Assuring the Safety, Security, and Reliability of Medical-Device Cyber- Physical Systems (MDCPS)
|
|
|
- Jasmin Booth
- 10 years ago
- Views:
Transcription
1 Assuring the Safety, Security, and Reliability of Medical-Device Cyber- Physical Systems (MDCPS) Insup Lee PRECISE Center Department of Computer and Information Science University of Pennsylvania November 16, 2012
2 Trend 1: Device Proliferation Embedded Everywhere! Smart Space
3 Trend 2: Integration at Scale World Wide Sensor Web Future Combat System Smart Building Environment Low End Ubiquitous embedded devices Large-scale networked embedded systems Seamless integration with a physical environment Interconnection, Interoperation, Integration & Scaling Challenges High End Complex systems with global integration Global Information Grid Smart Building Environment
4 Trend 3: Closing the Loop
5 Confluence of Trends #2. Integra2on at Scale #1. Data/Device Prolifera2on Cyber- Physical Systems (of Embedded Devices) Scale challenges Composi0on challenges The Overarching Challenge #3. Autonomy 5
6 Defense Healthcare Transportation Finance CPS Large-scale Infrastructure Process Control Energy
7 Overall Structure of MCPS Monitoring Medical Devices Administrative Support Patient Smart Interconnection (ICE Network Controller) Smart Controller Smart Alarm Caregiver Decision Support (ICE Supervisor) Treatment Delivery Medical Devices
8 MDCPS Research in a Nutshell Goal: Develop a new development paradigm for the effective design and implementation of MCPS that are safe, secure, and reliable Foundations of MCPS development Patient modeling Caregiver modeling Control-theoretic analysis of physiologically closed-loop scenarios High-confidence MCPS software development Model-based development Integration framework for MCPS Security for MCPS [Event recording for medical devices] MCPS validation and certification Assurance cases for evidence based certification Compositional techniques for MCPS and assurance cases Case studies GPCA, Closed-loop PCA, Pacemaker, Neurological decision support,
9 Some Software-related Failures Therac-25 ( ) Failure to understand software fault tolerance Numerous problems with radiation treatment ( Failures in the generation of treatment plans Pacemakers (500K recalls during ) St Jude pacemaker programmers (2006) Incorrect reporting of pacemaker state Difibtech external defibrillators (2007) Self-test resets low-battery status Baxter s Colleague Infusion Pumps (2010) Software update triggers buffer overflow, stops pump
10 Infusion Pump Safety Involved in many clinical accidents - During 2005 and 2009, FDA received approximately 56,000 reports of adverse events associated with the use of infusion pumps - 1% deaths, 34% serious injuries - 87 infusion pump recalls to address safety problems The most common types of problems Software Defect User Interface Issues Mechanical or Electrical Failure U.S. Food and Drug Administration, Center for Devices and Radiological Health. White Paper: Infusion Pump Improvement Initiative, April 2010
11 Generic PCA (GPCA) Project GPCA Hazard Analysis Assurance Case Hazard Analysis PCA Infusion Pump Model-Based Implementation Safety Requirements GPCA Model Reference Model GPCA Safety Requirements
12 GPCA reference implementation FDA initiated GPCA Safety Requirements GPCA Model (Simulink/Stateflow) Develop a GPCA reference implementation Model-based development Provide evidence that the implementation satisfies the safety requirements Safety cases Confidence cases All artifacts to be available as open source Safety Requirements Formal Modeling & Verification Automated Implementation Testing GPCA Reference Implementation GPCA Model Model-Based Development of GPCA Reference Implementation
13 Quantum related research Connectivity, Interoperability, and Compositonality VMD (virtual medical device), VMD app Smart Alarms & Decision Support Physiological Closed Loop Assurance and Certification
14 Supporting Medical Device Interoperability
15 Virtual Medical Devices (VMD) MD PnP (initiative for medical devices interoperability) enables a new kind of medical device, a Virtual Medical Device (VMD). VMD is a set of medical devices coordinating over a network for clinical scenario. VMD is a virtual system of systems. VMD does not physically exist until instantiated at a hospital. The Medical Device Coordination Framework (MDCF) is prototype middleware for managing the correct composition of medical devices into VMD. + = Device Coordination Algorithm Medical Device Types Virtual Medical Device (VMD) Clinician selects appropriate VMD MDCF binds appropriate devices into VMD instance MDCF MDCF displays VMD GUI for clinician
16 VMD Research Issues Real-time support Leverage current hospital networks Non-interference Assume-guarantee interface Development environment for VMD Apps Support for programming clinicalalgorithms with timing constraints MDCF Platform Implementation Device connection and configuration protocols VMD setup/tear-down algorithm Guarantee performance specified by VMD App or prevent clinician from unsafely instantiating VMD Safety analysis of the platform Correctness of the protocols Guarantees of communication VMD App Validation & Verification Generate simulation models directly from executable VMD App specification (for validation) Export specification to model-checker for verification Co-Developed with NSF CNS (PI: John Hatcliff) Medical Device NIH/NIBIB Quantum Grant (PI: Julian Goldman)
17 VMD Research Issues Formal VMD requirements and medical device capabilities language Automatic Device App compatibility checking by MD PnP platform Ensures correct devices used in any given VMD Reduces scope of standardization efforts to manageable size I.e. standardize the interface language but not the specific API Precise VMD development artifact Specs are executable Feed into VMD simulation (i.e. testing) Feed into verification (i.e. model checking) Formal semantics May, must, at-least-one of transitions Refinement relations between specification and implementation
18 Connectivity Support Open Health Connector (OHC) Connects legacy devices to modern networks and HIT systems Necessary for MD PnP research Open-source, standards-based connectivity Supports and HL7 messaging Customizable Simple patterns and interfaces for implementing new device drivers & network protocols Community Support Device Driver Stack Contains several device drivers supporting various data formats Device Driver compatible with Nelcor N-595 pulseoximeter Converts the raw data received from the PulseOximeter into OHCPhysioDescType Ontology Mappings for HL7 and IEEE11703 Maintains the mappings for various attributes defined by Drager, Phillips and GE Device Manager Responsible for getting the device specification and data Event Handler Manages queues for various data types and the list of subscribers for each queue and pushes data to each subscriber on specific channel Network Manager Responsible for registering the capabilities of the device and calling the suitable network module Connectivity to MDCF framework Interfaces with MDCF framework for further processing Network Module Stack Contains several network modules supporting various communication formats IEEE compatible network module Encapsulates the data into IEEE protocol data units and maintains a finite state machine for reliable communication JMS compatible network module Maintains a secured channel for publishersubscriber pairs using SSL and includes access control features Users contribute back device & network drivers
19 OHC Framework Software Architecture Ontology Mappings for HL7 and IEEE11703 Maintains the mappings for various attributes defined by Drager, Phillips and GE Connectivity to MDCF framework Interfaces with MDCF framework for further processing Device Driver Stack Contains several device drivers supporting various data formats Device Manager Responsible for getting the device specification and data Network Module Stack Contains several network modules supporting various communication formats Device Driver compatible with Nelcor N-595 pulseoximeter Converts the raw data received from the PulseOximeter into OHCPhysioDescType Event Handler Manages queues for various data types and the list of subscribers for each queue and pushes data to each subscriber on specific channel IEEE compatible network module Encapsulates the data into IEEE protocol data units and maintains a finite state machine for reliable communication Network Manager Responsible for registering the capabilities of the device and calling the suitable network module JMS compatible network module Maintains a secured channel for publishersubscriber pairs using SSL and includes access control features
20 Smart Alarms 85%-99% of alarms generated in ICUs are false alarms VMD of multiple devices and central smart controller Filter, combine, process, and present real-time medical information Suppress clinically irrelevant alarms Provide summaries of the patient's state and predictions of future trends Benefits Improves patient safety Reduces caregiver workload Facilitates practice of evidencebased medicine Challenges Filtering and combining data streams from multiple devices (clock synch?) Developing context-aware patient models Encoding hospital guidelines, extracting experts' models, learning models statistically Presenting data concisely and effectively
21 Case Study: CABG Smart Alarm CABG (Coronary Artery Bypass Graft) Monitoring of post-cabg patients 57% reduction in false alarms No missed true alarms Rule-based, from clinical guidelines and experts Joint work with Margaret Fortino- Mullen, RN Non-clinical implementation based on recorded data Barriers to realtime deployment 21
22 Case Study: Vasospasm Decision Caddy Post-brain surgery risk Hard to diagnose, deadly if not caught early Provide supporting information Context for alarms Give clinicians access to data 15 days of data 3-pronged approach Guideline driven Physician driven Data driven Current deployment barriers Few real-time data stream feeds No interfacing of streams to the systems Joint work with Soojin Park, MD Analyze data in new ways New device sources Trending Waveform analysis Clinician provide data Interpolate missing data 22
23 Technology Gaps in Smart Alarms/CDS 1. Integrating multiple streams of clinical data 2. Poor clock synchronization leads to timing uncertainty, making sensor fusion difficult 3. Safety analysis of Smart Alarms/CDS 4. Translating caregivers' needs into engineering requirements is difficult No "gold standard" for clinical alarms Effective presentation of CDS recommendations Interoperability platforms such as ICE standard needed for 1, 2, 3
24 Physiological Closed-Loop Systems Benefits Improved patient safety Improved clinical outcomes Reduced deployment cost Networking existing medical devices Clinical Use Cases Closed-loop Patient Controlled Analgesia (PCA) Closed-loop Blood Glucose (BG) control Ventilator weaning procedure Challenges Hazard identification and mitigation Network packet delay/drop, sensor disconnection, out-of-sync between controllers and devices Verification and Validation Proving safety properties at the model level Validating physiological models with clinical data QUANTUM gap Difficult to implement now due to lack of medical device interoperability
25 PCA Closed-loop System Quantum use case Goal: improve the safety of PCA Approach: Detect respiratory disturbance Provide a safety interlock by stopping the pump Activate nurse call Challenges: Patient modeling, large parameter variation New hazards due to network failures Parametric design improves safety but reduces effectiveness Safety analysis by formal verification The pump is stopped if patient enters alarming region The patient can not enter the critical region Safe Alarming Critical t crit Open-loop stability mitigates network hazards Instead of start/stop, allow pump to run for a fixed time t 1 t 2
26 Key Safety Property of Closed-Loop PCA Pump stops in time if total delay <= t crit Pulse Oximeter Signal Processing Time SpO 2 & HR Levels Supervisor Algorithm Processing Time Output Physiological Signals Pump Commands Total delay is the sum of: tpodel: worst case delay from PO (1s) tnet: worst case delay from network (0.5s) tsup: worst case delay from Supervisor (0.2s) tpump: worst case delay from pump (0.1s) tp2po: worst case latency for pump to stop (2s) Drug Absorption Function Drug Level Patient Model Drug Infusion Drug Request tcrit: shortest time the patient can spend in the alarming region before going critical Pump Processing Time PCA Pump
27 BG Closed-loop System Background Glycemic control is important for diabetics and ICU patients Current control guidelines are not adaptive to individual changes and can result in unsafe BG Goal: Improve BG control: more intarget time, less variability Minimize hypoglycemia incidents Approach: Design controllers on patient models and software simulators At runtime, automatically compute optimal insulin dose and alert caregivers to possible unsafe BG Challenges: Patient modeling Not enough data to monitor all physiological states Some factors (e.g., stress, physical activity) are hard to model Sensor measurement errors Actuation (infusion) delays Meal disturbances Safety vs. effectiveness Over-aggressive safety algorithm may trigger a lot of hyperglycemia Control design must address this trade-off
28 Safe Adaptive Exploration Adaptive control often involves learning the parameters by feeding in extreme inputs Example: aggressively turning a car Not safe for patient-in-the-loop systems Open issue: adaptive exploration with safety constraints
29 Regulatory Approval of MCPS Current approach to certification: Consider every configuration separately alarm coordinator supervisor Cannot be used for MCPS assembled at bedside Multiple devices in the same category Variation in clinical scenarios network
30 An MCPS instance is built to implement a clinical scenario Key idea: Modular Certification Treat clinical scenarios as virtual medical devices Replace approval of MCPS instances with Certify the scenario Assuming fixed interfaces to constituent devices Certify the interoperability platform Certify devices w.r.t. interfaces Joint work with J.M. Goldman, J. Hatcliff, A. King, O. Sokolsky, and many others
31 Assurance Cases Regulatory Challenge: evidence-based certification To construct an assurance case we need to: make an explicit set of claims about the system produce the supporting evidence provide a set of arguments that link the claims to the evidence make clear the assumptions and judgments underlying the arguments Challenges and on-going research: Effective ways of constructing assurance cases Evaluation strategies for regulators Certification of interoperating medical devices without N**2 problem Context Goal Strategy Sub-Goal Sub-Goal Evidence Evidence
32 Safety Case Pattern MDD Many devices are developed by similar methods and rely on similar safety claims Define a pattern for model-driven development (MDD) approaches (1) modeling the system (2) verifying this model (3) transformation the model into an implementation (4) validating the implementation MDD pattern Instantiation for the PCA safety case The PCA Safety Case Instance of the MDD pattern
33 Team members Penn, SEAS Insup Lee (PI) Rajeev Alur Rahul Mangharam George Pappas Rita Powell Oleg Sokolsky Penn, UPHS/SoM William Hanson, III, MD Margaret Mullen-Fortino, RN Soojin Park, MD Victoria Rich, RN Penn, Sociology, SAS Ross Koppel MGH/CIMIT Julian Goldman, MD Minnesota Mats Heimdahl Nicholas Hopper Yongdae Kim Michael Whalen Waterloo Sebastian Fischmeister Collaborators John Hatcliff, KSU Paul Jones, FDA Sandy Weininger, FDA Zhang Yi, FDA CPS: Large: Assuring the Safety, Security and Reliability of Medical Device Cyber Physical Systems (NSF CNS ) Affiliated Project: Medical Device NIH/NIBIB Quantum Grant: Development of a Prototype Healthcare Intranet for Improved Health Outcomes (PI: Julian Goldman)
34 THANK YOU!
Towards a Logical Foundation for Assurance Arguments for Plug & Play Systems
 Towards a Logical Foundation for Assurance Arguments for Plug & Play Systems Lu Feng, Andrew King, Insup Lee, Oleg Sokolsky PRECISE Center School of Engineering and Applied Science University of Pennsylvania
Towards a Logical Foundation for Assurance Arguments for Plug & Play Systems Lu Feng, Andrew King, Insup Lee, Oleg Sokolsky PRECISE Center School of Engineering and Applied Science University of Pennsylvania
Moderator: Julian M. Goldman, MD, Harvard Medical School, MGH, Partners HealthCare
 Panel IV: Challenges: The Digital Health Platform (System of Systems) Moderator: Julian M. Goldman, MD, Harvard Medical School, MGH, Partners HealthCare Regulatory Ambiguity and Requirements for New Devices
Panel IV: Challenges: The Digital Health Platform (System of Systems) Moderator: Julian M. Goldman, MD, Harvard Medical School, MGH, Partners HealthCare Regulatory Ambiguity and Requirements for New Devices
Medical devices are pervasive throughout modern
 Plug-and-Play for Medical Devices: Experiences from a Case Study David Arney, Sebastian Fischmeister, Julian M. Goldman, Insup Lee, Robert Trausmuth Medical devices are pervasive throughout modern healthcare,
Plug-and-Play for Medical Devices: Experiences from a Case Study David Arney, Sebastian Fischmeister, Julian M. Goldman, Insup Lee, Robert Trausmuth Medical devices are pervasive throughout modern healthcare,
Model Based Engineering and Its Integration with Safety Assurance Cases for Medical Device Software
 Model Based Engineering and Its Integration with Safety Assurance Cases for Medical Device Software Yi Zhang Ph.D., Paul Jones Office of Science and Engineering Laboratories Center for Devices and Radiological
Model Based Engineering and Its Integration with Safety Assurance Cases for Medical Device Software Yi Zhang Ph.D., Paul Jones Office of Science and Engineering Laboratories Center for Devices and Radiological
GSA: A Framework for Rapid Prototyping of Smart Alarm Systems
 GSA: A Framework for Rapid Prototyping of Smart Alarm Systems Andrew King, Alex Roederer, Dave Arney Sanjian Chen, Ana Giannareas, Margaret Mullen-Fortino R.N., C. William Hanson III M.D., Vanessa Kern
GSA: A Framework for Rapid Prototyping of Smart Alarm Systems Andrew King, Alex Roederer, Dave Arney Sanjian Chen, Ana Giannareas, Margaret Mullen-Fortino R.N., C. William Hanson III M.D., Vanessa Kern
Medical Device Interoperability Needs, Challenges, and Solutions
 Upenn PRECISE Center Cyber-Physical Systems Industry Day October 9 2014 Medical Device Interoperability Needs, Challenges, and Solutions Julian M. Goldman, MD Director, Program on Interoperability, Mass
Upenn PRECISE Center Cyber-Physical Systems Industry Day October 9 2014 Medical Device Interoperability Needs, Challenges, and Solutions Julian M. Goldman, MD Director, Program on Interoperability, Mass
Requirements Specification for Apps in Medical Application Platforms
 Requirements Specification for Apps in Medical Application Platforms Brian Larson, John Hatcliff, Sam Procter, Patrice Chalin Kansas State University {brl,hatcliff,samuel3,chalin}@ksu.edu Abstract Existing
Requirements Specification for Apps in Medical Application Platforms Brian Larson, John Hatcliff, Sam Procter, Patrice Chalin Kansas State University {brl,hatcliff,samuel3,chalin}@ksu.edu Abstract Existing
Medical Device Interoperability: the Medical Device Plug and Play Program
 C I M I T Medical Development Group Medical Software SIG 12 October 2011 Waltham, MA Medical Device Interoperability: the Medical Device Plug and Play Program David Arney Systems Engineer, CIMIT/MGH Program
C I M I T Medical Development Group Medical Software SIG 12 October 2011 Waltham, MA Medical Device Interoperability: the Medical Device Plug and Play Program David Arney Systems Engineer, CIMIT/MGH Program
CTX OVERVIEW. Ucentrik CTX
 CTX FACT SHEET CTX OVERVIEW CTX SDK API enables Independent Developers, VAR s & Systems Integrators and Enterprise Developer Teams to freely and openly integrate real-time audio, video and collaboration
CTX FACT SHEET CTX OVERVIEW CTX SDK API enables Independent Developers, VAR s & Systems Integrators and Enterprise Developer Teams to freely and openly integrate real-time audio, video and collaboration
A Study on Design of Health Device for U-Health System
 , pp.79-86 http://dx.doi.org/10.14257/ijbsbt.2015.7.2.08 A Study on Design of Health Device for U-Health System Am-Suk Oh Dept. of Media Engineering, Tongmyong University, Busan, Korea [email protected] Abstract
, pp.79-86 http://dx.doi.org/10.14257/ijbsbt.2015.7.2.08 A Study on Design of Health Device for U-Health System Am-Suk Oh Dept. of Media Engineering, Tongmyong University, Busan, Korea [email protected] Abstract
MindGent Healthcare Services. Addressing the Issues of Nursing Shortages and Patient Safety through Bio-Medical Device Integration (BMDI)
 MindGent Healthcare Services Addressing the Issues of Nursing Shortages and Patient Safety through Bio-Medical Device Integration (BMDI) December 2005 Page 1 of 7 Abstract Medical facilities are constantly
MindGent Healthcare Services Addressing the Issues of Nursing Shortages and Patient Safety through Bio-Medical Device Integration (BMDI) December 2005 Page 1 of 7 Abstract Medical facilities are constantly
Clinical Scenario #3: Home to Hospital. NIH Award U01EB012470-03 National Institute of Biomedical Imaging & Bioengineering
 Clinical Scenario #3: Home to Hospital Current State Clinical Scenario Textual Description & Graphical Workflow Diagrams Working Draft Version 5.0 NIH Award U01EB012470-03 National Institute of Biomedical
Clinical Scenario #3: Home to Hospital Current State Clinical Scenario Textual Description & Graphical Workflow Diagrams Working Draft Version 5.0 NIH Award U01EB012470-03 National Institute of Biomedical
Vortex White Paper. Simplifying Real-time Information Integration in Industrial Internet of Things (IIoT) Control Systems
 Vortex White Paper Simplifying Real-time Information Integration in Industrial Internet of Things (IIoT) Control Systems Version 1.0 February 2015 Andrew Foster, Product Marketing Manager, PrismTech Vortex
Vortex White Paper Simplifying Real-time Information Integration in Industrial Internet of Things (IIoT) Control Systems Version 1.0 February 2015 Andrew Foster, Product Marketing Manager, PrismTech Vortex
Synchronizing an X-ray and Anesthesia Machine Ventilator: A Medical Device Interoperability Case Study
 Department of Computer & Information Science Departmental Papers (CIS) University of Pennsylvania Year 2009 Synchronizing an X-ray and Anesthesia Machine Ventilator: A Medical Device Interoperability Case
Department of Computer & Information Science Departmental Papers (CIS) University of Pennsylvania Year 2009 Synchronizing an X-ray and Anesthesia Machine Ventilator: A Medical Device Interoperability Case
Medical Cyber Physical Systems and Bigdata Platforms
 Medical Cyber Physical Systems and Bigdata Platforms Don.S Department of Computer, Information and Communication Engineering Konkuk University S.Korea [email protected] ABSTRACT Over the past few decades
Medical Cyber Physical Systems and Bigdata Platforms Don.S Department of Computer, Information and Communication Engineering Konkuk University S.Korea [email protected] ABSTRACT Over the past few decades
Ensuring Patient Safety in Your Connected Hospital
 Ensuring Patient Safety in Your Connected Hospital Erin Sparnon, MEng Engineering Manager [email protected] (610) 825 6000, ext 5539 Learning Objectives Identify patient safety risks that have been mitigated
Ensuring Patient Safety in Your Connected Hospital Erin Sparnon, MEng Engineering Manager [email protected] (610) 825 6000, ext 5539 Learning Objectives Identify patient safety risks that have been mitigated
Flexible Architecture for Internet of Things Utilizing an Local Manager
 , pp.235-248 http://dx.doi.org/10.14257/ijfgcn.2014.7.1.24 Flexible Architecture for Internet of Things Utilizing an Local Manager Patrik Huss, Niklas Wigertz, Jingcheng Zhang, Allan Huynh, Qinzhong Ye
, pp.235-248 http://dx.doi.org/10.14257/ijfgcn.2014.7.1.24 Flexible Architecture for Internet of Things Utilizing an Local Manager Patrik Huss, Niklas Wigertz, Jingcheng Zhang, Allan Huynh, Qinzhong Ye
PERFORMANCE COMPARISON OF COMMON OBJECT REQUEST BROKER ARCHITECTURE(CORBA) VS JAVA MESSAGING SERVICE(JMS) BY TEAM SCALABLE
 PERFORMANCE COMPARISON OF COMMON OBJECT REQUEST BROKER ARCHITECTURE(CORBA) VS JAVA MESSAGING SERVICE(JMS) BY TEAM SCALABLE TIGRAN HAKOBYAN SUJAL PATEL VANDANA MURALI INTRODUCTION Common Object Request
PERFORMANCE COMPARISON OF COMMON OBJECT REQUEST BROKER ARCHITECTURE(CORBA) VS JAVA MESSAGING SERVICE(JMS) BY TEAM SCALABLE TIGRAN HAKOBYAN SUJAL PATEL VANDANA MURALI INTRODUCTION Common Object Request
System Aware Cyber Security
 System Aware Cyber Security Application of Dynamic System Models and State Estimation Technology to the Cyber Security of Physical Systems Barry M. Horowitz, Kate Pierce University of Virginia April, 2012
System Aware Cyber Security Application of Dynamic System Models and State Estimation Technology to the Cyber Security of Physical Systems Barry M. Horowitz, Kate Pierce University of Virginia April, 2012
The Problem: Automotive safety recalls, Control Systems Diagnostics, Stability Control, Traction Control, Anti-lock Braking, Adaptive Cruise Control
 AUTOPLUG: Remote Diagnostics Automotive Architecture for Control Software Safety Rahul Mangharam, Yash V. Pant and Truong X. Nghiem Department of Electrical & Systems Engineering University of Pennsylvania
AUTOPLUG: Remote Diagnostics Automotive Architecture for Control Software Safety Rahul Mangharam, Yash V. Pant and Truong X. Nghiem Department of Electrical & Systems Engineering University of Pennsylvania
White Paper. How Streaming Data Analytics Enables Real-Time Decisions
 White Paper How Streaming Data Analytics Enables Real-Time Decisions Contents Introduction... 1 What Is Streaming Analytics?... 1 How Does SAS Event Stream Processing Work?... 2 Overview...2 Event Stream
White Paper How Streaming Data Analytics Enables Real-Time Decisions Contents Introduction... 1 What Is Streaming Analytics?... 1 How Does SAS Event Stream Processing Work?... 2 Overview...2 Event Stream
Product Overview. DSL Xpert Advantages. Flexible Configuration Options. User-Friendly PC-Controlled GUI. Testing of ADSL, ADSL2 and ADSL2+
 Product Overview The DSL Xpert multi-layer analyzer (patent pending) breaks new ground as the only non-intrusive performance analysis solution for ADSL2+, ADSL2 and ADSL products. DSL Xpert is a modular
Product Overview The DSL Xpert multi-layer analyzer (patent pending) breaks new ground as the only non-intrusive performance analysis solution for ADSL2+, ADSL2 and ADSL products. DSL Xpert is a modular
Medical Application Platform Apps Requirements Engineering
 Medical Application Platform Apps Requirements Engineering Brian Larson, John Hatcliff, Sam Procter, Patrice Chalin Kansas State University {brl,hatcliff,samuel3,chalin}@ksu.edu Abstract Existing regulatory
Medical Application Platform Apps Requirements Engineering Brian Larson, John Hatcliff, Sam Procter, Patrice Chalin Kansas State University {brl,hatcliff,samuel3,chalin}@ksu.edu Abstract Existing regulatory
EL Program: Smart Manufacturing Systems Design and Analysis
 EL Program: Smart Manufacturing Systems Design and Analysis Program Manager: Dr. Sudarsan Rachuri Associate Program Manager: K C Morris Strategic Goal: Smart Manufacturing, Construction, and Cyber-Physical
EL Program: Smart Manufacturing Systems Design and Analysis Program Manager: Dr. Sudarsan Rachuri Associate Program Manager: K C Morris Strategic Goal: Smart Manufacturing, Construction, and Cyber-Physical
The Growing Concern Surrounding Medical Alarm Fatigue
 The Growing Concern Surrounding Medical Alarm Fatigue 01.15.2014 By Jillyan Morano Director of Clinical Engineering ABM Healthcare Support Services The Growing Concern Surrounding Medical Alarm Fatigue
The Growing Concern Surrounding Medical Alarm Fatigue 01.15.2014 By Jillyan Morano Director of Clinical Engineering ABM Healthcare Support Services The Growing Concern Surrounding Medical Alarm Fatigue
A Methodology for Safety Critical Software Systems Planning
 A Methodology for Safety Critical Software Systems Planning EHAB SHAFEI 1, IBRAHIM F. MOAWAD 2, HANY SALLAM 1, ZAKI TAHA 3, MOSTAFA AREF 3 1 Operation Safety and Human Factors Department, 2 Information
A Methodology for Safety Critical Software Systems Planning EHAB SHAFEI 1, IBRAHIM F. MOAWAD 2, HANY SALLAM 1, ZAKI TAHA 3, MOSTAFA AREF 3 1 Operation Safety and Human Factors Department, 2 Information
MD Link Integration. 2013 2015 MDI Solutions Limited
 MD Link Integration 2013 2015 MDI Solutions Limited Table of Contents THE MD LINK INTEGRATION STRATEGY...3 JAVA TECHNOLOGY FOR PORTABILITY, COMPATIBILITY AND SECURITY...3 LEVERAGE XML TECHNOLOGY FOR INDUSTRY
MD Link Integration 2013 2015 MDI Solutions Limited Table of Contents THE MD LINK INTEGRATION STRATEGY...3 JAVA TECHNOLOGY FOR PORTABILITY, COMPATIBILITY AND SECURITY...3 LEVERAGE XML TECHNOLOGY FOR INDUSTRY
Medical Device Software
 Medical Device Software Bakul Patel Senior Policy Advisor 1 Overview Medical devices and software Oversight principles and Current approach Trends, Challenges and opportunities Addressing challenges 2
Medical Device Software Bakul Patel Senior Policy Advisor 1 Overview Medical devices and software Oversight principles and Current approach Trends, Challenges and opportunities Addressing challenges 2
The Growing Concern Surrounding Medical Alarm Fatigue
 The Growing Concern Surrounding Medical Alarm Fatigue By: Jillyan Morano Director of Clinical Engineering, ABM Healthcare Support Services Executive Summary The issue of alarm fatigue and patient safety
The Growing Concern Surrounding Medical Alarm Fatigue By: Jillyan Morano Director of Clinical Engineering, ABM Healthcare Support Services Executive Summary The issue of alarm fatigue and patient safety
Connect care for early intervention
 IntelliVue Information Center ix Central monitoring station Connect care for early intervention See more. Know more. Do more. Connecting your teams to each other and to the information they need supports
IntelliVue Information Center ix Central monitoring station Connect care for early intervention See more. Know more. Do more. Connecting your teams to each other and to the information they need supports
From Data to Insight: Big Data and Analytics for Smart Manufacturing Systems
 From Data to Insight: Big Data and Analytics for Smart Manufacturing Systems Dr. Sudarsan Rachuri Program Manager Smart Manufacturing Systems Design and Analysis Systems Integration Division Engineering
From Data to Insight: Big Data and Analytics for Smart Manufacturing Systems Dr. Sudarsan Rachuri Program Manager Smart Manufacturing Systems Design and Analysis Systems Integration Division Engineering
Good Shepherd Medical Center Device Connectivity Case Study
 Good Shepherd Medical Center Device Connectivity Case Study How Nuvon Improved Time for Patient Care in the ED, Provided Better Patient Triage, and Supported Increased ED Throughput Capacity While Going
Good Shepherd Medical Center Device Connectivity Case Study How Nuvon Improved Time for Patient Care in the ED, Provided Better Patient Triage, and Supported Increased ED Throughput Capacity While Going
Open Source Business Rules Management System Enables Active Decisions
 JBoss Enterprise BRMS Open Source Business Rules Management System Enables Active Decisions What is it? JBoss Enterprise BRMS provides an open source business rules management system that enables active
JBoss Enterprise BRMS Open Source Business Rules Management System Enables Active Decisions What is it? JBoss Enterprise BRMS provides an open source business rules management system that enables active
The HYDRA project. Personal health monitoring
 The HYDRA project A middleware platform for personal health monitoring Peter Rosengren, Technical Coordinator [email protected] IST-2005-034891 Personal health monitoring Patient has some medical
The HYDRA project A middleware platform for personal health monitoring Peter Rosengren, Technical Coordinator [email protected] IST-2005-034891 Personal health monitoring Patient has some medical
Interoperabilität von patientennahen Medizingeräten An architecture for medical cyber physical systems in high acuity environments
 Interoperabilität von patientennahen Medizingeräten An architecture for medical cyber physical systems in high acuity environments KoSSE-Tag 2015, Lübeck 2015/06/03, Stefan Schlichting Agenda 1. Introduction
Interoperabilität von patientennahen Medizingeräten An architecture for medical cyber physical systems in high acuity environments KoSSE-Tag 2015, Lübeck 2015/06/03, Stefan Schlichting Agenda 1. Introduction
Open Source Interoperability
 Open Source Interoperability Problems, Prototypes, and Potential Jeffrey Peterson Clinical Engineer MD PnP Interoperability Program @ MGH 2 Agenda 1 Problems 2 Prototypes 3 Potential Problems 4 PCA Safety
Open Source Interoperability Problems, Prototypes, and Potential Jeffrey Peterson Clinical Engineer MD PnP Interoperability Program @ MGH 2 Agenda 1 Problems 2 Prototypes 3 Potential Problems 4 PCA Safety
Development of Integrated Management System based on Mobile and Cloud Service for Preventing Various Hazards
 , pp. 143-150 http://dx.doi.org/10.14257/ijseia.2015.9.7.15 Development of Integrated Management System based on Mobile and Cloud Service for Preventing Various Hazards Ryu HyunKi 1, Yeo ChangSub 1, Jeonghyun
, pp. 143-150 http://dx.doi.org/10.14257/ijseia.2015.9.7.15 Development of Integrated Management System based on Mobile and Cloud Service for Preventing Various Hazards Ryu HyunKi 1, Yeo ChangSub 1, Jeonghyun
GE Healthcare. Avance Carestation. Innovating with you, shaping exceptional care
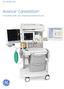 GE Healthcare Avance Carestation Innovating with you, shaping exceptional care Clinician inspired perioperative solutions GE s Avance Carestation was developed using an approach to perioperative solutions
GE Healthcare Avance Carestation Innovating with you, shaping exceptional care Clinician inspired perioperative solutions GE s Avance Carestation was developed using an approach to perioperative solutions
The U.S. FDA s Regulation and Oversight of Mobile Medical Applications
 The U.S. FDA s Regulation and Oversight of Mobile Medical Applications The U.S. FDA s Regulation and Oversight of Mobile Medical Applications As smart phones and portable tablet computers become the preferred
The U.S. FDA s Regulation and Oversight of Mobile Medical Applications The U.S. FDA s Regulation and Oversight of Mobile Medical Applications As smart phones and portable tablet computers become the preferred
Opportunities to Overcome Key Challenges
 The Electricity Transmission System Opportunities to Overcome Key Challenges Summary Results of Breakout Group Discussions Electricity Transmission Workshop Double Tree Crystal City, Arlington, Virginia
The Electricity Transmission System Opportunities to Overcome Key Challenges Summary Results of Breakout Group Discussions Electricity Transmission Workshop Double Tree Crystal City, Arlington, Virginia
Using Predictive Analytics to Improve Sepsis Outcomes 4/23/2014
 Using Predictive Analytics to Improve Sepsis Outcomes 4/23/2014 Ryan Arnold, MD Department of Emergency Medicine and Value Institute Christiana Care Health System, Newark, DE Susan Niemeier, RN Chief Nursing
Using Predictive Analytics to Improve Sepsis Outcomes 4/23/2014 Ryan Arnold, MD Department of Emergency Medicine and Value Institute Christiana Care Health System, Newark, DE Susan Niemeier, RN Chief Nursing
Development of Integrated Management System based on Mobile and Cloud service for preventing various dangerous situations
 Development of Integrated Management System based on Mobile and Cloud service for preventing various dangerous situations Ryu HyunKi, Moon ChangSoo, Yeo ChangSub, and Lee HaengSuk Abstract In this paper,
Development of Integrated Management System based on Mobile and Cloud service for preventing various dangerous situations Ryu HyunKi, Moon ChangSoo, Yeo ChangSub, and Lee HaengSuk Abstract In this paper,
University of Paderborn Software Engineering Group II-25. Dr. Holger Giese. University of Paderborn Software Engineering Group. External facilities
 II.2 Life Cycle and Safety Safety Life Cycle: The necessary activities involving safety-related systems, occurring during a period of time that starts at the concept phase of a project and finishes when
II.2 Life Cycle and Safety Safety Life Cycle: The necessary activities involving safety-related systems, occurring during a period of time that starts at the concept phase of a project and finishes when
Business-Driven Software Engineering Lecture 3 Foundations of Processes
 Business-Driven Software Engineering Lecture 3 Foundations of Processes Jochen Küster [email protected] Agenda Introduction and Background Process Modeling Foundations Activities and Process Models Summary
Business-Driven Software Engineering Lecture 3 Foundations of Processes Jochen Küster [email protected] Agenda Introduction and Background Process Modeling Foundations Activities and Process Models Summary
PROPOSAL FOR INTEGRATION OF ICU MEDICAL DEVICES WITH ELECTRONIC MEDICAL RECORD
 PROPOSAL FOR INTEGRATION OF ICU MEDICAL DEVICES WITH ELECTRONIC MEDICAL RECORD Presented on November 2011 to the Community Health Center Board of Directors Presented by: Jeana O Brien Sharon Perelman Maureen
PROPOSAL FOR INTEGRATION OF ICU MEDICAL DEVICES WITH ELECTRONIC MEDICAL RECORD Presented on November 2011 to the Community Health Center Board of Directors Presented by: Jeana O Brien Sharon Perelman Maureen
CHAPTER 7 SUMMARY AND CONCLUSION
 179 CHAPTER 7 SUMMARY AND CONCLUSION This chapter summarizes our research achievements and conclude this thesis with discussions and interesting avenues for future exploration. The thesis describes a novel
179 CHAPTER 7 SUMMARY AND CONCLUSION This chapter summarizes our research achievements and conclude this thesis with discussions and interesting avenues for future exploration. The thesis describes a novel
MDCF Tutorial Device Interface and App Development
 MDCF Tutorial Device Interface and App Development http://mdcf.santos.cis.ksu.edu/ Acknowledgements: Funding provided by US National Science Foundation awards 0734204, 0930647 Clinical documentation and
MDCF Tutorial Device Interface and App Development http://mdcf.santos.cis.ksu.edu/ Acknowledgements: Funding provided by US National Science Foundation awards 0734204, 0930647 Clinical documentation and
Pulse Oximetry Alarm Reduction Strategy at Boston Medical Center
 Pulse Oximetry Alarm Reduction Strategy at Boston Medical Center July 30, 2014 Boston Medical Center Alarm Initiative Reduction in alarms Recent focus on Pulse Oximetry Data review for supporting change
Pulse Oximetry Alarm Reduction Strategy at Boston Medical Center July 30, 2014 Boston Medical Center Alarm Initiative Reduction in alarms Recent focus on Pulse Oximetry Data review for supporting change
The SPES Methodology Modeling- and Analysis Techniques
 The SPES Methodology Modeling- and Analysis Techniques Dr. Wolfgang Böhm Technische Universität München [email protected] Agenda SPES_XT Project Overview Some Basic Notions The SPES Methodology SPES_XT
The SPES Methodology Modeling- and Analysis Techniques Dr. Wolfgang Böhm Technische Universität München [email protected] Agenda SPES_XT Project Overview Some Basic Notions The SPES Methodology SPES_XT
Clinical Decision Support
 Goals and Objectives Clinical Decision Support What Is It? Where Is It? Where Is It Going? Name the different types of clinical decision support Recall the Five Rights of clinical decision support Identify
Goals and Objectives Clinical Decision Support What Is It? Where Is It? Where Is It Going? Name the different types of clinical decision support Recall the Five Rights of clinical decision support Identify
Oracle Value Chain Planning Inventory Optimization
 Oracle Value Chain Planning Inventory Optimization Do you know what the most profitable balance is among customer service levels, budgets, and inventory cost? Do you know how much inventory to hold where
Oracle Value Chain Planning Inventory Optimization Do you know what the most profitable balance is among customer service levels, budgets, and inventory cost? Do you know how much inventory to hold where
Introduction to WebSphere Process Server and WebSphere Enterprise Service Bus
 Introduction to WebSphere Process Server and WebSphere Enterprise Service Bus Course materials may not be reproduced in whole or in part without the prior written permission of IBM. 4.0.3 Unit objectives
Introduction to WebSphere Process Server and WebSphere Enterprise Service Bus Course materials may not be reproduced in whole or in part without the prior written permission of IBM. 4.0.3 Unit objectives
Patient Monitor Gateway Implementation with EMR Case Review
 Patient Monitor Gateway Implementation with EMR Case Review Luis Melendez Partners Healthcare Biomedical Engineering Medical Device Integration and Informatics Medical Device Connectivity Conference Sept.
Patient Monitor Gateway Implementation with EMR Case Review Luis Melendez Partners Healthcare Biomedical Engineering Medical Device Integration and Informatics Medical Device Connectivity Conference Sept.
Model-Driven Software Development for Robotics: an overview
 Model-Driven Software Development for Robotics: an overview IEEE-ICRA2011 Workshop on Software Development and Integration in Robotics Jan F. Broenink, Maarten M. Bezemer Control Engineering, University
Model-Driven Software Development for Robotics: an overview IEEE-ICRA2011 Workshop on Software Development and Integration in Robotics Jan F. Broenink, Maarten M. Bezemer Control Engineering, University
MOBILE ARCHITECTURE FOR DYNAMIC GENERATION AND SCALABLE DISTRIBUTION OF SENSOR-BASED APPLICATIONS
 MOBILE ARCHITECTURE FOR DYNAMIC GENERATION AND SCALABLE DISTRIBUTION OF SENSOR-BASED APPLICATIONS Marco Picone, Marco Muro, Vincenzo Micelli, Michele Amoretti, Francesco Zanichelli Distributed Systems
MOBILE ARCHITECTURE FOR DYNAMIC GENERATION AND SCALABLE DISTRIBUTION OF SENSOR-BASED APPLICATIONS Marco Picone, Marco Muro, Vincenzo Micelli, Michele Amoretti, Francesco Zanichelli Distributed Systems
Patterns in Software Engineering
 Patterns in Software Engineering Lecturer: Raman Ramsin Lecture 7 GoV Patterns Architectural Part 1 1 GoV Patterns for Software Architecture According to Buschmann et al.: A pattern for software architecture
Patterns in Software Engineering Lecturer: Raman Ramsin Lecture 7 GoV Patterns Architectural Part 1 1 GoV Patterns for Software Architecture According to Buschmann et al.: A pattern for software architecture
Applying 4+1 View Architecture with UML 2. White Paper
 Applying 4+1 View Architecture with UML 2 White Paper Copyright 2007 FCGSS, all rights reserved. www.fcgss.com Introduction Unified Modeling Language (UML) has been available since 1997, and UML 2 was
Applying 4+1 View Architecture with UML 2 White Paper Copyright 2007 FCGSS, all rights reserved. www.fcgss.com Introduction Unified Modeling Language (UML) has been available since 1997, and UML 2 was
MOTEWORKS. Key Features. Overview
 MOTEWORKS SOFTWARE PLATFORM MoteWorks 2.0 provides a complete software development environment for wireless sensor network applications. Included is a collection of flexible software packages that enables
MOTEWORKS SOFTWARE PLATFORM MoteWorks 2.0 provides a complete software development environment for wireless sensor network applications. Included is a collection of flexible software packages that enables
Usability of Medical Applications Ved Line Kagenow Svenstrup, [email protected]
 Usability of Medical Applications Ved Line Kagenow Svenstrup, [email protected] What is usability? The user, rather than the system, at the center of the process. Risk of operating errors that can cause injury
Usability of Medical Applications Ved Line Kagenow Svenstrup, [email protected] What is usability? The user, rather than the system, at the center of the process. Risk of operating errors that can cause injury
Medical Device Interoperability: a wicked problem
 Medical Device Interoperability: a wicked problem Julian M. Goldman, MD Medical Director, Partners HealthCare Biomedical Engineering Anesthesiologist, Mass Gen Hospital/Harvard Medical School Director,
Medical Device Interoperability: a wicked problem Julian M. Goldman, MD Medical Director, Partners HealthCare Biomedical Engineering Anesthesiologist, Mass Gen Hospital/Harvard Medical School Director,
Early Evaluation Center
 www.winmedical.com Early Evaluation Center Early Evaluation Center - EEC is an intuitive and easy-to-use software for monitoring and evaluating a patient s clinical risk, and can acquire and process source
www.winmedical.com Early Evaluation Center Early Evaluation Center - EEC is an intuitive and easy-to-use software for monitoring and evaluating a patient s clinical risk, and can acquire and process source
INTERNET OF THE THINGS (IoT): An introduction to wireless sensor networking middleware
 1 INTERNET OF THE THINGS (IoT): An introduction to wireless sensor networking middleware Dr Antoine Bagula ISAT Laboratory, University of Cape Town, South Africa Goal of the lecture 2 The lecture intends
1 INTERNET OF THE THINGS (IoT): An introduction to wireless sensor networking middleware Dr Antoine Bagula ISAT Laboratory, University of Cape Town, South Africa Goal of the lecture 2 The lecture intends
Next Generation Grid Data Architecture & Analytics Required for the Future Grid
 & Analytics Required for the Future Grid Arjun Shankar and Russell Robertson Team: Lin Zhu, Frank Liu, Jim Nutaro, Yilu Liu, and Tom King 1 2 2 Project Purpose PURPOSE To foster open collaboration on issues,
& Analytics Required for the Future Grid Arjun Shankar and Russell Robertson Team: Lin Zhu, Frank Liu, Jim Nutaro, Yilu Liu, and Tom King 1 2 2 Project Purpose PURPOSE To foster open collaboration on issues,
Title: Using Formal Methods to Improve Safety of Home-Use Medical Devices
 Title: Using Formal Methods to Improve Safety of Home-Use Medical Devices Authors: Ayan Banerjee Impact Lab, Arizona State University [email protected] Yi Zhang Center for Devices and Radiological Health,
Title: Using Formal Methods to Improve Safety of Home-Use Medical Devices Authors: Ayan Banerjee Impact Lab, Arizona State University [email protected] Yi Zhang Center for Devices and Radiological Health,
Optimizing Masimo SET SpO2 Alarm Settings on Select GE Monitors
 whitepaper Optimizing Masimo SET SpO2 Alarm Settings on Select GE Monitors Summary Masimo SET pulse oximetry in GE patient monitors has different alarm configuration settings compared to standalone Masimo
whitepaper Optimizing Masimo SET SpO2 Alarm Settings on Select GE Monitors Summary Masimo SET pulse oximetry in GE patient monitors has different alarm configuration settings compared to standalone Masimo
Building Test-Sites with Simware
 Building Test-Sites with Simware TEL. +34 91 790 12 29 [email protected] www.simware.es 1 INTRODUCTION Construction of critical operational systems, like a Naval Combat Management (CMS) system are changing
Building Test-Sites with Simware TEL. +34 91 790 12 29 [email protected] www.simware.es 1 INTRODUCTION Construction of critical operational systems, like a Naval Combat Management (CMS) system are changing
Menu Case Study 3: Medication Administration Record
 Menu Case Study 3: Medication Administration Record Applicant Organization: Ontario Shores Centre for Mental Health Sciences Organization s Address: 700 Gordon Street, Whitby, Ontario, Canada, L1N5S9 Submitter
Menu Case Study 3: Medication Administration Record Applicant Organization: Ontario Shores Centre for Mental Health Sciences Organization s Address: 700 Gordon Street, Whitby, Ontario, Canada, L1N5S9 Submitter
Mitra Innovation Leverages WSO2's Open Source Middleware to Build BIM Exchange Platform
 Mitra Innovation Leverages WSO2's Open Source Middleware to Build BIM Exchange Platform May 2015 Contents 1. Introduction... 3 2. What is BIM... 3 2.1. History of BIM... 3 2.2. Why Implement BIM... 4 2.3.
Mitra Innovation Leverages WSO2's Open Source Middleware to Build BIM Exchange Platform May 2015 Contents 1. Introduction... 3 2. What is BIM... 3 2.1. History of BIM... 3 2.2. Why Implement BIM... 4 2.3.
KURA M2M/IoT Gateway. reducing the distance between embedded and enterprise technologies. Tiziano Modotti, October 28 th, 2014
 KURA M2M/IoT Gateway reducing the distance between embedded and enterprise technologies Tiziano Modotti, October 28 th, 2014 IoT Architecture @ M2M/IoT Integration Platform on Cloud Business Applications
KURA M2M/IoT Gateway reducing the distance between embedded and enterprise technologies Tiziano Modotti, October 28 th, 2014 IoT Architecture @ M2M/IoT Integration Platform on Cloud Business Applications
Stelios Sotiriadis, Euripides G.M. Petrakis, Stefan Covaci, Paolo Zampognaro, Eleni Georga, Christoph Thuemmler
 An architecture for designing Future Internet (FI) applications in sensitive domains: Expressing the Software to data paradigm by utilizing hybrid cloud technology Stelios Sotiriadis, Euripides G.M. Petrakis,
An architecture for designing Future Internet (FI) applications in sensitive domains: Expressing the Software to data paradigm by utilizing hybrid cloud technology Stelios Sotiriadis, Euripides G.M. Petrakis,
Chapter 1- Introduction. Lecture 1
 Chapter 1- Introduction Lecture 1 Topics covered Professional software development What is meant by software engineering. Software engineering ethics A brief introduction to ethical issues that affect
Chapter 1- Introduction Lecture 1 Topics covered Professional software development What is meant by software engineering. Software engineering ethics A brief introduction to ethical issues that affect
The Shifting Sands of Medical Software Regulation
 The Shifting Sands of Medical Software Regulation Suzanne O Shea Ralph Hall September 10, 2014 What Software is Regulated by FDA? FDA regulates medical devices. FDA regulates software that meets the definition
The Shifting Sands of Medical Software Regulation Suzanne O Shea Ralph Hall September 10, 2014 What Software is Regulated by FDA? FDA regulates medical devices. FDA regulates software that meets the definition
How To Integrate Medical Devices
 Developing a Strategy for Integrating Medical Device Data with Clinical Information Systems Developing a Strategy for Integrating Medical Device Data with Clinical Information Systems WHY NOW? The Current
Developing a Strategy for Integrating Medical Device Data with Clinical Information Systems Developing a Strategy for Integrating Medical Device Data with Clinical Information Systems WHY NOW? The Current
Full Link: http://www.modernhealthcare.com/article/20140816/magazi NE/308169986. Health IT poses safety problems if users not trained properly
 Full Link: http://www.modernhealthcare.com/article/20140816/magazi NE/308169986 Health IT poses safety problems if users not trained properly By Sabriya Rice August 16, 2014 To improve patient safety,
Full Link: http://www.modernhealthcare.com/article/20140816/magazi NE/308169986 Health IT poses safety problems if users not trained properly By Sabriya Rice August 16, 2014 To improve patient safety,
Developing Microsoft SharePoint Server 2013 Advanced Solutions MOC 20489
 Developing Microsoft SharePoint Server 2013 Advanced Solutions MOC 20489 Course Outline Module 1: Creating Robust and Efficient Apps for SharePoint In this module, you will review key aspects of the apps
Developing Microsoft SharePoint Server 2013 Advanced Solutions MOC 20489 Course Outline Module 1: Creating Robust and Efficient Apps for SharePoint In this module, you will review key aspects of the apps
Cloud Development of Medical Systems By Oleg Kruk, Embedded Research Lab Leader, DataArt
 Cloud Development of Medical Systems By Oleg Kruk, Embedded Research Lab Leader, DataArt Abstract Wireless electronic medical devices have made remote medicine a reality. Disease prevention, monitoring
Cloud Development of Medical Systems By Oleg Kruk, Embedded Research Lab Leader, DataArt Abstract Wireless electronic medical devices have made remote medicine a reality. Disease prevention, monitoring
Design of an Insulin Pump. Purpose of an Insulin Pump:
 Design of an Insulin Pump Purpose of an Insulin Pump: Insulin is a hormone central to regulating carbohydrate and fat metabolism in the body. It is secreted regularly within the body and aids in converting
Design of an Insulin Pump Purpose of an Insulin Pump: Insulin is a hormone central to regulating carbohydrate and fat metabolism in the body. It is secreted regularly within the body and aids in converting
i-pcgrid Workshop 2015 Cyber Security for Substation Automation The Jagged Line between Utility and Vendors
 March 25-27, 2014 Steven A. Kunsman i-pcgrid Workshop 2015 Cyber Security for Substation Automation The Jagged Line between Utility and Vendors ABB Inc. March 26, 2015 Slide 1 Cyber Security for Substation
March 25-27, 2014 Steven A. Kunsman i-pcgrid Workshop 2015 Cyber Security for Substation Automation The Jagged Line between Utility and Vendors ABB Inc. March 26, 2015 Slide 1 Cyber Security for Substation
Agent Languages. Overview. Requirements. Java. Tcl/Tk. Telescript. Evaluation. Artificial Intelligence Intelligent Agents
 Agent Languages Requirements Overview Java Tcl/Tk Telescript Evaluation Franz J. Kurfess, Cal Poly SLO 211 Requirements for agent Languages distributed programming large-scale (tens of thousands of computers)
Agent Languages Requirements Overview Java Tcl/Tk Telescript Evaluation Franz J. Kurfess, Cal Poly SLO 211 Requirements for agent Languages distributed programming large-scale (tens of thousands of computers)
Best Practices: Extending Enterprise Applications to Mobile Devices
 Best Practices: Extending Enterprise Applications to Mobile Devices by Kulathumani Hariharan Summary: Extending enterprise applications to mobile devices is increasingly becoming a priority for organizations
Best Practices: Extending Enterprise Applications to Mobile Devices by Kulathumani Hariharan Summary: Extending enterprise applications to mobile devices is increasingly becoming a priority for organizations
Cisco Context-Aware Mobility Solution: Put Your Assets in Motion
 Cisco Context-Aware Mobility Solution: Put Your Assets in Motion How Contextual Information Can Drastically Change Your Business Mobility and Allow You to Achieve Unprecedented Efficiency What You Will
Cisco Context-Aware Mobility Solution: Put Your Assets in Motion How Contextual Information Can Drastically Change Your Business Mobility and Allow You to Achieve Unprecedented Efficiency What You Will
