Carboplatin in the Treatment of Small Cell Lung Cancer
|
|
|
- Kerry Pope
- 8 years ago
- Views:
Transcription
1 Carboplatin in the Treatment of Small Cell Lung Cancer JULIE R. BRAHMER, DAVID S. ETTINGER The Johns Hopkins Oncology Center Baltimore, Maryland, USA Key Words. Carboplatin Small cell lung cancer Extensive disease Limited disease ABSTRACT Small cell lung cancer (SCLC) will account for approximately 20%-25% of the 171,500 estimated new lung cancer cases in Combination cytotoxic therapies have yielded the best response rates in SCLC patients. Cisplatin in combination with etoposide is used routinely in the treatment of SCLC. Because of cisplatin s nonhematologic toxicities, carboplatin was developed and has far fewer nonhematologic toxicities. Carboplatin in combination with etoposide has been shown to be as effective as, but less toxic than, cisplatin/etoposide. Moreover, carboplatin/etoposide is a viable combination in the treatment of the elderly with SCLC, who can readily tolerate this combination. Concurrent radiation therapy with carboplatin and etoposide in patients with limited SCLC disease can be given safely and effectively. Carboplatin has been combined with several different chemotherapeutic agents, including ifosfamide, and, more recently, paclitaxel in hopes of improving the response rates and overall survival. In order to try to dose intensify carboplatin-based regimens, peripheral blood stem cells have been used to decrease the hematologic toxicities. Further studies are warranted to investigate these therapies as well as newer carboplatin combinations. The Oncologist 1998;3: INTRODUCTION Lung cancer is the leading cause of death due to cancer in the United States [1]. Of new lung cancer cases, small cell lung cancer (SCLC) accounts for approximately 20%-25% of them [2]. Most cases of SCLC present with extensive disease due to the aggressive nature of SCLC. Extensive-stage disease (ED) and limited-stage disease (LD) are the two stages of SCLC that were identified by the Veterans Administration Lung Group (VALG). The VALG classifies LD as tumor growth limited to one hemithorax and its regional lymph nodes, while ED is tumor spread beyond these boundaries [2]. Combination cytotoxic therapies given at frequent intervals have yielded the best response rates in SCLC patients [2]. Although SCLC responds well to chemotherapy, the majority of patients will relapse and die within two years of diagnosis [3]. These poor survival rates are a result of the advanced stage of the disease at diagnosis, high recurrence rates associated with local therapy, and the inability of combination chemotherapy to prolong survival significantly [4]. Cisplatin in combination with etoposide is used routinely in the treatment of SCLC. The toxicity of cisplatin, including nausea, vomiting, nephrotoxicity, neurotoxicity, and ototoxicity, has led to the development and investigation of combination regimens with different toxicity profiles [5]. Carboplatin, a derivative of cisplatin, has far less nonhematologic toxicity, although myelosuppression may be slightly greater than that observed with cisplatin [5, 6]. The reduced toxicity and equivalent efficacy of carboplatin has resulted in increased use of carboplatin-based modalities for the treatment of SCLC [5]. Carboplatin, an analog of cisplatin, has similar activity in lung cancer but exhibits a more favorable toxicity profile and is easier to administer than cisplatin [5, 6]. Nonhematologic toxicities are less common in patients who receive carboplatin in comparison with those who receive cisplatin. The dose-limiting toxicity for carboplatin is myelosuppression, particularly thrombocytopenia [7]. ISSUES WITH CARBOPLATIN DOSING Approximately 70% of an administered dose of carboplatin is excreted in the urine. Renal clearance of carboplatin is closely correlated with glomerular filtration rate (GFR) [7]. Therefore, calculation of the carboplatin dose should consider patients renal function. The clear relationship between dose and hematologic toxicity for carboplatin justifies the need to pharmacokinetically determine the Correspondence: David S. Ettinger, M.D., F.A.C.P., Johns Hopkins Oncology Center, 600 North Wolfe Street, Baltimore, Maryland , USA. Telephone: ; Fax: ; ettinger@welchlink.welch.jhu.edu Accepted for publication April 23, AlphaMed Press /98/$5.00/0 The Oncologist 1998;3:
2 144 Carboplatin in the Treatment of Small Cell Lung Cancer dose of carboplatin to both minimize toxicity and increase therapeutic efficacy. The ability to predict the area under the concentration versus time curve (AUC) following administration of carboplatin will allow consistent drug exposure for patients with either normal or impaired renal function. Calvert and colleagues have validated the use of the following formula to calculate carboplatin dosage in adults based on GFR [7] measured as 51Cr EDTA clearance: Total dose (mg) = (target AUC) (GFR + 25) Note: With the Calvert formula, the total dose of carboplatin is calculated in mg, not mg/m 2. The AUC of carboplatin appears to relate more closely to the therapeutic and toxic effects of the drug than does dose calculated on the basis of body surface area. Therefore, the use of AUC dosing avoids subtherapeutic doses and minimizes overdosage. Additionally, with the use of AUC rather than toxicity as the measure of carboplatin exposure, previous or concurrent myelosuppressive therapy will not have a significant influence [7]. Studies that were conducted prior to the widespread use of the Calvert formula do not provide standardized doses of carboplatin to patients of varying ages, performance status, and renal function. The importance of standardizing carboplatin dose based on renal function cannot be overlooked in future trials. CARBOPLATIN/ETOPOSIDE REGIMENS A number of studies have been conducted to evaluate the combination of carboplatin and etoposide in the treatment of SCLC in previously untreated patients (Table 1) [8-15]. Overall response rates range from 73% to 93% of patients with LD SCLC and 50% to 85% of those with ED. Median duration of survival ranged from 11 to 15 months for LD and from 4.6 to 12 months for ED. Table 1. Carboplatin/etoposide (CE) regimens in the treatment of SCLC in previously untreated patients Overall Complete MTS Patients response (%) response (%) (mo) Study LD ED Dose (mg/m 2 ) LD ED LD ED LD ED Hellenic E 100 i.v. d b Cooperative C 300 i.v. d1 Oncology Group a [9] E 100 i.v. d b P 50 i.v. d1-2 CALGB, Luikart et al., 48 E 200 i.v. d c [10] C 125 i.v. d1-3 Viren et al., 1994 [11] 56 E 100 i.v. d C 450 i.v. d1 Pfeiffer et al., 1995 d [12] E 240 p.o. d C 300 i.v. d1 Ellis et al., 1995 a [13] E 120 i.v. d1-3 e C 600 i.v. d1 e Katakami et al., E 100 i.v. d a,f [14] C 400 i.v. d1 G-CSF d4-17 CALGB, Luikart et al., 44 C 125 d c [15] C 150 i.v. d1-3 E 200 i.v. d1-3 C 250 i.v. d1-3 C 300 i.v. d1-3 LD = limited-stage disease; ED = extensive-stage disease; MTS = median time of survival; E = etoposide; C = carboplatin; P = cisplatin; G-CSF = granulocyte colony-stimulating factor; SC = subcutaneously. a Thoracic irradiation was provided for LD patients, and prophylactic cranial irradiation was given to those LD patients who achieved a complete response. b Median time of survival of all patients in each treatment group; the difference was not statistically significant. c Prophylactic cranial irradiation was given only to patients who achieved a complete response. d At physician discretion, thoracic irradiation was given to 29 responding LD patients. e Dose could be reduced to E 100 mg/m 2 and C 400 mg/m 2 after two cycles for an additional two cycles in responding patients. f C dose was escalated in increments of 50 mg/m 2 according to toxicity; G-CSF dose = 2 µg/kg SC.
3 Brahmer, Ettinger 145 Carboplatin/etoposide therapy was associated with less renal, neurologic, and gastrointestinal toxicity than cisplatin/etoposide therapy, and both combinations were equally effective [9]. A randomized phase III trial was conducted by the Hellenic Cooperative Oncology Group that compared the efficacy and toxicity of etoposide/cisplatin (EP) versus etoposide/carboplatin (EC) in previously untreated patients with SCLC [9]. Patients less than 75 years of age with SCLC confirmed by histology or cytology and WHO (World Health Organization) performance status less than 3 were eligible. Disease stage was classified as LD or ED according to VALG criteria. Patients were randomized to receive etoposide 100 mg/m 2 i.v. on days 1 to 3 plus either cisplatin 50 mg/m 2 i.v. on days 1 and 2 or carboplatin 300 mg/m 2 i.v. day 1 [9]. Treatment cycles were administered every three weeks, for a maximum of six cycles. Following the third cycle, patients with LD received thoracic irradiation with the fourth cycle. For those LD patients who achieved a complete response, prophylactic cranial irradiation was given. The response rates of patients receiving etoposide/cisplatin versus etoposide/carboplatin are shown in Table 1. Patients with LD achieved an overall response rate of 73% with etoposide/cisplatin and 86% with etoposide/carboplatin, while patients with ED had overall response rates of 50% and 64%, respectively. Complete response rates also were comparable between the two treatment arms based on disease stage [9]. Median duration of survival was not dependent on the treatment regimen (12.5 months for etoposide/cisplatin and 11.8 months for etoposide/carboplatin); however, disease stage and performance status correlated strongly with survival. Median survival time was 14.1 months for patients with LD and 10.4 months for those with ED (p < 0.05). Patients Table 2. Carboplatin/etoposide in combination with other agents Overall Complete MTS Patients response (%) response (%) (mo) Study LD ED Dose (mg/m 2 ) LD ED LD ED LD ED Gatzemeier et al., 1992 [16] 35 C 300 mg/m 2 i.v. d E 140 mg/m 2 i.v. d1-3 V 1.4 mg/m 2 i.v. d1, 8, 15 versus 40 E 200 mg/m 2 i.v. d V 1.4 mg/m 2 i.v. d1, Janssen et al., 1997 [17] C 300 mg/m 2 i.v. d NR* 10 E 190 mg/m 2 i.v. d1-3 V 1.4 mg/m 2 i.v. d1, 8, 15 Gridelli et al., 1996 [18] C 300 mg/m 2 i.v. d1 EP 75 mg/m 2 i.v. d1 3 E 100 mg/m 2 i.v. d1-3 or NRP 6 E 120 mg/m 2 i.v. d1-3 or NRP 6 E 140 mg/m 2 i.v. d NRP Skarlos et al., 1997 [19] A = 29 C 150 mg/m 2 i.v. d1 q o wk A = 79.5 A = 28.6 A = 8.33 B = 37 E 75 mg/m 2 i.v. d1-2 q o wk B = 92 B = 46.9 B =10.6 alternating with EP 30 mg/m 2 i.v. d1 q o wk I 2 g/m 2 i.v. d1 q o wk A + B # Bishop et al., [20] E 120 mg/m 2 i.v. d1-3 NRP evaluable C 100 mg/m 2 i.v. d1-3 Cy 750 mg/m 2 i.v. d1 V 1.4 mg/m 2 i.v. d1 Sakurai et al., 1996 [21] 7 MTD C 250 mg/m 2 i.v. d MTD CS 60 mg/m 2 i.v. d1 E 100 mg/m 2 i.v. d1, 3, 5 LD = limited-stage disease; ED = extensive-stage disease; TI = thoracic irradiation; * = not reached; NRP = not reported; PCI = prophylactic cranial irradiation; CY = cyclophosphamide; MTS = median time of survival; EP = epirubicin; CS = cisplatin; A + = plus radiotherapy and PCI; MTD = maximum tolerated dose; B # = more therapy with increased doses by 25% except E plus radiotherapy and PCI; I = ifosfamide; E = etoposide; = LD patients who responded received TI, and those with complete response received PCI; C = carboplatin; ++ = LD patients who had not progressed received thoracic radiation (TI) - patients achieving CR received PCI.
4 146 Carboplatin in the Treatment of Small Cell Lung Cancer with LD and ED survived for more than two years 26% and 7%, respectively. In addition, patients who received etoposide/carboplatin experienced significantly less toxicity, both nonhematologic and hematologic [9]. WHO grade 3/4 leukopenia was seen in 37.5%/12.5% of the patients treated with etoposide and cisplatin in comparison with patients treated with etoposide and carboplatin, who only experienced 10.3%/6.8%. Thrombocytopenia (WHO grade 4) occurred in only 6% of patients receiving etoposide/cisplatin and 4% of patients receiving etoposide/carboplatin. Nausea and vomiting, nephrotoxicity, neurotoxicity, leukopenia, infection, mucositis, and allergic reactions were reported less frequently for patients in the carboplatin arm than for those in the cisplatin arm. Nausea and vomiting along with neurologic toxicities were significantly less in patients receiving etoposide and carboplatin. WHO grade 2/3 nausea and vomiting occurred in 71%/4% of patients receiving etoposide and cisplatin, where nausea and vomiting only occurred in 25%/0% of patients receiving etoposide and carboplatin (p = ). Neurologic toxicity (WHO grades 1/2) occurred in 41%/12% of patients receiving etoposide and cisplatin and only 18%/0% of patients receiving etoposide and carboplatin, which was statistically significant (p = ). Carboplatin was also easier to administer and resulted in fewer hospitalizations. Dose intensity was similar for both the carboplatin and the cisplatin regimens. This trial provides a rationale for the use of etoposide/carboplatin over etoposide/cisplatin because of similar efficacy and decreased toxicity. Similar toxicity profiles were reported in other trials using etoposide/carboplatin for the treatment of SCLC [10-15]. Leukopenia and thrombocytopenia were most frequently reported, dose limiting, and dependent on the dosage regimen used. In another clinical trial, when carboplatin 125 mg/m 2 /d in combination with etoposide 200 mg/m 2 /d was administered for three days, grade 3/4 leukopenia and thrombocytopenia were reported in 81% and 76% of patients, respectively. Four patients died from myelosuppression [10]. However, only 19% of patients reported grade 3/4 nausea and vomiting, and there was no neurotoxicity. In contrast, when carboplatin 300 mg/m 2 was administered on day 1, the incidence of grade 3/4 leukopenia and thrombocytopenia was reported at rates of 20% and 16%, respectively [12]. Similarly, when carboplatin 450 mg/m 2 was administered on day 1, the incidence of grade 3/4 leukopenia and thrombocytopenia was 8% and 11%, respectively [11]. Even higher single doses of carboplatin are associated with increased hematologic toxicity, with approximately twothirds of patients receiving carboplatin 600 mg/m 2 on day 1 developing grade 3/4 leukopenia and/or thrombocytopenia [13]. In a dose-escalation trial, the maximum tolerated dose of carboplatin was 650 mg/m 2 in patients younger than 70 years of age and 450 mg/m 2 for those 70 years or older [14]. As discussed earlier, the use of AUC dosing may help reduce the hematologic side effects. A recent CALGB study attempted to dose-intensify the etoposide/carboplatin combination using GM-CSF support. Several dose levels of GM-CSF, carboplatin, and etoposide were evaluated. Dose levels evaluated were: carboplatin 25 and 150 mg/m 2 /d 3; etoposide 200, 250, and 300 mg/m 2 /d 3, and GM-CSF 5, 10, 20 µg/kg/d and 5 µg/kg every 12 h. GM-CSF was found to support only the first cycle of carboplatin/etoposide with acceptable toxicities; further cycles were not given because of delayed platelet and neutrophil count recovery. The conclusion was that dose intensification of etoposide and carboplatin was not possible with GM-CSF support alone [15]. CARBOPLATIN/ETOPOSIDE IN COMBINATION WITH OTHER AGENTS A phase III trial designed to examine the role of carboplatin in SCLC randomized patients to receive either etoposide 200 mg/m 2 days 1-3 and vincristine 1.4 mg/m 2 (max 2 mg) days 1 and 8 or carboplatin 300 mg/m 2 i.v. day 1; etoposide 140 mg/m 2 days 1-3; and vincristine 1.4 mg/m 2 (maximum 2 mg) i.v. on days 1, 8, and 15 (CEV) (Table 2) [16]. These regimens were given every four weeks up to six cycles. Seventy-five patients with previously untreated ED were evaluable. Overall response rates were achieved in 83% of patients who received CEV and in 65% of those who received EV. Complete response rates were 20% and 15% for the two groups, respectively. Leukopenia and nonhematologic toxicities were similar for both treatment arms; however, thrombocytopenia was higher in patients who received carboplatin. Thus, the carboplatin-containing regimen appears to be more active at this time than the two-drug combination. Another study evaluated dose-intensified CEV given with G-CSF support. Fifty-two untreated patients (26 with LD and 26 with ED) were given carboplatin (300 mg/m 2 on day 1), vincristine (1.4 mg/m 2 on days 1, 8, 15) and etoposide (190 mg/m 2 on days 1-3) every three weeks. Of 45 evaluable patients (22 ED and 23 LD), the overall response rate was 86% and 95% for ED and LD patients, respectively. The median survival for ED patients was 10 months. In patients with LD, the median survival time has not been reached [17]. An Italian group evaluated the feasibility of dose-intensifying etoposide (i.v. on days 1-3) in combination with carboplatin (300 mg/m 2 i.v. on day 1) and epirubicin (75 mg/m 2 i.v. on day 1) with G-CSF support. Fifteen patients with ED SCLC received three dose levels of etoposide. Three patients received 100 mg/m 2, six patients received 120 mg/m 2, and
5 Brahmer, Ettinger 147 six patients received 140 mg/m 2. Of three patients who received VP-16 at 100 mg/m 2, two had a complete response and one had a partial response. Of six patients who received VP-16 at 120 mg/m 2, two of six achieved a complete response and three of six achieved a partial response. One of six patients had a complete response, and four of six had a partial response of those who received VP-16 at 140 mg/m 2. Survival was not reported. The maximum tolerated dose of etoposide was 140 mg/m 2. Hematologic toxicities (neutropenia and thrombocytopenia) were the dose-limiting toxicities [18]. Skarlos et al. evaluated the activity of a four-drug regimen containing carboplatin and etoposide in patients with LD SCLC [19]. The regimen consisted of carboplatin (150 mg/m 2 i.v. on day 1) with etoposide (75 mg/m 2 i.v. for two days) given every other week. These two drugs alternated with epirubicin (30 mg/m 2 i.v.) and ifosfamide (2 gm/m 2 with mesna) given every other week. After 77 weeks, patients were divided into two groups, A and B. If a complete response occurred, a patient could be put into either group A or group B. Patients in group A received only additional radiotherapy and PCI; patients in group B received more chemotherapy using the same chemotherapeutic agents, but the dosages were increased by 25% except for etoposide. The etoposide dose was kept at 75 mg/m 2, but given for three days instead of only two. Patients in group B were also given radiotherapy and PCI. Twenty-nine patients were included in group A. In group B, 37 patients were studied. The overall response rates in groups A and B were 79.5% and 92%, respectively. Group B had a higher complete response rate, 46.9%, compared with that of group A, at 28.6%. The median survival of 10.6 months for Group B was higher than that of Group A at 8.33 months. These results were not statistically significant. Thus, the study showed that this regimen was not superior to standard dose [19]. The Australian Lung Cancer Study Group evaluated etoposide 120 mg/m 2 and carboplatin 100 mg/m 2 (both i.v. days 1-3) plus cyclophosphamide 750 mg/m 2 and vincristine 1.4 mg/m 2 (both i.v. day 1) [20]. Patients were assessed after three cycles, and for those with LD who responded, mediastinal radiation was given. LD patients with a complete response also received PCI [20]. Of the 87 evaluable patients, complete response rates of 40% and partial response rates of 41% were observed for etoposide/carboplatin plus cyclophosphamide and vincristine. The median survival time was 13.3 months for patients with LD and 9.6 months for patients with ED [20]. Neutropenia was the dose-limiting toxicity, and nonhematologic toxicity was mild in these patients receiving the four-drug combination. A Japanese study evaluated the combination of carboplatin, etoposide, and cisplatin both in patients with LD and with ED [21]. Evaluation of increasing doses of carboplatin and cisplatin was performed in a stepwise fashion. The dose of etoposide was held at 100 mg/m 2 i.v. on days 1, 3, and 5. The maximum tolerated dose of carboplatin and cisplatin was 250 mg/m 2 i.v. on day 1 and 60 mg/m 2 i.v. on day 1, respectively. Hematologic toxicity was dose-limiting. At this dose level, four of seven patients experienced grade 4 thrombocytopenia, and the overall response rate was 100%, with 2/7 patients achieving a complete remission. More patients are needed to evaluate the tolerability, response, and survival in patients with SCLC [21]. CARBOPLATIN/ETOPOSIDE AND CONCURRENT XRT Recently, two different studies evaluating the carboplatin/etoposide combination with concurrent radiation therapy were presented at the IALSC Lung Cancer Conference held in Dublin in the summer of 1997 (Table 3). One study included only patients with LD SCLC who Table 3. Carboplatin/etoposide with concurrent radiotherapy Median Study Patients Dose (mg/m 2 ) OR (%) CR PR survival Koinumaru et al., 1997 [22] 21 C 300 i.v. d1 88 4/17 11/ mo. 17 evaluable E 60 i.v. d1-5 TRT d2 1.5 Gy BID for 5D/wk 3 wks Crino et al., 1997 [23] 64 Intensification cycle /39 9/ mo. 39 received C 150 i.v. d1-3 intensification E 100 i.v. d1-5 with TRT G-CSF TRT 45 Gy in BID fractions C = carboplatin; E = etoposide; TRT = thoracic radiation therapy; OR = overall response; CR = complete response; PR = partial response; Mo. = month.
6 148 Carboplatin in the Treatment of Small Cell Lung Cancer received carboplatin (300 mg/m 2 i.v. day 1) and etoposide (60 mg/m 2 on days 1-5). The regimen was given every 21 days for four cycles. Thoracic radiotherapy was started on day 2 at 1.5 Gy fraction two times a day, and was given five days a week for three weeks. Of 17 evaluable patients, there was an 88% overall response rate, with four patients achieving a complete remission. The median survival of those patients was 19.4 months. Two-year survival was 41%. The toxicities were relatively mild, however, with 13 patients needing G-CSF support. Grade 2 esophagitis and pneumonitis occurred in two patients each [22]. Another study evaluated induction chemotherapy followed by thoracic irradiation in LD SCLC patients [23]. The induction therapy consisted of cyclophosphamide, epirubicin, and vincristine. After two to three cycles of therapy, 46 of 64 patients achieved an objective response. Thirty-nine of those patients then went on to receive late intensification chemotherapy and concurrent radiotherapy. The chemotherapy consisted of carboplatin (150 mg/m 2 on days 1-3) and VP-16 (100 mg/m 2 i.v. days 1-5) given concurrently with thoracic radiation of 45 Gy in twice-daily fractions. Patients were supported with G-CSF (5 µg/kg/d) from days 7 to 16; this combination was given for three cycles. Of those 39 patients, 30 achieved a CR (77%) and nine achieved a partial response. The median survival was 18.9 months. There were no episodes of sepsis or bleeding complications recorded. However, grade 3 and 4 neutropenia and thrombocytopenia occurred in 22% and 27% of the courses, respectively. These studies show that concurrent thoracic radiotherapy can be given with carboplatin and etoposide safely and effectively. CARBOPLATIN/ETOPOSIDE IN THE ELDERLY Patients over 65 years of age frequently are not good candidates for systemic chemotherapy because of coexisting medical conditions, including chronic heart disease and renal impairment [24]. Furthermore, elderly individuals do not tolerate intensive chemotherapy as well as younger patients, and they attain only modest gains relative to increased toxicity [25]. As the population over the age of 70 continues to increase, and because one-third of lung cancers occur in this age group, treatment of elderly patients is increasingly important. Results of studies evaluating the use of etoposide/carboplatin in elderly or medically compromised patients are shown in Table 4. Response rates range from 66% to 71% for patients with ED and 88% to 93% for those with LD. Median survival time was approximately 8 to 11 months for patients with ED and approximately 11 to 15 months for those with LD [24-28]. The etoposide/carboplatin regimens were well tolerated in these patients [24, 25]. As might be expected, myelosuppression was the dose-limiting toxicity [24]. Grade 3/4 thrombocytopenia and leukopenia occurred in approximately 32% to 53% and 45% to 50% of these patients, respectively [26, 28]. Table 4. Carboplatin and etoposide in elderly and medically compromised patients Overall response (%) Complete response (%) MTS Study Patients/age Dose (mg/m 2 ) LD ED LD ED LD ED Evans et al., 1995 [24] 38 b E 100 p.o. d c wk 45 wk 8 LD C 150 i.v. d1 30 ED Byrne et al., 1994 [25] 70 a E 200 p.o. d wk C 300 i.v. d1 Kobayaski et al., 38 E 40 p.o. d mo 8.6 mo 1997 [26] 16 LD C 80% of calculated 22 ED dose i.v. d1 (> 70 years) Berzinec et al., 1997 [27] 10 E 120 i.v. d mo (> 75 years) C 250 i.v. d1 Shibata et al., 1997 [28] 22 E 70 i.v. d Not reported 11.2 mo 8.7 mo 8 LD C 100 i.v. d1,3,5 14 ED G-CSF MTS = median time of survival; LD = limited-stage disease; ED = extensive-stage disease; E = etoposide; C = carboplatin; TI = thoracic irradiation; PCI = prophylactic cranial irradiation. a Includes patients 65 years and older and patients with ED or an ECOG performance status of 3 or worse. Results were not reported on the basis of disease stage. b Includes patients 65 years and older with ED or patients of any age with LD and renal or cardiac disease. c LD patients who responded received TI, and those with complete or good partial remission received PCI.
7 Brahmer, Ettinger 149 CARBOPLATIN IN IFOSFAMIDE-CONTAINING REGIMENS Ifosfamide, an analog of cyclophosphamide, has a broad range of activity in various tumor types, including lung cancer, sarcomas, lymphomas, breast cancer, gynecologic malignancies, and germ cell tumors [29]. It is one of the most active agents in SCLC, with objective response rates to single-agent therapy of approximately 50% in previously untreated patients [4]. In addition, ifosfamide is relatively nonmyelosuppressive as compared with cyclophosphamide, allowing combinations of ifosfamide with other myelosuppressive agents [29]. Other side effects include renal and neurologic toxicities as well as hemorrhagic cystitis, which may be reduced or prevented by the administration of mesna [6, 29]. As noted below, a number of studies have evaluated ifosfamide in combination with carboplatin and etoposide, with or without other agents, in previously untreated patients with SCLC (Table 5). The ifosfamide/carboplatin/etoposide (ICE)- containing regimens produced overall response rates of 79% to 94% in patients with LD [30-34]. The median duration of survival ranged from 16.6 to 19 months and two-year survival rates ranged from 24% to 32%. In patients with ED, overall response rates ranged from 71% to 100%, the median duration of survival ranged from 8.3 to 13.4 months, and twoyear survival rates ranged from 14% to 22%. In one study, vincristine on day 14 of each course of carboplatin therapy was given in an attempt to prevent between-course relapse [30]. Based on the premise that there may be a lack of cross-resistance between cisplatin and carboplatin, Prendiville et al. alternated the two analogs in combination with ifosfamide and etoposide during successive cycles, combined with mid-cycle vincristine [33]. The major toxicities of the ICE-containing regimens were neutropenia and thrombocytopenia, with a tendency for these toxicities to increase with subsequent cycles of treatment, and a 6% to 10% rate of death from infection [30-33]. The severe myelosuppression observed in most patients requires close monitoring, supportive care, and dose reduction [30]. Nausea and vomiting, which can be severe, were also common [30-33]. Some investigators have attempted to dose-intensify regimens by increasing the doses of the chemotherapeutic agents, but Hanauske et al. evaluated dose intensification by decreasing the intervals between cycles from 27 to 17 days using a combination of carboplatin, ifosfamide, etoposide, and vincristine [35]. The specific regimen consisted of carboplatin 250 mg/m 2 i.v. on day 1, ifosfamide 2 g/m 2 on days 1 and 2, etoposide 120 mg/m 2 i.v. on days 1 and 2 with an oral dose on day 3, and vincristine 1.4 mg/m 2 i.v. on day 14. G-CSF was given, allowing cycle intervals to be reduced to every 17 days. The toxicities were moderate, with 21% of patients developing febrile episodes and 48% of patients being withdrawn from the study. The shorter the cycle interval, the better the response rate, with patients receiving cycles greater than every 23 days achieving a 71% response rate and patients receiving cycles equal to or less than every 17 days having a 100% response rate [35]. Shevlin et al. modified the ICE regimen dosages (carboplatin 6 (GFR + 25) mg, ifosfamide 3 g/m 2 plus mesna 3 g/m 2, mesna 1.8 g/m 2 bolus, and 50 mg oral etoposide twice daily for seven days) [36]. The chemotherapy regimen was given every 28 days. Thirty patients (27 with LD and three with ED) took part in the study. The overall response rate was 83%, and the median survival time was 12.6 months. Only one-half of the patients were able to receive treatment without dose reduction or delay. The deviation in most patients was caused by WHO grade 3 or 4 thrombocytopenia or neutropenia. The dose-intensified ICE chemotherapy regimen did result in acceptable survival and response rates, but had a substantial amount of toxicity associated with it. ICE-CONTAINING REGIMENS WITH PERIPHERAL BLOOD STEM CELL SUPPORT Several other studies have looked at whether dose-intensive ICE-containing regimens improve response rates [37-40]. Data from several ongoing studies were presented at the IASLC Lung Cancer Conference this past summer. These studies used peripheral blood stem cell (PBSC) support to theoretically reduce the cytopenias that occur with the doseintensive therapy (Table 6). One study showed that response rates were similar to those found in standard-dose therapy, but patients with dose-intensive ICE therapy supported by PBSC had decreased neutropenic sepsis [37]. It is still too early in this study to see if there is a survival advantage with dose-intensive ICE over standard-dose ICE. Another study evaluated what effect G-CSF or PBSC support had on patients receiving conventional-dose ICE. While receiving G-CSF, patients had a decreased incidence of neutropenia. On the other hand, while receiving PBSC support, patients only had decreased episodes of thrombocytopenia if more than CD34 cells/kg were given [38]. Dose-intensive therapy using PBSC transplant was studied using four drugs (etoposide, ifosfamide, carboplatin, and epirubicin). One such study took patients with LD SCLC and gave high-dose ifosfamide 4 gm/m 2 /d i.v. days 1-3 and epirubicin 30 mg/m 2 /d i.v. days 1-3 for cycles 1 and 3, then gave high-dose carboplatin AUC 5 mg/d i.v. days 1-2 and etoposide 120 mg/m 2 /d i.v. days 1-3 for cycles 2 and 4. These drugs were given currently with radiotherapy at fractions of 2 Gy once a day on days 1 through 5 of each cycle. G-CSF was given, and PBSC were reinfused on day 5 of cycles 2, 3, and 4. No prophylactic cranial irradiation was given, and no one died of acute toxicities. Of 35 evaluable patients, 65%
8 150 Carboplatin in the Treatment of Small Cell Lung Cancer Table 5. ICE regimens with or without other agents for previously untreated SCLC MTS 2-yr survival Patients OR (%) CR (%) (mo) rate (%) Study LD ED Dose (mg/m 2 ) LD ED LD ED LD ED LD ED Thatcher 1993 d [30] I 5,000 i.v. d C 300 i.v. d1 E 120 i.v. d1-2, 240 p.o. d3 V 1 mg i.v. d14 Thatcher et al., I 5,000 i.v. d a [31] C 300 i.v. d1 E 120 i.v. d1-2, 240 p.o. d3 V 0.5 i.v. d14 Smith et al., 1990 b [32] I 5,000 i.v. d C 400 i.v. d1 E 100 i.v. d1-3 Prendiville et al., 31 9 I 5,000 i.v. d c [33] C 300 i.v. d1 E 120 i.v. d1-2, 240 p.o. d3 V 1 mg i.v. d14 after C P 100 i.v. d1 Wolff et al., 1995 e [34] 35 I 3,750-5,000 i.v. d C 300 i.v. d1 E 50 p.o. d1-21 or 50 mg d1-14 Hanauske et al., 1997 [35] 2 27 I 2,000 i.v. d1,2 17 d interval Not Not Not C 250 i.v. d1 100 reported reported reported E 120 i.v. d1,2 p.o. d3 > 23 d interval V 1.4 i.v. d14 71 G-CSF Shevlin et al., 1997 [36] 27 3 I 3,000 d1 i.v. + mesna f 83 Not 12.6 Not C 6 (GFR + 25) mg i.v. d1 reported reported E 50 mg p.o. BID d1-7 OR = overall response; CR = complete response; MTS = median time of survival; LD = limited-stage disease; ED = extensive-stage disease; I = ifosfamide; i.v. = intravenous; C = carboplatin; E = etoposide, V = vincristine; p.o. = orally; P = cisplatin, NS = normal saline; TI = thoracic irradiation; PCI = prophylactic cranial irradiation; CIV = continuous intravenous infusion. a Mesna 5,000 mg/m 2 with ifosfamide infused in 2 L NS over 24 h following carboplatin and continued as a 3,000-mg/m 2 infusion in 1 L NS over the subsequent 12 h. TI was given to 36 patients four to five weeks after chemotherapy completion. Two-year survival was actuarial rate. b Mesna was given as follows: 1,000 mg/m 2 i.v. in 100 ml NS over 30 min before ifosfamide, then 5,000 mg/m 2 in 3 L dextrose saline over 24 h with the ifosfamide infusion, then 3,000 mg/m 2 i.v. in 1 L dextrose over 12 h immediately after the ifosfamide infusion (total dose 8,000 mg/m 2 over 36 h). Patients with LD received concurrent hyperfractionated TI with the first two cycles; PCI was given to those who achieved CR. Two-year survival rate was a predicted rate. c Mesna 5,000 mg/m 2 with ifosfamide in 2 L NS infused over 24 h, mesna continued as a 3,000-mg/m 2 infusion in 1 L NS over the subsequent 12 h. PCI was given with the first cycle of chemotherapy; TI was given after the third cycle. Carboplatin given cycles 2, 4, 6; cisplatin given cycles 1, 3, 5. Cycles repeated every three weeks after cisplatin and every four weeks after carboplatin. The dose of carboplatin alternated with the dose of cisplatin; the vincristine dose was not based on body surface area. Two-year survival rate was actual survival rate. d Mesna 5,000 mg/m 2 with ifosfamide infused over 24 h and continued as a 3,000-mg/m 2 infusion over the subsequent 12 h. TI and PCI were given [23]. e Mesna 5,065-6,730 mg/m 2 CIV given on day 1 beginning 15 min before and continuing until 12 h after ifosfamide, etoposide, and ifosfamide doses were reduced to decrease hematologic toxicity. PCI was given to all patients with CR. Two-year survival rate was actual survival rate. f Mesna 3 g/m 2 with mesna 1.8 g/m 2 bolus. achieved a complete response. However, 21 patients relapsed, mainly in the central nervous system. The median survival was 27 months [39]. Another study evaluated patients with LD and ED receiving first etoposide (500 mg/m 2 ), ifosfamide (400 mg/m 2 ), cisplatin (50 mg/m 2 ), and epirubicin (50 mg/m 2 ) with G-CSF. Then, thirty patients proceeded to receive high-dose chemotherapy consisting of etoposide 1,500 mg/m 2, ifosfamide 12 gm/m 2, carboplatin 750 mg/m 2, and epirubicin 150 mg/m 2, with autologous PBSC transplant. In contrast with the previous study, 13% of patients died of treatment-related complications. Median survival was 26 months in patients
9 Brahmer, Ettinger 151 Table 6. ICE-containing regimens with peripheral blood stem cell support (PBSC) Patients MTS Study LD ED Dose PBSC Response (%) LD ED Woll et al., 1997 b [37] 50 I 5 g/m 2 Given cycles 2-6 OR = 80 Ongoing study C 300 mg/m 2 d1 E 180 mg/m 2 d1-2 G-CSF b (all cycles) Dunlop et al., 1997 [38] 13/18 evaluable I 3 g/m 2 Reinfused 48 h Did not evaluate Did not evaluate C 400 mg/m 2 after cycles 4, 5 ± 6 E 700 mg/m 2 G-CSF (cycle 2, 3) Van de Velde et al., I 4 g/m 2 d1-3 Reinfused d5 of CR = mo [39] 35/39 evaluable Ep 30 mg/m 2 d1-3 on cycles cycles and 3 E 120 mg/m 2 d1-3 C AUC 5 d1-2 on cycles 2 and 4 G-CSF (all cycles) c Fetscher et al., 1997 a [40] E 1.5 g/m 2 Date of infusion 30/30 patients 26 mo 8 mo I 12 g/m 2 not noted improved or C 750 mg/m 2 maintained response a Ep 150 mg/m 2 LD = limited-stage disease; Ep = epirubicin; ED = extensive-stage disease; OR = overall response; E = etoposide; CR = complete response; I = ifosfamide; C = carboplatin. a After receiving induction chemotherapy, patients received the intensive therapy. Surgical resection was attempted, and thoracic radiation was given after chemotherapy. b Chemotherapy was given every two weeks for six cycles as the intensive arm of the study with G-CSF. In standard arm of study, chemotherapy was given every four weeks without G-CSF. c Patients were given four cycles of chemotherapy with radiotherapy on day 1 and 5 of each cycle. with LD SCLC and eight months with ED. Two-year survival also demonstrated the same trend, with 53% LD and 9% ED SCLC patients surviving at two years. Patients with LD were the only group that showed an improved survival from dose-intensive chemotherapy with PBSC support [40]. CARBOPLATIN/PACLITAXEL/ETOPOSIDE REGIMENS FOR SCLC Paclitaxel, isolated from the western yew tree (Taxus brevifolia), exerts its cytotoxic effect by interfering with microtubule structure and function. Dose-limiting toxicities include myelosuppression (neutropenia) and peripheral neuropathy [41]. In two clinical trials, as a single agent, paclitaxel 250 mg/m 2 given over 24 h every three weeks resulted in an overall response rate of 34% to 41% in previously untreated patients with ED SCLC [41, 42]. The combination of carboplatin/paclitaxel/etoposide has been evaluated in several trials of patients with SCLC (Table 7) [43-48]. The results of these clinical trials are encouraging, with overall response rates ranging from 61% to 84% in patients with ED and 81% to 98% in LD. Most of the trials are ongoing; therefore, median survival times and two-year survival rates are not yet available. Grade 3/4 leukopenia occurred in 24% to 81% of patients and was more frequent with higher doses of chemotherapy and concurrent radiotherapy. Nonhematologic toxicities such as myalgia, peripheral neuropathy, and nausea and vomiting were mild [45]. Additional trials are needed to evaluate the activity of paclitaxel-containing combination regimens in SCLC to establish its role in this disease. NEW CARBOPLATIN COMBINATIONS A complicated study done by Lassen et al. evaluated the efficacy and toxicity of cisplatin versus carboplatin in combination with teniposide and vincristine in patients with LD and ED SCLC [49]. Four hundred eighty-four patients were randomized to either arm 1 (three cycles of cisplatin) or arm II (three cycles of carboplatin), with teniposide and vincristine alternating with other combinations of chemotherapeutic agents such as: block A cyclophosphamide, etoposide, lomustine, and vincristine; block B doxorubicin and vincristine; and block C cisplatin,
10 152 Carboplatin in the Treatment of Small Cell Lung Cancer Table 7. Carboplatin/paclitaxel/etoposide regimens for SCLC Overall Complete MTS Patients response (%) response (%) (mo) Study LD ED Dose (mg/m 2 ) LD ED LD ED LD ED Reck et al., 1997 [43] 26 T 175 i.v. d Not evaluable C AUC 5 i.v. d1 reported E 100 mg p.o. d2-8 Hainsworth et al., T 135 i.v. d1 (1 h) * [44] C AUC 5 d1 E 50/100 mg alt p.o. d1-10 Hainsworth et al., 79 T 200 i.v. d1 (1 h) > [45, 46] C AUC 6 i.v. d1 Not E 50/100 mg alt reached p.o. d1-10 yet Gatzemeier et al., 26 T 175 i.v. d1 (1 h) NA 1997 [47] C AUC 5 i.v. d1 E 100 mg p.o. d2-8 Depperman et al., 23 T 200 i.v. d Not 1997 [48] evaluable C AUC 6 i.v. d1 reported MTS = median time of survival; LD = limited-stage disease; ED = extensive-stage disease; T = paclitaxel; C = carboplatin; AUC = area under the curve; E = etoposide; alt = alternating; p.o. = orally; NA = not available. a C dose calculated using the Calvert formula. Patients with limited-stage disease received thoracic irradiation with last two courses of chemotherapy. Median actuarial overall survival; first 13 patients received i.v. etoposide 25 mg/m 2 i.v. days 1-5, b Limited-stage disease patients received thoracic irradiation beginning day 42. hexamethylmelamine, and vindesine. Patients were also randomized to an arm III that only alternated blocks A, B, and C. While grade 3/4 leukopenia was more common in arm III, grade 3/4 thrombocytopenia was more common in arms I and II. The efficacy, toxicity, response rates, and survival rates were no different between arm I and II (cisplatin versus carboplatin). The platinum and teniposide combinations (arms I and II) showed only superior survival rates compared with arm III (without teniposide). For arms I, II, and III, the overall response rates were 63%, 72%, and 65%, respectively, while the two-year survival rates were 16%, 14%, and 9%, respectively [49]. A novel combination of carboplatin and vinorelbine has been evaluated recently [50]. Thirty-three chemo-naive patients with ED SCLC were given carboplatin (300 mg/m 2 ) i.v. on day 1 and vinorelbine (25 mg/m 2 ) i.v. on days 1 and 8 every four weeks. The overall response rate was 70%, with a complete response rate of 24%. The median survival rate has not yet been reached. This combination was relatively well tolerated. WHO grades 3 and 4 leukopenia were 14.2% and 3.5%, respectively. Grade 2 anemia was 10.5%, and grade 3 thrombocytopenia was 3.5%. Overall, the combination achieved a comparable response rate and was well tolerated. More studies of the carboplatin and vinorelbine combination are warranted. CONCLUSIONS The combination of carboplatin/etoposide results in comparable efficacy, has less nonhematologic toxicity, and is easier to administer than cisplatin/etoposide. In addition, carboplatin-based regimens are particularly useful for elderly patients and those who are medically compromised. Response rates with carboplatin/etoposide in patients with LD and ED range from 73% to 93% and from 50% to 85%, respectively. Median survival is up to 15 months in LD patients and up to 12 months in ED. Several investigators evaluated the ICE combination. The two-year survival rates with ICE-containing regimens were 24% to 32% in LD patients. The principal toxicity of ICE regimens is myelosuppression, particularly neutropenia and thrombocytopenia. Nausea and vomiting can be severe, but other nonhematologic toxicities are generally manageable. Dose-intensive ICE-containing regimens with PBSC support may decrease cytopenias related to this intensive therapy. Further studies are needed to evaluate this. Thus far, only one study showed that patients with LD may have an improved survival from dose-intensive chemotherapy with PBSC support. Further research has combined paclitaxel with carboplatin and etoposide in the treatment of SCLC. Preliminary results are encouraging, and they support additional, larger trials of this
11 Brahmer, Ettinger 153 combination. The study of the vinorelbine/carboplatin combination shows promising results but needs further investigation. These multiple approaches and new combination chemotherapy regimens with carboplatin may further determine the ability of systemic therapy to improve survival in patients with SCLC, and in combination with radiation therapy, potentially cure those patients with LD. REFERENCES 1 Landis SH, Murray T, Bolden S et al. Cancer statistics, CA Cancer J Clin 1998;48: Ihde DC, Pass HI, Glatstein EJ. Small cell lung cancer. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds. Cancer Principles and Practice of Oncology. Philadelphia: JB Lippincott Co. 1997: Lorigan P, Lee SM, Betticher D et al. Chemotherapy with vincristine/ifosfamide/carboplatin/etoposide in small cell lung cancer. Semin Oncol 1995;22(suppl 7): Ettinger DS. The place of ifosfamide in chemotherapy of small cell lung cancer: the Eastern Cooperative Oncology Group experience and a selected literature update. Semin Oncol 1995;22(suppl 2): Bunn PA Jr. Clinical experiences with carboplatin (paraplatin) in lung cancer. Semin Oncol 1992;19(suppl 2): Chang AY. Introduction of ifosfamide/carboplatin/etoposide chemotherapy. Semin Oncol 1995;22(suppl 7): Calvert AH, Newell DR, Gumbrell LA et al. Carboplatin dosage prospective evaluation of a simple formula based on renal function. J Clin Oncol 1989;7: Raghavan D, Perez R, Creaven P et al. Carboplatin for small cell lung cancer: progress toward greater efficacy and reduced toxicity. Semin Oncol 1994;21(suppl 6): Kosmidis PA, Samantas E, Fountzilas G et al. Cisplatin/etoposide versus carboplatin/etoposide chemotherapy and irradiation in small cell lung cancer: a randomized phase III study. Semin Oncol 1994;21(suppl 6): Luikart DS, Goutsou M, Mitchell ED et al. Phase I/II trial of etoposide and carboplatin in extensive small-cell lung cancer: a report from the Cancer and Leukemia Group B. Am J Clin Oncol 1993;16: Viren M, Liippo K, Ojala A et al. Carboplatin and etoposide in extensive small cell lung cancer. Acta Oncol 1994;33: Pfeiffer P, Sorensen P, Rose C. Is carboplatin and oral etoposide an effective and feasible regimen in patients with small cell lung cancer? Eur J Cancer 1995;31:64-69a. 13 Ellis PA, Talbot DC, Priest K et al. Dose intensification of carboplatin and etoposide as first-line combination chemotherapy in small cell lung cancer. Eur J Cancer 1995;31: a. 14 Katakami M, Takada M, Negoro S et al. Dose escalation study of carboplatin with fixed-dose etoposide plus granulocyte-colony stimulating factor in patients with small cell lung carcinoma: a study of the Lung Cancer Study Group of West Japan. Cancer 1996;77: Luikart SD, Herndon JE, Hollis DR et al. Phase I trial of etoposide, carboplatin, and GM-CSF in extensive small cell lung cancer: a Cancer and Leukemia Group B study. Am J Clin Oncol 1997;20: Gatzemeier U, Hossfeld DK, Neuhauss R et al. Phase II and III studies with carboplatin in small cell lung cancer. Semin Oncol 1992;19(suppl 2): Janssen B, Jagos U, Gatzemieir U et al. Phase II study of dose intensified chemotherapy with carboplatin/etoposide/vincristine (CEV) with lenograstim support in small cell lung cancer (SCLC). Lung Cancer 1997;18:31a. 18 Gridelli C, D Aprile M, Palmeri S et al. Phase I study of chemotherapy with carboplatin, epirubicin, and escalating dose of VP-16 with G-CSF support in extensive small cell lung cancer. Am J Clin Oncol 1996;19: Skarlos D, Samantas E, Pavlidis N et al. Weekly chemotherapy with alternating non-cross resistant chemotherapy regimens in good-prognosis small cell lung cancer (SCLC) (Final Results). Lung Cancer 1997;18:42a. 20 Bishop JF, Kefford R, Raghavan D et al. Etoposide, carboplatin, cyclophosphamide and vincristine in previously untreated patients with small cell lung cancer. Cancer Chemother Pharmacol 1990;25: Sakurai M, Ichiki M, Hayaski I. Phase I/II study of combination chemotherapy with cisplatin, carboplatin and etoposide in small cell lung cancer. Lung Cancer 1996;15: Koinumaru S, Saito J, Nakai Y. Concurrent hyperfractionated thoracic radiotherapy (TRT) with carboplatin and etoposide for the treatment of limited small cell lung cancer (LSCLC). Lung Cancer 1997;18:68a. 23 Crino L, De Marinis F, Corgna E et al. Concurrent chemoradiotherapy as intensification in limited (LD) small cell lung cancer (SCLC). A Phase II trial. Lung Cancer 1997;18:73a. 24 Evans WK, Radwi A, Tomiak E et al. Oral etoposide and carboplatin: effective therapy for elderly patients with small cell lung cancer. Am J Clin Oncol 1995;18: Byrne A, Carney DN. Small cell lung cancer in the elderly. Semin Oncol 1994;21(suppl 6): Kobayaski M, Matsui K, Masuda N et al. Phase II trial of carboplatin (CBDCA) plus oral etoposide (ETP) for elderly patients with small cell lung cancer (SCLC). Lung Cancer 1997;18:36a. 27 Berzinec P, Arpasova M, Kuzmova H. Carboplatin and etoposide in patients aged 75 years or older with small cell lung cancer. Lung Cancer 1997;18:46a.
12 154 Carboplatin in the Treatment of Small Cell Lung Cancer 28 Shibata K, Kasahara K, Nakatsumi Y et al. Carboplatin and etoposide in the treatment of elderly small cell lung cancer (SCLC). Lung Cancer 1997;18:54a. 29 Markman M. Ifosfamide: an important antineoplastic agent with a wide spectrum of activity. Semin Oncol 1996;23(suppl 6):1. 30 Thatcher N. Ifosfamide/carboplatin/etoposide (ICE) regimen in small cell lung cancer. Lung Cancer 1993;9(suppl 1): Thatcher N, Lind M, Stout R et al. Carboplatin, ifosfamide and etoposide with mid-course vincristine and thoracic radiotherapy for limited stage small cell carcinoma of the bronchus. Br J Cancer 1989;60: Smith IE, Perren TJ, Ashley SA et al. Carboplatin, etoposide, and ifosfamide as intensive chemotherapy for small cell lung cancer. J Clin Oncol 1990;8: Prendiville J, Radford J, Thatcher N et al. Intensive therapy for small-cell lung cancer using carboplatin alternating with cisplatin, ifosfamide, etoposide, mid-cycle vincristine, and radiotherapy. J Clin Oncol 1991;9: Wolff AC, Ettinger DS, Neuberg D et al. Phase II study of ifosfamide, carboplatin, and oral etoposide chemotherapy for extensive-disease small-cell lung cancer: an Eastern Cooperative Oncology group pilot study. J Clin Oncol 1995;13: Hanauske AR, Korfel A, Perker M et al. Dose intensity phase I/II trial with carboplatin, ifosfamide, etoposide and vincristine combined with filgrastim in patients with small-cell lung cancer. Oncology 1997;54: Shevlin PM, Stead ML, Brown JM et al. A phase II study of an intensive, modified ICE regimen in patients with good prognosis small cell lung cancer. Lung Cancer 1997;18:44a. 37 Woll PJ, Lomax L, Hodgetts J et al. Tolerability of doseintensive ICE chemotherapy for SCLC with filgrastim and reinfusion of haemopoietic progenitors in whole blood. Lung Cancer 1997;18:70a. 38 Dunlop DJ, Fitzsimons EJ. Peripheral blood stem cell (PBSC) support for ICE chemotherapy in small cell lung cancer (SCLC). Lung Cancer 1997;18:30a. 39 Van de Velde H, Bosquee L, Weynants P et al. Concomitant radiotherapy (RT) and high-dose chemotherapy (HDCT) with G-CSF and peripheral blood stem cell (PBSC) rescue for limited disease (LD) small cell lung cancer (SCLC): better local control but high central nervous system (CNS) relapse rate. Proc Am Soc Clin Oncol 1997;16:486a. 40 Fetscher S, Brugger W, Englehardt R et al. High-dose therapy with etoposide, ifosfamide, carboplatin, and epirubicin (VIP-E) in small cell lung cancer (SCLC). Lung Cancer 1997;18:31a. 41 Ettinger DS, Finkelstein DM, Sarma RR et al. Phase II study of paclitaxel in patients with extensive-disease small cell lung cancer. An Eastern Cooperative Group Study. J Clin Oncol 1995;13: Kirschling RJ, Jung SH, Jett JR. A phase II trial of taxol and G-CSF in previously untreated patients with extensive stage small cell lung cancer (SCC). Proc Am Soc Clin Oncol 1994;13:326a. 43 Reck M, Jagos U, Kaukel E et al. Chemotherapy of limited stage small cell lung cancer with carboplatin, paclitaxel, and oral etoposide. A phase II trial. Lung Cancer 1997;18:31a. 44 Hainsworth JD, Stroup SL, Greco FA. Paclitaxel, carboplatin, and extended schedule etoposide in the treatment of small cell lung carcinoma. Cancer 1996;77: Hainsworth JD, Gray JR, Hopkins LG et al. Paclitaxel (1-hour infusion), carboplatin, and extended schedule etoposide in small cell lung cancer (SCLC): a report on 117 patients (pts) treated by the Minnie Pearl Cancer Research Network. Proc Am Soc Clin Oncol 1997;16:451a. 46 Hainsworth JD, Gray JR, Stroup SL et al. Paclitaxel, carboplatin, and extended-schedule etoposide in the treatment of small-cell lung cancer: comparison of sequential phase II trials using different dose-intensities. J Clin Oncol 1997;15: Gatzemeier U, Jagos U, Kaukel E et al. Phase II study of paclitaxel, carboplatin and etoposide in patients with small cell lung cancer. Proc Am Soc Clin Oncol 1997;16:468a. 48 Deppermann KM, Kurzeja A, Lichey J et al. Paclitaxel (TAX) and carboplatin (CBDA) in advanced SCLC: a phase II study. Lung Cancer 1997;18:46a. 49 Lassen U, Kristjansen PEG, Osterlind K et al. Superiority of cisplatin or carboplatin in combination with teniposide and vincristine in the induction chemotherapy of small-cell lung cancer. A randomized trial with 5 years follow-up. Ann Oncol 1996;7: Gridelli C, Ianniello G, Brancaccio L et al. Carboplatin plus vinorelbine: a new active regimen in extensive small cell lung cancer. Results of a multicenter phase II study. Lung Cancer 1997;18:55a.
Lung Cancer - Combination Chemotherapy and the Risk of Failure
 Evidence-based Series 7-13-1 Version 2 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) The Role of Combination Chemotherapy in the Initial Management of Limited-Stage
Evidence-based Series 7-13-1 Version 2 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) The Role of Combination Chemotherapy in the Initial Management of Limited-Stage
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof.
 Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Small-Cell Lung Cancer: Is There a Standard Therapy?
 Small-Cell Lung Cancer: Is There a Standard Therapy? Review Article [1] January 02, 1998 By Pieter E. Postmus, MD, PhD [2] and Egbert F. Smit, MD [3] For more than 25 years, chemotherapy has been the cornerstone
Small-Cell Lung Cancer: Is There a Standard Therapy? Review Article [1] January 02, 1998 By Pieter E. Postmus, MD, PhD [2] and Egbert F. Smit, MD [3] For more than 25 years, chemotherapy has been the cornerstone
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Schedule: Drug Dose iv/infusion/oral q Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8
 Carboplatin/Gemcitabine Lung Cancer (non-small cell) - Advanced Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8 Cycle frequency: Every three weeks Total
Carboplatin/Gemcitabine Lung Cancer (non-small cell) - Advanced Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8 Cycle frequency: Every three weeks Total
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003)
 Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline
 Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Schedule: Drug Dose iv/infusion/oral q Doxorubicin 25mg/m 2 iv bolus Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/2hrs Days 1 & 2
 Doxorubicin/Cisplatin Osteosarcoma - neoadjuvant Doxorubicin 25mg/m 2 iv bolus Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/2hrs Days 1 & 2 (3 before surgery) Ensure adequate renal function Pre & post-hydration,
Doxorubicin/Cisplatin Osteosarcoma - neoadjuvant Doxorubicin 25mg/m 2 iv bolus Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/2hrs Days 1 & 2 (3 before surgery) Ensure adequate renal function Pre & post-hydration,
Out-Patient Chemotherapy for Lung Cancer
 Lung Cancer Out-Patient Chemotherapy for Lung Cancer Principles and practice JMAJ 46(12): 542 546, 2003 Shuichi YONEDA Director, Department of Pulmonary Medicine, Saitama Cancer Center Abstract: Recent
Lung Cancer Out-Patient Chemotherapy for Lung Cancer Principles and practice JMAJ 46(12): 542 546, 2003 Shuichi YONEDA Director, Department of Pulmonary Medicine, Saitama Cancer Center Abstract: Recent
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study
 Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Cycle frequency: Every four weeks Total number of cycles: 6-8
 Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
Small Cell Lung Cancer
 Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Small Cell Lung Cancer
 Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP)
 BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP) Protocol Code Tumour Group Contact Physician UGUTIP Genitourinary Dr.
BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP) Protocol Code Tumour Group Contact Physician UGUTIP Genitourinary Dr.
Chapter 7: Lung Cancer
 Chapter 7: Lung Cancer Contents Chapter 7: Lung Cancer... 1 Small Cell... 2 Good PS + Limited stage... 2 Cisplatin/etoposide... 2 Concurrent chemotherapy + XRT... 2 Good / Intermediate PS... 2 Carboplatin
Chapter 7: Lung Cancer Contents Chapter 7: Lung Cancer... 1 Small Cell... 2 Good PS + Limited stage... 2 Cisplatin/etoposide... 2 Concurrent chemotherapy + XRT... 2 Good / Intermediate PS... 2 Carboplatin
Management of stage III A-B of NSCLC. Hamed ALHusaini Medical Oncologist
 Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology. Endorsement of the American College of Chest Physicians (ACCP) Guideline
 Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline Table of Contents Data Supplement 1: Summary of included
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline Table of Contents Data Supplement 1: Summary of included
Cycle frequency: Every three weeks Total number of cycles: 3 or 4
 BEP 3-day (Bleomycin/Etoposide/Cisplatin) Germ cell tumours Bleomycin 30,000iu 200mls N. Saline/30mins Days 2, 8 & 15 Etoposide 165mg/m 2 1L N. Saline/1hr Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/4hrs
BEP 3-day (Bleomycin/Etoposide/Cisplatin) Germ cell tumours Bleomycin 30,000iu 200mls N. Saline/30mins Days 2, 8 & 15 Etoposide 165mg/m 2 1L N. Saline/1hr Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/4hrs
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases
 Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Controversies in Management of. Inoperable NSCLC. Inoperable NSCLC. Introduction:
 Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer
 Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
J Clin Oncol 22:1872-1877. 2004 by American Society of Clinical Oncology INTRODUCTION
 VOLUME 22 NUMBER 10 MAY 15 2004 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Results of a Phase II Study of Weekly Paclitaxel Plus Carboplatin in Patients With Extensive Small-Cell Lung Cancer
VOLUME 22 NUMBER 10 MAY 15 2004 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Results of a Phase II Study of Weekly Paclitaxel Plus Carboplatin in Patients With Extensive Small-Cell Lung Cancer
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
West of Scotland Cancer Network Chemotherapy Protocol. Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021)
 West of Scotland Cancer Network Chemotherapy Protocol Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021) Indication Palliative chemotherapy for malignant mesothelioma of the pleura Eligibility
West of Scotland Cancer Network Chemotherapy Protocol Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021) Indication Palliative chemotherapy for malignant mesothelioma of the pleura Eligibility
Network Chemotherapy Regimens
 Network Chemotherapy Regimens Lung Cancer Notes from the editor Thames Valley Cancer Network These protocols are available on the Network website www.tvcn.org.uk. Any correspondence about the protocols
Network Chemotherapy Regimens Lung Cancer Notes from the editor Thames Valley Cancer Network These protocols are available on the Network website www.tvcn.org.uk. Any correspondence about the protocols
Radiation Therapy in the Treatment of
 Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Small Cell Lung Cancer* State-of-the-Art Therapy 1994
 Small Cell Lung Cancer* State-of-the-Art Therapy 1994 Daniel C. Ihde, MD In the United States, small cell lung cancer (SCLC) accounts for about 20% of all cases of lung cancer. Without treatment, tumor
Small Cell Lung Cancer* State-of-the-Art Therapy 1994 Daniel C. Ihde, MD In the United States, small cell lung cancer (SCLC) accounts for about 20% of all cases of lung cancer. Without treatment, tumor
Cesare Gridelli 1, Francesca Casaluce 2, Assunta Sgambato 2, Fabio Monaco 3, Cesare Guida 4
 Editorial Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that s the question Cesare Gridelli 1, Francesca
Editorial Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that s the question Cesare Gridelli 1, Francesca
New Perspectives on an Old Friend: Optimizing Carboplatin for the Treatment of Solid Tumors
 New Perspectives on an Old Friend: Optimizing Carboplatin for the Treatment of Solid Tumors DAVID S. ALBERTS, ROBERT T. DORR University of Arizona, Arizona Cancer Center, Tucson, Arizona, USA Key Words.
New Perspectives on an Old Friend: Optimizing Carboplatin for the Treatment of Solid Tumors DAVID S. ALBERTS, ROBERT T. DORR University of Arizona, Arizona Cancer Center, Tucson, Arizona, USA Key Words.
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Type of intervention Treatment. Economic study type Cost-effectiveness analysis.
 Impact of uncertainty on cost-effectiveness analysis of medical strategies: the case of highdose chemotherapy for breast cancer patients Marino P, Siani C, Roche H, Moatti J P Record Status This is a critical
Impact of uncertainty on cost-effectiveness analysis of medical strategies: the case of highdose chemotherapy for breast cancer patients Marino P, Siani C, Roche H, Moatti J P Record Status This is a critical
Update on Small Cell Lung Cancer
 Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
More than half of all lung cancer patients present with. New Combinations in the Treatment of Lung Cancer* A Time for Optimism
 New Combinations in the Treatment of Lung Cancer* A Time for Optimism Paul A. Bunn, Jr., MD; and Karen Kelly, MD Strides have been made in the treatment of lung cancer in the last decade that warrant a
New Combinations in the Treatment of Lung Cancer* A Time for Optimism Paul A. Bunn, Jr., MD; and Karen Kelly, MD Strides have been made in the treatment of lung cancer in the last decade that warrant a
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
Pulmonary function. Is patient potentially operable? Yes. tests 3. Yes. Pulmonary function. tests 3, if clinically indicated. Yes
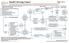 INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
Table of Contents. Data Supplement 1: Summary of ASTRO Guideline Statements. Data Supplement 2: Definition of Terms
 Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
U.S. Food and Drug Administration
 U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
Cycle frequency: Every three weeks Total number of cycles: 6
 Carboplatin/Paclitaxel Ovarian Cancer Adjuvant, Advanced Paclitaxel 175mg/m 2 500mls 5% dex/3hrs Day 1 Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Anti-emetic group - Moderately high Paclitaxel given first
Carboplatin/Paclitaxel Ovarian Cancer Adjuvant, Advanced Paclitaxel 175mg/m 2 500mls 5% dex/3hrs Day 1 Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Anti-emetic group - Moderately high Paclitaxel given first
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer
 Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
Avastin in Metastatic Breast Cancer
 Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
This 2-part article will discuss 9 anticancer. Commonly Used Chemotherapy Drugs Part 1. M e d i c a t i o n s. Clinician s Brief.
 M e d i c a t i o n s O N C O L O G Y Peer Reviewed Antony Moore, BVSc, MVSc, Diplomate ACVIM (Oncology) Veterinary Oncology Consultants, Sydney, Australia Commonly Used Chemotherapy Drugs Part 1 This
M e d i c a t i o n s O N C O L O G Y Peer Reviewed Antony Moore, BVSc, MVSc, Diplomate ACVIM (Oncology) Veterinary Oncology Consultants, Sydney, Australia Commonly Used Chemotherapy Drugs Part 1 This
London Cancer. Mesothelioma Lung Protocols
 London Cancer Mesothelioma Lung Protocols Version 0.9 Contents 1. Staging... 3 2. Mesothelioma Summary of Chemotherapy Protocols... 4 3. Mesothelioma Chemotherapy Protocols... 7 3.1. Pemetrexed (Alimta
London Cancer Mesothelioma Lung Protocols Version 0.9 Contents 1. Staging... 3 2. Mesothelioma Summary of Chemotherapy Protocols... 4 3. Mesothelioma Chemotherapy Protocols... 7 3.1. Pemetrexed (Alimta
Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy on limited small cell lung cancer
 [Chinese Journal of Cancer 27:10, 353-357; October 2008]; 2008 Sun Yat-Sen University Cancer Center Clinical Research Paper Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy
[Chinese Journal of Cancer 27:10, 353-357; October 2008]; 2008 Sun Yat-Sen University Cancer Center Clinical Research Paper Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy
SAKK Lung Cancer Group. Current activities and future projects
 SAKK Lung Cancer Group Current activities and future projects SAKK Lung Cancer Group Open group of physicians interested in lung cancer Mostly Medical Oncologists, but also Thoracic Surgeons Radiation
SAKK Lung Cancer Group Current activities and future projects SAKK Lung Cancer Group Open group of physicians interested in lung cancer Mostly Medical Oncologists, but also Thoracic Surgeons Radiation
4.8 Lung cancer. 4.8.5 In the UK, three fractionation regimens are most commonly used:
 4.8 Lung cancer 4.8.1 In 2005, both NICE (National Institute for Health and Clinical Excellence) and SIGN (Scottish Intercollegiate Guidelines Network) published guidelines on the management of lung cancer.
4.8 Lung cancer 4.8.1 In 2005, both NICE (National Institute for Health and Clinical Excellence) and SIGN (Scottish Intercollegiate Guidelines Network) published guidelines on the management of lung cancer.
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
Management of Platinum-Sensitive Recurrent Ovarian Cancer
 Management of Platinum-Sensitive Jacobus Pfisterer a and Jonathan A. Ledermann b The majority of patients with ovarian cancer will relapse despite state-of-the-art first-line surgery and chemotherapy.
Management of Platinum-Sensitive Jacobus Pfisterer a and Jonathan A. Ledermann b The majority of patients with ovarian cancer will relapse despite state-of-the-art first-line surgery and chemotherapy.
Bendamustine for the fourth-line treatment of multiple myeloma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine for the fourth-line treatment of multiple myeloma Contents Summary 1 Background 2 Epidemiology 3 Cost 6 References 7 Summary There is no standard
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine for the fourth-line treatment of multiple myeloma Contents Summary 1 Background 2 Epidemiology 3 Cost 6 References 7 Summary There is no standard
Advances In Chemotherapy For Hormone Refractory Prostate Cancer. TAX 327 study results & SWOG 99-16 study results presented at ASCO 2004
 Ronald de Wit Rotterdam Cancer Institute The Netherlands Advances In Chemotherapy For Hormone Refractory Prostate Cancer TAX 327 study results & SWOG 99-16 study results presented at Slide 1 Prostate Cancer
Ronald de Wit Rotterdam Cancer Institute The Netherlands Advances In Chemotherapy For Hormone Refractory Prostate Cancer TAX 327 study results & SWOG 99-16 study results presented at Slide 1 Prostate Cancer
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer
 Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER
 CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER INTRODUCTION This chapter provides an overview of treatment for small cell lung cancer (SCLC). Treatment options are presented based on the extent of disease.
CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER INTRODUCTION This chapter provides an overview of treatment for small cell lung cancer (SCLC). Treatment options are presented based on the extent of disease.
Post-operative intrapleural chemotherapy for mesothelioma
 Post-operative intrapleural chemotherapy for mesothelioma Robert Kratzke, MD John Skoglund Chair for Lung Cancer Research Section of Heme-Onc-Transplant University of Minnesota Medical School Efficacy
Post-operative intrapleural chemotherapy for mesothelioma Robert Kratzke, MD John Skoglund Chair for Lung Cancer Research Section of Heme-Onc-Transplant University of Minnesota Medical School Efficacy
Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment.
 1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
Once vs. Twice Daily Thoracic Irradiation in Limited Stage Small Cell Lung Cancer
 J. Korean Soc Ther Radiol Oncol : Vol. 16, No. 3, September, 1998 Once vs. Twice Daily Thoracic Irradiation in Limited Stage Small Cell Lung Cancer Jun Sang Kim, M.D.*, Jae Sung Kim, M.D.*, Ju Ock Kim,
J. Korean Soc Ther Radiol Oncol : Vol. 16, No. 3, September, 1998 Once vs. Twice Daily Thoracic Irradiation in Limited Stage Small Cell Lung Cancer Jun Sang Kim, M.D.*, Jae Sung Kim, M.D.*, Ju Ock Kim,
Adjuvant Therapy Non Small Cell Lung Cancer. Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015
 Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
Scottish Medicines Consortium
 Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
SECOND-LINE CHEMOTHERAPY in advanced non
 Gemcitabine as Second-Line Treatment for Advanced Non Small-Cell Lung Cancer: A Phase II Trial By Lucio Crinò, Anna Maria Mosconi, Giorgio Scagliotti, Giovanni Selvaggi, Silvia Novello, Massimo Rinaldi,
Gemcitabine as Second-Line Treatment for Advanced Non Small-Cell Lung Cancer: A Phase II Trial By Lucio Crinò, Anna Maria Mosconi, Giorgio Scagliotti, Giovanni Selvaggi, Silvia Novello, Massimo Rinaldi,
January 2013 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW. Summary. Contents
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS
 MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
Single-Agent Paclitaxel in the Treatment of Advanced Non-Small Cell Lung Cancer
 Single-Agent Paclitaxel in the Treatment of Advanced Non-Small Cell Lung Cancer MARK A. SOCINSKI The Multidisciplinary Thoracic Oncology Program, University of North Carolina, Chapel Hill, North Carolina,
Single-Agent Paclitaxel in the Treatment of Advanced Non-Small Cell Lung Cancer MARK A. SOCINSKI The Multidisciplinary Thoracic Oncology Program, University of North Carolina, Chapel Hill, North Carolina,
Chemotherapy in Ovarian Cancer. Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group
 Chemotherapy in Ovarian Cancer Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group Adjuvant chemotherapy for early stage EOC Fewer than 30% women present with FIGO stage
Chemotherapy in Ovarian Cancer Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group Adjuvant chemotherapy for early stage EOC Fewer than 30% women present with FIGO stage
BREAST CANCER - METASTATIC & LOCALLY ADVANCED CHEMOTHERAPY REGIMENS Capecitabine. Capecitabine + Docetaxel. Capecitabine + Vinorelbine
 Capecitabine Capecitabine 1000-1250mg/m 2 oral TWICE daily for 14 days Until disease progression Capecitabine + Docetaxel Capecitabine 750-1000mg/m 2 oral TWICE daily for 14 days Up to 6 cycles Capecitabine
Capecitabine Capecitabine 1000-1250mg/m 2 oral TWICE daily for 14 days Until disease progression Capecitabine + Docetaxel Capecitabine 750-1000mg/m 2 oral TWICE daily for 14 days Up to 6 cycles Capecitabine
Stage IIIB disease includes patients with T4 tumors,
 Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
Review. The combination of etoposide and cisplatin in non-small-cell lung cancer (NSCLC)
 Annals of Oncology 10 (Suppl. 5): S13-S17,1999. 1999 Kluwer Academic Publishers. Printed in the Netherlands. Review The combination of etoposide and cisplatin in non-small-cell lung cancer (NSCLC) A. Ardizzoni,
Annals of Oncology 10 (Suppl. 5): S13-S17,1999. 1999 Kluwer Academic Publishers. Printed in the Netherlands. Review The combination of etoposide and cisplatin in non-small-cell lung cancer (NSCLC) A. Ardizzoni,
Thames Valley Chemotherapy Regimens
 Chemotherapy Regimens Lung Cancer Notes from the editor These regimens are available on the Network website www.tvcn.org.uk. Any correspondence about the regimens should be addressed to: Sally Coutts,
Chemotherapy Regimens Lung Cancer Notes from the editor These regimens are available on the Network website www.tvcn.org.uk. Any correspondence about the regimens should be addressed to: Sally Coutts,
Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with relatively good performance status
 Available online at www.sciencedirect.com Journal of the Chinese Medical Association 74 (2011) 209e214 Original Article Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with
Available online at www.sciencedirect.com Journal of the Chinese Medical Association 74 (2011) 209e214 Original Article Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with
New Approaches for Small-Cell Lung Cancer: Local Treatments
 The current management of limited-disease small-cell lung cancer is reviewed, and strategies to improve local control are discussed. Gary Ernest Smith. Sorting Old Bales. Oil on canvas, 18 24. Courtesy
The current management of limited-disease small-cell lung cancer is reviewed, and strategies to improve local control are discussed. Gary Ernest Smith. Sorting Old Bales. Oil on canvas, 18 24. Courtesy
the standard of care 2009 5/1/2009 Mesothelioma: The standard of care take home messages PILC 2006 Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009
 Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
Long Term Low Dose Maintenance Chemotherapy in the Treatment of Acute Myeloid Leukemia
 Long Term Low Dose Chemotherapy in the Treatment of Acute Myeloid Leukemia Murat TOMBULO LU*, Seçkin ÇA IRGAN* * Department of Hematology, Faculty of Medicine, Ege University, zmir, TURKEY ABSTRACT In
Long Term Low Dose Chemotherapy in the Treatment of Acute Myeloid Leukemia Murat TOMBULO LU*, Seçkin ÇA IRGAN* * Department of Hematology, Faculty of Medicine, Ege University, zmir, TURKEY ABSTRACT In
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
Prior Authorization Guideline Guideline: PS Inj - Velcade Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antineoplastic Client: PS Inj Approval Date: 10/2/2004 Revision Date: 5/22/2007
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Feline Lymphoma Chemotherapy and Chemotherapy Protocols
 Feline Lymphoma Chemotherapy and Chemotherapy Protocols If you have reached this page, your cat probably has a definite diagnosis of feline lymphoma from your veterinarian. The information below is not
Feline Lymphoma Chemotherapy and Chemotherapy Protocols If you have reached this page, your cat probably has a definite diagnosis of feline lymphoma from your veterinarian. The information below is not
Practice of Interferon Therapy
 Interferon Therapy Practice of Interferon Therapy Multiple myeloma and other related hematological malignancies JMAJ 47(1): 32 37, 2004 Akihisa KANAMARU* and Takashi ASHIDA** *Professor, **Lecturer, Department
Interferon Therapy Practice of Interferon Therapy Multiple myeloma and other related hematological malignancies JMAJ 47(1): 32 37, 2004 Akihisa KANAMARU* and Takashi ASHIDA** *Professor, **Lecturer, Department
Treatment of Small Cell Lung Cancer
 Chapter 5 Treatment of Small Cell Lung Cancer Ariel Lopez-Chavez MD, MS Introduction There are two main types of lung cancer, non-small cell lung cancer and small cell lung cancer. 1 It is important that
Chapter 5 Treatment of Small Cell Lung Cancer Ariel Lopez-Chavez MD, MS Introduction There are two main types of lung cancer, non-small cell lung cancer and small cell lung cancer. 1 It is important that
Frequency of NHL Subtypes in Adults
 Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Hodgkin Lymphoma Disease Specific Biology and Treatment Options. John Kuruvilla
 Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Preliminary Results from a Phase 2 Study of ARQ 197 in Patients with Microphthalmia Transcription Factor Family (MiT) Associated Tumors
 Preliminary Results from a Phase 2 Study of ARQ 197 in Patients with Microphthalmia Transcription Factor Family (MiT) Associated Tumors John Goldberg 1 *, George Demetri 2, Edwin Choy 3, Lee Rosen 4, Alberto
Preliminary Results from a Phase 2 Study of ARQ 197 in Patients with Microphthalmia Transcription Factor Family (MiT) Associated Tumors John Goldberg 1 *, George Demetri 2, Edwin Choy 3, Lee Rosen 4, Alberto
Moving towards novel treatments in mesothelioma. Paul Baas, The Netherlands Cancer Institute Rolf Stahel, University Clinic Zurich
 Moving towards novel treatments in mesothelioma Paul Baas, The Netherlands Cancer Institute Rolf Stahel, University Clinic Zurich Malignant pleural mesothelioma (MPM) has proven to be quite resistant to
Moving towards novel treatments in mesothelioma Paul Baas, The Netherlands Cancer Institute Rolf Stahel, University Clinic Zurich Malignant pleural mesothelioma (MPM) has proven to be quite resistant to
NCCN Non-Small Cell Lung Cancer V.1.2011 Update Meeting 07/09/10
 Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
IMMUNOMEDICS, INC. February 2016. Advanced Antibody-Based Therapeutics. Oncology Autoimmune Diseases
 IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases February 2016 Forward-Looking Statements This presentation, in addition to historical information, contains certain
IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases February 2016 Forward-Looking Statements This presentation, in addition to historical information, contains certain
INFORMATION ON STEM CELLS/BONE MARROW AND REINFUSION/TRANSPLANTATION SUR703.002
 INFORMATION ON STEM CELLS/BONE MARROW AND REINFUSION/TRANSPLANTATION SUR703.002 COVERAGE: SPECIAL COMMENT ON POLICY REVIEW: Due to the complexity of the Peripheral and Bone Marrow Stem Cell Transplantation
INFORMATION ON STEM CELLS/BONE MARROW AND REINFUSION/TRANSPLANTATION SUR703.002 COVERAGE: SPECIAL COMMENT ON POLICY REVIEW: Due to the complexity of the Peripheral and Bone Marrow Stem Cell Transplantation
New Treatment Options for Breast Cancer
 New Treatment Options for Breast Cancer Brandon Vakiner, PharmD., BCOP Clinical Pharmacy Specialist - Oncology The University of Iowa Hospitals and Clinics Assistant Professor (Clinical) University of
New Treatment Options for Breast Cancer Brandon Vakiner, PharmD., BCOP Clinical Pharmacy Specialist - Oncology The University of Iowa Hospitals and Clinics Assistant Professor (Clinical) University of
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook
 National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
La Chemioterapia Adiuvante Dose-Dense. Lo studio GIM 2. Alessandra Fabi
 La Chemioterapia Adiuvante Dose-Dense Lo studio GIM 2 Alessandra Fabi San Antonio Breast Cancer Symposium -December 10-14, 2013 GIM 2 study Epirubicin and Cyclophosphamide (EC) followed by Paclitaxel (T)
La Chemioterapia Adiuvante Dose-Dense Lo studio GIM 2 Alessandra Fabi San Antonio Breast Cancer Symposium -December 10-14, 2013 GIM 2 study Epirubicin and Cyclophosphamide (EC) followed by Paclitaxel (T)
Key words: chemotherapy; evidence-based medicine; guidelines; non-small cell lung cancer
 Chemotherapeutic Management of Stage IV Non-small Cell Lung Cancer* Mark A. Socinski, MD, FCCP; David E. Morris, MD; Gregory A. Masters, MD, FCCP; and Rogerio Lilenbaum, MD Stage IV non-small cell lung
Chemotherapeutic Management of Stage IV Non-small Cell Lung Cancer* Mark A. Socinski, MD, FCCP; David E. Morris, MD; Gregory A. Masters, MD, FCCP; and Rogerio Lilenbaum, MD Stage IV non-small cell lung
Ovarian Cancer and Modern Immunotherapy: Regulatory Strategies for Drug Development
 Ovarian Cancer and Modern Immunotherapy: Regulatory Strategies for Drug Development Sanjeeve Bala, MD, MPH Ovarian Cancer Endpoints Workshop FDA White Oak September 3, 2015 Overview Immune agents from
Ovarian Cancer and Modern Immunotherapy: Regulatory Strategies for Drug Development Sanjeeve Bala, MD, MPH Ovarian Cancer Endpoints Workshop FDA White Oak September 3, 2015 Overview Immune agents from
Before, Frank's immune cells could
 Before, Frank's immune cells could barely recognize a prostate cancer cell. Now, they are focused on it. Stimulate an immune response against advanced prostate cancer Extend median survival beyond 2 years
Before, Frank's immune cells could barely recognize a prostate cancer cell. Now, they are focused on it. Stimulate an immune response against advanced prostate cancer Extend median survival beyond 2 years
Clair Clark, Cancer Care Pharmacist Beatson West of Scotland Cancer Centre
 Clair Clark, Cancer Care Pharmacist Beatson West of Scotland Cancer Centre An audit of neutropenic complications in breast cancer patients receiving adjuvant or neo-adjuvant chemotherapy with FEC-D in
Clair Clark, Cancer Care Pharmacist Beatson West of Scotland Cancer Centre An audit of neutropenic complications in breast cancer patients receiving adjuvant or neo-adjuvant chemotherapy with FEC-D in
Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
Aggressive lymphomas. Michael Crump Princess Margaret Hospital
 Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Sonneveld, P; de Ridder, M; van der Lelie, H; et al. J Clin Oncology, 13 (10) : 2530-2539 Oct 1995
 Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
Introduction. About 10,500 new cases of acute myelogenous leukemia are diagnosed each
 Introduction 1.1 Introduction: About 10,500 new cases of acute myelogenous leukemia are diagnosed each year in the United States (Hope et al., 2003). Acute myelogenous leukemia has several names, including
Introduction 1.1 Introduction: About 10,500 new cases of acute myelogenous leukemia are diagnosed each year in the United States (Hope et al., 2003). Acute myelogenous leukemia has several names, including
cancer cancer Hessamfar-Bonarek M et al. Int. J. Epidemiol. 2010;39:135-146
 Hematopoietic Stem Cell Transplant in HIV- related lymphoma Song Zhao, MD PhD Hematology-Oncology Program University of Washington/FHCRC Underlying Causes of Death in HIV-infected Adults 2000 2005 cancer
Hematopoietic Stem Cell Transplant in HIV- related lymphoma Song Zhao, MD PhD Hematology-Oncology Program University of Washington/FHCRC Underlying Causes of Death in HIV-infected Adults 2000 2005 cancer
How To Know If You Have Small Cell Lung Cancer
 Lung cancer Lung cancer TABLE 85 1. MALIGNANT PULMONARY NEOPLASMS INCIDENCE (%) Common 99 Non small cell lung cancer ~75 Adenocarcinoma ~35 Squamous cell carcinoma ~30 Large cell carcinoma ~10 Small cell
Lung cancer Lung cancer TABLE 85 1. MALIGNANT PULMONARY NEOPLASMS INCIDENCE (%) Common 99 Non small cell lung cancer ~75 Adenocarcinoma ~35 Squamous cell carcinoma ~30 Large cell carcinoma ~10 Small cell
