Pregnant Women. Double-Blind Placebo-Controlled Treatment Trial of Chlamydia trachomatis Endocervical Infections in
|
|
|
- Brittany Bennett
- 8 years ago
- Views:
Transcription
1 Infectious Diseases in Obstetrics and Gynecology 5:10-17 (1997) (C) 1997 Wiley-Liss, Inc. Double-Blind Placebo-Controlled Treatment Trial of Chlamydia trachomatis Endocervical Infections in Pregnant Women David H. Martin, 1 David A. Eschenbach, 2 Mary Frances Cotch, 3 Robert P. Nugent, 4. A. Vijaya Rao, 5 Mark A. Klebanoff, 4 Yu Lou, 5 Philip J. Rettig, 6 Ronald S. Gibbs, Joseph G. Pastorek II, 1 Joan A. Regan, 8 and Richard A. Kaslow 3 Department ofmedicine and Obstetrics and Gynecology, Louisiana State University, Nea Orleans, LA edepartment of Obstetrics and Gynecology, Universitj of lgasaington, Seattle, lga -National Institute of Allerg and Infectious Diseases, Betaesda, eid 4National Institute of Caild Healta and Human Development, tetaesda, elid -Researca Triangle Institute, Researca Triangle Parle, NC Departments of Pediatrics and Obstetrics and Gnecolog, University of Olelaaoma, O/laaoma Citj, OK 7Department of Obstetrics and Gnecolog, University of Texas, San Antonio, TX adepartment of Pediatrics, Columbia University, Nea Yor/, NY ABSTRACT Objective: The purpose of this study was to determine if treatment of pregnant women with Chlamydia trachomatis infection would lower the incidence of preterm delivery and/or low birth weight. Methods: Pregnant women between the 23rd and 29th weeks of gestation were randomized in double-blind fashion to receive either erythromycin 333 mg three times daily or an identical placebo. The trial continued until the end of the 35th week of gestation. Results: When the results were examined without regard to study site, erythromycin had little impact on reducing low birth weight (8% vs. 11%, P 0.4) or preterm delivery (13% vs. 15%, P 0.7). At the sites with high persistence of C. trachomatis in the placebo-treated women, low birth weight infants occurred in 9 (8%) of 114 erythromycin-treated and 18 (17%) of 105 placebo-treated women (P 0.04) and delivery <37 weeks occurred in 15 (13%) of 115 erythromycin-treated and 18 (17%) of 105 placebo-treated women (P 0.4). Conclusions: The results of this trial suggest that the risk of low birth weight can be decreased by giving erythromycin to some women with C. trachomatis. Due to the high clearance rate of C. Contract grant sponsor: National Institute of Child Health and Human Development; Contract grant numbers: HD through HD Contract grant sponsor: National Institute of Allergy and Infectious Diseases; Contract grant number: AI The authors are members of the Vaginal Infections and Prematurity (VIP) Study Group. Other members of the VIP Study Group are: Sumner J. Yaffe, Charlotte S. Catz, George G. Rhoads (now at the University of Medicine and Dentistry of New Jersey), Donald McNellis, Heinz W. Berendes, National Institute of Child Health and Human Development; Robert Edelman (now at the University of Maryland), William C. Blackwelder, George F. Reed, National Institutes of Allergy and Infectious Diseases; W. Kenneth Poole, Research Triangle Institute; Luann Wenthold, Department of Obstetrics and Gynecology, Louisiana State University; Molly Fischer, Department of Obstetrics and Gynecology, University of Washington; Arlene Meier, Departments of Pediatrics and Obstetrics and Gynecology, University of Oklahoma; Kathleen Lipscomb, Department of Obstetrics and Gynecology, University of Texas, San Antonio; Kim Geromanos, Department of Pediatrics, Columbia University. Dr. Ronald S. Gibbs is now at the Department of Obstetrics and Gynecology, University of Colorado, Denver, CO *Correspondence to: Dr. Robert P. Nugent, NIH, NICHD, PAMA Branch, 6100 Executive Boulevard, Room 4BllC, Bethesda, MD Received 19 January 1997 Clinical Symposium Accepted 31 March 1997
2 trachomatis in the placebo group, these data do not provide unequivocal evidence that erythromycin use in all C. trachomatis-infected women prevents low birth weight. Infect. Dis. Obstet. Gynecol. 5:10-17, (C) 1997Wiley-Liss, Inc. KEY WORDS chlamydial infection; premature delivery; low birth weight he prevalence of Chlamydia trachomatis varies widely depending upon the population, with 5-15% of pregnant women being infected. 1-3 During pregnancy, C. trachomatis is isolated 4-8 times more frequently than Neisseria gonorrhoeae, z,4,s C. trachomatis is particularly common among adolescents and individuals from low socioeconomic groups, 6,7 and it has been associated with adverse pregnancy outcomes in most, but not all, reports. 1,z,4,6,8, 9 Treatment of C. trachomatis in pregnancy has been associated with improved pregnancy outcome. In one study, the rate of abnormal pregnancy outcome was significantly lower in women with C. trachomatis who were treated with erythromycin over a 20 month study period compared to the rate in untreated women examined during the 16 month interval preceding the study period. 1 Furthermore, women infected with C. trachomatis who were treated had the same frequency of abnormal outcome as did uninfected women. 1 In another study, fewer abnormal pregnancy outcomes occurred among C. trachomatis-positivc women who were effectively treated than among those who remained culture positive. 1 Neither of these studies used a randomized or blinded treatment protocol nor did they account for other bacteria or obstetrical factors associated with poor pregnancy outcome. We report the results of a randomized placebocontrolled double-blinded trial of erythromycin treatment of C. trachomatis infection during pregnancy. Our study was able to adjust for the influence of demographic, sexual, and obstetrical factors as well as the presence of other potentially pathogenic organisms isolated from the lower genital tract. SUBJECTS AND METHODS Study Design As part of the Vaginal Infection and Prematurity (VIP) Study, pregnant women were enrolled from seven institutions utilizing six antepartum clinics (Harlem Hospital, New York, NY; Columbia University, New York, NY; Louisiana State University and Tulane University, New Orleans; University of Oklahoma, Oklahoma City; University of Texas Health Science Center, San Antonio; and University of Washington, Seattle). A uniform protocol had been agreed upon, including a standardized questionnaire and common laboratory methods. Women seeking prenatal care between the 23rd and 26th completed weeks of pregnancy were screened for eligibility to enter the observational phase of the study. Women were eligible if they were ->16 years old, were free of medical complications related to premature delivery, and were not taking selected medications. Details of inclusion and exclusion criteria have been published previously, lz,3 Research personnel obtained demographic, obstetric, sexual, contraceptive, and drug use history on standardized coded forms; performed a standardized physical and pelvic examination; and collected urogenital cultures in a standard manner. At delivery the medical records of the mother and infants were reviewed and information on intervening therapy, including non-trial antibiotic use, was abstracted. Women identified as colonized with Ureaplasma urealyticum, group B streptococci, and/or C. trachomatis were considered for randomization into the clinical trial. The clinical trial results for women positive for U. urealyticum but negative for group B streptococci and C. trachomatis have been published, as have the results for women with group B streptococci, ae Collection of Urogenital Specimens Following the insertion of sterile cotton swabs into the endocervix to obtain specimens for Gram s stain, and for N. gonorrhoeae and group B streptococcal culture, a sterile Dacron swab with a plastic shaft was then inserted within the endocervical canal and rotated for several seconds to obtain a INFECTIOUS DISEASES IN OBSTETRICS AND GYNECOLOGY II
3 specimen for C. trachomatis culture. The swab tip was broken off in a vial containing 1.5 ml of phosphate buffered 0.2 M sucrose solution. The specimen was maintained at refrigerator temperature until it reached the laboratory later that day. Details of specimen collection methods for the other microorganisms have been published. 13 Culture Methods The protocol for C. trachomatis culture followed by all centers was as follows: the swab was removed from the transport vial and a sample of the carrier media was inoculated onto McCoy cell monolayers grown in one dram shell vials at all centers as described 14 except for the University of Washington, which used 96-well microtiter plates. s At least two monolayers were inoculated for each specimen. Vials or plates were then centrifuged for h at 1,000-2,500 g. After replacement of the inoculum with cell culture media, vials and/or plates were incubated at 35C for h. One coverslip or one well was then stained with fluorescein isothiocyanate-conjugated C. trachomatis-specific monoclonal antibody and read with a fluorescent microscope. For positive specimens, C. trachomatis inclusions in random microscopic fields were counted to semiquantitate the number of viable organisms in each specimen. For negative specimens, the cells were scraped off the bottom of the second vial or well using a pipette tip and inoculated onto fresh monolayers. The culture procedure was then repeated. A specimen was considered negative only if inclusions were not observed in monolayers inoculated with the passed specimen. Clinical Trial Methods Women from whom C. trachomatis was recovered in the observational study were eligible to participate in the clinical trial. However, women receiving antibiotics since the screening examination, who were allergic to erythromycin or receiving theophylline were ineligible. Women with positive screening cultures for N. gonorrhoeae or > l0 s microorganisms/ml of urine were treated and thus ineligible for the trial. Eligible women who agreed to participate in the clinical trial entered a week placebo run-in. 6 Those who took less than two-thirds of the allotted placebo pills during the run-in, who did not return to the clinic or refused further participation were not randomized. Patients who successfully completed the run-in, had none of the exclusion criteria, and were <30 weeks gestation were randomized as described previously. 3 The randomization scheme stratified women by study site and microorganism combination to allow for subgroupspecific analyses. Participants were treated with either erythromycin base (333 mg) or identicalappearing placebo tablets 3 times daily from blister packs containing 21 pills each. The erythromycin and placebo were supplied by the Upjohn Company (Kalamazoo, MI). As a result of concerns that a daily dose of 2 g of erythromycin would be poorly tolerated over 6 weeks, the lower g dose was chosen. Earlier reports suggested that a g daily dose for 6 weeks in the third trimester decreased the rate of low birth weight infants among women colonized with genital mycoplasmas. 7 Previous experience of the investigators suggested that 500 mg erythromycin taken twice daily for 14 days effectively treated endocervical C. trachomatis in pregnant women (Martin and Eschenbach, unpublished data). Treatment of partners was recommended, but therapy was given to the study participants until the completion of the 35th week of pregnancy to reduce the likelihood of reinfection. Women were evaluated at each regular antenatal visit, at which time compliance (by pill count) and side effects were recorded by a research nurse using a standardized questionnaire, used blister packs were collected, and additional medication was supplied. Quality Control/Drug Efficacy Issues Repeat cultures were obtained 2-4 weeks after enrollment from the first 100 women enrolled into the clinical trial at each study site. In addition, a random sample of 12% of all study participants was selected to have repeat cultures (6% at weeks and 6% at weeks gestation). Repeat cultures also were obtained from women admitted for pregnancy complications at <37 weeks gestation (premature labor, rupture of membranes) and from all women admitted to the hospital in term labor during weekday daytime hours. Two methods were used for quality control of (7. trachomatis cultures. Each study site in turn sent 5 "unknowns" that included both positive and negative specimens to the other centers. In addition, a 12 INFECTIOUS DISEASES IN OBSTETRICS AND GYNECOLOGY
4 random sample of women had duplicate C. trachomatis specimens obtained and submitted to the laboratories for culture. Different study numbers were assigned to blind laboratory personnel to the duplicate specimens. Definitions Gestational age at study entry was estimated from the last menstrual period, results of the first pelvic examination, onset of fetal heart tone, and ultrasound data when available. Gestational age at delivery was calculated from the entry estimate and the time to delivery. All women had pertinent pregnancy, labor, and delivery information collected including the use of non-trial antibiotics. Premature rupture of membranes was defined as membrane rupture before the onset of regular uterine contractions. Antibiotics considered effective against C. trachomatis included erythromycin, ampicillin, tetracycline, oral sulfa preparations, and penicillin VK. Antibiotics considered ineffective included metronidazole, cephalosporins, nitrofurantoin, penicillins other than VK or ampicillin, and vaginal sulfa cream. Post-Delivery Trial participants with C trachomatis were retreated with doxycycline, tetracycline, or erythromycin immediately postpartum, regardless of which trial medication they received. Infants were either treated empirically after delivery or were followed, cultured at their first postnatal visit, and treated with antibiotics if indicated. Statistical Analysis Categorical variables were compared using the chisquare test or Fisher s exact test. 18 Significance was defined as a two-tailed P < All calculations were done using SAS with the exception of logistic regression analyses, which were done using BMDP procedures. 19 RESULTS Between November 1, 1984, and March 31, 1989, 13,914 women were enrolled into the VIP Study, of whom 13,750 (99%) had C. trachomatis results available. C. trachomatis was isolated from 1,239 (9.0%) women, 204 of whom were ineligible for the trial due to N. gonorrhoeae infection (n 59), asymptomatic bacteriuria (n 60), or other exclusion criteria. We were able to contact 933 of the 1,035 eligible women. Of those contacted, 218 women (23%) did not keep their enrollment appointment, 121 (13%) refused to participate, and 594 women were entered into the placebo run-in. The 180 women who did not comply with the run-in were not randomized, leaving 414 women who were randomized to receive either erythromycin (205) or placebo (209). After starting medication, 25 erythromycin-treated and 23 placebo-treated women withdrew from the trial but were included in the intent-to-treat analysis. The two groups were compared with respect to demographic, behavioral, and obstetrical characteristics (Table 1). No significant differences were seen in baseline characteristics between the groups (Table 1). Additional factors that did not differ between groups included living arrangement; method/source of medical payment; work during pregnancy; number of sexual partners during pregnancy, in the last year, and lifetime; frequency of intercourse; prior genital or kidney infection; history of a cone biopsy; difficulty becoming pregnant; hospitalization during pregnancy; general health; illicit drug use; mean height and weight; past chlamydial or gonococcal infection or present genital infection (group B streptococci, U. urealyticum, Trichomonas vaginalis, bacterial vaginosis, or endocervical mucopus). The pregnancy outcomes of women entered into the trial are summarized in Table 2. The mean birth weight of infants was similar in the two groups as was the distribution of birth weights. Birth weight <2,500 g occurred in 8% of erythromycin-treated and 11% of placebo-treated women (P 0.4) Additionally, there were no differences in the distribution of gestational age, gestational age at the time of premature rupture of membranes (PROM), or the total proportion of women experiencing PROM. The numbers of stillbirths and neonatal deaths were low in both groups. Thus, when data from all study sites were combined, there was no statistically significant impact of erythromycin on pregnancy outcome, although there were fewer low birth weight infants, fewer deliveries <37 weeks gestation, and fewer instances of PROM in the erythromycin compared to the placebo group. Compliance data were available for 199 of the 205 women in the erythromycin group and 206 of INFECTIOUS DISEASES IN OBSTETRICS AND GYNECOLOGY 13
5 TABLE I. Descriptive characteristics of patients with C. trachomatis randomized to receive either erythromycin or placebo Erythromycin Placebo (n 205) (n 209) Mean age + S.D. (years) Mean gravidity + S.D Mean gestational age when I I. screened + S.D. (weeks) Mean gestational age when randomized + S.D. (weeks) Ethnicity White, Asian, and Native 34 (I 7%) 33 (16%) American Black 126 (61%) 123 (59%) New York Hispanic 34 (17%) 47 (22%) Non-New York Hispanic II (5%) 6 (3%) Marital status Married 59 (29%) 48 (23%) Separated/divorced/widowed 18 (9%) 21 (10%) Never married 127 (62%) 140 (67%) Education (years) <12 90 (44%) 97 (47%) (41%) 73 (35%) >12 30 (I 5%) 38 (I 8%) Smoked cigarettes during the 50 (24%) 48 (23%) pregnancy Used alcohol during the 35 (I 7%) 37 (18%) pregnancy Prior low birth weight delivery Yes 46 (22%) 52 (25%) No 70 (34%) 55 (26%) First pregnancy 89 (43%) 102 (49%) Study site Harlem Hospital 6 (3%) 9 (4%) Columbia University 54 (26%) 59 (28%) University of Washington 6 (3%) 7 (3%) University of Oklahoma 29 (14%) 33 (16%) University of Texas 10 (5%) 3 (1%) Louisiana State University/ 100 (49%) 98 (47%) Tulane University alncluded patients who delivered an infant weighing <2,500 with a prior pregnancy. the 209 women in the placebo group. Twentythree percent of the 199 erythromycin-treated women took less than two-thirds of their pills compared to 16% of the 206 placebo-treated women (P 0.053). Nausea was the only side effect reported significantly more often among women in the erythromycin group (33%) compared to the placebo group (21%) (P 0.005). Appetite loss was also more frequent in the erythromycin than the placebo group (21% vs. 14%, P 0.08). Overall, 55% of women taking erythromycin experienced at least one side effect compared to 43% of women on placebo (P 0.01). The effect of erythromycin on low birth weight and preterm delivery was stratified by TABLE 2. Effect of erythromycin treatment on pregnancy outcome in patients with C. trachomatis for the total group in an intent-to-treat analysis Outcome/total Erythromycin Placebo (n 205) (n 209) Mean birth weight + S.D. (g) 3, , Low birth weight at delivery (g) <1,500 0/201 2/199 (I %) 1,500-2,499 17/201 (8%) 20/199 (I 0%) Total <2,500 17/201 (8%) 22/199 (I I%) Gestational age at delivery (weeks) <32 1/202 (0.5%) 1/203 (0.5%) /202 (I 3%) 29/203 (I 4%) Total <37 27/202 (I 3%) 30/203 (I 5%) Premature rupture of membranes (weeks) <37 5/196 (3%) 7/193 (4%) -->37 16/196 (8%) 18/193 (9%) Total PROM 21/196 (I I%) 25/193 (I 3%) Stillbirth 2/202 (1%) 1/203 (0.5%) Neonatal death 1/202 (0.5%) 0/203 athe difference between the number of women enrolled in each arm of the treatment trial and the numbers assessed for each outcome reflect the numbers of women for whom data could not be obtained. compliance with no apparent effect on pregnancy outcome. Endocervical C. trachomatis cultures obtained mid-study, while women were still receiving the study drug, remained positive in 20% of erythromycin-treated and 63% of placebo-treated women (P < 0.001). C. trachomatis was recovered from 8 (14%) of 56 erythromycin-treated women who took two-thirds or more of their pills compared to 7 (33%) of 19 women taking less than two-thirds of their erythromycin (P 0.03). Because of the unexpectedly high clearance of C. trachomatis in the placebo group (37%), the data were analyzed separately by study site (Table 3). The interim recovery rates were low (24-25%) in the placebo group at the New York and Oklahoma sites as opposed to the New Orleans and Seattle sites, where 89% and 83% of placebo-treated women continued to have positive cultures. Only one woman at San Antonio had repeat culture data. In an attempt to explain these results, we first examined the repeatability of culture results from the quality control data. Recovery rates of C. trachomatis from quality control specimens were similar at the various sites. Overall, laboratories correctly identified 70 of 75 positive and 84 of 85 negative C. trachomatis quality control specimens. 14 INFECTIOUS DISEASES IN OBSTETRICS AN/) GYNECOLOGY
6 TABLE 3. Bacteriologic effect of erythromycin treatment measured by interim endocervical C. trachomatis cultures No. C. trachomatis positive/no, treated Erythromycin Placebo P New Orleans 10/39 (25%) 39/44 (89%) <0.001 Seattle 0/4 5/6 (83%) San Antonio 0/I 0/0 New York I/21 (5%) 4/I 9 (24%) 0.17 Oklahoma 5/13 (38%) 3/12 (25%) 0.67 athe two New Orleans sites and the two New York,ites utilized the same laboratories and the data were combined. The results of blinded split specimens were also in agreement. The proportion of samples for which both specimens were positive among the total with at least one positive specimen were: Louisiana State University/Tulane, 24/29 (83%); Columbia University/Harlem, 16/22 (73%); University of Oklahoma, 3/10 (30%); University of Washington, 0/1 (0%); and University of Texas 1/1 (100%). We next examined data on the use of antibiotics prescribed outside of the clinical trial as a possible explanation for the high clearance rate in the placebo group. Significantly more use of non-trial antibiotics effective against C. trachomatis occurred in the placebo than the erythromycin group (22 vs. 8 women, P < 0.01). Harlem, Columbia, and Oklahoma showed a greater use of antibiotics in the placebo than the erythromycin group (33.3% vs. 0.0%, 17.0% vs. 1.9%, 18.8% vs. 3.5%, respectively). New Orleans had roughly similar rates in the two groups (5.6% vs. 2.8%), while San Antonio showed a greater use of non-trial antibiotics in the erythromycin group (0 vs. 20.0%). The number of women receiving effective antibiotics did not explain all of the differences in C. trachomatis positively among placebo-treated women. The trial outcome data were then stratified into two groups: data from study sites with low vs. high C. trachomatis clearance in the placebo group. In the sites with low clearance (New Orleans, Seattle, and San Antonio), low birth weight infants occurred in 9 of 114 erythromycin-treated and 18 of 105 placebo-treated women (P 0.04; Table 4) and delivery <37 weeks occurred in 15 of the 115 erythromycin and 18 of the 105 placebo group (P 0.4). In the sites with high clearance (New York and Oklahoma), low birth weight occurred in 8 of 87 erythromycin-treated and 4 of 94 placebo-treated women (P 0.18) and delivery <37 weeks occurred in 12 of 87 erythromycin-treated and 12 of 98 placebo-treated women (P 0.75). DISCUSSION In the intent-to-treat analyses, there were no apparent beneficial effects of erythromycin treatment on low birth weight, preterm delivery, PROM, or perinatal mortality. However, when the analysis took into account the clearance of C. trachomatis in the placebo group, erythromycin was associated with a 50% reduction in the rate of low birth weight in the centers with low clearance. The higher rate of low birth weight in the placebo group in these sites is consistent with the increased rate of low birth weight observed among untreated women with C. trachomatis in other studies. 1,z,4,5,1 The proportion of infants with low birth weight in the erythromycin group (8%) was consistent with that for C. trachomatis-negative women in the observational component of the VIP Study (7.6%), and the proportion of low birth weight infants in the placebo group (11%) was similar to that among C. trachomatis-positive women in the VIP Study (10.6%) who were not entered into the trial. There were at least three significant problems that interfered with our ability to detect an overall effect of erythromycin on pregnancy outcome. First, the trial included only 414 women, so the ability to detect small but potentially important differences between the erythromycin and placebo groups was limited. Second, approximately 20% of women receiving erythromycin remained C. trachomarls positive as determined from the random sample who were recultured. Third, at three study sites which contributed 46% of the cases to the trial, high clearance of C. trachomatis occurred in the placebo group. The 20% failure rate of erythromycin in our study suggests the g dose is less than optimal, possibly due to the 40% increase in blood and extracellular volume in pregnancy acting to reduce serum and tissue drug levels. While some investigators have reported cure rates in pregnancy of 98% with 1,500 mg of amoxicillin daily for 7 days, z and 95% with 1,000 mg of clindamycin daily for 14 days, zl cure rates with erythromycin seem to be more variable, perhaps related to gastrointestinal tolerance and, in turn, to non-compliance, z C. trachomatis cure rates after erythromycin have ranged INFECTIOUS DISEASES IN OBSTETRICS AND GYNECOLOGY 15
7 TABLE 4. Stratification of trial outcome by treatment and study center Study site stratified by C. trachomatis clearance in the placebo group Birth weight <2,500 g Delivery at <37 weeks Erythromycin Placebo Erythromycin Placebo (n 201) (n 199) (n 202) (n 203) High clearance New York-Harlem I/6 (I 7%) 0/9 (0%) I/6 (I 7%) I/9 (I I%) New York-Columbia 5/52 (10%) 2/53 (4%) 8/52 (I 5%) 6/57 (I I%) Oklahoma City 2/29 (7%) 2/32 (6%) 3/29 (10%) 5/32 (I 6%) Total 8/87 (9%) 4/94 (4%) 12/87 (I 4%) 12/98 (I 2%) Low clearance Seattle I/6 (I 7%) 0/7 (0%) 3/6 (50%) I/7 (I 4%) San Antonio 0/10 (0%) 0/3 (0%) 1/10 (I 0%) 0/3 (0%) New Orleans 8/98 (8%) 18/95 (I 9%) 1/99 (I I%) 17/95 (I 8%) Total 9/I 14 (8%) 18/105 (I 7%) 15/I 15 (I 3%) 18/105 (I 7%) from 92 to 95% with 1,600-2,000 mg daily doses for 7 days z,zz to 76-88% with 1,000-2,000 mg daily doses for 7-14 days. 11,zl Direct comparisons of 1,000 vs. 2,000 mg daily doses of erythromycin have not been performed during pregnancy, but in non-pregnant women, one study has suggested that the 1,000 mg dose appears to be less effective than the 2,000 mg daily dose. C. trachomatis was recovered after therapy in 27% of 45 non-pregnant women given 1,000 mg and 10% of 31 nonpregnant women given 2,000 mg. z3 The low recovery rate in the placebo group was unexpected since other studies have shown that C. trachomatis persists in 85-90% of untreated non-pregnant z-3,z4 and 71-75% of untreated pregnant women, z The effect of treating C. trachomatis has been reported in two studies. Ryan et al. 1 compared data on the pregnancy outcome of C. trachomatisinfected women treated with 2 g of erythromycin for 7 days with historical data on untreated women with C. trachomatis cared for in the previous 16 months in the same antenatal clinic. Treatment with erythromycin was associated with a 50% reduction of low birth weight, a 45% reduction of PROM, and a 400% increase in newborn survival. 1 Cohen et al. 1 reported the pregnancy outcome of women who were initially C. trachomatis positive and then underwent multiple repeat cultures after standard erythromycin therapy to detect treatment failures and reinfection. Low birth weight, PROM, and preterm delivery were more common in the women who remained positive than in the women who became C. trachomatis negative. 1 Neither of these studies employed a randomized trial design. What conclusions may be drawn from the studies of C. trachomatis and pregnancy outcome to date? Data from our observational study suggest an impact of untreated C. trachomatis on low birth weight, preterm delivery, and preterm PROM. Results of site-specific analyses from our clinical trial together with the results of Ryan et al. 1 and Cohen et al. suggest that the risk of low birth weight in C. trachomatis-positive women can be decreased by erythromycin treatment. Even in the absence of incontrovertible evidence of a role of C. trachomatis in abnormal pregnancy outcome, there is a strong rationale for the antepartum diagnosis and treatment of Chlamydia. First, treatment prevents transmission of C. trachomatis to the infant at delivery. The 15-20% rate of chlamydial conjunctivitis and chlamydial pneumonia zs appears to be reduced by 90% when the mother is treated, z,zz Second, treatment reduces the spread to sexual partners. Third, treatment can prevent fallopian tube damage and possible future tubal infertility among women who otherwise would be chronically infected or develop salpingitis postpartum, z6 Therefore, antepartum screening and treatment of C. trachomatis are recommended by the Centers for Disease Control (CDC). z7 Although treatment at 36 weeks of pregnancy would prevent vertical transmission to the 90% of infants born beyond this time, z,zz screening and treatment late in pregnancy would not be expected to prevent premature delivery. Treatment in the mid-trimester could both potentially reduce premature delivery rates and protect the infant from vertical transmission. In our opinion, these considerations tip the balance in favor of mid-trimester as the optimal time to treat C. trachomatis as opposed to the later time recommended by the CDC. z7 16 INFECTIOUS DISEASES IN OBSTETRICS AND GYNECOLOGY
8 ACKNOWLEDGMENTS This work was supported by contracts (HD through HD ) from the National Institute of Child Health and Human Development and the National Institute of Allergy and Infectious Diseases (AI ). REFERENCES 1. Martin DH, Koutsky LA, Eschenbach DA, Daling JR, Alexander ER, Holmes KK: Perinatal mortality and prematurity in pregnancies complicated by aritepartum maternal Chlamydia trachomatis infection. JAMA 247: , Sweet RL, Landers DV, Walker C, Schachter J: Ch/amydia trachornatis infection and pregnancy outcome. Am J Obstet Gynecol 156: , Hcggic AD, Lumicao GG, Stuart LA, Gyves MT: Chlamydia trachomatis infection in mothers and infants. Am J Dis Child 135:507, Gravett MG, Nelson HP, DeRoucn T, Critchlow CW, Eschenbach DA, Holmes KK: Independent association of bacterial vaginosis and Ch/amydia trachornatis infection with adverse pregnancy outcome. JAMA 256: , Alger LS, Lovchik JC, Hebcl JR, Blackmon LR, Crenshaw MC: The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynccol 159: , Sharer MA, Beck A, Blain B, ct al.: Chlamydia trachomatis: Important relationships to race, contraceptive use, lower genital tract infection and Papanicolaou smears. Pediatr 104: , Eager RM, Beach RK, Davidson AJ, Judson FN: Epidcmiologic and clinical factors of Chlamydia trachomatis in black, Hispanic and white female adolescents. West J Med 143:37, Harrison HR, Alexander ER, Weinstein L, Lewis M, Nash M, Sire DA: Cervical Ch/amydia trachomatis and mycoplasmal infections in pregnancy: Epidemiology and outcomes. JAMA 250: , Berman SM, Harrison HR, Boycc WT, Haffner WJ, Lewis M, Arthur JB: Low birth weight, prematurity, and postpartum endometritis. Association with prenatal cervical 3/lycop/asma hominis and Ch/amydia trachomatis infections. JAMA 257: , Ryan GM, Abdell RN, McNeeley G, ct al.: Ch/amydia trachomatis infection in pregnancy and effect of treatment on outcome. Am J Obstet Gynecol 162:34-39, Cohen I, Veille J, Colkins BM: Improved pregnancy outcome following successful treatment of chlamydial infection. JAMA 263: , Klebanoff MA, Rcgan JA, Rao AV, et al.: Outcome of the Vaginal Infections and Prematurity Study: Results of a clinical trial of erythromycin among pregnant women colonized with group B streptococci. Am J Obstet Gynecol 172: , Eschenbach DA, Nugent RP, Rao AV, et al., and Vaginal Infections and Prematurity Study Group: A randomized placebo-controlled trial of erythromycin for the treatment of U. urealyticum to prevent premature delivery. Am J Obstet Gynecol 164: , Schachter J: Chlamydiae. In Balows A, Hausler WJ, Herrmann KL, Isenbcrg HD, Shadomy HJ (eds): Clinical Microbiology. 5th ed. Washington, DC: American Society for Microbiology, Stamm WE, Tam MR, Koester M, Cles L: Detection of Chlamydia trachomatis inclusions in McCoy cell cultures with fluoresccin-conjugated monoclonal antibodies. Clin Microbiol 17: , Blackwelder WC, Hastings BK, Lee MF, et al.: Value of a run-in period in a drug trial during pregnancy. Controlled Clin Trial 11: , McCormack WM, Rosner B, Lee Y, Munoz A, Charles D, Kass EH: Effect on birth weight of erythromycin treatment of pregnant women. Obstet Gynecol 69: , Armitage P, Berry G (eds): Statistical Methods in Medical Research. Boston: Blackwell Scientific, pp , SAS Institute: SAS User s Guide. Version 5. Cary, NC: SAS Institute, Cromblcholmc W, Schachter J, Grossman M, Landers DV, Sweet RL: Amoxicillin therapy for Chlarnydia trachomatis in pregnancy. Obstet Gynecol 75:752, Alger LS, Lovchik JC: Comparative efficacy of clindamycin versus erythromycin in eradication of antenatal Ch/amydia trachomatis. Am J Obstct Gynccol 165: , Schachter J, Sweet RL, Grossman M, et al.: Experience with the routine use of erythromycin for chlamydial infections in pregnancy. N Engl Mcd 314: , Linnemann CC, Heaton CL, Ritchcy M: Treatment of Chlamydia trachomatis infections: Comparison of 1- and 2-g doses of erythromycin daily for seven days. Sex Transm Dis 14: , Handsfield HH, Murphy VL: Treatment of uncomplicated gonorrhea in women with single-dose cefonicid. Sex Transm Dis 12:90-92, Schachter J, Grossman M, Sweet RL, et al.: Prospective study of perinatal transmission of Chlamydia trachomatis. JAMA 255: , Plummer FA, Laga M, Brunham RC, et al.: Postpartum upper genital infections in Nairobi, Kenya: Epidcmiology, etiology and risk factors. Infect Dis 157:92-98, U.S. Public Health Service Centers for Disease Control: Sexually transmitted disease treatment guidelines. MMWR 38(S-8):1-40, INFECTIOUS DISEASES IN OBSTETRICS AND GYNECOLOGY 17
9 MEDIATORS of INFLAMMATION The Scientific World Journal Gastroenterology Research and Practice Diabetes Research International Endocrinology Immunology Research Disease Markers Submit your manuscripts at BioMed Research International PPAR Research Obesity Ophthalmology Evidence-Based Complementary and Alternative Medicine Stem Cells International Oncology Parkinson s Disease Computational and Mathematical Methods in Medicine AIDS Behavioural Neurology Research and Treatment Oxidative Medicine and Cellular Longevity
Treatment of Chlamydial Cervicitis During Pregnancy
 Infectious Diseases in Obstetrics and Gynecology 3:189-192 (1995) (C) 1996 Wiley-Liss, Inc. Efficacy and Tolerance of Single-Dose Azithromycin for Treatment of Chlamydial Cervicitis During Pregnancy Joseph
Infectious Diseases in Obstetrics and Gynecology 3:189-192 (1995) (C) 1996 Wiley-Liss, Inc. Efficacy and Tolerance of Single-Dose Azithromycin for Treatment of Chlamydial Cervicitis During Pregnancy Joseph
Etiology and treatment of chronic bacterial prostatitis the Croatian experience
 Etiology and treatment of chronic bacterial prostatitis the Croatian experience Višnja Škerk University Hospital for Infectious Diseases "Dr. Fran Mihaljevic" Zagreb Croatia Milano, Malpensa, 14 Nov 2008
Etiology and treatment of chronic bacterial prostatitis the Croatian experience Višnja Škerk University Hospital for Infectious Diseases "Dr. Fran Mihaljevic" Zagreb Croatia Milano, Malpensa, 14 Nov 2008
Specimen collection and transport for Chlamydia trachomatis and Neisseria gonorrhoeae testing
 Specimen collection and transport for Chlamydia trachomatis and Neisseria gonorrhoeae testing Overview Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections are two of the most common sexually
Specimen collection and transport for Chlamydia trachomatis and Neisseria gonorrhoeae testing Overview Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections are two of the most common sexually
The Minnesota Chlamydia Strategy: Action Plan to Reduce and Prevent Chlamydia in Minnesota Minnesota Chlamydia Partnership, April 2011
 The Minnesota Chlamydia Strategy: Action Plan to Reduce and Prevent Chlamydia in Minnesota Minnesota Chlamydia Partnership, April 2011 Section 5: Screening, Treating and Reporting Chlamydia While the information
The Minnesota Chlamydia Strategy: Action Plan to Reduce and Prevent Chlamydia in Minnesota Minnesota Chlamydia Partnership, April 2011 Section 5: Screening, Treating and Reporting Chlamydia While the information
Expedited Partner Therapy (EPT) for Sexually Transmitted Diseases Protocol for Health Care Providers in Oregon
 Expedited Partner Therapy (EPT) for Sexually Transmitted Diseases Protocol for Health Care Providers in Oregon Oregon Health Authority Center for Public Health Practice HIV/STD/TB Section Principles of
Expedited Partner Therapy (EPT) for Sexually Transmitted Diseases Protocol for Health Care Providers in Oregon Oregon Health Authority Center for Public Health Practice HIV/STD/TB Section Principles of
Frequently Asked Questions
 Guidelines for Testing and Treatment of Gonorrhea in Ontario, 2013 Frequently Asked Questions Table of Contents Background... 1 Treatment Recommendations... 2 Treatment of Contacts... 4 Administration
Guidelines for Testing and Treatment of Gonorrhea in Ontario, 2013 Frequently Asked Questions Table of Contents Background... 1 Treatment Recommendations... 2 Treatment of Contacts... 4 Administration
THIS IS AN OFFICIAL NH DHHS HEALTH ALERT
 THIS IS AN OFFICIAL NH DHHS HEALTH ALERT Distributed by the NH Health Alert Network Health.Alert@nh.gov August 13, 2015 1400 EDT (2:00 PM EDT) NH-HAN 20150813 Updated Centers for Disease Control (CDC)
THIS IS AN OFFICIAL NH DHHS HEALTH ALERT Distributed by the NH Health Alert Network Health.Alert@nh.gov August 13, 2015 1400 EDT (2:00 PM EDT) NH-HAN 20150813 Updated Centers for Disease Control (CDC)
A randomized controlled trial comparing amoxicillin and azithromycin for the treatment of Chlamydia trachomatis in pregnancy
 A randomized controlled trial comparing amoxicillin and azithromycin for the treatment of Chlamydia trachomatis in pregnancy Gavin F. Jacobson, MD, a Amy M. Autry, MD, b Russell S. Kirby, PhD, a Elaine
A randomized controlled trial comparing amoxicillin and azithromycin for the treatment of Chlamydia trachomatis in pregnancy Gavin F. Jacobson, MD, a Amy M. Autry, MD, b Russell S. Kirby, PhD, a Elaine
CDC 2015 STD Treatment Guidelines: Update for IHS Providers Sharon Adler M.D., M.P.H.
 CDC 2015 STD Treatment Guidelines: Update for IHS Providers Sharon Adler M.D., M.P.H. Clinical Faculty, CA Prevention Training Center Disclosure Information Sharon Adler MD, MPH I have no financial relationships
CDC 2015 STD Treatment Guidelines: Update for IHS Providers Sharon Adler M.D., M.P.H. Clinical Faculty, CA Prevention Training Center Disclosure Information Sharon Adler MD, MPH I have no financial relationships
Chlamydia THE FACTS. How do people get Chlamydia?
 What is Chlamydia? Chlamydia is a common bacterial infection that is sexually transmitted and often causes no symptoms. If not treated, chlamydia can damage reproductive organs and make it difficult for
What is Chlamydia? Chlamydia is a common bacterial infection that is sexually transmitted and often causes no symptoms. If not treated, chlamydia can damage reproductive organs and make it difficult for
TREATMENT OF STI CONTACTS
 This decision support tool is effective as of October 2014. For more information or to provide feedback on this or any other decision support tool, email certifiedpractice@crnbc.ca TREATMENT OF STI CONTACTS
This decision support tool is effective as of October 2014. For more information or to provide feedback on this or any other decision support tool, email certifiedpractice@crnbc.ca TREATMENT OF STI CONTACTS
signs suggesting chlamydia:
 Chlamydia - uncomplicated genital - Management View full scenario When should I suspect and test for chlamydia? Women: o Test for chlamydia if they are sexually active with symptoms and signs suggesting
Chlamydia - uncomplicated genital - Management View full scenario When should I suspect and test for chlamydia? Women: o Test for chlamydia if they are sexually active with symptoms and signs suggesting
California Guidelines for STD Screening and Treatment in Pregnancy
 California Guidelines for STD Screening and Treatment in Pregnancy These guidelines were developed by the California Department of Public Health (CDPH) Sexually Transmitted Diseases (STD) Control Branch
California Guidelines for STD Screening and Treatment in Pregnancy These guidelines were developed by the California Department of Public Health (CDPH) Sexually Transmitted Diseases (STD) Control Branch
Clinical Policy Title: Home uterine activity monitoring
 Clinical Policy Title: Home uterine activity monitoring Clinical Policy Number: 12.01.01 Effective Date: August 19, 2015 Initial Review Date: July 17, 2013 Most Recent Review Date: July 15, 2015 Next Review
Clinical Policy Title: Home uterine activity monitoring Clinical Policy Number: 12.01.01 Effective Date: August 19, 2015 Initial Review Date: July 17, 2013 Most Recent Review Date: July 15, 2015 Next Review
Frequently Asked Questions (FAQs)
 Frequently Asked Questions (FAQs) Research Rationale 1. What does PrEP stand for? There is scientific evidence that antiretroviral (anti-hiv) medications may be able to play an important role in reducing
Frequently Asked Questions (FAQs) Research Rationale 1. What does PrEP stand for? There is scientific evidence that antiretroviral (anti-hiv) medications may be able to play an important role in reducing
2014 CDC Treatment Guidelines for STDs What s New, What s Important, What s Essential. STD Treatment Guidelines. How are the guidelines prepared?
 2014 CDC Treatment Guidelines for STDs What s New, What s Important, What s Essential Bradley Stoner, MD, PhD Associate Professor, Washington University School of Medicine Medical Director, St. Louis STD/HIV
2014 CDC Treatment Guidelines for STDs What s New, What s Important, What s Essential Bradley Stoner, MD, PhD Associate Professor, Washington University School of Medicine Medical Director, St. Louis STD/HIV
Family Health Dataline
 October 1999 Vol 5, No 3 Corrected Feb. 2000 IN THIS ISSUE: In Alaska during 1996-97, 41% of live births were the result of unintended pregnancies. All racial, age, and education groups evaluated had high
October 1999 Vol 5, No 3 Corrected Feb. 2000 IN THIS ISSUE: In Alaska during 1996-97, 41% of live births were the result of unintended pregnancies. All racial, age, and education groups evaluated had high
Rhode Island Department of Health Division of Infectious Diseases and Epidemiology
 Rhode Island Department of Health Division of Infectious Diseases and Epidemiology STD (Sexually Transmitted Disease) PROGRAM Expedited Partner Therapy (EPT) for STDs Guidance for Medical Providers in
Rhode Island Department of Health Division of Infectious Diseases and Epidemiology STD (Sexually Transmitted Disease) PROGRAM Expedited Partner Therapy (EPT) for STDs Guidance for Medical Providers in
35-40% of GBS disease occurs in the elderly or in adults with chronic medical conditions.
 What is Group B Strep (GBS)? Group B Streptococcus (GBS) is a type of bacteria that is found in the lower intestine of 10-35% of all healthy adults and in the vagina and/or lower intestine of 10-35% of
What is Group B Strep (GBS)? Group B Streptococcus (GBS) is a type of bacteria that is found in the lower intestine of 10-35% of all healthy adults and in the vagina and/or lower intestine of 10-35% of
Frequently Asked Questions
 Frequently Asked Questions Testing for Gonorrhea Q1: What test should be completed for accurately diagnosing gonorrhea? A1: Testing is done with either a culture or a NAAT (nucleic acid amplification test).
Frequently Asked Questions Testing for Gonorrhea Q1: What test should be completed for accurately diagnosing gonorrhea? A1: Testing is done with either a culture or a NAAT (nucleic acid amplification test).
The Treatment ofnon-gonococcal Urethritis with Single Dose Oral Azithromycin
 The Journal of International Medical Research 1995; 23: 386-393 The Treatment ofnon-gonococcal Urethritis with Single Dose Oral Azithromycin T ERDOGRU\ A AGA9FIDAff, M ONELz,S BADUR z, 0 ANG 2 AND S TELALLOGLU
The Journal of International Medical Research 1995; 23: 386-393 The Treatment ofnon-gonococcal Urethritis with Single Dose Oral Azithromycin T ERDOGRU\ A AGA9FIDAff, M ONELz,S BADUR z, 0 ANG 2 AND S TELALLOGLU
Developed by: California Department of Public Health (CDPH) Sexually Transmitted Diseases (STD) Control Branch. In collaboration with:
 Best Practices for the Prevention and Early Detection of Repeat Chlamydial and Gonococcal Infections: Effective Partner Treatment and Patient Retesting Strategies for Implementation in California Health
Best Practices for the Prevention and Early Detection of Repeat Chlamydial and Gonococcal Infections: Effective Partner Treatment and Patient Retesting Strategies for Implementation in California Health
Preconception Clinical Care for Women Medical Conditions
 Preconception Clinical Care for Women All women of reproductive age are candidates for preconception care; however, preconception care must be tailored to meet the needs of the individual. Given that preconception
Preconception Clinical Care for Women All women of reproductive age are candidates for preconception care; however, preconception care must be tailored to meet the needs of the individual. Given that preconception
Title: Antibiotic Guideline for Acute Pelvic Inflammatory Disease
 Title: Antibiotic Guideline for Acute Pelvic Inflammatory Disease Version 3 Date ratified December 2007 Review date December 2009 Ratified by NUH Antimicrobial Guidelines Committee Gynaecology Directorate
Title: Antibiotic Guideline for Acute Pelvic Inflammatory Disease Version 3 Date ratified December 2007 Review date December 2009 Ratified by NUH Antimicrobial Guidelines Committee Gynaecology Directorate
Ana M. Viamonte Ros, M.D., M.P.H. State Surgeon General
 Florida Department of Health Division of Disease Control Bureau of Epidemiology Chronic Disease Epidemiology Section Charlie Crist Governor Ana M. Viamonte Ros, M.D., M.P.H. State Surgeon General Florida
Florida Department of Health Division of Disease Control Bureau of Epidemiology Chronic Disease Epidemiology Section Charlie Crist Governor Ana M. Viamonte Ros, M.D., M.P.H. State Surgeon General Florida
STI case management. Francis Ndowa - GFMER Theodora Wi - WHO
 Training Course in Sexual and Reproductive Health Research 2014 Module: Principles and Practice of Sexually Transmitted Infections Prevention and Care STI case management Francis Ndowa - GFMER Theodora
Training Course in Sexual and Reproductive Health Research 2014 Module: Principles and Practice of Sexually Transmitted Infections Prevention and Care STI case management Francis Ndowa - GFMER Theodora
Infection caused by the transmission of Trichomonas vaginalis (T. vaginalis) during sexual contact in which body fluids are exchanged.
 This decision support tool is effective as of November 2015. For more information or to provide feedback on this or any other decision support tool, email certifiedpractice@crnbc.ca TRICHOMONIASIS DEFINITION
This decision support tool is effective as of November 2015. For more information or to provide feedback on this or any other decision support tool, email certifiedpractice@crnbc.ca TRICHOMONIASIS DEFINITION
Vaccines in Pregnancy MARK H. SAWYER, MD UCSD SCHOOL OF MEDICINE RADY CHILDREN S HOSPITAL SAN DIEGO
 Vaccines in Pregnancy MARK H. SAWYER, MD UCSD SCHOOL OF MEDICINE RADY CHILDREN S HOSPITAL SAN DIEGO 1 Objectives List vaccines that should be given either during pregnancy or immediately post-partum in
Vaccines in Pregnancy MARK H. SAWYER, MD UCSD SCHOOL OF MEDICINE RADY CHILDREN S HOSPITAL SAN DIEGO 1 Objectives List vaccines that should be given either during pregnancy or immediately post-partum in
PROTOCOL FOR THE MANAGEMENT OF CLOSE CONTACTS OF PERTUSSIS INFECTION
 PROTOCOL FOR THE MANAGEMENT OF CLOSE CONTACTS OF PERTUSSIS INFECTION Printed copies must not be considered the definitive version DOCUMENT CONTROL PROTOCOL NO. 1.03 Policy Group Infection Control Committee
PROTOCOL FOR THE MANAGEMENT OF CLOSE CONTACTS OF PERTUSSIS INFECTION Printed copies must not be considered the definitive version DOCUMENT CONTROL PROTOCOL NO. 1.03 Policy Group Infection Control Committee
Provider Notification Obstetrical Billing
 Provider Notification Obstetrical Billing Date of Notification September 1, 20 Revision Date September 17, 2015 Plans Affected Mercy Care Plan and Mercy Care Long Term Care Plan Referrals As outlined in
Provider Notification Obstetrical Billing Date of Notification September 1, 20 Revision Date September 17, 2015 Plans Affected Mercy Care Plan and Mercy Care Long Term Care Plan Referrals As outlined in
Diseases that can be spread during sex
 Diseases that can be spread during sex Did you know... over 65 million people in the United States have a chronic, incurable sexually transmitted disease (STD)? and that every year another 19 million persons
Diseases that can be spread during sex Did you know... over 65 million people in the United States have a chronic, incurable sexually transmitted disease (STD)? and that every year another 19 million persons
Racial and Ethnic Disparities in Maternal Mortality in the United States
 Racial and Ethnic Disparities in Maternal Mortality in the United States KYRIAKOS S. MARKIDES, PHD UNIVERSITY OF TEXAS MEDICAL BRANCH GALVESTON, TEXAS PRESENTED AT THE HOWARD TAYLOR INTERNATIONAL SYMPOSIUM
Racial and Ethnic Disparities in Maternal Mortality in the United States KYRIAKOS S. MARKIDES, PHD UNIVERSITY OF TEXAS MEDICAL BRANCH GALVESTON, TEXAS PRESENTED AT THE HOWARD TAYLOR INTERNATIONAL SYMPOSIUM
Preventing Cervical Cancer with Gardasil Jana Ogden RN, MSN, MBA-HCA, IHCC Nursing Faculty. Upon Completion of the Lesson the student will be able to:
 Preventing Cervical Cancer with Gardasil Jana Ogden RN, MSN, MBA-HCA, IHCC Nursing Faculty Upon Completion of the Lesson the student will be able to: Review statistics related to cervical cancer and HPV
Preventing Cervical Cancer with Gardasil Jana Ogden RN, MSN, MBA-HCA, IHCC Nursing Faculty Upon Completion of the Lesson the student will be able to: Review statistics related to cervical cancer and HPV
GONORRHOEA. Use of lidocaine as a diluent when using ceftriaxone (see Appendix 1)
 Key practice notes: 1 st line therapy GONORRHOEA Decreasing sensitivity of gonorrhoea to cephalosporins is now a real threat: CEFTRIAXONE 500mg IM stat first-line for all gonococcal infections AZITHROMYCIN
Key practice notes: 1 st line therapy GONORRHOEA Decreasing sensitivity of gonorrhoea to cephalosporins is now a real threat: CEFTRIAXONE 500mg IM stat first-line for all gonococcal infections AZITHROMYCIN
Vaginal ph Test (ph Hydrion TM Paper 4.5-7.5)
 University of California, San Francisco Department of Laboratory Medicine San Francisco General Hospital 1001 Potrero Avenue, San Francisco CA 94110 Clinical Laboratory Eberhard Fiebig, M.D., Director
University of California, San Francisco Department of Laboratory Medicine San Francisco General Hospital 1001 Potrero Avenue, San Francisco CA 94110 Clinical Laboratory Eberhard Fiebig, M.D., Director
Oregon Birth Outcomes, by Planned Birth Place and Attendant Pursuant to: HB 2380 (2011)
 Oregon Birth Outcomes, by Birth Place and Attendant Pursuant to: HB 2380 (2011) In 2011, the Oregon Legislature passed House Bill 2380, which required the Oregon Public Health Division to add two questions
Oregon Birth Outcomes, by Birth Place and Attendant Pursuant to: HB 2380 (2011) In 2011, the Oregon Legislature passed House Bill 2380, which required the Oregon Public Health Division to add two questions
Changes in the 2010 STD Treatment Guidelines: What Adolescent Health Care Providers Should Know February 2011
 Changes in the 2010 STD Treatment Guidelines: What Adolescent Health Care Providers Should Know February 2011 Gale Burstein, MD, MPH, FAAP, FSAHM 1 Amanda Jacobs, MD, FAAP, 2 Dmitry Kissin, MD, MPH, 3
Changes in the 2010 STD Treatment Guidelines: What Adolescent Health Care Providers Should Know February 2011 Gale Burstein, MD, MPH, FAAP, FSAHM 1 Amanda Jacobs, MD, FAAP, 2 Dmitry Kissin, MD, MPH, 3
Slide 1: Chlamydia and Gonorrhea: What You and Your Clients Need to Know. Welcome to Chlamydia and Gonorrhea: What You and Your Clients Need to Know.
 Slide 1: Chlamydia and Gonorrhea: What You and Your Clients Need to Know Welcome to Chlamydia and Gonorrhea: What You and Your Clients Need to Know. This is a presentation for healthcare providers about
Slide 1: Chlamydia and Gonorrhea: What You and Your Clients Need to Know Welcome to Chlamydia and Gonorrhea: What You and Your Clients Need to Know. This is a presentation for healthcare providers about
General and Objectives Clinical Skills for. Nursing Students in Maternity and Gynecology. Nursing Department
 General and Objectives Clinical Skills for Nursing Students in Maternity and Gynecology Nursing Department Objectives and clinical skills of Antenatal unit Provide antenatal care to woman during normal
General and Objectives Clinical Skills for Nursing Students in Maternity and Gynecology Nursing Department Objectives and clinical skills of Antenatal unit Provide antenatal care to woman during normal
GLOBAL CONCERNS ABOUT HPV VACCINES FACT SHEET
 GLOBAL CONCERNS ABOUT HPV VACCINES FACT SHEET When detected, HPV infection is easily managed and rarely proceeds to cancer Very few women with HPV develop cervical cancer HPV infections are only one of
GLOBAL CONCERNS ABOUT HPV VACCINES FACT SHEET When detected, HPV infection is easily managed and rarely proceeds to cancer Very few women with HPV develop cervical cancer HPV infections are only one of
Denver County Births and Deaths 2013
 Denver County Births and Deaths 2013 Selected birth characteristics: County residents, 2013... 2 Selected birth characteristics by age group of mother: County residents, 2013... 3 Selected birth characteristics
Denver County Births and Deaths 2013 Selected birth characteristics: County residents, 2013... 2 Selected birth characteristics by age group of mother: County residents, 2013... 3 Selected birth characteristics
TEENAGE PREGNANCY. Arizona,2000-2010. Public Health Services Bureau of Public Health Statistics Health Status and Vital Statistics Section
 TEENAGE PREGNANCY Arizona,2000-2010 Public Health Services Bureau of Public Health Statistics Health Status and Vital Statistics Section ~ Leadership for a Healthy Arizona ~ Janice K. Brewer, Governor
TEENAGE PREGNANCY Arizona,2000-2010 Public Health Services Bureau of Public Health Statistics Health Status and Vital Statistics Section ~ Leadership for a Healthy Arizona ~ Janice K. Brewer, Governor
Yes, I know I have genital herpes:
 Counseling Messages for Herpes Simplex Type II (HSV-II) Genital herpes Always take the time to attend to the participant s feelings and emotional state; for some people, this is the most devastating news
Counseling Messages for Herpes Simplex Type II (HSV-II) Genital herpes Always take the time to attend to the participant s feelings and emotional state; for some people, this is the most devastating news
Assisted Reproductive Technologies at IGO
 9339 Genesee Avenue, Suite 220 San Diego, CA 92121 858 455 7520 Assisted Reproductive Technologies at IGO Although IGO no longer operates an IVF laboratory or program as such, we work closely with area
9339 Genesee Avenue, Suite 220 San Diego, CA 92121 858 455 7520 Assisted Reproductive Technologies at IGO Although IGO no longer operates an IVF laboratory or program as such, we work closely with area
Pregnant and Parenting Youth in Foster Care in Washington State: Comparison to Other Teens and Young Women who Gave Birth
 January 2014 RDA Report 11.202 Olympia, Washington Pregnant and Parenting in Care in Washington State: Comparison to Other and Women who Gave Birth Laurie Cawthon, MD, MPH Barbara Lucenko, PhD Peter Woodcox,
January 2014 RDA Report 11.202 Olympia, Washington Pregnant and Parenting in Care in Washington State: Comparison to Other and Women who Gave Birth Laurie Cawthon, MD, MPH Barbara Lucenko, PhD Peter Woodcox,
PERINATAL NUTRITION. Nutrition during pregnancy and lactation. Nutrition during infancy.
 PERINATAL NUTRITION Nutrition during pregnancy and lactation Nutrition during infancy. Rama Bhat, MD. Department of Pediatrics, University of Illinois Hospital Chicago, Illinois. Nutrition During Pregnancy
PERINATAL NUTRITION Nutrition during pregnancy and lactation Nutrition during infancy. Rama Bhat, MD. Department of Pediatrics, University of Illinois Hospital Chicago, Illinois. Nutrition During Pregnancy
Leader's Resource. Note: Both men and women can have an STD without physical symptoms.
 Leader's Resource Information on Sexually Transmitted Diseases (STDs) Signs and Symptoms of STDs Note: Both men and women can have an STD without physical symptoms. Any of the following can indicate to
Leader's Resource Information on Sexually Transmitted Diseases (STDs) Signs and Symptoms of STDs Note: Both men and women can have an STD without physical symptoms. Any of the following can indicate to
Urinary Tract Infections
 Urinary Tract Infections Overview A urine culture must ALWAYS be interpreted in the context of the urinalysis and patient symptoms. If a patient has no signs of infection on urinalysis, no symptoms of
Urinary Tract Infections Overview A urine culture must ALWAYS be interpreted in the context of the urinalysis and patient symptoms. If a patient has no signs of infection on urinalysis, no symptoms of
USPSTF Grade A B Recommendations
 USPSTF Grade Recommendations bdominal aortic aneurysm screening: men The USPSTF recommends one-time screening for abdominal aortic aneurysm by ultrasonography in men aged 65 to 75 who have ever smoked.
USPSTF Grade Recommendations bdominal aortic aneurysm screening: men The USPSTF recommends one-time screening for abdominal aortic aneurysm by ultrasonography in men aged 65 to 75 who have ever smoked.
Unplanned teenage pregnancy prevention. Introduction
 1 Unplanned teenage pregnancy prevention Introduction Unplanned Teenage pregnancy is becoming a serious problem in many countries, including Thailand. It creates many related impacts such as health, economic,
1 Unplanned teenage pregnancy prevention Introduction Unplanned Teenage pregnancy is becoming a serious problem in many countries, including Thailand. It creates many related impacts such as health, economic,
Laboratory confirmation requires isolation of Bordetella pertussis or detection of B. pertussis nucleic acid, preferably from a nasopharyngeal swab.
 Pertussis Epidemiology in New Zealand New Zealand has continued to experience outbreaks of pertussis in recent decades. This is in part due to historically low immunisation rates and in part because immunity
Pertussis Epidemiology in New Zealand New Zealand has continued to experience outbreaks of pertussis in recent decades. This is in part due to historically low immunisation rates and in part because immunity
The Clinical Content of Preconception Care: Alcohol, Tobacco, and Illicit Drug Exposures
 The Clinical Content of Preconception Care: Alcohol, Tobacco, and Illicit Drug Exposures by R. Louise Floyd, DSN, RN; Brian W. Jack, MD; Robert Cefalo, MD, PhD; Hani Atrash, MD, MPH; Jeanne Mahoney, BSN,
The Clinical Content of Preconception Care: Alcohol, Tobacco, and Illicit Drug Exposures by R. Louise Floyd, DSN, RN; Brian W. Jack, MD; Robert Cefalo, MD, PhD; Hani Atrash, MD, MPH; Jeanne Mahoney, BSN,
HIV (Human Immunodeficiency Virus) Screening and Pre-Exposure Prophylaxis Guideline
 HIV (Human Immunodeficiency Virus) Screening and Pre-Exposure Prophylaxis Guideline Background... 2 Screening... 2 Recommendations... 2 Ordering and consent... 2 Indications for Periodic HIV Screening...
HIV (Human Immunodeficiency Virus) Screening and Pre-Exposure Prophylaxis Guideline Background... 2 Screening... 2 Recommendations... 2 Ordering and consent... 2 Indications for Periodic HIV Screening...
Tdap booster vaccine for Health Care Workers Frequently Asked Questions for Health Professionals
 Tdap booster vaccine for Health Care Workers Frequently Asked Questions for Health Professionals NEW items in 2013 Immunisation Guidelines for Ireland are in RED What is Tdap booster vaccine? Tdap is a
Tdap booster vaccine for Health Care Workers Frequently Asked Questions for Health Professionals NEW items in 2013 Immunisation Guidelines for Ireland are in RED What is Tdap booster vaccine? Tdap is a
Improved Health and Lower Medical Costs: Why good dental care is important
 Improved Health and Lower Medical Costs: Why good dental care is important A white paper 840121 12/10 Research continues to associate oral health with overall health. Gum disease may have a potentially
Improved Health and Lower Medical Costs: Why good dental care is important A white paper 840121 12/10 Research continues to associate oral health with overall health. Gum disease may have a potentially
A Review of Genital Chlamydial Infections
 Clinical Review Article A Review of Genital Chlamydial Infections Daniel M. Breitkopf, MD Chlamydiae are obligate intracellular parasites containing both DNA and RNA. 1 Three species of the genus Chlamydia
Clinical Review Article A Review of Genital Chlamydial Infections Daniel M. Breitkopf, MD Chlamydiae are obligate intracellular parasites containing both DNA and RNA. 1 Three species of the genus Chlamydia
Chlamydial Abortions in Sheep and Goats
 Chlamydial Abortions in Sheep and Goats History Reported for the first time in 1959 in Germany, then diagnosis of the disease occurred in Bulgaria, Spain, USA, France, India, Japan, United Kingdom, Chad,
Chlamydial Abortions in Sheep and Goats History Reported for the first time in 1959 in Germany, then diagnosis of the disease occurred in Bulgaria, Spain, USA, France, India, Japan, United Kingdom, Chad,
The Basics of Drug Resistance:
 CONTACT: Lisa Rossi +1-412-641-8940 +1-412- 916-3315 (mobile) rossil@upmc.edu The Basics of Drug Resistance: QUESTIONS AND ANSWERS HIV Drug Resistance and ARV-Based Prevention 1. What is drug resistance?
CONTACT: Lisa Rossi +1-412-641-8940 +1-412- 916-3315 (mobile) rossil@upmc.edu The Basics of Drug Resistance: QUESTIONS AND ANSWERS HIV Drug Resistance and ARV-Based Prevention 1. What is drug resistance?
Women Who Quit Smoking During Pregnancy
 OKLAHOMA PREGNANCY RISK ASSESSMENT MONITORING SYSTEM VOL 7 NO2 Women Who Quit Smoking During Pregnancy Background Quitting smoking may be one of the most important steps pregnant women can take toward
OKLAHOMA PREGNANCY RISK ASSESSMENT MONITORING SYSTEM VOL 7 NO2 Women Who Quit Smoking During Pregnancy Background Quitting smoking may be one of the most important steps pregnant women can take toward
Wendy Martinez, MPH, CPH County of San Diego, Maternal, Child & Adolescent Health
 Wendy Martinez, MPH, CPH County of San Diego, Maternal, Child & Adolescent Health Describe local trends in birth Identify 3 perinatal health problems Identify 3 leading causes of infant death Age Class
Wendy Martinez, MPH, CPH County of San Diego, Maternal, Child & Adolescent Health Describe local trends in birth Identify 3 perinatal health problems Identify 3 leading causes of infant death Age Class
TEENAGE PREGNANCY. Arizona,1999-2009. Public Health Services Bureau of Public Health Statistics Health Status and Vital Statistics Section
 TEENAGE PREGNANCY Arizona,1999-2009 Public Health Services Bureau of Public Health Statistics Health Status and Vital Statistics Section ~ Leadership for a Healthy Arizona ~ Janice K. Brewer, Governor
TEENAGE PREGNANCY Arizona,1999-2009 Public Health Services Bureau of Public Health Statistics Health Status and Vital Statistics Section ~ Leadership for a Healthy Arizona ~ Janice K. Brewer, Governor
Trichomonas vaginalis. Looking after your sexual health
 Trichomonas vaginalis Looking after your sexual health 2 3 Trichomonas vaginalis Trichomonas vaginalis is a sexually transmitted infection (STI). It is sometimes referred to as trichomonas or trichomoniasis,
Trichomonas vaginalis Looking after your sexual health 2 3 Trichomonas vaginalis Trichomonas vaginalis is a sexually transmitted infection (STI). It is sometimes referred to as trichomonas or trichomoniasis,
Facts about Diabetes in Massachusetts
 Facts about Diabetes in Massachusetts Diabetes is a disease in which the body does not produce or properly use insulin (a hormone used to convert sugar, starches, and other food into the energy needed
Facts about Diabetes in Massachusetts Diabetes is a disease in which the body does not produce or properly use insulin (a hormone used to convert sugar, starches, and other food into the energy needed
49. INFANT MORTALITY RATE. Infant mortality rate is defined as the death of an infant before his or her first birthday.
 49. INFANT MORTALITY RATE Wing Tam (Alice) Jennifer Cheng Stat 157 course project More Risk in Everyday Life Risk Meter LIKELIHOOD of exposure to hazardous levels Low Medium High Consequences: Severity,
49. INFANT MORTALITY RATE Wing Tam (Alice) Jennifer Cheng Stat 157 course project More Risk in Everyday Life Risk Meter LIKELIHOOD of exposure to hazardous levels Low Medium High Consequences: Severity,
Home Births in the United States, 1990 2009
 Home Births in the United States, 1990 2009 Marian F. MacDorman, Ph.D.; T.J. Mathews, M.S.; and Eugene Declercq, Ph.D. Key findings After a decline from 1990 to 2004, the percentage of U.S. births that
Home Births in the United States, 1990 2009 Marian F. MacDorman, Ph.D.; T.J. Mathews, M.S.; and Eugene Declercq, Ph.D. Key findings After a decline from 1990 to 2004, the percentage of U.S. births that
Clinical Aspects of Diagnosis of Gonorrhea and Chlamydia Infection in an Acute Care Setting
 BRIEF REPORT Clinical Aspects of Diagnosis of Gonorrhea and Chlamydia Infection in an Acute Care Setting Supriya D. Mehta, 1 Richard E. Rothman, 2 Gabor D. Kelen, 2 Thomas C. Quinn, 3,4 and Jonathan M.
BRIEF REPORT Clinical Aspects of Diagnosis of Gonorrhea and Chlamydia Infection in an Acute Care Setting Supriya D. Mehta, 1 Richard E. Rothman, 2 Gabor D. Kelen, 2 Thomas C. Quinn, 3,4 and Jonathan M.
Chlamydia. Looking after your sexual health
 Chlamydia Looking after your sexual health 2 Chlamydia Chlamydia is one of the most common sexually transmitted infections (STIs). It is very easy to treat and cure. Up to one in 10 sexually active young
Chlamydia Looking after your sexual health 2 Chlamydia Chlamydia is one of the most common sexually transmitted infections (STIs). It is very easy to treat and cure. Up to one in 10 sexually active young
SCREENING FOR SEXUALLY TRANSMITTED INFECTIONS
 SCREENING FOR SEXUALLY TRANSMITTED INFECTIONS Take history:- History of presenting problem Full sexual history (refer to guideline on sexual history taking) Relevant past medical history, including previous
SCREENING FOR SEXUALLY TRANSMITTED INFECTIONS Take history:- History of presenting problem Full sexual history (refer to guideline on sexual history taking) Relevant past medical history, including previous
SYNDROMIC CASE MANAGEMENT OF RTIs Advantages, Limitations, Optimization
 WHO COLLABORATING CENTRE FOR RESEARCH IN HUMAN REPRODUCTION Hôpitaux Universitaires de Genève SYNDROMIC CASE MANAGEMENT OF RTIs Advantages, Limitations, Optimization Dr SALONEY NAZEER Department of Gynaecology
WHO COLLABORATING CENTRE FOR RESEARCH IN HUMAN REPRODUCTION Hôpitaux Universitaires de Genève SYNDROMIC CASE MANAGEMENT OF RTIs Advantages, Limitations, Optimization Dr SALONEY NAZEER Department of Gynaecology
National Chlamydia Screening Programme September 2012 PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF AZITHROMYCIN FOR CHLAMYDIA TRACHOMATIS
 PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF AZITHROMYCIN FOR CHLAMYDIA TRACHOMATIS Below is a template that can be used to produce a local patient group direction (PGD) for the administration of
PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF AZITHROMYCIN FOR CHLAMYDIA TRACHOMATIS Below is a template that can be used to produce a local patient group direction (PGD) for the administration of
GLOBAL OVERVIEW OF ANTIMICROBIAL RESISTANCE STD AGENTS
 GLOBAL OVERVIEW OF ANTIMICROBIAL RESISTANCE STD AGENTS IN Dr.P.Bhalla Director Professor and Head Dept. of Microbiology Maulana Azad Medical College New Delhi, INDIA STDs & RTIs l HIV l Gonococcal infections/
GLOBAL OVERVIEW OF ANTIMICROBIAL RESISTANCE STD AGENTS IN Dr.P.Bhalla Director Professor and Head Dept. of Microbiology Maulana Azad Medical College New Delhi, INDIA STDs & RTIs l HIV l Gonococcal infections/
Rural Health Advisory Committee s Rural Obstetric Services Work Group
 Rural Health Advisory Committee s Rural Obstetric Services Work Group March 15 th webinar topic: Rural Obstetric Patient and Community Issues Audio: 888-742-5095, conference code 6054760826 Rural Obstetric
Rural Health Advisory Committee s Rural Obstetric Services Work Group March 15 th webinar topic: Rural Obstetric Patient and Community Issues Audio: 888-742-5095, conference code 6054760826 Rural Obstetric
Meena Abraham, DrPH, MPH Director of Epidemiology Services Baltimore City Health Department
 Meena Abraham, DrPH, MPH Director of Epidemiology Services Baltimore City Health Department 271 Neighborhood Statistical Areas 55 Community Statistical Areas 26 Zip Codes Characteristic Baltimore City
Meena Abraham, DrPH, MPH Director of Epidemiology Services Baltimore City Health Department 271 Neighborhood Statistical Areas 55 Community Statistical Areas 26 Zip Codes Characteristic Baltimore City
Appendices. 2006 Bexar County Community Health Assessment Appendices Appendix A 125
 Appendices Appendix A Recent reports suggest that the number of mothers seeking dropped precipitously between 2004 and 2005. Tables 1A and 1B, below, shows information since 1990. The trend has been that
Appendices Appendix A Recent reports suggest that the number of mothers seeking dropped precipitously between 2004 and 2005. Tables 1A and 1B, below, shows information since 1990. The trend has been that
Medical criteria for IUCD s Based on the WHO MEC (2004- Annexure 3) system a woman s eligibility for IUCD insertion falls in 4 categories. These categ
 CLIENT ASSESSMENT Ensure that the woman is not pregnant Determine the length and direction of uterus. Ensure that she does not have gonorrhea and chlamydia, and is not a high risk case of STI s Identify
CLIENT ASSESSMENT Ensure that the woman is not pregnant Determine the length and direction of uterus. Ensure that she does not have gonorrhea and chlamydia, and is not a high risk case of STI s Identify
Lyme Disease in Pregnancy. Dr Sarah Chissell Consultant Obstetrician William Harvey Hospital, Kent
 Lyme Disease in Pregnancy Dr Sarah Chissell Consultant Obstetrician William Harvey Hospital, Kent Conflict of interest My son has chronic Lyme disease Infections in pregnancy Transplacental infection Perinatal
Lyme Disease in Pregnancy Dr Sarah Chissell Consultant Obstetrician William Harvey Hospital, Kent Conflict of interest My son has chronic Lyme disease Infections in pregnancy Transplacental infection Perinatal
TITLE V OF THE SOCIAL SECURITY ACT MATERNAL AND CHILD HEALTH INFANT MORTALITY EFFORTS
 TITLE V OF THE SOCIAL SECURITY ACT MATERNAL AND CHILD HEALTH INFANT MORTALITY EFFORTS Michele H. Lawler, M.S., R.D. Department of Health and Human Services Health Resources and Services Administration
TITLE V OF THE SOCIAL SECURITY ACT MATERNAL AND CHILD HEALTH INFANT MORTALITY EFFORTS Michele H. Lawler, M.S., R.D. Department of Health and Human Services Health Resources and Services Administration
A School-based Chlamydia Control Program Using DNA Amplification Technology
 A School-based Chlamydia Control Program Using DNA Amplification Technology Deborah A. Cohen, MD, MPH* ; Malanda Nsuami, MD, MPH*; Roger Bedimo Etame, MD, MSc*; Susanne Tropez-Sims, MD* ; Sue Abdalian,
A School-based Chlamydia Control Program Using DNA Amplification Technology Deborah A. Cohen, MD, MPH* ; Malanda Nsuami, MD, MPH*; Roger Bedimo Etame, MD, MSc*; Susanne Tropez-Sims, MD* ; Sue Abdalian,
California Gonorrhea Treatment Guidelines
 Califnia Gonrhea Treatment Guidelines These guidelines were developed by the Califnia Department of Public Health (CDPH) Sexually Transmitted Diseases (STD) Control Branch in conjunction with the Califnia
Califnia Gonrhea Treatment Guidelines These guidelines were developed by the Califnia Department of Public Health (CDPH) Sexually Transmitted Diseases (STD) Control Branch in conjunction with the Califnia
A Practical Guide to Diagnosis and Treatment of Infection in the Outpatient Setting Diagnosis and Treatment of Urinary Tract Infections
 A Practical Guide to Diagnosis and Treatment of Infection in the Outpatient Setting Diagnosis and Treatment of Urinary Tract Infections By Gary R. Skankey, MD, FACP, Infectious Disease, Las Vegas, NV Sponsored
A Practical Guide to Diagnosis and Treatment of Infection in the Outpatient Setting Diagnosis and Treatment of Urinary Tract Infections By Gary R. Skankey, MD, FACP, Infectious Disease, Las Vegas, NV Sponsored
UMBILICAL CORD BLOOD COLLECTION
 UMBILICAL CORD BLOOD COLLECTION by Frances Verter, PhD Founder & Director, Parent's Guide to Cord Blood Foundation info@parentsguidecordblood.org and Kim Petrella, RN Department of Obstetrics and Gynecology
UMBILICAL CORD BLOOD COLLECTION by Frances Verter, PhD Founder & Director, Parent's Guide to Cord Blood Foundation info@parentsguidecordblood.org and Kim Petrella, RN Department of Obstetrics and Gynecology
Zika Virus. Fred A. Lopez, MD, MACP Richard Vial Professor Department of Medicine Section of Infectious Diseases
 Zika Virus Fred A. Lopez, MD, MACP Richard Vial Professor Department of Medicine Section of Infectious Diseases What is the incubation period for Zika virus infection? Unknown but likely to be several
Zika Virus Fred A. Lopez, MD, MACP Richard Vial Professor Department of Medicine Section of Infectious Diseases What is the incubation period for Zika virus infection? Unknown but likely to be several
PROPERTY OF ELSEVIER SAMPLE CONTENT - NOT FINAL ABNORMAL PAP SMEAR (ABNORMAL CERVICAL CYTOLOGIC FINDINGS) Kathleen Dor
 1 ABNORMAL PAP SMEAR (ABNORMAL CERVICAL CYTOLOGIC FINDINGS) Kathleen Dor Cervical cytology screening has significantly decreased rates of mortality from cervical cancer; however, 400 women die each year
1 ABNORMAL PAP SMEAR (ABNORMAL CERVICAL CYTOLOGIC FINDINGS) Kathleen Dor Cervical cytology screening has significantly decreased rates of mortality from cervical cancer; however, 400 women die each year
THE KIDNEY. Bulb of penis Abdominal aorta Scrotum Adrenal gland Inferior vena cava Urethra Corona glandis. Kidney. Glans penis Testicular vein
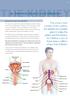 29 THE KIDNEY 9. Recurrent urinary tract infections Recurrent urinary tract infections The urinary tract consists of the urethra, the bladder, the ureters, the kidneys and in men the prostate gland. An
29 THE KIDNEY 9. Recurrent urinary tract infections Recurrent urinary tract infections The urinary tract consists of the urethra, the bladder, the ureters, the kidneys and in men the prostate gland. An
Professional Liability Insurance Changes in Practice as a Result of the Affordability or Availability of Professional Liability Insurance
 Overview of the 2015 ACOG Survey on Professional Liability By Andrea M. Carpentieri, MA, James J. Lumalcuri, MSW, Jennie Shaw, MPH, and Gerald F. Joseph, Jr., MD, FACOG The 2015 Survey on Professional
Overview of the 2015 ACOG Survey on Professional Liability By Andrea M. Carpentieri, MA, James J. Lumalcuri, MSW, Jennie Shaw, MPH, and Gerald F. Joseph, Jr., MD, FACOG The 2015 Survey on Professional
AUSTRALIA AND NEW ZEALAND FACTSHEET
 AUSTRALIA AND NEW ZEALAND FACTSHEET What is Stillbirth? In Australia and New Zealand, stillbirth is the death of a baby before or during birth, from the 20 th week of pregnancy onwards, or 400 grams birthweight.
AUSTRALIA AND NEW ZEALAND FACTSHEET What is Stillbirth? In Australia and New Zealand, stillbirth is the death of a baby before or during birth, from the 20 th week of pregnancy onwards, or 400 grams birthweight.
Impact of Diabetes on Treatment Outcomes among Maryland Tuberculosis Cases, 2004-2005. Tania Tang PHASE Symposium May 12, 2007
 Impact of Diabetes on Treatment Outcomes among Maryland Tuberculosis Cases, 2004-2005 Tania Tang PHASE Symposium May 12, 2007 Presentation Outline Background Research Questions Methods Results Discussion
Impact of Diabetes on Treatment Outcomes among Maryland Tuberculosis Cases, 2004-2005 Tania Tang PHASE Symposium May 12, 2007 Presentation Outline Background Research Questions Methods Results Discussion
Cocooning Strategies
 Cocooning Strategies Preventing Pertussis Infection in Infants Elizabeth Rosenblum, MD Department of Family & Preventive Medicine UCSD Medical Center COCOON noun \kə-kün\ 1 a : an envelope often largely
Cocooning Strategies Preventing Pertussis Infection in Infants Elizabeth Rosenblum, MD Department of Family & Preventive Medicine UCSD Medical Center COCOON noun \kə-kün\ 1 a : an envelope often largely
September 17, 2010. Dear Secretary Sebelius:
 September 17, 2010 Secretary Kathleen Sebelius Department of Health and Human Services Hubert H. Humphrey Building 200 Independence Avenue, SW Washington, DC 20201 RE: Comments on OCIIO- 9992- IFC, Interim
September 17, 2010 Secretary Kathleen Sebelius Department of Health and Human Services Hubert H. Humphrey Building 200 Independence Avenue, SW Washington, DC 20201 RE: Comments on OCIIO- 9992- IFC, Interim
Glossary. amenorrhea, primary - from the beginning and lifelong; menstruation never begins at puberty.
 Glossary amenorrhea - absence or cessation of menstrual periods. amenorrhea, primary - from the beginning and lifelong; menstruation never begins at puberty. A amenorrhea, secondary - due to some physical
Glossary amenorrhea - absence or cessation of menstrual periods. amenorrhea, primary - from the beginning and lifelong; menstruation never begins at puberty. A amenorrhea, secondary - due to some physical
Aetna Life Insurance Company
 Aetna Life Insurance Company Hartford, Connecticut 06156 Amendment Policyholder: Group Policy No.: Effective Date: UNIVERSITY OF PENNSYLVANIA POSTDOCTORAL INSURANCE PLAN GP-861472 This Amendment is effective
Aetna Life Insurance Company Hartford, Connecticut 06156 Amendment Policyholder: Group Policy No.: Effective Date: UNIVERSITY OF PENNSYLVANIA POSTDOCTORAL INSURANCE PLAN GP-861472 This Amendment is effective
Quality of Birth Certificate Data. Daniela Nitcheva, PhD Division of Biostatistics PHSIS
 Quality of Birth Certificate Data Daniela Nitcheva, PhD Division of Biostatistics PHSIS Data Quality SC State Law requires that you file the birth certificate within 5 days of a child s birth. Data needs
Quality of Birth Certificate Data Daniela Nitcheva, PhD Division of Biostatistics PHSIS Data Quality SC State Law requires that you file the birth certificate within 5 days of a child s birth. Data needs
Gonorrhoea. Looking after your sexual health
 Gonorrhoea Looking after your sexual health 2 Gonorrhoea Gonorrhoea is a bacterial sexually transmitted infection (STI). It can be painful and can cause serious health problems such as infertility in both
Gonorrhoea Looking after your sexual health 2 Gonorrhoea Gonorrhoea is a bacterial sexually transmitted infection (STI). It can be painful and can cause serious health problems such as infertility in both
Clostridium Difficile Colitis. Presented by Mark Skains August 2003
 Clostridium Difficile Colitis Presented by Mark Skains August 2003 What is Clostridium Difficile Gram positive rod Produces spores (hang out in diverticula) Forms Endotoxin A + B which cause diarrhea.
Clostridium Difficile Colitis Presented by Mark Skains August 2003 What is Clostridium Difficile Gram positive rod Produces spores (hang out in diverticula) Forms Endotoxin A + B which cause diarrhea.
4/30/2013 HPV VACCINE AND NORTH DAKOTA HPV IMMUNIZATION RATES HUMAN PAPILLOMAVIRUS (HPV) HUMAN PAPILLOMAVIRUS HPV CONTINUED
 HPV VACCINE AND NORTH DAKOTA HPV IMMUNIZATION RATES HUMAN PAPILLOMAVIRUS (HPV) HUMAN PAPILLOMAVIRUS What is human papillomavirus (HPV)? HPV is the most common sexually transmitted infection. There are
HPV VACCINE AND NORTH DAKOTA HPV IMMUNIZATION RATES HUMAN PAPILLOMAVIRUS (HPV) HUMAN PAPILLOMAVIRUS What is human papillomavirus (HPV)? HPV is the most common sexually transmitted infection. There are
HOSPITAL OF THE UNIVERSITY OF PENNSYLVANIA CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5322, Version 2.0, 01/28/15 Long-term Follow-up of Older HIV-infected Adults in the ACTG: Addressing
CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5322, Version 2.0, 01/28/15 Long-term Follow-up of Older HIV-infected Adults in the ACTG: Addressing
BUTTE COUNTY PUBLIC HEALTH DEPARTMENT POLICY & PROCEDURE
 BUTTE COUNTY PUBLIC HEALTH DEPARTMENT POLICY & PROCEDURE SUBJECT: Pregnancy Testing and Counseling Protocol P&P # APPROVED BY: EFFECTIVE DATE: Mark Lundberg MD Health Officer REVISION DATE: 2/20/2010 Phyllis
BUTTE COUNTY PUBLIC HEALTH DEPARTMENT POLICY & PROCEDURE SUBJECT: Pregnancy Testing and Counseling Protocol P&P # APPROVED BY: EFFECTIVE DATE: Mark Lundberg MD Health Officer REVISION DATE: 2/20/2010 Phyllis
Crohn's disease and pregnancy.
 Gut, 1984, 25, 52-56 Crohn's disease and pregnancy. R KHOSLA, C P WILLOUGHBY, AND D P JEWELL From the Gastroenterology Unit, Radcliffe Infirmary, Oxford SUMMARY Infertility and the outcome of pregnancy
Gut, 1984, 25, 52-56 Crohn's disease and pregnancy. R KHOSLA, C P WILLOUGHBY, AND D P JEWELL From the Gastroenterology Unit, Radcliffe Infirmary, Oxford SUMMARY Infertility and the outcome of pregnancy
Chlamydial cervicitis and urethritis: single dose treatment compared with doxycycline for seven
 Genitourin Med 1996;72:93-97 Chlamydial cervicitis and urethritis: single dose treatment compared with doxycycline for seven days in community based practises 93 E M Thorpe Jr, W E Stamm, E W Hook III,
Genitourin Med 1996;72:93-97 Chlamydial cervicitis and urethritis: single dose treatment compared with doxycycline for seven days in community based practises 93 E M Thorpe Jr, W E Stamm, E W Hook III,
