JOHNS HOPKINS HEALTHCARE
|
|
|
- Diane Ball
- 9 years ago
- Views:
Transcription
1 Page 1 of 15 ACTION: New Policy: Effective Date: 08/26/2003 Revising : Review Dates: 03/15/2004, 10/22/2004, Superseding : 10/21/2005, 10/19/2006, 01/07/2008, Archiving : 01/05/2009, 01/07/2011, 06/07/2013, 06/05/2015 Retiring : Johns Hopkins HealthCare (JHHC) provides a full spectrum of health care products and services for Employer Health Programs, Priority Partners, and US Family Health Plan. Each line of business possesses its own unique contract and guidelines which, for benefit and payment purposes, should be consulted to know what benefits are available for reimbursement. Specific contract benefits, guidelines, or policies supersede the information outlined in this policy. POLICY: For US Family Health Plan see TRICARE Policy Manual M, February 1, 2008, Diagnostic Genetic Testing and Counseling: Chapter 6, Section 3.1. I. All genetic testing, with the exception of routine karyotype and Fragile X, requires prior authorization whether performed in an inpatient or outpatient setting. II. All genetic tests must be ordered by a licensed physician, nurse practitioner or physician assistant who will ensure that the medical necessity criteria below are met. III. Johns Hopkins HealthCare (JHHC) considers genetic testing to establish a molecular diagnosis of an inheritable disease medically necessary when ALL of the following are met: A. After complete history, physical examination, family history and pedigree analysis, as well as laboratory, imaging or other diagnostic testing as indicated, a specific medical differential diagnosis is established; AND B. A specific genetic test is requested; AND C. The member displays clinical features, or is at direct risk of inheriting the mutation in question (pre-symptomatic); AND D. The result of the test will directly impact the current specific medical treatment being delivered to the member; AND E. The individual has not had the genetic testing done previously; AND F. The test requested has a Hayes Rating of A or B. IV. All genetic tests with a Hayes Rating of D will be considered investigational. V. Genetic Testing of Family Members A. Genetic testing of JHHC members is excluded from coverage under JHHC s benefit plans
2 Page 2 of 15 if the testing is performed primarily for the medical management of other family members who are not covered under a JHHC benefit plan. B. JHHC considers genetic testing for heritable disorders of family members who are not covered under JHHC benefit plans medically necessary when ALL of the following conditions are met: 1. The information is needed to adequately assess risk in the JHHC member; AND 2. The information will directly impact the current specific medical treatment being delivered to the JHHC member; AND 3. The non-jhhc member's benefit plan, if any, will not cover the test (a copy of the denial letter from the non-jhhc member's benefit plan must be provided). VI. Genetic Testing for Reproductive Planning and Prenatal Diagnosis A. For Non-Invasive Prenatal Testing for Fetal Aneuploidy (NIPT) see policy CMS B. Cystic Fibrosis (CF) Carrier Testing is considered medically necessary when EITHER of the following criteria are met: 1. The member is the reproductive partner of a person known to be a CF carrier; OR 2. The couple is planning a pregnancy or seeking prenatal care; AND EITHER a. The testing is for a first pregnancy; OR b. The provider has affirmatively documented that the testing was not performed during previous pregnancies. C. Spinal Muscular Atrophy (SMA) Carrier Testing is considered medically necessary when ALL of the following criteria are met: 1. The diagnosis of persons with hypotonia and muscle weakness who are suspected of having spinal muscular atrophy; OR 2. The identification of SMN1 deletion carriers in the families of persons with SMA (subject to limitations in Section V above); OR 3. The prenatal diagnosis of SMA in the pregnancy of two known carriers. For preimplantation genetic testing of embryos, criteria in Section VI.(F) must be met. D. Ashkenazi Jewish Panel Testing is considered medically necessary when ALL of the following criteria are met: 1. The testing is for the first pregnancy; OR 2. The provider has affirmatively documented that the testing was not performed during previous pregnancies; AND 3. The testing is limited to the following conditions: a. Tay Sachs disease b. Canavan disease c. Cystic fibrosis d. Familial dysautonomia e. Bloom syndrome f. Fanconi anemia g. Niemann-Pick disease h. Gaucher disease
3 Page 3 of 15 i. Mucolipidosis 4. The testing of the male partner is indicated if the female partner tests positive for any of the above conditions. E. Universal Carrier Screening Testing 1. Unless specific benefits apply, JHHC considers all Universal Carrier Screening tests, including but not limited to Counsyl and HereditT Universal, investigational, as they do not meet Technology Evaluation Criteria #2-5. F. Genetic Testing of Pre-implantation Embryos (Pre-implantation Genetic Diagnosis (PGD)) 1. When benefits for advanced reproductive technologies (ART), including but not limited to in-vitro fertilization (IVF), are available, JHHC considers pre-implantation genetic diagnosis medically necessary when testing is ordered for a single, recognized genetic disorder with a known inheritance pattern and ONE of the following criterion is met: a. Both partners (or member and donor) are known carriers of a single autosomal recessive disorder; OR b. One partner (or donor) is a known carrier of a single gene autosomal recessive disorder; AND c. The partners (or member and donor) have one offspring that has been diagnosed with that recessive disorder; OR d. One partner (or donor) is a known carrier of a single gene autosomal dominant disorder, OR e. One partner (or donor) is a known carrier of a single X-linked disorder; OR f. One partner (or donor) has a known balanced or unbalanced translocation. G. Genetic Testing of a Fetus Prior to In Utero Surgery 1. JHHC considers genetic testing of a fetus prior to proposed in-utero surgery medically necessary when ALL of the following criteria are met: a. The proposed fetal surgery is for ANY of the following indications: i. Ablation of anastomotic vessels in acardiac twins; ii. Insertion of pleuro-amniotic shunt for fetal pleural effusion; iii. Laser ablation of anastomotic vessels in early, severe twin-twin transfusion syndrome; iv. Removal of sacrococcygeal teratoma; v. Repair of myelomeningocele; vi. Resection of malformed pulmonary tissue, or placement of a thoracoamniotic shunt as a treatment of either of the following: Congenital cystic adenomatoid malformation; OR Extralobar pulmonary sequestration; vii. Twin reversed arterial perfusion (TRAP); viii. Vesico-amniotic shunting as a treatment of urinary tract obstruction. ix. Serial amnioreduction for twin-to-twin transfusion syndrome when the
4 Page 4 of 15 THIS POLICY REPLACES: BACKGROUND: following criteria are met: Women after 26 weeks of gestation; and Evidence of abnormal blood flow documented by Doppler studies in one or both fetuses; and Evidence of polyhydramnios in the recipient fetus; and Donor fetus is oligohydramniotic. AND x. ALL criteria in Section III above are met. CMS07.02 Genetic Counseling Genetic Testing for Colon Cancer With the advancement of technology, the medical field has seen a parallel in the advancement and utilization of genetic testing. Genetic testing is a type of medical test that allows medical professionals to detect changes in an individual s DNA. These changes can be detected in chromosomes, genes, and/or proteins. Genetic testing and next generation sequencing have a profound range of benefits. Such benefits may include: Diagnose disease and assess severity Associate changes in DNA with an already diagnosed disease Aid in clinical decision-making/personalized medicine Detect disease prevalence/risk Screen for abnormalities in children Single nucleotide polymorphisms (SNP s) can serve as biomarkers for many different diseases. Understanding and identifying changes in a patient s genome through genetic testing or nextgeneration sequencing can aid in clinical decision-making and prove to be cost-effective. The administration of drugs to a patient as a result of genetic testing can also reduce risk/side effects while maximizing benefits in the patient. Interpretation of genetic testing results transcends many disciplines within medicine. Laboratory scientists, pathologists, genetic counselors, physicians, and nurses all collaborate to ensure accurate interpretation of results and to discuss treatment options for patients. CODING INFORMATION: CPT Copyright 2015 American Medical Association. All rights reserved. CPT is a registered trademark of the American Medical Association.
5 Page 5 of 15 Note: The following CPT/HCPCS codes are included below for informational purposes. Inclusion or exclusion of a CPT/HCPCS code(s) below does not signify or imply member coverage or provider reimbursement. The member's specific benefit plan determines coverage and referral requirements. All inpatient admissions require pre-authorization. This list of CPT/HCPCS codes is not all inclusive: PRE-AUTHORIZATION REQUIRED Compliance with the provision in this policy may be monitored and addressed through post-payment data analysis and/or medical review audits Employer Health Programs (EHP) **See Specific Summary Plan Description (SPD) Priority Partners (PPMCO) refer to COMAR guidelines and PPMCO SPD then apply policy criteria US Family Health Plan (USFHP), TRICARE Medical Policy supersedes JHHC Medical Policy. If there is no Policy in TRICARE, apply the Medical Policy Criteria CPT DESCRIPTION CODES DMD (dystrophin) (eg, Duchenne/Becker muscular dystrophy) deletion analysis and duplication analysis, if performed ASPA (aspartoacylase) (eg,canavan disease) gene analysis, common variants APC (adenomatous polyposis coli) (eg, familial adenomatosis polyposis [FAP], attenuated FAP) gene analysis; full gene sequence APC (adenomatous polyposis coli) (eg, familial adenomatosis polyposis [FAP], attenuated FAP) gene analysis; known familial variants APC (adenomatous polyposis coli) (eg, familial adenomatosis polyposis [FAP], attenuated FAP) gene analysis; duplication/deletion variants BCKDHB (branched-chain keto acid dehydrogenase E1, beta polypeptide) (eg, Maple syrup urine disease) gene analysis, common variants (eg, R183P, G2785, E422X) BCR/ABL1 (t(9;22)) (eg, chronic myelogenous leukemia) translocation analysis; major breakpoint, qualitative or quantitative BCR/ABL1 (t(9;22)) (eg, chronic myelogenous leukemia) translocation analysis; minor breakpoint, qualitative or quantitative BCR/ABL1 (t(9;22)) (eg, chronic myelogenous leukemia) translocation analysis; other breakpoint, qualitative or quantitative BLM (Bloom syndrome, RecQ helicase-like) (eg, Bloom syndrome) gene analysis, 2281del6ins7 variant CFTR (cystic fibrosis transmembrane conductance regulator) (eg, cystic fibrosis) gene analysis; common variants (eg, ACMG/ACOG guidelines) CFTR (cystic fibrosis transmembrane conductance regulator) (eg, cystic fibrosis) gene analysis; known familial variants
6 Page 6 of CFTR (cystic fibrosis transmembrane conductance regulator) (eg, cystic fibrosis) gene analysis; duplication/deletion variants CFTR (cystic fibrosis transmembrane conductance regulator) (eg, cystic fibrosis) gene analysis; full gene sequence CFTR (cystic fibrosis transmembrane conductance regulator) (eg, cystic fibrosis) gene analysis; intron 8 poly-t analysis (eg, male infertility) CYP2C19 (cytochrome P450, family 2, subfamily C, polypeptide 19) (eg, drug metabolism), gene analysis, common variants (eg, *2, *3, *4, *8, *17) CYP2D6 (cytochrome P450, family 2, subfamily D, polypeptide 6) (eg, drug metabolism), gene analysis, common variants (eg, *2, *3, *4, *5, *6, *9, *10, *17, *19, *29, *35, *41, *1XN, *2XN, *4XN) CYP2C9 (cytochrome P450, family 2, subfamily C, polypeptide 9) (eg, drug metabolism), gene analysis, common variants (eg, *2, *3, *5, *6) Cytogenomic constitutional (genome-wide) microarray analysis; interrogation of genomic regions for copy number variants (eg, bacterial artificial chromosome [BAC] or oligo-based comparative genomic hybridization [CGH] microarray analysis) Cytogenomic constitutional (genome-wide) microarray analysis; interrogation of genomic regions for copy number and single nucleotide polymorphism (SNP) variants for chromosomal abnormalities EGFR (epidermal growth factor receptor) (eg, non-small cell lung cancer) gene analysis, common variants (eg, exon 19 LREA deletion, L858R, T790M, G719A, G719S, L861Q) F2 (prothrombin, coagulation factor II) (eg, hereditary hypercoagulability) gene analysis, 20210G>A variant F5 (coagulation Factor V) (eg, hereditary hypercoagulability gene analysis, Leiden variant FANCC (Fanconi anemia, complementation group C) (eg, Fanconi anemia type C) gene analysis, common variant (eg, IVS4+4A>T) FMR1 (Fragile X mental retardation 1) (eg, fragile X mental retardation) gene analysis; evaluation to detect abnormal (eg, expanded) alleles FMR1 (Fragile X mental retardation 1) (eg, fragile X mental retardation) gene analysis; characterization of alleles (eg, expanded size and methylation status) FLT3 (fms-related tyrosine kinase 3) (eg, acute myeloid leukemia), gene analysis; internal tandem duplication (ITD) variants (ie, exons 14, 15) FLT3 (fms-related tyrosine kinase 3) (eg, acute myeloid leukemia), gene analysis; tyrosine kinase domain (TKD) variants (eg, D835, I836) G6PC (glucose-6-phosphatase, catalytic subunit) (eg, Glycogen storage disease, Type 1a, von Gierke disease) gene analysis, common variants (eg, R83C, Q347X) GBA (glucosidase, beta, acid) (eg, Gaucher disease) gene analysis, common variants (eg, N370S, 84GG, L444P, IVS2+1G>A) GJB2 (gap junction protein, beta 2, 26kDa, connexin 26) (eg, nonsyndromic hearing loss) gene analysis; full gene sequence
7 Page 7 of GJB2 (gap junction protein, beta 2, 26kDa, connexin 26) (eg, nonsyndromic hearing loss) gene analysis; known familial variants GJB6 (gap junction protein, beta 6, 30kDa, connexin 30) (eg, nonsyndromic hearing loss) gene analysis, common variants (eg, 309kb [del(gjb6-d13s1830)] and 232kb [del(gjb6-d13s1854)]) HEXA (hexosaminidase A [alpha polypeptide]) (eg, Tay-Sachs disease) gene analysis, common variants (eg, 1278insTATC, G>C, G269S) HFE (hemochromatosis) (eg, hereditary hemochromatosis) gene analysis, common variants (eg, C282Y, H63D) HBA1/HBA2 (alpha globin 1 and alpha globin 2) (eg, alpha thalassemia, Hb Bart hydrops fetalis syndrome, HbH disease), gene analysis, for common deletions or variant (eg, Southeast Asian, Thai, Filipino, Mediterranean, alpha3.7, alpha4.2, alpha20.5, and Constant Spring) IKBKAP (inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase complexassociated protein) (eg, familial dysautonomia) gene analysis, common variants (eg, T>C, R696P) IGH@ (Immunoglobulin heavy chain locus) (eg, leukemias and lymphomas, B-cell), gene rearrangement analysis to detect abnormal clonal population(s); amplified methodology (eg, polymerase chain reaction) IGH@ (Immunoglobulin heavy chain locus) (eg, leukemias and lymphomas, B-cell), gene rearrangement analysis to detect abnormal clonal population(s); direct probe methodology (eg, Southern blot) IGH@ (Immunoglobulin heavy chain locus) (eg, leukemia and lymphoma, B-cell), variable region somatic mutation analysis IGK@ (Immunoglobulin kappa light chain locus) (eg, leukemia and lymphoma, B-cell), gene rearrangement analysis, evaluation to detect abnormal clonal population(s) Comparative analysis using Short Tandem Repeat (STR) markers; patient and comparative specimen (eg, pre-transplant recipient and donor germline testing, posttransplant non-hematopoietic recipient germline [eg, buccal swab or other germline tissue sample] and donor testing, twin zygosity testing, or maternal cell contamination of fetal cells) Comparative analysis using Short Tandem Repeat (STR) markers; each additional specimen (eg, additional cord blood donor, additional fetal samples from different cultures, or additional zygosity in multiple birth pregnancies) (List separately in addition to code for primary procedure) JAK2 (Janus kinase 2) (eg, myeloproliferative disorder) gene analysis, p.val617phe (V617F) variant KRAS (v-ki-ras2 Kirsten rat sarcoma viral oncogene) (eg, carcinoma) gene analysis, variants in codons 12 and 13
8 Page 8 of Long QT syndrome gene analysis (eg, KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, KCNJ2, CACNA1C, CAV3, SCN4B, AKAP, ANTA1 and AKN2); full sequence analysis Long QT syndrome gene analysis (eg, KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, KCNJ2, CACNA1C, CAV3, SCN4B, AKAP, ANTA1 and AKN2); known familial sequence variant Long QT syndrome gene analysis (eg, KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, KCNJ2, CACNA1C, CAV3, SCN4B, AKAP, ANTA1 and AKN2); duplication/deletion variants MLH1 (mutl homolog 1, colon cancer, nonpolyposis type 2) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) gene analysis; promoter methylation analysis MCOLN1 (mucolipin 1) (eg, Mucolipidosis, type IV) gene analysis, common variants (eg, IVS3-2A>G, del6.4kb) MTHFR (5,10-methylenetetrahydrofolate reductase) (eg, hereditary hypercoagulability) gene analysis, common variants (eg, 677T, 1298C) MLH1 (mutl homomlog 1, colon cancer, nonpolyposis type 2) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) gene analysis; full sequence analysis MLH1 (mutl homomlog 1, colon cancer, nonpolyposis type 2) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) gene analysis; known familial variants MLH1 (mutl homomlog 1, colon cancer, nonpolyposis type 2) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) gene analysis; duplication/deletion variants MSH2 (muts homolog 2, colon cancer, nonpolyposis type 1) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) gene analysis; full sequence analysis MSH2 (muts homolog 2, colon cancer, nonpolyposis type 1) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) gene analysis; known familial variants MSH2 (muts homolog 2, colon cancer, nonpolyposis type 1) (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) gene analysis; duplication/deletion variants MSH6 (muts homolog 6 [E. coli] (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) gene analysis; full sequence analysis MSH6 (muts homolog 6 [E. coli] (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) gene analysis; known familial variants MSH6 (muts homolog 6 [E. coli] (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) gene analysis; duplication/deletion variants Microsatellite instability analysis (eg, hereditary nonpolyposis colorectal cancer, Lynch syndrome) of markers for mismatch repair deficiency (eg, BAT25, BAT26), includes comparison of neoplastic and normal tissue, if performed MECP2 (methyl CpG binding protein 2) (eg, Rett syndrome) gene analysis; full sequence analysis
9 Page 9 of MECP2 (methyl CpG binding protein 2) (eg, Rett syndrome) gene analysis; known familial variant MECP2 (methyl CpG binding protein 2) (eg, Rett syndrome) gene analysis; duplication/deletion variants NPM1 (nucleophosmin) (eg, acute myeloid leukemia) gene analysis, exon 12 variants PCA3/KLK3 (prostate cancer antigen 3 [non-protein coding]/kallikrein-related peptidase 3 [prostate specific antigen]) ratio (eg, prostate cancer) PML/RARalpha, (t(15;17)), (promyelocytic leukemia/retinoic acid receptor alpha) (eg, promyelocytic leukemia) translocation analysis; common breakpoints (eg, intron 3 and intron 6), qualitative or quantitative PML/RARalpha, (t(15;17)), (promyelocytic leukemia/retinoic acid receptor alpha) (eg, promyelocytic leukemia) translocation analysis; single breakpoint (eg, intron 3, intron 6 or exon 6), qualitative or quantitative PMS2 (postmeiotic segregation increased 2 [S. cerevisiae]) (eg, hereditary non-polyoisus colorectal cancer, Lynch syndrome) gene analysis; full sequence analysis PMS2 (postmeiotic segregation increased 2 [S. cerevisiae]) (eg, hereditary non-polyposis colorectal cancer, Lynch syndrome) gene analysis; known familial variants PMS2 (postmeiotic segregation increased 2 [S. cerevisiae]) (eg, hereditary non-polyposis colorectal cancer, Lynch syndrome) gene analysis; duplication/deletion variants PTEN (phosphatase and tensin homolog) (eg, Cowden syndrome, PTEN hamartoma tumor syndrome) gene analysis; full sequence analysis PTEN (phosphatase and tensin homolog) (eg, Cowden syndrome, PTEN hamartoma tumor syndrome) gene analysis; known familial variant PTEN (phosphatase and tensin homolog) (eg, Cowden syndrome, PTEN hamartoma tumor syndrome) gene analysis; duplication/deletion variant PMP22 (peripheral myelin protein 22) (eg, Charcot-Marie-Tooth, hereditary neuropathy with liability to pressure palsies) gene analysis; duplication/deletion analysis PMP22 (peripheral myelin protein 22) (eg, Charcot-Marie-Tooth, hereditary neuropathy with liability to pressure palsies) gene analysis; full sequence analysis PMP22 (peripheral myelin protein 22) (eg, Charcot-Marie-Tooth, hereditary neuropathy with liability to pressure palsies) gene analysis; known familial variant SMPD1 (sphingomyelin phosphodiesterase 1, acid lysosomal) (eg, Niemann-Pick disease, Type A) gene analysis, common variants (eg, R496L, L302P, fsp330) SNRPN/UBE3A (small nuclear ribonucleoprotein polypeptide N and ubiquitin protein ligase E3A) (eg, Prader-Willi syndrome and/or Angelman syndrome), methylation analysis SERPINA1 (serpin peptidase inhibitor, clade A, alpha-1 antiproteinase, antitrypsin, member 1) (eg, alpha-1-antitrypsis deficiency), gene analysis, common variants (eg, *S and *Z)
10 Page 10 of (T cell antigen receptor, beta) (eg, leukemia and lymphoma), gene rearrangement analysis to detect abnormal clonal population(s); using amplification methodology (eg, polymerase chain reaction) (T cell antigen receptor, beta) (eg, leukemia and lymphoma), gene rearrangement analysis to detect abnormal clonal population(s); using direct probe methodology (eg, Southern blot) (T cell antigen receptor, gamma) (eg, leukemia and lymphoma), gene rearrangement analysis, evaluation to detect abnormal clonal population(s) UGT1A1 (UDP glucuronosyltransferase 1 family, polypeptide A1) (eg, irinotecan metabolism), gene analysis, common variants (eg, *28, *36, *37) VKORC1 (vitamin K epoxide reductase complex, subunit 1) (eg, warfarin metabolism), gene analysis, common variants (eg, -1639/3673) Molecular pathology procedure, Level 1 (eg, identification of single germline variant [eg,snp] by techniques such as restriction enzyme digestion or melt curve analysis) Molecular pathology procedure, Level 2 (eg, 2-10 SNP's, 1 methylated variant, or 1 somatic variant [typically using nonsequencing target variant analysis], or detection of a dynamic mutation disorder/triplet repeat) Molecular pathology procedure, Level 3 (eg, >10 SNPs, 2-10 methylated variants, or 2-10 somatic variants [typically using non-sequencing target variant analysis], immunoglobulin and T-cell receptor gene rearrangements, duplication/deletion variants of 1 exon loss of heterozygosity [LOH], uniparental disomy [UPD]) Molecular pathology procedure, Level 4 (eg, analysis of single exon by DNA sequence analysis, analysis of >10 amplicons using multiplex PCR in 2 or more independent reactions, mutation scanning or duplication/deletion variants of 2-5 exons) Molecular pathology procedure, Level 5 (eg, analysis of 2-5 exons by DNA sequence analysis, mutation scanning or duplication/deletion variants of 6-10 exons, or characterization of a dynamic mutation disorder/triplet repeat by Southern blot analysis) Molecular pathology procedure, Level 6 (eg, analysis of 6-10 exons by DNA sequence analysis, mutation scanning or duplication/deletion variants of exons, regionally targeted cytogenomic array analysis Molecular pathology procedure, Level 7 (eg, analysis of exons by DNA sequence analysis, mutation scanning or duplication/deletion variants of exons, cytogenomic array analysis for neoplasia) Molecular pathology procedure, Level 8 (eg, analysis of exons by DNA sequence analysis, mutation scannion or duplication/deletion variants of >50 exons, sequence analysis of multiple genes on one platform) Molecular pathology procedure, Level 9 (eg, analysis of >50 exons in a single gene by DNA sequence analysis)
11 Page 11 of Aortic dysfunction or dilation (eg, Marfan syndrome, Loeys Dietz syndrome, Ehler Danlos syndrome type IV, arterial tortuosity syndrome); genomic sequence analysis panel, must include sequencing of at least 9 genes, including FBN1, TGFBR1, TGFBR2, COL3A1, MYH11, ACTA2, SLC2A10, SMAD3, and MYLK Aortic dysfunction or dilation (eg, Marfan syndrome, Loeys Dietz syndrome, Ehler Danlos syndrome type IV, arterial tortuosity syndrome); duplication/deletion analysis panel, must include analyses for TGFBR1, TGFBR2, MYH11, and COL3A Exome (eg, unexplained constitutional or heritable disorder or syndrome); sequence analysis Exome (eg, unexplained constitutional or heritable disorder or syndrome); sequence analysis, each comparator exome (eg, parents, siblings) (List separately in addition to code for primary procedure) Exome (eg, unexplained constitutional or heritable disorder or syndrome); re-evaluation of previously obtained exome sequence (eg, updated knowledge or unrelated condition/syndrome) Fetal chromosomal aneuploidy (eg, trisomy 21, monosomy X) genomic sequence analysis panel, circulating cell-free fetal DNA in maternal blood, must include analysis of chromosomes 13, 18, and Genome (eg, unexplained constitutional or heritable disorder or syndrome); sequence analysis Genome (eg, unexplained constitutional or heritable disorder or syndrome); sequence analysis, each comparator genome (eg, parents, siblings) (List separately in addition to code for primary procedure) Genome (eg, unexplained constitutional or heritable disorder or syndrome); re-evaluation of previously obtained genome sequence (eg, updated knowledge or unrelated condition/syndrome) Hearing loss (eg, nonsyndromic hearing loss, Usher syndrome, Pendred syndrome); genomic sequence analysis panel, must include sequencing of at least 60 genes, including CDH23, CLRN1, GJB2, GPR98, MTRNR1, MYO7A, MYO15A, PCDH15, OTOF, SLC26A4, TMC1, TMPRSS3, USH1C, USH1G, USH2A, and WFS Hearing loss (eg, nonsyndromic hearing loss, Usher syndrome, Pendred syndrome); duplication/deletion analysis panel, must include copy number analyses for STRC and DFNB1 deletions in GJB2 and GJB6 genes Hereditary colon cancer syndromes (eg, Lynch syndrome, familial adenomatosis polyposis); genomic sequence analysis panel, must include analysis of at least 7 genes, including APC, CHEK2, MLH1, MSH2, MSH6, MUTYH, and PMS Hereditary colon cancer syndromes (eg, Lynch syndrome, familial adenomatosis polyposis); duplication/deletion gene analysis panel, must include analysis of at least 8 genes, including APC, MLH1, MSH2, MSH6, PMS2, EPCAM, CHEK2, and MUTYH
12 Page 12 of Nuclear encoded mitochondrial genes (eg, neurologic or myopathic phenotypes), genomic sequence panel, must include analysis of at least 100 genes, including BCS1L, C10orf2, COQ2, COX10, DGUOK, MPV17, OPA1, PDSS2, POLG, POLG2, RRM2B, SCO1, SCO2, SLC25A4, SUCLA2, SUCLG1, TAZ, TK2, and TYMP Targeted genomic sequence analysis panel, solid organ neoplasm, DNA analysis, 5-50 genes (eg, ALK, BRAF, CDKN2A, EGFR, ERBB2, KIT, KRAS, NRAS, MET, PDGFRA, PDGFRB, PGR, PIK3CA, PTEN, RET), interrogation for sequence variants and copy number variants or rearrangements, if performed Targeted genomic sequence analysis panel, hematolymphoid neoplasm or disorder, DNA and RNA analysis when performed, 5-50 genes (eg, BRAF, CEBPA, DNMT3A, EZH2, FLT3, IDH1, IDH2, JAK2, KRAS, KIT, MLL, NRAS, NPM1, NOTCH1), interrogation for sequence variants, and copy number variants or rearrangements, or isoform expression or mrna expression levels, if performed Targeted genomic sequence analysis panel, solid organ or hematolymphoid neoplasm, DNA and RNA analysis when performed, 51 or greater genes (eg, ALK, BRAF, CDKN2A, CEBPA, DNMT3A, EGFR, ERBB2, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MLL, NPM1, NRAS, MET, NOTCH1, PDGFRA, PDGFRB, PGR, PIK3CA, PTEN, RET), interrogation for sequence variants and copy number variants or rearrangements, if performed Whole mitochondrial genome (eg, Leigh syndrome, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes [MELAS], myoclonic epilepsy with ragged-red fibers [MERFF], neuropathy, ataxia, and retinitis pigmentosa [NARP], Leber hereditary optic neuropathy [LHON]), genomic sequence, must include sequence analysis of entire mitochondrial genome with heteroplasmy detection Whole mitochondrial genome large deletion analysis panel (eg, Kearns-Sayre syndrome, chronic progressive external ophthalmoplegia), including heteroplasmy detection, if performed X-linked intellectual disability (XLID) (eg, syndromic and non-syndromic XLID); genomic sequence analysis panel, must include sequencing of at least 60 genes, including ARX, ATRX, CDKL5, FGD1, FMR1, HUWE1, IL1RAPL, KDM5C, L1CAM, MECP2, MED12, MID1, OCRL, RPS6KA3, and SLC16A X-linked intellectual disability (XLID) (eg, syndromic and non-syndromic XLID); duplication/deletion gene analysis, must include analysis of at least 60 genes, including ARX, ATRX, CDKL5, FGD1, FMR1, HUWE1, IL1RAPL, KDM5C, L1CAM, MECP2, MED12, MID1, OCRL, RPS6KA3, and SLC16A Unlisted molecular pathology procedure Oncology (breast), mrna, gene expression profiling by real-time RT-PCR of 21 genes, utilizing formalin-fixed paraffin embedded tissue, algorithm reported as recurrence score Androsterone Growth stimulation expressed gene 2 (ST2, Interleukin 1 receptor like-1)
13 Page 13 of Chromosome analysis for breakage syndromes; baseline Sister Chromatid Exchange (SCE) cells Chromosome analysis for breakage syndromes; baseline breakage, score cells, count 20 cells, 2 karyotypes (eg, for ataxia telangiectasia, Fanconi anemia, fragile X) Chromosome analysis for breakage syndromes; score 100 cells, clastogen stress (eg, diepoxybutane, mitomycin C, ionizing radiation, UV radiation) Chromosome analysis; count 5 cells, 1 karyotype, with banding Chromosome analysis; count cells, 2 karyotypes, with banding Chromosome analysis; count 45 cells for mosaicism, 2 karyotypes, with banding Chromosome analysis; analyze cells Chromosome analysis, amniotic fluid or chorionic villus, count 15 cells, 1 karyotype, with banding Chromosome analysis, in situ for amniotic fluid cells, count cells from 6-12 colonies, 1 karyotype, with banding Molecular cytogenetics; DNA probe, each (eg, FISH) Molecular cytogenetics; chromosomal in situ hybridization, analyze 3-5 cells (eg, for derivatives and markers) Molecular cytogenetics; chromosomal in situ hybridization, analyze cells (eg, for microdeletions) Molecular cytogenetics; interphase in situ hybridization, analyze cells Molecular cytogenetics; interphase in situ hybridization, analyze cells Consultation and report on referred material requiring preparation of slides HCPCS CODE DESCRIPTION S3840 DNA analysis for germline mutations of the RET proto-oncogene for susceptibility to multiple endocrine neoplasia type 2 S3841 Genetic testing for retinoblastoma S3842 Genetic testing for Von Hippel-Lindau disease S3844 DNA analysis of the connexin 26 gene (GJB2) for susceptibility to congenital, profound deafness S3845 Genetic testing for alpha-thalassemia S3846 Genetic testing for hemoglobin E beta-thalassemia S3849 Genetic testing for Niemann-Pick disease S3850 Genetic testing for sickle cell anemia S3852 DNA analysis for APOE epsilon 4 allele for susceptibility to Alzheimer s disease S3853 Genetic testing for myotonic muscular dystrophy S3854 Gene expression profiling panel for use in the management of breast cancer treatment S3861 Genetic testing, sodium channel, voltage-gated, type V, alpha subunit (SCN5A) and variants for suspected Brugada syndrome S3865 Comprehensive gene sequence analysis for hypertrophic cardiomyopathy
14 Page 14 of 15 S3866 Genetic analysis for a specific gene mutation for hypertrophic cardiomyopathy (HCM) in an individual with a known HCM mutation in the family REFERENCES STATEMENT: Analyses of the scientific and clinical references cited below were conducted and utilized by the Johns Hopkins HealthCare (JHHC) Medical Policy Team during the development and implementation of this medical policy. Per NCQA standards, the Medical Policy Team will continue to monitor and review any newly published clinical evidence and adjust the references below accordingly if deemed necessary. CLINICAL: 1. Grada, A., Weinbrecht, K. (2013). Next-generation sequencing: Methodology and application. Journal of Investigative Dermatology, 133, e Robson, M., Storm, C., Weitzel, J., et al. (2010). American Society of Clinical Oncology Policy Statement Update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol., 28, Foster, M., Mulvihill, J., et al. (2009). Evaluating the utility of personal genomic information. Genet Medicine, 11, Domchek, S., Friebel, T., Singer, C., et al. (2010). Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA, 304, Wall, D.P., Tonellato, P.J. (2012). The future of genomics in pathology. F1000 Med Rep., 4, Hayes, Inc. Genetic Test Evaluation (GTE): HEALTH PLAN: 7. Aetna Clinical Policy Bulletin, (2015, May 6). Genetic Testing Policy; Number: Retrieved from: 8. Aetna Clinical Policy Bulletin, (2014, July 15). Fetal Surgery in Utero; Number: 0449 found at: REGULATORY: 9. TRICARE POLICY MANUAL M, February 1, Chapter 6, Section 3.1. Diagnostic Genetic Testing, Authority 32 CFR 199.4(a)(1)(i). Retrieved from:
15 Page 15 of Centers for Medicare and Medicaid (CMS), PUBLIC LAW MAY 21, Retrieved from: COMAR: 2010 Individual Medicare Supplement Policies-Plans A through D, F, G, K through N, (Revised 2010, April 28). Prohibition against Use of Genetic Information and Requests for Genetic Testing: C. Retrieved from: Shuren, J., (2010, July 22). 12. U.S. Food and Drug Administration (FDA). Direct-to-Consumer Genetic Testing and the Consequences to the Public. Retrieved from: Testimony/ucm htm. 13. U.S. Food and Drug Administration (FDA), (2011, July 8). Product Classification: Test Factor V Leiden DNA Mutations Detection Systems. Medical Device 510(k) Retrieved from: National Human Genome Research Institute. National Institute of Health. (2015). Access:
1199 SEIU Benefit Funds Laboratory Management Program PRIOR AUTHORIZATION CODE LIST FOR OUTPATIENT MOLECULAR AND GENOMIC LABORATORY TESTS
 1199 SEIU Benefit Funds Laboratory Management Program PRIOR AUTHORIZATION CODE LIST FOR OUTPATIENT MOLECULAR AND GENOMIC LABORATORY TESTS Effective January 1, 2016 Administered by evicore healthcare Refer
1199 SEIU Benefit Funds Laboratory Management Program PRIOR AUTHORIZATION CODE LIST FOR OUTPATIENT MOLECULAR AND GENOMIC LABORATORY TESTS Effective January 1, 2016 Administered by evicore healthcare Refer
MEDICAL POLICY No. 91540-R10 GENETICS: COUNSELING, TESTING, SCREENING
 GENETICS: COUNSELING, TESTING, SCREENING Effective Date: February 26, 2015 Review Dates: 8/07, 10/07, 8/08, 8/09, 10/09, 2/10, 8/10, 8/11, 10/11, 10/12, 10/13, 11/14 Date Of Origin: August 8, 2007 Status:
GENETICS: COUNSELING, TESTING, SCREENING Effective Date: February 26, 2015 Review Dates: 8/07, 10/07, 8/08, 8/09, 10/09, 2/10, 8/10, 8/11, 10/11, 10/12, 10/13, 11/14 Date Of Origin: August 8, 2007 Status:
July 14, 2014. Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244
 ASCP s Statements before the Centers for Medicare and Medicaid Services Regarding Recommendations for Crosswalking/Gapfilling New CPT s for the CLFS for CY 2015 and the Revaluation of the Clinical Laboratory
ASCP s Statements before the Centers for Medicare and Medicaid Services Regarding Recommendations for Crosswalking/Gapfilling New CPT s for the CLFS for CY 2015 and the Revaluation of the Clinical Laboratory
Molecular Pathology/Molecular Diagnostics/Genetic Testing
 Policy Number GEN01252012RP Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 01/28/2015 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable
Policy Number GEN01252012RP Approved By UnitedHealthcare Medicare Reimbursement Policy Committee Current Approval Date 01/28/2015 IMPORTANT NOTE ABOUT THIS REIMBURSEMENT POLICY This policy is applicable
Section: Genetic Testing Last Reviewed Date: October 2015. Policy No: 73 Effective Date: November 1, 2015
 Medical Policy Manual Topic: Genetic Cancer Susceptibility Panels Using Next Generation Sequencing Date of Origin: July 2014 Section: Genetic Testing Last Reviewed Date: October 2015 Policy No: 73 Effective
Medical Policy Manual Topic: Genetic Cancer Susceptibility Panels Using Next Generation Sequencing Date of Origin: July 2014 Section: Genetic Testing Last Reviewed Date: October 2015 Policy No: 73 Effective
CPT Generic Test Test Description
 CPT Generic Test Test Description Code Name 1 81200 ASPA Gene Test for leukodystrophies (Canavan disease), an autosomal recessive disease (most common in Ashkenazi Jewish population) with life expectancy
CPT Generic Test Test Description Code Name 1 81200 ASPA Gene Test for leukodystrophies (Canavan disease), an autosomal recessive disease (most common in Ashkenazi Jewish population) with life expectancy
Genetic Tests and Disease States Included in Humana s Genetic Guidance Program
 81161 81200 81201 Dystrophin (DMD) (e.g., Duchenne/Becker muscular dystrophy) deletion analysis and duplication analysis, if performed Aspartoacylase (ASPA) (e.g., Canavan disease) gene analysis, common
81161 81200 81201 Dystrophin (DMD) (e.g., Duchenne/Becker muscular dystrophy) deletion analysis and duplication analysis, if performed Aspartoacylase (ASPA) (e.g., Canavan disease) gene analysis, common
Health First Health Plans Authorization List
 Health First Health Plans Authorization List Effective April 15, 2016 IMPORTANT CONTACTS FOR AUTHORIZATIONS SUBMITTED TO THE HEALTH PLAN Submit online requests via your secure account at MyHFHP.org/login
Health First Health Plans Authorization List Effective April 15, 2016 IMPORTANT CONTACTS FOR AUTHORIZATIONS SUBMITTED TO THE HEALTH PLAN Submit online requests via your secure account at MyHFHP.org/login
NEW YORK STATE MEDICAID PROGRAM LABORATORY PROCEDURE CODES
 NEW YORK STATE MEDICAID PROGRAM LABORATORY PROCEDURE S Table of Contents GENERAL INFORMATION AND RULES...3 ORGAN OR DISEASE ORIENTED PANELS... 14 THERAPEUTIC DRUG ASSAYS... 15 EVOCATIVE/SUPPRESSION TESTING...
NEW YORK STATE MEDICAID PROGRAM LABORATORY PROCEDURE S Table of Contents GENERAL INFORMATION AND RULES...3 ORGAN OR DISEASE ORIENTED PANELS... 14 THERAPEUTIC DRUG ASSAYS... 15 EVOCATIVE/SUPPRESSION TESTING...
RMHP Prior Authorization List Effective October 1, 2015 rev 7/18/2016
 Effective October 1, 2015 rev 7/18/2016 *This list applies to all services for which RMHP is the primary payer. * Services that are not a benefit of the Member's Evidence of Coverage will not be authorized.
Effective October 1, 2015 rev 7/18/2016 *This list applies to all services for which RMHP is the primary payer. * Services that are not a benefit of the Member's Evidence of Coverage will not be authorized.
GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110
 GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110 COVERAGE: Pre- and post-genetic test counseling may be eligible for coverage in addition to the genetic
GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110 COVERAGE: Pre- and post-genetic test counseling may be eligible for coverage in addition to the genetic
The following chapter is called "Preimplantation Genetic Diagnosis (PGD)".
 Slide 1 Welcome to chapter 9. The following chapter is called "Preimplantation Genetic Diagnosis (PGD)". The author is Dr. Maria Lalioti. Slide 2 The learning objectives of this chapter are: To learn the
Slide 1 Welcome to chapter 9. The following chapter is called "Preimplantation Genetic Diagnosis (PGD)". The author is Dr. Maria Lalioti. Slide 2 The learning objectives of this chapter are: To learn the
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA
 CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
CLINICAL GUIDELINES. Lab Management Program. Effective January 15, 2016
 CLINICAL GUIDELINES Lab Management Program Effective January 15, 2016 CareCore National, LLC d/b/a evicore healthcare (evicore) Clinical guidelines for medical necessity review of lab management services.
CLINICAL GUIDELINES Lab Management Program Effective January 15, 2016 CareCore National, LLC d/b/a evicore healthcare (evicore) Clinical guidelines for medical necessity review of lab management services.
Carrier Screening For Genetic Diseases Preconception Consent (Female and/or Male Partner)
 Carrier Screening For Genetic Diseases Preconception Consent (Female and/or Male Partner) The goal of our practice at ARMS is to make sure that you receive optimal care to improve your chances of having
Carrier Screening For Genetic Diseases Preconception Consent (Female and/or Male Partner) The goal of our practice at ARMS is to make sure that you receive optimal care to improve your chances of having
Common Cancers & Hereditary Syndromes
 Common Cancers & Hereditary Syndromes Elizabeth Hoodfar, MS, LCGC Regional Cancer Genetics Coordinator Kaiser Permanente Northern California Detect clinical characteristics of hereditary cancer syndromes.
Common Cancers & Hereditary Syndromes Elizabeth Hoodfar, MS, LCGC Regional Cancer Genetics Coordinator Kaiser Permanente Northern California Detect clinical characteristics of hereditary cancer syndromes.
Corporate Medical Policy Carrier Testing for Genetic Disease
 Corporate Medical Policy Carrier Testing for Genetic Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: carrier_testing_for_genetic_disease 12/2013 8/2015 8/2016 8/2015 Description
Corporate Medical Policy Carrier Testing for Genetic Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: carrier_testing_for_genetic_disease 12/2013 8/2015 8/2016 8/2015 Description
Gene mutation and molecular medicine Chapter 15
 Gene mutation and molecular medicine Chapter 15 Lecture Objectives What Are Mutations? How Are DNA Molecules and Mutations Analyzed? How Do Defective Proteins Lead to Diseases? What DNA Changes Lead to
Gene mutation and molecular medicine Chapter 15 Lecture Objectives What Are Mutations? How Are DNA Molecules and Mutations Analyzed? How Do Defective Proteins Lead to Diseases? What DNA Changes Lead to
Becker Muscular Dystrophy
 Muscular Dystrophy A Case Study of Positional Cloning Described by Benjamin Duchenne (1868) X-linked recessive disease causing severe muscular degeneration. 100 % penetrance X d Y affected male Frequency
Muscular Dystrophy A Case Study of Positional Cloning Described by Benjamin Duchenne (1868) X-linked recessive disease causing severe muscular degeneration. 100 % penetrance X d Y affected male Frequency
Invasive Prenatal (Fetal) Diagnostic Testing
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Invasive Prenatal (Fetal) Diagnostic Testing Number 12.04.116
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Invasive Prenatal (Fetal) Diagnostic Testing Number 12.04.116
Genetic Testing for Lynch Syndrome/Colorectal Cancer and Polyposis Syndromes
 Genetic Testing for Lynch Syndrome/Colorectal Cancer and Polyposis Syndromes Policy Number: Original Effective Date: MM.02.007 09/01/2011 Line(s) of Business: Current Effective Date: HMO; PPO 09/01/2011
Genetic Testing for Lynch Syndrome/Colorectal Cancer and Polyposis Syndromes Policy Number: Original Effective Date: MM.02.007 09/01/2011 Line(s) of Business: Current Effective Date: HMO; PPO 09/01/2011
CPT Code Changes for 2015 PATHOLOGY/LABORATORY
 Changes for 2015 PATHOLOGY/LABORATORY Rick Oliver, CPCO, CPC, MT(ASCP) Compliance Director- Laboratory and Pathology McKesson Business Performance Services This commentary is a summary prepared by McKesson
Changes for 2015 PATHOLOGY/LABORATORY Rick Oliver, CPCO, CPC, MT(ASCP) Compliance Director- Laboratory and Pathology McKesson Business Performance Services This commentary is a summary prepared by McKesson
MEDICAL GENETICS GENERAL OBJECTIVE SPECIFIC OBJECTIVES
 SUBJECT MEDICAL GENETICS CREDITS Total: 4.5 Theory 2.5 Practical 2 GENERAL OBJECTIVE To provide students with terminology and knowledge from the field of human genetics that will enable them to understand
SUBJECT MEDICAL GENETICS CREDITS Total: 4.5 Theory 2.5 Practical 2 GENERAL OBJECTIVE To provide students with terminology and knowledge from the field of human genetics that will enable them to understand
Overview of Genetic Testing and Screening
 Integrating Genetics into Your Practice Webinar Series Overview of Genetic Testing and Screening Genetic testing is an important tool in the screening and diagnosis of many conditions. New technology is
Integrating Genetics into Your Practice Webinar Series Overview of Genetic Testing and Screening Genetic testing is an important tool in the screening and diagnosis of many conditions. New technology is
Corporate Medical Policy Genetic Testing for Fanconi Anemia
 Corporate Medical Policy Genetic Testing for Fanconi Anemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_fanconi_anemia 03/2015 3/2016 3/2017 3/2016 Description
Corporate Medical Policy Genetic Testing for Fanconi Anemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_fanconi_anemia 03/2015 3/2016 3/2017 3/2016 Description
GENETIC TESTING AND MARFAN SYNDROME
 GENETIC TESTING AND MARFAN SYNDROME Genetic testing for mutations in fibrillin-1 (FBN1) and other genes has become an important and reliable option to aid in the diagnosis of Marfan syndrome and related
GENETIC TESTING AND MARFAN SYNDROME Genetic testing for mutations in fibrillin-1 (FBN1) and other genes has become an important and reliable option to aid in the diagnosis of Marfan syndrome and related
targeted therapy a guide for the patient
 targeted therapy FOR LUNG CANCER a guide for the patient TABLE OF CONTENTS lung cancer basics... 2-3 Gene changes... 4-5 Testing... 7-8 Targeted therapy... 9-11 Drugs Targeting EGFR... 12 Drugs Targeting
targeted therapy FOR LUNG CANCER a guide for the patient TABLE OF CONTENTS lung cancer basics... 2-3 Gene changes... 4-5 Testing... 7-8 Targeted therapy... 9-11 Drugs Targeting EGFR... 12 Drugs Targeting
Test Information Sheet
 Test Information Sheet GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 888-729-1206 Fax: 301-710-6594 E-mail: [email protected] www.genedx.com/oncology OncoGene Dx: High/Moderate Risk Panel Sequence
Test Information Sheet GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 888-729-1206 Fax: 301-710-6594 E-mail: [email protected] www.genedx.com/oncology OncoGene Dx: High/Moderate Risk Panel Sequence
Objectives Role of Medical Genetics in Hearing Loss Evaluation. 5 y.o. boy with severe SNHL
 Objectives Role of Medical Genetics in Hearing Loss Evaluation Millan Patel, MD UBC Dept. of Medical Genetics October 22, 2010 Case presentation to illustrate importance of defining syndromic hearing loss
Objectives Role of Medical Genetics in Hearing Loss Evaluation Millan Patel, MD UBC Dept. of Medical Genetics October 22, 2010 Case presentation to illustrate importance of defining syndromic hearing loss
Mendelian inheritance and the
 Mendelian inheritance and the most common genetic diseases Cornelia Schubert, MD, University of Goettingen, Dept. Human Genetics EUPRIM-Net course Genetics, Immunology and Breeding Mangement German Primate
Mendelian inheritance and the most common genetic diseases Cornelia Schubert, MD, University of Goettingen, Dept. Human Genetics EUPRIM-Net course Genetics, Immunology and Breeding Mangement German Primate
Optional Tests Offered Before and During Pregnancy
 Plano Women s Healthcare Optional Tests Offered Before and During Pregnancy Alpha-Fetoprotein Test (AFP) and Quad Screen These are screening tests that can assess your baby s risk of having such birth
Plano Women s Healthcare Optional Tests Offered Before and During Pregnancy Alpha-Fetoprotein Test (AFP) and Quad Screen These are screening tests that can assess your baby s risk of having such birth
LAB MANAGEMENT CRITERIA. 1199SEIU Funds. Effective July 16, 2015
 LAB MANAGEMENT CRITERIA 1199SEIU Funds Effective July 16, 2015 Clinical criteria for medical necessity review of lab management services. Please note the following: CPT Copyright 2015 American Medical
LAB MANAGEMENT CRITERIA 1199SEIU Funds Effective July 16, 2015 Clinical criteria for medical necessity review of lab management services. Please note the following: CPT Copyright 2015 American Medical
National Medical Policy
 National Medical Policy Subject: Policy Number: Preimplantation Genetic Diagnosis in Assisted Reproduction NMP245 Effective Date*: October 2005 Updated: September 2015 This National Medical Policy is subject
National Medical Policy Subject: Policy Number: Preimplantation Genetic Diagnosis in Assisted Reproduction NMP245 Effective Date*: October 2005 Updated: September 2015 This National Medical Policy is subject
Overview of the new AMA Molecular Pathology CPT codes
 Overview of the new AMA Molecular Pathology CPT codes V.M. Pratt, PhD, FACMG Indiana University School of Medicine Reimbursement and CPT codes CPT code reimbursement List of services CPT is a registered
Overview of the new AMA Molecular Pathology CPT codes V.M. Pratt, PhD, FACMG Indiana University School of Medicine Reimbursement and CPT codes CPT code reimbursement List of services CPT is a registered
What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives
 What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives What does the career involve? Explore family histories to identify risks Reducing risks
What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives What does the career involve? Explore family histories to identify risks Reducing risks
PROVIDER POLICIES & PROCEDURES
 PROVIDER POLICIES & PROCEDURES BRCA GENETIC TESTING The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance Program (CMAP) on the requirements for
PROVIDER POLICIES & PROCEDURES BRCA GENETIC TESTING The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance Program (CMAP) on the requirements for
REI Pearls: Pitfalls of Genetic Testing in Miscarriage
 The Skinny: Genetic testing of miscarriage tissue is controversial and some people question if testing is helpful or not. This summary will: 1) outline the arguments for and against genetic testing; 2)
The Skinny: Genetic testing of miscarriage tissue is controversial and some people question if testing is helpful or not. This summary will: 1) outline the arguments for and against genetic testing; 2)
DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES
 DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES Extracts from a review article by KN North and KJ Jones: Recent advances in diagnosis of the childhood muscular dystrophies Journal of Paediatrics and Child Health
DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES Extracts from a review article by KN North and KJ Jones: Recent advances in diagnosis of the childhood muscular dystrophies Journal of Paediatrics and Child Health
Breast cancer and the role of low penetrance alleles: a focus on ATM gene
 Modena 18-19 novembre 2010 Breast cancer and the role of low penetrance alleles: a focus on ATM gene Dr. Laura La Paglia Breast Cancer genetic Other BC susceptibility genes TP53 PTEN STK11 CHEK2 BRCA1
Modena 18-19 novembre 2010 Breast cancer and the role of low penetrance alleles: a focus on ATM gene Dr. Laura La Paglia Breast Cancer genetic Other BC susceptibility genes TP53 PTEN STK11 CHEK2 BRCA1
MEDICAL POLICY Genetic Testing
 POLICY.........PG0041 EFFECTIVE......01/01/12 LAST REVIEW... 04/22/16 MEDICAL POLICY Genetic Testing GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by
POLICY.........PG0041 EFFECTIVE......01/01/12 LAST REVIEW... 04/22/16 MEDICAL POLICY Genetic Testing GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by
Genomic Medicine The Future of Cancer Care. Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America
 Genomic Medicine The Future of Cancer Care Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America Personalized Medicine Personalized health care is a broad term for interventions
Genomic Medicine The Future of Cancer Care Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America Personalized Medicine Personalized health care is a broad term for interventions
SICKLE CELL ANEMIA & THE HEMOGLOBIN GENE TEACHER S GUIDE
 AP Biology Date SICKLE CELL ANEMIA & THE HEMOGLOBIN GENE TEACHER S GUIDE LEARNING OBJECTIVES Students will gain an appreciation of the physical effects of sickle cell anemia, its prevalence in the population,
AP Biology Date SICKLE CELL ANEMIA & THE HEMOGLOBIN GENE TEACHER S GUIDE LEARNING OBJECTIVES Students will gain an appreciation of the physical effects of sickle cell anemia, its prevalence in the population,
Information leaflet. Centrum voor Medische Genetica. Version 1/20150504 Design by Ben Caljon, UZ Brussel. Universitair Ziekenhuis Brussel
 Information on genome-wide genetic testing Array Comparative Genomic Hybridization (array CGH) Single Nucleotide Polymorphism array (SNP array) Massive Parallel Sequencing (MPS) Version 120150504 Design
Information on genome-wide genetic testing Array Comparative Genomic Hybridization (array CGH) Single Nucleotide Polymorphism array (SNP array) Massive Parallel Sequencing (MPS) Version 120150504 Design
UNIT 13 (OPTION) Genetic Abnormalities
 Unit 13 Genetic Abnormailities 1 UNIT 13 (OPTION) Genetic Abnormalities Originally developed by: Hildur Helgedottir RN, MN Revised (2000) by: Marlene Reimer RN, PhD, CCN (C) Associate Professor Faculty
Unit 13 Genetic Abnormailities 1 UNIT 13 (OPTION) Genetic Abnormalities Originally developed by: Hildur Helgedottir RN, MN Revised (2000) by: Marlene Reimer RN, PhD, CCN (C) Associate Professor Faculty
LEUKODYSTROPHY GENETICS AND REPRODUCTIVE OPTIONS FOR AFFECTED FAMILIES. Leila Jamal, ScM Kennedy Krieger Institute, Baltimore MD
 LEUKODYSTROPHY GENETICS AND REPRODUCTIVE OPTIONS FOR AFFECTED FAMILIES Leila Jamal, ScM Kennedy Krieger Institute, Baltimore MD 2 Outline Genetics 101: Basic Concepts and Myth Busting Inheritance Patterns
LEUKODYSTROPHY GENETICS AND REPRODUCTIVE OPTIONS FOR AFFECTED FAMILIES Leila Jamal, ScM Kennedy Krieger Institute, Baltimore MD 2 Outline Genetics 101: Basic Concepts and Myth Busting Inheritance Patterns
Patient Information. for Childhood
 Patient Information Genetic Testing for Childhood Hearing Loss Introduction This document describes the most common genetic cause of childhood hearing loss and explains the role of genetic testing. Childhood
Patient Information Genetic Testing for Childhood Hearing Loss Introduction This document describes the most common genetic cause of childhood hearing loss and explains the role of genetic testing. Childhood
Test Information Sheet
 Test Information Sheet GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 888-729-1206 Fax: 301-710-6594 E-mail: [email protected] www.genedx.com/oncology OncoGene Dx: Breast/Ovarian Cancer Panel Sequence
Test Information Sheet GeneDx 207 Perry Parkway Gaithersburg, MD 20877 Phone: 888-729-1206 Fax: 301-710-6594 E-mail: [email protected] www.genedx.com/oncology OncoGene Dx: Breast/Ovarian Cancer Panel Sequence
Genetic Mutations. Indicator 4.8: Compare the consequences of mutations in body cells with those in gametes.
 Genetic Mutations Indicator 4.8: Compare the consequences of mutations in body cells with those in gametes. Agenda Warm UP: What is a mutation? Body cell? Gamete? Notes on Mutations Karyotype Web Activity
Genetic Mutations Indicator 4.8: Compare the consequences of mutations in body cells with those in gametes. Agenda Warm UP: What is a mutation? Body cell? Gamete? Notes on Mutations Karyotype Web Activity
Preimplantation Genetic Diagnosis. Evaluation for single gene disorders
 Preimplantation Genetic Diagnosis Evaluation for single gene disorders What is Preimplantation Genetic Diagnosis? Preimplantation genetic diagnosis or PGD is a technology that allows genetic testing of
Preimplantation Genetic Diagnosis Evaluation for single gene disorders What is Preimplantation Genetic Diagnosis? Preimplantation genetic diagnosis or PGD is a technology that allows genetic testing of
RECURRENT PREGNANCY LOSS DR.RAJALAKSHMI SRINIVASAN SPECIALIST GYNECOLOGIST ZULEKHA HOSPITAL DUBAI
 RECURRENT PREGNANCY LOSS DR.RAJALAKSHMI SRINIVASAN SPECIALIST GYNECOLOGIST ZULEKHA HOSPITAL DUBAI RECURRENT PREGNANCY LOSS -RM Clinically recognized consecutive or non consecutive pregnancy losses before
RECURRENT PREGNANCY LOSS DR.RAJALAKSHMI SRINIVASAN SPECIALIST GYNECOLOGIST ZULEKHA HOSPITAL DUBAI RECURRENT PREGNANCY LOSS -RM Clinically recognized consecutive or non consecutive pregnancy losses before
2014 CPT Code Updates
 2014 CPT Code Updates This publication is a summary of The American Medical Association Current Procedural Terminology (CPT) codes published in ARUP's Laboratory Test Directory effective January 1, 2014.
2014 CPT Code Updates This publication is a summary of The American Medical Association Current Procedural Terminology (CPT) codes published in ARUP's Laboratory Test Directory effective January 1, 2014.
What is Cancer? Cancer is a genetic disease: Cancer typically involves a change in gene expression/function:
 Cancer is a genetic disease: Inherited cancer Sporadic cancer What is Cancer? Cancer typically involves a change in gene expression/function: Qualitative change Quantitative change Any cancer causing genetic
Cancer is a genetic disease: Inherited cancer Sporadic cancer What is Cancer? Cancer typically involves a change in gene expression/function: Qualitative change Quantitative change Any cancer causing genetic
Genetics Review for USMLE (Part 2)
 Single Gene Disorders Genetics Review for USMLE (Part 2) Some Definitions Alleles variants of a given DNA sequence at a particular location (locus) in the genome. Often used more narrowly to describe alternative
Single Gene Disorders Genetics Review for USMLE (Part 2) Some Definitions Alleles variants of a given DNA sequence at a particular location (locus) in the genome. Often used more narrowly to describe alternative
CAP Accreditation Checklists 2015 Edition
 CAP Accreditation Checklists 2015 Edition The College of American Pathologists (CAP) accreditation checklists contain the CAP accreditation program requirements, developed on more than 50 years of insight
CAP Accreditation Checklists 2015 Edition The College of American Pathologists (CAP) accreditation checklists contain the CAP accreditation program requirements, developed on more than 50 years of insight
Basic Human Genetics: Reproductive Health and Chromosome Abnormalities
 Basic Human Genetics: Reproductive Health and Chromosome Abnormalities Professor Hanan Hamamy Department of Genetic Medicine and Development Geneva University Switzerland Training Course in Sexual and
Basic Human Genetics: Reproductive Health and Chromosome Abnormalities Professor Hanan Hamamy Department of Genetic Medicine and Development Geneva University Switzerland Training Course in Sexual and
Genetic Testing in Research & Healthcare
 We Innovate Healthcare Genetic Testing in Research & Healthcare We Innovate Healthcare Genetic Testing in Research and Healthcare Human genetic testing is a growing science. It is used to study genes
We Innovate Healthcare Genetic Testing in Research & Healthcare We Innovate Healthcare Genetic Testing in Research and Healthcare Human genetic testing is a growing science. It is used to study genes
Name of Policy: Genetic Testing for Inherited Cancer Predisposition and/or Pharmacogenetics related to Cancer Treatment
 Name of Policy: Genetic Testing for Inherited Cancer Predisposition and/or Pharmacogenetics related to Cancer Treatment Policy #: 133 Latest Review Date: April 2015 Category: Laboratory Policy Grade: D
Name of Policy: Genetic Testing for Inherited Cancer Predisposition and/or Pharmacogenetics related to Cancer Treatment Policy #: 133 Latest Review Date: April 2015 Category: Laboratory Policy Grade: D
Chromosomes, Mapping, and the Meiosis Inheritance Connection
 Chromosomes, Mapping, and the Meiosis Inheritance Connection Carl Correns 1900 Chapter 13 First suggests central role for chromosomes Rediscovery of Mendel s work Walter Sutton 1902 Chromosomal theory
Chromosomes, Mapping, and the Meiosis Inheritance Connection Carl Correns 1900 Chapter 13 First suggests central role for chromosomes Rediscovery of Mendel s work Walter Sutton 1902 Chromosomal theory
Contents. molecular biology techniques. - Mutations in Factor II. - Mutations in MTHFR gene. - Breast cencer genes. - p53 and breast cancer
 Contents Introduction: biology and medicine, two separated compartments What we need to know: - boring basics in DNA/RNA structure and overview of particular aspects of molecular biology techniques - How
Contents Introduction: biology and medicine, two separated compartments What we need to know: - boring basics in DNA/RNA structure and overview of particular aspects of molecular biology techniques - How
Reconsideration Code 86386. Reconsideration Code Description Nuclear Matrix Protein 22 (NMP22), qualitative
 Calendar Year 2013 Centers for Medicare and Medicaid Services (CMS) New and Reconsidered Clinical Laboratory Fee Schedule (CLFS) Test Codes And Final Payment Determinations Reconsideration Code 86386 Reconsideration
Calendar Year 2013 Centers for Medicare and Medicaid Services (CMS) New and Reconsidered Clinical Laboratory Fee Schedule (CLFS) Test Codes And Final Payment Determinations Reconsideration Code 86386 Reconsideration
Medical Policy Preimplantation Genetic Testing
 Medical Policy Preimplantation Genetic Testing Table of Contents Policy: Commercial Coding Information Information Pertaining to All Policies Policy: Medicare Description References Authorization Information
Medical Policy Preimplantation Genetic Testing Table of Contents Policy: Commercial Coding Information Information Pertaining to All Policies Policy: Medicare Description References Authorization Information
Gene Therapy and Genetic Counseling. Chapter 20
 Gene Therapy and Genetic Counseling Chapter 20 What is Gene Therapy? Treating a disease by replacing, manipulating or supplementing a gene The act of changing an individual s DNA sequence to fix a non-functional
Gene Therapy and Genetic Counseling Chapter 20 What is Gene Therapy? Treating a disease by replacing, manipulating or supplementing a gene The act of changing an individual s DNA sequence to fix a non-functional
1 Mutation and Genetic Change
 CHAPTER 14 1 Mutation and Genetic Change SECTION Genes in Action KEY IDEAS As you read this section, keep these questions in mind: What is the origin of genetic differences among organisms? What kinds
CHAPTER 14 1 Mutation and Genetic Change SECTION Genes in Action KEY IDEAS As you read this section, keep these questions in mind: What is the origin of genetic differences among organisms? What kinds
Prior to April, 2013, the preferred provider for outpatient labs was East Side Clinical Laboratory.
 Payment Policy BlueCHiP for Medicare Laboratory Network Exemption List EFFECTIVE DATE: 04 01 2013 POLICY LAST UPDATED: 05 06 2015 OVERVIEW Blue Cross Blue Shield of Rhode Island (BCBSRI) has developed
Payment Policy BlueCHiP for Medicare Laboratory Network Exemption List EFFECTIVE DATE: 04 01 2013 POLICY LAST UPDATED: 05 06 2015 OVERVIEW Blue Cross Blue Shield of Rhode Island (BCBSRI) has developed
Genomic Medicine Education Initiatives of the College of American Pathologists
 Genomic Medicine Education Initiatives of the College of American Pathologists Debra G.B. Leonard, MD, PhD, FCAP Chair, Personalized Healthcare Committee, CAP Professor of Pathology, Weill Cornell Medical
Genomic Medicine Education Initiatives of the College of American Pathologists Debra G.B. Leonard, MD, PhD, FCAP Chair, Personalized Healthcare Committee, CAP Professor of Pathology, Weill Cornell Medical
MCB41: Second Midterm Spring 2009
 MCB41: Second Midterm Spring 2009 Before you start, print your name and student identification number (S.I.D) at the top of each page. There are 7 pages including this page. You will have 50 minutes for
MCB41: Second Midterm Spring 2009 Before you start, print your name and student identification number (S.I.D) at the top of each page. There are 7 pages including this page. You will have 50 minutes for
MUTATION, DNA REPAIR AND CANCER
 MUTATION, DNA REPAIR AND CANCER 1 Mutation A heritable change in the genetic material Essential to the continuity of life Source of variation for natural selection New mutations are more likely to be harmful
MUTATION, DNA REPAIR AND CANCER 1 Mutation A heritable change in the genetic material Essential to the continuity of life Source of variation for natural selection New mutations are more likely to be harmful
Chromosomes, Karyotyping, and Abnormalities (Learning Objectives) Learn the components and parts of a metaphase chromosome.
 Chromosomes, Karyotyping, and Abnormalities (Learning Objectives) Learn the components and parts of a metaphase chromosome. Define the terms karyotype, autosomal and sex chromosomes. Explain how many of
Chromosomes, Karyotyping, and Abnormalities (Learning Objectives) Learn the components and parts of a metaphase chromosome. Define the terms karyotype, autosomal and sex chromosomes. Explain how many of
Obstetrical Ultrasound and Prenatal Diagnostic Center
 Obstetrical Ultrasound and Prenatal Diagnostic Center Prenatal Diagnosis: Options and Opportunities Learn about various screening options including Early Risk Assessment (ERA), now available to women of
Obstetrical Ultrasound and Prenatal Diagnostic Center Prenatal Diagnosis: Options and Opportunities Learn about various screening options including Early Risk Assessment (ERA), now available to women of
Genes and Cancer. What are genes? Dominant vs. recessive genes
 Genes and Cancer Advances in science have improved our knowledge of the inner workings of cells, the basic building blocks of the body. All living things are made of cells. Complex animals such as humans
Genes and Cancer Advances in science have improved our knowledge of the inner workings of cells, the basic building blocks of the body. All living things are made of cells. Complex animals such as humans
Genetic and Genomic Testing (Disease Specific) MPM 7.1
 Disclaimer Page 1 of 24 Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans or the plan may have broader or more limited benefits
Disclaimer Page 1 of 24 Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans or the plan may have broader or more limited benefits
Downstream Outcomes of New Molecular Diagnostics CPT Coding System Codes
 Downstream Outcomes of New Molecular Diagnostics CPT Coding System Codes Michael Watson, MS, PhD, FACMG Advisory Committee on Heritable Disorders of Newborns and Children April 9, 2014 Disclosures As Executive
Downstream Outcomes of New Molecular Diagnostics CPT Coding System Codes Michael Watson, MS, PhD, FACMG Advisory Committee on Heritable Disorders of Newborns and Children April 9, 2014 Disclosures As Executive
Diagnostic Scoring System for LQTS
 Medical Coverage Policy Genetic Testing: Congenital Long QT Syndrome Device/Equipment Drug Medical Surgery Test Other Effective Date: 2/15/2011 Policy Last Updated: 2/21/2012 Prospective review is recommended/required.
Medical Coverage Policy Genetic Testing: Congenital Long QT Syndrome Device/Equipment Drug Medical Surgery Test Other Effective Date: 2/15/2011 Policy Last Updated: 2/21/2012 Prospective review is recommended/required.
Umm AL Qura University MUTATIONS. Dr Neda M Bogari
 Umm AL Qura University MUTATIONS Dr Neda M Bogari CONTACTS www.bogari.net http://web.me.com/bogari/bogari.net/ From DNA to Mutations MUTATION Definition: Permanent change in nucleotide sequence. It can
Umm AL Qura University MUTATIONS Dr Neda M Bogari CONTACTS www.bogari.net http://web.me.com/bogari/bogari.net/ From DNA to Mutations MUTATION Definition: Permanent change in nucleotide sequence. It can
2013 Coding and Reimbursement Update
 2013 Coding and Reimbursement Update HCCA Compliance Institute 2013 Washington, DC April 21, 2013 2 Presented by Diana W. Voorhees, M.A., CLS, MT, SH, CLCP Principal/CEO DV & Associates, Inc. 5200 Highland
2013 Coding and Reimbursement Update HCCA Compliance Institute 2013 Washington, DC April 21, 2013 2 Presented by Diana W. Voorhees, M.A., CLS, MT, SH, CLCP Principal/CEO DV & Associates, Inc. 5200 Highland
Genetic Testing for Familial Adenomatous Polyposis and MYH-Associated Polyposis (Lynch Syndrome)
 Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
ACMG Practice Guideline
 June 2007 Vol. 9 No. 6 ACMG Practice Guideline Indications for genetic referral: a guide for healthcare providers Beth A. Pletcher, MD 1, Helga V. Toriello, PhD 2, Sarah J. Noblin, MS, CGC 3, Laurie H.
June 2007 Vol. 9 No. 6 ACMG Practice Guideline Indications for genetic referral: a guide for healthcare providers Beth A. Pletcher, MD 1, Helga V. Toriello, PhD 2, Sarah J. Noblin, MS, CGC 3, Laurie H.
Hereditary Breast Cancer Panels. High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel
 P A T I E N T G U I D E Hereditary Breast Cancer Panels High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel B a y l o r M i r a c a G e n e t i c s L a b o r a t
P A T I E N T G U I D E Hereditary Breast Cancer Panels High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel B a y l o r M i r a c a G e n e t i c s L a b o r a t
Consent to Perform Preimplantation Genetic Screening (PGS) using. Comparative Genomic Hybridization (acgh) or Next Generation Sequencing (NGS)
 Consent to Perform Preimplantation Genetic Screening (PGS) using Array Comparative Genomic Hybridization (acgh ) or Next Generation Sequencing (NGS) Purpose The purpose of Preimplantation Genetic Screening
Consent to Perform Preimplantation Genetic Screening (PGS) using Array Comparative Genomic Hybridization (acgh ) or Next Generation Sequencing (NGS) Purpose The purpose of Preimplantation Genetic Screening
Clinical Policy Title: Array comparative genomic hybridization testing
 Clinical Policy Title: Array comparative genomic hybridization testing Clinical Policy Number: 02.01.03 Effective Date: Sept 1, 2015 Initial Review Date: May 13, 2013 Most Recent Review Date: August 19,
Clinical Policy Title: Array comparative genomic hybridization testing Clinical Policy Number: 02.01.03 Effective Date: Sept 1, 2015 Initial Review Date: May 13, 2013 Most Recent Review Date: August 19,
Prior Authorization Updates
 Prior Authorization Updates Managed Health Services (MHS) requires prior authorization as a condition of payment for many services. This Notice contains information regarding such prior authorization requirements
Prior Authorization Updates Managed Health Services (MHS) requires prior authorization as a condition of payment for many services. This Notice contains information regarding such prior authorization requirements
Sequencing and microarrays for genome analysis: complementary rather than competing?
 Sequencing and microarrays for genome analysis: complementary rather than competing? Simon Hughes, Richard Capper, Sandra Lam and Nicole Sparkes Introduction The human genome is comprised of more than
Sequencing and microarrays for genome analysis: complementary rather than competing? Simon Hughes, Richard Capper, Sandra Lam and Nicole Sparkes Introduction The human genome is comprised of more than
JOHNS HOPKINS HEALTHCARE
 Page 1 of 5 ACTION: New Policy Revising Policy Number Superseding Policy Number Archiving Policy Number Retiring Policy Number Johns Hopkins HealthCare provides a full spectrum of health care products
Page 1 of 5 ACTION: New Policy Revising Policy Number Superseding Policy Number Archiving Policy Number Retiring Policy Number Johns Hopkins HealthCare provides a full spectrum of health care products
Overview of testing for Lynch syndrome/hnpcc
 Overview of testing for Lynch syndrome/hnpcc This overview provides detailed information about interpreting MSI/IHC testing and genetic testing for Lynch syndrome/hnpcc. It is intended to be a reference
Overview of testing for Lynch syndrome/hnpcc This overview provides detailed information about interpreting MSI/IHC testing and genetic testing for Lynch syndrome/hnpcc. It is intended to be a reference
Chapter 4 Pedigree Analysis in Human Genetics. Chapter 4 Human Heredity by Michael Cummings 2006 Brooks/Cole-Thomson Learning
 Chapter 4 Pedigree Analysis in Human Genetics Mendelian Inheritance in Humans Pigmentation Gene and Albinism Fig. 3.14 Two Genes Fig. 3.15 The Inheritance of Human Traits Difficulties Long generation time
Chapter 4 Pedigree Analysis in Human Genetics Mendelian Inheritance in Humans Pigmentation Gene and Albinism Fig. 3.14 Two Genes Fig. 3.15 The Inheritance of Human Traits Difficulties Long generation time
Non-Invasive Prenatal Testing (NIPT) Factsheet
 Introduction NIPT, which analyzes cell-free fetal DNA circulating in maternal blood, is a new option in the prenatal screening and testing paradigm for trisomy 21 and a few other fetal chromosomal aneuploidies.
Introduction NIPT, which analyzes cell-free fetal DNA circulating in maternal blood, is a new option in the prenatal screening and testing paradigm for trisomy 21 and a few other fetal chromosomal aneuploidies.
Genetic Mutations Cause Many Birth Defects:
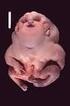 Genetic Mutations Cause Many Birth Defects: What We Learned from the FORGE Canada Project Jan M. Friedman, MD, PhD University it of British Columbia Vancouver, Canada I have no conflicts of interest related
Genetic Mutations Cause Many Birth Defects: What We Learned from the FORGE Canada Project Jan M. Friedman, MD, PhD University it of British Columbia Vancouver, Canada I have no conflicts of interest related
