June 25, Re: CMS-1588-P; Proposed Quality Reporting Requirements for Ambulatory Surgical Centers (ASCs)
|
|
|
- Bennett Hunter
- 8 years ago
- Views:
Transcription
1 VIA ELECTRONIC DELIVERY Marilyn Tavenner, Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1588-P Room 445-G Hubert H. Humphrey Building 200 Independence Avenue, SW Washington, DC Re: CMS-1588-P; Proposed Quality Reporting Requirements for Ambulatory Surgical Centers (ASCs) Dear Acting Administrator Tavenner: On behalf of the ASC Quality Collaboration (ASC QC), a cooperative effort of organizations and companies interested in ensuring ambulatory surgical center (ASC) quality data is appropriately developed and reported, please accept the following comments regarding CMS-1588-P, Section VIII.E. Proposed Quality Reporting Requirements for Ambulatory Surgical Centers (77 Fed. Reg , May 11, 2012) and the related ASC Quality Reporting Specifications Manual, Version 1.0 s include ASC corporations, ASC industry associations, physician and nursing professional societies, and accrediting bodies with an interest in ASCs. Please see Appendix A for a l participating organizations. The ASC QC strongly advocates quality reporting. This commitment is reflected in the steps we have taken independently to facilitate quality reporting by ASCs all without federal incentive or penalty. This includes developing six ASC facility-level quality measures and securing the endorsement of the National Quality Forum (NQF) for each, as well as developing and publishing a quarterly public report of ASC quality data that is freely available online. These quarterly reports are made possible through the voluntary efforts of participants in the ASC QC Over 1300 centers, representing more than 20 percent of all Medicare certified ASCs, participated in the most recent report. We recognize the significant effort the agency has invested in preparing for the implementation of the ASC Quality Reporting Program (ASC QRP). We also appreciate the consideration the agency has given to our prior comments on various aspects of the ASC QRP
2 Page 2 of 8 and are pleased to have this opportunity to provide additional insights, feedback and recommendations regarding the program. I. ASC Awareness of the Medicare ASC Quality Reporting Program Must Be Improved We believe, based on our experience at industry meetings and other events, that a significant number of ASCs remain unaware of the Medicare ASC QRP and its requirements. The ASC QC and other ASC industry organizations are committed to continuing our efforts to improve awareness. However, we believe that CMS should also step-up its efforts to inform ASCs about the ASC QRP and its requirements. Traditional outreach methods, such as the listserv have a limited following in the ASC community as a whole. On the other hand, ASCs are very likely to be aware of and respond to communications that reach them through their Medicare administrative contractors. We strongly recommend that CMS prepare an ASC communication that would be disseminated through its administrative contractors in order to achieve as wide an educational outreach as possible. This communication should alert ASCs to the imminent implementation of the program and the consequences of nonparticipation. It should invite ASCs to participate in a CMS-sponsored National Provider Call and direct ASCs to resources for detailed information regarding the ASC QRP. These resources should be accessible online at the QualityNet site. We also believe it would be helpful if CMS created a page on the cms.gov website devoted to the ASC QRP, similar to the Physician Quality Reporting System (PQRS) page it has created within its Quality Initiatives pages. Detailed resources posted here should include an explanation of program requirements and responses to frequently asked questions (FAQs) about the ASC QRP. We would be happy to provide input and/or feedback on the content of the direct communication and the resources CMS develops to support the ASC QRP. We believe such efforts are crucial and will improve participation rates, in addition to boosting the number of ASCs that achieve, or exceed, the minimum threshold for successful reporting. II. Proposed Quality Reporting Requirements for ASCs A. Proposed Minimum Thresholds for Claims-Base Measures Using QDCs We generally support the agen threshold for successful reporting for the CY 2014 and 2015 payment determinations at a level of at least 50 percent of Medicare fee-for-service claims. However, an issue has arisen with claims submission testing related to Medicare secondary payer (MSP) claims. Although CMS issued the G-codes for the ASC QRP with the April 2012 HCPCS release, private insurers will not have the files for use until January mpt ASCs from reporting G-codes on MSP claims from October 1, 2012 through December 31, We believe these secondary claims should be excluded from the population of claims considered in the determination of data completeness for the CY 2014 payment determination.
3 Page 3 of 8 B. Data Collection and Processing Period for CYs 2014 and 2015 Payment Determinations CMS proposes that, in order to be included in the quality reporting data used for the CY 2014 payment determination, claims for services furnished between October 1, 2102 and December 31, 2012 be paid by the administrative contractor by April 30, As the agency knows, ASCs have up to one year to submit claims for services rendered. While we understand the need for lead-time in order to process and analyze quality data, make payment determinations, and supply payment information to administrative contractors, we believe that the proposed period for the collection of claims data may be too abbreviated to capture all pertinent data, particularly for the outcome measures. We believe that as the agency gains experience with ASC quality data analysis, and the determination and implementation of any payment adjustments over time, it should seek ways to push the date by which claims must be processed back as close to the one-year mark as possible. For example, CMS could propose that, for the CY 2015 payment determination, claims for services furnished between January 1, 2013 and December 31, 2013 be processed by June 30, 2014, allowing for the capture of as many claims for services as possible. C. Proposed Reconsideration Timeline CMS proposes to implement a reconsideration process for the ASC Quality Reporting Program that is modeled on the reconsideration process it has implemented for its hospital quality reporting programs. Included is a proposal that ASCs submit a reconsideration request by March 17 of the affected payment year. This proposal is based on claims processing guidelines that allow Medicare administrative contractors 30 days to process clean claims, and 45 days to process claims other than clean ones. March 17 was chosen because it falls 45 days after January 31. We do not believe this is a sufficient period of time for a number of reasons. First, the proposed March 17 date appears to assume that all ASC claims are submitted on the same day as the date of service. While this sometimes happens, it is more often the case that the claim is submitted within a period of several days following the date of service. In addition, the month of January already brings other reimbursement changes that result from the Medicare ASC payment update for each calendar year. The proposed timeframe allows very little time for ASCs to analyze the full complement of their January payments from Medicare and determine the cause of any deviation from the expected payment (there are other issues that could universally drop payments, such as a downward adjustment of a local wage index), and subsequently prepare the reconsideration request, including copies of all the claims at issue which could be a substantial number of claims depending on the Medicare beneficiary volume at the ASC. We strongly recommend CMS allow ASCs until April 15 of the affected payment year to submit a request for reconsideration. This timeframe allows a number of days to pass between the beneficiary date of service and claim submission, allows sufficient time for ASC analysis of January claims, and allows a more reasonable amount of time for the preparation of the request
4 Page 4 of 8 for reconsideration, including gathering and copying the necessary supporting claims documentation. III. ASC Quality Reporting Specifications Manual A. Prophylactic Intravenous Antibiotic Timing Measure (ASC-5) As we have stated in comments to the agency in the past, case mix across ASCs is very diverse and should be considered when establishing reporting requirements for individual measures. The Prophylactic Intravenous (IV) Antibiotic Timing measure is an example of a measure that would not apply to all ASCs. For example, single specialty ASCs that provide gastrointestinal endoscopies do not administer IV prophylaxis for the prevention of surgical site infection (SSI). In addition, many single specialty ophthalmic ASCs administer topical, rather than IV, antibiotics for SSI prevention. CMS has determined that it will not offer an exemption for these ASCs and plans to require those facilities to submit G8918 on all their claims. We continue to believe that this approach imposes unnecessary burden for ASCs that do not administer prophylactic IV antibiotics for SSI. We recommend CMS this measure. The agency could develop a G-code to be submitted once annually by ASCs that do not administer intravenous antibiotic prophylaxis for SSI. This code would allow the ASC to claim an exemption from data submission for this measure. Another alternative would be to allow ASCs that do not administer IV antibiotic prophylaxis for SSI to claim an exemption through their QualityNet account. Absent the creation of such an exemption, we recommend that CMS clarify the need for all centers to report on this measure through explicit statements in the Specifications Manual. We believe it would be helpful to develop a heading (simila MS would indicate that all ASCs are required to report. In addition, we believe it would be helpful to include a statement under the coding options heading that makes it explicit that G8918 should be used to report on this measure when the patient is excluded from the measure denominator. B. Safe Surgery Checklist Use (ASC-7) We note that the Specifications Manual for the Safe Surgery Checklist Use measure asks ASCs to indicate whether the center used a safe surgery checklist based on accepted standards of at any time during the designated period (emphasis added). This is a change from the FC, in which it indicated an ASC would report whether their facility employed a safe surgery checklist that covered each of the three critical perioperative periods for the entire calendar year of 2012 (emphasis added). We appreciate the flexibility the agency has shown in relaxing the requirements for this measure for the initial year of reporting.
5 Page 5 of 8 As we stated in previous comments, it is our understanding that the agency intends to apply this measure to all ASCs, including centers that perform procedures. We continue to believe the agency should revise the descriptive statement of the measure so this intent is clear. We suggest the agency refer to the checklist as a its statement of the mea that the purpose is to assess whether ASCs are using a safe surgery/procedure checklist that addresses effective communication and helps ensure that safe practices are being performed at three critical perioperative or periprocedural periods: prior to the administration of anesthesia or sedation, prior to incision or the beginning of the procedure, and prior to the patient leaving the operating or procedure room. Absent this suggested revision, we recommend that CMS clarify the need for all centers to report on this measure through explicit statements in the Specifications Manual. As we have suggested above for the Prophylactic Intravenous Antibiotic Timing Measure, we believe it would be helpful to develop a heading similar phrase. Under this heading, CMS should indicate that all ASCs are required to report. C. ASC Facility Volume on Selected ASC Surgical Procedures (ASC-6) CMS has finalized the structural measure ASC Facility Volume Data on Selected ASC Surgical Procedures for the CY 2015 payment determination. When originally proposed, this measure was poorly specified. We appreciate the improvements that the current Specifications Manual has made as compared to the original description of the measure, but find that there are still many pertinent details lacking. There is a good deal of legitimate confusion surrounding how ASCs are to develop the aggregate count each of the categories and subcategories that CMS has specified for the measure. The measure specifications are not sufficiently detailed to allow for consistent preparation of procedure counts for reporting. There are several questions CMS should address in order to create clear specifications and ensure consistent data collection and reporting. For example, are aggregate procedure counts to be prepared for the nine categories alone, or are aggregate counts to be prepared for the thirty-four (34) subcategories, or both? In preparing the aggregate counts, are secondary procedures to be counted in addition to the primary procedure? How are bilateral procedures or those performed on multiple spinal levels to be counted? How should ASCs count cases that are cancelled either before or after the administration of anesthesia (e.g., those services that are submitted using a -73 or -74 modifier appended to the service code)? These types of questions should be addressed as soon as possible to allow ASCs to prepare for implementation. D. Appendix A The last paragraph of Appendix A to the Specification Manual Additional information and resources, such as sample data collection sheets or logs and frequently asked questions (FAQs) about the measures, can be found on the ASC Quality Collaboration website at While the reference to the ASC QC is appreciated, this implies that the ASC QC is a source of this type of information for all of the measures presented in the Manual, when in fact, we are only in a position to provide supplemental materials and information for the
6 Page 6 of 8 measures we have developed and for which we serve as the measure steward. Accordingly, we request that this statement be removed from Appendix A and that the agency instead include a similar statement at the end of each of the sections of the Manual that address measures developed and maintained by the ASC QC: 1. Measure Title: Patient Burn 2. Measure Title: Patient Fall 3. Measure Title: Wrong Site, Wrong Side, Wrong Patient, Wrong Procedure, Wrong Implant 4. Measure Title: Hospital Transfer/Admission 5. Measure Title: Prophylactic Intravenous (IV) Antibiotic Timing. We suggest the statement be a minor modification of the one now included at the end of Additional information and resources, such as sample data collection sheets and/or logs, and frequently asked questions (FAQs) about this measure, can be found on the ASC Quality Collaboration website at IV. Additional Considerations CMS has stated its intent to issue proposals pertaining to other aspects of the ASC QRP in future rulemaking. We offer the following comments regarding selected topics as the agency develops these additional proposals. We anticipate providing additional feedback on a broader range of topics in response to the upcoming CY 2013 OPPS/ASC proposed rule. A. Alternative Reporting Mechanisms As stated in the past, the ASC QC believes CMS should allow ASCs to meet the quality data reporting requirements under the ASC QRP using registry-based reporting as an alternative to the other mechanisms CMS has outlined for ASC use through CY We note that CMS has provided physicians with several data reporting options under PQRS and believe this flexibility should be extended to ASCs as well. The ASC QC has a strong interest in developing an ASC-specific registry. While we do not have a definitive timeline for a registry development project at this time, we are aware of other registries already in operation. Examples include the GIQuIC and Ophthalmic Patient Outcomes Database registries, which may currently be used to satisfy PQRS reporting requirements. We believe these registries have the potential for use in data collection and reporting from selected GI and ophthalmic ASCs as well. In addition to claims-based reporting and registry-based reporting, ASCs should also have the option of submitting quality data to CMS through an EHR-based reporting mechanism. While the penetration of EHRs in the ASC industry is limited at this time, there are centers that have implemented this technology that could benefit from this reporting option. B. Publication of ASC Quality Reporting Program Data
7 Page 7 of 8 The ASC QC supports transparency and welcomes a fair presentation of ASC quality and cost information that could assist consumers in making informed health care decisions. Consumers should be able to access this information on websites that are organized to allow easy comparisons across facilities that offer outpatient surgical services, while also protecting the rights of providers by assuring that the information made available is correct, current, and clearly presented. CMS should provide ASCs an opportunity to preview any data to be made public, in addition to providing contact information for program content areas experts that ASCs can contact to ask questions or raise concerns with any information prior to its publication. There should also be a provider narrative section for each provider-specific item presented to the consumer. This narrative box would allow the provider to advise the consumer of any concerns the provider has regarding the reliability or accuracy of the information presented. In addition to reporting quality data, other useful information such as facility accreditation status should be made available to the consumer. In addition, the site displaying ASC quality data should provide the consumer with basic information regarding each measure, including guidance regarding its interpretation and use in decision- quality measures includes the following information regarding the Hospital Transfer/Admission measure (ASC-4): Hospital Transfer/Admission ASCs provide surgical services to patients not requiring hospitalization. Therefore, ASCs screen patients referred to their facilities to ensure they can be safely cared for as an outpatient. The frequency of ASC admissions experiencing a transfer or admission to a hospital upon discharge from participating ASCs is shown below as a rate per 1000 admissions. Not all conditions requiring a hospital transfer or admission result from the care the patient received in the ASC, nor can all medical conditions requiring a hospital transfer or admission be anticipated in advance. Therefore, some level of hospital transfer or admission is expected. We believe that a similar narrative should be provided for each measure that is presented for public reporting. We look forward to the more detailed proposals on the publication of ASC quality program data in later rulemaking. determining the threshold at which data for centers with low Medicare volume should be deemed unreliable, and therefore unsuitable for public reporting. C. Feedback and Benchmarking Following the end of each the reporting periods, CMS should provide confidential feedback reports based on the quality measures reported by individual ASCs for services
8 Page 8 of 8 provided during the reporting period. These reports should address topics such as measure participation, data completeness, QDC submission errors and measure performance detail. In addition to its use for public reporting purposes, the data collected through the ASC QRP should also be made available to participating ASCs for benchmarking purposes. We urge CMS to develop a process for establishing ASC benchmarks on a measure-by-measure basis. This information would be valuable as individual ASCs assess their performance relative to their peers and determine if performance improvement activities are needed. The Hospital-Specific Reports (HSRs) CMS currently prepares for individual hospitals participating in the Hospital IQR program could serve as a model. *** Thank you for considering these comments. In closing, we wish to emphasize what we believe is a critical need for CMS educational outreach to all ASCs in order to encourage participation in the ASC QRP. We look forward to continuing our dialogue with the agency regarding the ASC QRP. We would be happy to assist with questions or provide additional information at your request. Sincerely, Donna Slosburg, BSN, LHRM, CASC Executive Director, ASC Quality Collaboration donnaslosburg@ascquality.org
9 Appendix A Page 1 of 1 Appendix A Current Participants in the Activities of the ASC Quality Collaboration Accreditation Association for Ambulatory HealthCare Ambulatory Surgery Foundation Ambulatory Surgical Centers of America American College of Surgeons American Osteopathic Association, Healthcare Facilities Accreditation Program AmSurg Association of perioperative Registered Nurses Florida Society of Ambulatory Surgery Centers Health Inventures Hospital Corporation of America, Ambulatory Surgery Division Nueterra Healthcare Outpatient Ophthalmic Surgery Society Surgical Care Affiliates Symbion The Joint Commission United Surgical Partners International
QUALITY DATA G-CODES
 QUALITY DATA G-CODES Corresponding Quality Measure G-code If no adverse events occurred, report the following: All four adverse events did not occur G8907 If one or more adverse events occurred, report
QUALITY DATA G-CODES Corresponding Quality Measure G-code If no adverse events occurred, report the following: All four adverse events did not occur G8907 If one or more adverse events occurred, report
This proposed rule clarifies and makes updates to details regarding this program that were finalized in
 2014 Ambulatory Surgery Center (ASC) and Outpatient Prospective Payment System (OPPS) A Summary of the Quality Provisions of the Proposed Rule Overview On July 8, 2013, the Centers for Medicare and Medicaid
2014 Ambulatory Surgery Center (ASC) and Outpatient Prospective Payment System (OPPS) A Summary of the Quality Provisions of the Proposed Rule Overview On July 8, 2013, the Centers for Medicare and Medicaid
April 8, 2013. Dear Ms. Tavenner:
 April 8, 2013 Marilyn B. Tavenner Acting Administrator and Chief Operating Officer Centers for Medicare & Medicaid Services Department of Health and Human Services Hubert H. Humphrey Building 200 Independence
April 8, 2013 Marilyn B. Tavenner Acting Administrator and Chief Operating Officer Centers for Medicare & Medicaid Services Department of Health and Human Services Hubert H. Humphrey Building 200 Independence
Preparing GI ASCs for October 2012
 Preparing GI ASCs for October 2012 Anita J. Bhatia, PHD, MPH, Centers for Medicare and Medicaid Services Lawrence B. Cohen, MD, FACG, AGAF, FASGE, New York Gastroenterology Associates Thomas M. Deas, Jr.,
Preparing GI ASCs for October 2012 Anita J. Bhatia, PHD, MPH, Centers for Medicare and Medicaid Services Lawrence B. Cohen, MD, FACG, AGAF, FASGE, New York Gastroenterology Associates Thomas M. Deas, Jr.,
CY 2016 OPPS/ASC Proposed Rule: Ambulatory Surgical Center Quality Reporting Program
 CY 2016 OPPS/ASC Proposed Rule: Ambulatory Surgical Center Quality Reporting Program Audio for this event is available via internet streaming. No telephone line is required. Computer speakers or headphones
CY 2016 OPPS/ASC Proposed Rule: Ambulatory Surgical Center Quality Reporting Program Audio for this event is available via internet streaming. No telephone line is required. Computer speakers or headphones
Preparing GI ASCs for October 2012
 Preparing GI ASCs for October 2012 Frank J. Chapman, MBA, Asheville Gastroenterology Associates, P.A. Lawrence B. Cohen, MD, FACG, AGAF, FASGE, New York Gastroenterology Associates Lawrence R. Kosinski,
Preparing GI ASCs for October 2012 Frank J. Chapman, MBA, Asheville Gastroenterology Associates, P.A. Lawrence B. Cohen, MD, FACG, AGAF, FASGE, New York Gastroenterology Associates Lawrence R. Kosinski,
April 22, 2013. Re: Advancing Interoperability and Health Information Exchange. Dear Dr. Mostashari,
 Farzad Mostashari, MD, ScM National Coordinator for Health Information Technology Department of Health and Human Services Office of the National Coordinator for Health Information Technology Hubert H.
Farzad Mostashari, MD, ScM National Coordinator for Health Information Technology Department of Health and Human Services Office of the National Coordinator for Health Information Technology Hubert H.
Re: CMS 3323 NC, Request for Information (RFI): Certification Frequency and Requirements for the Reporting of Quality Measures Under CMS Programs
 February 5, 2016 Andrew M. Slavitt Acting Administrator Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W., Room 445-G Washington, DC 20201 Re: CMS 3323
February 5, 2016 Andrew M. Slavitt Acting Administrator Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W., Room 445-G Washington, DC 20201 Re: CMS 3323
Re: CMS 3819-P, Medicare and Medicaid Programs; Conditions of Participation for Home Health Agencies; Proposed Rule, Oct. 9, 2014.
 Marilyn B. Tavenner Administrator Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W., Room 445-G Washington, D.C. 20201 Re: CMS 3819-P, Medicare and Medicaid
Marilyn B. Tavenner Administrator Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W., Room 445-G Washington, D.C. 20201 Re: CMS 3819-P, Medicare and Medicaid
CMS s framework for Value Modifier
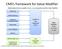 CMS s framework for Value Modifier Relationship between quality of care, cost composites and the Value Modifier Clinical Care Patient Experience Population/ Community Health Patient Safety Care Coordination
CMS s framework for Value Modifier Relationship between quality of care, cost composites and the Value Modifier Clinical Care Patient Experience Population/ Community Health Patient Safety Care Coordination
September 4, 2012. Submitted Electronically
 September 4, 2012 Ms. Marilyn Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-1589-P P.O. Box 8016 Baltimore, MD 21244-8016
September 4, 2012 Ms. Marilyn Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-1589-P P.O. Box 8016 Baltimore, MD 21244-8016
June 22, 2012. Dear Administrator Tavenner:
 Submitted Electronically Marilyn Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445-G Hubert H. Humphrey Building 200 Independence Avenue
Submitted Electronically Marilyn Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445-G Hubert H. Humphrey Building 200 Independence Avenue
Re: Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule, and Other Revisions to Part B for CY 2016 Proposed Rule
 September 8, 2015 Andy Slavitt Acting Administrator Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244 Re: Medicare Program; Revisions to Payment Policies Under the Physician
September 8, 2015 Andy Slavitt Acting Administrator Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244 Re: Medicare Program; Revisions to Payment Policies Under the Physician
Sustainable Growth Rate (SGR) Repeal and Replace: Comparison of 2014 and 2015 Legislation
 Sustainable Growth Rate (SGR) Repeal and Replace: Comparison of 2014 and 2015 Legislation Proposal 113 th Congress - - H.R.4015/S.2000 114 th Congress - - H.R.1470 SGR Repeal and Annual Updates General
Sustainable Growth Rate (SGR) Repeal and Replace: Comparison of 2014 and 2015 Legislation Proposal 113 th Congress - - H.R.4015/S.2000 114 th Congress - - H.R.1470 SGR Repeal and Annual Updates General
June 1, 2015 VIA ELECTRONIC SUBMISSION
 June 1, 2015 VIA ELECTRONIC SUBMISSION Howard Shelanski, OIRA Administrator Office of Management and Budget Office of Information and Regulatory Affairs Attention: CMS Desk Officer 725 17 th Street, NW
June 1, 2015 VIA ELECTRONIC SUBMISSION Howard Shelanski, OIRA Administrator Office of Management and Budget Office of Information and Regulatory Affairs Attention: CMS Desk Officer 725 17 th Street, NW
May 7, 2012. Submitted Electronically
 May 7, 2012 Submitted Electronically Secretary Kathleen Sebelius Department of Health and Human Services Office of the National Coordinator for Health Information Technology Attention: 2014 edition EHR
May 7, 2012 Submitted Electronically Secretary Kathleen Sebelius Department of Health and Human Services Office of the National Coordinator for Health Information Technology Attention: 2014 edition EHR
Re: CMS-1613-P Medicare Program; Proposed Changes to the Ambulatory Surgical Center Payment System and CY 2015 Payment Rates
 Marilyn Tavenner, MHA, BSN, RN Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1613-P Room 445-G Hubert H. Humphrey Building 200 Independence
Marilyn Tavenner, MHA, BSN, RN Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1613-P Room 445-G Hubert H. Humphrey Building 200 Independence
Announcements. Next Webinar
 QualityNet Reports and Utilization of the Secure File Transfer for the Ambulatory Surgical Center Quality Reporting (ASCQR) Program Reneé Parks, RN, BSN Project Lead, ASCQR Program October 22, 2014 Announcements
QualityNet Reports and Utilization of the Secure File Transfer for the Ambulatory Surgical Center Quality Reporting (ASCQR) Program Reneé Parks, RN, BSN Project Lead, ASCQR Program October 22, 2014 Announcements
CMS Proposals for Quality Reporting Programs under the 2015 Medicare Physician Fee Schedule Proposed Rule. July 24, 2014
 CMS Proposals for Quality Reporting Programs under the 2015 Medicare Physician Fee Schedule Proposed Rule July 24, 2014 Medicare Learning Network This MLN Connects National Provider Call (MLN Connects
CMS Proposals for Quality Reporting Programs under the 2015 Medicare Physician Fee Schedule Proposed Rule July 24, 2014 Medicare Learning Network This MLN Connects National Provider Call (MLN Connects
June 15, 2015. Submitted electronically via www.regulations.gov
 June 15, 2015 Marilyn Tavenner, R.N. Administrator Center for Medicare and Medicaid Services Department of Health and Human Services P.O. Box 8013 Baltimore, MD 21244-8013 Submitted electronically via
June 15, 2015 Marilyn Tavenner, R.N. Administrator Center for Medicare and Medicaid Services Department of Health and Human Services P.O. Box 8013 Baltimore, MD 21244-8013 Submitted electronically via
Medicare Learning Network
 CMS Proposals for the Physician Quality Reporting System (PQRS) and Physician Value-Based Payment Modifier (VM) under the Medicare Physician Fee Schedule 2014 July 25, 2013 Medicare Learning Network This
CMS Proposals for the Physician Quality Reporting System (PQRS) and Physician Value-Based Payment Modifier (VM) under the Medicare Physician Fee Schedule 2014 July 25, 2013 Medicare Learning Network This
RE: Proposed Establishment of Certification Programs for Health Information Technology Permanent Certification Program, RIN 0991-AB59
 Via Electronic Submission @ www.regulations.gov David Blumenthal, M.D., M.P.P. National Coordinator for Health Information Technology HHS/Office of the National Coordinator for Health Information Technology
Via Electronic Submission @ www.regulations.gov David Blumenthal, M.D., M.P.P. National Coordinator for Health Information Technology HHS/Office of the National Coordinator for Health Information Technology
December 3, 2010. Dear Administrator Berwick:
 Donald Berwick, M.D. Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445-G, Hubert H. Humphrey Building 200 Independence Avenue, SW Washington, DC 20201
Donald Berwick, M.D. Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445-G, Hubert H. Humphrey Building 200 Independence Avenue, SW Washington, DC 20201
CMS Proposed Electronic Health Record Incentive Program For Physicians
 May 7, 2012 Ms. Marilyn Tavenner Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-0044-P Mail Stop C4-26-05 7500 Security Boulevard
May 7, 2012 Ms. Marilyn Tavenner Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-0044-P Mail Stop C4-26-05 7500 Security Boulevard
Calendar Year 2014 Medicare Physician Fee Schedule Final Rule
 Calendar Year 2014 Medicare Physician Fee Schedule Final Rule Non-facility Cap after receiving many negative comments on this issue from physician groups along with the House GOP Doctors Caucus letter,
Calendar Year 2014 Medicare Physician Fee Schedule Final Rule Non-facility Cap after receiving many negative comments on this issue from physician groups along with the House GOP Doctors Caucus letter,
Establishment of a Temporary and Permanent Testing Program
 April 9, 2010 David Blumenthal, MD, MPP Office of the National Coordinator for Health Information Technology (ONCHIT) Attn: Certification Programs Proposed Rule Hubert H. Humphrey Building, Suite 729D
April 9, 2010 David Blumenthal, MD, MPP Office of the National Coordinator for Health Information Technology (ONCHIT) Attn: Certification Programs Proposed Rule Hubert H. Humphrey Building, Suite 729D
ADVANCING HIGHER EDUCATION IN NURSING
 September 4, 2012 Submitted via www.regulations.gov Marilyn Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attn: CMS 1590 P P.O. Box 8010
September 4, 2012 Submitted via www.regulations.gov Marilyn Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attn: CMS 1590 P P.O. Box 8010
Ambulatory Surgery Center (ASC) Track Session
 Ambulatory Surgery Center (ASC) Track Session 2012 HAI Data Summit Kansas City, MO May 30 31, 2012 Joe Perz, DrPH MA Team Leader, Ambulatory and Long Term Care Centers for Disease Control and Prevention
Ambulatory Surgery Center (ASC) Track Session 2012 HAI Data Summit Kansas City, MO May 30 31, 2012 Joe Perz, DrPH MA Team Leader, Ambulatory and Long Term Care Centers for Disease Control and Prevention
QUALITY BEGINNER. PQRS Training Module: QUALITY MEASUREMENT 101. Last Updated: August 2014
 QUALITY 01 BEGINNER PQRS Training Module: QUALITY MEASUREMENT 101 Last Updated: August 2014 TRAINING MODULE OBJECTIVES Quality Measurement 101 is a training module for providers who are interested in learning
QUALITY 01 BEGINNER PQRS Training Module: QUALITY MEASUREMENT 101 Last Updated: August 2014 TRAINING MODULE OBJECTIVES Quality Measurement 101 is a training module for providers who are interested in learning
Page 1 of 11. MLN Matters Number: SE1010 REVISED Related Change Request (CR) #: 6740. Related CR Release Date: N/A Effective Date: January 1, 2010
 News Flash Version 3.0 of the Measures Groups Specifications Manual released in November 2009 for 2010 PQRI has been revised. Version 3.1 of the 2010 PQRI Measures Groups Specifications Manual and Release
News Flash Version 3.0 of the Measures Groups Specifications Manual released in November 2009 for 2010 PQRI has been revised. Version 3.1 of the 2010 PQRI Measures Groups Specifications Manual and Release
Submitted Electronically RE: CMS-1609-P: ISSUE # 1: Solicitation of Comments on Definitions of Terminal Illness and Related Conditions :
 June 20, 2014 Submitted Electronically Ms. Marilyn B. Tavenner Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services 200 Independence Avenue, SW Washington, DC
June 20, 2014 Submitted Electronically Ms. Marilyn B. Tavenner Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services 200 Independence Avenue, SW Washington, DC
Physician Quality Reporting System What Neurosurgeons Need to Know for 2015
 Physician Quality System What Neurosurgeons Need to Know for 2015 Prepared by the: American Association of Neurological Surgeons Congress of Neurological Surgeons For More Information Contact: Rachel Groman,
Physician Quality System What Neurosurgeons Need to Know for 2015 Prepared by the: American Association of Neurological Surgeons Congress of Neurological Surgeons For More Information Contact: Rachel Groman,
March 15, 2010. Dear Dr. Blumenthal:
 March 15, 2010 David Blumenthal, MD, MPP National Coordinator Office of the National Coordinator for Health Information Technology (ONCHIT) Department of Health and Human Services ATTN: HITECH Initial
March 15, 2010 David Blumenthal, MD, MPP National Coordinator Office of the National Coordinator for Health Information Technology (ONCHIT) Department of Health and Human Services ATTN: HITECH Initial
RE: CMS-1416-P, Medicare Program; Medicare Shared Savings Program; Accountable Care Organizations; Proposed Rule
 Marilynn B. Tavenner Administrator Center for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445-G 200 Independence Avenue, SW Washington, DC
Marilynn B. Tavenner Administrator Center for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445-G 200 Independence Avenue, SW Washington, DC
June 28, 2013. Re: Medicare Program; Requirements for the Medicare Incentive Reward Program and Provider Enrollment. Dear Ms.
 June 28, 2013 Ms. Marilyn Tavenner, Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-6045-P PO Box 8013 Baltimore, MD 21244-8013 Re: Medicare
June 28, 2013 Ms. Marilyn Tavenner, Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-6045-P PO Box 8013 Baltimore, MD 21244-8013 Re: Medicare
RE: CMS-3310-P Electronic Health Record (EHR) Incentive Programs Stage 3
 May 29, 2015 Andy Slavitt Acting Administrator Centers for Medicare and Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445 G 200 Independence Avenue, SW
May 29, 2015 Andy Slavitt Acting Administrator Centers for Medicare and Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445 G 200 Independence Avenue, SW
February 24, 2012 (202) 690-6145 CMS PROPOSES DEFINITION OF STAGE 2 MEANINGFUL USE OF CERTIFIED ELECTRONIC HEALTH RECORDS (EHR) TECHNOLOGY
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 Office of Communications FACT SHEET FOR IMMEDIATE RELEASE Contact:
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 Office of Communications FACT SHEET FOR IMMEDIATE RELEASE Contact:
2014 Physician Quality Reporting System (PQRS): Implementation Guide 12/13/2013
 2014 Physician Quality Reporting System (PQRS): Implementation Guide 12/13/2013 CPT only copyright 2013 American Medical Association. All rights reserved. Page 1 of 41 Table of Contents Page Introduction
2014 Physician Quality Reporting System (PQRS): Implementation Guide 12/13/2013 CPT only copyright 2013 American Medical Association. All rights reserved. Page 1 of 41 Table of Contents Page Introduction
OVERALL IMPLEMENTATION CONSIDERATIONS
 Donald Berwick, M.D., M.P.H. Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Hubert H. Humphrey Building 200 Independence Avenue, S.W., Room 445-G Washington,
Donald Berwick, M.D., M.P.H. Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Hubert H. Humphrey Building 200 Independence Avenue, S.W., Room 445-G Washington,
2015 Physician Quality Reporting System (PQRS): Implementation Guide
 2015 Physician Quality Reporting System (PQRS): Implementation Guide 1/15/2015; Revised Table of Contents Introduction... 3 PQRS Measure Selection Considerations... 6 Satisfactorily Report Measures...
2015 Physician Quality Reporting System (PQRS): Implementation Guide 1/15/2015; Revised Table of Contents Introduction... 3 PQRS Measure Selection Considerations... 6 Satisfactorily Report Measures...
RE: CMS 1621 P, Medicare Clinical Diagnostic Laboratory Tests Payment System Proposed Rule; (Vol. 80, No.190), October 1, 2015.
 Andrew M. Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-1621-P P.O. Box 8016 Baltimore, MD 21244-8016 RE: CMS 1621 P, Medicare
Andrew M. Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-1621-P P.O. Box 8016 Baltimore, MD 21244-8016 RE: CMS 1621 P, Medicare
2015 Physician Quality Reporting System (PQRS): Implementation Guide
 2015 Physician Quality Reporting System (PQRS): Implementation Guide Table of Contents Introduction... 3 PQRS Measure Selection Considerations... 6 Satisfactorily Report Measures... 11 Reporting Electronically
2015 Physician Quality Reporting System (PQRS): Implementation Guide Table of Contents Introduction... 3 PQRS Measure Selection Considerations... 6 Satisfactorily Report Measures... 11 Reporting Electronically
June 2, 2014. RE: File Code CMS-1608-P. Dear Ms. Tavenner:
 . June 2, 2014 Marilyn Tavenner Centers for Medicare & Medicaid Services Room 445-G, Hubert H. Humphrey Building 200 Independence Avenue SW Washington, DC RE: File Code CMS-1608-P Dear Ms. Tavenner: The
. June 2, 2014 Marilyn Tavenner Centers for Medicare & Medicaid Services Room 445-G, Hubert H. Humphrey Building 200 Independence Avenue SW Washington, DC RE: File Code CMS-1608-P Dear Ms. Tavenner: The
2012 Physician Quality Reporting System (Physician Quality Reporting) Implementation Guide
 2012 Physician Quality Reporting System (Physician Quality Reporting) Implementation Guide CPT only copyright 2011 American Medical Association. All rights reserved. Page 1 of 26 Table of Contents Introduction
2012 Physician Quality Reporting System (Physician Quality Reporting) Implementation Guide CPT only copyright 2011 American Medical Association. All rights reserved. Page 1 of 26 Table of Contents Introduction
Frequently Asked Questions (FAQs) about the Home Health Compare (HHC) Star Ratings
 I. General IQ1: IA1: IQ2: IA2: IQ3: IA3: IQ4: IA4: What is the purpose of HHC Star Ratings and why is CMS choosing to add them to HHC now? The Affordable Care Act calls for transparent, easily-understood,
I. General IQ1: IA1: IQ2: IA2: IQ3: IA3: IQ4: IA4: What is the purpose of HHC Star Ratings and why is CMS choosing to add them to HHC now? The Affordable Care Act calls for transparent, easily-understood,
Re: Medicare and Medicaid Programs; Electronic Health Record Incentive Program Stage 3 (CMS-3310-P)
 May 29, 2015 Mr. Andrew Slavitt Acting Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445 G 200 Independence Avenue,
May 29, 2015 Mr. Andrew Slavitt Acting Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445 G 200 Independence Avenue,
April 17, 2014. Re: Evolution of ACO initiatives at CMS. Dear Dr. Conway:
 Patrick Conway, M.D. Acting Director of the Innovation Center Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W. Room 445-G Washington, DC 20201 Re: Evolution
Patrick Conway, M.D. Acting Director of the Innovation Center Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W. Room 445-G Washington, DC 20201 Re: Evolution
The undersigned provider groups would like to draw your attention to implementation concerns regarding two administrative simplification issues:
 January 30, 2015 Marilyn B. Tavenner Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445 G 200 Independence Avenue,
January 30, 2015 Marilyn B. Tavenner Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445 G 200 Independence Avenue,
Re: Medicare and Medicaid Programs; Electronic Health Record Incentive Program Stage 2 (CMS-0044-P)
 Ms. Marilyn B. Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-0044-P Room 445-G, Hubert H. Humphrey Building 200 Independence
Ms. Marilyn B. Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-0044-P Room 445-G, Hubert H. Humphrey Building 200 Independence
Re: Medicare Program; Medicare Shared Savings Program: Accountable Care Organizations
 February 6, 2015 Marilyn Tavenner, Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1461-P P.O. Box 8013 Baltimore, Md. 21244-8013 Re: Medicare
February 6, 2015 Marilyn Tavenner, Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1461-P P.O. Box 8013 Baltimore, Md. 21244-8013 Re: Medicare
(http://www.regulations.gov/#!documentdetail;d=cms-2013-0155-10181) File # CMS-2013-0155-10181
 January 27, 2014 Marilyn Tavenner, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-4159-P P.O. Box 8013 Baltimore, MD 21244-8013 Re: Final
January 27, 2014 Marilyn Tavenner, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-4159-P P.O. Box 8013 Baltimore, MD 21244-8013 Re: Final
March 28, 2012. Dear Acting Administrator Tavenner:
 March 28, 2012 Marilyn B. Tavenner Acting Administrator U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445-G 200 Independence Avenue, SW Washington, DC 20201 Dear Acting
March 28, 2012 Marilyn B. Tavenner Acting Administrator U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445-G 200 Independence Avenue, SW Washington, DC 20201 Dear Acting
Physician Quality Reporting System (PQRS)
 Physician Quality Reporting System (PQRS) and the Value-Based Payment Modifier Implementation guide for registry-based reporting for the Hepatitis C (HCV) Measures Group 2015 1 Overview of PQRS 1,2 What
Physician Quality Reporting System (PQRS) and the Value-Based Payment Modifier Implementation guide for registry-based reporting for the Hepatitis C (HCV) Measures Group 2015 1 Overview of PQRS 1,2 What
Physician Quality Reporting System (PQRS) Qualified Clinical Data Registry (QCDR) QCDR Reporting Overview. Program Year 2014
 Physician Quality Reporting System (PQRS) Qualified Clinical Data Registry (QCDR) QCDR Reporting Overview Program Year 2014 Disclaimers This presentation was current at the time it was published or uploaded
Physician Quality Reporting System (PQRS) Qualified Clinical Data Registry (QCDR) QCDR Reporting Overview Program Year 2014 Disclaimers This presentation was current at the time it was published or uploaded
Changes for Calendar Year 2015 Physician Quality Programs and Other Programs in the Medicare Physician Fee Schedule
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 FACT SHEET FOR IMMEDIATE RELEASE October 31, 2014 Contact: CMS
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 FACT SHEET FOR IMMEDIATE RELEASE October 31, 2014 Contact: CMS
May 7, 2012. Re: Medicare and Medicaid Programs; Electronic Health Record Incentive Program-- Stage 2 Proposed Rule CMS- 0044-P RIN 0938-AQ84
 May 7, 2012 Marilyn Tavenner Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Room 445-G, Hubert H. Humphrey Building 200 Independence Avenue, SW
May 7, 2012 Marilyn Tavenner Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Room 445-G, Hubert H. Humphrey Building 200 Independence Avenue, SW
2014 Medicare Physician Fee Schedule Proposed Rule Quality Provisions
 2014 Medicare Physician Fee Schedule Proposed Rule Quality Provisions The 2014 Medicare Physician Fee Schedule (MPFS) Notice of Proposed Rulemaking (NPRM) was published in the Federal Register on July
2014 Medicare Physician Fee Schedule Proposed Rule Quality Provisions The 2014 Medicare Physician Fee Schedule (MPFS) Notice of Proposed Rulemaking (NPRM) was published in the Federal Register on July
800 17th Street, NW Suite 1100, Washington, DC 20006
 800 17th Street, NW Suite 1100, Washington, DC 20006 September 3, 2015 Mr. Andrew Slavitt Acting Administrator, Centers for Medicare & Medicaid Services Department of Health and Human Services Hubert H.
800 17th Street, NW Suite 1100, Washington, DC 20006 September 3, 2015 Mr. Andrew Slavitt Acting Administrator, Centers for Medicare & Medicaid Services Department of Health and Human Services Hubert H.
RE: CMS-3819-P; Medicare and Medicaid Programs; Conditions of Participation for Home Health Agencies
 January 6, 2015 Marilyn Tavenner Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445 G Attention: CMS-3819-P Hubert H. Humphrey Building, 200 Independence
January 6, 2015 Marilyn Tavenner Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445 G Attention: CMS-3819-P Hubert H. Humphrey Building, 200 Independence
June 15, 2015 VIA ELECTRONIC SUBMISSION
 Charles N. Kahn III President & CEO June 15, 2015 VIA ELECTRONIC SUBMISSION Andrew M. Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention:
Charles N. Kahn III President & CEO June 15, 2015 VIA ELECTRONIC SUBMISSION Andrew M. Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention:
Meaningful Use Rules Proposed for Electronic Health Record Incentives Under HITECH Act By: Cherilyn G. Murer, JD, CRA
 Meaningful Use Rules Proposed for Electronic Health Record Incentives Under HITECH Act By: Cherilyn G. Murer, JD, CRA Introduction On December 30, 2009, The Centers for Medicare & Medicaid Services (CMS)
Meaningful Use Rules Proposed for Electronic Health Record Incentives Under HITECH Act By: Cherilyn G. Murer, JD, CRA Introduction On December 30, 2009, The Centers for Medicare & Medicaid Services (CMS)
DEPARTMENT OF HEALTH AND HUMAN SERVICES
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services 42 CFR Part 495 CMS-0052-P RIN 0938-AS30 Office of the Secretary 45 CFR Part 170 RIN 0991-AB97 Medicare and Medicaid Programs;
DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services 42 CFR Part 495 CMS-0052-P RIN 0938-AS30 Office of the Secretary 45 CFR Part 170 RIN 0991-AB97 Medicare and Medicaid Programs;
May 28, 2015. Dear Mr. Slavitt:
 May 28, 2015 The Honorable Andy Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445-G, Hubert H. Humphrey Building 200 Independence Ave,
May 28, 2015 The Honorable Andy Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445-G, Hubert H. Humphrey Building 200 Independence Ave,
Stage 2 of Meaningful Use: Ten Points of Interest
 November 8, 2012 Practice Group: Health Care Stage 2 of Meaningful Use: Ten Points of Interest By Patricia C. Shea On September 4, 2012, the Department of Health and Human Services, Centers for Medicare
November 8, 2012 Practice Group: Health Care Stage 2 of Meaningful Use: Ten Points of Interest By Patricia C. Shea On September 4, 2012, the Department of Health and Human Services, Centers for Medicare
CMS 1590-P: Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2013
 August 31, 2012 Marilyn Tavenner Acting Administrator and Chief Operating Officer Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1590-P P.O. Box 8013
August 31, 2012 Marilyn Tavenner Acting Administrator and Chief Operating Officer Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1590-P P.O. Box 8013
RE: CMS-0033-P, Medicare and Medicaid Programs; Electronic Health Record Incentive Program; Proposed Rule (Vol. 75, No. 8), January 13, 2010
 The Honorable Kathleen Sebelius Secretary Department of Health and Human Services 200 Independence Avenue, SW Washington, DC 20201 RE: CMS-0033-P, Medicare and Medicaid Programs; Electronic Health Record
The Honorable Kathleen Sebelius Secretary Department of Health and Human Services 200 Independence Avenue, SW Washington, DC 20201 RE: CMS-0033-P, Medicare and Medicaid Programs; Electronic Health Record
Quality Reporting and Registry Update: Challenges and Strategies for Success. Heather Smith, PT, MPH September 13, 2014
 Quality Reporting and Registry Update: Challenges and Strategies for Success Heather Smith, PT, MPH September 13, 2014 1 SETTING THE STAGE FOR TOMORROW 2014 American Physical Therapy Association. All rights
Quality Reporting and Registry Update: Challenges and Strategies for Success Heather Smith, PT, MPH September 13, 2014 1 SETTING THE STAGE FOR TOMORROW 2014 American Physical Therapy Association. All rights
Ambulatory Surgical Center Quality Reporting Program
 QualityNet Reports and Utilization of the Secure File Transfer: PM Questions and Answers Moderator: Mollie Carpenter, RN, BSN Educational Coordinator, ASCQR Program SC Speaker: Reneé Parks, RN, BSN ASCQR
QualityNet Reports and Utilization of the Secure File Transfer: PM Questions and Answers Moderator: Mollie Carpenter, RN, BSN Educational Coordinator, ASCQR Program SC Speaker: Reneé Parks, RN, BSN ASCQR
Medicare Shared Savings Program Final Rule
 Healthcare Committee Medicare Shared Savings Program Final Rule On June 9, 2015, the Centers for Medicare & Medicaid Services ( CMS ) published a final rule that, according to the agency, will update and
Healthcare Committee Medicare Shared Savings Program Final Rule On June 9, 2015, the Centers for Medicare & Medicaid Services ( CMS ) published a final rule that, according to the agency, will update and
March 15, 2010. Dear Ms. Frizzera,
 March 15, 2010 Ms. Charlene Frizzera Acting Administrator Centers for Medicare & Medicaid Services (CMS) Department of Health and Human Services Attention: CMS 0033 P P.O. Box 8013, Baltimore, MD 21244
March 15, 2010 Ms. Charlene Frizzera Acting Administrator Centers for Medicare & Medicaid Services (CMS) Department of Health and Human Services Attention: CMS 0033 P P.O. Box 8013, Baltimore, MD 21244
April 3, 2014. Submitted Electronically Via Federal Rulemaking Portal: www.regulations.gov
 April 3, 2014 Submitted Electronically Via Federal Rulemaking Portal: www.regulations.gov Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Attention: CMS-0037-P Room
April 3, 2014 Submitted Electronically Via Federal Rulemaking Portal: www.regulations.gov Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Attention: CMS-0037-P Room
2013 Physician Quality Reporting System (PQRS): Implementation Guide Claims-Based Reporting for Incentive
 2013 Physician Quality Reporting System (PQRS): Implementation Guide Claims-Based Reporting for Incentive CPT only copyright 2012 American Medical Association. All rights reserved. Page 1 of 28 Table of
2013 Physician Quality Reporting System (PQRS): Implementation Guide Claims-Based Reporting for Incentive CPT only copyright 2012 American Medical Association. All rights reserved. Page 1 of 28 Table of
Medicare and Medicaid Programs; Electronic Health Record Incentive Program Stage 2.
 May 7, 2012 Marilyn Tavenner Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS 0044 P P.O. Box 8013, Baltimore, MD 21244 8013 Re: Medicare
May 7, 2012 Marilyn Tavenner Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS 0044 P P.O. Box 8013, Baltimore, MD 21244 8013 Re: Medicare
September 4, 2012. Dear Acting Administrator Tavenner:
 Ms. Marilyn Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-1358-P P.O. Box 8016 Baltimore, MD 21244-8016 RE: CMS-1358-P; Medicare
Ms. Marilyn Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-1358-P P.O. Box 8016 Baltimore, MD 21244-8016 RE: CMS-1358-P; Medicare
CMS-14612-P Medicare Program; Medicare Shared Savings Program; Accountable Care Organizations Proposed Rule 79 Fed. Reg. 72760 (December 8, 2014)
 American Cancer Society Cancer Action Network 555 11 th Street, NW Suite 300 Washington, DC 20004 202.661.5700 www.acscan.org Marilyn Tavenner Administrator Centers for Medicare & Medicaid Services Department
American Cancer Society Cancer Action Network 555 11 th Street, NW Suite 300 Washington, DC 20004 202.661.5700 www.acscan.org Marilyn Tavenner Administrator Centers for Medicare & Medicaid Services Department
December 3, 2010 SUBMITTED ELECTRONICALLY
 December 3, 2010 SUBMITTED ELECTRONICALLY Donald M. Berwick, MD, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445 G Hubert H. Humphrey Building 200
December 3, 2010 SUBMITTED ELECTRONICALLY Donald M. Berwick, MD, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445 G Hubert H. Humphrey Building 200
Centers for Medicare & Medicaid Services Quality Measurement and Program Alignment
 Centers for Medicare & Medicaid Services Quality Measurement and Program Alignment 1 Conflict of Interest Disclosure Deborah Krauss, MS, BSN, RN Maria Michaels, MBA, CCRP, PMP Maria Harr, MBA, RHIA Have
Centers for Medicare & Medicaid Services Quality Measurement and Program Alignment 1 Conflict of Interest Disclosure Deborah Krauss, MS, BSN, RN Maria Michaels, MBA, CCRP, PMP Maria Harr, MBA, RHIA Have
RE: CMS 0033 P; Comments to Meaningful Use Notice of Proposed Rulemaking
 Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445 G Hubert H. Humphrey Building 200 Independence Avenue, SW. Washington, DC 20201 RE: CMS 0033 P; Comments to Meaningful
Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445 G Hubert H. Humphrey Building 200 Independence Avenue, SW. Washington, DC 20201 RE: CMS 0033 P; Comments to Meaningful
Mitigating Surgical Medical Malpractice Exposure in Ambulatory Surgery Centers
 Mitigating Surgical Medical Malpractice Exposure in Ambulatory Surgery Centers Making Your Ambulatory Surgery Center a Healthcare SafetyZone Even though Ambulatory Surgery Centers (ASCs) have been around
Mitigating Surgical Medical Malpractice Exposure in Ambulatory Surgery Centers Making Your Ambulatory Surgery Center a Healthcare SafetyZone Even though Ambulatory Surgery Centers (ASCs) have been around
FAQs for AMDA Members on the Medicare and Medicaid Electronic Health Record Incentive Programs, Including Medicare Payment Adjustments
 FAQs for AMDA Members on the Medicare and Medicaid Electronic Health Record Incentive Programs, Including Medicare Payment Adjustments Long Term Post-Acute Care Providers I am a physician or nurse practitioner
FAQs for AMDA Members on the Medicare and Medicaid Electronic Health Record Incentive Programs, Including Medicare Payment Adjustments Long Term Post-Acute Care Providers I am a physician or nurse practitioner
August 30, 2011. Dear Dr. Berwick:
 Donald Berwick, M.D. Administrator Centers for Medicare & Medicaid Services Department of Health & Human Services Attention: CMS-1524-P Mail Stop C4-26-05 7500 Security Boulevard Baltimore, MD 21244-1850
Donald Berwick, M.D. Administrator Centers for Medicare & Medicaid Services Department of Health & Human Services Attention: CMS-1524-P Mail Stop C4-26-05 7500 Security Boulevard Baltimore, MD 21244-1850
Re: Medicare and Medicaid Programs; Electronic Health Record Incentive Program Stage 2
 October 19, 2012 Marilyn Tavenner Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS 0044 P P.O. Box 8013 Baltimore, MD 21244 8013 Re:
October 19, 2012 Marilyn Tavenner Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS 0044 P P.O. Box 8013 Baltimore, MD 21244 8013 Re:
Fiscal Year 2016 proposed Inpatient and Long-term Care Hospital policy and payment changes (CMS-1632-P)
 Fiscal Year 2016 proposed Inpatient and Long-term Care Hospital policy and payment changes (CMS-1632-P) Date 2015-04-17 Title Fiscal Year 2016 proposed Inpatient and Long-term Care Hospital policy and
Fiscal Year 2016 proposed Inpatient and Long-term Care Hospital policy and payment changes (CMS-1632-P) Date 2015-04-17 Title Fiscal Year 2016 proposed Inpatient and Long-term Care Hospital policy and
Abstraction 101 An Introduction for New Abstractors
 California and Florida In the Know Webinar Series Abstraction 101 An Introduction for New Abstractors September 2011 Becky Ure, RN, BSN, MEd 1 Topics The driving forces behind abstraction and public reporting
California and Florida In the Know Webinar Series Abstraction 101 An Introduction for New Abstractors September 2011 Becky Ure, RN, BSN, MEd 1 Topics The driving forces behind abstraction and public reporting
Hospital Inpatient Quality Reporting Requirements
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services [CMS-3278-NC] Medicare Program; Request for Information on Hospital and Vendor Readiness for Electronic Health Records Hospital
DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services [CMS-3278-NC] Medicare Program; Request for Information on Hospital and Vendor Readiness for Electronic Health Records Hospital
12/5/2014. What is PQRS? Performance Measurement Committee Practical Theater. Historical concerns with the program (continued)
 What is PQRS? Navigating CMS Quality Initiatives: How to Successfully Report and Avoid Payment Adjustments Performance Measurement Committee Practical Theater A federally mandated Medicare Part B quality
What is PQRS? Navigating CMS Quality Initiatives: How to Successfully Report and Avoid Payment Adjustments Performance Measurement Committee Practical Theater A federally mandated Medicare Part B quality
December 3, 2010. Donald M. Berwick, M.D. Administrator Centers for Medicare and Medicaid Services Posted to Regulations.gov. File code CMS-1345-NC
 December 3, 2010 Donald M. Berwick, M.D. Administrator Centers for Medicare and Medicaid Services Posted to Regulations.gov File code CMS-1345-NC Dear Dr. Berwick: The American Urological Association (AUA),
December 3, 2010 Donald M. Berwick, M.D. Administrator Centers for Medicare and Medicaid Services Posted to Regulations.gov File code CMS-1345-NC Dear Dr. Berwick: The American Urological Association (AUA),
August 13, 2014. Dear Administrator Tavenner:
 Marilyn B. Tavenner Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445 G 200 Independence Avenue, SW Washington, DC
Marilyn B. Tavenner Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445 G 200 Independence Avenue, SW Washington, DC
Biodesign ADVANCED TISSUE REPAIR
 Biodesign ADVANCED TISSUE REPAIR 2013 CODING AND REIMBURSEMENT GUIDE FOR RECTOVAGINAL FISTULA The information provided herein reflects Cook Medical's understanding of the procedure(s) and/or devices(s)
Biodesign ADVANCED TISSUE REPAIR 2013 CODING AND REIMBURSEMENT GUIDE FOR RECTOVAGINAL FISTULA The information provided herein reflects Cook Medical's understanding of the procedure(s) and/or devices(s)
CMS is requesting information to aid in the planning and implementation of the MIPS in the following areas:
 Summary of Medicare s Request for Information on the Provisions in MACRA which Allow for Implementation of Alternative Payment Models and a Merit-Based Incentive Payment System On September 28, 2015, the
Summary of Medicare s Request for Information on the Provisions in MACRA which Allow for Implementation of Alternative Payment Models and a Merit-Based Incentive Payment System On September 28, 2015, the
The Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) Summary of SGR Repeal and Replacement Provisions
 ACOG Government Affairs May 2015 The Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) Summary of SGR Repeal and Replacement Provisions This landmark bipartisan legislation, signed into law
ACOG Government Affairs May 2015 The Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) Summary of SGR Repeal and Replacement Provisions This landmark bipartisan legislation, signed into law
Ambulatory Surgery Centers A P O S I T I V E T R E N D I N H E A L T H C A R E
 Ambulatory Surgery Centers A P O S I T I V E T R E N D I N H E A L T H C A R E Ambulatory surgery centers (ASCs) are health care facilities which offer patients the opportunity to have selected surgical
Ambulatory Surgery Centers A P O S I T I V E T R E N D I N H E A L T H C A R E Ambulatory surgery centers (ASCs) are health care facilities which offer patients the opportunity to have selected surgical
August 29, 2014. Dear Administrator Tavenner:
 Ms. Marilyn Tavenner Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1612-P 7500 Security Boulevard Baltimore, MD 21244-1850 Submitted electronically:
Ms. Marilyn Tavenner Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1612-P 7500 Security Boulevard Baltimore, MD 21244-1850 Submitted electronically:
Background. Requirements
 The Physician Quality Reporting System Maintenance of Certification Program Incentive Requirements for 2013 Background In accordance with section 1848(m) (7) of the Act ( Additional Incentive Payment ),
The Physician Quality Reporting System Maintenance of Certification Program Incentive Requirements for 2013 Background In accordance with section 1848(m) (7) of the Act ( Additional Incentive Payment ),
Health Care February 28, 2012. CMS Issues Proposed Rule on Stage 2 Meaningful Use,
 ROPES & GRAY ALERT Health Care February 28, 2012 CMS Issues Proposed Rule on Stage 2 Meaningful Use, ONC Issues Companion Proposed Rule on 2014 EHR Certification Criteria On February 23, 2012, the Centers
ROPES & GRAY ALERT Health Care February 28, 2012 CMS Issues Proposed Rule on Stage 2 Meaningful Use, ONC Issues Companion Proposed Rule on 2014 EHR Certification Criteria On February 23, 2012, the Centers
FY2015 Hospice Wage Index Proposed Rule
 FY2015 Hospice Wage Index Proposed Rule To: NHPCO Members From: NHPCO Health Policy Team Date: May 6, 2014 Summary of FY2015 Hospice Wage Index Proposed Rule On Friday, May 2 2014, CMS released the FY2015
FY2015 Hospice Wage Index Proposed Rule To: NHPCO Members From: NHPCO Health Policy Team Date: May 6, 2014 Summary of FY2015 Hospice Wage Index Proposed Rule On Friday, May 2 2014, CMS released the FY2015
The Honorable Sander Levin Ranking Member, Committee on Ways and Means
 33 W. Monroe, Suite 1700 Chicago, IL 60603 Phone: 312-915-9582 Fax: 312-915-9511 E-mail: himssehra@himss.org AllMeds, Inc. Allscripts Healthcare Solutions Amazing Charts Aprima Medical Software, Inc. athenahealth,
33 W. Monroe, Suite 1700 Chicago, IL 60603 Phone: 312-915-9582 Fax: 312-915-9511 E-mail: himssehra@himss.org AllMeds, Inc. Allscripts Healthcare Solutions Amazing Charts Aprima Medical Software, Inc. athenahealth,
Physician Compare Virtual Office Hour Questions and Answers
 Physician Compare Virtual Office Hour Questions and Answers The Physician Compare Virtual Office Hour session was held on January 22, 2015 via WebEx. The purpose of the session was to allow the Centers
Physician Compare Virtual Office Hour Questions and Answers The Physician Compare Virtual Office Hour session was held on January 22, 2015 via WebEx. The purpose of the session was to allow the Centers
Medicare Rules for Participation Agreements
 2015 Medicare Participation Kit Tools to help you understand your Medicare participation options and educate your patients. This kit includes: A summary of Medicare Participation Options Sample Patient
2015 Medicare Participation Kit Tools to help you understand your Medicare participation options and educate your patients. This kit includes: A summary of Medicare Participation Options Sample Patient
Quality Scores Monitoring and Reporting
 Section 5.1 Maintain Quality Scores Monitoring and Reporting This tool describes potential quality measurement and performance requirements for a communitybased care coordination (CCC) program, the process
Section 5.1 Maintain Quality Scores Monitoring and Reporting This tool describes potential quality measurement and performance requirements for a communitybased care coordination (CCC) program, the process
