Rituximab in non-hodgkin Lymphoma (NHL)
|
|
|
- Elijah Howard
- 10 years ago
- Views:
Transcription
1 Original Article ISSN: X Rituximab in non-hodgkin Lymphoma (NHL) Manal Elhabbash MD 1, Abukris Alwindi MD 2 (1) Assistant Professor, Tripoli University, (2) Medical consultant in oncology & hematology department Correspondence Author Manal Elhabbash MD Assistant Professor, Hematology and Oncology Department Tripoli Medical Center, Tripoli University, Tripoli, Libya Tel: [email protected] Key words: Non-Hodgkin Lymphoma (NHL), Rituximab, Overall response rate (ORR) Abstract Purpose: To study epidemiology of NHL in Tripoli Medical Center (TMC), to assess response rate to rituximab in CD20 positive NHL patients and compare our results with international results. Methods : Retrospective non-randomized study includes all patients registered as non Hodgkin Lymphoma in oncology clinic in TMC. 88 patients were registered in the period between Jan 2011 and Dec patients with CD20 positive B cell Non Hodgkin Lymphoma. Results: Median age was 56.5 years, SD± /88 (70%) were high grade NHL. 19/88 (21.3%) were low grade NHL. 5/88(5.7%) were T cell lymphomas. 70/88 (79.5%) were CD20 positive B cell type. 60/70 (86%) patients with CD20 positive B cell lymphomas received Rituximab either with CHOP or CVP. 43.3% were male and 56.7% were female. 23/60 (38.3%) have extra nodal lymphoma. Stomach is being the most common extra nodal organ involved in (56.2%). 37/60(61.7%) were nodal lymphoma. 48/60 (80%) were aggressive large B cell lymphomas, 12/60(20%)were low grade NHL. 7/60 (11.7%) of patients have bone marrow infiltration. In nodal lymphomas, 43.2% were stage III. According to International Prognostic Index (IPI) 41.7% of patients was low risk, 39%were intermediate risk and 16.7% were high risk. 31/37 (83.8%) received Rituximab as first line. The mean number of cycles was 6. Overall response rate was 89.2%, 64.9% of patients had complete response and 2 had partial response. No statistical difference in overall response rate between aggressive NHL and low grade lymphoma were noticed( p=0.2). In extra nodal NHL 87% were stage I. All received Rituximab as first line. Overall response rate was 82.6%, with 52.2% complete response and 30.4% partial response. Conclusion: Our results show that Rituximab had high response rate comparable with international results. More time is needed to assess event free survival and overall survival. Introduction Non-Hodgkin's lymphoma (NHL) is the most common hematological cancer in adults. (1, 2). NHL encompasses a heterogeneous group of lymphomas that have been classified in various ways. In 1995, the World Health Organization developed a classification that included a combination of morphology, immunotyping, genetic features, and clinical syndromes. The goal was to define disease entities of B cells, T cells, and natural killer (NK) cells that pathologists could recognize and had clinical relevance. Lymphomas were further subdivided into categories based on their behavior {indolent, aggressive or highly aggressive ). (3.4) B-cell lymphomas account for about 85% of all NHL diagnosis.(5) NHL are grouped as either indolent subtypes whish has prolonged median survival but considered incurable or aggressive subtypes whish has rapid growth with potential intent for cure. Conventional method of treatment including chemotherapy and radiotherapy. Cell surface proteins such as CD19, CD20, and CD 22 are highly expressed on B- cell lymphoma and represent key potential targets for treatment. Antibody therapy directed against CD 20 has had the most important clinical impact to date.
2 Rituximab is a chimeric antibody directed against the CD20 antigen which is a 297 amino acid phosphoprotein (33-35KD) found on the surface of B cells. CD 20 is highly expressed on the surface of B cells but not on stem cells, Pro-B cells, plasma cells or other cell types. It is the first monoclonal antibody licensed for treatment of NHL; it has been approved by Food and Drug Administration (FDA) in USA in Nov CD 20 is a transmembrane surface antigens expressed only by B-cell precursors and mature B cell. It is involved in the regulation of B-lymphocyte growth and differentiation. CD20 is expressed on more than 85% of B cell in NHL but not on stem cells or normal mature plasma cells or other normal tissues, and lost when normal B cells differentiate into antibody secreting plasma cells. (6,7). CD20 is present on malignant plasma cells in 20 % of patients with Multiple Myeloma and up to 50% of patients with plasma cell leukemia and 75%-100% of patients with Waldenstrom's macroglobulinaemia (8). CD20 positive cell can totally be eradicated without causing specific toxicity because normal B cell will re-emerge following differentiation from stem cells. CD20 is not internalized after binding to antibody, then anti CD20 antibody initiate immune response and apoptosis. Rituximab is genetically engineered human/mouse chimeric monoclonal antibody, that is specific for CD20 B cell surface antigen. (9) Rituximab consists of human IgG1, kappa constant region with variable region isolated from murine anti- CD20 antibody. It consists of two heavy chains and two light chains with molecular weight of 145 KD. Rituximab has low potential for immunogenicity, because the majority of the molecules are of human origin. Rituximab effectively reduces the circulating B-cell count in lymphoma patients by complement medicated cytotoxicity, Antibody-dependent cellular cytotoxicity (ADCC) and induction of apoptosis. Rituximab binds directly the C1q complement component, initiating complement mediated lysis of circulated B cell (9). Rituximab binds strongly to FC receptor on Macrophage and natural killer cells inducing ADCC (10). Rituximab induces apoptosis. (10) Recent studies suggest that complement - dependant cytotoxicity may be more important than ADCC. (11) Rituximab may be effective in patients who have failed to respond to chemotherapy or who have relapsed after chemotherapy. Regarding toxicity, no significant toxicological effects were observed at various doses and schedules. Only B cell depletion was observed and time to recovery needs 3 months with partial recovery most commonly occurring after 4-8 weeks. Pan Arab Journal of Oncology/Vol. 8/No. 2/ June 2015 More than 300,000 patients worldwide have been treated with rituximab. It is a well tolerated treatment. Rituximab administration is not associated with severe hematological or other adverse events, commonly seen with chemotherapy. Patients may experience infusion related reaction like fever and chills during the first 2 hour of the first infusion, these decrease substantially with subsequent infusions. Other side effects include, dyspnea, often with bronchospasm and hypoxia, flushing, angioedema, nausea, urticarial, rash, headache, throat irritation, rhinitis, vomiting and tumor pain. In 10% of patients these events are accompanied by hypotension. Tumor lysis syndrome has also been reported following rituximab administration. (12) Despite profound B cell depletion, the incidence of infection is not increased compared with chemotherapy alone. The (GELA -LNH-98.5) study, showed no additional toxicity with chemotherapy (13) Patients and method Retrospective non-randomized study includes all patients registered as Non-Hodgkin Lymphoma in oncology clinic in Tripoli Medical Center.88 patients were registered in the period between Jan 2011 and Dec patients with CD20 positive B cell Non-Hodgkin Lymphoma proved by immunohistochemistry had received Rituximab with chemotherapy. Study includes patient's characteristics as sex, age, extranodal or nodal involvement, stages of disease at diagnosis according to Ann Arbor staging System, and international Prognostic Index (age, LDH, more than 2 extranodal involvements, advanced stages as III, IV.). Histopathology depends on cell morphology and immune stain according WHO classification system. Rituximab was given to CD20 positive B cell NHL as 375 mg/ m 2 every 3 weeks for 6-8 cycles with chemotherapy as CHOP or CVP protocol. CHOP represent cyclophos-phamide, Adrimycin, Vincristine and predinsolone and CVP protocol stand for cyclophosphamide, vincrestine and predinsolone as first line treatment. Response was assessed by clinical examination and CT scan chest and abdomen as complete response or partial response. Disease free survival and overall survival was studied and we need more time to assess these variable. Statistical analysis: data was analyzed using SPSS computer software package, and Kaplan Meier curves used for survival analysis. Results 88 patients were diagnosed as Non-Hodgkin lymphoma in the period between Jan 2011-Dec Table (1) shows patients characteristics. Median age was (56.5 years, SD± 16.3), and mean age was 55.5 years with standard error of /88 (70%) were high grade NHL. 19/88 (21.3%) were low grade NHL as follicular, MALT cell, and small cell. 5/88 (5.7%) were
3 Table 1. Patients characteristics Nodal Extranodal Number of patients Median age (years) Male :female ratio 1:1.5 1:1.1 Sweating 32.4% 8.7% Fever 37.8% Wight loss 32.4% 39.1% Itching 10.8% - Peripheral lymphadenopathy 34/37 (91.9%) Gastric 56.5% Bone 13% Thyroid Nasopharynx Mediastinal lymphadenopathy 10/37 (27%) Small intestine Anorectal Soft tissue 8.6% Bulky mediastinum 10/37 (27%) - Splenomegaly 4/37 (10.8%) - Hepatomegaly 6/37 (16.2%) - Tonsil involvement 2/37 (5.4%) - Leukocytosis 7/37 (18.9%) 3/23 (13%) Median hemoglobin concentration 11.5 g/dl 11.8 g/dl ESR> 50mm/hour 7/37 (18.9%) 6/23 (26%) LDH high 19/37 (51.4%) 7/23 (30.7%) Bone marrow involvement 18.9% Stages according to Ann Arbor Stage I a Ib IIa IIb IIIa IIIb IVa IVb International prognostic index *low risk *intermediate risk *high risk Rituximab with chemotherapy as first line Rituximab with chemotherapy as second line Overall response rate *Complete response *Partial response 13.5% 5.4% 8.1% 5.4% 18.9% 2 8.1% 13.5% 41.7% 39% 16.7% 31/37 (83.8%) 6+2 (who relapsed after failure of first line) 89.2% 64.8% 24.4% Death 8/37(21.6%) 2/8 (25%) unrelated cause 56.5% 30.4% - 23/23 19/23(82.6%) 52.2% 30.4% 6/23 (26%)
4 T cell lymphomas. 70/88 (79.5%) are CD20 positive B cell type. 60/70 (85.7%) patients with CD20 positive B cell received Rituximab as first line with chemotherapy or after relapse after chemotherapy. 6/70 (8.6%) received chemotherapy only, 4/70 (5.7%) were unfit to receive any treatment. The 60 patients who were CD20 positive who received Rituximab, 26/60 (43.3%) were male, and 34/60 (56.7%) were female. 37/60 (61.7%) had nodal involvement and 23/60 (38.3%) had extra nodal involvement. Among patients with nodal involvement (37/60), the median age was 57 years and mean age was years. 22/37(59.5%) were female and 15/37(40.5%) were males. The main symptoms were as following: 32.4% had sweating, 37.8% had fever, 32.4% had history of weight loss, and 10.8% had itching. 34/37 (91.9%) present with peripheral lymphadenopathy, 10/37 (27%) had mediastinal lymphadenopathy with 4/10 (40%) bulky mediastinum. 4/37(10.8%) had splenomegaly, 6/37 (16.2%) had hepatomegaly and 2/37 (5.4%) had tonsils involvement. 7/37 (18.9%) had leukocytosis, median hemoglobin concentration was 11.5 g/dl, 7/37 (18.9%) had high ESR > 50 mm/1 st hour, and LDH were high in 19/37 (51.4%). 27/37 (73%) had aggressive lymphoma high grade NHL. 22/27 (81.4%) had diffuse large cell B cell type. 10/37 (27%) had indolent lymphoma low grade NHL, 3/10 (30%) had follicular subtype. According to Ann Arbor staging system; stages of patients were as follows Ia 13.5%, Ib 5.4%, IIa 8.1%, IIb 5.4%, IIIa 18.9%, IIIb 2, Iva 8.1%, IVb 13.5%. According to International Prognostic Index IPI, 41.7% were low risk, 39% were intermediate risk and 16.7% were high risk. 6/37 (18.9%) had positive bone marrow. 31/37 (83.8%) received rituximab as first line with chemotherapy as CHOP Cyclophosphamide, Vincristine, Adriamycin and prednisolone or CVP Cyclophosphamide, Vincristine, and prednisolone with mean Number of cycles was 6. Overall response rate (ORR) was 89.2%, with complete response in 64.8% and 2 had partial response. Among responders 2 patients relapsed. 8 patients received rituximab as second line 7/8 (87.5%) had response, 3/8 (37.5%) had complete response, and 4/8 (50%) had partial response. No statistical difference in overall response rate between aggressive non-hodgkin lymphoma and low grade Non Hodgkin Lymphoma p=0.2. 8/37(21.6%) patients died during follow up, 2 had unrelated death. Among extra anodal lymphomas, 23 patients with mean and Pan Arab Journal of Oncology/Vol. 8/No. 2/ June 2015 median age 54 years.12/23 (52.2%) were female and 11/23 (47.8%) were males. Main symptoms were as following: 2/23 (8.7%) had sweating, 1/23 () had fever, and 10/23(39.1%) had history of weight loss. Gastric NHL was the most common extra nodal involvement in 13/23(56.5%), bone in 3/23 (13%), thyroid in 2/23(8.7%), nasopharynx in 1/23 (), small intestine in1/23(), anorectal in 1/23(), and 2/23(8.6%) had soft tissue lymphoma. Regarding laboratory investigation mean hemoglobin was 11.8g/dl, 3/23 (13%) had leukocytosis, high ESR >50 mm/ hour in 6/23 (26%), and 7/23 (30.4%) had high LDH. Histological examination was 21/23(91.3%) has aggressive large cell B cell lymphoma, 2/23(8.7%) had low grade NHL of MALToma type. According to Ann Arbor staging system; stages of patients were as following Iea 56.5%, Ieb 30.4%, IIea, IIeb, IVea, IVeb. Among these patients 1/23 () had bone marrow infiltration. Rituximab was given in combination with chemotherapy as CHOP or CVP for all patients with extranodal lymphoma. Overall response rate was 19/23 (82.6%), with complete response in 52.2% and partial response in 30.4%. Among responders 2 patients had relapse. During follow up 6 patients had died. Progression free survival among all treated 60 patients (nodal and extranodal) at 3 years was 60% shown in Figure (1). Overall survival at 5 years was 40% Figure (2). Discussion Non-Hodgkin's lymphoma is a composite lymphoid malignancy with increased annual rate of 4% to 7% over the last 20 years in both USA and Europe (14) Low grade Non-Hodgkin lymphoma accounts approximately 40% of the incidence of NHL in the United State. While patients with intermediate and high grade are potentially curable with combination chemotherapy, low grade Non-Hodgkin's lymphoma are still considered to be essentially incurable with standard therapy, they respond to treatment but follows a course of recurrent relapse and shorter remission. Median survival for low grade lymphoma is 6.2 years and 5 years from time of first relapse. Diffuse large B cell Lymphoma is the most frequent, represent-
5 Figure 1. Overall progression free survival among all patients with NHL. Figure 2. Overall survival among all treated patients with NHL ing 40% of all lymphomas. For more than 25 years, CHOP has been the standard treatment of aggressive NHL but less than 50% of patients were cured. Vose et al. conducted a phase 2 study of Rituximab with CHOP chemotherapy in 33 previously untreated patients with advanced stage aggressive B cell lymphoma.the ORR was 94%; 61% of patients had complete response and 33% had partial response. (15). Eight cycles of Rituximab and CHOP are now the standard first line treatment for patients between years with aggressive NHL (previously untreated ) based on The Groupe d'etude des lymphomas de L'dulte (GELA.LNH-98.5) study (16). Rituximab is given in 375 mg/m2 on day 1 in each of eight cycles of chemotherapy every 3 weeks, the result were as follow: overall response rate was 63% in CHOP arm and 75% in CHOP and Rituximab (p=.005). Overall survival was as 57 months in CHOP arm vs. 70 months in CHOP and Rituximab arm. p= (0.007).(17) Subsequent analysis at 3 and 4 years follow- up confirmed that the significant survival advantage for Rituximab and CHOP was maintained. The addition of rituximab to CHOP increases the number of patients being cured of their disease. (17) According to International Prognostic Index (IPI), rituximab &CHOP significantly improved response rate, event free survival, and overall survival rate compared with CHOP alone in low risk as well as high risk patients. (17). Based on GELA-LNH 98.5 trial (British-Colombia cancer Agency) implemented a new policy recommending that 8 dose of rituximab &CHOP should be given to all newly diagnosed patients with aggressive NHL (18). Indolent type NHL follows a chronic relapsing and remitting course and remains incurable with chemotherapy. Thus, while the disease is responsive to conventional chemotherapy, no chemotherapy regimens have an impact on overall survival. Many patients with asymptomatic diseases may not be treated until their disease progresses, without any detrimental effect on survival (19). One of the first clinical trials of Rituximab plus chemotherapy was a phase II study (20), which used Rituximab plus CHOP chemotherapy, which showed complete response in 58% and partial response in 42%, and median time to progression was more than 7 years, then two large prospective phase III randomized trials have confirmed that addition of rituximab to chemotherapy yielded major improvement in overall response, complete response rate, and progression free survival. In Marcus et al study (21), 321 patients with advanced follicular lymphoma were treated with 8 cycles of CVP (Cyclophosphamide, Vincristine and prednisolone) plus Rituximab. The primary end point was time to treatment failure.at median follow- up of 18 months, time to treatment failure was significantly longer for patients treated with Rituximab plus CVP compared with CVP alone (26 months Vs 7 months P < ). Patients with Low grade Non-Hodgkin's Lymphoma treated with Rituximab plus CHOP Chemotherapy showed prolonged clinical and molecular remission. In one study, 9 years follow- up of 38 patients with NHL
6 previously untreated were included. Overall response rate was 100%. 87% of patients achieved a complete response. The median time to progression was 82.3 months. (22). Marcus et al 2005 (23) showed that overall and complete response rate were (81%, and 41%) in R-CVP arm versus (57% and 10%) in CVP arm (p=0.0001). Median follow- up of 30 months, median time to treatment failure was 27 months in patients receiving R - CVP and 7 month in patients receiving CVP alone.the addition of rituximab to CVP significantly improved the clinical outcome in patients with previously untreated advanced follicular Lymphoma, with- out increased toxicity. Another large randomized trial conducted by the German low grade study Group has compared rituximab plus CHOP with CHOP alone in previously untreated Follicular lymphoma patients which showed increased time to treatment failure, improvement in progression free survival and overall survival in the rituximab plus CHOP arm (24). Regarding our patients, 54 patients had received rituximab as first line with either CHOP or CVP,31 patient with nodal involvement and 23 patients with extranodal lymphoma. Overall response rate was 85%, complete response in 58% and partial response in 27% Conclusion Rituximab has changed the treatment paradigms and outcomes for all CD20+ NHL and represents the most noteworthy advance in lymphoma treatment over the past decade. In patients with NHL,the addition of rituximab to standard treatment significantly enhanced response to therapy and overall outcomes. Rituximab is currently approved for treatment of relapsed and refractory indolent lymphoma as single agent and in combination with chemotherapy, and approved in patients with aggressive lymphoma with chemotherapy in previously treated and untreated patients in both nodal and extranodal sites. References 1. Dotan E, Aggarwal C, Smith M. Impact of Rituximab on the treatment of B-cell Non-Hodgkin's Lymphoma. A peer reviewed journal for managed care and hospital formulary management 2010; 35(3): Jemal A, Siegal R, Ward E. Cancer statistics. Cancer J Clin. 2008; 58(2): Rudiger T, Muller-Hermelink HK. WHO classification of malignant lymphomas. Radiologe. 2002; 42(12): Harris NL, Jaffe ES, Diebold J, et al. The World Pan Arab Journal of Oncology/Vol. 8/No. 2/ June 2015 Organization Classification of Neoplastic Diseases of the Hematopoietic and Lymphoid Tissue. Report of the clinical Advisory Committee meeting.airlie House Virginia, Nov 1997; Ann Oncol; pp Armitage JO, Weisenburgrer DD. New approach to classify non-hodgkin's Lymphoma: Clinical features of the major histologic subtype. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998; 16(8) : Nadler LM, Ritz J, Hardy R, et al. A unique cell surface antigen identifying lymphoid malignancies of B cell origin. J Clin Invest 1981; 67: Anderson KC, Bates MP, Slaughenhoupt BL, et al. Expression of human B-cell associated antigens on Leukemia and Lymphoma: a model of human B cell differentiation. Blood 1984; 63: Treon SP, Kelliher A, Keele B et al. Expression of serotherapy target antigens in Waldenstrom's macroglobulinaemia: therapeutic application and consideration. Semin Oncol 2003; 30: Reff ME, Carner K, Chambers KS, PC, et al. Depletion of B cell in vivo by a Chimeric mouse human monoclonal antibody to CD20+. Blood 1994; 83: Maloney DG, Grillo-Lopez AJ, White CA et al. The antitumor effect of monoclonal anti-cd20 antibody (mab) therapy include direct anti-proliferative activity and induction of apoptosis in CD 20 positive non- Hodgkin's lymphoma cell lines. Blood 1996; 88 (suppl. 1):637a. 11. Di Gaetano N, Cittera E, Nota R, et al. Complement activation determines the therapeutics activity of Rituximab in vivo. J Immunol 2003; 171: Coiffier B, Lepage E, Briere J et al. CHOP chemotherapy plus Rituximab compared with CHOP alone in elderly patients with diffuse large B-cell lymphoma. N Engl J Med 2002; 346: Czuczman MS., Alexandra F, Alice M. Rituximab in combination with CHOP or Fludarabine in low grade lymphoma,seminar in oncology 2002; 29: Czuczman MS, Fallon A, Moher A, et al. Rituximab in combination with CHOP or Fludarabine in low grade lymphoma. Sem. Oncol. 2002; 29: Vose JM, Link BK, Grossbared ML, et al. Phase II study of Rituximab in combination with CHOP chemotherapy in patients with previously untreated aggressive Non- Hodgkin's lymphoma. J Clin Oncol 2001; 19(2): Kimby E, Geisler C., Hagberg H, et al. Rituximab as single agent and in combination with interferon-αfollicular or other low grade lymphoma. A randomised phase II study. Ann. Oncol. 2002; 13 (suppl. 2): Coiffier B, Herbrecht R, Morel P, et al. GELA study comparing CHOP and R-CHOP in elderly patients
7 with DLCL: 3 years median follow-up with an analysis according to co-morbidity factors. Hematol. J. 2003; 4 (suppl. 2): Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP Rituximab therapy dramatically improved outcome of diffuse large B- cell lymphoma (DLBC) in British Columbia. J Clin Oncol 2003; 2005: Ardeshna KM, Smith P, Norton A, et al. Long term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-hodgkin's lymphoma: a randomized controlled trial. Lancet 2003; 362: Czuczman MS, Grillo-López AJ, White CA, et al. Treatment of patients with low grade B cell lymphoma with the combination of chimeric anti-cd20 monoclonal antibody and CHOP chemotherapy. J. Clin. Oncol. 1999; 17: Marcus R, Imrie K, Belch A, et al. M39021-an international multicenter randomised, open label phase trial comparing Rituximab added to CVP chemotherapy alone in untreated stage III/IV follicular non-hodgkin's lymphoma: final analysis. Blood 2003; 102:28a. 22. Czuczman MS, Weaver R, Alkuzweny B, et al. Prolonged clinical and molecular remission in patients with low grade or follicular non Hodgkin's Lymphoma treated with Rituximab plus CHOP chemotherapy: 9 years follow-up. J. Clin. Oncol. 2004; 22; Marcus R, Imrie K, Blech A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood 2005; 105: Hiddemann W, Dreyling MH, Forstpointner R., et al. Combined immunochemotherapy (R-CHOP) significantly improve time to treatment failure in first line therapy of follicular lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). Blood 2003; 102:104a.
CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY
 CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY 26.1 Introduction rituximab Subsequent to the completion of drafts for the guidelines earlier in 2004,
CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY 26.1 Introduction rituximab Subsequent to the completion of drafts for the guidelines earlier in 2004,
MALIGNANT LYMPHOMAS. Dr. Olga Vujovic (Updated August 2010)
 MALIGNANT LYMPHOMAS Dr. Olga Vujovic (Updated August 2010) Malignant lymphomas consist of Hodgkin and non-hodgkin lymphomas. The current management of these diseases involves a multi-disciplinary approach.
MALIGNANT LYMPHOMAS Dr. Olga Vujovic (Updated August 2010) Malignant lymphomas consist of Hodgkin and non-hodgkin lymphomas. The current management of these diseases involves a multi-disciplinary approach.
Aggressive lymphomas. Michael Crump Princess Margaret Hospital
 Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on
 A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on examination. She also has fever, weight loss, and sweats. What
A 32 year old woman comes to your clinic with neck masses for the last several weeks. Masses are discrete, non matted, firm and rubbery on examination. She also has fever, weight loss, and sweats. What
FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma
 Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
What is non-hodgkin lymphoma, how is it treated, and what is the unmet need?
 What is non-hodgkin lymphoma, how is it treated, and what is the unmet need? Tim Illidge BSc PhD MRCP FRCR FRCPath Institute of Cancer Sciences, University of Manchester Manchester Cancer Research Centre,
What is non-hodgkin lymphoma, how is it treated, and what is the unmet need? Tim Illidge BSc PhD MRCP FRCR FRCPath Institute of Cancer Sciences, University of Manchester Manchester Cancer Research Centre,
Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma
 a report by Martin Dreyling Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma Head, Lymphoma Section, Department of Medicine III, University Hospital Großhadern, Ludwig Maximilians-University
a report by Martin Dreyling Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma Head, Lymphoma Section, Department of Medicine III, University Hospital Großhadern, Ludwig Maximilians-University
Lymphoma Diagnosis and Classification
 Lymphoma Diagnosis and Classification By Atef Shrit, MD, Pathology B- and T/NK-cell lymphomas are clonal neoplasms of immature and mature B-lymphocytes, T-lymphocytes or natural killer cells at various
Lymphoma Diagnosis and Classification By Atef Shrit, MD, Pathology B- and T/NK-cell lymphomas are clonal neoplasms of immature and mature B-lymphocytes, T-lymphocytes or natural killer cells at various
Guidelines for the Management of Follicular Lymphoma
 Guidelines for the Management of Follicular Lymphoma Scope The following guidance for first- and second-line therapy applies to follicular lymphoma histological grades 1, 2 and 3a according to the World
Guidelines for the Management of Follicular Lymphoma Scope The following guidance for first- and second-line therapy applies to follicular lymphoma histological grades 1, 2 and 3a according to the World
Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy
 Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy Yoichi Kitamura, MD Kazuhiko Hayashi, MD Kazumi Uchida,
Two Retroperitoneal Low-Grade B-Cell Lymphoma Successfully Treated With a Combination of Chimeric Anti-CD20 Monoclonal Antibody and CHOP Chemotherapy Yoichi Kitamura, MD Kazuhiko Hayashi, MD Kazumi Uchida,
Mantle Cell Lymphoma Understanding Your Treatment Options
 New Developments in Mantle Cell Lymphoma John P. Leonard, M.D. Richard T. Silver Distinguished Professor of Hematology and Medical Oncology Associate Dean for Clinical Research Vice Chairman, Department
New Developments in Mantle Cell Lymphoma John P. Leonard, M.D. Richard T. Silver Distinguished Professor of Hematology and Medical Oncology Associate Dean for Clinical Research Vice Chairman, Department
David Loew, LCL MabThera
 MabThera The star continues to rise David Loew, LCL MabThera MabThera the star continues to raise Group sales (CHF bn) 4,5 4,0 3,5 3,0 2,5 2,0 1,5 1,0 0,5 0,0 2001 2002 2003 2004 2005 Outstanding clinical
MabThera The star continues to rise David Loew, LCL MabThera MabThera the star continues to raise Group sales (CHF bn) 4,5 4,0 3,5 3,0 2,5 2,0 1,5 1,0 0,5 0,0 2001 2002 2003 2004 2005 Outstanding clinical
Lauren Berger: Why is it so important for patients to get an accurate diagnosis of their blood cancer subtype?
 Hello, I m Lauren Berger and I m the Senior Director of Patient Services Programs at The Leukemia & Lymphoma Society. I m pleased to welcome Dr. Rebecca Elstrom. Dr. Elstrom is an Assistant Professor in
Hello, I m Lauren Berger and I m the Senior Director of Patient Services Programs at The Leukemia & Lymphoma Society. I m pleased to welcome Dr. Rebecca Elstrom. Dr. Elstrom is an Assistant Professor in
Guidelines for the use of Rituximab in Non-Hodgkin s Lymphoma QEII Health Sciences Centre
 Guidelines for the use of Rituximab in Non-Hodgkin s Lymphoma QEII Health Sciences Centre Background Non-Hodgkin s lymphoma (NHL) makes up approximately 85% of all lymphomas. They are a heterogeneous collection
Guidelines for the use of Rituximab in Non-Hodgkin s Lymphoma QEII Health Sciences Centre Background Non-Hodgkin s lymphoma (NHL) makes up approximately 85% of all lymphomas. They are a heterogeneous collection
Malignant Lymphomas and Plasma Cell Myeloma
 Malignant Lymphomas and Plasma Cell Myeloma Dr. Bruce F. Burns Dept. of Pathology and Lab Medicine Overview definitions - lymphoma lymphoproliferative disorder plasma cell myeloma pathogenesis - translocations
Malignant Lymphomas and Plasma Cell Myeloma Dr. Bruce F. Burns Dept. of Pathology and Lab Medicine Overview definitions - lymphoma lymphoproliferative disorder plasma cell myeloma pathogenesis - translocations
Frequency of NHL Subtypes in Adults
 Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Non-Hodgkin s lymphomas (NHLs) are a
 Oncology 33 Non-Hodgkin s lymphoma in the elderly The incidence of non-hodgkin s lymphoma (NHL) is increasing, and this increase is even more rapid in the older population. Although treatment of NHL in
Oncology 33 Non-Hodgkin s lymphoma in the elderly The incidence of non-hodgkin s lymphoma (NHL) is increasing, and this increase is even more rapid in the older population. Although treatment of NHL in
Treatment of low-grade non-hodgkin lymphoma
 Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003)
 Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
Update in Hematology Oncology Targeted Therapies. Mark Holguin
 Update in Hematology Oncology Targeted Therapies Mark Holguin 25 years ago Why I chose oncology People How to help people with possibly the most difficult thing they may have to deal with Science Turning
Update in Hematology Oncology Targeted Therapies Mark Holguin 25 years ago Why I chose oncology People How to help people with possibly the most difficult thing they may have to deal with Science Turning
LYMPHOMA. BACHIR ALOBEID, M.D. HEMATOPATHOLOGY DIVISION PATHOLOGY DEPARTMENT Columbia University/ College of Physicians & Surgeons
 LYMPHOMA BACHIR ALOBEID, M.D. HEMATOPATHOLOGY DIVISION PATHOLOGY DEPARTMENT Columbia University/ College of Physicians & Surgeons Normal development of lymphocytes Lymphocyte proliferation and differentiation:
LYMPHOMA BACHIR ALOBEID, M.D. HEMATOPATHOLOGY DIVISION PATHOLOGY DEPARTMENT Columbia University/ College of Physicians & Surgeons Normal development of lymphocytes Lymphocyte proliferation and differentiation:
Hodgkin Lymphoma Disease Specific Biology and Treatment Options. John Kuruvilla
 Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
New Targets and Treatments for Follicular Lymphoma. Disclosures
 Winship Cancer Institute of Emory University New Targets and Treatments for Follicular Lymphoma Jonathon B. Cohen, MD, MS Assistant Professor Div of BMT, Emory University Disclosures Consulting fees from:
Winship Cancer Institute of Emory University New Targets and Treatments for Follicular Lymphoma Jonathon B. Cohen, MD, MS Assistant Professor Div of BMT, Emory University Disclosures Consulting fees from:
J Clin Oncol 23:8447-8452. 2005 by American Society of Clinical Oncology INTRODUCTION
 VOLUME 23 NUMBER 33 NOVEMBER 20 2005 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T New Treatment Options Have Changed the Survival of Patients With Follicular Lymphoma Richard I. Fisher, Michael
VOLUME 23 NUMBER 33 NOVEMBER 20 2005 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T New Treatment Options Have Changed the Survival of Patients With Follicular Lymphoma Richard I. Fisher, Michael
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
Rituximab in Non - Hodgkins Lymphoma. Fatima Bassa, Dept. of Haematology October 2008
 Rituximab in Non - Hodgkins Lymphoma Fatima Bassa, Dept. of Haematology October 2008 World Health Organization lymphoma classification (2001) Peripheral B-cell neoplasms: B-chronic lymphocytic leukemia/small
Rituximab in Non - Hodgkins Lymphoma Fatima Bassa, Dept. of Haematology October 2008 World Health Organization lymphoma classification (2001) Peripheral B-cell neoplasms: B-chronic lymphocytic leukemia/small
GLSG/OSHO Study Group. Supported by Deutsche Krebshilfe
 GLSG/OSHO Study Group Supported by Deutsche Krebshilfe GLSG/OSHO Study Group Study Concepts Follicular Lymphomas Mantel Cell Lymphomas Waldenstroem s Disease Key Steps in Improving Treatment for Follicular
GLSG/OSHO Study Group Supported by Deutsche Krebshilfe GLSG/OSHO Study Group Study Concepts Follicular Lymphomas Mantel Cell Lymphomas Waldenstroem s Disease Key Steps in Improving Treatment for Follicular
Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics
 Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics Disclosure(s) I do not intend to discuss an off-label use
Are CAR T-Cells the Solution for Chemotherapy Refractory Diffuse Large B-Cell Lymphoma? Umar Farooq, MD University of Iowa Hospitals and Clinics Disclosure(s) I do not intend to discuss an off-label use
rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited
 rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited 06 June 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
rituximab 1400mg solution for subcutaneous injection (Mabthera ) SMC No. (975/14) Roche Products Limited 06 June 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
An overview of CLL care and treatment. Dr Dean Smith Haematology Consultant City Hospital Nottingham
 An overview of CLL care and treatment Dr Dean Smith Haematology Consultant City Hospital Nottingham What is CLL? CLL (Chronic Lymphocytic Leukaemia) is a type of cancer in which the bone marrow makes too
An overview of CLL care and treatment Dr Dean Smith Haematology Consultant City Hospital Nottingham What is CLL? CLL (Chronic Lymphocytic Leukaemia) is a type of cancer in which the bone marrow makes too
CAR T cell therapy for lymphomas
 CAR T cell therapy for lymphomas Sattva S. Neelapu, MD Associate Professor and Deputy Chair ad interim Department of Lymphoma and Myeloma UT MD Anderson Cancer Center, Houston, TX CAR T cell therapy What
CAR T cell therapy for lymphomas Sattva S. Neelapu, MD Associate Professor and Deputy Chair ad interim Department of Lymphoma and Myeloma UT MD Anderson Cancer Center, Houston, TX CAR T cell therapy What
Severe rheumatoid arthritis (a disease that causes inflammation of the joints),where MabThera is given intravenously together with methotrexate.
 EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
亞 東 紀 念 醫 院 Follicular Lymphoma 臨 床 指 引
 前 言 : 惡 性 淋 巴 瘤 ( 或 簡 稱 淋 巴 癌 ) 乃 由 體 內 淋 巴 系 統 包 括 淋 巴 細 胞 淋 巴 管 淋 巴 腺 及 一 些 淋 巴 器 官 或 組 織 如 脾 臟 胸 腺 及 扁 桃 腺 等 所 長 出 的 惡 性 腫 瘤 依 腫 瘤 病 理 組 織 型 態 的 不 同 可 分 為 何 杰 金 氏 淋 巴 瘤 (Hodgkin s disease) 與 非 何 杰 金
前 言 : 惡 性 淋 巴 瘤 ( 或 簡 稱 淋 巴 癌 ) 乃 由 體 內 淋 巴 系 統 包 括 淋 巴 細 胞 淋 巴 管 淋 巴 腺 及 一 些 淋 巴 器 官 或 組 織 如 脾 臟 胸 腺 及 扁 桃 腺 等 所 長 出 的 惡 性 腫 瘤 依 腫 瘤 病 理 組 織 型 態 的 不 同 可 分 為 何 杰 金 氏 淋 巴 瘤 (Hodgkin s disease) 與 非 何 杰 金
Non-Hodgkin s Lymphoma
 Non-Hodgkin s Lymphoma Luis Fayad, MD Assistant Professor Clinical Medical Director Lymphoma/Myeloma Department Non-Hodgkin s Lymphoma Non-Hodgkin s lymphomas (NHL) are a heterogeneous group of malignant
Non-Hodgkin s Lymphoma Luis Fayad, MD Assistant Professor Clinical Medical Director Lymphoma/Myeloma Department Non-Hodgkin s Lymphoma Non-Hodgkin s lymphomas (NHL) are a heterogeneous group of malignant
Collaboration to collect Autologous transplant outcomes in Lymphoma and Myeloma (CALM) Additional Questionnaire (MED C) INCLUSION CRITERIA CALM STUDY
 Additional Questionnaire (MED C) CALM study Inclusion period: 01/01/2008 to 31/12/2011 PATIENT REGISTRATION FORM Disease Diagnosis Lymphoma S Non Hodgkin Lymphoma (NHL) Mature B-cell neoplasm Follicular
Additional Questionnaire (MED C) CALM study Inclusion period: 01/01/2008 to 31/12/2011 PATIENT REGISTRATION FORM Disease Diagnosis Lymphoma S Non Hodgkin Lymphoma (NHL) Mature B-cell neoplasm Follicular
PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA
 2012 1 31,, PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA Version 1.0 2012 DIVISION OF HAEMATOLOGY / ONCOLOGY DEPARTMENT OF MEDICINE KAOHSING VETERAN GENERAL HOSPTIAL General Guide Diagnosis 1.Adequate
2012 1 31,, PROTOCOLS FOR TREATMENT OF MALIGNANT LYMPHOMA Version 1.0 2012 DIVISION OF HAEMATOLOGY / ONCOLOGY DEPARTMENT OF MEDICINE KAOHSING VETERAN GENERAL HOSPTIAL General Guide Diagnosis 1.Adequate
Histopathologic results
 Self evaluation 1 Clinical Case 55-year-old woman Bilateral enlargement of cervical, axillary and inguinal lymph nodes, largest diameter > 6 cm Hepatosplenomegaly. Enlargement of retroperitoneal, mesenteric
Self evaluation 1 Clinical Case 55-year-old woman Bilateral enlargement of cervical, axillary and inguinal lymph nodes, largest diameter > 6 cm Hepatosplenomegaly. Enlargement of retroperitoneal, mesenteric
Chemoimmunotherapy resistant follicular lymphoma A single institutional study
 Cancer Research Journal 2014; 2(5): 93-97 Published online September 30, 2014 (http://www.sciencepublishinggroup.com/j/crj) doi: 10.11648/j.crj.20140205.13 ISSN: 2330-8192 (Print); ISSN: 2330-8214 (Online)
Cancer Research Journal 2014; 2(5): 93-97 Published online September 30, 2014 (http://www.sciencepublishinggroup.com/j/crj) doi: 10.11648/j.crj.20140205.13 ISSN: 2330-8192 (Print); ISSN: 2330-8214 (Online)
Lymphoma Overview Joseph Leach, MD
 Lymphoma Overview Joseph Leach, MD 71 year old male presents with complaints of mild fa5gue and a visible mass in the le: supraclavicular region PE demonstrates a firm easily palpable mass in the le: supraclavicular
Lymphoma Overview Joseph Leach, MD 71 year old male presents with complaints of mild fa5gue and a visible mass in the le: supraclavicular region PE demonstrates a firm easily palpable mass in the le: supraclavicular
In non-hodgkin s lymphoma, MabThera is used to treat two types of the disease, both of which affect B-lymphocytes:
 EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
6/3/2013. Follicular and Other Slow Growing Lymphomas. Stephen Ansell, MD, PhD Mayo Clinic
 Follicular and Other Slow Growing Lymphomas Stephen Ansell, MD, PhD Mayo Clinic 1 Learning Objectives Start with an overview of Follicular and other slow growing lymphomas Discuss current and emerging
Follicular and Other Slow Growing Lymphomas Stephen Ansell, MD, PhD Mayo Clinic 1 Learning Objectives Start with an overview of Follicular and other slow growing lymphomas Discuss current and emerging
chronic leukemia lymphoma myeloma differentiated 14 September 1999 Pre- Transformed Ig Surface Surface Secreted Myeloma Major malignant counterpart
 Disease Usual phenotype acute leukemia precursor chronic leukemia lymphoma myeloma differentiated Pre- B-cell B-cell Transformed B-cell Plasma cell Ig Surface Surface Secreted Major malignant counterpart
Disease Usual phenotype acute leukemia precursor chronic leukemia lymphoma myeloma differentiated Pre- B-cell B-cell Transformed B-cell Plasma cell Ig Surface Surface Secreted Major malignant counterpart
Rituximab in the Management of Follicular Lymphoma
 Hong Kong J Radiol. 2011;14(Suppl):S63-7 REVIEW ARTICLE Rituximab in the Management of Follicular Lymphoma YL Kwong Department of Medicine, Professorial Block, Queen Mary Hospital, Pokfulam Road, Hong
Hong Kong J Radiol. 2011;14(Suppl):S63-7 REVIEW ARTICLE Rituximab in the Management of Follicular Lymphoma YL Kwong Department of Medicine, Professorial Block, Queen Mary Hospital, Pokfulam Road, Hong
DIFFUSE LARGE B-CELL LYMPHOMA
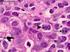 DIFFUSE LARGE B-CELL LYMPHOMA Executive Summary Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-hodgkin lymphoma (NHL), constituting up to 40% of all cases globally.[1] This subtype
DIFFUSE LARGE B-CELL LYMPHOMA Executive Summary Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-hodgkin lymphoma (NHL), constituting up to 40% of all cases globally.[1] This subtype
Update on Follicular Lymphoma. Brad Kahl, M.D.
 Update on Follicular Lymphoma Brad Kahl, M.D. Follicular Lymphoma: 25% of NHL Cases Other subtypes (9%) T and NK cell (12%) Burkitt (2.5%) Diffuse large B cell (DLBCL) (30%) Mantle cell (6%) Follicular
Update on Follicular Lymphoma Brad Kahl, M.D. Follicular Lymphoma: 25% of NHL Cases Other subtypes (9%) T and NK cell (12%) Burkitt (2.5%) Diffuse large B cell (DLBCL) (30%) Mantle cell (6%) Follicular
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cll_and_sll
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cll_and_sll
Lymphoma: The Roleof Nurses in the Treatment Process
 Lymphoma: The Roleof Nurses in the Treatment Process Sarah Liptrott MSc,BN(Hons), RN Istituto Europeo di Oncologia, Milan (IT) EBMT Swiss Study Day 2014, Zurich, Switzerland LymphomaManagement Watch &
Lymphoma: The Roleof Nurses in the Treatment Process Sarah Liptrott MSc,BN(Hons), RN Istituto Europeo di Oncologia, Milan (IT) EBMT Swiss Study Day 2014, Zurich, Switzerland LymphomaManagement Watch &
Many people with non-hodgkin lymphoma have found an educational support group helpful. Support
 Track 2: Treatment Options [Narrator] Many people with non-hodgkin lymphoma have found an educational support group helpful. Support groups take many forms: some meet the needs of people with all kinds
Track 2: Treatment Options [Narrator] Many people with non-hodgkin lymphoma have found an educational support group helpful. Support groups take many forms: some meet the needs of people with all kinds
1. Introduction. 2. Clinical aspects
 1. Introduction MabThera (rituximab) is a genetically engineered chimeric mouse/human monoclonal antibody which binds specifically to the transmembrane antigen, CD20. This antigen is located on pre-b-
1. Introduction MabThera (rituximab) is a genetically engineered chimeric mouse/human monoclonal antibody which binds specifically to the transmembrane antigen, CD20. This antigen is located on pre-b-
Waldenström Macroglobulinemia: The Burning Questions. IWMF Ed Forum May 18 2014 Morie Gertz MD, MACP
 Waldenström Macroglobulinemia: The Burning Questions IWMF Ed Forum May 18 2014 Morie Gertz MD, MACP Are my kids going to get this? Familial seen in approximately 5 10% of all CLL patients and can be associated
Waldenström Macroglobulinemia: The Burning Questions IWMF Ed Forum May 18 2014 Morie Gertz MD, MACP Are my kids going to get this? Familial seen in approximately 5 10% of all CLL patients and can be associated
CD22 Antigen Is Broadly Expressed on Lung Cancer Cells and Is a Target for Antibody-Based Therapy
 CD22 Antigen Is Broadly Expressed on Lung Cancer Cells and Is a Target for Antibody-Based Therapy Joseph M. Tuscano, Jason Kato, David Pearson, Chengyi Xiong, Laura Newell, Yunpeng Ma, David R. Gandara,
CD22 Antigen Is Broadly Expressed on Lung Cancer Cells and Is a Target for Antibody-Based Therapy Joseph M. Tuscano, Jason Kato, David Pearson, Chengyi Xiong, Laura Newell, Yunpeng Ma, David R. Gandara,
Michael Crump MD. Lymphoma Site Leader Princess Margaret Hospital University of Toronto
 Evolution of Lymphoma Therapy: What can we expect for the rest of the millenium decade? Michael Crump MD Lymphoma Site Leader Princess Margaret Hospital University of Toronto disclaimers Served on advisory
Evolution of Lymphoma Therapy: What can we expect for the rest of the millenium decade? Michael Crump MD Lymphoma Site Leader Princess Margaret Hospital University of Toronto disclaimers Served on advisory
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin s lymphoma
 DOI: 10.3310/hta13suppl2/06 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin s lymphoma A Boland, A Bagust,
DOI: 10.3310/hta13suppl2/06 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin s lymphoma A Boland, A Bagust,
Audience Response Question?
 Presenter Disclosure Information Session 4: 3:30 PM - 4:15 PM Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman, MD The following relationships
Presenter Disclosure Information Session 4: 3:30 PM - 4:15 PM Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman, MD The following relationships
Audience Response Question? Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management. Presenter Disclosure Information
 Welcome to Master Class for Oncologists Session 4: 10:00 AM - 10:45 AM Miami, FL December 18, 2009 Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman,
Welcome to Master Class for Oncologists Session 4: 10:00 AM - 10:45 AM Miami, FL December 18, 2009 Non-Hodgkin s Lymphomas: Optimizing Therapeutic Choices for Initial Management Speaker: Arnold S. Freedman,
Guidelines for the Management of Chronic Lymphocytic Leukaemia (CLL)
 Guidelines for the Management of Chronic Lymphocytic Leukaemia (CLL) Version History Version Date Summary of Change/Process 2.0 08.05.08 Endorsed by the Governance Committee 2.1 16.02.11 Circulated at
Guidelines for the Management of Chronic Lymphocytic Leukaemia (CLL) Version History Version Date Summary of Change/Process 2.0 08.05.08 Endorsed by the Governance Committee 2.1 16.02.11 Circulated at
Summary & Conclusion
 The prognostic value of angiogenesis markers in patients with non-hodgkin lymphoma. Summary & Conclusion The current study aims to asses the prognostic value of some angiogenesis markers in patients with
The prognostic value of angiogenesis markers in patients with non-hodgkin lymphoma. Summary & Conclusion The current study aims to asses the prognostic value of some angiogenesis markers in patients with
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
Social inequalities impacts of care management and survival in patients with non-hodgkin lymphomas (ISO-LYMPH)
 Session 3 : Epidemiology and public health Social inequalities impacts of care management and survival in patients with non-hodgkin lymphomas (ISO-LYMPH) Le Guyader-Peyrou Sandra Bergonie Institut Context:
Session 3 : Epidemiology and public health Social inequalities impacts of care management and survival in patients with non-hodgkin lymphomas (ISO-LYMPH) Le Guyader-Peyrou Sandra Bergonie Institut Context:
Hematopoietic Stem Cell Transplantation. Imad A. Tabbara, M.D. Professor of Medicine
 Hematopoietic Stem Cell Transplantation Imad A. Tabbara, M.D. Professor of Medicine Hematopoietic Stem Cells Harvested from blood, bone marrow, umbilical cord blood Positive selection of CD34 (+) cells
Hematopoietic Stem Cell Transplantation Imad A. Tabbara, M.D. Professor of Medicine Hematopoietic Stem Cells Harvested from blood, bone marrow, umbilical cord blood Positive selection of CD34 (+) cells
DE Tsai 1, HCF Moore 1, CL Hardy 2, DL Porter 1, EY Loh 1, DJ Vaughn 1, S Luger 1, SJ Schuster 1 and EA Stadtmauer 1
 Bone Marrow Transplantation, (1999) 24, 521 526 1999 Stockton Press All rights reserved 0268 3369/99 $15.00 http://www.stockton-press.co.uk/bmt Rituximab (anti-cd20 monoclonal antibody) therapy for progressive
Bone Marrow Transplantation, (1999) 24, 521 526 1999 Stockton Press All rights reserved 0268 3369/99 $15.00 http://www.stockton-press.co.uk/bmt Rituximab (anti-cd20 monoclonal antibody) therapy for progressive
Stem Cell Transplantation
 Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Indolent Lymphomas. American Academy of Insurance Medicine 121 st Annual Meeting. Hilton LaJolla October 2012
 Indolent Lymphomas American Academy of Insurance Medicine 121 st Annual Meeting Hilton LaJolla October 2012 Scottsdale, Arizona Rochester, Minnesota Jacksonville, Florida Joseph Mikhael, MD, MEd, FRCPC,
Indolent Lymphomas American Academy of Insurance Medicine 121 st Annual Meeting Hilton LaJolla October 2012 Scottsdale, Arizona Rochester, Minnesota Jacksonville, Florida Joseph Mikhael, MD, MEd, FRCPC,
Outline of thesis and future perspectives.
 Outline of thesis and future perspectives. This thesis is divided into two different sections. The B- section involves reviews and studies on B- cell non- Hodgkin lymphoma [NHL] and radioimmunotherapy
Outline of thesis and future perspectives. This thesis is divided into two different sections. The B- section involves reviews and studies on B- cell non- Hodgkin lymphoma [NHL] and radioimmunotherapy
Chronic Lymphocytic Leukemia. Case Study. AAIM Triennial October 2012 Susan Sokoloski, M.D.
 Chronic Lymphocytic Leukemia AAIM Triennial October 2012 Susan Sokoloski, M.D. Case Study 57 year old male, trial application for $1,000,000 Universal Life coverage Cover letter from sales agent indicates
Chronic Lymphocytic Leukemia AAIM Triennial October 2012 Susan Sokoloski, M.D. Case Study 57 year old male, trial application for $1,000,000 Universal Life coverage Cover letter from sales agent indicates
MULTIPLE MYELOMA. Dr Malkit S Riyat. MBChB, FRCPath(UK) Consultant Haematologist
 MULTIPLE MYELOMA Dr Malkit S Riyat MBChB, FRCPath(UK) Consultant Haematologist Multiple myeloma is an incurable malignancy that arises from postgerminal centre, somatically hypermutated B cells.
MULTIPLE MYELOMA Dr Malkit S Riyat MBChB, FRCPath(UK) Consultant Haematologist Multiple myeloma is an incurable malignancy that arises from postgerminal centre, somatically hypermutated B cells.
Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm
 Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm Name of Policy: Uses of Monoclonal Antibodies for the Treatment of Non-Hodgkin
Effective for dates of service on or after September 1, 2015, refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm Name of Policy: Uses of Monoclonal Antibodies for the Treatment of Non-Hodgkin
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD Boehringer Ingelheim International GmbH and Boehringer Ingelheim Pharmaceuticals, Inc. Petitioner, v. Biogen Idec, Inc.
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD Boehringer Ingelheim International GmbH and Boehringer Ingelheim Pharmaceuticals, Inc. Petitioner, v. Biogen Idec, Inc.
Follicular Lymphoma. Aruna K. Reddy, MD. Hematology& Oncology Peace Health Southwest Medical Center
 Follicular Lymphoma Aruna K. Reddy, MD Hematology& Oncology Peace Health Southwest Medical Center Follicular Lymphoma Malignant neoplasm resulting from clonal proliferation of malignant B-cells Second
Follicular Lymphoma Aruna K. Reddy, MD Hematology& Oncology Peace Health Southwest Medical Center Follicular Lymphoma Malignant neoplasm resulting from clonal proliferation of malignant B-cells Second
IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN
 + IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
+ IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma. Claire Vines, 2016 Pharm.D. Candidate
 + Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma Claire Vines, 2016 Pharm.D. Candidate + Disclosure I have no conflicts of interest to disclose. + Objectives Summarize NCCN
+ Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma Claire Vines, 2016 Pharm.D. Candidate + Disclosure I have no conflicts of interest to disclose. + Objectives Summarize NCCN
Cycle frequency: Every four weeks Total number of cycles: 6-8
 Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
Lymphomas after organ transplantation
 Produced 21.03.2011 Revision due 21.03.2011 Lymphomas after organ transplantation People who have undergone an organ transplant are more at risk of developing lymphoma known as post-transplant lymphoproliferative
Produced 21.03.2011 Revision due 21.03.2011 Lymphomas after organ transplantation People who have undergone an organ transplant are more at risk of developing lymphoma known as post-transplant lymphoproliferative
-Examination of key pipeline candidates with in-depth clinical and commercial profiles of Phase III candidates
 Brochure More information from http://www.researchandmarkets.com/reports/1215469/ Pipeline Insight: Lymphomas, Multiple Myeloma & Myelodysplastic Syndromes - Optimization of clinical practice creates opportunities
Brochure More information from http://www.researchandmarkets.com/reports/1215469/ Pipeline Insight: Lymphomas, Multiple Myeloma & Myelodysplastic Syndromes - Optimization of clinical practice creates opportunities
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
The Blood Cancer Twice As Likely To Affect African Americans: Multiple Myeloma
 The Blood Cancer Twice As Likely To Affect African Americans: Multiple Myeloma 11 th Annual National Leadership Summit on Health Disparities Innovation Towards Reducing Disparities Congressional Black
The Blood Cancer Twice As Likely To Affect African Americans: Multiple Myeloma 11 th Annual National Leadership Summit on Health Disparities Innovation Towards Reducing Disparities Congressional Black
Your NHL Journey. RITUXAN for Follicular Lymphoma and Diffuse Large B-cell Lymphoma (DLBCL) Indications. Important Safety Information
 * Your NHL Journey RITUXAN for Follicular Lymphoma and Diffuse Large B-cell Lymphoma (DLBCL) * Non-Hodgkin s Lymphoma Indications RITUXAN (rituximab) is indicated for the treatment of: Follicular CD20-positive
* Your NHL Journey RITUXAN for Follicular Lymphoma and Diffuse Large B-cell Lymphoma (DLBCL) * Non-Hodgkin s Lymphoma Indications RITUXAN (rituximab) is indicated for the treatment of: Follicular CD20-positive
Why discuss CLL? Common: 40% of US leukaemia. approx 100 pa in SJH / MWHB 3 inpatients in SJH at any time
 Why discuss CLL? Common: 40% of US leukaemia approx 100 pa in SJH / MWHB 3 inpatients in SJH at any time Median age of dx is 65 (30s. Incurable, survival 2-202 20 years Require ongoing supportive care
Why discuss CLL? Common: 40% of US leukaemia approx 100 pa in SJH / MWHB 3 inpatients in SJH at any time Median age of dx is 65 (30s. Incurable, survival 2-202 20 years Require ongoing supportive care
HOVON Staging and Response Criteria for Non-Hodgkin s Lymphomas Page 1
 HOVON Staging and Response Criteria for Non-Hodgkin s Lymphomas Page 1 This document describes the minimally required staging and evaluation procedures and response criteria that will be applied in all
HOVON Staging and Response Criteria for Non-Hodgkin s Lymphomas Page 1 This document describes the minimally required staging and evaluation procedures and response criteria that will be applied in all
Low grade non-hodgkin Lymphoma
 Low grade non-hodgkin Lymphoma www.lymphomas.org.uk The knowledge to challenge lymphatic cancers The Lymphoma Association provides: freephone helpline emotional support for those affected by lymphomas
Low grade non-hodgkin Lymphoma www.lymphomas.org.uk The knowledge to challenge lymphatic cancers The Lymphoma Association provides: freephone helpline emotional support for those affected by lymphomas
Imaging, Diagnosis, Prognosis. MinnaTaskinen, 1,2 Marja-Liisa Karjalainen-Lindsberg, 3 Heidi Nyman, 1,2 Leena-Maija Eerola, 1,2 1,2.
 Imaging, Diagnosis, Prognosis A HighTumor-Associated Macrophage Content Predicts Favorable Outcome in Follicular Lymphoma PatientsTreated with Rituximab and Cyclophosphamide-Doxorubicin-Vincristine-Prednisone
Imaging, Diagnosis, Prognosis A HighTumor-Associated Macrophage Content Predicts Favorable Outcome in Follicular Lymphoma PatientsTreated with Rituximab and Cyclophosphamide-Doxorubicin-Vincristine-Prednisone
CHAPTER 2. Neoplasms (C00-D49) March 2014. 2014 MVP Health Care, Inc.
 Neoplasms (C00-D49) March 2014 2014 MVP Health Care, Inc. CHAPTER SPECIFIC CATEGORY CODE BLOCKS C00-C14 Malignant neoplasms of lip, oral cavity and pharynx C15-C26 Malignant neoplasms of digestive organs
Neoplasms (C00-D49) March 2014 2014 MVP Health Care, Inc. CHAPTER SPECIFIC CATEGORY CODE BLOCKS C00-C14 Malignant neoplasms of lip, oral cavity and pharynx C15-C26 Malignant neoplasms of digestive organs
Future strategies for myeloma: An overview of novel treatments In development
 Future strategies for myeloma: An overview of novel treatments In development Dr. Matthew Streetly Guys and St. Thomas NHS Trust How far have we come? Melphalan and prednisolone VAD Autologous SCT Thalidomide
Future strategies for myeloma: An overview of novel treatments In development Dr. Matthew Streetly Guys and St. Thomas NHS Trust How far have we come? Melphalan and prednisolone VAD Autologous SCT Thalidomide
Interesting Case Series. Periorbital Richter Syndrome
 Interesting Case Series Periorbital Richter Syndrome MarkGorman,MRCS,MSc, a Julia Ruston, MRCS, b and Sarath Vennam, BMBS a a Division of Plastic Surgery, Royal Devon and Exeter Hospital, Exeter, Devon,
Interesting Case Series Periorbital Richter Syndrome MarkGorman,MRCS,MSc, a Julia Ruston, MRCS, b and Sarath Vennam, BMBS a a Division of Plastic Surgery, Royal Devon and Exeter Hospital, Exeter, Devon,
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS
 MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
Non-Hodgkin Lymphoma Richard Orlowski, MD
 Non-Hodgkin Lymphoma Richard Orlowski, MD The American Cancer Society (ACS) estimates that 69,740 Americans will be diagnosed with non-hodgkin lymphoma (NHL) in 2013. Excluding non-melanoma skin cancers,
Non-Hodgkin Lymphoma Richard Orlowski, MD The American Cancer Society (ACS) estimates that 69,740 Americans will be diagnosed with non-hodgkin lymphoma (NHL) in 2013. Excluding non-melanoma skin cancers,
Diffuse large B-cell lymphoma: the curable disease?
 Hematology Meeting Reports 2007; 1(5):77 86 Diffuse large B-cell lymphoma: the curable disease? Michael Pfreundschuh Med Klinik I, Saarland University Medical School, Homburg/Saar, Germany Corresponding
Hematology Meeting Reports 2007; 1(5):77 86 Diffuse large B-cell lymphoma: the curable disease? Michael Pfreundschuh Med Klinik I, Saarland University Medical School, Homburg/Saar, Germany Corresponding
