MULTILOAD Radio-Opaque Intrauterine Contraceptive Devices
|
|
|
- Frank Hancock
- 9 years ago
- Views:
Transcription
1 NEW ZEALAND DATA SHEET MULTILOAD Radio-Opaque Intrauterine Contraceptive Devices Presentation MULTILOAD consists of a small plastic rod wound with copper wire and provided with two flexible arms and a nylon string. MULTILOAD Cu375: length 35.0mm; width 20.5mm MULTILOAD Cu375 SL: length 29.5mm; width 20.5mm Uses Actions Although the exact mechanisms by which the IUDs exert their contraceptive effect are not completely clear, there is evidence that they act primarily to prevent sperm from fertilising ova and prevention of implantation. IUDs induce a local inflammatory response, which in turn leads to lysosomal activation and other inflammatory changes that are spermicidal. The presence of metallic copper improves the contraceptive efficacy of MULTILOAD. The plastic body of MULTILOAD contains barium sulphate to render it radio-opaque. The flexible side arms of MULTILOAD ensure that the IUD remains in position as high as possible against the fundus, without the uterine cavity being stretched. Pharmacokinetics The contraceptive influence of copper is related to the oxidation of copper atoms and the subsequent dissolution of the oxides in the intrauterine milieu. For MULTILOAD Cu375 and MULTILOAD Cu375 SL the mean daily release of copper amounts to about 30 micrograms. The intrauterine presence of copper-releasing IUDs causes neither an increase in serum copper concentrations to a measurable degree nor an increase in serum ceruloplasmin concentrations. This is to be expected since the average daily copper release of IUDs is only about one-hundredth of the average daily copper intake via food. Indications Intrauterine contraception. MULTILOAD is intended for single use only, and has to be inserted by a doctor. Do not re-use, reinsert or re-sterilize Multiload. Recommendations for the type of MULTILOAD to be fitted: MULTILOAD Cu375 for uteri with a sound length between 6 and 9 cm. MULTILOAD Cu375 SL for uteri with a sound length between 5 and 8 cm. Dosage And Administration Time For Insertion The optimum time for insertion of an IUD is the last days of the menstrual flow or in the first days afterwards (so-called interval insertion). This is to reduce the possibility of insertion in the presence of an existing undiagnosed pregnancy. However, it is essential that pregnancy is ruled out before insertion. An IUD may also be inserted immediately (within 10 minutes) post-abortion or post-partum, although in these cases the chances of pregnancy, translocation, and expulsion are higher. Immediate post-abortion or post-partum insertion does not adversely affect uterine involution or breastfeeding. If a post-abortion or post-partum insertion is not done immediately, it should be delayed until involution is complete i.e. at least 6 weeks after delivery or abortion (so-called delayed post-partum insertion). After caesarean section, insertion should not be attempted until 12 weeks after delivery. Postcoital IUD Use To prevent pregnancy after unprotected intercourse insertion of an IUD may be an effective measure, provided that intercourse has taken place no longer than 5 days before. NB. The physician should take account of the risk of pelvic infection associated with emergency IUD insertion and should adequately inform the patient in this respect. This is particularly relevant in cases of rape. Multiload A Version 4.0 Page 1
2 Recommended Insertion Procedure It is imperative that a no-touch technique is employed throughout the insertion procedure to ensure sterile handling. The intrauterine device should not be used in the event of the inner packaging being damaged. A. Interval Insertion Preparation 1. Ensure that the patient understands the contents of the patient leaflet. 2. Confirm that there are no contraindications to use of Multiload. 3. Perform a urine pregnancy test, if indicated. 4. Perform a careful bimanual examination to determine the version, flexion and uterine axis. 5. Insert a vaginal speculum to expose the cervix. Cleanse the cervix and vaginal walls with sterile cotton wool dipped in antiseptic solution (Figure a). Wipe all secretion away from the external os. 6. Grasp the anterior lip of the cervix with a single-tooth tenaculum, taking a good bite through the cervical lip so that steady downward traction to straighten the uterine axis can be maintained without risk of cervical laceration (Figure b). Reflex contraction, which causes cramp of the uterus when the tenaculum is applied, can be prevented by injection of a local anaesthetic into the anterior lip or a paracervical block. 7. Carefully sound the uterus to determine its depth and to confirm the direction of its axis. If the sound meets more than normal resistance at the internal os, it may be advisable to gently dilate the cervical canal to 4-5mm, using sterile, tapered rather than cylindrical dilators. In the absence of other instruments for measurement of the internal dimensions of the uterine cavity, the sound may be used to obtain an idea of its configuration. Figure a Figure b Inserting MULTILOAD The vertical stem of MULTILOAD is already preloaded in the introducer tube. The side arms do not require loading into the tube. They are sufficiently flexible to adapt to the shape of the cervical canal. 1. Tear the pouch open at the indicated notch (see Figure 1). Figure 1 Multiload A Version 4.0 Page 2
3 2. Peel the clear cover back so far that the introducer tube (with IUD) can be picked up at its distal end, grasping the tube and the threads, but without taking MULTILOAD out of the pouch (see Figure 2). Figure 2 3. Hold the cervical stop with the thumb of one hand and adjust the position of the top of MULTILOAD by moving the introducer tube with the other hand until it corresponds with the mark indicating, approximately, the sounded uterine length in centimetres (see Figure 3). Figure 3 4. The distal end of the introducer may be held without risk of contaminating the device. Holding the threads together with the tube ensures that the device does not fall out of the introducer tube. MULTILOAD can now be taken out of the pouch. 5. Carefully insert MULTILOAD into the uterus (Figure c) until it touches the fundus and the cervical stop rests against the external os (Figure d) while maintaining steady downward traction with the tenaculum to straighten the uterine axis. No attempt should be made to force insertion. Insufficient axial straightening may, on occasion, result in a sub-endometrial insertion. This risk may be reduced by exerting an adequate downward pulling force on the cervix, thereby fully straightening the axis of the uterus against its ligamentous supports. 6. When MULTILOAD touches the fundus, it is released into the uterine cavity by simply withdrawing the introducer tube (Figure e). During this procedure continue to apply downward traction with the tenaculum. No push-rod is required to insert MULTILOAD. Check the cervical canal with the sound to ensure that the tail of MULTILOAD is entirely within the uterine cavity. Trim the threads of MULTILOAD to 2 to 3 cm measured from the external os. Multiload A Version 4.0 Page 3
4 7. It is imperative to follow precisely the recommended insertion procedure in order to minimise the risk of a sub-endometrial insertion, which may, in turn, lead to full or partial endometrial embedding of the IUD. Should this occur, a higher than normal force may need to be applied to remove the IUD from this incorrect location, which may increase the risk of side-arm breakages. Furthermore, it may be clinically difficult to confirm the IUD s sub-endometrial location, since this is usually not obvious to the doctor during insertion of the device and the patient probably experiences no pain. It is anticipated that correctly inserting the device may reduce the incidence of both side-arm breakages and perforations. Figure c Figure d Figure e The MULTILOAD package contains both a User Card and a Physician Card. These cards record the Lot number, the date of insertion, the intended date of removal and the address of the local Merck Sharp & Dohme distributor. It is important to fill in both cards; the User Card should be given to the user and the Physician Card should be kept in the patient s file. In case of any complaints on MULTILOAD, it is important to always mention the Lot number. B. Immediate Post-Partum and Post-Abortion Insertions 1. Insert a bivalve (bayonet) speculum to expose the cervix after delivery of the placenta and membranes (no later than 10 minutes). Cleanse the cervix and vaginal walls with sterile cotton wool dipped in antiseptic solution. 2. Grasp both the anterior and the posterior lips with one or two ring forceps and draw the cervix down for close inspection. 3. Take the introducer tube (with pre-loaded IUD) and insert MULTILOAD along the palmar aspect of two fingers into the uterine cavity until it touches the fundus. Check the position of MULTILOAD with the flat hand on the abdominal wall covering the fundal region. 4. When MULTILOAD touches the fundus, it is released into the uterine cavity by very gentle withdrawal of the introducer tube. Take care not to pull on the threads which are left uncut until the first follow-up visit. Time of Removal The recommended in situ time is 5 years for each type of Cu375. In adult women in whom it is not expected that the uterus will grow, MULTILOAD Cu375 can remain in situ for the full 5 years. In young women in whom the uterus may still grow and in whom the short type of MULTILOAD has been fitted, it may be necessary to remove it within a shorter period and replace it with a larger type. Multiload A Version 4.0 Page 4
5 Removal Procedure Prepare the vulva, insert the speculum and cleanse the cervix as for insertion. To facilitate removal, a tenaculum should always be used to straighten out the uterine axis, thereby also minimising the risk of side-arm breakages. Use a forceps to grasp both threads of the MULTILOAD as near to the exit from the external os as possible. Using steady downward traction with the tenaculum to straighten the uterine axis, the MULTILOAD should be able to be easily withdrawn from the uterus. No excessive force must be used. After removal of Multiload verify that the device is intact, after this verification the IUD should be disposed if in accordance with local requirements. If Multiload is removed mid-cycle and the woman has had intercourse within the preceding week, she is at risk of pregnancy unless a new IUD is inserted immediately after removal. Difficult Removal And Breakage During Removal Sometimes difficulties are encountered when removing the IUD. In the event of a more than usual force being required for removal, consideration should be given to the probability that the MULTILOAD is embedded. There have been reports of part of an embedded device (in particular an embedded side-arm) breaking off within the uterine cavity, when a greater than normal force needs to be applied for removal. Retained fragments may be expelled painlessly with the menstrual period and embedded side-arms may be freed by uterine contractions. There have also been some rare reports of breakage not associated with embedding. If the device cannot be withdrawn by normal force or if a fragment has remained behind, diagnostic steps including X-rays or ultrasound, should be taken to exclude perforation or embedding. Plastic fragments, such as the side-arms, may be located using X-rays, ultrasound or hysteroscopy. The latter technique allows removal at the same time. However, reports indicate that routine curettage for removal of a fragment, whether or not located in advance, is successful in many cases. Removal of fragments should always be attempted. Contraindications Absolute Pregnancy (established or suspected) Malignant disease of the corpus uteri or cervix. Vaginal bleeding of undiagnosed etiology. Ectopic pregnancy in anamnesis or the presence of predisposing factors for this condition such as salpingitis, endometritis or pelvic peritonitis. Congenital or acquired malformations or distortions of the uterus or the cervix; large or multiple uterine fibromyomata in the presence of excessively heavy menstrual periods; endometrial hyperplasia; cervical dysplasia. Genital infection (with the exception of candidiasis). Sexually transmitted disease during the past 12 months (with the exception of bacterial vaginitis, candidiasis, recurrent herpes virus infection, hepatitis B or cytomegalovirus infection). Infected abortion in the past 3 months. Active pelvic inflammatory disease (PID) or history of recurrent PID. Hypersensitivity to any of the components of the product. Relative History of (partial) expulsion with Multiload or another IUD/IUS. Valvular heart disease. Use of an IUD in these cases may increase the risk of subacute bacterial endocarditis. Antibiotic prophylaxis should be given when inserting or removing the IUD. Anaemia or a history of excessive uterine bleeding. Coagulopathy or current administration of anti-coagulants. Severe dysmenorrhoea. Uterine scars from previous surgery other than caesarean section or previous perforation of the uterus. Small uterine fibromyomata, endometrial polyps, or endometriosis. Regular pelvic examination of a patient with fibroids is advised to assess any change in size. Long-lasting intensive treatment with corticosteroids or non-steroidal anti-inflammatory agents (see also Interactions). Long-lasting intensive immunosuppressive therapy (see also Interactions). Disorder of copper metabolism (e.g. Wilson s disease). Multiload A Version 4.0 Page 5
6 Current or recurrent lower genital tract infection. Multiple sexual partners (see also Interactions). Warnings And Precautions Medical Examination Prior to insertion of an IUD the medical contraindications for IUD use should be excluded on the basis of both the medical history and the physical examination of the woman. Physical examination should include a pelvic examination, cervical smear, and if possible, appropriate tests for sexually transmitted disease. After interval insertion IUD users should be re-examined shortly after the first period and after immediate post-abortion or post-partum insertion monthly during the first three months. Thereafter, appropriate examination should be carried out at regular intervals e.g. every six months. If MULTILOAD threads cannot be felt in a woman who has not noticed expulsion, examination is necessary to exclude perforation or unnoticed expulsion. Ultrasound or X-ray may be used to locate the device. Nulligravity/Nulliparity In nulliparous women the risks and benefits of intrauterine contraception should be assessed with special consideration of their future fertility. Pain during and after insertion is more likely to occur in nulliparous than in multiparous women. Pelvic Inflammatory Disease (PID) The risk of developing PID in women using an IUD is only increased in the first 20 days after insertion; thereafter the risk of PID is similar to the risk in women without an IUD. The occurrence of PID may also be due to other factors, such as contraction of a sexually transmitted disease. PID may lead to tubal occlusion resulting in impairment of future fertility, increase the risk of subsequent ectopic pregnancy and, if a tubo-ovarian abscess develops, may necessitate hysterectomy and oophorectomy. Therefore, in nulliparous women and in women with a recent history of treated pelvic infection, contraceptive benefits of IUD use have to be weighed against potential risks. The following clinical symptoms may be indicative of PID: pyrexia, lower abdominal tenderness or pain, abnormal vaginal discharge, deep dyspareunia, prolonged or heavy menstrual bleeding, pain on manipulation of the cervix, tenderness or pain on bi-manual examination of the uterus and uterine adnexae. If women fitted with an IUD are suspected of PID, the following is advised: In mild cases the diagnosis should be established and treatment with antibiotics started. If there is no response after 24 hours the IUD should be removed. In moderate cases with more definite clinical signs the IUD should be removed before starting antibiotic therapy and the patient referred for a gynaecological opinion. In severe cases associated with marked lower abdominal pain and fever the IUD should be removed and the patient should be admitted to hospital. Medical Reasons For Removal Pregnancy (see below) PID (see above) Excessive and persistent bleeding or cramping. Perforation of the cervical or uterine wall. This is extremely rare with MULTILOAD but if it should happen, the device should be removed. Downward displacement of MULTILOAD into the cervical canal. Translocation. NB. For the removal procedure and the possible complications encountered therewith see the Dosage and Administration section. Additional Warnings IUDs do not protect against HIV infection (AIDS) or any other sexually transmitted disease. Insertion of an IUD may precipitate a seizure in women suffering from epilepsy. Special care is therefore recommended during insertion. Multiload A Version 4.0 Page 6
7 In women fitted with an IUD menstrual blood loss is often increased and occasionally this may lead to iron deficiency anaemia. Use During Breastfeeding MULTILOAD may be inserted in breast-feeding women. However, in view of reports suggesting an increased risk of uterine perforation during lactation, particular care should be taken. Pregnancy Despite IUD Use IUDs are more protective against intrauterine than against extrauterine pregnancy, but there are indications that the chance of the latter is also decreased as a result of the use of an IUD. Should a pregnancy occur with an IUD in situ, it is necessary to determine, e.g. by ultrasonography, whether this pregnancy is intrauterine or ectopic. 1. Intrauterine Pregnancy If intrauterine pregnancy occurs with an IUD in situ the following is strongly recommended: up to 12 weeks gestation, the device should be removed if the threads are visible. beyond 12 weeks, or if no threads are visible, termination of pregnancy should be considered and offered to the woman as an option, bearing in mind that the risks associated with elective abortion increase with gestational age. If the woman insists on continuing the pregnancy with the IUD in situ, refer early for antenatal care. Some reports indicate an increased incidence of septic abortion in patients becoming pregnant with an IUD in situ. In some instances septic abortion has been complicated by septicaemia, sometimes with fatal outcome. The onset of septicaemia may be insidious. If pregnancy is continued with the IUD in situ, the woman must be closely observed and advised to report immediately all abnormal symptoms such as flu-like syndrome, fever, abdominal cramping and pain, dyspareunia, bleeding or vaginal discharge. If left in situ throughout pregnancy the device is usually expelled prior to or concomitantly with delivery of placenta and membranes. If not, X-ray or ultrasound should be used early in the puerperium to locate it. So far, there is no evidence that, when a pregnancy continues to term with an IUD in situ, this will lead to birth defects. 2. Ectopic Pregnancy If ectopic pregnancy is suspected early diagnosis is vital. If ectopic pregnancy is established immediate gynaecological intervention is required. Clinical features include amenorrhoea for 6-8 weeks accompanied by symptoms of pregnancy and (severe) unilateral pelvic pain with or without vaginal bleeding which is often scanty and black. However, these symptoms are not always present. The period may not even be late. In addition shouldertip pain or the recent onset of dizziness or fainting may be present. Effects on Ability to Drive and Use Machines No observed effects. Patient Counselling Every potential IUD user should be fully informed of the risks and benefits of IUDs. Any user of MULTILOAD should be given the Package insert containing the Information for the User. The physician should inform the user about: the necessity to read the Information for the User very carefully and to follow the instructions to the letter. This is particularly relevant with regard to the possibility of device failure and pregnancy and the paragraph in the section Possible complications, covering the early symptoms of genito-urinary infection. Women desiring future pregnancy should be made aware of the slightly increased risk of PID and its possible interference with future fertility. the necessity to carefully keep the User Card for future reference. how to feel for the threads of MULTILOAD after the end of each period and at any time she would experience unusual contractions in the lower abdomen during her period. how to feel the cervix each month after the end of her period and to ensure that no firm plastic is protruding. the necessity to contact a doctor: if she cannot feel the thread(s) of MULTILOAD. if she feels the plastic end of MULTILOAD protruding. Multiload A Version 4.0 Page 7
8 if she experiences heavy menstrual bleeding or troublesome intermenstrual bleeding or spotting as a new complaint occurring after the first few months after insertion, or postcoital bleeding. if she experiences dysmenorrhoea or dyspareunia. if she misses a period. if signs or symptoms of a genito-urinary infection or of an extrauterine pregnancy appear, i.e. prolonged or heavy menstrual bleeding, (persistent) lower abdominal pain or shouldertip pain, deep dyspareunia, fever in excess of 38 C and/or flu-like syndrome, abnormal vaginal discharge, dizziness or fainting. Adverse Effects The table* below lists the undesirable effects that have been reported in users of MULTILOAD. System Organ Class MedDRA term Cardiac disorders Bradycardia (1,2,4) Gastrointestinal disorders Abdominal pain (3) General disorders and administration site Complication of device insertion conditions Complication of device removal Device breakage Embedded IUD (Partial) device expulsion (5) Infections and infestations Genital infection Sepsis Urinary tract infection Injury, poisoning and procedural complications Uterine perforation (5) Musculoskeletal and connective tissue disorders Back pain Pain in extremity Nervous system disorders Syncope vasovagal (1,2,4) Pregnancy, puerperium and perinatal conditions Abortion spontaneous Ectopic pregnancy Reproductive system and breast disorders Dysmenorrhoea Dyspareunia Menorrhagia (6) Metrorrhagia (6) Vaginal discharge Skin and subcutaneous tissue disorders Dermatitis allergic Urticaria (1): during or immediately after insertion of IUD (2): during or immediately after removal of IUD (3): after insertion of IUD (4): particularly in nulliparous women (5): uterine perforation and IUD displacement into the abdomen have been followed by peritonitis, abdominal adhesions, intestinal penetration, intestinal obstruction and cystic masses in the pelvis. (6): mainly during first cycle(s) after insertion of IUD *: The most appropriate MedDRA term (version 13.0) to describe a certain adverse reaction is listed. Synonyms or related conditions are not listed, but should be taken into account as well. Interactions The foreign body reaction is crucial in the contraceptive activity of IUDs. Since this is a sterile inflammatory reaction of the endometrium, treatments that may interfere with this inflammatory process may reduce the contraceptive efficacy of IUDs. Accordingly, women needing long-lasting intensive treatment with corticosteroids, non-steroidal anti-inflammatory agents, or immunosuppressive therapy, preferably should opt for another contraceptive method. In case of occasional intensive treatment with anti-inflammatory or immunosuppressive agents, the IUD user should be advised to take additional contraceptive measures during these periods. It has been suggested that tetracyclines may reduce the contraceptive efficacy of coppercontaining IUDs. Multiload A Version 4.0 Page 8
9 Since the occurrence of pelvic inflammatory disease seems to be most strongly related to the background risk of sexually transmissible disease, multiple sexual partners is included as a relative contraindication. It has been suggested that medical diathermy (short-wave and microwave) of the abdominal and sacral areas might induce heat injuries because of the presence of metallic copper on the IUD. However, in situ measurements indicate that diathermic treatment of women with copper-bearing IUDs can be regarded to be safe when using dose intensities within the normal therapeutic range. The energetic status of copper will not be changed by Magnetic Resonance Imaging (MRI). Therefore, an effect on the IUD induced by MRI can be neglected. Furthermore, based on the non-ferro characteristics of copper the scan obtained with Magnetic Resonance Imaging (MRI) is considered not to be impaired by the presence of the IUD. Overdosage Not applicable. Pharmaceutical Precautions Incompatibilities No relevant incompatibilities are known. Shelf-Life The shelf-life of MULTILOAD, under the given storage conditions, is 5 years. MULTILOAD may be inserted until the expiration date indicated on the package. Special Precautions for Storage The intact package has to be stored dry and below 30 o C in the original carton box. Medicine Classification Prescription Medicine. Package Quantities Packed as single items in sterile containers (pouches). Further Information Adverse Event Reporting Details of any adverse event noticed, or reported, by a user should be reported immediately to the Medical Assessor, Medicines Adverse Reactions Committee, P.O. Box 913, Dunedin, on the standard ADR form (H1574). Customer Services In order to maintain the high quality of this product, the manufacturer is very interested in all complaints or remarks with respect to MULTILOAD. These can be reported to the local distributor of MULTILOAD (for address details see Physician Card). In case of complaints, it is important also to report the Lot number of the MULTILOAD. Qualitative and Quantitative Composition MULTILOAD Cu375/375 SL are intrauterine devices with flexible side-arms, made of a mixture of high density polyethylene, ethylene vinyl acetate copolymer and barium sulphate in a weight ratio of 44/36/20. A copper wire is wound around the stem, giving a total copper surface area of 375mm 2. A monofilament nylon thread is attached to the stem. The stem length of the MULTILOAD Cu375 standard device is 35mm; the stem of MULTILOAD Cu375 SL is 30mm. Except for stem length, these devices have identical dimensions. The introducer tube consists of: Tube made of polypropylene; Cervical stop consist of a 44/36/20 mixture of high density polyethylene, ethylene vinyl acetate copolymer and barium sulphate, or high density polyethylene only. Nature and Contents of Container MULTILOAD radio-opaque intrauterine devices (IUD) are packed individually in a transparent 3-sided sealed sachet, made of a laminated film comprising polyethylene terephthalate (polyester) and low density polyethylene. A paper ruler is inserted and the sachet is sealed on the fourth side. Multiload A Version 4.0 Page 9
10 Preclinical Safety Date No particulars. Name And Address of Sponsor Merck Sharp & Dohme (NZ) Ltd P O Box Newmarket Auckland 1149 Tel: Date Of Preparation November 2012 RA 0620 OS IFU S6 (Ref 5.0) Multiload A Version 4.0 Page 10
MULTILOAD cu 250 / cu 375
 MULTILOAD cu 250 / cu 375 CE 0336 Read all of this leaflet carefully before you decide to have Multiload Radiopaque inserted. This leaflet provides information that may help you in your decision to start
MULTILOAD cu 250 / cu 375 CE 0336 Read all of this leaflet carefully before you decide to have Multiload Radiopaque inserted. This leaflet provides information that may help you in your decision to start
Step-by-Step Insertion Instructions
 Step-by-Step Insertion Instructions Please read these instructions carefully and please visit LILETTAHCP.com for the full Prescribing Information. You may also visit LILETTAhcp.com/video for a video demonstration.
Step-by-Step Insertion Instructions Please read these instructions carefully and please visit LILETTAHCP.com for the full Prescribing Information. You may also visit LILETTAhcp.com/video for a video demonstration.
LIPPES LOOP TRADEMARK. your intrauterine contraceptive
 LIPPES LOOP TRADEMARK your intrauterine contraceptive LIPPES LOOP Patient Information This brochure provides information on the use of In trauterine Contraceptive Devices (lud s). There are other birth
LIPPES LOOP TRADEMARK your intrauterine contraceptive LIPPES LOOP Patient Information This brochure provides information on the use of In trauterine Contraceptive Devices (lud s). There are other birth
A Guide to Hysteroscopy. Patient Education
 A Guide to Hysteroscopy Patient Education QUESTIONS AND ANSWERS ABOUT HYSTEROSCOPY Your doctor has recommended that you have a procedure called a hysteroscopy. Naturally, you may have questions about
A Guide to Hysteroscopy Patient Education QUESTIONS AND ANSWERS ABOUT HYSTEROSCOPY Your doctor has recommended that you have a procedure called a hysteroscopy. Naturally, you may have questions about
Medical criteria for IUCD s Based on the WHO MEC (2004- Annexure 3) system a woman s eligibility for IUCD insertion falls in 4 categories. These categ
 CLIENT ASSESSMENT Ensure that the woman is not pregnant Determine the length and direction of uterus. Ensure that she does not have gonorrhea and chlamydia, and is not a high risk case of STI s Identify
CLIENT ASSESSMENT Ensure that the woman is not pregnant Determine the length and direction of uterus. Ensure that she does not have gonorrhea and chlamydia, and is not a high risk case of STI s Identify
MHRI IUD Protocol. Migraine with aura Current DVT or PE History of or current breast cancer Active viral hepatitis Severe cirrhosis or liver tumors
 Table of Contents A. Indications B. Contraindications C. Prior to Insertion D. Insertion E. Follow-Up Visit F. Removal G. Re-Insertion H. Complications/Side Effects I. Appendices MHRI IUD Protocol A. Indications
Table of Contents A. Indications B. Contraindications C. Prior to Insertion D. Insertion E. Follow-Up Visit F. Removal G. Re-Insertion H. Complications/Side Effects I. Appendices MHRI IUD Protocol A. Indications
Abnormal Uterine Bleeding FAQ Sheet
 Abnormal Uterine Bleeding FAQ Sheet What is abnormal uterine bleeding? Under normal circumstances, a woman's uterus sheds a limited amount of blood during each menstrual period. Bleeding that occurs between
Abnormal Uterine Bleeding FAQ Sheet What is abnormal uterine bleeding? Under normal circumstances, a woman's uterus sheds a limited amount of blood during each menstrual period. Bleeding that occurs between
Acute pelvic inflammatory disease: tests and treatment
 Acute pelvic inflammatory disease: tests and treatment Information for you Information for you Published August 2010 Published in August 2010 (next review date: 2014) Acute What is pelvic inflammatory
Acute pelvic inflammatory disease: tests and treatment Information for you Information for you Published August 2010 Published in August 2010 (next review date: 2014) Acute What is pelvic inflammatory
This is Jaydess. Patient Information. What is Jaydess? How does Jaydess work?
 , Patient Information This is Jaydess We hope that this brochure will answer your questions and concerns about Jaydess. What is Jaydess? Jaydess is an intrauterine device consisting of a hormone capsule
, Patient Information This is Jaydess We hope that this brochure will answer your questions and concerns about Jaydess. What is Jaydess? Jaydess is an intrauterine device consisting of a hormone capsule
Abnormal Uterine Bleeding
 Abnormal Uterine Bleeding WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Abnormal uterine bleeding is one of the most common reasons women see their doctors. It can occur at any age and has
Abnormal Uterine Bleeding WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Abnormal uterine bleeding is one of the most common reasons women see their doctors. It can occur at any age and has
Copper-Bearing Intrauterine Device
 CHAPTER 9 Copper-Bearing Intrauterine Device This chapter describes primarily the TCu-380A intrauterine device (for the Levonorgestrel Intrauterine Device, see p. 157). Key Points for Providers and Clients
CHAPTER 9 Copper-Bearing Intrauterine Device This chapter describes primarily the TCu-380A intrauterine device (for the Levonorgestrel Intrauterine Device, see p. 157). Key Points for Providers and Clients
Effective long-lasting strategy to prevent unintended pregnancy. The intrauterine system for contraception after abortion.
 Effective long-lasting strategy to prevent unintended pregnancy. The intrauterine system for contraception after abortion. After the abortion I started re-thinking my birth control method. I am looking
Effective long-lasting strategy to prevent unintended pregnancy. The intrauterine system for contraception after abortion. After the abortion I started re-thinking my birth control method. I am looking
STANDARD APRN PROTOCOL FOR IUD INSERTION: Levonorgestrel (LNG) Releasing Intrauterine System
 STANDARD APRN PROTOCOL FOR IUD INSERTION: Levonorgestrel (LNG) Releasing Intrauterine System DEFINITION The LNG-releasing intrauterine systems (Mirena, Liletta and Skyla ) are on the market. The LNG-releasing
STANDARD APRN PROTOCOL FOR IUD INSERTION: Levonorgestrel (LNG) Releasing Intrauterine System DEFINITION The LNG-releasing intrauterine systems (Mirena, Liletta and Skyla ) are on the market. The LNG-releasing
Uterine fibroids (Leiomyoma)
 Uterine fibroids (Leiomyoma) What are uterine fibroids? Uterine fibroids are fairly common benign (not cancer) growths in the uterus. They occur in about 25 50% of all women. Many women who have fibroids
Uterine fibroids (Leiomyoma) What are uterine fibroids? Uterine fibroids are fairly common benign (not cancer) growths in the uterus. They occur in about 25 50% of all women. Many women who have fibroids
Heavy menstrual bleeding and what you can do about it!
 Heavy menstrual bleeding and what you can do about it! The intrauterine system as an alternative to hysterectomy. What is heavy menstrual bleeding? Do I have it? A woman s menstrual periods are considered
Heavy menstrual bleeding and what you can do about it! The intrauterine system as an alternative to hysterectomy. What is heavy menstrual bleeding? Do I have it? A woman s menstrual periods are considered
Copper intra-uterine device (IUD)
 Oxford University Hospitals NHS Trust Copper intra-uterine device (IUD) Page What is an inter-uterine device? 3 How does it work? 4 Would an IUD be suitable for me? 5 Are there any risks or complications?
Oxford University Hospitals NHS Trust Copper intra-uterine device (IUD) Page What is an inter-uterine device? 3 How does it work? 4 Would an IUD be suitable for me? 5 Are there any risks or complications?
LILETTA CODING UPDATE
 Considerations before submitting claims Considerations after submitting claims LILETTA CODING UPDATE BEGINNING JANUARY 1, 2016, THE HCPCS* CODE (J-CODE) APPROPRIATE FOR LILETTA IS CHANGING FROM J7302 TO
Considerations before submitting claims Considerations after submitting claims LILETTA CODING UPDATE BEGINNING JANUARY 1, 2016, THE HCPCS* CODE (J-CODE) APPROPRIATE FOR LILETTA IS CHANGING FROM J7302 TO
EARLY PREGNANCY LOSS A Patient Guide to Treatment
 EARLY PREGNANCY LOSS A Patient Guide to Treatment You have a pregnancy that has stopped growing, or you have started to miscarry and the process has not completed. If so, there are four ways to manage
EARLY PREGNANCY LOSS A Patient Guide to Treatment You have a pregnancy that has stopped growing, or you have started to miscarry and the process has not completed. If so, there are four ways to manage
7 Intrauterine devices
 7 Intrauterine devices Meera Kishen Chapter contents Types of intrauterine device 111 Framed copper devices 112 Frameless copper devices 112 Mode of action 113 Effectiveness 114 Advantages 114 Compliance
7 Intrauterine devices Meera Kishen Chapter contents Types of intrauterine device 111 Framed copper devices 112 Frameless copper devices 112 Mode of action 113 Effectiveness 114 Advantages 114 Compliance
Safe & Unsafe. abortion
 Safe & Unsafe Facts About abortion WHAT IS THE DIFFERENCE BETWEEN UNSAFE AND SAFE ABORTION? What is unsafe abortion? Unsafe abortion is a procedure for terminating an unplanned pregnancy either by a person
Safe & Unsafe Facts About abortion WHAT IS THE DIFFERENCE BETWEEN UNSAFE AND SAFE ABORTION? What is unsafe abortion? Unsafe abortion is a procedure for terminating an unplanned pregnancy either by a person
Birth Control Options
 1 of 5 6/2/2014 9:46 AM Return to Web version Birth Control Options What is contraception? Contraception means preventing pregnancy, also called birth control. Most people know about options such as birth
1 of 5 6/2/2014 9:46 AM Return to Web version Birth Control Options What is contraception? Contraception means preventing pregnancy, also called birth control. Most people know about options such as birth
Hysterosalpingography
 Scan for mobile link. Hysterosalpingography Hysterosalpingography uses a real-time form of x-ray called fluoroscopy to examine the uterus and fallopian tubes of a woman who is having difficulty becoming
Scan for mobile link. Hysterosalpingography Hysterosalpingography uses a real-time form of x-ray called fluoroscopy to examine the uterus and fallopian tubes of a woman who is having difficulty becoming
Information for you Abortion care
 Information for you Abortion care Published in February 2012 This information is for you if you are considering having an abortion. It tells you: how you can access abortion services the care you can expect
Information for you Abortion care Published in February 2012 This information is for you if you are considering having an abortion. It tells you: how you can access abortion services the care you can expect
Heavy periods (menstrual bleeding)
 Heavy periods (menstrual bleeding) This information sheet has been given to you to help answer some of the questions you may have about heavy periods and the treatments that are available. This leaflet
Heavy periods (menstrual bleeding) This information sheet has been given to you to help answer some of the questions you may have about heavy periods and the treatments that are available. This leaflet
Glossary. amenorrhea, primary - from the beginning and lifelong; menstruation never begins at puberty.
 Glossary amenorrhea - absence or cessation of menstrual periods. amenorrhea, primary - from the beginning and lifelong; menstruation never begins at puberty. A amenorrhea, secondary - due to some physical
Glossary amenorrhea - absence or cessation of menstrual periods. amenorrhea, primary - from the beginning and lifelong; menstruation never begins at puberty. A amenorrhea, secondary - due to some physical
Out-patient hysteroscopy. Information for patients
 Out-patient hysteroscopy Information for patients Important If there is any chance you may be pregnant please tell a member of the team immediately. We will not be able to perform a hysteroscopy if you
Out-patient hysteroscopy Information for patients Important If there is any chance you may be pregnant please tell a member of the team immediately. We will not be able to perform a hysteroscopy if you
Objective. Indications for IUDs. IUDs 3 types. ParaGard IUD. Mirena IUD. Sonographic Evaluation of Intrauterine Devices (IUDs) Inert
 Sonographic Evaluation of Intrauterine Devices (IUDs) Anna S. Lev-Toaff, MD FACR Department of Radiology Hospital of the University of Pennsylvania Philadelphia, Pennsylvania Leading Edge in Diagnostic
Sonographic Evaluation of Intrauterine Devices (IUDs) Anna S. Lev-Toaff, MD FACR Department of Radiology Hospital of the University of Pennsylvania Philadelphia, Pennsylvania Leading Edge in Diagnostic
Clinical Interruption of Pregnancy (Medical/Surgical Abortion)
 Clinical Interruption of Pregnancy (Medical/Surgical Abortion) Approximately one fifth of all pregnancies in the United States end in abortion (Ventura et al., 2009). According to the CDC (2011a), there
Clinical Interruption of Pregnancy (Medical/Surgical Abortion) Approximately one fifth of all pregnancies in the United States end in abortion (Ventura et al., 2009). According to the CDC (2011a), there
% of Women Experiencing an Accidental Pregnancy within. Depo Provera 0.3 0.3 70 Norplant
 ParaGard T 80A INTRAUTERINE COPPER CONTRACEPTIVE P/N 101700 Rev. /14 Rx only PRESCRIBING INFORMATION Patients should be counseled that this product does not protect against HIV infection (AIDS) and other
ParaGard T 80A INTRAUTERINE COPPER CONTRACEPTIVE P/N 101700 Rev. /14 Rx only PRESCRIBING INFORMATION Patients should be counseled that this product does not protect against HIV infection (AIDS) and other
IUD training principles 2013
 IUD training principles 2013 Introduction These principles were developed in 2011 2012 by representatives and members of the following organisations: Sexual Health and Family Planning Australia (SHFPA)
IUD training principles 2013 Introduction These principles were developed in 2011 2012 by representatives and members of the following organisations: Sexual Health and Family Planning Australia (SHFPA)
IMAP Statement on Safe Abortion
 International Planned Parenthood Federation IMAP Statement on Safe Abortion Key points: When performed early in pregnancy by trained health personnel in adequate facilities, abortion is a very safe procedure
International Planned Parenthood Federation IMAP Statement on Safe Abortion Key points: When performed early in pregnancy by trained health personnel in adequate facilities, abortion is a very safe procedure
Laparoscopy and Hysteroscopy
 AMERICAN SOCIETY FOR REPRODUCTIVE MEDICINE Laparoscopy and Hysteroscopy A Guide for Patients PATIENT INFORMATION SERIES Published by the American Society for Reproductive Medicine under the direction of
AMERICAN SOCIETY FOR REPRODUCTIVE MEDICINE Laparoscopy and Hysteroscopy A Guide for Patients PATIENT INFORMATION SERIES Published by the American Society for Reproductive Medicine under the direction of
All methods of birth control are MUCH SAFER than being pregnant! If 100 women use each method for a year, how many of them get pregnant?
 The Correct Use of Birth Control: In order for any method of birth control to be effective, it must be used correctly ALL THE TIME. This means: One condom every time you have sex One pill every day One
The Correct Use of Birth Control: In order for any method of birth control to be effective, it must be used correctly ALL THE TIME. This means: One condom every time you have sex One pill every day One
Information for you A low-lying placenta (placenta praevia) after 20 weeks
 Information for you A low-lying placenta (placenta praevia) after 20 weeks Published in December 2011 Who is this information for? This information is intended to help you if you have, or have been told
Information for you A low-lying placenta (placenta praevia) after 20 weeks Published in December 2011 Who is this information for? This information is intended to help you if you have, or have been told
FDA-Approved Patient Labeling IMPLANON (etonogestrel implant) Subdermal Use
 FDA-Approved Patient Labeling IMPLANON (etonogestrel implant) Subdermal Use IMPLANON does not protect against HIV infection (the virus that causes AIDS) or other sexually transmitted diseases. Read this
FDA-Approved Patient Labeling IMPLANON (etonogestrel implant) Subdermal Use IMPLANON does not protect against HIV infection (the virus that causes AIDS) or other sexually transmitted diseases. Read this
Get the Facts, Be Informed, Make YOUR Best Decision. Pelvic Organ Prolapse
 Pelvic Organ Prolapse ETHICON Women s Health & Urology, a division of ETHICON, INC., a Johnson & Johnson company, is dedicated to providing innovative solutions for common women s health problems and to
Pelvic Organ Prolapse ETHICON Women s Health & Urology, a division of ETHICON, INC., a Johnson & Johnson company, is dedicated to providing innovative solutions for common women s health problems and to
Sterilisation for women and men: what you need to know
 Sterilisation for women and men: what you need to know Published January 2004 by the RCOG Contents Page number Key points 1 About this information 2 What are tubal occlusion and vasectomy? 2 What do I
Sterilisation for women and men: what you need to know Published January 2004 by the RCOG Contents Page number Key points 1 About this information 2 What are tubal occlusion and vasectomy? 2 What do I
This Protocol is adapted from the University of Colorado Protocol dated August 26, 2009.
 Protocol for Post-Placental IUD insertion July 14, 2010 This Protocol is adapted from the University of Colorado Protocol dated August 26, 2009. Background Post-placental intrauterine device (IUD) insertion
Protocol for Post-Placental IUD insertion July 14, 2010 This Protocol is adapted from the University of Colorado Protocol dated August 26, 2009. Background Post-placental intrauterine device (IUD) insertion
the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD your guide to
 your guide to Helping you choose the method of contraception that is best for you IUD IUD the e IUD IU IUD the IUD 2 The intrauterine device (IUD) An IUD is a small plastic and copper device that is put
your guide to Helping you choose the method of contraception that is best for you IUD IUD the e IUD IU IUD the IUD 2 The intrauterine device (IUD) An IUD is a small plastic and copper device that is put
PATIENTS SHOULD BE COUNSELED THAT THIS PRODUCT DOES NOT PROTECT AGAINST HIV INFECTION (AIDS) AND OTHER SEXUALLY TRANSMITTED DISEASES
 Mirena (levonorgestrel-releasing intrauterine system) PATIENTS SHOULD BE COUNSELED THAT THIS PRODUCT DOES NOT PROTECT AGAINST HIV INFECTION (AIDS) AND OTHER SEXUALLY TRANSMITTED DISEASES Rx only DESCRIPTION
Mirena (levonorgestrel-releasing intrauterine system) PATIENTS SHOULD BE COUNSELED THAT THIS PRODUCT DOES NOT PROTECT AGAINST HIV INFECTION (AIDS) AND OTHER SEXUALLY TRANSMITTED DISEASES Rx only DESCRIPTION
NORTHAMPTONSHIRE INTEGRATED SEXUAL HEALTH SERVICES IUD/IUS PROTOCOL
 NORTHAMPTONSHIRE INTEGRATED SEXUAL HEALTH SERVICES IUD/IUS PROTOCOL Policy Details NHFT document reference MMPr005 Version Final Date Ratified 19.01.16 Ratified by Medicines Management Committee Implementation
NORTHAMPTONSHIRE INTEGRATED SEXUAL HEALTH SERVICES IUD/IUS PROTOCOL Policy Details NHFT document reference MMPr005 Version Final Date Ratified 19.01.16 Ratified by Medicines Management Committee Implementation
Outpatient hysteroscopy
 Women s & Children s Outpatient hysteroscopy Information for patients Welcome to King s gynaecology service. The doctor who saw you in the outpatient clinic recently has recommended that you have a procedure
Women s & Children s Outpatient hysteroscopy Information for patients Welcome to King s gynaecology service. The doctor who saw you in the outpatient clinic recently has recommended that you have a procedure
OUTPATIENT HYSTEROSCOPY SERVICES JASMINE SUITE
 OUTPATIENT HYSTEROSCOPY SERVICES JASMINE SUITE Information Leaflet Your Health. Our Priority. Page 2 of 6 This information is for patients having a hysteroscopy (diagnostic or operative). It explains what
OUTPATIENT HYSTEROSCOPY SERVICES JASMINE SUITE Information Leaflet Your Health. Our Priority. Page 2 of 6 This information is for patients having a hysteroscopy (diagnostic or operative). It explains what
IUD. the IUD. the IUD. the IUD. the IUD. the IUD. the IUD. the IUD. the IUD. the IUD. your guide to
 your guide to Helping you choose the method of contraception that is best for you IUD he the the the 2 3 The intrauterine device (IUD) An IUD is a small plastic and copper device that is put into your
your guide to Helping you choose the method of contraception that is best for you IUD he the the the 2 3 The intrauterine device (IUD) An IUD is a small plastic and copper device that is put into your
IUD. the IUD. the IUD. the IUD. the IUD. the IUD. the IUD the IUD. the IUD. the the IUD. the IUD. the IUD. the IUD. the IUD. the IUD.
 your guide to Helping you choose the method of contraception that is best for you I the IUD 2 3 The intrauterine device (IUD) An IUD is a small plastic and copper device that is put into your uterus (womb).
your guide to Helping you choose the method of contraception that is best for you I the IUD 2 3 The intrauterine device (IUD) An IUD is a small plastic and copper device that is put into your uterus (womb).
NovaSure: A Procedure for Heavy Menstrual Bleeding
 NovaSure: A Procedure for Heavy Menstrual Bleeding The one-time, five-minute procedure Over a million women 1 have been treated with NovaSure. NovaSure Endometrial Ablation (EA) is the simple, one-time,
NovaSure: A Procedure for Heavy Menstrual Bleeding The one-time, five-minute procedure Over a million women 1 have been treated with NovaSure. NovaSure Endometrial Ablation (EA) is the simple, one-time,
Treating heavy menstrual bleeding caused by fibroids or polyps
 Treating heavy menstrual bleeding caused by fibroids or polyps With today s medical advances the outlook for successful treatment of fibroids and polyps has never been better. You don t have to live with
Treating heavy menstrual bleeding caused by fibroids or polyps With today s medical advances the outlook for successful treatment of fibroids and polyps has never been better. You don t have to live with
Artificial insemination with donor sperm
 Artificial insemination with donor sperm Ref. 123 / 2009 Reproductive Medicine Unit Servicio de Medicina de la Reproducción Gran Vía Carlos III 71-75 08028 Barcelona Tel. (+34) 93 227 47 00 Fax. (+34)
Artificial insemination with donor sperm Ref. 123 / 2009 Reproductive Medicine Unit Servicio de Medicina de la Reproducción Gran Vía Carlos III 71-75 08028 Barcelona Tel. (+34) 93 227 47 00 Fax. (+34)
Gonorrhoea. Looking after your sexual health
 Gonorrhoea Looking after your sexual health 2 Gonorrhoea Gonorrhoea is a bacterial sexually transmitted infection (STI). It can be painful and can cause serious health problems such as infertility in both
Gonorrhoea Looking after your sexual health 2 Gonorrhoea Gonorrhoea is a bacterial sexually transmitted infection (STI). It can be painful and can cause serious health problems such as infertility in both
ency emergency contra-
 your guide to emergency contraception Helping you choose the method of contraception that is best for you emergency cont gency contrace emergency contraception ency emergency contra- emergen mergency contraception
your guide to emergency contraception Helping you choose the method of contraception that is best for you emergency cont gency contrace emergency contraception ency emergency contra- emergen mergency contraception
Mirena prescribing information
 Mirena prescribing information This provides medical and scientific information on Mirena in general. Please refer to the local Summary of Product Characteristics for the information applicable in your
Mirena prescribing information This provides medical and scientific information on Mirena in general. Please refer to the local Summary of Product Characteristics for the information applicable in your
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Uterine Fibroid Symptoms, Diagnosis and Treatment
 Fibroids and IR Uterine Fibroid Symptoms, Diagnosis and Treatment Interventional radiologists use MRIs to determine if fibroids can be embolised, detect alternate causes for the symptoms and rule out misdiagnosis,
Fibroids and IR Uterine Fibroid Symptoms, Diagnosis and Treatment Interventional radiologists use MRIs to determine if fibroids can be embolised, detect alternate causes for the symptoms and rule out misdiagnosis,
TERMINATION OF PREGNANCY- MEDICAL
 TERMINATION OF PREGNANCY- MEDICAL Information Leaflet Your Health. Our Priority. Page 2 of 8 You have been offered a medical termination of pregnancy using mifepristone. You will have been given some verbal
TERMINATION OF PREGNANCY- MEDICAL Information Leaflet Your Health. Our Priority. Page 2 of 8 You have been offered a medical termination of pregnancy using mifepristone. You will have been given some verbal
COMPLICATIONS OF PREGNANCY, CHILDBIRTH AND THE PUERPERIUM
 COMPLICATIONS OF PREGNANCY, CHILDBIRTH AND THE PUERPERIUM PREGNANCY WITH ABORTIVE OUTCOME (630 639.9) 630 HYDATIDIFORM MOLE 631 OTHER ABNORMAL PRODUCT OF CONCEPTION 632 MISSED ABORTION 633 ECTOPIC PREGNANCY
COMPLICATIONS OF PREGNANCY, CHILDBIRTH AND THE PUERPERIUM PREGNANCY WITH ABORTIVE OUTCOME (630 639.9) 630 HYDATIDIFORM MOLE 631 OTHER ABNORMAL PRODUCT OF CONCEPTION 632 MISSED ABORTION 633 ECTOPIC PREGNANCY
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use MIRENA safely and effectively. See full prescribing information for MIRENA. MIRENA (levonorgestrel-releasing
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use MIRENA safely and effectively. See full prescribing information for MIRENA. MIRENA (levonorgestrel-releasing
Anatomy and Physiology of Human Reproduction. Module 10a
 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
WHAT YOU SHOULD KNOW ABOUT ABORTION
 WHAT YOU SHOULD KNOW ABOUT ABORTION It is the public policy of the state of Idaho to prefer live childbirth over abortion: "The Supreme Court of the United States having held that the states have a "profound
WHAT YOU SHOULD KNOW ABOUT ABORTION It is the public policy of the state of Idaho to prefer live childbirth over abortion: "The Supreme Court of the United States having held that the states have a "profound
Having an early medical abortion
 Having an early medical abortion Patient information PAGE 2 OF 15 Early medical abortion The following information is for women intending to have an abortion at Gynaecology Centres Australia. More detailed
Having an early medical abortion Patient information PAGE 2 OF 15 Early medical abortion The following information is for women intending to have an abortion at Gynaecology Centres Australia. More detailed
Gynecology Abnormal Pelvic Anatomy and Physiology: Cervix. Cervix. Nabothian cysts. cervical polyps. leiomyomas. Cervical stenosis
 Gynecology Abnormal Pelvic Anatomy and Physiology: (Effective February 2007) pediatric, reproductive, and perimenopausal/postmenopausal (24-28 %) Cervix Nabothian cysts result from chronic cervicitis most
Gynecology Abnormal Pelvic Anatomy and Physiology: (Effective February 2007) pediatric, reproductive, and perimenopausal/postmenopausal (24-28 %) Cervix Nabothian cysts result from chronic cervicitis most
CHAPTER 10 Uterine Synechiae
 CHAPTER 10 Uterine Synechiae Uterine synechiae are intrauterine adhesions. They may involve small focal areas of the endometrium (Figures 10.1a e), or they can be so extensive that they obliterate the
CHAPTER 10 Uterine Synechiae Uterine synechiae are intrauterine adhesions. They may involve small focal areas of the endometrium (Figures 10.1a e), or they can be so extensive that they obliterate the
A potential treatment for your abnormal uterine bleeding
 TRUCLEAR System A potential treatment for your abnormal uterine bleeding Do You Suffer from Abnormal Uterine Bleeding? What is a Hysteroscopy? What is the TRUCLEAR Procedure? What Happens Before Your
TRUCLEAR System A potential treatment for your abnormal uterine bleeding Do You Suffer from Abnormal Uterine Bleeding? What is a Hysteroscopy? What is the TRUCLEAR Procedure? What Happens Before Your
Ovarian Cyst. Homoeopathy Clinic. Introduction. Types of Ovarian Cysts. Contents. Case Reports. 21 August 2002
 Case Reports 21 August 2002 Ovarian Cyst Homoeopathy Clinic Check Yourself If you have any of the following symptoms call your doctor. Sense of fullness or pressure or a dull ache in the abdomen Pain during
Case Reports 21 August 2002 Ovarian Cyst Homoeopathy Clinic Check Yourself If you have any of the following symptoms call your doctor. Sense of fullness or pressure or a dull ache in the abdomen Pain during
WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500. Endometriosis
 Endometriosis WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 The lining of the uterus is called the endometrium. Sometimes, endometrial tissue grows elsewhere in the body. When this happens
Endometriosis WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 The lining of the uterus is called the endometrium. Sometimes, endometrial tissue grows elsewhere in the body. When this happens
implant contraceptiv contraceptive contraceptive raceptiv contraceptive implant contraceptive contraceptive ontraceptive implant ontraceptive im
 your guide to the contraceptive implant Helping you choose the method of contraception that is best for you contra ontraceptive im contraceptive implant mpl ceptive contraceptive contracepti ntraceptive
your guide to the contraceptive implant Helping you choose the method of contraception that is best for you contra ontraceptive im contraceptive implant mpl ceptive contraceptive contracepti ntraceptive
WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500. Birth Control Pills
 Birth Control Pills WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Birth control pills (also called oral contraceptives or "the pill") are used by millions of women in the United States to
Birth Control Pills WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Birth control pills (also called oral contraceptives or "the pill") are used by millions of women in the United States to
WHAT YOU SHOULD KNOW ABOUT ABORTION
 WHAT YOU SHOULD KNOW ABOUT ABORTION It is the public policy of the state of Idaho to prefer live childbirth over abortion: "The Supreme Court of the United States having held that the states have a "profound
WHAT YOU SHOULD KNOW ABOUT ABORTION It is the public policy of the state of Idaho to prefer live childbirth over abortion: "The Supreme Court of the United States having held that the states have a "profound
About the Uterus. Hysterectomy may be done to treat conditions that affect the uterus. Some reasons a hysterectomy may be needed include:
 Hysterectomy removal of the uterus is a way of treating problems that affect the uterus. Many conditions can be cured with hysterectomy. Because it is major surgery, your doctor may suggest trying other
Hysterectomy removal of the uterus is a way of treating problems that affect the uterus. Many conditions can be cured with hysterectomy. Because it is major surgery, your doctor may suggest trying other
Lippes Loop intrauterine device left in the uterus for 50 years. Case report
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Lippes Loop intrauterine device left in the uterus for 50 years Case report Background.The first Lippes Loop intrauterine device was distributed in 1962. It was a
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Lippes Loop intrauterine device left in the uterus for 50 years Case report Background.The first Lippes Loop intrauterine device was distributed in 1962. It was a
What Are Fertility Awareness Methods?
 CHAPTER 17 Fertility Awareness Methods Key Points for Providers and Clients Fertility awareness methods require partners' cooperation. Couple must be committed to abstaining or using another method on
CHAPTER 17 Fertility Awareness Methods Key Points for Providers and Clients Fertility awareness methods require partners' cooperation. Couple must be committed to abstaining or using another method on
Female Reproductive System. Unit 8 Lesson 2 Continued
 Female Reproductive System Unit 8 Lesson 2 Continued Female Reproductive System Female Reproductive System Female produce ovum or egg cells. The egg (ovum) cell is the female sex cell. Female Reproductive
Female Reproductive System Unit 8 Lesson 2 Continued Female Reproductive System Female Reproductive System Female produce ovum or egg cells. The egg (ovum) cell is the female sex cell. Female Reproductive
CHLAMYDIA SCREENING IN WOMEN
 CHLAMYDIA SCREENING IN WOMEN APPLICATIONS OBJECTIVE Purpose of Measure: ELIGIBLE POPULATION Which members are included? STANDARD OF CARE What screening should be done? NCQA ACCEPTED CODES DOCUMENTATION
CHLAMYDIA SCREENING IN WOMEN APPLICATIONS OBJECTIVE Purpose of Measure: ELIGIBLE POPULATION Which members are included? STANDARD OF CARE What screening should be done? NCQA ACCEPTED CODES DOCUMENTATION
Summa Health System. A Woman s Guide to Hysterectomy
 Summa Health System A Woman s Guide to Hysterectomy Hysterectomy A hysterectomy is a surgical procedure to remove a woman s uterus (womb). The uterus is the organ which shelters and nourishes a baby during
Summa Health System A Woman s Guide to Hysterectomy Hysterectomy A hysterectomy is a surgical procedure to remove a woman s uterus (womb). The uterus is the organ which shelters and nourishes a baby during
Chlamydia THE FACTS. How do people get Chlamydia?
 What is Chlamydia? Chlamydia is a common bacterial infection that is sexually transmitted and often causes no symptoms. If not treated, chlamydia can damage reproductive organs and make it difficult for
What is Chlamydia? Chlamydia is a common bacterial infection that is sexually transmitted and often causes no symptoms. If not treated, chlamydia can damage reproductive organs and make it difficult for
Women s Health Laparoscopy Information for patients
 Women s Health Laparoscopy Information for patients This leaflet is for women who have been advised to have a laparoscopy. It outlines the common reasons doctors recommend this operation, what will happen
Women s Health Laparoscopy Information for patients This leaflet is for women who have been advised to have a laparoscopy. It outlines the common reasons doctors recommend this operation, what will happen
abortion abortion abortion abortion abortion abortion abortion on abortio abortion ortion abortion abortion abortion abortion abortio
 Abortion Your questions answered abortio bortion ion ortion on abortio 2 Are you pregnant but not sure you want to have the baby? Do you need more information about your pregnancy choices? Unplanned pregnancy
Abortion Your questions answered abortio bortion ion ortion on abortio 2 Are you pregnant but not sure you want to have the baby? Do you need more information about your pregnancy choices? Unplanned pregnancy
From this site: http://www.energeticnutrition.com /vitalzym/fibroid_tumors.html Uterine Fibroid Tumors
 From this site: http://www.energeticnutrition.com /vitalzym/fibroid_tumors.html Uterine Fibroid Tumors Uterine Fibroid Tumors A woman s fibroisis condition usually associated with estrogen dominance. Uterine
From this site: http://www.energeticnutrition.com /vitalzym/fibroid_tumors.html Uterine Fibroid Tumors Uterine Fibroid Tumors A woman s fibroisis condition usually associated with estrogen dominance. Uterine
Hull & East Riding Prescribing Committee
 Hull & East Riding Prescribing Committee Guideline on Prescribing of Gonadorelin (GnRH) Analogues and Progesterone Receptor Modulators in treatment of Endometriosis or prior to Endometrial Ablation, or
Hull & East Riding Prescribing Committee Guideline on Prescribing of Gonadorelin (GnRH) Analogues and Progesterone Receptor Modulators in treatment of Endometriosis or prior to Endometrial Ablation, or
Menstruation and the Menstrual Cycle
 Menstruation and the Menstrual Cycle Q: What is menstruation? A: Menstruation is a woman s monthly bleeding, also called a period. When you menstruate, your body is shedding the lining of the uterus (womb).
Menstruation and the Menstrual Cycle Q: What is menstruation? A: Menstruation is a woman s monthly bleeding, also called a period. When you menstruate, your body is shedding the lining of the uterus (womb).
Migration of an intrauterine contraceptive device to the sigmoid colon: a case report
 The European Journal of Contraception and Reproductive Health Care 2003;8:229 232 Case Report Migration of an intrauterine contraceptive device to the sigmoid colon: a case report Ü. S. nceboz, H. T. Özçakir,
The European Journal of Contraception and Reproductive Health Care 2003;8:229 232 Case Report Migration of an intrauterine contraceptive device to the sigmoid colon: a case report Ü. S. nceboz, H. T. Özçakir,
abortion your questions answered
 abortion your questions answered About Marie Stopes International Marie Stopes International is a specialist reproductive healthcare organisation and a registered charity working in both the UK and overseas.
abortion your questions answered About Marie Stopes International Marie Stopes International is a specialist reproductive healthcare organisation and a registered charity working in both the UK and overseas.
OVARIAN CYSTS. Types of Ovarian Cysts There are many types of ovarian cysts and these can be categorized into functional and nonfunctional
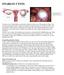 OVARIAN CYSTS Follicular Cyst Ovarian cysts are fluid-filled sacs that form within or on the ovary. The majority of these cysts are functional meaning they usually form during a normal menstrual cycle.
OVARIAN CYSTS Follicular Cyst Ovarian cysts are fluid-filled sacs that form within or on the ovary. The majority of these cysts are functional meaning they usually form during a normal menstrual cycle.
An operation for prolapse Sacrospinous Fixation Sacrospinous Hysteropexy
 Saint Mary s Hospital Gynaecology Service Warrell Unit An operation for prolapse Sacrospinous Fixation Sacrospinous Hysteropexy Information For Patients 1 What is a prolapse? Prolapse is a bulge or lump
Saint Mary s Hospital Gynaecology Service Warrell Unit An operation for prolapse Sacrospinous Fixation Sacrospinous Hysteropexy Information For Patients 1 What is a prolapse? Prolapse is a bulge or lump
Transcervical Resection of the Endometrium (TCRE)
 Oxford University Hospitals NHS Trust Women s Centre Transcervical Resection of the Endometrium (TCRE) Information for women This leaflet is for women who have been advised to have a transcervical resection
Oxford University Hospitals NHS Trust Women s Centre Transcervical Resection of the Endometrium (TCRE) Information for women This leaflet is for women who have been advised to have a transcervical resection
Vaginal hysterectomy and vaginal repair
 Women s Service Vaginal hysterectomy and vaginal repair Information for patients Vaginal hysterectomy and vaginal repair This leaflet is for women who have been advised to have a vaginal hysterectomy.
Women s Service Vaginal hysterectomy and vaginal repair Information for patients Vaginal hysterectomy and vaginal repair This leaflet is for women who have been advised to have a vaginal hysterectomy.
Understanding Endometriosis - Information Pack
 What is endometriosis? Endometriosis (pronounced en- doh mee tree oh sis) is the name given to the condition where cells like the ones in the lining of the womb (uterus) are found elsewhere in the body.
What is endometriosis? Endometriosis (pronounced en- doh mee tree oh sis) is the name given to the condition where cells like the ones in the lining of the womb (uterus) are found elsewhere in the body.
Ovarian Cystectomy / Oophorectomy
 Cystectomy and Ovarian Cysts Ovarian cysts are sacs filled with fluids or pockets located on or in an ovary. In some cases, these cysts need to be removed surgically. Types of Cysts Ovarian cysts are quite
Cystectomy and Ovarian Cysts Ovarian cysts are sacs filled with fluids or pockets located on or in an ovary. In some cases, these cysts need to be removed surgically. Types of Cysts Ovarian cysts are quite
Inferior Vena Cava filter and removal
 Inferior Vena Cava filter and removal What is Inferior Vena Cava Filter Placement and Removal? An inferior vena cava filter placement procedure involves an interventional radiologist (a specialist doctor)
Inferior Vena Cava filter and removal What is Inferior Vena Cava Filter Placement and Removal? An inferior vena cava filter placement procedure involves an interventional radiologist (a specialist doctor)
Artificial insemination
 Artificial insemination What is involved? Artificial insemination is an assisted reproduction technique that consists of inserting laboratory-treated spermatozoa into the woman s uterus or cervical canal.
Artificial insemination What is involved? Artificial insemination is an assisted reproduction technique that consists of inserting laboratory-treated spermatozoa into the woman s uterus or cervical canal.
All you need to know about Endometriosis. Nordica Fertility Centre, Lagos, Asaba, Abuja
 All you need to know about Endometriosis October, 2015 About The Author Nordica Lagos Fertility Centre is one of Nigeria's leading centres for world class Assisted Reproductive Services, with comfort centres
All you need to know about Endometriosis October, 2015 About The Author Nordica Lagos Fertility Centre is one of Nigeria's leading centres for world class Assisted Reproductive Services, with comfort centres
University College Hospital. Miscarriage Women s Health
 University College Hospital Miscarriage Women s Health 2 Introduction The purpose of this leafl et is to: Describe what a miscarriage is and why it happens What it means for your health What treatment
University College Hospital Miscarriage Women s Health 2 Introduction The purpose of this leafl et is to: Describe what a miscarriage is and why it happens What it means for your health What treatment
Hysterectomy. What is a hysterectomy? Why is hysterectomy done? Are there alternatives to hysterectomy?
 ROBERT LEVITT, MD JESSICA BERGER-WEISS, MD ADRIENNE POTTS, MD HARTAJ POWELL, MD, MPH COURTNEY LEVENSON, MD LAUREN BURNS, MSN, RN, WHNP OBGYNCWC.COM What is a hysterectomy? Hysterectomy Hysterectomy is
ROBERT LEVITT, MD JESSICA BERGER-WEISS, MD ADRIENNE POTTS, MD HARTAJ POWELL, MD, MPH COURTNEY LEVENSON, MD LAUREN BURNS, MSN, RN, WHNP OBGYNCWC.COM What is a hysterectomy? Hysterectomy Hysterectomy is
ALTERNATIVE TREATMENT PLAN AND CONSENT FOR MEDICAL ABORTION WITH MIFEPREX (MIFEPRISTONE) AND MISOPROSTOL
 ALTERNATIVE TREATMENT PLAN AND CONSENT FOR MEDICAL ABORTION WITH MIFEPREX (MIFEPRISTONE) AND MISOPROSTOL The FDA gave its approval status to Mifepristone in 1996 based on research up to that time. Extensive
ALTERNATIVE TREATMENT PLAN AND CONSENT FOR MEDICAL ABORTION WITH MIFEPREX (MIFEPRISTONE) AND MISOPROSTOL The FDA gave its approval status to Mifepristone in 1996 based on research up to that time. Extensive
