ph and ORP Electrode Manual
|
|
|
- Basil Preston
- 9 years ago
- Views:
Transcription
1 ph and ORP Electrode Manual Contents Page Electrode Applications Page Electrode Cable Page Installing Submersible Electrodes Page Electrode Care and Maintenance Page Calibration Page Electrode Problems
2 Electrode Applications ph and ORP (Redox) electrodes are sensitive measuring sensors and should be treated with care. The electrodes are replaceable items and the life span is dependent on the chemical and temperature conditions. A typical life span for an electrode is 2 to 3 years but this may be much less if the electrode is subjected to harsh conditions. The following manual is intended as a guide for maintaining common electrodes for laboratory, in-line and submersible applications. 1/ Laboratory Electrodes Laboratory electrodes usually have a body diameter of 12mm. The body material may be typically glass, epoxy or polymer. For specialised applications such as, measuring in a test tube or penetrating food products, the probe profile is specially designed. Laboratory probes are often adapted for in-line measurements, using a threaded adapter. 2/ In-line Electrodes For measuring ph or ORP in a pipe, a threaded in-line electrode is used. It is important to install the electrode so that an air cavity does not form around the sensing area of the electrode. Ideally install from the top of the pipe to maintain the fluid within the glass bulb. When installed the electrode should be at an angle of between 30 o and 90 o to the horizontal. 3/ Submersible Electrodes For ph and ORP measurements in an open tank or viaduct, a submersion electrode coupled with an immersion assembly is used. In this application it is important NOT to immerse the electrode cable. By using a submersion assembly, the electrode is supported and the cable contained within the immersion assembly. 1 of 6 PHORP
3 Electrode Cable The cable used for ph and ORP electrodes is a special screened low noise cable. The electrode is a high impedance device and is coupled to a high impedance measuring instrument. The cable incorporates a special conductive rubber material known as an anti-microphonic layer. This prevents voltages being generated when the cable is moved or vibrated. Special Note: Most electrodes are supplied with a BNC connector fitted. It is often necessary to remove the BNC connector or shorten the cable to make the cable suitable for connecting to screw terminals. IMPORTANT When re-terminating the cable, the anti-microphonic layer must be stripped back from the centre conductor to prevent a short circuit in the cable. Techniques for re-terminating the cable 2 of 6 PHORP
4 Installing Submersible Electrodes Electrodes may be submersed by using an immersion assembly. The electrode is fitted and thread sealed to the end of a pipe section. This provides a rigid assembly and protects and seals the cable. The assembly may be built from standard plumbing fittings, or purchased as a complete assembly. IMPORTANT - DO NOT IMMERSE THE CABLE Application Example In the example below additional protection is provided by the fixed open ended pipe. This is ideal for applications where turbulence is present. Example of immersion assembly The immersion assembly should be built to suit the application. In the example above a flange is incorporated to stabilise and support the assembly. The junction head makes electrode replacement easier, by allowing the cable to be accessed without undue twisting. In models with an inbuilt 4-20mA amplifier, the junction head contains the electronic circuit board, calibration adjustments and the 4-20mA terminals. 3 of 6 PHORP
5 Electrode Care and Maintenance New Electrodes When you receive your electrode the sensor zone is protected by a cover which contains a soaker solution. Before you use the electrode, rinse off the soaker solution and leave in clean tap water or a weak acid for 15 minutes. This will re-activate the reference junction. Occasionally during shipment air bubbles may form in the glass bulb of ph electrodes. Before use, visually check the electrode for trapped air bubbles. Should bubbles be present, then they can be removed by shaking the electrode in a downward motion. Important The electrode membranes must be kept wet at all times. For short term storage the electrode may be stored in a tap water solution. When the electrode is not in use for long periods, it should be stored in a 3M KCl soaker solution adjusted to 4 ph. Note: When storing double junction electrodes, use a solution saturated with the same salt type as the reference junction. Cleaning the electrode If the electrode becomes sluggish in response it may be necessary to clean the electrode. Usually cleaning with a mild detergent will remove the deposit and restore the electrode performance. Take care not to touch the glass at any time. Clean the electrode by washing in the mild detergent and then rinsing with clean tap water. More difficult deposits may be removed by rinsing with methyl alcohol, followed by a rinse in clean tap water. If the methyl alcohol rinse does not restore the response, carry out the following procedure: 1/ Soak the electrode in 0.1M HCl for 1 hour and then rinse the electrode with tap water. 2/ Soak the electrode in 0.1M NaOH for 1 hour and then rinse with tap water. If the electrode performance is not restored repeat the steps 1 and 2, but increase the soaking times to 24 hours. If this does not restore the electrode performance, replace the electrode. Re-fillable Electrodes Most combination electrodes contain a gelled electrolyte which is never replaced. In some specialised applications the electrode may have a flowing electrolyte. The electrolyte is contained in a chamber which must be constantly replenished. A variety of refillable solutions are available and should be selected to suit the application. Note: single junction AgCl lab electrodes should be topped up with 3 molar KCl/sat with AgCl, as KCl only can shorten the electrode life and cause an offset in ph 7 buffer. Points to Remember By following these points, it is possible to significantly increase the expected life of an electrode, and also greatly improve the quality of measurement results. ph and ORP electrodes must always be stored wet. For short term storage soak the electrode in KCl. For long term storage fill a container with the soaker solution immerse the electrode sensor zone and seal to prevent evaporation. Never store the electrode in: de-ionised water, solvents, hydrofluoric acid, ph buffers containing mercury based preservatives. Sensing tips should always be rinsed after use (laboratory electrodes). Reference cells should be kept regularly topped up with electrolyte (refillable laboratory electrodes). Connectors should be kept clean and dry. If the electrode needs to be cleaned physically, always use a soft tissue soaked in a mild detergent or methyl alcohol. Important: do not wipe the glass - use a dabbing technique. Never touch the electrode glass bulb with your fingers or other oily or abrasive objects. 4 of 6 PHORP
6 Calibration ph Calibration You will need: The equipment to be calibrated: comprising, ph electrode coupled to the ph meter. Buffer solutions: typically 1 x ph 7.00 and 1 x ph DI rinsing water: de-ionised or distilled water (ideally in a fast flow wash bottle). Tissue to dab electrode dry - do not wipe electrode - this can damage the glass surface. Thermometer to measure temperature of buffer solutions. Before you start: Make sure the ph meter is powered up and stabilised. Check the temperature of the buffer solutions (they should both be at ambient temperature). If the meter does not have automatic temperature compensation, set manually to the solution temperature. Allow the ph electrode to stabilise to the same ambient temperature as the buffer solutions. Calibration procedure: Rinse the electrode in de-ionised or distilled water - shake off excess water - dab dry with tissue. Immerse the electrode in a buffer of 7.00 ph - allow to stabilise for 1 to 2 minutes. Adjust the calibration of ph meter to read Remove electrode and rinse in DI water - shake off excess - dab dry with tissue. Immerse electrode in buffer of 4.00 ph - allow to stabilise for 1 to 2 minutes. Adjust ph meter to read Repeat procedure to confirm calibration - if necessary repeat until calibration is acceptable. (note: this should not be necessary on microprocessor based instruments). ORP (Redox) Calibration You will need: The equipment to be calibrated: comprising, ORP electrode coupled to the ORP meter. Buffer solutions: typically 1 x ph 7.00 and 1 x ph 4.00, a small quantity of quinhydrone. DI rinsing water: de-ionised or distilled water (ideally in a fast flow wash bottle). Tissue to dab electrode dry - do not wipe electrode - this can damage the glass surface. Thermometer to measure temperature of buffer solutions. Before you start: Make sure the ORP meter is powered up and stabilised. Check the temperature of the buffer solutions (they should both be at ambient temperature). Allow the ORP electrode to stabilise to the same ambient temperature as the buffer solutions. Add a pinch of quinhydrone to each buffer, mix well, add more quinhydrone until the solutions are saturated and will not absorb more quinhydrone. Calibration procedure: Rinse the electrode in de-ionised or distilled water - shake off excess water - dab dry with tissue. Immerse the electrode in the ORP 7.00 ph buffer - allow to stabilise for 1 to 2 minutes. Adjust the calibration of ORP meter to read 86mV. Remove electrode and rinse in DI water - shake off excess - dab dry with tissue. Immerse electrode in the ORP 4.00 ph buffer - allow to stabilise for 1 to 2 minutes. Adjust ORP meter to read 263mV. Repeat procedure to confirm calibration - if necessary repeat until calibration is acceptable. (note: this should not be necessary on microprocessor based instruments). Note: the mv values quoted are for electrodes with a silver-silver chloride reference. For electrodes with a calomel reference subtract 42mV from the above values. 5 of 6 PHORP
7 Electrode Problems ph and ORP electrodes continually age and will not last indefinitely. The life span cannot be accurately predicted and is dependent on the chemical and temperature conditions experienced by the electrode. As the electrode ages the slope (output) decreases. The electrode should be replaced when the electrode slope reduces below 90% of an ideal electrode. However it is often possible to rejuvenate a ph electrode by: 1/ Soak the electrode in 0.1M HCl for 5 minutes and then rinse the electrode with tap water. Soak the electrode in 0.1M NaOH for 5 minutes and then rinse with tap water. 2/ the process is detailed on the bottom of this page. Caution: this process uses hazardous chemicals and should only be carried out by a qualified chemist. See chapters Care and maintaining of your electrode for information on cleaning the electrodes and Electrode Cable for anti-microphonic layer. Slow Response Possible cause: Possible cause: Unstable Reading Possible cause: Electrode glass surface contaminated. Clean electrode. Reference junction or reference electrolyte contaminated. Clean electrode. Reference junction blocked or contaminated. Clean electrode. Low Slope (less than 90%) Possible cause: Electrode glass surface not clean. Clean electrode. Possible cause: Glass membrane aged. Try rejuvenating the electrode or replace electrode. Possible cause: Dampness or contamination around cable connections. Clean with methyl alcohol and allow to dry in a warm place. Displays approximately 7 ph (or 0mV for ORP) for all buffers Possible cause: Electrical short circuit. Check connector for short circuit. Check cable anti-microphonic layer. Displays 4 to 5 ph for all buffers Possible cause: Breakdown within electrode body. Replace electrode. Advanced information - Rejuvenating the glass electrode When the electrode is used at elevated temperatures the ph glass loses it s sensitivity. The following procedure may rejuvenate the glass surface and provide an extended electrode life. 1. Prepare a 20% solution of ammonium bifluoride. 2. Immerse the electrode in the solution for 15 to 20 seconds. 3. Rinse the electrode with tap water. 4. Immerse the electrode in 5-6M HCl for 5 minutes to remove the bifluoride. 5. Rinse electrode with tap water. 6. Immerse the electrode in ph buffer 4.00 for 1 to 2 hours. 6 of 6 PHORP
8 This page intentionally left blank 7 of 6 PHORP
ph Measurement and Control
 ph Measurement and Control Tech Tip #14 1997 I. The Need for ph II. ph System Requirements III. ph Electrode Conditioning Procedures IV. How the electrode works V. Identifying the Electrode Components
ph Measurement and Control Tech Tip #14 1997 I. The Need for ph II. ph System Requirements III. ph Electrode Conditioning Procedures IV. How the electrode works V. Identifying the Electrode Components
SenTix 950 SenTix 980
 SenTix 950 SenTix 980 ph combination electrodes with refillable liquid reference system Operating manual ba75843e01 08/2009 SenTix The latest version of the present operating manual can be found on the
SenTix 950 SenTix 980 ph combination electrodes with refillable liquid reference system Operating manual ba75843e01 08/2009 SenTix The latest version of the present operating manual can be found on the
Care, Maintenance, and Storage of YSI 6-Series Probes and Sondes
 Tech Note Care, Maintenance, and Storage of YSI 6-Series Probes and Sondes Probe Care and Maintenance Periodic cleaning and membrane changes are required to keep your sensors operating properly. Follow
Tech Note Care, Maintenance, and Storage of YSI 6-Series Probes and Sondes Probe Care and Maintenance Periodic cleaning and membrane changes are required to keep your sensors operating properly. Follow
ph Sensor Model 397 CAUTION SENSOR/PROCESS APPLICATION COMPATIBILITY WARNING ATEX DIRECTIVE Instruction Sheet PN 51A-397/rev.
 Instruction Sheet PN 51A-397/rev.F January 2011 Model 397 ph Sensor For additional information, please visit our website at www.emersonprocess.com/raihome/liquid/. SPECIFICATIONS MODEL 397 PERFORMANCE
Instruction Sheet PN 51A-397/rev.F January 2011 Model 397 ph Sensor For additional information, please visit our website at www.emersonprocess.com/raihome/liquid/. SPECIFICATIONS MODEL 397 PERFORMANCE
Direct ISE Method Method 8359 10 to 1000 mg/l Na + Sodium ISE
 Sodium DOC316.53.01240 Direct ISE Method Method 8359 10 to 1000 mg/l Na + Sodium ISE Scope and application: For drinking water and process water. Test preparation Instrument-specific information This procedure
Sodium DOC316.53.01240 Direct ISE Method Method 8359 10 to 1000 mg/l Na + Sodium ISE Scope and application: For drinking water and process water. Test preparation Instrument-specific information This procedure
REDOX AND REFERENCE ELECTRODE USER MANUAL
 REDOX AND REFERENCE ELECTRODE USER MANUAL 3 Redox and reference electrode manual Copyright 2012 Unisense A/S Version October 2012 REDOX AND REFERENCE ELECTRODE MANUAL UNISENSE A/S TABLE OF CONTENTS WARRANTY
REDOX AND REFERENCE ELECTRODE USER MANUAL 3 Redox and reference electrode manual Copyright 2012 Unisense A/S Version October 2012 REDOX AND REFERENCE ELECTRODE MANUAL UNISENSE A/S TABLE OF CONTENTS WARRANTY
OPERATION MANUAL. Pen type, separate electrode SOIL PH METER Model : PH-220S
 Pen type, separate electrode SOIL PH METER Model : PH-220S Your purchase of this SOIL PH METER marks a step forward for you into the field of precision measurement. Although this METER is a complex and
Pen type, separate electrode SOIL PH METER Model : PH-220S Your purchase of this SOIL PH METER marks a step forward for you into the field of precision measurement. Although this METER is a complex and
USEPA Electrode Method Method 8156
 ph DOC316.53.01245 USEPA Electrode Method Method 8156 Scope and application: For drinking water 1, wastewater 2 and process water. 1 Based on Standard Method 4500-H+B, ASTM Method D1293-95 and USEPA Method
ph DOC316.53.01245 USEPA Electrode Method Method 8156 Scope and application: For drinking water 1, wastewater 2 and process water. 1 Based on Standard Method 4500-H+B, ASTM Method D1293-95 and USEPA Method
Milwaukee USER MANUAL. Milwaukee. Smart DO Meter PORTABLE DISSOLVED OXYGEN METER MODEL: SM600. Authorized Dealer: ISMIL600 11/01
 Milwaukee Milwaukee USER MANUAL PORTABLE DISSOLVED OXYGEN METER MODEL: SM600 Smart DO Meter Authorized Dealer: ISMIL600 11/01 PROBE PREPARATION: The meter is supplied with a 9V battery. Slide off the battery
Milwaukee Milwaukee USER MANUAL PORTABLE DISSOLVED OXYGEN METER MODEL: SM600 Smart DO Meter Authorized Dealer: ISMIL600 11/01 PROBE PREPARATION: The meter is supplied with a 9V battery. Slide off the battery
Milwaukee USER MANUAL
 Milwaukee USER MANUAL PORTABLE DISSOLVED OXYGEN METER MODEL: MW600 Smart DO Meter PROBE PREPARATION: The meter is supplied with a 9V battery. Slide off the battery compartment cover on the back of the
Milwaukee USER MANUAL PORTABLE DISSOLVED OXYGEN METER MODEL: MW600 Smart DO Meter PROBE PREPARATION: The meter is supplied with a 9V battery. Slide off the battery compartment cover on the back of the
ph Measurements of Common Substances
 Chem 100 Section Experiment 10 Name Partner s Name Introduction ph Measurements of Common Substances The concentration of an acid or base is frequently expressed as ph. Historically, ph stands for the
Chem 100 Section Experiment 10 Name Partner s Name Introduction ph Measurements of Common Substances The concentration of an acid or base is frequently expressed as ph. Historically, ph stands for the
AIRMoN, ph and Conductivity Field Measurement
 AIRMoN, ph and Conductivity Field Measurement Items needed: Sample to be measured (sample volume 50 ml) Field Observer Form (FOF) for the sample to be measured conductance cell ph vials, 4 conductivity
AIRMoN, ph and Conductivity Field Measurement Items needed: Sample to be measured (sample volume 50 ml) Field Observer Form (FOF) for the sample to be measured conductance cell ph vials, 4 conductivity
ELECTROCHEMISTRY. Theory and Practice
 ELECTROCHEMISTRY Theory and Practice Dr. Axel Bier Lab Product Application Manager - Electrochemistry For more information, please visit: www.hach.com/smartprobes P a g e 2 CONTENT INTRODUCTION 3 HOW TO
ELECTROCHEMISTRY Theory and Practice Dr. Axel Bier Lab Product Application Manager - Electrochemistry For more information, please visit: www.hach.com/smartprobes P a g e 2 CONTENT INTRODUCTION 3 HOW TO
Nitrogen, Ammonia. Known Addition ISE Method 1 Method 10002 Minimum of 0.8 mg/l NH 3 N. Ammonia ISE. Test preparation. Instrument-specific table
 Nitrogen, Ammonia DOC316.53.01234 Known Addition ISE Method 1 Method 10002 Minimum of 0.8 mg/l NH 3 N Scope and application: For wastewater 2. Ammonia ISE 1 Adapted from the Standard Methods for the Examination
Nitrogen, Ammonia DOC316.53.01234 Known Addition ISE Method 1 Method 10002 Minimum of 0.8 mg/l NH 3 N Scope and application: For wastewater 2. Ammonia ISE 1 Adapted from the Standard Methods for the Examination
Measurement by Ion Selective Electrodes
 Measurement by Ion Selective Electrodes Adrian Vazquez May 8, 2012 Why Use Ion Selective Electrodes? Responsive over a wide concentration range Not affected by color or turbidity of sample Rugged and durable
Measurement by Ion Selective Electrodes Adrian Vazquez May 8, 2012 Why Use Ion Selective Electrodes? Responsive over a wide concentration range Not affected by color or turbidity of sample Rugged and durable
Professional Plus. Calibration Tips
 Professional Plus Calibration Tips Table of Contents Introduction...1 Calibration Worksheet...1 Temperature...3 Calibration Tips...3 Troubleshooting Tips...3 Conductivity...4 Calibration Tips...4 Troubleshooting
Professional Plus Calibration Tips Table of Contents Introduction...1 Calibration Worksheet...1 Temperature...3 Calibration Tips...3 Troubleshooting Tips...3 Conductivity...4 Calibration Tips...4 Troubleshooting
WEL Series ph/orp ELECTRODES
 Iwaki America Inc. WEL Series ph/orp ELECTRODES Instruction Manual Five Boynton Road Hopping Brook Park Holliston, MA 01746 TEL: 508-429-1110 Web: www.walchem.com Notice 2016 WALCHEM, An Iwaki America
Iwaki America Inc. WEL Series ph/orp ELECTRODES Instruction Manual Five Boynton Road Hopping Brook Park Holliston, MA 01746 TEL: 508-429-1110 Web: www.walchem.com Notice 2016 WALCHEM, An Iwaki America
Operator Quick Guide EC SENSOR
 Operator Quick Guide EC SENSOR Revision G - 24/04/2008 General Information About This Guide The information in this guide has been carefully checked and is believed to be accurate. However, Hach Ultra
Operator Quick Guide EC SENSOR Revision G - 24/04/2008 General Information About This Guide The information in this guide has been carefully checked and is believed to be accurate. However, Hach Ultra
General Purpose ph/orp Sensor
 Instruction Sheet General Purpose ph/orp Sensor Sensors LIQ_MAN_ABR_3900 October 2014 General Purpose ph/orp Sensor Specifications Figure 1. 3900 Insertion/Submersion Sensor Sensor Type: General purpose
Instruction Sheet General Purpose ph/orp Sensor Sensors LIQ_MAN_ABR_3900 October 2014 General Purpose ph/orp Sensor Specifications Figure 1. 3900 Insertion/Submersion Sensor Sensor Type: General purpose
DISSOLVED OXYGEN PROBE
 51970-88 DISSOLVED OXYGEN PROBE Introduction This instruction sheet provides information on the Hach Dissolved Oxygen Probe for use with the sension 6 Dissolved Oxygen Meter. The Dissolved Oxygen Probe
51970-88 DISSOLVED OXYGEN PROBE Introduction This instruction sheet provides information on the Hach Dissolved Oxygen Probe for use with the sension 6 Dissolved Oxygen Meter. The Dissolved Oxygen Probe
6 H2O + 6 CO 2 (g) + energy
 AEROBIC RESPIRATION LAB DO 2.CALC From Biology with Calculators, Vernier Software & Technology, 2000. INTRODUCTION Aerobic cellular respiration is the process of converting the chemical energy of organic
AEROBIC RESPIRATION LAB DO 2.CALC From Biology with Calculators, Vernier Software & Technology, 2000. INTRODUCTION Aerobic cellular respiration is the process of converting the chemical energy of organic
Features, Limitations & Potential Errors
 MINIMIZING USER ERRORS IN ph MEASUREMENTS Features, Limitations & Potential Errors of the Tools of ph Measurements Advanced Sensor Technologies, Inc. 603 North Poplar Street Orange CA 92868-1011 USA General
MINIMIZING USER ERRORS IN ph MEASUREMENTS Features, Limitations & Potential Errors of the Tools of ph Measurements Advanced Sensor Technologies, Inc. 603 North Poplar Street Orange CA 92868-1011 USA General
MODEL 970 DISSOLVED OXYGEN METER OPERATING MANUAL
 MODEL 970 DISSOLVED OXYGEN METER OPERATING MANUAL 970 350/REV A/10-03 MODEL 970 DISSOLVED OXYGEN METER OPERATING MANUAL CONTENTS Introduction 1 Specification 1 Installation 2 Displays 2-3 Controls 4 Preparation
MODEL 970 DISSOLVED OXYGEN METER OPERATING MANUAL 970 350/REV A/10-03 MODEL 970 DISSOLVED OXYGEN METER OPERATING MANUAL CONTENTS Introduction 1 Specification 1 Installation 2 Displays 2-3 Controls 4 Preparation
Recommended Procedures for Use of YSI Water Quality Monitoring Instruments during Oil Spills
 Recommended Procedures for Use of YSI Water Quality Monitoring Instruments during Oil Spills Overview: This is a guidance document that is intended for users of YSI 6-Series sondes and handheld instruments
Recommended Procedures for Use of YSI Water Quality Monitoring Instruments during Oil Spills Overview: This is a guidance document that is intended for users of YSI 6-Series sondes and handheld instruments
General Purpose ph/orp Sensors
 General Purpose ph/orp Sensors Product Data Sheet LIQ_PDS_3900 / Rev. F December 2014 General Purpose ph/orp Sensors SMART preamplifier-stores calibration and other data. Extended sensor life provided
General Purpose ph/orp Sensors Product Data Sheet LIQ_PDS_3900 / Rev. F December 2014 General Purpose ph/orp Sensors SMART preamplifier-stores calibration and other data. Extended sensor life provided
Resolution selectable from 0.01 and 0.001 ph. Range -2.000 16.000 ph. Accuracy ± 0.002 ph
 ph EC DO ph Resolution selectable from 0.01 and 0.001 ph Range -2.000 16.000 ph Accuracy ± 0.002 ph Data logging Manual log on demand manual log on stability Interval logging Temperature readout ( C or
ph EC DO ph Resolution selectable from 0.01 and 0.001 ph Range -2.000 16.000 ph Accuracy ± 0.002 ph Data logging Manual log on demand manual log on stability Interval logging Temperature readout ( C or
Measuring ph in Water or CaCl2 Using a ph Meter. Compiled by Darren Murray, June 30, 2011 TABLE OF CONTENTS OVERVIEW 3
 Measuring ph in Water or CaCl2 Using a ph Meter Compiled by Darren Murray, June 30, 2011 TABLE OF CONTENTS OVERVIEW 3 PREPARING THE ph METER FOR USE (Calibration and Buffer Solutions) 4 When is Calibration
Measuring ph in Water or CaCl2 Using a ph Meter Compiled by Darren Murray, June 30, 2011 TABLE OF CONTENTS OVERVIEW 3 PREPARING THE ph METER FOR USE (Calibration and Buffer Solutions) 4 When is Calibration
PART 1 - INTRODUCTION...
 Table of Contents PART 1 - INTRODUCTION... 3 1.1 General... 3 1.2 Sensor Features... 3 1.3 Sensor Specifications (CDE-45P)... 4 Figure 1-1 CDE-45P Sensor Dimensions (standard, convertible style)... 4 PART
Table of Contents PART 1 - INTRODUCTION... 3 1.1 General... 3 1.2 Sensor Features... 3 1.3 Sensor Specifications (CDE-45P)... 4 Figure 1-1 CDE-45P Sensor Dimensions (standard, convertible style)... 4 PART
MODEL 430 PORTABLE ph/conductivity METER OPERATING MANUAL
 MODEL 430 PORTABLE ph/conductivity METER OPERATING MANUAL 430 350/REV A/10-03 MODEL 430 PORTABLE ph/conductivity METER OPERATING MANUAL CONTENTS Introduction 1 Specification 2 Installation 3 Displays 3-4
MODEL 430 PORTABLE ph/conductivity METER OPERATING MANUAL 430 350/REV A/10-03 MODEL 430 PORTABLE ph/conductivity METER OPERATING MANUAL CONTENTS Introduction 1 Specification 2 Installation 3 Displays 3-4
Direct Measurement Method Method 8157 0 to 20.0 mg/l (or 0 to 200% saturation) O 2 Clark-type Amperometric Sensor
 Oxygen, Dissolved DOC316.53.01241 Direct Measurement Method Method 8157 0 to 20.0 mg/l (or 0 to 200% saturation) O 2 Clark-type Amperometric Sensor Scope and application: For water, wastewater and process
Oxygen, Dissolved DOC316.53.01241 Direct Measurement Method Method 8157 0 to 20.0 mg/l (or 0 to 200% saturation) O 2 Clark-type Amperometric Sensor Scope and application: For water, wastewater and process
Trace Dissolved Oxygen Sensor
 Instruction Sheet PN 51A-499ATRDO/rev.G September 2010 Model 499A TrDO Trace Dissolved Oxygen Sensor For additional information, please visit our website at www.emersonprocess.com/raihome/liquid/. CAUTION
Instruction Sheet PN 51A-499ATRDO/rev.G September 2010 Model 499A TrDO Trace Dissolved Oxygen Sensor For additional information, please visit our website at www.emersonprocess.com/raihome/liquid/. CAUTION
PN 44-6033/rev. D December 2010. Theory and Practice of ph Measurement
 PN 44-6033/rev. D December 2010 Theory and Practice of ph Measurement THEORY AND PRACTICE OF ph MEASUREMENT TABLE OF CONTENTS THEORY AND PRACTICE OF ph MEASUREMENT TABLE OF CONTENTS Section Title Page
PN 44-6033/rev. D December 2010 Theory and Practice of ph Measurement THEORY AND PRACTICE OF ph MEASUREMENT TABLE OF CONTENTS THEORY AND PRACTICE OF ph MEASUREMENT TABLE OF CONTENTS Section Title Page
Ion Selective Electrodes
 Ion Selective Electrodes OAKTON has a large selection of Ion Selective Electrodes () to suit a wide variety of applications. Each electrode has a typical response time of 20 to 30 seconds but will vary
Ion Selective Electrodes OAKTON has a large selection of Ion Selective Electrodes () to suit a wide variety of applications. Each electrode has a typical response time of 20 to 30 seconds but will vary
Common Problems with Online Water Quality Analyzers. Greg Macy Director AquatiPro LLC
 Common Problems with Online Water Quality Analyzers Greg Macy Director AquatiPro LLC Objectives Diagnosing Colorimetric Analyzers Identifying Common Problems with Sodium Analyzers Troubleshooting ph Analyzers
Common Problems with Online Water Quality Analyzers Greg Macy Director AquatiPro LLC Objectives Diagnosing Colorimetric Analyzers Identifying Common Problems with Sodium Analyzers Troubleshooting ph Analyzers
User Guide. ExStik TM ph (PH100 & PH110) and ORP (RE300) Waterproof Series Pens. Patent Pending
 User Guide ExStik TM ph (PH100 & PH110) and ORP (RE300) Waterproof Series Pens Patent Pending Getting Started Remove the cap from the bottom of the ExStik TM to expose the electrode bulb and reference
User Guide ExStik TM ph (PH100 & PH110) and ORP (RE300) Waterproof Series Pens Patent Pending Getting Started Remove the cap from the bottom of the ExStik TM to expose the electrode bulb and reference
Global Water Instrumentation, Inc.
 Global Water Instrumentation, Inc. 11390 Amalgam Way Gold River, CA 95670 T: 800-876-1172 Int l: (916) 638-3429, F: (916) 638-3270 Temperature Sensor: WQ101 ph Sensor: WQ201 Conductivity Sensor: WQ301
Global Water Instrumentation, Inc. 11390 Amalgam Way Gold River, CA 95670 T: 800-876-1172 Int l: (916) 638-3429, F: (916) 638-3270 Temperature Sensor: WQ101 ph Sensor: WQ201 Conductivity Sensor: WQ301
CONDUCTIVITY SENSOR. Description D0382. Figure 1. The Conductivity Sensor
 CONDUCTIVITY SENSOR Description D0382 Figure 1. The Conductivity Sensor Short Description The Conductivity Sensor can be used to measure either solution conductivity or total ion concentration of aqueous
CONDUCTIVITY SENSOR Description D0382 Figure 1. The Conductivity Sensor Short Description The Conductivity Sensor can be used to measure either solution conductivity or total ion concentration of aqueous
PROCEDURE COVERSHEET. Document Section: CHEMISTRY Author: JOANNE WONG Date Authored: JAN 2011. In Use Date: 2/1/2011 Discontinued Date:
 PROCEDURE COVERSHEET Procedure Title: ph (Urine, Body Fluid, Platelet Samples) Document Section: CHEMISTRY Author: JOANNE WONG Date Authored: JAN 2011 Laboratory Director: TED KURTZ, MD (PARNASSUS & CHINA
PROCEDURE COVERSHEET Procedure Title: ph (Urine, Body Fluid, Platelet Samples) Document Section: CHEMISTRY Author: JOANNE WONG Date Authored: JAN 2011 Laboratory Director: TED KURTZ, MD (PARNASSUS & CHINA
Compact ph Measuring Instruments
 testo 205, 206, 230 Compact ph Measuring Instruments With innovative probe engineering ph C The new ph measuring instruments with innovative probe engineering Measurement of the ph value plays an important
testo 205, 206, 230 Compact ph Measuring Instruments With innovative probe engineering ph C The new ph measuring instruments with innovative probe engineering Measurement of the ph value plays an important
Operating Instructions
 Operating Instructions FiveEasyPlus ph meter FEP20 Contents Contents 1. Introduction 3 2. Safety measures 3 3. Installation 4 3.1 Installing the electrode arm (optional for FEP20) 4 4. Operating the FiveEasyPlus
Operating Instructions FiveEasyPlus ph meter FEP20 Contents Contents 1. Introduction 3 2. Safety measures 3 3. Installation 4 3.1 Installing the electrode arm (optional for FEP20) 4 4. Operating the FiveEasyPlus
For additional information, please visit our website at www.emersonprocess.com/raihome/liquid/.
 Instruction Sheet PN 51A-499AOZ/rev.H September 2010 Model 499A OZ Ozone Sensor For additional information, please visit our website at www.emersonprocess.com/raihome/liquid/. CAUTION SENSOR/PROCESS APPLICATION
Instruction Sheet PN 51A-499AOZ/rev.H September 2010 Model 499A OZ Ozone Sensor For additional information, please visit our website at www.emersonprocess.com/raihome/liquid/. CAUTION SENSOR/PROCESS APPLICATION
A Potentiometric Analysis of Fluoride Ion in Toothpaste
 CHEM 311L Quantitative Analysis Laboratory Revision 2.0 A Potentiometric Analysis of Fluoride Ion in Toothpaste In this laboratory exercise, we will analyze a toothpaste sample for its Fluoride Ion (F
CHEM 311L Quantitative Analysis Laboratory Revision 2.0 A Potentiometric Analysis of Fluoride Ion in Toothpaste In this laboratory exercise, we will analyze a toothpaste sample for its Fluoride Ion (F
The Theory of ph Measurement
 Application Data Sheet ADS 43-002/rev.C November 2010 Theory The Theory of ph Measurement ph measurement has a wide variety of industrial applications in virtually every industry. These range from water
Application Data Sheet ADS 43-002/rev.C November 2010 Theory The Theory of ph Measurement ph measurement has a wide variety of industrial applications in virtually every industry. These range from water
User Guide. Heavy Duty Dissolved Oxygen Meter. Model 407510
 User Guide Heavy Duty Dissolved Oxygen Meter Model 407510 Introduction Congratulations on your purchase of Extech's Heavy Duty Dissolved Oxygen / Temperature Meter which simultaneously displays Dissolved
User Guide Heavy Duty Dissolved Oxygen Meter Model 407510 Introduction Congratulations on your purchase of Extech's Heavy Duty Dissolved Oxygen / Temperature Meter which simultaneously displays Dissolved
Instruction Manual HI 9146. Portable Waterproof Microprocessor Dissolved Oxygen Meter
 Instruction Manual HI 9146 Portable Waterproof Microprocessor Dissolved Oxygen Meter w w w. h a n n a i n s t. c o m Dear Customer, Thank you for choosing a Hanna Product. Please read this instruction
Instruction Manual HI 9146 Portable Waterproof Microprocessor Dissolved Oxygen Meter w w w. h a n n a i n s t. c o m Dear Customer, Thank you for choosing a Hanna Product. Please read this instruction
After the reactor has been running and the air in the chamber has been purged the co2 can be introduced and the dial in process can begin.
 About Calcium Reactors: A calcium reactor is a device that dissolves calcium carbonate by using co2 gas to create an acidic condition within the reaction chamber. A circulation pump is used to move the
About Calcium Reactors: A calcium reactor is a device that dissolves calcium carbonate by using co2 gas to create an acidic condition within the reaction chamber. A circulation pump is used to move the
UltraBasic Benchtop ph Meter Operation Manual
 UltraBasic Benchtop Meter Operation Manual UB-5 Denver Instrument Measuring 4 4 7 10 Meter 22.2 C 4.06 UltraBasic 902388.1 Rev. A UltraBasic Meter Quick Reference 1. Connect a AC adapter to the power connection.
UltraBasic Benchtop Meter Operation Manual UB-5 Denver Instrument Measuring 4 4 7 10 Meter 22.2 C 4.06 UltraBasic 902388.1 Rev. A UltraBasic Meter Quick Reference 1. Connect a AC adapter to the power connection.
ExStik TM ph Waterproof Meters
 User Guide ExStik TM ph Waterproof Meters Models PH100 & PH110 Patent Pending ExStik TM Description Front Panel Controls 1. Battery compartment cap 2. LCD Display 3. MODE / HOLD button 4. CAL / RECALL
User Guide ExStik TM ph Waterproof Meters Models PH100 & PH110 Patent Pending ExStik TM Description Front Panel Controls 1. Battery compartment cap 2. LCD Display 3. MODE / HOLD button 4. CAL / RECALL
Determining Equivalent Weight by Copper Electrolysis
 Purpose The purpose of this experiment is to determine the equivalent mass of copper based on change in the mass of a copper electrode and the volume of hydrogen gas generated during an electrolysis reaction.
Purpose The purpose of this experiment is to determine the equivalent mass of copper based on change in the mass of a copper electrode and the volume of hydrogen gas generated during an electrolysis reaction.
Care and use guide. Contents. page
 Care and use guide Contents page Features Bluelab Combo Meter Introduction Preparing for use Calibration Changing nutrient and temperature display units 6 Measuring hydroponic elements 7 Battery replacement
Care and use guide Contents page Features Bluelab Combo Meter Introduction Preparing for use Calibration Changing nutrient and temperature display units 6 Measuring hydroponic elements 7 Battery replacement
OxyGuard. Dissolved Oxygen Probe
 OxyGuard Dissolved Oxygen Probe Standard type USER MANUAL D02 Standard Probe manual gb 0903 CONTENTS 1. DESCRIPTION 1.1 The OxyGuard Probe... 1 1.3 Which kind of transmitter can be used with the OxyGuard
OxyGuard Dissolved Oxygen Probe Standard type USER MANUAL D02 Standard Probe manual gb 0903 CONTENTS 1. DESCRIPTION 1.1 The OxyGuard Probe... 1 1.3 Which kind of transmitter can be used with the OxyGuard
CYCLE CHEMISTRY ph MEASUREMENT
 CYCLE CHEMISTRY ph MEASUREMENT David M. Gray and Edward P. Santini Thornton Associates, Waltham MA Electric Utility Chemistry Workshop Champaign, Illinois May 12-14, 1998 Sponsored by University of Illinois
CYCLE CHEMISTRY ph MEASUREMENT David M. Gray and Edward P. Santini Thornton Associates, Waltham MA Electric Utility Chemistry Workshop Champaign, Illinois May 12-14, 1998 Sponsored by University of Illinois
Reaction of Magnesium with Hydrochloric Acid (Gas Laws) Chemicals Needed:
 Reaction of Magnesium with Hydrochloric Acid (Gas Laws) Your Name: Date: Partner(s) Names: Objectives: React magnesium metal with hydrochloric acid, collecting the hydrogen over water. Calculate the grams
Reaction of Magnesium with Hydrochloric Acid (Gas Laws) Your Name: Date: Partner(s) Names: Objectives: React magnesium metal with hydrochloric acid, collecting the hydrogen over water. Calculate the grams
Acid Dissociation Constants and the Titration of a Weak Acid
 Acid Dissociation Constants and the Titration of a Weak Acid One of the most important applications of equilibria is the chemistry of acids and bases. The Brønsted-Lowry acid-base theory defines an acid
Acid Dissociation Constants and the Titration of a Weak Acid One of the most important applications of equilibria is the chemistry of acids and bases. The Brønsted-Lowry acid-base theory defines an acid
SDX Submersible Depth Transmitter User Manual
 SDX Submersible Depth Transmitter User Manual January 2011 USER INFORMATION Stevens makes no warranty as to the information furnished in these instructions and the reader assumes all risk in the use thereof.
SDX Submersible Depth Transmitter User Manual January 2011 USER INFORMATION Stevens makes no warranty as to the information furnished in these instructions and the reader assumes all risk in the use thereof.
Handbook. Thermo Scientific. ph Electrode Selection Handbook
 Handbook Thermo Scientific ph Electrode Selection Handbook Table of Contents ph Theory... 4 ph Electrode Theory... 4 ph versus Temperature Theory... 5 ph Electrode Selection Guide... 6 Thermo Scientific
Handbook Thermo Scientific ph Electrode Selection Handbook Table of Contents ph Theory... 4 ph Electrode Theory... 4 ph versus Temperature Theory... 5 ph Electrode Selection Guide... 6 Thermo Scientific
Chapter2. Installation
 Chapter2. Installation Power Supply Requirements The ULM-1000C operates from a DC supply of 20 30V and will typically draw less than 0.08A. All electronic products are susceptible to electrostatic shock,
Chapter2. Installation Power Supply Requirements The ULM-1000C operates from a DC supply of 20 30V and will typically draw less than 0.08A. All electronic products are susceptible to electrostatic shock,
Ozone Sensor. Model 499A OZ CAUTION CAUTION. Instruction Sheet PN 51A-499AOZ/rev.F January 2007
 Instruction Sheet PN 51A-499AOZ/rev.F January 2007 Model 499A OZ Ozone Sensor For additional information, please refer to the Instruction Manuals CD shipped with this product, or visit our website at www.emersonprocess.com/raihome/liquid/.
Instruction Sheet PN 51A-499AOZ/rev.F January 2007 Model 499A OZ Ozone Sensor For additional information, please refer to the Instruction Manuals CD shipped with this product, or visit our website at www.emersonprocess.com/raihome/liquid/.
Care and use guide. Contents. a handy solution made easy. www.getbluelab.com. page
 Care and use guide Contents page Features Quick guide Before use To operate Important - ph pen probe care 4 Cleaning 5 Battery replacement 5 Hydration 6 Calibration 6 Error messages 7 Troubleshooting guide
Care and use guide Contents page Features Quick guide Before use To operate Important - ph pen probe care 4 Cleaning 5 Battery replacement 5 Hydration 6 Calibration 6 Error messages 7 Troubleshooting guide
Stainless Steel Dissolved Oxygen Electrodes
 Stainless Steel Dissolved Oxygen Electrodes DISSOLVED OXYGEN ELECTRODES The steam sterilizable family of dissolved oxygen electrodes from the phoenix Electrode Company is specifically designed for fermentation
Stainless Steel Dissolved Oxygen Electrodes DISSOLVED OXYGEN ELECTRODES The steam sterilizable family of dissolved oxygen electrodes from the phoenix Electrode Company is specifically designed for fermentation
The Control of ph and Oxidation Reduction Potential (ORP) in Cooling Tower Applications By Charles T. Johnson, Walchem Corporation
 The Control of ph and Oxidation Reduction Potential (ORP) in Cooling Tower Applications By Charles T. Johnson, Walchem Corporation Introduction The importance of keeping cooling tower water in proper chemical
The Control of ph and Oxidation Reduction Potential (ORP) in Cooling Tower Applications By Charles T. Johnson, Walchem Corporation Introduction The importance of keeping cooling tower water in proper chemical
Bluelab Soil ph Meter Instruction Manual
 Instruction Manual www.getbluelab.com Table of Contents 1.0 Information about measuring the ph of soils/media. 2 2.0 Introduction to Bluelab Soil ph Meter 4 3.0 Preparing the Bluelab Soil ph Meter for
Instruction Manual www.getbluelab.com Table of Contents 1.0 Information about measuring the ph of soils/media. 2 2.0 Introduction to Bluelab Soil ph Meter 4 3.0 Preparing the Bluelab Soil ph Meter for
Cadmium Reduction Method Method 8039 0.3 to 30.0 mg/l NO 3 N (HR) Powder Pillows or AccuVac Ampuls
 Nitrate DOC316.53.01066 Cadmium Reduction Method Method 8039 0.3 to 30.0 mg/l NO 3 N (HR) Powder Pillows or AccuVac Ampuls Scope and application: For water, wastewater and seawater. Test preparation Instrument-specific
Nitrate DOC316.53.01066 Cadmium Reduction Method Method 8039 0.3 to 30.0 mg/l NO 3 N (HR) Powder Pillows or AccuVac Ampuls Scope and application: For water, wastewater and seawater. Test preparation Instrument-specific
Figure 1. A voltaic cell Cu,Cu 2+ Ag +, Ag. gas is, by convention, assigned a reduction potential of 0.00 V.
 Voltaic Cells Introduction In this lab you will first prepare a set of simple standard half-cells and then measure the voltage between the half-cells with a voltmeter. From this data you will be able to
Voltaic Cells Introduction In this lab you will first prepare a set of simple standard half-cells and then measure the voltage between the half-cells with a voltmeter. From this data you will be able to
OXIGENO DISUELTO. Dissolved Oxygen. Install & forget. Sistema autolimpiable. Autoclean system
 Sistema autolimpiable Dissolved Oxygen Autoclean system Applications: - water treatment - activated sludge - de-nitrification - fish pond Install & forget For special applications, the D.O monitor can
Sistema autolimpiable Dissolved Oxygen Autoclean system Applications: - water treatment - activated sludge - de-nitrification - fish pond Install & forget For special applications, the D.O monitor can
Chloride and Salinity
 INTRODUCTION Chloride Chloride and Chloride, in the form of the Cl ion, is one of the major inorganic anions, or negative ions, in saltwater and freshwater. It originates from the dissociation of salts,
INTRODUCTION Chloride Chloride and Chloride, in the form of the Cl ion, is one of the major inorganic anions, or negative ions, in saltwater and freshwater. It originates from the dissociation of salts,
YSI Meter Protocol: Specific Conductivity, ph, & Dissolved Oxygen
 YSI Meter Protocol: Specific Conductivity, ph, & Dissolved Oxygen 1. Check the log-book to see if somebody else has already checked the meter for accuracy within the timeframes below. If the meter hasn
YSI Meter Protocol: Specific Conductivity, ph, & Dissolved Oxygen 1. Check the log-book to see if somebody else has already checked the meter for accuracy within the timeframes below. If the meter hasn
STANDARD OPERATING PROCEDURE Dissolved Oxygen Measurement, Meter Calibration and Maintenance
 Page 1 of 9 KEY WORDS- Dissolved Oxygen, calibration, probe preparation, meter preparation APPROVALS APPROVED BY: Kean S. Go, DATE: +/?? APPROVED BY: A Pement DATE: a// A/? f Lisa Ross, EHAP Senior Scientist
Page 1 of 9 KEY WORDS- Dissolved Oxygen, calibration, probe preparation, meter preparation APPROVALS APPROVED BY: Kean S. Go, DATE: +/?? APPROVED BY: A Pement DATE: a// A/? f Lisa Ross, EHAP Senior Scientist
SOLAR PV SYSTEM MAINTENANCE GUIDE
 SOLAR PV SYSTEM MAINTENANCE GUIDE GUYANA HINTERLANDS STAND-ALONE SOLAR PV INSTALLATIONS IMPROVING HEALTH FACILITY INFRASTRUCTURE (IHFI) GUYANA CONTRACT NO. EPP-I-00-03-00008-00, TASK ORDER 07 APRIL 2013
SOLAR PV SYSTEM MAINTENANCE GUIDE GUYANA HINTERLANDS STAND-ALONE SOLAR PV INSTALLATIONS IMPROVING HEALTH FACILITY INFRASTRUCTURE (IHFI) GUYANA CONTRACT NO. EPP-I-00-03-00008-00, TASK ORDER 07 APRIL 2013
SINGLE RANGE DS METERS Operation Instructions
 SINGLE RANGE DS METERS Operation Instructions User Manual for Models 512T2 512T3 512T4 512T5 512T10 512M2 512M3 512M4 512M5 512M10 6115 Corte del Cedro Carlsbad, CA 92009-1516 USA Tel 760-438-2021 Fax
SINGLE RANGE DS METERS Operation Instructions User Manual for Models 512T2 512T3 512T4 512T5 512T10 512M2 512M3 512M4 512M5 512M10 6115 Corte del Cedro Carlsbad, CA 92009-1516 USA Tel 760-438-2021 Fax
User Guide. 9708 Dissolved Oxygen Probes
 User Guide 9708 Dissolved Oxygen Probes Introduction The Thermo Scientific Orion 9708 dissolved oxygen probe simplifies measurements of dissolved oxygen, particularly Biochemical Oxygen Demand (BOD).
User Guide 9708 Dissolved Oxygen Probes Introduction The Thermo Scientific Orion 9708 dissolved oxygen probe simplifies measurements of dissolved oxygen, particularly Biochemical Oxygen Demand (BOD).
Pro1030 USER MANUAL. English
 Pro1030 USER MANUAL English Contents Warranty...i Introduction...1 Item #605182 Rev A, January 2013 For the latest version of this manual, visit ysi.com 2013 YSI Incorporated. The YSI logo is a registered
Pro1030 USER MANUAL English Contents Warranty...i Introduction...1 Item #605182 Rev A, January 2013 For the latest version of this manual, visit ysi.com 2013 YSI Incorporated. The YSI logo is a registered
Combination ph/redox (ORP) Sensors
 Data Sheet SS/AP00 Issue 4 Combination ph/redox (ORP) Sensors AP00 Series Long life large PTFE junction provides resistance to fouling double junction with gelled electrolyte provides better-defined junction
Data Sheet SS/AP00 Issue 4 Combination ph/redox (ORP) Sensors AP00 Series Long life large PTFE junction provides resistance to fouling double junction with gelled electrolyte provides better-defined junction
ELECTROCHEMISTRY. BOECO ELECTRODES The electrodes are not included with the ph meters and have to be ordered separately:
 BOECO PORTABLE PH/ORP/TEMP METER MODEL PT-380 A Simultaneous displays ph or mv and temperature. A Convenient calibration with automatic buffer recognition of European and US buffer sets. Calibration data
BOECO PORTABLE PH/ORP/TEMP METER MODEL PT-380 A Simultaneous displays ph or mv and temperature. A Convenient calibration with automatic buffer recognition of European and US buffer sets. Calibration data
Neutralizing an Acid and a Base
 Balancing Act Teacher Information Objectives In this activity, students neutralize a base with an acid. Students determine the point of neutralization of an acid mixed with a base while they: Recognize
Balancing Act Teacher Information Objectives In this activity, students neutralize a base with an acid. Students determine the point of neutralization of an acid mixed with a base while they: Recognize
BATTERY CHARGING GUIDELINES FOR 6-VOLT DEEP CYCLE BATTERIES
 BATTERY CHARGING GUIDELINES FOR 6-VOLT DEEP CYCLE BATTERIES Introduction Your 6-volt deep cycle battery set has been specially formulated for long cycle life and has particular voltage and specific gravity
BATTERY CHARGING GUIDELINES FOR 6-VOLT DEEP CYCLE BATTERIES Introduction Your 6-volt deep cycle battery set has been specially formulated for long cycle life and has particular voltage and specific gravity
Chemistry 119: Experiment 7. Potentiometric Titration of Ascorbic Acid in Vitamin C Tablets
 Chemistry 119: Experiment 7 Potentiometric Titration of Ascorbic Acid in Vitamin C Tablets Vitamin C is another name for ascorbic acid (C 6 H 8 O 6, see below ), a weak acid that can be determined by titration
Chemistry 119: Experiment 7 Potentiometric Titration of Ascorbic Acid in Vitamin C Tablets Vitamin C is another name for ascorbic acid (C 6 H 8 O 6, see below ), a weak acid that can be determined by titration
Experiment 4 (Future - Lab needs an unknown)
 Experiment 4 (Future - Lab needs an unknown) USING A ph TITRATION TO DETERMINE THE ACID CONTENT OF SOFT DRINKS 2 lab periods Reading: Chapter 9, 185-197; Chapter 10, pg 212-218; Chapter 14 pg 317-323,
Experiment 4 (Future - Lab needs an unknown) USING A ph TITRATION TO DETERMINE THE ACID CONTENT OF SOFT DRINKS 2 lab periods Reading: Chapter 9, 185-197; Chapter 10, pg 212-218; Chapter 14 pg 317-323,
MSFET 3332. MICROSENS Miniature ph Sensor Module
 Product Data Sheet MSFET 3332 MICROSENS Miniature ph Sensor Module Ta2O5 gate Ion Sensitive Field Effect transistor (ISFET) The MSFET3332 ph sensor module comprises a ph-isfet sensing element, an integrated
Product Data Sheet MSFET 3332 MICROSENS Miniature ph Sensor Module Ta2O5 gate Ion Sensitive Field Effect transistor (ISFET) The MSFET3332 ph sensor module comprises a ph-isfet sensing element, an integrated
MIST DECORATIVE LAMP WITH ESSENTIAL OIL DIFFUSER
 EN MIST DECORATIVE LAMP WITH ESSENTIAL OIL DIFFUSER OPERATING INSTRUCTIONS Read the instructions carefully before operating the appliance or carrying out maintenance operations. Observe all the safety
EN MIST DECORATIVE LAMP WITH ESSENTIAL OIL DIFFUSER OPERATING INSTRUCTIONS Read the instructions carefully before operating the appliance or carrying out maintenance operations. Observe all the safety
TEMPERATURE SENSOR USER MANUAL
 TEMPERATURE SENSOR USER MANUAL 1 Temperature sensor user manual Copyright 2012 Unisense A/S Version October 2012 TEMPERATURE SENSOR USER MANUAL UNISENSE A/S TABLE OF CONTENTS WARRANTY AND LIABILITY....8
TEMPERATURE SENSOR USER MANUAL 1 Temperature sensor user manual Copyright 2012 Unisense A/S Version October 2012 TEMPERATURE SENSOR USER MANUAL UNISENSE A/S TABLE OF CONTENTS WARRANTY AND LIABILITY....8
If you accidentally get battery acid on your skin, flush it with lots of water
 RV Battery Savvy To properly maintain and extend the life of your RV batteries you need to have a basic understanding of what a battery is and how it works. Batteries used in RVs are lead acid batteries.
RV Battery Savvy To properly maintain and extend the life of your RV batteries you need to have a basic understanding of what a battery is and how it works. Batteries used in RVs are lead acid batteries.
The table below lists the symbols used on the Clamp and/or in this manual. Important Information. See manual.
 i800 AC Current Clamp Instruction Sheet Introduction The i800 AC Current Clamp, the Clamp, has been designed for use with multimeters, recorders, power analyzers, safety testers, etc., for accurate non-intrusive
i800 AC Current Clamp Instruction Sheet Introduction The i800 AC Current Clamp, the Clamp, has been designed for use with multimeters, recorders, power analyzers, safety testers, etc., for accurate non-intrusive
User's Guide. ExStik DO600. Dissolved Oxygen Meter RECALL. Dissolved Oxygen DO600
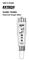 User's Guide ExStik DO600 Dissolved Oxygen Meter II RECALL Dissolved Oxygen DO600 Introduction Congratulations on your purchase of the ExStik DO600 dissolved oxygen / temperature meter which simultaneously
User's Guide ExStik DO600 Dissolved Oxygen Meter II RECALL Dissolved Oxygen DO600 Introduction Congratulations on your purchase of the ExStik DO600 dissolved oxygen / temperature meter which simultaneously
 2 (. 3 4 5 . -,.. -,. 3. : 1 2 3 > 4, :!! 1,, 17 Lt MAX 2 3 4,, > 5.,,, 6 :, :1)! 2)! 7,, 1, :,. 2,, Bottom of steam generator 7 8 9 10 11 12 automatically and this phenomenon will continue for
2 (. 3 4 5 . -,.. -,. 3. : 1 2 3 > 4, :!! 1,, 17 Lt MAX 2 3 4,, > 5.,,, 6 :, :1)! 2)! 7,, 1, :,. 2,, Bottom of steam generator 7 8 9 10 11 12 automatically and this phenomenon will continue for
white paper reviews why the chlor-alkali process is so damaging to ph electrodes and discusses a sensor design that has
 INGOLD White Paper Leading Process Analytics No More Tears Elevated temperatures, presence of chlorine and high salt concentration throughout the chlor-alkali process make stable and reliable ph measurement
INGOLD White Paper Leading Process Analytics No More Tears Elevated temperatures, presence of chlorine and high salt concentration throughout the chlor-alkali process make stable and reliable ph measurement
EXPERIMENT 7 Electrochemical Cells: A Discovery Exercise 1. Introduction. Discussion
 EXPERIMENT 7 Electrochemical Cells: A Discovery Exercise 1 Introduction This lab is designed for you to discover the properties of electrochemical cells. It requires little previous knowledge of electrochemical
EXPERIMENT 7 Electrochemical Cells: A Discovery Exercise 1 Introduction This lab is designed for you to discover the properties of electrochemical cells. It requires little previous knowledge of electrochemical
High Purity Water Resistivity/ Conductivity Measurement
 CONDUCTIVITY MEASUREMENT IN HIGH PURITY WATER SAMPLES below 10 µsiemens/cm High Purity Water Resistivity/ Conductivity Measurement Ultra-Pure Water without any chemical impurities will still have a conductivity
CONDUCTIVITY MEASUREMENT IN HIGH PURITY WATER SAMPLES below 10 µsiemens/cm High Purity Water Resistivity/ Conductivity Measurement Ultra-Pure Water without any chemical impurities will still have a conductivity
Appendix C. Vernier Tutorial
 C-1. Vernier Tutorial Introduction: In this lab course, you will collect, analyze and interpret data. The purpose of this tutorial is to teach you how to use the Vernier System to collect and transfer
C-1. Vernier Tutorial Introduction: In this lab course, you will collect, analyze and interpret data. The purpose of this tutorial is to teach you how to use the Vernier System to collect and transfer
Instruction Manual ph 5+ ph 6+ Ion 6+ Technology Made Easy...
 Instruction Manual ph 5+ ph/ C ph 6+ ph/ C/mV Ion 6+ ph/ C/mV/Ion Technology Made Easy... 68X243633 Rev 1-1 3/2011 Part of Thermo Fisher Scientific Preface This instruction manual serves to explain the
Instruction Manual ph 5+ ph/ C ph 6+ ph/ C/mV Ion 6+ ph/ C/mV/Ion Technology Made Easy... 68X243633 Rev 1-1 3/2011 Part of Thermo Fisher Scientific Preface This instruction manual serves to explain the
DeltaSpan Submersible Pressure Level Transmitter Manual LD31 Series 22 AUG 08 Rev A
 DeltaSpan Submersible Pressure Level Transmitter Manual LD31 Series 22 AUG 08 Flowline, Inc. 10500 Humbolt Street Los Alamitos, CA 90720 Tel: (562) 598-3015 Fax: (562) 431-8507 www.flowline.com 22 AUG
DeltaSpan Submersible Pressure Level Transmitter Manual LD31 Series 22 AUG 08 Flowline, Inc. 10500 Humbolt Street Los Alamitos, CA 90720 Tel: (562) 598-3015 Fax: (562) 431-8507 www.flowline.com 22 AUG
Acid Base Titrations
 Acid Base Titrations Introduction A common question chemists have to answer is how much of something is present in a sample or a product. If the product contains an acid or base, this question is usually
Acid Base Titrations Introduction A common question chemists have to answer is how much of something is present in a sample or a product. If the product contains an acid or base, this question is usually
JUMO BlackLine CR-GT/-EC/-GS Conductive 2-Electrode Conductivity Sensor
 Vorläufig V Page 1/8 JUMO BlackLine CR-GT/-EC/-GS Conductive 2-Electrode Conductivity Sensor Cell constants K = 0.01 1.0 202922 Series for universal application, e.g. drinking and surface water monitoring,
Vorläufig V Page 1/8 JUMO BlackLine CR-GT/-EC/-GS Conductive 2-Electrode Conductivity Sensor Cell constants K = 0.01 1.0 202922 Series for universal application, e.g. drinking and surface water monitoring,
Experiment 8 - Double Displacement Reactions
 Experiment 8 - Double Displacement Reactions A double displacement reaction involves two ionic compounds that are dissolved in water. In a double displacement reaction, it appears as though the ions are
Experiment 8 - Double Displacement Reactions A double displacement reaction involves two ionic compounds that are dissolved in water. In a double displacement reaction, it appears as though the ions are
EXPRESS STILL WARNING It is legal in New Zealand to own and operate a still for the purpose of producing alcohol for your own consumption.
 THE EXPRESS STILL This still represents the next generation in home distillation equipment. It eliminates the problem of having to spend all day processing a 25 litre wash. Taking 1 hour to heat up, then
THE EXPRESS STILL This still represents the next generation in home distillation equipment. It eliminates the problem of having to spend all day processing a 25 litre wash. Taking 1 hour to heat up, then
Dissolved Oxygen Probe (Order Code DO-BTA or DO-DIN)
 Dissolved Oxygen Probe (Order Code DO-BTA or DO-DIN) The Dissolved Oxygen Probe can be used to measure the concentration of dissolved oxygen in water samples tested in the field or in the laboratory. You
Dissolved Oxygen Probe (Order Code DO-BTA or DO-DIN) The Dissolved Oxygen Probe can be used to measure the concentration of dissolved oxygen in water samples tested in the field or in the laboratory. You
INSTALLATION & OPERATING INSTRUCTIONS
 INSTALLATION & OPERATING INSTRUCTIONS WARNING RISK OF ELECTRIC SHOCK. CONNECT ONLY TO A CIRCUIT PROTECTED BY A GROUND-FAULT CIRCUIT-INTERRUPTER. THE UNIT SHOULD BE INSTALLED BY A QUALIFIED SERVICE REPRESENTATIVE.
INSTALLATION & OPERATING INSTRUCTIONS WARNING RISK OF ELECTRIC SHOCK. CONNECT ONLY TO A CIRCUIT PROTECTED BY A GROUND-FAULT CIRCUIT-INTERRUPTER. THE UNIT SHOULD BE INSTALLED BY A QUALIFIED SERVICE REPRESENTATIVE.
PRETREATMENT TECHNICAL DATA SHEET WATER EMULSIFIABLE RUST PREVENTATIVE PRODUCT DESCRIPTION
 INDUSTRIAL COATINGS RA260 WATER EMULSIFIABLE RUST PREVENTATIVE PRODUCT DESCRIPTION is water emulsifiable rust preventative oil, which provides outstanding corrosion protection for all phosphate metals.
INDUSTRIAL COATINGS RA260 WATER EMULSIFIABLE RUST PREVENTATIVE PRODUCT DESCRIPTION is water emulsifiable rust preventative oil, which provides outstanding corrosion protection for all phosphate metals.
Electrical Conductivity of Aqueous Solutions
 Electrical Conductivity of Aqueous Solutions PRE-LAB ASSIGNMENT: Reading: Chapter 4.-4.3 in Brown, LeMay, Bursten & Murphy.. Using Table in this handout, determine which solution has a higher conductivity,.
Electrical Conductivity of Aqueous Solutions PRE-LAB ASSIGNMENT: Reading: Chapter 4.-4.3 in Brown, LeMay, Bursten & Murphy.. Using Table in this handout, determine which solution has a higher conductivity,.
