UK Guidance on Best Practice in Vaccine Administration
|
|
|
- Bonnie Paul
- 8 years ago
- Views:
Transcription
1 UK Guidance on Best Practice in Vaccine Administration
2 Copyright 2001 Applications for reproduction should be made to Shire Hall Communications, 3 Olaf Street, London, W11 4BE ISBN X Published by Shire Hall Communications For additional copies please write to: The Vaccine Administration Taskforce Shire Hall Communications PO Box London W11 4YZ This initiative has been supported by an educational grant from GlaxoSmithKline 2 UK Guidance on Best Practice in Vaccine Administration
3 Preface This guidance has been developed in the year 2001 after consultation with the following contributors, who formed the Vaccine Administration Taskforce (VAT): Jane Chiodini, Travel Health and Immunisation Specialist Nurse; Chair, Royal College of Nursing (RCN) Travel Health Forum Gail Cotton, President, Association of Occupational Health Nurse Practitioners (AOHNP); Head of Occupational Health, Leicestershire Fire and Rescue Service Fiona Genasi, Nurse Epidemiologist, Scottish Centre for Infection and Environmental Health (SCIEH) Karen Gupta, Independent Practice Nurse Advisor Mark Jones, Primary Care Policy and Practice Advisor, RCN Dr George Kassianos, GP; Spokesperson on Immunisation, Royal College of General Practitioners (RCGP); Primary Care Virology Group (PCVG) Judith Moreton, District Immunisation Co-ordinator, Oxfordshire Health Authority Dr Jane Zuckerman, Travel Medicine Consultant; Director, Academic Centre for Travel Medicine and Vaccines, Royal Free and University College Medical School, London Carolyn Driver, Travel Health Specialist Nurse and Chair, British Travel Health Association, has been instrumental in the development of this Guidance by authoring the first draft and working alongside the VAT members Special thanks also go to: Judith Moreton, who provided a copy of her work Immunisation Handbook (Oxfordshire HA 2000), which assisted in the development of this guidance Dr Helen Bedford, Institute of Child Health, for her input and advice Jeannett Martin for her help drafting the section on Consent Foreword: Reference has been made to the United Kingdom Central Council for Nursing, Midwifery and Health Visiting (UKCC) throughout this guidance. It is anticipated that the Nursing and Midwifery Council (NMC) will take over the role of the UKCC in April 2002 as well as taking on the work of the four national boards. UK Guidance on Best Practice in Vaccine Administration 3
4 Preface This guidance has been developed and endorsed by the following organisations: Association of Occupational Health Nurse Practitioners British Travel Health Association Community Practitioners and Health Visitors Association Royal College of General Practitioners Royal College of Nursing 4 UK Guidance on Best Practice in Vaccine Administration
5 Contents Introduction 7 Patient Group Directions and guidelines 9 Vaccine delivery, storage and stock control 13 Situations in which vaccination should be postponed or omitted 19 Consent 21 Preparing the vaccine equipment 25 Preparing and dealing with anxious people 29 Preparation of vaccine 31 Administration of vaccine 33 Choice of needle 39 Technique 43 Needle stick injuries 49 Disposal of needles, syringes and other waste products 51 Anaphylaxis and observation of patient after immunisation 53 Documentation 55 Local information form 57 Further reading 59 Further information sources 61 Appendix one 63 Appendix two 67 Index 69 UK Guidance on Best Practice in Vaccine Administration 5
6 6 UK Guidance on Best Practice in Vaccine Administration
7 Introduction Introduction The principal aims of immunisation are threefold: 1 To protect the individual from infectious diseases, with associated mortality, morbidity and long term sequelae To prevent outbreaks of disease Ultimately to eradicate infectious diseases world-wide, as in the case of smallpox Introduction Nurses are now the major force in administering vaccinations not only within the childhood immunisation programme but also increasingly in administering travel vaccines and in the annual influenza vaccination campaign. In agreeing to undertake this work, nurses assume professional accountability and should ensure that they keep up to date with all aspects of immunisation as outlined in the United Kingdom Central Council s (UKCC) Guidelines for Professional Practice. 2 A doctor who delegates the task of immunisation to a nurse also has a responsibility to see that they are adequately trained and have the opportunity for regular updates. 3 BOX 1 2 UKCC Guidelines for Professional Practice 1996 As a Registered Nurse you are personally accountable for your practice and, in the exercise of your professional accountability, must. Maintain and improve your professional knowledge and competence Acknowledge any limitations in your knowledge and competence and decline any duties or responsibilities unless able to perform them in a safe and skilled manner UK Guidance on Best Practice in Vaccine Administration 7
8 Introduction Immunisation is a safe and highly effective method of preventing infectious disease. Successful immunisation depends upon: Introduction Production of a safe and effective vaccine Maintenance of the cold chain during vaccine transportation and storage Injection into the correct anatomical site of an appropriate recipient Correct injection technique This guidance outlines the step by step process and techniques involved in vaccination, from taking a vaccine out of the refrigerator to disposal of the needle and syringe at the end of the procedure. The information provided should be used in conjunction with the Green Book 3 and will not therefore go into detail about individual vaccines. Nurses administering vaccines have a duty to be informed about each vaccine they administer and should use resources such as the Green Book and the most recent Summary of Product Characteristics (SPC) or Patient Information Leaflet (PIL - found within the vaccine packaging) to update themselves on information relating to a particular disease area or product. Practices should also keep on file any Chief Medical Officer (CMO) or Chief Nursing Officer (CNO) correspondence regarding vaccination. Such correspondence should always be shared at a Primary Care Organisation level (see section: Further information sources for CMO and CNO contact details). Note: The information contained in this document will be relevant to a range of healthcare professionals including practice nurses, occupational health staff practitioners, health visitors, midwives and general practitioners (GPs). Throughout this document the healthcare professional administering the vaccine is referred to as the 'nurse'. In addition, the person receiving the vaccine i.e. the vaccinee, client, child or adult, will be referred to as the 'patient'. References: 1. Moreton J. Immunisation Handbook. UK: Oxfordshire Health Authority October United Kingdom Central Council for Nursing, Midwifery and Health Visiting. Guidelines for Professional Practice Salisbury D M, Begg N T. Department of Health, Immunisation Against Infectious Disease. UK: HMSO 1996; Ch. 6: 17 8 UK Guidance on Best Practice in Vaccine Administration
9 Patient Group Directions and guidelines Patient Group Directions and guidelines 1,2,3,4,5 Nurses must be aware of their legal position when administering a vaccine that has not been individually prescribed by a doctor Since August 2000 Patient Group Directions (PGDs) have become a legal requirement throughout the UK for any prescription-only medicine (POM) administered to a patient for whom no individual prescription exists 2,4 In the absence of legislation allowing nurses to prescribe a wide range of medicines such as vaccines alternative means have been developed to facilitate their supply and administration. During the 1990s nurses utilised the ad hoc system of group protocols which was given more credence by the Crown Report of Legislation was introduced in 2000, 7,8,9 which made legal the supply and administration of medicines by nurses to undifferentiated patients under a formal agreement with a prescribing doctor. From August 9th 2000 such agreements became known as Patient Group Directions. PGDs are written agreements for the supply and administration of medicines to groups of patients who may not be individually identified before presentation for treatment. They can be used for homogeneous patient groups where presenting characteristics and requirements are sufficiently consistent to be catered for by such a non-specific direction, although patients who can be identified before they need a specific medicine should receive that medicine on a patient-specific basis. Patient Group Directions and guidelines The law governing the use of PGDs applies UK-wide since August The law is equally applicable to all countries within the UK and national guidance is available from the Department of Health (DoH) 2 for England, the Welsh Assembly 4 for Wales and the Scottish Executive Health Department 5 in Scotland. However, the Northern Ireland Assembly has yet to issue guidance on the use of PGDs. Throughout the UK, PGDs should now be drafted to conform with the prevailing legislation. The Royal College of Nursing produces additional guidance and explanation concerning the use of PGDs. 3 UK Guidance on Best Practice in Vaccine Administration 9
10 Patient Group Directions and guidelines Note that the law restricts the use of PGDs to the NHS or organisations providing care for NHS patients as part of a contract with the NHS. It is expected that legislation will be brought forward to cover the independent sector, but in the meantime it is suggested that where PGDs might be considered to be in the best interests of patients, they should be drawn up and used in line with the national guidance. Nurses using PGDs in non-nhs settings should seek guidance from their professional organisation/insurer. Patient Group Directions and guidelines Practices should ensure that PGDs applicable to their own surgery/workplace are in place these should be drawn up by a multidisciplinary team at either Primary Care Organisation or Health Authority (HA) level (please refer to Appendix one for a list of the information that must be included in a PGD) The PGD replaces a Group Protocol for the administration of a medicine it does not replace the need for a protocol (guidelines) which describes the standard of care given in a particular situation (note: all outdated protocols should be kept on file for future reference) Guidelines are templates for a consultation and are necessary to demonstrate the way in which a particular surgery or clinic undertakes immunisation The information must be available for new or deputising staff to familiarise themselves. In the event of a complaint or legal action the information will also demonstrate the standard of care that is given by a particular establishment Note: This guidance can form part of a protocol for immunisation or travel clinics, as can any other written material which a practice or clinic chooses to use during a consultation further information is available in Appendix one. 10 UK Guidance on Best Practice in Vaccine Administration
11 Patient Group Directions and guidelines BOX 2 1 Black Triangle Vaccines These can be given under a PGD, provided they are used in accordance with the schedules recommended by the Joint Committee on Vaccination and Immunisation (JCVI) 2 The PGD should state what action is to be taken if adverse events are reported Unlicensed Vaccines Vaccines that do not have a product licence in the UK cannot be given under a PGD They should have a specific prescription signed by a doctor Off-Label Use of a Licensed Vaccine A licensed product can be administered outside its licensed indications Patient Group Directions and guidelines under a PGD providing: 2 Such use is justified by best practice The status of the product is clearly described There is acceptable clinical evidence for the use of the product for the intended indication Note: Please refer to Appendix one for further information on PGDs and the information that should be included in them. Black Triangle Vaccines The black triangle symbol indicates newly licensed medicines that are monitored intensively by the Medicines Control Agency (MCA)/Committee on the Safety of Medicines (CSM). Such medicines include those that have been licensed for administration by a new route or drug delivery system, or for significant new indications which may alter the established risks and benefits of that drug. 10 For medicines showing the black triangle symbol, the MCA/CSM asks that all suspected reactions (including those not considered serious) are reported through the UK Guidance on Best Practice in Vaccine Administration 11
12 Patient Group Directions and guidelines Yellow Card scheme. An adverse reaction should be reported even if it is not certain that the drug has caused it, or if the reaction is well recognised, or if other drugs are given at the same time. 10 References: 1. United Kingdom Central Council for Nursing, Midwifery and Health Visiting website December 2000 ( 0protocols).asp) Patient Group Directions and guidelines 2. Department of Health. Health Service Circular 2000/026 9 August NHS Executive Royal College of Nursing. Patient Group Directions Guidance and Information. London: RCN The National Assembly for Wales. Review of Prescribing, Supply and Administration of Medicines Sale, Supply and Administration of Medicines by Health Professionals Under Patient Group Directions (PGD). CMOCNOCDOCSOCPhA-SALEMED3. 22 December NHS in Scotland Health Department website June NHS HDL (2001)7 ( 6. Department of Health. Review of prescribing supply and administration of medicines. Report on the supply and administration of medicines under group protocol arrangements. London: Department of Health. (Crown II part 1) Statutory Instrument. The Prescription only Medicines (Human Use) Amendment Order Statutory Instrument no London: HMSO Statutory Instrument. The Medicines (Sale or Supply)(Miscellaneous Provisions) Amendment (no.2) Regulations Statutory Instrument no London: HMSO Statutory Instrument. The Medicines (Pharmacy and General Sale Exemption) Amendment Order Statutory Instrument no London: HMSO British Medical Association and the Royal Pharmaceutical Society of Great Britain. BNF No.41. UK BMA. March UK Guidance on Best Practice in Vaccine Administration
13 Vaccine delivery, storage and stock control Vaccine delivery, storage and stock control BOX 3 1,2,3,4 All vaccines have a predetermined shelf life, and expiry dates will be clearly marked on the outer packaging of each product The expiry date is dependent upon the vaccine being stored in the correct manner and maintenance of the cold chain, throughout the shelf life of the product Breaks in the cold chain may result in loss of potency of a vaccine and ultimately to vaccine failure Most vaccines must be kept at temperatures between 2 and 8 C nurses should consult the product packaging to ensure vaccines are being stored at the correct temperature Vaccines are heat sensitive and may deteriorate if kept at temperatures higher than those recommended Freezing deteriorates some vaccines, and may fracture glass containers Vaccine Storage Protocol Practices should ensure that: There is a designated person (and deputy) responsible for ordering and storage of Vaccine delivery, storage and stock control vaccines 3 Reception staff are aware of the importance of ensuring that vaccines are handed over to the person responsible for them as soon as possible and know what action to take if either the person or their deputy is unavailable If the person receiving the vaccine delivery is unhappy about maintenance of the cold chain and the length of time spent in transit (i.e. more than 48 hours 1 ) then they should refuse to accept the order and return it to the supplier if the vaccine has been posted the date and time of dispatch should be clearly marked by the sender so it is clear when the package left optimum storage conditions 2 UK Guidance on Best Practice in Vaccine Administration 13
14 Vaccine delivery, storage and stock control The Refrigerator The refrigerator in which the vaccines are stored should: Be specifically designed for this purpose 4 (i.e. a pharmacy or vaccine refrigerator) Be large enough to hold the stock carried by the particular surgery, taking into consideration year-round needs (influenza programmes etc) Have sufficient room around the vaccine packages for air to circulate, thus enabling the temperature to remain constant Have a lock which complies with Control of Substances Hazardous to Health (COSHH) regulations a lock will also discourage people from opening the door unnecessarily 4 Domestic refrigerators are not suitable for storing vaccines as the temperature fluctuates considerably and the design is generally inappropriate (shelves in the doors for example should not be used for storing vaccines). 4 Best practice ensures that: Vaccine delivery, storage and stock control Electricity supply to the refrigerator is safeguarded against inadvertent breaks, 1 by use of a switchless electrical socket or a taped plug clearly marked: Pharmacy fridge do not switch off Practice guidelines state what action should be taken in the event of a power failure, 5 including a contact (name and number) Centrally supplied vaccines contact the district pharmacist (who should have a policy for dealing with this situation) Vaccines purchased by the practice contact the manufacturers or consult the BNF for further information about specific vaccines Note: Patient-held vaccines should be discouraged as the cold chain is interrupted. In cases where patient-held vaccines are used, the individual should be encouraged to bring the vaccine(s) into the surgery straight away for administration or storage until required. 14 UK Guidance on Best Practice in Vaccine Administration
15 Vaccine delivery, storage and stock control Monitoring the Refrigerator Temperature Best practice ensures that: The temperature of the refrigerator storage compartment is monitored, preferably daily but at least at the beginning of each immunisation session using an independent maximum and minimum thermometer located in the main body of the refrigerator, regardless of whether there is an alarm system or integral thermometer 3,4 A chart is kept to record this information each day s readings should be dated and timed and the thermometer reset after each reading 1 The vaccine protocol stipulates what action should be taken if the temperatures recorded fall outside the desired range and that such action taken is recorded Vaccines are transferred to another fridge or cool box while the fridge is defrosted and the temperature is continued to be monitored to maintain the cold chain Organisation of Stock Within Refrigerator Best practice ensures that: The refrigerator is not used to store anything other than vaccines or drugs 1 Certain shelves are designated for different vaccines this should be listed on the outside of the fridge to minimise the length of time the door is kept open when searching for a vaccine Stock is properly rotated (i.e. new stock is put at the back so the oldest stock is Vaccine delivery, storage and stock control always used first) 1 Vaccines are stored in the manufacturer s packaging many are sensitive to light and thus will deteriorate if taken out of boxes for any length of time Correct stocks are ordered over ordering can lead to problems with storage Note: Suppliers of influenza vaccines should stagger order deliveries as required. UK Guidance on Best Practice in Vaccine Administration 15
16 Vaccine delivery, storage and stock control Maintenance of Cold Chain During Clinic Sessions Vaccines should never be left out. They should be removed from the refrigerator just before use, or if a busy session is anticipated, then vaccines can be transferred to a cool box to prevent frequent opening and closing of the fridge door to maintain the cold chain. Vaccines needed for sessions in schools, outlying clinics or home visits should be transported in an appropriate cool box and returned to the vaccine refrigerator as soon as possible after the session. 1 It may be helpful for the nurse to record the amount of time that vaccines are kept out of the refrigerator. Note: Vaccine ampoules/vials should not come into direct contact with ice packs used in cool boxes. Stock Control Vaccine delivery, storage and stock control Vaccines are valuable and should not be wasted, regardless of whether they are centrally supplied or bought by the practice. Travel vaccinations potentially generate income for the practice so a system should be in place to ensure that all vaccines are accounted for and, where appropriate, vaccination claims are made. A stock control book/database makes this task much easier, and also helps maintain the cold chain as information is available without having to open the fridge door for long periods to examine stock. The book (preferably loose-leaf folder)/database should: Keep track of orders, expiry dates and running totals of vaccines Include a designated section for each vaccine with columns for patient details, date of administration and running totals Incorporate the refrigerator temperature-monitoring chart so that all the information is kept in one place Be dedicated to one fridge there should be a book for each fridge in practices or clinics where there is more than one Be completed by all staff who administer vaccines 16 UK Guidance on Best Practice in Vaccine Administration
17 Vaccine delivery, storage and stock control References: 1. Salisbury D M, Begg N T. Department of Health, Immunisation Against Infectious Disease. UK: HMSO 1996; Ch. 4: Shepherd D. Storage of vaccines: safe or hazardous? Practice Nursing 1995; 6: 4 3. Coppola J, Johnston R. Cold comfort. Nursing Times 1998; 94: Allcock A. Vaccine storage. Practice Nursing 1993 (7 Sept-20 Sept); Moreton J. Immunisation Handbook. UK: Oxfordshire Health Authority October 2000 Vaccine delivery, storage and stock control UK Guidance on Best Practice in Vaccine Administration 17
18 18 UK Guidance on Best Practice in Vaccine Administration
19 Situations in which vaccination should be postponed or omitted Situations in which vaccination should be postponed or omitted There are few contra-indications for the use of vaccines. Chapter 7 of the Green Book covers this issue in detail. 1 Nurses should be familiar with services in their area for dealing with individuals who may have a medical history that requires a cautious approach to vaccination (e.g. pregnant women) and encourage referral to the prescribing doctor rather than discourage vaccination. Most areas have systems in place to treat such individuals e.g. a paediatrician with a special interest in immunisation, travel medicine specialists or a Consultant in a Communicable Disease Control (CCDC) who may run specialised immunisation clinics. Nurses are often the first point of call for anxious parents and hence need to be well informed with all the facts about individual vaccines, particularly when the media have raised fears about the safety of vaccination. Nurses should present balanced information to parents and should not allow themselves to be influenced by ill-informed media coverage of an issue or their own personal opinion. If they feel unable to answer an individual s query, they should discuss it with the prescribing doctor or with other sources of specialist information (see above paragraph). BOX 4 1 Contraindications to Immunisation General Vaccines Acute febrile illness Severe local or systemic reaction to a preceding dose refer to the prescribing doctor where history is unclear Pregnancy preferable not to vaccinate except where the risk of disease may outweigh the risk of vaccination, in which case refer to the prescribing doctor Live Vaccines Immunosuppression either by chemotherapy, radiotherapy or steroids, or HIV infection Where possible, live vaccines should be administered either on the same day at different sites, or with a gap of three weeks between the vaccines (this also applies to Heaf/Mantoux tests) Situations in which vaccination should be postponed or omitted UK Guidance on Best Practice in Vaccine Administration 19
20 Situations in which vaccination should be postponed or omitted Exceptional Circumstances If any course of immunisation is interrupted, it should be resumed as soon as possible. 1 Where a course of vaccination has been interrupted it may be advisable to seek advice from the prescribing doctor or from the Medical Information Department of the relevant vaccine manufacturer (please refer to the contact details on the vaccine packaging). It is never necessary to re-start a course of vaccination. Good clinical practice dictates that where possible the same brand of vaccine should be used to complete a course. If the same brand is unavailable then the SPC should be consulted and an alternative brand of the same vaccine (and the same schedule) should be considered. 2 References: 1. Salisbury D M, Begg N T. Department of Health, Immunisation Against Infectious Disease. UK: HMSO 1996; Ch. 7: Kassianos G. Immunisation: Childhood and Travel Health. 4th Edn. London: Blackwell Science 2001 Situations in which vaccination should be postponed or omitted 20 UK Guidance on Best Practice in Vaccine Administration
21 Consent Consent Obtaining consent has two functions: Clinical function to foster trust and co-operation with patients Legal function to ensure that a person s right to autonomy has been addressed in order to prevent a charge of battery Consent Cases heard in the civil courts have confirmed that individuals who have capacity to consent have the legal right to autonomy within decision making. 1,2,3 A person would be held to have capacity (competence) to consent if they are able to meet the threestage test of being able to: 2 Comprehend and retain the information Believe it Consider the facts and make an informed decision Adults All adults are assumed to have capacity to consent unless it can be demonstrated that they do not meet the criteria outlined above. Therefore, unless a person is unconscious and in a life threatening situation, consent must be obtained before any healthcare treatment is provided. 2 It must be voluntary and not obtained under pressure or duress. 3 If an adult lacks the capacity to consent (e.g. flu vaccination for elderly people with severe dementia in nursing homes), the law does not allow for consent or refusal by one adult (e.g. relative) on behalf of another. The decision to treat would be made by the health professional in charge of their care on the basis of the patient s best interests. However, in more serious situations it is advisable to discuss the options with relatives as they may be able to give a view on what the patient would have wanted when they did have capacity to consent. UK Guidance on Best Practice in Vaccine Administration 21
22 Consent Children Consent The DoH states: Before examining, treating or caring for a child, you must also seek consent. Young people aged 16 and 17 are presumed to have the competence to give consent for themselves. Younger children who understand fully what is involved in the proposed procedure can also give consent (although their parents will ideally be involved). In other cases, someone with parental responsibility must give consent on the child s behalf, unless they cannot be reached in an emergency. If a competent child consents to treatment, a parent cannot over-ride that consent. Legally, a parent can consent if a competent child refuses, but it is likely that taking such a serious step will be rare. 4 Implications for Practice Guidance from the DoH 4 and statutory bodies 5,6 to healthcare professionals advises that in order to ensure consent is valid, patients must be given adequate information on which to base their decision to proceed with or refuse treatment. Consent can be given verbally, given in writing or implied. The process of obtaining consent for vaccination should be the same whether the consent obtained is written, verbal or implied (e.g. holding out an arm to be vaccinated). Before the patient can make their decision they need to have a clear explanation and an opportunity to ask questions about: The need for vaccination The vaccine and number of doses required The risks associated with the disease the patient is being immunised against The risks/side effects associated with the vaccine 22 UK Guidance on Best Practice in Vaccine Administration
23 Consent Written consent is not a legal requirement. However, one advantage of the consent form is that it can be constructed to demonstrate what information was provided before consent was obtained and also that the patient had been given the opportunity to ask questions. People are entitled to change their minds and withdraw consent. Therefore, if an adult attends a course of vaccinations, consent should be checked at each visit. Any refusal of recommended vaccination should be recorded. Consent Parental consent for a young child s vaccination also needs to be obtained at each visit. The person giving the immunisation should be satisfied that parental consent has been obtained. This may be written authorisation or verbal (i.e. a telephone instruction). If in doubt, vaccination should be postponed. Management of parental refusal of child vaccination should be in line with local policy and may involve offering a parent the opportunity of a referral to the GP or community paediatrician so that they can discuss their concerns. References: 1. St George s Healthcare NHS Trust v S: Rv Collins and Others ex part S [1998] 2 FLR 728, CA 2. Re C (adult: refusal of medical treatment) All ER Re T (consent to treatment) FLR 458 CA 4. Department of Health. 12 Key Points on Consent: the Law in England General Medical Council. Seeking Patients Consent; the Ethical Considerations UK: GMC United Kingdom Central Council for Nursing, Midwifery and Health Visiting website 14 June 2001 UK Guidance on Best Practice in Vaccine Administration 23
24 24 UK Guidance on Best Practice in Vaccine Administration
25 Preparing the vaccine equipment Preparing the vaccine equipment Before starting a vaccination session or travel consultation, best practice ensures that the nurse: Is conversant with local policy on needle stick injury (see section: Needle stick injuries ) Makes sure that someone is aware that a vaccine session is about to begin Has all the equipment e.g. telephone, vaccines and stationery required (see Box 5) Has ensured the room is equipped with suitable seating, ventilation, lighting and a sink/hand cleansing facilities Has ensured there are sufficient chairs for all patients and carers BOX 5 Material Required Stationery: Pre-vaccination checklist/risk assessment questionnaire Preparing the vaccine equipment Consent has been obtained Patient information sheet available from Health Promotion England (HPE), the Health Education Board for Scotland (HEBS), Public Health Protection Division (PHPD) of the National Assembly for Wales and the Health Promotion Agency for Northern Ireland (via local Health and Social Services Boards/Trusts) (see section: Further information sources for contact details) Advice sheet on possible post-vaccination side effects and their management - also available from the organisations listed above Patient-held record cards /Personal Child Health Record (PCHR) Certificate of Vaccination (for yellow fever or meningitis for the Hajj) Any existing patient records Data collection forms child health immunisation record sheets/unscheduled immunisation forms UK Guidance on Best Practice in Vaccine Administration 25
26 Preparing the vaccine equipment BOX 5 Material Required (continued) Equipment for vaccination: Appropriately sized syringes 1ml and 2ml Selection of needles 21G, 38mm (1 1 /2 inches): 23G, 25mm (1 inch): 25G, 16mm ( 5 /8 inch) (please refer to section: Choice of needle ) Preparing the vaccine equipment Cotton wool Sharps container Gloves if required (need should be assessed for individual situations) Resuscitation equipment/drugs for anaphylaxis Oxygen and appropriate masks if available Before Vaccination Nurses should ensure that the appointment is long enough to: Assess patient s suitability for immunisation following a risk assessment Advise on possible side effects Answer patient queries Obtain informed consent Consult the patient s records if available Administer the vaccine Complete all documentation Patients will be more distressed and anxious if they feel that the nurse is in a hurry. There is also a higher risk of procedural omissions if a session is rushed. These issues need to be discussed when protocols are being drawn up, and nurses must bear in mind their own professional responsibilities when time is allocated for clinic sessions. Primary care employers and managers should seriously consider these needs. 26 UK Guidance on Best Practice in Vaccine Administration
27 Preparing the vaccine equipment Pre-vaccination Checklist Before vaccination, best practice ensures that: The recipient is fit and well - children can be vaccinated if they have minor infections e.g. upper respiratory tract infection, diarrhoea or otitis media without fever 1 There are no contraindications The patient or parent understands all aspects of vaccination including possible side effects give written information where required Consent has been granted References: 1. Salisbury D M, Begg N T. Department of Health, Immunisation Against Infectious Disease. UK: HMSO 1996; Ch. 5: 14, Ch. 7: Preparing the vaccine equipment UK Guidance on Best Practice in Vaccine Administration 27
28 28 UK Guidance on Best Practice in Vaccine Administration
29 Preparing and dealing with anxious Contents people Preparing and dealing with anxious people Many people of all ages are anxious about injections. Nervous mothers can pass on their anxiety to their young children. Teenagers and young adults can become particularly nervous, often due to peer pressure. Nurses should consider the following when giving injections to anxious patients: Adopt a calm, sympathetic approach Use distraction techniques with both adults and particularly children as these can be very effective (i.e. engage them in a conversation about a subject of interest, sport, TV or current topical story) 1 Prepare the vaccine and administer the injection (where possible) out of sight of the patient Explain that an injection is a quick and simple procedure and not the same as taking blood often individuals who have had bad experiences with blood tests think the procedures are similar Fainting Infants and babies rarely faint, 2 but can be quite shocked after the experience. It is often appropriate for a patient over this age who is particularly anxious or has a history of fainting to be given an injection lying down. Fainting is more common in individuals who have not recently eaten. Consideration should be given in such situations to the positioning of the individual receiving the vaccine in order to reduce any risk of injury should the patient faint. Preparing and dealing with anxious people Young Children If a parent or carer is particularly anxious about observing their child receive injections it is advisable to have another member of staff to assist holding the child during the procedure. It is also important to recognise that children appear to suffer less trauma when parents value vaccinations and the child has been prepared for what is going to happen. 1 UK Guidance on Best Practice in Vaccine Administration 29
30 Preparing and dealing with anxious people Where a child needs to be given more than one injection at a visit it can be helpful for two members of staff to inject simultaneously, as the child is usually not aware that two injections have been given and hence distress is minimised. This is usually appropriate for children who are old enough to receive injections in the upper arm (see section: Administration of vaccine Box 7). The procedure could follow that outlined below: Sit the child on the parent s lap with both arms accessible Face the child forward, looking at something of interest Engage the child in conversation Give both injections simultaneously, using a prearranged signal (e.g. count of three) Local Anaesthetic Cream Preparing and dealing with anxious people The routine use of a local anaesthetic cream is not practical in the primary care setting but is an option for occasional use in situations where fear of pain is preventing immunisation from occurring. As the cream is a prescription-only medication and it takes up to an hour to be effective, its use needs to be planned in advance. References: 1. Jacobson R, et al. Making vaccines more acceptable methods to prevent and minimize pain and other common adverse events associated with vaccines. Vaccine 2001; 19: Salisbury D M, Begg N T. Department of Health, Immunisation Against Infectious Disease. UK: HMSO 1996; Ch. 10: UK Guidance on Best Practice in Vaccine Administration
31 Preparation of vaccine Preparation of vaccine Vaccination is an aseptic technique and thus thorough hand washing and cleanliness of all equipment used are imperative. The nurse should: Check storage conditions (temperature monitor chart) Wash hands thoroughly Check the name and expiry date on the vaccine package Check that the colour and appearance of the vaccine are correct 1 Check the dose is appropriate for the patient s age Reconstitution Where a vaccine has to be reconstituted prior to administration, use only the diluent supplied and note the time scale in which it must be used after dilution (usually one to four hours). Nurses should ensure that: Neither the vaccine nor the diluent has passed its expiry date Each dose is drawn up as required - if a multidose vial is to be used, do not draw up in advance for the whole session Only one type of vaccine is mixed in each syringe, unless specifically stated by the manufacturer Any vaccine which contains particles or whose colour differs from the SPC description is discarded BOX 6 Reconstitution A green needle (21G x 38mm (1 1 /2 inch)) should be used to draw up the diluent and to inject it slowly into the ampoule containing the vaccine 1 Injecting diluent rapidly into the vaccine may cause frothing, which can affect the dilution and consequent potency of the vaccine; shaking the ampoule may have a similar effect Preparation of vaccine UK Guidance on Best Practice in Vaccine Administration 31
32 Preparation of vaccine BOX 6 Reconstitution (continued) If the freeze-dried powder does not instantly dissolve in the diluent, gently rotate the ampoule until it dissolves rather than shaking it Draw the appropriate dose up into a clean syringe and change the needle to the appropriate size and gauge for administration to the specific patient When removing liquid from a vacuum-sealed ampoule, first inject the equivalent measure of air to the volume of liquid to be removed When drawing up from a glass ampoule, use a needle gauge no larger than 21G to eliminate the possibility of glass fragments being drawn up Where possible change a needle after it has passed through a rubber bung before administration to a patient For quantities less than 1ml use a graduated 1ml syringe Discard unused reconstituted vaccines at the end of the session References: 1. Salisbury D M, Begg N T. Department of Health, Immunisation Against Infectious Disease. UK: HMSO 1996; Ch.5: 14 Preparation of vaccine 32 UK Guidance on Best Practice in Vaccine Administration
33 Administration of vaccine Administration of vaccine Skin cleansing is not necessary in socially clean patients. Soap and water are adequate where a nurse feels skin cleansing is required. If spirit swabs are used the skin should be allowed to dry before the vaccine is administered (this is essential for live vaccines which may be inactivated by alcohol). 1 Adopting the correct technique for immunisation is vital for: Administration of vaccine Minimising patient discomfort during and after the procedure Ensuring the optimum immune response in the patient Following risk assessment of the patient, nurses should decide upon the appropriate anatomical site, needle size and product. Injection Site and Route of Administration Nurses should be familiar with the: Route of administration for each vaccine administered Local guidelines in place Reference can be made to either the Green Book, local guidelines or the product SPC. Further guidance should be obtained from the prescribing doctor. Vaccines should be administered: Via either the intramuscular (IM) or deep sub-cutaneous (SC) route. 1 The exceptions to this are the oral polio and oral typhoid vaccines and the BCG, which is given by an intradermal (ID) technique 1 In the deltoid muscle or anterolateral aspect of the thigh Using the appropriate size and length of needle Consideration should be given to the correct method of administration in people with coagulopathies. UK Guidance on Best Practice in Vaccine Administration 33
34 Administration of vaccine Administration of vaccine BOX 7 Route of Administration There is general agreement that infants under one year should receive all vaccines in the anterolateral aspect of the thigh (see Figures 1a, b and c) since the deltoid muscle is not sufficiently developed Over the age of one there is an element of choice For older children and adults, the deltoid muscle is the preferred site (see Figures 2a and b) Do not use the gluteal muscle for vaccination, as it is highly unlikely that the vaccine will reach the muscle, and this may result in poor immune response to the vaccine (this has been demonstrated with hepatitis B vaccine 1,3 ). In addition there is a risk of damage to underlying structures such as the sciatic nerve The deltoid muscle is also easier to access in most patients and results in less embarrassment for older children and adults 34 UK Guidance on Best Practice in Vaccine Administration
35 Administration of vaccine Administration of vaccine Figure 1a:The anterolateral aspect of the thigh Source: The Australian Immunisation Handbook 2 Rectus Femoris Vastus Intermedius and Vastus Medialis Sartorius Fat Vastus Lateralis Femoral Artery and Vein Abductors Femur Hamstrings Sciatic Nerve Figure 1b:Cross section - anterolateral aspect of the thigh Source: The Australian Immunisation Handbook 2 UK Guidance on Best Practice in Vaccine Administration 35
36 Administration of vaccine Administration of vaccine Figure 1c:Administration of an IM injection to an infant using the anterolateral aspect of the thigh Source: Mike Wyndham, Medical Picture Collection 36 UK Guidance on Best Practice in Vaccine Administration
37 Administration of vaccine Achromion process Radial nerve Administration of vaccine Brachial artery Figure 2a:Left lateral view of the deltoid muscle the dots indicate the area parameters recommended for administration of the vaccine Figure 2b: Administration of an IM injection into the deltoid muscle of an adult UK Guidance on Best Practice in Vaccine Administration 37
38 References: 1. Salisbury D M, Begg N T. Department of Health, Immunisation Against Infectious Disease. UK: HMSO 1996; Ch. 5: National Health and Medical Research Council. The Australian Immunisation Handbook. 7th Edn. Canberra: Pirie Printers March Shaw FE, et al. Effect of anatomic injection site, age and smoking on the immune response to hepatitis B vaccination. Vaccine 1989; 7: UK Guidance on Best Practice in Vaccine Administration
39 Choice of needle Choice of needle The correct length and gauge of the needle are key in ensuring that the vaccine is delivered to the correct location as painlessly as possible and with maximum immunogenicity. The colour on the hub of the needle refers to the gauge rather than the length of the needle. Thus, while different gauges can be obtained in different lengths, Box 8 outlines the standard sizes in the UK. The higher the number referring to the gauge, the narrower the lumen of the needle. BOX 8 Standard UK Needle Gauge and Length Orange = 10mm ( 3 /8 inch) long or 16mm ( 5 /8 inch) long or 25mm (1 inch) long 25 gauge Choice of needle Blue = 25mm (1 inch) long 23 gauge Green = 38mm (1 1 /2 inches) long 21 gauge Whenever the SPC suggests that the vaccine can be given by either IM or deep SC route, the IM route is to be preferred. When giving an IM injection, the needle must be long enough to reach the muscle mass. The needle should be 25mm long to ensure it reaches muscle (in all but the smallest of babies 1 ). A study of the deltoid fat pad thickness in adults suggests that bodyweight and gender can help to determine the needle length appropriate for IM injection in the deltoid muscle of adults (see Box 9). 2 UK Guidance on Best Practice in Vaccine Administration 39
40 Choice of needle BOX 9 Recommended Choice of Needle Lengths Children = 25mm (1 inch) needle 3 Women <90kg = 25mm (1 inch) needle Women >90kg = 38mm (1 1 /2 inch) needle 2 Choice of needle Men kg = 25mm (1 inch) needle 2 It is a common misconception that the longer the needle, the more painful the injection will be. This is not the case as muscle fibres have fewer pain receptors than subcutaneous tissue. 4,5 In addition to this, there is a growing body of evidence to suggest that post-vaccination discomfort is reduced by correct delivery of the vaccine into muscle. 6,7,8 If the vaccine is required to be given via the SC route a shorter needle may be adequate, but the gauge of the lumen should also be considered. There is evidence that a wider lumen allows the vaccine to disseminate over a wider area of tissue, thus reducing irritation to the site. 9 A blue needle (23G x 25mm (1 inch)) should be used both for its wider lumen and because it will ensure an IM or a deep SC injection if the correct technique is adopted. Another common misconception is that smaller doses (e.g. 0.5ml) of vaccine are better tolerated than larger doses (e.g. 1ml) and produce fewer local reactions. However, evidence suggests that both local and systemic reactions at the vaccination site are similar for both 0.5ml and 1ml doses. 10,11,12,13 40 UK Guidance on Best Practice in Vaccine Administration
41 Choice of needle References: 1. Hick J, et al. Optimum needle length for diphtheria-tetanus-pertussis inoculation of Infants. Paediatrics 1989; 84(1): Poland G, et al. Determination of deltoid fat pad thickness. Implications for needle length in adult immunization. JAMA 1997; 277(21): WHO. Global Programme for Vaccines and Immunisation, Expanded Programme on Immunisation. Module 8: Giving Immunisations. Geneva, WHO Greenblatt D, Koch-Weser J. Intramuscular injection of drugs. NEJM 1976; 295(10): Campbell J. Injections. Professional Nurse 1995; 10(7): Zuckerman JN. The importance of injecting vaccines into muscle. BMJ 2000; 321: Diggle L, Deeks J. Effect of needle length on incidence of local reactions to routine immunisation in infants aged 4 months: randomised controlled trial. BMJ 2000; 321: Choice of needle 8. Mark A, Carlsson R, Granstrom M. Subcutaneous versus intramuscular injection for booster DT vaccination of adolescents. Vaccine 1999; 17: Ipp M, et al. Adverse reactions to diptheria, tetanus, pertussis-polio vaccination at 18 months of age: Effect of injection site and needle length. Paediatrics 1989; 83(5): Department of Health. Vaccine Update April 1999; Begg N T, et al. Antibody responses and symptoms after DTP and either tetanus or diphtheria Haemophilus influenzae type B conjugate vaccines given for primary immunisation by separate or mixed injection. Vaccine 1995; 13: Goldblatt D, et al. Interchangeability of conjugated Haemopilus influenzae type b vaccines during primary immunisation in infants. BMJ 1996; 312: Goilav C, et al. Immunogenicity and safety of a new inactivated hepatitis A vaccine in a comparative study. Journal of Medical Virology 1995; 46: UK Guidance on Best Practice in Vaccine Administration 41
42 42 UK Guidance on Best Practice in Vaccine Administration
43 Technique Technique Technique is important in determining where the vaccine will be deposited and its resulting efficacy. Patient Positioning Best practice ensures that: The appropriate limb is fully exposed avoid having tight clothing above the injection site Patients are encouraged to sit while they are vaccinated (or to lie down if they are prone to fainting or particularly anxious) The upper arm is fully exposed, which may mean removing a shirt rather than rolling the sleeve up. This is important, because: Otherwise the vaccine may be given too low and end up as an SC rather than IM injection with subsequent local reactions and suboptimal immune response A tight shirt sleeve can act as a tourniquet and encourage bleeding at the injection site The muscle is relaxed encourage the patient to either: Let their arm hang by their side Rest their hand on their hip/lap When giving injections to babies and children, best practice ensures that: Technique Babies and infants are held on their parent/carer s knee (see Figure 1c) The parent understands the importance of not allowing the limb to move during the procedure and holds the child gently but firmly The child s free arm is tucked behind the parent and the child cuddled into their body when the injection is to be given in the deltoid The arm to be injected is held close to the child s body the parent can hold the forearm to prevent movement While older children may choose to sit on their own, the parent may still be required to help hold the limb still UK Guidance on Best Practice in Vaccine Administration 43
Patient Group Directions. Guidance and information for nurses
 Patient Group Directions Guidance and information for nurses Patient Group Directions Guidance and information for nurses Contents Introduction 4 What is a patient group direction (PGD)? 4 When can PGDs
Patient Group Directions Guidance and information for nurses Patient Group Directions Guidance and information for nurses Contents Introduction 4 What is a patient group direction (PGD)? 4 When can PGDs
Children & Young People s Directorate. School Nursing Procedure For Administration of Immunisations
 Children & Young People s Directorate School Nursing Procedure For Administration of Immunisations Author Mrs Gladys Bleakley & Mrs Barbara Ervine Directorate responsible for Children & Young Peoples this
Children & Young People s Directorate School Nursing Procedure For Administration of Immunisations Author Mrs Gladys Bleakley & Mrs Barbara Ervine Directorate responsible for Children & Young Peoples this
PROCEDURE FOR THE ADMINISTRATION OF MEDICATION BY INJECTION VIA THE INTRAMUSCULAR ROUTE OR VIA THE SUBCUTANEOUS ROUTE
 PROCEDURE FOR THE ADMINISTRATION OF MEDICATION BY INJECTION VIA THE INTRAMUSCULAR OR VIA THE SUBCUTANEOUS First Issued Issue Version Purpose of Issue/Description of Change Planned Review Date October 2012
PROCEDURE FOR THE ADMINISTRATION OF MEDICATION BY INJECTION VIA THE INTRAMUSCULAR OR VIA THE SUBCUTANEOUS First Issued Issue Version Purpose of Issue/Description of Change Planned Review Date October 2012
Draft guidance for registered pharmacies preparing unlicensed medicines
 Draft guidance for registered pharmacies preparing unlicensed medicines January 2014 1 The General Pharmaceutical Council is the regulator for pharmacists, pharmacy technicians and registered pharmacies
Draft guidance for registered pharmacies preparing unlicensed medicines January 2014 1 The General Pharmaceutical Council is the regulator for pharmacists, pharmacy technicians and registered pharmacies
An introduction to the principles and practice of safe and effective administration of injections
 An introduction to the principles and practice of safe and effective administration of injections Introduction Giving an injection safely is considered to be a routine nursing activity. However it requires
An introduction to the principles and practice of safe and effective administration of injections Introduction Giving an injection safely is considered to be a routine nursing activity. However it requires
NHS Professionals. Guidelines for the Administration of Medicines
 NHS Professionals Guidelines for the Administration of Medicines Introduction The control of medicines in the United Kingdom is primarily through the Medicines Act (1968) and associated British and European
NHS Professionals Guidelines for the Administration of Medicines Introduction The control of medicines in the United Kingdom is primarily through the Medicines Act (1968) and associated British and European
THE USE OF DRUGS BEYOND (OFF-LABEL) AND WITHOUT (UNLICENSED) MARKETING AUTHORIZATION
 THE USE OF DRUGS BEYOND (OFF-LABEL) AND WITHOUT (UNLICENSED) MARKETING AUTHORIZATION The use of drugs for off-label purposes is widespread. Surveys suggest that up to 1/4 of all prescriptions in palliative
THE USE OF DRUGS BEYOND (OFF-LABEL) AND WITHOUT (UNLICENSED) MARKETING AUTHORIZATION The use of drugs for off-label purposes is widespread. Surveys suggest that up to 1/4 of all prescriptions in palliative
Policy Document Control Page
 Policy Document Control Page Title Title: Policy for the storage, handling, distribution and disposal of vaccines Version: Version 2 Reference Number: CL98 Supersedes Supersedes: Version 1 Description
Policy Document Control Page Title Title: Policy for the storage, handling, distribution and disposal of vaccines Version: Version 2 Reference Number: CL98 Supersedes Supersedes: Version 1 Description
Vaccination against pertussis (whooping cough) - the replacement of Repevax with Boostrix -IPV an update for registered healthcare practitioners
 Vaccination against pertussis (whooping cough) - the replacement of Repevax with Boostrix -IPV an update for registered healthcare practitioners Questions and Answers May 2014 Health Protection Scotland
Vaccination against pertussis (whooping cough) - the replacement of Repevax with Boostrix -IPV an update for registered healthcare practitioners Questions and Answers May 2014 Health Protection Scotland
National Minimum Standards for Immunisation Training
 National Minimum Standards for Immunisation Training Contributors The following formed the advisory group which, hosted by the Health Protection Agency, produced these Minimum Standards for Immunisation
National Minimum Standards for Immunisation Training Contributors The following formed the advisory group which, hosted by the Health Protection Agency, produced these Minimum Standards for Immunisation
NHSG/PGD/lido_eton/MGPG412 Organisation Wide
 Title: Patient Group Direction For The Administration Of Lidocaine 2% Injection For The Insertion/Removal Of The 68mg Etonogestrel Contraceptive Implant By Nurses And Midwives Working Within NHS Grampian
Title: Patient Group Direction For The Administration Of Lidocaine 2% Injection For The Insertion/Removal Of The 68mg Etonogestrel Contraceptive Implant By Nurses And Midwives Working Within NHS Grampian
11 MEDICATION MANAGEMENT
 1 11 MEDICATION MANAGEMENT OVERVIEW OF MEDICATION MANAGEMENT Depending on the size, structure and functions of the health facility, there may be a pharmacy with qualified pharmacists to dispense medication,
1 11 MEDICATION MANAGEMENT OVERVIEW OF MEDICATION MANAGEMENT Depending on the size, structure and functions of the health facility, there may be a pharmacy with qualified pharmacists to dispense medication,
Emergency Treatment of an Anaphylactic Reaction in the Community Protocol
 Emergency Treatment of an Anaphylactic Reaction in the Community Protocol Reference Number: NHSCT/09/216 Responsible Directorate: Children s Services Replaces (if appropriate): Northern Trust Departmental
Emergency Treatment of an Anaphylactic Reaction in the Community Protocol Reference Number: NHSCT/09/216 Responsible Directorate: Children s Services Replaces (if appropriate): Northern Trust Departmental
Patient Group Direction For The Administration Of Rotavirus Vaccine Rotarix By Nurses, Midwives and Health Visitors Working Within NHS Grampian
 Patient Group Direction For The Administration Of Rotavirus Vaccine Rotarix By Nurses, Midwives and Health Visitors Working Within NHS Grampian Co-ordinators: Adapted from National PGD See Page 1 Consultation
Patient Group Direction For The Administration Of Rotavirus Vaccine Rotarix By Nurses, Midwives and Health Visitors Working Within NHS Grampian Co-ordinators: Adapted from National PGD See Page 1 Consultation
Professional Standards and Guidance for the Sale and Supply of Medicines
 Professional Standards and Guidance for the Sale and Supply of Medicines About this document The Code of Ethics sets out seven principles of ethical practice that you must follow as a pharmacist or pharmacy
Professional Standards and Guidance for the Sale and Supply of Medicines About this document The Code of Ethics sets out seven principles of ethical practice that you must follow as a pharmacist or pharmacy
Promoting safer use of injectable medicines
 Promoting safer use of injectable medicines A template standard operating procedure for: prescribing, preparing and administering injectable medicines in clinical areas Introduction The use of injectable
Promoting safer use of injectable medicines A template standard operating procedure for: prescribing, preparing and administering injectable medicines in clinical areas Introduction The use of injectable
Medicines Management
 Medicines Management Patient Group Direction for the Supply/administration of Adrenaline (Epinephrine) for Treatment of Anaphylaxis by accredited community Pharmacists. Rationale To enable a pharmacist,
Medicines Management Patient Group Direction for the Supply/administration of Adrenaline (Epinephrine) for Treatment of Anaphylaxis by accredited community Pharmacists. Rationale To enable a pharmacist,
Use of medicines outside of their UK marketing authorisation in pain management and palliative medicine
 The British Pain Society Use of medicines outside of their UK marketing authorisation in pain management and palliative medicine This is a consensus document prepared on behalf of the British Pain Society
The British Pain Society Use of medicines outside of their UK marketing authorisation in pain management and palliative medicine This is a consensus document prepared on behalf of the British Pain Society
Policy Document Control Page
 Policy Document Control Page Title Title: Covert Administration of Medicines Version: Version 6 Reference Number: CL37 Supersedes Supersedes: Version 5 Description of amendment(s): Originator 3.5 Clarification
Policy Document Control Page Title Title: Covert Administration of Medicines Version: Version 6 Reference Number: CL37 Supersedes Supersedes: Version 5 Description of amendment(s): Originator 3.5 Clarification
Immunisation Services - Authority for Registered Nurses and Midwives
 Policy Directive Ministry of Health, NSW 73 Miller Street North Sydney NSW 2060 Locked Mail Bag 961 North Sydney NSW 2059 Telephone (02) 9391 9000 Fax (02) 9391 9101 http://www.health.nsw.gov.au/policies/
Policy Directive Ministry of Health, NSW 73 Miller Street North Sydney NSW 2060 Locked Mail Bag 961 North Sydney NSW 2059 Telephone (02) 9391 9000 Fax (02) 9391 9101 http://www.health.nsw.gov.au/policies/
Document Title: PCT Doc Ref.: Version No.: 1/2014. Local Doc Ref.:
 Document Title: Patient Group Direction (PGD) for the vaccination of pregnant women (gestation week 28 onwards) and new mothers against pertussis (Whooping Cough) using Boostrix-IPV (Diphtheria, tetanus,
Document Title: Patient Group Direction (PGD) for the vaccination of pregnant women (gestation week 28 onwards) and new mothers against pertussis (Whooping Cough) using Boostrix-IPV (Diphtheria, tetanus,
Protocol for ordering, storing and handling vaccines. March 2014
 Protocol for ordering, storing and handling vaccines March 2014 Introduction This protocol applies to all staff involved in immunisation. It aims to ensure that vaccines are stored and managed properly
Protocol for ordering, storing and handling vaccines March 2014 Introduction This protocol applies to all staff involved in immunisation. It aims to ensure that vaccines are stored and managed properly
MEDICATION MANUAL Policy & Procedure
 MEDICATION MANUAL Policy & Procedure TITLE: Section: Initial Management of Anaphylaxis Following Immunization Medication Specific NUMBER: MM 20-005 Date Issued: October 2009 Source: Distribution: Capital
MEDICATION MANUAL Policy & Procedure TITLE: Section: Initial Management of Anaphylaxis Following Immunization Medication Specific NUMBER: MM 20-005 Date Issued: October 2009 Source: Distribution: Capital
Vaccination against pertussis (Whooping cough) for pregnant women- 2014. Information for healthcare professionals
 Vaccination against pertussis (Whooping cough) for pregnant women- 2014 Information for healthcare professionals About Public Health England Public Health England s mission is to protect and improve the
Vaccination against pertussis (Whooping cough) for pregnant women- 2014 Information for healthcare professionals About Public Health England Public Health England s mission is to protect and improve the
2 months Diptheria; Tetanus; Whooping Cough; Hib & Polio 1st dose Pneumococcal Conjugate Vaccination
 IMMUNISATIONS You may want to know if your child should have routine immunisations and whether there could be an increased risk of complications because of the heart condition. We have sought the opinions
IMMUNISATIONS You may want to know if your child should have routine immunisations and whether there could be an increased risk of complications because of the heart condition. We have sought the opinions
MULTI AGENCY POLICY FOR THE ADMINISTRATION OF MEDICATION AND HEALTH CARE PROCEDURES:
 MULTI AGENCY POLICY FOR THE ADMINISTRATION OF MEDICATION AND HEALTH CARE PROCEDURES: Early years provision, Educational Establishments and Voluntary Services Document reference number: C O R P O L O 0
MULTI AGENCY POLICY FOR THE ADMINISTRATION OF MEDICATION AND HEALTH CARE PROCEDURES: Early years provision, Educational Establishments and Voluntary Services Document reference number: C O R P O L O 0
Factsheet September 2012. Pertussis immunisation for pregnant women. Introduction
 Factsheet September 2012 Pertussis immunisation for pregnant women Introduction The routine childhood immunisation programme has been very effective in reducing the overall numbers of cases of pertussis.
Factsheet September 2012 Pertussis immunisation for pregnant women Introduction The routine childhood immunisation programme has been very effective in reducing the overall numbers of cases of pertussis.
Suturing Policy for Nurses in Emergency Departments
 This is an official Northern Trust policy and should not be edited in any way Suturing Policy for Nurses in Emergency Departments Reference Number: NHSCT/12/545 Target audience: Registered Nurses Sources
This is an official Northern Trust policy and should not be edited in any way Suturing Policy for Nurses in Emergency Departments Reference Number: NHSCT/12/545 Target audience: Registered Nurses Sources
TEMPORARY PROGRAMME PERTUSSIS VACCINATION FOR PREGNANT WOMEN
 TEMPORARY PROGRAMME PERTUSSIS VACCINATION FOR PREGNANT WOMEN Richard Smithson Neil Irvine Maureen McCartney Consultant Health Protection October 2012 Pertussis/whooping cough The disease Whooping Cough
TEMPORARY PROGRAMME PERTUSSIS VACCINATION FOR PREGNANT WOMEN Richard Smithson Neil Irvine Maureen McCartney Consultant Health Protection October 2012 Pertussis/whooping cough The disease Whooping Cough
Margaret Wing, B.Sc. Pharmacy, MBA Alberta Pharmacists Association (RxA) Director of Professional Services September 19, 2009
 Margaret Wing, B.Sc. Pharmacy, MBA Alberta Pharmacists Association (RxA) Director of Professional Services September 19, 2009 A health care provider who is administering drugs by injection, should only
Margaret Wing, B.Sc. Pharmacy, MBA Alberta Pharmacists Association (RxA) Director of Professional Services September 19, 2009 A health care provider who is administering drugs by injection, should only
Yes This controlled document shall not be copied in part or whole without the express permission of the author or the author s representative.
 Title: Patient Group Direction for the administration of lidocaine hydrochloride 1% injection as infiltration anaesthesia for insertion/removal of central venous catheters by nurses/radiographers working
Title: Patient Group Direction for the administration of lidocaine hydrochloride 1% injection as infiltration anaesthesia for insertion/removal of central venous catheters by nurses/radiographers working
Introduction 2. 1. The Role of Pharmacy Within a NHS Trust 3. 2. Pharmacy Staff 4. 3. Pharmacy Facilities 5. 4. Pharmacy and Resources 6
 Index Index Section Page Introduction 2 1. The Role of Pharmacy Within a NHS Trust 3 2. Pharmacy Staff 4 3. Pharmacy Facilities 5 4. Pharmacy and Resources 6 5. Prescription Charges 7 6. Communication
Index Index Section Page Introduction 2 1. The Role of Pharmacy Within a NHS Trust 3 2. Pharmacy Staff 4 3. Pharmacy Facilities 5 4. Pharmacy and Resources 6 5. Prescription Charges 7 6. Communication
Routine Immunization Procedures. Section 4. Newfoundland and Labrador Immunization Manual
 Newfoundland and Labrador Immunization Manual Section 4 Routine Immunization Procedures General Topics Related to Immunization Injection... 4.1-1 Procedure for Intramuscular Injection... 4.2-1 Procedure
Newfoundland and Labrador Immunization Manual Section 4 Routine Immunization Procedures General Topics Related to Immunization Injection... 4.1-1 Procedure for Intramuscular Injection... 4.2-1 Procedure
Medication Management Guidelines for Nurses and Midwives
 Medication Management Guidelines for Nurses and Midwives 1. Introduction As the statutory body responsible for the regulation of nursing and midwifery practice in Western Australia (WA), the Nurses & Midwives
Medication Management Guidelines for Nurses and Midwives 1. Introduction As the statutory body responsible for the regulation of nursing and midwifery practice in Western Australia (WA), the Nurses & Midwives
Guideline for the Safe Handling and Administration of Subcutaneous Cyotoxic Chemotherapy for Adults in the Community Setting
 Guideline for the Safe Handling and Administration of Subcutaneous Cyotoxic Chemotherapy for Adults in the Community Setting Guideline for the Safe Handling and Administration of Subcutaneous Cyotoxic
Guideline for the Safe Handling and Administration of Subcutaneous Cyotoxic Chemotherapy for Adults in the Community Setting Guideline for the Safe Handling and Administration of Subcutaneous Cyotoxic
Guideline for the Administration of Insulin by Nursing Staff
 Guideline for the Administration of Insulin by Nursing Staff Aims and objectives In Lanarkshire the number of people with Diabetes on insulin treatment is growing, as both the population ages and people
Guideline for the Administration of Insulin by Nursing Staff Aims and objectives In Lanarkshire the number of people with Diabetes on insulin treatment is growing, as both the population ages and people
HSE Guidelines for maintaining the vaccine cold-chain including maintenance of vaccine fridges and management of vaccines
 HSE Guidelines for maintaining the vaccine cold-chain including maintenance of vaccine fridges and management of vaccines Document reference number Revision number Approval date NIO01 Document developed
HSE Guidelines for maintaining the vaccine cold-chain including maintenance of vaccine fridges and management of vaccines Document reference number Revision number Approval date NIO01 Document developed
Good practice for the preparation of injectable medicines in clinical areas
 Further reading Dougherty L and Lister S (Eds). The Royal Marsden Hospital Manual of Clinical Nursing Procedures (th edition), Blackwell Publishing, Oxford, 00. NMC standards for medicines management (008).
Further reading Dougherty L and Lister S (Eds). The Royal Marsden Hospital Manual of Clinical Nursing Procedures (th edition), Blackwell Publishing, Oxford, 00. NMC standards for medicines management (008).
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
 SOMAVERT pegvisomant for injection PATIENT INFORMATION SOMAVERT (SOM-ah-vert) (pegvisomant for injection) Read the patient information that comes with SOMAVERT before you start using it and each time you
SOMAVERT pegvisomant for injection PATIENT INFORMATION SOMAVERT (SOM-ah-vert) (pegvisomant for injection) Read the patient information that comes with SOMAVERT before you start using it and each time you
INSULIN INJECTION KNOW-HOW
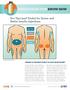 0-0- 0 INSULIN INJECTION KNOW-HOW pro tips (and tricks) for easier and better Insulin Injections ABDOMEN THIGHS BUTTOCKS ARMS recommended injection sites WHERE IS THE BEST PLACE TO GIVE INJECTIONS? 0-
0-0- 0 INSULIN INJECTION KNOW-HOW pro tips (and tricks) for easier and better Insulin Injections ABDOMEN THIGHS BUTTOCKS ARMS recommended injection sites WHERE IS THE BEST PLACE TO GIVE INJECTIONS? 0-
The Cold Chain and Vaccine storage
 The Cold Chain and Vaccine storage Cliona Kiersey Chief Pharmacist National Immunisation Office Objectives To explain the cold chain and vaccine delivery service including importance of using vaccine returns
The Cold Chain and Vaccine storage Cliona Kiersey Chief Pharmacist National Immunisation Office Objectives To explain the cold chain and vaccine delivery service including importance of using vaccine returns
Issue: June 2009 PROFESSIONAL STANDARDS AND GUIDANCE FOR THE SALE AND SUPPLY OF MEDICINES
 Issue: June 2009 PROFESSIONAL AND GUIDANCE FOR THE SALE AND SUPPLY OF MEDICINES PROFESSIONAL AND GUIDANCE FOR THE SALE AND SUPPLY OF MEDICINES CONTENTS Status of this document About this document 1 Pharmaceutical
Issue: June 2009 PROFESSIONAL AND GUIDANCE FOR THE SALE AND SUPPLY OF MEDICINES PROFESSIONAL AND GUIDANCE FOR THE SALE AND SUPPLY OF MEDICINES CONTENTS Status of this document About this document 1 Pharmaceutical
INJECTION TECHNIQUE. IVF NURSING OFFICE: (301) 400-2151 Darshana (301) 400-2146 Nicole
 IVF NURSING OFFICE: (301) 400-2151 Darshana (301) 400-2146 Nicole PLEASE NOTE: If you do not have medications for the next day s dose, you MUST go to the clinic that morning at 6:30 AM for more medications.
IVF NURSING OFFICE: (301) 400-2151 Darshana (301) 400-2146 Nicole PLEASE NOTE: If you do not have medications for the next day s dose, you MUST go to the clinic that morning at 6:30 AM for more medications.
NIH Clinical Center Patient Education Materials Giving a subcutaneous injection
 NIH Clinical Center Patient Education Materials What is a subcutaenous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous injection into the fatty
NIH Clinical Center Patient Education Materials What is a subcutaenous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous injection into the fatty
The Practice Nurse is accountable to the Managing Medical Principal for clinical issues and Practice Manager for non-clinical issues.
 Job Description Nurse Overview The Practice Nurse is a multi-skilled practitioner who, as part of a team, contributes to the provision of high-quality health care in the general practice setting. Her/his
Job Description Nurse Overview The Practice Nurse is a multi-skilled practitioner who, as part of a team, contributes to the provision of high-quality health care in the general practice setting. Her/his
Pertussis (whooping cough) immunisation for pregnant women
 Factsheet September 2012 Pertussis (whooping cough) immunisation for pregnant women Immunisation DEPARTMENT OF HEALTH Rheynn Slaynt The routine childhood immunisation programme has been very effective
Factsheet September 2012 Pertussis (whooping cough) immunisation for pregnant women Immunisation DEPARTMENT OF HEALTH Rheynn Slaynt The routine childhood immunisation programme has been very effective
1 Temperature Monitoring of Medication and Vaccine Fridges
 1 Policy Fridges used to store medications and vaccines are monitored to ensure the viability of the medications and vaccines. Food and medications must be stored in separate fridges to guard against contamination.
1 Policy Fridges used to store medications and vaccines are monitored to ensure the viability of the medications and vaccines. Food and medications must be stored in separate fridges to guard against contamination.
Guidelines and Procedure for the Safe Administration and Management of Medicines
 Appendix 7 Guidelines and Procedure for the Safe Administration and Management of Medicines 1. INTRODUCTION 1.1 This procedure must be read in conjunction with the Policy for the Administration of Medication
Appendix 7 Guidelines and Procedure for the Safe Administration and Management of Medicines 1. INTRODUCTION 1.1 This procedure must be read in conjunction with the Policy for the Administration of Medication
Patient Information Leaflet
 Patient Information Leaflet METHOTREXATE We hope this fact sheet will provide you with some information about Methotrexate and answer some of the questions you may have. Methotrexate is available in tablet
Patient Information Leaflet METHOTREXATE We hope this fact sheet will provide you with some information about Methotrexate and answer some of the questions you may have. Methotrexate is available in tablet
Information for you Abortion care
 Information for you Abortion care Published in February 2012 This information is for you if you are considering having an abortion. It tells you: how you can access abortion services the care you can expect
Information for you Abortion care Published in February 2012 This information is for you if you are considering having an abortion. It tells you: how you can access abortion services the care you can expect
Clinical Performance Director of Nursing Allison Bussey
 PGD 0314 Patient Group Direction Administration of Adrenaline (Epinephrine) 1:1000 (1mg/ml) Injection By Registered Nurses employed by South Staffordshire & Shropshire Healthcare Foundation NHS Trust This
PGD 0314 Patient Group Direction Administration of Adrenaline (Epinephrine) 1:1000 (1mg/ml) Injection By Registered Nurses employed by South Staffordshire & Shropshire Healthcare Foundation NHS Trust This
I B2.4. Design of the patient information leaflet for VariQuin
 (English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
(English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
patient group direction
 DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
Standards for medicines management Guidance for nurses and midwives
 Record keeping Standards for medicines management Guidance for nurses and midwives 1 15105_Record Keeping_A5_proof 3.indd 1 09/03/2010 09:47 We are the nursing and midwifery regulator for England, Wales,
Record keeping Standards for medicines management Guidance for nurses and midwives 1 15105_Record Keeping_A5_proof 3.indd 1 09/03/2010 09:47 We are the nursing and midwifery regulator for England, Wales,
Emergency Treatment of Anaphylactic Reactions
 Emergency Treatment of Anaphylactic Reactions Authorising Officer Signature Of Authorising Officer: Tom Cahill, Deputy Chief Executive Version: V3 Ratified By: HPFT Drugs and Therapeutic Committee Date
Emergency Treatment of Anaphylactic Reactions Authorising Officer Signature Of Authorising Officer: Tom Cahill, Deputy Chief Executive Version: V3 Ratified By: HPFT Drugs and Therapeutic Committee Date
Pertussis (whooping cough) immunisation for pregnant women the safest way to protect yourself and your baby
 Factsheet September 2012 Pertussis (whooping cough) immunisation for pregnant women the safest way to protect yourself and your baby The routine childhood immunisation programme has been very effective
Factsheet September 2012 Pertussis (whooping cough) immunisation for pregnant women the safest way to protect yourself and your baby The routine childhood immunisation programme has been very effective
Immunizing Children Against Haemophilus influenzae type B (Hib) A Training Module For Vaccinators
 Immunizing Children Against Haemophilus influenzae type B (Hib) A Training Module For Vaccinators Training Resource Series, Revised March 2006 Adapting This Training Module for Your Program This module
Immunizing Children Against Haemophilus influenzae type B (Hib) A Training Module For Vaccinators Training Resource Series, Revised March 2006 Adapting This Training Module for Your Program This module
Objectives At the completion of this module, unlicensed assistive personnel (UAP) should be able to:
 Objectives At the completion of this module, unlicensed assistive personnel (UAP) should be able to: 1. administer medications by subcutaneous injections. 2. document medication administration in the client
Objectives At the completion of this module, unlicensed assistive personnel (UAP) should be able to: 1. administer medications by subcutaneous injections. 2. document medication administration in the client
Importer / Manufacturer: MSD (THAILAND) LTD./ Merck & Co.,Inc., West Point, Pennsylvania 19486 SUMMARY OF PRODUCT CHARACTERISTICS
 Registration No. 1C 68/47(NC) Importer / Manufacturer: MSD (THAILAND) LTD./ Merck & Co.,Inc., West Point, Pennsylvania 19486 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICAL PRODUCT VARIVAX [Varicella
Registration No. 1C 68/47(NC) Importer / Manufacturer: MSD (THAILAND) LTD./ Merck & Co.,Inc., West Point, Pennsylvania 19486 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICAL PRODUCT VARIVAX [Varicella
FAQs on Influenza A (H1N1-2009) Vaccine
 FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
Guidelines for VACCINATIONS in General Practice. July 2015
 Guidelines for VACCINATIONS in General Practice July 2015 Table of Contents Executive Summary 3 1 Purpose 4 2 Scope of the Guidelines 4 3 Immunisation Schedules 5 3.1 Introduction 5 3.2 Primary Childhood
Guidelines for VACCINATIONS in General Practice July 2015 Table of Contents Executive Summary 3 1 Purpose 4 2 Scope of the Guidelines 4 3 Immunisation Schedules 5 3.1 Introduction 5 3.2 Primary Childhood
MENINGOCOCCAL GROUP B VACCINE (BEXSERO) Information for Health Professionals
 MENINGOCOCCAL GROUP B VACCINE (BEXSERO) Information for Health Professionals What is Bexsero vaccine? Bexsero is a meningococcal Group B (MenB) vaccine which is indicated for the active immunisation of
MENINGOCOCCAL GROUP B VACCINE (BEXSERO) Information for Health Professionals What is Bexsero vaccine? Bexsero is a meningococcal Group B (MenB) vaccine which is indicated for the active immunisation of
PRIMARY CARE PRACTICE GUIDELINES
 1 of 6 1. OUTCOME To identify anaphylaxis in the primary care setting and provide an evidence informed emergency response utilizing the most current provincial and federal practice guidelines. 2. DEFINITIONS
1 of 6 1. OUTCOME To identify anaphylaxis in the primary care setting and provide an evidence informed emergency response utilizing the most current provincial and federal practice guidelines. 2. DEFINITIONS
STANDARD OPERATING PROCEDURE. Administration of High Dose Muscular Vitamin Supplements for Undergoing Alcohol
 STANDARD OPERATING PROCEDURE Administration of High Dose Muscular Vitamin Supplements for Undergoing Alcohol DOCUMENT CONTROL: Version: 2 Ratified by: Clinical Effectiveness Committee Date ratified: 03
STANDARD OPERATING PROCEDURE Administration of High Dose Muscular Vitamin Supplements for Undergoing Alcohol DOCUMENT CONTROL: Version: 2 Ratified by: Clinical Effectiveness Committee Date ratified: 03
Draft guidance for registered pharmacies providing internet and distance sale, supply or service provision
 Draft guidance for registered pharmacies providing internet and distance sale, supply or service provision September 2014 1 The General Pharmaceutical Council is the regulator for pharmacists, pharmacy
Draft guidance for registered pharmacies providing internet and distance sale, supply or service provision September 2014 1 The General Pharmaceutical Council is the regulator for pharmacists, pharmacy
2. Characteristics of staff Qualifications required. Additional requirements. Continued education & training requirements
 Patient Group Direction The supply of Azithromycin 1g as a single dose by accredited Community Pharmacists to patients in receipt of a positive test result to Chlamydia trachomatis, and treatment of their
Patient Group Direction The supply of Azithromycin 1g as a single dose by accredited Community Pharmacists to patients in receipt of a positive test result to Chlamydia trachomatis, and treatment of their
National Chlamydia Screening Programme September 2012 PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF AZITHROMYCIN FOR CHLAMYDIA TRACHOMATIS
 PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF AZITHROMYCIN FOR CHLAMYDIA TRACHOMATIS Below is a template that can be used to produce a local patient group direction (PGD) for the administration of
PATIENT GROUP DIRECTION FOR THE ADMINISTRATION OF AZITHROMYCIN FOR CHLAMYDIA TRACHOMATIS Below is a template that can be used to produce a local patient group direction (PGD) for the administration of
BE SURE. BE SAFE. VACCINATE.
 DON T GET OR GIVE THE FLU THIS YEAR THANK YOU Vaccination is the only protection. www.immunisation.ie BE SURE. BE SAFE. VACCINATE. FLU VACCINE 2013-2014 Healthcare workers prevent the spread of flu and
DON T GET OR GIVE THE FLU THIS YEAR THANK YOU Vaccination is the only protection. www.immunisation.ie BE SURE. BE SAFE. VACCINATE. FLU VACCINE 2013-2014 Healthcare workers prevent the spread of flu and
Protecting your baby against meningitis and septicaemia
 Protecting your baby against meningitis and septicaemia caused by meningococcal B bacteria MenB vaccine now available! Information about the MenB vaccine and recommended paracetamol use mmunisation The
Protecting your baby against meningitis and septicaemia caused by meningococcal B bacteria MenB vaccine now available! Information about the MenB vaccine and recommended paracetamol use mmunisation The
Local Enhanced Service Specification for the Supply of Pharmaceutical Services to Care Homes through Community Pharmacy
 Local Enhanced Service Specification for the Supply of Pharmaceutical Services to Care Homes through Community Pharmacy Contents: 1. Introduction and purpose 2. Period of Service 3. Aim of the Service
Local Enhanced Service Specification for the Supply of Pharmaceutical Services to Care Homes through Community Pharmacy Contents: 1. Introduction and purpose 2. Period of Service 3. Aim of the Service
Parliament of Australia Department of Parliamentary Services
 Parliament of Australia Department of Parliamentary Services Operating Policies and Procedures No. 2 Role of the Nurses Centre Date: 17 February 2011 Expiry: December 2012 Approved: Secretary File No:
Parliament of Australia Department of Parliamentary Services Operating Policies and Procedures No. 2 Role of the Nurses Centre Date: 17 February 2011 Expiry: December 2012 Approved: Secretary File No:
Transition to Community Nursing Practice
 Transition to Community Nursing Practice Contents Section A - Thinking about working in the community Chapter 1 - What is community nursing Chapter 2 - Making the transition Section B - Working in the
Transition to Community Nursing Practice Contents Section A - Thinking about working in the community Chapter 1 - What is community nursing Chapter 2 - Making the transition Section B - Working in the
This document was published by: The National Department of Health. Editorial team: Provincial EPI Team National EPI Team Dr NJ Ngcobo
 REVISED : OCTOBER 2010 This document was published by: The National Department of Health Editorial team: Provincial EPI Team National EPI Team Dr NJ Ngcobo Copies may be obtained from: The National Department
REVISED : OCTOBER 2010 This document was published by: The National Department of Health Editorial team: Provincial EPI Team National EPI Team Dr NJ Ngcobo Copies may be obtained from: The National Department
PATIENT INFORMATION SHEET
 PATIENT INFORMATION SHEET Surgical and large bore pleural procedures in Malignant pleural Mesothelioma And Radiotherapy Trial (SMART trial) Stoke Mandeville Hospital Mandeville Road Aylesbury Buckinghamshire
PATIENT INFORMATION SHEET Surgical and large bore pleural procedures in Malignant pleural Mesothelioma And Radiotherapy Trial (SMART trial) Stoke Mandeville Hospital Mandeville Road Aylesbury Buckinghamshire
A patient guide to the use of insulin for diabetes
 A patient guide to the use of insulin for diabetes Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
A patient guide to the use of insulin for diabetes Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
TdaP-Booster (tee-dee-ay-pee boo-ster)
 New Zealand Consumer Medicine Information TdaP-Booster (tee-dee-ay-pee boo-ster) Tetanus, diphtheria and pertussis (acellular mono-component) vaccine (adsorbed, reduced antigen content) CONSUMER MEDICINE
New Zealand Consumer Medicine Information TdaP-Booster (tee-dee-ay-pee boo-ster) Tetanus, diphtheria and pertussis (acellular mono-component) vaccine (adsorbed, reduced antigen content) CONSUMER MEDICINE
IVF CLASS. IVF NURSE CONTACT INFORMATION: Darshana 301-400-2151, darshana.naik.ctr@health.mil Nicole 301-400-2146, nicole.l.sobers.ctr@health.
 IVF CLASS IVF NURSE CONTACT INFORMATION: Darshana 301-400-2151, darshana.naik.ctr@health.mil Nicole 301-400-2146, nicole.l.sobers.ctr@health.mil PLEASE NOTE: If you do not have medications for the next
IVF CLASS IVF NURSE CONTACT INFORMATION: Darshana 301-400-2151, darshana.naik.ctr@health.mil Nicole 301-400-2146, nicole.l.sobers.ctr@health.mil PLEASE NOTE: If you do not have medications for the next
Connection with other policy areas and (How does it fit/support wider early years work and partnerships)
 Illness such as gastroenteritis and upper respiratory tract infections, along with injuries caused by accidents in the home, are the leading causes of attendances at Accident & Emergency and hospitalisation
Illness such as gastroenteritis and upper respiratory tract infections, along with injuries caused by accidents in the home, are the leading causes of attendances at Accident & Emergency and hospitalisation
Heart problems - What are the possible side effects of AVONEX? What is AVONEX? Who should not take AVONEX?
 MEDICATION GUIDE AVONEX Interferon beta-1a (Including appendix with instructions for using AVONEX Vials) Please read this guide carefully before you start to use AVONEX (a-vuh-necks) and each time your
MEDICATION GUIDE AVONEX Interferon beta-1a (Including appendix with instructions for using AVONEX Vials) Please read this guide carefully before you start to use AVONEX (a-vuh-necks) and each time your
2014 Supporting Students at School with Medical Conditions Policy
 2014 Supporting Students at School with Medical Conditions Policy Review Framework The policy should be reviewed every four years This policy was created in: This issue was revised and released on: School
2014 Supporting Students at School with Medical Conditions Policy Review Framework The policy should be reviewed every four years This policy was created in: This issue was revised and released on: School
Administration of Medication Policy
 Administration of Medication Policy Best Practice Quality Area 2 PURPOSE This policy will clearly define the: procedures to be followed when a child requires medication while attending Montessori Early
Administration of Medication Policy Best Practice Quality Area 2 PURPOSE This policy will clearly define the: procedures to be followed when a child requires medication while attending Montessori Early
Childhood seasonal influenza vaccination programme
 Enhanced service specification Childhood seasonal influenza vaccination programme NHS England gateway reference: 01641 Introduction 1. All GMS practices are expected to provide essential and those additional
Enhanced service specification Childhood seasonal influenza vaccination programme NHS England gateway reference: 01641 Introduction 1. All GMS practices are expected to provide essential and those additional
Guidance for registered pharmacies providing pharmacy services at a distance, including on the internet
 Guidance for registered pharmacies providing pharmacy services at a distance, including on the internet April 2015 The General Pharmaceutical Council is the regulator for pharmacists, pharmacy technicians
Guidance for registered pharmacies providing pharmacy services at a distance, including on the internet April 2015 The General Pharmaceutical Council is the regulator for pharmacists, pharmacy technicians
INTO 39 GUIDANCE ON THE ADMINISTRATION OF MEDICINES IN SCHOOLS IMPLEMENTING BEST PRACTICE
 INTO 39 INTRODUCTION GUIDANCE ON THE ADMINISTRATION OF MEDICINES IN SCHOOLS IMPLEMENTING BEST PRACTICE There has been an increasing concern in recent years with regard to the extent to which teachers should
INTO 39 INTRODUCTION GUIDANCE ON THE ADMINISTRATION OF MEDICINES IN SCHOOLS IMPLEMENTING BEST PRACTICE There has been an increasing concern in recent years with regard to the extent to which teachers should
Waste Management Policy
 Waste Management Policy Revised April 2013 1 Contents Page Content Page No. Clinical Waste 3 - The handling and disposal of Clinical and Soiled 3 - Policy 3 - Warning - The collection of Clinical Waste
Waste Management Policy Revised April 2013 1 Contents Page Content Page No. Clinical Waste 3 - The handling and disposal of Clinical and Soiled 3 - Policy 3 - Warning - The collection of Clinical Waste
Standards of proficiency. Operating department practitioners
 Standards of proficiency Operating department practitioners Contents Foreword 1 Introduction 3 Standards of proficiency 7 Foreword We are pleased to present the Health and Care Professions Council s standards
Standards of proficiency Operating department practitioners Contents Foreword 1 Introduction 3 Standards of proficiency 7 Foreword We are pleased to present the Health and Care Professions Council s standards
Beginner's guide to Hepatitis C testing and immunisation against hepatitis A+B in general practice
 Beginner's guide to Hepatitis C testing and immunisation against hepatitis A+B in general practice Dr Chris Ford GP & SMMGP Clinical Lead Kate Halliday Telford & Wrekin Shared Care Coordinator Aims Discuss:
Beginner's guide to Hepatitis C testing and immunisation against hepatitis A+B in general practice Dr Chris Ford GP & SMMGP Clinical Lead Kate Halliday Telford & Wrekin Shared Care Coordinator Aims Discuss:
PATIENT INFORMATION. Medicine To Treat: D iabetes. What You Need to Know About. Insulin
 PATIENT INFORMATION Medicine To Treat: D iabetes What You Need to Know About Insulin INTRODUCTION The insulin preparations currently available in Singapore are mostly from human origin; pork or bovine
PATIENT INFORMATION Medicine To Treat: D iabetes What You Need to Know About Insulin INTRODUCTION The insulin preparations currently available in Singapore are mostly from human origin; pork or bovine
Parents and Grandparents
 Parents and Grandparents Vaccination for parents and grandparents is vital to help protect newborn babies against whooping cough. Protect your baby by being immunised too. To find out more about Boostrix
Parents and Grandparents Vaccination for parents and grandparents is vital to help protect newborn babies against whooping cough. Protect your baby by being immunised too. To find out more about Boostrix
Department of Health Commencing insulin therapy
 Department of Health Commencing insulin therapy Great state. Great opportunity. State of Queensland (Queensland Health) 2008 2013 This work is licensed under a Creative Commons Attribution No Derivatives
Department of Health Commencing insulin therapy Great state. Great opportunity. State of Queensland (Queensland Health) 2008 2013 This work is licensed under a Creative Commons Attribution No Derivatives
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION combined hepatitis A (inactivated) and hepatitis B (recombinant) vaccine This leaflet is part III of a three-part "Product Monograph" published when was approved for sale
PART III: CONSUMER INFORMATION combined hepatitis A (inactivated) and hepatitis B (recombinant) vaccine This leaflet is part III of a three-part "Product Monograph" published when was approved for sale
Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust.
 Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust. This Patient Group Direction (PGD) has been written
Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust. This Patient Group Direction (PGD) has been written
Medicines for Human Use (Clinical Trials Regulations) 2004. Informed consent in clinical trials
 Medicines for Human Use (Clinical Trials Regulations) 2004 Informed consent in clinical trials Introduction 1. This information note summarises the statutory requirements for informed consent of participants
Medicines for Human Use (Clinical Trials Regulations) 2004 Informed consent in clinical trials Introduction 1. This information note summarises the statutory requirements for informed consent of participants
Humulin R (U500) insulin: Prescribing Guidance
 Leeds Humulin R (U500) insulin: Prescribing Guidance Amber Drug Level 2 We have started your patient on Humulin R (U500) insulin for the treatment of diabetic patients with marked insulin resistance requiring
Leeds Humulin R (U500) insulin: Prescribing Guidance Amber Drug Level 2 We have started your patient on Humulin R (U500) insulin for the treatment of diabetic patients with marked insulin resistance requiring
AQUATIC ANIMAL MEDICINE RECORD BOOK
 AQUATIC ANIMAL MEDICINE RECORD BOOK FISH HEALTH INSPECTORATE www.efishbusiness.co.uk It is a legal requirement for you to retain these records for at least five years following the administration or other
AQUATIC ANIMAL MEDICINE RECORD BOOK FISH HEALTH INSPECTORATE www.efishbusiness.co.uk It is a legal requirement for you to retain these records for at least five years following the administration or other
Polio and the Introduction of IPV
 Polio and the Introduction of IPV Poliomyelitis (polio) is a highly infectious disease that is caused when a person is infected by the polio virus that invades the nervous system. Poliomyelitis can cause
Polio and the Introduction of IPV Poliomyelitis (polio) is a highly infectious disease that is caused when a person is infected by the polio virus that invades the nervous system. Poliomyelitis can cause
YOUR GUIDE TO THE LANTUS SOLOSTAR INSULIN PEN
 Important Safety Information for Lantus You must test your blood sugar levels while using insulin, such as Lantus. Do not make any changes to your dose or type of insulin without talking to your healthcare
Important Safety Information for Lantus You must test your blood sugar levels while using insulin, such as Lantus. Do not make any changes to your dose or type of insulin without talking to your healthcare
PATIENT INFORMATION SHEET KEY FACTS
 PATIENT INFORMATION SHEET KEY FACTS Please read this carefully and refer to the full information sheet You are invited to take part in a research study, comparing subcutaneously (injection under skin)
PATIENT INFORMATION SHEET KEY FACTS Please read this carefully and refer to the full information sheet You are invited to take part in a research study, comparing subcutaneously (injection under skin)
GUIDANCE ON THE SAFE HANDLING OF MONOCLONAL ANTIBODY (mab) PRODUCTS
 GUIDANCE ON THE SAFE HANDLING OF MONOCLONAL ANTIBODY (mab) PRODUCTS 5 th Edition November 2015 NHS Pharmaceutical Quality Assurance Committee 2015 British Oncology Pharmacists Association Pharmaceutical
GUIDANCE ON THE SAFE HANDLING OF MONOCLONAL ANTIBODY (mab) PRODUCTS 5 th Edition November 2015 NHS Pharmaceutical Quality Assurance Committee 2015 British Oncology Pharmacists Association Pharmaceutical
Medication Policy and Procedures
 Medication Policy and Procedures Policy Number: 2009/10 Approved by: Heritage Management Committee 10 November 2009 Last reviewed: October 2009 Next review due: November 2011 Policy Statement Studies of
Medication Policy and Procedures Policy Number: 2009/10 Approved by: Heritage Management Committee 10 November 2009 Last reviewed: October 2009 Next review due: November 2011 Policy Statement Studies of
