Magnetic Resonance Imaging Targeted Biopsy of the Prostate
|
|
|
- Garey Melton
- 8 years ago
- Views:
Transcription
1 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided in the applicable contract. Medical technology is constantly evolving, and we reserve the right to review and update Medical Policy periodically. Services Are Considered Investigational Coverage is not available for investigational medical treatments or procedures, drugs, devices or biological products. Based on review of available data, the Company considers magnetic resonance imaging (MRI) targeted biopsy of the prostate to be investigational.* Background/Overview Magnetic resonance imaging allows for targeted biopsy of suspicious lesions in the prostate, rather than blind biopsy, as is done with the current standard of care, transrectal ultrasound (TRUS) guided biopsy. The use of MRI-guided prostate biopsy may identify areas in the prostate that harbor high-grade tumor, minimizing the detection of clinically insignificant cancers and possible overtreatment and distinguishing men more appropriately managed by active surveillance from men recommended for definitive intervention. Prostate biopsy typically is performed in men who have an elevated prostate-specific antigen (PSA) level or who present with symptoms. The purpose of the biopsy is to determine whether cancer is present and to determine tumor grade. Tumor grade (Gleason score) is a major determinate in whether a patient is eligible for active surveillance (lower grade tumors) or factor for determining definitive intervention (higher grade tumors). Biopsies to diagnose prostate cancer are currently performed using TRUS guidance with a 12-core sampling strategy. TRUS was introduced in the late 1980s; with this technique, tissue cores are obtained systematically under ultrasound guidance throughout the whole prostate, although this approach still represents blind biopsy of the prostate as to the location of possible cancer. Prior to the 12-core sampling, 6-core (sextant) sampling was thought to miss too many cases of cancer. However, the 12-core sampling method may overdiagnose clinically insignificant disease and miss diagnosis of clinically significant disease. Compared with subsequent prostatectomy, TRUS underestimates tumor grade up to 40% of the time and too often detects clinically insignificant disease. Therefore, the ideal biopsy strategy would only identify men with prostate cancer of clinical significance to direct interventional therapy, and to minimize the detection of clinically insignificant prostate cancer and the risk of consequent overtreatment. For men undergoing an initial biopsy for an elevated PSA, the systematic 12-core TRUS biopsy detection rate for prostate cancer is approximately 40% to 45%. If an initial 12-core biopsy is negative, and there is still a clinical suspicion of cancer, subsequent serial 12-core biopsies may detect cancer, or, other biopsy techniques such as transperineal template guided saturation biopsy (in which cores are typically obtained) may be used. Saturation biopsy allows for anterior and apical sampling and may detect significant Page 1 of 8
2 cancer, but also results in oversampling of insignificant cancers. In addition, transperineal biopsy requires general anesthesia and is associated with increased morbidity. Multiparametric magnetic resonance imaging (mpmri) includes anatomic T2-weighted imaging for localization of the normal gland and cancer foci and 2 functional imaging techniques: diffusion-weighted and perfusion imaging. The mpmri evaluation permits identifying tumor location and extent, oversampling areas of interest, undersampling or not sampling nontarget areas, and sampling of clinically significant disease (higher grade tumor). T2-weighted images reflect water content of tissues and can define the zonal anatomy of the prostate and the presence of prostate cancer as focal areas of low-signal intensities. Degree of intensity decrease differs with Gleason score; higher Gleason score prostate cancer shows lower signal intensities.1 False-positive findings can occur with benign abnormalities, including prostatitis, atrophy, fibrosis, gland hyperplasia, or irradiation or hormonal treatment effects. Diffusion-weighted images measure the random motion of water molecules. Low diffusion coefficients are associated with prostate cancer, and there is an inverse correlation between these values and Gleason score; however, confidence intervals overlap. Perfusion imaging allows assessment of contrast kinetics in focal lesions; prostate cancer typically enhances faster and to a greater extent than the surrounding prostate; however, nonspecificity of patterns limits the usefulness of this technique in isolation. Several methods of MRI guidance are available for prostate biopsy: cognitive (or visual), direct ( in-bore ), and MRI-ultrasound (US) fusion (visual targeted or software-based targeted). Image fusion is the process of combining information from more than 1 image into a single image, which may be more informative than any of the images separately. To date, no prospective comparison of the 3 methods has been made. Based on MRI, suspicious areas are identified (ie, regions of interest) and subjected to targeted biopsy. With the visual method, the ultrasound operator simply aims the biopsy needle at the area of the prostate where prior MRI indicated the lesion. This method requires the MRI unit, a conventional TRUS facility, and an ultrasound operator with no additional training beyond TRUS biopsy. The disadvantage is the potential for human error in the extrapolation from MRI to TRUS without an overlay of the images. Direct (in-bore) MRI-targeted biopsy requires the MRI tube, fusion of a prior MRI demonstrating a lesion with a contemporaneous MRI to confirm biopsy needle location, and needles introduced into the regions of interest. Serial MRI scans are performed to confirm biopsy needle placement. Studies have demonstrated that in-bore MRI-targeted biopsies have a median cancer detection rate significantly higher than random biopsies; however, this technique is time-consuming and costly, including the in-bore time and the 2 MRI sessions necessary. In addition, only suspicious lesions are sampled, because tissues with a normal appearance on MRI are not obtained. MRI-TRUS fusion biopsy, done visually or using software, superimposes preprocedure (stored) MRI over an intraprocedure (real-time) ultrasound to direct the biopsy needle to an ultrasound region of interest defined by the mpmri. Proposed clinical indications for use of MRI-guided prostate biopsy include: (1) rebiopsy after a first negative standard biopsy in men with persistent suspicion of disease, including those with persistently Page 2 of 8
3 increased PSA, suspicious digital rectal exam, previous biopsy with an atypical focus on histology, or extensive high-grade prostatic intraepithelial neoplasia, (2) follow-up for active surveillance to determine initial eligibility for active surveillance and assessing progression disease over time, (3) as initial biopsy, and (4) for local recurrence post radical prostatectomy, post external beam radiotherapy, or after high-intensity focused ultrasound. FDA or Other Governmental Regulatory Approval U.S. Food and Drug Administration (FDA) Magnetic resonance imaging-guided or MRI/ultrasound (US) fusion biopsy is a medical procedure that uses MRI and ultrasound devices previously approved by the FDA. Prostate biopsy is a surgical procedure and, as such, it is not subject to regulation by FDA. FDA product code, ultrasound devices: IYN, ITX, IYO. FDA product code, MRI devices: LNH, LNI, MOS. Several MRI-US fusion software-based targeted prostate biopsy platform specifications have been cleared from marketing by FDA through the 510(k) marketing process. Fusion software and (manufacturers) include: Artemis (Eigen), BioJet (D&K Technologies), BiopSee (MedCom), Real-time Visual Sonography (Hitachi), UroNav (Invivo/Philips), Urostation (Koelis), and Virtual Navigator (Esaote). Centers for Medicare and Medicaid Services (CMS) There is no national coverage determination (NCD). In the absence of an NCD, coverage decisions are left to the discretion of local Medicare carriers. Rationale/Source Magnetic Resonance Imaging Targeted Biopsy Compared With Standard Biopsy for Detection of Prostate Cancer A 2015 systematic review and meta-analysis by Schoots et al (search through May 2014) assessed the diagnostic differences between MRI targeted biopsy (MRI-TBx) and TRUS guided biopsy (TRUS-Bx) in detecting overall prostate cancer (primary objective) and clinically significant and insignificant prostate cancer (secondary objective). Selected studies included men with suspected prostate cancer scheduled for transrectal biopsy because of increased prostate-specific antigen and/or positive digital rectal exam. Overall, according to QUADAS criteria, the methodologic quality of the studies was deemed to be fair. Only studies that included both MRI-TBx and conventional TRUS-Bx in each patient were included in order to compare the 2 technologies. Therefore, all men had a positive MRI, defined as a suspicious lesion on prostate MRI scan. Reports on transperineal or saturation biopsy were excluded. Relative sensitivity was the sensitivity ratio between MRI-TBx and TRUS-Bx. A relative sensitivity of greater than 1 indicated that MRI-TBx detected more cancers than TRUS-Bx, and a relative sensitivity less than 1 indicated that MRI- TBx detected fewer cancers than TRUS-Bx. Analyses were performed for 2 predefined subgroup categories: patient population was categorized as men undergoing initial biopsy, men with a previous negative biopsy, and reports in which results were mixed for initial versus subsequent biopsy; and navigational system for MRI as direct versus fusion biopsy with visual or software registration. Sixteen studies with 1926 men were eligible. MRI-TBx and TRUS-Bx did not differ significantly in overall prostate cancer detection (sensitivity, 0.85; 95% confidence interval [CI], 0.80 to 0.89 vs sensitivity, 0.81; 95% CI, Page 3 of 8
4 0.70 to 0.88, respectively). Ten studies presented data on the detection of significant versus insignificant prostate cancer detection. Of the 10 studies, 5 reported results for initial biopsy, 2 for a previous negative biopsy, and 3 with a mixed population. MRI-TBx had a higher rate of detection of significant prostate cancer than TRUS-Bx (sensitivity, 0.91; 95% CI, 0.87 to 0.94 vs sensitivity, 0.76; 95% CI, 0.64 to 0.84) and a lower rate of detection of insignificant prostate cancer (sensitivity, 0.44; 95% CI, 0.26 to 0.64 vs sensitivity, 0.83; 95% CI, 0.77 to 0.87). The improvement in significant prostate cancer detection by MRI-TBx was in men with previous negative biopsy, but not men undergoing initial biopsy (relative sensitivity, 1.54; 95% CI, 1.05 to 2.57 vs relative sensitivity, 1.10; 95% CI, 1.00 to 1.22). Two of 16 studies reported mixed data on MRIvisual-TBx and MRI-fusion-TBx. Therefore, 14 studies were available to compare outcomes of one navigational system versus another, of which only 8 presented data on the detection of significant and insignificant prostate cancer: 2 studies with MRI-visual-TBx, 5 with MRI-fusion-TBx, and 1 with MRI-in-bore- TBx. MRI-fusion-TBx and MRI-in-bore-TBx significantly improved prostate cancer detection compared with TRUS-Bx (relative sensitivity, 1.29; 95% CI, 1.16 to 1.43 vs relative sensitivity, 1.26; 95% CI, 1.08 to 1.46, respectively). MRI-visual-TBx did not show much improvement compared with TRUS-Bx (relative sensitivity, 1.03; 95% CI, 0.91 to 1.16). In summary, the study found that MRI-TBx had a 20% better detection rate for clinically significant prostate cancer than TRUS-Bx. A relative sensitivity of 0.56 was found for detection of insignificant prostate cancer, in that MRI-TBx showed an almost twofold better performance in avoiding detection of insignificant prostate cancer than TRUS-Bx. Strengths of this meta-analysis included the focus on reports that included comparison of biopsies performed by both MRI-guided and TRUS biopsy in the same patient. Limitations included the inclusion of small studies and publication bias, because the studies only included patients with a positive MRI. A 2015 systematic review by Valerio et al (search through December 2013) compared the detection rate of clinically significant cancer with software-based MRI-US fusion biopsy versus standard TRUS biopsy. Secondary outcomes were detection rate of all cancer, sampling efficiency, and utility and rate of serious adverse events. Fourteen articles were included in the final analysis. Most studies were considered to have a low risk of bias and low concern for applicability with respect to patient selection. Two studies were scored as potentially biased; one had a small sample size (n=13) and significant patient heterogeneity, and the other had a retrospective design and patient heterogeneity. All but 1 study were paired cohort design. Thirteen studies used 10- to 12-core TRUS biopsy as the reference test; 1 study used transperineal biopsy, however, systematic transperineal template cores were not taken from areas previously mapped using MRI- TRUS fusion biopsies and, therefore, the detection rate of clinically significant disease could not be compared. A total of 2293 men were included in the review, with sample sizes ranging from 13 to 582. Three studies were conducted in men undergoing first biopsy, 3 in men with a previous negative TRUS biopsy, 8 studies in a mixed cohort with either first biopsy or a previous biopsy, and 1 study with men with recurrent disease post radiation. Median detection of clinically significant cancer was 23.6% (range, 4.8%- 52%) for standard biopsy and 33.3% (range, 13.2%-50%) for MRI-TRUS fusion-targeted biopsy. Only 1 study did not report the criteria for defining clinically significant disease. In the remaining studies, the presence of Gleason pattern 4 was considered to be clinically significant disease. For secondary outcomes, median detection rate of any cancer was 43.4% for standard biopsy (range, 14.3%-59%) and 50.5% (range, 23.7%-82.1%) for MRI-TRUS fusion biopsy. Median number of cores needed to detect clinically significant Page 4 of 8
5 cancer was 37.1 and 9.2 for standard and MRI-TRUS fusion-targeted biopsy, respectively. Utility, defined as the number of clinically significant cancers detected by 1 strategy but missed by the other, was an absolute difference of 9.1% (range, 5%-16.2in favor of the MRI-TRUS biopsy. Limitations of the review included heterogeneity in study design and patient population, and variability across studies in terms of MRI characteristics and interpretation, threshold for biopsy, targeted biopsy conduct, and number of cores per target. MRI-Guided Biopsy in Active Surveillance In 2015, Schoots et al conducted a systematic review (search through April 2014) of the use of MRI in men on active surveillance for prostate cancer. The review assessed evidence for the use of MRI in men with low- or intermediate-risk prostate cancer diagnosed with TRUS-guided biopsy and were therefore deemed suitable for active surveillance. The reviewers addressed 2 main clinical questions: (1) Can MRI detect clinically significant disease in men on active surveillance (thereby prompting treatment intervention rather than remaining on active surveillance)?; and (2) Can MRI be used in place of repeat standard TRUS biopsy to detect disease progression over time? The studies included reports on 3 distinct populations of men: group 1: men with histologic suitability for active surveillance who chose to have radical prostatectomy and had an MRI performed preoperatively (n=10 studies); group 2: men on active surveillance who had an MRI before a confirmatory biopsy (n=7 studies); and group 3: men on active surveillance assessed for disease progression on further MRI scans after an initial baseline scan (n=2 studies). The accuracy of MRI findings was assessed using whole-mount histology from post prostatectomy specimens (group 1), repeat standard biopsy (groups 2 and 3), or biopsies targeted to any suspicious lesions on MRI (groups 2 and 3). The MRItargeted approach included in-bore targeting, visual registration, and software-assisted registration. Ten publications assessed radical prostatectomy data from men on active surveillance who had undergone preoperative MRI. Of men who chose surgery, 152 of 1070 (14%) were upstaged to T3 disease or worse, and 163 of 353 (43%) were upgraded to a Gleason score greater than 6. The likelihood of a positive MRI preoperatively was 73% (963/1326). Upgrading occurred in 43% (291/677) of cases with a positive preoperative MRI versus 27% (78/293) of men with a negative MRI preoperatively. (The denominators for these data differed because not all groups included reported data for upgrading.) Upstaging occurred in 10% (54/557) of positive MRI cases and 8% (16/194) with a negative MRI. Seven studies assessed repeat biopsy data for men on active surveillance who had undergone a prior MRI (group 2). Four studies performed MRI-targeted biopsies together with TRUS-guided biopsies, and 3 studies only performed repeat standard (TRUS) biopsy following MRI. MRI-targeted biopsies were performed using software-registered MRI/US fusion in 2 of the 4 studies, visual registered (cognitive) MRI/US fusion in 1 study, and direct in-bore in 1 study. The likelihood of a positive MRI in men undergoing active surveillance and an MRI and repeat standard (TRUS) biopsy was 70% (340/488). Following a positive MRI, reclassification occurred in 39% (115/298) of those who underwent MRI-targeted repeat biopsy with TRUS and those who underwent only TRUS for repeat biopsy versus 17% (18/107) reclassification in patients with a negative MRI before repeat biopsy. In the cases with a positive MRI and MRI-targeted and TRUS biopsy, reclassification occurred in 47% (84/179) of cases. Page 5 of 8
6 Two studies included in the review assessed whether men on active surveillance can be evaluated for disease progression over time with MRI using repeat standard biopsy. The studies defined progression differently and the criteria by which patients underwent repeat biopsy varied among study groups, making conclusions difficult. Ongoing and Unpublished Clinical Trials Some currently unpublished trials that might influence this policy are listed in Table 1. Table 1. Summary of Key Trials NCT No. Trial Name Planned Enrollment Ongoing NCT Comparison of MRI Fusion Biopsy Techniques in Men With Elevated PSA and Prior Negative Prostate Biopsy NCT MRI - Guided Biopsy for Suspicion of Locally Recurrent Prostate Cancer After External Beam Radiotherapy NCT An Evaluation of a Novel Imaging Based Complex Diagnostic and Therapeutic Pathway Intervention for Men Who Fail Radiotherapy for Prostate Cancer NCT MRI-Guided Biopsy Selection of Prostate Cancer Patients for Active Surveillance Versus Treatment: The Miami MAST Trial NCT: national clinical trial. Completion Date 400 Dec Oct Jan Oct 2017 Summary of Evidence The evidence for the use of magnetic resonance imaging targeted diagnostic or surveillance biopsy of the prostate includes numerous prospective and retrospective studies of paired cohorts and systematic reviews and meta-analyses of these studies. Relevant outcomes are overall survival, disease-specific survival, test accuracy, morbid events, and quality of life. Systematic reviews of the use of MRI-guided prostate biopsy have shown the technology may diagnose more high-grade cancers than TRUS biopsy and fewer lowgrade cancers, which may stratify patients for treatment versus active surveillance. In active surveillance, it has not been shown that this technique can detect patients who have progressed and need definitive intervention. It is unknown whether use of this technique will translate into positive clinically meaningful outcomes in terms of survival or quality of life. Further prospective evaluation of MRI-guided techniques is needed to determine whether this approach results in improved health outcomes, whether this approach would replace the standard biopsy protocol, or whether it would be performed in addition to TRUS-guided biopsy. The evidence is insufficient to determine the effects of the technology on health outcomes. References 1. Blue Cross and Blue Shield Association, Medical Policy Reference Manual, Magnetic Resonance Imaging Targeted Biopsy of the Prostate, Policy # , 10: Bjurlin MA, Meng X, Le Nobin J, et al. Optimization of prostate biopsy: the role of magnetic resonance imaging targeted biopsy in detection, localization and risk assessment. J Urol. Sep 2014;192(3): PMID Schoots IG, Roobol MJ, Nieboer D, et al. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and metaanalysis. Eur Urol. Sep 2015;68(3): PMID Page 6 of 8
7 4. Valerio M, Donaldson I, Emberton M, et al. Detection of clinically significant prostate cancer using magnetic resonance imagingultrasound fusion targeted biopsy: a systematic review. Eur Urol. Jul 2015;68(1):8-19. PMID Schoots IG, Petrides N, Giganti F, et al. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol. Apr 2015;67(4): PMID National Comprehensive Cancer Network (NCCN). Prostate Cancer V Accessed June 24, American College of Radiology (ACR). ACR Appropriateness Criteria; Prostate Cancer -Pretreatment Detection, Staging, and Surveillance. 2012; Accessed June 24, National Institute for Clinical Health and Care Excellence. Prostate Cancer: Diagnosis and Treatment, CG ; June 24, Policy History 03/03/2016 Medical Policy Committee review 03/16/2016 Medical Policy Implementation Committee approval. New Policy. Next Scheduled Review Date: 03/2017 Coding The five character codes included in the Blue Cross Blue Shield of Louisiana Medical Policy Coverage Guidelines are obtained from Current Procedural Terminology (CPT ), copyright 2015 by the American Medical Association (AMA). CPT is developed by the AMA as a listing of descriptive terms and five character identifying codes and modifiers for reporting medical services and procedures performed by physician. The responsibility for the content of Blue Cross Blue Shield of Louisiana Medical Policy Coverage Guidelines is with Blue Cross and Blue Shield of Louisiana and no endorsement by the AMA is intended or should be implied. The AMA disclaims responsibility for any consequences or liability attributable or related to any use, nonuse or interpretation of information contained in Blue Cross Blue Shield of Louisiana Medical Policy Coverage Guidelines. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for data contained or not contained herein. Any use of CPT outside of Blue Cross Blue Shield of Louisiana Medical Policy Coverage Guidelines should refer to the most current Current Procedural Terminology which contains the complete and most current listing of CPT codes and descriptive terms. Applicable FARS/DFARS apply. CPT is a registered trademark of the American Medical Association. Codes used to identify services associated with this policy may include (but may not be limited to) the following: Code Type Code CPT 55700, 55705, 55706, HCPCS No codes ICD-9 Diagnosis 185, 233.4, ICD-10 Diagnosis C61 D07.5 D40.0 *Investigational A medical treatment, procedure, drug, device, or biological product is Investigational if the effectiveness has not been clearly tested and it has not been incorporated into standard medical practice. Any determination we make that a medical treatment, procedure, drug, device, or biological product is Investigational will be based on a consideration of the following: Page 7 of 8
8 A. Whether the medical treatment, procedure, drug, device, or biological product can be lawfully marketed without approval of the FDA and whether such approval has been granted at the time the medical treatment, procedure, drug, device, or biological product is sought to be furnished; or B. Whether the medical treatment, procedure, drug, device, or biological product requires further studies or clinical trials to determine its maximum tolerated dose, toxicity, safety, effectiveness, or effectiveness as compared with the standard means of treatment or diagnosis, must improve health outcomes, according to the consensus of opinion among experts as shown by reliable evidence, including: 1. Consultation with the Blue Cross and Blue Shield Association technology assessment program (TEC) or other nonaffiliated technology evaluation center(s); 2. Credible scientific evidence published in peer-reviewed medical literature generally recognized by the relevant medical community; or 3. Reference to federal regulations. Indicated trademarks are the registered trademarks of their respective owners. NOTICE: Medical Policies are scientific based opinions, provided solely for coverage and informational purposes. Medical Policies should not be construed to suggest that the Company recommends, advocates, requires, encourages, or discourages any particular treatment, procedure, or service, or any particular course of treatment, procedure, or service. Page 8 of 8
Saturation Biopsy for Diagnosis and Staging of Prostate Cancer. Original Policy Date
 MP 7.01.101 Saturation Biopsy for Diagnosis and Staging of Prostate Cancer Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date /12/2013 Return to Medical Policy
MP 7.01.101 Saturation Biopsy for Diagnosis and Staging of Prostate Cancer Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date /12/2013 Return to Medical Policy
Corporate Medical Policy Saturation Biopsy for Diagnosis and Staging of Prostate Cancer
 Corporate Medical Policy Saturation Biopsy for Diagnosis and Staging of Prostate Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: saturation_biopsy_for_diagnosis_and_staging_of_prostate_cancer
Corporate Medical Policy Saturation Biopsy for Diagnosis and Staging of Prostate Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: saturation_biopsy_for_diagnosis_and_staging_of_prostate_cancer
Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical
 Summary. 111 Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical recurrence (BCR) is the first sign of recurrent
Summary. 111 Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical recurrence (BCR) is the first sign of recurrent
Saturation Biopsy vs. 3D Spatial Biopsy vs. Free Hand Ultrasound biopsy for Targeted Prostate Cancer Therapies
 Saturation Biopsy vs. 3D Spatial Biopsy vs. Free Hand Ultrasound biopsy for Targeted Prostate Cancer Therapies John F. Ward, MD Assistant Professor University of Texas M. D. Anderson Cancer Center Ablation
Saturation Biopsy vs. 3D Spatial Biopsy vs. Free Hand Ultrasound biopsy for Targeted Prostate Cancer Therapies John F. Ward, MD Assistant Professor University of Texas M. D. Anderson Cancer Center Ablation
7. Prostate cancer in PSA relapse
 7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
Historical Basis for Concern
 Androgens After : Are We Ready? Mohit Khera, MD, MBA Assistant Professor of Urology Division of Male Reproductive Medicine and Surgery Scott Department of Urology Baylor College of Medicine Historical
Androgens After : Are We Ready? Mohit Khera, MD, MBA Assistant Professor of Urology Division of Male Reproductive Medicine and Surgery Scott Department of Urology Baylor College of Medicine Historical
Cardiopulmonary Exercise Stress Test (CPET) Archived Medical Policy
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Health Policy Advisory Committee on Technology
 Health Policy Advisory Committee on Technology Technology Brief MRI screening for prostate cancer March 2015 State of Queensland (Queensland Department of Health) 2015 This work is licensed under a Creative
Health Policy Advisory Committee on Technology Technology Brief MRI screening for prostate cancer March 2015 State of Queensland (Queensland Department of Health) 2015 This work is licensed under a Creative
PSA Screening for Prostate Cancer Information for Care Providers
 All men should know they are having a PSA test and be informed of the implications prior to testing. This booklet was created to help primary care providers offer men information about the risks and benefits
All men should know they are having a PSA test and be informed of the implications prior to testing. This booklet was created to help primary care providers offer men information about the risks and benefits
Analysis of Prostate Cancer at Easter Connecticut Health Network Using Cancer Registry Data
 The 2014 Cancer Program Annual Public Reporting of Outcomes/Annual Site Analysis Statistical Data from 2013 More than 70 percent of all newly diagnosed cancer patients are treated in the more than 1,500
The 2014 Cancer Program Annual Public Reporting of Outcomes/Annual Site Analysis Statistical Data from 2013 More than 70 percent of all newly diagnosed cancer patients are treated in the more than 1,500
1. What is the prostate-specific antigen (PSA) test?
 1. What is the prostate-specific antigen (PSA) test? Prostate-specific antigen (PSA) is a protein produced by the cells of the prostate gland. The PSA test measures the level of PSA in the blood. The doctor
1. What is the prostate-specific antigen (PSA) test? Prostate-specific antigen (PSA) is a protein produced by the cells of the prostate gland. The PSA test measures the level of PSA in the blood. The doctor
Gleason Score. Oncotype DX GPS. identified for. about surveillance. time to get sophisticated
 patient: MARK SMITH PSA 6.2 Gleason Score 6 Oncotype DX GPS 8 identified for active surveillance time to get sophisticated about surveillance Accurate prediction of prostate cancer risk is needed at the
patient: MARK SMITH PSA 6.2 Gleason Score 6 Oncotype DX GPS 8 identified for active surveillance time to get sophisticated about surveillance Accurate prediction of prostate cancer risk is needed at the
Prostate cancer is the most common cause of death from cancer in men over age 75. Prostate cancer is rarely found in men younger than 40.
 A.D.A.M. Medical Encyclopedia. Prostate cancer Cancer - prostate; Biopsy - prostate; Prostate biopsy; Gleason score Last reviewed: October 2, 2013. Prostate cancer is cancer that starts in the prostate
A.D.A.M. Medical Encyclopedia. Prostate cancer Cancer - prostate; Biopsy - prostate; Prostate biopsy; Gleason score Last reviewed: October 2, 2013. Prostate cancer is cancer that starts in the prostate
PSA Testing 101. Stanley H. Weiss, MD. Professor, UMDNJ-New Jersey Medical School. Director & PI, Essex County Cancer Coalition. weiss@umdnj.
 PSA Testing 101 Stanley H. Weiss, MD Professor, UMDNJ-New Jersey Medical School Director & PI, Essex County Cancer Coalition weiss@umdnj.edu September 23, 2010 Screening: 3 tests for PCa A good screening
PSA Testing 101 Stanley H. Weiss, MD Professor, UMDNJ-New Jersey Medical School Director & PI, Essex County Cancer Coalition weiss@umdnj.edu September 23, 2010 Screening: 3 tests for PCa A good screening
Magnetic Resonance Imaging for Pre-Treatment Local Staging of Prostate Cancer
 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Magnetic Resonance Imaging for Pre-Treatment Local Staging of Prostate Cancer M Haider, J Salerno, A Finelli,
A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Magnetic Resonance Imaging for Pre-Treatment Local Staging of Prostate Cancer M Haider, J Salerno, A Finelli,
FAQ About Prostate Cancer Treatment and SpaceOAR System
 FAQ About Prostate Cancer Treatment and SpaceOAR System P. 4 Prostate Cancer Background SpaceOAR Frequently Asked Questions (FAQ) 1. What is prostate cancer? The vast majority of prostate cancers develop
FAQ About Prostate Cancer Treatment and SpaceOAR System P. 4 Prostate Cancer Background SpaceOAR Frequently Asked Questions (FAQ) 1. What is prostate cancer? The vast majority of prostate cancers develop
An Introduction to PROSTATE CANCER
 An Introduction to PROSTATE CANCER Being diagnosed with prostate cancer can be a life-altering experience. It requires making some very difficult decisions about treatments that can affect not only the
An Introduction to PROSTATE CANCER Being diagnosed with prostate cancer can be a life-altering experience. It requires making some very difficult decisions about treatments that can affect not only the
How To Know If You Should Get A Brachytherapy Or Radioactive Seed Implantation
 MEDICAL POLICY SUBJECT: BRACHYTHERAPY OR PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
MEDICAL POLICY SUBJECT: BRACHYTHERAPY OR PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
Targeted Biopsy in the Detection of Prostate Cancer Using an Office Based Magnetic Resonance Ultrasound Fusion Device
 Targeted Biopsy in the Detection of Prostate Cancer Using an Office Based Magnetic Resonance Ultrasound Fusion Device Geoffrey A. Sonn, Shyam Natarajan, Daniel J. A. Margolis,* Malu MacAiran, Patricia
Targeted Biopsy in the Detection of Prostate Cancer Using an Office Based Magnetic Resonance Ultrasound Fusion Device Geoffrey A. Sonn, Shyam Natarajan, Daniel J. A. Margolis,* Malu MacAiran, Patricia
Clinical Practice Guidelines
 Clinical Practice Guidelines Prostate Cancer Screening CareMore Quality Management CareMore Health System adopts Clinical Practice Guidelines for the purpose of improving health care and reducing unnecessary
Clinical Practice Guidelines Prostate Cancer Screening CareMore Quality Management CareMore Health System adopts Clinical Practice Guidelines for the purpose of improving health care and reducing unnecessary
Early Prostate Cancer: Questions and Answers. Key Points
 CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Early Prostate Cancer:
CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Early Prostate Cancer:
The 4Kscore blood test for risk of aggressive prostate cancer
 The 4Kscore blood test for risk of aggressive prostate cancer Prostate cancer tests When to use the 4Kscore Test? Screening Prior to 1 st biopsy Prior to negative previous biopsy Prognosis in Gleason 6
The 4Kscore blood test for risk of aggressive prostate cancer Prostate cancer tests When to use the 4Kscore Test? Screening Prior to 1 st biopsy Prior to negative previous biopsy Prognosis in Gleason 6
Prostate Cancer. What is prostate cancer?
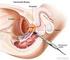 Scan for mobile link. Prostate Cancer Prostate cancer is a tumor of the prostate gland, which is located in front of the rectum and below the bladder. Your doctor may perform a physical exam, prostate-specific
Scan for mobile link. Prostate Cancer Prostate cancer is a tumor of the prostate gland, which is located in front of the rectum and below the bladder. Your doctor may perform a physical exam, prostate-specific
MEDICAL POLICY SUBJECT: PROSTATE CANCER SCREENING, DETECTION AND MONITORING
 MEDICAL POLICY PAGE: 1 OF: 8 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 8 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
Coverage Analysis: The Cornerstone of Clinical Research Billing Presented by: Mary L. Veazie, CPA, MBA, CHC, CHRC Executive Director, Clinical
 Coverage Analysis: The Cornerstone of Clinical Research Billing Presented by: Mary L. Veazie, CPA, MBA, CHC, CHRC Executive Director, Clinical Research Finance The University of Texas MD Anderson Cancer
Coverage Analysis: The Cornerstone of Clinical Research Billing Presented by: Mary L. Veazie, CPA, MBA, CHC, CHRC Executive Director, Clinical Research Finance The University of Texas MD Anderson Cancer
Role of MRI in the Diagnosis of Prostate Cancer, A Proposal
 Clinical and Experimental Medical Sciences, Vol. 1, 2013, no. 3, 111 116 HIKARI Ltd, www.m-hikari.com Role of MRI in the Diagnosis of Prostate Cancer, A Proposal W. Akhter Research Fellow Urology, Bartshealth
Clinical and Experimental Medical Sciences, Vol. 1, 2013, no. 3, 111 116 HIKARI Ltd, www.m-hikari.com Role of MRI in the Diagnosis of Prostate Cancer, A Proposal W. Akhter Research Fellow Urology, Bartshealth
Prostate Cancer. Treatments as unique as you are
 Prostate Cancer Treatments as unique as you are UCLA Prostate Cancer Program Prostate cancer is the second most common cancer among men. The UCLA Prostate Cancer Program brings together the elements essential
Prostate Cancer Treatments as unique as you are UCLA Prostate Cancer Program Prostate cancer is the second most common cancer among men. The UCLA Prostate Cancer Program brings together the elements essential
STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services
 STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v Blue Care Network of Michigan, Respondent. File
STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v Blue Care Network of Michigan, Respondent. File
NEW HYBRID IMAGING TECHNOLOGY MAY HAVE BIG POTENTIAL FOR IMPROVING DIAGNOSIS OF PROSTATE CANCER
 Media Release April 7, 2009 For Immediate Release NEW HYBRID IMAGING TECHNOLOGY MAY HAVE BIG POTENTIAL FOR IMPROVING DIAGNOSIS OF PROSTATE CANCER London, Ontario Improved hybrid imaging techniques developed
Media Release April 7, 2009 For Immediate Release NEW HYBRID IMAGING TECHNOLOGY MAY HAVE BIG POTENTIAL FOR IMPROVING DIAGNOSIS OF PROSTATE CANCER London, Ontario Improved hybrid imaging techniques developed
Prostate Cancer Treatment
 Scan for mobile link. Prostate Cancer Treatment Prostate cancer is a tumor of the prostate gland, which is located in front of the rectum and below the bladder. Your doctor may perform a physical exam,
Scan for mobile link. Prostate Cancer Treatment Prostate cancer is a tumor of the prostate gland, which is located in front of the rectum and below the bladder. Your doctor may perform a physical exam,
Advances in Diagnostic and Molecular Testing in Prostate Cancer
 Advances in Diagnostic and Molecular Testing in Prostate Cancer Ashley E. Ross MD PhD Assistant Professor Urology, Oncology, Pathology Johns Hopkins School of Medicine September 24, 2015 1 Disclosures
Advances in Diagnostic and Molecular Testing in Prostate Cancer Ashley E. Ross MD PhD Assistant Professor Urology, Oncology, Pathology Johns Hopkins School of Medicine September 24, 2015 1 Disclosures
Prostate Cancer Screening. Dr. J. McCracken, Urologist
 Prostate Cancer Screening Dr. J. McCracken, Urologist USPSTF Lifetime risk for diagnosis currently estimated at 15.9% Llifetime risk of dying of prostate cancer is 2.8% Seventy percent of deaths due to
Prostate Cancer Screening Dr. J. McCracken, Urologist USPSTF Lifetime risk for diagnosis currently estimated at 15.9% Llifetime risk of dying of prostate cancer is 2.8% Seventy percent of deaths due to
Local Coverage Determination (LCD): MolDX: Genomic Health Oncotype DX Prostate Cancer Assay (L36153)
 Local Coverage Determination (LCD): MolDX: Genomic Health Oncotype DX Prostate Cancer Assay (L36153) Contractor Information Contractor Name Palmetto GBA LCD Information Document Information LCD ID L36153
Local Coverage Determination (LCD): MolDX: Genomic Health Oncotype DX Prostate Cancer Assay (L36153) Contractor Information Contractor Name Palmetto GBA LCD Information Document Information LCD ID L36153
Guidelines for Cancer Prevention, Early detection & Screening. Prostate Cancer
 Guidelines for Cancer Prevention, Early detection & Screening Prostate Cancer Intervention Comments & Recommendations For primary prevention, it has been suggested that diets low in meat & other fatty
Guidelines for Cancer Prevention, Early detection & Screening Prostate Cancer Intervention Comments & Recommendations For primary prevention, it has been suggested that diets low in meat & other fatty
Diagnosis of Recurrent Prostate Tumor at Multiparametric Prostate MRI: Pearls and Pitfalls
 Diagnosis of Recurrent Prostate Tumor at Multiparametric Prostate MRI: Pearls and Pitfalls Mark Notley, MD; Jinxing Yu, MD; Ann S. Fulcher, MD; Mary A. Turner, MD; Don Nguyen, MD Virginia Commonwealth
Diagnosis of Recurrent Prostate Tumor at Multiparametric Prostate MRI: Pearls and Pitfalls Mark Notley, MD; Jinxing Yu, MD; Ann S. Fulcher, MD; Mary A. Turner, MD; Don Nguyen, MD Virginia Commonwealth
PROSTATE CANCER. Normal-risk men: No family history of prostate cancer No history of prior screening Not African-American
 PROSTATE CANCER 1. Guidelines for Screening Risk Factors Normal-risk men: No family history of prostate cancer No history of prior screening Not African-American High-risk men: Family history of prostate
PROSTATE CANCER 1. Guidelines for Screening Risk Factors Normal-risk men: No family history of prostate cancer No history of prior screening Not African-American High-risk men: Family history of prostate
CMScript. Member of a medical scheme? Know your guaranteed benefits! Issue 7 of 2014
 Background CMScript Member of a medical scheme? Know your guaranteed benefits! Issue 7 of 2014 Prostate cancer is second only to lung cancer as the leading cause of cancer-related deaths in men. It is
Background CMScript Member of a medical scheme? Know your guaranteed benefits! Issue 7 of 2014 Prostate cancer is second only to lung cancer as the leading cause of cancer-related deaths in men. It is
Prostate Cancer Guide. A resource to help answer your questions about prostate cancer
 Prostate Cancer Guide A resource to help answer your questions about prostate cancer Thank you for downloading this guide to prostate cancer treatment. We know that all the information provided online
Prostate Cancer Guide A resource to help answer your questions about prostate cancer Thank you for downloading this guide to prostate cancer treatment. We know that all the information provided online
Real Time MRI guided Focal Laser Ablation Therapy for Prostate Cancer
 Real Time MRI guided Focal Laser Ablation Therapy for Prostate Cancer A/Prof Celi Varol and Dr Orit Raz Uro-Oncologist Nepean and Macquarie Hospital Trial at Macquarie University Hospital Ethics board
Real Time MRI guided Focal Laser Ablation Therapy for Prostate Cancer A/Prof Celi Varol and Dr Orit Raz Uro-Oncologist Nepean and Macquarie Hospital Trial at Macquarie University Hospital Ethics board
PCa Commentary. Volume 73 January-February 2012 PSA AND TREATMENT DECISIONS:
 1101 Madison Street Suite 1101 Seattle, WA 98104 P 206-215-2480 www.seattleprostate.com PCa Commentary Volume 73 January-February 2012 CONTENTS PSA SCREENING & BASIC SCIENCE PSA AND TREATMENT 1 DECISIONS
1101 Madison Street Suite 1101 Seattle, WA 98104 P 206-215-2480 www.seattleprostate.com PCa Commentary Volume 73 January-February 2012 CONTENTS PSA SCREENING & BASIC SCIENCE PSA AND TREATMENT 1 DECISIONS
Prostatectomy, pelvic lymphadenect. Med age 63 years Mean followup 53 months No other cancer related therapy before recurrence. Negative.
 Adjuvante und Salvage Radiotherapie Ludwig Plasswilm Klinik für Radio-Onkologie, KSSG CANCER CONTROL WITH RADICAL PROSTATECTOMY ALONE IN 1,000 CONSECUTIVE PATIENTS 1983 1998 Clinical stage T1 and T2 Mean
Adjuvante und Salvage Radiotherapie Ludwig Plasswilm Klinik für Radio-Onkologie, KSSG CANCER CONTROL WITH RADICAL PROSTATECTOMY ALONE IN 1,000 CONSECUTIVE PATIENTS 1983 1998 Clinical stage T1 and T2 Mean
Us TOO University Presents: Understanding Diagnostic Testing
 Us TOO University Presents: Understanding Diagnostic Testing for Prostate Cancer Patients Today s speaker is Manish Bhandari, MD Program moderator is Pam Barrett, Us TOO International Made possible by
Us TOO University Presents: Understanding Diagnostic Testing for Prostate Cancer Patients Today s speaker is Manish Bhandari, MD Program moderator is Pam Barrett, Us TOO International Made possible by
HEALTH NEWS PROSTATE CANCER THE PROSTATE
 HEALTH NEWS PROSTATE CANCER THE PROSTATE Prostate comes from the Greek meaning to stand in front of ; this is very different than prostrate which means to lie down flat. The prostate is a walnut-sized
HEALTH NEWS PROSTATE CANCER THE PROSTATE Prostate comes from the Greek meaning to stand in front of ; this is very different than prostrate which means to lie down flat. The prostate is a walnut-sized
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
SIGNPOSTS. Along the Pathway of Prostate Cancer. Understanding Diagnostic Tests and Procedures to Monitor Prostate Cancer
 SIGNPOSTS Along the Pathway of Prostate Cancer Understanding Diagnostic Tests and Procedures to Monitor Prostate Cancer Your journey is unique like you. Signposts along the pathway can show you where you
SIGNPOSTS Along the Pathway of Prostate Cancer Understanding Diagnostic Tests and Procedures to Monitor Prostate Cancer Your journey is unique like you. Signposts along the pathway can show you where you
The 4Kscore blood test for risk of aggressive prostate cancer
 The 4Kscore blood test for risk of aggressive prostate cancer Early detection of aggressive prostate cancer Challenges Serum PSA has a high false positive rate Over 1 million prostate biopsies performed
The 4Kscore blood test for risk of aggressive prostate cancer Early detection of aggressive prostate cancer Challenges Serum PSA has a high false positive rate Over 1 million prostate biopsies performed
Jurisdiction Virginia
 PROPOSED/DRAFT Local Coverage Determination (LCD): MolDX: Prolaris Prostate Cancer Genomic Assay (DL35629) Please note: This is a Proposed/Draft policy. Proposed/Draft LCDs are works in progress that are
PROPOSED/DRAFT Local Coverage Determination (LCD): MolDX: Prolaris Prostate Cancer Genomic Assay (DL35629) Please note: This is a Proposed/Draft policy. Proposed/Draft LCDs are works in progress that are
Questions to ask my doctor: About prostate cancer
 Questions to ask my doctor: About prostate cancer Being diagnosed with prostate cancer can be scary and stressful. You probably have a lot of questions and concerns. Learning about the disease, how it
Questions to ask my doctor: About prostate cancer Being diagnosed with prostate cancer can be scary and stressful. You probably have a lot of questions and concerns. Learning about the disease, how it
Cancer research in the Midland Region the prostate and bowel cancer projects
 Cancer research in the Midland Region the prostate and bowel cancer projects Ross Lawrenson Waikato Clinical School University of Auckland MoH/HRC Cancer Research agenda Lung cancer Palliative care Prostate
Cancer research in the Midland Region the prostate and bowel cancer projects Ross Lawrenson Waikato Clinical School University of Auckland MoH/HRC Cancer Research agenda Lung cancer Palliative care Prostate
Corporate Medical Policy Intensity-Modulated Radiation Therapy (IMRT) of the Prostate
 Corporate Medical Policy Intensity-Modulated Radiation Therapy (IMRT) of the Prostate File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_the_prostate
Corporate Medical Policy Intensity-Modulated Radiation Therapy (IMRT) of the Prostate File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_the_prostate
Corporate Medical Policy
 Corporate Medical Policy Proteomics-based Testing Related to Ovarian Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteomics_based_testing_related_to_ovarian_cancer 7/2010
Corporate Medical Policy Proteomics-based Testing Related to Ovarian Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteomics_based_testing_related_to_ovarian_cancer 7/2010
Is it time for a new drug development paradigm?
 Is it time for a new drug development paradigm? Robert McDonough, M.D. Senior Director, Clinical Policy Research and Development 1 The Aetna Way Our Cause To make quality health care more affordable and
Is it time for a new drug development paradigm? Robert McDonough, M.D. Senior Director, Clinical Policy Research and Development 1 The Aetna Way Our Cause To make quality health care more affordable and
Cancer doesn t care but we do. 2010 Cancer Annual Report
 Cancer doesn t care but we do. 2010 Cancer Annual Report The Cancer Committee of CHRISTUS St. Patrick Hospital is proud to present its 2010 Annual Report. The Community Hospital Comprehensive Cancer Program
Cancer doesn t care but we do. 2010 Cancer Annual Report The Cancer Committee of CHRISTUS St. Patrick Hospital is proud to present its 2010 Annual Report. The Community Hospital Comprehensive Cancer Program
PSYCHOLOGICAL AND NEUROPSYCHOLOGICAL TESTING
 Status Active Medical and Behavioral Health Policy Section: Behavioral Health Policy Number: X-45 Effective Date: 01/22/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members
Status Active Medical and Behavioral Health Policy Section: Behavioral Health Policy Number: X-45 Effective Date: 01/22/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members
Update on Prostate Cancer: Screening, Diagnosis, and Treatment Making Sense of the Noise and Directions Forward
 Update on Prostate Cancer: Screening, Diagnosis, and Treatment Making Sense of the Noise and Directions Forward 33 rd Annual Internal Medicine Update December 5, 2015 Ryan C. Hedgepeth, MD, MS Chief of
Update on Prostate Cancer: Screening, Diagnosis, and Treatment Making Sense of the Noise and Directions Forward 33 rd Annual Internal Medicine Update December 5, 2015 Ryan C. Hedgepeth, MD, MS Chief of
Prostate Cancer Treatment: What s Best for You?
 Prostate Cancer Treatment: What s Best for You? Prostate Cancer: Radiation Therapy Approaches I. Choices There is really a variety of options in prostate cancer management overall and in radiation therapy.
Prostate Cancer Treatment: What s Best for You? Prostate Cancer: Radiation Therapy Approaches I. Choices There is really a variety of options in prostate cancer management overall and in radiation therapy.
Description of Procedure or Service. gene_based_tests_for_screening_detection_or_management_of_prostate_cancer 4/2009 8/2015 8/2016 8/2015
 Corporate Medical Policy Gene-Based Tests for Screening, Detection, and/or Management File Name: Origination: Last CAP Review: Next CAP Review: Last Review: gene_based_tests_for_screening_detection_or_management_of_prostate_cancer
Corporate Medical Policy Gene-Based Tests for Screening, Detection, and/or Management File Name: Origination: Last CAP Review: Next CAP Review: Last Review: gene_based_tests_for_screening_detection_or_management_of_prostate_cancer
Clinical Trial Design. Sponsored by Center for Cancer Research National Cancer Institute
 Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Status Active. Assistant Surgeons. This policy addresses reimbursement for assistant surgical procedures during the same operative session.
 Status Active Reimbursement Policy Section: Surgery/Interventional Procedure Policy Number: RP - Surgery/Interventional Procedure - 001 Assistant Surgeons Effective Date: June 1, 2015 Assistant Surgeons
Status Active Reimbursement Policy Section: Surgery/Interventional Procedure Policy Number: RP - Surgery/Interventional Procedure - 001 Assistant Surgeons Effective Date: June 1, 2015 Assistant Surgeons
Screening for Prostate Cancer
 Screening for Prostate Cancer It is now clear that screening for Prostate Cancer discovers the disease at an earlier and more curable stage. It is not yet clear whether this translates into reduced mortality
Screening for Prostate Cancer It is now clear that screening for Prostate Cancer discovers the disease at an earlier and more curable stage. It is not yet clear whether this translates into reduced mortality
Prostate cancer screening. It s YOUR decision!
 Prostate cancer screening It s YOUR decision! For many years now, a test has been available to screen for. The test is called the prostate-specific antigen blood test (or PSA test). It is used in combination
Prostate cancer screening It s YOUR decision! For many years now, a test has been available to screen for. The test is called the prostate-specific antigen blood test (or PSA test). It is used in combination
Robert Bristow MD PhD FRCPC
 Robert Bristow MD PhD FRCPC Clinician-Scientist and Professor, Radiation Oncology and Medical Biophysics, University of Toronto and Ontario Cancer Institute/ (UHN) Head, PMH-CFCRI Prostate Cancer Research
Robert Bristow MD PhD FRCPC Clinician-Scientist and Professor, Radiation Oncology and Medical Biophysics, University of Toronto and Ontario Cancer Institute/ (UHN) Head, PMH-CFCRI Prostate Cancer Research
PSA screening in asymptomatic men the debate continues www.bpac.org.nz keyword: psa
 PSA screening in asymptomatic men the debate continues www.bpac.org.nz keyword: psa Key messages: PSA is present in the benign and malignant prostate There is currently no national screening programme
PSA screening in asymptomatic men the debate continues www.bpac.org.nz keyword: psa Key messages: PSA is present in the benign and malignant prostate There is currently no national screening programme
GENERAL CODING. When you review old cases that were coded to unknown, make corrections based on guidelines in effect at the time of diagnosis.
 GENERAL CODING When you review old cases that were coded to unknown, make corrections based on guidelines in effect at the time of diagnosis. Exception: You must review and revise EOD coding for prostate
GENERAL CODING When you review old cases that were coded to unknown, make corrections based on guidelines in effect at the time of diagnosis. Exception: You must review and revise EOD coding for prostate
Oncology Annual Report: Prostate Cancer 2005 Update By: John Konefal, MD, Radiation Oncology
 Oncology Annual Report: Prostate Cancer 25 Update By: John Konefal, MD, Radiation Oncology Prostate cancer is the most common cancer in men, with 232,9 new cases projected to be diagnosed in the U.S. in
Oncology Annual Report: Prostate Cancer 25 Update By: John Konefal, MD, Radiation Oncology Prostate cancer is the most common cancer in men, with 232,9 new cases projected to be diagnosed in the U.S. in
2011 Radiology Diagnosis Coding Update Questions and Answers
 2011 Radiology Diagnosis Coding Update Questions and Answers How can we subscribe to the Coding Clinic for ICD-9 guidelines and updates? The American Hospital Association publishes this quarterly newsletter.
2011 Radiology Diagnosis Coding Update Questions and Answers How can we subscribe to the Coding Clinic for ICD-9 guidelines and updates? The American Hospital Association publishes this quarterly newsletter.
Bard: Prostate Cancer Treatment. Bard: Pelvic Organ Prolapse. Prostate Cancer. An overview of. Treatment. Prolapse. Information and Answers
 Bard: Prostate Cancer Treatment Bard: Pelvic Organ Prolapse Prostate Cancer An overview of Pelvic Treatment Organ Prolapse Information and Answers A Brief Overview Prostate Anatomy The prostate gland,
Bard: Prostate Cancer Treatment Bard: Pelvic Organ Prolapse Prostate Cancer An overview of Pelvic Treatment Organ Prolapse Information and Answers A Brief Overview Prostate Anatomy The prostate gland,
Issues Concerning Development of Products for Treatment of Non-Metastatic Castration- Resistant Prostate Cancer (NM-CRPC)
 Issues Concerning Development of Products for Treatment of Non-Metastatic Castration- Resistant Prostate Cancer (NM-CRPC) FDA Presentation ODAC Meeting September 14, 2011 1 Review Team Paul G. Kluetz,
Issues Concerning Development of Products for Treatment of Non-Metastatic Castration- Resistant Prostate Cancer (NM-CRPC) FDA Presentation ODAC Meeting September 14, 2011 1 Review Team Paul G. Kluetz,
Payers those financially responsible for the cost of
 Reimbursement Challenges with In Vitro Diagnostic Tests: Fitting a Square Peg into a Round Hole by Paul Radensky Payers those financially responsible for the cost of healthcare are critical market regulators
Reimbursement Challenges with In Vitro Diagnostic Tests: Fitting a Square Peg into a Round Hole by Paul Radensky Payers those financially responsible for the cost of healthcare are critical market regulators
Prostate cancer. Christopher Eden. The Royal Surrey County Hospital, Guildford & The Hampshire Clinic, Old Basing.
 Prostate cancer Christopher Eden The Royal Surrey County Hospital, Guildford & The Hampshire Clinic, Old Basing. Screening Screening men for PCa (prostate cancer) using PSA (Prostate Specific Antigen blood
Prostate cancer Christopher Eden The Royal Surrey County Hospital, Guildford & The Hampshire Clinic, Old Basing. Screening Screening men for PCa (prostate cancer) using PSA (Prostate Specific Antigen blood
Prostate Cancer Screening Guideline
 Prostate Cancer Screening Guideline Contents Prevention 2 Screening Recommendations 2 Shared Decision Making 2 Test Recommendations 3 Follow-up/Referral 3 Treatment Overview 4 Evidence Summary 4 References
Prostate Cancer Screening Guideline Contents Prevention 2 Screening Recommendations 2 Shared Decision Making 2 Test Recommendations 3 Follow-up/Referral 3 Treatment Overview 4 Evidence Summary 4 References
PHYSICIAN OFFICE BILLING INFORMATION SHEET FOR IMLYGIC (talimogene laherparepvec)
 PHYSICIAN OFFICE BILLING INFORMATION SHEET FOR IMLYGIC (talimogene laherparepvec) INDICATION IMLYGIC is a genetically modified oncolytic viral therapy indicated for the local treatment of unresectable
PHYSICIAN OFFICE BILLING INFORMATION SHEET FOR IMLYGIC (talimogene laherparepvec) INDICATION IMLYGIC is a genetically modified oncolytic viral therapy indicated for the local treatment of unresectable
Clinical Trials and Radiation Treatment. Gerard Morton Odette Cancer Centre Sunnybrook Research Institute University of Toronto
 Clinical Trials and Radiation Treatment Gerard Morton Odette Cancer Centre Sunnybrook Research Institute University of Toronto What I will cover.. A little about radiation treatment The clinical trials
Clinical Trials and Radiation Treatment Gerard Morton Odette Cancer Centre Sunnybrook Research Institute University of Toronto What I will cover.. A little about radiation treatment The clinical trials
Prostate Cancer. Patient Information
 Prostate Cancer Patient Information 1 The Prostate & Prostate Cancer The prostate is a small gland in the male reproductive system, approximately the size and shape of a walnut. It is located directly
Prostate Cancer Patient Information 1 The Prostate & Prostate Cancer The prostate is a small gland in the male reproductive system, approximately the size and shape of a walnut. It is located directly
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
The Centers for Medicare & Medicaid Services (CMS) seeks stakeholder comments on the following clinical quality measure under development:
 The Centers for Medicare & Medicaid Services (CMS) seeks stakeholder comments on the following clinical quality measure under development: Title: Non-Recommended PSA-Based Screening Description: The percentage
The Centers for Medicare & Medicaid Services (CMS) seeks stakeholder comments on the following clinical quality measure under development: Title: Non-Recommended PSA-Based Screening Description: The percentage
The PSA Controversy: Defining It, Discussing It, and Coping With It
 The PSA Controversy: Defining It, Discussing It, and Coping With It 11 TH ANNUAL SYMPOSIUM ON MEN S HEALTH June 12, 2013 The PSA Controversy Defining It, Discussing It and Coping With It As of May 2012,
The PSA Controversy: Defining It, Discussing It, and Coping With It 11 TH ANNUAL SYMPOSIUM ON MEN S HEALTH June 12, 2013 The PSA Controversy Defining It, Discussing It and Coping With It As of May 2012,
BAISHIDENG PUBLISHING GROUP INC
 Reviewer s code: 01714224 Reviewer s country: Italy Date reviewed: 2015-01-30 20:36 [ Y] Grade A: Priority publishing [ ] Accept [ ] Grade C: Good [ Y] Grade D: Fair language [ Y] Major revision The article
Reviewer s code: 01714224 Reviewer s country: Italy Date reviewed: 2015-01-30 20:36 [ Y] Grade A: Priority publishing [ ] Accept [ ] Grade C: Good [ Y] Grade D: Fair language [ Y] Major revision The article
Brain Tumor Treatment
 Scan for mobile link. Brain Tumor Treatment Brain Tumors Overview A brain tumor is a group of abnormal cells that grows in or around the brain. Tumors can directly destroy healthy brain cells. They can
Scan for mobile link. Brain Tumor Treatment Brain Tumors Overview A brain tumor is a group of abnormal cells that grows in or around the brain. Tumors can directly destroy healthy brain cells. They can
Newly Diagnosed Prostate Cancer: Understanding Your Risk
 Newly Diagnosed Prostate Cancer: Understanding Your Risk When the urologist calls with the life-changing news that your prostate biopsy is positive for prostate cancer, an office appointment is made to
Newly Diagnosed Prostate Cancer: Understanding Your Risk When the urologist calls with the life-changing news that your prostate biopsy is positive for prostate cancer, an office appointment is made to
4/8/13. Pre-test Audience Response. Prostate Cancer 2012. Screening and Treatment of Prostate Cancer: The 2013 Perspective
 Pre-test Audience Response Screening and Treatment of Prostate Cancer: The 2013 Perspective 1. I do not offer routine PSA screening, and the USPSTF D recommendation will not change my practice. 2. In light
Pre-test Audience Response Screening and Treatment of Prostate Cancer: The 2013 Perspective 1. I do not offer routine PSA screening, and the USPSTF D recommendation will not change my practice. 2. In light
Cancer in Primary Care: Prostate Cancer Screening. How and How often? Should we and in which patients?
 Cancer in Primary Care: Prostate Cancer Screening How and How often? Should we and in which patients? PLCO trial (Prostate, Lung, Colorectal and Ovarian) Results In the screening group, rates of compliance
Cancer in Primary Care: Prostate Cancer Screening How and How often? Should we and in which patients? PLCO trial (Prostate, Lung, Colorectal and Ovarian) Results In the screening group, rates of compliance
Science Highlights. To PSA or not to PSA: That is the Question.
 Science Highlights June 2012 by Ann A. Kiessling, PhD at the To PSA or not to PSA: That is the Question. The current raucous debate over the commonly used PSA blood test to screen for prostate cancer,
Science Highlights June 2012 by Ann A. Kiessling, PhD at the To PSA or not to PSA: That is the Question. The current raucous debate over the commonly used PSA blood test to screen for prostate cancer,
Implementation Date: April 2015 Clinical Operations
 National Imaging Associates, Inc. Clinical guideline PROSTATE CANCER Original Date: March 2011 Page 1 of 5 Radiation Oncology Last Review Date: March 2015 Guideline Number: NIA_CG_124 Last Revised Date:
National Imaging Associates, Inc. Clinical guideline PROSTATE CANCER Original Date: March 2011 Page 1 of 5 Radiation Oncology Last Review Date: March 2015 Guideline Number: NIA_CG_124 Last Revised Date:
Global Surgery Fact Sheet
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services R Global Surgery Fact Sheet Fact Sheet Definition of a Global Surgical Package Medicare established a national definition
DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services R Global Surgery Fact Sheet Fact Sheet Definition of a Global Surgical Package Medicare established a national definition
OBJECTIVES By the end of this segment, the community participant will be able to:
 Cancer 101: Cancer Diagnosis and Staging Linda U. Krebs, RN, PhD, AOCN, FAAN OCEAN Native Navigators and the Cancer Continuum (NNACC) (NCMHD R24MD002811) Cancer 101: Diagnosis & Staging (Watanabe-Galloway
Cancer 101: Cancer Diagnosis and Staging Linda U. Krebs, RN, PhD, AOCN, FAAN OCEAN Native Navigators and the Cancer Continuum (NNACC) (NCMHD R24MD002811) Cancer 101: Diagnosis & Staging (Watanabe-Galloway
EXHIBIT COORDINATING PROVISIONS-STATE/FEDERAL LAW, ACCREDITATION STANDARDS AND GEOGRAPHIC EXCEPTIONS MARYLAND
 EXHIBIT COORDINATING PROVISIONS-STATE/FEDERAL LAW, ACCREDITATION STANDARDS AND GEOGRAPHIC EXCEPTIONS MARYLAND I. INTRODUCTION: Scope. To the extent of any conflict between the Agreement and this State
EXHIBIT COORDINATING PROVISIONS-STATE/FEDERAL LAW, ACCREDITATION STANDARDS AND GEOGRAPHIC EXCEPTIONS MARYLAND I. INTRODUCTION: Scope. To the extent of any conflict between the Agreement and this State
Technologies To Detect Prostate Cancer
 CHAPTER 3 Technologies To Detect Prostate Cancer he most commonly used technologies for detecting and diagnosing prostate cancer are digital rectal examination (DRE), prostate-specific antigen (PSA) measurement,
CHAPTER 3 Technologies To Detect Prostate Cancer he most commonly used technologies for detecting and diagnosing prostate cancer are digital rectal examination (DRE), prostate-specific antigen (PSA) measurement,
In Practice Whole Body MR for Visualizing Metastatic Prostate Cancer
 In Practice Whole Body MR for Visualizing Metastatic Prostate Cancer Prostate cancer is the second most common cancer in men worldwide, accounting for 15% of all new cancer cases. 1 Great strides have
In Practice Whole Body MR for Visualizing Metastatic Prostate Cancer Prostate cancer is the second most common cancer in men worldwide, accounting for 15% of all new cancer cases. 1 Great strides have
Prostate Cancer Screening in Taiwan: a must
 Prostate Cancer Screening in Taiwan: a must 吳 俊 德 基 隆 長 庚 醫 院 台 灣 醫 學 會 105 th What is the PSA test? The blood level of PSA is often elevated in men with prostate cancer, and the PSA test was originally
Prostate Cancer Screening in Taiwan: a must 吳 俊 德 基 隆 長 庚 醫 院 台 灣 醫 學 會 105 th What is the PSA test? The blood level of PSA is often elevated in men with prostate cancer, and the PSA test was originally
Prostate Cancer 2014
 Prostate Cancer 2014 Eric A. Klein, M.D. Chairman Glickman Urological and Kidney Institute Professor of Surgery Cleveland Clinic Lerner College of Medicine Incidence rates, US Men Mortality Rates, US Men
Prostate Cancer 2014 Eric A. Klein, M.D. Chairman Glickman Urological and Kidney Institute Professor of Surgery Cleveland Clinic Lerner College of Medicine Incidence rates, US Men Mortality Rates, US Men
Prostate Cancer Screening. A Decision Guide
 Prostate Cancer Screening A Decision Guide This booklet was developed by the U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC). Is screening right for you?
Prostate Cancer Screening A Decision Guide This booklet was developed by the U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC). Is screening right for you?
Prostate Cancer In-Depth
 Prostate Cancer In-Depth Introduction Prostate cancer is the most common visceral malignancy among American men. In the year 2003, there are expected to be 220,000 new cases and nearly 29,000 deaths in
Prostate Cancer In-Depth Introduction Prostate cancer is the most common visceral malignancy among American men. In the year 2003, there are expected to be 220,000 new cases and nearly 29,000 deaths in
Corporate Medical Policy Brachytherapy Treatment of Breast Cancer
 Corporate Medical Policy Brachytherapy Treatment of Breast Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: brachytherapy_treatment_of_breast_cancer 7/1996 5/2015 5/2016 5/2015
Corporate Medical Policy Brachytherapy Treatment of Breast Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: brachytherapy_treatment_of_breast_cancer 7/1996 5/2015 5/2016 5/2015
Summary 1. KEY FINDINGS The Office of Technology Assessment (OTA) concludes that research has not yet been completed to determine CHAPTER
 CHAPTER 1 Summary 1 rostate cancer is a common and serious malignancy among Medicare-age men. 1 In 1995, 244,000 new cases and 40,400 deaths are anticipated from this disease; men age 65 and older bear
CHAPTER 1 Summary 1 rostate cancer is a common and serious malignancy among Medicare-age men. 1 In 1995, 244,000 new cases and 40,400 deaths are anticipated from this disease; men age 65 and older bear
MEDICARE PAYMENTS FOR DIAGNOSTIC RADIOLOGY SERVICES IN EMERGENCY DEPARTMENTS
 Department of Health and Human Services OFFICE OF INSPECTOR GENERAL MEDICARE PAYMENTS FOR DIAGNOSTIC RADIOLOGY SERVICES IN EMERGENCY DEPARTMENTS Daniel R. Levinson Inspector General April 2011 OEI-07-09-00450
Department of Health and Human Services OFFICE OF INSPECTOR GENERAL MEDICARE PAYMENTS FOR DIAGNOSTIC RADIOLOGY SERVICES IN EMERGENCY DEPARTMENTS Daniel R. Levinson Inspector General April 2011 OEI-07-09-00450
!"#$%&%'()&*+'"(,+"''*-*.
 /0'"0-'1!"#$%&%'()&*+'"(,+"''*-*.!"#$%&%'()&*)'"(+$(%,'($')#*-(.'&-+*/()&0$'(#1()&*)'"2"'.&%'-(-'&%,(+*(3'*(&*-(&4#0%(56(7'")'*%(#1(&..( -+&/*#$'-(7"#$%&%'()&*)'"$(&"'(1#0*-(+*(3'*(&/'(58(#"(#.-'"9 : (;'-+)&"'(7"#
/0'"0-'1!"#$%&%'()&*+'"(,+"''*-*.!"#$%&%'()&*)'"(+$(%,'($')#*-(.'&-+*/()&0$'(#1()&*)'"2"'.&%'-(-'&%,(+*(3'*(&*-(&4#0%(56(7'")'*%(#1(&..( -+&/*#$'-(7"#$%&%'()&*)'"$(&"'(1#0*-(+*(3'*(&/'(58(#"(#.-'"9 : (;'-+)&"'(7"#
DENOMINATOR: All patients aged 18 years and older with a diagnosis of chronic hepatitis C cirrhosis
 Measure #401: Hepatitis C: Screening for Hepatocellular Carcinoma (HCC) in Patients with Cirrhosis National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
Measure #401: Hepatitis C: Screening for Hepatocellular Carcinoma (HCC) in Patients with Cirrhosis National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
Q: What differentiates a diagnostic from a screening mammography procedure?
 The following Q&As address Medicare guidelines on the reporting of breast imaging procedures. Private payer guidelines may vary from Medicare guidelines and from payer to payer; therefore, please be sure
The following Q&As address Medicare guidelines on the reporting of breast imaging procedures. Private payer guidelines may vary from Medicare guidelines and from payer to payer; therefore, please be sure
Oncology Medical Home Measure Specification Data
 Oncology Medical Home Measure Specification Data Measure Name Chemotherapy pathway compliance Measure # 1 Measure Description % of chemotherapy treatments that have adhered to NCCN guidelines or pathways.
Oncology Medical Home Measure Specification Data Measure Name Chemotherapy pathway compliance Measure # 1 Measure Description % of chemotherapy treatments that have adhered to NCCN guidelines or pathways.
