Germany; Munich, Germany;
|
|
|
- Leslie Hicks
- 8 years ago
- Views:
Transcription
1 Treatment of limited stage follicular lymphoma with Rituximab immunotherapy and involved field radiotherapy in a prospective multicenter Phase II trial MIR trial Mathias Witzens-Harig 1, Manfred Hensel 2, Michael Unterhalt 3, Klaus Herfarth 4 1 Department of Internal Medicine V, University of Heidelberg, INF Heidelberg, Germany; mathias.witzens-harig@med.uni-heidelberg.de 2 Onkologische Schwerpunkt Praxis, Q , Mannheim, Germany; hensel@mannheimer-onkologie-praxis.de 3 Department Internal Medicine III, University of Munich (LMU), Marchioninistr. 15, Munich, Germany; michael.unterhalt@med.uni-muenchen.de 4 Department of Radiation Oncology, University of Heidelberg, INF 400; Heidelberg, Germany; klaus.herfarth@med.uni-heidelberg.de Corresponding author: Klaus Herfarth, MD Department of Radiation Oncology, University of Heidelberg INF Heidelberg, Germany phone: fax: klaus.herfarth@med.uni-heidelberg.de
2 Abstract Background The optimal treatment of early stage follicular Lymphoma is a matter of debate. Radiation therapy has frequently been applied with a curative approach beside watchful waiting. Involved field, extended field and total nodal radiation techniques are used in various protocols, but the optimal radiation field still has to be defined. Follicular lymphoma is characterized by stable expression of the CD20 antigen on the tumour cells surface. The anti CD20 antibody Rituximab (Mabthera ) has shown to be effective in systemic therapy of FL in primary treatment, relapse and maintenance therapy. Methods/design The MIR (Mabthera and Involved field Radiation) study is a prospective multicenter trial combining systemic treatment with the anti CD20 antibody Rituximab (Mabthera ) in combination with involved field radiotherapy (30-40 Gy). This trial aims at testing the combination s efficacy and safety with an accrual of 85 patients. Primary endpoint of the study is progression free survival. Secondary endpoints are response rate to Rituximab, complete remission rate at week 18, relapse rate, relapse pattern, relapse free survival, overall survival, toxicity and quality of life. Trial Registration: ClinicalTrials.gov Identifier: NCT
3 Background Worldwide, follicular lymphoma (FL) is the second most common type of non-hodgkin's lymphoma (NHL) 1 with a rapidly increasing incidence in the last decades 2. In the group of indolent lymphomas, FL is the most common type with 70% of cases. FL tumor cells are characterized by the translocation t (14;18)(q32;q21)and invariably express the CD20 antigen. The WHO Classification differentiates between grade I, II and III follicular lymphoma in respect to the number of centroblasts in the visual microscopy field. FL grade III is often regarded as an aggressive lymphoma and treated accordingly. Most patients are diagnosed in advanced disease stage and cannot be cured with conventional therapy. However, 15-30% patients are diagnosed in the limited Ann Arbor stages I and II and are potential candidates for a curative treatment approach. Standard of treatment for patients with limited disease with a curative intention is radiotherapy. However, no consensus of the required radiation field has been reached. Table 1 depicts the results of various radiotherapy trials in FL lymphoma in stage I and II. Most of the trials are retrospective analysis. Involved field (IF), extended field (EF) and total nodal (TN) irradiation techniques have been applied. The relapse free survival rate after 10 years was between 38% and 72% and the 10 year overall survival ranged in between 50% and 78%. Median survival was above 12 years, however, in some of the international trials which categorized lymphomas according to the Working Formulation also FL grade III and mantle cell lymphoma were part of the analysis. Forty-one percent of the patients had a relapse after 8 years in a prospective German observation trial with 117 patients with early stage FL who were treated with EF or TN. In stage I patients, there was no relapse in the irradiation field after 7 years, but 15% out of filed relapses. This trial led to the conclusion that large volume radiation techniques may prevent relapses. However, as extensive radiation protocols are associated with significant toxicities, grade 3 and grade 4 adverse events concerning the hematopoietic system were observed in 22% of patients 3.
4 There have been several studies which combined radiation therapy with systemic chemotherapy in early stage FL. Most studies failed to demonstrate a benefit of combined therapy 4, 5, 6, 7. In one study, the sequential administration of COP, CHOP-B and involved field irradiation improves relapse free survival, but not overall survival in comparison to historical cohort. Relapse free survival after 10 years was 72%, however 22% of patients experienced a grade IV neutropenia and 14 secondary malignancies were observed 8, 9. The monoclonal chimeric anti CD 20 antibody Rituximab has revolutionized the treatment of FL in the last decade. The pivotal phase II trial tested Rituximab monotherapy in 37 patients with refractory or relapsed FL. The overall response rate (ORR) was 46% with 8% complete responses (CR). The median time to progression (TTP) reached 10.2 months 10. These promising results as well as other phase II clinical trials demonstrated a significant single agent activity of Rituximab in pretreated as well as in previously untreated patients with FL 11, 12, 13. In combination with CHOP chemotherapy, Rituximab induced responses in all evaluable patients with a complete remission (CR) rate of 63% and a median PFS of 82 months in a Phase II trial 14. These results were confirmed in four prospective randomised phase III studies investigating Rituximab in combination chemotherapy versus chemotherapy alone in first line therapy which showed significant increase in initial response rates, a significant prolongation of response duration and a significantly longer overall survival 15, 16, 17, 18. Rituximab was added to FCM (Fludarabine, Cyclophosphamde, Mitoxantrone) chemotherapy in relapsed FL in a prospective randomized Phase III trial and showed a significantly longer PFS and OS compared with FCM alone 19. In addition to the impressing results of Rituximab as part of first line and relapse treatment, Rituximab maintenance therapy is effective in relapsed FL 20, 21, 22. Rituximab maintenance therapy has also been shown to prolong PFS after 3 years to 64% versus 33% in the control group after first-line
5 therapy in a recent trial 23 and is currently tested in two additional prospective randomized trials 24, 25. In the context of radiotherapy, the high efficacy of Rituximab in systemic treatment suggests that this drug may be of benefit in the eradication of minimal disease outside the radiation field. This concept is in line with the observation that Rituximab contributes to the elimination of minimal residual disease after cytotoxic therapy 26, 27, 28. In addition, Rituximab may enhance radiosensitivity of lymphoma cells and may thus improve the efficacy of radiotherapy Methods/design Trial organization The MIR trial has been designed by the Trial Center of the Department of Radiation Oncology and the Department of Internal Medicine in cooperation with the German Study Group for Low Grade Lymphoma (GLSG). The trial is an investigator initiated trial. Sponsor of the trial is the University Hospital of Heidelberg. The trial medication (Rituximab) is supplied by Roche, Basel, Switzerland. The trial receives financial support by Roche Pharma, Basel, Switzerland Coordination The trial is coordinated by the Department of Radiation Oncology of the University of Heidelberg in cooperation with the GLSG. The Dept. of Radiation Oncology is responsible for overall trial management, trial registration (ClinicalTrials.gov Identifier: NCT ), database management, quality assurance including monitoring, reporting and for the scientific program of all trial related meetings.
6 Participating Centres A total of 15 centers in Germany participate in this trial. The centers are (listed alphabetically): Universityhospital Charité, Berlin; HELIOS Hospital, Berlin-Buch; Universityhospital Dresden, Universityhospital Essen; Universityhospital Göttingen; Universityhospital Hannover; Universityhospital Heidelberg; Universityhospital Cologne; Universityhospital Mainz; Universityhospital Mannheim; Universityhospital Marburg; Universityhospital Munich LMU; Universityhospital Munich TU; Universityhospital Ulm. On-site monitoring During recruitment of patients monitoring on site is performed according to good clinical practice (GCP) guidelines. The data management will be performed by the Coordination Centre for Clinical Trials (KKS) of the University of Heidelberg. Ethics, informed consent and safety The final protocol was approved by the ethics committee of the University of Heidelberg, Medical School (AFmu-085/2007, and the Paul- Ehrlich-Institute (PEI-registration number 432/06). This study complies with the Helsinki Declaration in its recent German version, the Medical Association's professional code of conduct, the principles of Good Clinical Practice (GCP) guidelines and the Federal Data Protection Act. The trial will also be carried out in keeping with local legal and regulatory requirements. The medical secrecy and the Federal Data Protection Act will be followed. Written informed consent is obtained from each patient in oral and written form before inclusion in the trial and the nature, scope, and possible consequences of the trial have been explained by a physician. The investigator will not undertake any measures specifically required only for the clinical trial until valid consent has been obtained. Study design and endpoints
7 The MIR study is a prospective phase II study combining systemic Rituximab therapy with involved field irradiation in patients with FL WHO Grad 1 and 2 in Stage I and II. This trial aims at testing the combination s efficacy and toxicity with an accrual of 85 patients. Primary endpoint of the study is progression free survival (2 years). Secondary endpoints are complete remission rate after Rituximab monotherapy (week 7) and after complete treatment (week 18), relapse rate, relapse pattern, relapse free survival, overall survival, toxicity and quality of life. Patient selection In order to be included in the MIR trial, patients (18 to 75 years) are required to have pathologically documented FL Who grade 1 or 2 in Ann Arbor Stage I or II. The histology has to be confirmed by one of the reference pathologists of the GLSG. Evaluation of CD20 status is compulsory. Maximal tumour diameter is 7 cm. Accrued patients will be expected to demonstrate sufficient compliance, they should also live in relative proximity of the centre of care to ensure adequate follow-up after treatment. In addition, adequate haematological function is essential for inclusion in the trial (leukocytes 3000 x 10 3 / ml, platelets x 10 3 / ml, and haemoglobin 10 g/dl). Patients are required to use adequate contraception during and at least up to 3 months after treatment. Naturally, written informed consent is obtained prior to commencement of treatment in this trial. Patients are not eligible with previous irradiation, chemotherapy of immunotherapy. Patients with severe concurrent systemic disease or other malignant disease (apart from cervical carcinoma in-situ, basal cell carcinoma, or unless previously treated and in remission for 5 years without further treatment) can also not be included in this trial. Furthermore, clinical performance worse than ECOG 2, bulky disease (> 7cm), splenic involvement,
8 immunodeficiency, viral hepatitis, collagenosis, severe psychiatric illness, hypersensitivity to foreign proteins will prohibit inclusion as will pregnancy or breast feeding. Work up Patients with pathologically documented nodal FL Who grade 1 or 2 in stage I or II receive a complete work-up including, cervical, thoracic, abdominal and pelvic CT scans, bone marrow histology and extensive laboratory testing. An ENT examination is mandatory for supra diaphragmal disease and a kidney function test for abdominal disease. The FLIPI risk score will be calculated for each patient. All in- and exclusion criteria are examined. In the event of patients meeting the required inclusion criteria, information about participation in the study with possible risks and benefits is given to the patients and written informed consent is obtained. In the event of patients declining treatment within the MIR trial, irradiation alone is offered. Treatment The treatment lasts over 12 weeks and is divided in 3 sections as illustrated in Figure 1. The first section consists of 4 weekly i.v. infusions of Rituximab (Roche AG, Basel, Switzerland) at a standard dosage of 375 mg/m 2 given on day 1, 8, 15 and 22. There is a 4 week treatment break thereafter (week 5-8) which includes a radiation therapy planning CT of the involved body region in week 7. Response to the Rituximab therapy is also evaluated at this time. Another 4 weekly infusions of Rituximab are administered in week At the same time, radiation therapy of the involved lymph node region is applied, a three dimensional radiation planning is compulsory for all patients having abdominal or retroperitoneal disease. Involved field irradiation consists of a dose of 30 Gy in daily fractions of 2 Gy (Monday to Friday). Patients who still show enlarged lymph nodes in week 7 receive additional boost irradiation with a total dose of 40 Gy. Accepted tolerance doses of organs at risk must not be exceeded.
9 Safety and discontinuation of treatment Toxicities are classified by grade, type, duration, onset, and relationship to study treatment. Treatment of Rituximab-induced adverse reactions is carried according to recommendations by the provider. All patients will receive anti allergic prophylaxis (paracetamol, clemastin) and when appropriate, corticosteroids. In the case of fever (> 38,5 C), chills, bronchial constriction, hypotension (>30 mm Hg) it is recommended to discontinue treatment with Rituximab. Analysis of safety related data is performed with respect to frequency of: Serious Adverse Events and Adverse Events stratified by organ-system Adverse Events stratified by severity Adverse Events stratified by causality. Patient toxicities will be assessed using the NCI Common Toxicity Criteria (CTC). Toxicity will be evaluated prior to treatment, weekly prior to each course of infusional Rituximab and at follow-up. Unacceptable toxicity is defined as unpredictable, or irreversible Grade 4 toxicity. Decisions regarding Rituximab dose-adjustment will be made using the guidelines below and based on haematological parameters (ANC and platelets) monitored weekly during radiation before each dose of Rituximab. Supportive therapy Whenever necessary, metoclopramide or 5-HT 3 -antagonists are used for antiemesis. Antihistamines such as clemastine are administered prior to Rituximab application. Trial duration Patient recruitment is planned to be completed after 24 months. Patients will be monitored for two and a half years after study entry. The total duration of the trial is estimated to be 4 and a
10 half years. Recruitment started in March As of February 2010, 65 patients have been included in the trial. Individual exclusion criteria are serious adverse reactions or the patient s voluntary withdrawal from the trial. Assessment of therapeutic efficiency Methods/ Investigations CT or MRT of all involved regions in week 7. CT or MRT of all involved regions in week 18. Clinical examination with palpation all lymph node sites in after 6 months, 12 months, 18 months, 24 months and 30 months after start of therapy. Cervical, thoracic, abdominal and pelvic CT or MRI scans 6 months, 12 months, 18 months, 24 months and 30 months after start of therapy. Evaluation Local response is evaluated in accordance with the Cheson Criteria 31. Complete response (CR) is the complete disappearance of all detectable evidence of disease on CT, and all disease-related symptoms, and normalization of biochemical abnormalities, and normal bone marrow biopsy (BMB). Previously involved nodes on CT more than 1.5 cm in their greatest axial diameter must regress to less than 1.5 cm, and previously measured nodes of cm must decrease to less than 1 cm. CRu (uncertain) corresponds to CR criteria but with a residual mass more than 1.5 cm in greatest axial diameter that has regressed by more than 75%. Partial response (PR) is at least 50% reduction in the sum of the product of the greatest diameters (SPD) of the six largest nodes with no increase in the size of other nodes and no new sites of disease. Splenic and hepatic nodules must regress by at least 50% in the SPD. Stable disease (SD) is less than a PR but is not progressive disease. Progressive disease (PD) is more than 50% increase in the sum of the product of the greatest
11 diameters of any previously abnormal node, or appearance of any new lesions during or at the end of therapy. Relapsed disease (RD) is the appearance of any new lesion or increase in size of more than 50% of previously involved sites or nodes in patients who achieved CR or CRu. Quality assurance program All staging examinations are reviewed centrally as part of the quality assurance program. Completeness of the staging, quality of the imaging and extend of involvement are analysed. In addition, a proposal for the radiation site is sent out to the treatment centre. All radiation treatment plans are also centrally reviewed in a retrospective manner. Summary The MIR trial is evaluating the effectiveness and the safety of the combination of an involved field radiation therapy with the systemic anti CD20 antibody Rituximab for early stage follicular lymphoma grade 1 or 2 in a multi center setting. Competing interests There a no competing interests Authors contribution KKH and MH planned the study. KKH and MWH coordinated and conducted the study and wrote the manuscript. MU was responsible for the statistical design. Acknowledgments Study protocol committee: Martin Dreyling (Munich), Marianne Engelhard (Essen), Bertram Glass (Hamburg), Michael Kneba (Kiel), Heinz Schmidberger (Mainz), Stefan Stilgenbauer (Ulm), Martin Stuschke (Essen), Lothar Trümper (Göttingen), Andreas Viardot (Ulm), Thomas Wiegel (Ulm)
12 Trial medication (Rituximab, Mabthera ) is supplied by Roche AG, Basel, Switzerland. The trial is supported in part by Roche Pharma AG, Basel, Switzerland Figure Legend: Figure 1. Study Schema of the MIR trial (IF= involved-field; f/u = follow up) Reference List 1. Armitage JO, Weisenburger DD. New approach to classifying non-hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol 1998;16: Groves FD, Linet MS, Travis LB, Devesa SS. Cancer surveillance series: non-hodgkin's lymphoma incidence by histologic subtype in the United States from 1978 through J.Natl.Cancer Inst. 2000;92: Stuschke M, Hoederath A, Sack H et al. Extended field and total central lymphatic radiotherapy in the treatment of early stage lymph node centroblastic-centrocytic lymphomas: results of a prospective multicenter study. Study Group NHL- fruhe Stadien. Canc 1997;80: Monfardini S, Banfi A, Bonadonna G et al. Improved five year survival after combined radiotherapychemotherapy for stage I-II non-hodgkin's lymphoma. Int.J.Radiat.Oncol.Biol.Phys. 1980;6: Landberg TG, Hakansson LG, Moller TR et al. CVP-remission-maintenance in stage I or II non- Hodgkin's lymphomas: preliminary results of a randomized study. Canc 1979;44: Kelsey SM, Newland AC, Hudson GV, Jelliffe AM. A British National Lymphoma Investigation randomised trial of single agent chlorambucil plus radiotherapy versus radiotherapy alone in low grade, localised non-hodgkins lymphoma. Med.Oncol. 1994;11: Yahalom J, Varsos G, Fuks Z et al. Adjuvant cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy after radiation therapy in stage I low-grade and intermediate-grade non- Hodgkin lymphoma. Results of a prospective randomized study. Canc 1993;71: McLaughlin P, Fuller L, Redman J et al. Stage I-II low-grade lymphomas: a prospective trial of combination chemotherapy and radiotherapy. Ann.Oncol. 1991;2 Suppl 2:
13 9. Seymour JF, Pro B, Fuller LM et al. Long-term follow-up of a prospective study of combined modality therapy for stage I-II indolent non-hodgkin's lymphoma. J.Clin.Oncol. 2003;21: Maloney DG, Grillo LA, Bodkin DJ et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-hodgkin's lymphoma. J Clin.Oncol. 1997;15: McLaughlin P, Grillo-Lopez AJ, Link BK et al. Rituximab chimeric anti-cd20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin.Oncol 1998;16: Feuring-Buske M, Kneba M, Unterhalt M et al. IDEC-C2B8 (Rituximab) anti-cd20 antibody treatment in relapsed advanced-stage follicular lymphomas: results of a phase-ii study of the German Low-Grade Lymphoma Study Group. Ann.Hematol. 2000;79: Colombat P, Salles G, Brousse N et al. Rituximab (anti-cd20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood 2001;97: Czuczman MS, Weaver R, Alkuzweny B, Berlfein J, Grillo-Lopez AJ. Prolonged clinical and molecular remission in patients with low-grade or follicular non-hodgkin's lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J.Clin.Oncol. 2004;22: Herold M, Haas A, Srock S et al. Rituximab Added to First-Line Mitoxantrone, Chlorambucil, and Prednisolone Chemotherapy Followed by Interferon Maintenance Prolongs Survival in Patients With Advanced Follicular Lymphoma: An East German Study Group Hematology and Oncology Study. J Clin Oncol 2007;.25: Salles G, Mounier N, de GS et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the GELA-GOELAMS FL2000 study. Blood 2008;112: Hiddemann W, Kneba M, Dreyling M et al. Front-line therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) significantly improves the outcome of patients with advanced stage follicular lymphomas as compared to CHOP alone - results of a prospective randomized study of the german low grade lymphoma study group (GLSG). Blood 2005.Aug.25.;. 2005;.:
14 18. Marcus R, Imrie K, Solal-Celigny P et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J.Clin.Oncol. 2008;26: Forstpointner R, Dreyling M, Repp R et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2004;104: van Oers MH, Klasa R, Marcus RE et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood 2006;108: Ghielmini M, Schmitz SF, Cogliatti SB et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood 2004;103: Forstpointner R, Unterhalt M, Dreyling M et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood 2006;108: Hochster H, Weller E, Gascoyne RD et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 Study. J.Clin.Oncol. 2009;27: OSHO 70 Intergroup Study of the Eastern German Haematology/Oncology Study Group and the German Study Group for Indolent Lymphom Unpublished Work 25. PRIMA (primary rituximab in maintenance) International Intergroup Study (GELA, HOVON, GLSG, OSHO, ALLG, NCRI) Unpublished Work
15 26. Brugger W, Hirsch J, Grunebach F et al. Rituximab consolidation after high-dose chemotherapy and autologous blood stem cell transplantation in follicular and mantle cell lymphoma: a prospective, multicenter phase II study. Ann.Oncol. 2004;15: Galimberti S, Guerrini F, Morabito F et al. Quantitative molecular evaluation in autotransplant programs for follicular lymphoma: efficacy of in vivo purging by Rituximab. Bone Marrow Transplant 2003;32: Rambaldi A, Lazzari M, Manzoni C et al. Monitoring of minimal residual disease after CHOP and rituximab in previously untreated patients with follicular lymphoma. Blood 2002;99: Skvortsova I, Popper BA, Skvortsov S et al. Pretreatment with rituximab enhances radiosensitivity of non-hodgkin's lymphoma cells. J.Radiat.Res.(Tokyo) 2005;46: Skvortsova I, Skvortsov S, Popper BA et al. Rituximab enhances radiation-triggered apoptosis in non- Hodgkin's lymphoma cells via caspase-dependent and - independent mechanisms. J Radiat.Res.(Tokyo) 2006;47: Cheson BD, Horning SJ, Coiffier B et al. Report of an international workshop to standardize response criteria for non-hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244.
16 Figure 1
CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY
 CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY 26.1 Introduction rituximab Subsequent to the completion of drafts for the guidelines earlier in 2004,
CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY 26.1 Introduction rituximab Subsequent to the completion of drafts for the guidelines earlier in 2004,
Guidelines for the Management of Follicular Lymphoma
 Guidelines for the Management of Follicular Lymphoma Scope The following guidance for first- and second-line therapy applies to follicular lymphoma histological grades 1, 2 and 3a according to the World
Guidelines for the Management of Follicular Lymphoma Scope The following guidance for first- and second-line therapy applies to follicular lymphoma histological grades 1, 2 and 3a according to the World
Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma
 a report by Martin Dreyling Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma Head, Lymphoma Section, Department of Medicine III, University Hospital Großhadern, Ludwig Maximilians-University
a report by Martin Dreyling Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma Head, Lymphoma Section, Department of Medicine III, University Hospital Großhadern, Ludwig Maximilians-University
FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma
 Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
Frequency of NHL Subtypes in Adults
 Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003)
 Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
David Loew, LCL MabThera
 MabThera The star continues to rise David Loew, LCL MabThera MabThera the star continues to raise Group sales (CHF bn) 4,5 4,0 3,5 3,0 2,5 2,0 1,5 1,0 0,5 0,0 2001 2002 2003 2004 2005 Outstanding clinical
MabThera The star continues to rise David Loew, LCL MabThera MabThera the star continues to raise Group sales (CHF bn) 4,5 4,0 3,5 3,0 2,5 2,0 1,5 1,0 0,5 0,0 2001 2002 2003 2004 2005 Outstanding clinical
Treatment of low-grade non-hodgkin lymphoma
 Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN
 + IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
+ IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
Histopathologic results
 Self evaluation 1 Clinical Case 55-year-old woman Bilateral enlargement of cervical, axillary and inguinal lymph nodes, largest diameter > 6 cm Hepatosplenomegaly. Enlargement of retroperitoneal, mesenteric
Self evaluation 1 Clinical Case 55-year-old woman Bilateral enlargement of cervical, axillary and inguinal lymph nodes, largest diameter > 6 cm Hepatosplenomegaly. Enlargement of retroperitoneal, mesenteric
Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy
 Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy Yoichi Kitamura, MD Kazuhiko Hayashi, MD Kazumi Uchida,
Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy Yoichi Kitamura, MD Kazuhiko Hayashi, MD Kazumi Uchida,
Rituximab Maintenance for 2 Years in Patients with Untreated High Tumor Burden Follicular Lymphoma After Response to Immunochemotherapy
 Rituximab Maintenance for 2 Years in Patients with Untreated High Tumor Burden Follicular Lymphoma After Response to Immunochemotherapy G. A. Salles, J. F. Seymour, P. Feugier, F. Offner, A. Lopez-Guillermo,
Rituximab Maintenance for 2 Years in Patients with Untreated High Tumor Burden Follicular Lymphoma After Response to Immunochemotherapy G. A. Salles, J. F. Seymour, P. Feugier, F. Offner, A. Lopez-Guillermo,
Aggressive lymphomas. Michael Crump Princess Margaret Hospital
 Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Monoclonal Antibody Therapy for Lymphoma: Targeting CD20
 Monoclonal Antibody Therapy for Lymphoma: Targeting CD20 Session Chair: Jonathan W. Freidberg, MD Speakers: Michele Ghielmini, MD; Jonathan W. Friedberg, MD; and John P. Leonard, MD Multimodality Therapies
Monoclonal Antibody Therapy for Lymphoma: Targeting CD20 Session Chair: Jonathan W. Freidberg, MD Speakers: Michele Ghielmini, MD; Jonathan W. Friedberg, MD; and John P. Leonard, MD Multimodality Therapies
HOVON Staging and Response Criteria for Non-Hodgkin s Lymphomas Page 1
 HOVON Staging and Response Criteria for Non-Hodgkin s Lymphomas Page 1 This document describes the minimally required staging and evaluation procedures and response criteria that will be applied in all
HOVON Staging and Response Criteria for Non-Hodgkin s Lymphomas Page 1 This document describes the minimally required staging and evaluation procedures and response criteria that will be applied in all
Rituximab in the Management of Follicular Lymphoma
 Hong Kong J Radiol. 2011;14(Suppl):S63-7 REVIEW ARTICLE Rituximab in the Management of Follicular Lymphoma YL Kwong Department of Medicine, Professorial Block, Queen Mary Hospital, Pokfulam Road, Hong
Hong Kong J Radiol. 2011;14(Suppl):S63-7 REVIEW ARTICLE Rituximab in the Management of Follicular Lymphoma YL Kwong Department of Medicine, Professorial Block, Queen Mary Hospital, Pokfulam Road, Hong
New Targets and Treatments for Follicular Lymphoma. Disclosures
 Winship Cancer Institute of Emory University New Targets and Treatments for Follicular Lymphoma Jonathon B. Cohen, MD, MS Assistant Professor Div of BMT, Emory University Disclosures Consulting fees from:
Winship Cancer Institute of Emory University New Targets and Treatments for Follicular Lymphoma Jonathon B. Cohen, MD, MS Assistant Professor Div of BMT, Emory University Disclosures Consulting fees from:
Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
In the last decade, passive immunotherapy
 Mædica - a Journal of Clinical Medicine ORIGINAL PAPERS Immunotherapy with Rituximab in Follicular Lymphomas Carmen SAGUNA, MD a ; Ileana Delia MUT, MD, PhD a ; Anca Roxana LUPU, MD, PhD a ; Mihaela TEVET,
Mædica - a Journal of Clinical Medicine ORIGINAL PAPERS Immunotherapy with Rituximab in Follicular Lymphomas Carmen SAGUNA, MD a ; Ileana Delia MUT, MD, PhD a ; Anca Roxana LUPU, MD, PhD a ; Mihaela TEVET,
GLSG/OSHO Study Group. Supported by Deutsche Krebshilfe
 GLSG/OSHO Study Group Supported by Deutsche Krebshilfe GLSG/OSHO Study Group Study Concepts Follicular Lymphomas Mantel Cell Lymphomas Waldenstroem s Disease Key Steps in Improving Treatment for Follicular
GLSG/OSHO Study Group Supported by Deutsche Krebshilfe GLSG/OSHO Study Group Study Concepts Follicular Lymphomas Mantel Cell Lymphomas Waldenstroem s Disease Key Steps in Improving Treatment for Follicular
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
Rituximab in Non - Hodgkins Lymphoma. Fatima Bassa, Dept. of Haematology October 2008
 Rituximab in Non - Hodgkins Lymphoma Fatima Bassa, Dept. of Haematology October 2008 World Health Organization lymphoma classification (2001) Peripheral B-cell neoplasms: B-chronic lymphocytic leukemia/small
Rituximab in Non - Hodgkins Lymphoma Fatima Bassa, Dept. of Haematology October 2008 World Health Organization lymphoma classification (2001) Peripheral B-cell neoplasms: B-chronic lymphocytic leukemia/small
Avastin in Metastatic Breast Cancer
 Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases
 Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
亞 東 紀 念 醫 院 Follicular Lymphoma 臨 床 指 引
 前 言 : 惡 性 淋 巴 瘤 ( 或 簡 稱 淋 巴 癌 ) 乃 由 體 內 淋 巴 系 統 包 括 淋 巴 細 胞 淋 巴 管 淋 巴 腺 及 一 些 淋 巴 器 官 或 組 織 如 脾 臟 胸 腺 及 扁 桃 腺 等 所 長 出 的 惡 性 腫 瘤 依 腫 瘤 病 理 組 織 型 態 的 不 同 可 分 為 何 杰 金 氏 淋 巴 瘤 (Hodgkin s disease) 與 非 何 杰 金
前 言 : 惡 性 淋 巴 瘤 ( 或 簡 稱 淋 巴 癌 ) 乃 由 體 內 淋 巴 系 統 包 括 淋 巴 細 胞 淋 巴 管 淋 巴 腺 及 一 些 淋 巴 器 官 或 組 織 如 脾 臟 胸 腺 及 扁 桃 腺 等 所 長 出 的 惡 性 腫 瘤 依 腫 瘤 病 理 組 織 型 態 的 不 同 可 分 為 何 杰 金 氏 淋 巴 瘤 (Hodgkin s disease) 與 非 何 杰 金
Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics
 Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics Disclosure(s) I do not intend to discuss an off-label use
Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics Disclosure(s) I do not intend to discuss an off-label use
Early stage follicular lymphoma: what is the clinical impact of the first-line treatment strategy?
 Michallet et al. Journal of Hematology & Oncology 2013, 6:45 JOURNAL OF HEMATOLOGY & ONCOLOGY RESEARCH Open Access Early stage follicular lymphoma: what is the clinical impact of the first-line treatment
Michallet et al. Journal of Hematology & Oncology 2013, 6:45 JOURNAL OF HEMATOLOGY & ONCOLOGY RESEARCH Open Access Early stage follicular lymphoma: what is the clinical impact of the first-line treatment
PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA
 2012 1 31,, PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA Version 1.0 2012 DIVISION OF HAEMATOLOGY / ONCOLOGY DEPARTMENT OF MEDICINE KAOHSING VETERAN GENERAL HOSPTIAL General Guide Diagnosis 1.Adequate
2012 1 31,, PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA Version 1.0 2012 DIVISION OF HAEMATOLOGY / ONCOLOGY DEPARTMENT OF MEDICINE KAOHSING VETERAN GENERAL HOSPTIAL General Guide Diagnosis 1.Adequate
Non-Hodgkin s lymphomas (NHLs) are a
 Oncology 33 Non-Hodgkin s lymphoma in the elderly The incidence of non-hodgkin s lymphoma (NHL) is increasing, and this increase is even more rapid in the older population. Although treatment of NHL in
Oncology 33 Non-Hodgkin s lymphoma in the elderly The incidence of non-hodgkin s lymphoma (NHL) is increasing, and this increase is even more rapid in the older population. Although treatment of NHL in
Update on Follicular Lymphoma. Brad Kahl, M.D.
 Update on Follicular Lymphoma Brad Kahl, M.D. Follicular Lymphoma: 25% of NHL Cases Other subtypes (9%) T and NK cell (12%) Burkitt (2.5%) Diffuse large B cell (DLBCL) (30%) Mantle cell (6%) Follicular
Update on Follicular Lymphoma Brad Kahl, M.D. Follicular Lymphoma: 25% of NHL Cases Other subtypes (9%) T and NK cell (12%) Burkitt (2.5%) Diffuse large B cell (DLBCL) (30%) Mantle cell (6%) Follicular
Guidelines for the use of Rituximab in Non-Hodgkin s Lymphoma QEII Health Sciences Centre
 Guidelines for the use of Rituximab in Non-Hodgkin s Lymphoma QEII Health Sciences Centre Background Non-Hodgkin s lymphoma (NHL) makes up approximately 85% of all lymphomas. They are a heterogeneous collection
Guidelines for the use of Rituximab in Non-Hodgkin s Lymphoma QEII Health Sciences Centre Background Non-Hodgkin s lymphoma (NHL) makes up approximately 85% of all lymphomas. They are a heterogeneous collection
A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on
 A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on examination. She also has fever, weight loss, and sweats. What
A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on examination. She also has fever, weight loss, and sweats. What
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD Boehringer Ingelheim International GmbH and Boehringer Ingelheim Pharmaceuticals, Inc. Petitioner, v. Biogen Idec, Inc.
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD Boehringer Ingelheim International GmbH and Boehringer Ingelheim Pharmaceuticals, Inc. Petitioner, v. Biogen Idec, Inc.
MALIGNANT LYMPHOMAS. Dr. Olga Vujovic (Updated August 2010)
 MALIGNANT LYMPHOMAS Dr. Olga Vujovic (Updated August 2010) Malignant lymphomas consist of Hodgkin and non-hodgkin lymphomas. The current management of these diseases involves a multi-disciplinary approach.
MALIGNANT LYMPHOMAS Dr. Olga Vujovic (Updated August 2010) Malignant lymphomas consist of Hodgkin and non-hodgkin lymphomas. The current management of these diseases involves a multi-disciplinary approach.
Introduction of Rituximab in Front-Line and Salvage Therapies Has Improved Outcome of Advanced-Stage Follicular Lymphoma Patients
 2077 Introduction of Rituximab in Front-Line and Salvage Therapies Has Improved Outcome of Advanced-Stage Follicular Lymphoma Patients Stefano Sacchi, MD 1 Samantha Pozzi, MD 1 Luigi Marcheselli, MS 1
2077 Introduction of Rituximab in Front-Line and Salvage Therapies Has Improved Outcome of Advanced-Stage Follicular Lymphoma Patients Stefano Sacchi, MD 1 Samantha Pozzi, MD 1 Luigi Marcheselli, MS 1
Lauren Berger: Why is it so important for patients to get an accurate diagnosis of their blood cancer subtype?
 Hello, I m Lauren Berger and I m the Senior Director of Patient Services Programs at The Leukemia & Lymphoma Society. I m pleased to welcome Dr. Rebecca Elstrom. Dr. Elstrom is an Assistant Professor in
Hello, I m Lauren Berger and I m the Senior Director of Patient Services Programs at The Leukemia & Lymphoma Society. I m pleased to welcome Dr. Rebecca Elstrom. Dr. Elstrom is an Assistant Professor in
rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited
 rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited 06 June 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited 06 June 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Follicular lymphoma. What is follicular lymphoma? Freephone helpline 0808 808 5555 information@lymphomas.org.uk www.lymphomas.org.
 Freephone helpline 0808 808 5555 information@lymphomas.org.uk www.lymphomas.org.uk is a cancer of the lymphatic system, a type of non-hodgkin lymphoma. Even though more than 12,000 people are diagnosed
Freephone helpline 0808 808 5555 information@lymphomas.org.uk www.lymphomas.org.uk is a cancer of the lymphatic system, a type of non-hodgkin lymphoma. Even though more than 12,000 people are diagnosed
Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma
 Issue date: June 2011 Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma This guidance was developed using the the single technology appraisal process NICE technology
Issue date: June 2011 Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma This guidance was developed using the the single technology appraisal process NICE technology
1. Introduction. 2. Clinical aspects
 1. Introduction MabThera (rituximab) is a genetically engineered chimeric mouse/human monoclonal antibody which binds specifically to the transmembrane antigen, CD20. This antigen is located on pre-b-
1. Introduction MabThera (rituximab) is a genetically engineered chimeric mouse/human monoclonal antibody which binds specifically to the transmembrane antigen, CD20. This antigen is located on pre-b-
Réda Bouabdallah, MD Institut Paoli-Calmettes, Marseille, France. Oran, May, 8th, 2010
 FIRST LINE TREATMENT IN FOLLICULAR LYMPHOMA Current Status Réda Bouabdallah, MD Institut Paoli-Calmettes, Marseille, France Oran, May, 8th, 2010 FL is a complex disease One disease, but many profiles 20%
FIRST LINE TREATMENT IN FOLLICULAR LYMPHOMA Current Status Réda Bouabdallah, MD Institut Paoli-Calmettes, Marseille, France Oran, May, 8th, 2010 FL is a complex disease One disease, but many profiles 20%
Induction Chemotherapy (R-CHOP Vs. R-FC) Followed by Interferon Maintenance Versus Rituximab Maintenance in MCL (MCLelderly)
 1 von 7 31.07.2012 10:27 Home Search Study Topics Glossary Study 1 of 24 for search of: MCL elderly Previous Study Return to Search Results Next Study Full Text View Tabular View No Study Results Posted
1 von 7 31.07.2012 10:27 Home Search Study Topics Glossary Study 1 of 24 for search of: MCL elderly Previous Study Return to Search Results Next Study Full Text View Tabular View No Study Results Posted
Anti-HCV therapy in HCV-related NHL
 Gabriele Pozzato M.D. University of Trieste Anti-HCV therapy in HCV-related NHL Questions about HCV+ in NHL Is the NHL related with HCV infection? Which is the best therapeutic strategy? Is the antiviral
Gabriele Pozzato M.D. University of Trieste Anti-HCV therapy in HCV-related NHL Questions about HCV+ in NHL Is the NHL related with HCV infection? Which is the best therapeutic strategy? Is the antiviral
Outline of thesis and future perspectives.
 Outline of thesis and future perspectives. This thesis is divided into two different sections. The B- section involves reviews and studies on B- cell non- Hodgkin lymphoma [NHL] and radioimmunotherapy
Outline of thesis and future perspectives. This thesis is divided into two different sections. The B- section involves reviews and studies on B- cell non- Hodgkin lymphoma [NHL] and radioimmunotherapy
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin s lymphoma
 DOI: 10.3310/hta13suppl2/06 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin s lymphoma A Boland, A Bagust,
DOI: 10.3310/hta13suppl2/06 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin s lymphoma A Boland, A Bagust,
Agustin Avilés 1, Serafin Delgado 2, Alejandra Talavera 3, Natividad Neri 3, Judith Huerta-Guzmán 3
 Eur J Haematol 2002: 68: 144 149 Printed in UK. All rights reserved Copyright # Blackwell Munksgaard 2002 EUROPEAN JOURNAL OF HAEMATOLOGY ISSN 0902-4441 Combined therapy in advanced stages (III and IV)
Eur J Haematol 2002: 68: 144 149 Printed in UK. All rights reserved Copyright # Blackwell Munksgaard 2002 EUROPEAN JOURNAL OF HAEMATOLOGY ISSN 0902-4441 Combined therapy in advanced stages (III and IV)
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
ollicular Lymphoma The following investigations are indicated for most patients with follicular lymphoma:
 ollicular Lymphoma Drs. Kevin Imrie & Matthew Cheung Updated August 2007* Fximab Updates: Ritu maintenance therapy Introduction Follicular lymphoma is the second most frequent type of non-hodgkin s lymphoma
ollicular Lymphoma Drs. Kevin Imrie & Matthew Cheung Updated August 2007* Fximab Updates: Ritu maintenance therapy Introduction Follicular lymphoma is the second most frequent type of non-hodgkin s lymphoma
January 2013 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW. Summary. Contents
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
Mantle Cell Lymphoma Understanding Your Treatment Options
 New Developments in Mantle Cell Lymphoma John P. Leonard, M.D. Richard T. Silver Distinguished Professor of Hematology and Medical Oncology Associate Dean for Clinical Research Vice Chairman, Department
New Developments in Mantle Cell Lymphoma John P. Leonard, M.D. Richard T. Silver Distinguished Professor of Hematology and Medical Oncology Associate Dean for Clinical Research Vice Chairman, Department
Lymphoma: The Roleof Nurses in the Treatment Process
 Lymphoma: The Roleof Nurses in the Treatment Process Sarah Liptrott MSc,BN(Hons), RN Istituto Europeo di Oncologia, Milan (IT) EBMT Swiss Study Day 2014, Zurich, Switzerland LymphomaManagement Watch &
Lymphoma: The Roleof Nurses in the Treatment Process Sarah Liptrott MSc,BN(Hons), RN Istituto Europeo di Oncologia, Milan (IT) EBMT Swiss Study Day 2014, Zurich, Switzerland LymphomaManagement Watch &
The role of chemotherapy in follicular lymphomas Emanuele Zucca, M.D.
 The role of chemotherapy in follicular lymphomas Emanuele Zucca, M.D. Oncology Institute of Southern Switzerland (IOSI) Swiss Group for Clinical Cancer Research (SAKK) WHO grading of follicular lymphoma
The role of chemotherapy in follicular lymphomas Emanuele Zucca, M.D. Oncology Institute of Southern Switzerland (IOSI) Swiss Group for Clinical Cancer Research (SAKK) WHO grading of follicular lymphoma
Economic evaluation of rituximab plus cyclophosphamide, vincristine and prednisolone for advanced follicular lymphoma
 Leukemia & Lymphoma, February 2008; 49(2): 227 236 ORIGINAL ARTICLE: CLINICAL Economic evaluation of rituximab plus cyclophosphamide, vincristine and prednisolone for advanced follicular lymphoma JOHN
Leukemia & Lymphoma, February 2008; 49(2): 227 236 ORIGINAL ARTICLE: CLINICAL Economic evaluation of rituximab plus cyclophosphamide, vincristine and prednisolone for advanced follicular lymphoma JOHN
The Clinical Trials Process an educated patient s guide
 The Clinical Trials Process an educated patient s guide Gwen L. Nichols, MD Site Head, Oncology Roche TCRC, Translational and Clinical Research Center New York DISCLAIMER I am an employee of Hoffmann-
The Clinical Trials Process an educated patient s guide Gwen L. Nichols, MD Site Head, Oncology Roche TCRC, Translational and Clinical Research Center New York DISCLAIMER I am an employee of Hoffmann-
Audience Response Question?
 Presenter Disclosure Information Session 4: 3:30 PM - 4:15 PM Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman, MD The following relationships
Presenter Disclosure Information Session 4: 3:30 PM - 4:15 PM Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman, MD The following relationships
Follicular Lymphoma. 1. Introduction. 2. Diagnosis. 3. Baseline Investigations
 Follicular Lymphoma Follicular Lymphoma Treatment policy prepared by Dr. Louise Bordeleau 1. Introduction Follicular lymphoma is the second most frequent type of non-hodgkin s lymphoma. These lymphomas
Follicular Lymphoma Follicular Lymphoma Treatment policy prepared by Dr. Louise Bordeleau 1. Introduction Follicular lymphoma is the second most frequent type of non-hodgkin s lymphoma. These lymphomas
Chemoimmunotherapy resistant follicular lymphoma A single institutional study
 Cancer Research Journal 2014; 2(5): 93-97 Published online September 30, 2014 (http://www.sciencepublishinggroup.com/j/crj) doi: 10.11648/j.crj.20140205.13 ISSN: 2330-8192 (Print); ISSN: 2330-8214 (Online)
Cancer Research Journal 2014; 2(5): 93-97 Published online September 30, 2014 (http://www.sciencepublishinggroup.com/j/crj) doi: 10.11648/j.crj.20140205.13 ISSN: 2330-8192 (Print); ISSN: 2330-8214 (Online)
J Clin Oncol 23:8447-8452. 2005 by American Society of Clinical Oncology INTRODUCTION
 VOLUME 23 NUMBER 33 NOVEMBER 20 2005 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T New Treatment Options Have Changed the Survival of Patients With Follicular Lymphoma Richard I. Fisher, Michael
VOLUME 23 NUMBER 33 NOVEMBER 20 2005 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T New Treatment Options Have Changed the Survival of Patients With Follicular Lymphoma Richard I. Fisher, Michael
Audience Response Question? Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management. Presenter Disclosure Information
 Welcome to Master Class for Oncologists Session 4: 10:00 AM - 10:45 AM Miami, FL December 18, 2009 Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman,
Welcome to Master Class for Oncologists Session 4: 10:00 AM - 10:45 AM Miami, FL December 18, 2009 Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman,
DE Tsai 1, HCF Moore 1, CL Hardy 2, DL Porter 1, EY Loh 1, DJ Vaughn 1, S Luger 1, SJ Schuster 1 and EA Stadtmauer 1
 Bone Marrow Transplantation, (1999) 24, 521 526 1999 Stockton Press All rights reserved 0268 3369/99 $15.00 http://www.stockton-press.co.uk/bmt Rituximab (anti-cd20 monoclonal antibody) therapy for progressive
Bone Marrow Transplantation, (1999) 24, 521 526 1999 Stockton Press All rights reserved 0268 3369/99 $15.00 http://www.stockton-press.co.uk/bmt Rituximab (anti-cd20 monoclonal antibody) therapy for progressive
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cll_and_sll
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cll_and_sll
6/3/2013. Follicular and Other Slow Growing Lymphomas. Stephen Ansell, MD, PhD Mayo Clinic
 Follicular and Other Slow Growing Lymphomas Stephen Ansell, MD, PhD Mayo Clinic 1 Learning Objectives Start with an overview of Follicular and other slow growing lymphomas Discuss current and emerging
Follicular and Other Slow Growing Lymphomas Stephen Ansell, MD, PhD Mayo Clinic 1 Learning Objectives Start with an overview of Follicular and other slow growing lymphomas Discuss current and emerging
Hodgkin Lymphoma Disease Specific Biology and Treatment Options. John Kuruvilla
 Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
DIFFUSE LARGE B-CELL LYMPHOMA
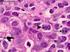 DIFFUSE LARGE B-CELL LYMPHOMA Executive Summary Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-hodgkin lymphoma (NHL), constituting up to 40% of all cases globally.[1] This subtype
DIFFUSE LARGE B-CELL LYMPHOMA Executive Summary Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-hodgkin lymphoma (NHL), constituting up to 40% of all cases globally.[1] This subtype
Lymphoma Diagnosis and Classification
 Lymphoma Diagnosis and Classification By Atef Shrit, MD, Pathology B- and T/NK-cell lymphomas are clonal neoplasms of immature and mature B-lymphocytes, T-lymphocytes or natural killer cells at various
Lymphoma Diagnosis and Classification By Atef Shrit, MD, Pathology B- and T/NK-cell lymphomas are clonal neoplasms of immature and mature B-lymphocytes, T-lymphocytes or natural killer cells at various
Follicular Lymphoma. Aruna K. Reddy, MD. Hematology& Oncology Peace Health Southwest Medical Center
 Follicular Lymphoma Aruna K. Reddy, MD Hematology& Oncology Peace Health Southwest Medical Center Follicular Lymphoma Malignant neoplasm resulting from clonal proliferation of malignant B-cells Second
Follicular Lymphoma Aruna K. Reddy, MD Hematology& Oncology Peace Health Southwest Medical Center Follicular Lymphoma Malignant neoplasm resulting from clonal proliferation of malignant B-cells Second
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Bendamustine for the fourth-line treatment of multiple myeloma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine for the fourth-line treatment of multiple myeloma Contents Summary 1 Background 2 Epidemiology 3 Cost 6 References 7 Summary There is no standard
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine for the fourth-line treatment of multiple myeloma Contents Summary 1 Background 2 Epidemiology 3 Cost 6 References 7 Summary There is no standard
What is non-hodgkin lymphoma, how is it treated, and what is the unmet need?
 What is non-hodgkin lymphoma, how is it treated, and what is the unmet need? Tim Illidge BSc PhD MRCP FRCR FRCPath Institute of Cancer Sciences, University of Manchester Manchester Cancer Research Centre,
What is non-hodgkin lymphoma, how is it treated, and what is the unmet need? Tim Illidge BSc PhD MRCP FRCR FRCPath Institute of Cancer Sciences, University of Manchester Manchester Cancer Research Centre,
NATIONAL CANCER DRUG FUND PRIORITISATION SCORES
 NATIONAL CANCER DRUG FUND PRIORITISATION SCORES Drug Indication Regimen (where appropriate) BORTEZOMIB In combination with dexamethasone (VD), or with dexamethasone and thalidomide (VTD), is indicated
NATIONAL CANCER DRUG FUND PRIORITISATION SCORES Drug Indication Regimen (where appropriate) BORTEZOMIB In combination with dexamethasone (VD), or with dexamethasone and thalidomide (VTD), is indicated
CAR T cell therapy for lymphomas
 CAR T cell therapy for lymphomas Sattva S. Neelapu, MD Associate Professor and Deputy Chair ad interim Department of Lymphoma and Myeloma UT MD Anderson Cancer Center, Houston, TX CAR T cell therapy What
CAR T cell therapy for lymphomas Sattva S. Neelapu, MD Associate Professor and Deputy Chair ad interim Department of Lymphoma and Myeloma UT MD Anderson Cancer Center, Houston, TX CAR T cell therapy What
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Farooqui MZH, Valdez J, Martyr S, et al. Ibrutinib
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Farooqui MZH, Valdez J, Martyr S, et al. Ibrutinib
Lymphomas after organ transplantation
 Produced 21.03.2011 Revision due 21.03.2011 Lymphomas after organ transplantation People who have undergone an organ transplant are more at risk of developing lymphoma known as post-transplant lymphoproliferative
Produced 21.03.2011 Revision due 21.03.2011 Lymphomas after organ transplantation People who have undergone an organ transplant are more at risk of developing lymphoma known as post-transplant lymphoproliferative
Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm
 Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm Name of Policy: Uses of Monoclonal Antibodies for the Treatment of Non-Hodgkin
Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm Name of Policy: Uses of Monoclonal Antibodies for the Treatment of Non-Hodgkin
New Evaluation Criteria for Response and Toxicity in Lung Cancer Treatment
 Lung Cancer New Evaluation Criteria for Response and Toxicity in Lung Cancer Treatment JMAJ 46(12): 554 558, 2003 Masahiko SHIBUYA Chief, Division of Respiratory Medicine, Tokyo Metropolitan Komagome Hospital
Lung Cancer New Evaluation Criteria for Response and Toxicity in Lung Cancer Treatment JMAJ 46(12): 554 558, 2003 Masahiko SHIBUYA Chief, Division of Respiratory Medicine, Tokyo Metropolitan Komagome Hospital
Rituximab in non-hodgkin Lymphoma (NHL)
 Original Article ISSN: 2070-254X Rituximab in non-hodgkin Lymphoma (NHL) Manal Elhabbash MD 1, Abukris Alwindi MD 2 (1) Assistant Professor, Tripoli University, (2) Medical consultant in oncology & hematology
Original Article ISSN: 2070-254X Rituximab in non-hodgkin Lymphoma (NHL) Manal Elhabbash MD 1, Abukris Alwindi MD 2 (1) Assistant Professor, Tripoli University, (2) Medical consultant in oncology & hematology
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Response Criteria for Malignant Lymphoma 2007. Cheson Criteria. Quick Reference Guide
 Response Criteria for Malignant Lymphoma 2007 Cheson Criteria Quick Reference Guide Table of Contents Summary of Assessments...3 Baseline Lesion Burden...4 What isameasurable Lesion?...5 Choosing Target
Response Criteria for Malignant Lymphoma 2007 Cheson Criteria Quick Reference Guide Table of Contents Summary of Assessments...3 Baseline Lesion Burden...4 What isameasurable Lesion?...5 Choosing Target
Severe rheumatoid arthritis (a disease that causes inflammation of the joints),where MabThera is given intravenously together with methotrexate.
 EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Non-Hodgkin Lymphoma Richard Orlowski, MD
 Non-Hodgkin Lymphoma Richard Orlowski, MD The American Cancer Society (ACS) estimates that 69,740 Americans will be diagnosed with non-hodgkin lymphoma (NHL) in 2013. Excluding non-melanoma skin cancers,
Non-Hodgkin Lymphoma Richard Orlowski, MD The American Cancer Society (ACS) estimates that 69,740 Americans will be diagnosed with non-hodgkin lymphoma (NHL) in 2013. Excluding non-melanoma skin cancers,
Clinical Trial Design. Sponsored by Center for Cancer Research National Cancer Institute
 Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
MOLOGEN AG. Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer. Berlin, 12 May 2015
 Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer Berlin, 12 May 2015 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future
Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer Berlin, 12 May 2015 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future
Sonneveld, P; de Ridder, M; van der Lelie, H; et al. J Clin Oncology, 13 (10) : 2530-2539 Oct 1995
 Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
7. Prostate cancer in PSA relapse
 7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
Supplementary Online Content
 Supplementary Online Content Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving
Supplementary Online Content Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving
Michael Crump MD. Lymphoma Site Leader Princess Margaret Hospital University of Toronto
 Evolution of Lymphoma Therapy: What can we expect for the rest of the millenium decade? Michael Crump MD Lymphoma Site Leader Princess Margaret Hospital University of Toronto disclaimers Served on advisory
Evolution of Lymphoma Therapy: What can we expect for the rest of the millenium decade? Michael Crump MD Lymphoma Site Leader Princess Margaret Hospital University of Toronto disclaimers Served on advisory
FOLLICULAR LYMPHOMA. Executive Summary
 FOLLICULAR LYMPHOMA Executive Summary Follicular lymphoma (FL) is the most common indolent lymphoma and the second most common non-hodgkin lymphoma accounting for about 10-20% of all lymphomas in Western
FOLLICULAR LYMPHOMA Executive Summary Follicular lymphoma (FL) is the most common indolent lymphoma and the second most common non-hodgkin lymphoma accounting for about 10-20% of all lymphomas in Western
Imaging, Diagnosis, Prognosis. MinnaTaskinen, 1,2 Marja-Liisa Karjalainen-Lindsberg, 3 Heidi Nyman, 1,2 Leena-Maija Eerola, 1,2 1,2.
 Imaging, Diagnosis, Prognosis A HighTumor-Associated Macrophage Content Predicts Favorable Outcome in Follicular Lymphoma PatientsTreated with Rituximab and Cyclophosphamide-Doxorubicin-Vincristine-Prednisone
Imaging, Diagnosis, Prognosis A HighTumor-Associated Macrophage Content Predicts Favorable Outcome in Follicular Lymphoma PatientsTreated with Rituximab and Cyclophosphamide-Doxorubicin-Vincristine-Prednisone
Follicular Lymphoma: Current Management and Future Directions
 Treatment decisions and outcomes for patients with follicular lymphoma may be improved with newer diagnostic tests and novel targeted agents. George Van Hook. Barns in Summer. Oil on linen, 22 28. Follicular
Treatment decisions and outcomes for patients with follicular lymphoma may be improved with newer diagnostic tests and novel targeted agents. George Van Hook. Barns in Summer. Oil on linen, 22 28. Follicular
Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015. (minutes for web publishing)
 Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the
Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer
 Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
bnmqwertyuiopasdfghjklzxcvbn mqwertyuiopasdfghjklzxcvbnm qwertyuiopasdfghjklzxcvbnmq ertyuiopasdfghjklzxcvbnmqwer tyuiopasdfghjklzxcvbnmqwerty
 bnmqwertyuiopasdfghjklzxcvbn mqwertyuiopasdfghjklzxcvbnm qwertyuiopasdfghjklzxcvbnmq wertyuiopasdfghjklzxcvbnmqw ertyuiopasdfghjklzxcvbnmqwer Follicular Lymphoma Overview tyuiopasdfghjklzxcvbnmqwerty Lymphoma
bnmqwertyuiopasdfghjklzxcvbn mqwertyuiopasdfghjklzxcvbnm qwertyuiopasdfghjklzxcvbnmq wertyuiopasdfghjklzxcvbnmqw ertyuiopasdfghjklzxcvbnmqwer Follicular Lymphoma Overview tyuiopasdfghjklzxcvbnmqwerty Lymphoma
Malignant Lymphomas and Plasma Cell Myeloma
 Malignant Lymphomas and Plasma Cell Myeloma Dr. Bruce F. Burns Dept. of Pathology and Lab Medicine Overview definitions - lymphoma lymphoproliferative disorder plasma cell myeloma pathogenesis - translocations
Malignant Lymphomas and Plasma Cell Myeloma Dr. Bruce F. Burns Dept. of Pathology and Lab Medicine Overview definitions - lymphoma lymphoproliferative disorder plasma cell myeloma pathogenesis - translocations
In non-hodgkin s lymphoma, MabThera is used to treat two types of the disease, both of which affect B-lymphocytes:
 EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
State-of-the-Art Treatment for Lymphoma December 19, 2007 Guests: David Maloney, M.D., Ph.D Hosted by Andrew Schorr INTRODUCTION
 State-of-the-Art Treatment for Lymphoma December 19, 2007 Guests: David Maloney, M.D., Ph.D Hosted by Andrew Schorr Please remember the opinions expressed on Patient Power are not necessarily the views
State-of-the-Art Treatment for Lymphoma December 19, 2007 Guests: David Maloney, M.D., Ph.D Hosted by Andrew Schorr Please remember the opinions expressed on Patient Power are not necessarily the views
Phase II Trial of R -C H O P V s C H O P C hem otherapy in Pakistani D iffuse Large B cell Lym phom a Patients
 Original Article Phase II Trial R -C H O P V s C H O P C hem otherapy in Pakistani D iffuse Large B cell Lym phom a Patients Objective: To determine response and relapse rates in diffuse large B cell lymphoma
Original Article Phase II Trial R -C H O P V s C H O P C hem otherapy in Pakistani D iffuse Large B cell Lym phom a Patients Objective: To determine response and relapse rates in diffuse large B cell lymphoma
The Di Bella Method (DBM) improves Survival, Objective Response and Performance Status in Breast Cancer
 BIT's 4th World Cancer Congress 2011 People s Republic of China Dalian The Di Bella Method (DBM) improves Survival, Objective Response and Performance Status in treated with DBM therapy Retrospective observational
BIT's 4th World Cancer Congress 2011 People s Republic of China Dalian The Di Bella Method (DBM) improves Survival, Objective Response and Performance Status in treated with DBM therapy Retrospective observational
Long Term Low Dose Maintenance Chemotherapy in the Treatment of Acute Myeloid Leukemia
 Long Term Low Dose Chemotherapy in the Treatment of Acute Myeloid Leukemia Murat TOMBULO LU*, Seçkin ÇA IRGAN* * Department of Hematology, Faculty of Medicine, Ege University, zmir, TURKEY ABSTRACT In
Long Term Low Dose Chemotherapy in the Treatment of Acute Myeloid Leukemia Murat TOMBULO LU*, Seçkin ÇA IRGAN* * Department of Hematology, Faculty of Medicine, Ege University, zmir, TURKEY ABSTRACT In
