Whole Exome and Whole Genome Sequencing for Diagnosis of Genetic Disorders
|
|
|
- Barry Johns
- 7 years ago
- Views:
Transcription
1 Whole Exome and Whole Genome Sequencing for Diagnosis of Genetic Disorders Policy Number: Last Review: 6/2016 Origination: 12/2015 Next Review: 12/2016 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for Whole Exome and Whole Genome Sequencing for Diagnosis of Genetic Disorders. This is considered investigational. Note: This is a type of genetic testing that may be excluded in some contracts. Verify benefits prior to review for Medical Necessity. When Policy Topic is covered n/a When Policy Topic is not covered Whole exome sequencing and whole genome sequencing are considered investigational for the diagnosis of genetic disorders. Considerations The policy statement is intended to address the use of whole exome and whole genome sequencing for the diagnosis of genetic disorders in patients with suspected genetic disorders and for population-based screening. This policy does not address the use of whole exome and whole genome sequencing for preimplantation genetic diagnosis or screening, prenatal (fetal) testing, or testing of cancer cells. Genetic Counseling Genetic counseling is primarily aimed at patients who are at risk for inherited disorders, and experts recommend formal genetic counseling in most cases when genetic testing for an inherited condition is considered. The interpretation of the results of genetic tests and the understanding of risk factors can be very difficult and complex. Therefore, genetic counseling will assist individuals in understanding the possible benefits and harms of genetic testing, including the possible impact of
2 the information on the individual s family. Genetic counseling may alter the utilization of genetic testing substantially and may reduce inappropriate testing. Genetic counseling should be performed by an individual with experience and expertise in genetic medicine and genetic testing methods. Description of Procedure or Service Populations Interventions Comparators Outcomes Individuals: With suspected genetic disorders Interventions of interest are: Whole exome sequencing or whole genome sequencing Comparators of interest are: Standard management Relevant outcomes include: Test accuracy Test validity Other test performance measures Changes in reproductive decision making Morbid events Health status measures Resource utilization Whole exome sequencing (WES) is targeted sequencing of the subset of the human genome that contains functionally important sequences of protein-coding DNA, while whole genome sequencing (WGS) uses next-generation sequencing (NGS) techniques to sequence both coding- and noncoding regions of the genome. The evidence for the use of WES/WGS in individuals with suspected genetic disorders includes multiple studies that describe detection of novel genetic variants and several relatively large cohort studies that report on the yield of WES/WGS. Relevant outcomes include test accuracy, test validity, other test performance measures, changes in reproductive decision making, morbid events, health status measures, and resource utilization. A potential major indication for their the use of WES/WGS is for molecular diagnosis of patients with a phenotype that is suspicious for a genetic disorder but when a specific mutation is not suspected or with suspected genetic disorders that have a large degree of genetic heterogeneity. Such patients may be left without a clinical diagnosis of their disorder, despite a lengthy diagnostic workup involving a variety of traditional molecular and other types of conventional diagnostic tests. For some of these patients, WES or WGS, after initial conventional testing has failed to make the diagnosis, may return a likely pathogenic variant. There is developing evidence about the use of WES/WGS in clinical practice, with studies describing yields of a molecular diagnosis in approximately 25% of patients without a diagnosis after a previous workup. No studies were identified that directly compared WES/WGS with alternative testing strategies in terms of the yield of testing for pathogenic variants associated with the phenotype being evaluated and variants of uncertain significance and incidental clinically-actionable findings. The evidence is insufficient to determine the effects of the technology on health outcomes.
3 Currently available clinical assays designed for the molecular diagnosis of rare Mendelian diseases are incomplete. This is due to genetic heterogeneity, the presence of unknown causative genes, and because only a portion of the known genes and mutations can be efficiently tested using conventional molecular methods. Recently, next-generation sequencing technologies have become more accessible in terms of cost and speed and have been adopted by a growing number of molecular genetic clinical laboratories. Depending on the disorder and the degree of genetic and clinical heterogeneity, the current diagnostic pathway for patients with suspected genetic disorders accompanied by multiple anomalies may depend on various combinations of lowyield radiographic, electrophysiologic, biochemical, biopsy, and targeted genetic evaluations. 1 The search for a diagnosis may thus become a time-consuming and expensive process. When a disease-causing gene(s) is established, assays based on polymerase chain reaction technology, for example, can be designed to specifically detect known mutations for clinical diagnosis. When many different point mutations in a gene are possible, Sanger sequencing, the current criterion standard for detecting unknown point mutations, can be employed to determine the entire sequence of the coding and intron/exon splice sites of gene regions where mutations are most likely to be found. However, when genes are large and mutations are possible in many or all exons (protein-coding regions of the gene), and when there is genetic (locus) heterogeneity, comprehensive Sanger sequencing may be prohibitively laborious and costly. Whole exome sequencing (WES) using next-generation sequencing technology is a relatively new approach to obtaining a genetic diagnosis in patients more efficiently compared with traditional methods. Exome sequencing has the capacity to determine a person s exomic variation profile in a single assay. This profile is limited to most of the protein coding sequence of an individual ( 85%), is composed of about 20,000 genes, and 180,000 exons (protein-coding segments of a gene), and constitutes approximately 1% of the whole genome. It is believed that the exome contains about 85% of heritable disease-causing mutations. Published exome sequencing studies show that the technology can be used to detect previously annotated pathogenic mutations and reveal new likely pathogenic mutations in known and unknown genes. The diagnostic yield, based on a limited number of studies, appears to be significantly increased above that of traditional Sanger sequencing, and exome sequencing has the advantage of speed and efficiency relative to Sanger sequencing of multiple genes. Whole genome sequencing (WGS) uses similar techniques to WES, but involves the sequencing of noncoding DNA in addition to the protein-coding segments of the genome.
4 Limitations of WES/WGS At this time, the limitations of WES/WGS include technical and implementation challenges. There are issues of error rates due to uneven sequencing coverage, gaps in exon capture before sequencing, and difficulties with narrowing the large initial number of variants to manageable numbers without losing likely candidate mutations. It is difficult to filter and interpret potential causative variants from the large number of variants of unknown significance generated for each patient. Variant databases are poorly annotated, and algorithms for annotating variants will need to be automated. Existing databases that catalog variants and putative disease associations are known to have significant entry error rates. Approaches for characterizing the functional impact of rare and novel variants (ie, achieving full-genome clinical interpretations that are scientifically sound and medically relevant) have to be improved. The variability contributed by the different platforms and procedures used by different clinical laboratories offering exome sequencing as a clinical service is unknown, and detailed guidance from regulatory and professional organizations is still under development. Finally, exome sequencing has some similar limitations as Sanger sequencing; eg, it will not identify the following: intronic sequences or gene regulatory regions, chromosomal changes, large deletions; duplications; or rearrangements within genes, nucleotide repeats, or epigenetic changes. WGS addresses some of these limitations, but is limited by the need for increased analytic power and the likelihood of greater identification of variants of uncertain significance. There are ethical questions about reporting incidental findings, such as identifying medically relevant mutations in genes unrelated to the diagnostic question, sex chromosome abnormalities, and nonpaternity when family studies are performed. Standards for the required components of informed consent before WES/WGS is performed have been proposed and include a description of confidentiality and a description of how incidental findings will be managed. 2 Methods of reporting findings from WES/WGS are under development. For example, McLaughlin et al, reporting on the MedSeq Project which is testing methods for evaluating and reporting WES/WGS data, described the development of a genome report that highlights results significant to the indication being evaluated. 3 Results of Testing With WES/WGS 4 1. A variant known to cause human disease is identified. This is a sequence variant that has been shown through prior genetic and clinical research to cause a disease. 2. A variant suspected to cause human disease is identified. Most variants detected by WES sequencing are uncharacterized and some are novel (ie, never known to have been observed in a human sample). Some variants allow for relatively easy and accurate clinical interpretation; however, for most there is little data on which to base an assessment of causality. Tools to facilitate the assessment of causality include bioinformatic analyses, predicted structural changes and others. While these tools may be useful, their predictive power is highly variable. 3. A variant of uncertain significance is identified.
5 Among the known 30,000 to 40,000 variants that reside in the proteincoding portions of the genome, the typical subject will have 3 to 8 actionable variants. (Most of these relate to reproductive risks, ie, heterozygous carrier alleles.) But the remaining thousands are either highly likely to be benign or of uncertain clinical significance. It can be equally as challenging to prove that a variant is benign as it is to prove it is pathogenic. Currently, nearly all of the variants among the tens of thousands must be considered of uncertain significance. Available WES/WGS Testing Services Although WES/WGS has been used as a research tool, it is less well-developed as a clinical service. Several laboratories offer WES/WGS as a clinical service. Illumina (San Diego, CA) offers 3 TruGenome tests: the TruGenome Undiagnosed Disease Test (indicated to find the underlying genetic cause of an undiagnosed rare genetic disease of single-gene etiology), TruGenome Predisposition Screen (indicated for healthy patients interested in learning about their carrier status and genetic predisposition toward adult-onset conditions), and the TruGenome Technical Sequence Data (WGS for labs and physicians who will make their own clinical interpretations.) Ambry Genetics (Aliso Viejo, CA) offers 2 WGS tests, the ExomeNext and ExomeNext-Rapid, both of which sequence both the nuclear and mitochondrial genomes. GeneDx (Gaithersburg, MD) offers WES with its XomeDx test. Medical centers may also offer WES/WGS as a clinical service. Examples of some of the laboratories offering WES as a clinical service and their indications for testing are summarized in Table 1. Table 1: Examples of Laboratories Offering Exome Sequencing as a Clinical Service Laboratory Ambry Genetics, Aliso Viejo, CA GeneDx, Gaithersburg, MD Baylor College of Medicine, Houston, TX University of California Los Angeles Health System EdgeBio, Gaithersburg, MD Children s Mercy Hospitals Laboratory Indications for Testing The patient's clinical presentation is unclear/atypical disease and there are multiple genetic conditions in the differential diagnosis. a patient with a diagnosis that suggests the involvement of one or more of many different genes, which would, if even available and sequenced individually, be prohibitively expensive used when a patient s medical history and physical exam findings strongly suggest that there is an underlying genetic etiology. In some cases, the patient may have had an extensive evaluation consisting of multiple genetic tests, without identifying an etiology. This test is intended for use in conjunction with the clinical presentation and other markers of disease progression for the management of patients with rare genetic disorders. Recommended In situations where there has been a diagnostic failure with no discernible path. In situations where there are currently no available tests to determine the status of a potential genetic disease. In situations with atypical findings indicative of multiple disease[s]. Provided as a service to families with children who have
6 and Clinics, Kansas City Emory Genetics Laboratory, Atlanta, GA had an extensive negative work-up for a genetic disease; also used to identify novel disease genes. Indicated when there is a suspicion of a genetic etiology contributing to the proband s manifestations. REGULATORY STATUS Clinical laboratories may develop and validate tests in-house and market them as a laboratory service; laboratory-developed tests (LDTs) must meet the general regulatory standards of the Clinical Laboratory Improvement Act (CLIA). Exome or genome sequencing tests as a clinical service are available under the auspices of CLIA. Laboratories that offer LDTs must be licensed by CLIA for high-complexity testing. To date, the U.S. Food and Drug Administration has chosen not to require any regulatory review of this test. Rationale The evidence review was created in August 2013 with review of the literature covering the period through August 27, 2013, and updated periodically with literature reviews. The most recent literature review covers the period through September 28, 2015 (see Appendix Table 2 for genetic testing categories). Analytic Validity Whole Exome Sequencing There is relatively little data specific to the analytic validity of whole exome sequencing (WES). The next-generation sequencing (NGS) techniques used for WES are generally expected to have high accuracy for mutation detection, NGS platforms differ in terms of the depth of sequence coverage, methods for base calling and read alignment, and other factors. These factors contribute to potential variability across the different platforms and procedures used by different clinical laboratories offering exome sequencing as a clinical service. The American College of Medical Genetics has clinical laboratory standards for NGS, including WES. 5 The guidelines outline the documentation of test performance measures that should be evaluated for NGS platforms, and note that typical definitions of analytic sensitivity and specificity do not apply for NGS. Depending on the platform and variant call method used, WES may not accurately detect large insertions and deletions, large copy number variants (CNVs), and structural chromosome rearrangements due to the short sequence read lengths. 5 WES may be less sensitive for the detection of CNVs than high-resolution microarray testing. 6 Whole Genome Sequencing Whole genome sequencing (WGS), is subject to the same considerations related to potential variability in technical performance as WES. In 2014, Dewey et al reported the coverage and concordance of clinically relevant genetic variation provided by WGS technologies in 12 healthy adult volunteers. 7 All subjects underwent WGS with the Illumina platform; 9 subjects also underwent WGS by the Complete Genomics platform to evaluate reproducibility of sequence data. Genome sequences were compared with several reference standards. Depending on the
7 sequencing platform, a median of 10% (Illumina Inc.; range, 5%-34%) to 19% (Complete Genomics; range, 18%-21%) of genes associated with inherited disease and a median of 9% (Illumina Inc.; range, 2%-27%) to 17% (Complete Genomics Inc.; range, 17%-19%) of American College of Medical Genetics (ACMG) reportable genes were not covered at a minimum threshold for genetic variant discovery. The genotype concordance between sequencing platforms was high for common genetic variants, for single nucleotide variants in protein coding regions of the genome, and among candidate variants for inherited disease risk. However, genotype concordance between sequencing platforms for small insertion/deletion variants was moderate overall (median, 57%; range, 53%-59%) and in protein coding regions of the genome (median, 66%; range, 64%-70%) but was substantially lower among genetic variants that were candidates for inherited disease risk (median, 33%; range, 10%-75%). Clinical Validity/Clinical Utility Overview: WES/WGS in Characterizing Mendelian Disorders The clinical utility of WES and WGS lies in the influence of the results on medical decision making and patient outcomes. There are several ways in which clinical utility can be demonstrated. WES/WGS may detect additional mutations that are missed by other testing methods, thus leading to a definitive diagnosis. o If the establishment of a definitive diagnosis leads to management changes that improve outcomes, then clinical utility has been established. o If the establishment of a definitive diagnosis leads to avoidance of other tests that are unnecessary, then this is another example of clinical utility. If WES/WGS is at least as accurate as other methods of sequencing, then an improvement in the efficiency of workup (diagnosis obtained more quickly and/or at less cost), then clinical utility has been established. Typically, when a phenotype/history suggests a genetic etiology, microdeletions/duplications should be excluded by chromosomal microarray analysis and, if clinically appropriate, other possible disorders like inborn errors of metabolism should also be excluded. If these tests are negative, the potential uses of WES/WGS include facilitating the accurate diagnosis of people with a suspected monogenic (Mendelian) disorder that presents with an atypical presentation or multiple congenital anomalies, is difficult to confirm with clinical or laboratory criteria alone (eg, when disease characteristics are shared among multiple disorders, leading to potentially overlapping differential diagnoses [clinical heterogeneity]), and when there is a long list of possible candidate genes. 8 An additional potential use of WES/WGS is when a clinical presentation is suggestive of a specific genetic condition, but targeted testing is negative or unavailable. In this situation, the yield of a definitive diagnosis can be used to evaluate the clinical utility of WES/WGS, also considering whether management changes occur that improve outcomes.
8 With advances in sequencing capacity, novel sequence variants associated with genetic disorders are rapidly being described. Sequence variants detected on WES/WGS, or any form of NGS, must be classified on a spectrum from almost certainly pathogenic to almost certainly benign. In 2013, the ACMG, Association for Molecular Pathology, and College of American Pathologists convened a workgroup to develop standard terminology for describing sequence variants. 9 Guidelines developed by this workgroup, published in 2015, describe criteria for classifying pathogenic and benign sequence variants based on a variety of population, computational and predictive, functional and data, segregation data into 5 categories: pathogenic, likely pathogenic, uncertain significance, likely benign, and benign. WES/WGS for Identifying Novel Mutations Since publication of the 2013 TEC Special Report, studies continue to demonstrate that WES can be used to identify novel genetic mutations in a range of clinical conditions. In particular, WES/WGS has been evaluated for disorders associated with significant genetic heterogeneity. A sample of such studies is shown in Table 2. Table 2: Studies Evaluating WES/WGS Study Clinical Condition Greenway Familial atrial (2014) 10 septal defect No. of Subjects for WES/WGS 2 family members with atrial septal defect Jiang ASD 32 patients (2013) 11 with ASD Kim Autosomal (2013) 12 dominant nonsyndromic hearing loss 21 family members with AD- NSHL Weeke dilqts 65 dilqts (2014) 13 patients and 148 drugexposed control subjects Zhou Syndrome of (2014) 14 intermittent fevers, earlyonset lacunar strokes, and other neurovascular manifestations, hepatosplenomegaly, and systemic 3 unrelated subjects with syndrome and unaffected parents Summary of Major Findings Identification of alpha-cardiac actin (ACTC1) as the candidate disease-causing gene Identification of deleterious de novo mutations in 6 families (19%) and X-linked or autosomal inherited alterations in 10 families (31%) Variants were found in 4 unrecognized, 9 known, and 8 candidate ASD risk genes Identification of a novel mutation in POU4F3 gene as the candidate disease-causing gene Identification of rare variants in KCNE1 and ACN9 as risk factors for dilqts Identification of mutations in CECR1 (cat eye syndrome chromosome region, candidate 1), encoding adenosine deaminase 2 as candidate gene
9 vasculopathy AD-NSHL: autosomal dominant nonsyndromic hearing loss; ASD: autism spectrum disorder; dilqts: drug-induced long-qt syndrome. WES/WGS in Clinical Practice Several studies have reported on the use of WES and, less frequently, WGS in clinical practice. Typically the populations included in these studies are those with suspected rare genetic disorders, although the specific patient populations varu. In 2015, Lee et al reported on a large (n=814) single-center cohort of patients with undiagnosed, suspected genetic conditions who underwent WES. 15 The investigators used a trio-ces [clinical exome sequencing] technique which involves sequencing of the proband and 2 family members, typically unaffected parents. For the first approximately 300 cases, all reported variants were confirmed by Sanger sequencing with more than 99% confirmation. After that, variants were evaluated with a QUAL score, a scaled probability of a variant existing at a given site, and only clinically significant mutations with a QUAL score lower than 500 were confirmed. Variants found were annotated to provide information about their effect on protein function, allele frequency in the general population, and prior evidence of disease causality and filtered to select likely pathogenic DNA variants. For variants in probands with family member testing available, variants were categorized as de novo (usually heterozygous in the patient and potentially causing an autosomal dominant condition), homozygous, compound heterozygous, and inherited variants. Variants were evaluated in the context of a primary gene list which was determined based on phenotypic key words included in referring clinician notes. Of the 814 patients included, 520 patients (64%) were children, and 254 of those were younger than 5 years at testing. The most common clinical indication for testing was developmental delay in the entire population and in the childhood group (37% and 53%, respectively). In the adult group, ataxia was the most common indication for testing (26%). Overall, a molecular diagnosis with a causative variant in a well-established clinical gene was provided for 213/814 cases (26%; 95% CI 23 to 29%). Of the 264 variants reported in 213 cases, 188 were reported as likely pathogenic and 73 were reported as pathogenic variants. In 2014, Yang et al reported on a single-center observational study that included 2000 consecutive patients referred for clinical WES for a suspected genetic disorder. 16 This report excluded 250 patients reported in an earlier publication. The majority of the samples were from pediatric patients, with 900 from children under 5 years (45.0%), 845 from children/adolescents aged 5 to 18 years (42.2%), 244 from adults (12.2%), and 11 fetal samples. The most common indications for testing were neurological disorders or developmental delay (87.8%). Molecular diagnoses were reported for 504 patients (25.2%, 95% CI 23.3% to 27.2%), with a total of 708 presumptive causative variants. Most of the identified diseaseassociated variants were novel (409/708; 57.8%). Overall, 95 medicallyactionable incidental findings were reported in 92 patients (4.6%), most of which
10 (n=59) were included in the American College of Medical Genetics list of 56 genes recommended to be disclosed to patients. In an earlier study from the same center as reported in Yang et al (2014), Yang et al reported results from the first 250 patients who underwent WES at a single institution. 17 Most patients (80%) were children presenting with phenotypes consistent with a neurologic disorder. Sixty-two patients were identified to have 86 mutated alleles that satisfied criteria for a molecular diagnosis for an overall rate of a positive molecular diagnosis of 25%. Thirty-nine of the patients with a molecular diagnosis had rare genetic diagnoses. In addition to diagnostic findings, 30 patients had medically actionable incidental findings in a total of 16 genes, and 13 had carrier-status mutations in genes from the ACMG-recommended population-screening panel. This study suggests that WES can have a high diagnostic yield in an appropriately-selected population. However, rates of incidental findings were also high, and the impact on clinical outcomes is unknown. Tammimies et al reported on the results of chromosomal microarray analysis (CMA) and WES in a sample of children with autism spectrum disorders. 18 The patient cohort included 258 consecutively enrolled patients, stratified into three groups based on the presence of major congenital abnormalities and minor physical anomalies (n=168, 37, and 53 considered essential, equivocal, and complex, respectively). All probands underwent CMA testing. WES was performed for 95 proband-parent trios. Among the 95 patients undergoing WES, 8 children (9 mutations) were received an ASD-related molecular diagnosis (8.4%, 95% CI 3.7% to 15.9%). Incidental or medically-actionable findings were reported in 8/95 (8.4%) probands tested with WES, 6 of which were considered medicallyactionable. Other studies have reported the yield of WES/WGS testing in clinical populations, varying depending on the population. Taylor et al report a yield of 21% for disease-causing variants with WES in a population of 217 patients with suspected genetic disorders with no pathogenic variants on prior screening. 19 Among 11 subjects with cardiomyopathy, Golbus et al reported a yield of 82% for pathogenic variants using WGS. 20 Clinical Utility: Change in Patient Management with WES/WGS As cited in a 2013 TEC Special Report on exome sequencing for patients with suspected genetic disorders, currently there are no published studies that systematically examine potential outcomes of interest such as changes in medical management (including revision of initial diagnoses), and changes in reproductive decision making after a diagnosis of a Mendelian disorder by WES. 21 A small number of studies of patient series, and a larger number of very small series or family studies report anecdotal examples of medical management and reproductive decision-making outcomes of exome sequencing in patients who were not diagnosed by traditional methods. These studies show that over and above traditional molecular and conventional diagnostic testing, exome sequencing can lead to a diagnosis that influences patient care and/or reproductive decisions, but give no indication of the proportion of patients for which this is true.
11 Since the publication of the 2013 TEC Special Report, several studies have reported on potential benefits, in terms of medical management changes or avoidance of alternative testing, following WES/WGS. Soden et al reported on the use of WGS and/or WES in parent-child trios for 119 children with neurodevelopmental disorders. 22 A definitive molecular diagnosis of an established genetic disorder was identified in 45 of the 100 families with children affected by neurodevelopmental disorders (53 of 119 affected children). Chart reviews and interviews with referring physicians were used to assess changes in short-term management following WES/WGS, and changed patient management and/or clinical impression was reported in 22/45 families (49%). In a retrospective study of 78 children with neurodevelopmental disorders with a prior unrevealing workup who underwent WES, Srivastava et al reported a presumptive diagnostic testing rate of 41%. 23 Results of WES changed patient management in all cases, most often related to reproductive planning (n=27), along with additional disease monitoring in 4 cases, further workup for systemic involvement in 6 cases, and 7 medication changes. Iglesias et al reported on clinical changes that occurred after WES/WGS in a broader population of 115 patients with a genetically undefined disorder. 24 The most common indications for WES evaluation were birth defects, developmental delay, and seizures, in 24.3%, 25.2%, and 14% of patients, respectively. A definitive diagnosis was made in 37 cases (32.2%). The clinical implications of testing are described qualitatively for patients with a genetic diagnosis. In 6 cases, it was noted that genetic information was used for reproductive planning; in 11 cases, patients were noted to have a change in medical management or surveillance or testing for related conditions. With wider genome coverage in sequencing comes an increased likelihood that testing will identify potentially clinically-significant gene mutations that may be unrelated to the phenotype being evaluated. The ACMG issued recommendations in 2013 outlining 56 genes associated with 24 conditions that should be reported to patients when known or likely pathogenic variants are detected. 25 The risks and benefits of WES/WGS in terms of detection of clinically actionable incidental findings should be evaluated on an individual basis. Ongoing and Unpublished Clinical Trials Some currently unpublished trials that might influence this review are listed in Table 3. Table 3. Summary of Key Trials NCT No. Trial Name Planned Enrollme nt Completio n Date Ongoing NCT NCGENES: North Carolina Clinical Genomic 750 Dec Evaluation by NextGen Exome Sequencing NCT Genetic Basis of Non Syndromic Congenital 78 Jul Diaphragmatic Hernia NCT Identification of Genes Involved in Juvenile 30 Dec 2017
12 2 Idiopathic Arthritis by Whole Exome Sequencing NCT Whole Exome and Whole Genome Sequencing 4 for Genotyping of Inherited and Congenital Eye Cond NCT Whole Genome Medical Sequencing for Gene 0 Discovery NCT Genetic Analysis of Hereditary Non-Syndromic 6 Oral Clefts NCT NCT NCT NCT: national clinical trial. 150 Sep No date 1000 No date Assessment of the Enrichment of Rare Coding Genetic Variants in Patients Affected by Neutrophil-Mediated Inflammatory Dermatoses 660 Jan 2020 Genetics of Epilepsy and Related Disorders 500 Dec 2020 Worm Study: Identification of Modifier Genes in a Unique Founder Population With Sudden Cardiac Death 223 Apr 2025 Summary of Evidence The evidence for the use of WES/WGS in individuals with suspected genetic disorders includes multiple studies that describe detection of novel genetic variants and several relatively large cohort studies that report on the yield of WES/WGS. Relevant outcomes include test accuracy, test validity, other test performance measures, changes in reproductive decision making, morbid events, health status measures, and resource utilization. A potential major indication for the use of WES/WGS is for molecular diagnosis of patients with a phenotype that is suspicious for a genetic disorder but when a specific mutation is not suspected or with suspected genetic disorders that have a large degree of genetic heterogeneity. Such patients may be left without a clinical diagnosis of their disorder, despite a lengthy diagnostic workup involving a variety of traditional molecular and other types of conventional diagnostic tests. For some of these patients, WES or WGS, after initial conventional testing has failed to make the diagnosis, may return a likely pathogenic variant. There is developing evidence about the use of WES/WGS in clinical practice, with studies describing yields of a molecular diagnosis in approximately 25% of patients without a diagnosis after a previous workup. No studies were identified that directly compared WES/WGS with alternative testing strategies in terms of the yield of testing for pathogenic variants associated with the phenotype being evaluated and variants of uncertain significance and incidental clinically-actionable findings. The evidence is insufficient to determine the effects of the technology on health outcomes. SUPPLEMENTAL INFORMATION The American College of Medical Genetics (ACMG) states that diagnostic testing with WES (and WGS) should be considered in the clinical diagnostic assessment of a phenotypically affected individual when: a. The phenotype or family history data strongly implicate a genetic etiology, but the phenotype does not correspond with a specific disorder for which a genetic test targeting a specific gene is available on a clinical basis.
13 b. A patient presents with a defined genetic disorder that demonstrates a high degree of genetic heterogeneity, making WES or WGS analysis of multiple genes simultaneously a more practical approach. c. A patient presents with a likely genetic disorder but specific genetic tests available for that phenotype have failed to arrive at a diagnosis. d. A fetus with a likely genetic disorder in which specific genetic tests, including targeted sequencing tests, available for that phenotype have failed to arrive at a diagnosis. The ACMG states that for screening purposes: WGS/WES may be considered in preconception carrier screening, using a strategy to focus on genetic variants known to be associated with significant phenotypes in homozygous or hemizygous progeny. ACMG states that WGS/WES should not be used at this time as an approach to prenatal screening, or as a first-tier approach for newborn screening. In March 2013, an ACMG board finalized approval of their recommends for reporting incidental findings in WGS and WES. 26 A working group determined that reporting some incidental findings would likely have medical benefit for the patients and families of patients undergoing clinical sequencing and recommended that when a report is issued for clinically indicated exome and genome sequencing, a minimum list of conditions, genes and variants should be routinely evaluated and reported to the ordering clinician. U.S. Preventive Services Task Force Recommendations Not applicable. Medicare National Coverage There is no national coverage determination (NCD). In the absence of an NCD, coverage decisions are left to the discretion of local Medicare carriers. References: 1. Dixon-Salazar TJ, Silhavy JL, Udpa N, et al. Exome sequencing can improve diagnosis and alter patient management. Sci Transl Med. Jun ;4(138):138ra178. PMID Ayuso C, Millan JM, Mancheno M, et al. Informed consent for whole-genome sequencing studies in the clinical setting. Proposed recommendations on essential content and process. Eur J Hum Genet. Oct 2013;21(10): PMID McLaughlin HM, Ceyhan-Birsoy O, Christensen KD, et al. A systematic approach to the reporting of medically relevant findings from whole genome sequencing. BMC Med Genet. 2014;15:134. PMID Biesecker LG. Opportunities and challenges for the integration of massively parallel genomic sequencing into clinical practice: lessons from the ClinSeq project. Genet Med. Apr 2012;14(4): PMID Rehm HL, Bale SJ, Bayrak-Toydemir P, et al. ACMG clinical laboratory standards for nextgeneration sequencing. Genet Med. Sep 2013;15(9): PMID de Ligt J, Boone PM, Pfundt R, et al. Detection of clinically relevant copy number variants with whole-exome sequencing. Hum Mutat. Oct 2013;34(10): PMID Dewey FE, Grove ME, Pan C, et al. Clinical interpretation and implications of whole-genome sequencing. JAMA. Mar ;311(10): PMID Bamshad MJ, Ng SB, Bigham AW, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. Nov 2011;12(11): PMID
14 9. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. May 2015;17(5): PMID Greenway SC, McLeod R, Hume S, et al. Exome sequencing identifies a novel variant in ACTC1 associated with familial atrial septal defect. Can J Cardiol. Feb 2014;30(2): PMID Jiang YH, Yuen RK, Jin X, et al. Detection of clinically relevant genetic variants in autism spectrum disorder by whole-genome sequencing. Am J Hum Genet. Aug ;93(2): PMID Kim HJ, Won HH, Park KJ, et al. SNP linkage analysis and whole exome sequencing identify a novel POU4F3 mutation in autosomal dominant late-onset nonsyndromic hearing loss (DFNA15). PLoS One. 2013;8(11):e PMID Weeke P, Mosley JD, Hanna D, et al. Exome sequencing implicates an increased burden of rare potassium channel variants in the risk of drug-induced long QT interval syndrome. J Am Coll Cardiol. Apr ;63(14): PMID Zhou Q, Yang D, Ombrello AK, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. Mar ;370(10): PMID Lee H, Deignan JL, Dorrani N, et al. Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA. Nov ;312(18): PMID Yang Y, Muzny DM, Xia F, et al. Molecular findings among patients referred for clinical wholeexome sequencing. JAMA. Nov ;312(18): PMID Yang Y, Muzny DM, Reid JG, et al. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N Engl J Med. Oct ;369(16): PMID Tammimies K, Marshall CR, Walker S, et al. Molecular Diagnostic Yield of Chromosomal Microarray Analysis and Whole-Exome Sequencing in Children With Autism Spectrum Disorder. JAMA. Sep ;314(9): PMID Taylor JC, Martin HC, Lise S, et al. Factors influencing success of clinical genome sequencing across a broad spectrum of disorders. Nat Genet. Jul 2015;47(7): PMID Golbus JR, Puckelwartz MJ, Dellefave-Castillo L, et al. Targeted analysis of whole genome sequence data to diagnose genetic cardiomyopathy. Circ Cardiovasc Genet. Dec 2014;7(6): PMID Soden SE, Saunders CJ, Willig LK, et al. Effectiveness of exome and genome sequencing guided by acuity of illness for diagnosis of neurodevelopmental disorders. Sci Transl Med. Dec ;6(265):265ra168. PMID Srivastava S, Cohen JS, Vernon H, et al. Clinical whole exome sequencing in child neurology practice. Ann Neurol. Oct 2014;76(4): PMID Iglesias A, Anyane-Yeboa K, Wynn J, et al. The usefulness of whole-exome sequencing in routine clinical practice. Genet Med. Dec 2014;16(12): PMID Green RC, Berg JS, Grody WW, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. Jul 2013;15(7): PMID Billing Coding/Physician Documentation Information Exome (eg, unexplained constitutional or heritable disorder or syndrome); sequence analysis Exome (eg, unexplained constitutional or heritable disorder or syndrome); sequence analysis, each comparator exome (eg, parents, siblings) (List separately in addition to code for primary procedure) Exome (eg, unexplained constitutional or heritable disorder or syndrome); re-evaluation of previously obtained exome sequence (eg, updated knowledge or unrelated condition/syndrome) Genome (eg, unexplained constitutional or heritable disorder or syndrome); sequence analysis
15 81426 Genome (eg, unexplained constitutional or heritable disorder or syndrome); sequence analysis, each comparator genome (eg, parents, siblings) (List separately in addition to code for primary procedure) Genome (eg, unexplained constitutional or heritable disorder or syndrome); re-evaluation of previously obtained genome sequence (eg, updated knowledge or unrelated condition/syndrome) Additional Policy Key Words N/A Policy Implementation/Update Information 12/1/15 New policy; considered investigational. 6/1/16 No policy statement changes. State and Federal mandates and health plan contract language, including specific provisions/exclusions, take precedence over Medical Policy and must be considered first in determining eligibility for coverage. The medical policies contained herein are for informational purposes. The medical policies do not constitute medical advice or medical care. Treating health care providers are independent contractors and are neither employees nor agents Blue KC and are solely responsible for diagnosis, treatment and medical advice. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, photocopying, or otherwise, without permission from Blue KC. Appendix Appendix Table 1. Categories of Genetic Testing Addressed in Category Addressed 1. Testing of an affected individual s germline to benefit the individual 1a. Diagnostic X 1b. Prognostic X 1c. Therapeutic X 2. Testing cancer cells from an affected individual to benefit the individual 2a. Diagnostic 2b. Prognostic 2c. Therapeutic 3. Testing an asymptomatic individual to determine future risk of disease 4. Testing of an affected individual s germline to benefit family members 5. Reproductive testing 5a. Carrier testing: preconception 5b. Carrier testing: prenatal 5c. In utero testing: aneuploidy 5d. In utero testing: mutations 5e. In utero testing: other 5f. Preimplantation testing with in vitro fertilization
Whole Exome and Whole Genome Sequencing for Diagnosis of Genetic Disorders
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Whole Exome and Whole Genome Sequencing for Diagnosis of Genetic
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Whole Exome and Whole Genome Sequencing for Diagnosis of Genetic
Corporate Medical Policy Genetic Testing for Fanconi Anemia
 Corporate Medical Policy Genetic Testing for Fanconi Anemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_fanconi_anemia 03/2015 3/2016 3/2017 3/2016 Description
Corporate Medical Policy Genetic Testing for Fanconi Anemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_fanconi_anemia 03/2015 3/2016 3/2017 3/2016 Description
Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss
 Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_hereditary_hearing_loss 10/2013 8/2015 8/2016
Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_hereditary_hearing_loss 10/2013 8/2015 8/2016
Title: Genetics and Hearing Loss: Clinical and Molecular Characteristics
 Session # : 46 Day/Time: Friday, May 1, 2015, 1:00 4:00 pm Title: Genetics and Hearing Loss: Clinical and Molecular Characteristics Presenter: Kathleen S. Arnos, PhD, Gallaudet University This presentation
Session # : 46 Day/Time: Friday, May 1, 2015, 1:00 4:00 pm Title: Genetics and Hearing Loss: Clinical and Molecular Characteristics Presenter: Kathleen S. Arnos, PhD, Gallaudet University This presentation
Information leaflet. Centrum voor Medische Genetica. Version 1/20150504 Design by Ben Caljon, UZ Brussel. Universitair Ziekenhuis Brussel
 Information on genome-wide genetic testing Array Comparative Genomic Hybridization (array CGH) Single Nucleotide Polymorphism array (SNP array) Massive Parallel Sequencing (MPS) Version 120150504 Design
Information on genome-wide genetic testing Array Comparative Genomic Hybridization (array CGH) Single Nucleotide Polymorphism array (SNP array) Massive Parallel Sequencing (MPS) Version 120150504 Design
Genetic Testing for CHEK2 Mutations for Breast Cancer
 Genetic Testing for CHEK2 Mutations for Breast Cancer Policy Number: 2.04.133 Last Review: 8/2015 Origination: 8/2015 Next Review: 8/2016 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Genetic Testing for CHEK2 Mutations for Breast Cancer Policy Number: 2.04.133 Last Review: 8/2015 Origination: 8/2015 Next Review: 8/2016 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Delivering the power of the world s most successful genomics platform
 Delivering the power of the world s most successful genomics platform NextCODE Health is bringing the full power of the world s largest and most successful genomics platform to everyday clinical care NextCODE
Delivering the power of the world s most successful genomics platform NextCODE Health is bringing the full power of the world s largest and most successful genomics platform to everyday clinical care NextCODE
Preimplantation Genetic Diagnosis (PGD) and Childhood Diagnostic Evaluation
 IG O Preimplantation Genetic Diagnosis (PGD) and Childhood Diagnostic Evaluation KD Carsten Bergmann carsten.bergmann@bioscientia.de carsten.bergmann@uniklinik-freiburg.de Controversies Conference on ADPKD
IG O Preimplantation Genetic Diagnosis (PGD) and Childhood Diagnostic Evaluation KD Carsten Bergmann carsten.bergmann@bioscientia.de carsten.bergmann@uniklinik-freiburg.de Controversies Conference on ADPKD
Leading Genomics. Diagnostic. Discove. Collab. harma. Shanghai Cambridge, MA Reykjavik
 Leading Genomics Diagnostic harma Discove Collab Shanghai Cambridge, MA Reykjavik Global leadership for using the genome to create better medicine WuXi NextCODE provides a uniquely proven and integrated
Leading Genomics Diagnostic harma Discove Collab Shanghai Cambridge, MA Reykjavik Global leadership for using the genome to create better medicine WuXi NextCODE provides a uniquely proven and integrated
G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics
 G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics One of every 500 newborns has bilateral permanent sensorineural hearing loss 40 db which makes it the most common
G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics One of every 500 newborns has bilateral permanent sensorineural hearing loss 40 db which makes it the most common
Genetic testing. The difference diagnostics can make. The British In Vitro Diagnostics Association
 6 Genetic testing The difference diagnostics can make The British In Vitro Diagnostics Association Genetic INTRODUCTION testing The Department of Health published Our Inheritance, Our Future - Realising
6 Genetic testing The difference diagnostics can make The British In Vitro Diagnostics Association Genetic INTRODUCTION testing The Department of Health published Our Inheritance, Our Future - Realising
Diagnostic Scoring System for LQTS
 Medical Coverage Policy Genetic Testing: Congenital Long QT Syndrome Device/Equipment Drug Medical Surgery Test Other Effective Date: 2/15/2011 Policy Last Updated: 2/21/2012 Prospective review is recommended/required.
Medical Coverage Policy Genetic Testing: Congenital Long QT Syndrome Device/Equipment Drug Medical Surgery Test Other Effective Date: 2/15/2011 Policy Last Updated: 2/21/2012 Prospective review is recommended/required.
Overview of Genetic Testing and Screening
 Integrating Genetics into Your Practice Webinar Series Overview of Genetic Testing and Screening Genetic testing is an important tool in the screening and diagnosis of many conditions. New technology is
Integrating Genetics into Your Practice Webinar Series Overview of Genetic Testing and Screening Genetic testing is an important tool in the screening and diagnosis of many conditions. New technology is
Genetic Mutations Cause Many Birth Defects:
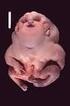 Genetic Mutations Cause Many Birth Defects: What We Learned from the FORGE Canada Project Jan M. Friedman, MD, PhD University it of British Columbia Vancouver, Canada I have no conflicts of interest related
Genetic Mutations Cause Many Birth Defects: What We Learned from the FORGE Canada Project Jan M. Friedman, MD, PhD University it of British Columbia Vancouver, Canada I have no conflicts of interest related
Number 12.04.516 Effective Date August 11, 2015 Revision Date(s) Replaces 2.04.133 (not adopted)
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDI HISTORY Genetic Testing for CHEK2 Mutations for Breast Cancer Number 12.04.516
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDI HISTORY Genetic Testing for CHEK2 Mutations for Breast Cancer Number 12.04.516
Corporate Medical Policy Genetic Testing for Alpha-1 Antitrypsin Deficiency
 Corporate Medical Policy Genetic Testing for Alpha-1 Antitrypsin Deficiency File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_alpha_1_antitrypsin_deficiency 5/2012
Corporate Medical Policy Genetic Testing for Alpha-1 Antitrypsin Deficiency File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_alpha_1_antitrypsin_deficiency 5/2012
Lecture 6: Single nucleotide polymorphisms (SNPs) and Restriction Fragment Length Polymorphisms (RFLPs)
 Lecture 6: Single nucleotide polymorphisms (SNPs) and Restriction Fragment Length Polymorphisms (RFLPs) Single nucleotide polymorphisms or SNPs (pronounced "snips") are DNA sequence variations that occur
Lecture 6: Single nucleotide polymorphisms (SNPs) and Restriction Fragment Length Polymorphisms (RFLPs) Single nucleotide polymorphisms or SNPs (pronounced "snips") are DNA sequence variations that occur
Targeted. sequencing solutions. Accurate, scalable, fast TARGETED
 Targeted TARGETED Sequencing sequencing solutions Accurate, scalable, fast Sequencing for every lab, every budget, every application Ion Torrent semiconductor sequencing Ion Torrent technology has pioneered
Targeted TARGETED Sequencing sequencing solutions Accurate, scalable, fast Sequencing for every lab, every budget, every application Ion Torrent semiconductor sequencing Ion Torrent technology has pioneered
Overview of testing for Lynch syndrome/hnpcc
 Overview of testing for Lynch syndrome/hnpcc This overview provides detailed information about interpreting MSI/IHC testing and genetic testing for Lynch syndrome/hnpcc. It is intended to be a reference
Overview of testing for Lynch syndrome/hnpcc This overview provides detailed information about interpreting MSI/IHC testing and genetic testing for Lynch syndrome/hnpcc. It is intended to be a reference
Mendelian inheritance and the
 Mendelian inheritance and the most common genetic diseases Cornelia Schubert, MD, University of Goettingen, Dept. Human Genetics EUPRIM-Net course Genetics, Immunology and Breeding Mangement German Primate
Mendelian inheritance and the most common genetic diseases Cornelia Schubert, MD, University of Goettingen, Dept. Human Genetics EUPRIM-Net course Genetics, Immunology and Breeding Mangement German Primate
The following chapter is called "Preimplantation Genetic Diagnosis (PGD)".
 Slide 1 Welcome to chapter 9. The following chapter is called "Preimplantation Genetic Diagnosis (PGD)". The author is Dr. Maria Lalioti. Slide 2 The learning objectives of this chapter are: To learn the
Slide 1 Welcome to chapter 9. The following chapter is called "Preimplantation Genetic Diagnosis (PGD)". The author is Dr. Maria Lalioti. Slide 2 The learning objectives of this chapter are: To learn the
A leader in the development and application of information technology to prevent and treat disease.
 A leader in the development and application of information technology to prevent and treat disease. About MOLECULAR HEALTH Molecular Health was founded in 2004 with the vision of changing healthcare. Today
A leader in the development and application of information technology to prevent and treat disease. About MOLECULAR HEALTH Molecular Health was founded in 2004 with the vision of changing healthcare. Today
LEUKODYSTROPHY GENETICS AND REPRODUCTIVE OPTIONS FOR AFFECTED FAMILIES. Leila Jamal, ScM Kennedy Krieger Institute, Baltimore MD
 LEUKODYSTROPHY GENETICS AND REPRODUCTIVE OPTIONS FOR AFFECTED FAMILIES Leila Jamal, ScM Kennedy Krieger Institute, Baltimore MD 2 Outline Genetics 101: Basic Concepts and Myth Busting Inheritance Patterns
LEUKODYSTROPHY GENETICS AND REPRODUCTIVE OPTIONS FOR AFFECTED FAMILIES Leila Jamal, ScM Kennedy Krieger Institute, Baltimore MD 2 Outline Genetics 101: Basic Concepts and Myth Busting Inheritance Patterns
REQUEST FOR IMAGe SYNDROME TESTING
 REQUEST FOR IMAGe SYNDROME TESTING Please provide the following information. We cannot perform your test without ALL of this information. PLEASE PRINT ALL ANSWERS PATIENT INFORMATION* FIRST NAME MI LAST
REQUEST FOR IMAGe SYNDROME TESTING Please provide the following information. We cannot perform your test without ALL of this information. PLEASE PRINT ALL ANSWERS PATIENT INFORMATION* FIRST NAME MI LAST
Objectives Role of Medical Genetics in Hearing Loss Evaluation. 5 y.o. boy with severe SNHL
 Objectives Role of Medical Genetics in Hearing Loss Evaluation Millan Patel, MD UBC Dept. of Medical Genetics October 22, 2010 Case presentation to illustrate importance of defining syndromic hearing loss
Objectives Role of Medical Genetics in Hearing Loss Evaluation Millan Patel, MD UBC Dept. of Medical Genetics October 22, 2010 Case presentation to illustrate importance of defining syndromic hearing loss
Patient Information. Ordering Physician Information. Indication for Testing (REQUIRED)
 EPILEPSY EXOME CLINICAL CHECKLIST REQUIRED Please check all clinical features that apply, and use the additional space provided at the bottom of the form if needed Patient Information Name: Last First
EPILEPSY EXOME CLINICAL CHECKLIST REQUIRED Please check all clinical features that apply, and use the additional space provided at the bottom of the form if needed Patient Information Name: Last First
Genetic diagnostics the gateway to personalized medicine
 Micronova 20.11.2012 Genetic diagnostics the gateway to personalized medicine Kristiina Assoc. professor, Director of Genetic Department HUSLAB, Helsinki University Central Hospital The Human Genome Packed
Micronova 20.11.2012 Genetic diagnostics the gateway to personalized medicine Kristiina Assoc. professor, Director of Genetic Department HUSLAB, Helsinki University Central Hospital The Human Genome Packed
G E N OM I C S S E RV I C ES
 GENOMICS SERVICES THE NEW YORK GENOME CENTER NYGC is an independent non-profit implementing advanced genomic research to improve diagnosis and treatment of serious diseases. capabilities. N E X T- G E
GENOMICS SERVICES THE NEW YORK GENOME CENTER NYGC is an independent non-profit implementing advanced genomic research to improve diagnosis and treatment of serious diseases. capabilities. N E X T- G E
Preimplantation Genetic Diagnosis. Evaluation for single gene disorders
 Preimplantation Genetic Diagnosis Evaluation for single gene disorders What is Preimplantation Genetic Diagnosis? Preimplantation genetic diagnosis or PGD is a technology that allows genetic testing of
Preimplantation Genetic Diagnosis Evaluation for single gene disorders What is Preimplantation Genetic Diagnosis? Preimplantation genetic diagnosis or PGD is a technology that allows genetic testing of
Genetic Testing: Scientific Background for Policymakers
 Genetic Testing: Scientific Background for Policymakers Amanda K. Sarata Specialist in Health Policy December 19, 2011 CRS Report for Congress Prepared for Members and Committees of Congress Congressional
Genetic Testing: Scientific Background for Policymakers Amanda K. Sarata Specialist in Health Policy December 19, 2011 CRS Report for Congress Prepared for Members and Committees of Congress Congressional
GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110
 GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110 COVERAGE: Pre- and post-genetic test counseling may be eligible for coverage in addition to the genetic
GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110 COVERAGE: Pre- and post-genetic test counseling may be eligible for coverage in addition to the genetic
Invasive Prenatal (Fetal) Diagnostic Testing
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Invasive Prenatal (Fetal) Diagnostic Testing Number 12.04.116
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Invasive Prenatal (Fetal) Diagnostic Testing Number 12.04.116
Patient Information. for Childhood
 Patient Information Genetic Testing for Childhood Hearing Loss Introduction This document describes the most common genetic cause of childhood hearing loss and explains the role of genetic testing. Childhood
Patient Information Genetic Testing for Childhood Hearing Loss Introduction This document describes the most common genetic cause of childhood hearing loss and explains the role of genetic testing. Childhood
Human Genome Organization: An Update. Genome Organization: An Update
 Human Genome Organization: An Update Genome Organization: An Update Highlights of Human Genome Project Timetable Proposed in 1990 as 3 billion dollar joint venture between DOE and NIH with 15 year completion
Human Genome Organization: An Update Genome Organization: An Update Highlights of Human Genome Project Timetable Proposed in 1990 as 3 billion dollar joint venture between DOE and NIH with 15 year completion
Sequencing and microarrays for genome analysis: complementary rather than competing?
 Sequencing and microarrays for genome analysis: complementary rather than competing? Simon Hughes, Richard Capper, Sandra Lam and Nicole Sparkes Introduction The human genome is comprised of more than
Sequencing and microarrays for genome analysis: complementary rather than competing? Simon Hughes, Richard Capper, Sandra Lam and Nicole Sparkes Introduction The human genome is comprised of more than
Chapter 20: Analysis of Surveillance Data
 Analysis of Surveillance Data: Chapter 20-1 Chapter 20: Analysis of Surveillance Data Sandra W. Roush, MT, MPH I. Background Ongoing analysis of surveillance data is important for detecting outbreaks and
Analysis of Surveillance Data: Chapter 20-1 Chapter 20: Analysis of Surveillance Data Sandra W. Roush, MT, MPH I. Background Ongoing analysis of surveillance data is important for detecting outbreaks and
GENETIC TESTING AND MARFAN SYNDROME
 GENETIC TESTING AND MARFAN SYNDROME Genetic testing for mutations in fibrillin-1 (FBN1) and other genes has become an important and reliable option to aid in the diagnosis of Marfan syndrome and related
GENETIC TESTING AND MARFAN SYNDROME Genetic testing for mutations in fibrillin-1 (FBN1) and other genes has become an important and reliable option to aid in the diagnosis of Marfan syndrome and related
DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES
 DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES Extracts from a review article by KN North and KJ Jones: Recent advances in diagnosis of the childhood muscular dystrophies Journal of Paediatrics and Child Health
DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES Extracts from a review article by KN North and KJ Jones: Recent advances in diagnosis of the childhood muscular dystrophies Journal of Paediatrics and Child Health
Genetic Testing for Duchenne and Becker Muscular Dystrophy
 Corporate Medical Policy Genetic Testing for Duchenne and Becker Muscular Dystrophy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_duchenne_and_becker_muscular_dystrophy
Corporate Medical Policy Genetic Testing for Duchenne and Becker Muscular Dystrophy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_duchenne_and_becker_muscular_dystrophy
Genetic Counseling and Testing: Cancer Genetics
 KAISER PERMANENTE HAWAII CLINICAL PRACTICE GUIDELINE Genetic Counseling and Testing: Cancer Genetics QUALITY COMMITTEE ADOPTION DATE: October 2015 LAST REVIEW DATE: September 2015 NEXT SCHEDULED REVIEW
KAISER PERMANENTE HAWAII CLINICAL PRACTICE GUIDELINE Genetic Counseling and Testing: Cancer Genetics QUALITY COMMITTEE ADOPTION DATE: October 2015 LAST REVIEW DATE: September 2015 NEXT SCHEDULED REVIEW
Nazneen Aziz, PhD. Director, Molecular Medicine Transformation Program Office
 2013 Laboratory Accreditation Program Audioconferences and Webinars Implementing Next Generation Sequencing (NGS) as a Clinical Tool in the Laboratory Nazneen Aziz, PhD Director, Molecular Medicine Transformation
2013 Laboratory Accreditation Program Audioconferences and Webinars Implementing Next Generation Sequencing (NGS) as a Clinical Tool in the Laboratory Nazneen Aziz, PhD Director, Molecular Medicine Transformation
Fact Sheet for Health Care Providers: Interpreting Results from the Aptima Zika Virus Assay. June 17, 2016
 Dear Health Care Provider: Fact Sheet for Health Care Providers: Interpreting Results from the Aptima Zika Virus Assay June 17, 2016 The U.S. Food and Drug Administration (FDA) has issued an Emergency
Dear Health Care Provider: Fact Sheet for Health Care Providers: Interpreting Results from the Aptima Zika Virus Assay June 17, 2016 The U.S. Food and Drug Administration (FDA) has issued an Emergency
Minimum standards for ICSI use, screening, patient information and follow-up in WA fertility clinics. January 2006
 Minimum standards for ICSI use, screening, patient information and follow-up in WA fertility clinics January 2006 1. BACKGROUND ICSI has been shown to be effective for male factor infertility and it also
Minimum standards for ICSI use, screening, patient information and follow-up in WA fertility clinics January 2006 1. BACKGROUND ICSI has been shown to be effective for male factor infertility and it also
Single-Cell Whole Genome Sequencing on the C1 System: a Performance Evaluation
 PN 100-9879 A1 TECHNICAL NOTE Single-Cell Whole Genome Sequencing on the C1 System: a Performance Evaluation Introduction Cancer is a dynamic evolutionary process of which intratumor genetic and phenotypic
PN 100-9879 A1 TECHNICAL NOTE Single-Cell Whole Genome Sequencing on the C1 System: a Performance Evaluation Introduction Cancer is a dynamic evolutionary process of which intratumor genetic and phenotypic
About The Causes of Hearing Loss
 About 1 in 500 infants is born with or develops hearing loss during early childhood. Hearing loss has many causes: some are genetic (that is, caused by a baby s genes) or non-genetic (such as certain infections
About 1 in 500 infants is born with or develops hearing loss during early childhood. Hearing loss has many causes: some are genetic (that is, caused by a baby s genes) or non-genetic (such as certain infections
Preparing for the Collection and Use of External Family History and Genetic Test Result Data
 Preparing for the Collection and Use of External Family History and Genetic Test Result Data HL7 - More Than You Think HIMSS March 5, 2013 Grant M. Wood Intermountain Healthcare Clinical Genetics Institute
Preparing for the Collection and Use of External Family History and Genetic Test Result Data HL7 - More Than You Think HIMSS March 5, 2013 Grant M. Wood Intermountain Healthcare Clinical Genetics Institute
MCB 4934: Introduction to Genetics and Genomics in Health Care Section 125D Fall 2012 2 Credits
 MCB 4934: Introduction to Genetics and Genomics in Health Care Section 125D Fall 2012 2 Credits Instructor Dr. Jennifer Drew Microbiology & Cell Science jdrew@ulf.edu Skype username: jennifercdrew I am
MCB 4934: Introduction to Genetics and Genomics in Health Care Section 125D Fall 2012 2 Credits Instructor Dr. Jennifer Drew Microbiology & Cell Science jdrew@ulf.edu Skype username: jennifercdrew I am
Roberto Ciccone, Orsetta Zuffardi Università di Pavia
 Roberto Ciccone, Orsetta Zuffardi Università di Pavia XIII Corso di Formazione Malformazioni Congenite dalla Diagnosi Prenatale alla Terapia Postnatale unipv.eu Carrara, 24 ottobre 2014 Legend:Bluebars
Roberto Ciccone, Orsetta Zuffardi Università di Pavia XIII Corso di Formazione Malformazioni Congenite dalla Diagnosi Prenatale alla Terapia Postnatale unipv.eu Carrara, 24 ottobre 2014 Legend:Bluebars
Heritability: Twin Studies. Twin studies are often used to assess genetic effects on variation in a trait
 TWINS AND GENETICS TWINS Heritability: Twin Studies Twin studies are often used to assess genetic effects on variation in a trait Comparing MZ/DZ twins can give evidence for genetic and/or environmental
TWINS AND GENETICS TWINS Heritability: Twin Studies Twin studies are often used to assess genetic effects on variation in a trait Comparing MZ/DZ twins can give evidence for genetic and/or environmental
TITLE: Next Generation DNA Sequencing: A Review of the Cost Effectiveness and Guidelines
 TITLE: Next Generation DNA Sequencing: A Review of the Cost Effectiveness and Guidelines DATE: 06 February 2014 CONTEXT AND POLICY ISSUES The use of genetic methodologies in health-care is not a novel
TITLE: Next Generation DNA Sequencing: A Review of the Cost Effectiveness and Guidelines DATE: 06 February 2014 CONTEXT AND POLICY ISSUES The use of genetic methodologies in health-care is not a novel
Corporate Medical Policy
 Corporate Medical Policy Quantitative Electroencephalography as a Diagnostic Aid for Attention File Name: Origination: Last CAP Review: Next CAP Review: Last Review: quantitative_electroencephalography_as_a_diagnostic_aid_for_adhd
Corporate Medical Policy Quantitative Electroencephalography as a Diagnostic Aid for Attention File Name: Origination: Last CAP Review: Next CAP Review: Last Review: quantitative_electroencephalography_as_a_diagnostic_aid_for_adhd
School of Nursing. Presented by Yvette Conley, PhD
 Presented by Yvette Conley, PhD What we will cover during this webcast: Briefly discuss the approaches introduced in the paper: Genome Sequencing Genome Wide Association Studies Epigenomics Gene Expression
Presented by Yvette Conley, PhD What we will cover during this webcast: Briefly discuss the approaches introduced in the paper: Genome Sequencing Genome Wide Association Studies Epigenomics Gene Expression
Corporate Medical Policy
 Corporate Medical Policy Proteomics-based Testing Related to Ovarian Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteomics_based_testing_related_to_ovarian_cancer 7/2010
Corporate Medical Policy Proteomics-based Testing Related to Ovarian Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteomics_based_testing_related_to_ovarian_cancer 7/2010
ACMG clinical laboratory standards for next-generation sequencing
 American College of Medical Genetics and Genomics ACMG Practice Guidelines ACMG clinical laboratory standards for next-generation sequencing Heidi L. Rehm, PhD 1,2, Sherri J. Bale, PhD 3, Pinar Bayrak-Toydemir,
American College of Medical Genetics and Genomics ACMG Practice Guidelines ACMG clinical laboratory standards for next-generation sequencing Heidi L. Rehm, PhD 1,2, Sherri J. Bale, PhD 3, Pinar Bayrak-Toydemir,
Influence of Sex on Genetics. Chapter Six
 Influence of Sex on Genetics Chapter Six Humans 23 Autosomes Chromosomal abnormalities very severe Often fatal All have at least one X Deletion of X chromosome is fatal Males = heterogametic sex XY Females
Influence of Sex on Genetics Chapter Six Humans 23 Autosomes Chromosomal abnormalities very severe Often fatal All have at least one X Deletion of X chromosome is fatal Males = heterogametic sex XY Females
Assuring the Quality of Next-Generation Sequencing in Clinical Laboratory Practice. Supplementary Guidelines
 Assuring the Quality of Next-Generation Sequencing in Clinical Laboratory Practice Next-generation Sequencing: Standardization of Clinical Testing (Nex-StoCT) Workgroup Principles and Guidelines Supplementary
Assuring the Quality of Next-Generation Sequencing in Clinical Laboratory Practice Next-generation Sequencing: Standardization of Clinical Testing (Nex-StoCT) Workgroup Principles and Guidelines Supplementary
Mendelian and Non-Mendelian Heredity Grade Ten
 Ohio Standards Connection: Life Sciences Benchmark C Explain the genetic mechanisms and molecular basis of inheritance. Indicator 6 Explain that a unit of hereditary information is called a gene, and genes
Ohio Standards Connection: Life Sciences Benchmark C Explain the genetic mechanisms and molecular basis of inheritance. Indicator 6 Explain that a unit of hereditary information is called a gene, and genes
INTRODUCTION TO THE UK CURRICULUM IN CLINICAL GENETICS
 INTRODUCTION TO THE UK CURRICULUM IN CLINICAL GENETICS Clinical Geneticists work in multidisciplinary regional genetic centres in the UK, in close collaboration with laboratory scientists, clinical co-workers
INTRODUCTION TO THE UK CURRICULUM IN CLINICAL GENETICS Clinical Geneticists work in multidisciplinary regional genetic centres in the UK, in close collaboration with laboratory scientists, clinical co-workers
Insurance. Chapter 7. Introduction
 65 Chapter 7 Insurance Introduction 7.1 The subject of genetic screening in relation to insurance is not new. In 1935 R A Fisher addressed the International Congress of Life Assurance Medicine on the topic,
65 Chapter 7 Insurance Introduction 7.1 The subject of genetic screening in relation to insurance is not new. In 1935 R A Fisher addressed the International Congress of Life Assurance Medicine on the topic,
Cigna Medical Coverage Policy
 Cigna Medical Coverage Policy Subject Whole Exome and Whole Genome Sequencing Table of Contents Coverage Policy... 1 Genetic Counseling... 1 Whole Exome Sequencing... 2 Whole Genome Sequencing... 3 Effective
Cigna Medical Coverage Policy Subject Whole Exome and Whole Genome Sequencing Table of Contents Coverage Policy... 1 Genetic Counseling... 1 Whole Exome Sequencing... 2 Whole Genome Sequencing... 3 Effective
The Developing Person Through the Life Span 8e by Kathleen Stassen Berger
 The Developing Person Through the Life Span 8e by Kathleen Stassen Berger Chapter 3 Heredity and Environment PowerPoint Slides developed by Martin Wolfger and Michael James Ivy Tech Community College-Bloomington
The Developing Person Through the Life Span 8e by Kathleen Stassen Berger Chapter 3 Heredity and Environment PowerPoint Slides developed by Martin Wolfger and Michael James Ivy Tech Community College-Bloomington
Just the Facts: A Basic Introduction to the Science Underlying NCBI Resources
 1 of 8 11/7/2004 11:00 AM National Center for Biotechnology Information About NCBI NCBI at a Glance A Science Primer Human Genome Resources Model Organisms Guide Outreach and Education Databases and Tools
1 of 8 11/7/2004 11:00 AM National Center for Biotechnology Information About NCBI NCBI at a Glance A Science Primer Human Genome Resources Model Organisms Guide Outreach and Education Databases and Tools
Genetic Testing for Hereditary Hearing Loss
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Genetic Testing for Hereditary Hearing Loss Number 12.04.87 Effective
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Genetic Testing for Hereditary Hearing Loss Number 12.04.87 Effective
Hereditary Breast Cancer Panels. High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel
 P A T I E N T G U I D E Hereditary Breast Cancer Panels High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel B a y l o r M i r a c a G e n e t i c s L a b o r a t
P A T I E N T G U I D E Hereditary Breast Cancer Panels High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel B a y l o r M i r a c a G e n e t i c s L a b o r a t
Medicare Coverage of Genomic Testing
 Medicare Coverage of Genomic Testing Louis B. Jacques, MD Director, DID/CAG/OCSQ With acknowledgements to Jeff Roche, MD Social Security Act 1862(a)(1)(A) Notwithstanding any other provision of this title,
Medicare Coverage of Genomic Testing Louis B. Jacques, MD Director, DID/CAG/OCSQ With acknowledgements to Jeff Roche, MD Social Security Act 1862(a)(1)(A) Notwithstanding any other provision of this title,
Preimplantation Genetic Diagnosis (PGD) in Western Australia
 Preimplantation Genetic Diagnosis (PGD) in Western Australia Human somatic cells have 46 chromosomes each, made up of the 23 chromosomes provided by the egg and the sperm cell from each parent. Each chromosome
Preimplantation Genetic Diagnosis (PGD) in Western Australia Human somatic cells have 46 chromosomes each, made up of the 23 chromosomes provided by the egg and the sperm cell from each parent. Each chromosome
Evaluation and Assessment and Eligibility Regulations 2011
 303.10 Developmental delay. As used in this part, developmental delay, when used with respect to an individual residing in a State, has the meaning given to that term under 303.300. 303.10 Developmental
303.10 Developmental delay. As used in this part, developmental delay, when used with respect to an individual residing in a State, has the meaning given to that term under 303.300. 303.10 Developmental
Disease gene identification with exome sequencing
 Disease gene identification with exome sequencing Christian Gilissen Dept. of Human Genetics Radboud University Nijmegen Medical Centre c.gilissen@antrg.umcn.nl Contents Infrastructure Exome sequencing
Disease gene identification with exome sequencing Christian Gilissen Dept. of Human Genetics Radboud University Nijmegen Medical Centre c.gilissen@antrg.umcn.nl Contents Infrastructure Exome sequencing
The Human Genome Project
 The Human Genome Project Brief History of the Human Genome Project Physical Chromosome Maps Genetic (or Linkage) Maps DNA Markers Sequencing and Annotating Genomic DNA What Have We learned from the HGP?
The Human Genome Project Brief History of the Human Genome Project Physical Chromosome Maps Genetic (or Linkage) Maps DNA Markers Sequencing and Annotating Genomic DNA What Have We learned from the HGP?
PROVIDER POLICIES & PROCEDURES
 PROVIDER POLICIES & PROCEDURES BRCA GENETIC TESTING The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance Program (CMAP) on the requirements for
PROVIDER POLICIES & PROCEDURES BRCA GENETIC TESTING The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance Program (CMAP) on the requirements for
Number 12.04.59 Effective Date August 1, 2016 Revision Date(s) 07/12/16; 10/13/15; 12/08/14; 12/09/13; 02/14/12 Replaces 2.04.59
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES CODING DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES APPENDIX HISTORY Genetic Testing, Including Chromosomal Microarray (CMA) Analysis
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES CODING DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES APPENDIX HISTORY Genetic Testing, Including Chromosomal Microarray (CMA) Analysis
What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives
 What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives What does the career involve? Explore family histories to identify risks Reducing risks
What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives What does the career involve? Explore family histories to identify risks Reducing risks
Genomic Medicine Education Initiatives of the College of American Pathologists
 Genomic Medicine Education Initiatives of the College of American Pathologists Debra G.B. Leonard, MD, PhD, FCAP Chair, Personalized Healthcare Committee, CAP Professor of Pathology, Weill Cornell Medical
Genomic Medicine Education Initiatives of the College of American Pathologists Debra G.B. Leonard, MD, PhD, FCAP Chair, Personalized Healthcare Committee, CAP Professor of Pathology, Weill Cornell Medical
TWO NEW DNA BASED TESTS AVAILABLE FOR THE NSDTR
 TWO NEW DNA BASED TESTS AVAILABLE FOR THE NSDTR Written by Danika Bannasch DVM PhD; Professor Department of Population Health and Reproduction, School of Veterinary Medicine, University of California-Davis
TWO NEW DNA BASED TESTS AVAILABLE FOR THE NSDTR Written by Danika Bannasch DVM PhD; Professor Department of Population Health and Reproduction, School of Veterinary Medicine, University of California-Davis
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE Q5B
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE QUALITY OF BIOTECHNOLOGICAL PRODUCTS: ANALYSIS
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE QUALITY OF BIOTECHNOLOGICAL PRODUCTS: ANALYSIS
MCB41: Second Midterm Spring 2009
 MCB41: Second Midterm Spring 2009 Before you start, print your name and student identification number (S.I.D) at the top of each page. There are 7 pages including this page. You will have 50 minutes for
MCB41: Second Midterm Spring 2009 Before you start, print your name and student identification number (S.I.D) at the top of each page. There are 7 pages including this page. You will have 50 minutes for
The National Institute of Genomic Medicine (INMEGEN) was
 Genome is...... the complete set of genetic information contained within all of the chromosomes of an organism. It defines the particular phenotype of an individual. What is Genomics? The study of the
Genome is...... the complete set of genetic information contained within all of the chromosomes of an organism. It defines the particular phenotype of an individual. What is Genomics? The study of the
Validation and Replication
 Validation and Replication Overview Definitions of validation and replication Difficulties and limitations Working examples from our group and others Why? False positive results still occur. even after
Validation and Replication Overview Definitions of validation and replication Difficulties and limitations Working examples from our group and others Why? False positive results still occur. even after
Clinical Policy Title: Genomic tests in sensorineural hearing loss
 Clinical Policy Title: Genomic tests in sensorineural hearing loss Clinical Policy Number: 02.01.18 Effective Date: January 1, 2016 Initial Review Date: September 16, 2015 Most Recent Review Date: September
Clinical Policy Title: Genomic tests in sensorineural hearing loss Clinical Policy Number: 02.01.18 Effective Date: January 1, 2016 Initial Review Date: September 16, 2015 Most Recent Review Date: September
Gene Therapy and Genetic Counseling. Chapter 20
 Gene Therapy and Genetic Counseling Chapter 20 What is Gene Therapy? Treating a disease by replacing, manipulating or supplementing a gene The act of changing an individual s DNA sequence to fix a non-functional
Gene Therapy and Genetic Counseling Chapter 20 What is Gene Therapy? Treating a disease by replacing, manipulating or supplementing a gene The act of changing an individual s DNA sequence to fix a non-functional
Guidance For Research Involving Human Embryonic Stem Cells, Germ Cells, And Cells Obtained From Cord Blood
 Guidance For Research Involving Human Embryonic Stem Cells, Germ Cells, And Cells Obtained From Cord Blood Supreme Council of Health Department of Research Guidance Regarding Research Involving Human Embryonic
Guidance For Research Involving Human Embryonic Stem Cells, Germ Cells, And Cells Obtained From Cord Blood Supreme Council of Health Department of Research Guidance Regarding Research Involving Human Embryonic
Innovation Platform: Sudden Cardiac Death
 Innovation Platform: Sudden Cardiac Death Prof. dr. Bart Loeys CRC Antwerp General Assembly VzW Board of Directors Strategic Advisory Board Staff: 1 executive director 1 ICT manager 1 financial administration
Innovation Platform: Sudden Cardiac Death Prof. dr. Bart Loeys CRC Antwerp General Assembly VzW Board of Directors Strategic Advisory Board Staff: 1 executive director 1 ICT manager 1 financial administration
Genetic Counseling: A Profession in the Making. Jessica Hooks, MS Genetic Counselor University of South Carolina
 Genetic Counseling: A Profession in the Making Jessica Hooks, MS Genetic Counselor University of South Carolina Definition the process of helping people understand and adapt to the medical, psychological
Genetic Counseling: A Profession in the Making Jessica Hooks, MS Genetic Counselor University of South Carolina Definition the process of helping people understand and adapt to the medical, psychological
Corporate Medical Policy Molecular Markers in Fine Needle Aspirates of the Thyroid
 Corporate Medical Policy Molecular Markers in Fine Needle Aspirates of the Thyroid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_markers_in_fine_needle_aspirates_of_the_thyroid
Corporate Medical Policy Molecular Markers in Fine Needle Aspirates of the Thyroid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_markers_in_fine_needle_aspirates_of_the_thyroid
Reimbursement for Molecular Diagnostics
 Reimbursement for Molecular Diagnostics Michael Pollock SLA Annual Conference June 11, 2013 San Diego Overview of Changes to Molecular Diagnostic Test Reimbursement Molecular pathology (MoPath) codes provide
Reimbursement for Molecular Diagnostics Michael Pollock SLA Annual Conference June 11, 2013 San Diego Overview of Changes to Molecular Diagnostic Test Reimbursement Molecular pathology (MoPath) codes provide
810.12. Federal Act on Human Genetic Testing (HGTA) Scope, Purpose and Definitions
 English is not an official language of the Swiss Confederation. This translation is provided for information purposes only and has no legal force. Federal Act on Human Genetic Testing (HGTA) 810.12 of
English is not an official language of the Swiss Confederation. This translation is provided for information purposes only and has no legal force. Federal Act on Human Genetic Testing (HGTA) 810.12 of
Array Comparative Genomic Hybridisation (CGH)
 Array Comparative Genomic Hybridisation (CGH) Exceptional healthcare, personally delivered What is array CGH? Array CGH is a new test that is now offered to all patients referred with learning disability
Array Comparative Genomic Hybridisation (CGH) Exceptional healthcare, personally delivered What is array CGH? Array CGH is a new test that is now offered to all patients referred with learning disability
REI Pearls: Pitfalls of Genetic Testing in Miscarriage
 The Skinny: Genetic testing of miscarriage tissue is controversial and some people question if testing is helpful or not. This summary will: 1) outline the arguments for and against genetic testing; 2)
The Skinny: Genetic testing of miscarriage tissue is controversial and some people question if testing is helpful or not. This summary will: 1) outline the arguments for and against genetic testing; 2)
Focusing on results not data comprehensive data analysis for targeted next generation sequencing
 Focusing on results not data comprehensive data analysis for targeted next generation sequencing Daniel Swan, Jolyon Holdstock, Angela Matchan, Richard Stark, John Shovelton, Duarte Mohla and Simon Hughes
Focusing on results not data comprehensive data analysis for targeted next generation sequencing Daniel Swan, Jolyon Holdstock, Angela Matchan, Richard Stark, John Shovelton, Duarte Mohla and Simon Hughes
Chromosomes, Mapping, and the Meiosis Inheritance Connection
 Chromosomes, Mapping, and the Meiosis Inheritance Connection Carl Correns 1900 Chapter 13 First suggests central role for chromosomes Rediscovery of Mendel s work Walter Sutton 1902 Chromosomal theory
Chromosomes, Mapping, and the Meiosis Inheritance Connection Carl Correns 1900 Chapter 13 First suggests central role for chromosomes Rediscovery of Mendel s work Walter Sutton 1902 Chromosomal theory
Applications of comprehensive clinical genomic analysis in solid tumors: obstacles and opportunities
 Applications of comprehensive clinical genomic analysis in solid tumors: obstacles and opportunities Vincent A. Miller, M.D. Foundation Medicine, Inc. AACR Annual Meeting 2012 Current Concepts session
Applications of comprehensive clinical genomic analysis in solid tumors: obstacles and opportunities Vincent A. Miller, M.D. Foundation Medicine, Inc. AACR Annual Meeting 2012 Current Concepts session
European registered Clinical Laboratory Geneticist (ErCLG) Core curriculum
 (February 2015; updated from paper issued by the European Society of Human Genetics Ad hoc committee for the accreditation of clinical laboratory geneticists, published in February 2012) Speciality Profile
(February 2015; updated from paper issued by the European Society of Human Genetics Ad hoc committee for the accreditation of clinical laboratory geneticists, published in February 2012) Speciality Profile
PGD: Genetic Testing of Embryos in the United States
 PGD: Genetic Testing of Embryos in the United States Susannah Baruch, JD Genetics and Public Policy Center Johns Hopkins University February 15, 2009 Preimplantation Genetic Diagnosis Genetic testing of
PGD: Genetic Testing of Embryos in the United States Susannah Baruch, JD Genetics and Public Policy Center Johns Hopkins University February 15, 2009 Preimplantation Genetic Diagnosis Genetic testing of
How To Change Medicine
 P4 Medicine: Personalized, Predictive, Preventive, Participatory A Change of View that Changes Everything Leroy E. Hood Institute for Systems Biology David J. Galas Battelle Memorial Institute Version
P4 Medicine: Personalized, Predictive, Preventive, Participatory A Change of View that Changes Everything Leroy E. Hood Institute for Systems Biology David J. Galas Battelle Memorial Institute Version
UNIT 13 (OPTION) Genetic Abnormalities
 Unit 13 Genetic Abnormailities 1 UNIT 13 (OPTION) Genetic Abnormalities Originally developed by: Hildur Helgedottir RN, MN Revised (2000) by: Marlene Reimer RN, PhD, CCN (C) Associate Professor Faculty
Unit 13 Genetic Abnormailities 1 UNIT 13 (OPTION) Genetic Abnormalities Originally developed by: Hildur Helgedottir RN, MN Revised (2000) by: Marlene Reimer RN, PhD, CCN (C) Associate Professor Faculty
Genetics Module B, Anchor 3
 Genetics Module B, Anchor 3 Key Concepts: - An individual s characteristics are determines by factors that are passed from one parental generation to the next. - During gamete formation, the alleles for
Genetics Module B, Anchor 3 Key Concepts: - An individual s characteristics are determines by factors that are passed from one parental generation to the next. - During gamete formation, the alleles for
European Medicines Agency
 European Medicines Agency July 1996 CPMP/ICH/139/95 ICH Topic Q 5 B Quality of Biotechnological Products: Analysis of the Expression Construct in Cell Lines Used for Production of r-dna Derived Protein
European Medicines Agency July 1996 CPMP/ICH/139/95 ICH Topic Q 5 B Quality of Biotechnological Products: Analysis of the Expression Construct in Cell Lines Used for Production of r-dna Derived Protein
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE E15
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE DEFINITIONS FOR GENOMIC BIOMARKERS, PHARMACOGENOMICS,
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE DEFINITIONS FOR GENOMIC BIOMARKERS, PHARMACOGENOMICS,
TRACKS GENETIC EPIDEMIOLOGY
 Dr. Priya Duggal, Director In the post-genomic era where larger amounts of genetic data are now readily available, it has become increasingly important to design studies and use analytical techniques that
Dr. Priya Duggal, Director In the post-genomic era where larger amounts of genetic data are now readily available, it has become increasingly important to design studies and use analytical techniques that
GENETIC CONSIDERATIONS IN CANCER TREATMENT AND SURVIVORSHIP
 GENETIC CONSIDERATIONS IN CANCER TREATMENT AND SURVIVORSHIP WHO IS AT HIGH RISK OF HEREDITARY CANCER? Hereditary Cancer accounts for a small proportion of all cancer or approximately 5-10% THE DEVELOPMENT
GENETIC CONSIDERATIONS IN CANCER TREATMENT AND SURVIVORSHIP WHO IS AT HIGH RISK OF HEREDITARY CANCER? Hereditary Cancer accounts for a small proportion of all cancer or approximately 5-10% THE DEVELOPMENT
