subcutaneous initially every 4 weeks then every 12 weeks Coverage Criteria: Express Scripts, Inc. monograph dated 02/24/2010
|
|
|
- Doris Cobb
- 9 years ago
- Views:
Transcription
1 BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Humira (adalimumab subcutaneous injection) Commercial HMO/PPO/CDHP HMO/PPO/CDHP: Rx (self-administered) Adalimumab is a recombinant human IgG1 monoclonal antibody specific for human tumor necrosis factor alpha (TNFα). Adalimumab neutralizes the biological activity of TNFα and inhibits binding of TNFα with its receptors. TNF, a naturally occurring cytokine, mediates inflammation and modulates cellular immune responses. Increased levels of TNF are found in the synovial fluid of patients with rheumatoid arthritis (RA), juvenile rheumatoid arthritis (JIA), psoriatic arthritis (PsA), and ankylosing spondylitis. J0135 Abbott Laboratories subcutaneous initially every 4 weeks then every 12 weeks Coverage Criteria: Express Scripts, Inc. monograph dated 02/24/2010 Approval Period: 12 months or as otherwise noted by indication Recommended Authorization Criteria Coverage of adalimumab is recommended in those who meet one of the following criteria: FDA-Approved Indications 1. Adults with rheumatoid arthritis. Approve for 12 months if the patient has tried one DMARD (brand or generic; oral or injectable) for at least 2 months, [this includes patients who have tried other biologic DMARDs for at least 2 months] OR is concurrently receiving MTX. Adalimumab is FDA-approved for moderate or severe active RA in adults and can be used alone or in combination with MTX or other DMARDs. Initiating DMARD therapy with a biologic agent such as adalimumab alone should be rare. Most patients will have received initial therapy with an oral DMARD(s) (e.g., hydroxychloroquine, leflunomide, sulfasalazine, MTX). If MTX is contraindicated, another oral DMARD should be tried. Some patients with important markers of poor prognosis (e.g., functional limitations, rheumatoid factor positivity and/or positive anticyclic citrullinated peptide (CCP) antibodies, extraarticular manifestations of RA [e.g., vasculitis, Sjögren s syndrome, RA lung disease]) or with joint erosions may be started early on biologic agents; patients will be evaluated by a pharmacist and/or physician on a case-by-case basis to determine a coverage recommendation for the client. This criterion is recommended based on the professional opinion of specialized physicians. 2. Juvenile idiopathic arthritis (JIA) or JRA, polyarticular course (regardless of type of onset). Approve for 12 months if the patient has tried MTX or will be 1
2 starting on adalimumab concurrently with MTX. Approve for 12 months without trying MTX if the patient has an absolute contraindication to MTX (e.g., pregnancy, breast feeding, alcoholic liver disease, immunodeficiency syndrome, blood dyscrasias). Adalimumab is FDA-approved for reducing signs and symptoms of moderately to severely active polyarticular JIA in patients ages 4 and older. The evidence for the effectiveness of DMARDs other than MTX for JRA is weak and no other therapy is the first choice once MTX is ineffective or does not produce an adequate response. Patients with aggressive disease, as determined by the prescribing physician, may be started early on a biologic agent (such as adalimumab); patients will be evaluated by a pharmacist and/or physician on a case-by-case basis to determine a coverage recommendation for the client. (professional opinion of specialist physicians) 3. Psoriatic arthritis (PsA). Approve for 12 months. Adalimumab is FDA-approved for PsA and can be used alone or in combination with DMARDs. In clinical trials, adalimumab was effective in patients with active PsA despite therapy with a NSAID. There are few well-controlled, prospective studies with adequate duration that have evaluated the efficacy of the oral DMARDs. In peripheral arthritis the traditional DMARDs, sulfasalazine, leflunomide, MTX, or cyclosporine, may be effective. For patients with peripheral arthritis who do not respond to a traditional DMARD, one of the TNF inhibitors (etanercept, adalimumab, or infliximab) is equally effective for treatment and also for inhibiting radiographic progression and improving physical function in patients with PsA. Patients with a poor prognosis could use a TNF inhibitor even though they have not failed on a traditional DMARD. The traditional DMARDs have not been shown to prevent the progression of radiographic (structural) damage or to have significant impact on axial disease, dactylitis, or enthesitis in PsA. This is in contrast with the newer biological DMARDs which have shown efficacy in well-controlled trials in reducing signs and symptoms of active arthritis, inhibiting the progression of structural damage, and improving physical function in patients with PsA. 4. Ankylosing spondylitis. Approve for 12 months. FDA-approved indication. According to ASAS/European League Against Rheumatism (EULAR) recommendations for ankylosing spondylitis, all patients should have an adequate trial of at least 2 NSAIDs for pain and stiffness. Recommendations for other therapies before receiving adalimumab (or etanercept or infliximab) vary according to the manifestations of the disease, level of current symptoms, clinical findings, etc. According to these recommendations, patients with pure axial manifestations do not have to try traditional DMARDs before anti-tnf agents such as adalimumab; patients with symptomatic peripheral arthritis should have an insufficient response to at least one local corticosteroid injection, if appropriate; patients with persistent peripheral arthritis must have a trial of sulfasalazine; and patients with enthesitis should try appropriate local therapy (corticosteroid injection in selected cases). 5. Plaque psoriasis in patients. Authorization can be given for 12 months for patients who meet all of the following criteria a and b: a. Patient has minimum body surface area (BSA) involvement with plaque psoriasis of 5%. Exceptions can be made to the requirement for 5% BSA involvement in the following instances (i or ii): i. Patients with plaque psoriasis of the palms, soles, head and neck, nails, intertriginous areas or genitalia are not required to have a minimum BSA involvement OR ii. The patient who meets all three of the following conditions is not required to have a minimum BSA involvement: (patient who meet this criteria are not required to meet 5b) 2
3 Patient has had an inadequate response to 3-month trial of either topical therapy OR localized phototherapy with ultraviolet B (UVB) or oral methoxsalen plus UVA light [PUVA]) for psoriasis and Patient has had an inadequate response to systemic therapy (See d. below) and Patient has significant disability or impairment in physical or mental functioning, according to the treating physician. b. Patient has tried a systemic therapy or phototherapy for 3 months with one of the following agents: MTX, cyclosporine, acitretin (Soriatane ), etanercept, alefacept (Amevive ), infliximab or ustekinumab (Stelara ) or has tried phototherapy with UVB or PUVA for psoriasis. Rarely, a patient may have contraindications to nearly all of these other therapies and patients will be evaluated by a pharmacist and/or a physician on a case-by-case basis to determine a coverage recommendation for the client. Due to its toxicity, adalimumab therapy should be reserved for patients who have not responded well or are intolerant to other standard systemic therapy. In addition, the NSF Clinical Consensus, states that there currently are no prognostic factors that ascertain which therapies will be most efficacious and least toxic.) 6. Crohn s Disease, active (to induce or maintain remission) in adults. Crohn s Disease, active (to induce remission). Approve adalimumab for 12 weeks of therapy in adults if the patient has tried corticosteroids or if corticosteroids are contraindicated or if the patient is currently on corticosteroids (to avoid increasing the dose of the corticosteroid). After 12 weeks (this is following 160 mg at week 0, 80 mg at week 2, and a maintenance dose of 40 mg every other week (EOW) beginning at week 4), patients are evaluated for response and further authorization for maintenance of remission. In patients who do not respond by week 12, additional therapy does not result in significantly more responses. In clinical trials, the first 2 doses of adalimumab were given to induce remission and if patients responded at week 4, then maintenance with EOW adalimumab was started. Crohn s Disease (to maintain remission). If the patient (adult) has received 2 doses of adalimumab to induce remission or has had 12 weeks of therapy with adalimumab (i.e., 160 mg at week 0, 80 mg at week 2, and a maintenance dose of 40 mg EOW) and has had a response to therapy, then authorization is recommended for 12 months. Further authorization is not recommended if there is no response by week 12. In patients who do not respond by week 12, additional therapy does not result in significantly more responses. OR If the patient (adult) has not received adalimumab for induction of remission, then authorize adalimumab for maintenance (i.e., for 12 months) if the patient has tried azathioprine, 6-mercaptopurine, or MTX OR if the patient has tried infliximab (Remicade) or certolizumab pegol (Cimzia ). Patient is already in remission and adalimumab is being used to maintain remission. Adalimumab is FDA-approved for reducing signs and symptoms and inducing and maintaining clinical remission in adults with moderately to severely active Crohn s disease who have had an inadequate response to conventional therapy. Adalimumab is also indicated for reducing signs and symptoms and inducing clinical remission in these patients if they have also lost response to or are intolerant to infliximab. Note: Patients with fistulizing Crohn s disease must meet the above criteria for Crohn s 3
4 disease, active (to induce or maintain remission), since adalimumab is not FDAapproved for fistulizing Crohn s disease. Other Uses with Supportive Evidence 7. Patient has already been started on adalimumab. Approve for any indication except Crohn s disease. (professional opinion of specialist physicians) For adults or adolescents 15 years of age with Crohn s disease, approve if the patient has had two doses for induction of remission and has responded by week 4 (standard induction dosing in adults for adalimumab is 160 mg at week 0 and then 80 mg at week 2 and is followed at week 4 with maintenance dosing) OR Approve if the patient with Crohn s disease has already received adalimumab, is already in remission, and is continuing therapy for maintenance of remission. Patients who are not in remission for Crohn s disease must meet the criteria #6 above for induction of remission. Level of evidence: Undifferentiated spondylarthritis (undifferentiated arthritis). Approve for 12 months. In a double-blind study conducted at 2 centers in Germany, 46 patients with active axial spondylarthritis without radiographically defined sacroiliitis were randomized to placebo or adalimumab 40 mg EOW for 12 weeks followed by an open-label extension for up to week 52. All patients in the open-label extension received adalimumab EOW. Patients were refractory to treatment with NSAIDs and had baseline BASDAI 4. The adalimumab dosage was increased to every week in 10 patients who did not attain ASAS 40 response after 12 weeks of therapy. At week 12 an ASAS 40 response (primary endpoint) was attained by 54.5% of the patients on adalimumab compared to 12.5% with placebo (P = vs. placebo). Efficacy was maintained at week 52. In the entire study group of 46 patients, 50% attained an ASAS 40 response at week 52. Of note, trials with ankylosing spondylitis include patients who have radiographic changes in the sacroiliac joints which are the consequences of previous inflammation and may take years to become evident. Level of evidence: Crohn s Disease, active (to induce or maintain remission) in adolescents. Crohn s Disease, active (to induce remission). Approve adalimumab for 12 weeks of therapy in adolescents aged 15 to < 18 years, if the patient has tried infliximab (Remicade) AND if the patient has tried corticosteroids or if corticosteroids are contraindicated or if the patient is currently on corticosteroids (to avoid increasing the dose of the corticosteroid). After 12 weeks (this is following an induction dose at week 0 and at week 2 followed by a maintenance dose EOW beginning at week 4), patients are evaluated for response and further authorization for maintenance of remission. In adults who do not respond by week 12, additional therapy does not result in significantly more responses. In clinical trials in adults, the first 2 doses of adalimumab were given to induce remission and if patients responded at week 4, then maintenance with EOW adalimumab was started. Crohn s Disease (to maintain remission). If the patient (adolescent aged 15 to < 18 years) has received 2 doses of adalimumab to induce remission or has had 12 weeks of therapy with adalimumab (i.e., induction dose at week 0 and at week 2, and a maintenance dose EOW) and has had a response to therapy, then authorization is recommended for 12 months. Further authorization is not recommended if there 4
5 is no response by week 12. In adults who do not respond by week 12, additional therapy does not result in significantly more responses. OR If the patient (adolescent aged 15 to < 18 years) has not received adalimumab for induction of remission, then authorize adalimumab for maintenance (i.e., for 12 months) if the patient has tried azathioprine, 6-mercaptopurine, or MTX OR if the patient has tried infliximab (Remicade). Patient is already in remission and adalimumab is being used to maintain remission. Note: there is no information on Cimzia in children. Adalimumab is FDA-approved in adults with moderately to severely active Crohn s disease who have had an inadequate response to conventional therapy and in these patients if they have lost response to or are intolerant to infliximab. Infliximab is FDAapproved in pediatric patients 6 years of age with Crohn s disease. Limited information is available on the use of adalimumab in children with Crohn s disease and published information mostly includes adolescents who have lost response to infliximab or are intolerant to infliximab. Note: Patients with fistulizing Crohn s disease must meet the above criteria for Crohn s disease, active (to induce or maintain remission), since adalimumab is not FDAapproved for fistulizing Crohn s disease. 10. Uveitis (noninfectious) in children or adults. Approve for 12 months if patient has tried topical (ophthalmic) or systemic corticosteroids, MTX, etanercept, infliximab, mycophenolate mofetil, or cyclosporine. Adalimumab has been effective in a small number of children with chronic uveitis (either with rheumatic disease (JIA) or idiopathic) refractory to other therapies. Adalimumab was also effective in the management of refractory uveitis in adults and allowed the dose of concomitant immunosuppressives to be reduced. Further long-term studies are needed. Level of evidence: Uveitis or other systemic manifestations of Behcet s disease in adults. Approve for 12 months if patient has tried topical (ophthalmic) or systemic corticosteroids, azathioprine, interferon alfa, MTX, etanercept, infliximab, mycophenolate mofetil, or cyclosporine. In 3 cases, adalimumab was effective in controlling uveitis in adults with Behcet s disease who were in remission after receiving infliximab. In another case series, 6 adults with Behcet s disease [uveitis (2), central nervous system disease (2), colitis (1), and severe oral ulcers and arthritis (1)] in whom immunosuppressive therapy had failed, adalimumab was effective. These patients had received prior therapy with infliximab which had been discontinued after complete response or acceptable improvement. EULAR recommendations for the management of Behcet disease include either infliximab or cyclosporine in combination with azathioprine and corticosteroids for refractory eye involvement. For gastrointestinal or parenchymal involvement, TNFα antagonists have been used in resistant and complicated case. More study is needed with adalimumab. Level of evidence: Sarcoidosis, cutaneous. Approve for 12 months if patient has tried corticosteroids and immunosuppressive agents (MTX, azathioprine, cyclosporine, chlorambucil) or infliximab, or chloroquine. Well-controlled studies are not available for any therapies. Adalimumab has been effective in case reports of patients who were refractory to standard therapy. A randomized, placebo-controlled trial is underway using adalimumab for cutaneous sarcoidosis. Level of evidence: Pyoderma gangrenosum. Approve for 12 months if patient has tried one other systemic therapy (e.g., intralesional injections of corticosteroids or cyclosporine [for localized pyoderma gangrenosum]; systemic corticosteroids or immunosuppressants 5
6 such as azathioprine, 6-mercaptopurine, cyclosporine, cyclophosphamide, chlorambucil, infliximab). In 4 case reports, adalimumab was effective in treating pyoderma gangrenosum. Additional studies are needed. Level of evidence: Hidradenitis suppurativa. Approve for 12 months if the patient has tried one other therapy (e.g., intralesional or oral corticosteroids, antibiotics, isotretinoin). In case reports, adalimumab has been effective in treating hidradenitis suppurativa that was refractory to other therapies. Level of evidence: 5. Exclusions Coverage of adalimumab is not recommended in the following circumstances: 1. Adalimumab should not be given in combination with anakinra or abatacept. Serious infections were seen with concurrent use of etanercept (a tumor necrosis factor (TNF)-blocking agent) and anakinra with no added benefit. Similar toxicities may also occur from combination therapy with anakinra and other TNF-blocking agents such as adalimumab. Increased rates of serious adverse events, including serious infections, have been reported with concomitant administration of TNF blocking agents and abatacept and there was no significant increased efficacy over using the TNF blocking agent alone. 2. Children with Crohn s disease who are less than 15 years of age. Safety and efficacy in pediatric patients have not been established. A phase 3 clinical trial is underway in children with Crohn s disease. Limited information is published on the use of adalimumab in children, mostly adolescents, who could not tolerate infliximab or who lost response to infliximab. 3. Osteoarthritis. In an open-label trial in 12 patients with moderate to severe erosive/inflammatory osteoarthritis of the hands despite therapy with NSAIDs, 12 weeks of therapy with adalimumab 40 mg EOW did not significantly improve signs and symptoms (number of tender joints, grip strength, disability, pain, or global disease assessment). Trends suggested modest improvement. Long-term, placebo-controlled trials that have a specific efficacy outcome for hand or generalized osteoarthritis are needed. 4. Ulcerative colitis. In a 4-week open-label study conducted at 2 centers in France, 10 adults with ulcerative colitis who had lost response or became intolerant to infliximab received adalimumab 160 mg at week 0 and 80 mg at week 2. Disease activity was assessed using the ulcerative colitis clinical activity index (CAI) with scores > 12 indicating severe disease and 4 to 12 representing mild to moderate activity. The primary outcome was a decrease in the CAI score of > 4 at week 4. Other concurrent therapies for ulcerative colitis were allowed. In the 6 patients with severe ulcerative colitis, no remissions resulted and only one patient had clinical improvement. One patient with mild to moderate disease achieved remission. A randomized placebocontrolled trial is needed to determine if adalimumab has a place in the therapy of ulcerative colitis. 6. Intra-articular injection. Large placebo- or corticosteroid-controlled trials are needed to determine which patients might benefit from intra-articular injection and if this therapy is safe and effective. 7. Recurrent spontaneous pregnancy loss (RSPL). In a retrospective analysis, TNF inhibitors (etanercept [n = 3] or adalimumab [n = 14]) were used in combination with 6
7 intravenous immune globulin (IVIG) and anticoagulants plus low dose aspirin in patients with recurrent spontaneous abortion. The authors concluded that the addition of IVIG or a TNF inhibitor plus IVIG to the anticoagulant regimen appears to improve live birth rates compared to therapy with anticoagulants alone. Further prospective clinical trials are needed. According to guidelines from the American College of Obstetricians and Gynecologists, recommended therapy for preventing recurrent early (< 15 weeks of gestation) pregnancy loss does not include IVIG. At the time of these guidelines were written anti- TNF agents were not being studied for this indication. Patients with a positive test for lupus anticoagulant or anticardiolipin antibodies should be treated with heparin and low dose aspirin during the next pregnancy attempt. The American Society for Reproductive Medicine also concluded after reviewing 5 randomized controlled trials which assessed IVIG treatment for RSPL, that IVIG is not effective for primary RSPL. For secondary (indicates an antecedent pregnancy) RSPL, there was a higher percentage of successful pregnancies with IVIG, but the number of patients was not sufficient to rule out a chance finding. They concluded that IVIG as a treatment for RSPL is experimental and should only be used in a randomized clinical trial setting. 8. In vitro fertilization (IVF). In an observational study, use of adalimumab and IVIG improved IVF outcome in young infertile women who were < 38 years of age with Th1/Th2 cytokine elevations who had > 2 IVF embryo transplant failures. In group I, 41 patients used both IVIG and adalimumab; in group II, 23 patients used IVIG; in group III, 6 patients used adalimumab alone; and in group IV, 5 patients did not use either IVIG or adalimumab. Patient groups were formed partly through self selection and partly through differences in initial work up and history. Patients who received adalimumab had two 40 mg doses at 2-week intervals and about 2 to 3 weeks after the second injection, a second Th1/Th assay was done. If the Th1/Th2 was still increased, 2 more dose of adalimumab were given. All patients received enoxapirin daily during their treatment cycles and 75 mg of aspirin daily. The implantation rate (number of gestational sacs per embryo transferred, with an average of two embryos transferred by cycle) was 59% (group I), 47% (group II), 31% (group III), and 0% (group IV). The respective clinical pregnancy rates (fetal heart activity per IVF cycle started) were 80%, 57%, 50%, and 0%. The respective live birth rates were 73%, 52%, 50%, and 0%. These improvements were significant for group I vs. group IV and for group II vs. group IV. Well controlled trials in a larger, well defined group of patients are needed. 9. Other indications. Exceptions not recommended. 10. Coverage is not recommended for circumstances not listed in the Recommended Authorization Criteria. Criteria will be updated as new published data are available. APPROVAL: ENDORSED BY: Pharmacy & Therapeutics Committee Original Date: Reviewed: 5/19/2010 APPROVED BY: Date 7
Biologic Treatments for Rheumatoid Arthritis
 Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Rheumatoid Arthritis. Outline. Treatment Goal 4/10/2013. Clinical evaluation New treatment options Future research Discussion
 Rheumatoid Arthritis Robert L. Talbert, Pharm.D., FCCP, BCPS University of Texas at Austin College of Pharmacy University of Texas Health Science Center at San Antonio Outline Clinical evaluation New treatment
Rheumatoid Arthritis Robert L. Talbert, Pharm.D., FCCP, BCPS University of Texas at Austin College of Pharmacy University of Texas Health Science Center at San Antonio Outline Clinical evaluation New treatment
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES `I. Requirements for Prior Authorization of Cytokine and CAM Antagonists
 MEDICAL ASSISTANCE HBOOK `I. Requirements for Prior Authorization of Cytokine and CAM Antagonists A. Prescriptions That Require Prior Authorization All prescriptions for Cytokine and CAM Antagonists must
MEDICAL ASSISTANCE HBOOK `I. Requirements for Prior Authorization of Cytokine and CAM Antagonists A. Prescriptions That Require Prior Authorization All prescriptions for Cytokine and CAM Antagonists must
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure
Drug Therapy Guidelines: Humira (adalimumab)
 Drug Therapy Guidelines: Humira (adalimumab) Effective Date: 5/1/08 Committee Review Date: 1/6/01, 9/18/01, 1/15/02, 1/7/03, 1/20/04, 1/18/05, 12/7/05, 10/15/06, 7/2/07, 11/5/07, 3/25/08 Policy Statements:
Drug Therapy Guidelines: Humira (adalimumab) Effective Date: 5/1/08 Committee Review Date: 1/6/01, 9/18/01, 1/15/02, 1/7/03, 1/20/04, 1/18/05, 12/7/05, 10/15/06, 7/2/07, 11/5/07, 3/25/08 Policy Statements:
Immune Modulating Drugs Prior Authorization Request Form
 Patient: HPHC member ID #: Requesting provider: Phone: Servicing provider: Diagnosis: Contact for questions (name and phone #): Projected start and end date for requested Requesting provider NPI: Fax:
Patient: HPHC member ID #: Requesting provider: Phone: Servicing provider: Diagnosis: Contact for questions (name and phone #): Projected start and end date for requested Requesting provider NPI: Fax:
SECTION 3. Criteria for Special Authorization of Select Drug Products. Section 3 Criteria for Special Authorization of Select Drug Products
 SECTION 3 Criteria for Special Authorization of Select Drug Products Section 3 Criteria for Special Authorization of Select Drug Products CRITERIA FOR SPECIAL AUTHORIZATION OF SELECT DRUG PRODUCTS The
SECTION 3 Criteria for Special Authorization of Select Drug Products Section 3 Criteria for Special Authorization of Select Drug Products CRITERIA FOR SPECIAL AUTHORIZATION OF SELECT DRUG PRODUCTS The
Evidence-based Management of Rheumatoid Arthritis (2009)
 CPLD reviews its distance learning programmes every twelve months to ensure currency. This update has been produced by an expert and should be read in conjunction with the Evidencebased Management of distance
CPLD reviews its distance learning programmes every twelve months to ensure currency. This update has been produced by an expert and should be read in conjunction with the Evidencebased Management of distance
Current Rheumatoid Arthritis Treatment Options: Update for Managed Care and Specialty Pharmacists
 Current Rheumatoid Arthritis Treatment Options: Update for Managed Care and Specialty Pharmacists 1. Which of the following matches of biologic targets that contribute to rheumatoid arthritis (RA) and
Current Rheumatoid Arthritis Treatment Options: Update for Managed Care and Specialty Pharmacists 1. Which of the following matches of biologic targets that contribute to rheumatoid arthritis (RA) and
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC)
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) September 2014 Review date: September 2017 Bulletin 203: Tocilizumab (subcutaneous) in combination with methotrexate or as monotherapy for the treatment
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) September 2014 Review date: September 2017 Bulletin 203: Tocilizumab (subcutaneous) in combination with methotrexate or as monotherapy for the treatment
DISEASE-MODIFYING ANTIRHEUMATIC DRUG THERAPY FOR RHEUMATOID ARTHRITIS
 DISEASE-MODIFYING ANTIRHEUMATIC DRUG THERAPY FOR RHEUMATOID ARTHRITIS APPLICATIONS OBJECTIVE Purpose of Measure: ELIGIBLE POPULATION Which members are included? STANDARD OF CARE NCQA APPROVED CODES HEDIS
DISEASE-MODIFYING ANTIRHEUMATIC DRUG THERAPY FOR RHEUMATOID ARTHRITIS APPLICATIONS OBJECTIVE Purpose of Measure: ELIGIBLE POPULATION Which members are included? STANDARD OF CARE NCQA APPROVED CODES HEDIS
Immune modulation in rheumatology. Geoff McColl University of Melbourne/Australian Rheumatology Association
 Immune modulation in rheumatology Geoff McColl University of Melbourne/Australian Rheumatology Association A traditional start to a presentation on biological agents in rheumatic disease is Plasma cell
Immune modulation in rheumatology Geoff McColl University of Melbourne/Australian Rheumatology Association A traditional start to a presentation on biological agents in rheumatic disease is Plasma cell
Guidelines for the Pharmaceutical Management of Rheumatoid Arthritis Swedish Society of Rheumatology, April 14, 2011
 Guidelines for the Pharmaceutical Management of Rheumatoid Arthritis Swedish Society of Rheumatology, April 14, 2011 Working party: Eva Baecklund, Helena Forsblad d Elia, Carl Turesson Background Our purpose
Guidelines for the Pharmaceutical Management of Rheumatoid Arthritis Swedish Society of Rheumatology, April 14, 2011 Working party: Eva Baecklund, Helena Forsblad d Elia, Carl Turesson Background Our purpose
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Multiple Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Multiple Technology Appraisal Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Multiple Technology Appraisal Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency London, 14 December 2006 Doc. Ref. CHMP/EWP/438/04 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS FOR THE TREATMENT
European Medicines Agency London, 14 December 2006 Doc. Ref. CHMP/EWP/438/04 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS FOR THE TREATMENT
ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd
 ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd 07 February 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the
ustekinumab 45mg solution for injection in pre-filled syringe (Stelara ) SMC No. (944/14) Janssen-Cilag Ltd 07 February 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Rheumatic Diseases, Psoriasis, and Crohn s Disease
 Rheumatic Diseases, Psoriasis, and Crohn s Disease What does this handout cover? This handout has information about rheumatic disease, psoriasis, and Crohn s disease. It also has information on how these
Rheumatic Diseases, Psoriasis, and Crohn s Disease What does this handout cover? This handout has information about rheumatic disease, psoriasis, and Crohn s disease. It also has information on how these
påçííáëü=jéçáåáåéë=`çåëçêíáìã==
 påçííáëü=jéçáåáåéë=`çåëçêíáìã== adalimumab 40mg pre-filled syringe for subcutaneous injection (Humira ) No. (218/05) Abbott New indication: treatment of active and progressive psoriatic arthritis in adults
påçííáëü=jéçáåáåéë=`çåëçêíáìã== adalimumab 40mg pre-filled syringe for subcutaneous injection (Humira ) No. (218/05) Abbott New indication: treatment of active and progressive psoriatic arthritis in adults
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
 European Medicines Agency Evaluation of Medicines for Human Use London, 23 June 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS
European Medicines Agency Evaluation of Medicines for Human Use London, 23 June 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS
How To Choose A Biologic Drug
 North Carolina Rheumatology Association Position Statements I. Biologic Agents A. Appropriate delivery, handling, storage and administration of biologic agents B. Indications for biologic agents II. III.
North Carolina Rheumatology Association Position Statements I. Biologic Agents A. Appropriate delivery, handling, storage and administration of biologic agents B. Indications for biologic agents II. III.
Remicade (infliximab)
 DRUG POLICY Remicade (infliximab) Policy Number: 2015D004P Effective Date: 12/1/2015 Table of Contents Page COVERAGE RATIONALE... 1 BENEFIT CONSIDERATIONS... 3 BACKGROUND... 3 CLINICAL EVIDENCE... 4 U.S.
DRUG POLICY Remicade (infliximab) Policy Number: 2015D004P Effective Date: 12/1/2015 Table of Contents Page COVERAGE RATIONALE... 1 BENEFIT CONSIDERATIONS... 3 BACKGROUND... 3 CLINICAL EVIDENCE... 4 U.S.
Arthritis and Rheumatology Clinics of Kansas Patient Education. Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis
 Arthritis and Rheumatology Clinics of Kansas Patient Education Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis Introduction: For as long as scientists have studied rheumatic disease,
Arthritis and Rheumatology Clinics of Kansas Patient Education Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis Introduction: For as long as scientists have studied rheumatic disease,
CLINICAL POLICY Department: Medical Management Document Name: Rheumatoid & Juvenile Arthritis and Ankylosing Spondylitis Treatments
 Page: 1 of 18 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Page: 1 of 18 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Is Monotherapy Treatment of Etanercept Effective Against Plaque Psoriasis?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 Is Monotherapy Treatment of Etanercept
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2011 Is Monotherapy Treatment of Etanercept
Improvement in Quality of Life of Rheumatoid Arthritis Patients on Biologic Therapy
 Improvement in Quality of Life of Rheumatoid Arthritis Patients on Biologic Therapy R Adams 1, Ct Ng 2, A Gibbs 2, L Tilson 1, D Veale 2, B Bresnihan 2, O FitzGerald 2, M Barry 1 1. National Centre for
Improvement in Quality of Life of Rheumatoid Arthritis Patients on Biologic Therapy R Adams 1, Ct Ng 2, A Gibbs 2, L Tilson 1, D Veale 2, B Bresnihan 2, O FitzGerald 2, M Barry 1 1. National Centre for
Let s talk about Arthritis
 Let s talk about Arthritis Osteoarthritis Rheumatoid Arthritis Kam Shojania, MD, FRCPC Clinical Professor and Head, St. Paul s, UBC and VGH Divisions of Rheumatology Slides with thanks to: Cheryl Koehn
Let s talk about Arthritis Osteoarthritis Rheumatoid Arthritis Kam Shojania, MD, FRCPC Clinical Professor and Head, St. Paul s, UBC and VGH Divisions of Rheumatology Slides with thanks to: Cheryl Koehn
Patient Input Information Clinical Trials Outcomes Common Drug Review
 CDEC FINAL RECOMMENDATION USTEKINUMAB (Stelara Janssen Inc.) Indication: Psoriatic Arthritis Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that ustekinumab not be listed at the submitted
CDEC FINAL RECOMMENDATION USTEKINUMAB (Stelara Janssen Inc.) Indication: Psoriatic Arthritis Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that ustekinumab not be listed at the submitted
SASKATCHEWAN FORMULARY BULLETIN Update to the 62nd Edition of the Saskatchewan Formulary
 November 1, 2014 Bulletin #150 ISSN 1923-0761 SASKATCHEWAN FORMULARY BULLETIN Update to the 62nd Edition of the Saskatchewan Formulary New Exception Drug Status (EDS) Listings Effective November 1, 2014
November 1, 2014 Bulletin #150 ISSN 1923-0761 SASKATCHEWAN FORMULARY BULLETIN Update to the 62nd Edition of the Saskatchewan Formulary New Exception Drug Status (EDS) Listings Effective November 1, 2014
Rheumatoid Arthritis:
 Rheumatoid Arthritis Update 2014 Mark Hulsey, MD FACR Rheumatoid Arthritis Key Features Symptoms >6 weeks duration Often lasts the remainder of the patient s life Inflammatory synovitis Palpable synovial
Rheumatoid Arthritis Update 2014 Mark Hulsey, MD FACR Rheumatoid Arthritis Key Features Symptoms >6 weeks duration Often lasts the remainder of the patient s life Inflammatory synovitis Palpable synovial
REFERENCE CODE GDHC503DFR PUBLICAT ION DATE DECEMBER 2014 METHOTREXATE (RHEUMATOID ARTHRITIS) - FORECAST AND MARKET ANALYSIS TO 2023
 REFERENCE CODE GDHC503DFR PUBLICAT ION DATE DECEMBER 2014 METHOTREXATE (RHEUMATOID ARTHRITIS) - Executive Summary The table below provides the key metrics for Methotrexate in the 10MM (US, France, Germany,
REFERENCE CODE GDHC503DFR PUBLICAT ION DATE DECEMBER 2014 METHOTREXATE (RHEUMATOID ARTHRITIS) - Executive Summary The table below provides the key metrics for Methotrexate in the 10MM (US, France, Germany,
Methotrexate Dose For Juvenile Rheumatoid Arthritis
 Methotrexate Dose For Juvenile Rheumatoid Arthritis should i take methotrexate for my ra methotrexate 50 mg/ml methotrexate sodium 2.5mg tablets what is the usual dosage of methotrexate for ra methotrexate
Methotrexate Dose For Juvenile Rheumatoid Arthritis should i take methotrexate for my ra methotrexate 50 mg/ml methotrexate sodium 2.5mg tablets what is the usual dosage of methotrexate for ra methotrexate
Can Rheumatoid Arthritis treatment ever be stopped?
 Can Rheumatoid Arthritis treatment ever be stopped? Robert L. DiGiovanni, DO, FACOI Program Director Largo Medical Center Rheumatology Fellowship [email protected] Do not pour strange medicines
Can Rheumatoid Arthritis treatment ever be stopped? Robert L. DiGiovanni, DO, FACOI Program Director Largo Medical Center Rheumatology Fellowship [email protected] Do not pour strange medicines
Psoriasis. Psoriasis. Mark A. Bechtel, M.D. Director of Dermatology The Ohio State University College of Medicine
 Psoriasis Mark A. Bechtel, M.D. Director of Dermatology The Ohio State University College of Medicine Psoriasis Psoriasis is a chronic skin disorder resulting from a polygenic predisposition combined with
Psoriasis Mark A. Bechtel, M.D. Director of Dermatology The Ohio State University College of Medicine Psoriasis Psoriasis is a chronic skin disorder resulting from a polygenic predisposition combined with
Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy
 Media Release Basel, 6 June 2012 Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced
Media Release Basel, 6 June 2012 Roche s RoACTEMRA improved rheumatoid arthritis signs and symptoms significantly more than adalimumab as single-agent therapy Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced
biologics for the treatment of psoriasis
 How to contact us The Psoriasis Association Dick Coles House 2 Queensbridge Northampton NN4 7BF tel: 08456 760 076 (01604) 251 620 fax: (01604) 251 621 email: [email protected] www.psoriasis-association.org.uk
How to contact us The Psoriasis Association Dick Coles House 2 Queensbridge Northampton NN4 7BF tel: 08456 760 076 (01604) 251 620 fax: (01604) 251 621 email: [email protected] www.psoriasis-association.org.uk
New Post Hoc Analyses of Phase 3b Data Examine Treatment with Orencia
 June 12, 2015 New Post Hoc Analyses of Phase 3b Data Examine Treatment with Orencia (abatacept) Plus Methotrexate (MTX) in Patients with Early Moderate to Severe Rheumatoid Arthritis (RA) and Markers of
June 12, 2015 New Post Hoc Analyses of Phase 3b Data Examine Treatment with Orencia (abatacept) Plus Methotrexate (MTX) in Patients with Early Moderate to Severe Rheumatoid Arthritis (RA) and Markers of
Rheumatoid Arthritis Information
 Rheumatoid Arthritis Information Definition Rheumatoid arthritis (RA) is a long-term disease that leads to inflammation of the joints and surrounding tissues. It can also affect other organs. Alternative
Rheumatoid Arthritis Information Definition Rheumatoid arthritis (RA) is a long-term disease that leads to inflammation of the joints and surrounding tissues. It can also affect other organs. Alternative
Spondyloarthritis is a general term for a group of rheumatic
 Rheumatic Disease Clinics of North America Supplement 1 11 Current Treatment for Psoriatic Arthritis and Other Spondyloarthritides Spondyloarthritis is a general term for a group of rheumatic diseases,
Rheumatic Disease Clinics of North America Supplement 1 11 Current Treatment for Psoriatic Arthritis and Other Spondyloarthritides Spondyloarthritis is a general term for a group of rheumatic diseases,
Critical Issues in School Health Arthritis in the School Setting. Lawrence Zemel MD Tegan Willard RN Connecticut Children s
 Critical Issues in School Health Arthritis in the School Setting Lawrence Zemel MD Tegan Willard RN Connecticut Children s Juvenile Rheumatoid Idiopathic Arthritis Definition of JRA (JIA) Persistent arthritis
Critical Issues in School Health Arthritis in the School Setting Lawrence Zemel MD Tegan Willard RN Connecticut Children s Juvenile Rheumatoid Idiopathic Arthritis Definition of JRA (JIA) Persistent arthritis
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
Pharmacotherapy of Autoimmune Disorders
 PHARMACY / MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Pharmacotherapy of Autoimmune Disorders Number 5.01.550
PHARMACY / MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Pharmacotherapy of Autoimmune Disorders Number 5.01.550
Speaking Plainly. Biologic treatment options for rheumatoid arthritis
 in association with Plain English Campaign Speaking Plainly Biologic treatment options for rheumatoid arthritis A guide to help healthcare professionals talking to patients with rheumatoid arthritis Foreword
in association with Plain English Campaign Speaking Plainly Biologic treatment options for rheumatoid arthritis A guide to help healthcare professionals talking to patients with rheumatoid arthritis Foreword
MEDICATION GUIDE REMICADE (Rem-eh-kaid) (infliximab) Read the Medication Guide that comes with REMICADE before you receive the first treatment, and
 MEDICATION GUIDE REMICADE (Rem-eh-kaid) (infliximab) Read the Medication Guide that comes with REMICADE before you receive the first treatment, and before each time you get a treatment of REMICADE. This
MEDICATION GUIDE REMICADE (Rem-eh-kaid) (infliximab) Read the Medication Guide that comes with REMICADE before you receive the first treatment, and before each time you get a treatment of REMICADE. This
Media Release. Basel, 11 June 2009. RA patients with enhanced response identified
 Media Release Basel, 11 June 2009 New data demonstrate the ability of MabThera to reduce the progression of joint damage when used as a first-line biologic treatment in rheumatoid arthritis RA patients
Media Release Basel, 11 June 2009 New data demonstrate the ability of MabThera to reduce the progression of joint damage when used as a first-line biologic treatment in rheumatoid arthritis RA patients
Rheumatoid Arthritis
 Rheumatoid Arthritis While rheumatoid arthritis (RA) has long been feared as one of the most disabling types of arthritis, the outlook has dramatically improved for many newly diagnosed patients. Certainly
Rheumatoid Arthritis While rheumatoid arthritis (RA) has long been feared as one of the most disabling types of arthritis, the outlook has dramatically improved for many newly diagnosed patients. Certainly
PART III: CONSUMER INFORMATION
 PART III: CONSUMER INFORMATION Pr REMICADE (Infliximab) This leaflet is part III of a three-part "Product Monograph" published when REMICADE was approved for sale in Canada and is designed specifically
PART III: CONSUMER INFORMATION Pr REMICADE (Infliximab) This leaflet is part III of a three-part "Product Monograph" published when REMICADE was approved for sale in Canada and is designed specifically
NATIONAL PSORIASIS FOUNDATION SYSTEMIC MEDICATIONS. for psoriasis and psoriatic arthritis including biologics and new oral treatments
 NATIONAL PSORIASIS FOUNDATION SYSTEMIC MEDICATIONS for psoriasis and psoriatic arthritis including biologics and new oral treatments Introduction to psoriasis and psoriatic arthritis WHAT IS PSORIASIS?
NATIONAL PSORIASIS FOUNDATION SYSTEMIC MEDICATIONS for psoriasis and psoriatic arthritis including biologics and new oral treatments Introduction to psoriasis and psoriatic arthritis WHAT IS PSORIASIS?
ABOUT RHEUMATOID ARTHRITIS
 MEDIA BACKGROUNDER ABOUT RHEUMATOID ARTHRITIS Rheumatoid arthritis (RA) is a type of arthritis (chronic inflammatory polyarthritis) that typically affects hands and feet, although any joint in the body
MEDIA BACKGROUNDER ABOUT RHEUMATOID ARTHRITIS Rheumatoid arthritis (RA) is a type of arthritis (chronic inflammatory polyarthritis) that typically affects hands and feet, although any joint in the body
SYNOPSIS. 2-Year (0.5 DB + 1.5 OL) Addendum to Clinical Study Report
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier (For National Authority Use
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier (For National Authority Use
Psoriasis Treatment Transition Pathway
 Psoriasis Treatment Transition Pathway A Treatment Support Tool Adapted from Circle Nottingham NHS Treatment Centre Psoriasis Pathway (under consultation) with support from Abbvie Ltd Treatment Pathways
Psoriasis Treatment Transition Pathway A Treatment Support Tool Adapted from Circle Nottingham NHS Treatment Centre Psoriasis Pathway (under consultation) with support from Abbvie Ltd Treatment Pathways
Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
 Moda Health Plan, Inc. Medical Necessity Criteria Subject: Actemra (tocilizumab) Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
Moda Health Plan, Inc. Medical Necessity Criteria Subject: Actemra (tocilizumab) Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Tysabri
 MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF I. Requirements for Prior Authorization of Tysabri A. Prescriptions That Require Prior Authorization All prescriptions for Tysabri must be prior authorized.
MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF I. Requirements for Prior Authorization of Tysabri A. Prescriptions That Require Prior Authorization All prescriptions for Tysabri must be prior authorized.
Top Down vs. Step Up Therapy Biologics in IBD: Treatment Algorithms. Stephen B. Hanauer, M.D. University of Chicago
 Top Down vs. Step Up Therapy Biologics in IBD: Treatment Algorithms Stephen B. Hanauer, M.D. University of Chicago Treatment Goals c.2008 Induce and maintain response/remission Prevent complications Disease
Top Down vs. Step Up Therapy Biologics in IBD: Treatment Algorithms Stephen B. Hanauer, M.D. University of Chicago Treatment Goals c.2008 Induce and maintain response/remission Prevent complications Disease
Rheumatoid Arthritis
 What is Rheumatoid Arthritis? Rheumatoid Arthritis (RA) is a chronic and systemic disease that causes pain, stiffness, swelling, and limitation in the motion and function of multiple joints. Though joints
What is Rheumatoid Arthritis? Rheumatoid Arthritis (RA) is a chronic and systemic disease that causes pain, stiffness, swelling, and limitation in the motion and function of multiple joints. Though joints
Cytokine and CAM Antagonists
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Actemra (Tocilizumab) Drugs requiring prior authorization: the list of drugs
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Actemra (Tocilizumab) Drugs requiring prior authorization: the list of drugs
PRODUCT MONOGRAPH. HUMIRA adalimumab 40 mg in 0.8 ml sterile solution (50 mg/ml) subcutaneous injection
 PRODUCT MONOGRAPH Pr HUMIRA adalimumab 40 mg in 0.8 ml sterile solution (50 mg/ml) subcutaneous injection Biological Response Modifier HUMIRA (adalimumab) treatment should be initiated and supervised by
PRODUCT MONOGRAPH Pr HUMIRA adalimumab 40 mg in 0.8 ml sterile solution (50 mg/ml) subcutaneous injection Biological Response Modifier HUMIRA (adalimumab) treatment should be initiated and supervised by
Early Diagnosis of Rheumatoid Arthritis & Axial Spondyloarthritis
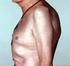 Early Diagnosis of Rheumatoid Arthritis & Axial Spondyloarthritis 奇 美 醫 院 過 敏 免 疫 風 濕 科 陳 宏 安 Rheumatoid arthritis Most common chronic inflammatory joint disease Multisystem autoimmune disease of unknown
Early Diagnosis of Rheumatoid Arthritis & Axial Spondyloarthritis 奇 美 醫 院 過 敏 免 疫 風 濕 科 陳 宏 安 Rheumatoid arthritis Most common chronic inflammatory joint disease Multisystem autoimmune disease of unknown
Rheumatoid Arthritis: Constantly Evolving Treatment Approaches
 Rheumatoid Arthritis: Constantly Evolving Treatment Approaches Jody Garry, Pharm.D. Primary Care Pharmacy Resident VA Medical Center - Iowa City Presentation Overview Pathophysiology & epidemiology Diagnostic
Rheumatoid Arthritis: Constantly Evolving Treatment Approaches Jody Garry, Pharm.D. Primary Care Pharmacy Resident VA Medical Center - Iowa City Presentation Overview Pathophysiology & epidemiology Diagnostic
Adalimumab for the treatment of psoriasis
 DOI: 10.3310/hta13suppl2/07 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Adalimumab for the treatment of psoriasis D Turner, J Picot,* K Cooper and E Loveman Southampton Health Technology Assessments
DOI: 10.3310/hta13suppl2/07 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Adalimumab for the treatment of psoriasis D Turner, J Picot,* K Cooper and E Loveman Southampton Health Technology Assessments
Once the immune system is triggered, cells migrate from the blood into the joints and produce substances that cause inflammation.
 HealthExchange Points For Your Joints An Arthritis Talk Howard Epstein, MD Orthopaedic & Rheumatologic Institute Rheumatic & Immunologic Disease Cleveland Clinic Beachwood Family Health & Surgery Center
HealthExchange Points For Your Joints An Arthritis Talk Howard Epstein, MD Orthopaedic & Rheumatologic Institute Rheumatic & Immunologic Disease Cleveland Clinic Beachwood Family Health & Surgery Center
Medicines for Psoriatic Arthritis. A Review of the Research for Adults
 Medicines for Psoriatic Arthritis A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has told you that you have psoriatic (pronounced
Medicines for Psoriatic Arthritis A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has told you that you have psoriatic (pronounced
Recognizing the Value of Innovation in the Treatment of Rheumatoid Arthritis
 White Paper March 2013 Recognizing the Value of Innovation in the Treatment of Rheumatoid Arthritis Catherine Augustyn, Brigham Walker, and Thomas F. Goss, PharmD Boston Healthcare Associates, Inc., Boston,
White Paper March 2013 Recognizing the Value of Innovation in the Treatment of Rheumatoid Arthritis Catherine Augustyn, Brigham Walker, and Thomas F. Goss, PharmD Boston Healthcare Associates, Inc., Boston,
GUIDELINES FOR THE TREATMENT OF PSORIATIC ARTHRITIS WITH BIOLOGICS
 GUIDELINES FOR THE TREATMENT OF PSORIATIC ARTHRITIS WITH BIOLOGICS The British Society for Rheumatology 2012 guidelines for the treatment of psoriatic arthritis with biologics pages 1 27 BSR guidelines
GUIDELINES FOR THE TREATMENT OF PSORIATIC ARTHRITIS WITH BIOLOGICS The British Society for Rheumatology 2012 guidelines for the treatment of psoriatic arthritis with biologics pages 1 27 BSR guidelines
2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
 Measure #337: Tuberculosis Prevention for Psoriasis,Psoriatic Arthritis and Rheumatoid Arthritis Patients on a Biological Immune Response Modifier National Quality Strategy Domain: Effective Clincal Care
Measure #337: Tuberculosis Prevention for Psoriasis,Psoriatic Arthritis and Rheumatoid Arthritis Patients on a Biological Immune Response Modifier National Quality Strategy Domain: Effective Clincal Care
Psoriatic arthritis FACTSHEET
 1 What is psoriatic arthritis? Psoriatic arthritis (PsA) is a disease where joints around the body become inflamed and sore. It can make moving about difficult and painful. People who have PsA also have
1 What is psoriatic arthritis? Psoriatic arthritis (PsA) is a disease where joints around the body become inflamed and sore. It can make moving about difficult and painful. People who have PsA also have
Methods for Measuring Dose Escalation in TNF Antagonists for Rheumatoid Arthritis Patients Treated in Routine Clinical Practice
 Methods for Measuring Dose Escalation in TNF Antagonists for Rheumatoid Arthritis Patients Treated in Routine Clinical Practice Gu NY 1, Huang XY 2, Globe D 2, Fox KM 3 1 University of Southern California,
Methods for Measuring Dose Escalation in TNF Antagonists for Rheumatoid Arthritis Patients Treated in Routine Clinical Practice Gu NY 1, Huang XY 2, Globe D 2, Fox KM 3 1 University of Southern California,
EVIDENCE BASED TREATMENT OF CROHN S DISEASE. Dr E Ndabaneze
 EVIDENCE BASED TREATMENT OF CROHN S DISEASE Dr E Ndabaneze PLAN 1. Case presentation 2. Topic on Evidence based Treatment of Crohn s disease - Introduction pathology aetiology - Treatment - concept of
EVIDENCE BASED TREATMENT OF CROHN S DISEASE Dr E Ndabaneze PLAN 1. Case presentation 2. Topic on Evidence based Treatment of Crohn s disease - Introduction pathology aetiology - Treatment - concept of
Rheumatoid Arthritis monitoring of DMARDs
 www.bpac.org.nz keyword: DMARDS Rheumatoid Arthritis monitoring of DMARDs Key reviewers: Professor John Highton, Head of Section, Department of Medical and Surgical Sciences, Dunedin School of Medicine,
www.bpac.org.nz keyword: DMARDS Rheumatoid Arthritis monitoring of DMARDs Key reviewers: Professor John Highton, Head of Section, Department of Medical and Surgical Sciences, Dunedin School of Medicine,
Rheumatoid Arthritis Medicines. A Guide for Adults
 Rheumatoid Arthritis Medicines A Guide for Adults Fast Facts Medicines for rheumatoid arthritis (RA) can slow down the disease and reduce damage to joints. They can relieve pain and make it easier to do
Rheumatoid Arthritis Medicines A Guide for Adults Fast Facts Medicines for rheumatoid arthritis (RA) can slow down the disease and reduce damage to joints. They can relieve pain and make it easier to do
UPDATED RECOMMENDATIONS FOR THE USE OF BIOLOGICAL AGENTS FOR THE TREATMENT OF RHEUMATIC DISEASES*
 UPDATED RECOMMENDATIONS FOR THE USE OF BIOLOGICAL AGENTS FOR THE TREATMENT OF RHEUMATIC DISEASES* * DISCLAIMER These recommendations are written to assist Australian rheumatologists prescribing biological
UPDATED RECOMMENDATIONS FOR THE USE OF BIOLOGICAL AGENTS FOR THE TREATMENT OF RHEUMATIC DISEASES* * DISCLAIMER These recommendations are written to assist Australian rheumatologists prescribing biological
A Survey of Barriers to Treatment Access in Rheumatoid Arthritis
 A Survey of Barriers to Treatment Access in Rheumatoid Arthritis in France, Germany, Italy, Spain and the UK October 2009 Funding for this report was provided by F. Hoffmann-La Roche Ltd Carolin Miltenburger,
A Survey of Barriers to Treatment Access in Rheumatoid Arthritis in France, Germany, Italy, Spain and the UK October 2009 Funding for this report was provided by F. Hoffmann-La Roche Ltd Carolin Miltenburger,
Giant Spondylitis and Its Effect on Globlogical Therapy
 Cytokine and CAM Antagonists and Related Agents Review 01/22/2010 Copyright 2005-2010 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No
Cytokine and CAM Antagonists and Related Agents Review 01/22/2010 Copyright 2005-2010 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No
The Most Common Autoimmune Disease: Rheumatoid Arthritis. Bonita S. Libman, M.D.
 The Most Common Autoimmune Disease: Rheumatoid Arthritis Bonita S. Libman, M.D. Disclosures Two googled comics The Normal Immune System Network of cells and proteins that work together Goal: protect against
The Most Common Autoimmune Disease: Rheumatoid Arthritis Bonita S. Libman, M.D. Disclosures Two googled comics The Normal Immune System Network of cells and proteins that work together Goal: protect against
How To Take Methotrexate By Injection
 How To Take Methotrexate By Injection 1 how long does it take for methotrexate to work for abortion 2 methotrexate 15 mg hair loss 3 methotrexate injection dosage for rheumatoid arthritis 4 order methotrexate
How To Take Methotrexate By Injection 1 how long does it take for methotrexate to work for abortion 2 methotrexate 15 mg hair loss 3 methotrexate injection dosage for rheumatoid arthritis 4 order methotrexate
PSORIATIC ARTHRITIS. » Diagnosis» Symptoms» Treatments» + more
 PSORIATIC ARTHRITIS» Diagnosis» Symptoms» Treatments» + more WHAT IS PSORIASIS? PSORIASIS is pronounced sore-eye-ah-sis. It is an autoimmune disease, meaning that certain triggers cause the immune system
PSORIATIC ARTHRITIS» Diagnosis» Symptoms» Treatments» + more WHAT IS PSORIASIS? PSORIASIS is pronounced sore-eye-ah-sis. It is an autoimmune disease, meaning that certain triggers cause the immune system
Psoriatic Arthritis. Title. Understanding and Managing. in All the Wrong Places. Clinical Features. Etiology of Psoriatic Arthritis
 Focus on CME at Memorial University Understanding and Managing Title Psoriatic Arthritis in All the Wrong Places Proton Rahman MD, MSc, FRCPC Although Baron Jean-Luis Aubert offered the first case description
Focus on CME at Memorial University Understanding and Managing Title Psoriatic Arthritis in All the Wrong Places Proton Rahman MD, MSc, FRCPC Although Baron Jean-Luis Aubert offered the first case description
PRACTICAL HELP FROM THE ARTHRITIS FOUNDATION. www.arthritis.org 800-283-7800. Psoriatic Arthritis
 Psoriatic Arthritis WHAT IS PSORIATIC ARTHRITIS? Psoriatic (sore-ee-aah-tick) arthritis is a condition that causes pain and swelling in joints and scaly patches on the skin. Psoriatic arthritis occurs
Psoriatic Arthritis WHAT IS PSORIATIC ARTHRITIS? Psoriatic (sore-ee-aah-tick) arthritis is a condition that causes pain and swelling in joints and scaly patches on the skin. Psoriatic arthritis occurs
Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis
 Etanercept, infliximab and adalimumab for the treatment of Issued: August 2010 guidance.nice.org.uk/ta199 NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to
Etanercept, infliximab and adalimumab for the treatment of Issued: August 2010 guidance.nice.org.uk/ta199 NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to
REMICADE (infliximab)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use REMICADE safely and effectively. See full prescribing information for REMICADE. REMICADE (infliximab)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use REMICADE safely and effectively. See full prescribing information for REMICADE. REMICADE (infliximab)
Psoriatic Arthritis www.arthritis.org.nz
 Psoriatic Arthritis www.arthritis.org.nz Did you know? Arthritis affects one in six New Zealanders over the age of 15 years. Psoriatic arthritis usually appears in people between the ages of 30 to 50.
Psoriatic Arthritis www.arthritis.org.nz Did you know? Arthritis affects one in six New Zealanders over the age of 15 years. Psoriatic arthritis usually appears in people between the ages of 30 to 50.
Rheumatoid Arthritis. Disease RA Final.indd 2 15. 6. 10. 11:23
 Rheumatoid Arthritis Disease RA Final.indd 2 15. 6. 10. 11:23 Understanding what to expect can help you prepare for your transition into treatment. Rheumatoid Arthritis What You Need To Know About Rheumatoid
Rheumatoid Arthritis Disease RA Final.indd 2 15. 6. 10. 11:23 Understanding what to expect can help you prepare for your transition into treatment. Rheumatoid Arthritis What You Need To Know About Rheumatoid
to Part of Dossier: Name of Active Ingredient: Title of Study: Quality of life study with adalimumab in rheumatoid arthritis. ESCALAR.
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Individual Study Table Referring to Part of Dossier: Adalimumab (HUMIRA) (For National Authority Use Only) Name of Active Ingredient: Adalimumab Title
2.0 Synopsis Abbott Laboratories Name of Study Drug: Individual Study Table Referring to Part of Dossier: Adalimumab (HUMIRA) (For National Authority Use Only) Name of Active Ingredient: Adalimumab Title
Psoriatic Arthritis Current Guidelines. Linda Sekhon, DHSc, PA-C
 Psoriatic Arthritis Current Guidelines Linda Sekhon, DHSc, PA-C Learning Objectives At the conclusion of this lecture, participants should be able to: Define Psoriatic Arthritis and briefly describe the
Psoriatic Arthritis Current Guidelines Linda Sekhon, DHSc, PA-C Learning Objectives At the conclusion of this lecture, participants should be able to: Define Psoriatic Arthritis and briefly describe the
Shared care protocol for the management of patients with Rheumatoid Arthritis treated with disease modifying antirheumatic drugs (DMARDs)
 Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared care protocol for the management of patients with Rheumatoid Arthritis treated with disease modifying antirheumatic drugs (DMARDs)
Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared care protocol for the management of patients with Rheumatoid Arthritis treated with disease modifying antirheumatic drugs (DMARDs)
X-Plain Psoriasis Reference Summary
 X-Plain Psoriasis Reference Summary Introduction Psoriasis is a long-lasting skin disease that causes the skin to become inflamed. Patches of thick, red skin are covered with silvery scales. It affects
X-Plain Psoriasis Reference Summary Introduction Psoriasis is a long-lasting skin disease that causes the skin to become inflamed. Patches of thick, red skin are covered with silvery scales. It affects
Key Findings. Use this report to... The Autoimmune Market Outlook to 2013
 Key Findings The global autoimmune market generated sales of $31.9bn in, an increase of 14.4% over 2006 sales. The market is forecast to grow at a CAGR of 8.1% to reach a total value of $51.0bn in 2013.
Key Findings The global autoimmune market generated sales of $31.9bn in, an increase of 14.4% over 2006 sales. The market is forecast to grow at a CAGR of 8.1% to reach a total value of $51.0bn in 2013.
Dr Sarah Levy Consultant Rheumatology Croydon University Hospital
 Dr Sarah Levy Consultant Rheumatology Croydon University Hospital Contents Definition/ epidemiology Diagnosis Importance of early diagnosis/ treatment Guidelines Evidence based treatment protocol Current
Dr Sarah Levy Consultant Rheumatology Croydon University Hospital Contents Definition/ epidemiology Diagnosis Importance of early diagnosis/ treatment Guidelines Evidence based treatment protocol Current
Do I need a physician referral? Yes, we see patients on referral from a health care provider.
 FAQS FOR OFFICE POLICIES How do I get an appointment? New appointments are made by physician referral only. Your referring health care provided will call for the appointment for you. What do I need to
FAQS FOR OFFICE POLICIES How do I get an appointment? New appointments are made by physician referral only. Your referring health care provided will call for the appointment for you. What do I need to
Autoimmune Diseases More common than you think Randall Stevens, MD
 Autoimmune Diseases More common than you think Randall Stevens, MD picture placeholder Autoimmune Diseases More than 60 different disorders Autoimmune disorders (AID) diseases caused by the immune system
Autoimmune Diseases More common than you think Randall Stevens, MD picture placeholder Autoimmune Diseases More than 60 different disorders Autoimmune disorders (AID) diseases caused by the immune system
