Drug-eluting/biodegradable stents
|
|
|
- Georgina Clarke
- 10 years ago
- Views:
Transcription
1 REPORT ON EMERGING TECHNOLOGY Drug-eluting/biodegradable stents The ASGE Technology Committee provides reviews of existing, new, or emerging endoscopic technologies that have an impact on the practice of GI endoscopy. Evidencebased methodology is used, performing a MEDLINE literature search to identify pertinent clinical studies on the topic and a MAUDE (U.S. Food and Drug Administration Center for Devices and Radiological Health) database search to identify the reported complications of a given technology. Both are supplemented by accessing the related articles feature of PubMed and by scrutinizing pertinent references cited by the identified studies. Controlled clinical trials are emphasized, but in many cases data from randomized, controlled trials are lacking. In such cases, large case series, preliminary clinical studies, and expert opinions are used. Technical data are gathered from traditional and Web-based publications, proprietary publications, and informal communications with pertinent vendors. Technology Status Evaluation Reports are drafted by 1 or 2 members of the ASGE Technology Committee, reviewed and edited by the Committee as a whole, and approved by the Governing Board of the ASGE. When financial guidance is indicated, the most recent coding data and list prices at the time of publication are provided. For this review, the MEDLINE database was searched through September 2010 for articles related to endoscopy by using the key words gastroscope, colonoscope, echoendoscope, duodenoscope, choledochoscope, ultraslim endoscope, variable stiffness colonoscope, and wide-angle colonoscope. Technology Status Evaluation Reports are scientific reviews provided solely for educational and informational purposes. Technology Status Evaluation Reports are not rules and should not be construed as establishing a legal standard of care or as encouraging, advocating, requiring, or discouraging any particular treatment or payment for such treatment. INTRODUCTION Metal and plastic stents have an established role in management of both benign and malignant strictures throughout the GI tract. Studies have demonstrated the Copyright 2011 by the American Society for Gastrointestinal Endoscopy /$36.00 doi: /j.gie clinical efficacy and safety in appropriately selected patients. Detailed reviews of commercially available stents have previously been published. 1-2 Alternative stent technology has been explored in an attempt to address some common problems including tissue ingrowth and need for removal when used in benign disease. This review discusses drug-eluting and biodegradable stents. BACKGROUND A wide variety of drugs or biologically active agents, including antineoplastic agents, antithrombins, immunosuppressants, and tissue growth or inhibitory factors can be incorporated into stents to exert a desired physiologic effect. 3-4 The cardiovascular stent market remains the dominant driving force for research and development of both drug-eluting and biodegradable stents. The requirements for biodegradable or drug-eluting stents in the GI tract, however, are vastly different than for coronary stents. Although much less is currently known about the clinical utility or ideal stent designs for use in the GI tract, these have been a topic of preclinical and preliminary studies over recent years. EMERGING TECHNOLOGY Biodegradable stents in the esophagus A case report and a small case series (including a total of 6 patients) demonstrated the feasibility of inserting a self-expandable stent made of a single wire of poly-llactide in a coil-spring configuration, designed to degrade over a 3 to 6 month period (InStent Inc, Eden Prairie, Minn). 5-6 Clinical results were inconsistent and suboptimal. Another biodegradable stent (Marui Textile Machinery Company Ltd, Osaka, Japan) made of machine-knitted poly-l-lactide monofilaments was developed more recently, with a configuration and mechanical radial force similar to those of commercially available esophageal stents. 7 Three subsequent reports by the same group of investigators demonstrated feasibility of use, with a low incidence of stent-related complications. 7-9 The largest cohort consisted of 6 patients undergoing treatment for benign esophageal strictures (caustic or anastomotic) and 7 patients undergoing prophylactic stenting following extensive endoscopic submucosal dissection (ESD) in an effort to prevent post-esd strictures. 8 Although the stent was deemed successful based on the absence of symptoms or need for further dilatations in all the study pa- 954 GASTROINTESTINAL ENDOSCOPY Volume 74, No. 5 :
2 tients, the majority (77%) of the stents had migrated out of the esophagus within 10 to 21 days of insertion, and only 3 patients had stents remain in position for more than 21 days. Because of this tendency for early stent migration in these 3 reports, the natural history of stent degradation within the esophagus and the tolerability of the degradation process over time were not adequately assessed. In 2007, the first (and currently the only) biodegradable stent with regulatory clearance for use in the GI tract became available in Europe only, indicated for use in benign refractory esophageal strictures (peptic, anastomotic, caustic) and achalasia. This stent comes in several sizes, with stent body diameters ranging from 18 to 25 mm and fully deployed lengths of 60 to 135 mm. The stent requires compression and assembly onto a 9.4-mm (28F) delivery system immediately prior to clinical use. The stent is self-expandable and is composed of polydioxanone fiber, which is a semicrystalline biodegradable polymer belonging to the polyester family. According to the manufacturer (ELLA-CS Ltd, Hradec Kralove, Czech Republic), the results of unpublished in vitro and animal tests demonstrate that the radial resistive force of the SX-ELLA-BD stent remains intact for approximately 4 weeks, dropping to 50% of the original level by week 9. Following insertion of the stent into the esophagus, the earliest signs of degradation are discoloration and single breaks of the stent mesh. Integrity and radial force are maintained for approximately 6 weeks following implantation, and the average time to complete degradation of the stent is reported to be 11 to 12 weeks. More rapid degradation occurs with acid exposure, hence acid-suppressing therapy is recommended. As the stent degrades, small fragments of the stent may pass distally through the GI tract and are further degraded, absorbed, or eliminated. Case series have described initial experience with the SX-ELLA-BD stent for treatment of a variety of esophageal strictures, including caustic, peptic, 10,13-14 malignant, anastomotic, 10,15-16 and radiation-induced strictures 10 as well as achalasia. 13 Clinical responses and outcomes were varied. In the largest cohort of patients with refractory benign esophageal strictures, 21 patients who had required an average of 2.2 endoscopic dilations per month underwent insertion of 25-mm SC-ELLA-BD stents. 10 There was 100% technical success and no intraprocedural complications. Postprocedural complications included severe thoracic pain (14%) sometimes lasting until the stent degraded, migration 4 to 7 weeks after placement (9.4%), and one case of significant tissue hyperplasia resulting in occlusion of the stent. In 19 patients, vestiges of the fragmented stent persisted in the esophagus at 3 months; the stents were completely eliminated by 6 months in all patients. After a median follow-up of 53 months, 45% of patients had significant relief of dysphagia and required no further therapy, whereas 55% failed to respond and required resumption of serial endoscopic dilations, albeit at less frequent intervals than they required before stenting. Other than the patient with hyperplasia-related stent occlusion, there were no longterm stent-related complications. Recently, SX-ELLA-BD stents modified with a nonbiodegradable covering made from polyurethane were used in 5 patients with esophageal leaks or perforations (4 anastomotic dehiscence, 1 iatrogenic perforation). 17 Initial clinical success was achieved in 4 of 5 (80%). The single treatment failure was caused by the polyurethane coating, which deformed as the stent degraded and caused acute aphagia requiring endoscopic extraction. Stent migration occurred in 3 patients within 5 to 7 days, but because of the biodegradable nature, retrieval was not performed. One of the 3 patients required placement of additional biodegradable stents, and successful closure of the leak was still achieved. Although total numbers of studied patients are small, published case series on SX-ELLA-BD stents encourage additional investigations into its use. However, this stent, not unlike commercially available self-expandable metal stents (SEMSs) and plastic stents, can induce significant hyperplastic tissue responses. 10,13,15-16,18 Efforts to define ways to prevent and treat this troublesome complication are ongoing. Biodegradable stents in the pancreaticobiliary tract Commercially available plastic and metal stents for the bile duct and pancreatic duct have many limitations, particularly when used for management of benign strictures. Although data suggest that endoscopic removal of covered SEMSs is feasible, their removal is not approved by the Food and Drug Administration, requires additional endoscopic procedures, and may be associated with complications. Biodegradable stents have the potential to address these limitations. Several animal trials of braided, woven, or helically configured biodegradable biliary stents have demonstrated feasibility of implantation, relative safety, and potential efficacy in a variety of experimental settings. Scenarios in which they have been studied include endoscopic 21 or surgical 22 placement into normal canine bile ducts, as reinforcement of hepaticojejunal or common bile duct anastomoses, for promotion of patency and growth of biliary epithelium within neo-bile ducts created by using explanted jugular vein grafts, and management of cystic duct leaks following cholecystectomy. 29 However, there have been a limited number of reports on their use in humans. In 2001, Haber et al 30 published (in abstract form) interim results from a prospective, multicenter study of a prototype bioabsorbable biliary Wallstent (Boston Scientific, Natick, Mass) made of poly-l-lactic acid monofilaments woven into a tubular mesh stent configuration (stent diameter, 10 mm; delivery system diameter, 11F) in 50 patients with inoperable malignant extrahepatic bile duct obstruction. Safe and successful stent deployment was achieved in the majority of patients, but data regard- Volume 74, No. 5 : 2011 GASTROINTESTINAL ENDOSCOPY 955
3 ing overall complications, patency, and survival are not available, and the study has not been published in full form. Another recent study described use of a version of the SX-ELLA-BD esophageal stent modified for the biliary tree to treat refractory intrahepatic bile duct stenoses (centrally located) in 2 patients who had previously undergone surgical bilioenteric reconstructions. 31 Durable clinical resolution of the biliary strictures was reported with up to 2-years of follow-up. In animal models, the histological changes in the duct and pancreatic parenchyma following placement of a biodegradable stent are reportedly negligible. 32 Adding barium sulfate to increase radiopacity of biodegradable stents helped facilitate both deployment under fluoroscopy and radiographic confirmation of complete degradation of the stent No human studies of pancreatic duct stenting with biodegradable stents have been performed. Biodegradable stents in the small intestine and colon Although use of enteral stents is an area of clinical interest, limited published information regarding biodegradable enteral stents exists. In one case report, 3 patients with Crohn s disease with stenosing complications (2 anastomotic strictures and 1 primary colonic Crohn s stricture) underwent endoscopy with balloon dilatation of the stenoses followed by placement of SX-ELLA-BD stents. 35 The stents degraded over a mean of 4 months. Despite one mechanical complication requiring endoscopic modification of the stent, no stent migrations or major complications occurred. However, long-term efficacy and safety data remain unknown. Drug-eluting stents in the GI tract Drug-eluting stents are composed of 3 main elements: a stent platform, a drug-carrier, and an active drug. 36 In vitro and in vivo studies (the latter using drug-coated SEMSs) have demonstrated that local exposure to paclitaxel, 5-fluorouracil, and gemcitabine can induce local responses when placed in contact with both benign and malignant GI tract tissues To date, there are only a few small published case series in humans. These demonstrated that partial responses in unresectable cholangiocarcinoma can be achieved with a carboplatincoated percutaneous biliary tube, 45 and endoscopically placed drug-eluting SEMS may be associated with improved stent patency and overall survival in patients with extrahepatic cholangiocarcinoma. 46 In the latter trial, 21 patients underwent placement of paclitaxel-coated biliary SEMSs for presumed extrahepatic cholangiocarcinoma; mean follow-up was 329 days. 46 There were no paclitaxel-specific toxicities, and systemic concentrations resulting from paclitaxel absorption were relatively low. Paclitaxel improved mean stent patency rates and overall patient survival compared with historical controls treated with conventional SEMSs. However, limitations of the trial include the use of a historic rather than an internal control arm, inclusion of patients (43%) without histopathologic confirmation of malignancy, the nonrandomized design, and an overall stent occlusion rate of 43%. POTENTIAL APPLICATIONS There are many clinical scenarios in which biodegradable stents may have potential advantages. Because biodegradable stents do not require endoscopic removal, even if they migrate, they may prove to be more useful than conventional SEMSs or plastic stents in many situations: Management of refractory benign stenoses and leaks (particularly if covered stents that are completely biodegradable become available) Palliation of malignant obstruction in patients undergoing chemoradiation for esophageal or pancreaticobiliary cancers When uncertainty exists about the diagnosis of malignancy (eg, indeterminate bile duct strictures) or about an individual s candidacy for surgery Prophylactic placement to prevent iatrogenic GI tract strictures in high-risk patients, such as those undergoing extensive endoscopic resections, enteral anastomoses, or pancreaticobiliary reconstructions In theory, an enormous array of biologically active agents and drugs could be incorporated into drug-eluting stents. Examples include chemotherapeutic drugs or other biologic modulators, such as radiation-sensitizing agents and mediators of angiogenesis, proliferation, inflammation, or fibrosis. Although the field is still in its infancy, evolution of drug-eluting stents could allow highly individualized, tailored, targeted therapy for a range of benign and malignant medical conditions. AREAS OF FUTURE RESEARCH The available series on drug-eluting and biodegradable stents in the GI tract are small series demonstrating technical feasibility and relative safety of these technologies. Additional reports from European endoscopists with experience using the SX-ELLA-BD stent are forthcoming, including trials comparing them to commercially available plastic stents and SEMSs (personal communication). Biodegradable GI tract stents probably will become available in the United States within the next few years. As with any emerging technology, it is advisable that initial use of these devices be limited to endoscopy centers with expertise in product assessment and development, because several important considerations need to be addressed before they reach the mainstream. Examples include the need for the following: Larger, prospective, well-designed studies must demonstrate long-term efficacy and safety of biodegradable 956 GASTROINTESTINAL ENDOSCOPY Volume 74, No. 5 :
4 stents at each applicable location within the GI tract and for a broad range of benign and malignant indications. Additional research is required to better understand the mechanisms of controlled-drug release and systemic absorption from drug-eluting GI tract stents, the complex interactions that occur, including drug-host response interactions and the effect that various drugs may have on the physicochemical and mechanical properties of stent platforms and biopolymers. 36,47-49 SUMMARY A biodegradable stent for GI applications is commercially available in Europe. In the upcoming years, these types of stents may enter the U.S. market. They have potential advantages over currently used plastic or metal stents for a range of clinical applications. Significant research on these as well as other new stent designs (eg, drug-eluting stents) is required. As with any novel technology, a tempered and evidence-based approach will be key in successfully establishing the role of these devices in clinical practice. DISCLOSURE J. Tokar is a consultant for Boston Scientific and is a speaker for and recipient of an educational grant from Fujinon. D. Pleskow is a consultant for Boston Scientific and is on the medical advisory board of Beacon Endoscopic. L.M. Wong Kee Song has received research support from Olympus and Fujinon. No other financial relationships relevant to this publication were disclosed. Abbreviations: ASGE, American Society for Gastrointestinal Endoscopy; ESD, endoscopic submucosal dissection; SEMS, self-expandable metal stent. REFERENCES 1. Somogyi L, Chuttani R, Croffie J, et al. Biliary and pancreatic stents. Gastrointest Endosc 2006;63: Tierney W, Chuttani R, Croffie J, et al. Enteral stents. Gastrointest Endosc 2006;63: Lee DK. Drug-eluting stent in malignant biliary obstruction. J Hepatobiliary Pancreat Surg 2009;16: Machan L. Clinical experience and applications of drug-eluting stents in the noncoronary vasculature, bile duct and esophagus. Adv Drug Deliv Rev 2006;58: Fry SW, Fleischer DE. Management of a refractory benign esophageal stricture with a new biodegradable stent. Gastrointest Endosc 1997;45: Goldin E, Fiorini A, Ratan Y, et al. A new biodegradable and selfexpandable stent for benign esophageal strictures [abstract]. Gastrointest Endosc 1996;43:AB Tanaka T, Takahashi M, Nitta N, et al. Newly developed biodegradable stents for benign gastrointestinal tract stenoses: a preliminary clinical trial. Digestion 2006;74: Saito Y, Tanaka T, Andoh A, et al. Usefulness of biodegradable stents constructed of poly-l-lactic acid monofilaments in patients with benign esophageal stenosis. World J Gastroenterol 2007;13: Saito Y, Tanaka T, Andoh A, et al. Novel biodegradable stents for benign esophageal strictures following endoscopic submucosal dissection. Dig Dis Sci 2008;53: Repici A, Vleggaar FP, Hassan C, et al. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the BEST (Biodegradable Esophageal Stent) study. Gastrointest Endosc 2010;72: Vandenplas Y, Hauser B, Devreker T, et al. A biodegradable esophageal stent in the treatment of a corrosive esophageal stenosis in a child. J Pediatr Gastroenterol Nutr 2009;49: Bychkova OV, Lazyuk II, Averin V. Bio-degradable stents a new approach to the treatment of caustic stenoses in children. Folia Gastroenterol Hepatol 2009;7: Hair CS, Devonshire DA. Severe hyperplastic tissue stenosis of a novel biodegradable esophageal stent and subsequent successful management with high-pressure balloon dilation. Endoscopy 2010;42(suppl 2): E Stivaros SM, Williams LR, Senger C, et al. Woven polydioxanone biodegradable stents: a new treatment option for benign and malignant oesophageal strictures. Eur Radiol 2010;20: van Hooft JE, van Berge Henegouwen MI, Rauws EA, et al. Endoscopic treatment of benign anastomostic esophageal strictures with a biodegradable stent (ESBIO). Endoscopy 2010;42:A Ibrahim M, Vandermeeren A, Van Maele V, et al. Belgian multicenter experience with the biodegradable Ella-stent in benign strictures of the digestive tract. Endoscopy 2010;42:A Cerna M, Kocher M, Valek V, et al. Covered biodegradable stent: new therapeutic option for the management of esophageal perforation or anastomotic leak. Cardiovasc Intervent Radiol. Epub 2011 Jan Orive-Calzada A, Alvarez-Rubio M, Romero-Izquierdo S, et al. Severe epithelial hyperplasia as a complication of a novel biodegradable stent. Endoscopy 2009;41:E Sauer B, Talreja J, Ellen K, et al. Temporary placement of a fully covered self-expandable metal stent in the pancreatic duct for management of symptomatic refractory chronic pancreatitis: preliminary data (with videos). Gastrointest Endosc 2008;68: Kahaleh M, Behm B, Clarke BW, et al. Temporary placement of covered self-expandable metal stents in benign biliary strictures: a new paradigm? (with video). Gastrointest Endosc 2008;67: Ginsberg G, Cope C, Shah J, et al. In vivo evaluation of a new bioabsorbable self-expanding biliary stent. Gastrointest Endosc 2003;58: Yamamoto K, Yoshioka T, Furuichi K, et al. Experimental study of poly-l: Lactic acid biodegradable stents in normal canine bile ducts. Cardiovasc Intervent Radiol. Epub 2010 Dec Laukkarinen JM, Sand JA, Chow P, et al. A novel biodegradable biliary stent in the normal duct hepaticojejunal anastomosis: an 18-month follow-up in a large animal model. J Gastrointest Surg 2007;11: Laukkarinen J, Sand J, Leppiniemi J, et al. A novel technique for hepaticojejunostomy for nondilated bile ducts: a purse-string anastomosis with an intra-anastomotic biodegradable biliary stent. Am J Surg 2010; 200: Tashiro H, Ogawa T, Itamoto T, et al. Synthetic bioabsorbable stent material for duct-to-duct biliary reconstruction. J Surg Res 2009;151: Xu X, Liu T, Liu S, et al. Feasibility of biodegradable PLGA common bile duct stents: an in vitro and in vivo study. J Mater Sci Mater Med 2009;20: Heistermann HP, Palmes D, Stratmann U, et al. A new technique for reconstruction of the common bile duct by an autologous vein graft and a biodegradable endoluminal stent. J Invest Surg 2006;19: Palmes D, Wolters H, Spiegel HU, et al. Morphological changes during creation of a neo-bile duct using a vein and a biodegradable endoluminal stent. J Invest Surg 2009;22: Laukkarinen J, Nordback I, Mikkonen J, et al. A novel biodegradable biliary stent in the endoscopic treatment of cystic-duct leakage after cholecystectomy. Gastrointest Endosc 2007;65: Haber GB, Freeman ML, Bedford R, et al. A prospective multi-center study of a bioabsorbable biliary Wallstent (BAS) in 50 patients with ma- Volume 74, No. 5 : 2011 GASTROINTESTINAL ENDOSCOPY 957
5 lignant obstructive jaundice (MOJ) [abstract]. Gastrointest Endosc 2001;53:AB Petrtyl J, Bruha R, Horak L, et al. Management of benign intrahepatic bile duct strictures: initial experience with polydioxanone biodegradable stents. Endoscopy 2010;42(suppl 2):E Parviainen M, Sand J, Harmoinen A, et al. A new biodegradable stent for the pancreaticojejunal anastomosis after pancreaticoduodenal resection: in vitro examination and pilot experiences in humans. Pancreas 2000;21: Laukkarinen J, Lamsa T, Nordback I, et al. A novel biodegradable pancreatic stent for human pancreatic applications: a preclinical safety study in a large animal model. Gastrointest Endosc 2008;67: Lamsa T, Jin H, Mikkonen J, et al. Biocompatibility of a new bioabsorbable radiopaque stent material (BaSO4 containing poly-l,d-lactide) in the rat pancreas. Pancreatology 2006;6: Rejchrt S, Kopacova M, Bartova J, et al. Intestinal biodegradable stents: initial experience in the Czech Republic. Folia Gastroenterol Hepatol 2009;7: Wykrzykowska JJ, Onuma Y, Serruys PW. Advances in stent drug delivery: the future is in bioabsorbable stents. Expert Opin Drug Deliv 2009; 6: Guo SR, Wang ZM, Zhang YQ, et al. In vivo evaluation of 5-fluorouracilcontaining self-expandable nitinol stent in rabbits: efficiency in longterm local drug delivery. J Pharm Sci 2010;99: Kalinowski M, Alfke H, Kleb B, et al. Paclitaxel inhibits proliferation of cell lines responsible for metal stent obstruction: possible topical application in malignant bile duct obstructions. Invest Radiol 2002;37: Lee DK, Kim HS, Kim KS, et al. The effect on porcine bile duct of a metallic stent covered with a paclitaxel-incorporated membrane. Gastrointest Endosc 2005;61: Guo Q, Guo S, Wang Z. A type of esophageal stent coating composed of one 5-fluorouracil-containing EVA layer and one drug-free protective layer: in vitro release, permeation and mechanical properties. J Control Release 2007;118: Jeon SR, Eun SH, Shim CS, et al. Effect of drug-eluting metal stents in benign esophageal stricture: an in vivo animal study. Endoscopy 2009; 41: Lee SS, Shin JH, Han JM, et al. Histologic influence of paclitaxel-eluting covered metallic stents in a canine biliary model. Gastrointest Endosc 2009;69: Chun MJ, Kyung SK, Park JY, et al. The effect on porcine bile duct with metallic stent covered with a gemcitabine-incorporated membrane [abstract]. Gastrointest Endosc 2010;71:AB Mezawa S, Homma H, Sato T, et al. A study of carboplatin-coated tube for the unresectable cholangiocarcinoma. Hepatology 2000;32: Suk KT, Kim JW, Kim HS, et al. Human application of a metallic stent covered with a paclitaxel-incorporated membrane for malignant biliary obstruction: multicenter pilot study. Gastrointest Endosc 2007;66: Hermawan H, Dube D, Mantovani D. Developments in metallic biodegradable stents. Acta Biomater 2010;6: Lendlein A, Behl M, Hiebl B, et al. Shape-memory polymers as a technology platform for biomedical applications. Expert Rev Med Devices 2010; 7: Wischke C, Lendlein A. Shape-memory polymers as drug carriers a multifunctional system. Pharm Res 2010;27: Wischke C, Neffe AT, Steuer S, et al. Comparing techniques for drug loading of shape-memory polymer networks effect on their functionalities. Eur J Pharm Sci 2010;41: Prepared by: ASGE TECHNOLOGY COMMITTEE Jeffrey L. Tokar, MD Subhas Banerjee, MD Bradley A. Barth, MD, NASPGHAN representative David J. Desilets, MD Vivek Kaul, MD Sripathi R. Kethi, MD Marcos C. Pedrosa, MD Patrick R. Pfau, MD Douglas K. Pleskow, MD Shyam Varadarajulu, MD Amy Wang, MD Louis-Michel Wong Kee Song, MD Sarah A. Rodriguez, MD, Committee Chair 958 GASTROINTESTINAL ENDOSCOPY Volume 74, No. 5 :
WallFlex Biliary RX Stent. Fully, Partially and Uncovered Self-Expanding Metal Stents
 WallFlex Biliary RX Stent Fully, Partially and Uncovered Self-Expanding Metal Stents WallFlex Biliary RX Stent Fully, Partially and Uncovered Self-Expanding Metal Stents The WallFlex Biliary RX Stent is
WallFlex Biliary RX Stent Fully, Partially and Uncovered Self-Expanding Metal Stents WallFlex Biliary RX Stent Fully, Partially and Uncovered Self-Expanding Metal Stents The WallFlex Biliary RX Stent is
Preoperative drainage is always indicated in malignant CBD strictures PRO. Horst Neuhaus Evangelisches Krankenhaus Düsseldorf, Germany
 Preoperative drainage is always indicated in malignant CBD strictures PRO Horst Neuhaus Evangelisches Krankenhaus Düsseldorf, Germany Background Jaundice is associated with high perioperative morbidity
Preoperative drainage is always indicated in malignant CBD strictures PRO Horst Neuhaus Evangelisches Krankenhaus Düsseldorf, Germany Background Jaundice is associated with high perioperative morbidity
Endoscopic therapy for obesity and complications of bariatric surgery
 Endoscopic therapy for obesity and complications of bariatric surgery Jacques Devière, MD, PhD Erasme University Hospital Brussels Belgium [email protected] Obesity Affects 300 millions
Endoscopic therapy for obesity and complications of bariatric surgery Jacques Devière, MD, PhD Erasme University Hospital Brussels Belgium [email protected] Obesity Affects 300 millions
ERCP in Post Surgical Anatomy
 ERCP in Post Surgical Anatomy ACG Western Regional Course, 2013 John G. Lee, MD Division of Gastroenterology University of California, Irvine Medical Center Common surgical alterations Intact pancreaticobiliary
ERCP in Post Surgical Anatomy ACG Western Regional Course, 2013 John G. Lee, MD Division of Gastroenterology University of California, Irvine Medical Center Common surgical alterations Intact pancreaticobiliary
Bile Leaks After Laparoscopic Cholecystectomy. Kings County Hospital Center Eliana A. Soto, MD
 Bile Leaks After Laparoscopic Cholecystectomy Kings County Hospital Center Eliana A. Soto, MD Biliary Injuries during Cholecystectomy In the 1990s, high rate of biliary injury was due in part to learning
Bile Leaks After Laparoscopic Cholecystectomy Kings County Hospital Center Eliana A. Soto, MD Biliary Injuries during Cholecystectomy In the 1990s, high rate of biliary injury was due in part to learning
Use of stents in esophageal cancer" Hans Gerdes, M.D. Director, GI Endoscopy Unit Memorial Sloan-Kettering Cancer Center
 Use of stents in esophageal cancer" Hans Gerdes, M.D. Director, GI Endoscopy Unit Memorial Sloan-Kettering Cancer Center Features of esophageal cancer Esophageal cancer is an abnormal growth that arises
Use of stents in esophageal cancer" Hans Gerdes, M.D. Director, GI Endoscopy Unit Memorial Sloan-Kettering Cancer Center Features of esophageal cancer Esophageal cancer is an abnormal growth that arises
Learning Luncheon 7: Endoscopic Mucosal Resection: When, Where and How?
 Endoscopic Mucosal Resection (EMR): When, Where, and Charles J. Lightdale, MD Columbia University New York, NY Endoscopic Mucosal Resection (EMR) EMR developed for removal of sessile or flat neoplasms
Endoscopic Mucosal Resection (EMR): When, Where, and Charles J. Lightdale, MD Columbia University New York, NY Endoscopic Mucosal Resection (EMR) EMR developed for removal of sessile or flat neoplasms
Endoscopic Management of Strictures and Leaks. Prepared by Aurora D. Pryor, MD Presented by Dana Portenier, MD Duke University Medical Center
 Endoscopic Management of Strictures and Leaks Prepared by Aurora D. Pryor, MD Presented by Dana Portenier, MD Duke University Medical Center What can go wrong? Bleeding (2%) Sleeve too big Angulated Too
Endoscopic Management of Strictures and Leaks Prepared by Aurora D. Pryor, MD Presented by Dana Portenier, MD Duke University Medical Center What can go wrong? Bleeding (2%) Sleeve too big Angulated Too
Esophageal Stenting: Role in strictures, leaks, fistulae, and malignancy
 Esophageal Stenting: Role in strictures, leaks, fistulae, and malignancy Jasmine L. Huang, MD General Thoracic Surgery St. Joseph s s Hospital and Medical Center Phoenix, AZ Jasmine Huang, MD I have no
Esophageal Stenting: Role in strictures, leaks, fistulae, and malignancy Jasmine L. Huang, MD General Thoracic Surgery St. Joseph s s Hospital and Medical Center Phoenix, AZ Jasmine Huang, MD I have no
Endoluminal stent placement core curriculum
 Communication from the ASGE Training Committee CORE CURRICULUM Endoluminal stent placement core curriculum This is one of a series of documents prepared by the American Society for Gastrointestinal Endoscopy
Communication from the ASGE Training Committee CORE CURRICULUM Endoluminal stent placement core curriculum This is one of a series of documents prepared by the American Society for Gastrointestinal Endoscopy
Contraindications: Malign or benign strictures in the upper part of esophagus close to the cricopharyngeal muscle.
 Manufactured by: ELLA CS, s.r.o. Milady Horákové 504 500 06 Hradec Králové 6 Czech Republic Phone: +420 49 527 91 11 Fax: +420 49 526 56 55 E-mail: [email protected] Instructions for Use FerX-ELLA Esophageal
Manufactured by: ELLA CS, s.r.o. Milady Horákové 504 500 06 Hradec Králové 6 Czech Republic Phone: +420 49 527 91 11 Fax: +420 49 526 56 55 E-mail: [email protected] Instructions for Use FerX-ELLA Esophageal
What is Barrett s esophagus? How does Barrett s esophagus develop?
 Barrett s Esophagus What is Barrett s esophagus? Barrett s esophagus is a pre-cancerous condition affecting the lining of the esophagus, the swallowing tube that carries foods and liquids from the mouth
Barrett s Esophagus What is Barrett s esophagus? Barrett s esophagus is a pre-cancerous condition affecting the lining of the esophagus, the swallowing tube that carries foods and liquids from the mouth
A Guide for Patients Living with a Biliary Metal Stent
 A Guide for Patients Living with a Biliary Metal Stent What is a biliary metal stent? A biliary metal stent (also known as a bile duct stent ) is a flexible metallic tube specially designed to hold your
A Guide for Patients Living with a Biliary Metal Stent What is a biliary metal stent? A biliary metal stent (also known as a bile duct stent ) is a flexible metallic tube specially designed to hold your
USE OF STENTS FOR UPPER GI DISASTERS. Michael Talbot. The St George Hospital, Sydney
 USE OF STENTS FOR UPPER GI DISASTERS Michael Talbot. The St George Hospital, Sydney Disclosures Educational grants by Coviden, Applied Medical, Endogastric Solutions and Allergan in the last 3 years Clinical
USE OF STENTS FOR UPPER GI DISASTERS Michael Talbot. The St George Hospital, Sydney Disclosures Educational grants by Coviden, Applied Medical, Endogastric Solutions and Allergan in the last 3 years Clinical
Evidence of Crohn s Disease. Case Presentation
 Witt Wait to Treat tutiled Until Endoscopic Evidence of Crohn s Disease Raymond Cross, MD, MS, AGAF Associate Professor of Medicine Director, IBD Program University of Maryland School of Medicine Co-Director,
Witt Wait to Treat tutiled Until Endoscopic Evidence of Crohn s Disease Raymond Cross, MD, MS, AGAF Associate Professor of Medicine Director, IBD Program University of Maryland School of Medicine Co-Director,
The digestive system. Medicine and technology. Normal structure and function Diagnostic methods Example diseases and therapies
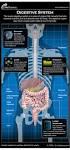 The digestive system Medicine and technology Normal structure and function Diagnostic methods Example diseases and therapies The digestive system An overview (1) Oesophagus Liver (hepar) Biliary system
The digestive system Medicine and technology Normal structure and function Diagnostic methods Example diseases and therapies The digestive system An overview (1) Oesophagus Liver (hepar) Biliary system
PANCREATIC AND PERIAMPULLARY TUMORS: PANCREATICODUODENECTOMY. Dr. Shailesh V. Shrikhande
 PANCREATIC AND PERIAMPULLARY TUMORS: PANCREATICODUODENECTOMY Dr. Shailesh V. Shrikhande Associate Professor & Consultant Surgeon GI and HPB Surgical Oncology Tata Memorial Hospital, Mumbai INDIA HELICAL
PANCREATIC AND PERIAMPULLARY TUMORS: PANCREATICODUODENECTOMY Dr. Shailesh V. Shrikhande Associate Professor & Consultant Surgeon GI and HPB Surgical Oncology Tata Memorial Hospital, Mumbai INDIA HELICAL
Evolution of Barrett s esophagus
 Endoscopic Treatment and Surveillance of Esophageal Cancer: GI Perspective Charles J. Lightdale, MD Columbia University New York, NY Evolution of Barrett s esophagus Squamous esophagus Chronic inflammation
Endoscopic Treatment and Surveillance of Esophageal Cancer: GI Perspective Charles J. Lightdale, MD Columbia University New York, NY Evolution of Barrett s esophagus Squamous esophagus Chronic inflammation
Localised Cancer Treatment. PCI Biotech. Amphinex a new product for localised cancer treatment
 Localised Cancer Treatment PCI Biotech Amphinex a new product for localised cancer treatment Disclaimer This document (the Presentation ) has been produced by PCI Biotech Holding ASA (the Company ). The
Localised Cancer Treatment PCI Biotech Amphinex a new product for localised cancer treatment Disclaimer This document (the Presentation ) has been produced by PCI Biotech Holding ASA (the Company ). The
Captivator EMR Device
 Device Clinical Article and Abstract Summary Endoscopic Mucosal Bergman et al: EMR Training Tips Bergman et al: EMR Learning Curve ASGE: EMR & ESD Guidelines Bergman et al: Captivator EMR vs Cook Duette
Device Clinical Article and Abstract Summary Endoscopic Mucosal Bergman et al: EMR Training Tips Bergman et al: EMR Learning Curve ASGE: EMR & ESD Guidelines Bergman et al: Captivator EMR vs Cook Duette
Open Ventral Hernia Repair
 Ventral Hernias Open Ventral Hernia Repair UCSF Postgraduate Course in General Surgery Maui, HI March 21, 2011 Hobart W. Harris, MD, MPH Ventral Hernias: National Experience Occur following 11-23% of laparotomies,
Ventral Hernias Open Ventral Hernia Repair UCSF Postgraduate Course in General Surgery Maui, HI March 21, 2011 Hobart W. Harris, MD, MPH Ventral Hernias: National Experience Occur following 11-23% of laparotomies,
Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI)
 Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) The American Society for Gastrointestinal Endoscopy PIVI on Endoscopic Bariatric Procedures (short form) Please see related White
Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) The American Society for Gastrointestinal Endoscopy PIVI on Endoscopic Bariatric Procedures (short form) Please see related White
WHAT S WRONG WITH MY GALL BLADDER? GALL BLADDER POLYPS
 WHAT S WRONG WITH MY GALL BLADDER? GALL BLADDER POLYPS This is a patient information booklet providing specific practical information about gall bladder polyps in brief. Its aim is to provide the patient
WHAT S WRONG WITH MY GALL BLADDER? GALL BLADDER POLYPS This is a patient information booklet providing specific practical information about gall bladder polyps in brief. Its aim is to provide the patient
Endoscopic Resection for Barrett s Esophagus and Early Cancer 2014 Masters of Minimally Invasive Surgery
 Endoscopic Resection for Barrett s Esophagus and Early Cancer 2014 Masters of Minimally Invasive Surgery Matthew Hartwig, M.D. Duke Cancer Institute Case Presentation: Patient ER 51 y/o man with schizophrenia
Endoscopic Resection for Barrett s Esophagus and Early Cancer 2014 Masters of Minimally Invasive Surgery Matthew Hartwig, M.D. Duke Cancer Institute Case Presentation: Patient ER 51 y/o man with schizophrenia
Acute Abdominal Pain following Bariatric Surgery. Disclosure. Objectives 8/17/2015. I have nothing to disclose
 Acute Abdominal Pain following Bariatric Surgery Kathy J. Morris, DNP, APRN, FNP C, FAANP University of Nebraska Medical Center College of Nursing Disclosure I have nothing to disclose Objectives Pathophysiology
Acute Abdominal Pain following Bariatric Surgery Kathy J. Morris, DNP, APRN, FNP C, FAANP University of Nebraska Medical Center College of Nursing Disclosure I have nothing to disclose Objectives Pathophysiology
The Role of Industry Representatives in the Endoscopy Unit
 The Role of Industry Representatives in the Endoscopy Unit Vivek Kaul, MD 1 and Douglas Faigel, MD, FASGE 2 Introduction The modern endoscopy unit is a busy workplace environment. With the patient as the
The Role of Industry Representatives in the Endoscopy Unit Vivek Kaul, MD 1 and Douglas Faigel, MD, FASGE 2 Introduction The modern endoscopy unit is a busy workplace environment. With the patient as the
LifeStent XL Biliary Stent System
 B05688 Rev.2/07-13 LifeStent XL Biliary Stent System Recommended Guidewire Length Table Catheter Working Length Recommended Guidewire Length 130 cm 300 cm 80 cm 260 cm 130 cm & 80 cm 160 cm & 110 cm Figure
B05688 Rev.2/07-13 LifeStent XL Biliary Stent System Recommended Guidewire Length Table Catheter Working Length Recommended Guidewire Length 130 cm 300 cm 80 cm 260 cm 130 cm & 80 cm 160 cm & 110 cm Figure
TECHNOLOGICAL INNOVATION FOR CORONARY STENTS Francesco Migliavacca
 LABORATORY OF BIOLOGICAL STRUCTURE MECHANICS www.labsmech.polimi.it TECHNOLOGICAL INNOVATION FOR CORONARY STENTS Francesco Migliavacca Erice, 1 maggio 2015 International School of Cardiac Surgery Introduction
LABORATORY OF BIOLOGICAL STRUCTURE MECHANICS www.labsmech.polimi.it TECHNOLOGICAL INNOVATION FOR CORONARY STENTS Francesco Migliavacca Erice, 1 maggio 2015 International School of Cardiac Surgery Introduction
How to Effectively Code for Endoscopic Procedures in Gastroenterology
 How to Effectively Code for Endoscopic Procedures in Gastroenterology Ariwan Rakvit, MD Associate Professor Interim Chief, Division of Gastroenterology Texas Tech University Health Science Center All rights
How to Effectively Code for Endoscopic Procedures in Gastroenterology Ariwan Rakvit, MD Associate Professor Interim Chief, Division of Gastroenterology Texas Tech University Health Science Center All rights
The hidden endoscopic burden of sleeve gastrectomy and its comparison with Roux-en-Y gastric bypass
 ORIGINAL ARTICLE Annals of Gastroenterology (2015) 28, 1-6 The hidden endoscopic burden of sleeve gastrectomy and its comparison with Roux-en-Y gastric bypass Katherine Arndtz a, Helen Steed b, James Hodson
ORIGINAL ARTICLE Annals of Gastroenterology (2015) 28, 1-6 The hidden endoscopic burden of sleeve gastrectomy and its comparison with Roux-en-Y gastric bypass Katherine Arndtz a, Helen Steed b, James Hodson
MEDICAL POLICY No. 91580-R1 DRUG-ELUTING STENTS FOR ISCHEMIC HEART DISEASE
 DRUG-ELUTING STENTS FOR ISCHEMIC HEART DISEASE Effective Date: October 1, 2015 Review Dates: 10/11, 10/12, 10/13, 8/14, 8/15 Date Of Origin: October 12, 2011 Status: Current Summary of Changes Clarifications:
DRUG-ELUTING STENTS FOR ISCHEMIC HEART DISEASE Effective Date: October 1, 2015 Review Dates: 10/11, 10/12, 10/13, 8/14, 8/15 Date Of Origin: October 12, 2011 Status: Current Summary of Changes Clarifications:
11/10/2014. I have nothing to Disclose. Covered Stents discussed are NOT FDA approved for the indications covered in my presentation
 I have nothing to Disclose Ramsey Dallal, MD, FACS Vice Chair Department of Surgery Chief Bariatric i and Minimally i Invasive Surgery Einstein Healthcare Network Nemacolin, PA 2014 Covered Stents discussed
I have nothing to Disclose Ramsey Dallal, MD, FACS Vice Chair Department of Surgery Chief Bariatric i and Minimally i Invasive Surgery Einstein Healthcare Network Nemacolin, PA 2014 Covered Stents discussed
Gary M. Annuniziata, D.O., F.A.C.P. Anh T. Duong, M.D. Jonathan C. Lin, M.D., MPH. Preparation for EGD, ERCP, Peg Placement.
 Gary M. Annuniziata, D.O., F.A.C.P. Anh T. Duong, M.D. Jonathan C. Lin, M.D., MPH Phone- (760) 321-2500 Fax- (760) 321-5720 Preparation for EGD, ERCP, Peg Placement Patient Name- Procedure Date and Time-
Gary M. Annuniziata, D.O., F.A.C.P. Anh T. Duong, M.D. Jonathan C. Lin, M.D., MPH Phone- (760) 321-2500 Fax- (760) 321-5720 Preparation for EGD, ERCP, Peg Placement Patient Name- Procedure Date and Time-
Endoscopy and infection: Prevention of infection during endoscopy Treatment of infection by endoscopy. M. Arvanitakis SRBG June 2009
 Endoscopy and infection: Prevention of infection during endoscopy Treatment of infection by endoscopy M. Arvanitakis SRBG June 2009 Outline Antibiotic prophylaxis during endoscopy Upper GI endoscopy Lower
Endoscopy and infection: Prevention of infection during endoscopy Treatment of infection by endoscopy M. Arvanitakis SRBG June 2009 Outline Antibiotic prophylaxis during endoscopy Upper GI endoscopy Lower
Lenox Hill Hospital Department of Surgery General Surgery Goals and Objectives
 Lenox Hill Hospital Department of Surgery General Surgery Goals and Objectives Medical Knowledge and Patient Care: Residents must demonstrate knowledge and application of the pathophysiology and epidemiology
Lenox Hill Hospital Department of Surgery General Surgery Goals and Objectives Medical Knowledge and Patient Care: Residents must demonstrate knowledge and application of the pathophysiology and epidemiology
Gastrointestinal Bleeding
 Gastrointestinal Bleeding Introduction Gastrointestinal bleeding is a symptom of many diseases rather than a disease itself. A number of different conditions can cause gastrointestinal bleeding. Some causes
Gastrointestinal Bleeding Introduction Gastrointestinal bleeding is a symptom of many diseases rather than a disease itself. A number of different conditions can cause gastrointestinal bleeding. Some causes
Cancer of the Cardia/GE Junction: Surgical Options
 Cancer of the Cardia/GE Junction: Surgical Options Michael A Smith, MD Associate Chief Thoracic Surgery Center for Thoracic Disease St Joseph s Hospital and Medical Center Phoenix, AZ Michael Smith, MD
Cancer of the Cardia/GE Junction: Surgical Options Michael A Smith, MD Associate Chief Thoracic Surgery Center for Thoracic Disease St Joseph s Hospital and Medical Center Phoenix, AZ Michael Smith, MD
Bile Duct Diseases and Problems
 Bile Duct Diseases and Problems Introduction A bile duct is a tube that carries bile between the liver and gallbladder and the intestine. Bile is a substance made by the liver that helps with digestion.
Bile Duct Diseases and Problems Introduction A bile duct is a tube that carries bile between the liver and gallbladder and the intestine. Bile is a substance made by the liver that helps with digestion.
Optimal Management of Splenic/Portal Vein Thrombosis. David Mauchley University of Colorado
 Optimal Management of Splenic/Portal Vein Thrombosis David Mauchley University of Colorado Overview Portal Vein Thrombosis (PVT) Etiology Presentation/Clinical Aspects Diagnosis Management Cirrhotic vs.
Optimal Management of Splenic/Portal Vein Thrombosis David Mauchley University of Colorado Overview Portal Vein Thrombosis (PVT) Etiology Presentation/Clinical Aspects Diagnosis Management Cirrhotic vs.
Facing Pancreatic Surgery? Learn about minimally invasive da Vinci Surgery
 Facing Pancreatic Surgery? Learn about minimally invasive da Vinci Surgery The Condition: Pancreatitis/Pancreatic Cancer The pancreas is an organ that produces enzymes and hormones to help your body digest
Facing Pancreatic Surgery? Learn about minimally invasive da Vinci Surgery The Condition: Pancreatitis/Pancreatic Cancer The pancreas is an organ that produces enzymes and hormones to help your body digest
Clinical Research Intracoronary Stenting with Crushing in Coronary Artery Bifurcation Lesions: Initial Results and Medium-Term Follow Up
 Hellenic J Cardiol 45: 379-383, 2004 Clinical Research Intracoronary Stenting with Crushing in Coronary Artery Bifurcation Lesions: Initial Results and Medium-Term Follow Up PETROS S. DARDAS, DIMITRIS
Hellenic J Cardiol 45: 379-383, 2004 Clinical Research Intracoronary Stenting with Crushing in Coronary Artery Bifurcation Lesions: Initial Results and Medium-Term Follow Up PETROS S. DARDAS, DIMITRIS
Endoscopic treatment of Common Esophageal disorders
 Endoscopic treatment of Common Esophageal disorders November 7, 2015 Shivangi T. Kothari, MD Assistant Professor, Medicine Associate Director of Endoscopy Co-Director Developmental Endoscopy Lab at UR
Endoscopic treatment of Common Esophageal disorders November 7, 2015 Shivangi T. Kothari, MD Assistant Professor, Medicine Associate Director of Endoscopy Co-Director Developmental Endoscopy Lab at UR
UCLA Asian Liver Program
 CLA Program Update Program Faculty Myron J. Tong, PhD, MD Professor of Medicine Hepatology Director, Asian Liver Program Surgery Ronald W. Busuttil, MD, PhD Executive Chair Department of Surgery Director,
CLA Program Update Program Faculty Myron J. Tong, PhD, MD Professor of Medicine Hepatology Director, Asian Liver Program Surgery Ronald W. Busuttil, MD, PhD Executive Chair Department of Surgery Director,
Center for Endoscopic Research & Therapeutics
 Center for Endoscopic Research & Therapeutics 5758 South Maryland Avenue (MC9028) Chicago, Illinois 60637 (773) 702-1459 www.uchospitals.edu Center for Endoscopic Research & Therapeutics To refer a patient
Center for Endoscopic Research & Therapeutics 5758 South Maryland Avenue (MC9028) Chicago, Illinois 60637 (773) 702-1459 www.uchospitals.edu Center for Endoscopic Research & Therapeutics To refer a patient
The Minvasys Amazonia Pax & Nile Pax Polymer Free Paclitaxel Eluting Stent Program
 The Minvasys Amazonia Pax & Nile Pax Polymer Free Paclitaxel Eluting Stent Program Jean Fajadet, MD, FESC Clinique Pasteur - Toulouse - France Disclosure statement Nothing to disclose Dedicated Delivery
The Minvasys Amazonia Pax & Nile Pax Polymer Free Paclitaxel Eluting Stent Program Jean Fajadet, MD, FESC Clinique Pasteur - Toulouse - France Disclosure statement Nothing to disclose Dedicated Delivery
SAMPLE. Asia-Pacific Interventional Cardiology Procedures Outlook to 2020. Reference Code: GDMECR0061PDB. Publication Date: May 2014
 Asia-Pacific Interventional Cardiology Procedures Outlook to 2020 Reference Code: GDMECR0061PDB Publication Date: May 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 4 1.2
Asia-Pacific Interventional Cardiology Procedures Outlook to 2020 Reference Code: GDMECR0061PDB Publication Date: May 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 4 1.2
Orsiro Hybrid Drug Eluting Stent Industry's first hybrid DES
 Vascular Intervention Drug Eluting Stents Orsiro Orsiro Hybrid Drug Eluting Stent Industry's first hybrid DES Orsiro An ideal combination of passive and active components The Orsiro Hybrid Drug Eluting
Vascular Intervention Drug Eluting Stents Orsiro Orsiro Hybrid Drug Eluting Stent Industry's first hybrid DES Orsiro An ideal combination of passive and active components The Orsiro Hybrid Drug Eluting
CORONARY ARTERY BYPASS GRAFTS, STENTS, AND EXTRACORONARY CARDIAC DZ. Charles White MD
 CORONARY ARTERY BYPASS GRAFTS, STENTS, AND EXTRACORONARY CARDIAC DZ Charles White MD Director of Thoracic Imaging Department of Radiology University of Maryland CORONARY ARTERY BYPASS GRAFTS First performed
CORONARY ARTERY BYPASS GRAFTS, STENTS, AND EXTRACORONARY CARDIAC DZ Charles White MD Director of Thoracic Imaging Department of Radiology University of Maryland CORONARY ARTERY BYPASS GRAFTS First performed
KIDNEY FUNCTION RELATION TO SIZE OF THE TUMOR IN RENAL CELL CANCINOMA
 KIDNEY FUNCTION RELATION TO SIZE OF THE TUMOR IN RENAL CELL CANCINOMA O.E. Stakhvoskyi, E.O. Stakhovsky, Y.V. Vitruk, O.A. Voylenko, P.S. Vukalovich, V.A. Kotov, O.M. Gavriluk National Canсer Institute,
KIDNEY FUNCTION RELATION TO SIZE OF THE TUMOR IN RENAL CELL CANCINOMA O.E. Stakhvoskyi, E.O. Stakhovsky, Y.V. Vitruk, O.A. Voylenko, P.S. Vukalovich, V.A. Kotov, O.M. Gavriluk National Canсer Institute,
The Whipple Procedure. Sally Hodges, Ph.D.(c) Given the length and difficulty of the procedure, regardless of the diagnosis, certain
 The Whipple Procedure Sally Hodges, Ph.D.(c) Preoperative procedures Given the length and difficulty of the procedure, regardless of the diagnosis, certain assurances must occur prior to offering a patient
The Whipple Procedure Sally Hodges, Ph.D.(c) Preoperative procedures Given the length and difficulty of the procedure, regardless of the diagnosis, certain assurances must occur prior to offering a patient
Cytoreduction and hyperthermic intraperitoneal chemotherapy for the treatment of pseudomyxoma
 Medical Policy Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Pseudomyxoma Peritonei and Peritoneal Carcinomatosis of Gastrointestinal Origin, and Peritoneal Mesothelioma
Medical Policy Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Pseudomyxoma Peritonei and Peritoneal Carcinomatosis of Gastrointestinal Origin, and Peritoneal Mesothelioma
Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products
 Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Introduction. 31 million Americans suffer from chronic sinusitis Surgical treatment for chronic sinusitis has evolved tremendously since its inception
 Balloon Sinuplasty Ki-Hong Kevin Ho, MD Faculty Advisor: Patricia Maeso, MD Department of Otolaryngology The University of Texas Medical Branch Grand Rounds Presentation December 16, 2009 Introduction
Balloon Sinuplasty Ki-Hong Kevin Ho, MD Faculty Advisor: Patricia Maeso, MD Department of Otolaryngology The University of Texas Medical Branch Grand Rounds Presentation December 16, 2009 Introduction
Principles of training in GI endoscopy
 Communication from the ASGE Training Committee REPORT ON TRAINING Principles of training in GI endoscopy This document, prepared by the American Society for Gastrointestinal Endoscopy Committee on Training,
Communication from the ASGE Training Committee REPORT ON TRAINING Principles of training in GI endoscopy This document, prepared by the American Society for Gastrointestinal Endoscopy Committee on Training,
Ching-Yao Yang, Yu-Wen Tien
 Ching-Yao Yang, Yu-Wen Tien Division of General Surgery, Department of Surgery, National Taiwan University Hospital Oct-30-2010 Pancreatic NET have poorer prognosis when presence of liver metastases at
Ching-Yao Yang, Yu-Wen Tien Division of General Surgery, Department of Surgery, National Taiwan University Hospital Oct-30-2010 Pancreatic NET have poorer prognosis when presence of liver metastases at
DATE: 29 August 2012 CONTEXT AND POLICY ISSUES
 TITLE: Dual Antiplatelet Therapy and Enoxaparin or Unfractionated Heparin for patients with ST-elevation Myocardial Infarction: A Review of the Clinical Evidence DATE: 29 August 2012 CONTEXT AND POLICY
TITLE: Dual Antiplatelet Therapy and Enoxaparin or Unfractionated Heparin for patients with ST-elevation Myocardial Infarction: A Review of the Clinical Evidence DATE: 29 August 2012 CONTEXT AND POLICY
Emerging Concepts in Bariatric Surgery
 Emerging Concepts in Bariatric Surgery C Y N T H I A L. L O N G, M D, F A C S S I N A I H O S P I T A L O F B A L T I M O R E D E P A R T M E N T O F S U R G E R Y D I V I S I O N O F M I N I M A L L Y
Emerging Concepts in Bariatric Surgery C Y N T H I A L. L O N G, M D, F A C S S I N A I H O S P I T A L O F B A L T I M O R E D E P A R T M E N T O F S U R G E R Y D I V I S I O N O F M I N I M A L L Y
Expect. Endoscopic Ultrasound Aspiration Needles. Your Patient. Your Needle. Your Preference.
 Expect Endoscopic Ultrasound Aspiration Needles Your Patient. Your Needle. Your Preference. Expect Slimline (SL) Endoscopic Ultrasound Aspiration Needles With applications for endoscopic ultrasound and
Expect Endoscopic Ultrasound Aspiration Needles Your Patient. Your Needle. Your Preference. Expect Slimline (SL) Endoscopic Ultrasound Aspiration Needles With applications for endoscopic ultrasound and
CPT COD1NG UPDATES Gastroenterology CPT Advisors
 2014 CPT COD1NG UPDATES Gastroenterology CPT Advisors Joel V. Brill, MD, AGA CPT Advisor Daniel C. DeMarco, MD, ACG CPT Advisor Glenn D. Littenberg, MD, ASGE CPT Advisor The American College of Gastroenterology
2014 CPT COD1NG UPDATES Gastroenterology CPT Advisors Joel V. Brill, MD, AGA CPT Advisor Daniel C. DeMarco, MD, ACG CPT Advisor Glenn D. Littenberg, MD, ASGE CPT Advisor The American College of Gastroenterology
Billing Guideline. Subject: Colorectal Cancer Screening Exams (Invasive Procedures) Effective Date: 1/1/2012 Last Update Effective: 4/16
 Subject: Colorectal Cancer Screening Exams (Invasive Procedures) Effective Date: 1/1/2012 Last Update Effective: 4/16 Billing Guideline Background Health First administers benefit packages with full coverage
Subject: Colorectal Cancer Screening Exams (Invasive Procedures) Effective Date: 1/1/2012 Last Update Effective: 4/16 Billing Guideline Background Health First administers benefit packages with full coverage
Post-operative intrapleural chemotherapy for mesothelioma
 Post-operative intrapleural chemotherapy for mesothelioma Robert Kratzke, MD John Skoglund Chair for Lung Cancer Research Section of Heme-Onc-Transplant University of Minnesota Medical School Efficacy
Post-operative intrapleural chemotherapy for mesothelioma Robert Kratzke, MD John Skoglund Chair for Lung Cancer Research Section of Heme-Onc-Transplant University of Minnesota Medical School Efficacy
Colocutaneous Fistula. Disclosures
 Colocutaneous Fistula Madhulika G. Varma MD Associate Professor Chief, Colorectal Surgery University of California, San Francisco Honoraria Applied Medical Covidien Disclosures 1 Colocutaneous Fistula
Colocutaneous Fistula Madhulika G. Varma MD Associate Professor Chief, Colorectal Surgery University of California, San Francisco Honoraria Applied Medical Covidien Disclosures 1 Colocutaneous Fistula
Tracheal Stent System
 nt Tracheal Stent System Available sizes: 08mm x 20mm NEW 08mm x 30mm 08mmx 50 mm 08mm x 60mm 08mm x 70mm 08mm x 80mm 08mm x 90mm 10mm x 20mm NEW 10 mm x 30 mm 10 mm x 50 mm 10 mm x 60 mm 10 mm x 70 mm
nt Tracheal Stent System Available sizes: 08mm x 20mm NEW 08mm x 30mm 08mmx 50 mm 08mm x 60mm 08mm x 70mm 08mm x 80mm 08mm x 90mm 10mm x 20mm NEW 10 mm x 30 mm 10 mm x 50 mm 10 mm x 60 mm 10 mm x 70 mm
SAGES 2015 Flexible Endoscopy Course for Fellows
 Goals and Objectives: At the end of the course, the MIS fellow will be familiar with GI endoscopes, towers, and the instruments used for endoscopy and endoscopic surgery. The fellow will also be able to
Goals and Objectives: At the end of the course, the MIS fellow will be familiar with GI endoscopes, towers, and the instruments used for endoscopy and endoscopic surgery. The fellow will also be able to
Advances in Stent Technology
 Nurse/Technologist Symposium Advances in Stent Technology Antonietta Tolentino, CCRN, MSN, ANP-C Adult Nurse Practitioner, Cardiac Catheterization Laboratory The Zena and Michael A. Weiner Cardiovascular
Nurse/Technologist Symposium Advances in Stent Technology Antonietta Tolentino, CCRN, MSN, ANP-C Adult Nurse Practitioner, Cardiac Catheterization Laboratory The Zena and Michael A. Weiner Cardiovascular
Endoscopic gastric pouch plication - a novel endoluminal incision free approach to revisional bariatric surgery
 Endoscopic gastric pouch plication - a novel endoluminal incision free approach to revisional bariatric surgery Authors: Chiranjiv S Virk, I Michael Leitman and Elliot R Goodman. Location: Beth Israel
Endoscopic gastric pouch plication - a novel endoluminal incision free approach to revisional bariatric surgery Authors: Chiranjiv S Virk, I Michael Leitman and Elliot R Goodman. Location: Beth Israel
ERBEJET 2. The versatility of waterjet surgery: ERBEJET 2 with hybrid instruments WATERJET SURGERY
 ERBEJET 2 The versatility of waterjet surgery: ERBEJET 2 with hybrid instruments WATERJET SURGERY Gentle interventions in surgery and endoscopy Waterjet surgery with hybrid technology Waterjet surgery
ERBEJET 2 The versatility of waterjet surgery: ERBEJET 2 with hybrid instruments WATERJET SURGERY Gentle interventions in surgery and endoscopy Waterjet surgery with hybrid technology Waterjet surgery
By Anne C. Travis, M.D., M.Sc. and John R. Saltzman, M.D., FACG Brigham and Women's Hospital Harvard Medical School Boston, MA
 SMALL BOWEL BLEEDING: CAUSES, DIAGNOSIS AND TREATMENT By Anne C. Travis, M.D., M.Sc. and John R. Saltzman, M.D., FACG Brigham and Women's Hospital Harvard Medical School Boston, MA 1. What is the small
SMALL BOWEL BLEEDING: CAUSES, DIAGNOSIS AND TREATMENT By Anne C. Travis, M.D., M.Sc. and John R. Saltzman, M.D., FACG Brigham and Women's Hospital Harvard Medical School Boston, MA 1. What is the small
Overview of Newer Stent Devices for Aneurysm Treatment
 Overview of Newer Stent Devices for Aneurysm Treatment Randall C. Edgell, M.D. Associate Professor Vascular and Interventional Neurology Saint Louis University Disclosure Outcome adjudication for THERAPY,
Overview of Newer Stent Devices for Aneurysm Treatment Randall C. Edgell, M.D. Associate Professor Vascular and Interventional Neurology Saint Louis University Disclosure Outcome adjudication for THERAPY,
Medical Therapies Limited EGM Presentation
 Medical Therapies Limited EGM Presentation Maria Halasz Chief Executive Officer 5 May 2009 1 Agenda 1. Company information 2. Recent developments 3. Business strategy 4. Key value inflection points for
Medical Therapies Limited EGM Presentation Maria Halasz Chief Executive Officer 5 May 2009 1 Agenda 1. Company information 2. Recent developments 3. Business strategy 4. Key value inflection points for
OHTAC Recommendation
 OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
ESD for colorectal lesions I am in favour. Alessandro Repici, MD Digestive Endoscopy Unit IRCCS Istituto Clinico Humanitas Milano, Italy
 ESD for colorectal lesions I am in favour Alessandro Repici, MD Digestive Endoscopy Unit IRCCS Istituto Clinico Humanitas Milano, Italy Surgery for early colonic lesions 51 pts referred for lap colectomy
ESD for colorectal lesions I am in favour Alessandro Repici, MD Digestive Endoscopy Unit IRCCS Istituto Clinico Humanitas Milano, Italy Surgery for early colonic lesions 51 pts referred for lap colectomy
POEM Procedure for. Esophageal Achalasia
 POEM Procedure for Esophageal Achalasia POEM (Per-Oral endoscopic myotomy) is an incisionless procedure to treat esophageal achalasia, totally performed by endoscopy, without cutting the surface of the
POEM Procedure for Esophageal Achalasia POEM (Per-Oral endoscopic myotomy) is an incisionless procedure to treat esophageal achalasia, totally performed by endoscopy, without cutting the surface of the
Targeting Specific Cell Signaling Pathways for the Treatment of Malignant Peritoneal Mesothelioma
 The Use of Kinase Inhibitors: Translational Lab Results Targeting Specific Cell Signaling Pathways for the Treatment of Malignant Peritoneal Mesothelioma Sheelu Varghese, Ph.D. H. Richard Alexander, M.D.
The Use of Kinase Inhibitors: Translational Lab Results Targeting Specific Cell Signaling Pathways for the Treatment of Malignant Peritoneal Mesothelioma Sheelu Varghese, Ph.D. H. Richard Alexander, M.D.
These parameters cannot, at the present time, be determined by non-invasive imaging techniques.
 Endoscopic Mucosal Resection for Upper Gastrointestinal Lesions Kenneth K. Wang, M.D. Chairman, WEO Publication and Guidelines Committee Professor of Medicine, Mayo Clinic Rochester, Minnesota Upper gastrointestinal
Endoscopic Mucosal Resection for Upper Gastrointestinal Lesions Kenneth K. Wang, M.D. Chairman, WEO Publication and Guidelines Committee Professor of Medicine, Mayo Clinic Rochester, Minnesota Upper gastrointestinal
Endoluminal Bariatric Revision. Todd David Wilson, MD
 Endoluminal Bariatric Revision Todd David Wilson, MD Surgical Endoscopy and the Bariatric Surgeon Preoperative Endoscopy Postoperative Endoscopy Revisional Endoscopy Primary Endoluminal Bariatrics Preoperative
Endoluminal Bariatric Revision Todd David Wilson, MD Surgical Endoscopy and the Bariatric Surgeon Preoperative Endoscopy Postoperative Endoscopy Revisional Endoscopy Primary Endoluminal Bariatrics Preoperative
New treatment options for chronic sinusitis
 New treatment options for chronic sinusitis Balloon Sinuplasty Technology Vishram Jalukar, MD Mason City Clinic ENT & Allergy MKT01014 Rev. D Sinusitis Overview Inflammation of the sinus lining caused
New treatment options for chronic sinusitis Balloon Sinuplasty Technology Vishram Jalukar, MD Mason City Clinic ENT & Allergy MKT01014 Rev. D Sinusitis Overview Inflammation of the sinus lining caused
Endoscopic Submucosal Dissection (E.S.D.) vs. Endoscopic Mucosal Resection (E.M.R.) in Colombia. Advocating E.M.R.
 Controversies in Gastroenterology Endoscopic Submucosal Dissection (E.S.D.) vs. Endoscopic Mucosal Resection (E.M.R.) in Colombia. Advocating E.M.R. Raúl Cañadas Garrido, MD. 1 1 Internist-Gastroenterologist.
Controversies in Gastroenterology Endoscopic Submucosal Dissection (E.S.D.) vs. Endoscopic Mucosal Resection (E.M.R.) in Colombia. Advocating E.M.R. Raúl Cañadas Garrido, MD. 1 1 Internist-Gastroenterologist.
Early Colonoscopy in Patients with Acute Diverticulitis Simon Bar-Meir, M.D.
 Early Colonoscopy in Patients with Acute Diverticulitis Simon Bar-Meir, M.D. Professor of Medicine Germanis Kaufman Chair of Gastroenterology Director, Dept. of Gastroenterology Chaim Sheba Medical Center,
Early Colonoscopy in Patients with Acute Diverticulitis Simon Bar-Meir, M.D. Professor of Medicine Germanis Kaufman Chair of Gastroenterology Director, Dept. of Gastroenterology Chaim Sheba Medical Center,
Management of the new antiplatelets and anticoagulants
 Management of the new antiplatelets and anticoagulants Session No.: 1 Name: C. Boustiere, T Ponchon Guidelines : Anti-thrombotic agents and digestive endoscopy 2006 : French guideline (SFED) 2007 : Japanese
Management of the new antiplatelets and anticoagulants Session No.: 1 Name: C. Boustiere, T Ponchon Guidelines : Anti-thrombotic agents and digestive endoscopy 2006 : French guideline (SFED) 2007 : Japanese
Carbohydrate antigen 19 9 (CA 19 9) (serum, plasma)
 Carbohydrate antigen 19 9 (CA 19 9) (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Carbohydrate antigen 19 9 (CA 19 9) 1.2 Alternative names Cancer antigen 19 9, cancer antigen GI
Carbohydrate antigen 19 9 (CA 19 9) (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Carbohydrate antigen 19 9 (CA 19 9) 1.2 Alternative names Cancer antigen 19 9, cancer antigen GI
Liver Transplantation for Hepatocellular Carcinoma. John P. Roberts, MD Chief, Division of Transplant Service University of California, San Francisco
 Liver Transplantation for Hepatocellular Carcinoma John P. Roberts, MD Chief, Division of Transplant Service University of California, San Francisco Hepatocellular Carcinoma HCC is the 5th most common
Liver Transplantation for Hepatocellular Carcinoma John P. Roberts, MD Chief, Division of Transplant Service University of California, San Francisco Hepatocellular Carcinoma HCC is the 5th most common
BSc in Medical Sciences with GASTROENTEROLOGY AND HEPATOLOGY
 BSc in Medical Sciences with GASTROENTEROLOGY AND HEPATOLOGY Introduction The BSc in Gastroenterology and Hepatology allows a science-based study of the physiology, cell biology, pathology and pharmacology
BSc in Medical Sciences with GASTROENTEROLOGY AND HEPATOLOGY Introduction The BSc in Gastroenterology and Hepatology allows a science-based study of the physiology, cell biology, pathology and pharmacology
Historical Basis for Concern
 Androgens After : Are We Ready? Mohit Khera, MD, MBA Assistant Professor of Urology Division of Male Reproductive Medicine and Surgery Scott Department of Urology Baylor College of Medicine Historical
Androgens After : Are We Ready? Mohit Khera, MD, MBA Assistant Professor of Urology Division of Male Reproductive Medicine and Surgery Scott Department of Urology Baylor College of Medicine Historical
Surgical & Nutritional Complications of Bariatric Surgery: What Every GI Doc Needs to Know Brian R. Smith, MD, FACS Associate Clinical Professor of
 Surgical & Nutritional Complications of Bariatric Surgery: What Every GI Doc Needs to Know Brian R. Smith, MD, FACS Associate Clinical Professor of Surgery & Associate Residency Program Director UC Irvine
Surgical & Nutritional Complications of Bariatric Surgery: What Every GI Doc Needs to Know Brian R. Smith, MD, FACS Associate Clinical Professor of Surgery & Associate Residency Program Director UC Irvine
DEVELOPING AND TESTING RETRIEVABLE DEVICES AND SCAFFOLDS FOR BETA CELL REPLACEMENT THERAPIES
 JDRF REQUESTS LETTERS OF INTENT FOR: DEVELOPING AND TESTING RETRIEVABLE DEVICES AND SCAFFOLDS FOR BETA CELL REPLACEMENT THERAPIES BACKGROUND & PURPOSE One of JDRF s therapeutic goals is to restore beta
JDRF REQUESTS LETTERS OF INTENT FOR: DEVELOPING AND TESTING RETRIEVABLE DEVICES AND SCAFFOLDS FOR BETA CELL REPLACEMENT THERAPIES BACKGROUND & PURPOSE One of JDRF s therapeutic goals is to restore beta
LIVER CANCER AND TUMOURS
 LIVER CANCER AND TUMOURS LIVER CANCER AND TUMOURS Healthy Liver Cirrhotic Liver Tumour What causes liver cancer? Many factors may play a role in the development of cancer. Because the liver filters blood
LIVER CANCER AND TUMOURS LIVER CANCER AND TUMOURS Healthy Liver Cirrhotic Liver Tumour What causes liver cancer? Many factors may play a role in the development of cancer. Because the liver filters blood
MU Inpatient Consult Rotation
 MU Inpatient Consult Rotation I. Description of Rotation and Educational Goal: This is a four-week rotation in which GI fellows gain exposure and acquire expertise in the evaluation and management of adult
MU Inpatient Consult Rotation I. Description of Rotation and Educational Goal: This is a four-week rotation in which GI fellows gain exposure and acquire expertise in the evaluation and management of adult
Preliminary Program 9th EDS Postgraduate Course Riga, Latvia May 14-16, 2015
 Preliminary Program 9th May 14-16, 2015 Venue: Riga city Bellevue Park Hotel Riga, Slokas iela 1, Rīga, Latvia Thursday, May 14, 2015 Time 14:00-14:20 Workshop: Evidence Based Medicine (limited to 35 participants)
Preliminary Program 9th May 14-16, 2015 Venue: Riga city Bellevue Park Hotel Riga, Slokas iela 1, Rīga, Latvia Thursday, May 14, 2015 Time 14:00-14:20 Workshop: Evidence Based Medicine (limited to 35 participants)
