Cigna Medical Coverage Policy
|
|
|
- Mabel Houston
- 9 years ago
- Views:
Transcription
1 Cigna Medical Coverage Policy Subject Injectable Bulking Agents for Urinary Conditions and Fecal Incontinence Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 9 References Effective Date... 2/15/2016 Next Review Date... 2/15/2017 Coverage Policy Number Related Coverage Resources Biofeedback Botulinum Therapy Electrical Stimulation Therapy and Devices Extracorporeal Electromagnetic Stimulation for Urinary Incontinence Invasive Treatments for Urinary Incontinence Physical Therapy Sacral Nerve Stimulation for Urinary Voiding Dysfunction and Fecal Incontinence INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna companies. Coverage Policies are intended to provide guidance in interpreting certain standard Cigna benefit plans. Please note, the terms of a customer s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer s benefit plan document always supersedes the information in the Coverage Policies. In the absence of a controlling federal or state coverage mandate, benefits are ultimately determined by the terms of the applicable benefit plan document. Coverage determinations in each specific instance require consideration of 1) the terms of the applicable benefit plan document in effect on the date of service; 2) any applicable laws/regulations; 3) any relevant collateral source materials including Coverage Policies and; 4) the specific facts of the particular situation. Coverage Policies relate exclusively to the administration of health benefit plans. Coverage Policies are not recommendations for treatment and should never be used as treatment guidelines. In certain markets, delegated vendor guidelines may be used to support medical necessity and other coverage determinations. Proprietary information of Cigna. Copyright 2016 Cigna Coverage Policy Cigna covers ANY of the following injectable bulking agents as medically necessary for the treatment of adult stress urinary incontinence (SUI) secondary to intrinsic sphincter deficiency (ISD) when there is failure, contraindication or intolerance to at least 12 months of conservative medical management: Glutaraldehyde cross-linked [GAX] bovine collagen (e.g., Contigen ) in men or women Carbon-coated zirconium oxide particles (e.g., Durasphere ), calcium hydroxylapatite [CaHA] particles (e.g., Coaptite ), or silicone elastomer (e.g., Macroplastique ) in women Cigna covers the endoscopic injection of Deflux as medically necessary for the treatment of severe vesicoureteral reflux (i.e., stage II IV) in children age one year or older. Cigna does not cover any other injectable urinary bulking agent other than those specified above as they are considered experimental, investigational or unproven. Cigna does not cover injectable bulking agents (e.g., Solesta ) for the treatment of fecal incontinence as they are considered experimental, investigational or unproven. Page 1 of 14
2 General Background Urinary incontinence is the involuntary loss of urine. It is not a disease but rather a symptom that can be caused by a wide range of conditions. There are several types of incontinence: Stress incontinence is the most common type of leakage. This occurs when urine is lost during activities such as walking, aerobics or even sneezing and coughing. The primary causes are urethral sphincter weakness "intrinsic sphincter deficiency" or a hypermobile urethra. Urethral hypermobility occurs when there is weakness of pelvic floor and poor support of the vesicourethral sphincter unit. The proximal urethra can be displaced outside the abdominal pressure zone during straining. Urge incontinence, often referred to as "overactive bladder," is another form of leakage. This can happen when a person has an uncontrollable urge to urinate but cannot reach the bathroom in time. Overflow incontinence occurs when the bladder is full, is unable to empty and yet leaks. Frequent small urinations and constant dribbling are symptoms. This is rare in women and more common in men with a history of surgery or prostate problems. Functional incontinence is the inability to access a proper facility or urinal container because of physical or intellectual disability. Mixed incontinence refers to a combination of types of incontinence; most commonly stress and urge incontinence. Stress Urinary Incontinence (SUI) If conservative medical treatments such as bladder training, pelvic floor muscle exercises, biofeedback, or medication fail to improve SUI, additional intervention may be necessary. Injectable therapy using bulking agents composed of synthetic materials, bovine collagen, or an autologous substance augments the urethral wall and increases urethral resistance to urinary flow. Injection of bulking agents to treat a dysfunctional urethra is a minimally invasive method of correcting intrinsic sphincteric deficiency (ISD) that results in SUI. ISD may be caused by aging or scarring. Bulking agents are materials that are injected into tissue surrounding the urethra to help keep the urethra closed and reduce urine leakage. A bulking agent procedure usually done in a doctor's office requires minimal anesthesia and takes about five minutes. Bulking up the bladder neck with particulate matter effectively closes the lumen of the urethra, which improves urethral coaptation and restores the mucosal seal mechanism of continence. The downside of the procedure is that most available bulking agents lose their effectiveness over time, and repeat injections are usually needed every six to 18 months. New bulking agents are being developed, as well as new ways to make the injection process easier and more efficient. The standard method of injecting a bulking agent is through a needle, which is inserted in different positions with the assistance of a cystoscope. Treatment-related adverse events are uncommon and relatively minor, the most common being dysuria, urinary urgency, transient urinary retention and acute (e.g., < seven days) urinary retention. Injectable agents are contraindicated in the presence of acute cystitis, urethritis, acute genitourinary infection, and bladder neck or urethral stricture. They should not be injected into blood vessels. Materials used as bulking agents for stress urinary incontinence include: glutaraldehyde cross-linked [GAX] bovine collagen (i.e., Contigen ). carbon-coated zirconium oxide particles (i.e., Durasphere ) calcium hydroxylapatite [CaHA] particles (i.e., Coaptite ) silicone elastomer/polydimethylsiloxane (i.e., Macroplastique ) polytetrafluoroethylene (PTFE; Teflon) Autologous Fat: Before the FDA approved collagen for use, fat injections were used to treat intrinsic sphincter deficiency (ISD) by bulking up the urethra. The short-term result of periurethral fat injection is extremely good. Over time, however, the injected adipocytes tend to be phagocytized by the patient s own body. This high degree of fat absorption is the major detriment to long-term cure. U.S. Food and Drug Administration (FDA): Contigen (glutaraldehyde cross-linked [GAX] bovine collagen) (C. R. Bard, Covington, GA, USA) received a premarket approval (PMA) in 1993 from the FDA. Once injected, this bovine collagen begins to degrade within 12 weeks and completely degrades in 9 19 months. One month before receiving the first treatment, the patient must undergo a skin test to exclude hypersensitivity. Per 2009 Page 2 of 14
3 PMA update, the device was modified and is marketed under the trade name Contigen Bard Collagen Implant and is indicated for use only in the treatment of urinary incontinence due to intrinsic sphincter deficiency (ISD) that may be helped by a locally injected bulking agent. Contigen implant therapy should be initiated only in patients who have shown no improvement in their incontinence for at least 12 months. Durasphere (carbon bead particles) (Carbon Medical Technologies, St. Paul, MN, USA) was approved by the FDA in 1999 for use in treating ISD in women age 21 and over. The use of carbon-coated zirconium oxide particles is restricted to women, as the studies that were submitted with the PMA application showed no improvement in the small number of male and child participants. Skin testing is not required prior to the use of this product. In 2002 there were bead modifications and a trade name revision to Durasphere EXP, also indicated for use in the treatment of adult women with stress urinary incontinence (SUI) due to ISD. Coaptite (calcium hydroxylapatite) (BioForm Medical, Inc., San Mateo, CA, USA) was granted PMA by the FDA in November Coaptite is an injectable, sterile implant composed of spherical particles of calcium hydroxylapatite (CaHA), suspended in an aqueous based gel carrier. The gel carrier is composed of sodium carboxymethyl cellulose, sterile water for injection, and glycerin. It is indicated for soft tissue augmentation in the treatment of SUI due to ISD in adult females. It is contraindicated in patients with 1) significant history of urinary tract infections without resolution, or 2) current or acute conditions of cystitis or urethritis, or 3) fragile urethral mucosal lining. Macroplastique Implants (silicone elastomer/polydimethylsiloxane) (Uroplasty, Inc., Minnetoka, MN, USA) was granted PMA by the FDA in October Macroplastique is a permanently implanted, injectable bulking agent composed of polydimethylsiloxanc particles suspended in a polyvinylpyrrolidone (PVP) carrier gel. It is indicated for transurethral injection in the treatment of adult women diagnosed with SUI primarily due to ISD. Macroplastique is contraindicated in patients with 1) acute urogenital tract inflammation or infection, or 2) fragile urethral mucosal lining (e.g., post-radiation therapy, post-surgery to the bladder neck). Teflon: Because of the risk of migration, polytetrafluoroethylene (PTFE; Teflon) is not approved by the US Food and Drug Administration (FDA) for treatment of female SUI. Tegress (C.R. Bard, Covington, GA, USA) formerly known as URYX (Genyx Medical, Inc., Aliso Viejo, CA) received PMA from the FDA in December 2004 for the treatment of SUI due to ISD in adult women. It is made of ethylene vinyl alcohol (EVOH) copolymers. Bard voluntarily discontinued sales of Tegress in 2007 due to reports of up to 37% erosion rates. Bulkamid (Contura International, Denmark), Zuidex (Q-MED Uppsala, Sweden) and Vantris (Promedon, Cordoba, Argentina) urethral bulking agents are currently not approved by the FDA. Polytetrafluoroethylene (PTFE, Teflon ) and autologous myoblasts are not addressed by the FDA as urinary bulking agents. Literature Review: The safety and clinical utility of most FDA-approved urethral bulking agents are wellsupported in the peer-reviewed scientific literature. While several agents have received approval for use through the FDA, their clinical efficacy has not been proven in all patient populations (e.g., women, men and/or children). Several manufacturers have printed warnings on their package inserts that their product has not been tested in women who are pregnant, in children or men. While some studies have included males in their study population, outcomes do not support the use of most agents for the treatment of male urinary incontinence. At this time, collagen is the only injectable agent that has been approved for use in men. Collagen injection has been used in the treatment of stress urinary incontinence (SUI) since Studies in the peer-reviewed scientific literature support the use of Contigen (collagen) injections for SUI with 50% - 60% success rates (social continence, 24-hour dry pad test) at months follow-up. Studies include men and women. Studies address SUI caused by intrinsic sphincter deficiency (ISD) only, or both ISD and urethral hypermobility. Although collagen injection is considered a safe and effective procedure, most patients need additional treatment sessions to achieve and maintain improvement or cure (Corcos, et al., 2005; Winters, et al., 2000; Smith, et al., 1998; Smith, et al., 1997). Ghoniem et al. (2012) conducted a systematic review and meta-analysis of the evidence (n= 23 cohort studies/958 patients) on the safety and effectiveness of Macroplastique (polydimethylsiloxane injection) SUI. Improvement rates were 75 % in the short-term, 73 % in the mid-term (6-18 months), and 64 % in the long-term Page 3 of 14
4 (>18 months). Cure/dry rates were 43 %, 37 %, and 36 % during the same respective follow-up periods. No serious adverse events were reported. According to the authors, this meta-analytic evidence is supportive of Macroplastique as a safe and effective urethral bulking agent for treating with SUI primarily due to ISD. A Cochrane review by Kirchin et al. (2012) assessed the effects of periurethral or transurethral injection therapy on the cure or improvement of urinary incontinence in women in randomized controlled trials (n=14 studies/2004 subjects). The limited data were not suitable for meta-analysis. Trials were small and generally of moderate quality. Of the 14 trials, eight compared different agents and all results had wide confidence intervals suggesting uncertainty. Silicone particles, calcium hydroxylapatite, ethylene vinyl alcohol, carbon spheres and dextranomer hyaluronic acid combination gave improvements which were not shown to be more or less efficacious than collagen. It was summarized that no clear-cut conclusions could be drawn from trials comparing agents. Insufficient evidence was found to show superiority of mid-urethral or bladder neck injection. Further comparative randomized trials with long term follow-up, involving a placebo or conservative treatment arm are required before injection therapy can be recommended as a standard first-line treatment for stress incontinence. Ghoniem et al. (2009) randomized 247 patients (122 received Macroplastique; 125 received Contigen). The Contigen group served as the control. At 12 months, the Macroplastique group the dry/cure rate was 36.9% compared to 24.8% in the control group (p <0.05). In the Macroplastique and control groups the 1-hour pad weight decrease was 25.4 and 22.8 ml from baseline (p = 0.64), and the mean improvement in Urinary Incontinence Quality of Life Scale score was 28.7 and 26.4 (p = 0.49), respectively. Mayer et al. (2007) compared the safety and effectiveness of Coaptite (calcium hydroxylapatite) to Contigen (collagen) in a randomized controlled trial (n=231). Up to five injections were performed in the first six months of the trial. At 12 months, 63.4% of Coaptite patients compared to 57.0% of Contigen patients showed improvement of 1 incontinence grade (not statistically significant). Most of the Coaptite and Contigen patients received two to three injections, and the mean number of injections was similar for the Coaptite and Contigen; however, a significantly greater percentage of Coaptite patients (38%) than collagen patients (26.1%) had only one injection (p=0.03). No statistically significant differences were found in the number of patients requiring greater than one injection of the test materials. A randomized controlled trial (n=45) compared the safety and clinical utility of Macroplastique (silicone elastomer) to pubovaginal sling procedure in the treatment of female SUI (Maher, et al., 2005). Within each group, there was a significant improvement in outcome as documented by the 1-hour pad test and validated urinary incontinence questionnaires. Macroplastique is associated with reduced morbidity when compared to the sling, including significantly decreased operating time, blood loss, hospital stay and a quicker return to normal activity. While the subjective, patient-determined and objective evaluations were all greater in the sling group, the objective evaluation was the only parameter in which the sling demonstrated a statistically superior outcome to the Macroplastique in the short term (81% versus 9%). Anderson (2002) also conducted a randomized controlled trial (n=46), with a longer average length of follow-up of 32.3 months. A total of 80% of Durasphere patients and 62% of Contigen patients demonstrated an improvement of 1 continence grade at 2.6 and 2.8 years, respectively. This difference was not statistically significant. A randomized controlled trial (n=129) compared the safety and clinical utility of Durasphere (carbon-coated zirconium oxide beads) to Contigen (collagen) in the treatment of SUI and found the two materials comparable with respect to the improvement in continence grade and pad weight testing at 12 months (Lightner, et al., 2001). Specifically, when examined one year after the date of the last treatment, 49 (80.3%) of the 61 women treated with Durasphere showed improvement of one continence grade or more compared to 47 (69.1%) of 68 women treated with bovine collagen (p=0.162, this difference was not statistically significant). Professional Societies/Organizations: In a systematic review by the International Consultation on Incontinence on Surgical Treatment of Stress Incontinence in Men, Herschorn et al. (2010) states the following regarding urethral bulking agents: Bulking agents remain the most minimally invasive treatment for post-rp incontinence after conservative measures. All agents for which there is peer-reviewed data available, show only modest success rates with low cure rates. Effects tend to deteriorate over time. It remains to be seen if improvements in outcomes can be achieved with alternative agents, or if the concept of urethral bulking has achieved its maximal benefit with the agents available now. Page 4 of 14
5 The American College of Obstetricians and Gynecologists (ACOG) guideline entitled Urinary incontinence in women lists the following Major Recommendations": Level B evidence: Bulking agents are a relatively noninvasive method of treatment for stress incontinence and can be used in women for whom any form of operative treatment is contraindicated. Levels of Recommendations: Level A Recommendations are based on good and consistent scientific evidence. Level B Recommendations are based on limited or inconsistent scientific evidence. Level C Recommendations are based primarily on consensus and expert opinion (ACOG, 2005). Vesicoureteral Reflux (VUR) VUR is the abnormal flow of urine from the bladder backwards towards the kidneys. Most commonly a condition of infancy and childhood, VUR increases the risk of urinary tract infections and can lead to kidney damage. Children with primary VUR are born with a defect in the valve that normally prevents urine from flowing backward from the bladder into the ureters. Some children with primary outgrow the condition. Secondary vesicoureteral reflux is due to a urinary tract blockage, often caused by infection. Treatment for both primary and secondary VUR is aimed at preventing kidney damage. Depending on the severity of the condition, treatment options include watchful waiting, medication and surgery. Surgery is typically reserved for those children for whom antibiotics are not successful. However, surgery may be a first line therapy option for grades IV and V or when a quicker, more definitive treatment than medication is appropriate. In endoscopic surgery, a bulking agent (e.g., Deflux, Oceana Therapeutics, Inc., Edison, NJ, USA) is injected around the opening of the affected ureter to try to strengthen the valve's ability to close properly. This method is minimally invasive and compared to open surgery and presents fewer surgical risks. This procedure also requires general anesthesia, but generally can be performed as outpatient surgery. The American Urological Association states the significantly lower morbidity associated with the use of Deflux, compared to open surgery, indicates Deflux must be considered as an important option in VUR management. The choice of management options remains with the informed family and the physician, based upon multiple factors including age, sex, reflux grade, voiding patterns, risk of renal injury, and parental preferences (2007). U.S. Food and Drug Administration (FDA): At the present time, Deflux (Oceana Therapeutics, Inc., Edison, NJ, USA) is the only FDA-approved injectable bulking agent for use in vesicoureteral reflux (VUR). Granted PMA by the FDA in September 2001, it is cross-linked dextran (dextranomer) microspheres in a carrier gel. It is intended for use in treating children age one year and over diagnosed with vesicoureteral reflux (stage II IV). It cannot be used in children with a urinary tract infection. Literature Review: Vesicoureteral Reflux (VUR): The overall success rate reported by different groups of authors for use of Deflux ranged between 68% and 92% depending mainly on the VUR grade (Chertin and Kocherov, 2009). Study results indicate that VUR can be treated successfully with Deflux, producing positive short- and long-term outcomes and providing an alternative to antibiotics or open surgery (Stredele, et al., 2013; Stenberg, et al., 2007; Puri, et al., 2006; Capozza, et al., 2002; Capozza, et al., 2001; Lackgren, et al., 2001). A meta-analysis of the literature on endoscopic therapy for vesicoureteral reflux was conducted by Elder et al. (2006). This analysis included the treatment of 5527 patients. The articles dealt with polytetrafluoroethylene (PTFE, Teflon), collagen, dextranomer/hyaluronic acid (Deflux), polydimethylsiloxane (Macroplastique), chondrocytes, blood and 2 or more injectables. In the database 47 articles (75%) pertained to children, 6 (10%) adults, and 10 (16%) children and adults. The number of studies, number of patients, and percent of reflux resolution by bulking agent was as follows: Teflon: 33 / 361 / 66.86% Collagen: 10 / 947 / 56.86% Deflux: 3 / 385 / 68.71% Macroplastique: 8 / 347 / 76.46% Chondrocytes: 1 / 47 / 50.48% Page 5 of 14
6 Overall, following one treatment, the reflux resolution rate (by ureter) for grades I and II reflux was 78.5%, grade III 72%, grade IV 63% and grade V 51%. If the first injection was unsuccessful, the second treatment had a success rate of 68%, and the third treatment 34%. The aggregate success rate with 1 or more injections was 85%. The success rate was similar among children and adults. It should be noted that relatively few studies included the incidence of urinary tract infection (UTI) in patients undergoing endoscopic therapy. The authors stated that further study of the rates of UTI and pyelonephritis after endoscopic and open antireflux surgery is necessary. It was concluded that future studies pertaining to endoscopic therapy should include data on rates of UTI and renal scarring, with prolonged follow-up (Elder, et al., 2006). Professional Societies/Organizations: The American Urological Association (AUA) Policy Statement on Use of Deflux in the Management of Vesicoureteral Reflux states It is the current position of the American Urological Association that endoscopic injection of the dextranomer/hyaluronic compound Deflux is an option in the management of pediatric vesicoureteral reflux (VUR). The absence of inclusion of Deflux in the 1997 Pediatric Reflux Guidelines simply reflects the fact that it had not been introduced at that time and therefore could not have been evaluated. The contention that Deflux has not been proven to reduce urinary infections associated with reflux is inappropriate to the same extent that no other treatment modality has been shown to reduce all urinary tract infections. The resolution of reflux has been shown to reduce the incidence of pyelonephritis. Therefore to the extent that Deflux can correct VUR, it will reduce the incidence of pyelonephristis. The significantly lower morbidity associated with the use of Deflux, compared to open surgery, indicates Deflux must be considered as an important option in VUR management. The choice of management options remains with the informed family and the physician, based upon multiple factors including age, sex, reflux grade, voiding patterns, risk of renal injury, and parental preferences. To attempt to dictate specific treatment modality based upon concrete evidence is simply impossible based upon the current state of evidence. Any claim that current evidence can guide such a decision reflects a lack of understanding of the state of current evidence. As more evidence emerges, selection of specific therapy for specific patients may become more appropriate. At present, Deflux must be considered an option in the care of the pediatric patient with VUR (October 2007). The AUA Management and Screening of Primary Vesicoureteral Reflux in Children Guideline (Peters, et al., 2010) states the following recommendation under Management of vesicoureteral reflux in the child over one year of age: Recommendation: It is recommended that in patients receiving continuous antibiotic prophylaxis with a febrile breakthrough urinary tract infection be considered for open surgical ureteral reimplantation or endoscopic injection of bulking agents for intervention with curative intent. In general, there is limited evidence to support polytetrafluoroethylene (PTFE, Teflon) or autologous myoblasts injections or other non-fda-approved agents, for use in SUI; or agents other than Deflux for use in VUR; or Deflux for use in SUI (Kotb, et al., 2009; Chertin and Kocherov, 2009; Dyer, et al., 2007; Yucel, et al., 2007; Lottmann, et al., 2006; Chapple, et al., 2005). Fecal Incontinence Fecal incontinence is the inability to control ones bowel movements in someone who is older than 4 years old. Common causes of fecal incontinence include constipation, diarrhea, and muscle or nerve damage. Fecal incontinence may be due to a weakened anal sphincter associated with aging or to damage to the nerves and muscles of the rectum and anus from giving birth. Treatment may include dietary changes, medications, special exercises to help better control the bowels, injection of bulking agents, or surgery. U.S. Food and Drug Administration (FDA): Solesta (Oceana Therapeutics, Inc., Edison, NJ, USA) received FDA-approval (P100014) in May It is approved for the treatment of fecal incontinence in patients 18 years and older who have failed conservative therapy (e.g., diet, fiber therapy, anti-motility medications). This product was developed under the name NASHA/Dx Fecal. Solesta is a sterile, viscous gel contained in a disposable 1 ml assembled glass syringe with a standard luerlock fitting. Solesta consists of dextranomer microspheres, 50 mg/ml, and stabilized sodium hyaluronate, 15 mg/ml, in phosphate buffered 0.9 % sodium chloride solution. Deflux, is the same material as Solesta. Literature Review: There is insufficient evidence in the peer-reviewed scientific literature to demonstrate long term safety and clinical utility of Solesta for fecal incontinence. There is a paucity of studies evaluating other bulking agents (e.g., Coaptite) for fecal incontinence. Page 6 of 14
7 A Hayes Brief reviewed the available literature on Solesta (NASHA/Dx) for fecal incontinence, which included RCTs (n=2 studies) and prospective uncontrolled trials (n=6 studies. Patient populations in studies ranged from and consisted of non-responders to conservative treatment. Outcomes included the change in the number of incontinence episodes and the number of days without incontinence. Follow-up ranged from three months to three years. According to the Hayes report, the available evidence showed that NASHA/Dx treatment for fecal incontinence is associated with modest but statistically significant improvements at follow-up compared with pretreatment status in 64%-74% of patients at one year, 59%-63% at two years, and 45% at three years. However, the overall quality of the evidence was found to be low due to the paucity of controlled studies and small sample sizes. Loss to follow-up was also found to be a limitation of the evidence. It was concluded that given the large placebo effect observed in studies of treatments for fecal incontinence, larger, independent, randomized, sham-controlled studies are needed to further evaluate the efficacy, durability, and safety of this treatment (Hayes, 2014; 2015). A randomized controlled trial (n=206) was conducted to compare injection of dextranomer in stabilized hyaluronic acid with a sham for treatment of fecal incontinence (Graf, et al., 2011). Patients were eligible for inclusion if they were aged years, had a Cleveland clinic Florida fecal incontinence score (CCFIS) of 10 or higher and at least four recorded incontinence episodes in two weeks, had symptoms for at least 12 months, had failure of medically supervised conservative treatment (at least one of dietary modification, fiber supplements, or loperamide hydrochloride treatment) ending at least two weeks before the screening period, were able to understand and comply with the requirements of the study, and were available for follow-up. Adults were randomized to receive NASHA Dx (n=136) or sham treatment (n=70). Of the NASHA Dx group, 132 were analyzed at six months, and 125 analyzed at 12 months. In the sham group, 65 were analyzed at six months. After six months, the trial was unmasked, and patients in the sham treatment group were offered active treatment and were thereafter excluded from further analysis. Data from the first six months were used to assess the effect of active treatment compared with sham treatment. The durability of injected NASHA Dx was assessed using 12-month data from individuals in the active treatment group. The primary endpoint was response to treatment based on the number of incontinence episodes. A 50% or greater reduction in the number of incontinence episodes was noted in 71 patients in the active treatment group (52%) compared with 22 (31%) in the sham treatment group (p=0.0089), this is statistically significant. However, the median decrease in number of incontinence episodes was not significantly greater in the active treatment group than in the sham treatment group at both three months and six months. Change in CCFIS compared with baseline did not differ between treatment groups at three months or at six months. Recorded were 128 treatment-related adverse events, of which two were serious (rectal abscess [n=1]; prostatic abscess [n=1]). Small sample size and short term follow-up are limitations of this study. MellGren et al. (2014) published follow-up results of the Graf et al. (2011) study at 36 months. The 136 patients randomized to NASHA Dx treatment in that study represented the patient population reported on in this extension study. A 36-month follow-up assessment was completed by112/136 patients. Re-treatment one month after initial treatment was performed in 112/136 patients (82%). The decrease of symptoms in 52% of patients at reported at six months was sustained at 36 months. Complete resolution of symptoms was experienced by 13% of patients at 36 months, however 15% of patients also experienced worsening of symptoms during this timeframe. A success rate of 59% was reported for all of 112 patients who were followed up at this time point; the success rate was 44.9% when all missing patients were treated as failures. The number of incontinence episodes recorded in a 14-day diary decreased significantly from baseline to 36-month follow-up (p<0.001). Likewise, the number of incontinence-free days increased significantly (p<0.001). Although this study provides medium-term success data, the results are limited by small sample size and loss to follow-up. Dodi et al. (2010) conducted a prospective observational study, treating 115 patients with fecal incontinence (FI) with NASHA/Dx gel. Of the 115 patients treated with NASHA/Dx gel, a total of 14 patients withdrew or were lost to follow-up at 6 months (n = 101), and additional 10 patients withdrew or were lost to follow-up at 12 months (n = 91). One month after the first treatment visit, the patients were offered retreatment of up to 4 treatments. The number of FI episodes per 24 hours was recorded in the diaries. Fever was fairly common after treatment. A total of 7% of patients reported pyrexia that was assessed by the investigator as related to treatment. A total of 6 cases of anorectal abscess were reported in the study. All of these events resolved after treatment. Results included a 50% reduction from baseline in the number of FI episodes in 57.1% of patients at 6months, and 64.0% at 12 months. The reduction from baseline in number of FI episodes, recorded in the 28-day diary, was Page 7 of 14
8 statistically significant at both 6 months (p<.001) and 12 months (p<.001) after last treatment. Limitations of this study include small sample size and no comparison group. A Cochrane systematic review (n= 4 RCTs/ 176 patients) by Maeda et al. (2010) evaluated perianal injectable bulking agents as treatment for fecal incontinence in adults. Most studies reported a short term benefit from injections regardless of the material used as outcome measures improved over time. A silicone biomaterial was found to provide some advantages and was safer in treating fecal incontinence than carbon-coated beads in the short term. There were also short term benefits from injections delivered under ultrasound guidance compared to digital guidance. The silicone biomaterial did not demonstrate clinical benefit compared to control injection of normal saline. Within the available data, the authors found no reliable evidence for effectiveness of one treatment over another in improving fecal incontinence. It was noted that a definitive conclusion cannot be drawn regarding the effectiveness of perianal injection of bulking agents for fecal incontinence due to the limited number of identified trials together with methodological weaknesses. Larger well-designed trials with adequate numbers of subjects using reliable validated outcome measures are needed to allow definitive assessment of the treatment for both effectiveness and safety (Maeda, et al., 2010). A 2013 update to this Cochrane review included one additional trial (n=5 RCTs/382 patients). Similar conclusions were made by the authors were similar in that although one large randomized controlled trial has shown that this form of treatment using dextranomer in stabilized hyaluronic acid (NASHA Dx) improves continence in the short term, the number of identified trials was limited and most had methodological weaknesses (Maeda, et al., 2013). Professional Societies/Organizations: The American Society of Colon and Rectal Surgeons (ASCRS) Practice parameters for the treatment of fecal incontinence (2007) notes that a surgical option includes Injectable therapy (silicone biomaterial, carbon-coated beads). The guideline states when passive fecal incontinence caused by internal sphincter dysfunction is the predominant symptom, injectable therapy seems to be effective and safe, although its long-term efficacy has yet to be defined. Level of Evidence: II; Grade of Recommendation: B. Levels of Evidence I. Meta-analysis of multiple well-designed, controlled studies, randomized trials with low false-positive and low false-negative errors (high power) II. At least one well-designed experimental study; randomized trials with high false-positive or high false negative errors or both (low power) III. Well-designed, quasi-experimental studies, such as nonrandomized, controlled, single-group, preoperative/postoperative comparison, cohort, time, or matched case-control series IV. Well-designed, nonexperimental studies, such as comparative and correlational descriptive and case studies V. Case reports and clinical examples Grades of Recommendations A. Evidence of Type I or consistent findings from multiple studies of Type II, III, or IV B. Evidence of Type II, III, or IV and generally consistent findings C. Evidence of Type II, III, or IV but inconsistent findings D. Little or no systematic empirical evidence Use Outside of the US The National Institute for Health and Clinical Excellence (NICE)and the National Collaborating Centre for Women s and Children s Health Guideline on urinary incontinence in women (September, 2013) notes that intramural bulking agents (glutaraldehyde cross-linked collagen, silicone, carboncoated zirconium beads or hyaluronic acid/dextran copolymer) should be considered for the management of SUI if conservative management has failed. Women should be made aware of the following: Repeat injections may be required to achieve efficacy. Efficacy diminishes with time. Efficacy is inferior to that of retropubic suspension or sling. Autologous fat and polytetrafluoroethylene used as intramural bulking agents are not recommended for the treatment of SUI. Page 8 of 14
9 The NICE guidance on the use of intramural urethral bulking procedures for SUI in women (November 2005) states Current evidence on the safety and short-term efficacy of intramural urethral bulking procedures for stress urinary incontinence is adequate to support the use for these procedures provided that normal arrangements are in place for clinical governance and for audit or research. The NICE guidance on the use of injectable bulking agents for fecal incontinence states that the Current evidence on the safety and efficacy does not appear adequate for this procedure to be used without special arrangements for consent and for audit or research, which should take place in the context of a clinical trial or formal audit protocol that includes information on well-defined patient groups (NICE, 2007). Summary Certain injectable bulking agents have been proven effective in treating stress urinary incontinence (SUI) due to intrinsic sphincter deficiency (ISD) in patients who meet specific selection criteria. Although the long-term efficacy of these agents is not known, studies have shown that the use of an injectable bulking agent may provide relief (improvement of one continence grade or more) of ISD in individuals refractory to conservative therapy in 50-80% of patients. Studies of children treated for severe vesicoureteral reflux (VUR) (grades, II IV) with Deflux injections have shown this bulking agent to be a safe and effective alternative to antibiotic prophylaxis or open surgical repair. Evidence in the peer-reviewed scientific literature demonstrating the long term safety and clinical utility of bulking agents for fecal incontinence including Solesta, is lacking. Well-designed, long-term trials are needed and the role of this technology in the treatment of fecal incontinence has not yet been demonstrated. There is insufficient evidence to support the use of bulking agents for the treatment of stress urinary incontinence (SUI) or vesicoureteral reflux (VUR) other than those specified above (e.g., the use of polytetrafluoroethylene [PTFE, Teflon] or autologous myoblasts injections or other non-fda-approved agents for SUI; the use of bulking agents other than Deflux for VUR; the use of Deflux for SUI). Coding/Billing Information Note: 1) This list of codes may not be all-inclusive. 2) Deleted codes and codes which are not effective at the time the service is rendered may not be eligible for reimbursement. Collagen/Carbon-coated Zirconium Oxide/Calcium Hydroxylapatite/Silicone Elastomer Agents: Covered when medically necessary: CPT * Description Codes Endoscopic injection of implant material into the submucosal tissues of the urethra and/or bladder neck Cystourethroscopy (including ureteral catheterization); with subureteric injection of implant material HCPCS Codes L8603 L8606 Description Injectable bulking agent, collagen implant, urinary tract, 2.5 ml syringe, includes shipping and necessary supplies Injectable bulking agent, synthetic implant, urinary tract, 1 ml syringe, includes shipping and necessary supplies Deflux : Covered when medically necessary: Page 9 of 14
10 CPT * Description Codes Endoscopic injection of implant material into the submucosal tissues of the urethra and/or bladder neck Cystourethroscopy (including ureteral catheterization); with subureteric injection of implant material HCPCS Codes L8604 Description Injectable bulking agent, dextranomer/hyaluronic acid copolymer implant, urinary tract, 1 ml, includes shipping and necessary supplies Bulking Agents for Fecal Incontinence (e.g., Solesta ): : Experimental/Investigational/Unproven/Not Covered when used to report the Solesta product or the administration of Solesta : CPT * Description Codes Unlisted procedure, anus 0377T Anoscopy with directed submucosal injection of bulking agent for fecal incontinence HCPCS Codes L8605 L8699 Description Injectable bulking agent, dextranomer/hyaluronic acid copolymer implant, anal canal, 1 ml, includes shipping and necessary supplies Prosthetic implant, not otherwise specified *Current Procedural Terminology (CPT ) 2014 American Medical Association: Chicago, IL. References 1. Agency for Healthcare Research and Quality (AHRQ). AHRQ Healthcare Horizon Scanning System Potential High-Impact Interventions Report. Functional Limitations and Disability. Accessed December 7, Available at URL address: ct_june_2012.pdf 2. American College of Obstetricians and Gynecologists (ACOG). ACOG Practice Bulletin No. 63: Urinary incontinence in women. Obstet Gynecol Jun;105(6): American Urological Association. Policy statement on the Use of Deflux in the Management of Vesicoureteral Reflux. October Accessed November No longer available at: 4. Andersen RC. Long-term follow-up comparison of durasphere and contigen in the treatment of stress urinary incontinence. J Low Genit Tract Dis Oct;6(4): Capozza N, Caione P. Dextranomer/hyaluronic acid copolymer implantation for vesicoureteral reflux: a randomized comparison with antibiotic prophylaxis. J Pediatr. 2002;140: Capozza N, Patricolo M, Lais A, Matarazzo E, Caione P. Endoscopic treatment of vesicoureteral reflux: twelve years' experience. Urol Int. 2001;67(3): Page 10 of 14
11 7. Chapple CR, Haab F, Cervigni M, Dannecker C, Fianu-Jonasson A, Sultan AH. An open, multicentre study of NASHA/Dx Gel (Zuidex) for the treatment of stress urinary incontinence. Eur Urol Sep;48(3): Chertin B, Kocherov S. Long-term results of endoscopic treatment of vesicoureteric reflux with different tissue-augmenting substances. J Pediatr Urol Nov 4. [Epub ahead of print] 9. Corcos J, Collet JP, Shapiro S, Herschorn S, Radomski SB, Schick E, et al. Multicenter randomized clinical trial comparing surgery and collagen injections for treatment of female stress urinary incontinence. Urology May;65(5): Dehli T, Stordahl A, Vatten LJ, et al. Sphincter training or anal injections of dextranomer for treatment of anal incontinence: a randomized trial. Scand J Gastroenterol. 2013;48(3): Dodi G, Jongen J, de la Portilla F, Raval M, Altomare DF, Lehur PA. An Open-Label, Noncomparative, Multicenter Study to Evaluate Efficacy and Safety of NASHA/Dx Gel as a Bulking Agent for the Treatment of Fecal Incontinence. Gastroenterol Res Pract. 2010;2010: Epub 2010 Dec Dyer L, Franco I, Firlit CF, Reda EF, Levitt SB, Palmer LS. Endoscopic injection of bulking agents in children with incontinence: dextranomer/hyaluronic acid copolymer versus polytetrafluoroethylene. J Urol Oct;178(4 Pt 2): Epub 2007 Aug Elder JS, Diaz M, Caldamone AA, Cendron M, Greenfield S, Hurwitz R, et al. Endoscopic Therapy for Vesicoureteral Reflux: A Meta-Analysis. I. Reflux Resolution and Urinary Tract Infection. J Urol : Ghoniem G, Corcos J, Comiter C, Bernhard P, Westney OL, Herschorn S. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol Jan;181(1): Epub 2008 Nov Ghoniem GM, Miller CJ. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J Jun 15. [Epub ahead of print] 16. Graf W, Mellgren A, Matzel KE, Hull T, Johansson C, NASHA Dx Study Group, et al. Efficacy of dextranomer in stabilised hyaluronic acid for treatment of faecal incontinence: a randomised, shamcontrolled trial. Lancet Mar 19;377(9770): Hayes, Inc. Hayes Health Technology Brief. Solesta (NASHA Dx; Q-Med AB) for Treatment of Fecal Incontinence. Lansdale, PA: Hayes, Inc.; October, 2014; Updated August Herschorn S, Bruschini H, Comiter C, Grise P, Hanus T, Kirschner-Hermanns R, Abrams P, Committee of the International Consultation on Incontinence, et al. Surgical treatment of stress incontinence in men. Neurourol Urodyn. 2010;29(1): Hodsen EM, Wheeler DM, Vimanchandra D, Smith GH, Craig JC. Interventions for primary vesicoureteric reflux. The Cochrane Database of Systematic Reviews 2007, Issue 3. Art No.: CD DOI: / CD pub Hoy SM. Dextranomer in stabilized sodium hyaluronate (Solesta ): in adults with faecal incontinence. Drugs Aug 20;72(12): doi: / Hurtado E, McCrery R, Appell R. The safety and efficacy of ethylene vinyl alcohol copolymer as an intraurethral bulking agent in women with intrinsic urethral deficiency. Int Urogynecol J Pelvic Floor Dysfunct Aug;18(8): Epub 2006 Nov Kirchin V, Page T, Keegan PE, Atiemo K, Cody JD, McClinton S. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev Feb 15;2:CD Page 11 of 14
12 23. Kotb AF, Campeau L, Corcos J. Urethral bulking agents: techniques and outcomes. Curr Urol Rep Sep;10(5): Lackgren G, Wahlin N, Skoldenberg E, Stenberg A. Long-term follow-up of children treated with Dextranomer/Hyaluronic Acid Copolymer for vesicoureteral reflux. J Urol. 2001;166: La Torre F, de la Portilla F. Long-term efficacy of dextranomer in stabilized hyaluronic acid (NASHA/Dx) for treatment of faecal incontinence. Colorectal Dis May;15(5): doi: /codi Leung FW. Treatment of fecal incontinence - review of observational studies (OS) and randomized controlled trials (RCT) related to injection of bulking agent into peri-anal tissue. J Interv Gastroenterol Oct;1(4): Epub 2011 Oct Lightner D, Calvosa C, Andersen R, Klimberg I, Brito CG, Snyder J, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled, doubleblind study of Durasphere. Urology Jul;58(1): Lottmann HB, Margaryan M, Lortat-Jacob S, Bernuy M, Läckgren G. Long-term effects of dextranomer endoscopic injections for the treatment of urinary incontinence: an update of a prospective study of 61 patients. J Urol Oct;176(4 Pt 2): Luo C, Samaranayake CB, Plank LD, Bissett IP. Systematic review on the efficacy and safety of injectable bulking agents for passive faecal incontinence. Colorectal Dis Apr;12(4): Epub 2009 Mar Maher CF, O Reilly BA, Dwyer PL, Carey MP, Cornish A, Schluter P. Pubovaginal sling versus transurethral Macroplastique for stress urinary incontinence and intrinsic sphincter deficiency: a prospective randomized controlled trial. BJOG Jun;112(6): Maeda Y, Laurberg S, Norton C. Perianal injectable bulking agents as treatment for faecal incontinence in adults. Cochrane Database Syst Rev Feb 28;2:CD doi: / CD pub Maeda Y, Laurberg S, Norton C. Perianal injectable bulking agents as treatment for faecal incontinence in adults. Cochrane Database Syst Rev May 12;(5):CD Mayer RD, Dmochowski R, Appell RA, Sand K, Klimberg IW, Jacoby K, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. J Urol. 2007;69: Mayo Clinic. Fecal incontinence. Updated August Accessed October Available at URL address: Mellgren A, Matzel KE, Pollack J, Hull T, Bernstein M, Graf W; Nasha Dx Study Group. Long-term efficacy of NASHA Dx injection therapy for treatment of fecal incontinence. Neurogastroenterol Motil Aug;26(8): doi: /nmo Epub 2014 May National Institute for Health and Clinical Excellence (NICE).Interventional procedures. Intramural urethral bulking procedures for stress urinary incontinence in women. Published November Accessed November Jan 7, Available at URL address: National Institute for Health and Clinical Excellence (NICE) and the National Collaborating Centre for Women s and Children s Health Guideline on Urinary Incontinence in Women (October, 2006); updated September, Accessed Jan. 7, Available at URL address: Page 12 of 14
13 38. National Institute for Health and Clinical Excellence (NICE). Guidance: Injectable bulking agents for faecal incontinence (February, 2007). Accessed Jan. 7, Available at URL address: Peters CA, Skoog SJ, Arant BS Jr, Copp HL, Elder JS, Hudson RG, et al. Summary of the AUA Guideline on Management of Primary Vesicoureteral Reflux in Children. J Urol Sep;184(3): Epub 2010 Jul Puri P, Pirker M, Mohanan N, Dawrant M, Dass L, Colhoun E. Subureteral Dextranomer/Hyaluronic Acid Injection as first line treatment in the management of high grade vesicoureteral reflux. J Urol. 2006;176: Smith DN, Appell RA, Rackley RR, Winters JC. Collagen injection therapy for post-prostatectomy incontinence. J Urol. 1998;160(2): Smith DN, Appell RA, Winters JC. Collagen injection therapy for female intrinsic sphincteric deficiency. J Urol. 1997;157(4): Stenberg A, Lackgren G. Treatment of vesicoureteral reflux in children using stabilized non-animal hyaluronic acid/dextranomer gel (NASHA/DX): A long-term observational study. J Pediatrc Urol. 2007; 3: Stredele RJ, Dietz HG, Stehr M. Long-term results of endoscopic treatment of vesicoureteral reflux in children: comparison of different bulking agents. J Pediatr Urol Feb;9(1):71-6. doi: /j.jpurol Epub 2011 Dec Tjandra JJ, Dykes SL, Kumar RR, Ellis CN, Gregorcyk SG, Standards Practice Task Force of The American Society of Colon and Rectal Surgeons, et al. Practice parameters for the treatment of fecal incontinence. Dis Colon Rectum Oct;50(10): U.S. Food and Drug Administration (FDA). PMA database. Coaptite. Last updated: 07/08/2009. Accessed November Available at URL address: cently-approveddevices/ucm htm U.S. Food and Drug Administration (FDA). PMA Database. Contigen. Updated 10/03/2011. Accessed November Available at URL address: U.S. Food and Drug Administration (FDA). PMA database. Deflux. Updated 06/29/2009. Accessed November Available at URL address: cently-approveddevices/ucm htm 49. U.S. Food and Drug Administration (FDA). PMA database. Durasphere injectable bulking agent Accessed November Available at URL address: U.S. Food and Drug Administration (FDA). PMA database. Macroplastique Implants. Last updated 08/20/2010. Accessed November Available at URL address: cently-approveddevices/ucm htm 51. U.S. Food and Drug Administration (FDA). PMA database. Solesta May Accessed November Available at URL address: Page 13 of 14
14 caldevicesadvisorycommittee/gastroenterology-urologydevicespanel/ucm pdf 52. U.S. Food and Drug Administration (FDA). Device Approvals and Clearances. URYX Urethral bulking agent. Tegress Urethral Implant. Last updated: 7/08/2009 Accessed November Available at URL address: cently-approveddevices/ucm htm 53. U.S. Food and Drug Administration (FDA). 510(k) Summary Permacol Porcine dermal implant. December 17, Accessed November Available at URL address: Winters JC, Chiverton A, Scarpero HM, Prats LJ Jr. Collagen injection therapy in elderly women: longterm results and patient satisfaction. Urol. 2000;55: Yucel S, Tarcan T, Simsek F. Durability of a Single Successful Endoscopic Polytetrafluororthylene Injection for Primary Vesicoureteral Reflux: 14-Year Follow-up Results. J Urol. 2007; 178: The registered marks "Cigna" and the "Tree of Life" logo are owned by Cigna Intellectual Property, Inc., licensed for use by Cigna Corporation and its operating subsidiaries. All products and services are provided by or through such operating subsidiaries and not by Cigna Corporation. Such operating subsidiaries include Connecticut General Life Insurance Company, Cigna Health and Life Insurance Company, Cigna Behavioral Health, Inc., Cigna Health Management, Inc., and HMO or service company subsidiaries of Cigna Health Corporation. Page 14 of 14
MEDICAL POLICY PERIURETHRAL BULKING AGENTS (I. E., COLLAGEN IMPLANT) FOR THE TREATMENT OF URINARY INCONTINENCE MP-1.027 POLICY TITLE POLICY NUMBER
 Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): Effective Date: July 17, 2007 May 5, 2008- RETIRED I. DESCRIPTION/BACKGROUND Urinary stress incontinence can be a result of
Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): Effective Date: July 17, 2007 May 5, 2008- RETIRED I. DESCRIPTION/BACKGROUND Urinary stress incontinence can be a result of
Refer to Coaptite Injectable Implant Instructions for Use provided with product for complete instructions for use.
 Questions for my Doctor Refer to Coaptite Injectable Implant Instructions for Use provided with product for complete instructions for use. INDICATIONS: Coaptite Injectable Implant is indicated for soft
Questions for my Doctor Refer to Coaptite Injectable Implant Instructions for Use provided with product for complete instructions for use. INDICATIONS: Coaptite Injectable Implant is indicated for soft
Normal bladder function requires a coordinated effort between the brain, spinal cord, and the bladder.
 .. Urinary Incontinence Urinary incontinence is not an inevitable part of aging, and it is not a disease. The loss of bladder control - called urinary incontinence - affects between 13 and 17 million adult
.. Urinary Incontinence Urinary incontinence is not an inevitable part of aging, and it is not a disease. The loss of bladder control - called urinary incontinence - affects between 13 and 17 million adult
URINARY INCONTINENCE
 URINARY INCONTINENCE What is urinary incontinence? Urinary incontinence is the uncontrollable loss of urine. The amount of urine leaked can vary from only a few drops when you cough or sneeze to entirely
URINARY INCONTINENCE What is urinary incontinence? Urinary incontinence is the uncontrollable loss of urine. The amount of urine leaked can vary from only a few drops when you cough or sneeze to entirely
surg urin Surgery: Urinary System 1
 Surgery: Urinary System 1 This section contains information to assist providers in billing for surgical procedures related to the urinary system. Extracorporeal Shock Wave Lithotripsy Medi-Cal covers Extracorporeal
Surgery: Urinary System 1 This section contains information to assist providers in billing for surgical procedures related to the urinary system. Extracorporeal Shock Wave Lithotripsy Medi-Cal covers Extracorporeal
Urinary Incontinence FAQ Sheet
 Urinary Incontinence FAQ Sheet Are you reluctant to talk to your doctor about your bladder control problem? Don t be. There is help. Loss of bladder control is called urinary incontinence. It can happen
Urinary Incontinence FAQ Sheet Are you reluctant to talk to your doctor about your bladder control problem? Don t be. There is help. Loss of bladder control is called urinary incontinence. It can happen
Bladder Health Promotion
 Bladder Health Promotion Community Awareness Presentation Content contributions provided by the Society of Urologic Nurses (SUNA) National Association for Continence (NAFC) Simon Foundation for Continence
Bladder Health Promotion Community Awareness Presentation Content contributions provided by the Society of Urologic Nurses (SUNA) National Association for Continence (NAFC) Simon Foundation for Continence
Bard: Continence Therapy. Stress Urinary Incontinence. Regaining Control. Restoring Your Lifestyle.
 Bard: Continence Therapy Stress Urinary Incontinence Regaining Control. Restoring Your Lifestyle. Stress Urinary Incontinence Becoming knowledgeable about urinary incontinence Uterus Normal Pelvic Anatomy
Bard: Continence Therapy Stress Urinary Incontinence Regaining Control. Restoring Your Lifestyle. Stress Urinary Incontinence Becoming knowledgeable about urinary incontinence Uterus Normal Pelvic Anatomy
Incontinence. What is incontinence?
 Incontinence What is incontinence? Broadly speaking, the medical term incontinence refers to any involuntary release of bodily fluids, but many people associate it strongly with the inability to control
Incontinence What is incontinence? Broadly speaking, the medical term incontinence refers to any involuntary release of bodily fluids, but many people associate it strongly with the inability to control
1 in 3 women experience Stress Urinary Incontinence.
 A PATIENT S GUIDE 1 in 3 women experience Stress Urinary Incontinence. It s time to talk about SUI Get the facts. This Patient s Guide is intended as a public resource on the issue of Stress Urinary Incontinence
A PATIENT S GUIDE 1 in 3 women experience Stress Urinary Incontinence. It s time to talk about SUI Get the facts. This Patient s Guide is intended as a public resource on the issue of Stress Urinary Incontinence
Bladder Health Promotion
 Bladder Health Promotion Community Awareness Presentation endorsed by the Society of Urologic Nurses (SUNA) National Association for Continence( NAFC) Simon Foundation for Continence This presentation
Bladder Health Promotion Community Awareness Presentation endorsed by the Society of Urologic Nurses (SUNA) National Association for Continence( NAFC) Simon Foundation for Continence This presentation
Female Urinary Incontinence
 Female Urinary Incontinence Molly Heublein, MD Assistant Professor Clinical Medicine UCSF Women s Health Primary Care Disclosures I have nothing to disclose. 1 Objectives Review the problem Feel confident
Female Urinary Incontinence Molly Heublein, MD Assistant Professor Clinical Medicine UCSF Women s Health Primary Care Disclosures I have nothing to disclose. 1 Objectives Review the problem Feel confident
Moda Health Medical Necessity Criteria
 Page 1 of 8 Approved: Neal Mills, MD Date: 03/23/2016 Description: A number of procedures have been investigated for the treatment of urinary incontinence, including pelvic floor muscle exercises, behavioral
Page 1 of 8 Approved: Neal Mills, MD Date: 03/23/2016 Description: A number of procedures have been investigated for the treatment of urinary incontinence, including pelvic floor muscle exercises, behavioral
Learning Resource Guide. Understanding Incontinence. 2000 Prism Innovations, Inc. All Rights Reserved
 Learning Resource Guide Understanding Incontinence 2000 Prism Innovations, Inc. All Rights Reserved ElderCare Online s Learning Resource Guide Understanding Incontinence Table of Contents Introduction
Learning Resource Guide Understanding Incontinence 2000 Prism Innovations, Inc. All Rights Reserved ElderCare Online s Learning Resource Guide Understanding Incontinence Table of Contents Introduction
Urinary Incontinence (Involuntary Loss of Urine) A Patient Guide
 Urinary Incontinence (Involuntary Loss of Urine) A Patient Guide Urinary Incontinence (Urine Loss) This booklet is intended to give you some facts on urinary incontinence - what it is, and is not, and
Urinary Incontinence (Involuntary Loss of Urine) A Patient Guide Urinary Incontinence (Urine Loss) This booklet is intended to give you some facts on urinary incontinence - what it is, and is not, and
FEMALE INCONTINENCE REVIEW
 200 S. Wenona Suite 298 Steven L. Jensen, M.D. 5400 Mackinaw, Suite 4302 Bay City, MI 48706 Frank H. Kim, M.D. Saginaw, MI 48604 Telephone (989) 895-2634 Adult & Pediatric Urologists (989) 791-4020 Fax
200 S. Wenona Suite 298 Steven L. Jensen, M.D. 5400 Mackinaw, Suite 4302 Bay City, MI 48706 Frank H. Kim, M.D. Saginaw, MI 48604 Telephone (989) 895-2634 Adult & Pediatric Urologists (989) 791-4020 Fax
Registered Charity No. 5365
 THE MULTIPLE SCLEROSIS SOCIETY OF IRELAND Dartmouth House, Grand Parade, Dublin 6. Telephone: (01) 269 4599. Fax: (01) 269 3746 MS Helpline: 1850 233 233 E-mail: [email protected] www.ms-society.ie
THE MULTIPLE SCLEROSIS SOCIETY OF IRELAND Dartmouth House, Grand Parade, Dublin 6. Telephone: (01) 269 4599. Fax: (01) 269 3746 MS Helpline: 1850 233 233 E-mail: [email protected] www.ms-society.ie
Overview of Urinary Incontinence in the Long Term Care Setting
 Overview of Urinary Incontinence in the Long Term Care Setting Management Strategies for the Nursing Assistant Ann M. Spenard RN, C, MSN Courtney Lyder ND, GNP Learning Objectives Describe common types
Overview of Urinary Incontinence in the Long Term Care Setting Management Strategies for the Nursing Assistant Ann M. Spenard RN, C, MSN Courtney Lyder ND, GNP Learning Objectives Describe common types
SOGC Recommendations for Urinary Incontinence
 The quality of evidence is rated, and recommendations are made using the criteria described by the Canadian Task Force on Preventive Health Care. Clinical Practice Guidelines: The Evaluation of Stress
The quality of evidence is rated, and recommendations are made using the criteria described by the Canadian Task Force on Preventive Health Care. Clinical Practice Guidelines: The Evaluation of Stress
1in 3. women experience bladder leakage.1. Do You? Reclaim your life. Your Resource Guide to Stress Urinary Incontinence
 1in 3 women experience bladder leakage.1 Do You? Reclaim your life Your Resource Guide to Stress Urinary Incontinence Urinary Incontinence Bladder leakage, also known as urinary incontinence is the loss
1in 3 women experience bladder leakage.1 Do You? Reclaim your life Your Resource Guide to Stress Urinary Incontinence Urinary Incontinence Bladder leakage, also known as urinary incontinence is the loss
Regain Control of Your Active Life Treatment Options for Incontinence and Pelvic Organ Prolapse
 Regain Control of Your Active Life Treatment Options for Incontinence and Pelvic Organ Prolapse Nearly one quarter of all women in the United States have some sort of pelvic floor disorder such as urinary
Regain Control of Your Active Life Treatment Options for Incontinence and Pelvic Organ Prolapse Nearly one quarter of all women in the United States have some sort of pelvic floor disorder such as urinary
LOSS OF BLADDER CONTROL IS TREATABLE TAKE CONTROL AND RESTORE YOUR LIFESTYLE
 LOSS OF BLADDER CONTROL IS TREATABLE TAKE CONTROL AND RESTORE YOUR LIFESTYLE TALKING ABOUT STRESS INCONTINENCE (SUI) Millions of women suffer from stress incontinence (SUI). This condition results in accidental
LOSS OF BLADDER CONTROL IS TREATABLE TAKE CONTROL AND RESTORE YOUR LIFESTYLE TALKING ABOUT STRESS INCONTINENCE (SUI) Millions of women suffer from stress incontinence (SUI). This condition results in accidental
Bowel Control Problems
 Bowel Control Problems WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Bowel control problems affect at least 1 million people in the United States. Loss of normal control of the bowels is
Bowel Control Problems WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Bowel control problems affect at least 1 million people in the United States. Loss of normal control of the bowels is
Fecal Incontinence. What is fecal incontinence?
 Scan for mobile link. Fecal Incontinence Fecal incontinence is the inability to control the passage of waste material from the body. It may be associated with constipation or diarrhea and typically occurs
Scan for mobile link. Fecal Incontinence Fecal incontinence is the inability to control the passage of waste material from the body. It may be associated with constipation or diarrhea and typically occurs
Consumer summary Minimally invasive techniques for the relief of stress urinary incontinence
 ASERNIP S Australian Safety and Efficacy Register of New Interventional Procedures Surgical Consumer summary Minimally invasive techniques for the relief of stress urinary incontinence (Adapted from the
ASERNIP S Australian Safety and Efficacy Register of New Interventional Procedures Surgical Consumer summary Minimally invasive techniques for the relief of stress urinary incontinence (Adapted from the
Urinary Incontinence
 Urinary Incontinence Q: What is urinary Urinary (YOOR-in-air-ee) incontinence (in-kahn-tih-nens) is when urine leaks out before you can get to a bathroom. If you have urinary incontinence, you re not alone.
Urinary Incontinence Q: What is urinary Urinary (YOOR-in-air-ee) incontinence (in-kahn-tih-nens) is when urine leaks out before you can get to a bathroom. If you have urinary incontinence, you re not alone.
Urinary Incontinence Dr. Leffler
 Urinary Incontinence Dr. Leffler The involuntary loss of urine at socially unacceptable times occurs in both women and men, but more commonly in women. It has multiple, far-reaching effects on daily activities,
Urinary Incontinence Dr. Leffler The involuntary loss of urine at socially unacceptable times occurs in both women and men, but more commonly in women. It has multiple, far-reaching effects on daily activities,
Transanal Radiofrequency Treatment of Fecal Incontinence
 Transanal Radiofrequency Treatment of Fecal Incontinence Policy Number: 2.01.58 Last Review: 12/2015 Origination: 1/2012 Next Review: 1/2016 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Transanal Radiofrequency Treatment of Fecal Incontinence Policy Number: 2.01.58 Last Review: 12/2015 Origination: 1/2012 Next Review: 1/2016 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
150640_Brochure_B 4/12/07 2:58 PM Page 2. Patient Information. Freedom From an Enlarged Prostate
 150640_Brochure_B 4/12/07 2:58 PM Page 2 Patient Information Freedom From an Enlarged Prostate 150640_Brochure_B 4/12/07 2:58 PM Page 3 GreenLight Laser Therapy 1 150640_Brochure_B 4/12/07 2:58 PM Page
150640_Brochure_B 4/12/07 2:58 PM Page 2 Patient Information Freedom From an Enlarged Prostate 150640_Brochure_B 4/12/07 2:58 PM Page 3 GreenLight Laser Therapy 1 150640_Brochure_B 4/12/07 2:58 PM Page
FAQ About Prostate Cancer Treatment and SpaceOAR System
 FAQ About Prostate Cancer Treatment and SpaceOAR System P. 4 Prostate Cancer Background SpaceOAR Frequently Asked Questions (FAQ) 1. What is prostate cancer? The vast majority of prostate cancers develop
FAQ About Prostate Cancer Treatment and SpaceOAR System P. 4 Prostate Cancer Background SpaceOAR Frequently Asked Questions (FAQ) 1. What is prostate cancer? The vast majority of prostate cancers develop
Patient. Frequently Asked Questions. Transvaginal Surgical Mesh for Pelvic Organ Prolapse
 Patient Frequently Asked Questions Transvaginal Surgical Mesh for Pelvic Organ Prolapse Frequently Asked Questions WHAT IS PELVIC ORGAN PROLAPSE AND HOW IS IT TREATED? Q: What is pelvic organ prolapse
Patient Frequently Asked Questions Transvaginal Surgical Mesh for Pelvic Organ Prolapse Frequently Asked Questions WHAT IS PELVIC ORGAN PROLAPSE AND HOW IS IT TREATED? Q: What is pelvic organ prolapse
What you should know about Stress Urinary Incontinence
 Gynecare TVT Stop coping. Start living. What you should know about Stress Urinary Incontinence Have you ever leaked urine when you laughed, coughed or sneezed? You are not alone. Many women suffer from
Gynecare TVT Stop coping. Start living. What you should know about Stress Urinary Incontinence Have you ever leaked urine when you laughed, coughed or sneezed? You are not alone. Many women suffer from
Urinary Incontinence
 Urinary Incontinence Q: What is urinary incontinence (UI)? A: UI is also known as loss of bladder control or urinary leakage. UI is when urine leaks out before you can get to a bathroom. If you have UI,
Urinary Incontinence Q: What is urinary incontinence (UI)? A: UI is also known as loss of bladder control or urinary leakage. UI is when urine leaks out before you can get to a bathroom. If you have UI,
URINARY INCONTINENCE IN WOMEN
 URINARY INCONTINENCE IN WOMEN Definition Urinary incontinence (UI) is defined as involuntary loss of urine that is a social or hygienic problem (International Continence Society, 1973) Magnitude of the
URINARY INCONTINENCE IN WOMEN Definition Urinary incontinence (UI) is defined as involuntary loss of urine that is a social or hygienic problem (International Continence Society, 1973) Magnitude of the
Female Urinary Disorders and Pelvic Organ Prolapse
 Female Urinary Disorders and Pelvic Organ Prolapse Richard S. Bercik, M.D. Director, Division of Urogynecology & Reconstruction Pelvic Surgery Department of Obstetrics, Gynecology & Reproductive Sciences
Female Urinary Disorders and Pelvic Organ Prolapse Richard S. Bercik, M.D. Director, Division of Urogynecology & Reconstruction Pelvic Surgery Department of Obstetrics, Gynecology & Reproductive Sciences
2/21/2016. Prolapse Surgery after Transvaginal Mesh: The Evolving Landscape. Disclosures. Objectives. No Relevant Disclosures
 Prolapse Surgery after Transvaginal Mesh: The Evolving Landscape David R. Ellington, MD, FACOG Assistant Professor Division of Urogynecology and Pelvic Reconstructive Surgery Disclosures No Relevant Disclosures
Prolapse Surgery after Transvaginal Mesh: The Evolving Landscape David R. Ellington, MD, FACOG Assistant Professor Division of Urogynecology and Pelvic Reconstructive Surgery Disclosures No Relevant Disclosures
Position Statement on Mesh Midurethral Slings for Stress Urinary Incontinence
 Position Statement on Mesh Midurethral Slings for Stress Urinary Incontinence The polypropylene mesh midurethral sling is the recognized worldwide standard of care for the surgical treatment of stress
Position Statement on Mesh Midurethral Slings for Stress Urinary Incontinence The polypropylene mesh midurethral sling is the recognized worldwide standard of care for the surgical treatment of stress
Mesh Erosion and What to do
 Disclosures Mesh Erosion and What to do None Michelle Y. Morrill, MD Chief of Urogynecology, TPMG Director of Urogynecology, Kaiser San Francisco Assistant Professor, Volunteer Faculty Dept of Ob/Gyn,
Disclosures Mesh Erosion and What to do None Michelle Y. Morrill, MD Chief of Urogynecology, TPMG Director of Urogynecology, Kaiser San Francisco Assistant Professor, Volunteer Faculty Dept of Ob/Gyn,
URINARY INCONTINENCE CASE PRESENTATION #1. Urinary Incontinence - History 2014/10/07. Structure of the Female Lower Urinary Tract
 Bladder pressure 2014/10/07 Structure of the Female Lower Urinary Tract Ureter URINARY INCONTINENCE Clinical Clerkship Lecture Series Outer peritoneal coat Detrusor smooth muscle Mucosa Trigone Proximal
Bladder pressure 2014/10/07 Structure of the Female Lower Urinary Tract Ureter URINARY INCONTINENCE Clinical Clerkship Lecture Series Outer peritoneal coat Detrusor smooth muscle Mucosa Trigone Proximal
Pyelonephritis: Kidney Infection
 Pyelonephritis: Kidney Infection National Kidney and Urologic Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is pyelonephritis? Pyelonephritis
Pyelonephritis: Kidney Infection National Kidney and Urologic Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is pyelonephritis? Pyelonephritis
Urinary Tract Infections in Children
 Urinary Tract Infections in Children National Kidney and Urologic Diseases Information Clearinghouse National Institute of Diabetes and Digestive and Kidney Diseases NATIONAL INSTITUTES OF HEALTH Urinary
Urinary Tract Infections in Children National Kidney and Urologic Diseases Information Clearinghouse National Institute of Diabetes and Digestive and Kidney Diseases NATIONAL INSTITUTES OF HEALTH Urinary
Women suffer in silence
 Women suffer in silence Stress urinary incontinence is the involuntary loss of urine resulting from increased intra-abdominal pressure. In people who suffer with this condition, forms of exertion such
Women suffer in silence Stress urinary incontinence is the involuntary loss of urine resulting from increased intra-abdominal pressure. In people who suffer with this condition, forms of exertion such
Urinary Incontinence in Women. Susan Hingle, M.D. Department of Medicine
 Urinary Incontinence in Women Susan Hingle, M.D. Department of Medicine Background Estimated 13 million Americans with urinary incontinence Women are affected twice as frequently as men Only 25% will seek
Urinary Incontinence in Women Susan Hingle, M.D. Department of Medicine Background Estimated 13 million Americans with urinary incontinence Women are affected twice as frequently as men Only 25% will seek
Get the Facts, Be Informed, Make YOUR Best Decision. Pelvic Organ Prolapse
 Pelvic Organ Prolapse ETHICON Women s Health & Urology, a division of ETHICON, INC., a Johnson & Johnson company, is dedicated to providing innovative solutions for common women s health problems and to
Pelvic Organ Prolapse ETHICON Women s Health & Urology, a division of ETHICON, INC., a Johnson & Johnson company, is dedicated to providing innovative solutions for common women s health problems and to
Urinary Incontinence. Types
 Urinary Incontinence Leakage of urine is called urinary incontinence. It is a common problem in women. Some women occasionally leak small amounts of urine. At other times, leakage of urine is frequent
Urinary Incontinence Leakage of urine is called urinary incontinence. It is a common problem in women. Some women occasionally leak small amounts of urine. At other times, leakage of urine is frequent
Classification of Mixed Incontinence
 european urology supplements 5 (2006) 837 841 available at www.sciencedirect.com journal homepage: www.europeanurology.com Classification of Mixed Incontinence Christopher Chapple * Sheffield Hallam University,
european urology supplements 5 (2006) 837 841 available at www.sciencedirect.com journal homepage: www.europeanurology.com Classification of Mixed Incontinence Christopher Chapple * Sheffield Hallam University,
Prevention and Recognition of Obstetric Fistula Training Package. Module 8: Pre-repair Care and Referral for Women with Obstetric Fistula
 Prevention and Recognition of Obstetric Fistula Training Package Module 8: Pre-repair Care and Referral for Women with Obstetric Fistula Early detection and treatment If a woman has recently survived a
Prevention and Recognition of Obstetric Fistula Training Package Module 8: Pre-repair Care and Referral for Women with Obstetric Fistula Early detection and treatment If a woman has recently survived a
Posterior Tibial Nerve Stimulation for Voiding Dysfunction. Original Policy Date
 MP 7.01.86 Posterior Tibial Nerve Stimulation for Voiding Dysfunction Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013
MP 7.01.86 Posterior Tibial Nerve Stimulation for Voiding Dysfunction Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013
Postoperative. Voiding Dysfunction
 Postoperative Voiding Trial Voiding Dysfunction Stephanie Pickett, MD Fellow Female Pelvic Medicine and Reconstructive Surgery Objectives Define postoperative voiding dysfunction Describe how to evaluate
Postoperative Voiding Trial Voiding Dysfunction Stephanie Pickett, MD Fellow Female Pelvic Medicine and Reconstructive Surgery Objectives Define postoperative voiding dysfunction Describe how to evaluate
Overactive Bladder (OAB)
 Overactive Bladder (OAB) Overactive bladder is a problem with bladder storage function that causes a sudden urge to urinate. The urge may be difficult to suppress, and overactive bladder can lead to the
Overactive Bladder (OAB) Overactive bladder is a problem with bladder storage function that causes a sudden urge to urinate. The urge may be difficult to suppress, and overactive bladder can lead to the
Urinary Incontinence. Anatomy and Terminology Overview. Moeen Abu-Sitta, MD, FACOG, FACS
 Urinary Incontinence Anatomy and Terminology Overview Moeen Abu-Sitta, MD, FACOG, FACS Purpose Locate and describe the anatomy of the Female Urinary System Define terminology related to Incontinence Describe
Urinary Incontinence Anatomy and Terminology Overview Moeen Abu-Sitta, MD, FACOG, FACS Purpose Locate and describe the anatomy of the Female Urinary System Define terminology related to Incontinence Describe
MANAGEMENT OF SLING COMPLICATIONS IN FEMALES. Jorge L. Lockhart M.D. Program Director Division of Urology University of South Florida
 MANAGEMENT OF SLING COMPLICATIONS IN FEMALES Jorge L. Lockhart M.D. Program Director Division of Urology University of South Florida INTRODUCTION The traditional gold standard treatments for stress urinary
MANAGEMENT OF SLING COMPLICATIONS IN FEMALES Jorge L. Lockhart M.D. Program Director Division of Urology University of South Florida INTRODUCTION The traditional gold standard treatments for stress urinary
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 18 December 2002 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 18 December 2002 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE
A Physical Therapist s Perspective
 You Can Do Something About INCONTINENCE A Physical Therapist s Perspective American Physical Therapy Association 1 You Can Do Something About Urinary Incontinence Incontinence, involuntary loss of bladder
You Can Do Something About INCONTINENCE A Physical Therapist s Perspective American Physical Therapy Association 1 You Can Do Something About Urinary Incontinence Incontinence, involuntary loss of bladder
Urinary Incontinence. Causes of Incontinence. What s Happening?
 National Institute on Aging AgePage Urinary Incontinence Sarah loves to spend time with her friends talking about her grandchildren and going to exercise classes with neighbors. But she s started to have
National Institute on Aging AgePage Urinary Incontinence Sarah loves to spend time with her friends talking about her grandchildren and going to exercise classes with neighbors. But she s started to have
Acticon. Neosphincter. Getting Back to Life. Information Guide treatment for fecal incontinence
 Acticon Neosphincter Information Guide treatment for fecal incontinence Getting Back to Life Introduction If you suffer from loss of bowel control, you are not alone. Did you know that over 2% of the worldwide
Acticon Neosphincter Information Guide treatment for fecal incontinence Getting Back to Life Introduction If you suffer from loss of bowel control, you are not alone. Did you know that over 2% of the worldwide
Managing Urinary Incontinence
 Patient & Family Guide 2016 Managing Urinary Incontinence www.nshealth.ca Managing Urinary Incontinence What is the urinary system? Urine (pee) is made in the kidneys. It flows through tubes called ureters.
Patient & Family Guide 2016 Managing Urinary Incontinence www.nshealth.ca Managing Urinary Incontinence What is the urinary system? Urine (pee) is made in the kidneys. It flows through tubes called ureters.
I can t empty my rectum without pressing my fingers in or near my vagina
 Since the birth of my baby, I can t control my bowel movements Normally bowel movements (stools) are stored in the rectum until the bowel sends a message to the brain that it is full, and the person finds
Since the birth of my baby, I can t control my bowel movements Normally bowel movements (stools) are stored in the rectum until the bowel sends a message to the brain that it is full, and the person finds
Female Urinary Incontinence
 Female Urinary Incontinence Molly Heublein, MD Assistant Professor Clinical Medicine UCSF Women s Health Primary Care Disclosures I have nothing to disclose. Objectives Which is most true? Review the problem
Female Urinary Incontinence Molly Heublein, MD Assistant Professor Clinical Medicine UCSF Women s Health Primary Care Disclosures I have nothing to disclose. Objectives Which is most true? Review the problem
Effects of Pregnancy & Delivery on Pelvic Floor
 Effects of Pregnancy & Delivery on Pelvic Floor 吳 銘 斌 M.D., Ph.D. 財 團 法 人 奇 美 醫 院 婦 產 部 婦 女 泌 尿 暨 骨 盆 醫 學 科 ; 台 北 醫 學 大 學 醫 學 院 婦 產 學 科 ; 古 都 府 城 台 南 Introduction Pelvic floor disorders (PFDs) include
Effects of Pregnancy & Delivery on Pelvic Floor 吳 銘 斌 M.D., Ph.D. 財 團 法 人 奇 美 醫 院 婦 產 部 婦 女 泌 尿 暨 骨 盆 醫 學 科 ; 台 北 醫 學 大 學 醫 學 院 婦 產 學 科 ; 古 都 府 城 台 南 Introduction Pelvic floor disorders (PFDs) include
Topic review: Clinical presentation and diagnosis of urinary incontinence in the elderly. Prapa Pattrapornpisut 7 June 2012
 1 Topic review: Clinical presentation and diagnosis of urinary incontinence in the elderly Prapa Pattrapornpisut 7 June 2012 2 Urinary incontinence Definition the complaint of any involuntary leakage of
1 Topic review: Clinical presentation and diagnosis of urinary incontinence in the elderly Prapa Pattrapornpisut 7 June 2012 2 Urinary incontinence Definition the complaint of any involuntary leakage of
Do I Need to Have Surgery for Urinary Incontinence? What Kinds of Surgery Can Treat Stress Incontinence?
 Do I Need to Have Surgery for Urinary Incontinence? Excerpts from The Incontinence Solution: Answers for Women of All Ages, by William H. Parker, MD, Amy E. Rosenman, MD and Rachel Parker (Simon and Schuster,
Do I Need to Have Surgery for Urinary Incontinence? Excerpts from The Incontinence Solution: Answers for Women of All Ages, by William H. Parker, MD, Amy E. Rosenman, MD and Rachel Parker (Simon and Schuster,
Bladder and Bowel Control
 Bladder and Bowel Control Dr Sue Woodward Lecturer, Florence Nightingale School of Nursing and Midwifery 2 Why do we need to understand anatomy? Normal physiology Normal adult bladder capacity = 450-500mls
Bladder and Bowel Control Dr Sue Woodward Lecturer, Florence Nightingale School of Nursing and Midwifery 2 Why do we need to understand anatomy? Normal physiology Normal adult bladder capacity = 450-500mls
Sacral Nerve Neuromodulation/Stimulation
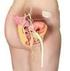 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Sacral Nerve Neuromodulation/Stimulation Number 7.01.69 Effective
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Sacral Nerve Neuromodulation/Stimulation Number 7.01.69 Effective
NHS. Surgical repair of vaginal wall prolapse using mesh. National Institute for Health and Clinical Excellence. 1 Guidance.
 Issue date: June 2008 NHS National Institute for Health and Clinical Excellence Surgical repair of vaginal wall prolapse using mesh 1 Guidance 1.1 The evidence suggests that surgical repair of vaginal
Issue date: June 2008 NHS National Institute for Health and Clinical Excellence Surgical repair of vaginal wall prolapse using mesh 1 Guidance 1.1 The evidence suggests that surgical repair of vaginal
Beverly E Hashimoto, M.D. Virginia Mason Medical Center, Seattle, WA
 Pelvic Floor Relaxation Beverly E Hashimoto, M.D. Virginia Mason Medical Center, Seattle, WA Disclosures Beverly Hashimoto: GE Medical Systems: research support and consultant (all fees given to Virginia
Pelvic Floor Relaxation Beverly E Hashimoto, M.D. Virginia Mason Medical Center, Seattle, WA Disclosures Beverly Hashimoto: GE Medical Systems: research support and consultant (all fees given to Virginia
Childhood Urinary Tract Infections
 Childhood Urinary Tract Infections What is a UTI? Urinary tract infection (UTI) is one of the most common infections in childhood. It can cause distress to the child, concerns to the parents, and may
Childhood Urinary Tract Infections What is a UTI? Urinary tract infection (UTI) is one of the most common infections in childhood. It can cause distress to the child, concerns to the parents, and may
symptoms of Incontinence
 Types, causes and symptoms of Urinary Incontinence Aims and Objectives Aim: To have an understanding of the types and causes of urinary incontinence. Objectives: To be aware of the incidence and prevalence
Types, causes and symptoms of Urinary Incontinence Aims and Objectives Aim: To have an understanding of the types and causes of urinary incontinence. Objectives: To be aware of the incidence and prevalence
Periurethral bulking agent for stress urinary incontinence (macroplastique)
 PLEASE PRINT WHOLE FORM DOUBLE SIDED ON YELLOW PAPER Patient Information to be retained by patient affix patient label Who is this leaflet for? This leaflet provides information about having an injection
PLEASE PRINT WHOLE FORM DOUBLE SIDED ON YELLOW PAPER Patient Information to be retained by patient affix patient label Who is this leaflet for? This leaflet provides information about having an injection
Palm Beach Obstetrics & Gynecology, PA
 Palm Beach Obstetrics & Gynecology, PA 4671 S. Congress Avenue, Lake Worth, FL 33461 561.434.0111 4631 N. Congress Avenue, Suite 102, West Palm Beach, FL 33407 Urinary Tract Infection About one of every
Palm Beach Obstetrics & Gynecology, PA 4671 S. Congress Avenue, Lake Worth, FL 33461 561.434.0111 4631 N. Congress Avenue, Suite 102, West Palm Beach, FL 33407 Urinary Tract Infection About one of every
GENUINE STRESS AND URGE INCONTINENCE PROTOCOL
 GENUINE STRESS AND URGE INCONTINENCE PROTOCOL Using the NeuroTrac ETSTM in combination of electrostimulation and EMG Biofeedback in the treatment of female urinary incontinence. Anna Pawlaczyk Specialist
GENUINE STRESS AND URGE INCONTINENCE PROTOCOL Using the NeuroTrac ETSTM in combination of electrostimulation and EMG Biofeedback in the treatment of female urinary incontinence. Anna Pawlaczyk Specialist
Treatments for Urinary Incontinence in Women
 Treatments for Urinary Incontinence in Women National Kidney and Urologic Diseases Information Clearinghouse National Institute of Diabetes and Digestive and Kidney Diseases NATIONAL INSTITUTES OF HEALTH
Treatments for Urinary Incontinence in Women National Kidney and Urologic Diseases Information Clearinghouse National Institute of Diabetes and Digestive and Kidney Diseases NATIONAL INSTITUTES OF HEALTH
Urinary Incontinence. Patient Information Sheet
 Urinary Incontinence Patient Information Sheet What is urinary incontinence (UI)? UI happens when you are not able to control when you urinate and you wet yourself. How common is urinary incontinence?
Urinary Incontinence Patient Information Sheet What is urinary incontinence (UI)? UI happens when you are not able to control when you urinate and you wet yourself. How common is urinary incontinence?
TIBIAL NERVE STIMULATION: ONE OF SEVERAL NEW OPTIONS FOR THE MANAGEMENT OF OVERACTIVE BLADDER IN WOMEN
 TIBIAL NERVE STIMULATION: ONE OF SEVERAL NEW OPTIONS FOR THE MANAGEMENT OF OVERACTIVE BLADDER IN WOMEN Scott A Farrell MD Professor Dept of Obstetrics and Gynaecology Dalhousie University Declaration of
TIBIAL NERVE STIMULATION: ONE OF SEVERAL NEW OPTIONS FOR THE MANAGEMENT OF OVERACTIVE BLADDER IN WOMEN Scott A Farrell MD Professor Dept of Obstetrics and Gynaecology Dalhousie University Declaration of
Patient Questionnaire for Men
 Patient Questionnaire for Men Please fill out the following questionnaire to the best of your ability prior to your first appointment. Your physical therapist will review your responses during your initial
Patient Questionnaire for Men Please fill out the following questionnaire to the best of your ability prior to your first appointment. Your physical therapist will review your responses during your initial
Mixed urinary incontinence - sling or not sling
 Mixed urinary incontinence - sling or not sling 吳 銘 斌 Ming-Ping Wu, M.D.,Ph.D. Director, Div. Urogynecology & Pelvic Floor Reconstruction, Chi Mei Foundation Hospital, Tainan, Taiwan Assistant Professor,
Mixed urinary incontinence - sling or not sling 吳 銘 斌 Ming-Ping Wu, M.D.,Ph.D. Director, Div. Urogynecology & Pelvic Floor Reconstruction, Chi Mei Foundation Hospital, Tainan, Taiwan Assistant Professor,
Vaginal Mesh: The FDA Decision and Repurcussions. Roger Dmochowski MD, FACS Dept of Urology Vanderbilt University Medical Center Nashville, TN
 Vaginal Mesh: The FDA Decision and Repurcussions Roger Dmochowski MD, FACS Dept of Urology Vanderbilt University Medical Center Nashville, TN 1 ANATOMY FUNCTION 2 Mesh vs No Mesh Outcomes Sivaslioglu 2007
Vaginal Mesh: The FDA Decision and Repurcussions Roger Dmochowski MD, FACS Dept of Urology Vanderbilt University Medical Center Nashville, TN 1 ANATOMY FUNCTION 2 Mesh vs No Mesh Outcomes Sivaslioglu 2007
Stress Urinary Incontinence
 Saint Mary s Hospital Gynaecology Service Warrell Unit Stress Urinary Incontinence Information for patients What is Stress Incontinence? Stress incontinence is a leakage of urine occurring on physical
Saint Mary s Hospital Gynaecology Service Warrell Unit Stress Urinary Incontinence Information for patients What is Stress Incontinence? Stress incontinence is a leakage of urine occurring on physical
Surgical Treatment for Female Stress Urinary. Continence. Consumer Education
 Surgical Treatment for Female Stress Urinary Incontinence By: Amy Rosenman, MD Geffen School of Medicine at UCLA Santa Monica, California Promoting Quality Continence Care through Consumer Education Always
Surgical Treatment for Female Stress Urinary Incontinence By: Amy Rosenman, MD Geffen School of Medicine at UCLA Santa Monica, California Promoting Quality Continence Care through Consumer Education Always
Dr Eva Fong. Urologist Auckland
 Dr Eva Fong Urologist Auckland Urinary incontinence: Treatment options GPCME 2013 Eva Fong Urologist Urinary incontinence Is not normal part of aging or childbearing We can make it better Urinary incontinence:
Dr Eva Fong Urologist Auckland Urinary incontinence: Treatment options GPCME 2013 Eva Fong Urologist Urinary incontinence Is not normal part of aging or childbearing We can make it better Urinary incontinence:
Transobturator tape sling Female sling system
 Transobturator tape sling Female sling system Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
Transobturator tape sling Female sling system Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
CONSENT FORM 12/19/08
 12/19/08 1001 University Place Evanston, Illinois 60201 www.northshore.org CONSENT FORM Phone (224) 364-7100 Fax (847) 570-8011 Intravesical Alkalized Lidocaine for the Treatment of Overactive Bladder
12/19/08 1001 University Place Evanston, Illinois 60201 www.northshore.org CONSENT FORM Phone (224) 364-7100 Fax (847) 570-8011 Intravesical Alkalized Lidocaine for the Treatment of Overactive Bladder
Case Based Urology Learning Program
 Case Based Urology Learning Program Resident s Corner: UROLOGY Case Number 21 CBULP 2011 068 Case Based Urology Learning Program Editor: Associate Editors: Manager: Case Contributors: Steven C. Campbell,
Case Based Urology Learning Program Resident s Corner: UROLOGY Case Number 21 CBULP 2011 068 Case Based Urology Learning Program Editor: Associate Editors: Manager: Case Contributors: Steven C. Campbell,
Spinal Cord and Bladder Management Male: Intermittent Catheter
 Spinal Cord and Bladder Management Male: Intermittent Catheter The 5 parts of the urinary system work together to get rid of waste and make urine. Urine is made in your kidneys and travels down 2 thin
Spinal Cord and Bladder Management Male: Intermittent Catheter The 5 parts of the urinary system work together to get rid of waste and make urine. Urine is made in your kidneys and travels down 2 thin
