Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma
|
|
|
- Arleen Pearson
- 8 years ago
- Views:
Transcription
1 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Contents Summary 1 Background 2 Epidemiology 3 Cost 4 References 6 Summary The combination of bendamustine and rituximab (BR) is not currently licensed for the first-line treatment of indolent NHL and mantle cell lymphoma (MCL); however a submission to the European Medicines Agency has been made and related NICE technology appraisals are in development. A submission to extend the license of bendamustine to include first-line treatment of indolent NHL has already been made to the US FDA but no decision has been made as yet, and the FDA has reportedly requested further data. According to the NICE final scope documents, the evaluation of BR in MCL will consider patients who are unsuitable for stem cell transplantation, who currently receive rituximab in combination with other chemotherapy regimens (e.g. R-CHOP [most frequently used], R-FC and R-CVP). The evaluation of BR in indolent NHL will consider patients with advanced disease who require therapy, and will include similar standard comparators (e.g. R-CHOP, R- CVP). For both indolent NHL and MCL, the main supporting data consist of the Phase III study (StiL NHL ), which compared BR to R-CHOP in the first-line treatment of advanced (stage III or IV) MCL (19%) or indolent NHL (mainly follicular [54%]). The primary endpoint was median progression-free survival (PFS) in the per protocol population, this was extended by around 38 months in the BR group (69.5 months versus 31.2 months; HR 0.58; 95% CI ; p<0.0001). Separate analyses according to histological subtype found the benefit of BR to be consistent in all apart from marginal zone lymphoma. For MCL patients, the PFS benefit was 13.3 months (35.4 months versus 22.1 months; HR 0.49; ; p=0.0044). Produced for the London New Drugs Group Contact: Nicky Pocock Medicines Information Pharmacist London & South East Medicines Information Service Guy s Hospital London SE1 9RT Tel: Fax: Nicola.pocock@gstt.nhs.uk Produced for use within the NHS. Not to be reproduced for commercial purposes R-CHOP was associated with a higher rate of serious adverse events (29% versus 19%; NNH of 10), including grade 3/4 neutropenia (69% versus 29%; p<0.0001; NNH 2-3) and leukocytopenia (72% versus 37%; p<0.0001; NNH 2-3). R-CHOP was also associated with a higher number of infectious episodes and more frequent use of G-CSF. BR was associated with a higher rate of drug-associated skin reactions (erythema or allergic reactions). The StiL study did not include any maintenance or consolidation treatment, and it is therefore not known how BR compares to R-CHOP followed by maintenance rituximab, or if rituximab maintenance has a role after first-line BR chemotherapy. Also data on the longer- safety of bendamustine are currently limited. There are no data comparing BR to other, alternative first-line treatment options (e.g. R-CVP), which clinicians may use if they wish to withhold anthracycline treatment. The results of the BRIGHT study have been presented in conference; this study however had a primary endpoint of complete response rate and timeto-event data (evaluated as secondary endpoints) were too immature for analysis at the time.
2 Background Indolent NHL The incidence of non-hodgkin s lymphoma (NHL) in the UK is approximately 18 per 100,000 people, and 40-50% of these have indolent (low-grade) disease, usually advanced (stage III/IV) at presentation (1). Types of NHL classified as indolent include follicular lymphoma (FL), small lymphocytic lymphoma (SLL), lymphoplasmacytic lymphoma and marginal zone lymphomas (MZL). Mantle cell lymphoma (MCL) has characteristics of aggressive as well indolent lymphoma (2). Indolent B cell lymphoid malignancies are characterised by slow and continuous growth, a high initial response rate, but a relapsing and progressive disease course (2). The clinical presentation, rate of disease progression and patterns of treatment for patients with indolent NHL vary widely. Active surveillance ( watchful waiting ) may be an appropriate treatment option for many, with appropriate interventions when symptoms develop. There may be multiple episodes of remission and relapse, and the nature of the disease can change at relapse, sometimes transforming to a more aggressive type (3). The most commonly used first-line treatment for symptomatic, advanced indolent NHL is rituximab plus combination chemotherapy, for example R- CVP (cyclophosphamide, vincristine, prednisolone and rituximab) or R-CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone and rituximab). Chlorambucil ± rituximab may be given to people who are unsuitable for these regimens (3). R-CHOP is the most widely used of these regimens (4). Mantle cell lymphoma MCL is a rare type of B-cell NHL that has a moderately aggressive course and is rarely curable with currently available standard treatment. The registered annual incidence of NHL in England and Wales is around 10,400, with MCL accounting for around 5 to 8% (around 670 new diagnoses per year, or 1.2 per 100,000). It usually occurs in older adults (the median age of presentation is 60 years) and has a male predominance (5). There is no current gold standard treatment for advanced-stage MCL, due to a lack of definitive data to guide treatment decisions (it is relatively uncommon and has often been included with other types of NHL in clinical trials). In the UK, patients unsuitable for autologous peripheral blood stem cell transplantation (ASCT) are most commonly treated first-line with R-CHOP or R-FC (cyclophosphamide in combination with rituximab) (final scope). Although MCL usually responds well to initial chemotherapy (response rates of 50-70% with many regimens), durations of response are short and overall survival is poor (median of 3 years) (5, 6). Licence status Bendamustine (Levact ) is currently licensed in the UK for use as monotherapy in the treatment of patients with indolent NHL who have progressed during or within 6 months following treatment with rituximab or a rituximab containing regimen. It is not licensed for use in combination with rituximab or in the first-line setting for indolent NHL, and is not licensed for the treatment of MCL (7). An application to extend the licence of bendamustine to include its use in combination with rituximab for the first-line treatment of indolent NHL and MCL has been submitted to the European Medicines Agency and a decision is expected towards the end of 2013 (1, 8). An application was filed in the US in December 2011 for use of bendamustine, in combination with rituximab, as a first-line therapy for indolent B-cell NHL. According to the New Drugs Online database, the FDA requested further data in October 2012 (no details of the data requested are given) (1). Rituximab (MabThera ) is licensed in the UK for the treatment of previously untreated patients with stage III-IV follicular lymphoma in combination with chemotherapy. The SPC discusses the results of three randomised trials using rituximab in combination with chemotherapy CVP, CHOP, MCP (melphalan, chlorambucil, and prednisone) and CHVP (cyclophosphamide, doxorubicin, etoposide, and prednisone)/ interferon-α (9). It is not licensed for use in the treatment of MCL or other indolent lymphomas. NICE guidance NICE is undertaking two separate technology appraisals on: 1. Bendamustine in combination with rituximab for the first-line treatment of advanced indolent non-hodgkin's lymphoma (expected in October 2013). This will look at its use in patients with previously untreated advanced, indolent NHL that requires therapy, with standard comparators including R- CVP, R-CHOP, and chlorambucil plus rituximab in those unsuitable for the former two regimens (10). 2. Bendamustine in combination with rituximab for the first-line treatment of mantle cell lymphoma (expected date of issue TBC). This will look at its use in patients who are unsuitable for stem cell transplantation, with standard comparators including R-CHOP, R-FC (plus R-CVP and chlorambucil plus rituximab in those unsuitable for the former two regimens) (11). Both are at an early stage of development and no draft recommendations are currently available.
3 Epidemiology Indolent NHL The incidence of NHL in the UK is approximately 18 per 100,000 people. If it is assumed that 45% have indolent disease, 85%* of these have stage III or IV disease, 94%* are eligible for chemotherapy, and 90% are considered suitable for bendamustine, then the population eligible for first-line treatment with BR can be estimated as around 6 per 100,000. [*These data were obtained from the costing statement from the NICE guidance on Rituximab for the for the first-line maintenance treatment of stage III- IV follicular non-hodgkin s lymphoma (TA 243) (12)]. MCL The incidence of MCL in the UK is approximately 1.2 per 100,000 people. If it is assumed that 85% have advanced disease, 90% are unsuitable for ASCT and 90% of these are considered suitable for bendamustine then the population eligible for firstline treatment with BR can be estimated as around 0.8 per 100,000. Published data StiL NHL This multicentre Phase III non-inferiority study compared bendamustine in combination with rituximab (BR) to R-CHOP in the first-line treatment of adults with advanced (stage III or IV) MCL (19%) or indolent NHL, the latter including follicular (54%), lymphoplasmacytic (Waldenstrom s macroglobulinaemia) (8%), small lymphocytic (4%), and marginal zone lymphoma (13%) (4). Participants were randomised to open-label treatment with BR (rituximab 375mg/m 2 on day 1 plus bendamustine 90mg/m 2 on days 1+2 every 4 weeks; n=274) or R-CHOP (rituximab 375mg/m 2 on day 1 plus cyclophosphamide 750mg/m 2, doxorubicin 50mg/m 2 and vincristine 1.4mg/m 2 on day 1 and prednisone 100mg daily for 5 days; n=275), for up to six cycles. No maintenance or consolidation treatment was administered. The primary endpoint was progression-free survival (PFS), and the aim was to demonstrate non-inferiority of BR to R-CHOP (the non-inferiority margin was set at 10%). A total of 549 patients were randomised to treatment and 514 were available for analysis (per protocol: 261 in the BR group and 253 in the R-CHOP group). The median age of the group was 64 years (range 34-83) and the majority had stage IV (77%) or III (19%) disease. A median of six cycles of treatment per patient was administered in both treatment arms, and full doses of treatment were given in 95.9% of BR cycles and 88.8% of R-CHOP cycles. The main results reported were as follows: At a median follow-up of 45 months, median PFS was 69.5 months (IQR 26.1-not yet reached) for BR versus 31.2 months ( ) for CHOP-R (HR 0.58; 95% CI ; p<0.0001). When separate analyses were conducted for histological subtypes, a significant benefit for BR in terms of PFS was shown for all except marginal zone lymphoma, with no significant treatment-bysubgroup interaction. For MCL, PFS was 35.4 months (IQR ) in BR and 22.1 months ( ) for R-CHOP (HR 0.49; ; p=0.0044) Overall response rates (ORR) did not differ between groups (93% with BR and 91% with R- CHOP), however more complete responses were seen in the BR group (40% versus 30%, respectively (p=0.021). The BR group also had a longer time to next antilymphoma treatment (not reached versus 42.3 months, respectively; HR 0.52; 95% CI ; p<0.0001). No difference in OS was seen (43 deaths in the B-R arm and 45 in the CHOP-R arm) but followup was limited, and the median OS had not been reached in either group. R-CHOP was more frequently associated with serious adverse events (29% versus 19% in the BR group), with a higher incidence of grade 3/4 neutropenia (69% versus 29%; p<0.0001) and leukocytopenia (72% versus 37%; p<0.0001). In addition it was associated with a higher overall rate of infectious episodes (any grade; 50% versus 37%; p=0.0025) and sepsis (3% versus <1%, respectively; p=0.019). One patient in the BR group and five in the R-CHOP group died from sepsis. G-CSF was more often used in CHOP-R treated pts (20% versus 4% of all cycles; p<0.0001). The BR regimen was associated with a lower rate of alopecia (0% versus 100%), stomatitis (6% versus 19%) and parasthesia (7% versus 29%), but a higher rate of drug-associated skin reactions (erythema [16% versus 9%] or allergic reactions [15% versus 6%]) than R-CHOP. Although R-CHOP is the most widely used chemotherapy regimen for the first-line treatment of indolent NHL and MCL, some clinicians may prefer to withhold the anthracycline, both to improve tolerability and to hold for use in the future event of histological transformation. There have been no randomised studies comparing BR to alternative treatments (e.g. R-CVP) in this setting. The StiL study did not include any maintenance or consolidation treatment, and it is therefore not known how BR compares to R-CHOP followed by maintenance rituximab, or if rituximab maintenance has a role after first-line BR chemotherapy (13).
4 Although bendamustine is well tolerated in the short term, its long-term toxicity profile may not yet be appreciated, particularly for the risk of myelodysplasia and leukaemia. There are scarce data on the effects of bendamustine on stem cell viability and collection for future ASCT, and whether its use affects response rates to subsequent treatments at relapse (13). BRIGHT study This Phase III study compared BR to standard treatment regimens (R-CHOP or R-CVP) in the first-line treatment for indolent NHL or MCL. The results of the study have not been fully published and these data have been taken from a conference abstract (presented at ASH in 2012) (14). A total of 477 participants were randomised to receive either BR (regimen as per StiL study) or R- CHOP/R-CVP (the choice of standard regimen was determined by the investigator prior to randomisation). The primary objective was to demonstrate non-inferiority of BR to standard treatment in terms of CR rate, with a non-inferiority margin (ratio) of Tumour response was determined by a blinded independent review committee (IRC). A total of 436 randomised patients received treatment and 419 were evaluable for efficacy (BR n=213; R-CHOP/R-CVP n=206 [R-CHOP 97; R- CVP n=109]). The median age of the groups was 59 years, 64% had an ECOG performance status of 0, 62% had Ann Arbor stage IV disease, and 83% had indolent NHL. Most patients completed six cycles of treatment (92% for BR and 91% for R-CHOP/R- CVP), with high relative dose intensity (>96%).The main results reported in the abstract are as follows: Among randomised patients, the rate of CR was 31% with BR and 23% with standard treatment (HR 1.34; ; p= for noninferiority). Results were similar in the evaluable population (31% versus 25%; HR 1.25; 95% CI ; p= for non-inferiority). Among randomised patients, the CR rate for those with indolent NHL was 27% for BR and 23% for standard treatment (HR 1.16; 95% CI ; p=0.1289). For those with MCL it was 51% and 24%, respectively (HR 1.95; 95% CI ; p= for superiority) For randomised patients, the ORR was 94% for BR and 84% for R-CHOP/R-CVP. Time-to-event data are immature and will be analysed at a later date. The most common adverse events for BR and R- CHOP/R-CVP, respectively, were nausea (139 vs. 102 patients), fatigue (113 vs. 107), neutropenia (76 vs. 85), constipation (65 vs. 90), and alopecia (8 vs. 74). Laboratory grade 3/4 haematological toxicities for BR and R-CHOP/R-CVP included lymphopenia (137 vs. 64), neutropenia (98 vs. 151), leukopenia (84 vs. 116), thrombocytopenia (16 vs. 15), and anaemia (6 vs. 9), respectively. The most frequent investigator-reported non-haematological grade 3/4 adverse events for BR and R-CHOP/R-CVP were infusion-related reactions (13 vs. 8 patients). G-CSF was administered at the discretion of the investigator (per ASCO recommendations) to 29% of the BR group and 43% of the R-CHOP/R-CVP group. Fatal adverse events occurred in six patients receiving BR patients (pneumonia, respiratory failure, and sepsis; acute respiratory failure; cardiac arrest; pneumonia; chronic obstructive pulmonary disease; lung cancer) and in one patient who received R-CHOP/R-CVP (sepsis). Cost Bendamustine is available in 25mg and 100mg vials at a cost of and , respectively (excluding VAT) (15). The total cost of bendamustine given at a dose of 90mg/m 2 on days 1 and 2 for six cycles (assuming an average BSA of 1.8m 2 ) can be calculated as follows: 162mg x 2 = 324mg per course 324mg = 3x100mg vials plus 1x25mg vial = 1,076 (inc. VAT) per cycle Total for six courses = 6,460 The dose of rituximab used in the BR regimen is the same as that employed in the current standard regimens (e.g. R-CHOP, R-CVP). The cost implications of treating eligible patients first-line with BR instead of these current standard regimens will therefore be based on the additional cost of bendamustine when compared with CHOP and CVP. The costing statement for TA243 (Rituximab for the first-line treatment of stage III IV follicular lymphoma) states that the CHOP component costs 229 per cycle (12), which would work out as a total cost of 1650 (inc. VAT) for six cycles. Therefore the use of BR instead of R-CHOP would be associated with additional costs of around 4,810 per patient treated. The total estimated population that could be eligible for BR in the first-line treatment of indolent NHL or MCL can be estimated at around 6.8 per 100,000 (see epidemiology section). Based on the above calculations, the additional costs associated with use of this instead of R-CHOP would be approximately 32,700 per 100,000 population. Service implications The use of BR instead of R-CHOP would be associated with increased service delivery costs [based upon the DH Chemotherapy Regimens List ].
5 Summary of Costings Drug Indication Incidence (number of patients per 100,000 eligible for this treatment) Average duration of treatment (taken from trial data) Cost per month/ cycle Cost per 100,000 population per month/ cycle Cost per 100,000 for average treatment duration Bendamustine plus rituximab First-line treatment of MCL and indolent NHL 6.8 Six cycles * 800 * 5,440 * 32,640 *costs presented are the additional costs, using R-CHOP as the standard comparator (i.e. the difference between bendamustine and CHOP, as the same rituximab dose is used in both regimens) Details of search strategy: EMBASE: exp BENDAMUSTINE/ AND exp RITUXIMAB/ AND exp *NONHODGKIN LYMPHOMA/ [Limit to: Human and English Language] MEDLINE: bendamustine.af AND rituximab.af and exp *LYMPHOMA, NON-HODGKIN [Limit to: English Language and Humans]
6 References 1. New Drugs Online database; accessed via 2. Vidal L et al (2012) Bendamustine for patients with indolent B cell lymphoid malignancies including chronic lymphocytic leukaemia. Cochrane Database of Systematic Reviews 2012, Issue 9 doi/ / cd pub2/ abstract 3. Lymphoma (non Hodgkin's) - bendamustine (with rituximab): appendix B - final scope Scope/pdf/English 4. Rummel MJ et al (2013) Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 noninferiority trial. The Lancet; published early online 20 th February Lymphoma (mantle cell) - bendamustine (1st line, with rituximab): final scope action=download&o= McKay P et al (2012) Guidelines for the investigation and management of mantle cell lymphoma. British Journal of Haematology doi: /bjh mcl_guideline.pdf 7. Bendamustine (Levact ) SPC (last revised 3/8/10) 8. Personal communication with Napp Pharmaceuticals Ltd medical information (8/4/2013) 9. Rituximab (Mabthera ) SPC (last revised 25/5/12) 10. NICE technology appraisal in development (ID434): Bendamustine in combination with rituximab for the first-line treatment of advanced indolent non-hodgkin's lymphoma NICE technology appraisal in development (ID609): Bendamustine in combination with rituximab for the first-line treatment of mantle cell lymphoma index.jsp?action=byid&o= Rituximab for the first-line treatment of stage III IV follicular lymphoma (review of NICE technology appraisal guidance 110) costing statement nicemedia/live/13650/57920/57920.pdf 13. Jacobson CA, Freedman AS (2013) Firstline treatment of indolent lymphoma: axing CHOP? The Lancet; published early online 20 th February Flinn IW et al (2012) An open-label, randomized study of bendamustine and rituximab (BR) compared with rituximab, cyclophosphamide, vincristine, and prednisone (R- CVP) or rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R- CHOP) in first-line treatment of patients with advanced indolent non-hodgkin's lymphoma (NHL) or mantle cell lymphoma (MCL): The bright study. Blood; 120(21); abstract content/abstract/120/21/902? maxtoshow=&hits=10&resultformat=&fulltext =bright&searchid=1&firstindex=0&volum e=120&issue=21&resourcetype=hwcit 15. British National Formulary edition 64 (September 2012) Please direct any comments to Nicola Pocock, London & South East Medicines Information Service, Guy s Hospital, Great Maze Pond, London SE1 9RT Tel: , Fax: mailto:nicola.pocok@gstt.nhs.uk The document reflects the views of LCNDG and may not reflect those of the reviewers
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
Bendamustine for the fourth-line treatment of multiple myeloma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine for the fourth-line treatment of multiple myeloma Contents Summary 1 Background 2 Epidemiology 3 Cost 6 References 7 Summary There is no standard
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine for the fourth-line treatment of multiple myeloma Contents Summary 1 Background 2 Epidemiology 3 Cost 6 References 7 Summary There is no standard
January 2013 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW. Summary. Contents
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003)
 Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY
 CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY 26.1 Introduction rituximab Subsequent to the completion of drafts for the guidelines earlier in 2004,
CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY 26.1 Introduction rituximab Subsequent to the completion of drafts for the guidelines earlier in 2004,
rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited
 rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited 06 June 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited 06 June 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma
 Issue date: June 2011 Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma This guidance was developed using the the single technology appraisal process NICE technology
Issue date: June 2011 Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma This guidance was developed using the the single technology appraisal process NICE technology
Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer
 LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma
 Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
David Loew, LCL MabThera
 MabThera The star continues to rise David Loew, LCL MabThera MabThera the star continues to raise Group sales (CHF bn) 4,5 4,0 3,5 3,0 2,5 2,0 1,5 1,0 0,5 0,0 2001 2002 2003 2004 2005 Outstanding clinical
MabThera The star continues to rise David Loew, LCL MabThera MabThera the star continues to raise Group sales (CHF bn) 4,5 4,0 3,5 3,0 2,5 2,0 1,5 1,0 0,5 0,0 2001 2002 2003 2004 2005 Outstanding clinical
GLSG/OSHO Study Group. Supported by Deutsche Krebshilfe
 GLSG/OSHO Study Group Supported by Deutsche Krebshilfe GLSG/OSHO Study Group Study Concepts Follicular Lymphomas Mantel Cell Lymphomas Waldenstroem s Disease Key Steps in Improving Treatment for Follicular
GLSG/OSHO Study Group Supported by Deutsche Krebshilfe GLSG/OSHO Study Group Study Concepts Follicular Lymphomas Mantel Cell Lymphomas Waldenstroem s Disease Key Steps in Improving Treatment for Follicular
New Targets and Treatments for Follicular Lymphoma. Disclosures
 Winship Cancer Institute of Emory University New Targets and Treatments for Follicular Lymphoma Jonathon B. Cohen, MD, MS Assistant Professor Div of BMT, Emory University Disclosures Consulting fees from:
Winship Cancer Institute of Emory University New Targets and Treatments for Follicular Lymphoma Jonathon B. Cohen, MD, MS Assistant Professor Div of BMT, Emory University Disclosures Consulting fees from:
Treatment of low-grade non-hodgkin lymphoma
 Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Rituximab for the first-line treatment of stage III IV. D Papaioannou,* R Rafia, J Rathbone, M Stevenson, H Buckley Woods and J Stevens
 Rituximab for the first-line treatment of stage III IV follicular lymphoma Rituximab for the first-line treatment of stage III IV follicular lymphoma (review of Technology Appraisal No. 110): a systematic
Rituximab for the first-line treatment of stage III IV follicular lymphoma Rituximab for the first-line treatment of stage III IV follicular lymphoma (review of Technology Appraisal No. 110): a systematic
Guidelines for the Management of Follicular Lymphoma
 Guidelines for the Management of Follicular Lymphoma Scope The following guidance for first- and second-line therapy applies to follicular lymphoma histological grades 1, 2 and 3a according to the World
Guidelines for the Management of Follicular Lymphoma Scope The following guidance for first- and second-line therapy applies to follicular lymphoma histological grades 1, 2 and 3a according to the World
Update on Follicular Lymphoma. Brad Kahl, M.D.
 Update on Follicular Lymphoma Brad Kahl, M.D. Follicular Lymphoma: 25% of NHL Cases Other subtypes (9%) T and NK cell (12%) Burkitt (2.5%) Diffuse large B cell (DLBCL) (30%) Mantle cell (6%) Follicular
Update on Follicular Lymphoma Brad Kahl, M.D. Follicular Lymphoma: 25% of NHL Cases Other subtypes (9%) T and NK cell (12%) Burkitt (2.5%) Diffuse large B cell (DLBCL) (30%) Mantle cell (6%) Follicular
Frequency of NHL Subtypes in Adults
 Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Michael Crump MD. Lymphoma Site Leader Princess Margaret Hospital University of Toronto
 Evolution of Lymphoma Therapy: What can we expect for the rest of the millenium decade? Michael Crump MD Lymphoma Site Leader Princess Margaret Hospital University of Toronto disclaimers Served on advisory
Evolution of Lymphoma Therapy: What can we expect for the rest of the millenium decade? Michael Crump MD Lymphoma Site Leader Princess Margaret Hospital University of Toronto disclaimers Served on advisory
Mantle Cell Lymphoma Understanding Your Treatment Options
 New Developments in Mantle Cell Lymphoma John P. Leonard, M.D. Richard T. Silver Distinguished Professor of Hematology and Medical Oncology Associate Dean for Clinical Research Vice Chairman, Department
New Developments in Mantle Cell Lymphoma John P. Leonard, M.D. Richard T. Silver Distinguished Professor of Hematology and Medical Oncology Associate Dean for Clinical Research Vice Chairman, Department
Rituximab in Non - Hodgkins Lymphoma. Fatima Bassa, Dept. of Haematology October 2008
 Rituximab in Non - Hodgkins Lymphoma Fatima Bassa, Dept. of Haematology October 2008 World Health Organization lymphoma classification (2001) Peripheral B-cell neoplasms: B-chronic lymphocytic leukemia/small
Rituximab in Non - Hodgkins Lymphoma Fatima Bassa, Dept. of Haematology October 2008 World Health Organization lymphoma classification (2001) Peripheral B-cell neoplasms: B-chronic lymphocytic leukemia/small
Audience Response Question?
 Presenter Disclosure Information Session 4: 3:30 PM - 4:15 PM Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman, MD The following relationships
Presenter Disclosure Information Session 4: 3:30 PM - 4:15 PM Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman, MD The following relationships
Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma
 a report by Martin Dreyling Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma Head, Lymphoma Section, Department of Medicine III, University Hospital Großhadern, Ludwig Maximilians-University
a report by Martin Dreyling Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma Head, Lymphoma Section, Department of Medicine III, University Hospital Großhadern, Ludwig Maximilians-University
Histopathologic results
 Self evaluation 1 Clinical Case 55-year-old woman Bilateral enlargement of cervical, axillary and inguinal lymph nodes, largest diameter > 6 cm Hepatosplenomegaly. Enlargement of retroperitoneal, mesenteric
Self evaluation 1 Clinical Case 55-year-old woman Bilateral enlargement of cervical, axillary and inguinal lymph nodes, largest diameter > 6 cm Hepatosplenomegaly. Enlargement of retroperitoneal, mesenteric
The role of chemotherapy in follicular lymphomas Emanuele Zucca, M.D.
 The role of chemotherapy in follicular lymphomas Emanuele Zucca, M.D. Oncology Institute of Southern Switzerland (IOSI) Swiss Group for Clinical Cancer Research (SAKK) WHO grading of follicular lymphoma
The role of chemotherapy in follicular lymphomas Emanuele Zucca, M.D. Oncology Institute of Southern Switzerland (IOSI) Swiss Group for Clinical Cancer Research (SAKK) WHO grading of follicular lymphoma
NATIONAL CANCER DRUG FUND PRIORITISATION SCORES
 NATIONAL CANCER DRUG FUND PRIORITISATION SCORES Drug Indication Regimen (where appropriate) BORTEZOMIB In combination with dexamethasone (VD), or with dexamethasone and thalidomide (VTD), is indicated
NATIONAL CANCER DRUG FUND PRIORITISATION SCORES Drug Indication Regimen (where appropriate) BORTEZOMIB In combination with dexamethasone (VD), or with dexamethasone and thalidomide (VTD), is indicated
Audience Response Question? Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management. Presenter Disclosure Information
 Welcome to Master Class for Oncologists Session 4: 10:00 AM - 10:45 AM Miami, FL December 18, 2009 Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman,
Welcome to Master Class for Oncologists Session 4: 10:00 AM - 10:45 AM Miami, FL December 18, 2009 Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman,
6/3/2013. Follicular and Other Slow Growing Lymphomas. Stephen Ansell, MD, PhD Mayo Clinic
 Follicular and Other Slow Growing Lymphomas Stephen Ansell, MD, PhD Mayo Clinic 1 Learning Objectives Start with an overview of Follicular and other slow growing lymphomas Discuss current and emerging
Follicular and Other Slow Growing Lymphomas Stephen Ansell, MD, PhD Mayo Clinic 1 Learning Objectives Start with an overview of Follicular and other slow growing lymphomas Discuss current and emerging
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin s lymphoma
 DOI: 10.3310/hta13suppl2/06 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin s lymphoma A Boland, A Bagust,
DOI: 10.3310/hta13suppl2/06 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin s lymphoma A Boland, A Bagust,
Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm
 Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm Name of Policy: Uses of Monoclonal Antibodies for the Treatment of Non-Hodgkin
Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm Name of Policy: Uses of Monoclonal Antibodies for the Treatment of Non-Hodgkin
DECISION AND SUMMARY OF RATIONALE
 DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Clofarabine in the treatment of relapsed acute myeloid leukaemia (AML) The application was for clofarabine to remain in
DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Clofarabine in the treatment of relapsed acute myeloid leukaemia (AML) The application was for clofarabine to remain in
MALIGNANT LYMPHOMAS. Dr. Olga Vujovic (Updated August 2010)
 MALIGNANT LYMPHOMAS Dr. Olga Vujovic (Updated August 2010) Malignant lymphomas consist of Hodgkin and non-hodgkin lymphomas. The current management of these diseases involves a multi-disciplinary approach.
MALIGNANT LYMPHOMAS Dr. Olga Vujovic (Updated August 2010) Malignant lymphomas consist of Hodgkin and non-hodgkin lymphomas. The current management of these diseases involves a multi-disciplinary approach.
Hodgkin Lymphoma Disease Specific Biology and Treatment Options. John Kuruvilla
 Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Follicular lymphoma. What is follicular lymphoma? Freephone helpline 0808 808 5555 information@lymphomas.org.uk www.lymphomas.org.
 Freephone helpline 0808 808 5555 information@lymphomas.org.uk www.lymphomas.org.uk is a cancer of the lymphatic system, a type of non-hodgkin lymphoma. Even though more than 12,000 people are diagnosed
Freephone helpline 0808 808 5555 information@lymphomas.org.uk www.lymphomas.org.uk is a cancer of the lymphatic system, a type of non-hodgkin lymphoma. Even though more than 12,000 people are diagnosed
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015. (minutes for web publishing)
 Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the
Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the
A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on
 A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on examination. She also has fever, weight loss, and sweats. What
A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on examination. She also has fever, weight loss, and sweats. What
Scottish Medicines Consortium
 Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases
 Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Follicular Lymphoma. Aruna K. Reddy, MD. Hematology& Oncology Peace Health Southwest Medical Center
 Follicular Lymphoma Aruna K. Reddy, MD Hematology& Oncology Peace Health Southwest Medical Center Follicular Lymphoma Malignant neoplasm resulting from clonal proliferation of malignant B-cells Second
Follicular Lymphoma Aruna K. Reddy, MD Hematology& Oncology Peace Health Southwest Medical Center Follicular Lymphoma Malignant neoplasm resulting from clonal proliferation of malignant B-cells Second
Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
 Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
National Horizon Scanning Centre. Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer. December 2007
 Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
In non-hodgkin s lymphoma, MabThera is used to treat two types of the disease, both of which affect B-lymphocytes:
 EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
Treatment results with Bortezomib in multiple myeloma
 Treatment results with Bortezomib in multiple myeloma Prof. Dr. Orhan Sezer Hamburg University Medical Center Circulating proteasome levels are an independent prognostic factor in MM 1.0 Probability of
Treatment results with Bortezomib in multiple myeloma Prof. Dr. Orhan Sezer Hamburg University Medical Center Circulating proteasome levels are an independent prognostic factor in MM 1.0 Probability of
IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN
 + IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
+ IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
Severe rheumatoid arthritis (a disease that causes inflammation of the joints),where MabThera is given intravenously together with methotrexate.
 EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
1. Introduction. 2. Clinical aspects
 1. Introduction MabThera (rituximab) is a genetically engineered chimeric mouse/human monoclonal antibody which binds specifically to the transmembrane antigen, CD20. This antigen is located on pre-b-
1. Introduction MabThera (rituximab) is a genetically engineered chimeric mouse/human monoclonal antibody which binds specifically to the transmembrane antigen, CD20. This antigen is located on pre-b-
FOLLICULAR LYMPHOMA. Executive Summary
 FOLLICULAR LYMPHOMA Executive Summary Follicular lymphoma (FL) is the most common indolent lymphoma and the second most common non-hodgkin lymphoma accounting for about 10-20% of all lymphomas in Western
FOLLICULAR LYMPHOMA Executive Summary Follicular lymphoma (FL) is the most common indolent lymphoma and the second most common non-hodgkin lymphoma accounting for about 10-20% of all lymphomas in Western
pan-canadian Oncology Drug Review Final Clinical Guidance Report Bortezomib (Velcade) for Multiple Myeloma March 25, 2013
 pan-canadian Oncology Drug Review Final Clinical Guidance Report Bortezomib (Velcade) for Multiple Myeloma March 25, 2013 DISCLAIMER Not a Substitute for Professional Advice This report is primarily intended
pan-canadian Oncology Drug Review Final Clinical Guidance Report Bortezomib (Velcade) for Multiple Myeloma March 25, 2013 DISCLAIMER Not a Substitute for Professional Advice This report is primarily intended
Lauren Berger: Why is it so important for patients to get an accurate diagnosis of their blood cancer subtype?
 Hello, I m Lauren Berger and I m the Senior Director of Patient Services Programs at The Leukemia & Lymphoma Society. I m pleased to welcome Dr. Rebecca Elstrom. Dr. Elstrom is an Assistant Professor in
Hello, I m Lauren Berger and I m the Senior Director of Patient Services Programs at The Leukemia & Lymphoma Society. I m pleased to welcome Dr. Rebecca Elstrom. Dr. Elstrom is an Assistant Professor in
Aggressive lymphomas. Michael Crump Princess Margaret Hospital
 Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Réda Bouabdallah, MD Institut Paoli-Calmettes, Marseille, France. Oran, May, 8th, 2010
 FIRST LINE TREATMENT IN FOLLICULAR LYMPHOMA Current Status Réda Bouabdallah, MD Institut Paoli-Calmettes, Marseille, France Oran, May, 8th, 2010 FL is a complex disease One disease, but many profiles 20%
FIRST LINE TREATMENT IN FOLLICULAR LYMPHOMA Current Status Réda Bouabdallah, MD Institut Paoli-Calmettes, Marseille, France Oran, May, 8th, 2010 FL is a complex disease One disease, but many profiles 20%
Lymphoma Diagnosis and Classification
 Lymphoma Diagnosis and Classification By Atef Shrit, MD, Pathology B- and T/NK-cell lymphomas are clonal neoplasms of immature and mature B-lymphocytes, T-lymphocytes or natural killer cells at various
Lymphoma Diagnosis and Classification By Atef Shrit, MD, Pathology B- and T/NK-cell lymphomas are clonal neoplasms of immature and mature B-lymphocytes, T-lymphocytes or natural killer cells at various
Background. t 1/2 of 3.7 4.7 days allows once-daily dosing (1.5 mg) with consistent serum concentration 2,3 No interaction with CYP3A4 inhibitors 4
 Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Lymphoma: The Roleof Nurses in the Treatment Process
 Lymphoma: The Roleof Nurses in the Treatment Process Sarah Liptrott MSc,BN(Hons), RN Istituto Europeo di Oncologia, Milan (IT) EBMT Swiss Study Day 2014, Zurich, Switzerland LymphomaManagement Watch &
Lymphoma: The Roleof Nurses in the Treatment Process Sarah Liptrott MSc,BN(Hons), RN Istituto Europeo di Oncologia, Milan (IT) EBMT Swiss Study Day 2014, Zurich, Switzerland LymphomaManagement Watch &
Future strategies for myeloma: An overview of novel treatments In development
 Future strategies for myeloma: An overview of novel treatments In development Dr. Matthew Streetly Guys and St. Thomas NHS Trust How far have we come? Melphalan and prednisolone VAD Autologous SCT Thalidomide
Future strategies for myeloma: An overview of novel treatments In development Dr. Matthew Streetly Guys and St. Thomas NHS Trust How far have we come? Melphalan and prednisolone VAD Autologous SCT Thalidomide
What is non-hodgkin lymphoma, how is it treated, and what is the unmet need?
 What is non-hodgkin lymphoma, how is it treated, and what is the unmet need? Tim Illidge BSc PhD MRCP FRCR FRCPath Institute of Cancer Sciences, University of Manchester Manchester Cancer Research Centre,
What is non-hodgkin lymphoma, how is it treated, and what is the unmet need? Tim Illidge BSc PhD MRCP FRCR FRCPath Institute of Cancer Sciences, University of Manchester Manchester Cancer Research Centre,
Cycle frequency: Every four weeks Total number of cycles: 6-8
 Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
Anti-HCV therapy in HCV-related NHL
 Gabriele Pozzato M.D. University of Trieste Anti-HCV therapy in HCV-related NHL Questions about HCV+ in NHL Is the NHL related with HCV infection? Which is the best therapeutic strategy? Is the antiviral
Gabriele Pozzato M.D. University of Trieste Anti-HCV therapy in HCV-related NHL Questions about HCV+ in NHL Is the NHL related with HCV infection? Which is the best therapeutic strategy? Is the antiviral
Cure versus control: Which is the best strategy?
 Cure versus control: Which is the best strategy? Barcelona 8-9-2012 Mario Boccadoro DIVISIONE UNIVERSITARIA DI EMATOLOGIA AZIENDA OSPEDALIERA SAN GIOVANNI TORINO, ITALY MULTIPLE MYELOMA Cure versus control
Cure versus control: Which is the best strategy? Barcelona 8-9-2012 Mario Boccadoro DIVISIONE UNIVERSITARIA DI EMATOLOGIA AZIENDA OSPEDALIERA SAN GIOVANNI TORINO, ITALY MULTIPLE MYELOMA Cure versus control
Chemotherapy in Ovarian Cancer. Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group
 Chemotherapy in Ovarian Cancer Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group Adjuvant chemotherapy for early stage EOC Fewer than 30% women present with FIGO stage
Chemotherapy in Ovarian Cancer Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group Adjuvant chemotherapy for early stage EOC Fewer than 30% women present with FIGO stage
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin's lymphoma: Review of technology appraisal guidance 37
 Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin's lymphoma: Review of technology appraisal Issued: February 2008 guidance.nice.org.uk/ta NICE 2008 Contents
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin's lymphoma: Review of technology appraisal Issued: February 2008 guidance.nice.org.uk/ta NICE 2008 Contents
Rituximab Maintenance for 2 Years in Patients with Untreated High Tumor Burden Follicular Lymphoma After Response to Immunochemotherapy
 Rituximab Maintenance for 2 Years in Patients with Untreated High Tumor Burden Follicular Lymphoma After Response to Immunochemotherapy G. A. Salles, J. F. Seymour, P. Feugier, F. Offner, A. Lopez-Guillermo,
Rituximab Maintenance for 2 Years in Patients with Untreated High Tumor Burden Follicular Lymphoma After Response to Immunochemotherapy G. A. Salles, J. F. Seymour, P. Feugier, F. Offner, A. Lopez-Guillermo,
New Treatment Options for Breast Cancer
 New Treatment Options for Breast Cancer Brandon Vakiner, PharmD., BCOP Clinical Pharmacy Specialist - Oncology The University of Iowa Hospitals and Clinics Assistant Professor (Clinical) University of
New Treatment Options for Breast Cancer Brandon Vakiner, PharmD., BCOP Clinical Pharmacy Specialist - Oncology The University of Iowa Hospitals and Clinics Assistant Professor (Clinical) University of
Rituximab in the Management of Follicular Lymphoma
 Hong Kong J Radiol. 2011;14(Suppl):S63-7 REVIEW ARTICLE Rituximab in the Management of Follicular Lymphoma YL Kwong Department of Medicine, Professorial Block, Queen Mary Hospital, Pokfulam Road, Hong
Hong Kong J Radiol. 2011;14(Suppl):S63-7 REVIEW ARTICLE Rituximab in the Management of Follicular Lymphoma YL Kwong Department of Medicine, Professorial Block, Queen Mary Hospital, Pokfulam Road, Hong
DIFFUSE LARGE B-CELL LYMPHOMA
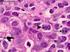 DIFFUSE LARGE B-CELL LYMPHOMA Executive Summary Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-hodgkin lymphoma (NHL), constituting up to 40% of all cases globally.[1] This subtype
DIFFUSE LARGE B-CELL LYMPHOMA Executive Summary Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-hodgkin lymphoma (NHL), constituting up to 40% of all cases globally.[1] This subtype
How valuable is a cancer therapy? It depends on who you ask.
 How valuable is a cancer therapy? It depends on who you ask. Comparing and contrasting the ESMO Magnitude of Clinical Benefit Scale with the ASCO Value Framework in Cancer Ram Subramanian Kevin Schorr
How valuable is a cancer therapy? It depends on who you ask. Comparing and contrasting the ESMO Magnitude of Clinical Benefit Scale with the ASCO Value Framework in Cancer Ram Subramanian Kevin Schorr
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Guidelines for the use of Rituximab in Non-Hodgkin s Lymphoma QEII Health Sciences Centre
 Guidelines for the use of Rituximab in Non-Hodgkin s Lymphoma QEII Health Sciences Centre Background Non-Hodgkin s lymphoma (NHL) makes up approximately 85% of all lymphomas. They are a heterogeneous collection
Guidelines for the use of Rituximab in Non-Hodgkin s Lymphoma QEII Health Sciences Centre Background Non-Hodgkin s lymphoma (NHL) makes up approximately 85% of all lymphomas. They are a heterogeneous collection
The NCPE has issued a recommendation regarding the use of pertuzumab for this indication. The NCPE does not recommend reimbursement of pertuzumab.
 Cost Effectiveness of Pertuzumab (Perjeta ) in Combination with Trastuzumab and Docetaxel in Adults with HER2-Positive Metastatic or Locally Recurrent Unresectable Breast Cancer Who Have Not Received Previous
Cost Effectiveness of Pertuzumab (Perjeta ) in Combination with Trastuzumab and Docetaxel in Adults with HER2-Positive Metastatic or Locally Recurrent Unresectable Breast Cancer Who Have Not Received Previous
lenalidomide, 5mg, 10mg, 15mg and 25mg hard capsules (Revlimid ) SMC No. (441/08) Celgene Limited
 Resubmission: lenalidomide, 5mg, 10mg, 15mg and 25mg hard capsules (Revlimid ) SMC No. (441/08) Celgene Limited 07 March 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Resubmission: lenalidomide, 5mg, 10mg, 15mg and 25mg hard capsules (Revlimid ) SMC No. (441/08) Celgene Limited 07 March 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the
If several different trials are mentioned in one publication, the data of each should be extracted in a separate data extraction form.
 General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
Non-Hodgkin s lymphomas (NHLs) are a
 Oncology 33 Non-Hodgkin s lymphoma in the elderly The incidence of non-hodgkin s lymphoma (NHL) is increasing, and this increase is even more rapid in the older population. Although treatment of NHL in
Oncology 33 Non-Hodgkin s lymphoma in the elderly The incidence of non-hodgkin s lymphoma (NHL) is increasing, and this increase is even more rapid in the older population. Although treatment of NHL in
Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics
 Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics Disclosure(s) I do not intend to discuss an off-label use
Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics Disclosure(s) I do not intend to discuss an off-label use
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW. FOLFIRINOX for first line treatment of advanced pancreatic cancer January 2012
 Background LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW FOLFIRINOX for first line treatment of advanced pancreatic cancer January 2012 The incidence of pancreatic cancer in the UK is 9.4/100,000. It is
Background LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW FOLFIRINOX for first line treatment of advanced pancreatic cancer January 2012 The incidence of pancreatic cancer in the UK is 9.4/100,000. It is
Collaboration to collect Autologous transplant outcomes in Lymphoma and Myeloma (CALM) Additional Questionnaire (MED C) INCLUSION CRITERIA CALM STUDY
 Additional Questionnaire (MED C) CALM study Inclusion period: 01/01/2008 to 31/12/2011 PATIENT REGISTRATION FORM Disease Diagnosis Lymphoma S Non Hodgkin Lymphoma (NHL) Mature B-cell neoplasm Follicular
Additional Questionnaire (MED C) CALM study Inclusion period: 01/01/2008 to 31/12/2011 PATIENT REGISTRATION FORM Disease Diagnosis Lymphoma S Non Hodgkin Lymphoma (NHL) Mature B-cell neoplasm Follicular
FLUDARABINE AS FIRST LINE THERAPY FOR CHRONIC LYMPHOCYTIC LEUKAEMIA
 FLUDARABINE AS FIRST LINE THERAPY FOR CHRONIC LYMPHOCYTIC LEUKAEMIA A West Midlands Health Technology Assessment Collaboration report Authors: Sarah Hancock, Research Reviewer and Analyst Beverley Wake,
FLUDARABINE AS FIRST LINE THERAPY FOR CHRONIC LYMPHOCYTIC LEUKAEMIA A West Midlands Health Technology Assessment Collaboration report Authors: Sarah Hancock, Research Reviewer and Analyst Beverley Wake,
Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy
 Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy Yoichi Kitamura, MD Kazuhiko Hayashi, MD Kazumi Uchida,
Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy Yoichi Kitamura, MD Kazuhiko Hayashi, MD Kazumi Uchida,
Sonneveld, P; de Ridder, M; van der Lelie, H; et al. J Clin Oncology, 13 (10) : 2530-2539 Oct 1995
 Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
HealthPACT Health Policy Advisory Committee on Technology Australia and New Zealand Technology Brief
 HealthPACT Health Policy Advisory Committee on Technology Australia and New Zealand Technology Brief 90Y Zevalin for the treatment of non-hodgkin s lymphoma (v1.0) August 2011 State of Queensland (Queensland
HealthPACT Health Policy Advisory Committee on Technology Australia and New Zealand Technology Brief 90Y Zevalin for the treatment of non-hodgkin s lymphoma (v1.0) August 2011 State of Queensland (Queensland
亞 東 紀 念 醫 院 Follicular Lymphoma 臨 床 指 引
 前 言 : 惡 性 淋 巴 瘤 ( 或 簡 稱 淋 巴 癌 ) 乃 由 體 內 淋 巴 系 統 包 括 淋 巴 細 胞 淋 巴 管 淋 巴 腺 及 一 些 淋 巴 器 官 或 組 織 如 脾 臟 胸 腺 及 扁 桃 腺 等 所 長 出 的 惡 性 腫 瘤 依 腫 瘤 病 理 組 織 型 態 的 不 同 可 分 為 何 杰 金 氏 淋 巴 瘤 (Hodgkin s disease) 與 非 何 杰 金
前 言 : 惡 性 淋 巴 瘤 ( 或 簡 稱 淋 巴 癌 ) 乃 由 體 內 淋 巴 系 統 包 括 淋 巴 細 胞 淋 巴 管 淋 巴 腺 及 一 些 淋 巴 器 官 或 組 織 如 脾 臟 胸 腺 及 扁 桃 腺 等 所 長 出 的 惡 性 腫 瘤 依 腫 瘤 病 理 組 織 型 態 的 不 同 可 分 為 何 杰 金 氏 淋 巴 瘤 (Hodgkin s disease) 與 非 何 杰 金
D Papaioannou, R Rafia, J Rathbone, M Stevenson, H Buckley Woods and J Stevens. Health Technology Assessment NIHR HTA programme www.hta.ac.
 Health Technology Assessment 2012; Vol. 16: No. 37 ISSN 1366-5278 Rituximab for the first-line treatment of stage III IV follicular lymphoma (review of Technology Appraisal No. 110): a systematic review
Health Technology Assessment 2012; Vol. 16: No. 37 ISSN 1366-5278 Rituximab for the first-line treatment of stage III IV follicular lymphoma (review of Technology Appraisal No. 110): a systematic review
Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012. (minutes for web publishing)
 Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
U.S. Food and Drug Administration
 U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD Boehringer Ingelheim International GmbH and Boehringer Ingelheim Pharmaceuticals, Inc. Petitioner, v. Biogen Idec, Inc.
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD Boehringer Ingelheim International GmbH and Boehringer Ingelheim Pharmaceuticals, Inc. Petitioner, v. Biogen Idec, Inc.
Treatment of Follicular Lymphoma
 JClin Exp Hematop Vol. 54, No. 1, June 2014 Review Article Treatment of Follicular Lymphoma Koji Izutsu 1,2) Follicular lymphoma (FL) is the most common subtype of indolent lymphomas. Several lines of
JClin Exp Hematop Vol. 54, No. 1, June 2014 Review Article Treatment of Follicular Lymphoma Koji Izutsu 1,2) Follicular lymphoma (FL) is the most common subtype of indolent lymphomas. Several lines of
Leukemias and Lymphomas: A primer
 Leukemias and Lymphomas: A primer Normal blood contains circulating white blood cells, red blood cells and platelets 700 red cells (oxygen) 1 white cell Neutrophils (60%) bacterial infection Lymphocytes
Leukemias and Lymphomas: A primer Normal blood contains circulating white blood cells, red blood cells and platelets 700 red cells (oxygen) 1 white cell Neutrophils (60%) bacterial infection Lymphocytes
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Effective on or after May 1, 2015,, refer to: Blue Cross and Blue Shield of Alabama Radiation Therapy Management RTM Policies
 Effective on or after May 1, 2015,, refer to: Blue Cross and Blue Shield of Alabama Radiation Therapy Management RTM Policies Name of Policy: Radioimmunotherapy in the Treatment of Non-Hodgkin Lymphoma
Effective on or after May 1, 2015,, refer to: Blue Cross and Blue Shield of Alabama Radiation Therapy Management RTM Policies Name of Policy: Radioimmunotherapy in the Treatment of Non-Hodgkin Lymphoma
boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd
 boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment
boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS
 MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
Treating myeloma. Dr Rachel Hall Royal Bournemouth Hospital
 Treating myeloma Dr Rachel Hall Royal Bournemouth Hospital Treatment overview When to treat? Aim of treatment Which treatment? Monitoring response to treatment Prevention of complications What happens
Treating myeloma Dr Rachel Hall Royal Bournemouth Hospital Treatment overview When to treat? Aim of treatment Which treatment? Monitoring response to treatment Prevention of complications What happens
Supplementary Online Content
 Supplementary Online Content Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving
Supplementary Online Content Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving
Treatment of follicular lymphoma
 Treatment of follicular lymphoma Mathias J. Rummel Med. Klinik IV/ V - Hämatologie Klinikum der Justus-Liebig Liebig-Universität Gießen MJR Therapy of Follicular Lymphoma wait and see policy alkylating
Treatment of follicular lymphoma Mathias J. Rummel Med. Klinik IV/ V - Hämatologie Klinikum der Justus-Liebig Liebig-Universität Gießen MJR Therapy of Follicular Lymphoma wait and see policy alkylating
DECISION AND SUMMARY OF RATIONALE
 DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Crizotinib as 2nd line treatment for patients with anaplastic lymphoma kinase (ALK) positive lung cancer Score The application
DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Crizotinib as 2nd line treatment for patients with anaplastic lymphoma kinase (ALK) positive lung cancer Score The application
Perspectives on Recent Non-Hodgkin s Lymphoma (NHL) Data
 Perspectives on Recent Non-Hodgkin s Lymphoma (NHL) Data Summary Article James O. Armitage, MD Presented through a strategic collaboration by A series of 5 expert interviews were conducted, focusing on
Perspectives on Recent Non-Hodgkin s Lymphoma (NHL) Data Summary Article James O. Armitage, MD Presented through a strategic collaboration by A series of 5 expert interviews were conducted, focusing on
Fludarabine based chemotherapy [Fludarabine, FC, FCR, FMD]
![Fludarabine based chemotherapy [Fludarabine, FC, FCR, FMD] Fludarabine based chemotherapy [Fludarabine, FC, FCR, FMD]](/thumbs/29/13852629.jpg) Approved Haematology Chemotherapy Protocol Fludarabine based chemotherapy [Fludarabine, FC, FCR, FMD] MCCN Ref OPCS Proc OPCS Del CLFLUD X70.5 X72.3 IV CLFC X70.4 X72.2 IV X73.1 O CLFCR X71.5 X72.1 LYFMD
Approved Haematology Chemotherapy Protocol Fludarabine based chemotherapy [Fludarabine, FC, FCR, FMD] MCCN Ref OPCS Proc OPCS Del CLFLUD X70.5 X72.3 IV CLFC X70.4 X72.2 IV X73.1 O CLFCR X71.5 X72.1 LYFMD
How do I find the best place to get treatment for my lymphoma?
 Produced November 2010 Next revision due November 2012 How do I find the best place to get treatment for my lymphoma? Introduction Fortunately this is not a question that patients with cancers of the blood
Produced November 2010 Next revision due November 2012 How do I find the best place to get treatment for my lymphoma? Introduction Fortunately this is not a question that patients with cancers of the blood
New Evidence reports on presentations given at EULAR 2012. Rituximab for the Treatment of Rheumatoid Arthritis
 New Evidence reports on presentations given at EULAR 2012 Rituximab for the Treatment of Rheumatoid Arthritis Report on EULAR 2012 presentations Long-term safety of rituximab: 10-year follow-up in the
New Evidence reports on presentations given at EULAR 2012 Rituximab for the Treatment of Rheumatoid Arthritis Report on EULAR 2012 presentations Long-term safety of rituximab: 10-year follow-up in the
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cll_and_sll
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cll_and_sll
