esubmission Guidelines
|
|
|
- Claribel Lewis
- 10 years ago
- Views:
Transcription
1 esubmission Guidelines New ways of working at FAMHP Version 2.11 Publication date: 2/12/2011 Implementation date: 2/12/2011
2 DOCUMENT HISTORY Date Author Version Comments 14/05/07 André Lhoir 2.6 Updated version xx/02/08 Ann Verhoye 2.7 Updated in order to add necessary information in view of the NeeS guideline 01/07/09 Ann Verhoye 2.8 Updated in order to comply to current European guidance and new mail size limit 01/05/2010 Ann Verhoye 2.9 Updated in order to comply to better regulation 07/07/2011 Ann Verhoye 2.10 Updated in order to comply to new ectd and NeeS validation rules 14/11/2011 Ann Verhoye 2.11 Updated in order to reflect EU requirements and in order to reflect validation reports to be saved in the workingdocuments folder. 2/31
3 Table of Content 1. Introduction Important reminder Glossary Different types of e-submission dossiers identified by the FAMHP... 8 A. ectd or electronic Common Technical Document... 8 B. Non-eCTD electronic Submission (NeeS) How to submit your dossier: ectd Directory structure and naming conventions Which communication channel is most appropriate for my esubmission? A B. CD-ROM/DVD: /31
4 1. Introduction One of the conclusions of the Business Process Reengineering project that was conducted by the DGMP in , was to implement a new back-office and case management system with document and workflow management capabilities in order to: - Automate administrative processes and improve the operational efficiency of the NCA - Ameliorate case management and decision-making processes - Eliminate paper handling (storage, copying, transportation, confidential shredding, etc.) - Increase communication and knowledge transfer - Enabling access to a centralized data model in line with European legislation - Further improve the quality carried out by the NCA - Build an electronic interface to industry stakeholders for electronic applications and communications This document completes and clarifies the previous Guidelines Ver. 1.0 and all FAQs already published. This document supersedes previous versions. 4/31
5 2. Important reminder The applicant is fully responsible for the correctness and completeness of the submitted file within any type of e-dossier submitted to the FAMHP. To this end the applicant must ensure that the submitted file will comply to the full compliance requirements as laid down in the full compliance road map, published on the FAMHP website and updated on regular basis. (full compliance webpage link: Moreover, the applicant needs to submit the following two statements: 1. A declaration about the content of the proposed leaflets (SmPC, PIL) & Labelling 2. The conformity statement of translation. (1) The applicant is responsible for the conformity of the national leaflets (SmPC and PIL) and the national labelling according to the file (registration or variation). As for the variations or files which cause change in the leaflets or labelling, the applicant has to declare that the content of the proposed leaflets and labelling is identical to the approved version and that the proposed changes only reflect the content of : 1. the previously submitted MRP variations type IA and IB, already approved by the RMS and/or the previously submitted national variations type IB for which the timeframes for implementation have passed, and the previously submitted type IA and administrative variations 2. if a MRP dossier is concerned: the previously submitted variations type II and notifications under Art. 61(3) (Directive 2001/83/EC) already approved by the RMS 3. if a NP dossier is concerned: the previously submitted variations type II and notifications under Art (RD ) already approved by the FAMHP (i.e. receipt of the round up mail) and notifications under Art (RD ) for which the timeframes for implementation have passed. 4. submitted file. For European procedures, this declaration should either be submitted at the closing of the variation. It is recommended to include this declaration in the cover letter. (2) The applicant should mention the intention to use the conformity statement on translation of SmPC into two national languages (Dutch and French), and PIL and labeling into the three national languages (Dutch, French, German) in the cover letter. A template of this conformity statement is available on the website. The conformity statement itself has to be submitted together with the translations at the end of the procedure. 5/31
6 3. Glossary Please find below a list of abbreviations and key words used in this Guidelines document: Key word or abbreviation AC AMM CHMP CP CTD DCP EAF ECTD EMA esubmission system EUDRALINK FAMHP FDA ICH MRP NCA NeeS NP Paper dossier PIL SmPC Tree structure Explanation or definition Administrative Change Autorisation de Mise sur le Marché (Marketing Authorisation) Committee on Human Medicinal Products of the EMA Centralised Procedure Common Technical Document (Document standard developed by ICH and supported by EMA) Decentralised Procedure electronic Application Form The electronic CTD European Medicines Agency ( The new document and workflow management system which is implemented at FAMHP that manages the electronically submitted applications for new drug registrations, variations, renewals, etc. System developed by EMA for secure electronic transfer of files Food and Drug Administration International Conference on Harmonisation ( The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) is a unique project that brings together the regulatory authorities of Europe, Japan and the United States and experts from the pharmaceutical industry in the three regions to discuss scientific and technical aspects of product registration. Mutual Recognition Procedure National Competent Authority Non-eCTD electronic Submission National Procedure The hard copy (paper copy) of a drug application Patient Information Leaflet Summary of Products Characteristics The term tree strcuture as used in this document indicates the sequence folder 6/31
7 XML and all underlying folders and documents. Extensible Markup Language (XML) is a simple, very flexible text format derived from SGML (ISO 8879). Originally designed to meet the challenges of large-scale electronic publishing, XML is also playing an increasingly important role in the exchange of a wide variety of data on the Web and elsewhere. 7/31
8 3. Different types of e-submission dossiers identified by the FAMHP The FAMHP has defined two types of dossiers for electronic submission. A. ectd or electronic Common Technical Document This is a standard agreed by the ICH and endorsed by the CHMP. Regarding Module I EU specifications are requested. It consists of 3 main elements: The electronic Application Form (eaf) in XML or PDF or RTF The directory structure, a tree structure with all the required documents for registration based on the CTD structure standard. an XML file ( XML backbone ) containing valuable and structured information on all documents in the dossier (e.g. meta data such as versioning information, check sum, table of contents, etc.). This format allows the FAMHP to import and classify the registration dossier. As it contains versioning data on the documents, it enables the FAMHP to manage different document versions (modified documents, unmodified documents, deleted documents, etc.). The ectd specification is available on (CTD modules 2 to 5) and for the European specifications (CTD Module 1) and more specifically for the application forms at There are ectd software tools on the market that can help the pharmaceutical industry to view and/or create ectd dossiers. Please pay attention to the following remarks: As the ectd format evolves it is important that the pharmaceutical industry respects the last ectd specification. The applicable ectd specifications version is always the current version. The XML versions applicable are: - For Modules 2 to 5: ich-ectd- DTD (current version) - For EU Module 1: EU-index. DTD (current version) - For the new application form: EU-application. DTD (current version ) and XSL version - For the variation form: DTD current version 8/31
9 Attention points when submitting an XML file When submitting an XML file (Application form) please be sure to add the DTD and the XSL properly linked to the XML file. Both DTD and XSL file are to be present in the UTIL directory. To be sure to have the correct information which help us when downloading the XML Form, please copy either the complete Util directory or the both mentioned files within an Util directory. Please also read point 4 described below for NeeS, this is applicable for ectd submissions as well!!!! B. Non-eCTD electronic Submission (NeeS) The FAMHP currently prefers the NeeS format. When an ectd is submitted, which is the recommended format in the near future, please assure that the ectd is backwards compatible with the NeeS format, meaning adherence to folder and filenaming convention which is still Best Practice. This type of electronic submission should comply to the recommendations as listed in the Guidance for Industry on Providing Regulatory Information in Electronic Format: Non-eCTD electronic Submissions (NeeS) current version. Notes: 1. the NeeS submission may not contain word documents. All documents requested by the FAMHP in word format (product information related documents and AMMs) are to be submitted at time of dossier submission /dossier closing (this is after the European phase for MRP and DCP procedures). At submission of the file PDF format of PI documents and AMMs need to be included in the NeeS. If you submit word documents (cfr translations of PI in case of IA variation), please gather them in a folder named sequence numberworkingdocuments (no hyphen), separated from the tree structure and mention this clearly in your cover letter. (see also nice to know point 5). The word documents required for the closing of files falling within the scope of the project change MAH (see circular letter 542, annex 7) have to be present at time of the original submission in this workingdocuments folder. deleted. 9/31
10 2. the TOCs as mentioned in the NeeS guideline are not required by the FAMHP. When the TOCs are included in the submission, they are accepted provided the naming convention is followed. 3. the sequence folder of the NeeS should be named The folder on top of the sequence folder ( 0000 ) should state the product specific variation procedure number prefix which is defined as follows: cc-h-xxxx-yy cc: country code or nat for national procedures xxxx: group counter yy: product counter Below this folder level one tree structure can be submitted.!!!always use - instead of / when indicating the procedurenumber in the intermediate level folder. No spaces are allowed in the procedurenumber! Caps cannot be used, the TIGes Harmonised Guidance for Non-eCTD electronic Submissions (NeeS) for human medicinal products in the EU version 3.0 mentions small letters for the procedure number in it s examples. Example Worksharing fr-h ema-h-c ema-h-c be-h be-h /31
11 In this example the submission concerns a worksharing procedure concerning different medicinal products: Top folder: prefix product specific variation procedure number: e.g. if be-h NeeS: the only tree structure allowed in the intermediate folder is applicable to all strengths/pharmaceutical forms of the specifc medicinal product. In this case, the one tree structure contained in it should concern products 01, 02, 03, 04 and 05 belonging to the medicinal group ectd: If an ectd submission is concerned, and lifecycle management allows to create one top folder for all strengths/pharmaceutical forms of a specifc medicnal product, then this is to be followed. Only in the cases where the lifecycle management requires one tree structure per strength/pharmaceutical form of a medicinal product, the top level folders are to be defined on that level. For example: if an ectd lifecycle exists for the x mg strength of Product P and a separate ectd exists for the y mg strength of product P then for each strength a seperate intermediate folder and corresponding ectd sequence is to be submitted. be-h be-h It is recommended to clearly mention in the cover letter which product data can be found in the different submitted tree structures. It is evident that the cover letter should mention that a worksharing or a grouping is concerned. Medicinal product (name +strength+form) 1: all info in fr-h Medicinal product (name +strength+form)2: all info in ema-h-c Medicinal product (name +strength+form) 3: all info in ema-h-c Medicinal product (name +strength+form) 4 (= product counter 01): all info in be-h Medicinal product (name +strength+form) 5 (= product counter 02): all info in be-h Medicinal product (name +strength+form) 6 (= product counter 03): all info in be-h Medicinal product (name +strength+form) 7 (= product counter 04): all info in be-h Medicinal product (name +strength+form) 8 (= product counter 05): all info in be-h Medicinal product (name +strength+form) 9: all info in be-h /31
12 Example grouping fr-h fr-h fr-h fr-h fr-h The explanation as mentioned under the example worksharing is valid for the grouping example as well. Please note that one CD/DVD/mail should be used per submission, if seperate variations and groupings are submitted simultaneously different CD/DVD/mail are required. Nice to know 1. Where to download the application form and get information on how to fill it? For new registrations, variations and European Renewals: For Art. 61(3) notifications and Art notifications: For National Renewals and parallel import dossiers the application forms as published on the FAMHP website (medicines formulieren/formularies, dutch and French version of website only) are to be used. 12/31
13 2. Are all non- volume based PDF formats accepted? No, only those which comply with section and annex I of the Guidance for Industry on Providing Regulatory Information in Electronic Format: Non-eCTD electronic Submissions (NeeS). Only PDF format 1.4 or higher will be allowed. As soon as the submission contains one pdf file of a lower version, the submission is rejected. 3. What about dossiers concerning several strengths/pharmaceutical forms of 1 medicinal product? ectd It is encouraged to submit one ectd for several strengths, all application forms can be listed in the 12- form/cc folder. The top folder, indicating the product specific procedure number is required. This foldernaming should clearly indicate if different forms or strength of a medicinal product are concerned by the submission. NeeS When submitting a dossier for different strengths/pharmaceutical forms of a medicinal product, 2 options are available : Option 1: applicable for new registrations Top folder: indicating the product specific procedure number. One tree structure for all concerned strengths/pharmaceutical forms of the concerned medicinal product. - Cover Letter should clearly specify the different forms or strengths of the concerned medicinal product. - The tree structure contains an overall retribution form. - The tree structure contains all information which is valid for all strengths and forms of the concerned medicinal product. - Empty folders can be deleted. - All application forms are to be in the 12-form/cc folder of the tree structure. Option 2: applicable for variations concerning different strengths/pharmaceutical of a specific medicinal products (grouping and work sharing excluded for this option) One tree structure is submitted including all information for all concerned strengths/pharmaceutical forms of the medicinal product. The top folder indicates the product specific variation number. The cover letter clearly states the strengths and pharmaceutical forms of the medicinal product involved by the dossier. The option of complex can not be used in combination with the option grouping or worksharing. 4. What about the need for signatures on the esubmitted dossier? A. Signature on cover letter: 13/31
14 Each submitted dossier needs to be foreseen of a handwritten signature (signed AND printed/scanned+ocr d cover letter) when submitted by mail/eudralink/cd-rom/dvd.! It is allowed to submit the cover letter in two-fold, as follows, if necessary: 0000\m1\eu\10-cover\be-cover.pdf = scanned image of the cover letter 0000\m1\eu\10-cover\be-cover-text.pdf = pdf text searchable document build directly from the initial Word document (without company header and signature) B. Signature on the application form: A signature on the application form is required for all dossiers where Belgium acts as RMS. 14/31
15 5. What about the submission of word files? If word files are to be submitted, please submit them out of the CTD-tree structure, within a separate folder, and mention the fact of submitting a separate word file folder in the cover letter of your submission. The folder should be named sequence number - workingdocuments (sequence number = 4 digits; no hyphen) in accordance with NeeS requirements. One such folder per submission ( one full application, one grouping,.! Several single variations are not considered to be one submission.) is allowed. For new registrations it has to be submitted out of all tree structures. For groupings and worksharings, and complex dossier it needs to be submitted out of the intermediate folders indicating the procedurenumbers, as indicated in the example below (option 1 1 overall working document folder, next to the folders above the sequencefolders, for the complete submission. Please use descriptive filenames! In case of groupings or different procedurenumbers involved the filename 0000-workingdocuments will be allowed.). However in order to comply to EU validation rules for NeeS submissions, the working document folder might also be a subfolder of the procedurenumber folder (option 2 1 working document folder next to each submitted sequencefolder): Option 1 (one submission concerned): Option 2 (one submission concerned): 15/31
16 6. What about esubmission in view of the better regulation ( A. The ectd submissions should follow the implementation timeline for the EU M1 1.4 ectd specifications: From the date of on: M1 v1.4: any European procedure that contains grouped variations or is subject to a worksharing agreement M1 v1.3: other applications From the date of on: M1 v1.4: all ectd submissions for all European procedures In general, once M1 v1.4 used, it is applicable to all future sequences of the ectd lifecycle! B. NEES submissions: For submission of grouped variations or variation in view of worksharing, one NEES tree structure per medicinal product group (= all strengths and forms of a specific medicinal product) is requested. This means that when introducing a group variations concerning different strengths or forms of a specifc medicinal product only one tree structure needs to be prepared. Create the necessary top folders on medicinal product level indicating the procedurenumber. For grouping and worksharing an overall, retribution form and application form is used, and should be present in each tree structure of the submission. It is recommended to detail the way of proceeding used to calculate the total fee. 16/31
17 NeeS New application concerning more than one strength/pharmaceutical form of a medicinal product: One Top folder: Always use one folder for all strengths/pharmaceutical forms of the medicinal product indicating the procedure number. ectd New application concerning more than one strength/pharmaceutical form of a medicinal product: (1) One top folder. Indicating the procedure number for all strengths/forms of the concerned medicinal product. One overall tree structure for the concerned strengths/pharmaceutical forms of the medicinal product. Example see left side. (2) Several top folders: one top folder per strength/pharmaceutical form of a specific medicinal product indicating the procedure number. One tree structure per strength/pharmaceutical form of the medicinal product per top folder. Example: NeeS new registration for three strengths of a specifc medicinal product. Example for ectd if life cycle management requires strength/pharmaceutical form specific tree structures.
18 NeeS: Variations concerning more than one strengths/pharmaceutical form of a medicinal product: 1. No grouping or worksharing is concerned Is more than one medicinal product concerned? YES: not accepted NO: Top folder: product specific variation number One tree structure covering all concerned strengths/pharmaceutical forms of the medicinal product. Example below: ectd: Variations concerning more than one strength/pharmaceutical form of a medicinal product: 1. No grouping or worksharing is concerned Is more than one medicinal product concerned? YES: not accepted NO: Top folder: product specifc variation number. One tree structure covering all strengths/pharmaceutical forms of the concerned medicinal product. Example see left side. OR *(2)Top folder per strength/pharmaceutical form indicating the Procedure number. One tree structure per strength/pharmaceutical form of the medicinal product. Example below: 18/31
19 1.2. NeeS: A grouping or worksharing is concerned One top folder for all strengths/pharmaceutical forms of each concerned medicinal product indicating the product specific variation procedure number One tree structure per the top folder. Example: grouping submission valid for five medicinal products, mention the concerned strengths/pharmaceutical forms of the medicinal product in the top folder name ectd: A grouping or worksharing is concerned *(1) One top folder for all strengths/pharmaceutical forms of each concerned medicinal product indicating the product specific variation procedure number : e.g. be-h One tree structure per top folder. Example see left side. *(2) One top folder per strength/pharmaceutical form of each concerned medicinal product indicating the product specific variation procedure number prefix. One tree structure per top folder. (* The choice is based on the LCM: if sequence 0000 was started with different tree structures per strength/pharmaceutical form, then option (2) is to be used, if it was started with one overall tree structure for all strengths/pharmaceutical forms of the medicinal product the option (1) is to be chosen.) Example below: Example see next page. Example see next page. 19/31
20 20/31
21 Note concerning annual reports: In an ectd, delaying the submission of type IA s for the annual report might seriously affect the life cyclemanagement of further sequences. As type IA s are issued in the FAMHP with an automated , applicants who prepare their submissions in the ectd format may be advised to submit the type IA s as soon as they are ready and not to collect them in an annual report. However this decision is left to the applicant. 21/31
22 4. How to submit your dossier: ectd Directory structure and naming conventions From 05/02/07, each electronic dossier concerning a human medicinal product and submitted to the FAMHP, is subject to two technical verifications. From September 2011, the technical validation rules which will be applicable are: (1) compliance towards the ectd tree structure for both NeeS and ectd submissions (2) compliance towards folder and file naming for NeeS submissions! Please remark that respect of folder and filenames is considered to be best practice for ectd submissions meaning that the applicant should be prepared to include justification for any Best Practice criteria, such as folder and filenaming, which are not met in the submission cover letter/reviewer's guide. This is called full compliance. NeeS submissions 1) For NeeS submission for which BE acts as RMS: instead of the Belgian checker NeeS report the Extedo actual version or the Lorenz NeeS actual version report needs to be included in the submission, this allows a 100% compliance check which will be valid in all concerned MS. Please file this validation report in the working documents folder. Even if you need to submit no word files, you still need to create the sequencenumber-workingdocuments folder in order to save the validation report within. If you choose option 1 as mentioned on page 15 of this guideline please use descriptive filenames for the validation reports of the different tree structures submitted, so the FAMHP can easily identify the tree structure reflected within each validation report. 2) For other submissions (where BE do not acts as RMS): Note that the Best report is now called NeeS report. Both technical verifications are based on the requirements described in the Notice to Applicants for module 1, and modules 2 to 5 (ICH requirements). For both technical verifications, a score is given to the dossier. This score will be included in the socalled NeeS report, which will be included in the mail sent to you after the dossier is uploaded in our system. This NeeS report will provide details on the errors concerning both the ectd structure and the folder/file naming. "full compliance" has been implemented in three stages : 1. adaptation stage (1/2/ /3/2007) 2. self-testing stage (27/3/2007 end june 2007)
23 3. start (September 2007) The score to which your dossier must comply, as far as both technical verifications are concerned, is determined by a road map. ( dex.jsp) It is available on our website. It mentions the minimum score for each technical verification, to which your dossier must comply to be accepted by the FAMHP. If one of the minimum scores is not obtained for your dossier, the NeeS report will be returned to you mentioning the rejection of your dossier. In that case, the dossier need to be resubmitted to the FAMHP and an administrative fee needs to be paid for the rejected dossier. If both minimum scores were obtained for your dossier, the NeeS report will be returned mentioning that the submission of your dossier was accepted by the FAMHP. Please consult the road map regularly before submitting your dossier in order to be aware of the latest updated information regarding minimumscores. If you use the checker available on our website before submitting the dossier, the NeeS report will automatically be placed outside/besides the NeeS structure. (*) ectd submissions For each ectd tree structure contained in the submission the Lorenz actual version, or Extedo actual versions of a valid ectd validation report must be included in your submission. A valid ectd validation report means that all Pass/Fail criteria are met. Absence of a valid validation report with all Pass/Fail criteria which are met means invalidation of the submission. The validation report must be made in presence of all preceding sequences and the new sequence you are to submit (*). Please name these reports ectd report and store them in the working documents folder. The Belgian NeeS checker will not be used for ectd s by the agency. The applicant should provide one ectd report per submitted ectd sequence. When clicking on sequencenumber-workingdocuments folder: The Extedo PDF version report or the Lorenz main html report should always be saved in the working documents folder. Even if you need to submit no word files, you still need to create the sequencenumber-workingdocuments folder in order to save the validation report within. There is no technical validation on the filename of the report. 23/31
24 (*)When launching the technical validation within Extedo or Lorenz be sure that the sequence to be submitted and all previous sequences are present within the ectd validator. On 27 march 2007 the FAMHP gave an infosession regarding full compliance, during which the CTD tree structure (NeeS Report) was discussed as well. The CTD structure including naming conventions can be consulted via our website: The full compliance webpage offers all necessary information for a correct esubmission: Which type of dossier is included in the esubmission full compliance (see further) requirement? All dossiers (new applications, variations, renewals) concerning human products (including products derived from herbals, excluding homeopathic products and parallel import) as far as they follow the national procedure, the MRP or DCP are included in the esubmission full compliance requirement. Dossiers concerning human products submitted by CP need to be submitted in ectd format as well, and should comply to the requirements mentioned here above (*) on the same page. Dossiers concerning veterinary products are excluded at this moment. PSURs are excluded. Response files are excluded as well except for response files for BE RMS dossiers submitted after 1/1/2012, these are to be fully compliant in compliance with EU requirements. ASMF can be submitted in electronic format, either in ectd or in NeeS. ASMF is out of scope of the technical validation. The content of module I such as it should be submitted including the FAMHP good practice remarks (in italics) is given below. Module 1 Please delete empty folders. 1.0 Cover Letter - The cover letter should be the original signed Cover Letter. The cover letter should be printed AND scanned+ocr d. The cover letter should be text searchable as indicated in the Guidance for Industry in Electronic Format: Non-eCTD electronic Submissions (NeeS). - Mention the dossier ID if known. - The 2 statements as listed on page 5 are to be retaken. - The cover letter (for both national and European procedures) should be in compliance with the Member States recommendation on the Cover Letter for new applications submitted through the MRP/DCP as published on the CMD website: 24/31
25 (the document can be found at the bottom of the page) Moreover, the templates for the cover letter for new applications in MR and DC procedures as published on the same web pages are to be used. For variations a template for the cover letter is now available on: Be sure to mention related variations. It is highly recommended to use the template of the variation cover letter since the template includes all necessary information to identify the application correctly and to support the validation The cover letter may be edited in English for national procedures as well. 1.1 Comprehensive Table of Contents The table of contents is allowed but is not required for the agency. If you decide to submit a ToC anyway, please take into account the following issues: - Include a Table of Contents with hyperlinks, if possible, to the related document in the respective ectd modules. When submitting a NeeS ensure that all the paths in the links are relative when building the hyperlink, ie: use /m1 instead of /0001/m1 - Make sure all filenames are clear and logical and respect the ectd nomenclature 1.2 Application Form - Application form should be stored in the be folder. - Annexes should be stored in the Common folder if applicable to more than one country for MRP. The annexes should be stored in the BE folder for NP. Annexes are: e.g ticked guideline, GMP-certificate, proof of establishment,dossier description, justification of type IA, other. For the application form itself the naming convention is to be followed (ex be-form.pdf), for the annexes a descriptive filename without hyphens or spaces should be added as a variable component (ex be-form-proofofpayment.pdf, common-formpheurcertificate.pdf, common-form-tickedguideline) - Always use the AF annex for the proof of payment (5.1 in case of new MAA). - For new registrations, one application form per medicinal product strength is expected - For grouping and worksharing one overall application form should be used. All tree structures involved by the grouping or worksharing should include the overall application form. - Signature on the application form is mandatory in case Belgium acts as RMS. - The Application form refers to any form (new applications, applications for variations or renewals). Always use the country directory at this level for all procedures even when only one file is submitted to only one country during the life cycle of the submission. - Delete empty folders. - The application form should be text searchable as recommended in the Guidance for Industry in Electronic Format: Non-eCTD electronic Submissions (NeeS). 25/31
26 1.3 Product Information SmPC, Labelling and Package Leaflet - The proposed European SmPC, PIL and labelling are to be included in the common subfolder for European dossiers. The proposed national SmPC, package leaflet and labelling are to be included in the be subfolder. - Please note that for type IA, IB and administrative variations affecting the Product Information all national translations should be present at the initial variation application, but the national proposals (European dossier)/translations (national dossier) for type II variations and renewals may be submitted at the end of the procedure. Word versions of translations at submission are to be included in the sequence number- workingdocument s folder. - The proposed labeling text should include braille in normal text format (this is in compliance with NtA requirements). - Please also check the file Note to finalise SPC-PIL-labelling (.WORD) as published on our website: x.jsp - The submitted electronic file should only contain pdf versions of the PI documents. The word versions of these documents are to be submitted at time of dossier closing (this is after the European phase for MRP and DCP procedures).if your word files are submitted at time of submission of your dossier please submit them in a separate word file folder, next to the tree structure.(see also nice to know point 5) Mock-up - The proposed mock-up should visualise braille using dots (this is in compliance with NtA requirements) Specimen Consultation with Target Patient Groups - Subfolder: be for national procedures, include here readability testing results. - Subfolder common : for European procedures include here readability testing results Product Information already approved in the Member States - The approved national SmPC, PIL (= version attached to most recent AMM version in your possession) and labelling have to be included here. Moreover, the last approved European final SmPC, harmonised PIL and labelling have to be included here as well Braille 1.4 Information about the Experts Quality Non-Clinical 26/31
27 1.4.3 Clinical 1.5 Specific Requirements for Different Types of Applications Information for Bibliographical Applications Information for Generic, Hybrid or Bio-similar Applications (Extended) Data/Market Exclusivity Exceptional Circumstances Conditional Marketing Authorisation 1.6 Environmental Risk Assessment Non-GMO GMO 1.7 Information relating to Orphan Market Exclusivity Similarity Market Exclusivity 1.8 Information relating to Pharmacovigilance Pharmacovigilance System Risk-management System 1.9 Information relating to Clinical Trials 1.10 Information relating to Paediatrics Responses to Questions - This folder is divided in CC folders.!be aware that the Guidance for Industry on Providing Regulatory Information in Electronic Format: Non-eCTD electronic Submissions (NeeS) requests responses to be introduced respecting the CTD tree structure of the original submission. - Currently, the FAMHP will not perform full compliance testing on responses to questions. Additional Data - Besides AMM and derogation forms the additional folder can be used for other documents that do not really fit in any other section (ex: list of dispatch dates for BE RMS files, transfer agreement, declaration of conformity of translations (The declaration of conformity is always to be submitted given that the SmPC is needed in Dutch and French and the PIL/labeling is needed in Dutch, French and German at the end of the procedure), ). - Include both the approved AMM and the proposal AMM. Note that submission of an AMM proposal is mandatory, if changes to the AMM are a consequence of the submitted dossier. 27/31
28 - This folder is divided in CC folders. - Please make sure that this folder only contains pdf format. The word format of these documents if requested by the FAMHP (e.g. word version of AMM is required) needs to be submitted at time of dossier closing (this is after the European phase for MRP and DCP procedures). If your word files are submitted at time of submission of your dossier please submit them corresponding to the requirements mentioned nice to know point 5.! For the project change MAH the following documents should be given within the folder additional data : AMM, transfer agreement, declaration of conformity of translations, declaration that no other changes have been included in the SmPC/PIL/labeling (if not already included in the cover letter) General remark regarding the use of CC and common subfolders: NP: always use BE folder MRP: use national country code folder for country specific documents, and use common folder for documents valid for ALL involved countries DCP: use the common folder only 28/31
29 5. Which communication channel is most appropriate for my esubmission? The following communication channels will be allowed for submitting electronic registration dossiers to the FAHMP. A. For NP, DCP, MRP registration dossiers as well as any type of correspondence related to the dossiers, s up to a size of 5,00 MB will be allowed. Please compress or zip the file. The general address for electronic dossier submission is [email protected] except for - PSUR for which the general address for electronic dossier submission is [email protected] (PSUR is not concerned by the FAHMP full compliance - CP dossiers where Belgium acts as Rapporteur or Co-rapporteur for which the general address for electronic submission is: [email protected] Do not forget to submit all sequences (including variations, ) for CP where FAMHP acts as (Co)- Rap. (CP is not concerned by the FAHMP full compliance ) ectds containing XML need to be submitted with Eudralink instead of en attachment. The general address for correspondence/additional data/post-approval commitments concerning an (ongoing) dossier is [email protected]. National documents (word version of PI documents in national languages, final AMM proposals) need to be submitted to [email protected].!!! Pay attention to the following: 1. The mail subject always needs to mention at least: (all fields should be separated by - ) 1. Type of procedure (MRP, DCP, NP) 2. Type of dossier (type IA, IB, AC, IInc, IIc, R (renewal), new, E (repeat-use), PSUR, P (notification)) 3. The name of the medicinal product in Belgium 4. Dossier ID (if known) 2. The content of the cover letter should be in the body of the mail. 29/31
30 3. Dossiers in a CTD directory structure that are sent via need to be compressed 1 ( zipped with WinZip) so that the relative ectd folder structure is respected. - If you are using a different compression software than WinZip, please mention it in the Cover Letter. - Do not use password protection, in line with the Guidance for Industry on Providing Regulatory Information in Electronic Format: Non-eCTD electronic Submissions (NeeS) Please use the ectd directory structure as it is defined in our empty folder structure tool. (published on the FAMHP website: Wonderpil CTD boomstructuur (.ZIP) on A compressed or zipped folder can keep its directory structure thanks to a function of WinZip (see below): a. First compress ( zip ) the dossier folder by using the WinZip program (see below) b. Then check the Option Save full path info. This will store the whole folder structure information. c. Verify the result in the compressed ( zipped ) archive d. Send the compressed archive via to FAMHP (only NeeS and size smaller than 5,00MB) 4. Eudralink (EMA): This option could be used to submit electronic dossiers to the FAMHP ( Dispatching EudraLink [email protected] ). This solution is a secure solution and allows to submit larger files. This option may also be used to submit additional info to the gestion team. As mentioned earlier, ectd submissions cannot be sent as en attachment as the filter does not allow it. Please use Eudralink in these cases always use the 90 day expiry date. The use of systems as YouSendIt will not be refused by the FAMHP on condition (i) that their use does not require a password and (ii) all responsibility is declined. However, the FAMHP stresses that the use of Eudralink is strongly preferred for security reasons. B. CD-ROM/DVD: When the size of the dossier exceeds 5.00 MB and is lower then 40,00 MB submission via mail is not supported, but Eudralink is still an option. When the size of the registration dossier exceeds the size of 40,00 MB, the pharmaceutical company needs to submit the dossier on a CD-ROM or DVD which needs to be shipped to the FAHMP (for DVD s all standards are accepted). 30/31
31 Dispatching unit!!! Pay attention to the following: 1. Please don t forget to print the signed cover letter and put it in the envelop when mailing the CD/DVD. 2. Do not use password protection, in line with the Guidance for Industry on Providing Regulatory Information in Electronic Format: Non-eCTD electronic Submissions (NeeS) 3. Do not send parts of dossiers by and parts on CD/DVD. 31/31
Harmonised Technical Guidance for ectd Submissions in the EU
 Guidance for Industry on Providing Regulatory Information in Electronic Format Harmonised Technical Guidance for ectd Submissions in the EU Version 3.0 August 2013 Page 1 of 60 Table of Contents Table
Guidance for Industry on Providing Regulatory Information in Electronic Format Harmonised Technical Guidance for ectd Submissions in the EU Version 3.0 August 2013 Page 1 of 60 Table of Contents Table
Bundesinstitut für Arzneimittel und Medizinprodukte Step-by-step Guide on e-only Submission at BfArM
 Step-by-step Guide on e-only Submission at BfArM Version 2.1 Date: December 30, 2014 Conditions for e-only submissions Electronic format only in ectd or NeeS regardless of whether submission concerns a
Step-by-step Guide on e-only Submission at BfArM Version 2.1 Date: December 30, 2014 Conditions for e-only submissions Electronic format only in ectd or NeeS regardless of whether submission concerns a
EMA esubmission Gateway: Questions and answers relating to practical and technical aspects of the implementation
 28 February 2014 EMA/609325/2011 EMA esubmission Gateway: Questions and answers relating to practical and technical aspects of the implementation This question and answer document aims to address the commonly-asked
28 February 2014 EMA/609325/2011 EMA esubmission Gateway: Questions and answers relating to practical and technical aspects of the implementation This question and answer document aims to address the commonly-asked
Questions & answers on signal management
 23 October 2015 EMA/261758/2013 Inspections and Human Medicines Pharmacovigilance Division This document addresses a number of questions which stakeholders, in particular marketing authorisation holders
23 October 2015 EMA/261758/2013 Inspections and Human Medicines Pharmacovigilance Division This document addresses a number of questions which stakeholders, in particular marketing authorisation holders
Volume 2B Notice to Applicants. Medicinal products for human use. Presentation and format of the dossier. Common Technical Document (CTD)
 Volume 2B Notice to Applicants Medicinal products for human use Presentation and format of the dossier Common Technical Document (CTD) Introduction Edition June 26 Module 1 Edition May 28 Module 2 Edition
Volume 2B Notice to Applicants Medicinal products for human use Presentation and format of the dossier Common Technical Document (CTD) Introduction Edition June 26 Module 1 Edition May 28 Module 2 Edition
Regulatory approval routes in the European System for Medicinal Products
 Regulatory approval routes in the European System for Medicinal Products Cardiovascular Combination Pharmacotherapy Global Summit, Melbourne, 8 th May 2014 Presented by: Kevin Blake Human Medicines Research
Regulatory approval routes in the European System for Medicinal Products Cardiovascular Combination Pharmacotherapy Global Summit, Melbourne, 8 th May 2014 Presented by: Kevin Blake Human Medicines Research
9th DGRA Annual Congress
 Bundesinstitut für Arzneimittel und Medizinprodukte 9th DGRA Annual Congress Electronic Regulatory Submission from the Point of View of the National Drug Agencies - Germany Dr. Klaus Menges, BfArM 13.06.2007,
Bundesinstitut für Arzneimittel und Medizinprodukte 9th DGRA Annual Congress Electronic Regulatory Submission from the Point of View of the National Drug Agencies - Germany Dr. Klaus Menges, BfArM 13.06.2007,
Guidelines. of 16.05.2013
 EUROPEAN COMMISSION Brussels, 16.05.2013 C (2013) 2804 Guidelines of 16.05.2013 on the details of the various categories of variations, on the operation of the procedures laid down in Chapters, a, I and
EUROPEAN COMMISSION Brussels, 16.05.2013 C (2013) 2804 Guidelines of 16.05.2013 on the details of the various categories of variations, on the operation of the procedures laid down in Chapters, a, I and
Report from the CMDh meeting held on 21-23 September 2015. !!! 3 months to go until the mandatory use of the electronic application form!!!
 Report from the meeting held on 21-23 September 2015!!! 3 months to go until the mandatory use of the electronic application form!!! Pharmacovigilance positions following PSUSA procedure for only nationally
Report from the meeting held on 21-23 September 2015!!! 3 months to go until the mandatory use of the electronic application form!!! Pharmacovigilance positions following PSUSA procedure for only nationally
CTD Dossier Preparation. Sr.Manager-Regulatory Affairs
 CTD Dossier Preparation K. Srikantha Reddy Sr.Manager-Regulatory Affairs Medreich Limited [email protected] CTD Dossier Preparation CTD (Common Technical Document) contains 5 modules Module 1 Module
CTD Dossier Preparation K. Srikantha Reddy Sr.Manager-Regulatory Affairs Medreich Limited [email protected] CTD Dossier Preparation CTD (Common Technical Document) contains 5 modules Module 1 Module
EMEA IMPLEMENTATION OF ELECTRONIC-ONLY SUBMISSION AND ectd SUBMISSION:
 European Medicines Agency Evaluation of Medicines for Human Use London, December 2008 EMEA/596881/2007 v0.5 EMEA IMPLEMENTATION OF ELECTRONIC-ONLY SUBMISSION AND ectd SUBMISSION: QUESTIONS AND ANSWERS
European Medicines Agency Evaluation of Medicines for Human Use London, December 2008 EMEA/596881/2007 v0.5 EMEA IMPLEMENTATION OF ELECTRONIC-ONLY SUBMISSION AND ectd SUBMISSION: QUESTIONS AND ANSWERS
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS (CVMP)
 European Medicines Agency Veterinary Medicines and Inspections London, 15 April 2005 EMEA/CVMP/134/02 Rev 1 CPMP/QWP/227/02 Rev 1 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) COMMITTEE FOR VETERINARY
European Medicines Agency Veterinary Medicines and Inspections London, 15 April 2005 EMEA/CVMP/134/02 Rev 1 CPMP/QWP/227/02 Rev 1 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) COMMITTEE FOR VETERINARY
Quick Response (QR) codes in the labelling and package leaflet of centrally authorised 1 medicinal products
 22 July 2015 EMA/493897/2015 Human Medicines Evaluation Division Quick Response (QR) codes in the labelling and package leaflet of centrally authorised 1 medicinal General principles of acceptability and
22 July 2015 EMA/493897/2015 Human Medicines Evaluation Division Quick Response (QR) codes in the labelling and package leaflet of centrally authorised 1 medicinal General principles of acceptability and
Overview of Authorisation Procedures for Medicinal Products
 Overview of Authorisation Procedures for Medicinal Products Updated by Martha Anna Bianchetto, PharmD, MBA OBJECTIVES q q Gain an understanding of the regulatory procedures necessary to grant a medicinal
Overview of Authorisation Procedures for Medicinal Products Updated by Martha Anna Bianchetto, PharmD, MBA OBJECTIVES q q Gain an understanding of the regulatory procedures necessary to grant a medicinal
MRP & DCP step by steb instructions how to apply and how the procedures are conducted
 MRP & DCP step by steb instructions how to apply and how the procedures are conducted 06 May 2013 Dubrovnik, Croatia Dr. Peter Bachmann Head of Unit Coordination Group European and International Affairs
MRP & DCP step by steb instructions how to apply and how the procedures are conducted 06 May 2013 Dubrovnik, Croatia Dr. Peter Bachmann Head of Unit Coordination Group European and International Affairs
A critical review of the current. marketing authorisation transfer procedure. in Europe
 A critical review of the current marketing authorisation transfer procedure in Europe Wissenschaftliche Prüfungsarbeit zur Erlangung des Titels Master of Drug Regulatory Affairs der Mathematisch-Naturwissenschaftlichen
A critical review of the current marketing authorisation transfer procedure in Europe Wissenschaftliche Prüfungsarbeit zur Erlangung des Titels Master of Drug Regulatory Affairs der Mathematisch-Naturwissenschaftlichen
Pharmacovigilance System Master File (PSMF), QPPV and audits
 Federal Agency for Medicines and Health Products (FAMHP) Pharmacovigilance System Master File (PSMF), QPPV and audits Matthijs Nele, PhV Inspector, DG Inspection BRAS - FAMHP/NM/ 1 Content Introduction
Federal Agency for Medicines and Health Products (FAMHP) Pharmacovigilance System Master File (PSMF), QPPV and audits Matthijs Nele, PhV Inspector, DG Inspection BRAS - FAMHP/NM/ 1 Content Introduction
VOLUME 2A Procedures for marketing authorisation CHAPTER 1 MARKETING AUTHORISATION. June 2013
 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health systems and products Medicinal products Revision 4 NOTICE TO APPLICANTS VOLUME 2A Procedures for marketing authorisation CHAPTER 1 MARKETING
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health systems and products Medicinal products Revision 4 NOTICE TO APPLICANTS VOLUME 2A Procedures for marketing authorisation CHAPTER 1 MARKETING
College ter Beoordeling van Geneesmiddelen
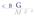 1 27-4-2012 CESP Bijeenkomst RegNed Zoetermeer, 19 april 2012 Stan van Belkum Agenda History and activities Conceptual model Practical use Metric of PoC and extended PoC Dutch experience Future steps 3
1 27-4-2012 CESP Bijeenkomst RegNed Zoetermeer, 19 april 2012 Stan van Belkum Agenda History and activities Conceptual model Practical use Metric of PoC and extended PoC Dutch experience Future steps 3
EU Change Control Process for Change Requests in the Entire Area of Electronic Submissions for Human Medicinal Products Version 2.
 EU Change Control Process for Change Requests in the Entire Area of Electronic Submissions for Human Medicinal Products Version 2.1 May 2011 Document Control Change Record Version Date Author(s) Comments
EU Change Control Process for Change Requests in the Entire Area of Electronic Submissions for Human Medicinal Products Version 2.1 May 2011 Document Control Change Record Version Date Author(s) Comments
REQUIREMENTS FOR NATIONAL AND MUTUAL RECOGNITIONS VMP APPLICATIONS
 1/13 REQUIREMENTS FOR NATIONAL AND MUTUAL RECOGNITIONS VMP APPLICATIONS 2/13 INDEX PAGE Applicable National Legislation and Community texts 3 Content of the application 3 Marketing authorisations 4 Language
1/13 REQUIREMENTS FOR NATIONAL AND MUTUAL RECOGNITIONS VMP APPLICATIONS 2/13 INDEX PAGE Applicable National Legislation and Community texts 3 Content of the application 3 Marketing authorisations 4 Language
CHAPTER 7 GENERAL INFORMATION
 Chapter 7 General Information CHAPTER 7 GENERAL INFORMATION Revision July 2008 Table of Content Page 1. FORMAT FOR APPLICATION IN THE EU 2 2. LANGUAGES TO BE USED FOR DOSSIER, RESPONSES, VARIATIONS AND
Chapter 7 General Information CHAPTER 7 GENERAL INFORMATION Revision July 2008 Table of Content Page 1. FORMAT FOR APPLICATION IN THE EU 2 2. LANGUAGES TO BE USED FOR DOSSIER, RESPONSES, VARIATIONS AND
CMDv/BPG/001. BEST PRACTICE GUIDE for Veterinary Mutual Recognition Procedure (MRP) Edition 04. Edition date: 19 July 2013
 EMA/CMDv/83618/2006 BEST PRACTICE GUIDE for Veterinary Mutual Recognition Procedure (MRP) Edition 04 Edition date: 19 July 2013 Implementation date: 23 November 2006 CMDv Secretariat: European Medicines
EMA/CMDv/83618/2006 BEST PRACTICE GUIDE for Veterinary Mutual Recognition Procedure (MRP) Edition 04 Edition date: 19 July 2013 Implementation date: 23 November 2006 CMDv Secretariat: European Medicines
INFARMED S Electronic System for the Management of Medicinal Products of Human Use (SMUH ALTER) Submission of Variation Applications
 INFARMED S Electronic System for the Management of Medicinal Products of Human Use (SMUH ALTER) Submission of Variation Applications Page 1 of 62 Contents 1 Introdution...4 2 SMUH ALTER External portal...5
INFARMED S Electronic System for the Management of Medicinal Products of Human Use (SMUH ALTER) Submission of Variation Applications Page 1 of 62 Contents 1 Introdution...4 2 SMUH ALTER External portal...5
VOLUME 2A Procedures for marketing authorisation CHAPTER 1 MARKETING AUTHORISATION. November 2005
 EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Brussels, ENTR/F2/BL D(2002) NOTICE TO APPLICANTS Revision 3 VOLUME 2A Procedures for marketing authorisation CHAPTER 1
EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Brussels, ENTR/F2/BL D(2002) NOTICE TO APPLICANTS Revision 3 VOLUME 2A Procedures for marketing authorisation CHAPTER 1
Best Archiving Practice Guidance
 Best Archiving Practice Guidance This document has been published under the auspices of the EU Telematics Implementation Group - electronic submissions (TIGes) Please note that this document has been published
Best Archiving Practice Guidance This document has been published under the auspices of the EU Telematics Implementation Group - electronic submissions (TIGes) Please note that this document has been published
End of consultation (deadline for comments) 14 October 2009. Adoption by Committee for advanced therapies 15 October 2010
 15 October 2010 EMA/CAT/418458/2008/corr. Committee for advanced therapies (CAT) Procedural advice on the certification of quality and nonclinical data for small and medium sized enterprises developing
15 October 2010 EMA/CAT/418458/2008/corr. Committee for advanced therapies (CAT) Procedural advice on the certification of quality and nonclinical data for small and medium sized enterprises developing
Standard operating procedure
 Standard operating procedure Title: How to conduct a procurement procedure Status: PUBLIC Document no.: SOP/EMA/0121 Lead author Approver Effective date: 04.09.14 Name: Caroline Maignen Name: Stefano Marino
Standard operating procedure Title: How to conduct a procurement procedure Status: PUBLIC Document no.: SOP/EMA/0121 Lead author Approver Effective date: 04.09.14 Name: Caroline Maignen Name: Stefano Marino
Our name ASPHALION derives from the Greek word asphaléia, alluding to values such as firmness, stability, certainty and reliability.
 Company Profile Our History ASPHALION is an International Drug Development and Regulatory Affairs consultancy firm founded in 2000, with an international team of over 40 people. Our Values Our name ASPHALION
Company Profile Our History ASPHALION is an International Drug Development and Regulatory Affairs consultancy firm founded in 2000, with an international team of over 40 people. Our Values Our name ASPHALION
BEST PRACTICE GUIDE for Handling of Periodic Safety Update Reports
 EMEA/CMDv/408477/2007 12 March 2009 BEST PRACTICE GUIDE for Handling of Periodic Safety Update Reports Edition number : 00 Edition date: 12 March 2009 Implementation date : 27 March 2009 CMD(v) Secretariat:
EMEA/CMDv/408477/2007 12 March 2009 BEST PRACTICE GUIDE for Handling of Periodic Safety Update Reports Edition number : 00 Edition date: 12 March 2009 Implementation date : 27 March 2009 CMD(v) Secretariat:
ectd Digital Handbook Table of Contents
 ectd Digital Handbook Table of Contents Introduction by Emily Ethridge, Editor, FDAnews Part 1 Tutorial Section 1.0 ectd Tutorial Table of Contents. This FDA tutorial, consisting of seven PowerPoint presentations,
ectd Digital Handbook Table of Contents Introduction by Emily Ethridge, Editor, FDAnews Part 1 Tutorial Section 1.0 ectd Tutorial Table of Contents. This FDA tutorial, consisting of seven PowerPoint presentations,
ACTIVE PHARMACEUTICAL INGREDIENTS COMMITTEE (APIC) ectd HOW TO DO DOCUMENT. July 2014
 ACTIVE PHARMACEUTICAL INGREDIENTS COMMITTEE (APIC) ectd HOW TO DO DOCUMENT July 2014 Table of Content 1. CHAPTER 1: REGULATORY FRAMEWORK & NATIONAL REQUIREMENTS... 5 1.1 Introduction: view of authorities
ACTIVE PHARMACEUTICAL INGREDIENTS COMMITTEE (APIC) ectd HOW TO DO DOCUMENT July 2014 Table of Content 1. CHAPTER 1: REGULATORY FRAMEWORK & NATIONAL REQUIREMENTS... 5 1.1 Introduction: view of authorities
PHV- 4 version 1 ELECTRONIC ADVERSE DRUG REACTION REPORTING
 PHV- 4 version 1 ELECTRONIC ADVERSE DRUG REACTION REPORTING This guideline supersedes guideline PHV 4 as of September 16 2008. 1. Introduction and general provisions 1.1 Purpose of the guideline The guideline
PHV- 4 version 1 ELECTRONIC ADVERSE DRUG REACTION REPORTING This guideline supersedes guideline PHV 4 as of September 16 2008. 1. Introduction and general provisions 1.1 Purpose of the guideline The guideline
EMA esignature capabilities: frequently asked questions relating to practical and technical aspects of the implementation
 August 2013 EMA/264709/2013 EMA esignature capabilities: frequently asked questions relating to practical and technical aspects of the implementation This question and answer document aims to address the
August 2013 EMA/264709/2013 EMA esignature capabilities: frequently asked questions relating to practical and technical aspects of the implementation This question and answer document aims to address the
EUROPEAN INDUSTRIAL PHARMACISTS GROUP
 EUROPEAN INDUSTRIAL PHARMACISTS GROUP Guide to Good Regulatory Practice Acknowledgments: Valérie LACAMOIRE, Conseil Central de la section B de l Ordre des Pharmaciens Kelsey MOWER, Industrial Pharmacists
EUROPEAN INDUSTRIAL PHARMACISTS GROUP Guide to Good Regulatory Practice Acknowledgments: Valérie LACAMOIRE, Conseil Central de la section B de l Ordre des Pharmaciens Kelsey MOWER, Industrial Pharmacists
Guideline on good pharmacovigilance practices (GVP)
 9 April 2013 EMA/816573/2011 Rev 1* Guideline on good pharmacovigilance practices (GVP) Module II Pharmacovigilance system master file (Rev 1) Draft of first version finalised by the Agency in collaboration
9 April 2013 EMA/816573/2011 Rev 1* Guideline on good pharmacovigilance practices (GVP) Module II Pharmacovigilance system master file (Rev 1) Draft of first version finalised by the Agency in collaboration
Questions and answers on post approval change management protocols
 27 October 2010 EMA/CHMP/CVMP/QWP/586330/2010 Questions and answers on post approval change management protocols Draft Draft Agreed by QWP September 2010 Adoption by CHMP for release for consultation 23
27 October 2010 EMA/CHMP/CVMP/QWP/586330/2010 Questions and answers on post approval change management protocols Draft Draft Agreed by QWP September 2010 Adoption by CHMP for release for consultation 23
Marketing Authorization Procedures in the European Union Making the Right Choice
 Life science i technical bulletin ISSUE N 33 /DECEMBER 2009 Marketing Authorization Procedures in the European Union Making the Right Choice AUTHOR: Arash Ghalamkarpour, PhD, Regulatory Affairs Associate,
Life science i technical bulletin ISSUE N 33 /DECEMBER 2009 Marketing Authorization Procedures in the European Union Making the Right Choice AUTHOR: Arash Ghalamkarpour, PhD, Regulatory Affairs Associate,
Questions and answers on post approval change management protocols
 30 March 2012 EMA/CHMP/CVMP/QWP/586330/2010 Committee for Medicinal Products for Human Use (CHMP) Questions and answers on post approval change management protocols Draft agreed by CHMP / CVMP Quality
30 March 2012 EMA/CHMP/CVMP/QWP/586330/2010 Committee for Medicinal Products for Human Use (CHMP) Questions and answers on post approval change management protocols Draft agreed by CHMP / CVMP Quality
Electronic exchanges of individual case safety reports (ICSRs) with ANSM (National Agency of Medicine and Health Product safety)
 Pharmacovigilance information for pharmaceutical companies Electronic exchanges of individual case safety reports (ICSRs) with ANSM (National Agency of Medicine and Health Product safety) This document
Pharmacovigilance information for pharmaceutical companies Electronic exchanges of individual case safety reports (ICSRs) with ANSM (National Agency of Medicine and Health Product safety) This document
A 4.5 Validity Period of a Marketing Authorisation
 104 A 4.5 Examples of situations in which the notification procedure according to Art. 61(3) of the Community Code will apply are as follows: Minor changes to the package leaflet resulting from readability
104 A 4.5 Examples of situations in which the notification procedure according to Art. 61(3) of the Community Code will apply are as follows: Minor changes to the package leaflet resulting from readability
Data Submission Manual
 Data Submission Manual Part 04 - How to Pass Business Rule Verification ("Enforce Rules") 2 Data Submission Manual Version: 3.1 Version Changes 3.1 04/2014 Minor textual revision P 5.3.3.2 Addition of
Data Submission Manual Part 04 - How to Pass Business Rule Verification ("Enforce Rules") 2 Data Submission Manual Version: 3.1 Version Changes 3.1 04/2014 Minor textual revision P 5.3.3.2 Addition of
White Paper The EU Clinical Trials Regulation Main Changes and Challenges
 White Paper The EU Clinical Trials Regulation Main Changes and Challenges Table of Contents 1. Introduction... 3 2. Main Changes and Associated Challenges... 4 2.1 Procedure for Initial Authorisation...
White Paper The EU Clinical Trials Regulation Main Changes and Challenges Table of Contents 1. Introduction... 3 2. Main Changes and Associated Challenges... 4 2.1 Procedure for Initial Authorisation...
training programme in pharmaceutical medicine Regulatory affairs
 training programme in pharmaceutical medicine Regulatory affairs INFARMED, Lisbon 19-21 january 2012 Regulatory affairs 19 21 january 2012 LocaL: INFARMED, Lisbon curricular unit Leader: Hélder Mota Filipe,
training programme in pharmaceutical medicine Regulatory affairs INFARMED, Lisbon 19-21 january 2012 Regulatory affairs 19 21 january 2012 LocaL: INFARMED, Lisbon curricular unit Leader: Hélder Mota Filipe,
How To Understand The Paediatric Regulation
 Paediatric Use Marketing Authorisation (PUMA) from a National Competent Authority Point of View DGRA Annual Congress 2006 Bonn, 10. May 2006 Dr. Susanne Keitel Content PUMA The Future Background Legislative
Paediatric Use Marketing Authorisation (PUMA) from a National Competent Authority Point of View DGRA Annual Congress 2006 Bonn, 10. May 2006 Dr. Susanne Keitel Content PUMA The Future Background Legislative
GUIDE FOR APPLICANTS SUPPORT FOR EUROPEAN COOPERATION PROJECTS
 Education, Audiovisual and Culture Executive Agency Culture Unit GUIDE FOR APPLICANTS SUPPORT FOR EUROPEAN COOPERATION PROJECTS CREATIVE EUROPE (2014-2020) CULTURE SUB-PROGRAMME VERSION: July 2014 Disclaimer:
Education, Audiovisual and Culture Executive Agency Culture Unit GUIDE FOR APPLICANTS SUPPORT FOR EUROPEAN COOPERATION PROJECTS CREATIVE EUROPE (2014-2020) CULTURE SUB-PROGRAMME VERSION: July 2014 Disclaimer:
Guide to Fees for Veterinary Products
 Guide to Fees for Veterinary Products FIN-G0003-14 04 FEBRUARY 2016 This guide does not purport to be an interpretation of law and/or regulations and is for guidance purposes only. CONTENTS ABBREVIATIONS
Guide to Fees for Veterinary Products FIN-G0003-14 04 FEBRUARY 2016 This guide does not purport to be an interpretation of law and/or regulations and is for guidance purposes only. CONTENTS ABBREVIATIONS
Official Journal of the European Union. (Acts whose publication is obligatory)
 27.12.2006 L 378/1 I (Acts whose publication is obligatory) REGULATION (EC) No 1901/2006 OF THE EUROPEAN PARLIAMT AND OF THE COUNCIL of 12 December 2006 on medicinal products for paediatric use and amending
27.12.2006 L 378/1 I (Acts whose publication is obligatory) REGULATION (EC) No 1901/2006 OF THE EUROPEAN PARLIAMT AND OF THE COUNCIL of 12 December 2006 on medicinal products for paediatric use and amending
Guideline on good pharmacovigilance practices (GVP)
 1 2 20 February 2012 EMA/541760/2011 3 4 Guideline on good pharmacovigilance practices (GVP) Module I Pharmacovigilance systems and their quality systems Draft finalised by the Agency in collaboration
1 2 20 February 2012 EMA/541760/2011 3 4 Guideline on good pharmacovigilance practices (GVP) Module I Pharmacovigilance systems and their quality systems Draft finalised by the Agency in collaboration
Management of Fees USER MANUAL. Version 4.01
 Management of Fees USER MANUAL Version 4.01 Index 1 ELECTRONIC PAYMENT... 3 1.1 ACCESS... 3 1.2 SECTION ON IDENTIFYING DATA: PAYER AND THE TAXABLE PERSON... 4 1.3 SECTION ON DATA RELATED TO THE CALCULATION
Management of Fees USER MANUAL Version 4.01 Index 1 ELECTRONIC PAYMENT... 3 1.1 ACCESS... 3 1.2 SECTION ON IDENTIFYING DATA: PAYER AND THE TAXABLE PERSON... 4 1.3 SECTION ON DATA RELATED TO THE CALCULATION
Guidance to applicants Submission of Marketing Authorisation Applications
 Guidance to applicants Submission of Marketing Authorisation Applications The purpose of this notice to applicants is to clarify aspects related with the submission to Infarmed Autoridade Nacional do Medicamento
Guidance to applicants Submission of Marketing Authorisation Applications The purpose of this notice to applicants is to clarify aspects related with the submission to Infarmed Autoridade Nacional do Medicamento
European Commission Directorate General for HOME AFFAIRS. Guide for applicants. Call for expression of interest HOME/2014/AMIH/001
 European Commission Directorate General for HOME AFFAIRS Guide for applicants Call for expression of interest HOME/2014/AMIH/001 for the establishment of a list of individual external experts to assist
European Commission Directorate General for HOME AFFAIRS Guide for applicants Call for expression of interest HOME/2014/AMIH/001 for the establishment of a list of individual external experts to assist
Agence fédérale des médicaments et des produits de santé
 Agence fédérale des médicaments et des produits de santé From Risk Management Plan to Risk Minimization Activities New Royal Decree: Status and General Explanation Role of the Pharmacovigilance Department
Agence fédérale des médicaments et des produits de santé From Risk Management Plan to Risk Minimization Activities New Royal Decree: Status and General Explanation Role of the Pharmacovigilance Department
Implementation strategy for ISO IDMP in EU
 Implementation strategy for ISO IDMP in EU Paolo Alcini Head of Data Standardisation and Analytics Business Data & Analytics Information Management EMA An agency of the European Union The EU ISO IDMP Task
Implementation strategy for ISO IDMP in EU Paolo Alcini Head of Data Standardisation and Analytics Business Data & Analytics Information Management EMA An agency of the European Union The EU ISO IDMP Task
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL
 Ref. Ares(2011)1044649-03/10/2011 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health systems and products Pharmaceuticals SANCO/D3/RSR/iv(2011)ddg1.d3. 1137738 Update 1 October 2011 HANDLING
Ref. Ares(2011)1044649-03/10/2011 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health systems and products Pharmaceuticals SANCO/D3/RSR/iv(2011)ddg1.d3. 1137738 Update 1 October 2011 HANDLING
Report to the European Commission on Pharmacovigilance audits carried out in the Medicines Evaluation Board, The Netherlands period of time from
 Report to the European Commission on Pharmacovigilance audits carried out in the Medicines Evaluation Board, The Netherlands period of time from September 2013 to September 2015 1. INTRODUCTION Applicable
Report to the European Commission on Pharmacovigilance audits carried out in the Medicines Evaluation Board, The Netherlands period of time from September 2013 to September 2015 1. INTRODUCTION Applicable
EudraVigilance stakeholder change management plan
 26 October 2015 EMA/797114/2014 Information Management Division Consultation of Project Maintenance Group 1 15 July 2015 Consultation of Eudravigilance Expert Working Group 23 September 2015 Consultation
26 October 2015 EMA/797114/2014 Information Management Division Consultation of Project Maintenance Group 1 15 July 2015 Consultation of Eudravigilance Expert Working Group 23 September 2015 Consultation
Marketing Authorisation: The Evaluation Process
 1 st EMEA Workshop for Micro, Small and Medium- Sized Enterprises (SMEs) Navigating the Regulatory Maze Marketing Authorisation: The Evaluation Process Dr Evdokia Korakianiti Scientific Administrator Centralised
1 st EMEA Workshop for Micro, Small and Medium- Sized Enterprises (SMEs) Navigating the Regulatory Maze Marketing Authorisation: The Evaluation Process Dr Evdokia Korakianiti Scientific Administrator Centralised
Elektronische Einreichung Wo steht die AGES PharmMed?
 Elektronische Einreichung Wo steht die AGES PharmMed? AGES-Gespräch 27. November 2008, Vienna Dr. Christa Wirthumer-Hoche AGES PharmMed 2 Electronic submission - aims Reduction of (internal) paper-flow
Elektronische Einreichung Wo steht die AGES PharmMed? AGES-Gespräch 27. November 2008, Vienna Dr. Christa Wirthumer-Hoche AGES PharmMed 2 Electronic submission - aims Reduction of (internal) paper-flow
Guide for Applicants. Call for Proposal:
 Guide for Applicants Call for Proposal: COSME Work Programme 2014 TABLE OF CONTENTS I. Introduction... 3 II. Preparation of the proposal... 3 II.1. Relevant documents... 3 II.2. Participants... 4 II.2.1.
Guide for Applicants Call for Proposal: COSME Work Programme 2014 TABLE OF CONTENTS I. Introduction... 3 II. Preparation of the proposal... 3 II.1. Relevant documents... 3 II.2. Participants... 4 II.2.1.
Data submission of authorised medicines in the European Union
 23 February 2015 EMA/471367/2014, Rev. 1 1 Business Data and Support Department Data submission of authorised medicines in the European Union Outlines on Article 57(2) of Regulation (EC) No 726/2004 1
23 February 2015 EMA/471367/2014, Rev. 1 1 Business Data and Support Department Data submission of authorised medicines in the European Union Outlines on Article 57(2) of Regulation (EC) No 726/2004 1
South East Europe (SEE) Implementation Manual
 South East Europe (SEE) Implementation Manual Version 5.0 Date of approval (v1.2): 8 th May 2009 1 st amendment (v2.1): 7th July 2009 2 nd amendment (v3.1): 21 st January 2011 3 rd amendment (v3.2): 27
South East Europe (SEE) Implementation Manual Version 5.0 Date of approval (v1.2): 8 th May 2009 1 st amendment (v2.1): 7th July 2009 2 nd amendment (v3.1): 21 st January 2011 3 rd amendment (v3.2): 27
Wissenschaftliche Prüfungsarbeit. zur Erlangung des Titels. Master of Drug Regulatory Affairs
 EMA s Product Information Management (PIM) project halted Impact from the perspective of biotech companies on strategies for structured product information management Wissenschaftliche Prüfungsarbeit zur
EMA s Product Information Management (PIM) project halted Impact from the perspective of biotech companies on strategies for structured product information management Wissenschaftliche Prüfungsarbeit zur
Reflection paper on the Use of Interactive Response Technologies (Interactive Voice/Web Response Systems) in Clinical Trials
 1 2 3 5 August 2011 EMA/INS/GCP/600788/2011 Compliance and Inspection 4 5 6 7 Reflection paper on the Use of Interactive Response Technologies (Interactive Voice/Web Response Systems) in Clinical Trials
1 2 3 5 August 2011 EMA/INS/GCP/600788/2011 Compliance and Inspection 4 5 6 7 Reflection paper on the Use of Interactive Response Technologies (Interactive Voice/Web Response Systems) in Clinical Trials
EUDRAVIGILANCE TELEMATICS IMPLEMENTATION GROUP
 European Medicines Agency Post-authorisation Evaluation of Medicines for Human Use London, 29 October 2004 Doc. Ref: EMEA/115735/2004 EUDRAVIGILANCE TELEMATICS IMPLEMENTATION GROUP NOTE FOR GUIDANCE ON
European Medicines Agency Post-authorisation Evaluation of Medicines for Human Use London, 29 October 2004 Doc. Ref: EMEA/115735/2004 EUDRAVIGILANCE TELEMATICS IMPLEMENTATION GROUP NOTE FOR GUIDANCE ON
The European regulatory system for medicines and the European Medicines Agency
 The European regulatory system for medicines and the European Medicines Agency A consistent approach to medicines regulation across the European Union An agency of the European Union This booklet is intended
The European regulatory system for medicines and the European Medicines Agency A consistent approach to medicines regulation across the European Union An agency of the European Union This booklet is intended
HMPWG in the view of the NCA
 EMEA Homeopathic Workshop Bundesinstitut für Arzneimittel London, 27.10.2006 HMPWG in the view of the NCA Objectives, Achievements, Roles and Responsibilities Werner Knöss Head of Division Complementary
EMEA Homeopathic Workshop Bundesinstitut für Arzneimittel London, 27.10.2006 HMPWG in the view of the NCA Objectives, Achievements, Roles and Responsibilities Werner Knöss Head of Division Complementary
Unedited version adopted by the 45th WHO Expert Committee on specifications for pharmaceutical preparations. World Health Organization 2010
 GUIDELINE ON SUBMISSION OF DOCUMENTATION FOR A MULTISOURCE (GENERIC) FINISHED PHARMACEUTICAL PRODUCT (FPP): PREPARATION OF PRODUCT DOSSIERS (PDS) IN COMMON TECHNICAL DOCUMENT (CTD) FORMAT Unedited version
GUIDELINE ON SUBMISSION OF DOCUMENTATION FOR A MULTISOURCE (GENERIC) FINISHED PHARMACEUTICAL PRODUCT (FPP): PREPARATION OF PRODUCT DOSSIERS (PDS) IN COMMON TECHNICAL DOCUMENT (CTD) FORMAT Unedited version
FAQs for SCHS License Application through DataFlow Group
 FAQs for SCHS License Application through DataFlow Group Q1: Why do I have to apply? A1: In accordance with the decision of the Board of Trustees of the Saudi Commission for Health Specialties No. 25 /
FAQs for SCHS License Application through DataFlow Group Q1: Why do I have to apply? A1: In accordance with the decision of the Board of Trustees of the Saudi Commission for Health Specialties No. 25 /
Clinical trials: from European perspective to National implementation. CTFG / FAMHP / pharma.be. Brussels, 19 November 2010
 Clinical trials: from European perspective to National implementation CTFG / FAMHP / pharma.be Brussels, 19 November 2010 Safety in clinical trials: From detection to decision How safety events are captured
Clinical trials: from European perspective to National implementation CTFG / FAMHP / pharma.be Brussels, 19 November 2010 Safety in clinical trials: From detection to decision How safety events are captured
How to Manage Email. Guidance for staff
 How to Manage Email Guidance for staff 1 Executive Summary Aimed at Note Purpose Benefits staff Necessary skills to All staff who use email This guidance does NOT cover basic IT literacy skills. Staff
How to Manage Email Guidance for staff 1 Executive Summary Aimed at Note Purpose Benefits staff Necessary skills to All staff who use email This guidance does NOT cover basic IT literacy skills. Staff
EU PAS Register Guide
 29 March 2016 EMA/613603/2012 (currently ENCePP E-Register of Studies) The EU PAS Register is temporarily hosted on the ENCePP website www.encepp.eu 1. Introduction... 2 2. ENCePP E-Register as a temporary
29 March 2016 EMA/613603/2012 (currently ENCePP E-Register of Studies) The EU PAS Register is temporarily hosted on the ENCePP website www.encepp.eu 1. Introduction... 2 2. ENCePP E-Register as a temporary
REACH-IT Industry User Manual
 REACH-IT Industry User Manual Part 03 - Login and message box 2 REACH-IT Industry User Manual Version 2.1 Version Changes 2.1 April 2014 Updates related to REACH-IT 2.7 regarding the Terms and Conditions,
REACH-IT Industry User Manual Part 03 - Login and message box 2 REACH-IT Industry User Manual Version 2.1 Version Changes 2.1 April 2014 Updates related to REACH-IT 2.7 regarding the Terms and Conditions,
EU Individual Case Safety Report (ICSR) 1 Implementation Guide
 4 December 2014 EMA/51938/2013 EU Individual Case Safety Report (ICSR) 1 Implementation Guide Start of Public Consultation 30 April 2014 End of Public Consultation 30 June 2014 Final draft agreed by Project
4 December 2014 EMA/51938/2013 EU Individual Case Safety Report (ICSR) 1 Implementation Guide Start of Public Consultation 30 April 2014 End of Public Consultation 30 June 2014 Final draft agreed by Project
Guide for Applicants COSME calls for proposals 2015
 Guide for Applicants COSME calls for proposals 2015 CONTENTS I. Introduction... 3 II. Preparation of the proposal... 3 II.1. Relevant documents... 3 II.2. Participants... 4 Consortium coordinator... 4
Guide for Applicants COSME calls for proposals 2015 CONTENTS I. Introduction... 3 II. Preparation of the proposal... 3 II.1. Relevant documents... 3 II.2. Participants... 4 Consortium coordinator... 4
Guidance for Industry
 #108 Guidance for Industry How to Register with the CVM Electronic Submission System To Submit Information in Electronic Format Using the FDA Electronic Submissions Gateway This version of the guidance
#108 Guidance for Industry How to Register with the CVM Electronic Submission System To Submit Information in Electronic Format Using the FDA Electronic Submissions Gateway This version of the guidance
eaf becomes mandatory for all procedures what to consider by applicants
 eaf becomes mandatory for all procedures what to consider by applicants Webinar for the usage of an eaf at applicants level Presented by: Georg Neuwirther, Klaus Menges and Kristiina Puusaari An agency
eaf becomes mandatory for all procedures what to consider by applicants Webinar for the usage of an eaf at applicants level Presented by: Georg Neuwirther, Klaus Menges and Kristiina Puusaari An agency
Full Compliance Contents
 Full Compliance for and EU Annex 11 With the regulation support of Contents 1. Introduction 2 2. The regulations 2 3. FDA 3 Subpart B Electronic records 3 Subpart C Electronic Signatures 9 4. EU GMP Annex
Full Compliance for and EU Annex 11 With the regulation support of Contents 1. Introduction 2 2. The regulations 2 3. FDA 3 Subpart B Electronic records 3 Subpart C Electronic Signatures 9 4. EU GMP Annex
Use of CESP to submit electronic documents to EDQM
 CFE/CB PUBLIC DOCUMENT (LEVEL 1) English only/anglais seulement PA/PH/CEP (13) 67, 1R Strasbourg, December 2013 Certification of suitability to Monographs of the European Pharmacopoeia Use of CESP to submit
CFE/CB PUBLIC DOCUMENT (LEVEL 1) English only/anglais seulement PA/PH/CEP (13) 67, 1R Strasbourg, December 2013 Certification of suitability to Monographs of the European Pharmacopoeia Use of CESP to submit
Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 1 Updated November 10, 2009
 Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 1 Updated November 10, 2009 The innovative pharmaceutical industry 2 is committed to the transparency
Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 1 Updated November 10, 2009 The innovative pharmaceutical industry 2 is committed to the transparency
Turnitin Blackboard 9.0 Integration Instructor User Manual
 Turnitin Blackboard 9.0 Integration Instructor User Manual Version: 2.1.3 Updated December 16, 2011 Copyright 1998 2011 iparadigms, LLC. All rights reserved. Turnitin Blackboard Learn Integration Manual:
Turnitin Blackboard 9.0 Integration Instructor User Manual Version: 2.1.3 Updated December 16, 2011 Copyright 1998 2011 iparadigms, LLC. All rights reserved. Turnitin Blackboard Learn Integration Manual:
GUIDE FOR APPLICANTS SUPPORT FOR LITERARY TRANSLATION PROJECTS
 Education, Audiovisual and Culture Executive Agency Culture Unit GUIDE FOR APPLICANTS SUPPORT FOR LITERARY TRANSLATION PROJECTS CREATIVE EUROPE (2014-2020) CULTURE SUB-PROGRAMME VERSION: December 2013
Education, Audiovisual and Culture Executive Agency Culture Unit GUIDE FOR APPLICANTS SUPPORT FOR LITERARY TRANSLATION PROJECTS CREATIVE EUROPE (2014-2020) CULTURE SUB-PROGRAMME VERSION: December 2013
CMD(v)/GUI/014. GUIDANCE for The Processing of Generic Applications Through MRP / DCP
 EMEA/CMDv/262452/2008 GUIDANCE Edition number : 00 Edition date: 19 June 2008 Implementation date : 04 July 2008 CMD(v) Secretariat: 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20) 74
EMEA/CMDv/262452/2008 GUIDANCE Edition number : 00 Edition date: 19 June 2008 Implementation date : 04 July 2008 CMD(v) Secretariat: 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20) 74
GUIDELINE ON ACTIVE PHARMACEUTICAL INGREDIENT MASTER FILE (APIMF) PROCEDURE 1 (The APIMF procedure guideline does not apply to biological APIs.
 15 January 2007 GUIDELINE ON ACTIVE PHARMACEUTICAL INGREDIENT MASTER FILE (APIMF) PROCEDURE 1 (The APIMF procedure guideline does not apply to biological APIs.) TABLE OF CONTENTS 1 INTRODUCTION... 2 2
15 January 2007 GUIDELINE ON ACTIVE PHARMACEUTICAL INGREDIENT MASTER FILE (APIMF) PROCEDURE 1 (The APIMF procedure guideline does not apply to biological APIs.) TABLE OF CONTENTS 1 INTRODUCTION... 2 2
Electronic Bank Account Management - EBAM
 Electronic Bank Account Management - EBAM This guide provides an overview of s EBAM offering. It includes a definition of the scope of the offering as well as a high level description of its building blocks.
Electronic Bank Account Management - EBAM This guide provides an overview of s EBAM offering. It includes a definition of the scope of the offering as well as a high level description of its building blocks.
Clinical Trials Facilitation Groups
 Clinical Trials Facilitation Groups Guidance document for a Voluntary Harmonisation Procedure (VHP) for the assessment of multinational Clinical Trial Applications Version 2 Table of contents Doc. Ref.:
Clinical Trials Facilitation Groups Guidance document for a Voluntary Harmonisation Procedure (VHP) for the assessment of multinational Clinical Trial Applications Version 2 Table of contents Doc. Ref.:
TRANSATLANTIC TRADE AND INVESTMENT PARTNERSHIP
 DISCLAIMER: The EU reserves the right to make subsequent modifications to this text and to complement its proposals at a later stage, by modifying, supplementing or withdrawing all, or any part, at any
DISCLAIMER: The EU reserves the right to make subsequent modifications to this text and to complement its proposals at a later stage, by modifying, supplementing or withdrawing all, or any part, at any
EFPIA Principles for the Development of the EU Clinical Trials Portal and Database
 Position Paper EFPIA Principles for the Development of the EU Clinical Trials Portal and Database Executive summary EFPIA sees the implementation of the Clinical Trials Regulation 1 as an opportunity to
Position Paper EFPIA Principles for the Development of the EU Clinical Trials Portal and Database Executive summary EFPIA sees the implementation of the Clinical Trials Regulation 1 as an opportunity to
The European Clinical Trials Framework Update on the Draft Clinical Trials Regulation
 The European Clinical Trials Framework Update on the Draft Clinical Trials Regulation Dr. Ilona Reischl BASG/AGES Austrian Agency for Health and Food Safety GmbH Content - References "Proposal for a Regulation
The European Clinical Trials Framework Update on the Draft Clinical Trials Regulation Dr. Ilona Reischl BASG/AGES Austrian Agency for Health and Food Safety GmbH Content - References "Proposal for a Regulation
EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL. Brussels, ENTR/F2/BL D(2003) CT 1 Revision 2
 EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single market : management & legislation for consumer goods Pharmaceuticals : regulatory framework and market authorisations Brussels, ENTR/F2/BL D(2003)
EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single market : management & legislation for consumer goods Pharmaceuticals : regulatory framework and market authorisations Brussels, ENTR/F2/BL D(2003)
Guideline on good pharmacovigilance practices (GVP)
 22 June 2012 EMA/541760/2011 Guideline on good pharmacovigilance practices (GVP) Module I Pharmacovigilance systems and their quality systems Draft finalised by the Agency in collaboration with Member
22 June 2012 EMA/541760/2011 Guideline on good pharmacovigilance practices (GVP) Module I Pharmacovigilance systems and their quality systems Draft finalised by the Agency in collaboration with Member
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL
 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health systems and products Medicinal products authorisations, EMA Brussels, 27.03.2014 ENTR/6283/00 Rev 4 orphan\guidelines\format content
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health systems and products Medicinal products authorisations, EMA Brussels, 27.03.2014 ENTR/6283/00 Rev 4 orphan\guidelines\format content
Compliance Response Edition 07/2009. SIMATIC WinCC V7.0 Compliance Response Electronic Records / Electronic Signatures. simatic wincc DOKUMENTATION
 Compliance Response Edition 07/2009 SIMATIC WinCC V7.0 Compliance Response Electronic Records / Electronic Signatures simatic wincc DOKUMENTATION Compliance Response Electronic Records / Electronic Signatures
Compliance Response Edition 07/2009 SIMATIC WinCC V7.0 Compliance Response Electronic Records / Electronic Signatures simatic wincc DOKUMENTATION Compliance Response Electronic Records / Electronic Signatures
Comparison of EU-Pharmacovigilance System Master File (PSMF) with US System
 Comparison of EU-Pharmacovigilance System Master File (PSMF) with US System Wissenschaftliche Prüfungsarbeit Zur Eralngung des Titels Master of Drug Regulatory Affairs der Mathematisch-Naturwissenschaftlichen
Comparison of EU-Pharmacovigilance System Master File (PSMF) with US System Wissenschaftliche Prüfungsarbeit Zur Eralngung des Titels Master of Drug Regulatory Affairs der Mathematisch-Naturwissenschaftlichen
PROGRAMME MANUAL 3. APPLICATION STAGE
 PROGRAMME MANUAL 3. APPLICATION STAGE 3. APPLICATION STAGE...1 Introduction...3 3.1. Application procedure...6 3.1.1. Application Procedure for modular projects...6 3.1.2. Application Procedure for horizontal
PROGRAMME MANUAL 3. APPLICATION STAGE 3. APPLICATION STAGE...1 Introduction...3 3.1. Application procedure...6 3.1.1. Application Procedure for modular projects...6 3.1.2. Application Procedure for horizontal
Work instructions. 1. Changes since last revision. 2. Records. 3. Instructions. Title: How to create reports from scientific memory database (SMD)
 Work instructions Title: How to create reports from scientific memory database (SMD) Applies to: Clinical and Non-Clinical Compliance Section Status: PUBLIC Document no.: WIN/INSP/2040 Lead Author Approver
Work instructions Title: How to create reports from scientific memory database (SMD) Applies to: Clinical and Non-Clinical Compliance Section Status: PUBLIC Document no.: WIN/INSP/2040 Lead Author Approver
PROGRAMME MANUAL 3. APPLICATION STAGE
 PROGRAMME MANUAL 3. APPLICATION STAGE 3. APPLICATION STAGE...1 Introduction...3 3.1. Application procedure...6 3.1.1. Application Procedure for modular projects...6 3.1.2. Application Procedure for horizontal
PROGRAMME MANUAL 3. APPLICATION STAGE 3. APPLICATION STAGE...1 Introduction...3 3.1. Application procedure...6 3.1.1. Application Procedure for modular projects...6 3.1.2. Application Procedure for horizontal
