Media Release. Basel, 8 October 2015
|
|
|
- Cuthbert Cox
- 10 years ago
- Views:
Transcription
1 Media Release Basel, 8 October 2015 Roche s ocrelizumab first investigational medicine to show positive pivotal study results in both relapsing and primary progressive forms of multiple sclerosis Ocrelizumab showed superiority to interferon beta-1a (Rebif ) in two identical Phase III studies in people with relapsing multiple sclerosis (MS), the most common form of the disease Ocrelizumab is the first investigational medicine to show efficacy in people with primary progressive MS in a large Phase III study Ocrelizumab Phase III data will be presented at the 31st congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) from 7th to 10th October in Barcelona, Spain Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced data from three positive, pivotal Phase III studies of ocrelizumab in people with relapsing multiple sclerosis (MS) and primary progressive multiple sclerosis (PPMS). Data from two identical studies (called OPERA I and OPERA II) in people with relapsing MS, which affects approximately 85 percent of people with MS at the time of diagnosis, showed ocrelizumab was superior to interferon beta-1a (Rebif ), a well-established MS therapy, in reducing the three major markers of disease activity over the two-year controlled treatment period. In a separate study (called ORATORIO) in people with PPMS, a form of the disease marked by steadily worsening symptoms and typically without distinct relapses or periods of remission, ocrelizumab significantly reduced the progression of clinical disability sustained for at least 12 weeks (the primary endpoint) and 24 weeks (a secondary endpoint) compared with placebo. Additionally, the study met other secondary endpoints of reducing the time required to walk 25 feet, the volume of chronic inflammatory brain lesions, and brain volume loss. The results of these three pivotal trials have the potential to transform the treatment of MS, said Sandra Horning, M.D., Roche s Chief Medical Officer and Head of Global Product Development. Ocrelizumab is the first investigational medicine to significantly reduce disability progression in people with relapsing MS and people with primary progressive MS a form of MS with no approved treatments. We are eager to work with F. Hoffmann-La Roche Ltd 4070 Basel Switzerland Group Communications Roche Group Media Relations Tel Fax /6
2 regulatory authorities to bring this investigational medicine to the MS community as soon as possible. These results redefine our understanding of MS by highlighting the central role of the B cell, said Stephen Hauser, M.D., Chair of the Scientific Steering Committee of the OPERA studies and Chair of the Department of Neurology at the University of California San Francisco School of Medicine. The findings may also encourage the MS community to look more closely at earlier treatment of the disease. Currently, many doctors reserve what are considered highly effective MS medicines until a patient s disease becomes more advanced. Patients and their doctors need new treatment options that offer the potential for greater efficacy than a standard-of-care interferon with a similar safety profile. This is an important moment for the MS community, said Xavier Montalban, M.D., Ph.D., Chair of the Scientific Steering Committee of the ORATORIO study and Professor of Neurology and Neuroimmunology at Vall d Hebron University Hospital and Research Institute, Barcelona, Spain. For decades, trial after trial has failed to show the benefit of any medicine for people with primary progressive MS. Now, for the first time, we have a positive Phase III study result for people with this debilitating form of the disease. Roche plans to pursue marketing authorisation for ocrelizumab in relapsing MS and in PPMS. Data from the ocrelizumab OPERA I and OPERA II studies and from the ORATORIO study will be submitted to global regulatory authorities in early About the OPERA I and OPERA II studies in relapsing MS Results from the OPERA I and OPERA II studies will be presented by Dr. Hauser on Friday, 9th October (Abstract #246, 14:40-14:52 CET). OPERA I and OPERA II are Phase III, randomised, double-blind, doubledummy, global multi-centre studies evaluating the efficacy and safety of ocrelizumab (600 mg administered by intravenous infusion every six months) compared with interferon beta-1a (44 mcg administered by subcutaneous injection three times per week) in 1,656 people with relapsing forms of MS (i.e., relapsingremitting MS and secondary-progressive MS with relapses). 1 In the OPERA I and OPERA II studies, ocrelizumab significantly reduced the annualised relapse rate (ARR) the primary endpoint of both studies by nearly 50 percent compared with interferon beta-1a over the two-year period. Additionally, ocrelizumab met secondary endpoints of the study, significantly delaying confirmed 2/6
3 disability progression (CDP; loss of physical abilities, measured by the Expanded Disability Status Scale, or EDSS) by approximately 40 percent sustained for both 12 and 24 weeks compared with interferon beta-1a in prespecified, pooled analyses of the two studies (p= and p=0.0025, respectively). Ocrelizumab also significantly reduced acute MS-related inflammation and brain injury (total number of T1-gadoliniumenhancing lesions measured by magnetic resonance imaging, or MRI) at 24, 48 and 96 weeks by more than 90 percent and the emergence of more chronic or growing areas of MS-related brain injury (T2 hyperintense lesions) at 24, 48 and 96 weeks by around 80 percent compared with interferon beta-1a. Data from the Phase III studies in patients with relapsing MS showed: A 46-percent and 47-percent reduction in the ARR compared with interferon beta-1a over the two-year period in OPERA I and OPERA II, respectively (p< and p<0.0001). A 43-percent and 37-percent risk reduction in CDP sustained for 12 weeks compared with interferon beta-1a in OPERA I and OPERA II, respectively (p= and p=0.0169). A 43-percent and 37-percent risk reduction in CDP sustained for 24 weeks compared with interferon beta-1a in OPERA I and OPERA II, respectively (p= and p=0.0370). A 94-percent and 95-percent reduction in the total number of T1 gadolinium-enhancing lesions compared with interferon beta-1a in OPERA I and OPERA II, respectively (p< and p<0.0001). A 77-percent and 83-percent reduction in the total number of new and/or enlarging hyperintense T2 lesions compared with interferon beta-1a in OPERA I and OPERA II, respectively (p< and p<0.0001). Overall, the proportion of patients in the ocrelizumab group with adverse events was similar to interferon beta- 1a in a pooled analysis of both studies (83.3 percent in each treatment group); the most common adverse event associated with ocrelizumab was infusion-related reactions (34.3 percent of patients who received ocrelizumab experienced at least one infusion-related reaction vs. 9.7 percent for interferon beta-1a). The proportion of patients in the ocrelizumab group with serious adverse events, including serious infections, was also similar to interferon beta-1a (6.9 percent vs. 8.7 percent, respectively). About the ORATORIO study in PPMS Results from the ORATORIO study will be presented as a late-breaking abstract by Professor Montalban on Saturday, 10th October (Abstract #2368, 8:52-9:03 CET). ORATORIO is a Phase III, randomised, double-blind, 3/6
4 global multi-centre study evaluating the efficacy and safety of ocrelizumab (600 mg administered by intravenous infusion every six months; given as two 300 mg infusions two weeks apart) compared with placebo in 732 people with PPMS. 2 In contrast to the OPERA I and OPERA II studies, where the blinded treatment period was two years, the blinded treatment period of the ORATORIO study continued beyond that until all patients had received at least 120 weeks of either ocrelizumab or placebo and a predefined number of CDP events was reached overall in the study. The ORATORIO study met its primary endpoint, showing treatment with ocrelizumab significantly reduced the risk of progression of clinical disability sustained for at least 12 weeks by 24 percent compared with placebo, as measured by the EDSS (p=0.0321). Additionally, ocrelizumab was superior to placebo in significantly reducing the risk of progression of clinical disability for at least 24 weeks by 25 percent (p=0.0365) and the time required to walk 25 feet (Timed 25-Foot Walk, or T25-FW) over 120 weeks by 29 percent (p=0.0404). Ocrelizumab decreased the volume of hyperintense T2 lesions by 3.4 percent over 120 weeks, compared to placebo which increased T2 volume by 7.4 percent (p<0.0001). Ocrelizumab reduced the rate of whole brain volume loss over 120 weeks by 17.5 percent compared to placebo (p=0.0206). Overall, the proportion of patients in the ocrelizumab group with adverse events was similar to placebo (95.1 percent vs percent, respectively); the most common adverse event associated with ocrelizumab was infusion-related reactions (39.9 percent vs percent for placebo). The proportion of patients in the ocrelizumab group with serious adverse events, including serious infections, was also similar to placebo (20.4 percent vs percent, respectively). Follow Roche on Twitter and keep up to date with 2015 congress of ECTRIMS news and updates by using the hashtag #ECTRIMS2015. About ocrelizumab Ocrelizumab is an investigational, humanised monoclonal antibody designed to selectively target CD20-positive B cells. CD20-positive B cells are a specific type of immune cell thought to be a key contributor to myelin (nerve cell insulation and support) and axonal (nerve cell) damage, which can result in disability in people with MS. Based on preclinical studies, ocrelizumab binds to CD20 cell surface proteins expressed on certain B cells, but not on stem cells or plasma cells, and therefore important functions of the immune system may be preserved. 4/6
5 The Phase III clinical development programme for ocrelizumab includes three studies: OPERA I, OPERA II and ORATORIO. About multiple sclerosis Multiple sclerosis (MS) is a chronic disease that affects an estimated 2.3 million people around the world, for which there is currently no cure. 3,4 MS occurs when the immune system abnormally attacks the insulation and support around nerve cells (myelin sheath) in the brain, spinal cord and optic nerves, causing inflammation and consequent damage. Damage to these nerves can cause a wide range of symptoms, including muscle weakness, fatigue and difficulty seeing, and may eventually lead to disability. 5,6,7 Most people with MS experience their first symptom between 20 and 40 years of age, making the disease the leading cause of non-traumatic disability in younger adults. 8 Relapsing MS is the most common form of the disease. Disease activity and progression can occur even when people do not show signs or symptoms of MS, despite available relapsing MS treatments. Primary progressive MS (PPMS) is a debilitating form of the disease marked by steadily worsening symptoms but typically without distinct relapses or periods of remission. 9 Approximately one in 10 people with MS are diagnosed with the primary progressive form of the disease. There are no approved treatments for PPMS. About Roche in neuroscience Neuroscience is a major focus of research and development at Roche. The company s goal is to develop treatment options based on the biology of the nervous system to help improve the lives of people with chronic and potentially devastating diseases. Roche has more than a dozen investigational medicines in clinical development for diseases that include multiple sclerosis, Alzheimer s disease, spinal muscular atrophy, Parkinson s disease, Down syndrome and autism. About Roche Headquartered in Basel, Switzerland, Roche is a leader in research-focused healthcare with combined strengths in pharmaceuticals and diagnostics. Roche is the world s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and neuroscience. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management. Roche s personalised 5/6
6 healthcare strategy aims at providing medicines and diagnostics that enable tangible improvements in the health, quality of life and survival of patients. Founded in 1896, Roche has been making important contributions to global health for more than a century. Twenty-nine medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and chemotherapy. In 2014, the Roche Group employed 88,500 people worldwide, invested 8.9 billion Swiss francs in R&D and posted sales of 47.5 billion Swiss francs. Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan. For more information, please visit roche.com. All trademarks used or mentioned in this release are protected by law. Rebif is a registered trademark of Merck KGaA and EMD Serono, Inc. Roche Group Media Relations Phone: / [email protected] - Nicolas Dunant (Head) - Ulrike Engels-Lange - Štěpán Kráčala - Nicole Rüppel - Claudia Schmitt References 1 F. Hoffmann-La Roche. ClinicalTrials.gov NCT and NCT National Library of Medicine. Available at: and 2 F. Hoffmann-La Roche. ClinicalTrials.gov NCT National Library of Medicine. Available at: 3 Multiple Sclerosis International Federation. (2013). Atlas of MS Available at: 4 National Institutes of Health-National Institute of Neurological Disorders and Stroke. (2015). Multiple Sclerosis: Hope Through Research. Available at: 5 Ziemssen T. (2005). Modulating processes within the central nervous system is central to therapeutic control of multiple sclerosis. J Neurol, 252(Suppl 5), v38-v45. 6 Hauser S.L. et al. (2012). Multiple sclerosis and other demyelinating diseases. In Harrison s Principles of Internal Medicine (pp ). New York, NY: McGraw Hill Medical. 7 Hadjimichael O. et al. (2007). Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain, 127(1-2), Multiple Sclerosis International Federation. What is MS? Available at Last accessed January MS International Federation. Types of MS. Available at: 6/6
Roche s marketing applications for review of OCREVUS (ocrelizumab) in two forms of multiple sclerosis accepted by EMA and FDA
 Media Release Basel, 28 June 2016 Roche s marketing applications for review of OCREVUS (ocrelizumab) in two forms of multiple sclerosis accepted by EMA and FDA OCREVUS is the first investigational medicine
Media Release Basel, 28 June 2016 Roche s marketing applications for review of OCREVUS (ocrelizumab) in two forms of multiple sclerosis accepted by EMA and FDA OCREVUS is the first investigational medicine
GENENTECH S OCRELIZUMAB FIRST INVESTIGATIONAL MEDICINE TO SHOW EFFICACY IN PEOPLE WITH PRIMARY PROGRESSIVE MULTIPLE SCLEROSIS IN LARGE PHASE III STUDY
 NEWS RELEASE Media Contact: Tara Iannuccillo (650) 467-6800 Investor Contacts: Stefan Foser Karl Mahler (650) 467-2016 011 41 61 687 8503 GENENTECH S OCRELIZUMAB FIRST INVESTIGATIONAL MEDICINE TO SHOW
NEWS RELEASE Media Contact: Tara Iannuccillo (650) 467-6800 Investor Contacts: Stefan Foser Karl Mahler (650) 467-2016 011 41 61 687 8503 GENENTECH S OCRELIZUMAB FIRST INVESTIGATIONAL MEDICINE TO SHOW
Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today that new data from three Phase III studies of the
 Media Release Basel, 12 April 2016 Roche to present new data at AAN showing superior efficacy of investigational medicine ocrelizumab versus comparators on disease activity and progression in two forms
Media Release Basel, 12 April 2016 Roche to present new data at AAN showing superior efficacy of investigational medicine ocrelizumab versus comparators on disease activity and progression in two forms
FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma
 Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy
 Media Release Basel, 6 June 2012 Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced
Media Release Basel, 6 June 2012 Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced
- Patients treated with alemtuzumab in CARE-MS II were more than twice as likely to experience disability improvement compared to Rebif -
 PRESS RELEASE Significant Improvement in Disability Scores Observed in Multiple Sclerosis Patients Who Received Lemtrada TM* (Alemtuzumab) Compared With Rebif in Phase lll Trial - Patients treated with
PRESS RELEASE Significant Improvement in Disability Scores Observed in Multiple Sclerosis Patients Who Received Lemtrada TM* (Alemtuzumab) Compared With Rebif in Phase lll Trial - Patients treated with
Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple Sclerosis
 Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Media Release. Basel, 11 June 2009. RA patients with enhanced response identified
 Media Release Basel, 11 June 2009 New data demonstrate the ability of MabThera to reduce the progression of joint damage when used as a first-line biologic treatment in rheumatoid arthritis RA patients
Media Release Basel, 11 June 2009 New data demonstrate the ability of MabThera to reduce the progression of joint damage when used as a first-line biologic treatment in rheumatoid arthritis RA patients
FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema
 Media Release Basel, 13 August 2012 FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema First major treatment advance in more than 25 years for sight-threatening condition
Media Release Basel, 13 August 2012 FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema First major treatment advance in more than 25 years for sight-threatening condition
Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis
 May 25, 2015 Contact: Shikha Virdi 905-919-0200 ext. 5504 Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis Rebif now reimbursed under Ontario Drug Benefit Program
May 25, 2015 Contact: Shikha Virdi 905-919-0200 ext. 5504 Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis Rebif now reimbursed under Ontario Drug Benefit Program
EMD Serono Presents New Data on Rebif (Interferon beta-1a) and Multiple Sclerosis Pipeline at Joint ACTRIMS-ECTRIMS Meeting in Boston
 Erin-Marie Beals Phone 1-781-681-2850 September 9, 2014 EMD Serono Presents New Data on Rebif (Interferon beta-1a) and Multiple Sclerosis Pipeline at Joint ACTRIMS-ECTRIMS Meeting in Boston Data include
Erin-Marie Beals Phone 1-781-681-2850 September 9, 2014 EMD Serono Presents New Data on Rebif (Interferon beta-1a) and Multiple Sclerosis Pipeline at Joint ACTRIMS-ECTRIMS Meeting in Boston Data include
Which injectable medication should I take for relapsing-remitting multiple sclerosis?
 Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Natalizumab (Tysabri)
 Natalizumab (Tysabri) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Natalizumab (Tysabri) Date of issue: July 2010 Review date: July 2011 Contents Section
Natalizumab (Tysabri) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Natalizumab (Tysabri) Date of issue: July 2010 Review date: July 2011 Contents Section
AUBMC Multiple Sclerosis Center
 AUBMC Multiple Sclerosis Center 1 AUBMC Multiple Sclerosis Center The vision of the American University of Beirut Medical Center (AUBMC) is to be the leading academic medical center in Lebanon and the
AUBMC Multiple Sclerosis Center 1 AUBMC Multiple Sclerosis Center The vision of the American University of Beirut Medical Center (AUBMC) is to be the leading academic medical center in Lebanon and the
Summary HTA. Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C. HTA-Report Summary
 Summary HTA HTA-Report Summary Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C Health policy background Multiple sclerosis (MS) is a chronic inflammatory disease
Summary HTA HTA-Report Summary Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C Health policy background Multiple sclerosis (MS) is a chronic inflammatory disease
Genzyme s Multiple Sclerosis Franchise Featured at AAN
 PRESS RELEASE Genzyme s Multiple Sclerosis Franchise Featured at AAN - Multiple Presentations Highlight Continuing Progress of AUBAGIO and LEMTRADA Programs - Paris, France March 13, 2013 Sanofi (EURONEXT:
PRESS RELEASE Genzyme s Multiple Sclerosis Franchise Featured at AAN - Multiple Presentations Highlight Continuing Progress of AUBAGIO and LEMTRADA Programs - Paris, France March 13, 2013 Sanofi (EURONEXT:
Biogen Idec Contacts: Media: Amy Brockelman (617) 914-6524 Investor: Eric Hoffman (617) 679-2812
 NEWS RELEASE Media: Nikki Levy (650) 225-1729 Investor: Susan Morris (650) 225-6523 Biogen Idec Contacts: Media: Amy Brockelman (617) 914-6524 Investor: Eric Hoffman (617) 679-2812 GENENTECH AND BIOGEN
NEWS RELEASE Media: Nikki Levy (650) 225-1729 Investor: Susan Morris (650) 225-6523 Biogen Idec Contacts: Media: Amy Brockelman (617) 914-6524 Investor: Eric Hoffman (617) 679-2812 GENENTECH AND BIOGEN
FORM 6-K. SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549. Report of Foreign Private Issuer
 FORM 6-K SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 under the Securities Exchange Act of 1934 For the month of August 2011
FORM 6-K SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 under the Securities Exchange Act of 1934 For the month of August 2011
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
Life with MS: Mastering Early Treatment
 Life with MS: Mastering Early Treatment Essential Information About MS Multiple sclerosis (MS) is a disease that attacks the central nervous system (CNS). Approximately 2.5 million people worldwide and
Life with MS: Mastering Early Treatment Essential Information About MS Multiple sclerosis (MS) is a disease that attacks the central nervous system (CNS). Approximately 2.5 million people worldwide and
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
What is Multiple Sclerosis? Gener al information
 What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
Clinical Trials of Disease Modifying Treatments
 MS CENTER CLINICAL RESEARCH The UCSF MS Center is an internationally recognized leader in multiple sclerosis clinical research. We conduct clinical trials involving the use of experimental treatments,
MS CENTER CLINICAL RESEARCH The UCSF MS Center is an internationally recognized leader in multiple sclerosis clinical research. We conduct clinical trials involving the use of experimental treatments,
A neurologist would assess your eligibility and suitability for the DMTs.
 Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Understanding How Existing and Emerging MS Therapies Work
 Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
Version History. Previous Versions. Policy Title. Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
A Definition of Multiple Sclerosis
 English 182 READING PRACTICE by Alyx Meltzer, Spring 2009 Vocabulary Preview (see bolded, underlined words) gait: (n) a particular way of walking transient: (adj) temporary; synonym = transitory remission:
English 182 READING PRACTICE by Alyx Meltzer, Spring 2009 Vocabulary Preview (see bolded, underlined words) gait: (n) a particular way of walking transient: (adj) temporary; synonym = transitory remission:
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS?
 Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
How to S.E.A.R.C.H. SM for the Right MS Therapy For You!
 How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
A Letter From the MS Coalition
 0 A Letter From the MS Coalition The treatment of multiple sclerosis (MS) requires a comprehensive management strategy. One important component of that strategy is modifying the disease course. When deciding
0 A Letter From the MS Coalition The treatment of multiple sclerosis (MS) requires a comprehensive management strategy. One important component of that strategy is modifying the disease course. When deciding
Programa Cooperación Farma-Biotech Neurociencias NT-KO-003
 Programa Cooperación Farma-Biotech Neurociencias NT-KO-003 A new oral treatment for Multiple Sclerosis based on a novel mechanism of action Barcelona, 15 de febrero 2011 Programa Cooperación Farma-Biotech
Programa Cooperación Farma-Biotech Neurociencias NT-KO-003 A new oral treatment for Multiple Sclerosis based on a novel mechanism of action Barcelona, 15 de febrero 2011 Programa Cooperación Farma-Biotech
A blood sample will be collected annually for up to 2 years for JCV antibody testing.
 Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
FORM 6-K. SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549. Report of Foreign Private Issuer
 FORM 6-K SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 under the Securities Exchange Act of 1934 For the month of September
FORM 6-K SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 under the Securities Exchange Act of 1934 For the month of September
Multiple Sclerosis (MS) is a disease of the central nervous system (including the brain and spinal cord) in which the nerves degenerate.
 What is Multiple Sclerosis? Multiple Sclerosis (MS) is a disease of the central nervous system (including the brain and spinal cord) in which the nerves degenerate. A disease of the central nervous system
What is Multiple Sclerosis? Multiple Sclerosis (MS) is a disease of the central nervous system (including the brain and spinal cord) in which the nerves degenerate. A disease of the central nervous system
Progress in MS: Current and Emerging Therapies
 Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Gold R, Giovannoni G, Selmaj K, et al, for
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Gold R, Giovannoni G, Selmaj K, et al, for
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
Teriflunomide (Aubagio)
 Teriflunomide (Aubagio) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 We hope you find the information in this factsheet helpful. If you would like to speak
Teriflunomide (Aubagio) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 We hope you find the information in this factsheet helpful. If you would like to speak
Understanding. Multiple Sclerosis. Tim, diagnosed in 2004.
 Understanding Multiple Sclerosis Tim, diagnosed in 2004. What Is Multiple Sclerosis? Multiple sclerosis (MS) is a neurologic disorder that affects the central nervous system (CNS). The CNS includes the
Understanding Multiple Sclerosis Tim, diagnosed in 2004. What Is Multiple Sclerosis? Multiple sclerosis (MS) is a neurologic disorder that affects the central nervous system (CNS). The CNS includes the
CBT IN THE CITY. adjusted to the news of being with MS? April 2013. Experts at your fingertips call now. Check out our new services in you local area
 April 2013 Experts at your fingertips call now CBT IN THE CITY Check out our new services in you local area contents. A message from Susie, Information Multiple Sclerosis CBT can make a difference on the
April 2013 Experts at your fingertips call now CBT IN THE CITY Check out our new services in you local area contents. A message from Susie, Information Multiple Sclerosis CBT can make a difference on the
fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK
 fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK 08 August 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK 08 August 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
Supplementary webappendix
 Supplementary webappendix This webappendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Giovannoni G, Gold R, Selmaj K, et al,
Supplementary webappendix This webappendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Giovannoni G, Gold R, Selmaj K, et al,
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU
 FOR MS RELAPSES Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU As a person with multiple sclerosis (MS), you know firsthand the profound impact MS relapses can have on your
FOR MS RELAPSES Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU As a person with multiple sclerosis (MS), you know firsthand the profound impact MS relapses can have on your
Version History. Previous Versions. for secondary progressive MS (SPMS) Policy Title. Drugs for MS.Drug facts box Interferon beta 1b
 Version History Policy Title Drugs for MS.Drug facts box Interferon beta 1b for secondary progressive MS (SPMS) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review
Version History Policy Title Drugs for MS.Drug facts box Interferon beta 1b for secondary progressive MS (SPMS) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review
Clinical Trials Need More Subjects
 Page 1 of 5 This copy is for your personal, non-commercial use only. To order presentation-ready copies for distribution to your colleagues, clients or customers visit http://www.djreprints.com. LIFE HEALTH
Page 1 of 5 This copy is for your personal, non-commercial use only. To order presentation-ready copies for distribution to your colleagues, clients or customers visit http://www.djreprints.com. LIFE HEALTH
Life with MS: Striving for Maximal Independence & Fulfillment
 Life with MS: Striving for Maximal Independence & Fulfillment St. Louis, May 7, 2005 Florian P. Thomas, MA, MD, PhD MS Center, Department of Neurology Associate Professor, Saint Louis University Brain
Life with MS: Striving for Maximal Independence & Fulfillment St. Louis, May 7, 2005 Florian P. Thomas, MA, MD, PhD MS Center, Department of Neurology Associate Professor, Saint Louis University Brain
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency London, 16 November 2006 Doc. Ref. CPMP/EWP/561/98 Rev. 1 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS FOR THE
European Medicines Agency London, 16 November 2006 Doc. Ref. CPMP/EWP/561/98 Rev. 1 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS FOR THE
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Managing Relapsing Remitting MS Risks & benefits of emerging therapies. Dr Mike Boggild The Walton Centre
 Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
News on modifying diseases therapies. Michel CLANET CHU Toulouse France ECTRIMS
 News on modifying diseases therapies Michel CLANET CHU Toulouse France ECTRIMS Current treatment strategies Future oral treatments Future non oral treatments Drug safety and risks CIS at risk of MS Active
News on modifying diseases therapies Michel CLANET CHU Toulouse France ECTRIMS Current treatment strategies Future oral treatments Future non oral treatments Drug safety and risks CIS at risk of MS Active
Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
 September 27, 2013 ONO PHARMACEUTICAL CO., LTD. Corporate Communications Phone: +81-6-6263-5670 Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
September 27, 2013 ONO PHARMACEUTICAL CO., LTD. Corporate Communications Phone: +81-6-6263-5670 Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
1. Comparative effectiveness of alemtuzumab
 Cost-effectiveness of alemtuzumab (Lemtrada ) for the treatment of adult patients with relapsing remitting multiple sclerosis with active disease defined by clinical or imaging features The NCPE has issued
Cost-effectiveness of alemtuzumab (Lemtrada ) for the treatment of adult patients with relapsing remitting multiple sclerosis with active disease defined by clinical or imaging features The NCPE has issued
IF YOU ARE RECEIVING TREATMENT WITH TYSABRI FOR RELAPSING-REMITTING MS (NATALIZUMAB)
 IF YOU ARE RECEIVING (NATALIZUMAB) TREATMENT WITH TYSABRI FOR RELAPSING-REMITTING MS Read the patient information leaflet that accompanies the medicine carefully. 1 This brochure is a supplement to the
IF YOU ARE RECEIVING (NATALIZUMAB) TREATMENT WITH TYSABRI FOR RELAPSING-REMITTING MS Read the patient information leaflet that accompanies the medicine carefully. 1 This brochure is a supplement to the
Lemtrada (alemtuzumab)
 Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook
 National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
How to evaluate medications in Multiple Sclerosis when placebo controlled RCTs are not feasible
 University of Florence Dept. of Neurosciences Careggi University Hospital Dept of Neurosciences How to evaluate medications in Multiple Sclerosis when placebo controlled RCTs are not feasible Luca Massacesi,
University of Florence Dept. of Neurosciences Careggi University Hospital Dept of Neurosciences How to evaluate medications in Multiple Sclerosis when placebo controlled RCTs are not feasible Luca Massacesi,
CNS DEMYLINATING DISORDERS
 CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON
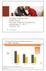 Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis Update. Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center
 Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Relapsing-remitting multiple sclerosis Ambulatory with or without aid
 AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Multiple sclerosis (MS)
 Multiple sclerosis (MS) Summary Multiple sclerosis (MS) is an incurable disease of the central nervous system that can affect the brain, spinal cord and optic nerves. The effects of MS are varied and unpredictable,
Multiple sclerosis (MS) Summary Multiple sclerosis (MS) is an incurable disease of the central nervous system that can affect the brain, spinal cord and optic nerves. The effects of MS are varied and unpredictable,
New Treatment Options for MS Patients: Understanding risks versus benefits
 New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
Understanding your Tecfidera treatment
 Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
Understanding your Tecfidera treatment
 Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
2.1 Who first described NMO?
 History & Discovery 54 2 History & Discovery 2.1 Who first described NMO? 2.2 What is the difference between NMO and Multiple Sclerosis? 2.3 How common is NMO? 2.4 Who is affected by NMO? 2.1 Who first
History & Discovery 54 2 History & Discovery 2.1 Who first described NMO? 2.2 What is the difference between NMO and Multiple Sclerosis? 2.3 How common is NMO? 2.4 Who is affected by NMO? 2.1 Who first
AMPYRA (dalfampridine) Important Safety Information
 NEWS RELEASE Acorda to Present New rhigm22 and AMPYRA (dalfampridine) Data at the 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) 10/7/2015 ARDSLEY, N.Y.--(BUSINESS
NEWS RELEASE Acorda to Present New rhigm22 and AMPYRA (dalfampridine) Data at the 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) 10/7/2015 ARDSLEY, N.Y.--(BUSINESS
New research in primary and secondary progressive multiple sclerosis. Dr Claire McCarthy MRCP PhD Neurology SpR Addenbrooke s Hospital
 New research in primary and secondary progressive multiple sclerosis Huntingdon MS Society Annual Meeting Sat 22nd March 2014 Dr Claire McCarthy MRCP PhD Neurology SpR Addenbrooke s Hospital 1 Overview
New research in primary and secondary progressive multiple sclerosis Huntingdon MS Society Annual Meeting Sat 22nd March 2014 Dr Claire McCarthy MRCP PhD Neurology SpR Addenbrooke s Hospital 1 Overview
The Prospect of Stem Cell Therapy in Multiple Sclerosis. Multiple sclerosis is a multifocal inflammatory disease of the central
 The Prospect of Stem Cell Therapy in Multiple Sclerosis Multiple sclerosis is a multifocal inflammatory disease of the central nervous system that generally affects young individuals, causing paralysis
The Prospect of Stem Cell Therapy in Multiple Sclerosis Multiple sclerosis is a multifocal inflammatory disease of the central nervous system that generally affects young individuals, causing paralysis
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 15 September 2005 Doc. Ref. EMEA/CHMP/EWP/561/98 Rev 1 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 15 September 2005 Doc. Ref. EMEA/CHMP/EWP/561/98 Rev 1 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
EMA and Progressive Multifocal Leukoencephalopathy.
 EMA and Progressive Multifocal Leukoencephalopathy. ENCePP Plenary, London 23 November 2011 Presented by: Henry Fitt Head of Coordination & Networking, Pharmacovigilance & Risk Management An agency of
EMA and Progressive Multifocal Leukoencephalopathy. ENCePP Plenary, London 23 November 2011 Presented by: Henry Fitt Head of Coordination & Networking, Pharmacovigilance & Risk Management An agency of
MS: from disease management to patient management
 2012 CME Annual meeting in multiple sclerosis MS: from disease management to patient management Valencia, Spain 18-19 May 2012 Dear Colleague On behalf of the Serono Symposia International Foundation we
2012 CME Annual meeting in multiple sclerosis MS: from disease management to patient management Valencia, Spain 18-19 May 2012 Dear Colleague On behalf of the Serono Symposia International Foundation we
OHTAC Recommendation
 OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
Using the MS Clinical Course Descriptions in Clinical Practice
 Using the MS Clinical Course Descriptions in Clinical Practice Mark J. Tullman, MD Director of Clinical Research The MS Center for Innovations in Care Missouri Baptist Medical Center Disclosures Consultant/speaking
Using the MS Clinical Course Descriptions in Clinical Practice Mark J. Tullman, MD Director of Clinical Research The MS Center for Innovations in Care Missouri Baptist Medical Center Disclosures Consultant/speaking
About MS. An introduction to. An introduction to multiple sclerosis for people who have recently been diagnosed. What is MS? Is it common?
 An introduction to multiple sclerosis for people who have recently been diagnosed When you have just been diagnosed with multiple sclerosis, you will probably have many questions about the condition and
An introduction to multiple sclerosis for people who have recently been diagnosed When you have just been diagnosed with multiple sclerosis, you will probably have many questions about the condition and
Trauma Insurance Claims Seminar Invitation
 Trauma Insurance Claims Seminar Invitation Introduction Since the development of Trauma Insurance in Australia in the 1980s, the product has evolved at a great pace. Some of the challenges faced by claims
Trauma Insurance Claims Seminar Invitation Introduction Since the development of Trauma Insurance in Australia in the 1980s, the product has evolved at a great pace. Some of the challenges faced by claims
Growth in revenue from MS drugs has been driven largely by price increases over the last several years.
 March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
The MS Disease- Modifying Medications GENERAL INFORMATION
 The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
NHS BOURNEMOUTH AND POOLE AND NHS DORSET
 NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
fingolimod (as hydrochloride), 0.5mg hard capsules (Gilenya ) SMC No. (763/12) Novartis Pharmaceuticals UK Ltd
 fingolimod (as hydrochloride), 0.5mg hard capsules (Gilenya ) SMC No. (763/12) Novartis Pharmaceuticals UK Ltd 10 February 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the
fingolimod (as hydrochloride), 0.5mg hard capsules (Gilenya ) SMC No. (763/12) Novartis Pharmaceuticals UK Ltd 10 February 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Clinical and Therapeutic Cannabis Information. Written by Cannabis Training University (CTU) All rights reserved
 Clinical and Therapeutic Cannabis Information Written by Cannabis Training University (CTU) All rights reserved Contents Introduction... 3 Chronic Pain... 6 Neuropathic Pain... 8 Movement Disorders...
Clinical and Therapeutic Cannabis Information Written by Cannabis Training University (CTU) All rights reserved Contents Introduction... 3 Chronic Pain... 6 Neuropathic Pain... 8 Movement Disorders...
Clinically isolated syndrome (CIS)
 Clinically isolated syndrome (CIS) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 We hope you find the information in this factsheet helpful. If you would
Clinically isolated syndrome (CIS) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 We hope you find the information in this factsheet helpful. If you would
Multiple Sclerosis: An imaging review and update on new treatments.
 Multiple Sclerosis: An imaging review and update on new treatments. Dr Marcus Likeman Consultant Neuroradiologist North Bristol NHS Trust Bristol Royal Hospital for Children MRI appearances - White Matter
Multiple Sclerosis: An imaging review and update on new treatments. Dr Marcus Likeman Consultant Neuroradiologist North Bristol NHS Trust Bristol Royal Hospital for Children MRI appearances - White Matter
