CONSENT FORM 12/19/08
|
|
|
- John Riley
- 9 years ago
- Views:
Transcription
1 12/19/ University Place Evanston, Illinois CONSENT FORM Phone (224) Fax (847) Intravesical Alkalized Lidocaine for the Treatment of Overactive Bladder (OAB), a Randomized, Prospective Double-blinded Controlled Trial Principal Investigator (name): Frank Tu, MD, MPH Principal Investigator telephone number: (847) Research Coordinator: Kristen Pozolo, BS (847) EXPLANATION OF STUDY: Nature and Purpose of Research Study: You are being asked whether you would be willing to volunteer to take part in this clinical research study because you have symptoms of an overactive bladder. This Consent Form gives information about the study that you will be able to discuss with your doctor. You are being given this information to help you decide if you would like to participate. If you have any questions, you can ask the study doctor and/or staff. The purpose of this study is to test whether a local anesthetic (Lidocaine) placed into the bladder is a rapid, sustained treatment for overactive bladder. The instillation of Lidocaine into the bladder will be compared with the instillation of a placebo (a nonactive substance). Although local anesthetics are used to treat many different pain disorders, they have not been approved for use inside the bladder by the FDA. Previous studies have found they may be a safe treatment for overactive bladder. You will undergo catheterization with instillation of fluid six times during a three week period. Because symptoms of overactive bladder often come and go, you will then be followed for improvement over a one year period afterwards. This study will include 100 subjects total. Of those subjects, 100 will be from NorthShore University HealthSystem. Explanation of Procedures: At an initial screening visit, you will be first asked to complete a basic medical history, focusing on your overactive bladder symptoms. You will have a small catheter placed into the bladder to test for infection or other causes of your symptoms. This screening study will determine if there are contraindications (such as untreated infection, unexplained blood in the urine, or pregnancy) for you to be in the study. Next, you will be asked to keep a diary of your normal EH Page 1/8 Amend. #6, Rev. dtd. 03/11/08 Consent Form is valid through 12/18/09
2 urinating patterns, which you will bring to the first treatment visit. The study doctors will perform standard tests of bladder function before the first treatment and also at three weeks after finishing the treatment (urodynamics). These tests include: - measuring if you retain any urine after emptying your bladder - measuring how much fluid your bladder can hold - measuring how fast you are able to empty your bladder - determining what volume of fluid makes you feel the need to void During the treatment part of the study, you will receive either instillation of local anesthetic or a saline solution (sterile salt water). The local anesthetic will have a buffered solution added to it to improve absorption into the bladder (sodium bicarbonate). These solutions will be placed into the bladder using a small bladder catheter. Which of the two solutions you receive will be determined at the first visit by random selection (like flipping a regular coin). Therefore, you will have a chance of receiving the local anesthetic solution. This means that 50% of the total patients will not receive treatment but will instead be given the placebo agent consisting of the saline solution. Whether you are receiving Lidocaine or the saline solution, you will be catheterized a total of eight times. These treatments will be scheduled for twice a week for three weeks in the gynecology clinic. This is a double-blinded study, which means that neither you, your doctor or the research staff, will know the identity of the solution you are treated with until after the analysis of the data is completed. (In the event of an emergency, the blind can be broken.) After each week of treatment, you will fill out brief questionnaires at home monitoring any change in your symptoms. We will also ask you to inform us of any unusual side effects you experience from the medication. At three weeks, three months, six months, and one year after completing treatment, you will be asked to return to the clinic for follow-up visits. You will also be asked to fill out additional questionnaires related to your bladder health. Also, at three weeks post-treatment you will undergo the bladder tests (urodynamics) that were done at your first visit again. This will determine if treatment has improved the urine capacity of your bladder. At any time if you are not satisfied with being part of this trial, you may discontinue your participation and discuss further treatment. Alternative Therapy: You do not have to take part in this study for treatment of your bladder problems. Other treatments are: oral medications, or bladder instillation of local anesthetic or other medications. You may choose to have instillation of local anesthetic without participating in this study. You may also choose no further treatment. EH Page 2/8 Amend. #6, Rev. dtd. 03/11/08 Consent Form is valid through 12/18/09
3 Your doctor can provide detailed information about your disease and the benefits of the options. You are free to discuss your disease and your prognosis with your doctor. POSSIBLE BENEFITS: This study will allow doctors to learn more about how this drug works in treating overactive bladder. Taking part in this study may or may not make your overactive bladder symptoms better. While doctors hope that this drug will be more useful in treating your disease than the usual treatment, there is no guarantee of this. If this is shown to be helpful, other patients with this disease may also benefit from treatment with this drug. There is the possibility that your condition may become worse while participating in this study. RISKS AND DISCOMFORTS: A. Catheterization: Urodynamics involves bladder catheterization (placing a sterile, narrow tube into your bladder to instill fluids or to remove fluids from the bladder). Instillation of the bladder medications (or placebo) will also require placement of this tube. Bladder infection is an uncommon risk from catheterization that can generally be treated with a few days of oral antibiotics. In addition, you may feel bladder or urethral discomfort, including urgency or frequency while the catheter is in place or while fluids are being instilled into your bladder. These sensations should not last more than a few hours. It is important to point out that half of the women who are in this study will undergo nine catheterizations without receiving any compounds with known medical properties (the placebo half will receive only sterile saline). B. Local anesthetic: The local anesthetic used in this study might have undesired effects. However, doctors do not know all the undesired effects that may happen. Undesired effects may be mild or very serious and can sometimes be life-threatening. Some undesired effects may go away soon after you stop taking the study drug. In some cases, the effects can be serious, long lasting or may never go away. The study doctor will watch you carefully for any undesired effects and will provide treatment for these effects. This may include stopping your participation in the study or giving you other medications. Although local anesthetic is poorly absorbed through the bladder, you may theoretically experience some systemic side effects. Side effects that have been reported by some patients using lidocaine for various medical reasons include confusion, dizziness, drowsiness, headache, constipation, nausea, vomiting, low blood pressure, urethral irritation, tingling sensations, or tremors. Rare, but EH Page 3/8 Amend. #6, Rev. dtd. 03/11/08 Consent Form is valid through 12/18/09
4 serious side effects include irregular heart rhythms, heart attack, methemoglobinemia (a serious condition where the blood is less able to carry oxygen to the tissues of the body), or seizures. Due to the investigational nature of this study, there may be other risks that are currently unknown. You should not become pregnant while on this study because local anesthetics could theoretically affect an unborn baby or a pregnant woman. You should also not be breastfeeding an infant/toddler, although the American Academy of Pediatrics does consider lidocaine to be compatible with breast-feeding. You should use effective birth control methods if you can become pregnant and you wish to participate in this study. If you become pregnant during this study, you should notify the study doctor for counseling and possibly to stop your participation in the study. Birth control may not prevent pregnancy; only total abstinence from sexual relations can guarantee prevention of pregnancy. If you suspect your birth control has failed, you should not rely on the result of one or several home pregnancy tests. Rather, you need to promptly contact your study doctor to arrange for more sensitive pregnancy tests. C. Non-treatment: Unfortunately, women receiving the placebo (nonactive substance) may potentially experience persistent or worsening overactive bladder symptoms. There is no evidence that a few months delay in treating symptoms predicts a worse outcome for women with this condition. Though the symptoms are not harmful, we cannot offer additional treatment for them while you are a subject. However, you can withdraw if you become too uncomfortable and receive additional treatment. EXPLANATION OF INVESTIGATOR'S AVAILABILITY TO ANSWER QUESTIONS: The study doctor will answer any questions you have based on current medical knowledge. Any new information or changes in the study that may affect your health or your willingness to continue in the study will be given to you as soon as they become available. CONFIDENTIALITY: If information from this study is published, presented at meetings or placed in a report, your name and other personal information will not be used. Every effort will be made to keep your personal medical information confidential. Your study related information may be examined by other researchers in this study, by the sponsoring organization (Berlex Foundation), by the NorthShore University HealthSystem Institutional Review Board, or by the Food and Drug Administration (FDA). EH Page 4/8 Amend. #6, Rev. dtd. 03/11/08 Consent Form is valid through 12/18/09
5 COMPENSATION DISCLAIMER: If you experience an injury/illness because of your participation in this research study, medical treatment for injuries or illness is available through NorthShore University HealthSystem. You and/or your health plan/insurance company will need to pay as no additional funds have been allocated to cover the costs for this treatment. Further information may be obtained from the Research Institute of NorthShore University HealthSystem. PAYMENT FOR PARTICIPATION: You will be paid $10 per visit for a total of $110 for 11 visits. ADDITIONAL COSTS: You should check with your insurance company to ensure that research related treatment is covered under your policy. There may be additional costs to you. EXPLANATION OF ABILITY TO WITHDRAW FROM STUDY: Your participation in this research study is voluntary. If you decide not to participate in this study, you can still receive medical care as usual. If you decide to participate now, you may leave the study at any time. No matter what decision you make, there will be no penalty to you and you will not lose any of your regular benefits. Your doctor may stop this study or your being a part of it without your consent. If this happens, it might be the result of a bad reaction you have to the therapy or new information that your physician learns about the safety or effectiveness of this treatment regimen. YOUR RIGHTS AS A RESEARCH SUBJECT: You may obtain additional information about your rights as a research subject from the Chairperson of the Institutional Review Board (IRB) or the IRB Coordinators, telephone - 224/ These are the individuals to whom you should report any problems or injuries that may be due to the research study. ******************************************************************************** EH Page 5/8 Amend. #6, Rev. dtd. 03/11/08 Consent Form is valid through 12/18/09
6 INDIVIDUAL PROVIDING EXPLANATION: The procedures and/or investigations described in the above paragraphs have been explained to you by (print): Name of Person Explaining Study Signature of Person Explaining Study Date Signed (PRINT) (Sign) (Date) If you have additional questions at any time during the study, you may contact the Project Director, Dr. Tu, at telephone (847) CONSENT TO PARTICIPATE: I understand that the activities will be supervised by Dr. Tu and whomever he may designate as his assistants. I have read this explanation of activities to be followed or have had it read to me. With this knowledge of the nature and purposes of the activities, treatment, the possible attendant risks and discomforts, the possible benefits and the possible alternative methods of treatment, I hereby authorize the performance of the activities described in this consent form. I have read and discussed the explanation of this study with the study director and/or staff. I have had enough time with the study director and/or staff to discuss all of my questions and concerns. I willingly consent to be a part of this study. I will receive a signed and dated copy of this Consent Form. Subject s Name (Please PRINT) Subject's Signature Date Signed EH Page 6/8 Amend. #6, Rev. dtd. 03/11/08 Consent Form is valid through 12/18/09
7 Authorization for the Release of Protected Health Information The Federal government created a rule called the Health Insurance Portability and Accountability Act of 1996 (HIPAA). This rule is designed to protect the confidentiality of your protected (private) health information (PHI). Your protected health information is information about you that could be used to find out who you are. For this research study, this includes information in your existing medical records needed for this study and new information created or collected during the study. This Data Privacy Statement explains how your PHI will be used and who it will be given to ("disclosed") for this research study. It also describes your privacy rights, including your right to see your protected health information. By signing the authorization document for this study, you will give permission ("authorization") for the uses and disclosures of your PHI that are described in this document. If you do not want to allow these uses, you should not participate in this study. Note, you will not be allowed to participate if you do not sign this form. If you agree to participate in the research study, your private health information will be used and disclosed in the following ways: The study Investigator and staff will use your medical records and information created or collected during the study to conduct the study. The Investigator will use the study data for research purposes to support the scientific objectives described in the consent form. Your study data may be shared with regulatory authorities in the United States and other countries, and the Institutional Review Board overseeing this study. Study data that does not directly identify you may be published in medical journals or shared with others as part of scientific discussions. Your original medical records, which may contain information that directly identifies you, may be reviewed by the Institutional Review Board overseeing this study, and regulatory authorities in the United States and other countries. The purpose of these reviews is to assure the quality of the study conduct and the study data, or for other uses authorized by law. The Investigator will not disclose PHI to insurance companies unless required to do so by law, or unless you provide separate written authorization to do so. Your medical records and study data may be held and processed on computers. Your personal health information may no longer be protected under the HIPAA privacy rule once it is disclosed by your study Investigator to these other parties. EH Page 7/8 Amend. #6, Rev. dtd. 03/11/08 Consent Form is valid through 12/18/09
8 You have the right to see and copy your personal health information related to the research study for as long as this information is held by the study Investigator or research institution. You may cancel your authorization at any time by providing written notice to the study Investigator. If you cancel your authorization, the study Investigator and staff will no longer use or disclose your personal health information in connection with this study, unless the study Investigator or staff needs to use or disclose some of your personal health information to preserve the scientific integrity of the study. The study Investigator will still use study data that was collected before you canceled your authorization. If you cancel your authorization, you will no longer be able to participate in the study. However, if you decide to cancel your authorization and withdraw from the study, you will not be penalized or lose any benefits to which you are otherwise entitled. Your authorization for the uses and disclosures described in this authorization does not have an expiration date. Subject s Name (Please PRINT) Subject's Signature Date Signed EH Page 8/8 Amend. #6, Rev. dtd. 03/11/08 Consent Form is valid through 12/18/09
Clinical Trials Network
 National Drug Abuse Treatment Clinical Trials Network STOP SMOKING STUDY Should I Join? NATIONAL INSTITUTES OF HEALTH U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Introduction Many people who abuse drugs
National Drug Abuse Treatment Clinical Trials Network STOP SMOKING STUDY Should I Join? NATIONAL INSTITUTES OF HEALTH U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Introduction Many people who abuse drugs
RESEARCH SUBJECT INFORMATION AND CONSENT FORM
 1 1 1 1 1 1 1 0 1 0 1 0 RESEARCH SUBJECT INFORMATION AND CONSENT FORM TITLE: PROTOCOL NR: SPONSOR: INVESTIGATOR: WIRB VCU tracking number This template is based on a drug or device research study. The
1 1 1 1 1 1 1 0 1 0 1 0 RESEARCH SUBJECT INFORMATION AND CONSENT FORM TITLE: PROTOCOL NR: SPONSOR: INVESTIGATOR: WIRB VCU tracking number This template is based on a drug or device research study. The
HOSPITAL OF THE UNIVERSITY OF PENNSYLVANIA CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5322, Version 2.0, 01/28/15 Long-term Follow-up of Older HIV-infected Adults in the ACTG: Addressing
CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5322, Version 2.0, 01/28/15 Long-term Follow-up of Older HIV-infected Adults in the ACTG: Addressing
Liver Disease & Hepatitis Program Providers: Brian McMahon, MD, Steve Livingston, MD, Lisa Townshend, ANP. Primary Care Provider:
 Liver Disease & Hepatitis Program Providers: Brian McMahon, MD, Steve Livingston, MD, Lisa Townshend, ANP Primary Care Provider: If you are considering hepatitis C treatment, please read this treatment
Liver Disease & Hepatitis Program Providers: Brian McMahon, MD, Steve Livingston, MD, Lisa Townshend, ANP Primary Care Provider: If you are considering hepatitis C treatment, please read this treatment
National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry
 National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry Instructions: This consent statement is to be signed and dated by the patient in the presence of a certified study staff
National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry Instructions: This consent statement is to be signed and dated by the patient in the presence of a certified study staff
MEDICATION GUIDE POMALYST (POM-uh-list) (pomalidomide) capsules. What is the most important information I should know about POMALYST?
 MEDICATION GUIDE POMALYST (POM-uh-list) (pomalidomide) capsules What is the most important information I should know about POMALYST? Before you begin taking POMALYST, you must read and agree to all of
MEDICATION GUIDE POMALYST (POM-uh-list) (pomalidomide) capsules What is the most important information I should know about POMALYST? Before you begin taking POMALYST, you must read and agree to all of
CAMARILLO AQUATICS AND REHABILITATION SERVICES
 CAMARILLO AQUATICS AND REHABILITATION SERVICES Last Name First M.I. Address Apt.# City State Zip Code Phone # SS# Date of Birth Sex M F Driver s License # Marital Status: S M D W Spouse s Name How did
CAMARILLO AQUATICS AND REHABILITATION SERVICES Last Name First M.I. Address Apt.# City State Zip Code Phone # SS# Date of Birth Sex M F Driver s License # Marital Status: S M D W Spouse s Name How did
How To Participate In A Bladder Cancer Study
 SCOTTSDALE HEALTHCARE INSTITUTIONAL REVIEW BOARD Consent to Participate in Research Protocol Name: A Study to Compare the Study Drug, Erlotinib or Polyphenon E, to a Placebo (a substance that looks like
SCOTTSDALE HEALTHCARE INSTITUTIONAL REVIEW BOARD Consent to Participate in Research Protocol Name: A Study to Compare the Study Drug, Erlotinib or Polyphenon E, to a Placebo (a substance that looks like
ALTERNATIVE TREATMENT PLAN AND CONSENT FOR MEDICAL ABORTION WITH MIFEPREX (MIFEPRISTONE) AND MISOPROSTOL
 ALTERNATIVE TREATMENT PLAN AND CONSENT FOR MEDICAL ABORTION WITH MIFEPREX (MIFEPRISTONE) AND MISOPROSTOL The FDA gave its approval status to Mifepristone in 1996 based on research up to that time. Extensive
ALTERNATIVE TREATMENT PLAN AND CONSENT FOR MEDICAL ABORTION WITH MIFEPREX (MIFEPRISTONE) AND MISOPROSTOL The FDA gave its approval status to Mifepristone in 1996 based on research up to that time. Extensive
Patient Information. Date: Date of Birth: / / Name: Social Security: _- - Address: Street City State Zip
 Personal Insurance Intake Form Patient Information Date of Birth: / / Social Security: _- - Address: Street City State Zip Email Address: Home Phone: Sex: M or F Work Phone:. Cell Phone: Height: Weight:
Personal Insurance Intake Form Patient Information Date of Birth: / / Social Security: _- - Address: Street City State Zip Email Address: Home Phone: Sex: M or F Work Phone:. Cell Phone: Height: Weight:
Research Ethics Review Committee (WHO ERC)
 Research Ethics Review Committee (WHO ERC) 20, AVENUE APPIA CH-1211 GENEVA 27 SWITZERLAND HTTP://INTRANET.WHO.INT/HOMES/RPC/ERC HTTP://WWW.WHO.INT/RPC/RESEARCH_ETHICS Informed Consent Template for Clinical
Research Ethics Review Committee (WHO ERC) 20, AVENUE APPIA CH-1211 GENEVA 27 SWITZERLAND HTTP://INTRANET.WHO.INT/HOMES/RPC/ERC HTTP://WWW.WHO.INT/RPC/RESEARCH_ETHICS Informed Consent Template for Clinical
RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM
 Patient I.D. Plate RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM Protocol Title: Application No.: Sponsor: The National Simulation Study: Evaluating Simulated Clinical Experiences
Patient I.D. Plate RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM Protocol Title: Application No.: Sponsor: The National Simulation Study: Evaluating Simulated Clinical Experiences
Informed Consent for Physical Therapy Services
 Informed Consent for Physical Therapy Services Physical therapy is a patient care service that is provided in order to manage a wide variety of conditions. Services are provided to individuals of all ages
Informed Consent for Physical Therapy Services Physical therapy is a patient care service that is provided in order to manage a wide variety of conditions. Services are provided to individuals of all ages
#3: SAMPLE CONSENT FORM
 #3: SAMPLE CONSENT FORM [Key Element #3: Who is conducting the study] UPMC University of Pittsburgh Medical Center Western Psychiatric Institute and Clinic CONSENT TO ACT AS A PARTICIPANT IN A RESEARCH
#3: SAMPLE CONSENT FORM [Key Element #3: Who is conducting the study] UPMC University of Pittsburgh Medical Center Western Psychiatric Institute and Clinic CONSENT TO ACT AS A PARTICIPANT IN A RESEARCH
CONSENT FORM. Cord Blood Banking for Transplantation
 Cord Blood Program Northwest Tissue Center/Puget Sound Blood Center 921 Terry Avenue Seattle, WA 98104 (206) 292-1896 Swedish Medical Center 747 Broadway Seattle, WA 98122 (206) 386-6000 CONSENT FORM Cord
Cord Blood Program Northwest Tissue Center/Puget Sound Blood Center 921 Terry Avenue Seattle, WA 98104 (206) 292-1896 Swedish Medical Center 747 Broadway Seattle, WA 98122 (206) 386-6000 CONSENT FORM Cord
What You Need to Know About LEMTRADA (alemtuzumab) Treatment: A Patient Guide
 For Patients What You Need to Know About LEMTRADA (alemtuzumab) Treatment: A Patient Guide Patients: Your doctor or nurse will go over this patient guide with you. It is important to ask any questions
For Patients What You Need to Know About LEMTRADA (alemtuzumab) Treatment: A Patient Guide Patients: Your doctor or nurse will go over this patient guide with you. It is important to ask any questions
Personal Injury Intake Form
 Personal Injury Intake Form Patient Information: Name Home Phone Address Work Phone Cell Phone Date of Birth Social Security # Sex Male Female Height Weight lbs Occupation Marital Status Employer No of
Personal Injury Intake Form Patient Information: Name Home Phone Address Work Phone Cell Phone Date of Birth Social Security # Sex Male Female Height Weight lbs Occupation Marital Status Employer No of
If you are signing for a minor child, you refers to your child throughout the consent document.
 CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY Adult Patient or Parent, for Minor Patient INSTITUTE: National Cancer Institute PRINCIPAL INVESTIGATOR: Raffit Hassan, M.D. STUDY TITLE: Tissue Procurement
CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY Adult Patient or Parent, for Minor Patient INSTITUTE: National Cancer Institute PRINCIPAL INVESTIGATOR: Raffit Hassan, M.D. STUDY TITLE: Tissue Procurement
MEDICATION GUIDE ACTOPLUS MET (ak-tō-plus-met) (pioglitazone hydrochloride and metformin hydrochloride) tablets
 MEDICATION GUIDE (ak-tō-plus-met) (pioglitazone hydrochloride and metformin hydrochloride) tablets Read this Medication Guide carefully before you start taking and each time you get a refill. There may
MEDICATION GUIDE (ak-tō-plus-met) (pioglitazone hydrochloride and metformin hydrochloride) tablets Read this Medication Guide carefully before you start taking and each time you get a refill. There may
THE 7:16 WAS ON TIME BUT MY LEGS WERE BEHIND SCHEDULE THAT S WHEN I ASKED FOR THE WALKING PILL
 THE 7:16 WAS ON TIME BUT MY LEGS WERE BEHIND SCHEDULE THAT S WHEN I ASKED FOR THE WALKING PILL See page 12 for FREE* TRIAL OFFER *Limitations and restrictions apply. Actor portrayal AMPYRA (dalfampridine)
THE 7:16 WAS ON TIME BUT MY LEGS WERE BEHIND SCHEDULE THAT S WHEN I ASKED FOR THE WALKING PILL See page 12 for FREE* TRIAL OFFER *Limitations and restrictions apply. Actor portrayal AMPYRA (dalfampridine)
University of Pennsylvania RESEARCH Subject Informed Consent Form AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 University of Pennsylvania RESEARCH Subject Informed Consent Form AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: Evaluating the impact of cocaine use and HIV infection in arterial wall inflammation
University of Pennsylvania RESEARCH Subject Informed Consent Form AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: Evaluating the impact of cocaine use and HIV infection in arterial wall inflammation
PREMIER PAIN CARE PA Carlos J Garcia MD 2435 W. Oak Street # 103 Denton, TX 76201 Phone 940-323-9404 Fax 940-323-9422 PATIENT REGISTRATION
 PREMIER PAIN CARE PA Carlos J Garcia MD 2435 W. Oak Street # 103 Denton, TX 76201 Phone 940-323-9404 Fax 940-323-9422 PATIENT REGISTRATION Last Name First Name MI Mailing Address City Zip code Home Phone
PREMIER PAIN CARE PA Carlos J Garcia MD 2435 W. Oak Street # 103 Denton, TX 76201 Phone 940-323-9404 Fax 940-323-9422 PATIENT REGISTRATION Last Name First Name MI Mailing Address City Zip code Home Phone
MODEL FORM. [Program s SART Name and Number] INFORMED CONSENT FOR EGG DONORS
![MODEL FORM. [Program s SART Name and Number] INFORMED CONSENT FOR EGG DONORS MODEL FORM. [Program s SART Name and Number] INFORMED CONSENT FOR EGG DONORS](/thumbs/39/19160563.jpg) MODEL FORM [Program s SART Name and Number] INFORMED CONSENT FOR EGG DONORS You are agreeing to undergo a cycle of egg donation at [program s SART name]. Do not sign this document if you have not received
MODEL FORM [Program s SART Name and Number] INFORMED CONSENT FOR EGG DONORS You are agreeing to undergo a cycle of egg donation at [program s SART name]. Do not sign this document if you have not received
1. Study title Is the title self explanatory to a layperson? If not, a simplified title should be included.
 These guidelines apply to all research projects where human subjects are involved in the study GUIDELINES FOR RESEARCHERS PATIENT INFORMATION SHEET & CONSENT FORM The guidance, which follows, applies primarily
These guidelines apply to all research projects where human subjects are involved in the study GUIDELINES FOR RESEARCHERS PATIENT INFORMATION SHEET & CONSENT FORM The guidance, which follows, applies primarily
Explanation of Research (Consent Form)
 Explanation of Research (Consent Form) Study Title: A Web-Based Self-Management Intervention for Intermittent Urinary Catheter Users (Pilot study) Principal Investigator: Mary H. Wilde, PhD, RN This is
Explanation of Research (Consent Form) Study Title: A Web-Based Self-Management Intervention for Intermittent Urinary Catheter Users (Pilot study) Principal Investigator: Mary H. Wilde, PhD, RN This is
Women s Continence and Pelvic Health Center
 Women s Continence and Pelvic Health Center Committed to Caring 580-590 Court Street Keene, New Hampshire 03431 (603) 354-5454 Ext. 6643 URINARY INCONTINENCE QUESTIONNAIRE The purpose of this questionnaire
Women s Continence and Pelvic Health Center Committed to Caring 580-590 Court Street Keene, New Hampshire 03431 (603) 354-5454 Ext. 6643 URINARY INCONTINENCE QUESTIONNAIRE The purpose of this questionnaire
Frequently Asked Questions (FAQs)
 Frequently Asked Questions (FAQs) Research Rationale 1. What does PrEP stand for? There is scientific evidence that antiretroviral (anti-hiv) medications may be able to play an important role in reducing
Frequently Asked Questions (FAQs) Research Rationale 1. What does PrEP stand for? There is scientific evidence that antiretroviral (anti-hiv) medications may be able to play an important role in reducing
Pl"OtocolDirector: Iris Schrijver _ IRB Approval Date: _June 20 2006 IRE Expiration Date: June 19, 2007 _ STANFORD SAMPLE CONSENT FORM
 Protocol Title: Molecular genetic basis of sensorineural hearing Pl"OtocolDirector: Iris Schrijver IRB Approval Date: June 20 2006 IRE Expiration Date: June 19, 2007 STANFORD SAMPLE CONSENT FORM Please
Protocol Title: Molecular genetic basis of sensorineural hearing Pl"OtocolDirector: Iris Schrijver IRB Approval Date: June 20 2006 IRE Expiration Date: June 19, 2007 STANFORD SAMPLE CONSENT FORM Please
MEDICATION GUIDE WELLBUTRIN (WELL byu-trin) (bupropion hydrochloride) Tablets
 MEDICATION GUIDE WELLBUTRIN (WELL byu-trin) (bupropion hydrochloride) Tablets Read this Medication Guide carefully before you start using WELLBUTRIN and each time you get a refill. There may be new information.
MEDICATION GUIDE WELLBUTRIN (WELL byu-trin) (bupropion hydrochloride) Tablets Read this Medication Guide carefully before you start using WELLBUTRIN and each time you get a refill. There may be new information.
WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500. Birth Control Pills
 Birth Control Pills WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Birth control pills (also called oral contraceptives or "the pill") are used by millions of women in the United States to
Birth Control Pills WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Birth control pills (also called oral contraceptives or "the pill") are used by millions of women in the United States to
MEDICATION GUIDE Savella (Sa-vel-la) (milnacipran HCl) Tablets
 MEDICATION GUIDE Savella (Sa-vel-la) (milnacipran HCl) Tablets Savella is not used to treat depression, but it acts like medicines that are used to treat depression (antidepressants) and other psychiatric
MEDICATION GUIDE Savella (Sa-vel-la) (milnacipran HCl) Tablets Savella is not used to treat depression, but it acts like medicines that are used to treat depression (antidepressants) and other psychiatric
University of Hawai i Human Studies Program. Guidelines for Developing a Clinical Research Protocol
 University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
Patient Guide. Important information for patients starting therapy with LEMTRADA (alemtuzumab)
 Patient Guide Important information for patients starting therapy with LEMTRADA (alemtuzumab) This medicinal product is subject to additional monitoring. This will allow quick identification of new safety
Patient Guide Important information for patients starting therapy with LEMTRADA (alemtuzumab) This medicinal product is subject to additional monitoring. This will allow quick identification of new safety
INFORMED CONSENT INFORMED CONSENT FOR PARTICIPATION IN A HEALTH AND FITNESS TRAINING PROGRAM
 INFORMED CONSENT INFORMED CONSENT FOR PARTICIPATION IN A HEALTH AND FITNESS TRAINING PROGRAM NAME: DATE: 1. PURPOSE AND EXPLANATION OF PROCEDURE I hereby consent to voluntarily engage in an acceptable
INFORMED CONSENT INFORMED CONSENT FOR PARTICIPATION IN A HEALTH AND FITNESS TRAINING PROGRAM NAME: DATE: 1. PURPOSE AND EXPLANATION OF PROCEDURE I hereby consent to voluntarily engage in an acceptable
CORD BLOOD TRANSPLANTATION STUDY EXPANDED ACCESS PROTOCOL APPENDIX A SAMPLE CONSENT FORM
 APPENDIX A SAMPLE CONSENT FORM CORD BLOOD TRANSPLANTATION (COBLT) STUDY SAMPLE CONSENT FORM FOR THE EXPANDED ACCESS PROTOCOL You (your child) are being asked to take part in a clinical research study.
APPENDIX A SAMPLE CONSENT FORM CORD BLOOD TRANSPLANTATION (COBLT) STUDY SAMPLE CONSENT FORM FOR THE EXPANDED ACCESS PROTOCOL You (your child) are being asked to take part in a clinical research study.
Manual: Policies Document ID: PI013 Date Created: Jun 08 Section: Investigator No. Pages: 7 Review Date: Aug 15 Future Review Date: Aug 17
 P0LICYI013 PREGNANCY AND SEXUAL HEALTH POLICY Manual: Policies Document ID: PI013 Date Created: Jun 08 Section: Investigator No. Pages: 7 Review Date: Aug 15 Future Review Date: Aug 17 PURPOSE To describe
P0LICYI013 PREGNANCY AND SEXUAL HEALTH POLICY Manual: Policies Document ID: PI013 Date Created: Jun 08 Section: Investigator No. Pages: 7 Review Date: Aug 15 Future Review Date: Aug 17 PURPOSE To describe
Dr. David Y. Liao, D.O. Orthopedic Center, LLC. Release of Information
 Release of Information The purpose of this form is to alert our office as to those family members and/or friends who may be scheduling or canceling appointments on your behalf and/or will need to have
Release of Information The purpose of this form is to alert our office as to those family members and/or friends who may be scheduling or canceling appointments on your behalf and/or will need to have
PCOM Letterhead [Substitute same from participating institution and, of course, change Department, PI, and Co-Investigators]
![PCOM Letterhead [Substitute same from participating institution and, of course, change Department, PI, and Co-Investigators] PCOM Letterhead [Substitute same from participating institution and, of course, change Department, PI, and Co-Investigators]](/thumbs/26/2087522.jpg) PCOM Letterhead [Substitute same from participating institution and, of course, change Department, PI, and Co-Investigators] Department of Neuroscience, Physiology and Pharmacology 215-871-6880 PATIENT
PCOM Letterhead [Substitute same from participating institution and, of course, change Department, PI, and Co-Investigators] Department of Neuroscience, Physiology and Pharmacology 215-871-6880 PATIENT
MS Treatments Aubagio TM
 1 MSology Essentials Series Aubagio TM (teriflunomide) Developed by MSology with the invaluable assistance of multiple sclerosis nurse advisors: Bonnie Blain Central Alberta MS Clinic, Red Deer, Alberta
1 MSology Essentials Series Aubagio TM (teriflunomide) Developed by MSology with the invaluable assistance of multiple sclerosis nurse advisors: Bonnie Blain Central Alberta MS Clinic, Red Deer, Alberta
ThinkTwice! Treating Alcohol Dependence with Topiramate: A Critical Appraisal Learning Activity JOURNAL ARTICLE TEI PLAIN LANGUAGE ANTHOLOGY
 JOURNAL ARTICLE Transformed into part of a plain language anthology Treating Alcohol Dependence with Topiramate: A Critical Appraisal Learning Activity Abstract: This study set out to test a drug, topiramate,
JOURNAL ARTICLE Transformed into part of a plain language anthology Treating Alcohol Dependence with Topiramate: A Critical Appraisal Learning Activity Abstract: This study set out to test a drug, topiramate,
National Hospital for Neurology and Neurosurgery
 National Hospital for Neurology and Neurosurgery Botulinum toxin injections for the bladder Department of Uro-Neurology If you would like this document in another language or format, or require the services
National Hospital for Neurology and Neurosurgery Botulinum toxin injections for the bladder Department of Uro-Neurology If you would like this document in another language or format, or require the services
Incontinence. What is incontinence?
 Incontinence What is incontinence? Broadly speaking, the medical term incontinence refers to any involuntary release of bodily fluids, but many people associate it strongly with the inability to control
Incontinence What is incontinence? Broadly speaking, the medical term incontinence refers to any involuntary release of bodily fluids, but many people associate it strongly with the inability to control
APPENDIX B SAMPLE INFORMED CONSENT FORM
 APPENDIX B SAMPLE INFORMED CONSENT FORM B.1 INFORMED CONSENT TO PARTICIPATE IN A RESEARCH STUDY Umbilical Cord Blood Banking for Transplantation You are being asked to take part in a research study that
APPENDIX B SAMPLE INFORMED CONSENT FORM B.1 INFORMED CONSENT TO PARTICIPATE IN A RESEARCH STUDY Umbilical Cord Blood Banking for Transplantation You are being asked to take part in a research study that
The science of medicine. The compassion to heal.
 A PATIENT S GUIDE TO ELECTROPHYSIOLOGY STUDIES OF THE HEART The science of medicine. The compassion to heal. This teaching booklet is designed to introduce you to electrophysiology studies of the heart.
A PATIENT S GUIDE TO ELECTROPHYSIOLOGY STUDIES OF THE HEART The science of medicine. The compassion to heal. This teaching booklet is designed to introduce you to electrophysiology studies of the heart.
What You Should Know
 What You Should Know Will this medicine work for me? The antidepressants presented in this decision aid all work the same for treating depression. Most people with depression can find one that can make
What You Should Know Will this medicine work for me? The antidepressants presented in this decision aid all work the same for treating depression. Most people with depression can find one that can make
Empower Physical Therapy
 PAYMENT AND COLLECTION POLICY Payment is expected at the time of service. Unpaid balances left by your insurance companies will be your responsibility. In the event you are unable to pay the balance in
PAYMENT AND COLLECTION POLICY Payment is expected at the time of service. Unpaid balances left by your insurance companies will be your responsibility. In the event you are unable to pay the balance in
CHOP Chemotherapy Regimen for Lymphoma Information for Patients
 CHOP Chemotherapy Regimen for Lymphoma Information for Patients The Regimen Contains: C: Cytoxan (cyclophosphamide) H: Adriamycin (hydroxy doxorubicin) O: vincristine (Oncovin ) P: Prednisone How Is This
CHOP Chemotherapy Regimen for Lymphoma Information for Patients The Regimen Contains: C: Cytoxan (cyclophosphamide) H: Adriamycin (hydroxy doxorubicin) O: vincristine (Oncovin ) P: Prednisone How Is This
Guidance on Withdrawal of Subjects from Research: Data Retention and Other Related Issues
 Office for Human Research Protections (OHRP) Department of Health and Human Services (HHS) Guidance on Withdrawal of Subjects from Research: Data Retention and Other Related Issues This guidance represents
Office for Human Research Protections (OHRP) Department of Health and Human Services (HHS) Guidance on Withdrawal of Subjects from Research: Data Retention and Other Related Issues This guidance represents
THANK YOU FOR CHOOSING QPT FOR YOUR PHYSICAL THERAPY NEEDS!
 THANK YOU FOR CHOOSING QPT FOR YOUR PHYSICAL THERAPY NEEDS! Please complete and sign all of the enclosed forms. Bring these forms, your physician s referral if required and any other documents required
THANK YOU FOR CHOOSING QPT FOR YOUR PHYSICAL THERAPY NEEDS! Please complete and sign all of the enclosed forms. Bring these forms, your physician s referral if required and any other documents required
Recognizing Signs and Symptoms of a Urinary Tract Infection (UTI)
 Use this checklist to help identify signs and symptoms of a or other illnesses. If the person you support has one or more of these signs and symptoms, call the doctor for advice and a medical appointment.
Use this checklist to help identify signs and symptoms of a or other illnesses. If the person you support has one or more of these signs and symptoms, call the doctor for advice and a medical appointment.
INFORMED CONSENT FOR EGG DONORS PRACTITIONER S GUIDE TO USING THE MODEL FORM
 INFORMED CONSENT FOR EGG DONORS PRACTITIONER S GUIDE TO USING THE MODEL FORM A. GENERAL GUIDELINES AND COMMENTS Purpose and Use of the Model Form This is a guide for practitioners to using the Model Informed
INFORMED CONSENT FOR EGG DONORS PRACTITIONER S GUIDE TO USING THE MODEL FORM A. GENERAL GUIDELINES AND COMMENTS Purpose and Use of the Model Form This is a guide for practitioners to using the Model Informed
Statistics of Type 2 Diabetes
 Statistics of Type 2 Diabetes Of the 17 million Americans with diabetes, 90 percent to 95 percent have type 2 diabetes. Of these, half are unaware they have the disease. People with type 2 diabetes often
Statistics of Type 2 Diabetes Of the 17 million Americans with diabetes, 90 percent to 95 percent have type 2 diabetes. Of these, half are unaware they have the disease. People with type 2 diabetes often
We are required to provide this Notice to you by the Health Insurance Portability and Accountability Act ("HIPAA")
 PRIVACY NOTICE We are required to provide this Notice to you by the Health Insurance Portability and Accountability Act ("HIPAA") THIS NOTICE DESCRIBES HOW PERSONAL AND MEDICAL INFORMATION ABOUT YOU MAY
PRIVACY NOTICE We are required to provide this Notice to you by the Health Insurance Portability and Accountability Act ("HIPAA") THIS NOTICE DESCRIBES HOW PERSONAL AND MEDICAL INFORMATION ABOUT YOU MAY
MEDICATION GUIDE POTIGA (po-tee-ga) tablets, CV (ezogabine)
 MEDICATION GUIDE POTIGA (po-tee-ga) tablets, CV (ezogabine) Read this Medication Guide before you start taking POTIGA and each time you get a refill. There may be new information. This Medication Guide
MEDICATION GUIDE POTIGA (po-tee-ga) tablets, CV (ezogabine) Read this Medication Guide before you start taking POTIGA and each time you get a refill. There may be new information. This Medication Guide
WORKERS COMPENSATION INTAKE FORM
 WORKERS COMPENSATION INTAKE FORM related injury? No Yes INSURANCE INFORMATION RELEASE By clicking this box,i hereby authorize ABA Physical Therapy Associates to release to my Insurance company/attorney,
WORKERS COMPENSATION INTAKE FORM related injury? No Yes INSURANCE INFORMATION RELEASE By clicking this box,i hereby authorize ABA Physical Therapy Associates to release to my Insurance company/attorney,
Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules
 Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules Read this Medication Guide before you start taking EQUETRO and each time you get a refill. There may be new information. This
Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules Read this Medication Guide before you start taking EQUETRO and each time you get a refill. There may be new information. This
Normal bladder function requires a coordinated effort between the brain, spinal cord, and the bladder.
 .. Urinary Incontinence Urinary incontinence is not an inevitable part of aging, and it is not a disease. The loss of bladder control - called urinary incontinence - affects between 13 and 17 million adult
.. Urinary Incontinence Urinary incontinence is not an inevitable part of aging, and it is not a disease. The loss of bladder control - called urinary incontinence - affects between 13 and 17 million adult
PATIENT INFORMATION SHEET. Last Name: First Name: MI: Home Address: Apt# City: State: Zip Code: Home Phone #: Cell Phone #:
 PATIENT INFORMATION SHEET PATIENT Last Name: First Name: MI: Gender: M F Date of Birth: / / SS# Home Address: Apt# City: State: Zip Code: Home Phone #: Cell Phone #: Employer Name: Work Phone #: Email
PATIENT INFORMATION SHEET PATIENT Last Name: First Name: MI: Gender: M F Date of Birth: / / SS# Home Address: Apt# City: State: Zip Code: Home Phone #: Cell Phone #: Employer Name: Work Phone #: Email
Palm Beach Obstetrics & Gynecology, PA
 Palm Beach Obstetrics & Gynecology, PA 4671 S. Congress Avenue, Lake Worth, FL 33461 561.434.0111 4631 N. Congress Avenue, Suite 102, West Palm Beach, FL 33407 Urinary Tract Infection About one of every
Palm Beach Obstetrics & Gynecology, PA 4671 S. Congress Avenue, Lake Worth, FL 33461 561.434.0111 4631 N. Congress Avenue, Suite 102, West Palm Beach, FL 33407 Urinary Tract Infection About one of every
Lake Oswego Eye Clinic 530 First ST, Suite A Lake Oswego, OR 97068 Office: (503) 636-9608 Fax: (503) 636-9600
 PAYMENT AGREEMENT: We accept most insurance plans as a courtesy. We encourage you to familiarize yourself with your individual plan. Insurance coverage is an agreement between patient and insurance company
PAYMENT AGREEMENT: We accept most insurance plans as a courtesy. We encourage you to familiarize yourself with your individual plan. Insurance coverage is an agreement between patient and insurance company
Information for Patients. Advance Health Care Directive Kit A guide to help you express your health care wishes
 Information for Patients Advance Health Care Directive Kit A guide to help you express your health care wishes Table of contents PART I - REQUIRED Selection of a decision maker Who will make health care
Information for Patients Advance Health Care Directive Kit A guide to help you express your health care wishes Table of contents PART I - REQUIRED Selection of a decision maker Who will make health care
STRATTERA (Stra-TAIR-a)
 1 PV 5859 AMP MEDICATION GUIDE STRATTERA (Stra-TAIR-a) (atomoxetine) Capsules Read the Medication Guide that comes with STRATTERA before you or your child starts taking it and each time you get a refill.
1 PV 5859 AMP MEDICATION GUIDE STRATTERA (Stra-TAIR-a) (atomoxetine) Capsules Read the Medication Guide that comes with STRATTERA before you or your child starts taking it and each time you get a refill.
150640_Brochure_B 4/12/07 2:58 PM Page 2. Patient Information. Freedom From an Enlarged Prostate
 150640_Brochure_B 4/12/07 2:58 PM Page 2 Patient Information Freedom From an Enlarged Prostate 150640_Brochure_B 4/12/07 2:58 PM Page 3 GreenLight Laser Therapy 1 150640_Brochure_B 4/12/07 2:58 PM Page
150640_Brochure_B 4/12/07 2:58 PM Page 2 Patient Information Freedom From an Enlarged Prostate 150640_Brochure_B 4/12/07 2:58 PM Page 3 GreenLight Laser Therapy 1 150640_Brochure_B 4/12/07 2:58 PM Page
CVP Chemotherapy Regimen for Lymphoma Information for Patients
 CVP Chemotherapy Regimen for Lymphoma Information for Patients The Regimen Contains: C: Cyclophosphamide (Cytoxan ) V: Vincristine (Oncovin ) P: Prednisone How Is This Regimen Given? CVP is given every
CVP Chemotherapy Regimen for Lymphoma Information for Patients The Regimen Contains: C: Cyclophosphamide (Cytoxan ) V: Vincristine (Oncovin ) P: Prednisone How Is This Regimen Given? CVP is given every
Medicare Patient Information. Patient Name: SS#: - - Date of Birth: / / Sex: Female Male. City: State: Zip Code:
 Medicare Patient Information Patient Name: SS#: - - Date of Birth: / / Sex: Female Male Address: Street: City: State: Zip Code: Home Phone: ( ) - Work/Mobile Phone: ( ) - Please print your name as it Appears
Medicare Patient Information Patient Name: SS#: - - Date of Birth: / / Sex: Female Male Address: Street: City: State: Zip Code: Home Phone: ( ) - Work/Mobile Phone: ( ) - Please print your name as it Appears
Medical History Questionnaire
 Medical History Questionnaire Name: Date: Allergies (including latex): List all medications that you are currently taking, either prescription or non- prescription. Please specify dosage and length of
Medical History Questionnaire Name: Date: Allergies (including latex): List all medications that you are currently taking, either prescription or non- prescription. Please specify dosage and length of
GOOD CLINICAL PRACTICE: CONSOLIDATED GUIDELINE
 ICH E6 GCP: Consolidated Guideline: Investigator 1/7 Institutional Review Board Services ICH HARMONIZED TRIPARTITE GUIDELINE E6: GOOD CLINICAL PRACTICE: CONSOLIDATED GUIDELINE 4. INVESTIGATOR 4.1 Investigator's
ICH E6 GCP: Consolidated Guideline: Investigator 1/7 Institutional Review Board Services ICH HARMONIZED TRIPARTITE GUIDELINE E6: GOOD CLINICAL PRACTICE: CONSOLIDATED GUIDELINE 4. INVESTIGATOR 4.1 Investigator's
Share the important information in this Medication Guide with members of your household.
 MEDICATION GUIDE BUPRENORPHINE (BUE-pre-NOR-feen) Sublingual Tablets, CIII IMPORTANT: Keep buprenorphine sublingual tablets in a secure place away from children. Accidental use by a child is a medical
MEDICATION GUIDE BUPRENORPHINE (BUE-pre-NOR-feen) Sublingual Tablets, CIII IMPORTANT: Keep buprenorphine sublingual tablets in a secure place away from children. Accidental use by a child is a medical
Long term observational study for multiple sclerosis patients using fingolimod
 Investigator: Dr Matthew Jackson (Consultant Neurologist) Centre number: 814 Contact for queries If you have any questions about this study, you can contact: Daytime: Elspeth Wolfenden on 01494 734312
Investigator: Dr Matthew Jackson (Consultant Neurologist) Centre number: 814 Contact for queries If you have any questions about this study, you can contact: Daytime: Elspeth Wolfenden on 01494 734312
Eastern Health MS Service. Tysabri Therapy. Information for People with MS and their Families
 Eastern Health MS Service Tysabri Therapy Information for People with MS and their Families The Eastern Health MS Service has developed this information for you as a guide through what will happen to you
Eastern Health MS Service Tysabri Therapy Information for People with MS and their Families The Eastern Health MS Service has developed this information for you as a guide through what will happen to you
Carter Physiotherapy, PLLC. Patient Contact Information
 Carter Physiotherapy, PLLC Patient Contact Information Patient Name Today s Date Address City State Zip Code DOB Gender Marital Status Occupation Home Phone Work Cell Other Fax Email Employer Work Address
Carter Physiotherapy, PLLC Patient Contact Information Patient Name Today s Date Address City State Zip Code DOB Gender Marital Status Occupation Home Phone Work Cell Other Fax Email Employer Work Address
OPIOID PAIN MEDICATION Agreement and Informed Consent
 OPIOID PAIN MEDICATION Agreement and Informed Consent I. Introduction Research and clinical experience show that opioid (narcotic) pain medications are helpful for some patients with chronic pain. The
OPIOID PAIN MEDICATION Agreement and Informed Consent I. Introduction Research and clinical experience show that opioid (narcotic) pain medications are helpful for some patients with chronic pain. The
Women s Health. The TVT procedure. Information for patients
 Women s Health The TVT procedure Information for patients What is a TVT procedure? A TVT (Tension-free Vaginal Tape) procedure is an operation to help women with stress incontinence the leakage of urine
Women s Health The TVT procedure Information for patients What is a TVT procedure? A TVT (Tension-free Vaginal Tape) procedure is an operation to help women with stress incontinence the leakage of urine
Consent for Frozen Donor Oocyte In Vitro Fertilization and Embryo Transfer (Recipient)
 Name of Patient: Name of Partner: We, the Patient and Partner (if applicable) named above, are each over the age of twenty-one (21) years. By our signatures below, I/we request and authorize the performance
Name of Patient: Name of Partner: We, the Patient and Partner (if applicable) named above, are each over the age of twenty-one (21) years. By our signatures below, I/we request and authorize the performance
THE KIDNEY. Bulb of penis Abdominal aorta Scrotum Adrenal gland Inferior vena cava Urethra Corona glandis. Kidney. Glans penis Testicular vein
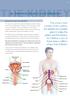 29 THE KIDNEY 9. Recurrent urinary tract infections Recurrent urinary tract infections The urinary tract consists of the urethra, the bladder, the ureters, the kidneys and in men the prostate gland. An
29 THE KIDNEY 9. Recurrent urinary tract infections Recurrent urinary tract infections The urinary tract consists of the urethra, the bladder, the ureters, the kidneys and in men the prostate gland. An
MEDICATION GUIDE. What is Morphine Sulfate Oral Solution?
 MEDICATION GUIDE Morphine Sulfate (mor-pheen) (CII) Oral Solution IMPORTANT: Keep Morphine Sulfate Oral Solution in a safe place away from children. Accidental use by a child is a medical emergency and
MEDICATION GUIDE Morphine Sulfate (mor-pheen) (CII) Oral Solution IMPORTANT: Keep Morphine Sulfate Oral Solution in a safe place away from children. Accidental use by a child is a medical emergency and
About one-half of all smokers die of a disease caused by smoking.the most common ones are lung cancer, heart disease, and strokes
 Patient information from the BMJ Group Stopping smoking Smoking harms your health, but it's difficult to stop. That s because most people who smoke have become addicted to nicotine, a chemical in tobacco.
Patient information from the BMJ Group Stopping smoking Smoking harms your health, but it's difficult to stop. That s because most people who smoke have become addicted to nicotine, a chemical in tobacco.
NOTICE TO THE INDIVIDUAL SIGNING THE ILLINOIS STATUTORY SHORT FORM POWER OF ATTORNEY FOR HEALTH CARE
 NOTICE TO THE INDIVIDUAL SIGNING THE ILLINOIS STATUTORY SHORT FORM POWER OF ATTORNEY FOR HEALTH CARE PLEASE READ THIS NOTICE CAREFULLY. The form that you will be signing is a legal document. It is governed
NOTICE TO THE INDIVIDUAL SIGNING THE ILLINOIS STATUTORY SHORT FORM POWER OF ATTORNEY FOR HEALTH CARE PLEASE READ THIS NOTICE CAREFULLY. The form that you will be signing is a legal document. It is governed
Glossary of Clinical Trial Terms
 Glossary of Clinical Trial Terms ADVERSE REACTION: (Adverse Event): Also known as side effects, adverse reactions include any undesired actions or effects of the experimental drug or treatment. Experimental
Glossary of Clinical Trial Terms ADVERSE REACTION: (Adverse Event): Also known as side effects, adverse reactions include any undesired actions or effects of the experimental drug or treatment. Experimental
APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167
 APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167 INFORMED CONSENT FOR USE OF DIPHTHERIA ANTITOXIN (DAT) FOR SUSPECTED DIPHTHERIA CASES Investigational New Drug (IND) BB 11184 Protocol
APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167 INFORMED CONSENT FOR USE OF DIPHTHERIA ANTITOXIN (DAT) FOR SUSPECTED DIPHTHERIA CASES Investigational New Drug (IND) BB 11184 Protocol
GONZABA MEDICAL GROUP PATIENT REGISTRATION FORM
 GONZABA MEDICAL GROUP PATIENT REGISTRATION FORM DATE: CHART#: GUARANTOR INFORMATION LAST NAME: FIRST NAME: MI: ADDRESS: HOME PHONE: ADDRESS: CITY/STATE: ZIP CODE: **************************************************************************************
GONZABA MEDICAL GROUP PATIENT REGISTRATION FORM DATE: CHART#: GUARANTOR INFORMATION LAST NAME: FIRST NAME: MI: ADDRESS: HOME PHONE: ADDRESS: CITY/STATE: ZIP CODE: **************************************************************************************
INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK)
 INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK) This information and the Patient Information booklet must be reviewed so you can make an informed decision regarding Photorefractive Keratectomy (PRK)
INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK) This information and the Patient Information booklet must be reviewed so you can make an informed decision regarding Photorefractive Keratectomy (PRK)
Ureteroscopy with Laser Lithotripsy
 Ureteroscopy with Laser Lithotripsy Introduction Kidney stones are fairly common. Although kidney stones can be very painful, they are treatable, and in many cases preventable. Your doctor may recommend
Ureteroscopy with Laser Lithotripsy Introduction Kidney stones are fairly common. Although kidney stones can be very painful, they are treatable, and in many cases preventable. Your doctor may recommend
EXCEL PHYSICAL THERAPY, INC.
 EXCEL PHYSICAL THERAPY, INC. Medical History Form Name: Date of Birth: Date: Are you employed? YES NO Right Handed Left Handed If NO, last day worked? Do you smoke? YES NO #of packs/day Occupation: Height:
EXCEL PHYSICAL THERAPY, INC. Medical History Form Name: Date of Birth: Date: Are you employed? YES NO Right Handed Left Handed If NO, last day worked? Do you smoke? YES NO #of packs/day Occupation: Height:
LIVING WILL AND DURABLE POWER OF ATTORNEY FOR HEALTH CARE
 LIVING WILL AND DURABLE POWER OF ATTNEY F HEALTH CARE Date of Directive: Name of person executing Directive: Address of person executing Directive: A Living Will A Directive to Withhold or to Provide Treatment
LIVING WILL AND DURABLE POWER OF ATTNEY F HEALTH CARE Date of Directive: Name of person executing Directive: Address of person executing Directive: A Living Will A Directive to Withhold or to Provide Treatment
