Effect of systemic antibiotics on clinical and patient-reported outcomes of implant therapy a multicenter randomized controlled clinical trial
|
|
|
- Jared Lawrence
- 8 years ago
- Views:
Transcription
1 Wah Ching Tan Marianne Ong Jie Han Nikos Mattheos Bjarni E. Pjetursson Alex Yi-Min Tsai Ignacio Sanz May C.M. Wong Niklaus P. Lang on Behalf of the ITI Antibiotic Study Group Effect of systemic antibiotics on clinical and patient-reported outcomes of implant therapy a multicenter randomized controlled clinical trial Authors affiliations: Wah Ching Tan, Marianne Ong, National Dental Centre Singapore, Singapore, Singapore Jie Han, Peking University School of Stomatology, Beijing, China Nikos Mattheos, Griffith University, Gold Coast, Queensland, Australia Nikos Mattheos, May C.M. Wong, Niklaus P. Lang, Faculty of Dentistry, The University of Hong Kong, Hong Kong SAR, China Bjarni E. Pjetursson, Faculty of Odontology, University of Iceland, Reykjavik, Iceland Alex Yi-Min Tsai, Department of Periodontology, School of Dental Medicine, National Taiwan University, Taipei, Taiwan Ignacio Sanz, Universidad Complutense de Madrid, Madrid, Spain ETEP Research Group Corresponding author: Dr. Wah Ching Tan, BDS, MDS, Dr.med.dent. Consultant, National Dental Centre Singapore 5 Second Hospital Avenue Singapore Singapore Tel.: Fax: drwahching@yahoo.com.sg Key words: complications, dental implants, failures, implant dentistry, patient-reported outcomes, success, survival, systemic antibiotics Abstract Objectives: To determine the effect of various systemic antibiotic prophylaxis regimes on patientreported outcomes and postsurgical complications in patients undergoing conventional implant installation. Material and methods: Three hundred and twenty-nine healthy adults in need of conventional implant installation were randomly assigned to one of four groups: (i) preoperatively 2 g of amoxycillin 1 h before surgery (positive control, ), (ii) postoperatively 2 g of amoxycillin immediately following surgery (test 1, ), (iii) preoperatively 2 g of amoxycillin 1 h before and 500 mg thrice daily on days 2 and 3 after surgery (test 2, ), (iv) preoperatively 2 g of placebo 1 h before surgery (negative control, ). Subjects were examined clinically by blinded examiners over 8 weeks after implant installation. In addition, Visual Analogue Scales (VAS) for pain, swelling, bruising and bleeding were obtained over 14 days. ANOVA was performed for the VAS. Chi-square tests were applied for postsurgical complications. Results: All VAS scores were low for all groups and decreased over time (P < 0.001). There were no significant differences for the VAS scores between the various groups at any time point (P > 0.05). There was only a significant difference in flap closure at week 4, where had 5% of the subjects not achieving complete wound closure compared to 0% for the three other groups (P = 0.01), with no other significant differences for any postsurgical complications (P > 0.05). Conclusion: For standard single implant placement, prophylactic systemic antibiotics either before or after, or before and after the surgical procedure do not improve patient-reported outcomes or prevalence of postsurgical complications. Conflicts of interest: The authors declare no conflict of interest. Date: Accepted 24 November 2012 To cite this article: Tan WC, Ong M, Han J, Mattheos N, Pjetursson BE, Tsai AY-M, Sanz I, Wong MCM, Lang NP. Effect of systemic antibiotics on clinical and patient-reported outcomes of implant therapy a multicenter randomized controlled clinical trial. Clin. Oral Impl. Res. 00, 2013, 1 9 doi: /clr The use of prophylactic antibiotics against postsurgical infection has largely been advocated. However, the effects of such measures remain obscure, and controversial beneficial outcomes have been reported in randomized controlled clinical trials so far for oral surgical procedures (Monaco et al. 2009; Siddiqi et al. 2010; Pasupathy & Alexander 2011). In the field of oral implant dentistry, the use of systemic antibiotics remains a controversial issue, and various antibiotic regimes have been propagated without providing scientific evidence for them. Some authors (Laskin et al. 2000) reported higher survival rates with the application of preoperative antibiotics, while others (Gynther et al. 1998) found no difference in postoperative infection and survival rates of implants with pre- and posttreatment antibiotics when compared to a control group without them. Moreover, no additional benefits were found with the use of antibiotics when compared to controls in a recent randomized controlled clinical trial (Abu-Ta a et al. 2008). No significant advan John Wiley & Sons A/S. Published by Blackwell Publishing Ltd 1
2 tages pertaining to postsurgical infection were found with the use of perioperative antibiotics for implant surgeries, provided proper asepsis was established. Furthermore, a multicenter placebo-controlled randomized clinical trial (RCT) (Anitua et al. 2009) on antibiotic prophylaxis with placement of single dental implants confirmed no statistically significant differences for postsurgical infection, adverse events, and implant failures between the groups. These two RCTs were analyzed together with two further RCTs from Italy (Esposito et al. 2008, 2010a) in a recent Cochrane systematic review (Esposito et al. 2010b) with a follow-up of at least 3 months comparing various prophylactic antibiotic regimes against administration of a placebo. A statistically significantly higher number of patients with implant failures was reported in the placebo group, with a risk ratio of 0.4. The authors concluded that there is some evidence suggesting that the use of preoperative antibiotics may reduce implant failures. It has to be realized, however, that the degree of oral cleanliness prior to implant installation was not well documented in these studies. At present, there is still a lack of largescale multicenter studies to support or refute the need of antibiotic prophylaxis with conventional implant placement. Some authors recommended antibiotic prophylaxis with the procedure based on cohort studies and anecdotal experience (Dent et al. 1997). With the increasing demand for oral implants worldwide and the development of antibiotic resistance due to indiscriminate usage, the use of antibiotics with conventional implant therapy should be reevaluated and proper guidelines for implant installation established. The question of whether or not the potential benefit of antibiotic prophylaxis with conventional implant therapy outweighs the risk of developing antibiotic resistance remains to be determined. Moreover, there are no data on the effect of perioperative administration of systemic antibiotics in implant surgery on patient-reported outcomes. As demonstrated in a retrospective cohort study (Powell et al. 2005), the prevalence of infection following periodontal surgery was low (2.09%). It was concluded that, although perioperative antibiotics were commonly used when performing regenerative and implant procedures, data from this and other studies suggested that there may be no benefit in using antibiotics for the sole purpose of preventing postsurgical infections. Likewise, it may be assumed that the use of perioperative antibiotics in implant installation may be of questionable value owing to the low prevalence of infection associated with implant installation. Based on the limitation of the present evidence available due to the lack of RCTs with a large subject pool, there is no clear evidence to recommend or contraindicate the use of antibiotics to prevent infections with oral implant placement. Neither is there any established protocol of pre-, peri-, or postoperative administration of antibiotics. Hence, the aims of the present multicenter RCT were to determine the effects of various systemic antibiotic prophylaxis regimes on patient-reported outcome measures (PROMs) and prevalence of postsurgical complications in patients undergoing conventional implant installation. Material and methods Subject population Three hundred and twenty-nine healthy adults were consecutively admitted to seven study centers (National Dental Centre Singapore (NDC), Singapore; The University of Hong Kong, Faculty of Dentistry, Hong Kong SAR; Peking University School of Stomatology, Beijing, PR China; Griffith University, Gold Coast, Queensland, Australia; Universidad Complutense de Madrid, Madrid, Spain; National Taiwan University, Taipei, Taiwan & University of Iceland, Reykjavik, Iceland) worldwide for conventional oral implant therapy. The subjects were recruited between August 2009 and October Ethical aspects The study protocol was submitted to and approved by the respective institutional review boards of the seven institutions. The subjects were all informed about the purpose of the study and the risks and benefits associated with it. Informed consent was obtained for all patients. The following admission criteria to the study were observed: Inclusion criteria a Medically healthy adults (ASA classification I II), aged 19 years b Preferably nonsmokers or previous smokers (quit 5 years), light smokers with <20 cigarettes/day c No allergies to amoxycillin or penicillin antibiotics d Single tooth edentulous space in the maxilla or mandible with adequate pristine bone for a standard oral implant placement without the need of simultaneous bone augmentation (bucco-lingual dimension 7 mm, mesio-distal dimension 7mm and height 8 mm) Exclusion criteria Subjects with any of the following exclusion criteria at baseline were excluded from the study: a Medically compromised subjects (ASA classification III V) b Subjects requiring antibiotic prophylaxis prior to dental treatment c Subjects aged <19 years d Heavy smokers or previous heavy smokers (quit <5 years; 20 cigarettes/day) e Allergic to amoxycillin or penicillin antibiotics f Use of any form of antibiotics in the last 3 months g Pregnant, intend to conceive or breast-feeding woman h Single tooth edentulous space in the maxilla or mandible with inadequate pristine bone volume for standard oral implant placement, with a possible need for bone augmentation (bucco-lingual dimension <7 mm, mesio-distal dimension <7 mm, and height <8 mm). The interventions involved conventional implant installation with or without antibiotic prophylaxis. The oral implants were placed into pristine bone, without any simultaneous bone augmentation. Only one implant system (Straumann â Institute, Basel, Switzerland) was used to minimize confounding factors that might affect the outcome. The implants used had a moderately rough (SLA) surface and were in the range of 8 12 mm in length. The diameters of the implants were 3.3, 4.1 or 4.8 mm. The implants were either Standard Plus â or Bone Level â implants. A one-stage implant installation protocol was employed with the placement of healing abutments, without the need for a second-stage procedure to expose the implant. Investigator calibration The study was a blinded randomized controlled clinical trial (RCT) with four treatment arms. Prior to commencement of the study, a 2-day investigator and examiner standardization and calibration meeting was held at the National Dental Centre Singapore, Singapore. Clinical procedures The subjects were randomly assigned to one of four groups (2 test and 2 control groups): 2 Clin. Oral Impl. Res. 0, 2013 / John Wiley & Sons A/S. Published by Blackwell Publishing Ltd
3 Group 1 (positive control, ): 2 g of amoxycillin preoperatively, 1 h prior to conventional implant placement. Group 2 (test 1, ): 2 g of amoxycillin immediately postoperatively. Group 3 (test 2, ): 2 g of amoxycillin preoperatively, 1 h prior to implant placement and 500 mg three times a day (8 hourly) on days 2 and 3. Group 4 (negative control, ): 2 g of a placebo preoperatively, 1 h prior to implant placement without any antibiotics. The medications were prescribed by a designated clinical coordinator in each center, who was not involved as a surgeon or examiner. With this scheme, the investigators, the examiners and the surgeons were blinded, although the patients were not. Randomization and Allocation concealment All patients were recruited for comprehensive dental care. Periodontal and endodontic health was established prior to surgical interventions. Following a hygienic phase, all patients were reevaluated for their oral health and healing response to periodontal therapy, and a surgical treatment plan was established. Following this, the patients were entered into the study using randomization tables allocating the patient a number with a corresponding envelope. The randomization tables were prepared for each center separately by a biostatistician (MCMW). Blocked randomization was performed in blocks of eight, whereby at every block of eight enrollments, there were two subjects randomly assigned to one of the four intervention groups. The envelope contained the allocation to one of the four groups and was opened 1 h prior to the surgical intervention by a registered dental surgery assistant owing to the fact that one test and the two control groups had to take the medication prior to implant placement. In this way, the allocation to one of the treatment groups remained obscure to the operating surgeon. Outcome variables Subjects were examined clinically by calibrated examiners at week 1, 2, 4, and 8 following implant installation for postoperative complications. In addition, Visual Analogue Scales (VAS) on a score of 0-10 were obtained from the patients from day 1 through 7 and day 14. The surgeons involved had no access to the data collection sheets or the group allocation, while the examiners had no access to the patients treatment records or group allocation. The parameters examined included: Primary outcome variable: Patientreported outcomes: VAS score on pain, swelling, bruising and bleeding. Secondary outcome variables: Clinical recordings of flap closure, pain, swelling, suppuration, and implant stability by calibrated examiners. The sequence of events is presented in Fig. 1. Statistical analysis Sample size calculation and power analysis For the sample size calculation, the power analysis was performed for a one-way fixed effects analysis of variance (ANOVA) with 4 levels. The criterion for significance was set at a = 0.05 (type I error) and at b = 0.20 (type II error). The analysis of variance is nondirectional (i.e., two-tailed) indicating that an Positive control () Pre-operative antibiotics effect in either direction will be interpreted. If an effect of 2.5 VAS units difference (largest mean smallest mean) among the four treatment groups is expected, the sample size is 45 cases per group. However, if an effect of 2.0 VAS units is expected, the sample size is 70 cases per group. Calculating a dropout rate of 20%, n = 84 per group resulting in a power of 0.80 for an effect size of 2.0 VAS units at a level of significance of a = Statistical and analytical methods Chi-square (or Chi-square exact) tests were used to compare the percentage distribution of postsurgical complications at weeks 1, 2, 4, and 8 among the 4 treatment groups. Repeated measures ANOVA were performed on the VAS scores with the use of multivariate tests (Wilk s Lambda) for the effect of time and the interaction effect between treatment groups and time (all effects considered to be fixed). The above analyses were per- Baseline (Recruitment) - consent - baseline probing depths on adjacent teeth - implant site analysis (stent fabrication, radiographs) - randomisation Test Group 1 () Post-operative antibiotics Standard Implant Placement - pre-operation 0.2 % chlorhexidine for 1 minute - crestal incision - 1-stage implant placement - 1 implant system: Straumann SLA - record: bone dimension, buccal bone thickness, soft tissue dimension, bone quality Review visits at 1 week, 2 weeks, 1 month - Complications (pain, swelling, suppuration, flap closure, implant stability) - VAS scale (swelling, bruising, bleeding, pain) (Day 7,14 only) Fig. 1. Sequence of Events. Test Group 2 () Pre- and postoperative antibiotics Day 1 Day 6, VAS scale on swelling, bruising, bleeding and pain (patient self-assessment) 1 month Prosthodontic management (at 8 weeks) - impression-taking, issue of crown - record: implant stability, pain, swelling, purulent discharge Negative Control () No antibiotics, preoperative placebo 2013 John Wiley & Sons A/S. Published by Blackwell Publishing Ltd 3 Clin. Oral Impl. Res. 0, 2013 / 1 9
4 formed using SPSS. To analyze the possible center effect, a random effect for center (different centers considered to be a random sample of centers) was added to the above repeated measures ANOVA using SAS. Results The surgical procedures of all 329 subjects were uneventful. 329 SLA Straumann â implants were installed in a nonsubmerged healing modality. 81 subjects were positive control (), 82 subjects were test group 1 (), 86 subjects were test group 2 (), and 80 subjects were the negative control (). Distribution of the subjects Upon analyzing the data, there were no significant differences (all P > 0.05) in subject profile among the four treatment groups in terms of age, gender, smoking status, sites of implantation profile, and implant dimensions (Table 1). The subjects were comparable in the four treatment groups in terms of demographic characteristics and clinical parameters as well. The percentage of patients who took analgesics did not differ significantly (P > 0.05) between the groups over the different postoperative days (Table 2). Postsurgical complications Flap closure There was only one statistically significant difference (P = 0.01) for flap closure at week 4, where had 5% of subjects not achieving complete wound closure compared to 0% for the three other groups. There was no significant difference in the other time points (P > 0.05) among the 4 groups either (Table 3). Pain There was no statistically significant difference in the experience of pain between the 4 treatment groups at any time (P > 0.05; Fig. 2). The experience of pain was present in 17.1% of all the subjects at 1 week. Pain perception decreased over time, and at week 8, none of the subjects complained of pain. Swelling There was no statistically significant difference in swelling of the operation site between the four treatment groups at any time (P > 0.05; Fig. 3). Swelling was present in 21.4% of all the subjects at 1 week, with having the highest proportion of 27.1% and having the lowest proportion of 17.5%. This decreased over time, and at week 8, only 0.6% of the subjects had swelling noted clinically. Table 1. Baseline characteristics of the subjects and clinical parameters in different groups (n = 81) (n = 82) (n = 86) (n = 80) Total (n = 329) P Age (Mean) Gender (%) Male Female Smoking status (%) Non-smoker * Previous smoker Light smoker Smoker Characteristics of the edentulous sites Alveolar Bone Width B-L (Mean, mm) Alveolar Bone Width M-D (Mean, mm) Buccal Bone Plate Thickness (Mean, mm) Soft Tissue Thickness (Mean, mm) Bone Quality (%) I * II III IV Diameter of the implants used (%) 3.3 mm * 4.1 mm mm Length of the implants used (%) 8 mm mm mm * Chi-square exact test. Suppuration There was no statistically significant difference in suppuration of the operation site between the four treatment groups at any time (P > 0.05; Table 3). Less than 1% of all the subjects yielded suppuration of the operation site. and groups showed no suppuration of the sites throughout the 8-week follow-up. Implant stability There was no statistically significant difference in implant stability between the four groups at any time (P > 0.05; Table 3). At week 1, one implant of the was slightly mobile, but became stable thereafter. In the group, two implants were slightly mobile after 2 weeks and one of them became stable thereafter. The second yielded slight mobility also after 4 weeks, but no more after 8 weeks. At 8 weeks, another implant in group was slightly mobile and was lost. Patient-reported outcome measures All the VAS scores reported by the patients were low. Results from the repeated measures ANOVA showed that there was no statistically significant difference between the 4 treatment groups for bleeding, swelling, pain, and bruising (all P > 0.05; Figs 4 7). There was a significant time effect, where the mean VAS scores decreased over time (P < 0.001). However, no significant interaction effect between the treatment groups and time suggested that the decrease in the mean VAS scores in different treatment groups was not significantly different from each other for all the variables assessed. When adjusted for the center effect, the same results remained that no statistically significant differences existed among the 4 treatment groups for all outcomes, but for a significant time effect (data not reported). Discussion The present study failed to identify any statistically significant differences in patientreported outcomes between the two treatment and the two control groups. This, in turn, means that the administration of pre-, peri-, or postsurgical prophylactic antibiotics did not influence the wound healing pattern and the subjective variables at all. It has to be kept in mind, however, that all the centers participating in this study applied high standards of infection control practices for the implant surgical procedures. Moreover, the patients had been treated periodontally prior to the implant installation, and chlorh- 4 Clin. Oral Impl. Res. 0, 2013 / John Wiley & Sons A/S. Published by Blackwell Publishing Ltd
5 Table 2. Percentages of subjects who took analgesics. Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7 Day Total P-value * 0.07 * 0.34 * * Chi-square exact test. Table 3. Percentages of subjects with post-surgical outcome variables in the different groups at weeks 1, 2, 4, and 8 after surgery (n = 81) (n = 82) (n = 86) (n = 80) Total (n = 329) P Flap Closure 1 week weeks weeks * 8 weeks Pain 1 week weeks weeks weeks N/A Swelling 1 week weeks weeks weeks Suppuration 1 week N/A 2 weeks weeks weeks Implant stability 1 week weeks weeks weeks *Statistically significant; Chi-square exact test. Fig. 2. Percentages of subjects with pain during examination at Weeks 1, 2, 4, and 8 after surgery. exidine mouthrinses were applied prior to surgery. Consequently, a high degree of oral cleanliness was achieved. Because there is no data on the effect of perioperative administration of systemic antibiotics in implant surgery on patient-reported outcomes in the literature, this study provides evidence that perisurgical antibiotic Fig. 3. Percentages of subjects with swelling during examination at Weeks 1, 2, 4 and 8 after surgeries. prophylaxis does not provide beneficial effects on bruising, swelling, bleeding, or pain. This statement applies to straightforward implant installations in patients with no residual periodontal disease and in a clean oral environment. The present study has further demonstrated that the perioperative administration of prophylactic antibiotics did not show any statistically significant differences in clinical parameters such as flap closures, suppuration, swelling, implant stability, and pain on palpation. Regarding implant stability, one implant in the group was mobile at 8 weeks and lost thereafter. Likewise, in the group, one implant was slightly mobile after 1 week and gained stability thereafter. While the two and groups did not reveal any mobile implants at any time, the group presented two more implants with slight mobility that reached stability thereafter. Hence, it may be speculated that these slightly mobile implants were in the process of gaining biological bonding (osseointegration) during the early weeks of healing, while mechanical stability was lost as a result of bone resorption in the area of implant threads (Abrahamsson et al. 2004; Lang et al. 2011). Because both positive and negative controls were affected by very few implants yielding slight mobility, this phenomenon may not be attributable to the administration of prophylactic antibiotics. Moreover, these findings are in agreement with results from two RCTs (Abu-Ta a et al. 2008; Anitua et al. 2009). One of these compared the effects of the antibiotic regime in the present study to the group, and the second RCT compared the group antibiotic regime of the present study to the group. In the first study (Abu-Ta a et al. 2008), 40 patients received 1 g of amoxycillin one hour before and 2 g postoperatively for 2 days, while the control group (n = 40) received a placebo drug. No significant advantages regarding postoperative infections were reported. The second RCT (Anitua et al. 2009) reported on 52 patients with and 53 patients without presurgical (one hour before) administration of 2 g of amoxycillin. Again, no statistically significant differences in postsurgical infection rates were observed. The results of the present study were not identical to those of additional two RCTs. In the third RCT (Esposito et al. 2008), 158 patients were given 2 g of amoxycillin one hour prior to implant installment, thus representing the group of the present study. They were compared with 158 placebocontrolled () patients. Three patients in the antibiotic test group and two patients in the placebo group presented with postsurgical infections. However, eight patients in the control group lost nine implants, while only two implants in two patients were lost in the test group. Although not statistically significant, a slight beneficial effect of perioperative antibiotic administration was claimed. The fact that 10 patients of 316 lost an implant 2013 John Wiley & Sons A/S. Published by Blackwell Publishing Ltd 5 Clin. Oral Impl. Res. 0, 2013 / 1 9
6 Day1 Day2 Day3 Day4 Day5 Day6 Day7 Day Total Effects p-value Treatment groups Time <0.001 Treatment groups * Time Fig. 4. Mean VAS of bleeding (Bleeding-VAS) from Day 1 to Day 14. Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7 Day Total Effects p-value Treatment groups Time <0.001 Treatment groups * Time Fig. 5. Mean VAS of swelling (Swelling -VAS) from Day 1 to Day 14. postoperatively represents a relatively high failure rate (3%) for the time being. The reasons for these infections may have been a suboptimal periodontal infection control prior to implant installation or unsatisfactory oral hygiene practices. In the present study with emphasis on comprehensive dental care and pre-implantation infection control, only one implant was lost of 329 resulting in a failure rate of 0.3%. Moreover, initial implant losses failing a successful incorporation may be attributed to other reasons than infection, such as confounders from the surgical procedure. The high failure rate (3%) would suggest that this was the case in the study discussed (Esposito et al. 2008), and hence, implant losses should not be taken as evidence for the beneficial effect of prophylactic antibiotic coverage. In the fourth and last RCT cited (Esposito et al. 2010a), a total of 252 test and 254 control patients were enrolled, again, testing the regime of the group in the present study against negative controls () receiving placebo. Four patients in the test and eight patients in the control group experienced signs of infection with no statistically significant difference. Five patients in the test group experienced the loss of seven implants, and 12 patients with 13 implant losses were encountered in the control group. Again, it has to be realized that the failure rate through infection was 4% in that study. As mentioned above, initial implant losses may be attributed to other reasons than infection, such as confounders from the surgical procedure. Hence, implant losses should not be taken as evidence for a beneficial effect of prophylactic antibiotic coverage as well, even though trends clearly favoured the antibiotic group (Esposito et al. 2010a). Although the prophylactic antibiotic regime was not the same in the four RCTs (one study compared of the present study to, three studies compared to ), they were meta-analyzed in a recent systematic review (Esposito et al. 2010b) for the survival (incorporation) rate of implants under prophylactic antibiotics. The number needed to treat (NNT) to prevent one patient from losing an implant was calculated as NNT = 33. In light of the fact that early implant failures may be attributed to a variety of aspects including host parasite, surgical or even prosthetic factors, one implant loss in the present study of over 300 implants may have occurred entirely by chance, and hence, a preventive effect of the perisurgical antibiotic regimen may be highly questionable. Because implant survival (incorporation rate) is very high in today s clinical practice, most of the studies addressing this parameter in relation to perioperative antibiotic prophylaxis must be considered as underpowered (Esposito et al. 2010b). Only the meta-analysis of four underpowered studies (Abu-Ta a 6 Clin. Oral Impl. Res. 0, 2013 / John Wiley & Sons A/S. Published by Blackwell Publishing Ltd
7 Day1 Day2 Day3 Day4 Day5 Day6 Day7 Day Total Effects Treatment groups Time Treatment groups * Time Fig. 6. Mean VAS of pain (Pain-VAS) from Day 1 to Day 14. et al. 2008; Esposito et al. 2008, 2010a; Anitua et al. 2009) revealed a risk ratio of 0.4 (95% CI: ) for early implant failures. p-value < Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7 Day Total Effects p-value Treatment groups Time <0.001 Treatment groups * Time Fig. 7. Mean VAS of bruising (Bruising -VAS) from Day 1 to Day 14. As indicated above, these implant failures cannot be taken as evidence of the beneficial effect of perisurgical prophylactic antibiotics, owing to the presumptive surgical confounders. In the meta-analysis of the four studies mentioned, the risk ratio for postsurgical infection was 0.74 (95% CI: ). In this relevant parameter for the effect of perioperative antibiotic administration, statistical significance was not reached indicating basically a lack of benefit from the antibiotic regimes. Because the primary goal of the present study was to determine the effects of various systemic antibiotic prophylaxis regimes on patient-reported outcome measures (PROMs) and postsurgical complications in patients undergoing conventional implant installation, the power calculation had to be based on clinically meaningful differences in the patient-reported outcome measures rather than on survival rates of implants. In the present study, no technical complications were diagnosed within the observation period irrespective of the group allocation of the patients. This may be due to the fact that only one implant system was used, thus minimizing the various possibilities of technical complications. On the other hand, biological complications (mucositis, peri-implantitis) have to be considered as generic and not related to implant systems (Abrahamsson et al. 1998; Albouy et al. 2008, 2009). In the present study, one case of surgical complication (flap opening), one case of suppuration, two cases of swelling, and one case of implant mobility were identified in over 300 implant installations amounting to a short-term success rate of 98.5%. This may be testimony of a very meticulous presurgical periodontal and carefully performed surgical procedures leaving the oral cavity in a status of cleanliness. In the present study, three regimens of amoxycillin were chosen as this drug is a suitable first-line antimicrobial agent demonstrating a high level of antimicrobial activity in patients with dento-alveolar infections (Kuriyama et al. 2007). Amoxycillin is indicated in the treatment of patients with mild to moderate infections caused by susceptible strains of microorganisms. The preoperative dosage is 2 g, one hour before treatment (Wilson et al. 2007). The preoperative dosage of amoxycillin applied in the present study was based on the antibiotic dosage recommendation by the American Heart Association (AHA) for prevention of infective endocarditis (Wilson et al. 2007). The single-dose regime used postoperatively was to test whether a postoperative dose is equally effective compared to the positive control. The regime used in the 2013 John Wiley & Sons A/S. Published by Blackwell Publishing Ltd 7 Clin. Oral Impl. Res. 0, 2013 / 1 9
8 group was modified from the old AHA recommendation for prevention of infective endocarditis. None of the patient-reported or clinical outcomes differed in any of the three antibiotic groups. This, in turn, means that there was no superiority for any of the prophylactic regimes. This is in agreement with previous studies (Morris et al. 2004; Binahmed et al. 2005), where the authors reported that the use of various antibiotic regimes did not affect clinical outcomes after implant installation. Up to now, predominantly 2 g of amoxycillin one hour prior to surgery has been recommended without scientific evidence. The results of the present study suggest that the post- and perisurgical administration of prophylactic antibiotics may elicit the same subjective and clinical effects. In conclusion, the results of the present study question the necessity of applying perisurgical antibiotic prophylaxis either before, at the time of or after implant installation. Because most of the patients recruited for the study belonged to the category of straightforward difficulty for surgical implant installation, the conclusions have to be limited to such patients. To what extent antibiotics may be indicated for augmentation procedures (advanced category) remains to be explored. Likewise, the potential influence of antibiotic prophylaxis in patients with untreated periodontitis and poor oral hygiene or poor operation room asepsis has to be determined. Acknowledgements: This study has been supported by a research grant of the ITI Foundation (Project ) and the Clinical Research Foundation (CRF) for the Promotion of Oral Health, Brienz, Switzerland. The cooperation of the staff of the centers involved in the study is highly appreciated: National Dental Centre Singapore, Department of Restorative Dentistry, ACORN secretariat; The University of Hong Kong, Faculty of Dentistry, Implant Dentistry; Peking University School of Stomatology, Beijing, PR China, Department of Periodontology; National Taiwan University, Taipei, Taiwan, Department of Periodontology; Griffith University, Gold Coast, Queensland, Australia, Department of Periodontology; University Complutense of Madrid, Spain, Department of Periodontology; University of Iceland, Reykjavik, Department of Reconstructive Dentistry. The cooperation of Mr. Martin Wacker, Straumann AG, Basel, Switzerland, is gratefully acknowledged. Appendix The ITI Antibiotic Study Group National Dental Centre Singapore, Singapore: Wah Ching Tan, Chu Guan Koh, Mervyn Ng, Marianne Ong, Ching Ching Tan, Li Beng Wong, Gui Feng Shen, Jia Yin Chen, Ping Liang, Audrey Ho The University of Hong Kong, Hong Kong SAR PR China: Niklaus P. Lang, Martina Lulic, Boyd W.K. Tsang, May C.M. Wong Peking University School of Stomatology, Beijing PR China: Jie Han, Huang Xin Meng Griffith University, Gold Coast, Queensland, Australia: Nikos Mattheos Universidad Complutense de Madrid, Spain: Ignacio Sanz, David Herrera, Mariano Sanz, Fabio Vignoletti National Taiwan University, Taipei, Taiwan: Alex Yi-Min Tsai, Cheing-Meei Liu, Po-Yao Chang, Che-Chang Tu University of Iceland, Reykjavik, Iceland: Bjarni E. Pjetursson References Abrahamsson, I., Berglundh, T., Linder, E., Lang, N. P. & Lindhe, J. (2004) Early bone formation adjacent to rough and turned endosseous implant surfaces. An experimental study in the dog. Clinical Oral Implants Research 15: Abrahamsson, I., Berglundh, T. & Lindhe, J. (1998) Soft tissue response to plaque formation at different implant systems. A comparative study in the dog. Clinical Oral Implants Research 9: 73 79, 281. Abu-Ta a, M., Quirynen, M., Teughels, W. & van Steenberghe, D. (2008) Asepsis during periodontal surgery involving oral implants and the usefulness of peri-operative antibiotics: a prospective, randomized, controlled clinical trial. Journal of Clinical Periodontology 35: Albouy, J.P., Abrahamsson, I., Persson, L.G. & Berglundh, T. (2008) Spontaneous progression of peri-implantitis at different types of implants. An experimental study in dogs. I: clinical and radiographic observations. Clinical Oral Implants Research 19: Albouy, J.P., Abrahamsson, I., Persson, L.G. & Berglundh, T. (2009) Spontaneous progression of ligatured induced peri-implantitis at implants with different surface characteristics. An experimental study in dogs II: histological observations. Clinical Oral Implants Research 20: Anitua, E., Aguirre, J.J., Gorosabel, A., Barrio, P., Errazquin, J.M., Roman, P., Pla, R., Carrete, J., de Petro, J. & Orive, G. (2009) A multicentre placebo-controlled randomised clinical trial of antibiotic prophylaxis for placement of single dental implants. European Journal Oral Implantology 2: Binahmed, A., Stoykewych, A. & Peterson, L. (2005) Single preoperative dose versus long-term prophylactic antibiotic regimens in dental implant surgery. International Journal Oral Maxillofacial Implants 20: Dent, C.D., Olson, J.W., Farish, S.E., Bellome, J., Casino, A.J., Morris, H.F. & Ochi, S. (1997) The influence of preoperative antibiotics on success of endosseous implants up to and including stage II surgery: a study of 2,641 implants. Journal Oral Maxillofacial Surgery 55: Esposito, M., Cannizzaro, G., Bozzoli, P., Checchi, L., Ferri, V., Landriani, S., Leone, M., Todisco, M., Torchio, C., Testori, T., Galli, F. & Felice, P. (2010a) Effectiveness of prophylactic antibiotics at placement of dental implants: a pragmatic multicentre placebo-controlled randomised clinical trial. European Journal Oral Implantology 3: Esposito, M., Cannizzaro, G., Bozzoli, P., Consolo, U., Felice, P., Ferri, V., Landriani, S., Leone, M., Magliano, A., Pellitteri, G., Todisco, M. & Torchio, C. (2008) Efficacy of prophylactic antibiotics for dental implants: a multicentre placebo-controlled randomised clinical trial. European Journal Oral Implantology 1: Esposito, M., Grusovin, M.G., Loli, V., Coulthard, P. & Worthington, H.V. (2010b) Does antibiotic prophylaxis at implant placement decrease early implant failures? A Cochrane systematic review. European Journal Oral Implantology 3: Gynther, G.W., K ondell, P.A., Moberg, L.E. & Heimdahl, A. (1998) Dental implant installation without antibiotic prophylaxis. Oral Surgery Oral Medicine Oral Pathology Oral Radiology Endodontics 85: Kuriyama, T., Williams, D.W., Yanagisawa, M., Iwahara, K., Shimizu, C., Nakagawa, K., Yamamoto, E. & Karasawa, T. (2007) Antimicrobial susceptibility of 800 anaerobic isolates from patients with dentoalveolar infection to 13 oral antibiotics. Oral Microbiology Immunology 22: Lang, N.P., Salvi, G.E., Huynh-Ba, G., Ivanovski, S., Donos, N. & Bosshardt, D.D. (2011) Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clinical Oral Implants Research 22: Laskin, D.M., Dent, C.D., Morris, H.F., Ochi, S. & Olson, J.W. (2000) The influence of preoperative 8 Clin. Oral Impl. Res. 0, 2013 / John Wiley & Sons A/S. Published by Blackwell Publishing Ltd
9 antibiotics on success of endosseous implants at 36 months. Annuals Periodontology 5: Monaco, G., Tavernese, L., Agostini, R. & Marchetti, C. (2009) Evaluation of antibiotic prophylaxis in reducing postoperative infection after mandibular third molar extraction in young patients. Journal Oral Maxillofacial Surgery 67: Morris, H.F., Ochi, S., Plezia, R., Gilbert, H., Dent, C.D., Pikulski, J. & Lambert, P.M. (2004) AICRG, Part III: the influence of antibiotic use on the survival of a new implant design. Journal Oral Implantology 30: Pasupathy, S. & Alexander, M. (2011) Antibiotic prophylaxis in third molar surgery. Journal Craniofacial Surgery 22: Powell, C.A., Mealey, B.L., Deas, D.E., McDonnell, H.T. & Moritz, A.J. (2005) Post-surgical infections: prevalence associated with various periodontal surgical procedures. Journal Periodontology 76: Siddiqi, A., Morkel, J.A. & Zafar, S. (2010) Antibiotic prophylaxis in third molar surgery: a randomized double-blind placebo-controlled clinical trial using split-mouth technique. International Journal Oral Maxillofacial Surgery 39: Wilson, W., Taubert, K.A., Gewitz, M., Lockhart, P. B., Baddour, L.M., Levison, M., Bolger, A., Cabell, C.H., Takahashi, M., Baltimore, R.S., Newburger, J.W., Strom, B.L., Tani, L.Y., Gerber, M., Bonow, R.O., Pallasch, T., Shulman, S.T., Rowley, A.H., Burns, J.C., Ferrieri, P., Gardner, T., Goff, D. & Durack, D.T.; American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; Council on Cardiovascular Surgery and Anesthesia; Quality of Care and Outcomes Research Interdisciplinary Working Group; American Dental Association. (2007) Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Journal American Dental Association 138: , John Wiley & Sons A/S. Published by Blackwell Publishing Ltd 9 Clin. Oral Impl. Res. 0, 2013 / 1 9
Antibiotic prophylaxis and early dental implant failure: a quasi-random controlled clinical trial.
 Antibiotic prophylaxis and early dental implant failure: a quasi-random controlled clinical trial. Karaky AE, Sawair FA, Al-Karadsheh OA, Eimar HA, Algarugly SA, Baqain ZH. Eur J Oral Implantol. 2011 Spring;4(1):31-8.
Antibiotic prophylaxis and early dental implant failure: a quasi-random controlled clinical trial. Karaky AE, Sawair FA, Al-Karadsheh OA, Eimar HA, Algarugly SA, Baqain ZH. Eur J Oral Implantol. 2011 Spring;4(1):31-8.
Prosthetic treatment planning on the basis of scientific evidence.
 Prosthetic treatment planning on the basis of scientific evidence. Pjetursson BE, Lang NP. J Oral Rehabil. 2008 Jan;35 Suppl 1:72-9. Faculty of Odontology, University of Iceland, Reykjavik, Iceland, and
Prosthetic treatment planning on the basis of scientific evidence. Pjetursson BE, Lang NP. J Oral Rehabil. 2008 Jan;35 Suppl 1:72-9. Faculty of Odontology, University of Iceland, Reykjavik, Iceland, and
Life Table Analysis for Evaluating Curative-effect of One-stage Non-submerged Dental Implant in Taiwan
 Journal of Data Science 6(2008), 591-599 Life Table Analysis for Evaluating Curative-effect of One-stage Non-submerged Dental Implant in Taiwan Miin-Jye Wen 1, Chuen-Chyi Tseng 2 and Cheng K. Lee 3 1 National
Journal of Data Science 6(2008), 591-599 Life Table Analysis for Evaluating Curative-effect of One-stage Non-submerged Dental Implant in Taiwan Miin-Jye Wen 1, Chuen-Chyi Tseng 2 and Cheng K. Lee 3 1 National
Supervisors: Dr. Farhan Raza Khan
 1 Presenter: Dr. Sana Ehsen Supervisors: Dr. Farhan Raza Khan 2 A dental implant (also known as an endosseous implant or fixture) is a surgical component that interfaces with the bone of the jaw to support
1 Presenter: Dr. Sana Ehsen Supervisors: Dr. Farhan Raza Khan 2 A dental implant (also known as an endosseous implant or fixture) is a surgical component that interfaces with the bone of the jaw to support
TRI Product NewsFlash. December 2015
 TRI Product NewsFlash December 2015 Study Overview 2015 Dear Partners Year in, year out, we are screening all major scientific journals to ensure that our TRI Performance Concept still reflects the latest
TRI Product NewsFlash December 2015 Study Overview 2015 Dear Partners Year in, year out, we are screening all major scientific journals to ensure that our TRI Performance Concept still reflects the latest
Interventions for replacing missing teeth: antibiotics at dental implant placement to prevent complications (Review)
 Interventions for replacing missing teeth: antibiotics at dental implant placement to prevent complications (Review) Esposito M, Worthington HV, Loli V, Coulthard P, Grusovin MG This is a reprint of a
Interventions for replacing missing teeth: antibiotics at dental implant placement to prevent complications (Review) Esposito M, Worthington HV, Loli V, Coulthard P, Grusovin MG This is a reprint of a
Long-Term dental Implant Survival In Fully Endentulous Patients: A 30-66 Month Follow-Up
 Article 1 Long-Term dental Implant Survival In Fully Endentulous Patients: A 30-66 Month Follow-Up Dr. Gadi Schneider DMD, Specialist in periodontics Dr. Yoram Bruckmayer DMD Long-Term dental Implant Survival
Article 1 Long-Term dental Implant Survival In Fully Endentulous Patients: A 30-66 Month Follow-Up Dr. Gadi Schneider DMD, Specialist in periodontics Dr. Yoram Bruckmayer DMD Long-Term dental Implant Survival
S ince Brånemark defined osseointegration
 IMPLANT DENTISTRY / VOLUME 0, NUMBER 0 2015 1 Postoperative Infections After Dental Implant Placement: Prevalence, Clinical Features, and Treatment Octavi Camps-Font, DDS,* Rui Figueiredo, DDS, MS, PhD,
IMPLANT DENTISTRY / VOLUME 0, NUMBER 0 2015 1 Postoperative Infections After Dental Implant Placement: Prevalence, Clinical Features, and Treatment Octavi Camps-Font, DDS,* Rui Figueiredo, DDS, MS, PhD,
Replacement of the upper left central incisor with a Straumann Bone Level Implant and a Straumann Customized Ceramic Abutment
 Replacement of the upper left central incisor with a Straumann Bone Level Implant and a Straumann Customized Ceramic Abutment by Dr. Ronald Jung and Master Dental Technician Xavier Zahno Initial situation
Replacement of the upper left central incisor with a Straumann Bone Level Implant and a Straumann Customized Ceramic Abutment by Dr. Ronald Jung and Master Dental Technician Xavier Zahno Initial situation
Advances in bone augmentation to enable dental implant placement: Consensus Report of the Sixth European Workshop on Periodontology
 J Clin Periodontol 2008; 35 (Suppl. 8): 168 172 doi: 10.1111/j.1600-051X.2008.01268.x Advances in bone augmentation to enable dental implant placement: Consensus Report of the Sixth European Workshop on
J Clin Periodontol 2008; 35 (Suppl. 8): 168 172 doi: 10.1111/j.1600-051X.2008.01268.x Advances in bone augmentation to enable dental implant placement: Consensus Report of the Sixth European Workshop on
LATERAL BONE EXPANSION FOR IMMEDIATE PLACEMENT OF ENDOSSEOUS DENTAL IMPLANTS
 LATERAL BONE EXPANSION FOR IMMEDIATE PLACEMENT OF ENDOSSEOUS DENTAL IMPLANTS Department of Oral Maxillofacial Surgery, Chisinau Abstract: The study included 10 using the split control expansion technique
LATERAL BONE EXPANSION FOR IMMEDIATE PLACEMENT OF ENDOSSEOUS DENTAL IMPLANTS Department of Oral Maxillofacial Surgery, Chisinau Abstract: The study included 10 using the split control expansion technique
The Immediate Placement of Dental Implants Into Extraction Sites With Periapical Lesions: A Retrospective Chart Review
 IMPLANTS The Immediate Placement of Dental Implants Into Extraction Sites With Periapical Lesions: A Retrospective Chart Review Christopher Lincoln Bell,* David Diehl, Brian Michael Bell, and Robert E.
IMPLANTS The Immediate Placement of Dental Implants Into Extraction Sites With Periapical Lesions: A Retrospective Chart Review Christopher Lincoln Bell,* David Diehl, Brian Michael Bell, and Robert E.
RESIDENT TRAINING GOALS AND OBJECTIVES STATEMENTS
 RESIDENT TRAINING GOALS AND OBJECTIVES STATEMENTS Evaluation and treatment of dental emergencies Recognize, anticipate and manage emergency problems related to the oral cavity. Differentiate between those
RESIDENT TRAINING GOALS AND OBJECTIVES STATEMENTS Evaluation and treatment of dental emergencies Recognize, anticipate and manage emergency problems related to the oral cavity. Differentiate between those
Straumann Dental Implant System. Implant Selection Guide.
 Straumann Dental Implant System. Implant Selection Guide. STRAUMANN's IMPLANT PORTFOLIO The Straumann Dental Implant System offers two implant lines with diverse body and neck designs ranging from the
Straumann Dental Implant System. Implant Selection Guide. STRAUMANN's IMPLANT PORTFOLIO The Straumann Dental Implant System offers two implant lines with diverse body and neck designs ranging from the
Saudi Fellowship In Dental Implant (SF-DI)
 Saudi Fellowship In Dental Implant (SF-DI) Prepared and Updated by Dr. Arwa AL-Sayed Consultant Periodontics and Dental Implants M E M B E R S Dr. Arwa AL-Sayed Dr. Abdulhadi Abanmy Dr. Ali AL-Ghamdi Dr.
Saudi Fellowship In Dental Implant (SF-DI) Prepared and Updated by Dr. Arwa AL-Sayed Consultant Periodontics and Dental Implants M E M B E R S Dr. Arwa AL-Sayed Dr. Abdulhadi Abanmy Dr. Ali AL-Ghamdi Dr.
Straumann Bone Level Tapered Implant Peer-to-peer communication
 Straumann Bone Level Tapered Implant Peer-to-peer communication Clinical cases April, 2015 Clinical Cases Case No. Site 1 Single unit; Anterior Maxilla 2 Multi-unit; Anterior Maxilla Implant placement
Straumann Bone Level Tapered Implant Peer-to-peer communication Clinical cases April, 2015 Clinical Cases Case No. Site 1 Single unit; Anterior Maxilla 2 Multi-unit; Anterior Maxilla Implant placement
INTERNATIONAL MEDICAL COLLEGE
 INTERNATIONAL MEDICAL COLLEGE Joint Degree Master Program: Implantology and Dental Surgery (M.Sc.) Basic modules: List of individual modules Basic Module 1 Basic principles of general and dental medicine
INTERNATIONAL MEDICAL COLLEGE Joint Degree Master Program: Implantology and Dental Surgery (M.Sc.) Basic modules: List of individual modules Basic Module 1 Basic principles of general and dental medicine
C HAPTER 4 O RAL AND M AXILLOFACIAL S URGERY
 C HAPTER 4 O RAL AND M AXILLOFACIAL S URGERY I. TREATMENT PLANNING GUIDELINES As part of informed consent, the clinician should carefully explain the risks and benefits of oral and maxillofacial surgery
C HAPTER 4 O RAL AND M AXILLOFACIAL S URGERY I. TREATMENT PLANNING GUIDELINES As part of informed consent, the clinician should carefully explain the risks and benefits of oral and maxillofacial surgery
Teeth and Dental Implants: When to save, and when to extract.
 Teeth and Dental Implants: When to save, and when to extract. One of the most difficult decisions a restorative dentist has to make is when to refer a patient for extraction and placement of dental implants.
Teeth and Dental Implants: When to save, and when to extract. One of the most difficult decisions a restorative dentist has to make is when to refer a patient for extraction and placement of dental implants.
Eastman Dental Hospital. Dental implants - general information for patients. Department of Restorative Dentistry
 Eastman Dental Hospital Dental implants - general information for patients Department of Restorative Dentistry First published: January 2004 Last review date: March 2014 Next review date: March 2016 Leafl
Eastman Dental Hospital Dental implants - general information for patients Department of Restorative Dentistry First published: January 2004 Last review date: March 2014 Next review date: March 2016 Leafl
More than a fixed rehabilitation.
 More than a fixed rehabilitation. A reason to smile. In combination with: Patient expectations drive dental treatments for fixed edentulous immediate restorations. Patients today have increasingly high
More than a fixed rehabilitation. A reason to smile. In combination with: Patient expectations drive dental treatments for fixed edentulous immediate restorations. Patients today have increasingly high
GUIDELINES. Educational Requirements & Professional Responsibilities for Implant Dentistry CONTENTS. The Guidelines of the Royal College of
 Educational Requirements & Professional GUIDELINES Approved by Council May 2013 This is replacing the document last published in August 2002. Educational Requirements & Professional The Guidelines of the
Educational Requirements & Professional GUIDELINES Approved by Council May 2013 This is replacing the document last published in August 2002. Educational Requirements & Professional The Guidelines of the
Dental Extractions, Antibiotics and Curettage First, Do no Harm
 Global Journal of Medical research: J Dentistry and Otolaryngology Volume 14 Issue 1 Version 1.0 Year 2014 Type: Double Blind Peer Reviewed International Research Journal Publisher: Global Journals Inc.
Global Journal of Medical research: J Dentistry and Otolaryngology Volume 14 Issue 1 Version 1.0 Year 2014 Type: Double Blind Peer Reviewed International Research Journal Publisher: Global Journals Inc.
IMPLANT CONSENT FORM WHAT ARE DENTAL IMPLANTS?
 IMPLANT CONSENT FORM WHAT ARE DENTAL IMPLANTS? Dental implants are a very successful and accepted treatment option to replace lost or missing teeth. A dental implant is essentially an artificial tooth
IMPLANT CONSENT FORM WHAT ARE DENTAL IMPLANTS? Dental implants are a very successful and accepted treatment option to replace lost or missing teeth. A dental implant is essentially an artificial tooth
Bone augmentation procedure without wound closure
 THE CREATION OF ATTACHED GINGIVA IMMEDIATELY AFTER EXTRACTION Bone augmentation procedure without wound closure One of the characteristics of wound healing after an extraction is that the alveolar process
THE CREATION OF ATTACHED GINGIVA IMMEDIATELY AFTER EXTRACTION Bone augmentation procedure without wound closure One of the characteristics of wound healing after an extraction is that the alveolar process
Survival Analysis of Dental Implants. Abstracts
 Survival Analysis of Dental Implants Andrew Kai-Ming Kwan 1,4, Dr. Fu Lee Wang 2, and Dr. Tak-Kun Chow 3 1 Census and Statistics Department, Hong Kong, China 2 Caritas Institute of Higher Education, Hong
Survival Analysis of Dental Implants Andrew Kai-Ming Kwan 1,4, Dr. Fu Lee Wang 2, and Dr. Tak-Kun Chow 3 1 Census and Statistics Department, Hong Kong, China 2 Caritas Institute of Higher Education, Hong
Current Concepts in American Dentistry: Advances in Implantology and Oral Rehabilitation
 2009 New York University College Of Dentistry Linhart Continuing Dental Education Program Presents Current Concepts in American Dentistry: Advances in Implantology and Oral Rehabilitation International
2009 New York University College Of Dentistry Linhart Continuing Dental Education Program Presents Current Concepts in American Dentistry: Advances in Implantology and Oral Rehabilitation International
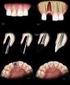
Single anterior tooth replacement: clinical approaches
 Single anterior tooth replacement: clinical approaches Paul Swanson examines the role of implant design in approaching a range of treatment protocols for replacing a single tooth Case 1 Figure 1: Patient
Single anterior tooth replacement: clinical approaches Paul Swanson examines the role of implant design in approaching a range of treatment protocols for replacing a single tooth Case 1 Figure 1: Patient
Clinical Study Report. Clinical Efficacy of the e-bright Tooth Whitening Accelerator Home Edition: a randomized placebo controlled clinical trial
 Clinical Study Report Clinical Efficacy of the e-bright Tooth Whitening Accelerator Home Edition: a randomized placebo controlled clinical trial (Clinical Study Protocol Number: EBRIGHT-2007-01) Study
Clinical Study Report Clinical Efficacy of the e-bright Tooth Whitening Accelerator Home Edition: a randomized placebo controlled clinical trial (Clinical Study Protocol Number: EBRIGHT-2007-01) Study
TRAINING STANDARDS IN IMPLANT DENTISTRY
 TRAINING STANDARDS IN IMPLANT DENTISTRY Introduction 2012 1 Dental implants are used to replace one or more missing teeth. Their insertion involves various surgical and restorative dental procedures and
TRAINING STANDARDS IN IMPLANT DENTISTRY Introduction 2012 1 Dental implants are used to replace one or more missing teeth. Their insertion involves various surgical and restorative dental procedures and
Straumann Dental implant system ONE SYSTEM ONE INSTRUMENT KIT ALL INDICATIONS*
 Straumann Dental implant system ONE SYSTEM ONE INSTRUMENT KIT ALL INDICATIONS* For all indications*! The Straumann Dental Implant System offers you and your patients efficient and esthetic implant-borne
Straumann Dental implant system ONE SYSTEM ONE INSTRUMENT KIT ALL INDICATIONS* For all indications*! The Straumann Dental Implant System offers you and your patients efficient and esthetic implant-borne
CLINICAL GOALS OF PATIENT CARE AND CLINIC MANAGEMENT. Philosophical Basis of the Patient Care System. Patient Care Goals
 University of Washington School of Dentistry CLINICAL GOALS OF PATIENT CARE AND CLINIC MANAGEMENT Philosophical Basis of the Patient Care System The overall mission of the patient care system in the School
University of Washington School of Dentistry CLINICAL GOALS OF PATIENT CARE AND CLINIC MANAGEMENT Philosophical Basis of the Patient Care System The overall mission of the patient care system in the School
Ridge Reconstruction for Implant Placement
 Volume 1, No. 5 July/August 2009 The Journal of Implant & Advanced Clinical Dentistry Ridge Reconstruction for Implant Placement 2 Hours of CE Credit Oral Implications of Cancer Chemotherapy Immediate
Volume 1, No. 5 July/August 2009 The Journal of Implant & Advanced Clinical Dentistry Ridge Reconstruction for Implant Placement 2 Hours of CE Credit Oral Implications of Cancer Chemotherapy Immediate
If several different trials are mentioned in one publication, the data of each should be extracted in a separate data extraction form.
 General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
The 2nd Emirates EDUCATION Week: A Master Course in Modern Evidence Based Implant Dentistry Focusing on Surgery & Prosthetics Aspects Le MERIDIEN
 The 2nd Emirates EDUCATION Week: A Master Course in Modern Evidence Based Implant Dentistry Focusing on Surgery & Prosthetics Aspects Le MERIDIEN Airport Hotel 20. -24. November, 2014 Course Description
The 2nd Emirates EDUCATION Week: A Master Course in Modern Evidence Based Implant Dentistry Focusing on Surgery & Prosthetics Aspects Le MERIDIEN Airport Hotel 20. -24. November, 2014 Course Description
What Dental Implants Can Do For You!
 What Dental Implants Can Do For You! Putting Smiles into Motion About Implants 01. What if a Tooth is Lost and the Area is Left Untreated? 02. Do You Want to Restore Confidence in Your Appearance? 03.
What Dental Implants Can Do For You! Putting Smiles into Motion About Implants 01. What if a Tooth is Lost and the Area is Left Untreated? 02. Do You Want to Restore Confidence in Your Appearance? 03.
Oftentimes, as implant surgeons, we are
 CLINICAL AVOIDING INJURY TO THE INFERIOR ALVEOLAR NERVE BY ROUTINE USE OF INTRAOPERATIVE RADIOGRAPHS DURING IMPLANT PLACEMENT Jeffrey Burstein, DDS, MD; Chris Mastin, DMD; Bach Le, DDS, MD Injury to the
CLINICAL AVOIDING INJURY TO THE INFERIOR ALVEOLAR NERVE BY ROUTINE USE OF INTRAOPERATIVE RADIOGRAPHS DURING IMPLANT PLACEMENT Jeffrey Burstein, DDS, MD; Chris Mastin, DMD; Bach Le, DDS, MD Injury to the
Residency Competency and Proficiency Statements
 Residency Competency and Proficiency Statements 1. REQUEST AND RESPOND TO REQUESTS FOR CONSULTATIONS Identify needs and make referrals to appropriate health care providers for the treatment of physiologic,
Residency Competency and Proficiency Statements 1. REQUEST AND RESPOND TO REQUESTS FOR CONSULTATIONS Identify needs and make referrals to appropriate health care providers for the treatment of physiologic,
Don t Let Life Pass You By Because Of Missing Teeth
 Don t Let Life Pass You By Because Of Missing Teeth Ask For Dental Implant Solutions From BIOMET 3i Scan With Your Smartphone! In order to scan QR codes, your mobile device must have a QR code reader installed.
Don t Let Life Pass You By Because Of Missing Teeth Ask For Dental Implant Solutions From BIOMET 3i Scan With Your Smartphone! In order to scan QR codes, your mobile device must have a QR code reader installed.
INFUSE Bone Graft (rhbmp-2/acs)
 1 INFUSE Bone Graft (rhbmp-2/acs) For patients who need more bone to place dental implants Enjoy living with INFUSE Bone Graft. www.medtronic.com Medtronic Spinal and Biologics Business Worldwide Headquarters
1 INFUSE Bone Graft (rhbmp-2/acs) For patients who need more bone to place dental implants Enjoy living with INFUSE Bone Graft. www.medtronic.com Medtronic Spinal and Biologics Business Worldwide Headquarters
4-1-2005. Dental Clinical Criteria and Documentation Requirements
 4-1-2005 Dental Clinical Criteria and Documentation Requirements Table of Contents Dental Clinical Criteria Cast Restorations and Veneer Procedures... Pages 1-3 Crown Repair... Page 3 Endodontic Procedures...
4-1-2005 Dental Clinical Criteria and Documentation Requirements Table of Contents Dental Clinical Criteria Cast Restorations and Veneer Procedures... Pages 1-3 Crown Repair... Page 3 Endodontic Procedures...
TREATMENT REFUSAL FORMS
 TREATMENT REFUSAL FORMS These forms are intended to be used when a patient refuses the treatment. These forms help confirm that the patient is informed and aware of the risks involved with not proceeding
TREATMENT REFUSAL FORMS These forms are intended to be used when a patient refuses the treatment. These forms help confirm that the patient is informed and aware of the risks involved with not proceeding
HEALTH SERVICES POLICY & PROCEDURE MANUAL. SUBJECT: Types of Dental Treatments Provided EFFECTIVE DATE: July 2014 SUPERCEDES DATE: January 2014
 PAGE 1 of 5 References Related ACA Standards 4 th Edition Standards for Adult Correctional Institutions 4-4369, 4-4375 PURPOSE To provide guidelines for determining appropriate levels of care and types
PAGE 1 of 5 References Related ACA Standards 4 th Edition Standards for Adult Correctional Institutions 4-4369, 4-4375 PURPOSE To provide guidelines for determining appropriate levels of care and types
Straumann. Time-tested
 Straumann Soft TiSSue Level implant System Time-tested Why a Soft Tissue Level implant? Simplicity and efficiency by integrated soft tissue management Straumann Soft Tissue Level implants have a built-in
Straumann Soft TiSSue Level implant System Time-tested Why a Soft Tissue Level implant? Simplicity and efficiency by integrated soft tissue management Straumann Soft Tissue Level implants have a built-in
Ideal treatment of the impaired
 RESEARCH IMPLANTS AS ANCHORAGE IN ORTHODONTICS: ACLINICAL CASE REPORT Dale B. Herrero, DDS KEY WORDS External anchorage Pneumatized Often, in dental reconstruction, orthodontics is required for either
RESEARCH IMPLANTS AS ANCHORAGE IN ORTHODONTICS: ACLINICAL CASE REPORT Dale B. Herrero, DDS KEY WORDS External anchorage Pneumatized Often, in dental reconstruction, orthodontics is required for either
Tuition and Fees Dentists - Full time (per annum): 20,000
 Diploma of Oral Surgery Residency Training Program in preparation for the Fachzahnarzt in Oral Surgery Specialty Examination in the Republic of Germany Degree awarded: - Diploma of Oral Surgery - Fachzahnarzt
Diploma of Oral Surgery Residency Training Program in preparation for the Fachzahnarzt in Oral Surgery Specialty Examination in the Republic of Germany Degree awarded: - Diploma of Oral Surgery - Fachzahnarzt
Dental Implant Treatment after Improvement of Oral Environment by Orthodontic Therapy
 Dental implant treatment after impr Title environment by orthodontic therapy. Sekine, H; Miyazaki, H; Takanashi, Author(s) Matsuzaki, F; Taguchi, T; Katada, H Journal Bulletin of Tokyo Dental College,
Dental implant treatment after impr Title environment by orthodontic therapy. Sekine, H; Miyazaki, H; Takanashi, Author(s) Matsuzaki, F; Taguchi, T; Katada, H Journal Bulletin of Tokyo Dental College,
PATIENT INFORM CONSENT for IMPLANT RESTORATION Rev 04.2012
 PATIENT INFORM CONSENT for IMPLANT RESTORATION Rev 04.2012 Implant placement and restoration involves two major stages: surgical placement of the implant(s) followed by the restoration of the implant after
PATIENT INFORM CONSENT for IMPLANT RESTORATION Rev 04.2012 Implant placement and restoration involves two major stages: surgical placement of the implant(s) followed by the restoration of the implant after
Course Curriculum for the Master Degree in Dentistry/Orthodontics
 Jordan University of Science and Technology Faculty of Graduate Studies Course Curriculum for the Master Degree in Dentistry/Orthodontics The Master Degree in Dentistry/ Orthodontics is awarded by the
Jordan University of Science and Technology Faculty of Graduate Studies Course Curriculum for the Master Degree in Dentistry/Orthodontics The Master Degree in Dentistry/ Orthodontics is awarded by the
DENTAL IMPLANT THERAPY
 DENTAL IMPLANT THERAPY PATIENT WELCOME PACK Dr. Syed Abdullah BDS, MSc (Dental Implants) What are dental implants? In the early 1950s, a Swedish Scientist, Per-Ingvar Branemark observed that titanium metal
DENTAL IMPLANT THERAPY PATIENT WELCOME PACK Dr. Syed Abdullah BDS, MSc (Dental Implants) What are dental implants? In the early 1950s, a Swedish Scientist, Per-Ingvar Branemark observed that titanium metal
More than an implant. A sense of trust. Straumann Dental Implant System
 More than an implant. A sense of trust. Straumann Dental Implant System More than an implant. A sense of trust. Patients today are more aware of their options when it comes to tooth replacement. Partner
More than an implant. A sense of trust. Straumann Dental Implant System More than an implant. A sense of trust. Patients today are more aware of their options when it comes to tooth replacement. Partner
2.0 Synopsis. Vicodin CR (ABT-712) M05-765 Clinical Study Report R&D/07/095. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
Develop a specialist who is capable of correlation of basic sciences and clinical sciences, and challenge the requirements for certification.
 Course Specification Faculty : Dentistry Department : Endodontics Program Specification: Diploma Degree A-Basic Information 1-Programme Title: Diploma in Endodontics 2-Departments (s): Endodontics 4-Coordinator:
Course Specification Faculty : Dentistry Department : Endodontics Program Specification: Diploma Degree A-Basic Information 1-Programme Title: Diploma in Endodontics 2-Departments (s): Endodontics 4-Coordinator:
Prosthodontist s Perspective
 Unless otherwise noted, the content of this course material is licensed under a Creative Commons Attribution - Non-Commercial - Share Alike 3.0 License. Copyright 2008, Dr. Jeff Shotwell. The following
Unless otherwise noted, the content of this course material is licensed under a Creative Commons Attribution - Non-Commercial - Share Alike 3.0 License. Copyright 2008, Dr. Jeff Shotwell. The following
Antibiotic Prophylaxis for the Prevention of Infective Endocarditis and Prosthetic Joint Infections for Dentists
 PRACTICE ADVISORY SERVICE FAQ 6 Crescent Road, Toronto, ON Canada M4W 1T1 T: 416.961.6555 F: 416.961.5814 Toll Free: 1.800.565.4591 www.rcdso.org Antibiotic Prophylaxis for the Prevention of Infective
PRACTICE ADVISORY SERVICE FAQ 6 Crescent Road, Toronto, ON Canada M4W 1T1 T: 416.961.6555 F: 416.961.5814 Toll Free: 1.800.565.4591 www.rcdso.org Antibiotic Prophylaxis for the Prevention of Infective
MEDICAID DENTAL PROGRAMS CODING, POLICY AND RELATED FEE REVISION INFORMATION
 MEDICAID DENTAL PROGRAMS CODING, POLICY AND RELATED FEE REVISION INFORMATION Effective for dates of service on and after November 1, 2005, the following dental coding, policy and related fee revisions
MEDICAID DENTAL PROGRAMS CODING, POLICY AND RELATED FEE REVISION INFORMATION Effective for dates of service on and after November 1, 2005, the following dental coding, policy and related fee revisions
Surgical protocols for early implant placement in post-extraction sockets: a systematic review
 Ignacio Sanz Maria Garcia-Gargallo David Herrera Conchita Martin Elena Figuero Mariano Sanz Surgical protocols for early implant placement in post-extraction sockets: a systematic review Authors affiliations:
Ignacio Sanz Maria Garcia-Gargallo David Herrera Conchita Martin Elena Figuero Mariano Sanz Surgical protocols for early implant placement in post-extraction sockets: a systematic review Authors affiliations:
Dental Implant Options in Atrophic Jaws
 Dental Implant Options in Atrophic Jaws Orthopedic Application Jay B. Reznick, D.M.D., M.D. Diplomate, American Board of Oral and Maxillofacial Surgery Tarzana, CA Endopore Dental Implant System Screw-Type
Dental Implant Options in Atrophic Jaws Orthopedic Application Jay B. Reznick, D.M.D., M.D. Diplomate, American Board of Oral and Maxillofacial Surgery Tarzana, CA Endopore Dental Implant System Screw-Type
Scottish Dental Clinical Effectiveness Programme SDcep. Prevention and Treatment of Periodontal Diseases in Primary Care Guidance in Brief
 Scottish Dental Clinical Effectiveness Programme SDcep Prevention and Treatment of Periodontal Diseases in Primary Care Guidance in Brief June 2014 Scottish Dental Clinical Effectiveness Programme SDcep
Scottish Dental Clinical Effectiveness Programme SDcep Prevention and Treatment of Periodontal Diseases in Primary Care Guidance in Brief June 2014 Scottish Dental Clinical Effectiveness Programme SDcep
Powertome Assisted Atraumatic Tooth Extraction
 Powertome Assisted Atraumatic Tooth Extraction White et al Jason White, DDS 1 2 3 Abstract Background: While traditional dental extraction techniques encourage minimal trauma, luxated elevation and forceps
Powertome Assisted Atraumatic Tooth Extraction White et al Jason White, DDS 1 2 3 Abstract Background: While traditional dental extraction techniques encourage minimal trauma, luxated elevation and forceps
Don t Let Life Pass You By Because Of Oral Bone Loss
 Don t Let Life Pass You By Because Of Oral Bone Loss Ask For Dental Implant Solutions From BIOMET 3i Scan With Your Smartphone! In order to scan QR codes, your mobile device must have a QR code reader
Don t Let Life Pass You By Because Of Oral Bone Loss Ask For Dental Implant Solutions From BIOMET 3i Scan With Your Smartphone! In order to scan QR codes, your mobile device must have a QR code reader
Accelerated recovery of post-operative dental implant patients by means of pulsed shortwave (SWT) therapy: An Observational Study
 Bartolomeo Operti, MD and Dr Tiziano Tealdo Accelerated recovery of post-operative dental implant patients by means of pulsed shortwave (SWT) therapy: Bartolomeo Operti, MD Chief of Anesthesia Department
Bartolomeo Operti, MD and Dr Tiziano Tealdo Accelerated recovery of post-operative dental implant patients by means of pulsed shortwave (SWT) therapy: Bartolomeo Operti, MD Chief of Anesthesia Department
education Although demographic factors and growing patient awareness of the benefits of dental implants
 education Increasing implant dentistry in undergraduate education using new technology: A pilot project Hugo De Bruyn, MDS, MsC, PhD ± & Stefan Vandeweghe, DDS Although demographic factors and growing
education Increasing implant dentistry in undergraduate education using new technology: A pilot project Hugo De Bruyn, MDS, MsC, PhD ± & Stefan Vandeweghe, DDS Although demographic factors and growing
HOW THE IMR FINAL DETERMINATION WAS MADE
 Case Number: CM13-0026016 Date Assigned: 11/22/2013 Date of Injury: 08/11/2005 Decision Date: 02/03/2014 UR Denial Date: 08/30/2013 Priority: Standard Application Received: 09/18/2013 HOW THE IMR FINAL
Case Number: CM13-0026016 Date Assigned: 11/22/2013 Date of Injury: 08/11/2005 Decision Date: 02/03/2014 UR Denial Date: 08/30/2013 Priority: Standard Application Received: 09/18/2013 HOW THE IMR FINAL
Immediate Molar Implant Placement: A Private Practice Clinical Investigation. Abstract
 Immediate Molar Implant Placement: A Private Practice Clinical Investigation Gargiulo et al Alphonse Gargiulo, DDS, MS 1 Thomas Manos, DDS, MS 2 Mark Kolozenski, DDS, MS 3 Alex Tzanos, DDS, MSD 3 Michael
Immediate Molar Implant Placement: A Private Practice Clinical Investigation Gargiulo et al Alphonse Gargiulo, DDS, MS 1 Thomas Manos, DDS, MS 2 Mark Kolozenski, DDS, MS 3 Alex Tzanos, DDS, MSD 3 Michael
Use of Antibiotics in Dental Implant Surgery: A Decision Based on Evidence from Systematic Review
 Int. J. Odontostomat., 9(1):137-147, 2015. Use of Antibiotics in Dental Implant Surgery: A Decision Based on Evidence from Systematic Review Uso de Antibióticos en la Cirugía de Implantes Dentales: una
Int. J. Odontostomat., 9(1):137-147, 2015. Use of Antibiotics in Dental Implant Surgery: A Decision Based on Evidence from Systematic Review Uso de Antibióticos en la Cirugía de Implantes Dentales: una
A Comprehensive Explanation
 Dental Implants A Comprehensive Explanation Overview Since the 1980s, dental implants have become more popular among dentists and patients. 1 In some clinical situations, implants may be the best treatment
Dental Implants A Comprehensive Explanation Overview Since the 1980s, dental implants have become more popular among dentists and patients. 1 In some clinical situations, implants may be the best treatment
TITLE: Metal-Ceramic and Porcelain Dental Crowns: A Review of Clinical and Cost- Effectiveness
 TITLE: Metal-Ceramic and Porcelain Dental Crowns: A Review of Clinical and Cost- Effectiveness DATE: 23 June 2009 CONTEXT AND POLICY ISSUES: Dental ceramics are widely used to repair damaged teeth and
TITLE: Metal-Ceramic and Porcelain Dental Crowns: A Review of Clinical and Cost- Effectiveness DATE: 23 June 2009 CONTEXT AND POLICY ISSUES: Dental ceramics are widely used to repair damaged teeth and
VASDHS MEDICAL CENTER
 VASDHS MEDICAL CENTER The General Practice Residency Program at the Veterans Affairs San Diego Healthcare System, Medical Center is a one year advanced training program accredited by the Commission on
VASDHS MEDICAL CENTER The General Practice Residency Program at the Veterans Affairs San Diego Healthcare System, Medical Center is a one year advanced training program accredited by the Commission on
Anatomic limitations in the maxilla provide challenges
 Osteotome Single-Stage Dental Implant Placement With and Without Sinus Elevation: A Clinical Report Orest G. Komarnyckyj, DDS*/Robert M. London, DDS** Forty-three sites in 16 patients were selected for
Osteotome Single-Stage Dental Implant Placement With and Without Sinus Elevation: A Clinical Report Orest G. Komarnyckyj, DDS*/Robert M. London, DDS** Forty-three sites in 16 patients were selected for
Comparison of survival and complication rates of toothsupported. fixed dental prostheses (FDPs) and implantsupported. crowns (SCs)
 Bjarni E. Pjetursson Urs Brägger Niklaus P. Lang Marcel Zwahlen Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns
Bjarni E. Pjetursson Urs Brägger Niklaus P. Lang Marcel Zwahlen Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns
Retrospective study on the survival rate of IBS implant
 Retrospective study on the survival rate of IBS implant Date : 30. 05. 2013 Written by : Dr. Je Won Wang, Director of research Approved by : Prof. Min Seung Ki - Contents - 1. Purpose Of Study 2. Materials
Retrospective study on the survival rate of IBS implant Date : 30. 05. 2013 Written by : Dr. Je Won Wang, Director of research Approved by : Prof. Min Seung Ki - Contents - 1. Purpose Of Study 2. Materials
Osseo-integrated Dental Implant Policy and Guidelines
 Osseo-integrated Dental Implant Policy and Guidelines 1. PURPOSE The purpose of this document is to outline the Department of Veterans Affairs (DVA) policy regarding the provision of dental implant treatment
Osseo-integrated Dental Implant Policy and Guidelines 1. PURPOSE The purpose of this document is to outline the Department of Veterans Affairs (DVA) policy regarding the provision of dental implant treatment
The use of a dental implant as an abutment in three unit implant-tooth supported fixed partial denture: a case report and 32 month follow-up
 CASE REPORT Australian Dental Journal 2006;51:(3):263-267 The use of a dental implant as an abutment in three unit implant-tooth supported fixed partial denture: a case report and 32 month follow-up DF
CASE REPORT Australian Dental Journal 2006;51:(3):263-267 The use of a dental implant as an abutment in three unit implant-tooth supported fixed partial denture: a case report and 32 month follow-up DF
Dental. Covered services and limitations module
 Dental Covered services and limitations module Dental Covered Services and Limitations Module Covered Dental Services for Patients Under the Age of 21...2 Examinations...2 Radiographs and Diagnostic Imaging...2
Dental Covered services and limitations module Dental Covered Services and Limitations Module Covered Dental Services for Patients Under the Age of 21...2 Examinations...2 Radiographs and Diagnostic Imaging...2
Spedding Dental Clinic. 73 Warwick Road Carlisle CA1 1EB T: 01228 521889 www.speddingdental.co.uk
 DENTAL IMPLANTS Spedding Dental Clinic 73 Warwick Road Carlisle CA1 1EB T: 01228 521889 www.speddingdental.co.uk SPEDDING DENTAL CLINIC Jack Spedding is a partner in Spedding dental clinic. He is a highly
DENTAL IMPLANTS Spedding Dental Clinic 73 Warwick Road Carlisle CA1 1EB T: 01228 521889 www.speddingdental.co.uk SPEDDING DENTAL CLINIC Jack Spedding is a partner in Spedding dental clinic. He is a highly
Emdogain. The reliable solution for periodontal treatment.
 Emdogain The reliable solution for periodontal treatment. Straumann is the exclusive industrial partner of the ITI (International Team for Implantology) in the areas of research, development, and education.
Emdogain The reliable solution for periodontal treatment. Straumann is the exclusive industrial partner of the ITI (International Team for Implantology) in the areas of research, development, and education.
2016 Buy Up Dental Care Plan Procedure List
 * This is in addition to the embedded Preventive Plan (see procedure list at deltadentalco.com/kp_preventive. BASIC SERVICES Minor Restorative Services D2140 Amalgam 1 surface, primary or permanent D2150
* This is in addition to the embedded Preventive Plan (see procedure list at deltadentalco.com/kp_preventive. BASIC SERVICES Minor Restorative Services D2140 Amalgam 1 surface, primary or permanent D2150
IMPLANT DENTISTRY CLINICAL SYLLABUS
 IMPLANT DENTISTRY CLINICAL SYLLABUS RESD 535 MATS H. KRONSTROM, DDS, PhD Course Director DEPARTMENT OF RESTORATIVE DENTISTRY SCHOOL OF DENTISTRY UNIVERSITY OF WASHINGTON 2008 2009 RD 535 - SCHEDULE FALL
IMPLANT DENTISTRY CLINICAL SYLLABUS RESD 535 MATS H. KRONSTROM, DDS, PhD Course Director DEPARTMENT OF RESTORATIVE DENTISTRY SCHOOL OF DENTISTRY UNIVERSITY OF WASHINGTON 2008 2009 RD 535 - SCHEDULE FALL
Flapless Implant Surgery for Replacement of Posterior Teeth
 Course Number: 108.2 Flapless Implant Surgery for Replacement of Posterior Teeth Authored by J. Steven Cloyd, DDS Upon successful completion of this CE activity 1 CE credit hour may be awarded A Peer-Reviewed
Course Number: 108.2 Flapless Implant Surgery for Replacement of Posterior Teeth Authored by J. Steven Cloyd, DDS Upon successful completion of this CE activity 1 CE credit hour may be awarded A Peer-Reviewed
Abutment fracture in a bridge supported by natural teeth and implants
 Abutment fracture in a bridge supported by natural teeth and implants Authors_Dr Gregory-George Zafiropoulos, Dr Giorgio Deli & Dr Rainer Valentin, Germany/Italy _Introduction Implant treatment has evolved
Abutment fracture in a bridge supported by natural teeth and implants Authors_Dr Gregory-George Zafiropoulos, Dr Giorgio Deli & Dr Rainer Valentin, Germany/Italy _Introduction Implant treatment has evolved
Outcomes of dental implant treatment in patients with generalized aggressive periodontitis: a systematic review
 ORIGINAL ARTICLE http://dx.doi.org/10.4047/jap.2012.4.4.210 Outcomes of dental implant treatment in patients with generalized aggressive periodontitis: a systematic review Kyoung-Kyu Kim*, DDS, MSD, Hun-Mo
ORIGINAL ARTICLE http://dx.doi.org/10.4047/jap.2012.4.4.210 Outcomes of dental implant treatment in patients with generalized aggressive periodontitis: a systematic review Kyoung-Kyu Kim*, DDS, MSD, Hun-Mo
Updated: February 2014. 1986-1990 Highland General Hospital, Certificate of Completion Oral & Maxillofacial Surgery Residency
 Edmond Bedrossian DDS, FACD, FACOMS, FAO Diplomate, American Board of Oral & Maxillofacial Surgery Director, Implant Surgery, Alameda Medical Center & UOP School of Dentistry Professor, Department of Oral
Edmond Bedrossian DDS, FACD, FACOMS, FAO Diplomate, American Board of Oral & Maxillofacial Surgery Director, Implant Surgery, Alameda Medical Center & UOP School of Dentistry Professor, Department of Oral
Replacement of a single front tooth Surgical procedure and three-year results
 Case Report 10 2011 Replacement of a single front tooth Surgical procedure and three-year results Dr Peter Randelzhofer Munich, Germany Prosthetics Dr Peter Randelzhofer studied dentistry in Munich, Germany,
Case Report 10 2011 Replacement of a single front tooth Surgical procedure and three-year results Dr Peter Randelzhofer Munich, Germany Prosthetics Dr Peter Randelzhofer studied dentistry in Munich, Germany,
Proven Techniques and New Developments in Partially Edentulous Patients
 Conjoint Programme In Implant Dentistry HKSPID - ADC Ltd. Proven Techniques and New Developments in Partially Edentulous Patients By Prof. Dr. Daniel Buser, Switzerland 3 July 2014, Thursday at LT1, Prince
Conjoint Programme In Implant Dentistry HKSPID - ADC Ltd. Proven Techniques and New Developments in Partially Edentulous Patients By Prof. Dr. Daniel Buser, Switzerland 3 July 2014, Thursday at LT1, Prince
PATIENT INFORMATION. A new quality of life with dental implants. www.straumann.com
 PATIENT INFORMATION A new quality of life with dental implants www.straumann.com A N E W Q U A L I T Y O F L I F E W I T H D E N T A L I M P L A N T S Contents Page 3 4 7 7 8 11 12 14 15 17 18 The beauty
PATIENT INFORMATION A new quality of life with dental implants www.straumann.com A N E W Q U A L I T Y O F L I F E W I T H D E N T A L I M P L A N T S Contents Page 3 4 7 7 8 11 12 14 15 17 18 The beauty
NAPCS Product List for NAICS 62121 (US, Mex): Offices of Dentists
 NAPCS List for NAICS 62121 (US, Mex): Offices of Dentists 62121 1 Services of dentists Providing dental medical attention by means of consultations, preventive services, and surgical and non-surgical interventions.
NAPCS List for NAICS 62121 (US, Mex): Offices of Dentists 62121 1 Services of dentists Providing dental medical attention by means of consultations, preventive services, and surgical and non-surgical interventions.
Final Result 1 year later. Patient Case 19. Preoperative: Main Complaint:
 Patient Case 19 Preoperative: Main Complaint: The patient presented to the practice with the 21 that according to her started to move forward. Dental History I have been treating this patient for many
Patient Case 19 Preoperative: Main Complaint: The patient presented to the practice with the 21 that according to her started to move forward. Dental History I have been treating this patient for many
Immediate versus early non-occlusal loading of dental implants placed flapless in partially edentulous patients: A 3-year randomized clinical trial
 J Clin Periodontol 2011; doi: 10.1111/j.1600-051X.2011.01821.x Immediate versus early non-occlusal loading of dental implants placed flapless in partially edentulous patients: A 3-year randomized clinical
J Clin Periodontol 2011; doi: 10.1111/j.1600-051X.2011.01821.x Immediate versus early non-occlusal loading of dental implants placed flapless in partially edentulous patients: A 3-year randomized clinical
Adramatic increase in the number of dental practitioners
 Risk Management Aspects of Implant Dentistry Navot Givol, DMD 1 /Shlomo Taicher, DMD 2 /Talia Halamish-Shani, LLB 3 /Gavriel Chaushu, DMD, MSc 4 Purpose: To categorize and review complications related
Risk Management Aspects of Implant Dentistry Navot Givol, DMD 1 /Shlomo Taicher, DMD 2 /Talia Halamish-Shani, LLB 3 /Gavriel Chaushu, DMD, MSc 4 Purpose: To categorize and review complications related
Understanding Dental Implants
 Understanding Dental Implants Comfort and Confidence Again A new smile It s no fun when you re missing teeth. You may not feel comfortable eating or speaking. You might even avoid smiling in public. Fortunately,
Understanding Dental Implants Comfort and Confidence Again A new smile It s no fun when you re missing teeth. You may not feel comfortable eating or speaking. You might even avoid smiling in public. Fortunately,
Tissue alterations at implant-supported single-tooth replacements: a 1-year prospective clinical study
 Giuseppe Cardaropoli Ulf Lekholm Jan L. Wennström Tissue alterations at implant-supported single-tooth res: a 1-year prospective clinical study Authors affiliations: Giuseppe Cardaropoli, Jan L. Wennström,
Giuseppe Cardaropoli Ulf Lekholm Jan L. Wennström Tissue alterations at implant-supported single-tooth res: a 1-year prospective clinical study Authors affiliations: Giuseppe Cardaropoli, Jan L. Wennström,
CDT 2015 Code Change Summary New codes effective 1/1/2015
 CDT 2015 Code Change Summary New codes effective 1/1/2015 Code Nomenclature Delta Dental Policy D0171 Re-Evaluation Post Operative Office Visit Not a Covered Benefit D0351 3D Photographic Image Not a Covered
CDT 2015 Code Change Summary New codes effective 1/1/2015 Code Nomenclature Delta Dental Policy D0171 Re-Evaluation Post Operative Office Visit Not a Covered Benefit D0351 3D Photographic Image Not a Covered
MAIN LINE DENTAL IMPLANT CENTER
 1257 Lancaster Ave Berwyn, PA 19312 Tel: 610-722-5542 CHIUN-LIN (STEVEN), LIU D.D.S., D.M.D. School of Dental Medicine CURRICULUM VITAE Summer, 2012 Education: 1987-1993 D.D.S. Kaohsiung Medical University
1257 Lancaster Ave Berwyn, PA 19312 Tel: 610-722-5542 CHIUN-LIN (STEVEN), LIU D.D.S., D.M.D. School of Dental Medicine CURRICULUM VITAE Summer, 2012 Education: 1987-1993 D.D.S. Kaohsiung Medical University
BioHorizons Education Programme 2015
 BioHorizons Education Programme 2015 SPMP14328GB Rev A November 2014 Contents The Role of Implants in Restorative Dentistry An Introduction to Contemporary Implant Prosthodontics Sinus Elevation Socket
BioHorizons Education Programme 2015 SPMP14328GB Rev A November 2014 Contents The Role of Implants in Restorative Dentistry An Introduction to Contemporary Implant Prosthodontics Sinus Elevation Socket
WMI Mutual Insurance Company
 Dental Policy WMI Mutual Insurance Company PO Box 572450 Salt Lake City, UT 84157 (801) 263-8000 & (800) 748-5340 Fax: (801) 263-1247 DENTAL POLICY A. Schedule of Benefits: Annual Maximum Dental Benefit
Dental Policy WMI Mutual Insurance Company PO Box 572450 Salt Lake City, UT 84157 (801) 263-8000 & (800) 748-5340 Fax: (801) 263-1247 DENTAL POLICY A. Schedule of Benefits: Annual Maximum Dental Benefit
vitality meets bone volume around dental implants Straumann BoneCeramic
 vitality meets bone volume around dental implants Straumann BoneCeramic The vitality you need around your implants Bone volume restoration and preservation for the desired esthetic outcome Image by PD
vitality meets bone volume around dental implants Straumann BoneCeramic The vitality you need around your implants Bone volume restoration and preservation for the desired esthetic outcome Image by PD
Dental Implants in Edentulism
 Academy of Medicine, Singapore Dental Implants in Edentulism AMS-MOH Clinical Practice Guidelines 1/2012 Aug 2012 Levels of evidence and grades of recommendation Levels of evidence Level Type of Evidence
Academy of Medicine, Singapore Dental Implants in Edentulism AMS-MOH Clinical Practice Guidelines 1/2012 Aug 2012 Levels of evidence and grades of recommendation Levels of evidence Level Type of Evidence
Central Carolina Dental Center Phone: (919) 777-7780 Dental Hygiene Program Fax: (919) 777-7788 900 S. Vance Street; Suite 220 Sanford, NC 27330
 Central Carolina Dental Center Phone: (919) 777-7780 Dental Hygiene Program Fax: (919) 777-7788 900 S. Vance Street; Suite 220 Sanford, NC 27330 Please Print All Information: Name of Patient: Sex: Male
Central Carolina Dental Center Phone: (919) 777-7780 Dental Hygiene Program Fax: (919) 777-7788 900 S. Vance Street; Suite 220 Sanford, NC 27330 Please Print All Information: Name of Patient: Sex: Male
DENTAL FOR EVERYONE SUMMARY OF BENEFITS, LIMITATIONS AND EXCLUSIONS
 DENTAL FOR EVERYONE SUMMARY OF BENEFITS, LIMITATIONS AND EXCLUSIONS DEDUCTIBLE The dental plan features a deductible. This is an amount the Enrollee must pay out-of-pocket before Benefits are paid. The
DENTAL FOR EVERYONE SUMMARY OF BENEFITS, LIMITATIONS AND EXCLUSIONS DEDUCTIBLE The dental plan features a deductible. This is an amount the Enrollee must pay out-of-pocket before Benefits are paid. The
