Technology. Brief. An assay
|
|
|
- Tyrone Ford
- 8 years ago
- Views:
Transcription
1 Health Policy Advisory Committee on Technology Technology Brief An assay to identify biopsy negative prostate cancer February 2013
2 State of Queensland (Queensland Department of Health) 2013 This work is licensed under a Creative Commons Attribution Non Commercial Noo Derivativess 2.5 Australiaa licence. In essence, you are free too copy and communicatee the work inn its current form f for non commercial purposes, as long as you attribute the authors and abide a by the licence terms. You may not alter or adapt the work in any way. To view a copy of this licence, visit nc nd/2.5/au/. For further information, contact the HealthPACT Secretariat at: HealthPACT Secretariat c/o Clinical Access and Redesign Unit, Healthh Service & Clinical Innovation Division Queensland Department of Health Lobby 2, Level 2, Citilink Business Centre 153 Campbell Street, Bowen Hills QLD 4006 Postal Address: GPO Box 48, Brisbane Qld gov.au Telephone: For permissions beyond the scope of this licence contact: Intellectual Property Officer, Queensland Health, GPO Box 48, Brisbane Qld 4001, phone (07) Electronic copies can be obtained from: DISCLAIMER: This brief is published with the intention of providing information of interest. Itt is based on information available at the time of research and cannot be expected to cover any developments arising from subsequent improvements to health technologies. This brief is based on a limited literature search and iss not a definitive statement on the safety, effectiveness or cost the effectiveness of the health technology covered. The State of Queensland acting through t Queensland Department of Health doess not guarantee accuracy, currency or completeness of the information in this brief. Information may containn or summarise the views of others, and not necessarily reflect the views of Queensland Department of Health. This brief is not intended to be used as medical advice and it is not intended to be used to diagnose, treat, cure or prevent any disease, nor should it be used for therapeutic purposes or as a substitute for a health professional's advice. It must not be relied upon withoutt verificationn from authoritative sources. Queensland Department of Health does not accept any liability, including for any injury, loss or damage, incurred by use of orr reliance onn the information. This brief was commissioned by Queenslandd Department of Health, in its role as the Secretariat of the Health Policy Advisory Committee on Technology (HealthPACT). The production of this brief was overseen by HealthPACT. HealthPACT comprises representatives from health departments in all States and Territories, the Australian and New Zealand governments s and MSAC. It is a sub to AHMAC s Hospital Principal Committee (HPC). AHMACC supports HealthPACT through funding. This brief was prepared by Dr. Vicki Foerster, Dr. Merrîccc Edgar Hughes, Deanne Forel and Ben Hoggan for the Australian Safety and Efficacyy Register of New Interventional Procedures Surgical (ASERNIP S). committee of the Australian Health Ministers Advisory Council (AHMAC), reporting
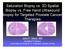
3 Technology, Company and Licensing Register ID Technology name Patientt indication WP 147 Assay to identify biopsy negative prostatee cancer: ConfirmMDx for Prostate Cancer Intended for use to assess tissue obtained from prostate biopsies, primarily to rule out false negative results. Description of the technology Prostate biopsy procedures generally collect 10 to 12 needle biopsy cores, sampling less l than one per cent of a prostate. This leads to a high rate of false negative results (25 30%) 1 and can lead to many repeat biopsies, often on men who are free of prostate cancer. ConfirmMDx TM for Prostate Cancer is an epigenetic assay to help distinguish patientss who have true negativee biopsies from those at risk for occult cancer. Epigenetics is the study of genomee alterations that do not involve a change in the nucleotide sequence but rather changes such as DNA methylation. The ConfirmMDx TM assay tests the DNAA methylation status of several genes associated with prostate cancer via a quantitative methylation specificc polymerase chain reaction (PCR) assay. Included in the assay are glutathione S transferase P1 (GSTP1), adenomatous polyposis coli (APC) and retinoic acid receptor B2. The assay, commercially available as of May 2012 in the Unitedd States of America, helps urologists to identify prostate cancer free men to avoid unnecessary repeat biopsies and also helps to identify high risk patients who may require repeat biopsies and potential treatment. The test is able to detect an epigenetic field effect or o halo associated with the cancerisation process at the DNA level in cells adjacent to cancer foci (Figure 1). This molecular halo around a cancer lesion can be present despite a normal microscopic appearance. 2 Currently, prostate biopsy specimens are submitted to the vendor s laboratories in the United States (US) or Belgium for DNA extraction and analysis. Figure 1 The halo effect of a tumour of the prostate and the possibilityy of missing tumour cells on biopsy (used with permission of MDxHealth) ConfirmMDx TM Assay for prostate cancer: February
4 Company or developer MDxHealth Inc., Belgium and US (California). 3 Reason for assessment A technology with the potential to reduce the number of false positive results related to biopsy for prostate cancer. Stage of development in Australia Yet to emerge Established Experimental Established but changed indication or modification of technique Investigational Should be taken out of use Nearly established Licensing, reimbursement and other approval In Australia, the ConfirmMDx TM assay would most likely be classified as an in vitro diagnostic and therefore would require inclusion on the Australian Register of Therapeutic Goods (ARTG) before marketing. No evidence of its presence on the ARTG was located. In the US, individual laboratory tests do not require US Food & Drug Administration approval. In a related process, the US Centers for Medicare & Medicaid Services regulates laboratory testing performed on humans through the Clinical Laboratory Improvement Amendments (CLIA) which cover 225,000 laboratory entities. The MDxHealth California laboratory received CLIA certification in January The laboratory is also accredited by the College of American Pathologists. Australian Therapeutic Goods Administration approval Yes ARTG number (s) No Not applicable Technology type Technology use Diagnostics Diagnostic Patient Indication and Setting Disease description and associated mortality and morbidity The causes of prostate cancer have not been established; however, the major risk factors for prostate cancer include age and family history. ConfirmMDx TM Assay for prostate cancer: February
5 In 2010, prostate cancer was the most common cancer in Australian men, with a five year prevalence of 72,582 (39% of males with cancer) followed by melanoma and bowel cancer. It was the second highest cause of cancer death after lung cancer at 3,235 deaths; mean age at death was 80 years. 5 Prostate cancer was associated with 16,935 overnight hospitalisations in , eight per cent of all those for cancer. It was also associated with 18,241 same day admissions, the highest of all cancers except non melanoma skin cancer. The overall benefits of screening for prostate cancer are currently uncertain, and there is no organised population screening program for prostate cancer in Australia or anywhere in the world. As to why there is no national screening program for prostate cancer in Australia: While prostate cancer is also an important health condition, current evidence is that the commonly used PSA test, either alone or combined with digital rectal examination, is not suitable for use in a population based screening program, and the harms of such screening outweigh the benefits. 5 Number of patients In Australia in 2012, prostate cancer was expected to be the most common cancer with an estimated incidence of 18,560 cases (age standardised rate [ASR] of per 100,000); the mean age at diagnosis was 67.4 years. Fluctuations in incidence occur year to year and are thought to be due mainly to methods of diagnosis. For example, prostate specific antigen (PSA) testing was listed in the Medicare Benefits Schedule (MBS) in 1989 and a diagnostic peak in the early 1990s reflected the large pool of undiagnosed cases identified using the PSA test (and subsequent biopsy). A second rise in incidence in 2008 (ASR peaked at 191) may have been due to changes in diagnostic procedures, including lowering the investigation threshold, that led to an increase in the use of biopsy. In a 2008 international comparison, Australia had the highest incidence rate of prostate cancer among all countries considered. 5 Speciality Technology setting Men s health / Surgery / Urology Specialist hospital / general hospital Impact Alternative and/or complementary technology ConfirmMDx TM would be used in combination with current diagnostic modalities, and would not replace them. A competitor test, the Prostate Core Mitomic Test TM (Mitomics, Ontario, Canada), is described as a highly advanced, proprietary test based on the science of mitochondrial DNA. Using existing prostate biopsy tissue, the test can determine the presence of malignant cells by detecting underlying molecular alterations in normal appearing tissue. 6 ConfirmMDx TM Assay for prostate cancer: February
6 Another emerging technology to detect malignant disease in patients with false negative biopsies of prostate tissue involves various new hematologic biomarkers such as PSA isoforms. 7 Current technology Currently, the most common methods of identifying prostate cancer are the PSA blood test, with or without digital rectal examination (DRE), and needle biopsy of the prostate with histopathological analysis of the samples. 8 The two former tests may be conducted as a screening manoeuvre or case finding as a result of symptoms. However, DRE and PSA have limited specificity (up to 75 per cent of men with elevated PSA levels do not have cancer), 8 and PSA screening has a high rate of false positive results. 9 This results in large numbers of men with elevated PSA and/or other high risk features, but a negative prostate biopsy. In this situation it is recommended that anterior zone biopsies also be taken. 10 Many patients undergo subsequent biopsy procedures, although only per cent of second biopsies detect cancer; this proportion decreases after each subsequent negative biopsy. Improved methods are needed to identify men who can forgo a repeat biopsy. The ideal test would have a high negative predictive value (NPV) and a low false negative rate. 11 Diffusion of technology in Australia Not yet available in Australia. International utilisation Country Belgium United Kingdom Trials underway or completed Level of Use Limited use United States Likely: MDxHealth says it is only targeting urologists in the US, given the size of the market sales were US$2.6 million in the first half of Widely diffused Cost infrastructure and economic consequences The cost of prostate biopsy (i.e. as a repeat procedure to follow up) was estimated to be approximately US$2,500 (approx. A$2,411). In contrast, the price of the ConfirmMDx TM test was reported as US$146 (approx. A$141) per tissue sample. 12 If 10 to 12 biopsy samples are generally obtained per patient, and all are tested, the cost of testing per patient would be US$1,500 to US$1,800 (approx. A$1,447 to A$1,736). However, clinical expert opinion suggests that many urologists will take between 16 and 30 cores for repeat or saturation ConfirmMDx TM Assay for prostate cancer: February
7 biopsies prior to placing someone on active surveillance, 13 which would increase the cost of testing to anywhere between US$2,300 and US$4,400 (approx. A$2218 to A$4244). MBS 14 costs associated with current tests for needle biopsy and PSA are listed in Table 1. Table 1 MBS fees and benefits associated with current tests for prostate cancer Item number Description Fee Benefit PROSTATE, needle biopsy of, or injection into, excluding for insertion of radiopaque markers (anaes.) PROSTATE, needle biopsy of, using prostatic ultrasound techniques and obtaining 1 or more prostatic specimens, being a service associated with a service to which item or applies (anaes.) (assist.) Prostate specific antigen - quantitation - 1 of this item in a 12-month period. (Item is subject to rule 25.) Prostate specific antigen - quantitation in the monitoring of previously diagnosed prostatic disease (including a test described in item 66655) Prostate specific antigen - quantitation of 2 or more fractions of PSA and any derived index including (if performed) a test described in item 66656, in the follow-up of a PSA result that lies at or above the agerelated median but below the age-related, method specific 97.5% reference limit - 1 of this item in a 12- month period. (Item is subject to rule 25.) Prostate specific antigen - quantitation of 2 or more fractions of PSA and any derived index including (if performed) a test described in item 66656, in the follow-up of a PSA result that lies at or above the agerelated, method specific 97.5% reference limit, but below a value of 10 ug/l - 4 of this item in a 12-month period. (Item is subject to rule 25.) MBS: Medicare Benefits Schedule; PSA: prostate-specific antigen. $ % = $ % = $ $ % = $ % = $ $ % = $ % = $17.15 $ % = $ % = $17.15 $ % = $ % = $31.75 $ % = $ % = $31.75 Ethical, cultural or religious considerations Men in rural and regional Australia have a 21 per cent higher prostate cancer mortality rate than men in capital cities. This is attributed to a combination of lack of awareness and education about prostate cancer, distance from testing and treatment, poor primary care provider awareness, and limited access to urologists and other specialists. 15 Evidence and Policy Safety and effectiveness Evidence for this brief comes from three diagnostic accuracy studies, one prospective 11 and 8, 16 two retrospective. Trock et al 11 An industry funded, prospective, three site study in the US (level II diagnostic accuracy study) enrolled consecutive men whose initial prostate biopsy was negative but who were at high risk for a missed cancer (based on suspicious DRE, high risk PSA level, or atypical cells ConfirmMDx TM Assay for prostate cancer: February
8 on initial biopsy). Of 162 enrolled men, 86 (53%) met inclusion criteria and had adequate testing samples (mean age 60 years, 80% white). These men were stratified into three subgroups: Group 1 (n=21) had suspicious DRE or high risk PSA level (PSA 8.0ng/ml and prostate volume < 50.0ml, PSA density 0.2ng/ml/cm 3 or % free PSA 10.0) Group 2 (n=39) had high grade prostatic intraepithelial neoplasia Group 3 (n=26) had atypical small acinar proliferation on initial biopsy. The men in these subgroups were estimated to have a per cent probability of prostate cancer detection on repeat biopsy, and it was hypothesised a priori that their prostate samples would exhibit different biomarker performance characteristics. The aim of the study was to provide a preliminary estimate of the NPV of two DNA methylation markers, APC and GSTP1. Slides from initial and repeat biopsies were read by a single blinded reader at an academic centre. Tissue sections were sent to the commercial laboratory for quantitative methylation testing, blinded to pathology outcome; this laboratory was owned by a company that provided some funding for the study. Safety Not applicable. Effectiveness Twenty four per cent (n=21) of the 86 men, with initial negative biopsy, included in this study had prostate cancer on repeat biopsy. Cancer incidence, on repeat biopsy, was 14%, 21% and 39% within subgroups 1, 2 and 3, respectively. For the three subgroups combined, the NPV (with methylation ratios below a pre defined threshold, predicting no cancer) was 0.96 for the APC marker and 0.80 for the GSTP1 marker. The sensitivity of methylation testing (with methylation ratios above the threshold) was 0.95 and 0.43 for the two markers, respectively, which indicates a significant advantage for the APC marker (p<0.0001). The false positive rate using APC methylation however was also significantly higher (0.60 vs. 0.25; p<0.001). This means that APC methylation was able to correctly identify more men with prostate cancer on repeat biopsy at the cost of wrongly diagnosing healthy men with a positive cancer finding. The sensitivity and NPV of the assay when both methylation markers were combined was similar to that of APC alone. APC methylation patterns were consistent with a possible field effect or occurrence early in carcinogenesis. Trock et al also compared the performance of APC methylation/gstp1 methylation with the use of per cent free PSA (%fpsa), which is a measure of the proportion of free PSA in the bloodstream compared with PSA bound to other proteins. In general, men with prostate cancer have a lower percentage of free PSA in their bloodstream; therefore, this measure ConfirmMDx TM Assay for prostate cancer: February
9 is often used to determine the need for biopsy. Of the patients included in this study, %fpsa was available for 37 patients (Table 2). Table 2 Performance characteristics for %fpsa compared with APC methylation and GSTP1 methylation Sensitivity Specificity Negative predictive value %fpsa* APC GSTP fpsa: free prostate-specific antigen; APC: adenomatous polyposis coli; GSTP1: glutathione S-transferase P1. *using a threshold of 15% Overall the authors concluded: And: Methylation markers could have the potential to avoid up to 30% of re biopsies after an initial negative biopsy. APC methylation provided a very high NPV with a low percentage of false negatives The potential of APC methylation to reduce unnecessary repeat biopsies warrants validation in a larger prospective cohort. Stewart et al 8 The industry funded, retrospective MATLOC (Methylation Analysis To Locate Occult Cancer) study (level III 1 diagnostic accuracy study) was conducted on archived prostate biopsy samples from patients in Scotland and Belgium (n=483; mean age 63). Using an epigenetic marker panel including APC, GSTP1 and RASSF1, researchers blindly tested histologynegative prostate biopsy samples that were followed by either a positive repeat biopsy (cases) or negative repeat biopsy (controls) within 30 months. Median time between biopsies was 7.3 months. The clinical performance of the marker panel was assessed and cross validated; NPV was of particular interest (to reduce unnecessary biopsies). Samples from test subjects were assayed randomly and blinded for subsequent cancer detection. Methylation ratios were determined for all three genes. Safety Not applicable. Effectiveness The assay performed on the initial negative biopsies resulted in an NPV of 90 per cent (95% CI [87, 93]), sensitivity of 68 per cent, and specificity of 64 per cent. In a multivariate model corrected for age, PSA, DRE and histopathological characteristics of the first biopsy, the ConfirmMDx TM Assay for prostate cancer: February
10 assay was a significant independent predictor of patient outcome with an odds ratio of 3.2 (95% CI [1.8, 5.5]). The assay identified 68 per cent of the cases that were false negatives based on histology alone. Negative methylation status also confirmed the negative diagnosis for 64 per cent of the men who had undergone two serial, cancer negative biopsies. Furthermore, of the 53 cases with benign histology in the first biopsy, 20 (38%) had confirmed significant cancer upon re biopsy; 13 (65%) were detected by the assay, resulting in a sensitivity of 65 per cent. The authors noted that their results were consistent with those of the smaller prospective study of Trock et al, 11 commenting that their own use of three markers rather than two (APC and GSTP1) resulted in a stable overall performance with a high NPV. They concluded that: Such a test could be a valuable addition to the current [prostate cancer] diagnostic process, reducing the unnecessary repeat biopsies by 64%. Cancer related epigenetic abnormalities can be detected up to 30 months prior to histopathological manifestation of [prostate cancer], using the initial, cancer negative biopsy tissue. Troyer et al 16 In an earlier industry funded, retrospective, multicentre study in the US (level III 1 diagnostic accuracy study), testing was completed on 971 archived paraffin embedded tissue blocks from 264 men screened for prostate cancer and considered to be at risk. Core biopsy tissue from men with serial negative biopsies (defined as three negative results or two negatives separated by 24 months) served as negative controls, whereas tissue from men diagnosed with prostate cancer within 24 months served as positive controls. All biopsy specimens were submitted to the manufacturer s laboratory in Belgium for quantitative methylation specific PCR assay (including GSTP1, APC, and others) and were also reinterpreted by blinded experts in the US. The goal of the study was to evaluate the ability to detect prostate cancer in histopathologically negative biopsy samples from men who were positively diagnosed during follow up biopsy. Safety Not applicable. Effectiveness The sensitivity and specificity of the assay used to test the initially negative biopsy tissue, in men who were later diagnosed with prostate cancer, was 52 per cent and 85 per cent, respectively. When the assay was used to test tissue biopsies containing histopathologically confirmed prostate cancer, sensitivity was 95 per cent. Economic evaluation Economic analyses were not available. ConfirmMDx TM Assay for prostate cancer: February
11 Ongoing research No ongoing or planned studies of ConfirmMDx TM for Prostate Cancer were identified. Other issues MDxHealth is currently developing a version of ConfirmMDx TM for lung cancer. An additional test under development aims to distinguish between aggressive and slow growing cancers. 12 On 1 December 2012, MDxHealth commenced a collaboration with Ghent University in Belgium to establish NXTGNT, a new Center in Pharmaco (Epi)genomics within the laboratory of Pharmaceutical Biotechnology. The Centre s mission is to accelerate innovation in personalized medicine by using advanced technology, knowledge and expertise in epigenetics. The Center will bring together scientific expertise from basic researchers, clinical validation teams, informaticians and cutting edge epigenomic technology. 4 Summary of findings Prostate cancer is common, yet the methods currently used to diagnose it have limitations. In particular, only a small proportion of the prostate is sampled via needle biopsy and the rate of false negative results is high. Many men are therefore subjected to additional, potentially unnecessary biopsy. The ConfirmMDx assay tests the DNA methylation status of several genes associated with prostate cancer in an attempt to lower the false negative rate of biopsy results. Evidence came from three fairly rigorous studies of diagnostic accuracy: A prospective study compared findings from quantitative methylation testing to the reference standard, blinded histology interpretation. NPVs of 96 and 80 per cent were obtained and the authors estimated that 30 per cent of re biopsies could be avoided with use of the testing. The retrospective MATLOC study was conducted using a DNA methylation assay on archived initial prostate biopsy samples, with results from subsequent repeat biopsies available. The NPV was 90 per cent; the assay identified 68 per cent of the cases that were false negatives; and negative assay results confirmed the negative diagnosis for 64 per cent of the men who had undergone two serial, cancer negative biopsies. An earlier study used a methylation assay to test archived prostate biopsy tissue blocks from at risk men screened for prostate cancer. Follow up biopsy samples were available and the histology was reinterpreted by blinded experts. Results showed that gene methylation showed promise as a tool for querying false negative results. ConfirmMDx TM Assay for prostate cancer: February
12 HealthPACT assessment From the evidence base available, prostate cancer detection using DNA methylation assays appears to offer some benefit over existing methods of diagnosis. In particular, the high NPVs and the low rates of false negatives observed indicate that DNA methylation assays may provide a means of reducing the number of healthy men incorrectly diagnosed and subjected to unnecessary biopsy; although, high quality comparative studies are needed before this can be truly determined. Further studies investigating the effects of prostate cancer detection using DNA methylation compared with conventional techniques on overall patient survival are also required. Despite these initial promising results, the need for ongoing monitoring of patients at highrisk of prostate cancer will remain and current patient management is unlikely to change; therefore, this technology will be archived. Number of studies included All evidence included for assessment in this Technology Brief has been assessed according to the revised NHMRC levels of evidence. A document summarising these levels may be accessed via the HealthPACT web site. Total number of studies 3 Total number of Level II diagnostic accuracy studies 1 Total number of Level III 1 diagnostic accuracy studies 2 References 1. Graif, T., Loeb, S. et al (2007). 'Under diagnosis and over diagnosis of prostate cancer', J Urol,178 (1), MDxHealth, Inc. (2012). MDxHealth s ConfirmMDx for prostate cancer test helps to identify men able to avoid repeat biopsies [press release]. [Internet]. MDxHealth, Inc. Available from: and events/press releases andevents?release=10 [Accessed 6 Jan 2013]. 3. MDxHealth, Inc. (2012). Company overview. [Internet]. MDxHealth, Inc. Available from: overview [Accessed 6 Jan 2013]. 4. MDxHealth, Inc. (2012). Ghent University and MDxHealth collaborate to establish a center in pharmaco (Epi)genomics. [Serial on the Internet]. StreetInsider.com. 30 November Available from: ollaborate+to+establish+a+center+in+pharmaco+%28epi%29genomics/ htm l [Accessed 17 Jan 2013]. 5. Australian Institute of Health and Welfare (2012). Cancer in Australia: an overview AIHW & Australasian Association of Cancer Registries, Canberra. Available from: [Accessed 17 Jan 2013]. ConfirmMDx TM Assay for prostate cancer: February
13 6. Mitomics (2010). The Prostate Core Mitomic Test: now you can know. [Internet]. Mitomics. Available from: core mitomictest/healthcare professionals.php [Accessed 7 Jan 2013]. 7. ClinicalTrials.gov (2013). Predictive value of prostate specific antigen isoform p2psa and its derivates in the diagnosis of prostate cancer [NCT ]. [Internet]. US National Institutes of Health. ClinicalTrials.gov. Available from: e+biopsy&rank=4 [Accessed 7 Jan 2013]. 8. Stewart, G. D., Van Neste, L. et al (2012). 'Clinical utility of an epigenetic assay to detect occult prostate cancer in histopathologically negative biopsies: results of the MATLOC Study', J Urol, (Sep 18), pii: S (12) Harvey, P., Basuita, A. et al (2009). 'A systematic review of the diagnostic accuracy of prostate specific antigen', BMC Urol,9, Hossack, T., Patel, M. I. et al (2012). 'Location and pathological characteristics of cancers in radical prostatectomy specimens identified by transperineal biopsy compared to transrectal biopsy', J Urol,188 (3), Trock, B. J., Brotzman, M. J. et al (2012). 'Evaluation of GSTP1 and APC methylation as indicators for repeat biopsy in a high risk cohort of men with negative initial prostate biopsies', BJU Int,110 (1), Kitamura, M. Prostate cancer test may reduce repeat biopsies: study. Bloomberg Businessweek [serial on the Internet]. 2012; (Oct 2): Available from: /prostate cancer test may reducerepeat biopsies study [Accessed 17 Jan 2013]. 13. Spernat, D., MB BS MPH FRACS (Urol). ASERNIP S Request for Clinical Review ConfirmMDx Assay for Prostate Cancer [Internet]. to Edgar Hughes M. 15 Jan 2013 [cited 15 Jan 2013]. 14. Australian Government Department of Health and Ageing (2013). Medicare Benefits Schedule. Available from: Benefits Schedule MBS 1 [Accessed 11 Jan 2012]. 15. Prostate Cancer Foundation of Australia (2013). Prostate cancer statistics. [Internet]. Prostate Cancer Foundation of Australia. Available from: Cancer Statistics.html [Accessed 7 Jan 2013]. 16. Troyer, D. A., Lucia, M. S. et al (2009). Prostate cancer detected by methylated gene markers in histopathologically cancer negative tissues from men with subsequent positive biopsies. Cancer Epidemiol Biomarkers and Prevention [serial on the Internet]. 2009; 18(10): Available from: [Accessed 17 Jan 2013]. Search criteria to be used (MeSH terms) MeSH: prostatic neoplasms, polymerase chain reaction, sensitivity and specificity Keywords: prostate cancer, polymerase chain reaction, PCR, false negative ConfirmMDx TM Assay for prostate cancer: February
Saturation Biopsy for Diagnosis and Staging of Prostate Cancer. Original Policy Date
 MP 7.01.101 Saturation Biopsy for Diagnosis and Staging of Prostate Cancer Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date /12/2013 Return to Medical Policy
MP 7.01.101 Saturation Biopsy for Diagnosis and Staging of Prostate Cancer Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date /12/2013 Return to Medical Policy
Screening for Prostate Cancer
 Screening for Prostate Cancer It is now clear that screening for Prostate Cancer discovers the disease at an earlier and more curable stage. It is not yet clear whether this translates into reduced mortality
Screening for Prostate Cancer It is now clear that screening for Prostate Cancer discovers the disease at an earlier and more curable stage. It is not yet clear whether this translates into reduced mortality
Advances in Diagnostic and Molecular Testing in Prostate Cancer
 Advances in Diagnostic and Molecular Testing in Prostate Cancer Ashley E. Ross MD PhD Assistant Professor Urology, Oncology, Pathology Johns Hopkins School of Medicine September 24, 2015 1 Disclosures
Advances in Diagnostic and Molecular Testing in Prostate Cancer Ashley E. Ross MD PhD Assistant Professor Urology, Oncology, Pathology Johns Hopkins School of Medicine September 24, 2015 1 Disclosures
Role of MRI in the Diagnosis of Prostate Cancer, A Proposal
 Clinical and Experimental Medical Sciences, Vol. 1, 2013, no. 3, 111 116 HIKARI Ltd, www.m-hikari.com Role of MRI in the Diagnosis of Prostate Cancer, A Proposal W. Akhter Research Fellow Urology, Bartshealth
Clinical and Experimental Medical Sciences, Vol. 1, 2013, no. 3, 111 116 HIKARI Ltd, www.m-hikari.com Role of MRI in the Diagnosis of Prostate Cancer, A Proposal W. Akhter Research Fellow Urology, Bartshealth
Historical Basis for Concern
 Androgens After : Are We Ready? Mohit Khera, MD, MBA Assistant Professor of Urology Division of Male Reproductive Medicine and Surgery Scott Department of Urology Baylor College of Medicine Historical
Androgens After : Are We Ready? Mohit Khera, MD, MBA Assistant Professor of Urology Division of Male Reproductive Medicine and Surgery Scott Department of Urology Baylor College of Medicine Historical
1. What is the prostate-specific antigen (PSA) test?
 1. What is the prostate-specific antigen (PSA) test? Prostate-specific antigen (PSA) is a protein produced by the cells of the prostate gland. The PSA test measures the level of PSA in the blood. The doctor
1. What is the prostate-specific antigen (PSA) test? Prostate-specific antigen (PSA) is a protein produced by the cells of the prostate gland. The PSA test measures the level of PSA in the blood. The doctor
PSA Screening for Prostate Cancer Information for Care Providers
 All men should know they are having a PSA test and be informed of the implications prior to testing. This booklet was created to help primary care providers offer men information about the risks and benefits
All men should know they are having a PSA test and be informed of the implications prior to testing. This booklet was created to help primary care providers offer men information about the risks and benefits
Cancer research in the Midland Region the prostate and bowel cancer projects
 Cancer research in the Midland Region the prostate and bowel cancer projects Ross Lawrenson Waikato Clinical School University of Auckland MoH/HRC Cancer Research agenda Lung cancer Palliative care Prostate
Cancer research in the Midland Region the prostate and bowel cancer projects Ross Lawrenson Waikato Clinical School University of Auckland MoH/HRC Cancer Research agenda Lung cancer Palliative care Prostate
PSA Testing 101. Stanley H. Weiss, MD. Professor, UMDNJ-New Jersey Medical School. Director & PI, Essex County Cancer Coalition. weiss@umdnj.
 PSA Testing 101 Stanley H. Weiss, MD Professor, UMDNJ-New Jersey Medical School Director & PI, Essex County Cancer Coalition weiss@umdnj.edu September 23, 2010 Screening: 3 tests for PCa A good screening
PSA Testing 101 Stanley H. Weiss, MD Professor, UMDNJ-New Jersey Medical School Director & PI, Essex County Cancer Coalition weiss@umdnj.edu September 23, 2010 Screening: 3 tests for PCa A good screening
Corporate Medical Policy Saturation Biopsy for Diagnosis and Staging of Prostate Cancer
 Corporate Medical Policy Saturation Biopsy for Diagnosis and Staging of Prostate Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: saturation_biopsy_for_diagnosis_and_staging_of_prostate_cancer
Corporate Medical Policy Saturation Biopsy for Diagnosis and Staging of Prostate Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: saturation_biopsy_for_diagnosis_and_staging_of_prostate_cancer
Guidelines for Cancer Prevention, Early detection & Screening. Prostate Cancer
 Guidelines for Cancer Prevention, Early detection & Screening Prostate Cancer Intervention Comments & Recommendations For primary prevention, it has been suggested that diets low in meat & other fatty
Guidelines for Cancer Prevention, Early detection & Screening Prostate Cancer Intervention Comments & Recommendations For primary prevention, it has been suggested that diets low in meat & other fatty
The 4Kscore blood test for risk of aggressive prostate cancer
 The 4Kscore blood test for risk of aggressive prostate cancer Prostate cancer tests When to use the 4Kscore Test? Screening Prior to 1 st biopsy Prior to negative previous biopsy Prognosis in Gleason 6
The 4Kscore blood test for risk of aggressive prostate cancer Prostate cancer tests When to use the 4Kscore Test? Screening Prior to 1 st biopsy Prior to negative previous biopsy Prognosis in Gleason 6
7. Prostate cancer in PSA relapse
 7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
Advice to patients about the PSA (prostate-specific antigen) blood test: frequently-asked questions
 Advice to patients about the PSA (prostate-specific antigen) blood test: frequently-asked questions What is the PSA blood test? If you want more information before deciding to have this test, it is important
Advice to patients about the PSA (prostate-specific antigen) blood test: frequently-asked questions What is the PSA blood test? If you want more information before deciding to have this test, it is important
Cancer in Primary Care: Prostate Cancer Screening. How and How often? Should we and in which patients?
 Cancer in Primary Care: Prostate Cancer Screening How and How often? Should we and in which patients? PLCO trial (Prostate, Lung, Colorectal and Ovarian) Results In the screening group, rates of compliance
Cancer in Primary Care: Prostate Cancer Screening How and How often? Should we and in which patients? PLCO trial (Prostate, Lung, Colorectal and Ovarian) Results In the screening group, rates of compliance
Epi procolon The Blood Test for Colorectal Cancer Screening
 Epi procolon The Blood Test for Colorectal Cancer Screening Epi procolon is an approved blood test for colorectal cancer screening. The US Preventive Services Task Force, the American Cancer Society and
Epi procolon The Blood Test for Colorectal Cancer Screening Epi procolon is an approved blood test for colorectal cancer screening. The US Preventive Services Task Force, the American Cancer Society and
Saturation Biopsy vs. 3D Spatial Biopsy vs. Free Hand Ultrasound biopsy for Targeted Prostate Cancer Therapies
 Saturation Biopsy vs. 3D Spatial Biopsy vs. Free Hand Ultrasound biopsy for Targeted Prostate Cancer Therapies John F. Ward, MD Assistant Professor University of Texas M. D. Anderson Cancer Center Ablation
Saturation Biopsy vs. 3D Spatial Biopsy vs. Free Hand Ultrasound biopsy for Targeted Prostate Cancer Therapies John F. Ward, MD Assistant Professor University of Texas M. D. Anderson Cancer Center Ablation
An Introduction to PROSTATE CANCER
 An Introduction to PROSTATE CANCER Being diagnosed with prostate cancer can be a life-altering experience. It requires making some very difficult decisions about treatments that can affect not only the
An Introduction to PROSTATE CANCER Being diagnosed with prostate cancer can be a life-altering experience. It requires making some very difficult decisions about treatments that can affect not only the
Early Prostate Cancer: Questions and Answers. Key Points
 CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Early Prostate Cancer:
CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Early Prostate Cancer:
MEDICAL POLICY SUBJECT: PROSTATE CANCER SCREENING, DETECTION AND MONITORING
 MEDICAL POLICY PAGE: 1 OF: 8 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 8 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
Description of Procedure or Service. gene_based_tests_for_screening_detection_or_management_of_prostate_cancer 4/2009 8/2015 8/2016 8/2015
 Corporate Medical Policy Gene-Based Tests for Screening, Detection, and/or Management File Name: Origination: Last CAP Review: Next CAP Review: Last Review: gene_based_tests_for_screening_detection_or_management_of_prostate_cancer
Corporate Medical Policy Gene-Based Tests for Screening, Detection, and/or Management File Name: Origination: Last CAP Review: Next CAP Review: Last Review: gene_based_tests_for_screening_detection_or_management_of_prostate_cancer
Name of Policy: Genetic and Protein Biomarkers for the Diagnosis and Cancer Risk Assessment of Prostate Cancer
 Name of Policy: Genetic and Protein Biomarkers for the Diagnosis and Cancer Risk Assessment of Prostate Cancer Policy #: 534 Latest Review Date: June 2015 Category: Laboratory Policy Grade: B Background/Definitions:
Name of Policy: Genetic and Protein Biomarkers for the Diagnosis and Cancer Risk Assessment of Prostate Cancer Policy #: 534 Latest Review Date: June 2015 Category: Laboratory Policy Grade: B Background/Definitions:
How prostate cancer is diagnosed
 How prostate cancer is diagnosed This information is an extract from the booklet Having tests for prostate cancer. You may find the full booklet helpful. We can send you a free copy see page 7. Contents
How prostate cancer is diagnosed This information is an extract from the booklet Having tests for prostate cancer. You may find the full booklet helpful. We can send you a free copy see page 7. Contents
The 4Kscore blood test for risk of aggressive prostate cancer
 The 4Kscore blood test for risk of aggressive prostate cancer Early detection of aggressive prostate cancer Challenges Serum PSA has a high false positive rate Over 1 million prostate biopsies performed
The 4Kscore blood test for risk of aggressive prostate cancer Early detection of aggressive prostate cancer Challenges Serum PSA has a high false positive rate Over 1 million prostate biopsies performed
Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical
 Summary. 111 Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical recurrence (BCR) is the first sign of recurrent
Summary. 111 Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical recurrence (BCR) is the first sign of recurrent
PSA screening in asymptomatic men the debate continues www.bpac.org.nz keyword: psa
 PSA screening in asymptomatic men the debate continues www.bpac.org.nz keyword: psa Key messages: PSA is present in the benign and malignant prostate There is currently no national screening programme
PSA screening in asymptomatic men the debate continues www.bpac.org.nz keyword: psa Key messages: PSA is present in the benign and malignant prostate There is currently no national screening programme
Health Policy Advisory Committee on Technology
 Health Policy Advisory Committee on Technology Technology Brief Molecular testing for prostate cancer prognosis November 2014 State of Queensland (Queensland Department of Health) 2014 This work is licensed
Health Policy Advisory Committee on Technology Technology Brief Molecular testing for prostate cancer prognosis November 2014 State of Queensland (Queensland Department of Health) 2014 This work is licensed
Health Policy Advisory Committee on Technology
 Health Policy Advisory Committee on Technology Technology Brief MRI screening for prostate cancer March 2015 State of Queensland (Queensland Department of Health) 2015 This work is licensed under a Creative
Health Policy Advisory Committee on Technology Technology Brief MRI screening for prostate cancer March 2015 State of Queensland (Queensland Department of Health) 2015 This work is licensed under a Creative
FAQ About Prostate Cancer Treatment and SpaceOAR System
 FAQ About Prostate Cancer Treatment and SpaceOAR System P. 4 Prostate Cancer Background SpaceOAR Frequently Asked Questions (FAQ) 1. What is prostate cancer? The vast majority of prostate cancers develop
FAQ About Prostate Cancer Treatment and SpaceOAR System P. 4 Prostate Cancer Background SpaceOAR Frequently Asked Questions (FAQ) 1. What is prostate cancer? The vast majority of prostate cancers develop
Cancer Screening. Robert L. Robinson, MD, MS. Ambulatory Conference SIU School of Medicine Department of Internal Medicine.
 Cancer Screening Robert L. Robinson, MD, MS Ambulatory Conference SIU School of Medicine Department of Internal Medicine March 13, 2003 Why screen for cancer? Early diagnosis often has a favorable prognosis
Cancer Screening Robert L. Robinson, MD, MS Ambulatory Conference SIU School of Medicine Department of Internal Medicine March 13, 2003 Why screen for cancer? Early diagnosis often has a favorable prognosis
PSA Testing for Prostate Cancer An information sheet for men considering a PSA Test
 PSA Testing for Prostate Cancer An information sheet for men considering a PSA Test What is the aim of this leaflet? Prostate cancer is a serious condition. The PSA test, which can give an early indication
PSA Testing for Prostate Cancer An information sheet for men considering a PSA Test What is the aim of this leaflet? Prostate cancer is a serious condition. The PSA test, which can give an early indication
Prostate Cancer Screening. A Decision Guide for African Americans
 Prostate Cancer Screening A Decision Guide for African Americans This booklet was developed by the U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC). Published
Prostate Cancer Screening A Decision Guide for African Americans This booklet was developed by the U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC). Published
Screening for Prostate Cancer
 Cancer Expert Working Group on Cancer Prevention and Screening Screening for Prostate Cancer Information for men and their families 1 What is the prostate? 2 What is prostate cancer? prostate The prostate
Cancer Expert Working Group on Cancer Prevention and Screening Screening for Prostate Cancer Information for men and their families 1 What is the prostate? 2 What is prostate cancer? prostate The prostate
Prostate Cancer Screening. A Decision Guide
 Prostate Cancer Screening A Decision Guide This booklet was developed by the U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC). Is screening right for you?
Prostate Cancer Screening A Decision Guide This booklet was developed by the U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC). Is screening right for you?
Prostate cancer in Australia
 Prostate cancer in Australia cancer series No. 79 CANCER SERIES Number 79 Prostate cancer in Australia Australian Institute of Health and Welfare Canberra Cat. no. CAN 76 The Australian Institute of Health
Prostate cancer in Australia cancer series No. 79 CANCER SERIES Number 79 Prostate cancer in Australia Australian Institute of Health and Welfare Canberra Cat. no. CAN 76 The Australian Institute of Health
Screening for Prostate Cancer
 Screening for Prostate Cancer Review against programme appraisal criteria for the UK National Screening Committee (UK NSC) Version 1: This document summarises the work of ScHARR 1 2 and places it against
Screening for Prostate Cancer Review against programme appraisal criteria for the UK National Screening Committee (UK NSC) Version 1: This document summarises the work of ScHARR 1 2 and places it against
Prostate Cancer. Treatments as unique as you are
 Prostate Cancer Treatments as unique as you are UCLA Prostate Cancer Program Prostate cancer is the second most common cancer among men. The UCLA Prostate Cancer Program brings together the elements essential
Prostate Cancer Treatments as unique as you are UCLA Prostate Cancer Program Prostate cancer is the second most common cancer among men. The UCLA Prostate Cancer Program brings together the elements essential
Magnetic Resonance Imaging Targeted Biopsy of the Prostate
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Update on Prostate Cancer: Screening, Diagnosis, and Treatment Making Sense of the Noise and Directions Forward
 Update on Prostate Cancer: Screening, Diagnosis, and Treatment Making Sense of the Noise and Directions Forward 33 rd Annual Internal Medicine Update December 5, 2015 Ryan C. Hedgepeth, MD, MS Chief of
Update on Prostate Cancer: Screening, Diagnosis, and Treatment Making Sense of the Noise and Directions Forward 33 rd Annual Internal Medicine Update December 5, 2015 Ryan C. Hedgepeth, MD, MS Chief of
Molecular Diagnos/c Solu/ons for Urologic Cancer
 Molecular Diagnos/c Solu/ons for Urologic Cancer 2016 Company Presentation Dr. Jan Groen, President & CEO Forward Looking Statement 2 This presentation contains forward-looking statements & estimates made
Molecular Diagnos/c Solu/ons for Urologic Cancer 2016 Company Presentation Dr. Jan Groen, President & CEO Forward Looking Statement 2 This presentation contains forward-looking statements & estimates made
Prostate Cancer Screening. Dr. J. McCracken, Urologist
 Prostate Cancer Screening Dr. J. McCracken, Urologist USPSTF Lifetime risk for diagnosis currently estimated at 15.9% Llifetime risk of dying of prostate cancer is 2.8% Seventy percent of deaths due to
Prostate Cancer Screening Dr. J. McCracken, Urologist USPSTF Lifetime risk for diagnosis currently estimated at 15.9% Llifetime risk of dying of prostate cancer is 2.8% Seventy percent of deaths due to
PROSTATE CANCER. Normal-risk men: No family history of prostate cancer No history of prior screening Not African-American
 PROSTATE CANCER 1. Guidelines for Screening Risk Factors Normal-risk men: No family history of prostate cancer No history of prior screening Not African-American High-risk men: Family history of prostate
PROSTATE CANCER 1. Guidelines for Screening Risk Factors Normal-risk men: No family history of prostate cancer No history of prior screening Not African-American High-risk men: Family history of prostate
Draft Clinical Practice Guidelines. PSA Testing and Early Management of Test-Detected Prostate Cancer
 Draft Clinical Practice Guidelines PSA Testing and Early Management of Test-Detected Prostate Cancer Draft November 2014 Prostate Cancer Foundation of Australia and Cancer Council Australia ISBN: This
Draft Clinical Practice Guidelines PSA Testing and Early Management of Test-Detected Prostate Cancer Draft November 2014 Prostate Cancer Foundation of Australia and Cancer Council Australia ISBN: This
Biomarkers for Prostate Cancer. Eric Wallen, MD Department of Urology
 Biomarkers for Prostate Cancer Eric Wallen, MD Department of Urology Disclosure MDxHealth Scientific Advisor 55-year-old man: Poor Guy Risk of prostate cancer? 1 in 6 Risk of prostate cancer death? 1 in
Biomarkers for Prostate Cancer Eric Wallen, MD Department of Urology Disclosure MDxHealth Scientific Advisor 55-year-old man: Poor Guy Risk of prostate cancer? 1 in 6 Risk of prostate cancer death? 1 in
Health Policy Advisory Committee on Technology Technology Brief Online programs for weight loss February 2014
 Health Policy Advisory Committee on Technology Technology Brief Online programs for weight loss February 2014 State of Queensland (Queensland Department of Health) 2014 This work is licensed under a Creative
Health Policy Advisory Committee on Technology Technology Brief Online programs for weight loss February 2014 State of Queensland (Queensland Department of Health) 2014 This work is licensed under a Creative
Screening for Cancer in Light of New Guidelines and Controversies. Christopher Celio, MD St. Jude Heritage Medical Group
 Screening for Cancer in Light of New Guidelines and Controversies Christopher Celio, MD St. Jude Heritage Medical Group Screening Tests The 2 major objectives of a good screening program are: (1) detection
Screening for Cancer in Light of New Guidelines and Controversies Christopher Celio, MD St. Jude Heritage Medical Group Screening Tests The 2 major objectives of a good screening program are: (1) detection
Science Highlights. To PSA or not to PSA: That is the Question.
 Science Highlights June 2012 by Ann A. Kiessling, PhD at the To PSA or not to PSA: That is the Question. The current raucous debate over the commonly used PSA blood test to screen for prostate cancer,
Science Highlights June 2012 by Ann A. Kiessling, PhD at the To PSA or not to PSA: That is the Question. The current raucous debate over the commonly used PSA blood test to screen for prostate cancer,
Analysis of Prostate Cancer at Easter Connecticut Health Network Using Cancer Registry Data
 The 2014 Cancer Program Annual Public Reporting of Outcomes/Annual Site Analysis Statistical Data from 2013 More than 70 percent of all newly diagnosed cancer patients are treated in the more than 1,500
The 2014 Cancer Program Annual Public Reporting of Outcomes/Annual Site Analysis Statistical Data from 2013 More than 70 percent of all newly diagnosed cancer patients are treated in the more than 1,500
Prostate Cancer Screening
 Prostate Cancer Screening The American Cancer Society and Congregational Health Ministry Team June Module To access this module via the Web, visit www.cancer.org and type in congregational health ministry
Prostate Cancer Screening The American Cancer Society and Congregational Health Ministry Team June Module To access this module via the Web, visit www.cancer.org and type in congregational health ministry
Prostate Health Index Literature
 Prostate Health Index Literature Update June 2013 Preoperative Prostate-Specific Antigen Isoform p2psa and Its Derivatives, %p2psa and Prostate Health Index, Predict Pathologic Outcomes in Patients Undergoing
Prostate Health Index Literature Update June 2013 Preoperative Prostate-Specific Antigen Isoform p2psa and Its Derivatives, %p2psa and Prostate Health Index, Predict Pathologic Outcomes in Patients Undergoing
Office of Population Health Genomics
 Office of Population Health Genomics Policy: Protocol for the management of female BRCA mutation carriers in Western Australia Purpose: Best Practice guidelines for the management of female BRCA mutation
Office of Population Health Genomics Policy: Protocol for the management of female BRCA mutation carriers in Western Australia Purpose: Best Practice guidelines for the management of female BRCA mutation
DECISION AID TOOL PROSTATE CANCER SCREENING WITH PSA TESTING
 DECISION AID TOOL PROSTATE CANCER SCREENING WITH PSA TESTING This booklet is what is often called a decision aid. The goals of a decision aid are to help people better understand their medical choices
DECISION AID TOOL PROSTATE CANCER SCREENING WITH PSA TESTING This booklet is what is often called a decision aid. The goals of a decision aid are to help people better understand their medical choices
Prostate Cancer. Patient Information
 Prostate Cancer Patient Information 1 The Prostate & Prostate Cancer The prostate is a small gland in the male reproductive system, approximately the size and shape of a walnut. It is located directly
Prostate Cancer Patient Information 1 The Prostate & Prostate Cancer The prostate is a small gland in the male reproductive system, approximately the size and shape of a walnut. It is located directly
Prostate Cancer Screening in Taiwan: a must
 Prostate Cancer Screening in Taiwan: a must 吳 俊 德 基 隆 長 庚 醫 院 台 灣 醫 學 會 105 th What is the PSA test? The blood level of PSA is often elevated in men with prostate cancer, and the PSA test was originally
Prostate Cancer Screening in Taiwan: a must 吳 俊 德 基 隆 長 庚 醫 院 台 灣 醫 學 會 105 th What is the PSA test? The blood level of PSA is often elevated in men with prostate cancer, and the PSA test was originally
NEW HYBRID IMAGING TECHNOLOGY MAY HAVE BIG POTENTIAL FOR IMPROVING DIAGNOSIS OF PROSTATE CANCER
 Media Release April 7, 2009 For Immediate Release NEW HYBRID IMAGING TECHNOLOGY MAY HAVE BIG POTENTIAL FOR IMPROVING DIAGNOSIS OF PROSTATE CANCER London, Ontario Improved hybrid imaging techniques developed
Media Release April 7, 2009 For Immediate Release NEW HYBRID IMAGING TECHNOLOGY MAY HAVE BIG POTENTIAL FOR IMPROVING DIAGNOSIS OF PROSTATE CANCER London, Ontario Improved hybrid imaging techniques developed
Prostate cancer statistics
 Prostate cancer in Australia The following material has been sourced from the Australian Institute of Health and Welfare Prostate cancer incorporates ICD-10 cancer code C61 (Malignant neoplasm of prostate).
Prostate cancer in Australia The following material has been sourced from the Australian Institute of Health and Welfare Prostate cancer incorporates ICD-10 cancer code C61 (Malignant neoplasm of prostate).
PSA screening: Controversies and Guidelines
 PSA screening: Controversies and Guidelines John Phillips, MD, FACS Department of Urology Urology Center of Westchester New York Medical College Historical PerspecGve Cancer of the prostate, although rare,
PSA screening: Controversies and Guidelines John Phillips, MD, FACS Department of Urology Urology Center of Westchester New York Medical College Historical PerspecGve Cancer of the prostate, although rare,
Robert Bristow MD PhD FRCPC
 Robert Bristow MD PhD FRCPC Clinician-Scientist and Professor, Radiation Oncology and Medical Biophysics, University of Toronto and Ontario Cancer Institute/ (UHN) Head, PMH-CFCRI Prostate Cancer Research
Robert Bristow MD PhD FRCPC Clinician-Scientist and Professor, Radiation Oncology and Medical Biophysics, University of Toronto and Ontario Cancer Institute/ (UHN) Head, PMH-CFCRI Prostate Cancer Research
HEALTH PREFACE. Introduction. Scope of the sector
 HEALTH PREFACE Introduction Government and non-government sectors provide a range of services including general practitioners, hospitals, nursing homes and community health services to support and promote
HEALTH PREFACE Introduction Government and non-government sectors provide a range of services including general practitioners, hospitals, nursing homes and community health services to support and promote
Health Policy Advisory Committee on Technology
 Health Policy Advisory Committee on Technology Technology Overview Blood and stool biomarker testing for colorectal cancer screening July 2015 State of Queensland (Queensland Department of Health) 2015
Health Policy Advisory Committee on Technology Technology Overview Blood and stool biomarker testing for colorectal cancer screening July 2015 State of Queensland (Queensland Department of Health) 2015
TO SCREEN OR NOT TO SCREEN: THE PROSTATE CANCER
 TO SCREEN OR NOT TO SCREEN: THE PROSTATE CANCER DILEMMA Thomas J Stormont MD January 2012 http://www.youtube.com/watch?v=8jd 7bAHVp0A&feature=related related INTRODUCTION A government health panel (the
TO SCREEN OR NOT TO SCREEN: THE PROSTATE CANCER DILEMMA Thomas J Stormont MD January 2012 http://www.youtube.com/watch?v=8jd 7bAHVp0A&feature=related related INTRODUCTION A government health panel (the
BJUI. Study Type Diagnosis (exploratory cohort) Level of Evidence 2b OBJECTIVE
 . 2010 BJU INTERNATIONAL Urological Oncology FREE-TO-TOTAL PSA RATIO AND PCA3 SCORE IN PREDICTING POSITIVE BIOPSIES PLOUSSARD ET AL. BJUI BJU INTERNATIONAL The prostate cancer gene 3 (PCA3) urine test
. 2010 BJU INTERNATIONAL Urological Oncology FREE-TO-TOTAL PSA RATIO AND PCA3 SCORE IN PREDICTING POSITIVE BIOPSIES PLOUSSARD ET AL. BJUI BJU INTERNATIONAL The prostate cancer gene 3 (PCA3) urine test
NCCN Prostate Cancer Early Detection Guideline
 NCCN Prostate Cancer Early Detection Guideline Joan McClure Senior Vice President National Comprehensive Cancer Network African American Prostate Cancer Disparity Summit September 22, 2006 Washington,
NCCN Prostate Cancer Early Detection Guideline Joan McClure Senior Vice President National Comprehensive Cancer Network African American Prostate Cancer Disparity Summit September 22, 2006 Washington,
STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services
 STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v Blue Care Network of Michigan, Respondent. File
STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v Blue Care Network of Michigan, Respondent. File
Prostate cancer: Removing the uncertainty
 Prostate cancer: Removing the uncertainty Conventional management of PCa relies primarily on digital rectal exams (DREs), blood tests for elevated and rising levels of prostate-specific antigen (PSA),
Prostate cancer: Removing the uncertainty Conventional management of PCa relies primarily on digital rectal exams (DREs), blood tests for elevated and rising levels of prostate-specific antigen (PSA),
HEALTH NEWS PROSTATE CANCER THE PROSTATE
 HEALTH NEWS PROSTATE CANCER THE PROSTATE Prostate comes from the Greek meaning to stand in front of ; this is very different than prostrate which means to lie down flat. The prostate is a walnut-sized
HEALTH NEWS PROSTATE CANCER THE PROSTATE Prostate comes from the Greek meaning to stand in front of ; this is very different than prostrate which means to lie down flat. The prostate is a walnut-sized
CMScript. Member of a medical scheme? Know your guaranteed benefits! Issue 7 of 2014
 Background CMScript Member of a medical scheme? Know your guaranteed benefits! Issue 7 of 2014 Prostate cancer is second only to lung cancer as the leading cause of cancer-related deaths in men. It is
Background CMScript Member of a medical scheme? Know your guaranteed benefits! Issue 7 of 2014 Prostate cancer is second only to lung cancer as the leading cause of cancer-related deaths in men. It is
Prostate cancer screening. It s YOUR decision!
 Prostate cancer screening It s YOUR decision! For many years now, a test has been available to screen for. The test is called the prostate-specific antigen blood test (or PSA test). It is used in combination
Prostate cancer screening It s YOUR decision! For many years now, a test has been available to screen for. The test is called the prostate-specific antigen blood test (or PSA test). It is used in combination
Therapeutic Goods Administration Orphan Drugs Program: Discussion paper
 Therapeutic Goods Administration Orphan Drugs Program: Discussion paper Submission from the Clinical Oncology Society of Australia and Cancer Council Australia March 2015 The Clinical Oncology Society
Therapeutic Goods Administration Orphan Drugs Program: Discussion paper Submission from the Clinical Oncology Society of Australia and Cancer Council Australia March 2015 The Clinical Oncology Society
Gleason Score. Oncotype DX GPS. identified for. about surveillance. time to get sophisticated
 patient: MARK SMITH PSA 6.2 Gleason Score 6 Oncotype DX GPS 8 identified for active surveillance time to get sophisticated about surveillance Accurate prediction of prostate cancer risk is needed at the
patient: MARK SMITH PSA 6.2 Gleason Score 6 Oncotype DX GPS 8 identified for active surveillance time to get sophisticated about surveillance Accurate prediction of prostate cancer risk is needed at the
There are many different types of cancer and sometimes cancer is diagnosed when in fact you are not suffering from the disease at all.
 About Cancer Cancer is a disease where there is a disturbance in the normal pattern of cell replacement. The cells mutate and become abnormal or grow uncontrollably. Not all tumours are cancerous (i.e.
About Cancer Cancer is a disease where there is a disturbance in the normal pattern of cell replacement. The cells mutate and become abnormal or grow uncontrollably. Not all tumours are cancerous (i.e.
Prostate Cancer Screening: Phantom menace to society. Folusho Ogunfiditimi, DM, MPH, PA-C Vattikuti Urology Institute Henry Ford Health System
 Prostate Cancer Screening: Phantom menace to society Folusho Ogunfiditimi, DM, MPH, PA-C Vattikuti Urology Institute Henry Ford Health System Objectives USPTF Recommendations on PSA testing Justification
Prostate Cancer Screening: Phantom menace to society Folusho Ogunfiditimi, DM, MPH, PA-C Vattikuti Urology Institute Henry Ford Health System Objectives USPTF Recommendations on PSA testing Justification
Us TOO University Presents: Understanding Diagnostic Testing
 Us TOO University Presents: Understanding Diagnostic Testing for Prostate Cancer Patients Today s speaker is Manish Bhandari, MD Program moderator is Pam Barrett, Us TOO International Made possible by
Us TOO University Presents: Understanding Diagnostic Testing for Prostate Cancer Patients Today s speaker is Manish Bhandari, MD Program moderator is Pam Barrett, Us TOO International Made possible by
Official reprint from UpToDate www.uptodate.com 2013 UpToDate
 Official reprint from UpToDate www.uptodate.com 2013 UpToDate The content on the UpToDate website is not intended nor recommended as a substitute for medical advice, diagnosis, or treatment. Always seek
Official reprint from UpToDate www.uptodate.com 2013 UpToDate The content on the UpToDate website is not intended nor recommended as a substitute for medical advice, diagnosis, or treatment. Always seek
Update on Prostate Cancer Screening Guidelines
 www.medscape.com Update on Prostate Cancer Screening Guidelines Christine Gonzalez, PharmD, CHHC US Pharmacist Abstract and Introduction Introduction In the United States, prostate cancer is the most common
www.medscape.com Update on Prostate Cancer Screening Guidelines Christine Gonzalez, PharmD, CHHC US Pharmacist Abstract and Introduction Introduction In the United States, prostate cancer is the most common
2010 SITE REPORT St. Joseph Hospital PROSTATE CANCER
 2010 SITE REPORT St. Joseph Hospital PROSTATE CANCER Humboldt County is located on the Redwood Coast of Northern California. U.S census data for 2010 reports county population at 134,623, an increase of
2010 SITE REPORT St. Joseph Hospital PROSTATE CANCER Humboldt County is located on the Redwood Coast of Northern California. U.S census data for 2010 reports county population at 134,623, an increase of
Cancer screening: indications, benefits and myths
 Cancer screening: indications, benefits and myths Silvia Deandrea Institute for Health and Consumer Protection Public Health Policy Support Unit Healthcare Quality Team Joint Research Centre The European
Cancer screening: indications, benefits and myths Silvia Deandrea Institute for Health and Consumer Protection Public Health Policy Support Unit Healthcare Quality Team Joint Research Centre The European
Cancer in Ireland 2013: Annual report of the National Cancer Registry
 Cancer in 2013: Annual report of the National Cancer Registry ABBREVIATIONS Acronyms 95% CI 95% confidence interval APC Annual percentage change ASR Age standardised rate (European standard population)
Cancer in 2013: Annual report of the National Cancer Registry ABBREVIATIONS Acronyms 95% CI 95% confidence interval APC Annual percentage change ASR Age standardised rate (European standard population)
If you are signing for a minor child, you refers to your child throughout the consent document.
 CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY Adult Patient or Parent, for Minor Patient INSTITUTE: National Cancer Institute PRINCIPAL INVESTIGATOR: Raffit Hassan, M.D. STUDY TITLE: Tissue Procurement
CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY Adult Patient or Parent, for Minor Patient INSTITUTE: National Cancer Institute PRINCIPAL INVESTIGATOR: Raffit Hassan, M.D. STUDY TITLE: Tissue Procurement
The PSA Test for Prostate Cancer Screening:
 For more information, please contact your local VA Medical Center or Health Clinic. U.S. Department of Veterans Affairs Veterans Health Administration Patient Care Services Health Promotion and Disease
For more information, please contact your local VA Medical Center or Health Clinic. U.S. Department of Veterans Affairs Veterans Health Administration Patient Care Services Health Promotion and Disease
Early Detection Research Network: Validation Infrastructure. Jo Ann Rinaudo, Ph.D. Division of Cancer Prevention
 Early Detection Research Network: Validation Infrastructure Jo Ann Rinaudo, Ph.D. Division of Cancer Prevention 15 October 2015 Mission The NCI Early Detection Research Network s (EDRN) mission is to implement
Early Detection Research Network: Validation Infrastructure Jo Ann Rinaudo, Ph.D. Division of Cancer Prevention 15 October 2015 Mission The NCI Early Detection Research Network s (EDRN) mission is to implement
How To Change Medicine
 P4 Medicine: Personalized, Predictive, Preventive, Participatory A Change of View that Changes Everything Leroy E. Hood Institute for Systems Biology David J. Galas Battelle Memorial Institute Version
P4 Medicine: Personalized, Predictive, Preventive, Participatory A Change of View that Changes Everything Leroy E. Hood Institute for Systems Biology David J. Galas Battelle Memorial Institute Version
Examples of good screening tests include: mammography for breast cancer screening and Pap smears for cervical cancer screening.
 CANCER SCREENING Dr. Tracy Sexton (updated July 2010) What is screening? Screening is the identification of asymptomatic disease or risk factors by history taking, physical examination, laboratory tests
CANCER SCREENING Dr. Tracy Sexton (updated July 2010) What is screening? Screening is the identification of asymptomatic disease or risk factors by history taking, physical examination, laboratory tests
Oncology Annual Report: Prostate Cancer 2005 Update By: John Konefal, MD, Radiation Oncology
 Oncology Annual Report: Prostate Cancer 25 Update By: John Konefal, MD, Radiation Oncology Prostate cancer is the most common cancer in men, with 232,9 new cases projected to be diagnosed in the U.S. in
Oncology Annual Report: Prostate Cancer 25 Update By: John Konefal, MD, Radiation Oncology Prostate cancer is the most common cancer in men, with 232,9 new cases projected to be diagnosed in the U.S. in
BREAST CANCER PATHOLOGY
 BREAST CANCER PATHOLOGY FACT SHEET Version 4, Aug 2013 This fact sheet was produced by Breast Cancer Network Australia with input from The Royal College of Pathologists of Australasia I m a nurse and know
BREAST CANCER PATHOLOGY FACT SHEET Version 4, Aug 2013 This fact sheet was produced by Breast Cancer Network Australia with input from The Royal College of Pathologists of Australasia I m a nurse and know
Epigene'c Diagnos'cs for Oncology
 Epigene'c Diagnos'cs for Oncology Dr. Jan Groen President & CEO Corporate Presenta1on 2015 Forward Looking Statement This presenta1on contains forward- looking statements & es1mates made by the management
Epigene'c Diagnos'cs for Oncology Dr. Jan Groen President & CEO Corporate Presenta1on 2015 Forward Looking Statement This presenta1on contains forward- looking statements & es1mates made by the management
4/8/13. Pre-test Audience Response. Prostate Cancer 2012. Screening and Treatment of Prostate Cancer: The 2013 Perspective
 Pre-test Audience Response Screening and Treatment of Prostate Cancer: The 2013 Perspective 1. I do not offer routine PSA screening, and the USPSTF D recommendation will not change my practice. 2. In light
Pre-test Audience Response Screening and Treatment of Prostate Cancer: The 2013 Perspective 1. I do not offer routine PSA screening, and the USPSTF D recommendation will not change my practice. 2. In light
The recommendations made throughout this book are by the National Health and Medical Research Council (NHMRC).
 INTRODUCTION This book has been prepared for people with bowel cancer, their families and friends. The first section is for people with bowel cancer, and is intended to help you understand what bowel cancer
INTRODUCTION This book has been prepared for people with bowel cancer, their families and friends. The first section is for people with bowel cancer, and is intended to help you understand what bowel cancer
Mesothelioma in Australia: Incidence (1982 to 2013) and Mortality (1997 to 2012)
 Mesothelioma in Australia: Incidence (1982 to 213) and Mortality (1997 to 212) 215 Disclaimer The information provided in this document can only assist you in the most general way. This document does not
Mesothelioma in Australia: Incidence (1982 to 213) and Mortality (1997 to 212) 215 Disclaimer The information provided in this document can only assist you in the most general way. This document does not
BRAF in the diagnostic evaluation of thyroid nodules
 Symposium 13 Molecular markers in thyroid cancer: current role in clinical practice BRAF in the diagnostic evaluation of thyroid nodules Laura Fugazzola University of Milan, Italy Papillary carcinoma BRAF
Symposium 13 Molecular markers in thyroid cancer: current role in clinical practice BRAF in the diagnostic evaluation of thyroid nodules Laura Fugazzola University of Milan, Italy Papillary carcinoma BRAF
The Centers for Medicare & Medicaid Services (CMS) seeks stakeholder comments on the following clinical quality measure under development:
 The Centers for Medicare & Medicaid Services (CMS) seeks stakeholder comments on the following clinical quality measure under development: Title: Non-Recommended PSA-Based Screening Description: The percentage
The Centers for Medicare & Medicaid Services (CMS) seeks stakeholder comments on the following clinical quality measure under development: Title: Non-Recommended PSA-Based Screening Description: The percentage
Establishing a Cohort of African-American Men to Validate a Method for using Serial PSA Measures to Detect Aggressive Prostate Cancers
 Establishing a Cohort of African-American Men to Validate a Method for using Serial PSA Measures to Detect Aggressive Prostate Cancers James R. Hebert, Sc.D., Cancer Prevention and Control Program, University
Establishing a Cohort of African-American Men to Validate a Method for using Serial PSA Measures to Detect Aggressive Prostate Cancers James R. Hebert, Sc.D., Cancer Prevention and Control Program, University
Summary 1. KEY FINDINGS The Office of Technology Assessment (OTA) concludes that research has not yet been completed to determine CHAPTER
 CHAPTER 1 Summary 1 rostate cancer is a common and serious malignancy among Medicare-age men. 1 In 1995, 244,000 new cases and 40,400 deaths are anticipated from this disease; men age 65 and older bear
CHAPTER 1 Summary 1 rostate cancer is a common and serious malignancy among Medicare-age men. 1 In 1995, 244,000 new cases and 40,400 deaths are anticipated from this disease; men age 65 and older bear
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials)
 ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials) 3 Integrated Trials Testing Targeted Therapy in Early Stage Lung Cancer Part of NCI s Precision Medicine Effort in
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials) 3 Integrated Trials Testing Targeted Therapy in Early Stage Lung Cancer Part of NCI s Precision Medicine Effort in
CONTENTS: WHAT S IN THIS BOOKLET
 Q Questions & A & Answers About Your Prostate Having a biopsy test to find out if you may have prostate cancer can bring up a lot of questions. This booklet will help answer those questions. CONTENTS:
Q Questions & A & Answers About Your Prostate Having a biopsy test to find out if you may have prostate cancer can bring up a lot of questions. This booklet will help answer those questions. CONTENTS:
PCA3 DETECTION TEST FOR PROSTATE CANCER DO YOU KNOW YOUR RISK OF HAVING CANCER?
 PCA3 DETECTION TEST FOR PROSTATE CANCER DO YOU KNOW YOUR RISK OF HAVING CANCER? PCA3 DETECTION TEST FOR PROSTATE CANCER There is a range of methods available to your healthcare professional to verify the
PCA3 DETECTION TEST FOR PROSTATE CANCER DO YOU KNOW YOUR RISK OF HAVING CANCER? PCA3 DETECTION TEST FOR PROSTATE CANCER There is a range of methods available to your healthcare professional to verify the
Prostate cancer. Christopher Eden. The Royal Surrey County Hospital, Guildford & The Hampshire Clinic, Old Basing.
 Prostate cancer Christopher Eden The Royal Surrey County Hospital, Guildford & The Hampshire Clinic, Old Basing. Screening Screening men for PCa (prostate cancer) using PSA (Prostate Specific Antigen blood
Prostate cancer Christopher Eden The Royal Surrey County Hospital, Guildford & The Hampshire Clinic, Old Basing. Screening Screening men for PCa (prostate cancer) using PSA (Prostate Specific Antigen blood
Corporate Medical Policy Molecular Markers in Fine Needle Aspirates of the Thyroid
 Corporate Medical Policy Molecular Markers in Fine Needle Aspirates of the Thyroid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_markers_in_fine_needle_aspirates_of_the_thyroid
Corporate Medical Policy Molecular Markers in Fine Needle Aspirates of the Thyroid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_markers_in_fine_needle_aspirates_of_the_thyroid
PRACTICE FRAMEWORK AND COMPETENCY STANDARDS FOR THE PROSTATE CANCER SPECIALIST NURSE
 PRACTICE FRAMEWORK AND COMPETENCY STANDARDS FOR THE PROSTATE CANCER SPECIALIST NURSE MARCH 2013 MONOGRAPHS IN PROSTATE CANCER OUR VISION, MISSION AND VALUES Prostate Cancer Foundation of Australia (PCFA)
PRACTICE FRAMEWORK AND COMPETENCY STANDARDS FOR THE PROSTATE CANCER SPECIALIST NURSE MARCH 2013 MONOGRAPHS IN PROSTATE CANCER OUR VISION, MISSION AND VALUES Prostate Cancer Foundation of Australia (PCFA)
PSA Screening and the USPSTF Understanding the Controversy
 PSA Screening and the USPSTF Understanding the Controversy Peter C. Albertsen Division of Urology University of Connecticut Farmington, CT, USA USPSTF Final Report 1 Four Key Questions 1. Does PSA based
PSA Screening and the USPSTF Understanding the Controversy Peter C. Albertsen Division of Urology University of Connecticut Farmington, CT, USA USPSTF Final Report 1 Four Key Questions 1. Does PSA based
