February 15,2005. Food and Drug Administration Dockets Management Branch Room Fishers Lane Rockville, MD 20852
|
|
|
- Lindsay Barrett
- 8 years ago
- Views:
Transcription
1 February 15,2005 Food and Drug Administration Dockets Management Branch Room Fishers Lane Rockville, MD Re: Citizen Petition to Request Risk Assessment of the Risks from SJS and TEN associated with ibuprofen; the Addition of Critical Safety Information relating to Serious Skin Reactions Associated with the Use of Ibuprofen to All Prescription and OTC Labeling; and Investigation into the withholding of critical safety information by McNeil Pharmaceuticals and Wyeth Consumer Healthcare. Dear Sir/Madam, Pursuant to 21 CFR 10.30, the enclosed Citizen Petition has been prepared to request that the FDA conduct a full risk assessment of the risks from SJS and TEN associated with ibuprofen, and an investigation into why McNeil Pharmaceuticals and Wyeth Consumer Healthcare withheld critical safety information from the FDA and the American public regarding the risks of SJS and TEN associated with ibuprofen. Remedies from that assessment should include the amplification of the current Ibuprofen prescription and either reconsider the OTC status of the pediatric formulation or, at minimum, enhance OTC labeling to properly reflect the increased risk of Stevens Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN) associated with ibuprofen. The current OTC labeling in the U.S. provides no information relating to the substantially increased risks of SJS and TEN associated with ibuprofen, and the prescription labeling in the U.S. fails to adequately and prominently describe the magnitude and severity of these adverse events. Neither label provides physicians or the patient information critical to reducing the risk of harm from SJS and TEN by identifying the early symptoms of SJS and TEN and to discontinue the medication at the first sign of a rash, unexplained or persistent fever, or any mucosal lesion. This instruction to physicians and patients is vital to reduce the risk and severity of SJS and TEN, and to prevent any further risks of morbidity and mortality associated with these exfoliating diseases. Please contact the undersigned if you have any questions or require additional information.
2 qc: Commissioner of DePqtment of Health and Hmum Services, AssiutantSec~~Heplth,DIIRS, Acting Director, CDEP (desk WPY) The Honombla US. Senator Ckistoplm Dodd The Bon-e U.S. 5knatur Char& E. Gmdey
3 THE UNITED STATES DEPARTMENT HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION OF February 15,2005 Citizen Petition to Request Risk Assessment of SJS and TEN; and Investigation of Withholding of Safety Information regarding Risks of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Ibuprofen products; and the Addition of Critical Safety Information Relating to Serious Skin Reactions Associated with the Use of Ibuprofen to All Prescription and OTC Labeling; Docket No. m%j Submitted by: Roger E. Salisbury, M.D. Professor of Surgery, Chief of Plastic Surgery New York Medical College Director of Burn Center at Westchester Medical Center Macy Pavillion Valhalla, New York Michael Rusty Nicar, Ph.D. Baylor University Medical Center Department of Pathology 3500 Gaston Avenue Dallas, Texas 75246
4 Randall Tackett, Ph.D. Department Clinical & Administrative Pharmacy College of Pharmacy, University of Georgia Athens, Georgia Steven Pliskow, M.D., FACOG, FACFE Clinical Professor of Obstetrics and Gynecology Nova South Eastern University 603 Village Blvd., Suite 201 West Palm Beach, Florida Darlene and Andrew Kiss 54 Overlea Lane Aberdeen, New Jersey Steve and Christy Esnard 3515 N. Ripples Court Missouri City, Texas LaSandra and Level1 Madden 4938 Mill Place, # 359 Dallas, Texas 75210
5 CITIZEN PETITION I. Introduction and Action Requested Roger E. Salisbury, M.D., Michael Rusty Nicar, Ph.D., Randall Tackett, Ph.D., Steven Pliskow, M.D., Darlene and Andrew Kiss, and other parents of victims of SJS and TEN caused by ibuprofen products submit this petition to request action by the Food and Drug Administration (FDA) relating to the drug product, ibuprofen (Motrin, Advil, etc.). Further, petitioners are requesting that a full risk assessment of SJS and TEN associated with ibuprofen be conducted by the FDA. Petitioners are further seeking the FDA to conduct an investigation into the withholding of critical safety information regarding the risks of SJS and TEN associated with ibuprofen by McNeil Pharmaceuticals, manufacturer of Motin products, and Wyeth Consumer Healthcare, manufacturer of Advil products, both from the FDA and the American public. Additionally, the petitioners request that FDA require the manufacturers of ibuprofen to amplify their prescription and OTC labeling to adequately warn prescribers, health care professionals and consumers of the increased risk of Stevens Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) associated with ibuprofen that has been established in the scientific literature since 1978 through the present. Further, petitioners request that additional warnings and instructions be provided in the Warnings and Precautions section of any prescription labeling for all ibuprofen products sold in 1
6 the U.S. to include a specific warning about the risk of serious skin reactions like SJS and TEN, and to disclose to physicians and consumers instructions to discontinue any/all ibuprofen product(s) at the first sign of a rash, mucosal blisters or sores in the mouth, eyes, throat, or genitalia, and any unexplained or persistent fever. The proposed actions include the addition of a bolded Black Box Warning in the prescription labeling to comply with 2 1 CFR , and the dissemination of Dear Doctor and Dear Healthcare Professional letters to alert prescribers, pharmacists, medical associations, burn centers, and hospitals to this critically important information. The Petition also requests amplification of multiple sections of the OTC label for ibuprofen products, including placement of critical information regarding the risks of SJS and TEN associated with ibuprofen in the Warnings and Stop Use sections on all ibuprofen products sold in the U.S. (21 CFR ) This Petition is submitted pursuant to 21 CFR 10.30, and relates to Sections 201(n), 502 (a), 502 (f)(l) and 505 of the Federal Food, Drug and Cosmetic Act; and 21 CFR and 21 CFR II. Statement of Factual Grounds A. The Current Ibuprofen Labeling Fails to Comply with FDA Labeling Requirements
7 The Federal Food, Drug and Cosmetic Act and the Code of Federal Regulations provide specific requirements for the content and format of labeling materials relating to prescription drug products. Pursuant to 2 1 CFR 20 1 S7, the Warnings section must describe all serious adverse reactions and potential safety hazards, limitations in use imposed by them, and actions to be taken if these events should occur. Events leading to serious injury or death may be placed in the Warnings section in a prominently displayed box for added emphasis. FDA regulations note that pharmaceutical product labeling must be revised to include a warning as soon as there is reasonable evidence of an association of a serious hazard with a drug. A causal relationship need not have been provided. In order to alert prescribers to new warnings as soon as possible, FDA also permits manufacturers to add new warnings to their labeling without first securing FDA approval (2 1 CFR ). The current labeling for prescription ibuprofen products does not provide any information relating to SJS and TEN in the Warnings (or even the Precautions) section of the insert. These sections are required to include the most critical information relating to product safety and the steps to be taken to ensure safe product use. Obviously, these sections of the package insert for prescription ibuprofen products should describe the increased risk of SJS and TEN associated with ibuprofen that has been established in the scientific literature, and in conjunction with the rising numbers of serious skin reactions associated with 3
8 ibuprofen use and precautionary procedures to halt progression of these events. Based on available literature and adverse event data, this information should have been added to the Warnings section of the labeling several years ago. Because toxic epidermal necrolysis has a 30% mortality rate and has been reported to be as high as 80% in certain populations, it is important to provide warnings and instructions in the prescription package inserts for ibuprofen products to alert the prescriber to these risks and steps to reduce the harm from these life-threatening and fatal reactions. Other prescription package inserts have such warnings and precautions, even though their risks of SJS and TEN are lower than ibuprofen. For example, Zithromax (azithromycin) carries a SJS and TEN warning, but the scientific literature reports that it has a lower relative risk for SJS and TEN than ibuprofen. Moreover, the FDA has approved a black box warning for the COX-II NSAID Bextra to specifically warn about the risk of SJS and TEN associated with Bextra, and we believe that the same warning should be placed on prescription ibuprofen products based on the scientific evidence that is available in the spontaneous adverse event databases and the scientific literature. SJS and TEN are adverse events that are noted in the package insert for prescription ibuprofen products in the in the Adverse Reactions section. However, there is no discussion or information relating to the magnitude and severity of these events in the Warnings section, or any discussion about the increased risks of S JS 4
9 and TEN that as been established in the scientific literature, as well as the rise in case reports in the scientific evidence observed over the last several years. The precautions to be taken to halt the progression of early symptoms of SJS and TEN are also not specifically described in any ibuprofen labeling, whether prescription or OTC. FDA laws and regulations also specify the format and content of OTC product labeling pursuant to 21 CFR Because medical intervention does not usually accompany OTC use, specific sections are available in OTC labeling to assist patients with recognition of potentially serious adverse events and to understand those situations in which drug use should be stopped and medical attention should be sought. Unfortunately, the current U.S. OTC labeling for ibuprofen products does not provide any description or information relating to EM, SJS, or TEN. As such, patients are not alerted to the severity of these skin reactions and are not instructed to discontinue all ibuprofen use if/when any of the early symptoms of SJS and TEN occur. Ibuprofen manufacturers cannot reasonably argue that the current Allergy Alert in the OTC label is in any way related to SJS and TEN. A review of the New Drug Applications submitted by these manufacturers confirms that the FDA considered the allergy alert to be intended for anaphylaxis related events which occur in aspirin sensitive patients. The allergy alert statements on the ibuprofen 5
10 labeling are not directed to SJS/TEN events. However, if aspirin sensitivity is deemed to necessitate a Warning statement, then the ibuprofen-related SJS/TEN events certainly warrant similar labeling attention. After all, there are significantly more skin-related events than allergic reactions associated with ibuprofen use. Both the prescription and OTC labels employed in the U.S. fail to comply with the labeling requirements needed to ensure patient safety. Paragraph C provides an analysis of the international labeling employed with ibuprofen products. It will be seen that U.S. prescribers and patients are provided with substantially less information relating to SJS and TEN than patients receiving ibuprofen outside the United States. Furthermore, to our knowledge, McNeil Pharmaceuticals and Wyeth Consumer Healthcare have not filed this foreign labeling with the FDA in their Annual reports for their respective pediatric ibuprofen products to alert the FDA that they employ different labeling in foreign countries. B. The Medical and Scientific Literature Confirm Causal Relationship between SJS and TEN associated with Ibuprofen, as well as increasing risks of Serious Skin Reactions Associated with Ibuprofen Use. Ibuprofen is an effective drug that is generally well tolerated in adults and children. It is effective for pain and inflammation in adults and for fever in children. FDA has rejected the applications for the pain indication for pediatric ibuprofen suspension submitted by McNeil and Wyeth in the past. However, using a new federal regulation instituted in 1994, both applications were approved by the 6
11 Agency based on extrapolation of data from adult studies of ibuprofen, and the pediatric formulation of ibuprofen was approved for prescription and OTC use for pain and fever. Under the supervision of a physician, the use of ibuprofen in treating fever should be reserved for those children with high or long-lasting fever. Under a physician s care, early signs of severe morbidity (eg., renal failure, toxic skin reactions) could be monitored for, and when they occur, the drug could be rapidly withdrawn and treatment commenced. OTC status for treatment of corm-non fever in children, especially otherwise healthy children, affords a different benefit-risk balance. This benefit-risk would be quite favorable, even given the discretionary nature of mild - moderate fever pharmacotherapy, if it were not for two rare, but very severe adverse drug reactions, SJS and TEN. Based on the review of NDAs, scientific literature and other relevant materials, we believe that substantial evidence for the causal relationship between ibuprofen and SJS and TEN existed when the first prescription pediatric formulations Pediaprofen and Children s Advil were approved in Substantial confirmation of that causal relationship has continued to accumulate over the ensuing years both in the scientific literature and in pharrnacoepiderniology studies. The casual relationship between NSAIDS and rare but severe skin reactions (SJS, TEN) is well-documented and recognized by the leaders of the dermatology community. Though the reactions rates are suspected to vary by sub-type of 7
12 NSAID, all have been implicated, including ibuprofen. Those facts have already been recognized by the FDA, McNeil and Wyeth so that the product labeling for prescription Children s Motrin and Children s Advil contains the following: Incidence less than 1% (Probable causal relationship) Skin and appendages Vesiculobullous eruptions, urticaria, erythema multiforme, Stevens-Johnson s Syndrome, alopecia, exfoliative dermatitis, Lyell s syndrome (toxic epidermal necrolysis), photosensitivity reactions. Evidence in support of this causal relationship between ibuprofen and SJS and TEN are from clinical case reports that have been reported since the late 70 s until present in adults and children. After 1995, substantial additional evidence of this relationship has been accumulated, much of it specific to ibuprofen. In addition to the case reports of ibuprofen induced SJS and TEN since 1995, the SCAR study group consisting of very well respected dermatologists and epidemiologists in the scientific community conducted a international case-control study that demonstrated a statistically significant causal relationship between ibuprofen and SJS and TEN with a RR of 5.3,95% CI (Mockenhaupt, et. al. 2003) McNeil and Wyeth sponsored studies such as the Boston University Fever Study (BUFS) and the Children s Analgesic Medicine Project (CAMP) which were used in support of their applications for the Rx. to OTC switch, which were 8
13 approved in 1995 and 1996, respectively. While these studies were informative about the common safety profile of ibuprofen in the pediatric population, they were uninformative toward the assessment of the risk for rare and severe reactions such as SJS or TEN. Despite the request from the FDA to the sponsors to design these postmarketing safety studies (BUFS & CAMP) to evaluate the rare, but serious adverse events associated with pediatric ibuprofen, we further believe that these issues were not adequately studied. Moreover, McNeil and Wyeth have failed to provide the FDA full information regarding the safety issues surrounding serious skin reactions, including SJS/TEN that were not presented in their applications for their OTC pediatric formulations. Evidence of causal relationship and increased risks were in existence prior to OTC switch of the pediatric formulation in and thru the present There was considerable experience with ibuprofen beginning with its U.S. approval for arthritis in adults in It rapidly became the most widely prescribed NSAID. Its common safety profile was excellent. There was, however, developing evidence for a number of rare but severe adverse outcomes associated with its use. Most notably they are; severe skin reactions and kidney failure. The cumulative evidence base on SJS and TEN are presented in Tables l-5. Regulatory milestones are noted on the relevant tables.
14 l It is clear that the labeling change in 1982 that added SJS as probably related was based on a substantial set of accumulated evidence. The addition of TEN as being probably related in 1994 was based on both a better understanding the SJS-TEN continuum and further accumulated evidence. Table 1: Accumulation of Knowledge of Risk of SJS/TEN in Adults associated with NSAIDs,978 thru 1989) WHAT NSAIDS (Adults) & S JS/TEN NSAIDs (Adults) & S JS/TEN NSAIDs (Adults) & SJS/TEN WHEN 1 HOW 1983 i S.se reports t Df SJS/TEN Published :ase reports of S JS/TEN in literature Published or sponsored studies How Strong Suggestive (Signal) Supportive Supportive References FDA review (Judith Jones, 1983) - FDA-FOI, WHO, CSM - Skin ADEs ( ) McNeil & Wyeth databases (l)stem R., et al., JAMA, Vol. 252, No. 11, pp , (1984); (2) Stem R., et al., Jour of Arner Acad Derrna, Vol. 12, No. 5, Part 1, pp , (1985); (3)Stem, RS, et al., Current Perspectives in Innnunodermatology, Chapter 6, pp (1984); (4) O Brien, WM, et. al., J Rheumatology, 12: pp (1985) (5) Roujeau J., Stand J Rheumatology, Suppl. 65, pp , (1987); (6) Roujeau J., et al., Arch Derrnatol, Vol. 123, pp , (1987); (7) Stem R., et al., Arch Dermatol, pp (1987) (1) Stem R., et al., J Am Academy of Derrnatology, Vol. 21, No. 2, Part 1, pp , (1989);(2) Bigby, M, et. al., Primary Care, Vol. 16, No. 3, pp (1989) Ibuprofen (Adults) & S JS/TEN 1983 Case reports of SJS/TEN Supportive - McNeil and Wyeth internal reports (1983); -UK CSM Roster of Skin 10
15 Ibuprofen (Adults) & S JS/TEN Published case reports Supportive ADEs ( ) -FDA-FOI, WHO, CSM - Skin ADEs ( ) Wveth databases (1) Stemlieb P., et al., Ny State Jour of Med, pp , (1978); (2) Stem R., et al., JAMA, Vol. 252, No. 11, pp (1984); (3) Stem R., et al., Jour of Arner Acad Derrna, Vol. 12, No. 5, Part 1, pp , (1985); (4) O Brien, WM, et. al., J Rheumatolonv 12: pp (1985); (5) Lai&, et. al. J Am Acad Derm, 19: pp (1988). Probable Causal Relationship for SJS in Adult RX Label for Motrin/Advil 1982 All of the Probable rate Addition to FPL for above less than 1% Motrin/Advil dated 1982 I Incidence less than I % (Probable causal relationship) Skin and appendages Vesiculobullous eruptions, urticaria, elythema multiforme, Stevens- Johnson s Syndrome, alopecia. Table #2 :Accumulation of Knowledge of Risk of SJS/TEN in adults (1990 thru 1996) WHAT WHEN HOW HOW STRONG REFERENCES NSAIDS (Adults) & S JUTEN NSAIDS (Adults) & S JS/TEN Case reports of SJS/TEN Published case reports in Literature Supportive Moderate FDA-FOI, WHO, CSM - Skin ADEs ( ) Wyeth databases (1) Roujeau, JC, J Am Acad Derm, 23:pp (1990); (2) Roujeau, JC, Arch Dermatol, Vol. 126, pp (1990); (3) Strom, BL et. al., Statistics in Medicine, vol. 10, pp (1991); (4) Stem, RS, et al., Immunoloay and Allerav 11
16 Yinics of North America, Vol. 11, no. 3, pp (1991);(5) Schopf, et. al., Arch Dermatol, Vol. 127, pp , (1991); (6) Roujeau, JC, et. al., Dermatology, 186:~~ (1993); (7) Roujeau, JC, et. al. NEJM, Vol. 331, pp (1994); (8) Roujeau, JC, J Amer Acad Dermatol. Vol. 31, pp (1994) NSAIDS (Adults) & S JS/TEN 1995 Published or sponsored studies Strong Roujeau, JC, et. al., NEJM, Vol. 333, pp (1995). Ibuprofen (Adults) & S JS/TEN Ibuprofen (Adults) & SJS/TEN Ibuprofen (Adults) & S JS/TEN Case Reports Published case reports in Literature Supportive Supportive -Published or Moderate sponsored studies FDA-FOI, WHO, CSM - Skin ADEs ( ) McNeil & Wyeth databases (1) Roujeau, JC, m Dermatol, Vol. 126, pp (1990); (2) Strom, BL et. al., Statistics in Medicine, vol. 10, pp (1991); (3) Strom, BL et. al., Arch Dermatol, Vol. 127, pp (1991); (4) Halpem, SM, et.al., Adverse Drug React. Toxic01 Rev., 12 (2): pp (1993); (5) Roujeau, JC, et. al. NEJM, Vol. 331, pp (1994); (6) Halpem, SM, et.al., Arch Derrnatol., vol. 130, pp (1994) Roujeau, JC, et. al., NEJM, Vol. 333, pp (1995). Probable Causal Relationship for TEN in Adult RX Label for 1994 All of the above Probable rate less than 1% Addition to FPL for Motrin/Advil dated
17 Motrin/Advil Probable Causal Relationship for TEN in Pediatric RX Label for Motrin/Advil 1994 All of the above Probable rate Addition to FPL for less than 1% Pediatric Motrin/Advil dated 1994 Pediatric OTC prep marketed 1996 N/A N/A N/A Incidence less than 1% (Probable causal relationship) Skin and appendages Vesiculobullous eruptions, urticaria, erythema multlforme, Stevens- Johnson s Syndrome, alopecia, exfoliative dermatitis, Lyell s syndrome (toxic epidermal necrolysis), photosensitivity reactions. (1994 FPL) Table 3: Accumulation of Knowledge of Risk of SJS/TEN in Adults associated with NSAIDs thru 6/2( 12) WHAT f WHEN HOW How Strong NSAIDS (Adults) & S JUTEN NSAIDs (Adults) & S JS/TEN Case reports of S JS/TEN Supportive Moderate NSAIDs (Adults) & 13 Published case reports/reviews of SJS/TEN in literature Published or sponsored References FDA-F01 & WHO databases McNeil & Wyeth databases (1) Paul, CN, et al., J Burn Care & Rehab., Vol. 19, pp (1998); (2) Bell, MJ, et. al., J Rheumatol, 25:~~ (1998); (3) Carucci, JA, et. al *, A Int J Dermatol, Vol. 38, pp (1999); (4) Fritsch, PO, et. al., Fitzpatrick s Dermatology in General Medicine, 5th. Ed., Vol. 1, Chap. 59, pp (1999); (5) Garcia-Doval;, I, et. al., Arch Dermatol., Vol. 136, pp (2000); (6) Wolkenstein, P, et. al., Dermatologic Clinics, Vol. 18, no. 3, pp (2000); (7) Svermson, CK, et. al., Pharmacol Rev., 53: pp (2000)
18 S JS/TEN Ibuprofen (Adults) & S JS/TEN Ibuprofen (Adults) & S JS/TEN studies Case reports of S JS/TEN Published case reports/reviews of SJS/TEN caused by ibuprofen Supportive Moderate FDA-FOI, WHO, McNeil & Wyeth databases (1) Salomon, D, et. al., Annals of Dermatology, 124 (Suppl.):S (1997); (2) Viard, I, et. al., Journal of Science, Vol. 282, pp (1998); (3) Becker, D, Lancet, Vol. 35 1, pg. 1417, (1998); (4) Fritsch, PO, et. al., Fitzpatrick s Dermatology in General Medicine, 5*. Ed., Vol. 1, Chap. 59, pp (1999); (5) Garcia-Doval;, I, et. al., Arch Dermatol., Vol. 136, pp (2000); (6) Wolkenstein, P, et. al., Dermatoloaic Clinics, Vol. 18, no. 3, pp (2000) Ibuprofen (Adults) & S JSITEN Significant subsequent evidence 2002 Published studies Strong Mockenhaupt, et. al., J Rheumatol, Vol. 30, pp (2003) Table #4 - Accumulation of Knowledge of Risk of SJS/TEN from NSAIDWIbuprofen in Pediatrics WHAT 1 WHEN 1 HOW 1 HOW 1 REFERENCES 1 STRONG 1 Higher risk of 1994 Literature on 3 fold higher Dooley, 1994 severe rash in Lamotrigine & than adults Roujeau 1995 pediatrics other anti- Besag, 1997 convulsants Guberman, 1999 Ibuprofen (Children) & S JS/TEN Ibuprofen (Children) & S JS/TEN Case reports Supportive FDA-FOI, WHO, McNeil & Wyeth databases Published case Strong (1) ); Roujeau, JC J Am 2002 reports in (1999) Acad Derm., Vol. 2pp. Literature (1990); (2) Roujeau, JC, et. al., NEJM, Vol. 331, pp
19 Ibuprofen (Children) & S JS/TEN Probable Causal Relationship to SJS in label Children s Rx Advil Pediatric OTC prep marketed Probable Causal Relationship to TEN in Rx /NDA label Children s Advil I Published or sponsored studies N/A N/A Noninformative N/A N/A (1994); (3) Power,et. al., Ophthalmolonv, Vol. 102, pp (1995); (4) Srivastava, M, et. al., Gastroenterologv, Vol. 115, pp (1998); (5) Sheridan, RL, et. al., J Burn Care Rehab., Vol. 20, pp (1999) (6) Fritsch, PO, et. al., Fitzpatrick s Dermatology in General Medicine, 5*. Ed., Vol. 1, Chap. 59, pp (1999); (7) Wolkenstein, P, et. al., Dermatolonic Clinics, Vol. 18, no. 3, pp (2000);(8) Sheridan, RL, et. al., Pediatrics, Vol. 109, pp (Jan.2000);(9) Spies, M, et. al., Pediatrics, Vol. 108, pp l 168 (2001) BUFS (Lesko, 1995) CAMP (submitted in NDA supplement only) Addition to FPL for Pediatric MotinIAdvil dated 1987 No SJS/lEN warning, nor any cease and seek MD a&ice Addition to FPL for Pediatric Motrin/Advil dated 1994 Constant risk on continued exposure 2000 Strong Garcia-Doval, 2000 Stern,
20 Recent evidence on Ibuprofen & S JS/TEN (pediatrics) % Published case series Extremely Strong Sheridan, 1999 & 2001 I The Severe Cutaneous Adverse Reaction (SCAR) study The SCAR study was a huge multinational effort to determine the etiology and risk factors for the most severe of the cutaneous reactions. The methodology was published by Kelly et.al, (1995), with the results published by Roujeau et.al. (1995), Auquier-Dunant et.al. (2002), and Mockenhaupt et.a1.,(2003). The Roujeau study identified cases then assessed exposure to all drugs. They found oxicam NSAIDS highly statistically significant but the proprionic acid NSAIDS, though having an increased point estimate, fell short of statistical significance. The point estimate for ibuprofen was 4.5 (21245 or over 2/l 147 or ) though statistical significance was not reached. This was clearly a signal in the adult population. Note that isoxicam was removed from the French marketplace after being associated with 13 cases of TEN (Roujeau JC, 1990). That followed the removal of benoxaprofen from the U.S. market in 1982 for toxicity that included cases of TEN (Stern 1984). Mockenhaupt et.al. (2003) reported on a component of the SCAR study, a population-based registry in Germany and on data from the US spontaneous reporting system. There were 373 diagnostically validated cases in the multi- 16
21 national case-control component and 950 in the German registry. The same questionnaire and definitions were used in both of these component studies. In the case-control component 112 of 373 were exposed to an NSAID (excluding aspirin). The oxicams (RR 34,95% CI: 1 l-105) were strongly associated with the greatest increase in risk for SJS and TEN. Of the non-oxicam NSAIDs with sufficient numbers of exposed cases, only diclofenac and ibuprofen has significantly increased risks of SJS and TEN. The relative risk for ibuprofen was 5.3 (95% CI: ). For the propionic acid NSAIDS (including ibuprofen), they estimated the excess risk to be less than one case in a million exposures.. Given the number of children exposed to the pediatric formulations of OTC ibuprofen, the expected number of ibuprofen associated cases worldwide, even at 3 cases per million, could be sizable and of public health import. The SCAR study (Roujeau et.al., 1995, and Mockenhaupt et.al., 2003) clearly implicates all NSAIDS, in varied intensity, in a causal relationship with SJSTEN. Based solely on the SCAR studies, Roujeau, et. al. s paper signaled ibuprofens causal relationship, and Mockenhaupt, et. al. s paper validated its statistical significance. C. The International Labeling/Proposed Labeling For Ibuprofen Provide More Warnings Relating To Serious Skin Reactions and The Need For Immediate Patient Care. 17
22 The Therapeutics Products Directorate commissioned an expert panel to draft an updated Guidance Document: Guidance for Industry - Basic Product Monograph Information for Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Health Canada/TPD have proposed that all prescription NSAID manufacturers, including manufacturers of prescription ibuprofen products, would have to provide a specific warning about SJS and TEN in their respective package inserts. Cornments were provided to Health Canada until September 9,2003. Final implementation of this document is expected shortly. A copy of this Notice is attached hereto, and incorporated herein fully by reference as Attachment 1. Labeling of foreign OTC ibuprofen products provides additional warnings that are not present on the domestic ibuprofen products. For example, McNeil/Johnson & Johnson market a product called Dolormin Ibuprofen Juice which is a non-prescription ibuprofen product sold in Germany. This product has a package insert that warns German consumers of side effects associated with this OTC product of rare but serious skin reactions, such as reddening and blister formation (e.g. erythema multiforme exudativum multiforme) which is bullous EM/SJS. Wyeth markets ibuprofen products known as Spalta in Germany that contain similar information to the public as well. Further, Wyeth markets ibuprofen products in European counties, including the Netherlands that specifically mention SJS and TEN on the OTC labeling. 18
23 American consumers deserve to be provided with appropriate warnings about serious life-threatening side effects as provided to foreign consumers. This foreign labeling was revealed in lawsuits that have been filed against the makers of Children s Motrin and Advil, however, the makers have insisted that these documents remain confidential and are not to be disclosed to anyone, except those persons associated with the law firms or their staff. Petitioners are requesting that the FDA obtain such foreign labels and that they be translated into English and disseminated to the public without delay so that such information can be viewed by the American public. D. Incidence and Frequency of all SJS and TEN events and for Ibuprofen are More Frequent and have a Higher Mortality Rate than Reye Syndrome The Aspirin and Reye Syndrome experience taught us that a low frequency event which is also life-threatening is critically important when a large and otherwise healthy population is exposed to a discretionary drug product. For decades, Baby aspirin was very important for treatment of childhood fevers. When a safer alternative that was just as effective, acetaminophen (APAP), was marketed, Baby aspirin became discretionary though still widely used. When the association with Reye Syndrome was discovered and confirmed, it made the benefit-risk balance unacceptable. That situation is almost exactly like this ibuprofen-sjsiten situation. 19
Recommended Warning for Overthe-Counter. Containing Drug Products and Labeling Statements Regarding Serious Skin Reactions
 Recommended Warning for Overthe-Counter Acetaminophen- Containing Drug Products and Labeling Statements Regarding Serious Skin Reactions Guidance for Industry DRAFT GUIDANCE This guidance document is being
Recommended Warning for Overthe-Counter Acetaminophen- Containing Drug Products and Labeling Statements Regarding Serious Skin Reactions Guidance for Industry DRAFT GUIDANCE This guidance document is being
Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada
 Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
DEPARTMENT OF HEALTH & HUMAN SERVICES NDA 17-854 NDA 21-793 NDA 17-862. [inside address] Dear :
![DEPARTMENT OF HEALTH & HUMAN SERVICES NDA 17-854 NDA 21-793 NDA 17-862. [inside address] Dear : DEPARTMENT OF HEALTH & HUMAN SERVICES NDA 17-854 NDA 21-793 NDA 17-862. [inside address] Dear :](/thumbs/24/3528188.jpg) DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 NDA 17-854 [inside address] Dear : Please refer to your new drug application NDA xx-xxx for
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 NDA 17-854 [inside address] Dear : Please refer to your new drug application NDA xx-xxx for
Opioid Prescribing Practices and Pain Management: Role of FDA Douglas C. Throckmorton, MD Deputy Director for Regulator Programs, CDER, FDA
 Opioid Prescribing Practices and Pain Management: Role of FDA Douglas C. Throckmorton, MD Deputy Director for Regulator Programs, CDER, FDA American Academy of Hospice and Palliative Care Medicine October,
Opioid Prescribing Practices and Pain Management: Role of FDA Douglas C. Throckmorton, MD Deputy Director for Regulator Programs, CDER, FDA American Academy of Hospice and Palliative Care Medicine October,
Guidance on adverse drug reactions
 Guidance on adverse drug reactions Classification of adverse drug reactions Adverse drug reactions are frequently classified as type A and type B reactions. An extended version of this classification system
Guidance on adverse drug reactions Classification of adverse drug reactions Adverse drug reactions are frequently classified as type A and type B reactions. An extended version of this classification system
Guidance for Industry Time and Extent Applications for Nonprescription Drug Products
 Guidance for Industry Time and Extent Applications for Nonprescription Drug Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
Guidance for Industry Time and Extent Applications for Nonprescription Drug Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
Nonsteroidal. Drugs (NSAIDs) Anti-Inflammatory. North American Spine Society Public Education Series
 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) North American Spine Society Public Education Series Nonsteroidal Anti- Inflammatory Drugs Your healthcare provider has recommended that you take a nonsteroidal
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) North American Spine Society Public Education Series Nonsteroidal Anti- Inflammatory Drugs Your healthcare provider has recommended that you take a nonsteroidal
GUaHEAR I N CORPORA TED
 GUaHEAR I N CORPORA TED 41 Martin Lane Elk Grove Village, IL 60007 Ph: 847.228.1113 August 82003 Fax: 847.228.1114 e-mail: gudhear@aol.com Dockets Management Branch Food and Drug Administration, Department
GUaHEAR I N CORPORA TED 41 Martin Lane Elk Grove Village, IL 60007 Ph: 847.228.1113 August 82003 Fax: 847.228.1114 e-mail: gudhear@aol.com Dockets Management Branch Food and Drug Administration, Department
Risk Management Plan (RMP) Guidance (Draft)
 Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare Translated by Pharmaceuticals and Medical Devices Agency Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour
Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare Translated by Pharmaceuticals and Medical Devices Agency Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour
US Health Statistics: Americans Most Over-Prescribed Country in the World
 US Health Statistics: Americans Most Over-Prescribed Country in the World A vitally important story reported in the April 15, 1998, issue of the Journal of the American Medical Association (JAMA), sums
US Health Statistics: Americans Most Over-Prescribed Country in the World A vitally important story reported in the April 15, 1998, issue of the Journal of the American Medical Association (JAMA), sums
GUIDELINE ON THE SUBMISSION OF RISK MANAGEMENT PLAN DOCUMENTS
 APPENDIX GUIDELINE ON THE SUBMISSION OF RISK MANAGEMENT PLAN DOCUMENTS This document is intended to provide guidance on the submission of risk management plan (RMP) documents in support of NDA, GDA and
APPENDIX GUIDELINE ON THE SUBMISSION OF RISK MANAGEMENT PLAN DOCUMENTS This document is intended to provide guidance on the submission of risk management plan (RMP) documents in support of NDA, GDA and
Skin reactions associated with the use of oral terbinafine
 Skin reactions associated with the use of oral terbinafine Introduction The orally and topically active allylamine antifungal agent terbinafine (Lamisil ) has been approved for the Dutch market in 1991.
Skin reactions associated with the use of oral terbinafine Introduction The orally and topically active allylamine antifungal agent terbinafine (Lamisil ) has been approved for the Dutch market in 1991.
WARNING LETTER DEPARTMENT OF HEALTH & HUMAN SERVICES TRANSMITTED BY FACSIMILE
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Peter A. Lankau President and CEO 100 Endo Boulevard Chadds Ford, PA
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Peter A. Lankau President and CEO 100 Endo Boulevard Chadds Ford, PA
A Middle Class in the United States? Behind the Counter (BTC) Drugs. Frederick H. Branding, R.Ph., J.D. Rahul Narula
 A Middle Class in the United States? Behind the Counter (BTC) Drugs Frederick H. Branding, R.Ph., J.D. Rahul Narula FDA s Regulation of Drugs Federal Food, Drug and Cosmetic Act (FDCA) passed by Congress
A Middle Class in the United States? Behind the Counter (BTC) Drugs Frederick H. Branding, R.Ph., J.D. Rahul Narula FDA s Regulation of Drugs Federal Food, Drug and Cosmetic Act (FDCA) passed by Congress
Choosing Pain Medicine for Osteoarthritis. A Guide for Consumers
 Choosing Pain Medicine for Osteoarthritis A Guide for Consumers Fast Facts on Pain Relievers Acetaminophen (Tylenol ) works on mild pain and has fewer risks than other pain pills. Prescription (Rx) pain
Choosing Pain Medicine for Osteoarthritis A Guide for Consumers Fast Facts on Pain Relievers Acetaminophen (Tylenol ) works on mild pain and has fewer risks than other pain pills. Prescription (Rx) pain
Learn More About Product Labeling
 Learn More About Product Labeling Product label The product label is developed during the formal process of review and approval by regulatory agencies of any medicine or medical product. There are specific
Learn More About Product Labeling Product label The product label is developed during the formal process of review and approval by regulatory agencies of any medicine or medical product. There are specific
Section II When you are finished with this section, you will be able to: Define medication (p 2) Describe how medications work (p 3)
 Section II When you are finished with this section, you will be able to: Define medication (p 2) Describe how medications work (p 3) List the different medication effects (p5) List the ways that medications
Section II When you are finished with this section, you will be able to: Define medication (p 2) Describe how medications work (p 3) List the different medication effects (p5) List the ways that medications
Opioids and the Injured Worker Tools for Successful Outcomes
 Opioids and the Injured Worker Tools for Successful Outcomes Tim Pokorney, RPh Director, Clinical Express Scripts Workers' Compensation Division Goals and Objectives Alarming statistics for narcotic utilization,
Opioids and the Injured Worker Tools for Successful Outcomes Tim Pokorney, RPh Director, Clinical Express Scripts Workers' Compensation Division Goals and Objectives Alarming statistics for narcotic utilization,
EMEA RM DRAFT GUIDANCE ISPE RESPONSE 1
 EMEA RM DRAFT GUIDANCE ISPE RESPONSE 1 October 4, 2005 Guideline on Risk Management Systems for Medicinal Products for Human Use DRAFT London, 6 September 2005. This guideline will be included as chapter
EMEA RM DRAFT GUIDANCE ISPE RESPONSE 1 October 4, 2005 Guideline on Risk Management Systems for Medicinal Products for Human Use DRAFT London, 6 September 2005. This guideline will be included as chapter
DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Guidance for Industry Format and Content of Proposed Risk Evaluation and Mitigation Strategies (REMS), REMS Assessments, and Proposed REMS Modifications DRAFT GUIDANCE This guidance document is being distributed
Guidance for Industry Format and Content of Proposed Risk Evaluation and Mitigation Strategies (REMS), REMS Assessments, and Proposed REMS Modifications DRAFT GUIDANCE This guidance document is being distributed
APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167
 APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167 INFORMED CONSENT FOR USE OF DIPHTHERIA ANTITOXIN (DAT) FOR SUSPECTED DIPHTHERIA CASES Investigational New Drug (IND) BB 11184 Protocol
APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167 INFORMED CONSENT FOR USE OF DIPHTHERIA ANTITOXIN (DAT) FOR SUSPECTED DIPHTHERIA CASES Investigational New Drug (IND) BB 11184 Protocol
Introduction to Post marketing Drug Safety Surveillance: Pharmacovigilance in FDA/CDER
 Introduction to Post marketing Drug Safety Surveillance: Pharmacovigilance in FDA/CDER LT Andrew Fine, Pharm.D., BCPS Safety Evaluator Division of Pharmacovigilance Office of Pharmacovigilance and Epidemiology
Introduction to Post marketing Drug Safety Surveillance: Pharmacovigilance in FDA/CDER LT Andrew Fine, Pharm.D., BCPS Safety Evaluator Division of Pharmacovigilance Office of Pharmacovigilance and Epidemiology
Program Approved by AoA, NCOA. Website: www.homemeds.org
 MEDICATION MANAGEMENT IMPROVEMENT SYSTEM: HomeMeds SM The HomeMeds SM system is a collaborative approach to identifying, assessing, and resolving medication problems in community-dwelling older adults.
MEDICATION MANAGEMENT IMPROVEMENT SYSTEM: HomeMeds SM The HomeMeds SM system is a collaborative approach to identifying, assessing, and resolving medication problems in community-dwelling older adults.
HEALTH SERVICES PROGRAM
 HEALTH SERVICES PROGRAM The Board of Education will provide for the health and physical well being of students through the establishment of a district wide student Health Services Program in the school
HEALTH SERVICES PROGRAM The Board of Education will provide for the health and physical well being of students through the establishment of a district wide student Health Services Program in the school
Ask Your Doctor if There May Be a SMARTER CHOICE
 If you have osteoarthritis, rheumatoid arthritis or ankylosing spondylitis, Could Your NSAID Pain Medicine Be Hurting Your Stomach? Ask Your Doctor if There May Be a SMARTER CHOICE 1 of 8 Making Smart
If you have osteoarthritis, rheumatoid arthritis or ankylosing spondylitis, Could Your NSAID Pain Medicine Be Hurting Your Stomach? Ask Your Doctor if There May Be a SMARTER CHOICE 1 of 8 Making Smart
Guidance for Industry
 Guidance for Industry Toll-Free Number Labeling and Related Requirements for Over-the-Counter and Prescription Drugs Marketed With Approved Applications Small Entity Compliance Guide U.S. Department of
Guidance for Industry Toll-Free Number Labeling and Related Requirements for Over-the-Counter and Prescription Drugs Marketed With Approved Applications Small Entity Compliance Guide U.S. Department of
JUL 2 2008. Ms. Kendra Basler Regulatory Affairs Associate Abbott Vascular Cardiac Therapies 3200 Lakeside Drive Santa Clara, CA 95054-2807
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service JUL 2 2008 Food and Drug Administration 9200 Corporate Boulevard Rockville MD 20850 Ms. Kendra Basler Regulatory Affairs Associate Abbott Vascular
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service JUL 2 2008 Food and Drug Administration 9200 Corporate Boulevard Rockville MD 20850 Ms. Kendra Basler Regulatory Affairs Associate Abbott Vascular
NOTICE OF PRIVACY PRACTICES Allergy Treatment Center of New Jersey, P.C. Effective Date: April 14, 2003
 Allergy Treatment Center of New Jersey, P.C. 388 Pompton Avenue 415 Avenel Street Cedar Grove, NJ 07009 Avenel, NJ 07001 (973) 857 9890 (732) 636-7030 NOTICE OF PRIVACY PRACTICES Allergy Treatment Center
Allergy Treatment Center of New Jersey, P.C. 388 Pompton Avenue 415 Avenel Street Cedar Grove, NJ 07009 Avenel, NJ 07001 (973) 857 9890 (732) 636-7030 NOTICE OF PRIVACY PRACTICES Allergy Treatment Center
IBUPROFEN vs. IBUPROFEN vs. ACETAMINOPHEN. Which Painkiller is Better for Children with Viral Hepatitis?
 IBUPROFEN vs. IBUPROFEN vs. ACETAMINOPHEN Which Painkiller is Better for Children with Viral Hepatitis? Adults and children with chronic viral hepatitis suffer the same fevers, colds and body aches as
IBUPROFEN vs. IBUPROFEN vs. ACETAMINOPHEN Which Painkiller is Better for Children with Viral Hepatitis? Adults and children with chronic viral hepatitis suffer the same fevers, colds and body aches as
United States Department of Health and Human Services Findings on the Permissibility of Reporting to Public Health Authorities under HIPAA
 United States Department of Health and Human Services Findings on the Permissibility of Reporting to Public Health Authorities under HIPAA Background DISCLOSURES FOR PUBLIC HEALTH ACTIVITIES [45 CFR 164.512(b)]
United States Department of Health and Human Services Findings on the Permissibility of Reporting to Public Health Authorities under HIPAA Background DISCLOSURES FOR PUBLIC HEALTH ACTIVITIES [45 CFR 164.512(b)]
100% WHEY PROTEIN PARTIALLY HYDROLYZED in Infant Formula and REDUCING THE RISK OF ALLERGY IN INFANTS EXECUTIVE SUMMARY
 QUALIFIED HEALTH CLAIM PETITION 100% WHEY PROTEIN PARTIALLY HYDROLYZED in Infant Formula and REDUCING THE RISK OF ALLERGY IN INFANTS EXECUTIVE SUMMARY The prevalence of allergic (atopic) diseases continues
QUALIFIED HEALTH CLAIM PETITION 100% WHEY PROTEIN PARTIALLY HYDROLYZED in Infant Formula and REDUCING THE RISK OF ALLERGY IN INFANTS EXECUTIVE SUMMARY The prevalence of allergic (atopic) diseases continues
Biologic Treatments for Rheumatoid Arthritis
 Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
February 25, 2014. Dear Dr. Hamburg:
 February 25, 2014 Margaret A. Hamburg, M.D. Commissioner Food and Drug Administration Department of Health and Human Services WO 2200 10903 New Hampshire Avenue Silver Spring, MD 20993-0002 Division of
February 25, 2014 Margaret A. Hamburg, M.D. Commissioner Food and Drug Administration Department of Health and Human Services WO 2200 10903 New Hampshire Avenue Silver Spring, MD 20993-0002 Division of
The Annual Direct Care of Asthma
 The Annual Direct Care of Asthma The annual direct health care cost of asthma in the United States is approximately $11.5 billion; indirect costs (e.g. lost productivity) add another $4.6 billion for a
The Annual Direct Care of Asthma The annual direct health care cost of asthma in the United States is approximately $11.5 billion; indirect costs (e.g. lost productivity) add another $4.6 billion for a
Communicating uncertainty about benefits and
 Communicating uncertainty about benefits and harms of pharmaceutical products Lisa M. Schwartz, MD, MS Steven Woloshin, MD, MS The Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth
Communicating uncertainty about benefits and harms of pharmaceutical products Lisa M. Schwartz, MD, MS Steven Woloshin, MD, MS The Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth
What You Don t Know Can Harm You
 A L C OHOL What You Don t Know Can Harm You National Institute on Alcohol Abuse and Alcoholism National Institutes of Health U.S. Department of Health and Human Services If you are like many Americans,
A L C OHOL What You Don t Know Can Harm You National Institute on Alcohol Abuse and Alcoholism National Institutes of Health U.S. Department of Health and Human Services If you are like many Americans,
Paracetamol apollo +9191 46 950 950. Paracetamol apollo +9191 46 950 950. Paracetamol
 Paracetamol apollo +9191 46 950 950 Paracetamol apollo +9191 46 950 950 Paracetamol CAS Number : 103-90-2 Molecular Weight : 151.17 g/mol Molecular Formula : C8H9NO2 Systematic (IUPAC) : N-(4- hydroxyphenyl)ethanamide
Paracetamol apollo +9191 46 950 950 Paracetamol apollo +9191 46 950 950 Paracetamol CAS Number : 103-90-2 Molecular Weight : 151.17 g/mol Molecular Formula : C8H9NO2 Systematic (IUPAC) : N-(4- hydroxyphenyl)ethanamide
Not All Clinical Trials Are Created Equal Understanding the Different Phases
 Not All Clinical Trials Are Created Equal Understanding the Different Phases This chapter will help you understand the differences between the various clinical trial phases and how these differences impact
Not All Clinical Trials Are Created Equal Understanding the Different Phases This chapter will help you understand the differences between the various clinical trial phases and how these differences impact
Ear Infections Chickenpox chickenpox
 Ear Chickenpox Infections chickenpox Chickenpox Chickenpox is a common, very contagious viral infection that over 90% of people get during childhood unless they have been immunised. After an infection,
Ear Chickenpox Infections chickenpox Chickenpox Chickenpox is a common, very contagious viral infection that over 90% of people get during childhood unless they have been immunised. After an infection,
Ethical Issues Surrounding the Conduct of Pediatric Clinical Studies
 Ethical Issues Surrounding the Conduct of Pediatric Clinical Studies Karsten A. Holm Saint Joseph s University Graduate Ethics Paper Competition, Fall 2013 (Word Count 3000) 1 Introduction The ethical
Ethical Issues Surrounding the Conduct of Pediatric Clinical Studies Karsten A. Holm Saint Joseph s University Graduate Ethics Paper Competition, Fall 2013 (Word Count 3000) 1 Introduction The ethical
EMEA PUBLIC STATEMENT ON LEFLUNOMIDE (ARAVA) - SEVERE AND SERIOUS HEPATIC REACTIONS -
 The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
o DOSAGE AND ADMINISTRATION Dosage in Special Populations: The recommended initial dose is 0.5 mg BID in patients who are elderly
 Some critics of Janssen, including plaintiff s lawyers, have stated it is improper for Risperdal to have been used to treat elderly dementia patients. As you consider that position, we suggest you consider
Some critics of Janssen, including plaintiff s lawyers, have stated it is improper for Risperdal to have been used to treat elderly dementia patients. As you consider that position, we suggest you consider
LEFLUNOMIDE (Adults)
 Shared Care Guideline DRUG: Introduction: LEFLUNOMIDE (Adults) Indication: Disease modifying drug for rheumatoid arthritis and psoriatic arthritis Licensing Information: Disease modifying drug for active
Shared Care Guideline DRUG: Introduction: LEFLUNOMIDE (Adults) Indication: Disease modifying drug for rheumatoid arthritis and psoriatic arthritis Licensing Information: Disease modifying drug for active
Health Literacy & Medication Safety
 Health Literacy & Medication Safety Can We Confuse Patients Less? Michael S. Wolf, MA MPH PhD Assistant Professor and Director Health Literacy and Learning Program (HeLP) Division of General Internal Medicine
Health Literacy & Medication Safety Can We Confuse Patients Less? Michael S. Wolf, MA MPH PhD Assistant Professor and Director Health Literacy and Learning Program (HeLP) Division of General Internal Medicine
Page 191 TITLE 21 FOOD AND DRUGS 355 1
 Page 191 TITLE 21 FOOD AND DRUGS 355 1 APPEALS TAKEN PRIOR TO OCTOBER 10, 1962 Section 104(d)(3) of Pub. L. 87 781 made amendments to subsec. (h) of this section inapplicable to any appeal taken prior
Page 191 TITLE 21 FOOD AND DRUGS 355 1 APPEALS TAKEN PRIOR TO OCTOBER 10, 1962 Section 104(d)(3) of Pub. L. 87 781 made amendments to subsec. (h) of this section inapplicable to any appeal taken prior
Medication Administration Training Overview. Miami-Dade County Public Schools Division of Student Services, Comprehensive Health Services
 Medication Administration Training Overview Miami-Dade County Public Schools Division of Student Services, Comprehensive Health Services Purpose of this Training To review the requirements of Florida Statues
Medication Administration Training Overview Miami-Dade County Public Schools Division of Student Services, Comprehensive Health Services Purpose of this Training To review the requirements of Florida Statues
ANALYSIS OF POISON CONTROL CENTER DATA FOR ACETAMINOPHEN-CONTAINING PRODUCTS 1998-2000
 ANALYSIS OF POISON CONTROL CENTER DATA FOR ACETAMINOPHEN-CONTAINING PRODUCTS 1998-2000 Prepared by Julie A. Skare, PhD. Health Care Research Center The Procter & Gamble Company 8700 Mason-Montgomery Road
ANALYSIS OF POISON CONTROL CENTER DATA FOR ACETAMINOPHEN-CONTAINING PRODUCTS 1998-2000 Prepared by Julie A. Skare, PhD. Health Care Research Center The Procter & Gamble Company 8700 Mason-Montgomery Road
105 CMR: DEPARTMENT OF PUBLIC HEALTH 105 CMR 210.000: THE ADMINISTRATION OF PRESCRIPTION MEDICATIONS IN PUBLIC AND PRIVATE SCHOOLS
 105 CMR 210.000: THE ADMINISTRATION OF PRESCRIPTION MEDICATIONS IN PUBLIC AND PRIVATE SCHOOLS Section 210.001: Purpose 210.002: Definitions 210.003: Policies Governing the Administration of Prescription
105 CMR 210.000: THE ADMINISTRATION OF PRESCRIPTION MEDICATIONS IN PUBLIC AND PRIVATE SCHOOLS Section 210.001: Purpose 210.002: Definitions 210.003: Policies Governing the Administration of Prescription
Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998
 Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998 Section I: Preamble The New Hampshire Medical Society believes that principles
Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998 Section I: Preamble The New Hampshire Medical Society believes that principles
Application Submitted on Received on Supplement type NDA 021875/S-005 July 24, 2008 July 25, 2008 Changes Being Effected
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 021875/S-005, S-008, S-015 SUPPLEMENT APPROVAL Cephalon Inc. Attention: Paul Kirsch Senior Director and Group
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 021875/S-005, S-008, S-015 SUPPLEMENT APPROVAL Cephalon Inc. Attention: Paul Kirsch Senior Director and Group
Drug development for children: how adequate is the current European ethical guidance?
 Chapter 7 Drug development for children: how adequate is the current European ethical guidance? ABSTRACT It is unacceptable that many drugs prescribed to children have not been proven safe and effective
Chapter 7 Drug development for children: how adequate is the current European ethical guidance? ABSTRACT It is unacceptable that many drugs prescribed to children have not been proven safe and effective
Lindenwold Board File Code # 5141.21 Of Education Page 1 of 7
 Of Education Page 1 of 7 The Board of Education disclaims any and all responsibility for the diagnosis and treatment of the illness be contingent upon the timely administration of medication duly prescribed
Of Education Page 1 of 7 The Board of Education disclaims any and all responsibility for the diagnosis and treatment of the illness be contingent upon the timely administration of medication duly prescribed
Wolfe v McNeil-PPC, Inc. A current application of the failure-to-warn doctrine.
 Wolfe v McNeil-PPC, Inc. A current application of the failure-to-warn doctrine. By Charles J. Crooks, Esquire, a member of Jackson Kelly PLLC For: Law360 s May 2011 Product Liability Guest Column New products
Wolfe v McNeil-PPC, Inc. A current application of the failure-to-warn doctrine. By Charles J. Crooks, Esquire, a member of Jackson Kelly PLLC For: Law360 s May 2011 Product Liability Guest Column New products
LIFESTREAM BEHAVIORAL CENTER, INC. JOINT NOTICE OF PRIVACY PRACTICES. Effective Date: April 14, 2003
 LIFESTREAM BEHAVIORAL CENTER, INC. JOINT NOTICE OF PRIVACY PRACTICES Effective Date: April 14, 2003 THIS DOCUMENT DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET
LIFESTREAM BEHAVIORAL CENTER, INC. JOINT NOTICE OF PRIVACY PRACTICES Effective Date: April 14, 2003 THIS DOCUMENT DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET
GAO ADVERSE EVENTS. Surveillance Systems for Adverse Events and Medical Errors. Testimony
 GAO For Release on Delivery Expected at 10:30 a.m. Wednesday, February 9, 2000 United States General Accounting Office Testimony Before the Subcommittees on Health and Environment, and Oversight and Investigations,
GAO For Release on Delivery Expected at 10:30 a.m. Wednesday, February 9, 2000 United States General Accounting Office Testimony Before the Subcommittees on Health and Environment, and Oversight and Investigations,
Medicines for Psoriatic Arthritis. A Review of the Research for Adults
 Medicines for Psoriatic Arthritis A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has told you that you have psoriatic (pronounced
Medicines for Psoriatic Arthritis A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* has told you that you have psoriatic (pronounced
Adverse Events in Clinical Trials: Definitions and Documentation
 Clinical and Translational Science Institute / CTSI at the University of California, San Francisco Welcome to Online Training for Clinical Research Coordinators Adverse Events in Clinical Trials: Definitions
Clinical and Translational Science Institute / CTSI at the University of California, San Francisco Welcome to Online Training for Clinical Research Coordinators Adverse Events in Clinical Trials: Definitions
PHARMACOVIGILANCE GUIDELINES
 PHARMACOVIGILANCE GUIDELINES What is Pharmacovigilance? Pharmacovigilance is defined as the science and activities concerned with the detection, assessment, understanding and prevention of adverse reactions
PHARMACOVIGILANCE GUIDELINES What is Pharmacovigilance? Pharmacovigilance is defined as the science and activities concerned with the detection, assessment, understanding and prevention of adverse reactions
Medications for chronic pain
 Medications for chronic pain When it comes to treating chronic pain with medications, there are many to choose from. Different types of pain medications are used for different pain conditions. You may
Medications for chronic pain When it comes to treating chronic pain with medications, there are many to choose from. Different types of pain medications are used for different pain conditions. You may
Guidance for Industry
 Guidance for Industry Applications Covered by Section 505(b)(2) DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document
Guidance for Industry Applications Covered by Section 505(b)(2) DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document
ADMINISTERING MEDICINES TO STUDENTS
 Adopted June 1977 Revised November 1985 Revised April 2004 Revised September 2007 APS Code: JLCD Students shall not be permitted to take medication while at school unless such medication is administered
Adopted June 1977 Revised November 1985 Revised April 2004 Revised September 2007 APS Code: JLCD Students shall not be permitted to take medication while at school unless such medication is administered
Guideline for Industry
 Guideline for Industry The Extent of Population Exposure to Assess Clinical Safety: For Drugs Intended for Longterm Treatment of Non-Life- Threatening Conditions ICH-E1A March 1995 GUIDELINE FOR INDUSTRY
Guideline for Industry The Extent of Population Exposure to Assess Clinical Safety: For Drugs Intended for Longterm Treatment of Non-Life- Threatening Conditions ICH-E1A March 1995 GUIDELINE FOR INDUSTRY
What you should know about treating your pain with opioids. Important information on the safe use of opioid pain medicine.
 What you should know about treating your pain with opioids Important information on the safe use of opioid pain medicine. If your healthcare provider has determined that opioid therapy is right for you,
What you should know about treating your pain with opioids Important information on the safe use of opioid pain medicine. If your healthcare provider has determined that opioid therapy is right for you,
Guidance for Industry
 Guidance for Industry Product Name Placement, Size, and Prominence in Advertising and Promotional Labeling DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
Guidance for Industry Product Name Placement, Size, and Prominence in Advertising and Promotional Labeling DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
ST. MICHAEL S HOSPITAL Guidelines for Reporting Serious Adverse Events / Unanticipated Problems to the SMH Research Ethics Board (REB) July 09, 2014
 ST. MICHAEL S HOSPITAL Guidelines for Reporting Serious Adverse Events / Unanticipated Problems to the SMH Research Ethics Board (REB) July 09, 2014 1. Introduction The St. Michael s Hospital (SMH) REB
ST. MICHAEL S HOSPITAL Guidelines for Reporting Serious Adverse Events / Unanticipated Problems to the SMH Research Ethics Board (REB) July 09, 2014 1. Introduction The St. Michael s Hospital (SMH) REB
Maintenance of abstinence in alcohol dependence
 Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Understanding Alberta s Drug Schedules
 Understanding Alberta s Drug Schedules Preface In May 2002, the provincial drug schedules to the Pharmaceutical Profession Act were amended. In April 2007, the Alberta Regulation 66/2007 to the Pharmacy
Understanding Alberta s Drug Schedules Preface In May 2002, the provincial drug schedules to the Pharmaceutical Profession Act were amended. In April 2007, the Alberta Regulation 66/2007 to the Pharmacy
Exposure. What Healthcare Personnel Need to Know
 Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
The Cost of Pain and Economic Burden of Prescription Misuse, Abuse and Diversion. Angela Huskey, PharmD, CPE
 The Cost of Pain and Economic Burden of Prescription Misuse, Abuse and Diversion Angela Huskey, PharmD, CPE Case Bill is a 47 year old man with a history of low back pain and spinal stenosis Not a real
The Cost of Pain and Economic Burden of Prescription Misuse, Abuse and Diversion Angela Huskey, PharmD, CPE Case Bill is a 47 year old man with a history of low back pain and spinal stenosis Not a real
Guidance for Industry Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation
 Guidance for Industry Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Guidance for Industry Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Stowe School Medications Policy
 INTRODUCTION Most pupils will need medication at some stage of their school life. Although this will mainly be for short periods there are a few pupils with chronic conditions who may require regular medication
INTRODUCTION Most pupils will need medication at some stage of their school life. Although this will mainly be for short periods there are a few pupils with chronic conditions who may require regular medication
Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules
 Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules Read this Medication Guide before you start taking EQUETRO and each time you get a refill. There may be new information. This
Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules Read this Medication Guide before you start taking EQUETRO and each time you get a refill. There may be new information. This
ADMINISTRATION OF MEDICATIONS POLICY
 Policy 6.007. ADMINISTRATION OF MEDICATIONS POLICY It is the policy of Cooperative Educational Services (C.E.S.) that students who require any medications to be administered during school hours, including
Policy 6.007. ADMINISTRATION OF MEDICATIONS POLICY It is the policy of Cooperative Educational Services (C.E.S.) that students who require any medications to be administered during school hours, including
HAWAII BOARD OF MEDICAL EXAMINERS PAIN MANAGEMENT GUIDELINES
 Pursuant to section 453-1.5, Hawaii Revised Statutes, the Board of Medical Examiners ("Board") has established guidelines for physicians with respect to the care and treatment of patients with severe acute
Pursuant to section 453-1.5, Hawaii Revised Statutes, the Board of Medical Examiners ("Board") has established guidelines for physicians with respect to the care and treatment of patients with severe acute
TAKING CARE OF YOUR RHEUMATOID ARTHRITIS
 TAKING CARE OF YOUR RHEUMATOID ARTHRITIS RHEUMATOID ARTHRITIS (RA) FAST FACTS What is Rheumatoid Arthritis? Rheumatoid arthritis (RA) is a chronic disease that can affect your ability to function and be
TAKING CARE OF YOUR RHEUMATOID ARTHRITIS RHEUMATOID ARTHRITIS (RA) FAST FACTS What is Rheumatoid Arthritis? Rheumatoid arthritis (RA) is a chronic disease that can affect your ability to function and be
Summary of the risk management plan (RMP) for Otezla (apremilast)
 EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
The Trans-Tasman Early Warning System. Processes in Australia and New Zealand
 The Trans-Tasman Early Warning System Processes in Australia and New Zealand Version 1.0 May 2013 About Medsafe Medsafe is the New Zealand Medicines and Medical Devices Safety Authority and is responsible
The Trans-Tasman Early Warning System Processes in Australia and New Zealand Version 1.0 May 2013 About Medsafe Medsafe is the New Zealand Medicines and Medical Devices Safety Authority and is responsible
Arthritis Foundation Position Statement on Biosimilar Substitution
 Arthritis Foundation Position Statement on Biosimilar Substitution The Affordable Care Act creates a regulatory pathway for the approval of a new generation of biologic medications called biosimilars.
Arthritis Foundation Position Statement on Biosimilar Substitution The Affordable Care Act creates a regulatory pathway for the approval of a new generation of biologic medications called biosimilars.
Exploring the Role of Vitamins in Achieving a Healthy Heart
 Exploring the Role of Vitamins in Achieving a Healthy Heart There are many avenues you can take to keep your heart healthy. The first step you should take is to have a medical professional evaluate the
Exploring the Role of Vitamins in Achieving a Healthy Heart There are many avenues you can take to keep your heart healthy. The first step you should take is to have a medical professional evaluate the
02 DEPARTMENT OF PROFESSIONAL AND FINANCIAL REGULATION
 Effective June 13, 2010 02-313, 02-373, 02-380, 02-383, 02-396 Chapter 21 page 1 02 DEPARTMENT OF PROFESSIONAL AND FINANCIAL REGULATION 313 BOARD OF DENTAL EXAMINERS 373 BOARD OF LICENSURE IN MEDICINE
Effective June 13, 2010 02-313, 02-373, 02-380, 02-383, 02-396 Chapter 21 page 1 02 DEPARTMENT OF PROFESSIONAL AND FINANCIAL REGULATION 313 BOARD OF DENTAL EXAMINERS 373 BOARD OF LICENSURE IN MEDICINE
Use of Electronic Health Record Data in Clinical Investigations
 Use of Electronic Health Record Data in Clinical Investigations Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Use of Electronic Health Record Data in Clinical Investigations Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Michigan Guidelines for the Use of Controlled Substances for the Treatment of Pain
 Michigan Guidelines for the Use of Controlled Substances for the Treatment of Pain Section I: Preamble The Michigan Boards of Medicine and Osteopathic Medicine & Surgery recognize that principles of quality
Michigan Guidelines for the Use of Controlled Substances for the Treatment of Pain Section I: Preamble The Michigan Boards of Medicine and Osteopathic Medicine & Surgery recognize that principles of quality
Public Assessment Report. Pharmacy to General Sales List Reclassification. Pirinase Hayfever Relief for Adults 0.05% Nasal Spray.
 Public Assessment Report Pharmacy to General Sales List Reclassification Pirinase Hayfever Relief for Adults 0.05% Nasal Spray (Fluticasone) PL 00079/0688 Glaxo Wellcome UK Limited TABLE OF CONTENTS Introduction
Public Assessment Report Pharmacy to General Sales List Reclassification Pirinase Hayfever Relief for Adults 0.05% Nasal Spray (Fluticasone) PL 00079/0688 Glaxo Wellcome UK Limited TABLE OF CONTENTS Introduction
Medication Guide TASIGNA (ta-sig-na) (nilotinib) Capsules
 Medication Guide TASIGNA (ta-sig-na) (nilotinib) Capsules Read this Medication Guide before you start taking Tasigna and each time you get a refill. There may be new information. This information does
Medication Guide TASIGNA (ta-sig-na) (nilotinib) Capsules Read this Medication Guide before you start taking Tasigna and each time you get a refill. There may be new information. This information does
Guidance for Industry and FDA Review Staff on Collection of Platelets by Automated Methods
 Guidance for Industry and FDA Review Staff on Collection of Platelets by Automated Methods Blood Products Advisory Committee, March 9, 2006 Steven Kleinman, MD Senior Medical Advisor, AABB Co-chair, AABB
Guidance for Industry and FDA Review Staff on Collection of Platelets by Automated Methods Blood Products Advisory Committee, March 9, 2006 Steven Kleinman, MD Senior Medical Advisor, AABB Co-chair, AABB
Formulary Management
 Formulary Management Formulary management is an integrated patient care process which enables physicians, pharmacists and other health care professionals to work together to promote clinically sound, cost-effective
Formulary Management Formulary management is an integrated patient care process which enables physicians, pharmacists and other health care professionals to work together to promote clinically sound, cost-effective
ROUTINE AND EXTRAORDINARY HEALTH CARE SERVICES. Frequently Asked Questions
 DEVAL L. PATRICK Governor TIMOTHY P. MURRAY Lieutenant Governor JUDYANN BIGBY, M.D. Secretary ANGELO MCCLAIN Commissioner The Commonwealth of Massachusetts Executive Office of Health and Human Services
DEVAL L. PATRICK Governor TIMOTHY P. MURRAY Lieutenant Governor JUDYANN BIGBY, M.D. Secretary ANGELO MCCLAIN Commissioner The Commonwealth of Massachusetts Executive Office of Health and Human Services
Risk Assessment and Management: A Regulatory Lawyer s Perspective
 Risk Assessment and Management: A Regulatory Lawyer s Perspective Presented By: Alan G. Minsk Partner and Leader, Food and Drug Practice Team Arnall Golden Gregory LLP 171 17 th Street, NW, Suite 2100
Risk Assessment and Management: A Regulatory Lawyer s Perspective Presented By: Alan G. Minsk Partner and Leader, Food and Drug Practice Team Arnall Golden Gregory LLP 171 17 th Street, NW, Suite 2100
Peptic Ulcer. Anatomy The stomach is a hollow organ. It is located in the upper abdomen, under the ribs.
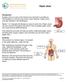 Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
CHAPTER 21 SPECIFIC AREAS OF NURSING NEGLIGENCE
 CHAPTER 21 SPECIFIC AREAS OF NURSING NEGLIGENCE I. INTRODUCTION The nursing profession is a specialized field in which the number of suits in which nurses are being named for negligence is growing. There
CHAPTER 21 SPECIFIC AREAS OF NURSING NEGLIGENCE I. INTRODUCTION The nursing profession is a specialized field in which the number of suits in which nurses are being named for negligence is growing. There
Law Offices of Christopher L. Nuland
 Law Offices of Christopher L. Nuland 1000 Riverside Avenue, Suite 115 Jacksonville, FL 32204 (904) 355-1555 nulandlaw@aol.com June 20, 2008 Over the past few years there has been increasing confusion as
Law Offices of Christopher L. Nuland 1000 Riverside Avenue, Suite 115 Jacksonville, FL 32204 (904) 355-1555 nulandlaw@aol.com June 20, 2008 Over the past few years there has been increasing confusion as
Keeps a physician up to date on all laws and regulations affecting medical practice.
 Dear Doctor: Thank you for the inquiry you made to the Cooperative of American Physicians, Inc. (CAP). The accompanying document that addresses your professional liability question is published by the
Dear Doctor: Thank you for the inquiry you made to the Cooperative of American Physicians, Inc. (CAP). The accompanying document that addresses your professional liability question is published by the
HOSPITAL DRUG SHORTAGES
 HOSPITAL DRUG SHORTAGES OVERVIEW More than 45,000 different prescription drug products are on the market today, originating from about 1,400 different manufacturers. Each year, the U.S. experiences a shortage
HOSPITAL DRUG SHORTAGES OVERVIEW More than 45,000 different prescription drug products are on the market today, originating from about 1,400 different manufacturers. Each year, the U.S. experiences a shortage
Arthritis Pain Management after Merck s Withdrawal of Vioxx Survey Results
 Arthritis Pain Management after Merck s Withdrawal of Vioxx/Survey Results MedicineNet Health Research Survey Report Arthritis Pain Management after Merck s Withdrawal of Vioxx Survey Results Prepared
Arthritis Pain Management after Merck s Withdrawal of Vioxx/Survey Results MedicineNet Health Research Survey Report Arthritis Pain Management after Merck s Withdrawal of Vioxx Survey Results Prepared
MINISTRY OF HEALTH PANDEMIC INFLUENZA A / H1N1 2009 VACCINE FREQUENTLY ASKED QUESTIONS
 Government of the Republic of Trinidad and Tobago MINISTRY OF HEALTH PANDEMIC INFLUENZA A / H1N1 2009 VACCINE FREQUENTLY ASKED QUESTIONS Influenza vaccines are one of the most effective ways to protect
Government of the Republic of Trinidad and Tobago MINISTRY OF HEALTH PANDEMIC INFLUENZA A / H1N1 2009 VACCINE FREQUENTLY ASKED QUESTIONS Influenza vaccines are one of the most effective ways to protect
Submitted via email to chronic_care@finance.senate.gov. June 22, 2015
 Submitted via email to chronic_care@finance.senate.gov June 22, 2015 Chronic Care Working Group Senate Finance Committee United States Senate 219 Dirksen Senate Office Building Washington, D.C. 20510 Re:
Submitted via email to chronic_care@finance.senate.gov June 22, 2015 Chronic Care Working Group Senate Finance Committee United States Senate 219 Dirksen Senate Office Building Washington, D.C. 20510 Re:
Excellence in Care: Over-the-Counter Drugs Update
 Excellence in Care: Over-the-Counter Drugs Update Renee Acosta, RPh INDEPENDENT STUDY Health Professions Institute for Continuing Education Austin Community College The Austin Community College Health
Excellence in Care: Over-the-Counter Drugs Update Renee Acosta, RPh INDEPENDENT STUDY Health Professions Institute for Continuing Education Austin Community College The Austin Community College Health
Mississippi Medicaid Enrollment Application (Ordering/Referring/Prescribing Provider)
 This application is for the sole purpose of ordering/referring/prescribing items and services for MS Medicaid beneficiaries. This type of enrollment does not allow MS Medicaid to reimburse the applicant/provider
This application is for the sole purpose of ordering/referring/prescribing items and services for MS Medicaid beneficiaries. This type of enrollment does not allow MS Medicaid to reimburse the applicant/provider
Guidance for Industry Labeling for Bronchodilators: Cold, Cough, Allergy, Bronchodilator,
 Reprinted from FDA s website by Guidance for Industry Labeling for Bronchodilators: Cold, Cough, Allergy, Bronchodilator, Guidance And Antiasthmatic for Drug Industry Products for Over-the-Counter Labeling
Reprinted from FDA s website by Guidance for Industry Labeling for Bronchodilators: Cold, Cough, Allergy, Bronchodilator, Guidance And Antiasthmatic for Drug Industry Products for Over-the-Counter Labeling
CONSENT FORM 12/19/08
 12/19/08 1001 University Place Evanston, Illinois 60201 www.northshore.org CONSENT FORM Phone (224) 364-7100 Fax (847) 570-8011 Intravesical Alkalized Lidocaine for the Treatment of Overactive Bladder
12/19/08 1001 University Place Evanston, Illinois 60201 www.northshore.org CONSENT FORM Phone (224) 364-7100 Fax (847) 570-8011 Intravesical Alkalized Lidocaine for the Treatment of Overactive Bladder
