WARNING LETTER DEPARTMENT OF HEALTH & HUMAN SERVICES TRANSMITTED BY FACSIMILE
|
|
|
- Michael Tate
- 8 years ago
- Views:
Transcription
1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD TRANSMITTED BY FACSIMILE Peter A. Lankau President and CEO 100 Endo Boulevard Chadds Ford, PA RE: Lidoderm (Lidocaine Patch 5%) MACMIS # Dear Mr. Lankau: WARNING LETTER The Division of Drug Marketing, Advertising, and Communications (DDMAC) in the U.S. Food and Drug Administration (FDA) has reviewed two professional direct mailing pieces (identified as LD-1204F and LD-1316B) for Lidoderm (Lidocaine Patch 5%) submitted by Endo Pharmaceuticals Inc. (Endo) under cover of Form FDA The direct mailing pieces are false or misleading for several reasons: first, they contain unsubstantiated effectiveness claims for Lidoderm; second, they omit and minimize serious risk information associated with Lidoderm; and third, they fail to communicate an important limitation in the drug's FDAapproved indication. Thus, the direct mailing pieces misbrand the drug within the meaning of the Federal Food, Drug, and Cosmetic Act (Act), 21 U.S.C. 352(a) and 321(n). These violations are a public health concern because they may encourage use of Lidoderm in circumstances other than those in which the drug has been shown to be safe and effective. Background Approved Product Labeling Lidoderm (Lidocaine Patch 5%) is a topical anesthetic patch comprised of an adhesive material containing 5% lidocaine. According to its FDA-approved product labeling (PI): LIDODERM is indicated for relief of pain associated with post-herpetic neuralgia [PHN]. It should be applied only to intact skin. (emphasis in original).
2 Page 2 The PI also states that Lidoderm is associated with numerous important risks, including the following (in pertinent part): CONTRAINDICATIONS LIDODERM is contraindicated in patients with a known history of sensitivity to local anesthetics of the amide type, or to any other component of the product. WARNINGS Accidental Exposure in Children Even a used LIDODERM patch contains a large amount of lidocaine (at least 665 mg). The potential exists for a small child or a pet to suffer serious adverse effects from chewing or ingesting a new or used LIDODERM patch, although the risk with this formulation has not been evaluated. It is important for patients to store and dispose of LIDODERM out of the reach of children and pets. Excessive Dosing Excessive dosing by applying LIDODERM to larger areas or for longer than the recommended wearing time could result in increased absorption of lidocaine and high blood concentrations, leading to serious adverse effects (see ADVERSE REACTIONS, Systemic Reactions). PRECAUTIONS General Hepatic Disease: Patients with severe hepatic disease are at greater risk of developing toxic blood concentrations of lidocaine, because of their inability to metabolize lidocaine normally. Drug Interactions Antiarrhythmic Drugs: LIDODERM should be used with caution in patients receiving Class I antiarrhythmic drugs (such as tocainide and mexiletine) since the toxic effects are additive and potentially synergistic. ADVERSE REACTIONS Application Site Reactions During or immediately after treatment with LIDODERM (lidocaine patch 5%), the skin at the site of application may develop erythema, edema, bruising, papules, vesicles, discoloration, depigmentation, burning sensation, pruritus, dermatitis, petechia, blisters, exfoliation, or may be the locus of abnormal sensation. These reactions are generally mild and transient, resolving spontaneously within a few minutes to hours. Regulatory History On November 24, 1999, DDMAC sent you an untitled letter regarding a previous sales aid for Lidoderm that omitted and minimized risk information, including important information from the Accidental Exposure in Children and the Excessive Dosing warnings (see above).
3 Page 3 Unsubstantiated Effectiveness Claims Both of the mailing pieces at issue in this letter make effectiveness claims for Lidoderm that, to our knowledge, have not been demonstrated by substantial evidence or substantial clinical experience. For example, the first piece (LD-1204F) presents the following claims: 66% of patients (n=310) reported improvement in pain intensity at week 1 78% of patients (n=310) reported improvement in quality of life at week 1 The second piece (LD-1316B) presents the following claims: 2 out of 3 patients (n=310) achieved reduction in pain intensity at week 1 - No serious systemic adverse events were seen in this predominantly elderly population (mean age 71 years) Continue with LIDODERM and more of your patients may respond 4 out of 5 patients (n=310) achieved reduction in pain intensity at week 2, with efficacy sustained for the duration of the trial These claims are misleading because they are not supported by substantial evidence or substantial clinical experience. The study cited for support of these claims was an openlabel, single-arm study with no concurrent control group, rather than a well controlled study. 1 Without a control group and blinding to reduce the possibility of bias, subjective endpoints such as pain intensity and quality of life cannot be properly assessed. It is therefore not possible to tell from the study whether patient outcomes on Lidoderm were better than what would be expected in the absence of treatment. Furthermore, we are not aware of any other evidence or clinical experience that substantiate your claims. Omission of Risk Both of the direct mailing pieces present numerous effectiveness claims for Lidoderm, including improvement in pain intensity, improvement in quality of life, relief of the neuropathic pain of PHN, Pain relief is within reach!, for localized pain relief, and With LIDODERM it all adds up to relief. Under the Act, whether a drug s labeling is misleading depends not only on the representations made or suggested in it, but also on the extent to which the labeling fails to reveal facts material in light of those representations. See 21 U.S.C. 321(n) and 352(a). In this instance, both pieces fail to include such information. Specifically, they fail to reveal important risks associated with use of Lidoderm as presented in the PI (above). For example, both pieces omit the serious warnings set forth above regarding accidental exposure in children and excessive dosing. 1 Katz NP, Gammaitoni AR, Davis MW, Dworkin RH and the Lidoderm Patch Study Group. Lidocaine patch 5% reduces pain intensity and interference with quality of life in patients with postherpetic neuralgia: an effectiveness trial. Pain Med. 2002;3(4):
4 Page 4 Minimization of Risk In addition to omitting important risk information, the direct mailing pieces also misleadingly minimize the risks they do mention. First, one of the pieces (LD-1204F) minimizes Lidoderm s risks by virtue of its physical configuration. In this piece, all of the risk information is only accessible after pulling a tab on the front of the piece and then turning the page over and reading the back of the tab. If the tab is reinserted prior to turning the page, this information is no longer visible. There are also no signals within the piece to alert readers to the presence of this important risk information inside the tab. Second, the information relating to risk that is presented in the pieces does not adequately describe the risks associated with Lidoderm. Specifically, the pieces claim: Most commonly reported adverse event in this study was localized rash, which was considered to be related to study treatment in majority of cases (LD-1204F) and Most commonly reported adverse event was localized rash (12%) (LD-1316B). These statements are insufficient to describe the myriad of application site reactions associated with Lidoderm use, as presented in the PI. Communication of Indication Both direct mailing pieces claim that Lidoderm is for the neuropathic pain of postherpetic neuralgia (PHN). This presentation is misleading because it fails to communicate an important limitation in Lidoderm s approved indication as reflected in the PI; specifically, that Lidoderm should be applied only to intact skin. (emphasis in original). By failing to adequately communicate Lidoderm s approved indication, the direct mailing pieces fail to appropriately caution against unsafe use of Lidoderm, including application to broken or inflamed skin, which, according to the PRECAUTIONS section of the PI, may result in higher blood concentrations of lidocaine from increased absorption. Conclusion and Requested Action For the reasons discussed above, the direct mailing pieces are false or misleading because they make unsubstantiated effectiveness claims for Lidoderm, they omit and minimize serious risk information associated with use of the drug, and they inadequately communicate an important limitation in Lidoderm s approved indication. Accordingly, the pieces misbrand Lidoderm under 21 U.S.C. 352(a) and 321(n).
5 Page 5 DDMAC requests that Endo immediately cease the dissemination of violative promotional materials for Lidoderm such as those described above. Please submit a written response to this letter on or before July 13, 2005 stating whether you intend to comply with this request, listing all violative promotional materials for Lidoderm such as those described above, and explaining your plan for discontinuing use of such materials. Because the violations described above are serious, we request, further, that your submission include a comprehensive plan of action to disseminate truthful, non-misleading, and complete corrective messages about the issues discussed in this letter to the audience(s) that received the violative promotional materials. Please direct your response to me at the Food and Drug Administration, Division of Drug Marketing, Advertising, and Communications HFD-42, Rm. 8B-45, 5600 Fishers Lane, Rockville, Maryland 20857, facsimile at In all future correspondence regarding this matter, please refer to MACMIS # in addition to the NDA number. We remind you that only written communications are considered official. The violations discussed in this letter do not necessarily constitute an exhaustive list. It is your responsibility to ensure that your promotional materials for Lidoderm comply with each applicable requirement of the Act and FDA implementing regulations. Failure to correct the violations discussed above may result in FDA regulatory action, including seizure or injunction, without further notice. Sincerely, {See appended electronic signature page} Thomas Abrams, RPh, MBA Director Division of Drug Marketing, Advertising, and Communications
6 This is a representation of an electronic record that was signed electronically and this page is the manifestation of the electronic signature /s/ Barbara Chong 6/28/05 04:38:40 PM Signed for Thomas Abrams
RE: NDA: 021876 DICLEGIS (doxylamine succinate and pyridoxine hydrochloride) delayed-release tablets, for oral use MA # 350 WARNING LETTER
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Eric Gervais, Executive Vice President 919 Conestoga Road Building
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Eric Gervais, Executive Vice President 919 Conestoga Road Building
WARNING LETTER. Multikine is an investigational new drug that does not have marketing authorization in the United States. (b) (4)
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Geert R. Kersten Director and Chief Executive Officer 8229 Boone
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Geert R. Kersten Director and Chief Executive Officer 8229 Boone
WARNING LETTER. Angus Russell Chief Executive Officer Shire Development Inc. 725 Chesterbrook Blvd. Wayne, PA 19087-5637
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Angus Russell Chief Executive Officer Shire Development Inc. 725 Chesterbrook
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Angus Russell Chief Executive Officer Shire Development Inc. 725 Chesterbrook
WARNING LETTER. Ian C. Reed Chairman and Chief Executive Officer Pfizer Inc. 235 East 42 nd Street New York, NY 10017
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 Ian C. Reed Chairman and Chief Executive Officer 235 East 42 nd Street New York, NY 10017
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 Ian C. Reed Chairman and Chief Executive Officer 235 East 42 nd Street New York, NY 10017
WARNING LETTER. According to its approved product labeling (PI) (in pertinent part, emphasis original):
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993-0002 TRANSMITTED BY FACSIMILE Sapan A. Shah, Ph.D. President and Chief Executive Officer
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993-0002 TRANSMITTED BY FACSIMILE Sapan A. Shah, Ph.D. President and Chief Executive Officer
Fadwa Almanakly, Pharm.D. Associate Director, Advertising and Promotions Bayer HealthCare Pharmaceuticals Inc. 6 West Belt Wayne, NJ 07470-6806
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Fadwa Almanakly, Pharm.D. Associate Director, Advertising and Promotions
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Fadwa Almanakly, Pharm.D. Associate Director, Advertising and Promotions
According to its FDA-approved product labeling (PI), Visipaque is indicated for the following (emphasis in original; footnotes omitted):
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Norma Vanderhorst Labeling Specialist 101 Carnegie Center Princeton,
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Norma Vanderhorst Labeling Specialist 101 Carnegie Center Princeton,
Application Submitted on Received on Supplement type NDA 021875/S-005 July 24, 2008 July 25, 2008 Changes Being Effected
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 021875/S-005, S-008, S-015 SUPPLEMENT APPROVAL Cephalon Inc. Attention: Paul Kirsch Senior Director and Group
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 021875/S-005, S-008, S-015 SUPPLEMENT APPROVAL Cephalon Inc. Attention: Paul Kirsch Senior Director and Group
Guidance for Industry. Further Amendments to General Regulations of the Food and Drug Administration to Incorporate Tobacco Products
 Guidance for Industry Further Amendments to General Regulations of the Food and Drug Administration to Incorporate Tobacco Products Small Entity Compliance Guide Written comments and suggestions may be
Guidance for Industry Further Amendments to General Regulations of the Food and Drug Administration to Incorporate Tobacco Products Small Entity Compliance Guide Written comments and suggestions may be
Carol Childers Director Teva Neuroscience, Inc. 901 East 104 th Street, Suite 900 Kansas City, MO 64131. RE: ANDA 076809 Clozapine tablets USP MA# 44
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 Carol Childers Director Teva Neuroscience, Inc. 901 East 104 th Street, Suite 900 Kansas
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 Carol Childers Director Teva Neuroscience, Inc. 901 East 104 th Street, Suite 900 Kansas
Guidance for Industry Consumer-Directed Broadcast Advertisements
 Guidance for Industry Consumer-Directed Broadcast Advertisements U.S. Department of Health and Human Services Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research
Guidance for Industry Consumer-Directed Broadcast Advertisements U.S. Department of Health and Human Services Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research
Guidance for Industry Direct-to-Consumer Television Advertisements FDAAA DTC Television Ad Pre- Dissemination Review Program
 Guidance for Industry Direct-to-Consumer Television Advertisements FDAAA DTC Television Ad Pre- Dissemination Review Program DRAFT GUIDANCE This guidance document is being distributed for comment purposes
Guidance for Industry Direct-to-Consumer Television Advertisements FDAAA DTC Television Ad Pre- Dissemination Review Program DRAFT GUIDANCE This guidance document is being distributed for comment purposes
Recommended Warning for Overthe-Counter. Containing Drug Products and Labeling Statements Regarding Serious Skin Reactions
 Recommended Warning for Overthe-Counter Acetaminophen- Containing Drug Products and Labeling Statements Regarding Serious Skin Reactions Guidance for Industry DRAFT GUIDANCE This guidance document is being
Recommended Warning for Overthe-Counter Acetaminophen- Containing Drug Products and Labeling Statements Regarding Serious Skin Reactions Guidance for Industry DRAFT GUIDANCE This guidance document is being
NDA 021879 NDA APPROVAL
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 021879 NDA APPROVAL Avanir Pharmaceuticals Attention: Randall Kaye, M.D. Vice President, Clinical and Medical
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 021879 NDA APPROVAL Avanir Pharmaceuticals Attention: Randall Kaye, M.D. Vice President, Clinical and Medical
DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Guidance for Industry Internet/Social Media Platforms with Character Space Limitations Presenting Risk and Benefit Information for Prescription Drugs and Medical Devices DRAFT GUIDANCE This guidance document
Guidance for Industry Internet/Social Media Platforms with Character Space Limitations Presenting Risk and Benefit Information for Prescription Drugs and Medical Devices DRAFT GUIDANCE This guidance document
Introduction to Compliance with FDA Labeling and Advertising Requirements
 Introduction to Compliance with FDA Labeling and Advertising Requirements Second Annual Pharmaceutical Industry Regulatory and Compliance Summit Dick Kenny FDA History Basic function of government Oldest
Introduction to Compliance with FDA Labeling and Advertising Requirements Second Annual Pharmaceutical Industry Regulatory and Compliance Summit Dick Kenny FDA History Basic function of government Oldest
Guidance for Industry
 Guidance for Industry Promoting Medical Products in a Changing Healthcare Environment; I. Medical Product Promotion by Healthcare Organizations or Pharmacy Benefits Management Companies (PBMs) DRAFT GUIDANCE
Guidance for Industry Promoting Medical Products in a Changing Healthcare Environment; I. Medical Product Promotion by Healthcare Organizations or Pharmacy Benefits Management Companies (PBMs) DRAFT GUIDANCE
NDA 202439/S-008 SUPPLEMENT APPROVAL
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 202439/S-008 SUPPLEMENT APPROVAL Janssen Pharmaceuticals, Inc. ATTENTION: Alla Rhoge Pharm.D., Associate
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 202439/S-008 SUPPLEMENT APPROVAL Janssen Pharmaceuticals, Inc. ATTENTION: Alla Rhoge Pharm.D., Associate
Guidance for Industry
 Guidance for Industry IND Exemptions for Studies of Lawfully Marketed Drug or Biological Products for the Treatment of Cancer U.S. Department of Health and Human Services Food and Drug Administration Center
Guidance for Industry IND Exemptions for Studies of Lawfully Marketed Drug or Biological Products for the Treatment of Cancer U.S. Department of Health and Human Services Food and Drug Administration Center
Guidance for Industry
 Guidance for Industry Internet/Social Media Platforms: Correcting Independent Third-Party Misinformation About Prescription Drugs and Medical Devices DRAFT GUIDANCE This guidance document is being distributed
Guidance for Industry Internet/Social Media Platforms: Correcting Independent Third-Party Misinformation About Prescription Drugs and Medical Devices DRAFT GUIDANCE This guidance document is being distributed
Regulation of Prescription Drug Promotion
 Regulation of Prescription Drug Promotion Amy Toscano, Pharm.D., RAC, CPA Team Leader Office of Prescription Drug Promotion Food and Drug Administration June 2013 Overview Office of Prescription Drug Promotion
Regulation of Prescription Drug Promotion Amy Toscano, Pharm.D., RAC, CPA Team Leader Office of Prescription Drug Promotion Food and Drug Administration June 2013 Overview Office of Prescription Drug Promotion
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Case Study Sanctura. (trospium chloride)
 Case Study Sanctura (trospium chloride) Bad Ad Case Study Sanctura (trospium chloride) Facilitator Guide Approximate Time: 30 minutes Exercise prerequisite: Students should view the Bad Ad e-learning CME/CE
Case Study Sanctura (trospium chloride) Bad Ad Case Study Sanctura (trospium chloride) Facilitator Guide Approximate Time: 30 minutes Exercise prerequisite: Students should view the Bad Ad e-learning CME/CE
Review of Pharmacological Pain Management
 Review of Pharmacological Pain Management CHAMP Activities are possible with generous support from The Atlantic Philanthropies and The John A. Hartford Foundation The WHO Pain Ladder The World Health Organization
Review of Pharmacological Pain Management CHAMP Activities are possible with generous support from The Atlantic Philanthropies and The John A. Hartford Foundation The WHO Pain Ladder The World Health Organization
Guidance for Industry Labeling for Bronchodilators: Cold, Cough, Allergy, Bronchodilator,
 Reprinted from FDA s website by Guidance for Industry Labeling for Bronchodilators: Cold, Cough, Allergy, Bronchodilator, Guidance And Antiasthmatic for Drug Industry Products for Over-the-Counter Labeling
Reprinted from FDA s website by Guidance for Industry Labeling for Bronchodilators: Cold, Cough, Allergy, Bronchodilator, Guidance And Antiasthmatic for Drug Industry Products for Over-the-Counter Labeling
Regulatory Affairs Professionals Society: Regulatory Affairs Certification (RAC) Study Group FDA Regulation of Advertising and Promotion
 Regulatory Affairs Professionals Society: Regulatory Affairs Certification (RAC) Study Group FDA Regulation of Advertising and Promotion Alex Toy July 14, 2013 1 Agenda FDA Regulatory Framework Office
Regulatory Affairs Professionals Society: Regulatory Affairs Certification (RAC) Study Group FDA Regulation of Advertising and Promotion Alex Toy July 14, 2013 1 Agenda FDA Regulatory Framework Office
An Introduction to the Improved FDA Prescription Drug Labeling
 An Introduction to the Improved FDA Prescription Drug Labeling 1 Introduction Mary E. Kremzner, Pharm.D. CDR, U.S. Public Health Service Deputy Director, Division of Drug Information Center for Drug Evaluation
An Introduction to the Improved FDA Prescription Drug Labeling 1 Introduction Mary E. Kremzner, Pharm.D. CDR, U.S. Public Health Service Deputy Director, Division of Drug Information Center for Drug Evaluation
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA UNITED STATES OF AMERICA : CRIMINAL NO. v.
 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA UNITED STATES OF AMERICA : CRIMINAL NO. v. : DATE FILED: NOVARTIS PHARMACEUTICALS : VIOLATION: CORPORATION 21 U.S.C. 331(a),
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA UNITED STATES OF AMERICA : CRIMINAL NO. v. : DATE FILED: NOVARTIS PHARMACEUTICALS : VIOLATION: CORPORATION 21 U.S.C. 331(a),
David M. Stern, M.D. JAN 2 9 7007 Dean, University of Cincinnati College of Medicine 231 Albert Sabin Way Cincinnati, OH 45267
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Serv ice Food and Drug Administration 9200 Corporate Boulevard Rockville, Ma ry land 20850 WARNING LETTER VIA FEDERAL EXPRESS David M. Stern, M.D. JAN
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Serv ice Food and Drug Administration 9200 Corporate Boulevard Rockville, Ma ry land 20850 WARNING LETTER VIA FEDERAL EXPRESS David M. Stern, M.D. JAN
Investigational Drugs: Investigational Drugs and Biologics
 : I. PURPOSE The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics to ensure that adequate safeguards are in place
: I. PURPOSE The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics to ensure that adequate safeguards are in place
Interplay Between FDA Advertising and Promotion Enforcement Activities, Product Liability, and Consumer Fraud Litigation
 Interplay Between FDA Advertising and Promotion Enforcement Activities, Product Liability, and Consumer Fraud Litigation Leslie M. Tector Quarles & Brady LLP September 30, 2014 Objectives Which federal
Interplay Between FDA Advertising and Promotion Enforcement Activities, Product Liability, and Consumer Fraud Litigation Leslie M. Tector Quarles & Brady LLP September 30, 2014 Objectives Which federal
DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Guidance for Industry Fulfilling Regulatory Requirements for Postmarketing Submissions of Interactive Promotional Media for Prescription Human and Animal Drugs and Biologics DRAFT GUIDANCE This guidance
Guidance for Industry Fulfilling Regulatory Requirements for Postmarketing Submissions of Interactive Promotional Media for Prescription Human and Animal Drugs and Biologics DRAFT GUIDANCE This guidance
DEPARTMENT OF HEALTH & HUMAN SERVICES NDA 17-854 NDA 21-793 NDA 17-862. [inside address] Dear :
![DEPARTMENT OF HEALTH & HUMAN SERVICES NDA 17-854 NDA 21-793 NDA 17-862. [inside address] Dear : DEPARTMENT OF HEALTH & HUMAN SERVICES NDA 17-854 NDA 21-793 NDA 17-862. [inside address] Dear :](/thumbs/24/3528188.jpg) DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 NDA 17-854 [inside address] Dear : Please refer to your new drug application NDA xx-xxx for
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 NDA 17-854 [inside address] Dear : Please refer to your new drug application NDA xx-xxx for
DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Providing Regulatory Submissions in Electronic and Non-Electronic Format Promotional Labeling and Advertising Materials for Human Prescription Drugs Guidance for Industry DRAFT GUIDANCE This guidance document
Providing Regulatory Submissions in Electronic and Non-Electronic Format Promotional Labeling and Advertising Materials for Human Prescription Drugs Guidance for Industry DRAFT GUIDANCE This guidance document
PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain
 P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
Guidance for Industry Circumstances that Constitute Delaying, Denying, Limiting, or Refusing a Drug Inspection
 Guidance for Industry Circumstances that Constitute Delaying, Denying, Limiting, or Refusing a Drug Inspection U.S. Department of Health and Human Services Food and Drug Administration Office of Regulatory
Guidance for Industry Circumstances that Constitute Delaying, Denying, Limiting, or Refusing a Drug Inspection U.S. Department of Health and Human Services Food and Drug Administration Office of Regulatory
CENTER FOR DRUG EVALUATION AND RESEARCH. 203551Orig1s000
 CENTER FOR DRUG EVALUATION AND RESEARCH Approval Package for: APPLICATION NUMBER: Trade Name: Generic Name: Sponsor: 203551Orig1s000 Docetaxel Injection Concentrate, 20 mg/ml, 80 mg/4 ml, 140 mg/7 ml.
CENTER FOR DRUG EVALUATION AND RESEARCH Approval Package for: APPLICATION NUMBER: Trade Name: Generic Name: Sponsor: 203551Orig1s000 Docetaxel Injection Concentrate, 20 mg/ml, 80 mg/4 ml, 140 mg/7 ml.
Guidance for Hospitals, Nursing Homes, and Other Health Care Facilities
 Guidance for Hospitals, Nursing Homes, and Other Health Care Facilities FDA PUBLIC HEALTH ADVISORY Comments and suggestions regarding this document should be submitted within 90 days of publication in
Guidance for Hospitals, Nursing Homes, and Other Health Care Facilities FDA PUBLIC HEALTH ADVISORY Comments and suggestions regarding this document should be submitted within 90 days of publication in
Goals & Objectives. Drug Development & the FDA Pharmacy 309. Outline. An History of Disasters. Be able to describe
 Drug Development & the FDA Pharmacy 309 Tom Hazlet, Pharm.D., Dr.P.H. 616-2732 thazlet@u... Goals & Objectives Be able to describe the major regulatory events in the drug development process the concepts
Drug Development & the FDA Pharmacy 309 Tom Hazlet, Pharm.D., Dr.P.H. 616-2732 thazlet@u... Goals & Objectives Be able to describe the major regulatory events in the drug development process the concepts
General Wellness: Policy for Low Risk Devices. Draft Guidance for Industry and Food and Drug Administration Staff
 General Wellness: Policy for Low Risk Devices Draft Guidance for Industry and Food and Drug Administration Staff DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Document
General Wellness: Policy for Low Risk Devices Draft Guidance for Industry and Food and Drug Administration Staff DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Document
Guidance for Industry and FDA Staff
 Guidance for Industry and FDA Staff Dear Health Care Provider Letters: Improving Communication of Important Safety Information U.S. Department of Health and Human Services Food and Drug Administration
Guidance for Industry and FDA Staff Dear Health Care Provider Letters: Improving Communication of Important Safety Information U.S. Department of Health and Human Services Food and Drug Administration
Guidance for Industry
 Guidance for Industry Consumer-Directed Broadcast Advertisements Questions and Answers U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research
Guidance for Industry Consumer-Directed Broadcast Advertisements Questions and Answers U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research
Center for Biologics Evaluation and Research Fiscal Year 2005
 The Warning Letters presented in this chapter were chosen to provide examples of the types of Warning Letters issued for violations of FDA laws. A complete list of Warning Letters issued is available on
The Warning Letters presented in this chapter were chosen to provide examples of the types of Warning Letters issued for violations of FDA laws. A complete list of Warning Letters issued is available on
Transcutaneous Electrical Nerve Stimulation Device LUMI-TENS
 Transcutaneous Electrical Nerve Stimulation Device LUMI-TENS Operation Manual Read Before Using LUMI-TENS-INS-LAB-RevA08 TABLE OF CONTENTS INTRODUCTION TO TENS INDICATIONS AND CONTRAINDICATIONS WARNINGS
Transcutaneous Electrical Nerve Stimulation Device LUMI-TENS Operation Manual Read Before Using LUMI-TENS-INS-LAB-RevA08 TABLE OF CONTENTS INTRODUCTION TO TENS INDICATIONS AND CONTRAINDICATIONS WARNINGS
WALKING THE COMPLIANCE LINE: A REGULATORY LAWYER S PERSPECTIVE
 WALKING THE COMPLIANCE LINE: A REGULATORY LAWYER S PERSPECTIVE Presented By: Alan B. Minsk Partner and Chair, Food and Drug Practice Team Arnall Golden Gregory LLP alan.minsk@agg.com Presented To: ISMPP
WALKING THE COMPLIANCE LINE: A REGULATORY LAWYER S PERSPECTIVE Presented By: Alan B. Minsk Partner and Chair, Food and Drug Practice Team Arnall Golden Gregory LLP alan.minsk@agg.com Presented To: ISMPP
Guidance for Industry Responding to Unsolicited Requests for Off-Label Information About Prescription Drugs and Medical Devices
 Guidance for Industry Responding to Unsolicited Requests for Off-Label Information About Prescription Drugs and Medical Devices DRAFT GUIDANCE This guidance document is being distributed for comment purposes
Guidance for Industry Responding to Unsolicited Requests for Off-Label Information About Prescription Drugs and Medical Devices DRAFT GUIDANCE This guidance document is being distributed for comment purposes
E-ALERT Food & Drug 2012 END-OF-YEAR SUMMARY OF FDA PROMOTIONAL ENFORCEMENT ACTIVITY OFFICE OF PRESCRIPTION DRUG PROMOTION (OPDP) ENFORCEMENT ACTIVITY
 E-ALERT Food & Drug March 18, 2013 2012 END-OF-YEAR SUMMARY OF FDA PROMOTIONAL ENFORCEMENT ACTIVITY This client alert reviews the warning and untitled letters issued in 2012 by the Office of Prescription
E-ALERT Food & Drug March 18, 2013 2012 END-OF-YEAR SUMMARY OF FDA PROMOTIONAL ENFORCEMENT ACTIVITY This client alert reviews the warning and untitled letters issued in 2012 by the Office of Prescription
Re: September 19,2002 meeting of the Nonprescription Drugs Advisory Committee to discuss safety issues related to the use of acetaminophen.
 Pfizer Inc 201 Tabor Road Moms Plains, NJ 07950 Tel 973 385 2000 Fax 973 385 3761 Sandra Titus Advisors and Consultants Staff FDAKDER 5630 Fishers Lane Rockville, MD 20857 Re: September 19,2002 meeting
Pfizer Inc 201 Tabor Road Moms Plains, NJ 07950 Tel 973 385 2000 Fax 973 385 3761 Sandra Titus Advisors and Consultants Staff FDAKDER 5630 Fishers Lane Rockville, MD 20857 Re: September 19,2002 meeting
Guidance for Industry Time and Extent Applications for Nonprescription Drug Products
 Guidance for Industry Time and Extent Applications for Nonprescription Drug Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
Guidance for Industry Time and Extent Applications for Nonprescription Drug Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
Guidance for Industry
 Guidance for Industry Charging for Investigational Drugs Under an IND Qs & As DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this
Guidance for Industry Charging for Investigational Drugs Under an IND Qs & As DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this
Leukocytoclastic Vasculitis and Stasis Dermatitis With Id Reaction
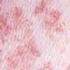 Id Reaction December 01, 2007 By David L. Kaplan, MD [1] A Photo Quiz to Hone Dermatologic Skills Case 1: A slightly pruritic eruption developed on the lower legs of a 39-year-old woman after she had an
Id Reaction December 01, 2007 By David L. Kaplan, MD [1] A Photo Quiz to Hone Dermatologic Skills Case 1: A slightly pruritic eruption developed on the lower legs of a 39-year-old woman after she had an
How To Weigh Data From A Study
 PRINCIPLES ON RESPONSIBLE SHARING OF TRUTHFUL AND NON-MISLEADING INFORMATION ABOUT MEDICINES WITH HEALTH CARE PROFESSIONALS AND PAYERS INTRODUCTION In the era of data-driven medicine, where all parties
PRINCIPLES ON RESPONSIBLE SHARING OF TRUTHFUL AND NON-MISLEADING INFORMATION ABOUT MEDICINES WITH HEALTH CARE PROFESSIONALS AND PAYERS INTRODUCTION In the era of data-driven medicine, where all parties
February 2006 Procedural
 Guidance for Industry Reports on the Status of Postmarketing Study Commitments Implementation of Section 130 of the Food and Drug Administration Modernization Act of 1997 U.S. Department of Health and
Guidance for Industry Reports on the Status of Postmarketing Study Commitments Implementation of Section 130 of the Food and Drug Administration Modernization Act of 1997 U.S. Department of Health and
interpretation of its Hazard Analysis Critical Control Point (I-IACCP) regulations for fish and fishery
 DEPARTMENT OF HEALTH ANb HUMAN SERVICES Food and Drug Administration [Docket No. OOD-15551 Guidance for Industry on Refusal of Inspection or Access to HACCP Records Pertaining to the Safe and Sanitary
DEPARTMENT OF HEALTH ANb HUMAN SERVICES Food and Drug Administration [Docket No. OOD-15551 Guidance for Industry on Refusal of Inspection or Access to HACCP Records Pertaining to the Safe and Sanitary
Guidance for Industry
 #108 Guidance for Industry How to Register with the CVM Electronic Submission System To Submit Information in Electronic Format Using the FDA Electronic Submissions Gateway This version of the guidance
#108 Guidance for Industry How to Register with the CVM Electronic Submission System To Submit Information in Electronic Format Using the FDA Electronic Submissions Gateway This version of the guidance
Guidance for Industry. Required Warnings for Cigarette Packages and Advertisements
 Guidance for Industry Required Warnings for Cigarette Packages and Advertisements Small Entity Compliance Guide Written comments and suggestions may be submitted at any time for Agency consideration to
Guidance for Industry Required Warnings for Cigarette Packages and Advertisements Small Entity Compliance Guide Written comments and suggestions may be submitted at any time for Agency consideration to
Guidance for Industry Formal Meetings Between the FDA and Sponsors or Applicants
 Guidance for Industry Formal Meetings Between the FDA and Sponsors or Applicants U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
Guidance for Industry Formal Meetings Between the FDA and Sponsors or Applicants U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
Nova Southeastern University Institutional Review Board Policies and Procedures
 Page 1 of 14 Purpose: To establish policy with respect to the emergency use of unapproved drugs and biologics. Definitions: 1. IRB approval means the determination of the IRB that the research has been
Page 1 of 14 Purpose: To establish policy with respect to the emergency use of unapproved drugs and biologics. Definitions: 1. IRB approval means the determination of the IRB that the research has been
Medical Devices; Cardiovascular Devices; Classification of the Esophageal Thermal Regulation
 This document is scheduled to be published in the Federal Register on 08/18/2015 and available online at http://federalregister.gov/a/2015-20317, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 08/18/2015 and available online at http://federalregister.gov/a/2015-20317, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
NDA 206321 SAXENDA (liraglutide [rdna origin] injection) Novo Nordisk Inc. 800 Scudders Mill Road, Plainsboro, NJ 08536 Phone: 609-786-4690
![NDA 206321 SAXENDA (liraglutide [rdna origin] injection) Novo Nordisk Inc. 800 Scudders Mill Road, Plainsboro, NJ 08536 Phone: 609-786-4690 NDA 206321 SAXENDA (liraglutide [rdna origin] injection) Novo Nordisk Inc. 800 Scudders Mill Road, Plainsboro, NJ 08536 Phone: 609-786-4690](/thumbs/30/14629112.jpg) Initial REMS approval: 12/2014 NDA 206321 SAXENDA (liraglutide [rdna origin] injection) Novo Nordisk Inc. 800 Scudders Mill Road, Plainsboro, NJ 08536 Phone: 609-786-4690 RISK EVALUATION AND MITIGATION
Initial REMS approval: 12/2014 NDA 206321 SAXENDA (liraglutide [rdna origin] injection) Novo Nordisk Inc. 800 Scudders Mill Road, Plainsboro, NJ 08536 Phone: 609-786-4690 RISK EVALUATION AND MITIGATION
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio
 ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
Risk Evaluation and Mitigation Strategies: Modifications and Revisions Guidance for Industry
 Risk Evaluation and Mitigation Strategies: Modifications and Revisions Guidance for Industry The portion of this guidance document setting forth the submission procedures for risk evaluation and mitigation
Risk Evaluation and Mitigation Strategies: Modifications and Revisions Guidance for Industry The portion of this guidance document setting forth the submission procedures for risk evaluation and mitigation
Guidance for Industry Bar Code Label Requirements Questions and Answers
 Guidance for Industry Bar Code Label Requirements Questions and Answers For questions on the content of this guidance, contact the Center for Drug Evaluation and Research or the Center for Biologics Evaluation
Guidance for Industry Bar Code Label Requirements Questions and Answers For questions on the content of this guidance, contact the Center for Drug Evaluation and Research or the Center for Biologics Evaluation
Guidance for Industry
 Guidance for Industry Labeling for Human Prescription Drug and Biological Products Implementing the PLR Content and Format Requirements U.S. Department of Health and Human Services Food and Drug Administration
Guidance for Industry Labeling for Human Prescription Drug and Biological Products Implementing the PLR Content and Format Requirements U.S. Department of Health and Human Services Food and Drug Administration
JAN 1 6 2007 WARNING LETTER CERTIFIED MAIL RETURN RECEIPT REQUESTED
 E DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville MD 20857 - JAN 1 6 2007 WARNING LETTER CERTIFIED MAIL RETURN RECEIPT REQUESTED Lawrence S. Kim, M.D.
E DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville MD 20857 - JAN 1 6 2007 WARNING LETTER CERTIFIED MAIL RETURN RECEIPT REQUESTED Lawrence S. Kim, M.D.
GENERAL INFORMATION. Adverse Event (AE) Definition (ICH GUIDELINES E6 FOR GCP 1.2):
 Make copies of the blank SAE report form as needed. Retain originals with confirmation of all information faxed to DMID Pharmacovigilance Group Clinical Research Operations and Management Support (CROMS
Make copies of the blank SAE report form as needed. Retain originals with confirmation of all information faxed to DMID Pharmacovigilance Group Clinical Research Operations and Management Support (CROMS
DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Guidance for Industry Format and Content of Proposed Risk Evaluation and Mitigation Strategies (REMS), REMS Assessments, and Proposed REMS Modifications DRAFT GUIDANCE This guidance document is being distributed
Guidance for Industry Format and Content of Proposed Risk Evaluation and Mitigation Strategies (REMS), REMS Assessments, and Proposed REMS Modifications DRAFT GUIDANCE This guidance document is being distributed
Guidance for Industry. 21 CFR Part 11; Electronic Records; Electronic Signatures. Electronic Copies of Electronic Records
 Guidance for Industry 21 CFR Part 11; Electronic Records; Electronic Signatures Electronic Copies of Electronic Records Draft Guidance This guidance document is being distributed for comment purposes only.
Guidance for Industry 21 CFR Part 11; Electronic Records; Electronic Signatures Electronic Copies of Electronic Records Draft Guidance This guidance document is being distributed for comment purposes only.
Guidance for Industry Investigator Responsibilities Protecting the Rights, Safety, and Welfare of Study Subjects
 Guidance for Industry Investigator Responsibilities Protecting the Rights, Safety, and Welfare of Study Subjects U.S. Department of Health and Human Services Food and Drug Administration Center for Drug
Guidance for Industry Investigator Responsibilities Protecting the Rights, Safety, and Welfare of Study Subjects U.S. Department of Health and Human Services Food and Drug Administration Center for Drug
Guidance for Industry Drug Supply Chain Security Act Implementation: Identification of Suspect Product and Notification
 Guidance for Industry Drug Supply Chain Security Act Implementation: Identification of Suspect Product and Notification DRAFT GUIDANCE This guidance document is being distributed for comment purposes only.
Guidance for Industry Drug Supply Chain Security Act Implementation: Identification of Suspect Product and Notification DRAFT GUIDANCE This guidance document is being distributed for comment purposes only.
Nurse Aide Training Program Application Checklist
 Nurse Aide Training Program Application Checklist The following checklist must be completed before enrolling in the Nurse Aide Training course: Complete, sign, and date the Application Form Have the physical
Nurse Aide Training Program Application Checklist The following checklist must be completed before enrolling in the Nurse Aide Training course: Complete, sign, and date the Application Form Have the physical
105 CMR: DEPARTMENT OF PUBLIC HEALTH 105 CMR 210.000: THE ADMINISTRATION OF PRESCRIPTION MEDICATIONS IN PUBLIC AND PRIVATE SCHOOLS
 105 CMR 210.000: THE ADMINISTRATION OF PRESCRIPTION MEDICATIONS IN PUBLIC AND PRIVATE SCHOOLS Section 210.001: Purpose 210.002: Definitions 210.003: Policies Governing the Administration of Prescription
105 CMR 210.000: THE ADMINISTRATION OF PRESCRIPTION MEDICATIONS IN PUBLIC AND PRIVATE SCHOOLS Section 210.001: Purpose 210.002: Definitions 210.003: Policies Governing the Administration of Prescription
Phoenix Thera-Lase Systems, LLC Ms. Diane Rutherford Ken Block Consulting 1201 Richardson Drive, Suite 280 Richardson, Texas 75080
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Phoenix Thera-Lase Systems,
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Phoenix Thera-Lase Systems,
Guidance for Industry
 Guidance for Industry Electronic Source Data in Clinical Investigations DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Guidance for Industry Electronic Source Data in Clinical Investigations DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Guidance for Industry Classifying Resubmissions in Response to Action Letters
 Guidance for Industry Classifying Resubmissions in Response to Action Letters U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
Guidance for Industry Classifying Resubmissions in Response to Action Letters U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
UNITED STATES OF AMERICA BEFORE THE FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES
 UNITED STATES OF AMERICA BEFORE THE FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES In the Matter of Dendy Foods, Inc. d/b/a Grocer s Pride ADMINISTRATIVE COMPLAINT FOR CIVIL MONEY
UNITED STATES OF AMERICA BEFORE THE FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES In the Matter of Dendy Foods, Inc. d/b/a Grocer s Pride ADMINISTRATIVE COMPLAINT FOR CIVIL MONEY
Triangle Compounding 11/2/15
 Triangle Compounding 11/2/15 Department of Health and Human Services Public Health Service Food and Drug Administration Atlanta District 60 Eighth Street NE Atlanta, GA 30309 November 2, 2015 VIA UNITED
Triangle Compounding 11/2/15 Department of Health and Human Services Public Health Service Food and Drug Administration Atlanta District 60 Eighth Street NE Atlanta, GA 30309 November 2, 2015 VIA UNITED
5.07.09. Aubagio. Aubagio (teriflunomide) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Marketed Unapproved Drugs: FDA to Take Immediate Enforcement Action at Any Time, Without Prior Notice
 Marketed Unapproved Drugs: FDA to Take Immediate Enforcement Action at Any Time, Without Prior Notice Kurt R. Karst Hyman, Phelps & McNamara, P.C. 700 Thirteenth Street, N.W., Suite 1200 Washington, D.C.
Marketed Unapproved Drugs: FDA to Take Immediate Enforcement Action at Any Time, Without Prior Notice Kurt R. Karst Hyman, Phelps & McNamara, P.C. 700 Thirteenth Street, N.W., Suite 1200 Washington, D.C.
JUL 2 2008. Ms. Kendra Basler Regulatory Affairs Associate Abbott Vascular Cardiac Therapies 3200 Lakeside Drive Santa Clara, CA 95054-2807
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service JUL 2 2008 Food and Drug Administration 9200 Corporate Boulevard Rockville MD 20850 Ms. Kendra Basler Regulatory Affairs Associate Abbott Vascular
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service JUL 2 2008 Food and Drug Administration 9200 Corporate Boulevard Rockville MD 20850 Ms. Kendra Basler Regulatory Affairs Associate Abbott Vascular
3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP
 PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
[DOCKET NO.96N-0002] DRAFT
![[DOCKET NO.96N-0002] DRAFT [DOCKET NO.96N-0002] DRAFT](/thumbs/26/7603707.jpg) [DOCKET NO.96N-0002] DRAFT DRAFT DOCUMENT CONCERNING THE REGULATION OF PLACENTAL/UMBILICAL CORD BLOOD STEM CELL PRODUCTS INTENDED FOR TRANSPLANTATION OR FURTHER MANUFACTURE INTO INJECTABLE PRODUCTS DECEMBER,
[DOCKET NO.96N-0002] DRAFT DRAFT DOCUMENT CONCERNING THE REGULATION OF PLACENTAL/UMBILICAL CORD BLOOD STEM CELL PRODUCTS INTENDED FOR TRANSPLANTATION OR FURTHER MANUFACTURE INTO INJECTABLE PRODUCTS DECEMBER,
Guidance for Industry
 Guidance for Industry FDA Export Certificates Submit comments and suggestions regarding this document at anytime to Dockets Management Branch (HFA-305), Food and Drug Administration, 5630 Fishers Lane,
Guidance for Industry FDA Export Certificates Submit comments and suggestions regarding this document at anytime to Dockets Management Branch (HFA-305), Food and Drug Administration, 5630 Fishers Lane,
POLICY STATEMENT. Guidelines for Refractive Surgery Advertising
 POLICY STATEMENT Guidelines for Refractive Surgery Advertising A joint statement of the American Academy of Ophthalmology, the American Society of Cataract and Refractive Surgery and the International
POLICY STATEMENT Guidelines for Refractive Surgery Advertising A joint statement of the American Academy of Ophthalmology, the American Society of Cataract and Refractive Surgery and the International
October 28, 2015. Cavex Holland Bv Mr. Richard Woortman Manager Technical Services Fustweg 5 Haarlem, 2031CJ The NETHERLANDS
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 October 28, 2015 Cavex
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 October 28, 2015 Cavex
160S01105, Page 1 of 7. Human Hepatitis B Immunoglobulin, solution for intramuscular injection.
 160S01105, Page 1 of 7 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF
160S01105, Page 1 of 7 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF
How To Use Merrimack Web Site
 TERMS AND CONDITIONS OF USE PLEASE READ THESE TERMS AND CONDITIONS OF USE CAREFULLY. THESE TERMS AND CONDITIONS OF USE MAY HAVE CHANGED SINCE YOUR LAST VISIT TO THIS WEB SITE. BY USING THIS WEB SITE, YOU
TERMS AND CONDITIONS OF USE PLEASE READ THESE TERMS AND CONDITIONS OF USE CAREFULLY. THESE TERMS AND CONDITIONS OF USE MAY HAVE CHANGED SINCE YOUR LAST VISIT TO THIS WEB SITE. BY USING THIS WEB SITE, YOU
RISK EVALUATION AND MITIGATION STRATEGY (REMS)
 Initial REMS approval: 08/2015 NDA 22526 Addyi TM (flibanserin) Serotonin 1A receptor agonist and a Serotonin 2A receptor antagonist Sprout Pharmaceuticals, Inc. 4208 Six Forks Road, Suite 1010 Raleigh,
Initial REMS approval: 08/2015 NDA 22526 Addyi TM (flibanserin) Serotonin 1A receptor agonist and a Serotonin 2A receptor antagonist Sprout Pharmaceuticals, Inc. 4208 Six Forks Road, Suite 1010 Raleigh,
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
EMEA PUBLIC STATEMENT ON LEFLUNOMIDE (ARAVA) - SEVERE AND SERIOUS HEPATIC REACTIONS -
 The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
Guideline for Industry
 Guideline for Industry The Extent of Population Exposure to Assess Clinical Safety: For Drugs Intended for Longterm Treatment of Non-Life- Threatening Conditions ICH-E1A March 1995 GUIDELINE FOR INDUSTRY
Guideline for Industry The Extent of Population Exposure to Assess Clinical Safety: For Drugs Intended for Longterm Treatment of Non-Life- Threatening Conditions ICH-E1A March 1995 GUIDELINE FOR INDUSTRY
Guidance. Useful Written Consumer Medication Information (CMI)
 Guidance Useful Written Consumer Medication Information (CMI) U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics
Guidance Useful Written Consumer Medication Information (CMI) U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics
May 5, 2015. Dear Mr. Courtney:
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 May 5, 2015 Terumo Cardiovascular
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 May 5, 2015 Terumo Cardiovascular
Inspections, Compliance, Enforcement, and Criminal Investigations
 Page 1 of 7 Home Inspections, Compliance, Enforcement, and Criminal Investigations Compliance Actions and Activities Warning Letters 2014 Inspections, Compliance, Enforcement, and Criminal Investigations
Page 1 of 7 Home Inspections, Compliance, Enforcement, and Criminal Investigations Compliance Actions and Activities Warning Letters 2014 Inspections, Compliance, Enforcement, and Criminal Investigations
Maintenance of abstinence in alcohol dependence
 Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Drug Development Process
 Drug Development Process Original Arthur: Addie D. Anderson CRB Consulting Engineers, Inc. Overview Important milestones establishing our current system of regulations Step-by-step overview of the drug
Drug Development Process Original Arthur: Addie D. Anderson CRB Consulting Engineers, Inc. Overview Important milestones establishing our current system of regulations Step-by-step overview of the drug
Q(K SVJM~jPagelIof 3
 K13 0460 Q(K SVJM~jPagelIof 3 JUL 1 12013 This 51 0(k) Summary is being submitted in accordance with the requirements of the Safe Medical Device Act (SMDA) of 1990. The content contained in this 510(k)
K13 0460 Q(K SVJM~jPagelIof 3 JUL 1 12013 This 51 0(k) Summary is being submitted in accordance with the requirements of the Safe Medical Device Act (SMDA) of 1990. The content contained in this 510(k)
CTC Technology Readiness Levels
 CTC Technology Readiness Levels Readiness: Software Development (Adapted from CECOM s Software Technology Readiness Levels) Level 1: Basic principles observed and reported. Lowest level of software readiness.
CTC Technology Readiness Levels Readiness: Software Development (Adapted from CECOM s Software Technology Readiness Levels) Level 1: Basic principles observed and reported. Lowest level of software readiness.
ANALYSIS OF MARKET RESEARCH DATA ON THE ICY HOT PATCH
 ANALYSIS OF MARKET RESEARCH DATA ON THE ICY HOT PATCH Manufacturers of over-the-counter products routinely conduct market research to identify consumer interest in and reaction to new products or modifications
ANALYSIS OF MARKET RESEARCH DATA ON THE ICY HOT PATCH Manufacturers of over-the-counter products routinely conduct market research to identify consumer interest in and reaction to new products or modifications
Medical Billing and Agency Formal Disputes
 Guidance for Industry Formal Dispute Resolution: Appeals Above the Division Level Additional copies of this Guidance are available from: Office of Training and Communications Division of Communications
Guidance for Industry Formal Dispute Resolution: Appeals Above the Division Level Additional copies of this Guidance are available from: Office of Training and Communications Division of Communications
