April 8, Dear Ms. Tavenner:
|
|
|
- Everett May
- 8 years ago
- Views:
Transcription
1 April 8, 2013 Marilyn B. Tavenner Acting Administrator and Chief Operating Officer Centers for Medicare & Medicaid Services Department of Health and Human Services Hubert H. Humphrey Building 200 Independence Ave., S.W. Washington, DC Re: CMS-3276-NC Medicare Program; Request for Information on the Use of Clinical Quality Measures (CQMs) Reported under the Physician Quality Reporting System (PQRS), the Electronic Health Record (EHR) Incentive Program, and Other Reporting Programs. Dear Ms. Tavenner: On behalf of the over 50,000 members of the American Society of Anesthesiologists (ASA), I appreciate the opportunity to comment on the Request for Information on the Use of Clinical Quality Measures (CQMs) Reported under the Physician Quality Reporting System (PQRS), the Electronic Health Record (EHR) Incentive Program, and Other Reporting Programs that was published in the Federal Register on February 7, As the Institute of Medicine (IOM) recognized leaders in patient safety, we look forward to continuing to work with CMS to improve the quality of care for all patients. Response to Request for Information 1. How are the current reporting requirements for the PQRS and the reporting requirements in 2014 for the EHR Incentive Program similar to the reporting requirements already established for the ABMS boards or to other non-federal quality reporting programs? How are they different? In what ways are these reporting requirements duplicative and can these reporting programs be integrated to reduce reporting burden on eligible professionals? PQRS currently has three measures applicable to the typical anesthesiologist. These measures may be reported by claims or registry. None of the clinical quality measures in the EHR incentive program are applicable to most anesthesiologists. The American Board of Anesthesiology (ABA) is one of 24 member boards of the American Boards of Medical Specialties, and we encourage CMS to work with ASA and ABA as you continue to modify the PQRS and EHR Incentive Programs. PQRS should align with rather than constrain current quality efforts at ASA and ABA. For example, Maintenance of Certification in Anesthesiology (MOCA) Part IV modules, which focus on high impact learning, are related to performance improvement but are not necessarily linked to a PQRS measures. The reporting requirements between MOCA and PQRS/EHR Incentive Program differ enough that it may not be possible to integrate them into one process. However, we hope that the PQRS could become more tightly aligned with MOCA such that integrated reporting is more feasible.
2 The ASA views the PQRS as too restrictive in scope. Currently the PQRS simply measures physician performance but does not involve active quality improvement. MOCA s comprehensive quality improvement approach includes active performance improvement through identifying and closing practice gaps. The ASA has developed tools to fulfill the Practice Performance Assessment and Improvement (PPAI) component of MOCA. These modules are case evaluation based performance improvement activities designed around identified practice gaps. Each PPAI module is designed to close the specified gap by linking education with performance improvement via implementation of an outcome improvement process. Quality of care is enhanced, not just measured. Likewise, the ASA believes federal quality reporting programs should promote and reward such activities that go beyond measurement to foster active quality improvement. 2. Are there examples of other non-federal programs under which eligible professionals report quality measures data? Multi-center registries of anesthesia care are now available to all anesthesiologists in the United States, enabling participants to compare their own outcomes to national and peer group benchmarks. The ASA, believing firmly that our members participation in such a registry will lead to improvements in safety and quality, has invested heavily in registry development. In October 2008, the ASA House of Delegates created the Anesthesia Quality Institute (AQI). The AQI is a separately incorporated organization, with its own Board of Directors, that receives most of its funding from the ASA. The vision of the AQI is to become the primary source of information for quality improvement in the clinical practice of anesthesiology. The mission of the AQI is to develop and maintain an ongoing registry of case data that helps anesthesiologists assess and improve patient care. This is achieved by organizing the registry such that anesthesiology practice groups may easily submit their case information. Individual anesthesiologists, practice groups, researchers, and professional societies currently use the resulting data for quality improvement activities. The AQI supports three active registries of clinical data with an additional one in development: The National Anesthesia Clinical Outcomes Registry (NACOR) has gathered data on millions of cases, hundreds of facilities, and thousands of providers. This number is growing every day as new practices submit data and existing practices contribute additional cases. Registry participants range from practices that record on paper documents to the most HIT-intensive academic centers in the country. NACOR is collecting the following types of data: billing/administrative, quality/perioperative events, Anesthesia Information Management Systems (AIMS) data and broader Electronic Health Records (EHRs), of which AIMS data is typically a component. The Anesthesia Incident Reporting System (AIRS) is a national collection of serious adverse events and near misses, collected confidentially. Any anesthesia provider can contribute to AIRS, by accessing the website at The AQI is an approved Patient Safety Organization (PSO). De-identified cases from AIRS provide teaching material for case reports featured in the ASA Newsletter, which is freely distributed to all ASA members and is available online to the general public. The Maintenance of Certification in Anesthesiology (MOCA) Practice Performance Assessment and Improvement (PPAI) registry is a joint effort of ASA, ABA and the AQI. Participants in MOCA are required to assess the quality of their practice including clinical data from real patients. ASA modules provide an easy-to-use format for doing this. The AQI registry collects and protects the clinical data entered by MOCA participants. For anesthesiologists in practices that participate in NACOR, the AQI plans to go a step further. We are piloting a voluntary program that helps providers more easily identify
3 the cases they need to review for their MOCA-PPAI project. In the long run this system will autopopulate much of the required data. The ASA's resources and AQI s performance improvement tools can be combined with ABA s Maintenance of Certification efforts to permit reporting MOCA data in ways the ABA cannot support alone. In addition, the ASA, AQI, and ABA are beginning to collaborate on a project to develop interactive learning and quality improvement modules each with a focus on a single quality measure. We hope this project will broaden the participation of anesthesiologists in the PQRS while improving the quality of patient care. The National Pain Registry will help anesthesiologists keep track of long-term pain management outcomes. Working with experts from the American Society for Regional Anesthesia and Pain Management (ASRA) we have laid out a template for data and definitions. We will help participants build these measures into existing EHRs such that the data can be periodically transferred to the AQI. AQI is listed as a Patient Safety Organization (PSO) by the Department of Health & Human Services (HHS), is a certified reporting registry for PQRS, and is a member of the National Quality Forum (NQF) and Multicenter Perioperative Outcomes Group (MPOG). Other professional anesthesia societies also maintain clinical data registries, such as the Society for Ambulatory Anesthesia Clinical Outcome Registry. For more information please visit the AQI website at 3. What would be the benefits and shortcomings involved with allowing third party entities to report quality data to CMS on behalf of physicians and other eligible professionals? Benefits of allowing third parties to report quality data to CMS are the convenience and ease of participation for physicians who choose to participate. For some, the decision to report to the third-party entity may be due in large part to a desire to participate in PQRS and perhaps other federal programs. However, significant shortcomings must be considered, and individual physicians or groups should be given the choice as to whether and to what extent to participate in federal reporting programs. The public reporting of federal benchmarking programs can have a major impact on a physician s practice as well as on the public perceptions of hospitals. Federal benchmarking programs often rely on administrative (billing) data, which, while relatively easy to gather and report, are of lower quality than the clinical data used for robust quality measures; furthermore, administrative data is subject to manipulation and has been shown to demonstrate reduced reported adverse event rates.[i] Administrative data often fail to distinguish between pre-existing conditions and complications that occur following hospital admission. [ii] Measures based on administrative data also may fail to identify significant clinical outcomes. In a comparison with clinical data from the National Surgical Quality Improvement Program, AHRQ s Patient Safety Indicators missed approximately 45-80% of the selected complications.[iii] Thus shortcomings exist in reporting quality data from third parties to CMS, to the extent that administrative data reported to third parties are not supplemented with robust clinical data, and to the extent to which CMS programs rely solely or even principally on administrative data. [i] Farmer SA, et al. JAMA 2013: 309: [ii] Glance LG, et al. Anesth Analg 2011; 112: [iii] Romano PS. Health Serv Res. 2009; 44: What entities have the capacity to report quality data similar to those reported under the PQRS, Value-based Payment Modifier, and/or EHR Incentive programs? If these entities were to report
4 such data to CMS, what requirements should we include in the reporting system used by such entities, including requirements to ensure high quality data? For anesthesiologists, the National Anesthesia Clinical Outcomes Registry described above can report such data. Because concerns exist (see above) about the validity of data used for some federal benchmarking, physicians who report data to third-party entities should need to initiate reporting to federal programs by opting in. If changes occur to federal requirements for third party reporting, individuals and groups reporting to the third party should be notified and given the option to change the extent to which their data are reported. PQRS and other federal reporting systems should emphasize clinical data and proper risk adjustment for any outcome measures. 5. How should our quality reporting programs change/evolve to reduce reporting burden on eligible professionals, while still receiving robust data on clinical quality? CMS could greatly improve the administrative burden on physicians by allowing specialty societies and their existing quality reporting mechanisms, such as ASA and AQI, to serve as the liaison between their clinical practice and all regulatory requirements. This would encourage collection and reporting of much more granular and specialty-appropriate measures (improving care more directly) while simultaneously reducing CMS's burden in developing and promulgating measures. If robust clinical data are desired, CMS should consider offsetting some of the costs associated with the expensive process of abstracting clinical data of reasonable quality. 6. What types of entities should be eligible to submit quality measures data on behalf of eligible professionals for PQRS and the EHR Incentive Program? Examples might include medical board registries, specialty society registries, regional quality collaboratives or other entities. What qualification requirements should be applicable to such entities? What functionalities should entities qualified to submit PQRS quality measures data possess? For example, for CQMs that can be electronically submitted and reported under PQRS and the EHR Incentive Program, should an entity s qualification to submit such measures be based on whether they have technology certified to ONC s certification criteria for CQM calculation and/or electronic submission? Several specialty specific registries exist and have demonstrated strong records in quality measures data management. These existing registries could and should funnel information to other entities (quality collaboratives, medical boards, etc.) rather than creating overlapping and duplicative systems. We are best served by medical boards and quality collaboratives analysis of the data not their aggregation of it. Specialty society registry participation should count for as many requirements as possible in PQRS, Meaningful Use, and Pay for Performance, at both the group and individual level. This assumes that the registry in question meets certain requirements, and is certified in some fashion. We recommend that the registry meets the following requirements: National in scope, non-profit/non-commercial Collect a range of clinical measures at the individual case level Publicly report what data elements are collected (not the data itself; the field definitions) Have mechanisms for data validation Provide regular feedback reports to all participants, which include peer group and national benchmarking data Share national level aggregated data with government agencies in a transparent fashion on behalf of all stakeholders
5 7. What criteria should we require of entities submitting quality measures data to us on behalf of eligible professionals? Examples might include transparency of measures available to EPs, specific frequency of feedback reports, tools to guide improvement efforts for EPs, ability to report aggregate data, agreement to data audits if requested, etc. For reporting by registries to CMS, CMS should specify the technical requirements of the data file, the way in which specialty-developed measures are defined and disseminated, and the frequency and granularity with which the registry must provide feedback to participants. 8. Should reporting entities be required to publicly post performance data? Until publicly reported benchmarks can be ensured to have as their basis robust clinical data and outcomes that are appropriately risk adjusted to avoid penalizing physicians who care for high-risk patients, public reporting should not be required. New publicly reported measures must be developed with the highest standards for data quality. It should also be recognized that entities use performance data for different purposes. For example medical specialty boards use the data to establish that participants meet their standards. Boards should report the participants who meet these standards, and what the criteria were; however, individual participant performance scores should not be reported. These participants either met or did not meet the aggregate criteria for certification. 9. Should we require an entity to submit a yearly self-nomination statement to participate in PQRS? Yearly statements may create unnecessary burden. However, if new requirements are put in place or existing reporting requirements significantly change in a given year, then there should be a straightforward and administratively simple attestation process confirming the entity's ability to meet these new requirements. 10. What oversight (for example, checks or audits) should be in place to ensure that data is submitted and calculated properly by entities? CMS should define standards for data integrity evaluation. Each entity should submit a self-administered validation plan as part of their initial application, and update it as requirements change. CMS could consider either performing or contracting for performance of random checks confirming that the data validation plan is being followed. 11. Should we require that a certain proportion of submitted measures have particular characteristics such as being NQF-endorsed or outcome-based? The current PQRS focus on chronic management of medical conditions fails to involve specialties such as ours sufficiently. Anesthesiology has long been a leader in patient safety and quality improvement, but many anesthesiologists struggle to find approved PQRS measures that apply to their practices, due to the focus in PQRS on measures designed for primary care and long-term medical management. The ASA encourages CMS to approve more measures with characteristics applicable to anesthesia care. In addition, specialty registries should not be limited to approved PQRS or NQF endorsed measures, but should be free to develop granular and specialty-specific quality measures through an internal process. Documentation and validation of this process might be an appropriate component of a CMS-guided protocol for accrediting Quality Management registries.
6 For most clinical decisions in medical care, definitive outcome data are lacking to guide choices between all reasonable options. Thus process measures will still have their place, especially when a given process is either strongly associated with improved primary or secondary outcomes or is widely accepted as a best practice based on the best available evidence. 12. Should we require that the quality measures data submitted cover a certain number of the six national quality strategy domains? This requirement would unduly constrain certain specialties in developing meaningful measures relevant to their practice. Many specialties do not align well with all of priorities of the National Quality Strategy. For instance, anesthesiology aligns well with the patient safety priority but does not fit the paradigm of the healthy living priority. We urge CMS to permit each specialty to align its measures appropriately within the National Quality Strategy for maximum impact on quality of care. 13. If we propose revised criteria for satisfactory reporting under PQRS and for meeting the CQM component of meaningful use under the EHR Incentive Program, how many measures should an eligible professional be required to report to collect meaningful quality data? If we were to align reporting criteria with reporting requirements for other non-federal reporting programs, in future years, should we propose to require reporting on a different number of measures than what is currently required for the PQRS in 2013 and the EHR Incentive Program under the Stage 2 final rule or should the non-federal reporting programs align with CMS criteria? Any proposal for a minimum number of measures to be reported needs to consider the number of measures available for use in each common practice setting for a given specialty. For instance, PQRS currently contains three measures approved for reporting based on procedures typically performed by anesthesiologists. One of them (PQRS #76, Central Venous Catheter Insertion Protocol) cannot be reported by most outpatient-based anesthesiologists, as this group rarely inserts central venous catheters. Section 601(b) was included in The Tax Payer Relief Act to address the inflexibility and low-participation rates in the PQRS and EHR Incentive Programs. ASA believes additional reporting options should be flexible and encourage participation, while collecting meaningful information. ASA urges CMS to not replicate the reporting structure of these programs in the rulemaking process. The ASA appreciates CMS efforts to improve federal quality reporting programs and the process of reporting data to them. At the same time, the ASA hopes that in doing so, CMS will embrace, not hinder, the use of robust tools already developed for active quality improvement, such as the ASA s PPAI modules. Again, we appreciate the opportunity to comment on this request for information. If you have any additional questions, please feel free to contact Grant Couch (g.couch@asawash.org) Federal Affairs Associate or Maureen Amos (m.amos@asawash.org) Director of Quality and Regulatory Affairs at (202) Sincerely, John M. Zerwas, M.D. President American Society of Anesthesiologists
June 25, 2012. Re: CMS-1588-P; Proposed Quality Reporting Requirements for Ambulatory Surgical Centers (ASCs)
 VIA ELECTRONIC DELIVERY Marilyn Tavenner, Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1588-P Room 445-G Hubert H. Humphrey Building
VIA ELECTRONIC DELIVERY Marilyn Tavenner, Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1588-P Room 445-G Hubert H. Humphrey Building
April 22, 2013. Re: Advancing Interoperability and Health Information Exchange. Dear Dr. Mostashari,
 Farzad Mostashari, MD, ScM National Coordinator for Health Information Technology Department of Health and Human Services Office of the National Coordinator for Health Information Technology Hubert H.
Farzad Mostashari, MD, ScM National Coordinator for Health Information Technology Department of Health and Human Services Office of the National Coordinator for Health Information Technology Hubert H.
Request for Feedback on the CMS Quality Strategy: 2013 Beyond
 Ms. Marilyn Tavenner Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services 7500 Security Boulevard Baltimore, MD 21244-1850 Request for Feedback on the CMS Quality
Ms. Marilyn Tavenner Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services 7500 Security Boulevard Baltimore, MD 21244-1850 Request for Feedback on the CMS Quality
May 7, 2012. Dear Dr. Mostashari:
 McKesson Corporation One Post Street San Francisco, CA 94104-5296 Ann Richardson Berkey Senior Vice President, Public Affairs May 7, 2012 Farzad Mostashari, M.D., ScM. Director Office of the National Coordinator
McKesson Corporation One Post Street San Francisco, CA 94104-5296 Ann Richardson Berkey Senior Vice President, Public Affairs May 7, 2012 Farzad Mostashari, M.D., ScM. Director Office of the National Coordinator
Re: CMS 3819-P, Medicare and Medicaid Programs; Conditions of Participation for Home Health Agencies; Proposed Rule, Oct. 9, 2014.
 Marilyn B. Tavenner Administrator Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W., Room 445-G Washington, D.C. 20201 Re: CMS 3819-P, Medicare and Medicaid
Marilyn B. Tavenner Administrator Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W., Room 445-G Washington, D.C. 20201 Re: CMS 3819-P, Medicare and Medicaid
Physician Quality Reporting System (PQRS) Qualified Clinical Data Registry (QCDR) QCDR Reporting Overview. Program Year 2014
 Physician Quality Reporting System (PQRS) Qualified Clinical Data Registry (QCDR) QCDR Reporting Overview Program Year 2014 Disclaimers This presentation was current at the time it was published or uploaded
Physician Quality Reporting System (PQRS) Qualified Clinical Data Registry (QCDR) QCDR Reporting Overview Program Year 2014 Disclaimers This presentation was current at the time it was published or uploaded
Centers for Medicare & Medicaid Services Quality Measurement and Program Alignment
 Centers for Medicare & Medicaid Services Quality Measurement and Program Alignment 1 Conflict of Interest Disclosure Deborah Krauss, MS, BSN, RN Maria Michaels, MBA, CCRP, PMP Maria Harr, MBA, RHIA Have
Centers for Medicare & Medicaid Services Quality Measurement and Program Alignment 1 Conflict of Interest Disclosure Deborah Krauss, MS, BSN, RN Maria Michaels, MBA, CCRP, PMP Maria Harr, MBA, RHIA Have
Re: Request for Information on Hospital and Vendor Readiness for Electronic Health Records Hospital Inpatient Quality Data Reporting
 20555 VICTOR PARKWAY LIVONIA, MI 48152 p 734-343-1000 trinity-health.org February 1, 2013 SUBMITTED ELECTRONICALLY Maria Harr Government Task Leader Centers for Medicare & Medicaid Services Department
20555 VICTOR PARKWAY LIVONIA, MI 48152 p 734-343-1000 trinity-health.org February 1, 2013 SUBMITTED ELECTRONICALLY Maria Harr Government Task Leader Centers for Medicare & Medicaid Services Department
May 28, 2015. Dear Mr. Slavitt:
 May 28, 2015 The Honorable Andy Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445-G, Hubert H. Humphrey Building 200 Independence Ave,
May 28, 2015 The Honorable Andy Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445-G, Hubert H. Humphrey Building 200 Independence Ave,
February 24, 2012 (202) 690-6145 CMS PROPOSES DEFINITION OF STAGE 2 MEANINGFUL USE OF CERTIFIED ELECTRONIC HEALTH RECORDS (EHR) TECHNOLOGY
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 Office of Communications FACT SHEET FOR IMMEDIATE RELEASE Contact:
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 Office of Communications FACT SHEET FOR IMMEDIATE RELEASE Contact:
Re: CMS 3323 NC, Request for Information (RFI): Certification Frequency and Requirements for the Reporting of Quality Measures Under CMS Programs
 February 5, 2016 Andrew M. Slavitt Acting Administrator Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W., Room 445-G Washington, DC 20201 Re: CMS 3323
February 5, 2016 Andrew M. Slavitt Acting Administrator Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W., Room 445-G Washington, DC 20201 Re: CMS 3323
Andy Slavitt Centers for Medicare & Medicaid Services
 January 29, 2016 Andy Slavitt Centers for Medicare & Medicaid Services Wellcentive comment, Request for Information: Certification Frequency and Requirements for the Reporting of Quality Measures under
January 29, 2016 Andy Slavitt Centers for Medicare & Medicaid Services Wellcentive comment, Request for Information: Certification Frequency and Requirements for the Reporting of Quality Measures under
Sustainable Growth Rate (SGR) Repeal and Replace: Comparison of 2014 and 2015 Legislation
 Sustainable Growth Rate (SGR) Repeal and Replace: Comparison of 2014 and 2015 Legislation Proposal 113 th Congress - - H.R.4015/S.2000 114 th Congress - - H.R.1470 SGR Repeal and Annual Updates General
Sustainable Growth Rate (SGR) Repeal and Replace: Comparison of 2014 and 2015 Legislation Proposal 113 th Congress - - H.R.4015/S.2000 114 th Congress - - H.R.1470 SGR Repeal and Annual Updates General
RE: Proposed Establishment of Certification Programs for Health Information Technology Permanent Certification Program, RIN 0991-AB59
 Via Electronic Submission @ www.regulations.gov David Blumenthal, M.D., M.P.P. National Coordinator for Health Information Technology HHS/Office of the National Coordinator for Health Information Technology
Via Electronic Submission @ www.regulations.gov David Blumenthal, M.D., M.P.P. National Coordinator for Health Information Technology HHS/Office of the National Coordinator for Health Information Technology
May 26, 2015. Dear Acting Administrator Slavitt:
 Andrew Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Hubert H. Humphrey Building, Room 445-G 200 Independence Avenue, SW Washington, DC 20201
Andrew Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Hubert H. Humphrey Building, Room 445-G 200 Independence Avenue, SW Washington, DC 20201
Hospital Inpatient Quality Reporting Requirements
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services [CMS-3278-NC] Medicare Program; Request for Information on Hospital and Vendor Readiness for Electronic Health Records Hospital
DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services [CMS-3278-NC] Medicare Program; Request for Information on Hospital and Vendor Readiness for Electronic Health Records Hospital
February 29, 2016. Andy Slavitt, Acting Administrator Centers for Medicare & Medicaid Services 200 Independence Ave., SW Washington, DC 20201
 Andy Slavitt, Acting Administrator Centers for Medicare & Medicaid Services 200 Independence Ave., SW Washington, DC 20201 Dear Acting Administrator Slavitt: On behalf of the American Academy of Family
Andy Slavitt, Acting Administrator Centers for Medicare & Medicaid Services 200 Independence Ave., SW Washington, DC 20201 Dear Acting Administrator Slavitt: On behalf of the American Academy of Family
HHS Issues Final Rule on Stage 2 of the Medicare and Medicaid EHR Incentive Programs. Amy M. Joseph, Esq. Paul T. Smith, Esq.
 HHS Issues Final Rule on Stage 2 of the Medicare and Medicaid EHR Incentive Programs Amy M. Joseph, Esq. Paul T. Smith, Esq. On August 23, 2012, the Department of Health and Human Services (HHS) released
HHS Issues Final Rule on Stage 2 of the Medicare and Medicaid EHR Incentive Programs Amy M. Joseph, Esq. Paul T. Smith, Esq. On August 23, 2012, the Department of Health and Human Services (HHS) released
Re: Medicare Program; Medicare Shared Savings Program: Accountable Care Organizations
 February 6, 2015 Marilyn Tavenner, Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1461-P P.O. Box 8013 Baltimore, Md. 21244-8013 Re: Medicare
February 6, 2015 Marilyn Tavenner, Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-1461-P P.O. Box 8013 Baltimore, Md. 21244-8013 Re: Medicare
May 7, 2012. Submitted Electronically
 May 7, 2012 Submitted Electronically Secretary Kathleen Sebelius Department of Health and Human Services Office of the National Coordinator for Health Information Technology Attention: 2014 edition EHR
May 7, 2012 Submitted Electronically Secretary Kathleen Sebelius Department of Health and Human Services Office of the National Coordinator for Health Information Technology Attention: 2014 edition EHR
Background. Requirements
 The Physician Quality Reporting System Maintenance of Certification Program Incentive Requirements for 2013 Background In accordance with section 1848(m) (7) of the Act ( Additional Incentive Payment ),
The Physician Quality Reporting System Maintenance of Certification Program Incentive Requirements for 2013 Background In accordance with section 1848(m) (7) of the Act ( Additional Incentive Payment ),
How to Report Once for 2015 Medicare Quality Reporting Programs: Individual Eligible Professionals
 Table of Contents How to Report Once for 2015 Medicare Quality Reporting Programs: Individual Eligible Professionals 3 How to Report Once for 2015 Medicare Quality Reporting Programs: Group Practices 5
Table of Contents How to Report Once for 2015 Medicare Quality Reporting Programs: Individual Eligible Professionals 3 How to Report Once for 2015 Medicare Quality Reporting Programs: Group Practices 5
June 15, 2015. Submitted electronically via www.regulations.gov
 June 15, 2015 Marilyn Tavenner, R.N. Administrator Center for Medicare and Medicaid Services Department of Health and Human Services P.O. Box 8013 Baltimore, MD 21244-8013 Submitted electronically via
June 15, 2015 Marilyn Tavenner, R.N. Administrator Center for Medicare and Medicaid Services Department of Health and Human Services P.O. Box 8013 Baltimore, MD 21244-8013 Submitted electronically via
Clinical Quality Measures for Providers
 Meaningful Use White Paper Series Paper no. 6a: Clinical Quality Measures for Providers Published September 15, 2010 Clinical Quality Measures for Providers Papers 5a and 5b in this series reviewed the
Meaningful Use White Paper Series Paper no. 6a: Clinical Quality Measures for Providers Published September 15, 2010 Clinical Quality Measures for Providers Papers 5a and 5b in this series reviewed the
Re: Medicare and Medicaid Programs; Electronic Health Record Incentive Program Stage 2
 October 19, 2012 Marilyn Tavenner Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS 0044 P P.O. Box 8013 Baltimore, MD 21244 8013 Re:
October 19, 2012 Marilyn Tavenner Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS 0044 P P.O. Box 8013 Baltimore, MD 21244 8013 Re:
APTA and Meaningful Use of HIT
 June 26, 2009 David Blumenthal, MD, MPP National Coordinator Office of the National Coordinator for Health Information Technology 200 Independence Avenue, SW Suite 729D Washington, DC 20201 RE: HIT Policy
June 26, 2009 David Blumenthal, MD, MPP National Coordinator Office of the National Coordinator for Health Information Technology 200 Independence Avenue, SW Suite 729D Washington, DC 20201 RE: HIT Policy
Re: Medicare and Medicaid Programs; Electronic Health Record Incentive Program Stage 3 (CMS-3310-P)
 May 29, 2015 Mr. Andrew Slavitt Acting Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445 G 200 Independence Avenue,
May 29, 2015 Mr. Andrew Slavitt Acting Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445 G 200 Independence Avenue,
October 15, 2010. Re: National Health Care Quality Strategy and Plan. Dear Dr. Wilson,
 October 15, 2010 Dr. Nancy Wilson, R.N., M.D., M.P.H. Senior Advisor to the Director Agency for Healthcare Research and Quality (AHRQ) 540 Gaither Road Room 3216 Rockville, MD 20850 Re: National Health
October 15, 2010 Dr. Nancy Wilson, R.N., M.D., M.P.H. Senior Advisor to the Director Agency for Healthcare Research and Quality (AHRQ) 540 Gaither Road Room 3216 Rockville, MD 20850 Re: National Health
March 15, 2010. Dear Dr. Blumenthal:
 March 15, 2010 David Blumenthal, MD, MPP National Coordinator Office of the National Coordinator for Health Information Technology (ONCHIT) Department of Health and Human Services ATTN: HITECH Initial
March 15, 2010 David Blumenthal, MD, MPP National Coordinator Office of the National Coordinator for Health Information Technology (ONCHIT) Department of Health and Human Services ATTN: HITECH Initial
Re: Medicare and Medicaid Programs: Electronic Health Record (EHR) Incentive Program- Stage 3 Proposed Rule, File Code CMS-3310-P
 Via Electronic Submission (www.regulations.gov) Mr. Andrew Slavitt Acting Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building,
Via Electronic Submission (www.regulations.gov) Mr. Andrew Slavitt Acting Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building,
Request for Information: Advancing Interoperability and Health Information Exchange
 April 22, 2013 Ms. Marilyn Tavenner Acting Administrator, Chief Operating Officer Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Farzad Mostashari, MD, ScM National
April 22, 2013 Ms. Marilyn Tavenner Acting Administrator, Chief Operating Officer Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Farzad Mostashari, MD, ScM National
Clinical Quality Measures (CQMs) What are CQMs?
 Clinical Quality Measures (CQMs) What are CQMs? What are CQMs? Clinical quality measures, or CQMs, are tools that help eligible providers (EPs) measure and track the quality of health care services provided
Clinical Quality Measures (CQMs) What are CQMs? What are CQMs? Clinical quality measures, or CQMs, are tools that help eligible providers (EPs) measure and track the quality of health care services provided
ADVANCING HIGHER EDUCATION IN NURSING
 September 4, 2012 Submitted via www.regulations.gov Marilyn Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attn: CMS 1590 P P.O. Box 8010
September 4, 2012 Submitted via www.regulations.gov Marilyn Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attn: CMS 1590 P P.O. Box 8010
(http://www.regulations.gov/#!documentdetail;d=cms-2013-0155-10181) File # CMS-2013-0155-10181
 January 27, 2014 Marilyn Tavenner, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-4159-P P.O. Box 8013 Baltimore, MD 21244-8013 Re: Final
January 27, 2014 Marilyn Tavenner, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-4159-P P.O. Box 8013 Baltimore, MD 21244-8013 Re: Final
RE: CMS 0033 P; Comments to Meaningful Use Notice of Proposed Rulemaking
 Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445 G Hubert H. Humphrey Building 200 Independence Avenue, SW. Washington, DC 20201 RE: CMS 0033 P; Comments to Meaningful
Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445 G Hubert H. Humphrey Building 200 Independence Avenue, SW. Washington, DC 20201 RE: CMS 0033 P; Comments to Meaningful
Establishment of a Temporary and Permanent Testing Program
 April 9, 2010 David Blumenthal, MD, MPP Office of the National Coordinator for Health Information Technology (ONCHIT) Attn: Certification Programs Proposed Rule Hubert H. Humphrey Building, Suite 729D
April 9, 2010 David Blumenthal, MD, MPP Office of the National Coordinator for Health Information Technology (ONCHIT) Attn: Certification Programs Proposed Rule Hubert H. Humphrey Building, Suite 729D
December 3, 2010. Donald M. Berwick, M.D. Administrator Centers for Medicare and Medicaid Services Posted to Regulations.gov. File code CMS-1345-NC
 December 3, 2010 Donald M. Berwick, M.D. Administrator Centers for Medicare and Medicaid Services Posted to Regulations.gov File code CMS-1345-NC Dear Dr. Berwick: The American Urological Association (AUA),
December 3, 2010 Donald M. Berwick, M.D. Administrator Centers for Medicare and Medicaid Services Posted to Regulations.gov File code CMS-1345-NC Dear Dr. Berwick: The American Urological Association (AUA),
Health Information Exchange of Post Acute Care Providers
 April 21, 2013 Ms. Marilyn Tavenner Acting Administrator, Chief Operating Officer Centers for Medicare and Medicaid Services Department of Health and Human Services 7500 Security Boulevard Baltimore, MD
April 21, 2013 Ms. Marilyn Tavenner Acting Administrator, Chief Operating Officer Centers for Medicare and Medicaid Services Department of Health and Human Services 7500 Security Boulevard Baltimore, MD
Crosswalk: CMS Shared Savings Rules & NCQA ACO Accreditation Standards 12/1/2011
 Crosswalk: CMS Shared Savings Rules & NCQA ACO Accreditation Standards 12/1/2011 The table below details areas where NCQA s ACO Accreditation standards overlap with the CMS Final Rule CMS Pioneer ACO CMS
Crosswalk: CMS Shared Savings Rules & NCQA ACO Accreditation Standards 12/1/2011 The table below details areas where NCQA s ACO Accreditation standards overlap with the CMS Final Rule CMS Pioneer ACO CMS
CMS Vision for Quality Measurement and Public Reporting
 CMS Vision for Quality Measurement and Public Reporting Annual Policy Conference Federation of American Hospitals Kate Goodrich, M.D., M.H.S. Quality Measurement & Health Assessment Group, Center for Clinical
CMS Vision for Quality Measurement and Public Reporting Annual Policy Conference Federation of American Hospitals Kate Goodrich, M.D., M.H.S. Quality Measurement & Health Assessment Group, Center for Clinical
Mar. 31, 2011 (202) 690-6145. Summary of proposed rule provisions for Accountable Care Organizations under the Medicare Shared Savings Program
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 Office of Media Affairs MEDICARE FACT SHEET FOR IMMEDIATE RELEASE
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 Office of Media Affairs MEDICARE FACT SHEET FOR IMMEDIATE RELEASE
Putting Reliable Health Care Performance Measurement Systems into Practice
 An NCQA Issue Brief 2000 L Street, NW Washington, DC 20036 888-275-7585 www.ncqa.org Putting Reliable Health Care Performance Measurement Systems into Practice By Joachim Roski, PhD MPH; Vice President,
An NCQA Issue Brief 2000 L Street, NW Washington, DC 20036 888-275-7585 www.ncqa.org Putting Reliable Health Care Performance Measurement Systems into Practice By Joachim Roski, PhD MPH; Vice President,
The Honorable Sander Levin Ranking Member, Committee on Ways and Means
 33 W. Monroe, Suite 1700 Chicago, IL 60603 Phone: 312-915-9582 Fax: 312-915-9511 E-mail: himssehra@himss.org AllMeds, Inc. Allscripts Healthcare Solutions Amazing Charts Aprima Medical Software, Inc. athenahealth,
33 W. Monroe, Suite 1700 Chicago, IL 60603 Phone: 312-915-9582 Fax: 312-915-9511 E-mail: himssehra@himss.org AllMeds, Inc. Allscripts Healthcare Solutions Amazing Charts Aprima Medical Software, Inc. athenahealth,
Changes for Calendar Year 2015 Physician Quality Programs and Other Programs in the Medicare Physician Fee Schedule
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 FACT SHEET FOR IMMEDIATE RELEASE October 31, 2014 Contact: CMS
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 FACT SHEET FOR IMMEDIATE RELEASE October 31, 2014 Contact: CMS
CMS s framework for Value Modifier
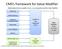 CMS s framework for Value Modifier Relationship between quality of care, cost composites and the Value Modifier Clinical Care Patient Experience Population/ Community Health Patient Safety Care Coordination
CMS s framework for Value Modifier Relationship between quality of care, cost composites and the Value Modifier Clinical Care Patient Experience Population/ Community Health Patient Safety Care Coordination
CMS QCDR (Qualified Clinical Data Registry) and Other Ways PPRNet Can Help with Value-Based Payment
 CMS QCDR (Qualified Clinical Data Registry) and Other Ways PPRNet Can Help with Value-Based Payment Cara Litvin MD, MS Assistant Professor MUSC Department of Medicine Agenda Provide an update of the current
CMS QCDR (Qualified Clinical Data Registry) and Other Ways PPRNet Can Help with Value-Based Payment Cara Litvin MD, MS Assistant Professor MUSC Department of Medicine Agenda Provide an update of the current
Demystifying Patient Satisfaction Surveys
 Demystifying Patient Satisfaction Surveys What you need to know about the H-CAHPS, CG-CAHPS, and PQRS and the benefits of performing patient satisfaction surveys. NCMGMA Lunch and Learn Webinar, June 11,
Demystifying Patient Satisfaction Surveys What you need to know about the H-CAHPS, CG-CAHPS, and PQRS and the benefits of performing patient satisfaction surveys. NCMGMA Lunch and Learn Webinar, June 11,
CMS Proposed Electronic Health Record Incentive Program For Physicians
 May 7, 2012 Ms. Marilyn Tavenner Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-0044-P Mail Stop C4-26-05 7500 Security Boulevard
May 7, 2012 Ms. Marilyn Tavenner Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-0044-P Mail Stop C4-26-05 7500 Security Boulevard
Welcome to the Data Analytics Toolkit PowerPoint presentation on clinical quality measures, meaningful use, and data analytics.
 Welcome to the Data Analytics Toolkit PowerPoint presentation on clinical quality measures, meaningful use, and data analytics. According to the Centers for Medicare and Medicaid Services, Clinical Quality
Welcome to the Data Analytics Toolkit PowerPoint presentation on clinical quality measures, meaningful use, and data analytics. According to the Centers for Medicare and Medicaid Services, Clinical Quality
Calendar Year 2014 Medicare Physician Fee Schedule Final Rule
 Calendar Year 2014 Medicare Physician Fee Schedule Final Rule Non-facility Cap after receiving many negative comments on this issue from physician groups along with the House GOP Doctors Caucus letter,
Calendar Year 2014 Medicare Physician Fee Schedule Final Rule Non-facility Cap after receiving many negative comments on this issue from physician groups along with the House GOP Doctors Caucus letter,
HIMSS Public Policy Initiatives in 2015: Using Health IT to Enable Healthcare Transformation Jeff Coughlin Senior Director Federal & State Affairs
 HIMSS Public Policy Initiatives in 2015: Using Health IT to Enable Healthcare Transformation Jeff Coughlin Senior Director Federal & State Affairs March 26, 2015 Agenda Meaningful Use Stage 3 NPRM 2015
HIMSS Public Policy Initiatives in 2015: Using Health IT to Enable Healthcare Transformation Jeff Coughlin Senior Director Federal & State Affairs March 26, 2015 Agenda Meaningful Use Stage 3 NPRM 2015
February 26, 2016. Dear Mr. Slavitt:
 February 26, 2016 Mr. Andy Slavitt Acting Administrator Centers for Medicare & Medicaid Services (CMS) Department of Health and Human Services Attention: CMS-3321-NC PO Box 8016 Baltimore, MD 21244 Re:
February 26, 2016 Mr. Andy Slavitt Acting Administrator Centers for Medicare & Medicaid Services (CMS) Department of Health and Human Services Attention: CMS-3321-NC PO Box 8016 Baltimore, MD 21244 Re:
Re: Comments on Proposed Establishment of Certification Programs for Health Information Technology (Health IT); Proposed Rule
 McKesson Corporation One Post Street San Francisco, CA 94104-5296 Ann Richardson Berkey Senior Vice President, Public Affairs April 9, 2010 David Blumenthal, M.D. Director Department of Health and Human
McKesson Corporation One Post Street San Francisco, CA 94104-5296 Ann Richardson Berkey Senior Vice President, Public Affairs April 9, 2010 David Blumenthal, M.D. Director Department of Health and Human
Re: Medicare and Medicaid Programs: Electronic Health Record Incentive Program- Stage 3
 Mr. Andrew M. Slavitt Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services 7500 Security Boulevard Baltimore, MD 21244-1850 {Submitted Electronically}
Mr. Andrew M. Slavitt Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services 7500 Security Boulevard Baltimore, MD 21244-1850 {Submitted Electronically}
OVERALL IMPLEMENTATION CONSIDERATIONS
 Donald Berwick, M.D., M.P.H. Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Hubert H. Humphrey Building 200 Independence Avenue, S.W., Room 445-G Washington,
Donald Berwick, M.D., M.P.H. Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Hubert H. Humphrey Building 200 Independence Avenue, S.W., Room 445-G Washington,
Re: Medicare and Medicaid Programs; Electronic Health Record Incentive Program Stage 2 (CMS-0044-P)
 Ms. Marilyn B. Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-0044-P Room 445-G, Hubert H. Humphrey Building 200 Independence
Ms. Marilyn B. Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-0044-P Room 445-G, Hubert H. Humphrey Building 200 Independence
DEPARTMENT OF HEALTH AND HUMAN SERVICES. Medicare Program; Changes to the Electronic Prescribing (erx) Incentive Program
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services 42 CFR Part 414 [CMS-3248-F] RIN 0938-AR00 Medicare Program; Changes to the Electronic Prescribing (erx) Incentive Program
DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services 42 CFR Part 414 [CMS-3248-F] RIN 0938-AR00 Medicare Program; Changes to the Electronic Prescribing (erx) Incentive Program
Medicare and Medicaid Programs; Electronic Health Record Incentive Program Stage 2.
 May 7, 2012 Marilyn Tavenner Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS 0044 P P.O. Box 8013, Baltimore, MD 21244 8013 Re: Medicare
May 7, 2012 Marilyn Tavenner Acting Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS 0044 P P.O. Box 8013, Baltimore, MD 21244 8013 Re: Medicare
QUALITY BEGINNER. PQRS Training Module: QUALITY MEASUREMENT 101. Last Updated: August 2014
 QUALITY 01 BEGINNER PQRS Training Module: QUALITY MEASUREMENT 101 Last Updated: August 2014 TRAINING MODULE OBJECTIVES Quality Measurement 101 is a training module for providers who are interested in learning
QUALITY 01 BEGINNER PQRS Training Module: QUALITY MEASUREMENT 101 Last Updated: August 2014 TRAINING MODULE OBJECTIVES Quality Measurement 101 is a training module for providers who are interested in learning
Clinical Quality Measures Physician Quality Reporting System 2014
 Clinical Quality Measures Physician Quality Reporting System 2014 Marcela Reyes, CHTS- CP Sevocity Product Manager 877-777-2298!! www.sevocity.com! 2014 CQMs CQMs are no longer a core objective of the
Clinical Quality Measures Physician Quality Reporting System 2014 Marcela Reyes, CHTS- CP Sevocity Product Manager 877-777-2298!! www.sevocity.com! 2014 CQMs CQMs are no longer a core objective of the
Prospective Attribution as a Single-Step Assignment Process
 Marilyn Tavenner, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS 1461 P P.O. Box 8013 Baltimore, MD 21244 8013 Dear Administrator Tavenner:
Marilyn Tavenner, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS 1461 P P.O. Box 8013 Baltimore, MD 21244 8013 Dear Administrator Tavenner:
June 15, 2015. Re: Electronic Health Record Incentive Program Modifications to Meaningful Use in 2015 through 2017. Dear Administrator Slavitt,
 June 15, 2015 Andy Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS 3311 P P.O. Box 8013 Baltimore, MD 21244 8013 Re: Electronic
June 15, 2015 Andy Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS 3311 P P.O. Box 8013 Baltimore, MD 21244 8013 Re: Electronic
Reporting Once for 2014 Medicare Quality Reporting Programs
 Reporting Once for 2014 Medicare Quality Reporting Programs Use this tool* to learn how to report quality measures one time in 2014 in order to: Become incentive eligible for 2014 Physician Quality Reporting
Reporting Once for 2014 Medicare Quality Reporting Programs Use this tool* to learn how to report quality measures one time in 2014 in order to: Become incentive eligible for 2014 Physician Quality Reporting
The undersigned provider groups would like to draw your attention to implementation concerns regarding two administrative simplification issues:
 January 30, 2015 Marilyn B. Tavenner Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445 G 200 Independence Avenue,
January 30, 2015 Marilyn B. Tavenner Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445 G 200 Independence Avenue,
DEPARTMENT OF HEALTH AND HUMAN SERVICES
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services 42 CFR Part 495 CMS-0052-P RIN 0938-AS30 Office of the Secretary 45 CFR Part 170 RIN 0991-AB97 Medicare and Medicaid Programs;
DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Medicare & Medicaid Services 42 CFR Part 495 CMS-0052-P RIN 0938-AS30 Office of the Secretary 45 CFR Part 170 RIN 0991-AB97 Medicare and Medicaid Programs;
May 26, 2011. Section 3022 of the Affordable Care Act. Dear Administrator Berwick:
 Donald M. Berwick, MD, MPP Administrator Attention: CMS-1345-P Mail Stop C4-26-05 7500 Security Boulevard Baltimore, MD 21244-1850 Re: Section 3022 of the Affordable Care Act Dear Administrator Berwick:
Donald M. Berwick, MD, MPP Administrator Attention: CMS-1345-P Mail Stop C4-26-05 7500 Security Boulevard Baltimore, MD 21244-1850 Re: Section 3022 of the Affordable Care Act Dear Administrator Berwick:
Re: CMS Notice of Proposed Rulemaking for Accountable Care Organizations
 Donald Berwick, MD, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-1345-P Submitted electronically Re: CMS Notice of Proposed Rulemaking for
Donald Berwick, MD, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-1345-P Submitted electronically Re: CMS Notice of Proposed Rulemaking for
Medicare Physician Reporting: Beyond PQRS. Mary Patton Wheatley Senior Specialist, AAMC August 17, 2011
 Medicare Physician Reporting: Beyond PQRS Mary Patton Wheatley Senior Specialist, AAMC August 17, 2011 Who is the AAMC? The Association of American Medical Colleges (AAMC) serves and leads the academic
Medicare Physician Reporting: Beyond PQRS Mary Patton Wheatley Senior Specialist, AAMC August 17, 2011 Who is the AAMC? The Association of American Medical Colleges (AAMC) serves and leads the academic
How Health Reform Will Affect Health Care Quality and the Delivery of Services
 Fact Sheet AARP Public Policy Institute How Health Reform Will Affect Health Care Quality and the Delivery of Services The recently enacted Affordable Care Act contains provisions to improve health care
Fact Sheet AARP Public Policy Institute How Health Reform Will Affect Health Care Quality and the Delivery of Services The recently enacted Affordable Care Act contains provisions to improve health care
CMS Quality Measurement and Value Based Purchasing Programs Kate Goodrich, MD MHS Director, Quality Measurement and Health Assessment Group, CMS
 CMS Quality Measurement and Value Based Purchasing Programs Kate Goodrich, MD MHS Director, Quality Measurement and Health Assessment Group, CMS American Urological Association Quality Improvement Summit
CMS Quality Measurement and Value Based Purchasing Programs Kate Goodrich, MD MHS Director, Quality Measurement and Health Assessment Group, CMS American Urological Association Quality Improvement Summit
CCO Incentive Metrics: Requirements for Reporting on EHR- Based Measures in 2015 GUIDANCE DOCUMENTATION
 CCO Incentive Metrics: Requirements for Reporting on EHR- Based Measures in 2015 GUIDANCE DOCUMENTATION Oregon Health Authority Page 1 of 22 Contents Section 1: Executive Summary... 4 1.1 Objective...
CCO Incentive Metrics: Requirements for Reporting on EHR- Based Measures in 2015 GUIDANCE DOCUMENTATION Oregon Health Authority Page 1 of 22 Contents Section 1: Executive Summary... 4 1.1 Objective...
19 June 2014. 888.879.7302 www.greenwayhealth.com
 Meaningful Use Timeline Changes and Penalties Explained By: Adele Allison, National Director of Industry and Government Affairs Greenway Health On May 20, 2014, CMS issued a proposed rule offering flexibility
Meaningful Use Timeline Changes and Penalties Explained By: Adele Allison, National Director of Industry and Government Affairs Greenway Health On May 20, 2014, CMS issued a proposed rule offering flexibility
April 17, 2014. Re: Evolution of ACO initiatives at CMS. Dear Dr. Conway:
 Patrick Conway, M.D. Acting Director of the Innovation Center Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W. Room 445-G Washington, DC 20201 Re: Evolution
Patrick Conway, M.D. Acting Director of the Innovation Center Centers for Medicare & Medicaid Services Hubert H. Humphrey Building 200 Independence Avenue, S.W. Room 445-G Washington, DC 20201 Re: Evolution
Demonstrating Meaningful Use of EHRs: The top 10 compliance challenges for Stage 1 and what s new with 2
 Demonstrating Meaningful Use of EHRs: The top 10 compliance challenges for Stage 1 and what s new with 2 Today s discussion A three-stage approach to achieving Meaningful Use Top 10 compliance challenges
Demonstrating Meaningful Use of EHRs: The top 10 compliance challenges for Stage 1 and what s new with 2 Today s discussion A three-stage approach to achieving Meaningful Use Top 10 compliance challenges
Meaningful Use Stage 2 2014
 Meaningful Use Stage 2 2014 Marcela Reyes, CHTS- CP Sevocity Product Manager 877-777-2298!! www.sevocity.com! ! What is Meaningful Use?! How do I parbcipate?! Medicare or Medicaid?! Register! ADest Meaningful
Meaningful Use Stage 2 2014 Marcela Reyes, CHTS- CP Sevocity Product Manager 877-777-2298!! www.sevocity.com! ! What is Meaningful Use?! How do I parbcipate?! Medicare or Medicaid?! Register! ADest Meaningful
RE: Medicare and Medicaid Programs; Electronic Health Record Incentive Program-Modifications to Meaningful Use in 2015 Through 2017
 June 15, 2015 SENT VIA ELECTRONIC MAIL Andrew M. Slavitt, Acting Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Attention: CMS-3311-P P.O. Box 8013
June 15, 2015 SENT VIA ELECTRONIC MAIL Andrew M. Slavitt, Acting Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Attention: CMS-3311-P P.O. Box 8013
Medicare Program; Request for Information Regarding Implementation of the Merit
 This document is scheduled to be published in the Federal Register on 10/20/2015 and available online at http://federalregister.gov/a/2015-26568, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 10/20/2015 and available online at http://federalregister.gov/a/2015-26568, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
EHR Incentive Programs in 2010 & Beyond
 CMS Listening Session: EHR Incentive Programs in 2018 & Beyond Kate Goodrich, MD, MHS, Director, Center for Clinical Standards and Quality Robert Anthony, Deputy Director, Quality Measurement & Value-Based
CMS Listening Session: EHR Incentive Programs in 2018 & Beyond Kate Goodrich, MD, MHS, Director, Center for Clinical Standards and Quality Robert Anthony, Deputy Director, Quality Measurement & Value-Based
Re: Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule, and Other Revisions to Part B for CY 2016 Proposed Rule
 September 8, 2015 Andy Slavitt Acting Administrator Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244 Re: Medicare Program; Revisions to Payment Policies Under the Physician
September 8, 2015 Andy Slavitt Acting Administrator Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244 Re: Medicare Program; Revisions to Payment Policies Under the Physician
Medicare & Medicaid EHR Incentive Programs
 Medicare & Medicaid EHR Incentive Programs Stage 2 NPRM Overview Robert Anthony Office of E-Health Standards and Services Marsha Smith Office of Clinical Standards and Quality March 21, 2012 Proposed Rule
Medicare & Medicaid EHR Incentive Programs Stage 2 NPRM Overview Robert Anthony Office of E-Health Standards and Services Marsha Smith Office of Clinical Standards and Quality March 21, 2012 Proposed Rule
DETAILED SUMMARY--MEDCIARE SHARED SAVINGS/ACCOUNTABLE CARE ORGANIZATION (ACO) PROGRAM
 1 DETAILED SUMMARY--MEDCIARE SHARED SAVINGS/ACCOUNTABLE CARE ORGANIZATION (ACO) PROGRAM Definition of ACO General Concept An ACO refers to a group of physician and other healthcare providers and suppliers
1 DETAILED SUMMARY--MEDCIARE SHARED SAVINGS/ACCOUNTABLE CARE ORGANIZATION (ACO) PROGRAM Definition of ACO General Concept An ACO refers to a group of physician and other healthcare providers and suppliers
Guidelines for Patient-Centered Medical Home (PCMH) Recognition and Accreditation Programs. February 2011
 American Academy of Family Physicians (AAFP) American Academy of Pediatrics (AAP) American College of Physicians (ACP) American Osteopathic Association (AOA) Guidelines for Patient-Centered Medical Home
American Academy of Family Physicians (AAFP) American Academy of Pediatrics (AAP) American College of Physicians (ACP) American Osteopathic Association (AOA) Guidelines for Patient-Centered Medical Home
RE: Comments on Discussion Draft Ensuring Interoperability of Qualified Electronic Health Records.
 April 8, 2015 The Honorable Michael Burgess, MD 2336 Rayburn House Office Building Washington, DC 20515 RE: Comments on Discussion Draft Ensuring Interoperability of Qualified Electronic Health Records.
April 8, 2015 The Honorable Michael Burgess, MD 2336 Rayburn House Office Building Washington, DC 20515 RE: Comments on Discussion Draft Ensuring Interoperability of Qualified Electronic Health Records.
ID: HHS-OS-2014-0002 / RIN: 0991-AB92)
 April 24, 2014 Attention: 2015 Edition EHR Standards and Certification Criteria Proposed Rule Office of the National Coordinator for Health Information Technology Department of Health and Human Services
April 24, 2014 Attention: 2015 Edition EHR Standards and Certification Criteria Proposed Rule Office of the National Coordinator for Health Information Technology Department of Health and Human Services
Medical Malpractice - Interoperability Measurement For physicians
 Karen DeSalvo, MD, MPH, MSc Acting Assistant Secretary for Health U.S. Department of Health and Human Services Office of the National Coordinator for Health Information Technology Attention, RFI Regarding
Karen DeSalvo, MD, MPH, MSc Acting Assistant Secretary for Health U.S. Department of Health and Human Services Office of the National Coordinator for Health Information Technology Attention, RFI Regarding
December 3, 2010. Dear Administrator Berwick:
 Donald Berwick, M.D. Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445-G, Hubert H. Humphrey Building 200 Independence Avenue, SW Washington, DC 20201
Donald Berwick, M.D. Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445-G, Hubert H. Humphrey Building 200 Independence Avenue, SW Washington, DC 20201
Medicare Shared Savings Program (ASN) and the kidney Disease Prevention Project
 December 3, 2010 Donald Berwick, MD Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Room 445-G, Hubert H. Humphrey Building 200 Independence Avenue, SW
December 3, 2010 Donald Berwick, MD Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Room 445-G, Hubert H. Humphrey Building 200 Independence Avenue, SW
Minnesota EHR Incentive Program (MEIP) 2015 2017 Program Year Timeline for EPs, EHs and CAHs. Updated November 2015
 Minnesota EHR Incentive Program (MEIP) 2015 2017 Program Year Timeline for EPs, EHs and CAHs Updated November 2015 Glossary CAH Critical access hospitals CEHRT Certified electronic health record technology
Minnesota EHR Incentive Program (MEIP) 2015 2017 Program Year Timeline for EPs, EHs and CAHs Updated November 2015 Glossary CAH Critical access hospitals CEHRT Certified electronic health record technology
Performance Measurement in CMS Programs Kate Goodrich, MD MHS Director, Quality Measurement and Health Assessment Group, CMS
 Performance Measurement in CMS Programs Kate Goodrich, MD MHS Director, Quality Measurement and Health Assessment Group, CMS Mind the Gap: Improving Quality Measures in Accountable Care Systems October
Performance Measurement in CMS Programs Kate Goodrich, MD MHS Director, Quality Measurement and Health Assessment Group, CMS Mind the Gap: Improving Quality Measures in Accountable Care Systems October
RE: Medicare and Medicaid Programs; Electronic Health Record Incentive Program; RIN 0938 AP78
 March 15, 2010 Charlene Frizzera Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445 G, Hubert H. Humphrey Building 200 Independence Avenue, SW
March 15, 2010 Charlene Frizzera Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445 G, Hubert H. Humphrey Building 200 Independence Avenue, SW
9/9/2015. Medicare/Medicaid Incentive Program. Medicare/Medicaid Incentive Program. Meaningful Use, Penalties and Audits
 Meaningful Use, Penalties and Audits SHERI SMITH, FACMPE STATE VOLUNTEER MUTUAL INSURANCE COMPANY Copyright 2014 State Volunteer Mutual Insurance Company Medicare/Medicaid Incentive Program Medicare/Medicaid
Meaningful Use, Penalties and Audits SHERI SMITH, FACMPE STATE VOLUNTEER MUTUAL INSURANCE COMPANY Copyright 2014 State Volunteer Mutual Insurance Company Medicare/Medicaid Incentive Program Medicare/Medicaid
June 18, 2012. Submitted Electronically Via ffecomments@cms.hhs.gov. Re: General Guidance on Federally Facilitated Exchanges. Dear Ms.
 June 18, 2012 Marilyn Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445-G, Hubert H. Humphrey Building 200 Independence Avenue, SW
June 18, 2012 Marilyn Tavenner Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Room 445-G, Hubert H. Humphrey Building 200 Independence Avenue, SW
Crosswalk of the Medicare Access and CHIP Reauthorization Act of 2015 (H.R. 2) April 21, 2015
 Crosswalk of the Medicare Access and CHIP Reauthorization Act of 2015 (H.R. 2) April 21, 2015 ACP has developed a cross-walk analysis of legislation in the 114 th Congress to permanently repeal Medicare
Crosswalk of the Medicare Access and CHIP Reauthorization Act of 2015 (H.R. 2) April 21, 2015 ACP has developed a cross-walk analysis of legislation in the 114 th Congress to permanently repeal Medicare
May 7, 2012. Re: RIN 0991-AB82. Dear Secretary Sebelius:
 May 7, 2012 Department of Health and Human Services Office of the National Coordinator for Health Information Technology Attention: 2014 Edition EHR Standards and Certification Proposed Rule Hubert H.
May 7, 2012 Department of Health and Human Services Office of the National Coordinator for Health Information Technology Attention: 2014 Edition EHR Standards and Certification Proposed Rule Hubert H.
Accountable Care Organizations (ACO) Proposed Rule Summary March 31, 2011
 Accountable Care Organizations (ACO) Proposed Rule Summary March 31, 2011 On March 31, 2011, the Centers for Medicare & Medicaid Services (CMS) released the longawaited proposed rule on Accountable Care
Accountable Care Organizations (ACO) Proposed Rule Summary March 31, 2011 On March 31, 2011, the Centers for Medicare & Medicaid Services (CMS) released the longawaited proposed rule on Accountable Care
B-327451. November 2, 2015. The Honorable Orrin G. Hatch Chairman The Honorable Ron Wyden Ranking Member Committee on Finance United States Senate
 441 G St. N.W. Washington, DC 20548 B-327451 November 2, 2015 The Honorable Orrin G. Hatch Chairman The Honorable Ron Wyden Committee on Finance United States Senate The Honorable Fred Upton Chairman The
441 G St. N.W. Washington, DC 20548 B-327451 November 2, 2015 The Honorable Orrin G. Hatch Chairman The Honorable Ron Wyden Committee on Finance United States Senate The Honorable Fred Upton Chairman The
MEANINGFUL USE STAGE 3 AND CERTIFICATION PROPOSED RULES
 MEANINGFUL USE STAGE 3 AND CERTIFICATION PROPOSED RULES The following provides a brief summary of the Meaningful Use (MU) Stage 3 and 2015 Edition certification proposed rules. Comments on the rules are
MEANINGFUL USE STAGE 3 AND CERTIFICATION PROPOSED RULES The following provides a brief summary of the Meaningful Use (MU) Stage 3 and 2015 Edition certification proposed rules. Comments on the rules are
Re: Medicare and Medicaid Programs; Electronic Health Record Incentive Program Modifications to Meaningful Use in 2015 through 2017; Proposed Rule
 Submitted Electronically Andrew M. Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attn: CMS-3311-P P.O. Box 8013 Baltimore, MD 21244-1850
Submitted Electronically Andrew M. Slavitt Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attn: CMS-3311-P P.O. Box 8013 Baltimore, MD 21244-1850
May 28, 2015. Centers for Medicare & Medicaid Services Department of Health and Human Services 7500 Security Boulevard Baltimore, MD 21244-1850
 May 28, 2015 Centers for Medicare & Medicaid Services Department of Health and Human Services 7500 Security Boulevard Baltimore, MD 21244-1850 Subject: (CMS-3310-P; 80 FR 16731) Medicare and Medicaid Programs;
May 28, 2015 Centers for Medicare & Medicaid Services Department of Health and Human Services 7500 Security Boulevard Baltimore, MD 21244-1850 Subject: (CMS-3310-P; 80 FR 16731) Medicare and Medicaid Programs;
April 22, 2013. RE: Advancing Interoperability and Health Information Exchange. Dear Dr. Mostashari:
 April 22, 2013 Farzad Mostashari, MD, ScM National Coordinator for Health Information Technology Department of Health and Human Services Hubert Humphrey Building, Suite 729-D Washington, DC 20201 RE: Advancing
April 22, 2013 Farzad Mostashari, MD, ScM National Coordinator for Health Information Technology Department of Health and Human Services Hubert Humphrey Building, Suite 729-D Washington, DC 20201 RE: Advancing
