Omadacycline: development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections
|
|
|
- Sibyl Morris
- 7 years ago
- Views:
Transcription
1 For reprint orders, please contact: Omadacycline: development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections Stephen Villano*,1, Judith Steenbergen 1 & Evan Loh 1 Omadacycline is a first-in-class aminomethylcycline antibiotic that circumvents common tetracycline resistance mechanisms. In vitro omadacycline has potent activity against Grampositive aerobic bacteria including methicillin-resistant Staphylococcus aureus, penicillinresistant and multidrug-resistant Streptococcus pneumoniae, and vancomycin-resistant Enterococcus spp. It is also active against common Gram-negative aerobes, some anaerobes and atypical bacteria including Legionella spp. and Chlamydia spp. Ongoing Phase III clinical trials with omadacycline are investigating once daily doses of 100 mg intravenously followed by once daily doses of 300 mg orally for the treatment of acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia. This paper provides an overview of the microbiology, nonclinical evaluations, clinical pharmacology and initial clinical experience with omadacycline. First draft submitted: 27 May 2016; Accepted for publication: 1 July 2016; Published online: 19 August 2016 Background The tetracycline family of therapeutic agents has been in commercial use since the late 1940s, although there is evidence that they may have provided antibiotic protection (through ingestion of grain contaminated by Streptomyces bacteria) even in ancient times (Cook et al., 1989) [1]. While the tetracyclines have represented a mainstay of broad-spectrum antibiotics for many years, but the increasing incidence of bacterial resistance has relegated older tetracyclines to a limited role for treating common infectious diseases [2,3]. Growing resistance to tetracyclines encouraged research into mechanisms of resistance and discovery of new generations of tetracycline. Tigecycline, a glycylcycline tetracycline, was introduced in the past decade and has been successfully used clinically, in part, because it circumvents the common tetracycline resistance mechanisms. However, tigecycline is only available as an intravenous (iv.) formulation, and is associated with a high incidence of dose-related nausea and vomiting, and safety concerns including increased all-cause mortality [4]. Thus, alternatives are needed for treating common community- and hospital-acquired infections. Omadacycline is a first-in-class aminomethylcycline antibiotic that overcomes the most common mechanisms of tetracycline [5,6]. Omadacycline is active in vitro against Gram-positive aerobes, many Gram-negative aerobes regardless of ESBL phenotype, some anaerobes and atypical bacteria including Legionella spp. and Chlamydia spp. [7]. In addition, omadacycline is active in vitro against many resistant Gram-positive pathogens including methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant and multidrug-resistant Streptococcus pneumoniae and vancomycin-resistant enterococcus [7]. Keywords aminomethylcycline bacterial infection omadacycline tetracyclines 1 Paratek Pharmaceuticals, 1000 First Avenue, Suite 400, King of Prussia, PA 19406, USA *Author for correspondence: Tel.: ; stephen.villano@paratekpharma.com part of 2016 Future Medicine Ltd Future Microbiol. (Epub ahead of print) ISSN
2 Villano, Steenbergen & Loh Omadacycline is undergoing clinical development as once daily oral and intravenous monotherapy for the treatment of acute bacterial skin and skin structure infections (ABSSSI) and community-acquired bacterial pneumonia (CABP). This review provides an overview of the microbiology, nonclinical data, clinical pharmacology and initial clinical experience with omadacycline. Chemistry As with other members of the tetracycline class, omadacycline binds to the tetracycline binding site on the 30S subunit of the bacterial ribosome and inhibits bacterial protein synthesis [6,8]. Omadacycline differs from tetracycline by modifications at both the C7 and presence of an aminomethyl group at the C9 position (Figure 1) [5]. Modifications at the C7 position were added to overcome the tetracycline efflux mechanism of resistance, and modifications at the C9 position were added to overcome ribosome protection mechanism of resistance [7]. Omadacycline is prepared by chemical modification of minocycline, and is a stable, well-characterized crystalline drug substance [5]. Omadacycline is administered as the tosylate salt for the intravenous formulation. Microbiology Omadacycline demonstrates antimicrobial activity in vitro against a range of Gram-positive and Gram-negative aerobes and some anaerobic bacteria that are commonly associated with ABSSSI and CABP [7,9 10]. Against S. aureus, omadacycline demonstrated an MIC 90 for all isolates collected of 0.25 mcg/ml [Paratek Pharmaceuticals, data on file]. Importantly, omadacycline was active in vitro against MRSA, penicillin-resistant Streptococcus pneumoniae, multidrug-resistant Streptococcus pneumoniae and vancomycinresistant enterococcus with an MIC 90 of 0.12 mcg/ml (Tables 1, 2 & 3) [11,12]. Additionally, omadacycline retained activity against tetracyclineresistant isolates (Table 4) [Paratek Pharmaceuticals, data on file]. Against Gram-negative pathogens, the MIC 90 of omadacycline for Haemophilus influenzae was 2 mcg/ml and for Moraxella catarrhalis was 0.25 mcg/ml [13]. In vitro, omadacycline also demonstrated activity with a MIC 90 at 4.0 mcg/ml against many Escherichia coli, Enterobacter aerogenes, Enterobacter cloacae, Serratia marcescens, Salmonella spp., Shigella spp. and Stenotrophomonas maltophilia (Table 5) [14]. In vitro, omadacycline demonstrates no notable activity (MIC 90 values >16 mcg/ml) against Proteus spp., Providencia spp., Pseudomonas spp. and Morganella spp. In vitro activity with omadacycline was demonstrated against atypical bacteria including Legionella pneumophila (Table 6) and Chlamydia spp. (MIC 90 of 0.25 mcg/ml) [15]. In vitro, omadacycline exhibits the following activity against the anaerobes tested: Bacteroides fragilis (MIC 90 = 2 mcg/ml), Clostridium difficile (MIC 90 = 0.12 mcg/ml), Clostridium perfringens (MIC 90 = 4 mcg/ml) and anaerobic Gram-positive cocci (MIC 90 = 0.5 mcg/ml) [Paratek Pharmaceuticals, data on file]. Two mechanisms, efflux pump and ribosomal protection, account for much of the resistance to tetracycline antibiotics [3,6]. While omadacycline is known to inhibit protein synthesis with a greater potency than tetracycline, definitive experiments performed with functional assays and macromolecular synthesis demonstrated that omadacycline inhibited protein synthesis in tetracycline-resistant bacterial strains that expressed both the efflux pump and ribosomal protection mechanisms [3,6,7]. Using a cell-free in vitro protein synthesis model, protein synthesis inhibition activity of omadacycline was investigated in both the presence and absence of the Tet(O), a ribosome protection protein. Omadacycline inhibited protein synthesis in a cell-free system regardless of whether Tet(O) was present or not. Importantly, omadacycline was able to overcome tetracycline resistance mechanisms and also was not affected by resistance mechanisms of other antibiotics [6]. Surveillance data from 2015 demonstrate that omadacycline retains activity (MIC 90 value 0.25 mcg/ml) against tetracycline resistant strains of S. aureus, E. faecalis and S. pneumoniae (Table 4) [Paratek Pharmaceuticals, data on file]. Additionally, the potential for the emergence of resistance to omadacycline was assessed by both single and multistep pressure selection. Resistance to omadacycline was not observed either following a single exposure to drug or after serial passage at sub-mic concentrations for any of the strains [Paratek Pharmaecuticals, data on file]. Nonclinical evaluations Metabolism The potential for enzymatic metabolism of omadacycline was evaluated in vitro using either Future Microbiol. (Epub ahead of print)
3 Omadacycline: a novel aminomethylcycline antibiotic for bacterial infections Review pooled human liver microsome preparations, S9, liver cytosol or recombinant flavin monooxygenases (FMO1, FMO3, FMO5) [16]. CYP450 isozymes evaluated included CYP 1A1, 1A2, 1B1, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 2J2 and 3A4/5. Omadacycline did not induce CYP isozymes and no or minimal (<40% of maximal positive control response) induction of their mrnas was observed. Omadacycline exhibited no significant inhibition of CYP isozyme activity and demonstrated no significant binding to human microsomes. These results indicate a low potential for drug drug interactions via enzymatic metabolism. Transporter effects Transport of [14C]omadacycline was determined in human embryonic kidney 293 cells stably expressing human organic anion transporters 1 or 3 (hoat1 or hoat3), human organic cation transporter 2 (hoct2) and human organic anion transport polypeptide transporters OATP1B1 and OATP1B3 as well as P-gp, MRP2 and Breast Cancer Resistance Protein (Hanna et al., 2012a) [17]. No difference was observed for accumulation of [14C]omadacycline into cells expressing hoat1, hoat3, hoct2, OATP1B1 or OATP1B3. Omadacycline appeared to be a substrate for P-gp but not Breast Cancer Resistance Protein or MRP-2. Omadacycline did not inhibit hoat3 function but inhibited hoat1 by approximately 32.1% at 25 μm. Transport of probes for OATP1B1 and OATP1B3 was reduced by 10.1% with omadacycline 100 μm. Omadacycline did not inhibit P-gp, BRCP or MRP-2 and did not induce H N N OH Figure 1. Chemical structure of omadacycline. Adapted with permission from [4]. O H H N OH OH OH O O NH 2 P-gp or MRP-2 mrna. Overall, the potential for omadacycline drug drug interactions via tr ansporter effects appears to be minimal. Nonclinical cardiovascular effects The cardiovascular risk potential for omadacycline was evaluated through a series of in vitro and in vivo studies, including mammalian pharmacologic receptor binding; human etherà-go-go-related Gene (herg) channel binding; effects on rabbit ex vivo sinoatrial node activity; and in vivo effects on cardiovascular function in the cynomolgus monkey [18]. No significant binding to the herg channel, beta-adrenergic receptor or any other receptors was observed that could result in a direct stimulatory effect on heart rates. Omadacycline binds in vitro to the muscarinic-2 (M 2 ) receptor subtype but not the M 1, M 3 or M 4 receptor subtypes and exhibited a concentration-dependent antagonism of the effect carbamylcholine (a muscarinic receptor agonist), which resulted in an increase in heart rate in the ex vivo sinoatrial node model that reached a peak at 4.5 h after the dose. Omadacycline exhibited no effect on herg channel activity Table 1. In vitro activity of omadacycline and comparators against methicillin-resistant Staphylococcus aureus strains isolated in 2014 (n = 402). Organism group (number tested) antimicrobial agent MIC 50 MIC 90 MIC range Omadacycline Tigecycline Doxycycline >8 Tetracycline >16 Clindamycin 0.25 > >2 Daptomycin Erythromycin >16 > >16 Gentamicin 1 >8 1 >8 Levofloxacin >4 > >4 Linezolid Trimethoprim-sulfamethoxazole >4 Vancomycin Adapted with permission from Flamm et al. (2015) [10].
4 Villano, Steenbergen & Loh Table 2. Susceptibility of bacterial strains to omadacycline. Organism Number of isolates MIC 50 MIC 90 Gram-positive pathogens Staphylococcus aureus (MSSA) Enterococcus faecalis (VSE) Enterococcus faecalis (VRE) Enterococcus faecium (VSE) Enterococcus faecium (VRE) Streptococcus pyogenes Streptococcus agalactiae Staphylococcus saprophyticus (MR) Gram-negative pathogens Haemophilus influenza Moraxella catarrhalis Citrobacter freundii Enterobacter cloacae Salmonella spp Serratia marcescens Shigella spp Acinetobacter baumannii Burkholderia cepacia Stentrophomonas maltophilia Anaerobic pathogens Bacteroides fragilis Bacteroides thetaiotaomicron Clostridium difficile Clostridium perfringens Anaerobic Gram-positive cocci [Paratek Pharmaceuticals, data on file] at a concentration of 100 ug/ml. In addition, omadacycline at doses up to 40 mg/kg had no effect on the QTc interval in conscious monkeys. Overall these nonclinical findings showed that omadacycline had a vagolytic effect on heart rate but had a low potential for cardiac arrhythmia or clinically significant cardiovascular toxicity. Clinical pharmacokinetics & pharmacodynamics The human pharmacokinetic profile of omadacycline was characterized in healthy subjects. Pharmacokinetic parameters are shown from a bioavailability study that compared single intravenous and oral administration using the formulations and doses being evaluated in ongoing Phase III studies (Table 7). Absorption, distribution, metabolism & excretion In a mass balance study, six healthy male subjects received a single oral 300 mg dose of [14C] omadacycline (mean radioactivity 36.6 μci) under fasting conditions [20]. Mean recovery of the radioactive dose was 95.5% after 7 days. In plasma, omadacycline and its C-4 epimer (which is formed spontaneously upon standing) accounted for 100% of the AUC. No enzymatically formed metabolites were detected. Based on radioactivity measurements, the main routes of elimination were fecal (81.1%) and urinary (14.4%). Given on an oral bioavailability of 35% for the tablet formulation, approximately 40% of the absorbed dose should be eliminated in the urine [20]. In a separate experiment, in vitro determination of protein binding in human plasma found no dose dependency over concentrations ranging from 0.1 to 10 mcg/ ml, and the mean bound protein fraction was 21% [21]. Intravenous dosing After single intravenous doses of omadacycline from 25 to 600 mg, mean AUC 0-inf was linear and ranged from 1.3 to 36.0 mcg*h/ml [19]. For single doses from 25 to 200 mg (0.5 mg/ml Future Microbiol. (Epub ahead of print)
5 Omadacycline: a novel aminomethylcycline antibiotic for bacterial infections Review infused over 30 min), mean C max was mcg/ml; for single doses ranging from 300 to 600 mg (1.0 mg/ml infused over 60 min), mean C max was mcg/ml. Omadacycline demonstrated accumulation following multiple-dose administration of 200 mg iv. once daily for 7 days. Between Days 1 and 7 mean C max increased from 2.8 to 3.4 mcg/ml, and mean AUC 0 24 increased from 11.2 to 17.4 mcg*h/ml. Therefore, based on AUC omadacycline accumulated by a pproximately 50% from Day 1 to steady-state. Oral dosing The earliest clinical studies of oral omadacycline were conducted with simple capsule formulations. Subsequently, various oral formulations have been evaluated in an effort to improve bioavailability and tolerability. The bioavailability of different oral formulations of omadacycline relative to an intravenous dose was investigated in an open-label, randomized, crossover study in healthy subjects [22]. Subjects received omadacycline 100 mg iv., two 300-mg tablet formulations Table 3. In vitro activity of omadacycline and comparators against Streptococcus pneumoniae strains isolated in Organism group (number tested) antimicrobial agent MIC 50 MIC 90 MIC range Streptococcus pneumoniae (1834) Omadacycline Tigecycline Doxycycline >8 Tetracycline 0.25 > >16 Amoxicillin-clavulanate >8 Ceftriaxone Clindamycin 0.25 > >2 Erythromycin 2 > >16 Levofloxacin >4 Penicillin Trimethoprim-sulfamethoxazole 0.5 >4 0.5 >4 MDR (434) Omadacycline Tigecycline Doxycycline 8 > >8 Tetracycline >16 > >16 Amoxicillin-clavulanate >8 Ceftriaxone Clindamycin >2 > >2 Erythromycin >16 > >16 Levofloxacin >4 Penicillin Trimethoprim-sulfamethoxazole 4 >4 0.5 >4 Ceftriaxone-NS (MIC 2 μg/ml) (129) Omadacycline Tigecycline Doxycycline >8 Tetracycline >16 > >16 Amoxicillin-clavulanate >8 Ceftriaxone Clindamycin >2 > >2 Erythromycin >16 > >16 Levofloxacin Penicillin Trimethoprim-sulfamethoxazole >4 >4 0.5 >4 MDR: Multidrug resistant; MIC: Minimum inhibitory concentration; NS: Not specified. Adapted with permission from Flamm et al. (2015) [11].
6 Villano, Steenbergen & Loh Table 4. In vitro activity of omadacycline against tetracycline-resistant strains isolated in Organism n MIC 50 MIC 90 MIC range Staphylococcus aureus Enterococcus faecalis Streptococcus pneumoniae [Paratek Pharmaceuticals, data on file] with different dissolution profiles and a 300 mg oral solution. Equivalent total exposure relative to the 100 mg iv. dose was observed with both 300 mg tablet formulations with geometric mean ratios (90% CI) for AUC 0-inf of 1.00 (0.93,1.07) and 0.96 (0.90,1.03), respectively. The coefficients of variation for AUC 0-inf for all studied formulations ranged from 16 to 24%. The absolute bioavailability of the tablet formulation selected for use in Phase III studies was approximately 35%. Thus, a 300 mg oral dose of the Phase III tablet formulation produced omadacycline exposure equivalent to that of a 100 mg iv. dose. With respect to pharmacodynamic assessments, animal models had identified AUC/MIC as the index that is most important for the efficacy of omadacycline [23]. In humans, the serum concentrations achieved following a 100 mg iv. or 300 mg oral dose are associated with AUC values (Table 7) that are expected to provide clinical activity against the bacteria commonly a ssociated with ABSSSI and CABP. Food effect A Phase I, random-sequence, open-label, 4-period crossover study evaluated the effects of food on the pharmacokinetics of omadacycline in healthy subjects (Tzanis et al., 2016) [24]. In each period subjects received a single 300 mg oral dose of omadacycline but the meal time varied relative to dosing: A) 6-h fast before dosing, B) standard, high fat, non-dairy meal 4 h before dosing, C) standard, high fat, non-dairy meal 2 h before dosing, and D) standard, high fat meal containing dairy 2 h before dosing. Compared with a fast of at least 6 h, omadacycline exposure (AUC and C max ) was reduced by 15 17% for the meal 4 h before dosing, 40 42% for the meal 2 h before dosing, and 59 63% for the meal with dairy 2 h before dosing. Thus, the food effect was more pronounced when a meal was consumed closer to oral dosing, with an even greater effect when dairy was included in the meal. The latter findings are consistent with the known tetracycline characteristic of binding to calcium as well as other multivalent cations. Accordingly, in general it is recommended that oral omadacycline should be administered in a fasted state, with avoidance of concomitant oral products containing calcium or other multivalent cations (e.g., dairy products, antacids, or multivitamins). Effect of gender & age Two Phase I studies were undertaken to evaluate the effect of age and gender on the PK of omadacycline after oral and intravenous administration in healthy volunteers [25]. Both were double-blind and placebo-controlled studies of single doses of omadacycline. Study 1 included four groups: young males; young females; elderly males; and elderly females all of whom received omadacycline 200 mg oral or placebo. Study 2 included healthy young male and female subjects who received a single 200 mg oral or 100 mg iv. dose of omadacycline or placebo. In Study 1, no effect of age on omadacycline absorption and PK profile was observed, but exposure (C max and AUC inf ) was at least 30% higher among females versus males in both age groups. In contrast, in study 2 omadacycline exposure based on AUCi nf was similar for both genders after the oral dose, but was approximately 30% higher among females versus males after the intravenous dose. Therefore, female subjects tended to have higher omadacycline exposure than male subjects, though this was not observed consistently in the two studies. In a population PK analysis of subjects across 10 different Phase I studies of omadacycline, the proportion of female subjects was low (19%), but the model showed that gender did not affect omadacycline clearance (the only subject characteristic that did so was renal function) [26]. Overall, no dosage adjustment for omadacycline is necessary on the basis of patient age or gender. ECG QT evaluation The effect of single therapeutic and supratherapeutic intravenous doses of omadacycline on ventricular repolarization and the relationship between plasma concentrations of omadacycline and QTc intervals was evaluated [27]. In this double-dummy, randomized, crossover study, healthy subjects were randomized to omadacycline Future Microbiol. (Epub ahead of print)
7 Omadacycline: a novel aminomethylcycline antibiotic for bacterial infections Review 100 mg iv., omadacycline 300 mg iv., moxifloxacin 400 mg or placebo. Mean AUC 0 24 and C max were dose proportional for omadacycline 100 mg and 300 mg. ECG results showed that omadacycline did not increase QTc; the largest one-sided upper 95% confidence bound (95% CB) on the difference between and omadacycline and placebo (ddqtcf) was 1.53 ms for omadacycline 100 mg and 0.83 ms for omadacycline 300 mg. Further, no relationship was observed between omadacycline plasma concentrations and ddqtcf. Assay sensitivity was confirmed with a >10 ms increase in ddqtcf with moxifloxacin. Within 1 h after dosing, mean peak increases in heart rate were observed for omadacycline (17 bpm for 100 mg iv. and 24 bpm for 300 mg iv.) versus 3 bpm Table 5. In vitro activity of omadacycline and comparators against Gram-negative pathogens isolated in Organism group (number tested) antimicrobial agent MIC 50 MIC 90 MIC range Enterobacteriaceae (301) Omadacycline Tigecycline Doxycycline Tetracycline Amoxicillin-clavulanate Aztreonam Ceftazidime >32 Ceftriaxone Gentamicin Imipenem Levofloxacin Trimethoprim-sulfamethoxazole Escherichia coli (138) Omadacycline Tigecycline Doxycycline Tetracycline Amoxicillin-clavulanate Aztreonam Ceftazidime >32 Ceftriaxone Gentamicin Imipenem Levofloxacin Trimethoprim-sulfamethoxazole Klebsiella spp. (60) Omadacycline Tigecycline Doxycycline Tetracycline Amoxicillin-clavulanate Aztreonam Ceftazidime 0.12 > >32 Ceftriaxone Gentamicin Imipenem Levofloxacin Trimethoprim-sulfamethoxazole Organisms include: K. oxytoca (8), K. pneumoniae (52). Adapted with permission from Flamm et al. (2015) [12].
8 Villano, Steenbergen & Loh Table 6. Susceptibility of Legionella pneumophila for all serogroups and for serogroup 1 collected from 1995 to Organism group (number tested) antimicrobial agent MIC 50 MIC 90 MIC range Legionella pneumophila, all serogroups (100 strains) Omadacycline Doxycycline Telithromycin Azithromycin Erythromycin Levofloxacin Moxifloxacin Legionella pneumophila, serogroup 1 (45 strains) Omadacycline Doxycycline Telithromycin Azithromycin Erythromycin Levofloxacin Moxifloxacin Adapted with permission from Dubois et al. (2015) [14]. for placebo and 5 bpm for moxifloxacin. These changes were asymptomatic, not associated with changes in blood pressure, and were comparable across all groups by h after dosing. Overall, this study is consistent with the results of preclinical studies demonstrating a low potential for adverse cardiac effects with omadacycline. Hepatic impairment The PK of omadacycline was evaluated in subjects with varying degrees of hepatic impairment (mild, moderate and severe as determined by Child Pugh classes A, B and C, respectively) and matched healthy subjects [28]. Both intravenous and oral doses of omadacycline were evaluated in these subjects. Results showed no effect of any degree of hepatic impairment on the PK of oral or intravenous omadacycline; geometric mean ratios for C max and AUC ranged from 0.89 to Further, pooled analysis of dosenormalized PK parameters demonstrated no clear relationship between exposure parameters and the severity of hepatic impairment. Thus, no dose adjustment for omadacycline is warranted in patients with hepatic impairment. Clinical efficacy for treatment of skin infections Two randomized, double-blind, multicenter studies (a Phase II study started in 2007 and a truncated Phase III study started in 2009) have been completed with omadacycline in patients with skin infections [29,30]. At the time that these studies were conducted, these infections were classified as complicated skin and skin structure infections (csssi). In the Phase II study, adult patients with csssi received omadacycline 100 mg iv. once daily followed by the option to switch to 200 mg oral once daily, or linezolid 600 mg iv. twice daily with the option to switch to 600 mg oral twice daily. Aztreonam 2 g iv. twice daily could be added to linezolid if an infection due to a Gram-negative pathogen was suspected. Treatment was administered for up to 14 days. A total of 219 patients were treated (111 omadacycline, 108 linezolid) for an average of 10 days in both treatment groups [29]. The primary efficacy assessment was performed at the test of cure (TOC) visit, which was to occur days after the last dose of study drug. Clinical success at that timepoint was defined (in abbreviated terms) as resolution of infection such that no additional antibiotics were needed for the skin infection at that time or used at any time between the end of study drug treatment and the TOC evaluation, and no antibiotics were given for another indication up to that time in the study. Clinical response in the intent-to-treat population was 88.3% with omadacycline and 75.9% with linezolid, and both drugs also were effective in patients known to be infected with MRSA. In the truncated Phase III study, enrollment was stopped early because of a decision by the US FDA to change the primary end point in studies of Future Microbiol. (Epub ahead of print)
9 Omadacycline: a novel aminomethylcycline antibiotic for bacterial infections Review the treatment of bacterial skin infection. However, patients who had been enrolled up to that point were followed as originally planned [30]. In the study, adult patients with csssi received omadacycline 100 mg iv. once daily followed by the option to switch to 300 mg oral once daily, or linezolid using same dosing regimen as the Phase II study. Moxifloxacin (IV or oral) could be added to linezolid if an infection due to a Gram-negative pathogen was suspected. Treatment was administered for up to 14 days. A total of 140 patients with csssi were treated (68 omadacycline, 72 linezolid) for an average of 10 days in both treatment groups [30]. Clinical success was defined similarly to that in the Phase II study. Clinical response at the TOC visit in the intent-to-treat population was comparable for omadacycline and linezolid (85 vs 89%), and again both drugs were effective in the patients known to be infected with MRSA. Clinical safety & tolerability In Phase I studies, single doses of omadacycline have been administered across a wide dose range ( mg iv., and mg oral). Multiple doses of up 200 mg iv. once daily (7 days) and 300 mg oral once daily (10 days) also have been investigated. Both the intravenous and oral formulations have been generally well tolerated in these studies. In the intravenous administration studies, modest and reversible alanine aminotransferase increases were seen most notably with intravenous doses of 300 mg or greater. Following oral administration of omadacycline, mild nausea was observed most commonly at oral doses of 400 mg or greater. However, the different oral formulations evaluated in early studies (e.g., capsule vs tablet) may have influenced the gastrointestinal profile. In both oral and intravenous Phase I studies, dose-dependent, transient increases in heart rate were observed for several hours following administration of omadacycline. The increases in heart rate were rarely reported as adverse events (palpitations) and were not associated with any ECG changes or other cardiac findings. Receptor binding studies suggest that omadacycline binds to the M2 subtype of the muscarinic receptor of the vagus nerve and this results in a short-lived nonadrenergic, vagolytic effect on heart rate, which is likely to be most notable in healthy volunteer subjects with relatively high vagal tone and lower resting heart rates (see the Nonclinical cardiovascular effects section). Importantly, omadacycline did not increase ECG QTc intervals (see the Electrocardiogram QT evaluation section). In the Phase II and truncated Phase III studies in csssi, the incidence and type of adverse event (AE) was comparable between omadacycline and linezolid (Table 8) [29,30]. In the Phase II study, gastrointestinal AEs were most common overall (19% omadacycline, 17% linezolid). Premature discontinuation of treatment due to an AE was very infrequent in both groups (1% omadacycline, 2% linezolid). There was no pattern of adverse changes in laboratory safety parameters among patients treated with omadacycline; linezolid patients showed a modest decrease in platelet count, which is a known potential effect of that drug. Increased serum transaminases were reported as AEs in 3% of omadacycline patients and 7% of linezolid patients. Measurement of Table 7. Summary of pharmacokinetic parameters for omadacycline after single intravenous and oral doses. Parameters 100 mg iv. (n = 21) 300 mg oral tablet (n = 21) AUC last (h*mcg/ml) %CV AUC inf (h**mcg/ml) %CV C max (mcg/ml) %CV T max (h) %CV T 1/2 (h) %CV CL (for iv.) or CL/F (for oral), (L/h) %CV 8.8 ± ± ± ± ± ± ± ± ± ± Values are mean ± standard deviation, except for T max, which is median. AUC: Area under the concentration-time curve; CL: Clearance; CV: Coefficient of variation; CL/F: Total clearance after oral administration; iv.: Intravenous. Data taken from Sun et al. (2011) [19].
10 Villano, Steenbergen & Loh Table 8. Incidence (%) of adverse events occurring in >3% of patients in either treatment group from Phase II and III studies in complicated skin and skin structure infections. Adverse event Patients n (%) Omadacycline (n = 179) Linezolid (n = 180) Nausea 31 (17.3) 27 (15.0) Headache 23 (12.9) 14 (7.8) Constipation 11 (6.2) 4 (2.2) Dizziness 11 (6.2) 11 (6.1) Vomiting 11 (6.2) 15 (8.3) CPK increased 10 (5.6) 3 (1.7) Fatigue 8 (4.5) 5 (2.8) Diarrhea 6 (3.4) 19 (10.6) Rash 6 (3.4) 6 (3.3) ALT increased 5 (2.8) 11 (6.1) AST increased 4 (2.2) 7 (3.9) Insomnia 4 (2.2) 8 (4.4) Decreased appetite 2 (1.1) 6 (3.3) Dysgeusia 1 (0.6) 6 (3.3) ALT: Alanine aminotransferase; AST: aspartate aminotransferase; CPK: creatine phosphokinase. Adapted with permission from [27,28]. vital signs was performed less frequently than in the Phase I studies, but changes from baseline in heart rate and blood pressure at the end of the course of treatment were clinically insignificant and similar between the treatment groups. Three omadacycline patients (3%) had AEs of tachycardia and one other patient reported palpitations; all of these AEs were mild in intensity, were assessed as unrelated to study drug, and none resulted in discontinuation. In the truncated Phase III study, gastrointestinal AEs again were most common overall (44% omadacycline, 40% linezolid) [28]. Premature discontinuation of treatment due to an AE was infrequent (3% omadacycline, 0 linezolid). Among laboratory-related events, creatine phosphokinase elevation (with no clinical manifestations) was reported in 9% of omadacycline patients compared with 3% for linezolid. Increased alanine aminotransferase was reported as an AE in one omadacycline patient (2%) and four linezolid patients (6%). Tachycardia was reported as an AE in two omadacycline patients (3%) and four linezolid patients (6%). Overall, the target therapeutic doses of omadacycline were very well tolerated in both oral and intravenous formulations. There were no serious AEs that were related to study drug in any of the completed clinical studies. Across both of the studies in csssi patients, nausea was the most common AE; all such events were of mild or moderate intensity and did not lead to treatment discontinuation in any of the completed studies. In contrast, dose-limiting nausea and vomiting occurs with intravenous tigecycline and with both intravenous and oral administration of eravacycline [31 36]. Because it is well tolerated, especially with regard to GI effects of nausea and vomiting that are common with many antibiotics, omadacycline may be particularly well suited for treatment of community-acquired bacterial infections, whether they are managed in hospital or as outpatients. Discussion Omadacycline is being evaluated in two Phase III randomized, double-blind studies in ABSSSI and CABP. The primary objective of these studies is to demonstrate the noninferiority of omadacycline to active comparators. The ABSSSI study is expected enroll approximately 650 patients with skin infections known or suspected to be due to Gram-positive pathogens. This study will evaluate the following two regimens, each of which is to be administered for 7 14 days: Omadacycline 100 mg iv. every 12 h for two doses and then 100 mg iv. every 24 h through at least Day 3, then an option to switch to 300 mg PO every 24 h. Linezolid 600 mg iv. every 12 h through at least Day 3, then an option to switch to 600 mg PO every 12 h. Future Microbiol. (Epub ahead of print)
11 Omadacycline: a novel aminomethylcycline antibiotic for bacterial infections Review Executive summary Mechanism of action Omadacycline exerts its primary effect by binding to the 30S subunit of the bacterial ribosome and inhibiting protein synthesis. Omadacycline is active against bacterial strains expressing efflux and ribosomal protection, which are the two main forms of tetracycline resistance. Microbiology Omadacycline demonstrates antimicrobial activity in vitro against a wide range of Gram-positive and Gram-negative pathogens. Omadacycline is active against methicillin-resistant Staphylococcus aureus, penicillin-resistant and multidrug-resistant Streptococcus pneumoniae and vancomycin-resistant enterococcus. Omadacycline exhibits in vitro activity against anerobes including Bacteroides fragilis, Clostridium difficile, Clostridium perfringens and anaerobic Gram-positive cocci. Omadacycline is active against atypical bacteria including Legionella pneumophila and Chlamydia spp. Pharmacokinetics Following intravenous (iv.) administration, omadacycline exhibits a linear pharmacokinetic profile over the dose range of mg. The oral tablet formulation is 35% bioavailable; a 300 mg oral dose is bioequivalent to a 100 mg iv. dose. Omadacycline has low plasma protein binding (21%) and no active metabolites have been identified; in vitro studies indicate a low potential for drug drug interactions. Omadacycline is eliminated predominantly by fecal elimination of parent drug; approximately 40% of an absorbed dose is excreted in the urine. Clinical efficacy In both a Phase II and a truncated Phase III clinical study in patients with complicated skin and skin structure infections, the efficacy of omadacycline was noninferior to linezolid. Safety & tolerability In the completed studies in patients with complicated skin and skin structure infections, the target therapeutic doses of omadacycline were well tolerated in both oral and intravenous formulations. In these studies the adverse event (AE) profile of omadacycline was comparable to linezolid; the most common AE was nausea, which occurred at similar rates for both omadacycline and linezolid and did not lead to any treatment discontinuations. Transient increases in heart rate (due to a vagolytic effect) were observed most notably in healthy volunteers with lower resting heart rates; there were no associated changes in blood pressure or other cardiac findings. Omadacycline does not prolong QTc intervals. Reversible increases in liver enzymes have been observed at relatively high doses. Dosage & administration For the indications of treatment of acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia, omadacycline is being evaluated as once daily doses of 100 mg iv. followed by once daily doses of 300 mg orally. Oral omadacycline should be administered in a fasted state. Among adults, no dosage adjustment is required for age, gender, or hepatic impairment.
12 Villano, Steenbergen & Loh The CABP study is expected to enroll approximately 750 patients with known or suspected bacterial pneumonia classified as Patient Outcomes Research Team (PORT) Risk Class II IV. This study will evaluate the following two regimens, each of which is to be administered for 7 14 days: Omadacycline 100 mg iv. every 12 h for two doses and then 100 mg iv. every 24 h through at least Day 3, then an option to switch to 300 mg PO every 24 h Moxifloxacin 400 mg iv. every 24 h through at least Day 3, then an option to switch to 400 mg PO every 24 h In addition to the Phase III studies described above, additional clinical pharmacology studies of omadacycline are ongoing to quantify lung penetration and urinary excretion, to evaluate the PK of omadacycline in subjects with renal impairment, and to assess the safety profile of multiple doses higher than those being used in the ongoing Phase III studies. These evaluations will inform decisions about potential development of omadacycline for indications beyond ABSSSI and CABP. Conclusion & future perspective Omadacycline may represent a novel antibiotic with a broad spectrum of activity against community-associated bacterial pathogens, once daily oral and intravenous dosing, favorably pharmacokinetics, low plasma protein binding, low potential for drug drug interactions, and early evidence of efficacy and tolerability. Ongoing and future clinical studies with both oral and intravenous formulations will help define the place of omadacycline in the armamentarium for treating common, serious bacterial infectious diseases originating in the community. Acknowledgements The authors acknowledge the editorial assistance of RS Perry in the preparation of this manuscript, which was supported by Paratek Pharmaceuticals, King of Prussia, PA. Financial & competing interests disclosure All authors are full-time employees of Paratek Pharmaceuticals (King of Prussia, PA, USA). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript. Open access This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit References Papers of special note have been highlighted as: of interest 1 Cook M, Molto E, Anderson C. Fluorochrome labeling in Roman period skeletons from Dakhleh Oasis, Egypt. Am. J. Phys. Anthropol. 80(2), (1989). 2 Zakeri B, Wright GD. Chemical biology of tetracycline antibiotics. Biochem. Cell Biol. 86(2), (2008). 3 Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65(2), (2001). 4 Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect. Dis. 11(11), (2011). 5 Honeyman L, Ismail M, Nelson M et al. Structure activity relationship of the aminomethylcyclines and the discovery of omadacycline. Antimicrob. Agents Chemother. 59(11), (2015). Describes the synthesis of a series of novel tetracycline derivatives from minocycline, the aminomethylcyclines, with the goal of creating new antibiotics that would be unaffected by the known tetracycline resistance mechanisms. Omadacycline was selected for clinical development based on this work. 6 Draper MP, Weir S, Macone A et al. Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob. Agents Chemother. 58(3), (2014). Describes the mechanism of action of omadacycline and differences in mechanisms of resistance from older tetracyclines. 7 Macone AB, Caruso BK, Leahy RG et al. In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline. Antimicrob. Agents Chemother. 58(2), (2014). Provides a comprehensive review of early in vitro and in vivo studies undertaken to characterize the antibacterial profile of omadacycline. 8 Weir S, Macone A, Donatelli J et al. The mechanism of action of BAY /PTK Presented at: 43rd Interscience Congress on Antimicrobial Agents and Chemotherapy. Chicago, IL, USA, September Bhatia B, Bowser T, Chen J et al. PTK 0796 and other novel tetracycline derivatives exhibiting potent in vitro and in vivo activities against antibiotic resistant Gram-positive bacteria. Presented at: 43rd Interscience Congress on Antimicrobial Agents and Chemotherapy. Chicago, IL, USA, September Biedenbach DJ, Mendes RE, Sader HS, Jones RN. In vitro evaluation of PTK796 activity tested against Staphylococcus aureus including Future Microbiol. (Epub ahead of print)
13 Omadacycline: a novel aminomethylcycline antibiotic for bacterial infections Review hospital- and community-associated MRSA strains from the USA and Europe. Presented at: 50th Interscience Congress on Antimicrobial Agents and Chemotherapy. Boston, MA, USA, September Flamm RK, Rhomberg PR, Huband MD, Farrell DJ. Activity of omadacycline tested against Staphylococcus aureus from a global surveillance program (2014). Presented at: 55th Interscience Congress on Antimicrobial Agents and Chemotherapy. San Diego, CA, USA, September Flamm RK, Rhomberg PR, Huband MD, Farrell DJ. Activity of omadacycline tested against Streptococcus pneumoniae from a global surveillance program (2014). Presented at: 55th Interscience Congress on Antimicrobial Agents and Chemotherapy. San Diego, CA, USA, September Flamm RK, Rhomberg PR, Huband MD, Farrell DJ. Activity of omadacycline tested against Enterobacteriaceae causing urinary tract infections from a global surveillance program (2014). Presented at: 55th Interscience Congress on Antimicrobial Agents and Chemotherapy. San Diego, CA, USA, September Traczewski MM, Brown SB. BAY / PTK 0796: in vitro potency and spectrum of activity compared with ten other antimicrobial compounds. Presented at: 43rd Interscience Congress on Antimicrobial Agents and Chemotherapy. Chicago, IL, USA, September DuBois J, Dubois M, Martel JF, Tanaka SK. In vitro activity of omadacycline against Legionella pneumophila. Presented at: 55th Interscience Congress on Antimicrobial Agents and Chemotherapy. San Diego, CA, USA, September Hanna I, Sun H, Zhu B et al. Metabolic stability of PTK 0796 (omadacycline). Presented at: 22nd European Congress of Clinical Microbiology and Infectious Diseases. London, UK, 31 March 2 April Hanna I, Sun H, Alexander N et al. Lack of interaction of PTK 0796 (omadacycline) with human transporter. Presented at: 22nd European Congress of Clinical Microbiology and Infectious Diseases. London, UK, 31 March 2 April Tanaka SK, Villano S. In vitro and in vivo assessment of cardiovascular effects with omadacycline. Antimicrob. Agents Chemther. doi: /aac (2016) (Epub ahead of print). Describes a series of nonclinical studies that were undertaken to fully characterize the cardiovascular effects of omadacycline. These nonclinical findings showed that omadacycline had a vagolytic effect on heart rate but had a low potential for cardiac arrhythmia or clinically significant cardiovascular toxicity. 19 Tanaka SK, Villano S, Tzanis E. Single and multiple dose pharmacokinetics and tolerability of intravenous omadacycline in healthy volunteers. Presented at: 26th European Congress of Clinical Microbiology and Infectious Diseases. Amsterdam, The Netherlands, 9 12 April Sun H, Ting L, Flarakos J et al. Pharmacokinetics of [14C]-labelled omadacycline (PTK 0796) in healthy male subjects. Presented at: 52nd Interscience Congress on Antimicrobial Agents and Chemotherapy. San Francisco, CA, USA, 9 12 September Villano S, Tzanis E, Tanaka SK. In vitro protein binding with omadacycline, a first in class aminomethylcycline antibiotic. Presented at: ASM Microbe Boston, MA, USA, June Sun H, Maietta R, Machineni S et al. A single-dose study to evaluate the pharmacokinetics, safety, and tolerability of multiple formulations of PTK 0796 in healthy subjects. Presented at: 21st European Congress of Clinical Microbiology and Infectious Diseases. Milan, Italy, 7 10 May Craig WA, Andes D, Odinecs A. In vivo pharmacodynamics of MK-2764/PTK 0796 against various Gram-positive and Gramnegative bacteria in the thighs of neutropenic and normal mice. Presented at: 46th Interscience Congress on Antimicrobial Agents and Chemotherapy. San Diego, CA, USA, September Tzanis E, Manley A, Villano S, Tanaka SK, Loh E. Effect of food on the bioavailability of omadacycline in healthy volunteers. Presented at: ASM Microbe Boston, MA, USA, June Tanaka SK, Tzanis E, Villano S. Effect of age and gender on the pharmacokinetics of the oral and IV omadacycline, a new class of aminomethylcyclines. Presented at: 26th European Congress of Clinical Microbiology and Infectious Diseases. Amsterdam, The Netherlands, 9 12 April Van Wart SA, Manley A, Bhavnani SM et al. Population pharmacokinetics (PPK) of omadacycline following intravenous (iv) or oral administration to Phase I subjects. Presented at: 26th European Congress of Clinical Microbiology and Infectious Diseases. Amsterdam, The Netherlands, 9 12 April Villano S, Tanaka SK. Double-blind, randomized study of the effects of a supratherapeutic iv dose of omadacycline on QT/QTc intervals in healthy subjects. Presented at: 26th European Congress of Clinical Microbiology and Infectious Diseases. Amsterdam, The Netherlands, 9 12 April This thorough QT study demonstrated that omadacycline has no significant effect on the QT interval in healthy subjects even after a supratherapeutic intravenous dose. 28 Ting L, Kovacs SJ, Prestgaard J et al. An open-label study of the pharmacokinetics and safety of single IV and oral doses of omadacycline in subjects with mild, moderate, and severe hepatic impairment. Presented at: 52nd Interscience Congress on Antimicrobial Agents and Chemotherapy. San Francisco, CA, USA, 9 12 September Noel GJ, Draper MP, Hait H, Tanaka SK, Arbeit RD. A randomized, evaluator-blind, Phase 2 study comparing the safety and efficacy of omadacycline to those of linezolid for treatment of complicated skin and skin structure infections. Antimicrob. Agents Chemother. 56(11), (2012). Reports the first clinical results with omadacycline for skin and skin structure infections, where omadacycline was comparable to linezolid. 30 Noel GJ, Draper MP, Hait H, Tanaka SK. Safety and efficacy of PTK 0796 (omadacycline) as treatment of complicated skin and soft tissue infection (cssti). Presented at: 22nd European Congress of Clinical Microbiology and Infectious Diseases. London, UK, 31 March 2 April Connors KP, Housman ST, Pope JS et al. Phase I, open-label, safety and pharmacokinetic study to assess bronchopulmonary disposition of intravenous eravacycline in healthy men and women. Antimicrob. Agents Chemother. 58(4), (2014). 32 Horn PT, Sutcliffe JA, Walpole SM, Leighton A. Pharmacokinetics, safety and tolerability of a novel fluorocycline, TP-434, following multiple dose administration. Presented at: 49th Infectious Diseases Society of American Annual Meeting. Boston, MA, USA, October Leighton A, Zupanets I, Bezugla N, Plamondon L, Macdonald G, Sutcliffe JA. Broad-spectrum fluorocycline TP-434 has oral bioavailability in human. Presented at: 21st European Congress of Clinical Microbiology and Infectious Diseases. Milan, Italy, 7 10 May
14 Villano, Steenbergen & Loh 34 Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. The pharmacokinetic and pharmacodynamic profile of tigecycline. Clin. Infect. Dis. 41(Suppl. 5), S333 S340 (2005). 35 Passarell J, Ludwig E, Liolios K et al. Exposure response analyses of tigecycline tolerability in healthy subjects. Diagn. Microbiol. Infect. Dis. 65(2), (2009). 36 Rubino CM, Bhavnani SM, Forrest A et al. Pharmacokinetics pharmacodynamics of tigecycline in patients with communityacquired pneumonia. Antimicrob. Agents Chemother. 56(1), (2012). Future Microbiol. (Epub ahead of print)
Late Stage, Novel Antibiotics
 Late Stage, Novel Antibiotics November, 2015 11/18/2015 1 Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information
Late Stage, Novel Antibiotics November, 2015 11/18/2015 1 Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
CEFA-DROPS AND CEFA-TABS
 Page 1 of 5 FORT DODGE ANIMAL HEALTH Division of Wyeth 800-5TH STREET N.W., P.O. BOX 518 FORT DODGE IA 50501 USA Telephone: 515-955-4600 Fax: 515-955-3730 Order Desk Telephone: 800-685-5656 Order Desk
Page 1 of 5 FORT DODGE ANIMAL HEALTH Division of Wyeth 800-5TH STREET N.W., P.O. BOX 518 FORT DODGE IA 50501 USA Telephone: 515-955-4600 Fax: 515-955-3730 Order Desk Telephone: 800-685-5656 Order Desk
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Application for a Marketing Authorisation: Requirements and Criteria for the Assessment of QT Prolonging Potential
 Application for a Marketing Authorisation: Requirements and Criteria for the Assessment of QT Prolonging Potential Bundesinstitut für Arzneimittel Dr. med. Clemens Mittmann Bundesinstitut für Arzneimittel
Application for a Marketing Authorisation: Requirements and Criteria for the Assessment of QT Prolonging Potential Bundesinstitut für Arzneimittel Dr. med. Clemens Mittmann Bundesinstitut für Arzneimittel
WARNING LETTER. According to its approved product labeling (PI) (in pertinent part, emphasis original):
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993-0002 TRANSMITTED BY FACSIMILE Sapan A. Shah, Ph.D. President and Chief Executive Officer
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993-0002 TRANSMITTED BY FACSIMILE Sapan A. Shah, Ph.D. President and Chief Executive Officer
PACKAGE LEAFLET. CLINDAMYCIN capsules Clidamycin. One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride).
 PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
A Peak at PK An Introduction to Pharmacokinetics
 Paper IS05 A Peak at PK An Introduction to Pharmacokinetics Hannah Twitchett, Roche Products Ltd, Welwyn Garden City, UK Paul Grimsey, Roche Products Ltd, Welwyn Garden City, UK ABSTRACT The aim of this
Paper IS05 A Peak at PK An Introduction to Pharmacokinetics Hannah Twitchett, Roche Products Ltd, Welwyn Garden City, UK Paul Grimsey, Roche Products Ltd, Welwyn Garden City, UK ABSTRACT The aim of this
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Humulin (LY041001) Page 1 of 1
 (LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
(LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
Guidance for Industry
 Guidance for Industry Food-Effect Bioavailability and Fed Bioequivalence Studies U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
Guidance for Industry Food-Effect Bioavailability and Fed Bioequivalence Studies U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
skin and soft tissue infections (skinfold pyoderma, impetigo, folliculitis, furunculosis, cellulitis) caused by susceptible strains of organisms.
 Part II SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL P 5 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredient Marbofloxacin 5mg per tablet
Part II SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL P 5 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredient Marbofloxacin 5mg per tablet
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Fexinidazole a new oral treatment for sleeping sickness update of development
 Fexinidazole a new oral treatment for sleeping sickness update of development SMe O 2 Me CH 2 O Antoine TARRAL Olaf Valverde Séverine Blesson Clélia Bardonneau Wilfried Mutumbo September 2011 Fexinidazole
Fexinidazole a new oral treatment for sleeping sickness update of development SMe O 2 Me CH 2 O Antoine TARRAL Olaf Valverde Séverine Blesson Clélia Bardonneau Wilfried Mutumbo September 2011 Fexinidazole
Phase of development:
 Title of study: A randomised, placebo-controlled, double-blind, double-dummy, four-way crossover, single-centre study to investigate the effects of 2 mg and 10 mg intravenously administered NRL972 on the
Title of study: A randomised, placebo-controlled, double-blind, double-dummy, four-way crossover, single-centre study to investigate the effects of 2 mg and 10 mg intravenously administered NRL972 on the
Sponsor. Novartis Generic Drug Name. Vildagliptin. Therapeutic Area of Trial. Type 2 diabetes. Approved Indication. Investigational.
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC) Indication: In combination with docetaxel in locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma
Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC) Indication: In combination with docetaxel in locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma
Urinary Tract Infections
 Urinary Tract Infections Overview A urine culture must ALWAYS be interpreted in the context of the urinalysis and patient symptoms. If a patient has no signs of infection on urinalysis, no symptoms of
Urinary Tract Infections Overview A urine culture must ALWAYS be interpreted in the context of the urinalysis and patient symptoms. If a patient has no signs of infection on urinalysis, no symptoms of
Guidance for Industry Antibacterial Therapies for Patients With Unmet Medical Need for the Treatment of Serious Bacterial Diseases
 Guidance for Industry Antibacterial Therapies for Patients With Unmet Medical Need for the Treatment of Serious Bacterial Diseases DRAFT GUIDANCE This guidance document is being distributed for comment
Guidance for Industry Antibacterial Therapies for Patients With Unmet Medical Need for the Treatment of Serious Bacterial Diseases DRAFT GUIDANCE This guidance document is being distributed for comment
Public Assessment Report Scientific discussion. Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC
 Public Assessment Report Scientific discussion Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC This module reflects the scientific discussion for the approval of Tenofovir disoproxil Teva.
Public Assessment Report Scientific discussion Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC This module reflects the scientific discussion for the approval of Tenofovir disoproxil Teva.
Lecture Outline. Quinolones
 Lecture Outline Quinolones Trimethoprim/Sulfamethoxazole Miscellaneous antimicrobials - Metronidazole Daptomycin Cases Quinolones Bactericidal broad spectrum antibiotics Increasingly used because of their
Lecture Outline Quinolones Trimethoprim/Sulfamethoxazole Miscellaneous antimicrobials - Metronidazole Daptomycin Cases Quinolones Bactericidal broad spectrum antibiotics Increasingly used because of their
COMPOSITION: Each capsule contains clindamycin hydrochloride equivalent to 150 mg clindamycin base.
 APPROVED PACKAGE INSERT DALACIN C 150 mg CAPSULES SCHEDULING STATUS: S4 PROPRIETARY NAME (and dosage form): DALACIN C TM 150 mg (Capsules) COMPOSITION: Each capsule contains clindamycin hydrochloride equivalent
APPROVED PACKAGE INSERT DALACIN C 150 mg CAPSULES SCHEDULING STATUS: S4 PROPRIETARY NAME (and dosage form): DALACIN C TM 150 mg (Capsules) COMPOSITION: Each capsule contains clindamycin hydrochloride equivalent
HUSRES Annual Report 2008 Martti Vaara. www.huslab.fi www.intra.hus.fi
 HUSRES Annual Report 2008 Martti Vaara www.huslab.fi www.intra.hus.fi The basis of this HUSRES 2008 report is the HUSLAB/Whonet database 2008, which contains susceptibility data on about 180.000 bacteria
HUSRES Annual Report 2008 Martti Vaara www.huslab.fi www.intra.hus.fi The basis of this HUSRES 2008 report is the HUSLAB/Whonet database 2008, which contains susceptibility data on about 180.000 bacteria
HUSRES Annual Report 2015
 HUSRES Annual Report 2015 1 This report is based on the HUSLAB/Whonet database 2015, which contains data on more than 190.000 microbes. EUCAST 2015 clinical breakpoints have mainly been used. The doses
HUSRES Annual Report 2015 1 This report is based on the HUSLAB/Whonet database 2015, which contains data on more than 190.000 microbes. EUCAST 2015 clinical breakpoints have mainly been used. The doses
Helsingin ja Uudenmaan alueen herkkyystilastoja 2004. www.huslab.fi www.intra.hus.fi
 Helsingin ja Uudenmaan alueen herkkyystilastoja 2004 www.huslab.fi www.intra.hus.fi Staph. aureus 2004 (%R+I) Pus and blood isolates at six hospitals (M,T,L,K,N,Ma) in Helsinki University Central Hospital,
Helsingin ja Uudenmaan alueen herkkyystilastoja 2004 www.huslab.fi www.intra.hus.fi Staph. aureus 2004 (%R+I) Pus and blood isolates at six hospitals (M,T,L,K,N,Ma) in Helsinki University Central Hospital,
GT-020 Phase 1 Clinical Trial: Results of Second Cohort
 GT-020 Phase 1 Clinical Trial: Results of Second Cohort July 29, 2014 NASDAQ: GALT www.galectintherapeutics.com 2014 Galectin Therapeutics inc. Forward-Looking Statement This presentation contains, in
GT-020 Phase 1 Clinical Trial: Results of Second Cohort July 29, 2014 NASDAQ: GALT www.galectintherapeutics.com 2014 Galectin Therapeutics inc. Forward-Looking Statement This presentation contains, in
Letter Date March 27, 2007 Stamp Date March 28, 2007 PDUFA Goal Date January 28, 2008
 CLINICAL REVIEW Application Type NDA Submission Number 22-157 Submission Code Letter Date March 27, 2007 Stamp Date March 28, 2007 PDUFA Goal Date January 28, 2008 Reviewer Name Review Completion Date
CLINICAL REVIEW Application Type NDA Submission Number 22-157 Submission Code Letter Date March 27, 2007 Stamp Date March 28, 2007 PDUFA Goal Date January 28, 2008 Reviewer Name Review Completion Date
How To Treat Mrsa From A Dead Body
 HUSRES Annual Report 2012 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2013 1 The basis of this HUSRES 2012 report is the HUSLAB/Whonet database 2012, which contains susceptibility data on
HUSRES Annual Report 2012 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2013 1 The basis of this HUSRES 2012 report is the HUSLAB/Whonet database 2012, which contains susceptibility data on
Statistics and Pharmacokinetics in Clinical Pharmacology Studies
 Paper ST03 Statistics and Pharmacokinetics in Clinical Pharmacology Studies ABSTRACT Amy Newlands, GlaxoSmithKline, Greenford UK The aim of this presentation is to show how we use statistics and pharmacokinetics
Paper ST03 Statistics and Pharmacokinetics in Clinical Pharmacology Studies ABSTRACT Amy Newlands, GlaxoSmithKline, Greenford UK The aim of this presentation is to show how we use statistics and pharmacokinetics
Antimicrobial Activity and Spectrum of PPI-0903M (T-91825), a Novel Cephalosporin, Tested against a Worldwide Collection of Clinical Strains
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2005, p. 3501 3512 Vol. 49, No. 8 0066-4804/05/$08.00 0 doi:10.1128/aac.49.8.3501 3512.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2005, p. 3501 3512 Vol. 49, No. 8 0066-4804/05/$08.00 0 doi:10.1128/aac.49.8.3501 3512.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
Introduction to Antimicrobial Therapy
 Introduction to Antimicrobial Therapy Christine Kubin, Pharm.D., BCPS Clinical Pharmacist, Infectious Diseases Case #1 L.G. is a 78 yo woman admitted for cardiac cath. 3-vessel disease was identified and
Introduction to Antimicrobial Therapy Christine Kubin, Pharm.D., BCPS Clinical Pharmacist, Infectious Diseases Case #1 L.G. is a 78 yo woman admitted for cardiac cath. 3-vessel disease was identified and
Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor)
 EMA/662624/2015 Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor) This is a summary of the risk management plan (RMP) for Orkambi, which details the measures to be taken
EMA/662624/2015 Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor) This is a summary of the risk management plan (RMP) for Orkambi, which details the measures to be taken
HUSRES Annual Report 2010 Martti Vaara www.huslab.fi www.intra.hus.fi
 HUSRES Annual Report 2010 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara, 2/2011 1 The basis of this HUSRES 2010 report is the HUSLAB/Whonet database 2010, which contains susceptibility data
HUSRES Annual Report 2010 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara, 2/2011 1 The basis of this HUSRES 2010 report is the HUSLAB/Whonet database 2010, which contains susceptibility data
Session 6 Clinical Trial Assessment Phase I Clinical Trial
 L1 Session 6 Clinical Trial Assessment Phase I Clinical Trial Presentation to APEC Preliminary Workshop on Review of Drug Development in Clinical Trials Celia Lourenco, PhD, Manager, Clinical Group I Office
L1 Session 6 Clinical Trial Assessment Phase I Clinical Trial Presentation to APEC Preliminary Workshop on Review of Drug Development in Clinical Trials Celia Lourenco, PhD, Manager, Clinical Group I Office
Summary of the risk management plan (RMP) for Sirturo (bedaquiline)
 EMA/16634/2014 Summary of the risk management plan (RMP) for Sirturo (bedaquiline) This is a summary of the risk management plan (RMP) for Sirturo, which details the measures to be taken in order to ensure
EMA/16634/2014 Summary of the risk management plan (RMP) for Sirturo (bedaquiline) This is a summary of the risk management plan (RMP) for Sirturo, which details the measures to be taken in order to ensure
2.0 Synopsis. Vicodin CR (ABT-712) M05-765 Clinical Study Report R&D/07/095. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
Helsingin ja Uudenmaan alueen herkkyystilastoja 2003. www.infektiofoorumi.fi www.huslab.fi www.intra.hus.fi
 Helsingin ja Uudenmaan alueen herkkyystilastoja 2003 www.infektiofoorumi.fi www.huslab.fi www.intra.hus.fi 12 10 Staph. aureus Pus and blood isolates % R+I, at HUCH (7 hospitals) [one isolate/patient (the
Helsingin ja Uudenmaan alueen herkkyystilastoja 2003 www.infektiofoorumi.fi www.huslab.fi www.intra.hus.fi 12 10 Staph. aureus Pus and blood isolates % R+I, at HUCH (7 hospitals) [one isolate/patient (the
Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults
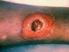 1 of 6 9/24/2010 11:16 AM Official reprint from UpToDate www.uptodate.com 2010 UpToDate Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults Author
1 of 6 9/24/2010 11:16 AM Official reprint from UpToDate www.uptodate.com 2010 UpToDate Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults Author
BIOAVAILABILITY & BIOEQUIVALENCE TRIALS
 BIOAVAILABILITY & BIOEQUIVALENCE TRIALS Shubha Rani,, Ph.D. Technical Director & Head-Biometrics and Data Management Synchron Research Services Pvt. Ltd. Ahmedabad 380 054 drshubha@synchronresearch.com
BIOAVAILABILITY & BIOEQUIVALENCE TRIALS Shubha Rani,, Ph.D. Technical Director & Head-Biometrics and Data Management Synchron Research Services Pvt. Ltd. Ahmedabad 380 054 drshubha@synchronresearch.com
Novartis Gilenya FDO Program Clinical Protocol and Highlights from Prescribing Information (PI)
 Novartis Gilenya FDO Program Clinical Protocol and Highlights from Prescribing Information (PI) Highlights from Prescribing Information - the link to the full text PI is as follows: http://www.pharma.us.novartis.com/product/pi/pdf/gilenya.pdf
Novartis Gilenya FDO Program Clinical Protocol and Highlights from Prescribing Information (PI) Highlights from Prescribing Information - the link to the full text PI is as follows: http://www.pharma.us.novartis.com/product/pi/pdf/gilenya.pdf
Public Assessment Report. Decentralised Procedure
 Public Assessment Report Decentralised Procedure Linezolid 2 mg/ml solution for infusion Linezolid 600mg film coated tablets Linezolid 100mg/5ml granules for oral suspension Procedure No: UK Licence No:
Public Assessment Report Decentralised Procedure Linezolid 2 mg/ml solution for infusion Linezolid 600mg film coated tablets Linezolid 100mg/5ml granules for oral suspension Procedure No: UK Licence No:
How To Treat Mrsa In Finnish
 HUSRES Annual Report 2013 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2014 1 The basis of this HUSRES 2013 report is the HUSLAB/Whonet database 2013, which contains susceptibility data on
HUSRES Annual Report 2013 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2014 1 The basis of this HUSRES 2013 report is the HUSLAB/Whonet database 2013, which contains susceptibility data on
TGN 1412 Welche Änderungen haben sich für die Erstanwendung am Menschen aus Sicht des BfArM ergeben?
 TGN 1412 Welche Änderungen haben sich für die Erstanwendung am Menschen aus Sicht des BfArM ergeben? PD Dr. med. Thomas Sudhop Bundesinstitut für Arzneimittel, Bonn Bundesinstitut für Arzneimittel IMP
TGN 1412 Welche Änderungen haben sich für die Erstanwendung am Menschen aus Sicht des BfArM ergeben? PD Dr. med. Thomas Sudhop Bundesinstitut für Arzneimittel, Bonn Bundesinstitut für Arzneimittel IMP
Vancomycin. Beta-lactams. Beta-lactams. Vancomycin (Glycopeptide) Rifamycins (rifampin) MID 4
 Antibiotic Classes Introduction to Antimicrobials Rachel J. Gordon, MD, MPH Assistant Professor of Clinical Medicine and Epidemiology Beta-lactams Inhibit cell wall synthesis Penicillins Cephalosporins
Antibiotic Classes Introduction to Antimicrobials Rachel J. Gordon, MD, MPH Assistant Professor of Clinical Medicine and Epidemiology Beta-lactams Inhibit cell wall synthesis Penicillins Cephalosporins
Clinical Trial Results Database Page 1
 Clinical Trial Results Database Page Sponsor Novartis Generic Drug Name BGT6 Therapeutic Area of Trial Advanced solid malignancies Approved Indication Investigational Study Number CBGT6A0 Title A phase
Clinical Trial Results Database Page Sponsor Novartis Generic Drug Name BGT6 Therapeutic Area of Trial Advanced solid malignancies Approved Indication Investigational Study Number CBGT6A0 Title A phase
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer
 Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
SYNOPSIS. Risperidone: Clinical Study Report CR003274
 SYNOPSIS Protocol No: CR003274 Title of Study: An Open-Label, Long-Term Trial of Risperidone Long-Acting Microspheres in the Treatment of Subjects Diagnosed with Schizophrenia Coordinating Investigator:
SYNOPSIS Protocol No: CR003274 Title of Study: An Open-Label, Long-Term Trial of Risperidone Long-Acting Microspheres in the Treatment of Subjects Diagnosed with Schizophrenia Coordinating Investigator:
NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL EVALUATION OF ANTICANCER MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 23 July 1998 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL
The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 23 July 1998 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
SAFETY PHARMACOLOGY STUDIES FOR HUMAN PHARMACEUTICALS S7A
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE SAFETY PHARMACOLOGY STUDIES FOR HUMAN PHARMACEUTICALS
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE SAFETY PHARMACOLOGY STUDIES FOR HUMAN PHARMACEUTICALS
Summary of the risk management plan (RMP) for Cerdelga (eliglustat)
 EMA/743948/2014 Summary of the risk management plan (RMP) for Cerdelga (eliglustat) This is a summary of the risk management plan (RMP) for Cerdelga, which details the measures to be taken in order to
EMA/743948/2014 Summary of the risk management plan (RMP) for Cerdelga (eliglustat) This is a summary of the risk management plan (RMP) for Cerdelga, which details the measures to be taken in order to
5.07.09. Aubagio. Aubagio (teriflunomide) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Lead optimization services
 Lead optimization services The WIL Research Company (WRC) has extensive experience in fast track tailor-made screening strategies to help you with the challenging task of selecting your best candidate
Lead optimization services The WIL Research Company (WRC) has extensive experience in fast track tailor-made screening strategies to help you with the challenging task of selecting your best candidate
Session 3 Topics. Argatroban. Argatroban. Drug Use and Adverse Effects. Laboratory Monitoring of Anticoagulant Therapy
 ~~Marshfield Labs Presents~~ Laboratory Monitoring of Anticoagulant Therapy Session 3 of 4 Michael J. Sanfelippo, M.S. Technical Director, Coagulation Services Session 3 Topics Direct Thrombin Inhibitors:
~~Marshfield Labs Presents~~ Laboratory Monitoring of Anticoagulant Therapy Session 3 of 4 Michael J. Sanfelippo, M.S. Technical Director, Coagulation Services Session 3 Topics Direct Thrombin Inhibitors:
C-Difficile Infection Control and Prevention Strategies
 C-Difficile Infection Control and Prevention Strategies Adrienne Mims, MD MPH VP, Chief Medical Officer Adrienne.Mims@AlliantQuality.org 1/18/2016 1 Disclosure This educational activity does not have commercial
C-Difficile Infection Control and Prevention Strategies Adrienne Mims, MD MPH VP, Chief Medical Officer Adrienne.Mims@AlliantQuality.org 1/18/2016 1 Disclosure This educational activity does not have commercial
Absorption of Drugs. Transport of a drug from the GI tract
 Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
PRIORITY RESEARCH TOPICS
 PRIORITY RESEARCH TOPICS Understanding all the issues associated with antimicrobial resistance is probably impossible, but it is clear that there are a number of key issues about which we need more information.
PRIORITY RESEARCH TOPICS Understanding all the issues associated with antimicrobial resistance is probably impossible, but it is clear that there are a number of key issues about which we need more information.
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Hunter syndrome is a rare genetic disease which mainly affects males of all ethnicities. The incidence rate ranges from 0.6 to
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Hunter syndrome is a rare genetic disease which mainly affects males of all ethnicities. The incidence rate ranges from 0.6 to
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
Ceftriaxone Therapy Vs. Ciprofloxacin In Treatment Of Typhoid Fever In Adult Patients.
 Dec QMJ VOL.5 No.8 Ceftriaxone Therapy Vs. Ciprofloxacin In Treatment Of Typhoid Fever In Adult Patients. Radhi F.Alshaibani* الخلاصھ لا زالت حمى التایفوید سببا مھما من اسباب الامراض وفقدان ساعات العمل
Dec QMJ VOL.5 No.8 Ceftriaxone Therapy Vs. Ciprofloxacin In Treatment Of Typhoid Fever In Adult Patients. Radhi F.Alshaibani* الخلاصھ لا زالت حمى التایفوید سببا مھما من اسباب الامراض وفقدان ساعات العمل
Guidance for Industry Safety Testing of Drug Metabolites
 Guidance for Industry Safety Testing of Drug Metabolites U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2008 Pharmacology
Guidance for Industry Safety Testing of Drug Metabolites U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2008 Pharmacology
Introduction to Enteris BioPharma
 Introduction to Enteris BioPharma Enteris BioPharma Intelligent Solutions for Oral Drug Delivery Privately held, New Jersey based biotech company Owned solely by Victory Park Capital, a large Chicago based
Introduction to Enteris BioPharma Enteris BioPharma Intelligent Solutions for Oral Drug Delivery Privately held, New Jersey based biotech company Owned solely by Victory Park Capital, a large Chicago based
Results of all scientific investigations with the A.Vogel Sore Throat Spray. Issue 2 - June 2008
 Results of all scientific investigations with the A.Vogel Sore Issue 2 - June 2008 Antiviral Antibacterial Anti-inflammatory Analgetic Immunemodulatory Authors: Andy Suter, Medical Department Roland Schoop,
Results of all scientific investigations with the A.Vogel Sore Issue 2 - June 2008 Antiviral Antibacterial Anti-inflammatory Analgetic Immunemodulatory Authors: Andy Suter, Medical Department Roland Schoop,
Guidance for Industry
 Guidance for Industry S9 Nonclinical Evaluation for Anticancer Pharmaceuticals U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
Guidance for Industry S9 Nonclinical Evaluation for Anticancer Pharmaceuticals U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
Human Clinical Study for Free Testosterone & Muscle Mass Boosting
 Human Clinical Study for Free Testosterone & Muscle Mass Boosting GE Nutrients, Inc. 920 E. Orangethorpe Avenue, Suite B Anaheim, California 92801, USA Phone: +1-714-870-8723 Fax: +1-732-875-0306 Contact
Human Clinical Study for Free Testosterone & Muscle Mass Boosting GE Nutrients, Inc. 920 E. Orangethorpe Avenue, Suite B Anaheim, California 92801, USA Phone: +1-714-870-8723 Fax: +1-732-875-0306 Contact
ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS LAUREN HYNICKA, PHARM.D.
 ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS LAUREN HYNICKA, PHARM.D. ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS ACTIVITY DESCRIPTION Antibiotic use in the safest
ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS LAUREN HYNICKA, PHARM.D. ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS ACTIVITY DESCRIPTION Antibiotic use in the safest
ANTIBIOTICS IN SEPSIS
 ANTIBIOTICS IN SEPSIS Jennifer Curello, PharmD, BCPS Clinical Pharmacist, Infectious Diseases Antimicrobial Stewardship Program Ronald Reagan UCLA Medical Center October 27, 2014 The power of antibiotics
ANTIBIOTICS IN SEPSIS Jennifer Curello, PharmD, BCPS Clinical Pharmacist, Infectious Diseases Antimicrobial Stewardship Program Ronald Reagan UCLA Medical Center October 27, 2014 The power of antibiotics
GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8 Current
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8 Current
APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES
 APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES Principles of prophylaxis 1) Use antimicrobials for surgical procedures where prophylactic antimicrobials have been found to be beneficial.
APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES Principles of prophylaxis 1) Use antimicrobials for surgical procedures where prophylactic antimicrobials have been found to be beneficial.
Biological importance of metabolites. Safety and efficacy aspects
 Biological importance of metabolites Safety and efficacy aspects Bernard Walther Technologie Servier Biological importance of metabolites Safety testing of drug metabolites Bioanalytical strategy Structural
Biological importance of metabolites Safety and efficacy aspects Bernard Walther Technologie Servier Biological importance of metabolites Safety testing of drug metabolites Bioanalytical strategy Structural
National Chlamydia Screening Programme September 2012 PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF AZITHROMYCIN FOR CHLAMYDIA TRACHOMATIS
 PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF AZITHROMYCIN FOR CHLAMYDIA TRACHOMATIS Below is a template that can be used to produce a local patient group direction (PGD) for the administration of
PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF AZITHROMYCIN FOR CHLAMYDIA TRACHOMATIS Below is a template that can be used to produce a local patient group direction (PGD) for the administration of
Analytical Specifications RIVAROXABAN
 Page 1 of 9 ANALYTE NAME AND STRUCTURE - RIVAROXABAN SYNONYMS Xarelto CATEGORY Anticoagulant TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND Xarelto (rivaroxaban) is an orally
Page 1 of 9 ANALYTE NAME AND STRUCTURE - RIVAROXABAN SYNONYMS Xarelto CATEGORY Anticoagulant TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND Xarelto (rivaroxaban) is an orally
Management of Extended Spectrum Beta- Lactamase (ESBL) Producing Enterobacteriaceae in health care settings
 Management of Extended Spectrum Beta- Lactamase (ESBL) Producing Enterobacteriaceae in health care settings Dr. Mary Vearncombe PIDAC-IPC February 2012 Objectives: To provide an overview of the RP/AP Annex
Management of Extended Spectrum Beta- Lactamase (ESBL) Producing Enterobacteriaceae in health care settings Dr. Mary Vearncombe PIDAC-IPC February 2012 Objectives: To provide an overview of the RP/AP Annex
Novel Anticoagulation Agents DISCLOSURES. Objectives ATRIAL FIBRILLATION TRIALS. NOAC Comparison 6/12/2015
 Novel Anticoagulation Agents DISCLOSURES James W. Haynes, MD Department of Family Medicine Univ of TN Health Science Center (Chattanooga) Objectives Understand mechanism of action behind the NOAC agents
Novel Anticoagulation Agents DISCLOSURES James W. Haynes, MD Department of Family Medicine Univ of TN Health Science Center (Chattanooga) Objectives Understand mechanism of action behind the NOAC agents
Guidance for Industry Migraine: Developing Drugs for Acute Treatment
 Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
ANTIBIOTIC RESISTANCE THREATS. in the United States, 2013
 ANTIBIOTIC RESISTANCE THREATS in the United States, 2013 TABLE OF CONTENTS Foreword... 5 Executive Summary.... 6 Section 1: The Threat of Antibiotic Resistance... 11 Introduction.... 11 National Summary
ANTIBIOTIC RESISTANCE THREATS in the United States, 2013 TABLE OF CONTENTS Foreword... 5 Executive Summary.... 6 Section 1: The Threat of Antibiotic Resistance... 11 Introduction.... 11 National Summary
Antibiotic resistance does it matter in paediatric clinical practice? Jette Bangsborg Department of Clinical Microbiology Herlev Hospital
 Antibiotic resistance does it matter in paediatric clinical practice? Jette Bangsborg Department of Clinical Microbiology Herlev Hospital Background The Department of Clinical Microbiology at Herlev Hospital
Antibiotic resistance does it matter in paediatric clinical practice? Jette Bangsborg Department of Clinical Microbiology Herlev Hospital Background The Department of Clinical Microbiology at Herlev Hospital
Agencia Española de Medicamentos y Productos Sanitarios C/Campezo 1, Edificio 8 28022 Madrid España
 DEPARTAMENTO DE MEDICAMENTOS VETERINARIOS Medicamentos y Productos C/Campezo 1, Edificio 8 28022 Madrid España DECENTRALISED PROCEDURE PUBLICLY AVAILABLE ASSESSMENT REPORT FOR A VETERINARY MEDICINAL PRODUCT
DEPARTAMENTO DE MEDICAMENTOS VETERINARIOS Medicamentos y Productos C/Campezo 1, Edificio 8 28022 Madrid España DECENTRALISED PROCEDURE PUBLICLY AVAILABLE ASSESSMENT REPORT FOR A VETERINARY MEDICINAL PRODUCT
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Antibiotic-Associated Diarrhea, Clostridium difficile- Associated Diarrhea and Colitis
 Antibiotic-Associated Diarrhea, Clostridium difficile- Associated Diarrhea and Colitis ANTIBIOTIC-ASSOCIATED DIARRHEA Disturbance of the normal colonic microflora Leading to alterations in bacterial degradation
Antibiotic-Associated Diarrhea, Clostridium difficile- Associated Diarrhea and Colitis ANTIBIOTIC-ASSOCIATED DIARRHEA Disturbance of the normal colonic microflora Leading to alterations in bacterial degradation
General Trends in Infectious Disease
 General Trends in Infectious Disease Four phenomena underline the increase in ID problems: Aging population Increasing numbers of immunocompromised patients Increased mobility of the population Newly emerging
General Trends in Infectious Disease Four phenomena underline the increase in ID problems: Aging population Increasing numbers of immunocompromised patients Increased mobility of the population Newly emerging
The Impact of Drug-Related QT Prolongation on FDA Regulatory Decisions
 The Impact of Drug-Related QT Prolongation on FDA Regulatory Decisions Eunjung Park, Ph.D. DBII / OGD / OPS / CDER / FDA SPS Feb 27 204 Regulatory Background Regulatory actions to QT-related cardiac proarrhythmia
The Impact of Drug-Related QT Prolongation on FDA Regulatory Decisions Eunjung Park, Ph.D. DBII / OGD / OPS / CDER / FDA SPS Feb 27 204 Regulatory Background Regulatory actions to QT-related cardiac proarrhythmia
Treatment for. Malaria: DHA/PQP. Science Day MMV Stakeholders Meeting. Defeating Malaria Together 1
 ANewOnce-a-day Treatment for Uncomplicated Malaria: DHA/PQP Science Day MMV Stakeholders Meeting Dr. Ambrose Talisuna Former Director Global Access, MMV & Field Coordinator, African Eurartesim Registration
ANewOnce-a-day Treatment for Uncomplicated Malaria: DHA/PQP Science Day MMV Stakeholders Meeting Dr. Ambrose Talisuna Former Director Global Access, MMV & Field Coordinator, African Eurartesim Registration
Newsletter. WntResearch AB, Medeon Science Park, Per Albin Hanssons väg 41, 205 12 Malmö, Sweden. Primary Objective:
 Newsletter This resume of the results from the phase 1 study with Foxy-5 is based on clinical and laboratory data from the study, and these data have now been locked into the database. The full report
Newsletter This resume of the results from the phase 1 study with Foxy-5 is based on clinical and laboratory data from the study, and these data have now been locked into the database. The full report
Questions and Answers for Health Care Providers: Renal Dosing and Administration Recommendations for Peramivir IV
 Questions and Answers for Health Care Providers: Renal Dosing and Administration Recommendations for Peramivir IV The purpose of this document is to provide additional clarification to the existing information
Questions and Answers for Health Care Providers: Renal Dosing and Administration Recommendations for Peramivir IV The purpose of this document is to provide additional clarification to the existing information
ZERBAXA (ceftolozane and tazobactam) for injection, for intravenous use Initial U.S. Approval: 2014
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZERBAXA safely and effectively. See full prescribing information for ZERBAXA. ZERBAXA (ceftolozane
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZERBAXA safely and effectively. See full prescribing information for ZERBAXA. ZERBAXA (ceftolozane
Phase: IV. Study Period: 20 Jan. 2006-17 Sep. 2008
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE S7B
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE THE NON-CLINICAL EVALUATION OF THE POTENTIAL FOR
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE THE NON-CLINICAL EVALUATION OF THE POTENTIAL FOR
EFFIMET 1000 XR Metformin Hydrochloride extended release tablet
 BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE THE COMMON TECHNICAL DOCUMENT FOR THE REGISTRATION
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE THE COMMON TECHNICAL DOCUMENT FOR THE REGISTRATION
QT analysis: A guide for statistical programmers. Prabhakar Munkampalli Statistical Analyst II Hyderabad, 7 th September 2012
 QT analysis: A guide for statistical programmers Prabhakar Munkampalli Statistical Analyst II Hyderabad, 7 th September 2012 Agenda ECG ICH E14 Thorough QT/QTc study Role of Statistical Programmer References
QT analysis: A guide for statistical programmers Prabhakar Munkampalli Statistical Analyst II Hyderabad, 7 th September 2012 Agenda ECG ICH E14 Thorough QT/QTc study Role of Statistical Programmer References
Etiology and treatment of chronic bacterial prostatitis the Croatian experience
 Etiology and treatment of chronic bacterial prostatitis the Croatian experience Višnja Škerk University Hospital for Infectious Diseases "Dr. Fran Mihaljevic" Zagreb Croatia Milano, Malpensa, 14 Nov 2008
Etiology and treatment of chronic bacterial prostatitis the Croatian experience Višnja Škerk University Hospital for Infectious Diseases "Dr. Fran Mihaljevic" Zagreb Croatia Milano, Malpensa, 14 Nov 2008
Chemical Name: (±)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6- carboxylic acid.
![Chemical Name: (±)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6- carboxylic acid. Chemical Name: (±)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6- carboxylic acid.](/thumbs/40/20977501.jpg) OCUFLOX (ofloxacin ophthalmic solution) 0.3% sterile DESCRIPTION OCUFLOX (ofloxacin ophthalmic solution) 0.3% is a sterile ophthalmic solution. It is a fluorinated carboxyquinolone anti-infective for topical
OCUFLOX (ofloxacin ophthalmic solution) 0.3% sterile DESCRIPTION OCUFLOX (ofloxacin ophthalmic solution) 0.3% is a sterile ophthalmic solution. It is a fluorinated carboxyquinolone anti-infective for topical
Basic Overview of Preclinical Toxicology Animal Models
 Basic Overview of Preclinical Toxicology Animal Models Charles D. Hebert, Ph.D., D.A.B.T. December 5, 2013 Outline Background In Vitro Toxicology In Vivo Toxicology Animal Models What is Toxicology? Background
Basic Overview of Preclinical Toxicology Animal Models Charles D. Hebert, Ph.D., D.A.B.T. December 5, 2013 Outline Background In Vitro Toxicology In Vivo Toxicology Animal Models What is Toxicology? Background
Regulatory decision-making for the assessment of ECG effects of new drugs: Exposure-response analyis
 Regulatory decision-making for the assessment of ECG effects of new drugs: Exposure-response analyis Kevin M. Krudys, Ph.D. Team Leader Division of Pharmacometrics Office of Clinical Pharmacology 1 Disclosures
Regulatory decision-making for the assessment of ECG effects of new drugs: Exposure-response analyis Kevin M. Krudys, Ph.D. Team Leader Division of Pharmacometrics Office of Clinical Pharmacology 1 Disclosures
Not All Clinical Trials Are Created Equal Understanding the Different Phases
 Not All Clinical Trials Are Created Equal Understanding the Different Phases This chapter will help you understand the differences between the various clinical trial phases and how these differences impact
Not All Clinical Trials Are Created Equal Understanding the Different Phases This chapter will help you understand the differences between the various clinical trial phases and how these differences impact
Guidance for Industry
 Guidance for Industry Codevelopment of Two or More New Investigational Drugs for Use in Combination U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry Codevelopment of Two or More New Investigational Drugs for Use in Combination U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
SR 5. W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1
 W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1 alfuzosin SR 5 mg (Alfuzosin HCI) COMPOSITION Each sustained-release, film-coated tablet contains: Alfuzosin hydrochloride......... 5 mg Excipients... q.s.
W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1 alfuzosin SR 5 mg (Alfuzosin HCI) COMPOSITION Each sustained-release, film-coated tablet contains: Alfuzosin hydrochloride......... 5 mg Excipients... q.s.
