encephalomyelitis: delineation of usage and mode
|
|
|
- Alexander Watts
- 10 years ago
- Views:
Transcription
1 3'ournal ofneurology, Neurosurgery, and Psychiatry 1994;57 (Supplement): III CLINICAL APPLICATIONS: CENTRAL NERVOUS SYSTEM DISEASES Intravenous gammaglobulin treatment in multiple sclerosis and experimental autoimmune encephalomyelitis: delineation of usage and mode of action A Achiron, R Gilad, R Margalit, U Gabbay, I Sarova-Pinhas, I R Cohen, E Melamed, O Lider, S Noy, I Ziv Department of Neurology, Beilinson Petah-Tiqva, Israel A Achiron R Margalit U Gabbay E Melamed S Noy I Ziv Department of Neurology, Wolfson Holon, Israel R Gilad I Sarova-Pinhas Department of Cell Biology, Weizmann Institute of Science, Rehovot, Israel I R Cohen Lider Correspondence to: Dr R Gilad, Department of Neurology, Beilinson Petah-Tiqva, Israel. Abstract Multiple sclerosis (MS) is a central nervous system demyelinating disease of implicated autoimmune aetiology. The effect was evaluated of intravenous gammaglobulin (IVIg), a successful therapy in various autoimmune diseases, in relapsing-remitting MS patients treated for three years. IVIg treatment significantly reduced the number and severity of acute exacerbations and resulted in a lesser neurological disability. There were no significant short or long-term adverse effects to IVIg treatment. To clarify the putative therapeutic effects of IVIg, this treatment was examined in the animal model of experimental autoimmune encephalomyelitis (EAE) in the rat. IVIg suppressed active EAE in relation to disease severity and duration, despite the presence of T-cell reactivity to specific antigens, while the treatment had no effect on passive EAE induced by adoptive transfer of myelin basic protein specific CD4 + T-cells. It is concluded that IVIg treatment may be a promising treatment in relapsing-remitting MS as it can alter the natural course of the disease. Multiple sclerosis (MS) is the most common central nervous system disease affecting the white matter. Numerous immunological abnormalities have been identified in patients with MS including increased immunoglobulin levels with oligoclonal bands in the CSF; reduction in the number of suppressor T cells (CD8) and loss of suppressor function; the presence of activated T cells (CD4) in the peripheral blood and the migration of inducer effector cells into the central nervous system to initiate the disease process; increased cytokine production capable of amplifying immune responses and mediating demyelination; enhanced expression of class II major histocompatibility complex (MHC), which results in increased immune reactivity; altered autologous mixed lymphocyte responses on myelin basic protein-coated target cells.'-5 The alternations in both cellular and humoral immune mechanisms in MS strongly suggest that immunoregulatory mechanisms mediating suppression are disturbed in the disease. Thus treatment with intravenous gammaglobulins (IVIg) as non-specific modulators of the immune response was suggested as a strategy to alter the grave course of the disease. We have recently reported the beneficial effects of IVIg treatment in relapsingremitting patients with MS.6 The present study summarises our long-term clinical experience of IVIg treatment in relapsingremitting MS patients for three years. In addition, we have investigated the mode of action of IVIg in experimental autoimmune encephalomyelitis (EAE), the animal model for MS induced by either active immunisation with myelin-associated proteins or adoptive transfer of syngeneic autoreactive encephalitogenic T cells.7-' Methods CLINICAL STUDIES The study was approved by the Medical Ethics Committee on Clinical Investigations of the Beilinson Petah- Tiqva, Israel. All patients gave informedconsent to participation in the study. Patients Twenty relapsing-remitting MS patients with definite disease," were included in the study. Selection of patients for participation in the study is described in detail elsewhere.6 Briefly, the major inclusion criteria were: age 2-55 years; annual exacerbation rate of at least two well documented exacerbations in the two years before the study; a score no higher than six (ambulatory with assistance) on the Kurtzke Expanded Disability Status Scale (EDSS)"I; emotionally stable and without severe cognitive impairments; no corticosteroid therapy for at least a month, and/or other immunosuppressive drugs during the previous year before entering the study; negative history of anaphylaxis after previous transfusions of blood or blood products; normal levels of IgA evaluated by plasma immu-
2 58 Achiron, Gilad, Margalit, Gabbay, Sarova-Pinhas, Cohen, Melamed, Lider, Noy, Ziv noelectrophoresis. Randomly, ten patients were assessed to receive IVIg treatment [mean (SD) age 39-8 (1.5); range 21-53] and 1 patients matched for age [41-4 (5 8); range 36-54], disease duration [9 5 (7.3); 8 (4 9), respectively] and annual exacerbation rate in the two years before the study, served as controls. IVIg treatment IVIg (Gamimune N, Miles Inc, Cutter Biological, Promedico, Israel) in a sterile % solution of human protein in 9-11 % maltose, was given intravenously once daily for five consecutive days at a dose of 4 g/kg/day at the beginning of the study. Thereafter, patients were further treated with booster doses of IVIg, -4 g/kg/day, once every two months, for the next three years. Clinical evaluation Patients were examined before starting the study, and once every month thereafter. Routine urinalysis, complete blood count and chemistry, serological survey of HTLV-1, HIV, and Hepatitis B, and immunoelectrophoresis were performed at entry to the study and at each six month visit. Evaluation of acute exacerbations Patients were seen at times of suspected exacerbations, that is, when reporting the rapid onset of new symptoms or a deterioration of pre-existing symptoms that persisted for 48 hours or more. An event was counted as an exacerbation only when the patient's symptoms were accompanied by observed objective changes on the neurological examination involving an increase of at least one grade in the score for one of the eight functional groups of the Kurtzke EDSS. Patients experiencing an acute exacerbation were evaluated at frequent intervals, usually every week, until a new, stable neurological base-line is established. Evaluation of severity of exacerbations 1) mild-change in one grade in the score for one of the eight functional groups of the Kurtzke EDSS; 2) moderate--change in two grades in the score for one of the eight functional groups of the Kurtzke EDSS or change in one grade in the score for two of the eight functional groups of the Kurtzke EDSS; 3) severe-change in two grades in the score for at least two of the eight functional groups of the Kurtzke EDSS. Treatment of acute exacerbations Severe exacerbations were treated with intravenous methylprednisolone (1 gr/day) for five consecutive days. Moderate relapses were treated with oral prednisone (6 mg/day), for five consecutive days. Mild exacerbations were not treated. EXPERIMENTAL STUDIES Animals Inbred Lewis rats, six to eight weeks old, were supplied by the Animal Breeding Center, Weizmann Institute of Science, Rehovot. Rats were matched for age and sex in each experiment. Active induction of EAE Rats were inoculated subcutaneously in each hind footpad with 25,ug of guinea-pig myelin basic protein (BP) in complete Freund's adjuvant containing 4,ig of Mycobacterium tuberculosis in 1 ml of oil. Passive induction ofeae Adoptive transfer of EAE was carried out with 2 x 16 activated anti-bp CD4+ Kl T- cells per rat, administrated intraperitoneally.7 Clinical assessment of EAE Clinical signs of EAE appeared days after active induction and five to seven days after adoptive transfer. Degree of clinical disease was scored as follows: -no signs; 1-loss of tail tonicity; 2-paralysis of hind limbs; 3-paralysis of all four limbs; 4-quadriplegic animal in a moribund state.89 IVIg treatment Human IgG in a sterile % solution in 9-11% maltose, (Gamimune N, Miles Inc., Cutter Biological, Promedico, Israel), was inoculated intravenously into a tail vein at a dose of 4 g/kg per dose. Controls included bovine serum albumin (BSA), at a dose of -4 g/kg/day, and Maltose 1%, 5 ml/day, given intravenously. Treatment was given from the day of disease induction (day ) until clinical signs appeared in the control group. Reinduction ofeae Secondary induction of EAE was performed one month after primary immunisation by subcutaneous inoculation in each hind footpad with 25,ug of guinea-pig BP in CFA containing 4,ug of Mycobacterium tuberculosis in 1 ml of oil. T-cell proliferative responses Ten days after active induction of EAE lymph node cells were isolated from treated or control rats and incubated in flat-bottomed microtitre plates in quadruplicate wells. Each well contained 2 x 15 cells in -2 ml of proliferation medium (Dulbecco's modified Eagle's medium (DMEM), supplemented with 1% syngeneic fresh rat serum, 5 x 1-5M 2-mercaptoethanol, 2mM glutamine, 1 U/ml penicillin and 1 mg/ml streptomycin), with the following antigens: Mycobacterium tuberculosis, ovalbumin, BP, and the encephalitogenic peptide 71-9, all used at 1,ug/ml; Con-A, 25,ug/ml; BSA, 2,ug/ml. After 72 hours of incubation at 37 C in a humidified atmosphere plus 1% CO,, each well was pulsed with 1 pci of ['H]thymidine for 16 hours. The cultures were then harvested on fibreglass filters and thymidine incorporation was measured using a liquid scintillation counter. The proliferative response was expressed as cpm (SD).
3 Intravenous gammaglobulin treatment in multiple sclerosis and experimental autoimmune encephalomyelitis 59 Figure 1 Effect ofivig on MS exacerbation rate. Figure 2 Effect of IVIg treatment on severity of acute exacerbations. Figure 3 Effect ofivig treatment on the EDSS. ~~~~~~~~li. 3 < z ~~~~Control.2 3 ) x 2 - X 2 C Xu 1_ O~~ o Years Statistical analysis Student's t test, Chi-square and Spearman's correlation test were used to evaluate differences between the various groups. Results Effect ofivig on MS acute exacerbations The major effect of IVIg on exacerbation rate was observed after the first year of treatment (fig 1, table 1). The mean (SD) annual exacerbation rate (AER) decreased from 3-7 (1-2) at baseline to 1 ((7) (p < -1) after one year of IVIg treatment, while in the control group AER before the study remained unchanged after one year [3-3 (1 4) and 3T Mild IVIg Total in 3 years Mild Moderate Severe u I -6 * IVIG l Control _ = Moderate IVIg n=23 26% 16) 478': (11) 26% (6) * * _ Severe Control Table 1 Effect of IVIg on acute exacerbations Exacerbation rate IVIg Control P value Before treatnent Mean (SD) 3-7 (1-4) 3-3 (1 2)* year Mean (SD) 1 ( 7) 3 (1-6) -2 2 years Mean (SD) -8 (-8) 2-1 (1) 5 3 years Mean (SD) -5 (-5) 1-7 (-7) 1 p < -1, IVIg before treatinent v one year. (1P6) respectively, p = -279]. The AER was similar in the two groups before treatment (p = -512); however the decrease in the number of acute exacerbations in the IVIg treated patients, resulted in a significant difference of the AER (p = -2) between the groups after one year. During the second and third years of the study, IVIg treated MS patients had significantly less exacerbations than untreated patients. The mean (SD) AER in the IVIg group after the second year was -8 (-8) and in the control untreated patients 2<1 (1), p = -5. The mean (SD) AER in the IVIg group after the third year of treatment was -5 ( 5) and 1P7 (-7) in the control MS patients (p < -1). The annual exacerbation rate was inversely correlated to duration of IVIg treatment (r = -74, p = -2), and to the neurological disability after three years (r = -7, p = -5). Severity of acute exacerbations was also influenced by IVIg treatment (fig 2). The majority of exacerbations in the IVIg treated patients were mild to moderate, while in untreated control patients most of the acute exacerbations were moderate to severe (Chisquare = 14-6, p = -7). Contrrol = - -3 (-58)] compared with the control n= ?'; ( 12) group [AEDSS = -2 (1P), p = -182]. At 38 2 ' (26) the three year endpoint the AEDSS became * i 2 A.I A Exacerbation \Effect ofivig treatment on neurological disability The mean EDSS score showed no significant change by IVIg treatment, although the numbers were slightly higher in the control untreated group at one and three years compared with baseline. The mean (SD) change in EDSS (AEDSS) calculated per patient showed a trend of lessened disability in the IVIg group at the one year endpoint [AEDSS 5 (3) significant between the two groups; in the IVIg group AEDSS was -35 (-58), compared with 1P7 (-98) in the control untreated patients (p = -1). Correlation between the change in acute exacerbations and neurological disability from baseline to the end of three years in each o patient, clearly showed that in IVIg treated MS patients the reduction in the number of acute exacerbations was correlated to a lesser neurological disability, while the opposite occurred in the untreated control group (fig 3). Effect ofivig treatment on actively induced EAE In IVIg treated animals, only 7% of rats J developed EAE with no mortality, while in 8 untreated rats all animals developed EAE with a mortality rate of 3% (table 2, fig 4).
4 6 Achiron, Gilad, Margalit, Gabbay, Sarova-Pinhas, Cohen, Melamed, Lider, Noy, Ziv Table 2 Effect of IVIg treatment on actively induced EAE Evaluation ofclinical symptoms ofeae Reinduction % Incidence Maximal (SD) Treatment (No of rats) % Mortality Onset (day) Duration (days) EAE score % Incidence None 1 (4) (1-4) /5 Maltose 1% 1 (1) (1-5)b ND BSA 1 (1) (1-2)b ND IVIg 7 (4) (-98)- /5 Following induction of EAE, rats were either untreated or treated with IVIg, 1% maltose or BSA. Reinduction was done one month after primary induction of EAE. app < -5 v untreated group. bpnot significant. ND-not determined. Figure 4 Effect ofivig treatment on the development of experimental autoimmune encephalomyelitis in rats. cn LLI LL C, a1) 4 Antigens None BSA Ovalbumine MBP Control ; ~~~~* IVIg _~~~~~N-- MBP peptide (71-9) r_ CPM Figure 6 Effect ofivig treatment on lymph node T-cell response. Days Effect of IVIg treatment on passively induced EAE IVIg had no effect in the treatment of passively induced EAE (fig 5), in relation to onset, duration and severity. Figure 5 Effect of IVIg treatment on the development ofpassively induced experimental autoimmune encephalomyelitis in rats. F Control * IVlg Effect of IVIg on reinduction ofeae Rats treated with IVIg were resistant to reinduction of EAE and did not develop secondary disease (table 2). Thus IVIg treatment induced suppression but did not prevent the acquisition of resistance. cil ) 21 1 T-cell proliferative responses The proliferative responses of lymph node T- cells to Con-A, ovalbumin and BSA were not affected by IVIg treatment. However, antigen-specific proliferative responses to BP and the 71-9 encephalitogenic peptide were increased in rats treated with IVIg compared with untreated controls (fig 6). Thus EAE suppression by IVIg treatment was associated with a strong T-cell response to the target encephalitogenic peptides. Days In the IVIg treated rats EAE was mild. The mean maximal EAE score was 1-6 compared with 3 in untreated animals (p < O5). Duration of EAE was shorter in the IVIg treated rats, seven days compared to nine days in untreated animals. Onset of EAE was not affected by IVIg treatment. The severity of disease in MBP-immunised rats treated with 1% maltose or BSA was similar to the results of untreated animals. Discussion The results of this study indicate that IVIg is well tolerated and has beneficial effects on the course of relapsing-remitting MS. Over a period of three year treatment, IVIg had significant effects on exacerbation rates, severity of exacerbations, and neurological disability. The major reduction in exacerbation rate was observed after the first year of IVIg treatment, and persisted during the next two years of therapy. The high exacerbation rate in the patients with MS that entered this study, enabled effective evaluation of IVIg treat-
5 Intravenous gammaglobulin treatment in multiple sclerosis and experimental autoimmune encephalomyelitis 61 ment, in spite of the small number of patients included. The decrease in exacerbation rate in the untreated group may be related to the natural course of the disease, reduced stress as a result of participation in a study protocol, or corticosteroid therapy during acute moderate and severe exacerbations. After three years the reduced exacerbation rate in IVIg treated MS patients was correlated with lesser neurological disability, while untreated patients had more exacerbations and increased EDSS score. The beneficial effects of IVIg were also evident in the animal model of EAE. IVIg suppressed active induction of EAE, while it had no effect on passively induced disease. Thus it appears that IVIg can alter immune response before T-cell activation. The results of increased proliferative T-cell responses to target antigens along with active EAE suppression suggest that the mechanism by which IVIg alter the natural course of the disease is not through inhibition of T-cell proliferation. This is further supported by the results of secondary EAE induction, where no disease developed, implying that IVIg suppression of primary induction had no effect on the acquisition of resistance. In conclusion, the use of IVIg in relapsingremitting MS has a significant beneficial effect on the natural course of the disease over time. Though the mechanism by which IVIg modulates the immune system is not yet clear, it probably involves T-cell interactions during the active phase of the disease. 1 Waksman BH, Reynolds WE. Multiple sclerosis as a disease of immune regulation. Proc Soc Exp Biol Med 1984;175: Golaz J, Steck A, Moretta L. Activated T lymphocytes in patients with multiple sclerosis. Neurology 1983;33: Reder AT, Arnason BGW. Immunology of multiple sclerosis. In: PJ Vinken, GW Bruyn, HC Klawans, eds. Handbook of clinical neurology. Amsterdam; Elsevier, 1985;47: Antel JP, Reder AT, Noronha AB. Cellular immunity and immune regulation in multiple sclerosis. Semin Neurol 1985;5: Noronha A, Toscas A, Jensen MA. Interferon beta augments suppressor cell function in multiple sclerosis. Ann Neurol 199;27: Achiron A, Pras E, Gilad R, Ziv I, Mendel M, Gordon CR, Noy S, Sarova-Pinhas I, Melamed E. Open controlled therapeutic trial of high-dose intravenous immunoglobulins in relapsing-remitting multiple sclerosis. Arch Neurol 1992;49: Mor F, Cohen IR. T cells in the lesion of experimental autoimmune encephalomyelitis. Enrichment for reactivities to myelin basic protein and heat shock proteins. J Clin Invest 1992;9: Mokhatarian VF, McFarlin DE, Raine CS. Adoptive transfer of myelin basic protein-synsitized T cells produce chronic relapsing demyelinating disease in mice. Nature 1984;39: Lemire JM, Weigle WO. Myelin basic protein-specific T cell clones and experimental allergic encephalomyelitis. Pathol Immunopathol Res 1986;5: Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol 199; 8: Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13: Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
RELAPSE MANAGEMENT. Pauline Shaw MS Nurse Specialist 25 th June 2010
 RELAPSE MANAGEMENT Pauline Shaw MS Nurse Specialist 25 th June 2010 AIMS OF SESSION Relapsing/Remitting MS Definition of relapse/relapse rate Relapse Management NICE Guidelines Regional Clinical Guidelines
RELAPSE MANAGEMENT Pauline Shaw MS Nurse Specialist 25 th June 2010 AIMS OF SESSION Relapsing/Remitting MS Definition of relapse/relapse rate Relapse Management NICE Guidelines Regional Clinical Guidelines
Effects of Two Proprietary Compounds in Multiple Sclerosis DATE
 Effects of Two Proprietary Compounds in a Model of Multiple Sclerosis DATE Authentication This study was conducted under the terms of a Services Agreement between Inc. and CLIENT dated DATE. Sponsor XXX
Effects of Two Proprietary Compounds in a Model of Multiple Sclerosis DATE Authentication This study was conducted under the terms of a Services Agreement between Inc. and CLIENT dated DATE. Sponsor XXX
The Nuts and Bolts of Multiple Sclerosis. Rebecca Milholland, M.D., Ph.D. Center for Neurosciences
 The Nuts and Bolts of Multiple Sclerosis Rebecca Milholland, M.D., Ph.D. Center for Neurosciences Objectives Discuss which patients are at risk for Multiple Sclerosis Discuss the diagnostic criteria for
The Nuts and Bolts of Multiple Sclerosis Rebecca Milholland, M.D., Ph.D. Center for Neurosciences Objectives Discuss which patients are at risk for Multiple Sclerosis Discuss the diagnostic criteria for
Clinical Study Synopsis
 Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Gold R, Giovannoni G, Selmaj K, et al, for
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Gold R, Giovannoni G, Selmaj K, et al, for
The Prospect of Stem Cell Therapy in Multiple Sclerosis. Multiple sclerosis is a multifocal inflammatory disease of the central
 The Prospect of Stem Cell Therapy in Multiple Sclerosis Multiple sclerosis is a multifocal inflammatory disease of the central nervous system that generally affects young individuals, causing paralysis
The Prospect of Stem Cell Therapy in Multiple Sclerosis Multiple sclerosis is a multifocal inflammatory disease of the central nervous system that generally affects young individuals, causing paralysis
Chapter 10. Summary & Future perspectives
 Summary & Future perspectives 123 Multiple sclerosis is a chronic disorder of the central nervous system, characterized by inflammation and axonal degeneration. All current therapies modulate the peripheral
Summary & Future perspectives 123 Multiple sclerosis is a chronic disorder of the central nervous system, characterized by inflammation and axonal degeneration. All current therapies modulate the peripheral
Version History. Previous Versions. Policy Title. Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
March 30, 2007. I. IVIg IS AN ACCEPTED THERAPY FOR RRMS, AND IS PRESCRIBED ALONG WITH A DISEASE-MODIFYING AGENT SUCH AS COPAXONE
 18 Timberline Drive Farmington, CT 06032 (860) 674-1370 (phone) (860) 674-1378 (fax) (860) 305-9835 (cell) www.advocacyforpatients.org [email protected] AZ Benefit Options ATTN: Appeals PO
18 Timberline Drive Farmington, CT 06032 (860) 674-1370 (phone) (860) 674-1378 (fax) (860) 305-9835 (cell) www.advocacyforpatients.org [email protected] AZ Benefit Options ATTN: Appeals PO
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook
 National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
Relapsing-remitting multiple sclerosis Ambulatory with or without aid
 AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
STANDARD OPERATING PROCEDURE
 Title: Lymphocyte Proliferation Assay (LPA) Using 3 H- Thymidine Incorporation Assay Core Name: Lloyd Mayer, Mount Sinai Medical Center Effective Date: 02/16/2012 Trial Number: ITN047AI SOP # ITN2800 SOP
Title: Lymphocyte Proliferation Assay (LPA) Using 3 H- Thymidine Incorporation Assay Core Name: Lloyd Mayer, Mount Sinai Medical Center Effective Date: 02/16/2012 Trial Number: ITN047AI SOP # ITN2800 SOP
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Clinical Department of Neurology, Innsbruck Medical University, Austria 1
 CHAPTER 6 LEVEL S OF SERUM IGM ANTIBODY AGAINST NATIVE REFOLDED MOG PREDICT RESPONSIVENESS TO CORTICOSTEROIDS FOR TRE ATMENT OF ACUTE MULTIPLE SCLEROSIS REL APSES Michael Khalil 1, Bettina Kuenz, Andreas
CHAPTER 6 LEVEL S OF SERUM IGM ANTIBODY AGAINST NATIVE REFOLDED MOG PREDICT RESPONSIVENESS TO CORTICOSTEROIDS FOR TRE ATMENT OF ACUTE MULTIPLE SCLEROSIS REL APSES Michael Khalil 1, Bettina Kuenz, Andreas
Reversibility of Acute Demyelinating Lesions in relapsingremitting
 Reversibility of Acute Demyelinating Lesions in relapsingremitting Multiple Sclerosis Omar A. Khan ( Division of Neuroimmunology, Department of Neurology, Neurology and Research Services. Veterans Affairs
Reversibility of Acute Demyelinating Lesions in relapsingremitting Multiple Sclerosis Omar A. Khan ( Division of Neuroimmunology, Department of Neurology, Neurology and Research Services. Veterans Affairs
Tuberculosis And Diabetes. Dr. hanan abuelrus Prof.of internal medicine Assiut University
 Tuberculosis And Diabetes Dr. hanan abuelrus Prof.of internal medicine Assiut University TUBERCULOSIS FACTS More than 9 million people fall sick with tuberculosis (TB) every year. Over 1.5 million die
Tuberculosis And Diabetes Dr. hanan abuelrus Prof.of internal medicine Assiut University TUBERCULOSIS FACTS More than 9 million people fall sick with tuberculosis (TB) every year. Over 1.5 million die
AUBMC Multiple Sclerosis Center
 AUBMC Multiple Sclerosis Center 1 AUBMC Multiple Sclerosis Center The vision of the American University of Beirut Medical Center (AUBMC) is to be the leading academic medical center in Lebanon and the
AUBMC Multiple Sclerosis Center 1 AUBMC Multiple Sclerosis Center The vision of the American University of Beirut Medical Center (AUBMC) is to be the leading academic medical center in Lebanon and the
acquired chronic immune-mediated inflammatory condition of CNS. MS in children: 10% +secondary progressive MS: rare +primary progressive MS: rare
 Immunomodulatory Therapies in Pediatric MS Vuong Chinh Quyen Neurology Department Medscape Mar 8, 2013 Multiple Sclerosis in Children. Iran J Child Neurol. 2013 Spring Introduction acquired chronic immune-mediated
Immunomodulatory Therapies in Pediatric MS Vuong Chinh Quyen Neurology Department Medscape Mar 8, 2013 Multiple Sclerosis in Children. Iran J Child Neurol. 2013 Spring Introduction acquired chronic immune-mediated
Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple Sclerosis
 Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
OHTAC Recommendation
 OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
PROPOSED REVISIONS TO THE CRITERIA. multiple sclerosis (RRMS)] Chapter 6 6 It is recognised that use is in only exceptional circumstances in RRMS.
![PROPOSED REVISIONS TO THE CRITERIA. multiple sclerosis (RRMS)] Chapter 6 6 It is recognised that use is in only exceptional circumstances in RRMS. PROPOSED REVISIONS TO THE CRITERIA. multiple sclerosis (RRMS)] Chapter 6 6 It is recognised that use is in only exceptional circumstances in RRMS.](/thumbs/33/16408576.jpg) Specialist Working Group for Neurology Proposed changes to the Criteria for the clinical use of intravenous immunoglobulin in Australia, Second Edition ITEM Condition Name Multiple Sclerosis (MS) Multiple
Specialist Working Group for Neurology Proposed changes to the Criteria for the clinical use of intravenous immunoglobulin in Australia, Second Edition ITEM Condition Name Multiple Sclerosis (MS) Multiple
Version History. Previous Versions. for secondary progressive MS (SPMS) Policy Title. Drugs for MS.Drug facts box Interferon beta 1b
 Version History Policy Title Drugs for MS.Drug facts box Interferon beta 1b for secondary progressive MS (SPMS) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review
Version History Policy Title Drugs for MS.Drug facts box Interferon beta 1b for secondary progressive MS (SPMS) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review
Chapter 3. Immunity and how vaccines work
 Chapter 3 Immunity and how vaccines work 3.1 Objectives: To understand and describe the immune system and how vaccines produce immunity To understand the differences between Passive and Active immunity
Chapter 3 Immunity and how vaccines work 3.1 Objectives: To understand and describe the immune system and how vaccines produce immunity To understand the differences between Passive and Active immunity
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
News on modifying diseases therapies. Michel CLANET CHU Toulouse France ECTRIMS
 News on modifying diseases therapies Michel CLANET CHU Toulouse France ECTRIMS Current treatment strategies Future oral treatments Future non oral treatments Drug safety and risks CIS at risk of MS Active
News on modifying diseases therapies Michel CLANET CHU Toulouse France ECTRIMS Current treatment strategies Future oral treatments Future non oral treatments Drug safety and risks CIS at risk of MS Active
Human CD4+T Cell Care Manual
 Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.02 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.02 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Chapter 43: The Immune System
 Name Period Our students consider this chapter to be a particularly challenging and important one. Expect to work your way slowly through the first three concepts. Take particular care with Concepts 43.2
Name Period Our students consider this chapter to be a particularly challenging and important one. Expect to work your way slowly through the first three concepts. Take particular care with Concepts 43.2
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Immunoablative therapy with autologous hematopoietic stem cell transplantation in the treatment of poor risk multiple sclerosis
 Immunoablative therapy with autologous hematopoietic stem cell transplantation in the treatment of poor risk multiple sclerosis T Kozák, P Lhotáková Department of Clinical Haematology, 3r d School of Medicine,
Immunoablative therapy with autologous hematopoietic stem cell transplantation in the treatment of poor risk multiple sclerosis T Kozák, P Lhotáková Department of Clinical Haematology, 3r d School of Medicine,
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON
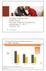 Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
New Treatment Options for MS Patients: Understanding risks versus benefits
 New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
9/16/2014. Anti-Immunoglobulin E (IgE) Omalizumab (Xolair ) Dosing Guidance
 Disclosure Statement of Financial Interest New Therapies for Asthma Including Omalizumab and Anti-Cytokine Therapies Marsha Dangler, PharmD, BCACP Clinical Pharmacy Specialist James H. Quillen VA Medical
Disclosure Statement of Financial Interest New Therapies for Asthma Including Omalizumab and Anti-Cytokine Therapies Marsha Dangler, PharmD, BCACP Clinical Pharmacy Specialist James H. Quillen VA Medical
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Multiple Sclerosis and Related Disorders
 Multiple Sclerosis and Related Disorders 1 (2012) 111 115 Contents lists available at SciVerse ScienceDirect Multiple Sclerosis and Related Disorders journal homepage: www.elsevier.com/locate/msard Checklist
Multiple Sclerosis and Related Disorders 1 (2012) 111 115 Contents lists available at SciVerse ScienceDirect Multiple Sclerosis and Related Disorders journal homepage: www.elsevier.com/locate/msard Checklist
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
Mariusz Stasiołek Neurology Department Polish Mother s Memorial Hospital Research Institute, Lodz
 Therapeutic Plasma Exchange in Multiple Sclerosis Relapses Mariusz Stasiołek Neurology Department Polish Mother s Memorial Hospital Research Institute, Lodz MS heterogeneity Multiple Sclerosis differences
Therapeutic Plasma Exchange in Multiple Sclerosis Relapses Mariusz Stasiołek Neurology Department Polish Mother s Memorial Hospital Research Institute, Lodz MS heterogeneity Multiple Sclerosis differences
Cancer Immunotherapy: Can Your Immune System Cure Cancer? Steve Emerson, MD, PhD Herbert Irving Comprehensive Cancer Center
 Cancer Immunotherapy: Can Your Immune System Cure Cancer? Steve Emerson, MD, PhD Herbert Irving Comprehensive Cancer Center Bodnar s Law Simple Things are Important Very Simple Things are Very Important
Cancer Immunotherapy: Can Your Immune System Cure Cancer? Steve Emerson, MD, PhD Herbert Irving Comprehensive Cancer Center Bodnar s Law Simple Things are Important Very Simple Things are Very Important
B Cells and Antibodies
 B Cells and Antibodies Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School Lecture outline Functions of antibodies B cell activation; the role of helper T cells in antibody production
B Cells and Antibodies Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School Lecture outline Functions of antibodies B cell activation; the role of helper T cells in antibody production
specific B cells Humoral immunity lymphocytes antibodies B cells bone marrow Cell-mediated immunity: T cells antibodies proteins
 Adaptive Immunity Chapter 17: Adaptive (specific) Immunity Bio 139 Dr. Amy Rogers Host defenses that are specific to a particular infectious agent Can be innate or genetic for humans as a group: most microbes
Adaptive Immunity Chapter 17: Adaptive (specific) Immunity Bio 139 Dr. Amy Rogers Host defenses that are specific to a particular infectious agent Can be innate or genetic for humans as a group: most microbes
Human Peripheral Blood Mononuclear Cell (PBMC) Manual
 Human Peripheral Blood Mononuclear Cell (PBMC) Manual INSTRUCTION MANUAL ZBM0063.04 SHIPPING CONDITIONS Human Peripheral Blood Mononuclear Cells, cryopreserved Cryopreserved human peripheral blood mononuclear
Human Peripheral Blood Mononuclear Cell (PBMC) Manual INSTRUCTION MANUAL ZBM0063.04 SHIPPING CONDITIONS Human Peripheral Blood Mononuclear Cells, cryopreserved Cryopreserved human peripheral blood mononuclear
Compositional Changes of B and T Cell Subtypes during Fingolimod Treatment in Multiple Sclerosis Patients: A 12-Month Follow-Up Study
 Compositional Changes of B and T Cell Subtypes during Fingolimod Treatment in Multiple Sclerosis Patients: A 12-Month Follow-Up Study Nele Claes 1 *; Tessa Dhaeze 1 *; Judith Fraussen PhD 1 ; Bieke Broux
Compositional Changes of B and T Cell Subtypes during Fingolimod Treatment in Multiple Sclerosis Patients: A 12-Month Follow-Up Study Nele Claes 1 *; Tessa Dhaeze 1 *; Judith Fraussen PhD 1 ; Bieke Broux
How To Get Ivig To Treat Neuromyelitis Optica
 Aetna Customer Resolution Team PO Box 14002 Lexington, KY 40512 RE: Patient Type of service: IVIg Dates of service: To be determined (prior authorization) Dear Sir or Madam: I am writing on behalf of your
Aetna Customer Resolution Team PO Box 14002 Lexington, KY 40512 RE: Patient Type of service: IVIg Dates of service: To be determined (prior authorization) Dear Sir or Madam: I am writing on behalf of your
- Patients treated with alemtuzumab in CARE-MS II were more than twice as likely to experience disability improvement compared to Rebif -
 PRESS RELEASE Significant Improvement in Disability Scores Observed in Multiple Sclerosis Patients Who Received Lemtrada TM* (Alemtuzumab) Compared With Rebif in Phase lll Trial - Patients treated with
PRESS RELEASE Significant Improvement in Disability Scores Observed in Multiple Sclerosis Patients Who Received Lemtrada TM* (Alemtuzumab) Compared With Rebif in Phase lll Trial - Patients treated with
The role of IBV proteins in protection: cellular immune responses. COST meeting WG2 + WG3 Budapest, Hungary, 2015
 The role of IBV proteins in protection: cellular immune responses COST meeting WG2 + WG3 Budapest, Hungary, 2015 1 Presentation include: Laboratory results Literature summary Role of T cells in response
The role of IBV proteins in protection: cellular immune responses COST meeting WG2 + WG3 Budapest, Hungary, 2015 1 Presentation include: Laboratory results Literature summary Role of T cells in response
Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual
 Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.03 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+ Progenitor Cells, cryopreserved Cryopreserved human umbilical
Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.03 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+ Progenitor Cells, cryopreserved Cryopreserved human umbilical
CNS DEMYLINATING DISORDERS
 CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: adoptive_immunotherapy 11/1993 3/2016 3/2017 3/2016 Description of Procedure or Service The spontaneous regression
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: adoptive_immunotherapy 11/1993 3/2016 3/2017 3/2016 Description of Procedure or Service The spontaneous regression
Multiple Sclerosis Update. Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center
 Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Immunex Corporation Novantrone (Mitoxantrone HCL) P&CNS Advisory Committee Briefing Document. Page 020
 Page 020 4.0 Efficacy of Mitoxantrone in Multiple Sclerosis The efficacy of mitoxantrone in MS was demonstrated in two well-designed, randomized trials: Studies 901 and 902. The study design and efficacy
Page 020 4.0 Efficacy of Mitoxantrone in Multiple Sclerosis The efficacy of mitoxantrone in MS was demonstrated in two well-designed, randomized trials: Studies 901 and 902. The study design and efficacy
Accuracy in Space and Time: Diagnosing Multiple Sclerosis. 2012 Genzyme Corporation, a Sanofi company.
 Accuracy in Space and Time: Diagnosing Multiple Sclerosis 2012 Genzyme Corporation, a Sanofi company. Brought All rights to reserved. you by www.msatrium.com, MS.US.PO876.1012 your gateway to MS knowledge.
Accuracy in Space and Time: Diagnosing Multiple Sclerosis 2012 Genzyme Corporation, a Sanofi company. Brought All rights to reserved. you by www.msatrium.com, MS.US.PO876.1012 your gateway to MS knowledge.
Objectives: Immunity Gone Wrong: Autoimmune Diseases in Dental Hygiene Practice
 Objectives: 1) Understand the concept of self- tolerance versus non- self- tolerance. 2) Recognize systemic and oral indicators of autoimmune diseases. 3) Identify various autoimmune diseases and their
Objectives: 1) Understand the concept of self- tolerance versus non- self- tolerance. 2) Recognize systemic and oral indicators of autoimmune diseases. 3) Identify various autoimmune diseases and their
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 15 September 2005 Doc. Ref. EMEA/CHMP/EWP/561/98 Rev 1 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 15 September 2005 Doc. Ref. EMEA/CHMP/EWP/561/98 Rev 1 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
SECTION 2. Section 2 Multiple Sclerosis (MS) Drug Coverage
 SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
Autoimmunity and immunemediated. FOCiS. Lecture outline
 1 Autoimmunity and immunemediated inflammatory diseases Abul K. Abbas, MD UCSF FOCiS 2 Lecture outline Pathogenesis of autoimmunity: why selftolerance fails Genetics of autoimmune diseases Therapeutic
1 Autoimmunity and immunemediated inflammatory diseases Abul K. Abbas, MD UCSF FOCiS 2 Lecture outline Pathogenesis of autoimmunity: why selftolerance fails Genetics of autoimmune diseases Therapeutic
AUBAGIO (teriflunomide) oral tablet
 AUBAGIO (teriflunomide) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
AUBAGIO (teriflunomide) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
Interferon- (1a and 1b) and glatiramer acetate (GA
 The Journal of Immunology Cutting Edge: Oral Type I IFN- Promotes a Th2 Bias and Enhances Suppression of Autoimmune Encephalomyelitis by Oral Glatiramer Acetate 1 Jeanne M. Soos, 2 * Olaf Stüve, Sawsan
The Journal of Immunology Cutting Edge: Oral Type I IFN- Promotes a Th2 Bias and Enhances Suppression of Autoimmune Encephalomyelitis by Oral Glatiramer Acetate 1 Jeanne M. Soos, 2 * Olaf Stüve, Sawsan
Autoimmunity. Autoimmunity. Genetic Contributions to Autoimmunity. Targets of Autoimmunity
 Autoimmunity Factors predisposing an individual to autoimmune disease Mechanisms of initiation of autoimmunity Pathogenesis of particular autoimmune disease Animal models of autoimmune disease Treatment
Autoimmunity Factors predisposing an individual to autoimmune disease Mechanisms of initiation of autoimmunity Pathogenesis of particular autoimmune disease Animal models of autoimmune disease Treatment
placebo-controlledcontrolled double-blind, blind,
 Clinical Potential of Minocycline for Depression with Psychotic Features Tsuyoshi Miyaoka Department of Psychiatry Shimane University School of Medicine Minocycline 1. Second-generation tetracycline which
Clinical Potential of Minocycline for Depression with Psychotic Features Tsuyoshi Miyaoka Department of Psychiatry Shimane University School of Medicine Minocycline 1. Second-generation tetracycline which
Study Design. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D. Ellis Unger, M.D. Ghanshyam Gupta, Ph.D. Chief, Therapeutics Evaluation Branch
 BLA: STN 103471 Betaseron (Interferon β-1b) for the treatment of secondary progressive multiple sclerosis. Submission dated June 29, 1998. Chiron Corp. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D.
BLA: STN 103471 Betaseron (Interferon β-1b) for the treatment of secondary progressive multiple sclerosis. Submission dated June 29, 1998. Chiron Corp. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D.
Managing Relapsing Remitting MS Risks & benefits of emerging therapies. Dr Mike Boggild The Walton Centre
 Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
A Case for Cure: Drug Portfolio Analysis. Team: PharmaKings Anirvan Chaudhuri Neha Madan Xiao Huang Jason Park Anjai Lal
 A Case for Cure: Drug Portfolio Analysis Team: PharmaKings Anirvan Chaudhuri Neha Madan Xiao Huang Jason Park Anjai Lal Executive Summary Our team recommends. A Recombinant Protein for treatment of Multiple
A Case for Cure: Drug Portfolio Analysis Team: PharmaKings Anirvan Chaudhuri Neha Madan Xiao Huang Jason Park Anjai Lal Executive Summary Our team recommends. A Recombinant Protein for treatment of Multiple
Rheumatoid arthritis: an overview. Christine Pham MD
 Rheumatoid arthritis: an overview Christine Pham MD RA prevalence Chronic inflammatory disease affecting approximately 0.5 1% of the general population Prevalence is higher in North America (approaching
Rheumatoid arthritis: an overview Christine Pham MD RA prevalence Chronic inflammatory disease affecting approximately 0.5 1% of the general population Prevalence is higher in North America (approaching
Carboxyfluorescein succinimidyl ester-based proliferative assays for assessment of T cell function in the diagnostic laboratory
 Immunology and Cell Biology (1999) 77, 559 564 Special Feature Carboxyfluorescein succinimidyl ester-based proliferative assays for assessment of T cell function in the diagnostic laboratory DA FULCHER
Immunology and Cell Biology (1999) 77, 559 564 Special Feature Carboxyfluorescein succinimidyl ester-based proliferative assays for assessment of T cell function in the diagnostic laboratory DA FULCHER
ANIMALS FORM & FUNCTION BODY DEFENSES NONSPECIFIC DEFENSES PHYSICAL BARRIERS PHAGOCYTES. Animals Form & Function Activity #4 page 1
 AP BIOLOGY ANIMALS FORM & FUNCTION ACTIVITY #4 NAME DATE HOUR BODY DEFENSES NONSPECIFIC DEFENSES PHYSICAL BARRIERS PHAGOCYTES Animals Form & Function Activity #4 page 1 INFLAMMATORY RESPONSE ANTIMICROBIAL
AP BIOLOGY ANIMALS FORM & FUNCTION ACTIVITY #4 NAME DATE HOUR BODY DEFENSES NONSPECIFIC DEFENSES PHYSICAL BARRIERS PHAGOCYTES Animals Form & Function Activity #4 page 1 INFLAMMATORY RESPONSE ANTIMICROBIAL
Laquinimod Polman, C. et al. Neurology 2005;64:987-991
 Laquinimod Polman, C. et al. Neurology 2005;64:987-991 Multicenter, double-blind, randomized trial, patients with RR MS received 0.1 mg or 0.3 mg laquinimod or placebo as three daily tablets for 24 weeks
Laquinimod Polman, C. et al. Neurology 2005;64:987-991 Multicenter, double-blind, randomized trial, patients with RR MS received 0.1 mg or 0.3 mg laquinimod or placebo as three daily tablets for 24 weeks
How To Use An Antibody
 GUIDELINES FOR THE PRODUCTION OF ANTIBODIES IN LABORATORY ANIMALS Table of Contents 1. Purpose 2. Choice of Species and Strain 3. Immunizing Antigen 4. Procedures for Polyclonal Antibody Production 5.
GUIDELINES FOR THE PRODUCTION OF ANTIBODIES IN LABORATORY ANIMALS Table of Contents 1. Purpose 2. Choice of Species and Strain 3. Immunizing Antigen 4. Procedures for Polyclonal Antibody Production 5.
What is Multiple Sclerosis? Gener al information
 What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
Prospects for Vaccines against Hepatitis C Viruses. T. Jake Liang. M.D. Liver Diseases Branch NIDDK, NIH, HHS
 Prospects for Vaccines against Hepatitis C Viruses T. Jake Liang. M.D. Liver Diseases Branch NIDDK, NIH, HHS HCV Vaccine Prevention strategies Protective immunity Barriers and solutions Vaccine candidates
Prospects for Vaccines against Hepatitis C Viruses T. Jake Liang. M.D. Liver Diseases Branch NIDDK, NIH, HHS HCV Vaccine Prevention strategies Protective immunity Barriers and solutions Vaccine candidates
Immunity and how vaccines work
 1 Introduction Immunity is the ability of the human body to protect itself from infectious disease. The defence mechanisms of the body are complex and include innate (non-specific, non-adaptive) mechanisms
1 Introduction Immunity is the ability of the human body to protect itself from infectious disease. The defence mechanisms of the body are complex and include innate (non-specific, non-adaptive) mechanisms
An introduction to modern MS treatments
 BETAFERON is a Prescription Medicine. Use strictly as directed. Consult your pharmacist or other health professional in case of side effects. BETAFERON is reimbursed for some patients. See your neurologist
BETAFERON is a Prescription Medicine. Use strictly as directed. Consult your pharmacist or other health professional in case of side effects. BETAFERON is reimbursed for some patients. See your neurologist
How To Use A Drug In Multiple Sclerosis
 Revised (2009) guidelines for prescribing in multiple sclerosis INTRODUCTION In January 2001, the (ABN) first published guidelines for the use of licensed disease modifying treatments (ß-interferon and
Revised (2009) guidelines for prescribing in multiple sclerosis INTRODUCTION In January 2001, the (ABN) first published guidelines for the use of licensed disease modifying treatments (ß-interferon and
MS ECHO: Update on MS treatment. Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology 10 14 2015
 MS ECHO: Update on MS treatment Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology 10 14 2015 Conflict of Interest Dr. Stobbe has no conflicts of interest to disclose
MS ECHO: Update on MS treatment Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology 10 14 2015 Conflict of Interest Dr. Stobbe has no conflicts of interest to disclose
NHS BOURNEMOUTH AND POOLE AND NHS DORSET
 NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
TREATING AUTOIMMUNE DISEASES WITH HOMEOPATHY. Dr. Stephen A. Messer, MSEd, ND, DHANP Professor and Chair of Homeopathic Medicine
 TREATING AUTOIMMUNE DISEASES WITH HOMEOPATHY Dr. Stephen A. Messer, MSEd, ND, DHANP Professor and Chair of Homeopathic Medicine AUTOIMMUNE DISEASES An autoimmune disorder occurs when the body s immune
TREATING AUTOIMMUNE DISEASES WITH HOMEOPATHY Dr. Stephen A. Messer, MSEd, ND, DHANP Professor and Chair of Homeopathic Medicine AUTOIMMUNE DISEASES An autoimmune disorder occurs when the body s immune
TITLE: Treatment of Patients with Multiple Sclerosis: A Review of Guidelines
 TITLE: Treatment of Patients with Multiple Sclerosis: A Review of Guidelines DATE: 13 March 2013 CONTEXT AND POLICY ISSUES Multiple sclerosis (MS) is an unpredictable, often disabling disease of the central
TITLE: Treatment of Patients with Multiple Sclerosis: A Review of Guidelines DATE: 13 March 2013 CONTEXT AND POLICY ISSUES Multiple sclerosis (MS) is an unpredictable, often disabling disease of the central
Biologic Treatments for Rheumatoid Arthritis
 Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Calming microglia: a future method for treating multiple sclerosis Jarred Younger, PhD University of Alabama at Birmingham
 Calming microglia: a future method for treating multiple sclerosis Jarred Younger, PhD University of Alabama at Birmingham Sonja Paetau, University of Helsinki Disclosures Will be discussing off-label
Calming microglia: a future method for treating multiple sclerosis Jarred Younger, PhD University of Alabama at Birmingham Sonja Paetau, University of Helsinki Disclosures Will be discussing off-label
Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012. Reference : NHSCB/D4/c/1
 Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012 Reference : NHSCB/D4/c/1 NHS Commissioning Board Clinical Commissioning Policy: Disease
Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012 Reference : NHSCB/D4/c/1 NHS Commissioning Board Clinical Commissioning Policy: Disease
EMD Serono Presents New Data on Rebif (Interferon beta-1a) and Multiple Sclerosis Pipeline at Joint ACTRIMS-ECTRIMS Meeting in Boston
 Erin-Marie Beals Phone 1-781-681-2850 September 9, 2014 EMD Serono Presents New Data on Rebif (Interferon beta-1a) and Multiple Sclerosis Pipeline at Joint ACTRIMS-ECTRIMS Meeting in Boston Data include
Erin-Marie Beals Phone 1-781-681-2850 September 9, 2014 EMD Serono Presents New Data on Rebif (Interferon beta-1a) and Multiple Sclerosis Pipeline at Joint ACTRIMS-ECTRIMS Meeting in Boston Data include
CMP Antibody Production Service
 CMP Antibody Production Service Polyclonal antibody production has become an essential part of many research projects at Texas A&M. Standardization of production procedures and the performance of the procedures
CMP Antibody Production Service Polyclonal antibody production has become an essential part of many research projects at Texas A&M. Standardization of production procedures and the performance of the procedures
Summary HTA. Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C. HTA-Report Summary
 Summary HTA HTA-Report Summary Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C Health policy background Multiple sclerosis (MS) is a chronic inflammatory disease
Summary HTA HTA-Report Summary Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C Health policy background Multiple sclerosis (MS) is a chronic inflammatory disease
ß-interferon and. ABN Guidelines for 2007 Treatment of Multiple Sclerosis with. Glatiramer Acetate
 ABN Guidelines for 2007 Treatment of Multiple Sclerosis with ß-interferon and Glatiramer Acetate Published by the Association of British Neurologists Ormond House, 27 Boswell Street, London WC1N 3JZ Contents
ABN Guidelines for 2007 Treatment of Multiple Sclerosis with ß-interferon and Glatiramer Acetate Published by the Association of British Neurologists Ormond House, 27 Boswell Street, London WC1N 3JZ Contents
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents
 New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
Hapten - a small molecule that is antigenic but not (by itself) immunogenic.
 Chapter 3. Antigens Terminology: Antigen: Substances that can be recognized by the surface antibody (B cells) or by the TCR (T cells) when associated with MHC molecules Immunogenicity VS Antigenicity:
Chapter 3. Antigens Terminology: Antigen: Substances that can be recognized by the surface antibody (B cells) or by the TCR (T cells) when associated with MHC molecules Immunogenicity VS Antigenicity:
