How are substances transported within cells and across cell membranes?
|
|
|
- Clifford Howard
- 9 years ago
- Views:
Transcription
1 EXERCISE 5 Name How are substances transported within cells and across cell membranes? Objectives After completing this exercise, you should be able to: Describe the kinetic theory of matter and explain what kinetic energy is. Distinguish between movement and net movement. Also, describe the factors that affect the direction and speed of molecular movement and net movement. Define and distinguish between: selectively permeable membrane, plasma membrane, dialysis tubing. For each of the following transport mechanisms, explain how the mechanism works and discuss the factors that affect the rate and direction of transport: simple diffusion, facilitated diffusion, osmosis, dialysis, active transport. Describe how chemical indicators can be used to test for the presence of starch and reducing sugars. Distinguish between isotonic, hypotonic, and hypertonic solutions. Also explain what happens when cells are placed in each type of solution. Prelab Before you come to lab, read this entire exercise. You must also answer all questions and complete all assignments on the first 8 pages of this exercise. Your instructor will give you directions on when and where to turn in your work. Cell survival depends on the movement of substances (such as enzymes, and chemical messengers) within the cell. In addition, certain materials (such as nutrients) must enter the cell, and other materials (such as waste products) must leave. In order to enter or leave a cell, materials must cross the plasma membrane. The movement of substances within the cell or across the plasma membrane is called cell transport. Mechanisms of cell transport can be divided into 2 main categories: passive transport and active transport. Both passive and active transport require energy. However, with passive transport, the cell itself does not expend any energy. In other words, the cell is passive. Energy for passive transport usually comes from random molecular motion. With active transport, on the other hand, the cell must expend some of its own energy, stored in molecules of ATP. In this lab, you will study various types of passive transport. To understand how passive transport works, you need to understand the kinetic theory of matter. According to this theory, all atoms and molecules are in constant random motion. This gives them energy of motion, also called kinetic energy. The kinetic energy of atoms and molecules is detected by humans as heat. As a substance is heated, the atoms and molecules move faster, and their kinetic energy increases. Furthermore, the constant random motion of atoms and molecules causes adjacent substances to become evenly mixed together, even if the substances are undisturbed by outside forces. As an example, suppose you place a sugar cube in a glass of water. Within a few minutes, the currents created by dropping the sugar cube into the water subside and the water is completely still - to the naked eye. Even if left undisturbed, however, each water molecule in the glass is actually moving in a straight line until it bumps into the sugar cube, the glass, or another water molecule; then it ricochets off in another direction. At the same time, individual sucrose molecules in the cube are also moving and being bombarded by water molecules from all directions. This bombardment eventually shakes individual sucrose molecules free of the sugar cube. 5-1
2 As each sucrose molecule dissolves in the water, it moves off in a straight line until it bumps into something and changes direction. Therefore, each sucrose molecule that separates from the sugar cube will move away from the cube sometimes, and toward it sometimes; its movement is random. However, when the cube is first placed in the water, more sucrose molecules will be moving away from the sugar cube (where the sugar concentration is higher) than are moving back toward the cube. Therefore, we say movement of the sucrose molecules is random, but net movement is from the area where the sugar concentration is higher to the area where the sugar concentration is lower (i.e. down a concentration gradient.) Likewise, the water molecules in the glass also move at random, but more water molecules will be moving toward the sugar cube (where the water concentration is lower) than are moving away from it. Therefore, net movement of the water molecules is toward the sugar cube. Note that each substance shows net movement down its own concentration gradient. As a result, net movement of water and sucrose are in opposite directions. This process continues until the sucrose and water molecules are evenly mixed a point called equilibrium. At equilibrium, the sucrose and water molecules continue to move at random but there is no longer any net movement of either substance. The net movement of a substance from a region where it has a higher concentration to a region where it has a lower concentration, due to random molecular motion, is called diffusion. Diffusion is a widespread and important process which occurs in both living and non-living systems. Because diffusion occurs under a variety of conditions, scientists have adopted the following terms to specify particular types of diffusion: Dialysis refers to the diffusion of solutes across a selectively permeable membrane. A selectively permeable membrane (also called a semipermeable membrane) is a membrane that allows some substances to pass through easily while other substances pass through very slowly or not at all. Osmosis, on the other hand, refers to the diffusion of the solvent across a selectively permeable membrane. Because water is the solvent in all living systems, biologists usually define osmosis as the diffusion of water across a selectively membrane. Facilitated diffusion refers to diffusion of a substance across the plasma membrane of a cell with the help of specific transport proteins. Simple diffusion refers to diffusion of a substance without the help of specific transport proteins. Although various types of diffusion have been recognized, all share the following characteristics: Net movement of each substance is caused by random molecular motion. Net movement of each substance involves passive transport. Net movement of each substance is down its own concentration gradient. At equilibrium, random molecular motion continues but there is no longer any net movement. In this lab, you will study several different types of diffusion using both non-living and living systems. First, you will conduct two experiments utilizing non-living systems: You will study the diffusion of 2 dyes, potassium permanganate and methylene blue, through a non-living, gel-like material called agar. Agar is prepared by boiling a complex polysaccharide (called agarose) in water until it dissolves and then allowing the solution to cool. As the solution cools, it turns into a semi-solid gel. You will study both dialysis and osmosis across a membrane made of dialysis tubing. Dialysis tubing is a commercially prepared, artificial membrane made of cellulose. The membrane contains microscopic pores that allow ions and molecules to pass through. The size of the pores in the membrane determines the size of the ions and molecules that can pass through. Finally, you will conduct one experiment utilizing a living system: You will study the process of osmosis across the plasma membrane of living cells found in the leaves of an aquatic plant called Elodea. 5-2
3 How do substances move across a selectively permeable membrane? A selectively permeable membrane allows some substances to pass through easily, while other substances pass through very slowly or not at all. The selective permeability of a membrane is related to its structure. For example, all cell membranes, including the plasma membrane, are selectively permeable. Cell membranes are composed of a lipid bilayer with embedded proteins. Because lipids are nonpolar, they attract other nonpolar molecules but repel polar molecules and ions. Proteins, on the other hand, often have large polar or charged regions that attract polar molecules and ions. These characteristics of lipids and proteins affect the ability of different types of substances to pass through the plasma membrane: Nonpolar molecules (such as fatty acids) can easily mix with and diffuse through the lipid bilayer of the plasma membrane. This process is called simple diffusion. Very small polar molecules (such as water) can also diffuse through the lipid bilayer. Even though nonpolar lipids tend to repel polar molecules, these molecules can pass through the lipid bilayer because of their small size. However, they pass through more slowly than nonpolar molecules. Larger polar molecules (such as amino acids and sugars) and ions of any size (such as Ca ++ ) cannot diffuse through the lipid bilayer. However, in some cases these substances can diffuse through the plasma membrane by passing through specific protein channels or carriers embedded in the membrane. Because these substances need the help of proteins to diffuse across the membrane, this process is called facilitated diffusion. Your Turn Assume there is a higher concentration of each of the following substances in the extracellular fluid surrounding a cell than in the cell s cytoplasm. Predict whether the substance would be more likely to enter the cell by simple diffusion or by facilitated diffusion, and explain why. O2 Explain why: Glucose Explain why: Na + Explain why: Insulin (a protein) Explain why: Estrogen (a lipid) Explain why: Hydrogen ions Explain why: 5-3
4 Dialysis tubing is another example of a selectively permeable membrane. However, its selectivity is much more limited than the selectivity of cell membranes. Microscopic holes, or pores, in the dialysis tubing allow substances to be separated only on the basis of size. Unlike with cell membranes, whether the substance is nonpolar, polar, or charged has little effect on its ability to cross the tubing. Molecules and ions that are smaller than the pores can move through the tubing, while those that are larger than the pores cannot. Dialysis tubing can be ordered from scientific supply companies in a variety of pore sizes. Dialysis is routinely used in biochemistry and molecular biology laboratories to separate and purify substances on the basis of size. Your Turn Of the substances listed in the exercise above (O2, glucose, Na +, insulin, estrogen, and hydrogen ions) which would not pass easily through the plasma membrane by simple diffusion, but probably would pass through the pores of dialysis tubing? (Hint: There are 2 correct answers.) Explain why: Diffusion of materials through cell membranes always involves net movement of a substance down its own concentration gradient (from higher concentration to lower concentration.) However, in some cases substances must be transported across cell membranes against their concentration gradient (from lower concentration to higher concentration.) This requires cellular energy and, therefore, a form of active transport. For example, the transfer of a phosphate group from an ATP molecule to a membrane protein may change the shape of the protein, causing it to pump a substance across the membrane against its concentration gradient. Whenever a substance is being moved against its concentration gradient, a form of active transport is required. Your turn Simple diffusion, facilitated diffusion, and active transport allow the cell to be very selective about what is allowed to cross its membranes. On each line below, write whether the indicated substance would enter the cell by simple diffusion, facilitated diffusion, or active transport. Higher concentration of glucose Lower concentration of glucose Lower concentration of K+ Higher concentration of K+ 5-4
5 How does solute concentration affect dialysis and osmosis? If two aqueous solutions are separated by a selectively permeable membrane, each solute that can pass through the membrane will show net movement down its own concentration gradient. The diffusion of solutes across a selectively permeable membrane is called dialysis. At the same time, if water (the solvent) can pass through the membrane, it will also show net movement down its own concentration gradient. Biologists call the diffusion of water across a selectively permeable membrane osmosis. Keep in mind that when we compare 2 aqueous solutions, the one with the higher concentration of solutes will have the lower concentration of water, and vice versa. Scientists use specific terms to compare the solute concentrations of different solutions. If 2 solutions have the same total concentration of solutes, the solutions are isotonic (or isosmotic.) On the other hand, if 2 solutions have different concentrations of solutes, the one with the lower concentration of solutes (higher concentration of water) is hypotonic (or hypoosmotic) and the one with the higher concentration of solutes (lower concentration of water) is hypertonic (or hyperosmotic.) Your Turn The following diagram represents a container with two aqueous solutions separated by a selectively permeable membrane. Water and both solutes can pass through the membrane freely. 1 M glucose 0.1 M glucose 1 M fructose 1.5 M fructose I. Before equilibrium is reached in this container: 1. Movement of glucose across the membrane can best be described as 2. Net movement of glucose across the membrane can best be described as 3. Movement of fructose across the membrane can best be described as 4. Net movement of fructose across the membrane can best be described as 5. Movement of water across the membrane can best be described as 6. Net movement of water across the membrane can best be described as 7. Compared to the solution on the right, the solution on the left is best described as A. isotonic B. hypotonic D. hypertonic 8. Compared to the solution on the left, the solution on the right is best described as A. isotonic B. hypotonic D. hypertonic 9. Net movement of water is A. from the hypertonic solution to the hypotonic solution B. from the hypotonic solution to the hypertonic solution C. both directions D. neither direction 5-5
6 II. After equilibrium is reached in this container: 1. Movement of glucose across the membrane can best be described as 2. Net movement of glucose across the membrane can best be described as 3. Movement of fructose across the membrane can best be described as 4. Net movement of fructose across the membrane can best be described as 5. Movement of water across the membrane can best be described as 6. Net movement of water across the membrane can best be described as 7. Compared to the solution on the right, the solution on the left is best described as A. isotonic B. hypotonic C. hypertonic 8. Compared to the solution on the left, the solution on the right is best described as A. isotonic B. hypotonic C. hypertonic III. If a cell is placed in an isotonic solution: 1. Movement of water will be A. into the cell B. out of the cell C. both in and out D. neither direction 2. Net movement of water will be A. into the cell B. out of the cell C. both in and out D. neither direction 3. The cell will tend to A. shrink B. swell C. both D. neither IV. If a cell is placed in a hypotonic solution: 1. Movement of water will be A. into the cell B. out of the cell C. both in and out D. neither direction 2. Net movement of water will be A. into the cell B. out of the cell C. both in and out D. neither direction 3. The cell will tend to A. shrink B. swell C. both D. neither V. If a cell is placed in a hypertonic solution: 1. Movement of water will be A. into the cell B. out of the cell C. both in and out D. neither direction 2. Net movement of water will be A. into the cell B. out of the cell C. both in and out D. neither direction 3. The cell will tend to A. shrink B. swell C. both D. neither 5-6
7 VI. Osmosis across membranes made of dialysis tubing In part III of the Lab Procedures, you will set up an experiment to study both dialysis and osmosis using bags made of dialysis tubing. During this experiment, four bags will be filled with different solutions and then placed in beakers also filled with different solutions. Each bag will be weighed at the beginning and end of the experiment in order to determine whether net movement of water was into the bag, out of the bag, or neither. If a bag increases in weight, that means net movement of water was into the bag; if a bag decreases in weight, that means net movement of water was out of the bag; and if there is no change in weight, that means there was no net movement of water. Read Part III of the Lab Procedures (pp ) and then label the arrows in the diagrams below to indicate the solution placed inside each bag and beaker. Then, based on your knowledge of how solute concentration affects osmosis, predict whether the weight of each dialysis bag will increase, decrease, or remain the same. Bag #1 prediction Weight of the bag will A. Increase B. Decrease C. Remain the same Bag #2 prediction Weight of the bag will A. Increase B. Decrease C. Remain the same Bag #3 prediction Weight of the bag will A. Increase B. Decrease C. Remain the same Bag #4 prediction Weight of the bag will A. Increase B. Decrease C. Remain the same 5-7
8 How can I determine if specific solutes are present in a solution? It is often a challenge to determine whether or not a certain solute is present in a solution. Many different solutes have the same color (most solutions found in cells are clear), most have no odor, tasting the solution could be hazardous, etc. Sometimes we can test for the presence of a particular solute is by adding a chemical indicator to the solution. An indicator is a chemical that will react with the solute being tested for and produce a visible change. This change could be a change in color, the formation of a solid material within the solution (a precipitate), or the formation of a gas. A negative control involves adding the indicator to a solution that does not have the solute being tested for. A positive control involves adding the indicator to a solution that has the solute being tested for. By running both negative and positive controls, you are able to rule out the possibilities that the test is defective, or that your glassware is contaminated. This will help avoid drawing the wrong conclusions from your test results. 5-8
9 Lab Procedures This lab requires a clock-watcher! Begin this lab exercise by setting up Parts II and III, and noting the time when you begin each activity. Then do Parts I, IV, and V while waiting for diffusion, osmosis, and dialysis to occur. The clock-watcher will be responsible for reminding the group when it is necessary to observe and measure the dye circles for Part II and when it is time to remove the dialysis bags for part III. I. Observe the effects of molecular motion Begin this activity after you have set up Parts II and III. 1. Place a drop of water on a clean microscope slide. Using a pair of forceps, break off a small piece of lichen (about the size of a match head) and place it in the drop of water. Use the blunt end of the forceps to thoroughly grind the lichen in the water into grayish green powder. NOTE: Lichens are organisms that superficially resemble plants but are not true plants. Instead, they are masses of interwoven cells from 2 different types of organisms a fungus and an alga. The fungus is a nonphotosynthetic organism and the alga is a photosynthetic protist. The fungal component and the algal component reproduce separately and remain distinct, yet interact as the lichen grows. Such a close, consistent co-existence is called symbiosis and the organisms involved are called symbionts. 2. Place a cover slip on the drop and view the ground-up lichen with the microscope. Find an area of the slide where the lichen powder is spread thinly, so there is plenty of light and you can see individual, separated grains of the powder. 3. Focus on low power first, and then switch to high power. Under high power, you should see tiny particles that are vibrating in place. The vibration is caused as water molecules on the slide move around and collide with the visible particles. This visible movement is called Brownian motion and is evidence for the kinetic theory of matter. Draw a diagram and describe your observations in your lab notebook. II. Determine how molecular size and temperature affect the rate of diffusion Begin this activity first. 1. Obtain the following materials: 2 room temperature agar plates, labeled A and B 1 refrigerated agar plate, labeled C Potassium permanganate solution Methylene blue solution Marking pen Ruler Micropipetters straw 2. Turn the agar plate labeled A upside down and make two small dots on the bottom of the plate with the marker. (Note: the bottom half of the agar plate is the side that contains the agar.) Each dot should be at least 2 cm from the edge of the plate and at least 3 cm away from the other dot on the bottom of the agar plate. Next to one dot, write PP (for potassium permanganate) and next to the other dot write MB (for methylene blue). Mark plates B and C the same way. 3. Remove the lid from agar plate A. Use the straw to make a well in the agar at each dot that you have marked. To do this, fold the straw over at one end to make a tight seal and hold it with one hand. Then, pinch the straw with your fingers to expel some air from the straw. Then, bore a well in the agar with the opposite end of the straw by pushing it into the agar. Finally, release the fingers pinching the straw. This will create a small vacuum in the straw and will pull a plug of agar up into the straw. Discard this and repeat until your agar plates each have two wells. 5-9
10 4. Using a micropipettor, withdraw 25 μl of potassium permanganate solution and transfer it to the well that you have made directly above the correspondingly labeled dot. Do not let the well overflow. Use the same procedure to transfer 25 μl of methylene blue dye solution to your plate A. 5. Repeat this for the potassium permanganate and methylene blue wells on plates B and C. Work quickly with the C plate so that it remains cold. 6. Keep plates A and B at room temperature, and place plate C back in the refrigerator (or on ice if a refrigerator is unavailable). For the remainder of your lab period, measure the diameter of the dye circles every 15 minutes. Record your measurements in your lab notebook, using a table such as the one below: Time (min) Potassium permanganate Methylene blue Agar A Agar B Agar C Agar A Agar B Agar C After you have prepared the agar plates with the dye solutions, begin Part III. Remember to observe your plates from this activity and record your measurements in your notebook every 15 minutes during the rest of the lab period. III. Observe how water and solutes move across an artificial semipermeable membrane (dialysis tubing) Begin this activity second. 1. Label four ml beakers with the numbers 1-4. Fill each beaker with about 150 ml of the solutions listed in the table below. Always wear gloves when handling dialysis tubing! Oils from your fingers will plug up the pores and ruin the tubing. 2. Cut 4 pieces of dialysis tubing if they haven't been provided precut for you. Each piece should be approximately 20 cm long. 3. Soak the dialysis tubing in a small beaker of distilled water for 1-2 minutes. Then use the following procedure to create 4 dialysis bags that you will fill with the solutions listed in the table below: a. Remove a piece of tubing from the distilled water and separate the sides to form a tube (similar to the way you separate the sides of a plastic produce bag at the grocery store.) Place a clip across the bottom of the tubing or carefully tie it closed. Take care to make a tight seal without tearing the bag. b. Fill the bag with approximately 10 ml of the appropriate solution (see the table below) using a 10 ml pipette. Be sure to use a clean pipette for each solution. c. Carefully squeeze air out of the bag and place a clip across the top of the bag, or tie it closed. The closed bag should not be tightly filled; leave enough room in the bag to allow it to swell as more water enters. Use a paper towel to carefully blot dry any spilled solution on the outside of the bag. Place the bag on a paper towel. You can write on the paper towel to identify the bags
11 Fill the beakers as follows: Beaker #1: distilled water Beaker #2: distilled water Beaker #3: distilled water Beaker #4: 10% glucose solution Fill the bags as follows: Bag #1: 1% starch solution Bag #2: 20% glucose solution Bag #3: 40% glucose solution Bag #4: 10% glucose solution 4. After you have filled all of the bags and blotted them dry, weigh them, and record their before dialysis weights in a clearly labeled table in your lab notebook. 5. Place all the bags in their corresponding beakers at the same time and record the time. 6. When the bags have been in the beakers for at least 90 minutes (take more time if you have it), remove all of the bags from the beakers at the same time. Do not discard the solutions in the beakers! Rinse the bags with tap water, carefully blot them dry with paper towels, and place them on a clean paper towel, labeling the bags by writing on the paper towel. 7. Weigh the bags and enter the data in the table in your lab notebook in order to compare this weight with the weight of the dialysis bags before incubating them in the beakers of solutions. 8. Carry out a series of tests, using the 2 indicators you studied in part IV, to determine which solute(s) crossed the dialysis membrane and which solute(s) did not. Record the results in your lab notebook and show them to your instructor before you discard the dialysis bags and the solutions in each beaker. IV. Determine which indicator to use to detect the presence of glucose or starch In this part of the lab, you will determine how to use indicators to detect the presence of two substances: starch and a reducing sugar (such as glucose). 1. a. Label three test tubes B1, B2, and B3. To tube B1 add 1.0 ml dh2o; to tube B2 add 1.0 ml 40% glucose solution; and to tube B3 add 1.0 ml 1% starch solution. b. Add 5 drops of the Benedict s solution to each test tube. Note the appearance of each tube, and then place all three tubes in the boiling water bath for about 1 minute. c. Retrieve your test tubes from the boiling water bath. Describe the appearance of each tube after it was removed from the boiling water bath: Tube #1 (dh2o) Tube #2 (glucose) Tube #3 (starch) d. Benedict s reagent can be used to detect the presence of which substance? e. The color for a positive test is f. The color for a negative test is 5-11
12 2. a. Label three test tubes L1, L2, and L3. To tube L1 add 1.0 ml dh2o; to tube L2 add 1.0 ml 40% glucose solution; and to tube L3 add 1.0 ml 1% starch solution. b. Add 5 drops of Lugol s iodine to each test tube. c. Describe the appearance of each tube after the Lugol s iodine was added: Tube #1 (dh2o) Tube #2 (glucose) Tube #3 (starch) d. Lugol s iodine could be used to detect the presence of which substance? e. The color for a positive test is f. The color for a negative test is V. Observe how osmosis affects living cells 1. Make a wet mount of an Elodea leaf. You will do all of this section of the lab activity using one wet mount of an Elodea leaf. Do not discard your wet mount until you have completed Part V! 2. Focus on the leaf using low power first, and then high power. While viewing the leaf under high power, draw a diagram of one cell labeling the chloroplasts, cytoplasm, plasma membrane, and cell wall. 3. Place a drop of the 10% NaCl solution on the right edge of the cover slip. While looking through the microscope, because you want to watch the cells response as it is occurring, touch the corner of a Kimwipe to the left edge of the cover slip. This will draw the salt solution under the cover slip by capillary action. 4. In your lab notebook, describe what happens to the cells as the 10% salt solution flows over them. After waiting several minutes, draw another diagram of one cell, again labeling the chloroplasts, cytoplasm, plasma membrane, and cell wall. Briefly describe the difference between the 2 diagrams. Ask your instructor to check both diagrams before proceeding to step Using the same slide, repeat step #3 using distilled water instead of 10% NaCl. In your lab notebook, describe what happens to the cells as the distilled water flows over them. Clean up Dispose of your solutions in the proper waste containers. Clean your glassware and place all equipment and solutions back where you found them. Leave your work area in the same order that you found it in. All disposable glassware goes into the special glass disposal receptacle. Dialysis tubing may be disposed of in the waste paper baskets. Wipe off your workspace with a damp paper towel. Make sure everything that you have used is clean, put away, or discarded. Ask your instructor to check your work area before you leave. 5-12
13 Postlab Part I 1. Describe what you observed while viewing the grains of lichen powder under the microscope. Were the movements of the grains random or in a specific direction? Was the rate of movement uniform or did it vary? 2. Explain what was happening at the molecular level to cause the grains of lichen powder to vibrate. Part II 3. Make a scatter diagram of your data from part II, plotting diameter of the dye circles against time. Make two separate graphs, one for potassium permanganate and one for methylene blue. On each graph, show the data for all three plates (A, B, and C). Use a different color or symbol for each plate. Make sure that each graph has a descriptive title and that all parts are clearly labeled. Also make sure that each graph occupies a full page and that you place the independent and dependent variables on the correct axes. Use the Graphing Check List on p. 18 of the Prelab for Exercise #2 to make sure you have included all necessary information on your graph. 4. Next, for each graph prepared in question 3 (one for potassium permanganate and one for methylene blue), use linear regression to determine the equation of the best-fit straight line for the data from each plate (A, B, and C). Plot the 3 best-fit straight lines on each graph, using a different color or style for each line. Also, write down the equation of each best-fit line and clearly show which line corresponds to each equation. When you are finished, you should have 3 best-fit lines and 3 equations displayed on each graph. 5. Examine the 6 linear regression equations that you calculated in question 4. Which part of each equation is a measure of how rapidly diffusion took place? 6. Agar forms a gel when heated in water and allowed to cool. The gel consists of water trapped within a network of polysaccharide threads. A denser network of polysaccharide threads will slow down the diffusion of the dye through the trapped water. In your experiment, plates A and C had the same concentration of agar while plate B had a different concentration. In addition, plates A and B were kept at room temperature while plate C was kept in a refrigerator. A. Which 2 plates should you compare to see the effect of agar concentration on the rate of diffusion? Based on this comparison, which plate had the higher concentration of agar? Explain your reasoning. B. Which 2 plates should you compare to determine the effect of temperature on the rate of diffusion? What does this comparison show? Explain your reasoning. 7. Potassium permanganate has a molecular weight of Daltons, and methylene blue has a molecular weight of Daltons. Based on your results, what can you conclude about the effect of molecular weight on the rate of diffusion? Explain your reasoning. Parts III & IV 8. For each of your dialysis set-ups, answer the following: Did the dialysis bag gain weight, lose weight, or stay about the same weight? Make a bar graph that shows weight change for each set-up. Use the Graphing Check List on p. 18 of the Prelab in Exercise #2 to make sure you have included all necessary information on your graph. 9. The change in weight of the dialysis bags during your experiment was mainly due to the net movement of water into or out of the bags. For each dialysis set-up, describe the direction of net movement of water during your experiment (into or out of the bag) and explain why this net movement occurred. Make sure you give four separate answers one for each dialysis set-up. 10. Indicate which solute(s) passed through the dialysis membrane and which solute(s) did not, and explain how you were able to determine this. 5-13
14 11. Explain why the solute(s) that passed through the dialysis membrane did, and why the solute(s) that didn t pass through the membrane didn t. 12. Suppose you have a solution containing both salt (NaCl) and glucose, and dialysis tubing with a pore size that allows the passage of Na + and Cl - ions, but doesn t allow the passage of glucose. How could you remove essentially all of the salt from the solution? Part V 13. When the Elodea cells were placed in 10% saline solution: A. Describe the visible change that took place and explain why it occurred. B. Describe the movement of water across the plasma membrane, and explain why it occurred. C. Describe the net movement of water across the plasma membrane, and explain why it occurred. 14. When the Elodea cells were placed back in distilled water: A. Describe the visible change that took place and explain why it occurred. B. Describe the movement of water across the plasma membrane, and explain why it occurred. C. Describe the net movement of water across the plasma membrane, and explain why it occurred. 15. If you repeated this experiment using animal cells instead of plant cells, what do you think you would have observed that was different from what you observed in plant cells? Explain your reasoning. 5-14
Diffusion, Osmosis, and Membrane Transport
 Diffusion, Osmosis, and Membrane Transport Introduction... 2 Diffusion and osmosis as related to cellular processes... 2 The hotter the medium, the faster the molecules diffuse... 2 TASK 1: TEMPERATURE
Diffusion, Osmosis, and Membrane Transport Introduction... 2 Diffusion and osmosis as related to cellular processes... 2 The hotter the medium, the faster the molecules diffuse... 2 TASK 1: TEMPERATURE
Lab 4: Osmosis and Diffusion
 Lab 4: Osmosis and Diffusion The plasma membrane enclosing every cell is the boundary that separates the cell from its external environment. It is not an impermeable barrier, but like all biological membranes,
Lab 4: Osmosis and Diffusion The plasma membrane enclosing every cell is the boundary that separates the cell from its external environment. It is not an impermeable barrier, but like all biological membranes,
Lab 4: Diffusion and Osmosis
 Lab 4: Diffusion and Osmosis Introduction The cell membrane encloses the contents of all cells, organelles and many cytoplasmic inclusions, and regulates what gets in and out. This is called selective
Lab 4: Diffusion and Osmosis Introduction The cell membrane encloses the contents of all cells, organelles and many cytoplasmic inclusions, and regulates what gets in and out. This is called selective
OSMOSIS AND DIALYSIS 2003 BY Wendy Weeks-Galindo with modifications by David A. Katz
 OSMOSIS AND DIALYSIS 2003 BY Wendy Weeks-Galindo with modifications by David A. Katz OSMOSIS Osmosis is the reason that a fresh water fish placed in the ocean desiccates and dies. Osmosis is the reason
OSMOSIS AND DIALYSIS 2003 BY Wendy Weeks-Galindo with modifications by David A. Katz OSMOSIS Osmosis is the reason that a fresh water fish placed in the ocean desiccates and dies. Osmosis is the reason
Cells, Diffusion, Osmosis, and Biological Membranes
 Cells, Diffusion, Osmosis, and Biological Membranes A. Objectives Upon completion of this lab activity, you should be able to: 1. Define and correctly use the following terms: solute, solvent, selectively
Cells, Diffusion, Osmosis, and Biological Membranes A. Objectives Upon completion of this lab activity, you should be able to: 1. Define and correctly use the following terms: solute, solvent, selectively
Diffusion and Osmosis
 Diffusion and Osmosis OBJECTIVES: 1. To explore how different molecules move by diffusion and osmosis through semi-permeable membranes. 2. To understand how different concentration gradients affect the
Diffusion and Osmosis OBJECTIVES: 1. To explore how different molecules move by diffusion and osmosis through semi-permeable membranes. 2. To understand how different concentration gradients affect the
Cell Membrane & Tonicity Worksheet
 NAME ANSWER KEY DATE PERIOD Cell Membrane & Tonicity Worksheet Composition of the Cell Membrane & Functions The cell membrane is also called the PLASMA membrane and is made of a phospholipid BI-LAYER.
NAME ANSWER KEY DATE PERIOD Cell Membrane & Tonicity Worksheet Composition of the Cell Membrane & Functions The cell membrane is also called the PLASMA membrane and is made of a phospholipid BI-LAYER.
4. Biology of the Cell
 4. Biology of the Cell Our primary focus in this chapter will be the plasma membrane and movement of materials across the plasma membrane. You should already be familiar with the basic structures and roles
4. Biology of the Cell Our primary focus in this chapter will be the plasma membrane and movement of materials across the plasma membrane. You should already be familiar with the basic structures and roles
Osmosis Demonstration Lab
 Osmosis Demonstration Lab Objectives The student will: 1) Observe the effects of different concentrations of salt solutions on potato cores. 2) Infer the relationship between weight loss and rate of osmosis.
Osmosis Demonstration Lab Objectives The student will: 1) Observe the effects of different concentrations of salt solutions on potato cores. 2) Infer the relationship between weight loss and rate of osmosis.
Membrane Structure and Function
 Membrane Structure and Function Part A Multiple Choice 1. The fluid mosaic model describes membranes as having A. a set of protein channels separated by phospholipids. B. a bilayer of phospholipids in
Membrane Structure and Function Part A Multiple Choice 1. The fluid mosaic model describes membranes as having A. a set of protein channels separated by phospholipids. B. a bilayer of phospholipids in
FIGURE 2.18. A. The phosphate end of the molecule is polar (charged) and hydrophilic (attracted to water).
 PLASMA MEMBRANE 1. The plasma membrane is the outermost part of a cell. 2. The main component of the plasma membrane is phospholipids. FIGURE 2.18 A. The phosphate end of the molecule is polar (charged)
PLASMA MEMBRANE 1. The plasma membrane is the outermost part of a cell. 2. The main component of the plasma membrane is phospholipids. FIGURE 2.18 A. The phosphate end of the molecule is polar (charged)
DIFFUSION (HYPERTONIC, HYPOTONIC, & ISOTONIC SOLUTIONS) THE GUMMY BEAR LAB PASS
 DIFFUSION (HYPERTONIC, HYPOTONIC, & ISOTONIC SOLUTIONS) THE GUMMY BEAR LAB PASS Have you ever wondered why your fingers have wrinkles after soaking in a bath tub? Your students have probably wondered the
DIFFUSION (HYPERTONIC, HYPOTONIC, & ISOTONIC SOLUTIONS) THE GUMMY BEAR LAB PASS Have you ever wondered why your fingers have wrinkles after soaking in a bath tub? Your students have probably wondered the
Activity Sheets Enzymes and Their Functions
 Name: Date: Activity Sheets Enzymes and Their Functions amylase What are Enzymes? starch glucose Enzymes are compounds that assist chemical reactions by increasing the rate at which they occur. For example,
Name: Date: Activity Sheets Enzymes and Their Functions amylase What are Enzymes? starch glucose Enzymes are compounds that assist chemical reactions by increasing the rate at which they occur. For example,
BACKGROUND (continued)
 BACKGROUND (continued) A cell must exchange materials with its surroundings, a process controlled by the plasma membrane. Plasma membranes are selectively permeable, regulating the cell s molecular traffic:
BACKGROUND (continued) A cell must exchange materials with its surroundings, a process controlled by the plasma membrane. Plasma membranes are selectively permeable, regulating the cell s molecular traffic:
Osmosis. Evaluation copy
 Osmosis Computer 5 In order to survive, all organisms need to move molecules in and out of their cells. Molecules such as gases (e.g., O 2, CO 2 ), water, food, and wastes pass across the cell membrane.
Osmosis Computer 5 In order to survive, all organisms need to move molecules in and out of their cells. Molecules such as gases (e.g., O 2, CO 2 ), water, food, and wastes pass across the cell membrane.
7. A selectively permeable membrane only allows certain molecules to pass through.
 CHAPTER 2 GETTING IN & OUT OF CELLS PASSIVE TRANSPORT Cell membranes help organisms maintain homeostasis by controlling what substances may enter or leave cells. Some substances can cross the cell membrane
CHAPTER 2 GETTING IN & OUT OF CELLS PASSIVE TRANSPORT Cell membranes help organisms maintain homeostasis by controlling what substances may enter or leave cells. Some substances can cross the cell membrane
Process of Science: Using Diffusion and Osmosis
 Process of Science: Using Diffusion and Osmosis OBJECTIVES: 1. To understand one way to approach the process of science through an investigation of diffusion and osmosis. 2. To explore how different molecules
Process of Science: Using Diffusion and Osmosis OBJECTIVES: 1. To understand one way to approach the process of science through an investigation of diffusion and osmosis. 2. To explore how different molecules
Cell Biology - Part 2 Membranes
 Cell Biology - Part 2 Membranes The organization of cells is made possible by membranes. Membranes isolate, partition, and compartmentalize cells. 1 Membranes isolate the inside of the cell from the outside
Cell Biology - Part 2 Membranes The organization of cells is made possible by membranes. Membranes isolate, partition, and compartmentalize cells. 1 Membranes isolate the inside of the cell from the outside
Six major functions of membrane proteins: Transport Enzymatic activity
 CH 7 Membranes Cellular Membranes Phospholipids are the most abundant lipid in the plasma membrane. Phospholipids are amphipathic molecules, containing hydrophobic and hydrophilic regions. The fluid mosaic
CH 7 Membranes Cellular Membranes Phospholipids are the most abundant lipid in the plasma membrane. Phospholipids are amphipathic molecules, containing hydrophobic and hydrophilic regions. The fluid mosaic
MEMBRANE FUNCTION CELLS AND OSMOSIS
 CELLS AND OSMOSIS MEMBRANE FUNCTION Consider placing a cell in a beaker of pure water (Fig. 1). The cell contains a water solution with many different kinds of dissolved molecules and ions so that it is
CELLS AND OSMOSIS MEMBRANE FUNCTION Consider placing a cell in a beaker of pure water (Fig. 1). The cell contains a water solution with many different kinds of dissolved molecules and ions so that it is
Section 7-3 Cell Boundaries
 Note: For the past several years, I ve been puzzling how to integrate new discoveries on the nature of water movement through cell membranes into Chapter 7. The Section below is a draft of my first efforts
Note: For the past several years, I ve been puzzling how to integrate new discoveries on the nature of water movement through cell membranes into Chapter 7. The Section below is a draft of my first efforts
Modes of Membrane Transport
 Modes of Membrane Transport Transmembrane Transport movement of small substances through a cellular membrane (plasma, ER, mitochondrial..) ions, fatty acids, H 2 O, monosaccharides, steroids, amino acids
Modes of Membrane Transport Transmembrane Transport movement of small substances through a cellular membrane (plasma, ER, mitochondrial..) ions, fatty acids, H 2 O, monosaccharides, steroids, amino acids
BIOL 305L Laboratory Two
 Please print Full name clearly: Introduction BIOL 305L Laboratory Two Osmosis, because it is different in plants! Osmosis is the movement of solvent molecules through a selectively permeable membrane into
Please print Full name clearly: Introduction BIOL 305L Laboratory Two Osmosis, because it is different in plants! Osmosis is the movement of solvent molecules through a selectively permeable membrane into
1.1.2. thebiotutor. AS Biology OCR. Unit F211: Cells, Exchange & Transport. Module 1.2 Cell Membranes. Notes & Questions.
 thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
Cell Transport and Plasma Membrane Structure
 Cell Transport and Plasma Membrane Structure POGIL Guided Inquiry Learning Targets Explain the importance of the plasma membrane. Compare and contrast different types of passive transport. Explain how
Cell Transport and Plasma Membrane Structure POGIL Guided Inquiry Learning Targets Explain the importance of the plasma membrane. Compare and contrast different types of passive transport. Explain how
Biology. STANDARD II: Objective 3. Osmosis Inquiry Labs
 Biology STANDARD II: Objective 3 Osmosis Inquiry Labs Background Knowledge: Students should have used a microscope before and be familiar with the parts. They should also know how to make a wet mount slide.
Biology STANDARD II: Objective 3 Osmosis Inquiry Labs Background Knowledge: Students should have used a microscope before and be familiar with the parts. They should also know how to make a wet mount slide.
Enzyme Action: Testing Catalase Activity
 Enzyme Action: Testing Catalase Activity Experiment 6A Many organisms can decompose hydrogen peroxide (H 2 O 2 ) enzymatically. Enzymes are globular proteins, responsible for most of the chemical activities
Enzyme Action: Testing Catalase Activity Experiment 6A Many organisms can decompose hydrogen peroxide (H 2 O 2 ) enzymatically. Enzymes are globular proteins, responsible for most of the chemical activities
CELLS An Introduction to Cell Structure & Function
 CELLS An Introduction to Cell Structure & Function Introduction: In this lab exercise we will be studying three general aspects of cellular structure and function. First, we will observe the anatomical
CELLS An Introduction to Cell Structure & Function Introduction: In this lab exercise we will be studying three general aspects of cellular structure and function. First, we will observe the anatomical
2 strong elastic bands holding beakers together. beaker representing the solution surrounding the cells. elastic band holding net onto one beaker.
 Using a pot model to represent osmosis Student sheet To do 1 Set up the potato investigation as instructed. 2 Record the mass of the potato which is then placed in distilled water... g 3 Record the mass
Using a pot model to represent osmosis Student sheet To do 1 Set up the potato investigation as instructed. 2 Record the mass of the potato which is then placed in distilled water... g 3 Record the mass
Cell Membrane Coloring Worksheet
 Cell Membrane Coloring Worksheet Composition of the Cell Membrane & Functions The cell membrane is also called the plasma membrane and is made of a phospholipid bilayer. The phospholipids have a hydrophilic
Cell Membrane Coloring Worksheet Composition of the Cell Membrane & Functions The cell membrane is also called the plasma membrane and is made of a phospholipid bilayer. The phospholipids have a hydrophilic
Cellular Membranes I. BACKGROUND MATERIAL
 Cellular Membranes Objectives: 1. To explore the nature of cellular membranes by investigating environmental conditions which stress them. 2. To learn methods for measuring the extent of stress on the
Cellular Membranes Objectives: 1. To explore the nature of cellular membranes by investigating environmental conditions which stress them. 2. To learn methods for measuring the extent of stress on the
Table of Content. Enzymes and Their Functions Teacher Version 1
 Enzymes and Their Functions Jeisa Pelet, Cornell University Carolyn Wilczynski, Binghamton High School Cornell Learning Initiative in Medicine and Bioengineering (CLIMB) Table of Content Title Page Abstract..
Enzymes and Their Functions Jeisa Pelet, Cornell University Carolyn Wilczynski, Binghamton High School Cornell Learning Initiative in Medicine and Bioengineering (CLIMB) Table of Content Title Page Abstract..
The Huntington Library, Art Collections, and Botanical Gardens. How Sweet It Is: Enzyme Action in Seed Germination
 The Huntington Library, Art Collections, and Botanical Gardens How Sweet It Is: Enzyme Action in Seed Germination Overview This experiment is intended to familiarize students with the macromolecule starch,
The Huntington Library, Art Collections, and Botanical Gardens How Sweet It Is: Enzyme Action in Seed Germination Overview This experiment is intended to familiarize students with the macromolecule starch,
Osmosis, Diffusion and Cell Transport
 Osmosis, Diffusion and Cell Transport Types of Transport There are 3 types of transport in cells: 1. Passive Transport: does not use the cell s energy in bringing materials in & out of the cell 2. Active
Osmosis, Diffusion and Cell Transport Types of Transport There are 3 types of transport in cells: 1. Passive Transport: does not use the cell s energy in bringing materials in & out of the cell 2. Active
EFFECT OF SALT ON CELL MEMBRANES
 EFFECT OF SALT ON CELL MEMBRANES LAB CELL 2 INTRODUCTION A eukaryotic cell, a cell with a nucleus, not only has a plasma membrane as its external boundary, but it also has a variety of membranes that divide
EFFECT OF SALT ON CELL MEMBRANES LAB CELL 2 INTRODUCTION A eukaryotic cell, a cell with a nucleus, not only has a plasma membrane as its external boundary, but it also has a variety of membranes that divide
PART I: Neurons and the Nerve Impulse
 PART I: Neurons and the Nerve Impulse Identify each of the labeled structures of the neuron below. A. B. C. D. E. F. G. Identify each of the labeled structures of the neuron below. A. dendrites B. nucleus
PART I: Neurons and the Nerve Impulse Identify each of the labeled structures of the neuron below. A. B. C. D. E. F. G. Identify each of the labeled structures of the neuron below. A. dendrites B. nucleus
Date: Student Name: Teacher Name: Jared George. Score: 1) A cell with 1% solute concentration is placed in a beaker with a 5% solute concentration.
 Biology Keystone (PA Core) Quiz Homeostasis and Transport - (BIO.A.4.1.1 ) Plasma Membrane, (BIO.A.4.1.2 ) Transport Mechanisms, (BIO.A.4.1.3 ) Transport Facilitation Student Name: Teacher Name: Jared
Biology Keystone (PA Core) Quiz Homeostasis and Transport - (BIO.A.4.1.1 ) Plasma Membrane, (BIO.A.4.1.2 ) Transport Mechanisms, (BIO.A.4.1.3 ) Transport Facilitation Student Name: Teacher Name: Jared
Cell and Membrane Practice. A. chromosome B. gene C. mitochondrion D. vacuole
 Name: ate: 1. Which structure is outside the nucleus of a cell and contains N?. chromosome. gene. mitochondrion. vacuole 2. potato core was placed in a beaker of water as shown in the figure below. Which
Name: ate: 1. Which structure is outside the nucleus of a cell and contains N?. chromosome. gene. mitochondrion. vacuole 2. potato core was placed in a beaker of water as shown in the figure below. Which
Experiment #10: Liquids, Liquid Mixtures and Solutions
 Experiment #10: Liquids, Liquid Mixtures and Solutions Objectives: This experiment is a broad survey of the physical properties of liquids. We will investigate solvent/solute mixtures. We will study and
Experiment #10: Liquids, Liquid Mixtures and Solutions Objectives: This experiment is a broad survey of the physical properties of liquids. We will investigate solvent/solute mixtures. We will study and
Paper Chromatography: Separation and Identification of Five Metal Cations
 Paper Chromatography: Separation and Identification of Five Metal Cations Objectives Known and unknown solutions of the metal ions Ag +, Fe 3+, Co 2+, Cu 2+ and Hg 2+ will be analyzed using paper chromatography.
Paper Chromatography: Separation and Identification of Five Metal Cations Objectives Known and unknown solutions of the metal ions Ag +, Fe 3+, Co 2+, Cu 2+ and Hg 2+ will be analyzed using paper chromatography.
CELL MEMBRANES, TRANSPORT, and COMMUNICATION. Teacher Packet
 AP * BIOLOGY CELL MEMBRANES, TRANSPORT, and COMMUNICATION Teacher Packet AP* is a trademark of the College Entrance Examination Board. The College Entrance Examination Board was not involved in the production
AP * BIOLOGY CELL MEMBRANES, TRANSPORT, and COMMUNICATION Teacher Packet AP* is a trademark of the College Entrance Examination Board. The College Entrance Examination Board was not involved in the production
Solubility Curve of Sugar in Water
 Solubility Curve of Sugar in Water INTRODUCTION Solutions are homogeneous mixtures of solvents (the larger volume of the mixture) and solutes (the smaller volume of the mixture). For example, a hot chocolate
Solubility Curve of Sugar in Water INTRODUCTION Solutions are homogeneous mixtures of solvents (the larger volume of the mixture) and solutes (the smaller volume of the mixture). For example, a hot chocolate
Lab: Observing Osmosis in Gummi Bears
 Name Period Date Points Lab: Observing Osmosis in Gummi Bears Haribo macht Kinder froh und Erwachsene ebenso! 1 Laboratory: Observing Osmosis in Gummy Bears (28 points) Purpose: To investigate the movement
Name Period Date Points Lab: Observing Osmosis in Gummi Bears Haribo macht Kinder froh und Erwachsene ebenso! 1 Laboratory: Observing Osmosis in Gummy Bears (28 points) Purpose: To investigate the movement
Chemistry B11 Chapter 6 Solutions and Colloids
 Chemistry B11 Chapter 6 Solutions and Colloids Solutions: solutions have some properties: 1. The distribution of particles in a solution is uniform. Every part of the solution has exactly the same composition
Chemistry B11 Chapter 6 Solutions and Colloids Solutions: solutions have some properties: 1. The distribution of particles in a solution is uniform. Every part of the solution has exactly the same composition
Metabolism: Cellular Respiration, Fermentation and Photosynthesis
 Metabolism: Cellular Respiration, Fermentation and Photosynthesis Introduction: All organisms require a supply of energy and matter to build themselves and to continue to function. To get that supply of
Metabolism: Cellular Respiration, Fermentation and Photosynthesis Introduction: All organisms require a supply of energy and matter to build themselves and to continue to function. To get that supply of
Biology: Osmosis and Diffusion Lab using Potato Cores Class: 3B Mr. Boyer Name: Simon Han
 Abstract: Biology: Osmosis and Diffusion Lab using Potato Cores Class: 3B Mr. Boyer Name: Simon Han In this experiment, we learnt about Osmosis and Diffusion through potato cores in different concentration
Abstract: Biology: Osmosis and Diffusion Lab using Potato Cores Class: 3B Mr. Boyer Name: Simon Han In this experiment, we learnt about Osmosis and Diffusion through potato cores in different concentration
Leaving Cert Biology. Conduct any Activity to Demonstrate Osmosis. Experiments
 Leaving Cert Biology Conduct any Activity to Demonstrate Osmosis Experiments CONDUCT ANY ACTIVITY TO DEMONSTRATE OSMOSIS Materials/Equipment Distilled water Electronic balance Sucrose solution (80%) Scissors
Leaving Cert Biology Conduct any Activity to Demonstrate Osmosis Experiments CONDUCT ANY ACTIVITY TO DEMONSTRATE OSMOSIS Materials/Equipment Distilled water Electronic balance Sucrose solution (80%) Scissors
1. 4. 1: Biochemistry of macromolecules and metabolic pathways
 1. 4 Investigating enzymes Many factors affect the activity of enzymes and it is very easy to investigate these factors using common enzymes. Enzymes work at their optimum temperature and ph. Any changes
1. 4 Investigating enzymes Many factors affect the activity of enzymes and it is very easy to investigate these factors using common enzymes. Enzymes work at their optimum temperature and ph. Any changes
Total body water ~(60% of body mass): Intracellular fluid ~2/3 or ~65% Extracellular fluid ~1/3 or ~35% fluid. Interstitial.
 http://www.bristol.ac.uk/phys-pharm/teaching/staffteaching/sergeykasparov.htmlpharm/teaching/staffteaching/sergeykasparov.html Physiology of the Cell Membrane Membrane proteins and their roles (channels,
http://www.bristol.ac.uk/phys-pharm/teaching/staffteaching/sergeykasparov.htmlpharm/teaching/staffteaching/sergeykasparov.html Physiology of the Cell Membrane Membrane proteins and their roles (channels,
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Cell Unit Practice Test #1
 ell Unit Practice Test #1 Name: ate: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell?. mitochondria.
ell Unit Practice Test #1 Name: ate: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell?. mitochondria.
Enzyme Action: Testing Catalase Activity
 Enzyme Action: Testing Catalase Activity Experiment 6A Many organisms can decompose hydrogen peroxide (H 2 O 2 ) enzymatically. Enzymes are globular proteins, responsible for most of the chemical activities
Enzyme Action: Testing Catalase Activity Experiment 6A Many organisms can decompose hydrogen peroxide (H 2 O 2 ) enzymatically. Enzymes are globular proteins, responsible for most of the chemical activities
PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY
 Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Amino Acids, Peptides, and Proteins
 1 Amino Acids, Peptides, and Proteins Introduction Amino Acids Amino acids are the building blocks of proteins. In class you learned the structures of the 20 common amino acids that make up proteins. All
1 Amino Acids, Peptides, and Proteins Introduction Amino Acids Amino acids are the building blocks of proteins. In class you learned the structures of the 20 common amino acids that make up proteins. All
Fig. 1. Background. Name: Class: Date:
 Background Bubbles make a great stand in for cell membranes. They re fluid, flexible, and can self-repair. Bubbles and cell membranes are alike because their parts are so similar. If you could zoom down
Background Bubbles make a great stand in for cell membranes. They re fluid, flexible, and can self-repair. Bubbles and cell membranes are alike because their parts are so similar. If you could zoom down
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
COMPARING PLANT AND ANIMAL CELLS
 COMPARING PLANT AND ANIMAL CELLS OBJECTIVES: Distinguish between plant and animals cells by their structures Demonstrate the benefit of stains Acquire ability to prepare wet mounts SAFETY: Methylene blue
COMPARING PLANT AND ANIMAL CELLS OBJECTIVES: Distinguish between plant and animals cells by their structures Demonstrate the benefit of stains Acquire ability to prepare wet mounts SAFETY: Methylene blue
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
Respiration and Photosynthesis
 Respiration and Photosynthesis Topic Plants and animals carry out cellular respiration, but only plants conduct photosynthesis. Introduction Cellular respiration is the process in which a cell uses oxygen
Respiration and Photosynthesis Topic Plants and animals carry out cellular respiration, but only plants conduct photosynthesis. Introduction Cellular respiration is the process in which a cell uses oxygen
Name Class Date Laboratory Investigation 4B Chapter 4: Cell Structure
 Name Class Date Laboratory Investigation 4B Chapter 4: Cell Structure The Microscope: A Tool of the Scientist You may refer to pages 66-67, 72-73 in your textbook for a general discussion of microscopes.
Name Class Date Laboratory Investigation 4B Chapter 4: Cell Structure The Microscope: A Tool of the Scientist You may refer to pages 66-67, 72-73 in your textbook for a general discussion of microscopes.
EFFECT OF ALCOHOL ON CELL MEMBRANES
 EFFECT OF ALCOHOL ON CELL MEMBRANES LAB CELL 1 INTRODUCTION A eukaryotic cell, a cell with a nucleus, not only has a plasma membrane as its external boundary, but it also has a variety of membranes that
EFFECT OF ALCOHOL ON CELL MEMBRANES LAB CELL 1 INTRODUCTION A eukaryotic cell, a cell with a nucleus, not only has a plasma membrane as its external boundary, but it also has a variety of membranes that
Comparing Plant and Animal Cells
 1.2 Comparing Plant and Animal Cells Here is a summary of what you will learn in this section: Plant and animal cell structures are called organelles. Plant and animal cells perform some similar functions,
1.2 Comparing Plant and Animal Cells Here is a summary of what you will learn in this section: Plant and animal cell structures are called organelles. Plant and animal cells perform some similar functions,
Biology for Science Majors
 Biology for Science Majors Lab 10 AP BIOLOGY Concepts covered Respirometers Metabolism Glycolysis Respiration Anaerobic vs. aerobic respiration Fermentation Lab 5: Cellular Respiration ATP is the energy
Biology for Science Majors Lab 10 AP BIOLOGY Concepts covered Respirometers Metabolism Glycolysis Respiration Anaerobic vs. aerobic respiration Fermentation Lab 5: Cellular Respiration ATP is the energy
Determination of a Chemical Formula
 1 Determination of a Chemical Formula Introduction Molar Ratios Elements combine in fixed ratios to form compounds. For example, consider the compound TiCl 4 (titanium chloride). Each molecule of TiCl
1 Determination of a Chemical Formula Introduction Molar Ratios Elements combine in fixed ratios to form compounds. For example, consider the compound TiCl 4 (titanium chloride). Each molecule of TiCl
Making Biodiesel from Virgin Vegetable Oil: Teacher Manual
 Making Biodiesel from Virgin Vegetable Oil: Teacher Manual Learning Goals: Students will understand how to produce biodiesel from virgin vegetable oil. Students will understand the effect of an exothermic
Making Biodiesel from Virgin Vegetable Oil: Teacher Manual Learning Goals: Students will understand how to produce biodiesel from virgin vegetable oil. Students will understand the effect of an exothermic
CELL MEMBRANE & CELL TRANSPORT (PASSIVE and ACTIVE) Webquest
 Name: Period: CELL MEMBRANE & CELL TRANSPORT (PASSIVE and ACTIVE) Webquest PART I: CELL MEMBRANES WEBSITE #1: http://www.wisc-online.com/objects/index_tj.asp?objid=ap1101 1. What is the BASIC UNIT of LIFE?
Name: Period: CELL MEMBRANE & CELL TRANSPORT (PASSIVE and ACTIVE) Webquest PART I: CELL MEMBRANES WEBSITE #1: http://www.wisc-online.com/objects/index_tj.asp?objid=ap1101 1. What is the BASIC UNIT of LIFE?
Unit 5 Photosynthesis and Cellular Respiration
 Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
Animal & Plant Cell Slides
 Animal & Plant Cell Slides Category: Biology Type: Class Experiment, 60 min class Materials: 2 Glass Slides 2 Cover Slips 1 Bottle of methylene blue (optional) 1 Plastic tray 1 Bottle of iodine 1 Plastic
Animal & Plant Cell Slides Category: Biology Type: Class Experiment, 60 min class Materials: 2 Glass Slides 2 Cover Slips 1 Bottle of methylene blue (optional) 1 Plastic tray 1 Bottle of iodine 1 Plastic
Chapter 3. Cellular Structure and Function Worksheets. 39 www.ck12.org
 Chapter 3 Cellular Structure and Function Worksheets (Opening image copyright by Sebastian Kaulitzki, 2010. Used under license from Shutterstock.com.) Lesson 3.1: Introduction to Cells Lesson 3.2: Cell
Chapter 3 Cellular Structure and Function Worksheets (Opening image copyright by Sebastian Kaulitzki, 2010. Used under license from Shutterstock.com.) Lesson 3.1: Introduction to Cells Lesson 3.2: Cell
Investigating cells. Cells are the basic units of living things (this means that all living things are made up of one or more cells).
 SG Biology Summary notes Investigating cells Sub-topic a: Investigating living cells Cells are the basic units of living things (this means that all living things are made up of one or more cells). Cells
SG Biology Summary notes Investigating cells Sub-topic a: Investigating living cells Cells are the basic units of living things (this means that all living things are made up of one or more cells). Cells
Cell Biology Prokaryotic and eukaryotic cells
 Cell Biology Prokaryotic and eukaryotic cells Observation of cells and organelles In this lab you will be looking at an example of a Prokaryotic cell (Bacillus cereus) and a some examples of Eukaryotic
Cell Biology Prokaryotic and eukaryotic cells Observation of cells and organelles In this lab you will be looking at an example of a Prokaryotic cell (Bacillus cereus) and a some examples of Eukaryotic
Name. Lab 3: ENZYMES. In this lab, you ll investigate some of the properties of enzymes.
 Name Lab 3: ENZYMES In this lab, you ll investigate some of the properties of enzymes. So what are enzymes? Enzymes are large protein molecules (macromolecules) They catalyze or speed up chemical reactions
Name Lab 3: ENZYMES In this lab, you ll investigate some of the properties of enzymes. So what are enzymes? Enzymes are large protein molecules (macromolecules) They catalyze or speed up chemical reactions
6 H2O + 6 CO 2 (g) + energy
 AEROBIC RESPIRATION LAB DO 2.CALC From Biology with Calculators, Vernier Software & Technology, 2000. INTRODUCTION Aerobic cellular respiration is the process of converting the chemical energy of organic
AEROBIC RESPIRATION LAB DO 2.CALC From Biology with Calculators, Vernier Software & Technology, 2000. INTRODUCTION Aerobic cellular respiration is the process of converting the chemical energy of organic
Chapter 5 Student Reading
 Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Plant and Animal Cells
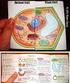 Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
Chapter 8. Movement across the Cell Membrane. AP Biology
 Chapter 8. Movement across the Cell Membrane More than just a barrier Expanding our view of cell membrane beyond just a phospholipid bilayer barrier phospholipids plus Fluid Mosaic Model In 1972, S.J.
Chapter 8. Movement across the Cell Membrane More than just a barrier Expanding our view of cell membrane beyond just a phospholipid bilayer barrier phospholipids plus Fluid Mosaic Model In 1972, S.J.
Chemistry 112 Laboratory Experiment 6: The Reaction of Aluminum and Zinc with Hydrochloric Acid
 Chemistry 112 Laboratory Experiment 6: The Reaction of Aluminum and Zinc with Hydrochloric Acid Introduction Many metals react with acids to form hydrogen gas. In this experiment, you will use the reactions
Chemistry 112 Laboratory Experiment 6: The Reaction of Aluminum and Zinc with Hydrochloric Acid Introduction Many metals react with acids to form hydrogen gas. In this experiment, you will use the reactions
Ch. 8 - The Cell Membrane
 Ch. 8 - The Cell Membrane 2007-2008 Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water Aaaah, one of those
Ch. 8 - The Cell Membrane 2007-2008 Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water Aaaah, one of those
SOLUBILITY OF A SALT IN WATER AT VARIOUS TEMPERATURES LAB
 SOLUBILITY OF A SALT IN WATER AT VARIOUS TEMPERATURES LAB Purpose: Most ionic compounds are considered by chemists to be salts and many of these are water soluble. In this lab, you will determine the solubility,
SOLUBILITY OF A SALT IN WATER AT VARIOUS TEMPERATURES LAB Purpose: Most ionic compounds are considered by chemists to be salts and many of these are water soluble. In this lab, you will determine the solubility,
Osmosis and Diffusion
 Spring Upshaw Lauren Beal Mary-Kate Perrone Kate Loftus Osmosis and Diffusion Students will explore the concepts of osmosis and diffusion through two days of lessons. Students will build upon prior knowledge
Spring Upshaw Lauren Beal Mary-Kate Perrone Kate Loftus Osmosis and Diffusion Students will explore the concepts of osmosis and diffusion through two days of lessons. Students will build upon prior knowledge
Membrane Structure and Function
 Membrane Structure and Function -plasma membrane acts as a barrier between cells and the surrounding. -plasma membrane is selective permeable -consist of lipids, proteins and carbohydrates -major lipids
Membrane Structure and Function -plasma membrane acts as a barrier between cells and the surrounding. -plasma membrane is selective permeable -consist of lipids, proteins and carbohydrates -major lipids
CHAPTER 5.1 5.2: Plasma Membrane Structure
 CHAPTER 5.1 5.2: Plasma Membrane Structure 1. Describe the structure of a phospholipid molecule. Be sure to describe their behavior in relationship to water. 2. What happens when a collection of phospholipids
CHAPTER 5.1 5.2: Plasma Membrane Structure 1. Describe the structure of a phospholipid molecule. Be sure to describe their behavior in relationship to water. 2. What happens when a collection of phospholipids
ANSWER KEY. Acids, Bases, and Solutions. Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries,
 Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries, and grass 2. Answers will vary. Sample: Cut 5 g of cherries into small pieces and place in blender. Blend for two minutes,
Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries, and grass 2. Answers will vary. Sample: Cut 5 g of cherries into small pieces and place in blender. Blend for two minutes,
RESTRICTION ENZYME ANALYSIS OF DNA
 University of Massachusetts Medical School Regional Science Resource Center SUPPORTING MATHEMATICS, SCIENCE AND TECHNOLOGY EDUCATION 222 Maple Avenue, Stoddard Building Shrewsbury, MA 01545-2732 508.856.5097
University of Massachusetts Medical School Regional Science Resource Center SUPPORTING MATHEMATICS, SCIENCE AND TECHNOLOGY EDUCATION 222 Maple Avenue, Stoddard Building Shrewsbury, MA 01545-2732 508.856.5097
Ions cannot cross membranes. Ions move through pores
 Ions cannot cross membranes Membranes are lipid bilayers Nonpolar tails Polar head Fig 3-1 Because of the charged nature of ions, they cannot cross a lipid bilayer. The ion and its cloud of polarized water
Ions cannot cross membranes Membranes are lipid bilayers Nonpolar tails Polar head Fig 3-1 Because of the charged nature of ions, they cannot cross a lipid bilayer. The ion and its cloud of polarized water
DNA SPOOLING 1 ISOLATION OF DNA FROM ONION
 DNA SPOOLING 1 ISOLATION OF DNA FROM ONION INTRODUCTION This laboratory protocol will demonstrate several basic steps required for isolation of chromosomal DNA from cells. To extract the chromosomal DNA,
DNA SPOOLING 1 ISOLATION OF DNA FROM ONION INTRODUCTION This laboratory protocol will demonstrate several basic steps required for isolation of chromosomal DNA from cells. To extract the chromosomal DNA,
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Homeostasis and Transport Module A Anchor 4
 Homeostasis and Transport Module A Anchor 4 Key Concepts: - Buffers play an important role in maintaining homeostasis in organisms. - To maintain homeostasis, unicellular organisms grow, respond to the
Homeostasis and Transport Module A Anchor 4 Key Concepts: - Buffers play an important role in maintaining homeostasis in organisms. - To maintain homeostasis, unicellular organisms grow, respond to the
In order to be useful, a smear must have the following qualities:
 Smear Preparation and Simple Stain Objectives: Make bacterial smear slides (usually called smears) Distinguish cells on these slides using a simple stain procedure Unstained microbial cells are nearly
Smear Preparation and Simple Stain Objectives: Make bacterial smear slides (usually called smears) Distinguish cells on these slides using a simple stain procedure Unstained microbial cells are nearly
BACKGROUND INFORMATION
 BACKGROUND INFORMATION It is often important to measure the concentration of glucose in a solution. The so-called ISOTONIC drinks can be tested to see if they are in fact isotonic with the blood. You may
BACKGROUND INFORMATION It is often important to measure the concentration of glucose in a solution. The so-called ISOTONIC drinks can be tested to see if they are in fact isotonic with the blood. You may
catalase 2H 2 O 2 (l) ----> 2H 2 O (l) + O 2 (g)
 ENZYME POST LAB QUIZ STUDY GUIDE Below are the answers to the post-lab (Data Analysis) questions. Make sure you UNDERSTAND all of these questions. The post-lab questions will, of course, be different,
ENZYME POST LAB QUIZ STUDY GUIDE Below are the answers to the post-lab (Data Analysis) questions. Make sure you UNDERSTAND all of these questions. The post-lab questions will, of course, be different,
THE ACTIVITY OF LACTASE
 THE ACTIVITY OF LACTASE Lab VIS-8 From Juniata College Science in Motion Enzymes are protein molecules which act to catalyze the chemical reactions in living things. These chemical reactions make up the
THE ACTIVITY OF LACTASE Lab VIS-8 From Juniata College Science in Motion Enzymes are protein molecules which act to catalyze the chemical reactions in living things. These chemical reactions make up the
Membrane Transport. Extracellular Concentration of X
 Use the following graph to answer questions 1 and 2. Rate of diffusion of X into the cell 1. Which of the following processes is represented by the above graph? c. Active transport 2. Molecule X is most
Use the following graph to answer questions 1 and 2. Rate of diffusion of X into the cell 1. Which of the following processes is represented by the above graph? c. Active transport 2. Molecule X is most
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL (Student Instructions) Determination of the Formula of a Hydrate A Greener Approach Objectives To experimentally determine the formula of a hydrate salt. To learn to think in terms
SUPPLEMENTARY MATERIAL (Student Instructions) Determination of the Formula of a Hydrate A Greener Approach Objectives To experimentally determine the formula of a hydrate salt. To learn to think in terms
Limiting Reagent (using an analogy and a learning cycle approach)
 Limiting Reagent (using an analogy and a learning cycle approach) Welcome: This is the fourth of a four- experiment sequence, covering four important aspects of chemistry, and utilizing a learning cycle
Limiting Reagent (using an analogy and a learning cycle approach) Welcome: This is the fourth of a four- experiment sequence, covering four important aspects of chemistry, and utilizing a learning cycle
Reaction of Magnesium with Hydrochloric Acid (Gas Laws) Chemicals Needed:
 Reaction of Magnesium with Hydrochloric Acid (Gas Laws) Your Name: Date: Partner(s) Names: Objectives: React magnesium metal with hydrochloric acid, collecting the hydrogen over water. Calculate the grams
Reaction of Magnesium with Hydrochloric Acid (Gas Laws) Your Name: Date: Partner(s) Names: Objectives: React magnesium metal with hydrochloric acid, collecting the hydrogen over water. Calculate the grams
Study the following diagrams of the States of Matter. Label the names of the Changes of State between the different states.
 Describe the strength of attractive forces between particles. Describe the amount of space between particles. Can the particles in this state be compressed? Do the particles in this state have a definite
Describe the strength of attractive forces between particles. Describe the amount of space between particles. Can the particles in this state be compressed? Do the particles in this state have a definite
CHAPTER 13: SOLUTIONS
 CHAPTER 13: SOLUTIONS Problems: 1-8, 11-15, 20-30, 37-88, 107-110, 131-132 13.2 SOLUTIONS: HOMOGENEOUS MIXTURES solution: homogeneous mixture of substances present as atoms, ions, and/or molecules solute:
CHAPTER 13: SOLUTIONS Problems: 1-8, 11-15, 20-30, 37-88, 107-110, 131-132 13.2 SOLUTIONS: HOMOGENEOUS MIXTURES solution: homogeneous mixture of substances present as atoms, ions, and/or molecules solute:
Chapter 7: Membrane Structure and Function
 Name Period Concept 7.1 Cellular membranes are fluid mosaics of lipids and proteins 1. The large molecules of all living things fall into just four main classes. Name them. 2. Explain what is meant when
Name Period Concept 7.1 Cellular membranes are fluid mosaics of lipids and proteins 1. The large molecules of all living things fall into just four main classes. Name them. 2. Explain what is meant when
10-ml Graduated cylinder 40 ml 3% Hydrogen peroxide solution (found in stores) Straight-edged razor blade Scissors and Forceps (tweezers)
 Name: Class: Date: Objectives * Measure the effects of changes in temperature, ph, and enzyme concentration on reaction rates of an enzyme catalyzed reaction in a controlled experiment. * Explain how environmental
Name: Class: Date: Objectives * Measure the effects of changes in temperature, ph, and enzyme concentration on reaction rates of an enzyme catalyzed reaction in a controlled experiment. * Explain how environmental
