PACKAGE INSERT TEMPLATE FOR ACETYLSALICYLIC ACID/ASPIRIN TABLET
|
|
|
- Kerry Poole
- 9 years ago
- Views:
Transcription
1 PACKAGE INSERT TEMPLATE FOR ACETYLSALICYLIC ACID/ASPIRIN TABLET Brand or Product Name [Product name] tablet 500mg Name and Strength of Active Substance(s) Acetylsalicylic acid.500mg Product Description [Visual description of the appearance of the product (eg colour etc)] eg White, circular flat beveled edge tablets marked 100 on one side. Pharmacodynamics Acetylsalicylic acid is an analgesic, anti-inflammatory, antipyretic and an inhibitor of platelet aggregation. It prolongs the bleeding time. It inhibits fatty acid cyclo-oxygenase by acetylation of the active site of the enzyme, and most of its pharmacological effects are due to inhibition of the formation of cyclo-oxygenase products including thromboxanes, prostaglandins and prostacyclin. Acetylsalicylic acid has an active metabolite (salicylate) which, in addition to possessing some antiinflammatory properties in its own right, also has important effects on respiration, acid-base balance and the stomach. Salicylates stimulate respiration by a direct effect on the medulla, and at high concentrations, uncouple oxidative phosphorylation in muscle, increasing oxygen consumption and carbon dioxide production. Hyperventilation causes respiratory alkalosis which is compensated by renal excretion of bicarbonate. Pharmacokinetics Absorption In general, immediate Acetylsalicylic acid is well and completely absorbed from the gastrointestinal (GI) tract. Following absorption, Acetylsalicylic acid is hydrolyzed to salicylic acid with peak plasma levels of salicylic acid occurring within 1-2 hours of dosing. The rate of absorption from the GI tract is dependent upon the dosage form, the presence or absence of food, gastric ph(the presence or absence of GI antacids or buffering agents), and other physiologic factors. Metabolism Acetylsalicylic acid is rapidly hydrolyzed in the plasma to salicylic acid such that plasma levels of Acetylsalicylic acid are essentially undetectable 1-2 hours after dosing. Salicylic acid is primarily conjugated in the liver to form salicyluric acid, a phenolic glucuronide, an acyl glucuronide, and a number of minor metabolites. Salicylic acid has a plasma half-life of approximately 6 hours. Salicylate metabolism is saturable and total body clearance decreases at higher serum concentrations due to the limited ability of the liver to form both salicyluric acid and phenolic glucuronide. Following toxic doses (10-20 grams (g)), the plasma half-life may be increased to over 20 hours.
2 Distribution Salicylic acid is widely distributed to all tissues and fluids In the body including the central nervous system(cns), breast milk and fetal tissues. The highest concentrations are found in the plasma, liver, renal cortex, heart and lungs. Excretion The elimination of salicylic acid is constant in relation to plasma concentration. Renal excretion of unchanged drug depends upon urine ph. As urinary ph rises above 6.5, the renal clearance of free salicylate increases from 5 percent to 80 percent. Elimination Half-life Salicylic acid: approximately 6 h. Indication It is indicated for temporary relief of headaches, toothache, neuralgia and muscular pains as well as colds, influenza complaints, and fever. Recommended Dosage Adult dosage 500mg to 1000mg Acetylsalicylic acid, taken every 4-8 hours as required up to a maximum of 3000mg daily. Pediatric and children dosage Not to be given to children under 16 years of age. Mode of Administration Oral Contraindications Known intolerance to Acetylsalicylic acid or any of the other ingredients in the tablet. Acetylsalicylic acid is contraindicated in patients with active peptic ulceration or a history of peptic ulceration and those with haemophilia or other clotting disorders or gout.known hypersensitivity reactions (e.g. bronchospasm, rhinitis, urticaria) in response to Acetylsalicylic acid or non-steroidal; antiinflammatory drugs. Acetylsalicylic acid should not be taken concurrently with anti-coagulants. Warnings and Precautions Serious GI toxicity such as bleeding, ulceration and perforation can occur at any time, with or without warning symptoms, in patients treated with NSAID therapy. Although minor upper GI problems (e.g. dyspepsia) are common, usually developing early in therapy, prescribers should remain alert for ulceration and bleeding in patients treated with NSAIDs even in the absence of previous GI tract symptoms.
3 Studies to date have not identified any subset of patients not at risk of developing peptic ulceration and bleeding. Patients with prior history of serious GI events and other risk factors associated with peptic ulcer disease (e.g. alcoholism, smoking, and corticosteroid therapy) are at increased risk. Elderly or debilitated patients seem to tolerate ulceration or bleeding less than other individuals and account for most spontaneous reports for fatal GI events. Hypersensitivity to other anti-inflammatory/antirheumatic drugs or other allergenic substances. Allergies (e.g. skin reactions, itching, nettle rash) or asthma, hay fever, nasal polyps, chronic respiratory tract infections. Concomitant treatment with anticoagulant drugs. Gastric or duodenal ulcers or a history of gastrointestinal bleeding. Impaired liver and kidney function. Before surgery (including minor surgery such as dental extractions); the bleeding time can be prolonged. Habitual use of analgesics can lead to permanent kidney damage with the risk of kidney failure.the risk is particularly great when several different analgesics are taken concomitantly. At low doses acetylsalicylic acid reduces the excretion of uric acid. This may cause a gout attack in predisposed patients. Interactions with Other Medicaments The effects of the medicines or groups of substances below may be affected by concomitant treatment with acetylsalicylic acid. Enhanced effects ranging up to an increased risk of side effects: Anticoagulants (e.g. coumarin, heparin) and thrombolytic drugs: acetylsalicylic acid can increase the risk of bleeding if taken before anticoagulant treatment. Other platelet aggregation inhibitors (drugs that inhibit the clumping together of blood platelets), e.g. ticlopidine, Clopidogrel: increased bleeding risk. Drug product containing cortisone or cortisone-like substances (with the exception of products that are applied topically or cortisone replacement therapy for Addison s disease): increased risk for gastrointestinal side effects. Alcohol: increased risk of gastrointestinal ulcers and bleeding. Other analgesics and anti-inflammatory medicines (nonsteroidal anti-inflammatory drugs) and other antirheumatic medicines in general: increased risk for gastrointestinal ulcers and bleeding.
4 Antidiabetics: the blood glucose level can be reduced. Digoxin (a drug to strengthen the heart muscle contraction). Methotrexate (a drug used to treat cancer and certain rheumatic disorders). Valproic acid (a drug used to treat convulsions of the brain). Selective Serotonin Re-uptake inhibitors (certain medicines for treatment of depression): increased risk for gastrointestinal bleeding. Certain antibiotics (sulphonamides and sulphonamide combinations, e.g. sulphamethoxazole / trimethoprim). Triiodothyronine (a medicine for treating hypothyroidism). Weakening of effects: Certain medicines that increase the excretion of urine (diuretics so-called aldosterone antagonists, e.g. spironolactone and canrenoate, and loop diuretics, e.g. furosemide). Certain antihypertension drugs (in particular angiotensin converting enzyme inhibitors). Gout remedies that promote the excretion of uric acid (e.g. probenecid, benzbromarone). Statement on Usage During Pregnancy and Lactation Pregnancy Acetylsalicylic acid used during the third trimester of pregnancy should be avoided because of the known effects of NSAIDs on the fetal cardiovascular system. Salicylate have also been associated with alterations in maternal and neonatal hemostasis mechanisms, decreased birth weight, and with perinatal mortality. Lactation Nursing mothers should avoid using Acetylsalicylic acid. Use of high doses may lead to rashes, platelet abnormalities, and bleeding in nursing infants. Adverse Effects / Undesirable Effects Gastrointestinal Effects Gastrointestinal disorders such as heartburn, nausea, vomiting, abdominal pain. Gastrointestinal bleeding which in very rare cases can lead to iron deficiency anaemia. Gastrointestinal ulcers which in very rare cases can lead to perforation. Elevated liver values have been reported. Neurologic Effects Headache, dizziness, impaired hearing ability, tinnitus can be signs of an overdose.
5 Hematologic effect: Bleeding, e.g. nosebleeds or bleeding gums, possibly with prolongation of the bleeding time. Dermatologic Effects: Hypersensitivity reactions such as skin reactions. Hepatic Effects Elevated liver values. Renal Effects Very rare cases of renal impairment and acute renal failure. Otic: Tinnitus Respiratory: Bronchospasm Other: Angioedema, Reye's syndrome Overdose and Treatment Symptoms Moderate intoxication: Tinnitus, hearing disorders, headache and vertigo are reported in all cases of over dosage Severe intoxication: Fever, hyperventilation, ketosis, respiratory alkalosis, metabolic acidosis, coma, cardiovascular shock, respiratory failure, severe hypoglycaemia. Treatment The moderate intoxication can be can be eliminated by reducing the dose. Meanwhile for the severe intoxication need to be treated at hospital. Gastric lavage and administration of activated carbon, monitoring of the acid-base balance. Alkaline diuresis to attain a urine ph of between 7.5 and 8; increased alkaline diuresis must be considered if the plasma Salicylate concentration exceeds 500 mg/l (3.6 mmol/l) in adults or 300 mg/l (2.2 mmol/l) in children.optionally haemodialysis in cases of severe intoxication. Fluid loss must be compensated and Symptomatic treatment. Storage Conditions [ Store below 30 C, protect from light and moisture ] Dosage Forms and Packaging Available [ Packaging type & pack size] Name and Address of Manufacturer [ Name & full address of manufacturer ] Name and Address of Marketing Authorization Holder [ Name & full address of marketing authorization holder ] Date of Revision of Package Insert [ day/month/year ]
PARACETAMOL REXIDOL. 600 mg Tablet. Analgesic-Antipyretic. Paracetamol 600 mg
 (Insert Text) UL Consumer Health PARACETAMOL REXIDOL 600 mg Tablet Analgesic-Antipyretic FORMULATION Each tablet contains: Paracetamol 600 mg PRODUCT DESCRIPTION Rexidol is a round, yellow, flat, bevel-edged
(Insert Text) UL Consumer Health PARACETAMOL REXIDOL 600 mg Tablet Analgesic-Antipyretic FORMULATION Each tablet contains: Paracetamol 600 mg PRODUCT DESCRIPTION Rexidol is a round, yellow, flat, bevel-edged
Package leaflet: Information for the user. < ASPROFLASH and associated names > 500 mg coated tablet Acetylsalicylic acid
 PACKAGE LEAFLET 1 Package leaflet: Information for the user < ASPROFLASH and associated names > 500 mg coated tablet Acetylsalicylic acid Read all of this leaflet carefully before you start using this
PACKAGE LEAFLET 1 Package leaflet: Information for the user < ASPROFLASH and associated names > 500 mg coated tablet Acetylsalicylic acid Read all of this leaflet carefully before you start using this
Doxylamine succinate belongs to the ethanolamine class of antihistamines with sedative properties.
 Data Sheet MERSYNDOL Tablet Paracetamol 450mg per tablet Codeine Phosphate 9.75mg per tablet Doxylamine Succinate 5mg per tablet MERSYNDOL FORTE Tablet Paracetamol 450mg per tablet Codeine Phosphate 30mg
Data Sheet MERSYNDOL Tablet Paracetamol 450mg per tablet Codeine Phosphate 9.75mg per tablet Doxylamine Succinate 5mg per tablet MERSYNDOL FORTE Tablet Paracetamol 450mg per tablet Codeine Phosphate 30mg
patient group direction
 DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
There is a risk of renal impairment in dehydrated children and adolescents.
 PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
NSAID PREPARATIONS. COMPOSITION : Each enteric coated tablet contains Diclofenac Sodium BP 25 mg. Clofenac 50. Clofenac SR. Sodium BP 50 mg.
 Diclofenac Analgesic & Anti-inflammatory COMPOSITION Clofenac 25 : Each enteric coated tablet contains Diclofenac Sodium BP 25 mg. Clofenac 50 : Each enteric coated tablet contains Diclofenac Sodium BP
Diclofenac Analgesic & Anti-inflammatory COMPOSITION Clofenac 25 : Each enteric coated tablet contains Diclofenac Sodium BP 25 mg. Clofenac 50 : Each enteric coated tablet contains Diclofenac Sodium BP
EFFIMET 1000 XR Metformin Hydrochloride extended release tablet
 BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
SUMMARY OF PRODUCT CHARACTERISTICS. Each tablet contains aspirin 75mg. Also contains lactose.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Dispersible Aspirin 75mg Tablets BP 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains aspirin 75mg. Also contains lactose.
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Dispersible Aspirin 75mg Tablets BP 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains aspirin 75mg. Also contains lactose.
P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER. 500 mg, film-coated tablet Active substance: metformin hydrochloride
 P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER Siofor 500 500 mg, film-coated tablet Active substance: metformin hydrochloride For use in children above 10 years and adults Read all of this leaflet
P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER Siofor 500 500 mg, film-coated tablet Active substance: metformin hydrochloride For use in children above 10 years and adults Read all of this leaflet
UpRight Aceclofenac 100 mg and Paracetamol 500 mg fixed dose combination
 For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only UpRight Aceclofenac 100 mg and Paracetamol 500 mg fixed dose combination DESCRIPTION UPRIGHT is a fixed dose combination
For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only UpRight Aceclofenac 100 mg and Paracetamol 500 mg fixed dose combination DESCRIPTION UPRIGHT is a fixed dose combination
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2016 FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK OR
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
PACKAGE LEAFLET: INFORMATION FOR THE USER. PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion. Paracetamol
 PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid
 Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
PACKAGE LEAFLET: INFORMATION FOR THE USER Paracetamol 500 mg Effervescent Tablets Paracetamol
 PACKAGE LEAFLET: INFORMATION FOR THE USER Paracetamol 500 mg Effervescent Tablets Paracetamol Read all of this leaflet carefully because it contains important information for you. This medicine is available
PACKAGE LEAFLET: INFORMATION FOR THE USER Paracetamol 500 mg Effervescent Tablets Paracetamol Read all of this leaflet carefully because it contains important information for you. This medicine is available
White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other.
 Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
Stowe School Medications Policy
 INTRODUCTION Most pupils will need medication at some stage of their school life. Although this will mainly be for short periods there are a few pupils with chronic conditions who may require regular medication
INTRODUCTION Most pupils will need medication at some stage of their school life. Although this will mainly be for short periods there are a few pupils with chronic conditions who may require regular medication
PIAX PLUS ASPIRIN 75/100 Film-Coated Tablets contains the active ingredients clopidogrel and aspirin
 PIAX PLUS ASPIRIN 75/100 Film-Coated Tablets contains the active ingredients clopidogrel and aspirin Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about
PIAX PLUS ASPIRIN 75/100 Film-Coated Tablets contains the active ingredients clopidogrel and aspirin Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about
DATA SHEET. This product is may not be interchangeable with other products containing this ingredient in the New Zealand Market.
 DATA SHEET METFORMIN GENERIC HEALTH Metformin hydrochloride 500mg and 850mg tablets This product is may not be interchangeable with other products containing this ingredient in the New Zealand Market.
DATA SHEET METFORMIN GENERIC HEALTH Metformin hydrochloride 500mg and 850mg tablets This product is may not be interchangeable with other products containing this ingredient in the New Zealand Market.
Clopidogrel Winthrop Plus Aspirin
 Aspirin Clopidogrel (clop-id-o(h)-grel) and Aspirin (as-per-in) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Clopidogrel Winthrop Plus Aspirin
Aspirin Clopidogrel (clop-id-o(h)-grel) and Aspirin (as-per-in) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Clopidogrel Winthrop Plus Aspirin
Acid-Base Balance and the Anion Gap
 Acid-Base Balance and the Anion Gap 1. The body strives for electrical neutrality. a. Cations = Anions b. One of the cations is very special, H +, and its concentration is monitored and regulated very
Acid-Base Balance and the Anion Gap 1. The body strives for electrical neutrality. a. Cations = Anions b. One of the cations is very special, H +, and its concentration is monitored and regulated very
Safety Information Card for Xarelto Patients
 Safety Information Card for Xarelto Patients 15mg Simply Protecting More Patients 20mg Simply Protecting More Patients Keep this card with you at all times Present this card to every physician or dentist
Safety Information Card for Xarelto Patients 15mg Simply Protecting More Patients 20mg Simply Protecting More Patients Keep this card with you at all times Present this card to every physician or dentist
PRODUCT INFORMATION PANAMAX
 NAME OF THE MEDICINE Non-proprietary Name Paracetamol PRODUCT INFORMATION PANAMAX DESCRIPTION Each tablet contains paracetamol 500 mg. The inactive ingredients are: maize starch, purified talc, pregelatinised
NAME OF THE MEDICINE Non-proprietary Name Paracetamol PRODUCT INFORMATION PANAMAX DESCRIPTION Each tablet contains paracetamol 500 mg. The inactive ingredients are: maize starch, purified talc, pregelatinised
SUMMARY OF PRODUCT CHARACTERISTICS BREXIN. 3. Pharmaceutical Form Tablet Pale yellow, hexagonal tablet with a median score line on one side
 פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר SUMMARY OF PRODUCT CHARACTERISTICS 1. Name of the Medicinal Product BREXIN 20 mg TABLETS BREXIN 2. Qualitative and Quantitative Composition Each tablet
פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר SUMMARY OF PRODUCT CHARACTERISTICS 1. Name of the Medicinal Product BREXIN 20 mg TABLETS BREXIN 2. Qualitative and Quantitative Composition Each tablet
Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust.
 Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust. This Patient Group Direction (PGD) has been written
Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust. This Patient Group Direction (PGD) has been written
4 Clinical Particulars
 SUMMARY OF PRODUCT CHARACTERISTICS 1 Name of the Medicinal Product Procyclidine Syrup 5mg/5ml 2. Qualitative and Quantitative Composition Each 5ml dose contains 5mg Procyclidine Hydrochloride BP. 3. Pharmaceutical
SUMMARY OF PRODUCT CHARACTERISTICS 1 Name of the Medicinal Product Procyclidine Syrup 5mg/5ml 2. Qualitative and Quantitative Composition Each 5ml dose contains 5mg Procyclidine Hydrochloride BP. 3. Pharmaceutical
The early symptoms of acute salicylism are the triad of gastrointestinal distress, tinnitus or altered hearing, and hyperventilation.
 POISONING SALICYLATES (ASPIRIN) Management Guidelines Emergency Department Princess Margaret Hospital for Children Perth, Western Australia Last reviewed: January 2007 Page 1 of 5 Dr Gary Geelhoed Dr Frank
POISONING SALICYLATES (ASPIRIN) Management Guidelines Emergency Department Princess Margaret Hospital for Children Perth, Western Australia Last reviewed: January 2007 Page 1 of 5 Dr Gary Geelhoed Dr Frank
PACKAGE LEAFLET: INFORMATION FOR THE USER Omeprazol XXX 40 mg powder for solution for infusion omeprazole
 PACKAGE LEAFLET: INFORMATION FOR THE USER Omeprazol XXX 40 mg powder for solution for infusion omeprazole Read all of this leaflet carefully before you start using this medicine. - Keep this leaflet. You
PACKAGE LEAFLET: INFORMATION FOR THE USER Omeprazol XXX 40 mg powder for solution for infusion omeprazole Read all of this leaflet carefully before you start using this medicine. - Keep this leaflet. You
What Alcohol Does to the Body. Chapter 25 Lesson 2
 What Alcohol Does to the Body Chapter 25 Lesson 2 Short-Term Effects of Drinking The short-term term effects of alcohol on the body depend on several factors including: amount of alcohol consumed, gender,
What Alcohol Does to the Body Chapter 25 Lesson 2 Short-Term Effects of Drinking The short-term term effects of alcohol on the body depend on several factors including: amount of alcohol consumed, gender,
This leaflet answers some common questions about ARROW - MELOXICAM.
 Arrow - Meloxicam Meloxicam Tablets 7.5mg What is in this leaflet This leaflet answers some common questions about ARROW - MELOXICAM. It does not contain all of the available information. It does not take
Arrow - Meloxicam Meloxicam Tablets 7.5mg What is in this leaflet This leaflet answers some common questions about ARROW - MELOXICAM. It does not contain all of the available information. It does not take
Warfarin therapy for stroke patients with atrial fibrillation
 Warfarin therapy for stroke patients with atrial fibrillation Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
Warfarin therapy for stroke patients with atrial fibrillation Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
Elderly: in elderly patients there is no need to reduce the daily dosage (see section 5.2).
 Mesulid-DL-Feb 2012-02 Doctor Leaflet 1. NAME OF THE MEDICINAL PRODUCT Mesulid 100 mg caplets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each caplet contains 100mg nimesulide. For excipients, see section
Mesulid-DL-Feb 2012-02 Doctor Leaflet 1. NAME OF THE MEDICINAL PRODUCT Mesulid 100 mg caplets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each caplet contains 100mg nimesulide. For excipients, see section
Elements for a public summary. VI.2.1 Overview of disease epidemiology. VI.2.2 Summary of treatment benefits
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Pain is one of the most common reasons for a patient to seek medical attention. Moderate or severe intensity pain can be acute
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Pain is one of the most common reasons for a patient to seek medical attention. Moderate or severe intensity pain can be acute
Nōdia DESCRIPTION PHARMACOLOGY. Loperamide hydrochloride USP 2 mg Tablets. Pharmacological classification - antidiarrhoeal.
 Nōdia Loperamide hydrochloride USP 2 mg Tablets DESCRIPTION Nōdia 2 mg tablets are green, capsule-shaped tablets with a break-line on one side. Each tablet contains 2 mg loperamide hydrochloride and typically
Nōdia Loperamide hydrochloride USP 2 mg Tablets DESCRIPTION Nōdia 2 mg tablets are green, capsule-shaped tablets with a break-line on one side. Each tablet contains 2 mg loperamide hydrochloride and typically
PATIENT INFORMATION LEAFLET. CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN
 PATIENT INFORMATION LEAFLET CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
PATIENT INFORMATION LEAFLET CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
A. Ketorolac*** B. Naproxen C. Ibuprofen D. Celecoxib
 1. A man, 66 years of age, with a history of knee osteoarthritis (OA) is experiencing increasing pain at rest and with physical activity. He also has a history of depression and coronary artery disease.
1. A man, 66 years of age, with a history of knee osteoarthritis (OA) is experiencing increasing pain at rest and with physical activity. He also has a history of depression and coronary artery disease.
Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada
 Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
Adjunctive psychosocial intervention. Conditions requiring dose reduction. Immediate, peak plasma concentration is reached within 1 hour.
 Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
PACKAGE LEAFLET: INFORMATION FOR THE USER. ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection. Adrenaline (Levorenine, Epinephrine)
 PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully before you start using this
PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully before you start using this
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1.3.1.3 PACKAGE LEAFLET
 Sandoz Business use only Page 1 of 8 1.3.1.3 PACKAGE LEAFLET Sandoz Business use only Page 2 of 8 Package leaflet: Information for the patient Cefuhexal 250 mg film-coated tablets Cefuhexal 500 mg film-coated
Sandoz Business use only Page 1 of 8 1.3.1.3 PACKAGE LEAFLET Sandoz Business use only Page 2 of 8 Package leaflet: Information for the patient Cefuhexal 250 mg film-coated tablets Cefuhexal 500 mg film-coated
Paracetamol apollo +9191 46 950 950. Paracetamol apollo +9191 46 950 950. Paracetamol
 Paracetamol apollo +9191 46 950 950 Paracetamol apollo +9191 46 950 950 Paracetamol CAS Number : 103-90-2 Molecular Weight : 151.17 g/mol Molecular Formula : C8H9NO2 Systematic (IUPAC) : N-(4- hydroxyphenyl)ethanamide
Paracetamol apollo +9191 46 950 950 Paracetamol apollo +9191 46 950 950 Paracetamol CAS Number : 103-90-2 Molecular Weight : 151.17 g/mol Molecular Formula : C8H9NO2 Systematic (IUPAC) : N-(4- hydroxyphenyl)ethanamide
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
Important: Please Read PART III: CONSUMER INFORMATION
 GlucoNorm 0.5 mg, 1 mg and 2 mg Repaglinide Tablets Oral anti-diabetic agent Important: Please Read PART III: CONSUMER INFORMATION Read this carefully before you start taking GlucoNorm and each time you
GlucoNorm 0.5 mg, 1 mg and 2 mg Repaglinide Tablets Oral anti-diabetic agent Important: Please Read PART III: CONSUMER INFORMATION Read this carefully before you start taking GlucoNorm and each time you
Emory Eye Center New Patient Questionnaire
 Patient Name: Date: Current Address: Current Phone: Date of Birth: Primary Care Physician: Referring Physician: (First & Last Name) (First & Last Name) Pharmacy Name: Phone #: ( ) Please answer all questions
Patient Name: Date: Current Address: Current Phone: Date of Birth: Primary Care Physician: Referring Physician: (First & Last Name) (First & Last Name) Pharmacy Name: Phone #: ( ) Please answer all questions
Essential Shared Care Agreement Drugs for Dementia
 Ref No. E040 Essential Shared Care Agreement Drugs for Dementia Please complete the following details: Patient s name, address, date of birth Consultant s contact details (p.3) And send One copy to: 1.
Ref No. E040 Essential Shared Care Agreement Drugs for Dementia Please complete the following details: Patient s name, address, date of birth Consultant s contact details (p.3) And send One copy to: 1.
Nurse Initiated Medications Procedure
 1. Purpose This Procedure is performed as a means of ensuring the safe administration of therapeutic medication to patients in accordance with all legislative and regulatory requirements. 2. Application
1. Purpose This Procedure is performed as a means of ensuring the safe administration of therapeutic medication to patients in accordance with all legislative and regulatory requirements. 2. Application
SR 5. W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1
 W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1 alfuzosin SR 5 mg (Alfuzosin HCI) COMPOSITION Each sustained-release, film-coated tablet contains: Alfuzosin hydrochloride......... 5 mg Excipients... q.s.
W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1 alfuzosin SR 5 mg (Alfuzosin HCI) COMPOSITION Each sustained-release, film-coated tablet contains: Alfuzosin hydrochloride......... 5 mg Excipients... q.s.
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension
 VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
An Introduction to the Improved FDA Prescription Drug Labeling
 An Introduction to the Improved FDA Prescription Drug Labeling 1 Introduction Mary E. Kremzner, Pharm.D. CDR, U.S. Public Health Service Deputy Director, Division of Drug Information Center for Drug Evaluation
An Introduction to the Improved FDA Prescription Drug Labeling 1 Introduction Mary E. Kremzner, Pharm.D. CDR, U.S. Public Health Service Deputy Director, Division of Drug Information Center for Drug Evaluation
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
PACKAGE LEAFLET VITAMIN C
 PACKAGE LEAFLET VITAMIN C ATC code: A11GA01 PHARMACOTHERAPEUTIC GROUP Vitamins. COMPOSITION Ascorbic acid 100 mg or 500 mg in 1 tablet; 600 mg or 1000 mg in 1 effervescent tablet. ACTION As a redoxy-compound,
PACKAGE LEAFLET VITAMIN C ATC code: A11GA01 PHARMACOTHERAPEUTIC GROUP Vitamins. COMPOSITION Ascorbic acid 100 mg or 500 mg in 1 tablet; 600 mg or 1000 mg in 1 effervescent tablet. ACTION As a redoxy-compound,
EMEA PUBLIC STATEMENT ON LEFLUNOMIDE (ARAVA) - SEVERE AND SERIOUS HEPATIC REACTIONS -
 The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
MEDICATION GUIDE ELIQUIS (ELL eh kwiss) (apixaban) tablets
 MEDICATION GUIDE ELIQUIS (ELL eh kwiss) (apixaban) tablets What is the most important information I should know about ELIQUIS? For people taking ELIQUIS for atrial fibrillation: People with atrial fibrillation
MEDICATION GUIDE ELIQUIS (ELL eh kwiss) (apixaban) tablets What is the most important information I should know about ELIQUIS? For people taking ELIQUIS for atrial fibrillation: People with atrial fibrillation
Perfalgan 10 mg/ml, solution for infusion
 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfalgan 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need
PACKAGE LEAFLET: INFORMATION FOR THE USER Perfalgan 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need
Adams Memorial Hospital Decatur, Indiana EXPLANATION OF LABORATORY TESTS
 Adams Memorial Hospital Decatur, Indiana EXPLANATION OF LABORATORY TESTS Your health is important to us! The test descriptions listed below are for educational purposes only. Laboratory test interpretation
Adams Memorial Hospital Decatur, Indiana EXPLANATION OF LABORATORY TESTS Your health is important to us! The test descriptions listed below are for educational purposes only. Laboratory test interpretation
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel
 Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
Paxil/Paxil-CR (paroxetine)
 Generic name: Paroxetine Available strengths: 10 mg, 20 mg, 30 mg, 40 mg tablets; 10 mg/5 ml oral suspension; 12.5 mg, 25 mg, 37.5 mg controlled-release tablets (Paxil-CR) Available in generic: Yes, except
Generic name: Paroxetine Available strengths: 10 mg, 20 mg, 30 mg, 40 mg tablets; 10 mg/5 ml oral suspension; 12.5 mg, 25 mg, 37.5 mg controlled-release tablets (Paxil-CR) Available in generic: Yes, except
Calcium Folinate Ebewe Data Sheet
 NAME OF THE MEDICINE Calcium folinate injection Composition Active: Calcium folinate (equivalent to 10 mg folinic acid per ml) Inactive: Sodium chloride (7.7mg/mL), qs Water for Injections. Preservative
NAME OF THE MEDICINE Calcium folinate injection Composition Active: Calcium folinate (equivalent to 10 mg folinic acid per ml) Inactive: Sodium chloride (7.7mg/mL), qs Water for Injections. Preservative
PATIENT HISTORY FORM
 PATIENT HISTORY FORM If you are new to the office, have not been seen in over one (1) year, or are returning for a new problem, please complete this form in full. If there have been any changes since your
PATIENT HISTORY FORM If you are new to the office, have not been seen in over one (1) year, or are returning for a new problem, please complete this form in full. If there have been any changes since your
2 What you need to know before you have Ampiclox
 Reason for update: GDS 14 & QRD Updates Response to questions for variation update section 4.1 of SPC MHRA Submission Date: 6 November 2014 MHRA Approval Date: Text Date: October 2014 Text Issue and Draft
Reason for update: GDS 14 & QRD Updates Response to questions for variation update section 4.1 of SPC MHRA Submission Date: 6 November 2014 MHRA Approval Date: Text Date: October 2014 Text Issue and Draft
It can therefore be bought without a prescription but should be used correctly to ensure its efficacy and to reduce side effects.
 140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
New Zealand Data Sheet. Tablet - 12.7mm round, flat bevelled edge, white, with "C" and "O" embossed on one side of break-bar
 CODALGIN New Zealand Data Sheet Paracetamol 500 mg, Codeine Phosphate 8 mg Presentation Tablet - 12.7mm round, flat bevelled edge, white, with "C" and "O" embossed on one side of break-bar Uses Actions
CODALGIN New Zealand Data Sheet Paracetamol 500 mg, Codeine Phosphate 8 mg Presentation Tablet - 12.7mm round, flat bevelled edge, white, with "C" and "O" embossed on one side of break-bar Uses Actions
Acid/Base Homeostasis (Part 4)
 Acid/Base Homeostasis (Part 4) Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) 5. The newly formed bicarbonate moves into the plasma.
Acid/Base Homeostasis (Part 4) Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) 5. The newly formed bicarbonate moves into the plasma.
MEDICATION GUIDE COUMADIN (COU-ma-din) (warfarin sodium)
 MEDICATION GUIDE COUMADIN (COU-ma-din) (warfarin sodium) Read this Medication Guide before you start taking COUMADIN (warfarin sodium) and each time you get a refill. There may be new information. This
MEDICATION GUIDE COUMADIN (COU-ma-din) (warfarin sodium) Read this Medication Guide before you start taking COUMADIN (warfarin sodium) and each time you get a refill. There may be new information. This
Medication Guide Plavix (PLAV-iks) (clopidogrel bisulfate) tablets
 Medication Guide Plavix (PLAV-iks) (clopidogrel bisulfate) tablets Read this Medication Guide before you start taking Plavix and each time you get a refill. There may be new information. This Medication
Medication Guide Plavix (PLAV-iks) (clopidogrel bisulfate) tablets Read this Medication Guide before you start taking Plavix and each time you get a refill. There may be new information. This Medication
1MFBTF GJMM PVU GPSNT BOE GBY 'PSNT XJMM CF TJHOFE BU ZPVS BQQPJOUNFOU
 CELL PHONE: PATIENT HISTORY FORM - CONFIDENTIAL DATE: PATIENT: (LAST NAME) (FIRST NAME) (Ml) (NICKNAME) DOB: Primary Physician/ Family Doctor: Phone: Past Medical History (Click all that apply) High blood
CELL PHONE: PATIENT HISTORY FORM - CONFIDENTIAL DATE: PATIENT: (LAST NAME) (FIRST NAME) (Ml) (NICKNAME) DOB: Primary Physician/ Family Doctor: Phone: Past Medical History (Click all that apply) High blood
DRUG INTERACTIONS: WHAT YOU SHOULD KNOW. Council on Family Health
 DRUG INTERACTIONS: WHAT YOU SHOULD KNOW Council on Family Health Drug Interactions There are more opportunities today than ever before to learn about your health and to take better care of yourself. It
DRUG INTERACTIONS: WHAT YOU SHOULD KNOW Council on Family Health Drug Interactions There are more opportunities today than ever before to learn about your health and to take better care of yourself. It
PACKAGE LEAFLET: INFORMATION FOR THE USER. Methotrexate 2.5 mg Tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER Methotrexate 2.5 mg Tablets Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. Keep this
PACKAGE LEAFLET: INFORMATION FOR THE USER Methotrexate 2.5 mg Tablets Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. Keep this
MEDICATION GUIDE Savella (Sa-vel-la) (milnacipran HCl) Tablets
 MEDICATION GUIDE Savella (Sa-vel-la) (milnacipran HCl) Tablets Savella is not used to treat depression, but it acts like medicines that are used to treat depression (antidepressants) and other psychiatric
MEDICATION GUIDE Savella (Sa-vel-la) (milnacipran HCl) Tablets Savella is not used to treat depression, but it acts like medicines that are used to treat depression (antidepressants) and other psychiatric
Rivaroxaban: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF
 Leeds Rivaroxaban: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF Amber Drug Level 3 (amber drug with monitoring requirements) We have started your
Leeds Rivaroxaban: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF Amber Drug Level 3 (amber drug with monitoring requirements) We have started your
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Methadone 10mg/ml Injection / Physeptone 10mg/ml Injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Contains: Methadone Hydrochloride
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Methadone 10mg/ml Injection / Physeptone 10mg/ml Injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Contains: Methadone Hydrochloride
Analytical Specifications RIVAROXABAN
 Page 1 of 9 ANALYTE NAME AND STRUCTURE - RIVAROXABAN SYNONYMS Xarelto CATEGORY Anticoagulant TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND Xarelto (rivaroxaban) is an orally
Page 1 of 9 ANALYTE NAME AND STRUCTURE - RIVAROXABAN SYNONYMS Xarelto CATEGORY Anticoagulant TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND Xarelto (rivaroxaban) is an orally
Informed Consent for Laparoscopic Vertical Sleeve Gastrectomy. Patient Name
 Informed Consent for Laparoscopic Vertical Sleeve Gastrectomy Patient Name Please read this form carefully and ask about anything you may not understand. I consent to have a laparoscopic Vertical Sleeve
Informed Consent for Laparoscopic Vertical Sleeve Gastrectomy Patient Name Please read this form carefully and ask about anything you may not understand. I consent to have a laparoscopic Vertical Sleeve
I B2.4. Design of the patient information leaflet for VariQuin
 (English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
(English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
Daily aspirin therapy: Understand the benefits and risks
 Daily aspirin therapy: Understand the benefits and risks Is an aspirin a day the right thing for you? It's not as easy a decision as it sounds. Know the benefits and risks before considering daily aspirin
Daily aspirin therapy: Understand the benefits and risks Is an aspirin a day the right thing for you? It's not as easy a decision as it sounds. Know the benefits and risks before considering daily aspirin
Borland-Groover Clinic PATIENT GENERATED MEDICAL HISTORY Name: DOB: Email: Primary Care Physician: Pharmacy: Pharmacy Phone #:
 PATIENT GENERATED MEDICAL HISTORY Name: DOB: Email: Primary Care Physician: Referring: Pharmacy: Pharmacy Phone #: Place Sticker Here Directions: Please circle any of the following you have personally
PATIENT GENERATED MEDICAL HISTORY Name: DOB: Email: Primary Care Physician: Referring: Pharmacy: Pharmacy Phone #: Place Sticker Here Directions: Please circle any of the following you have personally
Upstate University Health System Medication Exam - Version A
 Upstate University Health System Medication Exam - Version A Name: ID Number: Date: Unit: Directions: Please read each question below. Choose the best response for each of the Multiple Choice and Medication
Upstate University Health System Medication Exam - Version A Name: ID Number: Date: Unit: Directions: Please read each question below. Choose the best response for each of the Multiple Choice and Medication
Peptic Ulcer. Anatomy The stomach is a hollow organ. It is located in the upper abdomen, under the ribs.
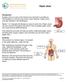 Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
Patient Medication Guide Brochure
 Patient Medication Guide Brochure 1 MEDICATION GUIDE TASIGNA (ta-sig-na) (nilotinib) Capsules Read this Medication Guide before you start taking TASIGNA and each time you get a refill. There may be new
Patient Medication Guide Brochure 1 MEDICATION GUIDE TASIGNA (ta-sig-na) (nilotinib) Capsules Read this Medication Guide before you start taking TASIGNA and each time you get a refill. There may be new
Medical Nutrition Therapy for Upper Gastrointestinal Tract Disorders. By: Jalal Hejazi PhD, MSc.
 Medical Nutrition Therapy for Upper Gastrointestinal Tract Disorders By: Jalal Hejazi PhD, MSc. Digestive Disorders Common problem; more than 50 million outpatient visits per year Dietary habits and nutrition
Medical Nutrition Therapy for Upper Gastrointestinal Tract Disorders By: Jalal Hejazi PhD, MSc. Digestive Disorders Common problem; more than 50 million outpatient visits per year Dietary habits and nutrition
SUMMARY OF PRODUCT CHARACTERISTICS. Paracetamol...10.00 mg for 1 ml of solution for infusion
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol...10.00 mg for 1 ml of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol...10.00 mg for 1 ml of
Dallas Neurosurgical and Spine Associates, P.A Patient Health History
 Dallas Neurosurgical and Spine Associates, P.A Patient Health History DOB: Date: Reason for your visit (Chief complaint): Past Medical History Please check corresponding box if you have ever had any of
Dallas Neurosurgical and Spine Associates, P.A Patient Health History DOB: Date: Reason for your visit (Chief complaint): Past Medical History Please check corresponding box if you have ever had any of
For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only OR for Specialist Use only
 For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only OR for Specialist Use only Cholecalciferol Granules VITOMIN D3 COMPOSITION Each sachet of 1 g contains: Cholecalciferol
For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only OR for Specialist Use only Cholecalciferol Granules VITOMIN D3 COMPOSITION Each sachet of 1 g contains: Cholecalciferol
Review of Pharmacological Pain Management
 Review of Pharmacological Pain Management CHAMP Activities are possible with generous support from The Atlantic Philanthropies and The John A. Hartford Foundation The WHO Pain Ladder The World Health Organization
Review of Pharmacological Pain Management CHAMP Activities are possible with generous support from The Atlantic Philanthropies and The John A. Hartford Foundation The WHO Pain Ladder The World Health Organization
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Librium 10mg Hard capsule Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 10 mg of chlordiazepoxide hydrochloride. Excipients:
1 NAME OF THE MEDICINAL PRODUCT Librium 10mg Hard capsule Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 10 mg of chlordiazepoxide hydrochloride. Excipients:
Digestive System (continued) Digestive System. Stomach. Peptic Ulcer Disease
 Digestive System Digestive System (continued) Responsible for breaking down food, absorbing nutrients, eliminating wastes Alimentary canal Also known as gastrointestinal tract Reaches from mouth to anus
Digestive System Digestive System (continued) Responsible for breaking down food, absorbing nutrients, eliminating wastes Alimentary canal Also known as gastrointestinal tract Reaches from mouth to anus
Medication Guide TASIGNA (ta-sig-na) (nilotinib) Capsules
 Medication Guide TASIGNA (ta-sig-na) (nilotinib) Capsules Read this Medication Guide before you start taking Tasigna and each time you get a refill. There may be new information. This information does
Medication Guide TASIGNA (ta-sig-na) (nilotinib) Capsules Read this Medication Guide before you start taking Tasigna and each time you get a refill. There may be new information. This information does
Public Assessment Report
 Public Assessment Report IBUPROFEN 200 MG TABLETS EXTRA STRENGTH IBUPROFEN 400 MG TABLETS (IBUPROFEN) PL 17907/0159-61 MHRA PAR Ibuprofen Tablets PL 17907/0159-61 - 1 - IBUPROFEN TABLETS (IBUPROFEN) PL
Public Assessment Report IBUPROFEN 200 MG TABLETS EXTRA STRENGTH IBUPROFEN 400 MG TABLETS (IBUPROFEN) PL 17907/0159-61 MHRA PAR Ibuprofen Tablets PL 17907/0159-61 - 1 - IBUPROFEN TABLETS (IBUPROFEN) PL
Allopurinol Allopurinol
 Drug information Allopurinol Allopurinol This leaflet provides information on allopurinol and will answer any questions you have about the treatment. Arthritis Research UK produce and print our booklets
Drug information Allopurinol Allopurinol This leaflet provides information on allopurinol and will answer any questions you have about the treatment. Arthritis Research UK produce and print our booklets
Perrigo Pharmaceuticals Co.
 Ibuprofen Page 1 of 7 Perrigo Pharmaceuticals Co. U.S. Pharmaceuticals Division MATERIAL SAFETY DATA SHEET NDC Product(s) Code(s): 0121-4774-05 0121-4774-10 Product Name: Ibuprofen Oral Suspension 100mg/5mL
Ibuprofen Page 1 of 7 Perrigo Pharmaceuticals Co. U.S. Pharmaceuticals Division MATERIAL SAFETY DATA SHEET NDC Product(s) Code(s): 0121-4774-05 0121-4774-10 Product Name: Ibuprofen Oral Suspension 100mg/5mL
MEDICATION GUIDE KOMBIGLYZE XR (kom-be-glyze X-R) (saxagliptin and metformin HCl extended-release) tablets
 MEDICATION GUIDE KOMBIGLYZE XR (kom-be-glyze X-R) (saxagliptin and metformin HCl extended-release) tablets Read this Medication Guide carefully before you start taking KOMBIGLYZE XR and each time you get
MEDICATION GUIDE KOMBIGLYZE XR (kom-be-glyze X-R) (saxagliptin and metformin HCl extended-release) tablets Read this Medication Guide carefully before you start taking KOMBIGLYZE XR and each time you get
Teriflunomide (Aubagio) 14mg once daily tablet
 Teriflunomide (Aubagio) 14mg once daily tablet Exceptional healthcare, personally delivered Your Consultant Neurologist has suggested that you may benefit from treatment with Teriflunomide. The decision
Teriflunomide (Aubagio) 14mg once daily tablet Exceptional healthcare, personally delivered Your Consultant Neurologist has suggested that you may benefit from treatment with Teriflunomide. The decision
Substance Abuse Treatment. Naltrexone for Extended-Release Injectable Suspension for Treatment of Alcohol Dependence
 Spring 2007 Volume 6 Issue 1 ADVISORY News for the Treatment Field Naltrexone for Extended-Release Injectable Suspension for Treatment of Alcohol Dependence What is naltrexone for extendedrelease injectable
Spring 2007 Volume 6 Issue 1 ADVISORY News for the Treatment Field Naltrexone for Extended-Release Injectable Suspension for Treatment of Alcohol Dependence What is naltrexone for extendedrelease injectable
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
