Study Details. Study Type: Observational Study Design: Observational Model: Cohort, Time Perspective: Prospective. Investigator Details
|
|
|
- Conrad Bridges
- 9 years ago
- Views:
Transcription
1 Registry For Temsirolimus, Sunitinib, And Axitinib Treated Patients With Metastatic Renal Cell Carcinoma (mrcc), Mantle Cell Lymphoma (MCL), And Gastro-Intestinal Stroma Tumor (GIST) [STAR-TOR] Status: Recruiting Study Phase: N/A Start Date: January 2013 Completion Date: December 2017 Condition(s): Carcinoma, Renal Cell, Advanced, Lymphoma, Mantle-Cell, Gastrointestinal Stroma Tumors Full Title of Study Star-tor - Registry For The Evaluation Of The Safety, Tolerability And Efficacy Of Temsirolimus (Torisel ), Sunitinib (Sutent ), And Axitinib (Inlyta ) For The Treatment Of Subjects With Advanced Renal Cell Carcinoma (MRCC), Mantle Cell Lymphoma (MCL), And Gastro-intestinal Stroma Tumor (GIST). Overview The purpose of this registry is to obtain a general view as regards efficacy, tolerability and safety issues of the Torisel, Sutent, and/or Inlyta therapies in patients with advanced renal cell carcinoma, recurrent / refractory mantle cell lymphoma (MCL) and gastro-intestinal stroma tumors (GIST) under the conditions of routine use Detailed Description Treatment of the metastatic renal cell carcinoma (mrcc) has experienced fundamental changes within a very short period of time. In the past few years, introduction of various new substances for the treatment of mrcc has therefore resulted in new scientific research questions. Temsirolimus and sunitinib are current standard therapies in the first-line treatment of mrcc. Inlyta is a new substance that was developed for the treatment of mrcc after failure of sunitinib or cytokines. Since August 2009, Torisel is available as another treatment option for patients with mantle cell lymphoma (MCL). In addition, Sutent is used for patients with non-
2 resectable / metastatic gastro-intestinal stroma tumors (GIST) after failure or intolerability of imatinib. The routine use of drugs in the usual clinical setting faces additional challenges that generally cannot be completely reflected by clinical trials. Therefore, the purpose of this registry is to obtain a general view as regards efficacy, tolerability and safety issues of the Torisel, Sutent, and/or Inlyta therapies in patients with advanced renal cell carcinoma, recurrent / refractory mantle cell lymphoma (MCL) and gastro-intestinal stroma tumors (GIST) under the conditions of routine use. Therefore, the following information is of particular interest in the course of the investigation: - Efficacy (best response, overall survival, progression-free survival) - Tolerability of the therapy (assessed by the physician) - Safety profile (overall incidence of adverse events as well as side-effect rate) of subjects with mrcc, rmcl, and GIST under treatment with Torisel, Sutent, and/or Inlyta - Profile, comorbidities, and characteristics of subjects treated with Torisel Sutent, and/or Inlyta - The sequence of using the systemic therapies for RCC, MCL, and GIST - Patient survey on the quality of life of mrcc patients Study Details Study Type: Observational Study Design: Observational Model: Cohort, Time Perspective: Prospective Investigator Details Lead Sponsor: Pfizer Study Director: Pfizer CT.gov Call Center Pfizer Trial Location Details
3 Facility: Dr. med. Harald Held Neumuenster, Germany Facility: Dr. med. Hans Wilhelm Duebbers Ahaus, Germany Facility: Dr. Ludwig Fischer von Weikersthal Amberg, Germany Facility: Studienzentrum Drs. Klausmann / Dr. Welslau, Haematologie-Onkologie- Diabetologie Aschaffenburg, Germany Facility: Carsten Lange Bernburg, Germany Facility: Office of Ulrich Kube Chemnitz, Germany Facility: Dr. Jens-Uwe Krieger Chemnitz, Germany Facility: Zeisigwaldklinikum Bethanien Chemnitz Chemnitz, Germany Facility: Leonhard Stark Deggendorf, Germany Facility: Prof. Dr. med. Udo Rebmann Dessau, Germany Facility: Dr.med Johannes Mohm Dresden, Germany Facility: Dr. med. Ralf Eckert Eisleben, Germany Facility: Specialist Urology Erfurt, Germany Facility: Goebell Erlangen, Germany Facility: Prof. Dr. med. Lothar Bergmann Frankfurt am Main, Germany Facility: Dr. med. Gunter Derigs Frankfurt am Main, Germany Facility: Hoffkes Fulda, Germany Facility: PD Dr. Uwe Zimmermann Greifswald, Germany Facility: Internistische Gemeinschaftspraxis Guestrow, Germany Facility: Dr. med. Arne Strauss Göttingen, Germany Facility: Dr. med. Michael Rink Hamburg, Germany Facility: Dr. med. Hanns-Detlev Harich Hof, Germany Facility: Universitaetsklinikum des Saarlandes, Klinik fuer Urologie und Kinderurologie Homburg/Saar, Germany Facility: Dr. med. Susan Foller Jena, Germany Facility: Steinmetz Koeln, Germany Facility: Klinisches Studienzentrum Urlogie Koeln, Germany Facility: Dr. med. Martina Stauch Kronach, Germany
4 Facility: Dr. med. Ursula Vehling-Kaiser Landshut, Germany Facility: Dr. Andreas Kohler Langen, Germany Facility: Dietel Leipzig, Germany Facility: Andreas Schwarzer Leipzig, Germany Facility: Lutz Kamann Leipzig, Germany Facility: Dr.med. Matthias Schulze Markkleeberg, Germany Facility: Institut of Healthcare Research Mayen, Germany Facility: Dr. med. Jan Klaus Schroder Mulheim, Germany Facility: Dr.med. Wolfgang Abenhardt München, Germany Facility: Herrmann Münster, Germany Facility: Dr. med. Thomas Gehring Neckarsulm, Germany Facility: Dres. Derouet Poenicke Becker Neunkirchen, Germany Facility: Physician for Internal Medicine Neuwied, Germany Facility: Dr. med. David Kunst Nienburg, Germany Facility: Dr.med. Christian Linder Nordhausen, Germany Facility: Dr. med. Joachim Zimber Nürnberg, Germany Facility: Ralf-Bodo Kühn Oldenburg, Germany Facility: Wolfgang Marz Osnabruck, Germany Facility: Dr. med. Torsten Geyer Ostfildern, Germany Facility: Dr. med. Ino Kietz Parchim, Germany Facility: Office of Friedrich Overkamp, Oncologia Nova Recklinghausen, Germany Facility: Andreas Hübner Rostock, Germany Facility: Diakonie-Klinikum ggmbh Schwäbisch Hall, Germany Facility: Dr. med. Thomas Geer Schwäbisch Hall, Germany Facility: Office of Judith Franz-Werner Speyer, Germany Facility: Dr. Matthias Groschek Stolberg, Germany Facility: Dr. med. Heinz Kirchen Trier, Germany Facility: Klinik für Urologie, Eberhard-Karls-Universitaet Tuebingen, Tuebingen, Germany Facility: Klotz Weiden, Germany
5 Facility: Dr.med. Jan Janssen Westerstede, Germany Facility: Universitaetsklinik Wuerzburg, Medizinische Poliklinik Wuerzburg, Germany Facility: Jochen Gleissner Wuppertal, Germany Facility: Mathias Schulze Zittau, Germany Facility: Scheffler Zwickau, Germany Interventions Drug: Temsirolimus Drug: Temsirolimus Drug: Sunitinib Drug: Sunitinib Drug: Axitinib Information Source ID Number: 3066K NCT Identifier: NCT Health Authority: Germany: BfArM (Bundesinstitut fuer Arzneimittel und Medizinprodukte) Full Source of Clinical Trial Data: ClinicalTrials.gov processed this data on June 30, 2016 Clinical trials entries are delivered from the US National Institutes of Health and are not reviewed separately by this site. Please see the full source link above for retrieving further details from the government database.
Prospective multicenter study for functional evaluation of transoral laser microsurgery (TLM) for supraglottic carcinomas - SUPRATOL
 Trial Description Title Prospective multicenter study for functional evaluation of transoral laser microsurgery (TLM) for supraglottic carcinomas - SUPRATOL Trial Acronym SUPRATOL URL of the trial [---]*
Trial Description Title Prospective multicenter study for functional evaluation of transoral laser microsurgery (TLM) for supraglottic carcinomas - SUPRATOL Trial Acronym SUPRATOL URL of the trial [---]*
Navigating GIST. The Life Raft Group June 12, 2008
 Navigating GIST Clinical Trials The Life Raft Group June 12, 2008 Some observations: Annually, only 3% of adult patients participate in cancer clinical trials. Lara et. al; Evaluation of factors affecting
Navigating GIST Clinical Trials The Life Raft Group June 12, 2008 Some observations: Annually, only 3% of adult patients participate in cancer clinical trials. Lara et. al; Evaluation of factors affecting
Clinical Management Guideline Management of locally advanced or recurrent Renal cell carcinoma. Protocol for Planning and Treatment
 Protocol for Planning and Treatment The process to be followed in the management of: LOCALLY ADVANCED OR METASTATIC RENAL CELL CARCINOMA Patient information given at each stage following agreed information
Protocol for Planning and Treatment The process to be followed in the management of: LOCALLY ADVANCED OR METASTATIC RENAL CELL CARCINOMA Patient information given at each stage following agreed information
2. Background This was the fourth submission for everolimus requesting listing for clear cell renal carcinoma.
 PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
PLEASE NOTE: This study has been imported from ClinicalTrials.gov without additional data checks.
 PLEASE NOTE: This study has been imported from ClinicalTrials.gov without additional data checks. Trial Description Title A Phase IIIb, Multicentre, Open-label Study of Nilotinib in Adult Patients With
PLEASE NOTE: This study has been imported from ClinicalTrials.gov without additional data checks. Trial Description Title A Phase IIIb, Multicentre, Open-label Study of Nilotinib in Adult Patients With
Stage IV Renal Cell Carcinoma. Changing Management in A Comprehensive Community Cancer Center. Susquehanna Health Cancer Center
 Stage IV Renal Cell Carcinoma Changing Management in A Comprehensive Community Cancer Center Susquehanna Health Cancer Center 2000 2009 Warren L. Robinson, MD, FACP January 27, 2014 Introduction 65,150
Stage IV Renal Cell Carcinoma Changing Management in A Comprehensive Community Cancer Center Susquehanna Health Cancer Center 2000 2009 Warren L. Robinson, MD, FACP January 27, 2014 Introduction 65,150
Avastin in Metastatic Breast Cancer
 Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center
 What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center The Case for Immunotherapy in mrcc 1. Achieves patient s goal 2.
What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center The Case for Immunotherapy in mrcc 1. Achieves patient s goal 2.
MOLOGEN AG. Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer. Berlin, 12 May 2015
 Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer Berlin, 12 May 2015 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future
Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer Berlin, 12 May 2015 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future
What is New in Oncology. Michael J Messino, MD Cancer Care of WNC An affiliate of Mission hospitals
 What is New in Oncology Michael J Messino, MD Cancer Care of WNC An affiliate of Mission hospitals Personalized Medicine Personalized Genomics Genomic Medicine Precision Medicine Definition Application
What is New in Oncology Michael J Messino, MD Cancer Care of WNC An affiliate of Mission hospitals Personalized Medicine Personalized Genomics Genomic Medicine Precision Medicine Definition Application
Investigation of the effect of isomaltulose (PalatinoseTM) on metabolic parameters in subjects with Type 2 Diabetes.
 PLEASE NOTE: This trial has been registered retrospectively. Trial Description Title Investigation of the effect of isomaltulose (PalatinoseTM) on metabolic parameters in subjects with Type 2 Diabetes.
PLEASE NOTE: This trial has been registered retrospectively. Trial Description Title Investigation of the effect of isomaltulose (PalatinoseTM) on metabolic parameters in subjects with Type 2 Diabetes.
Presenting the SUTENT Patient Call Center.
 Presenting the SUTENT Patient Call Center. Please see patient Medication Guide and full prescribing information attached. We re here to support you. Dealing with cancer is a journey. Along the way, you
Presenting the SUTENT Patient Call Center. Please see patient Medication Guide and full prescribing information attached. We re here to support you. Dealing with cancer is a journey. Along the way, you
Trial Description. Organizational Data. Secondary IDs
 Trial Description Title A randomized, double-blind, multicenter study to demonstrate equivalent efficacy and to compare safety and immunogenicity of a biosimilar etanercept (GP2015) and Enbrel in patients
Trial Description Title A randomized, double-blind, multicenter study to demonstrate equivalent efficacy and to compare safety and immunogenicity of a biosimilar etanercept (GP2015) and Enbrel in patients
Renal Cell Carcinoma (Event Driven)
 Brochure More information from http://www.researchandmarkets.com/reports/2365661/ Renal Cell Carcinoma (Event Driven) Description: The Renal Cell Carcinoma market is highly lucrative. We anticipate that
Brochure More information from http://www.researchandmarkets.com/reports/2365661/ Renal Cell Carcinoma (Event Driven) Description: The Renal Cell Carcinoma market is highly lucrative. We anticipate that
NEOPLASMS OF KIDNEY (RENAL CELL CARCINOMA) And RENAL PELVIS (TRANSITIONAL CELL CARCINOMA)
 NEOPLASMS OF KIDNEY (RENAL CELL CARCINOMA) And RENAL PELVIS (TRANSITIONAL CELL CARCINOMA) Merat Esfahani, MD Medical Oncologist, Hematologist Cancer Liaison Physician SwedishAmerican Regional Cancer Center
NEOPLASMS OF KIDNEY (RENAL CELL CARCINOMA) And RENAL PELVIS (TRANSITIONAL CELL CARCINOMA) Merat Esfahani, MD Medical Oncologist, Hematologist Cancer Liaison Physician SwedishAmerican Regional Cancer Center
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials)
 ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials) 3 Integrated Trials Testing Targeted Therapy in Early Stage Lung Cancer Part of NCI s Precision Medicine Effort in
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials) 3 Integrated Trials Testing Targeted Therapy in Early Stage Lung Cancer Part of NCI s Precision Medicine Effort in
http://clinicaltrials.gov/ct2/show/nct01462513
 Page 1 of 5 Home Search Study Topics Glossary Search Full Text View Tabular View No Study Results Posted Related Studies L-BLP25 in Patients With Colorectal Carcinoma After Curative Resection of Hepatic
Page 1 of 5 Home Search Study Topics Glossary Search Full Text View Tabular View No Study Results Posted Related Studies L-BLP25 in Patients With Colorectal Carcinoma After Curative Resection of Hepatic
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012. (minutes for web publishing)
 Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Effect of implanted device-based impedance monitoring with telemedicine alerts on mortality and morbidity in heart failure (OptiLink HF)
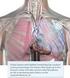 Effect of implanted device-based impedance monitoring with telemedicine alerts on mortality and morbidity in heart failure (OptiLink HF) Michael Böhm, Helmut Drexler, Hanno Oswald, Karin Rybak, Ralph Bosch,
Effect of implanted device-based impedance monitoring with telemedicine alerts on mortality and morbidity in heart failure (OptiLink HF) Michael Böhm, Helmut Drexler, Hanno Oswald, Karin Rybak, Ralph Bosch,
Accreditation, Certification and Quality Assurance Institute
 Accreditation, Certification and Quality Assurance Institute 1 Contents By HEIs for HEIs 4 Members 5 Organisational Structure 6 Objectives 8 The Quality Control Cycle 10 Further Development of the Accreditation
Accreditation, Certification and Quality Assurance Institute 1 Contents By HEIs for HEIs 4 Members 5 Organisational Structure 6 Objectives 8 The Quality Control Cycle 10 Further Development of the Accreditation
Clinical Trial Design. Sponsored by Center for Cancer Research National Cancer Institute
 Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Work-related diseases of occupational therapists - pilot study for creating an "occupational therapist-cohort" in Germany
 Trial Description Title Work-related diseases of occupational therapists - pilot study for creating an "occupational therapist-cohort" in Trial Acronym ERGO-Study URL of the trial [---]* Brief Summary
Trial Description Title Work-related diseases of occupational therapists - pilot study for creating an "occupational therapist-cohort" in Trial Acronym ERGO-Study URL of the trial [---]* Brief Summary
Across borders. Cross-border restructuring and insolvency. professions. English
 Cross-border restructuring and insolvency Cross-border Across borders and restructuring across professions and insolvency English Breadth of skills singular focus Schultze & Braun handles all German-related
Cross-border restructuring and insolvency Cross-border Across borders and restructuring across professions and insolvency English Breadth of skills singular focus Schultze & Braun handles all German-related
2014-06-10 Approved Bendamustine - Treatment of relapsed low grade NHL in patients unable to receive standard therapy 2014-05-
 Sub. Sub. Status -06-16 Approved Lenalidomide - 2nd line treatment of multiple myeloma in patients who have contraindications to the use of bortezomib -06-16 Approved Lenalidomide - 2nd line treatment
Sub. Sub. Status -06-16 Approved Lenalidomide - 2nd line treatment of multiple myeloma in patients who have contraindications to the use of bortezomib -06-16 Approved Lenalidomide - 2nd line treatment
Ask Us About Clinical Trials
 Ask Us About Clinical Trials Clinical Trials and You. Our specialists and researchers are at the forefront of their fields and are leading the way in developing new therapies and procedures for diagnosing
Ask Us About Clinical Trials Clinical Trials and You. Our specialists and researchers are at the forefront of their fields and are leading the way in developing new therapies and procedures for diagnosing
How To Make A New Dab Network In Germany Successful
 The German Re-launch of DAB: DMB Interactive Multimedia Radio and Mobile TV Making Digital Radio work: the German market perspective T-Systems Media&Broadcast Making Digital Radio work: the German market
The German Re-launch of DAB: DMB Interactive Multimedia Radio and Mobile TV Making Digital Radio work: the German market perspective T-Systems Media&Broadcast Making Digital Radio work: the German market
Decentralised Procedure. Public Assessment Report. Gadojaco Handvist Gadopentetat Dimeglumin Jacobsen 500 Mikromol/ml Injektionslösung
 Bundesinstitut für Arzneimittel und Medizinprodukte Decentralised Procedure Public Assessment Report Gadojaco Handvist Gadopentetat Dimeglumin Jacobsen 500 Mikromol/ml Injektionslösung Gadopentetate dimeglumine
Bundesinstitut für Arzneimittel und Medizinprodukte Decentralised Procedure Public Assessment Report Gadojaco Handvist Gadopentetat Dimeglumin Jacobsen 500 Mikromol/ml Injektionslösung Gadopentetate dimeglumine
Molecular markers and clinical trial design parallels between oncology and rare diseases?
 Molecular markers and clinical trial design parallels between oncology and rare diseases?, Harriet Sommer Institute for Medical Biometry and Statistics, University of Freiburg Medical Center 6. Forum Patientennahe
Molecular markers and clinical trial design parallels between oncology and rare diseases?, Harriet Sommer Institute for Medical Biometry and Statistics, University of Freiburg Medical Center 6. Forum Patientennahe
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
HMPWG in the view of the NCA
 EMEA Homeopathic Workshop Bundesinstitut für Arzneimittel London, 27.10.2006 HMPWG in the view of the NCA Objectives, Achievements, Roles and Responsibilities Werner Knöss Head of Division Complementary
EMEA Homeopathic Workshop Bundesinstitut für Arzneimittel London, 27.10.2006 HMPWG in the view of the NCA Objectives, Achievements, Roles and Responsibilities Werner Knöss Head of Division Complementary
News Release. Merck KGaA, Darmstadt, Germany, and Pfizer Receive FDA Breakthrough Therapy Designation for Avelumab in Metastatic Merkel Cell Carcinoma
 Your Contacts News Release Merck KGaA, Darmstadt, Germany Media: Markus Talanow +49 6151 72 7144 Investor Relations +49 6151 72 3321 Pfizer Inc., New York, USA Media: Sally Beatty +1 212 733 6566 Investor
Your Contacts News Release Merck KGaA, Darmstadt, Germany Media: Markus Talanow +49 6151 72 7144 Investor Relations +49 6151 72 3321 Pfizer Inc., New York, USA Media: Sally Beatty +1 212 733 6566 Investor
Individual Mobility Services: Closing the Gap between Public and Private Transport
 1st Eastern Mediterranean Conference on Intermodal Passenger Travel Individual Mobility Services: Closing the Gap between Public and Private Transport Christian Maertins & Dr. Hinrich Schmöe Nicosia, 14/11/2008
1st Eastern Mediterranean Conference on Intermodal Passenger Travel Individual Mobility Services: Closing the Gap between Public and Private Transport Christian Maertins & Dr. Hinrich Schmöe Nicosia, 14/11/2008
Registries: An alternative for clinical trials?
 Registries: An alternative for clinical trials? Prof. Dr. Joerg Hasford, M.D., Ph.D. Department of Medical Informatics, Biometry and Epidemiology Ludwig-Maximilians-Universität Email: [email protected]
Registries: An alternative for clinical trials? Prof. Dr. Joerg Hasford, M.D., Ph.D. Department of Medical Informatics, Biometry and Epidemiology Ludwig-Maximilians-Universität Email: [email protected]
Guidelines for Management of Renal Cancer
 Guidelines for Management of Renal Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Section 5 updated bullets 5.3 and 5.4 Section 6 updated
Guidelines for Management of Renal Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Section 5 updated bullets 5.3 and 5.4 Section 6 updated
Gruppo di lavoro: Malattie Tromboemboliche
 Gruppo di lavoro: Malattie Tromboemboliche 2381 Soluble Recombinant Thrombomodulin Ameliorates Hematological Malignancy-Induced Disseminated Intravascular Coagulation More Promptly Than Conventional Anticoagulant
Gruppo di lavoro: Malattie Tromboemboliche 2381 Soluble Recombinant Thrombomodulin Ameliorates Hematological Malignancy-Induced Disseminated Intravascular Coagulation More Promptly Than Conventional Anticoagulant
Hollie Goddard Sr. IRB Coordinator McKesson Specialty Health
 Hollie Goddard Sr. IRB Coordinator McKesson Specialty Health We are responsible for acquiring, analyzing, and protecting medical information vital to providing quality patient care HIM professionals ensure
Hollie Goddard Sr. IRB Coordinator McKesson Specialty Health We are responsible for acquiring, analyzing, and protecting medical information vital to providing quality patient care HIM professionals ensure
BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED
 ASX ANNOUNCEMENT 3 August 2011 ABN 53 075 582 740 BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED Data from renal cancer trial supports progression of the trial: o Combination
ASX ANNOUNCEMENT 3 August 2011 ABN 53 075 582 740 BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED Data from renal cancer trial supports progression of the trial: o Combination
PUBLIC ROADS. City-Light-Poster MEDIA DATA. Campaign media. Poster
 2015 Campaign media. Poster MEDIA DATA Campaign media. Poster s have a direct advertising impact all over the city, from downtown, at bus stops and in front of parking garages to pedestrian zones and in
2015 Campaign media. Poster MEDIA DATA Campaign media. Poster s have a direct advertising impact all over the city, from downtown, at bus stops and in front of parking garages to pedestrian zones and in
Breast cancer treatment for elderly women: a systematic review
 Breast cancer treatment for elderly women: a systematic review Gerlinde Pilkington Rumona Dickson Anna Sanniti Funded by the NCEI and POI Elderly people less likely to receive chemotherapy than younger
Breast cancer treatment for elderly women: a systematic review Gerlinde Pilkington Rumona Dickson Anna Sanniti Funded by the NCEI and POI Elderly people less likely to receive chemotherapy than younger
The Cancer Patient Journey. Dr. Jaco Fourie
 The Cancer Patient Journey Dr. Jaco Fourie The Cancer Patient Journey Prevention and health promotion Screening Diagnosis and staging Treatment Surveillance and survivorship End of life care The Cancer
The Cancer Patient Journey Dr. Jaco Fourie The Cancer Patient Journey Prevention and health promotion Screening Diagnosis and staging Treatment Surveillance and survivorship End of life care The Cancer
30th GMS. Russisches Haus der Wissenschaft und Kultur Friedrichstraße 176-179 10117 Berlin-Mitte
 30th GMS Symposium on Vascular Surgery Berlin 5 7 November 2015 Russisches Haus der Wissenschaft und Kultur Friedrichstraße 176-179 10117 Berlin-Mitte Scientific direction: PD Dr. med. R. I. Rückert Dr.
30th GMS Symposium on Vascular Surgery Berlin 5 7 November 2015 Russisches Haus der Wissenschaft und Kultur Friedrichstraße 176-179 10117 Berlin-Mitte Scientific direction: PD Dr. med. R. I. Rückert Dr.
CURRICULUM VITAE. M. Sc. Anne-Katharina Schiefele
 CURRICULUM VITAE Address: Department of Clinical Psychology and Psychotherapy, University of Trier, 54286 Trier, Germany TEL 0049 (0)651 201 2882 E-mail: [email protected] Birthday: November 30, 1987
CURRICULUM VITAE Address: Department of Clinical Psychology and Psychotherapy, University of Trier, 54286 Trier, Germany TEL 0049 (0)651 201 2882 E-mail: [email protected] Birthday: November 30, 1987
See you at the ACHEMA 2015 15. - 19.06.2015
 Sprache/ Language 15. 16. 17. 18. 19. Markus Dönni Geschäftsführer/ Managing Director Johannes Fliegen Geschäftsführer/ Managing Director Jochen Lindenberg Geschäftsführer/ Managing Director Christian
Sprache/ Language 15. 16. 17. 18. 19. Markus Dönni Geschäftsführer/ Managing Director Johannes Fliegen Geschäftsführer/ Managing Director Jochen Lindenberg Geschäftsführer/ Managing Director Christian
Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products
 Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
ASCO Initiatives in Personalized Medicine. Richard L. Schilsky, MD, FACP, FASCO Chief Medical Officer American Society of Clinical Oncology
 ASCO Initiatives in Personalized Medicine Richard L. Schilsky, MD, FACP, FASCO Chief Medical Officer American Society of Clinical Oncology Financial Disclosures No financial relationships to disclose.
ASCO Initiatives in Personalized Medicine Richard L. Schilsky, MD, FACP, FASCO Chief Medical Officer American Society of Clinical Oncology Financial Disclosures No financial relationships to disclose.
Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics
 Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics This trial is conducted in the United States of America (USA). The aim of this clinical
Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics This trial is conducted in the United States of America (USA). The aim of this clinical
TGN 1412 Welche Änderungen haben sich für die Erstanwendung am Menschen aus Sicht des BfArM ergeben?
 TGN 1412 Welche Änderungen haben sich für die Erstanwendung am Menschen aus Sicht des BfArM ergeben? PD Dr. med. Thomas Sudhop Bundesinstitut für Arzneimittel, Bonn Bundesinstitut für Arzneimittel IMP
TGN 1412 Welche Änderungen haben sich für die Erstanwendung am Menschen aus Sicht des BfArM ergeben? PD Dr. med. Thomas Sudhop Bundesinstitut für Arzneimittel, Bonn Bundesinstitut für Arzneimittel IMP
Vocational Education and Training (VET) and the Chambers of Commerce in Germany - and elsewhere
 Vocational Education and Training (VET) and the Chambers of Commerce in Germany - and elsewhere About us DIHK Registered Association Umbrella Organization of 80 Chambers of Commerce (IHK) in Germany All
Vocational Education and Training (VET) and the Chambers of Commerce in Germany - and elsewhere About us DIHK Registered Association Umbrella Organization of 80 Chambers of Commerce (IHK) in Germany All
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Nieuwe ontwikkelingen op het gebied van de angiogeneseremmers
 Nieuwe ontwikkelingen op het gebied van de angiogeneseremmers Emile Voest, MD, PhD Department of Medical Oncology University Medical Center Utrecht the Netherlands 4e Nascholing Targeted Therapy April
Nieuwe ontwikkelingen op het gebied van de angiogeneseremmers Emile Voest, MD, PhD Department of Medical Oncology University Medical Center Utrecht the Netherlands 4e Nascholing Targeted Therapy April
Immune Therapy for Pancreatic Cancer
 Immune Therapy for Pancreatic Cancer December 16, 2014 If you experience technical difficulty during the presentation: Contact WebEx Technical Support directly at: US Toll Free: 1-866-229-3239 Toll Only:
Immune Therapy for Pancreatic Cancer December 16, 2014 If you experience technical difficulty during the presentation: Contact WebEx Technical Support directly at: US Toll Free: 1-866-229-3239 Toll Only:
Integrating Chemotherapy and Liver Surgery for the Management of Colorectal Metastases
 I Congresso de Oncologia D Or July 5-6, 2013 Integrating Chemotherapy and Liver Surgery for the Management of Colorectal Metastases Michael A. Choti, MD, MBA, FACS Department of Surgery Johns Hopkins University
I Congresso de Oncologia D Or July 5-6, 2013 Integrating Chemotherapy and Liver Surgery for the Management of Colorectal Metastases Michael A. Choti, MD, MBA, FACS Department of Surgery Johns Hopkins University
5 th Heidelberg Myeloma Workshop
 Scientific program 5 th Heidelberg Myeloma Workshop Current status and developments in diagnosis and therapy of Multiple Myeloma Congress venue: Lecture hall, Hospital for Internal Medicine Friday, 24
Scientific program 5 th Heidelberg Myeloma Workshop Current status and developments in diagnosis and therapy of Multiple Myeloma Congress venue: Lecture hall, Hospital for Internal Medicine Friday, 24
NCCN Prostate Cancer Early Detection Guideline
 NCCN Prostate Cancer Early Detection Guideline Joan McClure Senior Vice President National Comprehensive Cancer Network African American Prostate Cancer Disparity Summit September 22, 2006 Washington,
NCCN Prostate Cancer Early Detection Guideline Joan McClure Senior Vice President National Comprehensive Cancer Network African American Prostate Cancer Disparity Summit September 22, 2006 Washington,
Analysis of KRAS/NRAS and BRAF mutations in FIRE-3
 Analysis of KRAS/NRAS and BRAF mutations in FIRE-3 A randomized phase III study of FOLFIRI plus cetuximab or bevacizumab as first-line treatment for wild-type KRAS (exon 2) metastatic colorectal cancer
Analysis of KRAS/NRAS and BRAF mutations in FIRE-3 A randomized phase III study of FOLFIRI plus cetuximab or bevacizumab as first-line treatment for wild-type KRAS (exon 2) metastatic colorectal cancer
List of participating Local Clinical Centers (LCCs) As of September 2013
 List of participating Local Clinical Centers (LCCs) As of September 2013 GERMANY sorted by Federal State Baden-Wurttemberg LCC Ulm Universitätsklinikum Ulm Studienzentrale Innere II Albert-Einstein-Allee
List of participating Local Clinical Centers (LCCs) As of September 2013 GERMANY sorted by Federal State Baden-Wurttemberg LCC Ulm Universitätsklinikum Ulm Studienzentrale Innere II Albert-Einstein-Allee
WARNING LETTER. Multikine is an investigational new drug that does not have marketing authorization in the United States. (b) (4)
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Geert R. Kersten Director and Chief Executive Officer 8229 Boone
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Geert R. Kersten Director and Chief Executive Officer 8229 Boone
DETECT-Register DocumEnTation and Evaluation of a COPD Combination Therapy
 DETECT-Register DocumEnTation and Evaluation of a COPD Combination Therapy Status: Recruiting Study Phase: N/A Start Date: September 2015 Completion Date: December 2017 Condition(s): Chronic Obstructive
DETECT-Register DocumEnTation and Evaluation of a COPD Combination Therapy Status: Recruiting Study Phase: N/A Start Date: September 2015 Completion Date: December 2017 Condition(s): Chronic Obstructive
Clinical Trials Reporting Program (CTRP) and Clinicaltrials.gov (CT.gov) Helpful tips and guidance
 Clinical Trials Reporting Program (CTRP) and Clinicaltrials.gov (CT.gov) Helpful tips and guidance Objectives NCI s Clinical Trials Reporting Program (CTRP) Overview CTRP What is it? CTRP is an NCI mandated
Clinical Trials Reporting Program (CTRP) and Clinicaltrials.gov (CT.gov) Helpful tips and guidance Objectives NCI s Clinical Trials Reporting Program (CTRP) Overview CTRP What is it? CTRP is an NCI mandated
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Targeted Molecular Therapy for Renal Cell Carcinoma: Impact on Existing Treatment Paradigms
 Targeted Molecular Therapy for Renal Cell Carcinoma: Impact on Existing Treatment Paradigms Wolfram Samlowski, MD For a CME/CEU version of this article please go to www.namcp.org/cmeonline.htm, and then
Targeted Molecular Therapy for Renal Cell Carcinoma: Impact on Existing Treatment Paradigms Wolfram Samlowski, MD For a CME/CEU version of this article please go to www.namcp.org/cmeonline.htm, and then
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS
 MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
Introduction of a Standard Drug Formulary in Hospital Authority
 Introduction of a Standard Drug Formulary in Hospital Authority PURPOSE This paper seeks Members views on the introduction of a Standard Hospital Authority Drug Formulary ( ) ( Standard Drug Formulary
Introduction of a Standard Drug Formulary in Hospital Authority PURPOSE This paper seeks Members views on the introduction of a Standard Hospital Authority Drug Formulary ( ) ( Standard Drug Formulary
The Role, Position and Funding of Laboratory Diagnostics in the German Health Care System
 The Role, Position and Funding of Laboratory Diagnostics in the German Health Care System Warsaw, 18. May 2007 Dr. med. Lothar Krimmel Bioscientia Institute for Medical Diagnostics Ingelheim, Germany Key
The Role, Position and Funding of Laboratory Diagnostics in the German Health Care System Warsaw, 18. May 2007 Dr. med. Lothar Krimmel Bioscientia Institute for Medical Diagnostics Ingelheim, Germany Key
Governance and Performance Management of Public Employment Services in the United States and the European Union: Lessons and issues for discussion
 Governance and Performance Management of Public Employment Services in the United States and the European Union: Lessons and issues for discussion 3 4 September 2015 Globalisation and demographic changes
Governance and Performance Management of Public Employment Services in the United States and the European Union: Lessons and issues for discussion 3 4 September 2015 Globalisation and demographic changes
Immunotherapy of Uveal Melanoma
 Eye Am Not Alone (EANA) Patient Retreat Saturday Session - March 3, 2012 Immunotherapy of Uveal Melanoma Do not distribute or copy without the express permission of the Ocular Melanoma Foundation (OMF).
Eye Am Not Alone (EANA) Patient Retreat Saturday Session - March 3, 2012 Immunotherapy of Uveal Melanoma Do not distribute or copy without the express permission of the Ocular Melanoma Foundation (OMF).
Genomic Medicine The Future of Cancer Care. Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America
 Genomic Medicine The Future of Cancer Care Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America Personalized Medicine Personalized health care is a broad term for interventions
Genomic Medicine The Future of Cancer Care Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America Personalized Medicine Personalized health care is a broad term for interventions
Therapy of decompensated cirrhosis Pre-transplant for HBV and HCV
 Therapy of decompensated cirrhosis Pre-transplant for HBV and HCV Universitätsklinikum Leipzig Thomas Berg Sektion Hepatologie Klinik und Poliklinik für Gastroenterologie und Rheumatologie Leber- und Studienzentrum
Therapy of decompensated cirrhosis Pre-transplant for HBV and HCV Universitätsklinikum Leipzig Thomas Berg Sektion Hepatologie Klinik und Poliklinik für Gastroenterologie und Rheumatologie Leber- und Studienzentrum
Background. t 1/2 of 3.7 4.7 days allows once-daily dosing (1.5 mg) with consistent serum concentration 2,3 No interaction with CYP3A4 inhibitors 4
 Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
JAPANESE-GERMAN CENTER BERLIN [JDZB] in cooperation with FEDERAL MINISTRY OF EDUCATION AND RESEARCH P R O GRAM
![JAPANESE-GERMAN CENTER BERLIN [JDZB] in cooperation with FEDERAL MINISTRY OF EDUCATION AND RESEARCH P R O GRAM JAPANESE-GERMAN CENTER BERLIN [JDZB] in cooperation with FEDERAL MINISTRY OF EDUCATION AND RESEARCH P R O GRAM](/thumbs/31/14818756.jpg) JAPANESE-GERMAN CENTER BERLIN [JDZB] in cooperation with FEDERAL MINISTRY OF EDUCATION AND RESEARCH P R O GRAM for the 2013 Junior Experts Exchange Program in Germany June 6 to June 17, 2013 Thursday,
JAPANESE-GERMAN CENTER BERLIN [JDZB] in cooperation with FEDERAL MINISTRY OF EDUCATION AND RESEARCH P R O GRAM for the 2013 Junior Experts Exchange Program in Germany June 6 to June 17, 2013 Thursday,
REFERENCE CODE GDHC212DFR PUBLICAT ION DATE JUNE 2013 GSK1572932A (NON-SMALL CELL LUNG CANCER) FORECAST AND MARKET ANALYSIS TO 2022
 REFERENCE CODE GDHC212DFR PUBLICAT ION DATE JUNE 2013 GSK1572932A (NON-SMALL CELL LUNG CANCER) GSK1572932A (NON-SMALL CELL LUNG CANCER) - Executive Summary GSK1572932A (MAGE-A3): Key Metrics in NSCLC Markets
REFERENCE CODE GDHC212DFR PUBLICAT ION DATE JUNE 2013 GSK1572932A (NON-SMALL CELL LUNG CANCER) GSK1572932A (NON-SMALL CELL LUNG CANCER) - Executive Summary GSK1572932A (MAGE-A3): Key Metrics in NSCLC Markets
Managing Data and Providing Competitive Insights from Clinical Trials Using BizInt Smart Charts
 Managing Data and Providing Competitive Insights from Clinical Trials Using BizInt Smart Charts Pharma-Bio-Med 2011 - Venice, Italy 21 Nov 2011 Diane Webb, President, BizInt Solutions Jennifer Friend-Huizer,
Managing Data and Providing Competitive Insights from Clinical Trials Using BizInt Smart Charts Pharma-Bio-Med 2011 - Venice, Italy 21 Nov 2011 Diane Webb, President, BizInt Solutions Jennifer Friend-Huizer,
Carcinoma papilar renal, cromófobo y otras histologías. Maria José Méndez Vidal Servicio de oncología Medica Hospital Reina Sofía Córdoba
 Carcinoma papilar renal, cromófobo y otras histologías. Maria José Méndez Vidal Servicio de oncología Medica Hospital Reina Sofía Córdoba Europe 121 629 new cases RCC 2012, 75 676 affected men Slide 3
Carcinoma papilar renal, cromófobo y otras histologías. Maria José Méndez Vidal Servicio de oncología Medica Hospital Reina Sofía Córdoba Europe 121 629 new cases RCC 2012, 75 676 affected men Slide 3
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Successes and Limitations of Targeted Therapies in Renal Cell Carcinoma
 g Tumor Res. Basel, Karger, 2014, vol 41, pp 98 112 (DOI: 10.1159/000355906) Successes and Limitations of Targeted Therapies in Renal Cell Carcinoma Marc Pracht Dominik Berthold Medical Oncology Unit,
g Tumor Res. Basel, Karger, 2014, vol 41, pp 98 112 (DOI: 10.1159/000355906) Successes and Limitations of Targeted Therapies in Renal Cell Carcinoma Marc Pracht Dominik Berthold Medical Oncology Unit,
REF/2011/06/002450 CTRI Website URL - http://ctri.nic.in
 Clinical Trial Details (PDF Generation Date :- Thu, 14 Jul 2016 07:52:01 GMT) CTRI Number Last Modified On 08/07/2013 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Clinical Trial Details (PDF Generation Date :- Thu, 14 Jul 2016 07:52:01 GMT) CTRI Number Last Modified On 08/07/2013 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Sorafenib. Bernard ESCUDIER Institut Gustave Roussy Villejuif, France
 Sorafenib for renal cell carcinoma Bernard ESCUDIER Institut Gustave Roussy Villejuif, France Renal Cell Carcinoma: Drugs and Targets pvhl = HIFα CCI-779 Bevacizumab VEGF KDR PDGF PDGFR TGFα EGFR Sunitinib,
Sorafenib for renal cell carcinoma Bernard ESCUDIER Institut Gustave Roussy Villejuif, France Renal Cell Carcinoma: Drugs and Targets pvhl = HIFα CCI-779 Bevacizumab VEGF KDR PDGF PDGFR TGFα EGFR Sunitinib,
Prostate Cancer Studies
 Prostate Cancer Studies STUDIES CURRENTLY RECRUITING MDV3100-14 A multinational, Phase 3, Randomized, Double- Blind, Placebo-Controlled, Efficacy and Safety Study of Enzalutamide in Patients With Nonmetastic
Prostate Cancer Studies STUDIES CURRENTLY RECRUITING MDV3100-14 A multinational, Phase 3, Randomized, Double- Blind, Placebo-Controlled, Efficacy and Safety Study of Enzalutamide in Patients With Nonmetastic
Seton Medical Center Hepatocellular Carcinoma Patterns of Care Study Rate of Treatment with Chemoembolization 2007 2012 N = 50
 General Data Seton Medical Center Hepatocellular Carcinoma Patterns of Care Study Rate of Treatment with Chemoembolization 2007 2012 N = 50 The vast majority of the patients in this study were diagnosed
General Data Seton Medical Center Hepatocellular Carcinoma Patterns of Care Study Rate of Treatment with Chemoembolization 2007 2012 N = 50 The vast majority of the patients in this study were diagnosed
German International Student Barometer, D ISB, Academic Year 2011/12 Executive Summary
 German International Student Barometer, D ISB, Academic Year 2011/12 Executive Summary Background The German International Student Barometer (D ISB) ran from 24 October to 9 December 2011, at 52 Hochschulen
German International Student Barometer, D ISB, Academic Year 2011/12 Executive Summary Background The German International Student Barometer (D ISB) ran from 24 October to 9 December 2011, at 52 Hochschulen
GOOD CLINICAL PRACTICE PROGRAM. Workshop 2006 DC THERA. November 27th 28th 2006, Dorint Hotel Erlangen, Germany
 DC THERA Dendritic Cells & New Immunotherapies EU Sixth Framework Programme Life Science, Genomics and Biotechnology for Health DC-THERA Dendritic Cells & New Immunotherapies Network of Excellence, Project
DC THERA Dendritic Cells & New Immunotherapies EU Sixth Framework Programme Life Science, Genomics and Biotechnology for Health DC-THERA Dendritic Cells & New Immunotherapies Network of Excellence, Project
