Review article: prevention of non-steroidal anti-inflammatory drug gastrointestinal complications review and recommendations based on risk assessment
|
|
|
- Lillian Pearson
- 7 years ago
- Views:
Transcription
1 Aliment Pharmacol Ther 2004; 19: doi: /j x Review article: prevention of non-steroidal anti-inflammatory drug gastrointestinal complications review and recommendations based on risk assessment F. K. L. CHAN* & D. Y. GRAHAM *Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong, China; Molecular Virology and Microbiology, Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, Houston, TX, USA Accepted for publication 4 March 2004 SUMMARY The incidence of non-steroidal anti-inflammatory drugrelated ulcer complications remains high despite the availability of potent anti-ulcer drugs and selective cyclo-oxygenase-2 inhibitors. Non-steroidal anti-inflammatory drug-related ulcer complications can be minimized by prospective assessment of patients baseline risk, rational choice and use of non-steroidal anti-inflammatory drugs, and selective use of co-therapy strategies with gastroprotectives. Current recommendations regarding strategies using anti-ulcer drugs and cyclooxygenase-2 inhibitors for prevention of clinical nonsteroidal anti-inflammatory drug upper gastrointestinal events are largely derived from studies using surrogates such as endoscopic ulcers, erosions, and symptoms in low- to average-risk patients. Conclusions based on surrogate and potentially manipulatable end-points are increasingly suspect with regard to applicability to clinical situations. This article reviews the risks associated with non-steroidal anti-inflammatory drugs including aspirin and includes the effect of the patients baseline risk, and the confounding effects of Helicobacter pylori infection. In addition, uncertainties regarding the clinical efficacy of anti-ulcer drugs and cyclo-oxygenase-2 inhibitors against non-steroidal anti-inflammatory drug-related ulcer complications are put into perspective. We propose management strategies based on the risk category: low risk (absence of risk factors) (least ulcerogenic non-steroidal anti-inflammatory drug at lowest effective dose), moderate risk (one to two risk factors) (as above, plus an antisecretory agent or misoprostol or a cyclo-oxygenase-2 inhibitor), high risk (multiple risk factors or patients using concomitant lowdose aspirin, steroids, or anticoagulants) (cyclo-oxygenase-2 inhibitor alone with steroids, plus misoprostol with warfarin, or plus a proton pump inhibitors or misoprostol with aspirin), and very high risk (history of ulcer complications) (avoid all non-steroidal antiinflammatory drugs, if possible or a cyclo-oxygenase-2 plus a proton pump inhibitors and/or misoprostol). The presence of H. pylori infection increases the risk of upper gastrointestinal complications in non-steroidal antiinflammatory drug users by two- to fourfold suggesting that all patients requiring regular non-steroidal antiinflammatory drug therapy be tested for H. pylori. INTRODUCTION Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most widely used drugs worldwide and are Correspondence to: Dr D. Y. Graham, Rm 3A-320 (111D), VA Medical Center, 2002 Holcombe Blvd, Houston, TX 77030, USA. dgraham@bcm.tmc.edu especially valued for their analgesic, antipyretic and anti-inflammatory properties. However, despite the great benefits associated with NSAID use, up to 25% of patients taking NSAIDs chronically experience upper gastrointestinal (UGI) adverse effects. 1 There are several management principles and options that can reduce the risk of NSAID-induced complications including issues regarding dose, type of NSAID, and gastroprotective Ó 2004 Blackwell Publishing Ltd 1051
2 1052 F. K. L. CHAN & D. Y. GRAHAM drug therapy consisting of either co-therapy with antisecretory drugs or prostaglandin replacement with misoprostol. It has also been recently recognized that Helicobacter pylori-infected individuals have about a twofold increased risk of developing complications while taking aspirin or traditional NSAIDs making H. pylori eradication another option to reduce risk. 2, 3 Case control studies have consistently shown that NSAIDs differ in their ulcerogenic risk and this fact alone suggests that care in selection of an NSAID to match the indication should reduce the incidence of untoward events. 4 7 ASSESSMENT OF ULCER RISK The strongest risk factor for a GI complication is the presence of prior ulcer disease with a history of ulcer complications. 8 Other important risk factors include old age, presence of cardiovascular diseases, concomitant use of aspirin or other antiplatelet drugs, steroids or warfarin. In clinical practice, patients can be stratified into three groups of progressive risk and a fourth group of very high-risk patients (i.e. the presence of prior ulcer complications). The three primary risk groups can be separated according to the number and nature of risk factors: low risk (absence of risk factors), moderate risk (one to two risk factors), and high risk (3 risk factors, or concomitant use of aspirin). According to the MUCOSA study, the estimated annualized incidence of NSAID-related ulcer complications was 0.8% for patients without risk factors, 2% with one risk factor, % with three risk factors and 18% with four risk factors. 9 CHOOSING THE RIGHT NSAID FOR THE RIGHT PATIENT The best and cheapest method to prevent an NSAIDrelated ulcer complication is to avoid NSAID use. Non- NSAID analgesics should be the first line treatment for patients with degenerative arthritis or other noninflammatory pain conditions. If NSAIDs are required, the important issues are related to cost, safety and effectiveness. Recently, a nomogram has been developed that incorporates factors including patients baseline risk, the added risk associated with the individual NSAID, and the cost of and protection conferred by cotherapy to estimate the cost-effectiveness of competing strategies for reducing the risk of clinical UGI events. 10 The nomogram is useful to rapidly calculate relative cost-effectiveness of the various trade-offs (Figure 1). In general, the GI toxicity of a cyclo-oxygenase (COX) non-selective NSAID correlates with its anti-inflammatory activity. NSAIDs with a high analgesic effect at Figure 1. The Figure represents nomogram designed to estimate the cost-effectiveness of competing strategies for reducing the risk of clinical upper gastrointestinal (GI) events. (a) Denotes the difference in the annual cost between any two strategies of non-steroidal anti-inflammatory drug (NSAID) use; (b) denotes the difference in the annual rate of clinical upper GI events; (c) denotes the incremental costeffectiveness ratio, which is the additional cost per clinical upper GI event prevented. Strategies with smaller incremental ratios are more cost-effective (reproduced with permission). 10
3 REVIEW: PREVENTION OF NSAID GI COMPLICATIONS 1053 doses with low anti-inflammatory activity, such as ibuprofen, are less ulcerogenic than NSAIDs that achieve adequate analgesic effect only at doses with high anti-inflammatory activity (e.g. piroxicam). 11 Ibuprofen appears safe among other non-selective NSAIDs in part because it is frequently prescribed for temporary painful conditions thus limiting both duration and dose. 12 However, when full anti-inflammatory doses are given (e.g. 2.4 g/day), the risk of GI bleeding with ibuprofen is comparable with other NSAIDs. 13 For pain relief, we recommend an NSAID with a high analgesic effect and low anti-inflammatory action (e.g. ibuprofen) and the lowest effective dose should be used. Selective cyclo-oxygenase-2 (COX-2) inhibitors provide good analgesia with increased safety but at greater expense. For a temporary problem (e.g. toothache or headache) it is unlikely that the expense of COX-2 selective drugs justifies it use in terms of the safety benefit. For patients who require NSAIDs for inflammatory conditions, NSAIDs with short half-lives (e.g. indometacin) provide good control of acute arthritis (e.g. gout) whereas NSAIDs with longer halflives (e.g. naproxen) are preferred for chronic conditions (e.g. rheumatoid arthritis). If all things including costs were equal, we would only prescribe selective COX-2 inhibitors for any indication where an NSAID is needed. GASTROPROTECTIVE THERAPY Studies evaluating different strategies to prevent NSAIDinduced ulcers with antisecretory drugs, misoprostol, or other agents have used endoscopic ulcers as surrogates for clinical ulcer disease or for clinical events such as bleeding or perforation. Initially, an endoscopic ulcer was arbitrarily defined as a circumscribed mucosal break with a diameter of at least 5 mm and a perceptible depth. 14 The 5 mm definition with apparent depth definition was chosen to try to minimize confusion with NSAID erosions. The definition of 3 mm diameter ulcer was introduced as part of the strategy to obtain Food and Drug Administration (FDA) approval for a low dose (800 mg) of cimetidine for use in healing of H. pylori duodenal ulcers. Approval of that dose of cimetidine with that definition resulted in it becoming the new FDA criteria for ulcers. 15 However, endoscopists often may have difficulty in distinguishing between the presence of NSAID-induced discrete acute mucosal lesions that come and go rapidly and have no known correlation to chronic ulcers or clinical events and a clinically significant NSAID ulcer. Most recent studies of NSAID ulcer prevention have used the definition of an endoscopic ulcer as any lesion 3 mm or greater in diameter with or without apparent depth. The significance of such trivial lesion is uncertain and ulcers are over diagnosed. For example, a blinded review of the teaching tape for investigators participating in NSAID ulcer prevention trials showed that a high proportion of lesions scored as ulcers would not be scored as ulcers by experienced endoscopists. 16 Below we discuss the potential impacts of the use of endoscopic ulcers, erosions, and symptoms as end-points, either individually or as a composite score. H 2 -receptor antagonists In a pooled analysis of five randomized-controlled trials of H 2 -receptor antagonists for the prevention of gastroduodenal ulcers associated with NSAID use, it was found that standard doses of H 2 -receptor antagonists reduced the risk of duodenal but not gastric ulcers. 17 Data from three randomized trials suggested that double-dose H 2 -receptor antagonists are effective against NSAID-related duodenal and gastric ulcers although the majority of the effect was among those with a history of prior ulcers One study in the USA used a protocol identical to the European trial and failed to show a statistical benefit. It was only published as an abstract (possibly publication bias). 21 The reported efficacy of double-dose H 2 -receptor antagonists has also varied among the studies. In one study, 18 those whose ulcers were healed at 12 weeks were continued in a 24-week maintenance trial comparing famotidine and placebo. The rates of gastric ulcers at 24 weeks were 26% (52 per 100 patient years) and 53.5% (107 per 100 patient years) in the groups receiving double-dose famotidine and placebo, respectively. Post hoc subgroup analysis showed that the effect was essentially limited to those with H. pylori infection as the recurrence rate among those without H. pylori infection was 23.8% with placebo compared with 21.4% for those receiving famotidine. 15 Overall, the data suggested that the major benefit from H 2 -receptor antagonists might be limited to those with H. pylori infection and provided important information regarding critical subgrouping that should have been taken into account when designing the subsequent proton pump inhibitor (PPI) ulcer prevention trials.
4 1054 F. K. L. CHAN & D. Y. GRAHAM Proton pump inhibitors The potential role of PPIs for prevention of NSAIDinduced gastroduodenal damage has been evaluated in a number of large-scale, drug company sponsored, multicentre studies. None of these trials used clinical events as end-points and all used surrogate end-points often as composite end-points that included endoscopic ulcers, erosions and symptoms. The tremendous difference in antisecretory activity of the comparators (half dose misoprostol or standard dose ranitidine) essentially ensured that if acid sensitive symptoms were present [i.e. gastro-oesophageal reflux disease (GERD) or GERDlike] that the PPI would appear superior. Two placebocontrolled endoscopic studies assessed the efficacy of omeprazole for the prevention of gastroduodenal ulcers associated with NSAIDs. 22, 23 The reported incidences of ulcer ranged from 7.2 to 18.8 per 100 patient years in the omeprazole group compared with per 100 patient years in the placebo group. Importantly, the proportion developing ulcers among those without H. pylori infection and no history of ulcer disease was 3% among omeprazole user compared with 2.6% among those receiving placebo. In the ASTRONAUT study 24 and the OMNIUM study, 25 omeprazole was compared with standard dose ranitidine (150 mg b.d.) (which parenthetically had repeatedly been shown to be ineffective for NSAID ulcer prevention 26 ) and to half dose misoprostol (200 lg b.d.) (equivalent to approximately 600 mg of cimetidine as an antisecretory drug) over a period of 24 weeks. The definition of an ulcer was a lesion >3 mm diameter. The primary end-point was a composite of endoscopic ulcers, multiple erosions and severe dyspepsia (mostly heartburn where the PPI was likely to be superior to either misoprostol or standard dose ranitidine). The investigators did not report detailed results with H. pylori-positive and -negative separately despite the evidence from the H 2 -receptor antagonist trials that was likely to be critical. 15 In the ASTRONAUT study, 24 28% (56 per 100 patient years) of patients in the omeprazole group compared with 41% (82 per 100 patient years) of patients in the ranitidine group had treatment failure. Post hoc subgroup analyses of the development of ulcer among those with and without H. pylori infection showed that the majority of the advantage among omeprazole users was in the H. pylori infected patients. 27 In fact, half dose misoprostol proved significantly superior to omeprazole for both healing of gastric ulcers and prevention of gastric ulcers among those without H. pylori infection. 27 There was a significant advantage for omeprazole over standard dose ranitidine among those with H. pylori infection but no statistical advantage among the H. pylori-negative patients. The advantage of omeprazole vs. ranitidine for prevention of gastric ulcer was 10.5% vs. 14.6% (21 vs. 30 per 100 patient years) and for duodenal ulcer was 1.2% vs. 2.1% (2.4 vs. 4.2 per 100 patient years). 27 Based on the data from the H. pylori-negative patients, the sample size to show a statistical advantage for the PPI over standard dose ranitidine would require more than 1800 patients for an outcome of both duodenal ulcer (DU) and gastric ulcer (GU), and more than 2200 for the outcome of GU alone. Planning head-to-head studies with fewer patients would likely risk failure to achieve a statistical advantage. The only potential advantage of H 2 -receptor antagonists has been cost. Whether these small differences are clinically important are another matter but the marked reduction in price for PPI expected now that the patent for omeprazole has expired may make cost differences moot. Misoprostol A meta-analysis of 33 randomized-controlled clinical trials of misoprostol, H 2 -receptor antagonists and PPIs revealed that all three classes of drugs reduce the incidence of NSAIDs-related gastroduodenal ulcers. 17 Only standard dose misoprostol (200 lg q.d.s.) has been tested for its ability to reduce NSAID-related ulcer complications 9 and it proved partially effective (approximately 40% reduction). However, side-effects such as abdominal cramps and diarrhoea were common such that the median dose in that study was only about 600 lg. Misoprostol provides physiologic replacement therapy and its effects extend beyond the stomach. Thus, it is theoretically better than therapy aimed at only reducing acid secretion. The fact that NSAIDs can produce ulcers and ulcer complications in patients with achlorhydria is rarely discussed but suggests that prostaglandin replacement therapy would be ideal approach if it could be offered in a way to minimize side-effects. 28 The effectiveness of misoprostol over standard and double doses of PPIs has been confirmed in a study 29 of standard dose misoprostol (200 lg q.d.s.) with two doses of lansoprazole (15 and 30 mg daily) among H. pylori-negative chronic NSAID users who had a history of gastric ulcer. Misoprostol proved superior
5 REVIEW: PREVENTION OF NSAID GI COMPLICATIONS 1055 for prevention of gastroduodenal ulcer. For example, at 12 weeks, 93% of patients in the misoprostol group compared with 80 82% of patients in the two groups receiving lansoprazole were protected from gastric ulcers. The ulcer rates were 15, 43, 47 per 100 patient years, for misoprostol, lansoprazole 15 mg, and 30 mg, respectively. However, because of the higher withdrawal rate in the misoprostol group, there was no practical advantage of misoprostol over lansoprazole. However, it is impossible to prospectively identify those who will experience symptoms from misoprostol making a clinical trial the only practical method of identifying those in whom it might be the preferred drug. Because misoprostol and antisecretory agents act via different mechanisms, it is possible that the combination of half dose misoprostol and an H 2 -receptor antagonist or a PPI (e.g. generic omeprazole) would provide inexpensive yet effective preventive therapy for high-risk patients. 27 The fact that the majority of patients took misoprostol without problems in the large clinical trials suggests that individual patients and not physicians should decide whether it can be used successfully. COX-2 INHIBITORS The discovery of a second isoform of COX-2 led to the rapid development and marketing of highly selective COX-2 inhibitors as gastric sparing anti-inflammatory analgesics and anti-inflammatory agents. There is good evidence that this new class of NSAIDs causes minimal endoscopic gastric damage. 30, 31 The extent to which these endoscopic findings translate into clinical benefits remains unclear. Two large-scale clinical outcome studies, CLASS 32 and VIGOR, 33 attempted to evaluate the GI safety of celecoxib and rofecoxib, respectively. The CLASS study was initially designed as a 12-month study of celecoxib vs. diclofenac or ibuprofen. Analysis of the first 6 months data showed that celecoxib was superior to non-selective NSAIDs among patients who did not receive concomitant low-dose aspirin. However, the long-term outcome of CLASS subsequently presented at the US FDA hearings failed to confirm a significant advantage of celecoxib over the comparators. Whether the failure of CLASS was due to flaws in the study design (for example, preferential dropout of patients receiving non-selective NSAIDs), some ulcerogenicity of celecoxib, or inclusion of patients with H. pylori ulcers or ulcer complications, remains uncertain. Unlike CLASS, VIGOR showed that patients receiving rofecoxib had a significantly lower incidence of clinical UGI events compared with patients receiving naproxen. However, significantly lower did not approximate the desired none. In the VIGOR study, patients requiring low-dose aspirin were excluded. This was in marked contrast to the CLASS study where 20% of patients received concomitant low-dose aspirin. Given the low incidence of events in these studies, the fact that a 1 2% incidence of events was to be expected among aspirin users, and that patients with prior H. pylori ulcers were entered, it is not surprising that there was difficulty showing superiority over any comparator. Part of the reason for poor designs was related to the pharmaceutical company not wanting a restricted claim (e.g. patients with no history of H. pylori ulcers, ulcer complications, not taking aspirin). Post hoc subgroup analysis 34 showed that patients with prior UGI events did not have a significant reduction in the incidence of new ulcer complications despite use of the COX-2 inhibitor, rofecoxib, instead of naproxen. This result is consistent with the fact that patients with prior ulcer complications either caused by H. pylori or NSAIDs represent a special high-risk group (e.g. the one where one would most prefer to use selective COX-2 inhibitors). The fact that these patients represent a special group was emphasized by a recent randomized, GI outcome study of celecoxib vs. omeprazole plus diclofenac in patients with a recent history of ulcer bleeding, 4.9% (9.8 per 100 patient years) of patients in the celecoxib group compared with 6.4% (12.8 per 100 patient years) in the omeprazole plus diclofenac group had recurrent ulcer bleeding. 35 Although the two treatments were comparable, the study could not address whether either was superior to placebo and neither strategy was sufficiently effective to be recommended for these very high-risk patients. In addition, up to 30% of these high-risk patients developed renal adverse events including hypertension, fluid retention and renal failure with either treatment. There was also one small bowel perforation resulting in a death in the omeprazole plus diclofenac group. 35 ROLE OF HELICOBACTER PYLORI INFECTION There are studies reporting that H. pylori infection increases, has no effect on, or even decreases the risk of NSAID-related ulcers. One reason for these conflicting findings is fact that the studies have rarely taken the natural history of H. pylori infection into account nor
6 1056 F. K. L. CHAN & D. Y. GRAHAM have the pre-existing risks been considered, stratified for, or specifically acknowledged. The risk for developing an ulcer among those with latent H. pylori infection is approximately 1% per year (1 per 100 patient years) independent of NSAID use. The risk of a GI complication among those with H. pylori ulcers who have never experienced a complication is also in the range of 1 per 100 patient years. Therefore, the risk for a new H. pylori ulcer complication among those with latent H. pylori infection is <1 per 1000 patient years and would remain invisible in most clinical trials. 36 In marked contrast, % of those with a history of H. pylorirelated ulcer will develop a recurrent ulcer within any 1 year (especially if evaluated by endoscopy) irrespective of NSAID use. NSAID users who had prior 36, 37 H. pylori ulcer disease would therefore be expected to develop ulcers during follow-up and, as it would be impossible to distinguish whether the ulcer was related to the NSAID use, the H. pylori infection, or to an interaction, entry of such patients only serve to bias the outcome. Ulcer complications among those with H. pylori ulcers are expected in 1 2% per year. 36 Thus, inclusion of H. pylori ulcer patients would result in an increase in the number of new complications unrelated to NSAID use and provide another source of major bias. Thus, inclusion of patients with prior H. pylori ulcers can only serve as a source of potential bias that can also markedly and potentially erroneously influence the results (see later). Any H. pylori NSAID interaction would also accentuate the number of events and complicate the interpretations. Patients at highest risk of developing an ulcer complication are those who have experienced an ulcer complication in the past. The risk of a new ulcer complication after one complication in H. pylori ulcers is in the range of 1 3% per month (12 36 per 100 patient 36, 38, 39 years) even without exposure to NSAIDs. Therefore, such patients must be excluded unless this issue is being specifically studied. The inclusion of these patients has resulted in them being repeatedly identified as the group at highest risk, which was neither useful nor new information in relation to NSAID risk. Importantly, their inclusion will predictably bias the outcome and can easily lead to misleading conclusions regarding the safety of a particular compound, the effectiveness of the gastroprotective therapy, or conversely, the comparative value of different therapies. The issue is further complicated as data suggest that patients with H. pylori infection who start NSAIDs are at a higher risk of ulcer than H. pylori-infected patients who are already on long-term NSAIDs. 40, 41 Eradication of H. pylori before starting NSAIDs has been shown to reduce the subsequent risk of ulcer (e.g. in one study from 26% to 7% over a period of 8 weeks). 40 Among patients with H. pylori infection who were already on long-term NSAIDs, however, curing the infection was not effective for prevention of NSAID ulcers 41 possibly because a prior bad experiences with NSAIDs may have served to identify those at special risk (e.g. those with latent H. pylori ulcers) and to have eliminated them. Eradication of H. pylori is not expected to greatly affect recurrence of NSAID ulcers among NSAID users. For example, the first studies of anti-h. pylori therapy showed that ulcer recurrence was eliminated by H. pylori eradication except in those where there was failure to cure the infection or where there was use of NSAIDs. 37 Thus, it is not surprising that eradication of H. pylori among chronic NSAID users would not affect the incidence of NSAID ulcers among those free of ulcer at the start of a clinical trial. ASPIRIN, AN NSAID WITH SPECIAL PROBLEMS Aspirin has been increasingly used for prevention of cardiovascular events and is now probably the most commonly used NSAID. An estimated of 50 million Americans have started taking aspirin over the past two decades. 42 The decision regarding whether the risks of GI haemorrhage with aspirin are clinically acceptable depends on an estimation of the expected benefits compared with the risks of vascular events. In a meta-analysis of the risk of GI bleeding (severity undefined) with long-term use of low-dose aspirin, it was estimated that about one in 248 subjects would develop GI bleeding with aspirin per year. 43 In the secondary prevention of stroke, the number needed to treat with aspirin to prevent a further cardiovascular event was only which means that more than two recurrent strokes could be prevented with aspirin at the cost of one GI bleed. By contrast, the number needed to treat for the primary prevention of myocardial infarction per year ranged from 555 in the US Physicians Health Study 45 to 794 in hypertensive patients. 46 Thus, in patients at low risk for cardiovascular events, aspirin use could cause five to seven GI bleeds for each episode of myocardial infarction prevented. The US Food and Drug Administration recently reviewed aspirin for primary prevention and
7 REVIEW: PREVENTION OF NSAID GI COMPLICATIONS 1057 declined to approve it. 47 There was a significant (approximately 25%) reduction in non-fatal myocardial infarction, no reduction in deaths and a slight increase in haemorrhagic stroke. This contrasts with secondary prevention where strokes, deaths, and myocardial infarctions were all reduced. 47 No dose of aspirin is free of bleeding risk. Even at a dose as low as 75 mg/day, the risk of UGI bleeding is two times higher than among non-users. 48 Recent post hoc analysis of observational studies examined the effect after grouping doses as 200, > and <100 mg/day among aspirin users randomized to receive clopidogrel or placebo in the treatment of acute coronary syndrome. 49 Although, the study was post hoc and observational, the data were internally consistent suggesting that the lowest dose was safer than high doses; mg are recommended. Long-term prevention trials with highly selected patients have reported severe bleeding or death to be in the range of per 100 patient years. This appears to be about 10-fold less than any bleeding. In contrast, the risk of a potentially life-threatening bleeding event in the CURE study 49 mentioned above was considerably greater (1.9 per 100 patient years with <100 mg to 3.9 per 100 patient years in those receiving mg of aspirin). These are at least 10-fold greater than seen in the long-term aspirin prevention trials and are more consistent with use in clinical practice. One should probably expect somewhere between the two extremes probably closer to 1% (1 per 100 patient years than to 0.3 per 100 patient years). Because of its widespread use and its effect on platelets, aspirin use must be considered separately when planning prevention strategies (Table 1). STRATEGIES FOR PREVENTION OF NSAID- RELATED ULCER COMPLICATIONS Management of low-risk patients Patients without risk factors are at low, but not insignificant, risk of ulcer complications with NSAID use. Because of the vast number of consumers, this group constitutes the majority of cases of NSAID-associated ulcer complications each year. The most cost-effective approach for prevention of ulcer complications in this low-risk category is rational use of NSAIDs. As mentioned earlier, a substantial proportion of cases of ulcer complications could be avoided by using simple analgesics or NSAIDs with low ulcerogenic potentials, avoiding high dose or multiple (prescription and non-prescription) NSAIDs. COX-2 inhibitors are safer than the least ulcerogenic conventional NSAIDs such as ibuprofen and diclofenac. However, the high cost of COX-2 inhibitors renders these drugs not cost-effective especially for short-term treatment of low-risk individuals. Management of moderate-risk patients This category consists of patients with one or two risk factors who are often elderly. We recommend the combination of a non-selective NSAID and misoprostol Table 1. Group Strategies recommended by authors Remarks Low-risk (no risk factors) Moderate-risk (one to two risk factors)* High-risk (3 risk factors or concomitant aspirin, steroids, or warfarin) Very high-risk patients (history of recent ulcer complications) Least ulcerogenic NSAIDs at lowest effective doses Least ulcerogenic NSAID plus an antisecretory agent or misoprostol COX-2 inhibitor COX-2 inhibitor plus PPI or misoprostol for concomitant aspirin COX-2 inhibitor plus misoprostol for concomitant warfarin COX-2 inhibitor for concomitant steroids Avoid NSAIDs all together COX-2 inhibitor plus PPI and/or misoprostol PPI is preferred to H 2 -receptor antagonist Misoprostol 200 lg t.i.s. is recommended Recommendations are based on post hoc subgroup analysis and preliminary data only Avoiding NSAIDs is the best approach Recommendation not tested clinically * Old age, presence of cardiovascular diseases, use of high dose or multiple NSAIDs, concomitant use of low-dose aspirin and other antiplatelet drugs, steroids or warfarin. NSAID, non-setroidal anti-infalmmatory drug; COX-2, cyclo-oxygenase-2; PPI, proton pump inhibitors.
8 1058 F. K. L. CHAN & D. Y. GRAHAM or an antisecretory agent. Alternatively, substitution for a COX-2 inhibitor alone is probably as effective as the combination therapy. The former has the advantage of taking fewer tablets. Emerging data suggest that treatment with a COX-2 inhibitor causes less lower GI complications than treatment with an 35, 50 NSAID plus an antisecretory agent. The magnitude of this problem is unclear and likely to be quite low. Whether it will eventually turn out to be more than a marketing point used to help differentiate drug remains uncertain. Management of specific groups of high-risk patients This category consist of patients with multiple risk factors or patients using concomitant low-dose aspirin, steroids, or anticoagulants. In general, NSAIDs should be avoided in these patients not only because of the high risk of ulcer complications but also due to the serious consequence of ulcer complications in the presence of comorbidities. Patients receiving concomitant aspirin. Aspirin at any dose increases the risk of ulcer complications, which is further increased by the presence of an H. pylori infection. It is not clear whether low-dose aspirin adds significantly to the risk of chronic NSAID therapy with non-selective NSAIDs. In contrast, there are data suggesting that chronic NSAID use may reduce the effectiveness of aspirin for cardiovascular protection. 51 The best data for an interaction comes from studies of interactions with ibuprofen. 52 In addition, post hoc subgroup analyses of the Physicians Health Study suggested that chronic use of traditional NSAIDs was associated with a reduction in aspirin s cardiovascular protective benefits. 51 We anticipate many more studies where pharmaceutical companies test their individual NSAID for this interaction in that lack of an effect provides a marketing advantage. The selective COX-2 inhibitors do not have this interaction and are therefore preferred for those who need secondary cardiovascular prophylaxis with aspirin and an NSAID. Unfortunately, as aspirin carries its own risks for ulcer and ulcer complications, gastric damage may occur despite the gastric sparing effect of COX-2 inhibitors. The best course of action remains unclear and as always depends on an analysis of benefits, risks and costs. For higher risk patients in whom a significant GI event would be expected to aggravate myocardial or cerebral ischaemia, we recommend the combination of a COX-2 inhibitor, low-dose aspirin (<100 mg) and a PPI or misoprostol if concomitant anti-inflammatory and antiplatelet therapies are both required. The recent introduction of generic omeprazole in the United States should result in costs becoming less of an issue. As noted above, co-therapy with misoprostol and aspirin looks very good in clinical trial but has not been evaluated in the setting where it would be of most benefit. Nitrous oxide donating aspirin (NO-aspirin) offers a theoretical advantage but has also not yet proven to be effective clinically. 53, 54 There is evidence that other antiplatelet agents such as ticlopidine or clopidogrel carry a higher risk of GI bleeding than aspirin at doses of <100 mg/day such that substitution of low-dose aspirin for other antiplatelet agents is not 49, 55 recommended. Patients receiving concomitant steroids or anticoagulants. Few clinical conditions require concomitant use of steroids and NSAIDs since short-term steroid therapy or disease-modifying drugs can usually control inflammatory arthritis. Nonetheless, concomitant use of steroids and NSAIDs is not rare and often consists of selfmedication with over-the-counter drugs containing aspirin or NSAIDs. Patients should be warned to avoid taking these drugs while taking prescription NSAIDs. Post hoc subgroup analysis of VIGOR suggested that concomitant steroid use did not increase the risk of clinical UGI events among patients receiving rofecoxib. 34 While this result suggests that COX-2 inhibitors are a potential alternative to conventional NSAIDs along with an antisecretory drug or misoprostol for patients receiving concomitant steroid therapy, the results of post hoc subgroup analyses must always be confirmed in prospective randomized-controlled clinical trials. It is prudent to always be somewhat wary regarding the conclusions of post hoc subgroup analyses dredged from large pharmaceutical trials in part because of the stringent entry criteria usually selects good risk patients that are often markedly different from the high-risk populations in whom the physician needs help. In addition, because many post hoc subgroup analyses are undergone, the companies are assured of obtaining some with positive and some with negative results. Generally, only the associations that are positive for the drug being tested are reported and we are essentially never given the number of comparisons done to find the reported association. Negative associations are also
9 REVIEW: PREVENTION OF NSAID GI COMPLICATIONS 1059 rarely reported robbing the physician of knowledge of subgroups where the new drug might not be the best choice. Studies based on healthy volunteers suggested there is no clinically important interaction between COX-2 inhibitors and anticoagulants. 56, 57 Patients who receive anticoagulants usually have serious medical conditions such as prosthetic heart valve replacement or deep vein thrombosis, such that the consequences of GI bleeding or withholding anticoagulants is potentially disastrous and NSAIDs including COX-2 inhibitors should be avoided. In rare occasions that long-term anti-inflammatory therapy is required, we recommend the combination of a COX-2 inhibitor and misoprostol. Misoprostol is preferred to antisecretory drugs because anticoagulants can provoke bleeding from pre-existing ulcers along with the whole GI tract. Theoretically, the combination of a COX-2 inhibitor and misoprostol reduces the risk of both upper and lower GI bleeding. Management of very high-risk patients Patients with prior ulcer complications. As noted earlier, patients with a history of ulcer complications have the highest risk of recurrent ulcer bleeding. 8 This also holds true for NSAID users. The outcome may depend in part on the cause of the ulcer disease and the time after ulcer healing and, in those with H. pylori infection, the duration since H. pylori eradication. In one study, about 19% (38 per 100 patient years) of NSAID users with H. pylori infection and a recent history of ulcer bleeding developed recurrent bleeding with naproxen in 6 months after eradication of H. pylori, which is within the 12 36% range expected from prior studies with H. pylori ulcers. 56 Current strategies for prevention of ulcer complications in these high-risk NSAID users include substitution of COX-2 inhibitors for non-selective NSAIDs and co-therapy of NSAIDs with a PPI, misoprostol, or both. As noted earlier, when these empiric recommendations were subjected to a randomized, head-to-head comparison of celecoxib vs. diclofenac plus omeprazole, neither strategy proved effective for eliminating the risk of recurrent bleeding in patients with prior ulcer bleeding. In that study the estimated annualized incidence of recurrent bleeding was about 10%. 35 That study emphasized that the current recommendations were not based on evidence but were based on the surrogate end-points from the endoscopic ulcer trials. The conclusion of trials using surrogate and end-points that potentially can be easily manipulated end-points are increasingly suspect with regard to applicability to clinical situations. We conclude that very high-risk patients should avoid NSAIDs. If shortterm anti-inflammatory therapy is required for acute, self-limiting arthritis (e.g. gout), we recommend the use of steroids, since steroids alone do not increase the ulcer risk. If regular anti-inflammatory therapy is required for chronic arthritis, the combination of a COX-2 inhibitor and misoprostol and possibly a PPI probably offers the best GI protection although this approach remains to be examined in prospective trials. CONCLUSION Ironically, despite the availability of potent anti-ulcer drugs and new developments in anti-inflammatory therapies, the incidence of NSAID-related morbidity and mortality remains high. Failure to take into account risk factors, inappropriate use of NSAIDs, and lack of anti-ulcer prophylaxis in high-risk patients account for a substantial proportion of preventable NSAID-related ulcer complications. The importance of education for primary care doctors and patients cannot be over-emphasized. Contrary to the findings in low- to average-risk individuals, current evidence suggests that neither co-therapy with an anti-ulcer drug or substitution of a COX-2 inhibitor for a traditional NSAID is a safe strategy for management of very high-risk patients or those with multiple-risk factors. Studies are needed to evaluate the gastric safety of anti-ulcer drugs and COX- 2 inhibitors in different risk groups using clinical outcomes rather than endoscopic mucosal injury as end-point. Since H. pylori significantly augments the efficacy of PPIs, the true efficacy of PPIs for prevention of NSAID-related ulcer complications must be reassessed without the confounding influence of H. pylori. ACKNOWLEDGMENTS AND POTENTIAL CONFLICTS OF INTEREST This material is based upon work supported in part by the Office of Research and Development Medical Research Service, Department of Veterans Affairs and by Public Health Service grant DK56338, which funds the Texas Gulf Coast Digestive Diseases Center. In the last 3 years, Dr Graham has received research support or honoraria for speaking engagements from AstraZen-
10 1060 F. K. L. CHAN & D. Y. GRAHAM eca, TAP, Pharmacia, Meretek, Otsuka, Prometheus and EISAI. In addition, Dr Graham is a paid consultant for Prometheus, Meretek and Otsuka. Dr Chan received a consulting fee from Pfizer in REFERENCES 1 Singh G, Triadafilopoulus G. Epidemiology of NSAIDinduced GI complications. J Rheumatol 1999; 26(Suppl. 26): Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in pepticulcer disease: a meta-analysis. Lancet 2002; 359: Graham DY. Celecoxib versus diclofenac and omeprazole to prevent recurrent ulcer bleeding Dr Graham replies [letter]. N Engl J Med 2003; 348: Griffin MR, Piper JM, Daugherty JR, et al. Nonsteroidal antiinflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Ann Intern Med 1991; 114: Gabriel SE, Jaakkimainen L, Bombardier C. Risks for serious gastrointestinal complications related to the use of nonsteroidal anti-inflammatory drugs: a meta-analysis. Ann Intern Med 1991; 115: Langman MJS, Weil J, Wainwright P, et al. Risks of bleeding peptic ulcer associated with individual non-steroidal antiinflammatory drugs. Lancet 1994; 343: Henry D, Lim LL, Rodriguez LAG, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative metaanalysis. BMJ 1996; 312: Rodriguez LAG, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal antiinflammatory drugs. Lancet 1994; 343: Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1995; 123: El-Serag HB, Graham DY, Richardson P, Inadomi JM. Prevention of complicated ulcer disease among chronic users of nonsteroidal anti-inflammatory drugs: the use of a nomogram in cost-effectiveness analysis. Arch Intern Med 2002; 162: Graham DY. Nonsteroidal anti-inflammatory drugs, Helicobacter pylori, and ulcers: where we stand. Am J Gastroenterol 1996; 91: LeLorier J. Patterns of prescription of nonsteroidal antiinflammatory drugs and gastroprotective agents. J Rheumatol Suppl. 1995; 43: Graham DY. NSAID ulcers: prevalence and prevention. Mod Rheumatol 2000; 10: Larkai EN, Smith JL, Lidsky MD, Graham DY. Gastroduodenal mucosa and dyspeptic symptoms in arthritic patients during chronic nonsteroidal anti-inflammatory drug use. Am J Gastroenterol 1987; 82: Graham DY. High-dose famotidine for prevention of NSAID ulcers? [editorial; comment]. Gastroenterology 1997; 112: Sung JY, Lau JY, Chan FK, Graham DY. How often are endoscopic ulcers in NSAID trials diagnosed as actual ulcers by experienced endoscopists [Abstract]. Gastroenterology 2001; 120(Suppl. 1): A Rostom A, Dube C, Wells G et al. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst Rev 2002; 4: CD Hudson N, Taha AS, Russell RI, et al. Famotidine for healing and maintenance in nonsteroidal anti-inflammatory drugassociated gastroduodenal ulceration. Gastroenterology 1997; 112: ten Wolde S, Dijkmans BA, Janssen M, Hermans J, Lamers CB. High-dose ranitidine for the prevention of recurrent peptic ulcer disease in rheumatoid arthritis patients taking NSAIDs. Aliment Pharmacol Ther 1996; 10: Taha AS, Hudson N, Hawkey CJ, et al. Famotidine for the prevention of gastric and duodenal ulcers caused by nonsteroidal anti-inflammatory drugs. N Engl J Med 1996; 334: Simon TJ, Berger ML, Hoover ME, Stauffer LA, Berline RG. A dose ranging study of famotidine in prevention of gastroduodenal lesions associated with non-steroidal anti-inflammatory drugs (NSAIDs): results of a US multicenter trial [Abstract]. Am J Gastroenterol 1994; 89: Ekstrom P, Carling L, Wetterhus S, et al. Prevention of peptic ulcer and dyspeptic symptoms with omeprazole in patients receiving continuous non-steroidal anti-inflammatory drug therapy. A Nordic Multicentre Study. Scand J Gastroenterol 1996; 31: Cullen D, Bardhan DK, Eisner M, et al. Primary gastroduodenal prophylaxis with omeprazole for non-steroidal antiinflammatory drug users. Aliment Pharmacol Ther 1998; 12: Yeomans ND, Tulassay Z, Juhasz L, et al. Omeprazole compared with ranitidine for ulcers associated with nonsteroidal antiinflammatory drugs. N Engl J Med 1998; 338: Hawkey CJ, Karrasch JA, Szczepanski L, et al. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. N Engl J Med 1998; 338: Ehsanullah RS, Page MC, Tildesley G, Wood JR. Prevention of gastroduodenal damage induced by non-steroidal antiinflammatory drugs: controlled trial of ranitidine. BMJ 1988; 297: Graham DY. Critical effect of Helicobacter pylori infection on the effectiveness of omeprazole for prevention of gastric or duodenal ulcers among chronic NSAID users. Helicobacter 2002; 7: Janssen M, Dijkmans BA, Vandenbroucke JP, Biemond I, Lamers CB. Achlorhydria does not protect against benign upper gastrointestinal ulcers during NSAID use. Dig Dis Sci 1994; 39: Graham DY, Agrawal NM, Campbell DR, et al. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory
11 REVIEW: PREVENTION OF NSAID GI COMPLICATIONS 1061 drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs. lansoprazole. Arch Intern Med 2002; 162: Simon LS, Weaver AL, Graham DY, et al. Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA 1999; 282: Laine L, Harper S, Simon T, et al. A randomized trial comparing the effect of rofecoxib, a cyclooxygenase 2-specific inhibitor, with that of ibuprofen on the gastroduodenal mucosa of patients with osteoarthritis. Rofecoxib Osteoarthritis Endoscopy Study Group. Gastroenterology 1999; 117: Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis. JAMA 2000; 284: Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med 2000; 343: Laine L, Bombardier C, Hawkey CJ, et al. Stratifying the risk of NSAID-related upper gastrointestinal clinical events: results of a double-blind outcomes study in patients with rheumatoid arthritis. Gastroenterology 2002; 123: Chan FK, Hung LC, Suen BY, et al. Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N Engl J Med 2002; 347: Graham DY. Ulcer complications and their nonoperative treatment. In: Sleisenger, MH, Fordtran, JS, eds. Gastrointestinal Disease. Pathophysiology, Diagnosis, Management, 5th edn. Philadelphia: WB Saunders Co, 1993: Graham DY, Lew GM, Klein PD, et al. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med 1992; 116: Graham DY, Hepps KS, Ramirez FC, Lew GM. Treatment of H. pylori reduces the rate of rebleeding in complicated peptic ulcer disease. Scand J Gastroenterol 1993; 28: Labenz J, Borsch G. Role of Helicobacter pylori eradication in the prevention of peptic ulcer bleeding relapse. Digestion 1994; 55: Chan FK, Sung JJ, Chung SC, et al. Randomised trial of eradication of Helicobacter pylori before non-steroidal anti-inflammatory drug therapy to prevent peptic ulcers. Lancet 1997; 350: Hawkey CJ, Tulassay Z, Szczepanski L, et al. Randomised controlled trial of Helicobacter pylori eradication in patients on non-steroidal anti-inflammatory drugs: HELP NSAIDs study. Helicobacter Eradication for Lesion Prevention. Lancet 1998; 352: Bayer Pharmaceuticals. Facts About Aspirin. fact sheet.pdf, accessed 28 July Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. BMJ 2000; 321: Swedish Aspirin Low-Dose Trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. The SALT Collaborative Group. Lancet 1991; 338: Final Report on the Aspirin Component of the Ongoing Physicians Health Study. Steering Committee of the Physicians Health Study Research Group. N Engl J Med 1989; 321: Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomized trial. Lancet 1998; 351: htm, accessed 8 December Weil J, Colin-Jones D, Langman M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ 1995; 310: Peters RJ, Mehta SR, Fox KA, et al. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the clopidogrel in unstable angina to prevent recurrent events (CURE) study. Circulation 2003; 108: Laine L, Connors LG, Reicin A, et al. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology 2003; 124: Kurth T, Glynn RJ, Walker AM, et al. Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal antiinflammatory drugs. Circulation 2003; 108: Catella-Lawson F, Reilly MP, Kapoor SC, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med 2001; 345: Fiorucci S, Santucci L, Gresele P, Faccino RM, Del Soldato P, Morelli A. Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study. Gastroenterology 2003; 124: Fiorucci S, Del Soldato P. NO-aspirin: mechanism of action and gastrointestinal safety. Dig Liver Dis 2003; 35(Suppl. 2): S Ng FH, Wong SY, Chang CM, et al. High incidence of clopidogrel-associated gastrointestinal bleeding in patients with previous peptic ulcer disease. Aliment Pharmacol Ther 2003; 18: Schwartz JI, Bugianesi KJ, Ebel DL, et al. The effect of rofecoxib on the pharmacodynamics and pharmcokinetics of warfarin. Clin Pharmacol Ther 2000; 68: Karim A, Tolbert D, Piergies A, et al. Celecoxib does not significantly alter the pharmacokinetics or hypoprothrombinemic effect of warfarin in healthy subjects. J Clin Pharmacol 2000; 40:
A. Ketorolac*** B. Naproxen C. Ibuprofen D. Celecoxib
 1. A man, 66 years of age, with a history of knee osteoarthritis (OA) is experiencing increasing pain at rest and with physical activity. He also has a history of depression and coronary artery disease.
1. A man, 66 years of age, with a history of knee osteoarthritis (OA) is experiencing increasing pain at rest and with physical activity. He also has a history of depression and coronary artery disease.
Articles Presented. Journal Presentation. Dr Albert Lo. Dr Albert Lo
 * This presentation is prepared by the author in one s personal capacity for the purpose of academic exchange and does not represent the views of his/her organisations on the topic discussed. Journal Presentation
* This presentation is prepared by the author in one s personal capacity for the purpose of academic exchange and does not represent the views of his/her organisations on the topic discussed. Journal Presentation
What s the Deal with NSAIDs?
 Focus on CME at the Memorial xxx University of Newfoundland What s the Deal with NSAIDs? Majed M. Khraishi, MB, BCh, FRCPC Presented at Wednesday at Noon Ask the Consultant Arthritis is one of the most
Focus on CME at the Memorial xxx University of Newfoundland What s the Deal with NSAIDs? Majed M. Khraishi, MB, BCh, FRCPC Presented at Wednesday at Noon Ask the Consultant Arthritis is one of the most
What can I eat? Peptic ulcers. What are peptic ulcers? What tests are needed? Will the ulcer come back? What causes a peptic ulcer?
 In association with: INFORMATION ABOUT Peptic ulcers www.corecharity.org.uk What are peptic ulcers? What causes a peptic ulcer? How are NSAIDs and aspirin involved? How do I know if I ve got an ulcer?
In association with: INFORMATION ABOUT Peptic ulcers www.corecharity.org.uk What are peptic ulcers? What causes a peptic ulcer? How are NSAIDs and aspirin involved? How do I know if I ve got an ulcer?
understanding GI bleeding
 understanding GI bleeding a consumer education brochure American College of Gastroenterology 4900B South 31st Street, Arlington, VA 22206 703-820-7400 www.acg.gi.org American College of Gastroenterology
understanding GI bleeding a consumer education brochure American College of Gastroenterology 4900B South 31st Street, Arlington, VA 22206 703-820-7400 www.acg.gi.org American College of Gastroenterology
Canadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risks
 Alimentary Pharmacology & Therapeutics Canadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risks A. ROSTOM*, P. MOAYYEDI
Alimentary Pharmacology & Therapeutics Canadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risks A. ROSTOM*, P. MOAYYEDI
Aspirin to Prevent Heart Attack and Stroke: What s the Right Dose?
 The American Journal of Medicine (2006) 119, 198-202 REVIEW Aspirin to Prevent Heart Attack and Stroke: What s the Right Dose? James E. Dalen, MD, MPH Professor Emeritus, University of Arizona, Tucson
The American Journal of Medicine (2006) 119, 198-202 REVIEW Aspirin to Prevent Heart Attack and Stroke: What s the Right Dose? James E. Dalen, MD, MPH Professor Emeritus, University of Arizona, Tucson
Alimentary Pharmacology & Therapeutics
 Alimentary Pharmacology & Therapeutics Changing perceptions and practices regarding aspirin, nonsteroidal anti-inflammatory drugs, and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs among
Alimentary Pharmacology & Therapeutics Changing perceptions and practices regarding aspirin, nonsteroidal anti-inflammatory drugs, and cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs among
Gloucestershire Hospitals
 TRUST GUIDELINE In the case of hard copies of this policy the content can only be assured to be accurate on the date of issue marked on the document. The Policy framework requires that the policy is fully
TRUST GUIDELINE In the case of hard copies of this policy the content can only be assured to be accurate on the date of issue marked on the document. The Policy framework requires that the policy is fully
NIMULID MD. 1. Introduction. 2. Nimulid MD Drug delivery system
 NIMULID MD 1. Introduction Nimulid MD is a flavoured dispersible Nimesulide tablet with fast mouth dissolving characteristics thereby providing immediate relief. Nimesulide is a non-steroidal antiinflammatory
NIMULID MD 1. Introduction Nimulid MD is a flavoured dispersible Nimesulide tablet with fast mouth dissolving characteristics thereby providing immediate relief. Nimesulide is a non-steroidal antiinflammatory
Guidelines for Prevention of NSAID-Related Ulcer Complications
 728 ACG PRACTICE GUIDELINES nature publishing group Guidelines for Prevention of NSAID-Related Ulcer Complications Frank L. L anza, MD, FACG 1,2, Fr anc is K.L. C han, M D, FRCP, FACG 3, E amonn M.M. Q
728 ACG PRACTICE GUIDELINES nature publishing group Guidelines for Prevention of NSAID-Related Ulcer Complications Frank L. L anza, MD, FACG 1,2, Fr anc is K.L. C han, M D, FRCP, FACG 3, E amonn M.M. Q
National Institute for Clinical Excellence
 National Institute for Clinical Excellence 11 Strand London WC2N 5HR Web: www.nice.org.uk N0016 50k 1P July 01 (ABA) Technology Appraisal Guidance - No.27 Guidance on the use of cyclo-oxygenase (Cox) II
National Institute for Clinical Excellence 11 Strand London WC2N 5HR Web: www.nice.org.uk N0016 50k 1P July 01 (ABA) Technology Appraisal Guidance - No.27 Guidance on the use of cyclo-oxygenase (Cox) II
New Anticoagulants and GI bleeding
 New Anticoagulants and GI bleeding DR DANNY MYERS MD FRCP(C) CLINICAL ASSISTANT PROFESSOR OF MEDICINE, UBC Conflicts of Interest None I am unbiased in the use of NOAC s vs Warfarin based on risk benefit
New Anticoagulants and GI bleeding DR DANNY MYERS MD FRCP(C) CLINICAL ASSISTANT PROFESSOR OF MEDICINE, UBC Conflicts of Interest None I am unbiased in the use of NOAC s vs Warfarin based on risk benefit
Comparative Effectiveness Review Number 4. Comparative Effectiveness and Safety of Analgesics for Osteoarthritis
 This report is based on research conducted by the Oregon Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-02-0024).
This report is based on research conducted by the Oregon Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-02-0024).
VIOXX Gastrointestinal Outcomes Research Trial (VIGOR) Bonnie Goldmann, M.D. Regulatory Affairs Merck Research Laboratories
 VIOXX Gastrointestinal Outcomes Research Trial (VIGOR) Bonnie Goldmann, M.D. Regulatory Affairs Merck Research Laboratories 1 Arachidonic Acid CO 2 H COX-1 NSAIDs COX-2 Prostanoids Prostanoids Protection
VIOXX Gastrointestinal Outcomes Research Trial (VIGOR) Bonnie Goldmann, M.D. Regulatory Affairs Merck Research Laboratories 1 Arachidonic Acid CO 2 H COX-1 NSAIDs COX-2 Prostanoids Prostanoids Protection
FOR THE PREVENTION OF ATRIAL FIBRILLATION RELATED STROKE
 www.bpac.org.nz keyword: warfarinaspirin FOR THE PREVENTION OF ATRIAL FIBRILLATION RELATED STROKE Key Concepts In atrial fibrillation (AF) warfarin is more effective than aspirin for stroke prevention.
www.bpac.org.nz keyword: warfarinaspirin FOR THE PREVENTION OF ATRIAL FIBRILLATION RELATED STROKE Key Concepts In atrial fibrillation (AF) warfarin is more effective than aspirin for stroke prevention.
Rivaroxaban for acute coronary syndromes
 Northern Treatment Advisory Group Rivaroxaban for acute coronary syndromes Lead author: Nancy Kane Regional Drug & Therapeutics Centre (Newcastle) May 2014 2014 Summary Current long-term management following
Northern Treatment Advisory Group Rivaroxaban for acute coronary syndromes Lead author: Nancy Kane Regional Drug & Therapeutics Centre (Newcastle) May 2014 2014 Summary Current long-term management following
Duration of Dual Antiplatelet Therapy After Coronary Stenting
 Duration of Dual Antiplatelet Therapy After Coronary Stenting C. DEAN KATSAMAKIS, DO, FACC, FSCAI INTERVENTIONAL CARDIOLOGIST ADVOCATE LUTHERAN GENERAL HOSPITAL INTRODUCTION Coronary artery stents are
Duration of Dual Antiplatelet Therapy After Coronary Stenting C. DEAN KATSAMAKIS, DO, FACC, FSCAI INTERVENTIONAL CARDIOLOGIST ADVOCATE LUTHERAN GENERAL HOSPITAL INTRODUCTION Coronary artery stents are
Dual Antiplatelet Therapy. Stephen Monroe, MD FACC Chattanooga Heart Institute
 Dual Antiplatelet Therapy Stephen Monroe, MD FACC Chattanooga Heart Institute Scope of Talk Identify the antiplatelet drugs and their mechanisms of action Review dual antiplatelet therapy in: The medical
Dual Antiplatelet Therapy Stephen Monroe, MD FACC Chattanooga Heart Institute Scope of Talk Identify the antiplatelet drugs and their mechanisms of action Review dual antiplatelet therapy in: The medical
Analgesics for Osteoarthritis: An Update of the
 Comparative Effectiveness Review Number 38 Analgesics for Osteoarthritis: An Update of the 2006 Comparative Effectiveness Review Comparative Effectiveness Review Number 38 Analgesics for Osteoarthritis:
Comparative Effectiveness Review Number 38 Analgesics for Osteoarthritis: An Update of the 2006 Comparative Effectiveness Review Comparative Effectiveness Review Number 38 Analgesics for Osteoarthritis:
Guidelines for Use of Clopidogrel (Plavix )
 East Lancashire Medicines Management Board representing East Lancashire Hospitals NHS Trust, Lancashire Care Trust, Blackburn with Darwen PCT, East Lancs PCT Licensed Indications Guidelines for Use of
East Lancashire Medicines Management Board representing East Lancashire Hospitals NHS Trust, Lancashire Care Trust, Blackburn with Darwen PCT, East Lancs PCT Licensed Indications Guidelines for Use of
DUAL ANTIPLATELET THERAPY. Dr Robert S Mvungi, MD(Dar), Mmed (Wits) FCP(SA), Cert.Cardio(SA) Phy Tanzania Cardiac Society Dar es Salaam Tanzania
 DUAL ANTIPLATELET THERAPY Dr Robert S Mvungi, MD(Dar), Mmed (Wits) FCP(SA), Cert.Cardio(SA) Phy Tanzania Cardiac Society Dar es Salaam Tanzania DUAL ANTIPLATELET THERAPY (DAPT) Dual antiplatelet regimen
DUAL ANTIPLATELET THERAPY Dr Robert S Mvungi, MD(Dar), Mmed (Wits) FCP(SA), Cert.Cardio(SA) Phy Tanzania Cardiac Society Dar es Salaam Tanzania DUAL ANTIPLATELET THERAPY (DAPT) Dual antiplatelet regimen
Apixaban Plus Mono vs. Dual Antiplatelet Therapy in Acute Coronary Syndromes: Insights from the APPRAISE-2 Trial
 Apixaban Plus Mono vs. Dual Antiplatelet Therapy in Acute Coronary Syndromes: Insights from the APPRAISE-2 Trial Connie N. Hess, MD, MHS, Stefan James, MD, PhD, Renato D. Lopes, MD, PhD, Daniel M. Wojdyla,
Apixaban Plus Mono vs. Dual Antiplatelet Therapy in Acute Coronary Syndromes: Insights from the APPRAISE-2 Trial Connie N. Hess, MD, MHS, Stefan James, MD, PhD, Renato D. Lopes, MD, PhD, Daniel M. Wojdyla,
A. Approval for the indications of osteoarthritis and rheumatoid arthritis at a dose of 10mg/day and dysmenorrhea at a dose of 20-mg bid as needed.
 Agent: Valdecoxib Indication: Analgesia, Dysmenorrhea Osteoarthritis, and Rheumatoid Arthritis Reviewer: Kent Johnson, MD Date: November 7, 2001 NDA: 21,341 EXECUTIVE SUMMARY 1-RECOMMENDATIONS A. Approval
Agent: Valdecoxib Indication: Analgesia, Dysmenorrhea Osteoarthritis, and Rheumatoid Arthritis Reviewer: Kent Johnson, MD Date: November 7, 2001 NDA: 21,341 EXECUTIVE SUMMARY 1-RECOMMENDATIONS A. Approval
The largest clinical study of Bayer's Xarelto (rivaroxaban) Wednesday, 14 November 2012 07:38
 Bayer HealthCare has announced the initiation of the COMPASS study, the largest clinical study of its oral anticoagulant Xarelto (rivaroxaban) to date, investigating the prevention of major adverse cardiac
Bayer HealthCare has announced the initiation of the COMPASS study, the largest clinical study of its oral anticoagulant Xarelto (rivaroxaban) to date, investigating the prevention of major adverse cardiac
Gastroesophageal Reflux Disease (GERD) and Barrett s Esophagus (BE)
 Gastroesophageal Reflux Disease (GERD) and Barrett s Esophagus (BE) Hashem El-Serag, M.D., M.P.H. Dan L. Duncan Professor of Medicine Chief, Gastroenterology and Hepatology Baylor College of Medicine Houston,
Gastroesophageal Reflux Disease (GERD) and Barrett s Esophagus (BE) Hashem El-Serag, M.D., M.P.H. Dan L. Duncan Professor of Medicine Chief, Gastroenterology and Hepatology Baylor College of Medicine Houston,
Medication Management Improvement System Protocol #4 Potentially Inappropriate Use of NSAIDs
 Medication Management Improvement System Protocol #4 Potentially Inappropriate Use of NSAIDs Problem: Use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in clients with any one of the following risk
Medication Management Improvement System Protocol #4 Potentially Inappropriate Use of NSAIDs Problem: Use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in clients with any one of the following risk
Psoriasis and Psoriatic Arthritis Alliance
 Psoriasis and Psoriatic Arthritis Alliance A principal source of information on psoriasis and psoriatic arthritis ) Treatments for Psoriatic Arthritis overview Although psoriatic arthritis is a chronic
Psoriasis and Psoriatic Arthritis Alliance A principal source of information on psoriasis and psoriatic arthritis ) Treatments for Psoriatic Arthritis overview Although psoriatic arthritis is a chronic
New Treatments for Stroke Prevention in Atrial Fibrillation. John C. Andrefsky, MD, FAHA NEOMED Internal Medicine Review course May 5 th, 2013
 New Treatments for Stroke Prevention in Atrial Fibrillation John C. Andrefsky, MD, FAHA NEOMED Internal Medicine Review course May 5 th, 2013 Classification Paroxysmal atrial fibrillation (AF) Last < 7
New Treatments for Stroke Prevention in Atrial Fibrillation John C. Andrefsky, MD, FAHA NEOMED Internal Medicine Review course May 5 th, 2013 Classification Paroxysmal atrial fibrillation (AF) Last < 7
ABOUT XARELTO CLINICAL STUDIES
 ABOUT XARELTO CLINICAL STUDIES FAST FACTS Xarelto (rivaroxaban) is a novel, oral direct Factor Xa inhibitor. On September 30, 2008, the European Commission granted marketing approval for Xarelto for the
ABOUT XARELTO CLINICAL STUDIES FAST FACTS Xarelto (rivaroxaban) is a novel, oral direct Factor Xa inhibitor. On September 30, 2008, the European Commission granted marketing approval for Xarelto for the
Survey conducted into patient experience of life with osteoarthritis. A collaboration between Pro Bono Bio, Arthritis Research UK, and LloydsPharmacy
 Survey conducted into patient experience of life with osteoarthritis. A collaboration between Pro Bono Bio, Arthritis Research UK, and LloydsPharmacy Executive summary Over 400 people with joint conditions
Survey conducted into patient experience of life with osteoarthritis. A collaboration between Pro Bono Bio, Arthritis Research UK, and LloydsPharmacy Executive summary Over 400 people with joint conditions
patient group direction
 DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT
 THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT Stroke Prevention in Atrial Fibrillation Gregory Albers, M.D. Director Stanford Stroke Center Professor of Neurology and Neurological
THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT Stroke Prevention in Atrial Fibrillation Gregory Albers, M.D. Director Stanford Stroke Center Professor of Neurology and Neurological
Digestive System (continued) Digestive System. Stomach. Peptic Ulcer Disease
 Digestive System Digestive System (continued) Responsible for breaking down food, absorbing nutrients, eliminating wastes Alimentary canal Also known as gastrointestinal tract Reaches from mouth to anus
Digestive System Digestive System (continued) Responsible for breaking down food, absorbing nutrients, eliminating wastes Alimentary canal Also known as gastrointestinal tract Reaches from mouth to anus
Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes
 Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes U.S. Department of Health and Human Services Food and Drug Administration Center
Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes U.S. Department of Health and Human Services Food and Drug Administration Center
National Digestive Diseases Information Clearinghouse
 Gastritis National Digestive Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is gastritis? Gastritis is a condition in which the stomach
Gastritis National Digestive Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is gastritis? Gastritis is a condition in which the stomach
Bayer Initiates Rivaroxaban Phase III Study to Support Dose Selection According to Individual Benefit-Risk Profile in Long- Term VTE Prevention
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Long-term prevention of venous blood clots (VTE): Bayer Initiates Rivaroxaban
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Long-term prevention of venous blood clots (VTE): Bayer Initiates Rivaroxaban
Cilostazol versus Clopidogrel after Coronary Stenting
 Cilostazol versus Clopidogrel after Coronary Stenting Seong-Wook Park, MD, PhD, FACC Division of Cardiology, Asan Medical Center University of Ulsan College of Medicine Seoul, Korea AMC, 2004 Background
Cilostazol versus Clopidogrel after Coronary Stenting Seong-Wook Park, MD, PhD, FACC Division of Cardiology, Asan Medical Center University of Ulsan College of Medicine Seoul, Korea AMC, 2004 Background
A Guideline for the Treatment and Prevention of NSAID-Induced Ulcers
 THE AMERICAN JOURNAL OF GASTROENTEROLOGY Vol. 93, No. 11, 1998 Copyright 1998 by Am. Coll. of Gastroenterology ISSN 0002-9270/98/$19.00 Published by Elsevier Science Inc. PII S0002-9270(98)00469-9 A Guideline
THE AMERICAN JOURNAL OF GASTROENTEROLOGY Vol. 93, No. 11, 1998 Copyright 1998 by Am. Coll. of Gastroenterology ISSN 0002-9270/98/$19.00 Published by Elsevier Science Inc. PII S0002-9270(98)00469-9 A Guideline
STROKE PREVENTION IN ATRIAL FIBRILLATION. TARGET AUDIENCE: All Canadian health care professionals. OBJECTIVE: ABBREVIATIONS: BACKGROUND:
 STROKE PREVENTION IN ATRIAL FIBRILLATION TARGET AUDIENCE: All Canadian health care professionals. OBJECTIVE: To guide clinicians in the selection of antithrombotic therapy for the secondary prevention
STROKE PREVENTION IN ATRIAL FIBRILLATION TARGET AUDIENCE: All Canadian health care professionals. OBJECTIVE: To guide clinicians in the selection of antithrombotic therapy for the secondary prevention
PATIENTS AND METHODS
 symptoms 5. Heartburn is also common and the sufferers attribute symptoms to various lifestyle events, including diet and stress. In all these cases antacid usage is the commonest mode of therapy 6. Antacid
symptoms 5. Heartburn is also common and the sufferers attribute symptoms to various lifestyle events, including diet and stress. In all these cases antacid usage is the commonest mode of therapy 6. Antacid
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM
 Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
Perspectives on the Selection and Duration of Dual Antiplatelet Therapy
 Perspectives on the Selection and Duration of Dual Antiplatelet Therapy Dominick J. Angiolillo, MD, PhD, FACC, FESC, FSCAI Director of Cardiovascular Research Associate Professor of Medicine University
Perspectives on the Selection and Duration of Dual Antiplatelet Therapy Dominick J. Angiolillo, MD, PhD, FACC, FESC, FSCAI Director of Cardiovascular Research Associate Professor of Medicine University
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and
 Health Technology Assessment 2008; Vol. 12: No. 11 Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for
Health Technology Assessment 2008; Vol. 12: No. 11 Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for
Drug treatments in upper gastrointestinal bleeding: value of endoscopic findings as surrogate end points
 372 Gut 2001;49:372 379 Drug treatments in upper gastrointestinal bleeding: value of endoscopic findings as surrogate end points G M Hawkey, A T Cole, A S McIntyre, R G Long, C J Hawkey Division of Gastroenterology,
372 Gut 2001;49:372 379 Drug treatments in upper gastrointestinal bleeding: value of endoscopic findings as surrogate end points G M Hawkey, A T Cole, A S McIntyre, R G Long, C J Hawkey Division of Gastroenterology,
Committee Approval Date: September 12, 2014 Next Review Date: September 2015
 Medication Policy Manual Policy No: dru361 Topic: Pradaxa, dabigatran Date of Origin: September 12, 2014 Committee Approval Date: September 12, 2014 Next Review Date: September 2015 Effective Date: November
Medication Policy Manual Policy No: dru361 Topic: Pradaxa, dabigatran Date of Origin: September 12, 2014 Committee Approval Date: September 12, 2014 Next Review Date: September 2015 Effective Date: November
A rapid-release 50-mg tablet-based 13 C-urea breath test for the diagnosis of Helicobacter pylori infection
 Aliment Pharmacol Ther 2003; 17: 253 257. doi: 10.1046/j.0269-2813.2003.01417.x A rapid-release 50-mg tablet-based 13 C-urea breath test for the diagnosis of Helicobacter pylori infection W.M.WONG*,S.K.LAM*,K.C.LAI*,K.M.CHU,
Aliment Pharmacol Ther 2003; 17: 253 257. doi: 10.1046/j.0269-2813.2003.01417.x A rapid-release 50-mg tablet-based 13 C-urea breath test for the diagnosis of Helicobacter pylori infection W.M.WONG*,S.K.LAM*,K.C.LAI*,K.M.CHU,
Tapered Withdrawal of a Proton Pump Inhibitor to Prevent Rebound Acid-Related Symptoms
 Akin Oyalowo CRC Rotation IRB Proposal August 8, 2012 Tapered Withdrawal of a Proton Pump Inhibitor to Prevent Rebound Acid-Related Symptoms A. Study Purpose and Rationale Proton pump inhibitors (PPIs)
Akin Oyalowo CRC Rotation IRB Proposal August 8, 2012 Tapered Withdrawal of a Proton Pump Inhibitor to Prevent Rebound Acid-Related Symptoms A. Study Purpose and Rationale Proton pump inhibitors (PPIs)
Arthritis A CLOSER L OOK AT. One in six Americans suffers from arthritis, and the CDC projects that number will grow to one in five by 2020.
 Arthritis A CLOSER L OOK AT One in six Americans suffers from arthritis, and the CDC projects that number will grow to one in five by 2020. A COLLABORATION BETWEEN THE A RTHRITIS F OUNDATION AND T HE N
Arthritis A CLOSER L OOK AT One in six Americans suffers from arthritis, and the CDC projects that number will grow to one in five by 2020. A COLLABORATION BETWEEN THE A RTHRITIS F OUNDATION AND T HE N
WOEST TRIAL- NO ASPIRIN IN STENTED PATIENTS REQUIRING ANTICOAGULATION. Van Crisco, MD, FACC, FSCAI First Coast
 WOEST TRIAL- NO ASPIRIN IN STENTED PATIENTS REQUIRING ANTICOAGULATION Van Crisco, MD, FACC, FSCAI First Coast Conflicts of Interest I have been a paid consultant and speaker for AstraZeneca, makers of
WOEST TRIAL- NO ASPIRIN IN STENTED PATIENTS REQUIRING ANTICOAGULATION Van Crisco, MD, FACC, FSCAI First Coast Conflicts of Interest I have been a paid consultant and speaker for AstraZeneca, makers of
Peptic Ulcer. Anatomy The stomach is a hollow organ. It is located in the upper abdomen, under the ribs.
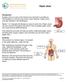 Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
Management of the new antiplatelets and anticoagulants
 Management of the new antiplatelets and anticoagulants Session No.: 1 Name: C. Boustiere, T Ponchon Guidelines : Anti-thrombotic agents and digestive endoscopy 2006 : French guideline (SFED) 2007 : Japanese
Management of the new antiplatelets and anticoagulants Session No.: 1 Name: C. Boustiere, T Ponchon Guidelines : Anti-thrombotic agents and digestive endoscopy 2006 : French guideline (SFED) 2007 : Japanese
What is an NNT? What is...? series Second edition Statistics. Supported by sanofi-aventis
 ...? series Second edition Statistics Supported by sanofi-aventis What is an NNT? Andrew Moore MA DPhil DSc CChem FRSC Senior Research Fellow, Pain Research and Nuffield Department of Anaesthetics, University
...? series Second edition Statistics Supported by sanofi-aventis What is an NNT? Andrew Moore MA DPhil DSc CChem FRSC Senior Research Fellow, Pain Research and Nuffield Department of Anaesthetics, University
L'aspirina è diventata obsoleta nell'era dei nuovi inbitori P2Y12? Leonardo Bolognese MD, FESC, FACC Cardiovascular Department, Arezzo, Italy ISO 9001
 L'aspirina è diventata obsoleta nell'era dei nuovi inbitori P2Y12? Leonardo Bolognese MD, FESC, FACC Cardiovascular Department, Arezzo, Italy Scientific Advances and Cardiovascular Mortality Nabel and
L'aspirina è diventata obsoleta nell'era dei nuovi inbitori P2Y12? Leonardo Bolognese MD, FESC, FACC Cardiovascular Department, Arezzo, Italy Scientific Advances and Cardiovascular Mortality Nabel and
Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada
 Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
Long term anticoagulant therapy in patients with atrial fibrillation at high risk of stroke: a new scenario after RE-LY trial
 Long term anticoagulant therapy in patients with atrial fibrillation at high risk of stroke: a new scenario after RE-LY trial Camillo Autore Università di Roma Sapienza II Facoltà di Medicina e Chirurgia
Long term anticoagulant therapy in patients with atrial fibrillation at high risk of stroke: a new scenario after RE-LY trial Camillo Autore Università di Roma Sapienza II Facoltà di Medicina e Chirurgia
CMAJ JAMC. An evidence-based approach to the management of uninvestigated dyspepsia in the era of Helicobacter pylori
 CANADIAN M EDICAL A SSOCIATION J OURNAL J OURNAL DE L ASSOCIATION MÉDICALE CANADIENNE CMAJ JAMC Return to June 13, 2000 Table of Contents An evidence-based approach to the management of uninvestigated
CANADIAN M EDICAL A SSOCIATION J OURNAL J OURNAL DE L ASSOCIATION MÉDICALE CANADIENNE CMAJ JAMC Return to June 13, 2000 Table of Contents An evidence-based approach to the management of uninvestigated
gastrointestinal ulcers and ulcer complications 2 (COX2) enzyme might reduce this burden. 8 To date, two groups of investigators have attempted to
 Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial Thomas
Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial Thomas
Antiplatelet therapy:
 Balanced information for better care Antiplatelet therapy: Aggregating the latest evidence Evaluating the choices for a preventive therapy with impressive benefits and important risks Antiplatelet agents
Balanced information for better care Antiplatelet therapy: Aggregating the latest evidence Evaluating the choices for a preventive therapy with impressive benefits and important risks Antiplatelet agents
ADVANCE: a factorial randomised trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes
 ADVANCE: a factorial randomised trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes Effects of a fixed combination of the ACE inhibitor, perindopril,
ADVANCE: a factorial randomised trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes Effects of a fixed combination of the ACE inhibitor, perindopril,
What are peptic ulcers?
 Information about Peptic ulcers www.corecharity.org.uk What are the symptoms? What are the causes? What are peptic ulcers? When should I consult a doctor? What will the doctor do? How should I treat peptic
Information about Peptic ulcers www.corecharity.org.uk What are the symptoms? What are the causes? What are peptic ulcers? When should I consult a doctor? What will the doctor do? How should I treat peptic
Rivaroxaban (Xarelto) for preventing venous thromboembolism after hip or knee replacement surgery
 for preventing venous thromboembolism after hip or knee replacement surgery (riv-ah-rocks-ah-ban) Summary Rivaroxaban is an oral anticoagulant and the first direct factor Xa inhibitor. Rivaroxaban has
for preventing venous thromboembolism after hip or knee replacement surgery (riv-ah-rocks-ah-ban) Summary Rivaroxaban is an oral anticoagulant and the first direct factor Xa inhibitor. Rivaroxaban has
cme/ce article series
 This activity is supported by an unrestricted educational grant from the Western Pain Society. Release Date: November 1, 2013 Expiration Date: November 1, 2014 LEARNING OBJECTIVE Identify monitoring parameters
This activity is supported by an unrestricted educational grant from the Western Pain Society. Release Date: November 1, 2013 Expiration Date: November 1, 2014 LEARNING OBJECTIVE Identify monitoring parameters
Apixaban for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation
 Apixaban for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation ERRATUM This report was commissioned by the NIHR HTA Programme as project number 11/49 This document
Apixaban for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation ERRATUM This report was commissioned by the NIHR HTA Programme as project number 11/49 This document
Clopidogrel versus Aspirin and Esomeprazole to Prevent Recurrent Ulcer Bleeding
 The new england journal of medicine original article Clopidogrel versus Aspirin and Esomeprazole to Prevent Recurrent Ulcer Bleeding Francis K.L. Chan, M.D., Jessica Y.L. Ching, M.P.H., Lawrence C.T. Hung,
The new england journal of medicine original article Clopidogrel versus Aspirin and Esomeprazole to Prevent Recurrent Ulcer Bleeding Francis K.L. Chan, M.D., Jessica Y.L. Ching, M.P.H., Lawrence C.T. Hung,
Getting smart about dyspnea and life saving drug therapy in ACS patients. Kobi George Kaplan Medical Center Rehovot
 Getting smart about dyspnea and life saving drug therapy in ACS patients Kobi George Kaplan Medical Center Rehovot 78 year old female Case description Presented with resting chest pain and dyspnea Co morbidities:
Getting smart about dyspnea and life saving drug therapy in ACS patients Kobi George Kaplan Medical Center Rehovot 78 year old female Case description Presented with resting chest pain and dyspnea Co morbidities:
PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain
 P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
Bayer Pharma AG 13342 Berlin Germany Tel. +49 30 468-1111 www.bayerpharma.com. News Release. Not intended for U.S. and UK Media
 News Release Not intended for U.S. and UK Media Bayer Pharma AG 13342 Berlin Germany Tel. +49 30 468-1111 www.bayerpharma.com Bayer Forms Collaboration with Academic and Governmental Institutions for Rivaroxaban
News Release Not intended for U.S. and UK Media Bayer Pharma AG 13342 Berlin Germany Tel. +49 30 468-1111 www.bayerpharma.com Bayer Forms Collaboration with Academic and Governmental Institutions for Rivaroxaban
Ask Your Doctor if There May Be a SMARTER CHOICE
 If you have osteoarthritis, rheumatoid arthritis or ankylosing spondylitis, Could Your NSAID Pain Medicine Be Hurting Your Stomach? Ask Your Doctor if There May Be a SMARTER CHOICE 1 of 8 Making Smart
If you have osteoarthritis, rheumatoid arthritis or ankylosing spondylitis, Could Your NSAID Pain Medicine Be Hurting Your Stomach? Ask Your Doctor if There May Be a SMARTER CHOICE 1 of 8 Making Smart
The Consequences of Missing Data in the ATLAS ACS 2-TIMI 51 Trial
 The Consequences of Missing Data in the ATLAS ACS 2-TIMI 51 Trial In this white paper, we will explore the consequences of missing data in the ATLAS ACS 2-TIMI 51 Trial and consider if an alternative approach
The Consequences of Missing Data in the ATLAS ACS 2-TIMI 51 Trial In this white paper, we will explore the consequences of missing data in the ATLAS ACS 2-TIMI 51 Trial and consider if an alternative approach
Prevention of nonsteroidal anti-inflammatory drug-induced gastropathy
 J Gastroenterol 2009; 44[Suppl XIX]:44 52 DOI 10.1007/s00535-008-2275-5 Prevention of nonsteroidal anti-inflammatory drug-induced gastropathy BARRY SCHLANSKY and JOO HA HWANG University of Washington School
J Gastroenterol 2009; 44[Suppl XIX]:44 52 DOI 10.1007/s00535-008-2275-5 Prevention of nonsteroidal anti-inflammatory drug-induced gastropathy BARRY SCHLANSKY and JOO HA HWANG University of Washington School
Gruppo di lavoro: Malattie Tromboemboliche
 Gruppo di lavoro: Malattie Tromboemboliche 2381 Soluble Recombinant Thrombomodulin Ameliorates Hematological Malignancy-Induced Disseminated Intravascular Coagulation More Promptly Than Conventional Anticoagulant
Gruppo di lavoro: Malattie Tromboemboliche 2381 Soluble Recombinant Thrombomodulin Ameliorates Hematological Malignancy-Induced Disseminated Intravascular Coagulation More Promptly Than Conventional Anticoagulant
CHAPTER 1 INTRODUCTION
 CHAPTER 1 INTRODUCTION Rheumatoid Arthritis (RA) is a chronic syndrome characterised by non-specific, usually symmetric inflammation of the peripheral joints, potentially resulting in progressive destruction
CHAPTER 1 INTRODUCTION Rheumatoid Arthritis (RA) is a chronic syndrome characterised by non-specific, usually symmetric inflammation of the peripheral joints, potentially resulting in progressive destruction
Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care
 Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Public Assessment Report. Pharmacy to General Sales List Reclassification. Nexium Control 20mg Gastro-Resistant Tablets.
 Public Assessment Report Pharmacy to General Sales List Reclassification Nexium Control 20mg Gastro-Resistant Tablets (Esomeprazole) EMA Agency number: Pfizer Consumer Healthcare Ltd TABLE OF CONTENTS
Public Assessment Report Pharmacy to General Sales List Reclassification Nexium Control 20mg Gastro-Resistant Tablets (Esomeprazole) EMA Agency number: Pfizer Consumer Healthcare Ltd TABLE OF CONTENTS
The author has no disclosures
 Mary Bradbury, PharmD, BCPS Clinical Pharmacy Specialist, Cardiac Surgery September 18, 2012 Mary.bradbury@inova.org This presentation will discuss unlabeled and investigational use of products The author
Mary Bradbury, PharmD, BCPS Clinical Pharmacy Specialist, Cardiac Surgery September 18, 2012 Mary.bradbury@inova.org This presentation will discuss unlabeled and investigational use of products The author
NSAIDs and Peptic Ulcers
 NSAIDs and Peptic Ulcers National Digestive Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is a peptic ulcer? A peptic ulcer is a sore
NSAIDs and Peptic Ulcers National Digestive Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is a peptic ulcer? A peptic ulcer is a sore
US Health Statistics: Americans Most Over-Prescribed Country in the World
 US Health Statistics: Americans Most Over-Prescribed Country in the World A vitally important story reported in the April 15, 1998, issue of the Journal of the American Medical Association (JAMA), sums
US Health Statistics: Americans Most Over-Prescribed Country in the World A vitally important story reported in the April 15, 1998, issue of the Journal of the American Medical Association (JAMA), sums
Medical Nutrition Therapy for Upper Gastrointestinal Tract Disorders. By: Jalal Hejazi PhD, MSc.
 Medical Nutrition Therapy for Upper Gastrointestinal Tract Disorders By: Jalal Hejazi PhD, MSc. Digestive Disorders Common problem; more than 50 million outpatient visits per year Dietary habits and nutrition
Medical Nutrition Therapy for Upper Gastrointestinal Tract Disorders By: Jalal Hejazi PhD, MSc. Digestive Disorders Common problem; more than 50 million outpatient visits per year Dietary habits and nutrition
What is Rheumatoid Arthritis?
 Name: A Case Study in Pharmaceutical Research Date: Class Work: Part I Aim: How do Biologists Select their Projects? What is Rheumatoid Arthritis? Rheumatoid arthritis (rue-ma-toyd arth-write-tis) is a
Name: A Case Study in Pharmaceutical Research Date: Class Work: Part I Aim: How do Biologists Select their Projects? What is Rheumatoid Arthritis? Rheumatoid arthritis (rue-ma-toyd arth-write-tis) is a
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medical management of CHF: A New Class of Medication. Al Timothy, M.D. Cardiovascular Institute of the South
 Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Treating AF: The Newest Recommendations. CardioCase presentation. Ethel s Case. Wayne Warnica, MD, FACC, FACP, FRCPC
 Treating AF: The Newest Recommendations Wayne Warnica, MD, FACC, FACP, FRCPC CardioCase presentation Ethel s Case Ethel, 73, presents with rapid heart beating and mild chest discomfort. In the ED, ECG
Treating AF: The Newest Recommendations Wayne Warnica, MD, FACC, FACP, FRCPC CardioCase presentation Ethel s Case Ethel, 73, presents with rapid heart beating and mild chest discomfort. In the ED, ECG
NHS FORTH VALLEY Guidelines for use of high dose Intravenous Esomeprazole in Adults (Previously called the Hong Kong Protocol)
 NHS FORTH VALLEY Guidelines for use of high dose Intravenous Esomeprazole in Adults (Previously called the Hong Kong Protocol) Date of First Issue 10/05/2010 Approved 16/06/2010 Current Issue Date 18/11/2015
NHS FORTH VALLEY Guidelines for use of high dose Intravenous Esomeprazole in Adults (Previously called the Hong Kong Protocol) Date of First Issue 10/05/2010 Approved 16/06/2010 Current Issue Date 18/11/2015
U.S. Food and Drug Administration
 U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
Cardiovascular Subcommittee of PTAC Meeting held 27 February 2014. (minutes for web publishing)
 Cardiovascular Subcommittee of PTAC Meeting held 27 February 2014 (minutes for web publishing) Cardiovascular Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Cardiovascular Subcommittee of PTAC Meeting held 27 February 2014 (minutes for web publishing) Cardiovascular Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
at our institution, was $1.8 million in 1999. While some patients require PPI therapy, many could control their symptoms with a histamine H 2
 A Practice-Based Approach for Converting from Proton Pump Inhibitors to Less Costly Therapy CASE REPORT LINDA M. LUCAS, MD MARTHA S. GERRITY, MD, PhD THOMAS ANDERSON, MD Department of Veterans Affairs
A Practice-Based Approach for Converting from Proton Pump Inhibitors to Less Costly Therapy CASE REPORT LINDA M. LUCAS, MD MARTHA S. GERRITY, MD, PhD THOMAS ANDERSON, MD Department of Veterans Affairs
New Real-World Evidence Reaffirms Low Major Bleeding Rates for Bayer s Xarelto in Patients with Non-Valvular Atrial Fibrillation
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Late-Breaking Science at ESC Congress 2015: New Real-World Evidence Reaffirms
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Late-Breaking Science at ESC Congress 2015: New Real-World Evidence Reaffirms
University of Ulsan College of Medicine, Asan Medical Center on behalf of the REAL-LATE and the ZEST-LATE trial
 Duration of Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation A Pooled Analysis of the REAL-LATE and the ZEST-LATE Trial Seung-Jung Park MD PhD Seung-Jung Park, MD, PhD, University of Ulsan
Duration of Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation A Pooled Analysis of the REAL-LATE and the ZEST-LATE Trial Seung-Jung Park MD PhD Seung-Jung Park, MD, PhD, University of Ulsan
9/5/14. Objectives. Atrial Fibrillation (AF)
 Novel Anticoagulation for Prevention of Stroke in Patients with Atrial Fibrillation Objectives 1. Review current evidence on use of warfarin in individuals with atrial fibrillation 2. Compare the three
Novel Anticoagulation for Prevention of Stroke in Patients with Atrial Fibrillation Objectives 1. Review current evidence on use of warfarin in individuals with atrial fibrillation 2. Compare the three
1/7/2012. Objectives. Epidemiology of Atrial Fibrillation(AF) Stroke in AF. Stroke Risk Stratification in AF
 Objectives Atrial Fibrillation and Prevention of Thrombotic Complications: Therapeutic Update Andrea C. Flores Pharm.D Pharmacy Resident at the Miami VA Healthcare System Review the epidemiology, pathophysiology
Objectives Atrial Fibrillation and Prevention of Thrombotic Complications: Therapeutic Update Andrea C. Flores Pharm.D Pharmacy Resident at the Miami VA Healthcare System Review the epidemiology, pathophysiology
Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty
 Round Table: Antithrombotic therapy beyond ACS Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty M. Matsagkas, MD, PhD, EBSQ-Vasc Associate Professor
Round Table: Antithrombotic therapy beyond ACS Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty M. Matsagkas, MD, PhD, EBSQ-Vasc Associate Professor
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators
 Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
A Patient s Guide to Primary and Secondary Prevention of Cardiovascular Disease Using Blood-Thinning (Anticoagulant) Drugs
 A Patient s Guide to Primary and Secondary Prevention of PATIENT EDUCATION GUIDE What Is Cardiovascular Disease? Cardiovascular disease (CVD) is a broad term that covers any disease of the heart and circulatory
A Patient s Guide to Primary and Secondary Prevention of PATIENT EDUCATION GUIDE What Is Cardiovascular Disease? Cardiovascular disease (CVD) is a broad term that covers any disease of the heart and circulatory
Inhibit terminal acid secretion from parietal cells by blocking H + /K + - ATPase pump
 Chris J. Taylor, Pharm.D., BCPS Clinical Pharmacist Phoenix VA Health Care System Review pharmacology of PPIs Discuss possible association between PPI use and development of the following: Pneumonia (community-acquired
Chris J. Taylor, Pharm.D., BCPS Clinical Pharmacist Phoenix VA Health Care System Review pharmacology of PPIs Discuss possible association between PPI use and development of the following: Pneumonia (community-acquired
New Oral Anticoagulants
 New Oral Anticoagulants Tracy Minichiello, MD Associate Professor of Medicine Chief, San FranciscoVA Anticoagulation and Thrombosis Service Ansell, J. Hematology Copyright 2010 American Society of Hematology.
New Oral Anticoagulants Tracy Minichiello, MD Associate Professor of Medicine Chief, San FranciscoVA Anticoagulation and Thrombosis Service Ansell, J. Hematology Copyright 2010 American Society of Hematology.
Series 1 Case Studies Adverse Events that Represent Unanticipated Problems: Reporting Required
 Welcome! This document contains three (3) series of Case Study examples that will demonstrate all four OHSU reporting categories (#1 4) as well as examples of events that are considered not reportable.
Welcome! This document contains three (3) series of Case Study examples that will demonstrate all four OHSU reporting categories (#1 4) as well as examples of events that are considered not reportable.
2. Background This drug had not previously been considered by the PBAC.
 PUBLIC SUMMARY DOCUMENT Product: Ambrisentan, tablets, 5 mg and 10 mg, Volibris Sponsor: GlaxoSmithKline Australia Pty Ltd Date of PBAC Consideration: July 2009 1. Purpose of Application The submission
PUBLIC SUMMARY DOCUMENT Product: Ambrisentan, tablets, 5 mg and 10 mg, Volibris Sponsor: GlaxoSmithKline Australia Pty Ltd Date of PBAC Consideration: July 2009 1. Purpose of Application The submission
Atrial Fibrillation, Chronic - Antithrombotic Treatment - OBSOLETE
 Atrial Fibrillation, Chronic - Antithrombotic Treatment - OBSOLETE Clinical practice guidelines serve as an educational reference, and do not supersede the clinical judgment of the treating physician with
Atrial Fibrillation, Chronic - Antithrombotic Treatment - OBSOLETE Clinical practice guidelines serve as an educational reference, and do not supersede the clinical judgment of the treating physician with
Figure 2: Recurrent chest pain of suspected esophageal origin
 Figure 2: Recurrent chest pain of suspected esophageal origin 1 patient with chest pain of suspected esophageal origin 2 history and physical exam. suggestive of n-esophageal etiology? 3 evaluate and treat
Figure 2: Recurrent chest pain of suspected esophageal origin 1 patient with chest pain of suspected esophageal origin 2 history and physical exam. suggestive of n-esophageal etiology? 3 evaluate and treat
