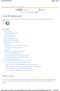
Start Here. Figure 1: Program-Analyze-Write-Review Process
|
|
|
- Allan Jennings
- 8 years ago
- Views:
Transcription
1 Supporting the Program-Analyze-Write-Review Process with a Development Environment for Base SAS and the Macro Language Barry R. Cohen, Planning Data Systems, Inc. ABSTRACT Pharmaceutical companies do much Base SAS and Macro Language programming in the process of analyzing their clinical trials data. This is one part in their larger process of collecting, managing, analyzing, and presenting this data. Many companies w provide considerable system support for the early part of this process (data collection and management) and for the later part (document publishing and presentation of results). But less has been done to support the analysis part in between; i.e., the process of developing and executing SAS programs for statistical analysis, and then writing and reviewing an analysis document based upon the results of program execution. This Program- Analyze-Write-Review process is a major, timeconsuming process in any research organization, and I address automated support for it in this paper. After a top-level discussion of the full Program-Analyze-Write- Review process, I focus in more detail on support for the SAS program development component within this process. INTRODUCTION As pharmaceutical companies conduct clinical trials, they generally follow the process presented in Table 1 regarding the data and analysis: Process Activity Prepare, manage data Develop analysis programs Run programs, analyze data Write analysis document Review document Publish document Present document, data, programs Major System Support yes yes yes Table 1: Clinical Trial Process Regarding Data and Analysis Much attention has been given in this industry to system support for data preparation and management, for document publication, and for document and data presentation. For example: 1 Large-scale Clinical Trials Systems have been and are being developed to handle clinical data collection, preparation, and management. Document Management Systems are being used to publish New Drug Application (NDA) documents. CANDAs (Computer Assisted New Drug Applications) are being used to present the documents, statistical analysis, and data. But perhaps surprisingly the middle of this process, i.e., the program, analyze, write, and review steps, (shaded in Table 1) has received less system support. I will refer to this middle part as the PAWR process (from Program, Analyze, Write, Review). So, for example, often: SAS programmers are on their own to develop programs. No facilitating environment beyond SAS Display Manager is available to support them as they code, test, debug, store, and retrieve the generations of their programs. Statisticians are on their own to analyze data and write the statistical portion of NDA documents. No facilitating environment is available for them to retrieve and execute the generations of SAS programs, retrieve and review the program outputs matched to these generations, and move the program outputs (tables and graphs) into the analysis document they are writing. Clinical staff are similarly on their own to analyze data and write the medical portion of analysis documents. Statisticians and clinical staff are on their own to review the document. No facilitating environment is available for them to access the written document, to antate and then share their review comments in the document, to move from the document text and tables to view the particular patient group data behind them, to re-create a table via changes to program parameter settings in order to explore alternative approaches to the analysis, or to view additional data about the patient group involved in the table. The limited amount of integrated system support for the PAWR process is somewhat surprising because the PAWR process is a major part of the larger clinical trials process, and effective system support could have a commensurate major impact. But I do see two
2 possible reasons why such support has t yet been forthcoming. First, it requires integrating activities that are disparate from the information techlogy perspective. Specifically, the PAWR process is part software-development oriented, part documentdevelopment oriented, (and even has a data handling component). Second, each activity has received at least some system support within that activity, which has allowed the industry to reach an acceptable level of functioning by tying these activities together manually, while putting their system development resources instead into more pressing needs. But I feel that an organization can get much benefit from providing integrated system support for the PAWR process. I also feel that w is a good time to do so because, for many organizations, the more pressing needs of the greater clinical trials process have been or are w being addressed. Also, I feel that more recent advances in the IT industry w make it more feasible to integrate these technically disparate activities. (I am thinking specifically of the ability to tie together a document environment and a program/data environment). This, then, is the subject of my paper. I will present some thoughts about the activities of the PAWR process --- thoughts that describe the process and are a necessary precursor to building an effective software application to integrate and support the activities of this process. I will also present some thoughts about what such a software application might look like, at least in part. But even more basically, and perhaps more importantly for w, I will simply talk about the PAWR process as a process. We cant effectively support a process if we do t readily see it as a process, as a business cycle with a cycle time, and with activities within it where time can be saved if appropriate support is provided. I hope to foster more dialog within the SAS community on this subject through this paper. Hopefully, we can collectively generate ideas that might lead to additions to the SAS System in this regard and to development of our own applications in this area. But I do feel that since so much of the SAS System is about analysis, that if we generate information that describes the activities of the analysis process we conduct, and describes where these activities are and are t currently supported from a system viewpoint, then we will probably be generating information which will eventually be reflected in new features of the SAS System. PROGRAM-ANALYZE-WRITE-REVIEW PROCESS The activities of the PAWR process and their flow are illustrated in Figure 1. The process begins in the lower 2 left of the diagram with the Program Development Environment (PDE), where Base SAS/Macro Language programs are developed and executed. The activities in the PDE include writing, testing, and debugging code, and eventually executing the code in production to produce the tabular and graphic outputs which express the analysis in raw form and which will become part of the analysis document. Analysis Text and other text Word Processing Sys, SAS Program Outputs (Tables, Graphs) SAS Program Development Environment (PDE) Start Here Document Environment (Assemble, Manage) Word Processing Sys. or Document Mgt Sys Document Review Electronic Submission Document Statistical Figure 1: Program-Analyze-Write-Review Process The process continues with the analyst writing the text which discusses the results of the analysis, referring to the tables and graphs. This typically occurs in a word processing environment. Other text is typically also written, generally before the analysis even occurs. The analysis text, other text, and the tables and graphs are next assembled and managed. If the word processing environment is used for this function, the tables and graphs, which are SAS outputs, are incorporated into the word processing environment. Alternatively, a document management system, (a different genre than word processing software), may be the place where the text and the SAS outputs are assembled and managed. If so, the SAS outputs must be loaded into this software-controlled environment instead. The next activity of the PAWR process concerns review of the document. Typically in the pharmaceutical industry many people beyond the
3 author are involved in the review. This activity includes feedback and questions to the authors from the reviewers, and can also involve revising and re-running programs to re-produce SAS outputs and re-write analysis text, which then must be re-loaded to the word processing environment or document management environment. Finally, the dotted-line box in the lower right of Figure 1 indicates that once the analysis is complete and the document is complete and published, it is often submitted electronically (as well as in hard copy) to a regulatory agency for review. This is sometimes referred to as a CANDA, for Computer Assisted New Drug Application. And the statistical analysis (programs and data) may be submitted in a Statistical CANDA, too. I show this activity in a dotted-line because some (many?) companies do t treat it as part of the PAWR process, but as more of a follow-on activity. PAWR Activities Un-integrated From the software application point of view, in the pharmaceutical industry today, I feel the individual activities of the PAWR process have quite varying levels of support within the activity. And I see them as fairly un-integrated across activities; typically, there is one software application which manages and ties together this PAWR process. The one partial exception to this I am aware of is the SAS/PH-Clinical product. PH-Clinical has begun to provide integrated support for the PAWR process along the lines I suggest herein (see especially Villiers, 1997). But I feel the following is still generally true in the industry today, even with PH- Clinical available (at least with the current version). Regarding program development and execution, the SAS System is used to develop and execute the programs, but it does t provide a robust PDE to support this process. This will be further discussed in the next section of this document. Regarding document creation, the process of populating documents with SAS outputs is t a feature of the SAS System today. Users have had to develop their own solutions which include: manually inserting SAS output tables into documents as pictures (which are un-editable as text); using custom written SAS software to engage the DDE facility to send the output to spreadsheets, which are then loaded to the documents using facilities available between the spreadsheet software and word processing software; using custom written SAS software to generate commands in the word processing software s language which are then executed to load the SAS outputs to the word processing document as tables (which are w editable as text). The Output Delivery System planned in version 7 of the SAS System will take a major step forward by rendering its outputs in formats compatible with document environments. This will truly be a major, long awaited advance. But, as I understand it, even then it will be incumbent upon the user to add features to fully automate this SAS-to-document process as part of an integrated application. Regarding the document review process, some work has already been done in this industry to integrate document management systems into the review of NDA documents, both for in-house review and particularly for external review at a government regulatory agency. Specifically, such systems w make documents come alive during review through use of hyperlinks from text to text, search functions, dynamic tables of contents, automated antation, etc. And these systems support creating and sharing antations in the document by multiple parties during review. Further, we are w also seeing document management systems provide web-enabled and web-exploited documents to enhance the review process. So I would t describe the document management and review activities of the PAWR process as unsupported, from the software application point of view. But I would still call them un-integrated activities of the PAWR process. Specifically, much less thinking has occurred to date about how an automated analysis document might also be linked to the data and programs behind the analysis during the review. If this were done, then if a reviewer had questions about a particular table in the document, he/she could drill down into a facilitated environment to view the exact data involved in the table (i.e., the patient group involved), to view other data for the same patient group, and even have the ability to re-run the table with the data for other sets of patients or with the same patient data but with other specifications for the table (e.g., different cut points between categories of an item being reported on in the table). And a SAS PDE ---- whose initial purpose is to help SAS programmers create, access, manage, and execute SAS programs, outputs, and data ---- could also be used as the facilitated environment where document reviewers drill 3
4 down to, in order to gain access to the data and programs to answer their own questions about the analysis during review of an automated document. Finally, a comment regarding electronic document submissions. If a company develops a document management application for internal assembly, management, and review of a document during the PAWR process, there is potential to use the same application as the CANDA tool for external review at a regulatory agency. If done this way, it is an excellent integration of software application support activities within the PAWR process. But I think the more typical situation today is that a document management system is t used in the in-house PAWR process. Rather, when document management systems are used, they tend to be part of a document publication process that occurs separately, after the PAWR process. And this way, there is integration of document publication with internal document writing and review, from a system s perspective. Similarly, when a Statistical CANDA is produced, I believe it is more typically developed as a separate software application following the PAWR process, and t integrated with it. I believe that a significant opportunity for time and cost savings is being lost by approaching it this way. Specifically, a robust SAS PDE would be a facilitated environment for retrieving and executing SAS programs and data, and for retrieving and reviewing the outputs of those programs and data. And this is largely what a Statistical CANDA environment is, too. So, if a company developed its NDA by having its SAS programmers operate within a robust SAS PDE, they would also be building their Statistical CANDA at the same time. This would save a considerable amount of time that is w spent building the Statistical CANDA after the fact. And it would represent a strong integration, from the system s perspective, of the program development activity with ather activity of the PAWR process --- the Statistical CANDA. So, a SAS PDE can serve multiple functions within integrated system support for the PAWR process of the pharmaceutical industry. We might even say that by integrating the PDE fully into this process, we are moving toward the concept of an application called an analysis development environment. That is, an environment which facilitates all aspects of the analysis process, t just the creation of the raw analysis tables and graphics. And the PDE portion of this full analysis development environment would have these roles: 1. Make the Base SAS/Macro Language program development process more efficient. 2. Facilitate the automated process of populating analysis documents with SAS table and graph outputs during document writing. 3. Provide the facilitated environment where reviewers arrive, when drilling down through the automated document during review, to examine the data and re-run the programs that were used to produce the analysis tables and graphs described in the document. 4. Provide a Statistical CANDA software application. PROGRAM DEVELOPMENT ENVIRONMENT The PAWR process is a large one. I have only been able to describe it at the top-level, and make some toplevel suggestions for how it can be integrated from a system s point of view, within the space constraints of this paper. But I am able, herein, to discuss one activity within the PAWR process, specifically SAS program development, in somewhat more detail. Much program development in the software industry today occurs within a PDE. A PDE, simply put, is a software application that provides an environment that facilitates the development of software. PDE s are provided today for many program languages and for many application development tools --- especially for object-oriented GUI tools. Program languages using PDE s today include C, C++, and Smalltalk. Development tools using PDE s today include Visual Basic, Power Builder, and Oracle Developer/2000. The SAS Display Manager environment is a PDE for the Base SAS/Macro Language, albeit it is less robust than the better PDE s seen today. In this section, I will present ideas about what features would be valuable in a more robust SAS PDE for the Base SAS/Macro Language. My ultimate context for this PDE is its use within the pharmaceutical industry s PAWR process. But most of what pharmaceutical SAS programmers do during program development is quite generic, so these ideas basically fit a general PDE for the SAS System. Also, I te that a SAS PDE is well worth pursuing, even if it is never integrated with software support for the other activities of the PAWR process. It will shorten cycle time for the program development activity, regardless. The SAS Institute has already developed some PDEtype support for application development in the AF/SCL environment. It is called Source Control Manager and it is experimental in release But 4
5 there is similar support provided yet for program development in the Base SAS/Macro Language environment, where most pharmaceutical SAS programming occurs. I am aware today that the SAS Institute is planning to provide some sort of PDE for Base SAS programming as part of version 7 of the SAS System. But I still want to include this section in my paper to foster a dialog that can lead to a full expression of desired features by the user community. Also, I understand that the Institute s PDE will t pay special attention to Macro Language programming, which is a central part of the program development process conducted by pharmaceutical SAS programmers. I will discuss additional PDE support for macro programming herein. Common PDE Components In general, the thinking behind PDE s has been to identify and provide automated support for those programming activities that are done repeatedly, that tend to be tedious, and that can feasibly be supported by a software application. Here is my list of such programming activities in the SAS environment: Write code Store code (especially including generations of same program) Retrieve code (by generation) Execute code to test for syntax errors Execute code with data, to test for logic errors Debug code with logic errors Compare generations of code for differences Share code with co-developers Save outputs, including logs, procedure outputs (Output window), and custom generated tables and graphs sent to external files --- all by generation Retrieve these same outputs for viewing Document code Validate code Orient others to the development environment Given this list of activities, I see the SAS PDE centered around these chief components: 1. A Custom Editor 2. A Library Manager for Source Code and Outputs The Custom Editor would have these features: Syntax sensitive editing - It would examine all code from the syntax perspective, and correct it, before the code was even executed. Color coding - It would display the code in colors, according to rules, to better reveal the structure, and thereby add clarity and meaning to the program. Automatic indentation - It would automatically indent code, according to rules, as ather means of revealing the structure. Documentation window - It would provide a separate place (e.g., a pop-up window) where the programmer could write tes that describe the program. This information would be saved along with the code, and would always be available in this editor. Change history window - This feature would be similar to the documentation window, but it would contain tes about the changes within and across generations of the program. Debug facility - It would allow you to submit code for execution from the editor, and put traces on the values of variables, and then see the code scroll up line by line as it executes, and display trace variable values on-demand. The Library Manager for Source Code and Outputs would have these features: Flexibility in defining libraries, such as on a per project basis. Ability to define private libraries and shared libraries, with a facility to promote and demote entries between the two types of libraries. Storage of all SAS programs as entries in a SAS catalog, with regular SAS catalog support for saving, retrieving, deleting, browsing, etc. Option to store code as the next generation of a given program, or as a new program. Similar catalog storage for all outputs when the program was executed, linked with the associated source code item. Outputs include: Program logs Statistical procedure output (text and graphic) Externals files (text and graphic) Facilitated searching of log entries for tes, warnings, errors, etc. Storage of the source code Documentation Window contents, as described just above. A checkin/checkout facility allowing multiple programmers to obtain and work on a program in a shared library, without overwriting the other programmers work. A facility to compare and report differences on two source code entries in the library (e.g., two generations for a program). Storage of the source code Change History Window contents, as described above. This feature would be related to ather feature of the library which would record for each entry in the library the time and date of last change, and the identity of the last user to make the change. 5
6 Output entries would be read-only by default. When output items were editable, then checkin/checkout support and change history support would be provided, as just above. Additional SAS PDE Components for Macros Many of the PDE components above might be considered standard or common support for program development, even beyond just SAS program development. But SAS programmers, certainly including pharmaceutical SAS programers, write many programs in the SAS Macro Language instead of just the Base SAS Language. As such, I envision other components in the SAS PDE to support development of macro programs. These would be in addition to having all the above common components: The Custom Editor would provide for a macro program (in one or more pop-up windows): a list of all its macro parameters the default value of each parameter, if any the current value of each parameter, if any a text description of each parameter, as supplied by the programmer GUI type support (point and click, drag and drop) for setting the current value of each parameter The Library Manager would handle all this macro parameter information, along with the macro source code, as one or more catalog entries, with all support for save, retrieve, delete, browse, etc. The Library Manager would save the full set of parameter values as an additional output item when macro programs were executed. The Library Manager would have a macro documentation facility that would, for any given macro: (1) report what other macros are called from within this macro, and whether or t they are also in the same library or other available libraries; (2) report what other macros in the same library or other available libraries call this macro. including an audit trail on change history. These features would be inherent parts of a SAS PDE, and as such, any organization developing its SAS programs within this environment would be automatically addressing these aspects of program validation. REFERENCES Villiers, P. (1997), "New Architecture for Linkage of SAS/PH-Clinical Software with Electronic Document Management Systems, SAS Institute, Cary, NC (in the Proceedings of the PharmaSUG 97 Conference). Barry R. Cohen, Planning Data Systems, Inc. PO Box 666, Ardmore, PA Mr. Cohen is an independent information systems consultant, specializing in application development and other support for analytic processing. He has been using SAS software since 1980 in a variety of industries, including a focus on the pharmaceutical industry. Program Validation There are major requirements in the pharmaceutical industry for validation of programs used in data analysis. The effort put into this program validation is substantial. A SAS PDE would make a major contribution both toward insuring that validation was done, and toward getting it done more easily. This is because part of the program validation process includes providing well defined, well organized handling of the storage and retrieval of programs, and providing tight control over access to and editing of programs, 6
Source Code Translation
 Source Code Translation Everyone who writes computer software eventually faces the requirement of converting a large code base from one programming language to another. That requirement is sometimes driven
Source Code Translation Everyone who writes computer software eventually faces the requirement of converting a large code base from one programming language to another. That requirement is sometimes driven
How To Write A Clinical Trial In Sas
 PharmaSUG2013 Paper AD11 Let SAS Set Up and Track Your Project Tom Santopoli, Octagon, now part of Accenture Wayne Zhong, Octagon, now part of Accenture ABSTRACT When managing the programming activities
PharmaSUG2013 Paper AD11 Let SAS Set Up and Track Your Project Tom Santopoli, Octagon, now part of Accenture Wayne Zhong, Octagon, now part of Accenture ABSTRACT When managing the programming activities
Paper-less Reporting: On-line Data Review and Analysis Using SAS/PH-Clinical Software
 Paper-less Reporting: On-line Data Review and Analysis Using SAS/PH-Clinical Software Eileen Ching, SmithKline Beecham Pharmaceuticals, Collegeville, PA Rosemary Oakes, SmithKline Beecham Pharmaceuticals,
Paper-less Reporting: On-line Data Review and Analysis Using SAS/PH-Clinical Software Eileen Ching, SmithKline Beecham Pharmaceuticals, Collegeville, PA Rosemary Oakes, SmithKline Beecham Pharmaceuticals,
Applications Development
 User-Interface Tool Choice and Audit Trail Tool Choice for a SAS Based Data Entry/Verify System for Clinical Trials Data Barry R. Cohen, Planning Data Systems, Inc., Ardmore, PA ABSTRACT A double-key data
User-Interface Tool Choice and Audit Trail Tool Choice for a SAS Based Data Entry/Verify System for Clinical Trials Data Barry R. Cohen, Planning Data Systems, Inc., Ardmore, PA ABSTRACT A double-key data
Guide to SAS/AF Applications Development
 Guide to SAS/AF Applications Development SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2012. Guide to SAS/AF Applications Development. Cary, NC:
Guide to SAS/AF Applications Development SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2012. Guide to SAS/AF Applications Development. Cary, NC:
Enhancing the SAS Enhanced Editor with Toolbar Customizations Lynn Mullins, PPD, Cincinnati, Ohio
 PharmaSUG 016 - Paper QT1 Enhancing the SAS Enhanced Editor with Toolbar Customizations Lynn Mullins, PPD, Cincinnati, Ohio ABSTRACT One of the most important tools for SAS programmers is the Display Manager
PharmaSUG 016 - Paper QT1 Enhancing the SAS Enhanced Editor with Toolbar Customizations Lynn Mullins, PPD, Cincinnati, Ohio ABSTRACT One of the most important tools for SAS programmers is the Display Manager
Release 2.1 of SAS Add-In for Microsoft Office Bringing Microsoft PowerPoint into the Mix ABSTRACT INTRODUCTION Data Access
 Release 2.1 of SAS Add-In for Microsoft Office Bringing Microsoft PowerPoint into the Mix Jennifer Clegg, SAS Institute Inc., Cary, NC Eric Hill, SAS Institute Inc., Cary, NC ABSTRACT Release 2.1 of SAS
Release 2.1 of SAS Add-In for Microsoft Office Bringing Microsoft PowerPoint into the Mix Jennifer Clegg, SAS Institute Inc., Cary, NC Eric Hill, SAS Institute Inc., Cary, NC ABSTRACT Release 2.1 of SAS
Using SAS Data Integration Studio to Convert Clinical Trials Data to the CDISC SDTM Standard Barry R. Cohen, Octagon Research Solutions, Wayne, PA
 Using SAS Data Integration Studio to Convert Clinical Trials Data to the CDISC SDTM Standard Barry R. Cohen, Octagon Research Solutions, Wayne, PA ABSTRACT A new industry standard for clinical trials data,
Using SAS Data Integration Studio to Convert Clinical Trials Data to the CDISC SDTM Standard Barry R. Cohen, Octagon Research Solutions, Wayne, PA ABSTRACT A new industry standard for clinical trials data,
A Hybrid Modeling Platform to meet Basel II Requirements in Banking Jeffery Morrision, SunTrust Bank, Inc.
 A Hybrid Modeling Platform to meet Basel II Requirements in Banking Jeffery Morrision, SunTrust Bank, Inc. Introduction: The Basel Capital Accord, ready for implementation in force around 2006, sets out
A Hybrid Modeling Platform to meet Basel II Requirements in Banking Jeffery Morrision, SunTrust Bank, Inc. Introduction: The Basel Capital Accord, ready for implementation in force around 2006, sets out
Using Pharmacovigilance Reporting System to Generate Ad-hoc Reports
 Using Pharmacovigilance Reporting System to Generate Ad-hoc Reports Jeff Cai, Amylin Pharmaceuticals, Inc., San Diego, CA Jay Zhou, Amylin Pharmaceuticals, Inc., San Diego, CA ABSTRACT To supplement Oracle
Using Pharmacovigilance Reporting System to Generate Ad-hoc Reports Jeff Cai, Amylin Pharmaceuticals, Inc., San Diego, CA Jay Zhou, Amylin Pharmaceuticals, Inc., San Diego, CA ABSTRACT To supplement Oracle
KEY FEATURES OF SOURCE CONTROL UTILITIES
 Source Code Revision Control Systems and Auto-Documenting Headers for SAS Programs on a UNIX or PC Multiuser Environment Terek Peterson, Alliance Consulting Group, Philadelphia, PA Max Cherny, Alliance
Source Code Revision Control Systems and Auto-Documenting Headers for SAS Programs on a UNIX or PC Multiuser Environment Terek Peterson, Alliance Consulting Group, Philadelphia, PA Max Cherny, Alliance
Quality Assurance: Best Practices in Clinical SAS Programming. Parag Shiralkar
 Quality Assurance: Best Practices in Clinical SAS Programming Parag Shiralkar eclinical Solutions, a Division of Eliassen Group Abstract SAS programmers working on clinical reporting projects are often
Quality Assurance: Best Practices in Clinical SAS Programming Parag Shiralkar eclinical Solutions, a Division of Eliassen Group Abstract SAS programmers working on clinical reporting projects are often
ABSTRACT INTRODUCTION EXERCISE 1: EXPLORING THE USER INTERFACE GRAPH GALLERY
 Statistical Graphics for Clinical Research Using ODS Graphics Designer Wei Cheng, Isis Pharmaceuticals, Inc., Carlsbad, CA Sanjay Matange, SAS Institute, Cary, NC ABSTRACT Statistical graphics play an
Statistical Graphics for Clinical Research Using ODS Graphics Designer Wei Cheng, Isis Pharmaceuticals, Inc., Carlsbad, CA Sanjay Matange, SAS Institute, Cary, NC ABSTRACT Statistical graphics play an
2 SQL in iseries Navigator
 2 SQL in iseries Navigator In V4R4, IBM added an SQL scripting tool to the standard features included within iseries Navigator and has continued enhancing it in subsequent releases. Because standard features
2 SQL in iseries Navigator In V4R4, IBM added an SQL scripting tool to the standard features included within iseries Navigator and has continued enhancing it in subsequent releases. Because standard features
Search help. More on Office.com: images templates
 Page 1 of 14 Access 2010 Home > Access 2010 Help and How-to > Getting started Search help More on Office.com: images templates Access 2010: database tasks Here are some basic database tasks that you can
Page 1 of 14 Access 2010 Home > Access 2010 Help and How-to > Getting started Search help More on Office.com: images templates Access 2010: database tasks Here are some basic database tasks that you can
Switching from PC SAS to SAS Enterprise Guide Zhengxin (Cindy) Yang, inventiv Health Clinical, Princeton, NJ
 PharmaSUG 2014 PO10 Switching from PC SAS to SAS Enterprise Guide Zhengxin (Cindy) Yang, inventiv Health Clinical, Princeton, NJ ABSTRACT As more and more organizations adapt to the SAS Enterprise Guide,
PharmaSUG 2014 PO10 Switching from PC SAS to SAS Enterprise Guide Zhengxin (Cindy) Yang, inventiv Health Clinical, Princeton, NJ ABSTRACT As more and more organizations adapt to the SAS Enterprise Guide,
PharmaSUG 2014 Paper CC23. Need to Review or Deliver Outputs on a Rolling Basis? Just Apply the Filter! Tom Santopoli, Accenture, Berwyn, PA
 PharmaSUG 2014 Paper CC23 Need to Review or Deliver Outputs on a Rolling Basis? Just Apply the Filter! Tom Santopoli, Accenture, Berwyn, PA ABSTRACT Wouldn t it be nice if all of the outputs in a deliverable
PharmaSUG 2014 Paper CC23 Need to Review or Deliver Outputs on a Rolling Basis? Just Apply the Filter! Tom Santopoli, Accenture, Berwyn, PA ABSTRACT Wouldn t it be nice if all of the outputs in a deliverable
Double-Key Data EntryNerification in SASe Software Part of Downsizing a Clinical Data System to the OS/2" Operating System
 , i: Double-Key Data EntryNerification in SASe Software Part of Downsizing a Clinical Data System to the OS/2" Operating System Barry R. Cohen, Planning Data Systems ABSTRACT The pharmaceutical industry,
, i: Double-Key Data EntryNerification in SASe Software Part of Downsizing a Clinical Data System to the OS/2" Operating System Barry R. Cohen, Planning Data Systems ABSTRACT The pharmaceutical industry,
Automate Data Integration Processes for Pharmaceutical Data Warehouse
 Paper AD01 Automate Data Integration Processes for Pharmaceutical Data Warehouse Sandy Lei, Johnson & Johnson Pharmaceutical Research and Development, L.L.C, Titusville, NJ Kwang-Shi Shu, Johnson & Johnson
Paper AD01 Automate Data Integration Processes for Pharmaceutical Data Warehouse Sandy Lei, Johnson & Johnson Pharmaceutical Research and Development, L.L.C, Titusville, NJ Kwang-Shi Shu, Johnson & Johnson
Supporting a Global SAS Programming Envronment? Real World Applications in an Outsourcing Model
 Supporting a Global SAS Programming Envronment? Real World Applications in an Outsourcing Model Karen Curran and Mark Penniston, Omnicare Clinical Research, King of Prussia, PA ABSTRACT Talented individuals
Supporting a Global SAS Programming Envronment? Real World Applications in an Outsourcing Model Karen Curran and Mark Penniston, Omnicare Clinical Research, King of Prussia, PA ABSTRACT Talented individuals
SAS Marketing Automation 5.1. User s Guide
 SAS Marketing Automation 5.1 User s Guide The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2007. SAS Marketing Automation 5.1: User s Guide. Cary, NC: SAS Institute
SAS Marketing Automation 5.1 User s Guide The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2007. SAS Marketing Automation 5.1: User s Guide. Cary, NC: SAS Institute
Implementing an Audit Trail within a Clinical Reporting Tool Paul Gilbert, Troy A. Ruth, Gregory T. Weber DataCeutics, Inc.
 Paper AD12 Implementing an Audit Trail within a Clinical Reporting Tool Paul Gilbert, Troy A. Ruth, Gregory T. Weber DataCeutics, Inc., Pottstown, PA ABSTRACT This paper is a follow-up to Overview of a
Paper AD12 Implementing an Audit Trail within a Clinical Reporting Tool Paul Gilbert, Troy A. Ruth, Gregory T. Weber DataCeutics, Inc., Pottstown, PA ABSTRACT This paper is a follow-up to Overview of a
Scatter Chart. Segmented Bar Chart. Overlay Chart
 Data Visualization Using Java and VRML Lingxiao Li, Art Barnes, SAS Institute Inc., Cary, NC ABSTRACT Java and VRML (Virtual Reality Modeling Language) are tools with tremendous potential for creating
Data Visualization Using Java and VRML Lingxiao Li, Art Barnes, SAS Institute Inc., Cary, NC ABSTRACT Java and VRML (Virtual Reality Modeling Language) are tools with tremendous potential for creating
SAS/GRAPH Network Visualization Workshop 2.1
 SAS/GRAPH Network Visualization Workshop 2.1 User s Guide SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc 2009. SAS/GRAPH : Network Visualization Workshop
SAS/GRAPH Network Visualization Workshop 2.1 User s Guide SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc 2009. SAS/GRAPH : Network Visualization Workshop
Appendix A How to create a data-sharing lab
 Appendix A How to create a data-sharing lab Creating a lab involves completing five major steps: creating lists, then graphs, then the page for lab instructions, then adding forms to the lab instructions,
Appendix A How to create a data-sharing lab Creating a lab involves completing five major steps: creating lists, then graphs, then the page for lab instructions, then adding forms to the lab instructions,
Word 2010: Mail Merge to Email with Attachments
 Word 2010: Mail Merge to Email with Attachments Table of Contents TO SEE THE SECTION FOR MACROS, YOU MUST TURN ON THE DEVELOPER TAB:... 2 SET REFERENCE IN VISUAL BASIC:... 2 CREATE THE MACRO TO USE WITHIN
Word 2010: Mail Merge to Email with Attachments Table of Contents TO SEE THE SECTION FOR MACROS, YOU MUST TURN ON THE DEVELOPER TAB:... 2 SET REFERENCE IN VISUAL BASIC:... 2 CREATE THE MACRO TO USE WITHIN
While You Were Sleeping - Scheduling SAS Jobs to Run Automatically Faron Kincheloe, Baylor University, Waco, TX
 Paper 276-27 While You Were Sleeping - Scheduling SAS Jobs to Run Automatically Faron Kincheloe, Baylor University, Waco, TX ABSTRACT If you are tired of running the same jobs over and over again, this
Paper 276-27 While You Were Sleeping - Scheduling SAS Jobs to Run Automatically Faron Kincheloe, Baylor University, Waco, TX ABSTRACT If you are tired of running the same jobs over and over again, this
Intellect Platform - The Workflow Engine Basic HelpDesk Troubleticket System - A102
 Intellect Platform - The Workflow Engine Basic HelpDesk Troubleticket System - A102 Interneer, Inc. Updated on 2/22/2012 Created by Erika Keresztyen Fahey 2 Workflow - A102 - Basic HelpDesk Ticketing System
Intellect Platform - The Workflow Engine Basic HelpDesk Troubleticket System - A102 Interneer, Inc. Updated on 2/22/2012 Created by Erika Keresztyen Fahey 2 Workflow - A102 - Basic HelpDesk Ticketing System
Paper PO03. A Case of Online Data Processing and Statistical Analysis via SAS/IntrNet. Sijian Zhang University of Alabama at Birmingham
 Paper PO03 A Case of Online Data Processing and Statistical Analysis via SAS/IntrNet Sijian Zhang University of Alabama at Birmingham BACKGROUND It is common to see that statisticians at the statistical
Paper PO03 A Case of Online Data Processing and Statistical Analysis via SAS/IntrNet Sijian Zhang University of Alabama at Birmingham BACKGROUND It is common to see that statisticians at the statistical
SPSS: Getting Started. For Windows
 For Windows Updated: August 2012 Table of Contents Section 1: Overview... 3 1.1 Introduction to SPSS Tutorials... 3 1.2 Introduction to SPSS... 3 1.3 Overview of SPSS for Windows... 3 Section 2: Entering
For Windows Updated: August 2012 Table of Contents Section 1: Overview... 3 1.1 Introduction to SPSS Tutorials... 3 1.2 Introduction to SPSS... 3 1.3 Overview of SPSS for Windows... 3 Section 2: Entering
Config Guide. Gimmal Smart Tiles (SharePoint-Hosted) Software Release 4.4.0
 Config Guide Gimmal Smart Tiles (SharePoint-Hosted) Software Release 4.4.0 November 2014 Title: Gimmal Smart Tiles (SharePoint-Hosted) Configuration Guide Copyright 2014 Gimmal, All Rights Reserved. Gimmal
Config Guide Gimmal Smart Tiles (SharePoint-Hosted) Software Release 4.4.0 November 2014 Title: Gimmal Smart Tiles (SharePoint-Hosted) Configuration Guide Copyright 2014 Gimmal, All Rights Reserved. Gimmal
Business Insight Report Authoring Getting Started Guide
 Business Insight Report Authoring Getting Started Guide Version: 6.6 Written by: Product Documentation, R&D Date: February 2011 ImageNow and CaptureNow are registered trademarks of Perceptive Software,
Business Insight Report Authoring Getting Started Guide Version: 6.6 Written by: Product Documentation, R&D Date: February 2011 ImageNow and CaptureNow are registered trademarks of Perceptive Software,
Benefits of Upgrading to Phoenix WinNonlin 6.2
 Benefits of Upgrading to Phoenix WinNonlin 6.2 Pharsight, a Certara Company 5625 Dillard Drive; Suite 205 Cary, NC 27518; USA www.pharsight.com March, 2011 Benefits of Upgrading to Phoenix WinNonlin 6.2
Benefits of Upgrading to Phoenix WinNonlin 6.2 Pharsight, a Certara Company 5625 Dillard Drive; Suite 205 Cary, NC 27518; USA www.pharsight.com March, 2011 Benefits of Upgrading to Phoenix WinNonlin 6.2
ABSTRACT THE ISSUE AT HAND THE RECIPE FOR BUILDING THE SYSTEM THE TEAM REQUIREMENTS. Paper DM09-2012
 Paper DM09-2012 A Basic Recipe for Building a Campaign Management System from Scratch: How Base SAS, SQL Server and Access can Blend Together Tera Olson, Aimia Proprietary Loyalty U.S. Inc., Minneapolis,
Paper DM09-2012 A Basic Recipe for Building a Campaign Management System from Scratch: How Base SAS, SQL Server and Access can Blend Together Tera Olson, Aimia Proprietary Loyalty U.S. Inc., Minneapolis,
Tips and Tricks SAGE ACCPAC INTELLIGENCE
 Tips and Tricks SAGE ACCPAC INTELLIGENCE 1 Table of Contents Auto e-mailing reports... 4 Automatically Running Macros... 7 Creating new Macros from Excel... 8 Compact Metadata Functionality... 9 Copying,
Tips and Tricks SAGE ACCPAC INTELLIGENCE 1 Table of Contents Auto e-mailing reports... 4 Automatically Running Macros... 7 Creating new Macros from Excel... 8 Compact Metadata Functionality... 9 Copying,
The structured application of advanced logging techniques for SystemVerilog testbench debug and analysis. By Bindesh Patel and Amanda Hsiao.
 Logging makes sense for testbench debug The structured application of advanced logging techniques for SystemVerilog testbench debug and analysis. By Bindesh Patel and Amanda Hsiao. SystemVerilog provides
Logging makes sense for testbench debug The structured application of advanced logging techniques for SystemVerilog testbench debug and analysis. By Bindesh Patel and Amanda Hsiao. SystemVerilog provides
Business Process. Automation. Automation. David Chernicoff Susan Perschke. sponsored by
 Business Process Automation Managing Cost in Your Automation Enterprise David Chernicoff Susan Perschke sponsored by i Contents Business Process Automation Managing Cost in Your Enterprise Chapter 4: BPA
Business Process Automation Managing Cost in Your Automation Enterprise David Chernicoff Susan Perschke sponsored by i Contents Business Process Automation Managing Cost in Your Enterprise Chapter 4: BPA
DCA. Document Control & Archiving USER S GUIDE
 DCA Document Control & Archiving USER S GUIDE Decision Management International, Inc. 1111 Third Street West Suite 250 Bradenton, FL 34205 Phone 800-530-0803 FAX 941-744-0314 www.dmius.com Copyright 2002,
DCA Document Control & Archiving USER S GUIDE Decision Management International, Inc. 1111 Third Street West Suite 250 Bradenton, FL 34205 Phone 800-530-0803 FAX 941-744-0314 www.dmius.com Copyright 2002,
MICROSOFT OFFICE ACCESS 2007 - NEW FEATURES
 MICROSOFT OFFICE 2007 MICROSOFT OFFICE ACCESS 2007 - NEW FEATURES Exploring Access Creating and Working with Tables Finding and Filtering Data Working with Queries and Recordsets Working with Forms Working
MICROSOFT OFFICE 2007 MICROSOFT OFFICE ACCESS 2007 - NEW FEATURES Exploring Access Creating and Working with Tables Finding and Filtering Data Working with Queries and Recordsets Working with Forms Working
Oracle Warehouse Builder 10g
 Oracle Warehouse Builder 10g Architectural White paper February 2004 Table of contents INTRODUCTION... 3 OVERVIEW... 4 THE DESIGN COMPONENT... 4 THE RUNTIME COMPONENT... 5 THE DESIGN ARCHITECTURE... 6
Oracle Warehouse Builder 10g Architectural White paper February 2004 Table of contents INTRODUCTION... 3 OVERVIEW... 4 THE DESIGN COMPONENT... 4 THE RUNTIME COMPONENT... 5 THE DESIGN ARCHITECTURE... 6
MOVING THE CLINICAL ANALYTICAL ENVIRONMENT INTO THE CLOUD
 MOVING THE CLINICAL ANALYTICAL ENVIRONMENT INTO THE CLOUD STIJN ROGIERS, SENIOR INDUSTRY CONSULTANT, LIFE SCIENCES/HEALTH CARE (EMEA/AP) SANDEEP JUNEJA CONSULTING MANAGER (SSOD) AGENDA Move towards cloud
MOVING THE CLINICAL ANALYTICAL ENVIRONMENT INTO THE CLOUD STIJN ROGIERS, SENIOR INDUSTRY CONSULTANT, LIFE SCIENCES/HEALTH CARE (EMEA/AP) SANDEEP JUNEJA CONSULTING MANAGER (SSOD) AGENDA Move towards cloud
Appointment Scheduler
 EZClaim Appointment Scheduler User Guide Last Update: 11/19/2008 Copyright 2008 EZClaim This page intentionally left blank Contents Contents... iii Getting Started... 5 System Requirements... 5 Installing
EZClaim Appointment Scheduler User Guide Last Update: 11/19/2008 Copyright 2008 EZClaim This page intentionally left blank Contents Contents... iii Getting Started... 5 System Requirements... 5 Installing
Producing Listings and Reports Using SAS and Crystal Reports Krishna (Balakrishna) Dandamudi, PharmaNet - SPS, Kennett Square, PA
 Producing Listings and Reports Using SAS and Crystal Reports Krishna (Balakrishna) Dandamudi, PharmaNet - SPS, Kennett Square, PA ABSTRACT The SAS Institute has a long history of commitment to openness
Producing Listings and Reports Using SAS and Crystal Reports Krishna (Balakrishna) Dandamudi, PharmaNet - SPS, Kennett Square, PA ABSTRACT The SAS Institute has a long history of commitment to openness
OpenWells Software. DecisionSpace Drilling & Completions. Streamline activity and morning reporting. Improve drilling operations DATA SHEET
 DATA SHEET OpenWells Software OVERVIEW DecisionSpace Drilling & Completions KEY FEATURES Comprehensive operations reporting from site selection to abandonment Simple, visual data entry saves time and reduces
DATA SHEET OpenWells Software OVERVIEW DecisionSpace Drilling & Completions KEY FEATURES Comprehensive operations reporting from site selection to abandonment Simple, visual data entry saves time and reduces
Vendor: Crystal Decisions Product: Crystal Reports and Crystal Enterprise
 1 Ability to access the database platforms desired (text, spreadsheet, Oracle, Sybase and other databases, OLAP engines.) Y Y 2 Ability to access relational data base Y Y 3 Ability to access dimensional
1 Ability to access the database platforms desired (text, spreadsheet, Oracle, Sybase and other databases, OLAP engines.) Y Y 2 Ability to access relational data base Y Y 3 Ability to access dimensional
SAS Task Manager 2.2. User s Guide. SAS Documentation
 SAS Task Manager 2.2 User s Guide SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2015. SAS Task Manager 2.2: User's Guide. Cary, NC: SAS Institute
SAS Task Manager 2.2 User s Guide SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2015. SAS Task Manager 2.2: User's Guide. Cary, NC: SAS Institute
Macros in Word & Excel
 Macros in Word & Excel Description: If you perform a task repeatedly in Word or Excel, you can automate the task by using a macro. A macro is a series of steps that is grouped together as a single step
Macros in Word & Excel Description: If you perform a task repeatedly in Word or Excel, you can automate the task by using a macro. A macro is a series of steps that is grouped together as a single step
CRGroup Whitepaper: Digging through the Data. www.crgroup.com. Reporting Options in Microsoft Dynamics GP
 CRGroup Whitepaper: Digging through the Data Reporting Options in Microsoft Dynamics GP The objective of this paper is to provide greater insight on each of the reporting options available to you within
CRGroup Whitepaper: Digging through the Data Reporting Options in Microsoft Dynamics GP The objective of this paper is to provide greater insight on each of the reporting options available to you within
Macros allow you to integrate existing Excel reports with a new information system
 Macro Magic Macros allow you to integrate existing Excel reports with a new information system By Rick Collard Many water and wastewater professionals use Microsoft Excel extensively, producing reports
Macro Magic Macros allow you to integrate existing Excel reports with a new information system By Rick Collard Many water and wastewater professionals use Microsoft Excel extensively, producing reports
MicroStrategy Products
 MicroStrategy Products Bringing MicroStrategy Reporting, Analysis, and Monitoring to Microsoft Excel, PowerPoint, and Word With MicroStrategy Office, business users can create and run MicroStrategy reports
MicroStrategy Products Bringing MicroStrategy Reporting, Analysis, and Monitoring to Microsoft Excel, PowerPoint, and Word With MicroStrategy Office, business users can create and run MicroStrategy reports
Migration of SAS Software From VMS to Windows NT : A Real Life Story
 Migration of Software From VMS to Windows NT : A Real Life Story Paul Gilbert & Steve Light at DataCeutics, Inc., John Scott Grainger at ClinTrials Research Introduction At ClinTrials Research, Inc. clinical
Migration of Software From VMS to Windows NT : A Real Life Story Paul Gilbert & Steve Light at DataCeutics, Inc., John Scott Grainger at ClinTrials Research Introduction At ClinTrials Research, Inc. clinical
A simple tool to catalogue statistical outputs developed for submission by linking two in-house systems experience from a submission project
 Paper PP11 A simple tool to catalogue statistical outputs developed for submission by linking two in-house systems experience from a submission project Katja Diezel, Novartis Pharma AG, Basel, Switzerland
Paper PP11 A simple tool to catalogue statistical outputs developed for submission by linking two in-house systems experience from a submission project Katja Diezel, Novartis Pharma AG, Basel, Switzerland
I Didn t Know SAS Enterprise Guide Could Do That!
 Paper SAS016-2014 I Didn t Know SAS Enterprise Guide Could Do That! Mark Allemang, SAS Institute Inc., Cary, NC ABSTRACT This presentation is for users who are familiar with SAS Enterprise Guide but might
Paper SAS016-2014 I Didn t Know SAS Enterprise Guide Could Do That! Mark Allemang, SAS Institute Inc., Cary, NC ABSTRACT This presentation is for users who are familiar with SAS Enterprise Guide but might
StreamServe Persuasion SP5 Ad Hoc Correspondence and Correspondence Reviewer
 StreamServe Persuasion SP5 Ad Hoc Correspondence and Correspondence Reviewer User Guide Rev B StreamServe Persuasion SP5 Ad Hoc Correspondence and Correspondence Reviewer User Guide Rev B 2001-2010 STREAMSERVE,
StreamServe Persuasion SP5 Ad Hoc Correspondence and Correspondence Reviewer User Guide Rev B StreamServe Persuasion SP5 Ad Hoc Correspondence and Correspondence Reviewer User Guide Rev B 2001-2010 STREAMSERVE,
STATEMENT OF WORK. NETL Cooperative Agreement DE-FC26-02NT41476
 STATEMENT OF WORK NETL Cooperative Agreement DE-FC26-02NT41476 Database and Analytical Tool for the Management of Data Derived from U. S. DOE (NETL) Funded Fine Particulate (PM 2.5 ) Research PROJECT SCOPE
STATEMENT OF WORK NETL Cooperative Agreement DE-FC26-02NT41476 Database and Analytical Tool for the Management of Data Derived from U. S. DOE (NETL) Funded Fine Particulate (PM 2.5 ) Research PROJECT SCOPE
Digital Asset Management
 A collaborative digital asset management system for marketing organizations that improves performance, saves time and reduces costs. MarketingPilot provides powerful digital asset management software for
A collaborative digital asset management system for marketing organizations that improves performance, saves time and reduces costs. MarketingPilot provides powerful digital asset management software for
National Child Measurement Programme 2015/16. IT System User Guide Part 3. Pupil Data Management
 National Child Measurement Programme 2015/16 IT System User Guide Part 3 Pupil Data Management Published May 2016 Version 2.1 We are the trusted source of authoritative data and information relating to
National Child Measurement Programme 2015/16 IT System User Guide Part 3 Pupil Data Management Published May 2016 Version 2.1 We are the trusted source of authoritative data and information relating to
SAS System and SAS Program Validation Techniques Sy Truong, Meta-Xceed, Inc., San Jose, CA
 SAS System and SAS Program Validation Techniques Sy Truong, Meta-Xceed, Inc., San Jose, CA ABSTRACT This course will teach methodologies of performing SAS system and SAS program validation including new
SAS System and SAS Program Validation Techniques Sy Truong, Meta-Xceed, Inc., San Jose, CA ABSTRACT This course will teach methodologies of performing SAS system and SAS program validation including new
How To Use The Correlog With The Cpl Powerpoint Powerpoint Cpl.Org Powerpoint.Org (Powerpoint) Powerpoint (Powerplst) And Powerpoint 2 (Powerstation) (Powerpoints) (Operations
 orrelog SQL Table Monitor Adapter Users Manual http://www.correlog.com mailto:info@correlog.com CorreLog, SQL Table Monitor Users Manual Copyright 2008-2015, CorreLog, Inc. All rights reserved. No part
orrelog SQL Table Monitor Adapter Users Manual http://www.correlog.com mailto:info@correlog.com CorreLog, SQL Table Monitor Users Manual Copyright 2008-2015, CorreLog, Inc. All rights reserved. No part
The Power Loader GUI
 The Power Loader GUI (212) 405.1010 info@1010data.com Follow: @1010data www.1010data.com The Power Loader GUI Contents 2 Contents Pre-Load To-Do List... 3 Login to Power Loader... 4 Upload Data Files to
The Power Loader GUI (212) 405.1010 info@1010data.com Follow: @1010data www.1010data.com The Power Loader GUI Contents 2 Contents Pre-Load To-Do List... 3 Login to Power Loader... 4 Upload Data Files to
Microsoft Access 2010- Introduction
 Microsoft Access 2010- Introduction Access is the database management system in Microsoft Office. A database is an organized collection of facts about a particular subject. Examples of databases are an
Microsoft Access 2010- Introduction Access is the database management system in Microsoft Office. A database is an organized collection of facts about a particular subject. Examples of databases are an
BI Project Management Software
 BI Project Management Software Plan and Manage with Confidence When planning a new project, program managers can search past projects to leverage project assets and resources. With complete project and
BI Project Management Software Plan and Manage with Confidence When planning a new project, program managers can search past projects to leverage project assets and resources. With complete project and
Clinical Data Warehouse Functionality Peter Villiers, SAS Institute Inc., Cary, NC
 Clinical Warehouse Functionality Peter Villiers, SAS Institute Inc., Cary, NC ABSTRACT Warehousing is a buzz-phrase that has taken the information systems world by storm. It seems that in every industry
Clinical Warehouse Functionality Peter Villiers, SAS Institute Inc., Cary, NC ABSTRACT Warehousing is a buzz-phrase that has taken the information systems world by storm. It seems that in every industry
Appendix M INFORMATION TECHNOLOGY (IT) YOUTH APPRENTICESHIP
 Appendix M INFORMATION TECHNOLOGY (IT) YOUTH APPRENTICESHIP PROGRAMMING & SOFTWARE DEVELOPMENT AND INFORMATION SUPPORT & SERVICES PATHWAY SOFTWARE UNIT UNIT 5 Programming & and Support & s: (Unit 5) PAGE
Appendix M INFORMATION TECHNOLOGY (IT) YOUTH APPRENTICESHIP PROGRAMMING & SOFTWARE DEVELOPMENT AND INFORMATION SUPPORT & SERVICES PATHWAY SOFTWARE UNIT UNIT 5 Programming & and Support & s: (Unit 5) PAGE
Product Summary of XLReporter with OPC Servers
 Product Summary of XLReporter with OPC Servers SyTech, Inc. Page 1 Contents Summary...3 SYTECH is THE REPORT COMPANY... 3 Product Overview...4 XLREPORTER EDITIONS... 4 DATA INTERFACES... 5 ARCHITECTURES...
Product Summary of XLReporter with OPC Servers SyTech, Inc. Page 1 Contents Summary...3 SYTECH is THE REPORT COMPANY... 3 Product Overview...4 XLREPORTER EDITIONS... 4 DATA INTERFACES... 5 ARCHITECTURES...
Visual Studio 2008 Express Editions
 Visual Studio 2008 Express Editions Visual Studio 2008 Installation Instructions Burning a Visual Studio 2008 Express Editions DVD Download (http://www.microsoft.com/express/download/) the Visual Studio
Visual Studio 2008 Express Editions Visual Studio 2008 Installation Instructions Burning a Visual Studio 2008 Express Editions DVD Download (http://www.microsoft.com/express/download/) the Visual Studio
Seamless Dynamic Web Reporting with SAS D.J. Penix, Pinnacle Solutions, Indianapolis, IN
 Seamless Dynamic Web Reporting with SAS D.J. Penix, Pinnacle Solutions, Indianapolis, IN ABSTRACT The SAS Business Intelligence platform provides a wide variety of reporting interfaces and capabilities
Seamless Dynamic Web Reporting with SAS D.J. Penix, Pinnacle Solutions, Indianapolis, IN ABSTRACT The SAS Business Intelligence platform provides a wide variety of reporting interfaces and capabilities
SAS Add-In 2.1 for Microsoft Office: Getting Started with Data Analysis
 SAS Add-In 2.1 for Microsoft Office: Getting Started with Data Analysis The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2007. SAS Add-In 2.1 for Microsoft Office: Getting
SAS Add-In 2.1 for Microsoft Office: Getting Started with Data Analysis The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2007. SAS Add-In 2.1 for Microsoft Office: Getting
ABSTRACT TECHNICAL DESIGN INTRODUCTION FUNCTIONAL DESIGN
 Overview of a Browser-Based Clinical Report Generation Tool Paul Gilbert, DataCeutics, Pottstown PA Greg Weber, DataCeutics Teofil Boata, Purdue Pharma ABSTRACT In an effort to increase reporting quality
Overview of a Browser-Based Clinical Report Generation Tool Paul Gilbert, DataCeutics, Pottstown PA Greg Weber, DataCeutics Teofil Boata, Purdue Pharma ABSTRACT In an effort to increase reporting quality
Statistical Operations: The Other Half of Good Statistical Practice
 Integrating science, technology and experienced implementation Statistical Operations: The Other Half of Good Statistical Practice Alan Hopkins, Ph.D. Theravance, Inc. Presented at FDA/Industry Statistics
Integrating science, technology and experienced implementation Statistical Operations: The Other Half of Good Statistical Practice Alan Hopkins, Ph.D. Theravance, Inc. Presented at FDA/Industry Statistics
SAS BI Dashboard 4.3. User's Guide. SAS Documentation
 SAS BI Dashboard 4.3 User's Guide SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2010. SAS BI Dashboard 4.3: User s Guide. Cary, NC: SAS Institute
SAS BI Dashboard 4.3 User's Guide SAS Documentation The correct bibliographic citation for this manual is as follows: SAS Institute Inc. 2010. SAS BI Dashboard 4.3: User s Guide. Cary, NC: SAS Institute
Application Note Windows 2008 and IBM Tape Diagnostic Tool (ITDT-GE)
 Application Note Windows 2008 and IBM Tape Diagnostic Tool (ITDT-GE) PREFACE This document describes how to install and use IBM Tape Diagnostic Tool GUI Edition (ITDT-GE) on a Windows 2008 x 64 server
Application Note Windows 2008 and IBM Tape Diagnostic Tool (ITDT-GE) PREFACE This document describes how to install and use IBM Tape Diagnostic Tool GUI Edition (ITDT-GE) on a Windows 2008 x 64 server
Using SQL Server Management Studio
 Using SQL Server Management Studio Microsoft SQL Server Management Studio 2005 is a graphical tool for database designer or programmer. With SQL Server Management Studio 2005 you can: Create databases
Using SQL Server Management Studio Microsoft SQL Server Management Studio 2005 is a graphical tool for database designer or programmer. With SQL Server Management Studio 2005 you can: Create databases
Validating Methods using Waters Empower TM 2 Method. Validation. Manager
 Validating Methods using Waters Empower TM 2 Method Validation Manager (MVM) Anders Janesten Nordic Informatics Sales Specialist 2008 Waters Corporation Decision-centric centric method development information
Validating Methods using Waters Empower TM 2 Method Validation Manager (MVM) Anders Janesten Nordic Informatics Sales Specialist 2008 Waters Corporation Decision-centric centric method development information
IN THE WORKSHOP Tip #14
 IN THE WORKSHOP Tip #14 Smart Grid tools accelerate your component creation Summary May 2007 Author: Phil Loughhead Component creation is a fundamental part of the design process, and must be done accurately.
IN THE WORKSHOP Tip #14 Smart Grid tools accelerate your component creation Summary May 2007 Author: Phil Loughhead Component creation is a fundamental part of the design process, and must be done accurately.
JOB SUBMISSION MENU (JSM): A PROGRAMMING TOOL TO STANDARDIZE CLINICAL TRIAL SUMMARIZATION
 JO SUMISSION MENU (JSM): A PROGRAMMING TOOL TO STANDARDIZE CLINICAL TRIAL SUMMARIZATION Iraj Mohebalian, Parke-Davis Pharmaceutical Research Mark ecker, STATPROE, Inc. Lora H. Schwab, STATPROE, Inc. ASTRACT
JO SUMISSION MENU (JSM): A PROGRAMMING TOOL TO STANDARDIZE CLINICAL TRIAL SUMMARIZATION Iraj Mohebalian, Parke-Davis Pharmaceutical Research Mark ecker, STATPROE, Inc. Lora H. Schwab, STATPROE, Inc. ASTRACT
ClinPlus. Report. Technology Consulting Outsourcing. Create high-quality statistical tables and listings. An industry-proven authoring tool
 Technology Consulting Outsourcing ClinPlus Report Create high-quality statistical tables and listings An industry-proven authoring tool Ensure consistency across different programmers Extensive Template
Technology Consulting Outsourcing ClinPlus Report Create high-quality statistical tables and listings An industry-proven authoring tool Ensure consistency across different programmers Extensive Template
Vendor: Brio Software Product: Brio Performance Suite
 1 Ability to access the database platforms desired (text, spreadsheet, Oracle, Sybase and other databases, OLAP engines.) yes yes Brio is recognized for it Universal database access. Any source that is
1 Ability to access the database platforms desired (text, spreadsheet, Oracle, Sybase and other databases, OLAP engines.) yes yes Brio is recognized for it Universal database access. Any source that is
CA Clarity PPM. Demand Management User Guide. v13.0.00
 CA Clarity PPM Demand Management User Guide v13.0.00 This documentation, which includes embedded help systems and electronically distributed materials, (hereinafter referred to as the Documentation ) is
CA Clarity PPM Demand Management User Guide v13.0.00 This documentation, which includes embedded help systems and electronically distributed materials, (hereinafter referred to as the Documentation ) is
White Paper: 5GL RAD Development
 White Paper: 5GL RAD Development After 2.5 hours of training, subjects reduced their development time by 60-90% A Study By: 326 Market Street Harrisburg, PA 17101 Luis Paris, Ph.D. Associate Professor
White Paper: 5GL RAD Development After 2.5 hours of training, subjects reduced their development time by 60-90% A Study By: 326 Market Street Harrisburg, PA 17101 Luis Paris, Ph.D. Associate Professor
Applications Development
 Paper 21-25 Using SAS Software and Visual Basic for Applications to Automate Tasks in Microsoft Word: An Alternative to Dynamic Data Exchange Mark Stetz, Amgen, Inc., Thousand Oaks, CA ABSTRACT Using Dynamic
Paper 21-25 Using SAS Software and Visual Basic for Applications to Automate Tasks in Microsoft Word: An Alternative to Dynamic Data Exchange Mark Stetz, Amgen, Inc., Thousand Oaks, CA ABSTRACT Using Dynamic
Data Management, Analysis Tools, and Analysis Mechanics
 Chapter 2 Data Management, Analysis Tools, and Analysis Mechanics This chapter explores different tools and techniques for handling data for research purposes. This chapter assumes that a research problem
Chapter 2 Data Management, Analysis Tools, and Analysis Mechanics This chapter explores different tools and techniques for handling data for research purposes. This chapter assumes that a research problem
3 What s New in Excel 2007
 3 What s New in Excel 2007 3.1 Overview of Excel 2007 Microsoft Office Excel 2007 is a spreadsheet program that enables you to enter, manipulate, calculate, and chart data. An Excel file is referred to
3 What s New in Excel 2007 3.1 Overview of Excel 2007 Microsoft Office Excel 2007 is a spreadsheet program that enables you to enter, manipulate, calculate, and chart data. An Excel file is referred to
Programming in Access VBA
 PART I Programming in Access VBA In this part, you will learn all about how Visual Basic for Applications (VBA) works for Access 2010. A number of new VBA features have been incorporated into the 2010
PART I Programming in Access VBA In this part, you will learn all about how Visual Basic for Applications (VBA) works for Access 2010. A number of new VBA features have been incorporated into the 2010
Choosing the Best Method to Create an Excel Report Romain Miralles, Clinovo, Sunnyvale, CA
 Choosing the Best Method to Create an Excel Report Romain Miralles, Clinovo, Sunnyvale, CA ABSTRACT PROC EXPORT, LIBNAME, DDE or excelxp tagset? Many techniques exist to create an excel file using SAS.
Choosing the Best Method to Create an Excel Report Romain Miralles, Clinovo, Sunnyvale, CA ABSTRACT PROC EXPORT, LIBNAME, DDE or excelxp tagset? Many techniques exist to create an excel file using SAS.
Unemployment Insurance Data Validation Operations Guide
 Unemployment Insurance Data Validation Operations Guide ETA Operations Guide 411 U.S. Department of Labor Employment and Training Administration Office of Unemployment Insurance TABLE OF CONTENTS Chapter
Unemployment Insurance Data Validation Operations Guide ETA Operations Guide 411 U.S. Department of Labor Employment and Training Administration Office of Unemployment Insurance TABLE OF CONTENTS Chapter
How to create and personalize a PDF portfolio
 How to create and personalize a PDF portfolio Creating and organizing a PDF portfolio is a simple process as simple as dragging and dropping files from one folder to another. To drag files into an empty
How to create and personalize a PDF portfolio Creating and organizing a PDF portfolio is a simple process as simple as dragging and dropping files from one folder to another. To drag files into an empty
WebSphere Business Monitor
 WebSphere Business Monitor Dashboards 2010 IBM Corporation This presentation should provide an overview of the dashboard widgets for use with WebSphere Business Monitor. WBPM_Monitor_Dashboards.ppt Page
WebSphere Business Monitor Dashboards 2010 IBM Corporation This presentation should provide an overview of the dashboard widgets for use with WebSphere Business Monitor. WBPM_Monitor_Dashboards.ppt Page
Oracle Data Integrator: Administration and Development
 Oracle Data Integrator: Administration and Development What you will learn: In this course you will get an overview of the Active Integration Platform Architecture, and a complete-walk through of the steps
Oracle Data Integrator: Administration and Development What you will learn: In this course you will get an overview of the Active Integration Platform Architecture, and a complete-walk through of the steps
DIABLO VALLEY COLLEGE CATALOG 2014-2015
 COMPUTER SCIENCE COMSC The computer science department offers courses in three general areas, each targeted to serve students with specific needs: 1. General education students seeking a computer literacy
COMPUTER SCIENCE COMSC The computer science department offers courses in three general areas, each targeted to serve students with specific needs: 1. General education students seeking a computer literacy
Microsoft Office System Tip Sheet
 Experience the 2007 Microsoft Office System The 2007 Microsoft Office system includes programs, servers, services, and solutions designed to work together to help you succeed. New features in the 2007
Experience the 2007 Microsoft Office System The 2007 Microsoft Office system includes programs, servers, services, and solutions designed to work together to help you succeed. New features in the 2007
Importing Excel File using Microsoft Access in SAS Ajay Gupta, PPD Inc, Morrisville, NC
 ABSTRACT PharmaSUG 2012 - Paper CC07 Importing Excel File using Microsoft Access in SAS Ajay Gupta, PPD Inc, Morrisville, NC In Pharmaceuticals/CRO industries, Excel files are widely use for data storage.
ABSTRACT PharmaSUG 2012 - Paper CC07 Importing Excel File using Microsoft Access in SAS Ajay Gupta, PPD Inc, Morrisville, NC In Pharmaceuticals/CRO industries, Excel files are widely use for data storage.
PharmaSUG 2013 - Paper DG06
 PharmaSUG 2013 - Paper DG06 JMP versus JMP Clinical for Interactive Visualization of Clinical Trials Data Doug Robinson, SAS Institute, Cary, NC Jordan Hiller, SAS Institute, Cary, NC ABSTRACT JMP software
PharmaSUG 2013 - Paper DG06 JMP versus JMP Clinical for Interactive Visualization of Clinical Trials Data Doug Robinson, SAS Institute, Cary, NC Jordan Hiller, SAS Institute, Cary, NC ABSTRACT JMP software
CONVERSION GUIDE PPC s Engagement Manager to Engagement CS
 CONVERSION GUIDE PPC s Engagement Manager to Engagement CS Introduction... 1 Conversion program overview... 1 Minimum operating system and software requirements... 2 Installing the conversion program from
CONVERSION GUIDE PPC s Engagement Manager to Engagement CS Introduction... 1 Conversion program overview... 1 Minimum operating system and software requirements... 2 Installing the conversion program from
A Complete Drug Stability Program. Stability Lab Information Manager SL IM
 A Complete Drug Stability Program Stability Lab Information Manager SL IM SLIM is a Fully Validated, World-Class Stability LIMS. SLIM combines the ease of use of browser style user interface and industry
A Complete Drug Stability Program Stability Lab Information Manager SL IM SLIM is a Fully Validated, World-Class Stability LIMS. SLIM combines the ease of use of browser style user interface and industry
B.Sc (Computer Science) Database Management Systems UNIT-V
 1 B.Sc (Computer Science) Database Management Systems UNIT-V Business Intelligence? Business intelligence is a term used to describe a comprehensive cohesive and integrated set of tools and process used
1 B.Sc (Computer Science) Database Management Systems UNIT-V Business Intelligence? Business intelligence is a term used to describe a comprehensive cohesive and integrated set of tools and process used
1-04-10 Configuration Management: An Object-Based Method Barbara Dumas
 1-04-10 Configuration Management: An Object-Based Method Barbara Dumas Payoff Configuration management (CM) helps an organization maintain an inventory of its software assets. In traditional CM systems,
1-04-10 Configuration Management: An Object-Based Method Barbara Dumas Payoff Configuration management (CM) helps an organization maintain an inventory of its software assets. In traditional CM systems,
Lesson 07: MS ACCESS - Handout. Introduction to database (30 mins)
 Lesson 07: MS ACCESS - Handout Handout Introduction to database (30 mins) Microsoft Access is a database application. A database is a collection of related information put together in database objects.
Lesson 07: MS ACCESS - Handout Handout Introduction to database (30 mins) Microsoft Access is a database application. A database is a collection of related information put together in database objects.
PharmaSUG 2016 Paper IB10
 ABSTRACT PharmaSUG 2016 Paper IB10 Moving from Data Collection to Data Visualization and Analytics: Leveraging CDISC SDTM Standards to Support Data Marts Steve Kirby, JD, MS, Chiltern, King of Prussia,
ABSTRACT PharmaSUG 2016 Paper IB10 Moving from Data Collection to Data Visualization and Analytics: Leveraging CDISC SDTM Standards to Support Data Marts Steve Kirby, JD, MS, Chiltern, King of Prussia,
Optimizing Performance of the SAS Enterprise Guide User Interface
 Optimizing Performance of the SAS Enterprise Guide User Interface Beginning with version 4.3, the new Program Editor in SAS Enterprise Guide offers several compelling features for SAS programmers, including
Optimizing Performance of the SAS Enterprise Guide User Interface Beginning with version 4.3, the new Program Editor in SAS Enterprise Guide offers several compelling features for SAS programmers, including
