SECOND-LINE CHEMOTHERAPY in advanced non
|
|
|
- Jonathan Howard
- 8 years ago
- Views:
Transcription
1 Gemcitabine as Second-Line Treatment for Advanced Non Small-Cell Lung Cancer: A Phase II Trial By Lucio Crinò, Anna Maria Mosconi, Giorgio Scagliotti, Giovanni Selvaggi, Silvia Novello, Massimo Rinaldi, Marina Della Giulia, Cesare Gridelli, Antonio Rossi, Cesare Calandri, Filippo De Marinis, Mariantonietta Noseda, and Maurizio Tonato Purpose: To investigate the activity and toxicity of gemcitabine as a single agent in patients with advanced non small-cell lung cancer (NSCLC) after recurrence or failure of previous treatment with a platinum-containing regimen. Patients and Methods: From November 1995 to October 1997, 83 patients with stage IIIB or IV NSCLC received gemcitabine 1,000 mg/m 2 once a week for 3 weeks every 28 days. Responses were assessed every two treatment courses. The median age of the patients was 63 years; Eastern Cooperative Oncology Group performance status was 0 to 1 in 62 patients and 2 in 21 patients. The predominant histology was squamous (39 patients); 49 patients had stage IV disease and 34 patients had stage III disease (33 stage IIIB and one stage IIIA). Results: Sixteen patients (19%) achieved a partial response to treatment; the median duration of response was 29 weeks (range, 6 to 50 weeks). Treatment was well tolerated: grade 2 to 3 (World Health Organization standardized response criteria) leukopenia and thrombocytopenia occurred in 23% and 20% of patients, respectively. Mild asthenia was observed in 16% of patients, and peripheral edema in 5% of patients. Nausea and vomiting were present in 16% of patients. Conclusion: In this experience, gemcitabine showed significant activity without relevant toxicity, mainly in patients who were previously responsive to chemotherapy. This suggests a possible role for gemcitabine as a second-line treatment in patients who had a previous response or achieved stable disease with a platinumcontaining regimen. J Clin Oncol 17: by American Society of Clinical Oncology. SECOND-LINE CHEMOTHERAPY in advanced non small-cell lung cancer (NSCLC) is not usually indicated, as all attempts have been, until recently, almost completely unsuccessful. 1 On the other hand, several metaanalyses have shown that the survival benefit obtained with first-line platinum-based chemotherapy for advanced NSCLC is limited to a few weeks, 2,3 and in pretreated patients with recurrent or progressive NSCLC, the rapid worsening of general conditions often contraindicates further treatment. However, thanks to the availability of a number of new drugs with favorable efficacy/toxicity profiles and that seem to have a positive impact on survival for patients with NSCLC, 4 there now seem to be increasing opportunities for single-agent treatment in patients with advanced disease when first-line chemotherapy has failed. Among these new drugs, gemcitabine (2,2 difluorodeoxycytidine), a novel pyrimidine antimetabolite, has been shown in several phase II trials of more than 500 advanced NSCLC patients to induce as a single agent a response rate of 20%, as well as an improvement of major disease symptoms (cough, dyspnea, hemoptysis, pleural effusion, and pain) in a large portion of this patient population. 5 Gemcitabine has a unique mechanism of action with multiple cellular targets and shows a peculiar development of drug resistance. The most widely observed in vitro resistance mechanism is deoxycytidine kinase deficiency; this was achieved for the first time through continuous exposure of A2780 human ovarian cancer cells to gemcitabine, which resulted in a 150,000-fold resistant cell line. 6 The main molecular defect was a deficiency of deoxycytidine kinase, and resistance was associated with the complete absence of gemcitabine triphosphate accumulation. Resistance to gemcitabine in vivo is quite difficult to achieve. Its development mechanism is not yet clear and seems largely to depend on drug infusion scheduling, with continuous infusion being the most active and toxic schedule. 7 On the basis of the clinical activity of gemcitabine, its peculiar mechanism of resistance (which is not mediated by P170 glycoprotein), and its activity in platinum-resistant cancer cell lines, 8,9 we decided to evaluate the efficacy and From the Medical Oncology Division, Policlinico Hospital, Perugia; Department of Clinical and Biological Sciences, University of Torino, Torino; Medical Oncology Division, Regina Elena, Rome; Medical Oncology B, National Cancer Institute G. Pascale, Naples; and III Pneumology Division, Forlanini Hospital, Rome, Italy. Submitted July 23, 1998; accepted March 17, The preliminary results of this trial were presented at the 33rd Annual Meeting of the American Society of Clinical Oncology, Denver, CO, May 17-20, Address reprint requests to Lucio Crinò, MD, Medical Oncology Division, Policlinico Hospital, Perugia, Italy; oncmedpg@krenet.it by American Society of Clinical Oncology X/99/ Journal of Clinical Oncology, Vol 17, No 7 (July), 1999: pp
2 2082 CRINÒ ET AL toxicity profile of gemcitabine as a single agent in a phase II trial in advanced NSCLC patients who have recurrent or refractory disease after one or more chemotherapy regimens. PATIENTS AND METHODS Patients with histologically or cytologically confirmed unresectable or metastatic NSCLC entered the trial, provided that they had a performance status (PS) of 0 to 2 (Eastern Cooperative Oncology Group), a life expectancy of 3 months, and adequate bone marrow, hepatic, and renal function. Patients with concurrent severe cardiac, metabolic, or infectious diseases were excluded. Patients were eligible if they had received one or more platinum-based chemotherapy regimens, regardless of their response to previous treatment. Definitions of response (ie, partial or complete response), stable disease, and progressive disease were based on the standardized response criteria established by the World Health Organization (WHO). Patients who either relapse after adjuvant chemotherapy or experience recurrent disease after an initial response, or who have resistant or stable disease, were enrolled after giving informed consent. Patients who had received radiotherapy were entered provided that measurable lesions were outside of radiotherapy fields. Patients with brain metastases were enrolled if they did not require emergency radiotherapy treatment. Within 2 weeks of study registration, disease was staged with chest x-ray, abdomen ultrasound, and/or total body computed tomography (CT) scan. During treatment courses, patients were monitored with a weekly blood count on the day of planned gemcitabine administration. Toxicities were graded according to WHO criteria before each therapy course. WHO response criteria were used for efficacy analysis; responses were assessed in alternate therapy cycles with CT scan or radiologic and ultrasound evaluation of the lesions. Survival and response were both determined according to the intention-to-treat principle in all enrolled patients. Duration of response and survival were calculated starting from the beginning of second-line chemotherapy treatment. Treatment Plan Second-line gemcitabine treatment was administered after a minimum 4-week interval after previous chemotherapy (range, 4 to 119 weeks) in patients showing progressive disease. Gemcitabine was administered at a 1,000 mg/m 2 dose intravenously over a 30-minute period on days 1, 8, and15, followed by a 1-week rest (28-day cycles). Doses were reduced by 50% if patients experienced leukopenia (WBC count 1,500/dL) and thrombocytopenia (platelet count 100,000/ dl). Chemotherapy was omitted if WBC count and platelet count were less than 1,000/dL and 50,000/dL, respectively. Treatment was discontinued if disease progression or major toxicities occurred, or according to patient s and/or physician s decision. Characteristic Table 1. Patient Characteristics Patients Total no. of patients 83 Male 73 Female 10 Age, years Median 63 Range PS Stage IIIA 1 IIIB 33 IV 49 Histology Squamous 39 Adenocarcinoma 37 Large cell 4 Unclassified non small-cell 3 Previous chemotherapy Adjuvant 3 First-line 67 Second-line 11 Third-line 2 RESULTS From November 1995 to October 1997, 83 patients entered the study. Patient characteristics are listed in Table 1. Most patients (75%) had a favorable PS (0 to 1); 59% of patients had stage IV disease, and the most common histologic type was squamous (47%). Prior chemotherapy regimens are listed in Table 2; three patients had relapsed after adjuvant chemotherapy, 13 were pretreated patients who had received more than one chemotherapy regimen, and 67 patients had received only first-line chemotherapy. Twenty-four of the 83 patients had initially responded to platinum-based chemotherapy. Thirty-two patients had obtained stable disease and 24 had experienced progressive disease while on first-line chemotherapy. Three patients had relapsed after adjuvant cisplatin-based chemotherapy. The median time between the completion of firstline treatment and starting of second-line gemcitabine was 22 weeks (range, 4 to 119 weeks). Responses were evaluated in all 83 patients. Sixteen patients (19.28%; 95% confidence interval, 10.79% to 27.77%) achieved partial responses, 26 patients achieved stable disease, and 41 experienced progressive disease (Table 3). Regimen* Table 2. Prior Chemotherapy Patients MIC 26 Cisplatin or carboplatin paclitaxel 13 Cisplatin or carboplatin vinorelbine 29 Cisplatin-gemcitabine 7 MVP 6 Cisplatin or carboplatin etoposide 8 Vinorelbine alone 7 Anthracycline-based regimens 3 Carboplatin-ifosfamide 1 Abbreviations: MIC, mitomycin, ifosfamide, cisplatin; MVP, mitomycin, vindesine, cisplatin. *Each patient could have received more than one regimen. All of these patients had also received platinum-based regimens.
3 SECOND-LINE GEMCITABINE IN NSCLC 2083 Table 3. Response to Treatment (all patients) Complete response 0 Partial response Overall response Stable disease Progression 41 Median Duration of response, weeks 29 Range 6-50 The median number of cycles administered was three (range, one to 12 cycles). A total of 290 chemotherapy courses were given, with a potential 870 gemcitabine injections. Thirty-four injections (4%) were given at a reduced dose, 54 (6%) were omitted. Median duration of response (from start of treatment to first documented disease progression) was 29 weeks (range, 6 to 50 weeks). Eleven of the 16 responses occurred in chemosensitive patients (ie, patients who were responsive to previous treatment); four responses occurred in patients with stable disease, and only one response was achieved in a patient who experienced disease progression during platinum treatment. The responses occurred with equal frequency in all histologic subtypes, in patients with stage IIIB disease as well as in patients with stage IV disease. Of 11 responding patients with stage IV disease, five had metastases only in the contralateral lung, and two had brain metastases in continuous complete remission after previous treatments and achieved partial response in lung lesions. Of the remaining responding patients, two achieved objective responses not only in the lung, but also in the brain and in the skin; two other patients achieved partial responses in lung lesions, with minor response or stable disease in the adrenals and bone (one patient each). Three (14%) of 21 patients having performance status of 2 (two with stage IV disease and one with stage IIIB disease) had partial responses. The responses were observed in all but one participating center. Three of seven patients previously treated with a gemcitabinecisplatin combination had partial responses to second-line gemcitabine. All three patients had achieved partial response to the first-line combination. Two started second-line gemcitabine after 12 and 56 weeks from completion of first-line treatment because disease progression occurred. The third patient started gemcitabine after a 5-week interval from completion of first-line treatment for progressive disease and obtained a partial remission after three courses of secondline treatment. The median survival time for all patients from the start of gemcitabine chemotherapy was 34 weeks (range, 5 to 112 weeks) and the 1-year survival rate was 45%. The median survival time calculated for all patients from initial chemotherapy was 72 weeks (range, 19 to 331 weeks). Toxicity Toxicity was evaluated for all patients (Table 4). Hematologic toxicity was the main side effect in this trial. WHO grade 3 and 4 leucopenia occurred in five (6%) and one (1%) patient, respectively. Six patients (7%) had grade 3 thrombocytopenia at some time during the treatment. There was no evidence of cumulative hematologic toxicity. Duration of leucopenia was brief, no infectious episodes occurred, and growth factors were never administered during the trial. Nonhematologic toxicity consisted mainly of mild transient fever (27%) and fatigue (16%) after gemcitabine administration. DISCUSSION In recent years, the role of chemotherapy has seemed to extend to the treatment of advanced NSCLC, mainly thanks to new drugs with innovative mechanisms of action and mild toxicity profiles, which have widened the indications for chemotherapy in advanced and disseminated disease. Although there is no evidence that second-line chemotherapy can influence survival in nonresponding advanced NSCLC patients or in those who experience disease progression, there is some suggestion that second-line treatment may be appropriate for patients with good PS who experience disease progression or stable disease after first-line chemotherapy or for patients who responded to initial chemotherapy and then experienced a progression-free interval off treatment. 10 Parameter Table 4. WHO Toxicity Worst WHO Grade Attained Hemoglobin White blood cells Platelets Nausea/vomiting Renal Hepatic Pulmonary Fever Fatigue Hairloss Cutaneous Edema Hemorrhage
4 2084 CRINÒ ET AL Recently, Fossella et al 11 showed in a phase II trial of 44 patients the significant activity of docetaxel (100 mg/m 2 every 21 days) as second-line treatment in platinumrefractory NSCLC. In this study, a response rate of 21% (partial response), a median survival of 42 weeks, and a 1-year survival rate of 41% were achieved in a relatively favorable population of patients with good PS. These results have been confirmed by Gandara et al 12 in a Southwest Oncology Group phase II trial with docetaxel: in 80 patients with disease progression or recurrence after platinum-based therapy, 12 (16%) of 77 patients achieved partial responses, the median survival time was 7 months, and the 1-year survival rate was 25%. In these studies, the activity of docetaxel was shown in previously chemosensitive patients as well as in platinumrefractory patients. Dose-limiting side effects were reported to be neutropenia, fluid retention, and dermatitis. Other drugs have been evaluated in this setting with conflicting results. Paclitaxel and vinorelbine showed activity in some small studies, but this was not later confirmed in other similar trials. 13,14 Paclitaxel showed contrasting results in two phase II trials with a response rate of 3% in one study 15 and 30% in the other. 16 In this phase II study of 83 extensively pretreated patients, we showed that gemcitabine as a single agent is active as second-line treatment. Response rate (19% of patients achieved partial responses) was comparable to that obtained in phase II studies of chemotherapy-naive patients, suggesting that specific mechanisms of action, together with the peculiar development of drug resistance, can be involved in this prolonged activity in some patients. Our results are similar to those of Fossella et al, 11 both for activity and for median survival (34 weeks), although our study included a less favorable patient population (16% of patients with more than one line of chemotherapy) because of more extensive previous treatment and poorer PS (25% of patients had PS of 2). An important difference is that in our experience, all but one response was achieved in patients who had previous response or stable disease as a result of first-line platinum-based regimens. Recently, Langer et al 17 confirmed our results in a phase II study of 28 patients who relapsed after or were resistant to first-line carboplatin-paclitaxel treatment. Five of 24 assessable patients have sustained partial responses in pulmonary or nodal sites, in addition to four minor responses. The good median survival (in excess of 30 weeks) shown in all these studies would seem to indicate that some selection of patients with favorable PS could be an important predictive variable for response in second-line treatment, as well. In conclusion, we believe that the reported phase II experiences, together with our own trial, have provided some new insights into the difficult area of second-line treatment of advanced NSCLC. Selected previously responsive patients with good PS could benefit from a second-line treatment with new active drugs. Gemcitabine seems to be particularly promising because of its low toxicity profile, which allows a prolonged administration of the drug, and because of the proven difficulty for cancer cells to develop drug resistance. 18 Randomized trials that evaluate second-line chemotherapy with new drugs, either by direct comparison or versus best supportive care, are warranted. 1. Fossella FV, Lee SS, Kory WK: Management strategies for recurrent non-small cell lung cancer. Semin Oncol 24: , Soquet PJ, Chauvin F, Boissel JP, et al: Polychemotherapy in advanced non-small cell lung cancer: A meta-analysis. Lancet 342:19-21, Non-Small Cell Lung Collaborative Group: Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data on individual patients from 52 randomized clinical trials. BMJ 311: , Bunn PA, Kelly K: New chemotherapeutic agents prolong survival and improve quality of life in non-small cell lung cancer: A review of the literature and future directions. Clin Cancer Res 5: , Kaye SB: Gemcitabine: Current status of phase I and II trials. J Clin Oncol 12: , Ruiz van Haperen VWT, Veerman G, Eriksson S, et al: Development and characterization of a resistant variant of the human ovarian cancer cell line A2780. Cancer Res 54: , Ruiz van Haperen VWT, Veerman G, Boven E, et al: Scheduledependence of sensitivity to 2,2 -difluorodeoxycytidine (gemcitabine) in relation to accumulation and retention of its triphosphate in solid tumor cell lines and solid tumors. Biochem Pharmacol 48: , 1994 REFERENCES 8. Veerman G, Van Moorsel CJA, Ruiz Von Haperen VWT, et al: Induction of in vivo resistance against gemcitabine (2 2 difluorodeoxycytidine). Ann Oncol 7:122, 1996 (abstr 433) (suppl 1) 9. Bergman AM, Ruiz van Haperen, Veerman G, et al: Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res 2: , American Society of Clinical Oncology: Clinical Practice Guidelines for the treatment of unresectable non-small-cell lung cancer. J Clin Oncol 15: , Fossella FV, Lee JS, Shin DM, et al: Phase II study of docetaxel for advanced or metastatic platinum-refractory non-small cell lung cancer. J Clin Oncol 13: , Gandara DR, Vokes E, Green M, et al: Multicenter trial of Docetaxel (taxotere) in platinum-treated non-small cell lung cancer (NSCLC): Confirmation of prolonged survival. Lung Cancer 18:21, 1997 (abstr 72) (suppl 1) 13. Pronzato P, Landucci M, Vaira F, et al: Failure of vinorelbine to produce responses in pretreated non-small cell lung cancer patients. Anticancer Res 14: , Rinaldi M, Della Giulia M, Venturo I, et al: Vinorelbine as single agent in the treatment of advanced non-small cell lung cancer. Proc Am Soc Clin Oncol 13:360, 1994 (abstr 12129)
5 SECOND-LINE GEMCITABINE IN NSCLC Murphy WK, Win RJ, Huber M, et al: Phase II study of Taxol in patients with non-small cell lung cancer who have failed platinumcontaining chemotherapy. Proc Am Soc Clin Oncol 13:363, 1994 (abstr 1224) 16. Hainsworth JD, Thompson DS, Greco FA: Paclitaxel by 1-hour infusion: An active drug in metastatic non-small cell lung cancer. J Clin Oncol 13: , Rosvold E, Langer CJ, Schilder R, et al: Salvage therapy with gemcitabine in advanced non-small cell lung cancer (NSCLC) progressing after prior carboplatin-paclitaxel (C-P). Proc Am Soc Clin Oncol 17:467a, 1998 (abstr 1797) 18. Peters GJ, Ruiz van Haperen V, Bergman A, et al: Preclinical combination therapy with gemcitabine and mechanism of resistance. Semin Oncol 23:16-24, 1996 (suppl 10)
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
NCCN Non-Small Cell Lung Cancer V.1.2011 Update Meeting 07/09/10
 Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study
 Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with relatively good performance status
 Available online at www.sciencedirect.com Journal of the Chinese Medical Association 74 (2011) 209e214 Original Article Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with
Available online at www.sciencedirect.com Journal of the Chinese Medical Association 74 (2011) 209e214 Original Article Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer
 Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Out-Patient Chemotherapy for Lung Cancer
 Lung Cancer Out-Patient Chemotherapy for Lung Cancer Principles and practice JMAJ 46(12): 542 546, 2003 Shuichi YONEDA Director, Department of Pulmonary Medicine, Saitama Cancer Center Abstract: Recent
Lung Cancer Out-Patient Chemotherapy for Lung Cancer Principles and practice JMAJ 46(12): 542 546, 2003 Shuichi YONEDA Director, Department of Pulmonary Medicine, Saitama Cancer Center Abstract: Recent
Emerging Drug List GEFITINIB
 Generic (Trade Name): Manufacturer: Gefitinib (Iressa ) formerly referred to as ZD1839 AstraZeneca NO. 52 JANUARY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence:
Generic (Trade Name): Manufacturer: Gefitinib (Iressa ) formerly referred to as ZD1839 AstraZeneca NO. 52 JANUARY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence:
Taxotere: Clinical Trials in Non-Small Cell Lung Cancer -- Ornstein and Rigas 3 (2): 86...
 Page 1 of 11 HOME HELP CONTACT US SUBSCRIPTIONS ARCHIVE SEARCH SEARCH RESULT The Oncologist, Vol. 3, No. 2, 86-93, April 1998 1998 AlphaMed Press This Article Taxotere: Clinical Trials in Non-Small Cell
Page 1 of 11 HOME HELP CONTACT US SUBSCRIPTIONS ARCHIVE SEARCH SEARCH RESULT The Oncologist, Vol. 3, No. 2, 86-93, April 1998 1998 AlphaMed Press This Article Taxotere: Clinical Trials in Non-Small Cell
THE RESULTS OF A large meta-analysis of 52 randomized
 Prospective Randomized Trial of Docetaxel Versus Best Supportive Care in Patients With Non Small-Cell Lung Cancer Previously Treated With Platinum-Based Chemotherapy By Frances A. Shepherd, Janet Dancey,
Prospective Randomized Trial of Docetaxel Versus Best Supportive Care in Patients With Non Small-Cell Lung Cancer Previously Treated With Platinum-Based Chemotherapy By Frances A. Shepherd, Janet Dancey,
More than half of all lung cancer patients present with. New Combinations in the Treatment of Lung Cancer* A Time for Optimism
 New Combinations in the Treatment of Lung Cancer* A Time for Optimism Paul A. Bunn, Jr., MD; and Karen Kelly, MD Strides have been made in the treatment of lung cancer in the last decade that warrant a
New Combinations in the Treatment of Lung Cancer* A Time for Optimism Paul A. Bunn, Jr., MD; and Karen Kelly, MD Strides have been made in the treatment of lung cancer in the last decade that warrant a
Medication Policy Manual. Topic: Alimta, pemetrexed Date of Origin: May 12, 2010
 Medication Policy Manual Policy No: dru213 Topic: Alimta, pemetrexed Date of Origin: May 12, 2010 Committee Approval Date: February 17, 2015 Next Review Date: February 2016 Effective Date: March 1, 2015
Medication Policy Manual Policy No: dru213 Topic: Alimta, pemetrexed Date of Origin: May 12, 2010 Committee Approval Date: February 17, 2015 Next Review Date: February 2016 Effective Date: March 1, 2015
Controversies in Management of. Inoperable NSCLC. Inoperable NSCLC. Introduction:
 Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Shifting the Paradigm for Maintenance Therapy for Non small-cell Lung Cancer
 J Hong Kong Col Radiol. 2010;13(Suppl):S16-21 ORIGINAL ARTICLE Shifting the Paradigm for Maintenance Therapy for Non small-cell Lung Cancer VHF Lee Department of Clinical Oncology, Queen Mary Hospital,
J Hong Kong Col Radiol. 2010;13(Suppl):S16-21 ORIGINAL ARTICLE Shifting the Paradigm for Maintenance Therapy for Non small-cell Lung Cancer VHF Lee Department of Clinical Oncology, Queen Mary Hospital,
POLICY A. INDICATIONS
 Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin
 COMPENDIA TRANSPARENCY TRACKING FORM DRUG: Docetaxel INDICATION: Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin COMPENDIA TRANSPARENCY REQUIREMENTS 1 Provide
COMPENDIA TRANSPARENCY TRACKING FORM DRUG: Docetaxel INDICATION: Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin COMPENDIA TRANSPARENCY REQUIREMENTS 1 Provide
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer
 Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
Scottish Medicines Consortium
 Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Management of stage III A-B of NSCLC. Hamed ALHusaini Medical Oncologist
 Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Treatment of Advanced Non Small-Cell Lung Cancer: A Review of Current Randomized Clinical Trials and an Examination of Emerging Therapies
 Progress in the treatment of advanced non small-cell lung cancer allows patients to live longer and with better quality of life. Milton Rochman. Mother s Comfort. Acrylic on canvas, 24 30. Courtesy of
Progress in the treatment of advanced non small-cell lung cancer allows patients to live longer and with better quality of life. Milton Rochman. Mother s Comfort. Acrylic on canvas, 24 30. Courtesy of
Advances In Chemotherapy For Hormone Refractory Prostate Cancer. TAX 327 study results & SWOG 99-16 study results presented at ASCO 2004
 Ronald de Wit Rotterdam Cancer Institute The Netherlands Advances In Chemotherapy For Hormone Refractory Prostate Cancer TAX 327 study results & SWOG 99-16 study results presented at Slide 1 Prostate Cancer
Ronald de Wit Rotterdam Cancer Institute The Netherlands Advances In Chemotherapy For Hormone Refractory Prostate Cancer TAX 327 study results & SWOG 99-16 study results presented at Slide 1 Prostate Cancer
Effects of Chemotherapy on Quality of Life for Patients with Lung Cancer
 CLINICAL STUDY Effects of Chemotherapy on Quality of Life for Patients with Lung Cancer Ahmet Bircan, MD 1 ; M. Bahad r Berktafl, MD 1 ; Hülya Bay z, MD 1 ; Nihal Baflay, MD 1 ; Sema Bircan, MD 2 ; Mine
CLINICAL STUDY Effects of Chemotherapy on Quality of Life for Patients with Lung Cancer Ahmet Bircan, MD 1 ; M. Bahad r Berktafl, MD 1 ; Hülya Bay z, MD 1 ; Nihal Baflay, MD 1 ; Sema Bircan, MD 2 ; Mine
Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART
 Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART Goals Discuss treatment options for stage 4 lung cancer: New and old Discuss new developments in personalized
Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART Goals Discuss treatment options for stage 4 lung cancer: New and old Discuss new developments in personalized
Radiation Therapy in the Treatment of
 Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
 September 27, 2013 ONO PHARMACEUTICAL CO., LTD. Corporate Communications Phone: +81-6-6263-5670 Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
September 27, 2013 ONO PHARMACEUTICAL CO., LTD. Corporate Communications Phone: +81-6-6263-5670 Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
Stage 3 Lung Cancer - Know YourSurviving Conditions
 Survival of Stage IIIb and IV Non-Small Cell Lung Cancer Patients on Best Supportive Care in Manitoba, Canada Erich Kliewer Alain Demers Sri Navaratnam Coreen Hildebrand Grace Musto Report for AstraZeneca
Survival of Stage IIIb and IV Non-Small Cell Lung Cancer Patients on Best Supportive Care in Manitoba, Canada Erich Kliewer Alain Demers Sri Navaratnam Coreen Hildebrand Grace Musto Report for AstraZeneca
Summary ID# 13095. Clinical Study Summary: Study H3E-EW-B012
 Page 1 Summary ID# 13095 Clinical Study Summary: Study H3E-EW-B012 First-line Treatment of Non-Small Cell Lung Cancer under Routine Conditions: Observational Study on Overall Survival Date summary electronically
Page 1 Summary ID# 13095 Clinical Study Summary: Study H3E-EW-B012 First-line Treatment of Non-Small Cell Lung Cancer under Routine Conditions: Observational Study on Overall Survival Date summary electronically
Chapter 7: Lung Cancer
 Chapter 7: Lung Cancer Contents Chapter 7: Lung Cancer... 1 Small Cell... 2 Good PS + Limited stage... 2 Cisplatin/etoposide... 2 Concurrent chemotherapy + XRT... 2 Good / Intermediate PS... 2 Carboplatin
Chapter 7: Lung Cancer Contents Chapter 7: Lung Cancer... 1 Small Cell... 2 Good PS + Limited stage... 2 Cisplatin/etoposide... 2 Concurrent chemotherapy + XRT... 2 Good / Intermediate PS... 2 Carboplatin
INTRODUCTION. liver neoplasms, adrenal gland neoplasms, non small-cell lung cancer, radionuclide imaging, bisphosphonates,
 VOLUME 22 NUMBER 2 JANUARY 15 2004 JOURNAL OF CLINICAL ONCOLOGY A S C O S P E C I A L A R T I C L E American Society of Clinical Oncology Treatment of Unresectable Non Small-Cell Lung Cancer Guideline:
VOLUME 22 NUMBER 2 JANUARY 15 2004 JOURNAL OF CLINICAL ONCOLOGY A S C O S P E C I A L A R T I C L E American Society of Clinical Oncology Treatment of Unresectable Non Small-Cell Lung Cancer Guideline:
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline
 Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
People Living with Cancer
 Patient Guide ASCOInformation for People Living with Cancer ADVANCED LUNG CANCER TREATMENT Recommendations of the American Society of Clinical Oncology Welcome The American Society of Clinical Oncology
Patient Guide ASCOInformation for People Living with Cancer ADVANCED LUNG CANCER TREATMENT Recommendations of the American Society of Clinical Oncology Welcome The American Society of Clinical Oncology
National Horizon Scanning Centre. Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer. December 2007
 Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
SYSTEMIC THERAPY FOR STAGE IV NON-SMALL CELL LUNG CANCER: AMERICAN SOCIETY OF CLINICAL ONCOLOGY CLINICAL PRACTICE GUIDELINE UPDATE
 Which patients with stage IV NSCLC should be treated with chemotherapy? NSCLC with nonsquamous cell carcinoma, negative or unknown EGFR-sensitizing mutation and ALK gene rearrangement status, and PS 0-1
Which patients with stage IV NSCLC should be treated with chemotherapy? NSCLC with nonsquamous cell carcinoma, negative or unknown EGFR-sensitizing mutation and ALK gene rearrangement status, and PS 0-1
Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
 Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Summary of treatment benefits
 Risk Management Plan PEMETREXED Powder for concentrate for Solution for infusion Pemetrexed is also indicated as monotherapy for the maintenance treatment of locally advanced or metastatic non small cell
Risk Management Plan PEMETREXED Powder for concentrate for Solution for infusion Pemetrexed is also indicated as monotherapy for the maintenance treatment of locally advanced or metastatic non small cell
Review. The combination of etoposide and cisplatin in non-small-cell lung cancer (NSCLC)
 Annals of Oncology 10 (Suppl. 5): S13-S17,1999. 1999 Kluwer Academic Publishers. Printed in the Netherlands. Review The combination of etoposide and cisplatin in non-small-cell lung cancer (NSCLC) A. Ardizzoni,
Annals of Oncology 10 (Suppl. 5): S13-S17,1999. 1999 Kluwer Academic Publishers. Printed in the Netherlands. Review The combination of etoposide and cisplatin in non-small-cell lung cancer (NSCLC) A. Ardizzoni,
Management of Platinum-Sensitive Recurrent Ovarian Cancer
 Management of Platinum-Sensitive Jacobus Pfisterer a and Jonathan A. Ledermann b The majority of patients with ovarian cancer will relapse despite state-of-the-art first-line surgery and chemotherapy.
Management of Platinum-Sensitive Jacobus Pfisterer a and Jonathan A. Ledermann b The majority of patients with ovarian cancer will relapse despite state-of-the-art first-line surgery and chemotherapy.
Future Directions in Clinical Research. Karen Kelly, MD Associate Director for Clinical Research UC Davis Cancer Center
 Future Directions in Clinical Research Karen Kelly, MD Associate Director for Clinical Research UC Davis Cancer Center Outline 1. Status of Cancer Treatment 2. Overview of Clinical Research at UCDCC 3.
Future Directions in Clinical Research Karen Kelly, MD Associate Director for Clinical Research UC Davis Cancer Center Outline 1. Status of Cancer Treatment 2. Overview of Clinical Research at UCDCC 3.
Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment.
 1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
Avastin: Glossary of key terms
 Avastin: Glossary of key terms Adenocarcinoma Adenoma Adjuvant therapy Angiogenesis Anti-angiogenics Antibody Antigen Avastin (bevacizumab) Benign A form of carcinoma that originates in glandular tissue.
Avastin: Glossary of key terms Adenocarcinoma Adenoma Adjuvant therapy Angiogenesis Anti-angiogenics Antibody Antigen Avastin (bevacizumab) Benign A form of carcinoma that originates in glandular tissue.
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
Key words: chemotherapy; evidence-based medicine; guidelines; non-small cell lung cancer
 Chemotherapeutic Management of Stage IV Non-small Cell Lung Cancer* Mark A. Socinski, MD, FCCP; David E. Morris, MD; Gregory A. Masters, MD, FCCP; and Rogerio Lilenbaum, MD Stage IV non-small cell lung
Chemotherapeutic Management of Stage IV Non-small Cell Lung Cancer* Mark A. Socinski, MD, FCCP; David E. Morris, MD; Gregory A. Masters, MD, FCCP; and Rogerio Lilenbaum, MD Stage IV non-small cell lung
Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer
 LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
Background. t 1/2 of 3.7 4.7 days allows once-daily dosing (1.5 mg) with consistent serum concentration 2,3 No interaction with CYP3A4 inhibitors 4
 Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
January 2013 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW. Summary. Contents
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases
 Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER
 CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER INTRODUCTION This chapter provides an overview of treatment for small cell lung cancer (SCLC). Treatment options are presented based on the extent of disease.
CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER INTRODUCTION This chapter provides an overview of treatment for small cell lung cancer (SCLC). Treatment options are presented based on the extent of disease.
DOXETAXEL IN PREVIOUSLY TREATED NON-SMALL CELL LUNG CANCER PATIENTS: CLINICAL EFFICACY AND QUALITY OF LIFE
 SOUTHEAST ASIAN J TROP MED PUBLIC HEALTH DOXETAXEL IN PREVIOUSLY TREATED NON-SMALL CELL LUNG CANCER PATIENTS: CLINICAL EFFICACY AND QUALITY OF LIFE Thitiya Sirisinha 1, Suwanee Sirilertrakul 2, Manmana
SOUTHEAST ASIAN J TROP MED PUBLIC HEALTH DOXETAXEL IN PREVIOUSLY TREATED NON-SMALL CELL LUNG CANCER PATIENTS: CLINICAL EFFICACY AND QUALITY OF LIFE Thitiya Sirisinha 1, Suwanee Sirilertrakul 2, Manmana
Chemotherapy in Ovarian Cancer. Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group
 Chemotherapy in Ovarian Cancer Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group Adjuvant chemotherapy for early stage EOC Fewer than 30% women present with FIGO stage
Chemotherapy in Ovarian Cancer Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group Adjuvant chemotherapy for early stage EOC Fewer than 30% women present with FIGO stage
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof.
 Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Principal Investigator: Valerie W. Rusch, MD, FACS, Chief, Thoracic Surgery Memorial Sloan-Kettering Cancer Center
 Protocol 1101-1088 Phase I study of intra-pleural administration of GL-ONC1 in patients with malignant pleural effusion: primary, metastases and mesothelioma Principal Investigator: Valerie W. Rusch, MD,
Protocol 1101-1088 Phase I study of intra-pleural administration of GL-ONC1 in patients with malignant pleural effusion: primary, metastases and mesothelioma Principal Investigator: Valerie W. Rusch, MD,
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Schedule: Drug Dose iv/infusion/oral q Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8
 Carboplatin/Gemcitabine Lung Cancer (non-small cell) - Advanced Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8 Cycle frequency: Every three weeks Total
Carboplatin/Gemcitabine Lung Cancer (non-small cell) - Advanced Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8 Cycle frequency: Every three weeks Total
Small Cell Lung Cancer
 Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Second-Line Chemotherapy
 40 Second-Line Chemotherapy Nick Pavlakis and Nicholas J. Vogelzang Chemotherapy trials for malignant pleural mesothelioma have almost exclusively focused on chemotherapy-naive patients. Until 2000, the
40 Second-Line Chemotherapy Nick Pavlakis and Nicholas J. Vogelzang Chemotherapy trials for malignant pleural mesothelioma have almost exclusively focused on chemotherapy-naive patients. Until 2000, the
Sur les nouveaux médicaments et les perspectives qu ils offrent (traitement à la carte et survie longue)
 Sur les nouveaux médicaments et les perspectives qu ils offrent (traitement à la carte et survie longue) Professeur Jean Trédaniel Unité de cancérologie thoracique Hôpital Saint-Louis Comparison of Four
Sur les nouveaux médicaments et les perspectives qu ils offrent (traitement à la carte et survie longue) Professeur Jean Trédaniel Unité de cancérologie thoracique Hôpital Saint-Louis Comparison of Four
Role of Gemcitabine in the Treatment of Advanced and Metastatic Breast Cancer
 Review Oncology 2003;64:191 206 DOI: 10.1159/000069315 Role of Gemcitabine in the Treatment of Advanced and Metastatic Breast Cancer Volker Heinemann Medical Clinic III, Klinikum Grosshadern, Munich, Germany
Review Oncology 2003;64:191 206 DOI: 10.1159/000069315 Role of Gemcitabine in the Treatment of Advanced and Metastatic Breast Cancer Volker Heinemann Medical Clinic III, Klinikum Grosshadern, Munich, Germany
Second-Line Therapy in Non Small-Cell Lung Cancer: The DELTA Between Different Genotypes Widens
 VOLUME 32 NUMBER 18 JUNE 20 2014 JOURNAL OF CLINICAL ONCOLOGY ONCOLOGY GRAND ROUNDS Second-Line Therapy in Non Small-Cell Lung Cancer: The DELTA Between Different Genotypes Widens Alona Zer and Natasha
VOLUME 32 NUMBER 18 JUNE 20 2014 JOURNAL OF CLINICAL ONCOLOGY ONCOLOGY GRAND ROUNDS Second-Line Therapy in Non Small-Cell Lung Cancer: The DELTA Between Different Genotypes Widens Alona Zer and Natasha
Cetuximab (Erbitux) MM.04.005 05/10/2005. HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s) of Service: Office: Outpatient
 Cetuximab (Erbitux) Policy Number: Original Effective Date: MM.04.005 05/10/2005 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s)
Cetuximab (Erbitux) Policy Number: Original Effective Date: MM.04.005 05/10/2005 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s)
Cytotoxic Therapy in Metastatic Breast Cancer
 Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Cytotoxic Therapy in Metastatic Breast Cancer Cytotoxic Therapy in Metastatic Breast Cancer Version 2002: von Minckwitz Versions
Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Cytotoxic Therapy in Metastatic Breast Cancer Cytotoxic Therapy in Metastatic Breast Cancer Version 2002: von Minckwitz Versions
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS
 MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
Before, Frank's immune cells could
 Before, Frank's immune cells could barely recognize a prostate cancer cell. Now, they are focused on it. Stimulate an immune response against advanced prostate cancer Extend median survival beyond 2 years
Before, Frank's immune cells could barely recognize a prostate cancer cell. Now, they are focused on it. Stimulate an immune response against advanced prostate cancer Extend median survival beyond 2 years
the standard of care 2009 5/1/2009 Mesothelioma: The standard of care take home messages PILC 2006 Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009
 Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
Epidemiology, Staging and Treatment of Lung Cancer. Mark A. Socinski, MD
 Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
Recent Trends in Management of Unresectable Non-Small Cell Lung Cancer (NSCLC)
 Bahrain Medical Bulletin, Vol.23, No.4, December 2001 Recent Trends in Management of Unresectable Non-Small Cell Lung Cancer (NSCLC) Jalal Al-Maskati, MBChB, ABIM * Lung cancer is a major health problem
Bahrain Medical Bulletin, Vol.23, No.4, December 2001 Recent Trends in Management of Unresectable Non-Small Cell Lung Cancer (NSCLC) Jalal Al-Maskati, MBChB, ABIM * Lung cancer is a major health problem
Stage I, II Non Small Cell Lung Cancer
 Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
 Department of Veterans Affairs Health Services Research & Development Service Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
Department of Veterans Affairs Health Services Research & Development Service Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
Small-Cell Lung Cancer: Is There a Standard Therapy?
 Small-Cell Lung Cancer: Is There a Standard Therapy? Review Article [1] January 02, 1998 By Pieter E. Postmus, MD, PhD [2] and Egbert F. Smit, MD [3] For more than 25 years, chemotherapy has been the cornerstone
Small-Cell Lung Cancer: Is There a Standard Therapy? Review Article [1] January 02, 1998 By Pieter E. Postmus, MD, PhD [2] and Egbert F. Smit, MD [3] For more than 25 years, chemotherapy has been the cornerstone
a Phase 2 prostate cancer clinical trial is ongoing. Table 2: Squalamine vs Standard-of-care literature
 PRODUCT FACT SHEET Spring 2007 MISSION STATEMENT Genaera Corporation is a biopharmaceutical company with a focus on metabolic and respiratory diseases. The compounds in the Genaera pipeline address signal
PRODUCT FACT SHEET Spring 2007 MISSION STATEMENT Genaera Corporation is a biopharmaceutical company with a focus on metabolic and respiratory diseases. The compounds in the Genaera pipeline address signal
Hodgkin Lymphoma Disease Specific Biology and Treatment Options. John Kuruvilla
 Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Phase III trials in oncology
 Phase III trials in oncology Setting standards of care? Siegfried Seeber and Ada H Braun* Survival data from phase III trials can be very misleading because patients are not offered the best follow-up
Phase III trials in oncology Setting standards of care? Siegfried Seeber and Ada H Braun* Survival data from phase III trials can be very misleading because patients are not offered the best follow-up
Recommendation Strength Strong, supported by the evidence and expert consensus. Recommendation Benefit/Harm Evidence Quality
 CHEMO- AND TARGETED THERAPY FOR WOMEN WITH HER2 NEGATIVE (OR UNKNOWN) ADVANCED BREAST Benefit/Harm Evidence Quality 1: Endocrine therapy, rather than chemotherapy, should be offered as the standard firstline
CHEMO- AND TARGETED THERAPY FOR WOMEN WITH HER2 NEGATIVE (OR UNKNOWN) ADVANCED BREAST Benefit/Harm Evidence Quality 1: Endocrine therapy, rather than chemotherapy, should be offered as the standard firstline
Active centers: 2. Number of patients/subjects: Planned: 20 Randomized: Treated: 20 Evaluated: Efficacy: 13 Safety: 20
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
Elderly Patients with Advanced Non-Small Cell Lung Cancer: What Treatment?
 4 The Open Lung Cancer Journal, 2011, 4, 4-9 Open Access Elderly Patients with Advanced Non-Small Cell Lung Cancer: What Treatment? Antonio Rossi * Division of Medical Oncology, S.G. Moscati Hospital,
4 The Open Lung Cancer Journal, 2011, 4, 4-9 Open Access Elderly Patients with Advanced Non-Small Cell Lung Cancer: What Treatment? Antonio Rossi * Division of Medical Oncology, S.G. Moscati Hospital,
Chemotherapy for advanced non small-cell lung cancer: role of paclitaxel and gemcitabine
 Chemotherapy for advanced non small-cell lung cancer: role of paclitaxel and gemcitabine WK Lam, KWT Tsang, MSM Ip Objective. To review the role of chemotherapy in advanced non small-cell lung cancer,
Chemotherapy for advanced non small-cell lung cancer: role of paclitaxel and gemcitabine WK Lam, KWT Tsang, MSM Ip Objective. To review the role of chemotherapy in advanced non small-cell lung cancer,
Small Cell Lung Cancer
 Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Second-Line Treatment Options in Non Small Cell Lung Cancer: A Comparison of Cytotoxic Agents and Targeted Therapies
 Second-Line Treatment Options in Non Small Cell Lung Cancer: A Comparison of Cytotoxic Agents and Targeted Therapies Filippo de Marinis, a Stefano De Santis, a and Luigi De Petris b Current options for
Second-Line Treatment Options in Non Small Cell Lung Cancer: A Comparison of Cytotoxic Agents and Targeted Therapies Filippo de Marinis, a Stefano De Santis, a and Luigi De Petris b Current options for
Avastin in Metastatic Breast Cancer
 Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
London Cancer. Mesothelioma Lung Protocols
 London Cancer Mesothelioma Lung Protocols Version 0.9 Contents 1. Staging... 3 2. Mesothelioma Summary of Chemotherapy Protocols... 4 3. Mesothelioma Chemotherapy Protocols... 7 3.1. Pemetrexed (Alimta
London Cancer Mesothelioma Lung Protocols Version 0.9 Contents 1. Staging... 3 2. Mesothelioma Summary of Chemotherapy Protocols... 4 3. Mesothelioma Chemotherapy Protocols... 7 3.1. Pemetrexed (Alimta
Stage IIIB disease includes patients with T4 tumors,
 Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
What is non-small-cell lung cancer?
 Non-Small-Cell Lung Cancer What is non-small-cell lung cancer? Let us explain it to you. www.anticancerfund.org www.esmo.org ESMO/ACF Patient Guide Series based on the ESMO Clinical Practice Guidelines
Non-Small-Cell Lung Cancer What is non-small-cell lung cancer? Let us explain it to you. www.anticancerfund.org www.esmo.org ESMO/ACF Patient Guide Series based on the ESMO Clinical Practice Guidelines
Update on Small Cell Lung Cancer
 Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy
 17 (Supplement 10): x108 x112, 2006 doi:10.1093/annonc/mdl247 The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy E. Felip & E. Vilar Oncology Department, Vall d
17 (Supplement 10): x108 x112, 2006 doi:10.1093/annonc/mdl247 The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy E. Felip & E. Vilar Oncology Department, Vall d
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
Single-Agent Paclitaxel in the Treatment of Advanced Non-Small Cell Lung Cancer
 Single-Agent Paclitaxel in the Treatment of Advanced Non-Small Cell Lung Cancer MARK A. SOCINSKI The Multidisciplinary Thoracic Oncology Program, University of North Carolina, Chapel Hill, North Carolina,
Single-Agent Paclitaxel in the Treatment of Advanced Non-Small Cell Lung Cancer MARK A. SOCINSKI The Multidisciplinary Thoracic Oncology Program, University of North Carolina, Chapel Hill, North Carolina,
Clinical Study Report
 An Open, Multi-Center, Phase II Clinical Trial to Evaluate Efficacy and Safety of Taxol (), UFT, and Leucovorin in Patients with Advanced Gastric Cancer Clinical Study Report 4F, No. 156, Jiankang Rd.,
An Open, Multi-Center, Phase II Clinical Trial to Evaluate Efficacy and Safety of Taxol (), UFT, and Leucovorin in Patients with Advanced Gastric Cancer Clinical Study Report 4F, No. 156, Jiankang Rd.,
Drug/Drug Combination: Bevacizumab in combination with chemotherapy
 AHFS Final Determination of Medical Acceptance: Off-label Use of Bevacizumab in Combination with Chemotherapy for the Treatment of Metastatic Breast Cancer Previously Treated with Cytotoxic Chemotherapy
AHFS Final Determination of Medical Acceptance: Off-label Use of Bevacizumab in Combination with Chemotherapy for the Treatment of Metastatic Breast Cancer Previously Treated with Cytotoxic Chemotherapy
Chemotherapy in Advanced Non-Small Cell Lung Cancer: Optimal Treatment Approach for Elderly and Patients With Poor Performance Status
 Chemotherapy in Advanced Non-Small Cell Lung Cancer: Optimal Treatment Approach for Elderly and Patients With Poor Performance Status Tracey L. Evans, MD Abstract In spite of advances in molecular profiling
Chemotherapy in Advanced Non-Small Cell Lung Cancer: Optimal Treatment Approach for Elderly and Patients With Poor Performance Status Tracey L. Evans, MD Abstract In spite of advances in molecular profiling
Non-Small Cell Lung Cancer
 Non-Small Cell Lung Cancer John delcharco, MD (Statistics based on CVMC data 2009-2013) Statistics Lung cancer is the leading cause of cancer deaths in the United States. The American Cancer Society estimates
Non-Small Cell Lung Cancer John delcharco, MD (Statistics based on CVMC data 2009-2013) Statistics Lung cancer is the leading cause of cancer deaths in the United States. The American Cancer Society estimates
ADJUVANT TREATMENT CLINICAL EVALUATION NEOADJUVANT TREATMENT
 te: Consider Clinical Trials as treatment options for eligible patients. Referral to a center with both pediatric oncology and orthopedic surgery is essential. CLINICAL EVALUATION This practice algorithm
te: Consider Clinical Trials as treatment options for eligible patients. Referral to a center with both pediatric oncology and orthopedic surgery is essential. CLINICAL EVALUATION This practice algorithm
MALIGNANT MESOTHELIOMA is an aggressive
 Cisplatin and Gemcitabine Treatment for Malignant Mesothelioma: A Phase II Study By M.J. Byrne, J.A. Davidson, A.W. Musk, J. Dewar, G. van Hazel, M. Buck, N.H. de Klerk, and B.W.S. Robinson Purpose: We
Cisplatin and Gemcitabine Treatment for Malignant Mesothelioma: A Phase II Study By M.J. Byrne, J.A. Davidson, A.W. Musk, J. Dewar, G. van Hazel, M. Buck, N.H. de Klerk, and B.W.S. Robinson Purpose: We
Pulmonary function. Is patient potentially operable? Yes. tests 3. Yes. Pulmonary function. tests 3, if clinically indicated. Yes
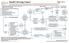 INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness
 Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness Investigators: Paul G. Shekelle, MD, PhD, Director Alicia R. Maher, MD Clinical
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness Investigators: Paul G. Shekelle, MD, PhD, Director Alicia R. Maher, MD Clinical
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians
 Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
