Kvartalets)informationsbrev)till)aktieägarna)från)Protein)Sciences)
|
|
|
- David Gary Wheeler
- 10 years ago
- Views:
Transcription
1 ! M Mertiva!! Pressmeddelande den 4 april 2015 Kvartalets)informationsbrev)till)aktieägarna)från)Protein)Sciences) Protein Sciences Corporation (Protein Sciences) har distribuerat ett informationsbrev till sina aktieägare ( Shareholder Letter March 2015 ). Protein Sciences har godkänt att Mertiva offentliggör detta informationsbrev. Informationsbrevet innehåller en redogörelse för de viktigaste händelserna i bolaget, samt oreviderade siffror för 2014 och är bifogat till detta pressmeddelande. Informationsbrevet innehåller även pressmeddelanden, vilka tidigare publicerats på Protein Sciences hemsida. Protein Sciences avser att distribuera informationsbrev kvartalsvis till sina aktieägare. Om Mertiva erhåller sådana informationsbrev avser Mertiva publicera dessa. För ytterligare information, vänligen kontakta: Andreas Bergsten, VD Mertiva AB [email protected] Om Mertiva Mertiva AB är ett investeringsföretag som i huvudsak består av innehav i Protein Sciences Corporation och Mercodia AB. Mertiva-aktien är listad på NGM:s handelsplats Nordic MTF (kortnamn: MERT MTF). Mer information finns på Denna information offentliggörs enligt lagen om värdepappersmarknaden, lagen om handel med finansiella instrument eller krav ställda i noteringsavtal. Mertiva!AB!(publ.)! Upplandsgatan!67,!113!28!Stockholm,!Sverige! EEpost:[email protected]! Org.!Nr:!556530E1420!
2 To Our Shareholders: March 2015 The year ending December 31, 2014 represents another milestone in the growth of your Company. Both clinical trials of the quadrivalent version of Flublok were fully enrolled more quickly than expected and we are eagerly awaiting the data from these trials. FDA inspected our Pearl River facility, and while there were several observations we anticipate approval in May, a two-month delay due to requests for additional information. We brought in several new GeneXpress customers and signed an expressf+ (SF+) cell line license with a major pharmaceutical company. From a financial standpoint, we continued to be profitable despite the expenses associated with launching Flublok, and we were able to add modestly to cash during the year. In 2014 we laid the foundation for the commercialization of Flublok. We addressed four critical factors described below that limited uptake in the market. We added a second distributor, however continued limited accessibility of Flublok to the general public remained our major challenge. Our marketing focus for the upcoming influenza season has been to further broaden our distribution network two new distributors added - and to reach agreement (successfully) with large retail chains to offer Flublok. In addition, we have been working with government agencies to purchase Flublok we signed a purchase contract with the Centers for Disease Control and Prevention (CDC) and are cautiously optimistic about purchases from the Department of Defense (DoD). We continue to strengthen our marketing efforts through additions to the team and co-marketing. Our priorities for 2015 are: Successful execution of the three components of the BARDA contract: Pearl River licenure, quadrivalent studies and PSC26 pandemic study. Commercial manufacturing: produce lots of Flublok drug substance in Meriden that will be filled at Hospira and 6-8 lots in Pearl River that will be filled at MassBio. Secure sales of million doses. Critical Success Factor: early availability of Flublok. Secure additional non-dilutive funding for new products - stock-piling and/or expansion of Flublok sales. We are looking forward to a successful year, and our goal is to sell-out Flublok before the end of October. Flublok: In 2014 we laid the foundation for the commercialization of Flublok. We addressed the four critical factors that limited uptake of Flublok: 1) We reached agreement with FDA in August to extend the shelf life of Flublok from four to six months. 2) FDA expanded the age indication to include adults over 50 years old. 3) Information on Flublok reimbursement is now available from Medicare and multiple insurance companies that should mitigate resistance to the higher price point of Flublok. 4) The first doses of Flublok will be available in retail chains this August despite two strain changes. Flublok sales 2014/15: Hospitals continued to be the most important buyers albeit in small quantities with approximately 30% of U.S. hospitals placing orders that accounted for about 80% of our sales. Sales through retail chains were very limited (less than 1%) with Price Chopper and Publix shipping doses on
3 an individual basis based on customers requests. About 2% of the doses were delivered through clinics operated by Health-At-Work and another 2% through Passport Health clinics. Pre-booking for 2015 began at the end of February, and sales increased somewhat compared to last season, but since pre-booking is done primarily on securing discounted pricing we are not overly concerned. We added two new distributors, McKesson and Cardinal Health, and expect to add one or two more distributors this year. They will join FFF Enterprises and will further expand our access points (see attached press release). As we prepare for the 2015/16 flu season, an important focus is to secure approval to ship Flublok direct to larger customers. This will enable us to split lots and distribute Flublok even more widely and in a cost effective way. We are pleased to announce that we signed an agreement with the CDC providing them with the option to purchase up to 300,000 doses of Flublok. We continue to work with DoD to secure part of their business and are optimistic that we will succeed. We are also excited to announce that multiple retail chains including Target Pharmacies and Walmart will carry Flublok and expect more chains will follow. We will be undertaking marketing efforts with these retails chains to create public awareness and more actively promote demand for Flublok. Price Chopper and Publix will continue to carry Flublok in their distribution centers and will ship to their pharmacies upon request. We continue to work with them to ensure availability in each store. Health-At-Work is in the process of obtaining authorization to be in-network with most of the large insurance providers in New England and will serve CT, MA and NY next season. The goal is to increase the doses delivered by Health-At-Work and Passport Health clinics ten-fold. We are also working with other mass immunizers to have Flublok included in their formularies. This will allow us to set up clinics with employers outside of the areas serviced by Health-At-Work. We are strengthening our relations with insurance companies and other stakeholders to create awareness around Flublok through multiple channels. As an example of our initiatives, we developed an educational piece in collaboration with the American Association of Pharmacists. This One-Minute Counselor contains Flublok information tailored to pharmacists and consumers and was distributed to 155,000 pharmacists in the US. Finally, our outreach to shareholders has resulted in several important initiatives ranging from convincing primary care physicians to purchase Flublok and direct purchasing of Flublok to providing leads in retail chains and contact with possible future partners and stakeholders. Thank you for that! Our Flublok licensee in Mexico, Liomont, plans to file for approval of Flublok in June. If all goes well, Flublok could be on the market in time for the 2015/16 influenza season. Initially Liomont will buy vialed Flublok from us but in one to two years will move to purchasing bulk product to be filled/finished in Mexico and may eventually establish manufacturing there to serve the greater Latin American market. Upon licensure in Mexico, we are entitled to receive a milestone payment and royalties on sales. 2
4 Astellas, UMN Pharma s marketing partner in Japan, continues to pursue licensure of Flublok (it will be marketed under a different name in Japan) with PMDA (the Japanese equivalent of FDA in the U.S.) and expects to secure licensure later this year. We are entitled to major milestone payments and royalties on sales when Flublok is approved for the Japan market. Connecticut State Senator Danté Bartolomeo continues to be an enormous help to us both in bringing the public s and the State s attention to Flublok and in tirelessly working to secure purchases by the State. She recently conducted an interview highlighting the good work we are doing to vaccinate the poor in partnership with our neighbor Hunter s Ambulance and Transportation Service that can be viewed at Hunter s is a superb partner that should be your choice for limousine and airport transportation needs. Clinical Trials: Two major clinical trials of Flublok Quadrivalent vaccine (PSC12 and PSC16) are progressing according to plan (see attached press release). Subject retention is over 98% in both trials and Serious Adverse Events (SAEs) are consistent with or lower than expected in studies in these age groups. None of the SAEs has been considered related to study vaccine. Data entry, monitoring and data cleaning are on-track. We expect the last subjects to complete follow-up in late May 2015 and anticipate top-line results in early to mid-july. We plan to submit a supplemental BLA for Flublok Quadrivalent in adults 18 years by late October 2015 that should lead to FDA approval by August Pearl River: At the beginning of March Pearl River underwent a Pre-Approval Inspection by the FDA that resulted in a total of 13 observations. The inspectors were complimentary of the team, noting their hard work and high level of dedication. They specifically acknowledged improvements in all quality systems. The week-long inspection was generally positive and we now expect approval of the facility on May 12 th. In preparation for approval the Pearl River team has been generating inventory with production of H1 California rha in January and February. To attain our goal of producing over 1 million doses for next season, we will need to achieve a success rate of >90% in manufacturing, delivering one batch every week from January to August. Filling at Hospira and MassBio is scheduled to begin in June to allow us to deliver product to distributors in August, which is two months earlier than last season. Early distribution is critical for pharmacies that begin their vaccination campaigns at the end of August. Collaborations: We signed a license agreement with a major pharmaceutical company to use our SF+ cell line for research purposes with the option to elect an exclusive commercial license for two defined products. We have an excellent working relationship with the company, and this license is further testament to the value of our BEVS platform. We completed two 40L production runs of GMP material in our Pilot Plant for one of our Japanese customers. This has been a multi-year project with many deliverables and the customer commented that the material received this time was the best quality they had received. We also completed a project this winter for a customer to generate recombinant adeno-associated viruses (raavs) in our cell line that have gene therapy applications. The material was produced and delivered on schedule. Preparations are underway to manufacture additional clinical-grade fibroblast growth factor (FGF1) for BioArctic this summer. The FGF1 we produce is inserted into a biodegradable device that is used under 3
5 evaluation by BioArctic as a surgical treatment for complete spinal cord injury. This treatment has succeeded in one patient, and BioArctic is optimistic that their product will cure this devastating injury. We have completed the initial phase of the National Cancer Institute-supported vault nanoparticle lung cancer project under which we produced recombinant baculoviruses to express the nanoparticles and the associated chemokine in collaboration with UCLA and Vault Nano Inc. We successfully produced both the vault particles and the chemokine, and the purification process is being developed. Ebola Vaccine: A non-human primate study on our Ebola vaccine is ongoing in collaboration with NIH, and results will be available soon. Rabies Vaccine: We are continuing to develop our rabies vaccine and are initiating immunogenicity studies with our collaborators at Erasmus University in The Netherlands. These studies will be followed by challenge studies in animals later this year. Japanese Encephalitis (JE) Vaccine. JE is a serious disease in much of Asia and we have agreed to develop a modern vaccine for a collaborator in Asia. Traditional JE vaccines are made by growing the JE virus in the brains of mice or in cell culture. Middle East Respiratory Syndrome (MERS) Vaccine. MERS continues to be a significant health hazard especially in Saudi Arabia. We submitted a proposal to develop a vaccine and have been invited to participate in a BARDA-sponsored conference in early April Financial Results: For the year ended December 31, 2014 revenues increased 18% to $46.4 million from $39.5 million in This increase reflects a steady contribution from our BARDA contract that accounted for approximately 78% of revenues, up from 70% in 2013 primarily as a result of the initiation of two large clinical trials that are funded by BARDA. Product sales increased 38% to $3.7 million from $2.7 million in but our Collaborative Agreements and Technology Licenses that together accounted for 14% of revenues were down somewhat from As a reminder, revenues from Collaborative Agreements are based on timelines for and success of products being development by our customers and, therefore, significantly increase or decrease on a quarterly basis. Operating expenses increased by 18% to $44.7 million from $37.9 million in 2013 primarily because of expenses associated with clinical trials. We had an operating profit of $1.6 million in line with Net income before tax benefit in 2014 declined 66% to $1.6 million compared to $4.8 million in 2013 primarily because of the sale in 2013 of a portion of the UMN Pharma shares we own. Cash and receivables after deducting payables and accrued expenses associated with receivables but including the value of the remaining UMN Pharma shares we own were essentially the same as at the end of Cash flow continued to be positive in 2014 and we added approximately $900,000 to available cash. We continue to be highly dependent on revenues from our BARDA contract that continues through the end of this year. We do expect to become less dependent on this contract as we build Flublok sales and secure royalties from licenses and collaborative agreements. In addition, we have applied for several other contracts with BARDA and other governmental agencies and are optimistic that at least some will be approved with revenues equal to or greater than our BARDA contract. We elected to change auditors to Marcum LLC because of personnel departures at our previous auditor and significantly lower cost of the audit. The audit of our 2014 financial statements is underway and is expected to be available around mid
6 Shareholder Call In and Annual Meeting: The next shareholder call in will occur on Friday, April 17, 2015 at 10:00 am. The call in number is and the access code is If you are calling from a public place or a car please place your phone on mute unless and until you want to ask a question. The annual meeting of shareholders will be held at our Pearl River facility located at 401 North Middletown Road, Pearl River, NY on Thursday, June 25, 2015 at 11:00 am. Because this is located on the Pfizer campus special arrangements have to be made to secure access and it is very important that you let us know if you plan to attend the meeting. Cordially, Manon M.J. Cox President & CEO Daniel D. Adams Executive Chairman LET S GO GREEN. Would you like to receive these shareholder updates and other correspondence from Protein Sciences via ? You ll be sent the latest news quickly and help the Company conserve resources. Please respond with your name and address to Nadine Francis West ([email protected]). We will add your address to the correspondence list. Thank you! 5
7 Protein Sciences Corporation Balance Sheets (Unaudited) December 31, December 31, ASSETS CURRENT ASSETS: Cash and cash equivalents $ 5,818,164 $ 4,936,355 Short term investment 3,630,057 4,323,771 BARDA funds receivable -- including unbilled of $4,563,599 and $3,834,701, respectively 13,253,403 5,098,194 Accounts receivable -- including unbilled of $199,876 and $1,067,473, respectively and net of allowance for doubtful accounts of $50,000 and $50,000, respectively 2,233,560 2,319,022 Inventory 2,207,079 1,807,028 Deferred tax asset 2,946,126 3,311,661 Other current assets 742, ,958 Total current assets 30,830,960 22,420,989 PROPERTY, PLANT AND EQUIPMENT -- Net 4,020,026 6,265,254 Long term deferred tax asset 4,979,616 4,979,616 Restricted cash 638, ,258 Other assets 350,859 13,642 TOTAL ASSETS $ 40,819,720 $ 34,317,759 LIABILITIES AND STOCKHOLDERS' EQUITY CURRENT LIABILITIES: Deferred revenue, current portion $ 757,578 $ 3,570,232 Accounts payable 7,294,735 2,525,018 Accrued expenses 5,177,764 2,043,658 Other current liabilities 147, ,038 Total current liabilities 13,377,368 8,577,946 LONG TERM LIABILITIES: Deferred revenue 1,226, ,951 Other liabilities 343, ,968 Total long term liabilities 1,569, ,919 COMMITMENTS AND CONTINGENCIES - - STOCKHOLDERS' EQUITY Common Stock, $0.001 par value; 150,000,000 shares authorized; 77,549,402 and 77,512,769 shares issued and outstanding at December 31, 2014 and December 31, 2013, respectively 77,549 77,513 Additional paid-in capital 63,569,789 63,210,458 Accumulated other comprehensive income, net of tax 640,850 1,070,861 Accumulated deficit (38,415,668) (39,609,938) Total stockholders' equity 25,872,520 24,748,894 TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY $ 40,819,720 $ 34,317,759
8 Twelve Months Ending December 31, 2014 % of % Change Revenue REVENUES: BARDA contract $ 36,519,636 $ 27,765,624 32% 78.7% Collaborative agreements 2,744,508 4,275,153-36% 5.9% Technology licenses 3,465,448 4,777,723-27% 7.5% Product sales 3,690,849 2,672,524 38% 8.0% Total revenues 46,420,441 39,491,024 18% 100.0% OPERATING EXPENSES: Protein Sciences Corporation Statements of Operations (Unaudited) Research and development 32,735,861 32,354,008 1% 70.5% Cost of goods sold 6,807, , % 14.7% General and administrative 5,254,102 4,822,249 9% 11.3% Total operating expenses 44,797,360 37,856,287 18% 96.5% INCOME FROM OPERATIONS 1,623,081 1,634,737-1% 3.5% (INCOME) OTHER EXPENSE: Interest expense - 1, % 0.0% Interest income (10,961) (15,200) -28% 0.0% Other income/expense 20,611 (3,153,150) Other assets 9,650 (3,167,208) -100% 0.0% Net income before tax expense and benefit 1,613,431 4,801,945-66% 3.5% Tax (expense) benefit (419,162) (2,237,283) -81% -0.9% Net Income $ 1,194,270 $ 2,564,662-53% 2.6%
9 Protein Sciences Corporation Statements of Cash Flow (Unaudited) Twelve Months Ending December 31, CASH FLOWS FROM OPERATING ACTIVITIES: Net income $ 1,194,270 $ 2,564,662 Adjustments to reconcile net income to net cash provided by operating activities: Realized gain on short term investment - (3,152,915) Depreciation and amortization 2,441,085 1,532,659 Share-based compensation 347, ,326 Excess tax benefit from stock compensation - (18,329) Loss on disposal of assets 17, Deferred taxes 365,535 2,129,395 Changes in operating assets and liabilities: Inventory (400,051) (1,504,930) Accounts receivable 85,462 (1,547,256) BARDA funds receivable (8,155,209) (2,670,717) Restricted cash Other assets (454,830) 373,937 Accounts payable and accrued expenses 7,903,822 1,549,790 Other liabilities (439,039) 490,968 Other assets Deferred accounts (2,086,448) (3,769,858) Net cash provided by operating activities 819,588 (3,646,813) CASH FLOWS FROM INVESTING ACTIVITIES: Proceed from sale of short term investment - 4,468,625 Purchase of investments 263,703 (3,895,194) Purchases of property and equipment (213,753) (2,297,335) Net cash used in investing activities 49,950 (1,723,904) CASH FLOWS FROM FINANCING ACTIVITIES: Proceeds from exercise of stock options 12, ,005 Payments of capital lease obligations - (2,497) Net cash used in financing activities 12, ,508 NET INCREASE IN CASH AND CASH EQUIVALENTS 881,809 (5,044,209) CASH AND CASH EQUIVALENTS - Beginning of period 4,936,355 9,980,564 CASH AND CASH EQUIVALENTS - End of period $ 5,818,164 $ 4,936,355 SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: Cash paid for interest $ - $ 1,142 Cash paid for taxes $ 81,672 $ 45,478
10 Protein Sciences Corporation Statements of Comprehensive Income (Unaudited) Twelve Months Ending December 31, Net Income $ 1,194,270 2,564,662 Other comprehensive income: Net change in unrealized gain in investments $ 640,850 1,070,861 Total Comprehensive Income $ 1,835,120 $ 3,635,523
11 Pre-Booking for Flublok Influenza Vaccine Now Available from Cardinal Health and McKesson For Immediate Release February 24, 2015 Contact: Courtney Goodwin Communications Associate Phone: (203) ext. 301 Meriden, CT Protein Sciences Corporation announced today it has expanded distribution options for its revolutionary Flublok influenza vaccine and that pre-booking for the influenza season is underway. Cardinal Health and McKesson have been added as new Flublok distributors joining FFF Enterprises. Together this broad array of distributors makes Flublok more widely available to healthcare professionals across the United States. Accessibility to Flublok by healthcare professionals and consumers has been limited in previous seasons, said Manon Cox, President and CEO of Protein Sciences. We are committed to making the vaccine easier to access, and our first step is to create multiple options for healthcare professionals to make Flublok available to their patients. Healthcare professionals wishing to pre-order Flublok should contact one of the following distributors: FFF Enterprises: Cardinal Health: McKesson: 877-MCK-4FLU mms.mckesson.com Flublok is fully reimbursed by most insurance companies, including Medicare. Healthcare professionals should use the Flublok-specific CPT code to ensure compensation occurs at an appropriate rate. For more information about Flublok, please visit About Protein Sciences Protein Sciences specializes in vaccine development and protein production. Our mission is our inspiration: to save lives and improve health through the creation of innovative vaccines and biopharmaceuticals. Flublok, the world s first recombinant protein-based vaccine for the prevention of seasonal influenza disease, was approved by FDA in January Flublok is the only flu vaccine made in a 100% egg-free system using modern cell culture technology, making it unnecessary to use an infectious influenza virus or antibiotics in manufacturing. Flublok is highly purified and does not contain any preservatives (e.g., thimerosal, a mercury derivative), egg proteins, gelatin or FB14052
12 latex. In addition, Flublok contains three times more antigen than traditional flu vaccines (3x45mcg hemagglutinin protein versus 3x15mcg hemagglutinin protein)*. Flublok is a perfect copy of the virus coat and is not subject to the egg-adapted mutations associated with low vaccine effectiveness (see Skowronski et al. (2014) PLOS ONE 9(3), e92153). Healthcare professionals can order Flublok for the 2014/15 season by contacting FFF Enterprises at Learn more at and Flublok Safety Information Flublok is approved for people 18 and older to prevent influenza disease. The most common side effect from Flublok is pain at the site of injection. Headache, fatigue or muscle ache may occur. Tell the doctor if you have ever experienced Guillain-Barré syndrome (severe muscle weakness) or have had a severe allergic reaction to any component of Flublok vaccine. Vaccination with Flublok may not protect all individuals. Clinical effectiveness in adults 50 and older is based on the immune response elicited by Flublok and not on demonstration of decreased influenza disease. Please see the complete Package Insert available at or call for more information. *Flublok demonstrated a higher antibody response to the A strains during 2 clinical trials in adults 50 years old. The B strain antibody response was comparable to traditional trivalent vaccines. ### FB14052
13 Clinical Efficacy Study of Flublok Quadrivalent Compares Flublok to a Traditional Egg-based Flu Vaccine For Immediate Release January 29, 2015 Contact: Courtney Goodwin Communications Associate Phone: (203) ext. 301 Meriden, CT Protein Sciences Corporation announced today that results from a doubleblinded comparative efficacy study of Flublok Quadrivalent in adults over 50 are expected in June. The study is designed to demonstrate whether Flublok s higher antigen content and its modern production process that avoids the introduction of egg-based mutations into the vaccine s active ingredients will result in better efficacy than the recently announced flu vaccine efficacy of 14% in adults older than 50. PSC12 is a large trial in adults 50 years of age and older intended to demonstrate the protective efficacy of Flublok Quadrivalent in prevention of influenza disease. The trial enrolled 9,000 participants at sites across the U.S. The efficacy of Flublok Quadrivalent is being compared to that of a licensed conventional egg-based inactivated quadrivalent vaccine. Flublok Quadrivalent is a quadrivalent version of Flublok influenza vaccine that is designed to protect against two A subtypes and two B lineages of influenza. Trivalent Flublok that protects against two A subtypes and one B lineage of influenza is licensed by the FDA for all adults 18 and older and is available for general use. Flublok has gained popularity due to its purity, eggfree nature and high antigen content and is considered especially important in a flu season such as this that is severe due to the predominance and drift in the H3N2 virus. The recombinant technology used to make Flublok is able to perfectly match circulating flu strains and, therefore, may prove more effective than traditional vaccines. Clinical trials have suggested that the higher antigen content of Flublok provides significant protection against drifted influenza viruses. A separate Phase 3 clinical trial of Flublok Quadrivalent is being conducted in adults years of age. PSC16 is intended to demonstrate that satisfactory immune responses are elicited for all four influenza proteins by Flublok Quadrivalent. The trial enrolled 1,350 adults across the U.S. and will compare Flublok Quadrivalent to a licensed conventional egg-based inactivated quadrivalent vaccine. Results are also expected in June. There has been a shift in influenza vaccines to quadrivalent formulations, commented Manon Cox, President and CEO of Protein Sciences. Our recombinant platform technology that we use to make Flublok and all other vaccines allows us to rapidly and predictably manufacture antigens that are perfectly matched to circulating influenza strains, whether it be the addition FB14052
14 of a fourth antigen as in the case of Flublok Quadrivalent, or to update the annual formulation of Trivalent Flublok. PSC12 and PSC16 put us on track for receiving regulatory approval of Flublok Quadrivalent in the next year or two. Trivalent Flublok is available now. Healthcare professionals seeking the vaccine should contact FFF Enterprises at or online at Consumers seeking Flublok should visit About Protein Sciences Protein Sciences specializes in vaccine development and protein production. Our mission is our inspiration: to save lives and improve health through the creation of innovative vaccines and biopharmaceuticals. Flublok, the world s first recombinant protein-based vaccine for the prevention of seasonal influenza disease, was approved by FDA in January Flublok is the only flu vaccine made in a 100% egg-free system using modern cell culture technology, making it unnecessary to use an infectious influenza virus or antibiotics in manufacturing. Flublok is highly purified and does not contain any preservatives (e.g., thimerosal, a mercury derivative), egg proteins, gelatin or latex. In addition, Flublok contains three times more antigen than traditional flu vaccines (3x45mcg hemagglutinin protein versus 3x15mcg hemagglutinin protein)*. Flublok is a perfect copy of the virus coat and is not subject to the egg-adapted mutations associated with low vaccine effectiveness (see Skowronski et al. (2014) PLOS ONE 9(3), e92153). Healthcare professionals can order Flublok by contacting FFF Enterprises at Learn more at and Flublok Safety Information Flublok is approved for people 18 and older to prevent influenza disease. The most common side effect from Flublok is pain at the site of injection. Headache, fatigue or muscle ache may occur. Tell the doctor if you have ever experienced Guillain-Barré syndrome (severe muscle weakness) or have had a severe allergic reaction to any component of Flublok vaccine. Vaccination with Flublok may not protect all individuals. Clinical effectiveness in adults 50 and older is based on the immune response elicited by Flublok and not on demonstration of decreased influenza disease. Please see the complete Package Insert available at or call for more information. FB14052
15 *Flublok demonstrated a higher antibody response to the A strains during 2 clinical trials in adults 50 years old. The B strain antibody response was comparable to traditional trivalent vaccines. ### FB14052
Kvartalets)informationsbrev)till)aktieägarna)från)Protein)Sciences)
 ! M Mertiva!! Pressmeddelande!den!24!september!2015! Kvartalets)informationsbrev)till)aktieägarna)från)Protein)Sciences) Protein(Sciences(Corporation((Protein(Sciences)(har(distribuerat(ett(informationsbrev(till(sina(
! M Mertiva!! Pressmeddelande!den!24!september!2015! Kvartalets)informationsbrev)till)aktieägarna)från)Protein)Sciences) Protein(Sciences(Corporation((Protein(Sciences)(har(distribuerat(ett(informationsbrev(till(sina(
HSA Consumer Guide. Understanding Vaccines, Vaccine Development and Production. www.hsa.gov.sg November 2009. How a Vaccine Works.
 November 2009 Understanding Vaccines, Vaccine Development and Production Vaccines, in general, help protect people from harmful infections before they come in contact with the disease. Vaccines may also
November 2009 Understanding Vaccines, Vaccine Development and Production Vaccines, in general, help protect people from harmful infections before they come in contact with the disease. Vaccines may also
Zebra Technologies Announces Record Sales for Second Quarter of 2006
 FOR IMMEDIATE RELEASE Zebra Technologies Announces Record Sales for Second Quarter of 2006 Vernon Hills, IL, July 26, 2006 Zebra Technologies Corporation (NASDAQ: ZBRA) today announced that net income
FOR IMMEDIATE RELEASE Zebra Technologies Announces Record Sales for Second Quarter of 2006 Vernon Hills, IL, July 26, 2006 Zebra Technologies Corporation (NASDAQ: ZBRA) today announced that net income
China Pharma Holdings, Inc. Reports Second Quarter 2010 Financial Results
 China Pharma Holdings, Inc. Reports Second Quarter 2010 Financial Results HAIKOU CITY, China, August 10, 2010 China Pharma Holdings, Inc. (NYSE AMEX: CPHI) ( China Pharma or the Company ), a leading fully
China Pharma Holdings, Inc. Reports Second Quarter 2010 Financial Results HAIKOU CITY, China, August 10, 2010 China Pharma Holdings, Inc. (NYSE AMEX: CPHI) ( China Pharma or the Company ), a leading fully
Recombinant protein vaccines produced in insect cells
 Recombinant protein vaccines produced in insect cells Manon M.J. Cox Hong Kong 25JAN2013 Flublok BREAKING NEWS 16JAN2013 FDA approves Flublok for the prevention of influenza in adults 18-49 years old The
Recombinant protein vaccines produced in insect cells Manon M.J. Cox Hong Kong 25JAN2013 Flublok BREAKING NEWS 16JAN2013 FDA approves Flublok for the prevention of influenza in adults 18-49 years old The
AMAZON.COM, INC. CONSOLIDATED STATEMENTS OF CASH FLOWS (in millions)
 CONSOLIDATED STATEMENTS OF CASH FLOWS CASH AND CASH EQUIVALENTS, BEGINNING OF PERIOD $ 8,084 $ 5,269 $ 3,777 OPERATING ACTIVITIES: Net income (loss) 274 (39) 631 Adjustments to reconcile net income (loss)
CONSOLIDATED STATEMENTS OF CASH FLOWS CASH AND CASH EQUIVALENTS, BEGINNING OF PERIOD $ 8,084 $ 5,269 $ 3,777 OPERATING ACTIVITIES: Net income (loss) 274 (39) 631 Adjustments to reconcile net income (loss)
Contact: Don Zerio 5:00 EDT Vice President, Finance, Chief Financial Officer July 21, 2015 (408) 432-1900 NATIONAL DISTRIBUTION
 e News Release Contact: Don Zerio 5:00 EDT Vice President, Finance, Chief Financial Officer July 21, (408) 432-1900 NATIONAL DISTRIBUTION LINEAR TECHNOLOGY REPORTS INCREASES IN REVENUE AND NET INCOME OVER
e News Release Contact: Don Zerio 5:00 EDT Vice President, Finance, Chief Financial Officer July 21, (408) 432-1900 NATIONAL DISTRIBUTION LINEAR TECHNOLOGY REPORTS INCREASES IN REVENUE AND NET INCOME OVER
Half Year 2015 Results
 Half Year 2015 Results Letter to shareholders LifeWatch First Half Highlights Revenue growth of 9.1% to USD 52.5 million Above-market growth of over 12% in core monitoring services resulting in market
Half Year 2015 Results Letter to shareholders LifeWatch First Half Highlights Revenue growth of 9.1% to USD 52.5 million Above-market growth of over 12% in core monitoring services resulting in market
NORWEGIAN CRUISE LINE HOLDINGS LTD. CONSOLIDATED STATEMENTS OF OPERATIONS (unaudited, in thousands, except share and per share data)
 CONSOLIDATED STATEMENTS OF OPERATIONS (unaudited, in thousands, except share and per share data) Revenue Passenger ticket $ 583,923 $ 490,322 $ 1,400,470 $ 1,257,871 Onboard and other 213,962 184,089 569,479
CONSOLIDATED STATEMENTS OF OPERATIONS (unaudited, in thousands, except share and per share data) Revenue Passenger ticket $ 583,923 $ 490,322 $ 1,400,470 $ 1,257,871 Onboard and other 213,962 184,089 569,479
News Release. 3D Systems Reports Second Quarter and Six Months 2014 Financial Results
 News Release 333 Three D Systems Circle Rock Hill, SC 29730 www.3dsystems.com NYSE: DDD Investor Contact: Stacey Witten Email: [email protected] Media Contact: Alyssa Reichental Email: [email protected]
News Release 333 Three D Systems Circle Rock Hill, SC 29730 www.3dsystems.com NYSE: DDD Investor Contact: Stacey Witten Email: [email protected] Media Contact: Alyssa Reichental Email: [email protected]
MEDTRONIC, INC. WORLD WIDE REVENUE (Unaudited)
 WORLD WIDE REVENUE ($ millions) 1 2 3 4 1 2 3 4 FY11 FY11 FY11 FY11 FY11 FY12 FY12 FY12 FY12 FY12 QTR 1 QTR 2 QTR 3 QTR 4 Total QTR 1 QTR 2 QTR 3 QTR 4 Total REPORTED REVENUE : CARDIAC RHYTHM DISEASE MANAGEMENT
WORLD WIDE REVENUE ($ millions) 1 2 3 4 1 2 3 4 FY11 FY11 FY11 FY11 FY11 FY12 FY12 FY12 FY12 FY12 QTR 1 QTR 2 QTR 3 QTR 4 Total QTR 1 QTR 2 QTR 3 QTR 4 Total REPORTED REVENUE : CARDIAC RHYTHM DISEASE MANAGEMENT
FOR IMMEDIATE RELEASE
 FOR IMMEDIATE RELEASE March 19, 2014 NEWS NYSE MKT: GORO GOLD RESOURCE CORPORATION REPORTS $16.2M NET INCOME AND RECORD ANNUAL METAL PRODUCTION FOR 2014; PROVIDES 2015 PRODUCTION OUTLOOK COLORADO SPRINGS
FOR IMMEDIATE RELEASE March 19, 2014 NEWS NYSE MKT: GORO GOLD RESOURCE CORPORATION REPORTS $16.2M NET INCOME AND RECORD ANNUAL METAL PRODUCTION FOR 2014; PROVIDES 2015 PRODUCTION OUTLOOK COLORADO SPRINGS
NETFLIX REPORTS PRO-FORMA NET INCOME ON RECORD 4 th QUARTER 2002 REVENUE
 FOR RELEASE AT 1:02 PM PDT IR CONTACT: Barry McCarthy Wednesday, January 15, 2003 CFO 408 399-3740 PR CONTACT: Lynn Brinton Director of Corporate Communications 408 399-3726 NETFLIX REPORTS PRO-FORMA NET
FOR RELEASE AT 1:02 PM PDT IR CONTACT: Barry McCarthy Wednesday, January 15, 2003 CFO 408 399-3740 PR CONTACT: Lynn Brinton Director of Corporate Communications 408 399-3726 NETFLIX REPORTS PRO-FORMA NET
Thomas A. Bessant, Jr. (817) 335-1100
 Additional Information: Thomas A. Bessant, Jr. (817) 335-1100 For Immediate Release ********************************************************************************** CASH AMERICA FIRST QUARTER NET INCOME
Additional Information: Thomas A. Bessant, Jr. (817) 335-1100 For Immediate Release ********************************************************************************** CASH AMERICA FIRST QUARTER NET INCOME
Cytec Announces First Quarter 2010 Results. As-Adjusted EPS of $0.66, Significantly Above Prior Year As-Adjusted EPS of $0.06
 cytec News & Information Cytec Industries Inc. Five Garret Mountain Plaza Woodland Park, New Jersey 07424 www.cytec.com Contact: Jodi Allen (Investor Relations) (973) 357-3283 Release Date: Immediate Cytec
cytec News & Information Cytec Industries Inc. Five Garret Mountain Plaza Woodland Park, New Jersey 07424 www.cytec.com Contact: Jodi Allen (Investor Relations) (973) 357-3283 Release Date: Immediate Cytec
FAQs on Influenza A (H1N1-2009) Vaccine
 FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
FDA Update on the H1N1 Flu Vaccine and Antiviral Medications
 FDA Update on the H1N1 Flu Vaccine and Antiviral Medications Beth Fabian Fritsch, R.Ph., M.B.A. Commander, U.S. Public Health Service Health Programs Coordinator Office of Special Health Issues Food and
FDA Update on the H1N1 Flu Vaccine and Antiviral Medications Beth Fabian Fritsch, R.Ph., M.B.A. Commander, U.S. Public Health Service Health Programs Coordinator Office of Special Health Issues Food and
Atthapol Charoenkietkrai ACG2021 Section 2
 Atthapol Charoenkietkrai ACG2021 Section 2 Amazon has been in a good position for the last few years and still on a rise. The assets have increased, and the debt has decreased. The company has exponentially
Atthapol Charoenkietkrai ACG2021 Section 2 Amazon has been in a good position for the last few years and still on a rise. The assets have increased, and the debt has decreased. The company has exponentially
Consolidated Balance Sheets
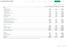 Consolidated Balance Sheets March 31 2015 2014 2015 Assets: Current assets Cash and cash equivalents 726,888 604,571 $ 6,057,400 Marketable securities 19,033 16,635 158,608 Notes and accounts receivable:
Consolidated Balance Sheets March 31 2015 2014 2015 Assets: Current assets Cash and cash equivalents 726,888 604,571 $ 6,057,400 Marketable securities 19,033 16,635 158,608 Notes and accounts receivable:
CARDIOME PHARMA CORP.
 Consolidated Financial Statements (Expressed in thousands of United States (U.S.) dollars) (Prepared in accordance with generally accepted accounting principles used in the United States of America (U.S.
Consolidated Financial Statements (Expressed in thousands of United States (U.S.) dollars) (Prepared in accordance with generally accepted accounting principles used in the United States of America (U.S.
MGC Diagnostics Corporation Reports Fiscal Year 2015 Financial Results
 MGC Diagnostics Corporation 350 Oak Grove Parkway Saint Paul, MN 55127 Telephone: (651) 484-4874 Facsimile: (651) 484-4826 FOR IMMEDIATE RELEASE MGC Diagnostics Corporation Reports Fiscal Year 2015 Financial
MGC Diagnostics Corporation 350 Oak Grove Parkway Saint Paul, MN 55127 Telephone: (651) 484-4874 Facsimile: (651) 484-4826 FOR IMMEDIATE RELEASE MGC Diagnostics Corporation Reports Fiscal Year 2015 Financial
2-8. Identify whether each of the following items increases or decreases cash flow:
 Problems 2-8. Identify whether each of the following items increases or decreases cash flow: Increase in accounts receivable Increase in notes payable Depreciation expense Increase in investments Decrease
Problems 2-8. Identify whether each of the following items increases or decreases cash flow: Increase in accounts receivable Increase in notes payable Depreciation expense Increase in investments Decrease
Key Facts about Influenza (Flu) & Flu Vaccine
 Key Facts about Influenza (Flu) & Flu Vaccine mouths or noses of people who are nearby. Less often, a person might also get flu by touching a surface or object that has flu virus on it and then touching
Key Facts about Influenza (Flu) & Flu Vaccine mouths or noses of people who are nearby. Less often, a person might also get flu by touching a surface or object that has flu virus on it and then touching
Cynk Technology Corp. (A Development Stage Company) (formerly Introbuzz) Balance Sheets
 Cynk Technology Corp. (A Development Stage Company) (formerly Introbuzz) Balance Sheets ASSETS March 31, December 2014 31, 2013 ------- --------- Current Assets Cash and cash equivalents $ 39 $ 39 --------
Cynk Technology Corp. (A Development Stage Company) (formerly Introbuzz) Balance Sheets ASSETS March 31, December 2014 31, 2013 ------- --------- Current Assets Cash and cash equivalents $ 39 $ 39 --------
Influenza Vaccine Frequently Asked Questions. Influenza Control Program
 Influenza Vaccine Frequently Asked Questions Influenza Control Program Influenza or the flu can be a serious contagious disease, which is spread by droplet transmission through close contact with an infected
Influenza Vaccine Frequently Asked Questions Influenza Control Program Influenza or the flu can be a serious contagious disease, which is spread by droplet transmission through close contact with an infected
About TurboChef. Forward-Looking Statements
 TurboChef Reports Results for Second Quarter 2006 Cites Strong Momentum in Commercial Oven Business, Sees Increasing Revenues in Q3 and Q4; Significant Progress Cited Regarding Residential Oven Atlanta,
TurboChef Reports Results for Second Quarter 2006 Cites Strong Momentum in Commercial Oven Business, Sees Increasing Revenues in Q3 and Q4; Significant Progress Cited Regarding Residential Oven Atlanta,
Walmart reports Q3 FY 16 EPS of $1.03, Walmart U.S. added $2.7 billion in sales, comp sales of 1.5%
 Walmart reports Q3 FY 6 EPS of.03, Walmart U.S. added 2.7 billion in sales, comp sales of.5% Q3 diluted EPS from continuing operations was.03, benefited by approximately 0.04 from an adjustment for certain
Walmart reports Q3 FY 6 EPS of.03, Walmart U.S. added 2.7 billion in sales, comp sales of.5% Q3 diluted EPS from continuing operations was.03, benefited by approximately 0.04 from an adjustment for certain
ACXIOM ANNOUNCES FIRST QUARTER RESULTS. Audience Operating System to Launch September 24 at AdWeek
 For more information, contact: Katharine Boyce Acxiom Investor Relations 501-342-1321 [email protected] EACXM ACXIOM ANNOUNCES FIRST QUARTER RESULTS Audience Operating System to Launch September
For more information, contact: Katharine Boyce Acxiom Investor Relations 501-342-1321 [email protected] EACXM ACXIOM ANNOUNCES FIRST QUARTER RESULTS Audience Operating System to Launch September
Great Basin Reports 2015 Second Quarter Results and Business Update
 Great Basin Reports 2015 Second Quarter Results and Business Update Company Reports 122 Revenue-Generating Customers, Reaffirms Guidance of 170-180 Customers by Year End SALT LAKE CITY, August 12, 2015
Great Basin Reports 2015 Second Quarter Results and Business Update Company Reports 122 Revenue-Generating Customers, Reaffirms Guidance of 170-180 Customers by Year End SALT LAKE CITY, August 12, 2015
Boss Holdings, Inc. 2015 Fiscal Year End Report
 Boss Holdings, Inc. 1221 Page Street Kewanee, IL 61443 PH: 800.447.4581 F: 309.852.0848 2015 Fiscal Year End Report Consolidated revenues of Boss Holdings, Inc. (the Company ) for the fourth quarter of
Boss Holdings, Inc. 1221 Page Street Kewanee, IL 61443 PH: 800.447.4581 F: 309.852.0848 2015 Fiscal Year End Report Consolidated revenues of Boss Holdings, Inc. (the Company ) for the fourth quarter of
Westmoreland Reports First Quarter 2016 Results and Affirms Full-year Guidance
 News Release Westmoreland Reports First Quarter 2016 Results and Affirms Full-year Guidance Englewood, CO May 10, 2016 - Westmoreland Coal Company (NasdaqGM:WLB) today reported financial results for the
News Release Westmoreland Reports First Quarter 2016 Results and Affirms Full-year Guidance Englewood, CO May 10, 2016 - Westmoreland Coal Company (NasdaqGM:WLB) today reported financial results for the
BlackBerry Reports 2015 Fiscal First Quarter GAAP Profitability
 NEWS RELEASE FOR IMMEDIATE RELEASE June 19, BlackBerry Reports 2015 Fiscal First Quarter GAAP Profitability Waterloo, ON BlackBerry Limited (NASDAQ: BBRY; TSX: BB), a global leader in mobile communications,
NEWS RELEASE FOR IMMEDIATE RELEASE June 19, BlackBerry Reports 2015 Fiscal First Quarter GAAP Profitability Waterloo, ON BlackBerry Limited (NASDAQ: BBRY; TSX: BB), a global leader in mobile communications,
Performance Food Group Company Reports First-Quarter Fiscal 2016 Earnings
 NEWS RELEASE For Immediate Release November 4, 2015 Investors: Michael D. Neese VP, Investor Relations (804) 287-8126 [email protected] Media: Joe Vagi Manager, Corporate Communications (804) 484-7737
NEWS RELEASE For Immediate Release November 4, 2015 Investors: Michael D. Neese VP, Investor Relations (804) 287-8126 [email protected] Media: Joe Vagi Manager, Corporate Communications (804) 484-7737
XENOPORT INC FORM 8-K. (Current report filing) Filed 05/08/14 for the Period Ending 05/08/14
 XENOPORT INC FORM 8-K (Current report filing) Filed 05/08/14 for the Period Ending 05/08/14 Address 3410 CENTRAL EXPRESSWAY SANTA CLARA, CA 95051 Telephone 4086167200 CIK 0001130591 Symbol XNPT SIC Code
XENOPORT INC FORM 8-K (Current report filing) Filed 05/08/14 for the Period Ending 05/08/14 Address 3410 CENTRAL EXPRESSWAY SANTA CLARA, CA 95051 Telephone 4086167200 CIK 0001130591 Symbol XNPT SIC Code
Statement Of. The National Association of Chain Drug Stores. For. U.S. Senate Committee On Armed Services. Personnel Subcommittee.
 Statement Of The National Association of Chain Drug Stores For U.S. Senate Committee On Armed Services Personnel Subcommittee Hearing on FY2014 Budget April 17, 2012 2:00p.m. Russell Senate Office Building
Statement Of The National Association of Chain Drug Stores For U.S. Senate Committee On Armed Services Personnel Subcommittee Hearing on FY2014 Budget April 17, 2012 2:00p.m. Russell Senate Office Building
Numerex Reports First Quarter 2015 Financial Results
 May 11, 2015 Numerex Reports First Quarter 2015 Financial Results ATLANTA, May 11, 2015 (GLOBE NEWSWIRE) -- Numerex Corp (Nasdaq:NMRX), a leading provider of on-demand and interactive machine-to-machine
May 11, 2015 Numerex Reports First Quarter 2015 Financial Results ATLANTA, May 11, 2015 (GLOBE NEWSWIRE) -- Numerex Corp (Nasdaq:NMRX), a leading provider of on-demand and interactive machine-to-machine
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of earliest event
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of earliest event
Capmark Financial Group Inc. Announces Stand Alone Third Quarter 2014 Earnings Results for its Wholly Owned Subsidiary, Bluestem Brands, Inc.
 Capmark Financial Group Inc. Announces Stand Alone Third Quarter 2014 Earnings Results for its Wholly Owned Subsidiary, Bluestem Brands, Inc. Horsham, PA December 23, 2014 Capmark Financial Group Inc.
Capmark Financial Group Inc. Announces Stand Alone Third Quarter 2014 Earnings Results for its Wholly Owned Subsidiary, Bluestem Brands, Inc. Horsham, PA December 23, 2014 Capmark Financial Group Inc.
SYNOPSYS POSTS FINANCIAL RESULTS FOR FOURTH QUARTER AND FISCAL YEAR 2007
 PRESS RELEASE INVESTOR CONTACT: Lisa L. Ewbank Synopsys, Inc. 650-584-1901 EDITORIAL CONTACT: Yvette Huygen Synopsys, Inc. 650-584-4547 [email protected] SYNOPSYS POSTS FINANCIAL RESULTS FOR FOURTH
PRESS RELEASE INVESTOR CONTACT: Lisa L. Ewbank Synopsys, Inc. 650-584-1901 EDITORIAL CONTACT: Yvette Huygen Synopsys, Inc. 650-584-4547 [email protected] SYNOPSYS POSTS FINANCIAL RESULTS FOR FOURTH
Sample: Policy for the Administration of Influenza Vaccine Employees in Health Care Settings
 Sample: Policy for the Administration of Influenza Vaccine Employees in Health Care Settings 1.0 PURPOSE The purpose of this policy is to minimize transmission of the influenza virus in the workplace by
Sample: Policy for the Administration of Influenza Vaccine Employees in Health Care Settings 1.0 PURPOSE The purpose of this policy is to minimize transmission of the influenza virus in the workplace by
VAALCO ENERGY ANNOUNCES FIRST QUARTER 2015 RESULTS
 VAALCO ENERGY ANNOUNCES FIRST QUARTER 2015 RESULTS HOUSTON MAY 7, 2015 VAALCO Energy, Inc. (NYSE: EGY) today reported results for the first quarter of 2015. First Quarter 2015 highlights: Successfully
VAALCO ENERGY ANNOUNCES FIRST QUARTER 2015 RESULTS HOUSTON MAY 7, 2015 VAALCO Energy, Inc. (NYSE: EGY) today reported results for the first quarter of 2015. First Quarter 2015 highlights: Successfully
ACCESS TO THE SEASONAL FLU VACCINE IN CANADA. How the flu shot makes its way from the laboratory to the doctor s office.
 ACCESS TO THE SEASONAL FLU VACCINE IN CANADA How the flu shot makes its way from the laboratory to the doctor s office. Health Canada is the federal department responsible for helping Canadians maintain
ACCESS TO THE SEASONAL FLU VACCINE IN CANADA How the flu shot makes its way from the laboratory to the doctor s office. Health Canada is the federal department responsible for helping Canadians maintain
LightPath Technologies Reports 61% Revenue Increase with Fiscal 2016 First Quarter Financial Results
 For Immediate Release LightPath Technologies Reports 61% Revenue Increase with Fiscal 2016 First Quarter Financial Results Continued Momentum for Global Sales of Specialty and Infrared Products ORLANDO,
For Immediate Release LightPath Technologies Reports 61% Revenue Increase with Fiscal 2016 First Quarter Financial Results Continued Momentum for Global Sales of Specialty and Infrared Products ORLANDO,
Inventory Management Tracking, Distribution and Delivery
 Inventory Management Tracking, Distribution and Delivery Ben Erickson Public Health Analyst Office of Public Health Preparedness and Response Division of Strategic National Stockpile Agenda Inventory Management
Inventory Management Tracking, Distribution and Delivery Ben Erickson Public Health Analyst Office of Public Health Preparedness and Response Division of Strategic National Stockpile Agenda Inventory Management
Garmin Reports Best Quarter and Best Year in Company History, Announces Share Repurchase Program, Offers 2008 Guidance
 INVESTOR CONTACT: Polly Schwerdt Phone 913/397-8200 E-Mail [email protected] MEDIA CONTACT: Ted Gartner Phone 913/397-8200 E-Mail [email protected] Garmin Reports Best Quarter and
INVESTOR CONTACT: Polly Schwerdt Phone 913/397-8200 E-Mail [email protected] MEDIA CONTACT: Ted Gartner Phone 913/397-8200 E-Mail [email protected] Garmin Reports Best Quarter and
APPLE INC FORM 8-K. (Current report filing) Filed 10/27/15 for the Period Ending 10/27/15
 APPLE INC FORM 8-K (Current report filing) Filed 10/27/15 for the Period Ending 10/27/15 Address ONE INFINITE LOOP CUPERTINO, CA 95014 Telephone (408) 996-1010 CIK 0000320193 Symbol AAPL SIC Code 3571
APPLE INC FORM 8-K (Current report filing) Filed 10/27/15 for the Period Ending 10/27/15 Address ONE INFINITE LOOP CUPERTINO, CA 95014 Telephone (408) 996-1010 CIK 0000320193 Symbol AAPL SIC Code 3571
Village Farms International Announces Year End 2014 Results and Adoption of Advance Notice By-Law
 March 18, 2015 TRADING SYMBOL: The Toronto Stock Exchange: Village Farms International, Inc. VFF Village Farms International Announces Year End 2014 Results and Adoption of Advance Notice By-Law Vancouver,
March 18, 2015 TRADING SYMBOL: The Toronto Stock Exchange: Village Farms International, Inc. VFF Village Farms International Announces Year End 2014 Results and Adoption of Advance Notice By-Law Vancouver,
FREEDOM INVESTMENTS, INC. STATEMENT OF FINANCIAL CONDITION AS OF JUNE 30, 2015 (UNAUDITED)
 FREEDOM INVESTMENTS, INC. STATEMENT OF FINANCIAL CONDITION AS OF JUNE 30, 2015 (UNAUDITED) ****** Index Page(s) Statement of Financial Condition. 2 Notes to Statement of Financial Condition. 3-5 Statement
FREEDOM INVESTMENTS, INC. STATEMENT OF FINANCIAL CONDITION AS OF JUNE 30, 2015 (UNAUDITED) ****** Index Page(s) Statement of Financial Condition. 2 Notes to Statement of Financial Condition. 3-5 Statement
EDWARDS LIFESCIENCES REPORTS STRONG FIRST QUARTER RESULTS DRIVEN BY SALES GROWTH OF 18.8 PERCENT
 Edwards Lifesciences Corporation One Edwards Way Irvine, CA USA 92614 Phone: 949.250.2500 Fax: 949.250.2525 www.edwards.com FOR IMMEDIATE RELEASE Media Contact: Amanda C. Fowler, 949-250-5070 Investor
Edwards Lifesciences Corporation One Edwards Way Irvine, CA USA 92614 Phone: 949.250.2500 Fax: 949.250.2525 www.edwards.com FOR IMMEDIATE RELEASE Media Contact: Amanda C. Fowler, 949-250-5070 Investor
China Distance Education Holdings Limited Reports First Quarter Fiscal Year 2016 Financial Results
 China Distance Education Holdings Limited Reports First Quarter Fiscal Year 2016 Financial Results - Revenue Up 13.5% Year-Over-Year to $24.4 Million, Exceeding Guidance - Cash Receipts From Online Course
China Distance Education Holdings Limited Reports First Quarter Fiscal Year 2016 Financial Results - Revenue Up 13.5% Year-Over-Year to $24.4 Million, Exceeding Guidance - Cash Receipts From Online Course
WILLIAMS-SONOMA, INC.
 WILLIAMS-SONOMA, INC. 3250 Van Ness Avenue San Francisco, CA 94109 CONT: Julie P. Whalen EVP, Chief Financial Officer (415) 616-8524 Gabrielle L. Rabinovitch Vice President, Investor Relations (415) 616-7727
WILLIAMS-SONOMA, INC. 3250 Van Ness Avenue San Francisco, CA 94109 CONT: Julie P. Whalen EVP, Chief Financial Officer (415) 616-8524 Gabrielle L. Rabinovitch Vice President, Investor Relations (415) 616-7727
China Clean Energy Announces Third Quarter 2011 Financial Results
 China Clean Energy Inc. ccontact: China Clean Energy Inc. William Chen, CFO Email: [email protected] Website: http://www.chinacleanenergyinc.com CCG Investor Relations Inc. David Rudnick,
China Clean Energy Inc. ccontact: China Clean Energy Inc. William Chen, CFO Email: [email protected] Website: http://www.chinacleanenergyinc.com CCG Investor Relations Inc. David Rudnick,
BlackBerry Reports Strong Software Revenue and Positive Cash Flow for the Fiscal 2016 First Quarter
 FOR IMMEDIATE RELEASE June 23, BlackBerry Reports Strong Software Revenue and Positive Cash Flow for the Fiscal 2016 First Quarter Waterloo, ON BlackBerry Limited (NASDAQ: BBRY; TSX: BB), a global leader
FOR IMMEDIATE RELEASE June 23, BlackBerry Reports Strong Software Revenue and Positive Cash Flow for the Fiscal 2016 First Quarter Waterloo, ON BlackBerry Limited (NASDAQ: BBRY; TSX: BB), a global leader
PAYCHEX, INC. REPORTS FOURTH QUARTER AND FISCAL 2015 RESULTS
 PAYCHEX, INC. REPORTS FOURTH QUARTER AND FISCAL 2015 RESULTS July 1, 2015 FOURTH QUARTER AND FULL YEAR FISCAL 2015 HIGHLIGHTS Total service revenue increased 8% to $681.4 million for the fourth quarter;
PAYCHEX, INC. REPORTS FOURTH QUARTER AND FISCAL 2015 RESULTS July 1, 2015 FOURTH QUARTER AND FULL YEAR FISCAL 2015 HIGHLIGHTS Total service revenue increased 8% to $681.4 million for the fourth quarter;
LANCASTER COLONY REPORTS SECOND QUARTER SALES AND EARNINGS
 FOR IMMEDIATE RELEASE January 28, 2016 SYMBOL: LANC TRADED: Nasdaq LANCASTER COLONY REPORTS SECOND QUARTER SALES AND EARNINGS COLUMBUS, Ohio, January 28 - Lancaster Colony Corporation (Nasdaq: LANC) today
FOR IMMEDIATE RELEASE January 28, 2016 SYMBOL: LANC TRADED: Nasdaq LANCASTER COLONY REPORTS SECOND QUARTER SALES AND EARNINGS COLUMBUS, Ohio, January 28 - Lancaster Colony Corporation (Nasdaq: LANC) today
Consolidated Financial Statements. FUJIFILM Holdings Corporation and Subsidiaries. March 31, 2015 with Report of Independent Auditors
 Consolidated Financial Statements FUJIFILM Holdings Corporation and Subsidiaries March 31, 2015 with Report of Independent Auditors Consolidated Financial Statements March 31, 2015 Contents Report of Independent
Consolidated Financial Statements FUJIFILM Holdings Corporation and Subsidiaries March 31, 2015 with Report of Independent Auditors Consolidated Financial Statements March 31, 2015 Contents Report of Independent
From Research Services and Process Development to GMP Manufacturing
 From Research Services and Process Development to GMP Manufacturing P a r ag o n B i o s e r v i c e s, I n c. A contract research and GMP manufacturing organization (CMO) with a focus on the development
From Research Services and Process Development to GMP Manufacturing P a r ag o n B i o s e r v i c e s, I n c. A contract research and GMP manufacturing organization (CMO) with a focus on the development
Walmart reports Q1 FY 16 EPS of $1.03
 Walmart reports Q FY 6 EPS of.03 Q diluted EPS from continuing operations was.03, within guidance of 0.95 to.0. Currency negatively impacted EPS by approximately 0.03. Walmart U.S..% comp includes positive
Walmart reports Q FY 6 EPS of.03 Q diluted EPS from continuing operations was.03, within guidance of 0.95 to.0. Currency negatively impacted EPS by approximately 0.03. Walmart U.S..% comp includes positive
Diodes Incorporated Reports Record Fourth Quarter and Full Year 2005 Results
 FOR IMMEDIATE RELEASE Diodes Incorporated Reports Record Fourth Quarter and Full Year 2005 Results Annual revenues up 15.6% to a record $214.8 million Annual net income increases 30.4% to a record $33.3
FOR IMMEDIATE RELEASE Diodes Incorporated Reports Record Fourth Quarter and Full Year 2005 Results Annual revenues up 15.6% to a record $214.8 million Annual net income increases 30.4% to a record $33.3
TransUnion Reports Third Quarter 2014 Results
 TransUnion Reports Third Quarter 2014 Results Revenue of $338 million, an increase of 13 percent on a GAAP basis (14 percent on a constant currency basis) compared with the third quarter of 2013 Adjusted
TransUnion Reports Third Quarter 2014 Results Revenue of $338 million, an increase of 13 percent on a GAAP basis (14 percent on a constant currency basis) compared with the third quarter of 2013 Adjusted
Burlington Stores, Inc. Announces Operating Results for the Fourth Quarter and Fiscal Year Ended February 1, 2014
 FOR IMMEDIATE RELEASE Burlington Stores, Inc. Announces Operating Results for the Fourth Quarter and Fiscal Year Ended February 1, 2014 Comparable store sales increased 4.0% and 4.7%, for the fourth quarter
FOR IMMEDIATE RELEASE Burlington Stores, Inc. Announces Operating Results for the Fourth Quarter and Fiscal Year Ended February 1, 2014 Comparable store sales increased 4.0% and 4.7%, for the fourth quarter
WAL-MART STORES, INC. 800-331-0085 www.walmartstores.com/news/
 WAL-MART STORES, INC. 800-331-0085 www.walmartstores.com/news/ FOR IMMEDIATE RELEASE Investor Relations Contacts Investor Relations 479-273-8446 Carol Schumacher 479-277-1498 Pauline Tureman 479-277-9558
WAL-MART STORES, INC. 800-331-0085 www.walmartstores.com/news/ FOR IMMEDIATE RELEASE Investor Relations Contacts Investor Relations 479-273-8446 Carol Schumacher 479-277-1498 Pauline Tureman 479-277-9558
HubSpot's Momentum Accelerates in Q4 2014 with 53% Revenue Growth and 35% Customer Growth
 HubSpot's Momentum Accelerates in Q4 2014 with 53% Revenue Growth and 35% Customer Growth CAMBRIDGE, MA (February 11, 2015) HubSpot, Inc. (NYSE: HUBS), a leading inbound marketing and sales software company,
HubSpot's Momentum Accelerates in Q4 2014 with 53% Revenue Growth and 35% Customer Growth CAMBRIDGE, MA (February 11, 2015) HubSpot, Inc. (NYSE: HUBS), a leading inbound marketing and sales software company,
Enclosed is a press release announcing the 2015 second quarter results for:
 Enclosed is a press release announcing the 2015 second quarter results for: A conference call to discuss these results is scheduled for tomorrow, Wednesday, August 5, 2015, at 11:00 a.m. (Eastern Time)
Enclosed is a press release announcing the 2015 second quarter results for: A conference call to discuss these results is scheduled for tomorrow, Wednesday, August 5, 2015, at 11:00 a.m. (Eastern Time)
Almost Family Reports First Quarter 2016 Results
 Exhibit 99.1 Almost Family, Inc. Steve Guenthner (502) 891-1000 FOR IMMEDIATE RELEASE Almost Family Reports First Quarter 2016 Results Louisville, KY, Almost Family, Inc. (Nasdaq: AFAM), a leading regional
Exhibit 99.1 Almost Family, Inc. Steve Guenthner (502) 891-1000 FOR IMMEDIATE RELEASE Almost Family Reports First Quarter 2016 Results Louisville, KY, Almost Family, Inc. (Nasdaq: AFAM), a leading regional
H1 2015 Audio webcast RESULTS PRESENTATION. July 30, 2015
 H1 2015 Audio webcast RESULTS PRESENTATION July 30, 2015 DISCLOSURE This presentation contains no confidential material and may include publicly available market information which has not been independently
H1 2015 Audio webcast RESULTS PRESENTATION July 30, 2015 DISCLOSURE This presentation contains no confidential material and may include publicly available market information which has not been independently
WEYCO REPORTS THIRD QUARTER SALES AND EARNINGS
 WEYCO REPORTS THIRD QUARTER SALES AND EARNINGS (Milwaukee, Wisconsin---November 4, 2014) Weyco Group, Inc. (NASDAQ:WEYS) (the Company ) today announced financial results for the quarter ended September
WEYCO REPORTS THIRD QUARTER SALES AND EARNINGS (Milwaukee, Wisconsin---November 4, 2014) Weyco Group, Inc. (NASDAQ:WEYS) (the Company ) today announced financial results for the quarter ended September
PANHANDLE OIL AND GAS INC. REPORTS SECOND QUARTER AND SIX MONTHS 2009 RESULTS. Second Quarter Production Increases 38%
 FOR IMMEDIATE RELEASE PLEASE CONTACT: Michael C. Coffman 405.948.1560 Website: www.panhandleoilandgas.com May 8, 2009 PANHANDLE OIL AND GAS INC. REPORTS SECOND QUARTER AND SIX MONTHS 2009 RESULTS Second
FOR IMMEDIATE RELEASE PLEASE CONTACT: Michael C. Coffman 405.948.1560 Website: www.panhandleoilandgas.com May 8, 2009 PANHANDLE OIL AND GAS INC. REPORTS SECOND QUARTER AND SIX MONTHS 2009 RESULTS Second
BE SURE. BE SAFE. VACCINATE.
 DON T GET OR GIVE THE FLU THIS YEAR THANK YOU Vaccination is the only protection. www.immunisation.ie BE SURE. BE SAFE. VACCINATE. FLU VACCINE 2013-2014 Healthcare workers prevent the spread of flu and
DON T GET OR GIVE THE FLU THIS YEAR THANK YOU Vaccination is the only protection. www.immunisation.ie BE SURE. BE SAFE. VACCINATE. FLU VACCINE 2013-2014 Healthcare workers prevent the spread of flu and
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-Q
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-Q (Mark One) QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-Q (Mark One) QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period
3Q 2012 EARNINGS For Immediate Release
 3Q 2012 EARNINGS For Immediate Release Contact: Angela Skinner NIC Inc. 913-754-7054 [email protected] NIC Grows Total Revenues 14 Percent in the Third Quarter; Declares Special Cash Dividend of $0.25
3Q 2012 EARNINGS For Immediate Release Contact: Angela Skinner NIC Inc. 913-754-7054 [email protected] NIC Grows Total Revenues 14 Percent in the Third Quarter; Declares Special Cash Dividend of $0.25
NOTICE OF NO AUDITOR REVIEW OF INTERIM FINANCIAL STATEMENTS
 Condensed Interim Consolidated Financial Statements of THE BRICK LTD. For the three months ended March 31, 2013 NOTICE OF NO AUDITOR REVIEW OF INTERIM FINANCIAL STATEMENTS Under National Instrument 51-102,
Condensed Interim Consolidated Financial Statements of THE BRICK LTD. For the three months ended March 31, 2013 NOTICE OF NO AUDITOR REVIEW OF INTERIM FINANCIAL STATEMENTS Under National Instrument 51-102,
FINANCIAL SUMMARY. (All financial information has been prepared in accordance with U.S. generally accepted accounting principles)
 FINANCIAL SUMMARY FY2015 First Quarter (April 1, 2014 through June 30, 2014) English translation from the original Japanese-language document TOYOTA MOTOR CORPORATION FY2015 First Quarter Consolidated
FINANCIAL SUMMARY FY2015 First Quarter (April 1, 2014 through June 30, 2014) English translation from the original Japanese-language document TOYOTA MOTOR CORPORATION FY2015 First Quarter Consolidated
Kansas City 1Life Insurance Company
 Kansas City 1Life Insurance Company 2011 First Quarter Report Includes our subsidiaries: Sunset Life Insurance Company of America Old American Insurance Company Sunset Financial Services, Inc. Post Office
Kansas City 1Life Insurance Company 2011 First Quarter Report Includes our subsidiaries: Sunset Life Insurance Company of America Old American Insurance Company Sunset Financial Services, Inc. Post Office
HOPKINTON, Mass., April 24, 2013 - HIGHLIGHTS:
 HOPKINTON, Mass., April 24, 2013 - HIGHLIGHTS: Record first-quarter revenue, non-gaap net income, non-gaap EPS, operating cash flow and free cash flow Year-over-year revenue growth across U.S. and major
HOPKINTON, Mass., April 24, 2013 - HIGHLIGHTS: Record first-quarter revenue, non-gaap net income, non-gaap EPS, operating cash flow and free cash flow Year-over-year revenue growth across U.S. and major
Vaccine Production Strategies: Ensuring Alignment and Sustainability
 Vaccine Production Strategies: Ensuring Alignment and Sustainability Second WHO Consultation on Global Action Plan for Influenza Vaccines (GAP-II) Robert W Malone, MD, MS Consulting, Vaccines and Biologicals
Vaccine Production Strategies: Ensuring Alignment and Sustainability Second WHO Consultation on Global Action Plan for Influenza Vaccines (GAP-II) Robert W Malone, MD, MS Consulting, Vaccines and Biologicals
Unaudited Interim Consolidated Financial Statements and Footnotes July 3, 2011
 Unaudited Interim Consolidated Financial Statements and Footnotes July 3, 2011 1 MEXICAN RESTAURANTS, INC. AND SUBSIDIARIES CONSOLIDATED BALANCE SHEETS (unaudited) 7/3/2011 1/2/2011 ASSETS Current assets:
Unaudited Interim Consolidated Financial Statements and Footnotes July 3, 2011 1 MEXICAN RESTAURANTS, INC. AND SUBSIDIARIES CONSOLIDATED BALANCE SHEETS (unaudited) 7/3/2011 1/2/2011 ASSETS Current assets:
H1N1 Flu Vaccine Available to All Virginia Beach City Public Schools Students
 V i r g i n i a B e a c h C i t y P u b l i c S c h o o l s apple-a-day F o r O u r F a m i l y o f I n t e r e s t e d C i t i z e n s Special Edition H1N1 Flu Vaccine Available to All Virginia Beach
V i r g i n i a B e a c h C i t y P u b l i c S c h o o l s apple-a-day F o r O u r F a m i l y o f I n t e r e s t e d C i t i z e n s Special Edition H1N1 Flu Vaccine Available to All Virginia Beach
Audit Report of Independent Certified Public Accountants
 Audit Report of Independent Certified Public Accountants The Board of Directors Acer Incorporated: We have audited the non-consolidated balance sheets of Acer Incorporated (the Company ) as of June 30,
Audit Report of Independent Certified Public Accountants The Board of Directors Acer Incorporated: We have audited the non-consolidated balance sheets of Acer Incorporated (the Company ) as of June 30,
Financial Statements and Report of Independent Certified Public Accountants. The California Wellness Foundation. December 31, 2014 and 2013
 Financial Statements and Report of Independent Certified Public Accountants The California Wellness Foundation December 31, 2014 and 2013 Contents Page Report of Independent Certified Public Accountants
Financial Statements and Report of Independent Certified Public Accountants The California Wellness Foundation December 31, 2014 and 2013 Contents Page Report of Independent Certified Public Accountants
FLEET MANAGEMENT SOLUTIONS INC.
 FLEET MANAGEMENT SOLUTIONS INC. (Formerly: Silverton Mining Corp.) CONSOLIDATED FINANCIAL STATEMENTS (unaudited prepared by management) March 31, 2013 (Expressed in US Dollars) 1 FLEET MANAGEMENT SOLUTIONS
FLEET MANAGEMENT SOLUTIONS INC. (Formerly: Silverton Mining Corp.) CONSOLIDATED FINANCIAL STATEMENTS (unaudited prepared by management) March 31, 2013 (Expressed in US Dollars) 1 FLEET MANAGEMENT SOLUTIONS
rv.wbn.rec.020 Recording entries using company disclosures, determining their financial-statement effects and analyzing related risks (HP)
 Revenue and Related Balance Sheet Concepts» What s Behind the numbers» Receivables» Exercises www.navigatingaccounting.com EXERCISES rv.wbn.rec.020 Recording entries using company disclosures, determining
Revenue and Related Balance Sheet Concepts» What s Behind the numbers» Receivables» Exercises www.navigatingaccounting.com EXERCISES rv.wbn.rec.020 Recording entries using company disclosures, determining
Theravance Biopharma, Inc. Reports Second Quarter 2015 Financial Results and Provides Business Update
 August 10, 2015 Theravance Biopharma, Inc. Reports Second Quarter 2015 Financial Results and Provides Business Update DUBLIN, IRELAND -- (Marketwired) -- 08/10/15 -- Theravance Biopharma, Inc. (NASDAQ:
August 10, 2015 Theravance Biopharma, Inc. Reports Second Quarter 2015 Financial Results and Provides Business Update DUBLIN, IRELAND -- (Marketwired) -- 08/10/15 -- Theravance Biopharma, Inc. (NASDAQ:
FOR INFORMATION CONTACT:
 NEWS RELEASE FOR INFORMATION CONTACT: Caroline Calderone Baisley Deborah C. Travers Director of Health Director of Family Health Tel [203] 622-7836 Tel [203] 622-7854 September 10, 2014 For Immediate Release
NEWS RELEASE FOR INFORMATION CONTACT: Caroline Calderone Baisley Deborah C. Travers Director of Health Director of Family Health Tel [203] 622-7836 Tel [203] 622-7854 September 10, 2014 For Immediate Release
