Faster Cancer Treatment Indicators: Business Rules and Data Definitions
|
|
|
- Melina Pierce
- 8 years ago
- Views:
Transcription
1 Faster Cancer Treatment Indicators: Business Rules and Data Definitions Date: arch 2014 Version: 3 Owner: inistry of Health Cancer Services Status Final
2 Contents Purpose of this document... 4 Intended audience... 4 Authority for collection of health information... 4 Faster cancer treatment indicators... 5 Rationale... 5 Reporting against the FCT indicators... 6 Retrospective reporting... 6 Data collection responsibility... 6 Data reporting process... 7 Inclusions and exclusions for the 62 day indicator... 8 Inclusions and exclusions for the 31 day indicator... 9 FCT cancer pathway entry points Reportable data items for the FCT indicators andatory data items Primary key Data definitions Record type definition National Health Index (NHI) number definition First name definition Family name definition Date of birth definition Sex definition DHB of domicile definition Date of diagnosis definition Primary site International Classification of Diseases (ICD) definition Date of receipt of referral definition DHB of receipt of referral definition Date patient informed of diagnosis First multidisciplinary meeting (D) date definition Date of Decision-to-treat definition Date of first treatment definition Type of first treatment definition DHB of service for first treatment definition Source of referral definition SCAN definition W definition Delay code definition
3 Extract file requirements Batch file name Batch file format Null fields Dates, partial dates and date times Batch processing Batch process overview Batch send process Creating the FCT batch (input) file Header record FCT event record Sending the batch to the inistry inistry batch pre-processing Pre-processing Batch passes pre-processing Batch fails pre-processing Load into the FCT database and validate (pre-processing passed) Automated return files Data quality checks Appendix A: DHB acronyms for file naming Appendix B: Primary site ICD codes
4 Purpose of this document This document outlines the requirements for reporting the faster cancer treatment indicators (the FCT indicators). DHBs began collecting baseline data on the FCT indicators during 2012/13. During the 2014/15 financial year the 62 day indicator will replace the current Shorter waits for cancer health target. Reporting is required on both the 62 day indicator and the 31 day indicator. This document provides the updated Business Rules and Data Definitions for the FCT indicators. This sits alongside the inistry of Health s (the inistry) faster cancer treatment initiatives supporting quality care, including the development of tumour standards and pathways. The definitions for the FCT indicators are based on: National Cancer Core Data Definitions Interim Standard HISO (2011) National Health Service (Scotland) New Cancer Waiting Times Targets Data and Definitions anual (2010) National Health Service (Wales) Definitions to support the Cancer Waiting Times Service and Financial Framework (SaFF) Target (2004). National Health Index Data Dictionary v5.3 (July 2009). This document also describes the business rules required to ensure that: data from DHBs can be loaded into the inistry of Health s (the inistry s) FCT database data in the inistry s FCT database can be analysed. Intended audience The intended audience for this document are: anyone working in a DHB who is responsible for collecting and submitting FCT indicator data to the inistry Software developers designing, implementing and altering provider systems to ensure they export information in a format suitable for loading into the FCT database Business Analysts verifying that all required data elements are present and specified correctly. Authority for collection of health information The inistry may collect health information where this is necessary to carry out lawful purposes connected with the inistry s functions and activities. These purposes, functions and activities may be set out in legislation, such as the Health Act 1956, or may be derived from lawful instructions from the inister of Health. The collection, storage and use of health information is also governed by the Privacy Act 1993 and the Health Information Privacy Code
5 Faster cancer treatment indicators There are two indicators collectively referred to as the FCT indicators. Collection of data to calculate the indicators is mandatory and will be used to measure the timeliness of key points in a patient s cancer pathway. The FCT indicators are: The 62 day indicator: The maximum target length of time taken for a patient referred with o a high-suspicion 1 of cancer 2 (without a confirmed pathological diagnosis of cancer at referral) o where the triaging clinician believes the patient needs to be seen within two weeks to receive their first treatment 3 (or other management) for cancer. The 31 day indicator: The maximum target length of time a patient should have to wait from date of decision-to-treat 4 to receive their first treatment (or other management) for cancer. The 31 day indicator includes all patients who receive their first cancer treatment, irrespective of how they were initially referred. Rationale The Government is committed to Better, Sooner, ore Convenient Health Care. For the National Cancer Programme this means improving access to, and shorter waiting times for cancer treatment. Streamlined pathways of care that are based on wellcoordinated services are crucial to timely diagnosis and management of cancer. This programme takes a patient pathway approach that covers surgical and non-surgical cancer treatment. The programme aims to improve services so that over time, all patients will have access to the same quality care within the same timeframes, no matter where they live. 1 High-suspicion: means the person presents with clinical features typical of cancer, or has less typical signs and symptoms but the triaging clinician suspects that there is a high probability of cancer. 2 For the purposes of the FCT project, the term cancer is defined as the ICD10 primary diagnosis codes set out in Appendix B. 3 A patient s first treatment for cancer must be one that is publicly funded if it is to be included in FCT data reporting. 4 Decision-to-treat: The date on which the treatment plan was agreed between the patient and the clinician responsible for first treatment. 5
6 Reporting against the FCT indicators Reporting against the FCT indicators will be based on three data points. Figure 1 shows the data points that make up the start and stop points of the FCT indicators. Figure 1: Data points for the two FCT indicators Retrospective reporting The reporting to the inistry on the FCT indicators data is retrospective 5. For both indicators, reporting to the inistry occurs at the time of first treatment (or other management) for cancer. DHBs should establish their data collection and reporting systems to collect information on patients as they track through the care pathway (ie, prospectively). This information is then reported retrospectively to the inistry. Any changes to the nature of the retrospective reporting will be on an annual basis. Data collection responsibility The DHB of domicile is responsible for collecting and collating information on the data items for their patients and submitting this information via submission of a text file to the inistry, see Data reporting process below. The DHB of domicile is responsible for collecting and reporting information on their domiciled population even if it is not the DHB of: receipt of referral service. 5 Retrospective means that reporting the length of time it takes for each patient against the FCT indicators happens once the patient has started their first treatment. 6
7 Data reporting process Data is reported retrospectively, by the DHB of domicile, via a pipe delimited text file and must be submitted to the inistry via secure File Transfer Protocol (FTP). All data is reported monthly and must reach the inistry within 20 days of the end of the month in which the patient s first treatment took place. 7
8 Inclusions and exclusions for the 62 day indicator The following inclusions and exclusions are detailed for the FCT 62 day indicator. Grouping Patients are included in the 62 day indicator if they Eligibility Are eligible for treatment in New Zealand Cancer Have a pathway that pathway begins inside the NZ start point health system Have a cancer that was diagnosed publicly Have entered the cancer pathway through an acute presentation, as long as there is a subsequent referral to an outpatient clinic Have received treatment for metastatic cancer where the primary site is unknown Age of patient Are under the care of adult services and are 16 or older at the date of first cancer treatment Treatment Have received a first treatment (or other management) for cancer that is publicly funded, in the reporting period Triage Have been triaged as having a high suspicion of cancer and as having a need to be seen within two weeks Primary diagnosis Have a primary diagnosis of cancer that is included in Appendix B of this document Patients are excluded from the 62 day indicator if they Are not eligible for treatment in New Zealand Have a pathway that begins outside the New Zealand health system Have a cancer that has been diagnosed through a screening programme Have a cancer that was diagnosed privately Have entered the cancer pathway through an acute presentation, which does not subsequently result in a referral to an outpatient clinic Have a recurrent cancer (irrespective of the time frame of recurrence) Have metastatic cancer, but the patient s primary cancer has already been included in FCT reporting 6 Are not under the care of adult services and/or are not 16 or older at the date of first cancer treatment Have not received a first treatment (or other management) for cancer that is publicly funded, in the reporting period Have a first treatment for cancer that was undertaken privately Have been triaged as not having a high suspicion of cancer Have a confirmed diagnosis of cancer at triage of referral Have been triaged as not needing to be seen within two weeks Have a primary diagnosis that is not included in Appendix B of this document 6 etastatic cancers should not be recorded in FCT reporting unless the patient s primary cancer site is unknown. Such cancers should be recorded as C80 - alignant neoplasm without specification of site. 8
9 Inclusions and exclusions for the 31 day indicator The following inclusions and exclusions are detailed for the FCT 31 day indicator. Grouping Patients are included in the 31 day indicator if they Eligibility Are eligible for treatment in New Zealand Cancer Have a treatment pathway start pathway that begins point inside the New Zealand health system Have a cancer that has been diagnosed through a screening programme Have entered the cancer pathway through an acute presentation Have a cancer that was treated following it being detected incidentally 7 Have received treatment for metastatic cancer where the primary site is unknown Age of patient Are under the care of adult services and are 16 or older at the date of first cancer treatment Treatment Have received a first treatment (or other management) for cancer that is publicly funded, in the reporting period Primary diagnosis Have a primary diagnosis of cancer that is included in Appendix B of this document Patients are excluded from the 31 day indicator if they Are not eligible for treatment in New Zealand. Have a treatment pathway that begins outside the New Zealand health system Have a recurrent cancer (irrespective of the time frame of recurrence) Have metastatic cancer and the patient s primary cancer has already been included in FCT reporting Are not under the care of adult services and/or are not 16 or older at the date of first cancer treatment Have not received a first treatment (or other management) for cancer that is publicly funded, in the reporting period Have a first treatment for cancer that was undertaken privately Have a primary diagnosis that is not included in Appendix B of this document 7 An incidentally-found cancer may be diagnosed and treated on the same day. If this is the case the patient should be reported for the 31 day indicator, with the decision-to-treat date being the date that the decision-to-treat as cancer was made (which may also be the date of surgery). 9
10 FCT cancer pathway entry points Patients enter their cancer pathway through a variety of different entry points. Figure 2 shows how some of the different pathway entry points should be related to the two FCT indicators. Figure 2: Cancer pathway entry points and the FCT indicators NB - All records submitted for the 62 day indicator, should, by definition, also contain data that enables the 31 day indicator to be calculated. 10
11 Reportable data items for the FCT indicators andatory data items The mandatory () data items to be reported to the inistry for the two FCT indicators are identified as follows: andatory fields required for processing and calculation of the 62 day indicator Record type National Health Index (NHI) number First name Family name Date of birth Sex DHB of domicile Date of diagnosis Primary site ICD10 Date of receipt of referral DHB of receipt of referral Date patient informed of diagnosis Date of first multidisciplinary meeting (D) Date of decision-to-treat Date of first treatment Type of first treatment DHB of service for first treatment Source of referral Clinician defined suspicion of cancer 2 Week flag Delay code 62 Delay code 31 11
12 andatory fields required for processing and calculation of the 31 day indicator Record type National Health Index (NHI) number First name Family name Date of birth Sex DHB of domicile Date of diagnosis Primary site ICD10 Date of receipt of referral DHB of receipt of referral Date patient informed of diagnosis Date of first multidisciplinary meeting (D) Date of decision-to-treat Date of first treatment Type of first treatment DHB of service for first treatment Source of referral Clinician defined suspicion of cancer 2 Week flag Delay code 62 Delay code 31 Primary key Each record has a unique primary key consisting of: NHI_Number Primary_site_ID. The primary key is used to check for duplicates on insert, or check for existence of a record for update or delete. During the load process, the FCT database checks that the data key is unique for the records with an A (add) record type. It is possible that a record may legitimately have the same primary key as a record already stored within the FCT database, due to a patient developing a second primary cancer with the same ICD10 code. This is a rare occurrence, however if it occurs, please speak to your inistry FCT contact who can ensure the legitimate duplicate record is not rejected from the FCT database. 12
13 Data definitions Record type definition Source standards: Each FCT event record in an input file must have a record type in order to be loaded correctly N/A andatory for any submitted record Data domain: Value eaning A Add U D Update Delete Guide for use: Record_type A(1) This field denotes whether a record is to be added to, updated within, or deleted from the FCT database Add: Creates a new event if no existing record with the same primary key 8 is found on the FCT database. Update: Replaces any event with the same primary key. Delete: Deletes the record from the FCT database based on the primary key. National Health Index (NHI) number definition The NHI is a unique 7-character identification number assigned to a healthcare user by the NHI database. Source standards: National Health Index Data Dictionary v5.3 (July 2009). Guide for use: andatory for any submitted record NHI_Number AAANNNN The NHI number forms part of the primary key for the faster cancer treatment record. 8 The Primary Key is made up of two fields: NHI and primary site 13
14 First name definition The first given name of a healthcare user. Source standards: National Health Index Data Dictionary v5.3 (July 2009). Data domain: Non-mandatory N/A First_Name A(50) Family name definition The family name (surname) of a healthcare user. Source standards: National Health Index Data Dictionary v5.3 (July 2009). Non-mandatory Family_Name A(50) Date of birth definition Source standards: Data domain: The date on which the healthcare user was born. HL7 v2.4 DT date. andatory for any submitted record Valid date Date_of_Birth DDCCYY Sex definition The person s biological sex Source standards: National Health Index Data Dictionary v5.3 (July 2009). andatory for any submitted record Data domain: Value eaning F Female I U Indeterminate ale Unknown Sex A(1) 14
15 DHB of domicile definition The DHB of domicile is the code of the DHB responsible for the patient. Source standards: National Health Index Data Dictionary v5.3 (July 2009). andatory for any submitted record Data domain: Value eaning 011 Northland 021 Waitemata 022 Auckland 023 Counties anukau 031 Waikato 042 Lakes 047 Bay of Plenty 051 Tairawhiti 061 Hawkes Bay 071 Taranaki 081 idcentral 082 Whanganui 091 Capital and Coast 092 Hutt Valley 093 Wairarapa 101 Nelson arlborough 111 West Coast 121 Canterbury 123 South Canterbury 160 Southern DHB_of_ domicile NNN 15
16 Date of diagnosis definition Source standards: The date on which the patient was definitively diagnosed with a particular condition or disease. National Cancer Core Data Definitions Interim Standard HISO October (2011). Data domain: Guide for use: Non-mandatory Valid date Date_of_diagnosis DDCCYY The date of diagnosis is the date of the pathology report, if any, that first confirmed the diagnosis of cancer. This date may be found attached to a letter of referral or a patient's medical record from another institution or hospital. If this date is unavailable, or if no pathological test was done, then the date may be determined from one of the sources listed in the following sequence: 1. Date of the consultation at, or admission to, the hospital, clinic or institution when the cancer was first diagnosed. Note: do not use the admission date of the current admission if the patient had a prior diagnosis of this cancer. 2. Date of first diagnosis as stated by a recognised medical practitioner or dentist. Note: This date may be found attached to a letter of referral or a patient's medical record from an institution or hospital. 3. Date the patient states they were first diagnosed with cancer. Note: This may be the only date available in a few cases (for example, patient was first diagnosed in a foreign country). If a patient is admitted for another condition (for example a broken leg or pregnancy), and a cancer is diagnosed incidentally then the date of diagnosis is the date that the decision-to-treat as cancer was made. Primary site International Classification of Diseases (ICD) definition The Primary site ICD is the code that describes the primary site of the cancer for which the patient is being seen. Source standards: National Health Index Data Dictionary v5.3 (July 2009). Data domain: Guide for use: andatory for any submitted record Valid ICD (10th edition) codes recorded to the third digit (see appendix B). Primary_Site ANN The 3 digit ICD10 code forms part of the primary key for the faster cancer treatment record. See appendix B of this document for the list of the ICD10 codes included in the FCT initiative. 16
17 Date of receipt of referral definition Source standards: The date of receipt of referral is the date the referral is initially received into secondary care. If the referral is transferred to another district health board (DHB) the date of referral remains the date that the referral was received by the first DHB. National Cancer Core Data Definitions Interim Standard HISO October (2011). HL7 v2.4 DT date. Data domain: Guide for use: andatory for the 62 day indicator. Non-mandatory for the 31 day indicator (as the patient may not have been referred with a high suspicion of cancer with a need to be seen within two weeks). Valid date Date_of_receipt_of_referral DDCCYY As referrals are received in different formats the following provides a guide for consistency purposes Electronic referrals Best practice is for referrals to be submitted electronically. Where referrals are submitted electronically the date of receipt of referral is the date that initiates the handling / processing of the electronic referral. Letter or faxed referrals When referrals are made by letter or fax the date of receipt of referral is the date with which the referral is stamped as having first being received in secondary care. Telephone or verbal referrals When referrals are made by telephone or a face to face conversation there is a need for information to be recorded or documented so that the booking process can be initiated. The date of receipt of referral is the date recorded on that documentation of the conversation with secondary care. On occasion, there will be multiple referrals for an individual patient. Where a patient has been accepted into another care pathway a clinical decision will need to be made as to whether the newer referral overrides the current pathway or not. The clinical decision needs to be documented and will determine the date of receipt of referral. Electronic referrals must consider the Referrals, Status and Discharge Referrals (RSD) suite of standards. These provide guidance for electronic information exchange when all or part of patient care is transferred from one health care provider to another as based on HL7 V
18 DHB of receipt of referral definition The DHB of receipt of referral is the code of the DHB that received the initial referral. Source standards: National Health Index Data Dictionary v5.3 (July 2009). Data domain: Guide for use: andatory for the 62 day indicator. Non-mandatory for the 31 day indicator (as the patient may not have been referred with a high suspicion of cancer with a need to be seen within two weeks). Valid 3-digit DHB code (refer to DHB of domicile definition). DHB_of_receipt_of_referral NNN On occasion, an individual patient will have multiple referrals. Where a patient has been referred from one DHB to another DHB use the code for the DHB that first received the referral that initiated the treatment for cancer. Date patient informed of diagnosis Source standards: Data domain: Guide for use: The date the patient was informed of their diagnosis HL7 v2.4 DT date. Non-mandatory for all records Valid date Date_patient_informed_of_diagnosis DDCCYY N/A First multidisciplinary meeting (D) date definition Source standards: Data domain: Guide for use: Date on which the patient was first discussed at a D. National Cancer Core Data Definitions Interim Standard HISO October (2011). Non-mandatory for all records Valid date Date_of_First_D DDCCYY N/A 18
19 Date of Decision-to-treat definition Source standards: Data domain: Guide for use: The decision-to-treat is the date when the decision was made for the patient s treatment plan or other management plan, following discussion between the patient and the clinician responsible for treatment. National Health Service Scotland New Cancer Waiting Times Targets Data and Definitions anual (2010). National Patient Flow File Specification andatory for all submitted records Valid date Date_of_Decision_to_treat DDCCYY Where there are two possible dates, the earliest date applies. When a patient has been discussed in a D, it is in the best interests of the patient that the decision-to-treat discussion with the patient takes place as soon as possible after the D. Where decision-to-treat is not routinely collected, the date that a booking request for treatment is made can be used as a surrogate for decision-to-treat. The National Patient Flow collection requires outpatient attendance outcome decision to be reported. The date that this is recorded is to be used in the first instance. Where there is no outpatient attendance outcome decision recorded then the following dates can be used as date of decision-to-treat (for the associated treatment type) Surgery - Date booking for surgery was requested Chemotherapy / Radiotherapy (or concurrent) - Date chemotherapy or radiotherapy booking was requested Targeted therapy - Date prescription was written Non intervention management date the decision of nonintervention management was recorded in the patient s record Best supportive care date referral was written Patient declined treatment date of outpatient visit Patient died date of death 19
20 Date of first treatment definition Source standards: Data domain: Guide for use: The date for first treatment is the date that the first treatment was provided for that patient for that cancer. HL7 v2.4 DT date. andatory for all submitted records Valid date Date-of_First_Treatment DDCCYY The date of first treatment is the date the first treatment was provided or attempted but not carried out or completed for clinical reasons. For example open and shut surgery would be coded under 01 surgery. Where a patient s diagnostic biopsy is included as first treatment, because the whole tumour has been removed and the margins are clear, the date of the biopsy is the date of first treatment. Where first treatment is targeted therapy hormone therapy, the date of first treatment is considered to be the date the prescription is written for the treatment. Where the treatment is best supportive care and there is a referral to a palliative or supportive care service outside the DHB, the date the referral was written can be used as date of first treatment. If a patient dies before treatment occurs, the date of death is used as the date of first treatment. Where first treatment is an ethically approved clinical trial, the date of first treatment is considered to be the date the patient consents to be put forward for the clinical trial. Note that the following treatments could have the same date as the date of decision-to-treat: Non-intervention management Best supportive care Patient declined treatment Targeted therapy. 20
21 Type of first treatment definition Source standards: The type of first treatment is defined as the treatment or other management that attempts to begin the patient s first treatment, including palliative care or non-intervention management. Based on the: National Cancer Core Data Definitions Interim Standard HISO October (2011). National Health Service Scotland New Cancer Waiting Times Targets Data and Definitions anual (2010). andatory for all submitted records Data domain: Value eaning 00 Other 01 Surgery: exclude diagnostic procedures such as punch, incisional, needle or core biopsy 02 Radiation therapy 03 Chemotherapy 04 Targeted therapy: refers to a medication / drug that targets a specific pathway in the growth and development of a tumour 05 Non-intervention management: an expectant or observational approach pending change in the patient s circumstances. It is a period of active management not unmanaged non-treatment 06 Palliative care (including best supportive care): covers the essential services provided to patients that are not surgical, chemotherapy or radiotherapy based. These are likely to be delivered by staff trained in delivering palliative and/or supportive care. The care maybe delivered in the patient s home or in a palliative care setting. 07 Patient declined treatment 08 Patient died before treatment 09 Concurrent radiation therapy and chemotherapy 10 Clinical trial: where a patient is being treated as part of a clinical trial, irrespective of modality of treatment 99 Not recorded Type_of_First_Treatment NN 21
22 Guide for use: Patients should be included if first treatment is attempted but not carried out or completed for clinical reasons. For example open and shut surgery would be coded under 01 surgery. A diagnostic biopsy should only be included as first treatment when the whole tumour has been removed and the margins are clear. Where the treatment is a decision not to treat the record should be allocated the most appropriate of either the best supportive care or non-intervention management codes. Concurrent radiation therapy and chemotherapy refers to where both radiation and chemotherapy are given simultaneously. This is distinct to when both are given in sequence where a course of chemotherapy is followed by a course of radiotherapy (or vice versa). 22
23 DHB of service for first treatment definition The DHB of service for first treatment is the code of the DHB that provided the patient s first cancer treatment. Source standards: National Health Index Data Dictionary v5.3 (July 2009). Data domain: Guide for use: andatory for all submitted records Valid 3-digit DHB code (refer to DHB of domicile definition). DHB_of_First_Treatment NNN This is the DHB that provided the first treatment recorded in the type of first treatment field. Where first treatment is a clinical trial, the DHB of first treatment is considered to the DHB where the patient was enrolled in the clinical trial. Where first treatment is targeted therapy hormone therapy, the DHB of first treatment is considered to be the DHB where the prescription is written for the treatment. Source of referral definition The source of the referral is defined by the facility / health professional that made the referral. Source standards: National Health Service Scotland New Cancer Waiting Times Targets Data and Definitions anual (2010). Non-mandatory for all records Data domain: Value eaning 00 Other 01 Primary care clinician / practice 02 Primary dental clinician / practice 03 Accident and medical / after-hours 04 Emergency department 05 Other hospital department 06 Other hospital 07 Private specialist / hospital 08 National screening programme 09 Unknown Source_of_Referral NN 23
24 SCAN definition Source standards: Clinician defined suspicion of cancer National Patient Flow documentation as at 28 February 2014 andatory for the 62 day indicator. Non-mandatory for the 31 day indicator (as the patient may not have been referred with a high suspicion of cancer). Data domain: Value eaning The patient had a confirmed diagnosis of 10 cancer at triage 20 There is not a high suspicion of cancer 30 There is a high suspicion of cancer Guide for use: SCAN NN If set to 10 this indicates that the patient already had a confirmed pathological diagnosis of cancer at the point of triage. If set to 20 this indicates that the patient was triaged as not having a high suspicion of cancer. If set to 30 this indicates that the patient was triaged as having a high suspicion of cancer. These decisions are at the discretion of the triaging clinician. If not captured by the DHB, then set 30 as default for all records where you wish the 62 day indicator to be calculated. 2W definition Source standards: The triaging clinician has assessed that the patient needs to be seen within two weeks of the initial referral. andatory for the 62 day indicator Non-mandatory for the 31 day indicator (as the patient may not need to be seen within two weeks). Data domain: Value eaning 0 No 1 Yes 2W_flag N 24
25 Guide for use: If set to 1 this indicates that the patient was triaged as needing to be seen within two weeks. This decision is at the discretion of the triaging clinician. A DHB may be able to derive this flag from the optimal date of assessment field described in the draft National Patient Flow file specification document (as at February 2014). Otherwise if not captured by the DHB, then set 1 as default for all records where you wish the 62 day indicator to be calculated. Delay code definition When the time taken for a patient to track through the patient pathway is outside the time identified for the indicator the main reason for the delay must be reported. There is a separate delay code for each indicator, stored in two individual fields representing the two indicators, as required. Source standards: Non-mandatory for all records. Only include where there was a delay for that particular indicator. Data domain: Value eaning 1 Patient reason (chosen to delay) 2 Clinical consideration (co-morbidities) 3 Capacity constraint (resulting from lack of resources (theatre, equipment, facilities or workforce) or process constraint including administrative errors) Field Names: Delay_code_62 Delay_code_31 Guide for use: N This field should be used to indicate the reason why the timeframe was not met. The main reason for delay is the reason that contributed to the longest delay, or if there are two delays of equal length, use the first delay that occurred. Although this field is currently non-mandatory, DHBs are encouraged to report this information for all records that exceed the indicator timeframes. It is intended that reporting of this field becomes mandatory in the future. The situation will be reviewed in early If a record contains the fields to enable both the 62 day indicator and the 31 day indicator to be calculated, and both of the indicator timeframes have been exceeded, both the delay_code_62 and delay_code 31 fields should be completed for that record. 25
26 Extract file requirements Batch file name The file naming convention used to supply batches to the FCT database must consist of the following elements: three-letter acronym allocated to each sending agency by the inistry (see Appendix A for a list of the acronyms) sequential number to uniquely identify each batch file extension allocated by the inistry (.fct for FCT database upload files). For example, a typical file name for Capital Coast District Health Board would be CCH00001.fct. Batch file format The file is in ASCII format, where: only ASCII characters 32 through 127 (except for 34) are permitted records are delimited by carriage return and line feed (ASCII 13 and ASCII 10) fields are variable in length and delimited by the pipe character. Null fields If a field is not mandatory and no data is being sent, a field delimiter must be present. Dates, partial dates and date times Dates are DDCCYY unless otherwise specified Partial dates are not permitted Dates are sent as char Dates must be formatted with leading zeros. Batch processing Batch process overview The FCT data collection is the responsibility of the provider (submitting DHB). The provider is to set up and maintain batch processes to supply the data to the inistry via secure FTP. The inistry will send an acknowledgement of the data processing via an . The inistry validates and loads data, and reports from the database. Batch send process This section describes batch reporting, which will be carried out on a monthly basis. The data should reach the inistry within 20 days after the end of the month in which the patient s first treatment took place. 26
27 Creating the FCT batch (input) file The provider extracts data from their local FCT repository into a batch file (also known as the input file) to send to the inistry. Each input file must contain a header record and an unlimited number of event details records. All fields should be pipe delimited. Commas, carriage returns or other formatting must not appear in any field. (however, commas are allowed in text fields but not carriage returns or other formatting). Text fields should not be in quotes. No leading or trailing spaces are permitted unless otherwise stated. Header record Field Type Format Notes record type Char 6 A (6) HEADER file name Char 12 A (12) Including.fct extension number of records Integer The number of records, including the header, in the file eg, date sent Date 8 DDCCYY ust be a valid date and must be on or before the current date file version Char 5 ANN.N ust be V02.0 if using the specifications set out in this document. FCT event record Field Type Format Notes All files must be V02.0 for patients who are treated on or after 1 July 2014, as V01.0 files will not be accepted after this time. Records for patients with a date of first treatment prior to 1 July 2014 can be sent using either of the V01.0 or VO2.0 formats both will be loaded into the FCT database. Record_Type Text A(6) ADD, UPDATE or DELETE NHI_Number Text AAANNNN First_Name Text A(40) Optional DHB choice to include Family_Name Text A(50) Optional DHB choice to include Date_of_Birth Date/Time DDCCYY Sex Text A(1) DHB_of_Domicile Integer NNN Date_of_Diagnosis Date/Time DDCCYY Primary_Site Text ANN Date_of_Receipt_of_Referral Date/Time DDCCYY DHB_of_Receipt_of_Referral Long Integer NNN Date_Patient_Informed_of_Dx Date/Time DDCCYY Date_of_First_D Date/Time DDCCYY Date_of_Decision_to_Treat Date/Time DDCCYY Date_of_First_Treatment Date/Time DDCCYY Type_of_First_Treatment Integer NN 27
28 DHB_of_First_Treatment Integer NNN Source_of_Referral Integer N SCAN Integer NN Clinician defined suspicion of cancer 2W_flag Integer N 2 week flag Delay_Code_62 Integer N Delay_Code_31 Integer N An example of the format of the file is shown below. In this example, Nelson arlborough DHB (NH) has submitted four records using the new format (version V02.0). HEADER NH00007.fct V02.0 A ABC1234 Bob Brown C D HIJ1234 Ruth Red F C U KL5678 Gary Green C A XYZ7890 Betty Black F C Sending the batch to the inistry The batch file is sent to the inistry via secure FTP. Quarterly or monthly files are placed on the inistry s FTP server following the same mechanisms and software currently used when providing files for similar collection processes (eg, National inimum Data Set (NDS) or National Booking Reporting System (NBRS)). For FCT data, a separate directory structure called FCT has been created: \\[dhb]\fct\ inistry batch pre-processing Pre-processing The input file is initially pre-processed. This checks that the: batch is in sequence the count of records in the header equals the actual number of records in the file (including the header record) number of fields per record complies with FCT database requirements. Batch passes pre-processing If the batch passes pre-processing the data is loaded into the FCT database and an is generated to indicate how many records have been inserted, updated and deleted. 28
29 Batch fails pre-processing If the batch fails pre-processing, an is generated containing error messages indicating the cause of failure. Load into the FCT database and validate (pre-processing passed) When pre-processing is passed, the batch is loaded into the FCT database. The records are processed as follows: each delete (D) record is applied, removing it from the database each new (A) record is added to the database each update (U) record is processed by first deleting the existing record with the same primary key, and then adding the new update record. During the load process, if any record in a batch is found to contain an error, that record will not be loaded. Records with the following errors will not be loaded: if the NHI number is validated against the NHI repository and is not able to be matched, the record will not be loaded if the record is deemed to be a duplicate (based on the primary key of NHI and primary site) the record will not be loaded 9 if the record does not have a date of first treatment, or the date of first treatment is prior to 01 January 2012, or is greater than the load date the record will not be loaded if there is a missing primary site, or the primary site is out of range then the record will not be loaded. Automated return files Details to follow. Data quality checks Once each batch of data is successfully loaded into the FCT database, a series of routine data quality checks are performed, and the results are reported back to the submitting DHB. Examples include checking that: mandatory fields have been completed codes supplied for each field are within the expected code range dates are sensible and are in chronological order a range of tumour groups have been supplied a range of treatment types have been supplied a range of source of referral and delay codes have been supplied. 9 In some instances a legitimate duplicate record will need to be reported. Such a record would have a primary site and NHI combination (ie a primary key) that already exists in the database. This would occur if a patient has been reported as having two primary cancers with the same site code, which is rare, but does occur. Such a record would be rejected using the current rules. If this is the case and a DHB has a legitimate duplicate record to submit, they should inform their inistry FCT contact. 29
30 Appendix A: DHB acronyms for file naming DHB 3 letter acronym NHL YT AHC SAH HYK LLH BOP TRW HBH THL PNH GHW CCH HVH YRR NH WCO CHC HSC SRN DHB_Description Northland Waitemata Auckland Counties anukau Waikato Lakes Bay of Plenty Tairawhiti Hawkes Bay Taranaki idcentral Whanganui Capital and Coast Hutt Valley Wairarapa Nelson arlborough West Coast Canterbury South Canterbury Southern 30
31 Appendix B: Primary site ICD codes The ICD 10 codes should be recorded to the third digit for all cancers. The ICD codes to be reported are provided in the following table. Note that D codes are excluded from the FCT indicators because they relate to cancers that are low-risk, or noninvasive, or non-malignant, or low-grade, asymptomatic or indolent. ICD codes to third digit Description Cancer site group 11 Effective date C00 alignant neoplasm of lip Head and neck 01/01/2012 C01 alignant neoplasm of base of tongue Head and neck 01/01/2012 C02 alignant neoplasm of other and unspecified Head and neck 01/01/2012 parts of tongue C03 alignant neoplasm of gum Head and neck 01/01/2012 C04 alignant neoplasm floor of mouth Head and neck 01/01/2012 C05 alignant neoplasm of palate Head and neck 01/01/2012 C06 alignant neoplasm other and unspecified Head and neck 01/01/2012 parts of the mouth C07 alignant neoplasms of parotid gland Head and neck 01/01/2012 C08 alignant neoplasm other and unspecific part Head and neck 01/01/2012 of salivary gland C09 alignant neoplasm of tonsil Head and neck 01/01/2012 C10 alignant neoplasm of oropharynx Head and neck 01/01/2012 C11 alignant neoplasm of nasopharynx Head and neck 01/01/2012 C12 alignant neoplasm of pyriform sinus Head and neck 01/01/2012 C13 alignant neoplasm of hypopharynx Head and neck 01/01/2012 C14 alignant neoplasm of other and ill-defined Head and neck 01/01/2012 sites in the lip, oral cavity and pharynx C15 alignant neoplasm of oesophagus Upper gastrointestinal 01/01/2012 C16 alignant neoplasm of stomach Upper gastrointestinal 01/01/2012 C17 alignant neoplasm of small intestine Upper gastrointestinal 01/01/2012 C18 alignant neoplasm of colon Lower gastrointestinal 01/01/2012 C19 alignant neoplasm of rectosigmoid junction Lower gastrointestinal 01/01/2012 C20 alignant neoplasm of rectum Lower gastrointestinal 01/01/2012 C21 alignant neoplasm of anus and anal canal Lower gastrointestinal 01/01/2012 C22 alignant neoplasm of liver and intrahepatic Upper gastrointestinal 01/01/2012 bile ducts C23 alignant neoplasm of gallbladder Upper gastrointestinal 01/01/2012 C24 alignant neoplasm of other and unspecific Upper gastrointestinal 01/01/2012 parts of biliary tract C25 alignant neoplasm of pancreas Upper gastrointestinal 01/01/2012 C26 alignant neoplasm of other and ill-defined Lower gastrointestinal 01/01/2012 digestive organs C31 alignant neoplasm of accessory sinuses Head and neck 01/01/2012 C32 alignant neoplasm of larynx Head and neck 01/01/2012 C33 alignant neoplasm of trachea Lung 01/01/ ICD10 (A) version The cancer site group is used to group data for reporting purposes 31
32 ICD codes to third digit Description Cancer site group 11 Effective date C34 alignant neoplasm of bronchus and lung Lung 01/01/2012 C37 alignant neoplasm of thymus Other 01/01/2012 C38 alignant neoplasm of heart, mediastinum Lung 01/01/2012 and pleura, heart C39 alignant neoplasm of other and ill-defined Lung 01/01/2012 sites in the respiratory system and intrathoracic organs C40 alignant neoplasm of bone and articular Sarcoma 01/01/2012 cartilage of limbs C41 alignant neoplasm of bone and articular Sarcoma 01/01/2012 cartilage of other and unspecific sites C43 alignant melanoma of skin Skin 01/01/2012 C45 esothelioma Lung 01/01/2012 C46 Kaposi s sarcoma Sarcoma 01/01/2012 C47 alignant neoplasm of peripheral nervous Brain/CNS 01/01/2012 and autonomic nervous system C48 alignant neoplasm of retroperiotneum or Sarcoma 01/01/2012 peritoneum C49 alignant neoplasm of other connective or Sarcoma 01/01/2012 soft tissue C50 alignant neoplasm of breast Breast 01/01/2012 C51 alignant neoplasm of vulva Gynaecological 01/01/2012 C52 alignant neoplasm of vagina Gynaecological 01/01/2012 C53 alignant neoplasm of cervix uteri Gynaecological 01/01/2012 C54 alignant neoplasm of corpus uteri Gynaecological 01/01/2012 C55 alignant neoplasm of uterus, part Gynaecological 01/01/2012 unspecified C56 alignant neoplasm of ovary Gynaecological 01/01/2012 C57 alignant neoplasm of other and unspecified Gynaecological 01/01/2012 female genital organs C58 alignant neoplasm of placenta Gynaecological 01/01/2012 C60 alignant neoplasm of penis Urological 01/01/2012 C61 alignant neoplasm of prostate Urological 01/01/2012 C62 alignant neoplasm of testis Urological 01/01/2012 C63 alignant neoplasm of other and unspecified Urological 01/01/2012 male genital organs C64 alignant neoplasm of kidney, except renal Urological 01/01/2012 pelvis C65 alignant neoplasm of renal pelvis Urological 01/01/2012 C66 alignant neoplasm of ureter Urological 01/01/2012 C67 alignant neoplasm of bladder Urological 01/01/2012 C68 alignant neoplasm of other and unspecified Urological 01/01/2012 urinary organs C69 alignant neoplasm of eye and adnexa Brain/CNS 01/01/2012 C70 alignant neoplasm of meninges Brain/CNS 01/01/2012 C71 alignant neoplasm of brain Brain/CNS 01/01/2012 C72 alignant neoplasm of spinal cord, cranial Brain/CNS 01/01/2012 nerves and other parts of central nervous system C73 alignant neoplasm of thyroid gland Head and neck 01/01/
33 ICD codes to third digit Description Cancer site group 11 Effective date C74 alignant neoplasm of adrenal gland Other 01/01/2012 C75 alignant neoplasm of other endocrine Other 01/01/2012 glands and related structures C76 alignant neoplasm of other and ill-defined Other 01/01/2012 sites C80 alignant neoplasm without specification of Other 01/01/2012 site C81 Hodgkin s disease Haematological 01/01/2012 C82 Follicular lymphoma Haematological 01/04/2014 C83 Diffuse non-hodgkin s lymphoma Haematological 01/01/2012 C84 Peripheral and cutaneous T-cell lymphomas Haematological 01/01/2012 C85 Other and unspecified types of non-hodgkin s Haematological 01/01/2012 lymphoma C86 Other specified types of T/NK-cell lymphoma Haematological 01/04/2014 C88 alignant immunoproliferative diseases Haematological 01/01/2012 C90 ultiple myeloma and malignant plasma cell Haematological 01/01/2012 neoplasms C91 Lymphoid leukaemia Haematological 01/01/2012 C92 yeloid leukaemia Haematological 01/01/2012 C93 onocytic leukaemia Haematological 01/01/2012 C94 Other leukaemia of specified cell type Haematological 01/01/2012 C95 Leukaemia of unspecified cell type Haematological 01/01/2012 C96 Other and unspecified malignant neoplasms of lymphoid, haematopoietic and related tissue Haematological 01/01/
Chapter I Overview Chapter Contents
 Chapter I Overview Chapter Contents Table Number Contents I-1 Estimated New Cancer Cases and Deaths for 2005 I-2 53-Year Trends in US Cancer Death Rates I-3 Summary of Changes in Cancer Incidence and Mortality
Chapter I Overview Chapter Contents Table Number Contents I-1 Estimated New Cancer Cases and Deaths for 2005 I-2 53-Year Trends in US Cancer Death Rates I-3 Summary of Changes in Cancer Incidence and Mortality
C a nc e r C e nter. Annual Registry Report
 C a nc e r C e nter Annual Registry Report 214 214 Cancer Registry Report Larraine A. Tooker, CTR Please note that the 214 Cancer Registry Annual Report is created in 214, but it reflects data on cases
C a nc e r C e nter Annual Registry Report 214 214 Cancer Registry Report Larraine A. Tooker, CTR Please note that the 214 Cancer Registry Annual Report is created in 214, but it reflects data on cases
Table 2.2. Cohort studies of consumption of alcoholic beverages and cancer in special populations
 North America Canada Canadian 1951 Schmidt & Popham (1981) 1951 70 9 889 alcoholic men, aged 15 years, admitted to the clinical service of the Addiction Research Foundation of Ontario between Death records
North America Canada Canadian 1951 Schmidt & Popham (1981) 1951 70 9 889 alcoholic men, aged 15 years, admitted to the clinical service of the Addiction Research Foundation of Ontario between Death records
Hospital-Based Tumor Registry. Srinagarind Hospital, Khon Kaen University
 Hospital-Based Tumor Registry Srinagarind Hospital, Khon Kaen University Statistical Report 2012 Cancer Unit, Faculty of Medicine Khon Kaen University Khon Kaen, Thailand Tel & Fax:+66(43)-202485 E-mail:
Hospital-Based Tumor Registry Srinagarind Hospital, Khon Kaen University Statistical Report 2012 Cancer Unit, Faculty of Medicine Khon Kaen University Khon Kaen, Thailand Tel & Fax:+66(43)-202485 E-mail:
The effect of the introduction of ICD-10 on cancer mortality trends in England and Wales
 The effect of the introduction of ICD-10 on cancer mortality trends in Anita Brock, Clare Griffiths and Cleo Rooney, Offi ce for INTRODUCTION From January 2001 deaths in have been coded to the Tenth Revision
The effect of the introduction of ICD-10 on cancer mortality trends in Anita Brock, Clare Griffiths and Cleo Rooney, Offi ce for INTRODUCTION From January 2001 deaths in have been coded to the Tenth Revision
Number. Source: Vital Records, M CDPH
 Epidemiology of Cancer in Department of Public Health Revised April 212 Introduction The general public is very concerned about cancer in the community. Many residents believe that cancer rates are high
Epidemiology of Cancer in Department of Public Health Revised April 212 Introduction The general public is very concerned about cancer in the community. Many residents believe that cancer rates are high
Cancer in Ireland 2013: Annual report of the National Cancer Registry
 Cancer in 2013: Annual report of the National Cancer Registry ABBREVIATIONS Acronyms 95% CI 95% confidence interval APC Annual percentage change ASR Age standardised rate (European standard population)
Cancer in 2013: Annual report of the National Cancer Registry ABBREVIATIONS Acronyms 95% CI 95% confidence interval APC Annual percentage change ASR Age standardised rate (European standard population)
ENTERAL NUTRITION Policy/CPT ICD-10 ICD-10 Description ICD-9 ICD-9 Description EPA 870001100 N18.6 End stage renal disease 585.
 ENTERAL NUTRITION Policy/CPT ICD-10 ICD-10 Description ICD-9 ICD-9 Description EPA 870001100 N18.6 End stage renal disease 585.6 End stage renal disease EPA 870001101 C00.0 Malignant neoplasm of external
ENTERAL NUTRITION Policy/CPT ICD-10 ICD-10 Description ICD-9 ICD-9 Description EPA 870001100 N18.6 End stage renal disease 585.6 End stage renal disease EPA 870001101 C00.0 Malignant neoplasm of external
DELRAY MEDICAL CENTER. Cancer Program Annual Report
 DELRAY MEDICAL CENTER Cancer Program Annual Report Cancer Statistical Data From 2010 TABLE OF CONTENTS Chairman s Report....3 Tumor Registry Statistical Report Summary...4-11 Lung Study.12-17 Definitions
DELRAY MEDICAL CENTER Cancer Program Annual Report Cancer Statistical Data From 2010 TABLE OF CONTENTS Chairman s Report....3 Tumor Registry Statistical Report Summary...4-11 Lung Study.12-17 Definitions
THE CANCER CENTER 2013 ANNUAL REPORT CONTAINING 2012 STATISTICS
 THE CANCER CENTER 2013 ANNUAL REPORT CONTAINING 2012 STATISTICS Northside Medical Center Cancer Committee Mission Statement It is the mission of the Cancer Committee to evaluate and monitor the care of
THE CANCER CENTER 2013 ANNUAL REPORT CONTAINING 2012 STATISTICS Northside Medical Center Cancer Committee Mission Statement It is the mission of the Cancer Committee to evaluate and monitor the care of
CHAPTER 2. Neoplasms (C00-D49) March 2014. 2014 MVP Health Care, Inc.
 Neoplasms (C00-D49) March 2014 2014 MVP Health Care, Inc. CHAPTER SPECIFIC CATEGORY CODE BLOCKS C00-C14 Malignant neoplasms of lip, oral cavity and pharynx C15-C26 Malignant neoplasms of digestive organs
Neoplasms (C00-D49) March 2014 2014 MVP Health Care, Inc. CHAPTER SPECIFIC CATEGORY CODE BLOCKS C00-C14 Malignant neoplasms of lip, oral cavity and pharynx C15-C26 Malignant neoplasms of digestive organs
Description Code Recommendation Description Code. All natural death 001-799 IPH All natural death A00-R99
 Natural death Description Code Recommendation Description Code All natural death 001-799 IPH All natural death A00-R99 Infectious and parasitic diseases 001-139 CDC, EUROSTAT, CBS & VG Infectious and parasitic
Natural death Description Code Recommendation Description Code All natural death 001-799 IPH All natural death A00-R99 Infectious and parasitic diseases 001-139 CDC, EUROSTAT, CBS & VG Infectious and parasitic
2010 National Survey. Newham University Hospital NHS Trust
 National Cancer Patient Experience Programme 2010 National Survey Published January 2011 The National Cancer Patient Experience Survey Programme is being undertaken by Quality Health on behalf of the Department
National Cancer Patient Experience Programme 2010 National Survey Published January 2011 The National Cancer Patient Experience Survey Programme is being undertaken by Quality Health on behalf of the Department
New Zealand mortality statistics: 1950 to 2010
 Contents New Zealand mortality statistics: 1950 to 2010 Purpose 1 Overview of mortality in New Zealand 2 Deaths, raw numbers and age-standardised rates, total population, 1950 to 2010 2 Death rates from
Contents New Zealand mortality statistics: 1950 to 2010 Purpose 1 Overview of mortality in New Zealand 2 Deaths, raw numbers and age-standardised rates, total population, 1950 to 2010 2 Death rates from
REPORTABLE NEOPLASMS. Reportable List
 to Based on FY2014 codes REPORTABLE NEOPLASMS and subcategory codes are shaded in grey and marked with an ^ Cells shaded in pink and marked with an *indicate the preferred code when a single code maps
to Based on FY2014 codes REPORTABLE NEOPLASMS and subcategory codes are shaded in grey and marked with an ^ Cells shaded in pink and marked with an *indicate the preferred code when a single code maps
Cancer Conferences 2008
 2009 Annual Report Cancer Registry Report The Cancer Registry collects data on all cancer patients who were diagnosed and/or treated at East Alabama Medical Center. Diagnostic, therapeutic and outcome
2009 Annual Report Cancer Registry Report The Cancer Registry collects data on all cancer patients who were diagnosed and/or treated at East Alabama Medical Center. Diagnostic, therapeutic and outcome
CANCER COMMITTEE MEMBERS NORTHWESTERN LAKE FOREST HOSPITAL\ 2010
 CANCER COMMITTEE MEMBERS NORTHWESTERN LAKE FOREST HOSPITAL\ 2010 Michael Cochran, MD Susan Balling R.N. Karline Peal RT Stephen Ganshirt M.D. Nancy Bulzoni Emily Rosecrans Joseph Imperato M.D Linda Dickson
CANCER COMMITTEE MEMBERS NORTHWESTERN LAKE FOREST HOSPITAL\ 2010 Michael Cochran, MD Susan Balling R.N. Karline Peal RT Stephen Ganshirt M.D. Nancy Bulzoni Emily Rosecrans Joseph Imperato M.D Linda Dickson
Measure Name: Low Back Pain Imaging Studies Measure Code: LBP Lab Data: N
 Measure Name: Low Back Pain Imaging Studies Owner: NCQA (LBP) Measure Code: LBP Lab Data: N Rule Description: General Criteria Summary The percentage of patients 18-50 years of age who had a principal
Measure Name: Low Back Pain Imaging Studies Owner: NCQA (LBP) Measure Code: LBP Lab Data: N Rule Description: General Criteria Summary The percentage of patients 18-50 years of age who had a principal
NCI Community Cancer Centers Program Program Overview Ascension Health St. Vincent Indianapolis Hospital
 A. Name and location of hospital:, Indianapolis, IN B. Name of cancer center: St. Vincent Oncology Center C. Identify PI and key personnel with contact information for each pilot focus areas: a. Disparities
A. Name and location of hospital:, Indianapolis, IN B. Name of cancer center: St. Vincent Oncology Center C. Identify PI and key personnel with contact information for each pilot focus areas: a. Disparities
The Cancer Center of Chester County Annual Report 2006 With statistical data from 2005
 06 The Cancer Center of Chester County Annual Report 2006 With statistical data from 2005 2005 Statistical Report Community Screenings Screening Participants Normal Results Referred for Follow-up Colon
06 The Cancer Center of Chester County Annual Report 2006 With statistical data from 2005 2005 Statistical Report Community Screenings Screening Participants Normal Results Referred for Follow-up Colon
NEOPLASMS C00 D49. Presented by Jan Halloran CCS
 NEOPLASMS C00 D49 Presented by Jan Halloran CCS 1 INTRODUCTION A neoplasm is a new or abnormal growth. In the ICD-10-CM classification system, neoplastic disease is classified in categories C00 through
NEOPLASMS C00 D49 Presented by Jan Halloran CCS 1 INTRODUCTION A neoplasm is a new or abnormal growth. In the ICD-10-CM classification system, neoplastic disease is classified in categories C00 through
The Ontario Cancer Registry moves to the 21 st Century
 The Ontario Cancer Registry moves to the 21 st Century Rebuilding the OCR Public Health Ontario Grand Rounds Oct. 14, 2014 Diane Nishri, MSc Mary Jane King, MPH, CTR Outline 1. What is the Ontario Cancer
The Ontario Cancer Registry moves to the 21 st Century Rebuilding the OCR Public Health Ontario Grand Rounds Oct. 14, 2014 Diane Nishri, MSc Mary Jane King, MPH, CTR Outline 1. What is the Ontario Cancer
Cancer in Norway 2008
 Cancer in Norway 8 Cancer incidence, mortality, survival and prevalence in Norway Special issue: The Janus Serum Bank From sample collection to cancer research Cancer in Norway 8 Editor-in-chief: Freddie
Cancer in Norway 8 Cancer incidence, mortality, survival and prevalence in Norway Special issue: The Janus Serum Bank From sample collection to cancer research Cancer in Norway 8 Editor-in-chief: Freddie
ORGAN SYSTEMS OF THE BODY
 ORGAN SYSTEMS OF THE BODY DEFINITIONS AND CONCEPTS A. Organ a structure made up of two or more kinds of tissues organized in such a way that they can together perform a more complex function that can any
ORGAN SYSTEMS OF THE BODY DEFINITIONS AND CONCEPTS A. Organ a structure made up of two or more kinds of tissues organized in such a way that they can together perform a more complex function that can any
How To Know If You Have Cancer At Mercy Regional Medical Center
 MERCY REGIONAL CANCER CENTER 2012 CANCER PROGRAM ANNUAL REPORT Using 2011 Data Mercy Regional Cancer Center When you have cancer, you might think first of treatments chemotherapy and radiation. You want
MERCY REGIONAL CANCER CENTER 2012 CANCER PROGRAM ANNUAL REPORT Using 2011 Data Mercy Regional Cancer Center When you have cancer, you might think first of treatments chemotherapy and radiation. You want
September 2013. District health board mental health and addictions services
 September 2013 District health board mental health and addictions services Serious adverse events reported to the Health Quality & Safety Commission 1 July 2012 to 30 June 2013 Contents Introduction...
September 2013 District health board mental health and addictions services Serious adverse events reported to the Health Quality & Safety Commission 1 July 2012 to 30 June 2013 Contents Introduction...
Singapore Cancer Registry Annual Registry Report Trends in Cancer Incidence in Singapore 2009 2013. National Registry of Diseases Office (NRDO)
 Singapore Cancer Registry Annual Registry Report Trends in Cancer Incidence in Singapore 2009 2013 National Registry of Diseases Office (NRDO) Released November 3, 2014 Acknowledgement This report was
Singapore Cancer Registry Annual Registry Report Trends in Cancer Incidence in Singapore 2009 2013 National Registry of Diseases Office (NRDO) Released November 3, 2014 Acknowledgement This report was
Critical Illness with Term Assurance
 AIG Life Critical Illness with Term Assurance Our comprehensive Critical Illness with Term Assurance delivers more value and quality to the customer and their family than ever before. It is designed to
AIG Life Critical Illness with Term Assurance Our comprehensive Critical Illness with Term Assurance delivers more value and quality to the customer and their family than ever before. It is designed to
What is a Low Back Pain Imaging Tour?
 Measure Name: Low Back Pain Imaging Studies Owner: NCQA (LBP) Measure : LBP Lab Data: N Rule : Applicable Provider Specialty: General Criteria Summary The percentage of patients 18-50 years of age who
Measure Name: Low Back Pain Imaging Studies Owner: NCQA (LBP) Measure : LBP Lab Data: N Rule : Applicable Provider Specialty: General Criteria Summary The percentage of patients 18-50 years of age who
Cancer research in the Midland Region the prostate and bowel cancer projects
 Cancer research in the Midland Region the prostate and bowel cancer projects Ross Lawrenson Waikato Clinical School University of Auckland MoH/HRC Cancer Research agenda Lung cancer Palliative care Prostate
Cancer research in the Midland Region the prostate and bowel cancer projects Ross Lawrenson Waikato Clinical School University of Auckland MoH/HRC Cancer Research agenda Lung cancer Palliative care Prostate
Cancer in Cumbria Jennifer Clay Public Health Intelligence Analyst November 2008 www.cumbria.nhs.uk
 Cancer in Cumbria Jennifer Clay Public Health Intelligence Analyst November 2008 www.cumbria.nhs.uk 2 Table of contents: Summary... 4 Introduction..6 Cancer Incidence 7 Cancer Mortality....13 Cancer Survival
Cancer in Cumbria Jennifer Clay Public Health Intelligence Analyst November 2008 www.cumbria.nhs.uk 2 Table of contents: Summary... 4 Introduction..6 Cancer Incidence 7 Cancer Mortality....13 Cancer Survival
2011 ANNUAL REPORT C.H. Chub O Reilly Cancer Center. The cancer journey
 2011 ANNUAL REPORT C.H. Chub O Reilly Cancer Center The cancer journey The C.H. Chub O Reilly Cancer Center is home to the latest technology and treatments, including davinci surgery, the region s only
2011 ANNUAL REPORT C.H. Chub O Reilly Cancer Center The cancer journey The C.H. Chub O Reilly Cancer Center is home to the latest technology and treatments, including davinci surgery, the region s only
MedStar Montgomery Medical Center. Cancer Center 2014 Annual Report
 MedStar Montgomery Medical Center Cancer Center 2014 Annual Report 2014 MedStar Montgomery Medical Center Cancer Center Committee Report Cancer Committee Chair s Report According to a recent report from
MedStar Montgomery Medical Center Cancer Center 2014 Annual Report 2014 MedStar Montgomery Medical Center Cancer Center Committee Report Cancer Committee Chair s Report According to a recent report from
European Board of Radiotherapy Logbook
 European Board of Radiotherapy Logbook X:\Projekte\2005\EURALOG2005\doc\EBRLogbookUsage_en2008-07-26.doc Autor: wf Seite 1 von 24 Content Content... 2 Using the System as a Trainee... 3 Accessing the EBR
European Board of Radiotherapy Logbook X:\Projekte\2005\EURALOG2005\doc\EBRLogbookUsage_en2008-07-26.doc Autor: wf Seite 1 von 24 Content Content... 2 Using the System as a Trainee... 3 Accessing the EBR
The Price of Cancer The public price of registered cancer in New Zealand
 The Price of Cancer The public price of registered cancer in New Zealand Citation: The Price of Cancer: The public price of registered cancer in New Zealand. Wellington: Ministry of Health. Published in
The Price of Cancer The public price of registered cancer in New Zealand Citation: The Price of Cancer: The public price of registered cancer in New Zealand. Wellington: Ministry of Health. Published in
Treatment Part Two 1 FLORIDA CANCER DATA SYSTEM Treatment - Part Two
 Treatment Part Two 1 Prerequisites 2 Completion of FCDS, Introduction to Abstracting module Completion of FCDS, Treatment Part One Learning Objectives 3 Recognize cancer treatment modalities Acquire a
Treatment Part Two 1 Prerequisites 2 Completion of FCDS, Introduction to Abstracting module Completion of FCDS, Treatment Part One Learning Objectives 3 Recognize cancer treatment modalities Acquire a
Investigating Community Cancer Concerns--Deer Park Community Advisory Council, 2008
 Investigating Community Cancer Concerns--Deer Park Community Advisory Council, 2008 David R. Risser, M.P.H., Ph.D. David.Risser@dshs.state.tx.us Epidemiologist Cancer Epidemiology and Surveillance Branch
Investigating Community Cancer Concerns--Deer Park Community Advisory Council, 2008 David R. Risser, M.P.H., Ph.D. David.Risser@dshs.state.tx.us Epidemiologist Cancer Epidemiology and Surveillance Branch
Foreword. Kitisak Thepsuwan, M.D. Director of Chonburi Cancer Hospital. Ministry of Public Health. Chonburi, Thailand, 20000
 Foreword The reliable of data on the magnitude of the cancer problems are significant for monitoring the health status in the community, assessing the performance of the health care system and allowing
Foreword The reliable of data on the magnitude of the cancer problems are significant for monitoring the health status in the community, assessing the performance of the health care system and allowing
Cancer Survival - How Long Do People Survive?
 A research briefing paper by Macmillan Cancer Support Introduction Key findings 3 People with cancer are surviving longer 4 Median survival time has seen dramatic improvement for some cancers 5 Median
A research briefing paper by Macmillan Cancer Support Introduction Key findings 3 People with cancer are surviving longer 4 Median survival time has seen dramatic improvement for some cancers 5 Median
District health boards: Availability and accessibility of after-hours services
 District health boards: Availability and accessibility of after-hours services Progress in responding to the Auditor-General s recommendations Published under section 21 of the Public Audit Act 2001. June
District health boards: Availability and accessibility of after-hours services Progress in responding to the Auditor-General s recommendations Published under section 21 of the Public Audit Act 2001. June
BIO 137: CHAPTER 1 OBJECTIVES
 BIO 137: CHAPTER 1 OBJECTIVES 1. Define the terms anatomy and physiology, and explain their relationship using an example of a human structure with its corresponding function. A. ANATOMY = the study of
BIO 137: CHAPTER 1 OBJECTIVES 1. Define the terms anatomy and physiology, and explain their relationship using an example of a human structure with its corresponding function. A. ANATOMY = the study of
Diagnosis and Treatment of Common Oral Lesions Causing Pain
 Diagnosis and Treatment of Common Oral Lesions Causing Pain John D. McDowell, DDS, MS University of Colorado School of Dentistry Chair, Oral Diagnosis, Medicine and Radiology Director, Oral Medicine and
Diagnosis and Treatment of Common Oral Lesions Causing Pain John D. McDowell, DDS, MS University of Colorado School of Dentistry Chair, Oral Diagnosis, Medicine and Radiology Director, Oral Medicine and
PET (FDG) Scan for Solid Tumors PET (FDG) SCAN FOR SOLID TUMORS HS-112. Policy Number: HS-112. Original Effective Date: 6/18/2009
 Easy Choice Health Plan, Inc. Exactus Pharmacy Solutions, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Incorporated WellCare Health Insurance of Arizona, Inc., operating in Hawai i as Ohana
Easy Choice Health Plan, Inc. Exactus Pharmacy Solutions, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Incorporated WellCare Health Insurance of Arizona, Inc., operating in Hawai i as Ohana
IHS Area Cancer Mortality Rate
 CANCER MORTALITY Among Native Americans in the United States + Regional Differences in Indian Health, 984988 & Trends Over Time, 968987 Department of Health and Human Services Public Health Service Indian
CANCER MORTALITY Among Native Americans in the United States + Regional Differences in Indian Health, 984988 & Trends Over Time, 968987 Department of Health and Human Services Public Health Service Indian
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Jurisdiction Virginia. Retirement Date N/A
 Local Coverage Determination (LCD): Computerized Axial Tomography of the Chest/Thorax (L34416) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor
Local Coverage Determination (LCD): Computerized Axial Tomography of the Chest/Thorax (L34416) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor
Report series: General cancer information
 Fighting cancer with information Report series: General cancer information Eastern Cancer Registration and Information Centre ECRIC report series: General cancer information Cancer is a general term for
Fighting cancer with information Report series: General cancer information Eastern Cancer Registration and Information Centre ECRIC report series: General cancer information Cancer is a general term for
World Trade Center Health Program; Addition of Certain Types of Cancer to the List of WTC-Related Health Conditions
 This document is scheduled to be published in the Federal Register on 09/12/2012 and available online at http://federalregister.gov/a/2012-22304, and on FDsys.gov BILLING CODE: 4163-18-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 09/12/2012 and available online at http://federalregister.gov/a/2012-22304, and on FDsys.gov BILLING CODE: 4163-18-P DEPARTMENT OF
2014 Asthma Information
 2014 Asthma Information This document contains: Asthma facts and figures Table with estimated children under 15 years old who take asthma medication by region Table showing total asthma admissions and
2014 Asthma Information This document contains: Asthma facts and figures Table with estimated children under 15 years old who take asthma medication by region Table showing total asthma admissions and
.org. Metastatic Bone Disease. Description
 Metastatic Bone Disease Page ( 1 ) Cancer that begins in an organ, such as the lungs, breast, or prostate, and then spreads to bone is called metastatic bone disease (MBD). More than 1.2 million new cancer
Metastatic Bone Disease Page ( 1 ) Cancer that begins in an organ, such as the lungs, breast, or prostate, and then spreads to bone is called metastatic bone disease (MBD). More than 1.2 million new cancer
Local Coverage Determination (LCD): Immunohistochemistry (L34369)
 Local Coverage Determination (LCD): Immunohistochemistry (L34369) Contractor Information Contractor Name CGS Administrators, LLC LCD Information Document Information LCD ID L34369 Original ICD9 LCD ID
Local Coverage Determination (LCD): Immunohistochemistry (L34369) Contractor Information Contractor Name CGS Administrators, LLC LCD Information Document Information LCD ID L34369 Original ICD9 LCD ID
Our Facility. Advanced clinical center with the newest and highly exact technology for treatment of patients with cancer pencil beam
 PTC Czech The main goal of radiotherapy is to irreversibly damage tumor cells, whereas the cells of healthy tissue are damaged only reversibly or not at all. Proton therapy currently comes closest to this
PTC Czech The main goal of radiotherapy is to irreversibly damage tumor cells, whereas the cells of healthy tissue are damaged only reversibly or not at all. Proton therapy currently comes closest to this
Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012
 an Journal of Cancer (2013) 49, 1374 1403 Available at www.sciencedirect.com journalhomepage:www.ejcancer.info Cancer incidence and mortality patterns in : Estimates for 40 countries in 2012 J. Ferlay
an Journal of Cancer (2013) 49, 1374 1403 Available at www.sciencedirect.com journalhomepage:www.ejcancer.info Cancer incidence and mortality patterns in : Estimates for 40 countries in 2012 J. Ferlay
Cancer in Northeastern Pennsylvania: Incidence and Mortality of Common Cancers
 Cancer in Northeastern Pennsylvania: Incidence and Mortality of Common Cancers Samuel M. Lesko, MD, MPH Medical Director Karen Ryczak, RN Surveillance Coordinator November 2015 334 Jefferson Avenue, Scranton,
Cancer in Northeastern Pennsylvania: Incidence and Mortality of Common Cancers Samuel M. Lesko, MD, MPH Medical Director Karen Ryczak, RN Surveillance Coordinator November 2015 334 Jefferson Avenue, Scranton,
Mužík J., Dušek L., Blaha M., Klika P. Institute of Biostatistics and Analyses, Masaryk University, Brno, Czech republic
 Hospital-based data in regional and local strategies optimizing CRC control and management: real-world outcomes of the Czech National Cancer Control Programme Mužík J., Dušek L., Blaha M., Klika P. Institute
Hospital-based data in regional and local strategies optimizing CRC control and management: real-world outcomes of the Czech National Cancer Control Programme Mužík J., Dušek L., Blaha M., Klika P. Institute
CANCER WAITING TARGETS A GUIDE (VERSION 5)
 National Cancer Waits Project CANCER WAITING TARGETS A GUIDE (VERSION 5) Cancer Action Team Amendments from version 4 to version 5 The waiting times guide has been updated from version 4. Sections which
National Cancer Waits Project CANCER WAITING TARGETS A GUIDE (VERSION 5) Cancer Action Team Amendments from version 4 to version 5 The waiting times guide has been updated from version 4. Sections which
R E X C A N C E R C E N T E R. Annual Report 2012. Rex Cancer Care Committee 2012 On behalf of the Rex Cancer Center & Rex Health Care
 R E X C A N C E R C E N T E R Annual Report 2012 Rex Cancer Care Committee 2012 On behalf of the Rex Cancer Center & Rex Health Care An American College of Surgeons Commission on Cancer Accredited Comprehensive
R E X C A N C E R C E N T E R Annual Report 2012 Rex Cancer Care Committee 2012 On behalf of the Rex Cancer Center & Rex Health Care An American College of Surgeons Commission on Cancer Accredited Comprehensive
CARDCARE PROTECTOR CERTIFICATE OF INSURANCE
 CARDCARE PROTECTOR CERTIFICATE OF INSURANCE DBS Bank Ltd ( DBS ) is the master policyholder under Group Policy No. MD00000002 (the Policy ), underwritten by Manulife (Singapore) Pte. Ltd. (the Insurer
CARDCARE PROTECTOR CERTIFICATE OF INSURANCE DBS Bank Ltd ( DBS ) is the master policyholder under Group Policy No. MD00000002 (the Policy ), underwritten by Manulife (Singapore) Pte. Ltd. (the Insurer
Measure Name: URI Treatment without Antibiotics for Children Measure Code: URI Lab Data: N
 Measure Name: URI Treatment without Antibiotics for Children Owner: NCQA (URI) Measure Code: URI Lab Data: N Rule Description: General Criteria Summary The percentage of children 3 months -18 years of
Measure Name: URI Treatment without Antibiotics for Children Owner: NCQA (URI) Measure Code: URI Lab Data: N Rule Description: General Criteria Summary The percentage of children 3 months -18 years of
MEA 131 MEDICAL CODING II (CPT/HCPCS)
 MEA 131 MEDICAL CODING II (CPT/HCPCS) PRESENTED FEBRUARY 3, 2012 EFFECTIVE FALL 2012-13 Prefix & Number MEA 131 Course Title: Medical Coding II- CPT/HCPCS Purpose of this submission: New Change/Updated
MEA 131 MEDICAL CODING II (CPT/HCPCS) PRESENTED FEBRUARY 3, 2012 EFFECTIVE FALL 2012-13 Prefix & Number MEA 131 Course Title: Medical Coding II- CPT/HCPCS Purpose of this submission: New Change/Updated
BRAIN TUMOUR RESEARCH FUNDING FLOWS
 BRAIN TUMOUR RESEARCH FUNDING FLOWS Ellen Harries, Iona Joy v London, April 2013 (updated) CONTENTS 1 Research brief and headline findings 2 Research funding for cancer 3 Brain tumour funding compared
BRAIN TUMOUR RESEARCH FUNDING FLOWS Ellen Harries, Iona Joy v London, April 2013 (updated) CONTENTS 1 Research brief and headline findings 2 Research funding for cancer 3 Brain tumour funding compared
Ovarian Cancer. in Georgia, 1999-2003. Georgia Department of Human Resources Division of Public Health
 Ovarian Cancer in Georgia, 1999-23 Georgia Department of Human Resources Division of Public Health Acknowledgments Georgia Department of Human Resources......B. J. Walker, Commissioner Division of Public
Ovarian Cancer in Georgia, 1999-23 Georgia Department of Human Resources Division of Public Health Acknowledgments Georgia Department of Human Resources......B. J. Walker, Commissioner Division of Public
2008-2009 2009 ANNUAL REPORT
 2008-2009 2009 ANNUAL REPORT Utilizing 2008 Statistics MEMORIAL CANCER INSTITUTE The Leading Cancer Institute in Broward County CANCER COMMITTEE ROSTER 2008-2009 Chairman: Atif Hussein, MD Medical Director,
2008-2009 2009 ANNUAL REPORT Utilizing 2008 Statistics MEMORIAL CANCER INSTITUTE The Leading Cancer Institute in Broward County CANCER COMMITTEE ROSTER 2008-2009 Chairman: Atif Hussein, MD Medical Director,
NUMERIC INDEX... 13 CHAPTER 1: GENERAL MEDICARE INFORMATION...
 B March 3, 2015 TABLE OF CONTENTS NUMERIC INDEX... 13 CHAPTER 1: GENERAL MEDICARE INFORMATION... 31 Section 1: Conditions of Participation... 31 1.1 Definition of a Practitioner... 31 1.2 Participating
B March 3, 2015 TABLE OF CONTENTS NUMERIC INDEX... 13 CHAPTER 1: GENERAL MEDICARE INFORMATION... 31 Section 1: Conditions of Participation... 31 1.1 Definition of a Practitioner... 31 1.2 Participating
Project Scope. Background
 Project Scope Project Name: Cancer Centre Collaboration Project Project Description: Project Leader: Robert Bull Project Code: Project End Date: May 2010 Version: 6 (Draft) Version Date: 19/01/10 Background
Project Scope Project Name: Cancer Centre Collaboration Project Project Description: Project Leader: Robert Bull Project Code: Project End Date: May 2010 Version: 6 (Draft) Version Date: 19/01/10 Background
Pediatric Oncology for Otolaryngologists
 Pediatric Oncology for Otolaryngologists Frederick S. Huang, M.D. Division of Hematology/Oncology Department of Pediatrics The University of Texas Medical Branch Grand Rounds Presentation to Department
Pediatric Oncology for Otolaryngologists Frederick S. Huang, M.D. Division of Hematology/Oncology Department of Pediatrics The University of Texas Medical Branch Grand Rounds Presentation to Department
Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Paediatric Cancer Treatment
 Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Paediatric Cancer Treatment Reference: NHS England xxx/x/x 1 Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy)
Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Paediatric Cancer Treatment Reference: NHS England xxx/x/x 1 Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy)
Cancer Screening and Early Detection Guidelines
 Cancer Screening and Early Detection Guidelines Guillermo Tortolero Luna, MD, PhD Director Cancer Control and Population Sciences Program University of Puerto Rico Comprehensive Cancer Center ASPPR Clinical
Cancer Screening and Early Detection Guidelines Guillermo Tortolero Luna, MD, PhD Director Cancer Control and Population Sciences Program University of Puerto Rico Comprehensive Cancer Center ASPPR Clinical
Policy Wording. Together, all the way.
 Cancer cover Policy Wording Together, all the way. Cancer Cover Insurance Policy Wording 1. Introducing your Policy 2. What is Cancer Cover Insurance? 3. About your Policy 4. Some terms defined 5. What
Cancer cover Policy Wording Together, all the way. Cancer Cover Insurance Policy Wording 1. Introducing your Policy 2. What is Cancer Cover Insurance? 3. About your Policy 4. Some terms defined 5. What
Cancer Surgery Volume Study: ICD-9 and CPT Codes
 This paper contains the ICD-9 diagnostic and procedure codes and the CPT procedure codes used by researchers for a project of the California HealthCare Foundation (CHCF) and the California Office of Statewide
This paper contains the ICD-9 diagnostic and procedure codes and the CPT procedure codes used by researchers for a project of the California HealthCare Foundation (CHCF) and the California Office of Statewide
Advancing Health Economics, Services, Policy and Ethics. Reka Pataky, MSc ARCC Conference May 25, 2015
 Advancing Health Economics, Services, Policy and Ethics Reka Pataky, MSc ARCC Conference May 25, 2015 Acknowledgements: Ontario Team: Dr. Murray Krahn (PI) Dr. Claire de Oliveira Karen Bremner Dr. Kelvin
Advancing Health Economics, Services, Policy and Ethics Reka Pataky, MSc ARCC Conference May 25, 2015 Acknowledgements: Ontario Team: Dr. Murray Krahn (PI) Dr. Claire de Oliveira Karen Bremner Dr. Kelvin
Work-Related Disease in New Zealand
 LABOUR AND COMMERCIAL ENVIRONMENT Work-Related Disease in New Zealand The state of play in 2010 MB 12548 AUG 13 Ministry of Business, Innovation and Employment (MBIE) Hīkina Whakatutuki Lifting to make
LABOUR AND COMMERCIAL ENVIRONMENT Work-Related Disease in New Zealand The state of play in 2010 MB 12548 AUG 13 Ministry of Business, Innovation and Employment (MBIE) Hīkina Whakatutuki Lifting to make
Thymus Cancer. This reference summary will help you better understand what thymus cancer is and what treatment options are available.
 Thymus Cancer Introduction Thymus cancer is a rare cancer. It starts in the small organ that lies in the upper chest under the breastbone. The thymus makes white blood cells that protect the body against
Thymus Cancer Introduction Thymus cancer is a rare cancer. It starts in the small organ that lies in the upper chest under the breastbone. The thymus makes white blood cells that protect the body against
October is Breast Cancer Awareness Month!
 October is Breast Cancer Awareness Month! A STUDY OF CHARACTERISTICS AND MANAGEMENT OF BREAST CANCER IN TAIWAN Eric Kam-Chuan Lau, OMS II a, Jim Yu, OMSII a, Christabel Moy, OMSII a, Jian Ming Chen, MD
October is Breast Cancer Awareness Month! A STUDY OF CHARACTERISTICS AND MANAGEMENT OF BREAST CANCER IN TAIWAN Eric Kam-Chuan Lau, OMS II a, Jim Yu, OMSII a, Christabel Moy, OMSII a, Jian Ming Chen, MD
Regional Cancer Center
 Southwest Washington Medical Center Regional Cancer Center 2009 Annual Report Site Review Prostate Cancer The Paradox of Prostate Cancer Peter Wasserman, MD; Kathryn Richert-Boe, MD Southwest Regional
Southwest Washington Medical Center Regional Cancer Center 2009 Annual Report Site Review Prostate Cancer The Paradox of Prostate Cancer Peter Wasserman, MD; Kathryn Richert-Boe, MD Southwest Regional
Lung Cancer Multidisciplinary Meeting Toolkit. National Lung Cancer Working Group
 Lung Cancer Multidisciplinary Meeting Toolkit National Lung Cancer Working Group September 2014 Contents Introduction 1 Multidisciplinary meetings 1 Toolkit for implementing high-quality lung cancer MDMs
Lung Cancer Multidisciplinary Meeting Toolkit National Lung Cancer Working Group September 2014 Contents Introduction 1 Multidisciplinary meetings 1 Toolkit for implementing high-quality lung cancer MDMs
Selected Health Status Indicators DALLAS COUNTY. Jointly produced to assist those seeking to improve health care in rural Alabama
 Selected Health Status Indicators DALLAS COUNTY Jointly produced to assist those seeking to improve health care in rural Alabama By The Office of Primary Care and Rural Health, Alabama Department of Public
Selected Health Status Indicators DALLAS COUNTY Jointly produced to assist those seeking to improve health care in rural Alabama By The Office of Primary Care and Rural Health, Alabama Department of Public
PROTOCOL OF THE RITA DATA QUALITY STUDY
 PROTOCOL OF THE RITA DATA QUALITY STUDY INTRODUCTION The RITA project is aimed at estimating the burden of rare malignant tumours in Italy using the population based cancer registries (CRs) data. One of
PROTOCOL OF THE RITA DATA QUALITY STUDY INTRODUCTION The RITA project is aimed at estimating the burden of rare malignant tumours in Italy using the population based cancer registries (CRs) data. One of
Healthcare services requiring prior authorisation
 Annex 2 Healthcare requiring prior authorisation This list does not include organ transplants and does also not apply to long-term care and the primary purpose of which is to support people in need of
Annex 2 Healthcare requiring prior authorisation This list does not include organ transplants and does also not apply to long-term care and the primary purpose of which is to support people in need of
Phone: 1-877-336-3736 Fax: 1-877-556-3737 M F 8:00 am 9:00 pm ET
 QUICK REFERENCE CODING & BILLING GUIDE PHYSICIAN OFFICE CMS National Coverage Determination and Q-Code for PROVENGE Simplifies patient coverage criteria Clarifies coding requirements Expedites electronic
QUICK REFERENCE CODING & BILLING GUIDE PHYSICIAN OFFICE CMS National Coverage Determination and Q-Code for PROVENGE Simplifies patient coverage criteria Clarifies coding requirements Expedites electronic
Total Cost of Cancer Care by Site of Service: Physician Office vs Outpatient Hospital
 Total Cost of Cancer Care by Site of Service: Physician Office vs Outpatient Hospital Prepared by Avalere Health, LLC Page 2 Executive Summary Avalere Health analyzed three years of commercial health plan
Total Cost of Cancer Care by Site of Service: Physician Office vs Outpatient Hospital Prepared by Avalere Health, LLC Page 2 Executive Summary Avalere Health analyzed three years of commercial health plan
Community Health Assessment 2012
 Community Health Assessment 2012 Licking County Health Department 675 Price Road Newark, Ohio 43055 www.lickingcohealth.org Assessment Commissioned By: R. Joseph Ebel, RS, MS, MBA Health Commissioner Assessment
Community Health Assessment 2012 Licking County Health Department 675 Price Road Newark, Ohio 43055 www.lickingcohealth.org Assessment Commissioned By: R. Joseph Ebel, RS, MS, MBA Health Commissioner Assessment
Cancer in Wales. People living longer increases the number of new cancer cases
 Welsh Cancer Intelligence and Surveillance Unit, Health Intelligence Division, Public Health Wales Iechyd Cyhoeddus Cymru Public Health Wales Am y fersiwn Gymraeg ewch i Cancer in Wales A summary report
Welsh Cancer Intelligence and Surveillance Unit, Health Intelligence Division, Public Health Wales Iechyd Cyhoeddus Cymru Public Health Wales Am y fersiwn Gymraeg ewch i Cancer in Wales A summary report
STUDY PLAN FOR THE CERTIFICATE OF THE HIGHER SPECIALIZATION IN ( Diagnostic Radiology)
 STUDY PLAN FOR THE CERTIFICATE OF THE HIGHER SPECIALIZATION IN ( Diagnostic Radiology) plan number :15/11/97/NT I-GENERAL RULES AND CONDITIONS: 1- This plan conforms to the regulations of granting the
STUDY PLAN FOR THE CERTIFICATE OF THE HIGHER SPECIALIZATION IN ( Diagnostic Radiology) plan number :15/11/97/NT I-GENERAL RULES AND CONDITIONS: 1- This plan conforms to the regulations of granting the
Cancer Statistics, 2013
 CA CANCER J CLIN 2013;63:11 30 Cancer Statistics, 2013 Rebecca Siegel, MPH 1 ; Deepa Naishadham, MA, MS 2 ; Ahmedin Jemal, DVM, PhD 3 Each year, the American Cancer Society estimates the numbers of new
CA CANCER J CLIN 2013;63:11 30 Cancer Statistics, 2013 Rebecca Siegel, MPH 1 ; Deepa Naishadham, MA, MS 2 ; Ahmedin Jemal, DVM, PhD 3 Each year, the American Cancer Society estimates the numbers of new
Metastasis. Brookdale Hospital, Brooklyn, New York 11212, USA; 2 Cambridge, MA 02138, USA ma8080@gmail.com
 Metastasis Ma Hongbao 1, Margaret Ma 2, Yang Yan 1 1 Brookdale Hospital, Brooklyn, New York 11212, USA; 2 Cambridge, MA 02138, USA ma8080@gmail.com Abstract: Cancer begins when cells in a part of the body
Metastasis Ma Hongbao 1, Margaret Ma 2, Yang Yan 1 1 Brookdale Hospital, Brooklyn, New York 11212, USA; 2 Cambridge, MA 02138, USA ma8080@gmail.com Abstract: Cancer begins when cells in a part of the body
52,929,390 paid out in critical illness claims in the first six months of 2013*
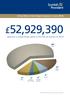 Critical Illness Report (January to June 2013) 52,929,390 paid out in critical illness claims in the first six months of 2013* 66 % Cancer 12 % Heart Attack 9 % Other 3 % Benign Brain Tumour 4 % Multiple
Critical Illness Report (January to June 2013) 52,929,390 paid out in critical illness claims in the first six months of 2013* 66 % Cancer 12 % Heart Attack 9 % Other 3 % Benign Brain Tumour 4 % Multiple
National Cancer Rehabilitation Pathways. Rachel Atkinson AHP Lead May 2011
 National Cancer Rehabilitation Pathways Rachel Atkinson AHP Lead May 2011 National Cancer and Palliative Care Rehabilitation workforce Project One of the objectives was to produce 9 tumour specific, evidence
National Cancer Rehabilitation Pathways Rachel Atkinson AHP Lead May 2011 National Cancer and Palliative Care Rehabilitation workforce Project One of the objectives was to produce 9 tumour specific, evidence
Life Insurance Application Form
 Life Insurance Application Form INSTRUCTION To be completed by all applicants PERSONAL DETAILS Surname First name Middle name Sex Female Male Marital status (please tick) Single Married Other Current residential
Life Insurance Application Form INSTRUCTION To be completed by all applicants PERSONAL DETAILS Surname First name Middle name Sex Female Male Marital status (please tick) Single Married Other Current residential
Lymphoma. Measurability of Quality Performance Indicators Version 2.0
 Lymphoma Measurability of Quality Performance Indicators Version 2.0 To be read in conjunction with: Lymphoma Clinical Quality Performance Indicators (September 2013) Lymphoma Data Definitions V2.0 (September
Lymphoma Measurability of Quality Performance Indicators Version 2.0 To be read in conjunction with: Lymphoma Clinical Quality Performance Indicators (September 2013) Lymphoma Data Definitions V2.0 (September
Patient Electronic Alert to Key-worker System (PEAKS) Guidelines
 Patient Electronic Alert to Key-worker System (PEAKS) Guidelines This procedural document supersedes: PAT/EC 4 v.1 Guidelines for Patient Electronic Alert to Key-worker systems (PEAKS). Did you print this
Patient Electronic Alert to Key-worker System (PEAKS) Guidelines This procedural document supersedes: PAT/EC 4 v.1 Guidelines for Patient Electronic Alert to Key-worker systems (PEAKS). Did you print this
AG CancerCare Platinum Plus Initial Diagnosis Benefit First occurrence Internal cancer only (one-time payment)
 AG CancerCare from American General Life Insurance Company (American General Life) is a specified disease policy that pays benefits for expenses related to the diagnosis and treatment of cancer, as well
AG CancerCare from American General Life Insurance Company (American General Life) is a specified disease policy that pays benefits for expenses related to the diagnosis and treatment of cancer, as well
Cancer Program 2008 Annual Report (With 2007 Statistical Data)
 Cancer Program 2008 Annual Report (With 2007 Statistical Data) Cancer Program Western Maryland Health System Memorial Hospital & Braddock Hospital Cumberland, Maryland MISSION The mission of the Western
Cancer Program 2008 Annual Report (With 2007 Statistical Data) Cancer Program Western Maryland Health System Memorial Hospital & Braddock Hospital Cumberland, Maryland MISSION The mission of the Western
Finnish Cancer Registry Institute for Statistical and Epidemiological Cancer Research. Survival ratios of cancer patients by area in Finland
 Survival ratios of cancer patients by area in Finland Pages 2 14 present the relative survival ratios for patients diagnosed in 2005 2012 and followed-up in 2010 2012 (see Methods p. 15) on different university
Survival ratios of cancer patients by area in Finland Pages 2 14 present the relative survival ratios for patients diagnosed in 2005 2012 and followed-up in 2010 2012 (see Methods p. 15) on different university
www.e-mercy.com Mercy Health Fairfield Hospital Annual Report on 2010 Activities Non-Hodgkin Lymphoma Outcomes Study
 www.e-mercy.com Mercy Health Fairfield Hospital Annual Report on 2010 Activities Non-Hodgkin Lymphoma Outcomes Study Cancer Program Summary The Cancer Program at Mercy Health - Fairfield Hospital has maintained
www.e-mercy.com Mercy Health Fairfield Hospital Annual Report on 2010 Activities Non-Hodgkin Lymphoma Outcomes Study Cancer Program Summary The Cancer Program at Mercy Health - Fairfield Hospital has maintained
New Hampshire Childhood Cancer
 Introduction: New Hampshire Childhood Cancer New Hampshire, Childhood Cancer, January 2009 Issue Brief Cancer in children is relatively uncommon, impacting fewer than twenty two of every 100,000 children
Introduction: New Hampshire Childhood Cancer New Hampshire, Childhood Cancer, January 2009 Issue Brief Cancer in children is relatively uncommon, impacting fewer than twenty two of every 100,000 children
How To Fill Out A Health Declaration
 The English translation has no legal force and is provided to the customer for convenience only. The Dutch health declaration should be filled in. Health declaration for occupational disability insurance
The English translation has no legal force and is provided to the customer for convenience only. The Dutch health declaration should be filled in. Health declaration for occupational disability insurance
Progressive Care Insurance for life A NEW TYPE OF INSURANCE
 Progressive Care Insurance for life A NEW TYPE OF INSURANCE New Progressive Care from Sovereign Progressive Care is a type of insurance that is new to New Zealand. It s not a traditional all-or-nothing
Progressive Care Insurance for life A NEW TYPE OF INSURANCE New Progressive Care from Sovereign Progressive Care is a type of insurance that is new to New Zealand. It s not a traditional all-or-nothing
Corporate Medical Policy
 Corporate Medical Policy Intensity Modulated Radiation Therapy (IMRT) of Head and Neck File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_head_and_neck
Corporate Medical Policy Intensity Modulated Radiation Therapy (IMRT) of Head and Neck File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_head_and_neck
