Guideline for good evaluation practice in health informatics (GEP-HI)
|
|
|
- Sibyl Barbra Floyd
- 8 years ago
- Views:
Transcription
1 i n t e r n a t i o n a l j o u r n a l o f m e d i c a l i n f o r m a t i c s 8 0 ( ) j ourna l homepage: Guideline for good evaluation practice in health informatics (GEP-HI) Pirkko Nykänen a,, Jytte Brender b, Jan Talmon c, Nicolette de Keizer d, Michael Rigby e, Marie-Catherine Beuscart-Zephir f, Elske Ammenwerth g a University of Tampere, School of Information Sciences, ehealth Research, Tampere, Finland b Aalborg University, Department of Health Science and Technology, and Virtual Center for Health Informatics, Aalborg, Denmark c Maastricht University, School for Public Health and Primary Care: Caphri, Maastricht, The Netherlands d Academic Medical Center, Department of Medical Informatics, Amsterdam, The Netherlands e Keele University, School of Public Policy and Professional Practice, Keele, United Kingdom f University of Lille Nord de France, INSERM-CIC-IT EVALAB, Lille, France g UMIT, University for Health Sciences, Medical Informatics and Technology, Institute for Health Informatics, Hall in Tyrol, Austria a r t i c l e i n f o Article history: Received 21 January 2011 Received in revised form 15 August 2011 Accepted 16 August 2011 Keywords: Health informatics Evaluation Guideline Research design a b s t r a c t Objective: Development of a good practice guideline to plan and perform scientifically robust evaluation studies in health informatics. Methods: Issues to be addressed in evaluation studies were identified and guidance drafted based on the evaluation literature and on experiences by key players. Successive drafts of the guideline were discussed in several rounds by an increasing number of experts during conferences and by . At a fairly early point the guideline was put up for comments on the web. Results: Sixty issues were identified that are of potential relevance for planning, implementation and execution of an evaluation study in the health informatics domain. These issues cover all phases of an evaluation study: Preliminary outline, study design, operationalization of methods, project planning, execution and completion of the evaluation study. Issues of risk management and project control as well as reporting and publication of the evaluation results are also addressed. Conclusion: A comprehensive list of issues is presented as a guideline for good evaluation practice in health informatics (GEP-HI). The strengths and weaknesses of the guideline are discussed. Application of this guideline will support better handling of an evaluation study, potentially leading to a higher quality of evaluation studies. This guideline is an important step towards building stronger evidence and thus to progress towards evidence-based health informatics Elsevier Ireland Ltd. All rights reserved. 1. Introduction Corresponding author. address: Pirkko.Nykanen@uta.fi (P. Nykänen) /$ see front matter 2011 Elsevier Ireland Ltd. All rights reserved. doi: /j.ijmedinf Development and use of health informatics applications offer tremendous opportunities to improve health care, its delivery and outcomes. There is an increasing political urge to implement available information technology (IT) solutions, as discussed by Refs. [1 4]. However, there are also hazards and
2 816 i n t e r n a t i o n a l j o u r n a l o f m e d i c a l i n f o r m a t i c s 8 0 ( ) problems related to the use of IT in health care, for example IT may be inappropriately specified, unreliable, user-unfriendly or the organization may not be properly prepared to adopt IT within the clinical work flows and processes, as shown by Ref. [5]. Health IT may also be ill-functioning, for example inducing medical errors by presenting faulty displays of the electronic health records, see Ref. [6], or having negative impacts on the outcome of care in a specialised care unit, as documented by Ref. [7]. The effectiveness of health IT is still limited and inconsistent, across a wide range of fields, as reviewed by Ref. [8]. In the primary care the only benefits reliably documented on health information exchange were those on efficiency, including improved access to test results and other data from outside the practice, and decreased staff time for handling referrals and claims processing. Barriers included cost, privacy and liability concerns, organizational characteristics, and technical barriers, see Ref. [9]. This situation calls for evaluation to provide robust evidence about the impacts and actual efficiency, quality, and safety gains of health IT that can be achieved. Evaluation is the means to assess the quality, value, effects and impacts of IT in the health care environment, as stated by Refs. [10,11]. Evaluation is defined as the act of measuring or exploring properties of a health information system (in planning, development, implementation, or operation), the result of which informs a decision to be made concerning that system in a specific context [[12], p. 480]. The white paper [13] suggests extensive and systematic pre-implementation evaluation studies to inform difficult decisions prior to starting any programme initiative as well as post-implementation evaluation studies. The present contribution provides a guideline for conducting health IT evaluations. It goes beyond preand post-impact evaluation to support evaluation studies in general, formative as well as summative studies. Formative evaluation is performed throughout the systems lifecycle and it provides information for improving the system under development. Summative evaluation is focused on assessing the effect or outcome of the evaluation object at a certain point of time after implementation [14]. The exploratory workshop HIS-EVAL by European Science Foundation (ESF) was instrumental in identifying the state of affairs with respect to evaluation of health IT applications and in defining the necessary steps to further evaluations [12]. An important result from the workshop was the Declaration of Innsbruck [15] summarizing the importance of evaluation as: Health information systems are intended to improve the functioning of health professionals and organizations in managing health and delivering healthcare. Given the significance of this type of intervention, and the intended beneficial effect on patients and professionals, it is morally imperative to ensure that the optimum results are achieved and any unanticipated outcomes identified. The necessary process is evaluation and this should be considered an essential adjunct to design and implementation of information systems. Reflective deliberations at the HIS-EVAL workshop led to the conclusion of a need for two guidelines: One for structured reporting of evaluation studies (STARE-HI 1 ) and one for good practice for planning and execution of evaluation studies (GEP-HI). The STARE-HI statement is finalized and published in Ref. [16]. It is endorsed by the board of the European Federation of Medical Informatics (EFMI) and adopted as an official document by the International Medical Informatics Association (IMIA), and is cited by the Equator network for good reporting of health informatics studies [17]. The present paper describes the GEP-HI guideline as well as its development process Background The current state of evaluation studies was explored in a review of problems and pitfalls [[11], pp ] resulting in a listing of several biases one should be aware of when designing and executing an evaluation study. These biases may result in incorrect results, wrong interpretations and under- or overestimation of the findings. In the review [11] many examples were found for most of the identified biases while it was difficult to find publications on high quality studies that properly addressed all of these potential biases. The conclusion is that evaluation of health IT is more than the deployment of a collection of methods, it is both a discipline and a specialty, and requires an overarching objectives-led design and operational management. Evaluation can be compared with health technology assessment (HTA), but HTA is based on a pre-defined formal framework and usually has a summative nature using general evaluation methods and approaches, often quantitative in nature, see Ref. [18]. Reviews of the health IT evaluations commissioned for the IMIA Yearbook showed the importance of evaluation, the challenges, and the currently very limited response, both in recognising integrated methodologies and in endorsing the importance of robust studies, as argued by Refs. [19,20]. The need to develop and publish a guideline was identified in the health IT evaluation domain concurrent with the GEP- HI guideline development. In a literature review on discourses, dimensions and methods of health IT evaluation the conclusion was that the identified evaluation frameworks differed in terms of generality, specificity, timing related to system development phases and theoretical underpinning, see Ref. [21]. GEP-HI adds to this knowledge by presenting a guideline for design and execution of an evaluation study. A Model for ASsessment of Telemedicine applications (MAST) was developed in an EU-project Metho-Telemed to give advice to users on what to consider before an evaluation study. The MAST-model lists aspects of telemedicine evaluation within seven domains of outcomes: Health problem and characteristics of the application, safety, clinical effectiveness, patient perspectives, economic aspects, organizational aspects and socio-cultural, ethical and legal aspects, see Ref. [22]. It is planned as a toolkit, a checklist of issues that need to be considered in evaluation. MAST is based on a HTA approach and focuses on telemedicine systems, not on health IT generally. In contrast, this GEP-HI guideline is wider and applicable to evaluation of health IT in general. Additionally, MAST does 1 Abbreviation for STAtement on Reporting of Evaluation studies in Health Informatics.
3 i n t e r n a t i o n a l j o u r n a l o f m e d i c a l i n f o r m a t i c s 8 0 ( ) not consider the execution and management of an evaluation project. Cusack et al. developed for the US Agency for Healthcare Research and Quality (AHRQ) a toolkit to provide step-by-step guidance for developing evaluation plans for health IT projects [23]. The toolkit assists evaluators in defining the goals for evaluation, what is important to stakeholders, what needs to be measured to satisfy stakeholders, what is realistic and feasible to measure, and how to measure these items. Examples are presented with suggested evaluation methods for each item. The toolkit is very useful from the methodological point of view and it can be applied within the GEP-HI guideline. The toolkit does not, however, give guidance on the evaluation project itself, how to manage it, how to carry out the project, or how to complete and report the study. Thus it details only part of the GEP-HI guideline. Life cycle frameworks for evaluation have been proposed for example by Catwell and Sheikh [24] and Clarke et al. [25]. These are focused on how to evaluate health IT interventions while being designed, developed and deployed. These schematic models are formative and relate evaluation to the phases of the system development. The frameworks propose various evaluation measures and checkpoints during the system phases. The lifecycle evaluation frameworks are valuable tools to monitor the development process and the deployment of a new system. The frameworks do not, however, give guidance for the planning and execution of an evaluation project, and do not support evaluation as an independent activity. Additional reports on various approaches and frameworks on evaluation are published, for example in Refs. [26 31]. These frameworks can be applied together with the over-arching GEP-HI guideline as they provide support for methodical choices but do not deal with the overall design and execution of an evaluation study as do the GEP-HI guideline Objective of the guideline The objective of the GEP-HI guideline is to give advice on how to design and carry out evaluation studies in various health IT contexts. The guideline aims to be general and practical, and to provide evaluators with a set of structured principles for good evaluation practice. The principles are inspired by the best evaluation practices as implicitly and explicitly described in the literature. In the guideline we point at issues and list recommendations on how to design evaluation studies, how to make methodological choices, how to conduct studies, and how to define evaluation criteria at specific phases of the health IT applications life cycle. 2. Method The method applied to develop GEP-HI guideline was a consensus-seeking process within the community of health IT evaluation experts. The primary authors of GEP-HI, all were participants of the ESF HIS-EVAL Workshop (see Ref. [15]) and active in the EFMI and IMIA working groups dealing with evaluation of health IT applications and HTA; and they all have long experience in planning and conducting evaluation studies [see for example Refs. [5,11,12,16,19,20,25,30,32,33,36,38]]. The starting point for the guideline development was the existing knowledge, experience and literature on evaluation studies, methodologies, guidelines development, codes of ethics and good implementation practices. In particular the following recent review material, encyclopaedias and textbooks provided the foundation for preparation of the guideline: [11,12,16,32 38]. An initial list of important items was drafted based on the literature analysis as well as on the experiences of the authors. The guideline also took inspiration from other types of guidelines within health informatics and medical research, such as guidelines cited by the Equator Network [17], the Agree Collaboration [39], the CONSORT statement [40 42], the QUOROM statement [43], the STARD statement [44], the STROBE [45] and an extension of the CONSORT [46]. Overviews of the various guidelines are published in Refs. [17,47]. At regular intervals, the GEP-HI guideline was presented and/or submitted for discussion among an increasing number of evaluation experts through EFMI s EVAL-working group mailing list, see Ref. [48]. This mailing list includes a comprehensive list of people with expertise in evaluation (340 in total as per December 2010) ranging from evaluation experts to healthcare practitioners, and coming from universities and health organizations, from industries, and elsewhere. Initial ideas for the guideline were presented and discussed in workshops during the World Congress on Medical Informatics (MEDINFO) in 2004, and Medical Informatics Europe (MIE) in 2005 and 2006 congresses. Feedback was collected in these workshops to inform the core team for further elaboration of the guideline. The guideline items were not formally rated or balloted, but agreements were achieved during consensus discussions in working sessions and by s. A first full draft of the guideline was presented at MEDINFO in 2007 and elaborated further at workshops in MIE 2008 and MIE 2009 as well as at a dedicated Amsterdam working conference in 2008 juxtaposing GEP-HI with the needs of usability evaluation studies. The close-to-final version of GEP-HI was presented in a workshop in MEDINFO 2010 for discussion and led to further feedback included in this current version. 3. GEP-HI guideline for evaluation STUDIES This GEP-HI guideline presents the essential aspects and activities to be taken into account in the design and execution of an evaluation study, including the study s management. The specific methods to be used are not explicitly mentioned. It is up to the users of the guideline to identify which method is applicable in their specific concrete situation, for instance by means of the latest edition of handbooks and textbooks like Refs. [11,35,49 54], and other relevant literature such as Refs. [21,31,37,38,55] as well as websites collecting evaluation studies [48]. The use of models and theories in methodological implementation is strongly recommended. The guideline is divided into parts corresponding to the phases of an evaluation study (Fig. 1). The theoretical background for the study phases is analogous to a traditional approach in information systems development models, here presented in a cascade fashion. Implementation may equally well be seen as an iterative spiral because the topics are in
4 818 i n t e r n a t i o n a l j o u r n a l o f m e d i c a l i n f o r m a t i c s 8 0 ( ) Fig. 1 Phases of the GEP-HI guideline. general repeated in depth or breadth to achieve progress during all phases, and because of the feedback loops urging to revisit earlier phases when new aspects, additional information, or changes in context appear. The phases include items that are coherent and meaningful components for the given phase.the phases in GEP-HI guideline are: Preliminary outline presenting the purpose of the study and the first ideas on why, for whom, and how the evaluation should take place, Study design clarifying the design issues for the evaluation study, Operationalization 2 of methods making the methodological approach and methods concrete and compliant with the system type, the organization and the information need, Project planning developing plans and procedures for the evaluation project, Execution of the evaluation study accomplishing the designed evaluation study, Completion of the evaluation study reporting, accounting, archiving of evaluation study results, finalization of outstanding issues and formal closure of the evaluation study. The phases and their related issues are described one by one in more detail in the following sections according to the overview in Table Preliminary outline This phase describes the purpose of the evaluation and the evaluation question, the primary hypothesis stimulating or initiating the study, and the subsequent explorative activities to establish its feasibility and relevance. This phase is the strategic planning level and it forms the basis for the entire study. 2 The act of making the method operational (ready for use) for its specific purpose and context. The evaluator should get acquainted with the prior research and evaluation studies that address similar topics as the study at hand to get an overview of the approaches and to learn from previous work. The following articles and textbooks provide good background [10 12,16,19 21,30,31,35,38]. During the preliminary outline phase the following items should be addressed: Purpose of the study: Establish the purpose of the study, the preliminary hypothesis reflecting the information need. As stated in the Declaration of Innsbruck [15]: Evaluation of IT in health care only has a value when there is a purpose, i.e. there is a question to be answered, for example improvement of knowledge and generation of insight from a scientific perspective, or making informed decisions about design, procurement, development or routine operation of a health information system. Establishing the purpose and the preliminary hypothesis is relevant, as these will guide almost all of the study design decisions and will secure that the results obtained are clear and relevant to those who will be involved in the decision or affected by it. Primary audience: Identify the recipients, readers and users of the evaluation results. Identification of the study funding party(ies): Identify the payers, funders of the evaluation. They may, for instance, be the evaluators, their employer, a system development project, a university, a company, a health care organization or institution, an external research foundation, or a governmental organization. It should be noted from the beginning what potential bias the given funder may create, explicitly or unintentionally, and hence what measures need to be taken demonstrably to minimize such bias. First identification of stakeholders: Identify, broadly, who are the stakeholders in this evaluation study. By stakeholders we mean the interest groups such as IT developers, users in this or in another organization, management, policy makers, healthcare funding bodies, patients, and so on, all those who may be directly or indirectly affected by the study itself,
5 i n t e r n a t i o n a l j o u r n a l o f m e d i c a l i n f o r m a t i c s 8 0 ( ) Table 1 The phases and items of the GEP-HI guideline. Phase no Phase Items of the phase 1 Preliminary outline - Purpose of the study - Primary audience - Identification of the study funding party(ies) - First identification of stakeholders - Identification of required expertise - The organizational and user context of the evaluation study - Object of evaluation, type of health IT - First exploration of evaluation methods to be used - Ethical and legal issues - Budget - Preliminary permissions for publication - Result of preliminary outline - Formal acceptance to proceed to the next phase 2 Study design - Detailed rationale and objectives for the study - Key evaluation issues, questions, indicators - Stakeholder analysis/social Network analysis - Study methods - Organizational context, the study setting - Technical setting, the type of health IT - Participants from the organization - Project timeline - Material and practical resources - Establishment of the study team - Risk analysis and quality management - Budget - Ethical and legal issues - Strategy for reporting and disseminating the results - Result of study design - Formal acceptance to proceed to the next phase 3 Operationalization of methods - Study type - Approach - Assumptions and feasibility assessment - Frame of reference - Timing - Justification of the methodological approach - Expertise - Outcome measures - Avoiding Bias - Quality control on data (measures) - Participants - Ethical and legal issues - Result of operationalization of methods - Approval of operationalization of methods 4 Project planning - Project management - Study flow - Evaluation activity mapping - Quality management - Risk management - Recruitment of necessary staff - Inform all relevant stakeholders - Result of project planning - Approval of project planning 5 Execution of the evaluation study - Undertake the study, collect data and interpret observations - Quality control of findings and observation of changes - Continuous project management, quality and risk management - Regular reports Final result of execution of the evaluation study by its anticipated outcome, by the system being evaluated, or by future implementations/revisions. Identification of required expertise: Identify the required evaluation expertise dependent on the type and scope of the study. In some cases it may be necessary to involve an experienced evaluator or an external consultant, e.g. a statistician, either as a partner, as a discussant/mentor, as an actor or as actual participant in the study. It is important
6 820 i n t e r n a t i o n a l j o u r n a l o f m e d i c a l i n f o r m a t i c s 8 0 ( ) that the neutrality of evaluators is already established at this early phase, and evaluators should preferably be impartial in relation to the object of evaluation. If they are not neutral and independent, it should be made clear from the beginning what kind of relations they have and what measures are taken to minimize the potential resulting bias. Organizational and user context of the evaluation study: Outline a preliminary picture of the organizational context where the evaluation study will be performed. Describe roughly the organization (unit/department, and the type of care provider and health system) and the user types that will be involved in the evaluation study. Object of evaluation, type of health IT: Prepare a first description of the object of the study, i.e. description of the system, application, method or service to be evaluated. It is important to present which features of the system are in focus at the evaluation. First exploration of evaluation methods to be used: At this stage it is important to develop an overview of applicable and suitable methods to serve the purpose, given the context and the conditions of the study. Selection of methods is to be based on the study purpose and objectives, the study type (for example objective or subjective, summative or formative) and the information need (quantitative or qualitative, objective or subjective, prognostic or diagnostic, etc.). To search for available methods see for example the review in Ref. [11]. Ethical and legal issues: Seek informal advice on which legal and ethical aspects need to be considered and solved, examples are: o The ethical and legal aspects related to patient data access and usage (data privacy and confidentiality)? How will anonymity of confidential patient (and end user) data be handled? Are patient and/or staff consents necessary? Is it mandatory to get approval from ethical committees or similar bodies? o Are there any conflicts of interest or likely issues or restrictions, publication-wise, financially, or personally, or by a particular stakeholder that needs to be taken into account upfront? o How are the intellectual property rights (IPR) to be managed? Will IPR in terms of business secrets impact the study execution and/or reporting? Budget: Outline a draft budget, or alternative budgets, based on the purpose, objectives, scope and the desired depth of the study, and the list of applicable methods. The budget should present the estimates of the needed resources. Preliminary permissions for publication: Ask always the appropriate authority(ies) for permission to publish the results before a study starts, thus ensuring right to report the study results. The main goal is to be able to present the study results openly without pressure from any of the stakeholder groups, and if there are publication constraints, for instance from a sponsor, you need to know those conditions for publication in detail. Result of the preliminary outline phase: Document the result as an outline report that presents a draft design of the study, including description of the study purpose, preliminary hypothesis and information need, the evaluation object, the system under study and its organizational environment, the aspects of interest in evaluation, publication constraints, the draft budget and the resources and co-operation needed. The outline document should also indicate whether the outlined study is feasible from the perspectives of stakeholders, funding parties and the organizational and user contexts. Formal acceptance to proceed to the next phase: Present the outline report for political or administrative bodies within the information system s organizational environment, for the sponsor of the study, and for staff/professional committees and/or ethics committees. Which bodies to approach depends on who has to authorise these kinds of decisions as well as on which party could potentially influence the execution of later phases of the evaluation study Study design This phase builds the foundation for developing the detailed project plan. During the study design phase the following items should be addressed: Detailed rationale and objectives for the study: Formulate the rationale for the study and reasons to carry out the study, and describe the detailed study objectives. Key evaluation issues, questions, indicators: Describe the key issues, main questions and evaluation criteria or indicators to be addressed in the evaluation. Are there any desirable secondary evaluation questions? Make sure that the list of key issues corresponds to the study objectives. Stakeholder analysis/social network analysis: Elaborate the stakeholder analysis while addressing both the formal and informal aspects of the organization to get the full picture of the stakeholders, their relations, and their possible support or resistance. Study methods: Make a shortlist of methods for the study, for example with which methods it is possible to acquire the data and information necessary to provide the answers to the key evaluation questions. Get acquainted with the assumptions and perspectives behind these candidate methods, the validity of methods and how easy or difficult they are to use. Organizational context, the study setting: Develop a more detailed description of the organizational context where the evaluation study will be carried out. Important issues to consider are the kinds of organizational units that will be involved, their type and size and their roles and tasks in the evaluation study. The implementation of IT systems in an organization usually impacts the distribution of tasks between healthcare professionals, the communication and cooperation procedures, and the distribution of information across individual minds and tangible supports. Moreover, the quality and completeness of the training process also has an impact on the resulting work situation. Unfortunately those intermediary (hidden) variables are usually not controlled and their impact on the results of the evaluation study not taken into account. As a result, a given IT system implemented in similar organizations may end up generating fundamentally different work systems as far as collective and individual cognitive and work processes are concerned. Depending on the type of IT application under
7 i n t e r n a t i o n a l j o u r n a l o f m e d i c a l i n f o r m a t i c s 8 0 ( ) evaluation, the description of the organizational context may need to be completed by a human factors (HF) based analysis of the work system to be able to identify the relevant HF intermediary variables and their potential impact on the results of the evaluation study. Technical setting, the type of health IT: Prepare a description of the evaluation object, system, application, service or method to be evaluated covering sufficient system details, system type and phase of development, and any other aspects of interest. It is recommended that trainers and users of the health IT system under study verify the technical description. This is to ensure the right level of understanding by all involved parties. Describe also how the object of study fits within the larger health IT environment of the organization or unit, and where relevant within the wider health sector. Participants from the organization: Describe the participants to be recruited from the organization, system users and other health professionals. Describe their roles and tasks in the organization and in the study, and the arrangements and permissions needed to have these participants involved in the evaluation study, and how potential bias will be mitigated. Project timeline: Outline one or more alternative schedules, and calendars, for the project duration, project events and milestones. Material and practical resources: Describe what is needed in the health care organization and what are the materials and resources that need to be brought in by the evaluation team. Establishment of the study team: Identify the person-related resources and the personal competencies and powers that are needed from the organizational environment and what is available from external sources. Identify and appoint members of the team to carry out the evaluation study. Make clear that evaluators must be impartial and unbiased in relation to the object of the evaluation. If not transparently achievable, describe their relations and what measures are taken to minimize the risk that these relations influence the outcome of the study. Risk analysis and quality management: List elements that comprise significant risks such as potential changes in key personnel, potential unexpected events in the study environment, ongoing adaptation of the IT application, process changes, potential failures of the health IT system installation and other systems being installed during the study period. Prepare a preliminary quality management plan that describes the organization of the project, roles and responsibilities of the participants, control actions and tools for monitoring the progress and for reporting. Budget: Refine the budget of the study to the necessary level of detail based on the current study design, estimated participants and resources and planned scope and study duration. Take into account that some variants depended on the methods to be chosen. Ethical and legal issues: Pay attention to legal and ethical issues. Data protection, security and privacy principles and laws must be obeyed. Some study types may require legal registration, e.g. registration in a clinical trial registry. Ethical issues are related to consents needed to manage confidential patient (and possibly practitioner and organization) data and permissions from the organization to involve users (nurses, doctors) in the study. Further, formulate a strategy, policy and principles for how to handle problematic issues that may be identified or observed during the project period examples may be procedures for how to deal with observation of human errors, observed adverse events, and similar events that will expose individuals or the organization. It is advisable to engage an Ethical or Internal Review Board (IRB) at this stage to identify potential barriers that should be dealt with in the final study plan. Strategy for reporting and disseminating results: Pay attention to the following two issues: (i) How the results of the study will be communicated locally to the various study stakeholders; and (ii) how the study results will be reported and disseminated in the public domain. These should be encapsulated and shared in a communication strategy. For reporting, define the reporting schedule with the type, scope and contents of the reports. Use the STARE-HI reporting principles for the definition of the report content [16]. Specify the target audience, the responsible authors, the strategy for handling ethical issues in reporting, the other publication and dissemination means, for example scientific journals, conference papers, reports, presentations, web-based publications, etc. Define whether any approval is needed and clarify what acknowledgements and disclaimers should be used in various publication types. Result of the study design phase: Document the study design as a first evaluation study plan, which forms a basis to achieve an agreement with the appropriate decisionmaking authorities and a commitment as regards to the organization s engagement and resources. Formal acceptance to proceed to the next phase: Seek formal acceptance from the necessary stakeholders in order to proceed with evaluation planning Operationalization of methods This phase deals with the selection of appropriate methods to answer the evaluation questions in accordance with the study context and setting, the physical and financial resources and the objectives of the study. It is essential to consult textbooks and handbooks on evaluation methods, since there are many potential impediments to the desired level of quality, accuracy and precision of the outcome. Examples of relevant textbooks, handbooks and articles in this respect are Refs. [11,21,23,35,37,49 54] Methodological aspects The following methodological aspects should be addressed: 3 3 Methodology signifies the science of methods. In functional terms it is concerned with the knowledge of how to prepare and use methods in some context [[11], pp ]. A method is a formal description of the procedures involved to accomplish an actual task, based on a well-defined theory and a set of coherent and consistent techniques, tools and principles for organizing it.
8 822 i n t e r n a t i o n a l j o u r n a l o f m e d i c a l i n f o r m a t i c s 8 0 ( ) Study type: Specify in detail the characteristics of this evaluation study, for example quantitative or qualitative, subjective or objective, formative or summative. Approach: Make an informed choice on the approach and methods. Describe in detail the selected methodological approach(es) (for example observational, case-study, action research, conceptual research, empirical study). Assumptions: Specific approaches may imply various assumptions for example on their scope, specificity and application range, on the results interpretation or on the data set. Identify the consequences of these assumptions and consider how they may affect the study outcomes. Frames of reference: Specify the frame of reference for the interpretation of the findings of the study. In some evaluation approaches, the establishment of a frame of reference is an integrated feature of the method/methodology, such as in before-and-after studies and controlled studies. For other methods requiring a frame of reference, this may be compiled from several sources, for example from the organization s pre-implementation work flow and performance, but also from standards, normative rules and laws, from theoretical optimal values and generally accepted good practices or guidelines, or from specific study objectives. Explore if the frame of reference already exists, and if so, how was it established, where/what is it derived from, is it reliable? Consider if it is applicable in this situation, or is there a risk that it may have a regressive bias by being based on previous technologies and their related safeguards. Timing: Explore if the planned time schedule for evaluation is in agreement with other plans in the health care organization and in the research organization. Consider also if the timing is adequate for measuring as intended, for example are there enough users with adequate experience available? Justification of the methodological approach: Justify that the methodological approach is feasible for the study and its context and that it complies with the objectives of the study. Confirm that this approach allows adequate measurement of all relevant aspects with sufficient confidence for the study purpose Methodical aspects The following items should be addressed with respect to the methodical aspects: Expertise: Identify whether relevant professional competencies for applying the selected methods are available for the study. If not, identify the actions to be taken to acquire the necessary expertise. Outcome measures, evaluation criteria: Identify which specific measures and evaluation criteria will provide the answers to the information need and study objectives, and identify which specific methods for data acquisition/elicitation and data analysis are needed. Define the threshold values, success/failure levels for the evaluation criteria. Examples of methods are interviews, questionnaires, observations, surveys, log file analysis, document analysis. Specify how the data collection is planned, i.e. methods, setting, types and number of cases needed for the study. When appropriate, HF measures should be added to other outcome measures and evaluation criteria. Avoiding bias: Consider whether using specific methods might yield differential response rates, or promotion of specific views or interests; identify what measures could be introduced to prevent or minimize such risk, see for instance Ref. [11]. Quality control on data (measures): Explore whether the actual measures have been tested as regards to syntactic and semantic validity, whether the calculations are working properly, and whether the questionnaires have been tested for validity, etc. Identify what means can be taken to ensure that the collected data suffice for the purpose. Participants: Identify which participants (evaluators/observers, patients/clients, and/or staff participants) are involved and when, which specific stakeholder groups or organizational units, skill types, and specific individuals are essential or necessary or useful for the study. Identify for which tasks and for what purposes they are involved, and how the participation is organized. The inclusion and exclusion criteria should be explicitly and comprehensibly defined for the participants that are to be included in the study. Communicate information on the study to the users and other study participants and stakeholders. Ethical and legal issues: Prepare all practical details, such as necessary approvals, consents and permissions, dedicated forms, etc. Result of operationalization of methods: Document the methodological and methodical aspects of the study. The plan should include sufficient details to serve as a protocol for the project planning and execution phases of the study. Approval of operationalization of methods: Seek approval from the relevant stakeholders to proceed to the detailed study plan Project planning During this phase a detailed project plan is developed. The following items should be addressed: Project management: Establish a project management plan according to the size and complexity of the evaluation study and the organization s preferences as regards the project management model, values and principles. Use dedicated tools and approaches for management. Define a quality management strategy and related action plan. Define a communication strategy and a dissemination and publication strategy and related action plans and responsibilities. Identify means and tools for conflict resolution. Identify mechanisms for incorporation of lessons learned and identify mechanisms for interim corrections of the project plan and handling of ethical and legal issues. Study flow: Identify the start-criteria, end-criteria and success-criteria for each activity, periods, interventions, participation, and reporting deadlines. Specify milestones in order to be able to monitor and control the progress of the evaluation study. Evaluation activity mapping: Divide evaluation tasks into activities and sub-activities, map them on to a time table and put named resources and responsibilities to them.
9 i n t e r n a t i o n a l j o u r n a l o f m e d i c a l i n f o r m a t i c s 8 0 ( ) Identify dependencies between the activities. Make a path analysis to identify the critical path(s) and activities. Match all activities with responsible activity managers, allocated personnel, affiliated experts/scientists and third party actors. Establish a budget monitoring and mitigation strategy. Quality management: Elaborate further the preliminary quality management plan according to the preferred quality management model and principles, for example ISO 9000 standards can be applied. Risk management: Elaborate a list of significant risks and reassess them with respect to likelihood of occurrence, with relevant measures for monitoring, means for remedy and anticipated impacts of risks. Some risks may relate to the organization or the system under study, others may relate to the evaluation itself such as loss of key personnel or failures/induced biases of the data collection methods. Specify a mitigation strategy and potential means and corrective actions in case a given risk manifests. Nominate the person in charge of each risk factor. Identify also risks related to confidentiality and conflict of interest principles, processes, and sanctions. Recruitment of necessary staff: Specify what types of competencies are needed, such as statistical, organizational insights, cognitive/psychological competences, sociological, etc., and necessary training. Employ the project team. Ensure the evaluators neutrality and their commitment to confidentiality of data within the studied system as well as of the analyses and pre-publication findings. Inform all relevant stakeholders: Inform all relevant stakeholders sufficiently about the study and about all relevant aspects, but in such a way that the behaviour or performance of the participants will not be changed due to the fact that they know they are observed (the Hawthorne effect). Result of project planning phase: Document the project plan with incorporated project management tools. Approval of project planning: Present the project plan to relevant stakeholders to seek approval to proceed for the study implementation Execution of the evaluation study Now the evaluation study can be executed. During the study execution, however, a situation may arise where the study design has to be redefined due to unforeseen events or conditions and then the study plan has to be modified accordingly. When executing the evaluation study the following items should be addressed: Undertake the study, collect data and interpret observations: Implement the prescribed methods for the study. Adherence to the methodological and methodical agreements is important. Verify the assumptions by means of the data, and interpret the resulting findings. It is necessary to analyse and identify the causal relations behind unexpected events and unexpected observations, in order to secure that none of these will interfere with the conclusion. Quality control of findings and observation of changes: Implement the quality management plan to its finest detail. Organizations, people, processes, and contexts may change as a function of time and opportunities. Any such changes with a potential to affect the study need to be documented. Continuous project management, quality and risk management: Implement continuous management and control of the project. Evaluation studies are often large and formal projects, and they may take a long time and involve a large group of personnel. Regular reports: Prepare and deliver regular reports to the relevant parties and stakeholders. Final result of execution of the evaluation study phase: Prepare a final report for the commissioner of the study summarizing the main information on objectives, the setting, the conditions, the method, the outcome data and information and the results and conclusions Completion of the evaluation study In this phase the study should be reported and made public for the benefit of similar studies and the potential future users of similar systems, as recommended by the Declaration of Innsbruck [15]. Reporting also supports development of reflective practice, learning from professional experiences. During the completion phase the following items should be addressed: Accounting: Prepare and deliver accounts when external funds were acquired and/or specific demands exist with respect to accounting. Archiving: Keep the data and other collected materials of the study in storage, and accessible to others if and when needed for later follow-on analyses. Archiving has to follow the local and national legislation, regulations and good practices on archiving and disclosure of medical and/or research data. If privileged types of data were acquired, for example extracts from medical records, regulations for these must be followed. Reports and publications: Produce the project s operational reports and publications. They may be of many types and potentially for many recipients. Detailed recommendations on writing reports and publications are elaborated more fully in the STARE-HI statement [16]. See also appropriate guidance for the authorship in Ref. [56] and for ethical and moral aspects of publication in Ref. [57]. 4. Discussion This guideline for good evaluation practice in health informatics was developed to support evaluators, health care professionals, decision makers and other health IT stakeholders in design, planning and execution of an evaluation study. The guideline gives support and advice on what to do and on aspects needing attention at each evaluation phase. Existing methodological textbooks will give the details of which methods to apply and how. The guideline has been developed through an informal consensus-seeking process in the community of health IT evaluation experts, and it was put regularly into open discussion through the HISEVAL website and many conference workshops. The final list of items was achieved through
10 824 i n t e r n a t i o n a l j o u r n a l o f m e d i c a l i n f o r m a t i c s 8 0 ( ) informal discussions and feedback; no formal rating or balloting has been applied for the items. The timeline for this guideline development has been long, from 2004 until 2010, and this made it possible to collect wide feedback and elaborate revised versions for further discussion. The purpose of this guideline is to raise the level of quality of evaluation studies through careful planning, and thus contribute to the accumulation of the scientific evidence base for health informatics Applicability of the GEP-HI guideline This guideline can be applied to any health IT evaluation study, either a small scale or a large scale study, and irrespective of whether the object of study is an IT application or a method like nursing classification or data security practice. The guideline is applicable at various phases of a health IT project, starting from design and development, over application or system implementation and installation, and ending with the study of effects and impacts in routine use. This GEP-HI guideline can be used for different types of health IT evaluation studies such as feasibility, effectiveness, efficiency and impact evaluation. In small or circumscribed evaluation studies not all phases of the guideline may be needed. The user should consider in each situation which phases are necessary and relevant for the design and execution of the given study. However, it is recommended that even if all the guideline items are not documented, they should be considered during the design and the reason for non-inclusion documented. For health informatics usability evaluation studies there are specific conditions due to legal regulations, such as the European Commission s prescription of usability evaluation of all medical devices applying for CE marking. This prescription could be extended to health IT applications used for medical diagnosis and therapeutic purposes. Moreover, usability studies rely on specific standards and usability heuristics applied as frames of reference in usability evaluation studies [see Refs. [58 62]]. Economic evaluation has also specific conditions that need to be considered due to the approaches and methods required to measure economic aspects [see Refs. [55,63]]. These domains require a topical technical specificity. GEP-HI provides a generic guideline, therefore, for certain types of highly standardized studies such as RCTs, the GEP-HI guideline can be complemented with specific guidelines such as the CONSORT statement (see for example Refs. [42,44,45,47]) Strengths and weaknesses of the guideline The present guideline was intentionally made as general as possible and with an awareness of cultural aspects. Fortunately, the consensus approach involving experts from all over the world gave some assurance that global diversity will not hinder appropriate application of the present guideline. In different cultural environments the evaluation study principles are likely to be the same, but the actual study design could be influenced by the local cultural preferences, however, no major conflicts could be expected. The rigor of this GEP-HI guideline may initially bring some minor additional costs and other additional resource usage to the evaluation study, but should ensure higher quality, reduced risks, and higher utility to the general health IT and health community through production of stronger evidence. Potential organizational barriers to apply the guideline may arise as some organizations may find it somewhat threatening to expose their practices to a robust methodology and open scrutiny. However, thorough approaches for evaluation are necessary to fulfil the ethical imperative (see Ref. [15]) and to collect reliable information on the health IT intervention in order to make decisions. The strength of the guideline is that it forces the user to go through a checklist of relevant issues that might otherwise only act informally as tacit knowledge, or even be overlooked. This systematic approach will increase the likelihood of an outcome with the desired level of accuracy and precision and hence an increased effectiveness, and additionally encourage the adoption of a scientifically valid approach in an evaluation study Further development and validation of GEP-HI guideline Further development and assessment of the validity, clarity and completeness of the guideline will take place through its application and use, through pilot health IT evaluations and by peer reviews in panels of evaluation experts. 5. Conclusions A comprehensive lifecycle of phases has been developed, strengthened by world-wide iteration, to plan and execute health IT evaluations effectively. The phases contain some 60 detailed items, which are presented in relation to the evaluation study phases. When designers and executers of evaluation studies address these items, the plan, structure, objectives and results of the studies will become more robust and consequently the studies contribute an important step towards evidence-based health informatics. Authors contributions This GEP-HI work has been a shared activity of EFMI s Working Group EVAL and IMIA s Working Group on Technology Assessment and Quality Development. The idea of this standard/guideline contribution crystallised during the ESF working conference 2003 in Innsbruck, organized by E Ammenwerth and attended among others by J Brender, P Nykänen, M Rigby, and J Talmon. P Nykänen and J Brender drafted a first list of issues, while in later versions P Nykänen and J Brender together as the responsible coordinators integrated the various contributions and further completed and refined the successive versions of the manuscript. E Ammenwerth, J Talmon, N de Keizer, M Rigby and MC Beuscart-Zephir contributed actively by critically assessing the items and their descriptions, making suggestions for directions and expansion, as well as contributed to the writing of the various versions of the document. All authors have read and approved this final version.
11 i n t e r n a t i o n a l j o u r n a l o f m e d i c a l i n f o r m a t i c s 8 0 ( ) Summary points What was already known on the topic Health IT applications are widely used to achieve benefits and improvements in health care practices. However, there are problems and hazards related to adoption of health IT applications for clinical use. The significance and volume of health IT applications in clinical practice calls for a need to evaluate health IT applications to collect evidence on their efficiency, effectiveness and impacts. Evaluation of health IT is a discipline and a specialty that requires good practices for design, execution and management of evaluation studies. The need to develop an evaluation guideline has been identified and guidelines to develop guidelines have matured. What this study added to our knowledge The GEP-HI guideline adds on to existing evaluation approaches and guidelines by providing guidance for systematic design, execution and management of an evaluation study. The GEP-HI guideline is applicable to different types of health informatics evaluation studies independent on whether the object of the study is an IT application, or a method, a service or a practice. The GEP-HI guideline may serve as a basis for developing guidelines for specific niches, like usability evaluation studies. Conflict of interests P Nykänen and J Brender are co-chairs of the EFMI working group on Evaluation (EVAL) that has E Ammenwerth as the chairperson. N de Keizer is the chairperson of IMIA s working group on Technology Assessment and Quality Development. J Talmon has been chair of this IMIA working group till May 2008, and J Brender was his co-chair until the end of Besides this all authors have positions as university employed researchers with no commercial interests and no conflicting interests financially or otherwise in relation to the GEP-HI guidelines. r e f e r e n c e s [1] Commission of the European Communities, COM 356: Communication from the Commission to the Council, the European Parliament, the European Economic and Social Committee and the Committee of the Regions: e-health making health care better for European citizens: An action plan for a European e-health Area (COM (2004) 356, Brussels, [2] European Commission, ICT for Health & i2010 Transforming the European Healthcare Landscape Towards a Strategy for ICT for Health, June 2006, Office for Official Publications of the European Communities, Luxembourg, 2006, society/activities/health/ docs/publications/ictforhealth-and-i2010-final.pdf (accessed ). [3] United States Congress, American Recovery and Reinvestment Act of 2009, United States Government Printing Office, Washington, DC, [4] D.F. Sittig, D.C. Classen, Safe electronic health record use requires a comprehensive monitoring and evaluation framework, JAMA 303 (5) (2010) [5] E. Ammenwerth, N. Shaw, Bad health informatics can kill is evaluation the answer. Editorial, Methods Inf. Med. 44 (2005) 1 3. [6] Veterans given wrong drug doses due to glitch, Health care at msnbc.com, (accessed ). [7] Y.Y. Han, J.A. Carcillo, S.T. Venkataraman, R.S.B. Clark, R.S. Watson, T.C. Nguyen, H. Bayir, R.A. Orr, Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system, Pediatrics 116 (6) (2005) [8] A.G. Ekeland, A. Bowes, S. Flottorp, Effectiveness of telemedicine: a systematic review of reviews, Int. J. Med. Inform. 79 (2010) [9] P. Fontaine, S.E. Ross, T. Zink, L.M. Schilling, Systematic review of health information exchange in primary care practices, J. Am. Board Fam. Med. 23 (5) (2010) [10] J.R. Moehr, Evaluation of health information systems: beyond efficiency and effectiveness, Comput. Biol. Med. 32 (3) (2002) [11] J. Brender, Handbook of Evaluation Methods for Health Informatics, Academic Press, New York, USA, [12] E. Ammenwerth, J. Brender, P. Nykänen, H.U. Prokosch, M. Rigby, J. Talmon, Visions and strategies to improve evaluation of health information systems. Reflections and lessons based on the HIS-EVAL workshop in Innsbruck, Int. J. Med. Inform. 73 (2004) [13] A.D. Oxman, A. Bjørndal, F. Becerra-Posada, M. Gibson, M.A. Block, A. Haines, et al., A framework for mandatory impact evaluation to ensure well informed public policy decisions, Lancet 375 (9712) (2010) [14] M. Scriven, Evaluation Thesaurus, Fourth edition, Sage Publications, Thousand Oaks, USA, [15] Declaration of Innsbruck Results from the European Science Foundation sponsored Workshop on Systematic Evaluation of Health Information Systems (HIS-EVAL), April 4 6th, 2003, (accessed ). [16] J. Talmon, E. Ammenwerth, J. Brender, N. de Keizer, P. Nykänen, M. Rigby, STARE-HI statement on reporting of evaluation studies in health informatics, Int. J. Med. Inform. 78 (2009) 1 9. [17] Equator Network, Reporting Guidelines, (accessed ). [18] A. Kazanjian, C.J. Green, Beyond effectiveness: the evaluation of information systems using a comprehensive health technology assessment framework, Comput. Biol. Med. 32 (3) (2002) [19] M. Rigby, Evaluation the Cinderella science of ICT in health, in: C. Kulikowski, R. Haux (Eds.), IMIA Yearbook of Medical Informatics 2006, Schattauer Verlagsgeschellschaft, Stuttgart, 2006, pp [20] J. Talmon, Evaluation and implementation: a call for action, in: C. Kulikowski, R. Haux (Eds.), IMIA Yearbook of Medical Informatics 2006, Schattauer Verlagsgeschellschaft, Stuttgart, 2006, pp
12 826 i n t e r n a t i o n a l j o u r n a l o f m e d i c a l i n f o r m a t i c s 8 0 ( ) [21] M.M. Yusof, A. Papazafeiropoulou, R.J. Paul, K. Stergioulas, Investigating evaluation frameworks for health information systems, Int. J. Med. Inform. 77 (2008) [22] K. Kidholm, A. Bowes, S. Dyrehauge, A. Granstrøm Ekeland, S.A. Flottorp, L. Kvistgaard, et al., The MAST Manual, MAST-Model for Assessment of Telemedicine, (accessed ). [23] C.M. Cusack, C. Byrne, J.M. Hook, J. McGowan, E.G. Poon, A. Zafar, Health information technology evaluation toolkit: 2009 Update (Prepared for the AHRQ National Resource Center for Health Information Technology under Contract No ), AHRQ Publication No EF, Agency for Healthcare Research and Quality, Rockville, MD, 2009, (accessed ). [24] L. Catwell, A. Sheikh, Evaluating ehealth interventions: the need for continuous systemic evaluation, PLoS Med. 6 (8) (2009) e , doi: /journal.pmed [25] K. Clarke, R. O Moore, R. Smeets, J. Talmon, J. Brender, P. McNair, et al., A methodology for evaluation of knowledge-based systems in medicine, Artif. Intell. Med. 6 (2) (1994) [26] B. Kaplan, Evaluating informatics applications some alternative approaches: theory, social interactionism, and call for methodological pluralism, Int. J. Med. Inform. 64 (2001) [27] N.T. Shaw, CHEATS: a generic information communication technology (ICT) evaluation framework, Comput. Biol. Med. 32 (2002) [28] J.I. Westbrook, J. Braithwaite, R. Iedema, E.W. Coiera, Evaluating the impact of information communication technologies on complex organizational systems: a multi-disciplinary, multi-method framework, in: M. Fieschi, E. Coiera, Y.-C. Jack Li (Eds.), Proceedings of the 11th World Congress on Medical Informatics (MEDINFO 2004), IOS Press, Amsterdam, 2004, pp [29] H. Hyppönen, L. Salmivalli, P. Nykänen, P. Ruotsalainen, M. Pajukoski, Testing a theoretical framework for interdisciplinary IS evaluation: the case of Finnish electronic prescription, Int. J. Healthc. Technol. Manag. 8 (1 2) (2007) [30] J. Brender, Evaluation methods to monitor success and failure factors in health information system s development, in: A.W. Kushniruk, E.M. Borycki (Eds.), The Human, Social, and Organizational Aspects of Health Information Systems, Medical Information Science Reference, Hershey, 2008, pp [31] M.M. Yusof, J. Kuljis, A. Papazafeiropoulou, K. Stergioulas, An evaluation framework for health information systems: human, organization and technology-fit factors (HOT-fit), Int. J. Med. Inform. 77 (2008) [32] J. Talmon, J. Enning, G. Castadena, F. Eurlings, D. Hoyer, P. Nykänen, et al., The VATAM guidelines, Int. J. Med. Inform. 56 (1999) [33] P. Nykänen, J. Enning, J. Talmon, D. Hoyer, F. Sanz, C. Thayer, R. Roine, M. Vissers, F. Eurlings, Inventory of validation approaches in selected health telematics projects, Int. J. Med. Inform. 56 (1999) [34] B. Kaplan, N. Shaw, Future directions in evaluations research: people, organisational and social issues, Methods Inf. Med. 43 (2004) [35] C.F. Friedman, J. Wyatt, Evaluation Methods in Medical Informatics, 2nd edition, Springer Verlag, New York, [36] J. Brender, E. Ammenwerth, P. Nykänen, J. Talmon, Factors influencing success and failure of health informatics systems a pilot Delphi study, Methods Inf. Med. 45 (2006) [37] J.L. Westbrook, J. Braithwaite, A. Georgiou, A. Ampt, N. Creswick, E. Coiera, R. Iedema, Multimethod evaluation of information and communication technologies in health in the context of wicked problems and sociotechnical theory, J. Am. Med. Inform. Assoc. 14 (2007) [38] E. Ammenwerth, N. de Keizer, An inventory of evaluation studies of information technology in health care. Trends in evaluation research , Methods Inf. Med. 44 (2005) [39] AGREE-Criteria, (accessed ). [40] C. Begg, M. Cho, S. Eastwood, R. Horton, D. Moher, I. Olkin, et al., Improving the quality of reporting of randomized controlled trials. The CONSORT statement, JAMA 276 (8) (1996) [41] D.G. Altman, K.F. Schulz, D. Moher, M. Egger, F. Davidoff, D.D. Elbourne, et al., The revised CONSORT statement for reporting randomized trials: explanation and elaboration, Ann. Intern. Med. 134 (8) (2001) [42] M.K. Campbell, D.R. Elbourne, D.G. Altman, CONSORT statement: extension to cluster randomised trials, BMJ 328 (7441) (2004) [43] D. Moher, D.J. Cook, S. Eastwood, I. Olkin, D. Rennie, D.F. Stroup, Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses, Lancet 354 (1993) (1999) [44] P.M. Bossuyt, J.B. Reitsma, D.E. Bruns, C.A. Gatsonis, P.P. Glasziou, L.M. Irwig, et al., Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative, Clin. Chem. 49 (2003) 1 6. [45] E.A. von Elm, D. Altman, M. Egger, S.J. Pockock, P.C. Gøtzsche, J.P. Vandenbroucke, et al., The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies, Bull. World Health Organ. 85 (11) (2007) , (accessed ). [46] I. Boutron, D. Moher, D.G. Altman, K.F. Schulz, P. Ravaud, Extending the CONSORT statement to randomized trials of non-pharmacologic treatment: explanation and elaboration, Ann. Intern. Med. 148 (4) (2008) [47] M.E. Falagas, E.I. Pitsouni, Guidelines and consensus statements regarding the conduction and reporting of clinical research studies, Arch. Intern. Med. 167 (9) (2007) [48] Website of the EFMI Working Group: assessment of health information systems (EMFI-WG EVAL), (accessed ). [49] H. Coolican, Introduction to Research Methods and Statistics in Psychology, 2nd edition, Hodder & Stoughton, London, [50] R.L. Schalock, Outcome-Based Evaluation, 2nd edition, Kluwer Academic Plenum Publishers, New York, [51] M. Quinn Patton, Qualitative Research and Evaluation Methods, 3rd edition, Sage Publications, Thousand Oaks, USA, [52] E.J. Davidson, Evaluation Methodology Basics, the Nuts and Bolts of Sound Evaluation, Sage Publications, Thousand Oaks, USA, [53] A. Fink, Evaluation Fundamentals, Insights into the Outcomes, Effectiveness and Quality of Health Programs, Sage Publications, Thousand Oaks, USA, [54] J.M. Owen, Program Evaluation, Forms and Approaches, 3rd edition, The Guilford Press, New York, USA, 2007.
13 i n t e r n a t i o n a l j o u r n a l o f m e d i c a l i n f o r m a t i c s 8 0 ( ) [55] V. Vimarlund, N.G. Olve, Economic analyses for ICT in elderly healthcare: questions and challenges, Health Inform. J. 11 (4) (2005) [56] Uniform requirements for manuscripts submitted to biomedical journals: writing and editing for biomedical publications, (accessed ). [57] IMIA Code of Ethics for health information professionals, (accessed ). [58] A. Abban, A. Khelif, W. Suryn, Usability meanings and interpretations in ISO standards, Softw. Qual. J. 11 (4) (2003) [59] J. Nielsen, Ten usability heuristics, list.html (accessed ). [60] M.C. Beuscart-Zephir, P. Elkin, S. Pelayo, R. Beuscart, The human factors engineering approach to biomedical informatics projects: state of the art, results, benefits and challenges, in: A. Geissbuhler, R. Haux, C. Kulikowski (Eds.), IMIA Yearbook of Medical Informatics 2007, Schattauer Verlagsgeschellschaft, Stuttgart, 2007, pp [61] ANSI/AAMI HE75:2009, Human factors engineering design of medical devices. [62] ANSI/AAMI/IEC 62366:2007, Medical devices application of usability engineering to medical devices (Rep No EN62366). [63] M. Drummond, A. McGuire (Eds.), Economic Evaluation in Health Care: Merging Theory with Practice, Oxford University Press, USA, 2001.
Introducing Guidelines for Good Evaluation Practice in Health Informatics
 958 Medical Informatics in a United and Healthy Europe K.-P. Adlassnig et al. (Eds.) IOS Press, 2009 2009 European Federation for Medical Informatics. All rights reserved. doi:10.3233/978-1-60750-044-5-958
958 Medical Informatics in a United and Healthy Europe K.-P. Adlassnig et al. (Eds.) IOS Press, 2009 2009 European Federation for Medical Informatics. All rights reserved. doi:10.3233/978-1-60750-044-5-958
Stakeholder Guide 2014 www.effectivehealthcare.ahrq.gov
 Stakeholder Guide 2014 www.effectivehealthcare.ahrq.gov AHRQ Publication No. 14-EHC010-EF Replaces Publication No. 11-EHC069-EF February 2014 Effective Health Care Program Stakeholder Guide Contents Introduction...1
Stakeholder Guide 2014 www.effectivehealthcare.ahrq.gov AHRQ Publication No. 14-EHC010-EF Replaces Publication No. 11-EHC069-EF February 2014 Effective Health Care Program Stakeholder Guide Contents Introduction...1
National Occupational Standards. Compliance
 National Occupational Standards Compliance NOTES ABOUT NATIONAL OCCUPATIONAL STANDARDS What are National Occupational Standards, and why should you use them? National Occupational Standards (NOS) are statements
National Occupational Standards Compliance NOTES ABOUT NATIONAL OCCUPATIONAL STANDARDS What are National Occupational Standards, and why should you use them? National Occupational Standards (NOS) are statements
Information Governance and Management Standards for the Health Identifiers Operator in Ireland
 Information Governance and Management Standards for the Health Identifiers Operator in Ireland 30 July 2015 About the The (the Authority or HIQA) is the independent Authority established to drive high
Information Governance and Management Standards for the Health Identifiers Operator in Ireland 30 July 2015 About the The (the Authority or HIQA) is the independent Authority established to drive high
Annex 1. Call for proposals EACEA No 34/2015 Erasmus+ Key Action 3: Support for policy reform - Initiatives for policy innovation
 Annex 1 Call for proposals EACEA No 34/2015 Erasmus+ Key Action 3: Support for policy reform - Initiatives for policy innovation European policy experimentations in the fields of Education, Training and
Annex 1 Call for proposals EACEA No 34/2015 Erasmus+ Key Action 3: Support for policy reform - Initiatives for policy innovation European policy experimentations in the fields of Education, Training and
Guide for Clinical Audit Leads
 Guide for Clinical Audit Leads Nancy Dixon and Mary Pearce Healthcare Quality Quest March 2011 Clinical audit tool to promote quality for better health services Contents 1 Introduction 1 1.1 Who this
Guide for Clinical Audit Leads Nancy Dixon and Mary Pearce Healthcare Quality Quest March 2011 Clinical audit tool to promote quality for better health services Contents 1 Introduction 1 1.1 Who this
Haulsey Engineering, Inc. Quality Management System (QMS) Table of Contents
 Haulsey Engineering, Inc. Quality Management System (QMS) Table of Contents 1.0 Introduction 1.1 Quality Management Policy and Practices 2.0 Quality System Components 2.1 Quality Management Plans 2.2 Quality
Haulsey Engineering, Inc. Quality Management System (QMS) Table of Contents 1.0 Introduction 1.1 Quality Management Policy and Practices 2.0 Quality System Components 2.1 Quality Management Plans 2.2 Quality
International Consortium for Harmonization of Clinical Laboratory Results. Operating Procedures
 International Consortium for Harmonization of Clinical Laboratory Results Operating Procedures Approved: February 11, 2014 Background Results from clinical laboratory measurement procedures should be comparable
International Consortium for Harmonization of Clinical Laboratory Results Operating Procedures Approved: February 11, 2014 Background Results from clinical laboratory measurement procedures should be comparable
TABLE OF CONTENTS. Accreditation and Educational Outcomes 1. Curriculum of the Post-Master s DNP Program 1-2. Program of Study 2-3
 DNP 2015 2016 TABLE OF CONTENTS Accreditation and Educational Outcomes 1 Curriculum of the Post-Master s DNP Program 1-2 Program of Study 2-3 Course Descriptions for Core Courses 4-6 The E-Portfolio 6-7
DNP 2015 2016 TABLE OF CONTENTS Accreditation and Educational Outcomes 1 Curriculum of the Post-Master s DNP Program 1-2 Program of Study 2-3 Course Descriptions for Core Courses 4-6 The E-Portfolio 6-7
Guidance Note on Developing Terms of Reference (ToR) for Evaluations
 Evaluation Guidance Note Series UNIFEM Evaluation Unit October 2009 Guidance Note on Developing Terms of Reference (ToR) for Evaluations Terms of Reference (ToR) What? Why? And How? These guidelines aim
Evaluation Guidance Note Series UNIFEM Evaluation Unit October 2009 Guidance Note on Developing Terms of Reference (ToR) for Evaluations Terms of Reference (ToR) What? Why? And How? These guidelines aim
PORTFOLIO, PROGRAMME & PROJECT MANAGEMENT MATURITY MODEL (P3M3)
 PORTFOLIO, PROGRAMME & PROJECT MANAGEMENT MATURITY MODEL (P3M3) 1st February 2006 Version 1.0 1 P3M3 Version 1.0 The OGC logo is a Registered Trade Mark of the Office of Government Commerce This is a Value
PORTFOLIO, PROGRAMME & PROJECT MANAGEMENT MATURITY MODEL (P3M3) 1st February 2006 Version 1.0 1 P3M3 Version 1.0 The OGC logo is a Registered Trade Mark of the Office of Government Commerce This is a Value
BOSTON UNIVERSITY SCHOOL OF PUBLIC HEALTH PUBLIC HEALTH COMPETENCIES
 BOSTON UNIVERSITY SCHOOL OF PUBLIC HEALTH PUBLIC HEALTH COMPETENCIES Competency-based education focuses on what students need to know and be able to do in varying and complex situations. These competencies
BOSTON UNIVERSITY SCHOOL OF PUBLIC HEALTH PUBLIC HEALTH COMPETENCIES Competency-based education focuses on what students need to know and be able to do in varying and complex situations. These competencies
Integrated Risk Management:
 Integrated Risk Management: A Framework for Fraser Health For further information contact: Integrated Risk Management Fraser Health Corporate Office 300, 10334 152A Street Surrey, BC V3R 8T4 Phone: (604)
Integrated Risk Management: A Framework for Fraser Health For further information contact: Integrated Risk Management Fraser Health Corporate Office 300, 10334 152A Street Surrey, BC V3R 8T4 Phone: (604)
EVALUATION REPORT TITLE: Independent evaluation of the Demand-Driven Impact Evaluations for Decisions (3DE) Pilot
 EVALUATION REPORT TITLE: Independent evaluation of the Demand-Driven Impact Evaluations for Decisions (3DE) Pilot RESPONSE TO EVALUATION REPORT (overarching narrative) Overall we are pleased with the findings
EVALUATION REPORT TITLE: Independent evaluation of the Demand-Driven Impact Evaluations for Decisions (3DE) Pilot RESPONSE TO EVALUATION REPORT (overarching narrative) Overall we are pleased with the findings
Request for feedback on the revised Code of Governance for NHS Foundation Trusts
 Request for feedback on the revised Code of Governance for NHS Foundation Trusts Introduction 8 November 2013 One of Monitor s key objectives is to make sure that public providers are well led. To this
Request for feedback on the revised Code of Governance for NHS Foundation Trusts Introduction 8 November 2013 One of Monitor s key objectives is to make sure that public providers are well led. To this
European Forum for Good Clinical Practice Audit Working Party
 European Forum for Good Clinical Practice Audit Working Party REVISION OF THE ENGAGE 1 AUDITING GUIDELINE. AN OPTIONAL GUIDELINE FOR GCP COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS AUDITING This document
European Forum for Good Clinical Practice Audit Working Party REVISION OF THE ENGAGE 1 AUDITING GUIDELINE. AN OPTIONAL GUIDELINE FOR GCP COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS AUDITING This document
Putting Reliable Health Care Performance Measurement Systems into Practice
 An NCQA Issue Brief 2000 L Street, NW Washington, DC 20036 888-275-7585 www.ncqa.org Putting Reliable Health Care Performance Measurement Systems into Practice By Joachim Roski, PhD MPH; Vice President,
An NCQA Issue Brief 2000 L Street, NW Washington, DC 20036 888-275-7585 www.ncqa.org Putting Reliable Health Care Performance Measurement Systems into Practice By Joachim Roski, PhD MPH; Vice President,
PROJECT MANAGEMENT FRAMEWORK
 PROJECT MANAGEMENT FRAMEWORK DOCUMENT INFORMATION DOCUMENT TYPE: DOCUMENT STATUS: POLICY OWNER POSITION: INTERNAL COMMITTEE ENDORSEMENT: APPROVED BY: Strategic document Approved Executive Assistant to
PROJECT MANAGEMENT FRAMEWORK DOCUMENT INFORMATION DOCUMENT TYPE: DOCUMENT STATUS: POLICY OWNER POSITION: INTERNAL COMMITTEE ENDORSEMENT: APPROVED BY: Strategic document Approved Executive Assistant to
The Research Design Service Yorkshire and the Humber CONTENTS TOP TIPS FOR QUALITATIVE RESEARCH TOP TIPS FOR QUANTITATIVE PROPOSALS
 The Research Design Service Yorkshire and the Humber CONTENTS TOP TIPS FOR QUALITATIVE RESEARCH TOP TIPS FOR QUANTITATIVE PROPOSALS TOP TIPS FOR SYSTEMATIC REVIEWS TOP TIPS FOR ECONOMIC EVALUATION TOP
The Research Design Service Yorkshire and the Humber CONTENTS TOP TIPS FOR QUALITATIVE RESEARCH TOP TIPS FOR QUANTITATIVE PROPOSALS TOP TIPS FOR SYSTEMATIC REVIEWS TOP TIPS FOR ECONOMIC EVALUATION TOP
STARE-HI - Statement on Reporting of Evaluation Studies in Health Informatics
 STARE-HI - Statement on Reporting of Evaluation Studies in Health Informatics Jan Talmon 1*, Elske Ammenwerth 2, Jytte Brender 3, Nicolette de Keizer 4, Pirkko Nykänen 5, Michael Rigby 6 1 Care and Public
STARE-HI - Statement on Reporting of Evaluation Studies in Health Informatics Jan Talmon 1*, Elske Ammenwerth 2, Jytte Brender 3, Nicolette de Keizer 4, Pirkko Nykänen 5, Michael Rigby 6 1 Care and Public
Finding the Right People for Your Program Evaluation Team: Evaluator and Planning Team Job Descriptions
 : Evaluator and Planning Team Job Descriptions I. Overview II. Sample Evaluator Job Description III. Evaluator Competencies IV. Recruiting members of your strategic evaluation planning team V. Recruiting
: Evaluator and Planning Team Job Descriptions I. Overview II. Sample Evaluator Job Description III. Evaluator Competencies IV. Recruiting members of your strategic evaluation planning team V. Recruiting
Part B1: Business case developing the business case
 Overview Part A: Strategic assessment Part B1: Business case developing the business case Part B2: Business case procurement options Part B3: Business case funding and financing options Part C: Project
Overview Part A: Strategic assessment Part B1: Business case developing the business case Part B2: Business case procurement options Part B3: Business case funding and financing options Part C: Project
FINLAND ON A ROAD TOWARDS A MODERN LEGAL BIOBANKING INFRASTRUCTURE
 Postrefereed, preprint version of the text published at European Journal of Health Law 2013(3)28994. Link to the publisher s website: http://www.brill.com/europeanjournalhealthlaw Sirpa Soini FINLAND ON
Postrefereed, preprint version of the text published at European Journal of Health Law 2013(3)28994. Link to the publisher s website: http://www.brill.com/europeanjournalhealthlaw Sirpa Soini FINLAND ON
Procurement Programmes & Projects P3M3 v2.1 Self-Assessment Instructions and Questionnaire. P3M3 Project Management Self-Assessment
 Procurement Programmes & Projects P3M3 v2.1 Self-Assessment Instructions and Questionnaire P3M3 Project Management Self-Assessment Contents Introduction 3 User Guidance 4 P3M3 Self-Assessment Questionnaire
Procurement Programmes & Projects P3M3 v2.1 Self-Assessment Instructions and Questionnaire P3M3 Project Management Self-Assessment Contents Introduction 3 User Guidance 4 P3M3 Self-Assessment Questionnaire
1. FORMULATING A RESEARCH QUESTION
 W R I T I N G A R E S E A R C H G R A N T P R O P O S A L 1. FORMULATING A RESEARCH QUESTION 1.1. Identify a broad area of interest through literature searches, discussions with colleagues, policy makers
W R I T I N G A R E S E A R C H G R A N T P R O P O S A L 1. FORMULATING A RESEARCH QUESTION 1.1. Identify a broad area of interest through literature searches, discussions with colleagues, policy makers
CHECKLIST ISO/IEC 17021:2011 Conformity Assessment Requirements for Bodies Providing Audit and Certification of Management Systems
 Date(s) of Evaluation: CHECKLIST ISO/IEC 17021:2011 Conformity Assessment Requirements for Bodies Providing Audit and Certification of Management Systems Assessor(s) & Observer(s): Organization: Area/Field
Date(s) of Evaluation: CHECKLIST ISO/IEC 17021:2011 Conformity Assessment Requirements for Bodies Providing Audit and Certification of Management Systems Assessor(s) & Observer(s): Organization: Area/Field
jytte.brender@v-chi.dk "Success and failure factors in health information systems development" Jytte Brender Associate Research Professor
 "Success and failure factors in health information systems development" Jytte Brender Associate Research Professor Factors influencing success and failure of Health Informatics Systems, a pilot Delphi
"Success and failure factors in health information systems development" Jytte Brender Associate Research Professor Factors influencing success and failure of Health Informatics Systems, a pilot Delphi
Use advanced techniques for summary and visualization of complex data for exploratory analysis and presentation.
 MS Biostatistics MS Biostatistics Competencies Study Development: Work collaboratively with biomedical or public health researchers and PhD biostatisticians, as necessary, to provide biostatistical expertise
MS Biostatistics MS Biostatistics Competencies Study Development: Work collaboratively with biomedical or public health researchers and PhD biostatisticians, as necessary, to provide biostatistical expertise
A Question of Balance
 A Question of Balance Independent Assurance of Information Governance Returns Audit Requirement Sheets Contents Scope 4 How to use the audit requirement sheets 4 Evidence 5 Sources of assurance 5 What
A Question of Balance Independent Assurance of Information Governance Returns Audit Requirement Sheets Contents Scope 4 How to use the audit requirement sheets 4 Evidence 5 Sources of assurance 5 What
INSTITUTE OF FINANCIAL ADVISERS INC. P2 - PRACTICE STANDARDS
 INSTITUTE OF FINANCIAL ADVISERS INC. P2 - PRACTICE STANDARDS EFFECTIVE 1 JANUARY 2012 TABLE OF CONTENTS INTRODUCTION... 2 Professional Financial Advice... 2 The Six-Step Process... 2 The Core Components...
INSTITUTE OF FINANCIAL ADVISERS INC. P2 - PRACTICE STANDARDS EFFECTIVE 1 JANUARY 2012 TABLE OF CONTENTS INTRODUCTION... 2 Professional Financial Advice... 2 The Six-Step Process... 2 The Core Components...
INTERNAL AUDIT MANUAL
 དང ལ ར ས ལ ན ཁག Internal Audit Manual INTERNAL AUDIT MANUAL Royal Government of Bhutan 2014 i i ii ii Internal Audit Manual དང ལ ར ས ལ ན ཁག ROYAL GOVERNMNET OF BHUTAN MINISTRY OF FINANCE TASHICHHO DZONG
དང ལ ར ས ལ ན ཁག Internal Audit Manual INTERNAL AUDIT MANUAL Royal Government of Bhutan 2014 i i ii ii Internal Audit Manual དང ལ ར ས ལ ན ཁག ROYAL GOVERNMNET OF BHUTAN MINISTRY OF FINANCE TASHICHHO DZONG
Instructional Technology Capstone Project Standards and Guidelines
 Instructional Technology Capstone Project Standards and Guidelines The Committee recognizes the fact that each EdD program is likely to articulate some requirements that are unique. What follows are a
Instructional Technology Capstone Project Standards and Guidelines The Committee recognizes the fact that each EdD program is likely to articulate some requirements that are unique. What follows are a
Note that the following document is copyright, details of which are provided on the next page.
 Please note that the following document was created by the former Australian Council for Safety and Quality in Health Care. The former Council ceased its activities on 31 December 2005 and the Australian
Please note that the following document was created by the former Australian Council for Safety and Quality in Health Care. The former Council ceased its activities on 31 December 2005 and the Australian
National Clinical Effectiveness Committee. Prioritisation and Quality Assurance Processes for National Clinical Audit. June 2015
 National Clinical Effectiveness Committee Prioritisation and Quality Assurance Processes for National Clinical Audit June 2015 0 P age Table of Contents Glossary of Terms... 2 Purpose of this prioritisation
National Clinical Effectiveness Committee Prioritisation and Quality Assurance Processes for National Clinical Audit June 2015 0 P age Table of Contents Glossary of Terms... 2 Purpose of this prioritisation
How To Be A Successful Supervisor
 Quick Guide For Administrators Based on TIP 52 Clinical Supervision and Professional Development of the Substance Abuse Counselor Contents Why a Quick Guide?...2 What Is a TIP?...3 Benefits and Rationale...4
Quick Guide For Administrators Based on TIP 52 Clinical Supervision and Professional Development of the Substance Abuse Counselor Contents Why a Quick Guide?...2 What Is a TIP?...3 Benefits and Rationale...4
Guidance for Peer Reviewers. The Journal of the American Osteopathic Association (JAOA)
 Guidance for Peer Reviewers The Journal of the American Osteopathic Association (JAOA) JAOA Editorial Staff This module is available online at http://jaoa.org/documentlibrary/prmodule.pdf Guidance for
Guidance for Peer Reviewers The Journal of the American Osteopathic Association (JAOA) JAOA Editorial Staff This module is available online at http://jaoa.org/documentlibrary/prmodule.pdf Guidance for
PROJECT AUDIT METHODOLOGY
 PROJECT AUDIT METHODOLOGY 1 "Your career as a project manager begins here!" Content Introduction... 3 1. Definition of the project audit... 3 2. Objectives of the project audit... 3 3. Benefit of the audit
PROJECT AUDIT METHODOLOGY 1 "Your career as a project manager begins here!" Content Introduction... 3 1. Definition of the project audit... 3 2. Objectives of the project audit... 3 3. Benefit of the audit
Actuarial Standard of Practice No. 23. Data Quality. Revised Edition
 Actuarial Standard of Practice No. 23 Data Quality Revised Edition Developed by the General Committee of the Actuarial Standards Board and Applies to All Practice Areas Adopted by the Actuarial Standards
Actuarial Standard of Practice No. 23 Data Quality Revised Edition Developed by the General Committee of the Actuarial Standards Board and Applies to All Practice Areas Adopted by the Actuarial Standards
DOCTOR OF PHILOSOPHY DEGREE. Educational Leadership Doctor of Philosophy Degree Major Course Requirements. EDU721 (3.
 DOCTOR OF PHILOSOPHY DEGREE Educational Leadership Doctor of Philosophy Degree Major Course Requirements EDU710 (3.0 credit hours) Ethical and Legal Issues in Education/Leadership This course is an intensive
DOCTOR OF PHILOSOPHY DEGREE Educational Leadership Doctor of Philosophy Degree Major Course Requirements EDU710 (3.0 credit hours) Ethical and Legal Issues in Education/Leadership This course is an intensive
Process for reporting and learning from serious incidents requiring investigation
 Process for reporting and learning from serious incidents requiring investigation Date: 9 March 2012 NHS South of England Process for reporting and learning from serious incidents requiring investigation
Process for reporting and learning from serious incidents requiring investigation Date: 9 March 2012 NHS South of England Process for reporting and learning from serious incidents requiring investigation
Guidance Note: Corporate Governance - Board of Directors. March 2015. Ce document est aussi disponible en français.
 Guidance Note: Corporate Governance - Board of Directors March 2015 Ce document est aussi disponible en français. Applicability The Guidance Note: Corporate Governance - Board of Directors (the Guidance
Guidance Note: Corporate Governance - Board of Directors March 2015 Ce document est aussi disponible en français. Applicability The Guidance Note: Corporate Governance - Board of Directors (the Guidance
General Dental Council Website Review: Dental Professionals Survey Invitation to Tender
 General Dental Council Website Review: Dental Professionals Survey Invitation to Tender Summary 1. The General Dental Council (GDC) invites tender proposals for a research project on the usability of the
General Dental Council Website Review: Dental Professionals Survey Invitation to Tender Summary 1. The General Dental Council (GDC) invites tender proposals for a research project on the usability of the
Quality Assurance of Medical Appraisers
 Quality Assurance of Medical Appraisers Recruitment, training, support and review of medical appraisers in England www.revalidationsupport.nhs.uk Contents 1. Introduction 3 2. Purpose and overview 4 3.
Quality Assurance of Medical Appraisers Recruitment, training, support and review of medical appraisers in England www.revalidationsupport.nhs.uk Contents 1. Introduction 3 2. Purpose and overview 4 3.
Electronic Health Record System Evaluation Based on Patient Safety
 Electronic Health Record System Evaluation Based on Patient Safety Yung Yu (Paul) SU, Khin Than WIN & John FULCHER Health Informatics Research Centre, University of Wollongong, Australia Correspondence:
Electronic Health Record System Evaluation Based on Patient Safety Yung Yu (Paul) SU, Khin Than WIN & John FULCHER Health Informatics Research Centre, University of Wollongong, Australia Correspondence:
A Systematic Review Process for Software Engineering
 A Systematic Review Process for Software Engineering Paula Mian, Tayana Conte, Ana Natali, Jorge Biolchini and Guilherme Travassos COPPE / UFRJ Computer Science Department Cx. Postal 68.511, CEP 21945-970,
A Systematic Review Process for Software Engineering Paula Mian, Tayana Conte, Ana Natali, Jorge Biolchini and Guilherme Travassos COPPE / UFRJ Computer Science Department Cx. Postal 68.511, CEP 21945-970,
Standards of proficiency. Occupational therapists
 Standards of proficiency Occupational therapists Contents Foreword 1 Introduction 3 Standards of proficiency 7 Foreword We are pleased to present the Health and Care Professions Council s standards of
Standards of proficiency Occupational therapists Contents Foreword 1 Introduction 3 Standards of proficiency 7 Foreword We are pleased to present the Health and Care Professions Council s standards of
MAKING YOUR ORGANISATION S INFORMATION ACCESSIBLE FOR ALL IMPLEMENTING THE GUIDELINES FOR ACCESSIBLE INFORMATION
 MAKING YOUR ORGANISATION S INFORMATION ACCESSIBLE FOR ALL IMPLEMENTING THE GUIDELINES FOR ACCESSIBLE INFORMATION project has been funded with support from the European Union. publication reflects the views
MAKING YOUR ORGANISATION S INFORMATION ACCESSIBLE FOR ALL IMPLEMENTING THE GUIDELINES FOR ACCESSIBLE INFORMATION project has been funded with support from the European Union. publication reflects the views
Undergraduate Psychology Major Learning Goals and Outcomes i
 Undergraduate Psychology Major Learning Goals and Outcomes i Goal 1: Knowledge Base of Psychology Demonstrate familiarity with the major concepts, theoretical perspectives, empirical findings, and historical
Undergraduate Psychology Major Learning Goals and Outcomes i Goal 1: Knowledge Base of Psychology Demonstrate familiarity with the major concepts, theoretical perspectives, empirical findings, and historical
REPORTING ACCOUNTANTS WORK ON FINANCIAL REPORTING PROCEDURES. Financing Change initiative
 REPORTING ACCOUNTANTS WORK ON FINANCIAL REPORTING PROCEDURES consultation PAPER Financing Change initiative inspiring CONFIdENCE icaew.com/financingchange ICAEW operates under a Royal Charter, working
REPORTING ACCOUNTANTS WORK ON FINANCIAL REPORTING PROCEDURES consultation PAPER Financing Change initiative inspiring CONFIdENCE icaew.com/financingchange ICAEW operates under a Royal Charter, working
Nurse Practitioner Mentor Guideline NPAC-NZ
 Nurse Practitioner Mentor Guideline NPAC-NZ Purpose To provide a framework for the mentorship of registered nurses to prepare for Nurse Practitioner (NP) registration from the Nursing Council of New Zealand.
Nurse Practitioner Mentor Guideline NPAC-NZ Purpose To provide a framework for the mentorship of registered nurses to prepare for Nurse Practitioner (NP) registration from the Nursing Council of New Zealand.
Award STANDARDS - Nursing and midwifery
 Award STANDARDS - Nursing and midwifery www.qqi.ie July 2014/HS10 QQI Foreword The Qualifications (Education & Training) Act 1999 required the Higher Education and Training Awards Council to determine
Award STANDARDS - Nursing and midwifery www.qqi.ie July 2014/HS10 QQI Foreword The Qualifications (Education & Training) Act 1999 required the Higher Education and Training Awards Council to determine
Comparison table showing 2015 accreditation standards for specialist medical programs and professional development programs against the 2010 standards
 Comparison table showing 2015 accreditation standards for specialist medical programs and professional development programs against the 2010 standards Medical Programs and Professional Development Programs
Comparison table showing 2015 accreditation standards for specialist medical programs and professional development programs against the 2010 standards Medical Programs and Professional Development Programs
Healthcare Coalition on Data Protection
 Healthcare Coalition on Data Protection Recommendations and joint statement supporting citizens interests in the benefits of data driven healthcare in a secure environment Representing leading actors in
Healthcare Coalition on Data Protection Recommendations and joint statement supporting citizens interests in the benefits of data driven healthcare in a secure environment Representing leading actors in
Planning, Monitoring, Evaluation and Learning Program
 Planning, Monitoring, Evaluation and Learning Program Program Description Engineers Without Borders USA EWB-USA Planning, Monitoring, Evaluation 02/2014 and Learning Program Description This document describes
Planning, Monitoring, Evaluation and Learning Program Program Description Engineers Without Borders USA EWB-USA Planning, Monitoring, Evaluation 02/2014 and Learning Program Description This document describes
National Commission for Academic Accreditation & Assessment. Standards for Quality Assurance and Accreditation of Higher Education Institutions
 National Commission for Academic Accreditation & Assessment Standards for Quality Assurance and Accreditation of Higher Education Institutions November 2009 Standards for Institutional Accreditation in
National Commission for Academic Accreditation & Assessment Standards for Quality Assurance and Accreditation of Higher Education Institutions November 2009 Standards for Institutional Accreditation in
Maturity Model. March 2006. Version 1.0. P2MM Version 1.0 The OGC logo is a Registered Trade Mark of the Office of Government Commerce
 Maturity Model March 2006 Version 1.0 P2MM Version 1.0 The OGC logo is a Registered Trade Mark of the Office of Government Commerce This is a Value Added product which is outside the scope of the HMSO
Maturity Model March 2006 Version 1.0 P2MM Version 1.0 The OGC logo is a Registered Trade Mark of the Office of Government Commerce This is a Value Added product which is outside the scope of the HMSO
Department of Infrastructure and Planning: Governance Framework for Infrastructure Delivery Special Purpose Vehicles
 Department of Infrastructure and Planning: Governance Framework for Infrastructure Delivery Special Purpose Vehicles Governance Framework for Special Purpose Vehicles Table of Contents Executive Summary...3
Department of Infrastructure and Planning: Governance Framework for Infrastructure Delivery Special Purpose Vehicles Governance Framework for Special Purpose Vehicles Table of Contents Executive Summary...3
National Commission for Academic Accreditation & Assessment. Standards for Quality Assurance and Accreditation of Higher Education Programs
 National Commission for Academic Accreditation & Assessment Standards for Quality Assurance and Accreditation of Higher Education Programs November 2009 Standards for Quality Assurance and Accreditation
National Commission for Academic Accreditation & Assessment Standards for Quality Assurance and Accreditation of Higher Education Programs November 2009 Standards for Quality Assurance and Accreditation
INTERNATIONAL STANDARDS FOR THE PROFESSIONAL PRACTICE OF INTERNAL AUDITING (STANDARDS)
 INTERNATIONAL STANDARDS FOR THE PROFESSIONAL PRACTICE OF INTERNAL AUDITING (STANDARDS) Introduction to the International Standards Internal auditing is conducted in diverse legal and cultural environments;
INTERNATIONAL STANDARDS FOR THE PROFESSIONAL PRACTICE OF INTERNAL AUDITING (STANDARDS) Introduction to the International Standards Internal auditing is conducted in diverse legal and cultural environments;
STAGE 1 COMPETENCY STANDARD FOR PROFESSIONAL ENGINEER
 STAGE 1 STANDARD FOR PROFESSIONAL ENGINEER ROLE DESCRIPTION - THE MATURE, PROFESSIONAL ENGINEER The following characterises the senior practice role that the mature, Professional Engineer may be expected
STAGE 1 STANDARD FOR PROFESSIONAL ENGINEER ROLE DESCRIPTION - THE MATURE, PROFESSIONAL ENGINEER The following characterises the senior practice role that the mature, Professional Engineer may be expected
Middlesbrough Manager Competency Framework. Behaviours Business Skills Middlesbrough Manager
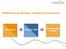 Middlesbrough Manager Competency Framework + = Behaviours Business Skills Middlesbrough Manager Middlesbrough Manager Competency Framework Background Middlesbrough Council is going through significant
Middlesbrough Manager Competency Framework + = Behaviours Business Skills Middlesbrough Manager Middlesbrough Manager Competency Framework Background Middlesbrough Council is going through significant
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON DATA MONITORING COMMITTEES
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 27 July 2005 Doc. Ref. EMEA/CHMP/EWP/5872/03 Corr COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 27 July 2005 Doc. Ref. EMEA/CHMP/EWP/5872/03 Corr COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
Specification Document (11/1023)
 Responsive funding in the NIHR SDO programme: open call (with special interest in studies with an economic/costing component) Closing date 1.00pm on 15 September 2011 1. Introduction This is the fifth
Responsive funding in the NIHR SDO programme: open call (with special interest in studies with an economic/costing component) Closing date 1.00pm on 15 September 2011 1. Introduction This is the fifth
Current reporting in published research
 Current reporting in published research Doug Altman Centre for Statistics in Medicine, Oxford, UK and EQUATOR Network Research article A published research article is a permanent record that will be used
Current reporting in published research Doug Altman Centre for Statistics in Medicine, Oxford, UK and EQUATOR Network Research article A published research article is a permanent record that will be used
STANDARDS OF PRACTICE (2013)
 STANDARDS OF PRACTICE (2013) COLLEGE OF ALBERTA PSYCHOLOGISTS STANDARDS OF PRACTICE (2013) 1. INTRODUCTION The Health Professions Act (HPA) authorizes and requires the College of Alberta Psychologists
STANDARDS OF PRACTICE (2013) COLLEGE OF ALBERTA PSYCHOLOGISTS STANDARDS OF PRACTICE (2013) 1. INTRODUCTION The Health Professions Act (HPA) authorizes and requires the College of Alberta Psychologists
CFP Certification Global excellence in financial planning
 CFP Certification Global excellence in financial planning VISION STATEMENT To establish financial planning as a recognized profession through the promotion of excellence in financial planning for the benefit
CFP Certification Global excellence in financial planning VISION STATEMENT To establish financial planning as a recognized profession through the promotion of excellence in financial planning for the benefit
FINANCIAL PLANNING PRACTICE STANADARDS
 FINANCIAL PLANNING PRACTICE STANADARDS Introduction IFPHK has adopted the Financial Planning Practice Standards ( Practice Standards ) in order to: Establish the level of practice expected of a financial
FINANCIAL PLANNING PRACTICE STANADARDS Introduction IFPHK has adopted the Financial Planning Practice Standards ( Practice Standards ) in order to: Establish the level of practice expected of a financial
P3M3 Portfolio Management Self-Assessment
 Procurement Programmes & Projects P3M3 v2.1 Self-Assessment Instructions and Questionnaire P3M3 Portfolio Management Self-Assessment P3M3 is a registered trade mark of AXELOS Limited Contents Introduction
Procurement Programmes & Projects P3M3 v2.1 Self-Assessment Instructions and Questionnaire P3M3 Portfolio Management Self-Assessment P3M3 is a registered trade mark of AXELOS Limited Contents Introduction
PROJECT MANAGEMENT PLAN Outline VERSION 0.0 STATUS: OUTLINE DATE:
 PROJECT MANAGEMENT PLAN Outline VERSION 0.0 STATUS: OUTLINE DATE: Project Name Project Management Plan Document Information Document Title Version Author Owner Project Management Plan Amendment History
PROJECT MANAGEMENT PLAN Outline VERSION 0.0 STATUS: OUTLINE DATE: Project Name Project Management Plan Document Information Document Title Version Author Owner Project Management Plan Amendment History
A Guide to Clinical Coding Audit Best Practice 2015-16
 A Guide to Clinical Coding Audit Best Practice 2015-16 Authors: Clinical Classifications Service Contents 1 Introduction 3 1.1 Purpose of Document 3 1.2 Audience 3 1.3 Background 3 1.3.1 Information Governance
A Guide to Clinical Coding Audit Best Practice 2015-16 Authors: Clinical Classifications Service Contents 1 Introduction 3 1.1 Purpose of Document 3 1.2 Audience 3 1.3 Background 3 1.3.1 Information Governance
Doctor of Education Program Handbook
 Doctor of Education Program Handbook Student Copy Revised Summer 2013 1 Introduction The Johns Hopkins University (JHU) School of Education (SOE) attracts the most innovative and progressive scholars without
Doctor of Education Program Handbook Student Copy Revised Summer 2013 1 Introduction The Johns Hopkins University (JHU) School of Education (SOE) attracts the most innovative and progressive scholars without
IT Professional Standards. Information Security Discipline. Sub-discipline 605 Information Security Testing and Information Assurance Methodologies
 IT Professional Standards Information Security Discipline Sub-discipline 605 Information Security Testing and Information Assurance Methodologies December 2012 Draft Version 0.6 DOCUMENT REVIEW Document
IT Professional Standards Information Security Discipline Sub-discipline 605 Information Security Testing and Information Assurance Methodologies December 2012 Draft Version 0.6 DOCUMENT REVIEW Document
Using Public Health Evaluation Models to Assess Health IT Implementations
 Using Public Health Evaluation Models to Assess Health IT Implementations September 2011 White Paper Prepared for Healthcare Information and Management Systems Society 33 West Monroe Street Suite 1700
Using Public Health Evaluation Models to Assess Health IT Implementations September 2011 White Paper Prepared for Healthcare Information and Management Systems Society 33 West Monroe Street Suite 1700
ASSESSING THE EFFECTIVENESS OF COMPANY GRIEVANCE MECHANISMS
 REPORT SUMMARY ASSESSING THE EFFECTIVENESS OF COMPANY GRIEVANCE MECHANISMS CSR Europe s Management of Complaints Assessment (MOC-A) Results THE FULL VERSION OF THIS REPORT IS AVAILABLE AT: HTTP://WWW.CSREUROPE.ORG/COMPANY_MECHANISMS_FOR_ADDRESSING_HUMAN_RIGHTS_COMPLAINTS.HTML
REPORT SUMMARY ASSESSING THE EFFECTIVENESS OF COMPANY GRIEVANCE MECHANISMS CSR Europe s Management of Complaints Assessment (MOC-A) Results THE FULL VERSION OF THIS REPORT IS AVAILABLE AT: HTTP://WWW.CSREUROPE.ORG/COMPANY_MECHANISMS_FOR_ADDRESSING_HUMAN_RIGHTS_COMPLAINTS.HTML
STAGE 1 COMPETENCY STANDARD FOR ENGINEERING ASSOCIATE
 STAGE 1 STANDARD FOR ENGINEERING ASSOCIATE ROLE DESCRIPTION THE MATURE ENGINEERING ASSOCIATE The following characterises the senior practice role that the mature, Engineering Associate may be expected
STAGE 1 STANDARD FOR ENGINEERING ASSOCIATE ROLE DESCRIPTION THE MATURE ENGINEERING ASSOCIATE The following characterises the senior practice role that the mature, Engineering Associate may be expected
FINANCIAL SERVICES TRAINING PACKAGE FNB99
 FINANCIAL SERVICES TRAINING PACKAGE FNB99 This is Volume 12 of a 13-volume set. This volume should not be used in isolation but in the context of the complete set for the Financial Services Training Package.
FINANCIAL SERVICES TRAINING PACKAGE FNB99 This is Volume 12 of a 13-volume set. This volume should not be used in isolation but in the context of the complete set for the Financial Services Training Package.
Inter-American Development Bank KNOWLEDGE AND LEARNING SECTOR (KNL) TECHNICAL NOTES. No. IDB-TN-421 AFTER ACTION REVIEW
 Inter-American Development Bank KNOWLEDGE AND LEARNING SECTOR (KNL) TECHNICAL NOTES AFTER ACTION REVIEW No. IDB-TN-421 June 2012 AFTER ACTION REVIEW Inter-American Development Bank 2012 http://www.iadb.org
Inter-American Development Bank KNOWLEDGE AND LEARNING SECTOR (KNL) TECHNICAL NOTES AFTER ACTION REVIEW No. IDB-TN-421 June 2012 AFTER ACTION REVIEW Inter-American Development Bank 2012 http://www.iadb.org
Serious Incident Framework 2015/16- frequently asked questions
 Serious Incident Framework 2015/16- frequently asked questions NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations Patients and Information Nursing Trans. & Corp. Ops. Commissioning
Serious Incident Framework 2015/16- frequently asked questions NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations Patients and Information Nursing Trans. & Corp. Ops. Commissioning
Standard 1. Governance for Safety and Quality in Health Service Organisations. Safety and Quality Improvement Guide
 Standard 1 Governance for Safety and Quality in Health Service Organisations Safety and Quality Improvement Guide 1 1 1October 1 2012 ISBN: Print: 978-1-921983-27-6 Electronic: 978-1-921983-28-3 Suggested
Standard 1 Governance for Safety and Quality in Health Service Organisations Safety and Quality Improvement Guide 1 1 1October 1 2012 ISBN: Print: 978-1-921983-27-6 Electronic: 978-1-921983-28-3 Suggested
Gateway review guidebook. for project owners and review teams
 Gateway review guidebook for project owners and review teams The State of Queensland (Queensland Treasury and Trade) 2013. First published by the Queensland Government, Department of Infrastructure and
Gateway review guidebook for project owners and review teams The State of Queensland (Queensland Treasury and Trade) 2013. First published by the Queensland Government, Department of Infrastructure and
THE APPLICATION OF A VALUE ASSURANCE SYSTEM TO OIL & GAS DEVELOPMENT PROJECTS (Guido Mattu, Franca Marini)
 PAGE 1 THE APPLICATION OF A VALUE ASSURANCE SYSTEM TO OIL & GAS DEVELOPMENT PROJECTS (Guido Mattu, Franca Marini) Ing. Guido Mattu More than 25 years experience in Engineering and Project Management activities
PAGE 1 THE APPLICATION OF A VALUE ASSURANCE SYSTEM TO OIL & GAS DEVELOPMENT PROJECTS (Guido Mattu, Franca Marini) Ing. Guido Mattu More than 25 years experience in Engineering and Project Management activities
Standards of proficiency. Operating department practitioners
 Standards of proficiency Operating department practitioners Contents Foreword 1 Introduction 3 Standards of proficiency 7 Foreword We are pleased to present the Health and Care Professions Council s standards
Standards of proficiency Operating department practitioners Contents Foreword 1 Introduction 3 Standards of proficiency 7 Foreword We are pleased to present the Health and Care Professions Council s standards
Fundamental Principles of Public-Sector Auditing
 ISSAI 100 The International Standards of Supreme Audit Institutions, or ISSAIs, are issued by INTOSAI, the International Organisation of Supreme Audit Institutions. For more information visit www.issai.org
ISSAI 100 The International Standards of Supreme Audit Institutions, or ISSAIs, are issued by INTOSAI, the International Organisation of Supreme Audit Institutions. For more information visit www.issai.org
A17.0 Research Higher Degree Programs Policy
 A17.0 Research Higher Degree Programs Policy Policy Category Academic Document Owner Vice Chancellor and President Responsible Officer Academic Director Review Date 1 January 2015 File Number Staff Code
A17.0 Research Higher Degree Programs Policy Policy Category Academic Document Owner Vice Chancellor and President Responsible Officer Academic Director Review Date 1 January 2015 File Number Staff Code
PHASE 8: IMPLEMENTATION PHASE
 PHASE 8: IMPLEMENTATION PHASE The Implementation Phase has one key activity: deploying the new system in its target environment. Supporting actions include training end-users and preparing to turn the
PHASE 8: IMPLEMENTATION PHASE The Implementation Phase has one key activity: deploying the new system in its target environment. Supporting actions include training end-users and preparing to turn the
The ICMCI CMC Competence Framework - Overview
 This CMC Competence Framework specifies the cluster of related abilities, commitments, knowledge, and skills that a management consultant should demonstrate in practice in order to successfully complete
This CMC Competence Framework specifies the cluster of related abilities, commitments, knowledge, and skills that a management consultant should demonstrate in practice in order to successfully complete
Module Management Information
 Approved Module Information for BHM353, 2014/5 Module Title/Name: Research Methods in HRM and OB Module Code: BHM353 School: Aston Business School Module Type: Standard Module New Module? No Module Credits:
Approved Module Information for BHM353, 2014/5 Module Title/Name: Research Methods in HRM and OB Module Code: BHM353 School: Aston Business School Module Type: Standard Module New Module? No Module Credits:
Information Governance Strategy & Policy
 Information Governance Strategy & Policy March 2014 CONTENT Page 1 Introduction 1 2 Strategic Aims 1 3 Policy 2 4 Responsibilities 3 5 Information Governance Reporting Structure 4 6 Managing Information
Information Governance Strategy & Policy March 2014 CONTENT Page 1 Introduction 1 2 Strategic Aims 1 3 Policy 2 4 Responsibilities 3 5 Information Governance Reporting Structure 4 6 Managing Information
PROGRAMMME SPECIFICATION FOR MA in LEADERSHIP AND MANAGEMENT (HEALTH AND SOCIAL CARE SERVICES)
 PROGRAMMME SPECIFICATION FOR MA in LEADERSHIP AND MANAGEMENT (HEALTH AND SOCIAL CARE SERVICES) MA in LEADERSHIP AND MANAGEMENT (HEALTH AND SOCIAL CARE SERVICES) 1. Award 2. Route Management (Health and
PROGRAMMME SPECIFICATION FOR MA in LEADERSHIP AND MANAGEMENT (HEALTH AND SOCIAL CARE SERVICES) MA in LEADERSHIP AND MANAGEMENT (HEALTH AND SOCIAL CARE SERVICES) 1. Award 2. Route Management (Health and
A Guide to Learning Outcomes, Degree Level Expectations and the Quality Assurance Process in Ontario
 A Guide to Learning Outcomes, Degree Level Expectations and the Quality Assurance Process in Ontario A Guide to Learning Outcomes, Degree Level Expectations and the Quality Assurance Process in Ontario
A Guide to Learning Outcomes, Degree Level Expectations and the Quality Assurance Process in Ontario A Guide to Learning Outcomes, Degree Level Expectations and the Quality Assurance Process in Ontario
Standards of proficiency. Arts therapists
 Standards of proficiency Arts therapists Contents Foreword 1 Introduction 3 Standards of proficiency 7 Foreword We are pleased to present the Health and Care Professions Council s standards of proficiency
Standards of proficiency Arts therapists Contents Foreword 1 Introduction 3 Standards of proficiency 7 Foreword We are pleased to present the Health and Care Professions Council s standards of proficiency
The roles of Graduate Studies Committees and the operation of progress reviews for research students
 Approved by Academic Council 26th November 2010 The roles of Graduate Studies Committees and the operation of progress reviews for research students The roles of Graduate Studies Committees and the operation
Approved by Academic Council 26th November 2010 The roles of Graduate Studies Committees and the operation of progress reviews for research students The roles of Graduate Studies Committees and the operation
FINAL DOCUMENT. Guidelines for Regulatory Auditing of Quality Management Systems of Medical Device Manufacturers Part 1: General Requirements
 GHTF/SG4/N28R4:2008 FINAL DOCUMENT Title: Guidelines for Regulatory Auditing of Quality Management Systems of Medical Device Manufacturers Authoring Group: GHTF Study Group 4 Endorsed by: The Global Harmonization
GHTF/SG4/N28R4:2008 FINAL DOCUMENT Title: Guidelines for Regulatory Auditing of Quality Management Systems of Medical Device Manufacturers Authoring Group: GHTF Study Group 4 Endorsed by: The Global Harmonization
SECTION B DEFINITION, PURPOSE, INDEPENDENCE AND NATURE OF WORK OF INTERNAL AUDIT
 SECTION B DEFINITION, PURPOSE, INDEPENDENCE AND NATURE OF WORK OF INTERNAL AUDIT Through CGIAR Financial Guideline No 3 Auditing Guidelines Manual the CGIAR has adopted the IIA Definition of internal auditing
SECTION B DEFINITION, PURPOSE, INDEPENDENCE AND NATURE OF WORK OF INTERNAL AUDIT Through CGIAR Financial Guideline No 3 Auditing Guidelines Manual the CGIAR has adopted the IIA Definition of internal auditing
Standards and Guidelines for Quality Assurance in the European Higher Education Area (ESG)
 Standards and Guidelines for Quality Assurance in the European Higher Education Area (ESG) Approved by the Ministerial Conference in May 2015 by European Association for Quality Assurance in Higher Education
Standards and Guidelines for Quality Assurance in the European Higher Education Area (ESG) Approved by the Ministerial Conference in May 2015 by European Association for Quality Assurance in Higher Education
The Netherlands response to the public consultation on the revision of the European Commission s Impact Assessment guidelines
 The Netherlands response to the public consultation on the revision of the European Commission s Impact Assessment guidelines Introduction Robust impact assessment is a vital element of both the Dutch
The Netherlands response to the public consultation on the revision of the European Commission s Impact Assessment guidelines Introduction Robust impact assessment is a vital element of both the Dutch
RE AP QUE PLIC FOR IONS. Compa CPRIT RFA C 12 FORM 2. Company. p.1/17
 RE AP QUE ST PLIC AT RFA C 12 FORM 2 FOR IONS Compa any Formation Awards FY 2012 Fiscal Year Award Period September 1, 2011 Augustt 31, 2012 CPRIT RFA C 12 FORM 2 (Rev 6/ /30/11) Company Formation Awards
RE AP QUE ST PLIC AT RFA C 12 FORM 2 FOR IONS Compa any Formation Awards FY 2012 Fiscal Year Award Period September 1, 2011 Augustt 31, 2012 CPRIT RFA C 12 FORM 2 (Rev 6/ /30/11) Company Formation Awards
Achieve. Performance objectives
 Achieve Performance objectives Performance objectives are benchmarks of effective performance that describe the types of work activities students and affiliates will be involved in as trainee accountants.
Achieve Performance objectives Performance objectives are benchmarks of effective performance that describe the types of work activities students and affiliates will be involved in as trainee accountants.
RKI workshop, Evidence based immunisation. Evidence-based methods for public health
 RKI workshop, Evidence based immunisation Evidence-based methods for public health ECDC report Evidence-based methods for public health How to assess the best available evidence when time is limited and
RKI workshop, Evidence based immunisation Evidence-based methods for public health ECDC report Evidence-based methods for public health How to assess the best available evidence when time is limited and
Controlling Our Critical Path: A CDOT Guide to Better Project Management Practices
 : A CDOT Guide to Better Project Management Practices Project Management practices have been a part of the CDOT culture for many years. However, with a transitioning workforce and increasing demands, it
: A CDOT Guide to Better Project Management Practices Project Management practices have been a part of the CDOT culture for many years. However, with a transitioning workforce and increasing demands, it
