) who randomized patients with FL or mantle cell lymphoma (MCL) to the standard 1-month treatment or to the same treatment ( induction ) followed by 1 infusion of rituximab every 2 months for 4 cycles; the latter prolonged schedule resulted in a treatment of 9 months and an approximate rituximab exposure of 1 year. In this study it was shown that for patients with FL responding to induction the duration of remission was doubled with the prolonged schedule, 13 while in MCL the difference did not reach statistical significance. 14 The SAKK is now conducting a trial comparing the 9-month schedule with a treatment of 5 years to investigate the benefit of prolonged rituximab treatment in maintaining long-term remission. Interval between administrations The half-life of rituximab is about 1 week, but the median duration of persistence in the blood at active levels is of about 3 months: 4 the need to infuse the drug at 1-week intervals is therefore questionable. Even though some have claimed that the first doses of rituximab should be administered at short intervals to saturate all the CD20 receptors on malignant B-cells and render chemoresistant cells chemosensitive, 15 this hypothesis has not been substantiated. Because the amount of rituximab given at the current schedule is probably in excess, a schedule of every 3 weeks or even longer intervals could possibly be as active. A prospective PK-based study by Gordan et al addressed the optimal interval between administrations. Having defined a serum concentration of 25 mg/ml as the target, they measured the rituximab blood level in treated patients monthly and infused 1 dose every time the concentration fell below the 25 mg/ml threshold. 16 With this strategy the great majority of patients could maintain active levels of drug with an infusion interval of 3 months, and all of the patients could be kept with blood levels in this range if they were treated every 2 months. We can therefore assume that an infusion of rituximab every 2 3 months should be sufficient to maintain tumors constantly exposed to active concentrations of the drug. This is actually the schedule that was chosen by many cooperative study groups for their maintenance strategy. Speed of administration About 1 of 4 patients receiving rituximab for the first time experiences infusion-related side effects. This has led to a policy of slow and gradually increasing speed of infusion, resulting in the first infusion being given over approximately 4 hours. The reaction is mainly due to the acute lysis of circulating B-cells, which have almost all disappeared by the time the second infusion is due: from the Hematology
4 second administration on, the infusion-related symptoms are therefore much milder or even absent. 2 Despite this, and although other human immunoglobulins are administered at a much higher rate, a duration of infusion of about 3 hours was recommended for further drug administrations as a measure of caution. Investigators in British Columbia have experimented with infusing rituximab in 1½-hour intervals from the second infusion on, with steroid premedication, and have shown the feasibility of this approach. 17 A phase I speed escalation study ongoing in Switzerland has shown that rituximab can be given in 1-hour intervals even without steroid pre-medication, provided the first 2 infusions were given without serious reactions (Ghielmini, abstract ASH 2005). As discussed above, several schedules of rituximab administration have equivalent or better activity, but observations in a single disease can not necessarily be generalized to all lymphomas. For instance, prolonged treatment according to the SAKK schedule showed benefit in FL but not in MCL. 13,14 Consequently, the effect of different dosage schedules of single-agent rituximab requires further study. Furthermore, while equivalent activity was demonstrated for several schedules, we are still not certain of their respective impact on side effects. For example, prolonged treatment causes a longer B-cell depletion and a significant reduction in IgM levels. Even though this has not yet resulted in increased morbidity, 13,14 we don t know the possible impact of this immunosuppression if the treatment is prolonged beyond 2 years, as is the case in ongoing clinical studies. Response to rituximab can appear rapidly, but usually the time to maximum response is measured in months. These observations can be explained by the multiple mechanisms of action of the drug, which have potentially sequential effects. 18 Rituximab in combination with chemotherapy Rituximab and chemotherapy are both active against lymphomas: their different mechanisms of action and spectrum of toxicity made it natural to attempt to combine them. The combination of the two modalities could have additive, synergistic or antagonistic effects, and several trials have addressed the question of whether the two modalities should be given concomitantly or sequentially, in which sequence, and on what schedule. Additive or synergistic effect The in vitro combination of rituximab and chemotherapy could demonstrate additive or synergistic effects, 19 while in patients the effect might differ depending on which drugs are combined and which disease is being treated (Table 1). In diffuse large B-cell lymphoma (DLBCL), a disease in which rituximab is not curative as single agent, the GELA and the MINT trials showed that the addition of rituximab to CHOP increases the proportion of cured patients: 20,21 this suggests synergy. On the other hand, the ECOG trial in a comparable group of patients showed that rituximab obtains the same effect on time-to-treatment failure (TTF) if given together with chemotherapy or after chemotherapy in the form of maintenance: 22 this suggests an additive effect. In previously untreated patients with FL, the duration of remission after prolonged single-agent rituximab is approximately 18 months; 13 while the combination of prolonged rituximab with CVP (cyclophosphamide, vincristine, prednisone), CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone), or FCM (fludarabine, cyclophosphamide, mitoxantrone) chemotherapy increases the eventfree survival of respectively months: this could suggest an additive effect with some chemotherapy combinations and a synergistic effect with others. On the other hand, the addition of rituximab to MCP (mitoxantrone, chlorambucil, predisolone) prolongs the median survival Table 1. Event-free survival in randomized trials of chemotherapy +/- rituximab. Disease First Author Ref. Chemotherapy n Line of Treatment No Rituximab + Rituximab P-value Follicular Marcus (23) CVP st 7 m 27 m Hiddemann (32) CHOP* st 30 m Not reached < Herold (33) MCP st 19 m Not reached < Hagenbeck (24) CHOP** 369 Relapsed 20 m 47 m 0.03 Dreyling (25) FCM 67 Relapsed 20 m 51 m DLBCL Feugier (20) CHOP st 13 m 45 m Mantle cell Lenz* (31) CHOP st 14 m 21 m Herold (33) MCP 90 1 st 14 m 20 m 0.24 Forstpointner (27) FCM 48 Relapsed 4 m 8 m 0.4 * Patients underwent a second randomization stratified by age to either HDCT/SCT versus IFN or between two doses of IFN ** Patients underwent a second randomization to +/- rituximab maintenance. PFS from time of randomization (add approx 6 m for induction CVP). responding patients received IFN maintenance. Abbreviations: DLBCL, diffuse large B cell lymphoma; CVP, cyclophosphamide, vincristine, prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; MCP, mitoxantrone, chlorambucil, predisolone; FCM, fludarabine, cyclophosphamide, mitoxantrone; HDCT, high-dose chemotherapy; SCT, stem cell transplantation; IFN, interferon; PFS, progression-free survival 324 American Society of Hematology
5 of first-line FL patients, suggesting again a synergistic effect. 26 In MCL the median EFS of single-agent prolonged rituximab is 12 months, 14 but rituximab added to chemotherapy improves EFS by only an additional 4-6 months, suggesting a lack of synergy. Combined or sequential treatment Because rituximab depends on the host immune system for part of its activity, 18 and because chemotherapy is toxic to the immune system, 13 it might be preferable to give rituximab first, then consolidate the result with chemotherapy. On the other hand, because rituximab is more active when the disease load is smaller, 13 it could be better to give it after chemotherapy. Finally, because in vitro the two modalities have shown to be in part synergistic, 3,19 it could be wiser to give them together. Using chemotherapy to consolidate a response to rituximab has not been tested clinically (and should in fact be tried), but using rituximab to consolidate a response to chemotherapy has been tested and shown to be effective. In a study by Zinzani et al patients with FL who were not in complete clinical or molecular response after chemotherapy, received a consolidation treatment with rituximab and obtained an improvement of the complete response and of the molecular response rate. 28 The same observation was made by Brugger et al in first-line FL and MCL patients after autologous transplantation, 100% of whom were converted to molecular remission only after consolidating the remission with post-transplant rituximab. 29 On the other hand similar clinical and molecular response rates are obtained when rituximab and chemotherapy (both at standard and at high doses) are administered concomitantly. 15,30 In a randomized phase 2 trial of fludarabine combined with rituximab given either concomitantly or sequentially, the PFS was similar in both arms. 38 Schedule with chemotherapy What is the best dose, interval and sequence of rituximab when combined with chemotherapy? Because the dose of 325 mg/m 2 allows a persistence of active levels in the blood for a much longer time than chemotherapy, a lower rituximab dose could possibly be sufficient for the synergistic effect to be exploited. On the other hand, due to this long persistence in the blood, administering rituximab with every chemotherapy cycle (i.e., every 3 weeks) could be excessive, and administration of a loading dose followed by 1 infusion every 2-3 months during chemotherapy, independently of the day of chemotherapy administration, could be another option. This was the schedule originally piloted by Czuczman et al. 15 Nevertheless, in studies using rituximab in combination with CHOP in DLBCL, the clinical results of giving the combination on day 1 of each cycle produced better results than the administration according to the Czuczman schedule. 20,22 This suggests again that the effect of the combination is more than additive and that it is higher when high concentrations of rituximab are present in the patients blood at the time of chemotherapy. Irrespective of the additive or synergistic effect, many trials have shown that the addition of rituximab to chemotherapy improves both response rate and response duration (Table 1) and, in some studies, translates into an improvement in survival. Because practically nearly all the trials have shown a better activity of the combination compared to chemotherapy alone, it is difficult to assess which, if any, chemotherapy is more potentiated than another by rituximab. Therefore, since receiving rituximab together with chemotherapy is more practical for the patient than receiving it afterwards as a maintenance (shorter duration of treatment, same side-effects), it could be recommended to administer rituximab on the same day as chemotherapy. Maintenance Treatment with Rituximab Maintaining remission has been a long-standing goal in hematology-oncology: in ALL, in AML, in breast cancer and in many other tumors, investigators have tried for decades to prolong the disease-free interval by administering maintenance treatment. In only a few cases does this strategy appear worthwhile, due to the short- and long-term side effects of prolonged chemotherapy, such as fatigue or secondary leukemia. Rituximab on the other hand is almost devoid of acute side effects, making it an ideal candidate for a prolonged treatment without interference with quality of life. We should caution that data on long-term administration of rituximab are scarce and we need to await the results of prospective randomized trials of maintenance versus no maintenance before we can recommend this treatment as standard. The appropriateness of rituximab maintenance may depend on whether the antibody was or was not included in the remission induction regimen. The data on rituximab maintenance after rituximab monotherapy were described above, while below, we will consider the data of rituximab maintenance after standard chemotherapy, after high-dose chemotherapy and after combined rituximab-chemotherapy. Maintenance after chemotherapy Patients responding to chemotherapy benefit from rituximab maintenance, at least in terms of remission duration. This was demonstrated for patients with indolent and aggressive lymphomas. The ECOG conducted a trial in untreated FL patients treated with CVP: patients in remission were randomized to observation or maintenance with a 4-week course of rituximab every 6 months for 4 cycles. Patients in the latter arm experienced a median remission of 30 months compared to 18 months for controls. 34 In an EORTC-HOVON study patients with relapsed or resistant FL were treated with CHOP, followed or not by 1 infusion of rituximab every 3 months for 2 years: again the maintenance arm experienced a significantly longer remission (31% vs 67% at 3 years, P < ) without excessive side effects. 24 Finally the ECOG randomized trial in DLBCL patients responding to CHOP compared observation with Hematology
6 Table 2. When and how to use rituximab in B-cell lymphoproliferative diseases. When With first line chemotherapy in curable B-cell lymphomas With chemotherapy in rituximab-naïve indolent lymphomas requiring rapid response As a single agent for grade I-II follicular lymphoma with FLIPI low-intermediate, not in need of a rapid response As a single agent for any type of lymphoma requiring therapy, but unsuitable for chemotherapy (e.g., age, co-morbidity, refusal) How 375 mg/m 2 on d1 of each chemotherapy cycle 375 mg/m 2 on d1 of each chemotherapy cycle 375 mg/m 2 weekly x 4, then 375 mg/m 2 every 3 months for 1-2 years 375 mg/m 2 weekly x 4, then 375 mg/m 2 every 3 months for 1-2 years rituximab maintenance: the latter cohort experienced a significantly longer remission. 22 We can therefore affirm that in general, for patients with B-cell lymphomas experiencing remission after chemotherapy, maintenance with rituximab is likely to prolong remission duration. The costeffectiveness of this strategy and its impact on survival still remain to be demonstrated. Maintenance after high-dose chemotherapy Because rituximab has been shown to be more active in patients with less disease burden, it is appealing to give maintenance in patients with minimal residual disease following high-dose chemotherapy and autologous stem cell transplantation (ASCT). A few pilot studies showed the feasibility of the treatment but were not designed to compare their efficacy with observation. 29 An ongoing study by the EBMT is investigating the role of rituximab maintenance (1 infusion every 3 months for 2 years) after ASCT for relapsed FL. Another trial in Germany is investigating the role of maintenance after ASCT for MCL. Because these trials are still ongoing and patients treated with this modality usually experience long remissions, it will be several years until it can be demonstrated whether maintenance in this setting improves outcome. Maintenance after combined rituximab-chemotherapy The rationale behind using rituximab both during and after chemotherapy lies in the possibility that it first acts as a chemosensitizer, synergizing with chemotherapy, and later works on minimal residual disease through immunologically mediated mechanisms. In first-line FL the ongoing PRIMA study investigates the role of rituximab maintenance (once every 2 months for 2 years) after chemotherapy and rituximab, while an EORTC-HOVON trial studies the role of rituximab maintenance (once every 3 months for 2 years) in patients with relapsed FL responding to R-CHOP. Schedule for maintenance With regard to both monotherapy and combination treatment, we still do not know the best schedule for maintenance: dose, duration and interval between doses. The only prospectively studied options are planned re-treatment every 6 months (the Hainsworth schedule 10 ) or a single dose every 2 to 3 months for 1 to 2 years. 13,24 None of the ongoing studies is comparing different maintenance schedules after chemotherapy, but the data discussed above for singleagent rituximab suggest as the best choice the schedule of 375 mg/m 2 every 3 months for at least 2 years. To summarize the data to date, maintenance rituximab should be recommended for patients who were not exposed to rituximab during remission induction, but the cost-effectiveness of this approach must still be established. Rituximab Retreatment Patients relapsing after single-agent rituximab can be retreated at relapse and respond again in about 50% of the cases. 39 These data were obtained in a small retrospective trial and have not been confirmed by other studies. We do not have consistent information on the efficacy of re-treatment with rituximab combined with chemotherapy in patients who were already exposed to rituximab. Because rituximab with chemotherapy improves response rate and survival in a number of B-cell neoplasias, this combination is rapidly becoming the standard first-line treatment. When these patients relapse, the usefulness of re-administering rituximab with further chemotherapy regimens must be addressed. Although many clinicians tend to add rituximab to further lines of chemotherapy, no studies have addressed this issue and data from retrospective observations are scanty and inconclusive. 40 Does it make sense to re-challenge patients with rituximab if they relapse after a previous rituximab-containing regimen? Given the cost of the drug, the question is not trivial and should be addressed in a randomized trial. Conclusion In conclusion, 10 years after the appearance of rituximab in clinical practice, we can say that this antibody should be used in the following situations (see Table 2 for the suggested schedules): With first-line chemotherapy for curable B-cell neoplasias With the first chemotherapy in rituximab-naive indolent lymphomas requiring chemotherapy As a single agent with a prolonged schedule for follicular lymphomas not in need of rapid response As single agent for patients with B-cell neoplasias who are unsuitable for chemotherapy 326 American Society of Hematology
7 References 1. Maloney DG, Grillo-López AJ, Bodkin DJ, et al. IDEC-C2B2: results of a Phase I multiple-dose trial in patients with relapsed non-hodgkin s lymphoma. J Clin Oncol. 1997;15: McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-cd20 monoclonal antibody therapy for relapsed indolent lymphoma: hald of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16: Rose AL, Smith BE, Maloney DG. Glucocorticoids and rituximab in vitro; synergistic direct antiproliferative and apoptotic effects. Blood. 2002;100: Berinstein NL, Grillo-López AJ, White CA, et al. Association of serum Rituximab (IDEC-C2B8) concentration and antitumor response in the treatment of recurrent low-grade or follicular non-hodgkin s lymphoma. Ann Oncol. 1998;9: Coiffier B, Haioun C, Ketterer N, et al. Rituximab (anti-cd20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92: O Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19: Manshouri T, Do K, Wang X, et al. Circulating CD20 is detectable in the plasma of patients with chronic lymphocytic leukemia and is of prognostic significance. Blood. 2003;101: Piro LD, White CA, Grillo-López AJ, et al. Extended rituximab (anti-cd20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-hodgkin s lymphoma. Ann Oncol. 1999;10: Bremer K. Semi-extended, six weekly rituximab infusions in pre-treated advanced low-grade B cell non-hodgkin s lymphoma: a phase II study. Anti-cancer Drugs. 2003;14: Hainsworth JD, Litschy S, Burris III HA, et al. Rituximab as first-line and maintenance therapy for patients with indolent non-hodgkin s lymphoma. J Clin Oncol. 2002;20: Hainsworth JD, Litsch S, Barton JH, et al. Singe-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21: Hainsworth JD, Litchy S, Shaffer DW, et al. Maximizing therapeutic benefit of Rituximab: maintenance therapy versus re-treatment at progression in patients with indolent non-hodgkin s lymphoma a randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2005;23: Ghielmini M, Hsu-Schmitz SF, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood. 2004;103: Ghielmini M, Hsu-Schmitz S, Cogliatti S, et al. Effect of single-agent Rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: a study of the Swiss Group for Clinical Cancer Research (SAKK). J Clin Oncol. 2005;4: Czuczman MS, Grillo-López AJ, White CA, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-cd20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol. 1999;17: Gordan LN, Grow WB, Pusateri A, et al. Phase II trial of individualized rituximab dosing for patients with CD20- positive lymphoproliferative disorders. J Clin Oncol. 2005;6: Sehn LH, Donaldson J, Filewich A, et al. Rapid infusion rituximab in combination with steroid containing chemotherapy can be given safely and substantially reduces resource utilization [abstract]. Blood. 2004;104:394a. Abstract # Cartron G, Watier H, Golay, et al. From the bench to the bedside; ways to improve rituximab efficacy. Blood. 2004;104: Demidem A, Lam T, Alas S, et al. Chimeric anti-cd20 (IDEC- C2B8) monoclonal antibody sensitizes a B-cell lymphoma cell line to cell killing by cytotoxic drugs. Cancer Biother Radiopharm. 1997;12: Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d Etudes des Lymphomes de l Adulte. J Clin Oncol. 2005;23: Pfreundschuh M, Truemper L, Gill Devinder, et al. First analysis of the completed Mabthera International (MInT) trial in young patients with low-risk diffuse large B-cell lymphoma (DLBCL): addition of rituximab to a CHOP-like regimen significantly improves outcome of all patients with the identification of a very favorable subgroup with IPI=0 and no bulky disease [abstract]. Blood. 2004;104:48a. Abstract # Habermann TM, Weller E, Morrison VA, et al. Rituximab- CHOP versus CHOP with or without maintenance rituximab in patients 60 years of age or older with diffuse large B-cell lymphoma (DLBCL): an update [abstract]. Blood. 2004;104:40a. Abstract # Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105: Hagenbeek A, Van Glabbeke M, Teodorovic I, et al. The role of rituximab maintenance treatment in relapsed follicular NHL: in interim analysis of the EORTC randomized intergroup trial [abstract]. Ann Oncol. 2005;16:v52. Abstract # Dreyling M, Forstpointner R, Ludwig W, et al. The addition of rituximab to fludarabine combination (R-FCM) significantly improves remission rates and overall survival in recurrent follicular as well as mantle cell lymphoma - follow-up of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) [abstract]. Ann Oncol. 2005;16:110. Abstract # Herold M, Pasold R, Srock S, et al. Rituximab plus Mitoxantrone, Chlorambucil, Prednisolon (R-MCP) is superior to MCP alone in advanced indolent and follicular lymphoma results of a phase III study (OSHO39) [abstract]. Ann Oncol. 2005;16:51. Abstract # Forstpointer R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104: Zinzani PL, Pulsoni A, Perrotti A, et al. Fludarabine plus mitoxantrone with and without rituximab versus CHOP with and without rituximab as front-line treatment for patients with follicular lymphoma. J Clin Oncol. 2004;22: Brugger W, Hirsch J, Grünebach F, et al. Rituximab consolidation after high-dose chemotherapy and autologous blood stem cell transplantation in follicular and mantle cell lymphoma: a prospective, multicenter phase II study. Ann Oncol. 2004;15: Gianni AM, Magni M, Martelli M, et al. Long-term remission in mantle cell lymphoma following high-dose sequential Hematology
8 chemotherapy and in vivo rituximab-purged stem cell autografting (R-HDS regimen). Blood. 2003;102: Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol. 2005;23: Hiddeman W, Forstpointer R, Kneba M, et al. The addition of rituximab with chemotherapy with CHOP has a long lasting impact on subsequent treatment in remission in follicular lymphoma but not in mantle cell lymphoma: results of two prospective randomized studies of the German Low Grade Study Group (GLSG) [abstract]. Blood. 2004;104:50a. Abstract # Herold M, Pasold R, Srock S, et al. Results of a prospective randomised open label phase III study comparing rituximab plus mitoxantrone, chlorambucile, prednisolone chemotherapy (R-MCP) versus MCP alone in untreated advanced indolent non-hodgkin s lymphoma (NHL) and mantle-celllymphoma (MCL) [abstract]. Blood. 2004;104:169a; Abstract # Hochster HS, Weller E, Ryan T, et al. Results of E1496: a phase III trial of CVP with or without maintenance rituximab in advanced indolent lymphoma (NHL) [abstract]. J Clin Oncol. 2004;22:558s. Abstract # Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19: Czuczman, Koryzna A, Mohr A, et al. Rituximab in combination with Fludarabine chemotherapy in low-grade or follicular lymphoma. J Clin Oncol. 2005;23: Schulz H, Klein SK, Rehwald U, et al. Phase 2 study of a combined immunochemotherapy using rituximab and fludarabine in patients with chronic lymphocytic leukemia. Blood. 2002;100: Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B9712 (CALGB 9712). Blood. 2003;101: Davis TA, Grillo-López AJ, White CA, et al. Rituximab anti- CD-20 monoclonal antibody therapy in non-hodgkin s lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18: Lemieux B, Bouafia F, Thieblemont C, et al. Second treatment with rituximab in B-cell non-hodgkin s lymphoma: efficacy and toxicity on 41 patients treated at CHU-Lyon Sud. Hematol J. 2004;5: American Society of Hematology
CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY
 CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY 26.1 Introduction rituximab Subsequent to the completion of drafts for the guidelines earlier in 2004,
CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY 26.1 Introduction rituximab Subsequent to the completion of drafts for the guidelines earlier in 2004,
Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma
 a report by Martin Dreyling Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma Head, Lymphoma Section, Department of Medicine III, University Hospital Großhadern, Ludwig Maximilians-University
a report by Martin Dreyling Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma Head, Lymphoma Section, Department of Medicine III, University Hospital Großhadern, Ludwig Maximilians-University
David Loew, LCL MabThera
 MabThera The star continues to rise David Loew, LCL MabThera MabThera the star continues to raise Group sales (CHF bn) 4,5 4,0 3,5 3,0 2,5 2,0 1,5 1,0 0,5 0,0 2001 2002 2003 2004 2005 Outstanding clinical
MabThera The star continues to rise David Loew, LCL MabThera MabThera the star continues to raise Group sales (CHF bn) 4,5 4,0 3,5 3,0 2,5 2,0 1,5 1,0 0,5 0,0 2001 2002 2003 2004 2005 Outstanding clinical
FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma
 Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
Guidelines for the Management of Follicular Lymphoma
 Guidelines for the Management of Follicular Lymphoma Scope The following guidance for first- and second-line therapy applies to follicular lymphoma histological grades 1, 2 and 3a according to the World
Guidelines for the Management of Follicular Lymphoma Scope The following guidance for first- and second-line therapy applies to follicular lymphoma histological grades 1, 2 and 3a according to the World
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003)
 Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
Frequency of NHL Subtypes in Adults
 Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Rituximab in Non - Hodgkins Lymphoma. Fatima Bassa, Dept. of Haematology October 2008
 Rituximab in Non - Hodgkins Lymphoma Fatima Bassa, Dept. of Haematology October 2008 World Health Organization lymphoma classification (2001) Peripheral B-cell neoplasms: B-chronic lymphocytic leukemia/small
Rituximab in Non - Hodgkins Lymphoma Fatima Bassa, Dept. of Haematology October 2008 World Health Organization lymphoma classification (2001) Peripheral B-cell neoplasms: B-chronic lymphocytic leukemia/small
1. Introduction. 2. Clinical aspects
 1. Introduction MabThera (rituximab) is a genetically engineered chimeric mouse/human monoclonal antibody which binds specifically to the transmembrane antigen, CD20. This antigen is located on pre-b-
1. Introduction MabThera (rituximab) is a genetically engineered chimeric mouse/human monoclonal antibody which binds specifically to the transmembrane antigen, CD20. This antigen is located on pre-b-
Réda Bouabdallah, MD Institut Paoli-Calmettes, Marseille, France. Oran, May, 8th, 2010
 FIRST LINE TREATMENT IN FOLLICULAR LYMPHOMA Current Status Réda Bouabdallah, MD Institut Paoli-Calmettes, Marseille, France Oran, May, 8th, 2010 FL is a complex disease One disease, but many profiles 20%
FIRST LINE TREATMENT IN FOLLICULAR LYMPHOMA Current Status Réda Bouabdallah, MD Institut Paoli-Calmettes, Marseille, France Oran, May, 8th, 2010 FL is a complex disease One disease, but many profiles 20%
Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
Anti-HCV therapy in HCV-related NHL
 Gabriele Pozzato M.D. University of Trieste Anti-HCV therapy in HCV-related NHL Questions about HCV+ in NHL Is the NHL related with HCV infection? Which is the best therapeutic strategy? Is the antiviral
Gabriele Pozzato M.D. University of Trieste Anti-HCV therapy in HCV-related NHL Questions about HCV+ in NHL Is the NHL related with HCV infection? Which is the best therapeutic strategy? Is the antiviral
Treatment of low-grade non-hodgkin lymphoma
 Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Guidelines for the use of Rituximab in Non-Hodgkin s Lymphoma QEII Health Sciences Centre
 Guidelines for the use of Rituximab in Non-Hodgkin s Lymphoma QEII Health Sciences Centre Background Non-Hodgkin s lymphoma (NHL) makes up approximately 85% of all lymphomas. They are a heterogeneous collection
Guidelines for the use of Rituximab in Non-Hodgkin s Lymphoma QEII Health Sciences Centre Background Non-Hodgkin s lymphoma (NHL) makes up approximately 85% of all lymphomas. They are a heterogeneous collection
Audience Response Question?
 Presenter Disclosure Information Session 4: 3:30 PM - 4:15 PM Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman, MD The following relationships
Presenter Disclosure Information Session 4: 3:30 PM - 4:15 PM Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman, MD The following relationships
Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma
 Issue date: June 2011 Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma This guidance was developed using the the single technology appraisal process NICE technology
Issue date: June 2011 Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma This guidance was developed using the the single technology appraisal process NICE technology
Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm
 Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm Name of Policy: Uses of Monoclonal Antibodies for the Treatment of Non-Hodgkin
Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm Name of Policy: Uses of Monoclonal Antibodies for the Treatment of Non-Hodgkin
GLSG/OSHO Study Group. Supported by Deutsche Krebshilfe
 GLSG/OSHO Study Group Supported by Deutsche Krebshilfe GLSG/OSHO Study Group Study Concepts Follicular Lymphomas Mantel Cell Lymphomas Waldenstroem s Disease Key Steps in Improving Treatment for Follicular
GLSG/OSHO Study Group Supported by Deutsche Krebshilfe GLSG/OSHO Study Group Study Concepts Follicular Lymphomas Mantel Cell Lymphomas Waldenstroem s Disease Key Steps in Improving Treatment for Follicular
Audience Response Question? Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management. Presenter Disclosure Information
 Welcome to Master Class for Oncologists Session 4: 10:00 AM - 10:45 AM Miami, FL December 18, 2009 Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman,
Welcome to Master Class for Oncologists Session 4: 10:00 AM - 10:45 AM Miami, FL December 18, 2009 Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman,
Non-Hodgkin s lymphomas (NHLs) are a
 Oncology 33 Non-Hodgkin s lymphoma in the elderly The incidence of non-hodgkin s lymphoma (NHL) is increasing, and this increase is even more rapid in the older population. Although treatment of NHL in
Oncology 33 Non-Hodgkin s lymphoma in the elderly The incidence of non-hodgkin s lymphoma (NHL) is increasing, and this increase is even more rapid in the older population. Although treatment of NHL in
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD Boehringer Ingelheim International GmbH and Boehringer Ingelheim Pharmaceuticals, Inc. Petitioner, v. Biogen Idec, Inc.
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD Boehringer Ingelheim International GmbH and Boehringer Ingelheim Pharmaceuticals, Inc. Petitioner, v. Biogen Idec, Inc.
rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited
 rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited 06 June 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited 06 June 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Mantle Cell Lymphoma Understanding Your Treatment Options
 New Developments in Mantle Cell Lymphoma John P. Leonard, M.D. Richard T. Silver Distinguished Professor of Hematology and Medical Oncology Associate Dean for Clinical Research Vice Chairman, Department
New Developments in Mantle Cell Lymphoma John P. Leonard, M.D. Richard T. Silver Distinguished Professor of Hematology and Medical Oncology Associate Dean for Clinical Research Vice Chairman, Department
Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics
 Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics Disclosure(s) I do not intend to discuss an off-label use
Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics Disclosure(s) I do not intend to discuss an off-label use
New Targets and Treatments for Follicular Lymphoma. Disclosures
 Winship Cancer Institute of Emory University New Targets and Treatments for Follicular Lymphoma Jonathon B. Cohen, MD, MS Assistant Professor Div of BMT, Emory University Disclosures Consulting fees from:
Winship Cancer Institute of Emory University New Targets and Treatments for Follicular Lymphoma Jonathon B. Cohen, MD, MS Assistant Professor Div of BMT, Emory University Disclosures Consulting fees from:
ollicular Lymphoma The following investigations are indicated for most patients with follicular lymphoma:
 ollicular Lymphoma Drs. Kevin Imrie & Matthew Cheung Updated August 2007* Fximab Updates: Ritu maintenance therapy Introduction Follicular lymphoma is the second most frequent type of non-hodgkin s lymphoma
ollicular Lymphoma Drs. Kevin Imrie & Matthew Cheung Updated August 2007* Fximab Updates: Ritu maintenance therapy Introduction Follicular lymphoma is the second most frequent type of non-hodgkin s lymphoma
Update on Follicular Lymphoma. Brad Kahl, M.D.
 Update on Follicular Lymphoma Brad Kahl, M.D. Follicular Lymphoma: 25% of NHL Cases Other subtypes (9%) T and NK cell (12%) Burkitt (2.5%) Diffuse large B cell (DLBCL) (30%) Mantle cell (6%) Follicular
Update on Follicular Lymphoma Brad Kahl, M.D. Follicular Lymphoma: 25% of NHL Cases Other subtypes (9%) T and NK cell (12%) Burkitt (2.5%) Diffuse large B cell (DLBCL) (30%) Mantle cell (6%) Follicular
Chemoimmunotherapy resistant follicular lymphoma A single institutional study
 Cancer Research Journal 2014; 2(5): 93-97 Published online September 30, 2014 (http://www.sciencepublishinggroup.com/j/crj) doi: 10.11648/j.crj.20140205.13 ISSN: 2330-8192 (Print); ISSN: 2330-8214 (Online)
Cancer Research Journal 2014; 2(5): 93-97 Published online September 30, 2014 (http://www.sciencepublishinggroup.com/j/crj) doi: 10.11648/j.crj.20140205.13 ISSN: 2330-8192 (Print); ISSN: 2330-8214 (Online)
Rituximab in the Management of Follicular Lymphoma
 Hong Kong J Radiol. 2011;14(Suppl):S63-7 REVIEW ARTICLE Rituximab in the Management of Follicular Lymphoma YL Kwong Department of Medicine, Professorial Block, Queen Mary Hospital, Pokfulam Road, Hong
Hong Kong J Radiol. 2011;14(Suppl):S63-7 REVIEW ARTICLE Rituximab in the Management of Follicular Lymphoma YL Kwong Department of Medicine, Professorial Block, Queen Mary Hospital, Pokfulam Road, Hong
CAR T cell therapy for lymphomas
 CAR T cell therapy for lymphomas Sattva S. Neelapu, MD Associate Professor and Deputy Chair ad interim Department of Lymphoma and Myeloma UT MD Anderson Cancer Center, Houston, TX CAR T cell therapy What
CAR T cell therapy for lymphomas Sattva S. Neelapu, MD Associate Professor and Deputy Chair ad interim Department of Lymphoma and Myeloma UT MD Anderson Cancer Center, Houston, TX CAR T cell therapy What
In the last decade, passive immunotherapy
 Mædica - a Journal of Clinical Medicine ORIGINAL PAPERS Immunotherapy with Rituximab in Follicular Lymphomas Carmen SAGUNA, MD a ; Ileana Delia MUT, MD, PhD a ; Anca Roxana LUPU, MD, PhD a ; Mihaela TEVET,
Mædica - a Journal of Clinical Medicine ORIGINAL PAPERS Immunotherapy with Rituximab in Follicular Lymphomas Carmen SAGUNA, MD a ; Ileana Delia MUT, MD, PhD a ; Anca Roxana LUPU, MD, PhD a ; Mihaela TEVET,
IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN
 + IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
+ IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
Lauren Berger: Why is it so important for patients to get an accurate diagnosis of their blood cancer subtype?
 Hello, I m Lauren Berger and I m the Senior Director of Patient Services Programs at The Leukemia & Lymphoma Society. I m pleased to welcome Dr. Rebecca Elstrom. Dr. Elstrom is an Assistant Professor in
Hello, I m Lauren Berger and I m the Senior Director of Patient Services Programs at The Leukemia & Lymphoma Society. I m pleased to welcome Dr. Rebecca Elstrom. Dr. Elstrom is an Assistant Professor in
Economic evaluation of rituximab plus cyclophosphamide, vincristine and prednisolone for advanced follicular lymphoma
 Leukemia & Lymphoma, February 2008; 49(2): 227 236 ORIGINAL ARTICLE: CLINICAL Economic evaluation of rituximab plus cyclophosphamide, vincristine and prednisolone for advanced follicular lymphoma JOHN
Leukemia & Lymphoma, February 2008; 49(2): 227 236 ORIGINAL ARTICLE: CLINICAL Economic evaluation of rituximab plus cyclophosphamide, vincristine and prednisolone for advanced follicular lymphoma JOHN
What is non-hodgkin lymphoma, how is it treated, and what is the unmet need?
 What is non-hodgkin lymphoma, how is it treated, and what is the unmet need? Tim Illidge BSc PhD MRCP FRCR FRCPath Institute of Cancer Sciences, University of Manchester Manchester Cancer Research Centre,
What is non-hodgkin lymphoma, how is it treated, and what is the unmet need? Tim Illidge BSc PhD MRCP FRCR FRCPath Institute of Cancer Sciences, University of Manchester Manchester Cancer Research Centre,
Histopathologic results
 Self evaluation 1 Clinical Case 55-year-old woman Bilateral enlargement of cervical, axillary and inguinal lymph nodes, largest diameter > 6 cm Hepatosplenomegaly. Enlargement of retroperitoneal, mesenteric
Self evaluation 1 Clinical Case 55-year-old woman Bilateral enlargement of cervical, axillary and inguinal lymph nodes, largest diameter > 6 cm Hepatosplenomegaly. Enlargement of retroperitoneal, mesenteric
DIFFUSE LARGE B-CELL LYMPHOMA
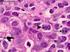 DIFFUSE LARGE B-CELL LYMPHOMA Executive Summary Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-hodgkin lymphoma (NHL), constituting up to 40% of all cases globally.[1] This subtype
DIFFUSE LARGE B-CELL LYMPHOMA Executive Summary Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-hodgkin lymphoma (NHL), constituting up to 40% of all cases globally.[1] This subtype
The role of chemotherapy in follicular lymphomas Emanuele Zucca, M.D.
 The role of chemotherapy in follicular lymphomas Emanuele Zucca, M.D. Oncology Institute of Southern Switzerland (IOSI) Swiss Group for Clinical Cancer Research (SAKK) WHO grading of follicular lymphoma
The role of chemotherapy in follicular lymphomas Emanuele Zucca, M.D. Oncology Institute of Southern Switzerland (IOSI) Swiss Group for Clinical Cancer Research (SAKK) WHO grading of follicular lymphoma
Update in Hematology Oncology Targeted Therapies. Mark Holguin
 Update in Hematology Oncology Targeted Therapies Mark Holguin 25 years ago Why I chose oncology People How to help people with possibly the most difficult thing they may have to deal with Science Turning
Update in Hematology Oncology Targeted Therapies Mark Holguin 25 years ago Why I chose oncology People How to help people with possibly the most difficult thing they may have to deal with Science Turning
Induction Chemotherapy (R-CHOP Vs. R-FC) Followed by Interferon Maintenance Versus Rituximab Maintenance in MCL (MCLelderly)
 1 von 7 31.07.2012 10:27 Home Search Study Topics Glossary Study 1 of 24 for search of: MCL elderly Previous Study Return to Search Results Next Study Full Text View Tabular View No Study Results Posted
1 von 7 31.07.2012 10:27 Home Search Study Topics Glossary Study 1 of 24 for search of: MCL elderly Previous Study Return to Search Results Next Study Full Text View Tabular View No Study Results Posted
Diffuse large B-cell lymphoma: the curable disease?
 Hematology Meeting Reports 2007; 1(5):77 86 Diffuse large B-cell lymphoma: the curable disease? Michael Pfreundschuh Med Klinik I, Saarland University Medical School, Homburg/Saar, Germany Corresponding
Hematology Meeting Reports 2007; 1(5):77 86 Diffuse large B-cell lymphoma: the curable disease? Michael Pfreundschuh Med Klinik I, Saarland University Medical School, Homburg/Saar, Germany Corresponding
DE Tsai 1, HCF Moore 1, CL Hardy 2, DL Porter 1, EY Loh 1, DJ Vaughn 1, S Luger 1, SJ Schuster 1 and EA Stadtmauer 1
 Bone Marrow Transplantation, (1999) 24, 521 526 1999 Stockton Press All rights reserved 0268 3369/99 $15.00 http://www.stockton-press.co.uk/bmt Rituximab (anti-cd20 monoclonal antibody) therapy for progressive
Bone Marrow Transplantation, (1999) 24, 521 526 1999 Stockton Press All rights reserved 0268 3369/99 $15.00 http://www.stockton-press.co.uk/bmt Rituximab (anti-cd20 monoclonal antibody) therapy for progressive
Michael Crump MD. Lymphoma Site Leader Princess Margaret Hospital University of Toronto
 Evolution of Lymphoma Therapy: What can we expect for the rest of the millenium decade? Michael Crump MD Lymphoma Site Leader Princess Margaret Hospital University of Toronto disclaimers Served on advisory
Evolution of Lymphoma Therapy: What can we expect for the rest of the millenium decade? Michael Crump MD Lymphoma Site Leader Princess Margaret Hospital University of Toronto disclaimers Served on advisory
Aggressive non-hodgkin s lymphoma long-term survival for all patients?
 Hematology Meeting Reports 2007; 1(4):27 41 Aggressive non-hodgkin s lymphoma long-term survival for all patients? Bertrand Coiffier 1 Jacques Tabacof 2 Zhi-Xiang Shen 3 Ulrich Jäger 4 1 Hospices Civils
Hematology Meeting Reports 2007; 1(4):27 41 Aggressive non-hodgkin s lymphoma long-term survival for all patients? Bertrand Coiffier 1 Jacques Tabacof 2 Zhi-Xiang Shen 3 Ulrich Jäger 4 1 Hospices Civils
Imaging, Diagnosis, Prognosis. MinnaTaskinen, 1,2 Marja-Liisa Karjalainen-Lindsberg, 3 Heidi Nyman, 1,2 Leena-Maija Eerola, 1,2 1,2.
 Imaging, Diagnosis, Prognosis A HighTumor-Associated Macrophage Content Predicts Favorable Outcome in Follicular Lymphoma PatientsTreated with Rituximab and Cyclophosphamide-Doxorubicin-Vincristine-Prednisone
Imaging, Diagnosis, Prognosis A HighTumor-Associated Macrophage Content Predicts Favorable Outcome in Follicular Lymphoma PatientsTreated with Rituximab and Cyclophosphamide-Doxorubicin-Vincristine-Prednisone
Lymphomas after organ transplantation
 Produced 21.03.2011 Revision due 21.03.2011 Lymphomas after organ transplantation People who have undergone an organ transplant are more at risk of developing lymphoma known as post-transplant lymphoproliferative
Produced 21.03.2011 Revision due 21.03.2011 Lymphomas after organ transplantation People who have undergone an organ transplant are more at risk of developing lymphoma known as post-transplant lymphoproliferative
Rituximab Maintenance for 2 Years in Patients with Untreated High Tumor Burden Follicular Lymphoma After Response to Immunochemotherapy
 Rituximab Maintenance for 2 Years in Patients with Untreated High Tumor Burden Follicular Lymphoma After Response to Immunochemotherapy G. A. Salles, J. F. Seymour, P. Feugier, F. Offner, A. Lopez-Guillermo,
Rituximab Maintenance for 2 Years in Patients with Untreated High Tumor Burden Follicular Lymphoma After Response to Immunochemotherapy G. A. Salles, J. F. Seymour, P. Feugier, F. Offner, A. Lopez-Guillermo,
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cll_and_sll
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cll_and_sll
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin s lymphoma
 DOI: 10.3310/hta13suppl2/06 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin s lymphoma A Boland, A Bagust,
DOI: 10.3310/hta13suppl2/06 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin s lymphoma A Boland, A Bagust,
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy
 Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy Yoichi Kitamura, MD Kazuhiko Hayashi, MD Kazumi Uchida,
Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy Yoichi Kitamura, MD Kazuhiko Hayashi, MD Kazumi Uchida,
Germany; email:mathias.witzens-harig@med.uni-heidelberg.de. Munich, Germany; email: michael.unterhalt@med.uni-muenchen.de
 Treatment of limited stage follicular lymphoma with Rituximab immunotherapy and involved field radiotherapy in a prospective multicenter Phase II trial MIR trial Mathias Witzens-Harig 1, Manfred Hensel
Treatment of limited stage follicular lymphoma with Rituximab immunotherapy and involved field radiotherapy in a prospective multicenter Phase II trial MIR trial Mathias Witzens-Harig 1, Manfred Hensel
Introduction of Rituximab in Front-Line and Salvage Therapies Has Improved Outcome of Advanced-Stage Follicular Lymphoma Patients
 2077 Introduction of Rituximab in Front-Line and Salvage Therapies Has Improved Outcome of Advanced-Stage Follicular Lymphoma Patients Stefano Sacchi, MD 1 Samantha Pozzi, MD 1 Luigi Marcheselli, MS 1
2077 Introduction of Rituximab in Front-Line and Salvage Therapies Has Improved Outcome of Advanced-Stage Follicular Lymphoma Patients Stefano Sacchi, MD 1 Samantha Pozzi, MD 1 Luigi Marcheselli, MS 1
Treatment of follicular lymphoma
 Treatment of follicular lymphoma Mathias J. Rummel Med. Klinik IV/ V - Hämatologie Klinikum der Justus-Liebig Liebig-Universität Gießen MJR Therapy of Follicular Lymphoma wait and see policy alkylating
Treatment of follicular lymphoma Mathias J. Rummel Med. Klinik IV/ V - Hämatologie Klinikum der Justus-Liebig Liebig-Universität Gießen MJR Therapy of Follicular Lymphoma wait and see policy alkylating
Rituximab in non-hodgkin Lymphoma (NHL)
 Original Article ISSN: 2070-254X Rituximab in non-hodgkin Lymphoma (NHL) Manal Elhabbash MD 1, Abukris Alwindi MD 2 (1) Assistant Professor, Tripoli University, (2) Medical consultant in oncology & hematology
Original Article ISSN: 2070-254X Rituximab in non-hodgkin Lymphoma (NHL) Manal Elhabbash MD 1, Abukris Alwindi MD 2 (1) Assistant Professor, Tripoli University, (2) Medical consultant in oncology & hematology
Aggressive lymphomas. Michael Crump Princess Margaret Hospital
 Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Hematopoietic Stem Cell Transplantation. Imad A. Tabbara, M.D. Professor of Medicine
 Hematopoietic Stem Cell Transplantation Imad A. Tabbara, M.D. Professor of Medicine Hematopoietic Stem Cells Harvested from blood, bone marrow, umbilical cord blood Positive selection of CD34 (+) cells
Hematopoietic Stem Cell Transplantation Imad A. Tabbara, M.D. Professor of Medicine Hematopoietic Stem Cells Harvested from blood, bone marrow, umbilical cord blood Positive selection of CD34 (+) cells
State-of-the-Art Treatment for Lymphoma December 19, 2007 Guests: David Maloney, M.D., Ph.D Hosted by Andrew Schorr INTRODUCTION
 State-of-the-Art Treatment for Lymphoma December 19, 2007 Guests: David Maloney, M.D., Ph.D Hosted by Andrew Schorr Please remember the opinions expressed on Patient Power are not necessarily the views
State-of-the-Art Treatment for Lymphoma December 19, 2007 Guests: David Maloney, M.D., Ph.D Hosted by Andrew Schorr Please remember the opinions expressed on Patient Power are not necessarily the views
Practice of Interferon Therapy
 Interferon Therapy Practice of Interferon Therapy Multiple myeloma and other related hematological malignancies JMAJ 47(1): 32 37, 2004 Akihisa KANAMARU* and Takashi ASHIDA** *Professor, **Lecturer, Department
Interferon Therapy Practice of Interferon Therapy Multiple myeloma and other related hematological malignancies JMAJ 47(1): 32 37, 2004 Akihisa KANAMARU* and Takashi ASHIDA** *Professor, **Lecturer, Department
Biogen Idec Contacts: Media: Amy Brockelman (617) 914-6524 Investor: Eric Hoffman (617) 679-2812
 NEWS RELEASE Media: Nikki Levy (650) 225-1729 Investor: Susan Morris (650) 225-6523 Biogen Idec Contacts: Media: Amy Brockelman (617) 914-6524 Investor: Eric Hoffman (617) 679-2812 GENENTECH AND BIOGEN
NEWS RELEASE Media: Nikki Levy (650) 225-1729 Investor: Susan Morris (650) 225-6523 Biogen Idec Contacts: Media: Amy Brockelman (617) 914-6524 Investor: Eric Hoffman (617) 679-2812 GENENTECH AND BIOGEN
DECISION AND SUMMARY OF RATIONALE
 DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Clofarabine in the treatment of relapsed acute myeloid leukaemia (AML) The application was for clofarabine to remain in
DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Clofarabine in the treatment of relapsed acute myeloid leukaemia (AML) The application was for clofarabine to remain in
Clinical Trial Design. Sponsored by Center for Cancer Research National Cancer Institute
 Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases
 Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
J Clin Oncol 23:8447-8452. 2005 by American Society of Clinical Oncology INTRODUCTION
 VOLUME 23 NUMBER 33 NOVEMBER 20 2005 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T New Treatment Options Have Changed the Survival of Patients With Follicular Lymphoma Richard I. Fisher, Michael
VOLUME 23 NUMBER 33 NOVEMBER 20 2005 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T New Treatment Options Have Changed the Survival of Patients With Follicular Lymphoma Richard I. Fisher, Michael
NATIONAL CANCER DRUG FUND PRIORITISATION SCORES
 NATIONAL CANCER DRUG FUND PRIORITISATION SCORES Drug Indication Regimen (where appropriate) BORTEZOMIB In combination with dexamethasone (VD), or with dexamethasone and thalidomide (VTD), is indicated
NATIONAL CANCER DRUG FUND PRIORITISATION SCORES Drug Indication Regimen (where appropriate) BORTEZOMIB In combination with dexamethasone (VD), or with dexamethasone and thalidomide (VTD), is indicated
Bendamustine for the fourth-line treatment of multiple myeloma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine for the fourth-line treatment of multiple myeloma Contents Summary 1 Background 2 Epidemiology 3 Cost 6 References 7 Summary There is no standard
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine for the fourth-line treatment of multiple myeloma Contents Summary 1 Background 2 Epidemiology 3 Cost 6 References 7 Summary There is no standard
Salvage Chemotherapy with Dexamethasone, Etoposide, Ifosfamide and Cisplatin (DVIP) for Relapsing and Refractory Non-Hodgkin s Lymphoma
 IMAJ VOL 11 January 9 Salvage Chemotherapy with Dexamethasone, Etoposide, Ifosfamide and Cisplatin (DVIP) for Relapsing and Refractory Non-Hodgkin s Lymphoma Ariela Dortort Lazar MD 1,, Ofer Shpilberg
IMAJ VOL 11 January 9 Salvage Chemotherapy with Dexamethasone, Etoposide, Ifosfamide and Cisplatin (DVIP) for Relapsing and Refractory Non-Hodgkin s Lymphoma Ariela Dortort Lazar MD 1,, Ofer Shpilberg
Rituximab for the first-line treatment of stage III IV. D Papaioannou,* R Rafia, J Rathbone, M Stevenson, H Buckley Woods and J Stevens
 Rituximab for the first-line treatment of stage III IV follicular lymphoma Rituximab for the first-line treatment of stage III IV follicular lymphoma (review of Technology Appraisal No. 110): a systematic
Rituximab for the first-line treatment of stage III IV follicular lymphoma Rituximab for the first-line treatment of stage III IV follicular lymphoma (review of Technology Appraisal No. 110): a systematic
Outline of thesis and future perspectives.
 Outline of thesis and future perspectives. This thesis is divided into two different sections. The B- section involves reviews and studies on B- cell non- Hodgkin lymphoma [NHL] and radioimmunotherapy
Outline of thesis and future perspectives. This thesis is divided into two different sections. The B- section involves reviews and studies on B- cell non- Hodgkin lymphoma [NHL] and radioimmunotherapy
Avastin in Metastatic Breast Cancer
 Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Long Term Low Dose Maintenance Chemotherapy in the Treatment of Acute Myeloid Leukemia
 Long Term Low Dose Chemotherapy in the Treatment of Acute Myeloid Leukemia Murat TOMBULO LU*, Seçkin ÇA IRGAN* * Department of Hematology, Faculty of Medicine, Ege University, zmir, TURKEY ABSTRACT In
Long Term Low Dose Chemotherapy in the Treatment of Acute Myeloid Leukemia Murat TOMBULO LU*, Seçkin ÇA IRGAN* * Department of Hematology, Faculty of Medicine, Ege University, zmir, TURKEY ABSTRACT In
Lymphoma: The Roleof Nurses in the Treatment Process
 Lymphoma: The Roleof Nurses in the Treatment Process Sarah Liptrott MSc,BN(Hons), RN Istituto Europeo di Oncologia, Milan (IT) EBMT Swiss Study Day 2014, Zurich, Switzerland LymphomaManagement Watch &
Lymphoma: The Roleof Nurses in the Treatment Process Sarah Liptrott MSc,BN(Hons), RN Istituto Europeo di Oncologia, Milan (IT) EBMT Swiss Study Day 2014, Zurich, Switzerland LymphomaManagement Watch &
亞 東 紀 念 醫 院 Follicular Lymphoma 臨 床 指 引
 前 言 : 惡 性 淋 巴 瘤 ( 或 簡 稱 淋 巴 癌 ) 乃 由 體 內 淋 巴 系 統 包 括 淋 巴 細 胞 淋 巴 管 淋 巴 腺 及 一 些 淋 巴 器 官 或 組 織 如 脾 臟 胸 腺 及 扁 桃 腺 等 所 長 出 的 惡 性 腫 瘤 依 腫 瘤 病 理 組 織 型 態 的 不 同 可 分 為 何 杰 金 氏 淋 巴 瘤 (Hodgkin s disease) 與 非 何 杰 金
前 言 : 惡 性 淋 巴 瘤 ( 或 簡 稱 淋 巴 癌 ) 乃 由 體 內 淋 巴 系 統 包 括 淋 巴 細 胞 淋 巴 管 淋 巴 腺 及 一 些 淋 巴 器 官 或 組 織 如 脾 臟 胸 腺 及 扁 桃 腺 等 所 長 出 的 惡 性 腫 瘤 依 腫 瘤 病 理 組 織 型 態 的 不 同 可 分 為 何 杰 金 氏 淋 巴 瘤 (Hodgkin s disease) 與 非 何 杰 金
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES
 BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Clinical Development Program Prof. Moshe Phillip, MD VP Clinical & Medical Affairs 1 Rationale for BL-8040 Development
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Clinical Development Program Prof. Moshe Phillip, MD VP Clinical & Medical Affairs 1 Rationale for BL-8040 Development
Follicular Lymphoma. 1. Introduction. 2. Diagnosis. 3. Baseline Investigations
 Follicular Lymphoma Follicular Lymphoma Treatment policy prepared by Dr. Louise Bordeleau 1. Introduction Follicular lymphoma is the second most frequent type of non-hodgkin s lymphoma. These lymphomas
Follicular Lymphoma Follicular Lymphoma Treatment policy prepared by Dr. Louise Bordeleau 1. Introduction Follicular lymphoma is the second most frequent type of non-hodgkin s lymphoma. These lymphomas
MEDICAL COVERAGE POLICY
 Important note Even though this policy may indicate that a particular service or supply is considered covered, this conclusion is not necessarily based upon the terms of your particular benefit plan. Each
Important note Even though this policy may indicate that a particular service or supply is considered covered, this conclusion is not necessarily based upon the terms of your particular benefit plan. Each
Cure versus control: Which is the best strategy?
 Cure versus control: Which is the best strategy? Barcelona 8-9-2012 Mario Boccadoro DIVISIONE UNIVERSITARIA DI EMATOLOGIA AZIENDA OSPEDALIERA SAN GIOVANNI TORINO, ITALY MULTIPLE MYELOMA Cure versus control
Cure versus control: Which is the best strategy? Barcelona 8-9-2012 Mario Boccadoro DIVISIONE UNIVERSITARIA DI EMATOLOGIA AZIENDA OSPEDALIERA SAN GIOVANNI TORINO, ITALY MULTIPLE MYELOMA Cure versus control
Future strategies for myeloma: An overview of novel treatments In development
 Future strategies for myeloma: An overview of novel treatments In development Dr. Matthew Streetly Guys and St. Thomas NHS Trust How far have we come? Melphalan and prednisolone VAD Autologous SCT Thalidomide
Future strategies for myeloma: An overview of novel treatments In development Dr. Matthew Streetly Guys and St. Thomas NHS Trust How far have we come? Melphalan and prednisolone VAD Autologous SCT Thalidomide
cancer cancer Hessamfar-Bonarek M et al. Int. J. Epidemiol. 2010;39:135-146
 Hematopoietic Stem Cell Transplant in HIV- related lymphoma Song Zhao, MD PhD Hematology-Oncology Program University of Washington/FHCRC Underlying Causes of Death in HIV-infected Adults 2000 2005 cancer
Hematopoietic Stem Cell Transplant in HIV- related lymphoma Song Zhao, MD PhD Hematology-Oncology Program University of Washington/FHCRC Underlying Causes of Death in HIV-infected Adults 2000 2005 cancer
Perspectives on Recent Non-Hodgkin s Lymphoma (NHL) Data
 Perspectives on Recent Non-Hodgkin s Lymphoma (NHL) Data Summary Article James O. Armitage, MD Presented through a strategic collaboration by A series of 5 expert interviews were conducted, focusing on
Perspectives on Recent Non-Hodgkin s Lymphoma (NHL) Data Summary Article James O. Armitage, MD Presented through a strategic collaboration by A series of 5 expert interviews were conducted, focusing on
Lymphoma Diagnosis and Classification
 Lymphoma Diagnosis and Classification By Atef Shrit, MD, Pathology B- and T/NK-cell lymphomas are clonal neoplasms of immature and mature B-lymphocytes, T-lymphocytes or natural killer cells at various
Lymphoma Diagnosis and Classification By Atef Shrit, MD, Pathology B- and T/NK-cell lymphomas are clonal neoplasms of immature and mature B-lymphocytes, T-lymphocytes or natural killer cells at various
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
The Role of Cytotoxic Therapy with Hematopoietic Stem Cell Transplantation in the Treatment of Follicular Lymphoma: An Evidence-Based Review
 REVIEW The Role of Cytotoxic Therapy with Hematopoietic Stem Cell Transplantation in the Treatment of Follicular Lymphoma: An Evidence-Based Review Denise M. Oliansky, 1 Leo I. Gordon, 2 Jerry King, 3
REVIEW The Role of Cytotoxic Therapy with Hematopoietic Stem Cell Transplantation in the Treatment of Follicular Lymphoma: An Evidence-Based Review Denise M. Oliansky, 1 Leo I. Gordon, 2 Jerry King, 3
Follicular Lymphoma: Current Management and Future Directions
 Treatment decisions and outcomes for patients with follicular lymphoma may be improved with newer diagnostic tests and novel targeted agents. George Van Hook. Barns in Summer. Oil on linen, 22 28. Follicular
Treatment decisions and outcomes for patients with follicular lymphoma may be improved with newer diagnostic tests and novel targeted agents. George Van Hook. Barns in Summer. Oil on linen, 22 28. Follicular
bnmqwertyuiopasdfghjklzxcvbn mqwertyuiopasdfghjklzxcvbnm qwertyuiopasdfghjklzxcvbnmq ertyuiopasdfghjklzxcvbnmqwer tyuiopasdfghjklzxcvbnmqwerty
 bnmqwertyuiopasdfghjklzxcvbn mqwertyuiopasdfghjklzxcvbnm qwertyuiopasdfghjklzxcvbnmq wertyuiopasdfghjklzxcvbnmqw ertyuiopasdfghjklzxcvbnmqwer Follicular Lymphoma Overview tyuiopasdfghjklzxcvbnmqwerty Lymphoma
bnmqwertyuiopasdfghjklzxcvbn mqwertyuiopasdfghjklzxcvbnm qwertyuiopasdfghjklzxcvbnmq wertyuiopasdfghjklzxcvbnmqw ertyuiopasdfghjklzxcvbnmqwer Follicular Lymphoma Overview tyuiopasdfghjklzxcvbnmqwerty Lymphoma
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on
 A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on examination. She also has fever, weight loss, and sweats. What
A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on examination. She also has fever, weight loss, and sweats. What
Clinical Features, Prognosis and Treatment of Follicular Lymphoma
 Clinical Features, Prognosis and Treatment of Follicular Lymphoma Gilles A. Salles Hospices Civils de Lyon; Université Lyon 1, Pierre-Bénite, France Follicular lymphoma constitutes the most frequent indolent
Clinical Features, Prognosis and Treatment of Follicular Lymphoma Gilles A. Salles Hospices Civils de Lyon; Université Lyon 1, Pierre-Bénite, France Follicular lymphoma constitutes the most frequent indolent
Pro Cure in Multiple Myeloma. Nicolaus Kröger Dept. of Stem Cell Transplantation University Hospital Hamburg Hamburg, Germany
 Pro Cure in Multiple Myeloma Nicolaus Kröger Dept. of Stem Cell Transplantation University Hospital Hamburg Hamburg, Germany Pro Cure in Multiple Myeloma Several hematological malignancies can be cured
Pro Cure in Multiple Myeloma Nicolaus Kröger Dept. of Stem Cell Transplantation University Hospital Hamburg Hamburg, Germany Pro Cure in Multiple Myeloma Several hematological malignancies can be cured
Effective on or after May 1, 2015,, refer to: Blue Cross and Blue Shield of Alabama Radiation Therapy Management RTM Policies
 Effective on or after May 1, 2015,, refer to: Blue Cross and Blue Shield of Alabama Radiation Therapy Management RTM Policies Name of Policy: Radioimmunotherapy in the Treatment of Non-Hodgkin Lymphoma
Effective on or after May 1, 2015,, refer to: Blue Cross and Blue Shield of Alabama Radiation Therapy Management RTM Policies Name of Policy: Radioimmunotherapy in the Treatment of Non-Hodgkin Lymphoma
In non-hodgkin s lymphoma, MabThera is used to treat two types of the disease, both of which affect B-lymphocytes:
 EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma. Claire Vines, 2016 Pharm.D. Candidate
 + Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma Claire Vines, 2016 Pharm.D. Candidate + Disclosure I have no conflicts of interest to disclose. + Objectives Summarize NCCN
+ Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma Claire Vines, 2016 Pharm.D. Candidate + Disclosure I have no conflicts of interest to disclose. + Objectives Summarize NCCN
Radioimmunotherapy in the Treatment of Non-Hodgkin Lymphoma. Original Policy Date
 MP 8.01.28 Radioimmunotherapy in the Treatment of Non-Hodgkin Lymphoma Medical Policy Section Therapy Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013
MP 8.01.28 Radioimmunotherapy in the Treatment of Non-Hodgkin Lymphoma Medical Policy Section Therapy Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013
Guidelines of the Belgian Hematological Society for newly diagnosed and relapsed follicular lymphoma 2012
 2 Guidelines of the Belgian Hematological Society for newly diagnosed and relapsed follicular lymphoma 2012 S. Debussche, A. Van Hoof, A. Sonnet, C. Bonnet, A. Janssens, G. Verhoef, D. Dierickx, V. De
2 Guidelines of the Belgian Hematological Society for newly diagnosed and relapsed follicular lymphoma 2012 S. Debussche, A. Van Hoof, A. Sonnet, C. Bonnet, A. Janssens, G. Verhoef, D. Dierickx, V. De
6/3/2013. Follicular and Other Slow Growing Lymphomas. Stephen Ansell, MD, PhD Mayo Clinic
 Follicular and Other Slow Growing Lymphomas Stephen Ansell, MD, PhD Mayo Clinic 1 Learning Objectives Start with an overview of Follicular and other slow growing lymphomas Discuss current and emerging
Follicular and Other Slow Growing Lymphomas Stephen Ansell, MD, PhD Mayo Clinic 1 Learning Objectives Start with an overview of Follicular and other slow growing lymphomas Discuss current and emerging
This information can also be found in the Summit 2011 Program on page 8.
 If you would like to receive CME credit for this activity, please visit: http://www.pesgce.com/pvasummit2011/ This information can also be found in the Summit 2011 Program on page 8. Newer Treatments:
If you would like to receive CME credit for this activity, please visit: http://www.pesgce.com/pvasummit2011/ This information can also be found in the Summit 2011 Program on page 8. Newer Treatments:
MALIGNANT LYMPHOMAS. Dr. Olga Vujovic (Updated August 2010)
 MALIGNANT LYMPHOMAS Dr. Olga Vujovic (Updated August 2010) Malignant lymphomas consist of Hodgkin and non-hodgkin lymphomas. The current management of these diseases involves a multi-disciplinary approach.
MALIGNANT LYMPHOMAS Dr. Olga Vujovic (Updated August 2010) Malignant lymphomas consist of Hodgkin and non-hodgkin lymphomas. The current management of these diseases involves a multi-disciplinary approach.
Supplementary Online Content
 Supplementary Online Content Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving
Supplementary Online Content Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving
TITLE: Rituximab for Non-Hodgkin s Lymphoma: A Review of the Clinical and Cost- Effectiveness and Guidelines
 TITLE: Rituximab for Non-Hodgkin s Lymphoma: A Review of the Clinical and Cost- Effectiveness and Guidelines DATE: 11 January 2010 CONTEXT AND POLICY ISSUES: Non-Hodgkin s lymphoma is the most common hematological
TITLE: Rituximab for Non-Hodgkin s Lymphoma: A Review of the Clinical and Cost- Effectiveness and Guidelines DATE: 11 January 2010 CONTEXT AND POLICY ISSUES: Non-Hodgkin s lymphoma is the most common hematological
