Helicobacter Pylori RAPU08V400
|
|
|
- Jeremy Freeman
- 7 years ago
- Views:
Transcription
1 Helicobacter Pylori RAPU08V400 DIAsource ImmunoAssays S.A. Rue du Bosquet, 2 - B-1348 Louvain-La-Neuve - Belgium
2 : /1
3 H.Pylori Cassette Test en A rapid test for the qualitative detection of antibodies to Helicobacter Pylori in whole blood, serum or plasma For professional in vitro diagnostic use only RAPU08V400 IN VITRO DIAGNOSTIC DIAsource ImmunoAssays SA - Rue du Bosquet 2, B-1348 Louvain-la-Neuve, Belgium - Tel: Fax : INTENDED USE The DIAsource Helicobacter pylori Test is a rapid chromatographic immunoassay for the qualitative detection of antibodies to H. pylori in whole blood, serum, or plasma to aid in the diagnosis of H. pylori infection. SUMMARY H. pylori is a small, spiral-shaped bacterium that lives in the surface of the stomach and duodenum. It is implicated in the etiology of a variety of gastrointestinal diseases, including duodenal and gastric ulcer, non-ulcer dyspepsia and active and chronic gastritis. 1,2 Both invasive and non-invasive methods are used to diagnose H. pylori infection in patients with symptoms of gastrointestinal disease. Specimen dependent and costly invasive diagnostic methods include gastric or duodenal biopsy followed by urease testing (presumptive), culture, and/or histological staining. 3 Non-invasive techniques include the urea breath test, which requires expensive laboratory equipment and moderate radiation exposure, and serological methods. 4,5 Individuals infected with H. pylori develop antibodies which correlate strongly with histologically confirmed H. pylori infection. 6,7,8 The DIAsource Helicobacter pylori Test is a simple test that utilizes a combination of H. Pylori antigen coated particles and anti-human IgG to qualitatively and selectively detect H. pylori antibodies in whole blood, serum, or plasma. TEST PRINCIPLE The DIAsource Helicobacter pylori Test is a qualitative membrane based immunoassay for the detection of H. pylori antibodies in whole blood, serum, or plasma. In this test procedure, anti-human IgG is immobilized in the test line region of the test. After specimen is added to the specimen well of the device, it reacts with H. pylori antigen coated particles in the test. This mixture migrates chromatographically along the length of the test and interacts with the immobilized anti-human IgG. If the specimen contains H. pylori antibodies, a colored line will appear in the test line region indicating a positive result. If the specimen does not contain H. pylori antibodies, a colored line will not appear in this region indicating a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that proper volume of specimen has been added and membrane wicking has occurred. REAGENTS The test contains H. pylori antigen coated particles and anti-human IgG coated on the membrane. PRECAUTIONS For professional in vitro diagnostic use only. The test device should remain in the sealed pouch until use. Do not use the test if the foil pouch is damaged Read the entire procedure carefully prior testing. Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout the procedure and follow the standard procedures for proper disposal of specimens. Humidity and temperature can adversely affect results. Do not use after the expiration date. Do not reuse tests Do not eat, drink or smoke in the area where the specimens and kits are handled. The used test device should be discarded according to federal state and local regulations. Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are being tested. STORAGE AND STABILITY Store as packaged at room temperature or refrigerated (2-30 C). The test is stable through the expiration date printed on the sealed pouch. The test must remain in the sealed pouch until use. Tests should be kept out of direct sunlight. MATERIALS PROVIDED CARD PIPETTE BUF Package insert 20 H. Pylori test cassette 20 Sample droppers 1 Buffer (Tris-buffer), 3 ml 20 Capillary tubes 75 µl and dispensing bulb (for fingersticks Whole blood only ) MATERIAL REQUIRED BUT NOT PROVIDED Specimen collection containers Centrifuge Timer Lancets (for fingerstick whole blood only) SPECIMEN COLLECTION AND PREPARATION The DIAsource Helicobacter pylori Test can be performed using whole blood (from venipuncture or fingerstick) serum or plasma To collect Fingerstick whole blood specimens: Wash the patient s hand with soap and warm water or clean with an alcohol swab. Allow to dry. Massage the hand without touching the puncture site by rubbing down the hand towards the fingertip of the middle or ring finger. Puncture the skin with a sterile lancet. Wipe away the first sign of blood Gently rub the hand from wrist to palm to finger to form a rounded drop of blood over the puncture site. Add the Fingerstick whole blood specimen to the test by using a capillary tube: Touch the end of the capillary tube to the blood until filled to approximately 75 L. Avoid air bubbles. Place the bulb onto the top end of the capillary tube, then squeeze the bulb to dispense the whole blood to the specimen area of the test cassette. Add the Fingerstick whole blood specimen to the test by using hanging drops: Position the patient s finger so that the drop of blood is just above the specimen area of the test cassette. Allow 3 hanging drops of fingerstick whole blood to fall into the center of the specimen area on the test cassette, or move the patient s finger so that the hanging drop touches the center of the specimen area. Avoid touching the finger directly to the specimen area. Separate serum or plasma from blood as soon as possible to avoid hemolysis. Use only clear non-hemolyzed specimens. Do not freeze Do not use beyond the expiration date.
4 Testing should be performed immediately after the specimens have been collected. Do not leave the specimens at room temperature for prolonged periods. Serum and plasma specimens may be stored at 2-8 C for up to 3 days. For long term storage, specimens should be kept below -20 C. Whole blood collected by venipuncture should be stored at 2-8 C if the test is to be run within 2 days of collection. Do not freeze whole bloodspecimens. Whole blood collected by fingerstick should be tested immediately. Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. specimens should not be frozen and thawed repeatedly. If specimens are to be shipped, they should be packed in compliance with local regulations covering the transportation of etiologic agents. DIRECTIONS FOR USE 1. Allow the test, specimen, buffer and/or controls to reach room temperature (15-30 C) prior to testing. 2. Bring the pouch to room temperature before opening it. Remove the test cassette from the sealed pouch and use it as soon as possible. 3. Place the cassette on a clean and level surface. 4.a For serum or plasma specimen: Hold the dropper vertically and transfer 3 drops of serum or plasma (approximately 75 L) to the specimen well of test cassette and start the timer. 4.b For venipuncture whole blood specimen: Hold the dropper vertically and transfer 3 drops of whole blood (approximately 75 L) to the specimen well, then add 1 drop of buffer (approximately 40 L), and start the timer. 4.c For fingerstick whole blood specimen: To use a capillary tube: Fill the capillary tube and transfer approximately 75 L of fingerstick whole blood specimen to the specimen area of test cassette, then add 1 drop of buffer (approximately 40 L) and start the timer. To use hanging drops: Allow 3 hanging drops of fingerstick whole blood specimen (approximately 75 L) to fall into the specimen area of test cassette, then add 1 drop of buffer (approximately 40 L) and start the timer. 5. Wait for the colored line(s) to appear. Read results at 10 minutes. Do not interpret the result after 20 minutes. INTERPRETATION OF RESULTS Negative Positive Invalid Negative: One colored line appears in the control region (C). No apparent colored line appears in the test region (T). Positive:* Two distinct colored lines appear. One colored line should be in the control region (C) and another colored line should be in the test region (T). Invalide: Control lin Control lines fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor. *Note: The intensity of the color in the test line region (T) will vary depending on the concentration of the DIAsource Helicobacter pylori Test in the specimen. Therefore, any shade of color in the test region (T) should be considered positive. QUALITY CONTROL A procedural control is included in the test. A colored line appearing in the control region (C) is the internal procedural control. It confirms sufficient specimen volume and correct procedural technique. Control standards are not supplied with this kit; however, it is recommended that positive and negative controls be tested as a good laboratory practice to confirm the test procedure and to verify proper test performance LIMITATIONS The DIAsource Helicobacter pylori Test is for in vitro diagnostic use only. The test should be used for the detection of H. pylori antibodies in whole blood, serum or plasma specimens only. Neither the quantitative value nor the rate of increase in H. pylori antibody concentration can be determined by this qualitative test. The H. pylori Antibody Test will only indicate the presence of H. pylori antibodies in the specimen and should not be used as the sole criteria for the diagnosis of H. pylori infection. As with all diagnostic tests, all results must be interpreted together with other clinical information available to the physician. If the test result is negative and clinical symptoms persist, additional testing using other clinical methods is recommended. A negative result does not at any time preclude the possibility of H. pylori infection. EXPECTED VALUES The DIAsource Helicobacter pylori Test has been compared with Culture/Histology, demonstrating an overall accuracy of 94.6%. PERFORMANCE CHARACTERISTICS Clinical Sensitivity, Specificity and Accuracy The DIAsource Helicobacter pylori Test has been evaluated with specimens obtained from a population of symptomatic and asymptomatic individuals who presented for endoscopic examination. Biopsy (Culture) served as the reference method for the DIAsource Helicobacter pylori Test. Histology and a Rapid Urease Test (RUT) were performed on all negative culture specimens. The specimen was considered positive if Culture was positive. The specimen was also considered positive if the Culture was negative, but both Histology and RUT were positive. The result shows that the sensitivity of the H. pylori Antibody Test is 96.8% and the specificity is 93.0% relative to Biopsy/Histology/RUT. DIAsource Helicobacter pylori Test vs. Biopsy/Histology/RUT Reproductions may vary from original! Method Biopsy/Histology/RUT Total H.pylori Test Results Positive Negative Results Cassette Positive Negative Total Results Relative Sensitivity: 96.8% (95%CI*: 92.6%-98.9%) Relatively Specificity: 93.0% (95%CI*: 88.8%-96.0%) Accuracy: 94.6% (95%CI*: 91.8%-96.7%) *Confidence Interval
5 Precision Intra-Assay Within-run precision has been determined by using 10 replicates of four specimens: a negative, a low positive, a medium positive and a high positive. The negative, low positive, medium positive and high positive values were correctly identified >99% of the time. Inter-Assay Between-run precision has been determined by 10 independent assays on the same four specimens: a negative, a low positive, a medium positive and a high positive. Three different lots of the H. pylori Antibody have been tested using negative, low positive, medium positive and high positive specimens. The specimens were correctly identified >99% of the time. SYMBOLS Consult instructions for use Storage temperature Manufacturer Contains sufficient for n tests Use by In vitro diagnostic medical device Cross-reactivity Sera containing known amounts of antibodies to H. pylori have been tested with Hepatitis A, B, C, E, HIV and Syphilis. No cross-reactivity was observed, indicating that the H. pylori Antibody Test has a high degree of specificity for antibodies to H. pylori. Interfering Substances The DIAsource Helicobacter pylori Test has been tested for possible interference from visibly hemolyzed and lipemic specimens, as well as serum specimens containing high bilirubin levels. In addition, no interference was observed in specimens containing up to 1,000 mg/dl hemoglobin; up to 1,000 mg/dl bilirubin; and up to 2,000 mg/dl human serum albumin.. Batch code Catalogue number For single use only PIPETTE Dropper CARD CONTENT BUF Card Test Content Expiry date Buffer BIBLIOGRAPHY 1. Marshall, BJ, McGechie, DB, Rogers, PAR and Glancy, RG. Pyloric Campylobacter infection and gastroduodenal disease. Med. J. Australia. (1985), 149: Soll, AH. Pathogenesis of peptic ulcer and implications for therapy. New England J. Med. (1990), 322: Hazell, SL, et al. Campylobacter pyloridis and gastritis I: Detection of urease as a marker of bacterial colonization and gastritis. Amer. J. Gastroenterology. (1987), 82(4): Loffeld, RJLF, et al. Usefulness of several commercial enzyme-linked immunoassays for detection of Helicobacter pylori infection in clinical medicine. Euro. J. Gastroen. Hepa. (1993) 5: Cutler, AF, et al. Accuracy of invasive and non-invasive tests to diagnose Helicobacter pylori infection. Gastroenterology.(1995), 109: Ansorg, R, Von Recklinghausen, G, Pomarius, R and Schmid, EN. Evaluation of techniques for isolation, subcultivation and preservation of Helicobacter pylori. J. Clin. Micro. (1991), 29: Pronovost, AP, Rose, SL, Pawlak, J, Robin, H and Schneider, R. Evaluation of a new immunodiagnostic assay for Helicobacter pylori antibody detection: Correlation with histopathological and microbiological results. J. Clin. Micro. (1994), 32: Capillary Tubes Keep Dry Keep away from direct sunlight Revision date : Diasource
CK-MB TEST CARD 004A302
 A rapid test for the qualitative detection of CK-MB in whole blood, serum or plasma. For professional in vitro diagnostic use only. INTENDED USE The CK-MB test card is a rapid chromatographic immunoassay
A rapid test for the qualitative detection of CK-MB in whole blood, serum or plasma. For professional in vitro diagnostic use only. INTENDED USE The CK-MB test card is a rapid chromatographic immunoassay
OneStep Fecal Occult Blood RapiDip InstaTest. Cat # 13020-1
 CORTEZ DIAGNOSTICS, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 USA Tel: (818) 591-3030 Fax: (818) 591-8383 E-mail: onestep@rapidtest.com Web site: www.rapidtest.com See external label
CORTEZ DIAGNOSTICS, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 USA Tel: (818) 591-3030 Fax: (818) 591-8383 E-mail: onestep@rapidtest.com Web site: www.rapidtest.com See external label
Mouse IgM ELISA. Cat. No. KT-407 K-ASSAY. For the quantitative determination of IgM in mouse biological samples. For Research Use Only. 1 Rev.
 K-ASSAY Mouse IgM ELISA For the quantitative determination of IgM in mouse biological samples Cat. No. KT-407 For Research Use Only. 1 Rev. 072309 K-ASSAY PRODUCT INFORMATION Mouse IgM ELISA Cat. No. KT-407
K-ASSAY Mouse IgM ELISA For the quantitative determination of IgM in mouse biological samples Cat. No. KT-407 For Research Use Only. 1 Rev. 072309 K-ASSAY PRODUCT INFORMATION Mouse IgM ELISA Cat. No. KT-407
IgM ELISA. For the quantitative determination of IgM in human serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures.
 IgM ELISA For the quantitative determination of IgM in human serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calibrator
IgM ELISA For the quantitative determination of IgM in human serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calibrator
Toxoplasma gondii IgM ELISA Kit Protocol
 Toxoplasma gondii IgM ELISA Kit Protocol (Cat. No.:EK-310-87) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com Toxoplasma IgM ELISA
Toxoplasma gondii IgM ELISA Kit Protocol (Cat. No.:EK-310-87) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com Toxoplasma IgM ELISA
ABORhCard. ABORhCard Package Insert ABO and Rh Blood Grouping Device
 ABORhCard Package Insert ABO and Rh Blood Grouping Device ABORhCard Intended Use The ABORhCard is a qualitative in vitro test that provides a simultaneous ABO and Rh determination of an individual s ABO
ABORhCard Package Insert ABO and Rh Blood Grouping Device ABORhCard Intended Use The ABORhCard is a qualitative in vitro test that provides a simultaneous ABO and Rh determination of an individual s ABO
STEP-BY-STEP INSTRUCTIONS FOR INVESTIGATIONAL USE. Rapid HCV Antibody Test FOR ORAQUICK RAPID HCV ANTIBODY TEST
 Before performing testing, all operators MUST read and become familiar with Universal Precautions for Prevention of Transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and other Blood-borne
Before performing testing, all operators MUST read and become familiar with Universal Precautions for Prevention of Transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and other Blood-borne
SAMPLE PROCEDURE 1029-3, 09/11
 SAMPLE PROCEDURE This Sample Procedure is not intended as a substitute for your facility s Procedure Manual or reagent labeling, but rather as a model for your use in customizing for your laboratory s
SAMPLE PROCEDURE This Sample Procedure is not intended as a substitute for your facility s Procedure Manual or reagent labeling, but rather as a model for your use in customizing for your laboratory s
Measles (Rubeola) IgM ELISA Catalog No. CB40-101-325099 (96 Tests)
 For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE Measles test is an enzyme linked immunosorbent assay (ELISA) for the detection of IgG class antibodies to Measles (Rubeola) in
For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE Measles test is an enzyme linked immunosorbent assay (ELISA) for the detection of IgG class antibodies to Measles (Rubeola) in
Mouse Insulin ELISA. For the quantitative determination of insulin in mouse serum and plasma
 Mouse Insulin ELISA For the quantitative determination of insulin in mouse serum and plasma Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research Use Only. Not For Use
Mouse Insulin ELISA For the quantitative determination of insulin in mouse serum and plasma Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research Use Only. Not For Use
Uni-Gold Recombigen HIV-1/2
 Uni-Gold Recombigen 1206506 Pour d'autres langues Für andere Sprachen Para otras lenguas Per le altre lingue Dla innych języków Para outras línguas Για τις άλλες λώσσες För andra språk For andre språk
Uni-Gold Recombigen 1206506 Pour d'autres langues Für andere Sprachen Para otras lenguas Per le altre lingue Dla innych języków Para outras línguas Για τις άλλες λώσσες För andra språk For andre språk
HBV Quantitative Real Time PCR Kit
 Revision No.: ZJ0002 Issue Date: Aug 7 th, 2008 HBV Quantitative Real Time PCR Kit Cat. No.: HD-0002-01 For Use with LightCycler 1.0/LightCycler2.0/LightCycler480 (Roche) Real Time PCR Systems (Pls ignore
Revision No.: ZJ0002 Issue Date: Aug 7 th, 2008 HBV Quantitative Real Time PCR Kit Cat. No.: HD-0002-01 For Use with LightCycler 1.0/LightCycler2.0/LightCycler480 (Roche) Real Time PCR Systems (Pls ignore
WHO Prequalification of Diagnostics Programme PUBLIC REPORT
 WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: ABON HIV 1/2/O Tri-Line Human Immunodeficiency Virus Rapid Test Device Number: PQDx 0141-051-00 Abstract ABON HIV 1/2/O Tri-Line Human
WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: ABON HIV 1/2/O Tri-Line Human Immunodeficiency Virus Rapid Test Device Number: PQDx 0141-051-00 Abstract ABON HIV 1/2/O Tri-Line Human
Anti-Zona Pellucida Antibody Latex Agglutination Test
 Instructions for use Anti-Zona Pellucida Antibody Latex Agglutination Test Cat. No.: BS-20-10 Size: 50 Determinations Storage: 2 C 8 C (36 F 46 F) Screening test for the determination of anti-zona pellucida
Instructions for use Anti-Zona Pellucida Antibody Latex Agglutination Test Cat. No.: BS-20-10 Size: 50 Determinations Storage: 2 C 8 C (36 F 46 F) Screening test for the determination of anti-zona pellucida
How Does a Doctor Test for AIDS?
 Edvo-Kit #S-70 How Does a Doctor Test for AIDS? S-70 Experiment Objective: The Human Immunodefi ciency Virus (HIV) is an infectious agent that causes Acquired Immunodefi ciency Syndrome (AIDS) in humans.
Edvo-Kit #S-70 How Does a Doctor Test for AIDS? S-70 Experiment Objective: The Human Immunodefi ciency Virus (HIV) is an infectious agent that causes Acquired Immunodefi ciency Syndrome (AIDS) in humans.
Cold Agglutination Titer detecting Cold Reacting Antibodies
 Objectives: Cold Agglutination Titer detecting Cold Reacting Antibodies 1. Perform a serial dilution to determine the amount of cold reacting antibody present in a patient specimen with the results obtained
Objectives: Cold Agglutination Titer detecting Cold Reacting Antibodies 1. Perform a serial dilution to determine the amount of cold reacting antibody present in a patient specimen with the results obtained
Human Peripheral Blood Mononuclear Cell (PBMC) Manual
 Human Peripheral Blood Mononuclear Cell (PBMC) Manual INSTRUCTION MANUAL ZBM0063.04 SHIPPING CONDITIONS Human Peripheral Blood Mononuclear Cells, cryopreserved Cryopreserved human peripheral blood mononuclear
Human Peripheral Blood Mononuclear Cell (PBMC) Manual INSTRUCTION MANUAL ZBM0063.04 SHIPPING CONDITIONS Human Peripheral Blood Mononuclear Cells, cryopreserved Cryopreserved human peripheral blood mononuclear
Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA
 KAMIYA BIOMEDICAL COMPANY Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA For the quantitative determination of mouse CKMB in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-57681
KAMIYA BIOMEDICAL COMPANY Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA For the quantitative determination of mouse CKMB in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-57681
Title: Panbio Dengue IgG Indirect ELISA Revision Date : Sep. 03, 2013
 Page No. : 1 / 6 I. Product / Company Identification Product Name & Synonyms: Panbio Dengue IgG Indirect ELISA Chemical Family: In-Vitro diagnostics Manufacturer : Standard Diagnostics, Inc. Manufacturer's
Page No. : 1 / 6 I. Product / Company Identification Product Name & Synonyms: Panbio Dengue IgG Indirect ELISA Chemical Family: In-Vitro diagnostics Manufacturer : Standard Diagnostics, Inc. Manufacturer's
INSTRUCTIONS FOR USE HUMIRA 40 MG/0.8 ML, 20 MG/0.4 ML AND 10 MG/0.2 ML SINGLE-USE PREFILLED SYRINGE
 INSTRUCTIONS FOR USE HUMIRA (Hu-MARE-ah) (adalimumab) 40 MG/0.8 ML, 20 MG/0.4 ML AND 10 MG/0.2 ML SINGLE-USE PREFILLED SYRINGE Do not try to inject HUMIRA yourself until you have been shown the right way
INSTRUCTIONS FOR USE HUMIRA (Hu-MARE-ah) (adalimumab) 40 MG/0.8 ML, 20 MG/0.4 ML AND 10 MG/0.2 ML SINGLE-USE PREFILLED SYRINGE Do not try to inject HUMIRA yourself until you have been shown the right way
Lyme (IgG and IgM) Antibody Confirmation
 Pathology & Laboratory Medicine Lyme (IgG and IgM) Antibody Confirmation TEST UPDATE: New Test Notification Date: 1/9/2013 Effective Date: 1/7/2013 CONTACT INFO Call 802-847-5121 800-991-2799 email labmarketing@vtmednet.org
Pathology & Laboratory Medicine Lyme (IgG and IgM) Antibody Confirmation TEST UPDATE: New Test Notification Date: 1/9/2013 Effective Date: 1/7/2013 CONTACT INFO Call 802-847-5121 800-991-2799 email labmarketing@vtmednet.org
LIA-VIDITEST anti-borrelia IgM
 LIA-VIDITEST anti-borrelia IgM ODZ - 317 Instruction manual PRODUCER: VIDIA s.r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice u Prahy, Czech Republic, tel.: +420 261 090 565, www.vidia.cz 1. TITLE: LIA-VIDITEST
LIA-VIDITEST anti-borrelia IgM ODZ - 317 Instruction manual PRODUCER: VIDIA s.r.o., Nad Safinou II 365, Vestec, 252 42 Jesenice u Prahy, Czech Republic, tel.: +420 261 090 565, www.vidia.cz 1. TITLE: LIA-VIDITEST
Vitamin B12. ADVIA Centaur System
 Vitamin B12 Principle of the Test ADVIA Centaur System The ADVIA Centaur VB12 assay is a competitive immunoassay using direct chemiluminescent technology in which vitamin B 12 from the patient sample competes
Vitamin B12 Principle of the Test ADVIA Centaur System The ADVIA Centaur VB12 assay is a competitive immunoassay using direct chemiluminescent technology in which vitamin B 12 from the patient sample competes
Dengue IgM ELISA. For the quantitative determination of IgM-class antibodies to Dengue Virus in serum.
 Dengue IgM ELISA For the quantitative determination of IgM-class antibodies to Dengue Virus in serum. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 20-DEMHU-E01 Size: 96
Dengue IgM ELISA For the quantitative determination of IgM-class antibodies to Dengue Virus in serum. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 20-DEMHU-E01 Size: 96
Carcinoembryonic Antigen (CEA)
 Principle of the Test Carcinoembryonic Antigen (CEA) ADVIA Centaur System The ADVIA Centaur CEA assay is a two-site sandwich immunoassay using direct chemiluminometric technology, which uses constant amounts
Principle of the Test Carcinoembryonic Antigen (CEA) ADVIA Centaur System The ADVIA Centaur CEA assay is a two-site sandwich immunoassay using direct chemiluminometric technology, which uses constant amounts
Revised 3 Oct. 2012 rm (Vers. 16.1)
 Intended Use This kit is intended for Research Use Only. Not for use in diagnostic procedures. The DRG Dengue Virus IgM Enzyme Immunoassay Kit provides materials for the qualitative and semiquantitative
Intended Use This kit is intended for Research Use Only. Not for use in diagnostic procedures. The DRG Dengue Virus IgM Enzyme Immunoassay Kit provides materials for the qualitative and semiquantitative
IgE (Human) ELISA Kit
 IgE (Human) ELISA Kit Catalog Number KA0216 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
IgE (Human) ELISA Kit Catalog Number KA0216 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Title: Fingerstick Glucose by Accu-Chek Inform
 Title: Fingerstick Glucose by Accu-Chek Inform Target Audience: This module is available to aid in assessing competency for all clinical staff who perform fingerstick glucose testing. Contents Instructions...
Title: Fingerstick Glucose by Accu-Chek Inform Target Audience: This module is available to aid in assessing competency for all clinical staff who perform fingerstick glucose testing. Contents Instructions...
INSTRUCTIONS FOR USE HUMIRA 40 MG/0.8 ML SINGLE-USE PEN
 INSTRUCTIONS FOR USE HUMIRA (Hu-MARE-ah) (adalimumab) 40 MG/0.8 ML SINGLE-USE PEN Do not try to inject HUMIRA yourself until you have been shown the right way to give the injections and have read and understand
INSTRUCTIONS FOR USE HUMIRA (Hu-MARE-ah) (adalimumab) 40 MG/0.8 ML SINGLE-USE PEN Do not try to inject HUMIRA yourself until you have been shown the right way to give the injections and have read and understand
This is a Waived Complexity test for Urine, and Moderately Complex for Serum.
 Sure-Vue Serum/Urine hcg-stat CLSI + More Packet Laboratory Name: Laboratory Address: Date of this packet: Sure-Vue Serum/Urine hcg-stat Laboratory Procedure This procedure is intended to provide a ready
Sure-Vue Serum/Urine hcg-stat CLSI + More Packet Laboratory Name: Laboratory Address: Date of this packet: Sure-Vue Serum/Urine hcg-stat Laboratory Procedure This procedure is intended to provide a ready
Albumin ELISA Kit. Albumin ELISA Kit. Zur in vitro Bestimmung des Albumin in Urin und Stuhl
 ELISA Kit Super sensitiv Zur in vitro Bestimmung des in Urin und Stuhl ELISA Kit Super sensitive For the in vitro determination of in urine and stool Gültig ab / Valid from 06.03.2007 +8 C IMM-K 6330 96
ELISA Kit Super sensitiv Zur in vitro Bestimmung des in Urin und Stuhl ELISA Kit Super sensitive For the in vitro determination of in urine and stool Gültig ab / Valid from 06.03.2007 +8 C IMM-K 6330 96
Biochemistry Validation Form
 Biochemistry Validation Form Method: Piccolo Xpress Chemistry 13 panel Manufacturer: Abaxis Cat no: 400-0029 CE marked: Yes: x No: Location of bench book: X:\Bio\Patricia\Piccollo Xpress evaluation (August
Biochemistry Validation Form Method: Piccolo Xpress Chemistry 13 panel Manufacturer: Abaxis Cat no: 400-0029 CE marked: Yes: x No: Location of bench book: X:\Bio\Patricia\Piccollo Xpress evaluation (August
Vaginal ph Test (ph Hydrion TM Paper 4.5-7.5)
 University of California, San Francisco Department of Laboratory Medicine San Francisco General Hospital 1001 Potrero Avenue, San Francisco CA 94110 Clinical Laboratory Eberhard Fiebig, M.D., Director
University of California, San Francisco Department of Laboratory Medicine San Francisco General Hospital 1001 Potrero Avenue, San Francisco CA 94110 Clinical Laboratory Eberhard Fiebig, M.D., Director
STANDARD OPERATING PROCEDURE
 TAKING BLOOD FROM INFANTS FOR THE HIV DNA PCR TEST STANDARD OPERATING PROCEDURE ACRONYMS ARV CCMT DBS Antiretrovirals Comprehensive care, management and treatment Dried blood spots This Standard Operating
TAKING BLOOD FROM INFANTS FOR THE HIV DNA PCR TEST STANDARD OPERATING PROCEDURE ACRONYMS ARV CCMT DBS Antiretrovirals Comprehensive care, management and treatment Dried blood spots This Standard Operating
RealLine HCV PCR Qualitative - Uni-Format
 Instructions for use PCR KIT FOR EXTRACTION OF RNA AND REAL TIME PCR DETECTION KIT FOR HEPATITIS C VIRUS RNA Research Use Only Qualitative Uni Format VBD0798 48 tests valid from: December 2013 Rev11122013
Instructions for use PCR KIT FOR EXTRACTION OF RNA AND REAL TIME PCR DETECTION KIT FOR HEPATITIS C VIRUS RNA Research Use Only Qualitative Uni Format VBD0798 48 tests valid from: December 2013 Rev11122013
Human CD4+T Cell Care Manual
 Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.02 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.02 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
HUMULIN 70/30 KwikPen
 1 Instructions for Use HUMULIN 70/30 KwikPen (70% human insulin isophane suspension 30% human insulin injection [rdna origin]) Read the Instructions for Use before you start taking HUMULIN 70/30 and each
1 Instructions for Use HUMULIN 70/30 KwikPen (70% human insulin isophane suspension 30% human insulin injection [rdna origin]) Read the Instructions for Use before you start taking HUMULIN 70/30 and each
EBV-VCA IgA Cat # 1414-11
 See external label 2 C-8 C Σ=96 tests Cat # 1414-11 EBV-VCA IgA Cat # 1414-11 NAME AND INTENDED USE The Diagnostic Automation Epstein-Barr Virus-Viral Capsid Antigen (EBV-VCA) IgA Enzyme-linked Immunosorbent
See external label 2 C-8 C Σ=96 tests Cat # 1414-11 EBV-VCA IgA Cat # 1414-11 NAME AND INTENDED USE The Diagnostic Automation Epstein-Barr Virus-Viral Capsid Antigen (EBV-VCA) IgA Enzyme-linked Immunosorbent
WIESLAB Complement system
 Instruction WIESLAB Complement system Alternative pathway Enzyme immunoassay for assessment of Complement functional activity Break apart microtitration strips (12x8) 96 wells Store the kit at +2-8 C Store
Instruction WIESLAB Complement system Alternative pathway Enzyme immunoassay for assessment of Complement functional activity Break apart microtitration strips (12x8) 96 wells Store the kit at +2-8 C Store
LAB 14 ENZYME LINKED IMMUNOSORBENT ASSAY (ELISA)
 STUDENT GUIDE LAB 14 ENZYME LINKED IMMUNOSORBENT ASSAY (ELISA) GOAL The goal of this laboratory lesson is to explain the concepts and technique of enzyme linked immunosorbent assay (ELISA). OBJECTIVES
STUDENT GUIDE LAB 14 ENZYME LINKED IMMUNOSORBENT ASSAY (ELISA) GOAL The goal of this laboratory lesson is to explain the concepts and technique of enzyme linked immunosorbent assay (ELISA). OBJECTIVES
Inc. Wuhan. Quantity Pre-coated, ready to use 96-well strip plate 1 Plate sealer for 96 wells 4 Standard (liquid) 2
 Uscn Life Science Inc. Wuhan Website: www.uscnk.com Phone: +86 27 84259552 Fax: +86 27 84259551 E-mail: uscnk@uscnk.com ELISA Kit for Human Prostaglandin E1(PG-E1) Instruction manual Cat. No.: E0904Hu
Uscn Life Science Inc. Wuhan Website: www.uscnk.com Phone: +86 27 84259552 Fax: +86 27 84259551 E-mail: uscnk@uscnk.com ELISA Kit for Human Prostaglandin E1(PG-E1) Instruction manual Cat. No.: E0904Hu
Cancer Antigen CA 125
 Principle of the Test Cancer Antigen CA 125 ADVIA Centaur System The ADVIA Centaur CA 125 II assay is a two-site sandwich immunoassay using direct chemiluminometric technology, which uses two monoclonal
Principle of the Test Cancer Antigen CA 125 ADVIA Centaur System The ADVIA Centaur CA 125 II assay is a two-site sandwich immunoassay using direct chemiluminometric technology, which uses two monoclonal
Direct Testing Systems and Serology
 Direct Testing Systems and Serology Rapid Manual Tests 6-2 Serology Diagnostics 6-6 BD Diagnostics Diagnostic Systems Catalog 2005/2006 6-1 Rapid Manual Tests Meningitis Test Systems 252360 Directigen
Direct Testing Systems and Serology Rapid Manual Tests 6-2 Serology Diagnostics 6-6 BD Diagnostics Diagnostic Systems Catalog 2005/2006 6-1 Rapid Manual Tests Meningitis Test Systems 252360 Directigen
MEDICAL DEVICE GUIDANCE
 May 2014 MEDICAL DEVICE GUIDANCE GN-23: Guidance on Labelling for Medical Devices PREFACE This document is intended to provide general guidance. Although we have tried to ensure that the information contained
May 2014 MEDICAL DEVICE GUIDANCE GN-23: Guidance on Labelling for Medical Devices PREFACE This document is intended to provide general guidance. Although we have tried to ensure that the information contained
HUMULIN (HU-mu-lin) N
 Instructions for Use HUMULIN (HU-mu-lin) N (human insulin [rdna origin] isophane suspension) vial (100 Units/mL, U-100) Read the Instructions for Use before you start taking HUMULIN N and each time you
Instructions for Use HUMULIN (HU-mu-lin) N (human insulin [rdna origin] isophane suspension) vial (100 Units/mL, U-100) Read the Instructions for Use before you start taking HUMULIN N and each time you
Dot Blot Analysis. Teacher s Guidebook. (Cat. # BE 502) think proteins! think G-Biosciences www.gbiosciences.com
 PR110 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Dot Blot Analysis Teacher s Guidebook (Cat. # BE 502) think proteins! think G-Biosciences
PR110 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Dot Blot Analysis Teacher s Guidebook (Cat. # BE 502) think proteins! think G-Biosciences
FINAL DOCUMENT. Global Harmonization Task Force. Title: Label and Instructions for Use for Medical Devices
 GHTF/SG1/N70:2011 FINAL DOCUMENT Global Harmonization Task Force Title: Label and Instructions for Use for Medical Devices Authoring Group: Study Group 1 of the Global Harmonization Task Force Endorsed
GHTF/SG1/N70:2011 FINAL DOCUMENT Global Harmonization Task Force Title: Label and Instructions for Use for Medical Devices Authoring Group: Study Group 1 of the Global Harmonization Task Force Endorsed
Hemoglobin Testing using the Data Management HemoCue Analyzer
 The Johns Hopkins Medical Institutions Hemoglobin Testing using the Data Management HemoCue Analyzer A Self-Study Packet for The Johns Hopkins Hospital Point-of-Care Testing Program Revision: 12/02 copyright
The Johns Hopkins Medical Institutions Hemoglobin Testing using the Data Management HemoCue Analyzer A Self-Study Packet for The Johns Hopkins Hospital Point-of-Care Testing Program Revision: 12/02 copyright
URINE DIPSTICK Multistix 10SG
 The Johns Hopkins Hospital Point-of-Care Testing Program URINE DIPSTICK Multistix 10SG Operator Competency Only operators who have completed a defined training program and can demonstrate competence will
The Johns Hopkins Hospital Point-of-Care Testing Program URINE DIPSTICK Multistix 10SG Operator Competency Only operators who have completed a defined training program and can demonstrate competence will
MANITOBA PATIENT SERVICE CENTRE STANDARDS
 MANITOBA PATIENT SERVICE CENTRE STANDARDS February 2015 INTRODUCTION These Standards are derived from Z316.7-12 and are approved by the Council of the College of Physicians and Surgeons of Manitoba. These
MANITOBA PATIENT SERVICE CENTRE STANDARDS February 2015 INTRODUCTION These Standards are derived from Z316.7-12 and are approved by the Council of the College of Physicians and Surgeons of Manitoba. These
FLORENCE TOWNSHIP BOARD OF EDUCATION FILE CODE: 4112.4/4212.4 Florence, New Jersey
 FLORENCE TOWNSHIP BOARD OF EDUCATION FILE CODE: 4112.4/4212.4 Florence, New Jersey Regulation Exposure Control Administration BLOODBORNE PATHOGENS A. The district safety and health program officer, district
FLORENCE TOWNSHIP BOARD OF EDUCATION FILE CODE: 4112.4/4212.4 Florence, New Jersey Regulation Exposure Control Administration BLOODBORNE PATHOGENS A. The district safety and health program officer, district
Tick-Borne Encephalitis (TBE/FSME) Virus IgG Human ELISA Kit
 ab108773 Tick-Borne Encephalitis (TBE/FSME) Virus IgG Human ELISA Kit Instructions for Use For the quantitative measurement of IgG class antibodies against Tick-Borne Encephalitis (TBE/FSME) Virus in Human
ab108773 Tick-Borne Encephalitis (TBE/FSME) Virus IgG Human ELISA Kit Instructions for Use For the quantitative measurement of IgG class antibodies against Tick-Borne Encephalitis (TBE/FSME) Virus in Human
Malondialdehyde (MDA) ELISA
 Malondialdehyde (MDA) ELISA For the quantitative determination of MDA in serum, plasma and other biological fluids Cat. No. KT-21493 For Research Use Only. Not for use in diagnostic procedures. Page 1
Malondialdehyde (MDA) ELISA For the quantitative determination of MDA in serum, plasma and other biological fluids Cat. No. KT-21493 For Research Use Only. Not for use in diagnostic procedures. Page 1
Cancer Antigen CA125 Human ELISA Kit
 ab108653 Cancer Antigen CA125 Human ELISA Kit Instructions for Use For the quantitative measurement of Human Cancer Antigen CA125 concentrations in serum. This product is for research use only and is not
ab108653 Cancer Antigen CA125 Human ELISA Kit Instructions for Use For the quantitative measurement of Human Cancer Antigen CA125 concentrations in serum. This product is for research use only and is not
Immunoglobulin E (IgE) concentrations in Human. Immunoglobulin E (IgE) Human ELISA Kit
 ab108650 Immunoglobulin E (IgE) Human ELISA Kit Instructions for Use For the quantitative measurement of Immunoglobulin E (IgE) concentrations in Human serum. This product is for research use only and
ab108650 Immunoglobulin E (IgE) Human ELISA Kit Instructions for Use For the quantitative measurement of Immunoglobulin E (IgE) concentrations in Human serum. This product is for research use only and
Development and Validation of In Vitro Diagnostic Tests. YC Lee, Ph.D. CEO
 Development and Validation of In Vitro Diagnostic Tests YC Lee, Ph.D. CEO 1 Validation of In Vitro Diagnostic Tests Validated d Diagnostic Test should: Provides test results that identify if positive i
Development and Validation of In Vitro Diagnostic Tests YC Lee, Ph.D. CEO 1 Validation of In Vitro Diagnostic Tests Validated d Diagnostic Test should: Provides test results that identify if positive i
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. PRODUCT IDENTIFICATION 1.1 Product Name: Cyanogen Bromide Fibrinogen Fragments 1.2 Product REF: 459 1.3 Configuration: One (1) vial, 5.0 mg, lyophilized 1.4 Use of Product:
MATERIAL SAFETY DATA SHEET 1. PRODUCT IDENTIFICATION 1.1 Product Name: Cyanogen Bromide Fibrinogen Fragments 1.2 Product REF: 459 1.3 Configuration: One (1) vial, 5.0 mg, lyophilized 1.4 Use of Product:
TRIIODOTHYRONINE (T3) ELISA Kit Protocol
 TRIIODOTHYRONINE (T3) ELISA Kit Protocol (Cat. No.:EK-310-05) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com INTENDED USE For the
TRIIODOTHYRONINE (T3) ELISA Kit Protocol (Cat. No.:EK-310-05) 330 Beach Road, Burlingame CA Tel: 650-558-8898 Fax: 650-558-1686 E-Mail: info@phoenixpeptide.com www.phoenixpeptide.com INTENDED USE For the
Nova Max Blood Glucose Monitor Owner s Guide
 Nova Max Blood Glucose Monitor Owner s Guide Nova Biomedical 200 Prospect Street Waltham, MA 02454-9141 U.S.A. Telephone: 1-800-681-7390 Web site: www.novacares.com Made in the USA by Nova Biomedical Corporation
Nova Max Blood Glucose Monitor Owner s Guide Nova Biomedical 200 Prospect Street Waltham, MA 02454-9141 U.S.A. Telephone: 1-800-681-7390 Web site: www.novacares.com Made in the USA by Nova Biomedical Corporation
PCR and Sequencing Reaction Clean-Up Kit (Magnetic Bead System) 50 preps Product #60200
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com PCR and Sequencing Reaction Clean-Up Kit (Magnetic Bead System)
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com PCR and Sequencing Reaction Clean-Up Kit (Magnetic Bead System)
ClikSTAR - Important facts about your new insulin delivery device.
 ClikSTAR - Important facts about your new insulin delivery device. Instruction for Use ClikSTAR Insulin delivery device Before you start: Read these instructions and follow them completely each time you
ClikSTAR - Important facts about your new insulin delivery device. Instruction for Use ClikSTAR Insulin delivery device Before you start: Read these instructions and follow them completely each time you
Anti-ss-DNA Screen (IgA, IgG, IgM) ELISA
 Anti-ss-DNA Screen (IgA, IgG, IgM) ELISA For the quantitative/qualitative determination of IgA/G/M against single stranded DNA in serum For Research Use Only. Not For Use In Diagnostic Procedures. Catalog
Anti-ss-DNA Screen (IgA, IgG, IgM) ELISA For the quantitative/qualitative determination of IgA/G/M against single stranded DNA in serum For Research Use Only. Not For Use In Diagnostic Procedures. Catalog
Mouse glycated hemoglobin A1c(GHbA1c) ELISA Kit
 Mouse glycated hemoglobin A1c(GHbA1c) ELISA Kit Catalog Number. CSB-E08141m For the quantitative determination of mouse glycated hemoglobin A1c(GHbA1c) concentrations in lysate for RBC. This package insert
Mouse glycated hemoglobin A1c(GHbA1c) ELISA Kit Catalog Number. CSB-E08141m For the quantitative determination of mouse glycated hemoglobin A1c(GHbA1c) concentrations in lysate for RBC. This package insert
Bovine Vitamin B12 (VB12) ELISA Kit
 Bovine Vitamin B12 (VB12) ELISA Kit Catalog Number. CSB-E14089B For the quantitative determination of endogenic bovine vitamin B12 (VB12) concentrations in serum, plasma, tissue homogenates. This package
Bovine Vitamin B12 (VB12) ELISA Kit Catalog Number. CSB-E14089B For the quantitative determination of endogenic bovine vitamin B12 (VB12) concentrations in serum, plasma, tissue homogenates. This package
HiPer Blood Grouping Teaching Kit
 HiPer Blood Grouping Teaching Kit Product Code: HTI008 Number of experiments that can be performed: 100 Duration of Experiment: Protocol: 15 minutes Storage Instructions: The kit is stable for 6 months
HiPer Blood Grouping Teaching Kit Product Code: HTI008 Number of experiments that can be performed: 100 Duration of Experiment: Protocol: 15 minutes Storage Instructions: The kit is stable for 6 months
Rat Fibronectin ELISA Kit
 AssayMax TM Rat Fibronectin ELISA Kit Assaypro LLC 3400 Harry S Truman Blvd St. Charles, MO 63301 T (636) 447-9175 F (636) 395-7419 www.assaypro.com For any questions regarding troubleshooting or performing
AssayMax TM Rat Fibronectin ELISA Kit Assaypro LLC 3400 Harry S Truman Blvd St. Charles, MO 63301 T (636) 447-9175 F (636) 395-7419 www.assaypro.com For any questions regarding troubleshooting or performing
Contents. Approved by: Kent Lewandrowski, M.D. 6/1/2005 Written/Updated by: Gino Pagnani Date: 4/3/09
 POCT Program Massachusetts General Hospital - Pathology Service 55 Fruit Street, Boston, MA 02114 Title: Hemoccult Sensa Fecal Occult Blood Procedure Cross References: Hemoccult Sensa Fecal Occult Blood
POCT Program Massachusetts General Hospital - Pathology Service 55 Fruit Street, Boston, MA 02114 Title: Hemoccult Sensa Fecal Occult Blood Procedure Cross References: Hemoccult Sensa Fecal Occult Blood
Peptic Ulcer. Anatomy The stomach is a hollow organ. It is located in the upper abdomen, under the ribs.
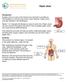 Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
Hepatitis and Retrovirus. LIAISON XL Accurate detection of HIV infection. HIV Ab/Ag FOR OUTSIDE THE US AND CANADA ONLY
 Hepatitis and Retrovirus LIAISON XL Accurate detection of HIV infection HIV Ab/Ag FOR OUTSIDE THE US AND CANADA ONLY LIAISON XL HIV Ab/Ag is Your solution LIAISON XL murex HIV Ab/Ag main features The LIAISON
Hepatitis and Retrovirus LIAISON XL Accurate detection of HIV infection HIV Ab/Ag FOR OUTSIDE THE US AND CANADA ONLY LIAISON XL HIV Ab/Ag is Your solution LIAISON XL murex HIV Ab/Ag main features The LIAISON
Introduction 1 The system 1 The meter 2 The display 3 The mode 3 The measurement 4 Coding the meter 4 How to obtain a drop of blood 6 Application of
 Introduction 1 The system 1 The meter 2 The display 3 The mode 3 The measurement 4 Coding the meter 4 How to obtain a drop of blood 6 Application of the blood 7 Procedure to test glucose 7 Procedure to
Introduction 1 The system 1 The meter 2 The display 3 The mode 3 The measurement 4 Coding the meter 4 How to obtain a drop of blood 6 Application of the blood 7 Procedure to test glucose 7 Procedure to
ELISA-VIDITEST-MICROCYSTIN LR Lot: XX
 ELISA-VIDITEST-MICROCYSTIN LR Lot: XX Instruction manual PRODUCER : VIDIA Ltd., Nad Safinou II/365, Vestec, 252 42 Jesenice u Prahy, Czech Republic, Tel.: +420261090566 1. TITLE: ELISA-VIDITEST MICROCYSTIN
ELISA-VIDITEST-MICROCYSTIN LR Lot: XX Instruction manual PRODUCER : VIDIA Ltd., Nad Safinou II/365, Vestec, 252 42 Jesenice u Prahy, Czech Republic, Tel.: +420261090566 1. TITLE: ELISA-VIDITEST MICROCYSTIN
Intact PTH. system. Intact PTH 84-6434/R5. Key to symbols used 8K25
 system en Intact PTH 8K25 84-6434/R5 Intact PTH Read Highlighted Changes Revised November, 2008 Customer Service United States: 1-877-4ABBOTT International: Call your Abbott Representative This package
system en Intact PTH 8K25 84-6434/R5 Intact PTH Read Highlighted Changes Revised November, 2008 Customer Service United States: 1-877-4ABBOTT International: Call your Abbott Representative This package
Rat Creatine Kinase MB isoenzyme,ck-mb ELISA Kit
 Rat Creatine Kinase MB isoenzyme,ck-mb ELISA Kit Catalog No: E0479r 96 Tests Operating instructions www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Rat Creatine Kinase MB isoenzyme,ck-mb ELISA Kit Catalog No: E0479r 96 Tests Operating instructions www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
ab108711 Anti- Borrelia burgdorferi IgM Human ELISA Kit
 ab108711 Anti- Borrelia burgdorferi IgM Human ELISA Kit Instructions for Use For the qualitative measurement of IgM class antibodies against Borrelia burgdorferi in Human serum, plasma and CSF (cerebrospinal
ab108711 Anti- Borrelia burgdorferi IgM Human ELISA Kit Instructions for Use For the qualitative measurement of IgM class antibodies against Borrelia burgdorferi in Human serum, plasma and CSF (cerebrospinal
Human Free Testosterone(F-TESTO) ELISA Kit
 Human Free Testosterone(F-TESTO) ELISA Kit Catalog Number. MBS700040 For the quantitative determination of human free testosterone(f-testo) concentrations in serum, plasma. This package insert must be
Human Free Testosterone(F-TESTO) ELISA Kit Catalog Number. MBS700040 For the quantitative determination of human free testosterone(f-testo) concentrations in serum, plasma. This package insert must be
STANDARD OPERATING PROCEDURE FOR NOVA STAT STRIP CONNECTIVITY BLOOD GLUCOSE METERS FOR USE IN LEEDS NHS TRUST
 STANDARD OPERATING PROCEDURE FOR NOVA STAT STRIP CONNECTIVITY BLOOD GLUCOSE METERS FOR USE IN LEEDS NHS TRUST This Standard Operating Procedure explains the protocol for measuring blood glucose concentration
STANDARD OPERATING PROCEDURE FOR NOVA STAT STRIP CONNECTIVITY BLOOD GLUCOSE METERS FOR USE IN LEEDS NHS TRUST This Standard Operating Procedure explains the protocol for measuring blood glucose concentration
Unaccredited Point-of-Care Laboratory Testing Guideline for Physicians
 Unaccredited Point-of-Care Laboratory Testing Guideline for Physicians Prepared by the Advisory Committee on Laboratory Medicine College of Physicians & Surgeons of Alberta Serving the Public by guiding
Unaccredited Point-of-Care Laboratory Testing Guideline for Physicians Prepared by the Advisory Committee on Laboratory Medicine College of Physicians & Surgeons of Alberta Serving the Public by guiding
Hand Hygiene and Infection Control
 C Hand Hygiene and Infection Control Sirius Business Services Ltd www.siriusbusinessservices.co.uk Tel 01305 769969 info@siriusbusinessservices.co.uk Whatever your First Aid, Fire Safety or Health & Safety
C Hand Hygiene and Infection Control Sirius Business Services Ltd www.siriusbusinessservices.co.uk Tel 01305 769969 info@siriusbusinessservices.co.uk Whatever your First Aid, Fire Safety or Health & Safety
Borrelia burgdorferi IgM Human ELISA Kit
 ab108711 Borrelia burgdorferi IgM Human ELISA Kit Instructions for Use For the qualitative determination of IgM class antibodies against Borrelia burgdorferi in Human serum or plasma (citrate). This product
ab108711 Borrelia burgdorferi IgM Human ELISA Kit Instructions for Use For the qualitative determination of IgM class antibodies against Borrelia burgdorferi in Human serum or plasma (citrate). This product
SMF Awareness Seminar 2014
 SMF Awareness Seminar 2014 Clinical Evaluation for In Vitro Diagnostic Medical Devices Dr Jiang Naxin Health Sciences Authority Medical Device Branch 1 In vitro diagnostic product means Definition of IVD
SMF Awareness Seminar 2014 Clinical Evaluation for In Vitro Diagnostic Medical Devices Dr Jiang Naxin Health Sciences Authority Medical Device Branch 1 In vitro diagnostic product means Definition of IVD
VACUETTE RNAgard Blood Tubes BioMaxi Blood RNA Purification Kit. For the collection, preservation and purification of RNA from whole blood
 RNAgard Blood System VACUETTE RNAgard Blood Tubes BioMaxi Blood RNA Purification Kit For the collection, preservation and purification of RNA from whole blood Instructions For Use Protocol blood collection,
RNAgard Blood System VACUETTE RNAgard Blood Tubes BioMaxi Blood RNA Purification Kit For the collection, preservation and purification of RNA from whole blood Instructions For Use Protocol blood collection,
Algorithm for detecting Zika virus (ZIKV) 1
 Algorithm for detecting Zika virus (ZIKV) 1 This algorithm is addressed to laboratories with established capacity (molecular, antigenic and/or serological) to detect dengue (DENV), Zika (ZIKV) 2, and chikungunya
Algorithm for detecting Zika virus (ZIKV) 1 This algorithm is addressed to laboratories with established capacity (molecular, antigenic and/or serological) to detect dengue (DENV), Zika (ZIKV) 2, and chikungunya
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY
 A. 510(k) Number: K092353 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY B. Purpose for Submission: This is a new 510k application for a new indication for the MONOLISA Anti-HAV IgM EIA
A. 510(k) Number: K092353 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY B. Purpose for Submission: This is a new 510k application for a new indication for the MONOLISA Anti-HAV IgM EIA
Mouse krebs von den lungen 6 (KL-6) ELISA
 KAMIYA BIOMEDICAL COMPANY Mouse krebs von den lungen 6 (KL-6) ELISA For the quantitative determination of mouse KL-6 in serum, plasma, cell culture supernatants, body fluid and tissue homogenate Cat. No.
KAMIYA BIOMEDICAL COMPANY Mouse krebs von den lungen 6 (KL-6) ELISA For the quantitative determination of mouse KL-6 in serum, plasma, cell culture supernatants, body fluid and tissue homogenate Cat. No.
Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum
 SUPPLEMENTAL PROTOCOL WHITE PAPER Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum Bee Na Lee, Ph.D., Beckman Coulter Life Sciences Process
SUPPLEMENTAL PROTOCOL WHITE PAPER Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum Bee Na Lee, Ph.D., Beckman Coulter Life Sciences Process
Identifying Celiac Disease and Gluten Sensitivity with Minimally Invasive Testing
 Identifying Celiac Disease and Gluten Sensitivity with Minimally Invasive Testing Enjoy this white paper by Jack A. Maggiore, PhD, MT(ASCP), Assistant Director, Chemistry Laboratory and R&D at Doctor s
Identifying Celiac Disease and Gluten Sensitivity with Minimally Invasive Testing Enjoy this white paper by Jack A. Maggiore, PhD, MT(ASCP), Assistant Director, Chemistry Laboratory and R&D at Doctor s
FBS06 P30 Antigen Test for the Presence of Seminal Fluid
 FBS06 P30 Antigen Test for the Presence of Seminal Fluid Table of Contents 1. Scope 2. Background 3. Safety 4. Materials Required 5. Standards and Controls 6. Calibration 7. Procedures 8. Sampling 9. Calculations
FBS06 P30 Antigen Test for the Presence of Seminal Fluid Table of Contents 1. Scope 2. Background 3. Safety 4. Materials Required 5. Standards and Controls 6. Calibration 7. Procedures 8. Sampling 9. Calculations
Mercyhurst University Athletic Training Program Bloodborne Pathogens Exposure Control Plan
 Mercyhurst University Athletic Training Program Bloodborne Pathogens Exposure Control Plan In accordance with the Occupational Safety Health Administration (OSHA) Bloodborne Pathogens Standard, 29 CFR
Mercyhurst University Athletic Training Program Bloodborne Pathogens Exposure Control Plan In accordance with the Occupational Safety Health Administration (OSHA) Bloodborne Pathogens Standard, 29 CFR
MYCOLOGY PARASITOLOGY RHEUMATOID FACTORS VIROLOGY SAMPLE COLLECTION
 C A T A L O G U E C L I N I C A L D I A G N O S T I C S couv BACTERIOLOGYOGY MYCOLOGY PARASITOLOGY RHEUMATOID FACTORS VIROLOGY SAMPLE COLLECTION couv EXPORT DEPARTMENT Orders, technical and commercial
C A T A L O G U E C L I N I C A L D I A G N O S T I C S couv BACTERIOLOGYOGY MYCOLOGY PARASITOLOGY RHEUMATOID FACTORS VIROLOGY SAMPLE COLLECTION couv EXPORT DEPARTMENT Orders, technical and commercial
Inform II. Quick Reference Guide. for Healthcare Professionals BLOOD GLUCOSE MONITORING SYSTEM
 Inform II BLOOD GLUCOSE MONITORING SYSTEM Quick Reference Guide for Healthcare Professionals 2 Table of Contents Important... 4 General Information... 6 ACCU-CHEK Inform II Meter...10 How to Perform Patient
Inform II BLOOD GLUCOSE MONITORING SYSTEM Quick Reference Guide for Healthcare Professionals 2 Table of Contents Important... 4 General Information... 6 ACCU-CHEK Inform II Meter...10 How to Perform Patient
SYSTEM. For In Vitro Diagnostic Use. Store at 2-8 C
 SYSTEM E HAVAb-IgG 6C29 B6C290 36-6800/R1 HAVAb-IgG This package insert must be read carefully prior to use. Package insert instructions must be followed accordingly. Reliability of assay results cannot
SYSTEM E HAVAb-IgG 6C29 B6C290 36-6800/R1 HAVAb-IgG This package insert must be read carefully prior to use. Package insert instructions must be followed accordingly. Reliability of assay results cannot
BioResearch. RAFT 3D Cell Culture Kit Protocol
 BioResearch RAFT 3D Cell Culture Kit Protocol RAFT 3D Cell Culture Kit This protocol describes in detail how to produce RAFT 3D Cell Cultures in 96-well plates, 24-well plates and 24-well inserts. It is
BioResearch RAFT 3D Cell Culture Kit Protocol RAFT 3D Cell Culture Kit This protocol describes in detail how to produce RAFT 3D Cell Cultures in 96-well plates, 24-well plates and 24-well inserts. It is
Anti-M2 (IgG) ELISA. For the quantitative and qualitative detection of antibodies against M2 in serum.
 Anti-M2 (IgG) ELISA For the quantitative and qualitative detection of antibodies against M2 in serum. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 35-M2GHU-E01 Size: 96
Anti-M2 (IgG) ELISA For the quantitative and qualitative detection of antibodies against M2 in serum. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 35-M2GHU-E01 Size: 96
Canine Creatine Kinase MM isoenzyme(ck-mm) ELISA. kit
 BlueGene Biotech. Tel: 0086-21-61471242 Fax: 0086-21-61471242 ext 806 E-mail: sales@bluegene.cc tech@bluegene.cc www.elisakit.cc www.bluegene.cc Canine Creatine Kinase MM isoenzyme(ck-mm) ELISA kit 96
BlueGene Biotech. Tel: 0086-21-61471242 Fax: 0086-21-61471242 ext 806 E-mail: sales@bluegene.cc tech@bluegene.cc www.elisakit.cc www.bluegene.cc Canine Creatine Kinase MM isoenzyme(ck-mm) ELISA kit 96
THE PREPARATION OF SINGLE DONOR CRYOPRECIPITATE
 FACTS AND FIGURES JUNE 2004 NO 2 THE PREPARATION OF SINGLE DONOR CRYOPRECIPITATE Revised Edition Shân Lloyd National Blood Transfusion Service Zimbabwe Published by the World Federation of Hemophilia (WFH);
FACTS AND FIGURES JUNE 2004 NO 2 THE PREPARATION OF SINGLE DONOR CRYOPRECIPITATE Revised Edition Shân Lloyd National Blood Transfusion Service Zimbabwe Published by the World Federation of Hemophilia (WFH);
OraQuick HCV Rapid Antibody Test Customer Letter
 OraQuick HCV Rapid Antibody Test Customer Letter Dear Customer, Thank you for deciding to use the OraQuick HCV Rapid Antibody Test. The sale, distribution, and use of this product is restricted as described
OraQuick HCV Rapid Antibody Test Customer Letter Dear Customer, Thank you for deciding to use the OraQuick HCV Rapid Antibody Test. The sale, distribution, and use of this product is restricted as described
HiPer RA Test Teaching Kit
 HiPer RA Test Teaching Kit Product Code: HTI019 Number of experiments that can be performed: 20 Duration of Experiment: 1hour Storage Instructions: The kit is stable for 6 months from the date of receipt
HiPer RA Test Teaching Kit Product Code: HTI019 Number of experiments that can be performed: 20 Duration of Experiment: 1hour Storage Instructions: The kit is stable for 6 months from the date of receipt
Prevention and control of infection in care homes. Summary for staff
 Prevention and control of infection in care homes Summary for staff 1 DH INFORMATION READER BOX Policy Clinical Estates HR / Workforce Commissioner Development IM & T Management Provider Development Finance
Prevention and control of infection in care homes Summary for staff 1 DH INFORMATION READER BOX Policy Clinical Estates HR / Workforce Commissioner Development IM & T Management Provider Development Finance
ECL Western Blotting Substrate INSTRUCTIONS FOR USE OF PRODUCTS W1001 AND W1015.
 Technical Manual ECL Western Blotting Substrate INSTRUCTIONS FOR USE OF PRODUCTS W1001 AND W1015. PRINTED IN USA. 6/09 ECL Western Blotting Substrate All technical literature is available on the Internet
Technical Manual ECL Western Blotting Substrate INSTRUCTIONS FOR USE OF PRODUCTS W1001 AND W1015. PRINTED IN USA. 6/09 ECL Western Blotting Substrate All technical literature is available on the Internet
Human Parathyroid Hormone (PTH) ELISA Kit
 AssayMax TM Human Parathyroid Hormone (PTH) ELISA Kit Assaypro LLC 3400 Harry S Truman Blvd St. Charles, MO 63301 T (636) 447-9175 F (636) 395-7419 www.assaypro.com For any questions regarding troubleshooting
AssayMax TM Human Parathyroid Hormone (PTH) ELISA Kit Assaypro LLC 3400 Harry S Truman Blvd St. Charles, MO 63301 T (636) 447-9175 F (636) 395-7419 www.assaypro.com For any questions regarding troubleshooting
