Amino Acid Metabolism (Chapter 20) Lecture 8:
|
|
|
- Agatha Nash
- 9 years ago
- Views:
Transcription
1 Amino Acid Metabolism (Chapter 20) Lecture 8: Nitrogen Fixation (20.7); Nitrite Assimilation (not in text?); Protein Digestion in the Gut (5.3b, 11.5, 20.2); Amino Acid Degradation in Cells (20.2); Next: The Urea Cycle (20.3) Breakdown of AAs (20.4) AA Biosynthesis (20.5)
2 Amino acid roles: 1) protein monomeric units (primary purpose) 2) energy metabolites (about 10% of energy) 3) precursors of many biologically important nitrogencontaining compounds such as: HEME physiologically active AMINES [(nor)epinephrine, dopamine, GABA (γ-aminobutyric acid), serotonin, histamine] GLUTATHIONE NUCLEOTIDES nucleotide COENZYMES
3 Amino acid metabolism is intimately intertwined with nitrogen acquisition (see 20-7). All organisms need bioavailable sources of nitrogen for proteins and nucleic acids. The major form of nitrogen in the atmosphere is N 2, an extremely stable compound: the N N triple bond has a bond energy of 945kJ/mol. N H e ATP + 16 H 2 O 2 NH 3 + H ADP + 16 Pi N 2 is converted to metabolically useful forms (is "fixed") only by a few species of prokaryotes, called diazatrophs. Diazatrophs of the genus Rhizobium live symbiotically in the root nodules of legumes, where they convert N 2 to NH 3 (ammonia) in a process called NITROGEN FIXATION: NITROGENASE
4 But, less than 1% of N entering the biosphere comes from N- fixation. Another oxidized form of nitrogen, NO 3 - (nitrate ion) is also found in the soils and oceans. It is converted to NH 4 + through... NITRATE ASSIMILATION: The reduction of NO 3 - to NH 4 + (ammonium ion) occurs in green plants, various fungi, and certain bacteria in a two-step pathway: (1) The 2-electron reduction of nitrate to nitrite: NO H e - NO H 2 O This reaction is catalyzed by nitrate reductase, a soluble α2-type homodimer protein that has an unusual molybdenum-containing cofactor (named "MoCo") that is involved in the transfer of the electrons to nitrate. (2) This is followed by the 6-electron reduction of nitrite to ammonium: NO H e - NH H 2 O This reaction is catalyzed by nitrite reductase. THESE REACTIONS ALSO REQUIRE LARGE ENERGY INPUTS (not shown).
5 NH 3 /NH 4 + can be incorporated into the amino acids glutamate by glutamate dehydrogenase (20-2B) and glutamine by glutamine synthetase (20-5A). No animals are capable of either N-fixation or nitrate assimilation, so they (we!) are totally dependent on plants and microorganisms for the synthesis of organic nitrogenous compounds, such as amino acids and proteins, to provide this essential nutrient.
6 Dietary protein digestion: Most amino acids which we eat are in the form of proteins, which must be digested into amino acid monomers. This digestion occurs in the gut: stomach: pancreas to small intestine: intestinal wall: pepsin trypsin dipeptidases chymotrypsin carboxypeptidase A carboxypeptidase B elastase Monomers (free amino acids) pass through the wall and into the bloodstream and are taken up by cells. Excess dietary amino acids are converted to common metabolites that are precursors of glucose, fatty acids, and ketone bodies (metabolic fuels).
7 Amino acid degradation (section 20-2): There are 3 common stages of amino acid degradation: 1) DEAMINATION = amino group removal: amino groups converted to A) ammonia or B) the amino group of aspartate. 2) Incorporation of ammonia or aspartate nitrogen into UREA for excretion (20.3). 3) Conversion of the AA carbon skeletons (i.e., the α-keto acids that result from deamination) to the common intermediates (20.4)
8 AMINO ACID DEAMINATION The first step in AA breakdown usually is removal of an α-amino group. Most AAs are deaminated by "TRANSAMINATION" the transfer of the amino group to an -keto acid. The OVERALL STOICHIOMETRY of a generalized transamination reaction is: amino acid 1 + -keto acid 2 -keto acid 1 + amino acid 2 The acceptor -keto acid 2 is usually -ketoglutarate (or oxaloacetate to a lesser extent). The enzymes that catalyze these reactions are now generally referred to as aminotransferases (the older name still in use is transaminases).
9 Typical first transamination reaction: The usual AA acceptor is α-ketoglutarate, producing GLUTAMATE and the new α-keto acid. What is produced if oxaloacetate is the acceptor?
10 What is produced if oxaloacetate is the acceptor? It is one C shorter than α-ketoglutarate, so produces.. X X ASPARTATE
11 The second typical step in AA deamination involves transfer of the amino group from GLU to oxaloacetate, yeilding α-ketoglutarate and ASP: glutamate-aspartate aminotransferase
12 Oxidative deamination (20.2b): The transamination steps do not result in any NET deamination. GLUTAMATE is oxidatively deaminated by GLUTAMATE DEHYDROGENASE, yielding NH 3 and regenerating α-ketoglutarate (p. 619):
13 Urea Cycle (20.3): Animals are the only organisms that normally have a dietary excess of N, but... [NH 4+ ] is toxic to animals, so it must be gotten rid of. Excess N from AA breakdown is excreted from animals in one of 3 forms, depending on the availability of water: Ammonia (book shows it as NH 3 or NH 4+ ). Aquatic animals simply release it into the surrounding water where it gets diluted to non-toxic concentrations. Animals that do this are called AMMONOTELIC (from the Greek, meaning "end"! they did not know metabolism, so I don't think that this refers to the "end product"!!).
AP BIOLOGY CHAPTER 7 Cellular Respiration Outline
 AP BIOLOGY CHAPTER 7 Cellular Respiration Outline I. How cells get energy. A. Cellular Respiration 1. Cellular respiration includes the various metabolic pathways that break down carbohydrates and other
AP BIOLOGY CHAPTER 7 Cellular Respiration Outline I. How cells get energy. A. Cellular Respiration 1. Cellular respiration includes the various metabolic pathways that break down carbohydrates and other
The Urea Cycle. April 11, 2003 Bryant Miles
 The Urea ycle April 11, 2003 Bryant Miles I. Ammonia Toxicity Every amino acid contains at least one amino group. Therefore every amino acid degradation pathway has a key step where the amino group is
The Urea ycle April 11, 2003 Bryant Miles I. Ammonia Toxicity Every amino acid contains at least one amino group. Therefore every amino acid degradation pathway has a key step where the amino group is
Copyright 2000-2003 Mark Brandt, Ph.D. 35
 Amino acid breakdown Amino acids comprise one of the three major energy sources for animals. They are an especially important energy source for carnivorous animals, and for all animals during early starvation
Amino acid breakdown Amino acids comprise one of the three major energy sources for animals. They are an especially important energy source for carnivorous animals, and for all animals during early starvation
Regulation of the Citric Acid Cycle
 Regulation of the itric Acid ycle I. hanges in Free Energy February 17, 2003 Bryant Miles kj/mol 40 20 0 20 40 60 80 Reaction DGo' DG TA Free Energy hanges 1 2 3 4 5 6 7 8 9 1.) itrate Synthase 2.) Aconitase
Regulation of the itric Acid ycle I. hanges in Free Energy February 17, 2003 Bryant Miles kj/mol 40 20 0 20 40 60 80 Reaction DGo' DG TA Free Energy hanges 1 2 3 4 5 6 7 8 9 1.) itrate Synthase 2.) Aconitase
Nitrogen Fixing Bacteria in Agriculture Now a Real Option Guy Webb B.Sc. REM Agricultural Consultant
 Nitrogen Fixing Bacteria in Agriculture Now a Real Option Guy Webb B.Sc. REM Agricultural Consultant The Pursuit of Protein and Profit All agricultural enterprises, in essence, are based on the pursuit
Nitrogen Fixing Bacteria in Agriculture Now a Real Option Guy Webb B.Sc. REM Agricultural Consultant The Pursuit of Protein and Profit All agricultural enterprises, in essence, are based on the pursuit
CITRIC ACID (KREB S, TCA) CYCLE
 ITRI AID (KREB S, TA) YLE Date: September 2, 2005 * Time: 10:40 am 11:30 am * Room: G202 Biomolecular Building Lecturer: Steve haney 515A Mary Ellen Jones Building [email protected] 9663286 *Please
ITRI AID (KREB S, TA) YLE Date: September 2, 2005 * Time: 10:40 am 11:30 am * Room: G202 Biomolecular Building Lecturer: Steve haney 515A Mary Ellen Jones Building [email protected] 9663286 *Please
CHAPTER 15: ANSWERS TO SELECTED PROBLEMS
 CHAPTER 15: ANSWERS T SELECTED PRBLEMS SAMPLE PRBLEMS ( Try it yourself ) 15.1 ur bodies can carry out the second reaction, because it requires less energy than we get from breaking down a molecule of
CHAPTER 15: ANSWERS T SELECTED PRBLEMS SAMPLE PRBLEMS ( Try it yourself ) 15.1 ur bodies can carry out the second reaction, because it requires less energy than we get from breaking down a molecule of
AMINO ACIDS & PEPTIDE BONDS STRUCTURE, CLASSIFICATION & METABOLISM
 AMINO ACIDS & PEPTIDE BONDS STRUCTURE, CLASSIFICATION & METABOLISM OBJECTIVES At the end of this session the student should be able to, recognize the structures of the protein amino acid and state their
AMINO ACIDS & PEPTIDE BONDS STRUCTURE, CLASSIFICATION & METABOLISM OBJECTIVES At the end of this session the student should be able to, recognize the structures of the protein amino acid and state their
PRACTICE SET 6. A. Questions on Lipid Metabolism and Glyoxylate Cycle
 PRATIE SET 6 A. Questions on Lipid Metabolism and Glyoxylate ycle 1. The hydroxy acid given below can be completely oxidized to acetyl-oa by betaoxidation. Write the series of individual reactions that
PRATIE SET 6 A. Questions on Lipid Metabolism and Glyoxylate ycle 1. The hydroxy acid given below can be completely oxidized to acetyl-oa by betaoxidation. Write the series of individual reactions that
Chapter 16 The Citric Acid Cycle
 Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Production of acetyl-coa (activated acetate) Page: 603 Difficulty: 2 Ans: A Which of the following is not true of the reaction catalyzed by
Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Production of acetyl-coa (activated acetate) Page: 603 Difficulty: 2 Ans: A Which of the following is not true of the reaction catalyzed by
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
The Citric Acid Cycle
 The itric Acid ycle February 14, 2003 Bryant Miles I. itrate Synthase + 3 SoA The first reaction of the citric acid cycle is the condensation of acetyloa and oxaloacetate to form citrate and oas. The enzyme
The itric Acid ycle February 14, 2003 Bryant Miles I. itrate Synthase + 3 SoA The first reaction of the citric acid cycle is the condensation of acetyloa and oxaloacetate to form citrate and oas. The enzyme
Lactic Acid Dehydrogenase
 Lactic Acid Dehydrogenase Pyruvic Acid Dehydrogenase Complex Pyruvate to ACETYL coa CC CoA + CO 2 Mitochondria 3 carbon Pyruvate to 2 carbon ACETYL Coenzyme A Pyruvate Acetyl CoA + CO 2 + NADH + H + CO2
Lactic Acid Dehydrogenase Pyruvic Acid Dehydrogenase Complex Pyruvate to ACETYL coa CC CoA + CO 2 Mitochondria 3 carbon Pyruvate to 2 carbon ACETYL Coenzyme A Pyruvate Acetyl CoA + CO 2 + NADH + H + CO2
Citric Acid Cycle. Cycle Overview. Metabolic Sources of Acetyl-Coenzyme A. Enzymes of the Citric Acid Cycle. Regulation of the Citric Acid Cycle
 Citric Acid Cycle Cycle Overview Metabolic Sources of Acetyl-Coenzyme A Enzymes of the Citric Acid Cycle Regulation of the Citric Acid Cycle The Amphibolic Nature of the Citric Acid Cycle Cycle Overview
Citric Acid Cycle Cycle Overview Metabolic Sources of Acetyl-Coenzyme A Enzymes of the Citric Acid Cycle Regulation of the Citric Acid Cycle The Amphibolic Nature of the Citric Acid Cycle Cycle Overview
Which of the following can be determined based on this model? The atmosphere is the only reservoir on Earth that can store carbon in any form. A.
 Earth s Cycles 1. Models are often used to explain scientific knowledge or experimental results. A model of the carbon cycle is shown below. Which of the following can be determined based on this model?
Earth s Cycles 1. Models are often used to explain scientific knowledge or experimental results. A model of the carbon cycle is shown below. Which of the following can be determined based on this model?
Summary of Metabolism. Mechanism of Enzyme Action
 Summary of Metabolism Mechanism of Enzyme Action 1. The substrate contacts the active site 2. The enzyme-substrate complex is formed. 3. The substrate molecule is altered (atoms are rearranged, or the
Summary of Metabolism Mechanism of Enzyme Action 1. The substrate contacts the active site 2. The enzyme-substrate complex is formed. 3. The substrate molecule is altered (atoms are rearranged, or the
Nitrogen Cycling in Ecosystems
 Nitrogen Cycling in Ecosystems In order to have a firm understanding of how nitrogen impacts our ecosystems, it is important that students fully understand how the various forms of nitrogen cycle through
Nitrogen Cycling in Ecosystems In order to have a firm understanding of how nitrogen impacts our ecosystems, it is important that students fully understand how the various forms of nitrogen cycle through
Chapter 16 The Citric Acid Cycle
 Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Which of the following is not true of the reaction catalyzed by the pyruvate dehydrogenase complex? A) Biotin participates in the decarboxylation.
Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Which of the following is not true of the reaction catalyzed by the pyruvate dehydrogenase complex? A) Biotin participates in the decarboxylation.
Cellular Respiration: Practice Questions #1
 Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Regulation of enzyme activity
 1 Regulation of enzyme activity Regulation of enzyme activity is important to coordinate the different metabolic processes. It is also important for homeostasis i.e. to maintain the internal environment
1 Regulation of enzyme activity Regulation of enzyme activity is important to coordinate the different metabolic processes. It is also important for homeostasis i.e. to maintain the internal environment
THE WATER CYCLE. Ecology
 THE WATER CYCLE Water is the most abundant substance in living things. The human body, for example, is composed of about 70% water, and jellyfish are 95% water. Water participates in many important biochemical
THE WATER CYCLE Water is the most abundant substance in living things. The human body, for example, is composed of about 70% water, and jellyfish are 95% water. Water participates in many important biochemical
Microbial Metabolism. Chapter 5. Enzymes. Enzyme Components. Mechanism of Enzymatic Action
 Chapter 5 Microbial Metabolism Metabolism is the sum of all chemical reactions within a living organism, including anabolic (biosynthetic) reactions and catabolic (degradative) reactions. Anabolism is
Chapter 5 Microbial Metabolism Metabolism is the sum of all chemical reactions within a living organism, including anabolic (biosynthetic) reactions and catabolic (degradative) reactions. Anabolism is
Anabolic and Catabolic Reactions are Linked by ATP in Living Organisms
 Chapter 5: Microbial Metabolism Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living a living organism. These chemical reactions are generally of two types: Catabolic:
Chapter 5: Microbial Metabolism Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living a living organism. These chemical reactions are generally of two types: Catabolic:
Chem 306 Chapter 21 Bioenergetics Lecture Outline III
 Chem 306 Chapter 21 Bioenergetics Lecture Outline III I. HOW IS ATP GENERATED IN THE FINAL STAGE CATABOLISM? A. OVERVIEW 1. At the end of the citric acid cycle, all six carbons of glucose have been oxidized
Chem 306 Chapter 21 Bioenergetics Lecture Outline III I. HOW IS ATP GENERATED IN THE FINAL STAGE CATABOLISM? A. OVERVIEW 1. At the end of the citric acid cycle, all six carbons of glucose have been oxidized
General Protein Metabolism
 General Protein Metabolism Protein Digestion Dietary proteins are very large complex molecules that cannot be absorbed from the intestine. To be absorbed, dietary proteins must be digested to small simple
General Protein Metabolism Protein Digestion Dietary proteins are very large complex molecules that cannot be absorbed from the intestine. To be absorbed, dietary proteins must be digested to small simple
Cellular Respiration and Fermentation
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 9 Cellular Respiration and Fermentation
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 9 Cellular Respiration and Fermentation
Photosynthesis (Life from Light)
 Photosynthesis Photosynthesis (Life from Light) Energy needs of life All life needs a constant input of energy o Heterotrophs (consumers) Animals, fungi, most bacteria Get their energy from other organisms
Photosynthesis Photosynthesis (Life from Light) Energy needs of life All life needs a constant input of energy o Heterotrophs (consumers) Animals, fungi, most bacteria Get their energy from other organisms
Chapter 25: Metabolism and Nutrition
 Chapter 25: Metabolism and Nutrition Chapter Objectives INTRODUCTION 1. Generalize the way in which nutrients are processed through the three major metabolic fates in order to perform various energetic
Chapter 25: Metabolism and Nutrition Chapter Objectives INTRODUCTION 1. Generalize the way in which nutrients are processed through the three major metabolic fates in order to perform various energetic
Energy Production In A Cell (Chapter 25 Metabolism)
 Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
008 Chapter 8. Student:
 008 Chapter 8 Student: 1. Some bacteria are strict aerobes and others are strict anaerobes. Some bacteria, however, are facultative anaerobes and can live with or without oxygen. If given the choice of
008 Chapter 8 Student: 1. Some bacteria are strict aerobes and others are strict anaerobes. Some bacteria, however, are facultative anaerobes and can live with or without oxygen. If given the choice of
Microbial Metabolism. Biochemical diversity
 Microbial Metabolism Biochemical diversity Metabolism Define Requirements Energy Enzymes Rate Limiting step Reaction time Types Anabolic Endergonic Dehydration Catabolic Exergonic Hydrolytic Metabolism
Microbial Metabolism Biochemical diversity Metabolism Define Requirements Energy Enzymes Rate Limiting step Reaction time Types Anabolic Endergonic Dehydration Catabolic Exergonic Hydrolytic Metabolism
Digestive System Lecture 5 Winter 2014
 Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Enzymes and Metabolic Pathways
 Enzymes and Metabolic Pathways Enzyme characteristics Made of protein Catalysts: reactions occur 1,000,000 times faster with enzymes Not part of reaction Not changed or affected by reaction Used over and
Enzymes and Metabolic Pathways Enzyme characteristics Made of protein Catalysts: reactions occur 1,000,000 times faster with enzymes Not part of reaction Not changed or affected by reaction Used over and
Chapter 14- RESPIRATION IN PLANTS
 Chapter 14- RESPIRATION IN PLANTS Living cells require a continuous supply of energy for maintaining various life activities. This energy is obtained by oxidizing the organic compounds (carbohydrates,
Chapter 14- RESPIRATION IN PLANTS Living cells require a continuous supply of energy for maintaining various life activities. This energy is obtained by oxidizing the organic compounds (carbohydrates,
1. Essay: The Digestive and Absorption Processes of Macronutrients
 Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Integration of Metabolism
 I. Central Themes of Metabolism 1. ATP is the universal energy carrier. Integration of Metabolism Bryant Miles 2. ATP is generated by the oxidation of metabolic fuels Glucose Fatty Acids Amino Acids 3.
I. Central Themes of Metabolism 1. ATP is the universal energy carrier. Integration of Metabolism Bryant Miles 2. ATP is generated by the oxidation of metabolic fuels Glucose Fatty Acids Amino Acids 3.
CELLULAR RESPIRATION. Chapter 19 & 20. Biochemistry by Campbell and Farell (7 th Edition) By Prof M A Mogale
 CELLULAR RESPIRATION Chapter 19 & 20 Biochemistry by Campbell and Farell (7 th Edition) By Prof M A Mogale 1. Cellular respiration (energy capture) The enzymatic breakdown of food stuffs in the presence
CELLULAR RESPIRATION Chapter 19 & 20 Biochemistry by Campbell and Farell (7 th Edition) By Prof M A Mogale 1. Cellular respiration (energy capture) The enzymatic breakdown of food stuffs in the presence
Bioenergetics. Free Energy Change
 Bioenergetics Energy is the capacity or ability to do work All organisms need a constant supply of energy for functions such as motion, transport across membrane barriers, synthesis of biomolecules, information
Bioenergetics Energy is the capacity or ability to do work All organisms need a constant supply of energy for functions such as motion, transport across membrane barriers, synthesis of biomolecules, information
Amides and Amines: Organic Nitrogen Compounds
 Chapter 25 Amides and Amines: Organic Nitrogen Compounds Nylon is one of the materials used to give these colorful sails their strength and durability. Introduction to General, Organic, and Biochemistry,
Chapter 25 Amides and Amines: Organic Nitrogen Compounds Nylon is one of the materials used to give these colorful sails their strength and durability. Introduction to General, Organic, and Biochemistry,
Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look
 OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
Todays Outline. Metabolism. Why do cells need energy? How do cells acquire energy? Metabolism. Concepts & Processes. The cells capacity to:
 and Work Metabolic Pathways Enzymes Features Factors Affecting Enzyme Activity Membrane Transport Diffusion Osmosis Passive Transport Active Transport Bulk Transport Todays Outline -Releasing Pathways
and Work Metabolic Pathways Enzymes Features Factors Affecting Enzyme Activity Membrane Transport Diffusion Osmosis Passive Transport Active Transport Bulk Transport Todays Outline -Releasing Pathways
Copyright 2000-2003 Mark Brandt, Ph.D. 59
 The Tricarboxylic Acid Cycle Background (why are eight enzymes necessary?) In principle, acetyl-coa could be converted to carbon dioxide very simply. However, doing so has three potential problems: 1)
The Tricarboxylic Acid Cycle Background (why are eight enzymes necessary?) In principle, acetyl-coa could be converted to carbon dioxide very simply. However, doing so has three potential problems: 1)
Energy & Enzymes. Life requires energy for maintenance of order, growth, and reproduction. The energy living things use is chemical energy.
 Energy & Enzymes Life requires energy for maintenance of order, growth, and reproduction. The energy living things use is chemical energy. 1 Energy exists in two forms - potential and kinetic. Potential
Energy & Enzymes Life requires energy for maintenance of order, growth, and reproduction. The energy living things use is chemical energy. 1 Energy exists in two forms - potential and kinetic. Potential
The correct answer is d C. Answer c is incorrect. Reliance on the energy produced by others is a characteristic of heterotrophs.
 1. An autotroph is an organism that a. extracts energy from organic sources b. converts energy from sunlight into chemical energy c. relies on the energy produced by other organisms as an energy source
1. An autotroph is an organism that a. extracts energy from organic sources b. converts energy from sunlight into chemical energy c. relies on the energy produced by other organisms as an energy source
O 2. What is anaerobic digestion?
 What is anaerobic digestion? Microbial degradation of organic material under anaerobic conditions Ubiquitous, naturally-occurring process Occurs in swamps, hydric soils, landfills, digestive tracks of
What is anaerobic digestion? Microbial degradation of organic material under anaerobic conditions Ubiquitous, naturally-occurring process Occurs in swamps, hydric soils, landfills, digestive tracks of
1- Fatty acids are activated to acyl-coas and the acyl group is further transferred to carnitine because:
 Section 10 Multiple Choice 1- Fatty acids are activated to acyl-coas and the acyl group is further transferred to carnitine because: A) acyl-carnitines readily cross the mitochondrial inner membrane, but
Section 10 Multiple Choice 1- Fatty acids are activated to acyl-coas and the acyl group is further transferred to carnitine because: A) acyl-carnitines readily cross the mitochondrial inner membrane, but
Cellular Respiration & Metabolism. Metabolism. Coupled Reactions: Bioenergetics. Cellular Respiration: ATP is the cell s rechargable battery
 Cellular Respiration & Metabolism Metabolic Pathways: a summary Metabolism Bioenergetics Flow of energy in living systems obeys: 1 st law of thermodynamics: Energy can be transformed, but it cannot be
Cellular Respiration & Metabolism Metabolic Pathways: a summary Metabolism Bioenergetics Flow of energy in living systems obeys: 1 st law of thermodynamics: Energy can be transformed, but it cannot be
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
Chapter 2. The Nitrogen Cycle
 Chapter 2 Plants need at least seventeen elements to grow. Three of these elements carbon, oxygen, and hydrogen are referred to as "building blocks." Plants get these elements from air and water. The other
Chapter 2 Plants need at least seventeen elements to grow. Three of these elements carbon, oxygen, and hydrogen are referred to as "building blocks." Plants get these elements from air and water. The other
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
B12 & Cobalamin. Learning objectives
 Learning objectives B12 & Cobalamin Define vitamins Classify fat soluble and water soluble vitamins. Study chemical structure and biological active coenzyme form of vitamin B12. List the dietary sources
Learning objectives B12 & Cobalamin Define vitamins Classify fat soluble and water soluble vitamins. Study chemical structure and biological active coenzyme form of vitamin B12. List the dietary sources
Chapter 8: An Introduction to Metabolism
 Chapter 8: An Introduction to Metabolism Name Period Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. The totality of an organism
Chapter 8: An Introduction to Metabolism Name Period Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. The totality of an organism
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Chapter 7 Active Reading Guide Cellular Respiration and Fermentation
 Name: AP Biology Mr. Croft Chapter 7 Active Reading Guide Cellular Respiration and Fermentation Overview: Before getting involved with the details of cellular respiration and photosynthesis, take a second
Name: AP Biology Mr. Croft Chapter 7 Active Reading Guide Cellular Respiration and Fermentation Overview: Before getting involved with the details of cellular respiration and photosynthesis, take a second
1. Enzymes. Biochemical Reactions. Chapter 5: Microbial Metabolism. 1. Enzymes. 2. ATP Production. 3. Autotrophic Processes
 Chapter 5: Microbial Metabolism 1. Enzymes 2. ATP Production 3. Autotrophic Processes 1. Enzymes Biochemical Reactions All living cells depend on biochemical reactions to maintain homeostasis. All of the
Chapter 5: Microbial Metabolism 1. Enzymes 2. ATP Production 3. Autotrophic Processes 1. Enzymes Biochemical Reactions All living cells depend on biochemical reactions to maintain homeostasis. All of the
Chemistry 20 Chapters 15 Enzymes
 Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Microbial Nutrition And bacterial Classification Microbiology Unit-I. Muhammad Iqbal Lecturer KMU
 Microbial Nutrition And bacterial Classification Microbiology Unit-I Muhammad Iqbal Lecturer KMU Objectives At the end of this lecture the students will be able to: Define key terms. Identify the basic
Microbial Nutrition And bacterial Classification Microbiology Unit-I Muhammad Iqbal Lecturer KMU Objectives At the end of this lecture the students will be able to: Define key terms. Identify the basic
2. Which type of macromolecule contains high-energy bonds and is used for long-term energy storage?
 Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Cellular Energy. 1. Photosynthesis is carried out by which of the following?
 Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
MULTIPLE CHOICE QUESTIONS
 MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
Copyright 2000-2003 Mark Brandt, Ph.D. 54
 Pyruvate Oxidation Overview of pyruvate metabolism Pyruvate can be produced in a variety of ways. It is an end product of glycolysis, and can be derived from lactate taken up from the environment (or,
Pyruvate Oxidation Overview of pyruvate metabolism Pyruvate can be produced in a variety of ways. It is an end product of glycolysis, and can be derived from lactate taken up from the environment (or,
SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman
 SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman An Introduction to Metabolism Most biochemical processes occur as biochemical pathways, each individual reaction of which is catalyzed
SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman An Introduction to Metabolism Most biochemical processes occur as biochemical pathways, each individual reaction of which is catalyzed
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Mammalian digestive tracts
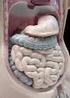 Mammalian digestive tracts Mouth: mastication, some digestive enzymes Esophagus: simple transport tube Stomach: most initial digestion, some physical processing Small intestine: digestion continues, some
Mammalian digestive tracts Mouth: mastication, some digestive enzymes Esophagus: simple transport tube Stomach: most initial digestion, some physical processing Small intestine: digestion continues, some
The Aerobic Fate of Pyruvate
 The Aerobic Fate of yruvate February 12, 2003 Bryant Miles I could tell that some of you were not impressed by the mere 2 ATs produced per glucose by glycolysis. The 2 AT s produced are only a small fraction
The Aerobic Fate of yruvate February 12, 2003 Bryant Miles I could tell that some of you were not impressed by the mere 2 ATs produced per glucose by glycolysis. The 2 AT s produced are only a small fraction
Multiple Choice Identify the choice that best completes the statement or answers the question.
 AP bio fall 2014 final exam prep Multiple Choice Identify the choice that best completes the statement or answers the question. 1. According to the first law of thermodynamics, a. the energy of a system
AP bio fall 2014 final exam prep Multiple Choice Identify the choice that best completes the statement or answers the question. 1. According to the first law of thermodynamics, a. the energy of a system
CHAPTER 6 AN INTRODUCTION TO METABOLISM. Section B: Enzymes
 CHAPTER 6 AN INTRODUCTION TO METABOLISM Section B: Enzymes 1. Enzymes speed up metabolic reactions by lowering energy barriers 2. Enzymes are substrate specific 3. The active site in an enzyme s catalytic
CHAPTER 6 AN INTRODUCTION TO METABOLISM Section B: Enzymes 1. Enzymes speed up metabolic reactions by lowering energy barriers 2. Enzymes are substrate specific 3. The active site in an enzyme s catalytic
Essentials of Anatomy and Physiology, 5e (Martini/Nath) Chapter 17 Nutrition and Metabolism. Multiple-Choice Questions
 Essentials of Anatomy and Physiology, 5e (Martini/Nath) Chapter 17 Nutrition and Metabolism Multiple-Choice Questions 1) The sum of all of the biochemical processes going on within the human body at any
Essentials of Anatomy and Physiology, 5e (Martini/Nath) Chapter 17 Nutrition and Metabolism Multiple-Choice Questions 1) The sum of all of the biochemical processes going on within the human body at any
Special organ structures and functions conduct these tasks through the successive parts of the overall system.
 Chapter 5 Digestion, Absorption, and Metabolism Chapter 5 Lesson 5.1 Key Concepts Through a balanced system of mechanical and chemical digestion, food is broken down into smaller substances and the nutrients
Chapter 5 Digestion, Absorption, and Metabolism Chapter 5 Lesson 5.1 Key Concepts Through a balanced system of mechanical and chemical digestion, food is broken down into smaller substances and the nutrients
BEC Feed Solutions. Steve Blake BEC Feed Solutions
 BEC Feed Solutions Presenter: Steve Blake BEC Feed Solutions Nutritional Role of Phosphorus Phosphorus (P) is present in all cells in the body Essential for many digestive and metabolic processes, including
BEC Feed Solutions Presenter: Steve Blake BEC Feed Solutions Nutritional Role of Phosphorus Phosphorus (P) is present in all cells in the body Essential for many digestive and metabolic processes, including
H.W. 1 Bio 101 Prof. Fournier
 H.W. 1 Bio 101 Prof. Fournier 1. What is a similarity between all bacteria and plants? A) They both have a nucleus B) They are both composed of cells C) They both have chloroplasts D) They both lack a
H.W. 1 Bio 101 Prof. Fournier 1. What is a similarity between all bacteria and plants? A) They both have a nucleus B) They are both composed of cells C) They both have chloroplasts D) They both lack a
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture-11 Enzyme Mechanisms II
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture-11 Enzyme Mechanisms II In the last class we studied the enzyme mechanisms of ribonuclease A
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture-11 Enzyme Mechanisms II In the last class we studied the enzyme mechanisms of ribonuclease A
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
pathway that involves taking in heat from the environment at each step. C.
 Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Nucleotides and Nucleic Acids
 Nucleotides and Nucleic Acids Brief History 1 1869 - Miescher Isolated nuclein from soiled bandages 1902 - Garrod Studied rare genetic disorder: Alkaptonuria; concluded that specific gene is associated
Nucleotides and Nucleic Acids Brief History 1 1869 - Miescher Isolated nuclein from soiled bandages 1902 - Garrod Studied rare genetic disorder: Alkaptonuria; concluded that specific gene is associated
Chapter 14 Glycolysis. Glucose. 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) TCA Cycle
 Chapter 14 Glycolysis Requires mitochondria and O 2 Glucose glycolysis anaerobic respiration 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) pyruvate dehydrogenase acetyl-coa TCA Cycle
Chapter 14 Glycolysis Requires mitochondria and O 2 Glucose glycolysis anaerobic respiration 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) pyruvate dehydrogenase acetyl-coa TCA Cycle
Methods of Grading S/N Style of grading Percentage Score 1 Attendance, class work and assignment 10 2 Test 20 3 Examination 70 Total 100
 COURSE: MIB 303 Microbial Physiology and Metabolism (3 Units- Compulsory) Course Duration: Three hours per week for 15 weeks (45 hours). Lecturer: Jimoh, S.O. B.Sc., M.Sc, Ph.D Microbiology (ABU, Zaria)
COURSE: MIB 303 Microbial Physiology and Metabolism (3 Units- Compulsory) Course Duration: Three hours per week for 15 weeks (45 hours). Lecturer: Jimoh, S.O. B.Sc., M.Sc, Ph.D Microbiology (ABU, Zaria)
Chapter 8: Energy and Metabolism
 Chapter 8: Energy and Metabolism 1. Discuss energy conversions and the 1 st and 2 nd law of thermodynamics. Be sure to use the terms work, potential energy, kinetic energy, and entropy. 2. What are Joules
Chapter 8: Energy and Metabolism 1. Discuss energy conversions and the 1 st and 2 nd law of thermodynamics. Be sure to use the terms work, potential energy, kinetic energy, and entropy. 2. What are Joules
10.2 The Human Digestive System pg. 411
 10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
10.2 The Human Digestive System pg. 411 The human digestive system is made up of a group of organs working together. The digestive tract is made up of the mouth, esophagus, stomach, small intestine, and
Amino Acid Degradation
 Amino Acid Degradation April 14, 2003 Bryant Miles The carbon skeletons of amino acids are broken down into metabolites that can either be oxidized into 2 and H 2 to generate ATP, or can be used for gluconeogenesis.
Amino Acid Degradation April 14, 2003 Bryant Miles The carbon skeletons of amino acids are broken down into metabolites that can either be oxidized into 2 and H 2 to generate ATP, or can be used for gluconeogenesis.
1. What has a higher stored energy potential per gram, glycogen or triglycerides? Explain.
 Lipid Metabolism 1. What has a higher stored energy potential per gram, glycogen or triglycerides? Explain. 2. How can excess acetyl CoA trapped in the mitochondria, be utilized as a substrate for fatty
Lipid Metabolism 1. What has a higher stored energy potential per gram, glycogen or triglycerides? Explain. 2. How can excess acetyl CoA trapped in the mitochondria, be utilized as a substrate for fatty
Unsaturated and Odd-Chain Fatty Acid Catabolism
 Unsaturated and dd-hain Fatty Acid atabolism March 24, 2003 Bryant Miles The complete oxidation of saturated fatty acids containing an even number of carbon atoms is accomplished by the β-oxidation pathway.
Unsaturated and dd-hain Fatty Acid atabolism March 24, 2003 Bryant Miles The complete oxidation of saturated fatty acids containing an even number of carbon atoms is accomplished by the β-oxidation pathway.
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Working With Enzymes. a world of learning. Introduction. How Enzymes Work. Types and Sources of Enzymes
 Working With Enzymes a world of learning Presented by Peter J Ball, Southern Biological. For further information, please contact the author by phone (03) 9877-4597 or by email [email protected].
Working With Enzymes a world of learning Presented by Peter J Ball, Southern Biological. For further information, please contact the author by phone (03) 9877-4597 or by email [email protected].
Photosynthesis and Sucrose Production
 Photosynthesis and Sucrose Production 2 Starch and sucrose, key substrates for the development of dental caries, are exclusively synthesized by plants. They are made in plant leaves by a process called
Photosynthesis and Sucrose Production 2 Starch and sucrose, key substrates for the development of dental caries, are exclusively synthesized by plants. They are made in plant leaves by a process called
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
Copyright 2010 Pearson Education, Inc. Chapter Twenty Three 1
 23.2 Glucose Metabolism: An Overview When glucose enters a cell from the bloodstream, it is immediately converted to glucose 6- phosphate. Once this phosphate is formed, glucose is trapped within the cell
23.2 Glucose Metabolism: An Overview When glucose enters a cell from the bloodstream, it is immediately converted to glucose 6- phosphate. Once this phosphate is formed, glucose is trapped within the cell
How to measure Ammonia and Organic Nitrogen: Kjeldahl Method
 World Bank & Government of The Netherlands funded Training module # WQ - 38 How to measure Ammonia and Organic Nitrogen: Kjeldahl Method New Delhi, March 2000 CSMRS Building, 4th Floor, Olof Palme Marg,
World Bank & Government of The Netherlands funded Training module # WQ - 38 How to measure Ammonia and Organic Nitrogen: Kjeldahl Method New Delhi, March 2000 CSMRS Building, 4th Floor, Olof Palme Marg,
What affects an enzyme s activity? General environmental factors, such as temperature and ph. Chemicals that specifically influence the enzyme.
 CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
How To Understand The Chemistry Of An Enzyme
 Chapt. 8 Enzymes as catalysts Ch. 8 Enzymes as catalysts Student Learning Outcomes: Explain general features of enzymes as catalysts: Substrate -> Product Describe nature of catalytic sites general mechanisms
Chapt. 8 Enzymes as catalysts Ch. 8 Enzymes as catalysts Student Learning Outcomes: Explain general features of enzymes as catalysts: Substrate -> Product Describe nature of catalytic sites general mechanisms
The Human Digestive System
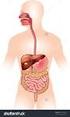 The Human Digestive System Name: Section: Date: Page 1 of 10 Page 2 of 10 Page 3 of 10 Page 4 of 10 Page 5 of 10 Page 6 of 10 Putting it All Together Digestive Enzymes Page 7 of 10 Page 8 of 10 Page 9
The Human Digestive System Name: Section: Date: Page 1 of 10 Page 2 of 10 Page 3 of 10 Page 4 of 10 Page 5 of 10 Page 6 of 10 Putting it All Together Digestive Enzymes Page 7 of 10 Page 8 of 10 Page 9
The Nitrogen Cycle. What is Nitrogen? Human Alteration of the Global Nitrogen Cycle. How does the nitrogen cycle work?
 Human Alteration of the Global Nitrogen Cycle Heather McGraw, Mandy Williams, Suzanne Heinzel, and Cristen Whorl, Give SIUE Permission to Put Our Presentation on E-reserve at Lovejoy Library. What is Nitrogen?
Human Alteration of the Global Nitrogen Cycle Heather McGraw, Mandy Williams, Suzanne Heinzel, and Cristen Whorl, Give SIUE Permission to Put Our Presentation on E-reserve at Lovejoy Library. What is Nitrogen?
The chemical reactions inside cells are controlled by enzymes. Cells may be specialised to carry out a particular function.
 12.1 What are animals and plants built from? All living things are made up of cells. The structures of different types of cells are related to their functions. to relate the structure of different types
12.1 What are animals and plants built from? All living things are made up of cells. The structures of different types of cells are related to their functions. to relate the structure of different types
Bioremediation. Biodegradation
 Bioremediation A technology that encourages growth and reproduction of indigenous microorganisms (bacteria and fungi) to enhance biodegradation of organic constituents in the saturated zone Can effectively
Bioremediation A technology that encourages growth and reproduction of indigenous microorganisms (bacteria and fungi) to enhance biodegradation of organic constituents in the saturated zone Can effectively
AP Bio Photosynthesis & Respiration
 AP Bio Photosynthesis & Respiration Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the term used for the metabolic pathway in which
AP Bio Photosynthesis & Respiration Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the term used for the metabolic pathway in which
RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES. Bio 171 Week 6
 RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES Bio 171 Week 6 Procedure Label test tubes well, including group name 1) Add solutions listed to small test tubes 2) For
RESPIRATION AND FERMENTATION: AEROBIC AND ANAEROBIC OXIDATION OF ORGANIC MOLECULES Bio 171 Week 6 Procedure Label test tubes well, including group name 1) Add solutions listed to small test tubes 2) For
